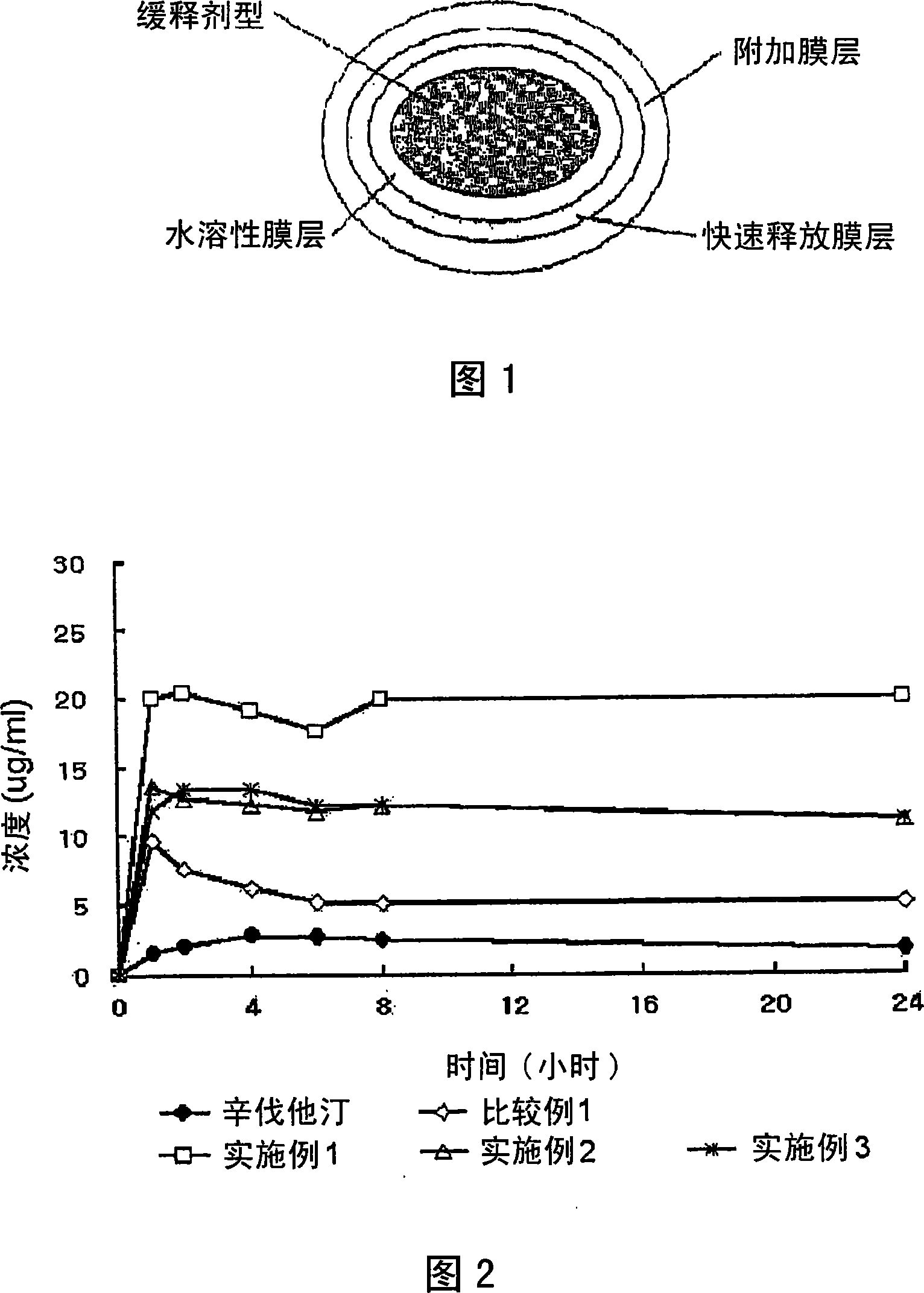
Complex formulation of 3-hydroxy-3-methyl glutaryl coa reductase inhibitor and antihypertensive agent, and process for preparing same
A reductase inhibitor and anti-hypertensive technology, applied in pill delivery, drug combination, pharmaceutical formula, etc., can solve the problems that the therapeutic effect cannot be maintained for a long time, liver toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
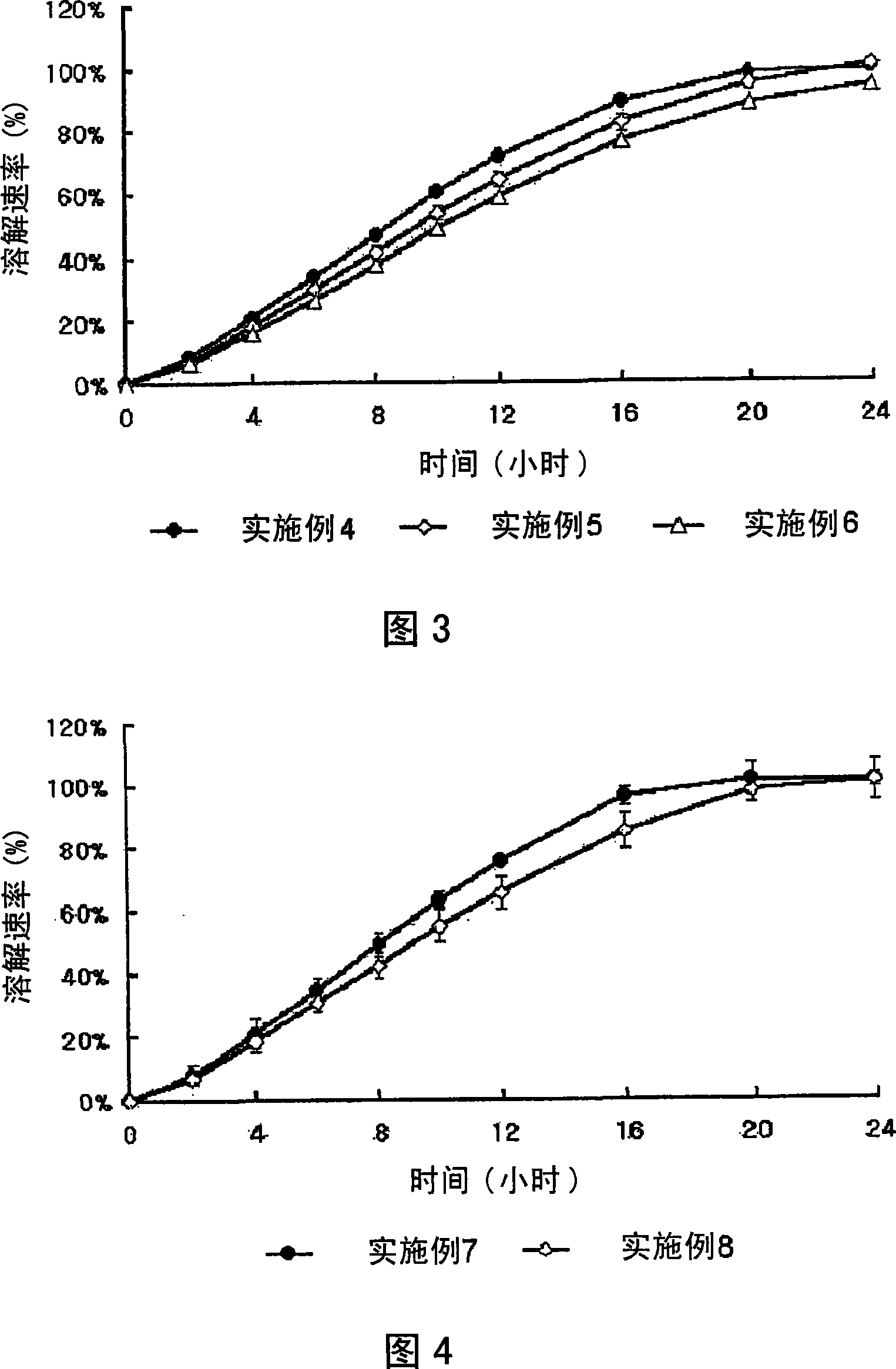
Embodiment 1 to 3 and comparative example 1
[0062] Examples 1 to 3 and Comparative Example 1: Preparation of Solid Dispersion
[0063]Simvastatin (Hanmi Fine Chemical Co., Ltd., Korea), MYRJ (ICI, USA), HPMC 2910 (viscosity: 3 to 15cps, Shin-Etsu, Japan), BHT (UENO Fine Chemical, USA) and light Quality anhydrous silicic acid (as a dispersant) was dissolved in a mixture of ethanol and dichloromethane according to the amounts described in Table 1, and each resulting mixture was spray-dried to obtain particles with an average particle size of 100 μm or less. solid dispersion. The solid dispersions of Examples 1 to 3 and Comparative Example 1 thus obtained are shown in Table 1.
