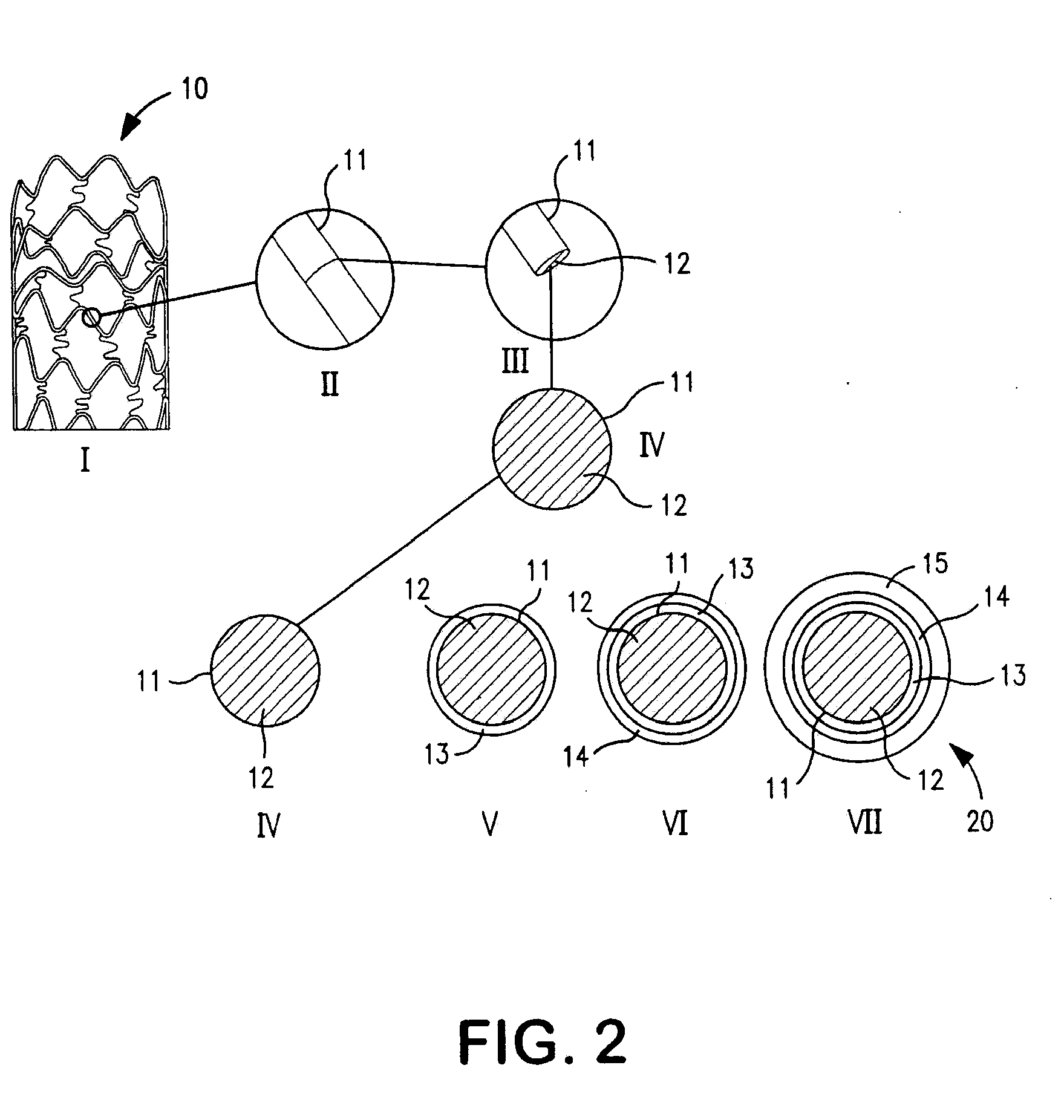
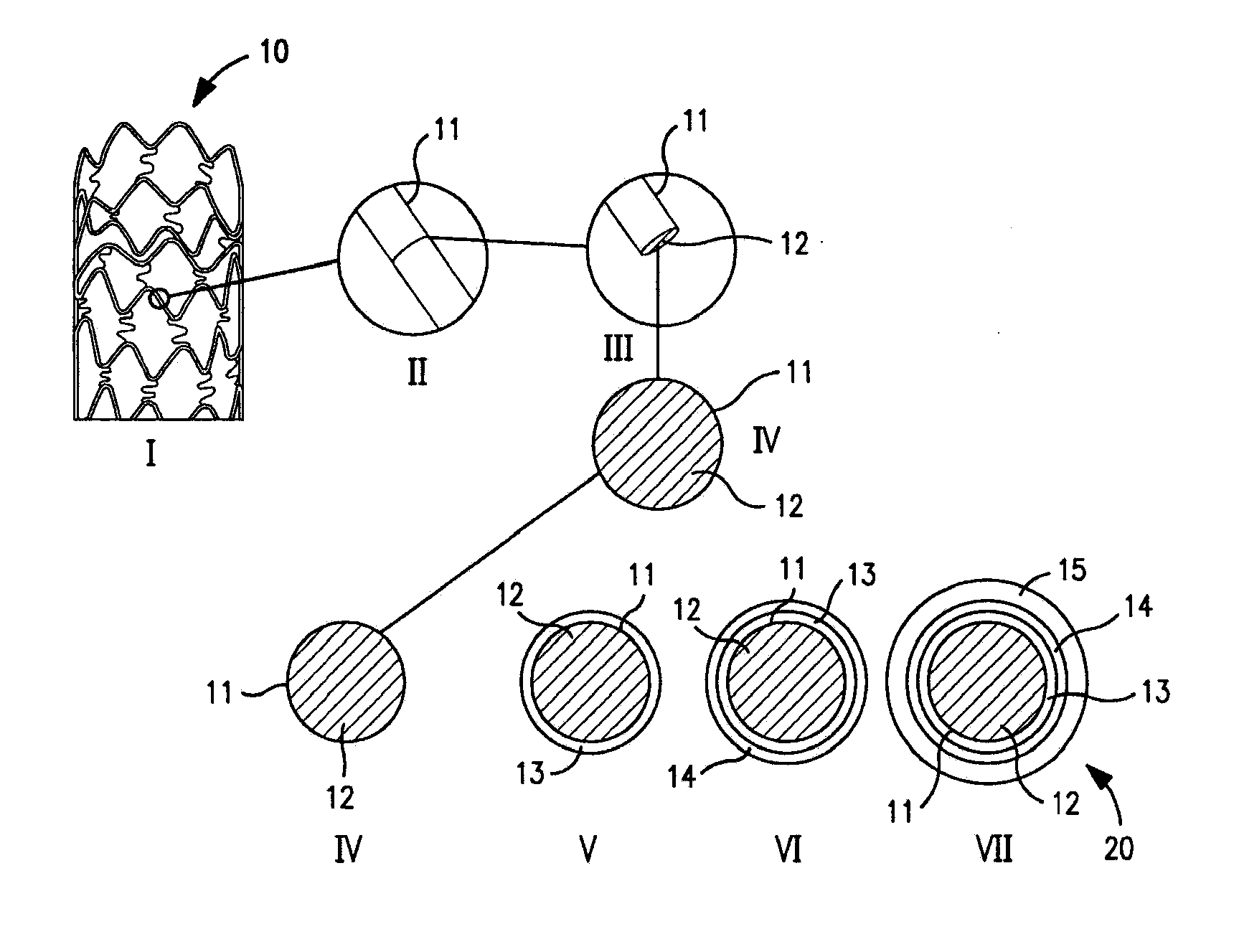
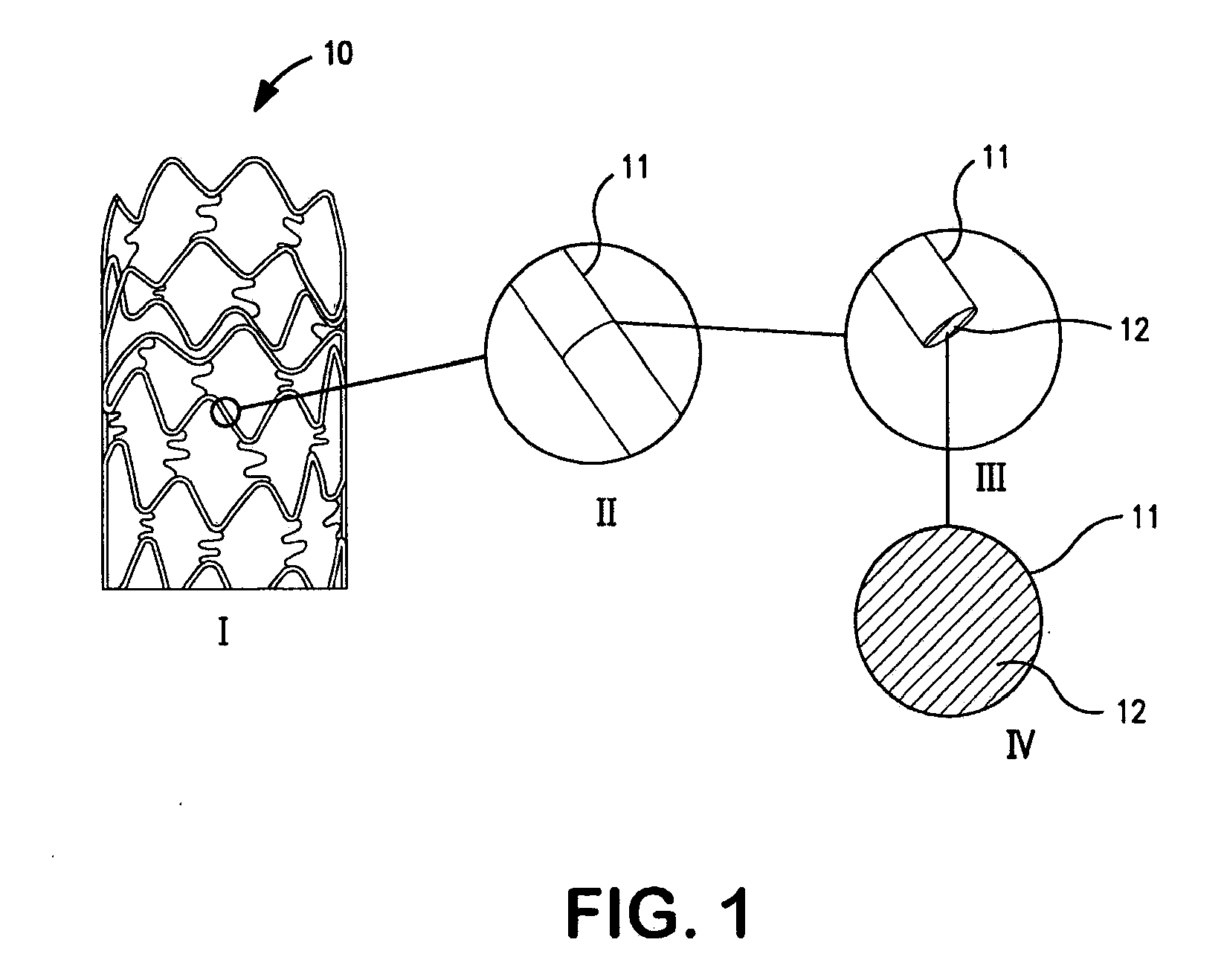
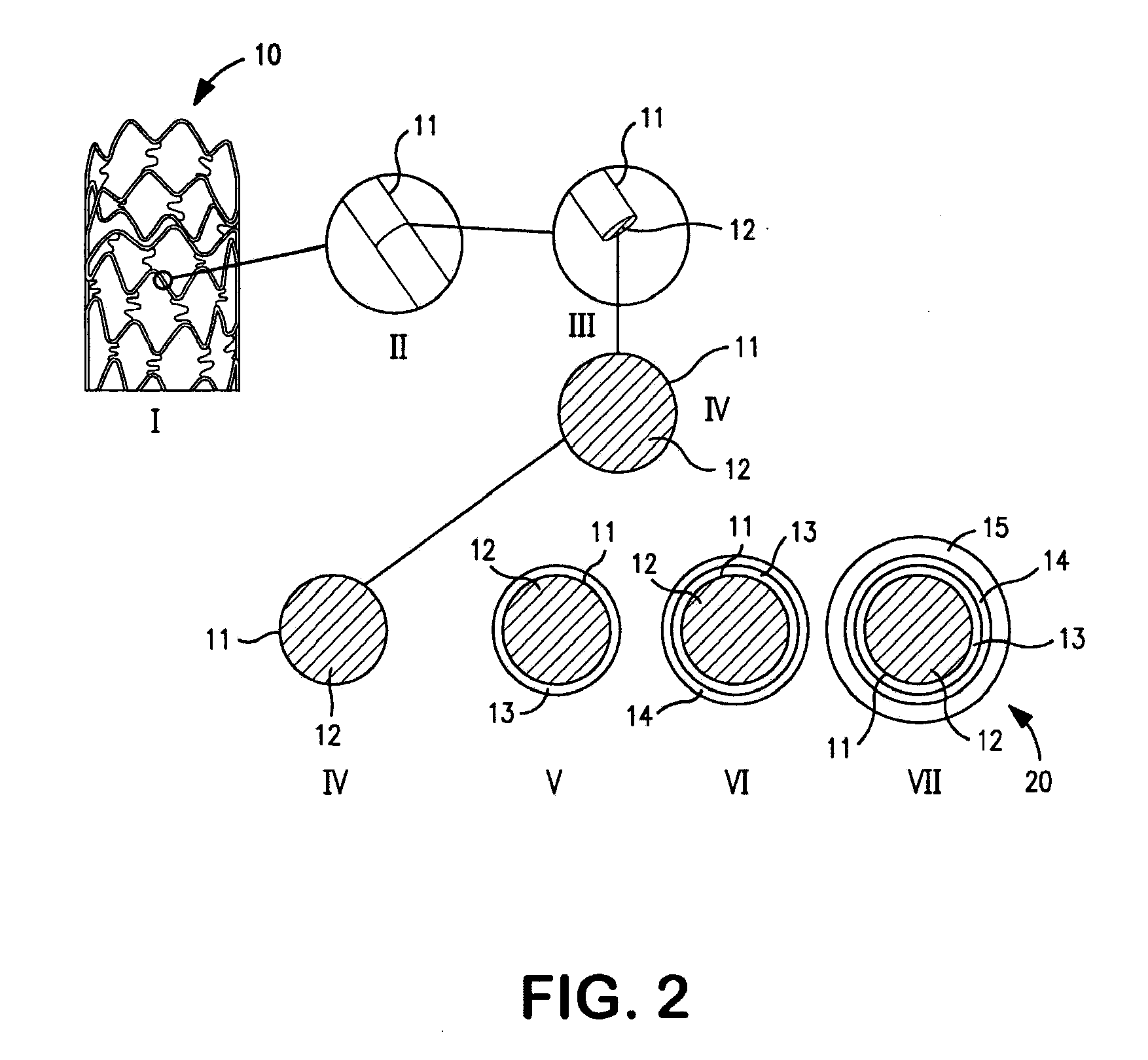
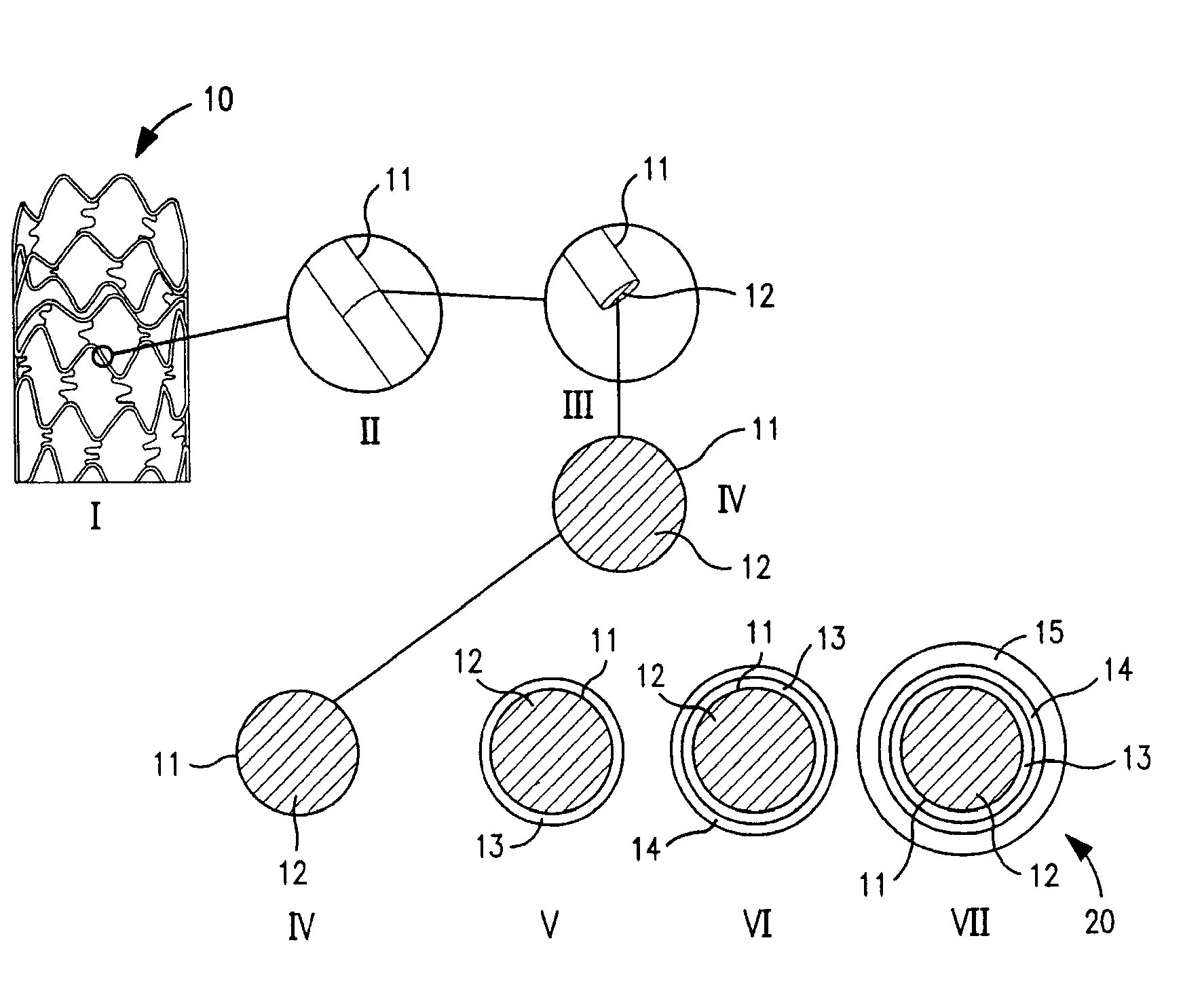
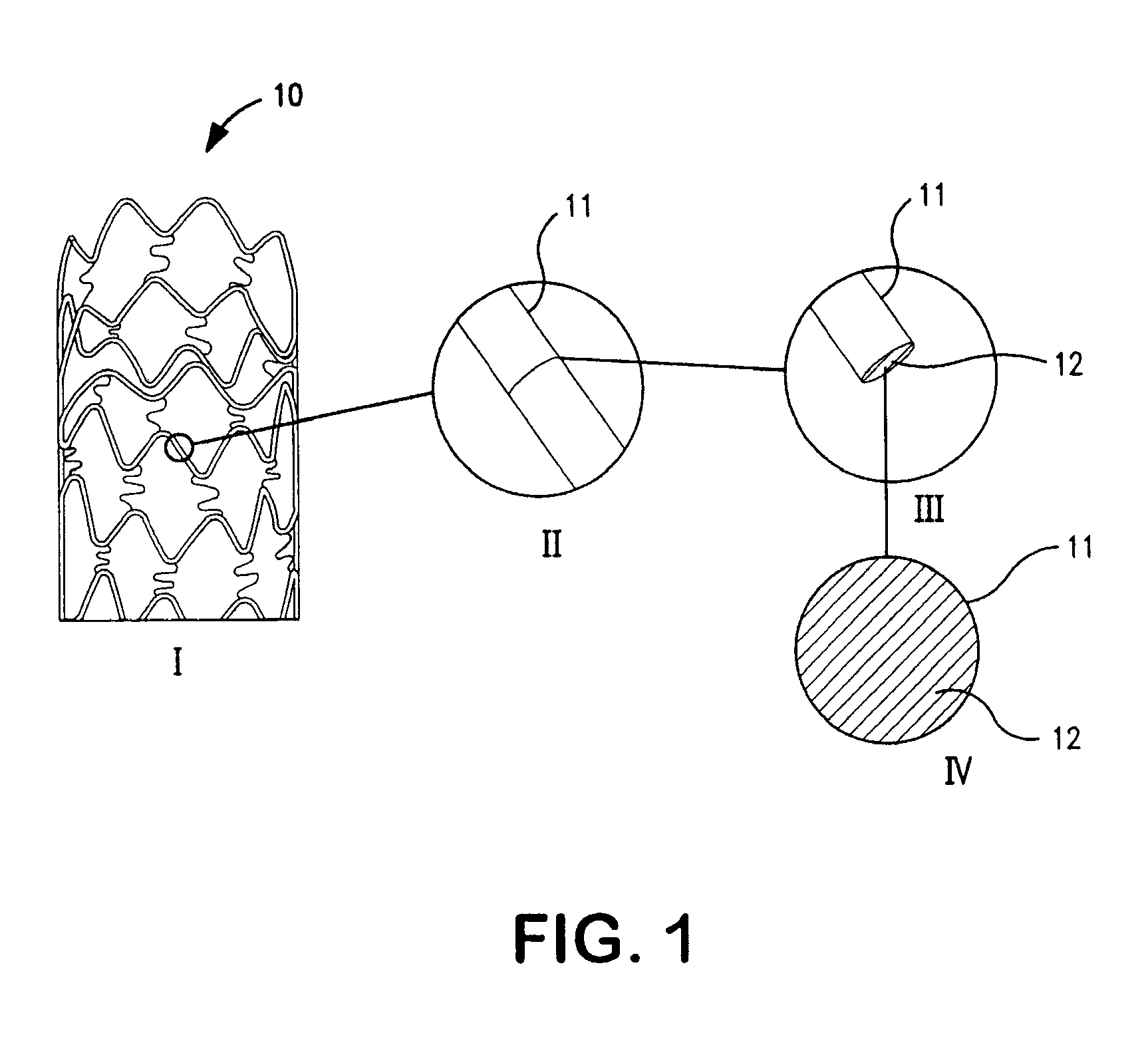
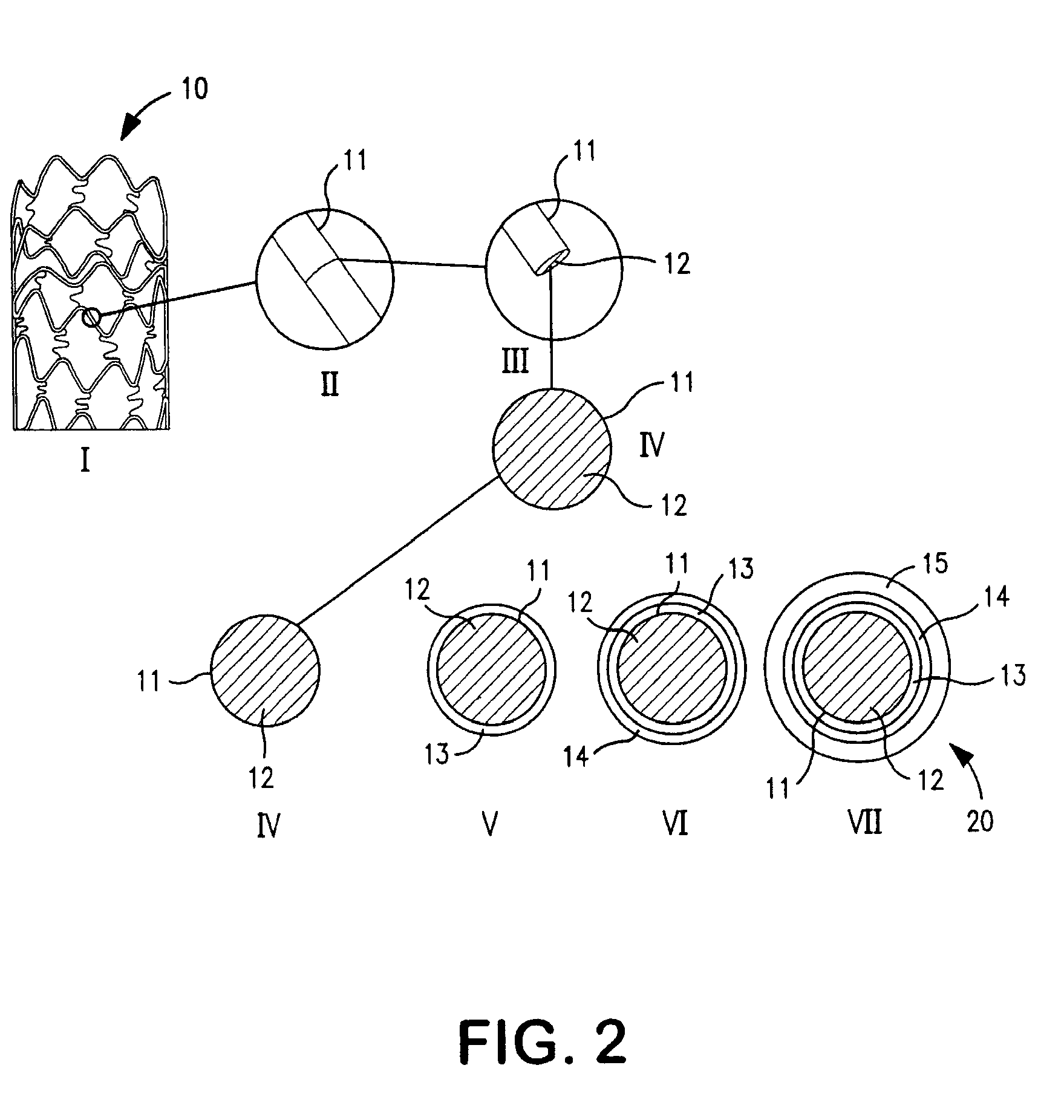
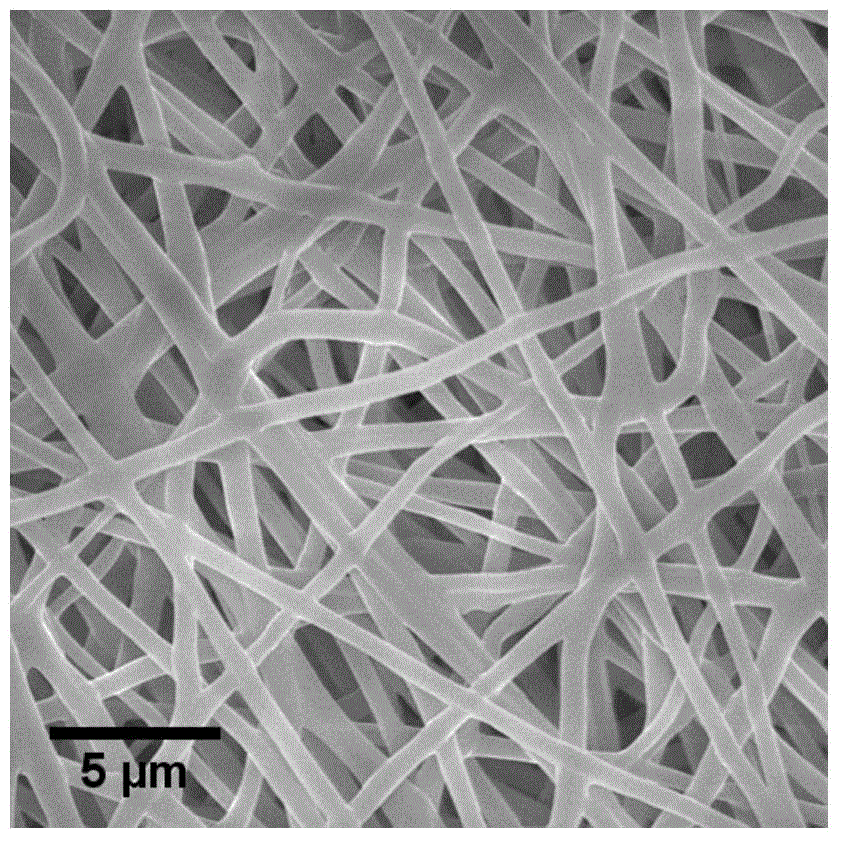
Patents
Literature
33 results about "Acute thrombosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acute vein thrombosis means there is a blood clot in your veins. Acute vein thrombosis most commonly starts in the legs. Acute vein thrombosis usually causes sudden pain or swelling in the leg without another obvious cause like an injury. Acute vein thrombosis is potentially dangerous.
