Ticagrelor sustained-release tablet system and preparation method thereof
A technology for ticagrelor and sustained-release tablets, which is applied in the field of medicine and can solve problems such as half-life of only 12 hours and difficulty in development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
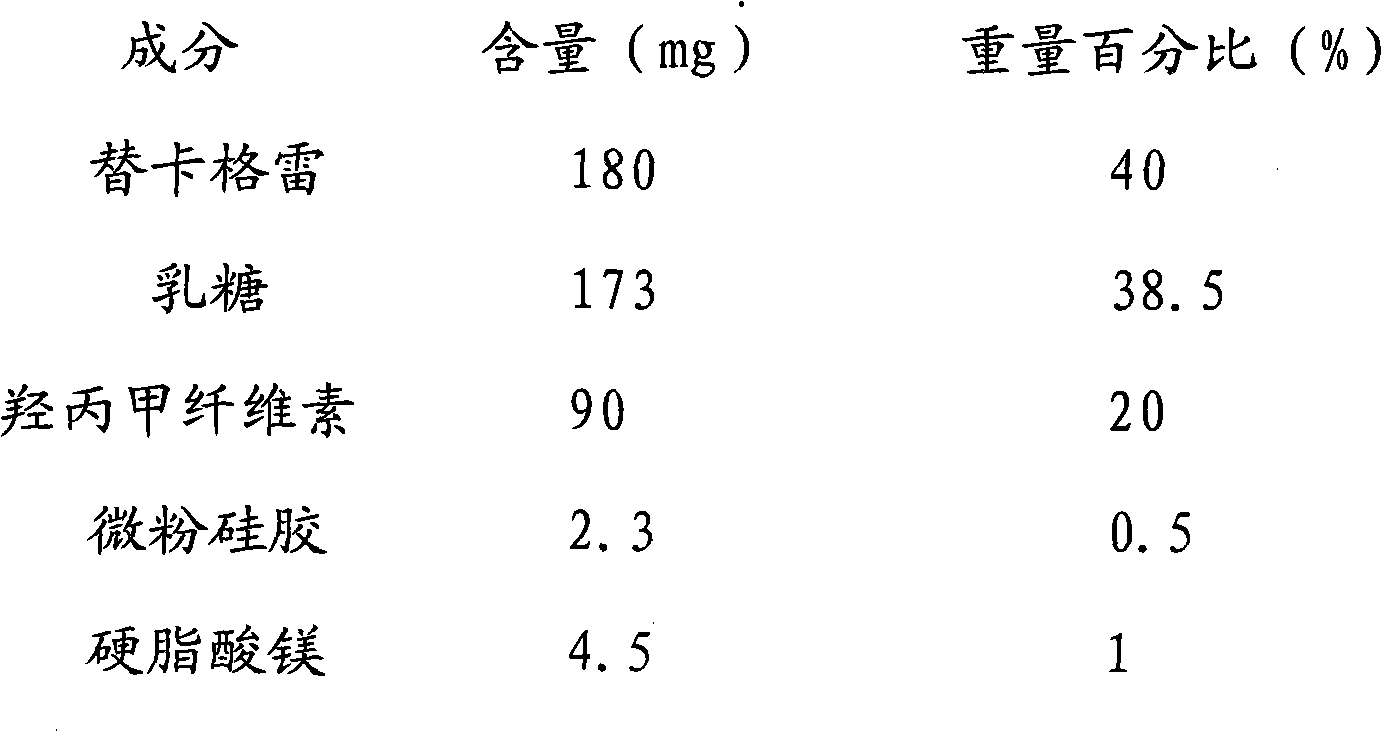
[0031] Ticagrelor Sustained Release Tablet System Formula Composition:
[0032]
[0033] The particle size of ticagrelor: 30 μm.
[0034] To prepare ticagrelor extended-release tablets:
[0035] Mix ticagrelor, lactose, and hypromellose first, add appropriate amount of water to granulate; fully dry the prepared granules at 50-60°C, and mix the sieved granules with magnesium stearate and micropowder silica gel Post-compression to make ticagrelor matrix sustained-release tablets.
Embodiment 2
[0037] Ticagrelor Sustained Release Tablet System Formula Composition:
[0038]
[0039] The particle size of ticagrelor: 15 μm.
[0040] To prepare ticagrelor extended-release tablets:
[0041] Mix ticagrelor, lactose, and hypromellose first, add appropriate amount of water to granulate; fully dry the prepared granules at 50-60°C, and mix the sieved granules with magnesium stearate and micropowder silica gel Post-compression to make ticagrelor matrix sustained-release tablets.
Embodiment 3
[0043] Ticagrelor Sustained Release Tablet System Formula Composition:
[0044]
[0045] The particle size of ticagrelor: 5 μm.
