Patents
Literature
38 results about "Pain responses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
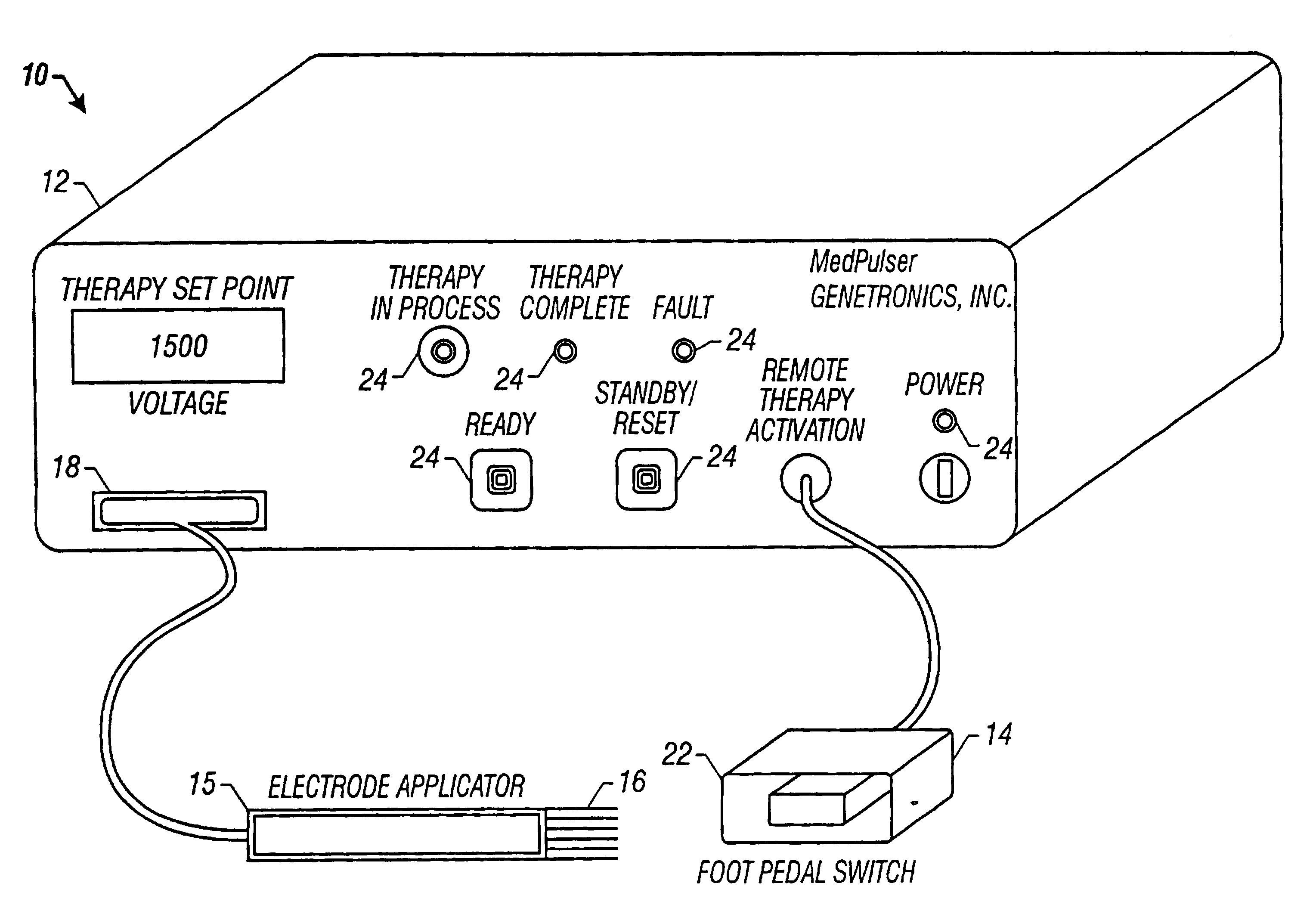
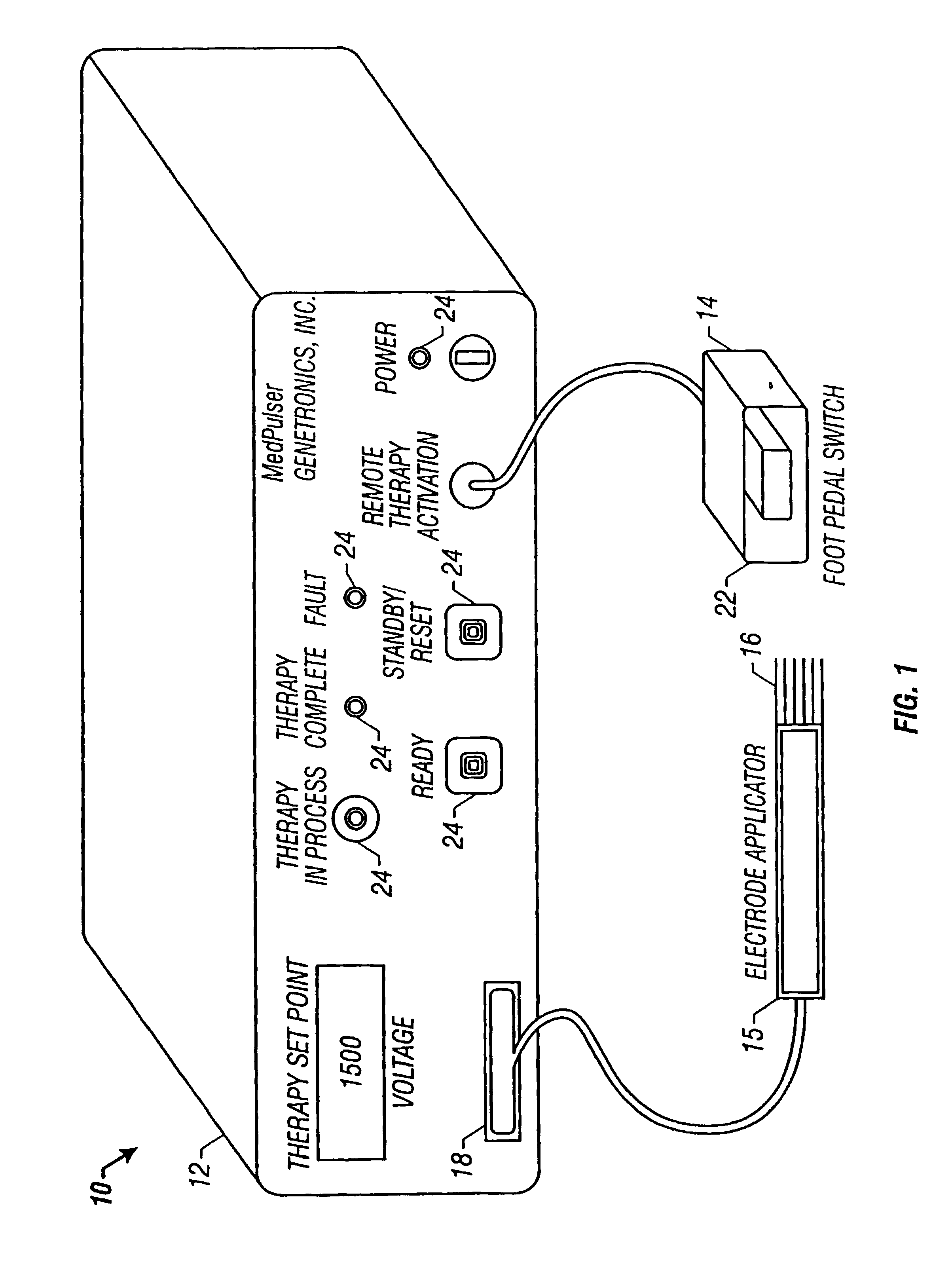
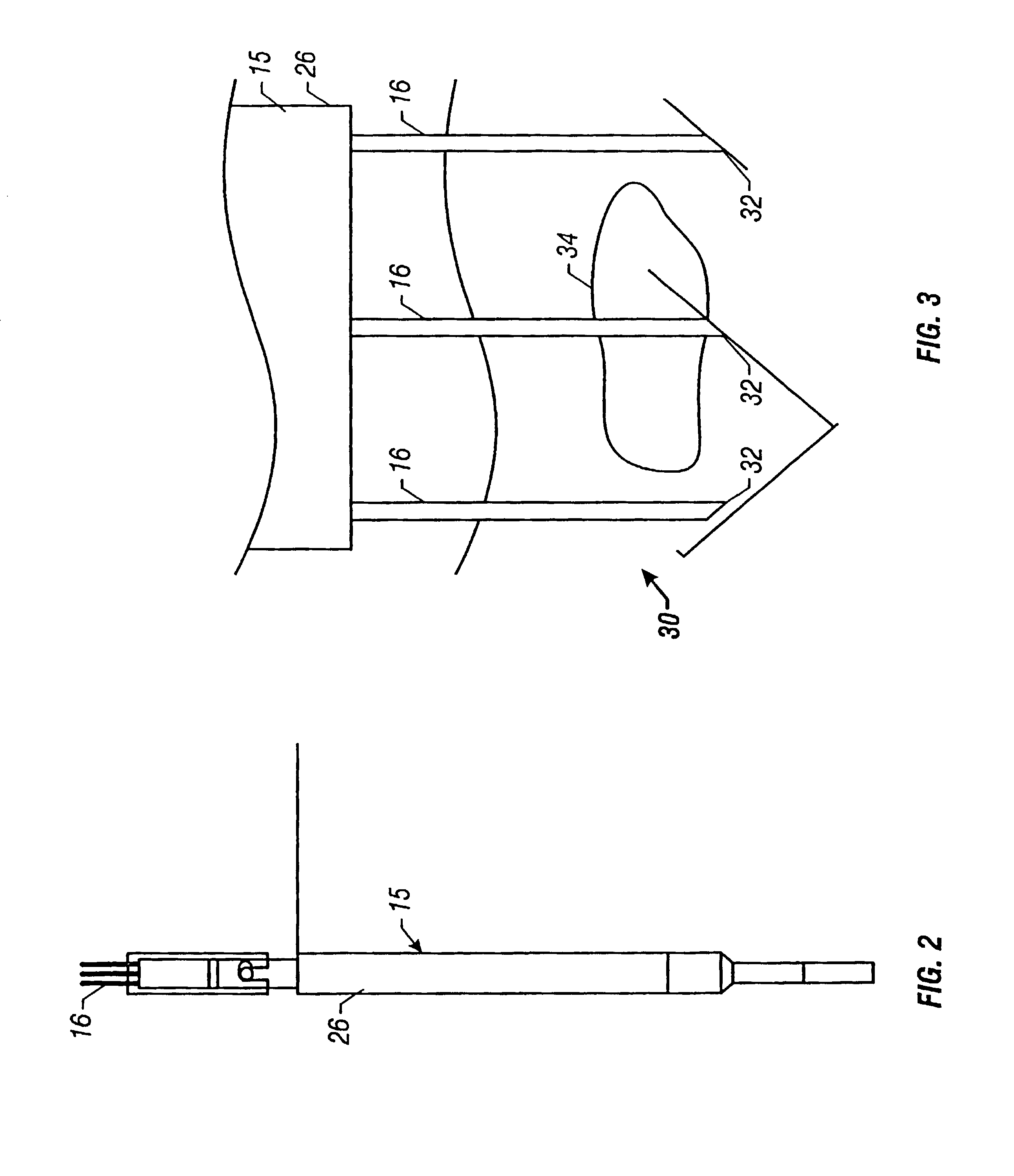
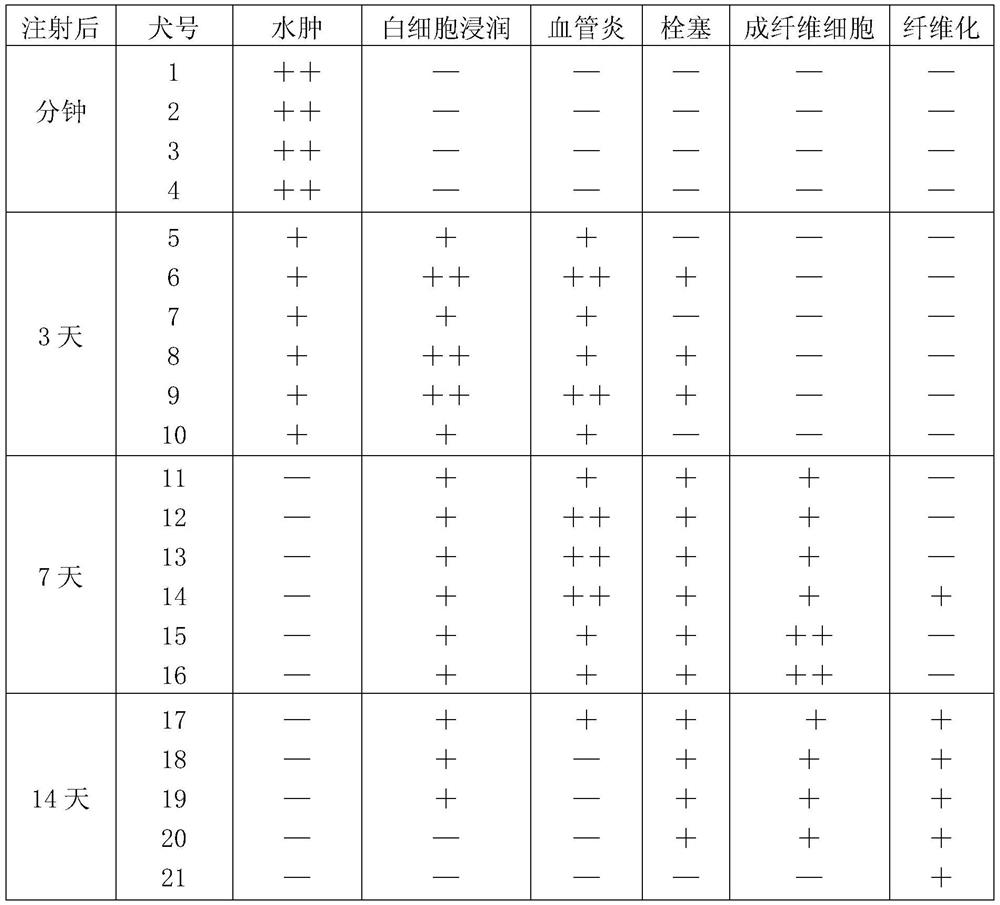
Method and apparatus for reducing electroporation-mediated muscle reaction and pain response
InactiveUS7054685B2Relieve painLower Level RequirementsHeart stimulatorsElectricityElectrical battery
A method for delivery of an agent to a cell using electroporation is disclosed. The method includes positioning a first electrode and a second electrode such that an electrical signal passed between the first electrode and the second electrode passes through the cell. The method also includes passing an electrical signal between the first electrode and the second electrode, the electrical signal having a frequency greater than about 10 kHz. In one embodiment of the method, the electrical signal has a bipolar waveform. In another embodiment of the method, the electrodes are positioned at a treatment site, e.g., a tumor, for in vivo delivery of an agent.
Owner:INOVIO PHARMA
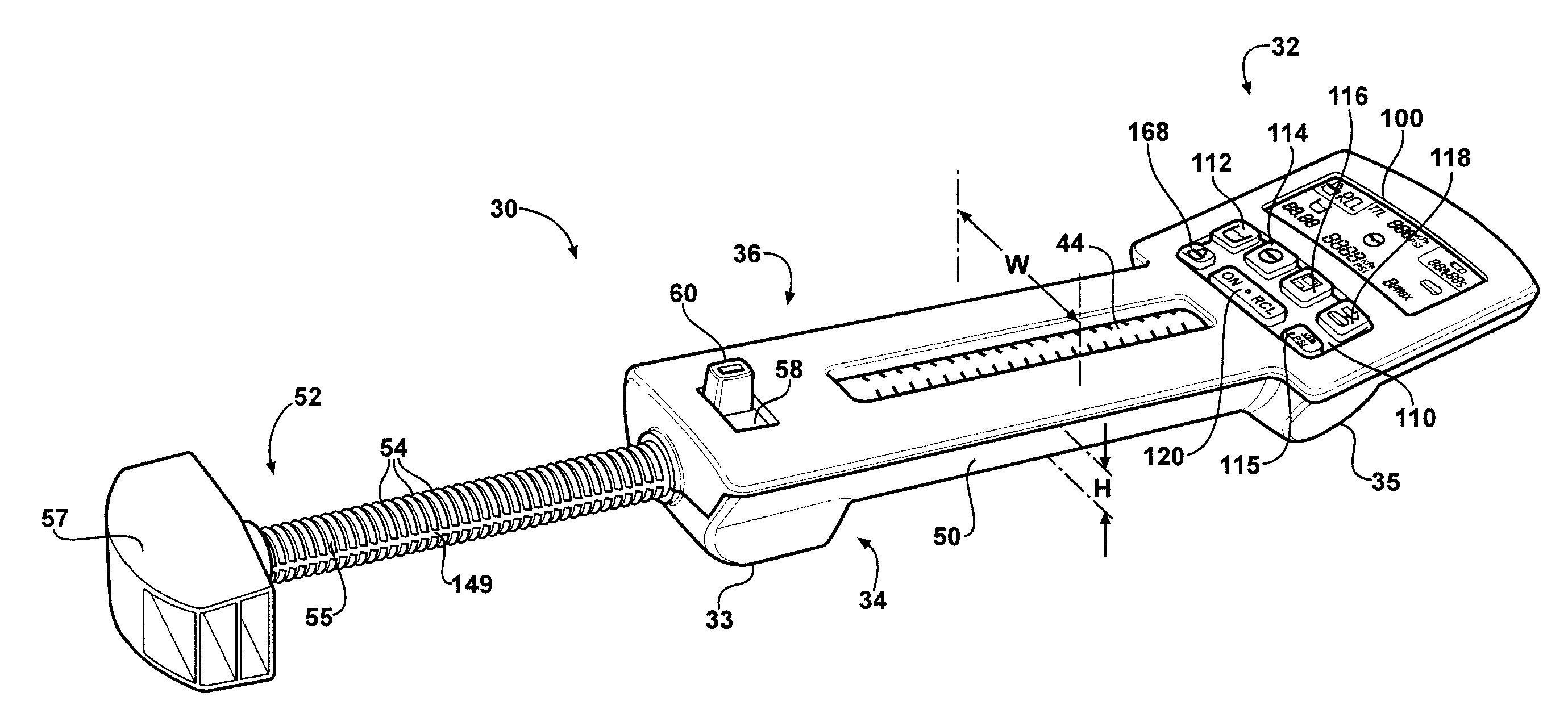
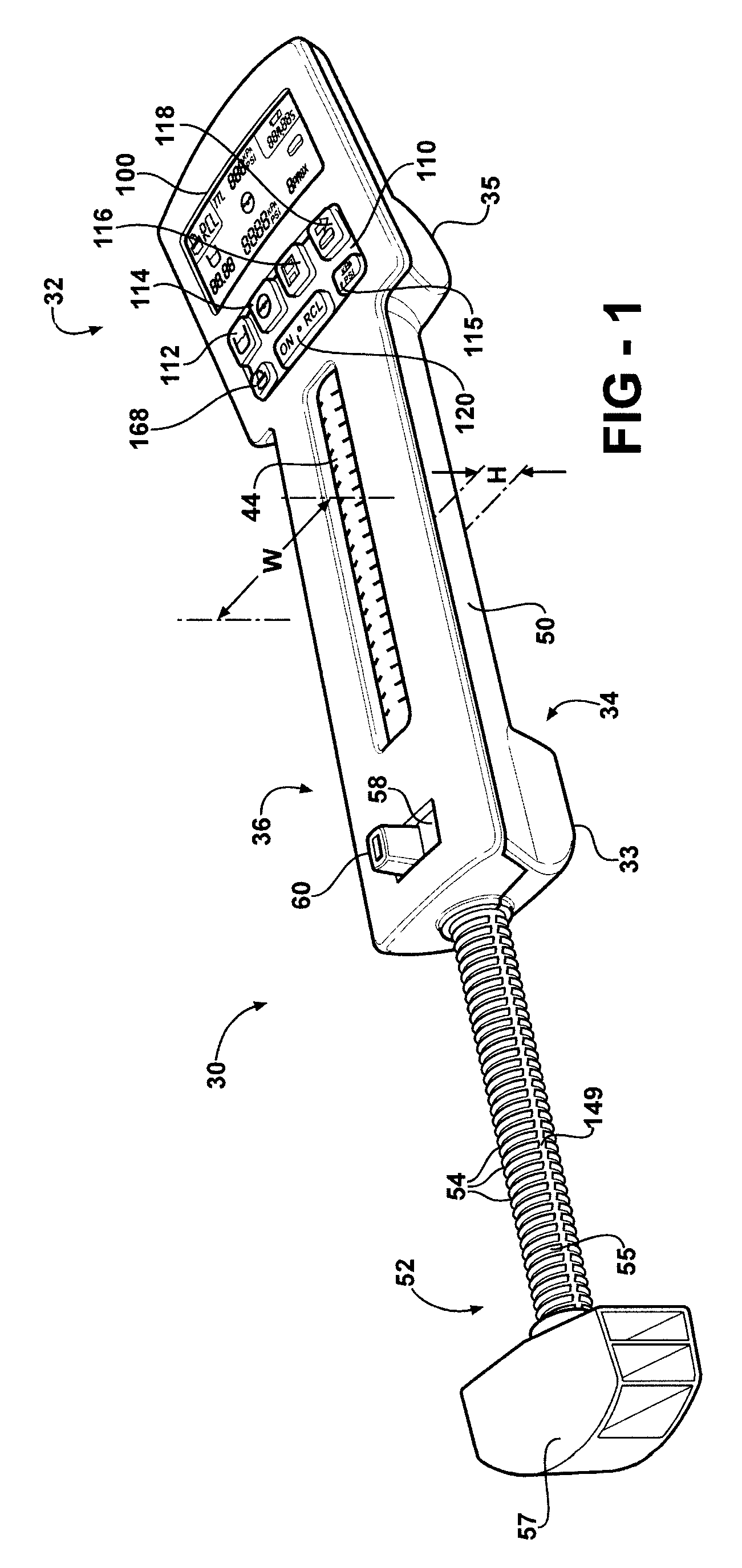
