Injectable hydrogel hemostatic based on marine-derived gelatin as well as application and application method of hemostatic
A technology of hydrogel hemostatic agent and injection water, which can be applied in applications, pharmaceutical formulations, surgical adhesives, etc. It can solve problems such as sealing failure, mechanical property decline, and surrounding tissue damage, and achieves small curing swelling deformation and short curing time , The effect of fast hemostasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] A preparation method of an injectable hydrogel hemostatic agent based on marine source gelatin, comprising the following steps:
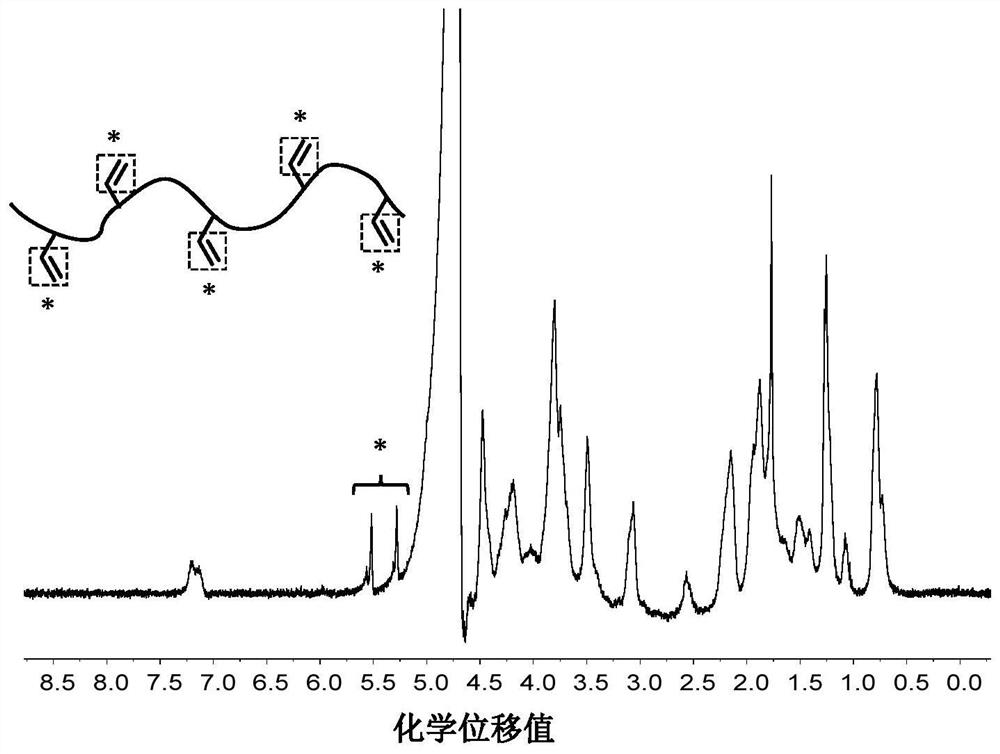
[0064] (1). Synthesis of chemically modified marine source gelatin:
[0065] Dissolve 5.0g of marine fish skin gelatin in 200mL of deionized water and heat to dissolve at 60°C;
[0066] Cool down to 50°C, add 1.0 mL of methacrylic anhydride dropwise, and adjust the pH value to about 8.6 with 2 mol / L sodium hydroxide solution; stir for 2 hours;
[0067] After the reaction was completed, the reaction solution was added to 1000 mL of absolute ethanol to precipitate, the precipitate was collected by centrifugation and dissolved in deionized water, and the solution was dialyzed in deionized water for 3 days, and freeze-dried to obtain marine source fish skin gelatin modified with methacrylic anhydride , collected for later use.
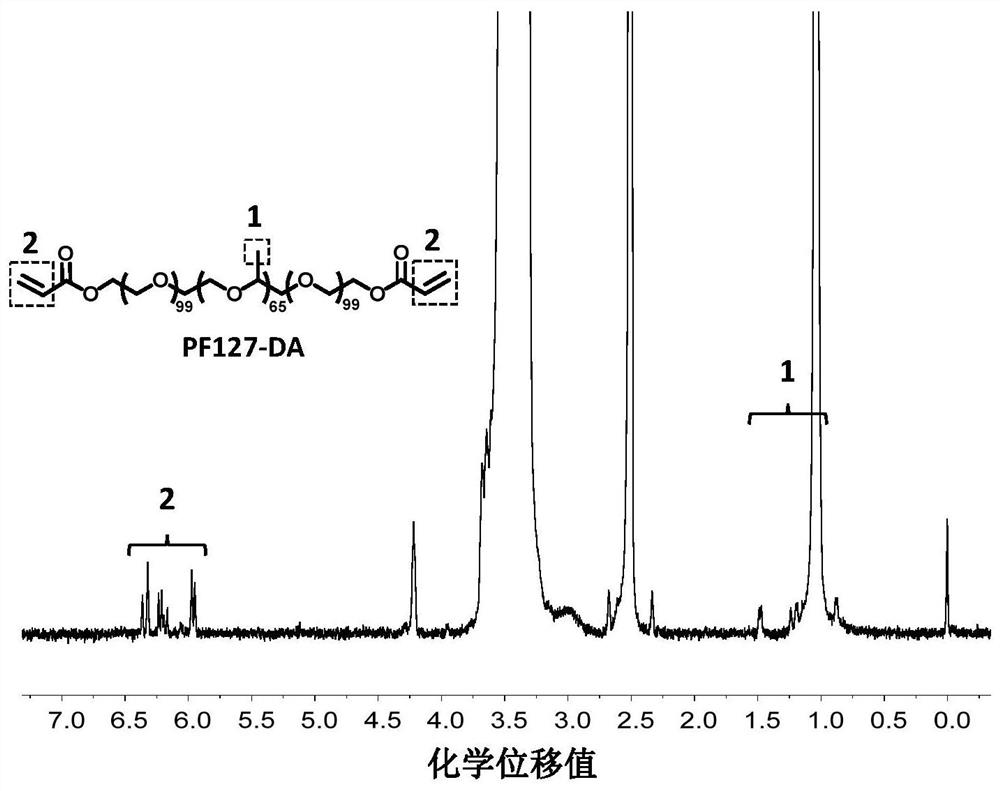
[0068] (2). Synthesis of PF127-DA:
[0069] Dissolve 12.6g of Pluronics F127 in 100mL of anhydrous dichloromethane, ...
