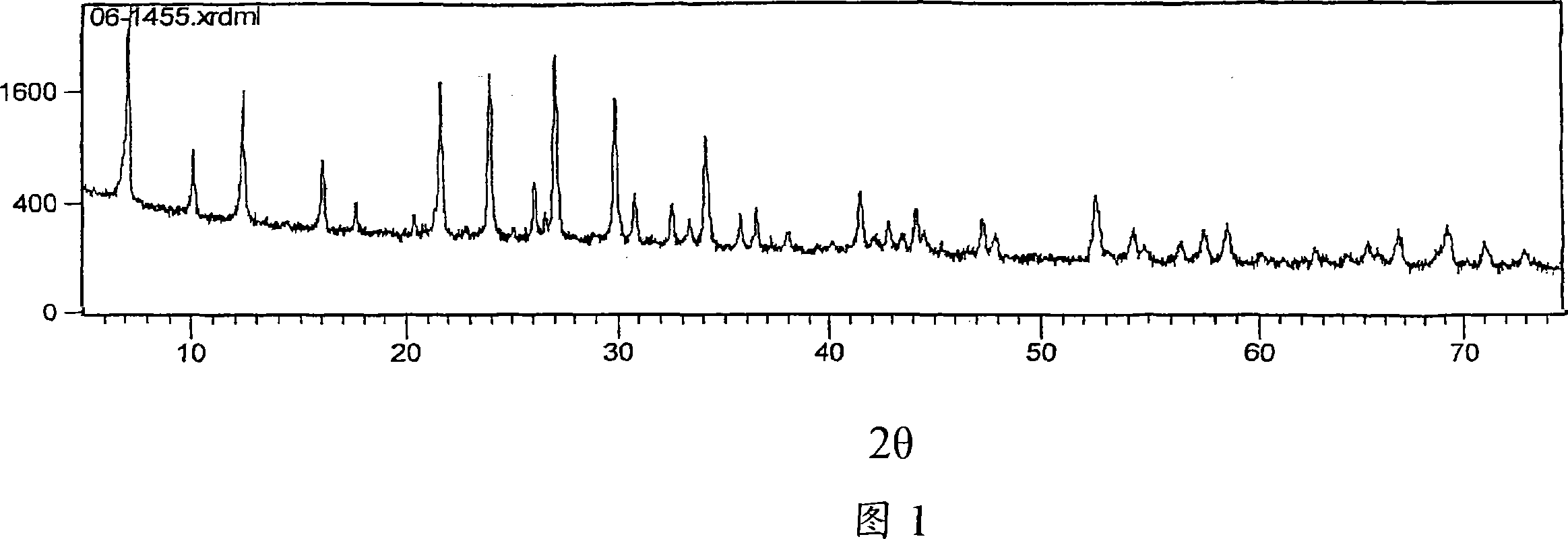
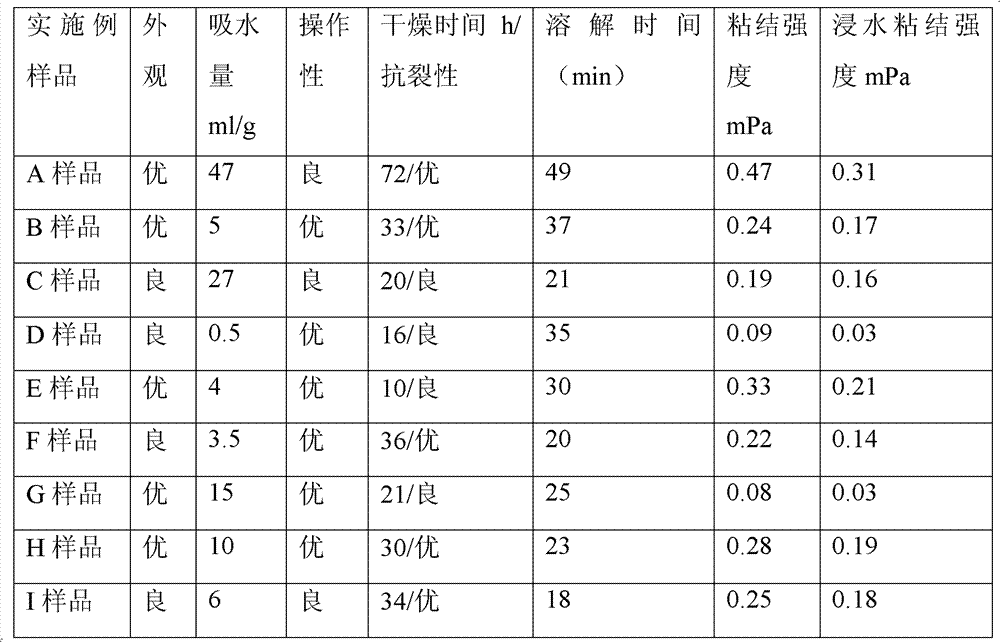
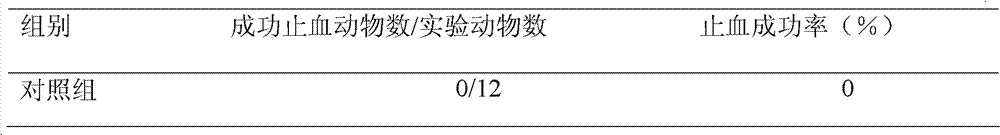
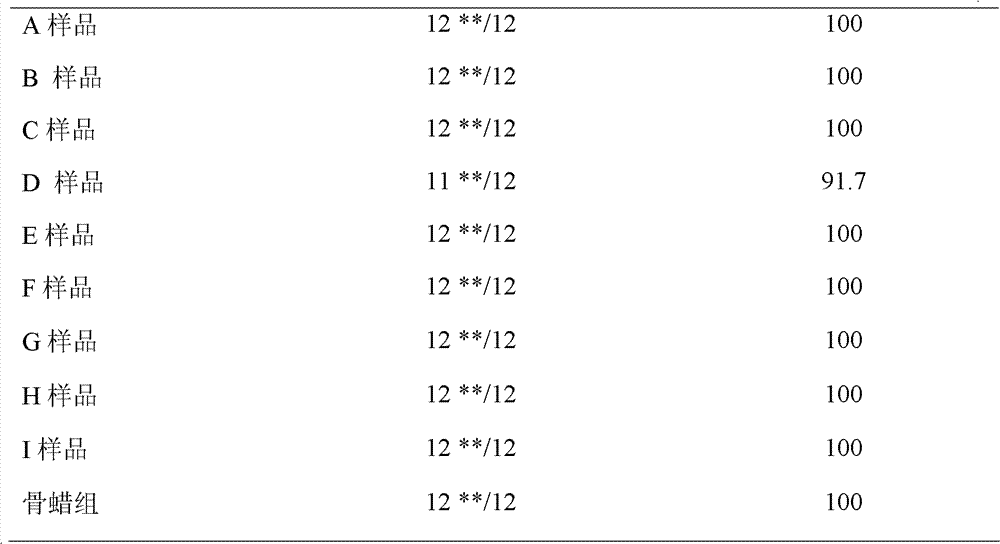
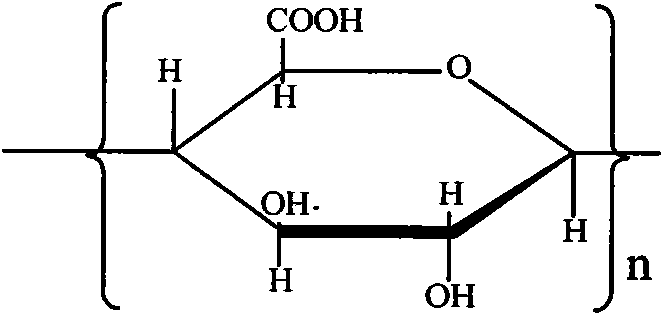
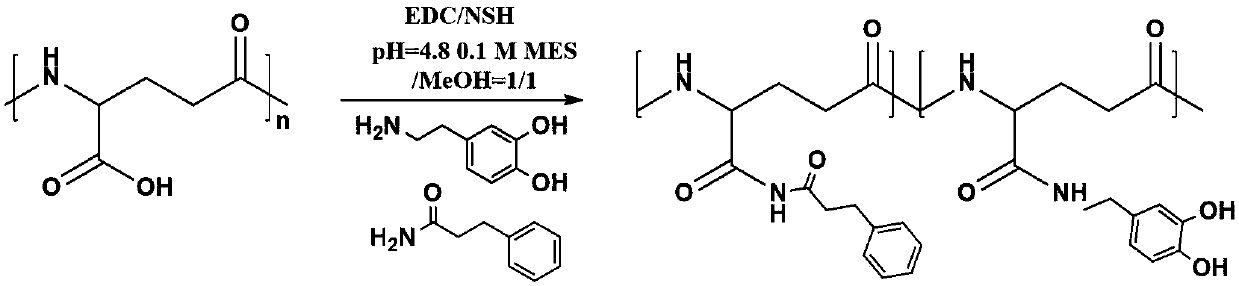
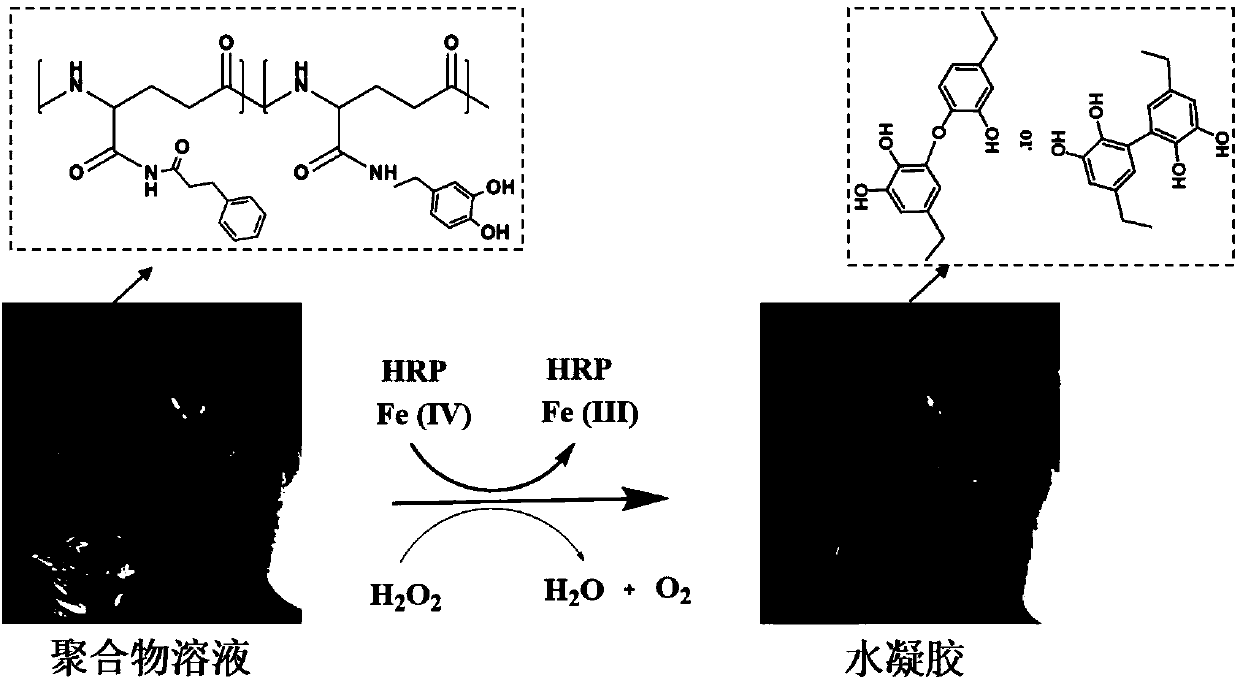
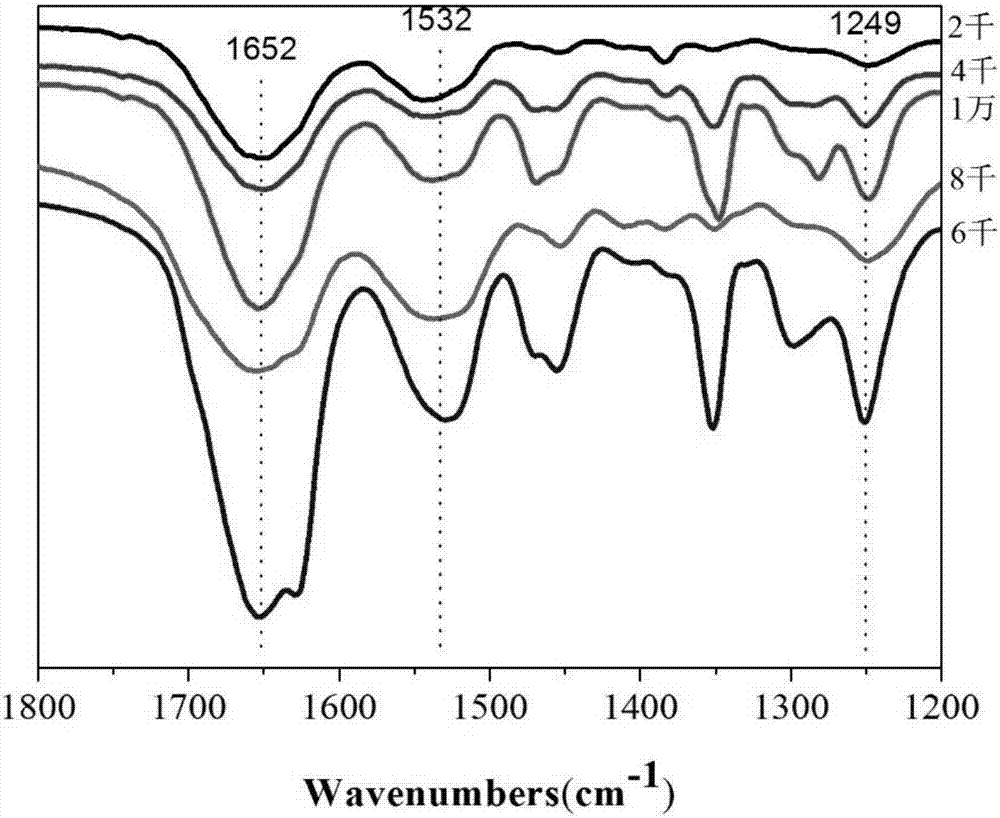
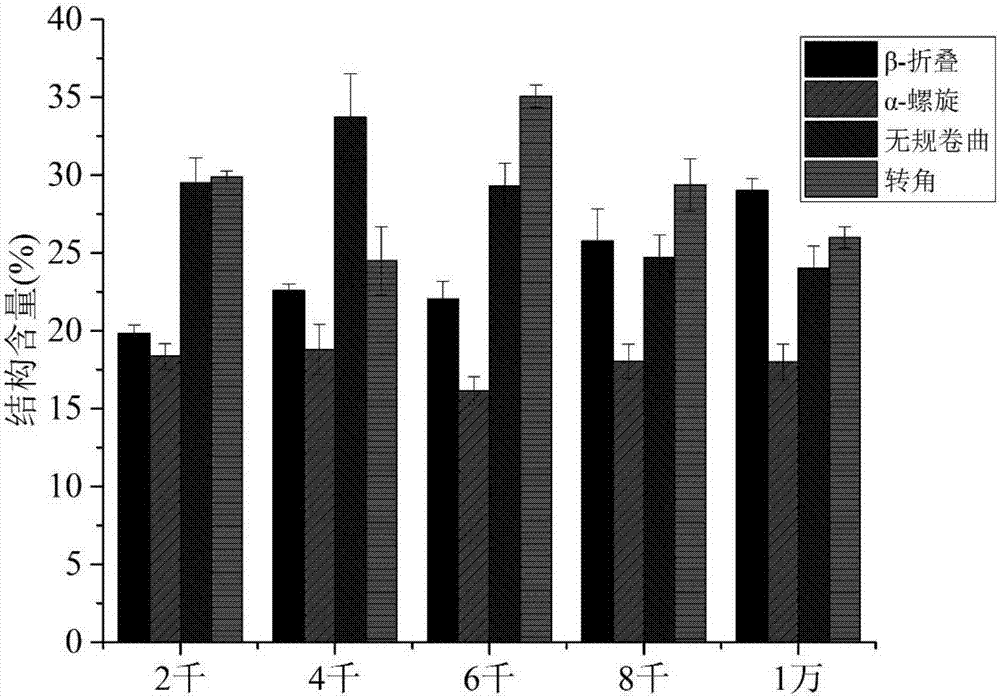
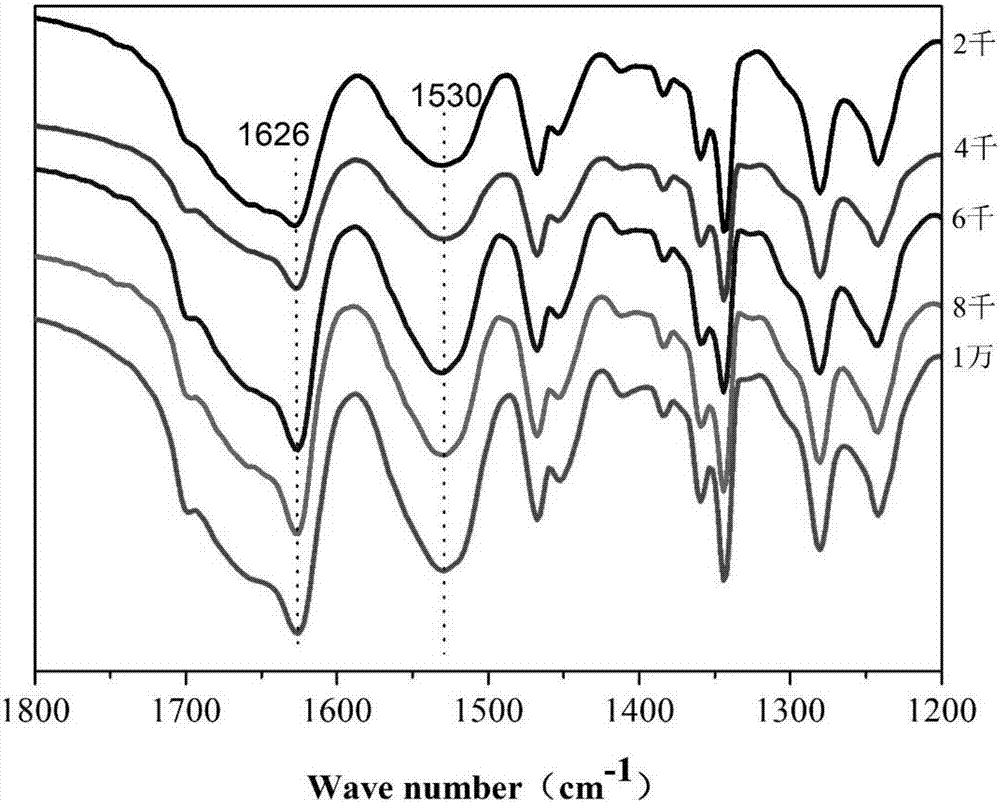
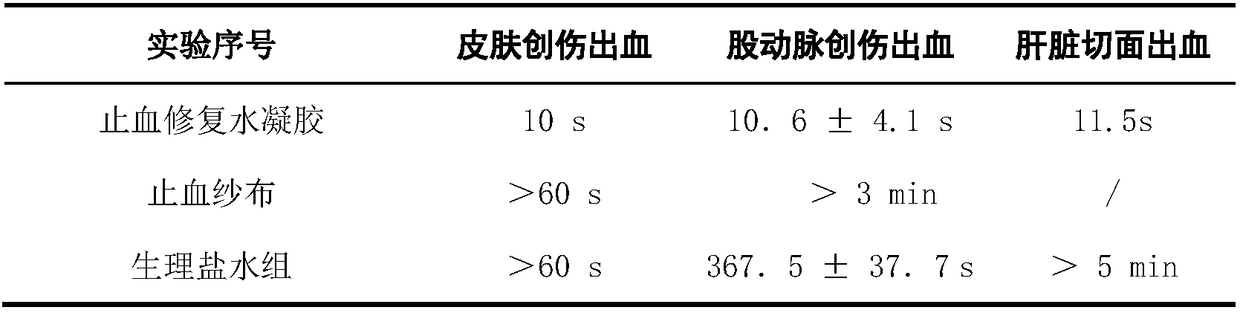
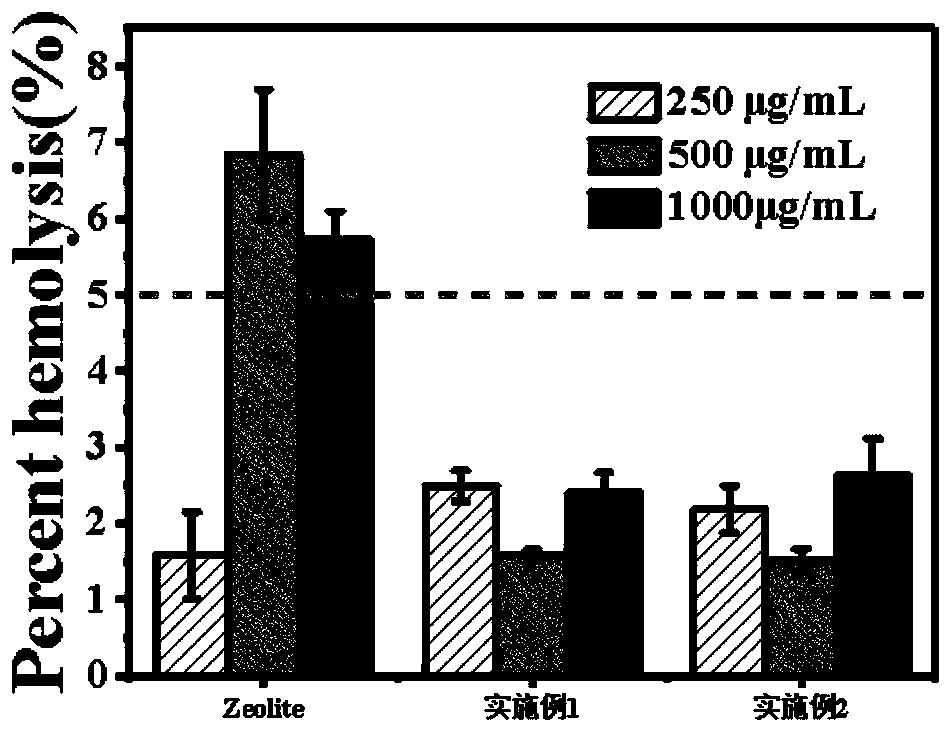
Patents
Literature
120results about How to "Fast hemostasis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Zeolite hemostatic dressings and preparation method and application thereof
The invention relates to the high degree of exchange Ca-A type zeolite hemostasis dressing and the preparation method and the purpose. The zeolite hemostasis dressing of the invention containing the zeolite has fast hemostasis speed, and has no bacterium, no pyrogen, no cell toxicity, no hypersensitive reaction and no skin irritation, the using is convenient and the cost is low.
Owner:深圳泰明嘉业药业有限公司
Medical absorbable skeletal wound hemostatic material and preparation method thereof
InactiveCN102727930AEasy to useHigh hardnessSurgical adhesivesPharmaceutical delivery mechanismWound healingAlcohol
The invention relates to a medical absorbable skeletal wound hemostatic material with accelerating wound healing and a preparation method thereof. The absorbable skeletal wound hemostatic material comprises 40-95 wt% of matrix material and 5-60 wt% of auxiliary material, wherein the matrix material comprises oligosaccharide, polysaccharide or a mixture of oligosaccharide and polysaccharide, and the auxiliary material comprises (1) one or more of polyhydroxy alcohols, (2) one or more of plant oil, and (3) one or more of emulsifiers. The preparation method comprises the following steps: carrying out chemical blending and latex blending on the matrix material and the auxiliary material with the prescribed dose, cooling to form solid clumps, packaging, and then conducting disinfection.
Owner:ZHUHAI ORTUS BIOTECH
Preparation method of oxidized regenerated cellulose hemostatic material with micro-nano composite structure
ActiveCN102908651APromote absorptionShort hemostatic timeArtificial filaments from cellulose derivativesAbsorbent padsMicro nanoDecomposition
