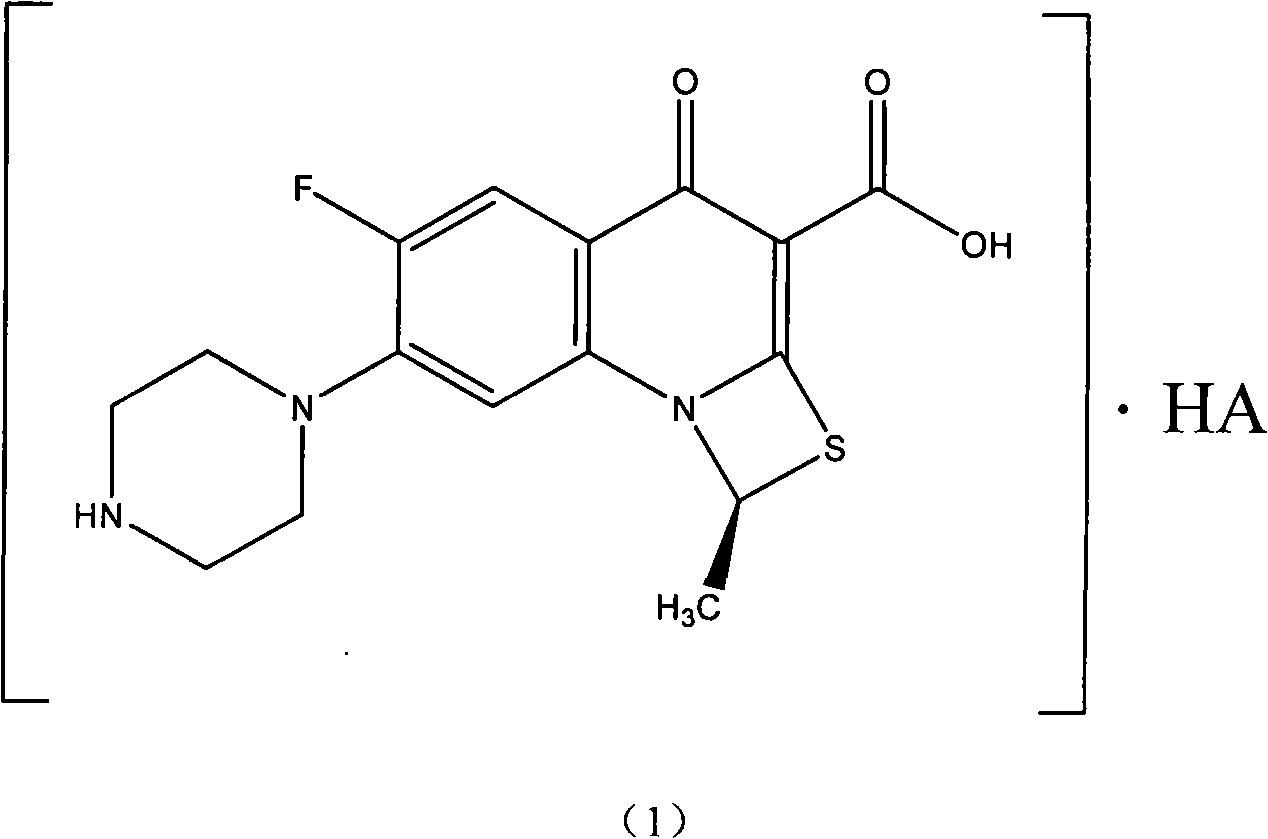
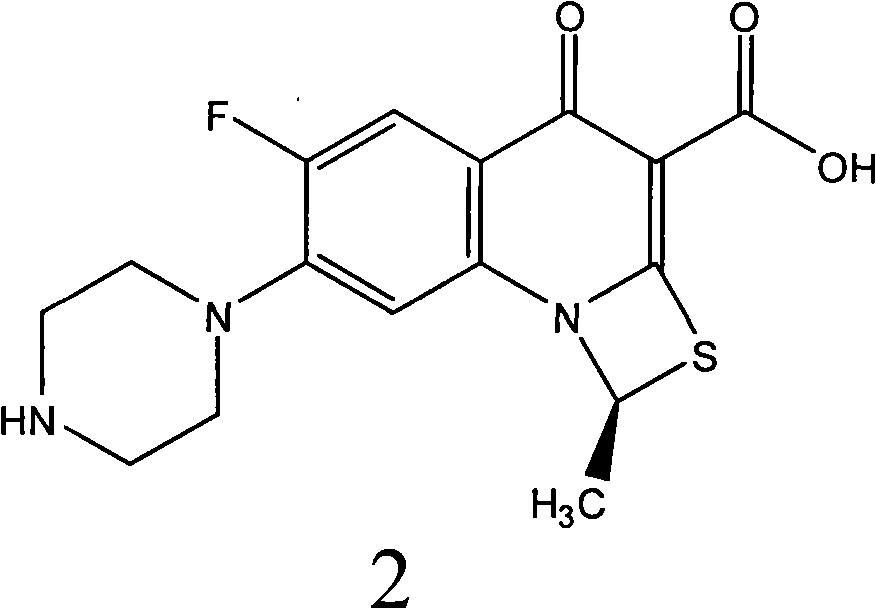
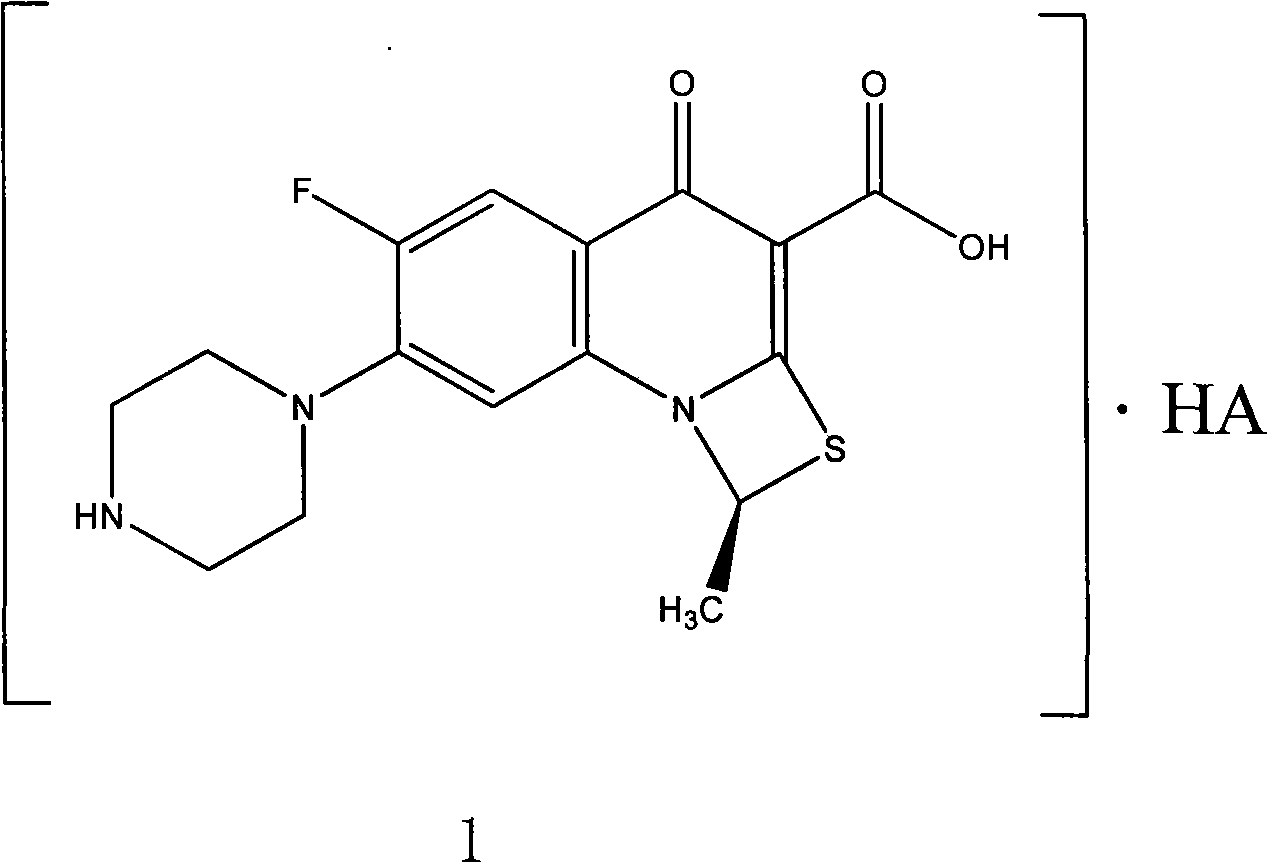
Patents
Literature
46 results about "Drug levels" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
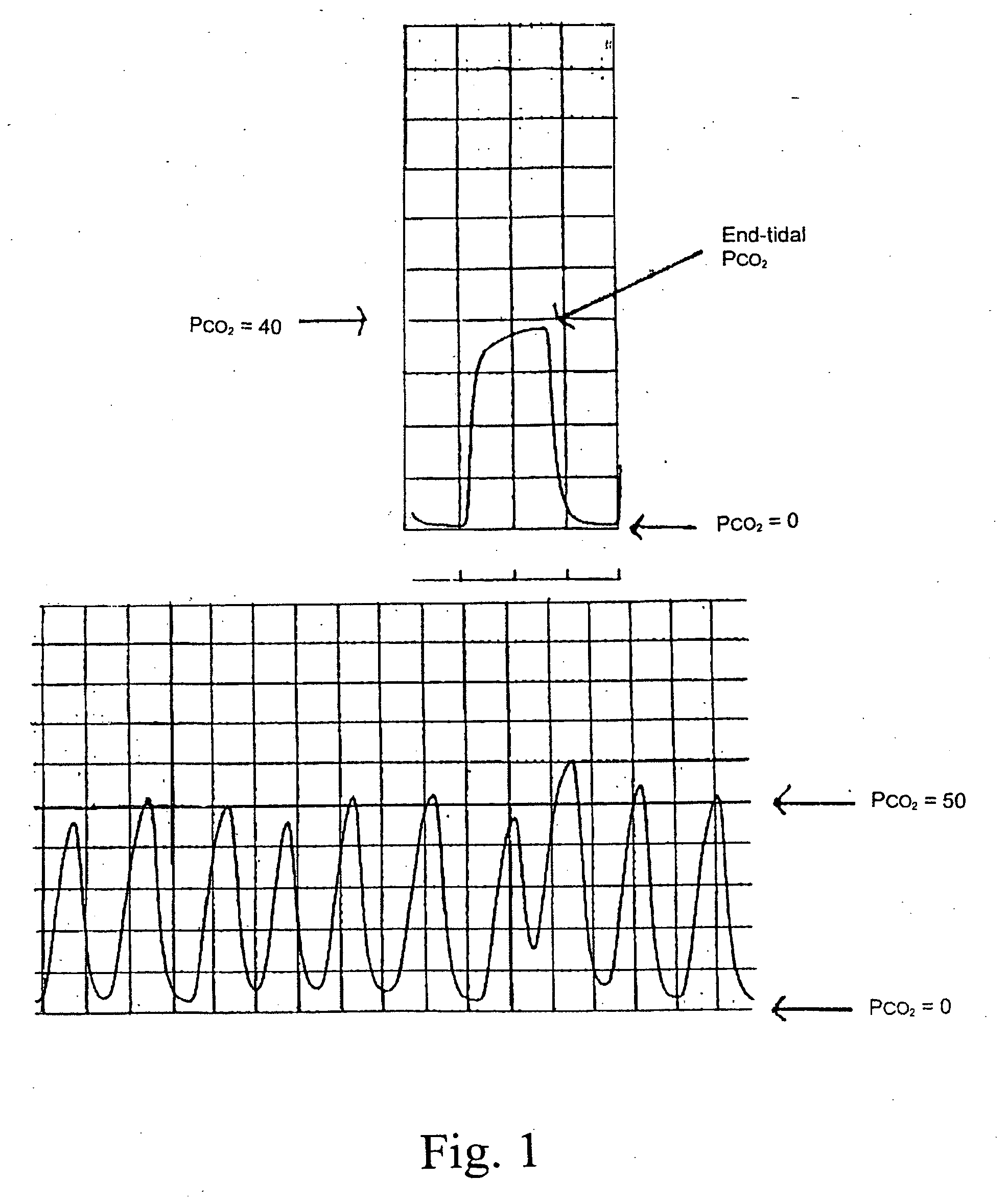
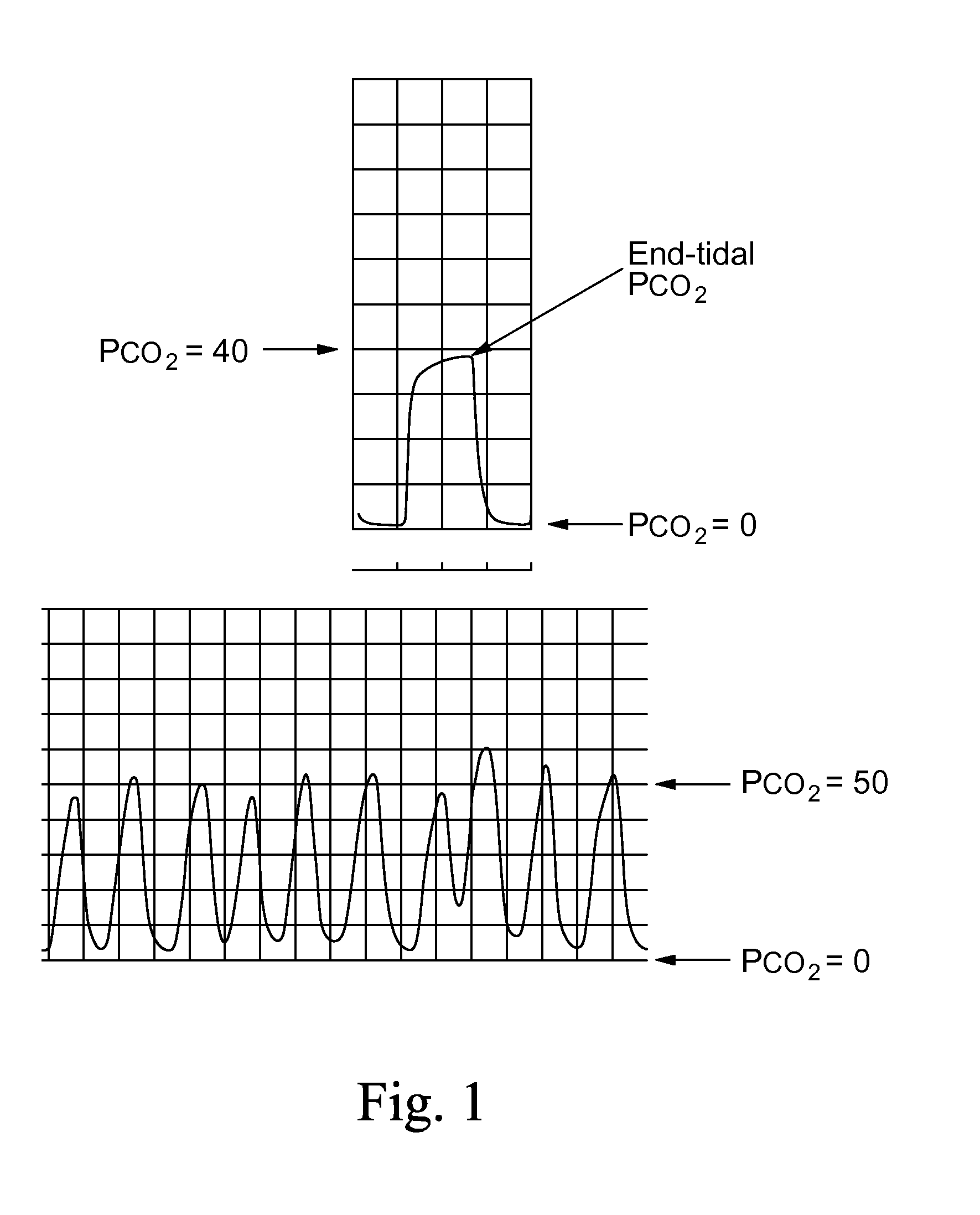
The therapeutic level of a drug in the bloodstream is the range within which that drug is expected to be effective without causing any serious problems to the patient. Your doctor can request a test to measure the amount of a specific drug in the serum portion of your blood.
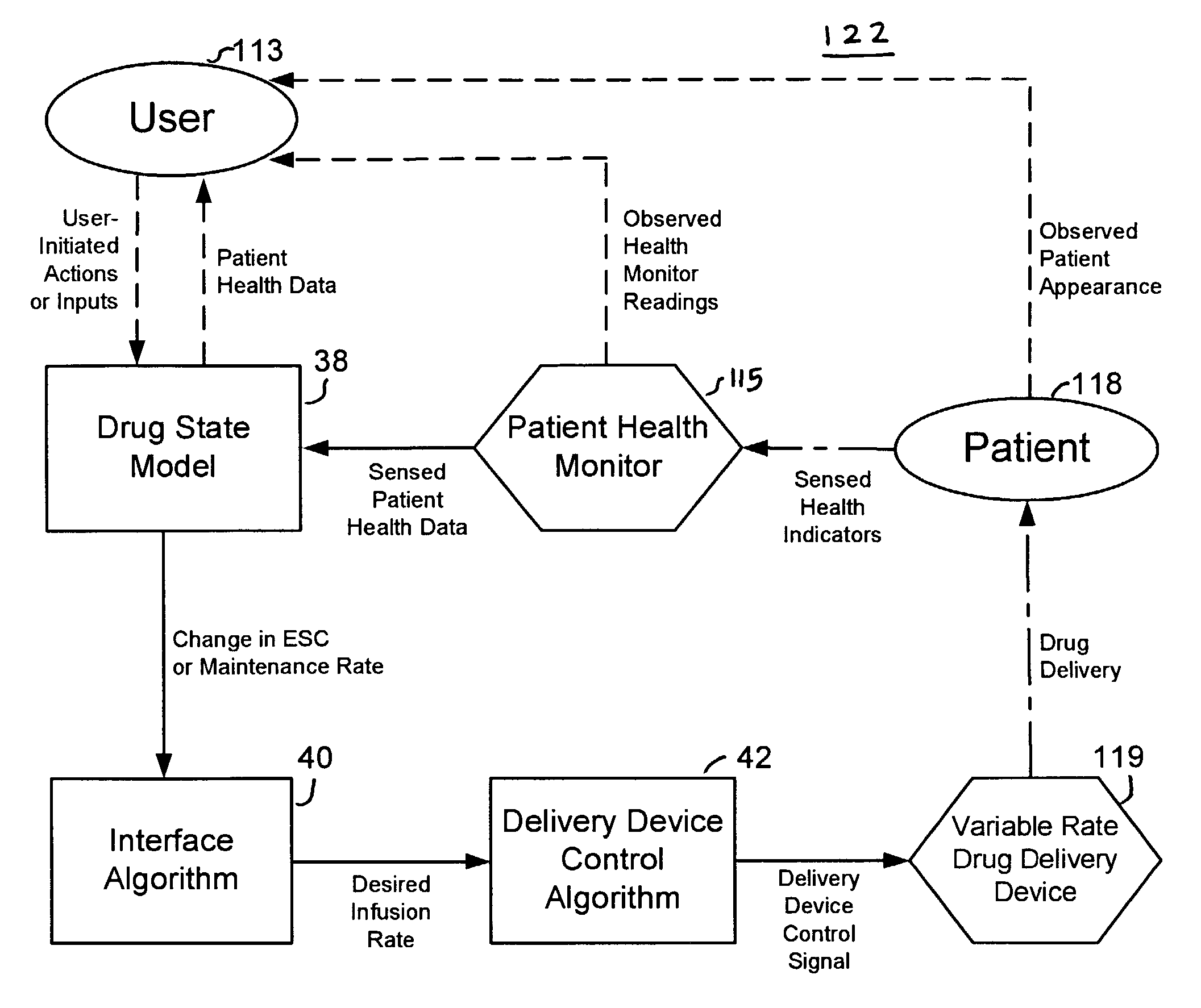
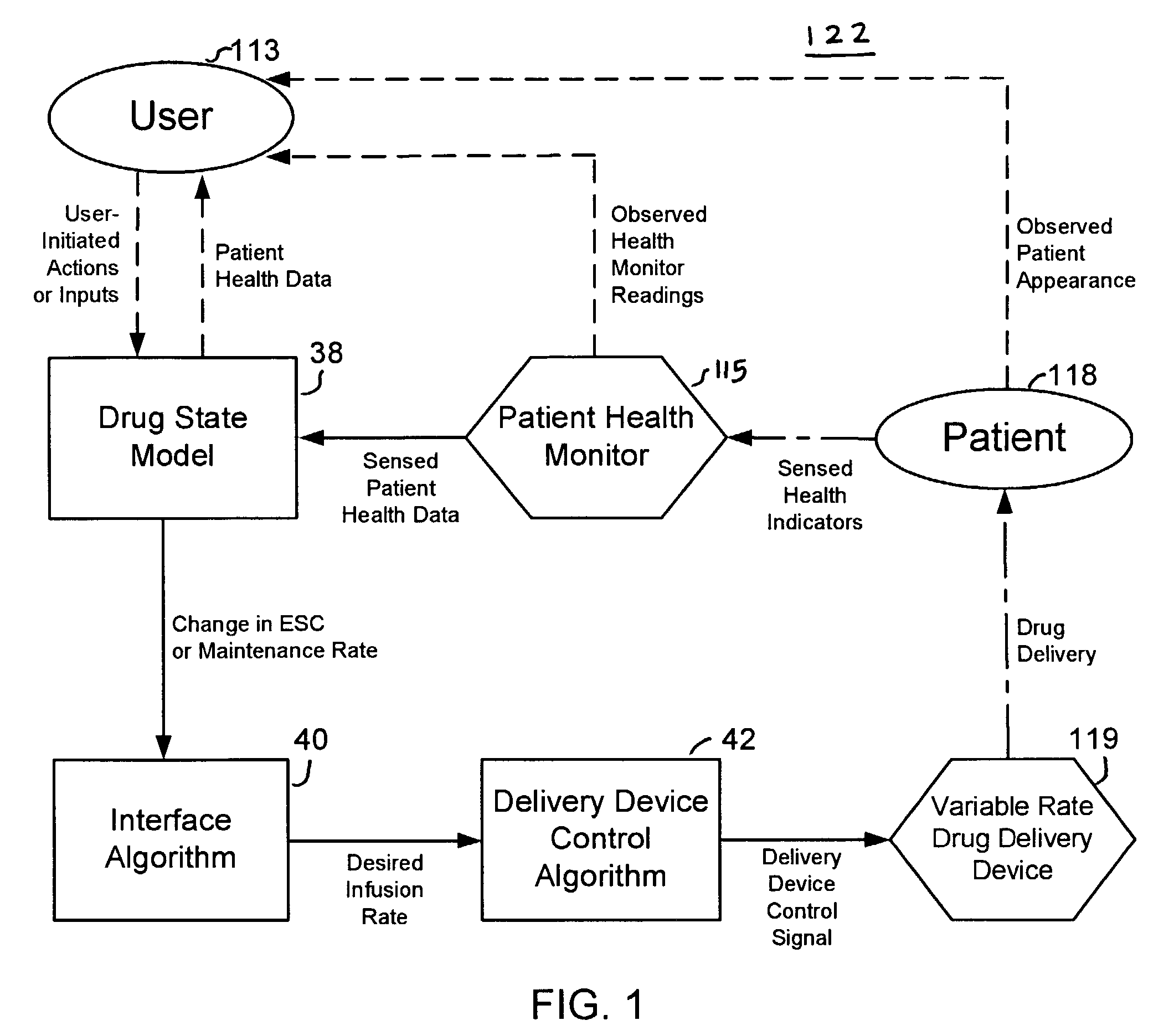
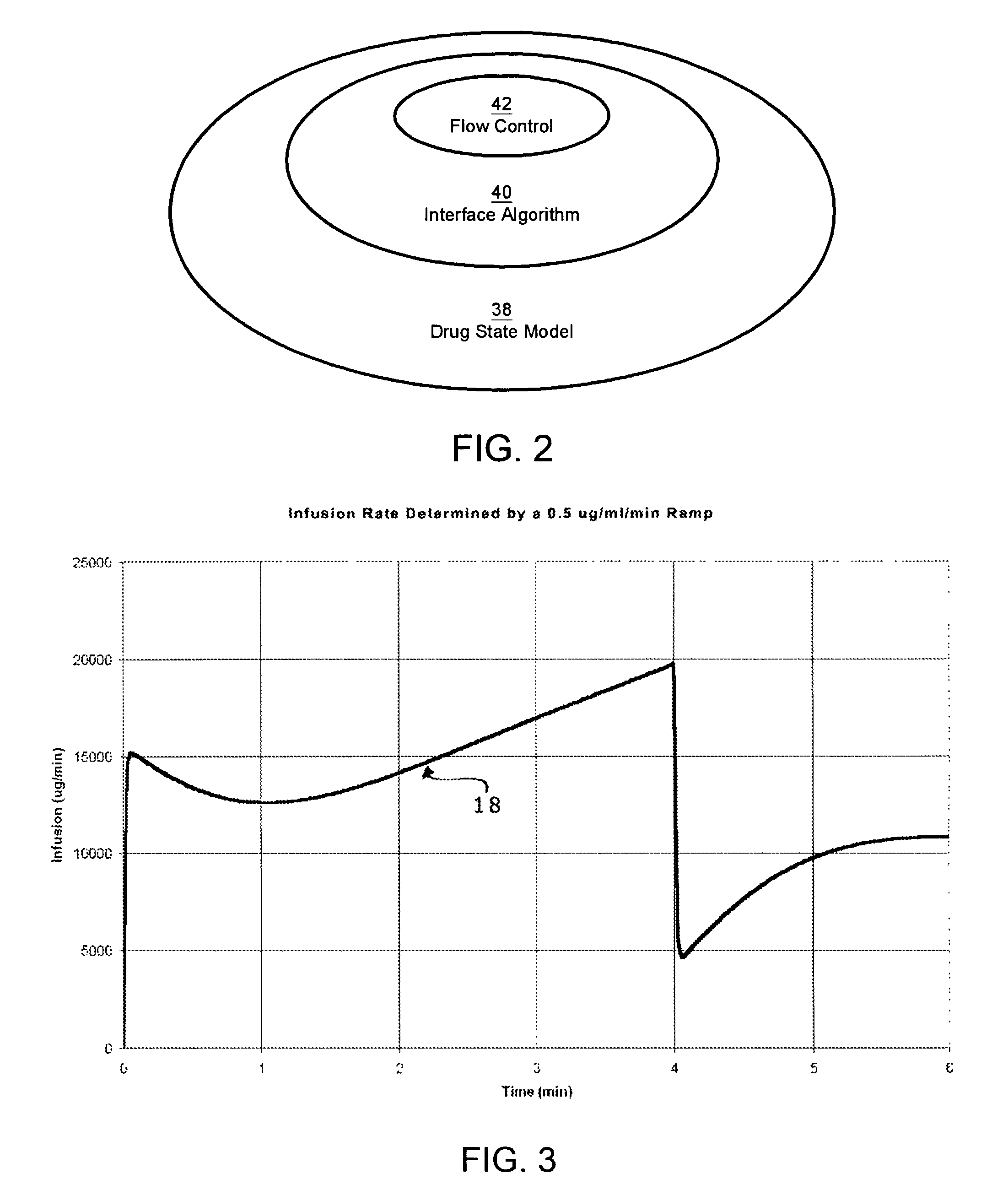
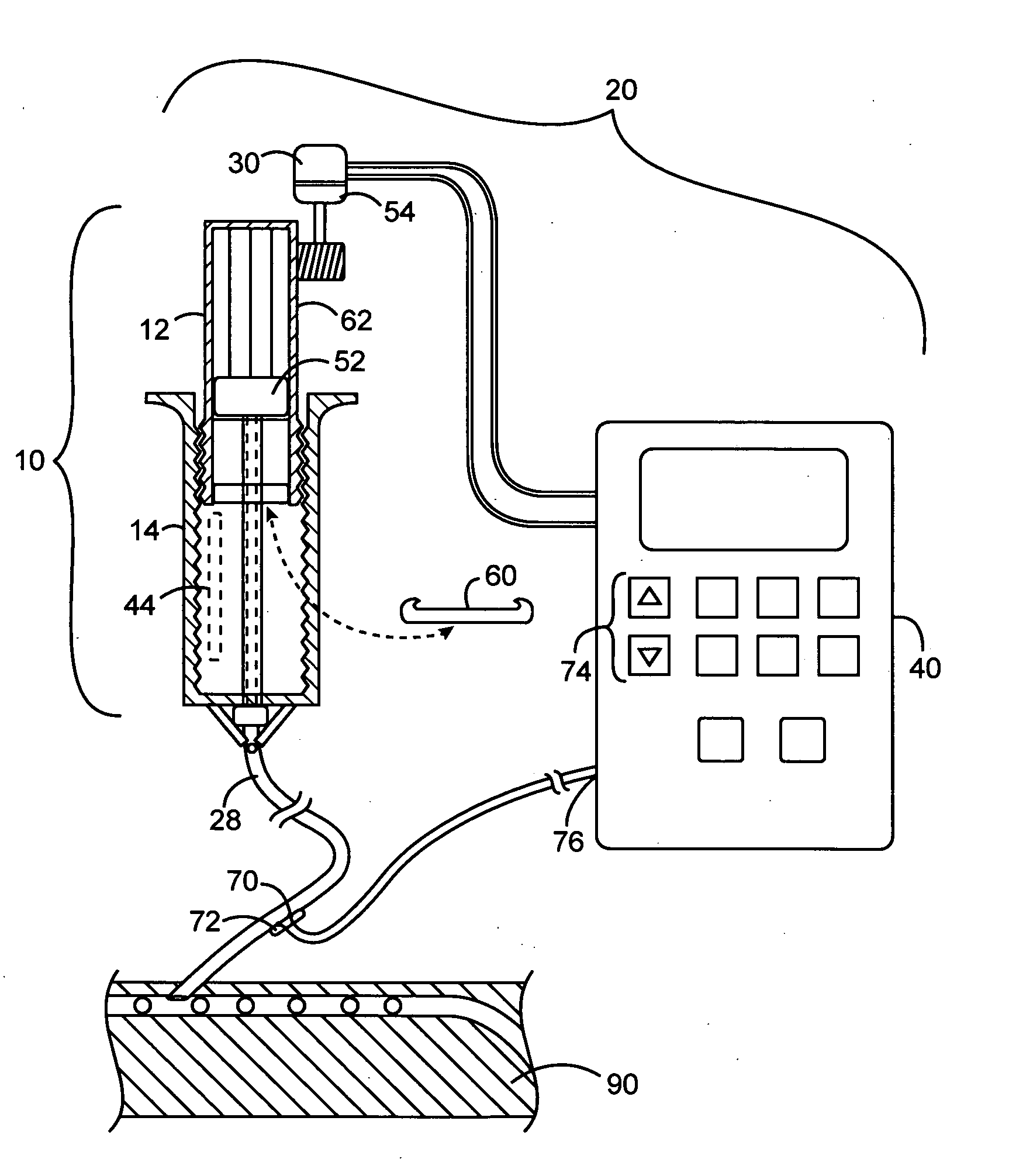
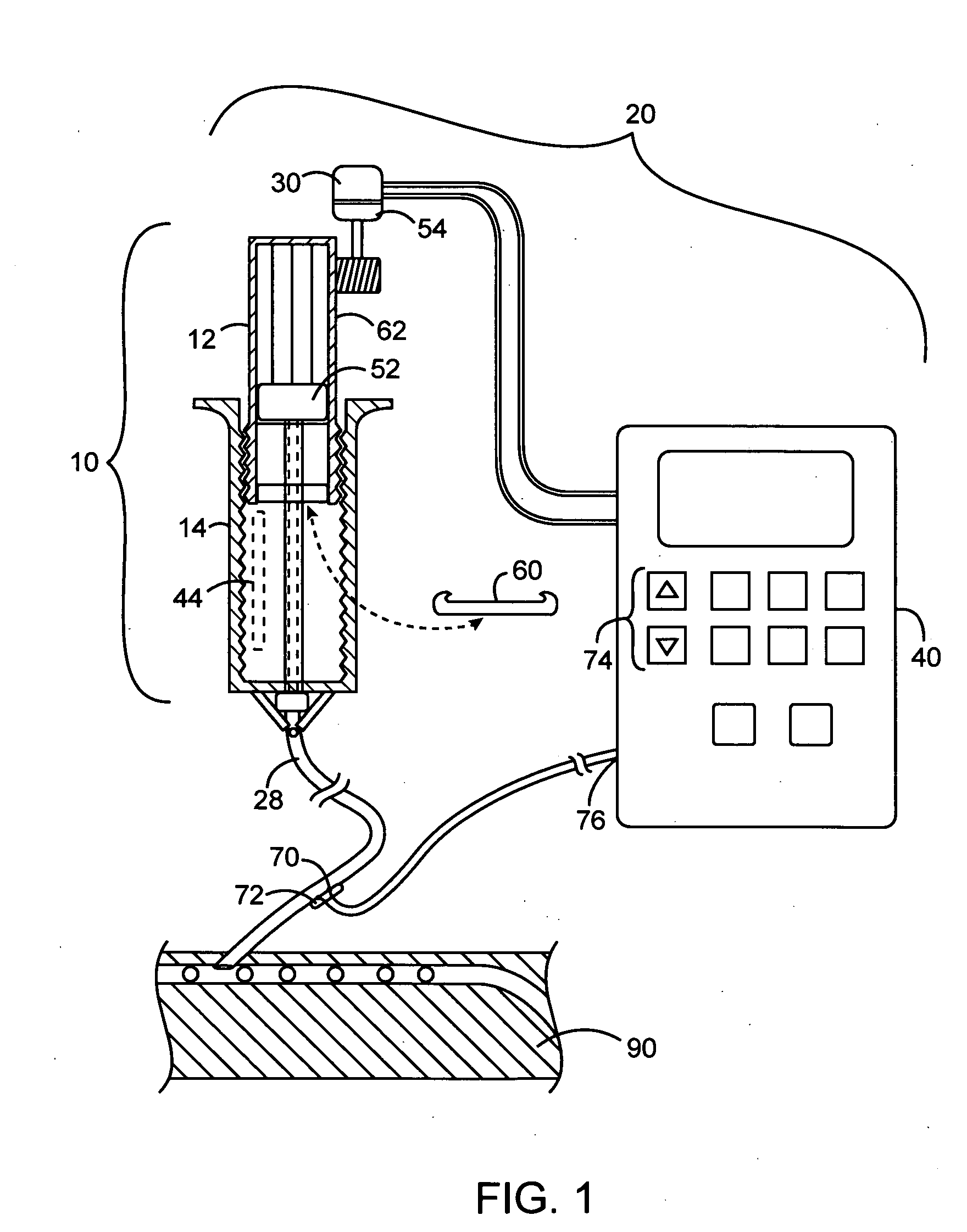
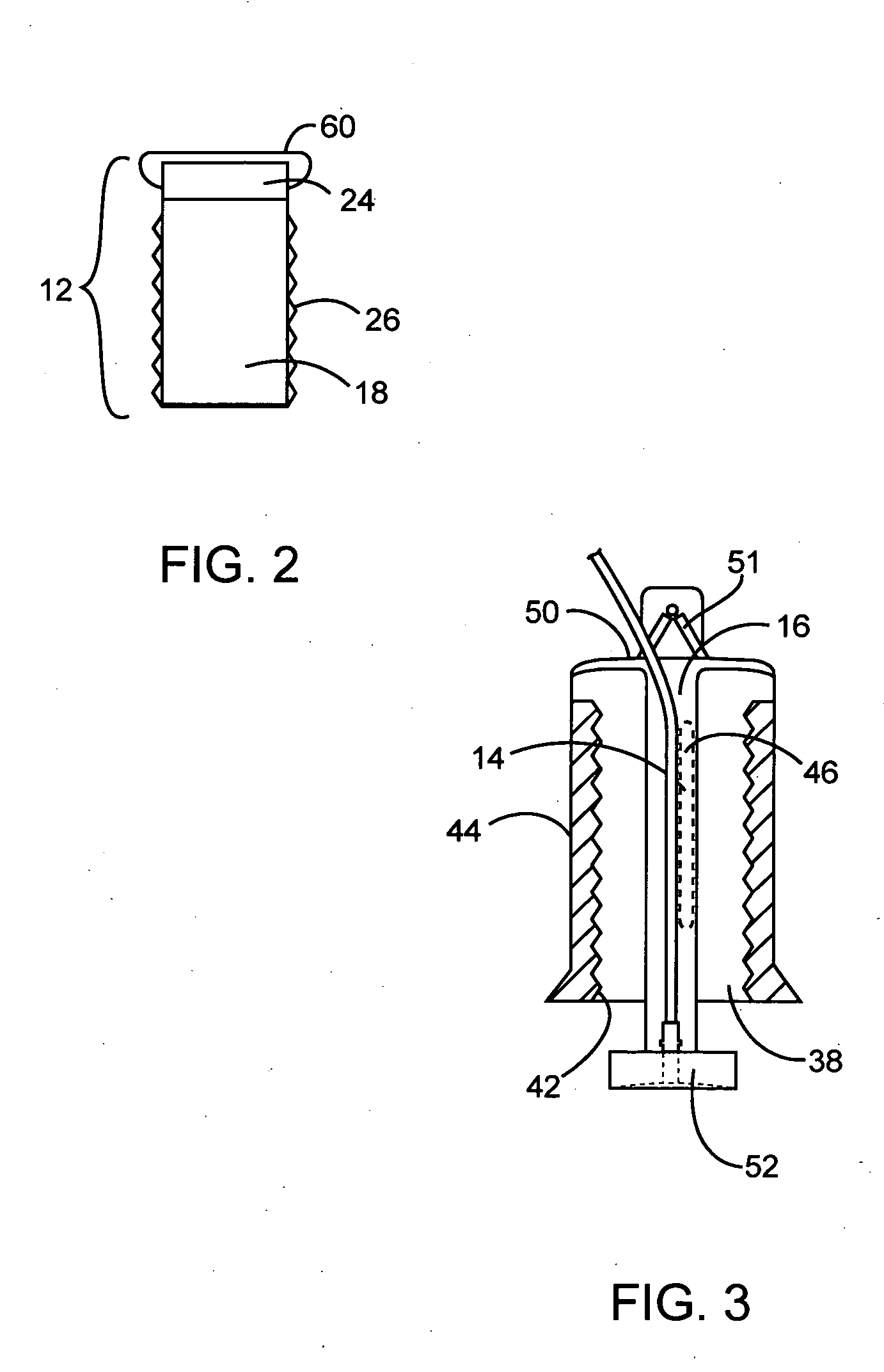
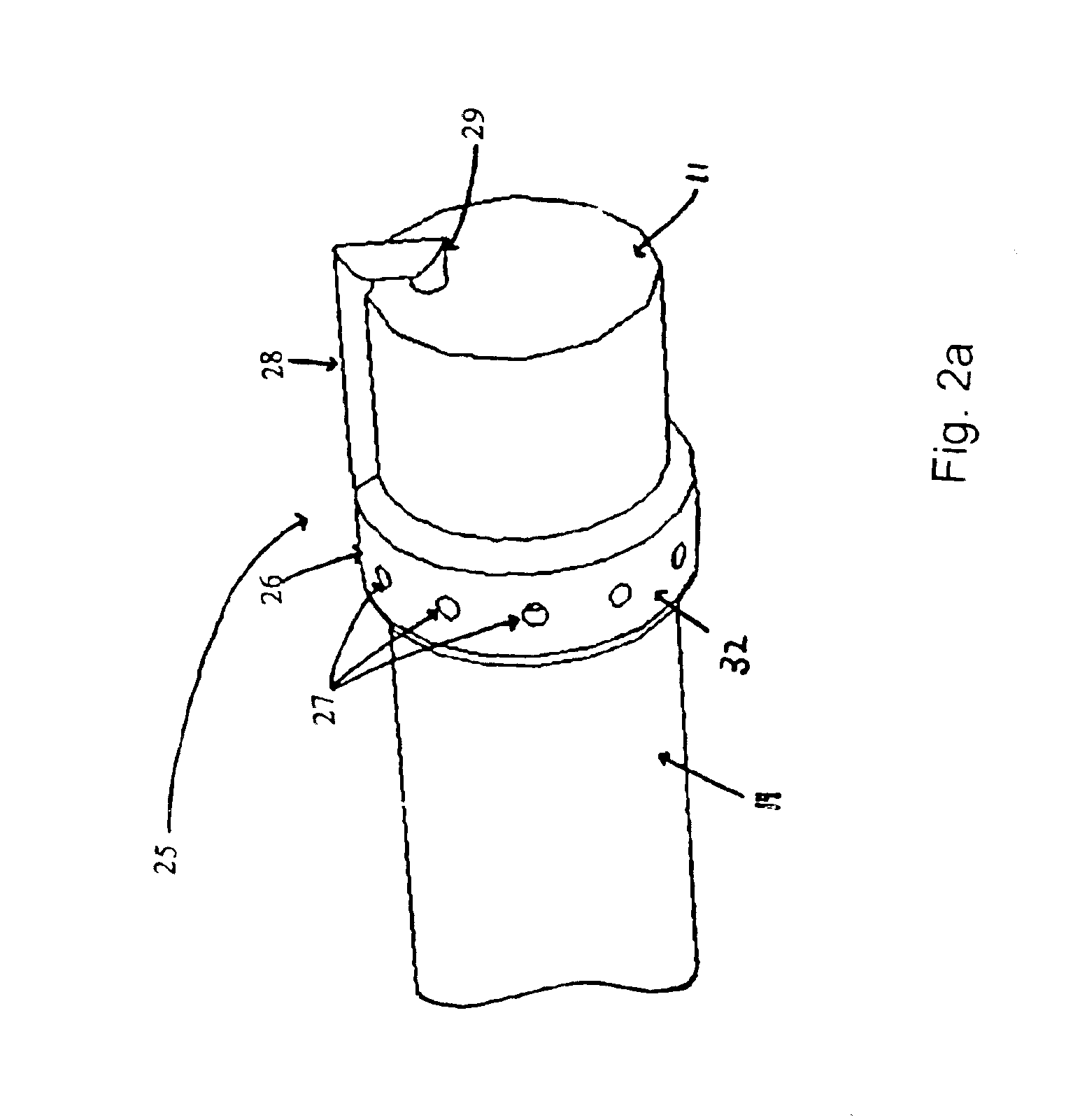
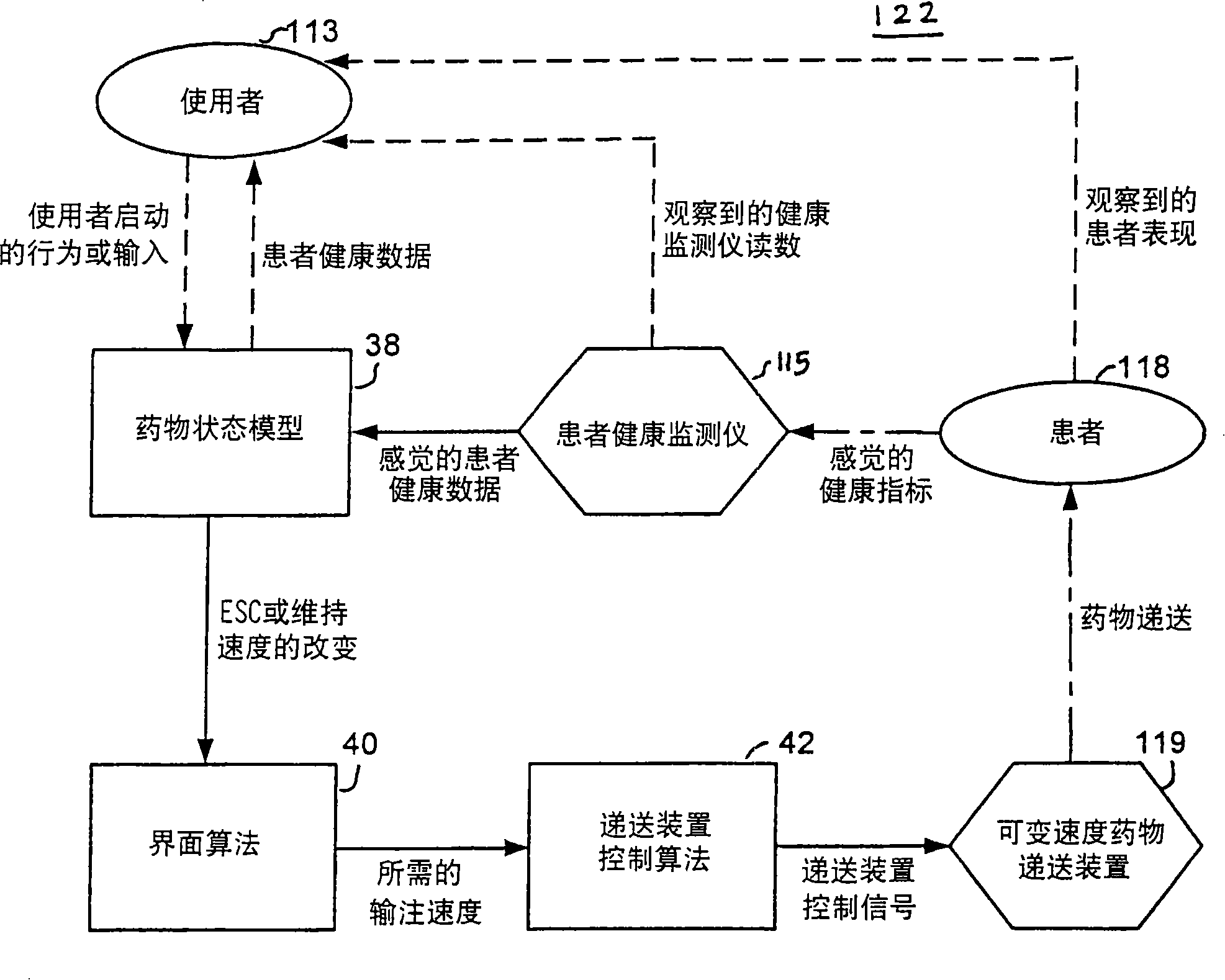
Apparatuses and methods for titrating drug delivery
InactiveUS7229430B2Reduce clinical useSafety managementMedical simulationDrug and medicationsState modelTime profile
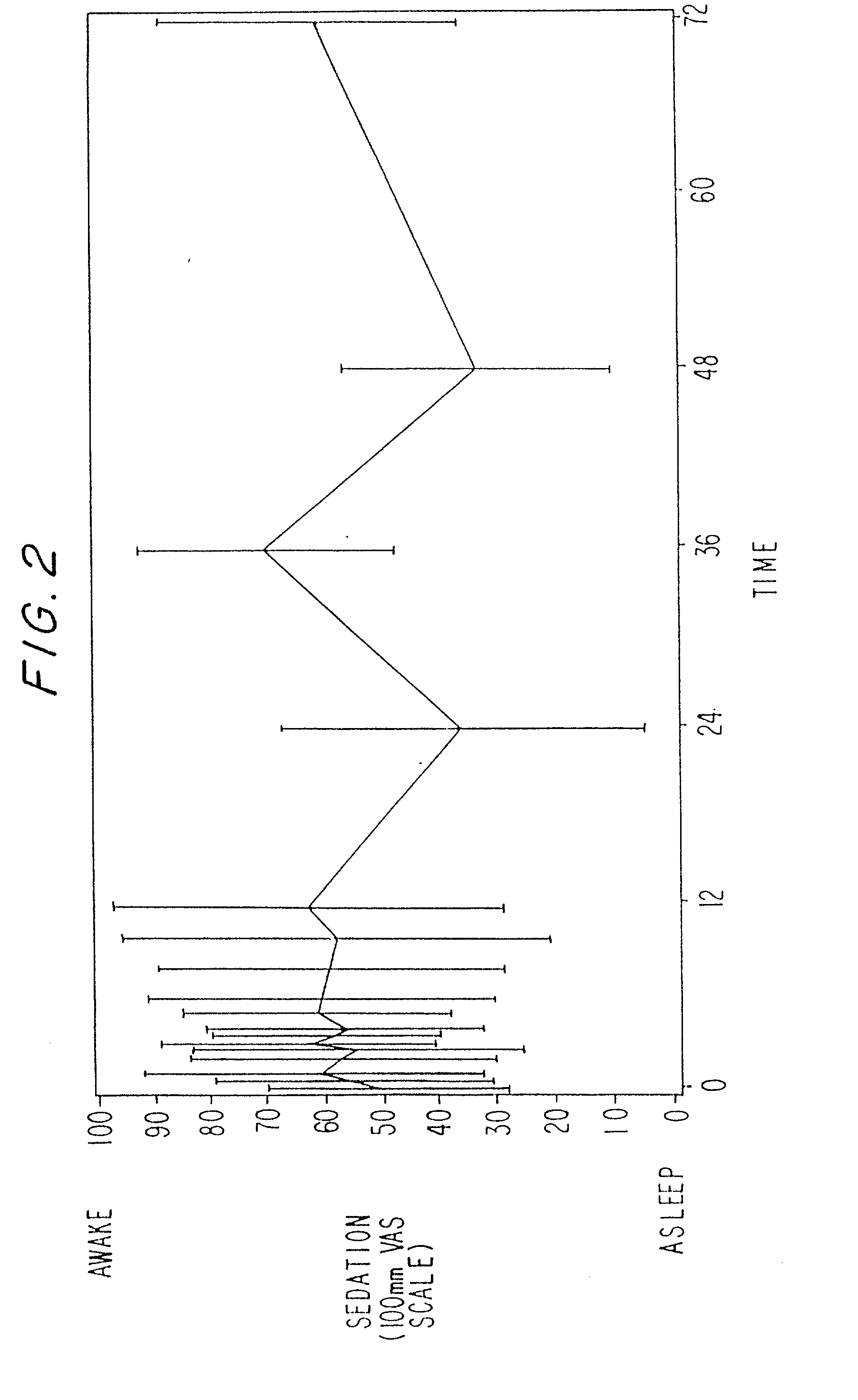
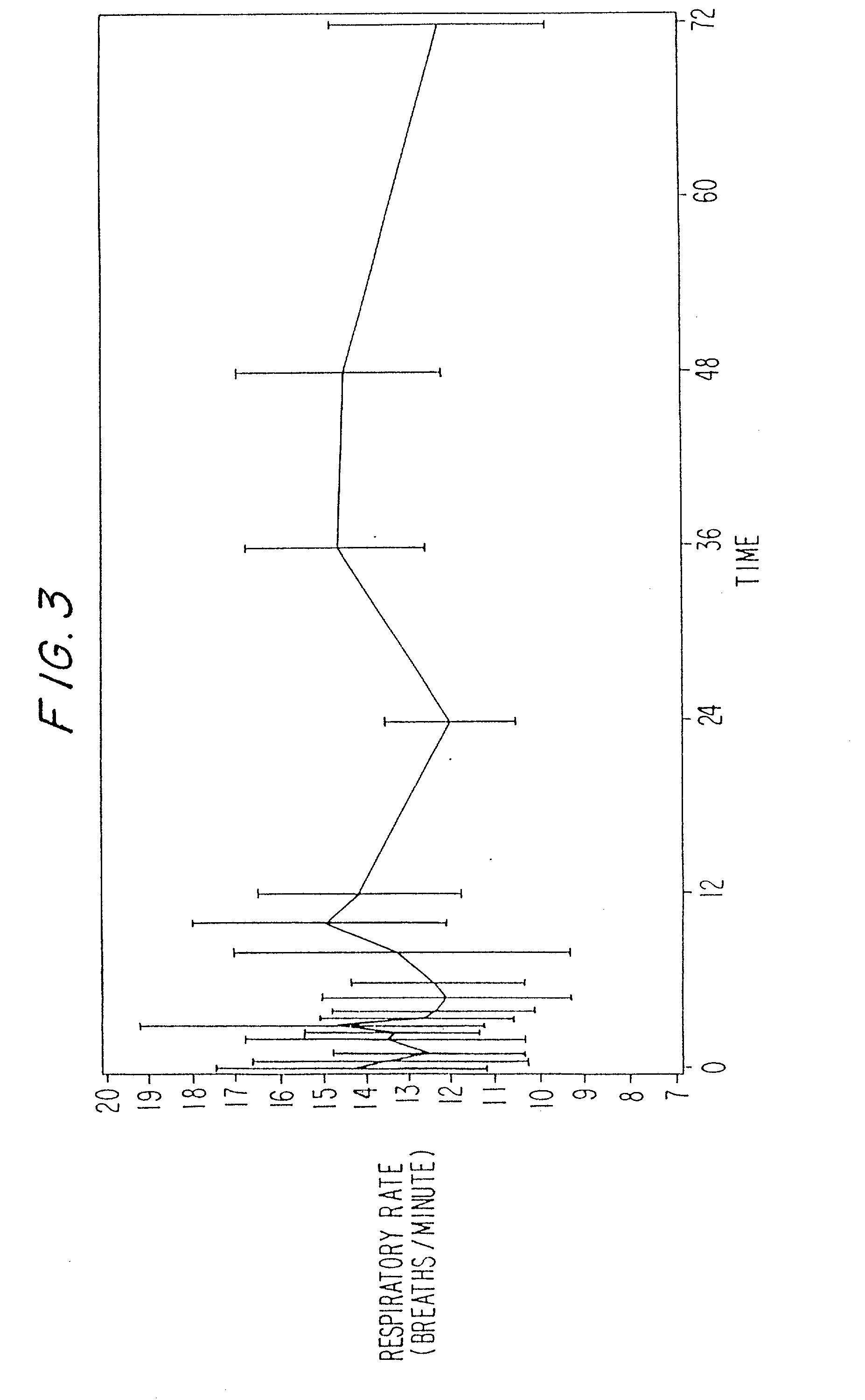
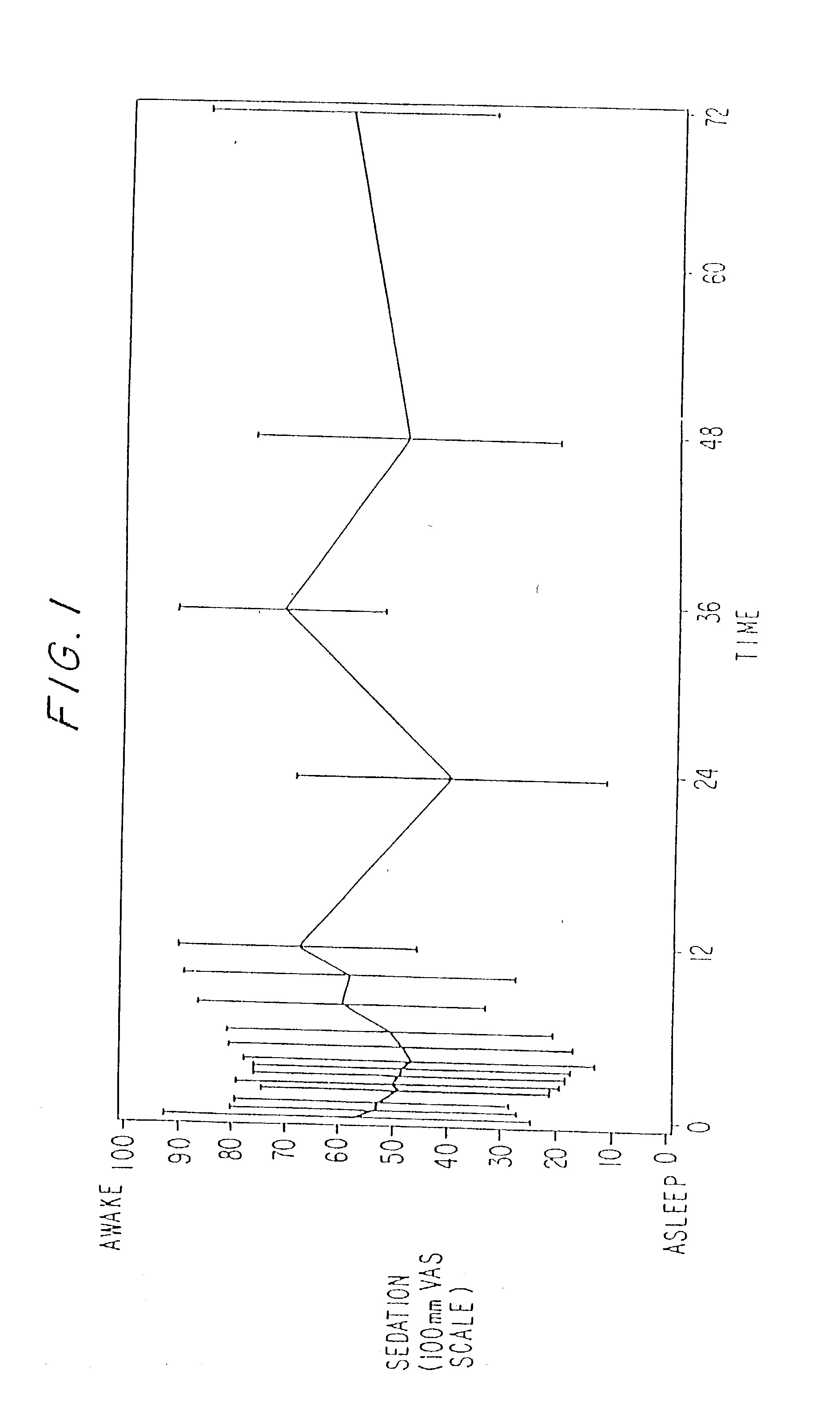
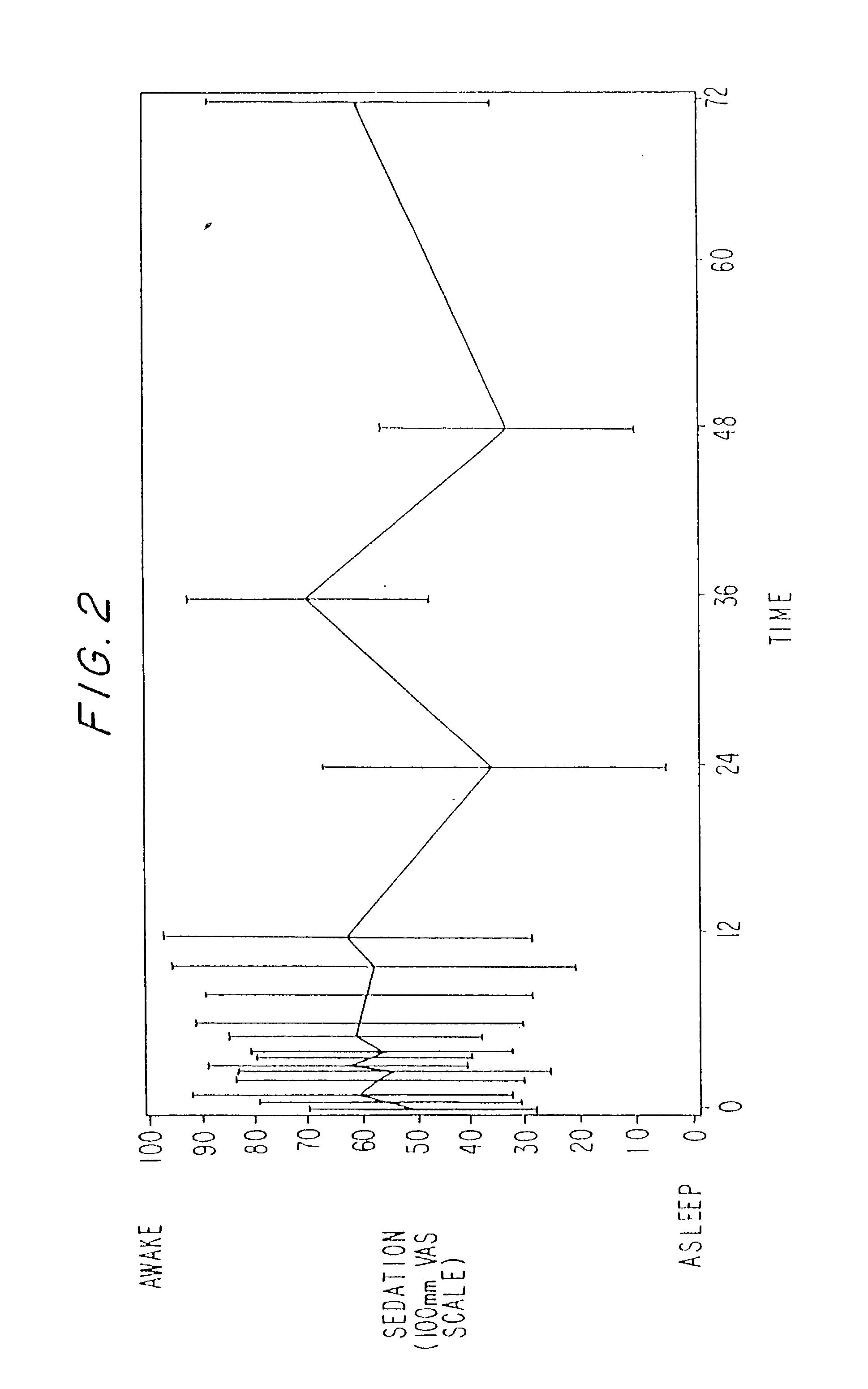
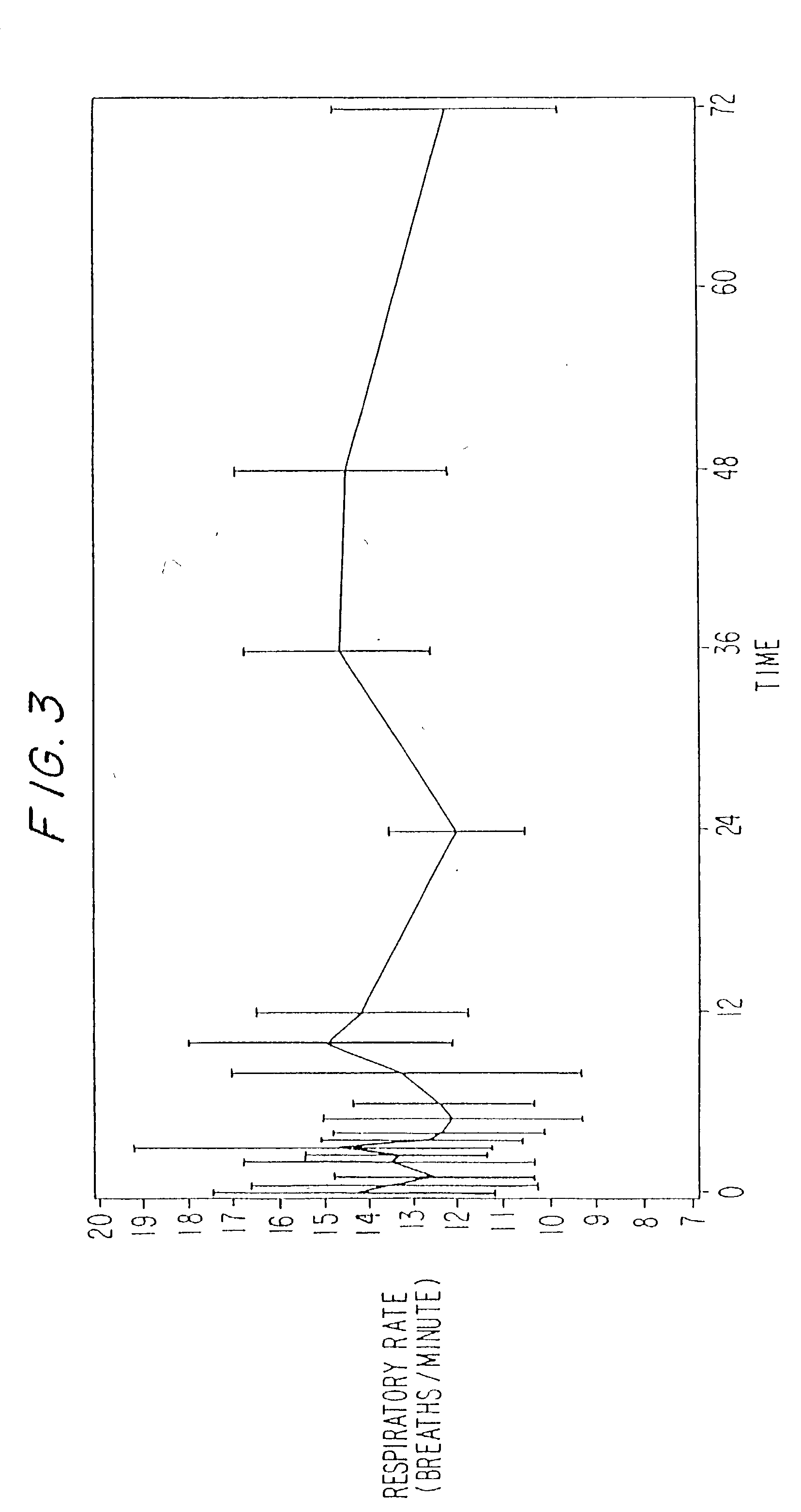
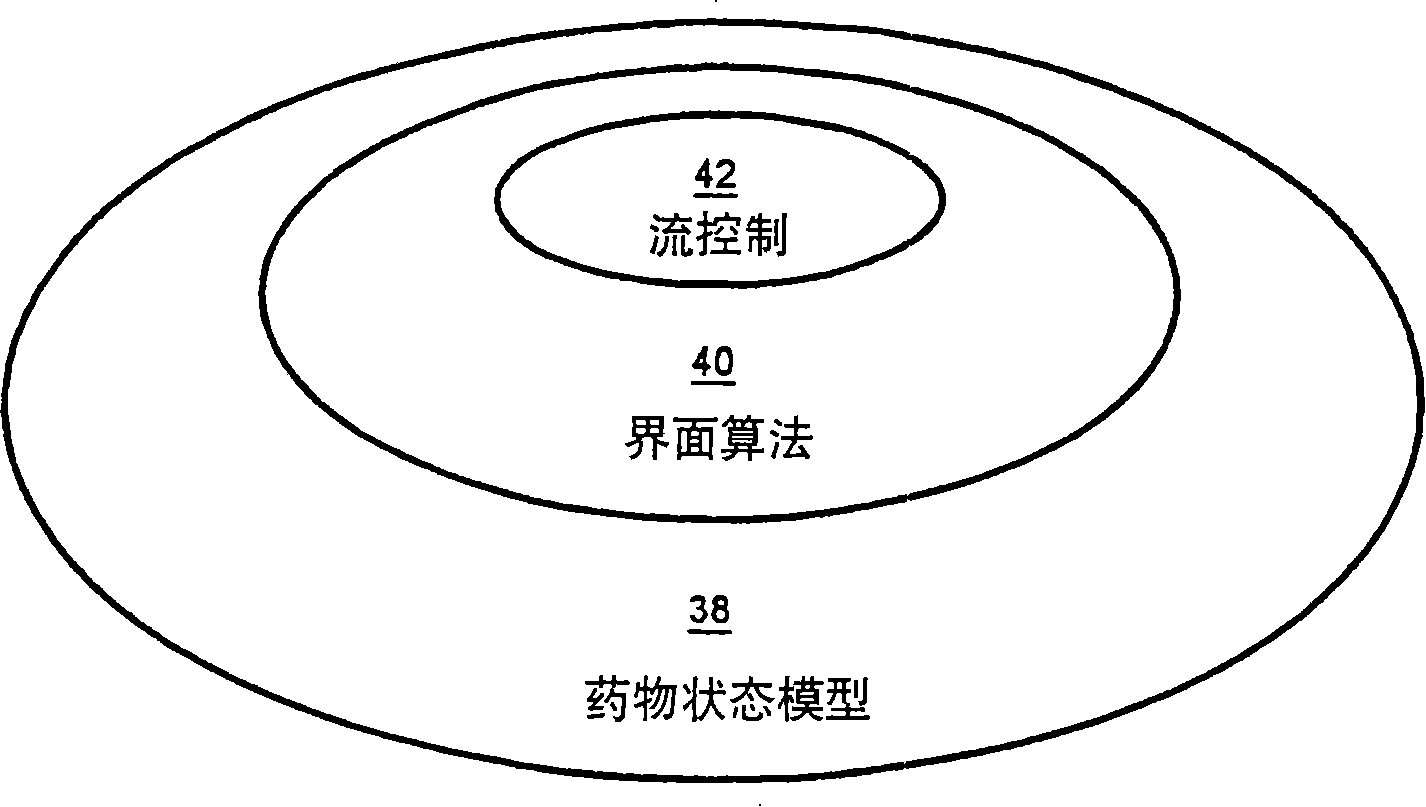
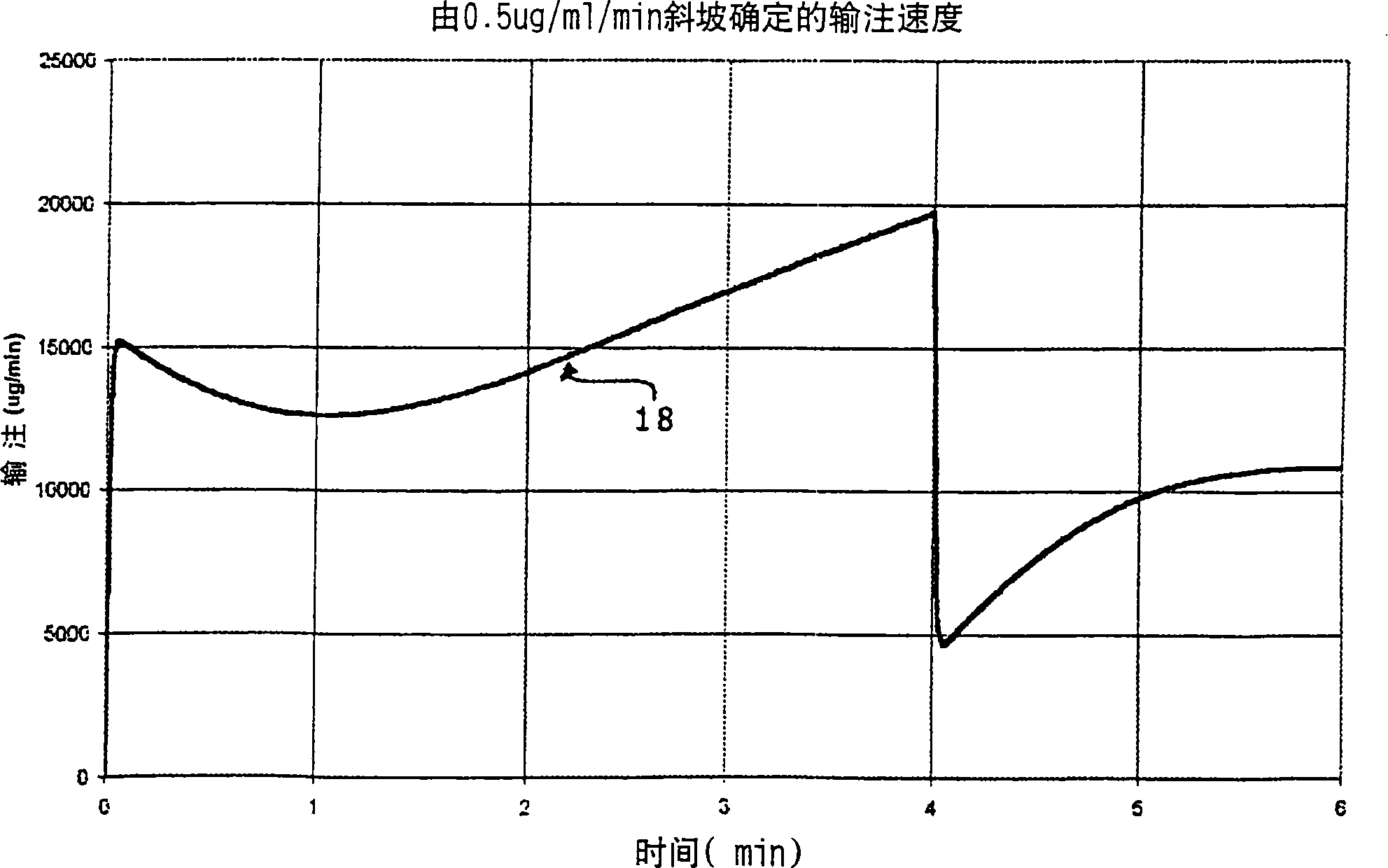
A method and apparatus for reducing the workload of titrating drug to effect while leaving clinician users in control of a related procedure is described. A drug delivery device is controlled to achieve a target drug concentration at a selected site in the patient or a predetermined infusion rate waveform. The time profile of the target drug concentration or a predetermined infusion rate waveform is controlled by a drug state model that uses clinical heuristics to implement safe, pre-defined changes in the target drug concentration or infusion rate and user-commanded changes in target drug concentration or infusion rate. The invention allows time to assess the response of the patient to changes in drug level by making small incremental and conservative changes in drug level over time.
Owner:SCOTT LAB
System and method for therapeutic drug monitoring
InactiveUS20050054942A1Accurate assessmentCost-effective and frequentNervous disorderElectrotherapyNoseEnvironmental health
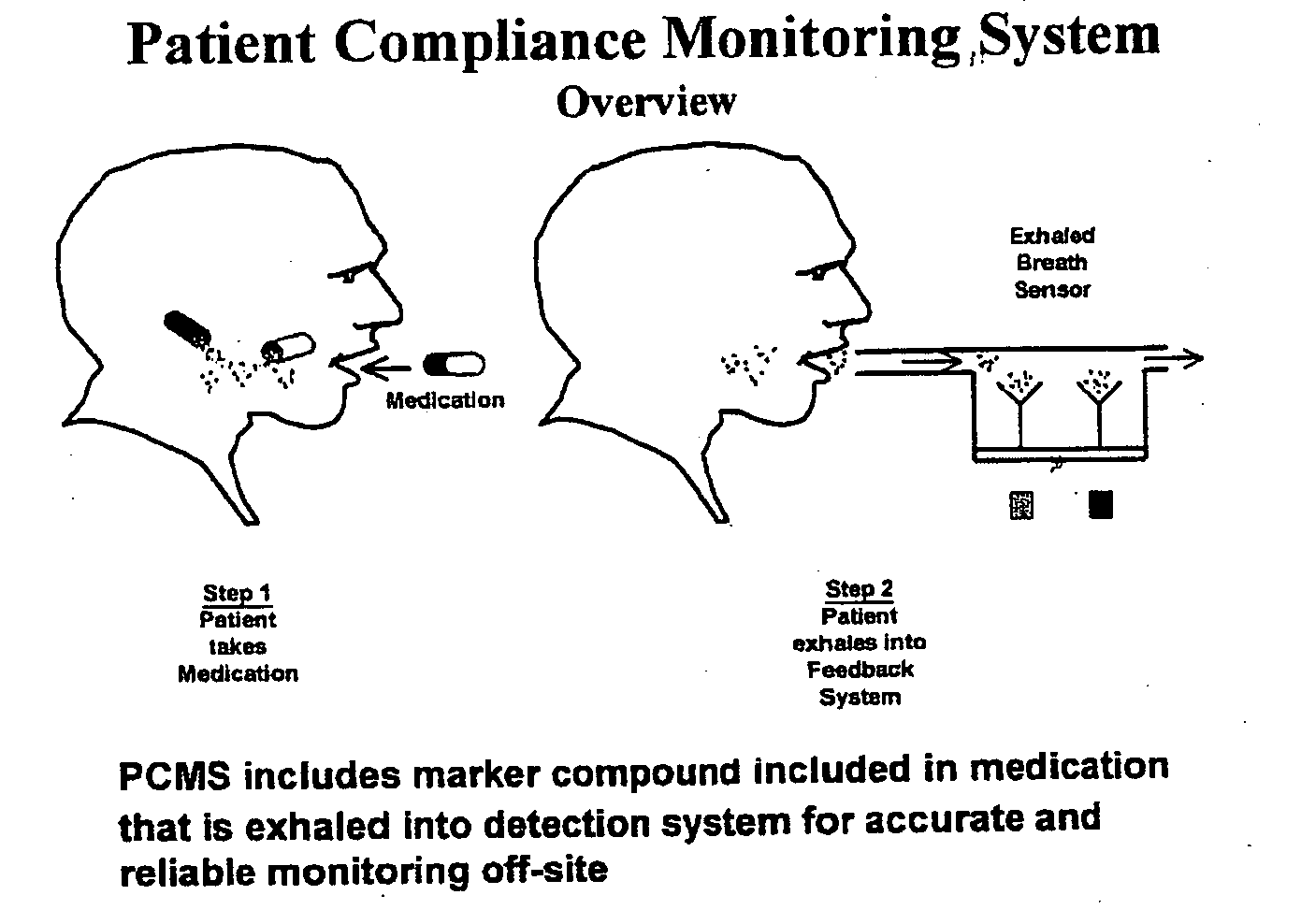
