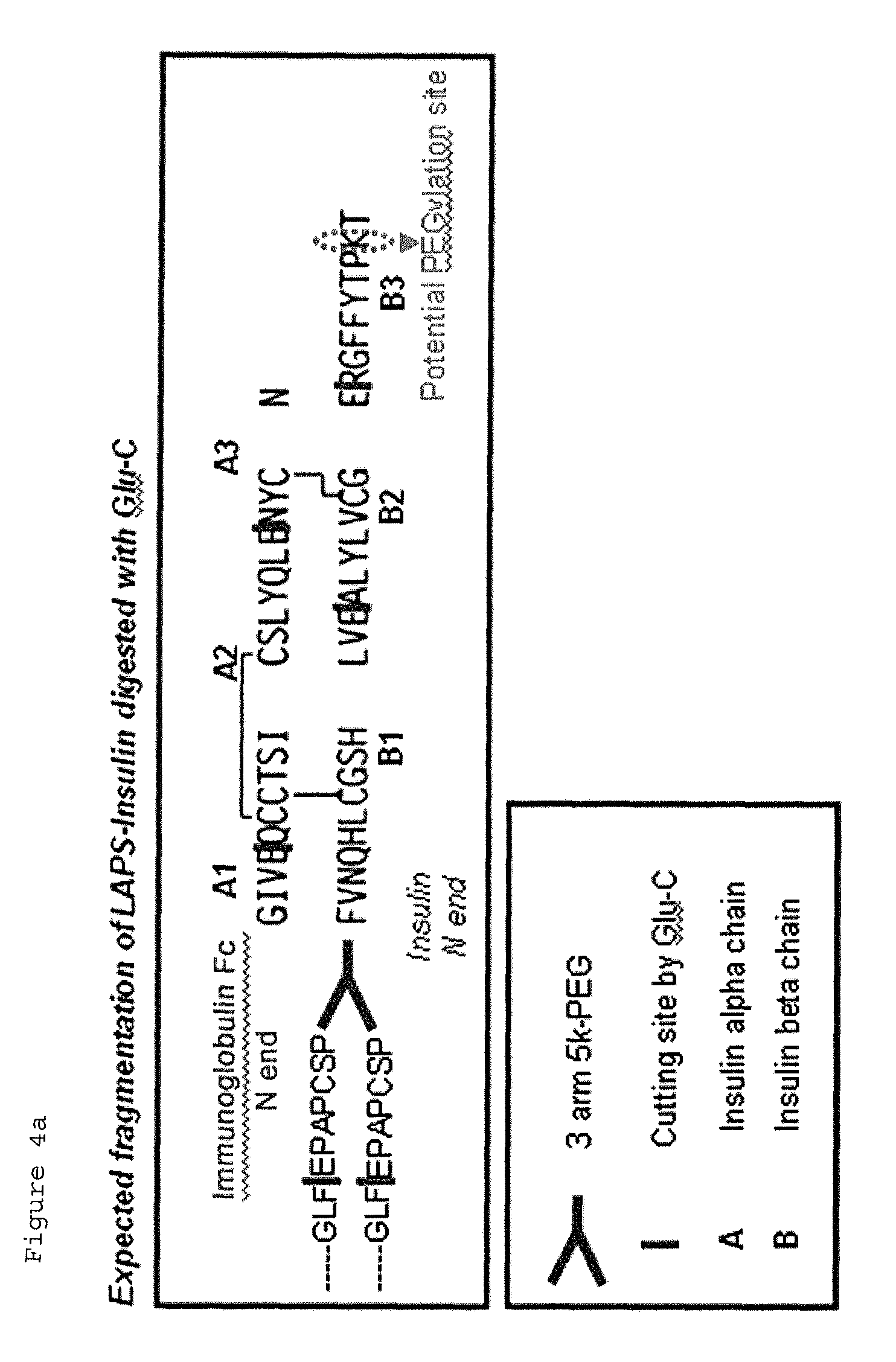
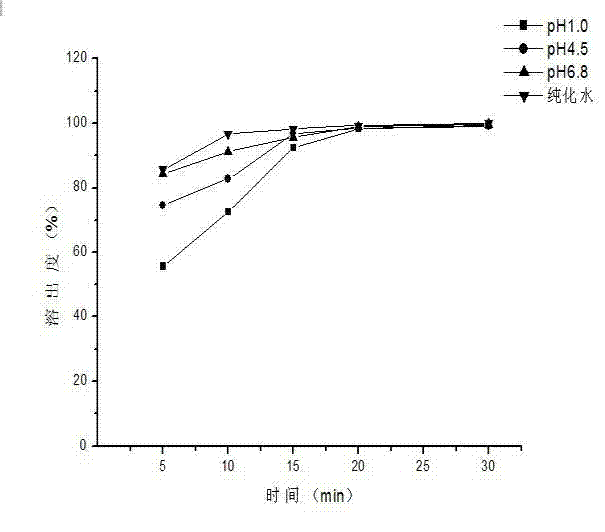
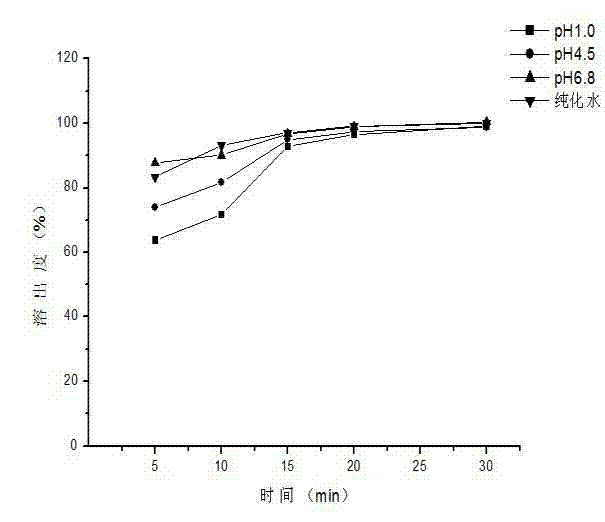
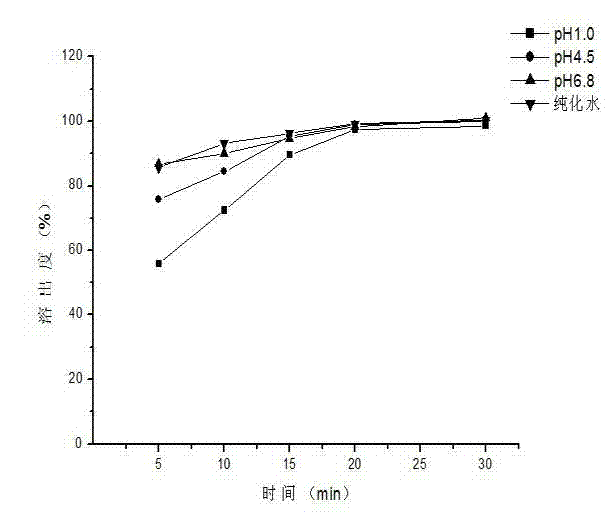
Patents
Literature
190 results about "Drug compliance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug compliance. the reliability of the patient in using a prescribed medication exactly as ordered by the physician. Noncompliance occurs when a patient forgets or neglects to take the prescribed dosages at the recommended times or decides to discontinue the drug without consulting the physician.
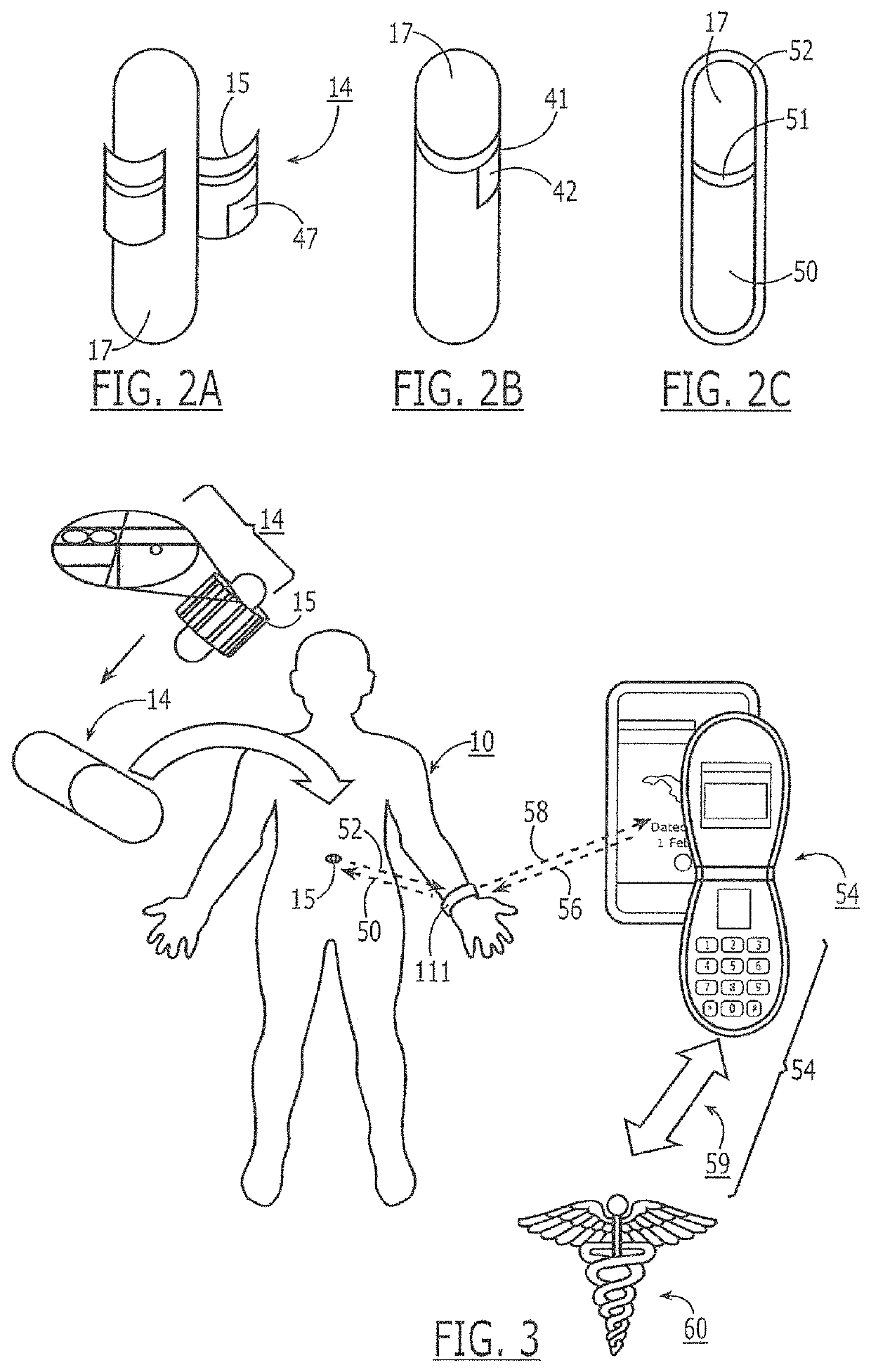
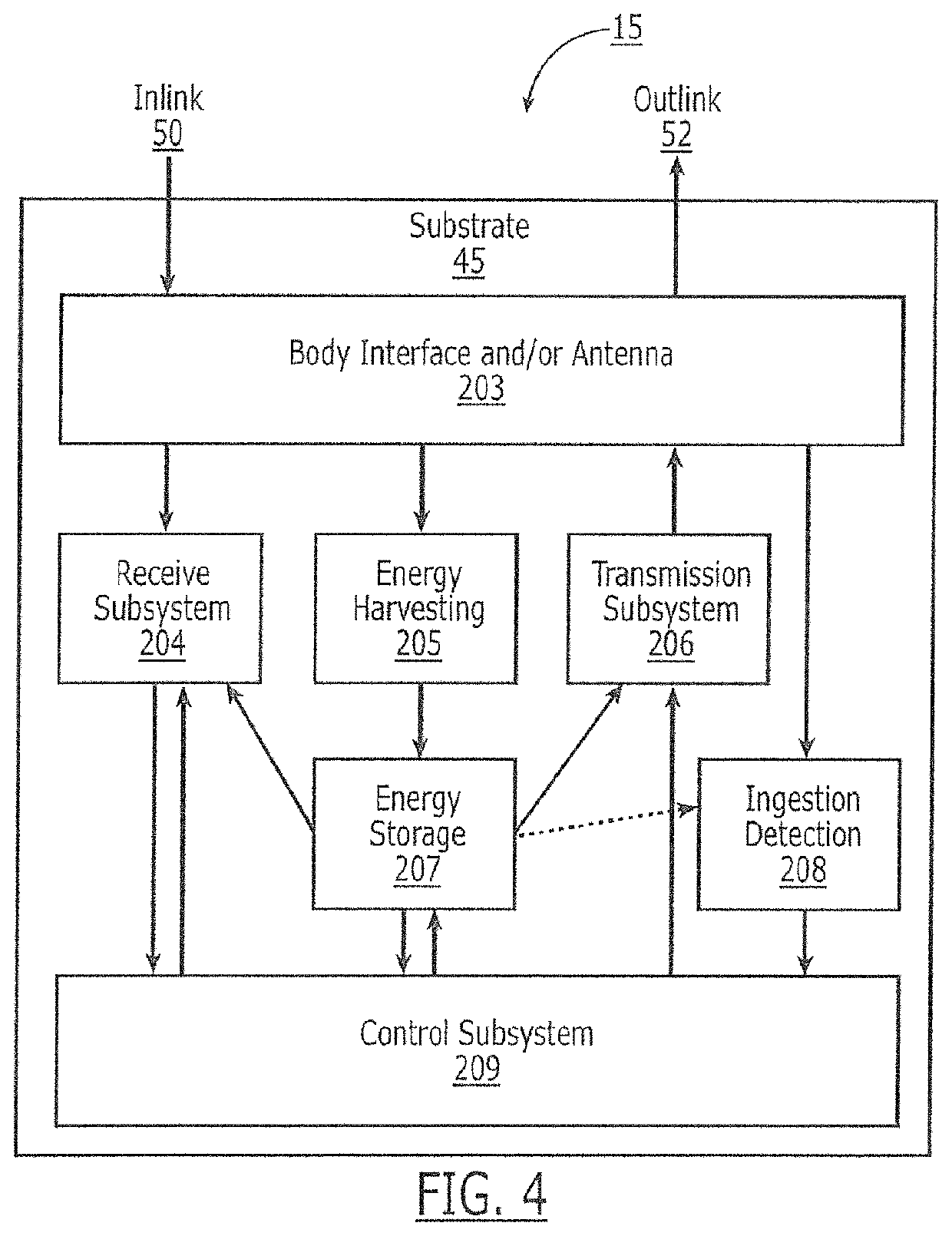
Oral drug compliance monitoring using radio frequency identification tags
A device useful for oral drug delivery device consisting of: (a) a capsule, tablet or pill designed to disperse in the gastrointestinal system; (b) an RFID tag positioned in the capsule, tablet or pill, the RFID tag comprising an antenna; (c) an object selected from the group consisting of a magnet, a ferromagnetic object, a ferrite object and an electromagnetic shielding object positioned within, over or adjacent the antenna of the RFID tag to alter the antenna characteristics of the RFID tag so that if the RFID tag is interrogated before the capsule, tablet or pill disperses in the gastrointestinal system, the response of the RFID tag is sufficiently altered or attenuated to determine that the capsule, tablet or pill has not dispersed in the gastrointestinal system and so that if the RFID tag is interrogated after the capsule, tablet or pill has dispersed in the gastrointestinal system, the object separates from the RFID tag so that the response of the RFID tag is sufficiently detectable to determine that the capsule, tablet or pill has dispersed in the gastrointestinal system. Alternatively, a switch can be used to signal ingestion of the device, and change the response of the device. In another embodiment, the instant invention is a device useful for oral drug delivery, consisting of: (a) a capsule, tablet or pill designed to disperse in the gastrointestinal system; (b) a first non-anti-collision RFID tag positioned in the capsule; (c) a second non-anti-collision RFID tag positioned in the capsule, so that if the RFID tags are interrogated by an RFID reader before the capsule, tablet or pill disperses in the gastrointestinal system, the response of the RFID tags collide and so that after the dispersible material of the capsule has dispersed in the gastrointestinal system thereby allowing the first and second non-anti-collision tags to separate from each other, then the response of the RFID tags is sufficiently different from each other to determine that the capsule has dispersed in the gastrointestinal system
Owner:DOW GLOBAL TECH LLC
Oral drug compliance monitoring using radio frequency identification tags