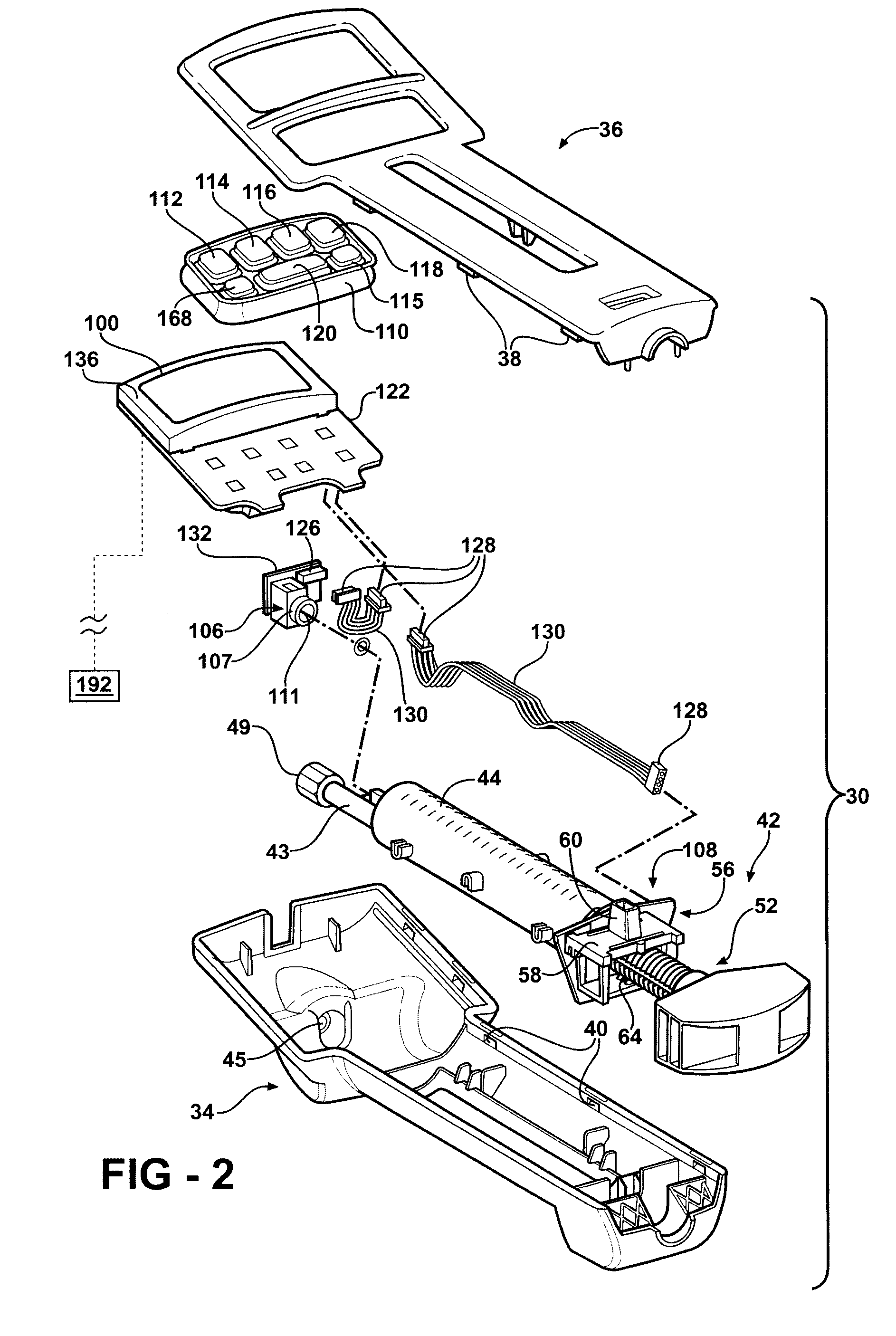
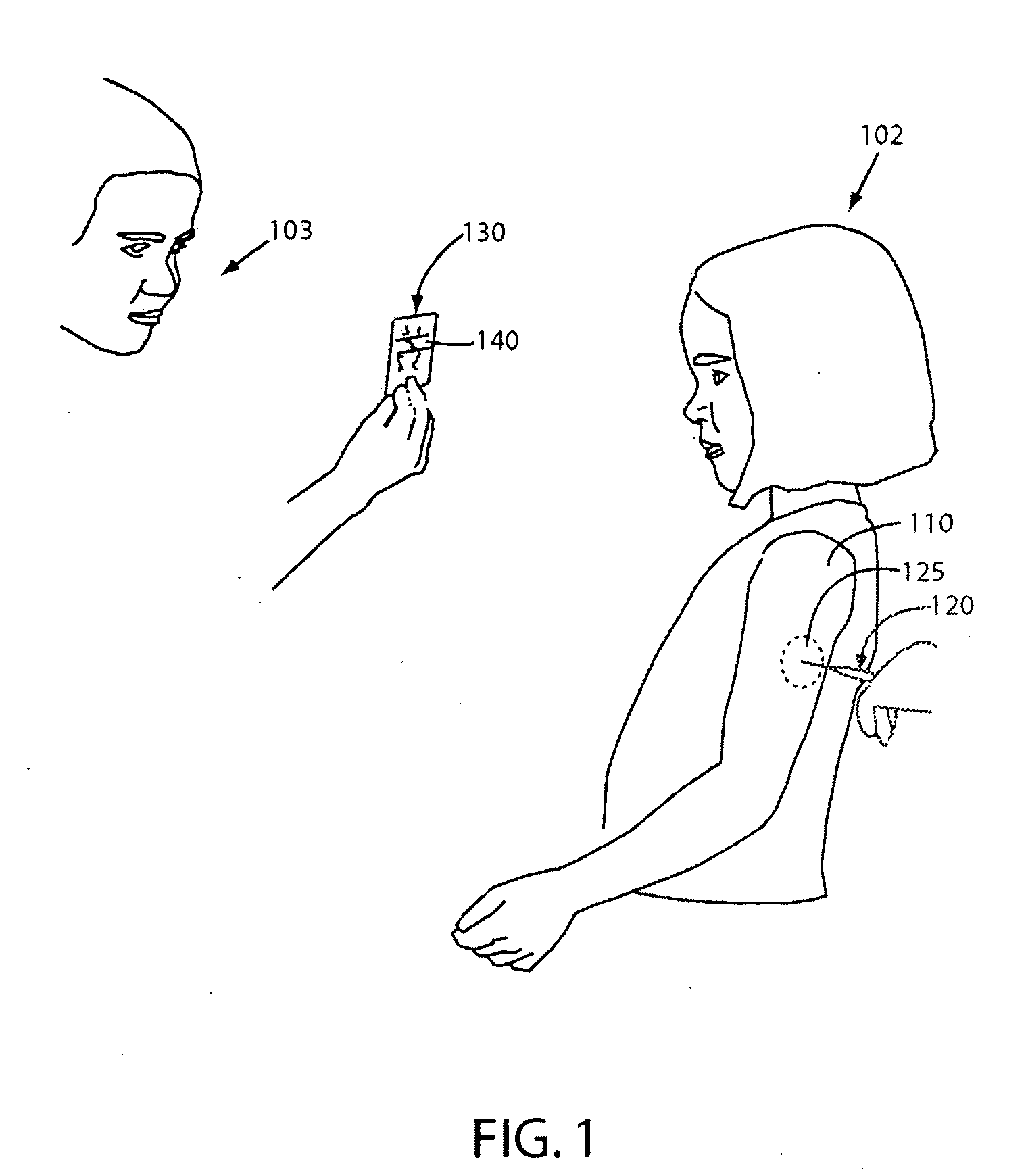
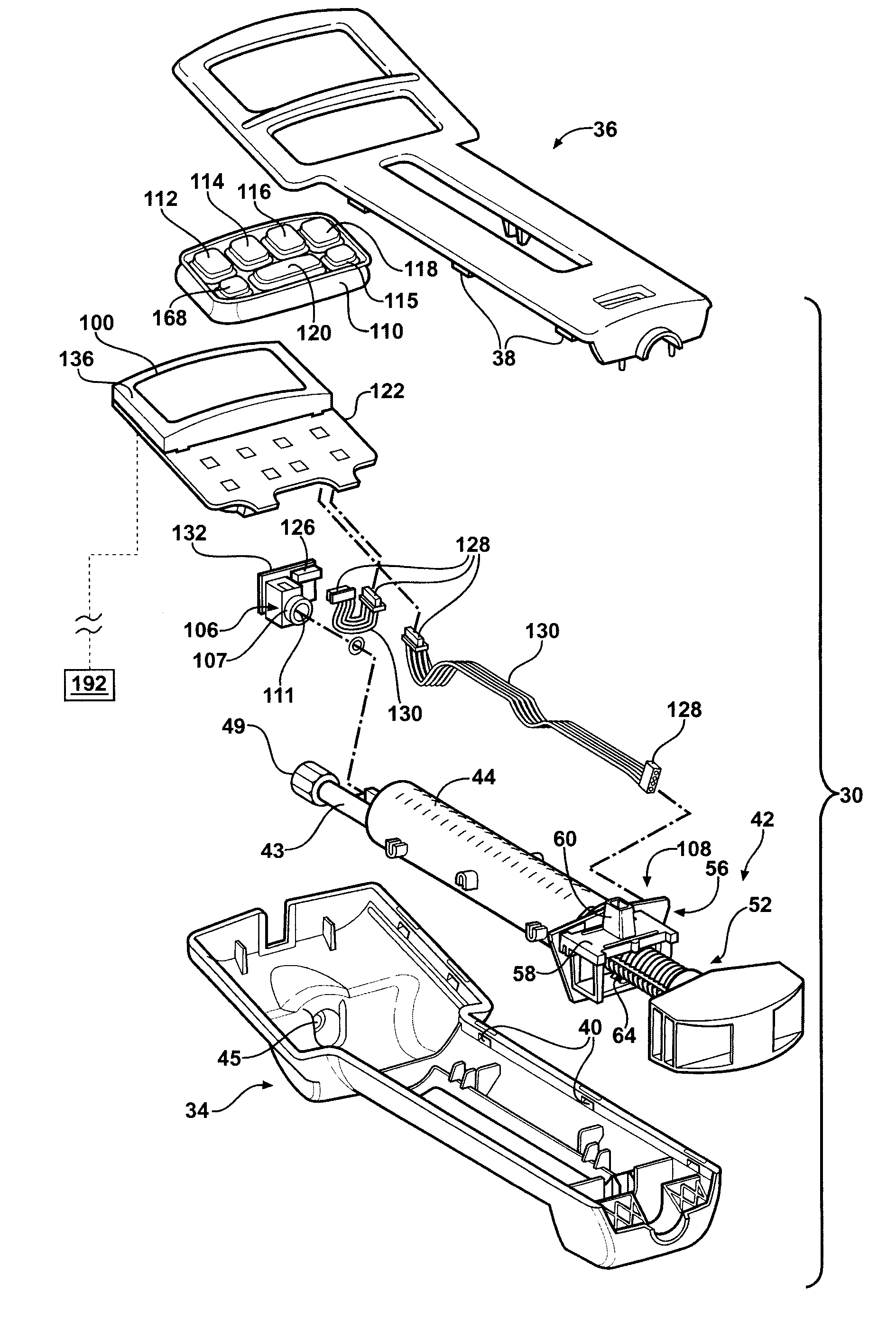
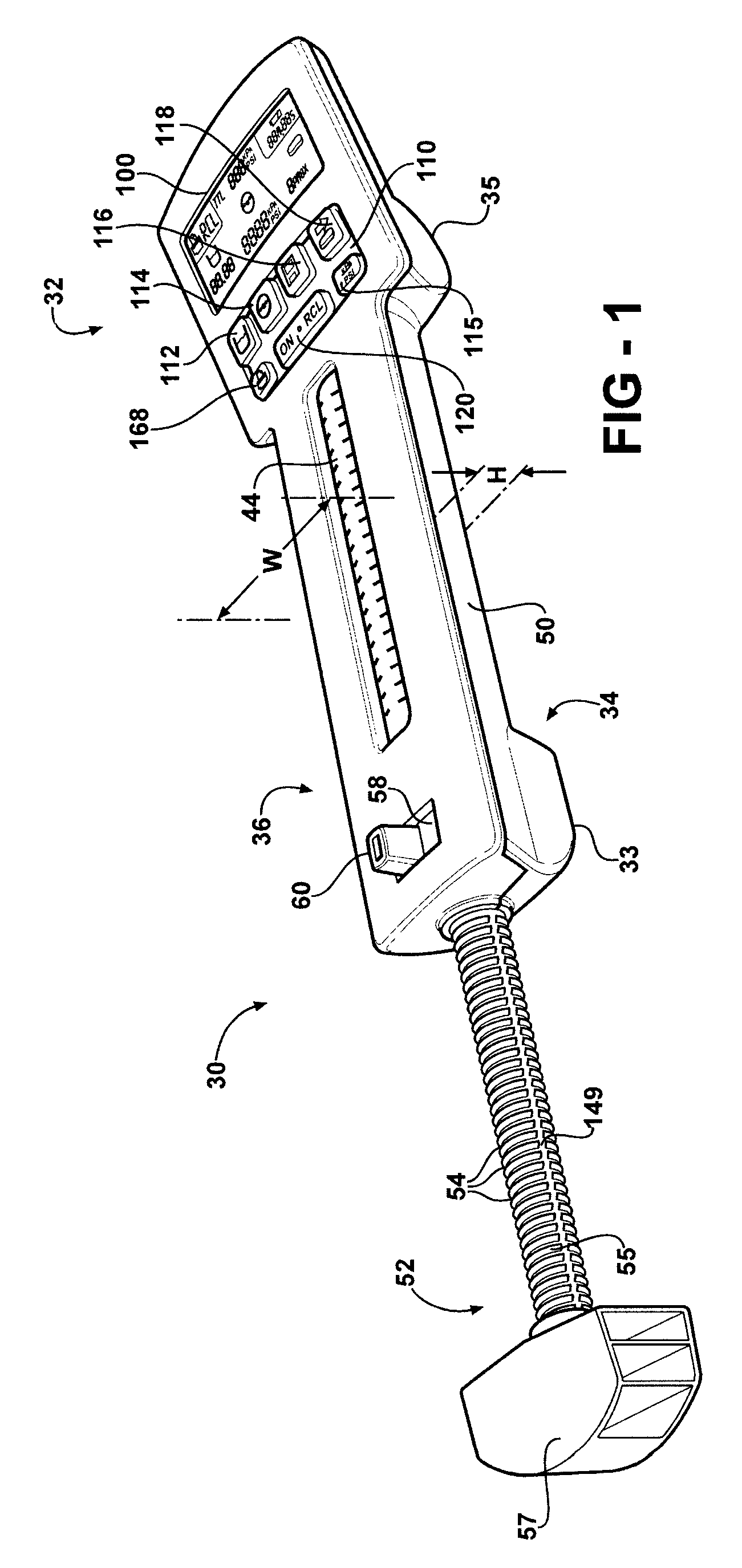
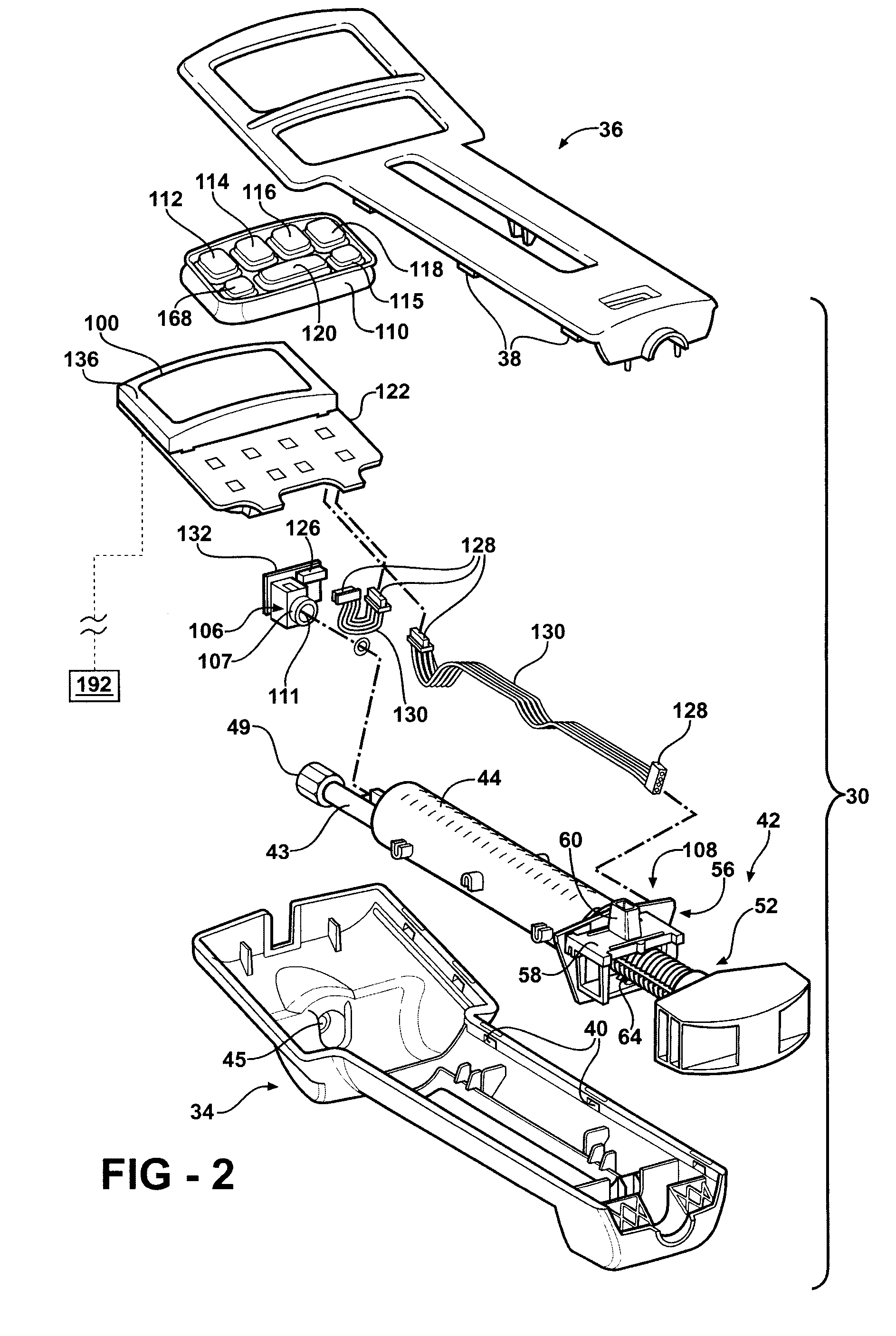
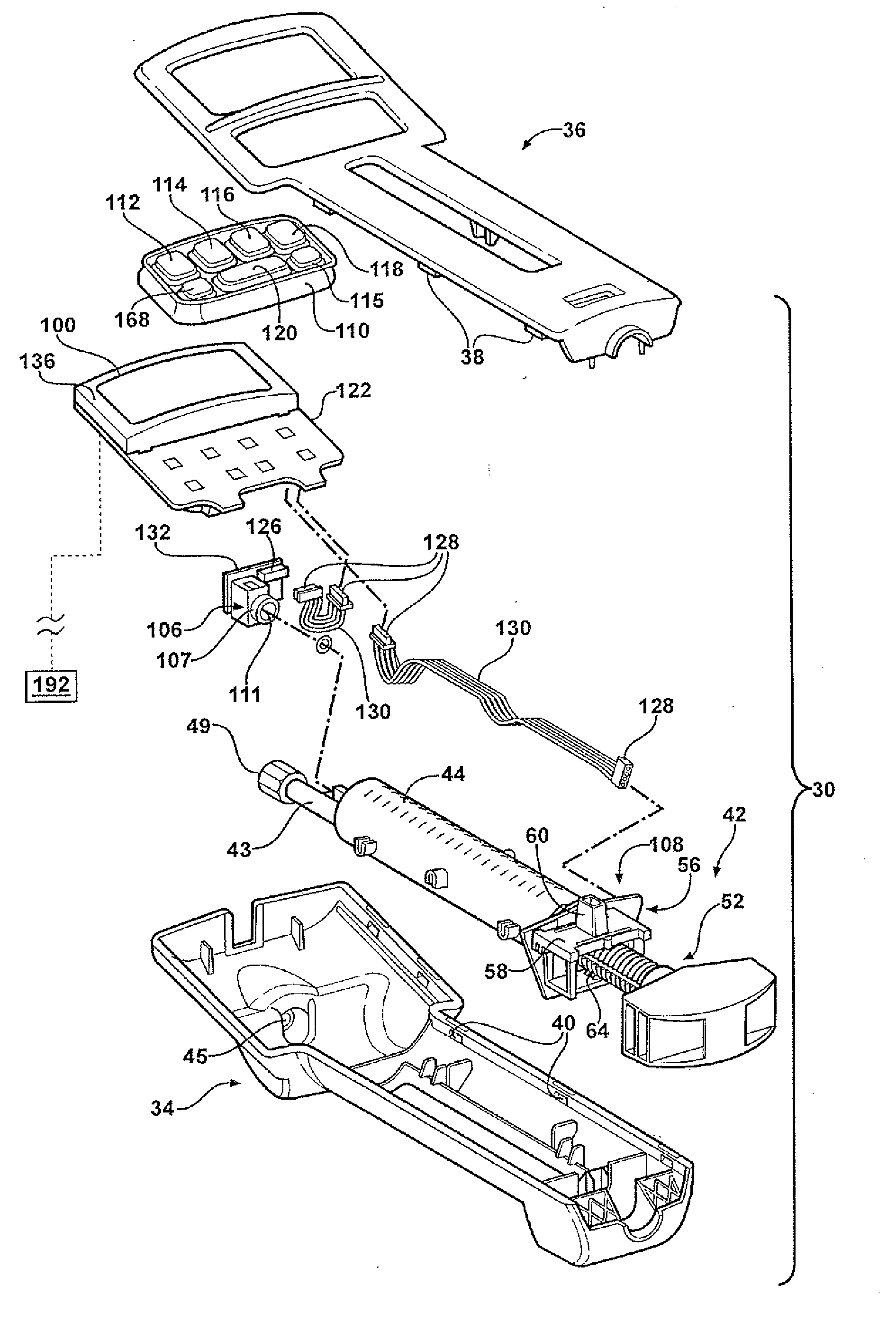
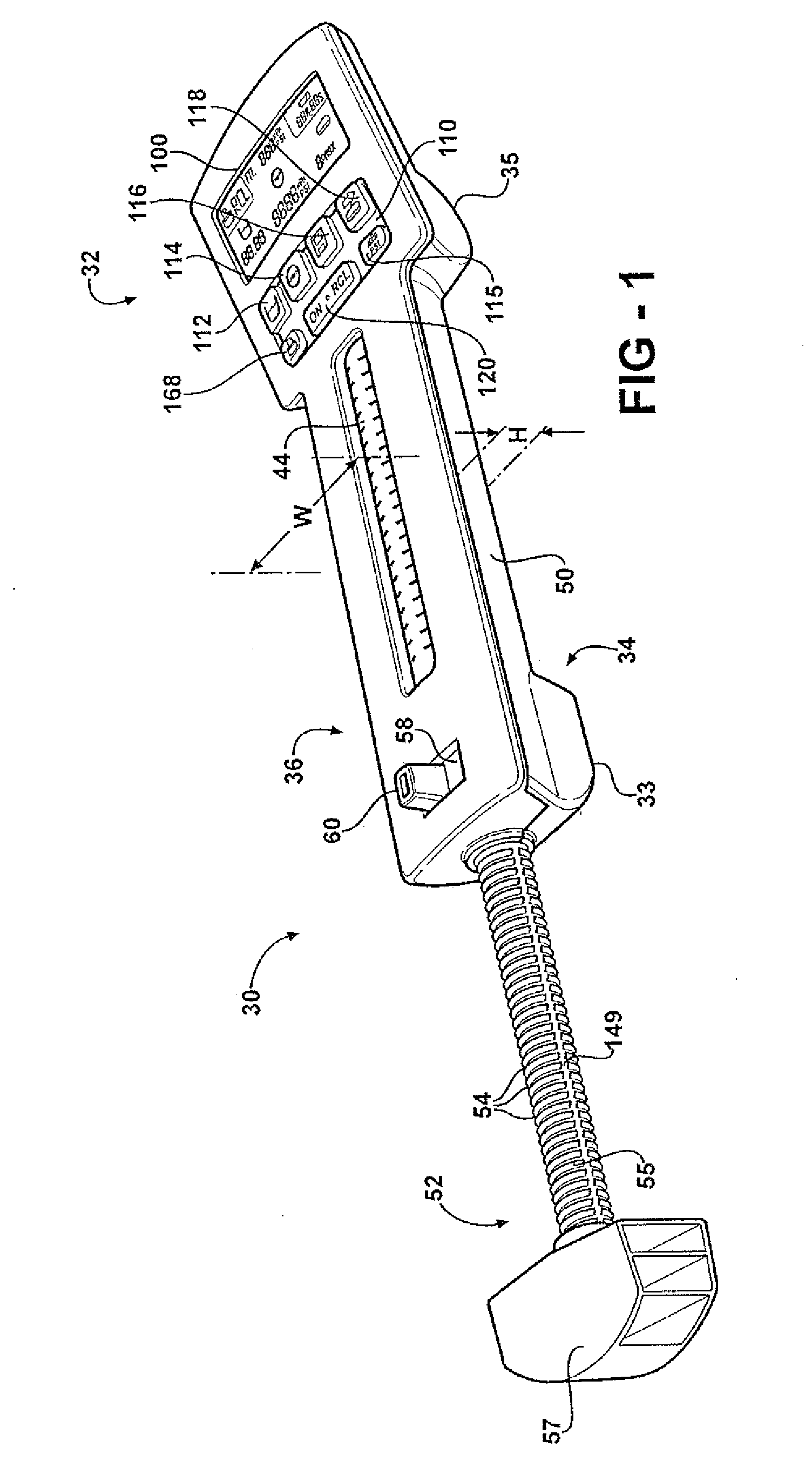
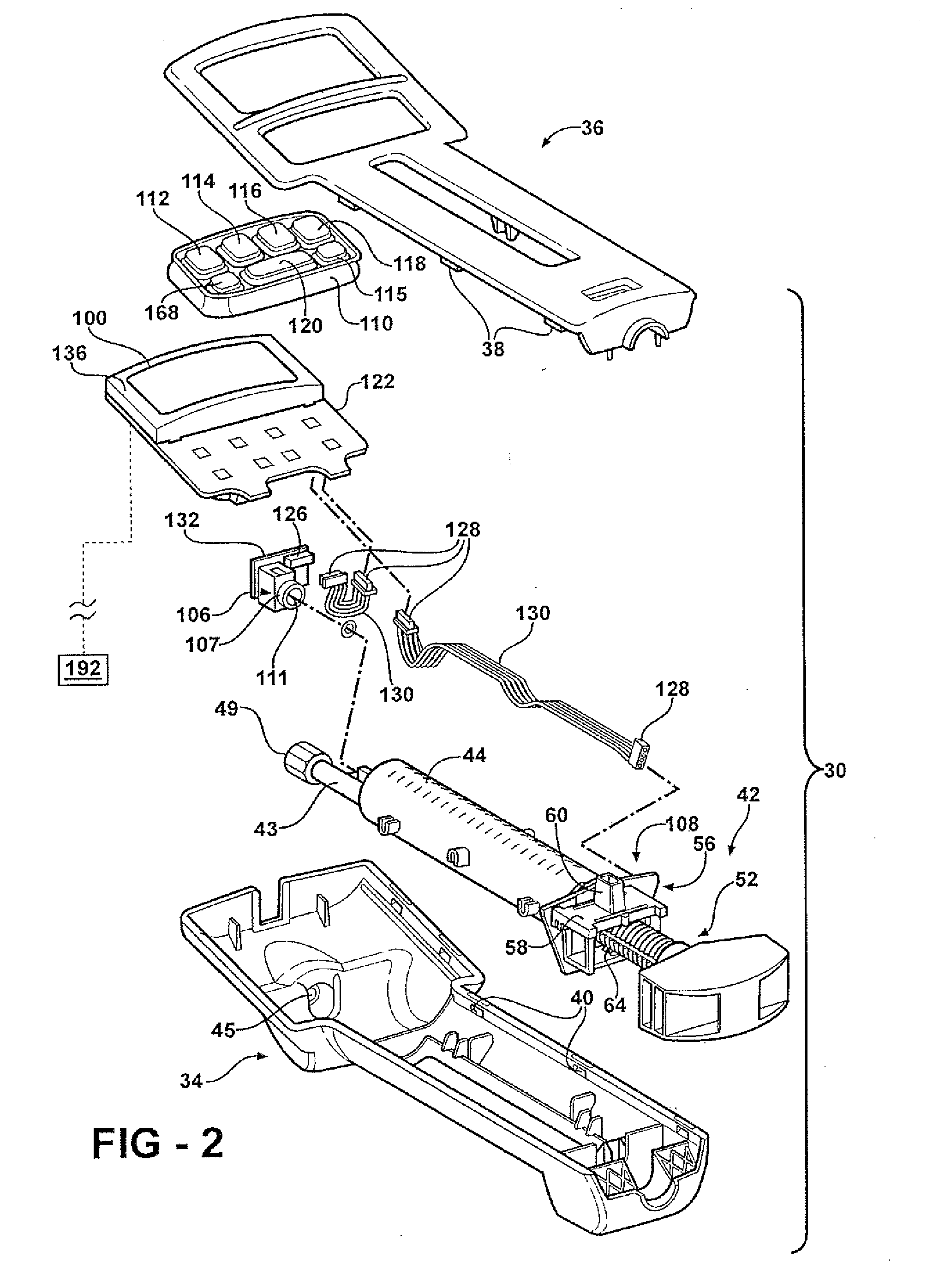
Hand-held fluid delivery device with sensors to determine fluid pressure and volume of fluid delivered to intervertebral discs during discography
ActiveUS20070112299A1Easy to operateContinuous monitoringPerson identificationMedical devicesHand heldSTERILE FIELD
A fluid delivery device is provided for delivering fluid to a target site such as an intervertebral disc during discography. The fluid delivery device includes pressure and volume sensors to determine the pressure and the volume of the fluid delivered to the intervertebral disc. The fluid delivery device is hand-held during use and includes a syringe assembly having a plunger with threads that enables controlled discharge of the fluid from the fluid delivery device by rotating the plunger in a housing of the fluid delivery device. The fluid delivery device may also include a communication module to wirelessly transfer data to an external