Patents
Literature
38 results about "Severe trauma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
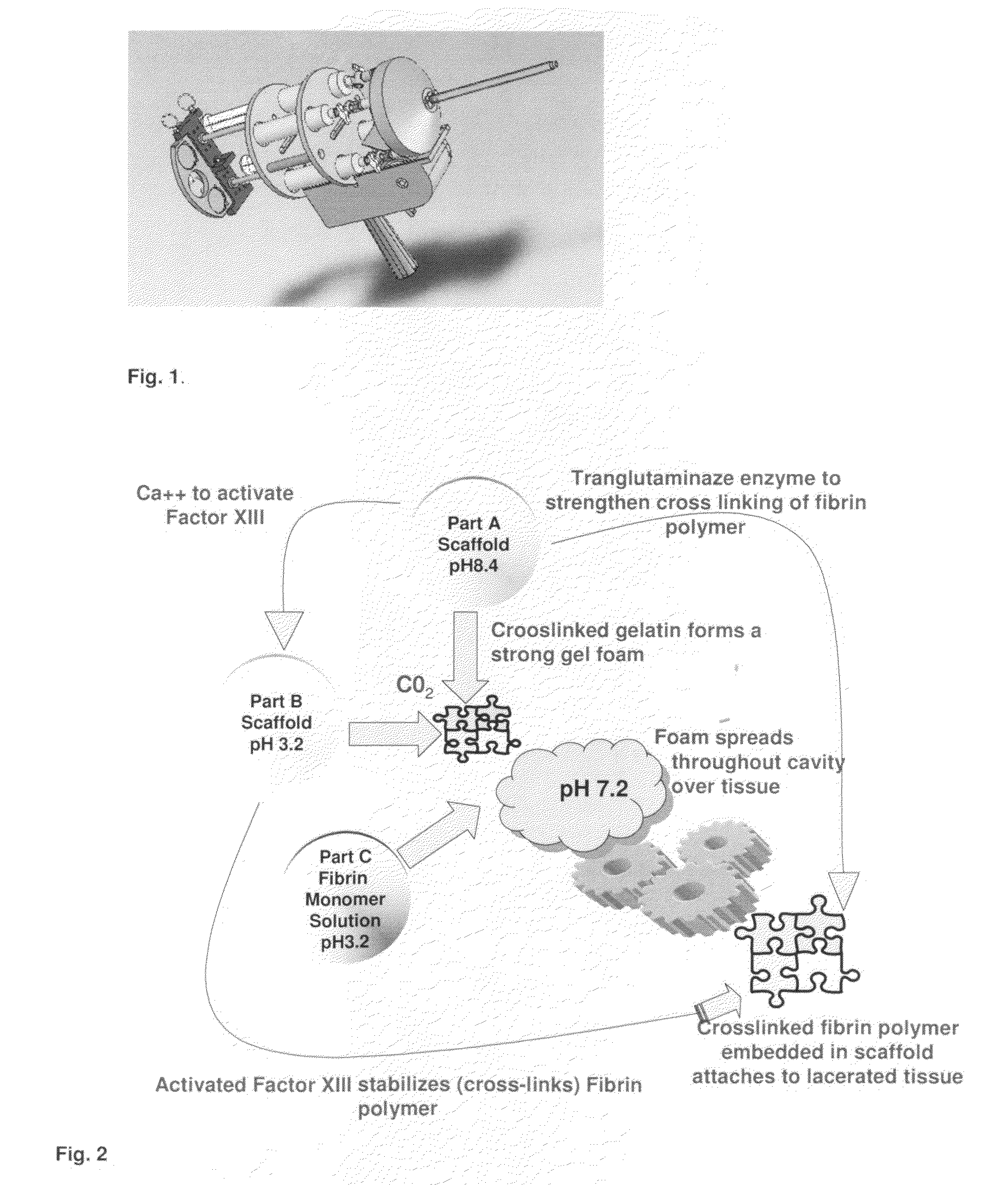
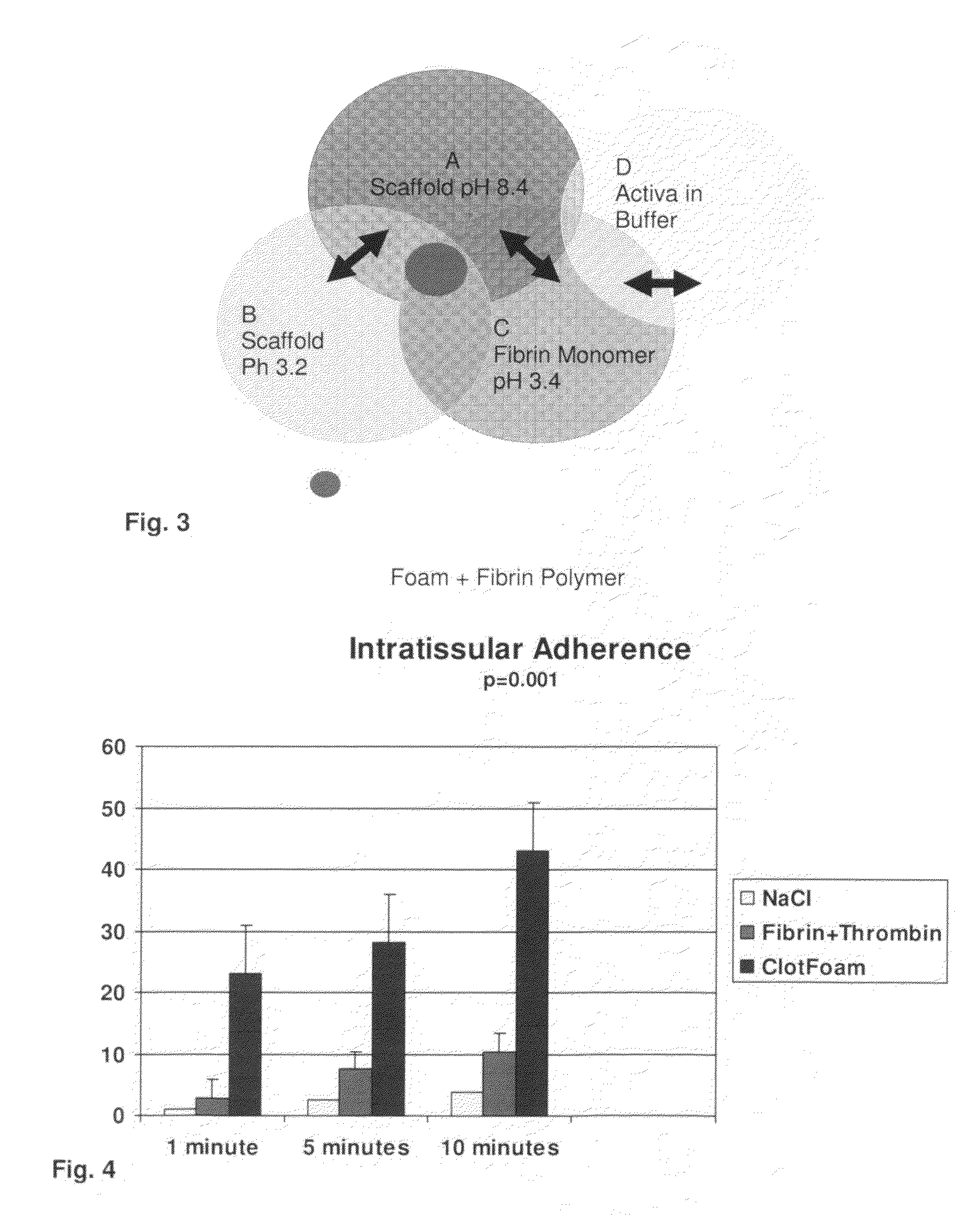
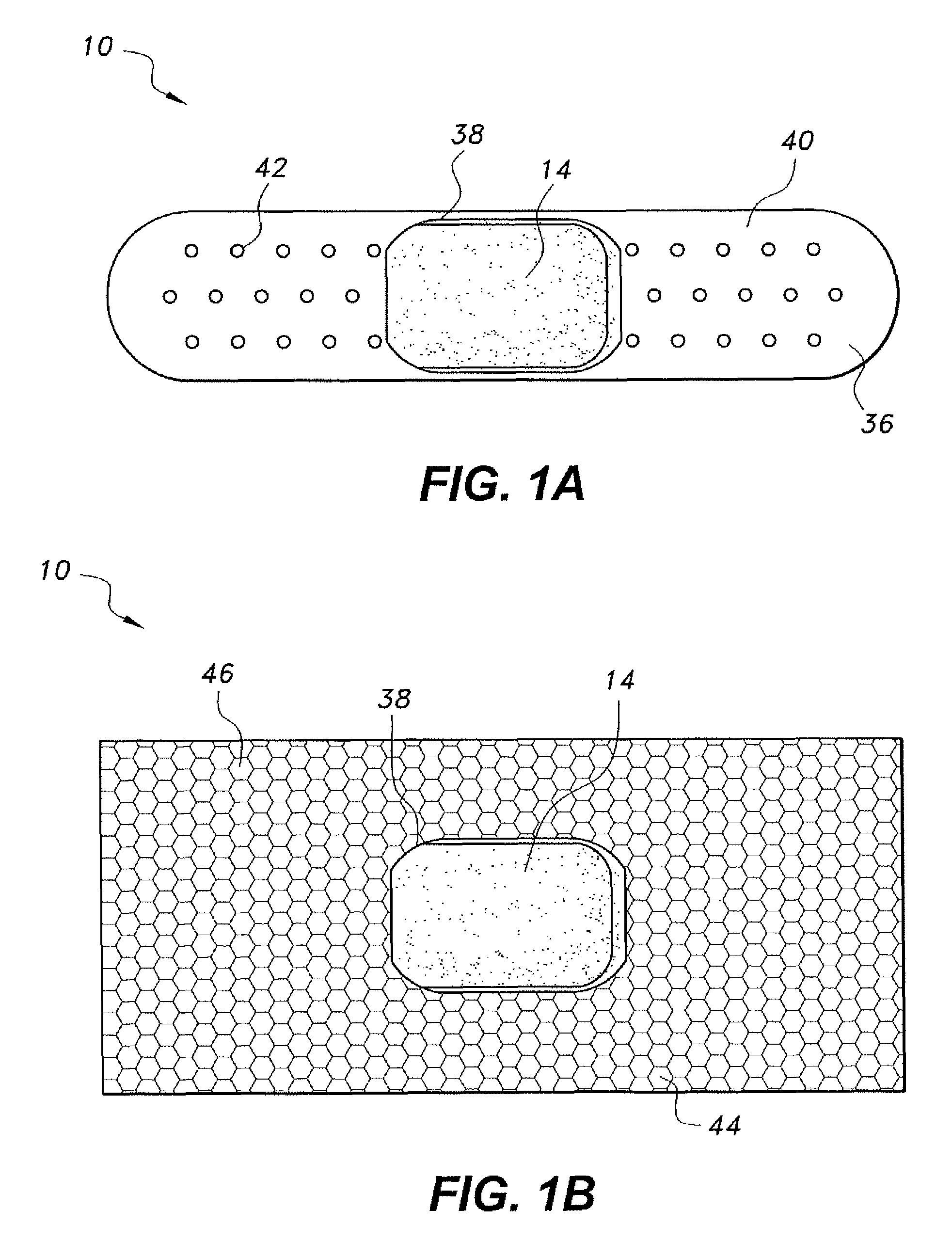
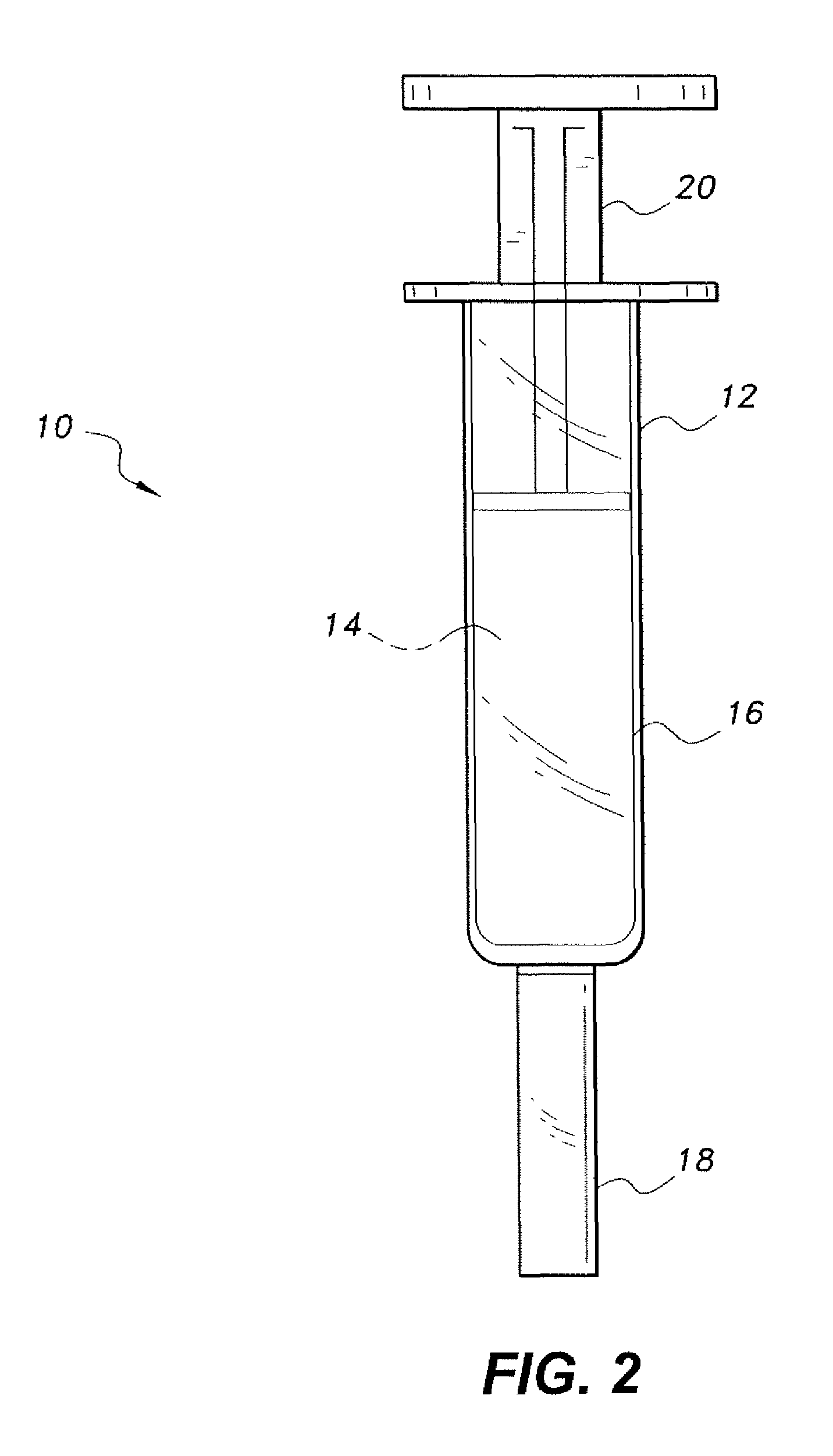
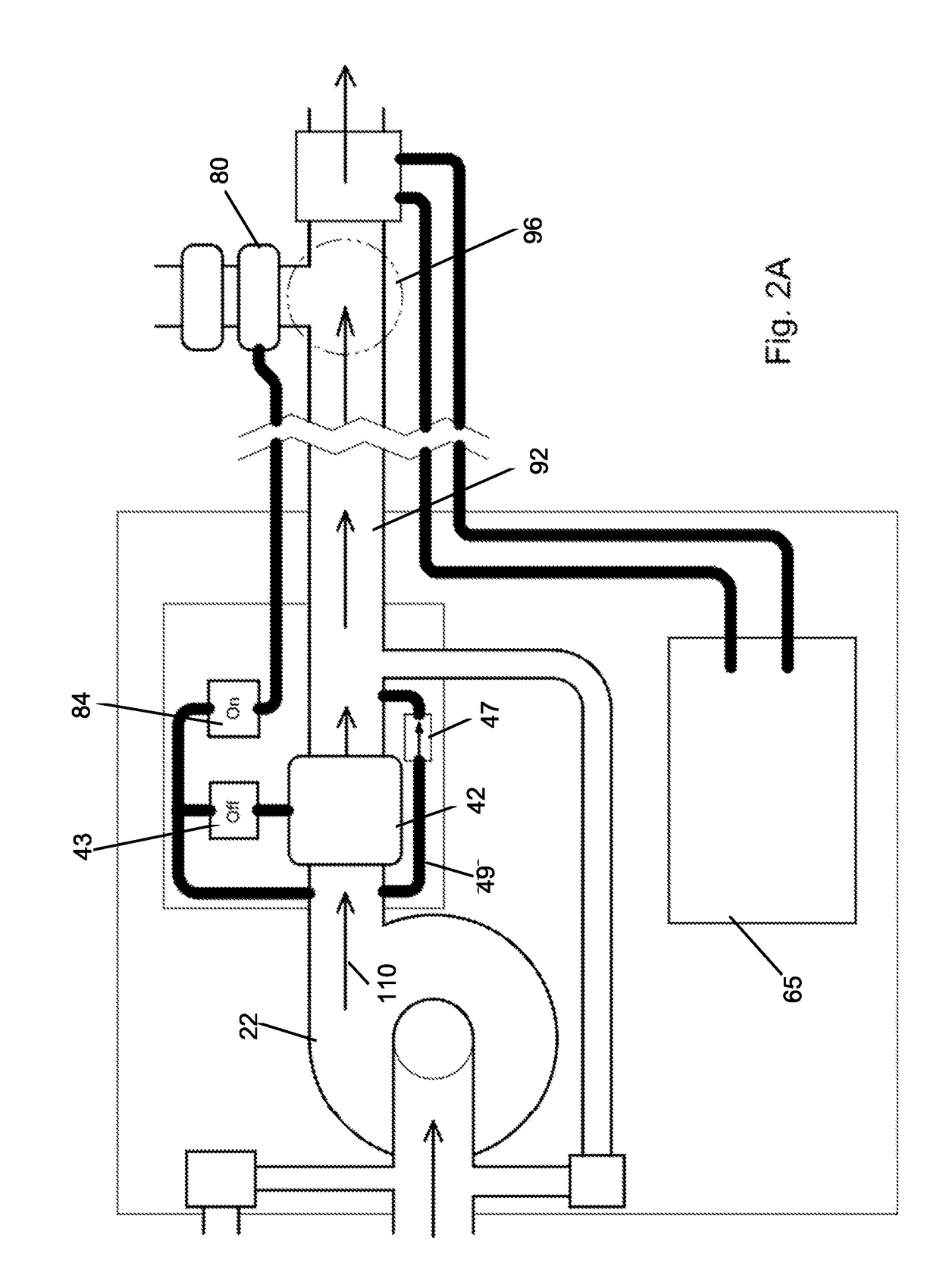
Tissue Sealant for Use in Non Compressible Hemorrhage
InactiveUS20110066182A1Excellent hemostatic agent candidateLeast riskSuture equipmentsPowder deliveryTissue sealantThoracic cavity
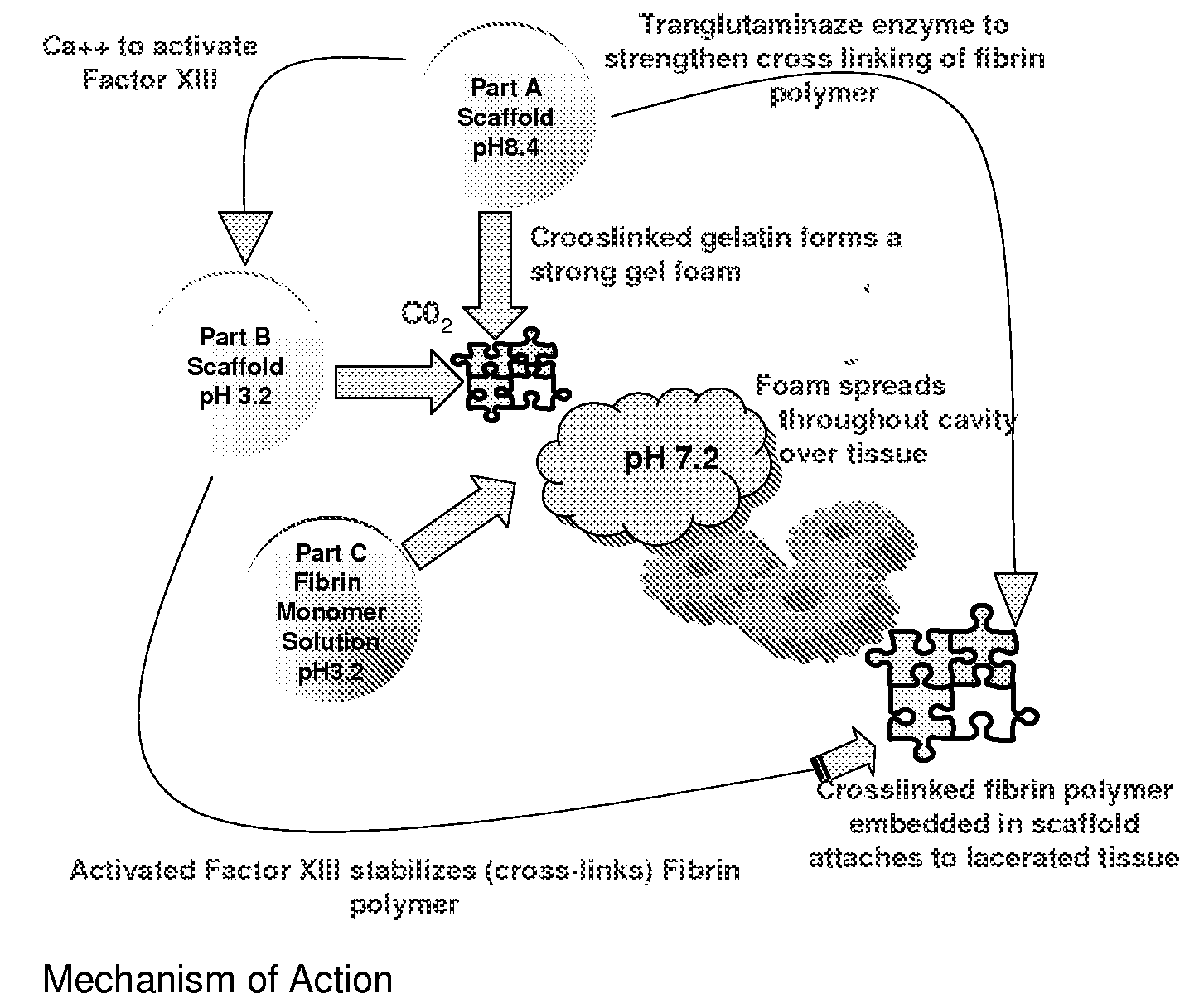
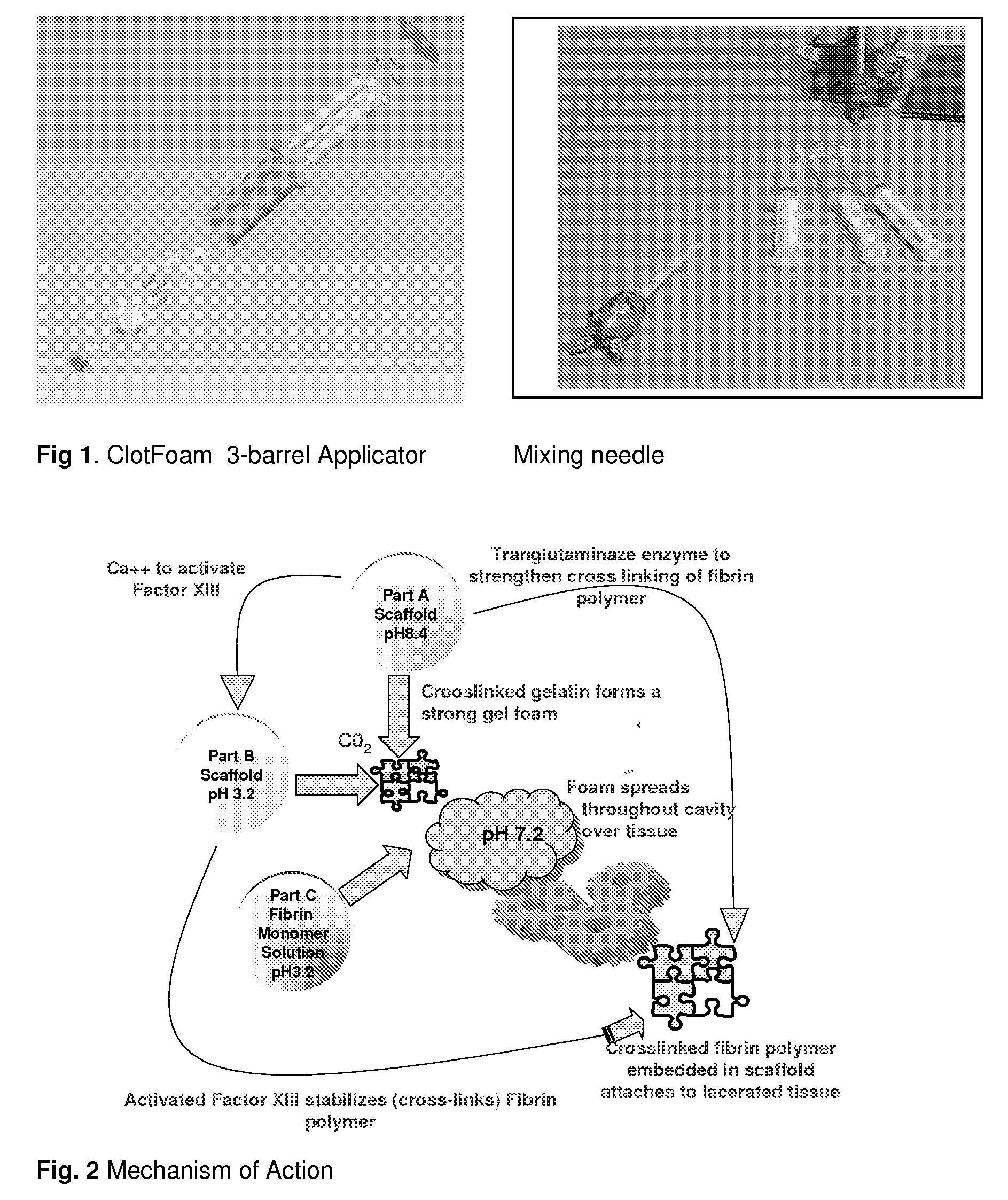
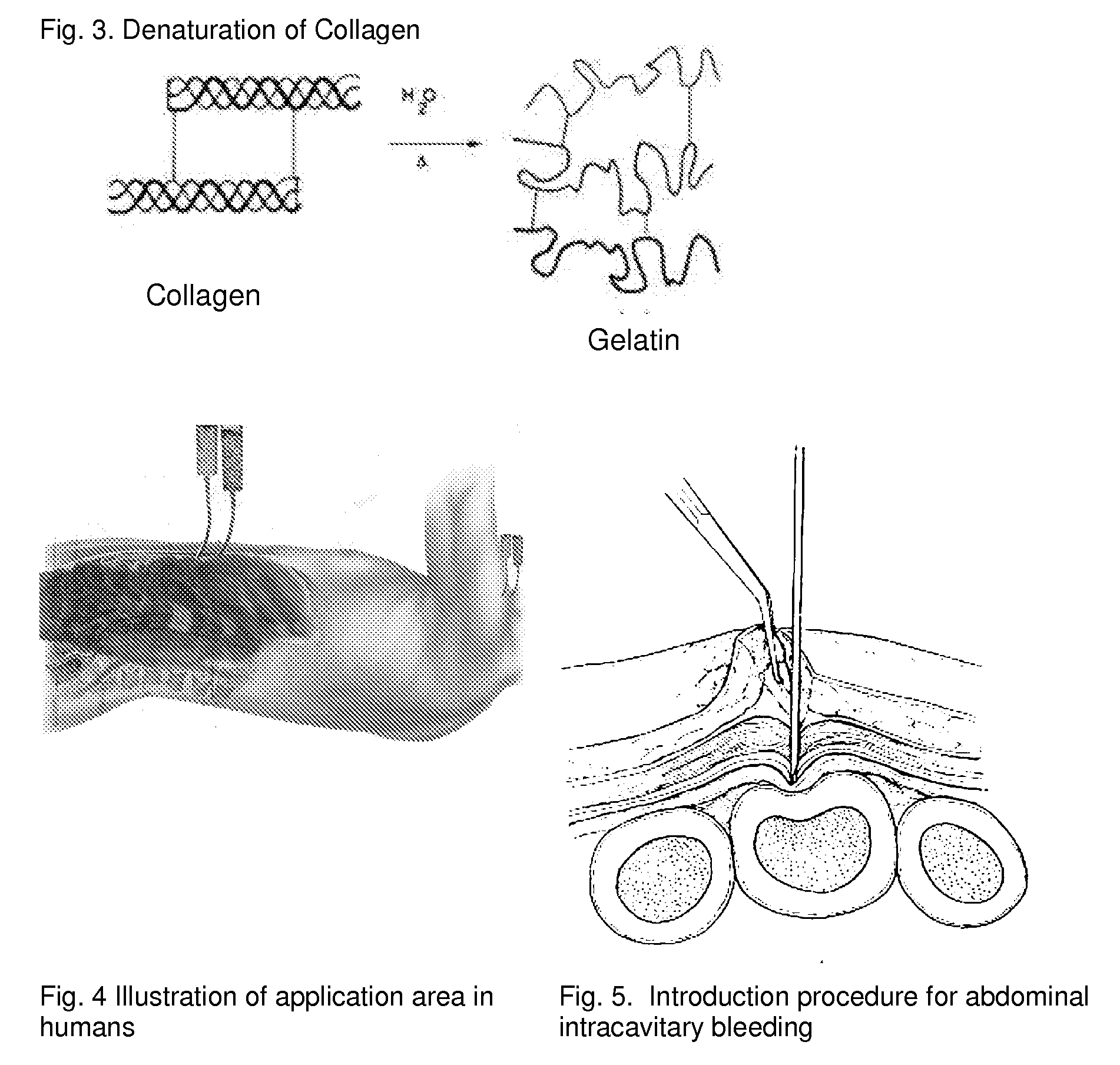
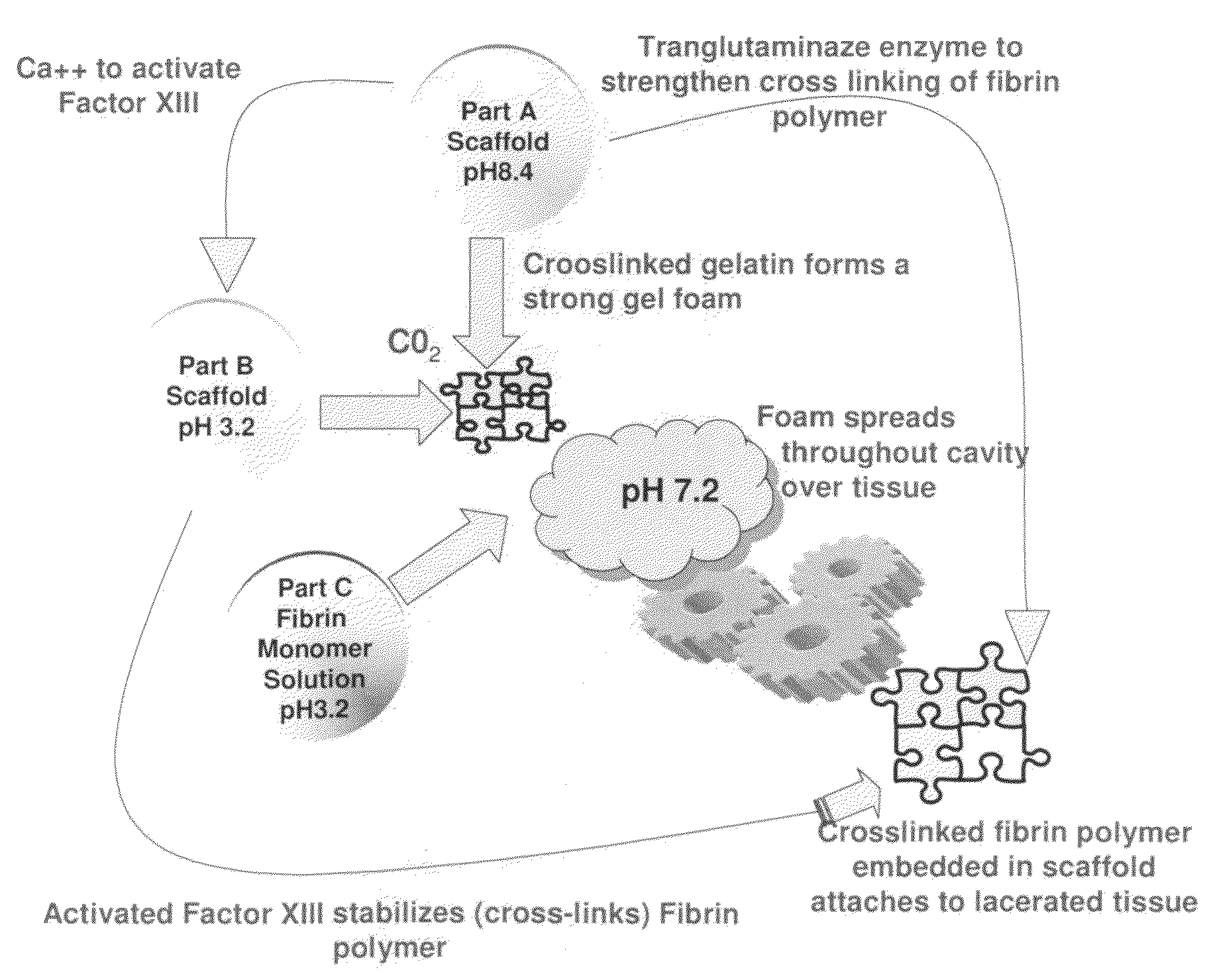
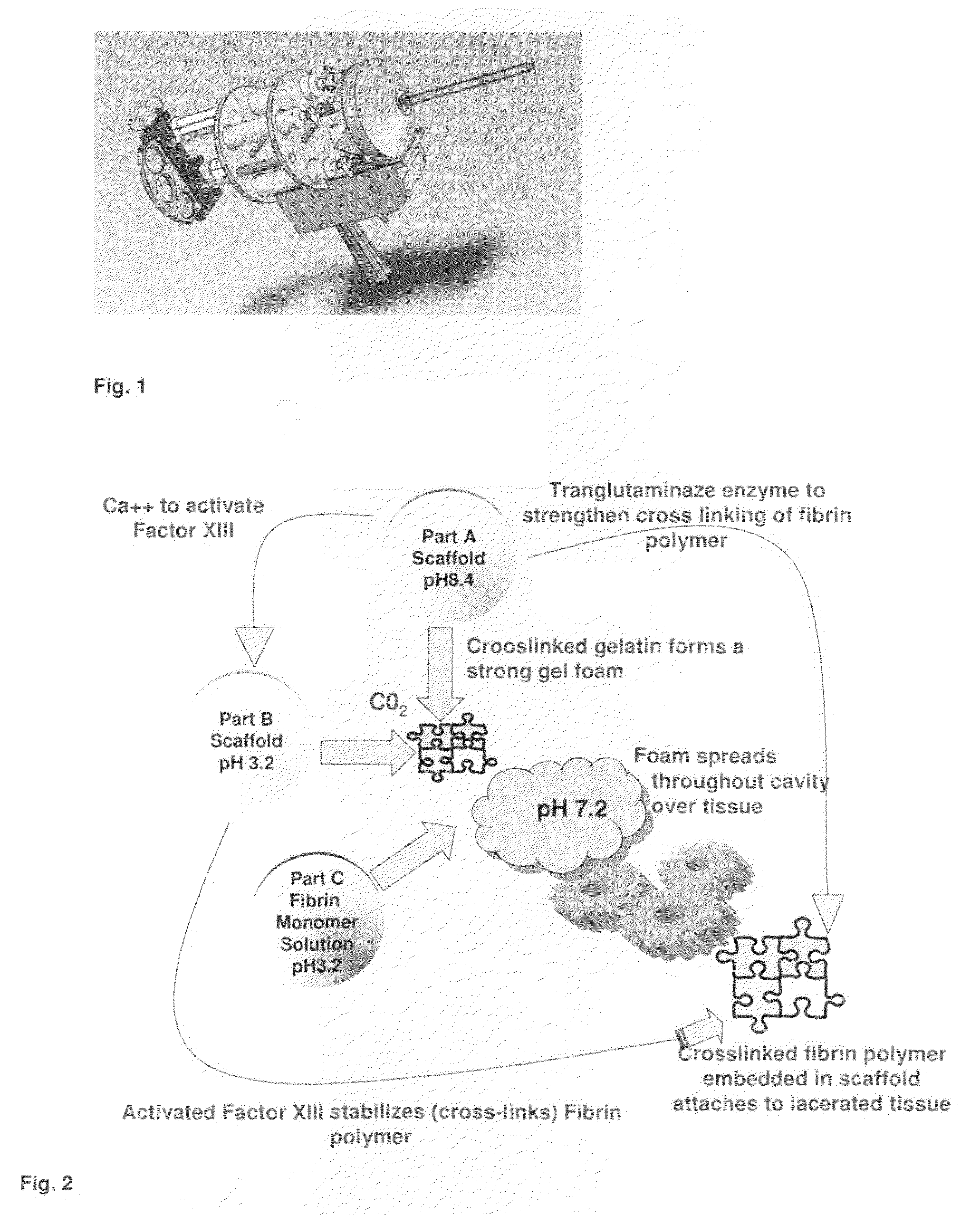
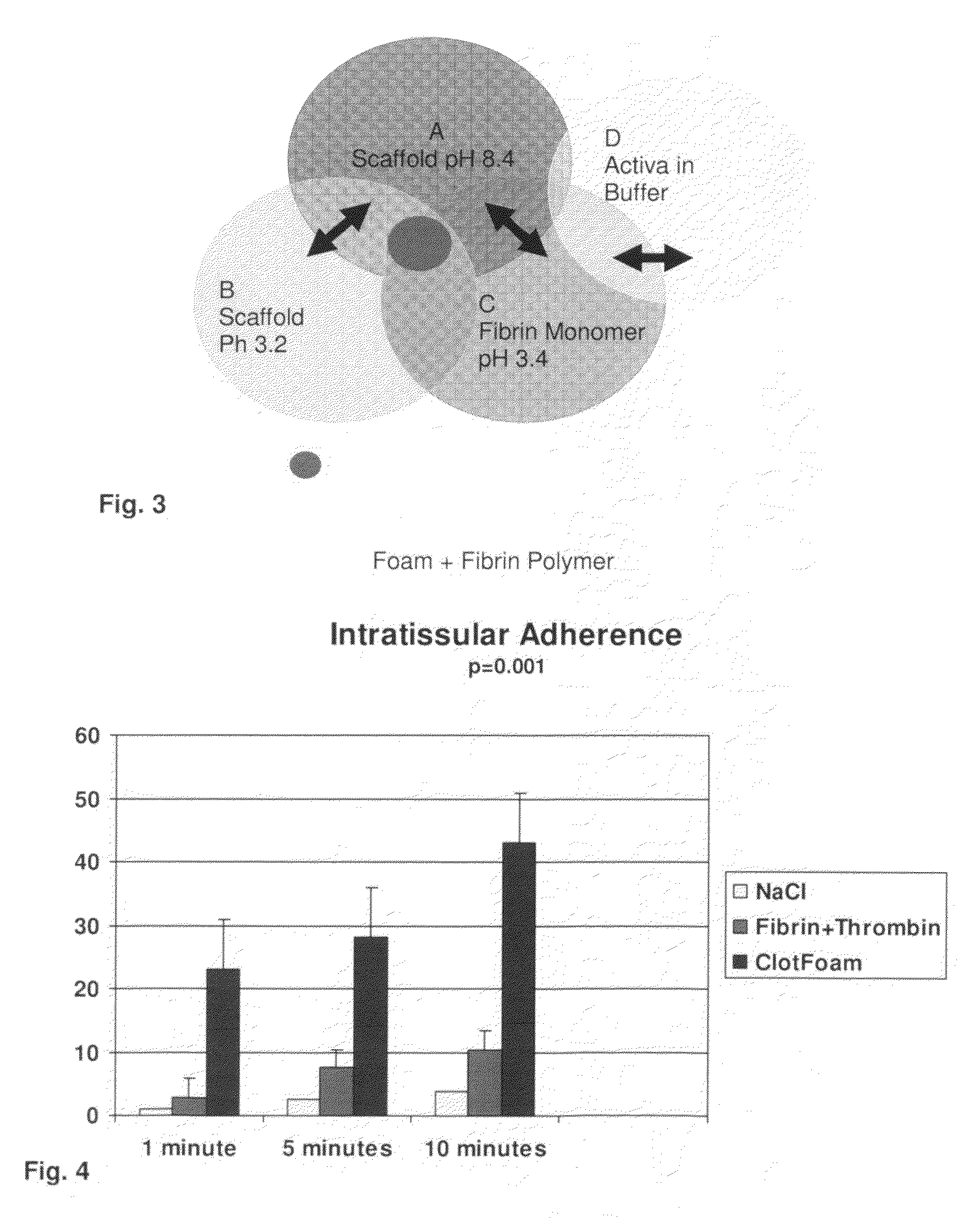
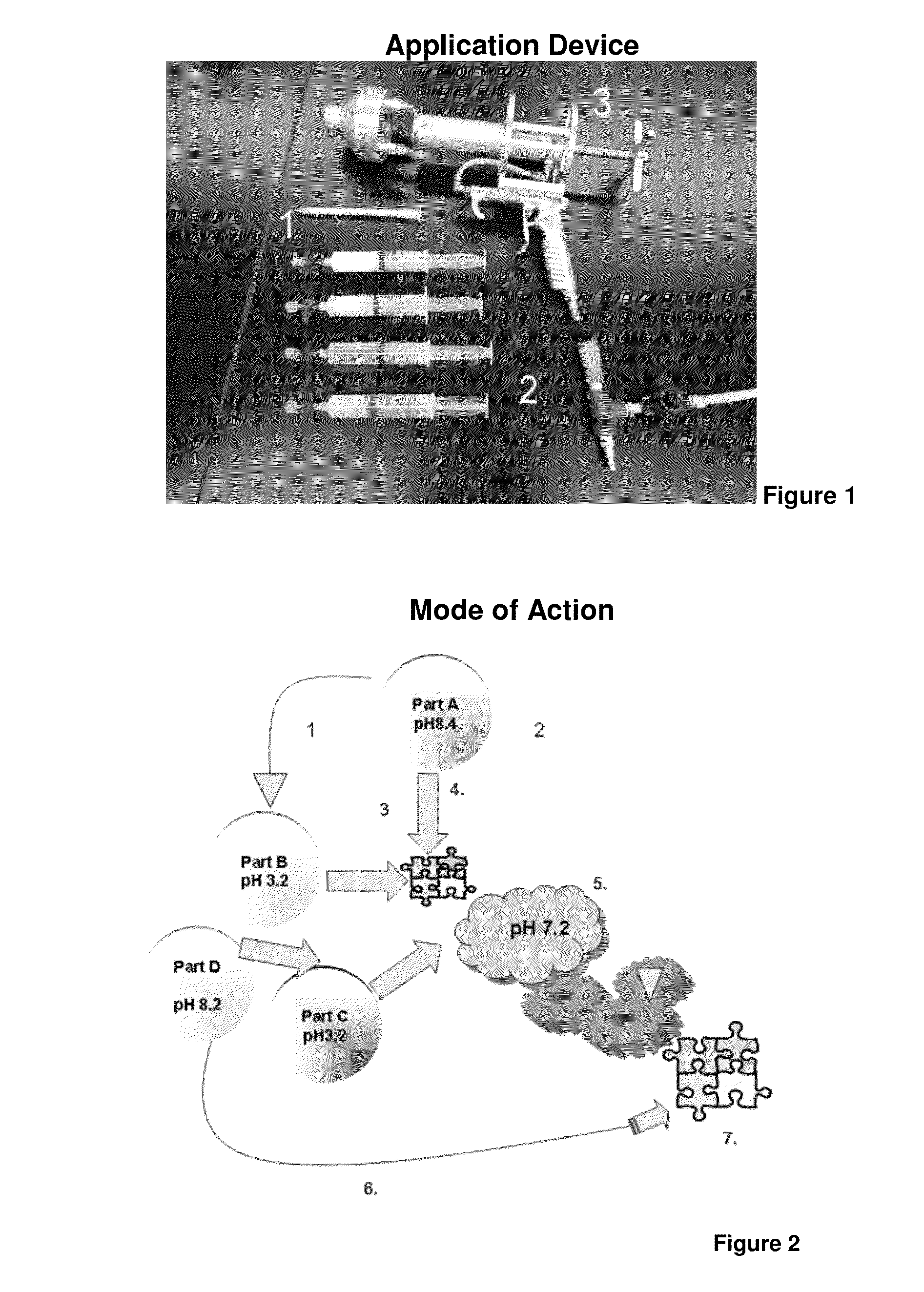
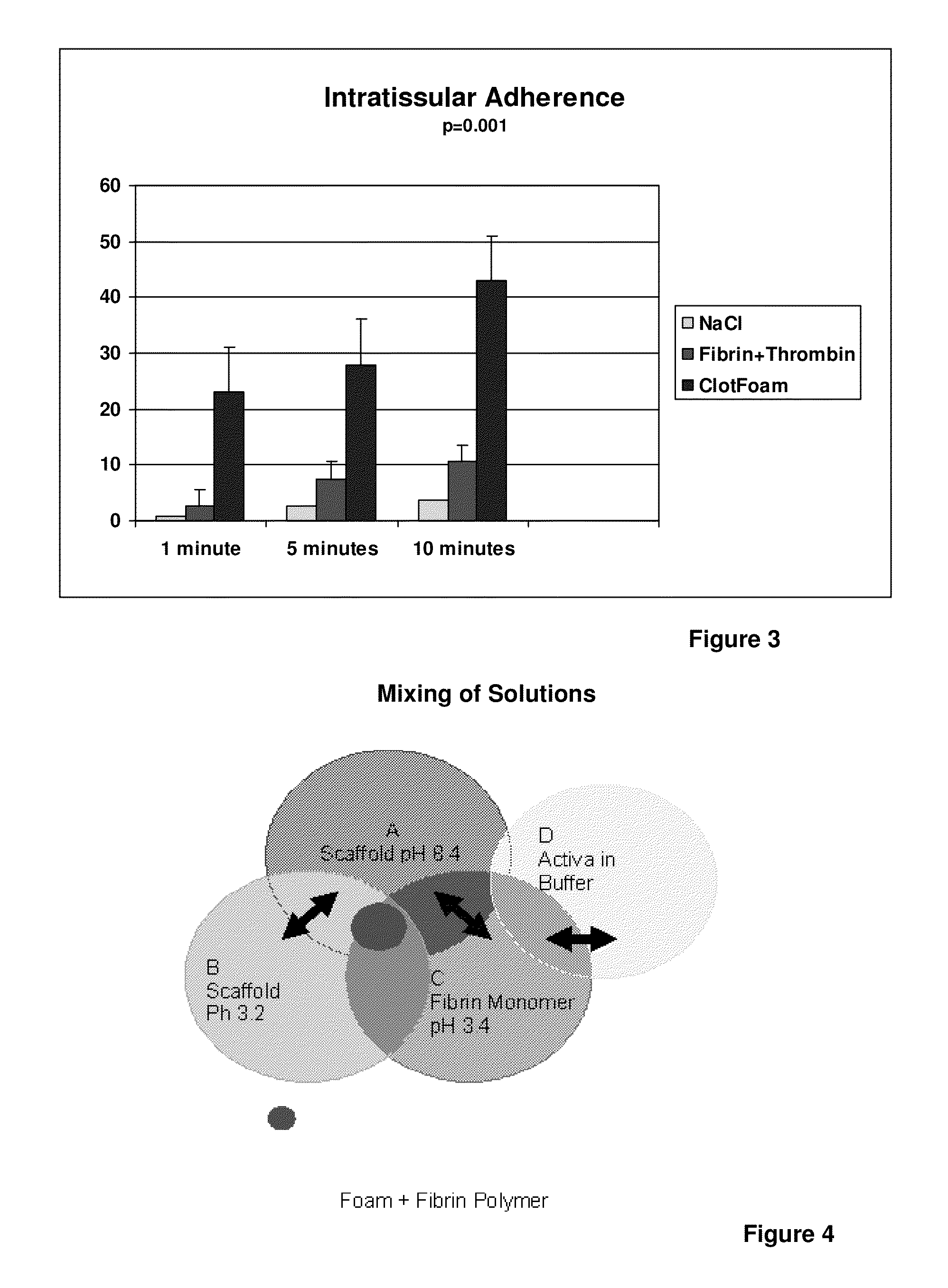
ClotFoam is a surgical sealant and hemostatic agent designed to be used in cases of non-compressible hemorrhage. It can be applied in the operating room through laparoscopic ports, or directly over lacerated tissue in laparotomy procedures or outside the operating room through a mixing needle and / or a spray injection method following abdominal, chest, extremities or other intracavitary severe trauma to promote hemostasis. Its crosslinking technology generates an adhesive three-dimensional polymeric network or scaffold that carries a fibrin sealant required for hemostasis. When mixed, Clotfoam produces a foam that spreads throughout a body cavity reaching the lacerated tissue to seal tissue and promote the coagulation cascade.The viscoelastic attachment properties of the foam as well as the rapid formation of a fibrin clot that ensure that the sealant remains at the site of application without being washed away by blood or displaced by movement of the target tissue .