A device useful for oral drug delivery device consisting of: (a) a capsule, tablet or pill designed to disperse in the gastrointestinal system; (b) an RFID tag positioned in the capsule, tablet or pill, the RFID tag comprising an antenna; (c) an object selected from the group consisting of a magnet, a ferromagnetic object, a ferrite object and an electromagnetic shielding object positioned within, over or adjacent the antenna of the RFID tag to alter the antenna characteristics of the RFID tag so that if the RFID tag is interrogated before the capsule, tablet or pill disperses in the gastrointestinal system, the response of the RFID tag is sufficiently altered or attenuated to determine that the capsule, tablet or pill has not dispersed in the gastrointestinal system and so that if the RFID tag is interrogated after the capsule, tablet or pill has dispersed in the gastrointestinal system, the object separates from the RFID tag so that the response of the RFID tag is sufficiently detectable to determine that the capsule, tablet or pill has dispersed in the gastrointestinal system. Alternatively, a switch can be used to signal ingestion of the device, and change the response of the device.
Owner:DOW GLOBAL TECH LLC
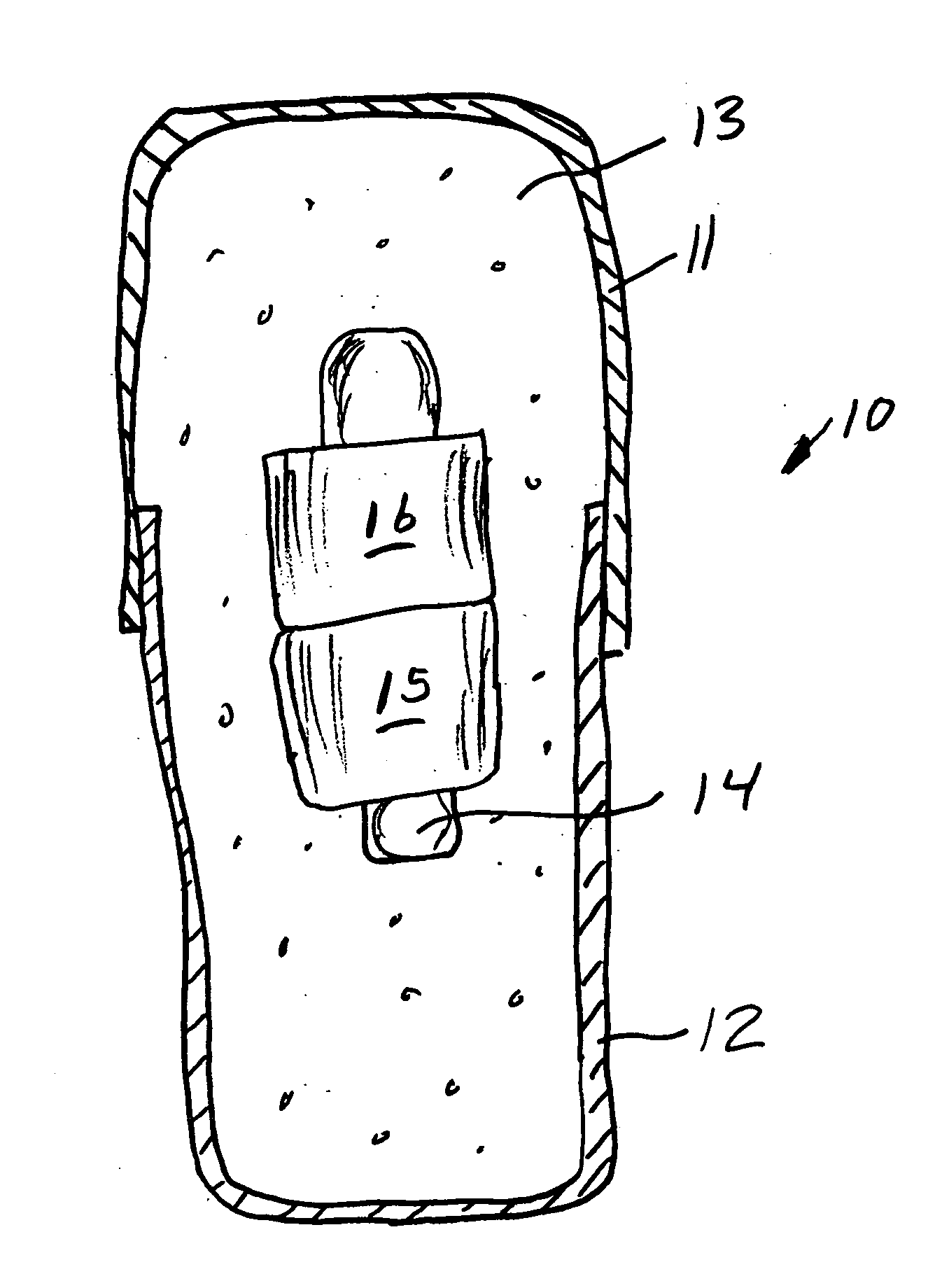
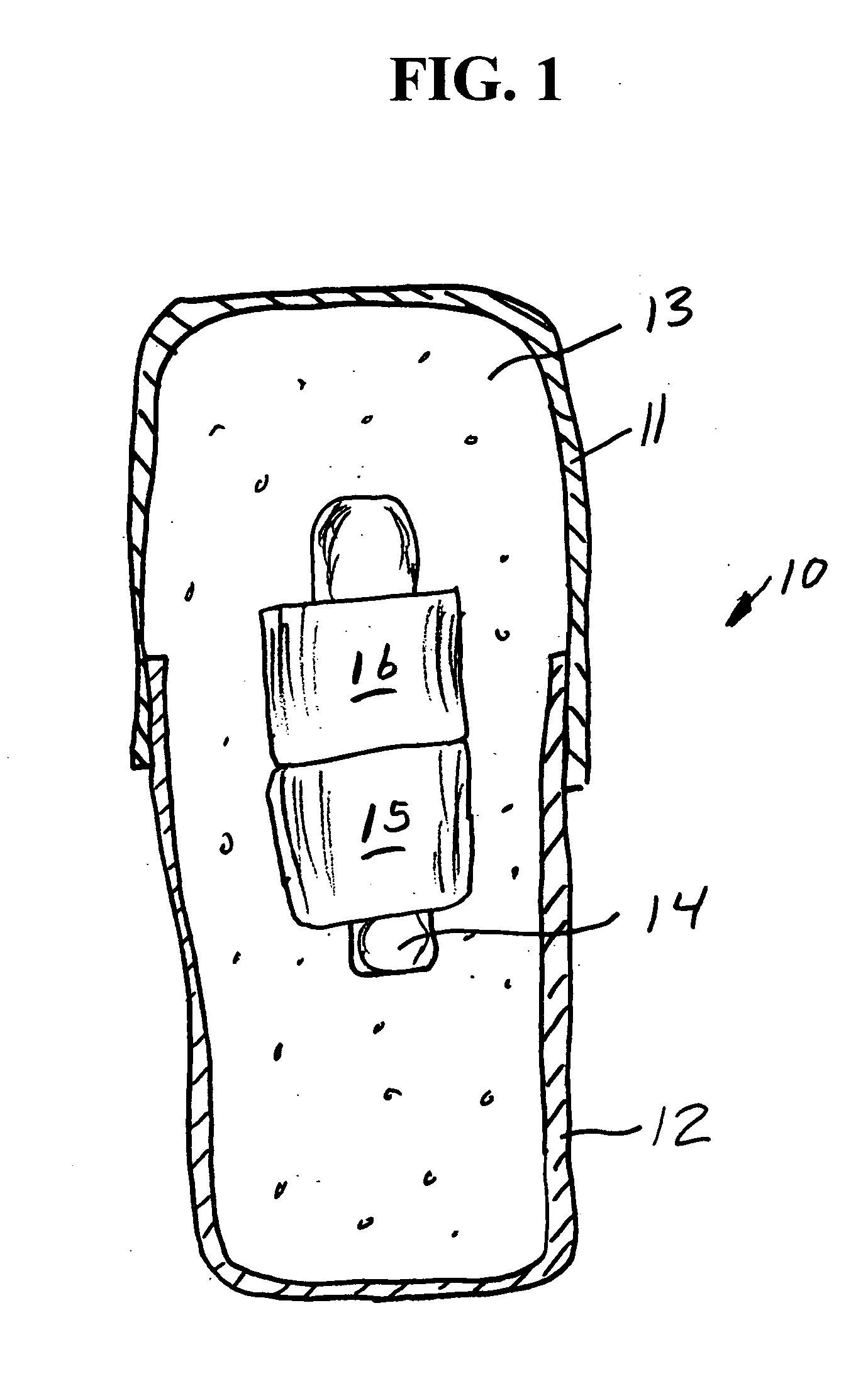
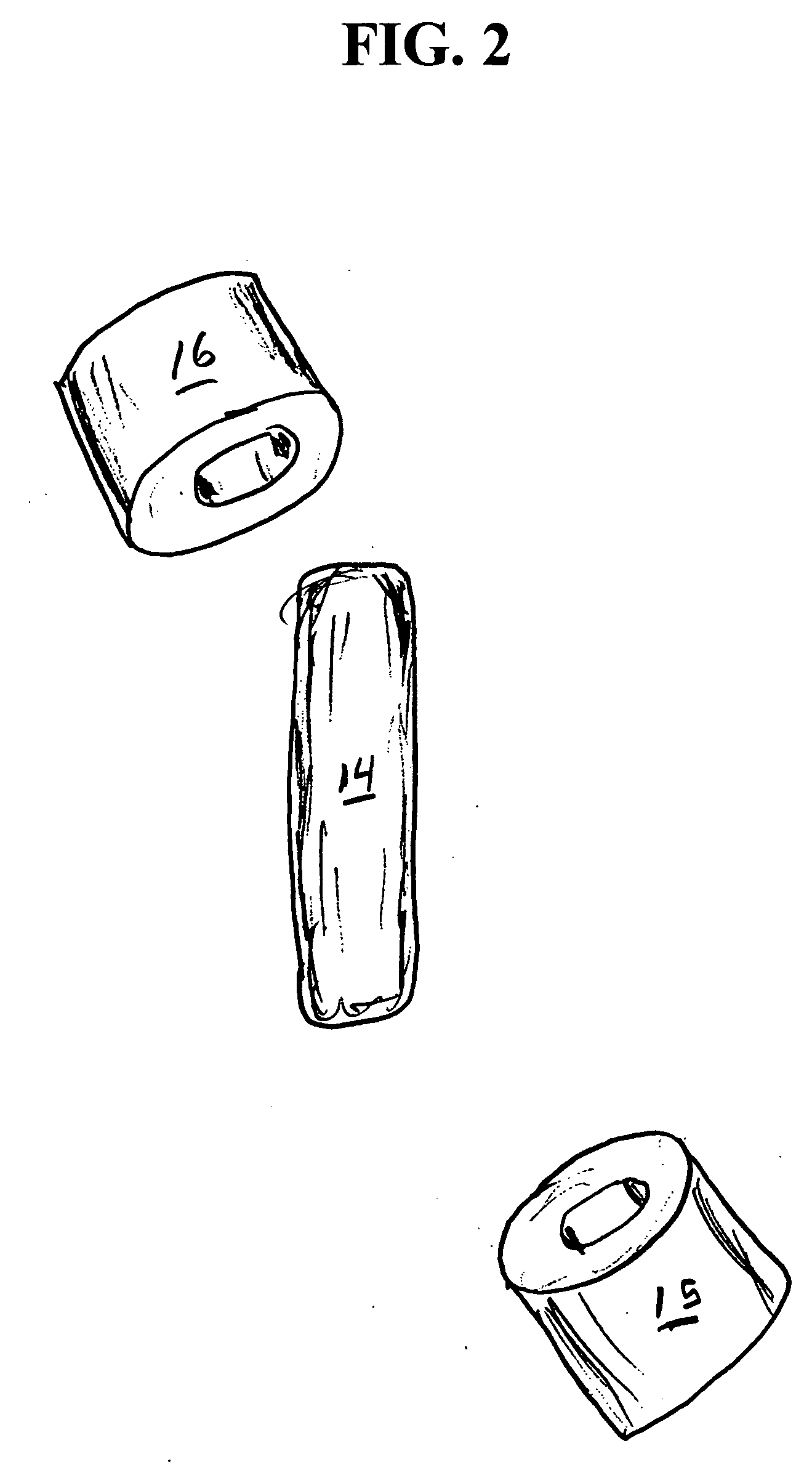
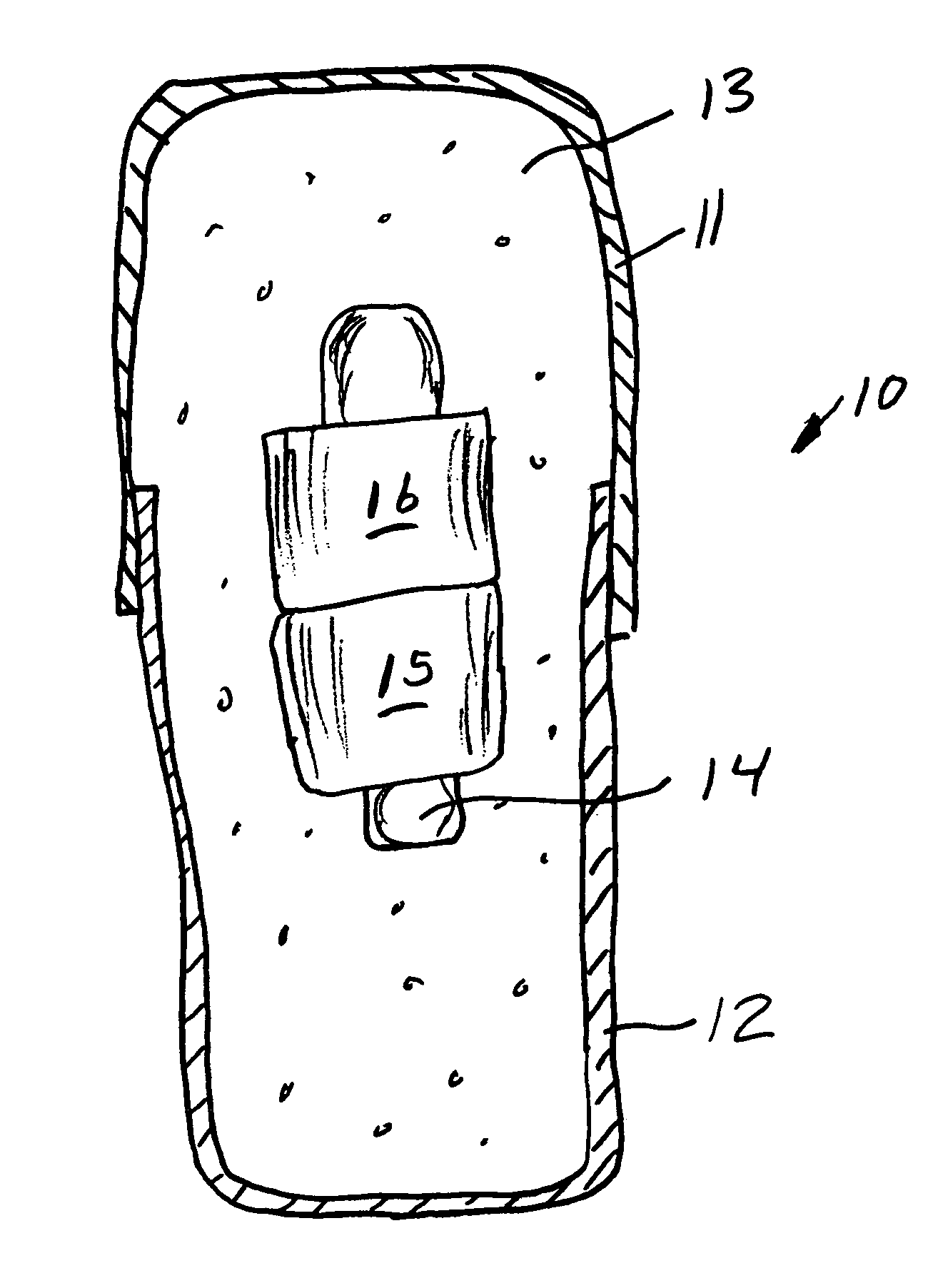
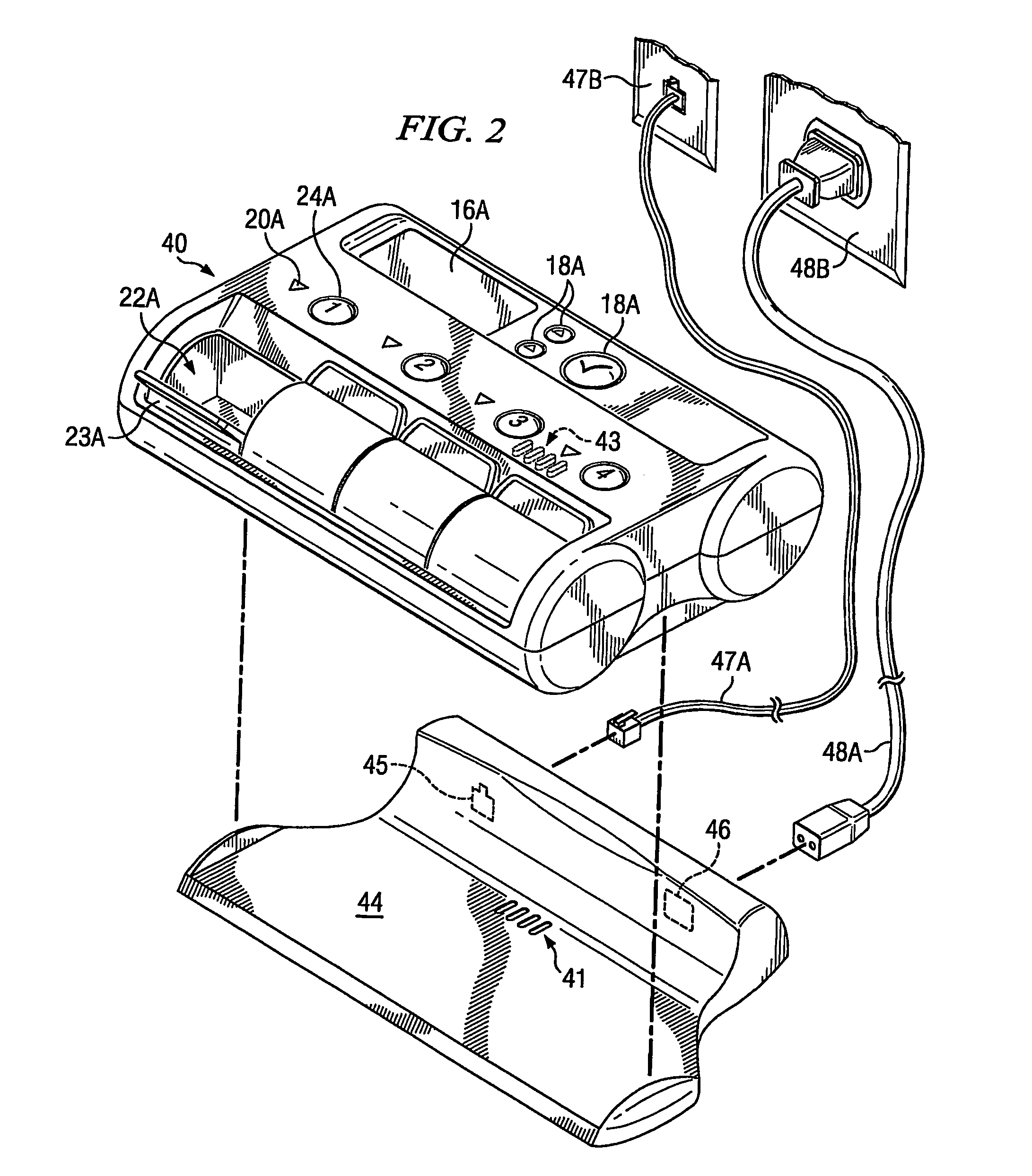
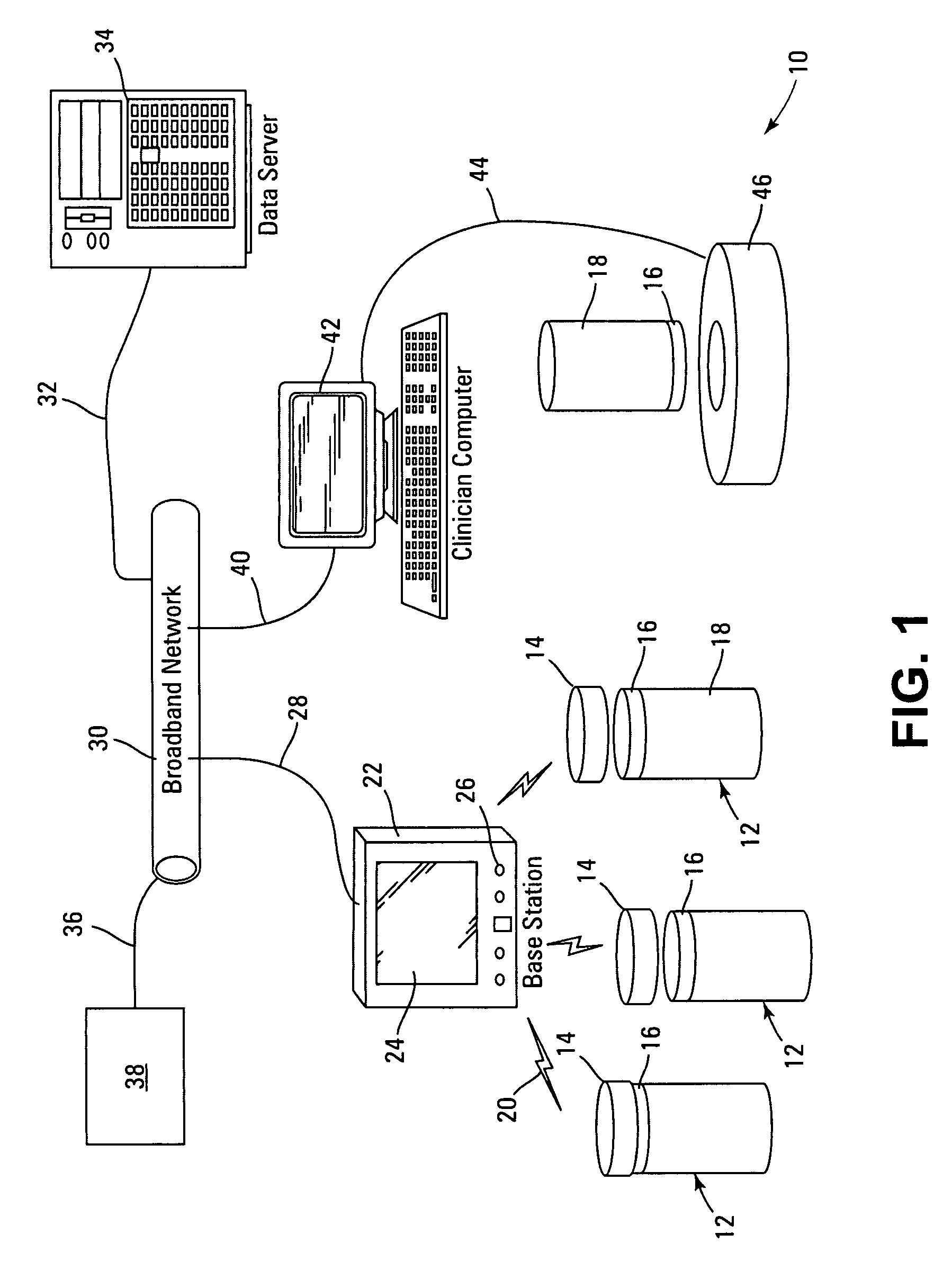
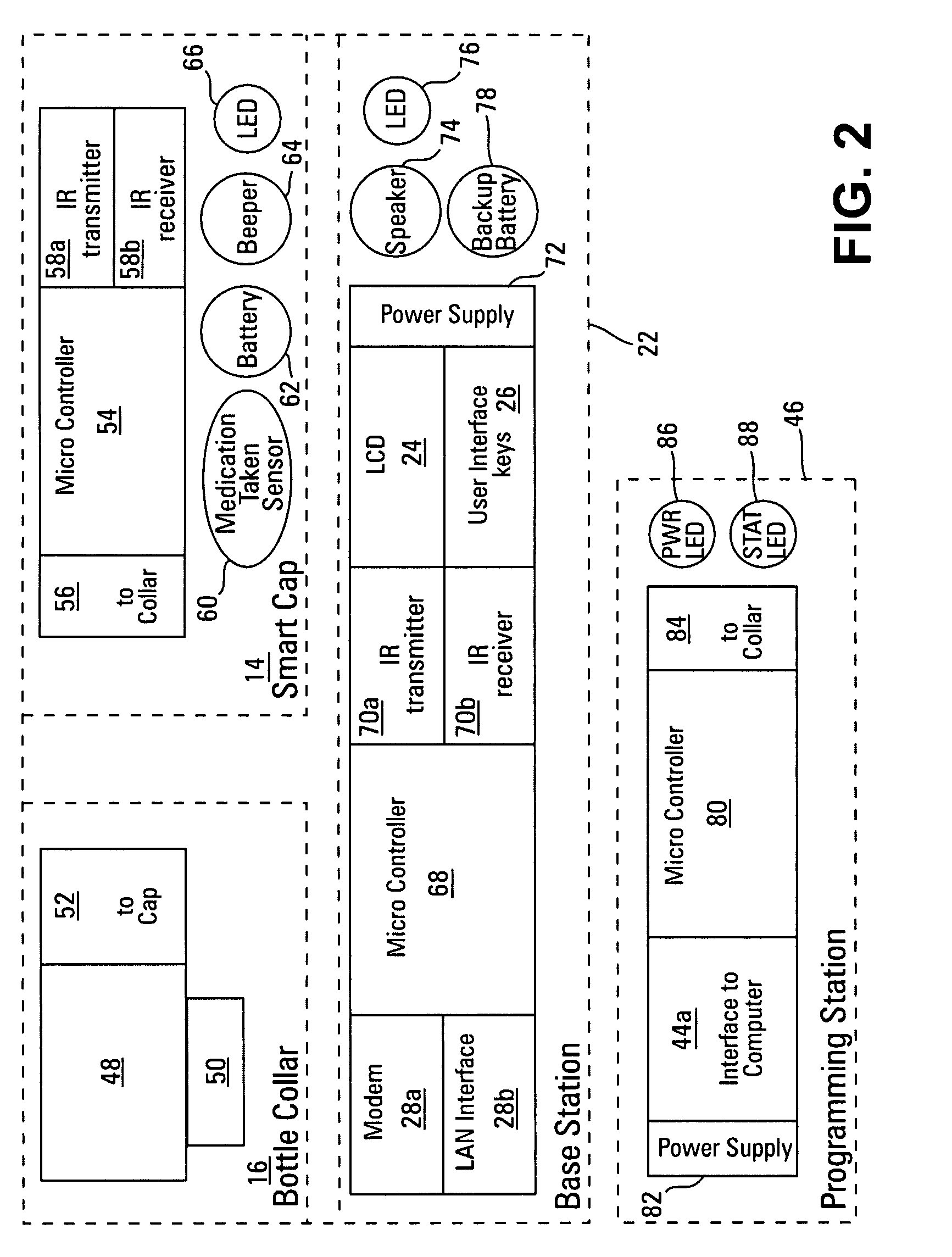
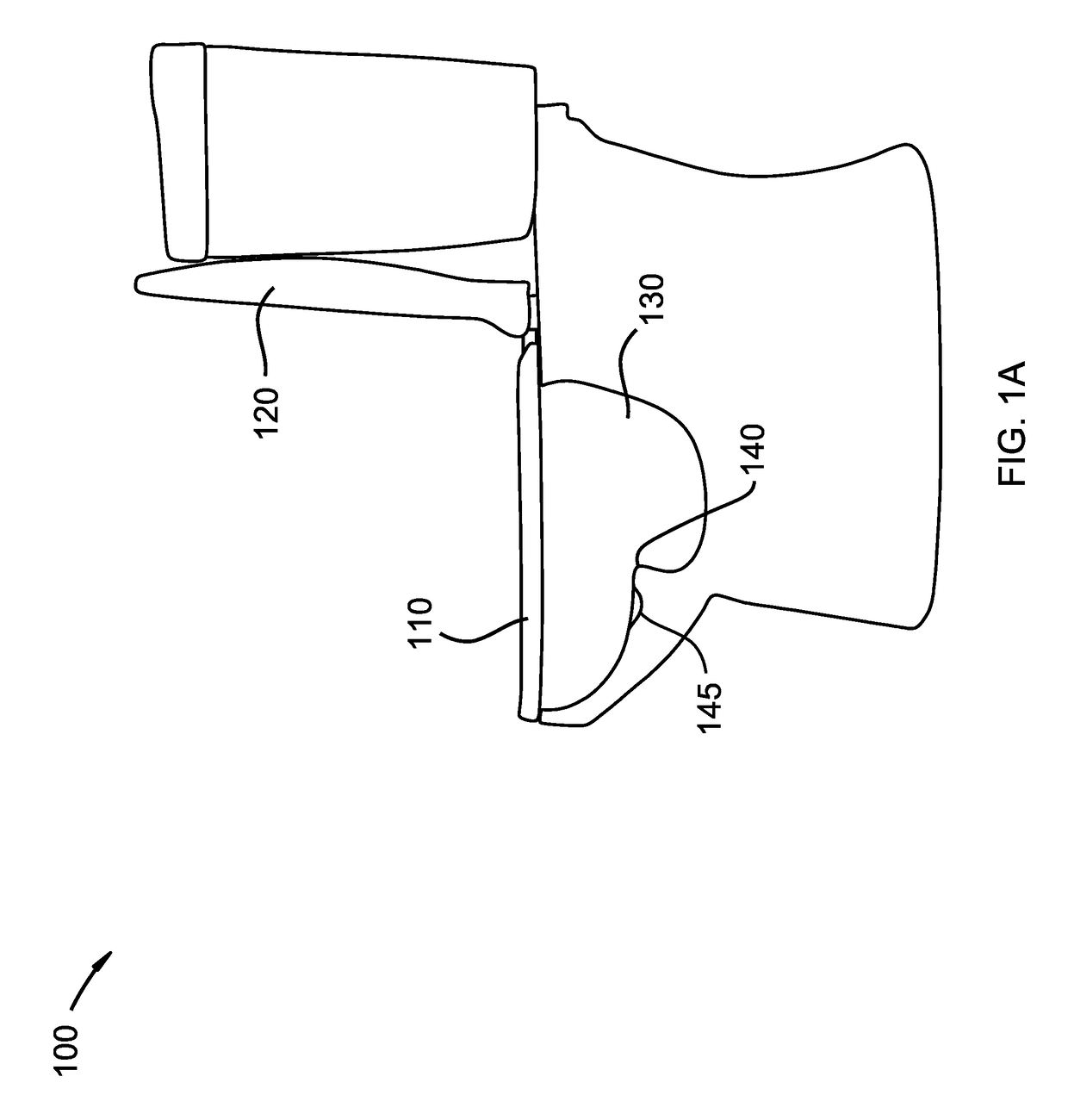
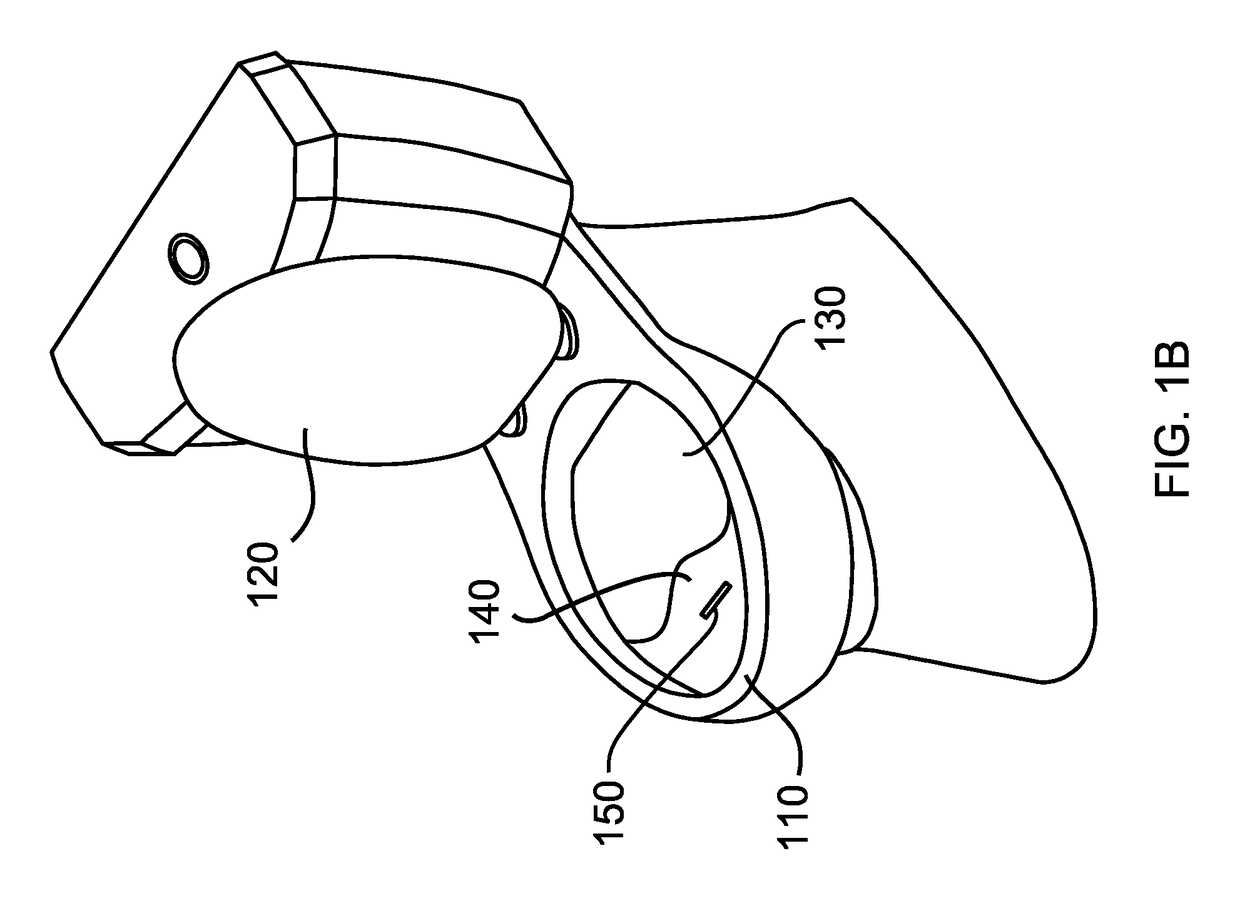
Medication compliance device
InactiveUS7158011B2Frequency-division multiplex detailsDrug and medicationsProximateLiquid-crystal display
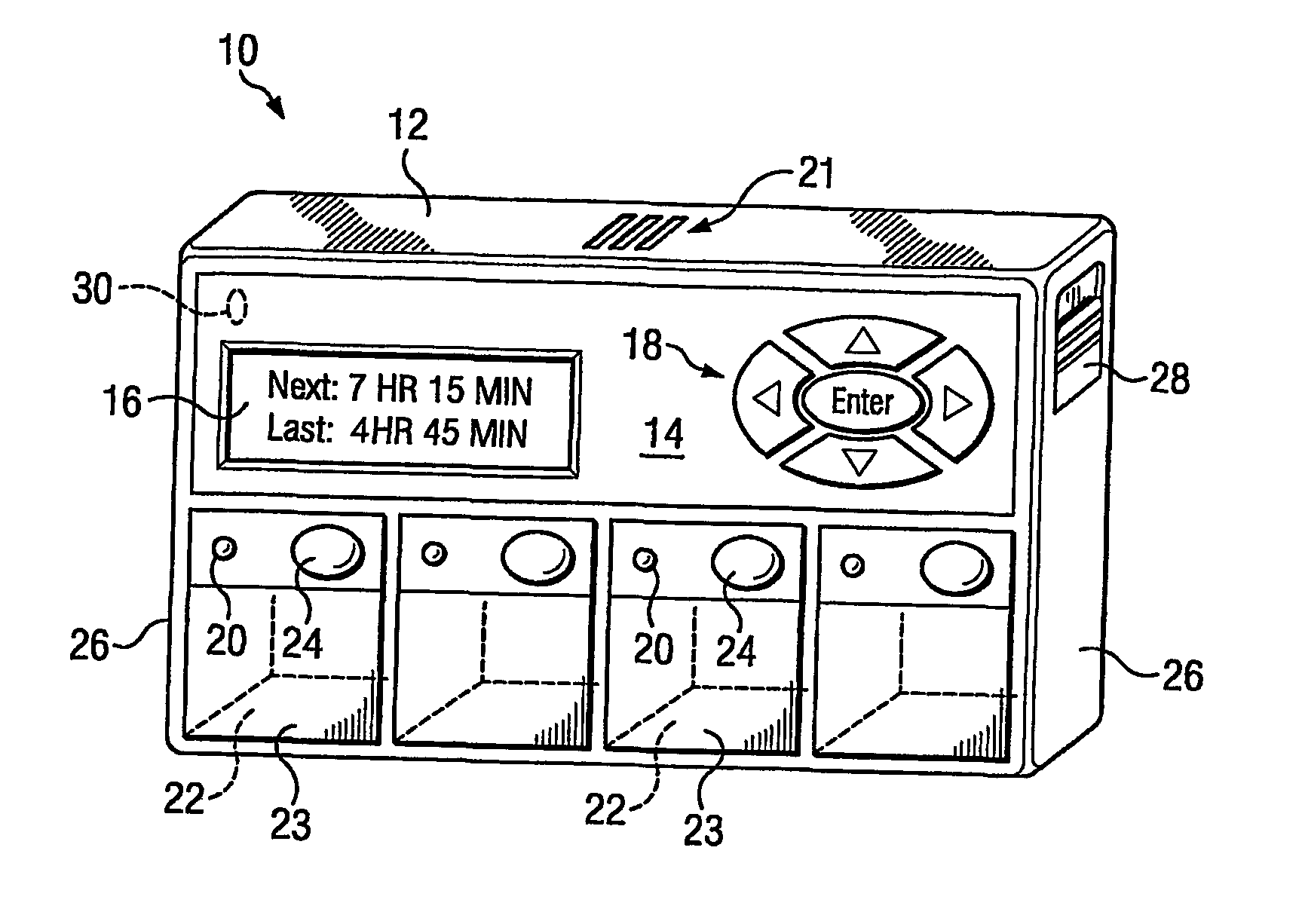
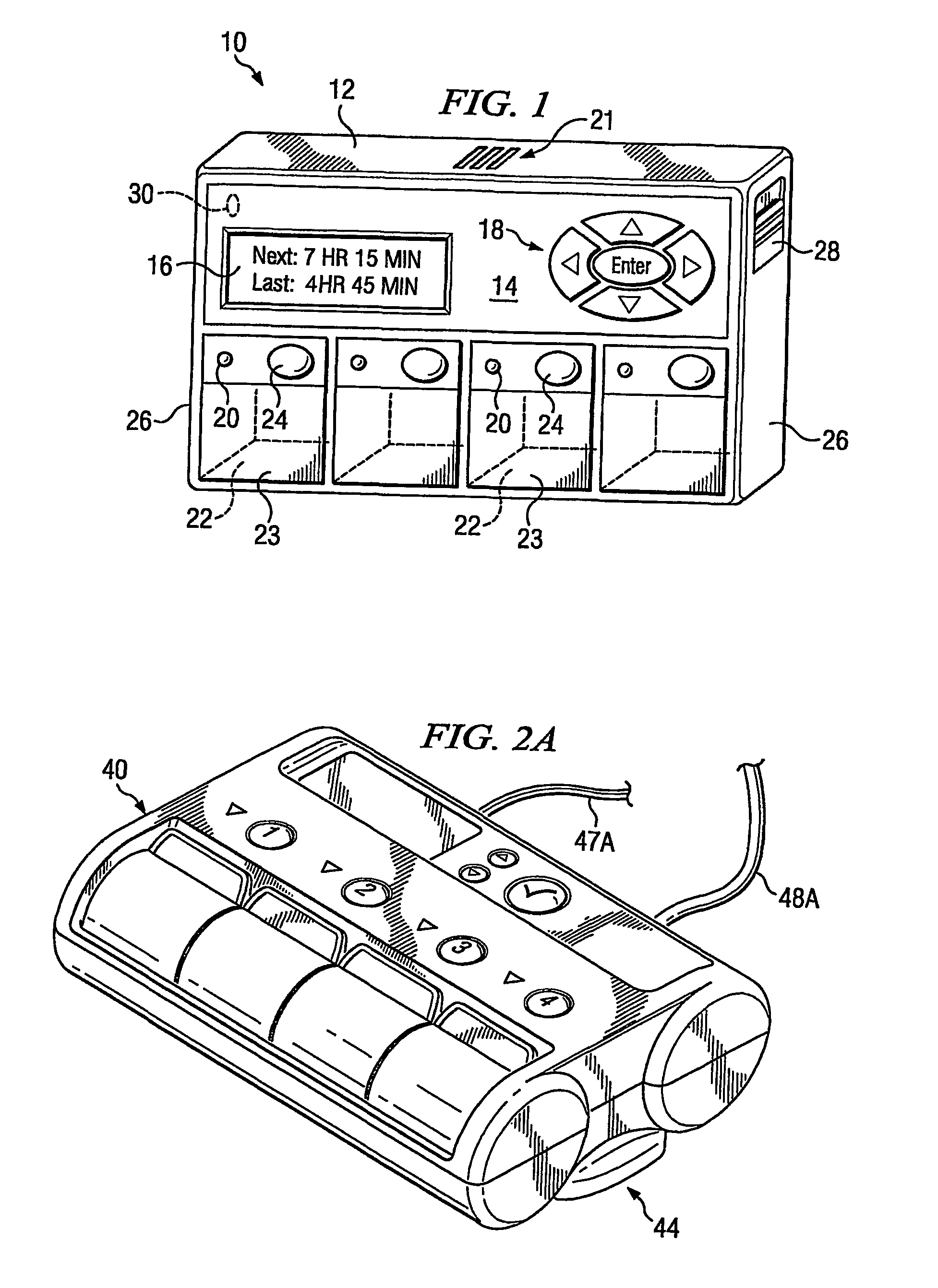
A portable medication compliance device (10) having a body (12) and compartments (22) formed in the body. A microprocessor (120) is disposed in the body (12) and is coupled to each of the compartments (22). The microprocessor (120) is programmable to determine relative time intervals for dispensing medication from each of the compartments, to notify the user when a dose of medication is to be taken from each of the compartments, and to record the opening of each of the compartments in a memory. Programming buttons (24) are positioned proximate the body and are coupled to the microprocessor (120). The programming buttons (24) enable a user to program the microprocessor. The device may also be programmed via a remote computer through communication means (47A). A display means (16) such as a Liquid Crystal Display (LCD) is positioned on the body and is coupled to the microprocessor (120). The LCD (16) displays data including the relative time intervals for dispensing medication determined by the microprocessor (120).
