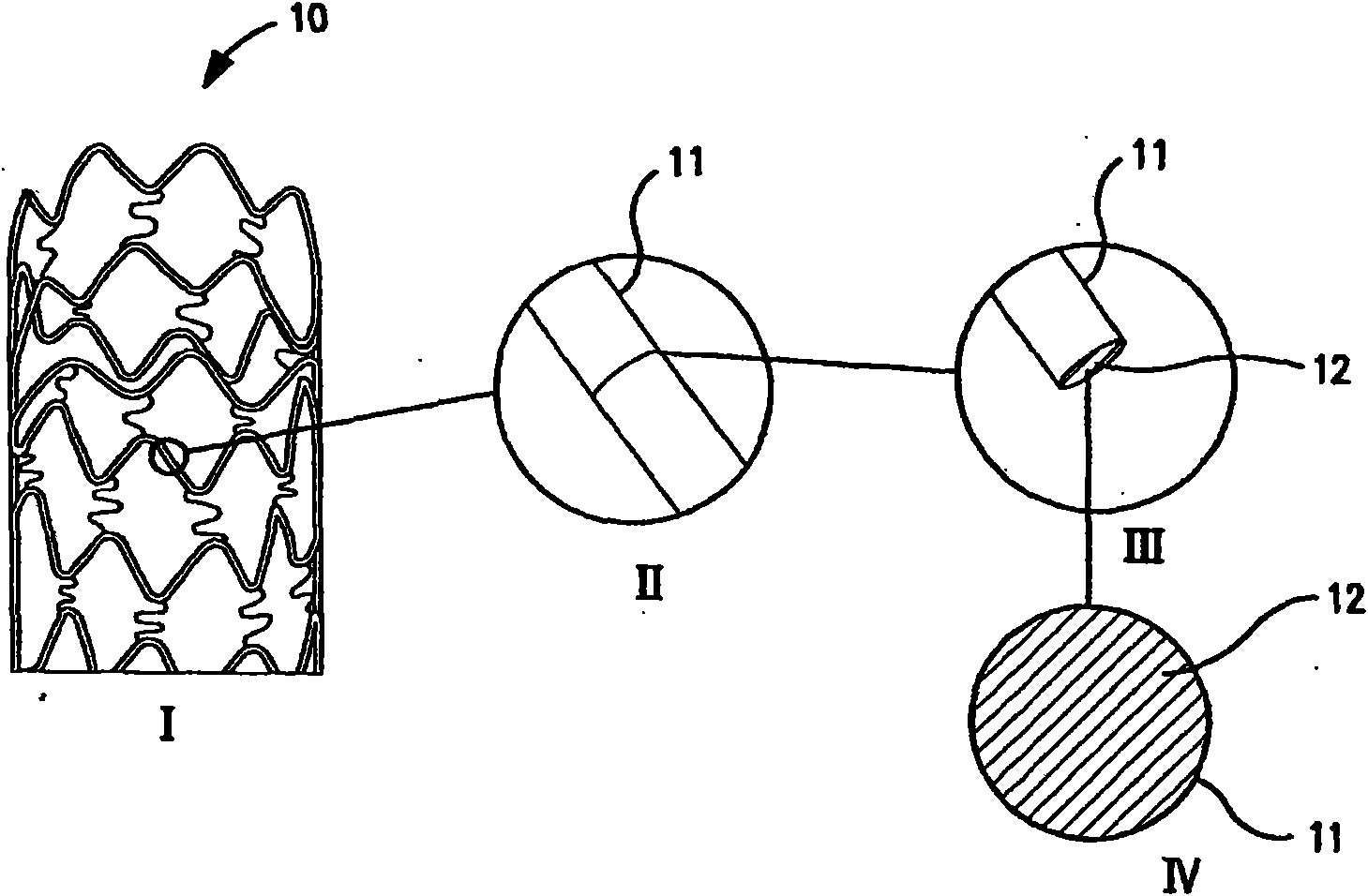
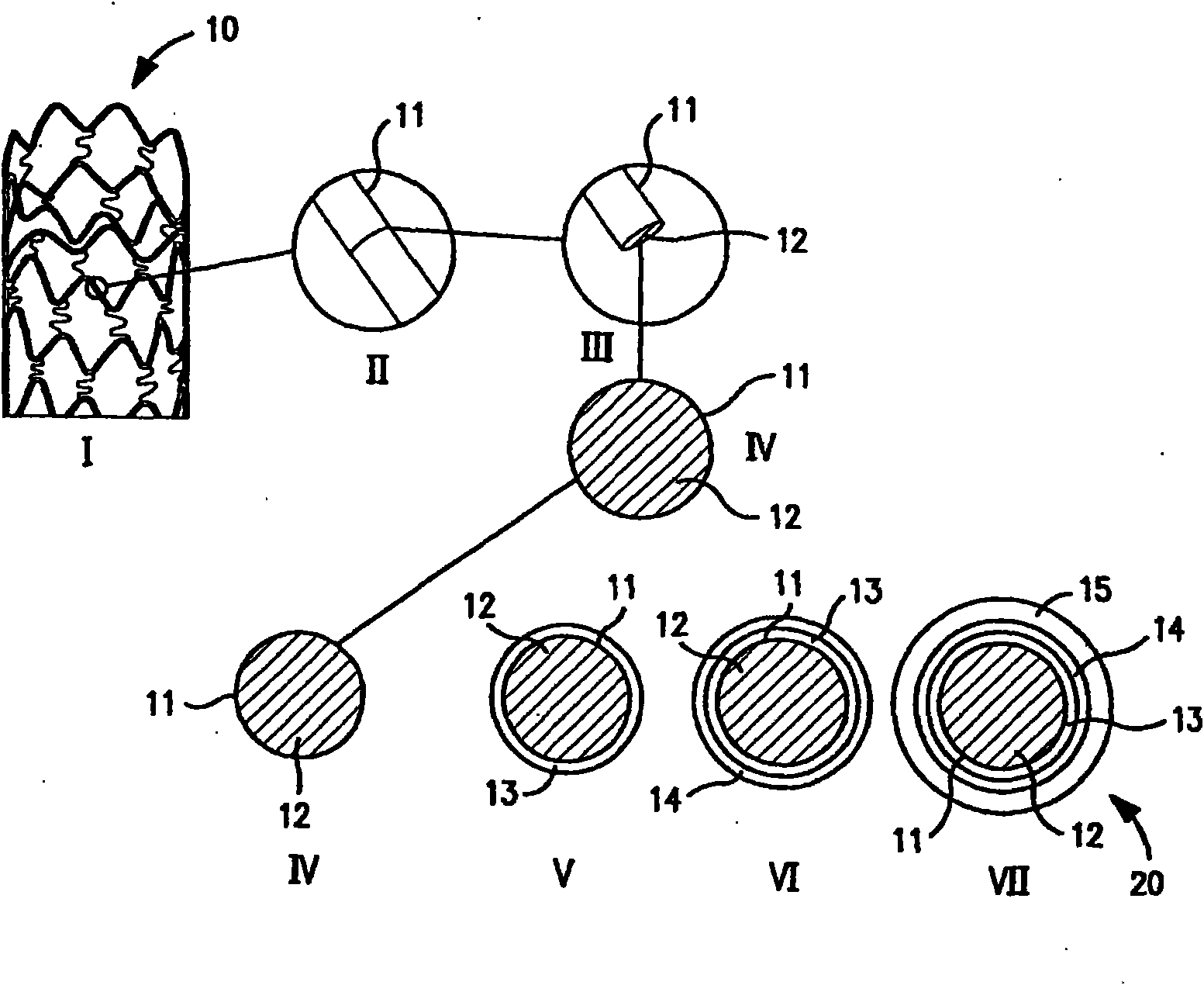
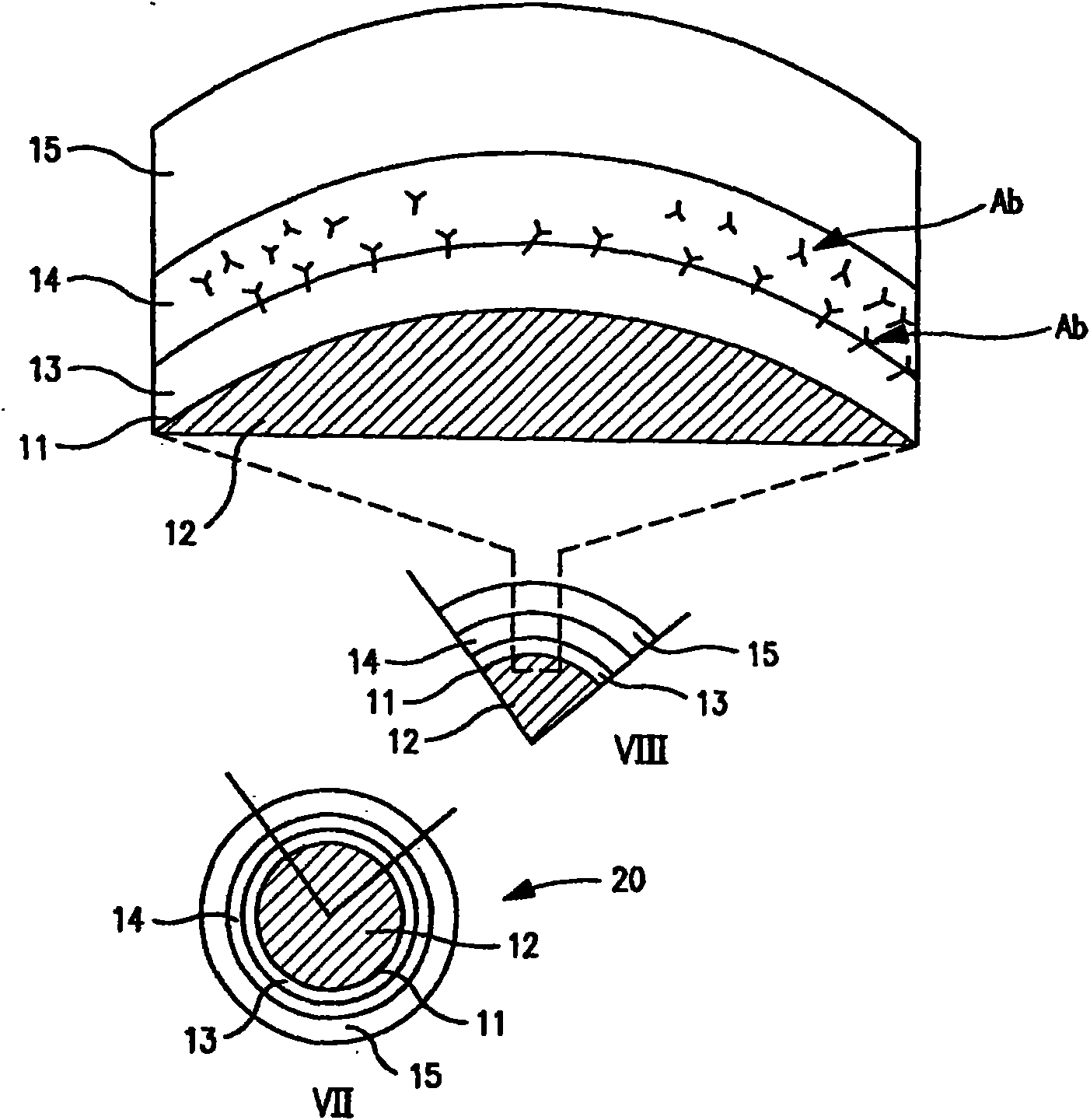
Multiple drug-eluting coronary artery stent for percutaneous coronary artery intervention
A technology of implants and vessels, applied in the field of percutaneous coronary angioplasty to treat heart disease and other vascular diseases, multi-drug eluting tubular vascular implants, can solve insignificant BM stents, Issues such as poor clinical outcomes and low open rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Agents: paclitaxel, sirolimus, fibronectin, and anti-PDGF monoclonal antibody.
[0145] [0143] Delivery method:
[0146] 1. Experimental stent delivery method - delivery from polymer matrix:
[0147] [0145] Agent solutions for the different layers prepared in a solvent miscible with the polymer carrier solution were mixed with the polymer solution at final concentrations ranging from 0.001 wt% to 30 wt% paclitaxel and sirolimus. Polymers that are biocompatible (i.e., do not elicit any adverse tissue reactions or promote mural thrombus formation) and degradable, such as lactone-based polyesters or copolyesters, e.g., polylactide, polycaprolactone - Glycolides, polyorthoesters; polyanhydrides; polyamino acids; polysaccharides; polyphosphazenes; poly(ether-ester) copolymers such as PEO-PLLA, or blends thereof. Nonabsorbable biocompatible polymers are also suitable candidates. Polymers such as polydimethylsiloxane; poly(ethylene-vinyl acetate); acrylate-based polymers or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com