Medical absorbable skeletal wound hemostatic material and preparation method thereof
A hemostatic material and wound surface technology, applied in the field of medicine, can solve the problems of non-degradable bone wax and affecting the healing of bone tissue, and achieve reliable safety and clinical promotion value, good hemostatic effect, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
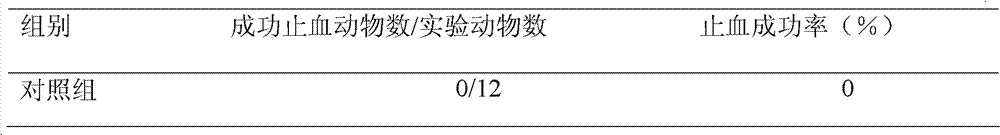
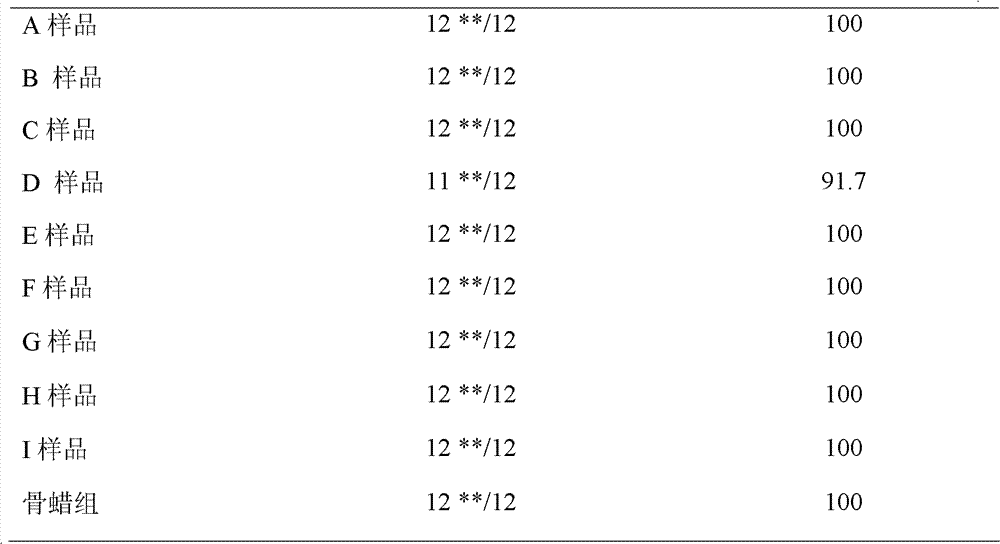
Embodiment 1
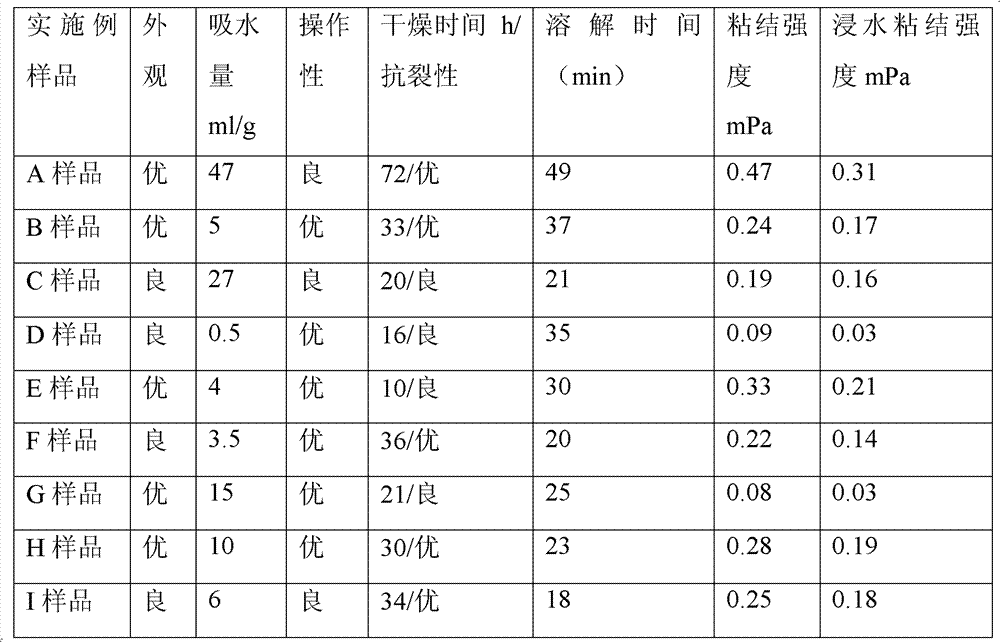
[0043] The carboxymethyl cellulose is washed with alcohol and then dried into powder, and maltose is added into distilled water to dissolve and filtered, and then concentrated into maltose syrup with a cured product ≥ 75%.
[0044] Put 23g of medical glycerin, 3g of Tween 80 and 10g of olive oil into the reaction kettle, seal it and vacuum it to a degree of 10KPa, heat it up to 50°C, keep it warm and stir for 30 minutes; then put 38g of carboxymethyl Base cellulose and 26g of maltose syrup in terms of maltose were put into a reaction kettle, sealed and vacuumed, heated to 80°C and kept at a vacuum of 10KPa, and continuously stirred at a low speed for 2 hours. The paste in the reaction kettle was taken out and poured into polytetrafluoroethylene molds and quickly placed in a refrigerator at 4°C for 120 minutes. A solid block (sample A) that can be shaped arbitrarily and has certain mechanical strength is prepared. Finally, the obtained products are sealed and packaged separate...
Embodiment 2
[0046] Wash the hydroxypropyl starch with alcohol and dry it into powder, add sucrose into distilled water to dissolve and filter to remove impurities, and dry it into powder. Put 12g of glycerin, 0.5g of polysorbate and 7.5g of refined corn oil into the reaction kettle, seal and evacuate, the vacuum degree is 20KPa, heat up to 30°C, keep warm and stir for 120 minutes; then put 63g of hydroxypropyl starch and Put 17g of sucrose into the reaction kettle, seal and vacuumize, raise the temperature to 40°C and keep the vacuum at 20KPa, and stir continuously at low speed for 6 hours. The paste in the reaction kettle was taken out and poured into polytetrafluoroethylene molds, and quickly placed in a refrigerator at 2°C for 90 minutes. A solid block (sample B) that can be shaped arbitrarily and has a certain mechanical strength is prepared. Finally, the obtained products are sealed and packaged separately, and sterilized by ethylene oxide.
Embodiment 3
[0048] Carboxymethyl starch was washed with alcohol and dried into powder. Put 31g of glycerin, 2g of Tween 80 and 9g of soybean oil into the reaction kettle, seal and evacuate, the vacuum degree is 30KPa, heat up to 30°C, keep warm and stir for 60 minutes; then put 58g of carboxymethyl starch into the reaction kettle , sealed and evacuated, the temperature was raised to 50° C. and the vacuum was maintained at 30 KPa, and the mixture was continuously stirred at a low speed for 4.5 hours. Take out the paste in the reaction kettle and pour it into a polytetrafluoroethylene mold, and quickly place it in a refrigerator at 0°C for 30 minutes. A solid block (sample C) that can be shaped arbitrarily and has a certain mechanical strength is prepared. Finally, the obtained products are sealed and packaged separately, and sterilized by ethylene oxide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com