Owner:BRUE VESTA L
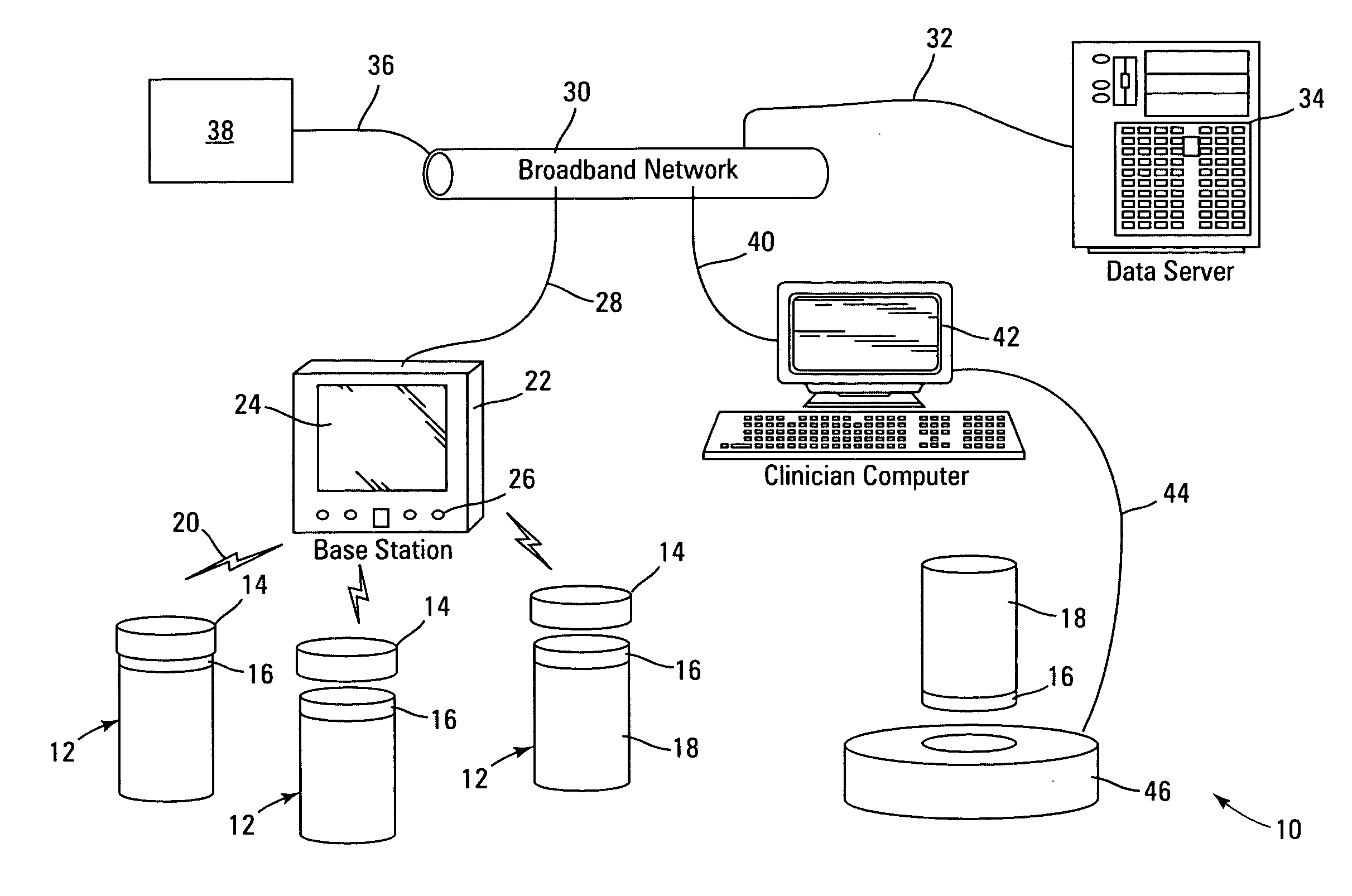
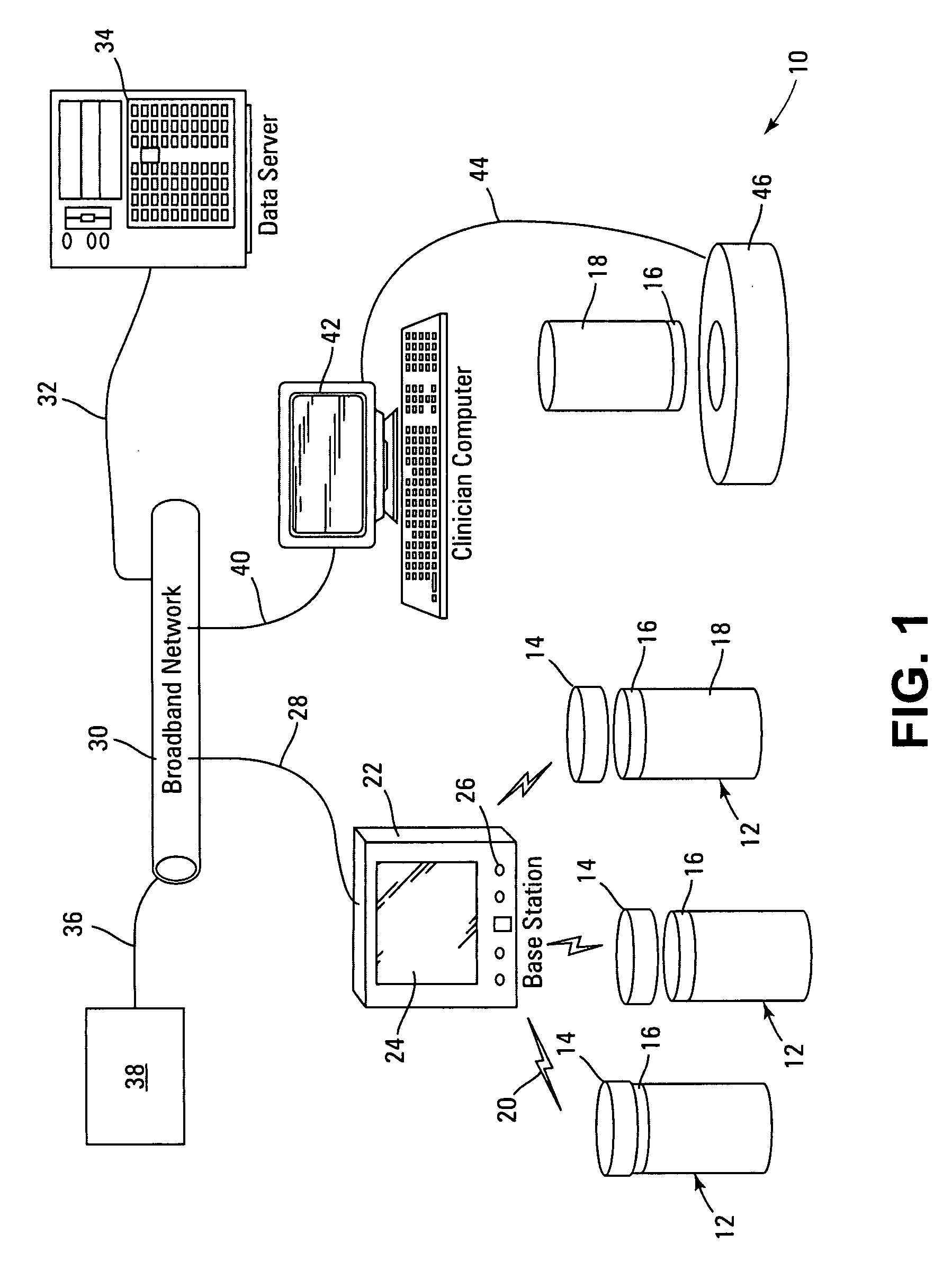
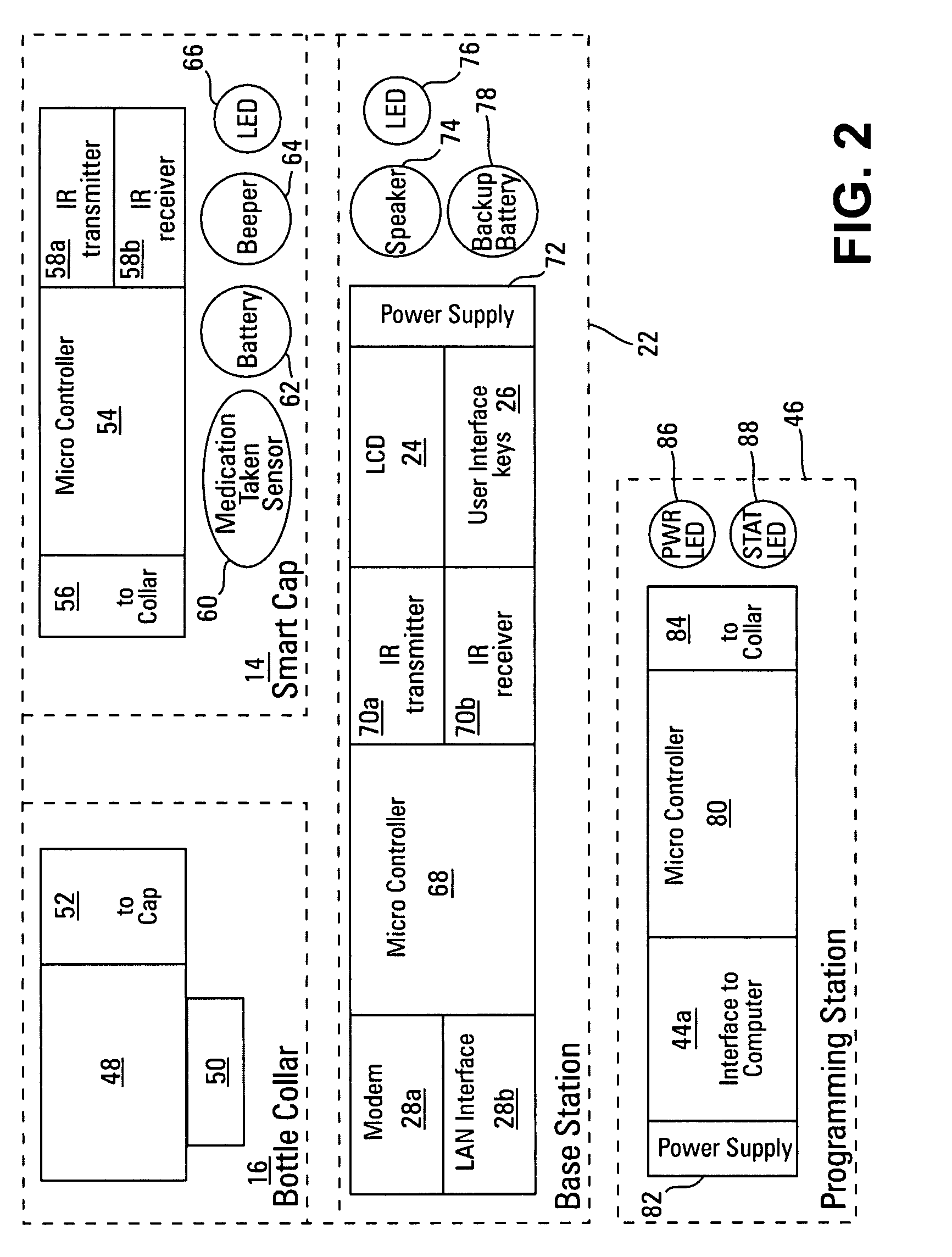
Prescription drug compliance monitoring system
InactiveUS20080114490A1Improve self-relianceIncrease independenceData processing applicationsDrug and medicationsMedication DispenserCaregiver person
The drug compliance monitoring system provides a patient with a portable medication dispenser programmed with medication-taking data. The dispenser alerts the patient to take a dose of medication and gathers compliance data relating to the medication-taking data. The compliance data is accessible to a physician, or other caregiver, etc., via a network database.