The present invention includes systems and methods for monitoring therapeutic drug concentration in blood by detecting markers, such as odors, upon exhalation by a patient after the drug is taken, wherein such markers result either directly from the drug itself or from an additive combined with the drug. In the case of olfactory markers, the invention preferably utilizes electronic sensor technology, such as the commercial devices referred to as “artificial” or “electronic” noses or tongues, to non-invasively monitor drug levels in blood. The invention further includes a reporting system capable of tracking drug concentrations in blood (remote or proximate locations) and providing the necessary alerts with regarding to ineffective or toxic drug dosages in a patient.
Owner:UNIV OF FLORIDA
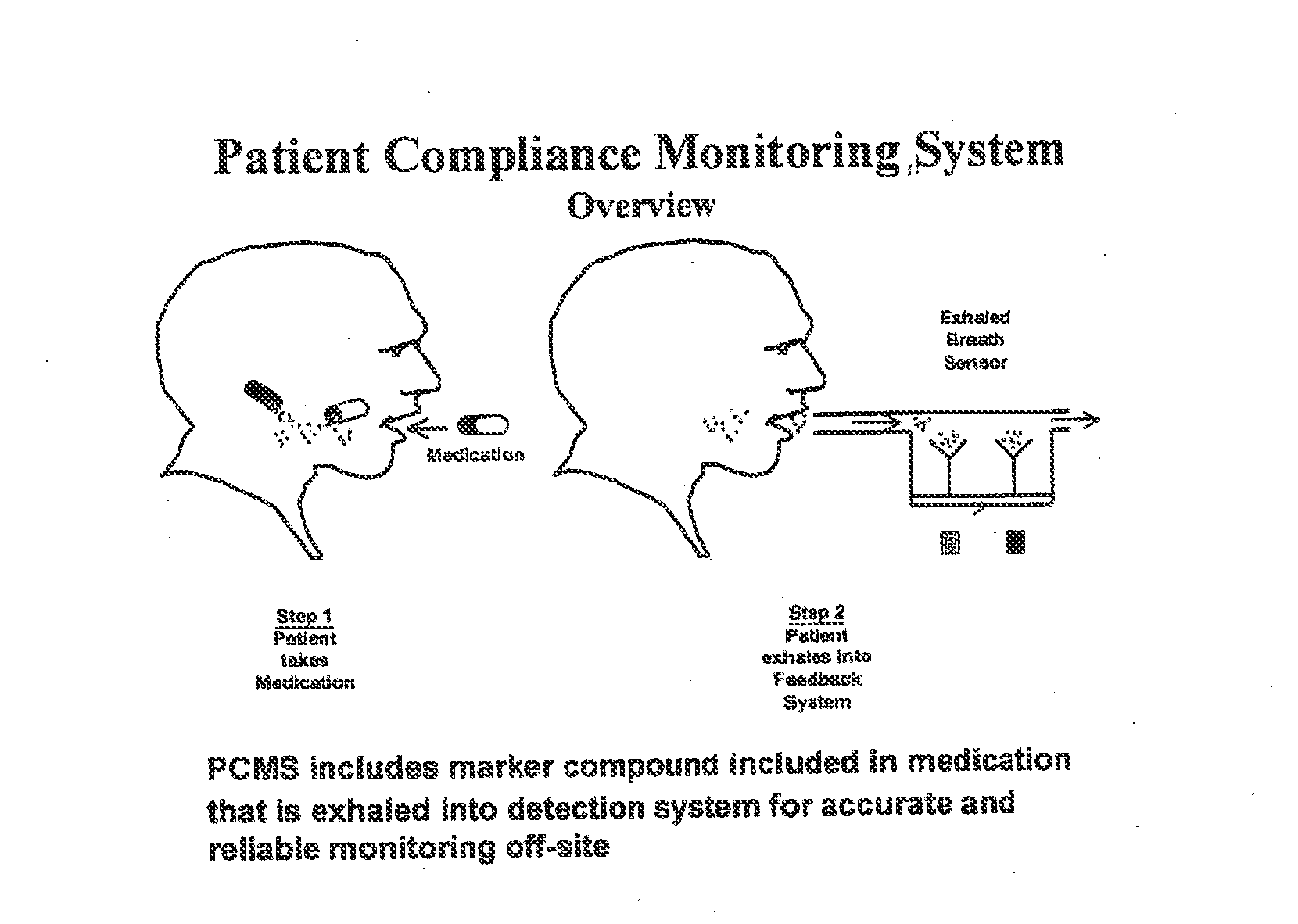
Marker detection method and apparatus to monitor drug compliance
InactiveUS20050233459A1Accurate assessmentPatient complianceDiagnostic recording/measuringSensorsNoseEnvironmental health
The present invention includes systems and methods for monitoring therapeutic drug concentration in blood by detecting markers, such as odors, upon exhalation by a patient after the drug is taken, wherein such markers result either directly from the drug itself or from an additive combined with the drug. In the case of olfactory markers, the invention preferably utilizes electronic sensor technology, such as the commercial devices referred to as “artificial” or “electronic” noses or tongues, to non-invasively monitor drug levels in blood. The invention further includes a reporting system capable of tracking drug concentrations in blood (remote or proximate locations) and providing the necessary alerts with regarding to ineffective or toxic drug dosages in a patient.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Quantitative chronological medical infusion device
InactiveUS20060079831A1Reduce riskReduced glucose levelMedical devicesIntravenous devicesLine sensorHigh rate
The present invention is a medical infusion and aspiration system delivering precisely timed and accurately calculated, adjusted pulsated delivery in high rates of flow delivering an effective profile of pulses tailored to provide momentary spikes of levels of freely available medicines based upon the uptake of the medicine and optimally on real time measurements of the medicine or response of the patient, termed Quantitative Chronological Delivery. The system comprises any pumping mechanism, and optimally a pumping mechanism, and a cassette or cartridge having a reservoir area where the plunger rotates as it advances in reference to the cartridge to provide additional accuracy and overcome the forces of inertia and slip-stick as well as eliminate backlash. Optimally, the systems incorporates an encoded