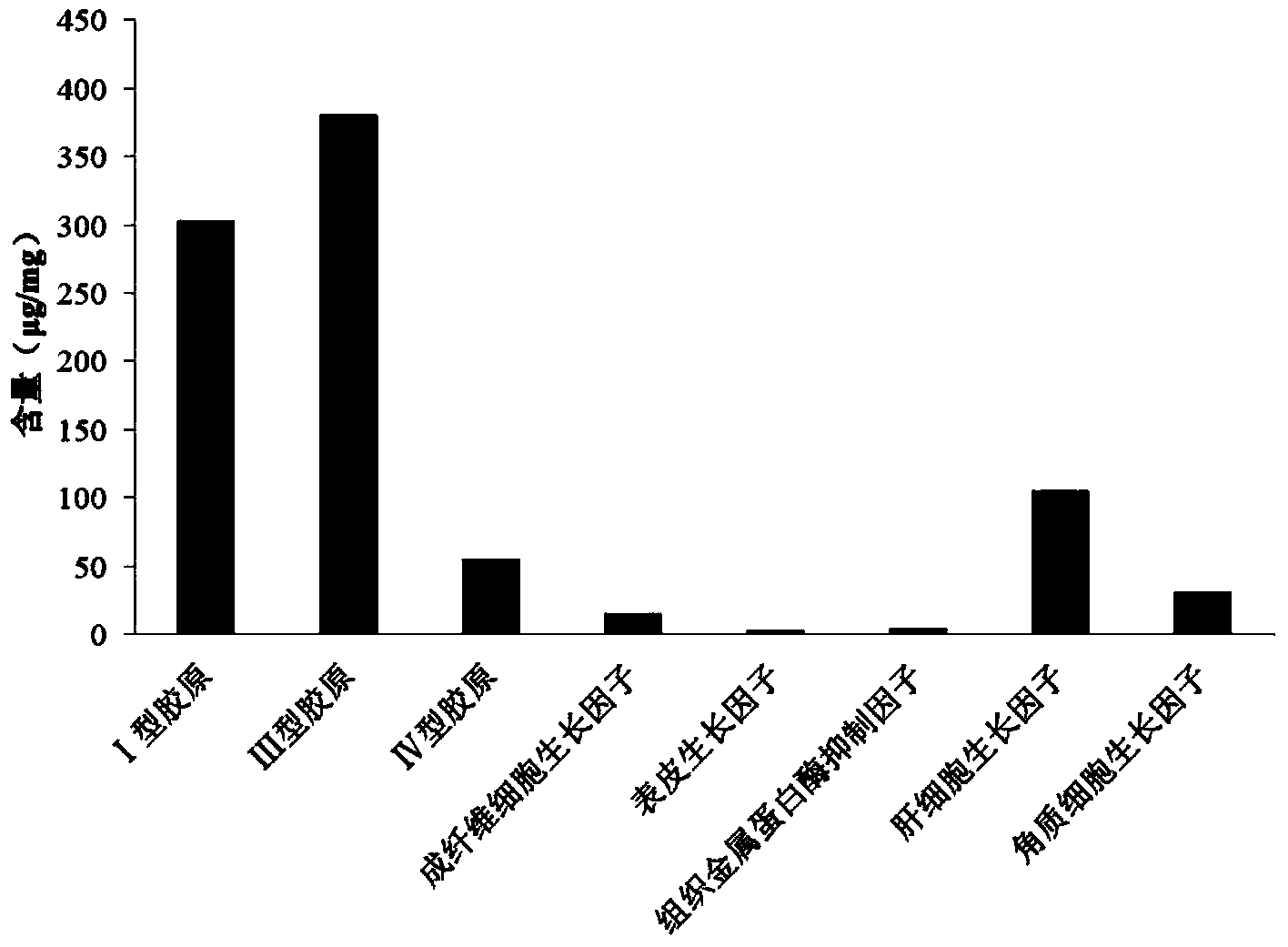Patents
Literature
83 results about "Moderate to severe" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
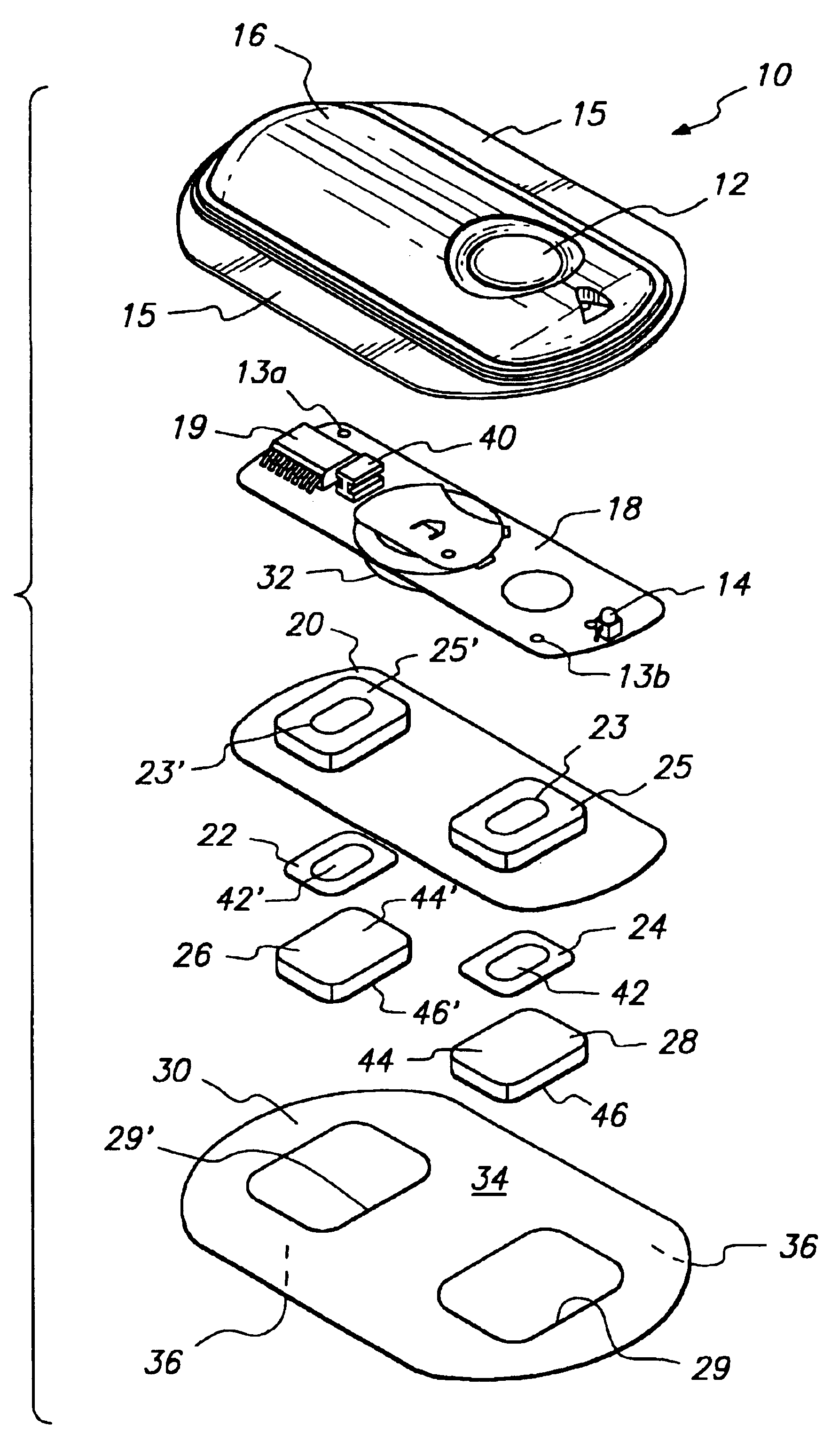
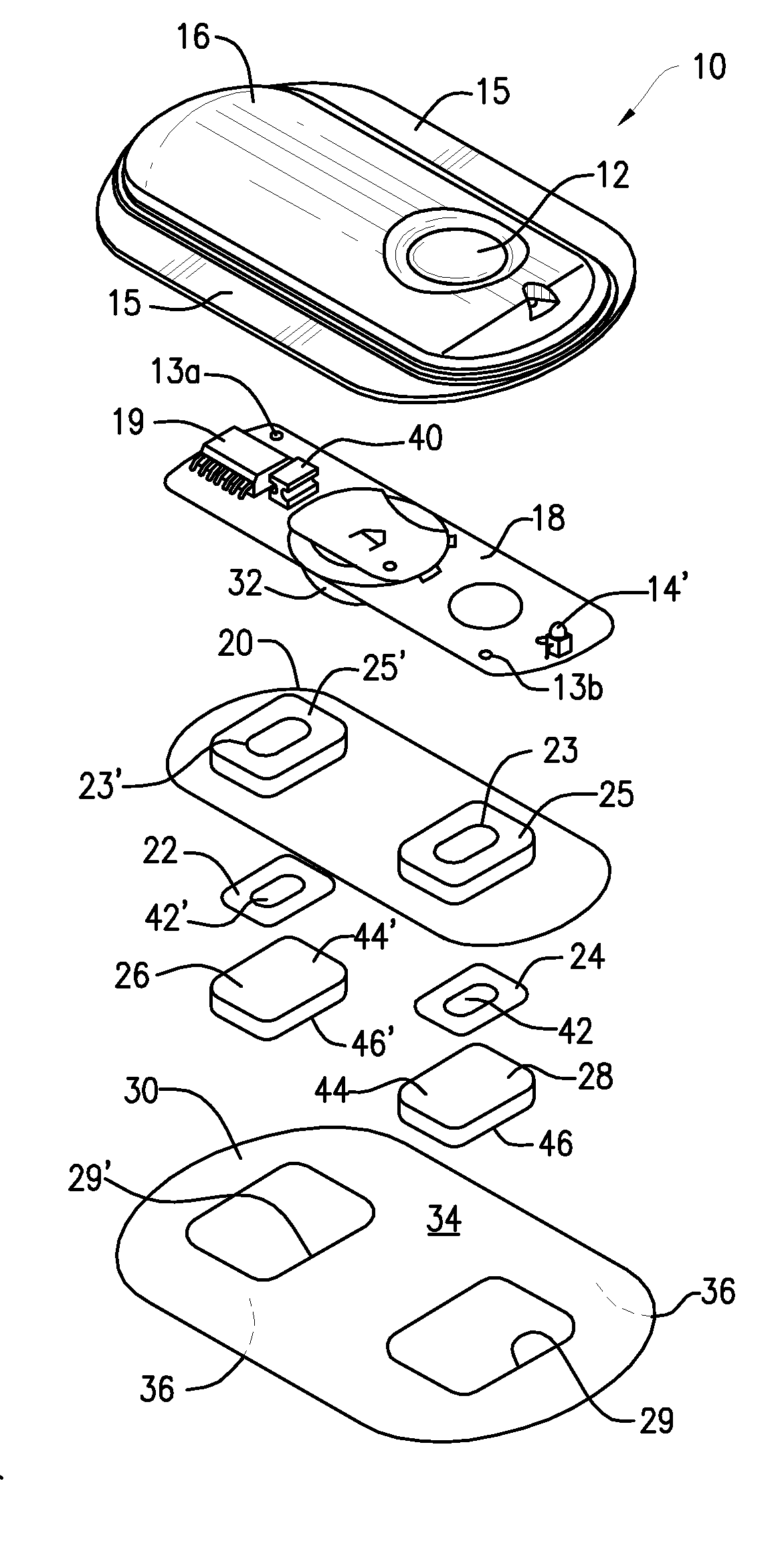
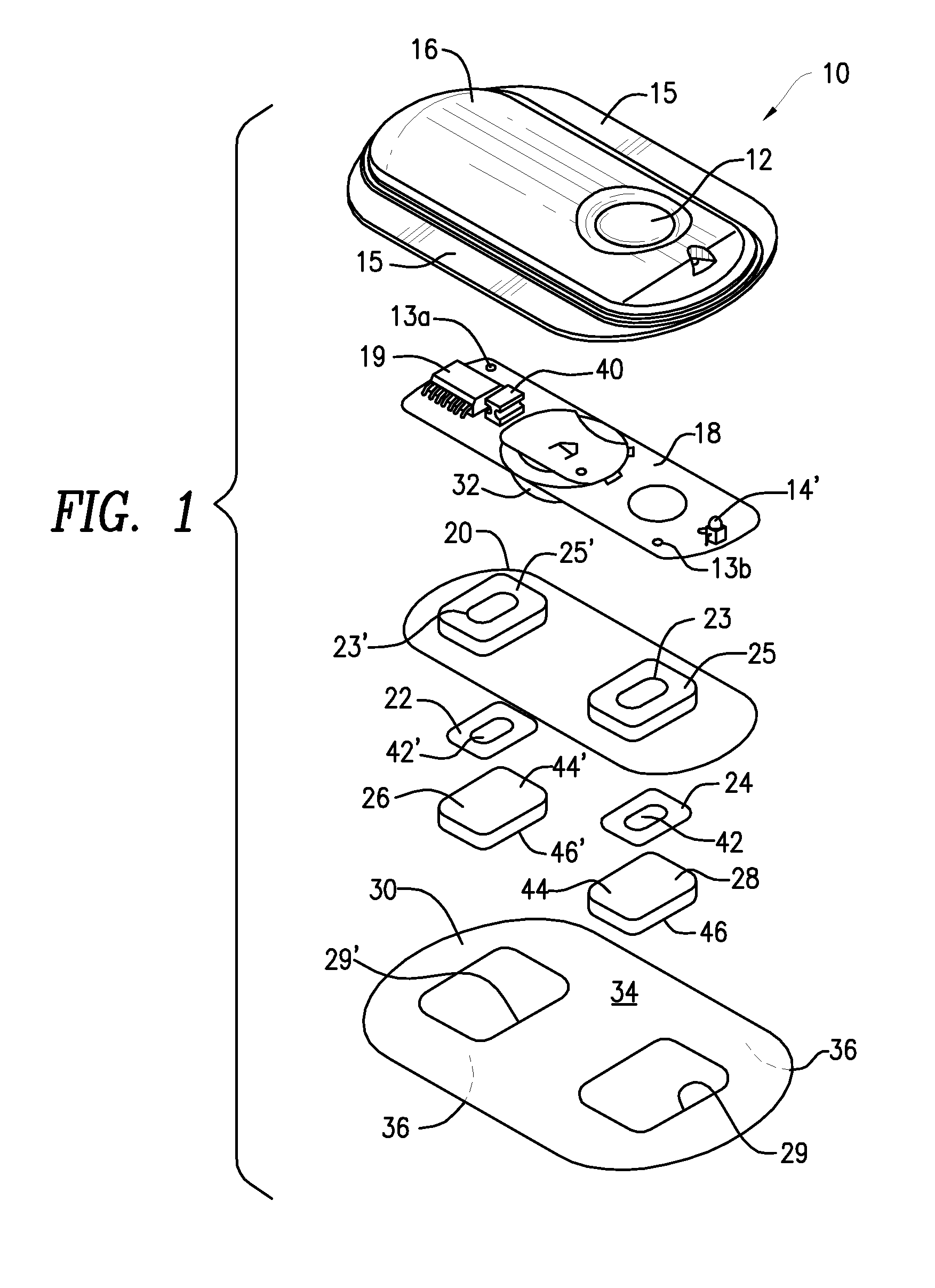
Device for transdermal electrotransport delivery of fentanyl and sufentanil
InactiveUS7018370B2Improved transdermal electrotransport deliveryImprove efficiencyOrganic active ingredientsElectrotherapyAnalgesics drugsHuman patient
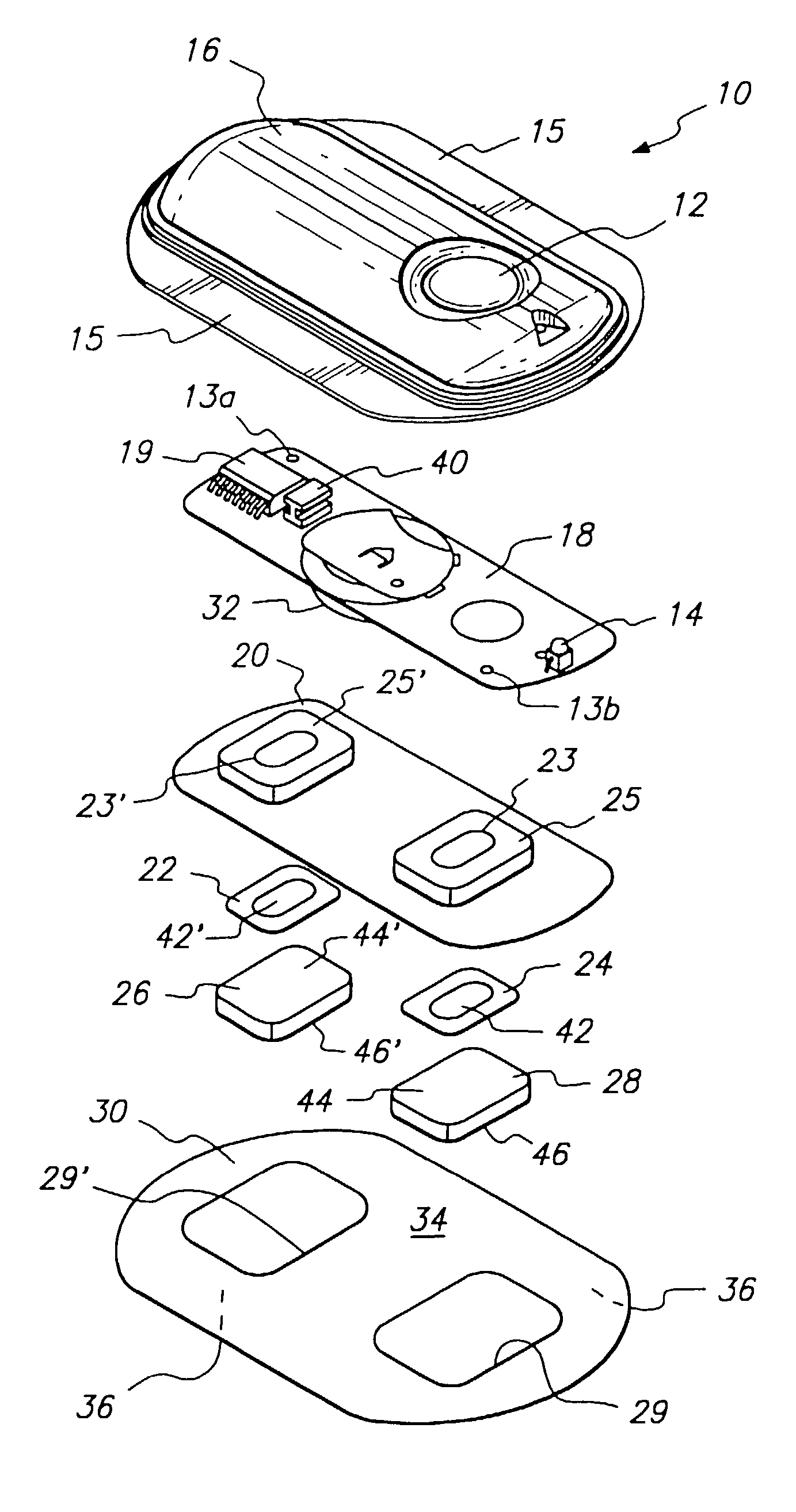
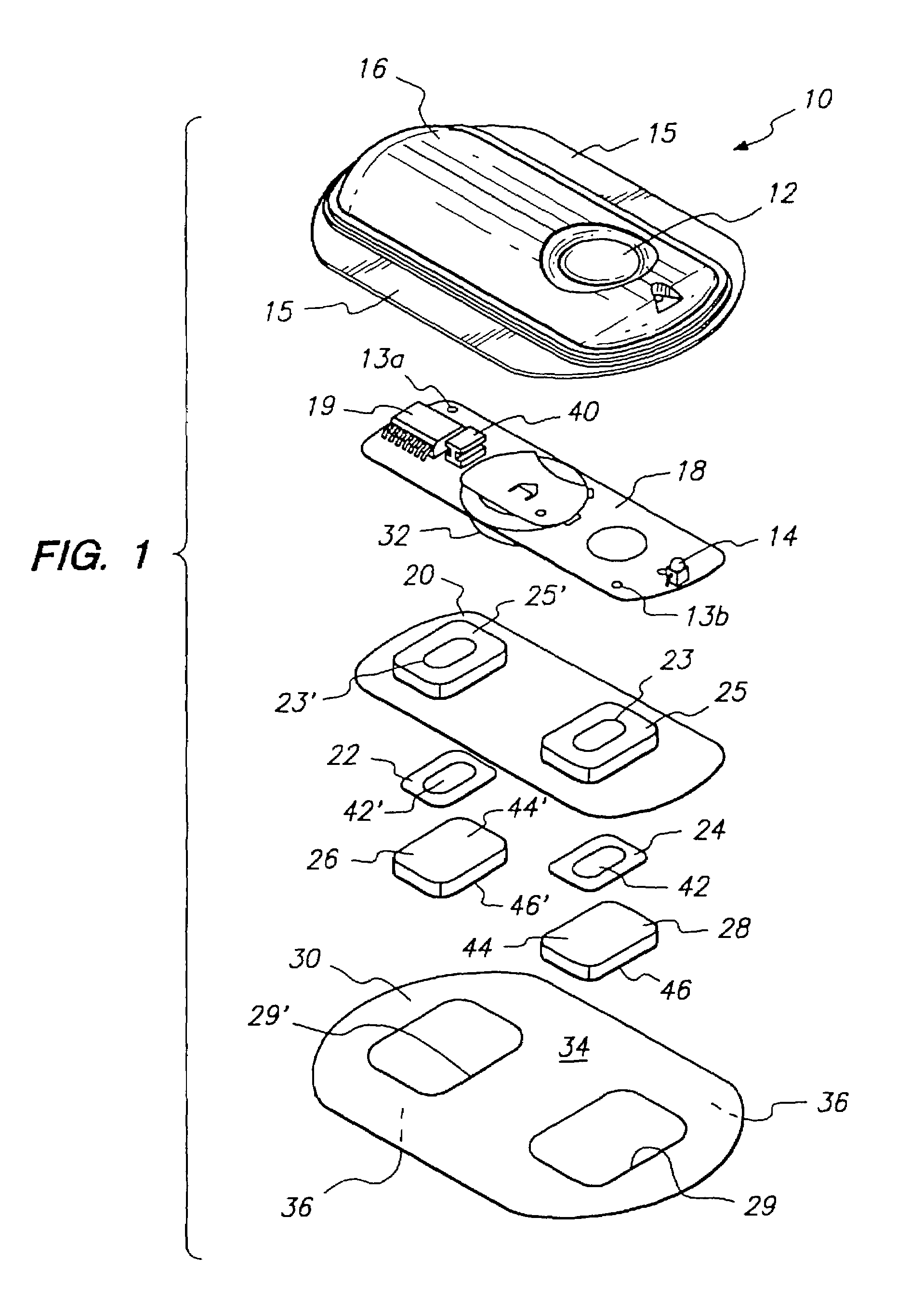
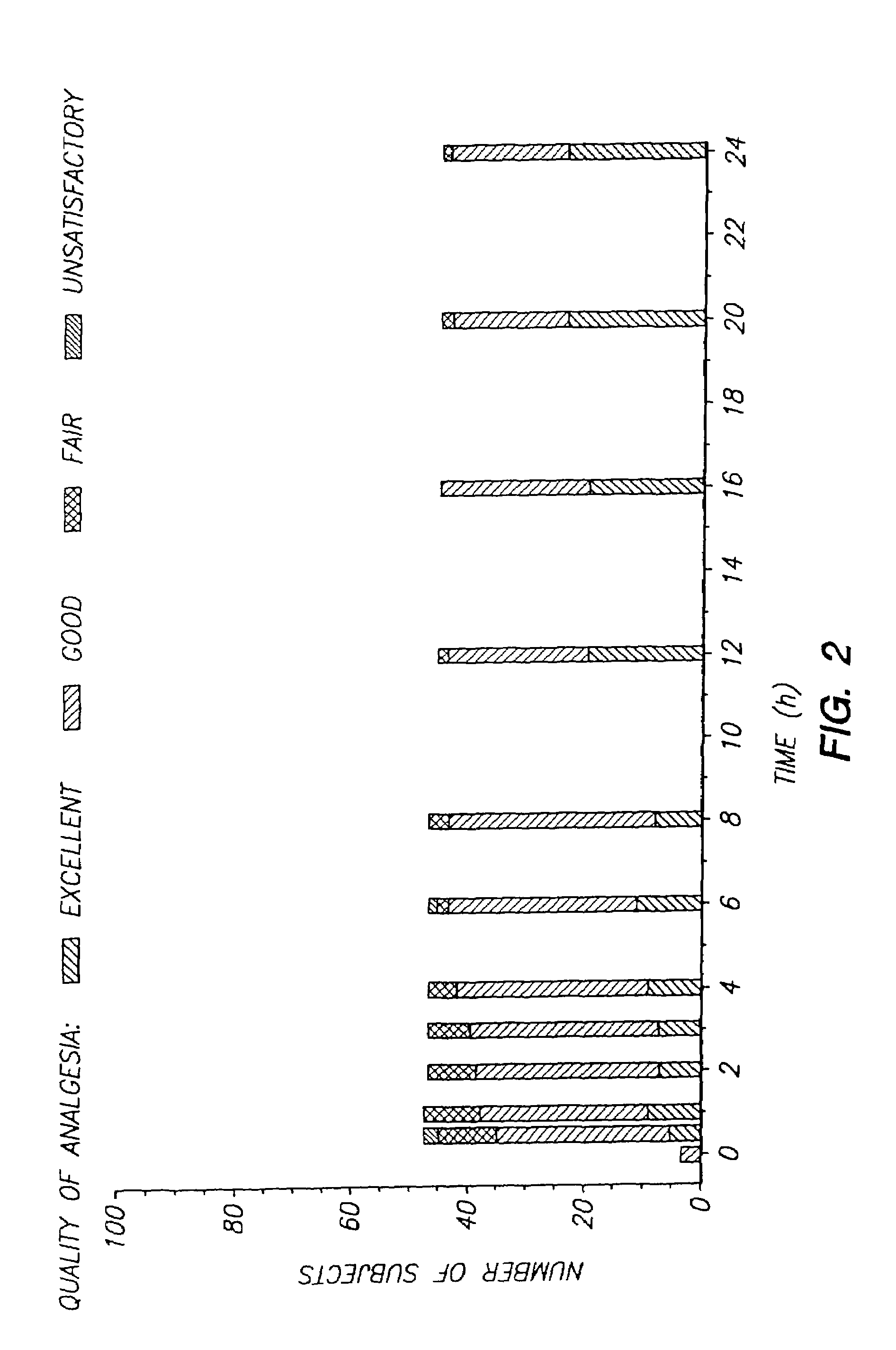
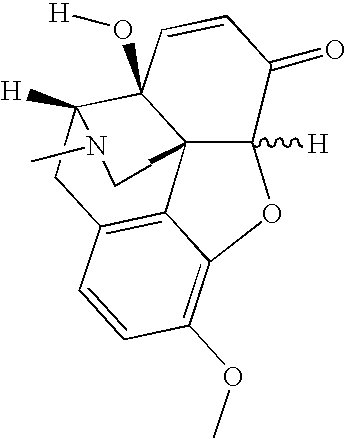
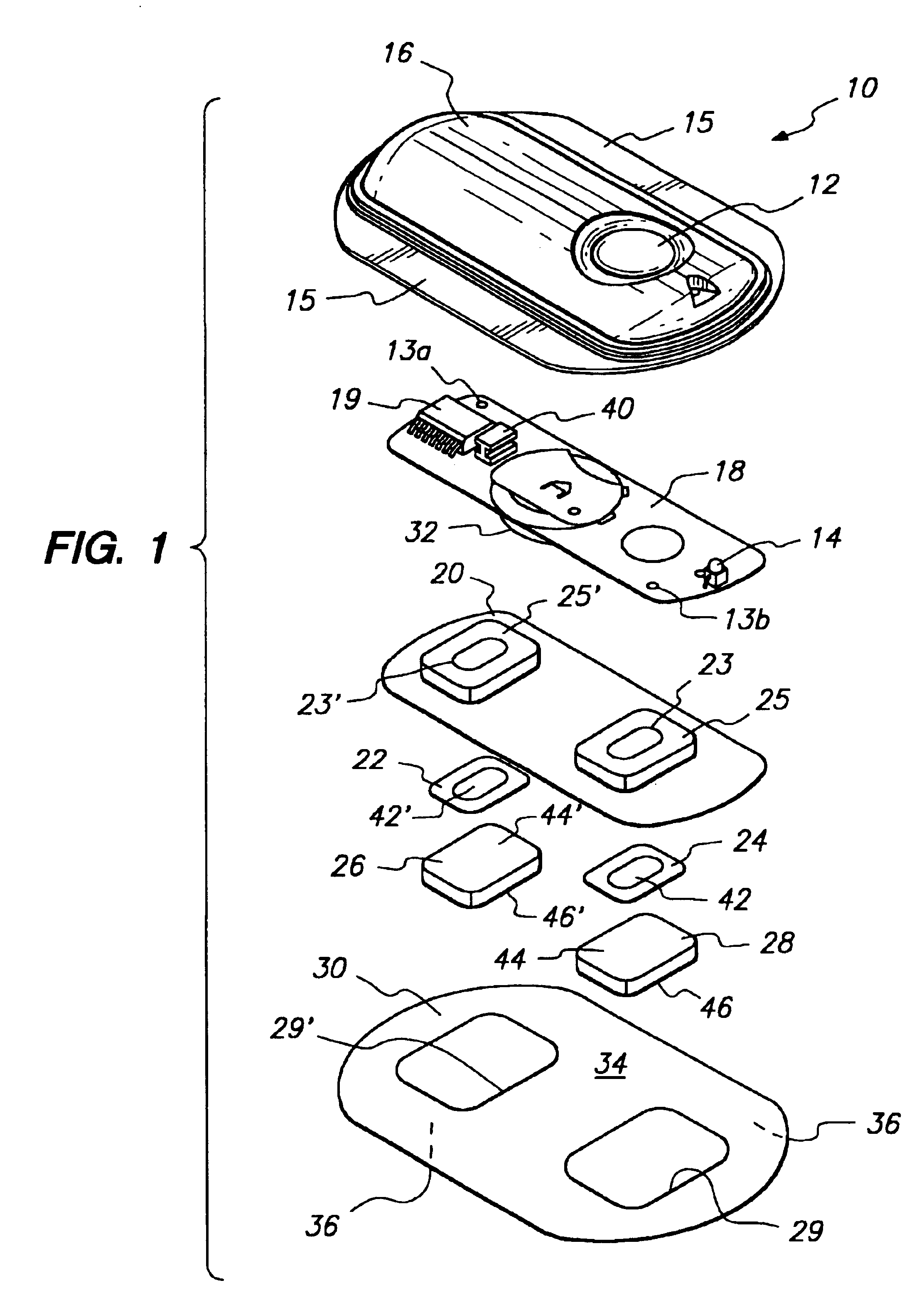
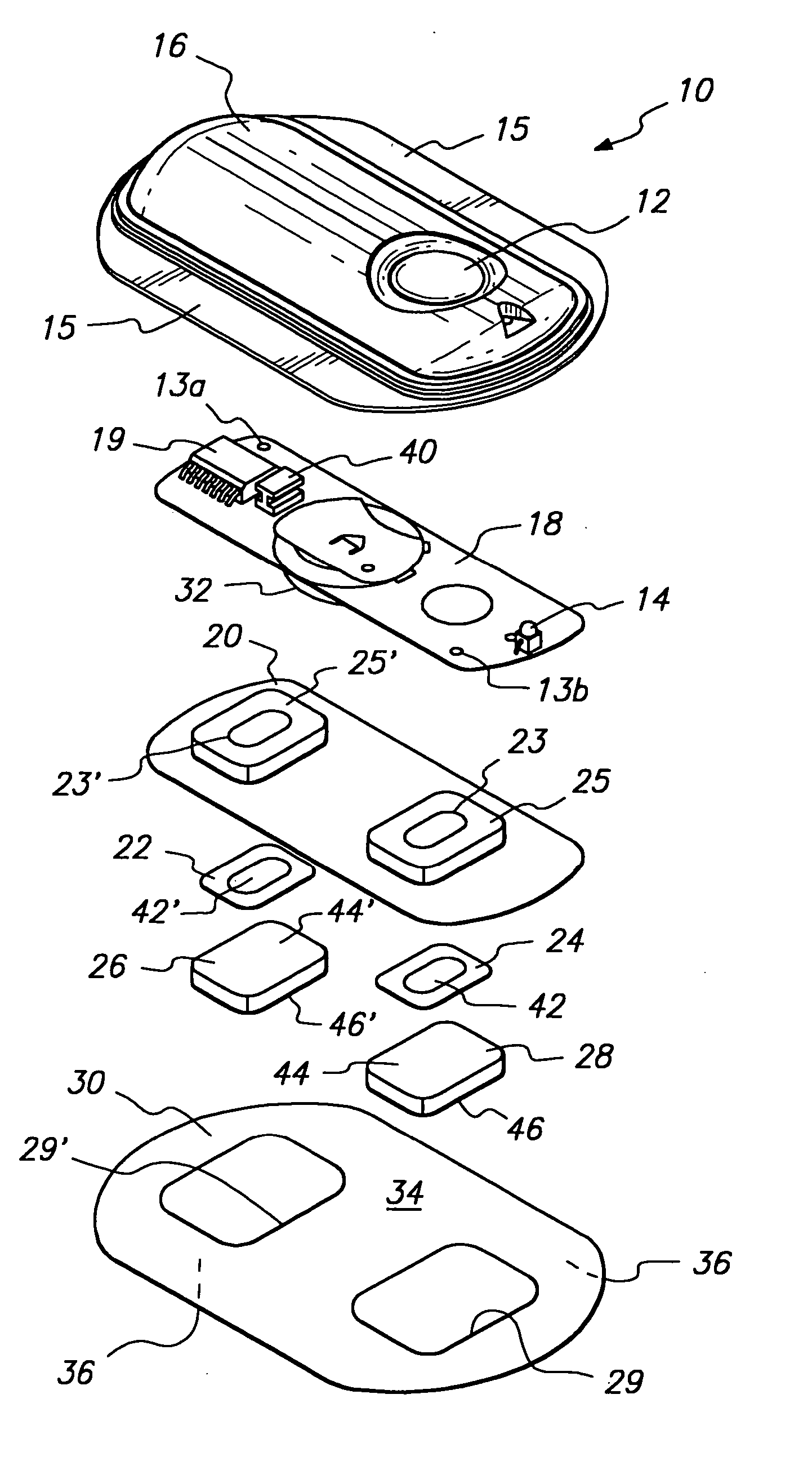
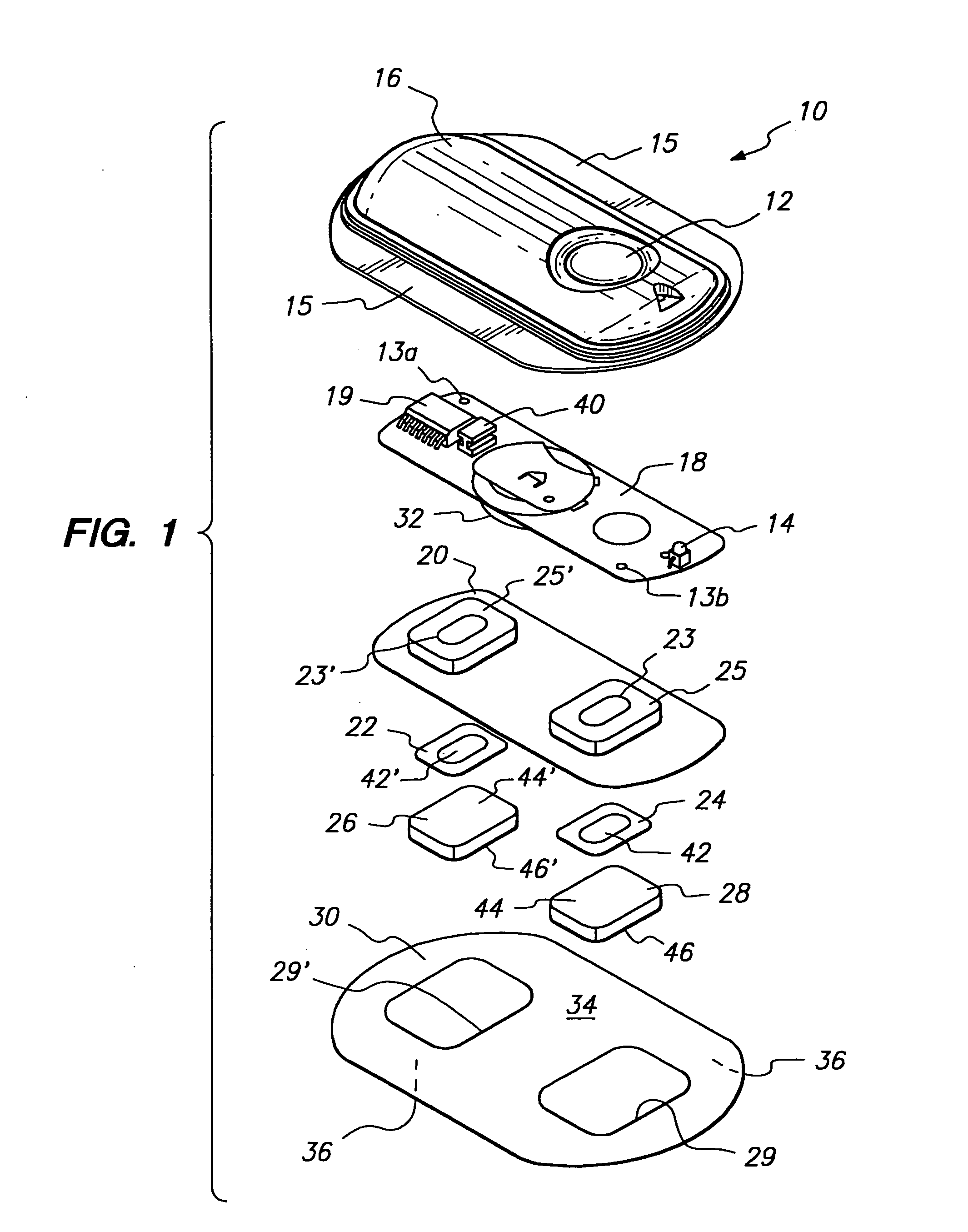
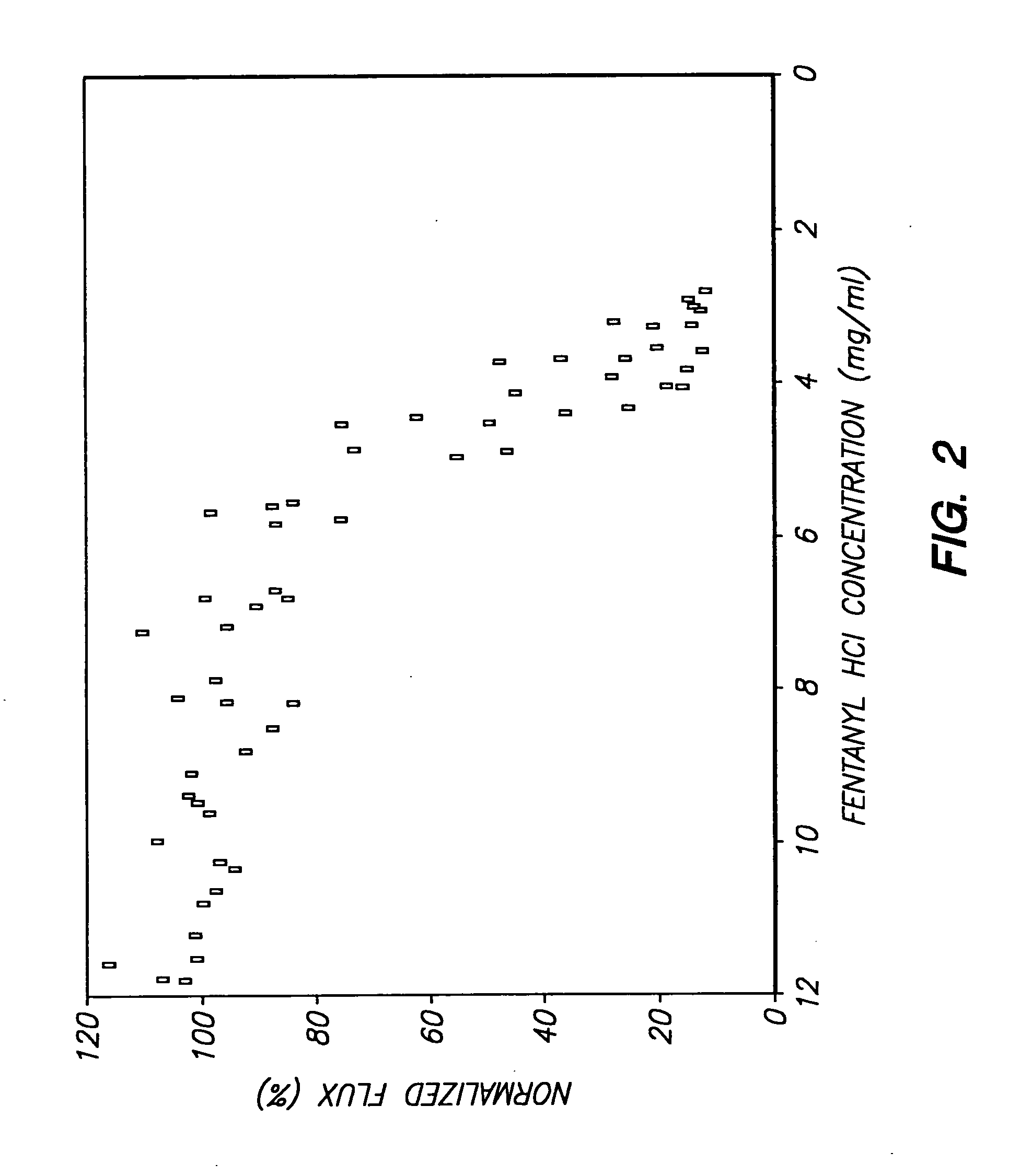
The invention provides an improved electrotransport drug delivery system for analgesic drugs, namely fentanyl and sufentanil. The fentanyl / sufentanil is provided as a water soluble salt (e.g., fentanyl hydrochloride), preferably in a hydrogel formulation, for use in an electrotransport device (10). In accordance with the present invention, a transdermal electrotransport delivered dose of fentanyl / sufentanil is provided which is sufficient to induce analgesia in (e.g., adult) human patients suffering from moderate-to-severe pain associated with major surgical procedures.
Owner:ALZA CORP
Pharmaceutical formulations comprising ibuprofen, oxycodone, and 14-hydroxycodeinone
The present invention relates to pharmaceutical formulations of ibuprofen, oxycodone and 14-hydroxycodeinone and their use for the treatment of acute, moderate to severe pain.