Owner:STRATAMED LABS
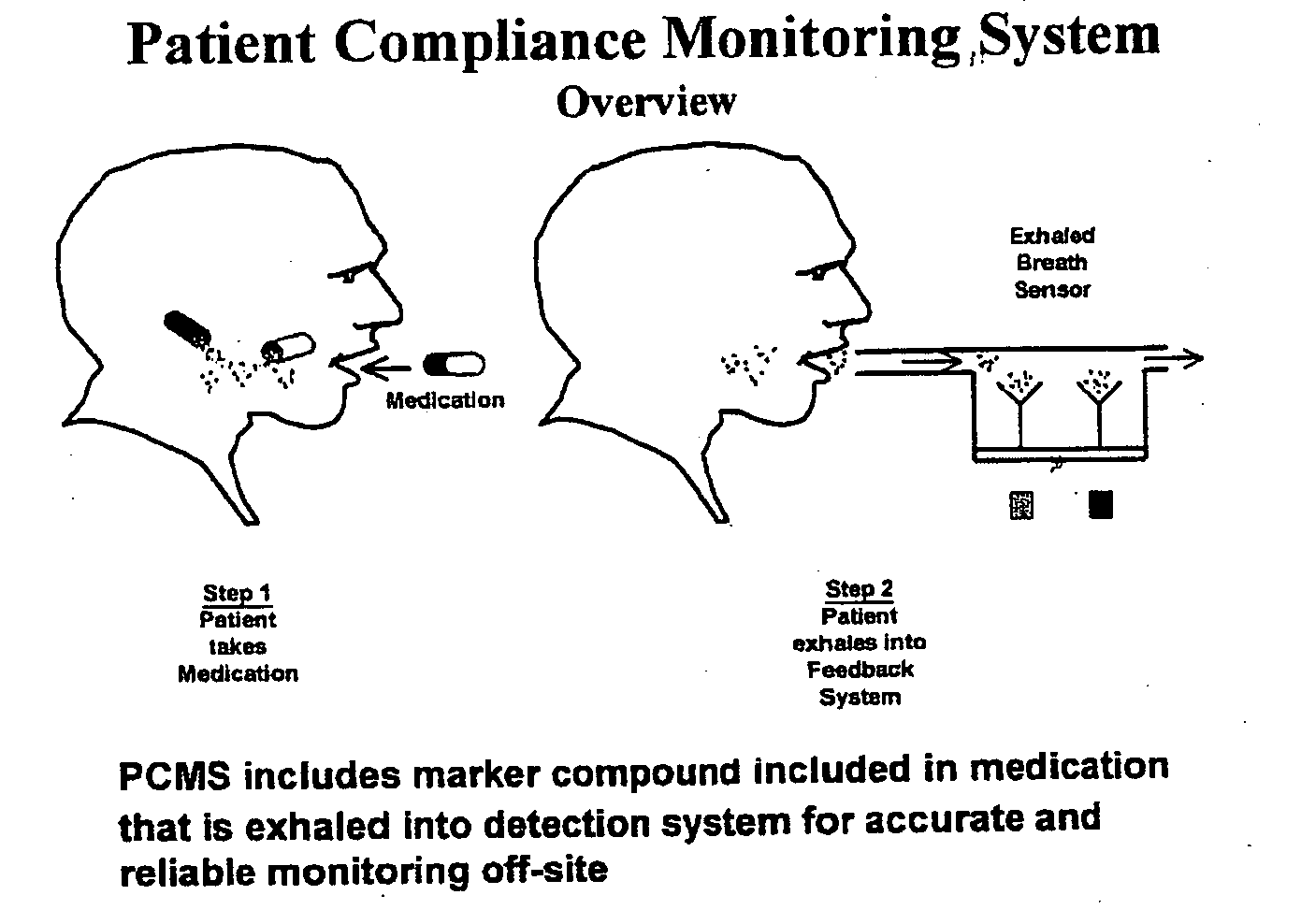
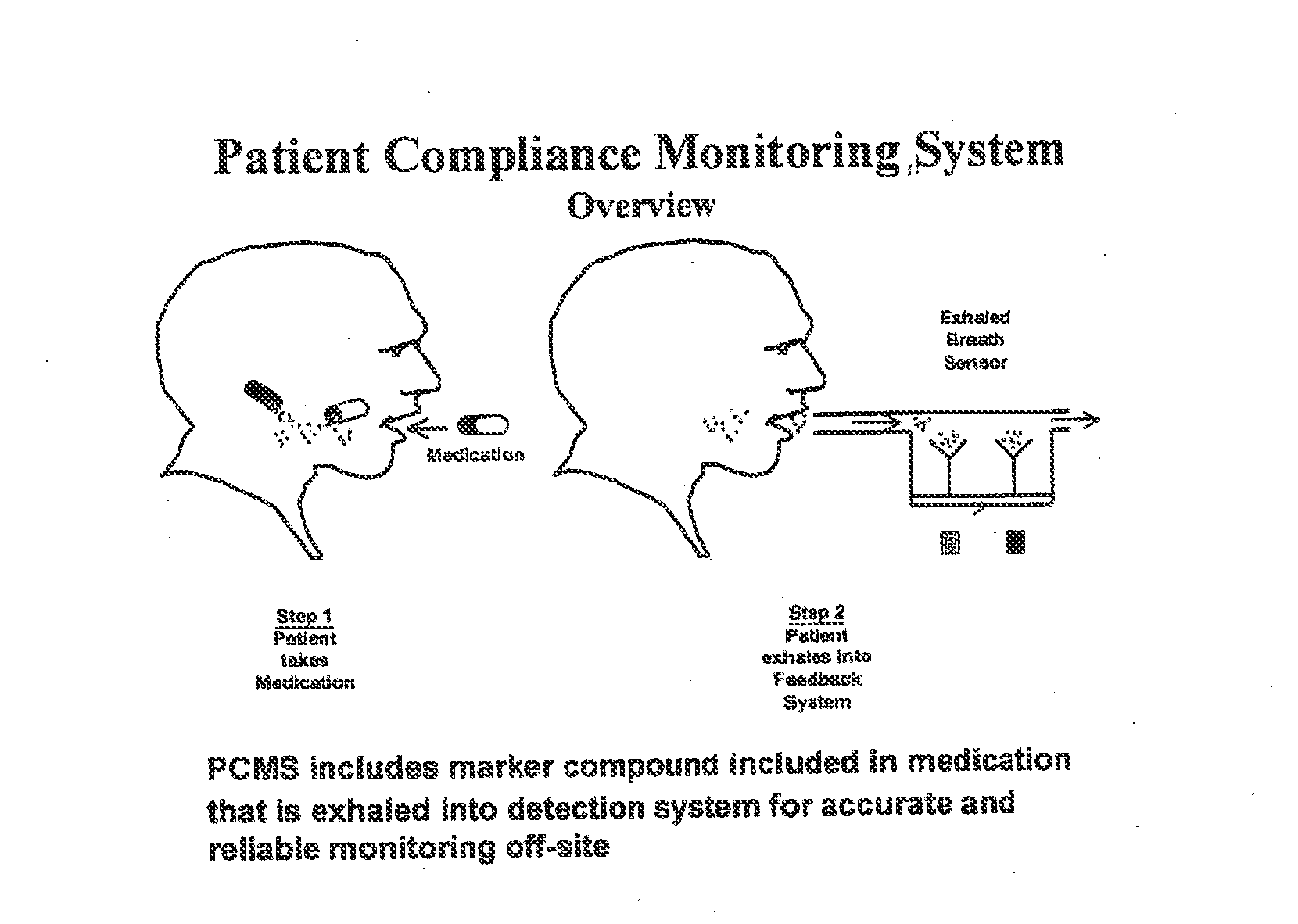
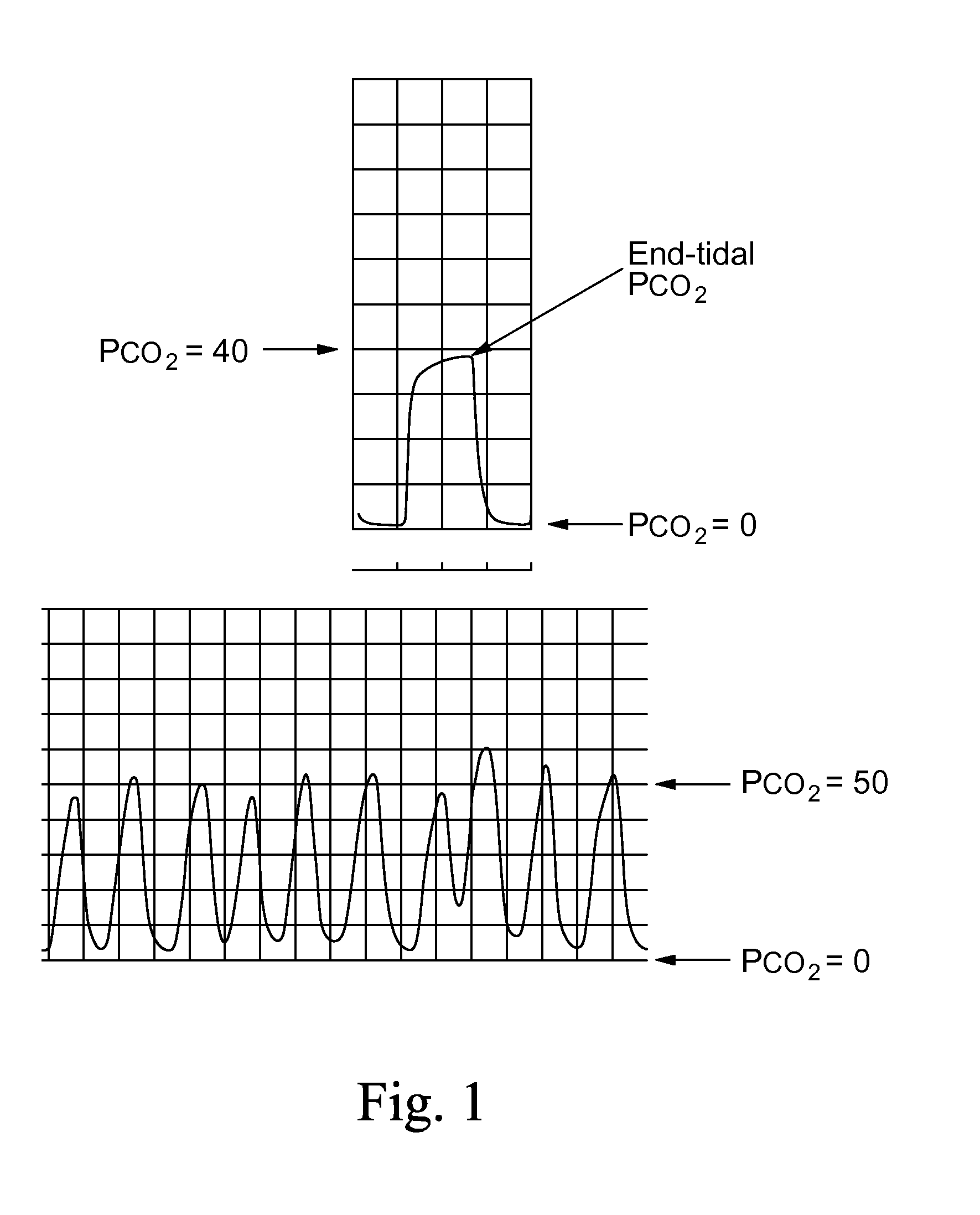
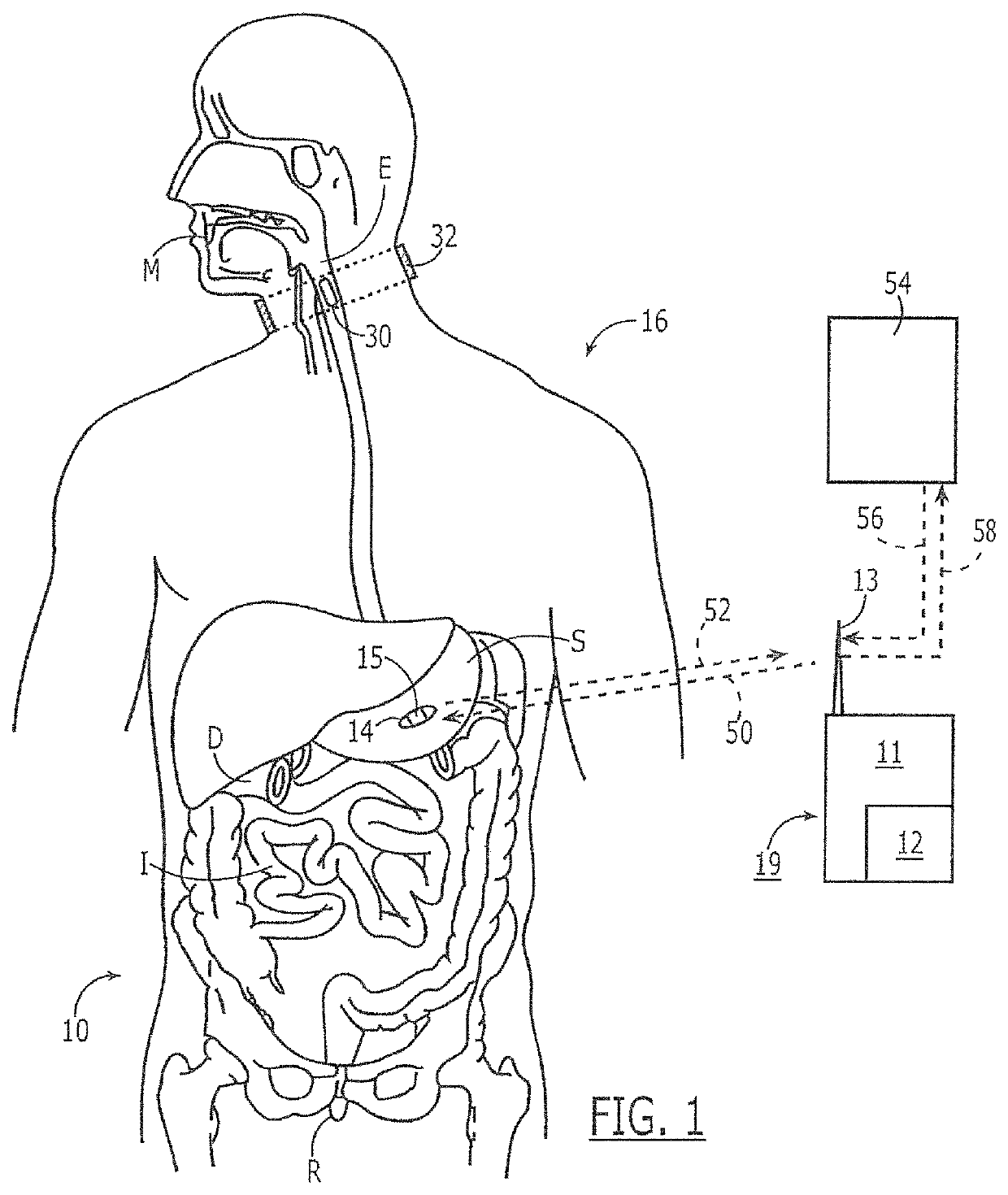
Marker detection method and apparatus to monitor drug compliance
InactiveUS20050233459A1Accurate assessmentPatient complianceDiagnostic recording/measuringSensorsNoseEnvironmental health
The present invention includes systems and methods for monitoring therapeutic drug concentration in blood by detecting markers, such as odors, upon exhalation by a patient after the drug is taken, wherein such markers result either directly from the drug itself or from an additive combined with the drug. In the case of olfactory markers, the invention preferably utilizes electronic sensor technology, such as the commercial devices referred to as “artificial” or “electronic” noses or tongues, to non-invasively monitor drug levels in blood. The invention further includes a reporting system capable of tracking drug concentrations in blood (remote or proximate locations) and providing the necessary alerts with regarding to ineffective or toxic drug dosages in a patient.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Prescription drug compliance monitoring system
InactiveUS7844361B2Improving self-reliance and independenceFacilitate complianceDrug and medicationsCoin-freed apparatus detailsMedication DispenserCaregiver person
The drug compliance monitoring system provides a patient with a portable medication dispenser programmed with medication-taking data. The dispenser alerts the patient to take a dose of medication and gathers compliance data relating to the medication-taking data. The compliance data is accessible to a physician, or other caregiver, etc., via a network database.
Owner:STRATAMED LABS
Monitoring drug compliance, food-intake or toxin-intake using non-invasively-read labels
InactiveUS20080213904A1Diagnostics using lightChemiluminescene/bioluminescenceEnvironmental healthToxic material
A system is disclosed for monitoring a property of an ingested or in-taken drug, food, drink or toxic substance, non-invasively or minimally invasively, which can also identify the subject person being monitored, if desired. The system comprises: a means of labeling the substance with a labeling media to have a useful signature indicative of, or bearing a relation to the property; a means to allow the signature to be read non-invasively or minimally invasively; and a means to identify, in any manner, who is being monitored.
Owner:SLIWA JOHN W +3
Systems and methods for drug compliance monitoring
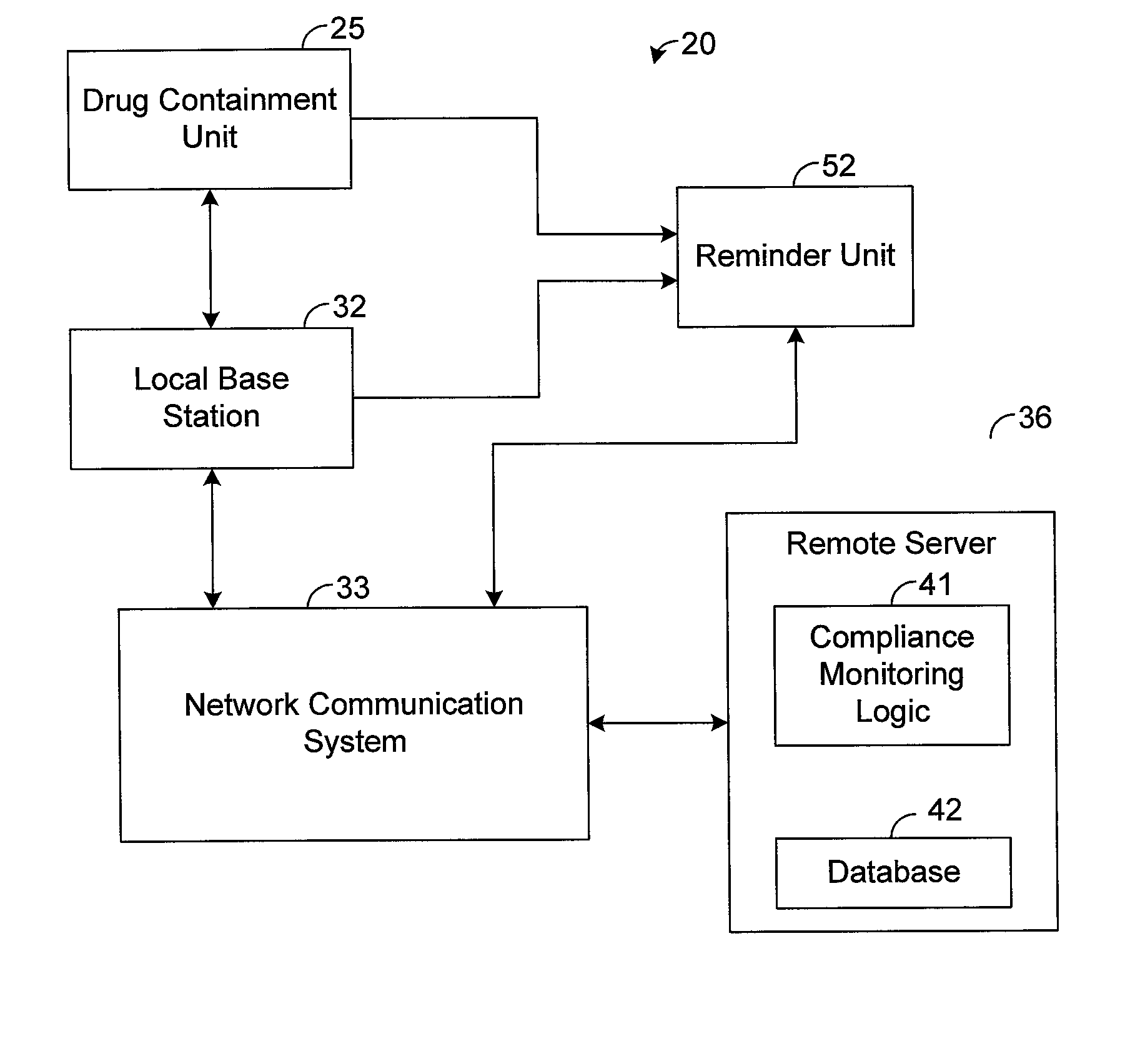
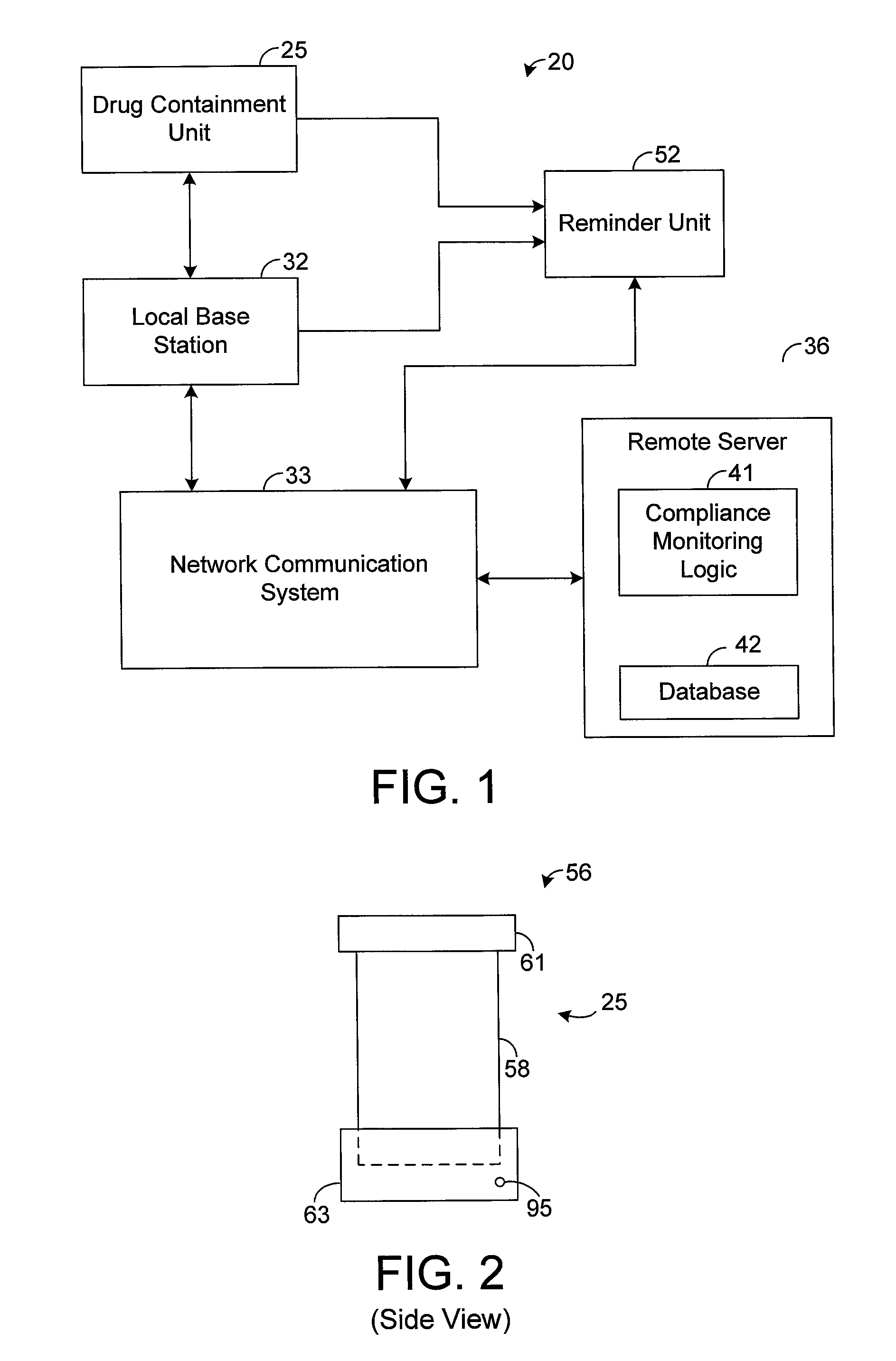
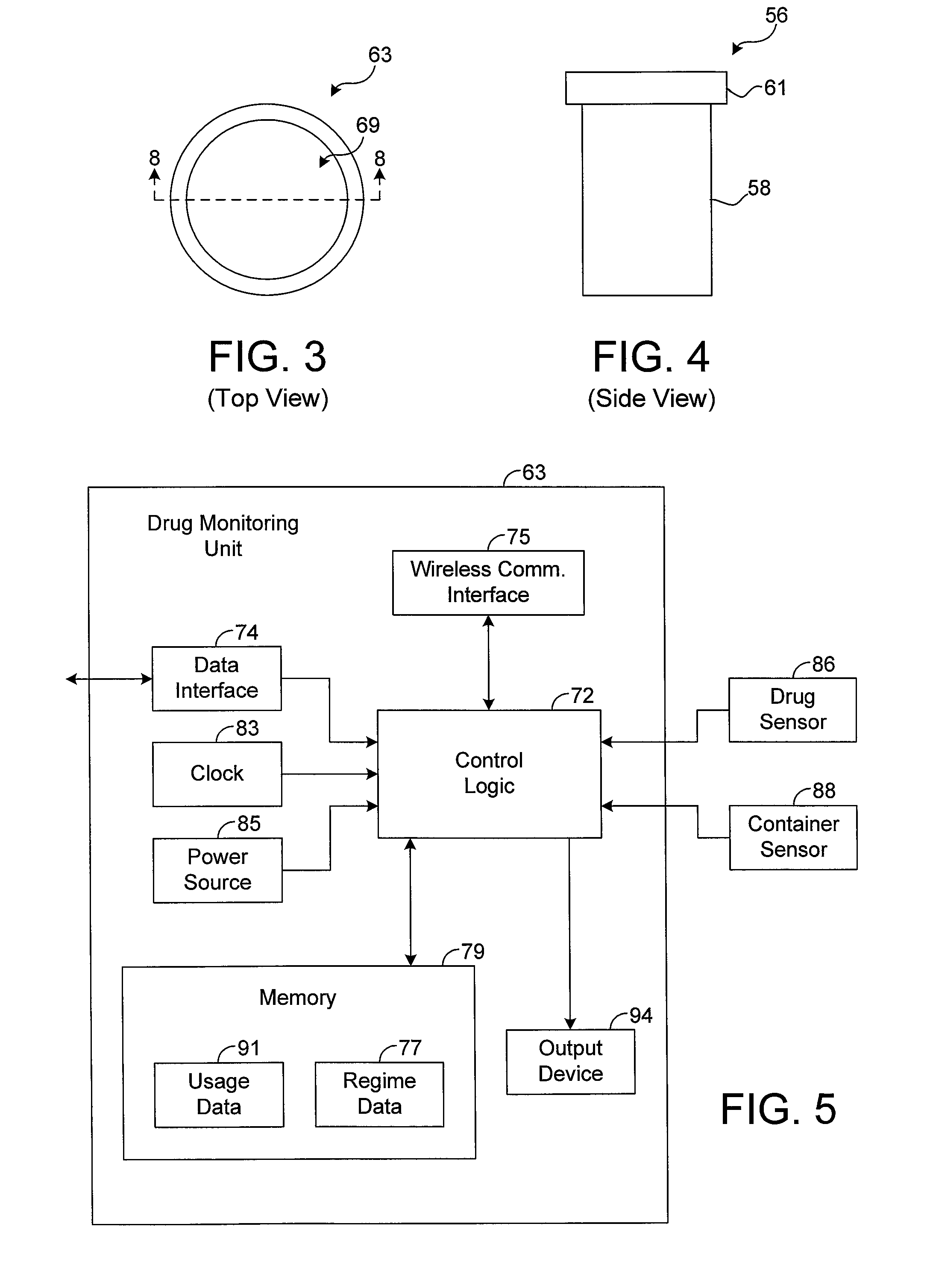
The present disclosure generally pertains to systems and methods for drug compliance monitoring. A drug compliance monitoring system in accordance with one exemplary embodiment of the present disclosure comprises a portable drug containment unit, which has a drug container, such as a pill bottle, for holding prescription or non-prescription drugs. The drug containment unit also comprises at least one sensor and control logic. The sensor is configured to automatically sense a parameter indicating when a drug, such as one or more pills or an amount of liquid, has been or is about to be removed from the drug container. The system, based on the sensor, automatically estimates and tracks drug consumption and provides a patient with reminders when a dosage is currently due. If the patient deviates from an expected drug regime, the system automatically senses this event and provides a notification to the patient or caregiver. In addition, the system stores a usage history indicating the approximate time and amount of each sensed dosage.