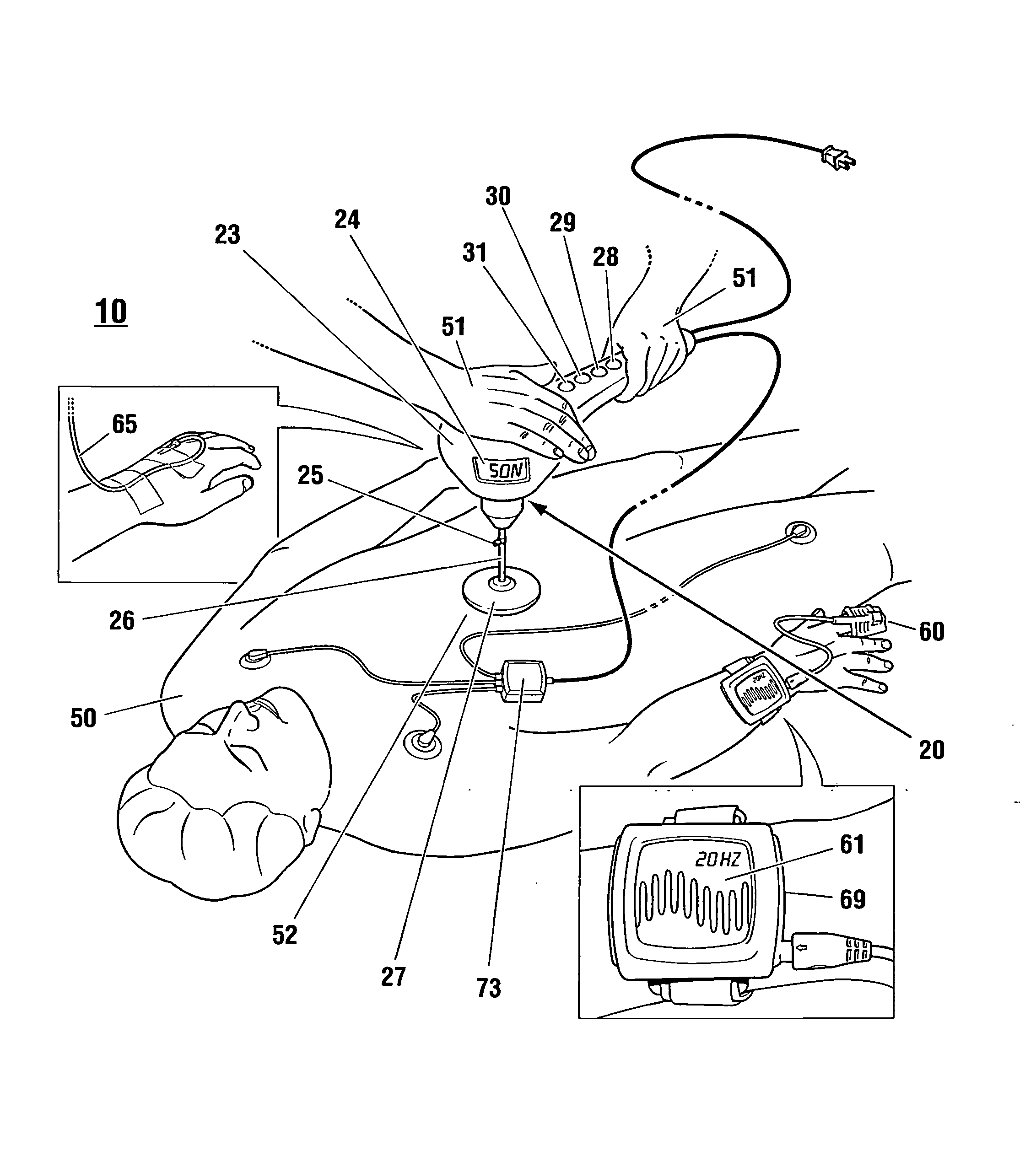
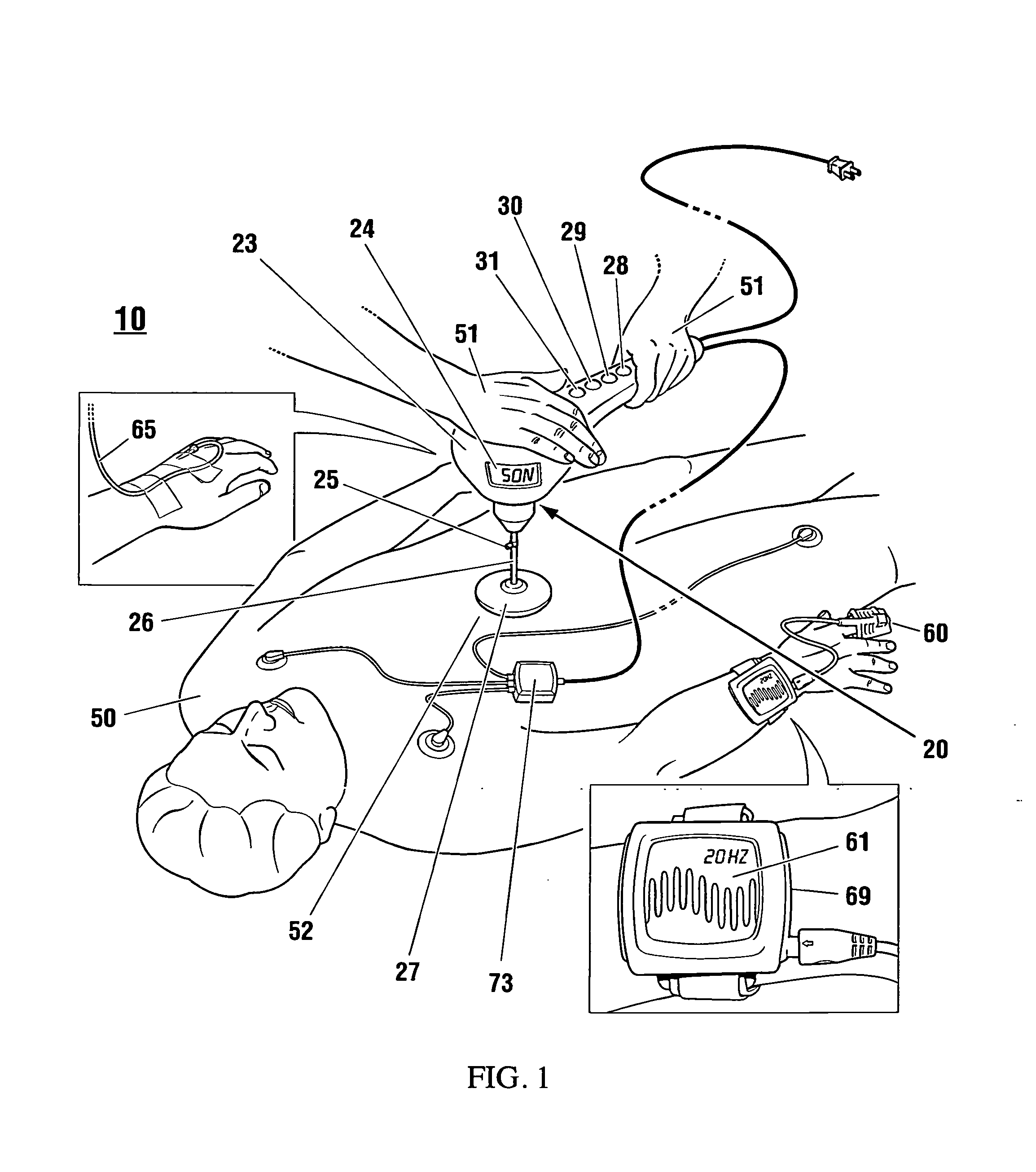
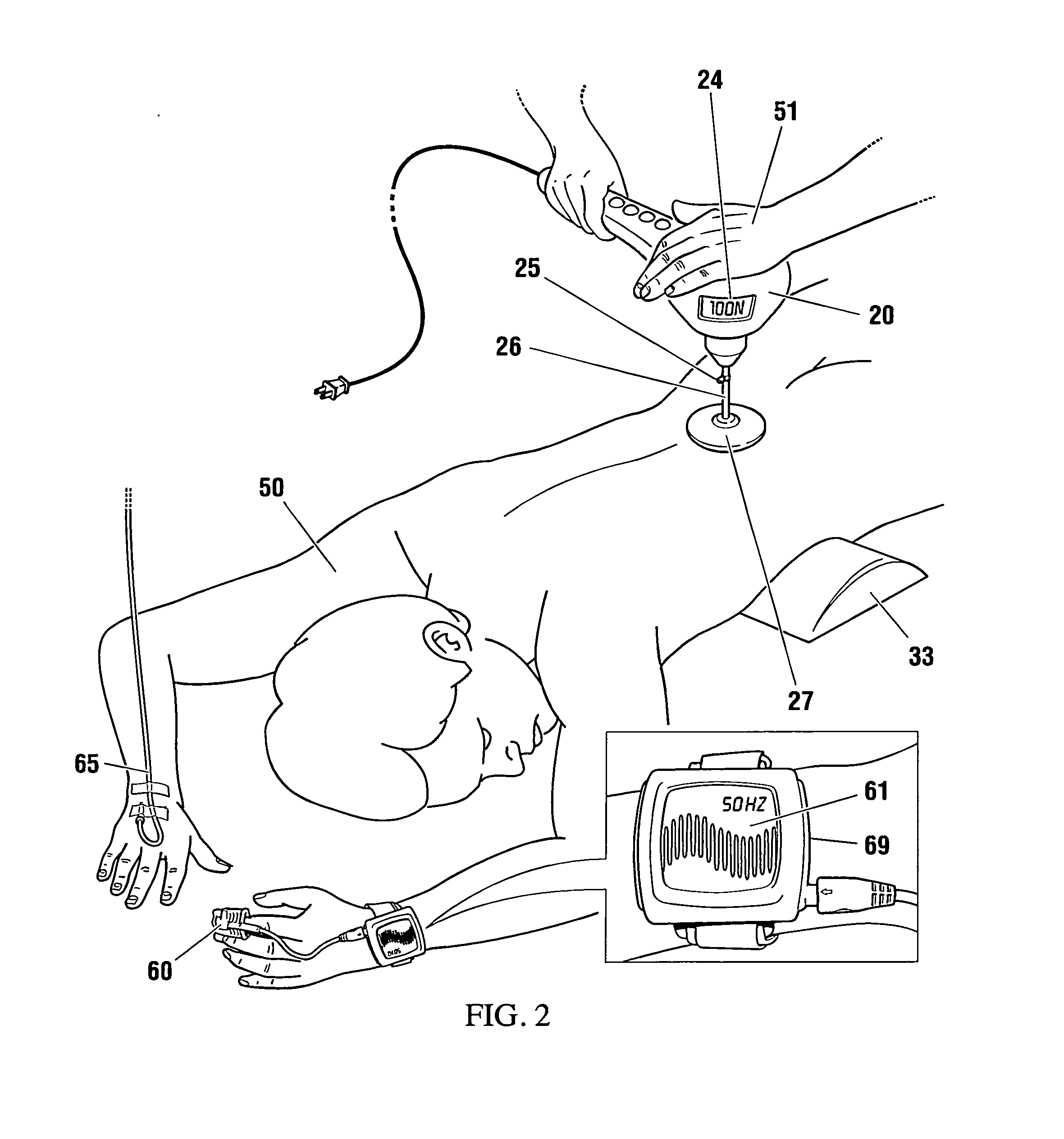
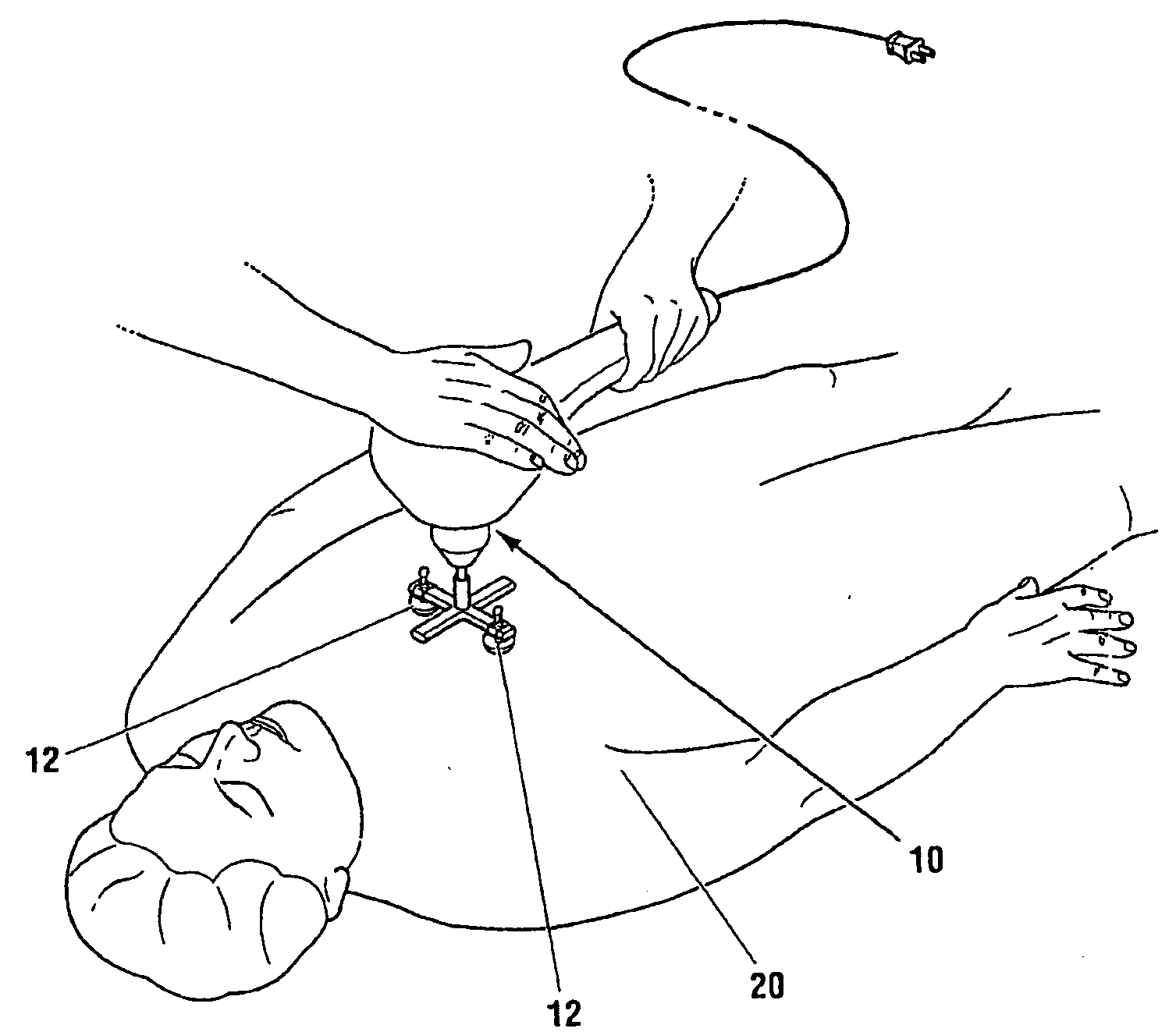
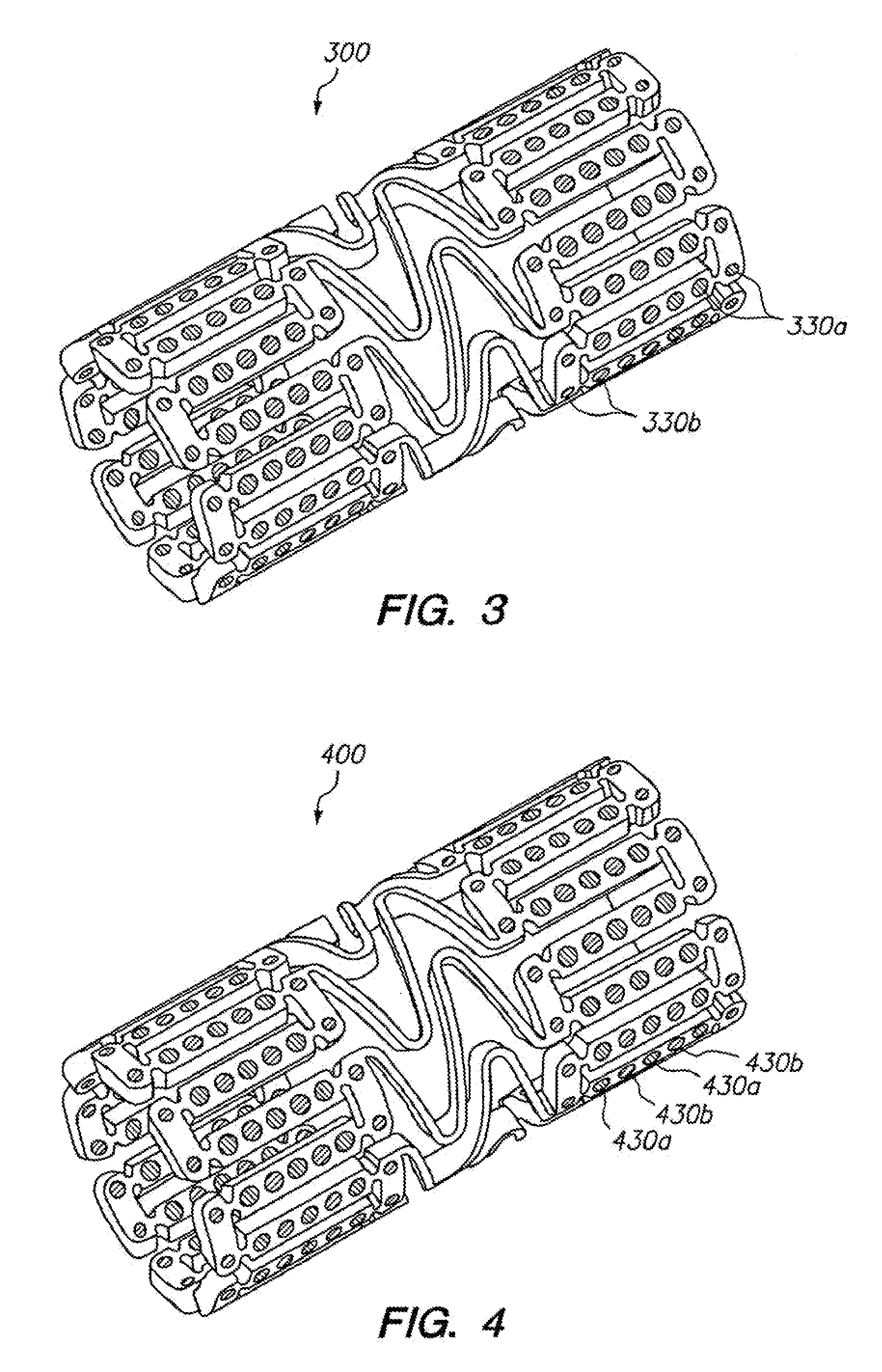
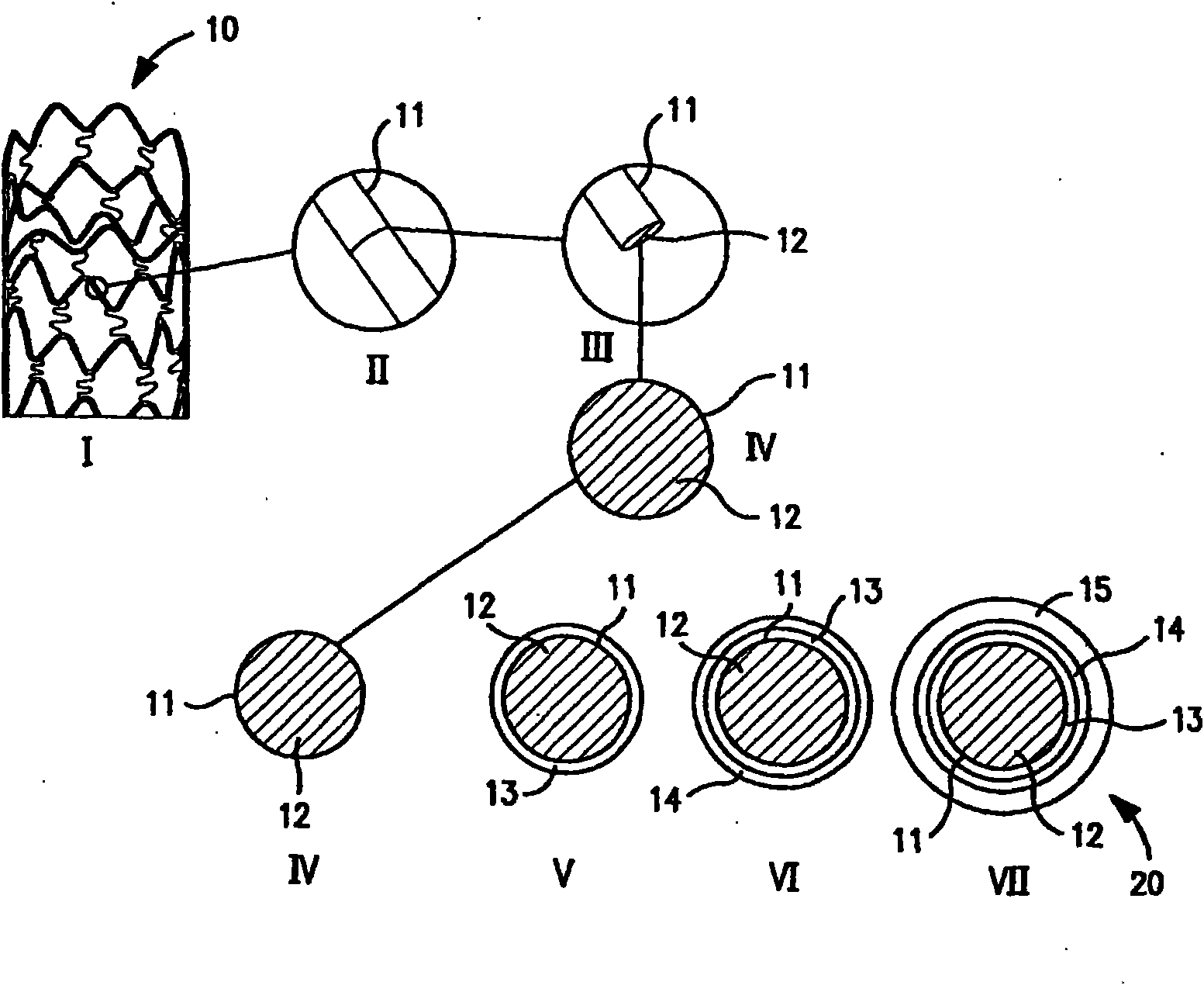
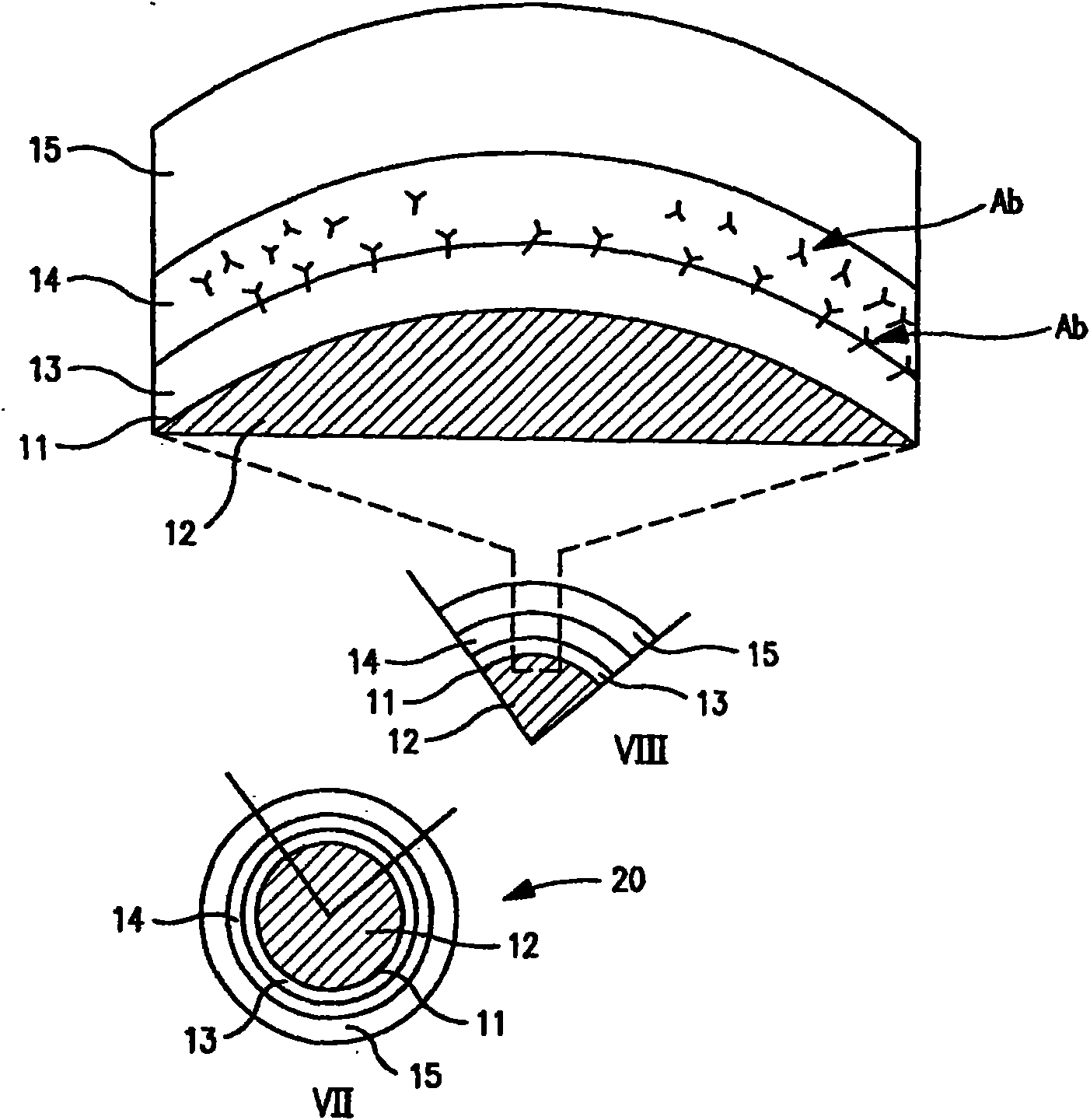
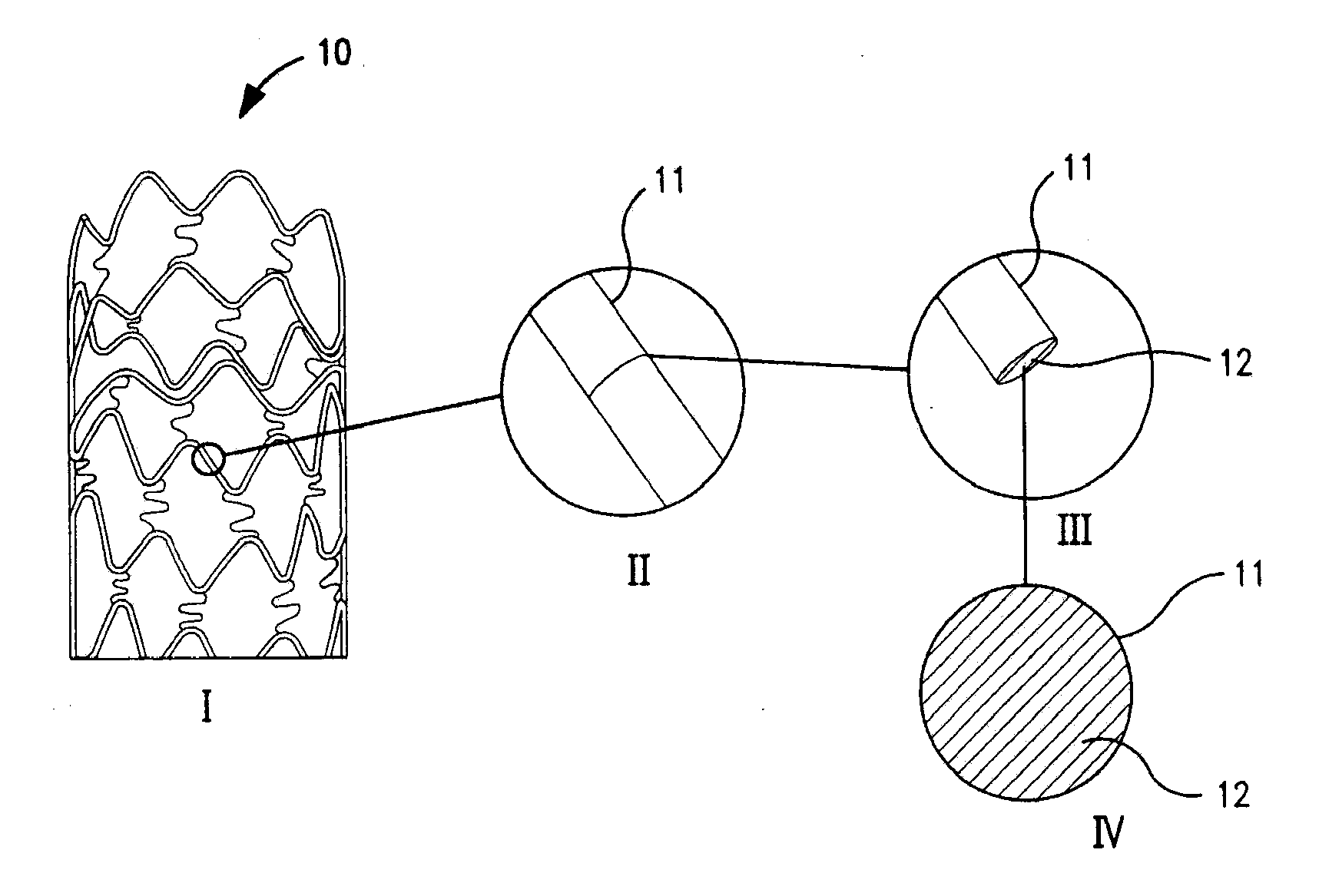
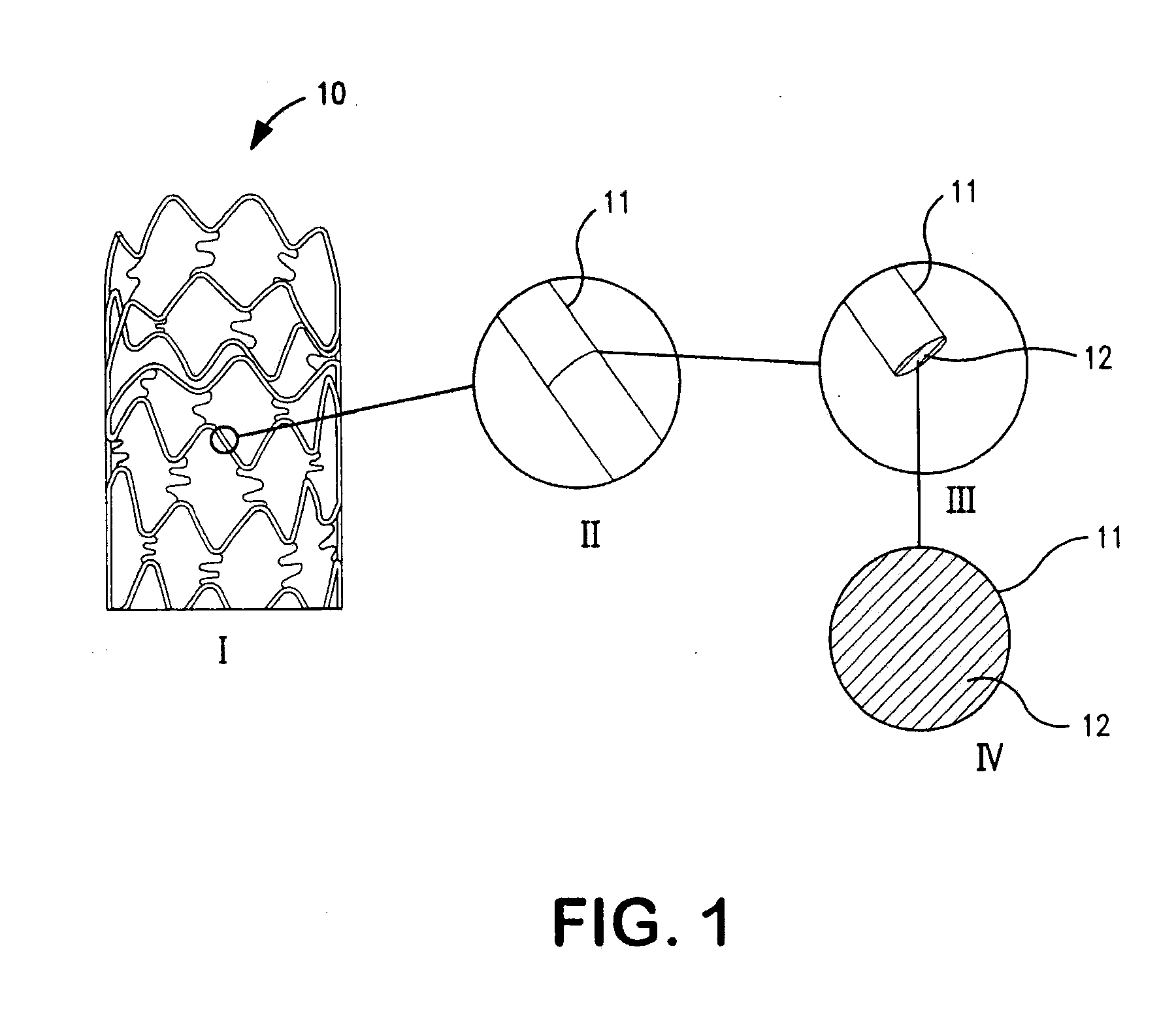
Non-invasive reperfusion system by deformation of remote, superficial arteries at a frequency much greater than the pulse rate
InactiveUS20130281897A1Effective researchEffective clinical toolUltrasound therapyOrgan movement/changes detectionPULMONARY EMBOLUSThrombus
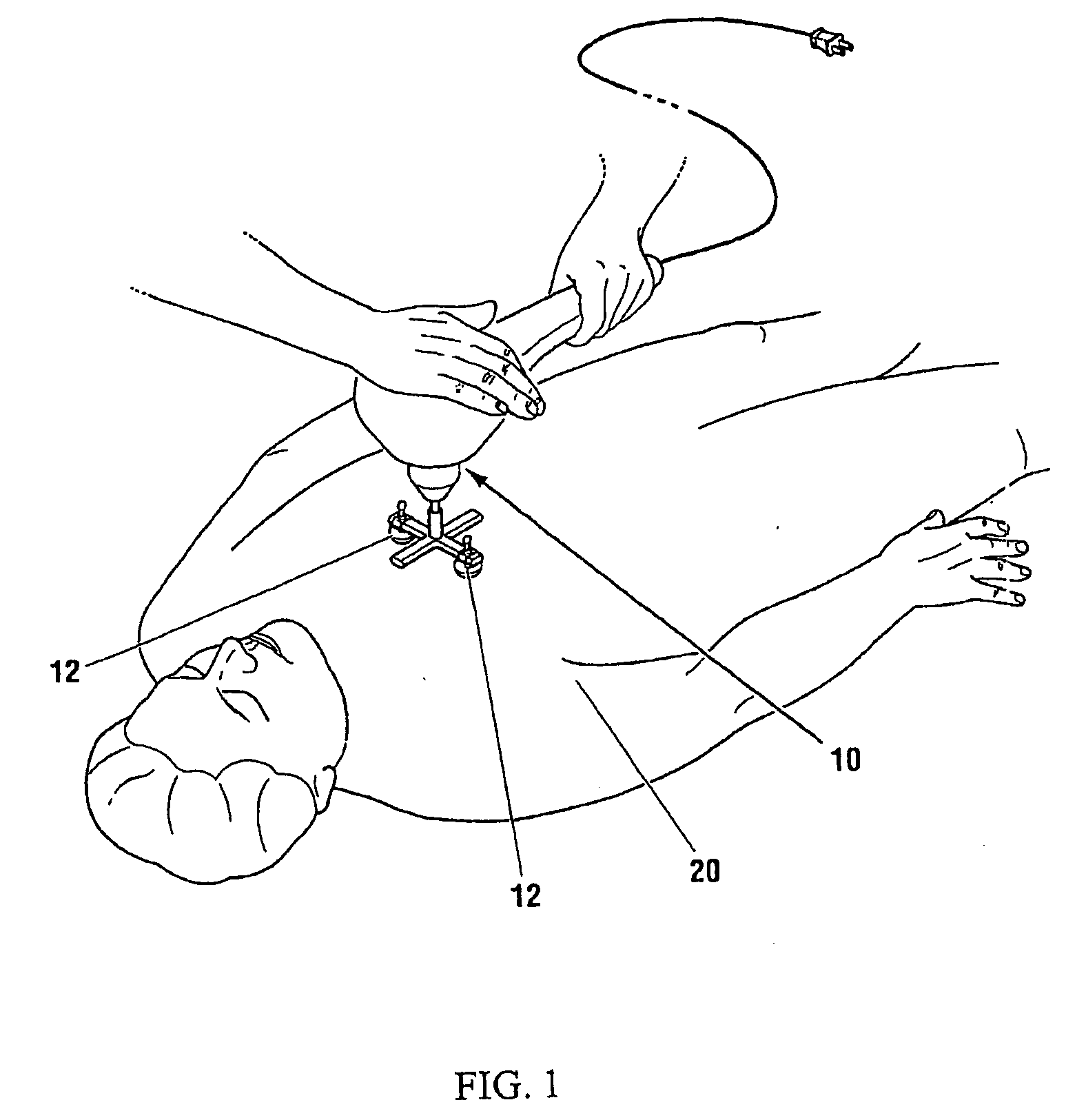
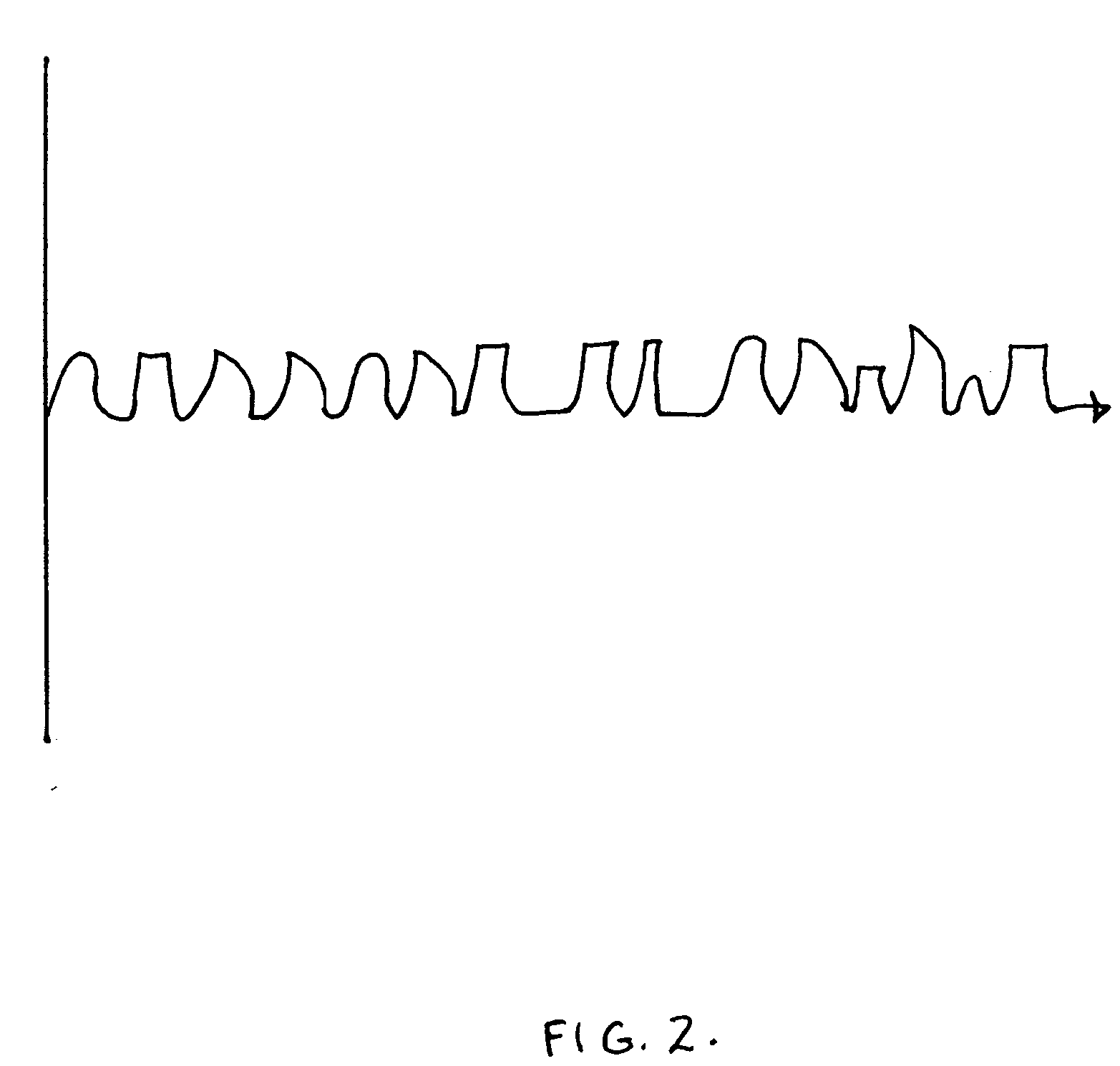
Preferred systems for assisting clearance of an acutely thrombosed artery substantially surrounded by boney external body surfaces which are resistant to deformative displacement relative to the thrombosed artery by the application of external percussive force are described. The method consists of applying targeted, localized, non-invasive, high infrasonic to low sonic frequency vibratory percussion with a serial impact frequency much greater than the pulse rate of a patient being treated, the percussion directed towards a remote, preferably superficial “target vessel” residing palpably close to the skin surface. Marked vessel deformations with resultant blood pressure and flow fluctuations are thereby induced by the percussion within the target vessel which propagate to the acutely thrombosed artery to provide localized agitation and turbulence to assist thrombolytic and / or IV microbubble delivery and effectiveness in facilitating reperfusion. Preferred apparatus for treatment of ST elevation myocardial infarction, acute ischemic stroke and acute pulmonary embolus are presented.