Owner:FOREST LABORATORIES
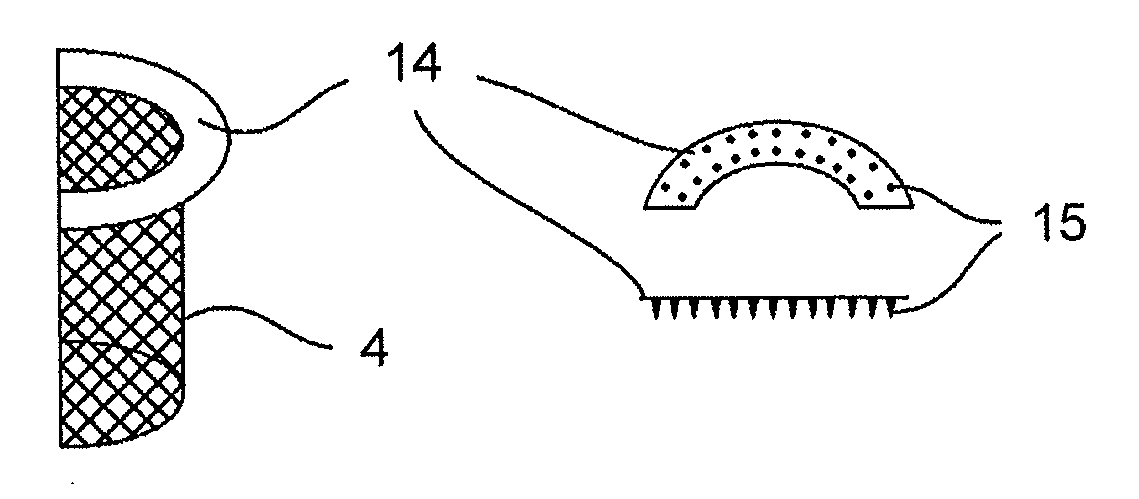
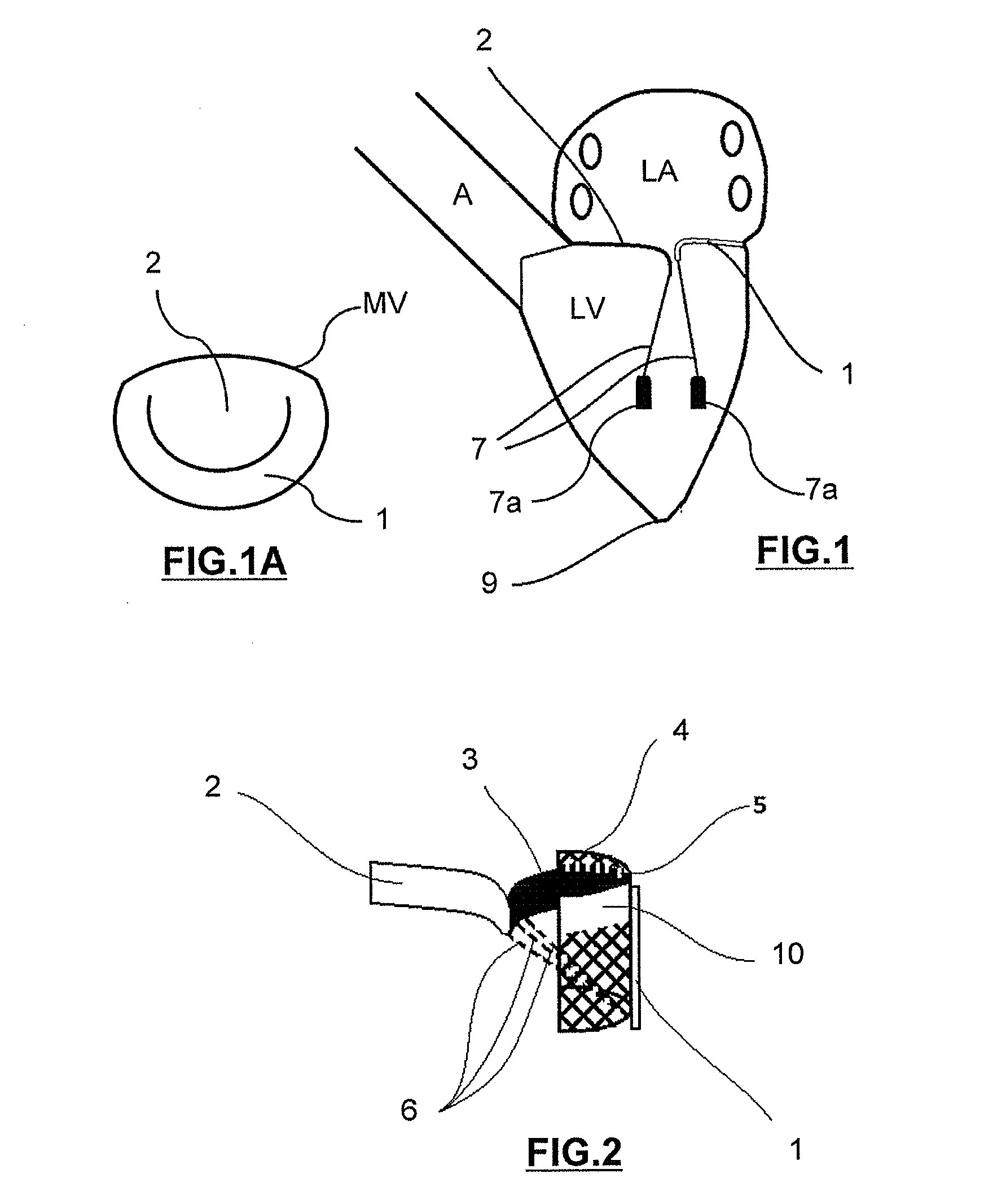
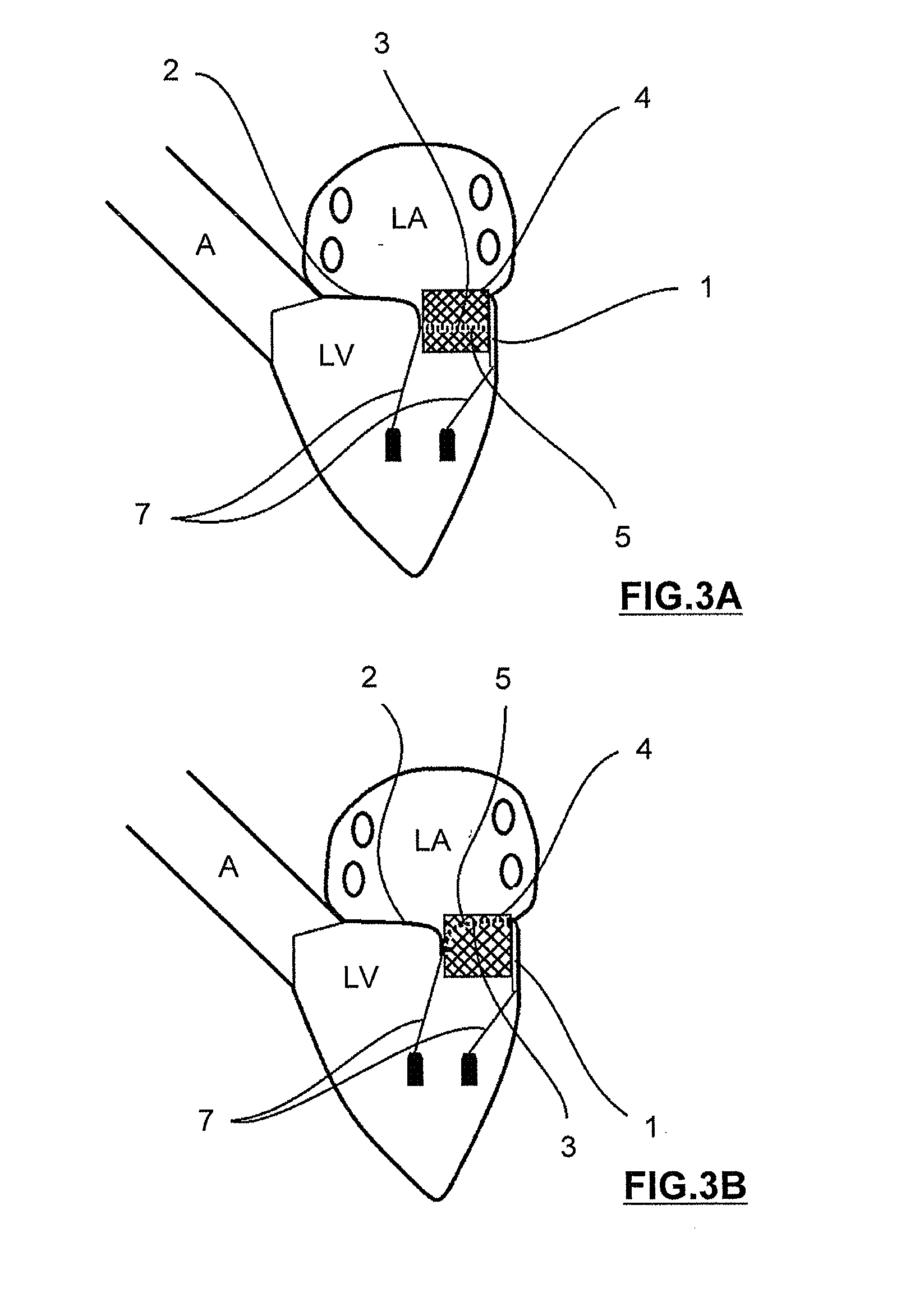
Mitral heart valve prosthesis and associated delivery catheter
InactiveUS20140309727A1Prevent paravalvular leakagePrevent leakageHeart valvesDocking stationPosterior leaflet
The invention relates to a mitral heart valve prosthesis and a delivery catheter to carry and deploy such a prosthesis. The invention allows to effectively treat a pathology related to moderate to severe mitral regurgitation. Such a prosthesis implantable by catheterism includes mainly a docking station and a leaflet cooperating with the docking station. The leaflet is advantageously arranged in a configuration close to a posterior leaflet of a native mitral valve of a patient.
Owner:ST GEORGE MEDICAL INC BVI
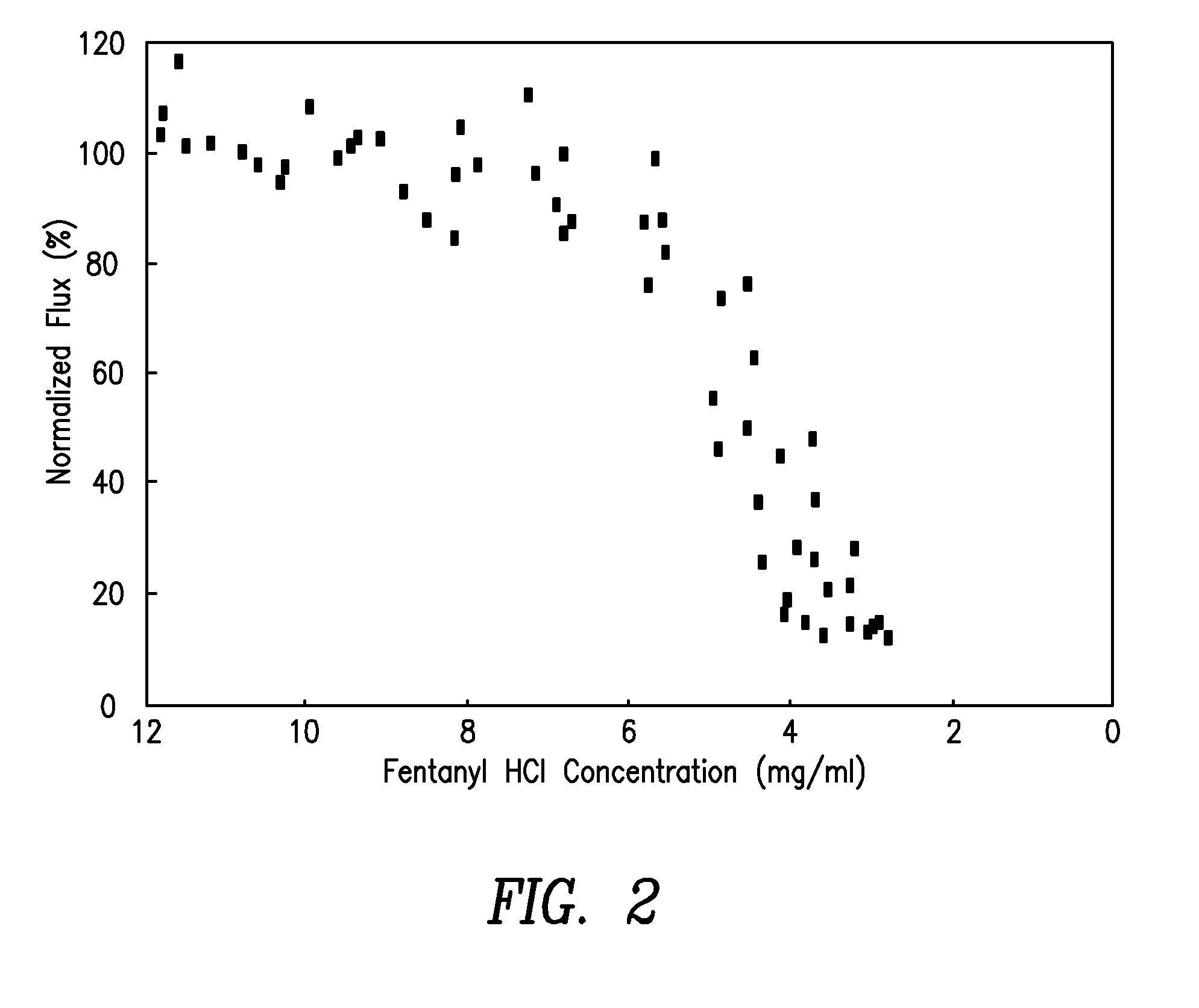
Method and device for transdermal electrotransport delivery of fentanyl and sufentanil
InactiveUS6881208B1Improved transdermal electrotransport deliveryImprove efficiencyOrganic active ingredientsNervous disorderAnalgesics drugsMedicine
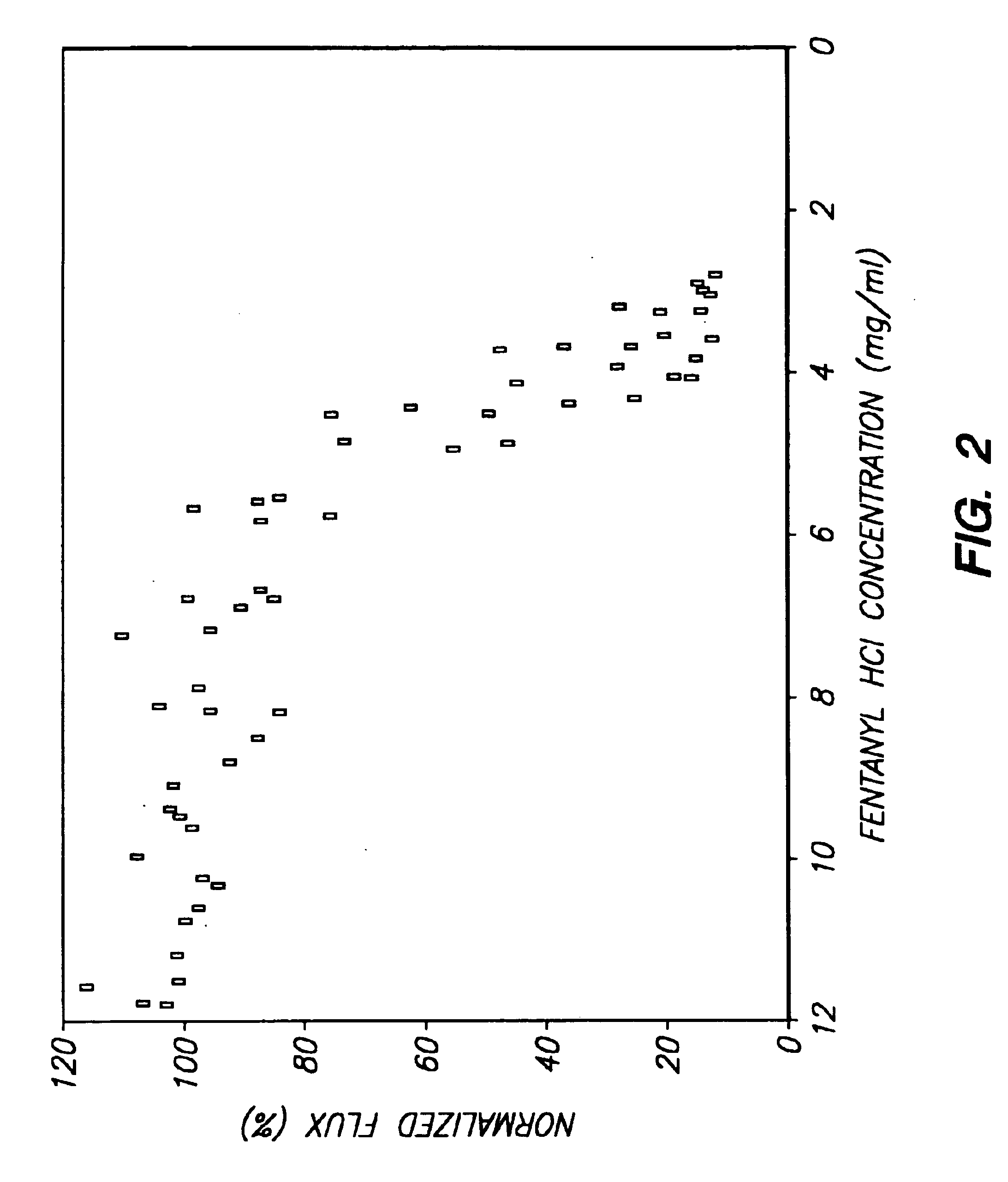
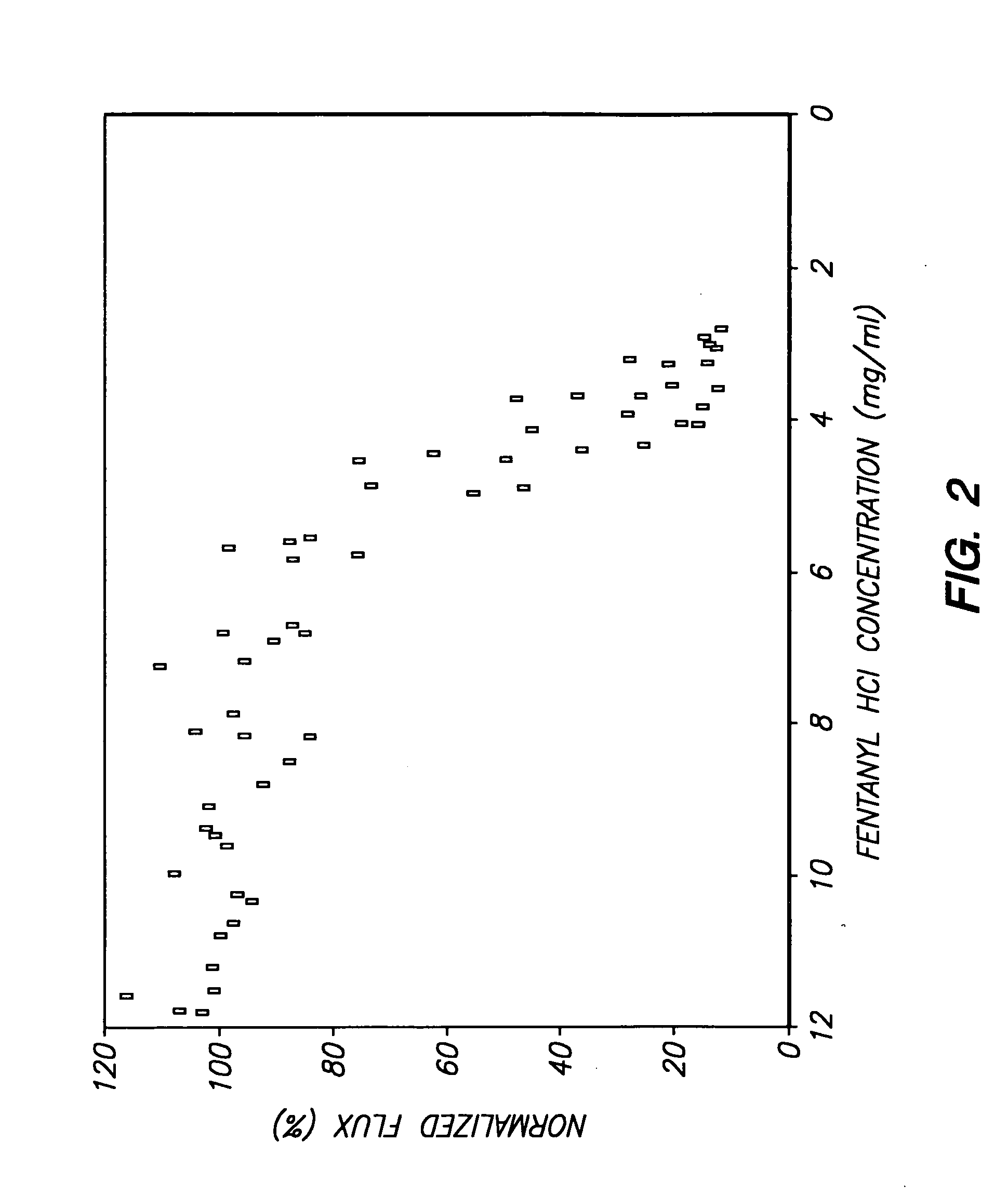
The invention provides an improved electrotransport drug delivery system for analgesic drugs, namely fentanyl and sufentanil. The fentanyl / sufentanil is provided as a water soluble salt (eg, fentanyl hydrochloride) dispersed in a hydrogel formulation for use in an electrotransport device (10). In accordance with one aspect of the invention, the concentration of fentanyl / sufentanil in the donor reservoir (26) solution is above a predetermined minimum concentration, whereby the transdermal electrotransport flux of fentanyl / sufentanil is maintained independent of the concentration of fentanyl / sufentanil in solution. In accordance with a second aspect of the present invention, the donor reservoir (26) of the electrotransport delivery device (10) is comprised of silver and the donor reservoir (26) contains a predetermined “excess” loading of fentanyl / sufentanil halide to prevent silver ion migration with attendant skin discoloration. In accordance with a third aspect of the present invention, a transdermal electrotransport delivered dose of fentanyl / sufentanil is provided which is sufficient to induce analgesia in (eg, adult) human patients suffering from moderate-to-severe pain associated with major surgical procedures.
Owner:ALZA CORP
Injectable crosslinked hyaluronic acid gel and preparation method thereof
The invention relates to an injectable crosslinked hyaluronic acid gel and a preparation method thereof. The injectable crosslinked hyaluronic acid gel is prepared by blending hyaluronic acid gel granules and chlorinated sodium phosphate physiological buffer solution; the hyaluronic acid gel granules are prepared by comprising the steps of crosslinking treatment, emulsion crosslinking granulation, purification and drying and swelling, filling and sterilization technologies. The hyaluronic acid gel granules are uniform in granule size, the residual of the crosslinking agent is less than 0.2ppm, the injection pushing is proper, and the in-vivo degradation time can last more than 8-12 months. The implant has an excellent esthetical restoration effect, is applicable to being injected to and subcutaneous dermis deep layer to subcutaneous superficial layer, restoration of moderate to severe wrinkles or folds, and can satisfy the restoration demand of wrinkles or folds caused due to skin aging.