Owner:UNIVERSITY OF ALABAMA
Method of providing comprehensive drug compliance information
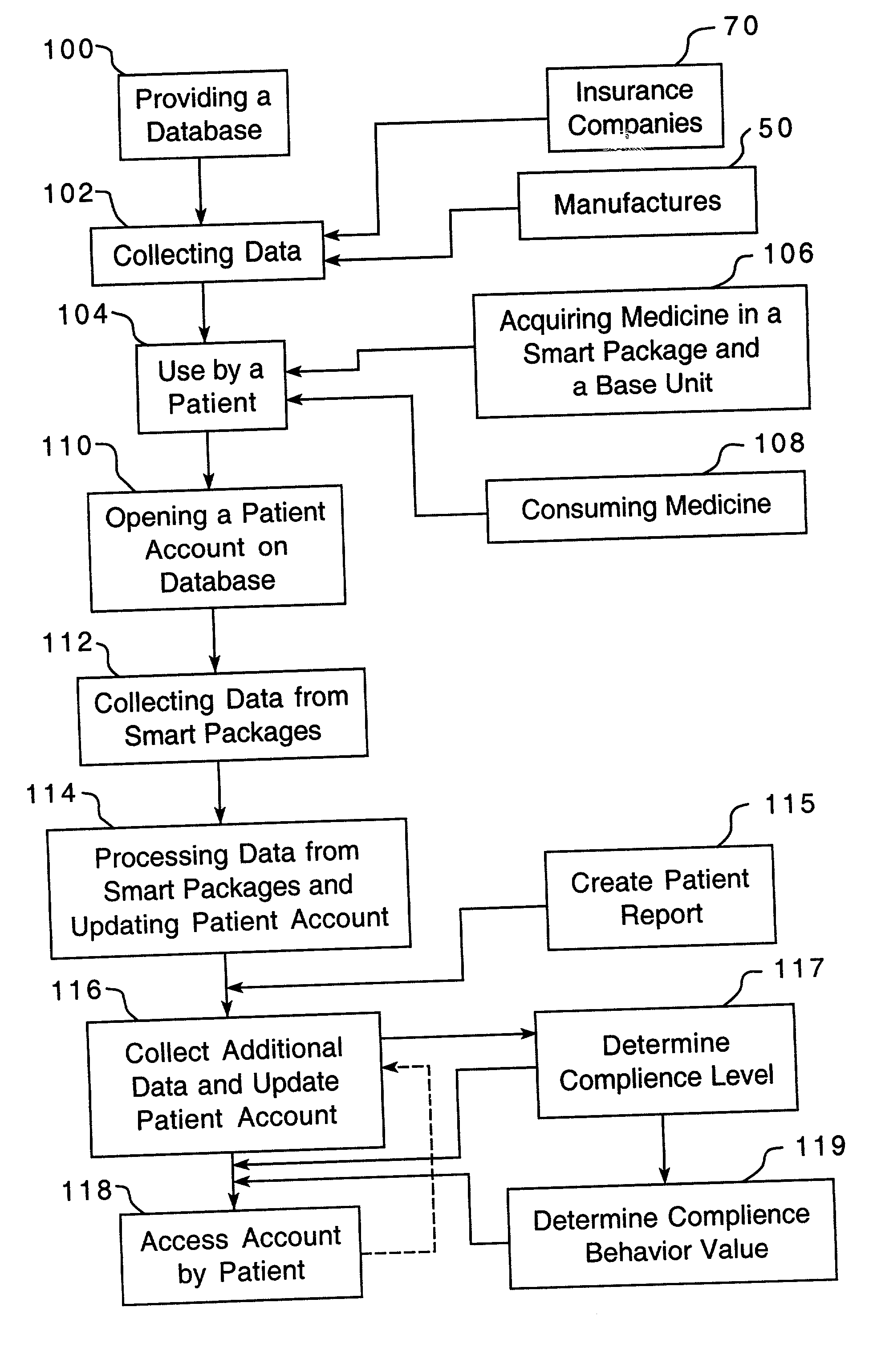
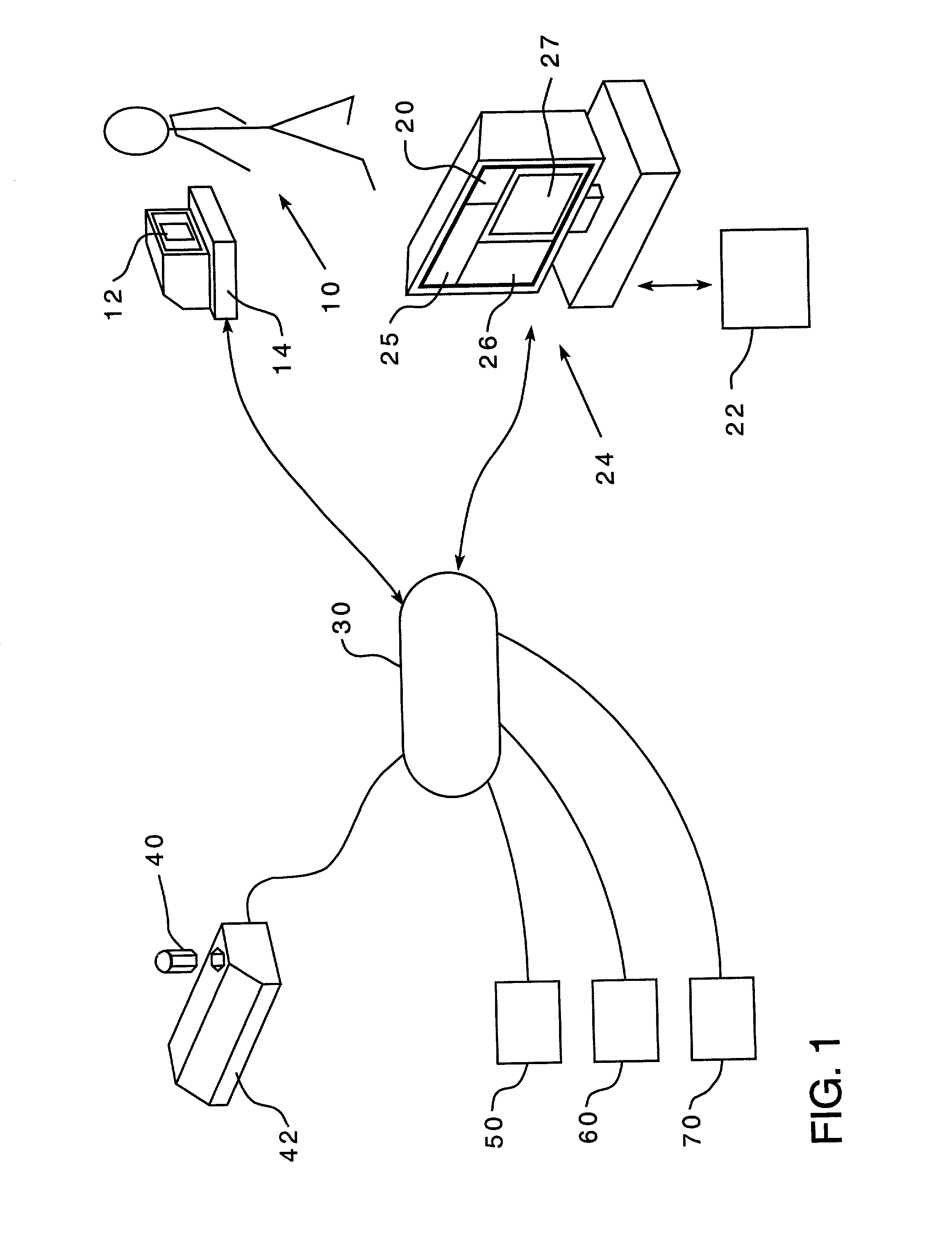
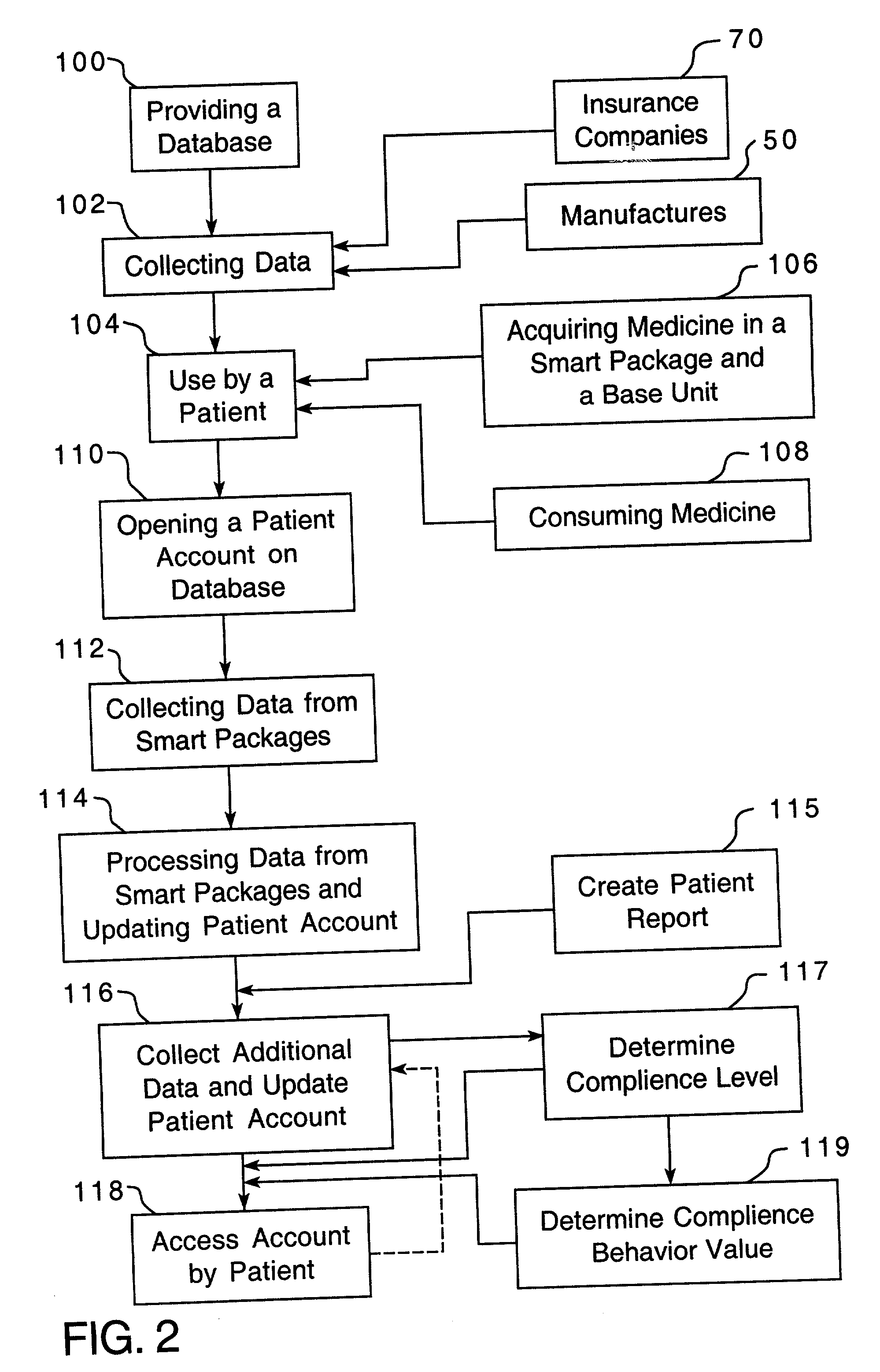
A method is provided for encouraging patient compliance with a medicine regimen that includes interaction with the patient. The method includes the steps of providing a database which includes information regarding each patient. Information is supplied to the database by smart packages and other stakeholders. The information in the database is used to construct a patient report that may be viewed by the patient and others. Information in the patient report includes data on the patient's compliance, side effects of the medicine, expected results, interactions with other medicines, statements complimenting compliance, and warnings discouraging non-compliance. The patient report may be accessed by a common communication means such as the Internet.
Owner:CLEMENTI & ASSOCS
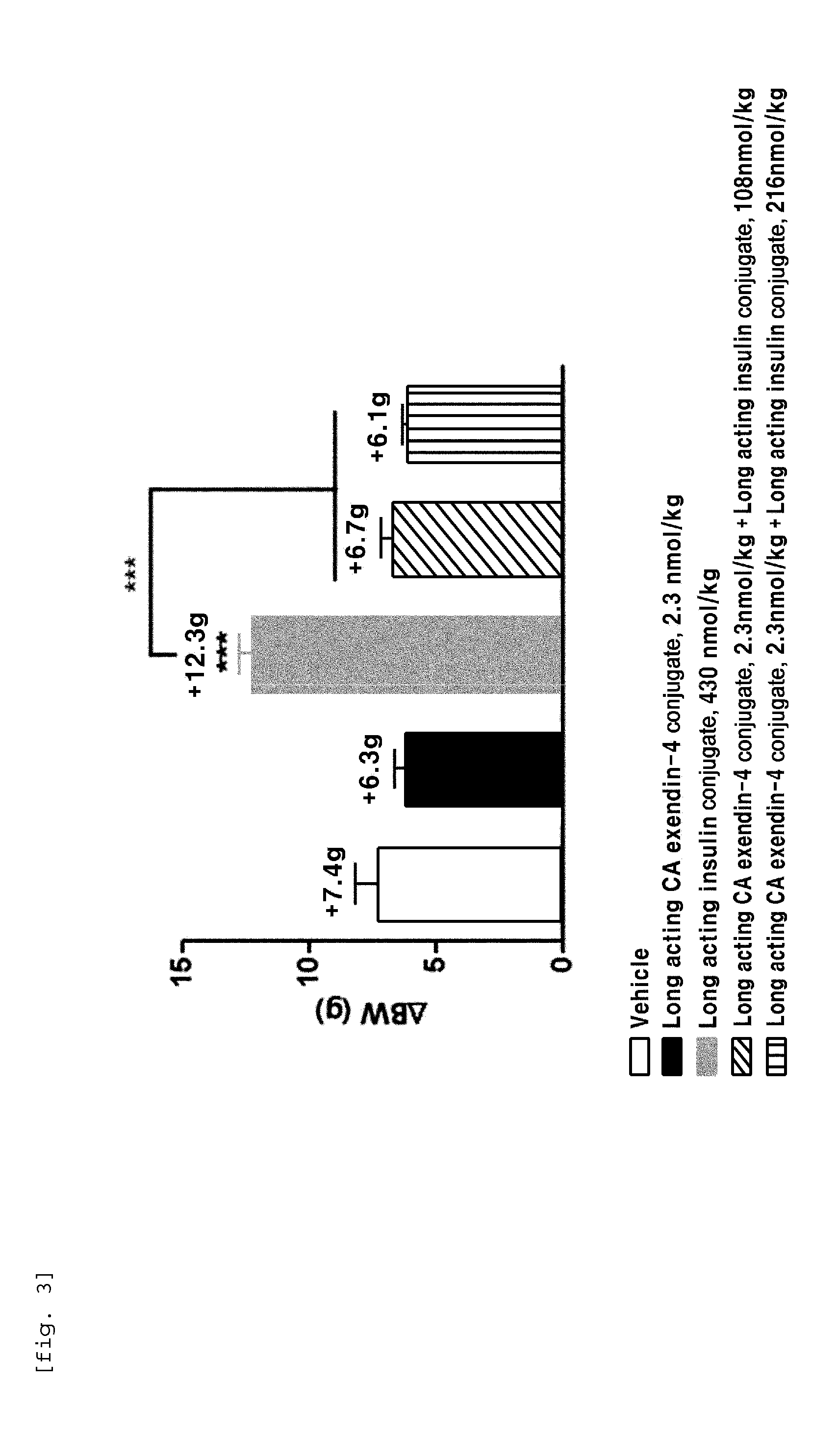
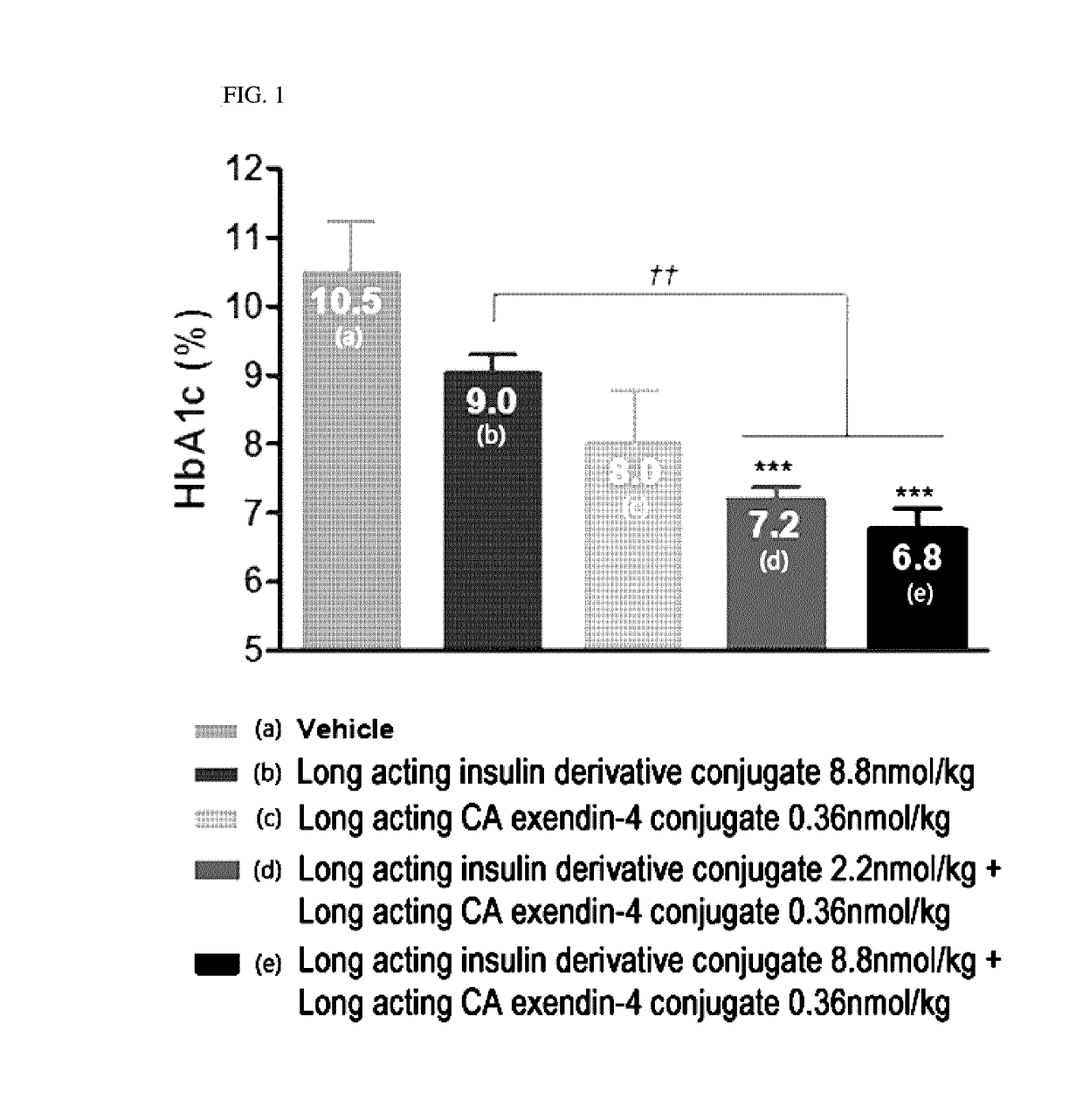
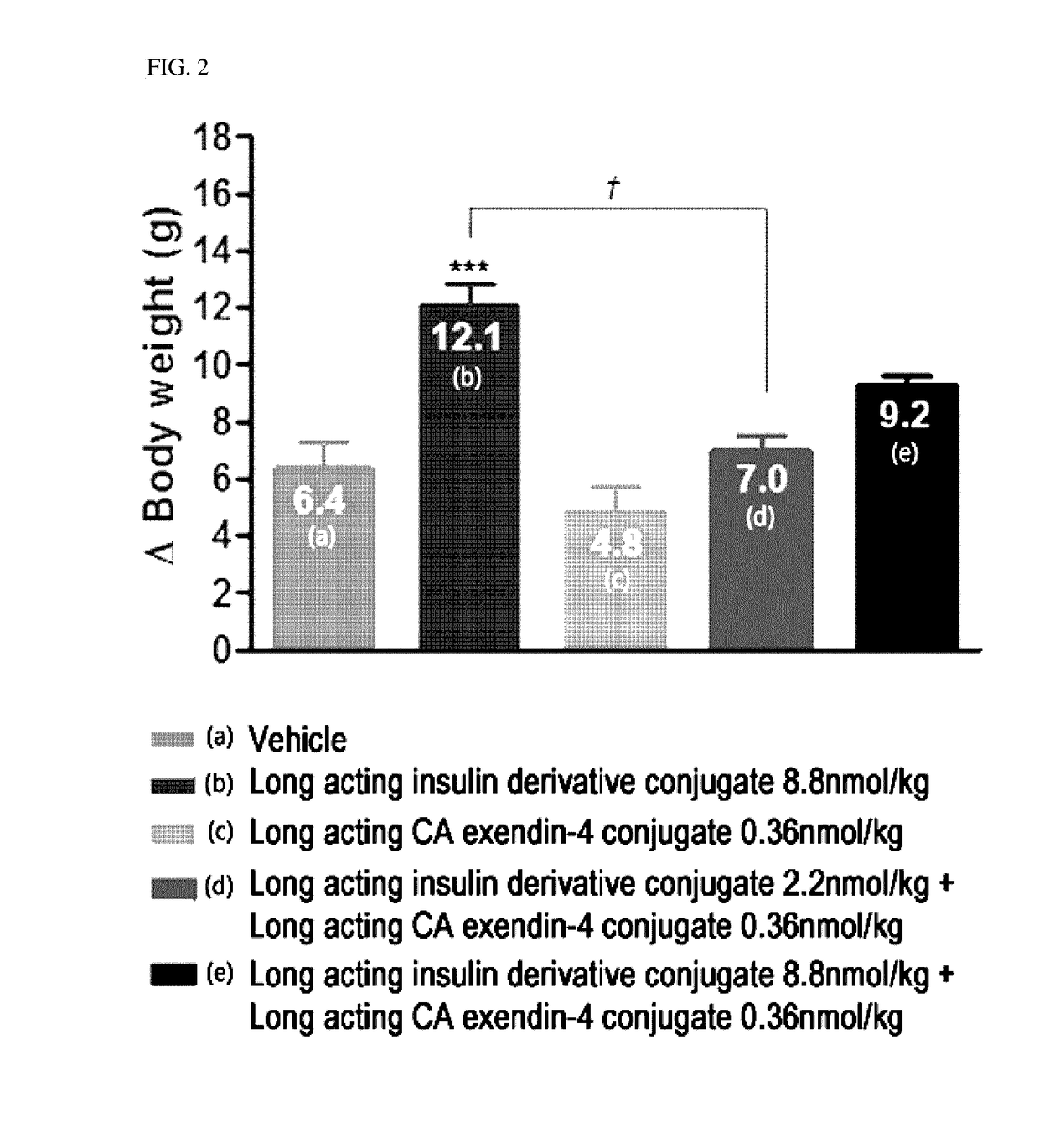
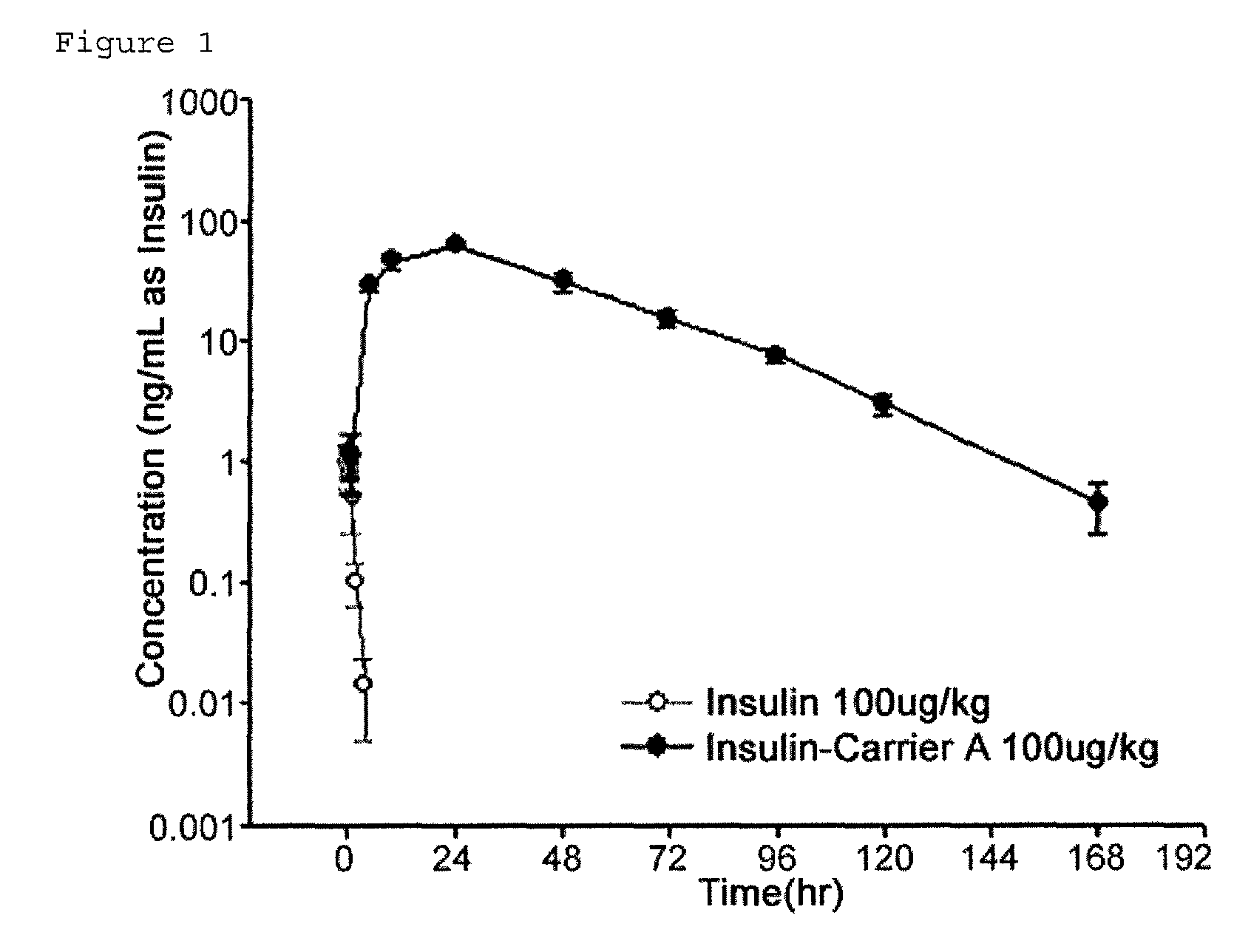
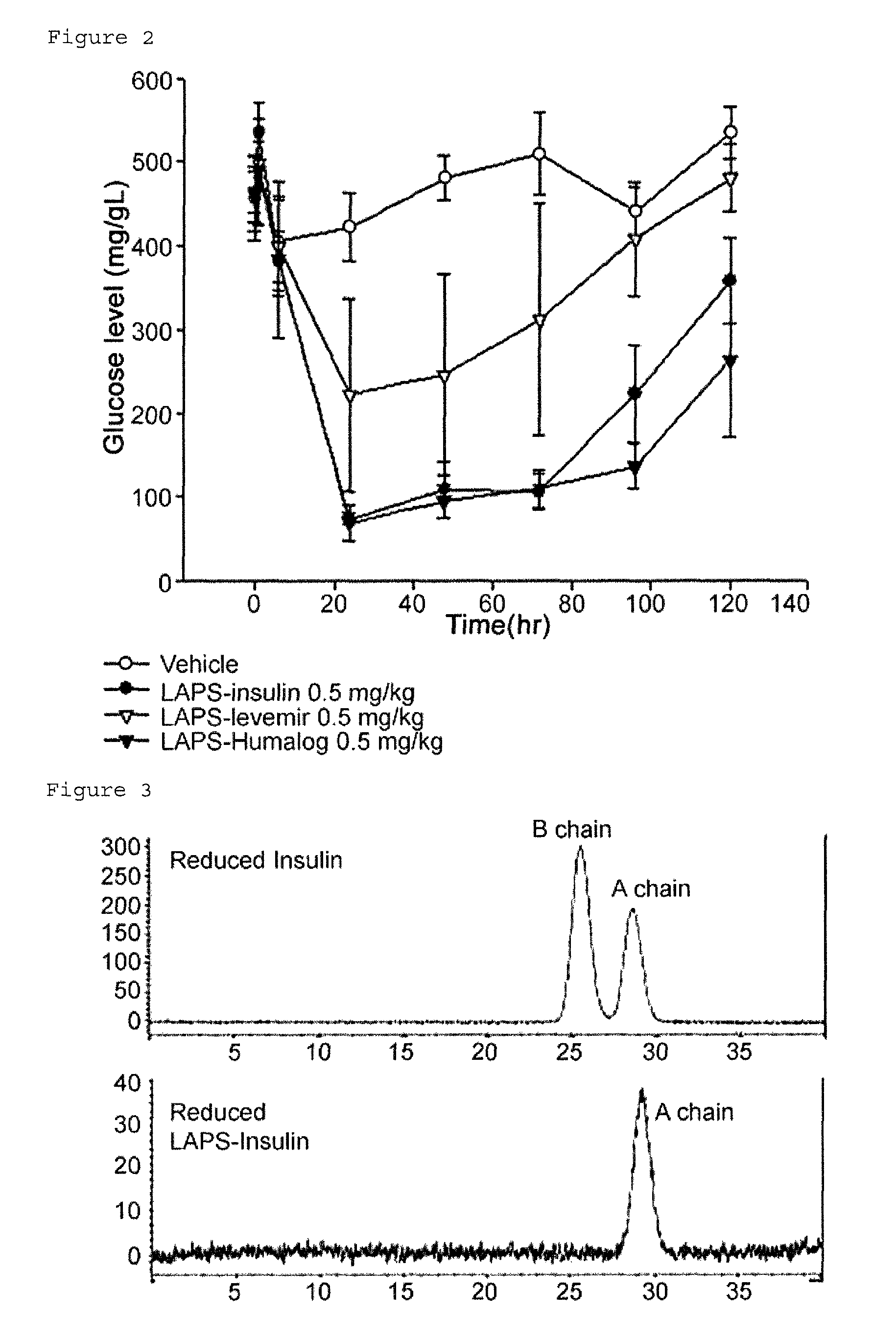
Composition for treating diabetes comprising long-acting insulin conjugate and long-acting insulinotropic peptide conjugate
ActiveUS20140120120A1Good treatment effectImprove stabilityPeptide/protein ingredientsMetabolism disorderIn vivoWeight gain
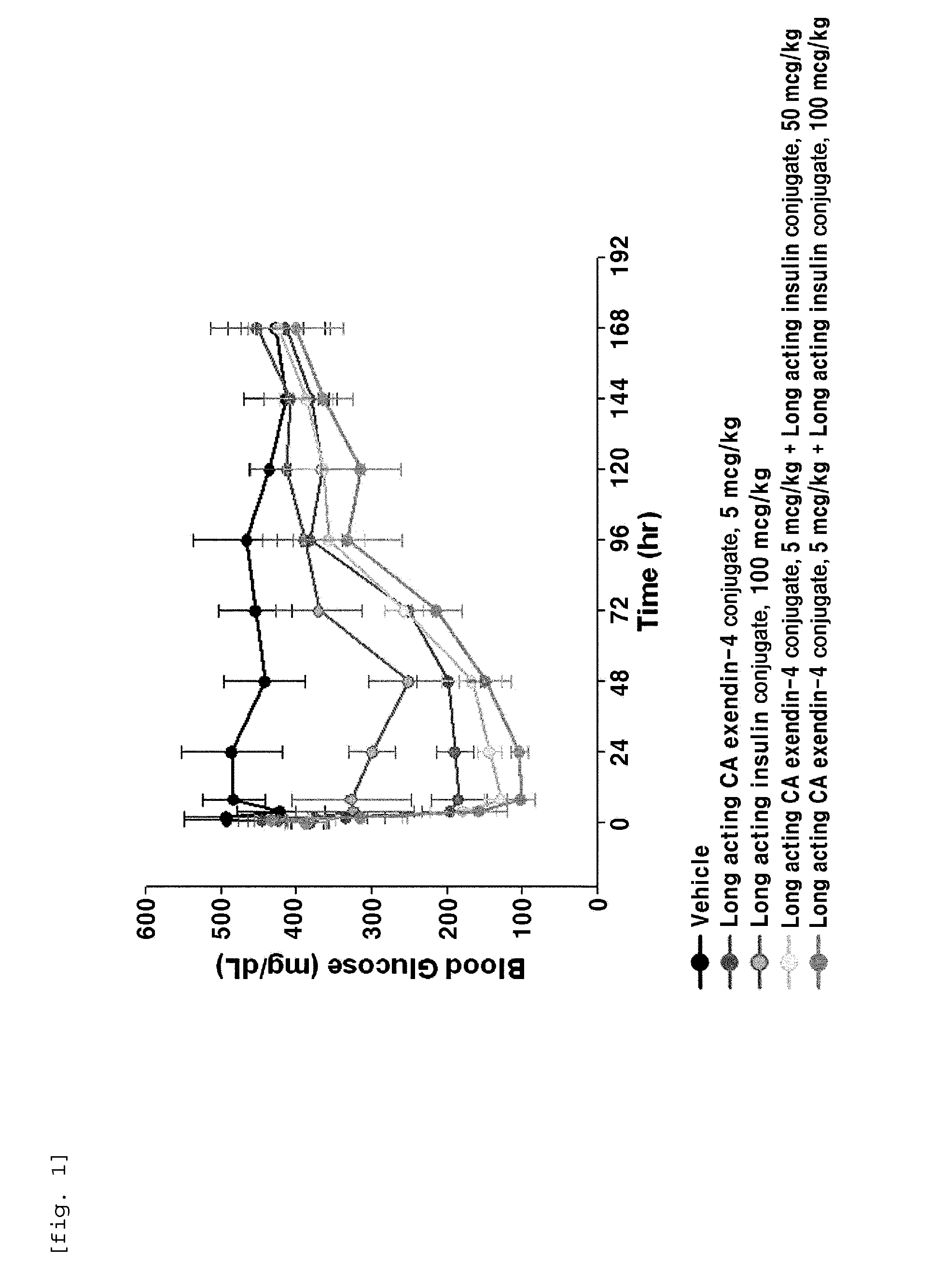
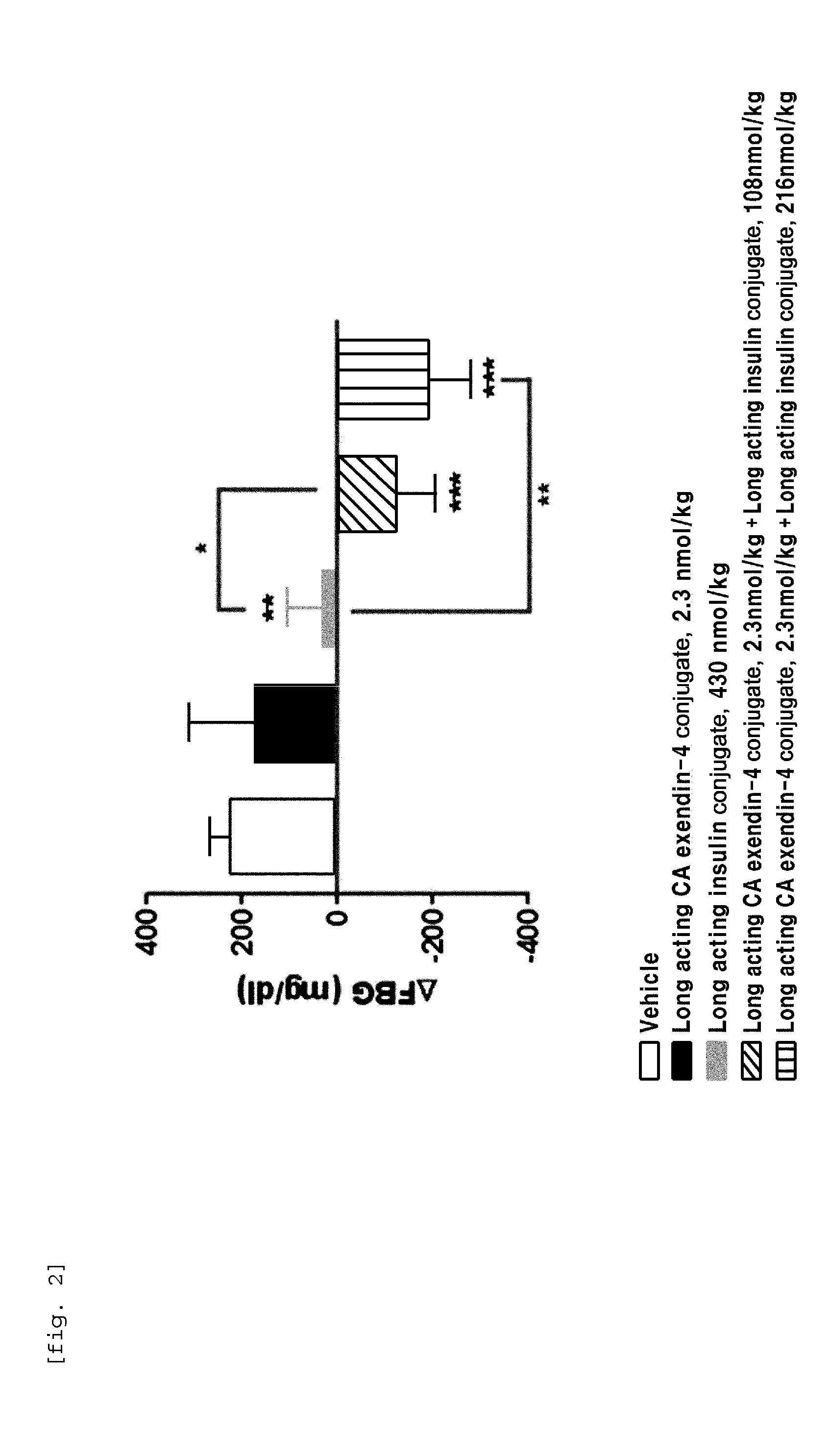
The present invention relates to a composition for the prevention or treatment of diabetes comprising a long-acting insulin conjugate and a long-acting insulinotropic peptide conjugate, and a therapeutic method for the treatment of diabetes, and more particularly, concurrent administration of the long-acting insulin conjugate and the long-acting insulinotropic peptide conjugate inhibits weight gain caused by insulin treatment, and vomiting and nausea caused by insulinotropic peptide treatment, and reduces the required dose of insulin, thereby remarkably improving drug compliance. Moreover, each of the long-acting insulin conjugate and the long-acting insulinotropic peptide conjugate of the present invention is prepared by linking insulin or insulinotropic peptide with an immunoglobulin Fc region via a non-peptidyl linker, thereby showing improved in-vivo duration of efficacy and stability.
Owner:HANMI SCI CO LTD
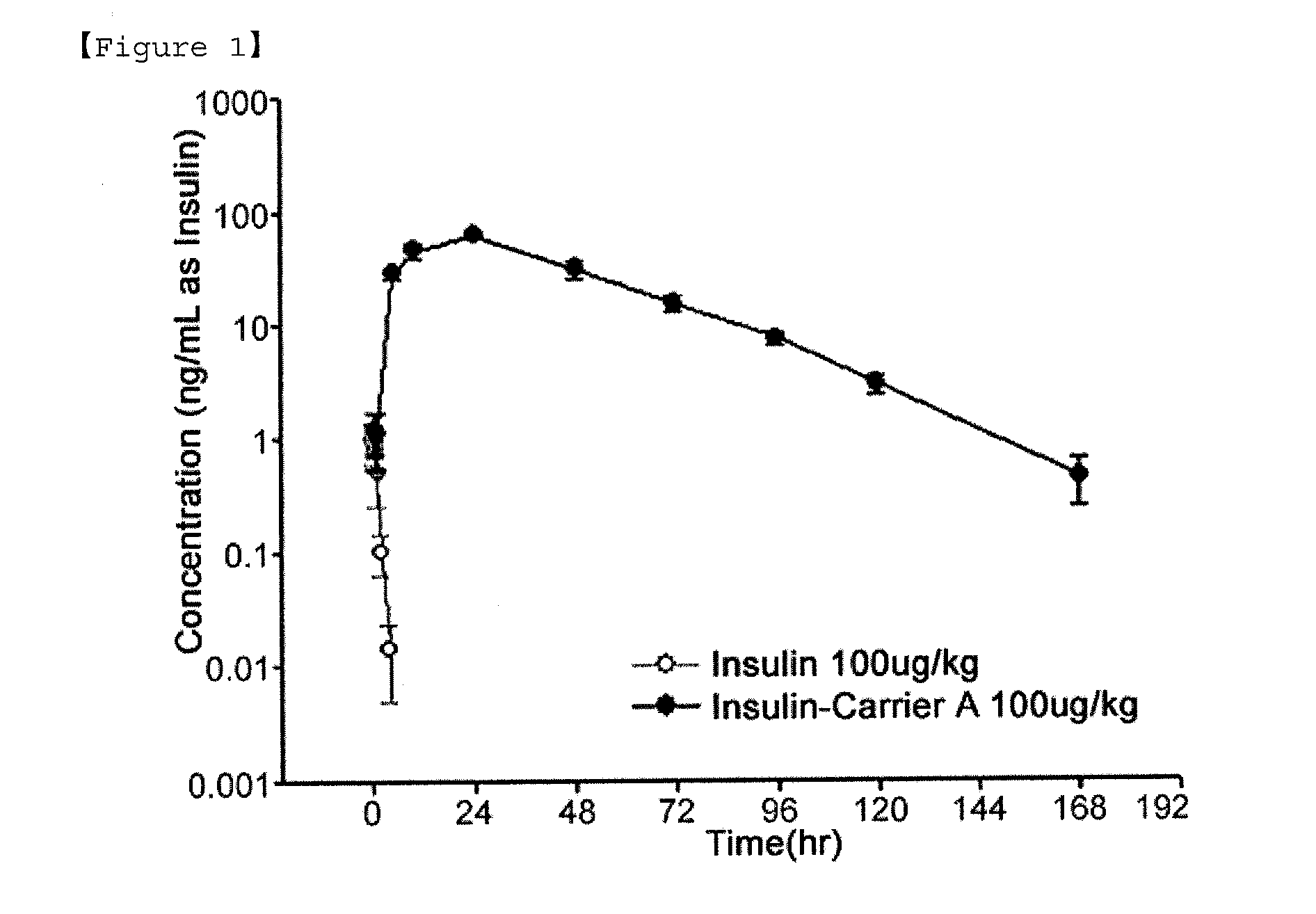
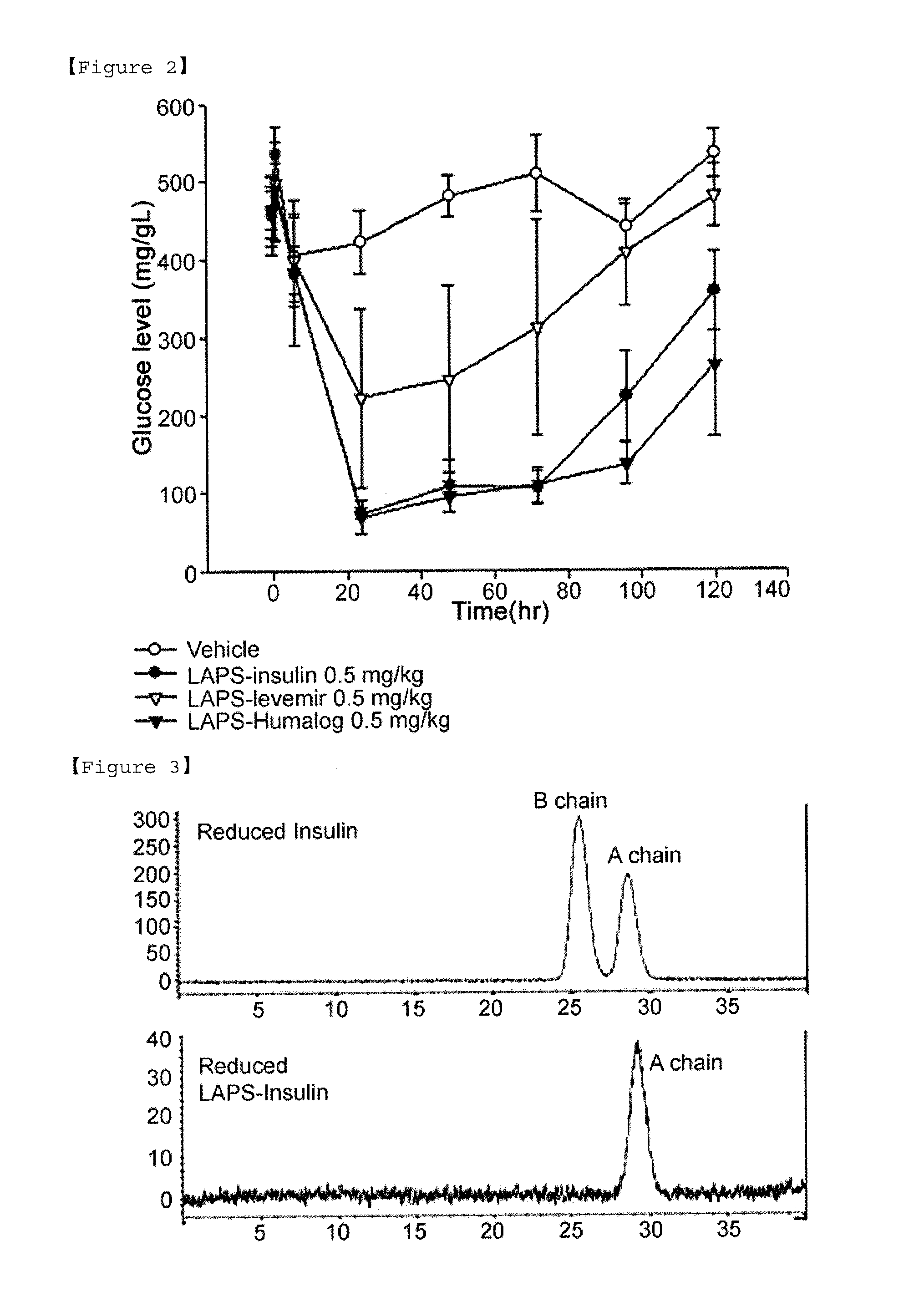
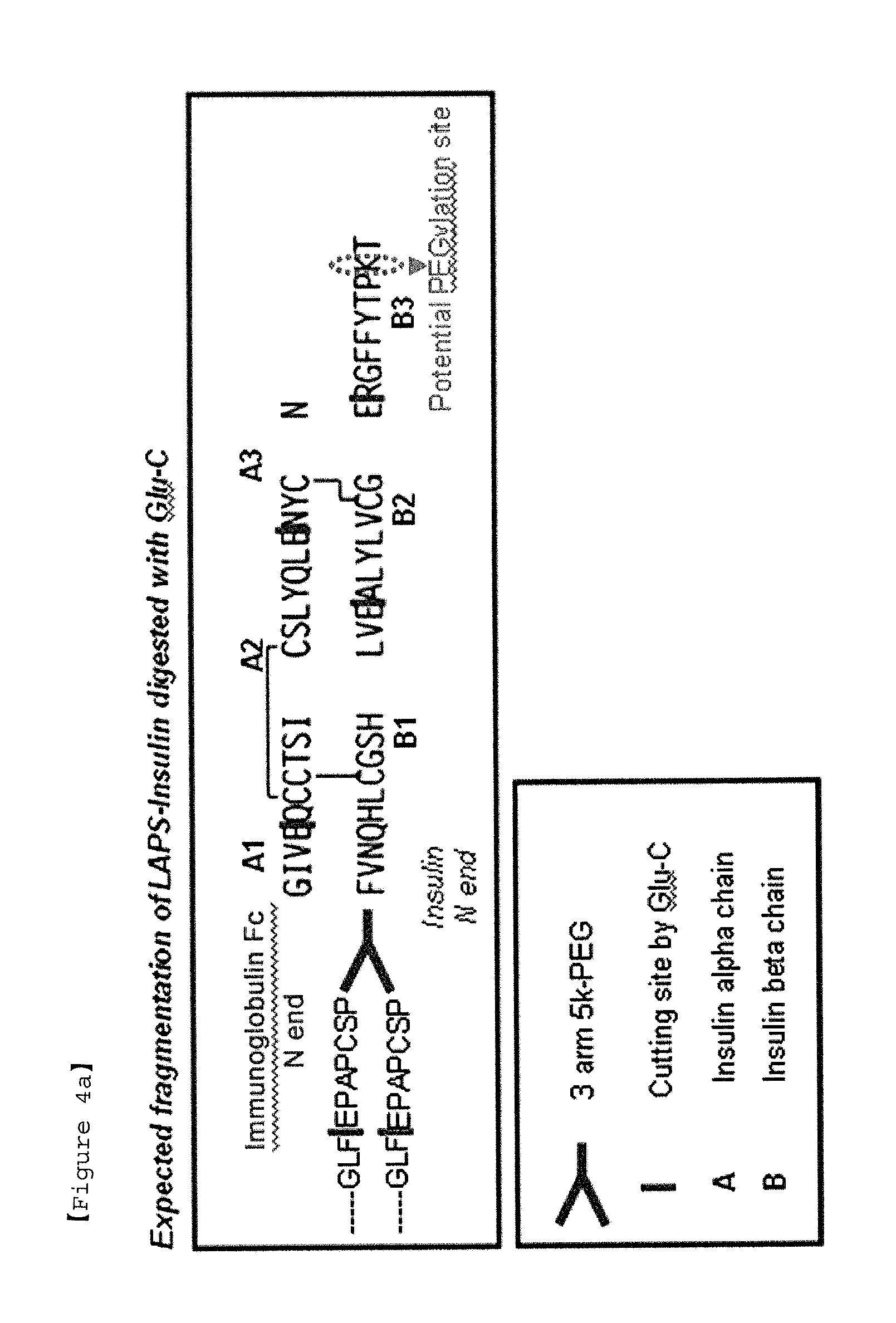
Insulin conjugate using an immunoglobulin fragment
ActiveUS20130028918A1Improve the level ofExtended half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeIn vivo
The present invention relates to an insulin conjugate having improved in vivo duration and stability, which is prepared by covalently linking insulin with an immunoglobulin Fc region via a non-peptidyl polymer, a long-acting formulation comprising the same, and a preparation method thereof. The insulin conjugate of the present invention maintains in vivo activity of the peptide at a relatively high level and remarkably increases the serum half-life thereof, thereby greatly improving drug compliance upon insulin treatment.
