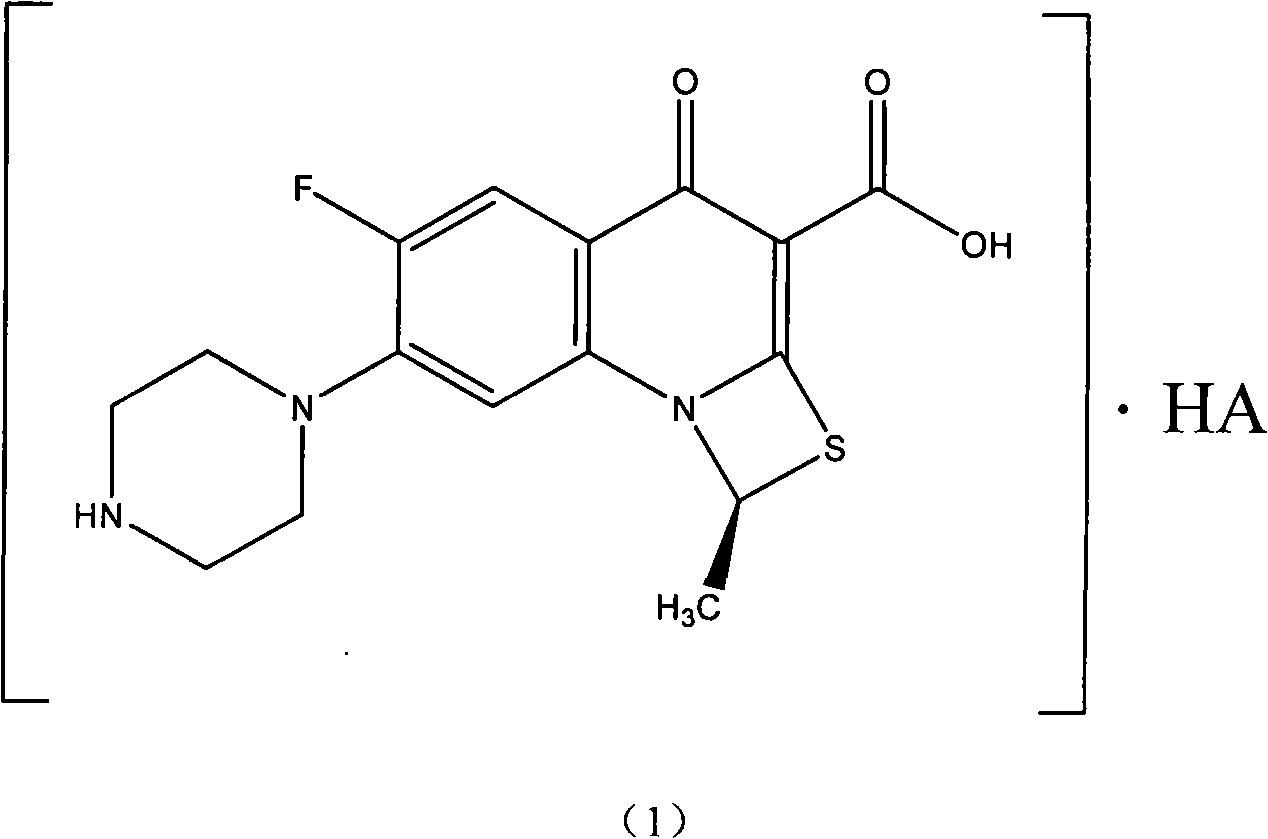
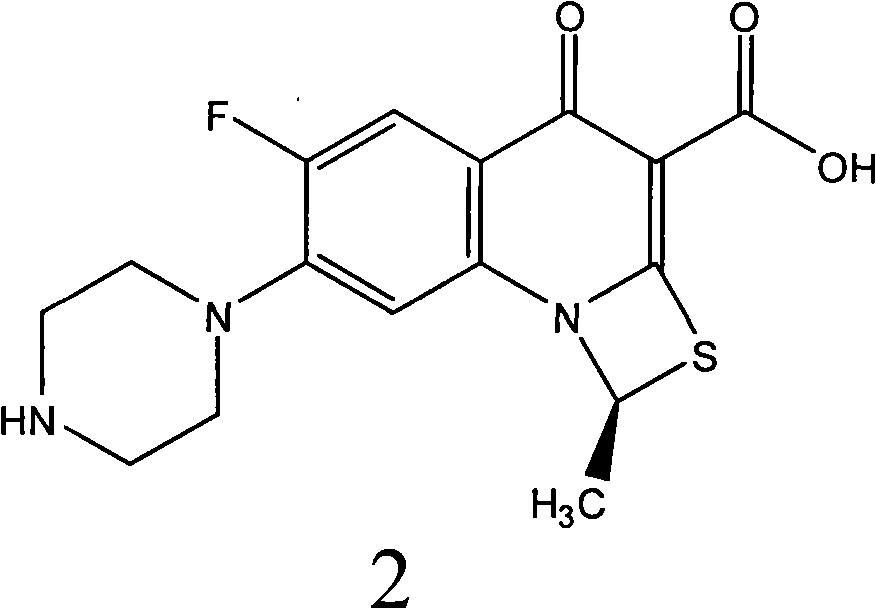
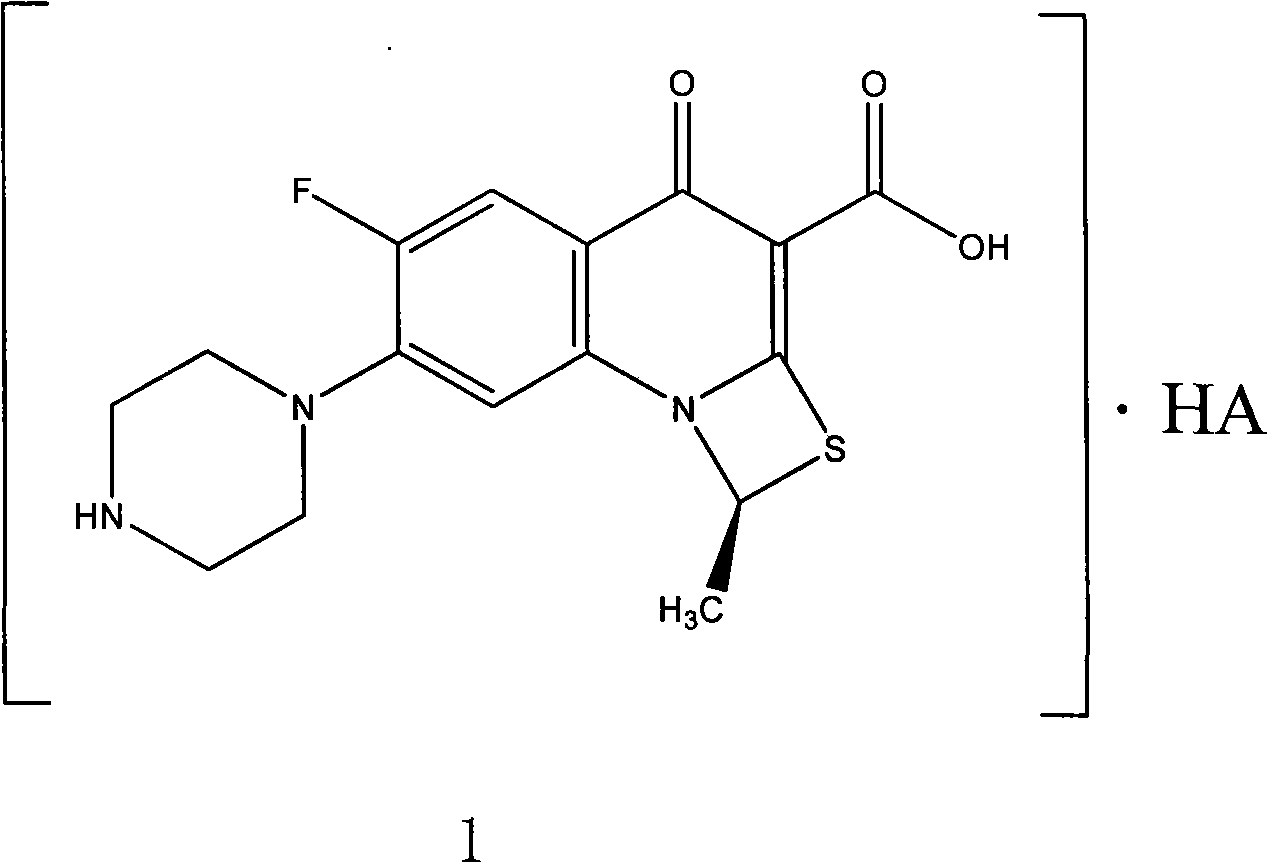
Fluorine-containing optically active composition for anti-infection
A compound and anti-infection technology, applied in anti-infective drugs, organic active ingredients, organic chemistry, etc., can solve the problems of no clinical application significance, high toxicity, and large irritation of racemic ulifloxacin, and achieve no skin and muscle Stimulation, low side effects, strong biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1, (S)-6-fluoro-1-methyl-4-oxo-(1-piperazinyl)-1H,4H-[1,3]thiazetidino[3,2 -a] the preparation of quinoline-3-carboxylic acid (abbreviation compound 2)
[0029] Dissolve 105 g of racemic ulifloxacin in 1500 mL of dimethyl sulfoxide, add dropwise a solution of 27 g of D-tartaric acid dissolved in 405 mL of dimethyl sulfoxide under stirring, turbidity and precipitation appear, and stir at room temperature for 20 hours , filtered, and the resulting solid was dried in vacuo to obtain 86 g. This solid was recrystallized and purified in dimethyl sulfoxide to obtain (S)-6-fluoro-1-methyl-4-oxo-(1-piper Azinyl)-1H, 