Owner:SHAANXI BIO REGENERATIVE MEDICINE CO LTD
Methods of treating pain
The present invention is directed to methods and compositions for inducing, promoting or otherwise facilitating pain relief. More particularly the present invention discloses the combination of a nitric oxide donor and an opioid analgesic in the therapeutic management of vertebrate animals including humans, for the prevention or alleviation of pain, particularly moderate to severe pain. In particular, the nitric oxide donor is a slow-release nitric oxide donor or is formulated to provide a sustained release of a low dose of nitric oxide.
Owner:THE UNIV OF QUEENSLAND
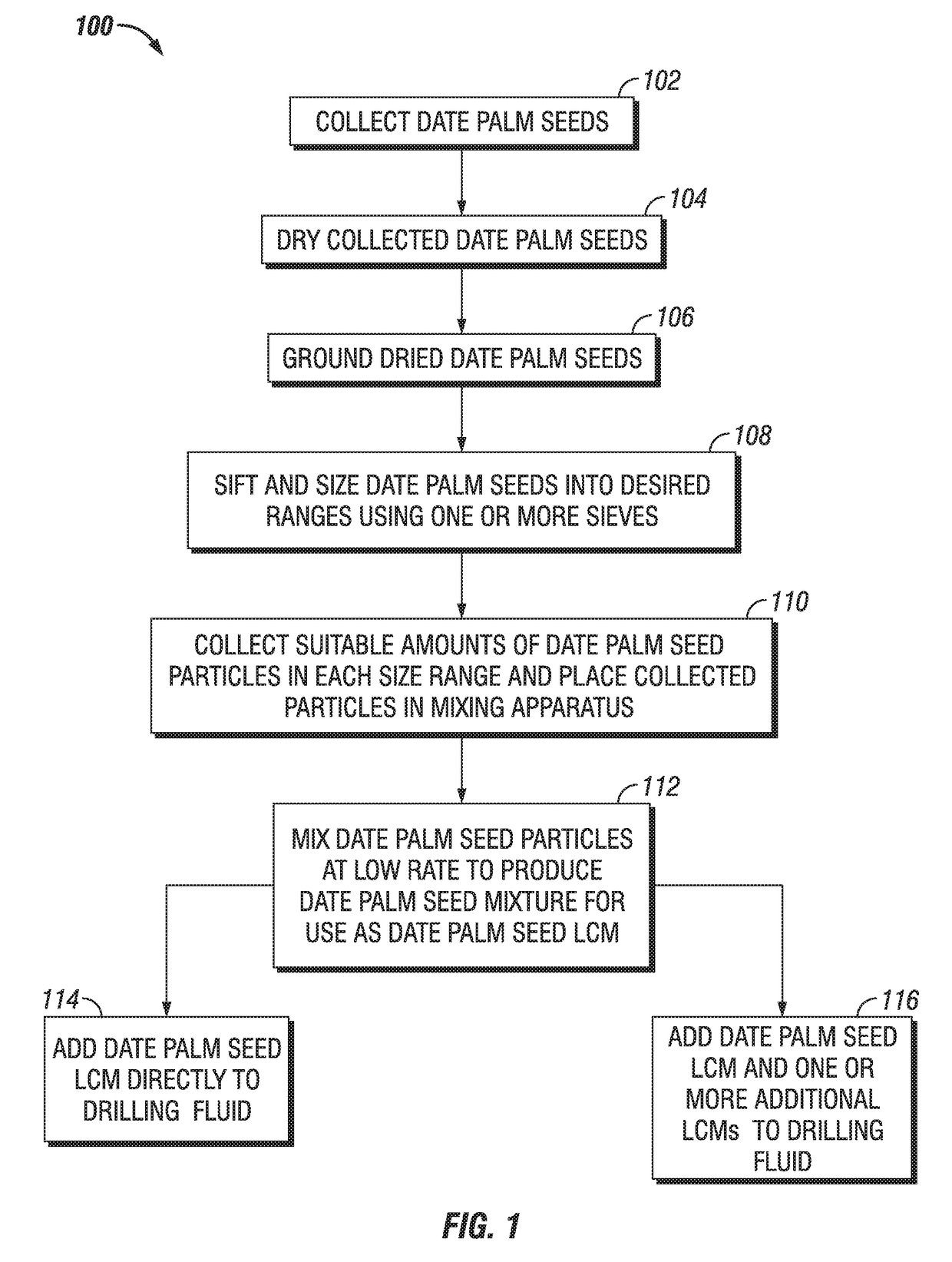
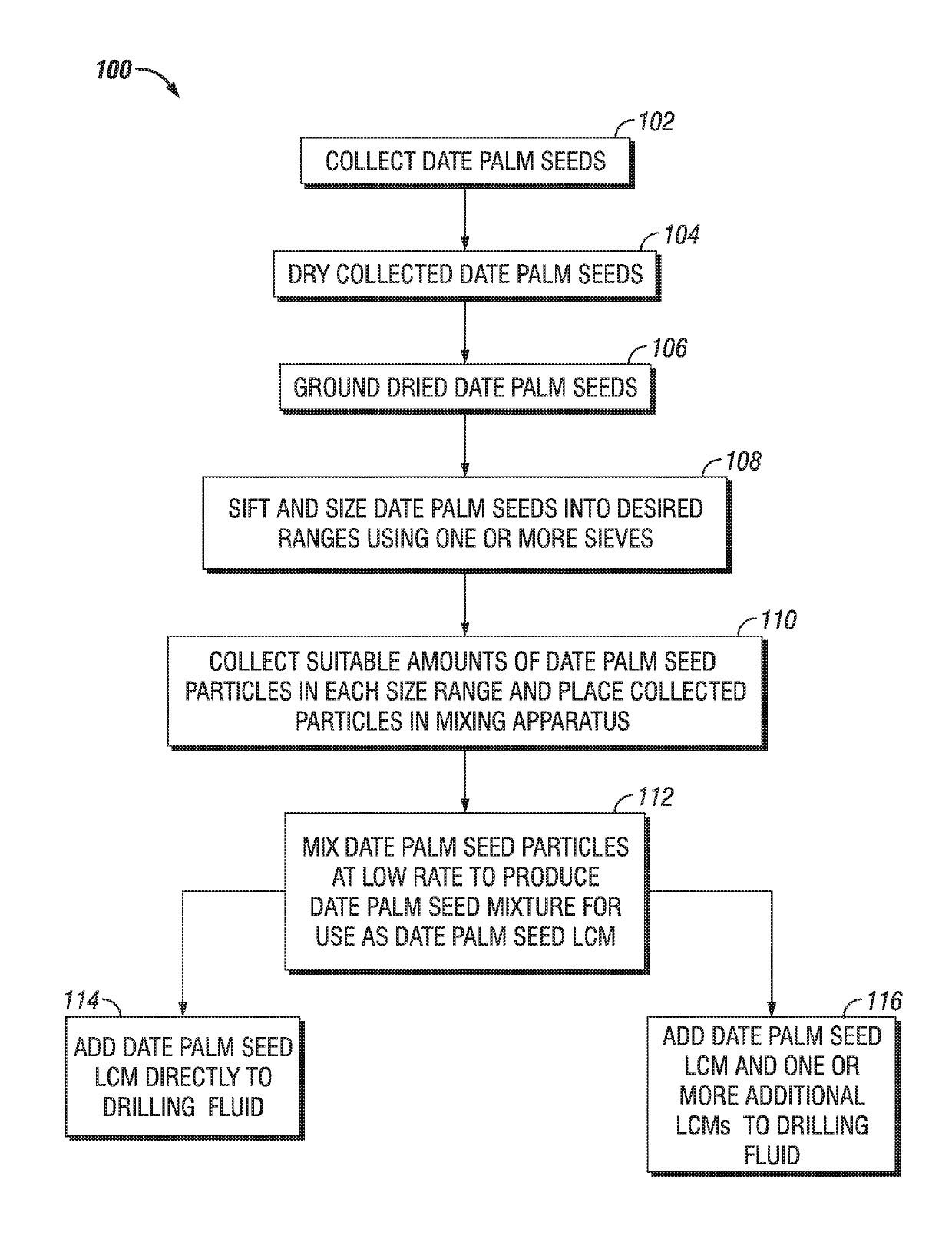
Date Seed-based Multi-Modal Particulate Admixture for Moderate to Severe Loss Control
A date palm seed lost circulation material (LCM) is provided having a date palm seed admixture of date palm seed particles of various sizes. The date palm seed particles may have a size greater than about 2380 microns in a range of about 40% to about 42% by weight, particles having a size greater than about 595 microns and less than about 2381 microns in a range of about 46% to about 48% by weight, particles having a size greater than about 400 microns and less than about 596 microns in a range of about 4% to about 6% by weight, particles having a size less than about 210 microns in a range of about 4% to about 6% by weight, and particles having a size less than about 149 microns in a range of about 1% to about 3% by weight. Methods of lost circulation control using and manufacture of a date palm seed LCM are also provided.
Owner:SAUDI ARABIAN OIL CO
Jak1 selective inhibitor and uses thereof
InactiveUS20150118229A1High selectivityLow potencyBiocideSenses disorderInflammatory bowel diseaseDosage schedule
Owner:ABBVIE INC
Method and device for transdermal delivery of fentanyl and sufentanil
InactiveUS20050131337A1Improved transdermal electrotransport deliveryImprove efficiencyBiocideNervous disorderAnalgesics drugsMedicine
Owner:ALZA CORP
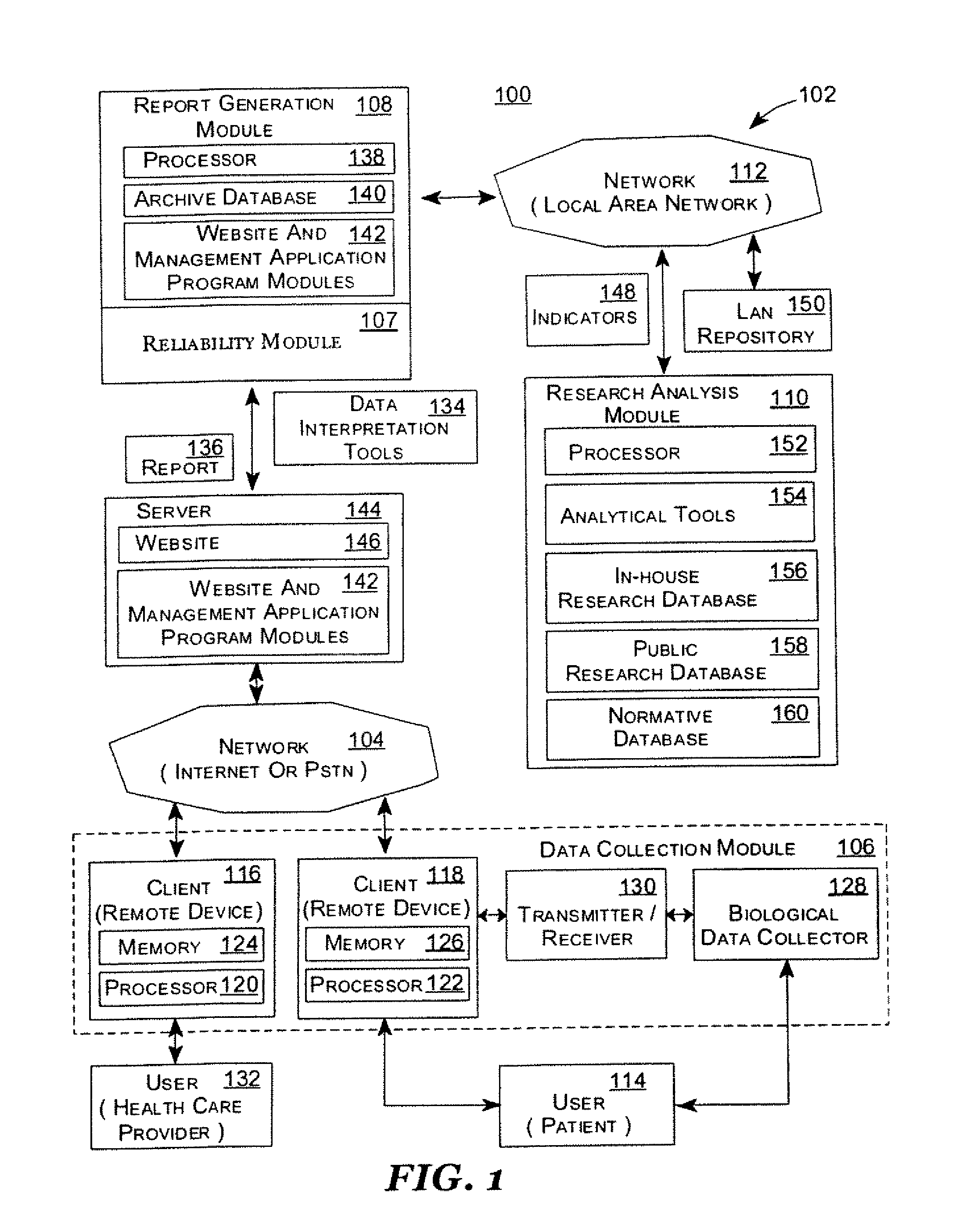
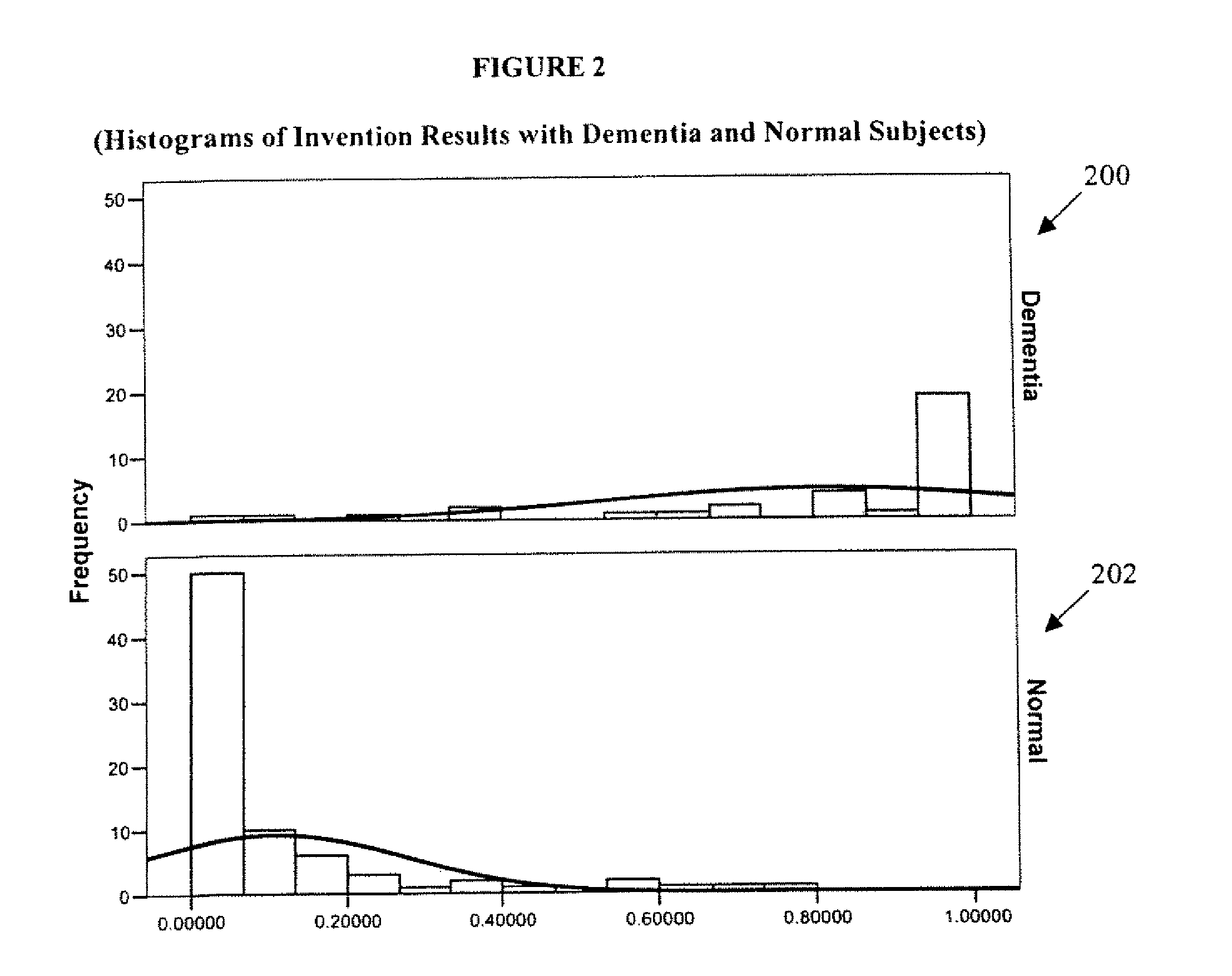
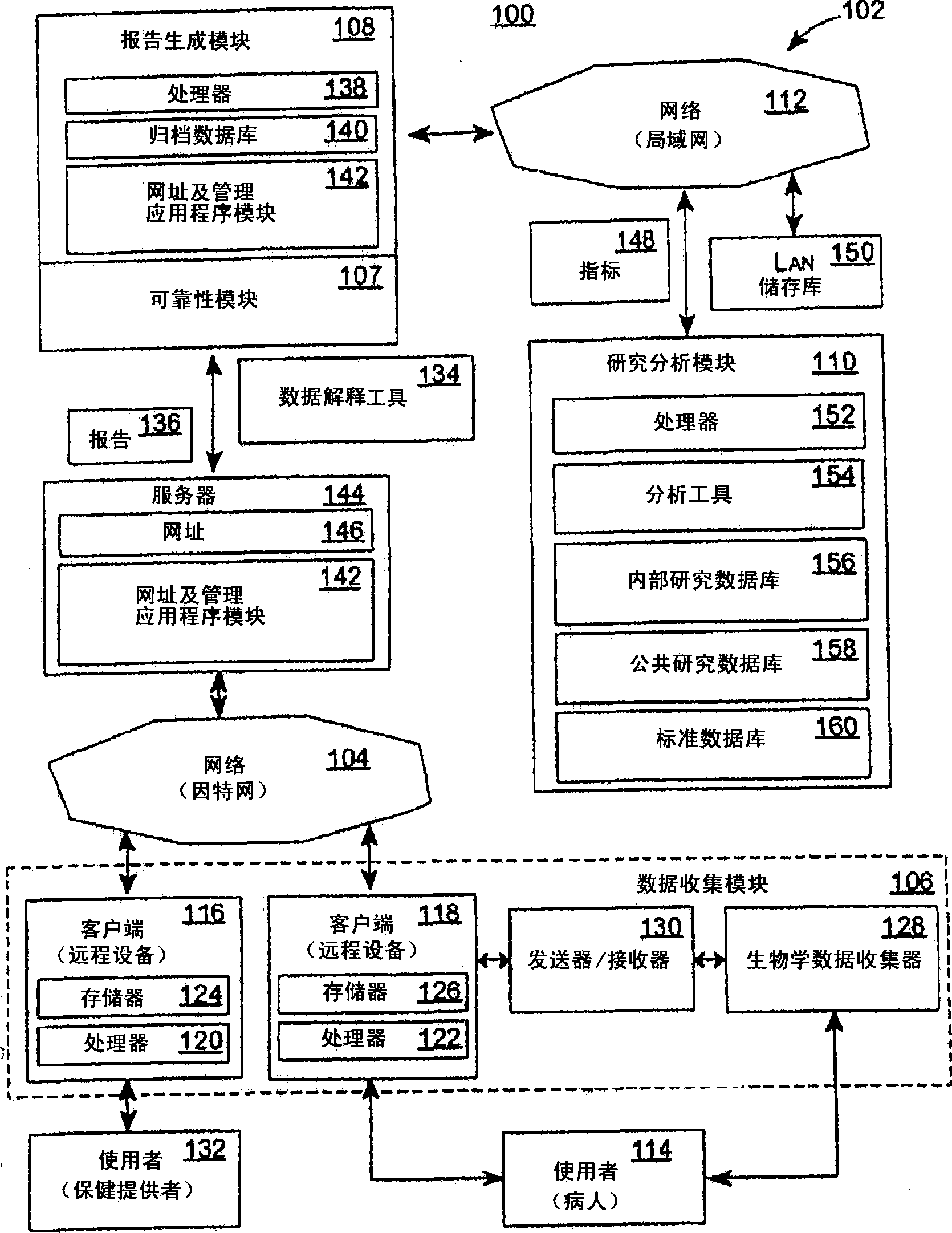
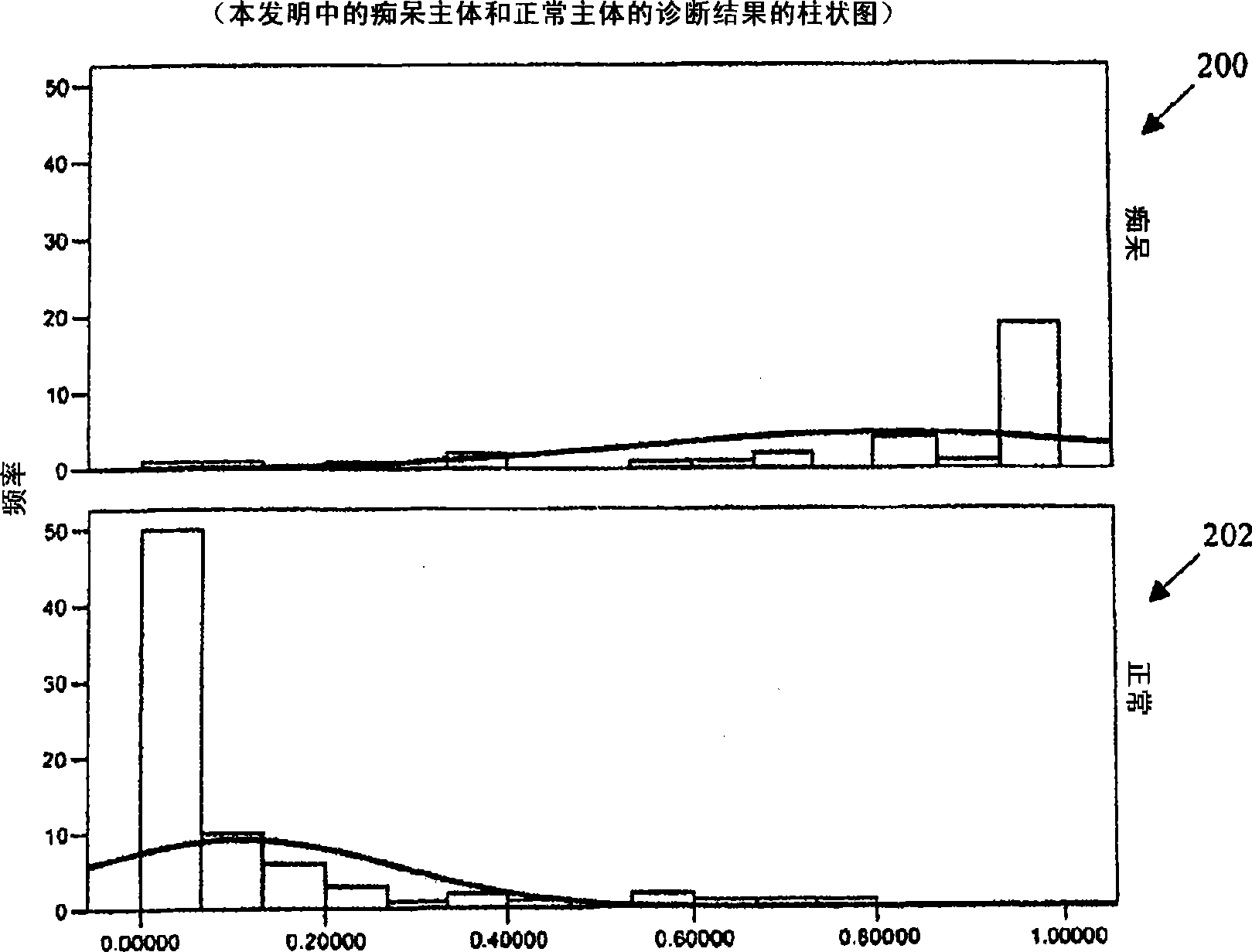