Owner:HANMI SCI CO LTD
Marker Detection Method And Apparatus To Monitor Drug Compliance
InactiveUS20140294675A1Accurate assessmentPatient complianceWithdrawing sample devicesDiagnostic recording/measuringNoseEnvironmental health
The present invention includes systems and methods for monitoring therapeutic drug concentration in blood by detecting markers, such as odors, upon exhalation by a patient after the drug is taken, wherein such markers result either directly from the drug itself or from an additive combined with the drug. In the case of olfactory markers, the invention preferably utilizes electronic sensor technology, such as the commercial devices referred to as “artificial” or “electronic” noses or tongues, to non-invasively monitor drug levels in blood. The invention further includes a reporting system capable of tracking drug concentrations in blood (remote or proximate locations) and providing the necessary alerts with regarding to ineffective or toxic drug dosages in a patient.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
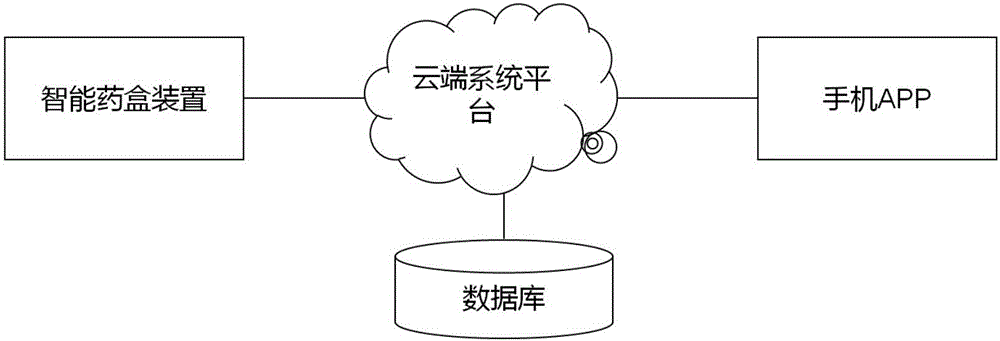
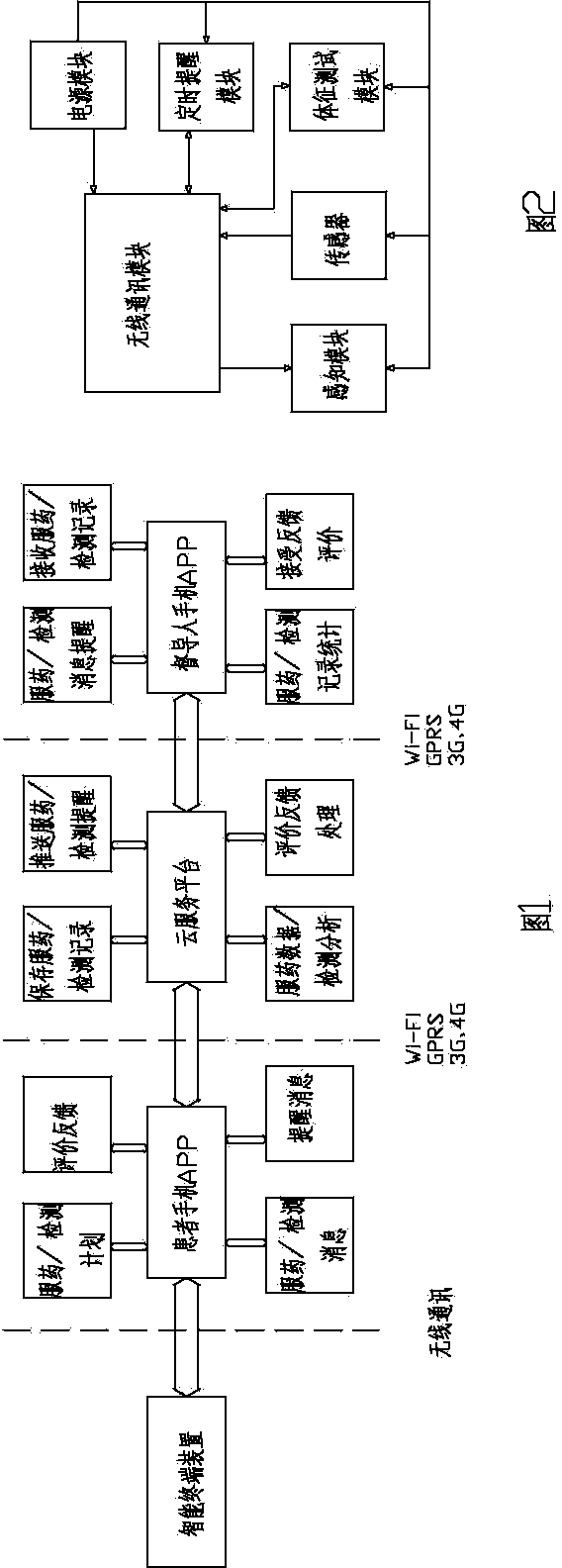
Method for improving medication compliance of patient and intelligent medicine box system
ActiveCN106420347AImprove Medication AdherenceSolve the problem of forgettingPharmaceutical containersMedical packagingRegimenLoudspeaker
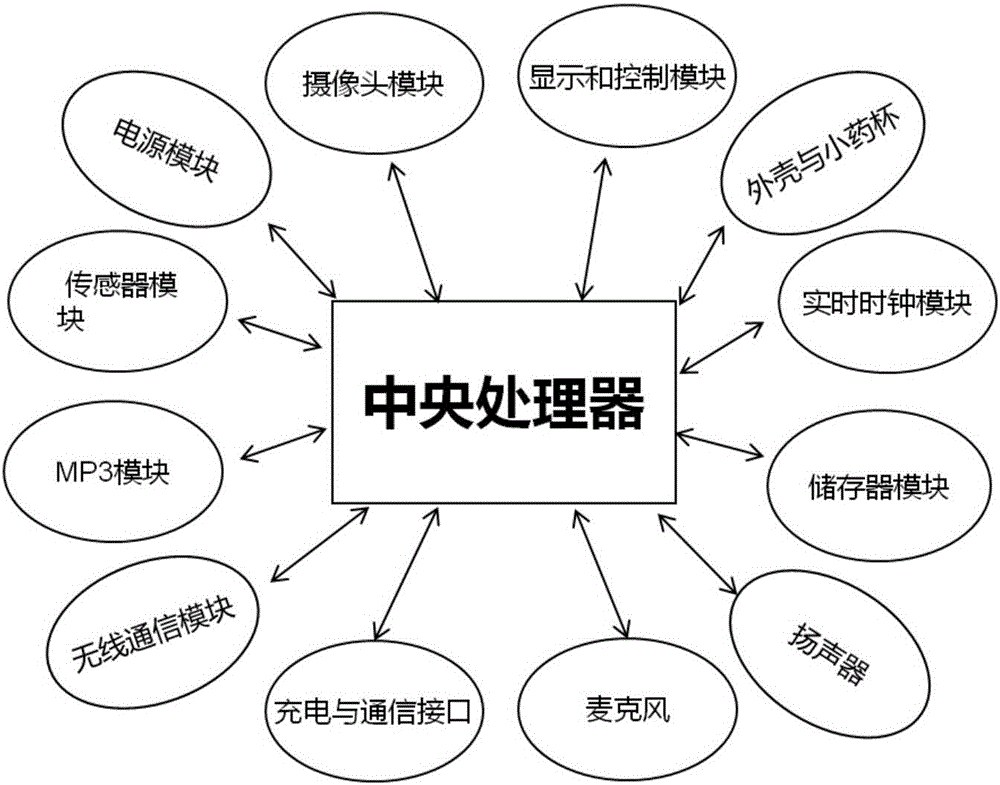
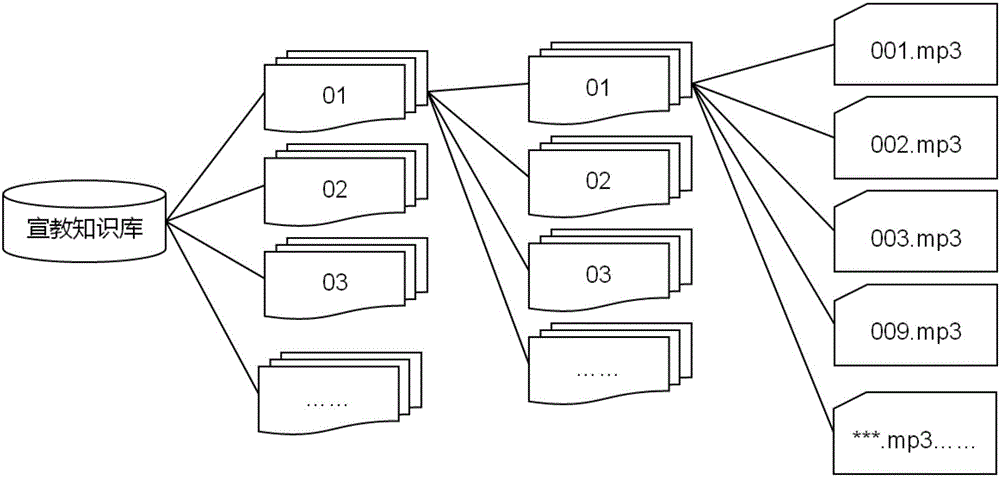
The invention relates to a method for improving medication compliance of a patient and an intelligent medicine box system. The method comprises the following steps: arranging the intelligent medicine box system which comprises an intelligent medicine box device, a cloud platform and a mobile phone APP, wherein the intelligent medicine box device comprises a central processing module, a real-time clock module, a camera module, a wireless communication module, an MP3 play module, a sensor module, a memory module and a loudspeaker; establishing a knowledge base, and arranging contents of the knowledge base at different time points of the course of treatment to form a stepped health education knowledge base; recording knowledge contents of the knowledge base into a voice file, then translating the file into different language versions, and performing classified hierarchical storage; copying the voice file together with a catalogue to a memory card of the intelligent medicine box device; calculating the medication time when the patient takes medicine every time, retrieving the voice file with the same name of times or days in the corresponding catalogue in the knowledge base on the memory card, and finally achieving the synchronous health education in the process of medication of the patient in a way of playing or prompting the patient to listen.
