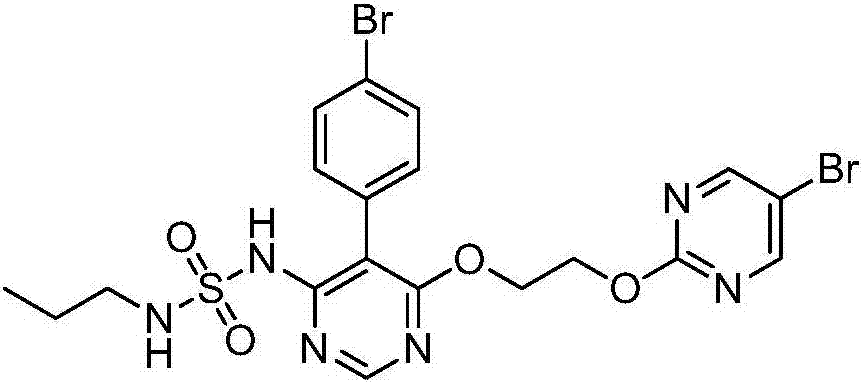
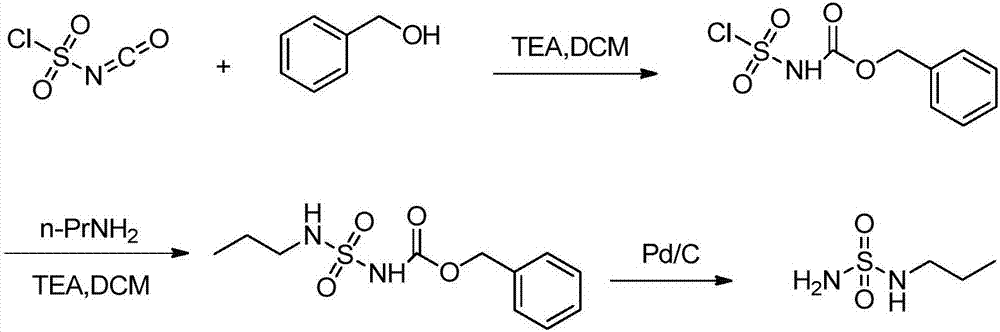
Preparation method of macitentan intermediate
A technology for macitentan and intermediates, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of subsequent purification difficulties, high production costs, and long reaction steps, and achieve short reaction steps and reaction time, low cost, and easy purification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
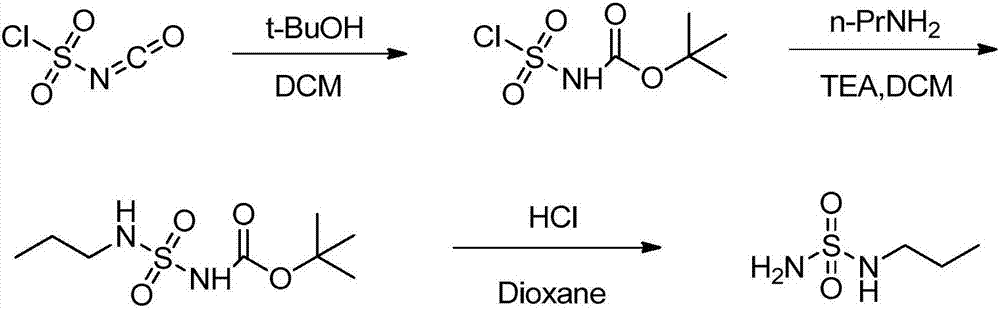
Embodiment 1
[0027] (1) Synthesis of n-propylaminosulfonyl chloride
[0028] Under the protection of nitrogen, put 135g of compound of formula I (1mol) into a clean four-necked reaction flask, add 250ml of dichloromethane, stir and cool down to -10°C, slowly add 59.1g of compound of formula II (1mol) and triethyl A mixed solution of 101.2 g of amine was added dropwise for about 60 minutes. After the dropwise addition was completed, the temperature was controlled and stirred at 10° C. for about 3 hours. The reaction solution was concentrated under reduced pressure to obtain 155 g of the compound of formula III, which could be directly used in the next step.
[0029] (2) Synthesis of n-propylaminosulfonamide
[0030] Under the protection of nitrogen, put 155g of the compound of formula III obtained in the previous step into a clean four-necked reaction flask, add 300ml of dichloromethane, stir, control the temperature at about 20°C, and slowly introduce ammonia gas for about 1 hour. The re...
Embodiment 2
[0034] (1) Synthesis of n-propylaminosulfonyl chloride
[0035] Under the protection of nitrogen, put 135g of the compound of formula I into a clean four-necked reaction flask, add 250ml of dichloromethane, stir and cool down to 0°C, slowly add the mixed solution of 59.1g of the compound of formula II and 79.1g of pyridine dropwise, and control the dropwise The addition time is about 60 minutes, the dropwise addition is completed, the temperature is controlled at 10°C and the mixture is stirred for about 2 hours. The reaction solution was concentrated under reduced pressure to obtain 150 g of the compound of formula III, which could be directly used in the next step.
[0036] (2) Synthesis of n-propylaminosulfonamide
[0037] Under the protection of nitrogen, put 150g of the compound of formula III obtained in the previous step into a clean four-necked reaction flask, add 300ml of dichloromethane, stir, control the temperature at about 10°C, and slowly introduce ammonia gas f...
Embodiment 3
[0041] (1) Synthesis of n-propylaminosulfonyl chloride
[0042] Under the protection of nitrogen, put 135g of the compound of formula I into a clean four-necked reaction flask, add 250ml of dichloromethane, stir and cool down to 10°C, and slowly drop the mixed solution of 59.1g of the compound of formula II and 101.2g of triethylamine, Control the dropwise addition time for about 60 minutes, after the dropwise addition is completed, stir at 10° C. for about 2 hours under temperature control, and complete. The reaction solution was concentrated under reduced pressure to obtain 146 g of the compound of formula III, which could be directly used in the next step.
[0043] (2) Synthesis of n-propylaminosulfonamide
[0044] Under the protection of nitrogen, put 146g of the compound of formula III obtained in the previous step into a clean four-necked reaction flask, add 300ml of dichloromethane, stir, control the temperature at about 30°C, and slowly inject ammonia gas for about 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com