Ticagrelor and aspirin compound tablet and preparation method thereof
A technology of aspirin and ticagrelor, which is applied in the directions of drug combination, pill delivery, pharmaceutical formulation, etc., can solve the problems of poor stability, high viscosity, serious sticking of tableting devices, etc., and achieves reduction of stickiness and less generation of impurities. , the effect of prolonging the storage time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
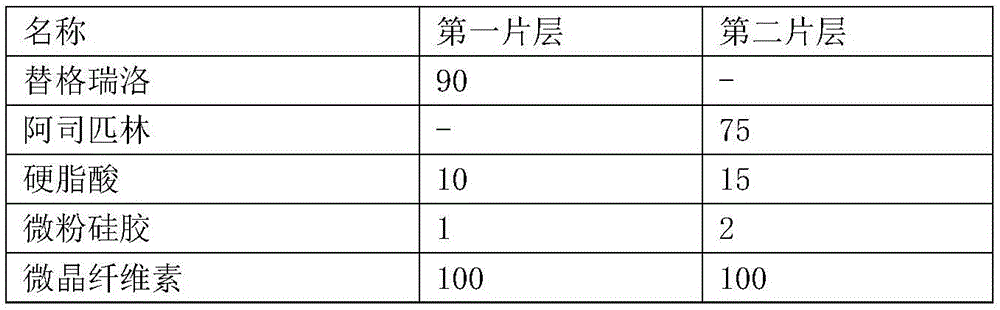
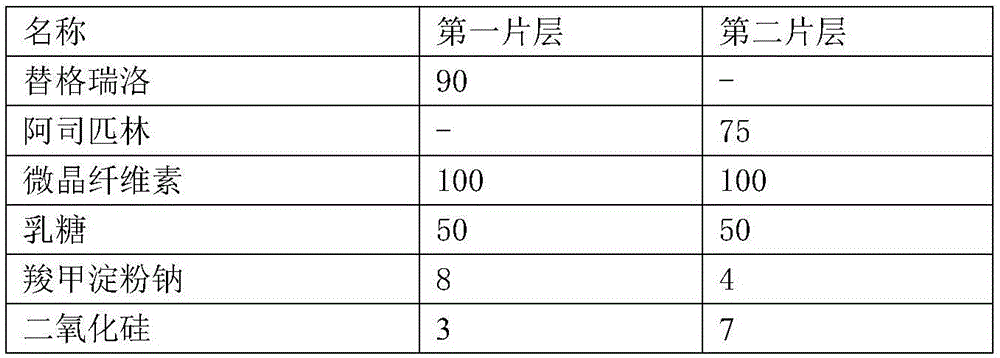
[0045] This embodiment provides a composite tablet of ticagrelor and aspirin, which includes the following raw materials:
[0046] name The first sheet (mg) can be Second sheet (mg) Ticagrelor 90 - aspirin - 75 stearic acid 7.5 15 Micropowder silica gel 1.5 1.5 Mannitol 120 45 corn starch - 75 Low-substituted hydroxypropyl cellulose 9 - hydrogenated castor oil 3 1.5 Magnesium stearate 3 1.5
[0047] The preparation method of the ticagrelor and aspirin composite tablet of the present embodiment comprises the steps:
[0048] (1) According to the amount of raw materials described in the first sheet in the above table, the required ticagrelor, stearic acid, micronized silica gel, mannitol, low-substituted hydroxypropyl cellulose, hydrogenated castor oil and stearin Magnesium acid mixed to prepare the first granules; according to the amount of raw materials described in the second sheet in the above tab...
Embodiment 2
[0051] This embodiment provides a composite tablet of ticagrelor and aspirin, which includes the following raw materials:
[0052] name The first layer can second sheet Ticagrelor 90 - aspirin - 100 stearic acid 10 20 Micropowder silica gel 1.5 1.5 Mannitol 120 45 pregelatinized starch 80 75 Crospovidone 4 - talcum powder 7 3 Magnesium stearate 2 8
[0053] The present embodiment provides a kind of preparation method of ticagrelor and aspirin composite tablet, comprising the following steps:
[0054](1) According to the amount of raw materials described in the first sheet in the above table, mix the required ticagrelor, stearic acid, micropowder silica gel, mannitol and pregelatinized starch, add absolute ethanol and place Granulated in a wet granulator, the obtained granules were dried at 60°C, mixed with crospovidone, talc powder and magnesium stearate, rolled into tablets, and crushed into granu...
Embodiment 3
[0058] This embodiment provides a composite tablet of ticagrelor and aspirin, which includes the following raw materials:
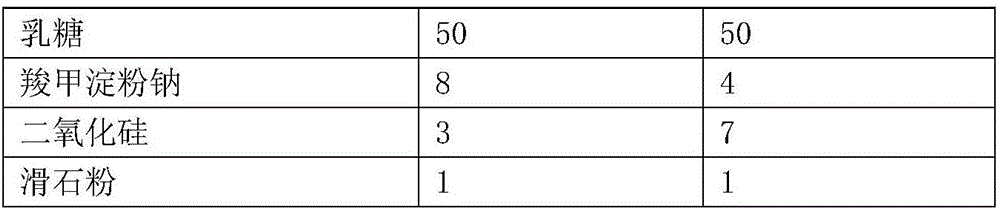
[0059] name The first layer can second sheet Ticagrelor 90 - aspirin - 100 stearic acid 1 4 Micropowder silica gel 1 2 Mannitol 100 50 lactose 50 70 Carboxymethyl Starch Sodium 12 6 polyethylene glycol 6000 - 4 Magnesium stearate 1 1
[0060] The present embodiment provides a kind of preparation method of ticagrelor and aspirin composite tablet, comprising the following steps:
[0061] (1) According to the amount of raw materials described in the first sheet in the above table, mix the required ticagrelor, stearic acid, micronized silica gel, mannitol, lactose, and sodium carboxymethyl starch, and then add stearin Magnesium acid, stirred evenly, and dry granulated to obtain the first granule.
[0062] (2) According to the amount of raw materials described in the second sheet ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com