The invention provides a preparation method of an oxidized regenerated cellulose hemostatic material with a micro-nano composite structure and relates to a preparation method of an oxidized regenerated cellulose hemostatic material. The invention aims to solve the problems of low hemostatic speed, great effect on environment and uneasiness in decomposition of the existing hemostatic material. The preparation method comprises the following steps: firstly, preparing cellulose solution; secondly, netting fibers in a wet state and reinforcing into a nano layer cellulose non-woven fabric; thirdly, preparing an oxidized nano cellulose non-woven fabric and oxidized viscose staple fibers; and fourthly, cotton-slitting and cotton-carding the oxidized viscose staple fibers prepared in the step 3 and compounding to obtain the oxidized regenerated cellulose hemostatic material with the micro-nano composite structure. The preparation method is applied to the preparation field of the oxidized regenerated cellulose hemostatic material.
Owner:WEIGAO HLDG +1
Preparation method of medical hemostatic occlusion dressing
The invention discloses a preparation method of a medical hemostatic occlusion dressing. The preparation method comprises the following steps: (1), taking and pouring deionized water into a reactor, heating the reactor, slowly adding lemon peel pectin and gelatin, and mechanically stirring at the same time; (2), after the addition of the lemon peel pectin and gelatin, stirring continuously, then adding other components into the reactor, and carrying out mechanical stirring uniformly at the same time; (3), after the mixing of the materials, cooling hydrogel to be at a room temperature, so as to obtain the medical hemostatic occlusion dressing. The medical hemostatic occlusion dressing prepared through the method provided by the invention is high in bleeding stopping speed, has auxiliary functions of promoting wound surface healing, preventing adhesion and the like, is also better in biomechanics and air permeability, and has an important clinical application value; the preparation method provided by the invention has the advantages that the process is simple, requirements for equipment are relaxed, raw materials are low in cost and readily available, the cost is low, and industrialization is easy to realize.
Owner:SUZHOU BEC BIOLOGICAL TECH
Powder absorbable haemostat and its preparation method
InactiveCN102309776AFast hemostasisGood hemostatic effectAbsorbent padsBandagesMicrosphereCross linker