Owner:AHOF BIOPHYSICAL SYST
Randomic vibration for treatment of blood flow disorders
InactiveUS20090069728A1Significant positive effectFacilitate and improve emergency treatmentUltrasound therapyPneumatic massageVascular obstructionTherapeutic Devices
A therapeutic device and method for use thereof for treatment of blood flow disorders is disclosed. In one embodiment, a first line emergency response system for treatment of acute thrombotic and / or vasospastic vascular obstructions via the noninvasive application of low frequency vibration with at least one, and preferably a plurality of randomly administered vibratory waveform characteristics (herein after “Randomic Vibration”) is detailed. The disclosed apparatus and methods are based on the intuition that transcutaneously imparted low frequency randomic vibration can provide enhanced clot disruption and mixing of clot disruptive agents to acutely thrombosed vessels, due to the addition of mechanical chaos via non-regular, multi-vectored convection currents. In a preferred embodiment, the disclosed apparatus and methods preferably utilize randomic vibration as an adjunct to systemically administered drug therapy, most preferably intravenously administered thrombolytic drug therapy.
Owner:SIMON FRASER UNIVERSITY
Mixtures of particular LMW heparinic polysaccharides for the prophylaxis/treatment of acute thrombotic events
InactiveUSRE38743E1Improve bioavailabilityAdministered can be reducedOrganic active ingredientsBiocideThrombotic episodesSulfated polysaccharides