area and an opening for connection to an infusion tube with an in-line sensor area where sampling probes are located. The infusion is adjusted in both amount and duration between pulses to provide quantitatively controlled, chronologically optimized infusion. A motor causes bi-directional pumping to allow for samples to be presented to the sensor area. The system accuracy allows for more concentrated medicines, as a sealed container can eliminate the need for diluting or withdrawing medicine to load a reservoir, and achieves extraordinary accuracy without error correcting software or expensive volumetric measurement and control systems.
Owner:BIONICA INT
Treating pain by administering 24 hours opioid formulations exhibiting rapid rise of drug level
InactiveUS20020058050A1Great analgesic efficacyQuick releaseOrganic active ingredientsCosmetic preparationsAbsorption Half-LifeOral medication
Patients are treated with 24-hour oral sustained release opioid formulations which, upon administration, provide an initially rapid opioid absorption such that the minimum effective analgesic concentration of the opioid is more quickly achieved. These sustained release opioid formulations include an effective amount of at least one retardant material to cause said opioid analgesic to be released at a such a rate as to provide an analgesic effect after oral administration to a human patient for at least about 24 hours, and are characterized by providing an absorption half-life from 1 to about 8 hours. A method of titrating a human patient utilizing these sustained release opioid formulations is also disclosed.
Owner:PURDUE PHARMA LP
Method of treating pain by administering 24 hour oral opioid formulations exhibiting rapid rate of initial rise of plasma drug level
InactiveUS20030035837A1Good analgesic effectQuick releaseBiocidePowder deliveryAbsorption Half-LifeOral medication
Patients are treated with 24-hour oral sustained release opioid formulations which, upon administration, provide an initially rapid opioid absorption such that the minimum effective analgesic concentration of the opioid is more quickly achieved. These sustained release opioid formulations include an effective amount of at least one retardant material to cause said opioid analgesic to be released at a such a rate as to provide an analgesic effect after oral administration to a human patient for at least about 24 hours, and are characterized by providing an absorption half-life from 1 to about 8 hours. A method of titrating a human patient utilizing these sustained release opioid formulations is also disclosed.
