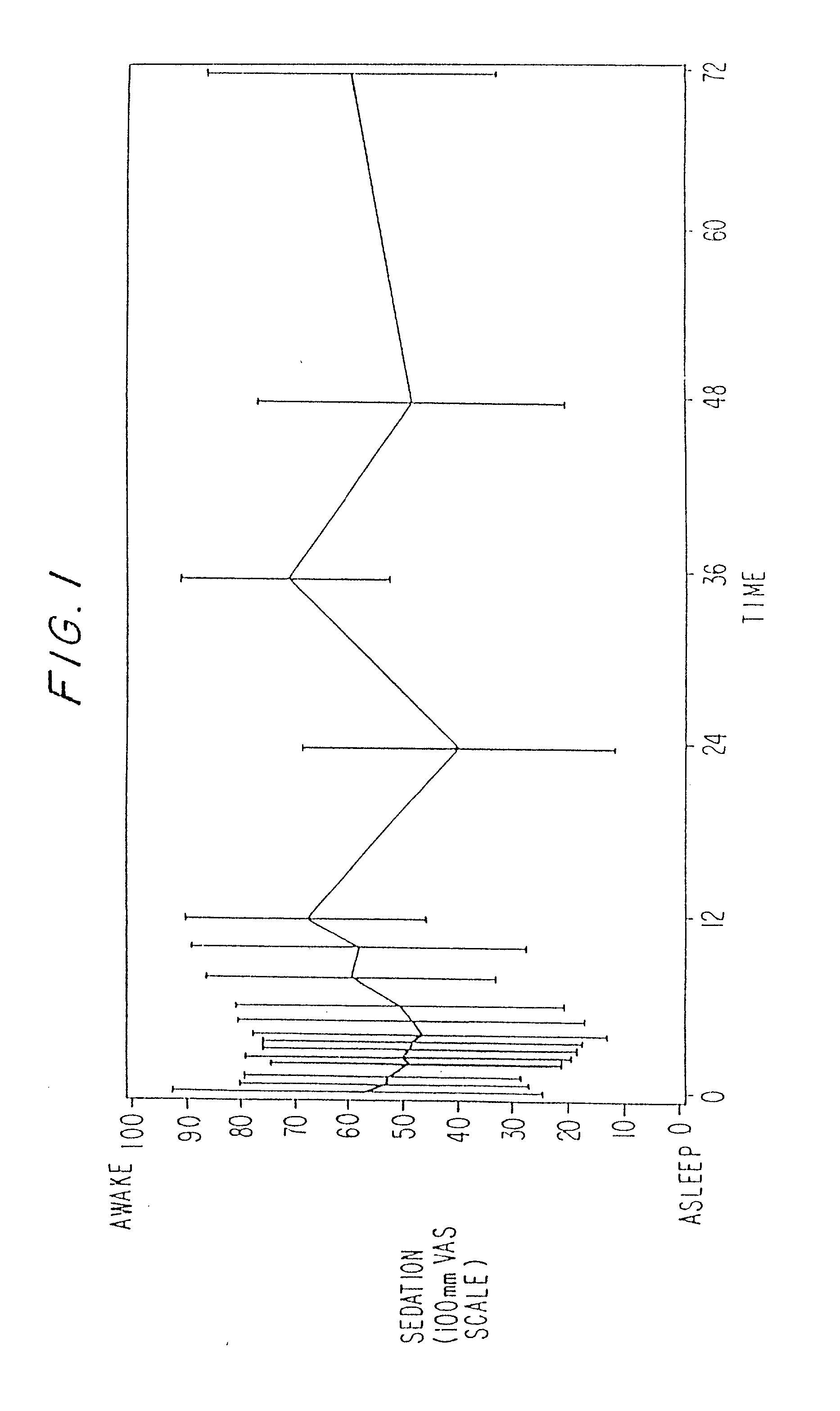
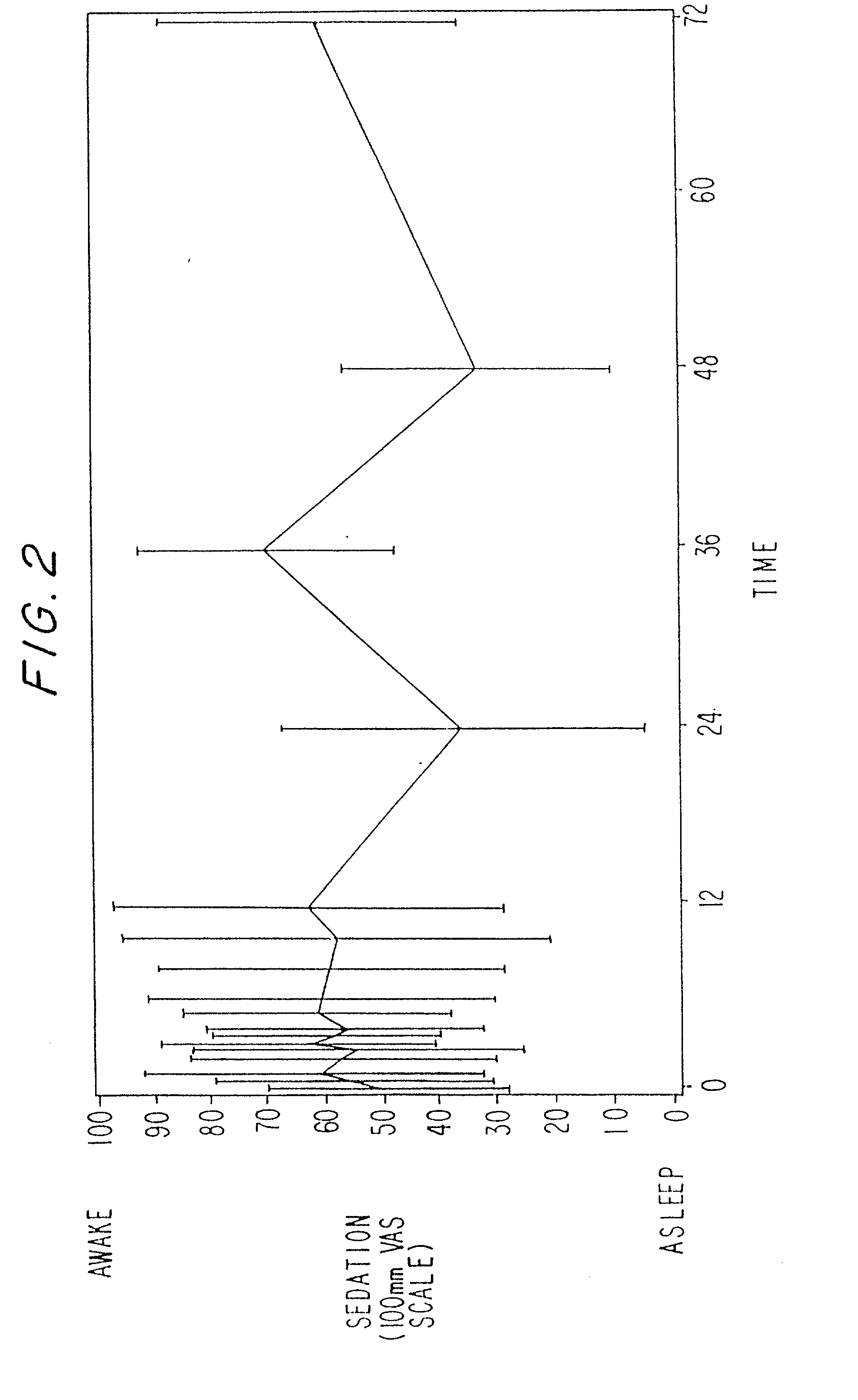
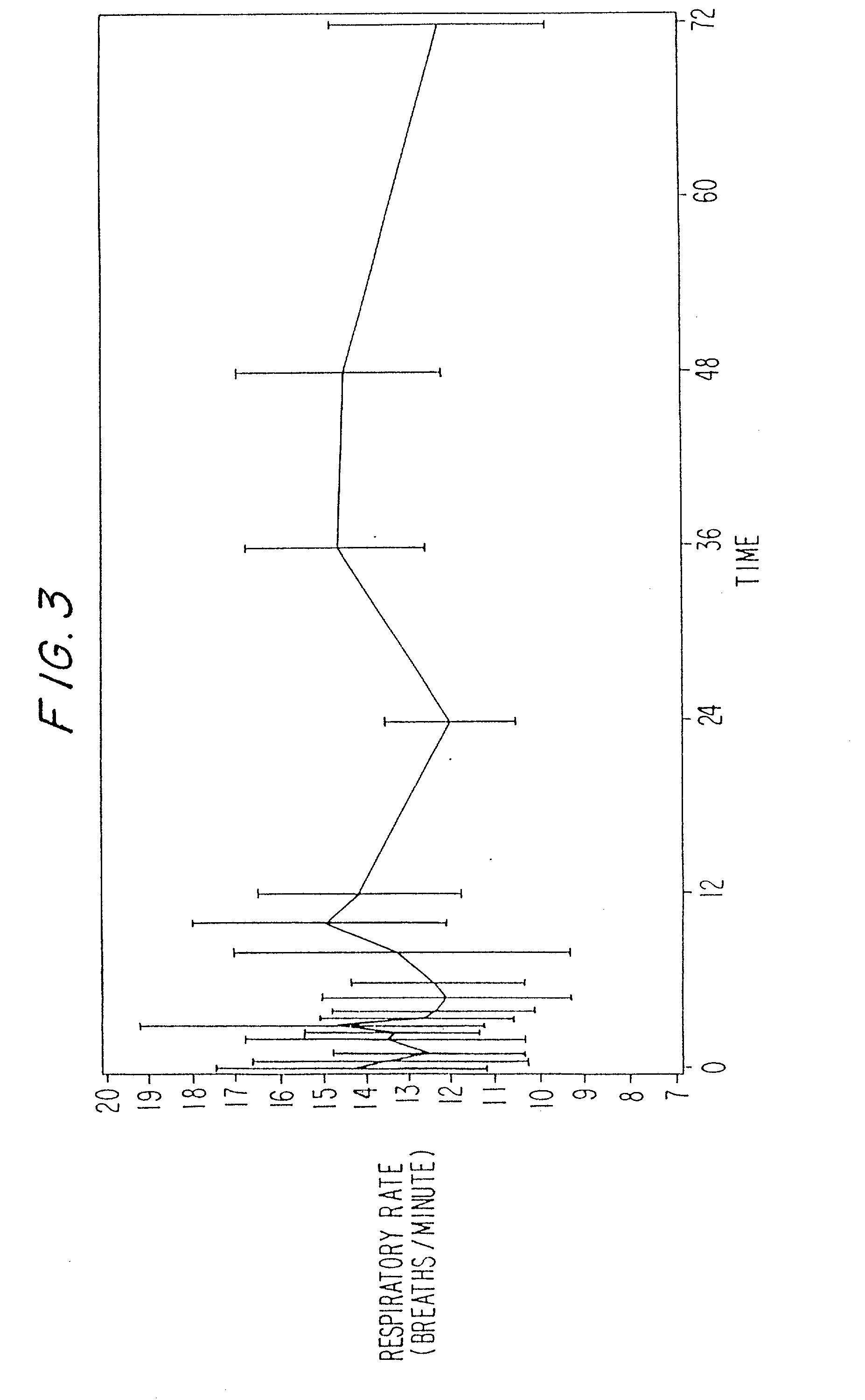
Treating pain by administering 24 hours opioid formulations exhibiting rapid rise of drug level
a technology of opioids and formulations, applied in the direction of drug compositions, oil/fat/waxes non-active ingredients, microcapsules, etc., can solve the problems of special problems in maintaining analgesic efficacy, insure bioavailability of gastrointestinal fluids, and inability to withstand 24 hours, and achieve rapid rise and greater analgesic efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-2
[0102] In Example 1, morphine sulfate sustained-release beads with a 5% w / w sustained release coating comprising Eudragit.RTM. RS were prepared, including a 10% immediate release morphine sulfate overcoat. In Example 2, morphine sulfate sustained-release beads with an 8% w / w sustained-release coating comprising Eudragit.RTM. RS were prepared, including a 10% immediate release morphine sulfate overcoat.