The invention discloses a powder absorbable haemostat and its preparation method, which belongs to the technical field of novel hemostatic material. The main components are chitosan, sodium alginate and hyaluronic acid. The weight ratio of chitosan to sodium alginate to hyaluronic acid is (0.1-10): (0.1-10): 1, The mass fraction of a cross-linking agent calcium chloride solution is 0.5-5 wt%. The invention also discloses a preparation method of the powder absorbable haemostat. According to the invention, the soybean oil is taken as an oil phase, calcium chloride is taken as a cross-linking agent, composite microspheres of chitosan, sodium alginate and hyaluronic acid can be obtained through a simple emulsification crosslinking process, the composite microspheres is dried to obtain the powder absorbable haemostat. According to the invention, the performance advantages of chitosan, sodium alginate and hyaluronic acid are combined by the product, so that the product has a plurality of haemostasis mechanisms which can rapidly suck water and swell, and the powder absorbable haemostat has the good anti-adhesion performance and characteristic of promoting the heal of the wound.
Owner:北京博恩康生物科技有限公司
Preparation method and application of quick absorption saturation gelation seaweed hemostatic dressing
InactiveCN104338173ARaw materials are easy to getLow priceFibre treatmentDry-cleaning apparatus for textilesSurgical operationFiber
The invention discloses a preparation method of a quick absorption saturation gelation seaweed hemostatic dressing. The method includes the steps of: soaking, rinsing and drying on calcium fiber textile or non-woven products containing alginate, and the carrying out cutting, packaging and sterilizing to obtain the quick absorption saturation gelation hemostatic dressing. The invention also discloses the application of the quick absorption saturation gelation seaweed hemostatic dressing prepared by the above method. The invention is applicable to rapid absorption and removal of a large amount of surgical diffusate in surgical operation process, so as to realize the effects of rapid hemostasis and operation wound clean; the hemostatic dressing is used for burn, scald and other acute and chronic wounds, can cover the wound and prevent moisture loss in body fluids, provide a positive moist environment for wound healing, prevent the wound from effusion and erosion, isolate bacterial infection, and play the effects of stopping bleeding, relieving pain, promoting wound healing, and reducing scar.