Owner:SACKLER RICHARD S +2
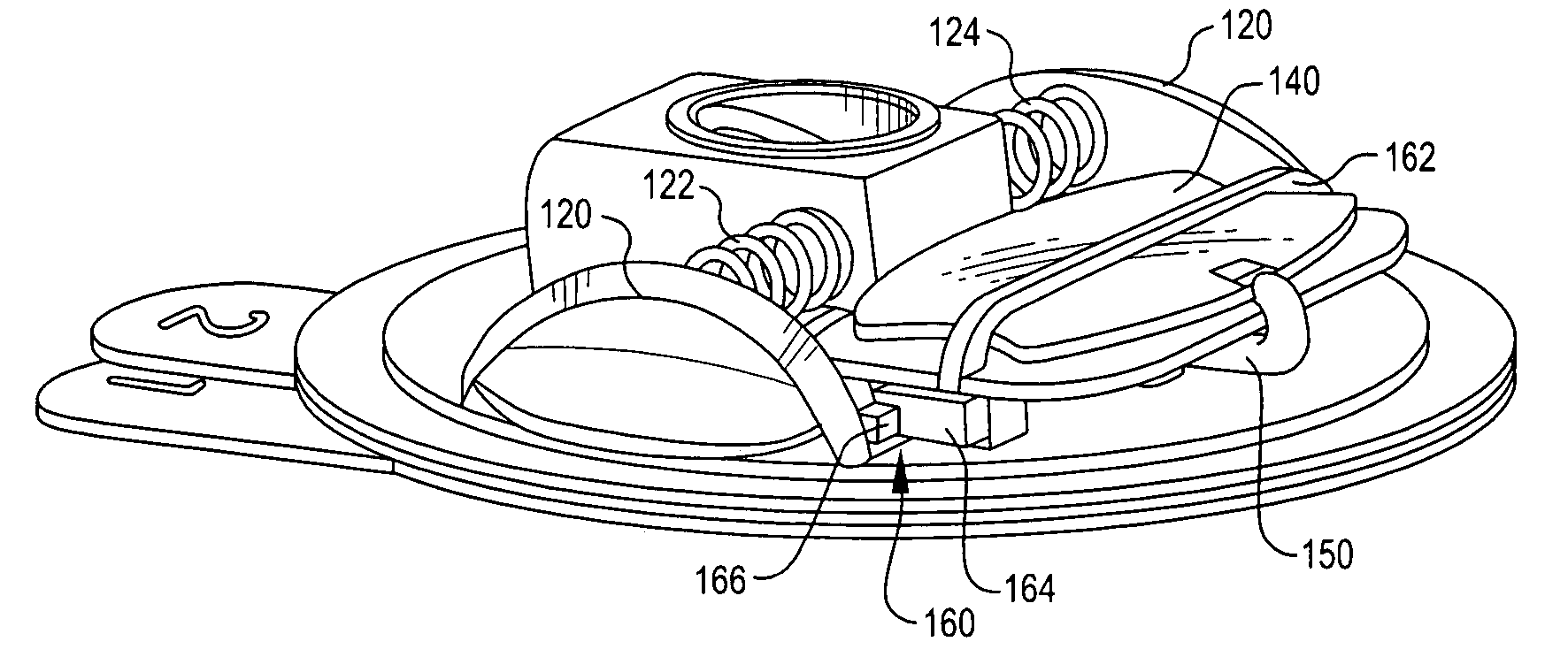
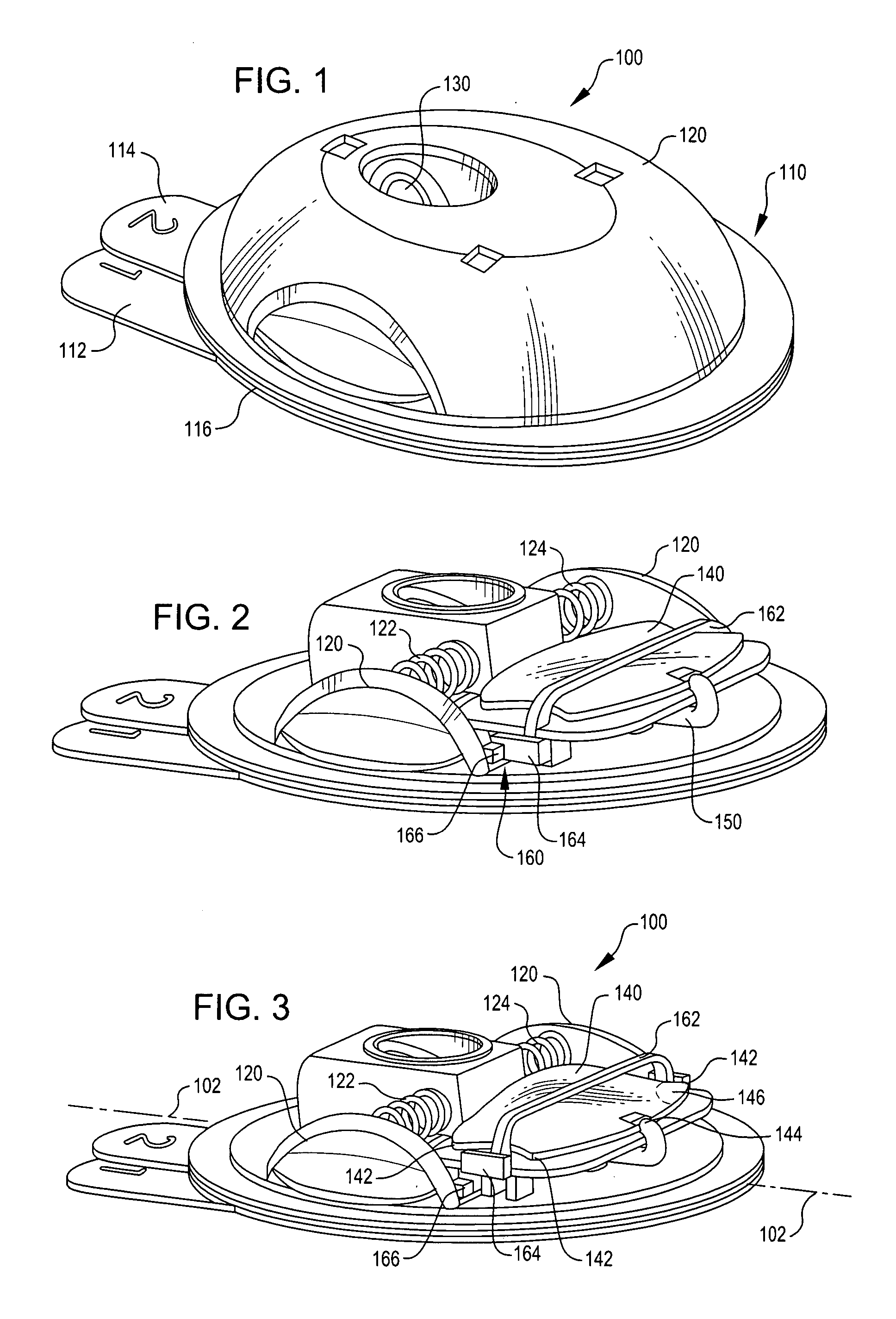
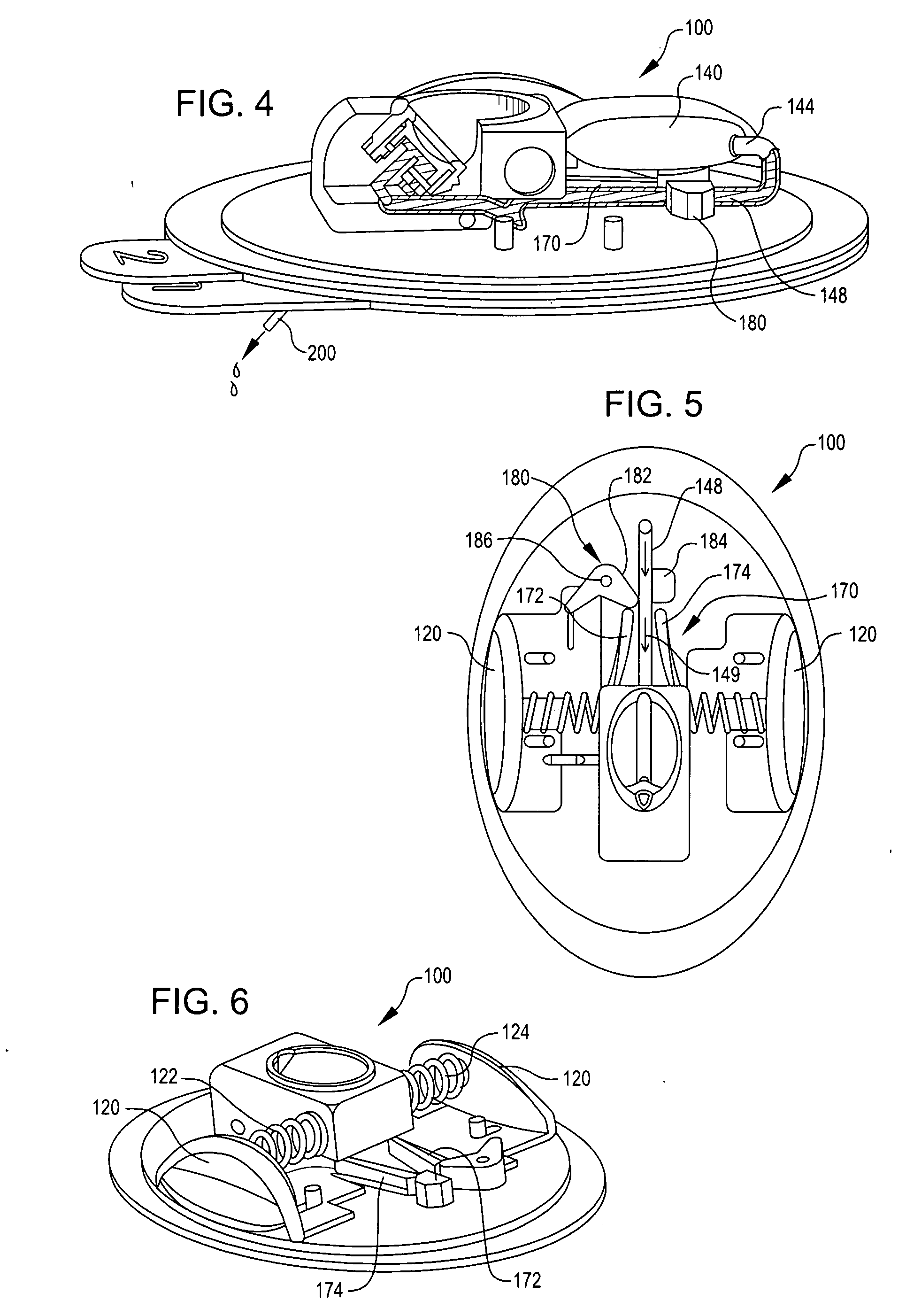
Disposable infusion device with medicament level indicator
A wearable infusion device delivers a liquid medicament, such as insulin, to a patient. The device comprises a base that contacts a patient's skin, a reservoir arranged to contain a liquid medicament to be delivered to beneath a patient's skin, a pump that causes the medicament to flow from the reservoir, and a level indicator that provides an indication of medicament level.
Owner:CALIBRA MEDICAL
Methods and compositions for a multipurpose, lab-on-chip device
InactiveUS20140030800A1Sturdy in constructionReduce usageBioreactor/fermenter combinationsBiological substance pretreatmentsDisaster areaTerrain
Methods and compositions for developing a series of microfluidic, USB-enabled, wireless-enabled, lab-on-chip devices, designed to reduce the chain-of-custody handling of samples between sample acquisition and final reporting of data, to a single individual. These devices provide on-the-spot testing for micro- and nanoscale (molecular) analysis of blood, urine, infectious agents, toxins, measurement of therapeutic drug levels, purity-of-sample testing and presence of contaminants (toxic and non-toxic, volatile and non-volatile); and for the identification of individual components and formal compounds—elemental, biological, organic and inorganic—inclusive of foodstuffs, air, water, soil, oil and gas samples. These devices may be relatively inexpensive, ruggedly designed, lightweight and capable of being employed—depending upon the specific application—by individuals with limited training, in remote and extreme environments and settings: including combat zones, disaster areas, rural communities, tropical / arctic / desert and other inhospitable climates and challenging terrains. The device may be comprised of materials that are reclaimed, are re-usable and are recyclable.
Owner:MOSES JONAS +1
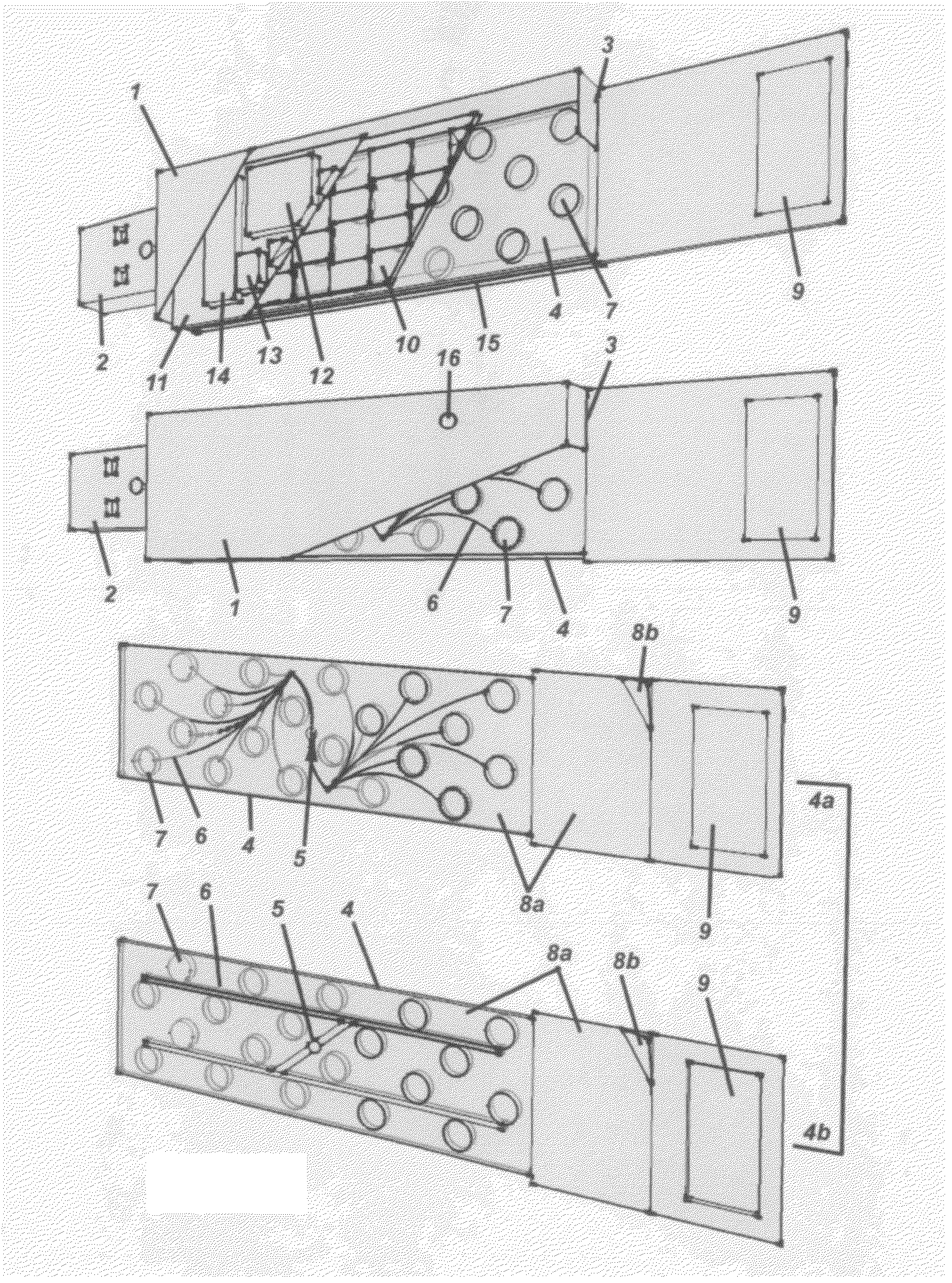
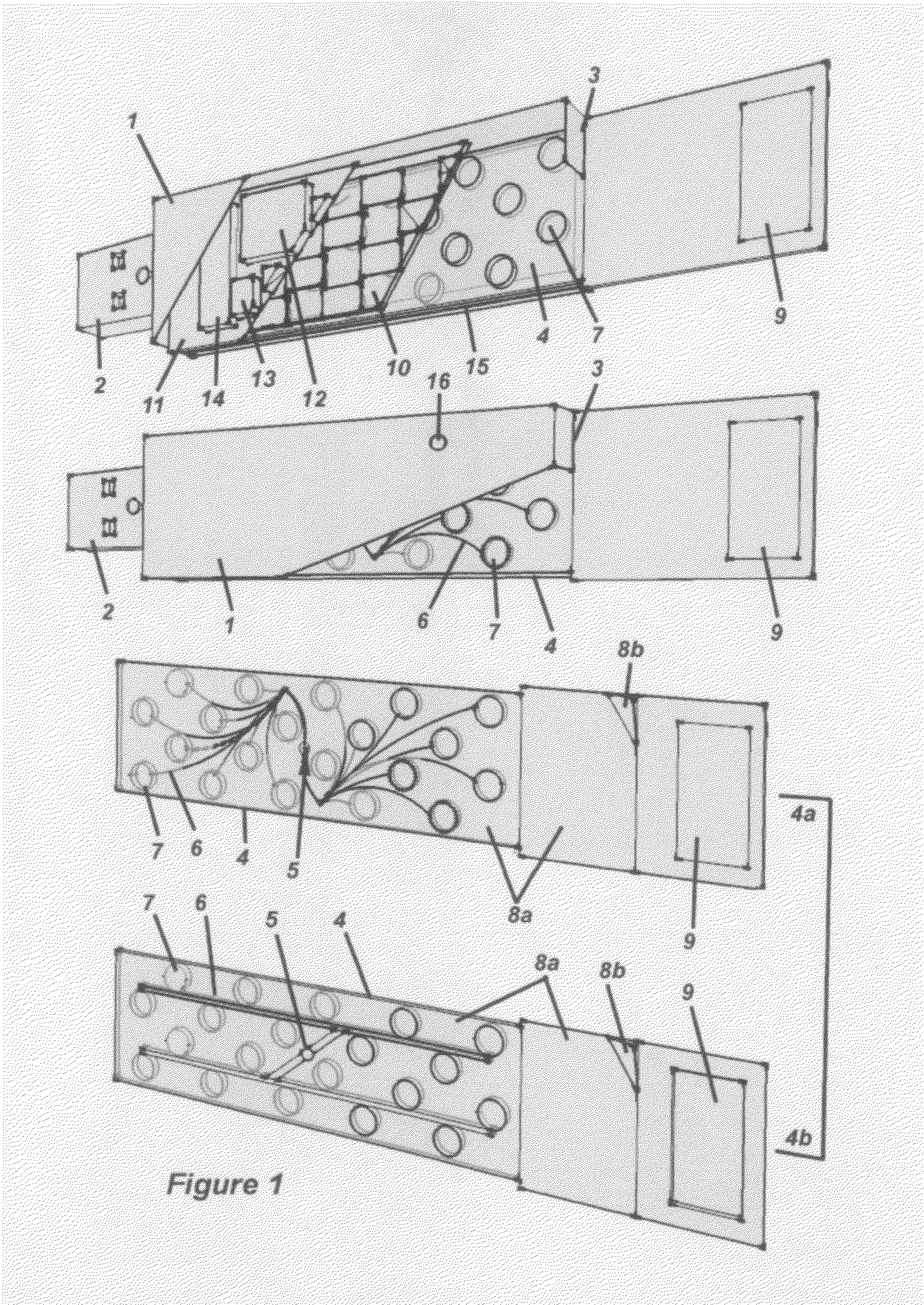
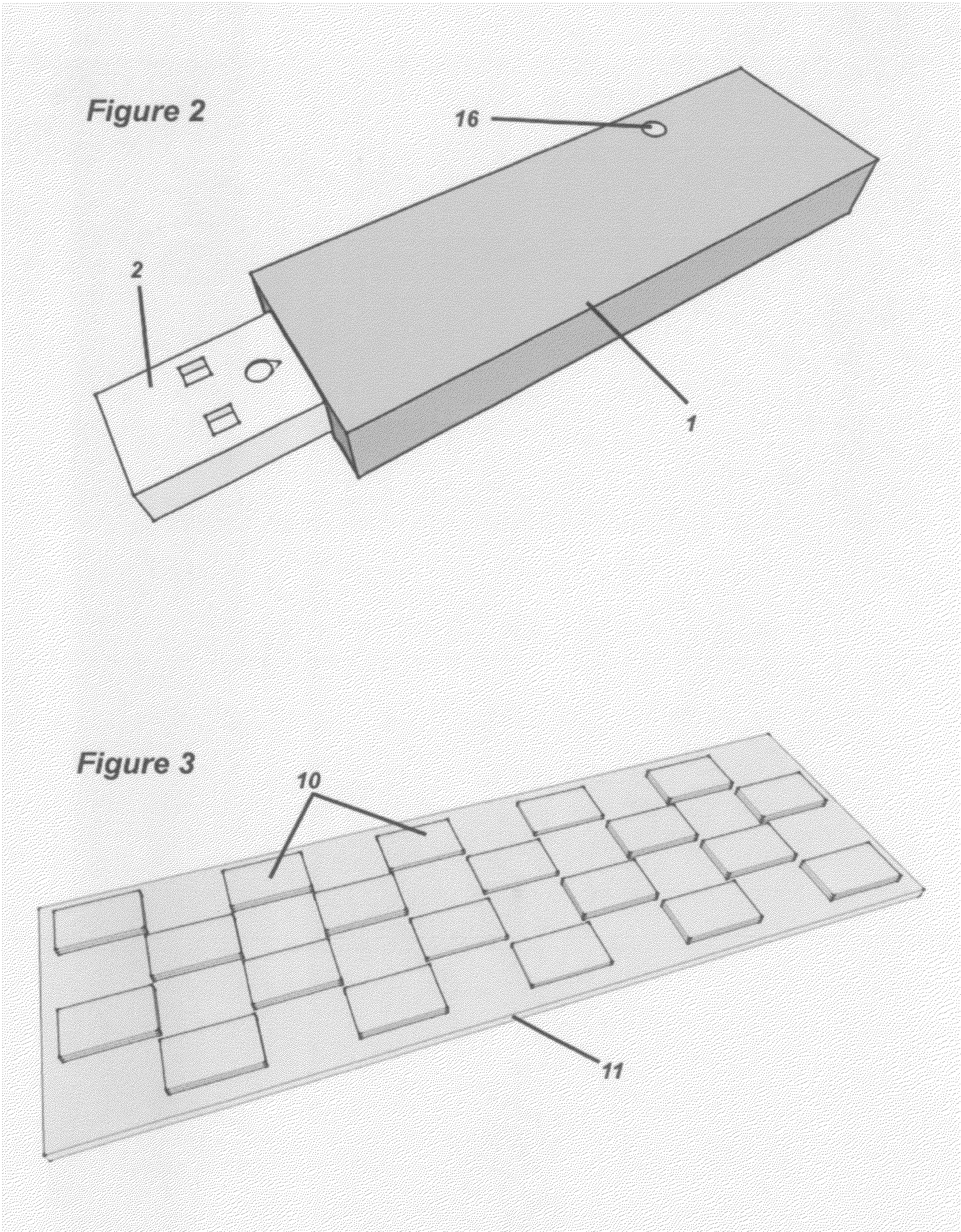
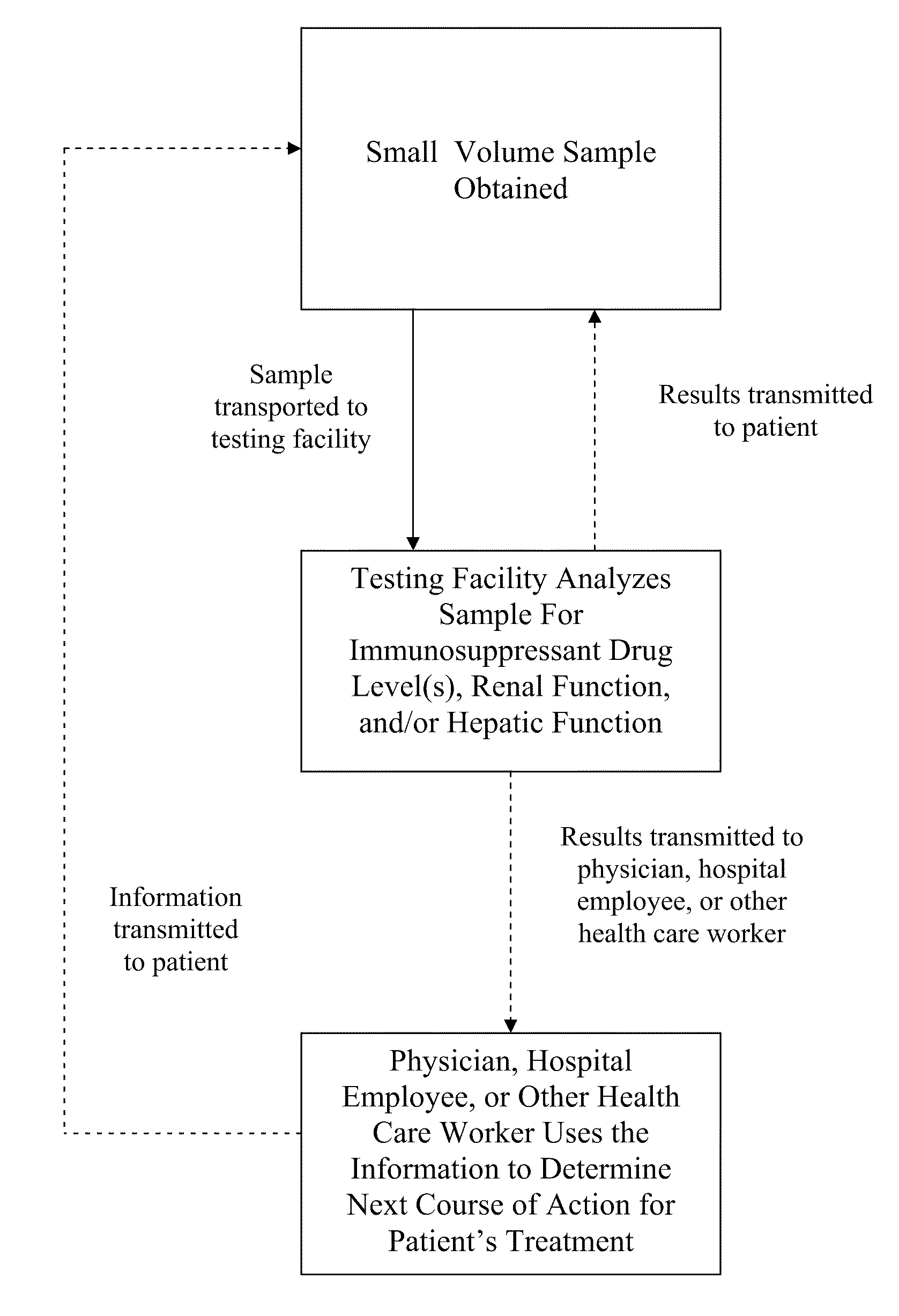
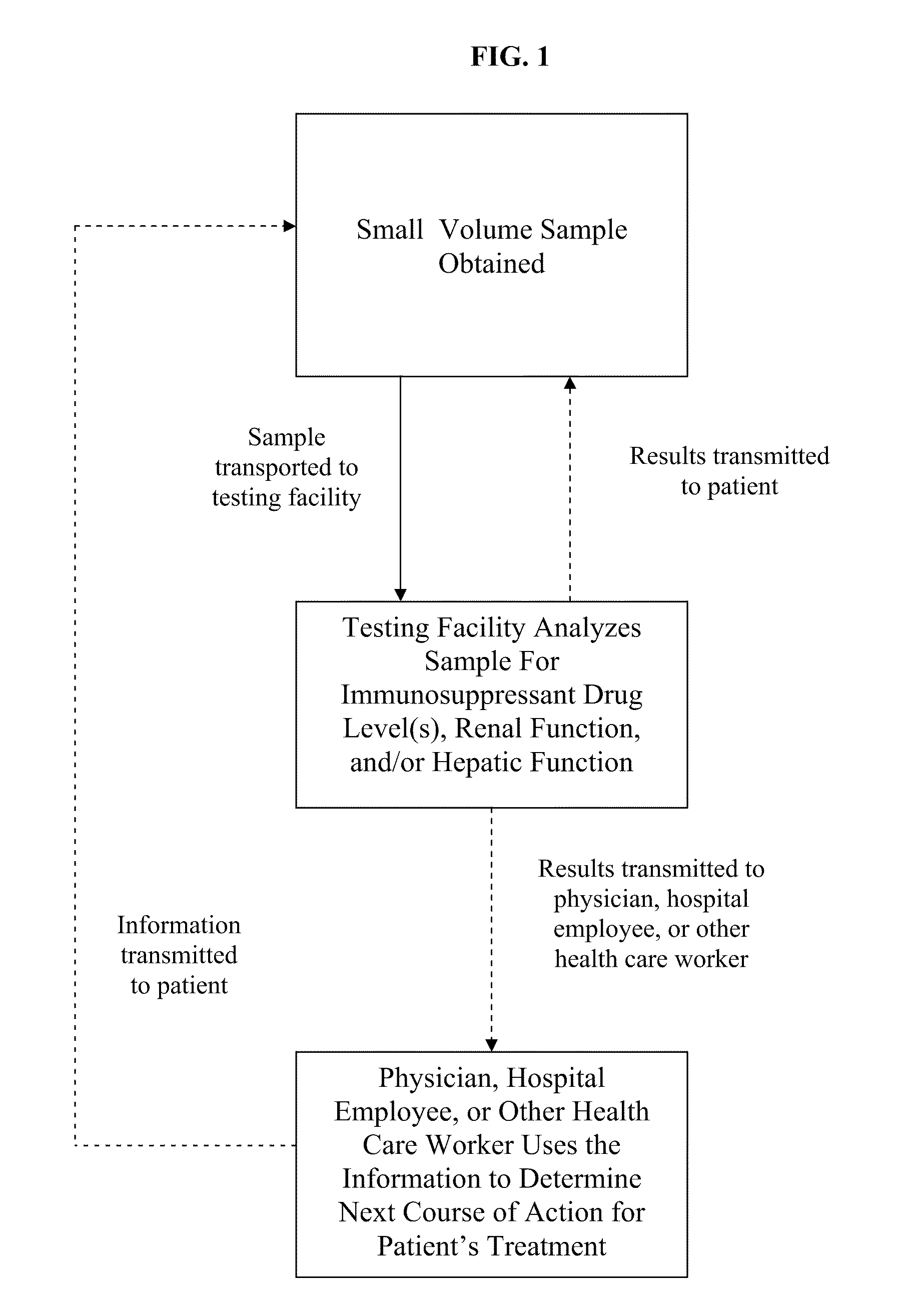
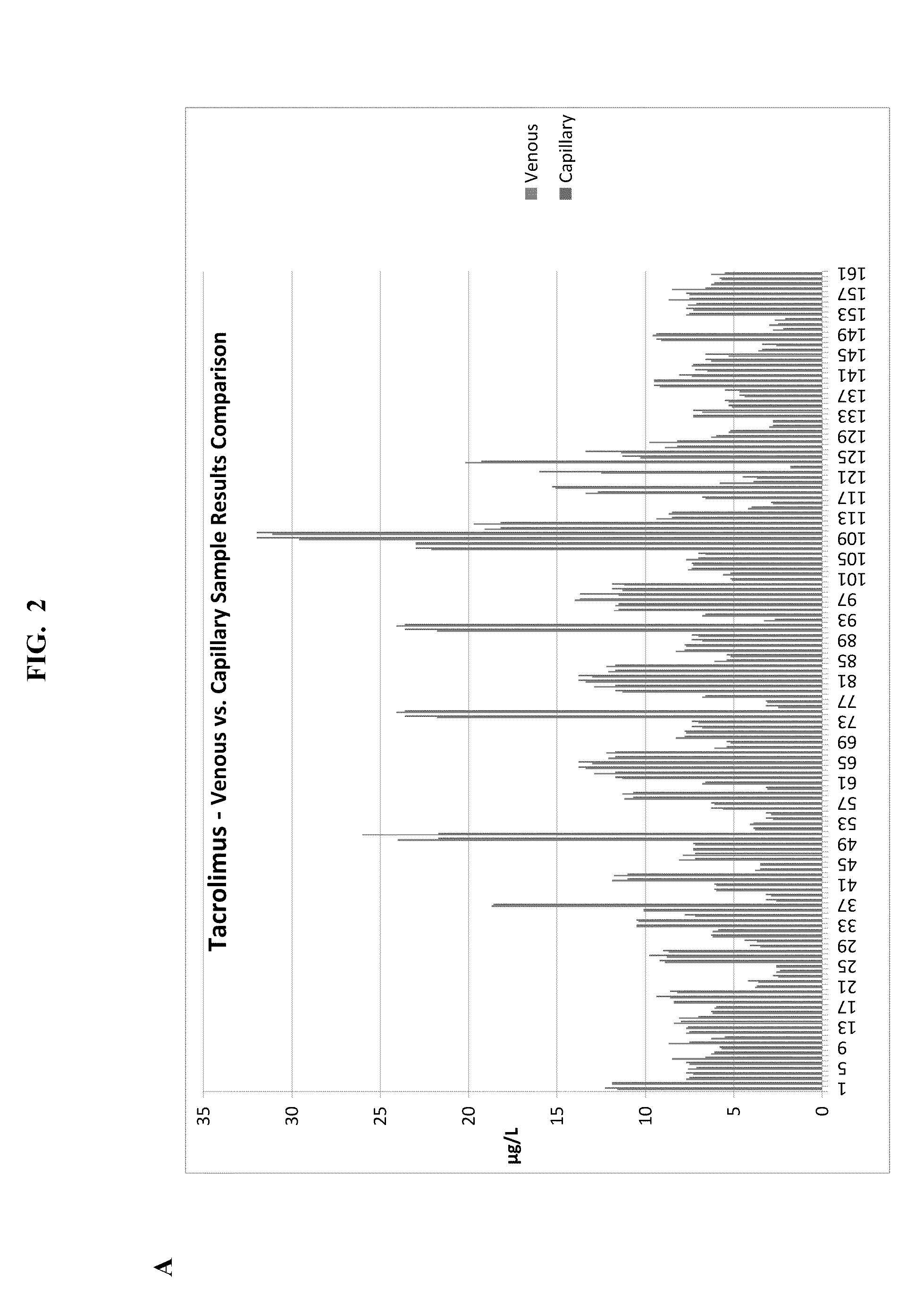
Methods for Monitoring Immunosuppressant Drug Levels, Renal Function, and Hepatic Function Using Small Volume Samples
InactiveUS20090298106A1Analysis using chemical indicatorsComponent separationIntensive care medicineMass spectrometric
Systems and methods are provided for monitoring a immunosuppressant drug level and renal function, hepatic function, or a combination thereof in a patient, comprising obtaining a small volume blood sample from the patient; determining the level of at least one immunosuppressant drug in the small volume blood sample and determining the level of a second immunosuppressant drug or analyzing the renal function, the hepatic function, or a combination thereof in the patient. In some embodiments, the immunosuppressant drug levels are determined using a liquid chromatography tandem mass spectrometry (LC-MS / MS) procedure. Also provided are kits for use in any of the systems and methods described herein.