Heterogeneous intimate admixtures of sulfated heparinic polysaccharides, well suited for the prophylaxis / treatment of acute thrombotic episodes in a human patient, comprise immixture of sulfated polysaccharides having a weight average molecular weight less than that of heparin and which include from 9% to 20% of polysaccharide chains having a molecular weight less than 2,000 daltons and from 5% to 20% of polysaccharide chains having a molecular weight greater than 8,000 daltons, the ratio between the weight average molecular weight and the number average molecular weight thereof ranging from 1.3 to 1.6.
Owner:AVENTIS PHARMA SA (US)
Antithrombotic and Anti-restenotic drug eluting stent
ActiveUS20080188925A1Increasing effective wall thicknessAdversely impactingStentsSurgeryDiseasePercent Diameter Stenosis
An expandable medical device includes a plurality of elongated struts, forming a substantially cylindrical device which is expandable from a cylinder having a first diameter to a cylinder having a second diameter. A plurality of different beneficial agents may be loaded into different openings within the struts for delivery to the tissue. For treatment of conditions such as restenosis, different beneficial agents are loaded into different openings in the device to address different biological processes involved in restenosis and are delivered at different release kinetics matched to the biological process treated. The different beneficial agents may also be used to address different diseases, such as restenosis and acute myocardial infarction from the same drug delivery device. In addition, anti-thrombotic agents may be affixed to at least a portion of the surfaces of the medical device for the prevention of sub-acute thrombosis.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