device outside of a sterile field, while the fluid delivery device is in the sterile field. The fluid delivery device may also include a physiological sensor to monitor involuntary pain responses based on changing physiological parameters such as increases in body temperature or blood pressure.
Owner:STRYKER CORP
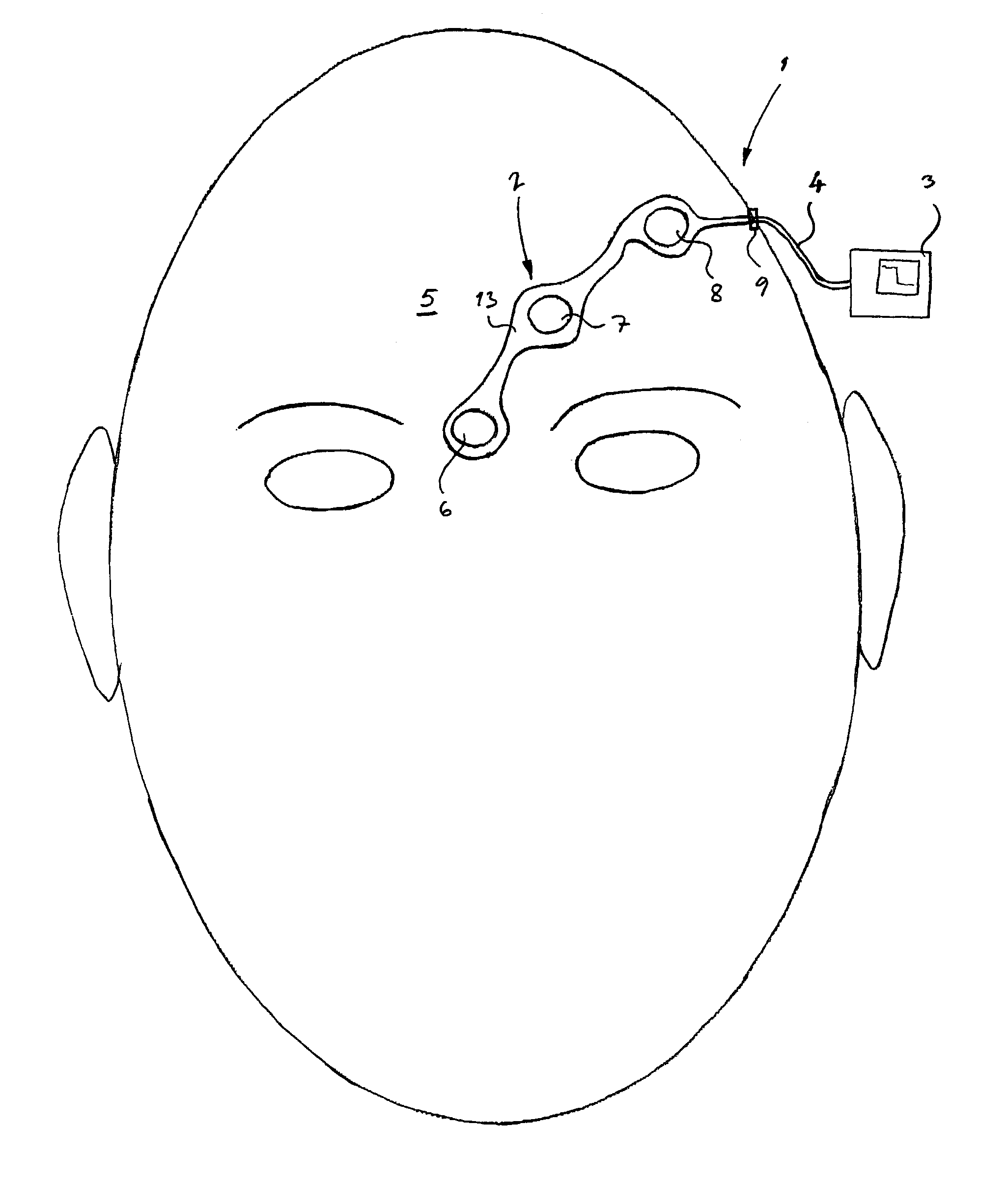
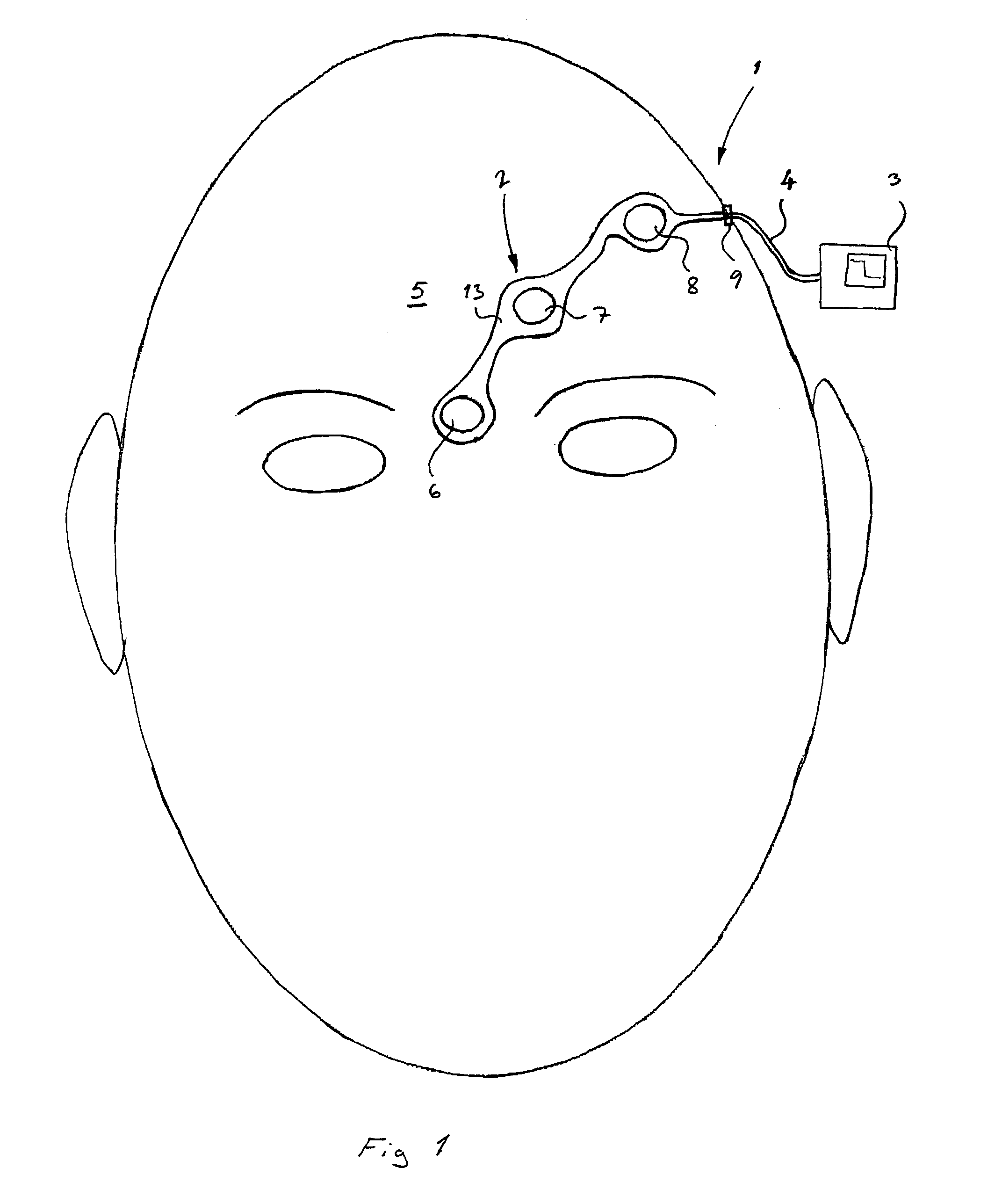
Method of positioning electrodes for central nervous system monitoring and sensing pain reactions of a patient
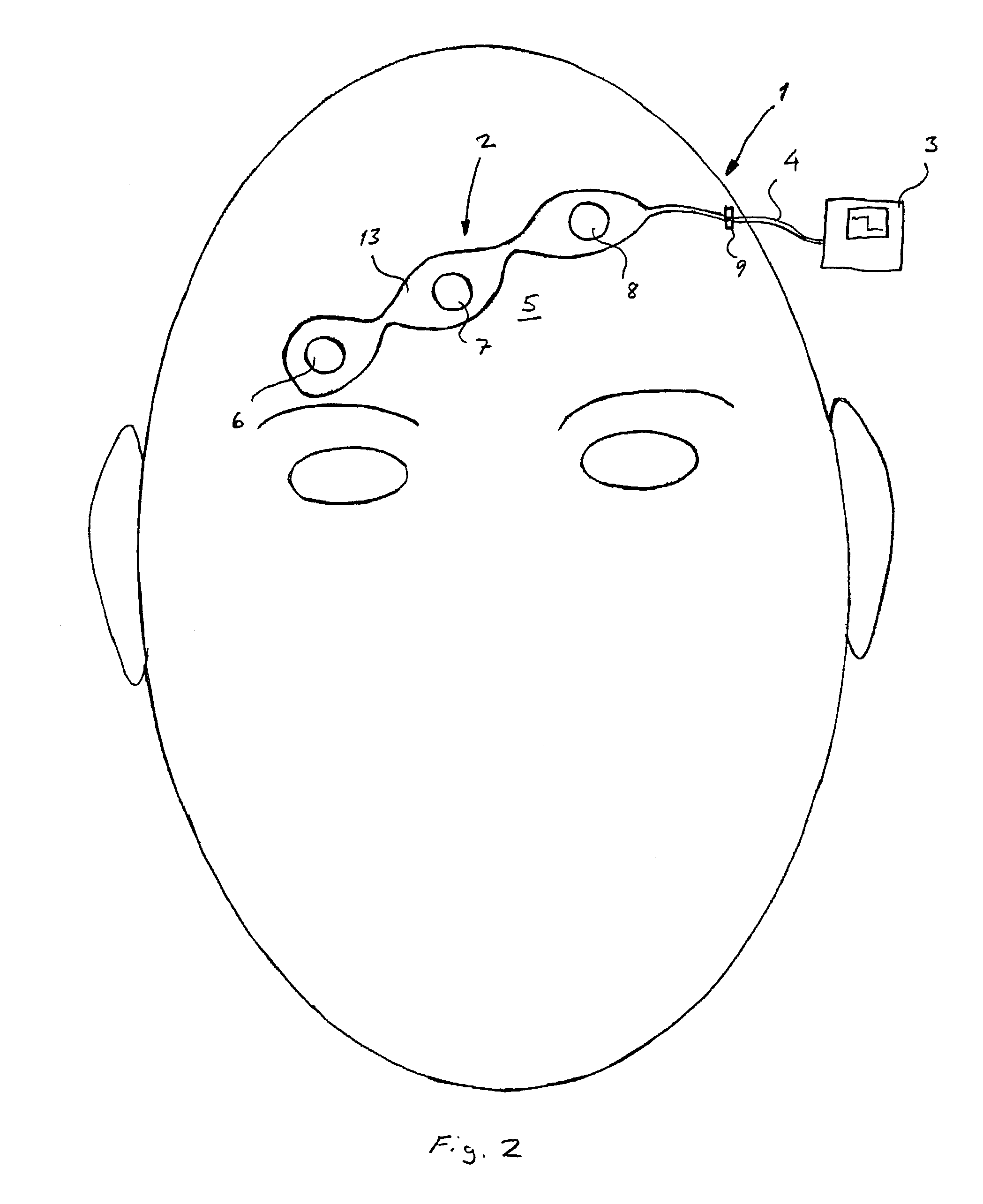
ActiveUS7130673B2Better measurement resultsPractical and convenientElectroencephalographyElectromyographyMedicineForehead
A method concerning measurements by using an electrode array comprising three electrodes for central nervous system (CNS) monitoring from the forehead of a patient's head. A first electrode of said three electrodes is positioned between the eyebrows or immediately above the eyebrows of the patient. A third electrode of said three electrodes is positioned apart from the first electrode on the hairless fronto-lateral area of the forehead the patient. A second electrode is positioned between the first and the third electrodes on the forehead of the patient.