Owner:THERAPEUTIC MONITORING SERVICES
Systems and methods for providing gastrointestinal pain management
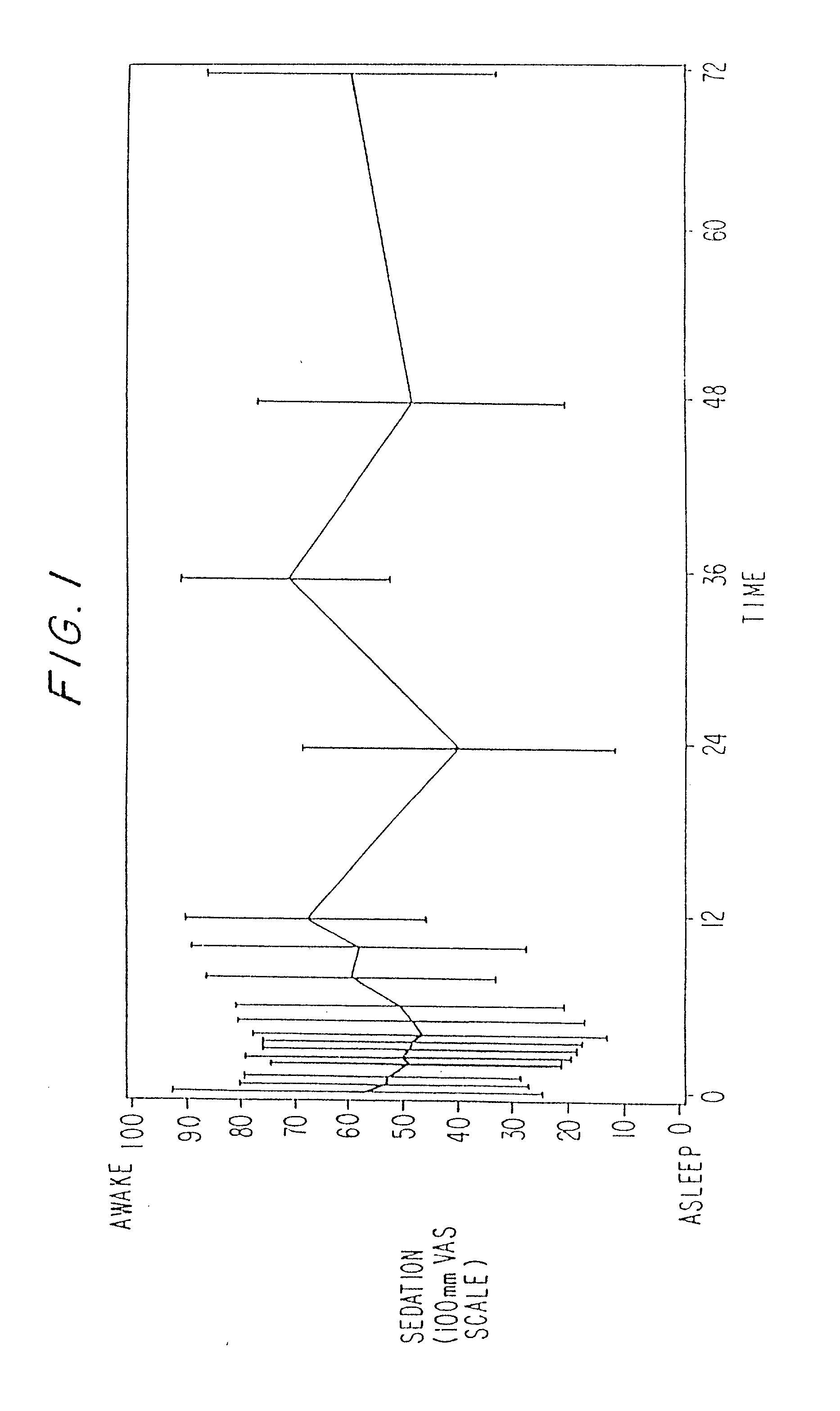
The present invention includes systems and methods for decreasing the pain and discomfort commonly associated with endoscopic procedures, where such procedures may be performed with lower dosage levels of sedative and analgesic drugs. The invention includes use of an anesthetic collar coupled to an endoscope with a flexible shaft. The anesthetic collar allows lubricants, local anesthetics, dyes, and / or other desirable fluids to be passed through the existing lumen of the flexible shaft into an annulus, where the fluid may be distributed through expulsion pores into the gastrointestinal tract. Utilizing the existing lumens found in endoscopes, the present invention allows those fluids that may reduce the pain and discomfort associated with endoscopies such as, for example, local anesthetics and lubricants, to be distributed in an even fashion throughout the gastrointestinal tract or throughout the length and circumference of the endoscope, where such fluids may reduce the drug level requirements for sedative and analgesic agents. Alternatively, the endoscope may be redesigned for streamlined integration with the anesthetic collar or to accomplish the same function of distributing local anesthetics and lubricants, in an even fashion throughout the gastrointestinal tract or throughout the length and circumference of the endoscope, The invention can also be used with endoscopes without existing lumens.
Owner:SCOTT LAB
Marker Detection Method And Apparatus To Monitor Drug Compliance
InactiveUS20140294675A1Accurate assessmentPatient complianceWithdrawing sample devicesDiagnostic recording/measuringNoseEnvironmental health
The present invention includes systems and methods for monitoring therapeutic drug concentration in blood by detecting markers, such as odors, upon exhalation by a patient after the drug is taken, wherein such markers result either directly from the drug itself or from an additive combined with the drug. In the case of olfactory markers, the invention preferably utilizes electronic sensor technology, such as the commercial devices referred to as “artificial” or “electronic” noses or tongues, to non-invasively monitor drug levels in blood. The invention further includes a reporting system capable of tracking drug concentrations in blood (remote or proximate locations) and providing the necessary alerts with regarding to ineffective or toxic drug dosages in a patient.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Fluorine-containing optically active composition for anti-infection
ActiveCN101550153AStable in natureLower pHOrganic active ingredientsOrganic chemistrySolubilitySide effect
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE +1
Anti-cancer medicine composition containing antimetabolite
InactiveCN1634584AAntineoplastic agentsHeterocyclic compound active ingredientsWhole bodyTherapeutic effect
An anticancer pharmaceutical composition composed of pharmaceutic adjuvant and anti-metabolism medicine is disclosed. Wherein, the anti-metabolism medicine can effectively destroy DNA and / or protein synthesis and repairing function inside the tumor cell so as to inhibit the tumor cell growth, while the pharmaceutic adjuvant can mainly be biological compatible, degradable and absorbable macromolecule polymer, which can make the anti-metabolism drug to release slowly in the local tumor region in the degradation and absorption process, therefore it can both decrease considerably the whole body toxic reaction and sustain the local tumor effective drug level.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
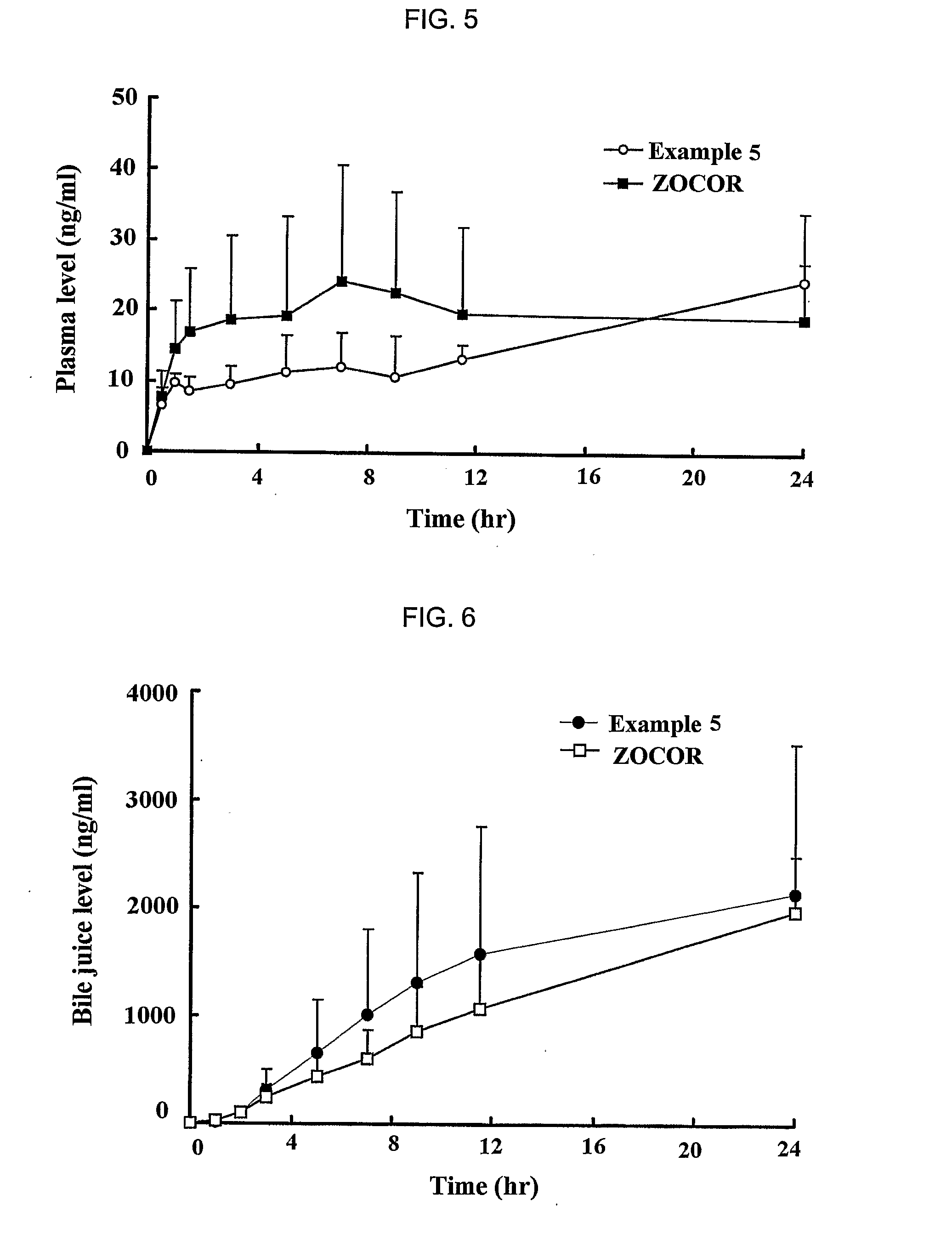
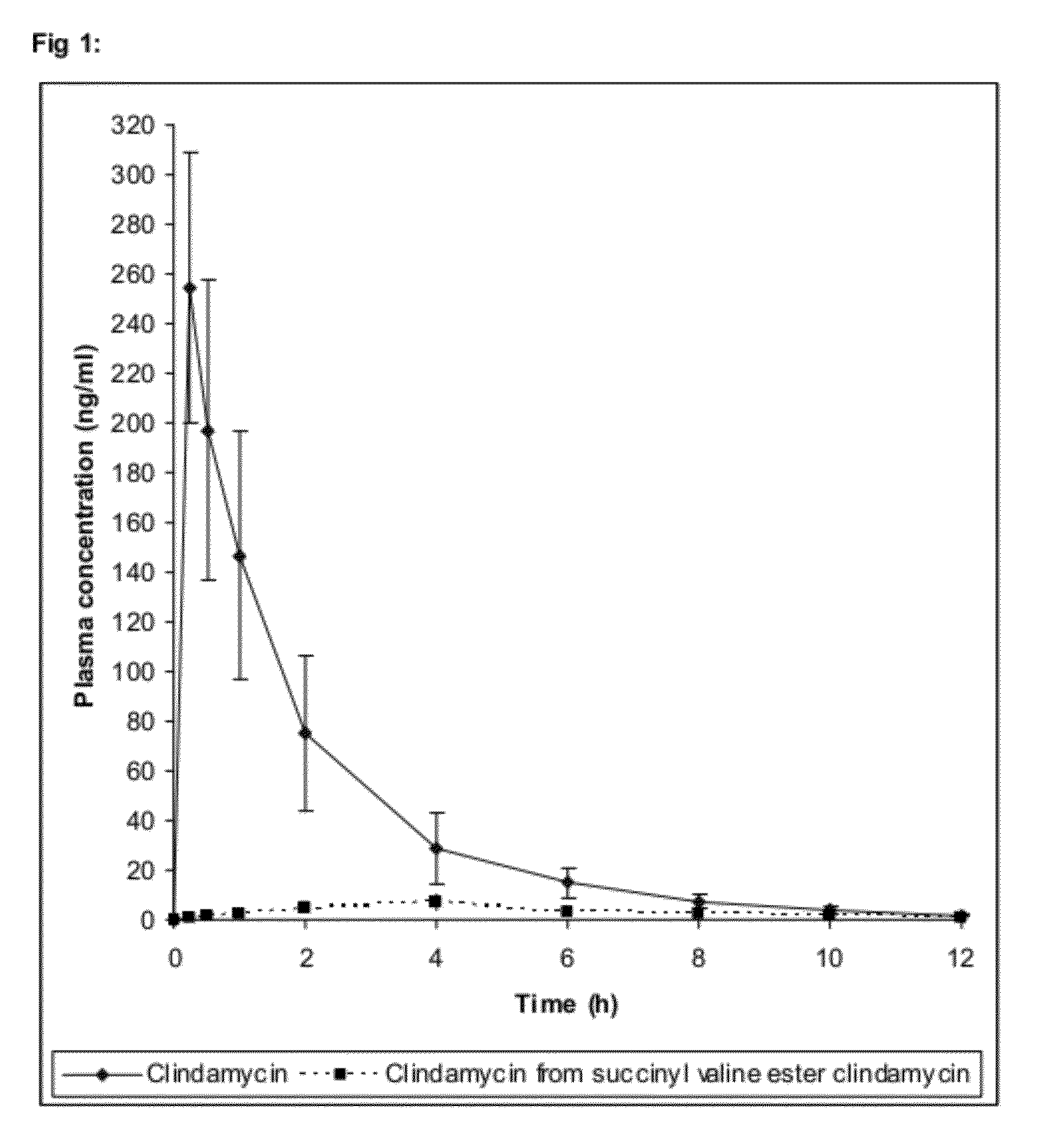
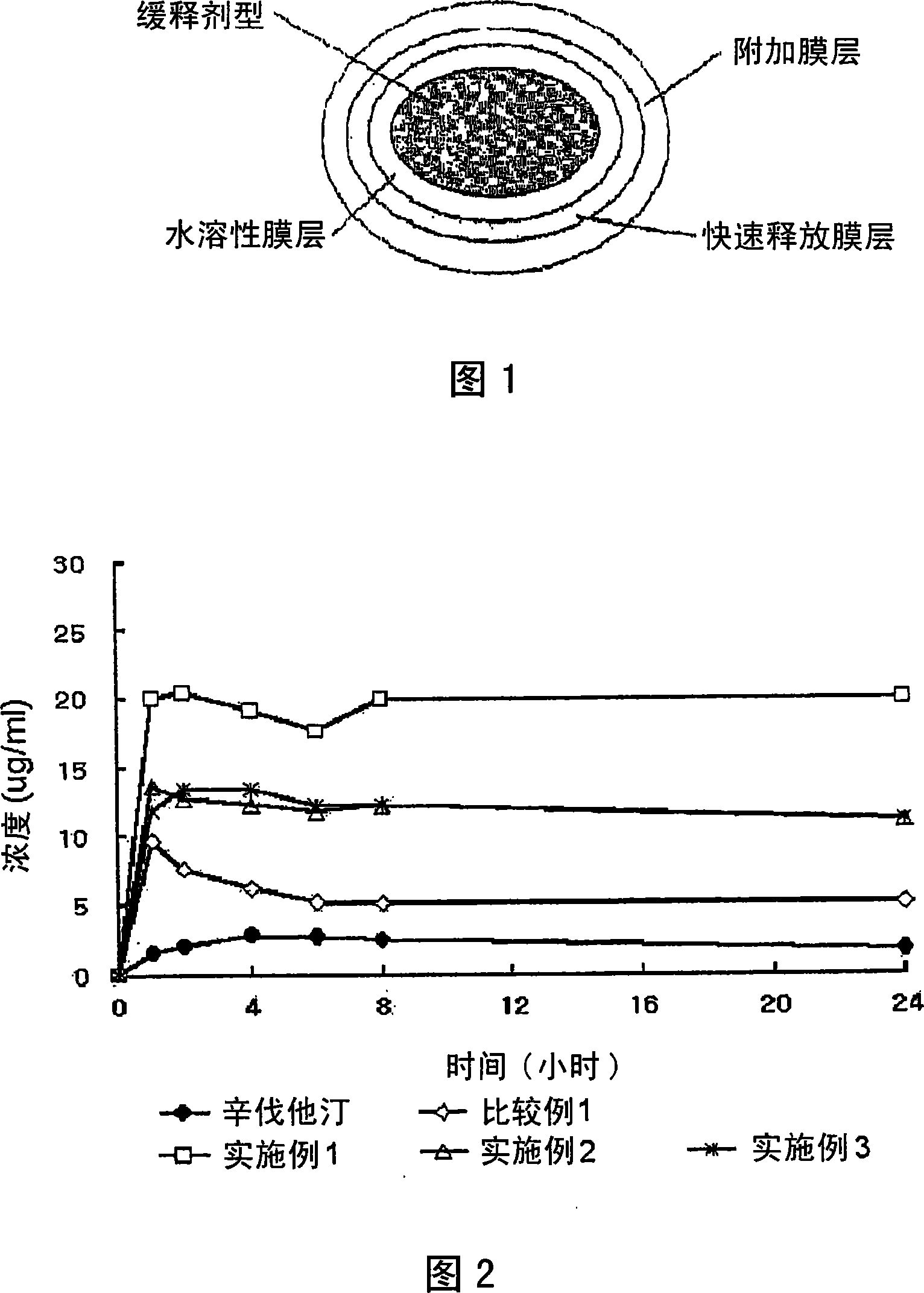
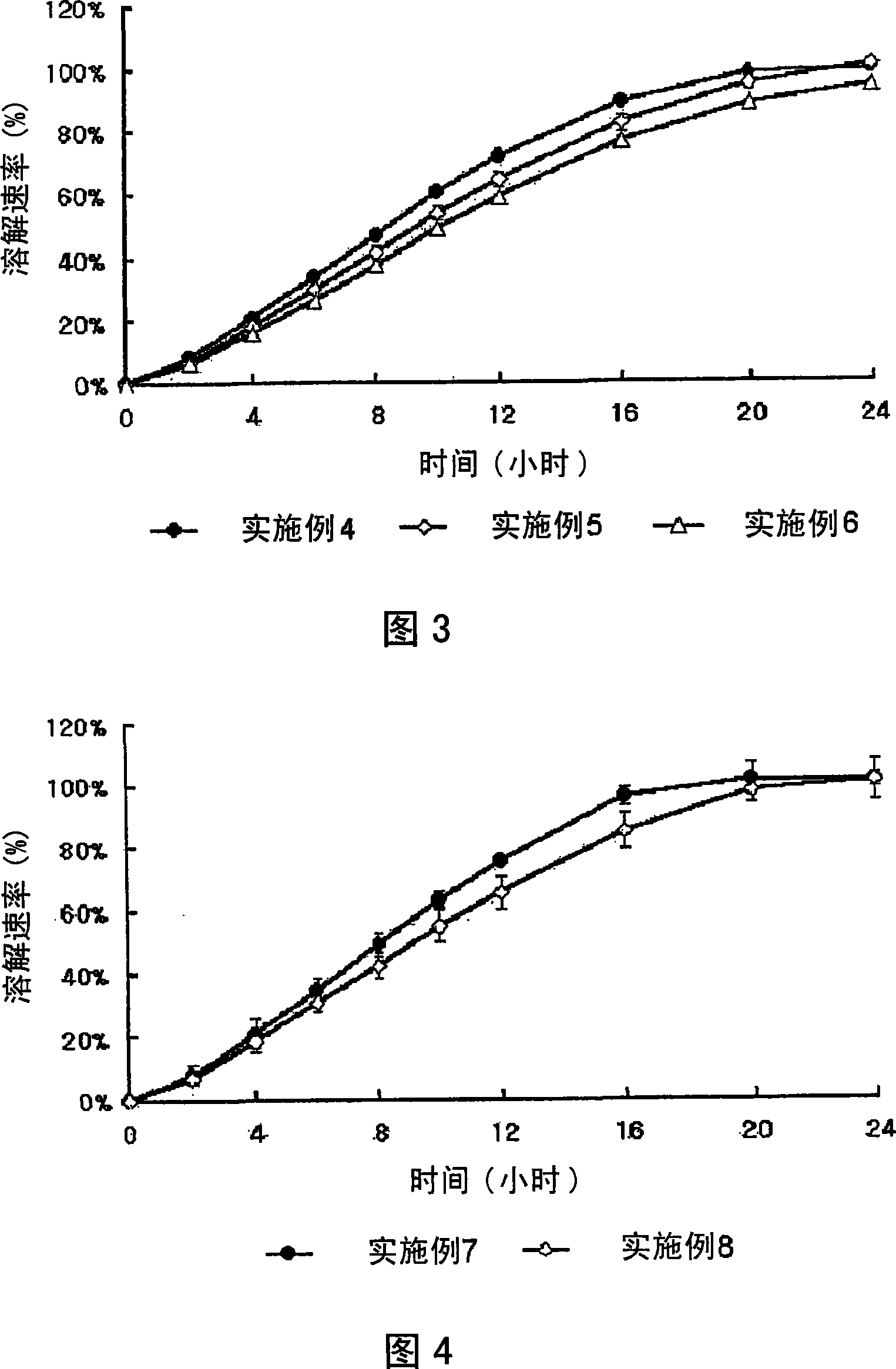
Complex Formulation Of 3-Hydroxy-3-Methyl Glutaryl Coa Reductace Inhibitor And Antihypertensive Agent, And Process For Preparing Same
InactiveUS20080096866A1Minor side effectsBiocideMetabolism disorderHMG-CoA reductaseHypertension medications
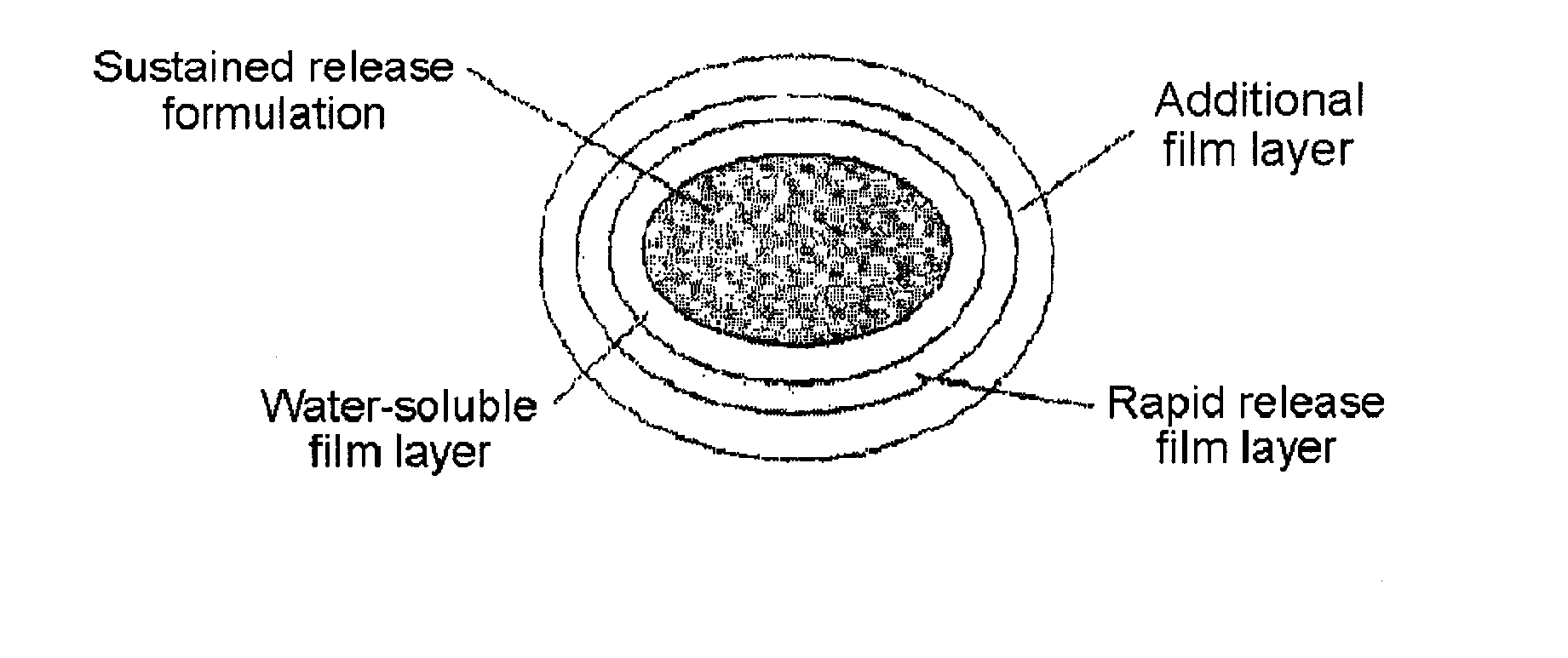
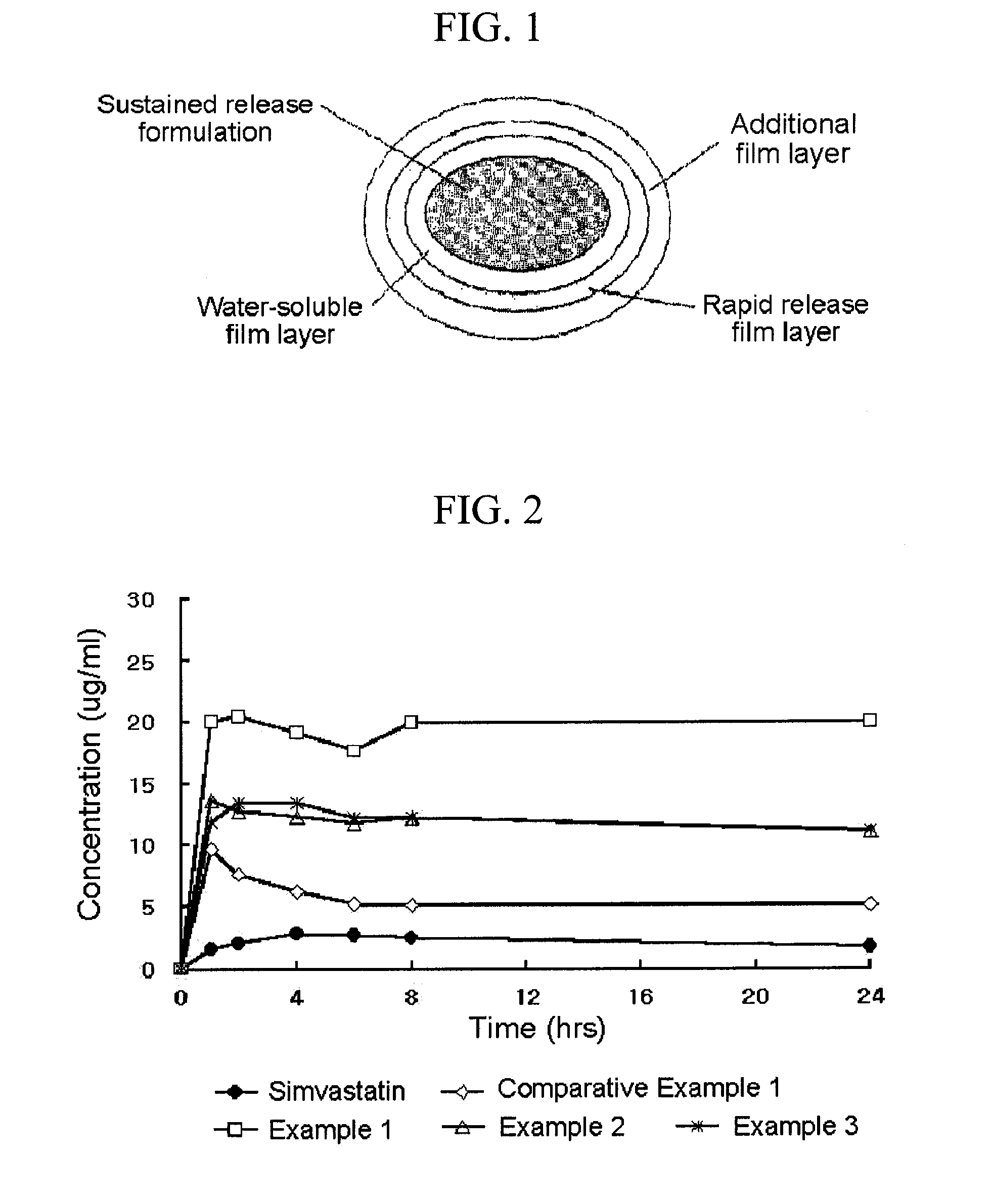
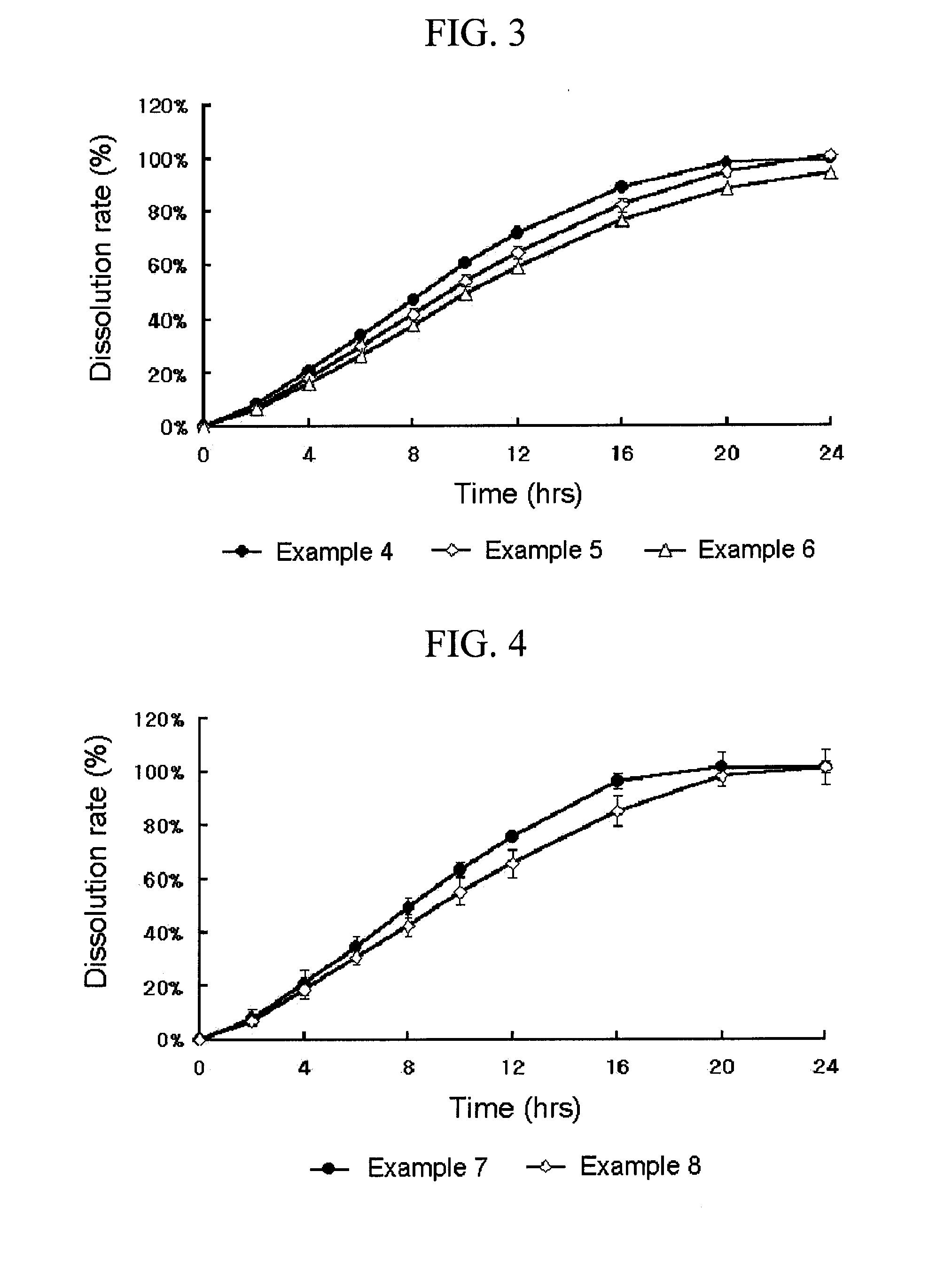
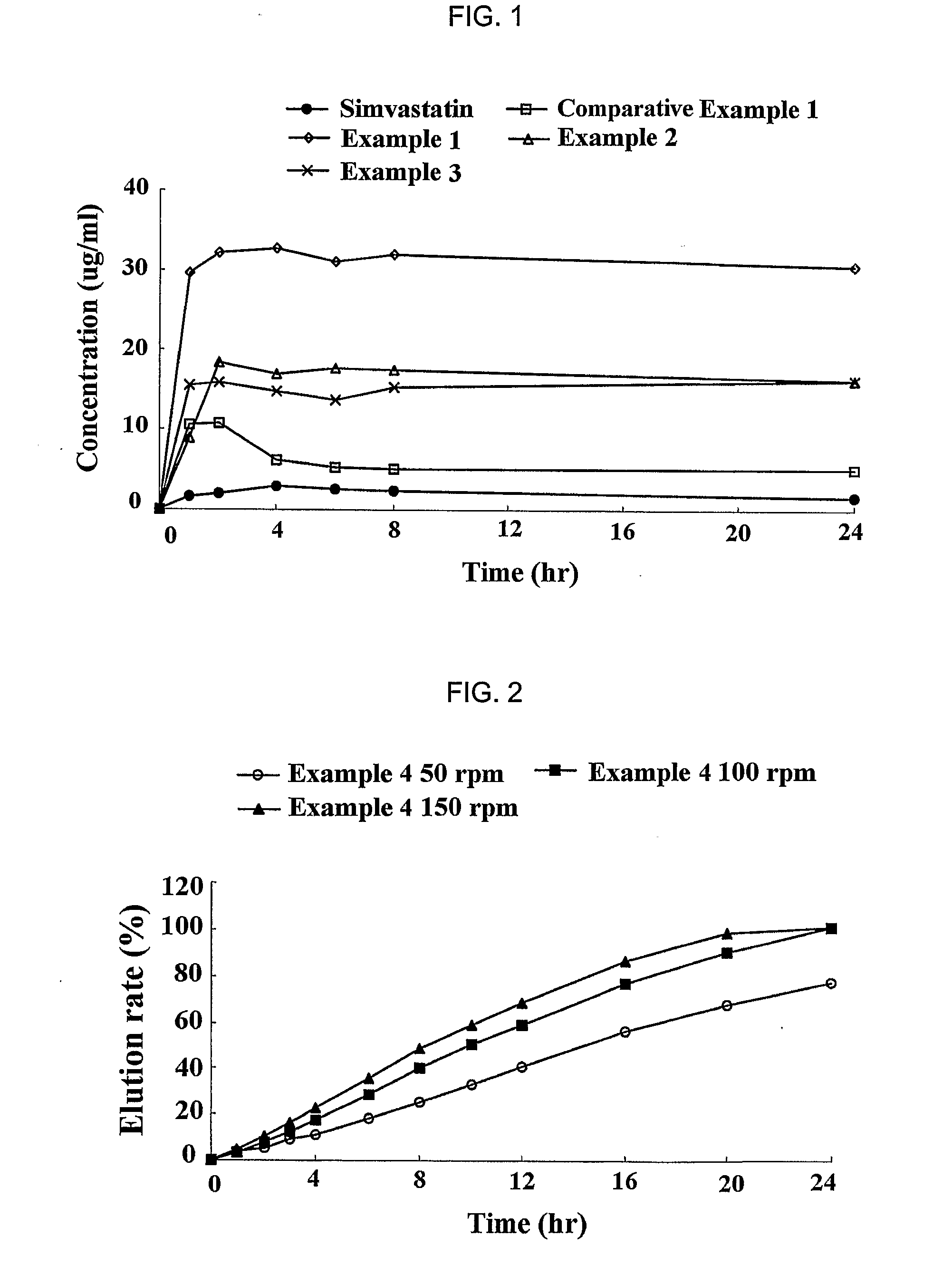
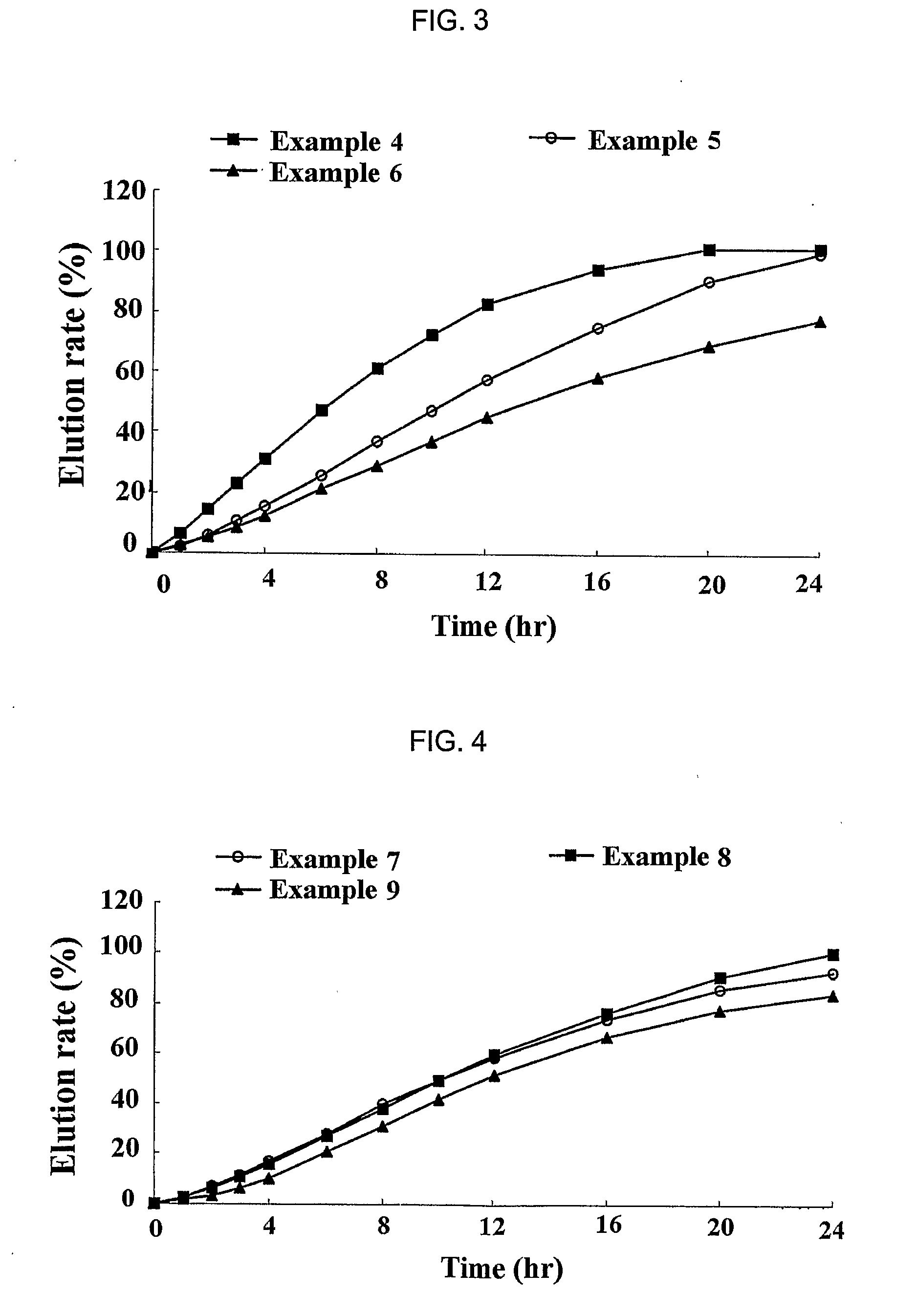