Embodiment 2
[0087] A preparation method of an injectable hydrogel hemostatic agent based on marine source gelatin, comprising the following steps:
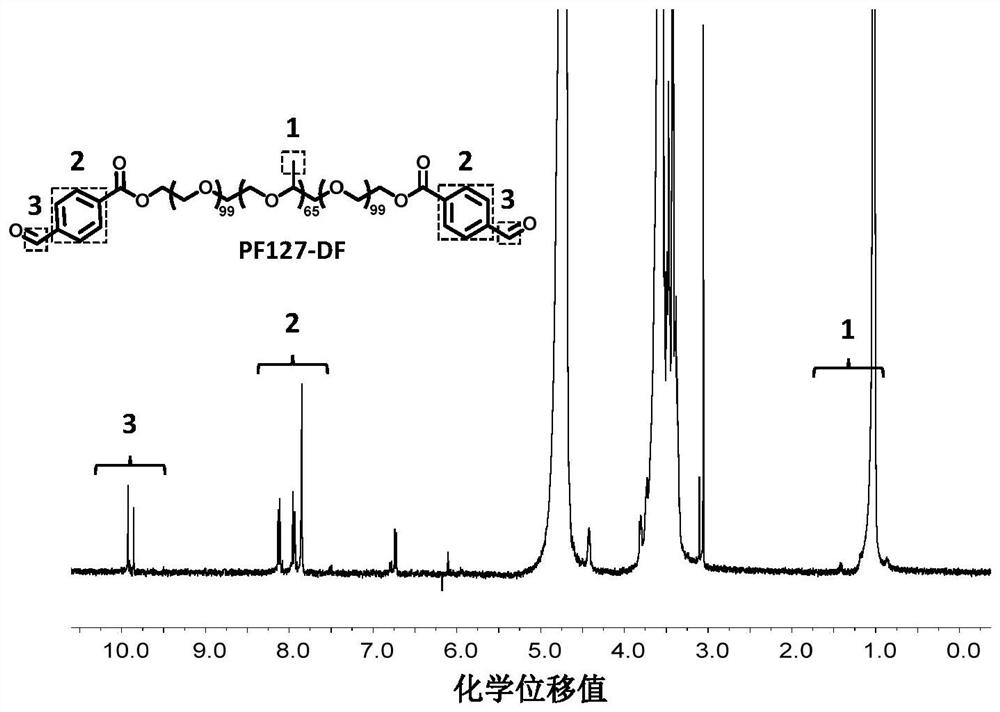
[0088] (1). The synthesis of chemically modified marine source gelatin, PF127-DA and PF127-DF is the same as that in Example 1, except that in the chemically modified marine source gelatin, the marine source gelatin is derived from marine source fish scale gelatin.
[0089] (2). Preparation of injectable hydrogel hemostatic agent based on marine gelatin:
[0090] Add 100 mg of methacrylic anhydride-modified marine fish phosphogelatin, 100 mg of PF127-DA, 50 mg of PF127-DF and 2.5 mg of phenyl(2,4,6-trimethylbenzoyl)phosphate lithium salt into 750 μL of PBS buffer solution (pH=7.4), vortex until fully dissolved, and store away from ultraviolet light.
Embodiment 3
[0092] A preparation method of an injectable hydrogel hemostatic agent based on marine source gelatin, comprising the following steps:
[0093] (1). The synthesis of chemically modified marine gelatin, PF127-DA and PF127-DF is the same as in Example 1.
[0094] (2). Preparation of injectable hydrogel hemostatic agent based on marine gelatin:
[0095]Add 100 mg of methacrylic anhydride-modified marine fish skin gelatin, 100 mg of PF127-DA, 100 mg of PF127-DF and 5 mg of phenyl(2,4,6-trimethylbenzoyl)phosphate lithium salt into 695 μL of normal saline , vortex until fully dissolved, and store away from ultraviolet light.
PUM
| Property | Measurement | Unit |
|---|---|---|
| bursting strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com