Owner:GE HEALTHCARE FINLAND
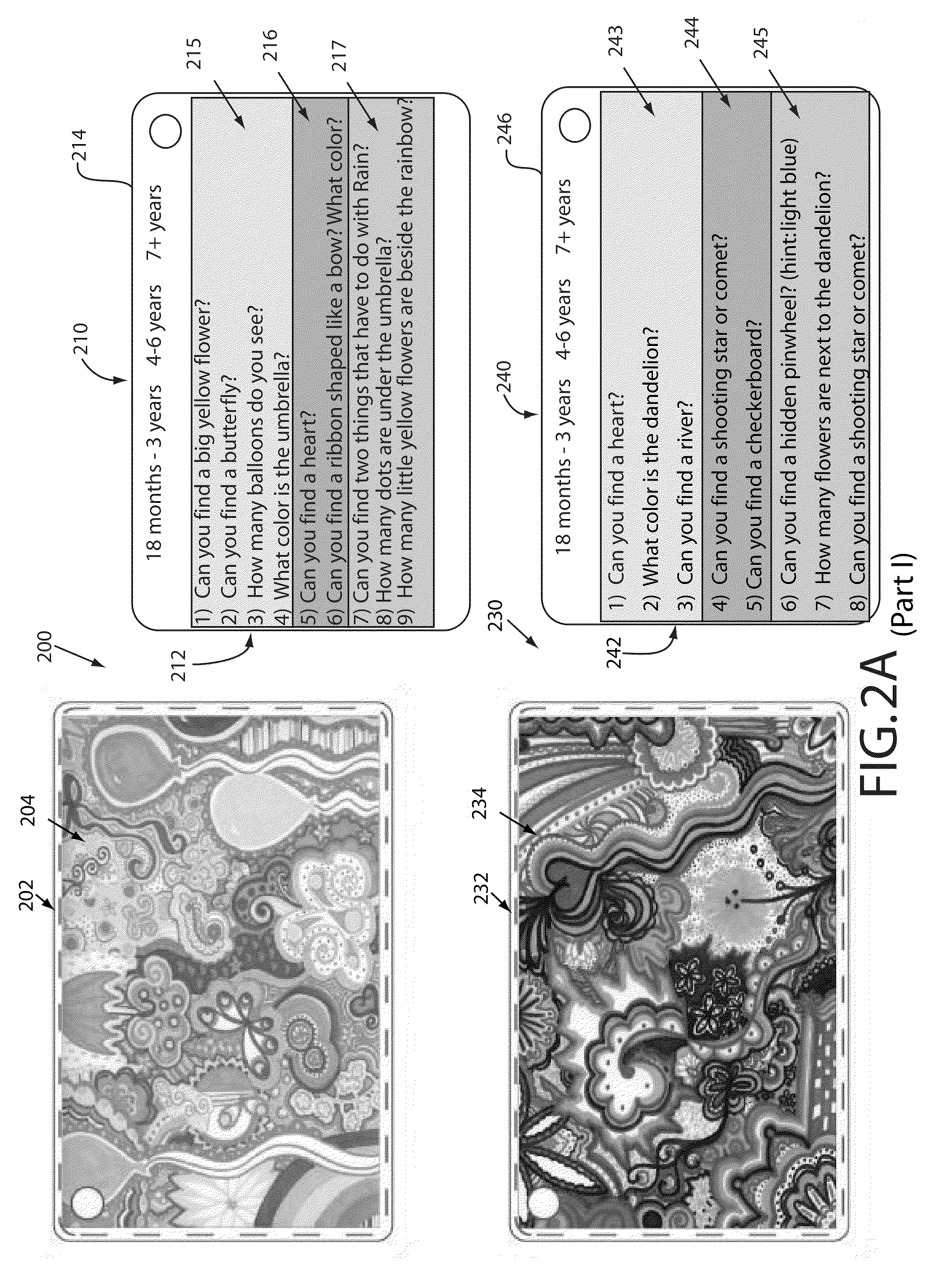
Focused attention and pain reduction
InactiveUS20090151737A1Easy to optimizeMaximize analgesic effectViewersDiagnosticsGraphicsPain reduction
A focus card evokes a neurological response in a user to dampen the user's response to a negative stimulus. A focus card has a first face comprising visual graphics such as lenticular graphics, and a second face comprising indicia pertaining to the visual graphics. Indicia are configured for a target population, and can include novel questions, tasks or directions. A parent or other untrained individual can direct a patient as indicated by the indicia. A single focus card can have indicia for several target populations. A focus card can be used in combination with an analgesia means to further reduce a patient's pain response. By focusing the patient's attention with a focus card, behavior of the patient's anterior cingulate gyrus can be modified to reduce the patient's response to a pain-causing stimulus. It can also be used to reduce agitation, anxiety or distress outside of a medical environment.
Owner:MMJ LABS
Hand-held fluid delivery device with sensors to determine fluid pressure and volume of fluid delivered to intervertebral discs during discography
ActiveUS7959607B2Easy to operateContinuous monitoringPerson identificationMedical devicesSTERILE FIELDHand held
A fluid delivery device is provided for delivering fluid to a target site such as an intervertebral disc during discography. The fluid delivery device includes pressure and volume sensors to determine the pressure and the volume of the fluid delivered to the intervertebral disc. The fluid delivery device is hand-held during use and includes a syringe assembly having a plunger with threads that enables controlled discharge of the fluid from the fluid delivery device by rotating the plunger in a housing of the fluid delivery device. The fluid delivery device may also include a communication module to wirelessly transfer data to an external device outside of a sterile field, while the fluid delivery device is in the sterile field. The fluid delivery device may also include a physiological sensor to monitor involuntary pain responses based on changing physiological parameters such as increases in body temperature or blood pressure.
Owner:STRYKER CORP
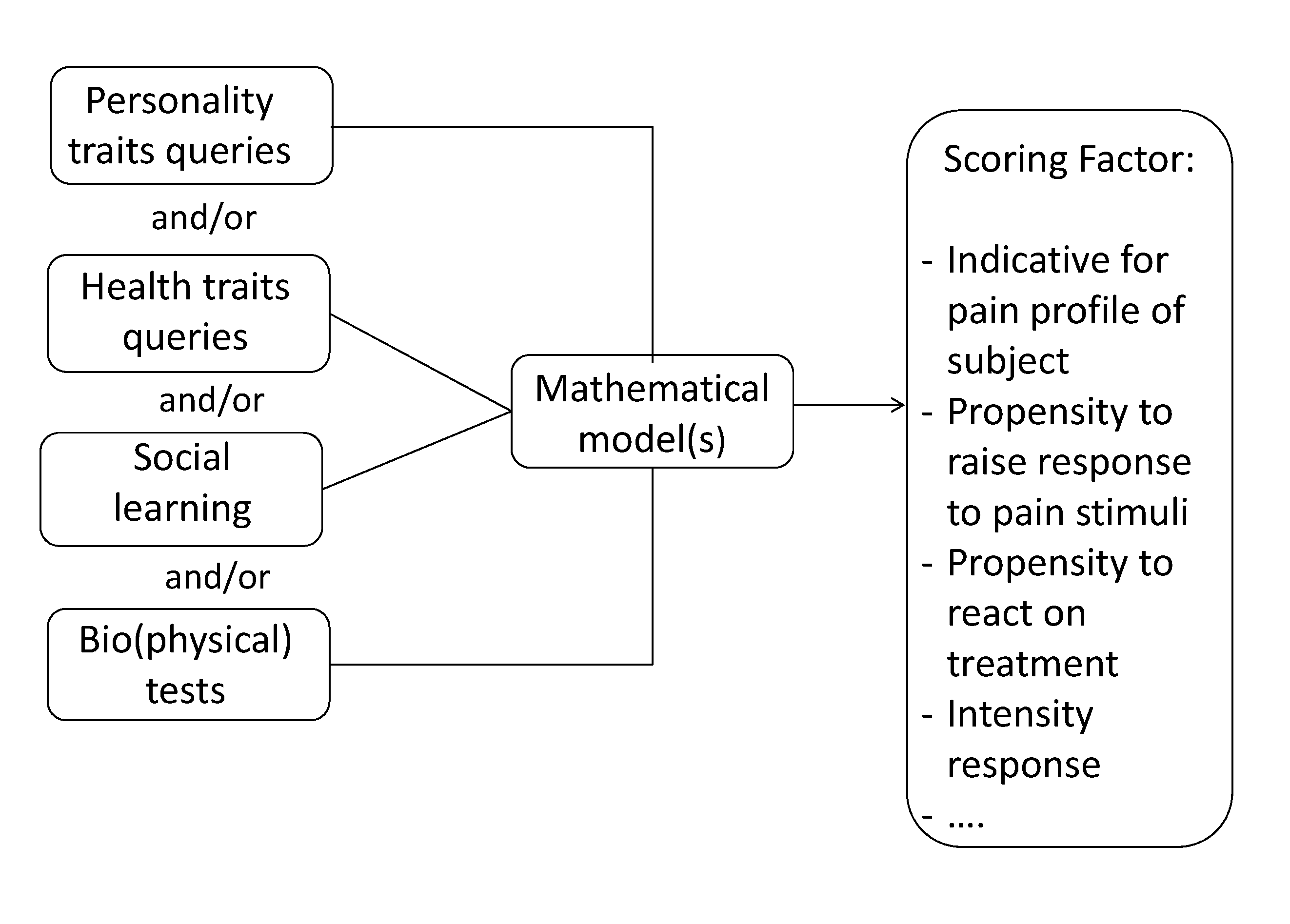
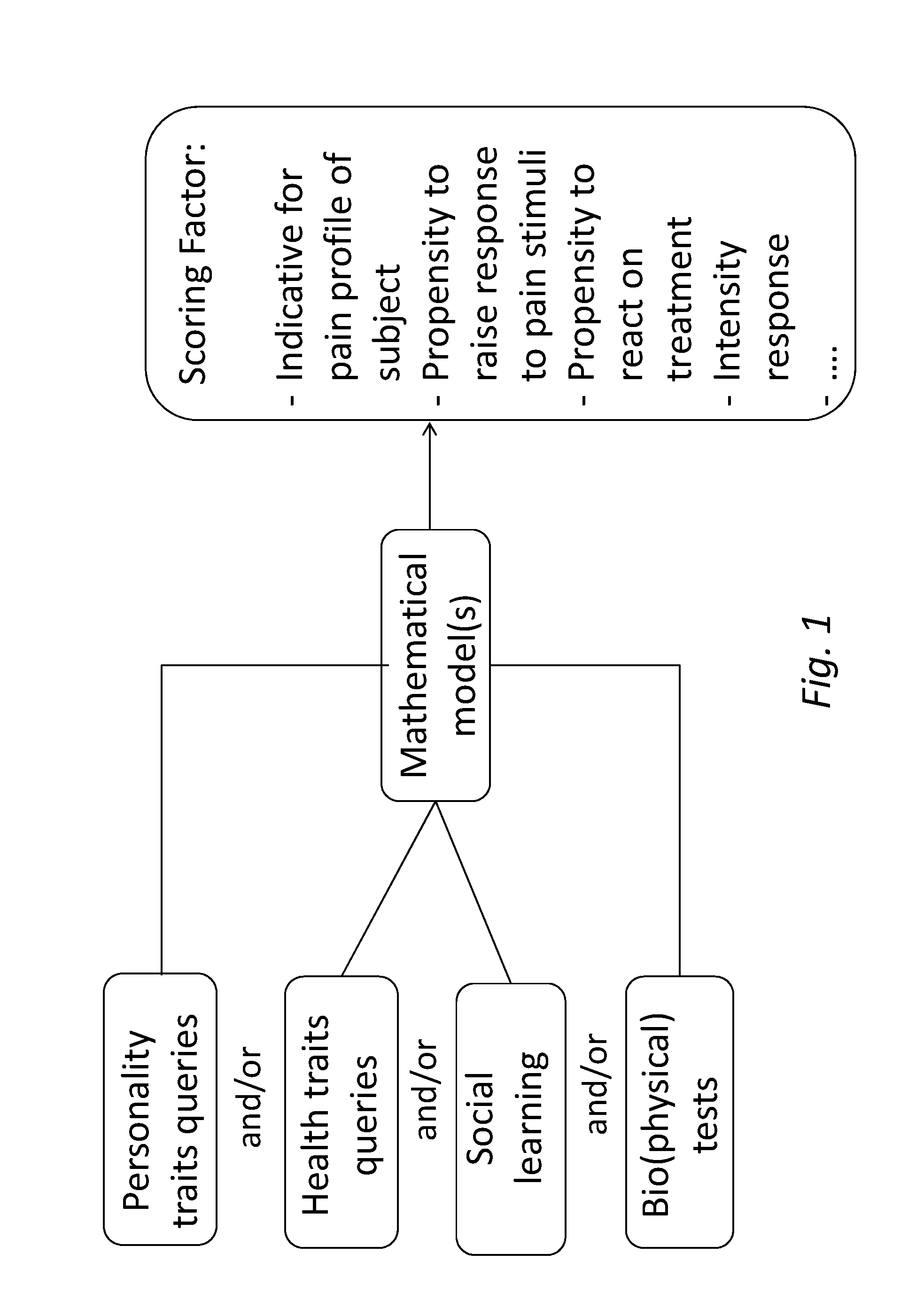
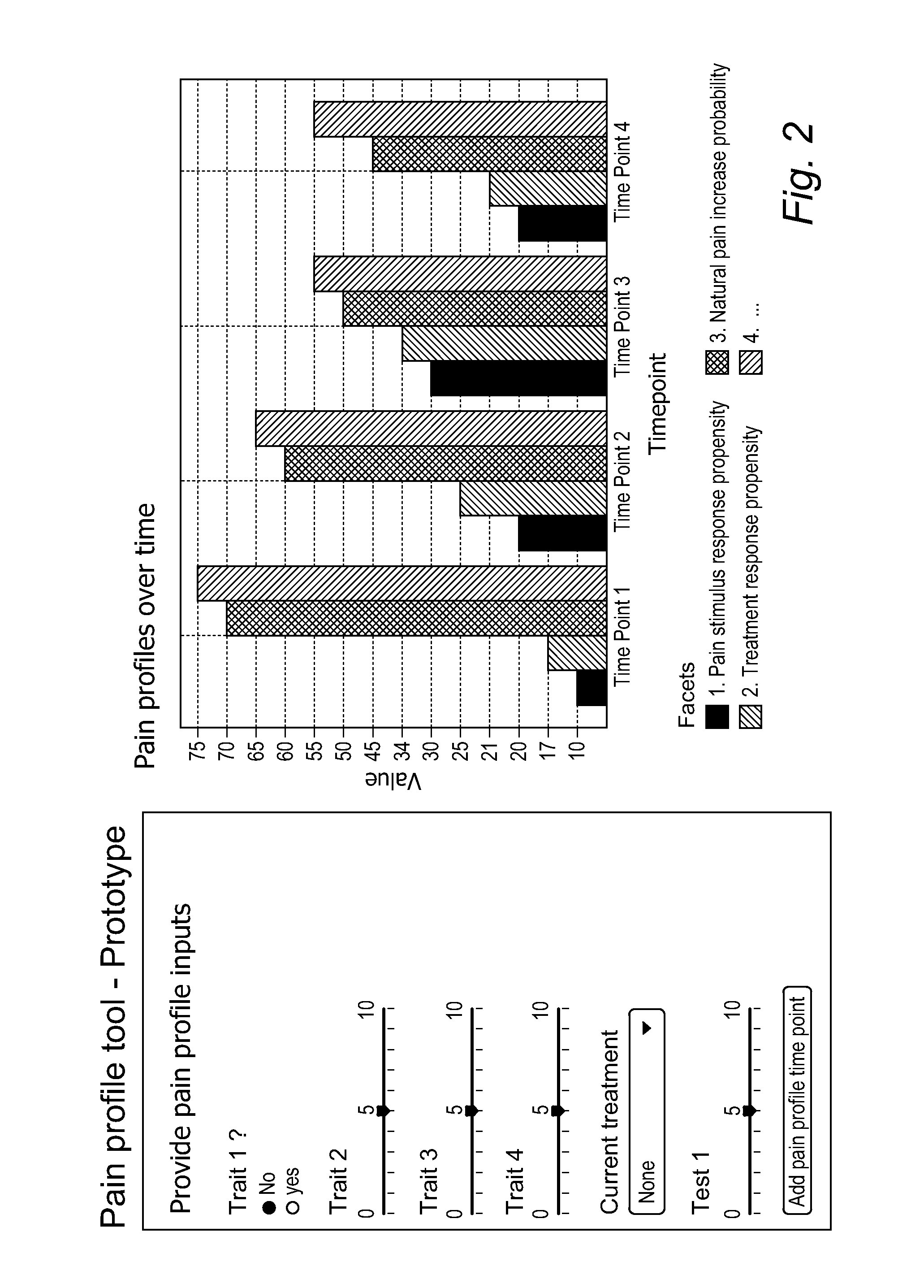
Method and tools for predicting a pain response in a subject suffering from cancer-induced bone pain
InactiveUS20160048659A1Not pose burdenMedical automated diagnosisMedical report generationMathematical modelClinical psychology
A method is described for predicting a pain response in a subject suffering from cancer-induced bone pain (CIBP). Data is collected from the subject by querying the subject on personality and / or health traits, and / or performing one or more social learning and / or (bio)physical tests by or on the subject. The data is used in a set of mathematical models to attribute one or more Scoring Factors to the subject. The Scoring Factor is a measure of the propensity of the subject to raise a response to a pain stimulus or a treatment strategy; and / or a measure of the intensity of the response of the subject.