Owner:THE FIRST TEACHING HOSPITAL OF XINJIANG MEDICAL UNIVERCITY +1
Pharmaceutical composition having activity of anticancer
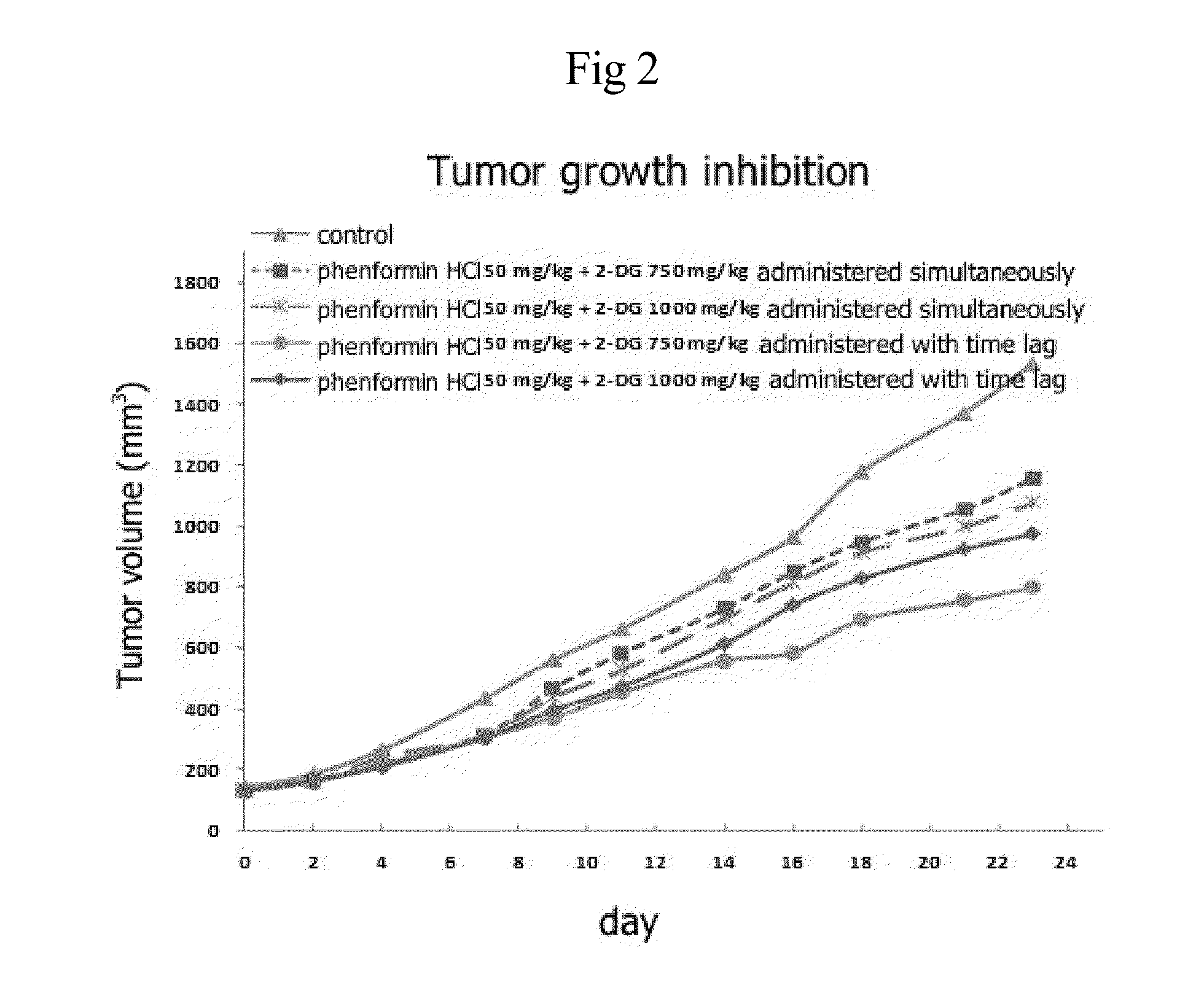
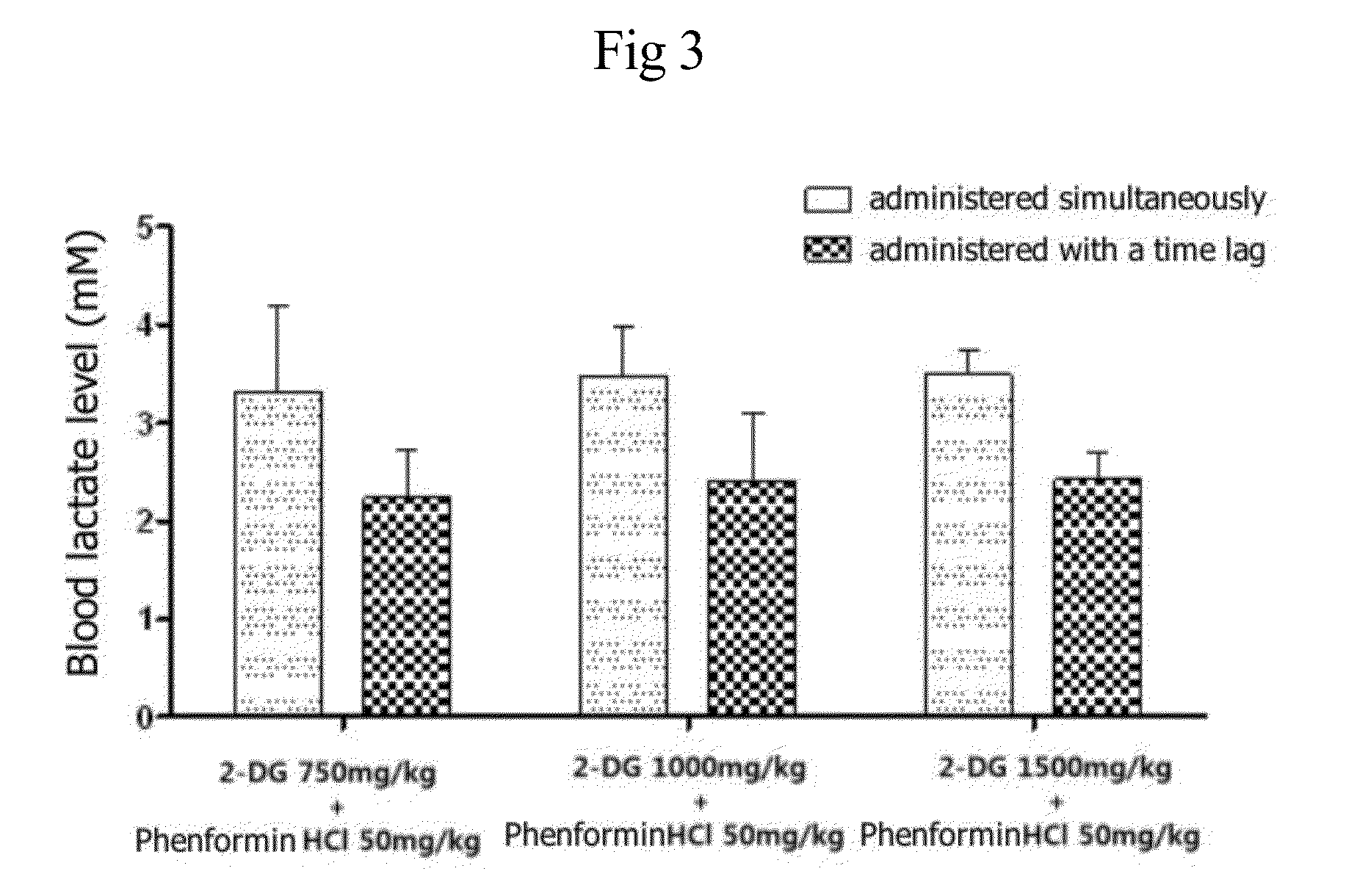
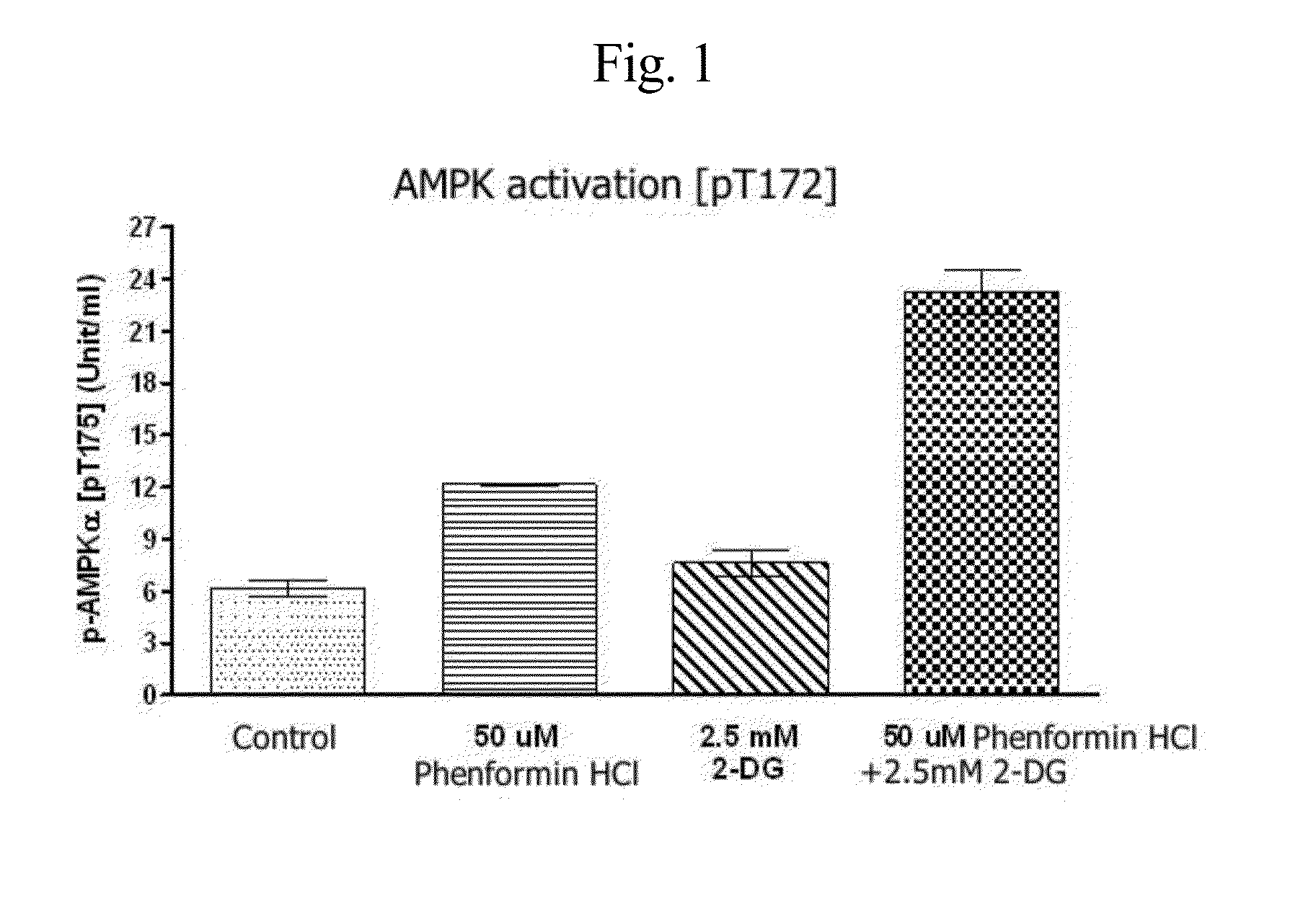
InactiveUS20140235558A1Improve anti-cancer effectDoses necessary for the therapy of cancer can be decreasedBiocideCarbohydrate active ingredientsTime lagBULK ACTIVE INGREDIENT
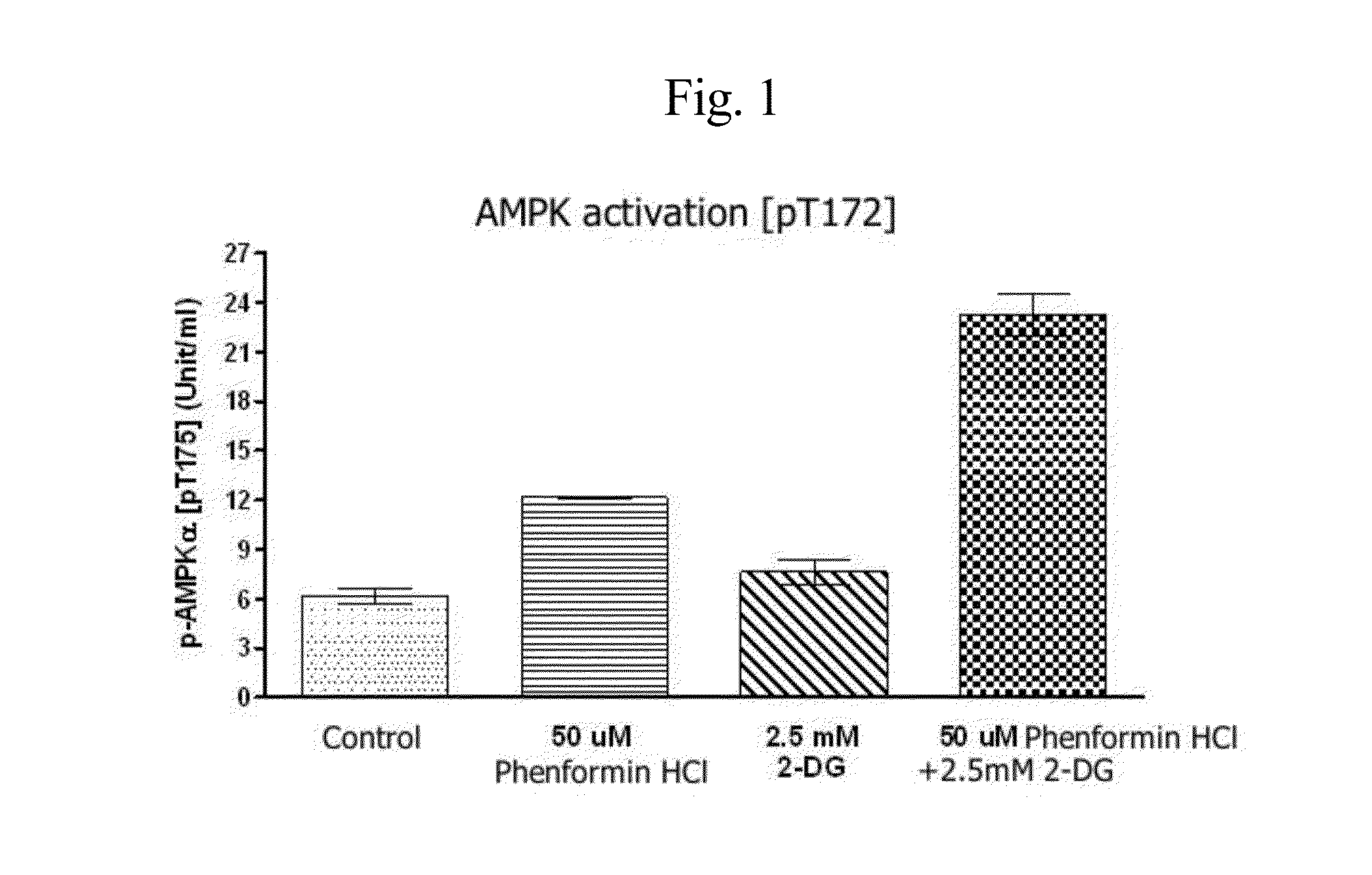
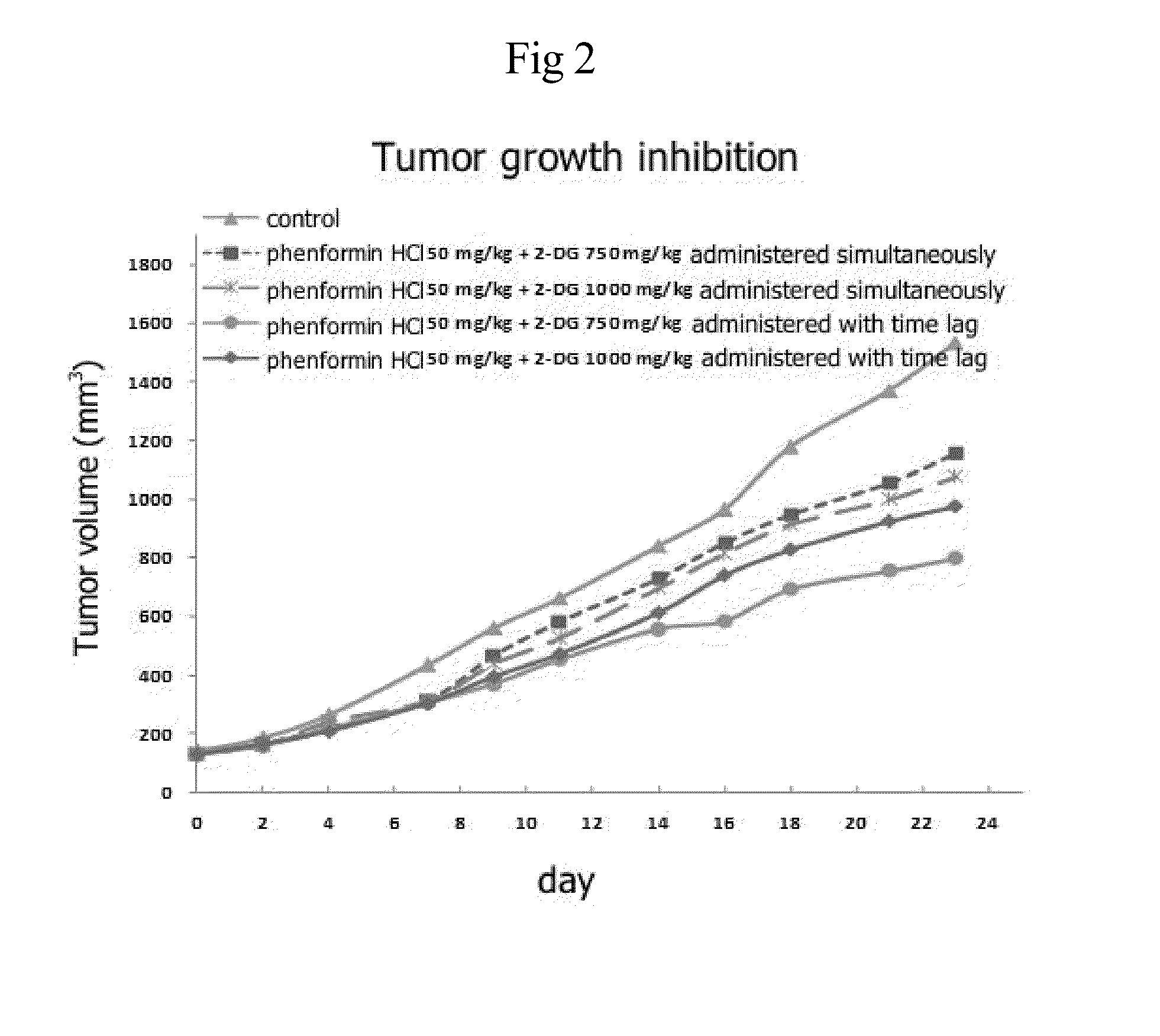
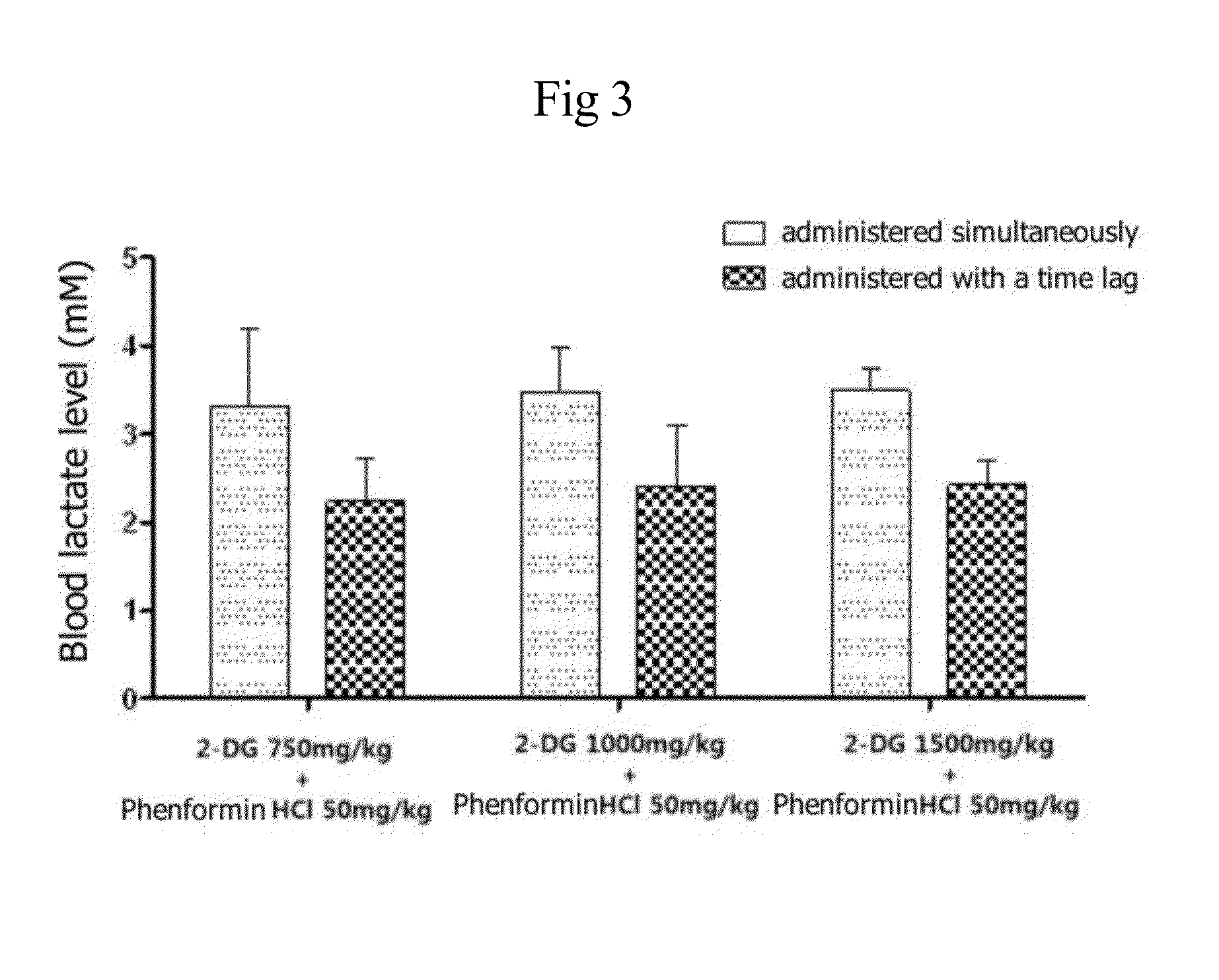
Disclosed is an anticancer pharmaceutical composition comprising phenformin or a pharmaceutically acceptable salt thereof, and a glycolysis inhibitor, particularly, 2-deoxy-D-glucose as active ingredients. These ingredients act in synergy with each other, thus exhibiting more potent inhibitory activity against the growth of cancer cells, compared to individual ingredients. Also, the synergistic anticancer activity allows the individual drugs to be used in lower amounts, which leads to a reduction in the occurrence of adverse effects. In addition, the time-lag release or administration of the ingredients decreases blood lactic acid levels to significantly mitigate the adverse effect of lactic acidosis, as well as exerting high anticancer effects. Particularly, the pharmaceutical composition can be formulated to dosage forms effective for therapy, increasing the drug compliance of the subject.
