Preparation of ticagrelor or pharmaceutical salt thereof
A technology for ticagrelor and preparations, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, and pill delivery, etc., can solve problems such as no conformational change and signal transmission, and achieve good reproducibility , High degree of industrialization, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
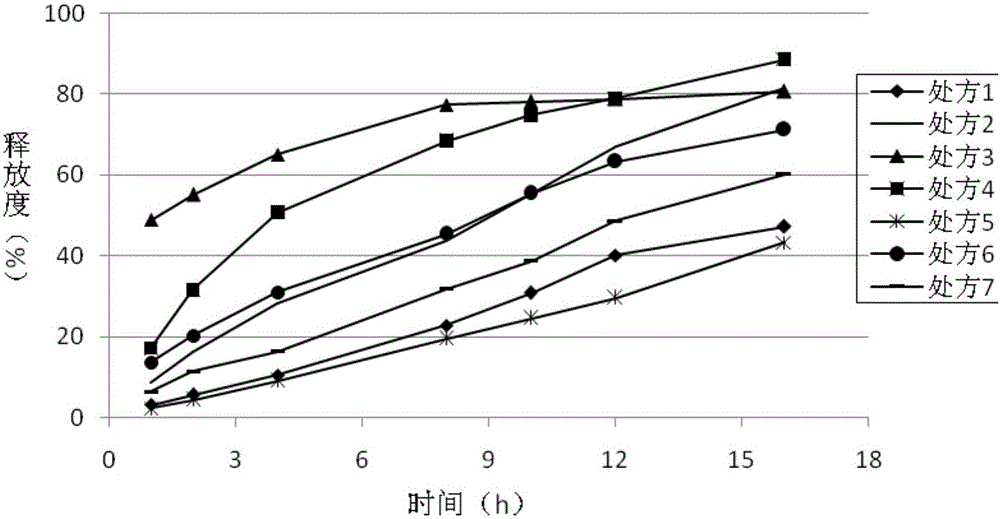
[0081] Polyoxyethylene, sodium alginate, and Kollidon SR were used as skeleton materials to prepare sustained-release tablets, and hypromellose in CN1102657629A was used to prepare ticagrelor sustained-release tablets for comparative study, and the release rate was evaluated.
[0082] Element
Prescription 1
Prescription 2
Prescription 3
Prescription 4
Prescription 5
Prescription 6
Prescription 7
180mg
180mg
180mg
180mg
180mg
180mg
180mg
PEO N80
100mg
-
-
-
-
-
-
PEO N10
-
50mg
-
-
-
-
Kollidon SR
-
-
50mg
-
-
-
-
sodium alginate
-
-
-
50mg
-
-
-
HPMC K4M
-
-
-
-
100mg
-
-
HPMC K100LV
-
-
-
-
-
100mg
150mg
100mg
100mg
100mg
100mg
100mg
100mg
100mg
...
Embodiment 2
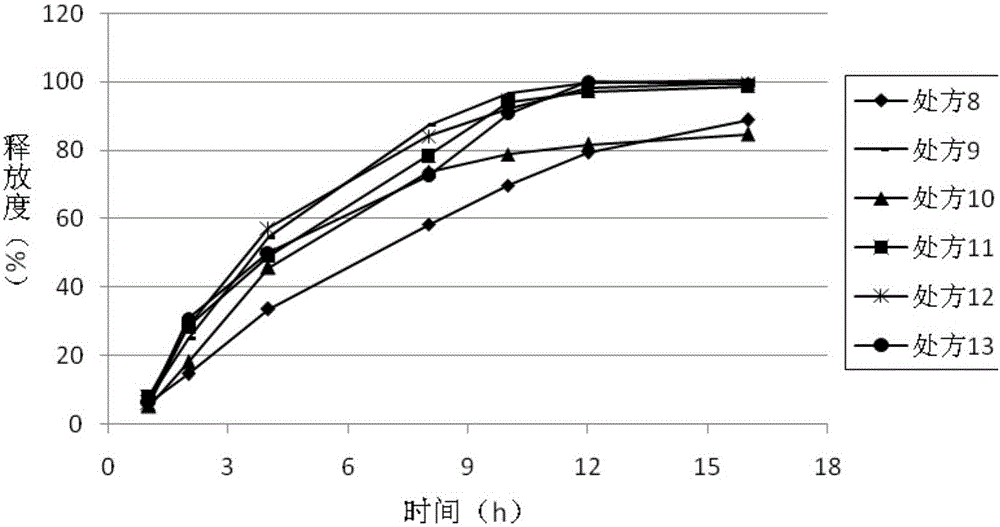
[0090] Considering that ticagrelor is almost insoluble in water, and there is a phenomenon of incomplete dissolution in the later stage when it is directly mixed with sustained-release materials, in order to make the release rate in vitro more controllable and meet the requirements for later release, the present invention proposes "immediate release first and then The design idea of "sustained release" is that firstly, the solid dispersion technology is used to increase the release rate of the raw material, so that the raw material can be released quickly; then the solid dispersion is mixed with the sustained release material, and compressed into tablets to prepare ticagrelor sustained release tablets.