Owner:许春晖 +1
Preparation method for chitosan porous hemostatic sponge
The invention discloses a preparation method for a chitosan porous hemostatic sponge, which includes the following steps: (Step 1) preparation of solutions; (Step 2) preparation of the chitosan porous hemostatic sponge: the chitosan solution, the gelatin solution and the glycerine solution are sufficiently agitated to be mixed according to the volume ratio of 3:3:1 under the water bath condition of 25DEG C, so that a mixed milky gel solution is obtained, the gel solution is then added with a calcium chloride solution, sufficiently agitated under the water bath condition of 25DEG C for 30 minutes and then added with a tannin solution and continues to be agitated under the water bath condition of 25DEG C for 20 minutes, so that a mixed red gel solution is obtained, and the calcium chloride solution and the tannin solution in the obtained mixed red gel solution respectively account for 0.5 percent and 0.01 percent; after the mixed red gel solution is poured into a mold, the mixed red gel solution is prefrozen for more than 12 hours to form an ice block and then freeze-dried under vacuum for 48 hours, the chitosan porous hemostatic sponge is obtained. The preparation method has the following advantages: preparation is simple, and the finished product has high chitosan content, high staunching speed and good antibacterial effect, and can naturally degrade.
Owner:苏州和其美生物材料有限公司
Manufacturing method of chitosan hemostatic membrane with high water-absorbing swelling performance
InactiveCN102028966AImprove water absorptionGood adhesion to wound surfaceAbsorbent padsBandagesAcetic acidGlycerol
The invention discloses a manufacturing method of a chitosan hemostatic membrane with high water-absorbing swelling performance. The method comprises the following steps of: dissolving chitosan with the deacetylation degree of between 55 and 99 percent and the molecular weight of between 100,000 and 500,000 in an appropriate amount of dilute solution of methanoic acid, acetic acid or hydrochloric acid, preparing 0.5 to 5 percent (m / v) chitosan solution, standing and defoaming; injection the solution into a mold, covering a layer of one-way permeable membrane on the mold, soaking the mold in an alkali medium, preparing chitosan gel by a one-way penetration and neutralization method and washing the gel with a large amount of deionized water until the gel is neutral; performing N-position or O-position carboxylation, soaking the gel in 1 to 5 percent CaCl2 dilute solution for a short period of time, crosslinking appropriately, taking the gel out and washing; soaking the gel in 1 to 10 percent (m / v) glycerol solution for 1 hour, taking the gel out and draining; and drying and molding at the temperature of 60 DEG C so as to obtain the hemostatic membrane. Chitosan has excellent hemostatic effect and Ca2+ is one of blood coagulation factors and has the effect of promoting blood coagulation, so that hemostasis is promoted under the synergism of the chitosan and the Ca2+.
Owner:ZHEJIANG HAIZHI INVESTMENT MANAGEMENT
Method for preparing bleeding stopping oxidized celluloses in ramie oxidation degumming procedures
ActiveCN106478825AChange structureWith hemostatic functionSurgical adhesivesPharmaceutical delivery mechanismOrganic solventColloid
The invention provides a method for preparing bleeding stopping oxidized celluloses in ramie oxidation degumming procedures. The method includes smashing raw ramie to obtain short fibers and soaking the short fibers to sufficiently moisten the short fibers and allow the short fibers to sufficiently swell; mixing the pretreated raw ramie short fibers and degumming solution with one another to obtain mixtures, heating the mixtures, then preserving heat, sufficiently removing colloid in the raw ramie by the aid of oxidability of specific oxidizing agents and simultaneously oxidizing celluloses with active hydroxyl to obtain oxidized celluloses with a large quantity of carboxyl; soaking oxidized and treated fibers by the aid of organic solvent aqueous solution and removing the unreacted oxidizing agents; separating the oxidized celluloses from water and carrying out drying treatment on the oxidized celluloses to ultimately obtain the powdery bleeding stopping oxidized celluloses. The method has the advantages that processes are simple, the original two working procedures are combined into a single working procedure, accordingly, the total reaction time can be shortened, medicines and the cost can be saved, and the efficiency can be greatly improved; the bleeding stopping oxidized celluloses which are products prepared by the aid of the method are high in bleeding stopping speed and bleeding stopping efficiency and can be widely applied to quickly stopping bleeding under conditions of war wound, trauma and the like, and stable effects can be realized.
