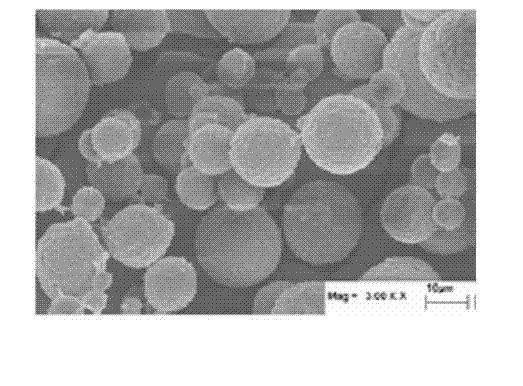
Powder absorbable haemostat and its preparation method
A hemostatic agent and powdered technology, applied in the field of new hemostatic materials, can solve the problem of no hemostatic material found, and achieve the effects of good hemostatic performance, moderate viscosity and fast hemostasis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] ①Dissolve 1g of chitosan, 1g of sodium alginate, and 1g of hyaluronic acid in 100ml of 2wt% acetic acid solution to obtain solution A;
[0034] ② Add A dropwise to 500ml of soybean oil B, stir at room temperature for 30 minutes at high speed, then add 100ml of calcium chloride solution with a concentration of 1 wt%, continue to stir at room temperature for 30 minutes, let stand for precipitation, and obtain cross-linked composite microspheres;
[0035] ③Wash the precipitate with 50ml of petroleum ether for 3 times, and distill the precipitate under reduced pressure at 60°C, and finally blow dry it at 80°C to form a powder C;
[0036] ④ After C was sterilized by ultraviolet rays for 15 minutes, the finished product D was obtained.
Embodiment 2
[0038] ① Dissolving 2g of chitosan, 2g of sodium alginate, and 1g of hyaluronic acid in 100ml of acetic acid solution with a concentration of 3wt% to obtain solution A;
[0039] ② Add A dropwise to 400ml of soybean oil B, stir at room temperature for 30 minutes at high speed, then add 100ml of calcium chloride solution with a concentration of 2wt%, continue to stir at room temperature for 30 minutes, let stand for precipitation, and obtain cross-linked composite microspheres;
[0040] ③Wash the precipitate with 50ml of petroleum ether for 3 times, and distill the precipitate under reduced pressure at 60°C, and finally blow dry it at 80°C to form a powder C;
[0041] ④ After C was sterilized by ultraviolet rays for 10 minutes, the finished product D was obtained.
Embodiment 3
[0043] ①Dissolve 3g chitosan, 3g sodium alginate, and 1g hyaluronic acid in 100ml of 3wt% acetic acid solution to obtain solution A;
[0044] ② Add A dropwise to 600ml of soybean oil B, stir at room temperature for 30 minutes at high speed, then add 100ml of calcium chloride solution with a concentration of 3wt%, continue to stir at room temperature for 30 minutes at high speed, let it stand for precipitation, and obtain cross-linked composite microspheres;
[0045] ③Wash the precipitate with 60ml of petroleum ether for 3 times, and distill the precipitate under reduced pressure at 60°C, and finally blow dry it at 80°C to form a powder C;
[0046] ④ After C was sterilized by ultraviolet rays for 20 minutes, the finished product D was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com