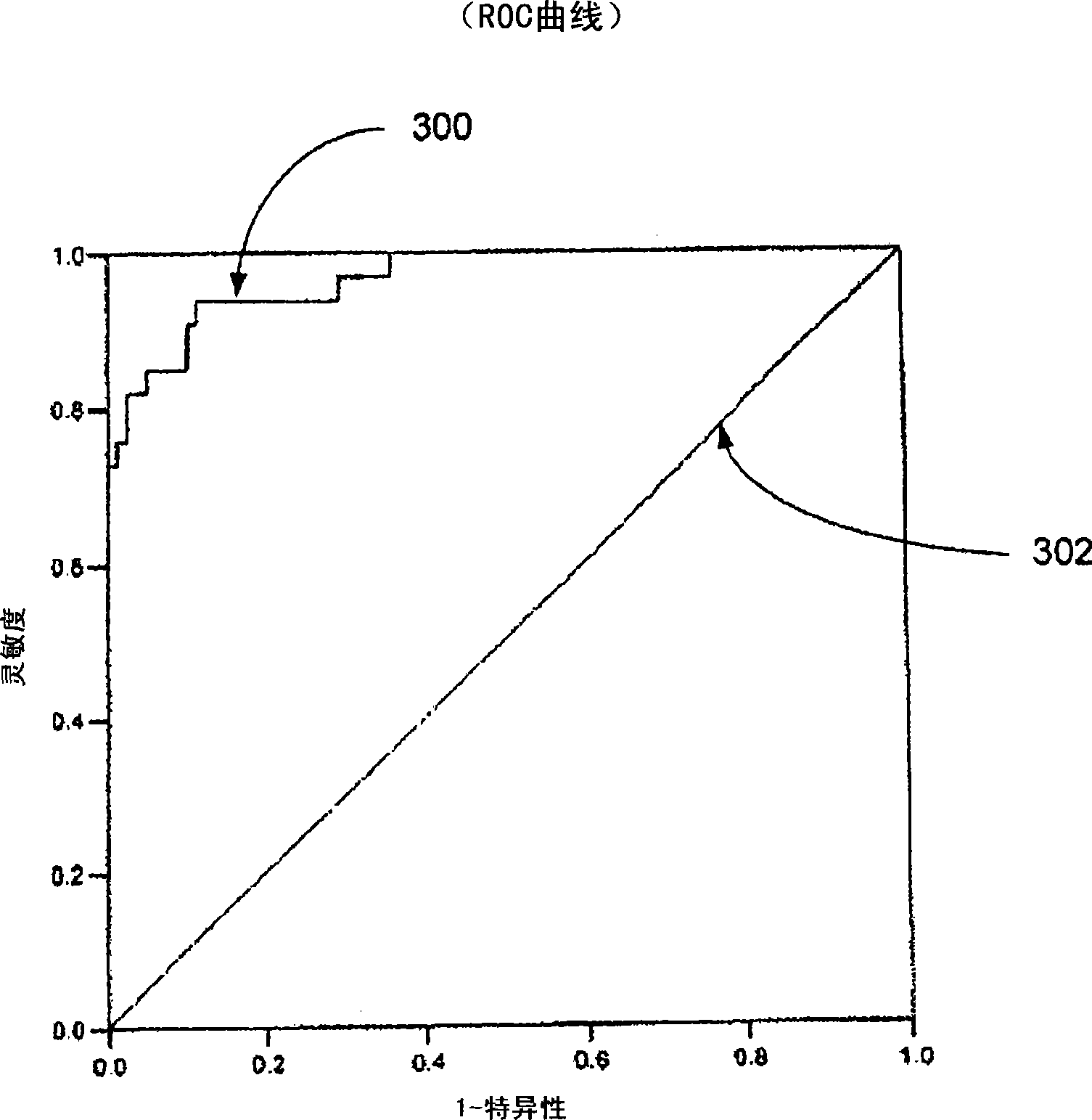
Systems and Methods for Analyzing and Assessing Dementia and Dementia-Type Disorders
InactiveUS20070299360A1High sensitivityStrong specificityElectroencephalographyMedical automated diagnosisMixed dementiaElectroencephalography
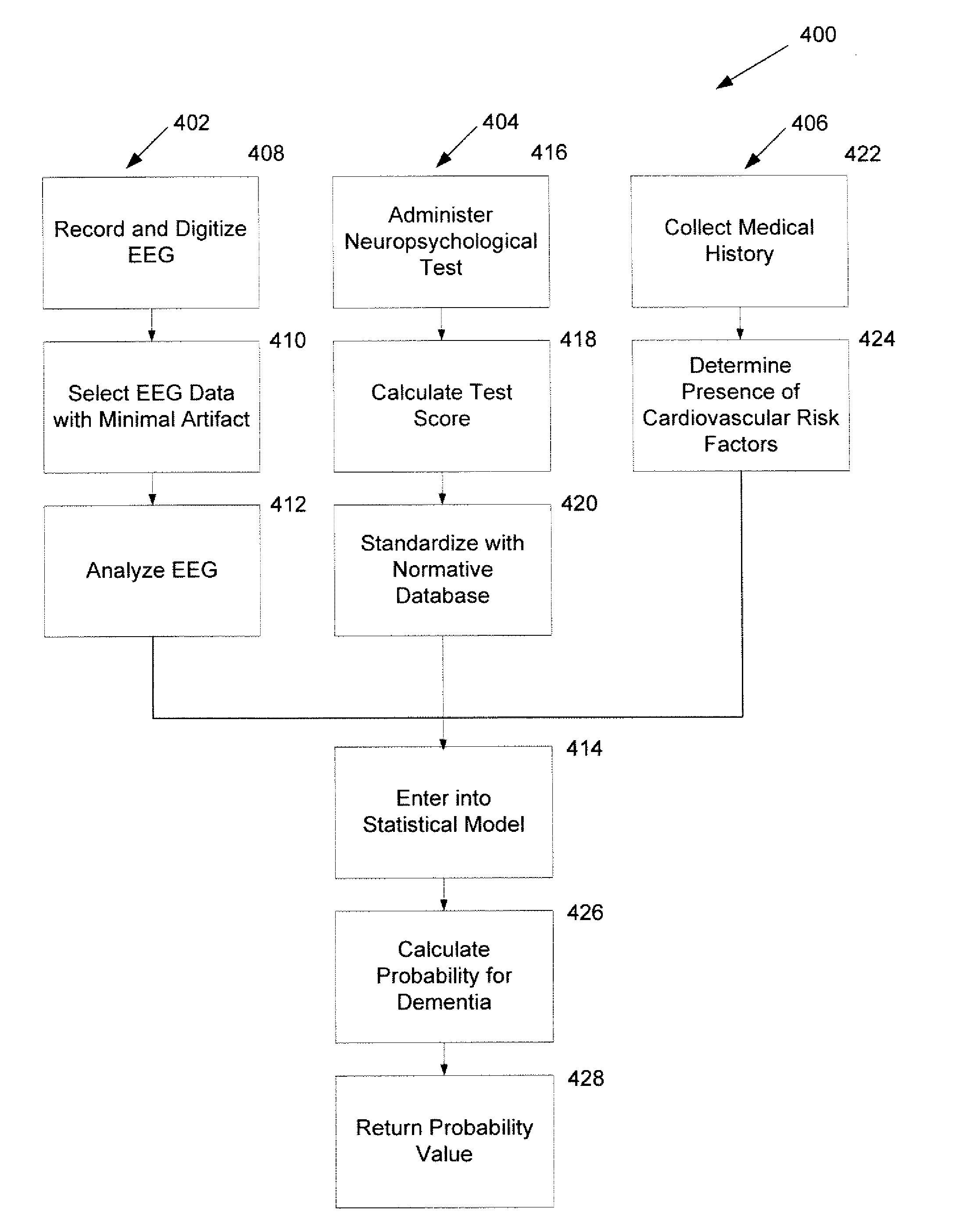
Embodiments of the invention can provide systems and methods for analyzing and assessing dementia and dementia-type disorders by integrating the use of electroencephalography (EEG), neuropsychological or cognitive testing data, and cardiovascular risk factor data. Embodiments of the invention can provide systems and methods for early detection of dementia, including Alzheimer's disease (AD), vascular dementia (VAD), mixed dementia (AD and VAD), MCI, and other dementia-type disorders. Embodiments of the invention can provide some or all of the following improvements over conventional systems and methods, including: (1) Increased sensitivity, specificity, and overall accuracy; (2) Detection of AD, VAD and mixed dementia; and (3) Accurate detection of mild dementia and some cases of mild cognitive impairment in addition to the detection of moderate to severe dementia.
Owner:LEXICOR MEDICAL TECH
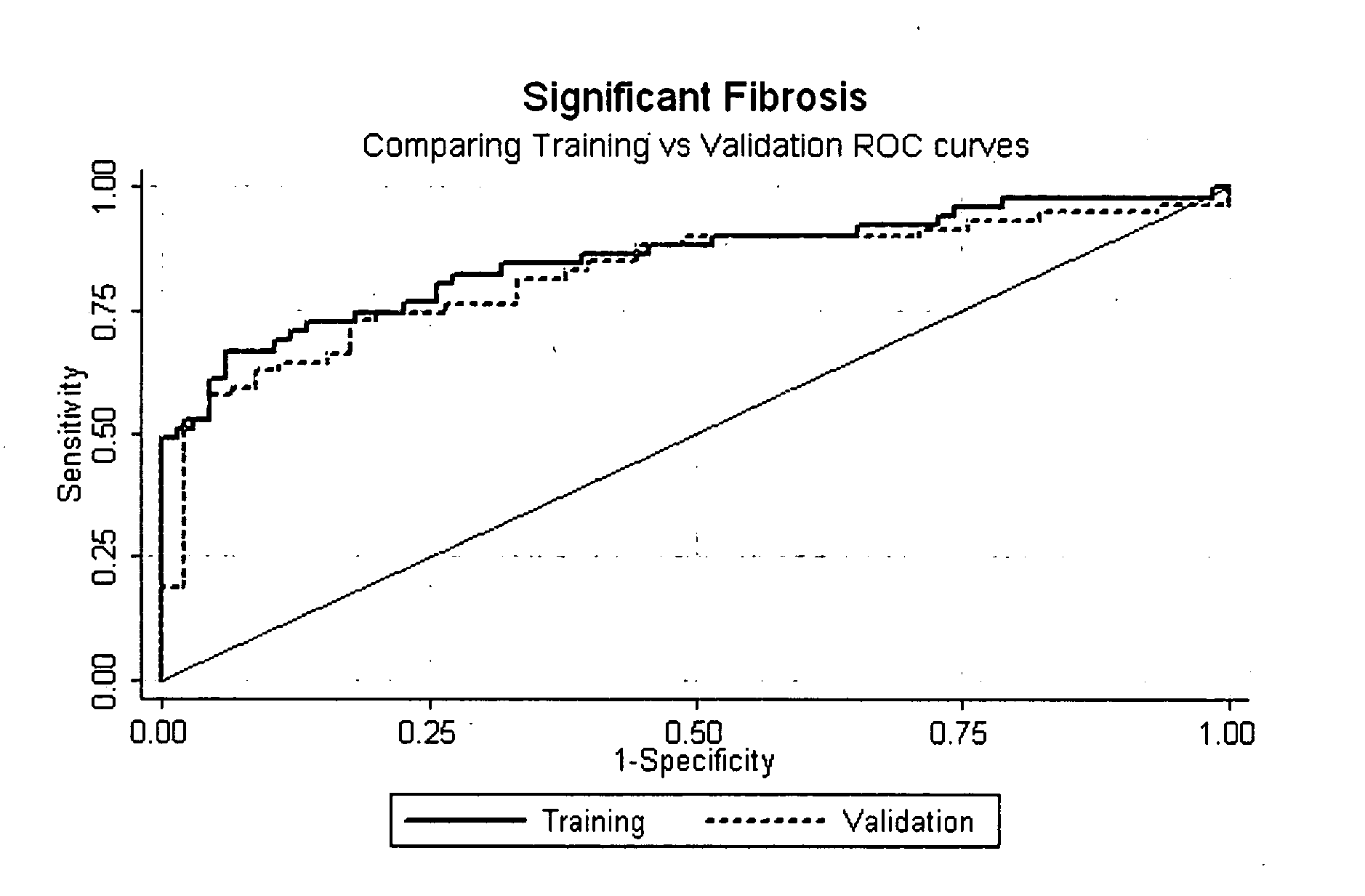
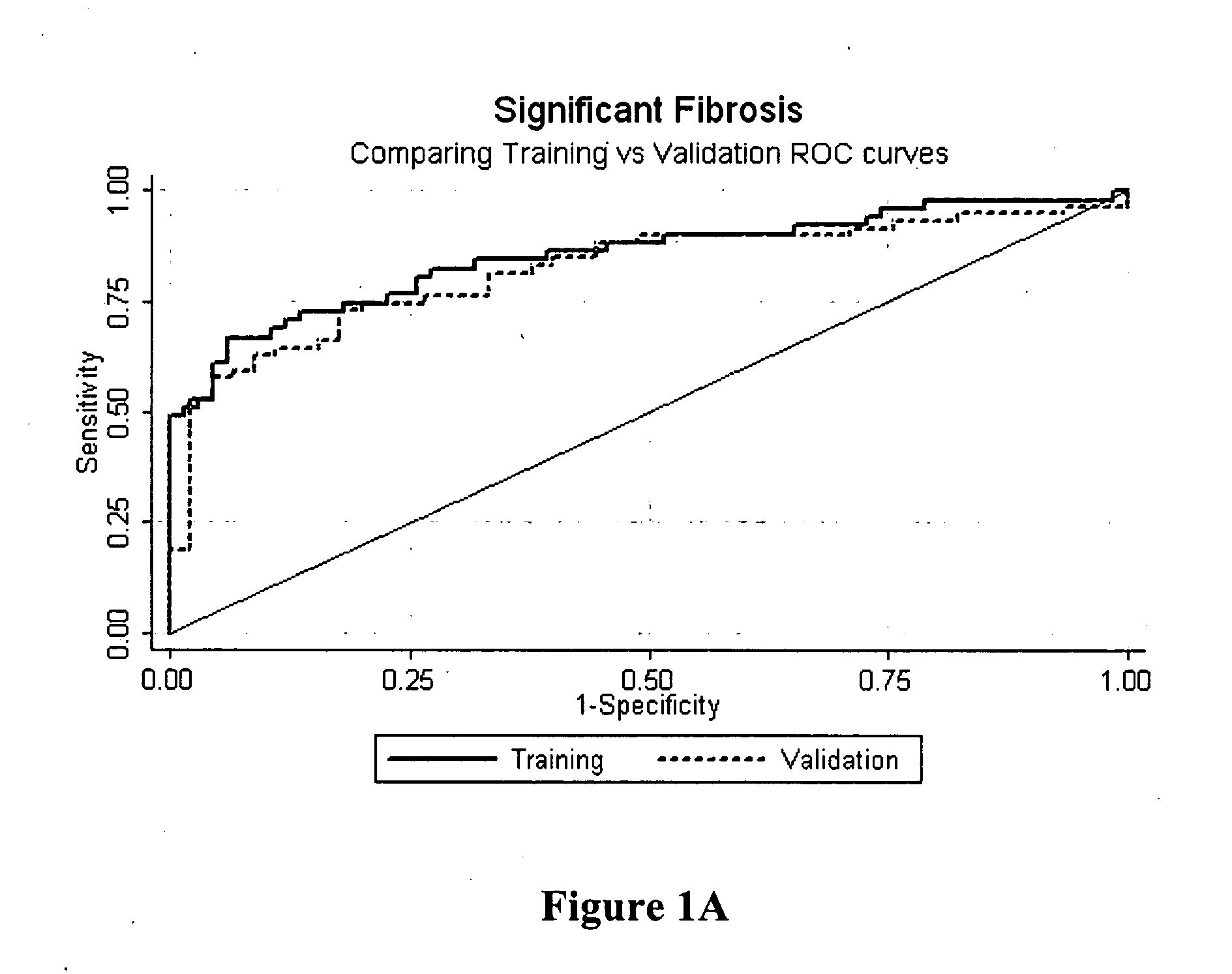
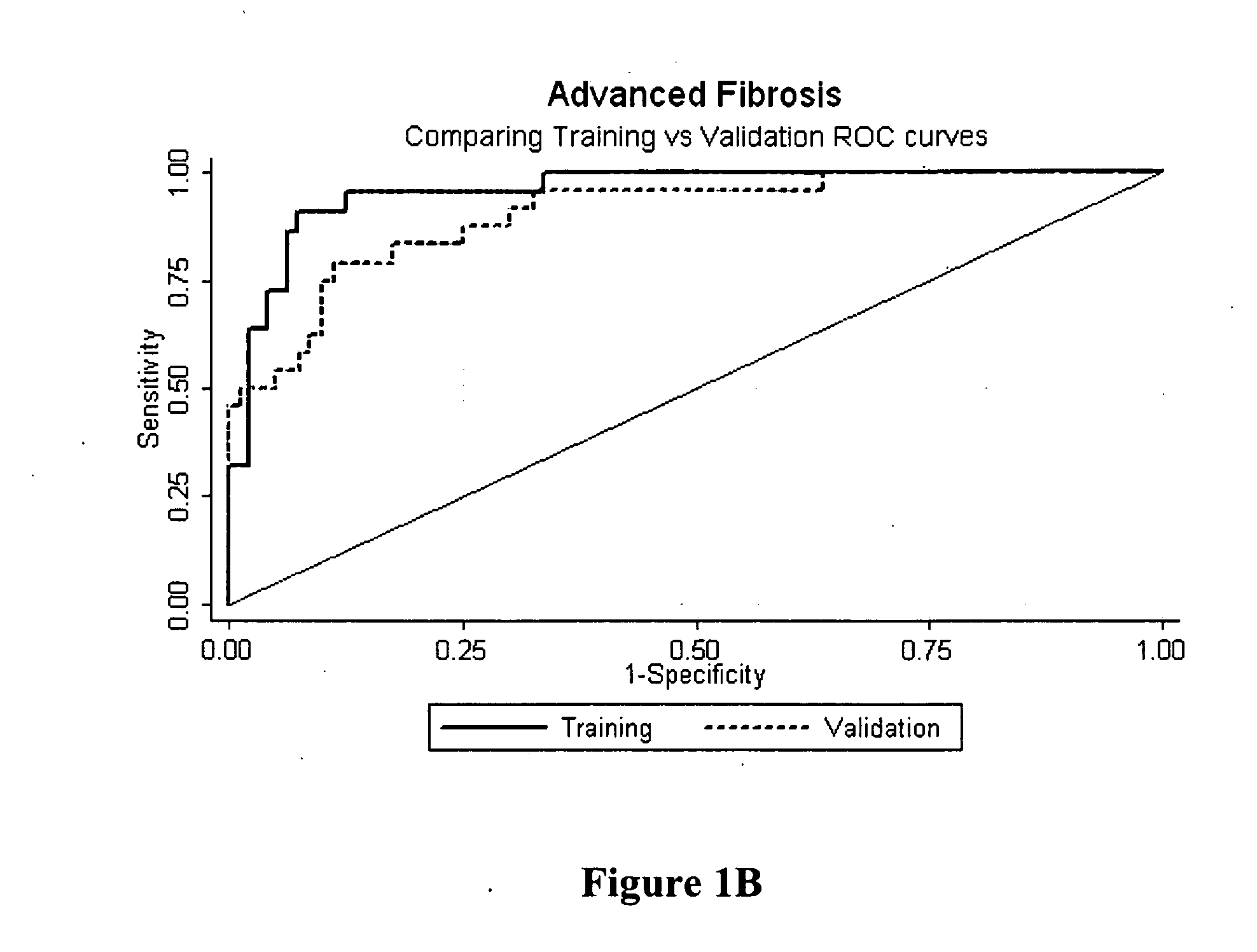
Method for predicting liver fibrosis and related pathologies
ActiveUS20070225919A1Microbiological testing/measurementDisease diagnosisSignificant fibrosisSerum markers
Provided herein are methods of detecting and staging liver fibrosis in an individual with liver disease. Also provided are methods of detecting necroinflammatory activity. Invention methods utilize four serum markers, age, and gender to determine an end value. The end value is compared to a cut-off value, in order to identify significant fibrosis (METAVIR stages F2 to F4), or an absence of advanced fibrosis (stages F3 and F4) or cirrhosis (stage F4). In particular aspects, progression or treatment of liver fibrosis can be monitored by invention methods. The end value is also used to distinguish between no to mild necroinflammatory activity (METAVIR grade A0 to A1) and moderate to severe necroinflammatory activity (grade A2 to A3).