Owner:TOOLS4PATIENT
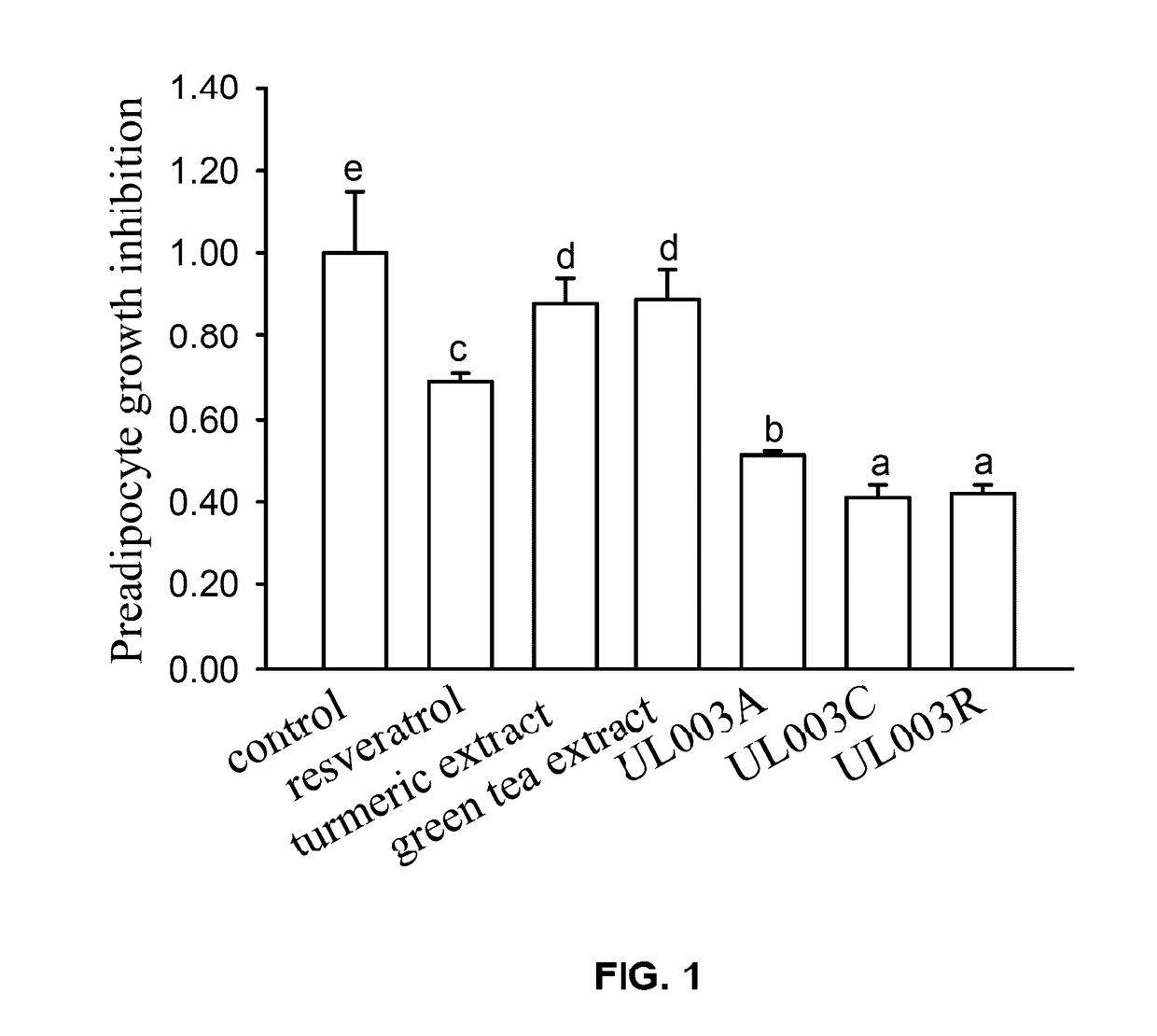
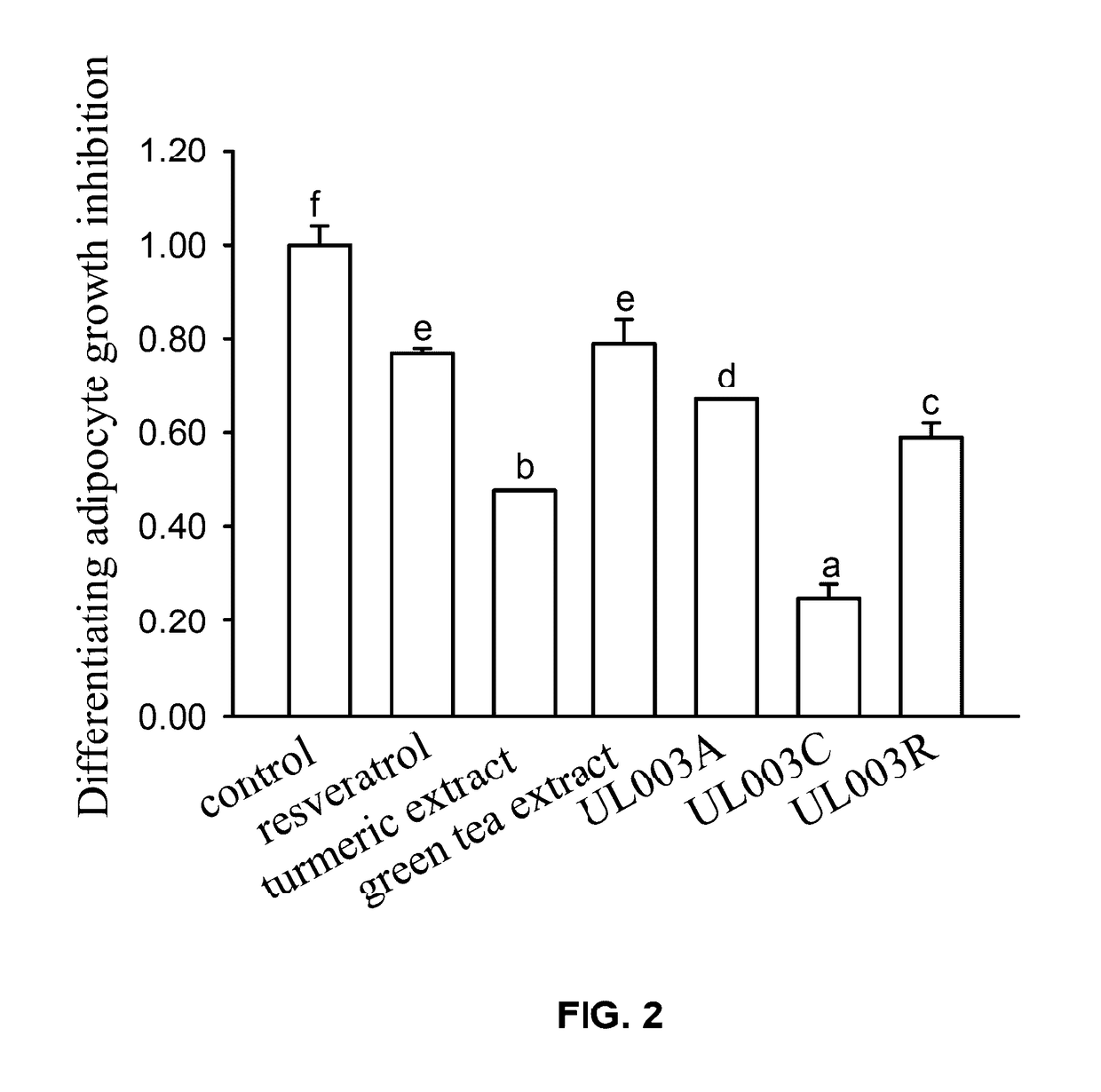
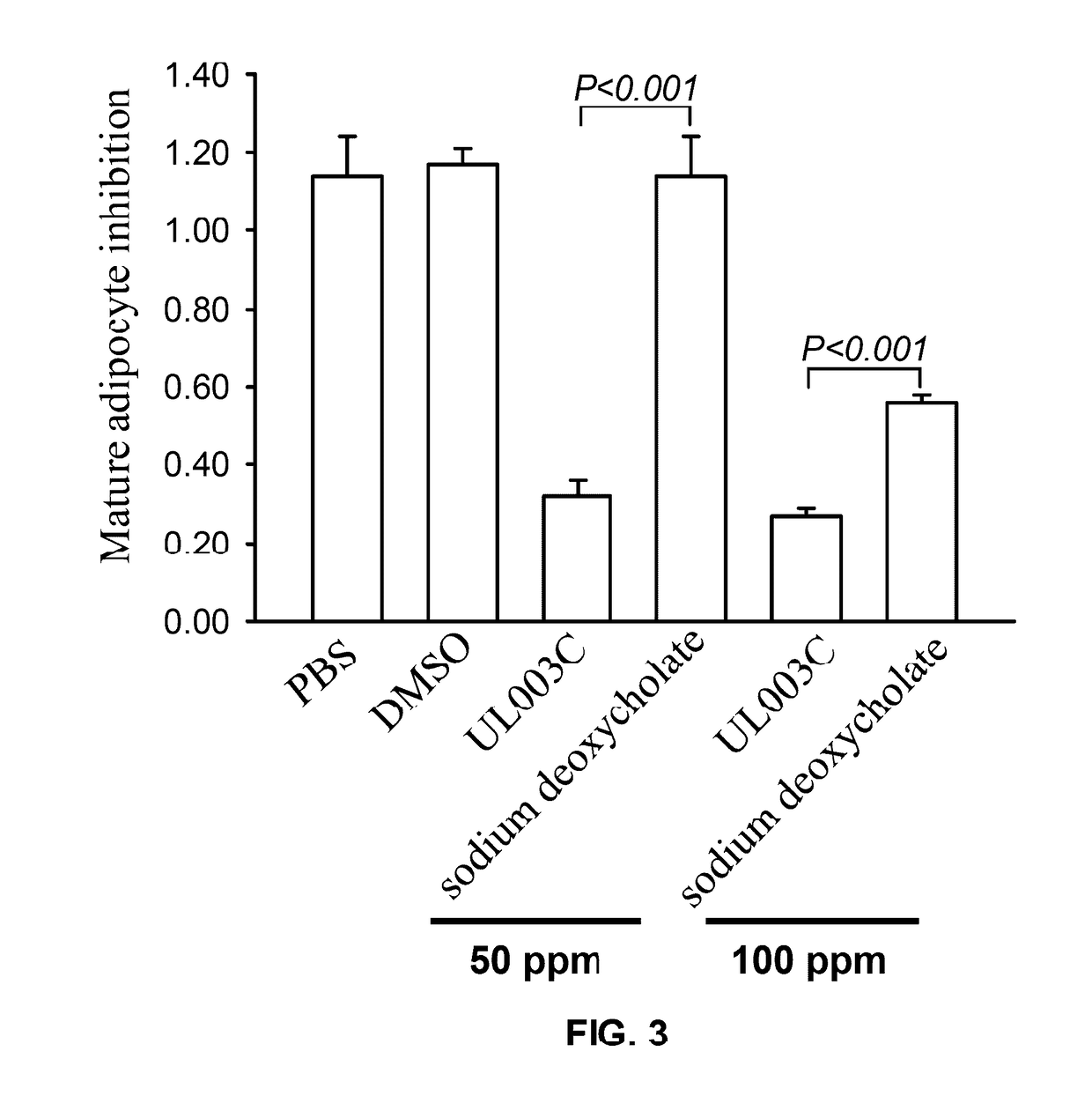
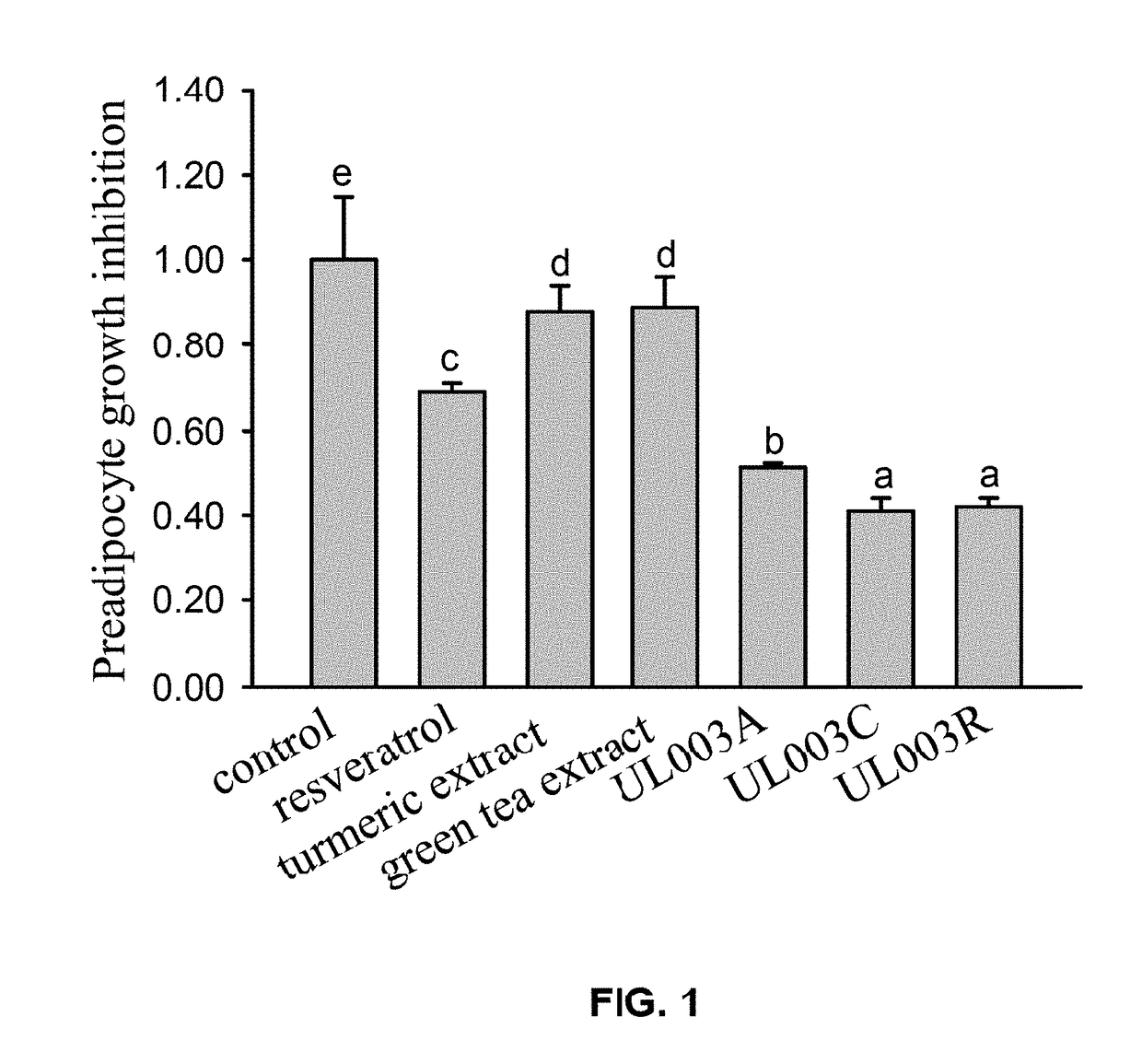
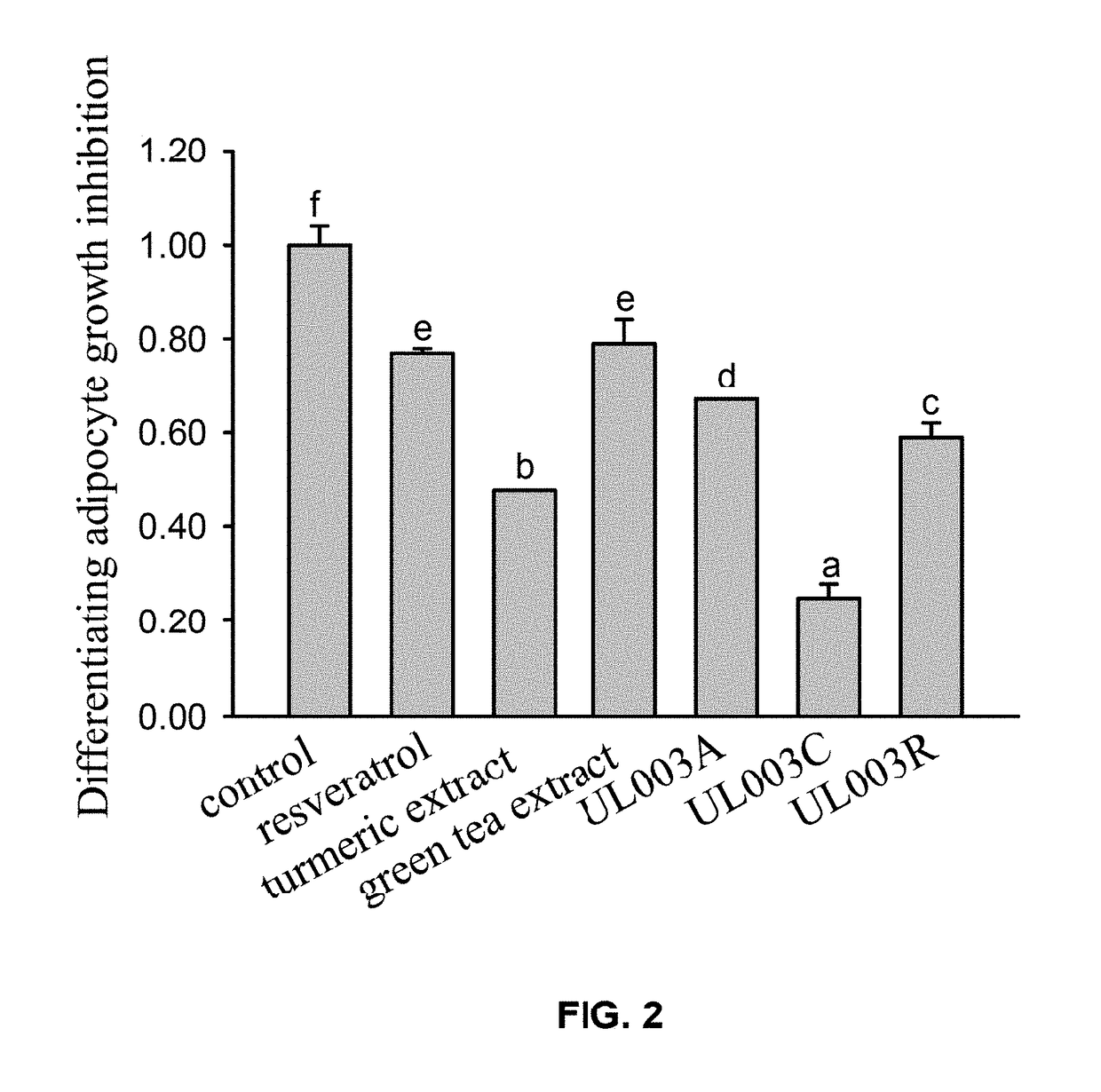
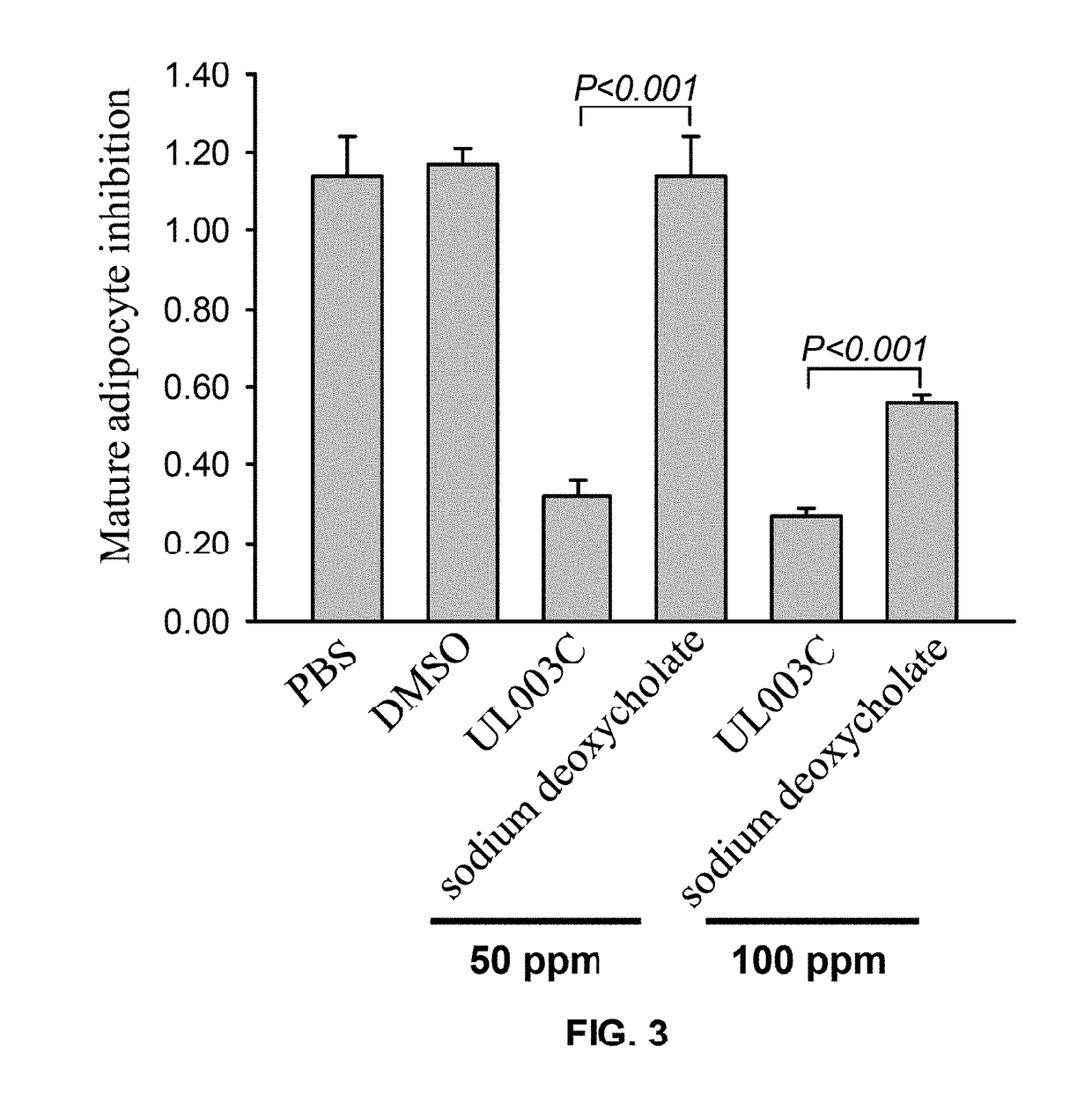
Plant extract composition for reducing topical fat and promoting weight loss as well as applications thereof
ActiveUS20170157195A1Inhibit adipocyte growthPromote adipocyte apoptosisHydroxy compound active ingredientsPharmaceutical delivery mechanismApoptosisPain responses
Disclosed in the present invention is the composition for reducing local fat and body weight, and pharmaceuticals and use thereof. The composition contains resveratrol and curcumin extract at a weight ration from 1:30 to 10:1. The composition and pharmaceuticals thereof of the invention can inhibit the growth of fat cells, cause planned apoptosis of fat cells, achieve the effects of reducing fat cells, and reducing local fat deposition and body weight without causing inflammations or necrosis of surrounding cells or tissues and inflammations or pain reactions of surrounding tissues, thereby avoiding the problems of tissue damage and inflammatory pains caused by liposuction or low invasion fat-dissolving apparatus used in the prior art and the problems such as surrounding tissue inflammations and necrosis infections triggered by cell disruption and necrosis caused by components of a fat-dissolving injection, phosphatidylcholine or sodium deoxycholate.