A complex formulation for oral administration comprising a sustained release formulation of an HMG-CoA reductase inhibitor and a film layer for rapid release of an anti-hypertensive agent, the film layer being coated on the sustained release formulation, can achieve improved therapeutic effects of the anti-hypertensive agent by promptly releasing it, while maintaining a constant drug level of the HMG-CoA reductase inhibitor in blood through a slow release. Accordingly, the complex formulation is useful for preventing and treating diseases such as hyperlipidemia, atherosclerosis, hypertension and cardiovascular disease.
Owner:HANMI PHARMA
Sustained release formulation for oral administration of hmg-coa reductase inhibitor and method for the preparation thereof
The sustained release formulation for oral administration of an HMG-CoA reductase inhibitor of the present invention can be easily and economically prepared and is capable of maintaining a constant drug level in blood by slowly releasing the HMG-CoA reductase inhibitor at a uniform rate for 24 hrs. Accordingly, the sustained release formulation of the present invention can be effectively used for lowering blood cholesterol and triglyceride levels.
Owner:HANMI SCI CO LTD
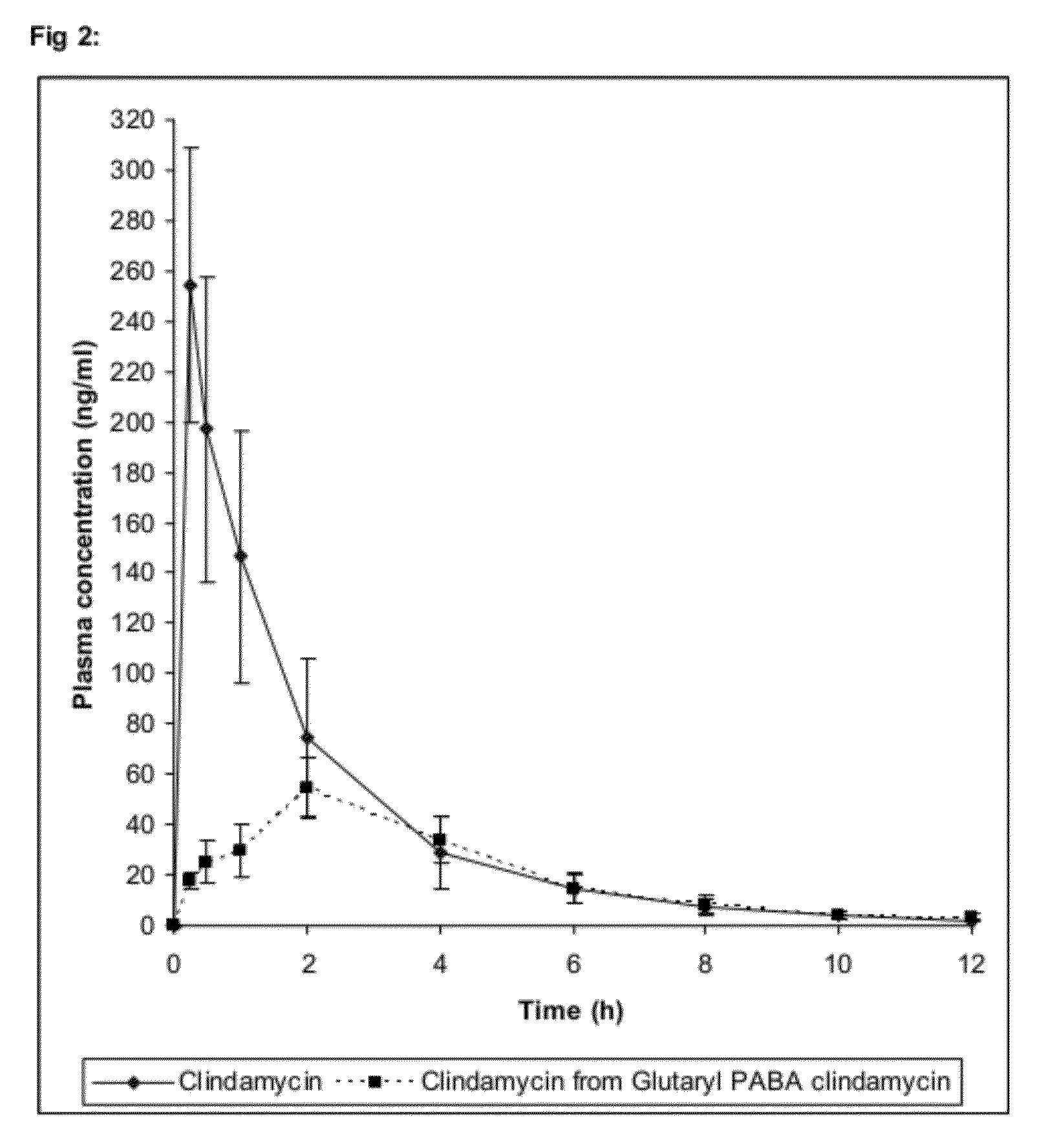
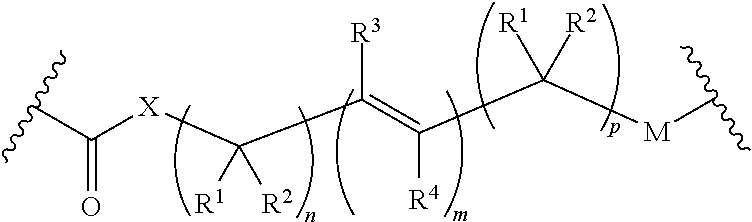
Use of prodrugs to avoid gi mediated adverse events
InactiveUS20120202756A1Minimizing gastrointestinal side effectReduce frequencyAntibacterial agentsBiocideSide effectBlood plasma
The present invention relates to prodrugs of a wide variety of drugs and pharmaceutical compositions containing such prodrugs. Methods for minimizing locally mediated (from within the gut lumen) adverse gastrointestinal events associated with the underivatised drug and increasing the sustainment of plasma drug levels with the aforementioned prodrugs are also provided. Thus, the present invention relates to the use of prodrugs of a wide diversity of drugs (other than opioids) to transiently inactivate them and so reduce directly, locally mediated adverse gastrointestinal (GI) side-effects normally evident after administration of the parent compound. Additionally, such prodrugs may confer improved pharmacokinetics.
Owner:FRANKLIN RICHARD +3
Transdermal drug administering system
InactiveCN1663560ALow costReduce wasteMedical devicesPharmaceutical non-active ingredientsVerapamil HydrochlorideWater soluble drug
The invention discloses a percutaneous give drug system, which comprises: a drug storeroom, the gel drug layer comprises drug, solvent, transdermal accelerating agent and gelatinizing agent; one adsorption layer comprised solvent absorption material of solvent in absorbable drug storeroom; one isolating layer between drug storeroom and adsorption layer, which is of non-woven fabrics or semipermeable membrane to make solvent of gas or liquid pass through successfully; adsorption layer can joint isolating layer of drug storeroom in opposite directions of closing up to skin of drug storeroom. The percutaneous give drug system in this invention can increase drug level in drug storeroom of the system to elevate drug transdermal speed, as well as increase controlled release effect of percutaneous give drug and absolute bioavailability, enhance drug curative effect, decrease drug waste, and depress preparation cost of the system. The invention is fit particular to percutaneous give drug system for water-soluble drug of propranolol hydrochloride, verapamil hydrochloride and chlorpromazine hydrochloride.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Complex formulation of 3-hydroxy-3-methyl glutaryl coa reductase inhibitor and antihypertensive agent, and process for preparing same
InactiveCN101090718AMaximize the effect of treatmentGood treatment effectMetabolism disorderPill deliveryHMG-CoA reductaseHypertension medications
A complex formulation for oral administration comprising a sustained release formulation of an HMG-CoA reductase inhibitor and a film layer for rapid release of an anti-hypertensive agent, the film layer being coated on the sustained release formulation, can achieve improved therapeutic effects of the anti-hypertensive agent by promptly releasing it, while maintaining a constant drug level of the HMG-CoA reductase inhibitor in blood through a slow release. Accordingly, the complex formulation is useful for preventing and treating diseases such as hyperlipidemia, atherosclerosis, hypertension and cardiovascular disease.
Owner:HANMI PHARMA
Compositions for the treatment of neoplasms
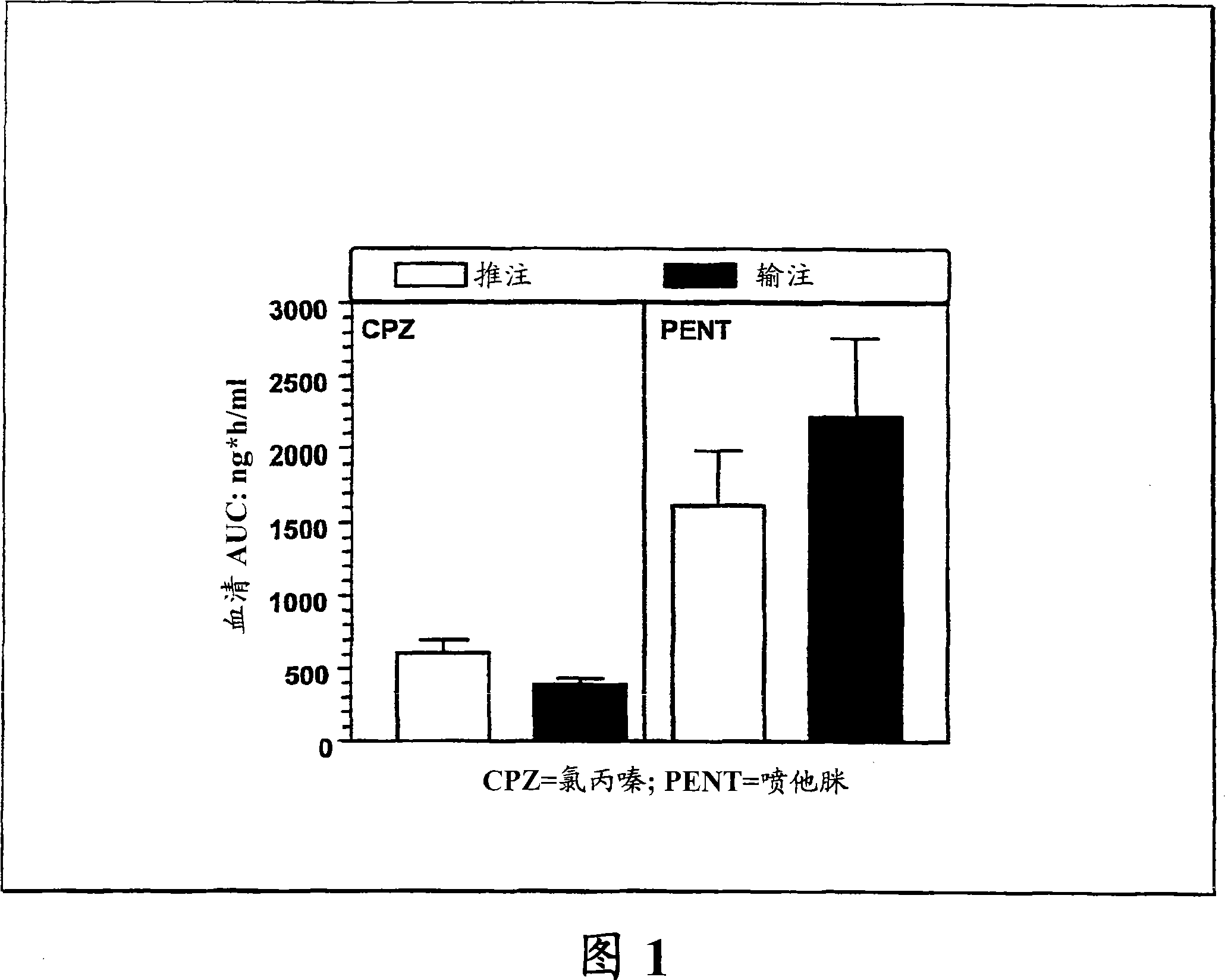
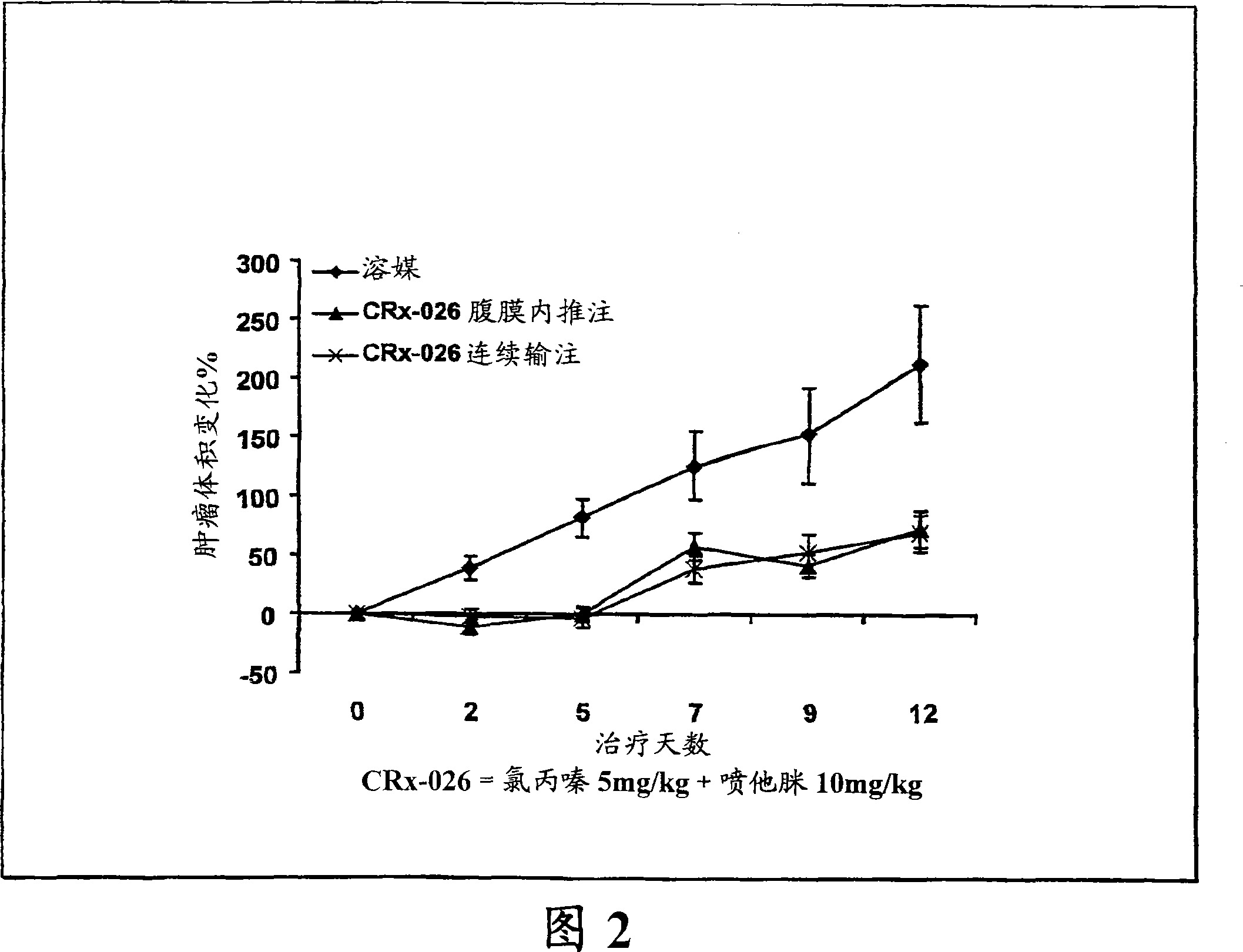
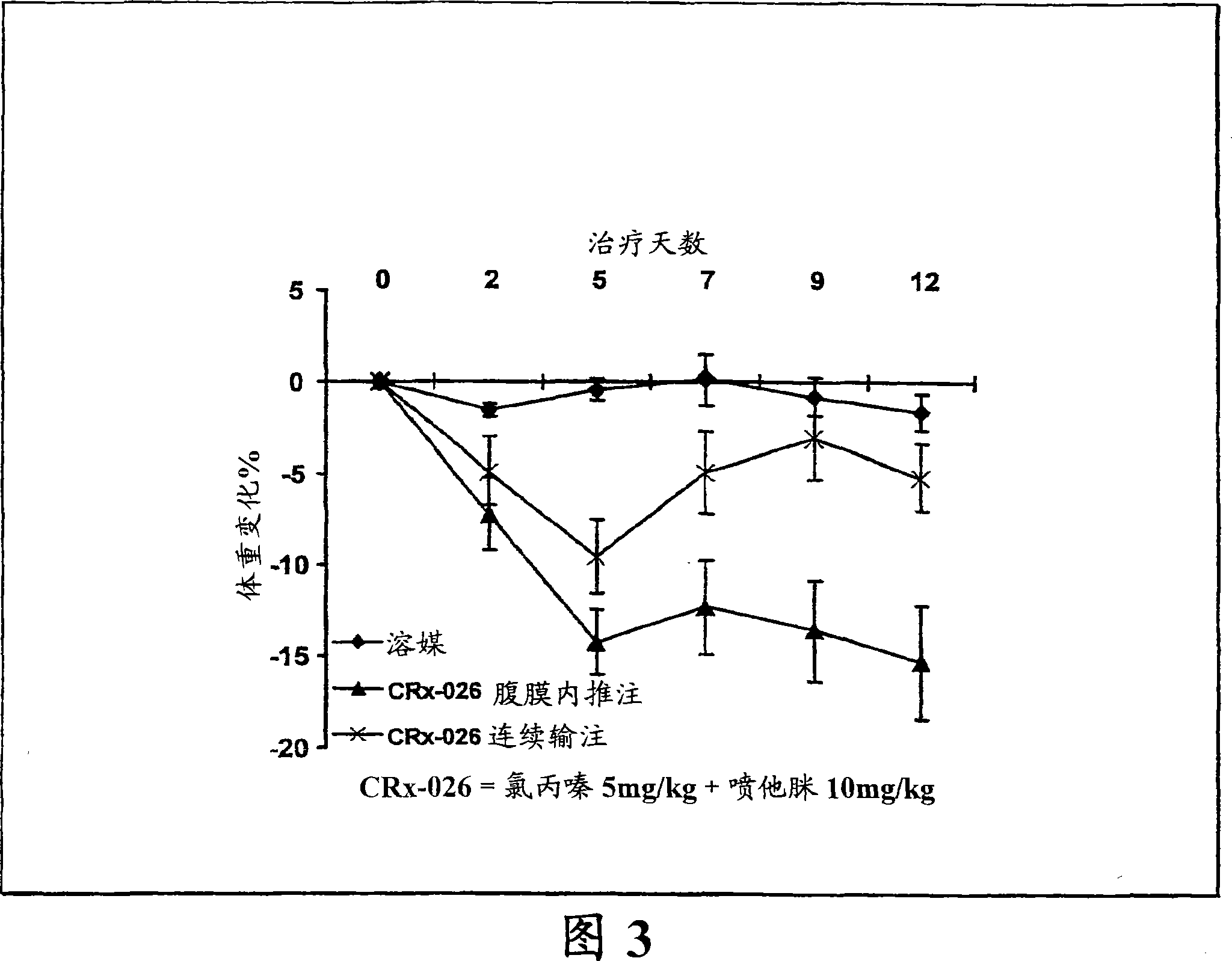
The invention features a method for treating a patient having a cancer or other neoplasm by administering to the patient a composition that includes a phenothiazine and another active agent, where predetermined plasma drug levels are achieved and maintained for 12 hours or more.