Owner:DONGHUA UNIV
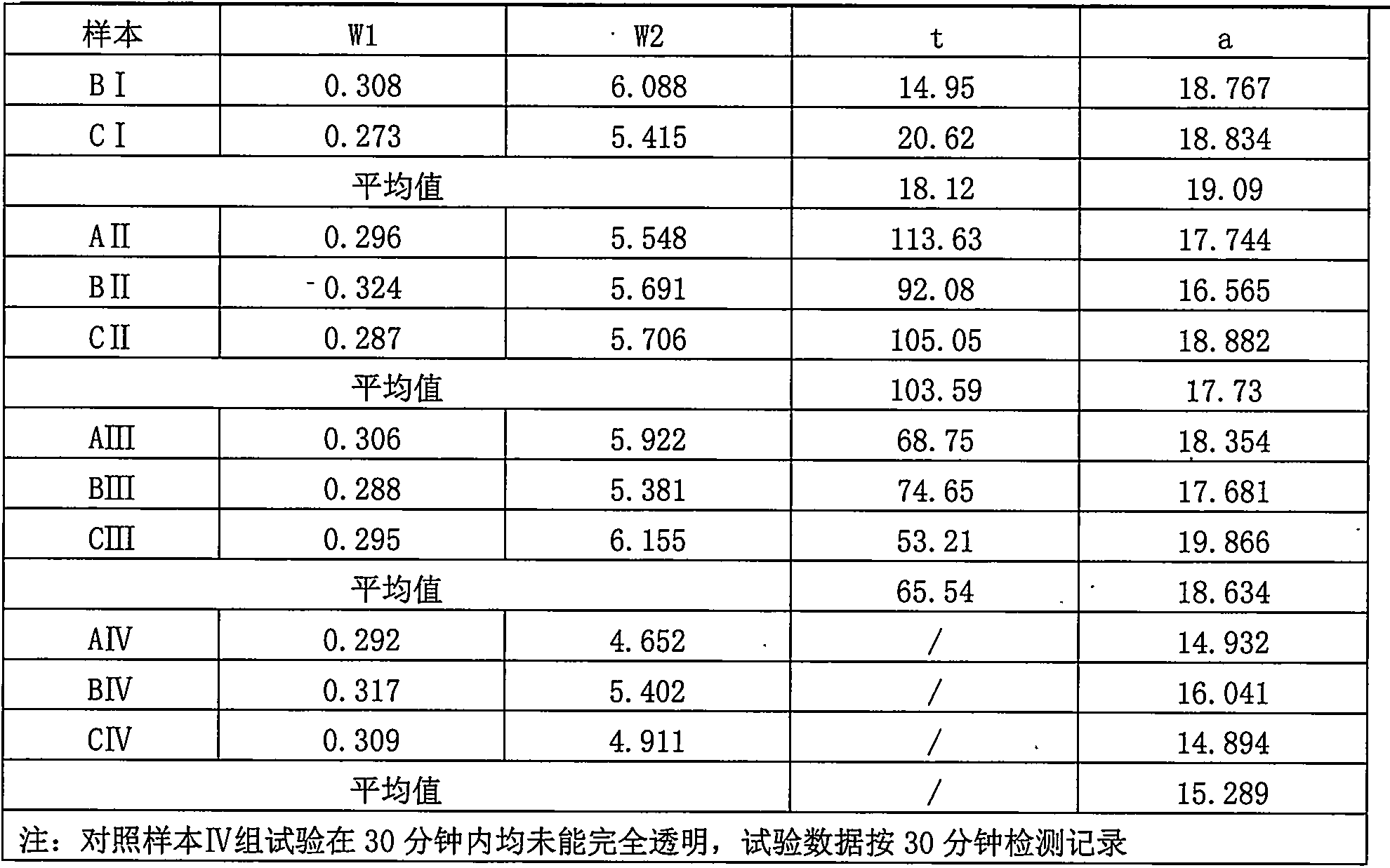
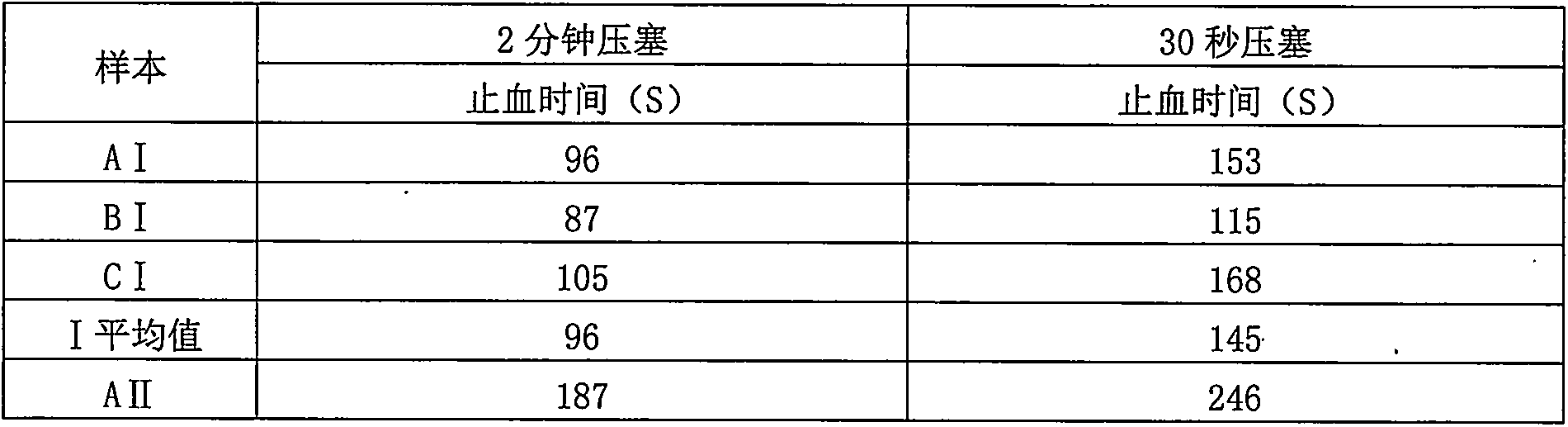
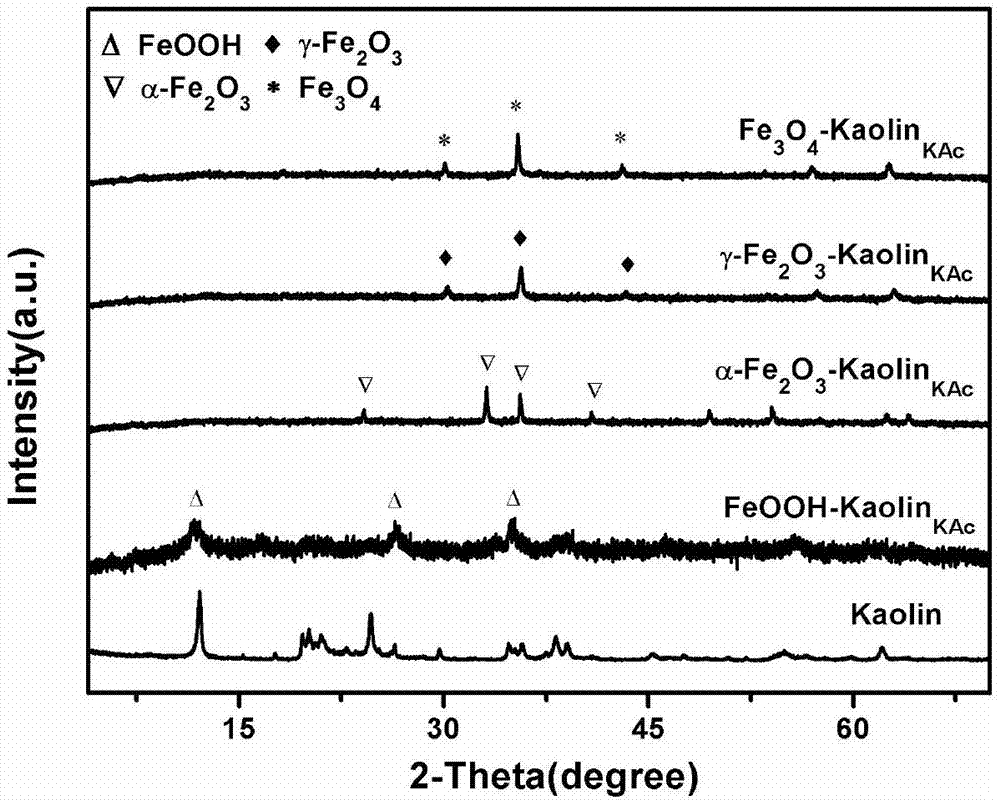
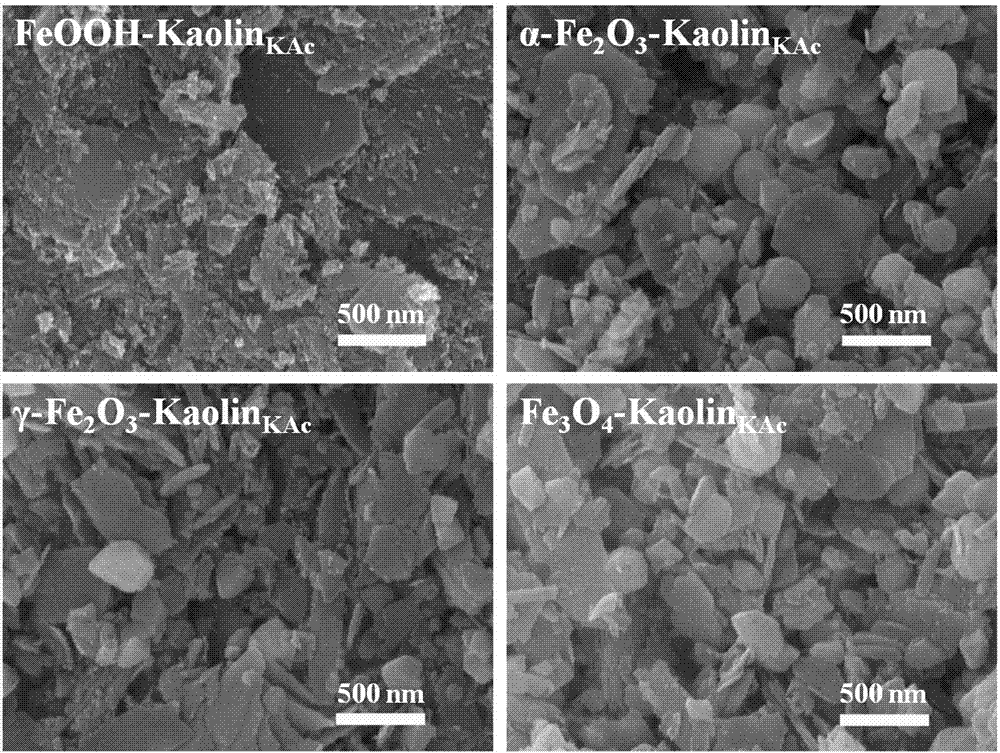
Iron oxide/nano kaolin-containing composite hemostatic and preparation method thereof
ActiveCN107213508AGood hemostatic effectFast hemostasisHeavy metal active ingredientsSurgical adhesivesBiocompatibility TestingIron oxide
The invention discloses an iron oxide / nano kaolin-containing composite hemostatic and a preparation method thereof. The composite hemostatic consists of nano kaolin and iron oxide, wherein the nano kaolin is adopted as a carrier; and the iron oxide is loaded on the surface of the nano kaolin. The composite hemostatic is prepared through a precipitation method, and has the advantages of being good in hemostatic effect, rapid in wound heal speed, free of conspicuous cytotoxicity, free of hemolysis, high in biocompatibility and the like.
Owner:CENT SOUTH UNIV
Porous gelatin sponge and preparation method thereof
ActiveCN108530671AIncrease internal porosityIncrease the areaPharmaceutical delivery mechanismAbsorbent padsCross-linkPorosity
The invention discloses a preparation method of porous gelatin sponge. The preparation method comprises the following steps: S1, dissolving a crosslinking agent; adding gelatin in a stirring process and carrying out crosslinking reaction; S2, transferring a cross-linked gelatin solution into a sealed reaction container; introducing gas until the pressure in the reaction container reaches one atmospheric pressure or more, and then keeping for 10min or more; S3, carrying out programmed cooling on the gelatin solution treated by step S2 to be completely cured; S4, freezing and drying the cured gelatin; S5, immersing the freeze-dried gelatin into absolute ethyl alcohol and purified water in sequence to remove the crosslinking agent; S6, carrying out secondary freeze-drying on the immersed gelatin in step S5 and sterilizing to obtain the porous gelatin sponge. According to the preparation method of the porous gelatin sponge, disclosed by the invention, gas introduction and pressurizing, andprogrammed cooling curing treatment are carried out after the gelatin is crosslinked, and the inner porosity and specific surface area of the gelatin sponge are effectively increased; the water absorption performance of the gelatin sponge is improved so that the bleeding-stopping speed of the gelatin sponge is improved and the bleeding-stopping time is shortened.
Owner:MEDPRIN REGENERATIVE MEDICAL TECH
Traditional Chinese medicine combination for treating gynecologic functional uterine bleeding diseases, preparation method and capsule quality control method thereof
ActiveCN101919942ADefinite curative effectFast hemostasisComponent separationSexual disorderPollenUterine bleeding
The invention relates to a traditional Chinese medicine combination for treating gynecologic functional uterine bleeding diseases, which comprises the following raw medicinal materials in portions by weight: 900-1100 portions of red paeonia, 900-1100 portions of motherwort, 200-400 portions of pseudo-ginseng, 900-1100 portions of agrimony, 500-700 portions of charred radix sanguisorbae and 500-700 portions of charred pollen typhae. The preparation method of the traditional Chinese medicine combination comprises the following steps: extracting from the red paeonia and the pseudo-ginseng with 80% ethanol, extracting from the motherwort, the agrimony, the charred radix sanguisorbae and the charred pollen typhae with water, and finally preparing the traditional Chinese medicine combination byan appropriate process. The invention also relates to a capsule quality control method of the traditional Chinese medicine combination, which comprises an identification step and a content determination step, wherein in the identification step, identification is carried out by using red paeonia control medicinal materials, paeoniflorin controls, pseudo-ginseng control medicinal materials, agrimony control medicinal materials, motherwort control medicinal materials and stachydrine hydrochloride controls based on a thin-layer chromatography; and in the content determination step, the content ofthe red paeonia should be not less than 12.0mg per capsule (measured by penoniflorin, C23H28O11 and determined by the red paeonia). The traditional Chinese medicine combination has the advantages of high hemostasis speed, good effect and the like. The capsule quality control method can conveniently and visually identify the preparation and can well ensure the effect of the traditional Chinese medicine combination.