Owner:WESTERN AUSTRALIA UNIV OF THE +1
Method and Device for Transdermal Electrotransport Delivery of Fentanyl and Sufentanil
InactiveUS20090264855A1Improved transdermal electrotransport deliveryImprove efficiencyOrganic active ingredientsNervous disorderAnalgesics drugsMedicine
The invention provides an improved electrotransport drug delivery system for analgesic drugs, namely fentanyl and sufentanil. The fentanyl / sufentanil is provided as a water soluble salt (eg, fentanyl hydrochloride) dispersed in a hydrogel formulation for use in an electrotransport device (10). In accordance with one aspect of the invention, the concentration of fentanyl / sufentanil in the donor reservoir (26) solution is above a predetermined minimum concentration, whereby the transdermal electrotransport flux of fentanyl / sufentanil is maintained independent of the concentration of fentanyl / sufentanil in solution. In accordance with a second aspect of the present invention, the donor reservoir (26) of the electrotransport delivery device (10) is comprised of silver and the donor reservoir (26) contains a predetermined “excess” loading of fentanyl / sufentanil halide to prevent silver ion migration with attendant skin discoloration. In accordance with a third aspect of the present invention, a transdermal electrotransport delivered dose of fentanyl / sufentanil is provided which is sufficient to induce analgesia in (eg, adult) human patients suffering from moderate-to-severe pain associated with major surgical procedures.
Owner:ALZA CORP
Methods of treating moderate to severe hidradenitis suppurativa with anti-TNF-alpha antibodies
ActiveUS8747854B2Treatment safetyStrong specificityAntipyreticAutomatic syringesAntigen bindingHidradenitis suppurativa
The invention provides methods, uses and compositions for the treatment of hidradenitis suppurativa. The invention describes methods and uses for treating hidradenitis suppurativa, wherein a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof, is used to treat hidradenitis suppurativa in a subject. Also described are methods for determining the efficacy of a TNFα inhibitor for treating hidradenitis suppurativa in a subject.
Owner:ABBVIE BIOTECHNOLOGY LTD
Method and device for transdermal electrotransport delivery of fentanyl and sufentanil
InactiveUS20050171464A1Improved transdermal electrotransport deliveryImprove efficiencyOrganic active ingredientsNervous disorderAnalgesics drugsMedicine
The invention provides an improved electrotransport drug delivery system for analgesic drugs, namely fentanyl and sufentanil. The fentanyl / sufentanil is provided as a water soluble salt (eg, fentanyl hydrochloride) dispersed in a hydrogel formulation for use in an electrotransport device (10). In accordance with one aspect of the invention, the concentration of fentanyl / sufentanil in the donor reservoir (26) solution is above a predetermined minimum concentration, whereby the transdermal electrotransport flux of fentanyl / sufentanil is maintained independent of the concentration of fentanyl / sufentanil in solution. In accordance with a second aspect of the present invention, the donor reservoir (26) of the electrotransport delivery device (10) is comprised of silver and the donor reservoir (26) contains a predetermined “excess” loading of fentanyl / sufentanil halide to prevent silver ion migration with attendant skin discoloration. In accordance with a third aspect of the present invention, a transdermal electrotransport delivered dose of fentanyl / sufentanil is provided which is sufficient to induce analgesia in (eg, adult) human patients suffering from moderate-to-severe pain associated with major surgical procedures.
Owner:ALZA CORP
Methods for Treating Severe Atopic Dermatitis by Administering an IL-4R Inhibitor
ActiveUS20180078603A1Reduce dependenceReduce the amount requiredPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsWhole bodySevere atopic dermatitis
The present invention provides methods for treating moderate-to-severe or severe atopic dermatitis (AD). The methods of the present invention comprise administering to a subject in need thereof one or more doses of an interleukin-4 receptor (IL-4R) inhibitor such as an anti-IL-4R antibody. In certain embodiments, the methods of the present invention are used to treat severe AD in a patient whose disease is not controlled with systemic therapy (e.g., cyclosporine A) or when such therapy is inadvisable.
Owner:SANOFI BIOTECH SAS +1
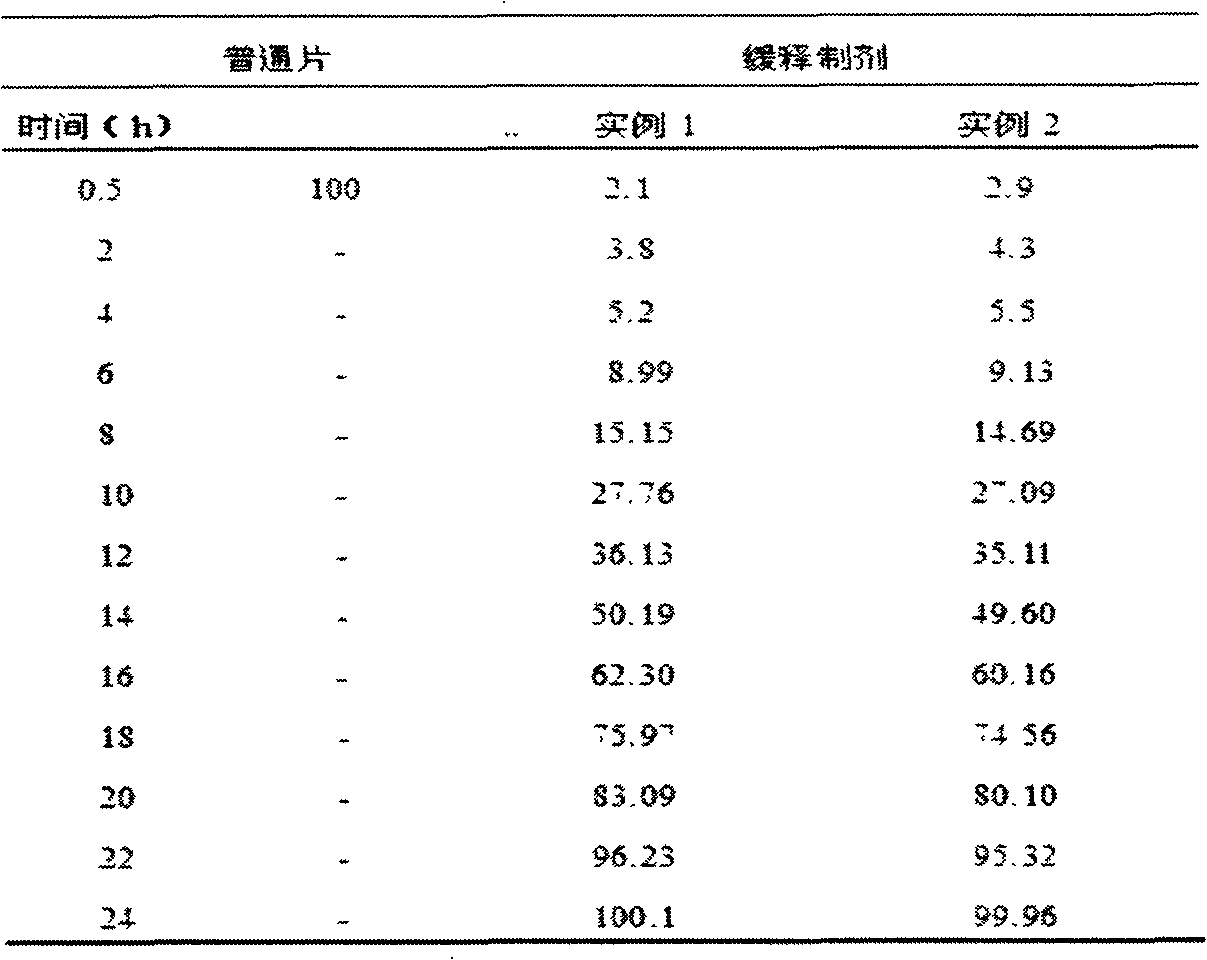
Memantine hydrochloride capsule sustained-release preparation and preparation method for same
InactiveCN102552218ALarge distribution areaImprove bioavailabilityNervous disorderPharmaceutical delivery mechanismControl layerSide effect
The invention discloses a memantine hydrochloride capsule sustained-release preparation, wherein the preparation is composed of two parts, namely, immediate-release grains and sustained-release grains; the sustained-release part is composed of a blank pellet core, a medicine layer and a release control layer; and the immediate-release part is composed of a blank pellet core and a medicine layer. The capsule is uniform in content, good in release effect, stable in blood concentration, good for reducing the toxic and side effects of medicines, and is capable of being used for treating moderate-to-severe Alzheimer-type dementia, and the medicine-taking times of the patient are reduced,.
Owner:无锡万全医药技术有限公司
Date seed-based multi-modal particulate admixture for moderate to severe loss control
A date palm seed lost circulation material (LCM) is provided having a date palm seed admixture of date palm seed particles of various sizes. The date palm seed particles may have a size greater than about 2380 microns in a range of about 40% to about 42% by weight, particles having a size greater than about 595 microns and less than about 2381 microns in a range of about 46% to about 48% by weight, particles having a size greater than about 400 microns and less than about 596 microns in a range of about 4% to about 6% by weight, particles having a size less than about 210 microns in a range of about 4% to about 6% by weight, and particles having a size less than about 149 microns in a range of about 1% to about 3% by weight. Methods of lost circulation control using and manufacture of a date palm seed LCM are also provided.
Owner:SAUDI ARABIAN OIL CO
Injectable implant and preparation method thereof
ActiveCN104055795AKeep buildSuppress generationSenses disorderPeptide/protein ingredientsPhosphateActive component
The invention relates to an injectable implant and a preparation method thereof. The implant is amnion microparticles mixed in a sodium chloride phosphate physiological solution with a pH of 7.0-7.5. The preparation method comprises the following steps of disinfection processing, decellularization processing, modification processing, and amnion microparticle preparation, and in particular, comprises: taking amnion microparticles as a component A and a sodium chloride phosphate physiological solution as a component B, performing vibration and well mixing according to a mass volume ratio of 20:1-100:1 to prepare an amnion microparticle suspension with a concentration of 20-100 mg / mL by high-speed microjet equipment, wherein the particle size of the amnion microparticles is 50-200 microns. The injectable implant of the invention maintains the natural three dimensional stereo structure and a lot of natural active components of human amnion, reduces immunological rejection reaction caused by raw material source after clinical application of cosmetic injection products, is suitable for injection into deep subcutaneous dermal layer to subcutaneous superficial layer to repair moderate to severe wrinkles or folds, and has good cosmetic repair effect.
Owner:SHAANXI RUISHENG BIOTECH
Systems and methods for analyzing and assessing dementia and dementia -type disorders
Embodiments of the invention can provide systems and methods for anaiyzing and assessing dementia and dementia-type disorders by integrating the use of electroencephalography (EEG), neuropsychological or cognitive testing data, and cardiovascular risk factor data. Embodiments of the invention can provide systems and methods for early detection of dementia, including Alzheimer's disease (AD), vascular dementia (VAD), mixed dementia (AD and VAD), MCI, and other dementia-type disorders. Embodiments of the invention can provide some or all of the following improvements over conventional systems and methods, including: (1) Increased sensitivity, specificity, and overall accuracy; (2) Detection of AD, VAD and mixed dementia; and (3) Accurate detection of mild dementia and some cases of mild cognitive impairment in addition to the detection of moderate to severe dementia.
Owner:LEXICOR MEDICAL TECH
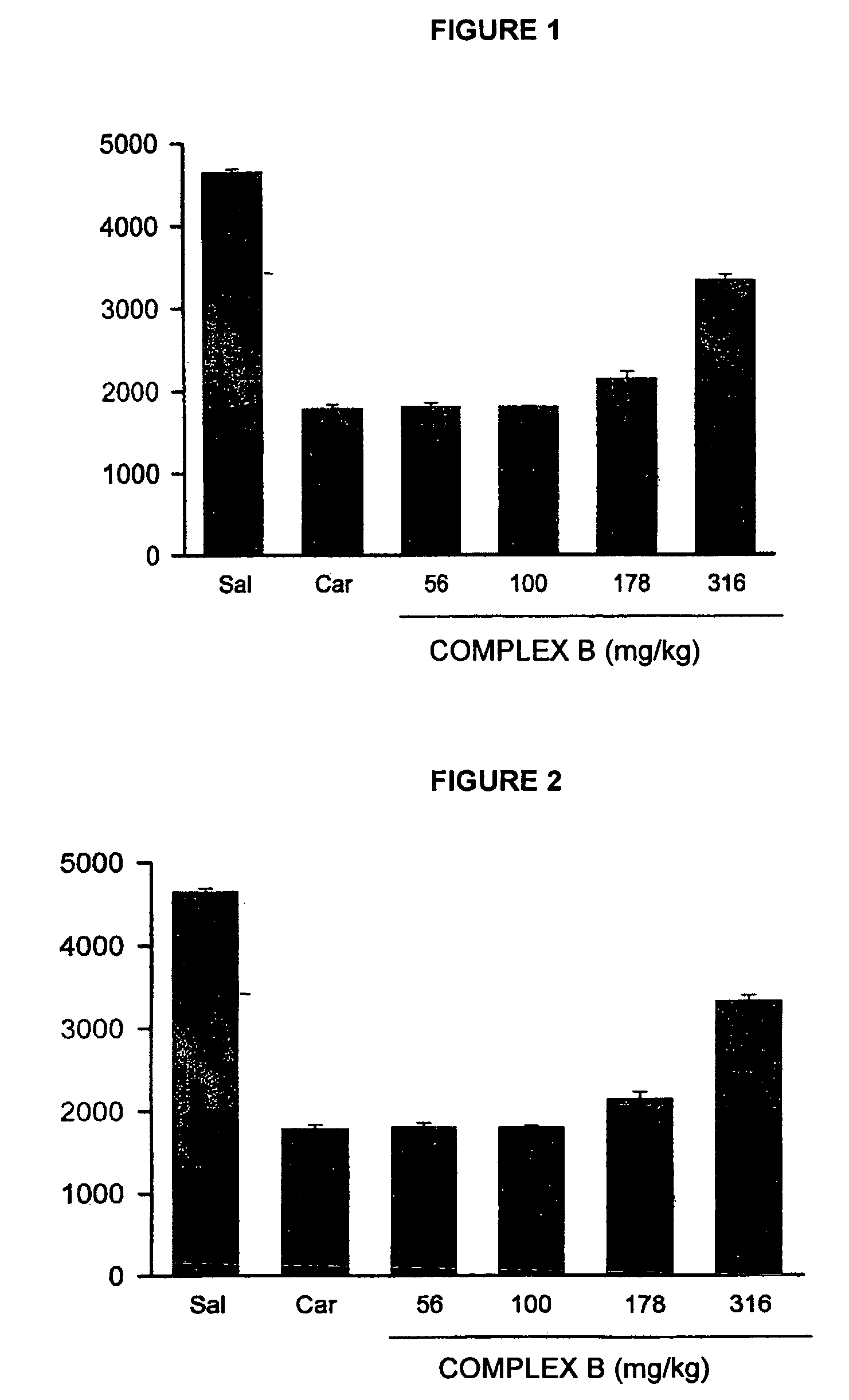
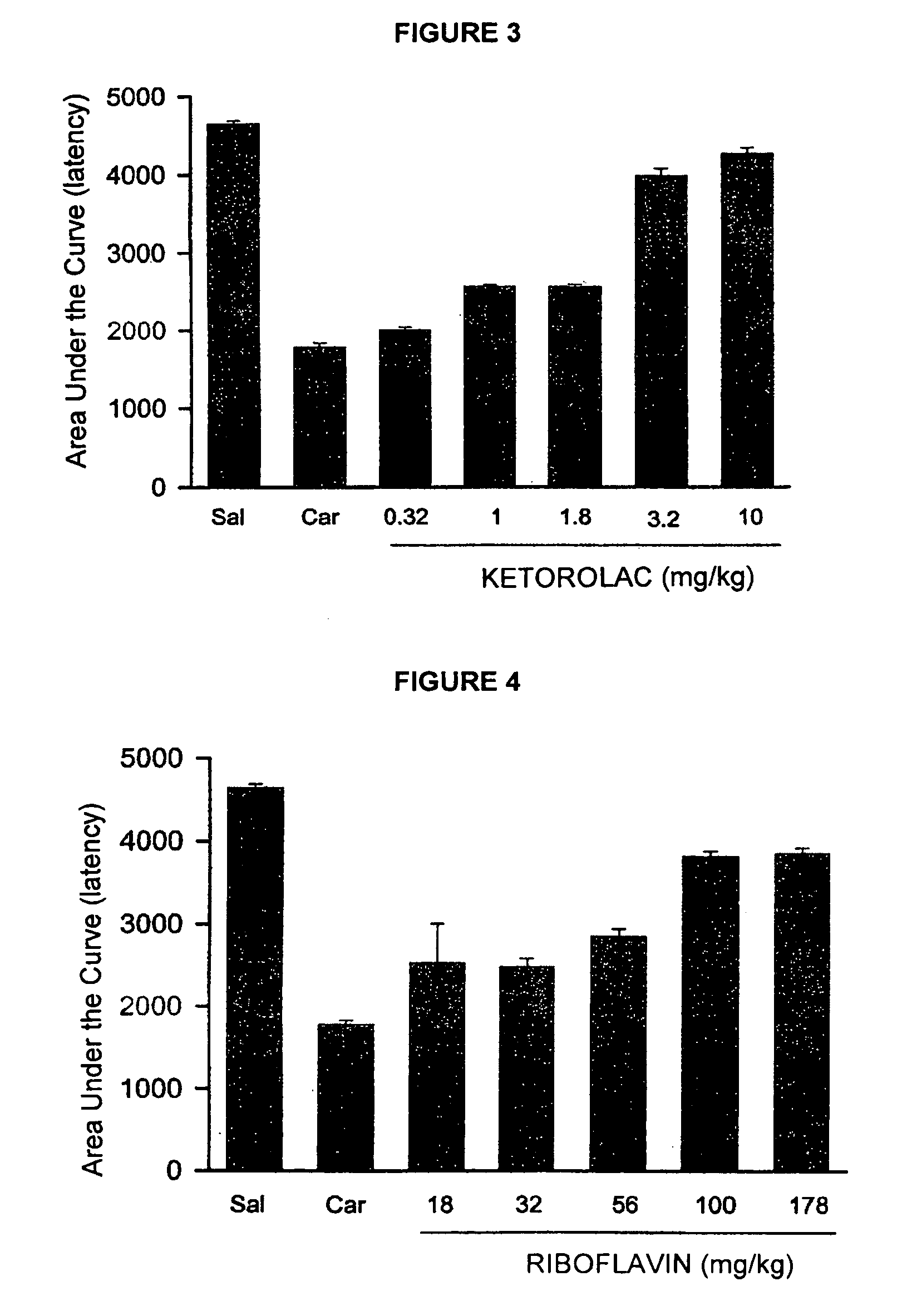
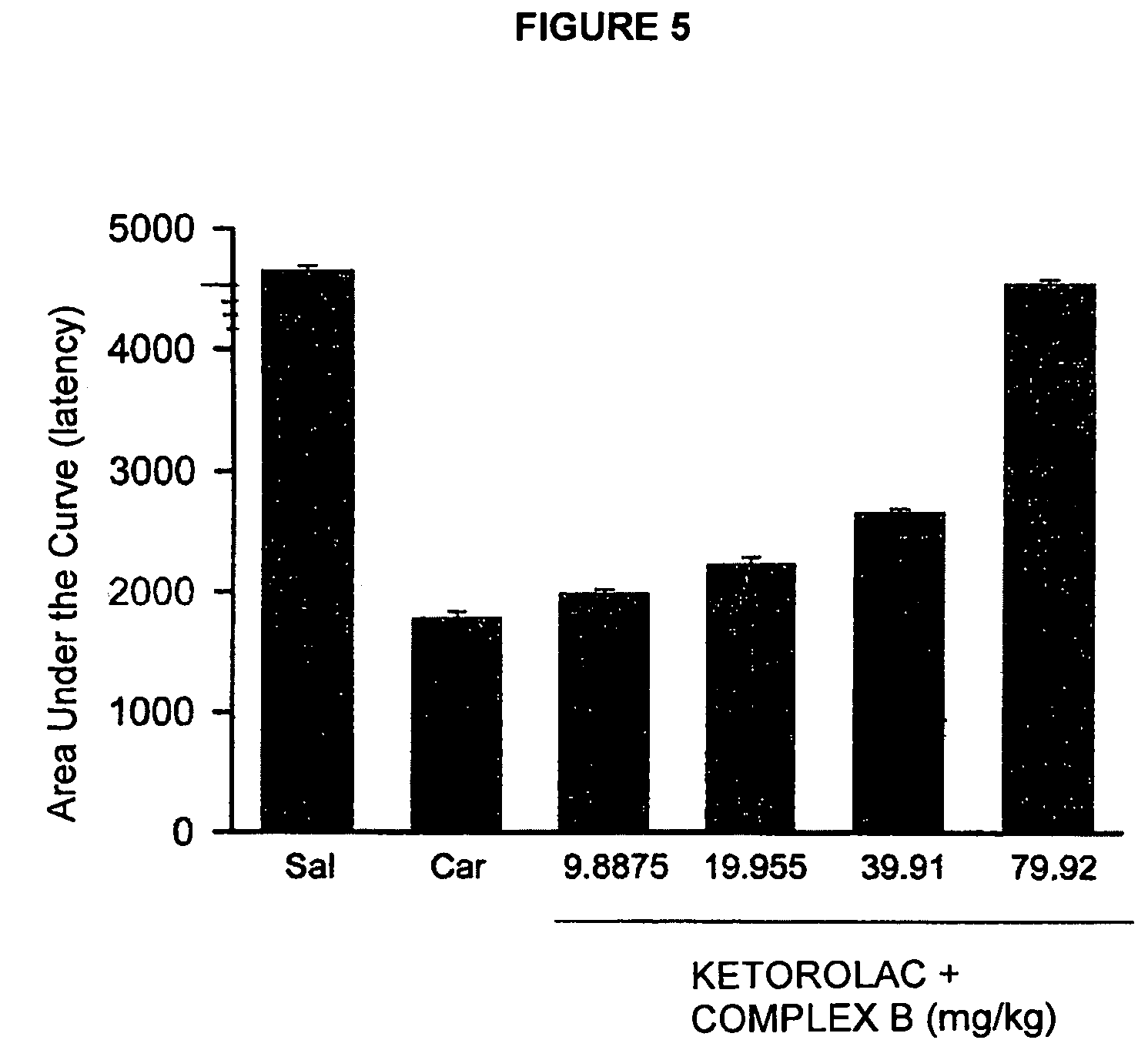
Pharmaceutical Composition Comprising the Combination of a Ketorolac Salt and Vitamins of the-B-Complex for the Treatment of Neuralgia
This invention refers to the pharmaceutical combinations of Ketorolac salts and B-complex; to the methods used to make said combinations; and particularly, to ketorolac and B-complex synergic combinations useful in the treatment of patients that suffer from moderate to severe pain and neuralgias in different body sites.