Owner:COMBINATORX
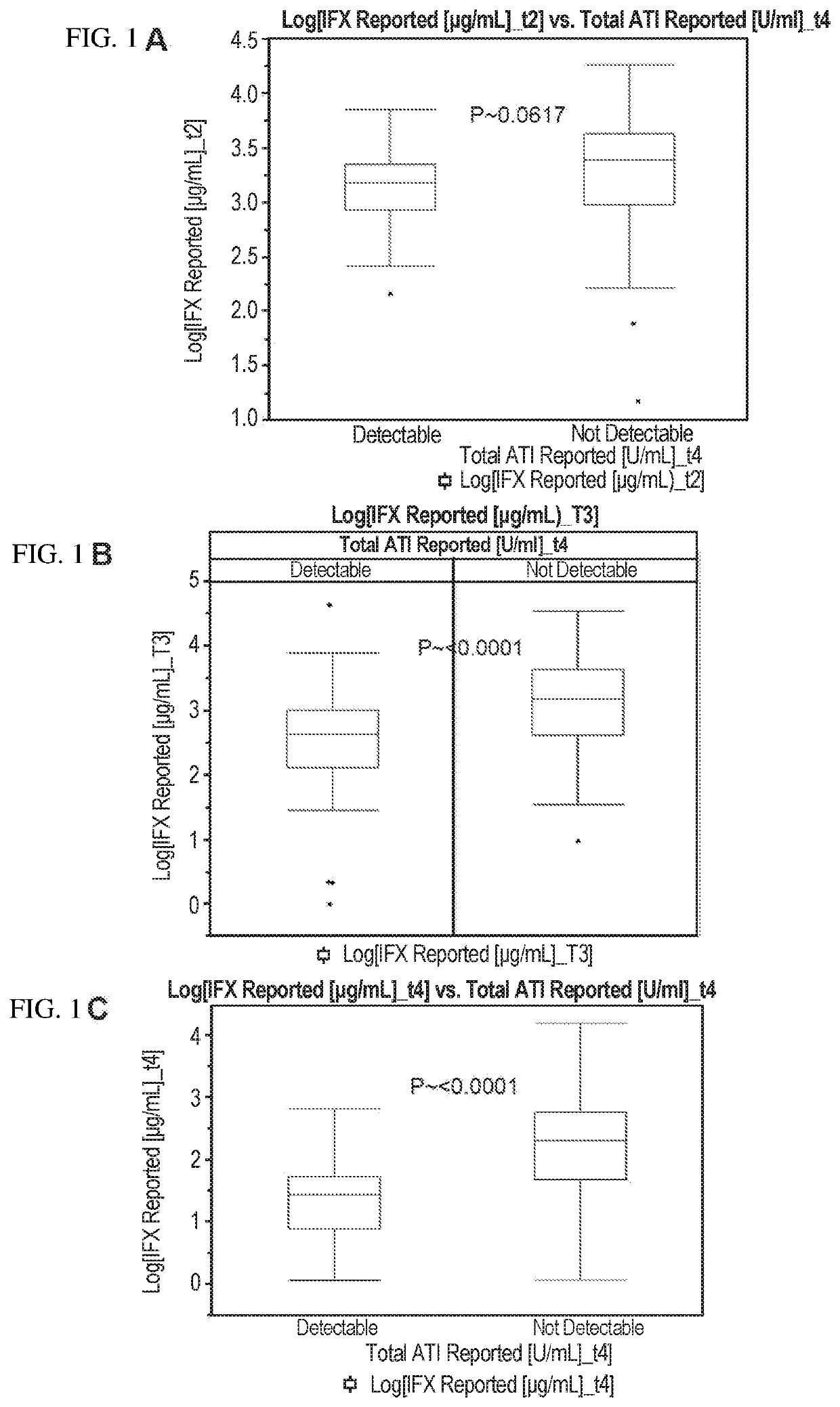
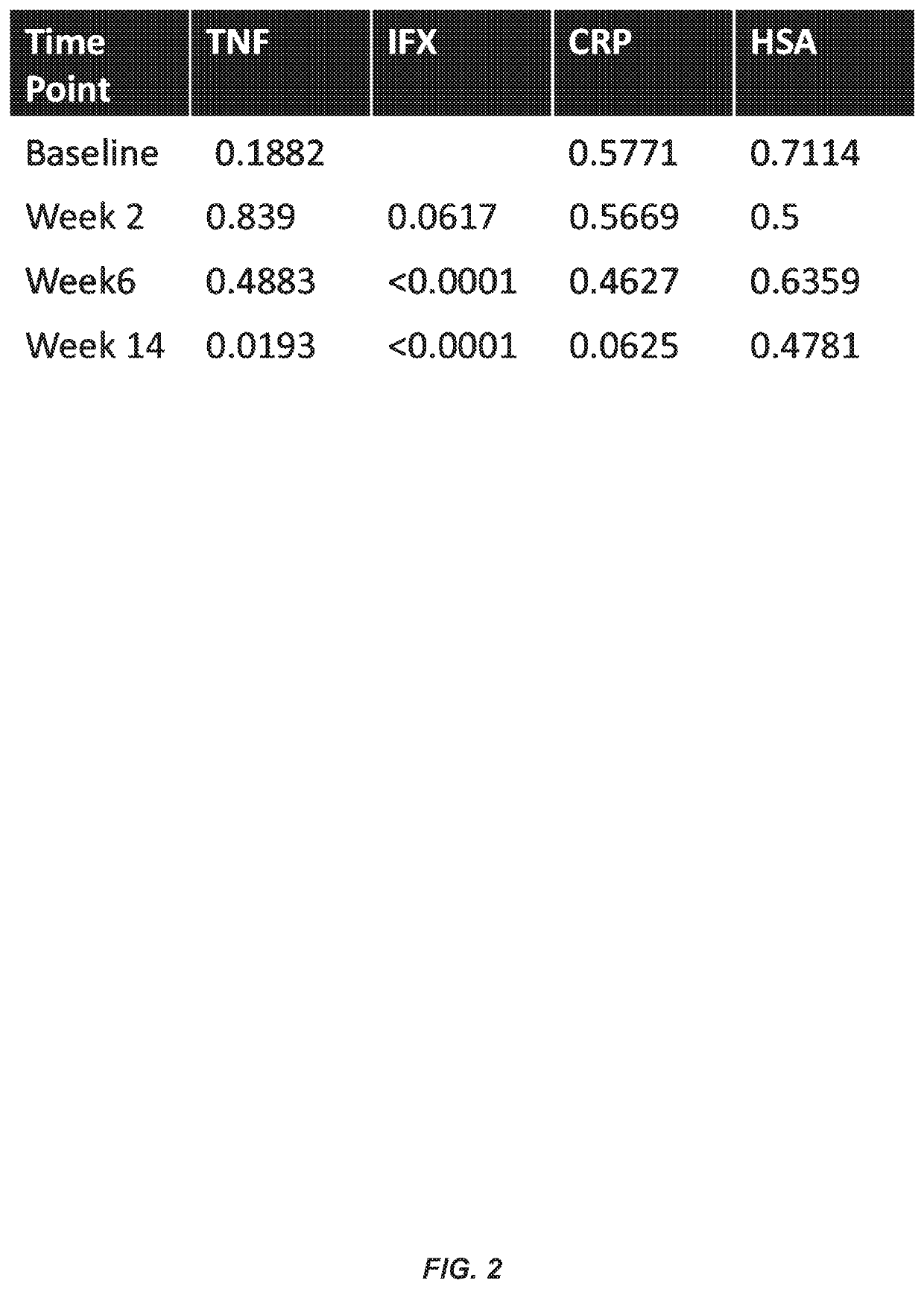
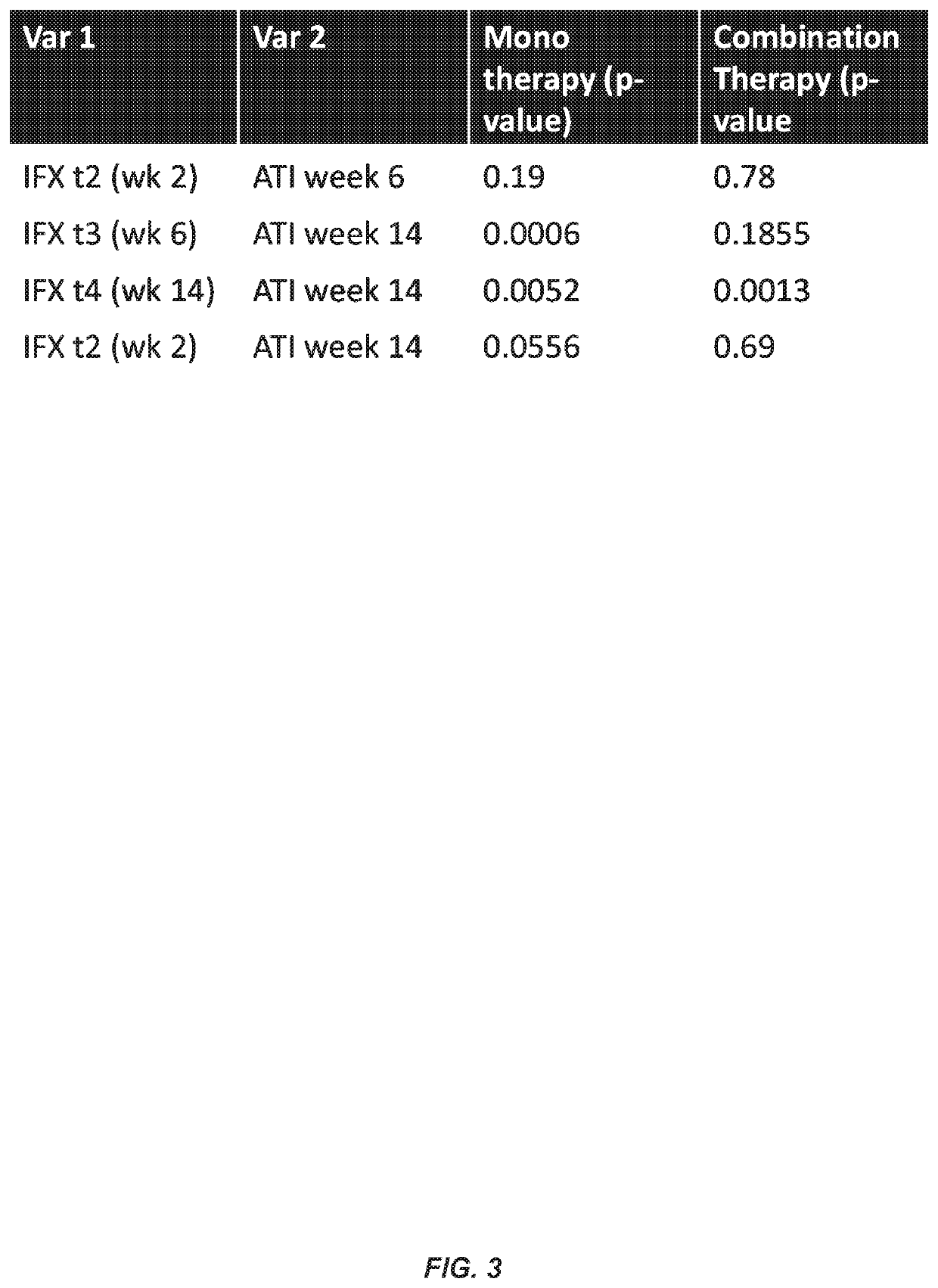
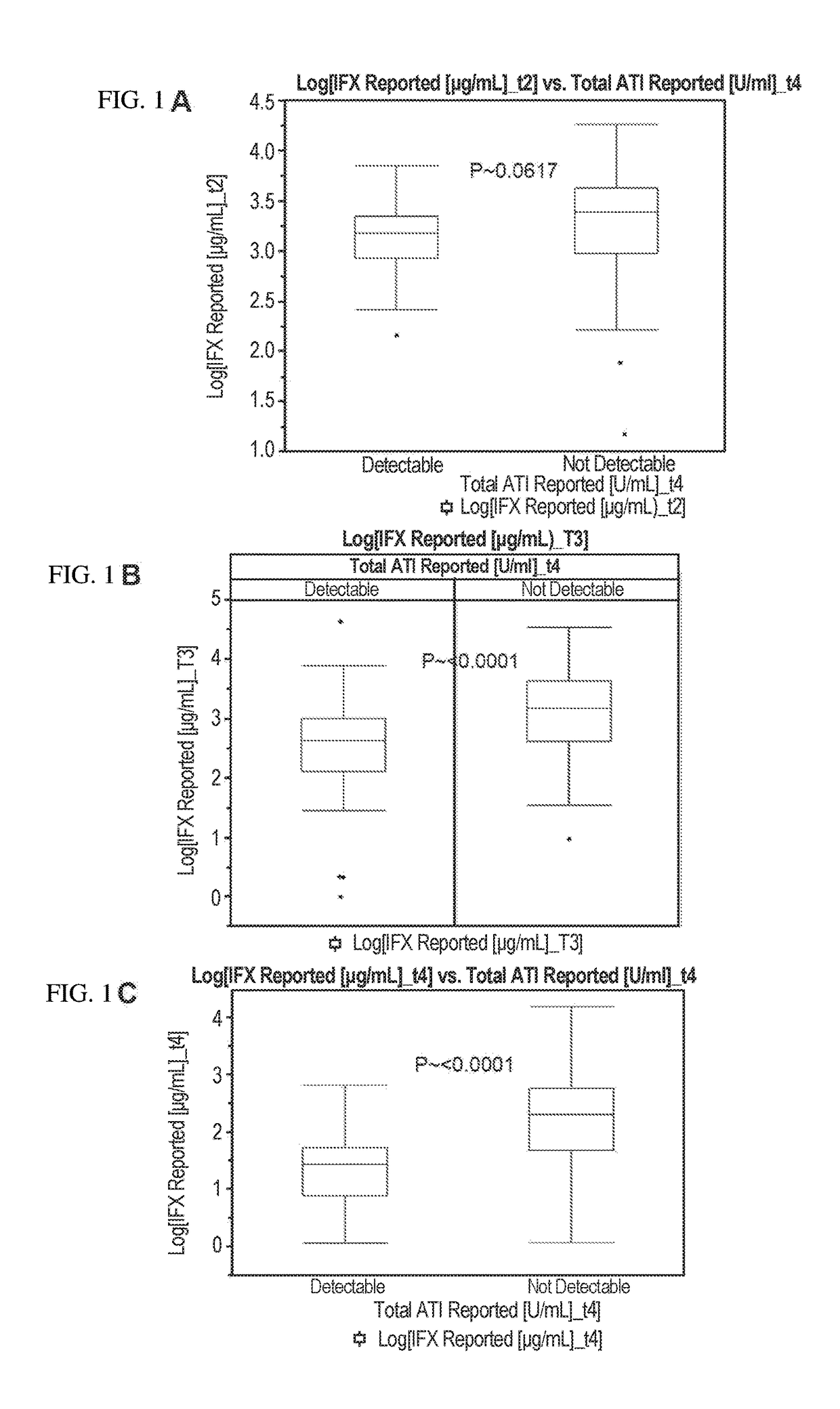
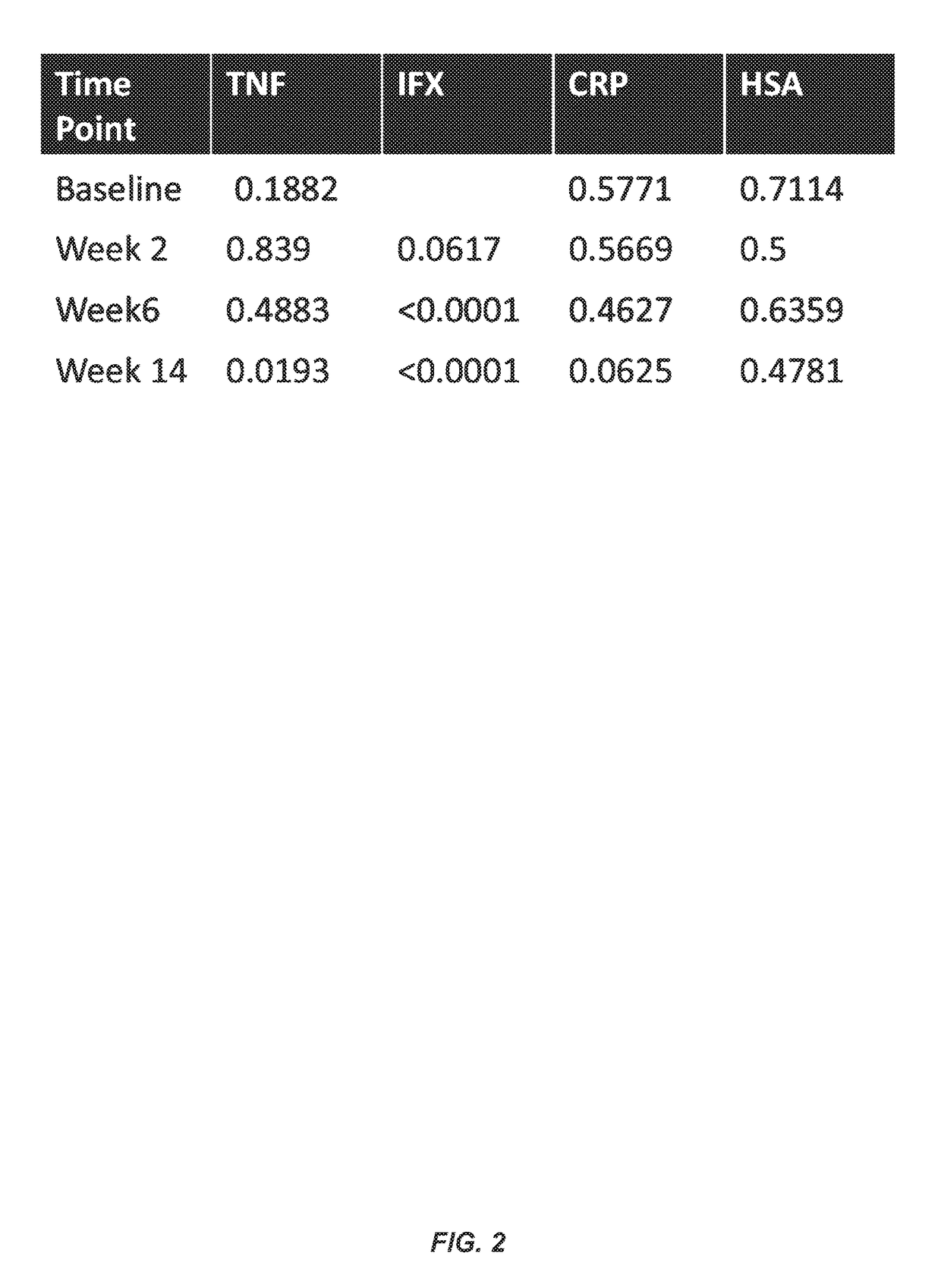
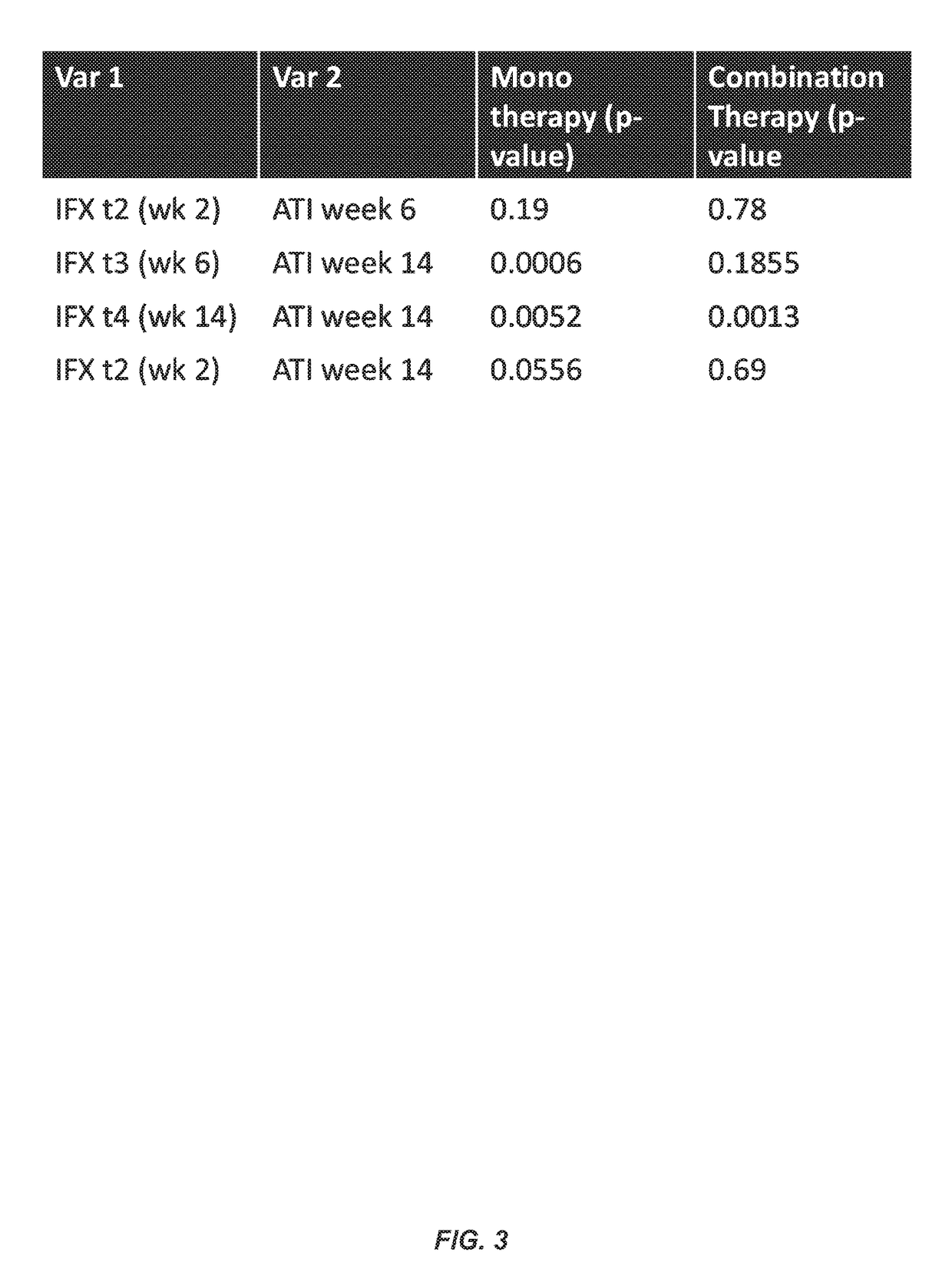
Methods for prediction of anti-TNFα drug levels and autoantibody formation
In some aspects, the present invention provides methods for predicting whether a subject will develop autoantibodies to an anti-TNFα drug during the course of anti-TNFα drug therapy. In other aspects, the present invention provides methods for predicting the level of an anti-TNFα drug in a subject during the course of anti-TNFα drug therapy. Systems for predicting anti-TNFα drug levels and the likelihood of autoantibody formation during the course of anti-TNFα drug therapy are also provided herein. The present invention further provides methods for predicting a clinical outcome (e.g., endoscopic response) of a subject on anti-TNFα drug therapy.
Owner:PROMETHEUS LAB
Apparatuses and methods for titrating drug delivery
The present invention describes methods and devices for reducing the workload of instilling medication and allowing the clinician-user to control the associated procedure. The drug delivery device 122 is controlled to achieve a target drug concentration or a predetermined infusion rate waveform at a selected site of the patient. The time profile of the target drug concentration or predetermined infusion rate waveform is controlled by a drug state model (38) using clinical heuristics to achieve safe, predetermined changes in target drug concentration or infusion rate and target drug concentration or infusion rate. User-controlled change of note speed. The present invention allows time to assess a patient's response to changes in drug levels by making small increments and steady changes in drug levels over time.