Owner:FALUS GEORGE D PHD
Methods for minimally invasive vascular access
InactiveUS20060224110A1Maximize sizeMinimally invasiveControl devicesBlood pumpsPresent methodRadiology
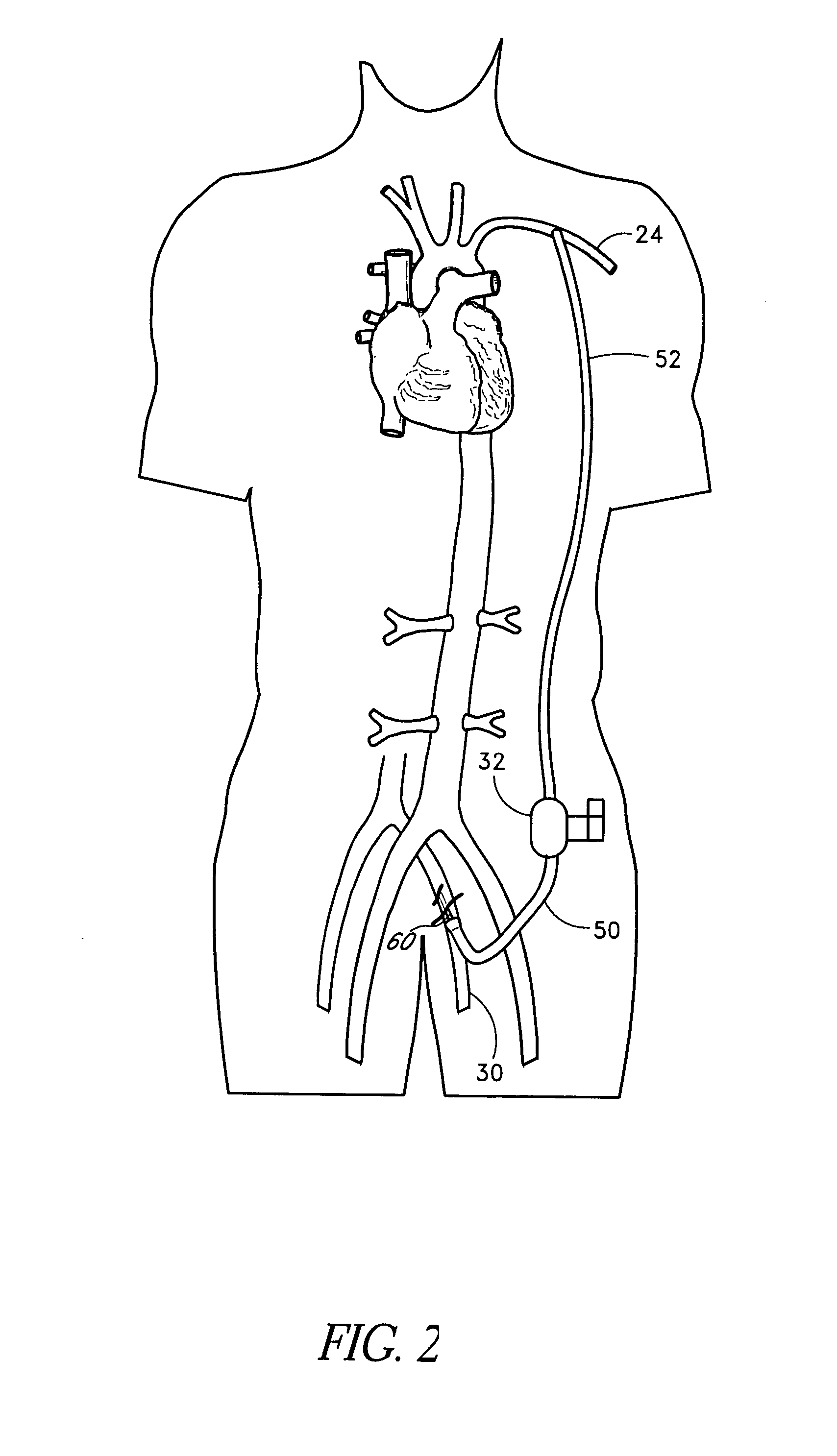
The present methods provide access to high flow vessels without causing severe trauma for the patient. At the same time, the methods maximize the size of a vascular instrument that can be deployed at the target location. The methods involve tunneling through the patient's tissue to create an access path between a percutaneous access site and the target location by using a second percutaneous site that is generally subcutaneous.
Owner:ORQIS MEDICAL
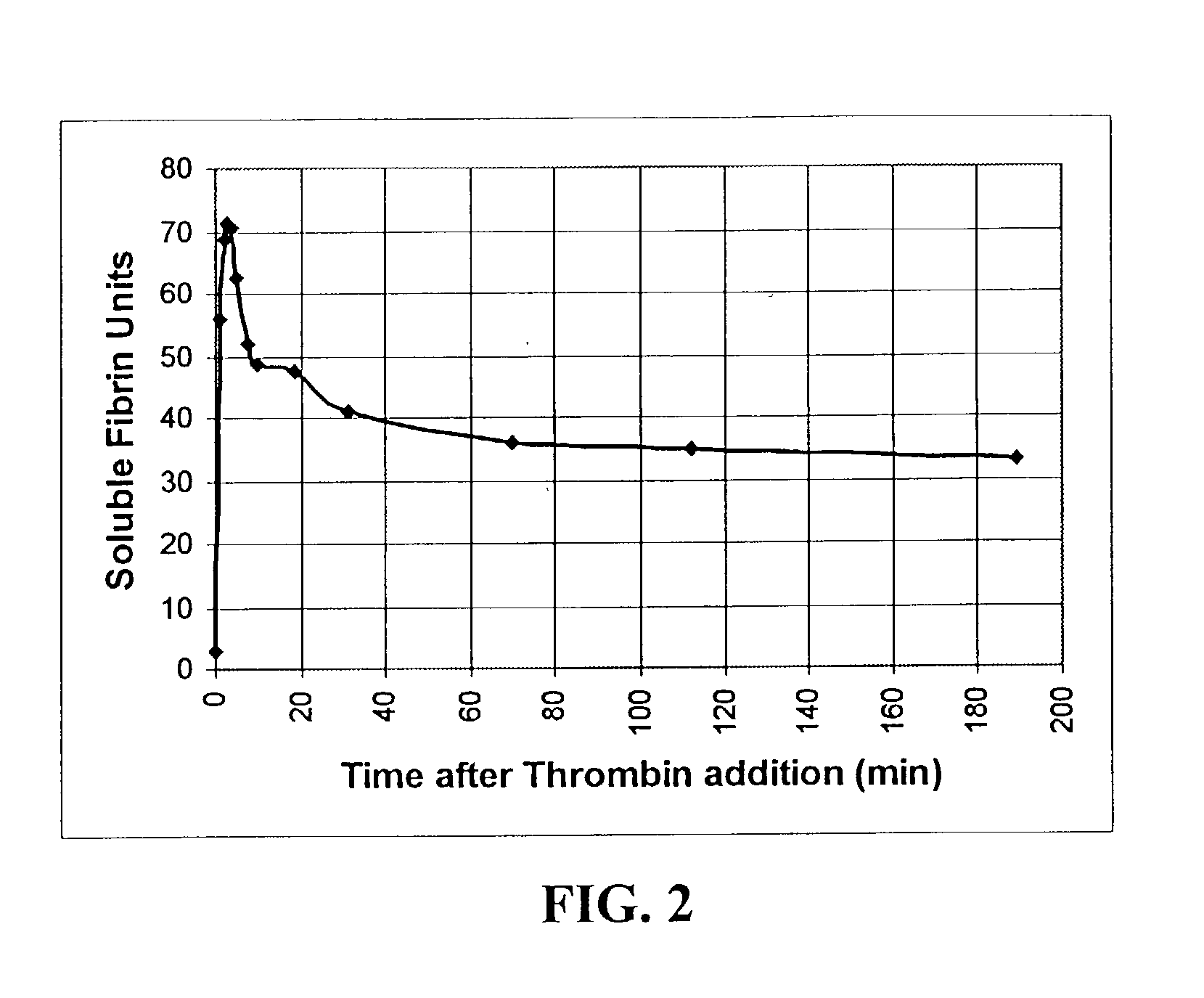
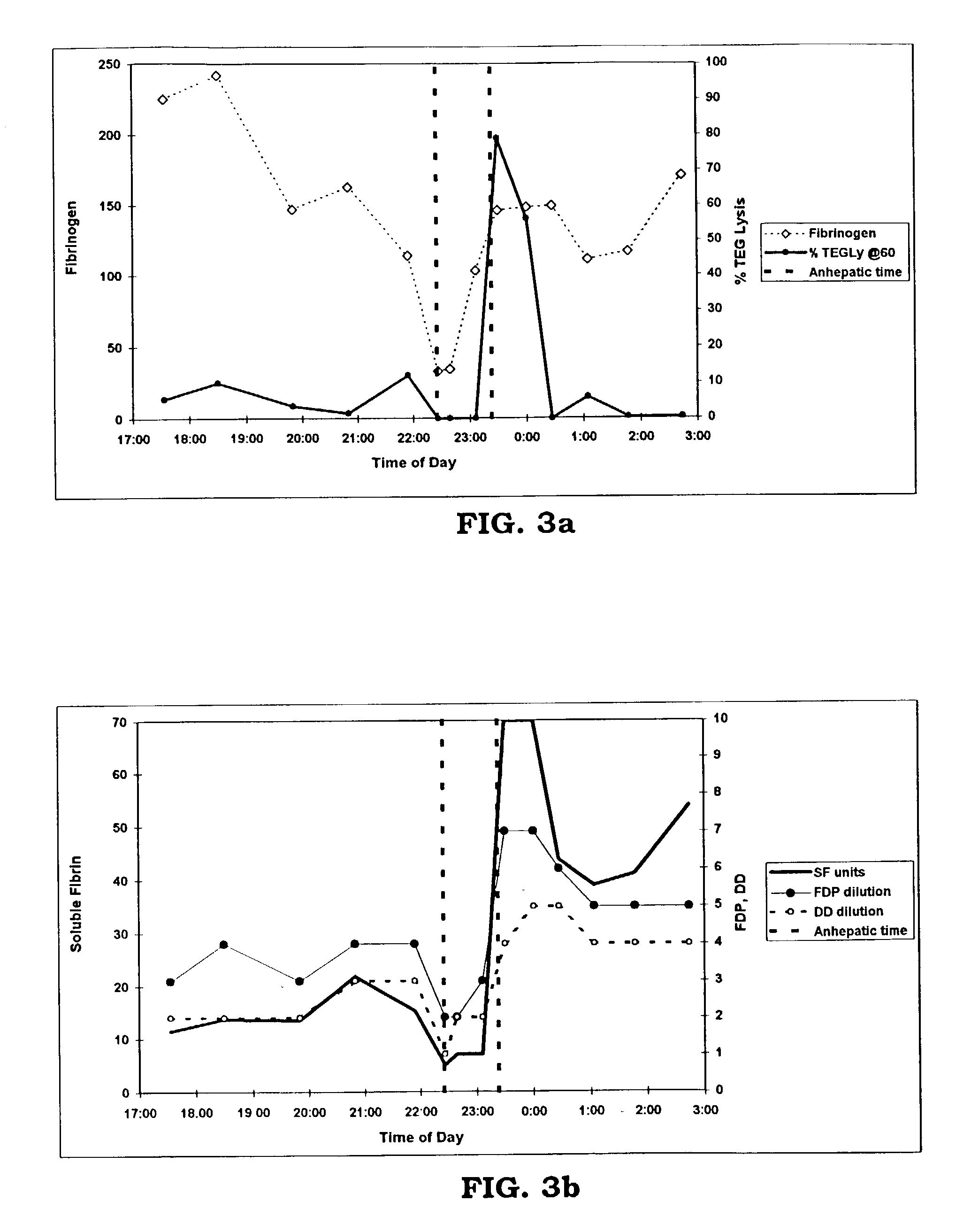
Rapid quantitative measurement of soluble fibrin in opaque body fluids
InactiveUS6436655B1Easy Optical InspectionAccurate detectionPeptide/protein ingredientsMicrobiological testing/measurementOperating theatresBody fluid
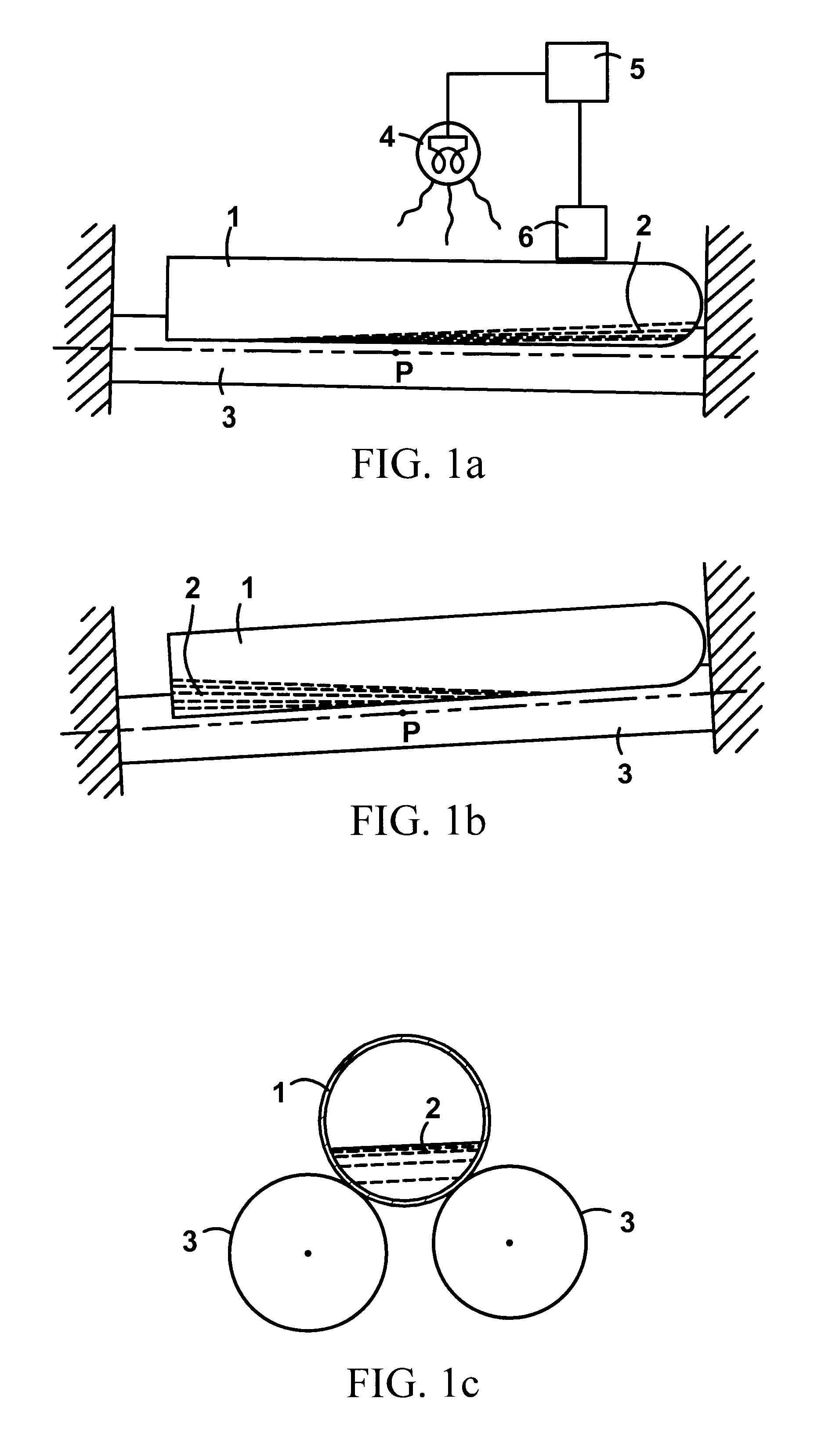
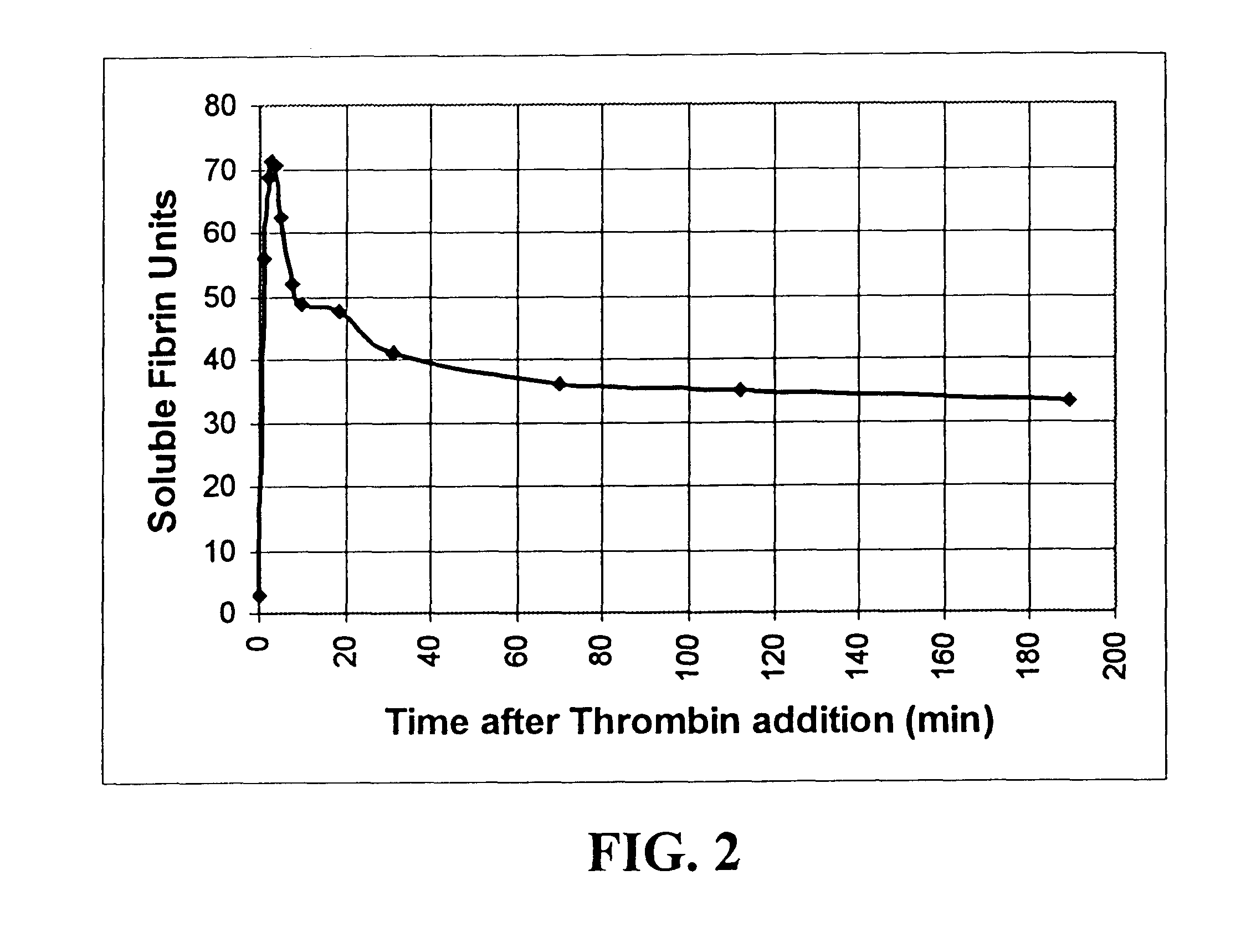
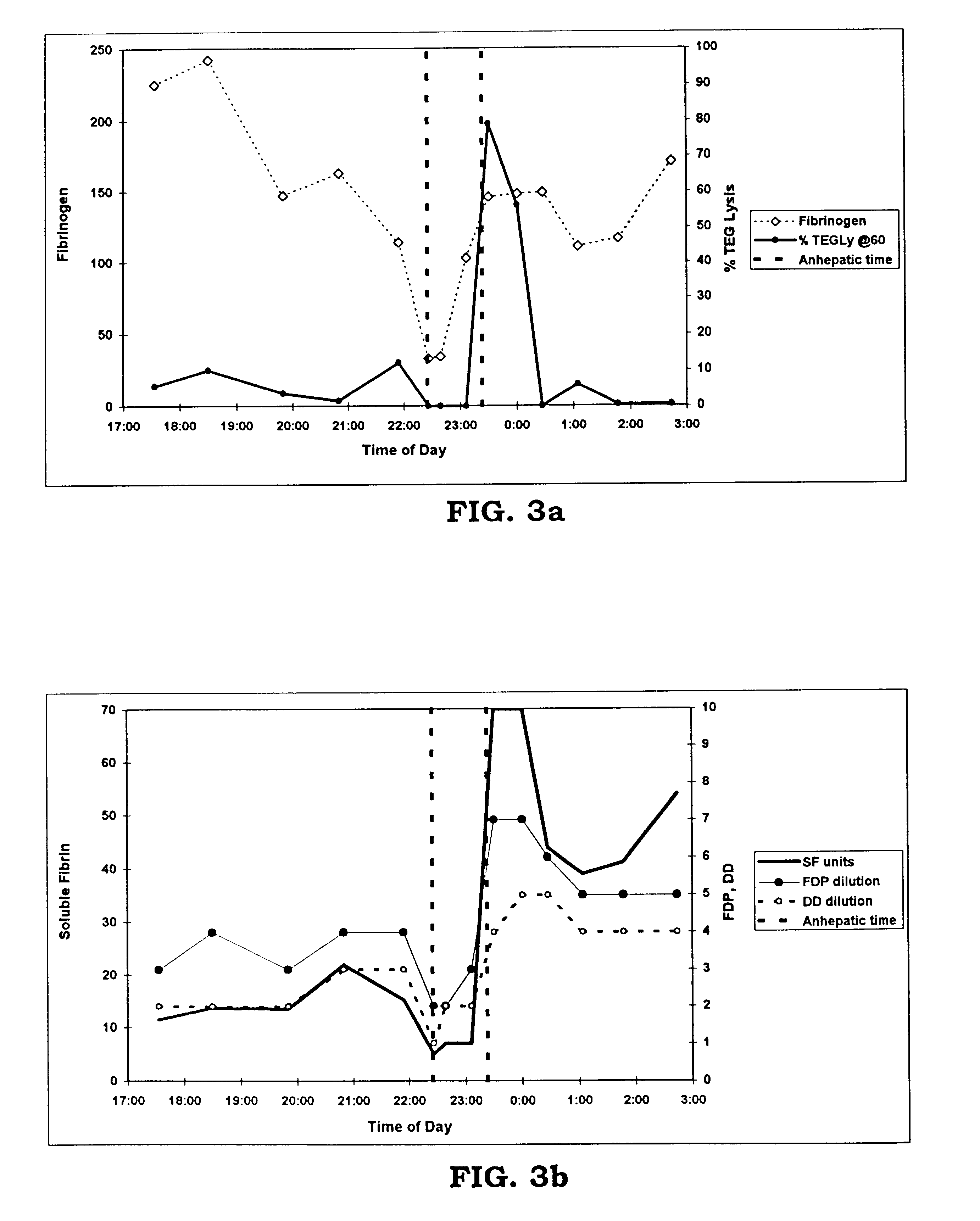
A method for determining the existence and the amount of soluble fibrin contained in a specimen fluid is provided. The method includes the steps of precipitating soluble fibrin out of the opaque specimen fluid, aggregating the soluble fibrin precipitates in a limited region of a transparent container so as to render the precipitates optically detectable in the opaque specimen fluid, and optically detecting the precipitates. The amount of soluble fibrin may be determined by measuring the time from the addition of the precipitating regent to the detection of the soluble fibrin precipitates. Methods of the present invention allow one to measure soluble fibrin in whole blood, and therefore render the test useful in the operating room under conditions of major surgery and in the presence of severe trauma wherein DIC is likely to supervene.
