Application of (E)-2-(3,5-dimethoxybenzylidene)-cyclopentanone in preparing medicines used for treating pain diseases
A disease and drug technology, applied in drug combinations, nervous system diseases, antipyretics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
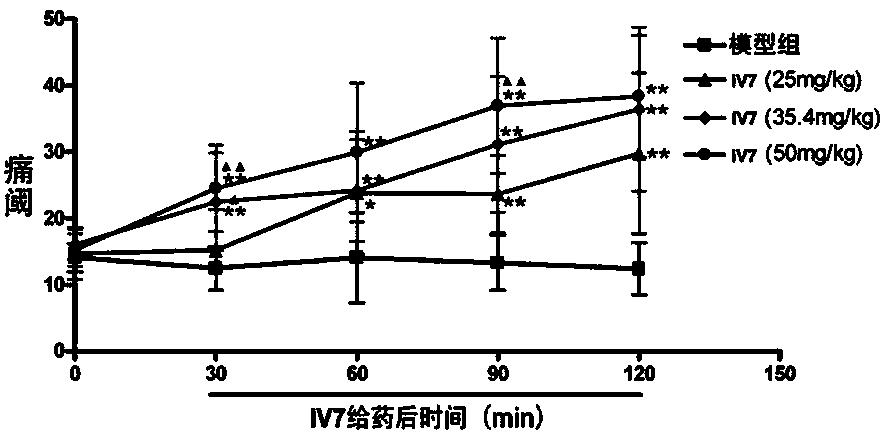
[0023] Compound Effect on the hot plate response of mice, the compound is referred to as IV in the following 7 .
[0024] 40 male mice were randomly divided into vehicle control group (model group, model) and IV 7 High, medium and low dose groups (50mg / kg, 35.4mg / kg and 25mg / kg), each group contains 10 animals. Each group of mice were injected with vehicle or IV into the abdominal cavity 7 After 30, 60, 90 and 120 minutes hot plate reaction. The shorter the hot plate reaction time, the more severe the pain in mice.
[0025] Attached figure 1 For IV 7 The effect on the hot plate reaction of mice. mean±SD, n=10; compared with the model group, **p 7 25mg / kg comparison, ▲ P﹤0.05, ▲▲ P<0.01.
[0026] figure 1 The results show that: Compared with the model group (vehicle control group), IV 7 50mg / kg, 35.4mg / kg and 25mg / kg significantly prolong the hot plate reaction time of mice, and IV 7 The analgesic effect of 50mg / kg and 35.4mg / kg is significantly stronger than that of IV 7 25mg...
Embodiment 2
[0028] Compound IV 7 Effect on writhing response in mice
[0029] 40 mice, half male and half male, randomly divided into vehicle control group (model group, model) and IV 7 High, medium and low dose groups (50mg / kg, 35.4mg / kg and 25mg / kg), each group contains 10 animals. Mice in each group were injected with vehicle or IV into the abdominal cavity 7 After 50 minutes, 1% acetic acid solution was intraperitoneally injected, and the number of writhing times of mice in each group was recorded for 10-20 minutes. Inhibition rate of writhing response = (number of writhing in the vehicle control group-IV 7 Number of writhing) / Number of writhing in the solvent control group×100%. The more writhing times, the more severe the pain in mice.
[0030] Attached figure 2 For IV 7 The effect on the writhing response of mice. Mean ± SD, n=10; compared with the model group, **p <0.01.
[0031] Attached figure 2 Show: Compared with the model group (vehicle control group), IV 7 50mg / kg, 35.4mg / kg ...
Embodiment 3
[0034] Cathepsin L specific inhibitor Clik 148 versus compound IV 7 Analgesic effects
[0035] 60 mice, half male and half male, were randomly divided into 6 groups, namely the vehicle control group (model group, model), IV 7 50mg / kg group, Clik 148 10mg / kg group, Clik 148 20mg / kg group, IV 7 50mg / kg + Clik 148 10mg / kg group and IV 7 50mg / kg + Clik 148 20mg / kg group, 10 animals in each group. Each group of mice was injected with vehicle or Clik 148 into the abdominal cavity and injected with vehicle or IV into the abdominal cavity. 7 After 50 minutes, 1% acetic acid solution was intraperitoneally injected, and the number of writhing times of mice in each group was recorded for 10-20 minutes. Inhibition rate of writhing response = (number of writhing in the vehicle control group-IV 7 The number of writhing) / the number of writhing in the solvent control group × 100%. The more writhing times, the more severe the pain in mice.
[0036] Attached image 3 For Clik 148 to compound IV 7 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com