Owner:SCOTT LAB
Zaocys dhumnades oil product and preparation method thereof
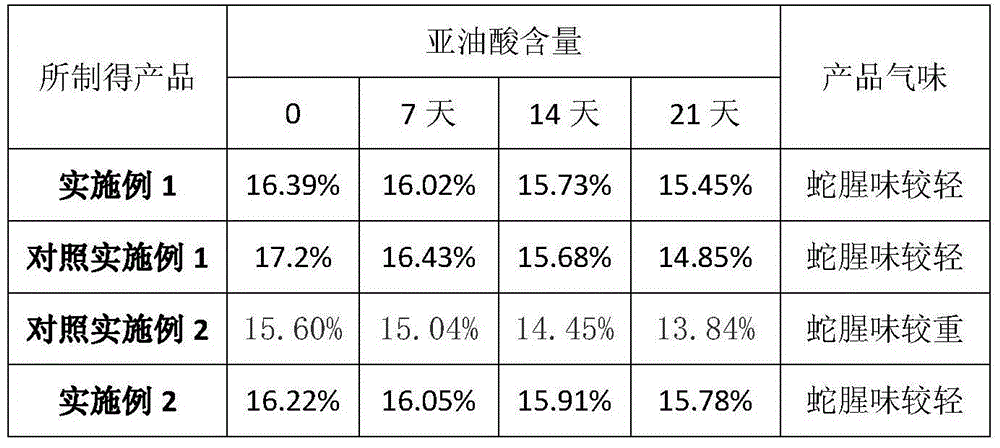
InactiveCN104689296AAbundant raw materialsImprove stabilityPeptide/protein ingredientsUnknown materialsNeutral proteaseTrypsin
The invention relates to a product which is primarily based on zaocys dhumnades oil and a preparation method thereof. The preparation method comprises the following steps: by taking drug level zaocys dhumnades as a raw material, crushing the raw materials, adding acetone, extracting under nitrogen protection and deodorizing and decoloring through active carbon to obtain refined zaocys dhumnades oil; and adding neutral protease and trypsin into acetone extraction residues for enzymolysis, centrifugalizing after high-temperature treatment, adding ether into a supernate to be precipitated, wherein the precipitate is a small peptide dried object, and mixing with the refined zaocys dhumnades oil to obtain the zaocys dhumnades oil product. The method can effectively prevent damage of linoleic acid and is high in oil yield, shallow in luster, high in transparency, free of precipitate and suspended solids and relatively small in viscosity. The content of linoleic acid can be over 16% and the expiration date of the product is long.
Owner:向华
Huganning tablets for treating chronic and urgent liver disease and preparation method thereof
InactiveCN101385783AGuaranteed uptimeRestore normal detoxification functionDigestive systemAntiviralsSelf-healingLactate dehydrogenase
The invention relates to a liver protecting and calming pill which has the efficacy of clearing heat and promoting diuresis, benefiting liver and removing blood stasis, soothing liver and relieving pain, removing jaundice and lowering glutamic-pyruvic transaminase, and is suitable for treating acute hepatitis and chromic hepatitis and the active components of which consists of the medicinal materials of stringy stonecrop, giant knotweed, red sage root and ganoderma lucidum, and a preparation method thereof. The liver protecting and calming pill prompts an organism to play the normal immunologic role, start a liver self-healing system, block autoimmune reaction and strength physique and anti-virus ability; recovers the normal detoxification function of the liver to cause normal operation of human body; resists liver damage to effectively inhibit the increase of serum glutamic-pyruvic transaminase (SGPT), serum glutamic-oxaloacetic transaminase (SGOT) and lactate dehydrogenase (LDH); and increases hemoglobin to obviously increase the Hb content of blood plasma. The liver protecting and calming pill keeps stable blood-drug level and fully play the drug effect according to the theory of long-term administration for liver disease and the 'sluggish characteristic of pills' .
Owner:李浪辉
Transdermal drug administering system
InactiveCN100386073CLow costReduce wasteMedical devicesPharmaceutical non-active ingredientsVerapamil HydrochlorideWater soluble drug
The invention discloses a percutaneous give drug system, which comprises: a drug storeroom, the gel drug layer comprises drug, solvent, transdermal accelerating agent and gelatinizing agent; one adsorption layer comprised solvent absorption material of solvent in absorbable drug storeroom; one isolating layer between drug storeroom and adsorption layer, which is of non-woven fabrics or semipermeable membrane to make solvent of gas or liquid pass through successfully; adsorption layer can joint isolating layer of drug storeroom in opposite directions of closing up to skin of drug storeroom. The percutaneous give drug system in this invention can increase drug level in drug storeroom of the system to elevate drug transdermal speed, as well as increase controlled release effect of percutaneous give drug and absolute bioavailability, enhance drug curative effect, decrease drug waste, and depress preparation cost of the system. The invention is fit particular to percutaneous give drug system for water-soluble drug of propranolol hydrochloride, verapamil hydrochloride and chlorpromazine hydrochloride.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Compound preparation for treating cold and preparation method thereof
InactiveCN101683527AMaximize the effect of treatmentFast onsetOrganic active ingredientsAntipyreticTreatment effectAdditive ingredient
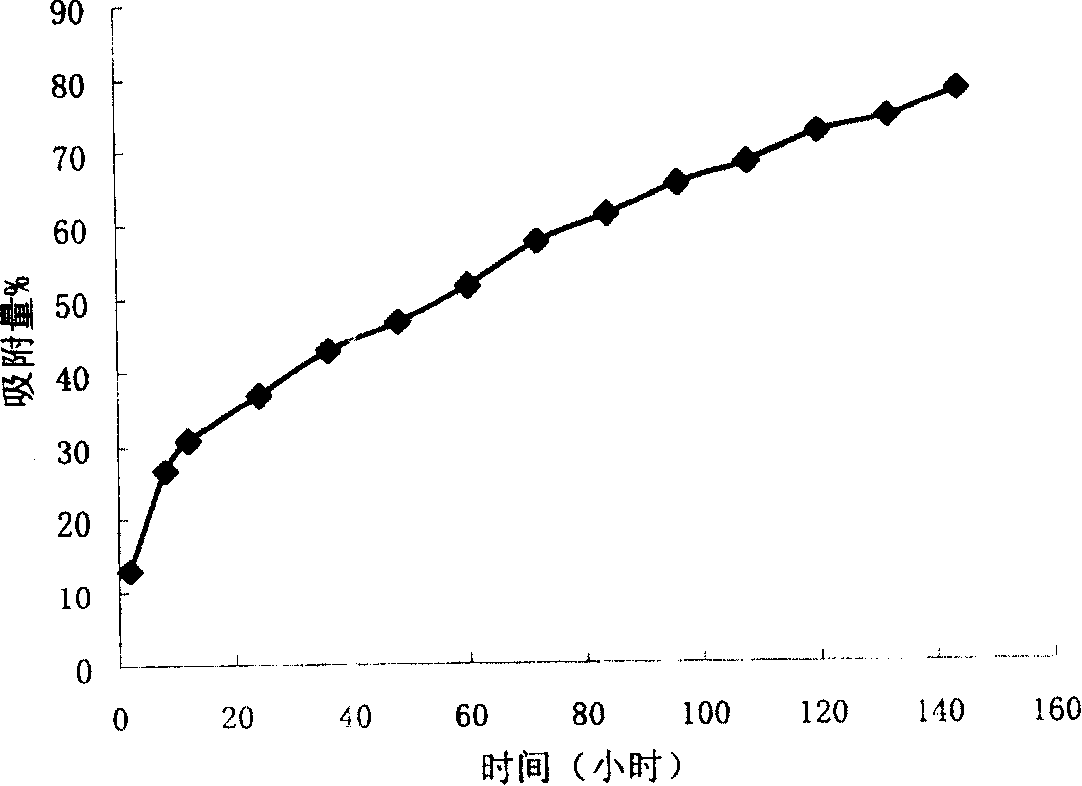
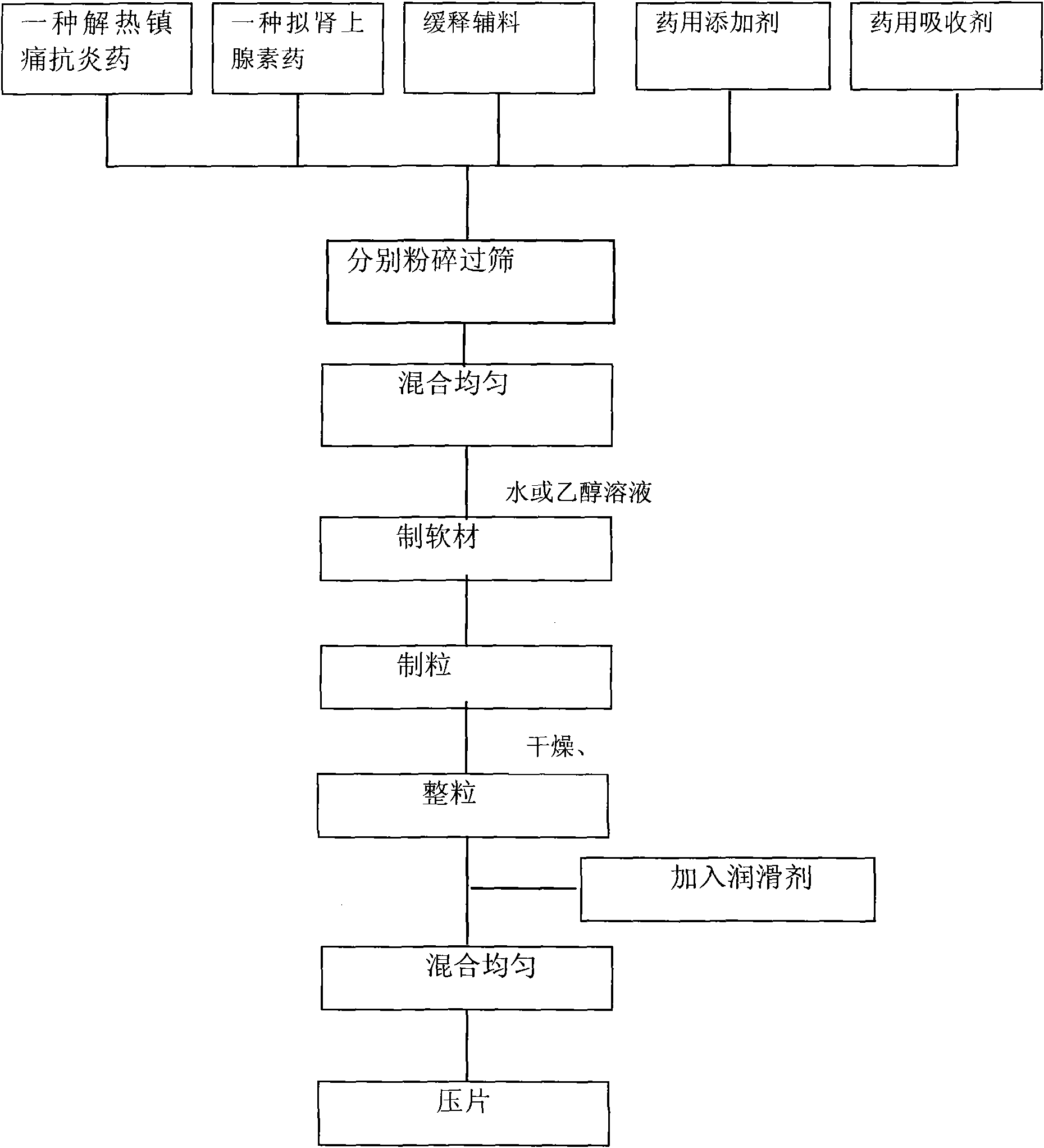
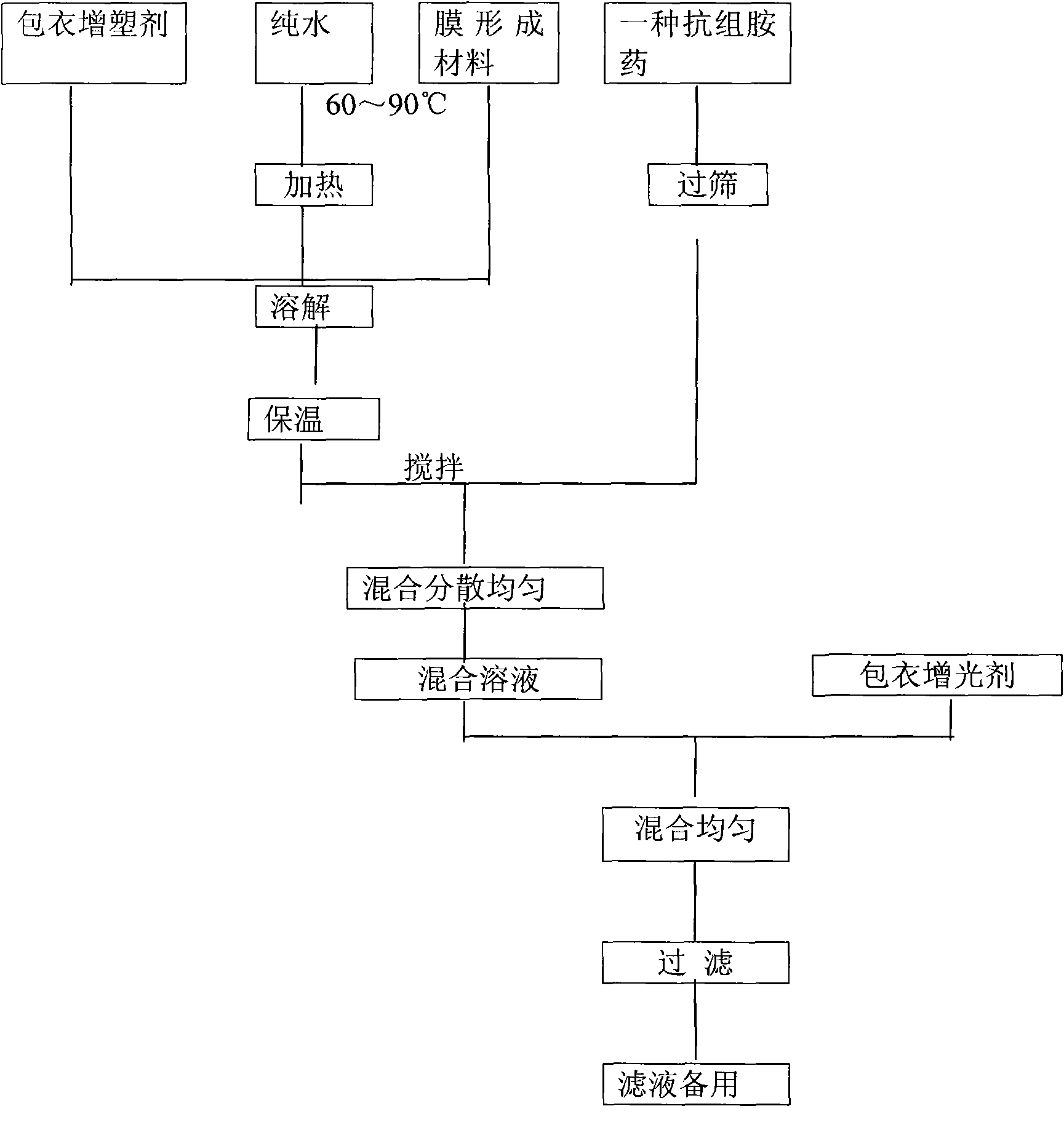
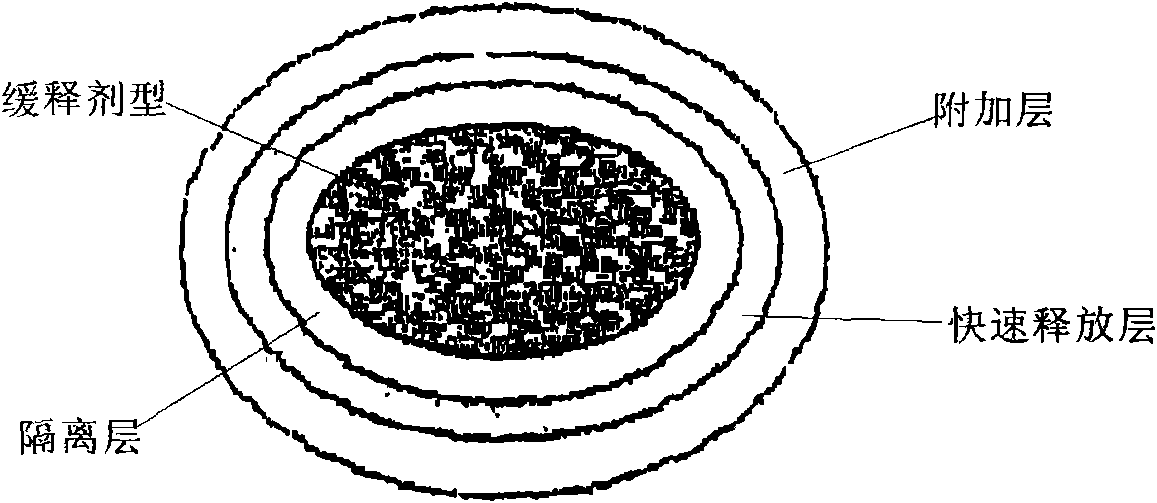
The invention relates to a compound preparation for treating cold and a preparation method thereof, belonging to the technical field of medicines. The compound preparation contains a sustained-releasedosage form prepared by respectively selecting an ingredient from an adrenomimetic drug and an antipyretic-analgesic and anti-inflammatory drug, and a fast-release membranous layer formed by any ingredient of an antihistamine drug, wherein the membranous layer is coated on the sustained-release dosage form, the antihistamine drug has fast effect and long time of drug effect, better treating effect can be achieved by fast release of the fast-release membranous layer, and simultaneously, the constant drug level of the adrenomimetic drug and the antipyretic-analgesic and anti-inflammatory drug is maintained in blood by sustained-release releasing. Therefore, the compound preparation can overcome the symptoms related to the cold, including the symptoms of nasal obstruction, sneeze, nasal bleeding, sputum itching, lacrimation and the like with pain and fever.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Compositions for the treatment of tumour
InactiveCN101203226ASedation preventionAntineoplastic agentsHeterocyclic compound active ingredientsActive agentNeoplasm
The invention features a method for treating a patient having a cancer or other neoplasm by administering to the patient a composition that includes a phenothiazine and another active agent, where predetermined plasma drug levels are achieved and maintained for 12 hours or more.
Owner:COMBINATORX
Methods for ophthalmic delivery of roflumilast
PendingUS20220249451A1Elevated level of drugImprove the level ofInorganic non-active ingredientsSolution deliveryConjunctivaAqueous humor
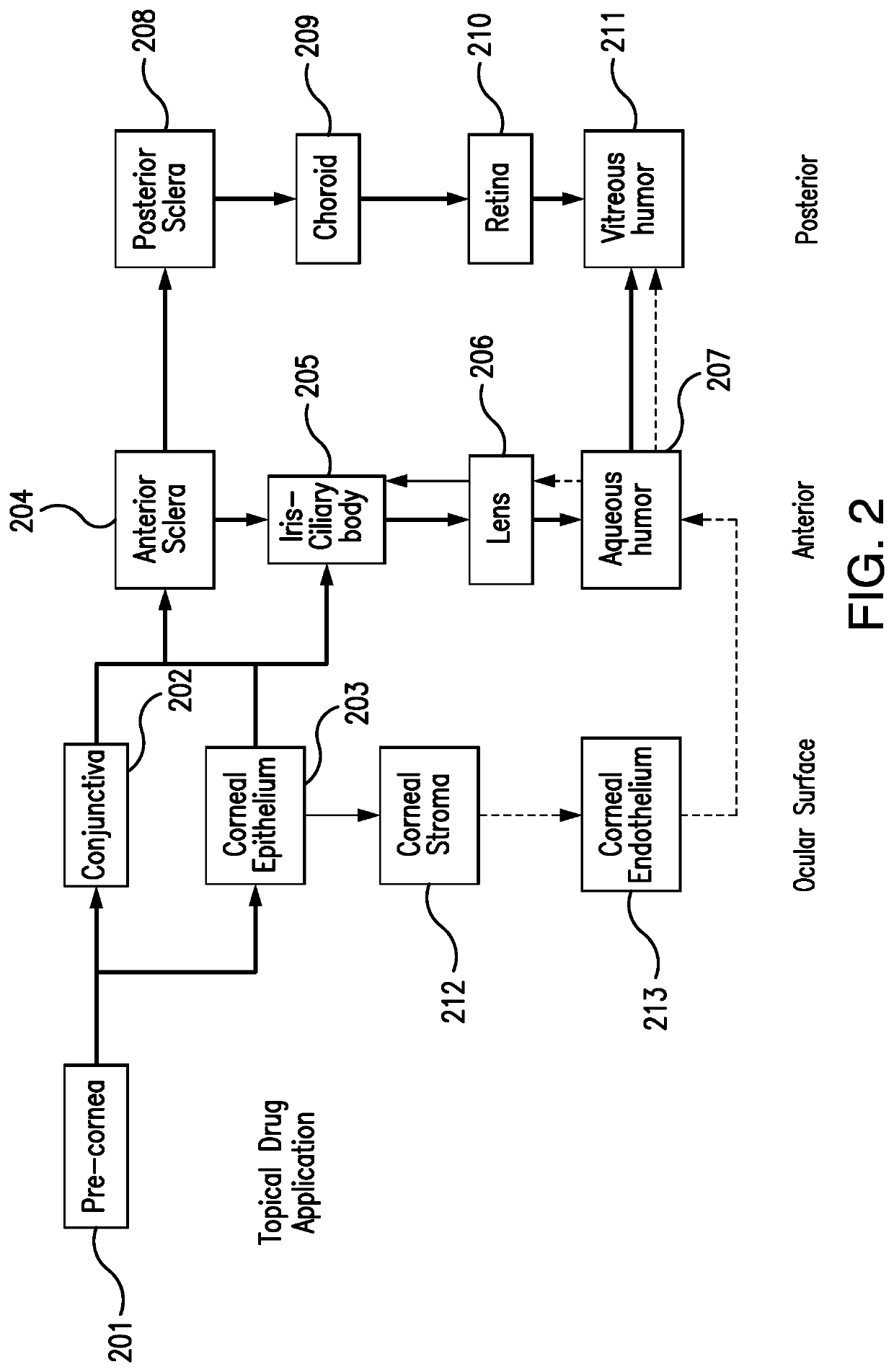
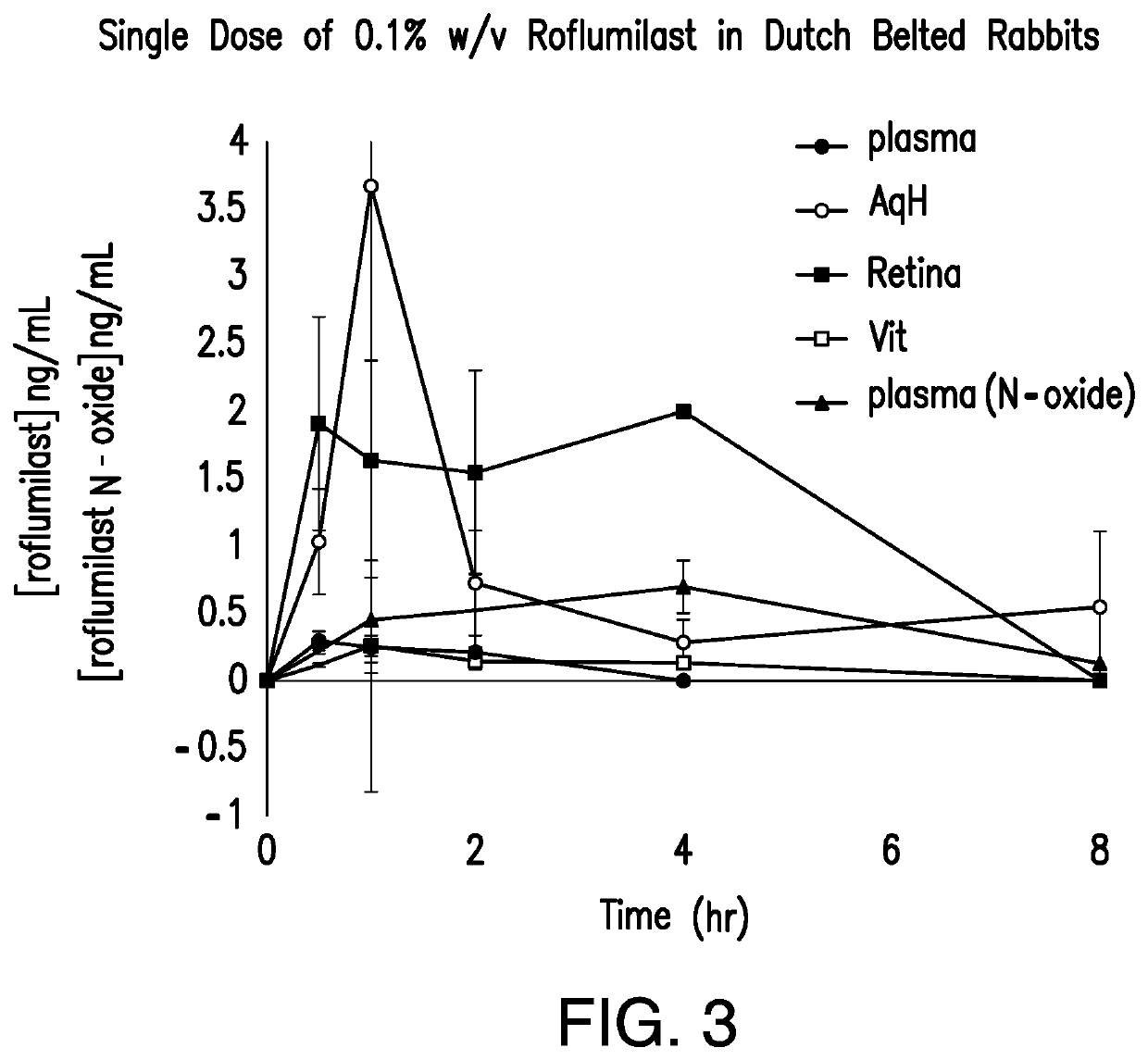
Methods for the ophthalmic delivery of roflumilast. The inventors have made the surprising discovery that the administration of pharmaceutical compositions comprising roflumilast to the cornea preferentially delivers the drug laterally through the ocular surface and anterior tissues of the eye. In contrast to the delivery of roflumilast to the skin, which primarily travels transversely through the various tissues of the skin, or many other topical ocular pharmaceutical compositions which travel through the cornea to the aqueous humor, the delivery of drug to the eye to the cornea travels laterally to the ocular surface and anterior tissues of the eye. Such methods can result in elevated levels of the drug in the cornea and other ocular surface and anterior tissues of the eye (e.g., iris-ciliary body, sclera, conjunctiva, and aqueous humor) relative to the interior or posterior tissues of the eye.
Owner:IOLYX THERAPEUTICS INC
Methods for prediction of Anti-tnf alpha drug levels and autoantibody formation
In some aspects, the present invention provides methods for predicting whether a subject will develop autoantibodies to an anti-TNFα drug during the course of anti-TNFα drug therapy. In other aspects, the present invention provides methods for predicting the level of an anti-TNFα drug in a subject during the course of anti-TNFα drug therapy. Systems for predicting anti-TNFα drug levels and the likelihood of autoantibody formation during the course of anti-TNFα drug therapy are also provided herein. The present invention further provides methods for predicting a clinical outcome (e.g., endoscopic response) of a subject on anti-TNFα drug therapy.
Owner:PROMETHEUS LAB
Anti-cancer medicine composition containing antineoplastic antibiotics
An anticancer pharmaceutical composition composed of pharmaceutic adjuvant and enwrapped in anti-tumor drug is provided. Wherein the anti-tumor drug is tumor resistant antibiotic, the pharmaceutic adjuvant is mainly biological compatible, degradable and absorbable macromolecule polymer. The anticancer drug can be sustained released to local tumor region in the degradation and absorption process, as a result, it can both decrease whole body toxic reaction considerably and maintain tumor local effective drug level.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
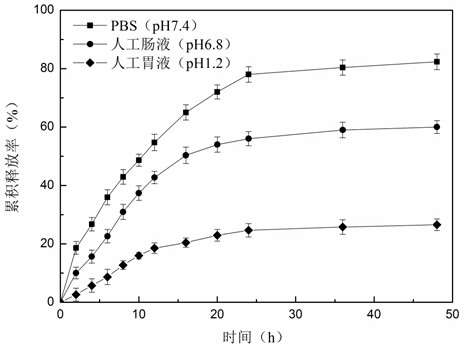
A kind of preparation method of oleanolic acid sustained-release nano-microcapsules
ActiveCN110327311BSimple designGood choiceAntibacterial agentsOrganic active ingredientsCholic acidGastric fluid
The invention provides a preparation method of oleanolic acid slow-release nano-microcapsules. The aim was to design and optimize oleanolic acid (OA) nanoparticles of poorly water-soluble drugs to improve their oral bioavailability and prolong the duration of therapeutic drug levels. The nanoparticle wall material is an amphiphilic polymer formed by hydrophilic chitosan oligosaccharide and hydrophobic deoxycholic acid. The particle size of nanoparticles of different wall materials is between 200-400nm, and the distribution is relatively uniform. In vitro release experiments were carried out in a simulated gastrointestinal tract environment, and the results showed that oleanolic acid nanocapsules released slowly in simulated gastric juice, and gradually released in intestinal juice; in the initial stage of release in PBS solution, it showed a burst release, and then released slowly; The sustained-release effect of oleanolic acid nano-microcapsules in each solution is remarkable. The oleanolic acid slow-release nano-microcapsules of the invention improve the bioavailability of the oleanolic acid, have obvious sustained-release effect in vitro, and can be used as an effective oral preparation for future liver injury treatment.
Owner:HARBIN INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com