Owner:ZHUZHOU QIANJIN PHARMA
Medical absorbable oxycellulose material and preparation method thereof
The invention belongs to the technical field of a biologically medical material, and concretely relates to a medical absorbable oxycellulose material and a preparation method thereof. The material has dual functions of stopping bleeding and restoring wound. The material takes natural cellulose as a raw material, and is oxidized by an oxidation system under certain technology condition, the obtained product is subjected to acid treatment, washing by water and drying to prepare the medical absorbable oxycellulose material. The oxidation system has the advantages of strong selectivity, mild and controllable condition, and environmental protection of preparation technology. The prepared material has the advantages of rapidly stopping bleeding and rapidly being absorbed, has wound restoration function, can promote the healing of wound, and has wide application value on medical science.
Owner:HEBEI AINENG BIOTECH CO LTD
Blood protein responsive type gamma-PGA (polyglutamic acid) hydrogel hemostasis material as well as preparation method and application thereof
ActiveCN107596429AGuarantee "anchor" abilityGood hemostatic effectSurgical adhesivesBiocompatibility TestingSolvent
The invention provides a blood protein responsive type gamma-PGA (polyglutamic acid) hydrogel hemostasis material as well as a preparation method and an application thereof. The hemostasis material isprepared from a PHPA and DA (dopamine) molecule functionalized gamma-PGA polymer, a solvent with water as a main body, horseradish peroxidase and hydrogen peroxide, the synergetic enhancement effectof gamma-PGA, PHPA and DA on integration of wet high-strength wound is realized and the efficient blocking and hemostasis effect is achieved through Fe<3+> in gamma-PGA complexing blood, adsorption and blood coagulation of blood protein under the inducing action of benzene ring hydrophobic functional groups in PHPA and gel forming through oxidation and crosslinking by catechol groups in DA. The provided gamma-PGA hydrogel hemostasis material can effectively overcome the limitation of conventional pressing hemostasis method in application fields, besides, the problems that numerous hemostasis materials have poor blood responsiveness, poor integration capability for the wet bleeding wound and the like at present are solved, and the blood protein responsive type gamma-PGA hydrogel hemostasismaterial has the advantages of being high in hemostasis efficiency, good in biocompatibility, capable of allowing orthotopic injection of matched complicated wound types and the like and has broad market application prospects.
Owner:NANJING SHINEKING BIOTECH CO LTD
Silk fibroin hemostatic material and preparation method thereof
ActiveCN106913900AFast water absorptionImprove adhesionSurgical adhesivesFreeze-dryingPolyethylene glycol
The invention relates to the field of application of silk fibroin and especially relates to a silk fibroin hemostatic material and a preparation method thereof. The preparation method includes the following steps: S1) preparing a silk fibroin solution with mass percentage being 1-6%; S2) preparing a polyethylene glycol solution with mass percentage being 10-40%, wherein molecular weight of the polyethylene glycol is 1,000-10,000; S3) mixing the silk fibroin solution and the polyethylene glycol solution according to the volume ratio of 10:0.25-10:3, and performing freeze drying to prepare the silk fibroin hemostatic material. The silk fibroin hemostatic material has good water absorption, is tightly combined with a wound and is not liable to fall off, has excellent hemostatic effect on body surface and in-vivo hemorrhage, is degradable and absorbable and can prevent tissue adhesion due to operation, has no inflammatory reactions and is low in cost.
Owner:SUZHOU SIMEITE BIOTECH CO LTD
Silicate clay modified hemostatic material and preparation method thereof
ActiveCN110464868AEasy to useCan be closely attached to the wound surfaceSurgical adhesivesPharmaceutical delivery mechanismHalloysiteBiocompatibility Testing
The invention provides a silicate clay modified hemostatic material and a preparation method thereof. The hemostatic material is composed of silicate clay and a high polymer by electrostatic spinning;the mass ratio of the silicate clay and the high polymer is (0.5 to 2.5):1; the silicate clay includes kaolinite, halloysite, attapulgite or montmorillonite; part of silicon hydroxyl of the silicateclay combines with hydrogen bond of the high polymer, and the rest of silicon hydroxyl plays the role of hemostasis and hydrophilicity on the surface of the hemostatic material. The preparation methodincludes the following steps that first, the silicate clay is pretreated and broken by ultrasound and then mixed with a high polymer ethanol solution to obtain an electrostatic spinning solution, andfinally the electrostatic spinning is conducted to obtain the silicate clay modified hemostatic material. The hemostatic material has the advantages of fast hemostasis, convenient use, wound healingfacilitation, good biocompatibility and low cost.
Owner:CENT SOUTH UNIV
Preparation of natural polymer base hemostasis dressing
The invention provides a method of preparing a natural polymer base composite polyelectrolyte hemostasis dressing. A natural polymer base composite polyelectrolyte solution with traces of thrombin is prepared by preparing polyanion and polycation solutions with different concentrations. The composite polyelectrolyte solution with the traces of thrombin is transferred to a liquid nitrogen refrigeration device and is enabled to be frozen in a liquid nitrogen environment. Under the conditions that the temperature is -80 DEG C and the vacuum degree is -1 pascal, freeze-drying treatment is conducted for more than 48 hours by a freezer dryer, and a high porosity composite polyelectrolyte fiber dressing with the traces of thrombin is obtained. The high porosity natural composite polyelectrolyte fiber dressing with the traces of thrombin has wide application values in aspects such as hemostasis, bacteriostat and wound healing promotion.
Owner:ROOSIN MEDICAL CO LTD
Injectable hydrogel hemostatic based on marine-derived gelatin as well as application and application method of hemostatic
ActiveCN111632189AFast and durable closureEffects without additional traumaSurgical adhesivesPharmaceutical delivery mechanismPhotoinitiatorHaemostatic agent
The invention relates to the technical field of hemostatics, and particularly provides an injectable hydrogel hemostatic based on marine-derived gelatin as well as application and application method of the hemostatic. According to the weight percentage of 100%, the injectable hydrogel hemostatic based on the marine-derived gelatin is prepared from the following components in percentage by weight:0.1%-10% of chemically-modified marine-derived gelatin, 10%-20% of a photo-crosslinking gelator, 0.1%-15% of a tissue adhesion factor, 0.1%-0.5% of a photoinitiator, and a dissolved amount of solvent.The injectable hydrogel hemostatic disclosed by the invention has a rapid and good hemostatic effect, has no extra trauma to surrounding tissues in a hemostatic process, has a degradable characteristic, and can be widely applied to hemostasis of human or animal tissues, visceral accidental wounds or surgical wounds.
Owner:SHENZHEN INST OF ADVANCED TECH
Rapid hemostatic powder for injury and preparation method of rapid hemostatic powder
The invention relates to the technical field of medical materials and particularly relates to rapid hemostatic powder for an injury and a preparation method of the rapid hemostatic powder. The rapid hemostatic powder for the injury comprises the following raw materials in parts by weight: 40-56 parts of chitosan quaternary ammonium salt, 28-36 parts of puffball extracts, 8-12 parts of sodium alginate, 1-5 parts of ramie root extracts, 1-5 parts of cirsium setosum extracts, 1-5 parts of madder extracts and 0.5-1.5 parts of carbonized hair extracts. The rapid hemostatic powder for the injury can take a hemostasis effect through various approaches and remarkably shorten the blood coagulation time, also has the effects of inhibiting and resisting bacteria, relieving pain and promoting wound healing and can be widely applied to rapid hemostasis, emergency treatment and treatment of skin scratch, injury and haemorrhoids and relief of scar formation.
Owner:DONGGUAN DAQING MEDICAL DEVICES CO LTD +2
Preparing method of hemostasis repair hydrogel
InactiveCN108159484AFast hemostasisGood biocompatibilitySurgical adhesivesPharmaceutical delivery mechanismCross-linkMedicine
The invention discloses a preparing method of hemostasis repair hydrogel, and belongs to the field of hemostasis hydrogel. The preparing method comprises the following steps of weighing, by mass, 10-40 parts of snail mucus, 60-90 parts of silk protein sol containing hemostasis traditional Chinese medicine and 0.1-0.5 part of natural cross-linking agent, conducting a reaction at 25-40 DEG C for 3-24 h and conducting freezing and drying to obtain the hemostasis repair hydrogel. By compounding the natural cross-linking agent, the snail mucus, silk protein and the hemostasis traditional Chinese medicine, the hemostasis speed of the hemostasis repair hydrogel can be effectively increased, the repair performance to a wound can be effectively improved, and meanwhile, the attaching performance between the hemostasis repair hydrogel and the skin can be improved.
Owner:HUNAN UNIV OF TECH
Trauma hemostatic sponge and preparing method and application thereof
ActiveCN109999216AGood hemostatic effectFast hemostasisSurgical adhesivesPharmaceutical delivery mechanismGrapheneExothermic reaction
The invention relates to the technical field of hemostasis, in particular to a trauma hemostatic sponge and a preparing method thereof. The trauma hemostatic sponge comprises zeolite and graphene; thegraphene is of a three-dimensional crosslinking structure; the zeolite is dispersed in the three-dimensional crosslinking structure; the mass ratio of the zeolite to the graphene is 1:(0.2-5). The trauma hemostatic sponge has a distinctive hemostasis mechanism and excellent hemostasis performance. According to records of the embodiment, the trauma hemostatic sponge has a high hemostasis speed, amild exothermic reaction and high biological safety.