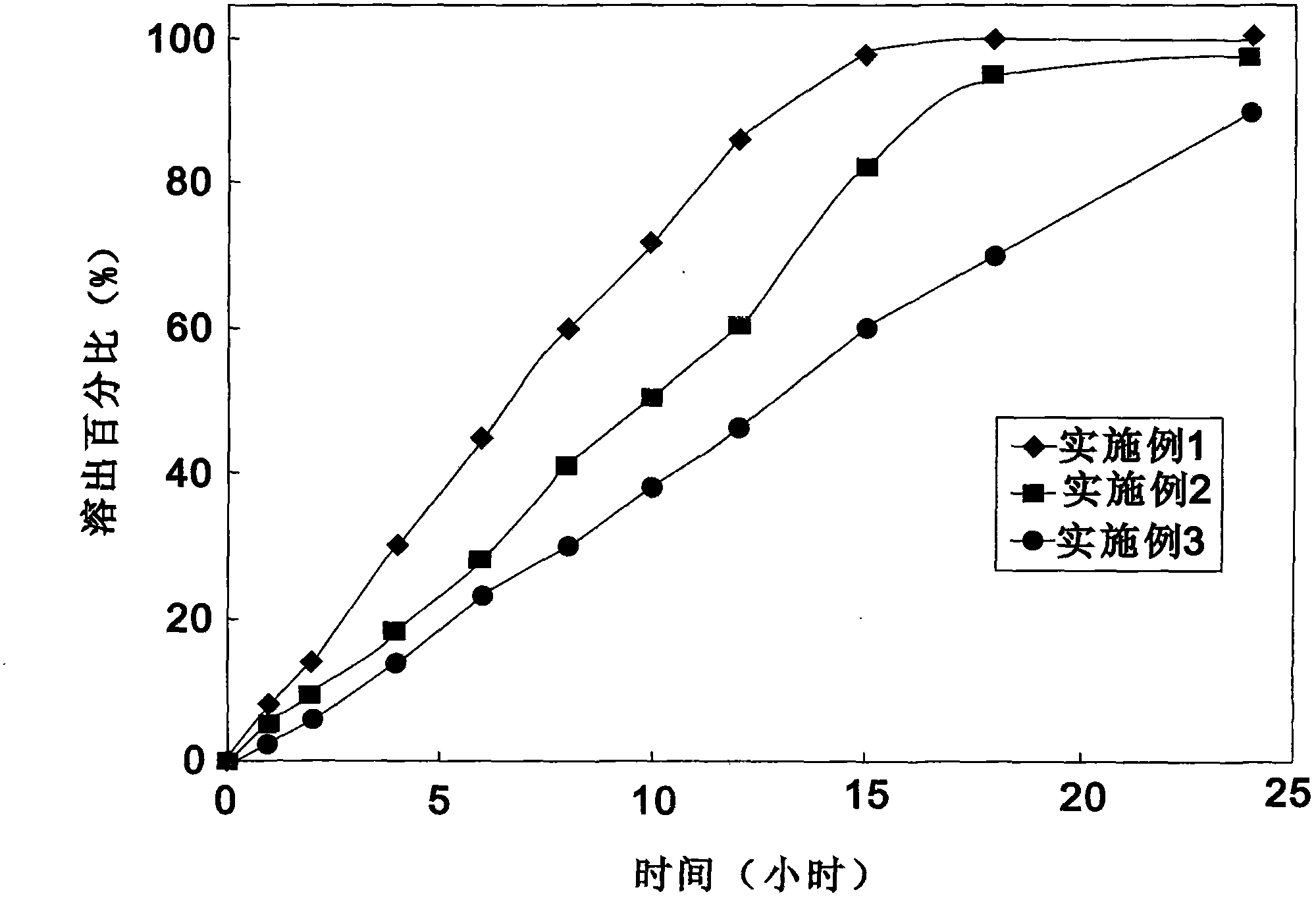
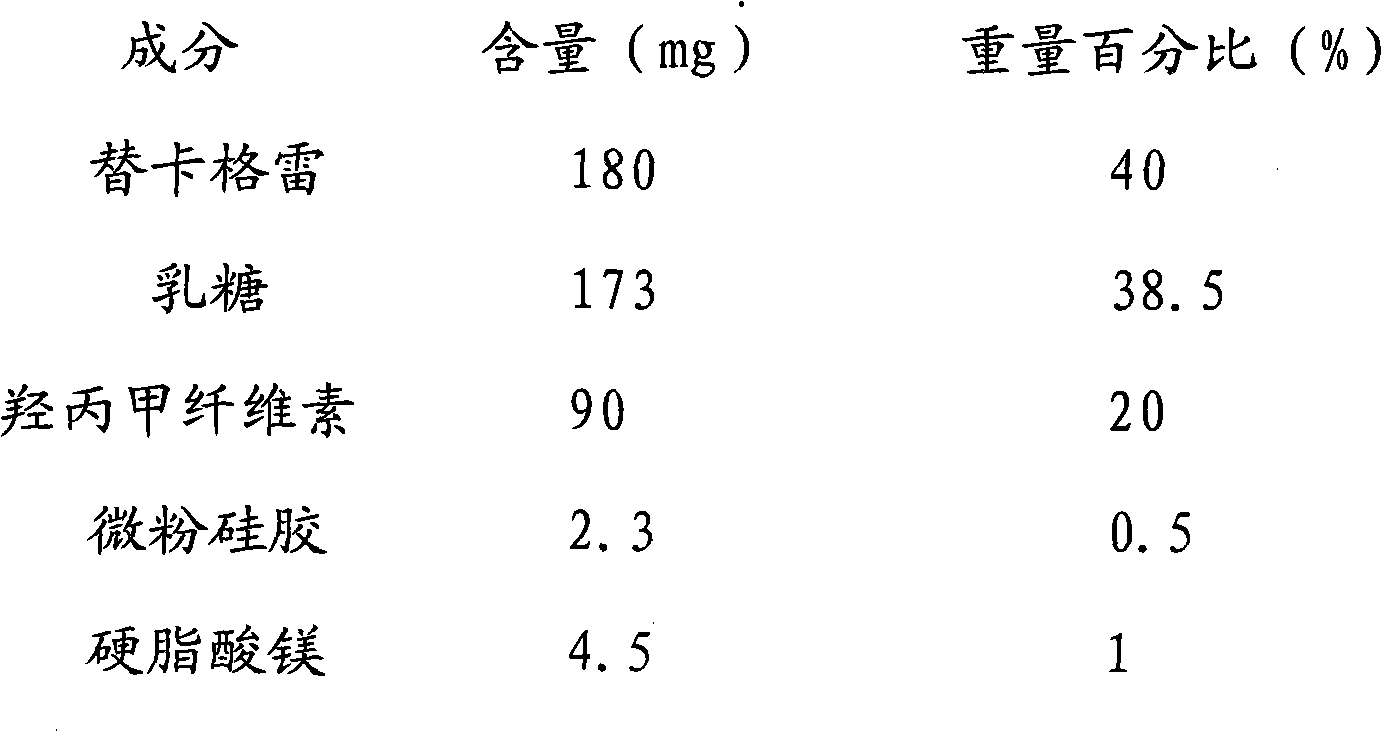
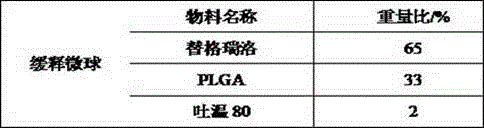
Ticagrelor sustained-release tablet system and preparation method thereof
InactiveCN102657629AMaintain blood levelsOrganic active ingredientsPharmaceutical delivery mechanismTicagrelorPediatrics
The invention provides a ticagrelor sustained-release tablet system and a preparation method of the ticagrelor sustained-release tablet system. The preparation method comprises the following steps of: firstly uniformly mixing 10-60% of ticagrelor, 5-60% of a filler and 5-60% of high molecular polymer in percentage by weight, adding a granulating solution to granulate; fully drying the obtained granules at a temperature of 50-60 DEG C, uniformly mixing the sieved granules with 0.25-10% of a lubricant and / or 0-10% of a flow aid, carrying out tabletting to obtain the ticagrelor matrix type sustained-release tablets, wherein the granulating solution is preferably water, an alcohol-water solution or absolute ethyl alcohol; the granule size of the ticagrelor is below 100 micrometers; and the content of the ticagrelor is 50-300mg in the preparation process. The ticagrelor matrix type sustained-release tablet system provided by the invention has the advantages that a patient can take the ticagrelor once a day to ensure that the drug dependence of the patient can be improved and the risk of myocardial infarction or apoplexy caused by acute thrombosis due to a dose of the ticagrelor missing of the patient is reduced.