[0064]
[0065] components
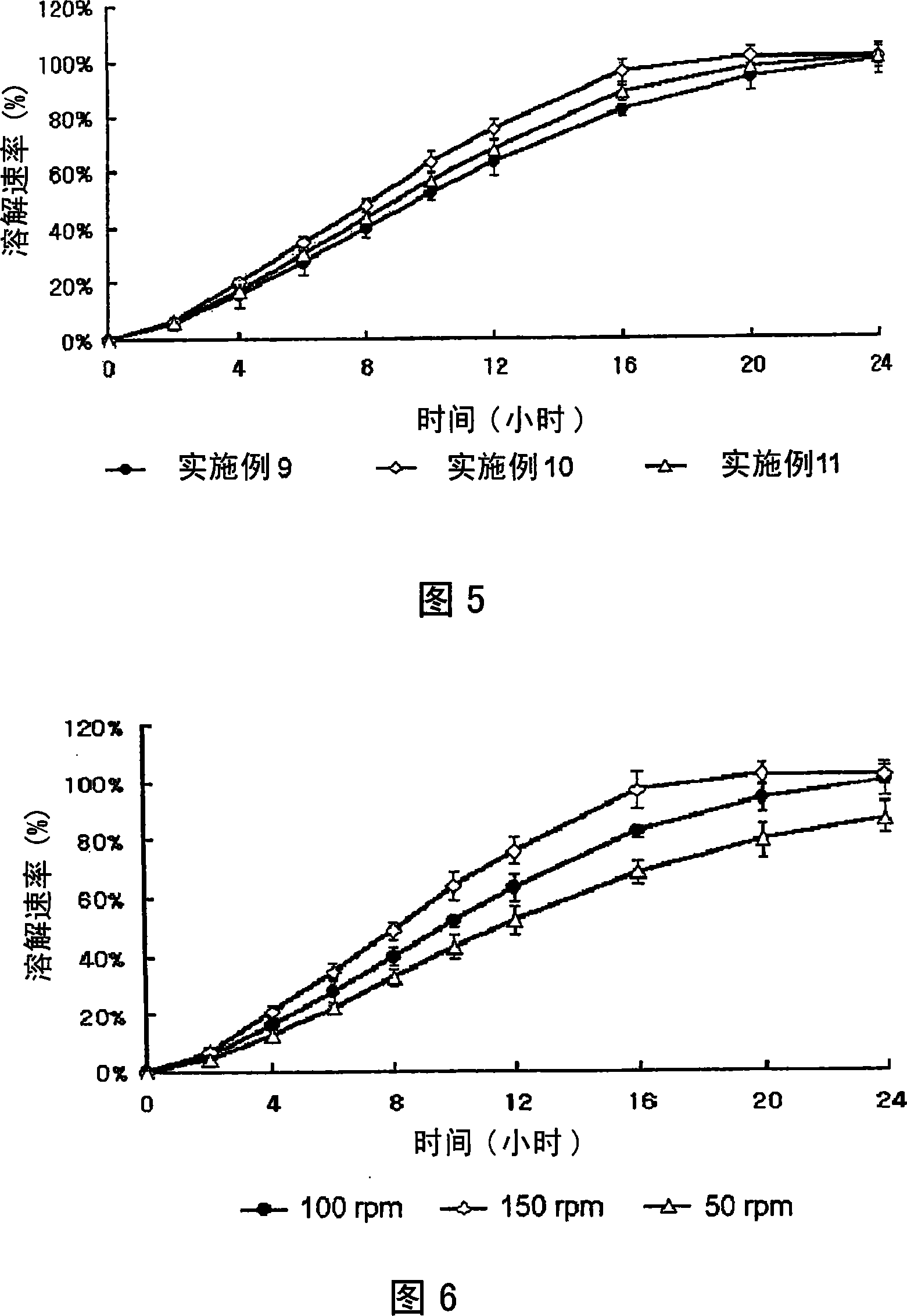
Embodiment 4 to 8
[0066] Examples 4 to 8: Preparation of sustained-release dosage forms for oral administration
[0067] According to the amounts described in Tables 2 to 4, respectively utilizing simvastatin, lovastatin or fluvastatin as active ingredients, together with MYRJ, HPMC 2910, BHT, and light anhydrous silicic acid, the procedure of Example 1 was repeated to obtain A solid dispersion is obtained. Then, each solid dispersion was mixed with xanthan gum (Kelco, USA), locust bean gum (Cesalpinia, Italy), propylene glycol alginate (ISP, USA), HPMC 2208 (viscosity: 4,000 to 100,000 cps, Shin -Etsu, Japan) and erythorbic acid were mixed for about 30 minutes; and sucrose fatty acid ester and light anhydrous silicic acid powder (finer than 40 mesh) were added and mixed for 5 minutes. Each of the resulting mixtures was molded into a block using a molding assembly, and the block was pulverized into particles having a mesh size ranging from 20 to 80. The granules are then formulated into tab...
Embodiment 9 to 11
[0074] Examples 9 to 11: Preparation of combination dosage forms for oral administration
[0075] Each sustained-release dosage form obtained in Examples 5, 7 and 8 was coated with Opadry AMB (Colorcon) film. Amlodipine camphorsulfonate (Hanmi Fine Chemical Co., Ltd., Korea), HPMC 2910 (viscosity: 3 to 15 cps) and acetylated monoglyceride (Myvacet) were dissolved respectively according to the amounts described in Table 5 In a mixture of ethanol and dichloromethane, it was applied to the film-coated formulations described above.
[0076]
[0077] components
(mg / tablet)
Example 9
Example 10
Example 11
Sustained-release dosage form core
Example 5
Example 7
Example 8
Active into
Minute
Ammonium D-camphorsulfonate
Clodipine
7.9
7.9
7.9
coating agent
HPMC 2910
10
10
10
Myvacet
1.6
1.6
1.6
stabilizer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com