Zeolite hemostatic dressings and preparation method and application thereof
The technology of hemostatic dressing and zeolite is applied in the field of high exchange degree Ca-A type zeolite hemostatic dressing and its preparation, which can solve the problems such as the undisclosed synthetic zeolite process, the pharmacological effect and the function disclosure, and achieve no sensitization reaction, no cell Toxic, non-skin irritating effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] One embodiment of the present invention relates to a method for preparing the zeolite hemostatic dressing, the method comprising the following steps:
[0040] 1) Mix 4A zeolite, binder, lignin and optional molecular sieve activation powder together to make seeds and granulate, polish, and then sieve particles with a particle size of 0.2-1.0 mm;
[0041] 2) After drying the particles obtained in step 1), activate and calcinate at about 300-800 °C;
[0042] 3) reacting the calcined particles with an alkaline solution for a suitable period of time at about 50-150° C., followed by washing;
[0043] 4) repeatedly exchange the particles obtained in step 3) with the calcium ion-containing solution, and then repeatedly wash and dry with deionized water;
[0044] 5) The particles obtained in step 4) are activated and calcined at about 300-800° C. for a second time.
[0045] Step 1) can be granulated by a granulation technique well known to those skilled in the art. The granul...
Embodiment 1
[0054] Example 1 Preparation of zeolite hemostatic dressing
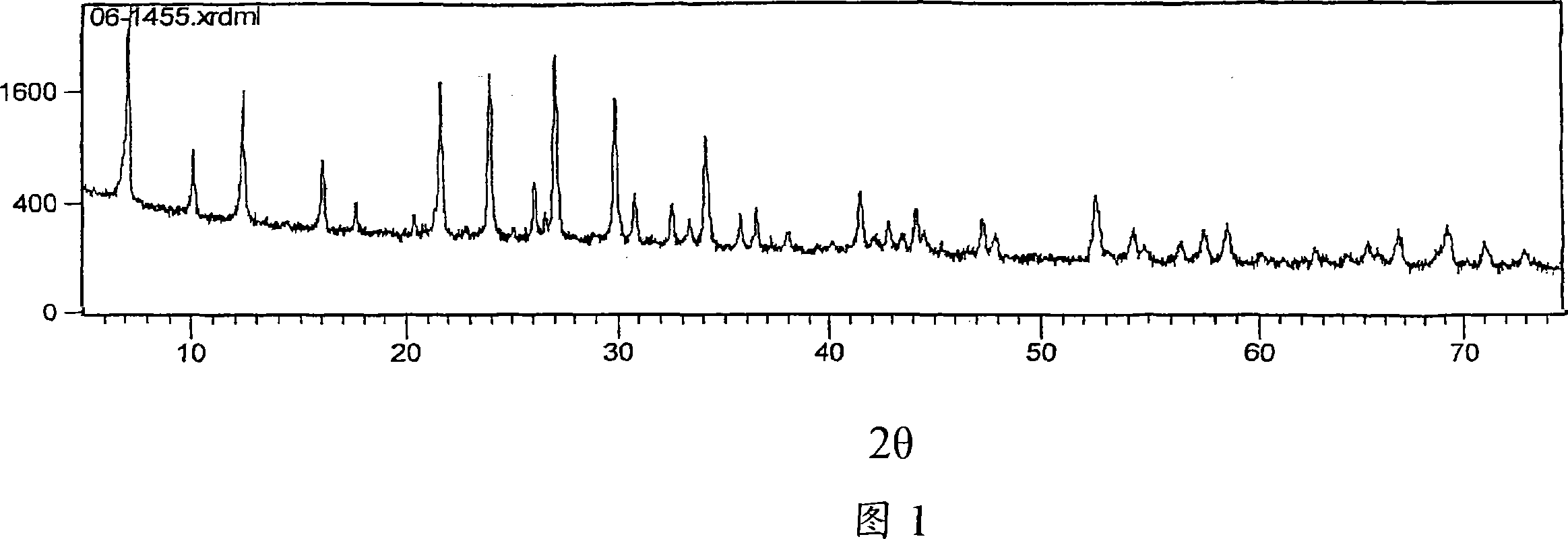
[0055] The zeolite used in the present invention is the 4A zeolite provided by China Kaolin Co., Ltd. The zeolite was tested by X-ray diffractometer in the testing laboratory of Zhengzhou Light Metal Research Institute. See Figure 1 for the X-ray diffraction pattern.
[0056] In gained 1000g zeolite, add 400g kaolin (product of China Kaolin Company), 40g lignin (Jilin Province Chenning Paper Co., Ltd.) and add 120g molecular sieve activation powder as dispersant, spray sodium carboxymethyl cellulose in the pelletizer The solution is made into spherical particles, sieved with a 0.2mm sieve and a 1.0mm sieve into particles of 0.2-1.0mm, and then activated and calcined at 600°C.
[0057] The product obtained by activation calcination was reacted in sodium hydroxide solution at 95°C for 2 hours. After solid-liquid separation, and then washing with hot water, the residual alkali contained in it is washed clean.
[00...
Embodiment 2
[0059] Embodiment 2 Hemostatic powder composition test
[0060] The testing laboratory of Zhengzhou Light Metal Research Institute was responsible for testing, and X-fluorescence spectrometer was used to test the composition of zeolite hemostatic dressings. The analysis result of X-fluorescence spectrometer shows that the zeolite hemostatic dressing of the present invention is mainly composed of Al 2 O 3 About 25% to 35%, SiO 2 About 30% to 40%, and CaO about 10% to 18%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com