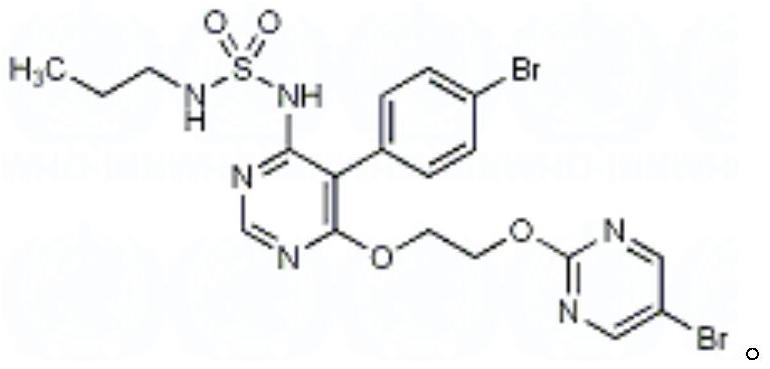
Macitentan solution for inhalation and preparation method of macitentan solution
A macitentan and solution technology, applied in the field of medicine, can solve the problems of no macitentan, etc., and achieve the effect of good curative effect, simple operation and accurate measurement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
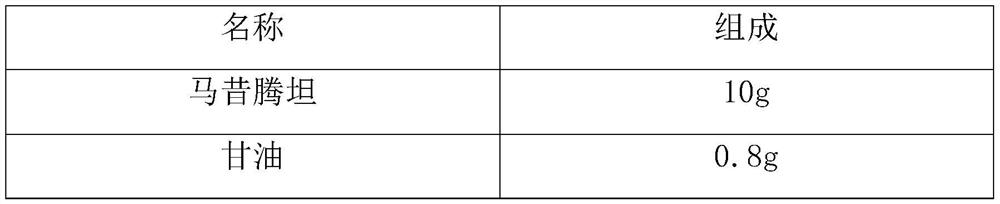
[0031] Prescription composition:
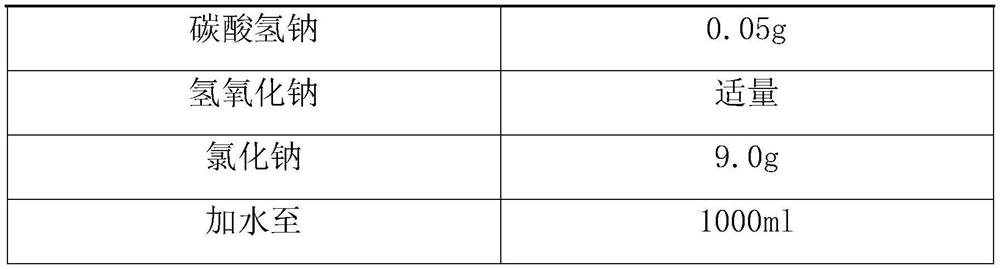
[0032] name composition macitentan 10.0g Tween 80 0.1g citric acid 0.03g Sodium citrate 2g Sodium chloride 8.0g Add water to 1000ml
[0033] Preparation:
[0034] Take 800ml of water for injection, control the water temperature at 45-55°C, add Tween 80 and stir to dissolve, adjust the pH to about 8.0 with citric acid-sodium citrate, add macitentan, stir to dissolve. Continue to add the prescribed amount of sodium chloride, stir to dissolve, set the volume to 1000ml, filter the resulting solution through a sterile filter, and then fill the filtered medicinal solution in a 2ml ampoule to obtain macitentan for inhalation solution.
[0035] The above solutions need to be protected from light during the production process.
Embodiment 2
[0037] Prescription composition:
[0038] name composition macitentan 8.0g Tween 80 0.08g citric acid 0.02g Sodium citrate 1.8g Sodium chloride 8.0g Add water to 1000ml
[0039] Preparation:
[0040] Take 800ml of water for injection, control the water temperature at 45-55°C, add Tween 80 and stir to dissolve, adjust the pH to about 8.0 with citric acid-sodium citrate, add macitentan, stir to dissolve. Continue to add the prescribed amount of sodium chloride, stir to dissolve, set the volume to 1000ml, filter the resulting solution through a sterile filter, and then fill the filtered medicinal solution in a 2ml ampoule to obtain macitentan for inhalation solution.
[0041] The above solutions need to be protected from light during the production process.
Embodiment 3
[0043] Prescription composition:
[0044] name composition macitentan 5.0g Tween 80 0.05g citric acid 0.02g Sodium citrate 1.2g Sodium chloride 8.0g Add water to 1000ml
[0045] Preparation:
[0046] Take 800ml of water for injection, control the water temperature at 45-55°C, add Tween 80 and stir to dissolve, adjust the pH to about 8.0 with citric acid-sodium citrate, add macitentan, stir to dissolve. Continue to add the prescribed amount of sodium chloride, stir to dissolve, set the volume to 1000ml, filter the resulting solution through a sterile filter, and then fill the filtered medicinal solution in a 2ml ampoule to obtain macitentan for inhalation solution.
[0047] The above solutions need to be protected from light during the production process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com