Owner:MEDICAL DEVICES CORP
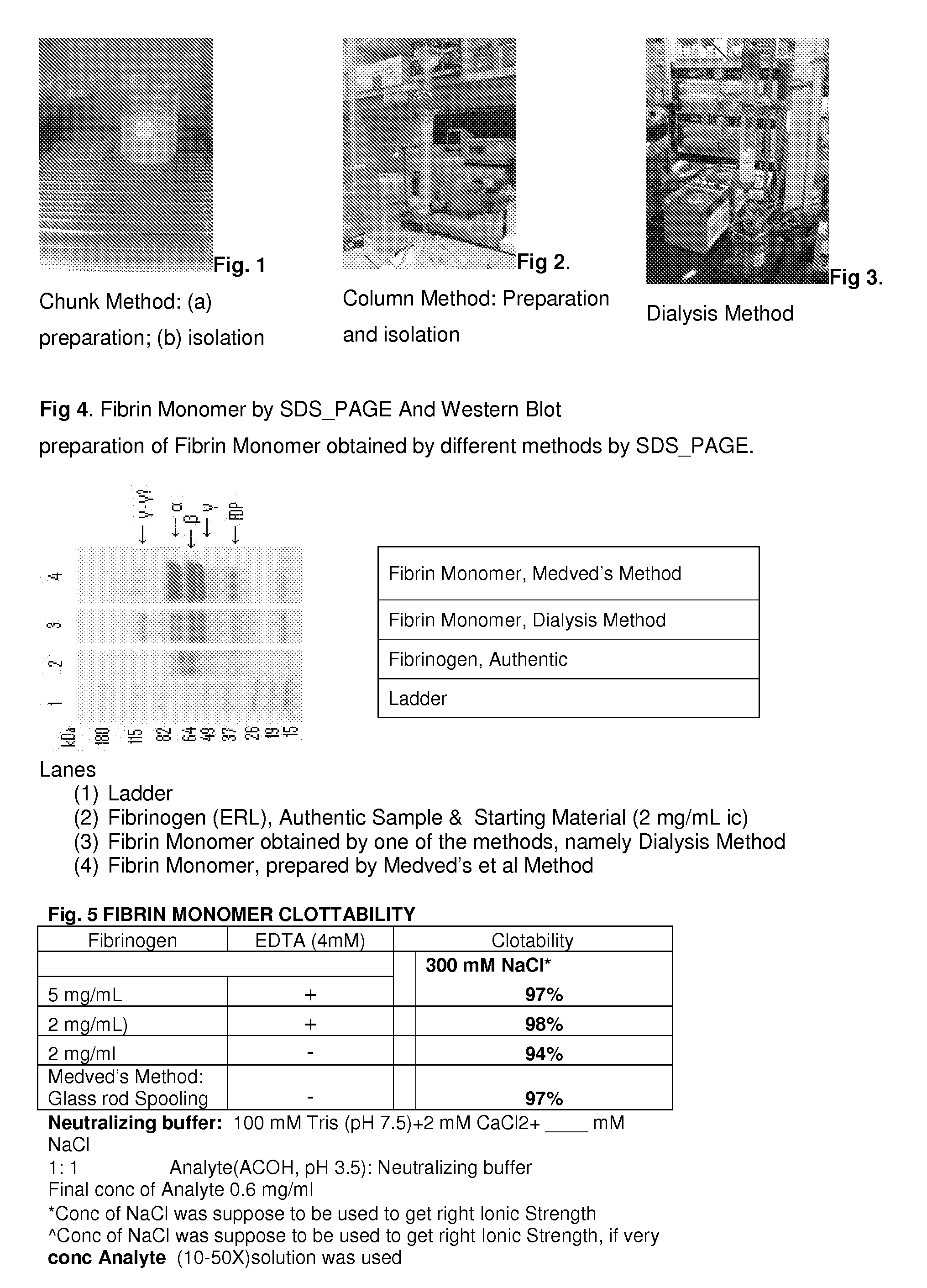
Method to produce fibrin monomer in acid media for use as tissue sealant
InactiveUS8367802B2Prevent re-bleedingReduce riskPeptide/protein ingredientsMammal material medical ingredientsFiberTissue sealant
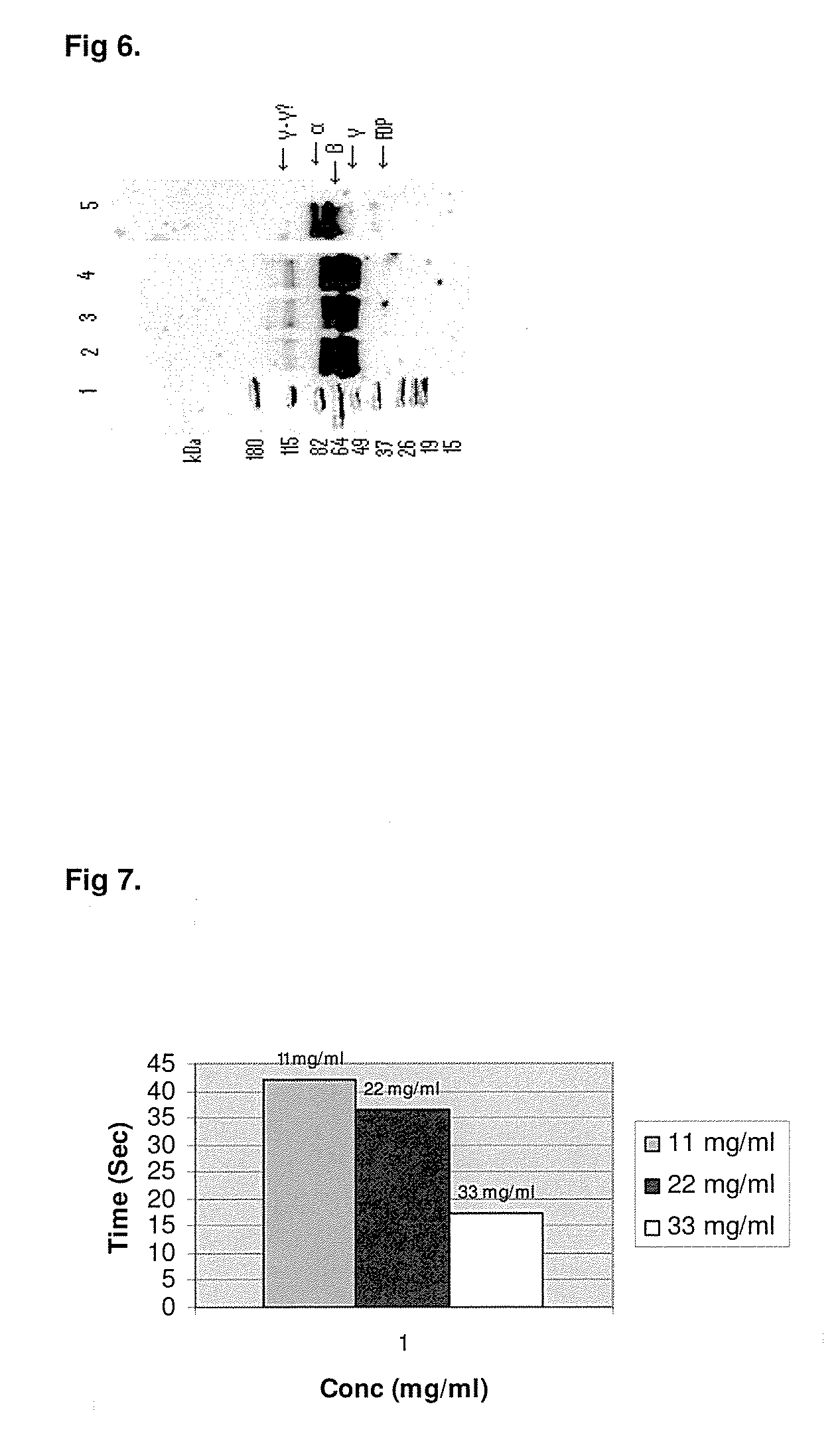
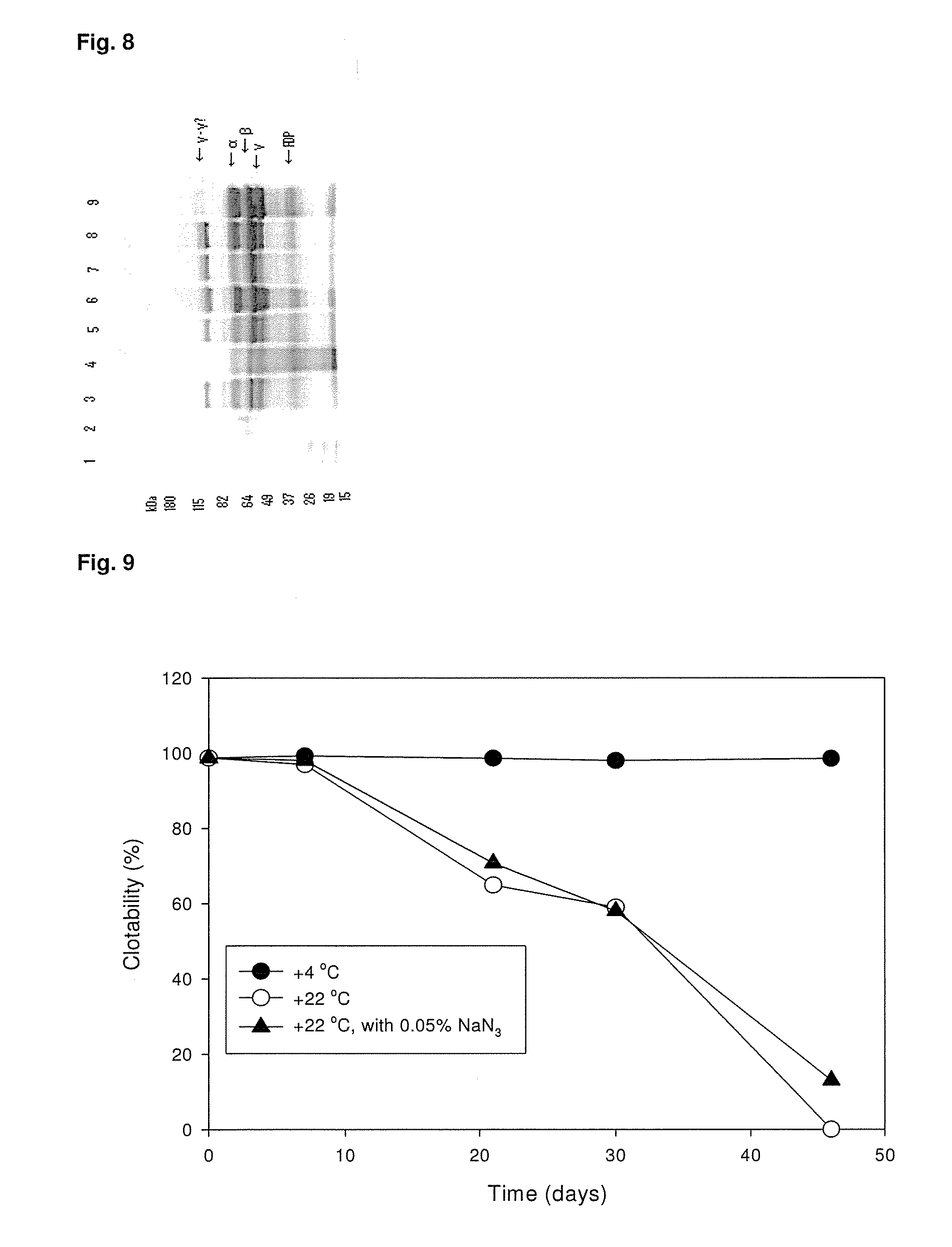
A hemostatic agent designed for use in cases of non-compressible hemorrhage. It can be applied through a mixing needle and / or a spray injection method following abdominal, chest, extremities or other intracavitary severe trauma to promote hemostasis, or it can be used for laparoscopic procedures or other surgical procedures in which compression is not possible or recommended. Its crosslinking technology generates an adhesive three-dimensional polymeric network or scaffold that carries a fibrin sealant required for hemostasis. When mixed, it produces a foam that spreads throughout a body cavity reaching the lacerated tissue to seal tissue and promote the coagulation cascade. The fibrin components are produced by a novel dialysis method which does not present thrombin to the immune system and can be maintained in solution for six weeks without significant proteolytic degradation.
Owner:MEDVED LEONID PHD +1
Tissue sealant for use in noncompressible hemorrhage
InactiveUS20100256671A1Excellent hemostatic agent candidateLeast riskBiocidePowder deliveryLaparoscopyTissue sealant
ClotFoam is an hemostatic agent designed for non-compressible hemorrhage. It can be applied outside the operating room through a mixing needle and / or a spray injection method following abdominal, chest, extremities or other intracavitary severe trauma to promote hemostasis, or it can be used in the operating room for laparoscopic procedures or other surgical procedures in which compression is not possible or recommended. Its crosslinking technology generates an adhesive three-dimensional polymeric network or scaffold that carries a fibrin sealant required for hemostasis. When mixed, Clotfoam produces a foam that spreads throughout a body cavity reaching the lacerated tissue to seal tissue and promote the coagulation cascade. The viscoelastic attachment properties of the foam as well as the rapid formation of a fibrin clot that ensure that the sealant remains at the site of application without being washed away by blood or displaced by movement of the target tissue .
Owner:BIOMEDICA MANAGEMENT
Tissue sealant for use in non compressible hemorrhage
InactiveUS8314211B2Excellent hemostatic agent candidateLeast riskSuture equipmentsPowder deliveryTissue sealantLaparoscopes
Owner:FALUS GEORGE D PHD
Severe trauma treatment warning communication system
InactiveCN103565430ACompression waiting time for medical treatmentRespiratory organ evaluationSensorsData centerTreatment team
The invention discloses a severe trauma treatment warning communication system which comprises a vehicle-mounted traumatic condition assessment warning information release terminal, a data center, a first-aid center and a medical first-aid station. The vehicle-mounted terminal comprises a central processing unit (CPU) control module, a man-machine interface module, a high-definition camera, a positing module. The CPU control module transmits collected or reflected information to the data center through a communication system; the data center carries out real-time detection over warning information uploaded by each vehicle-mounted traumatic condition assessment warning information release terminal and uploads the warning information to the first-aid center; the fist-aid center gives out the warning information to the hospital first-aid station. The trauma treatment warning communication system enables an ambulance, a treatment hospital and a specialized treatment team to acquire an early information communication and a warning channel, so that each procedure of treating patients is closely linked together, treatment and waiting time is shortened to an extreme, all procedures for treating and curing the patients can be prepared in advance, and valuable golden rescuing time for curing the severe trauma patients is available.
Owner:PEOPLES HOSPITAL PEKING UNIV
Methods and devices for biomechanical assessment of pelvic floor including perineum prior to childbirth
The transvaginal tactile probe is configured to obtain a high resolution mapping of pressures and strains within the vagina of a pregnant woman prior to birth. The device provides real-time data visualization, analysis tools and information. This data may be used to assist with clinical decisions regarding selecting a preferred method of prevention of severe childbirth injury or altering delivery management, e.g. early induction at term, elective caesarean section in patients with a history of obstetric anal sphincter injury, water-birth, warm compresses to the perineum. The device is intended for use by medically trained personnel who counsels patients regarding risk of severe trauma at childbirth (such as urogynecologists and obstetricians) and regarding the effect of perineal massage and childbirth training device (such as physical therapists).
Owner:ADVANCED TACTILE IMAGING
1,4,6-androstatriene-3,17-dione ("ATD") for therapeutic uses
InactiveUS20060154909A1Good for healthEnhance and stimulate growthBiocideOrganic active ingredientsExercise performanceGynecomastia
A composition having modified or derivative of 1,4,6-androstatriene-3,17-dione (“ATD”) will improve the health of mammalian subjects. The improvement of health is achieved with the administration of an effective amount of the at least one modified or derivative of 1,4,6-androstatriene-3,17-dione. Particularly, health is improved for a subject suffering with a gynecomastia, and / or estrogen-dependent cancer. Also, subjects recovering from steroid misuse / abuse with treatment in accordance with the present invention. Other improvements found to occur with an administration of ATD is that growth is enhanced and / or stimulated in developing mammals, recovery is shortened in cases of severe trauma or burns, mood levels are improved, male fertility is improved, and athletic performance is improved by increasing testosterone and lean muscle mass.
Owner:KNELLER BRUCE W
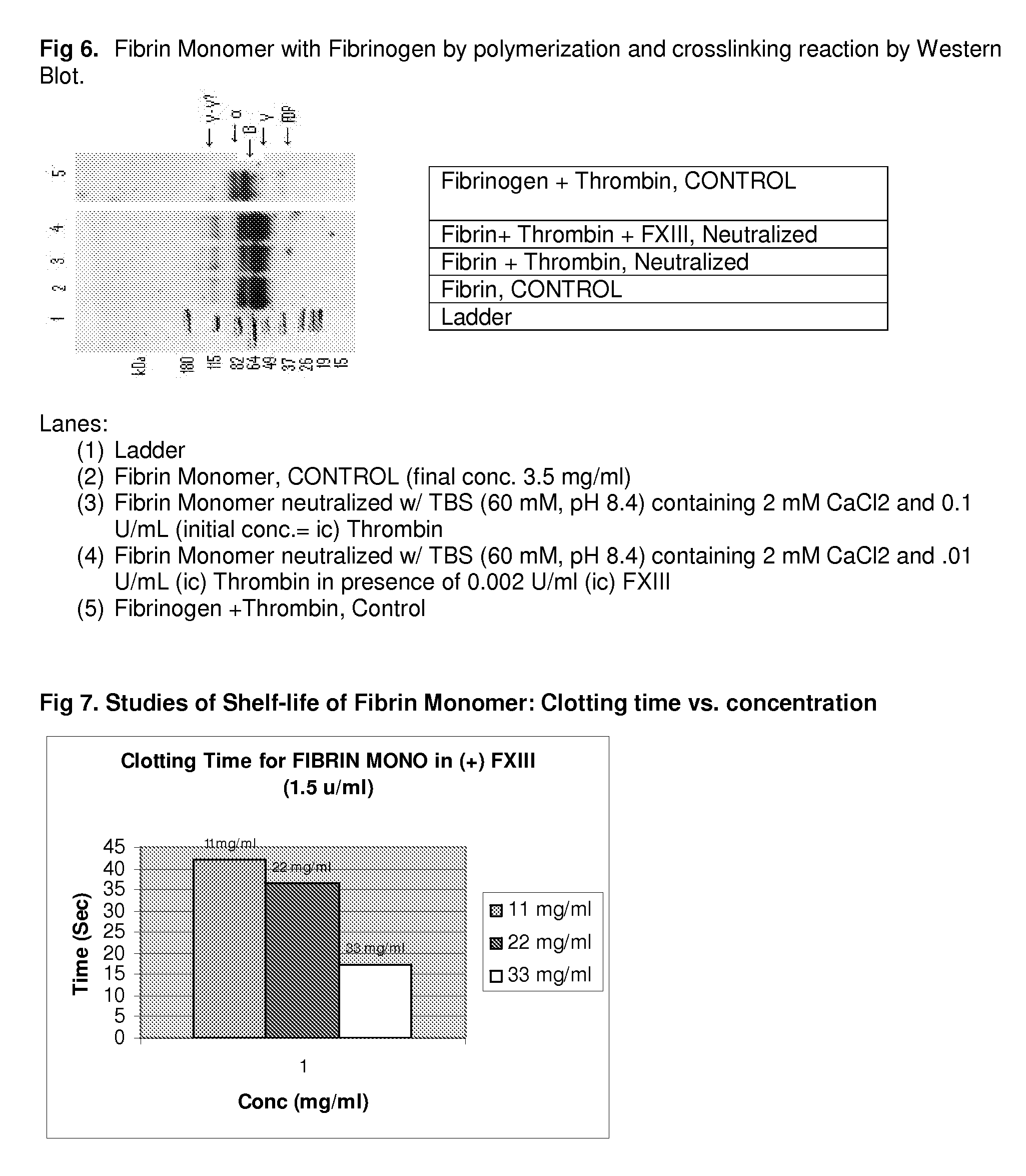
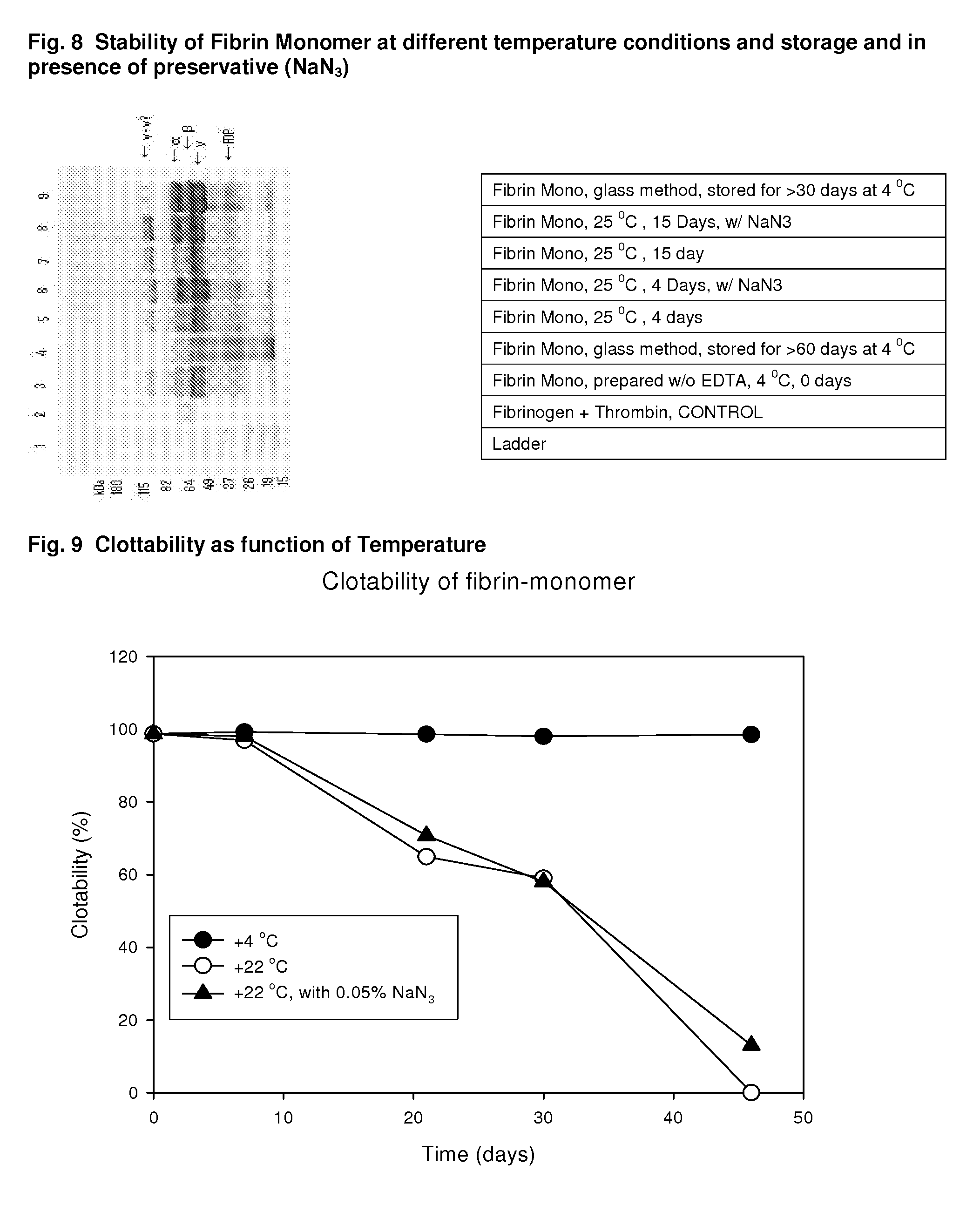
Method to produce fibrin monomer in acid media for use as tissue sealant
InactiveUS20100197893A1Prevent re-bleedingReduce riskPeptide/protein ingredientsMammal material medical ingredientsFiberTissue sealant
ClotFoam is an hemostatic agent designed for use in cases of non-compressible hemorrhage. It can be applied outside the operating room through a mixing needle and / or a spray injection method following abdominal, chest, extremities or other intracavitary severe trauma to promote hemostasis, or it can be used in the operating room for laparoscopic procedures or other surgical procedures in which compression is not possible or recommended. Its crosslinking technology generates an adhesive three-dimensional polymeric network or scaffold that carries a fibrin sealant required for hemostasis. When mixed, Clotfoam produces a foam that spreads throughout a body cavity reaching the lacerated tissue to seal tissue and promote the coagulation cascade. The viscoelastic attachment properties of the foam as well as the rapid formation of a fibrin clot that ensure that the sealant remains at the site of application without being washed away by blood or displaced by movement of the target tissue. The fibrin components are produced by a novel method which does not present thrombin to immune system and can be maintained in solution for six weeks without significant proteolytic degradation.