Owner:HANALL PHARMA CO LTD
Pharmaceutical compositions and preparations for treatment of metabolic bone disease
The present invention relates to a pharmaceutical composition for the treatment of metabolic bone disease and the method of preparation thereof, and more particularly, to an improved pharmaceutical composition for the therapeutic treatment of metabolic disease and the method of preparation thereof, wherein said composition is prepared as a composite pharmaceutical agent which comprises calcitriol; which reduces the rate of spine fractures and increases bone density; alendronate, a bone resorption inhibitor, as two main active ingredients in an optimal mixing ratio to exert the greatest synergistic therapeutic effect; and adequate amount of other additives such as a resorption fortifier of alendronate. Thereof, the pharmaceutical composition according to the present invention can inhibit hypercalcemia caused when administered by calcitriol alone, compensate the inhibitory activity of bone remodeling caused by alendronate due to the presence of calcitriol, and improve drug compliance associated with the usual difficulty in administration as well as a side effect in esophagus, thus effectively preventing the occurence of osteoporosis.
Owner:YUYU IND
Slow/controlled-release preparation of ticagrelor
InactiveCN103860504AImprove complianceGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismTicagrelorThrombus
The invention provides a slow / controlled-release preparation of ticagrelor. The slow / controlled-release preparation of ticagrelor is an oral drug. The slow / controlled-release preparation contains ticagrelor or its pharmaceutically acceptable salt. A mass percent of ticagrelor to controlled-release accessory materials is in a range of 1: 0.2 to 1: 20 and preferably, the mass percent is in a range of 1: 0.1 to 1: 10, and a proper amount of other accessory materials are used. The slow / controlled-release base comprises one or more of cellulose, cellulose derivatives, alginate, starch, starch derivatives, polypropylene resins, carboxyvinyl polymers and other controlled-release accessory materials. Compared with a fast-release preparation, the slow / controlled-release preparation of ticagrelor provides a ticagrelor slow / controlled-release preparation system, can be eaten by a patient once each day, can change drug compliance of patients, can reduce the risk of myocardial infarction or stroke caused by acute thrombosis caused by missing of ticagrelor, and provides the easily-prepared slow / controlled-release preparation of ticagrelor or its pharmaceutically acceptable salt.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Anti-cancer pharmaceutical composition
InactiveUS20140235559A1Inhibitory activityDecrease blood lactic acid levelBiocideMetabolism disorderTime lagCancer cell
Disclosed is an anticancer pharmaceutical composition comprising phenformin or a pharmaceutically acceptable salt thereof, and 2-deoxy-D-glucose as active ingredients. These ingredients act in synergy with each other, thus exhibiting more potent inhibitory activity against the growth of cancer cells, compared to individual ingredients. Also, the synergistic anticancer activity allows the individual drugs to be used in lower amounts, which leads to a reduction in the occurrence of adverse effects. In addition, the time-lag release or administration of the ingredients decreases blood lactic acid levels to significantly mitigate the adverse effect of lactic acidosis, as well as exerting high anticancer effects. Particularly, the pharmaceutical composition can be formulated to dosage forms effective for therapy, increasing the drug compliance of the subject.
Owner:HANALL PHARMA CO LTD
Carragheenan and potassium chloride gelled hydroxypropyl methylcellulose enteric-coated hollow capsule
ActiveCN103394093AReduced risk of hygroscopicityReduce the impactCosmetic preparationsToilet preparationsPutrefactionVegetable fibers
The invention relates to a carragheenan and potassium chloride gelled hydroxypropyl methylcellulose enteric-coated hollow capsule, and belongs to the technical field of the production of hollow capsules. The hollow capsule is characterized in that the hollow capsule is prepared through mixing a main raw material hydroxypropyl methylcellulose with carragheenan and potassium chloride under a special condition, the hollow capsule has the characteristics of timely disintegration, low water content, suitableness for medicines having strong hygroscopic property and being sensitive to water, small influences of a dissolve-out medium to the shell of the capsule, high drug compliance, long storage duration data, no deterioration within 3-4 years, low condition requirements on the storage and the transportation, difficult crushing in the low humidity environment, difficult deformation, putrefaction and deterioration in the high temperature environment, according with the requirements of populations having all culture backgrounds because of pure vegetable fibers, almost no pollution in the production process, secondary re-dissolving utilization of tailings, omission of the commonly-used surfactants and plasticizers, acceleration of the gelling speed by reducing the gelling temperature only through utilizing potassium chloride to assist the carragheenan, additive reduction, production cost reduction, and suitableness for the mass popularization.
Owner:SICHUAN TIANSHENG PHARMA
Device for supervising medication compliance of patient and tracking medication effects and using method of device
InactiveCN104224550AMonitor Medication AdherenceImprove Medication AdherenceOral administration deviceMedication effectsTerminal equipment
The invention relates to the technical field of devices and methods for supervising the medicine taking of patients, and discloses a device for supervising the medication compliance of a patient and tracking medication effects and a using method of the device. The device for supervising the medication compliance of the patient and tracking the medication effects comprises a medicine box, an intelligent terminal device, a patient mobile phone and a cloud service platform, wherein the intelligent terminal device is fixedly arranged in the medicine box, and comprises a shell; a sensor capable of sensing the opening of the medicine box, a wireless communication module, a timed reminding module, a sensing module and a power module are arranged in the shell. According to the device for supervising the medication compliance of the patient and tracking the medication effects and the using method of the device, the patient can effectively execute a medication plan, and can be effectively supervised to take medicine, the medication effects of the patient can be effectively tracked, the medication compliance of the patient is improved, and an important basis is provided for the subsequent treatment of the patient.
Owner:THE FIRST TEACHING HOSPITAL OF XINJIANG MEDICAL UNIVERCITY +1
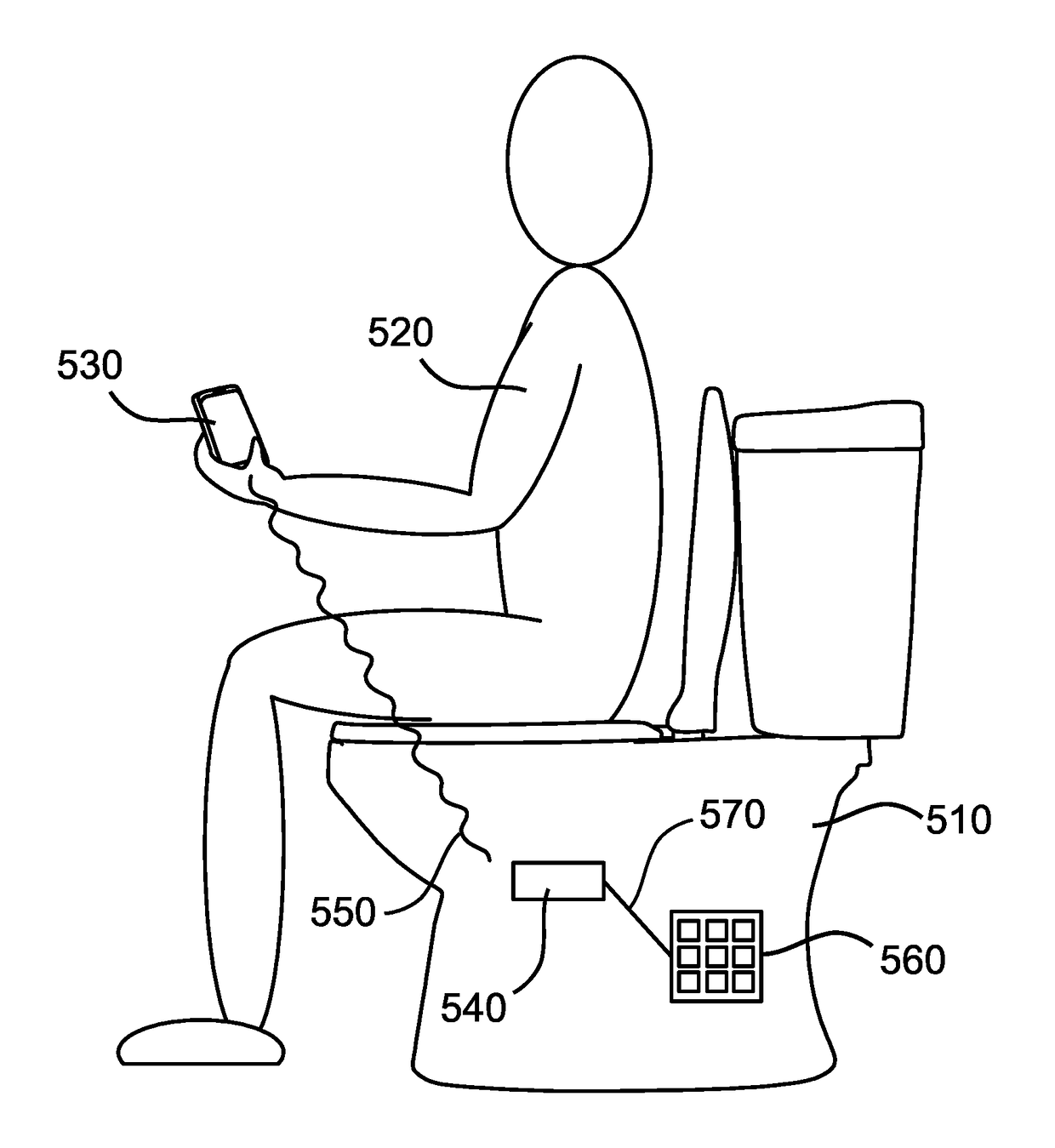
Toilet that detects drug markers and methods of use thereof
The present disclosure describes a method of detecting a drug marker in urine a urine sample using a toilet. The drug markers are fluorophores each of which emits a unique fluorescence spectra. Accordingly, the method does not detect the drug but rather, the drug marker. A user who has consumed the drug with its unique drug marker then urinates into the toilet and a urine sample is captured. The toilet includes a mechanism for fluid handling which diverts urine into a fluorescence spectrometer. The fluorescence spectrometer screens the urine for drug markers based on their unique fluorescent spectra. The toilet may include a controller which quantifies the drug marker. The fluorescent spectrometer may detect multiple drug markers in a single urine sample. This method may be used to confirm drug compliance, test for illicit drugs, identify amounts of drugs consumed, and other uses described herein.
Owner:MEDIC INC
Orally disintegrating tablet of ambroxol hydrochloride and preparation method thereof
InactiveCN101904827AGreat tasteImprove Medication AdherenceOrganic active ingredientsPharmaceutical non-active ingredientsDiseaseSide effect
The invention discloses an orally disintegrating tablet of ambroxol hydrochloride and a preparation method thereof, which can effectively treat acute and chronic respiratory diseases (such as thick sputum and expectoration difficulty due to acute and chronic bronchitis, bronchial asthma, bronchiectasis, tuberculosis and the like.) The invention aims to provide an orally disintegrating tablet of ambroxol hydrochloride for wide patients and medical staff, i.e. a new preparation of a clinical medicine, which has rapid absorption, high bioavailability, no water for taking, little side effect and more convenience and is prepared by using ambroxol hydrochloride as a raw material, adding some special kinds of accessories in a certain proportion according to a technical measure disclosed in the invention. The product of the invention has sweet and fragrant taste, rapid effect and high bioavailability and is particularly easy to improve the drug compliance of patients.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Gellan gum and calcium chloride gelled hydroxypropyl methylcellulose enteric-coated hollow capsule
ActiveCN103394092AReduced risk of hygroscopicityReduce adhesionCosmetic preparationsToilet preparationsPutrefactionVegetable fibers
The invention relates to a gellan gum and calcium chloride gelled hydroxypropyl methylcellulose enteric-coated hollow capsule, and belongs to the technical field of the production of hollow capsules. The hollow capsule is characterized in that the hollow capsule is prepared through mixing a main raw material hydroxypropyl methylcellulose with the gellan gum and calcium chloride under a special condition, the hollow capsule has the characteristics of timely disintegration, low water content, suitableness for medicines having strong hygroscopic property and being sensitive to water, small influences of a dissolve-out medium to the shell of the capsule, high drug compliance, long storage duration data, no deterioration within 3-4 years, low condition requirements on the storage and the transportation, difficult crushing in the low humidity environment, difficult deformation, putrefaction and deterioration in the high temperature environment, according with the requirements of populations having all culture backgrounds and religious faiths because of pure vegetable fibers, almost no pollution in the production process, secondary re-dissolving utilization of tailings, omission of the commonly-used surfactants and plasticizers, acceleration of the gelling speed by reducing the gelling temperature only through utilizing calcium chloride to assist the gellan gum, additive reduction, production cost reduction, and suitableness for the mass popularization.
Owner:SICHUAN TIANSHENG PHARMA
Electronic compliance system and associated methods
ActiveUS10521561B1Low costMinimizing required patient monitoring/interactionDrug and medicationsMedical devicesElectronic transmissionMonitoring system
An electronic drug compliance monitoring system and associated methods utilize a pill having an electronic transmission capability and external means for receiving that transmission to sense the presence of the pill in the patient's body or digestive tract.
Owner:ETECTRX INC
Macitentan oral disintegrating tablet for treating PAH (pulmonary arterial hypertension) and preparation method of macitentan oral disintegrating tablet
InactiveCN107913256ADisintegrates quicklyGreat tasteOrganic active ingredientsRespiratory disorderDiseaseSolubility
The invention discloses a macitentan orally disintegrating tablet and a preparation method thereof. The orally disintegrating tablet is a drug mixture comprising macitentan, flavoring agent, filler, disintegrating agent, lubricant, wetting agent and binder, wherein the physiologically active ingredient macitentan is The weight percentage of disintegrating tablets is 5-10%. The solid pharmaceutical preparation provided by the invention adopts solid dispersion technology. By mixing and grinding the main drug and the filler, the solubility of the main drug is improved, the dissolution effect is improved, and the dissolution rate of the preparation is ensured. High content uniformity and high bioavailability. In addition, the macitentan orally disintegrating tablet with convenient administration, rapid onset of effect and high bioavailability of the present invention can improve the drug compliance of patients and is beneficial to the treatment of diseases.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
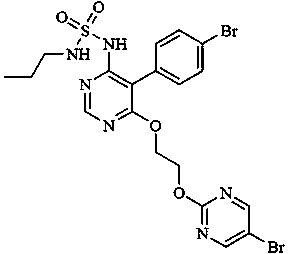
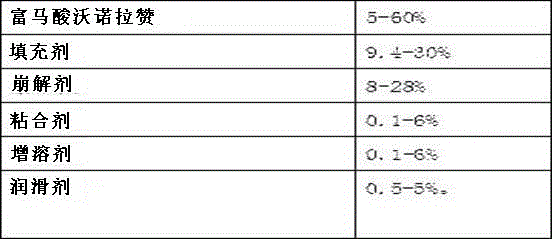
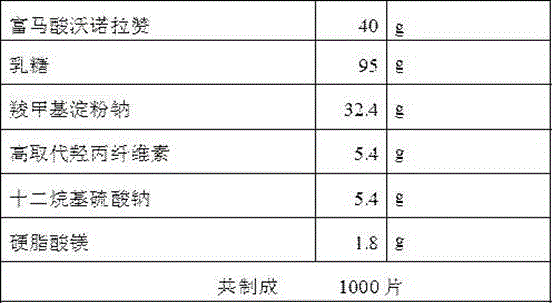
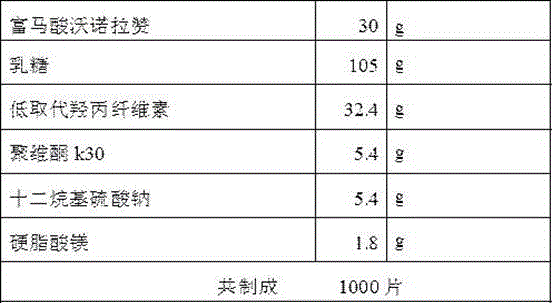
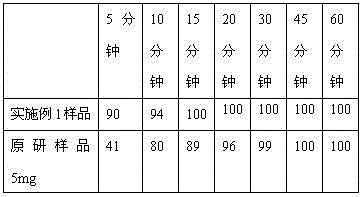
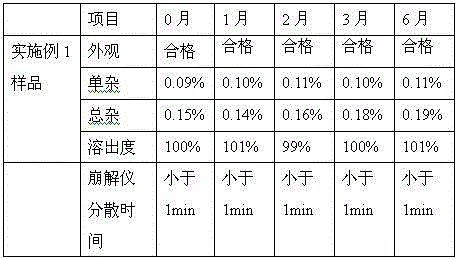
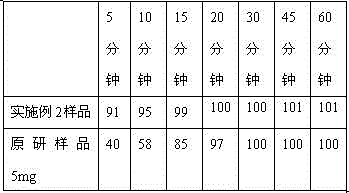
Vonoprazan fumarate dispersible tablets and preparation method thereof
InactiveCN106074406ADisintegrates quicklyFast absorptionOrganic active ingredientsDigestive systemSide effectVonoprazan
The invention discloses vonoprazan fumarate dispersible tablets, used for treating gastric and duodenal ulcers. The vonoprazan fumarate dispersible tablets are prepared from vonoprazan fumarate, with the addition of adjuvant materials. The vonoprazan fumarate dispersible tablets are rapid in disintegration and absorption, high in bioavailability, convenient to take, low in content of intestinal residues, light in side effects, sweet in taste and fragrant; and especially, the dispersible tablets are easy to improve the medication compliance of patients.
Linagliptin composition and preparation method thereof
InactiveCN104644563ADisintegrates quicklyMask bitternessMetabolism disorderPharmaceutical non-active ingredientsMedicineDrug compliance
The invention discloses linagliptin particles and a preparation method thereof. Linagliptin is used for treating type 2 diebetes. At present, only linagliptin tablets are sold on the market. Aiming at dysphagia patients, compared with the above two dosage forms, the linagliptin particles have obvious advantages and can improve drug compliance of patients.
Owner:TIANJIN HANKANG PHARMA BIOTECH
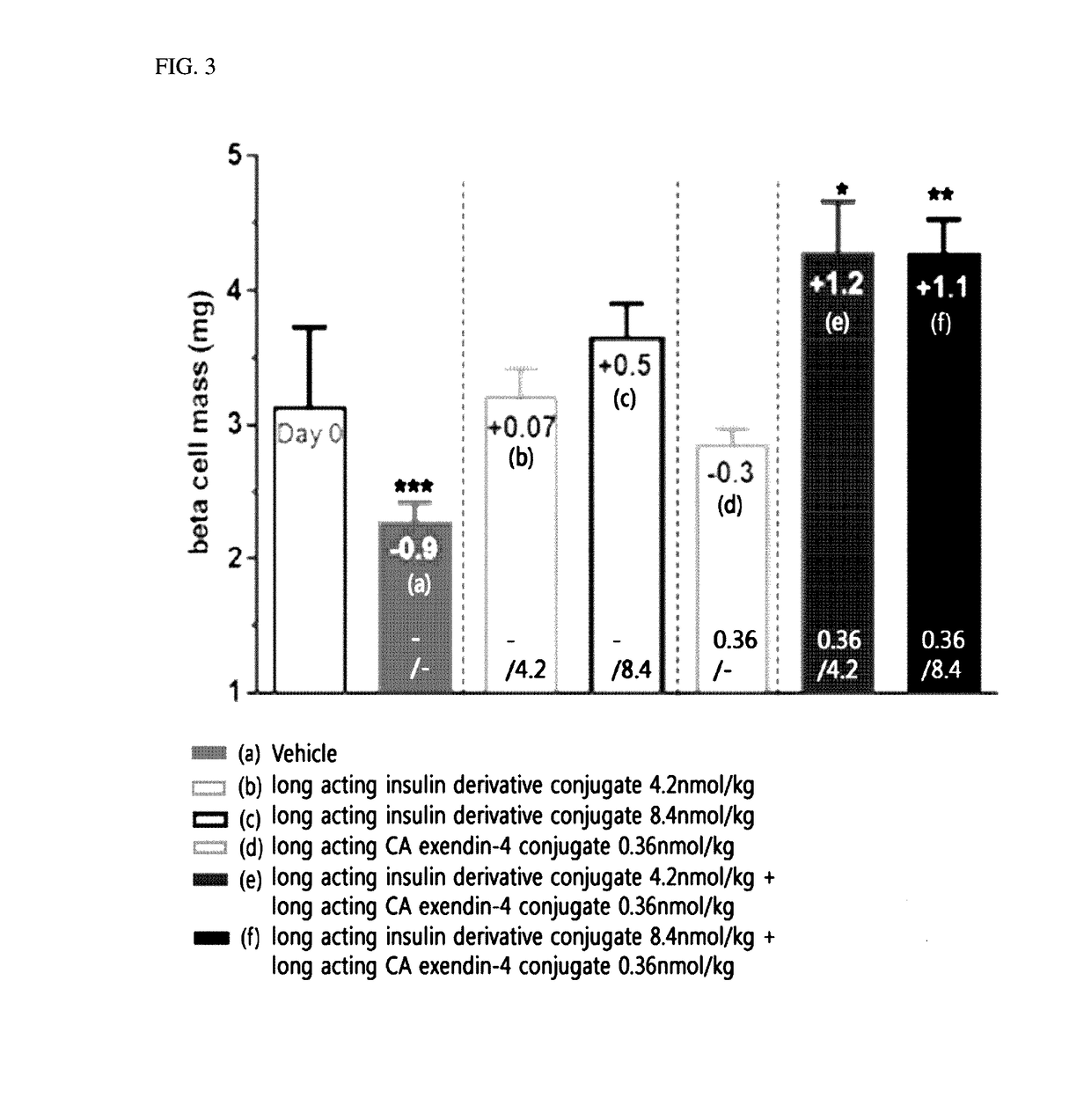
Composition for treating diabetes comprising long-acting insulin analogue conjugate and long-acting insulinotropic peptide conjugate
ActiveUS20170143802A1Good treatment effectImprove in duration of efficacyPeptide/protein ingredientsMetabolism disorderSide effectWeight gain
The present invention relates to a composition for the prevention or treatment of diabetes including a long-acting insulin conjugate and a long-acting insulinotropic peptide conjugate, and a method for treating diabetes. More specifically, combination administration of the long-acting analogue conjugate and the long-acting insulinotropic peptide conjugate inhibits weight gain due to administration of insulin, and vomiting and nausea due to administration of the insulinotropic peptide, and also reduces the required doses of insulin, thereby remarkably improving drug compliance. In addition, the present invention relates to administering a pharmaceutical composition for reducing side effects of pancreatic beta cells in diabetic patients, including a long-acting insulin analogue conjugate and a long-acting insulinotropic peptide analogue conjugate, and to a method for reducing side effects of pancreatic beta cells in diabetic patients, including the step of administering the composition. Specifically, the present invention is characterized in reducing side effects such as abnormality in the function of pancreatic beta cells associated with the development of diabetes, reduction in the pancreatic beta cell mass, lipotoxicity, or glucotoxicity.
Owner:HANMI PHARMA
Medication adherence monitoring system and method
Owner:AIC INNOVATIONS GRP
Insulin conjugate using an immunoglobulin fragment
ActiveUS9492507B2Extended half-lifeImprove Medication AdherencePeptide/protein ingredientsMetabolism disorderHalf-lifeIn vivo
Owner:HANMI SCI CO LTD
Capecitabine granule and preparation method thereof
ActiveCN103356488AImprove stabilityMask bad tasteOrganic active ingredientsGranular deliverySide effectAdhesive
The invention relates to an oral anti-tumor drug capecitabine granule and a preparation method thereof. The capecitabine granule exists in the form of a cyclodextrin inclusion compound and is prepared from capecitabine serving as a raw material drug and auxiliary materials comprising cyclodextrin, a diluting agent, a disintegrating agent, an adhesive and a flavoring agent by wet granulation. The capecitabine granule disclosed by the invention not only covers the bitterness of a capecitabine drug and enhances the drug compliance of a cancerous person, but also enhances the stability of the capecitabine in gastrointestinal tracts and accelerates the digestion velocity, thereby preventing the peak valley phenomenon of the blood concentration of a drug, outstandingly reducing the adverse reactions of the drug, such as irritation and toxic side effect, on the gastrointestinal tracts due to the stable release of capecitabine in the gastrointestinal tracts, enhancing the drug safety and better taking the anti-tumor effect.
Owner:QILU PHARMA HAINAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com