Owner:BEIJING UNIV OF CHEM TECH
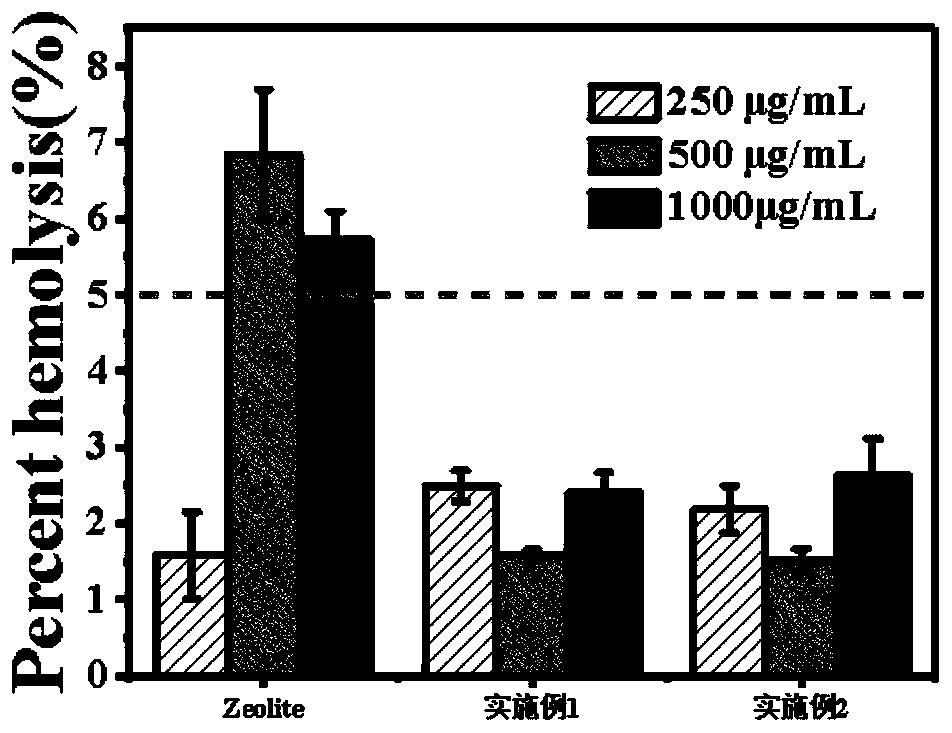
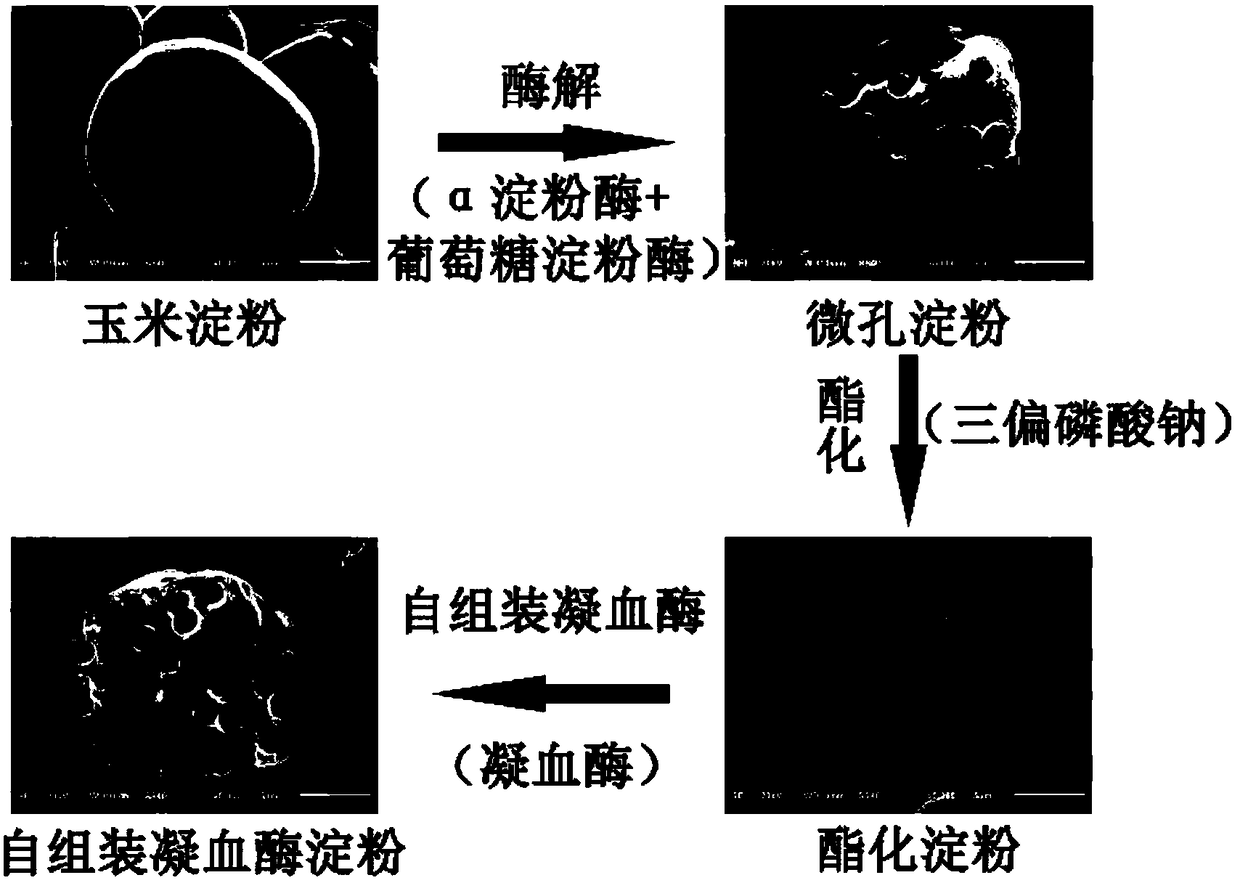
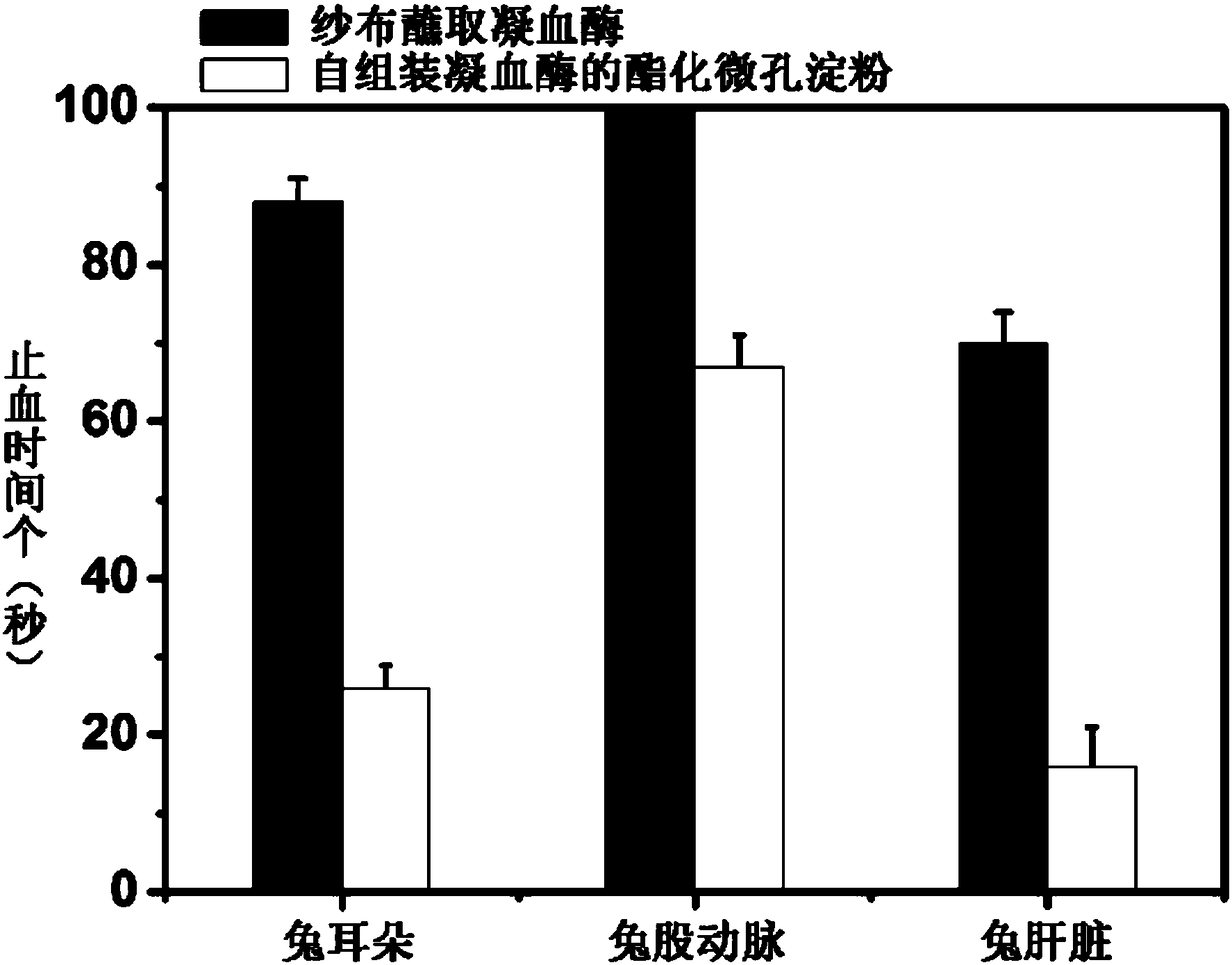
Preparation method and application of self-assembled thrombin esterified microporous hemostatic starch
PendingCN108355163AImprove hydrophilic abilityIncrease the surface negative potentialSurgical adhesivesPharmaceutical delivery mechanismChemistryThrombin activity
The invention relates to a preparation method and application of self-assembled thrombin esterified microporous hemostatic starch. The preparation method comprises the following steps: carrying out heating reaction on microporous starch and sodium trimetaphosphate under the alkaline condition to obtain esterified microporous starch with starch phospholipid on the surface; mixing the esterified microporous hemostatic starch with a thrombin aqueous solution and then carrying out low-temperature drying to obtain the self-assembled thrombin esterified microporous hemostatic starch. Firstly, the hemostatic starch in the invention has a larger surface area and a higher surface negative charge performance, and can be stably combined with a large amount of thrombin through hydrogen bonds. Secondly, the hemostatic starch in the invention can quickly and extensively suck blood, as well as aggregate and activate platelets so as to achieve a rapid hemostatic effect. Thirdly, the hemostatic starchin the invention has no biotoxicity and can be rapidly degraded and absorbed by tissue cells.