Owner:MEDVED LEONID PHD +1
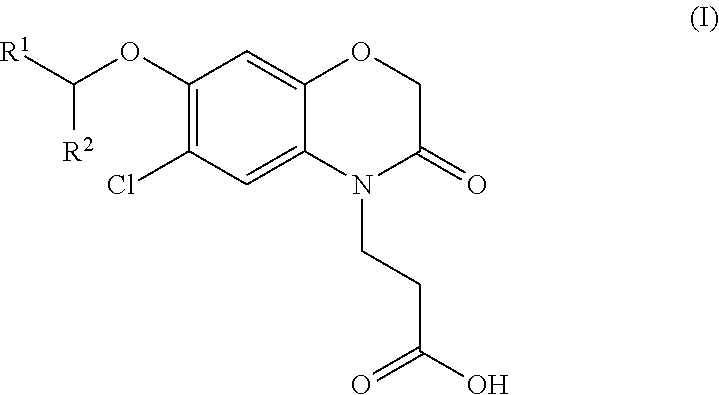
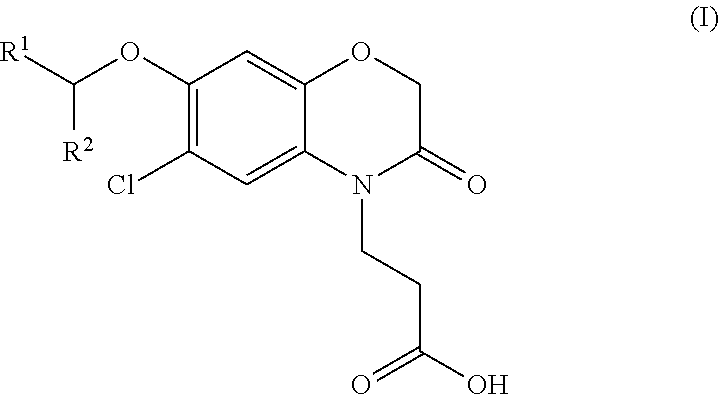
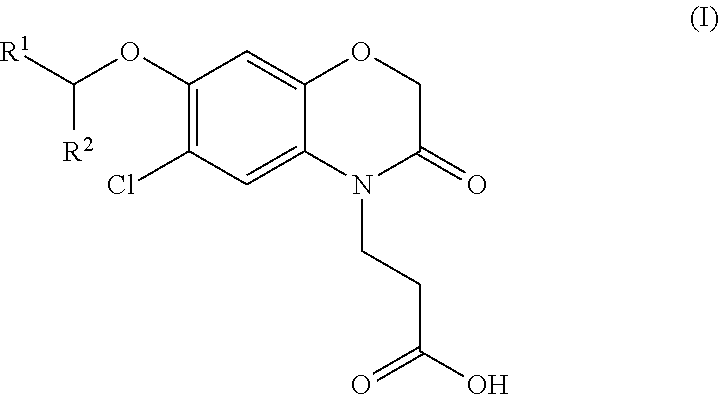
3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors
InactiveUS20160318884A1Antibacterial agentsOrganic active ingredientsAIDS dementia complexNeonatal respiratory distress syndrome
A compound of formula (I) or a salt thereof are provided:wherein R1, X and R3 are defined in the specification, useful in the treatment of disorders mediated by KMO such as acute pancreatitis, chronic kidney disease, other conditions associated with systemic inflammatory response syndrome (SIRS), Huntington's disease, Alzheimer's disease, spinocerebellar ataxias, Parkinson's disease, AIDS-dementia complex, amylotrophic lateral sclerosis (ALS), depression, schizophrenia, sepsis, cardiovascular shock, severe trauma, acute lung injury, acute respiratory distress syndrome, acute cholecystitis, severe burns, pneumonia, extensive surgical procedures, ischemic bowel, severe acute hepatic disease, severe acute hepatic encephalopathy or acute renal failure.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Blood clotting composition and method of use
InactiveUS20130022552A1Easily identifiableEasily and immediately accessibleOrganic active ingredientsPeptide/protein ingredientsPublic placeMedicine
The composition is a blood clotting composition including at least one hemostatic agent and an indicator agent that adheres to the skin on or around the wound, the indicator agent being specifically identifiable to the hemostatic composition. The composition can be adapted to be applied to a wound as an emergency trauma treatment in homes, public places, trauma treatment centers, at accident locations, in the field of military combat, or any other potential site of severe trauma as a first response to reduce or stop bleeding. The composition can include at least one clotting agent, an analgesic, a bactericide (such as silver), and at least one antibiotic. An applicator device for the composition is included.
Owner:SOLOMON CLIFFORD T +1
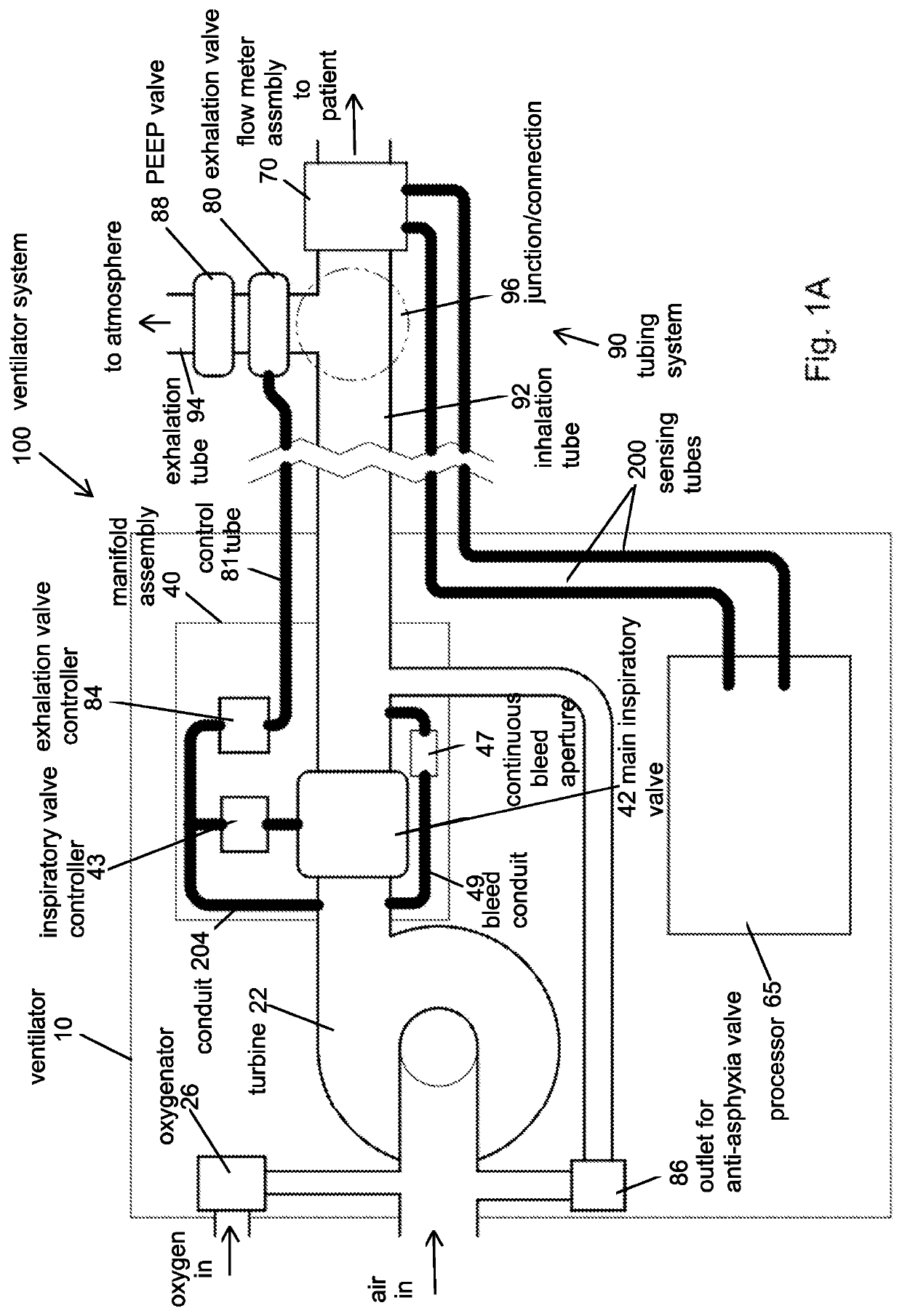
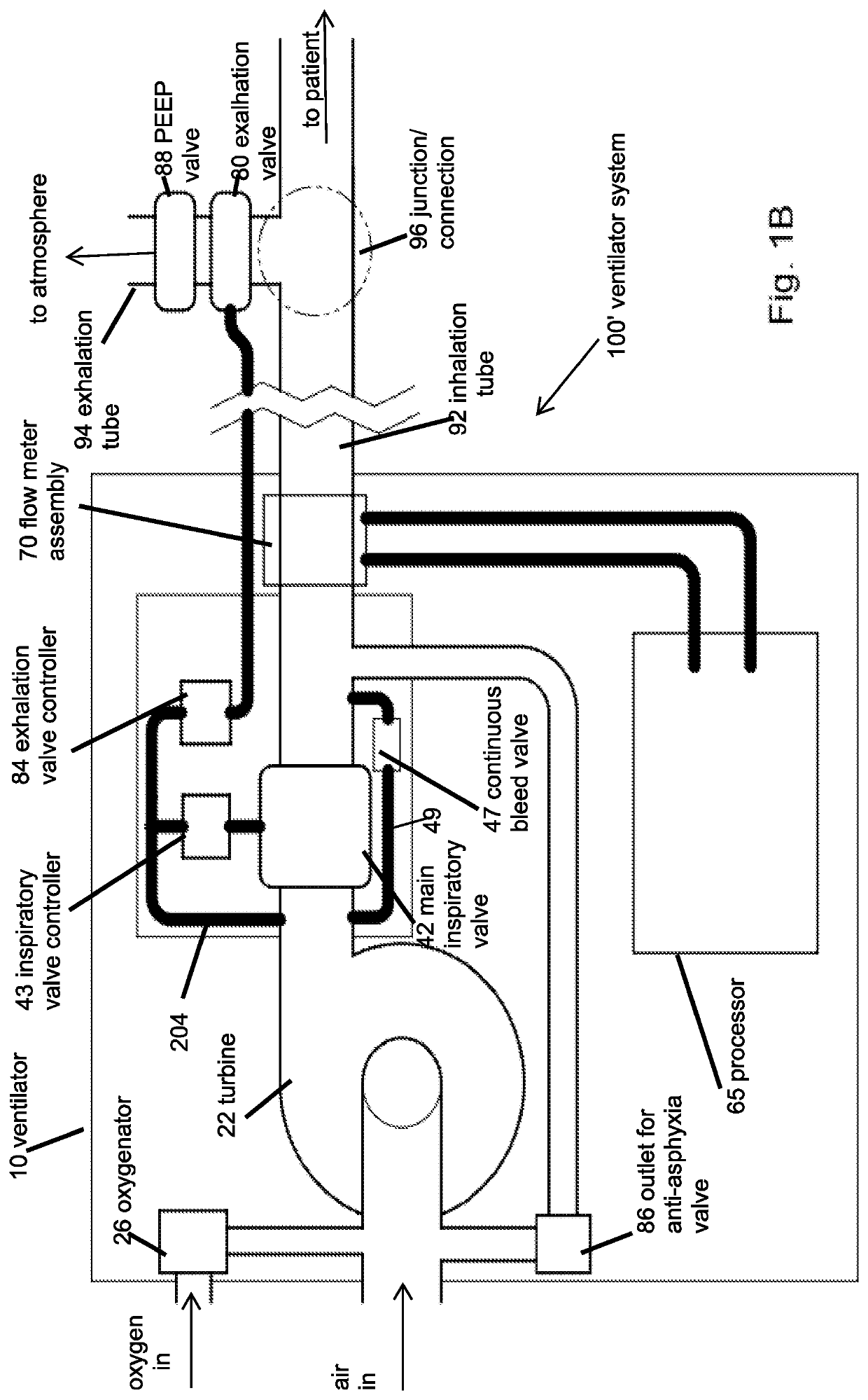
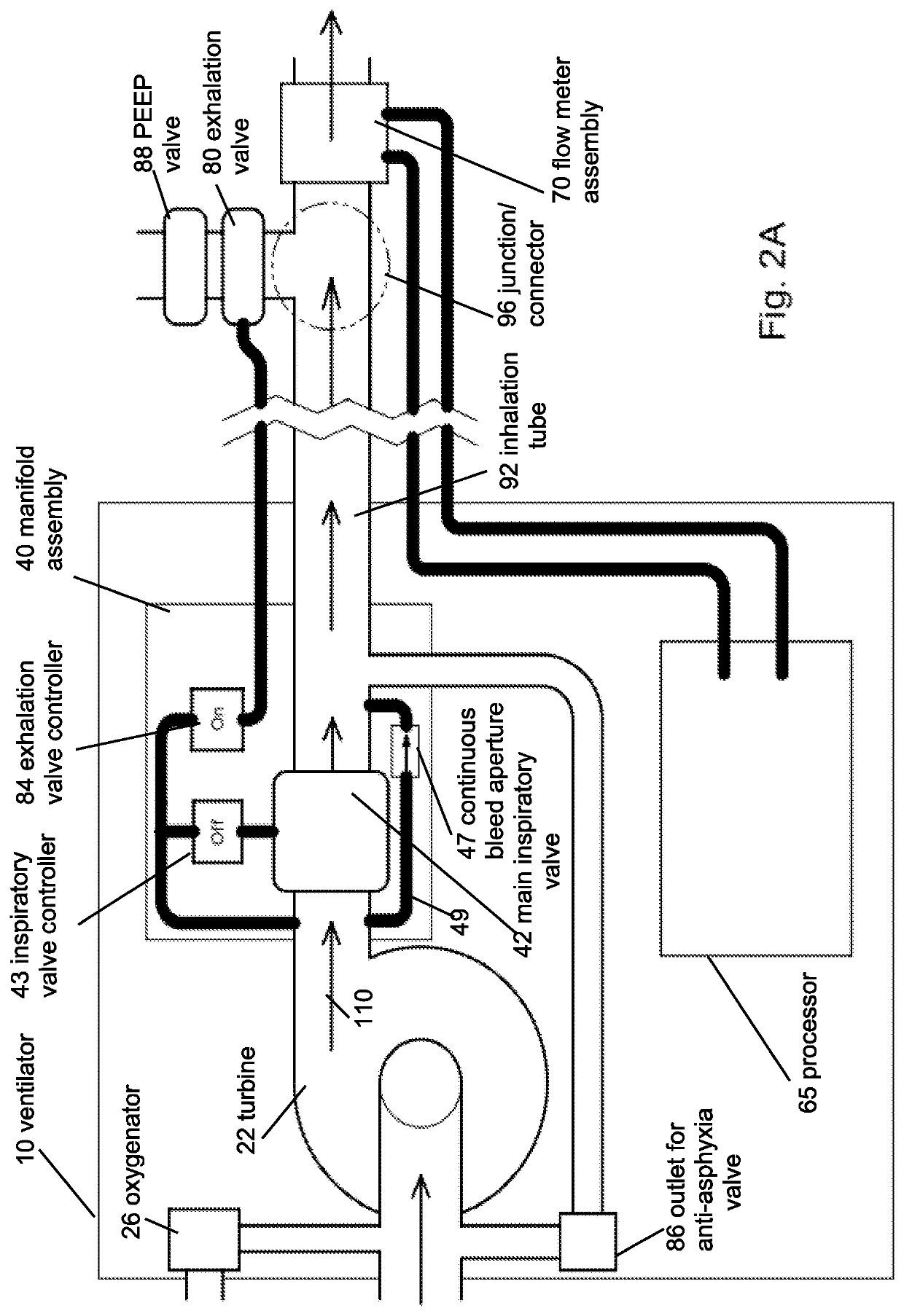
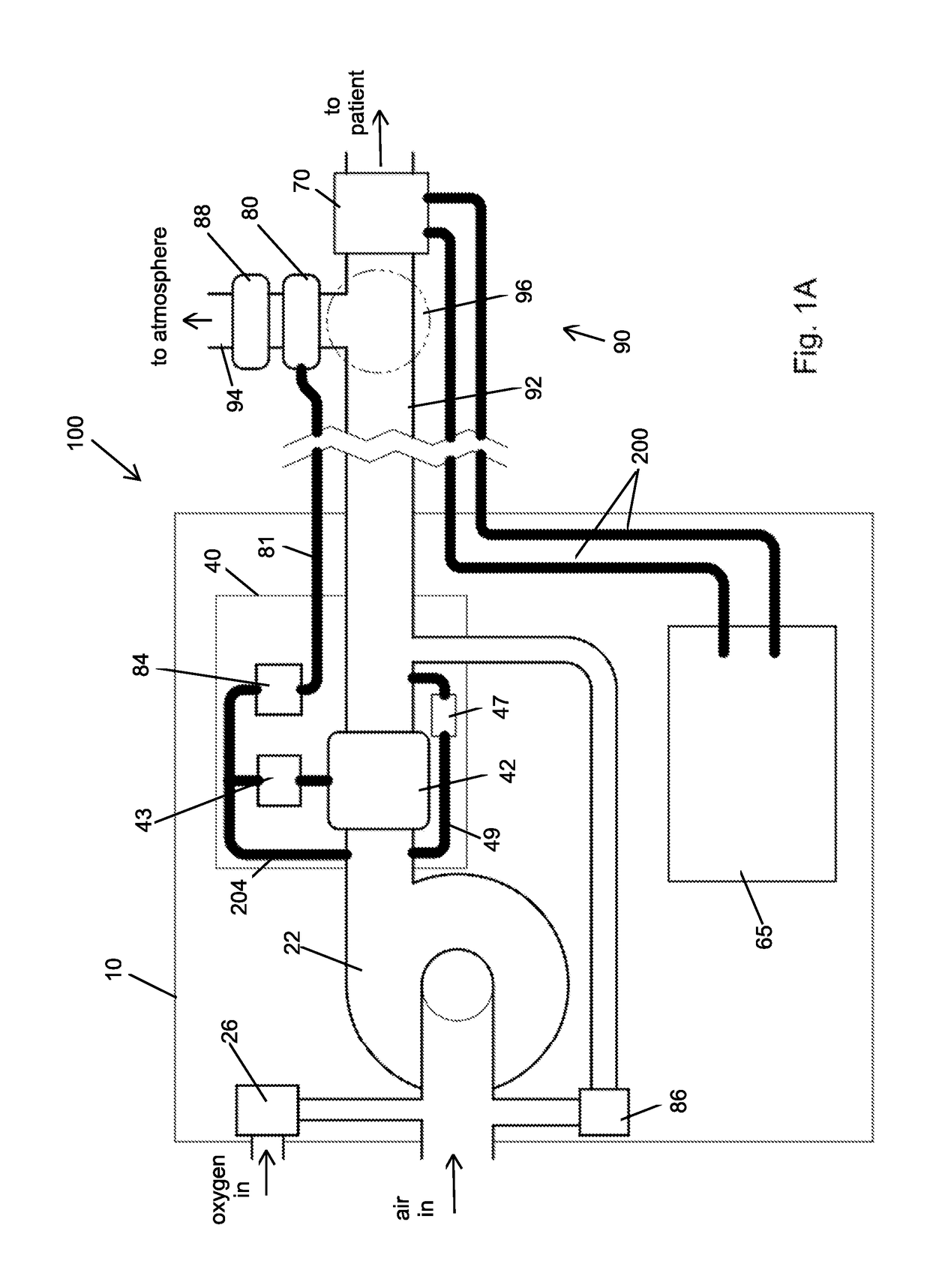
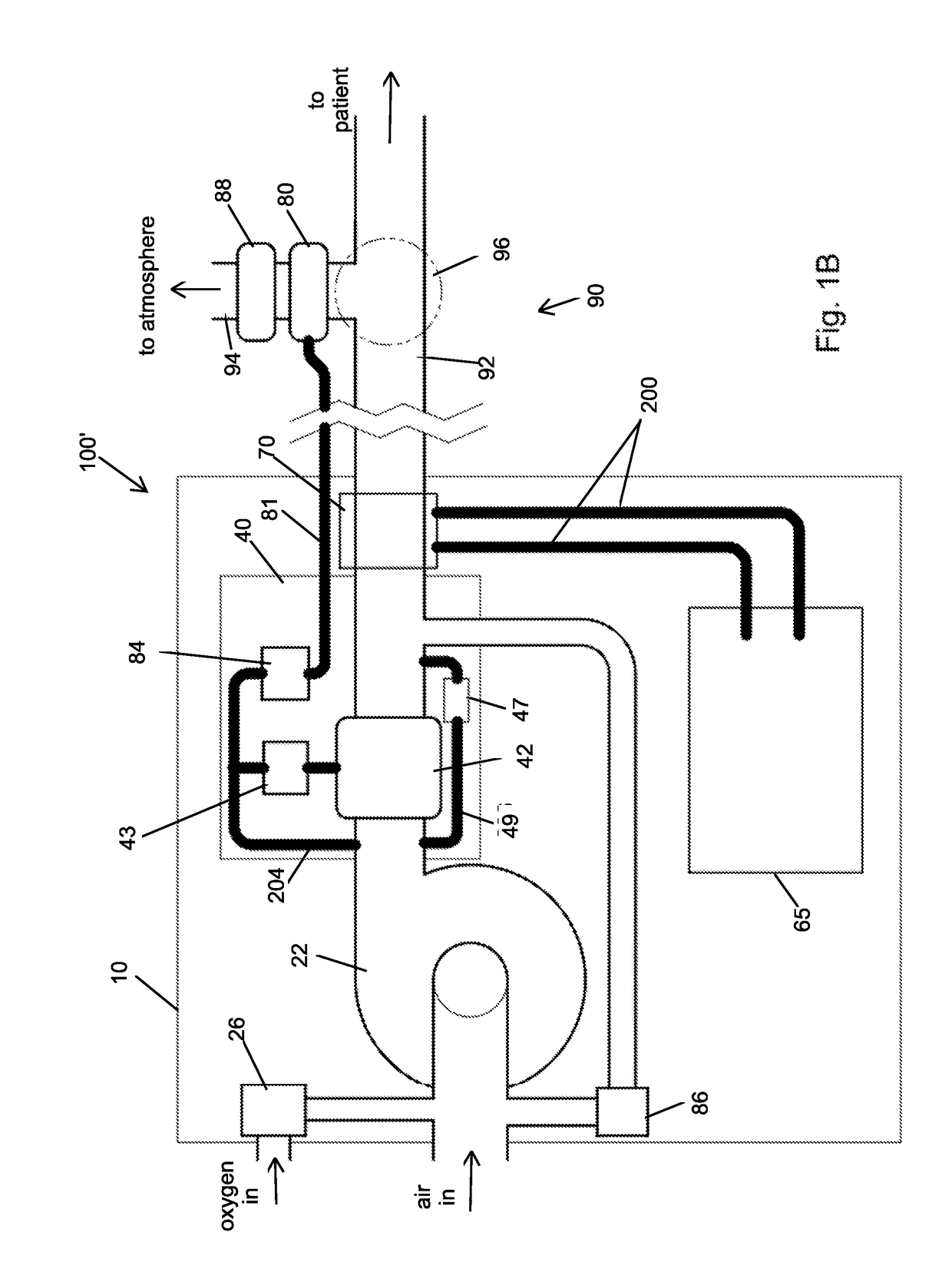
Portable light-weight ventilator system
A ventilator system for providing respiratory support in cases of acute respiratory failure or severe trauma is described. The ventilator system comprises a ventilator and a tubing system. The system is characterized in that the ventilator comprises a continuous bleed valve configured to be open to air flow from the blower at all times when the blower is operating during both inspiration and expiration; thereby providing a minimal amount of pressure within a patient's lungs at the end of each exhalation positive end expiratory pressure (PEEP). In an embodiment of the invention the system comprises a manifold block configured to hold the main operating elements of ventilator.