Owner:LAB SENOSIAIN DE C V
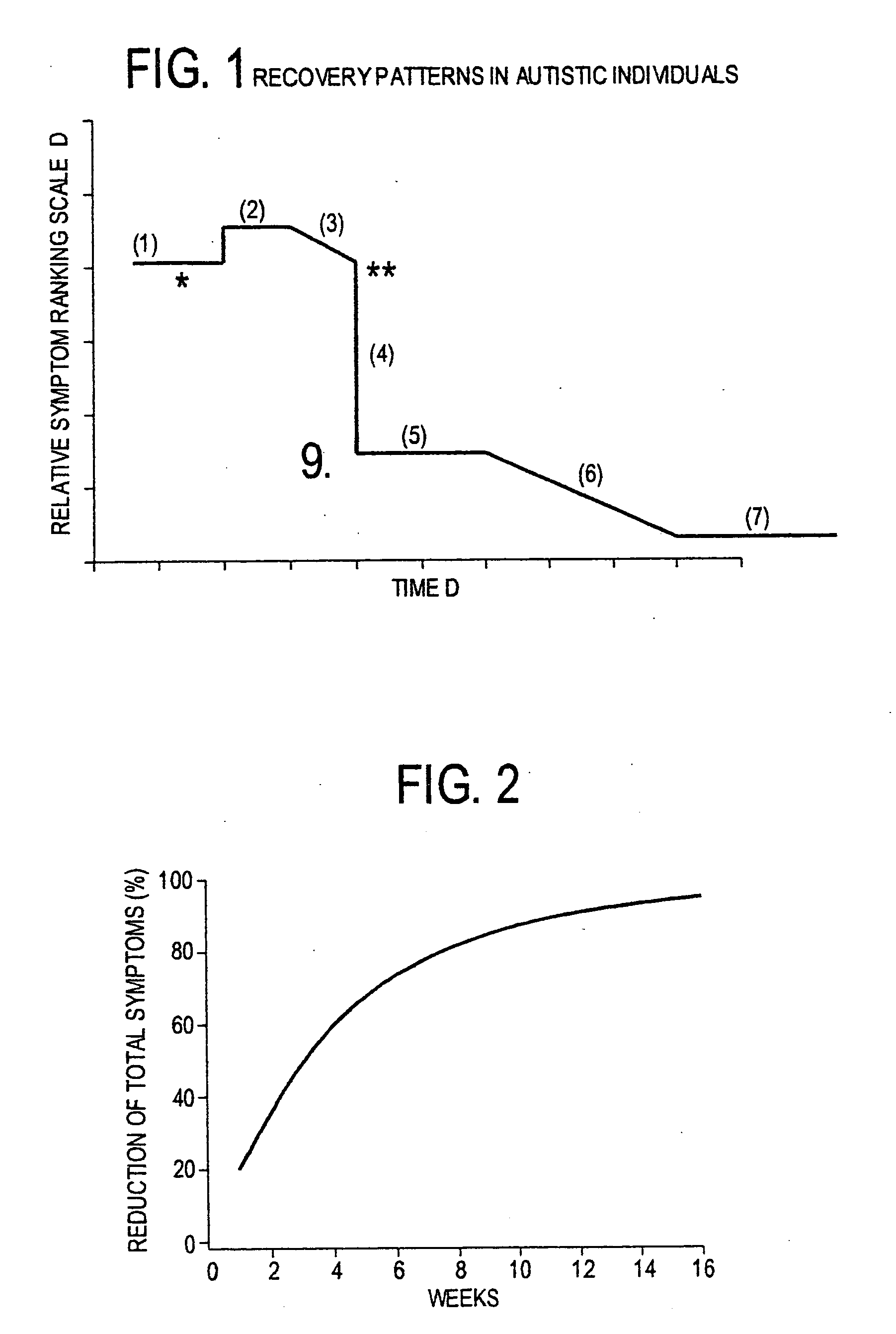
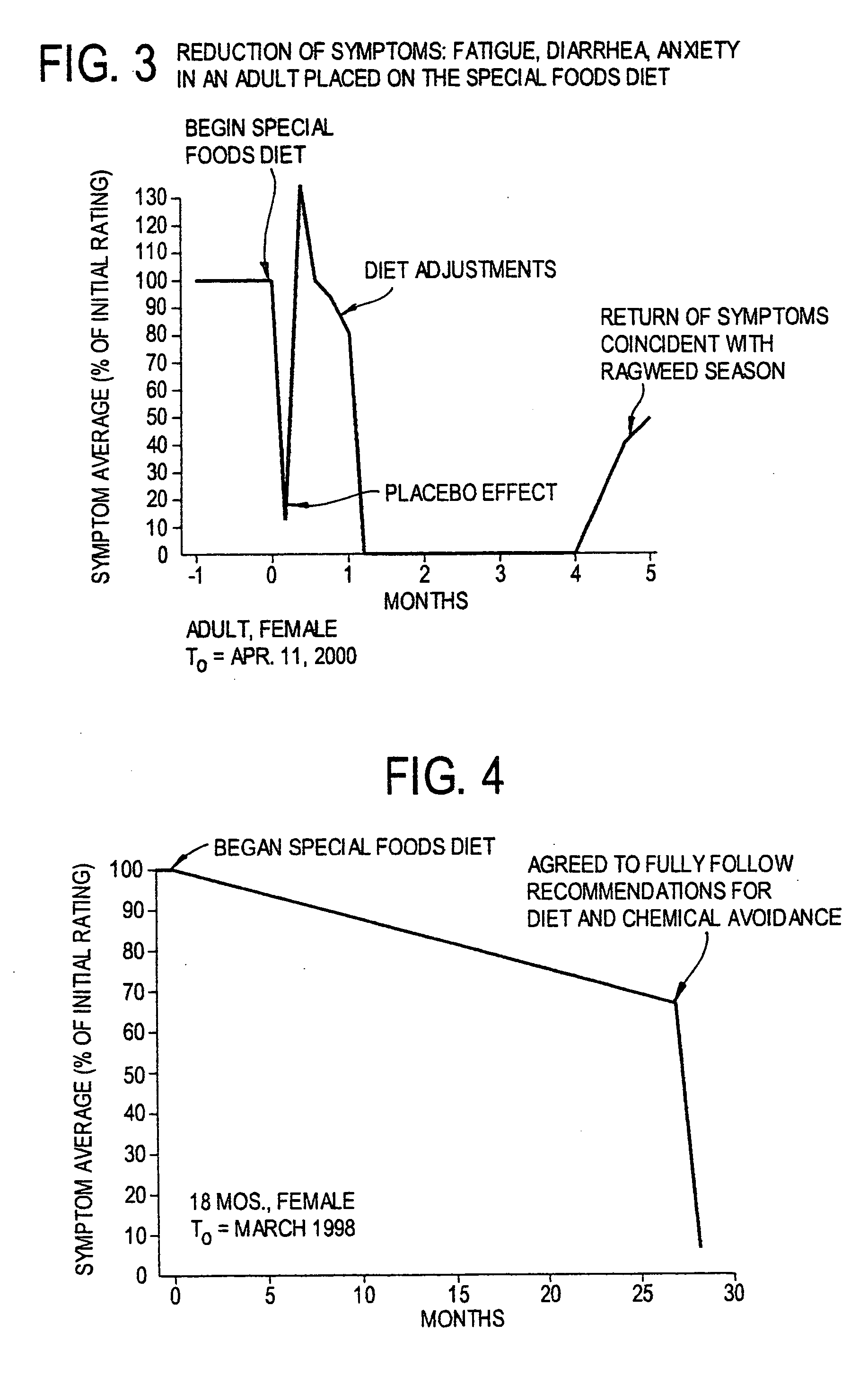
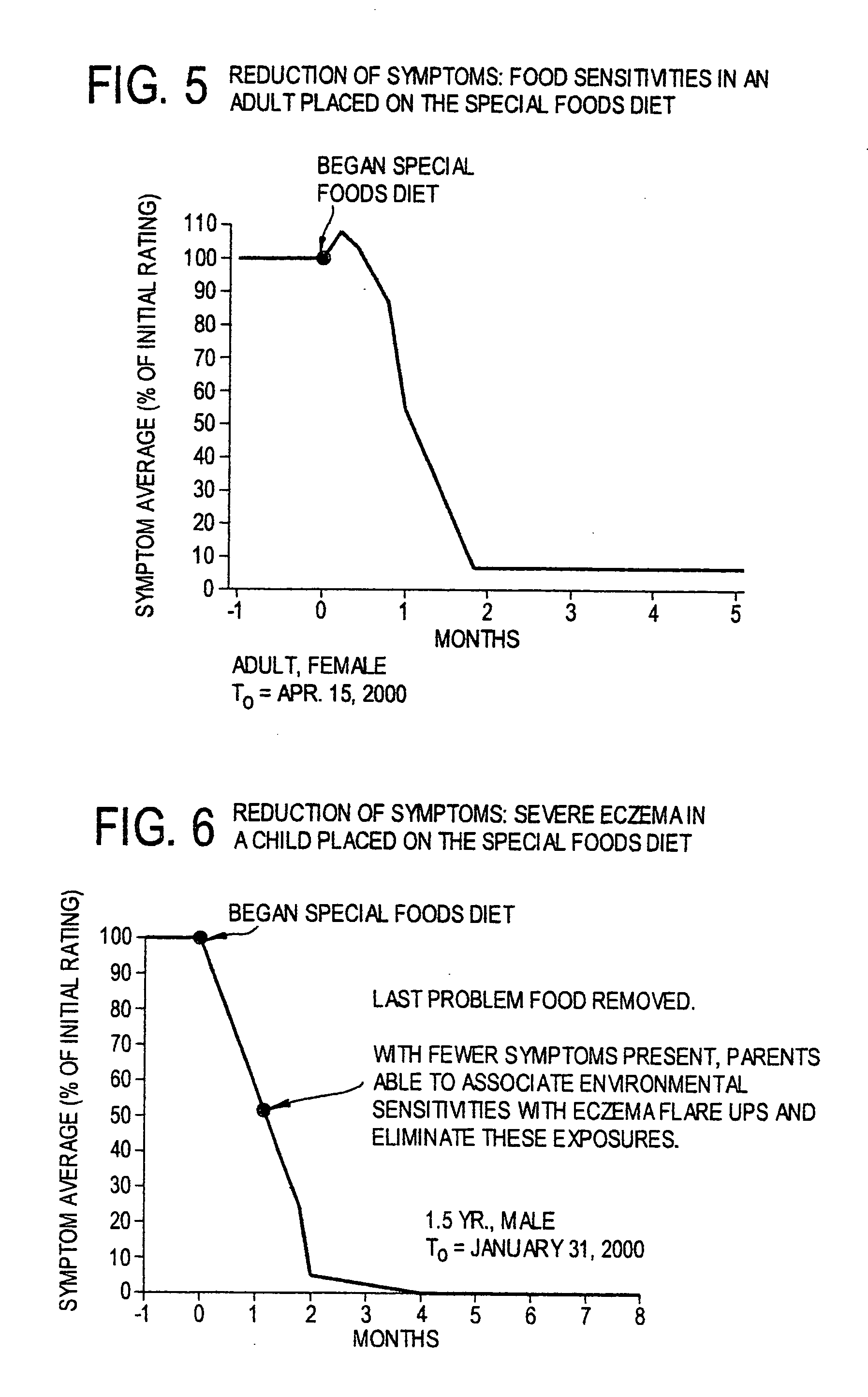
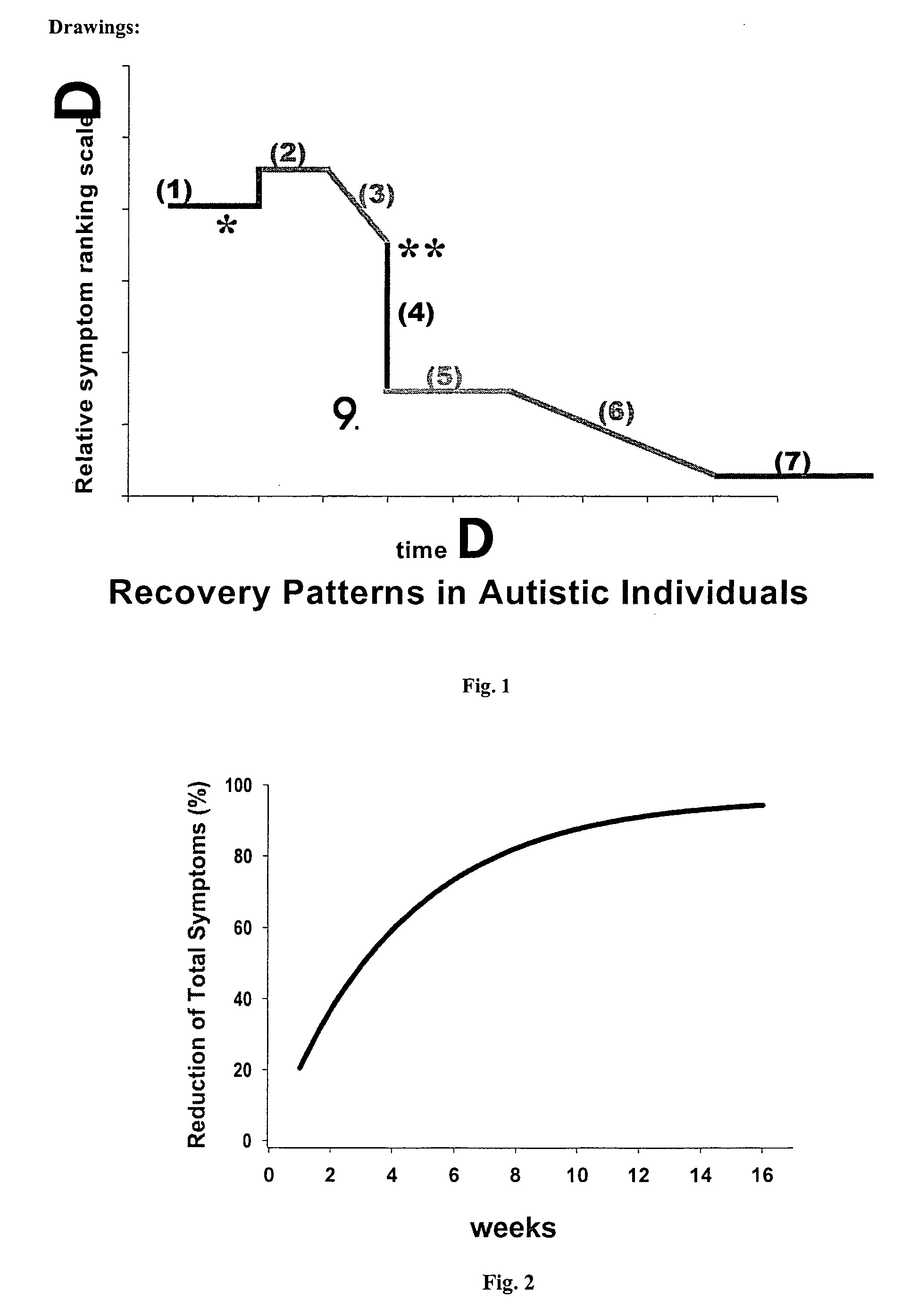
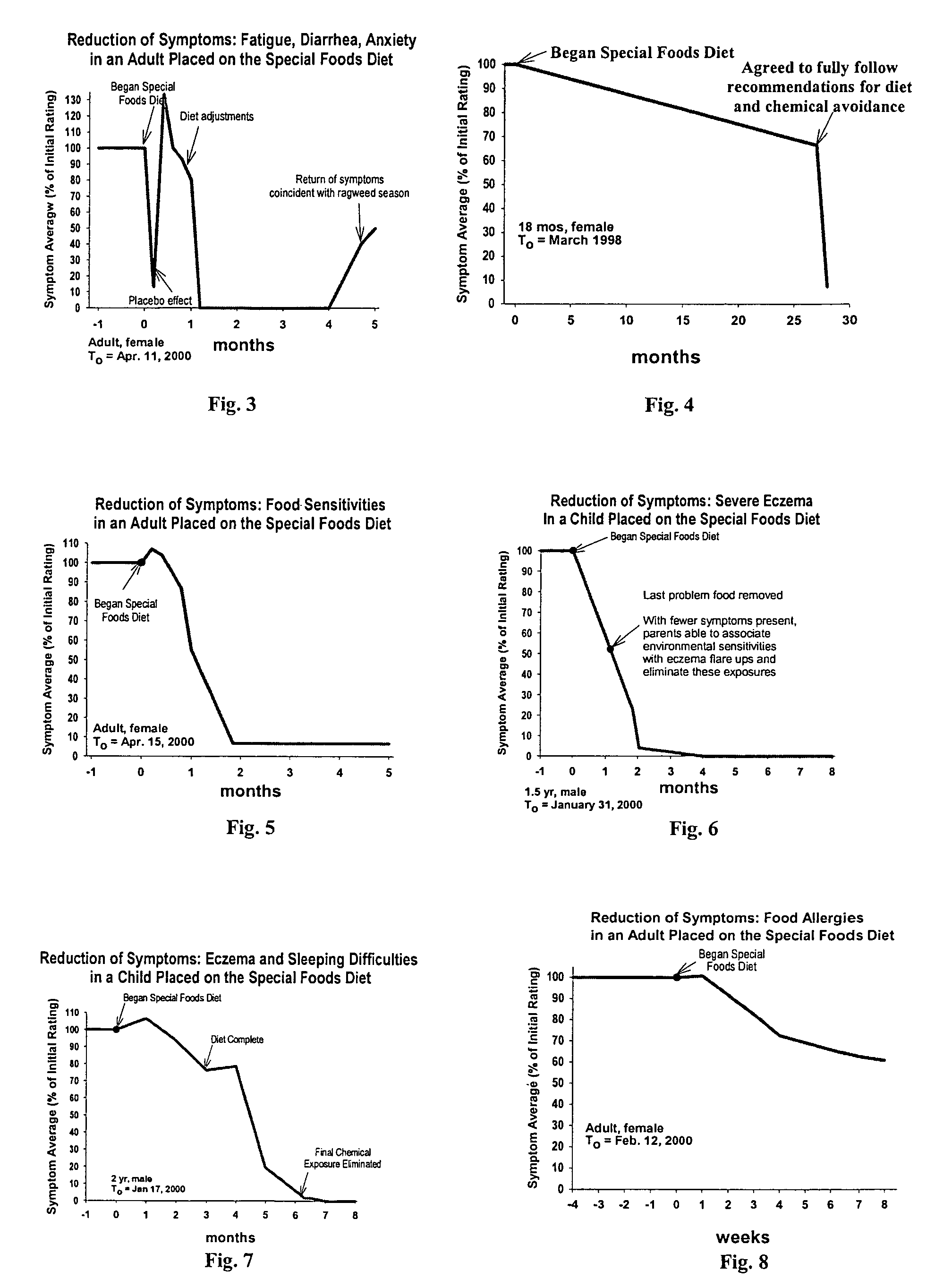
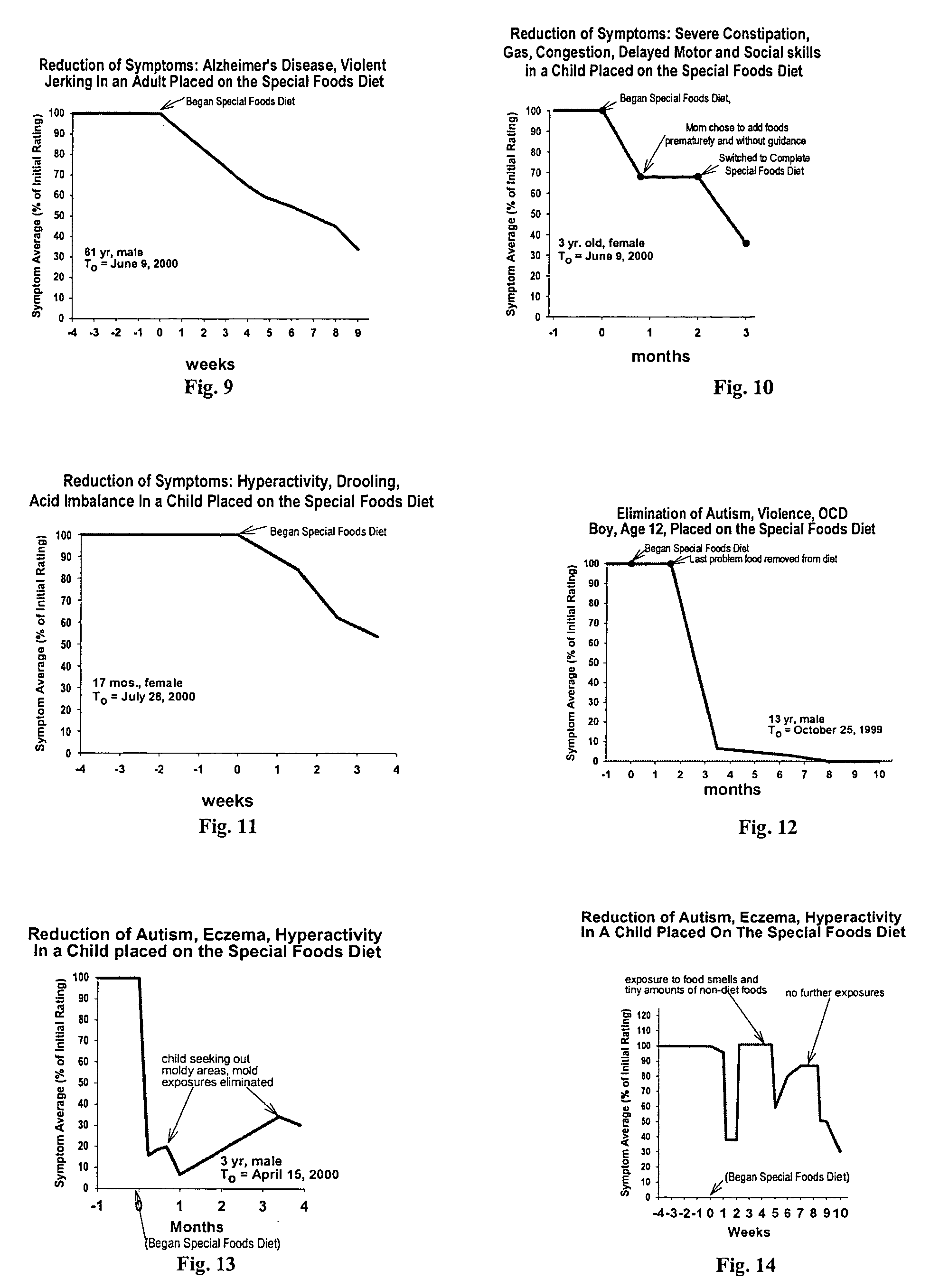
Use of tropical root crops in effective intervention strategies for treating difficult and complex cases and chronic diseases
This invention relates to an effective dietary intervention plan. In one aspect all food is withheld for a period of at least 5 days, except for tropical root crops. In another aspect the invention relates to the treatment of various symptoms, conditions or diseases such as Diarrhea, constipation, congestion, eczema, asthma, fatigue, muscle weakness, tension, and spasms, irritable bowel syndrome, swelling, anxiety, multiple chemical sensitivities, moderate to extensive and moderate to severe symptoms due to food allergies, sensitivities, and intolerances, bloating, pain, headaches, leaky gut, hyperactivity, sleeping difficulties, severe underweight, eating disorders, obsessive, compulsive disorders, panic attacks, sensory sensitivities, Alzheimer's disease, acid reflux, irritability, delayed motor skills, delayed social skills, autism, PDD, infantile spasms, seizures by withholding from the patient for a period of at least 5 days all food except for concentrated forms of concentrated tropical root crops. Preferably the patient is also removed from external environmental sources of allergens. After the initial withholding period new foods may be introduced according to a particular selection and schedule. In another aspect of the invention the subject undergoes an effective dietary intervention plan in which at least five (5) tropical root crops are selected, each eaten on a successive day, along with selected other meat, vegetables, and oils that the subject has never eaten before, eating a different selection of meat, vegetables, and oils each from different food families each day, with no food or food family being repeated for at least 5 days. In another aspect the invention relates to the treatment of various symptoms, conditions or diseases such as Diarrhea, constipation, congestion, eczema, asthma, fatigue, muscle weakness, tension, and spasms, irritable bowel syndrome, swelling, anxiety, multiple chemical sensitivities, moderate to extensive and moderate to severe symptoms due to food allergies, sensitivities, and intolerances, bloating, pain, headaches, leaky gut, hyperactivity, sleeping difficulties, severe underweight, eating disorders, obsessive, compulsive disorders, panic attacks, sensory sensitivities, Alzheimer's disease, acid reflux, irritability, delayed motor skills, delayed social skills, autism, PDD, infantile spasms, seizures by withholding from the patient for a period of at least 5 days all food except for concentrated forms of concentrated tropical root crops. Preferably the patient is also removed from external environmental sources of allergens. After the initial withholding period new foods may be introduced according to a particular selection and schedule. In another aspect of the invention the subject undergoes an effective dietary intervention plan in which at least seven (7) tropical root crops are selected, each eaten on a successive day, along with selected other meat, vegetables, and oils that the subject has never eaten before, eating a different selection of meat, vegetables, and oils each from different food families each day, with no food or food family being repeated for at least 7 days. In another aspect the invention relates to the treatment of various symptoms, conditions or diseases such as Diarrhea, constipation, congestion, eczema, asthma, fatigue, muscle weakness, tension, and spasms, irritable bowel syndrome, swelling, anxiety, multiple chemical sensitivities, moderate to extensive and moderate to severe symptoms due to food allergies, sensitivities, and intolerances, bloating, pain, headaches, leaky gut, hyperactivity, sleeping difficulties, severe underweight, eating disorders, obsessive, compulsive disorders, panic attacks, sensory sensitivities, Alzheimer's disease, acid reflux, irritability, delayed motor skills, delayed social skills, autism, PDD, infantile spasms, seizures by withholding from the patient for a period of at least 5 days all food except for concentrated forms of concentrated tropical root crops. Preferably the patient is also remo
Owner:SLIMAK K M
Hemostatic Devices with Improved Properties and Methods of Making Same
ActiveUS20160121019A1Effective maintenanceProviding such maintenanceBiocideOrganic active ingredientsMedicineOxidized regenerated cellulose
Hemostatic devices and methods of making same are disclosed. Disclosed hemostatic devices include biocompatible non-oxidized regenerated cellulose. The disclosed hemostatic devices are effective in providing and maintaining hemostasis in cases of moderate to severe bleeding caused by non-compressional and / or non-tourniquetable injuries, among other things. The disclosed methods enable manufacture of a bioabsorbable, biocompatible, biodegradable carboxylmethyl cellulose having high stability and high adherence.