Owner:CALIWAY BIOPHARM
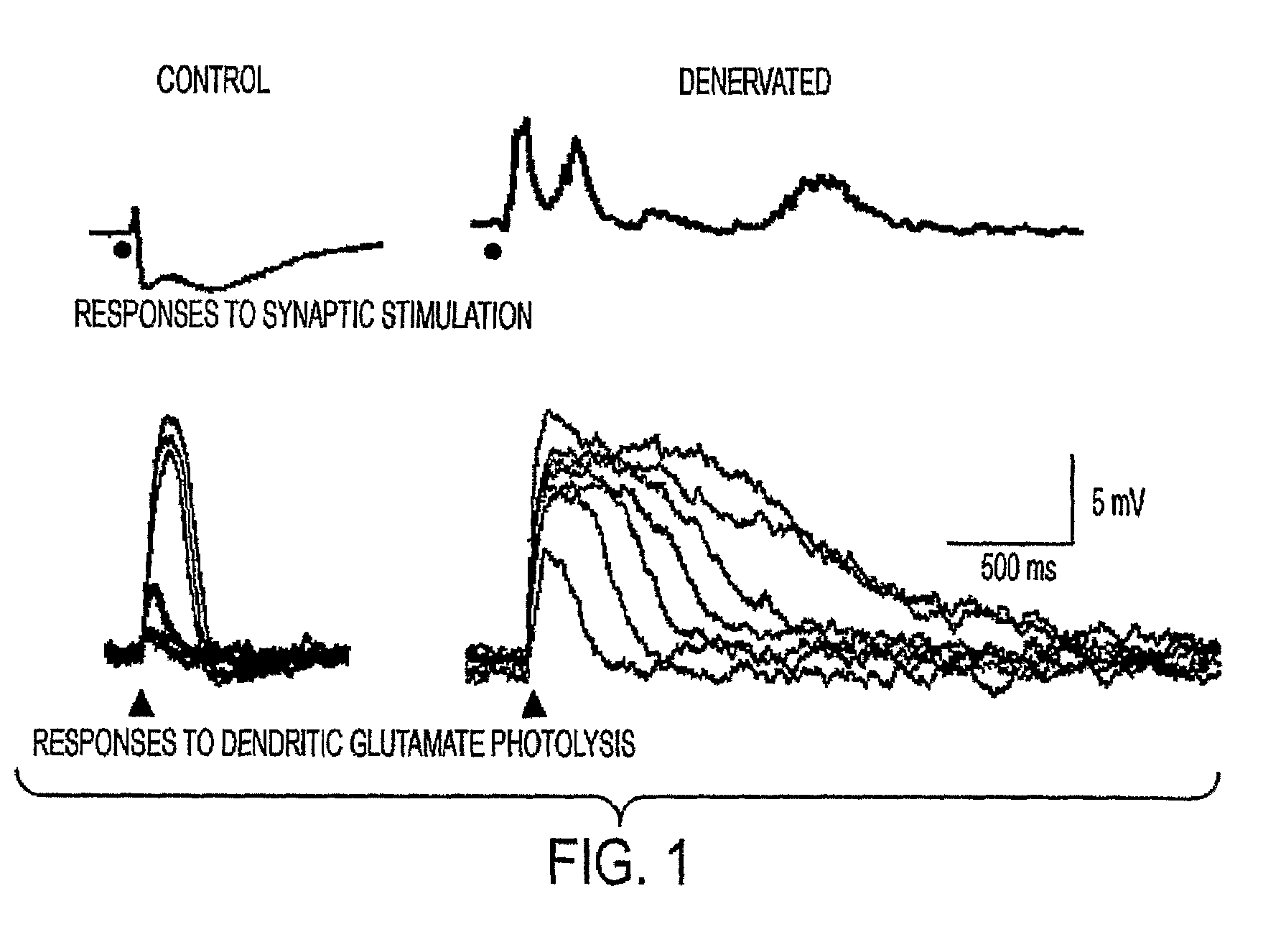
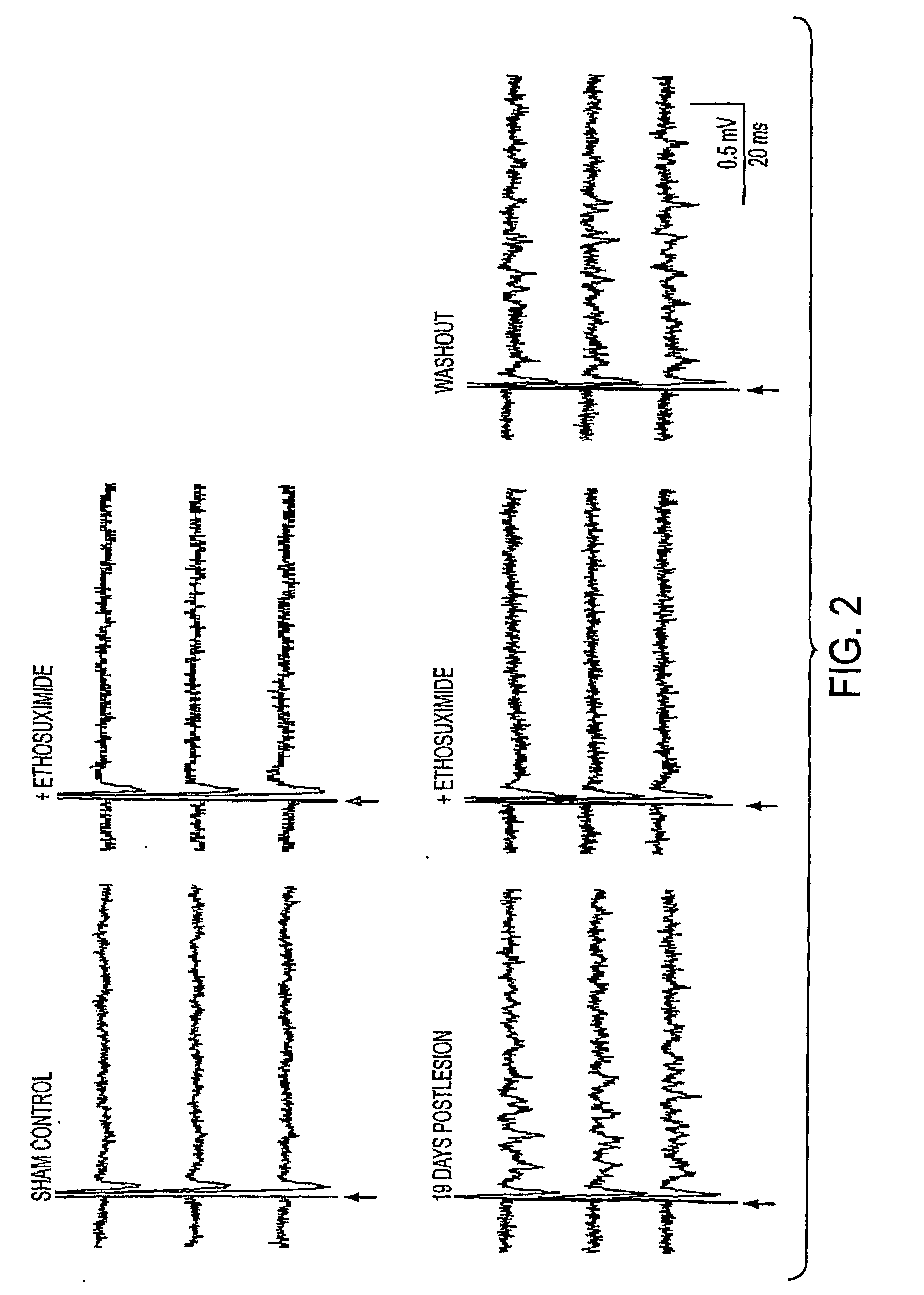
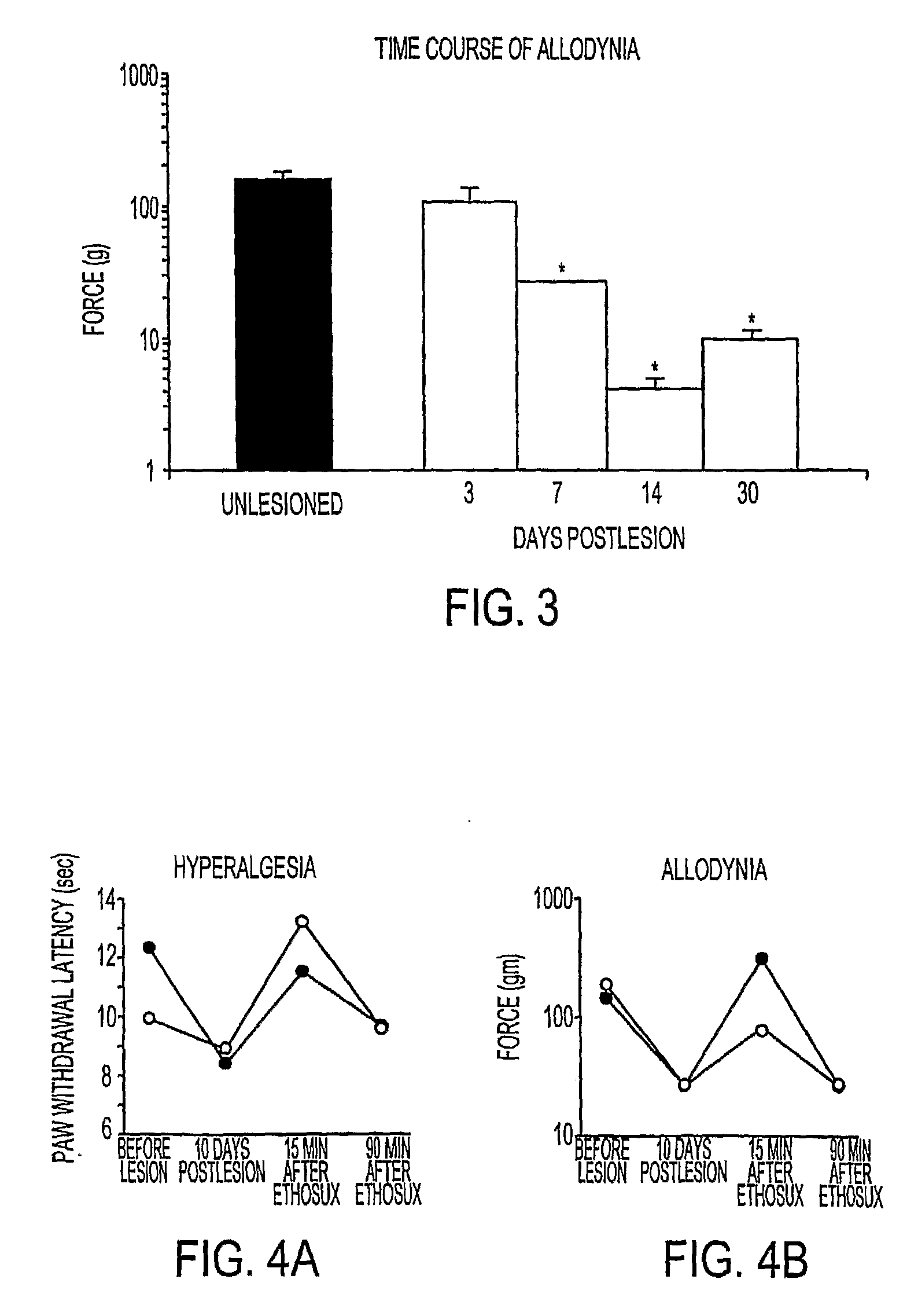
Method for treating central pain syndrom or for inducing centrally generated pain in an animal model
The present invention provides a method for treating central pain syndrome in a mammal by administering an effective amount of a thalamic anticonvulsant compound. Also provided are methods for inducing centrally generated pain responses in an animal model and for screening and identifying a compound that inhibits T-type calcium channels.
Owner:UNIV OF MARYLAND
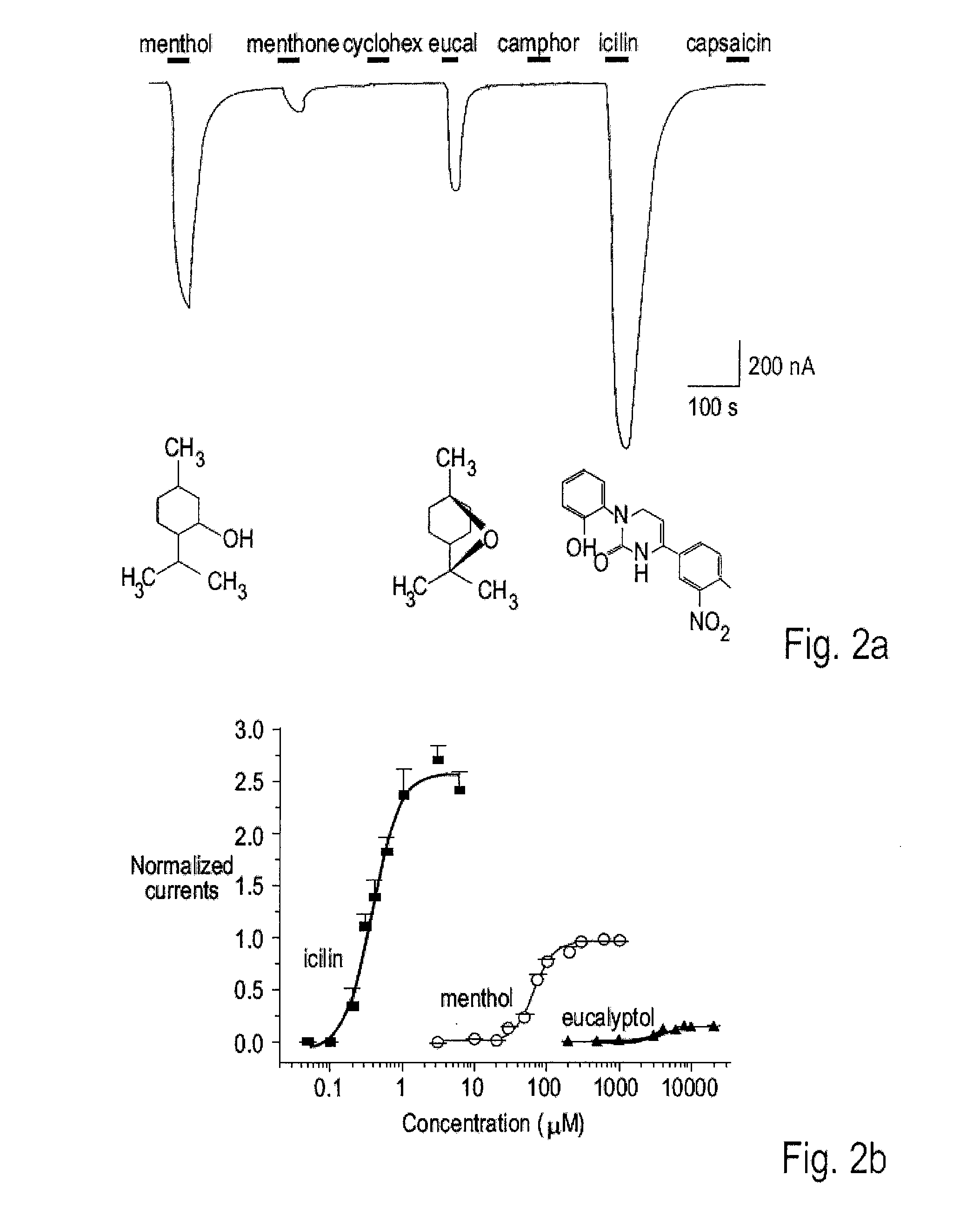
Methods of modulating cold sensory perception
ActiveUS7371841B2Peptide/protein ingredientsAntibody mimetics/scaffoldsPattern perceptionCold sensation
The present invention relates to regulation of cold sensation and pain. More particularly, the present invention is directed to nucleic acids encoding a member of the transient regulatory protein family, CMR1, which is involved in modulation of the perception of cold sensations and pain. The invention further relates to methods for identifying and using agents that modulate cold responses and pain responses stimulated by cold via modulation of CMR1 and CMR1-related signal transduction.
Owner:RGT UNIV OF CALIFORNIA
Methods of modulating cold sensory perception
ActiveUS20080241872A1Cell receptors/surface-antigens/surface-determinantsBacteriaPattern perceptionCold sensation
The present invention relates to regulation of cold sensation and pain. More particularly, the present invention is directed to nucleic acids encoding a member of the transient regulatory protein family, CMR1, which is involved in modulation of the perception of cold sensations and pain. The invention further relates to methods for identifying and using agents that modulate cold responses and pain responses stimulated by cold via modulation of CMR1 and CMR1-related signal transduction.
Owner:RGT UNIV OF CALIFORNIA
Methods of modulating cold sensory perception
ActiveUS20080242841A1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsPattern perceptionCold sensation
Owner:RGT UNIV OF CALIFORNIA
Plant extract composition for reducing topical fat and promoting weight loss as well as applications thereof
ActiveUS10226503B2Reducing localized fat depositsDecrease adipocytesHydroxy compound active ingredientsPharmaceutical delivery mechanismApoptosisPain responses
Disclosed in the present invention is the composition for reducing local fat and body weight, and pharmaceuticals and use thereof. The composition contains resveratrol and curcumin extract at a weight ration from 1:30 to 10:1. The composition and pharmaceuticals thereof of the invention can inhibit the growth of fat cells, cause planned apoptosis of fat cells, achieve the effects of reducing fat cells, and reducing local fat deposition and body weight without causing inflammations or necrosis of surrounding cells or tissues and inflammations or pain reactions of surrounding tissues, thereby avoiding the problems of tissue damage and inflammatory pains caused by liposuction or low invasion fat-dissolving apparatus used in the prior art and the problems such as surrounding tissue inflammations and necrosis infections triggered by cell disruption and necrosis caused by components of a fat-dissolving injection, phosphatidylcholine or sodium deoxycholate.
Owner:CALIWAY BIOPHARM
Stable progesterone water-soluble injection and preparation method thereof
InactiveCN109381424AMild pain responseLess irritatingOrganic active ingredientsPharmaceutical delivery mechanismIrritationCyclodextrin Derivatives
The invention relates to a progesterone water-soluble injection and a preparation method thereof. The progesterone water-soluble injection comprises progesterone, a cyclodextrin derivative solubilizer, an inclusion accelerator and injection water. Sodium citrate and citric acid are taken as the inclusion accelerator, and a complex is formed with cyclodextrin by using the polyhydric structural features of sodium citrate and citric acid, so that the inclusion constant of the complex is improved, the problem that the using amount is excessive when the progesterone water-soluble injection is prepared by using a cyclodextrin derivative as the solubilizer is solved effectively, the using amount is reduced to less than 10%, and the renal toxicity, hemolytic activity and carcinogenicity risk of the cyclodextrin derivative are reduced obviously through such using amount, so that the safety is improved. The pain reaction is relatively lower when the progesterone water-soluble injection is used for injection, the injection is low in irritation, unlikely to be allergic and high in adaptability for long-time use of a patient, and relative to clinically frequently-used progesterone oil-soluble injection in the present stage, the injection has the advantages of dosage form.
Owner:南京泽恒医药技术开发有限公司
Application of mastic and myrrh in preparing medicine for preventing and treating nerve pathological pain diseases
InactiveCN106074660ASuppress pain responseOvert pain responseNervous disorderPlant ingredientsDiseaseMyrrh
The invention discloses application of mastic and myrrh in preparing medicine for preventing and treating nerve pathological pain diseases. Based on the existing pharmacological activity of mastic and myrrh, new clinical effects of mastic and myrrh are screened through deep research via a large quantity of experiments. According to the application, an animal model of nerve pathological chronic pain caused by ligation of the sciatic nerve is used for researching the suppression effect of mastic-myrrh aqueous extract on the pain sense of mice, and experiment results show that after the model for ligation of the sciatic nerve is implemented for 7 days, obvious pain reactions of mice can be caused, while the pain reactions of mice can be remarkably suppressed through oral administration of the mastic-myrrh aqueous extract. The mastic-myrrh aqueous extract provided in the application can be prepared into high-efficiency and low-toxicity treatment medicine for preventing and treating nerve pathological pain, has significant economic value and is worthy of application and popularization.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
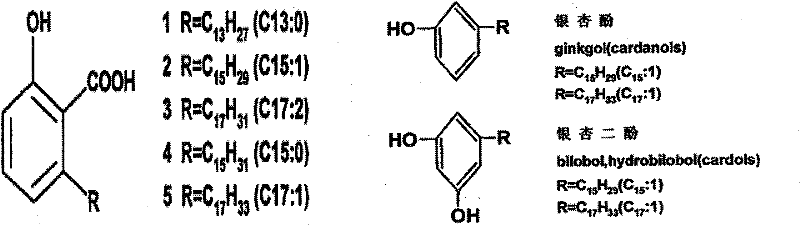
Application of ginkgolic acid in pain alleviation
InactiveCN102641309AGood inhibitory effectRaise pain thresholdNervous disorderAntipyreticSide effectDisinfectant
The invention relates to application of ginkgolic acid in pain alleviation. The ginkgolic acid comprises ginkgo acid, ginkgol, ginkgo diphenol and the like, and can be obtained from an extract of leaves, fruits, testa, rhizomes and the like of ginkgo by a chemical separation method. The pain comprises nerve pain, bone pain, muscle pain, tendon ligament pain, cancer pain and the like. A medicament, a medical instrument, a cosmetic or a disinfectant containing the ginkgolic acid and having a purpose of alleviating pain contains 0.001 to 80 weight percent of ginkgolic acid and pharmaceutically common external preparation matrix or acceptable carrier; and the preparation is any one of pharmaceutical external formulations. Pharmacological experimental research shows that the ginkgolic acid has an obvious effect of improving pain threshold on pain response caused by physical, chemical and cancerous stimulus. Clinical trials show that the ginkgolic acid has a remarkable alleviating effect on various pains and is predominant in curative effect, the effective rate reaches over 95 percent, and the ginkgolic acid is safe in use and does not have side effect.
Owner:王青
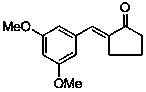
Application of (E)-2-(3,5-dimethoxybenzylidene)-cyclopentanone in preparing medicines used for treating pain diseases
ActiveCN103385866AObvious analgesic effectReduce pain responseOrganic active ingredientsNervous disorderThermal stimulationNeuralgia
The invention discloses an application of (E)-2-(3,5-dimethoxybenzylidene)-cyclopentanone in preparing medicines used for treating pain diseases. Related studies show that the compound has a significant abirritation, and can effectively reduce pain reactions caused by thermal stimulation and chemical stimulation. The compound can be used for preparing medicines used for relieving acute pains, headache, toothache, neuralgia, tumor-type pain, and pains caused by inflammatory diseases of muscles and bone joints, wherein the acute pains are caused by severe injury, burns, etc., and the pains caused by the inflammatory diseases of the muscles and the bone joints comprise rheumatic and rheumatoid arthritis pains, dysmenorrheal and other inflammatory pains, and neuropathic pains.