Owner:INOVYTEC MEDICAL SOLUTIONS
Improvements on tissue sealant for use in non-compressible hemorrhage
InactiveCN104918606ASafe evacuationSurgical adhesivesPeptide/protein ingredientsTissue sealantPERITONEOSCOPE
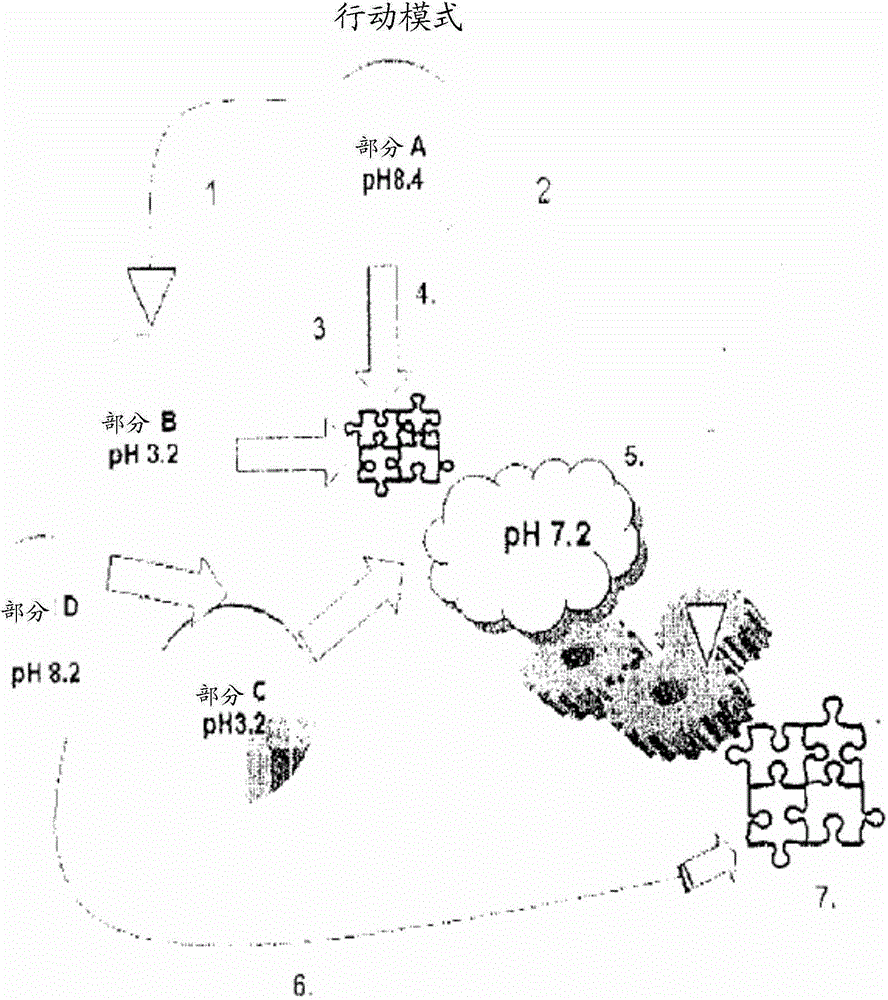
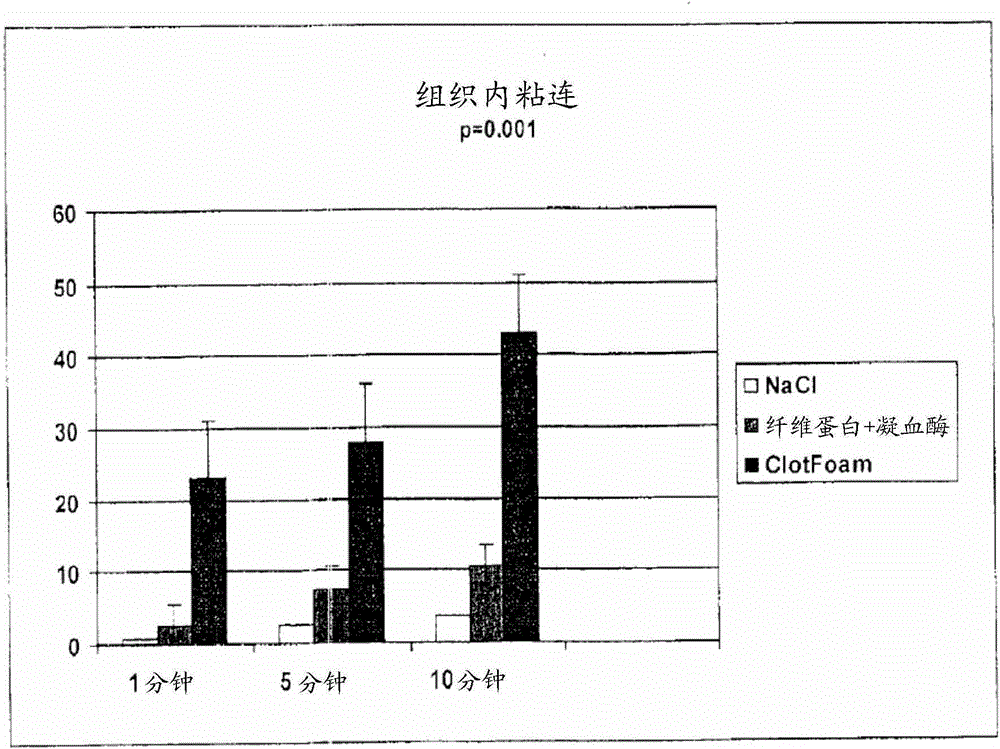
ClotFoam is a sealant and hemostatic agent for use in cases of non-compressible hemorrhage for moderate to severe bleeding. It can be applied in the operating room through laparoscopic ports, or directly over lacerated tissue in laparotomy procedures or outside the operating room through a mixing needle and / or a spray injection method following intracavitary severe trauma or surgery. Its crosslinking technology generates an adhesive scaffold that carries a fibrin sealant required for hemostasis. Clotfoam produces a foam that spreads throughout a body cavity reaching the lacerated tissue to seal tissue and promote the coagulation cascade. The composition is biocompatible and non-inflammatory. The invention uses fibrin monomer polymerized by a change of pH as active sealing component. The viscoelastic properties of the foam as well as the rapid formation of a fibrin clot ensure that the sealant remains at the site of application without being washed away by blood.
Owner:乔治 D. 福卢什
3-(6-chloro-3-oxo-3,4-dihydro-(2H)-1,4-benzoxazin-4-yl) propanoic acid derivatives and their use as KMO inhibitors
Compounds of formula (I)wherein:R1 is heteroaryl optionally substituted by methyl, ethyl, halo or ═O; andR2 is H, methyl or ethyl.and salts thereof are KMO inhibitors and may be useful in the treatment of various disorders, for example acute pancreatitis, chronic kidney disease, other conditions associated with systemic inflammatory response syndrome (SIRS), Huntington's disease, Alzheimer's disease, spinocerebellar ataxias, Parkinson's disease, AIDS-dementia complex, HIV infection, amylotrophic lateral sclerosis (ALS), depression, schizophrenia, sepsis, cardiovascular shock, severe trauma, acute lung injury, acute respiratory distress syndrome, acute cholecystitis, severe burns, pneumonia, extensive surgical procedures, ischemic bowel disease, severe acute hepatic disease, severe acute hepatic encephalopathy or acute renal failure.
Owner:THE UNIV COURT OF THE UNIV OF EDINBURGH
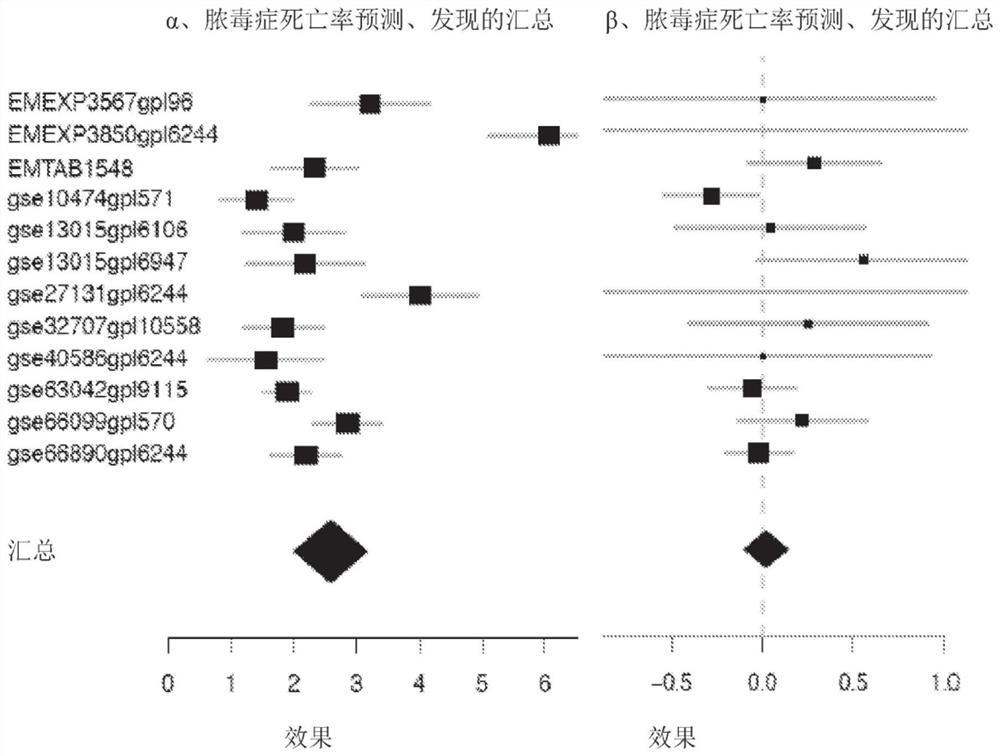
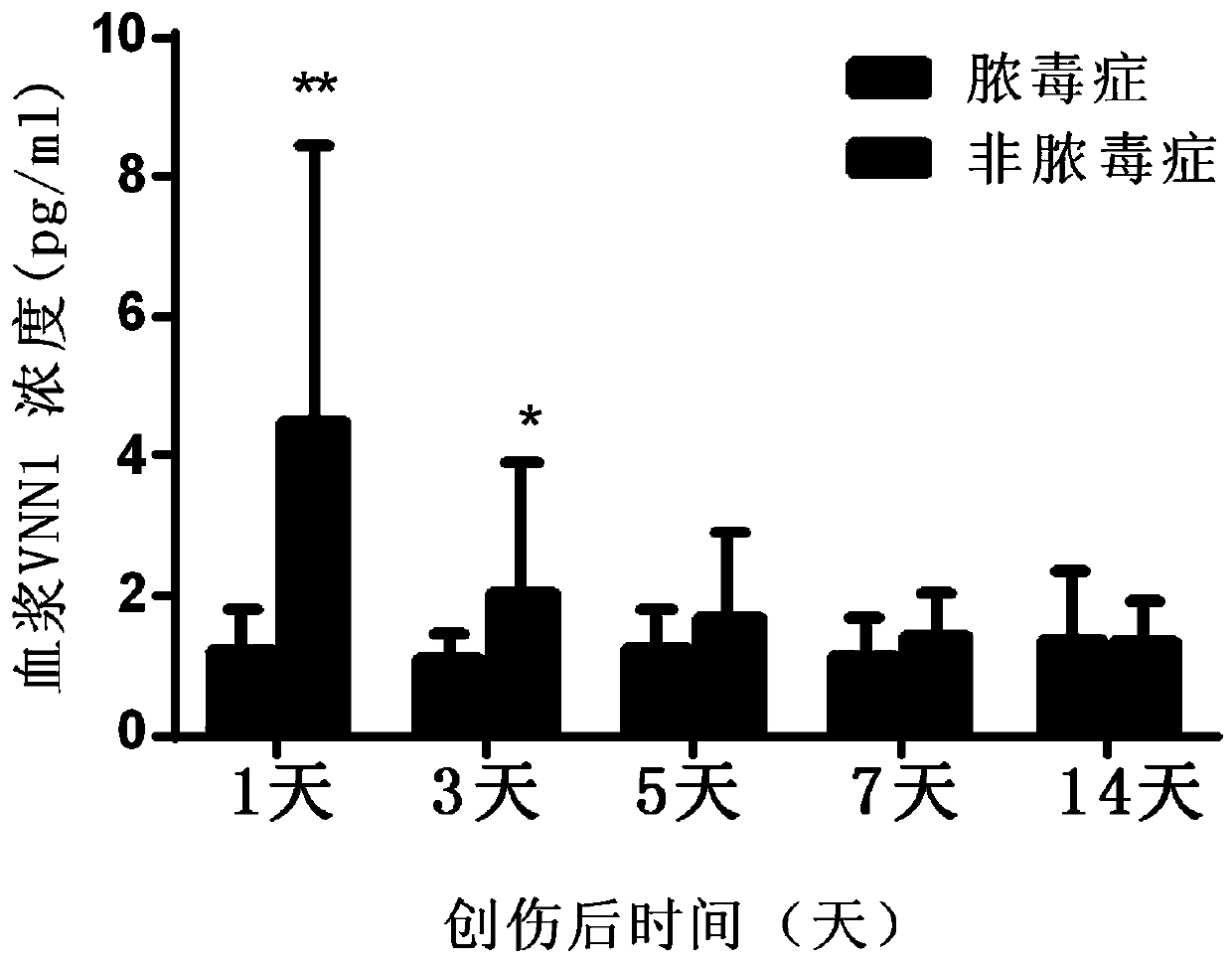
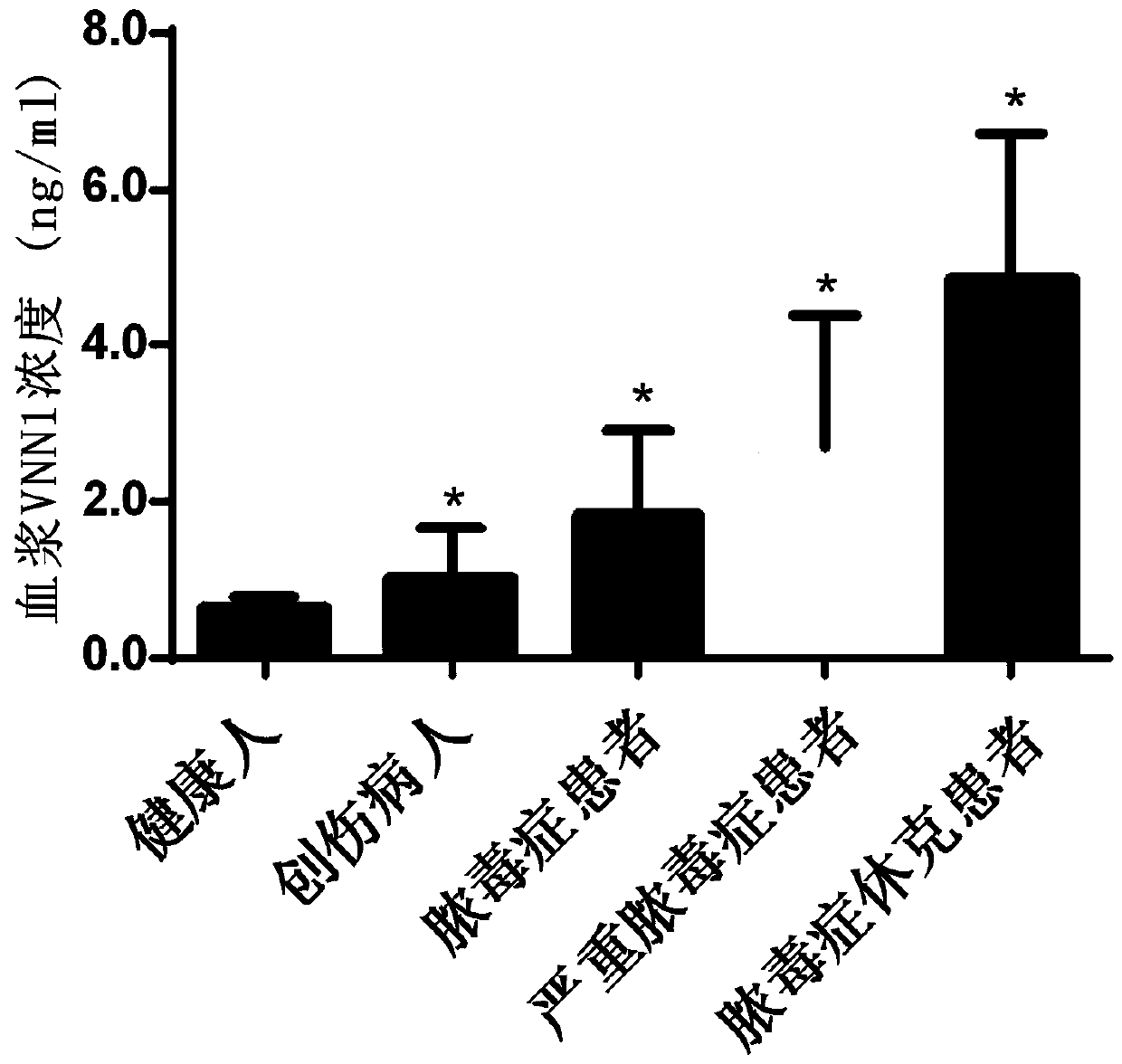
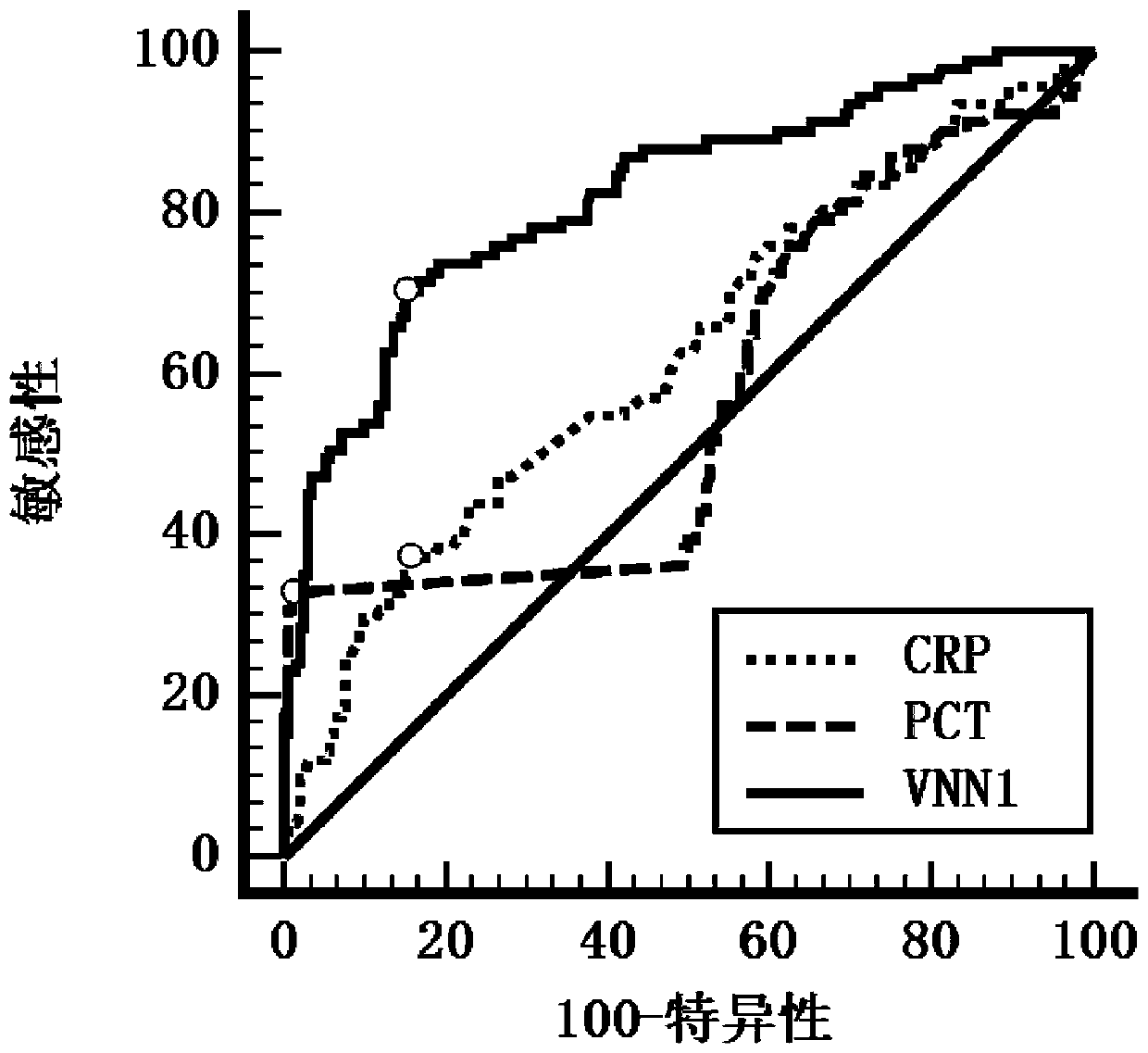
Application of VNN1 in plasma or serum for markers in early warning of sepsis
ActiveCN110095603AHigh clinical application valueReduce incidenceDisease diagnosisInflammatory factorsNormal people
The invention relates to an application of a vascular non-inflammatory factor (VNN1) in plasma or serum for markers in early warning of sepsis. According to the application, the VNN1 is adopted as a biomarker, an applicant has verified through experiments that the level of VNN1 in plasma of patients with severe trauma is significantly higher than that of normal people, and the level of VNN1 in plasma of patients with sepsis occurred after trauma is significantly higher than that in patients without sepsis. By detecting the level of plasma or serum VNN1, early prediction of the sepsis occurrence risk of severely traumatized patients is performed, so as to give individualized intervention, thereby being capable of reducing the occurrence rate of post-traumatic sepsis, and having an importantclinical application value in improving the prognosis of patients with severe trauma.
