Method for rapidly controlling and evaluating release of macitentan tablet
A macitentan, rapid control technology, applied in the field of medicine, can solve the problems of no patents for controlling and evaluating the release of macitentan tablets, etc., to ensure effectiveness and safety, improve production quality and requirements, and test quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In production, the raw material of macitentan obtained through the refining process of organic reagents generally has a larger particle size. Samples were taken for microscopic observation, such as figure 1 The physical form of the raw materials shown is flaky crystals, which are relatively brittle.
Embodiment 2
[0036] The macitentan raw material is pulverized by a jet mill to obtain raw materials with different particle sizes. Measure the particle size separately.
[0037] project d(0.1)μm d(0.5)μm d(0.9)μm raw material 1 9.043 43.841 91.298 raw material 2 7.395 25.901 51.082 raw material 3 1.236 9.777 36.03 raw material 4 0.751 2.882 9.807
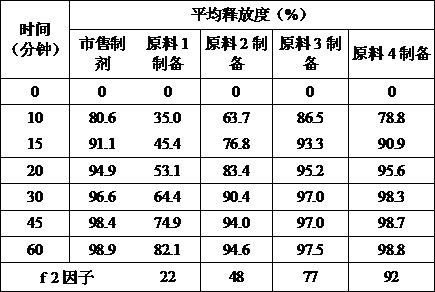
[0038] Adopt above-mentioned raw material of different granularity to prepare macitentan tablet through identical process, carry out dissolution rate and release rate measurement respectively, take pH6.8 phosphate buffer solution 900ml as dissolution medium, rotating speed is 75 revolutions per minute, in 10 minutes, Sampling and testing at 15 minutes, 20 minutes, 30 minutes, 45 minutes and 60 minutes. Compared with the samples of commercially available preparations, comparative research was carried out.
[0039] Table 2 shows the release rate of macitentan tablets in Example 2.
[0040] ...
Embodiment 3
[0043] Dissolution determination is an important index to evaluate the dissolution behavior in vitro. The choice of the dissolution medium has an extremely important effect on the dissolution results. The same batch of macitentan tablets was adopted for dissolution and release determination, respectively adopting pH6.8 phosphate buffer solution containing different proportions of cetyltrimethylammonium bromide as the dissolution medium, and the rotating speed was 75 revolutions per minute, Samples were taken at 10 minutes, 15 minutes, 20 minutes, 30 minutes, 45 minutes and 60 minutes.
[0044] Table 3 shows the release rate of macitentan tablets in Example 3.
[0045]
[0046] Conclusion: The results of dissolution curves are quite different with different concentrations of cetyltrimethylammonium bromide in the dissolution medium. The discriminating power was weaker under the conditions of 0.05% and 0.1% cetyltrimethylammonium bromide in the dissolution medium, and the di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com