Owner:SUZHOU UNIV
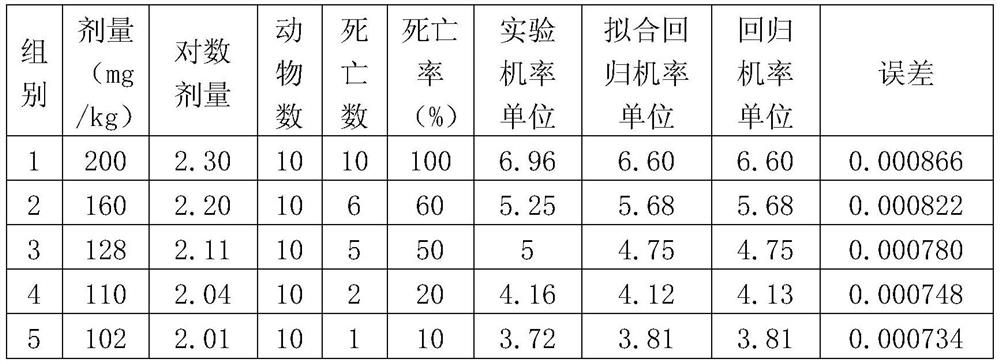
Method and device for evoking temporal pain summation
InactiveUS20120323138A1Effective treatmentDiagnostics using vibrationsDiagnostics using pressureThermal stimulationControl signal
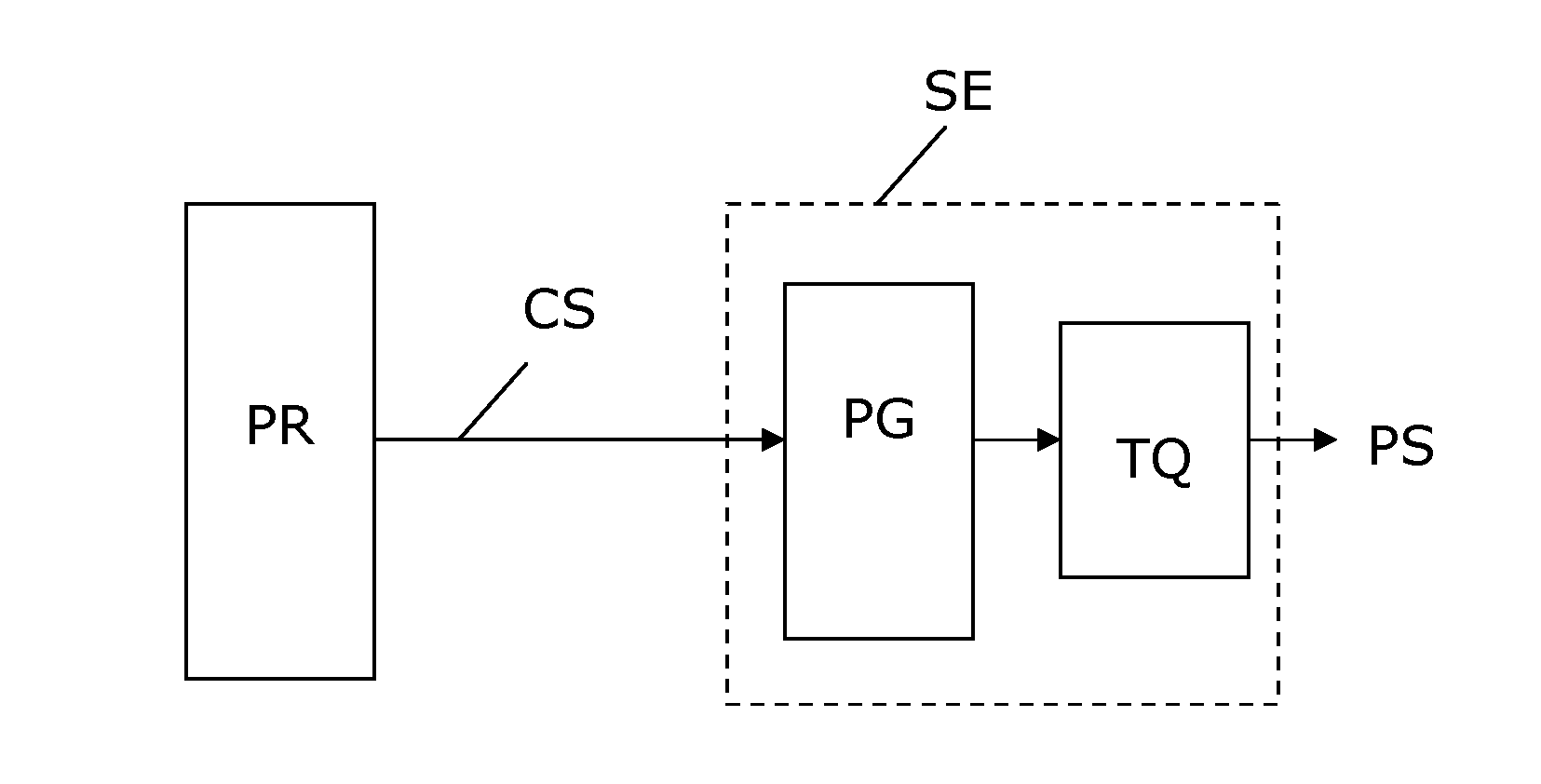
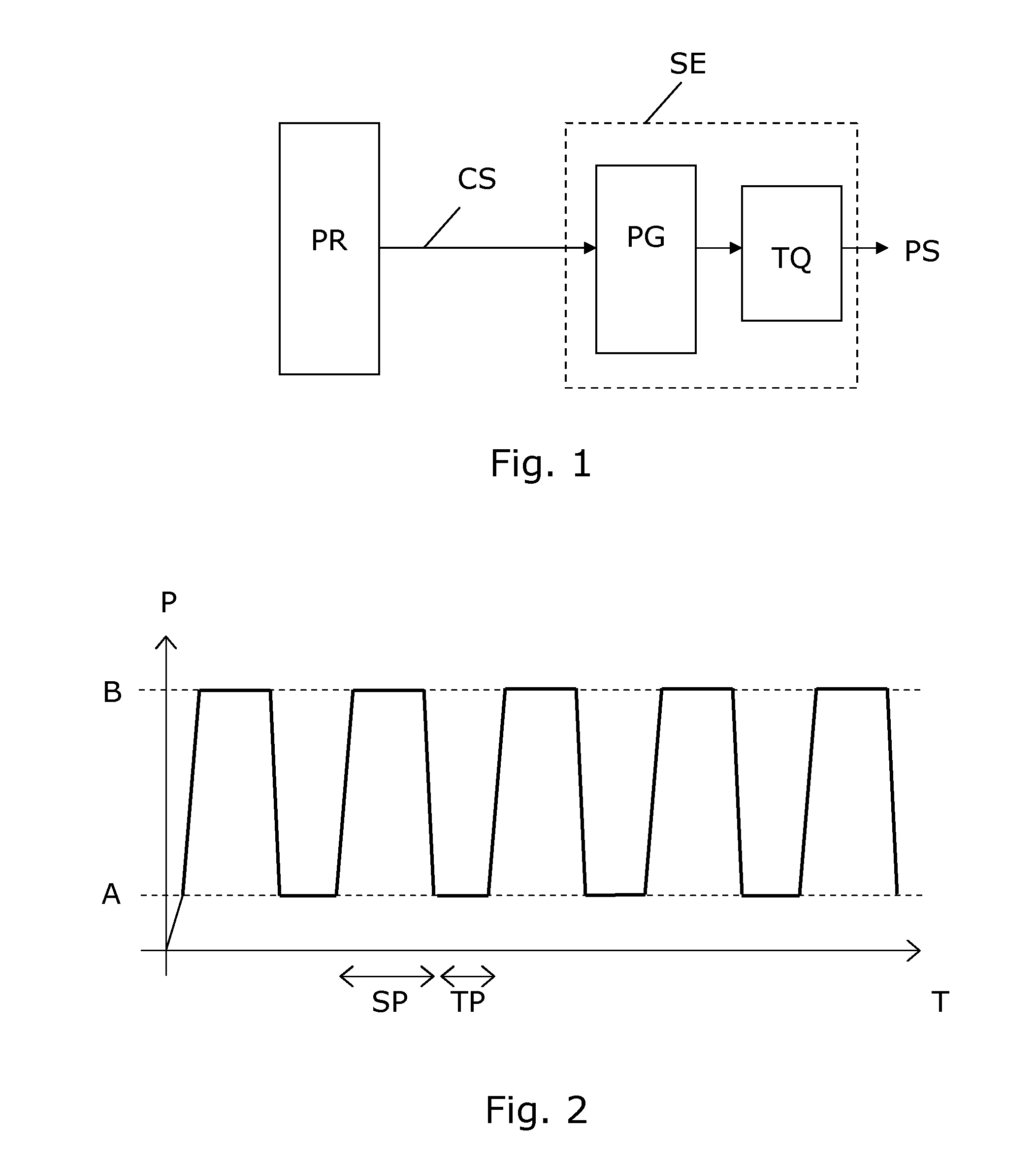
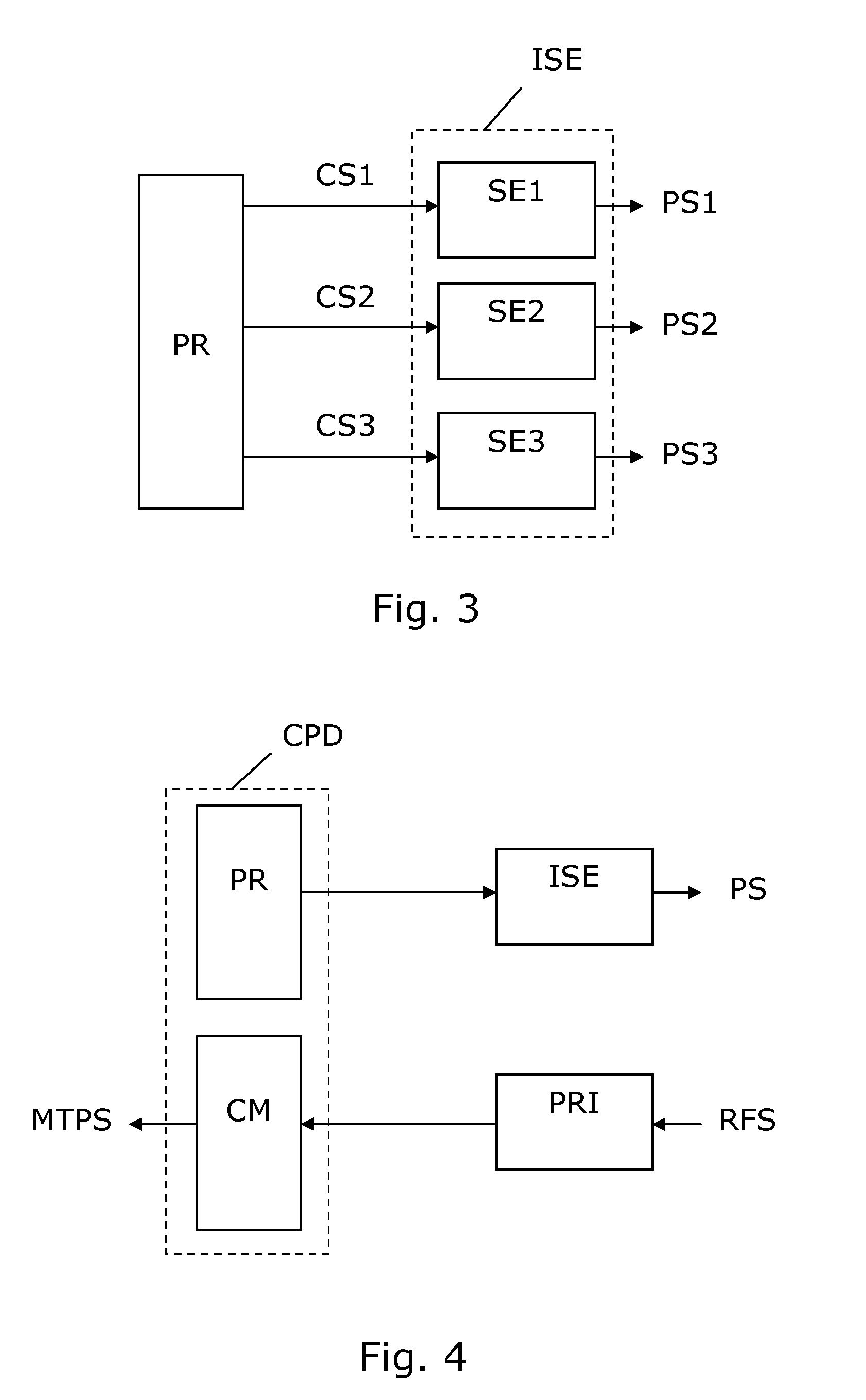
A device for evoking a temporal summation of pain in a subject. The device comprises a stimulation element (SE) arranged generating a physical stimulation on a skin surface area of at least 20 cm2 according to a control signal (CS), where the stimulation (PS) evokes pain in the subject. A processor (PR) generates the control signal (CS) to the stimulation element (SE) so as to provide a repetitive stimulation pattern comprising at least repetitions of: a)applying stimulation for a period (SP) of 0.5-120 seconds, b)stopping stimulation for a period (TP) of 0.5-20 seconds. Preferred periods are 1-3 seconds for both stimulation and intermediate stopping periods. The stimulation element may include an inflatable tourniquet arranged for providing a compressional stimulation of an arm or a leg. However, other types of stimulation elements may be used such as electric, heating, mechanical stimulation or the like may be used. The device is suited for providing a measure of temporal summation in a subject with different intensities, and this measure can be used for determining if the subject suffers from central nervous sensitization which is an important diagnosis with respect to effective analgesic treatment. In some embodiments, the device is arranged to collect pain responses during the stimulation and to calculate a measure of temporal summation of the subject accordingly, e.g. in the form of a rate of pain change (VAS) versus time (T).
Owner:NOCITECH APS
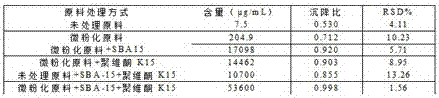
Progesterone suspending injection and preparation method thereof
InactiveCN107441039AGood dispersionGood suspensionOrganic active ingredientsSolution deliveryCrystal structureProgesterones
The invention relates to progesterone suspending injection and a preparation method thereof. The progesterone suspending injection comprises progesterone, SBA-15, povidone K15 and injection water. According to the invention, under the effect of physical mechanical force, the well-organized crystal structure of the progesterone is damaged but is not suffered from chemical degradation and SBA-15 and progesterone molecule are interacted through hydrogen bond, so that the amorphous progesterone molecule is loaded onto a nanometer structure duct, can be attached to the SBA-15 loading a large quantity of drug molecules and can be maintained under amorphous state; the amorphous progesterone molecule reacts with the povidone K15 through the hydrogen bond, so that the amorphous state is more stable and a certain suspending assisting function is achieved; the technical problems that the progesterone is difficult to dissolve in water, the oil-soluble progesterone injection brings a strong injection pain response and a conventional solubilizing method is not suitable for preparing the progesterone into the injection can be solved; and the progesterone is developed into the injection with stable nature and without pain response in the injection process.
Owner:南京斯泰尔医药科技有限公司
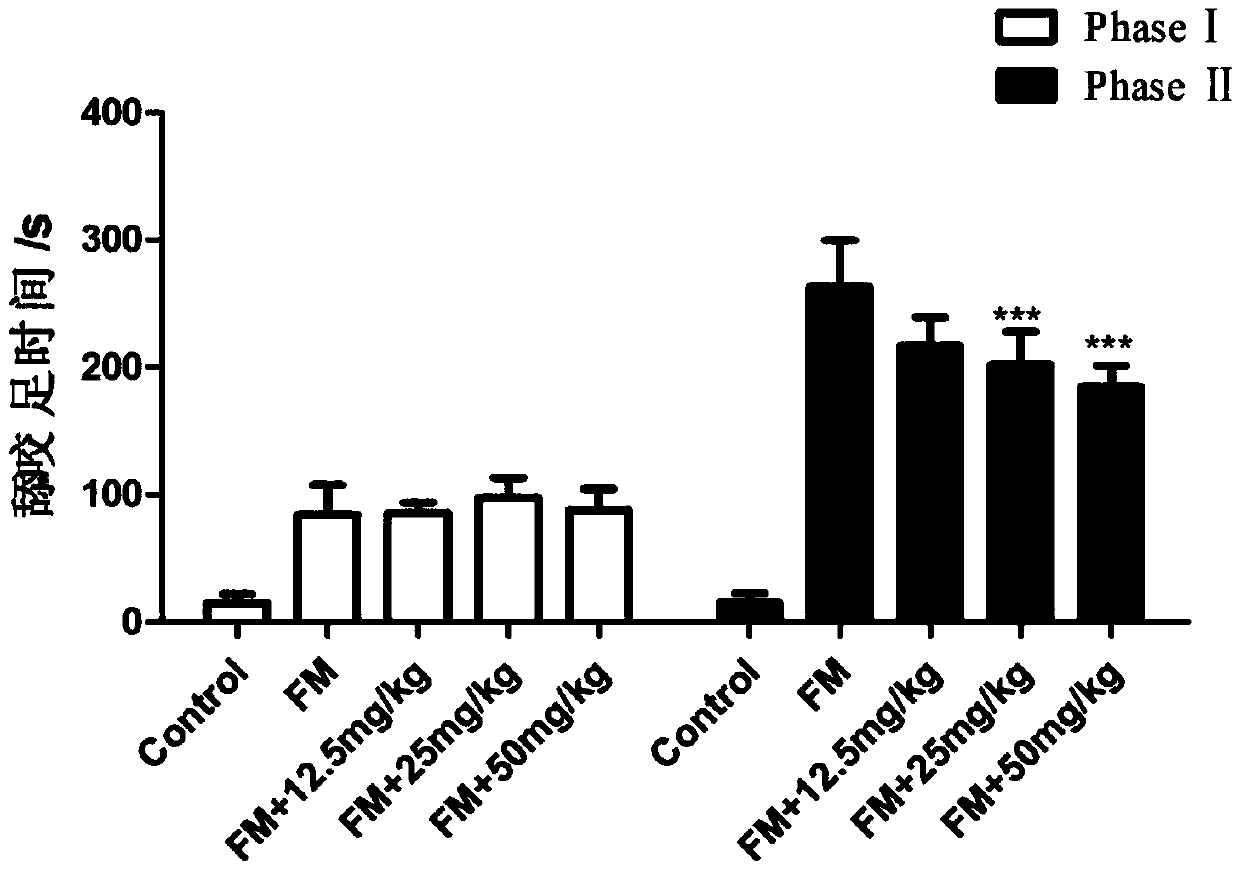
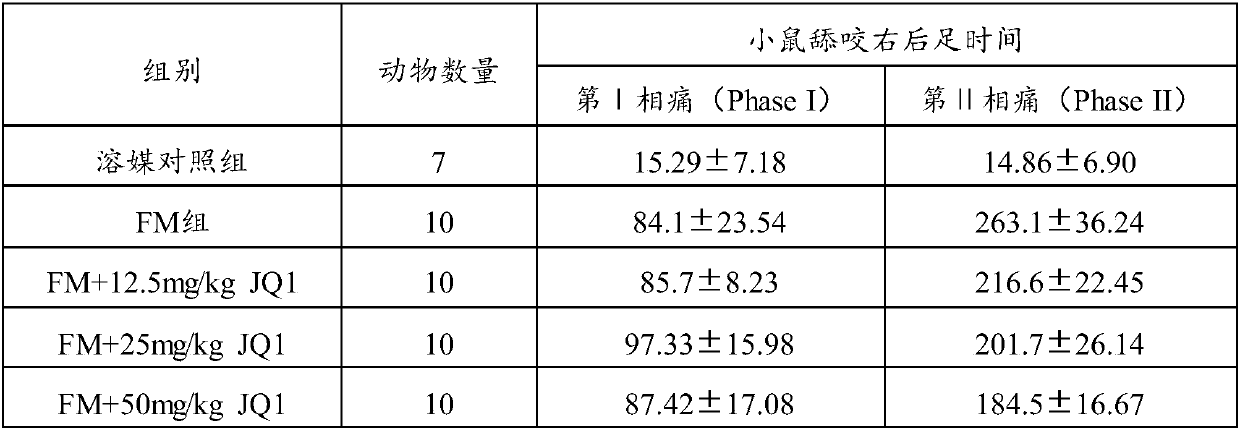
Application of JQ1 or derivative of JQ1 in preparation of analgesic drugs
InactiveCN109568324AGood analgesic effectRelieve acute inflammatory painOrganic active ingredientsAntipyreticAnalgesics drugsBromodomain
The invention relates to the technical field of medicines, in particular to application of JQ1 or a derivative of the JQ1 in preparation of analgesic drugs. A test result shows that the BRD4 (bromodomain-containing protein 4) inhibitor JQ1 can effectively relieve acute inflammatory pain induced by formalin, and the expression lies in that the duration time of the pain response of mice is obviouslyshortened after JQ1 pretreatment. Experiments prove that the bromodomain-containing protein 4 inhibitor JQ1 has an obvious analgesic effect and can be used for preparation of the analgesic drugs.
Owner:NANHUA UNIV
Bone-setting traditional Chinese medicine decoction and preparation method thereof
InactiveCN103393895BPromote circulationHeal fastAntipyreticInorganic active ingredientsMyrrhAinsliaea
The invention discloses a bone-setting traditional Chinese medicine decoction and a preparation method thereof. The decoction comprises the following materials by weight:10-12 parts of Dragon's blood, 8-10 parts of natural copper, 4-6 parts of pseudo-ginseng, 10-12 parts of Rhizoma Drynariae, 4-6 parts of radix angelicae, 6-8 parts of angelica, 3-5 parts of frankincense, 4-6 parts of myrrh, 1-2 parts of red ginseng, 2-3 parts of safflower, 10-12 parts of Speranskia tuberculata, 2-3 parts of Ligusticum wallichii, 8-10 parts of melon seeds, 4-6 parts of Selaginella delicatula, 5-8 parts of Ainsliaea, 9-11 parts of Primula szechuanica, 6-9 parts of teasel root, 2-3 parts of epimedium, 5-8 parts of shrimp shell and 10-12 parts of sheep bone. The traditional Chinese medicine provided by the invention can significantly improve blood circulation, promote rapid healing of bone fracture, quickly repair bone fracture damage, shorten healing time and rapidly ease the pain response in the healing process, and has the advantages of short course of treatment, good curative effect and convenient usage; therefore, the decoction can fast and effectively treat bone fracture, sprain and contusion, etc.