Owner:中国人民解放军陆军特色医学中心
Tissue sealant for use in non-compressible hemorrhage
InactiveUS8680240B1Least riskLittle useSurgical adhesivesPeptide/protein ingredientsTissue sealantPeritoneoscopes
ClotFoam is a sealant and hemostatic agent for use in cases of non-compressible hemorrhage for moderate to severe bleeding. It can be applied in the operating room through laparoscopic ports, or directly over lacerated tissue in laparotomy procedures or outside the operating room through a mixing needle and / or a spray injection method following intracavitary severe trauma or surgery. Its crosslinking technology generates an adhesive scaffold that carries a fibrin sealant required for hemostasis. Clotfoam produces a foam that spreads throughout a body cavity reaching the lacerated tissue to seal tissue and promote the coagulation cascade. The composition is biocompatible and non-inflammatory.The invention uses fibrin monomer polymerized by a change of pH as active sealing component. The viscoelastic properties of the foam as well as the rapid formation of a fibrin clot ensure that the sealant remains at the site of application without being washed away by blood.
Owner:FALUS GEORGE DAVID
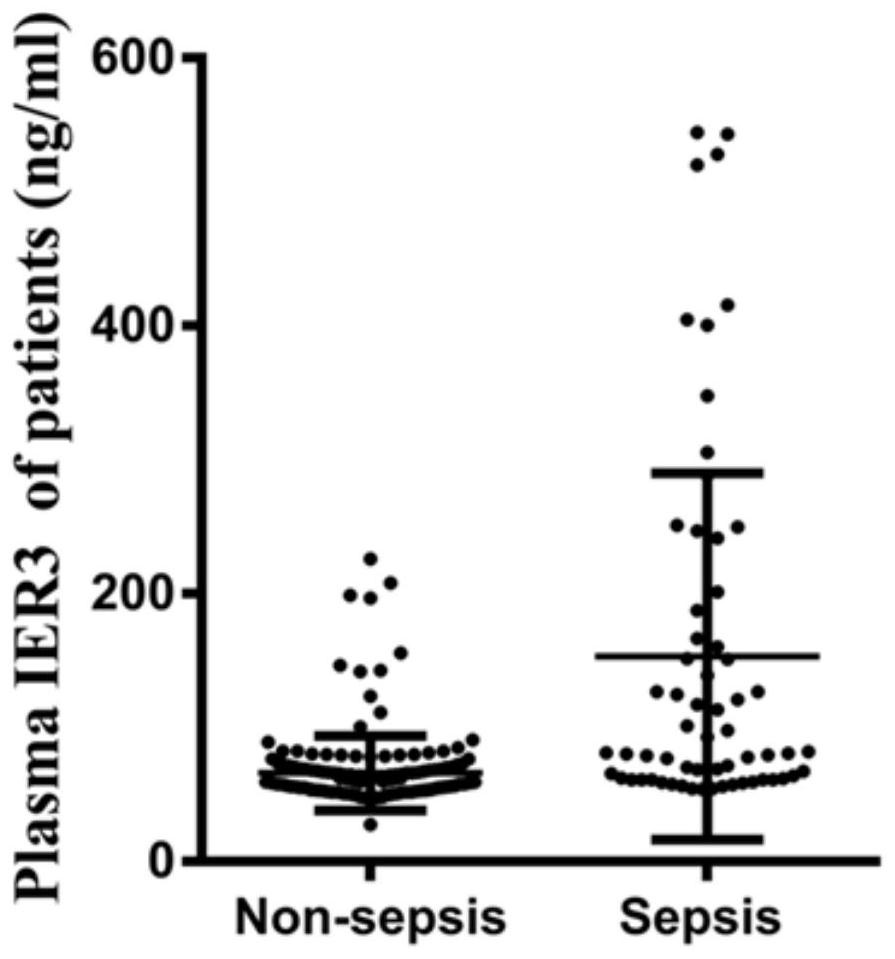
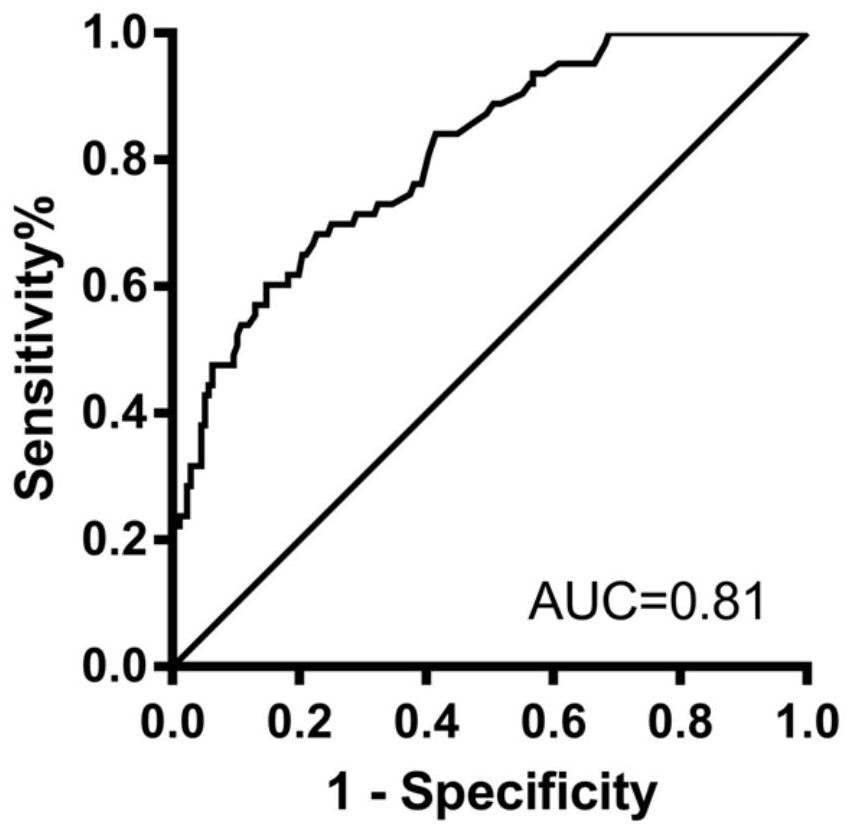
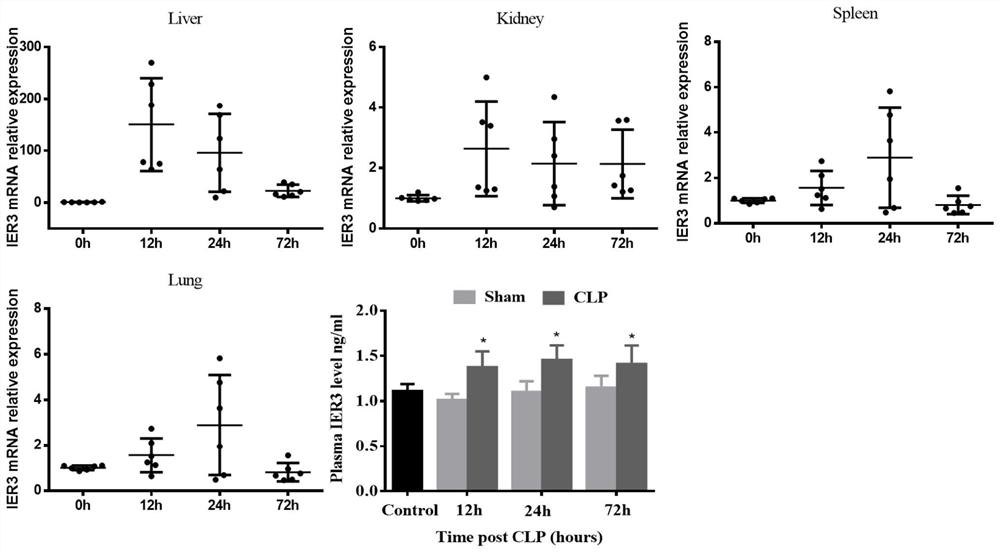
Application of IER3 as biomarker in early prediction of sepsis
PendingCN112011603AGood early predictive valueReduce incidenceMicrobiological testing/measurementDisease diagnosisEarly predictionIntervention treatment
The invention relates to the technical field of biology, in particular to application of an early response gene 3 (IER3) as a biomarker in a reagent or a kit for early prediction of sepsis. The IER3 is adopted as a biomarker, and crowd experiments prove that the IER3 level of plasma of a patient suffering from severe trauma sepsis is remarkably higher than that of a patient without sepsis. By detecting the plasma IER3 level, early prediction is conducted on the sepsis occurrence risk of a severely wounded patient so as to give individualized interventional therapy, the occurrence rate of post-traumatic sepsis can be reduced, and the important clinical application value can be achieved for improving prognosis of the severely wounded patient.
Owner:中国人民解放军陆军特色医学中心
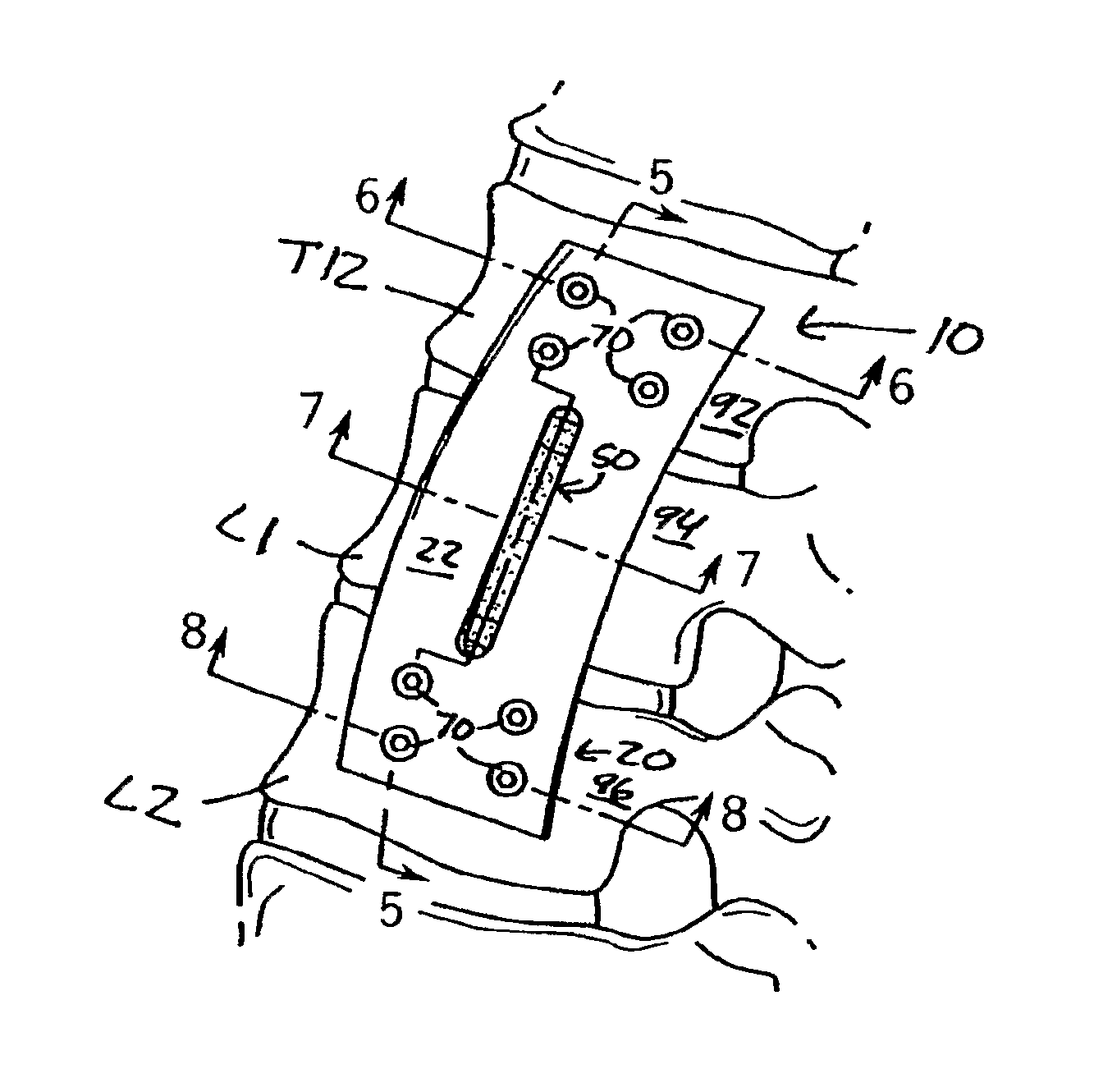
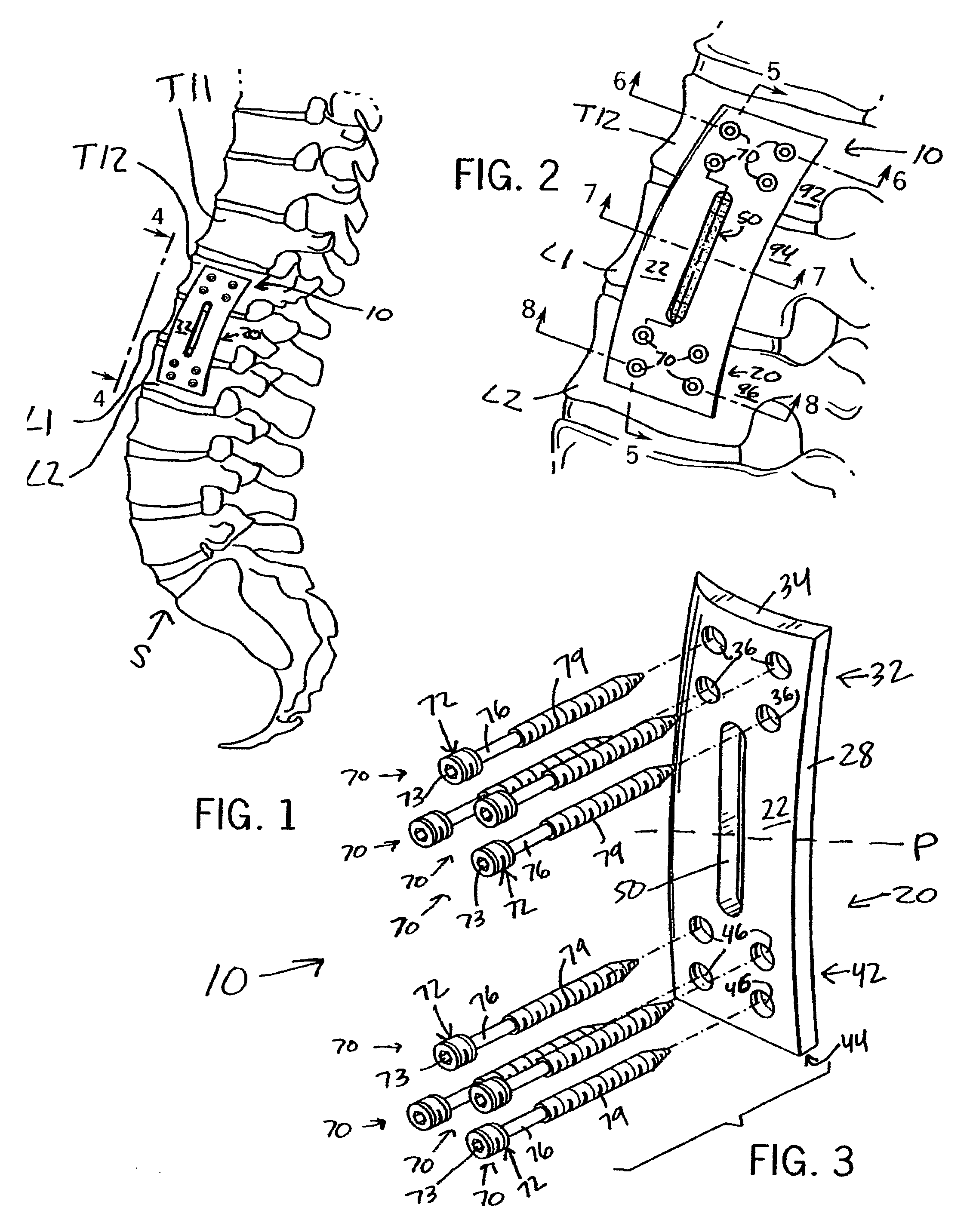
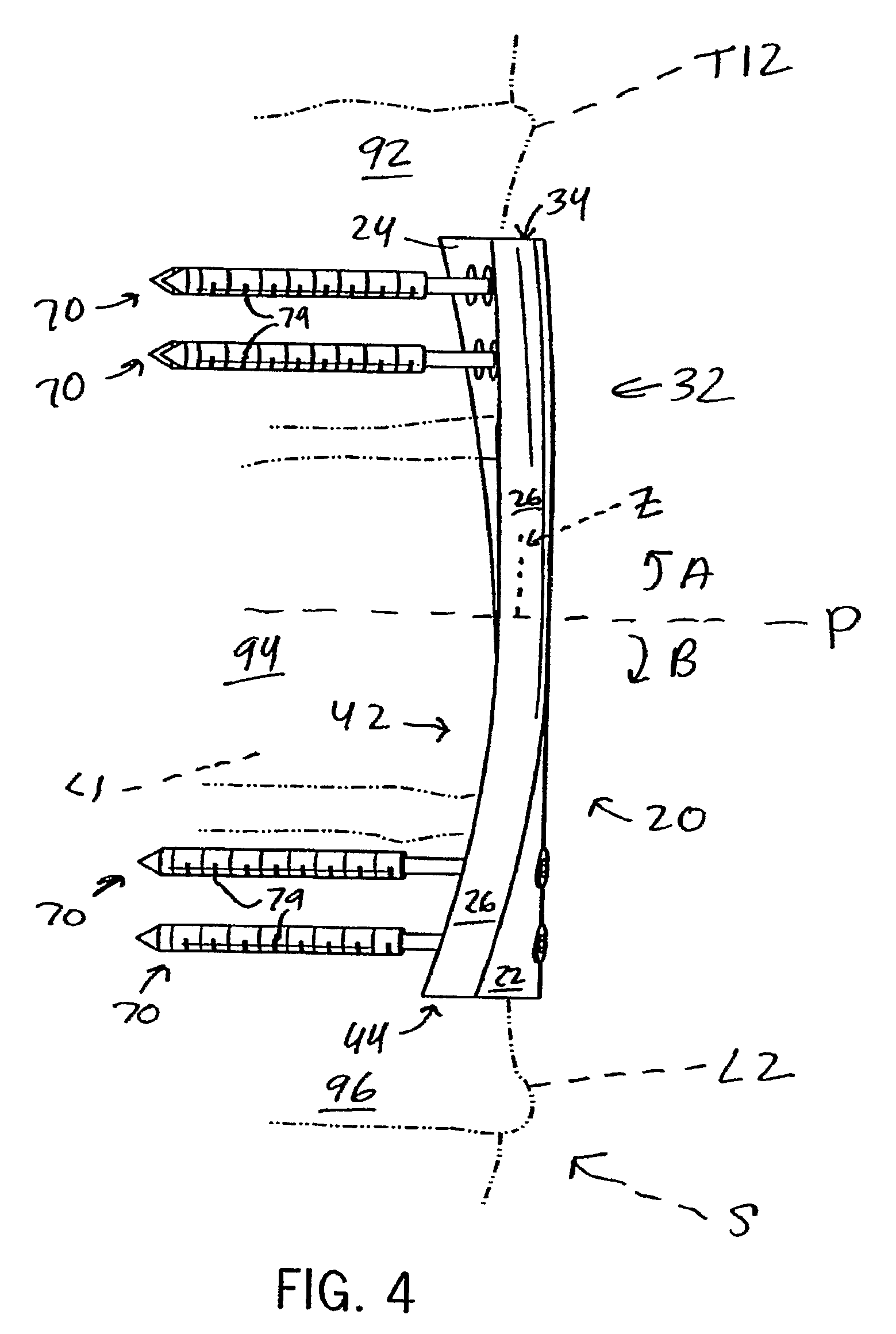
Anterior adherent thoracolumbar spine plate
ActiveUS8551145B2Accurate anatomic designFacilitates close possible applicationInternal osteosythesisBone platesThoracolumbar spineNon union
The implant is a fixation device shaped in the form of a plate for use in the fixation and stabilization of the thoracolumbar spine. The design and the configuration of the device allows for an anatomic fit of the plate in the spine. The improved fixation allows for an anatomical and biomechanicat advantage in stabilizing thoracolumbar spine trauma both in a primary fashion and / or in conjunction with vertebral body replacement. Its anatomic design allows the application of an ingrowth surface at the host / implant interface and encourages an additional point of fixation in cases at high risk for delayed or non-union such as severe trauma or malignancy. The thoracolumbar spine plate is anatomically designed to fit snugly against the thoracolumbar vertebral bodies through a twist around the Z (longitudinal) axis of the generally rectangular plate.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
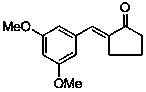
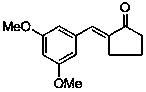
Application of (E)-2-(3,5-dimethoxybenzylidene)-cyclopentanone in preparing medicines used for treating pain diseases
ActiveCN103385866AObvious analgesic effectReduce pain responseOrganic active ingredientsNervous disorderThermal stimulationNeuralgia
The invention discloses an application of (E)-2-(3,5-dimethoxybenzylidene)-cyclopentanone in preparing medicines used for treating pain diseases. Related studies show that the compound has a significant abirritation, and can effectively reduce pain reactions caused by thermal stimulation and chemical stimulation. The compound can be used for preparing medicines used for relieving acute pains, headache, toothache, neuralgia, tumor-type pain, and pains caused by inflammatory diseases of muscles and bone joints, wherein the acute pains are caused by severe injury, burns, etc., and the pains caused by the inflammatory diseases of the muscles and the bone joints comprise rheumatic and rheumatoid arthritis pains, dysmenorrheal and other inflammatory pains, and neuropathic pains.
Owner:SUZHOU UNIV
Rapid quantitative measurement of soluble fibrin in opaque body fluids
InactiveUS20030059836A1Easy Optical InspectionAccurate detectionMicrobiological testing/measurementScattering properties measurementsBody fluidSevere trauma
A method for determining the existence and the amount of soluble fibrin contained in a specimen fluid is provided. The method includes the steps of precipitating soluble fibrin out of the opaque specimen fluid, aggregating the soluble fibrin precipitates in a limited region of a transparent container so as to render the precipitates optically detectable in the opaque specimen fluid, and optically detecting the precipitates. The amount of soluble fibrin may be determined by measuring the time from the addition of the precipitating regent to the detection of the soluble fibrin precipitates. Methods of the present invention allow one to measure soluble fibrin in whole blood, and therefore render the test useful in the operating room under conditions of major surgery and in the presence of severe trauma wherein DIC is likely to supervene.