Owner:SHENZHEN HUALIKANG BIOLOGICAL MEDICINE
Preparation technique of three-layer artficial blood vessel
ActiveCN106075596AEnhanced Axial MechanicsEnhanced radial mechanicsProsthesisHemodialysisCell-Extracellular Matrix
The invention provides a preparation technique of a three-layer (inner, middle and outer layers) artificial blood vessel. The inner layer is composed of a smooth compact thin layer prepared by an ink printing method and can inhibit plasma protein and platelet adhesion, prevent acute thrombosis formation and provide axial mechanical support. The middle layer is formed by winding oriented micro fiber prepared by wet spinning or melt spinning, and mainly functions in guiding tissue cells to grow into gaps of the oriented fiber to realize oriented deposition arrangement of extracellular matrix, and the oriented micro fiber can provide radial mechanical support. The outer layer is formed by winding thick polymer fiber and closely bonded with the middle layer and mainly functions in preventing folding when the artificial blood vessel is bent. The artificial blood vessel prepared by the method can remarkably increase patency rate, can utilize microenvironment at an implanting position to realize quasi-natural reshaping and regeneration and has good application prospect in the aspects of coronary artery bypass surgery, hemodialysis and cerebral and peripheral blood vessel replacement.
Owner:NANKAI UNIV
Slow/controlled-release preparation of ticagrelor
InactiveCN103860504AImprove complianceGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismTicagrelorThrombus
The invention provides a slow / controlled-release preparation of ticagrelor. The slow / controlled-release preparation of ticagrelor is an oral drug. The slow / controlled-release preparation contains ticagrelor or its pharmaceutically acceptable salt. A mass percent of ticagrelor to controlled-release accessory materials is in a range of 1: 0.2 to 1: 20 and preferably, the mass percent is in a range of 1: 0.1 to 1: 10, and a proper amount of other accessory materials are used. The slow / controlled-release base comprises one or more of cellulose, cellulose derivatives, alginate, starch, starch derivatives, polypropylene resins, carboxyvinyl polymers and other controlled-release accessory materials. Compared with a fast-release preparation, the slow / controlled-release preparation of ticagrelor provides a ticagrelor slow / controlled-release preparation system, can be eaten by a patient once each day, can change drug compliance of patients, can reduce the risk of myocardial infarction or stroke caused by acute thrombosis caused by missing of ticagrelor, and provides the easily-prepared slow / controlled-release preparation of ticagrelor or its pharmaceutically acceptable salt.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Multiple drug-eluting coronary artery stent for percutaneous coronary artery intervention
The present invention relates to a combination of agents, including an anti-proliferative agent, an anti-inflammatory agent, an anti-growth factor, and an extracellular matrix (ECM) molecule coated on a stent to prevent acute and subacute thrombosis, enhance endothelial in-growth, and prevent neointimal hyperplasia, and / or suppress neovascularization, and thereby reduce restenosis rates for drug eluting stents. The present invention also relates to methods of using such multiple drug eluting stents for the treatment of heart disease and other vascular conditions.
Owner:R·L·小比约克
Novel Drug-Eluting Coronary Artery Stent Coated With Anti-Platelet-Derived Growth Factor Antibodies Overlaying Extracellular Matrix Proteins With an Outer Coating of Anti-Inflammatory (Calcineurin Inhibitor) and/or Anti-Proliferatives
ActiveUS20090157173A1Prevent acute thrombosisPromote growthStentsSurgeryCell-Extracellular MatrixPercent Diameter Stenosis
The present invention relates to a combination of agents, including an anti-proliferative agent, an anti-inflammatory agent, an anti-growth factor, and an extracellular matrix (ECM) molecule coated on a stent to prevent acute and subacute thrombosis, enhance endothelial in-growth, and prevent neointimal hyperplasia, and / or suppress neovascularization, and thereby reduce restenosis rates for drug eluting stents. The present invention also relates to methods of using such multiple drug eluting stents for the treatment of heart disease and other vascular conditions.
Owner:BJORK JR ROBERT L
Multiple drug-eluting coronary artery stent for percutaneous coronary artery intervention
InactiveUS20080172124A1Promote growthReduce restenosis rateStentsSurgeryCell-Extracellular MatrixRenal artery stent
The present invention relates to a combination of agents, including an anti-proliferative agent, an anti-inflammatory agent, an anti-growth factor, and an extracellular matrix (ECM) molecule coated on a stent to prevent acute and subacute thrombosis, enhance endothelial in-growth, and prevent neointimal hyperplasia, and / or suppress neovascularization, and thereby reduce restenosis rates for drug eluting stents. The present invention also relates to methods of using such multiple drug eluting stents for the treatment of heart disease and other vascular conditions.
Owner:BJORK ROBERT LAMAR
Slow-release preparation of ticagrelor
InactiveCN108210498ATo meet the need for the first loading doseAvoid wastingOrganic active ingredientsPill deliveryOral medicationTicagrelor
The invention relates to a slow-release preparation of ticagrelor. Specifically, the slow-release preparation of the ticagrelor, provided by the invention, is suitable for being orally taken one timeevery day; meanwhile, a rapid drug releasing effect can be realized after tablets are damaged. The releasing tablets which are administrated one time every day can reduce the number of times of oral administration, so that the oral drug taking compliance of patients can be improved and risks that myocardial infarction or stroke is caused when acute thrombus formation is caused by the fact that thepatients forget to orally take the ticagrelor are reduced; meanwhile, when the tablets provided by the invention are orally taken for the first time, the tablets can be opened to meet the requirementof giving a load dosage to the patients, so as to rapidly take effect.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Preparation of ticagrelor or pharmaceutical salt thereof
ActiveCN106074357AOrganic active ingredientsInorganic non-active ingredientsTicagrelorAcute thrombosis
The invention relates to a preparation of ticagrelor or a pharmaceutical salt thereof. Specifically, the invention relates to the improved preparation of ticagrelor or the pharmaceutical salt thereof for administration for once daily. The ticagrelor plasma concentration of a tested person can reach about more than 0.2 [mu]g / mL within 2 hours; the ticagrelor plasma concentration of the tested person can still reach about more than 0.2 [mu]g / mL 12 hours later after the drug is taken; and the maximum plasma concentration (Cmax) of the ticagrelor or the pharmaceutical salt thereof between about 0.2 [mu]g / mL-about 0.8 [mu]g / mL is generated in the tested person. The preparation of ticagrelor or the pharmaceutical salt thereof can make the administration times reduced, thereby improving the administration compliance of patients, reducing the risks of myocardial infarction or stroke caused by acute thrombosis caused because the patients miss taking ticagrelor.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Antithrombotic and anti-restenotic drug eluting stent
An expandable medical device includes a plurality of elongated struts, forming a substantially cylindrical device which is expandable from a cylinder having a first diameter to a cylinder having a second diameter. A plurality of different beneficial agents may be loaded into different openings within the struts for delivery to the tissue. For treatment of conditions such as restenosis, different beneficial agents are loaded into different openings in the device to address different biological processes involved in restenosis and are delivered at different release kinetics matched to the biological process treated. The different beneficial agents may also be used to address different diseases, such as restenosis and acute myocardial infarction from the same drug delivery device. In addition, anti-thrombotic agents may be affixed to at least a portion of the surfaces of the medical device for the prevention of sub-acute thrombosis.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Embolectomy apparatus for intravascular embolectomy and application of embolectomy apparatus
InactiveCN106388902ARemoval of mural thrombusCause blockageSurgeryTectorial membraneReticular formation
The invention relates to an embolectomy apparatus for intravascular embolectomy and an application of the embolectomy apparatus. The embolectomy apparatus comprises a stent portion, a tectorial membrane portion, a proximal portion, a push-pull rod and a micro catheter. The stent portion is of a meshed structure, and the stent, under a release state, is expanded as a hollow tubular structure; many holes are formed in the far end of the tectorial membrane, so as to facilitate passing of a blood flow; the proximal portion is of a triangular or elliptic meshed opening structure with the end connected to the push-pull rod; and by virtue of the micro catheter, the embolectomy apparatus for the intravascular embolectomy is transported. With the application of the tectorial membrane stent-type embolectomy apparatus provided by the invention, acute thrombosis formed in minute blood vessels, in particular in cerebral blood vessels, can be removed conveniently; the embolectomy apparatus can prevent the prolapse of the thrombosis from meshes of the stent and protect openings of branch vessels from getting blocked; and in addition, damage to the inner walls of the blood vessels caused by steel strips of the stent. The embolectomy apparatus provided by the invention is simple, convenient and safe to operate and is convenient for popularization; and the embolectomy apparatus is applicable to broad ordinary people at salaried class.
Owner:高不郎
Reservoir eluting stent
The invention discloses a reservoir eluting stent, especially an expandable medical device including a plurality of elongated struts, forming a substantially cylindrical device which is expandable from a first diameter to a second diameter. A plurality of different beneficial agents may be loaded into different openings within the struts for delivery to the tissue. For treatment of conditions such as restenosis, different agents are loaded into different openings in the device to address different biological processes involved in restenosis and are delivered at different release kinetics matched to the biological process treated. The different agents may also be used to address different diseases from the same drug delivery device. In addition, anti-thrombotic agents may be affixed to at least a portion of the surfaces of the medical device for the prevention of sub-acute thrombosis. Primer layers may be used to ensure that the different agents remain affixed to the device as well as to each other.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Adhesion promoting primer for coated surfaces
An expandable medical device includes a plurality of elongated struts, forming a substantially cylindrical device which is expandable from a first diameter to a second diameter. A plurality of different beneficial agents may be loaded into different openings within the struts for delivery to the tissue. For treatment of conditions such as restenosis, different agents are loaded into different openings in the device to address different biological processes involved in restenosis and are delivered at different release kinetics matched to the biological process treated. The different agents may also be used to address different diseases from the same drug delivery device. In addition, anti-thrombotic agents may be affixed to at least a portion of the surfaces of the medical device for the prevention of sub-acute thrombosis. To ensure that the different agents remain affixed to the device as well as to each other, primer layers may be utilized.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Adhesion promoting temporary mask for coated surfaces
InactiveUS20100161039A1Increasing effective wall thicknessAdversely impactingStentsSurgeryDiseaseCoated surface
An expandable medical device includes a plurality of elongated struts, forming a substantially cylindrical device which is expandable from a first diameter to a second diameter. A plurality of different beneficial agents may be loaded into different openings within the struts for delivery to the tissue. For treatment of conditions such as restenosis, different agents are loaded into different openings in the device to address different biological processes involved in restenosis and are delivered at different release kinetics matched to the biological process treated. The different agents may also be used to address different diseases from the same drug delivery device. In addition, anti-thrombotic agents may be affixed to at least a portion of the surfaces of the medical device for the prevention of sub-acute thrombosis. To ensure that the different agents remain affixed to the device as well as to each other, masking and de-masking processes may be utilized.
