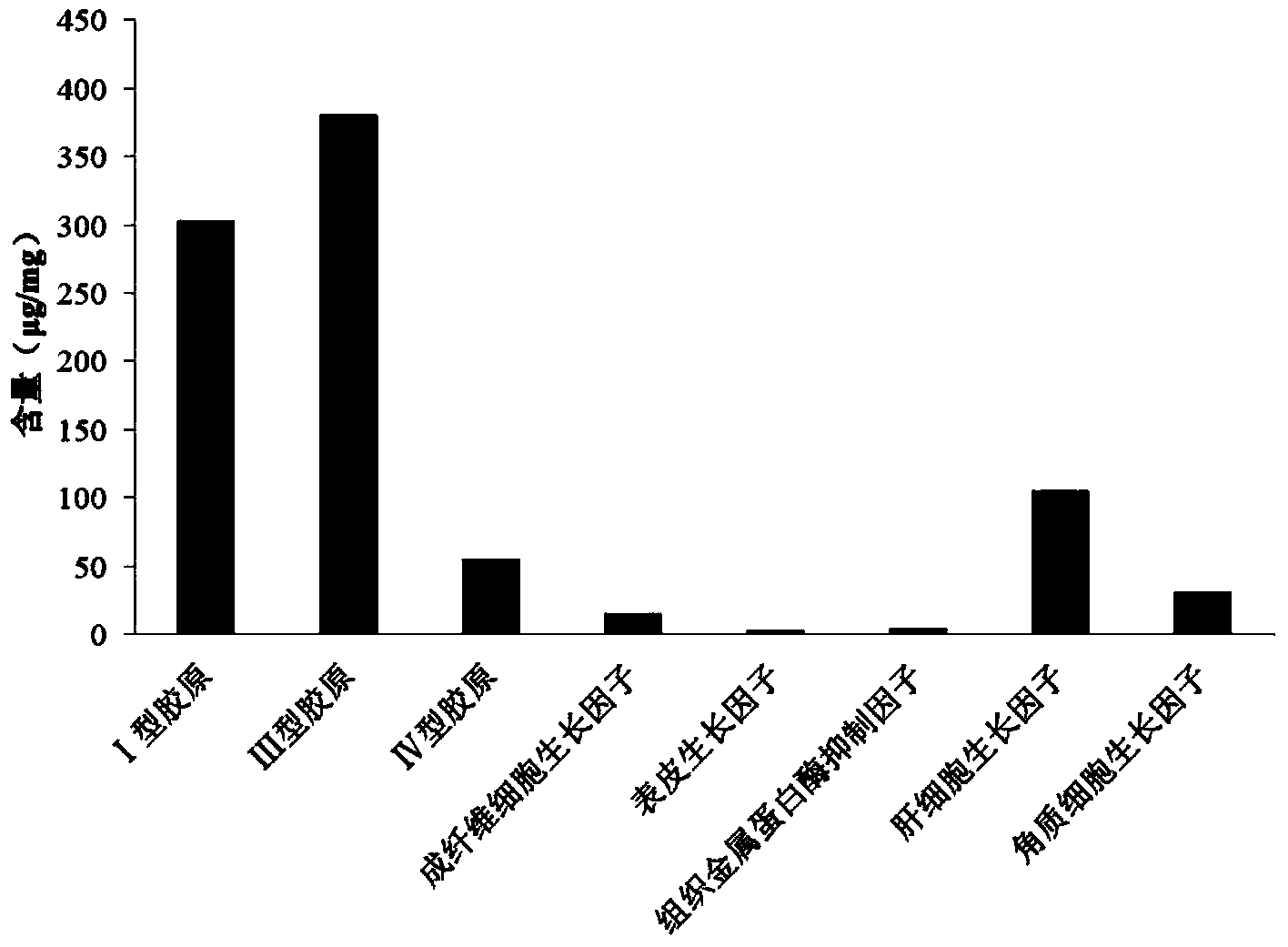Injectable implant and preparation method thereof
A technology for injecting implants and implants, applied in anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., can solve delayed allergic reactions, no patents and literature reports, destroy the original structure of animal tissues and Other ingredients and other issues, to achieve good histocompatibility, good cell compatibility, good three-dimensional repair effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Step 1. Disinfection treatment: Under aseptic conditions, soak the amniotic membrane in PBS until it is free of blood color and dirt, then wash it once with purified water, and then disinfect the amniotic membrane with 75% ethanol aqueous solution for 30 minutes minute;
[0075] Step 2, decellularization treatment: wash the amniotic membrane sterilized in step 1 with purified water for 3 times, then oscillate the amniotic membrane with 0.05mol / L sodium hydroxide aqueous solution for 30 minutes, the oscillation frequency is 50rpm, and finally use purified water Wash 3 times;
[0076] Step 3, modification treatment: the amniotic membrane obtained in step 2 was treated with 0.1 mol / L 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride in an aqueous solution with a pH of 5 at 2° C. Under treatment for 6 hours;
[0077] Step 4. Preparation of amniotic membrane microparticles: Wash the amniotic membrane modified in step 3 with purified water for 3 times, then vacuum f...
Embodiment 2
[0081] Step 1. Disinfection treatment: Under aseptic conditions, soak the amniotic membrane in PBS until it is free of blood color and dirt, then wash it with purified water for 5 times, and then disinfect the amniotic membrane with 75% ethanol aqueous solution for 15 minutes , and then disinfect the amnion with 0.1% peracetic acid for 15 minutes;
[0082] Step 2, decellularization treatment: wash the amnion after the disinfection treatment in step 1 with purified water once, and then use 0.01mol / L sodium hydroxide aqueous solution to shake the amnion for 10 minutes at a frequency of 80rpm, and then use 3mol / L sodium hydroxide solution to shake the amnion for 10 minutes. 1. L sodium chloride aqueous solution was shaken for 100 minutes, and finally cleaned 10 times with purified water;
[0083] Step 3, modification treatment: the amniotic membrane obtained in step 2 was treated with 0.5 mg / mL ribose aqueous solution at 2°C for 3 days;
[0084] Step 4. Preparation of amniotic m...
Embodiment 3
[0088] Step 1. Disinfection treatment: Under aseptic conditions, soak the amniotic membrane in PBS until it is free of blood color and dirt, then wash it with purified water for 3 times, and then disinfect the amniotic membrane with 75% ethanol aqueous solution for 10 minutes ; Disinfect the amniotic membrane with 1% hydrogen peroxide solution for 10 minutes;
[0089] Step 2, decellularization treatment: first wash the sterilized amniotic membrane twice with purified water, then oscillate the amniotic membrane with 1 mg / mL EDTA aqueous solution for 60 minutes, and oscillate with 0.1 mg / mL trypsin aqueous solution for 10 minutes. Both are at 60rpm, and finally washed with purified water for 6 times;
[0090] Step 3, modification treatment: treating the amniotic membrane obtained in step 2 with an aqueous glutaraldehyde solution with a volume concentration of 1% at room temperature for 6 hours;
[0091] Step 4. Preparation of amniotic membrane microparticles: Wash the amniotic ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com