New preparation method of macitentan intermediate
A technology for macitentan and intermediates, which is applied in the new preparation field of macitentan intermediates, can solve the problems of low product purity, can not be well solved, serious moisture absorption problems, etc., and achieves high product purity. , Improve production efficiency and reduce the effect of salt-forming steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
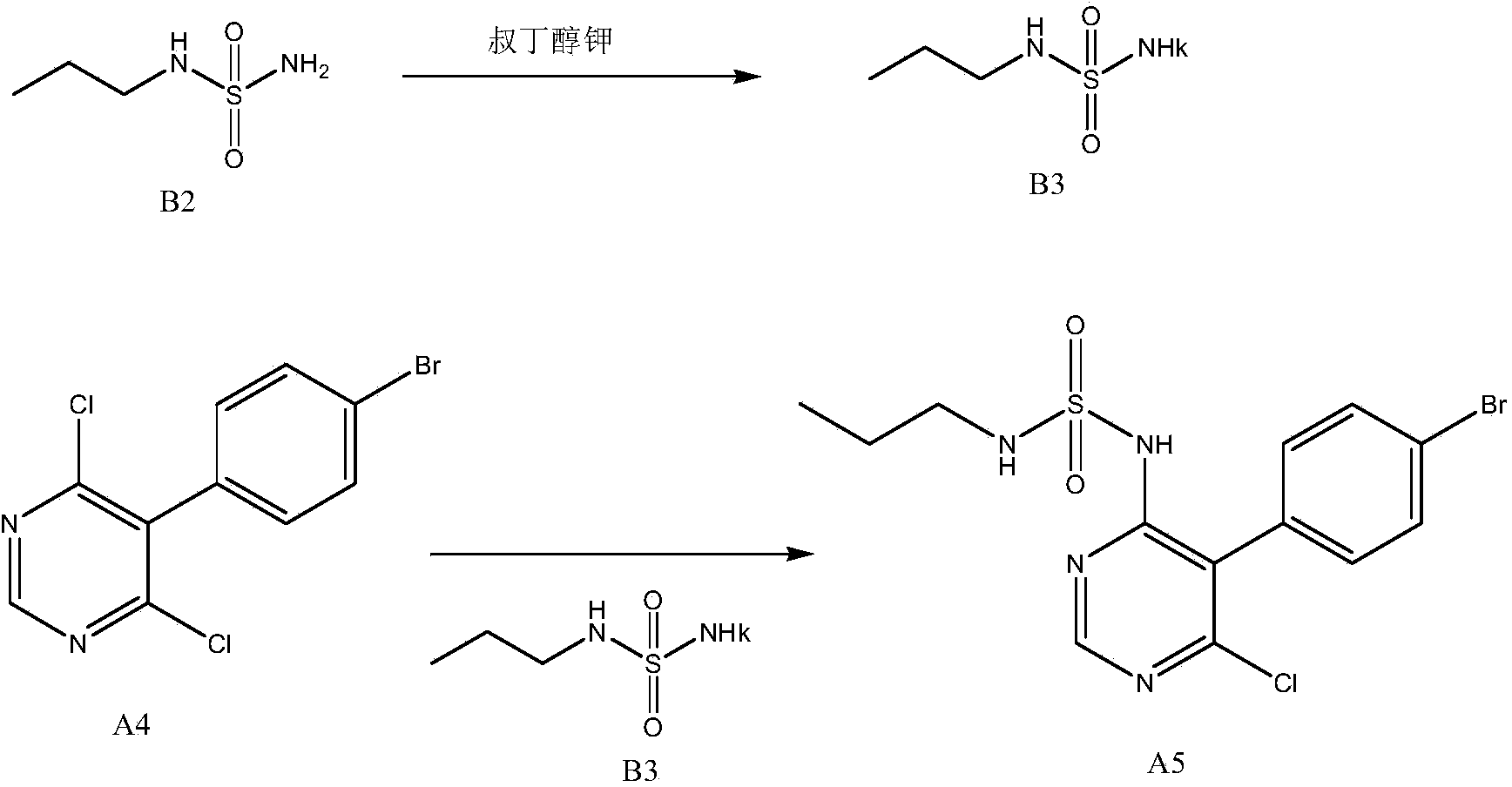
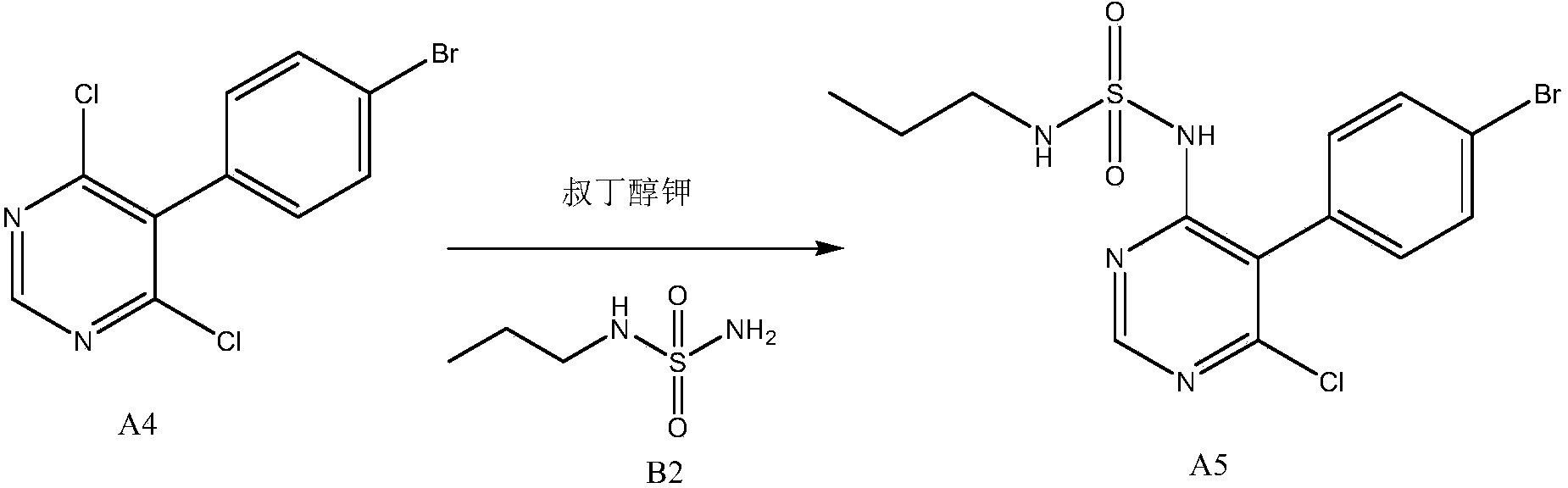
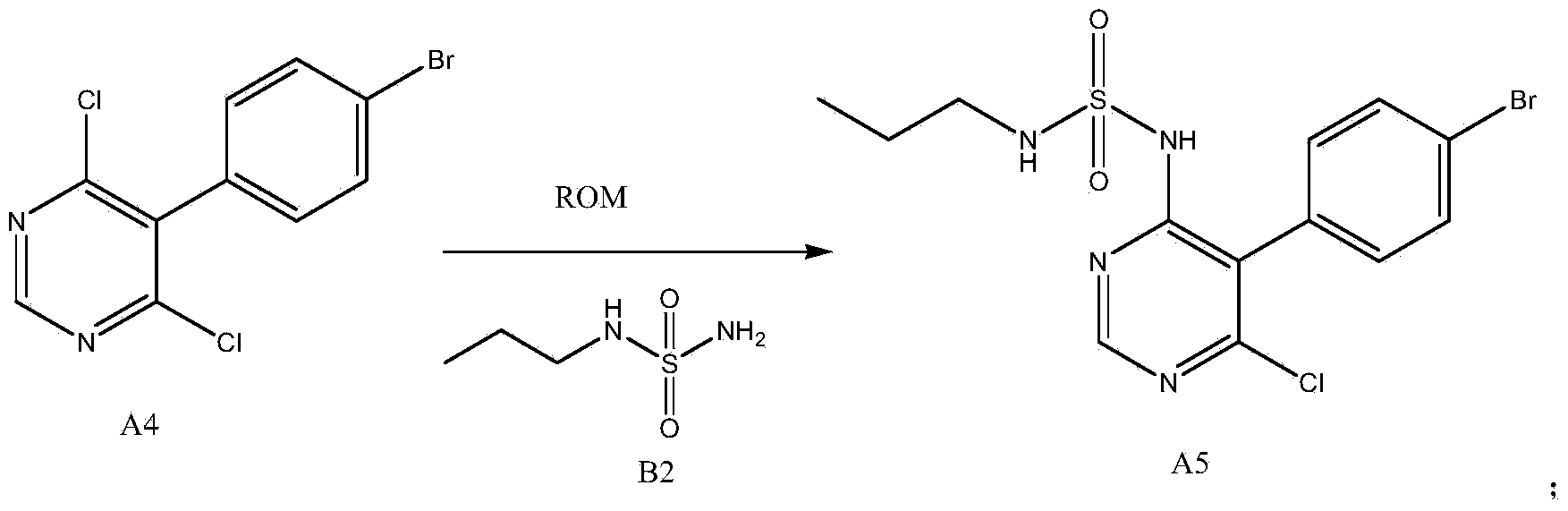
[0035] Such as figure 2 Shown, a kind of new preparation method of macitentan intermediate, this intermediate is N-(5-(4-bromophenyl)-6-chloro-4-pyrimidinyl)-N'-propylsulfonamide , including the following steps:
[0036] N-propylsulfonamide and 5-(4-bromophenyl)-4,6-dichloropyrimidine are added to dimethyl sulfoxide, then alkoxy metal compound ROM is added, and N-( 5-(4-bromophenyl)-6-chloro-4-pyrimidinyl)-N'-propylsulfonamide, its chemical reaction formula is as follows:
[0037]
[0038] in:
[0039] B2: N-propylsulfonamide;
[0040] A4: 5-(4-bromophenyl)-4,6-dichloropyrimidine;
[0041] A5: N-(5-(4-bromophenyl)-6-chloro-4-pyrimidinyl)-N'-propylsulfonamide.
[0042] As mentioned above, R is a C1-C4 alkane, preferably a tert-butyl group. M is an element of the first main group in the periodic table, preferably potassium. Such as figure 2 As shown, ROM is potassium tert-butoxide, because potassium tert-butoxide is a commercially available commodity. The molar rat...
Embodiment 1
[0047] Such as figure 2 Shown, a kind of preparation method of macitentan intermediate N-(5-(4-bromophenyl)-6-chloro-4-pyrimidinyl)-N'-propylsulfonamide comprises the following steps:
[0048] Step 1, add 272g (2.00mol) of N-propylsulfonamide B2 to 500g (1.65mol) of 5-(4-bromophenyl)-4,6-dichloropyrimidine A4 and 5000ml of dimethyl sulfide in sulfone;
[0049] Step 2, adding 220 g (2.00 mol) of potassium tert-butoxide to the reaction liquid, stirring and reacting at room temperature for 4 hours;
[0050]Step 3, adding 25000ml of saturated brine and 25000ml of ethyl acetate to the reaction liquid for extraction, and recrystallizing the organic layer with 2000ml of methanol to obtain N-(5-(4-bromophenyl)-6-chloro-4-pyrimidine Base)-N'-propylsulfonamide A5, 473g; based on A4, the molar yield was 71%; the HPLC purity was 99.2%.
Embodiment 2
[0052] A preparation method of macitentan intermediate N-(5-(4-bromophenyl)-6-chloro-4-pyrimidinyl)-N'-propylsulfonamide, comprising the following steps:
[0053] Step 1, add 272g (2.00mol) of N-propylsulfonamide B2 to 500g (1.65mol) of 5-(4-bromophenyl)-4,6-dichloropyrimidine A4 and 5000ml of dimethyl sulfide in sulfone;
[0054] Step 2, adding 168g (2.00mol) of potassium ethoxide to the reaction solution, stirring and reacting at room temperature for 4 hours;
[0055] Step 3, adding 25000ml of saturated brine and 25000ml of ethyl acetate to the reaction liquid for extraction, and recrystallizing the organic layer with 2000ml of methanol to obtain N-(5-(4-bromophenyl)-6-chloro-4-pyrimidine Base)-N'-propylsulfonamide A5, 446g; based on A4, the molar yield was 67%; HPLC purity was 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com