Macitentan related substances, and preparing methods and uses thereof
A technology of macitentan and its use, which is applied in the field of drug synthesis and can solve problems affecting product quality, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
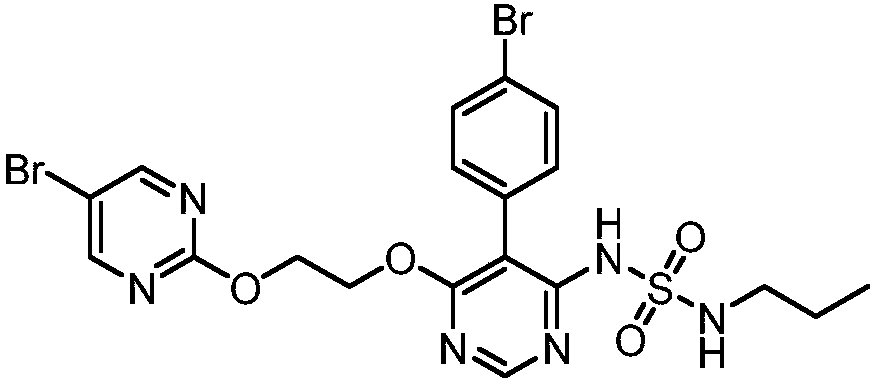
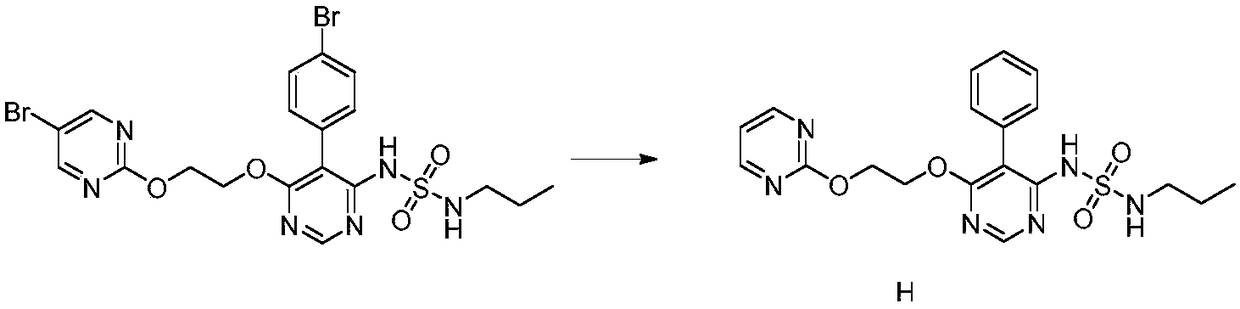
[0036] The preparation of embodiment 1 related substance H
[0037]
[0038] Weigh 3.0g of macitentan into a single-necked bottle, add 30ml of absolute ethanol, 1.5g of Pd / C and 0.6g of ammonium formate, reflux for 4 hours, cool down to room temperature, pour the reaction solution into 300ml of citric acid aqueous solution, stir After 2h, filter with suction, and dry the filter cake to obtain 1.6g of solid. The crude product was recrystallized from methanol to obtain 1.0 g of solid. HPLC purity 100%. 1 H NMR (DMSO-d 6 ): δ9.35(1H,s),8.57(2H,d),8.50(1H,s),7.37(3H,d),7.23(3H,d),7.13(1H,t),4.66(2H, s),4.57(2H,s),2.81(2H,q),1.42(2H,m),0.80(3H,t).ESI(+)m / z 431.3
Embodiment 2
[0039] The preparation of embodiment 2 related substance H
[0040]
[0041] Weigh 3.0g of macitentan into a single-necked bottle, add 30ml of anhydrous methanol and 1.5g of Pd / C, react under hydrogen for 3h, cool down to room temperature, pour the reaction solution into 300ml of citric acid aqueous solution, and stir for 2h , filtered with suction, and the filter cake was dried to obtain 1.5 g of solid. Add 8ml of methanol, reflux for 2h, drop to room temperature, filter with suction, and dry the filter cake to obtain 0.8g of solid, HPLC purity 99.5%.
Embodiment 3
[0042] The preparation of embodiment 3 related substance J
[0043]
[0044] Add 10.0g of intermediate V, 5.1g of ammonium formate, 5.0g of Pd / C and 100ml of absolute ethanol into the reaction flask, heat to reflux for 2h, filter with suction, and rinse with 20ml of absolute ethanol. Pour the filtrate into 300ml of 10% citric acid aqueous solution, add 50ml of ethyl acetate to extract 3 times, dry the organic phase with 40.2g of anhydrous magnesium sulfate, concentrate by suction filtration to obtain a solid, add 10ml of anhydrous methanol to the solid, heat to reflux, and cool to Stir and crystallize at 20°C, filter with suction, rinse with 5 ml of anhydrous methanol, and dry the filter cake to obtain 2.1 g of Ⅴ-6.
[0045] Add 0.7g of sodium hydrogen, 21ml of tetrahydrofuran and 2.1g of Ⅴ-6 to the one-necked flask, stir for 1 hour, add 5ml of DMF and 1.4g of 5-bromo-2-chloropyrimidine, heat to 60°C, and react for 2 hours. Cool down to 25°C, pour the filtrate into 60ml of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com