[0091] Using povidone and copovidone as carriers respectively, the solid dispersion was prepared by solvent method, and the prepared solid dispersion was uniformly mixed with polyoxyethylene, microcrystalline cellulose, and magnesium stearate, and pressed into tablets to prepa...
Embodiment 3
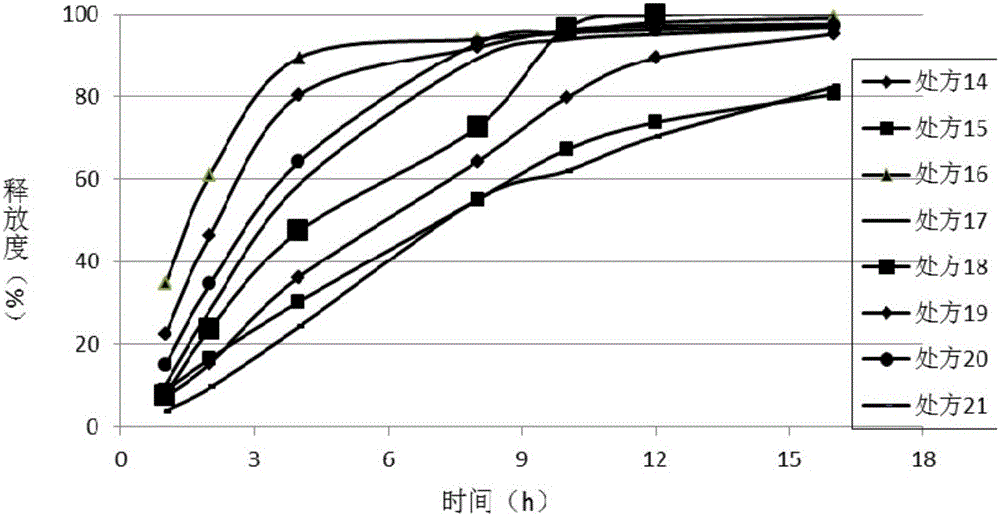
[0100] The solid dispersion of ticagrelor was mixed with sodium alginate, Kollidon SR, and polyoxyethylene as sustained-release matrix materials to prepare sustained-release formulation compositions, and the release behavior of the formulations was investigated.
[0101] Element
Prescription 14
Prescription 15
Prescription 16
Prescription 17
Prescription 18
Prescription 19
Prescription 20
Prescription 21
180mg
180mg
180mg
180mg
180mg
180mg
180mg
180mg
Copovidone
180mg
180mg
180mg
180mg
180mg
180mg
180mg
180mg
PEO N80
-
-
75mg
75mg
150mg
112.5mg
-
PEO 205
-
-
-
-
-
-
-
100mg
PEO N60K
-
-
-
-
-
-
-
50mg
Kollidon SR
75mg
150mg
-
75mg
112.5mg
112.5mg
-
112.5mg
-
-
150mg
-
15...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com