4H-[1,3]thiazetidino[3,2-a]quinoline-3-carboxylic acid-D-tartrate 37 grams, elemental analysis C49.08%, H5.06%, N9.50%, S7.44% (molecular composition: C 16 h 16 FN 3 o 3 S.1 / 2C 4 h 6 o 6 .H 2 O, calculated value C48.86%, H4.78, N9.50%, S7.25%); add this salt into water to form a suspension, adjust the pH value to 7-8 with 2% NaOH aqueous solution und...
Embodiment 2
[0030] Example 2, (R)-6-fluoro-1-methyl-4-oxo-(1-piperazinyl)-1H,4H-[1,3]thiazetidine[3,2 -a] Preparation of quinoline-3-carboxylic acid (compound 3 for short) Dissolve 105 grams of racemic ulifloxacin in 1500 mL of dimethyl sulfoxide, add 27 grams of L-tartaric acid dropwise under stirring and dissolve in 405 mL di The solution of methyl sulfoxide appeared turbid and precipitated, stirred at room temperature for 20 hours, filtered, and the obtained solid was dried under vacuum to obtain 82 grams, and the solid was recrystallized and purified in dimethyl sulfoxide to obtain (R)- 6-fluoro-1-methyl-4-oxo-(1-piperazinyl)-1H,4H-[1,3]thiazetidino[3,2-a]quinoline-3- Carboxylic acid-L-tartrate 34 grams, add this salt into water to form a suspension, adjust the pH value to 7-8 with 2% NaOH aqueous solution under stirring, precipitate, filter and dry to obtain (R)-6-fluoro-1-methyl Base-4-oxo-(1-piperazinyl)-1H, 4H-[1,3]thiazetidino[3,2-a]quinoline-3-carboxylic acid 22 grams, specific...
Embodiment 3
[0031] Example 3 Lactic acid (S)-6-fluoro-1-methyl-4-oxo-(1-piperazinyl)-1H,4H-[1,3]thiazetidino[3,2 -a] the preparation of quinoline-3-carboxylic acid (abbreviated as compound 4)
[0032] At room temperature 20°C, add 30 mL of water to the reaction flask, add 1.6 g of lactic acid while stirring, and then add 5 g of (S)-6-fluoro-1-methyl-4-oxo-(1-piperazinyl)- 1H, 4H-[1,3]thiazetidino[3,2-a]quinoline-3-carboxylic acid, after stirring for 60 minutes, a substantially clear solution was obtained, which was decolorized by adding 5% activated carbon for 30 minutes and then filtered , the filtrate was added dropwise with 200 ml of absolute ethanol under stirring, and a solid precipitated at this time, continued to stir for 2 hours, filtered, the filter cake was crushed, and dried in vacuo at 50°C to obtain 3.6 grams of lactic acid (S)-6-fluoro-1-formazan Base-4-oxo-(1-piperazinyl)-1H,4H-[1,3]thiazetidino[3,2-a]quinoline-3-carboxylic acid, assay: [α ] D 20 =-116.5 (c=1.0, water);...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com