[0103] Morphine sulfate beads were first manufactured using a rotor processing technique. The formula of the morphine sulfate beads to which the sustained-release coating was applied is set forth in Table 1 below:
1 TABLE 1 Amt / Unit Ingredient (mg) Percent (%) Morphine Sulfate Powder 30 mg 14.3% Lactose Hydrous Impalpable 42.5 mg 20.2% PVP 2.5 mg 1.2% Sugar Beads 18 / 20 125 mg 59.4% Purified Water qs mg -- Opadry Red YS-1-1841 10.5 mg 4.9% Total 210.5 mg 100.0%
[0104] A sustained-release coating was then applied to the morphine sulfate beads. The formula for the sustained release coating ...
example 3
[0167] Beads with a higher loading of morphine sulfate were produced with the use of the powder layering technique in the Glatt Rotor Processor. The formulation of the high load beads is set forth in Table 8 below:
8 TABLE 8 High Load Bead Percent Ingredient mg / unit (%) Morphine Sulfate Powder 30.0 mg 63.3% Lactose 6.0 mg 12.7% Povidone C-30 1.25 mg 2.6% Sugar Beads 7.75 mg 16.4% Opadry 2.37 mg 5.0% Purified Water qs -- Total 47.37 mg 100.0%
[0168] The sustained-release coating comprised an acrylic polymer (i.e., Eudragit.RTM. RL). A HPMC protective coat was also included between the Eudragit layer and the morphine immediate release layer to further enhance stability. The formula of the sustained release coating of Example 1 is set forth in Table 9 below:
9 TABLE 9 Amt / Unit Percent Ingredient (mg) (%) Morphine (high load) base beads 42.63 mg 78.8% Retardant Coating Eudragit RS 30D 2.1 mg 3.9% Eudragit RL 30D 0.05 mg 0.1% Triethyl Citrate 0.45 mg 0.8% Talc 0.85 mg 1.6% Overcoatings Opad...
example 4
[0180] Beads with a higher loading of morphine sulfate were produced with the use of the powder layering technique in the Glatt Rotor Processor. The formulation of the high load beads is set forth in Table 15 below.
15 TABLE 15 High Load Percent Ingredient Bead mg / unit (%) Morphine Sulfate Powder 60.0 mg 56.4% Lactose 12.0 mg 11.3% Eudragit RS30D 4.16 mg 3.9% Povidone C-30 8.31 mg 7.8% Sugar Beads 16.80 mg 15.8% Opadry 5.06 mg 4.8% Purified Water qs -- Total 106.33 mg 100%
[0181] These immediate release base beads were manufactured using the powder layering technique in the Glatt Rotor Processor.
[0182] The sustained release coating comprised an ethylcellulose acrylic polymer (i.e., Aquacoat ECD 30). A HPMC protective coat was also included after the Aquacoat layer to further enhance stability. The formula of the sustained-release coating of Example 1 is set forth in Table 16 below.
16 TABLE 16 Ingredient Amt / Unit (mg) Percent (%) Morphine (high load) 106.33 mg 73.1% based beads Retarda...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com