[0046] To prepare ticagrelor extended-release tablets:
[0047] Mix ticagrelor, lactose, and hypromellose first, add appropriate amount of water to granulate; fully dry the prepared granules at 50-60°C, mix the sieved granules with magnesium stearate, and press into tablets , made of ticagrelor matrix sustained-release tablets.
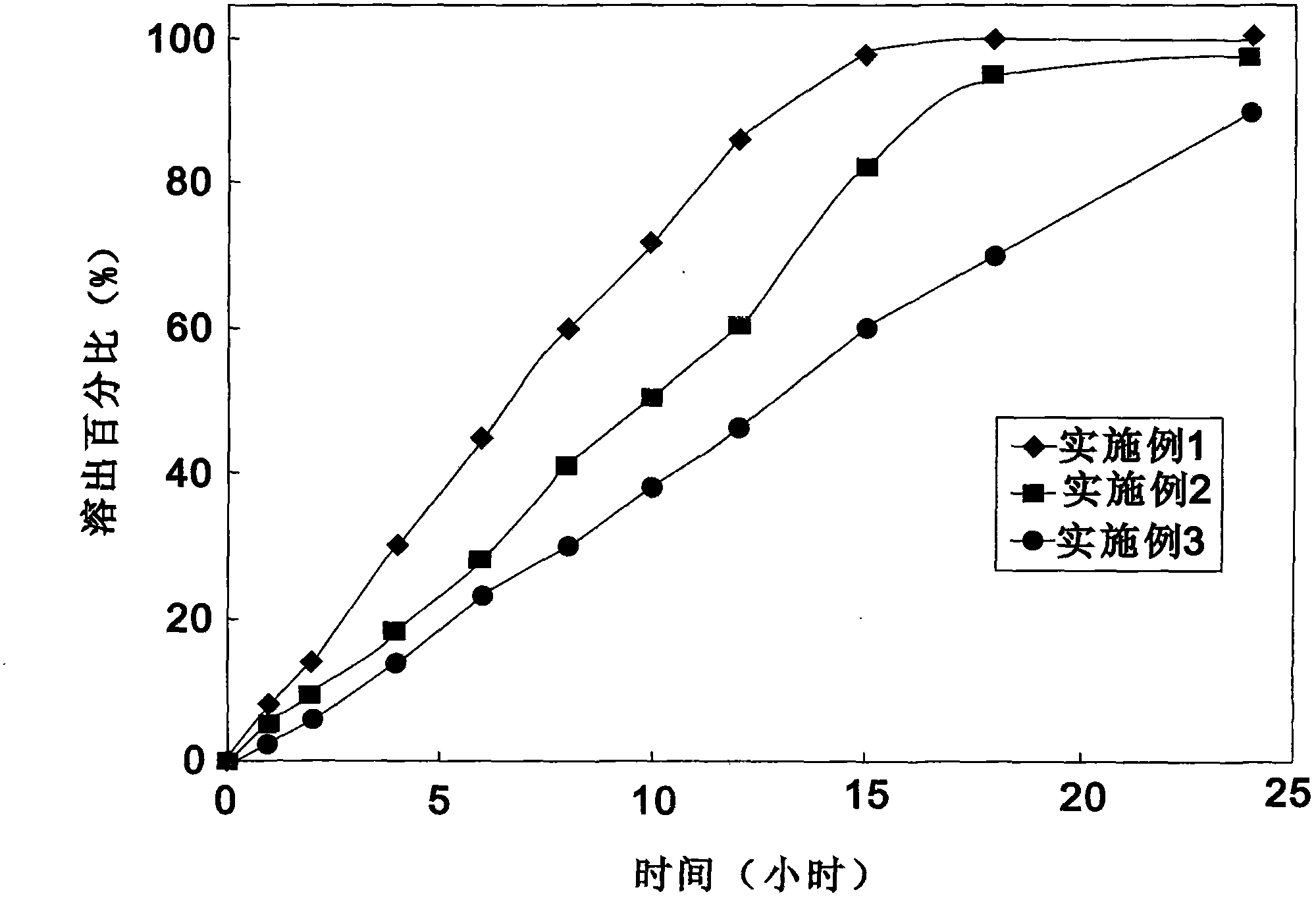
[0048] With 900ml adding 1% sodium dodecyl sulfate (SDS) pH6.8 phosphate buffer saline as stripping medium, constant temperature is to 37 ℃, in the paddle method that 50 turns, embodiment 1 to embodiment 3 are measured, stripping Curve see attached figure 1 .
[0049] from figure 1 It shows that the ticagrelor sustained-release tablet of the present invention makes the drug continuously effective within 12-24 hours, and can release slowly under physiological conditions, so that the patient can take it once a day, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com