Owner:CORE SCI CREATIONS
Method and composition for the treatment of moderate to severe keratoconjunctivitis sicca
InactiveUS20120141410A1Slow changeProtect drySenses disorderPeptide/protein ingredientsKCS - Keratoconjunctivitis siccaModerate to severe
Embodiments of the invention relate to compositions and methods of dry eye or keratoconjunctivitis sicca.
Owner:INVITRX
Methods for treating atopic dermatitis by administering an il-4r inhibitor
ActiveUS20190345253A1High unmet medical needTreatment safetyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSevere atopic dermatitisAD - Atopic dermatitis
The present invention provides methods for treating moderate-to-severe or severe atopic dermatitis (AD). The methods of the present invention comprise administering to a subject in need thereof one or more doses of an interleukin-4 receptor (IL-4R) inhibitor such as an anti-IL-4R antibody.
Owner:REGENERON PHARM INC
Use of tropical root crops in dietary intervention strategies
InactiveUS7854948B2Nourish wellLarge populationBiocidePharmaceutical delivery mechanismCompulsive disordersPanic
This invention relates to an effective dietary intervention plan. In one aspect all food is withheld for a period of at least 5 days, except for tropical root crops. In another aspect the invention relates to the treatment of various symptoms, conditions or diseases such as Diarrhea, constipation, congestion, eczema, asthma, fatigue, muscle weakness, tension, and spasms, irritable bowel syndrome, swelling, anxiety, multiple chemical sensitivities, moderate to extensive and moderate to severe symptoms due to food allergies, sensitivities, and intolerances, bloating, pain, headaches, leaky gut, hyperactivity, sleeping difficulties, severe underweight, eating disorders, obsessive, compulsive disorders, panic attacks, sensory sensitivities, Alzheimer's disease, acid reflux, irritability, delayed motor skills, delayed social skills, autism, PDD, infantile spasms, seizures by withholding from the patient for a period of at least 5 days all food except for concentrated forms of concentrated tropical root crops. Preferably the patient is also removed from external environmental sources of allergens. After the initial withholding period new foods may be introduced according to a particular selection and schedule.
Owner:SLIMAK K M
Ketorolac tromethamine freezing-dried power injection and preparation method thereof
ActiveCN101167722AOvercoming low bioavailabilityOvercome curative effectOrganic active ingredientsPowder deliveryVisceral painAnalgesics effects
Ketorolac tromethamine is a new type of non-steroidal analgesic and anti-inflammatory drug with strong analgesic effect, which can be administered orally or by injection. It is mainly used to relieve moderate to severe postoperative pain, and Acute renal colic related to trauma, visceral pain related to cancer, anti-inflammatory of local inflammation, etc., the present invention provides a preparation method of ketorolac tromethamine freeze-dried powder preparation, which greatly improves the stability of the main drug, It not only overcomes the shortcoming of slow onset of oral administration, but more importantly, overcomes the problems of unstable main drug of liquid injection and unstable process in actual production.
Owner:LUNAN PHARMA GROUP CORPORATION
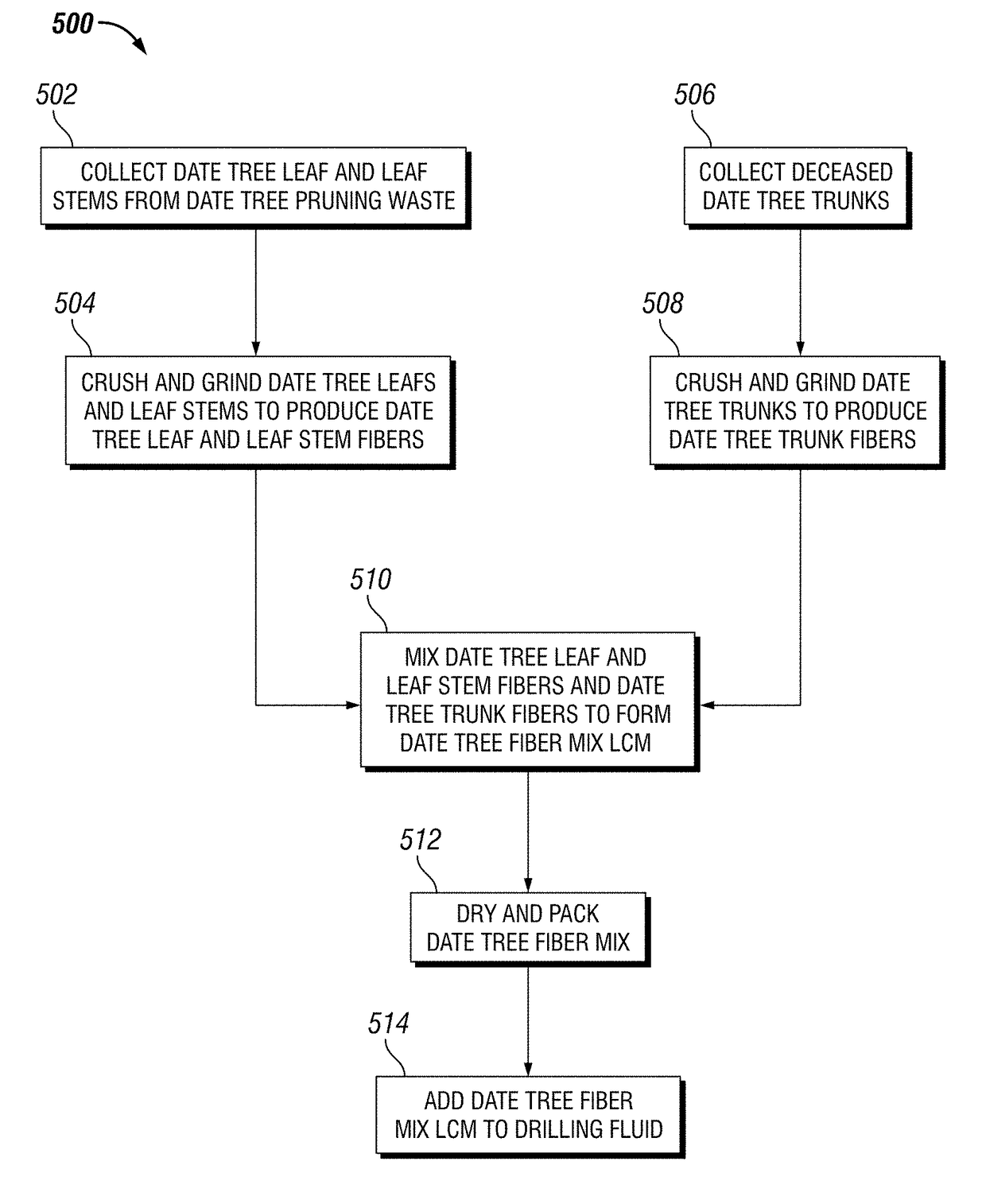
Date Tree Waste-Based Binary Fibrous Mix for Moderate to Severe Loss Control
A date tree fiber mix lost circulation material (LCM) is provided. The date tree fiber mix LCM may include includes date tree trunk fibers produced from date tree trunks and date tree leaf and leaf stem fibers produced from date tree leaves and leaf stems. The LCM include a mix of 30% by weight date tree trunk fibers and 70% date tree leaf and leaf stem fibers, 40% by weight date tree trunk fibers and 60% date tree leaf and leaf stem fibers, 50% by weight date tree trunk fibers and 50% date tree leaf and leaf stem fibers. Methods of lost circulation control using and manufacture of a date tree fiber mix LCM are also provided
Owner:SAUDI ARABIAN OIL CO
Topical pain reliever
Analgesic formulations to be applied topically that are reported to relieve moderate to severe pain due to tendonitis, fibromyalgia, shingles, insect bites and bee stings, ACL, arthritis, and sinus headaches. In many cases, test subjects report immediate pain relief with a single application and complete pain elimination with only a few applications. The formulations are in a coconut oil base which is skin friendly. The primary active ingredient is wrightia tinctoria, which when combined with other ingredients, causes the formulation to exhibit a synergistic effect on pain relief unexpected from using the ingredients alone or in combination.
Owner:APPTEC
Oxycodone controlled release tablets
InactiveCN101467977ASimple methodImprove stabilityOrganic active ingredientsNervous disorderControlled Release TabletDosage form
The invention belongs to the technical field of medicament, disclosing an oxycodone controlled release tablet for easing durative moderate to severe pain. The characteristic of the novel formulation is that the invention is controlled release tablet which slowly and constantly or nearly constantly release medicament in water or determined releasing substrate.
Owner:BEIJING HOPE HUGE PHARM SCI
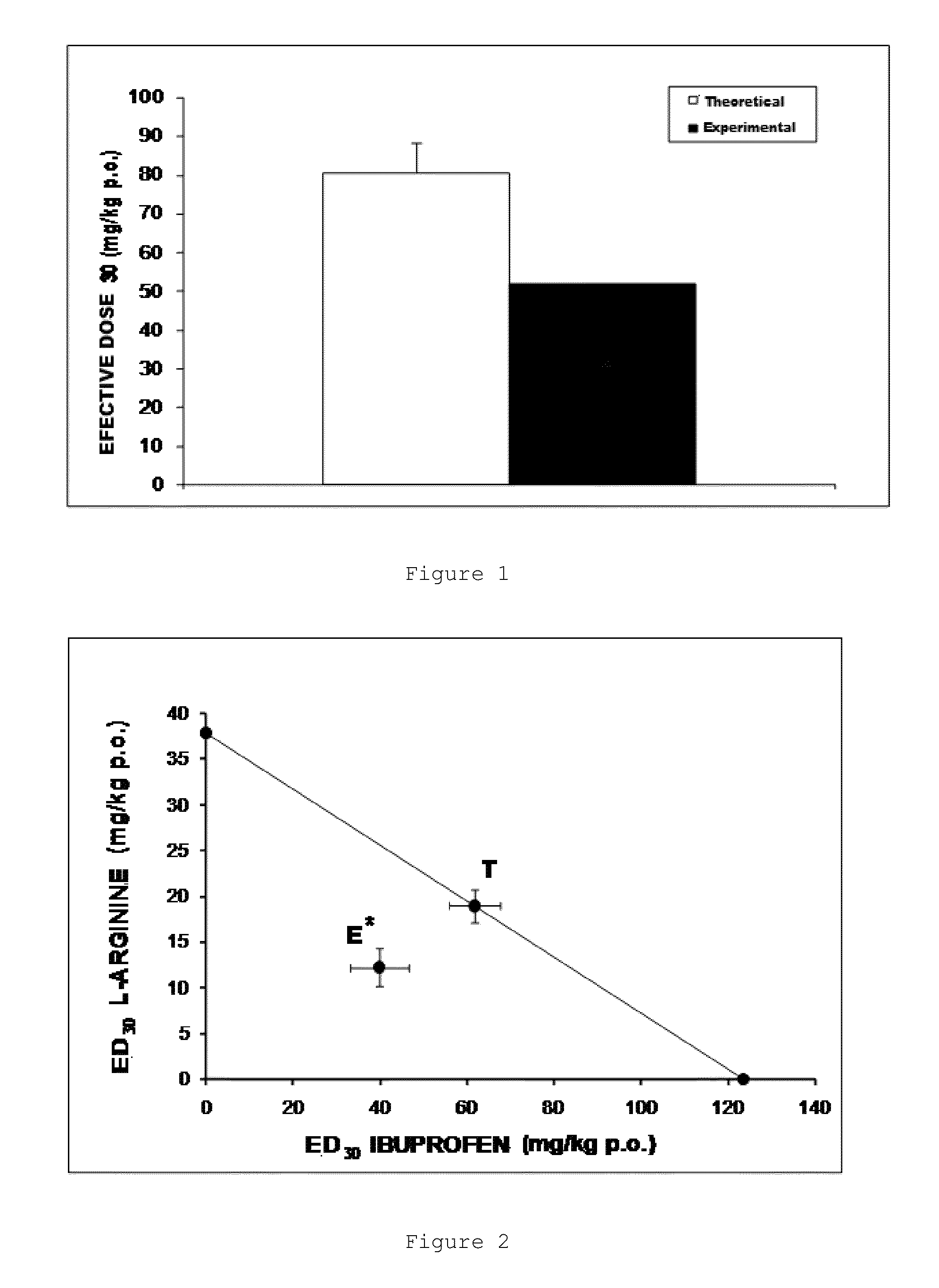
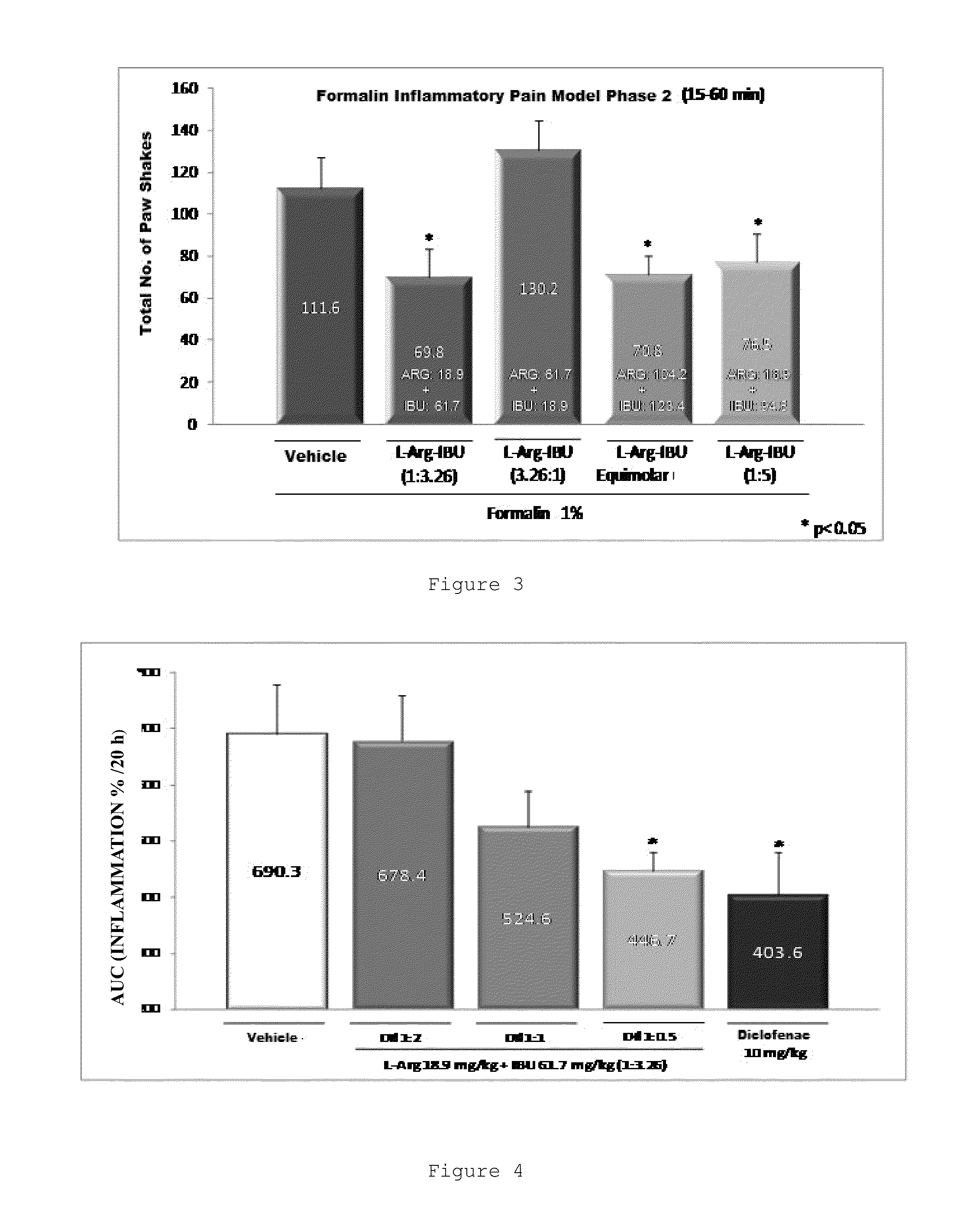
Combination of an nsaid and an amino acid
ActiveUS20130109754A1Maintain therapeutic effectReduce the amount requiredBiocideOrganic active ingredientsArginineTreatment fever
The invention relates to an oral pharmaceutical composition comprising the combination of an ibuprofen salt or S(+)-ibuprofen with L-arginine and / or the pharmaceutically acceptable salts thereof, and additionally pharmaceutically acceptable vehicles and / or excipients. The invention also relates to the method of producing the composition and to the use of said composition having synergic therapeutic activity, for treating moderate to severe inflammatory pain, fever and inflammation.
Owner:LAB SENOSIAIN DE C V
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com