Owner:苏州和其美生物材料有限公司
Preparation method of modified loofah sponge fiber dressing with cell regeneration function
InactiveCN107050500AImprove adsorption capacityEfficient removalAbsorbent padsBandagesPolymer scienceEggshell membrane
The invention discloses a preparation method of a modified loofah sponge fiber dressing with a cell regeneration function. The modified loofah sponge fiber dressing is prepared by molding modified loofah sponge fibers through a spunlace non-woven fabric technology, and compounding eggshell membrane powder. The preparation method comprises the following steps: firstly, performing plasma treatment on the surface of the loofah sponge fibers by selecting a proper discharging condition in order to improve the flexibility, skin fitness and adhesion of the dressing; secondly, loading calcium on the surface of the chemically-modified loofah sponge fibers by using waste egg shell calcified liquid in order to effectively prevent heavy metal poisoning and shorten the wound incrustation time; lastly, spraying the eggshell membrane powder on the surface of a non-woven fabric by using natural honey to improve the hygroscopicity of the dressing. The modified loofah sponge fiber dressing has the advantages of easiness in operation, adoption of simple, readily-available and environment-friendly raw materials, and high comfort and great convenience in use, and has the effects of activating blood, promoting cell regeneration, high hemostasis speed, and high anti-bacterial and anti-infection performance.
Owner:安徽月娇家具有限公司
Medical quick biological rhinal styptic powder and preparation method thereof
ActiveCN105126154APromote healingRapid hemostasisAbsorbent padsBandagesCirsium japonicumSodium carboxymethylcellulose
The invention relates to the technical field of medical materials, in particular to medical quick biological rhinal styptic powder and a preparation method thereof. The medical quick biological rhinal styptic powder is composed of, by weight, 65-85 parts of lasiosphaera seu calvatia extracts, 10-14 parts of sodium carboxymethylcellulose, 1-5 parts of cirsium japonicum extracts, 1-5 parts of lalang grass rhizome extracts, 1-5 parts of flos sophorae extracts, 1-5 parts of Indian mockstrawberry extracts and 0.5-1.5 parts of cacumen platycladi extracts. The medical quick biological rhinal styptic powder is capable of realizing stypticity by various means and evidently shortening blood clotting time, further has functions of bacteriostasis, antibiosis, analgesia and acceleration of wound healing and is widely applicable to postoperation quick hemostasis, pain relief and wound repair for nasal cavities, oral cavities and throats.
Owner:DONGGUAN DAQING MEDICAL DEVICES CO LTD +2
Absorptivity hemostatic micropowder of hydroxyethyl modified cotton fiber and preparation method thereof
InactiveCN101716365AIncrease the speed of wound hemostasisFast hemostasisAbsorbent padsBandagesFiberSide effect
The invention provides an absorptivity hemostatic micropowder of hydroxyethyl modified cotton fiber and a preparation method thereof, mainly solving the problem that the present hemostatic materials can not effectively stanch the wound caused by minimally invasive surgery. The particle size of the absorptivity hemostatic micropowder of hydroxyethyl modified cotton fiber is between 50mu m and 100mu m. The preparation method of the absorptivity hemostatic micropowder of hydroxyethyl modified cotton fiber comprises the main steps of alkalization treatment of cotton fiber, modification treatment of cotton fiber, supermicro grinding, crosslink sterilization and the like. The hemostatic micropowder made from hydroxyethyl modified cotton fiber can be directly applied to the wound of minimally invasive surgery in human body, and can be directly absorbed by human body without toxic or side effect, thus improving wound stanching speed and avoiding the problem of easy agglomeration in the process of micropowder storage.
Owner:郑明义
Hydroxypropyl-beta-cyclodextrin hemostatic gauze and preparation method thereof
InactiveCN104474579AFast hemostasisPromote wound healingAbsorbent padsBandagesSodium carboxymethylcelluloseBroad spectrum
The invention discloses hydroxypropyl-beta-cyclodextrin hemostatic gauze. The hydroxypropyl-beta-cyclodextrin hemostatic gauze is characterized by being prepared from the following raw materials in parts by weight: 3-5 parts of luffa leaves, 1-2 parts of cacumen biotae, 1-2 parts of sangusis draconis, 1-2 parts of mock strawberry, 0.5-1 part of pine pollen, 6-8 parts of hydroxypropyl-beta-cyclodextrin, 6-8 parts of gelatin, 3-5 parts of sodium alginate, 0.1-0.2 part of polysorbate-80, 0.5-1 part of caprylic capric triglyceride, 2-4 parts of sodium carboxymethylcellulose, 1-2 parts of auxiliary materials, and 200-250 parts of water. The soluble hemostatic gauze disclosed by the invention is collaboratively prepared from natural high polymer materials of hydroxypropyl-beta-cyclodextrin and the like, is fast in hemostatic effect, is capable of effectively promoting wound healing, is good in antibacterial effect, is easily absorbed by human body, is good in biocompatibility, and is free from toxic or side effects; the soluble hemostatic gauze is capable of resisting bacteria in a broad spectrum, and diminishing inflammation and easing pain by extracting and compounding natural plant effective ingredients, has a controlled-release function, effectively prolongs the action time, and achieves a better use effect.
Owner:ANHUI JIANYUAN MEDICAL APP & INSTR EQUIP
Wound dressing capable of stopping bleeding and resisting infection and preparation method of wound dressing
ActiveCN109847085ASignificant coagulationFast hemostasisPharmaceutical delivery mechanismAbsorbent padsEnzymeAntimicrobial
The invention provides wound dressing capable of stopping bleeding and resisting infection and a preparation method of the wound dressing, and belongs to the technical field of medical dressing. According to the wound dressing disclosed by the invention, N-alkylated chitosan of which the substitution degree is 8%-39% is used as a bleeding stopping material, so that the blood coagulation effect isnotable, and the bleeding stopping speed is high. Manuka honey is used as an antibacterial agent, so that the antibacterial efficacy is powerful, the activity is stable, and the Manuka honey is not liable to decompose at normal temperature and not liable to decompose by bioactive enzymes. According to the wound dressing, the N-alkylated chitosan and the Manuka honey are combined for use, and the wound dressing which can quickly and effectively stop bleeding, concurrently has the effects of restraining bacterial reproduction and preventing wound from being infected and is good in biocompatibility can be obtained.
Owner:军事科学院系统工程研究院军用标准研究中心 +2
Zeolite hemostatic dressings and preparation method and application thereof
The invention relates to the high degree of exchange Ca-A type zeolite hemostasis dressing and the preparation method and the purpose. The zeolite hemostasis dressing of the invention containing the zeolite has fast hemostasis speed, and has no bacterium, no pyrogen, no cell toxicity, no hypersensitive reaction and no skin irritation, the using is convenient and the cost is low.
Owner:深圳泰明嘉业药业有限公司
A kind of production method and application of quick-absorbing gelatinized seaweed hemostatic dressing
InactiveCN104338173BFast absorptionFast hemostasisFibre treatmentDry-cleaning apparatus for textilesSurgical operationFiber
The invention discloses a preparation method of a quick absorption saturation gelation seaweed hemostatic dressing. The method includes the steps of: soaking, rinsing and drying on calcium fiber textile or non-woven products containing alginate, and the carrying out cutting, packaging and sterilizing to obtain the quick absorption saturation gelation hemostatic dressing. The invention also discloses the application of the quick absorption saturation gelation seaweed hemostatic dressing prepared by the above method. The invention is applicable to rapid absorption and removal of a large amount of surgical diffusate in surgical operation process, so as to realize the effects of rapid hemostasis and operation wound clean; the hemostatic dressing is used for burn, scald and other acute and chronic wounds, can cover the wound and prevent moisture loss in body fluids, provide a positive moist environment for wound healing, prevent the wound from effusion and erosion, isolate bacterial infection, and play the effects of stopping bleeding, relieving pain, promoting wound healing, and reducing scar.
Owner:许春晖 +1
PRP-chitosan-silk fibroin composite material and preparation method therefor
ActiveCN110812526AGood biocompatibilityIncrease the speed of solidificationPharmaceutical delivery mechanismAbsorbent padsBleeding timeFreeze-drying
The invention belongs to the technical field of synthesis of biomaterials and particularly relates to a novel PRP-chitosan-silk fibroin composite material capable of rapidly arresting bleeding and a preparation method therefor. The composite material contains a 2%-4% chitosan solution and a 3% silk fibroin solution; and the PRP-chitosan-silk fibroin composite material is prepared by a freeze-drying method through introducing human-derived platelet rich plasma containing a variety of growth factors and a fixed platelet concentration. According to the material disclosed by the invention, ingredients contained are safe and non-irritating; and compared with the prior art, the material has the advantages that the PRP containing the fixed platelet concentration is added, and a substrate structure is changed through adding silk fibroin, so that the rate of coagulation of whole blood is increased, the bleeding time and amount of bleeding of a wound are reduced, the bleeding arresting propertyof the material is improved, and the bleeding arresting speed, pain alleviating and bacterial resistance of the wound are promoted.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com