Owner:MEDICAL DEVICES CORP
3-(6-alkoxy-5-chlorobenzo[d]isoxazol-3-yl)propanoic acid useful as kynurenine monooxygenase inhibitors
Owner:THE UNIV COURT OF THE UNIV OF EDINBURGH
Portable light-weight ventilator system
A ventilator system for providing respiratory support in cases of acute respiratory failure or severe trauma is described. The ventilator system has a ventilator and a tubing system. The system is characterized in that the ventilator has a continuous bleed valve configured to be open to air flow from the blower at all times when the blower is operating during both inspiration and expiration; thereby providing a minimal amount of pressure within a patient's lungs at the end of each exhalation—positive end expiratory pressure (PEEP). In an embodiment of the invention the system comprises a manifold block configured to hold the main operating elements of the ventilator.
Owner:INOVYTEC MEDICAL SOLUTIONS
Uygur medicine calculus removing powder and preparation method thereof
InactiveCN106619809AEasy to acceptEffective treatmentPowder deliveryOrganic active ingredientsKidneyChinese patent medicine
The invention relates to Uygur medicine composition, in particular to Uygur medicine calculus removing powder capable of treating urinary calculus and a preparation method thereof. Urinary calculus comprising kidney, ureter, urinary bladder and gallstone is a common disease. At present, the diseases are generally treated with traditional Chinese medicines, Chinese patent medicines, western medicines, calculus breakage and surgery treatment, and the curative effects of these medicines are not ideal; calculus breaking has poor or even ineffective treatment effects during treatment of calculus larger than 1 cm; the surgery treatment causes severe trauma to human bodies Raw material components of the powder comprise scorpion ash, tribulus terrestris, cucumber seeds, plegiocidaris pome, salt, semen cuscutae, mint, celery seeds, fennel, cichorium intybus and white granulated sugar. Uygur medical knowledge is adopted to distinguish diseases and symptoms, calculus dissolution and removal are integrated, and the curative effect is obviously superior to that of other proved recipes; the powder is economical, cheap, efficient, simple, practical, safe, non-toxic and acceptable among patients and is a relatively effective Uygur medicine for treating urinary calculus diseases at present.
Owner:乌鲁木齐特比美拉斯国际贸易有限公司
Application of (E)-2-(3,5-dimethoxybenzylidene)-cyclopentanone in preparing medicines used for treating pain diseases
ActiveCN103385866BObvious analgesic effectReduce pain responseOrganic active ingredientsNervous disorderThermal stimulationNeuralgia
The invention discloses an application of (E)-2-(3,5-dimethoxybenzylidene)-cyclopentanone in preparing medicines used for treating pain diseases. Related studies show that the compound has a significant abirritation, and can effectively reduce pain reactions caused by thermal stimulation and chemical stimulation. The compound can be used for preparing medicines used for relieving acute pains, headache, toothache, neuralgia, tumor-type pain, and pains caused by inflammatory diseases of muscles and bone joints, wherein the acute pains are caused by severe injury, burns, etc., and the pains caused by the inflammatory diseases of the muscles and the bone joints comprise rheumatic and rheumatoid arthritis pains, dysmenorrheal and other inflammatory pains, and neuropathic pains.
Owner:SUZHOU UNIV
Biomarkers for mortality prognosis in critically ill patients
ActiveCN109451744BAntibacterial agentsMicrobiological testing/measurementCritically illBiologic marker
Biomarkers and methods of using the same to aid in the diagnosis, prognosis and treatment of critically ill patients are disclosed. In particular, the present invention relates to the use of biomarkers for prognosis of death in critically ill patients suffering from sepsis, severe trauma or burns.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
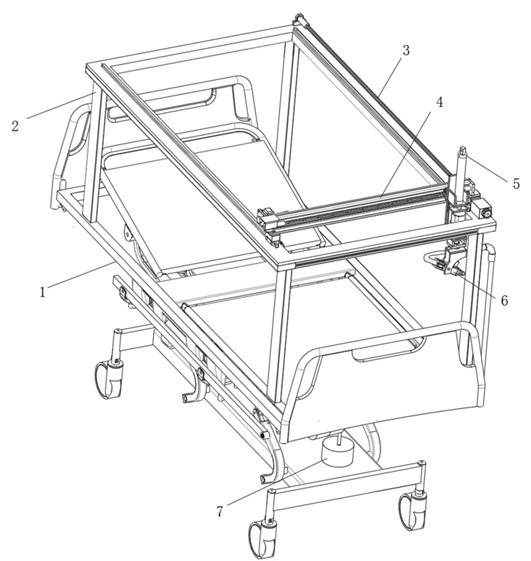
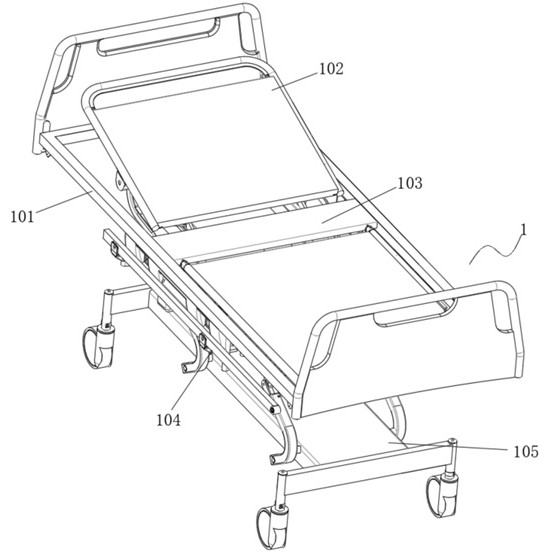
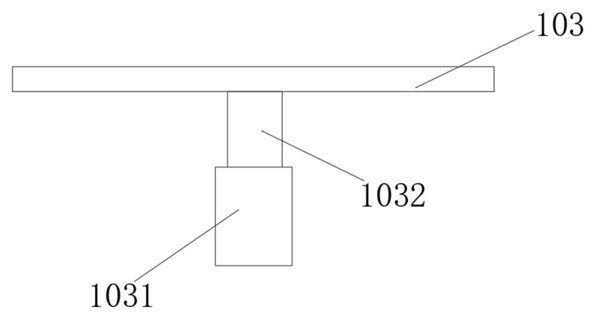
Seepage drainage system for patient with severe trauma
InactiveCN114366881AEasy to infiltrate and drainGood for osmotic drainageNursing bedsSuction devicesPhysical medicine and rehabilitationNursing care
The seepage drainage system comprises a nursing bed, a supporting bottom frame, a moving assembly, an adjusting assembly, a lifting assembly, a drainage assembly and a collecting assembly, the bottom of the moving assembly is welded to the top of the supporting bottom frame, and the front-back position of the drainage assembly is conveniently adjusted through the moving assembly; secondly, through cooperation with the adjusting assembly at the top of the moving assembly, the left-right position of the drainage assembly is adjusted through the adjusting assembly, and the drainage assembly can be conveniently used for conducting permeation drainage on the front and back different positions of the body of a patient on the nursing bed, so that the use flexibility of the permeation drainage system is improved; the permeable drainage system is simple in structure and convenient to use, the drainage assembly can conduct memory permeable drainage on the left and right trauma parts of the body of the patient, the movement area of the drainage assembly is wider, it is guaranteed that the permeable drainage system can conduct permeable drainage on different positions of the trauma parts of the patient, and the permeable drainage system can conduct permeable drainage on the trauma parts of the patient more comprehensively.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
A kind of preparation method of modified starch hemostatic microsphere
ActiveCN108434510BImprove stabilityQuick focusSurgical adhesivesPharmaceutical delivery mechanismMajor bleedingButanedioic acid
The invention discloses a method for preparing modified starch hemostatic microspheres. The method is characterized in that with starch as a medium material, succinic anhydride modification and chemical crosslinking are conducted on the starch, and the starch is prepared into the microspheres, so that the surface contact area is greatly increased, the method facilitates increasing the liquid absorption rate and speed of the starch, and the stability of the hemostatic microspheres prepared by the method is high; moreover, by utilizing the characteristic that the hemostatic microspheres quicklyabsorb blood and rapidly swell, the blood vessels are quickly and centralizedly blocked, and the effect of quick hemostasis is achieved; since the hemostasis effect of the modified starch hemostatic microspheres is physical, no chemical or pharmaceutical reagent is used in the function process, the modified starch hemostatic microspheres are safe and reliable and have no side effects on the livingbodies, and the modified starch hemostatic microspheres are not only suitable for hemostasis during in-vivo or body-surface bleeding but also suitable for effective hemostasis for severe traumas caused by sudden disasters or life-threatening major bleeding caused by clinically large surgery.
Owner:NINGBO BAOTING BIOTECHNOLOGY CO LTD
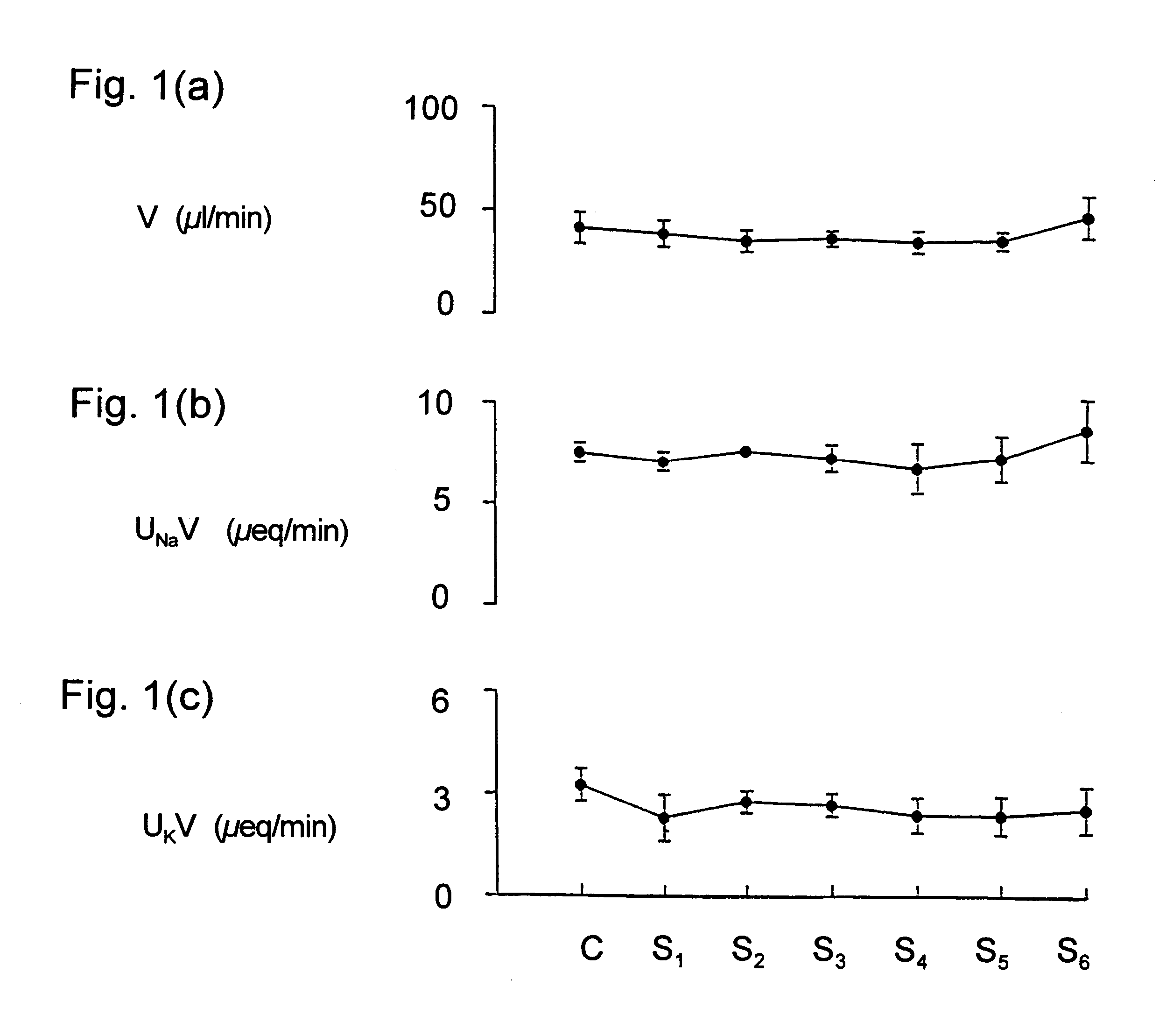
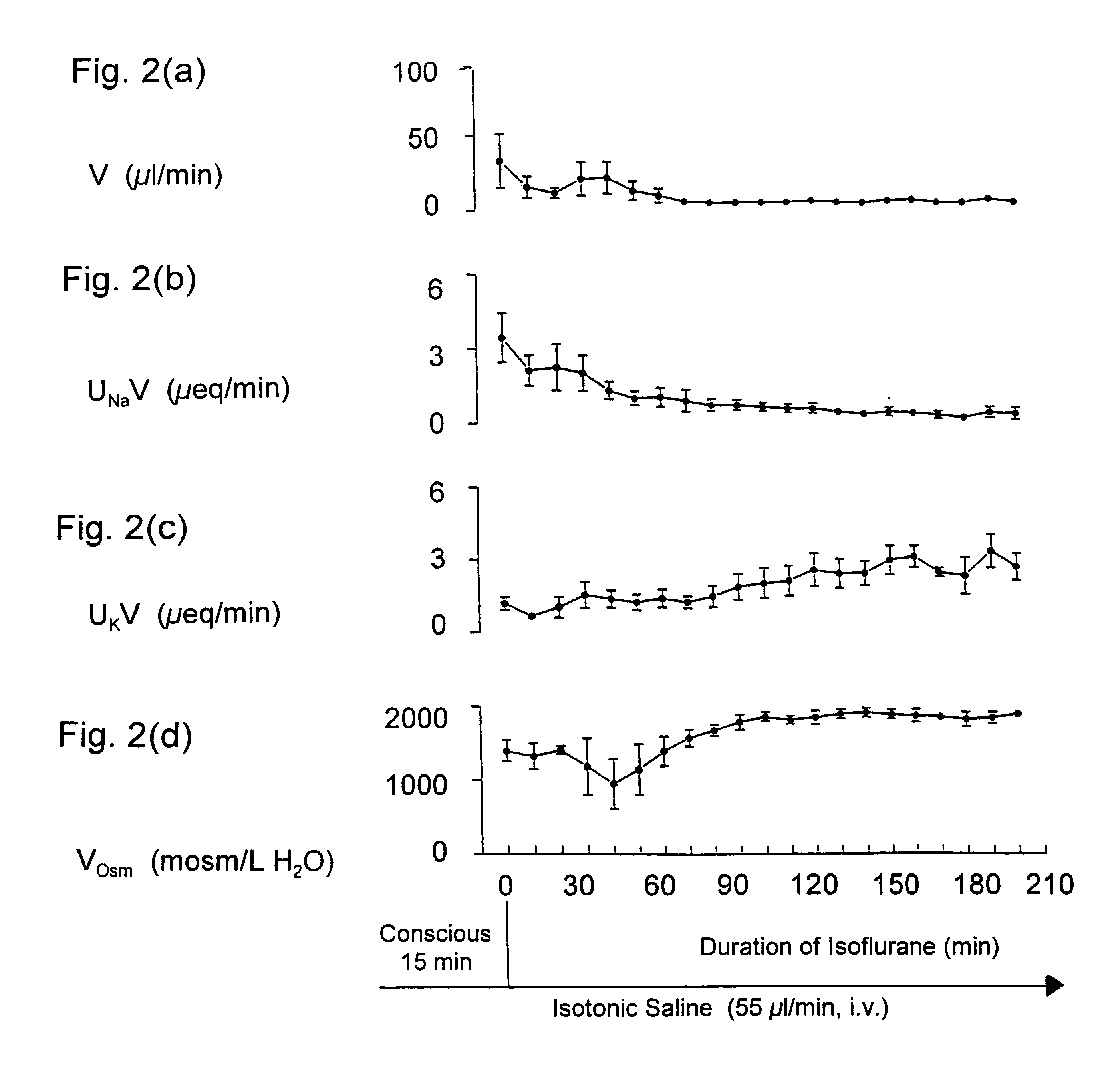
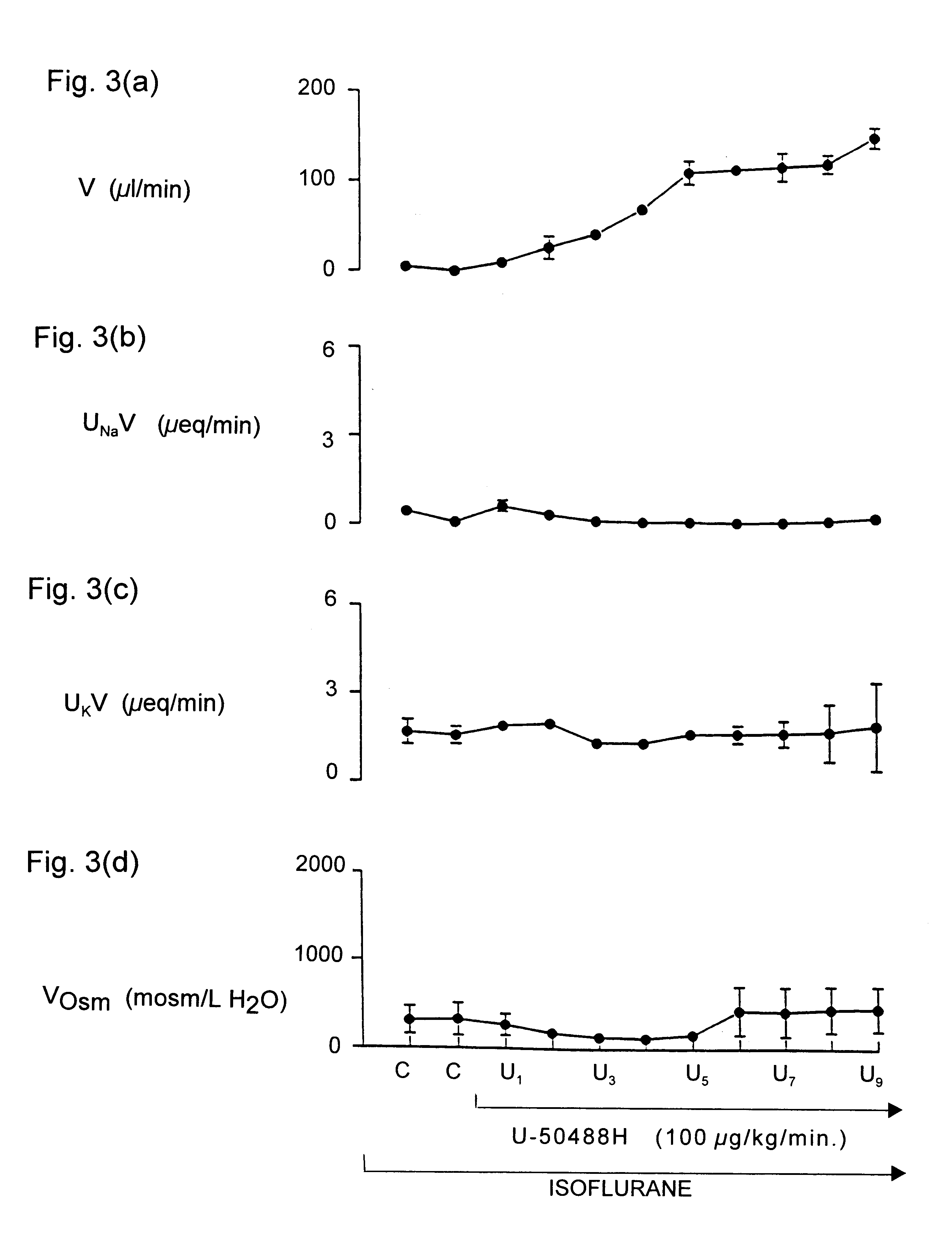
Maintaining kidney function during surgery or trauma
InactiveUS6468971B1Prevent impairment of renal functionProtect kidney functionBiocideNervous disorderAnaesthesia inductionPotassium
Kappa-opioid agonists prevent the impairment of renal function otherwise caused by the combination of gaseous anesthesia and surgery or severe trauma. Not only do these agents preserve renal function and maintain urine output, they also maintain plasma electrolyte concentration and osmolality by reducing renal loss of sodium and potassium when compared to other diuretic agents. The preservation of urine flow as well as the ability to retain body sodium, potassium, calcium, and osmolality during surgery or severe trauma under gaseous anesthesia are novel and unique properties associated only with kappa opioid agonists. To date, no other clinically-used diuretic agent has been shown to provide constant urine flow, or to retain electrolytes during anesthesia and surgery. The kappa opioid agonists may be used in surgical patients with normal cardiovascular function, but are particularly useful in patients with compromised cardiovascular and / or renal function. Continuous intravenous infusion of the kappa agonist preferably begins about 30 to 90 minutes before induction of anesthesia, and continues throughout anesthesia and surgery. The result is a constant and adequate output of urine while maintaining homeostasis of blood volume, electrolyte concentration, and osmolality throughout surgery and anesthesia. The dose of the kappa opioid agonist needed to induce diuresis during anesthesia is significantly higher than the dose needed to induce diuresis in a conscious patient.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Arginine acetate oral preparation and clinical application thereof
InactiveCN1209102CSafe and effective doseOrganic active ingredientsDigestive systemArgininePhosphate
The present invention belongs to non-specific immunoenhancing drugs, which is an oral preparation of arginine acetate. Road will not produce irritation, long-term use will not produce hyperchloremia. As an immune-enhancing drug, it is mainly used clinically for patients with severe trauma, burns, major surgery, and cancer patients whose immune function is suppressed after radiotherapy and chemotherapy. It is safe, convenient, and effective.
Owner:YIFAN XINFU PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors 3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors](https://images-eureka.patsnap.com/patent_img/41458c72-594e-4502-b3f4-2bd465aa0ad6/US20160318884A1-20161103-C00001.PNG)
![3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors 3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors](https://images-eureka.patsnap.com/patent_img/41458c72-594e-4502-b3f4-2bd465aa0ad6/US20160318884A1-20161103-C00002.PNG)
![3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors 3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors](https://images-eureka.patsnap.com/patent_img/41458c72-594e-4502-b3f4-2bd465aa0ad6/US20160318884A1-20161103-C00003.PNG)
![3-(6-alkoxy-5-chlorobenzo[d]isoxazol-3-yl)propanoic acid useful as kynurenine monooxygenase inhibitors 3-(6-alkoxy-5-chlorobenzo[d]isoxazol-3-yl)propanoic acid useful as kynurenine monooxygenase inhibitors](https://images-eureka.patsnap.com/patent_img/8d5bce21-3861-494f-8ef5-2dc00b6ce7c8/US09932328-20180403-C00001.png)
![3-(6-alkoxy-5-chlorobenzo[d]isoxazol-3-yl)propanoic acid useful as kynurenine monooxygenase inhibitors 3-(6-alkoxy-5-chlorobenzo[d]isoxazol-3-yl)propanoic acid useful as kynurenine monooxygenase inhibitors](https://images-eureka.patsnap.com/patent_img/8d5bce21-3861-494f-8ef5-2dc00b6ce7c8/US09932328-20180403-C00002.png)
![3-(6-alkoxy-5-chlorobenzo[d]isoxazol-3-yl)propanoic acid useful as kynurenine monooxygenase inhibitors 3-(6-alkoxy-5-chlorobenzo[d]isoxazol-3-yl)propanoic acid useful as kynurenine monooxygenase inhibitors](https://images-eureka.patsnap.com/patent_img/8d5bce21-3861-494f-8ef5-2dc00b6ce7c8/US09932328-20180403-C00003.png)