Owner:CORDIS CORP
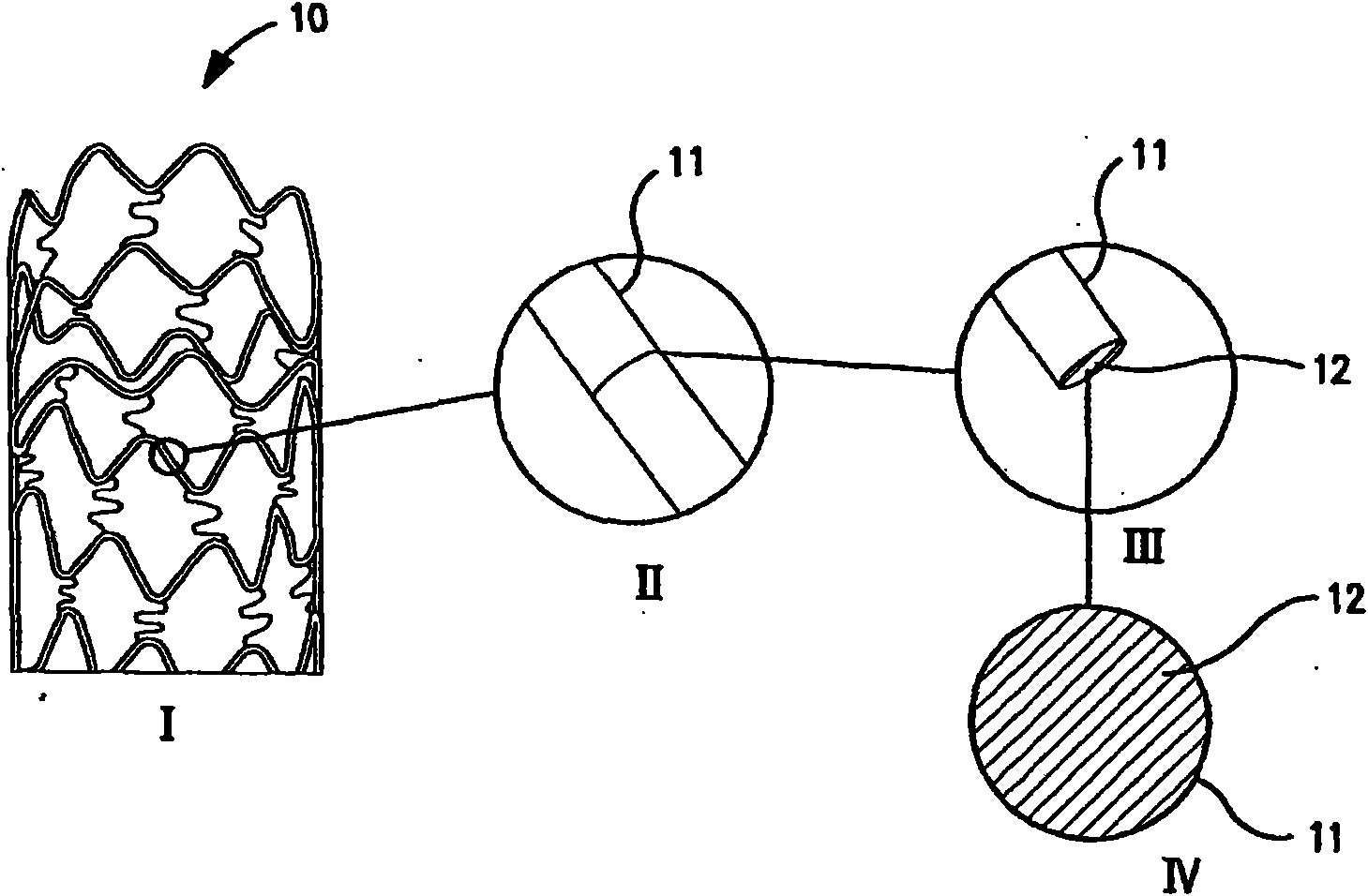
Intravascular stent
The invention relates to the field of medical instruments, in particular to an intravascular stent for treating cardiovascular and cerebrovascular diseases. In order to solve the problem that stent rings of an existing intravascular stent are prone to deformation, overturning and twisting, the intravascular stent is provided and comprises stent rings and a linking rod, each stent ring comprises asupporting rod section and an arc-shaped crown, and each supporting rod section and the corresponding crown are connected with each other to form a wavy ring structure; and two ends of the linking rodare connected with the crowns of the two adjacent stent rings, and the linking rod is of an arc-shaped structure. The intravascular stent is attached to the inner wall of a blood vessel after being placed into the blood vessel of a patient, so that the blood vessel loss rate is effectively reduced, and the problems of intravascular restenosis and intravascular acute thrombosis after the intravascular stent is implanted are reduced; and the crowns at the corresponding positions on the two adjacent stent rings are connected with each other through the linking rod which is of the arc-shaped structure, and when the intravascular stent is expanded, the circumstance that the linking rod deforms, overturns and twists, and consequently the wall attaching effect of the intravascular stent is affected can be avoided.
Owner:SHANGHAI JUNLIAN MEDICAL EQUIP
Woven vascular drug stent
The invention belongs to the field of medical apparatuses, and in particular relates to a vascular woven stent. The vascular woven stent comprises a woven stent main body and a coating, wherein the woven stent main body is constituted by metal wires having a shape memory function; and the coating comprises a drug coating bottom course and a protective top course. The vascular woven stent provided by the invention, by combining the woven stent, can effectively relieve coating falling, so that an acute thrombosis risk is reduced, stimulation to vascular walls and blood vessels is relieved, an occurrence rate of vascular restenosis is reduced, thrombogenesis is relieved, influence of patients' complications to the drug coating is relieved, and finally, product's effectiveness is improved.
Owner:LEO MEDICAL
Adhesion promoting primer for coated surfaces
ActiveUS20100152841A1Increasing effective wall thicknessAdversely impactingStentsSurgeryDiseaseCoated surface
An expandable medical device includes a plurality of elongated struts, forming a substantially cylindrical device which is expandable from a first diameter to a second diameter. A plurality of different beneficial agents may be loaded into different openings within the struts for delivery to the tissue. For treatment of conditions such as restenosis, different agents are loaded into different openings in the device to address different biological processes involved in restenosis and are delivered at different release kinetics matched to the biological process treated. The different agents may also be used to address different diseases from the same drug delivery device. In addition, anti-thrombotic agents may be affixed to at least a portion of the surfaces of the medical device for the prevention of sub-acute thrombosis. To ensure that the different agents remain affixed to the device as well as to each other, primer layers may be utilized.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Preparation method of medicine elution type blood vessel stent
A medicine eluting blood vessel scaffold is composed of blood vessel scaffold, TiO2 layer and medicine-polymer layer. It is prepared through roughening the surface of blood vessel scaffold, generating TiO2 layer on the rough surface, coating polymer layer, and combining medicine to the polymer layer. It can prevent said scaffold from renarrowing and thrombosis.
Owner:CHONGQING UNIV
Ticagrelor oral-disintegrating sustained release tablet and preparation method thereof
InactiveCN105853385AReduce the risk of acute thrombosisImprove medication complianceOrganic active ingredientsPill deliverySustained Release TabletTicagrelor
The invention discloses a ticagrelor oral-disintegrating sustained release tablet, belongs to the field of medicinal preparations, and concretely relates to a sustained release preparation rapidly disintegrating in the oral cavity after being taken. The ticagrelor oral-disintegrating sustained release tablet is prepared through the following steps: preparing microcapsules with a sustained release effect from a main medicine and a suitable capsule material, proportioning the microcapsules and auxiliary materials, and carrying out wet granulation, wherein the auxiliary materials comprise a filler, an adhesive, a disintegrating agent, a lubricant and a flavoring. The ticagrelor oral-disintegrating sustained release tablet can rapidly disintegrate in the oral cavity after being orally taken, can continuously and stably release in vivo after being orally taken, can reduce the acute thrombus formation risk of patients, increases the compliance of the patients, and has important clinic application values.
Owner:BEIJING VENTUREPHARM BIOTECH
Adhesion promoting temporary mask for coated surfaces
An expandable medical device includes a plurality of elongated struts, forming a substantially cylindrical device which is expandable from a first diameter to a second diameter. A plurality of different beneficial agents may be loaded into different openings within the struts for delivery to the tissue. For treatment of conditions such as restenosis, different agents are loaded into different openings in the device to address different biological processes involved in restenosis and are delivered at different release kinetics matched to the biological process treated. The different agents may also be used to address different diseases from the same drug delivery device. In addition, anti-thrombotic agents may be affixed to at least a portion of the surfaces of the medical device for the prevention of sub-acute thrombosis. To ensure that the different agents remain affixed to the device as well as to each other, masking and de-masking processes may be utilized.
Owner:CORDIS CORP
Ticagrelor sustained-release tablet system and preparation method thereof
InactiveCN102657629BMaintain blood levelsOrganic active ingredientsPharmaceutical delivery mechanismTicagrelorPediatrics
The invention provides a ticagrelor sustained-release tablet system and a preparation method of the ticagrelor sustained-release tablet system. The preparation method comprises the following steps of: firstly uniformly mixing 10-60% of ticagrelor, 5-60% of a filler and 5-60% of high molecular polymer in percentage by weight, adding a granulating solution to granulate; fully drying the obtained granules at a temperature of 50-60 DEG C, uniformly mixing the sieved granules with 0.25-10% of a lubricant and / or 0-10% of a flow aid, carrying out tabletting to obtain the ticagrelor matrix type sustained-release tablets, wherein the granulating solution is preferably water, an alcohol-water solution or absolute ethyl alcohol; the granule size of the ticagrelor is below 100 micrometers; and the content of the ticagrelor is 50-300mg in the preparation process. The ticagrelor matrix type sustained-release tablet system provided by the invention has the advantages that a patient can take the ticagrelor once a day to ensure that the drug dependence of the patient can be improved and the risk of myocardial infarction or apoplexy caused by acute thrombosis due to a dose of the ticagrelor missing of the patient is reduced.
Owner:SHENZHEN HUALIKANG BIOLOGICAL MEDICINE
Drug-eluting coronary artery stent coated with anti-platelet-derived growth factor antibodies overlaying extracellular matrix proteins with an outer coating of anti-inflammatory (calcineurin inhibitor) and/or anti-proliferatives
ActiveUS9339593B2Promote growthReduce restenosis rateStentsSurgeryAnti plateletCalcineurin phosphatase
Owner:BJORK JR ROBERT L
Dipyridamole-loaded polyurethane anticoagulative material and preparation process thereof
InactiveCN104548198AGood blood compatibilityImprove the success rate of transplantationBlood vesselsDipyridamoleIntimal proliferation
Owner:胡作军 +3
Formulation of ticagrelor or pharmaceutically acceptable salt thereof
The invention relates to a formulation of ticagrelor or a pharmaceutically acceptable salt thereof. In particular, the present invention relates to an improved formulation of ticagrelor, or a pharmaceutically acceptable salt thereof, administered once a day. The present invention can achieve a plasma concentration of ticagrelor of greater than about 0.2 [mu] g / mL within 2 hours in a subject; and aplasma concentration of ticagrelor of greater than about 0.2 [mu] g / mL can still be achieved after administration in a subject for 12 hours; and generating a maximum plasma concentration (Cmax) of ticagrelor or a pharmaceutically acceptable salt thereof between about 0.2 [mu] g / mL and about 0.8 [mu] g / mL in the subject. According to the preparation of the ticagrelor or the medicinal salt thereof,the administration frequency can be reduced, so that the administration compliance of a patient can be improved, and the risk of myocardial infarction or stroke caused by acute thrombosis due to missing administration of the ticagrelor by the patient is reduced.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Preparation process of dipyridamole-loaded polyurethane anticoagulant material
InactiveCN104548198BGood blood compatibilityImprove the success rate of transplantationBlood vesselsDipyridamoleAcute thrombosis
The invention discloses a dipyridamole-loaded polyurethane anticoagulative material and a preparation process thereof. The material adopts a core-shell structure, the inner layer is a dipyridamole loaded layer, and the outer layer is a polyurethane main body layer. When the material is used as an artificial blood vessel to be transplanted, the dipyridamole can be slowly released in a long term, adhesion, aggregation and activation of platelets on the surface of the artificial blood vessel are inhibited, the blood compatibility of the artificial blood vessel is improved, the acute thrombosis and intima proliferation can be effectively avoided, and the transplanting success rate of the artificial blood vessel can be effectively improved.
Owner:胡作军 +3
Reservoir eluting stent
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
A drug eluting balloon
ActiveCN105105892BPromote absorptionPrevent thrombosisStentsMedical devicesVascular endotheliumThrombus
Owner:DONGZHIMEN HOSPITAL OF BEIJING UNIV OF CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com