Owner:王文轩
Application of melatonin to preparation of medicine for treating autoimmune prostatitis and medicine for treating autoimmune prostatitis
PendingCN112891344AEffective treatmentReduce inflammatory changesOrganic active ingredientsAntipyreticPharmaceutical drugAutoimmunity
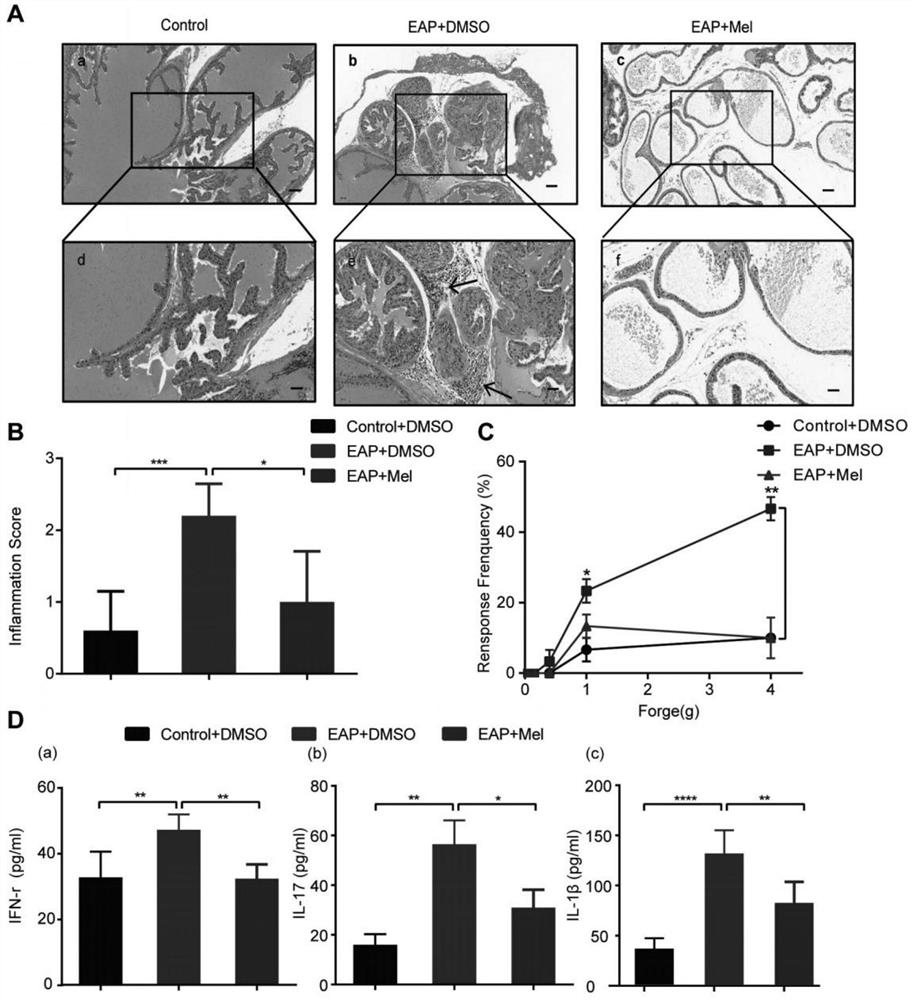
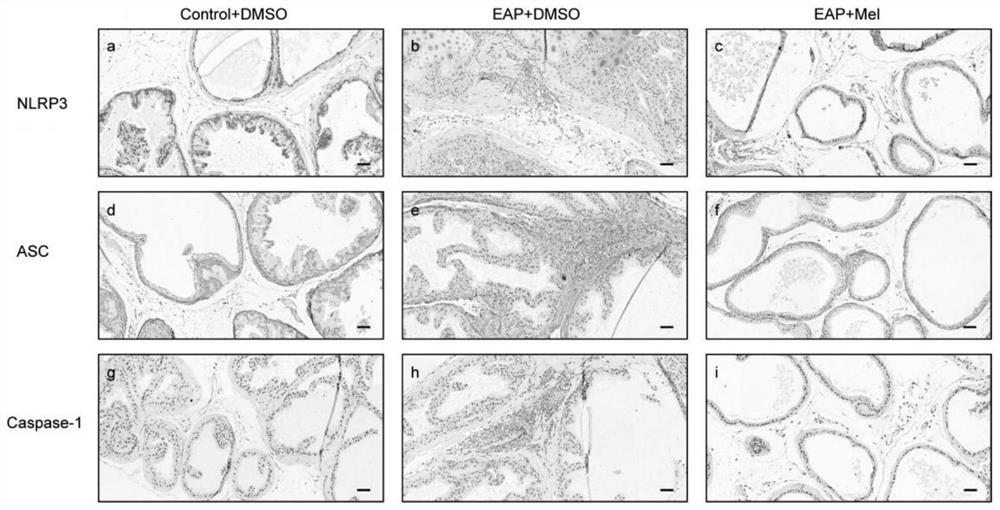
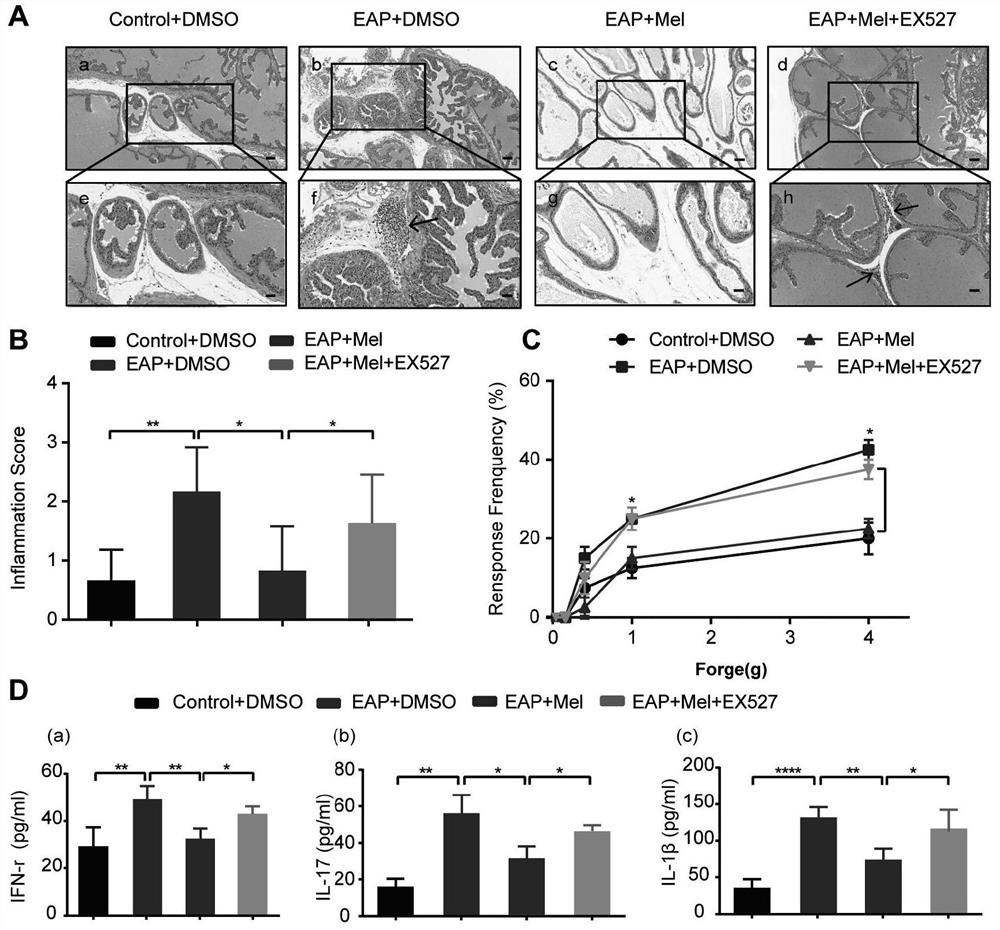
The invention provides application of melatonin to preparation of a medicine for treating autoimmune prostatitis and the medicine for treating the autoimmune prostatitis, and belongs to the technical field of medicine application. According to the invention, by enhancing a Sirt1 pathway and inhibiting the NLRP3 inflammasome, the inflammatory change and pain response of prostate tissue and the content of inflammatory cytokines in peripheral blood are reduced. According to the invention, an autoimmune prostatitis mouse model is taken as a test object, and research finds that the melatonin obviously reduces the inflammatory change and pain response of the prostate tissue and the content of inflammatory cytokines in the peripheral blood by enhancing the Sirt1 pathway and inhibiting the NLRP3 inflammasome, namely that the melatonin inhibits the expression of the NLRP3 inflammasome through Sirt1 dependency to reduce prostatitis, and the melatonin is feasible and effective for treating prostatitis.
Owner:THE FIRST AFFILIATED HOSPITAL OF ANHUI MEDICAL UNIV
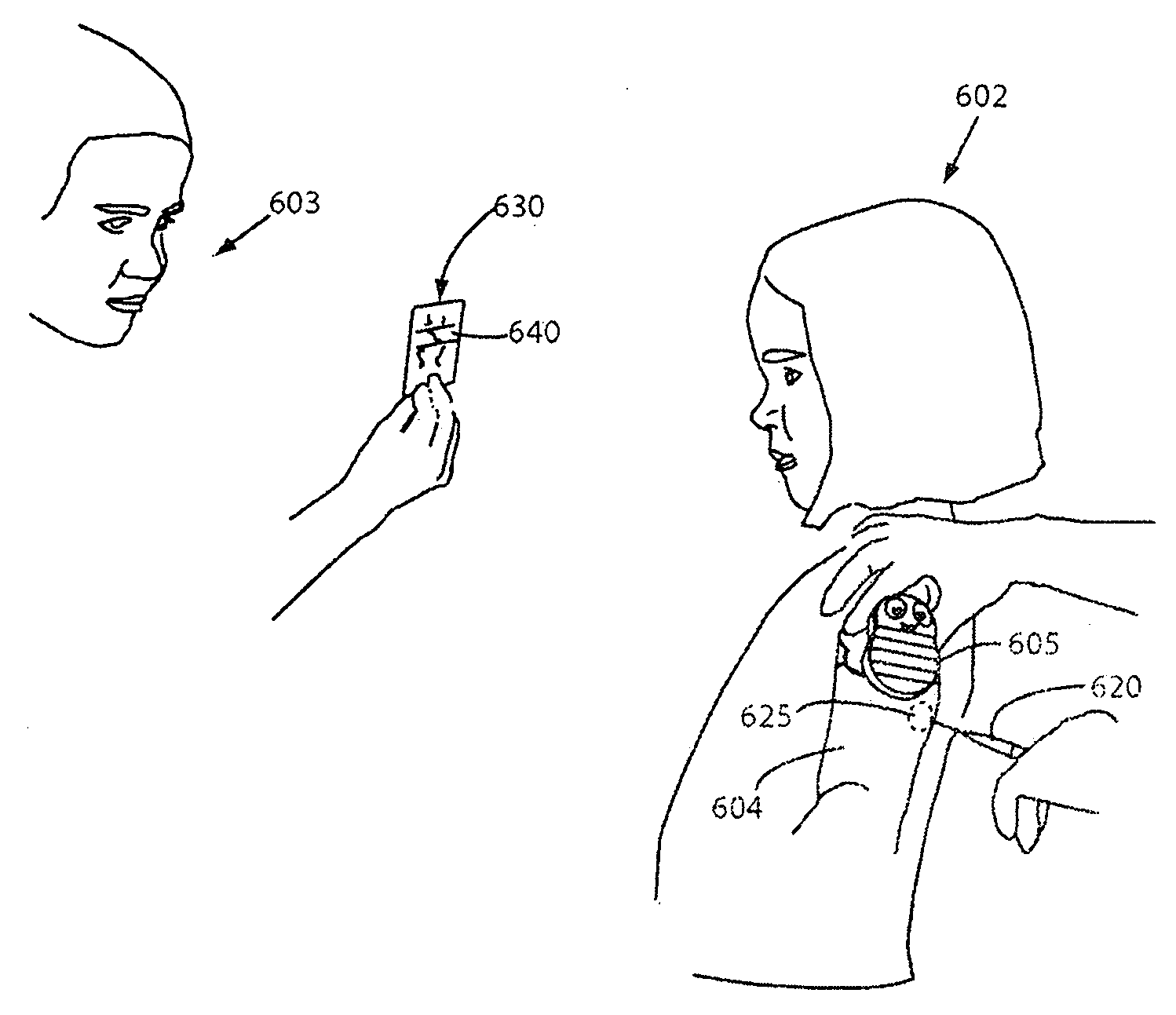
System for use during discography to wirelessly transmit data from hand-held fluid delivery device inside sterile field to device outside sterile field
ActiveUS20110245760A1Easy to operateContinuous monitoringMedical devicesPressure infusionIntervertebral discBlood pressure
A fluid delivery device is provided for delivering fluid to a target site such as an intervertebral disc during discography. The fluid delivery device includes pressure and volume sensors to determine the pressure and the volume of the fluid delivered to the intervertebral disc. The fluid delivery device is hand-held during use and includes a syringe assembly having a plunger with threads that enables controlled discharge of the fluid from the fluid delivery device by rotating the plunger in a housing of the fluid delivery device. The fluid delivery device includes a communication module to wirelessly transfer data to an external device outside of a sterile field, while the fluid delivery device is in the sterile field. The fluid delivery device may also include a physiological sensor to monitor involuntary pain responses based on changing physiological parameters such as increases in body temperature or blood pressure.
Owner:STRYKER CORP
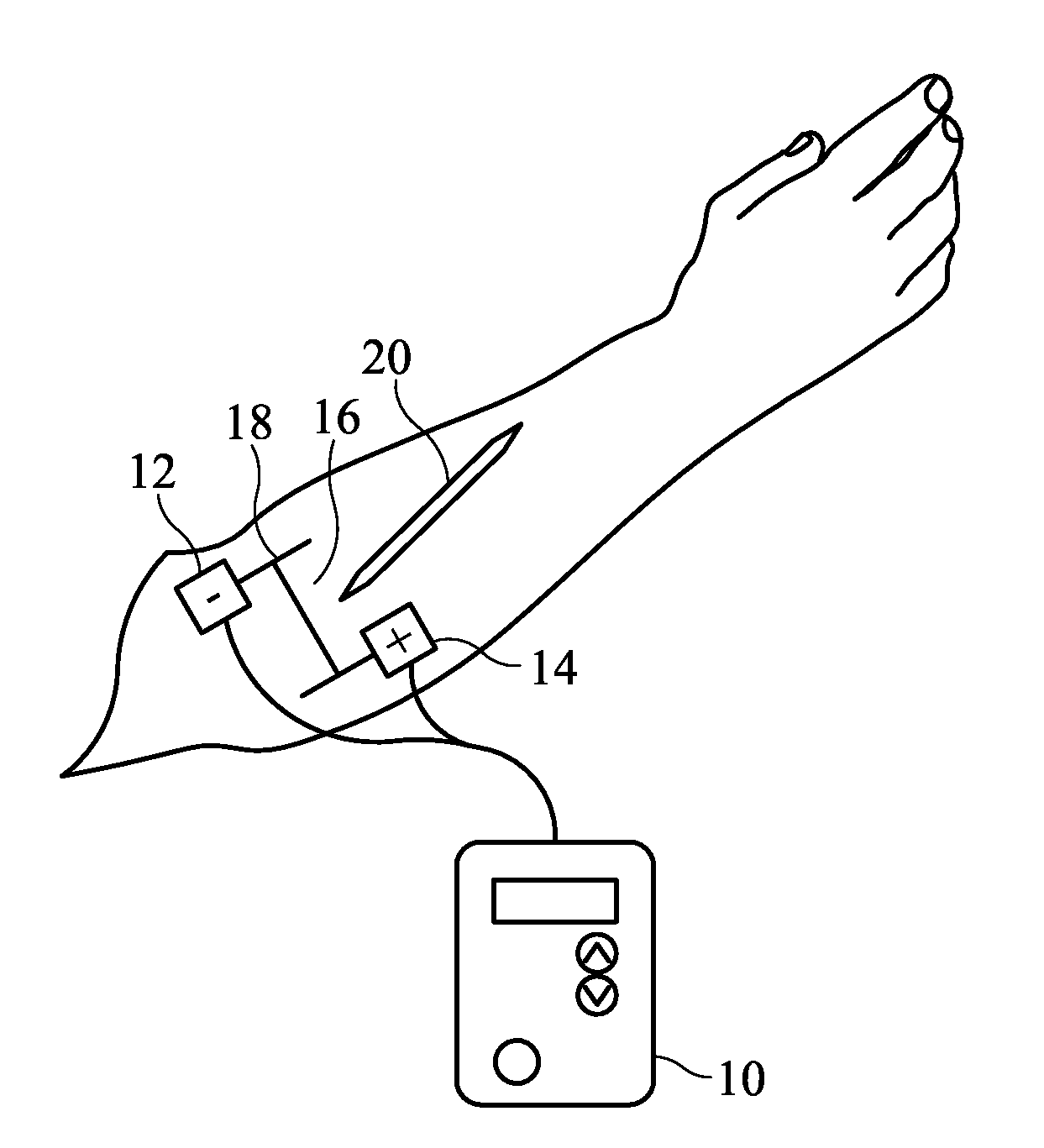
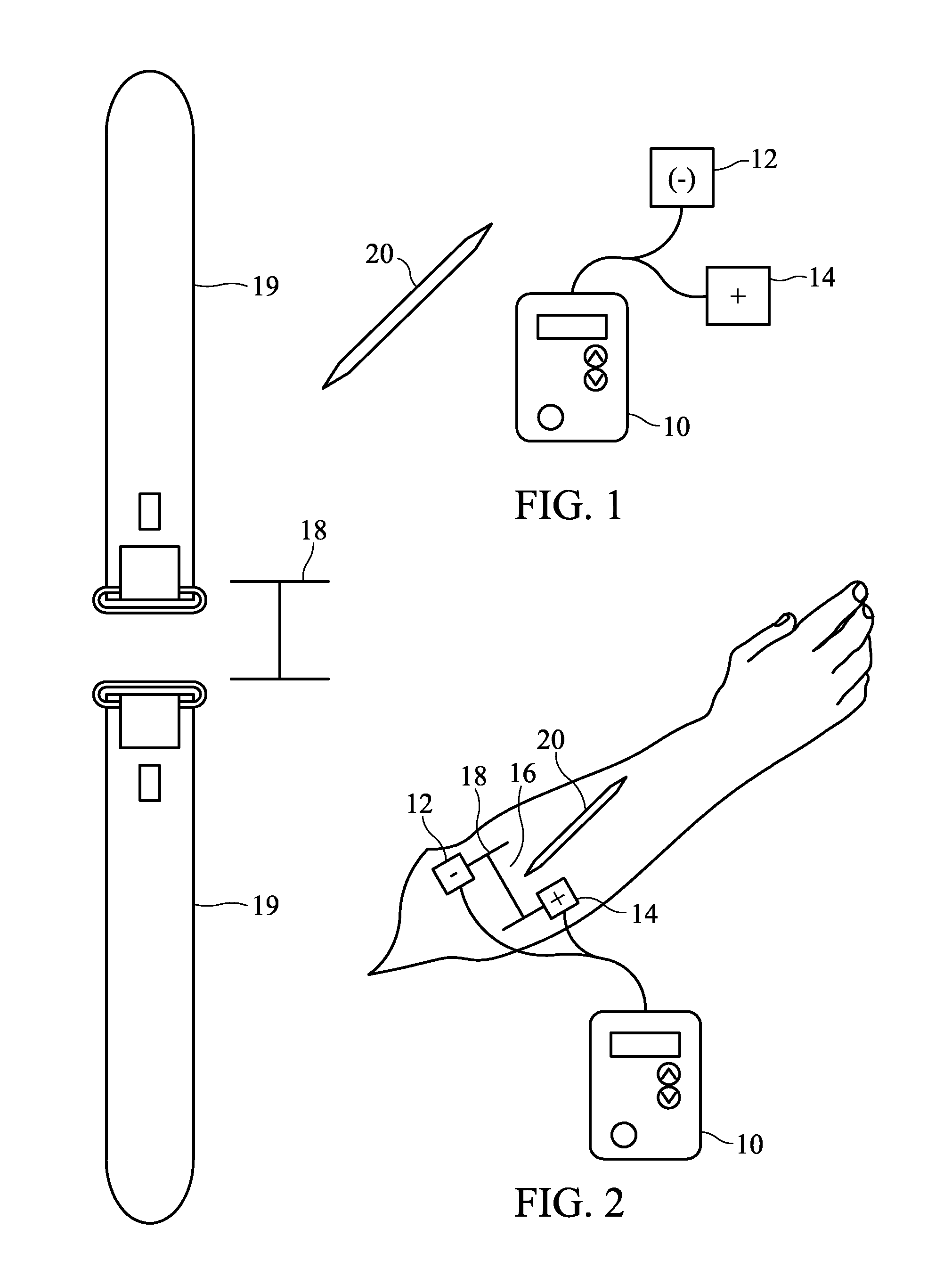
System and Method for Pain-Free Injections
InactiveUS20120209246A1Cost- and performance-effectiveElectrotherapyMedical devicesInjection siteEngineering
A system for administering pain-free injections includes an electric current source having at least two electrodes attached adjacent an injection site, the electric current source producing a variable numbness pattern and providing a frame of reference for identifying a plurality of sub-areas of the injection site; and a probe applied to the plurality of sub-areas of the injection site for pain-response testing, wherein one or more areas of numbness in the variable numbness pattern are identifiable according to the plurality of sub-areas of the injection site. A fence may be removably applied to the injection site and provide a frame of reference for identifying a plurality of sub-areas of the injection site, and may aid in identifying one or more areas of numbness in the variable numbness pattern. The fence may be removably applied to the injection site via a belt or manual pressure.
Owner:PERKINS WALTER T
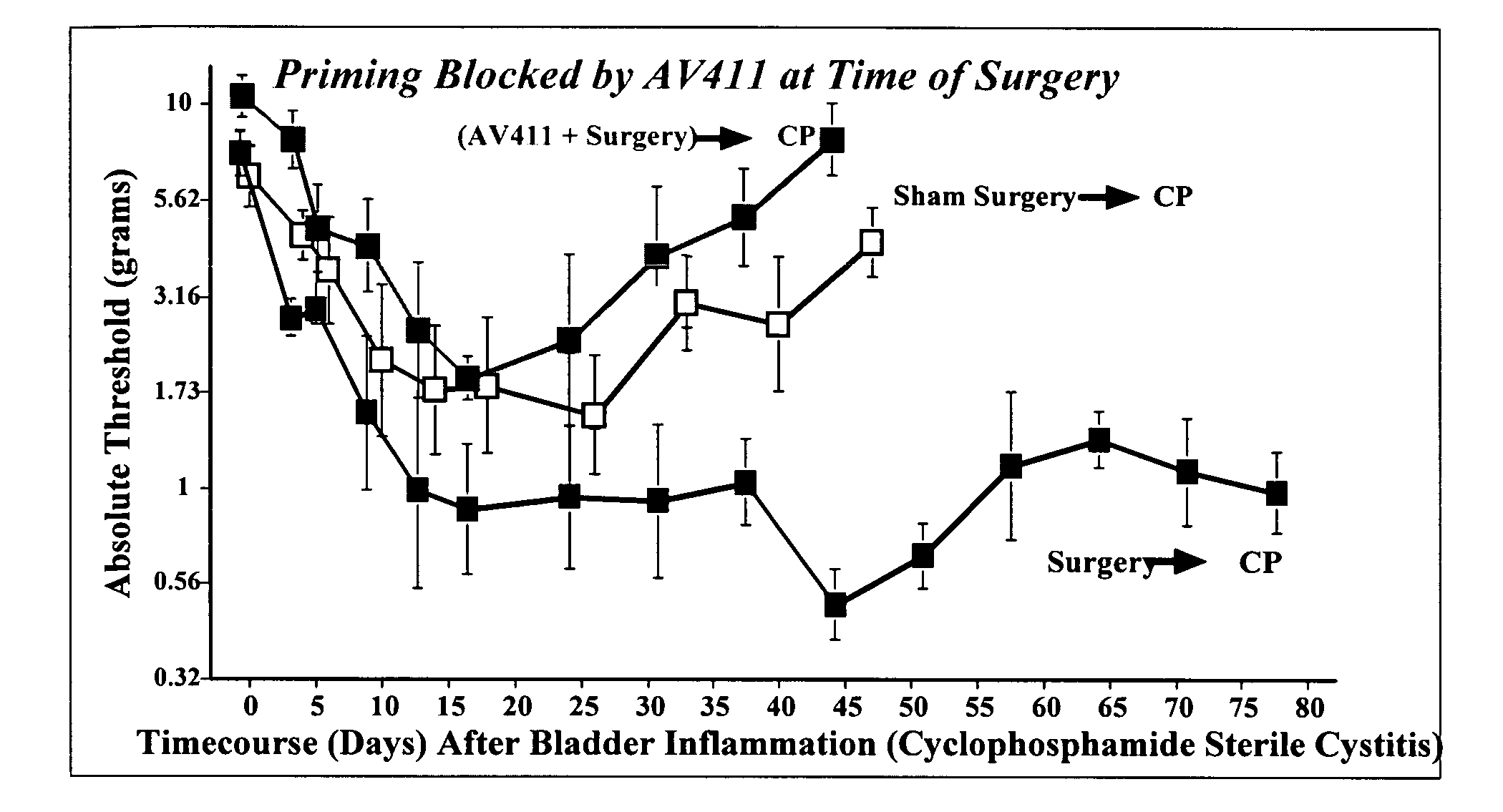
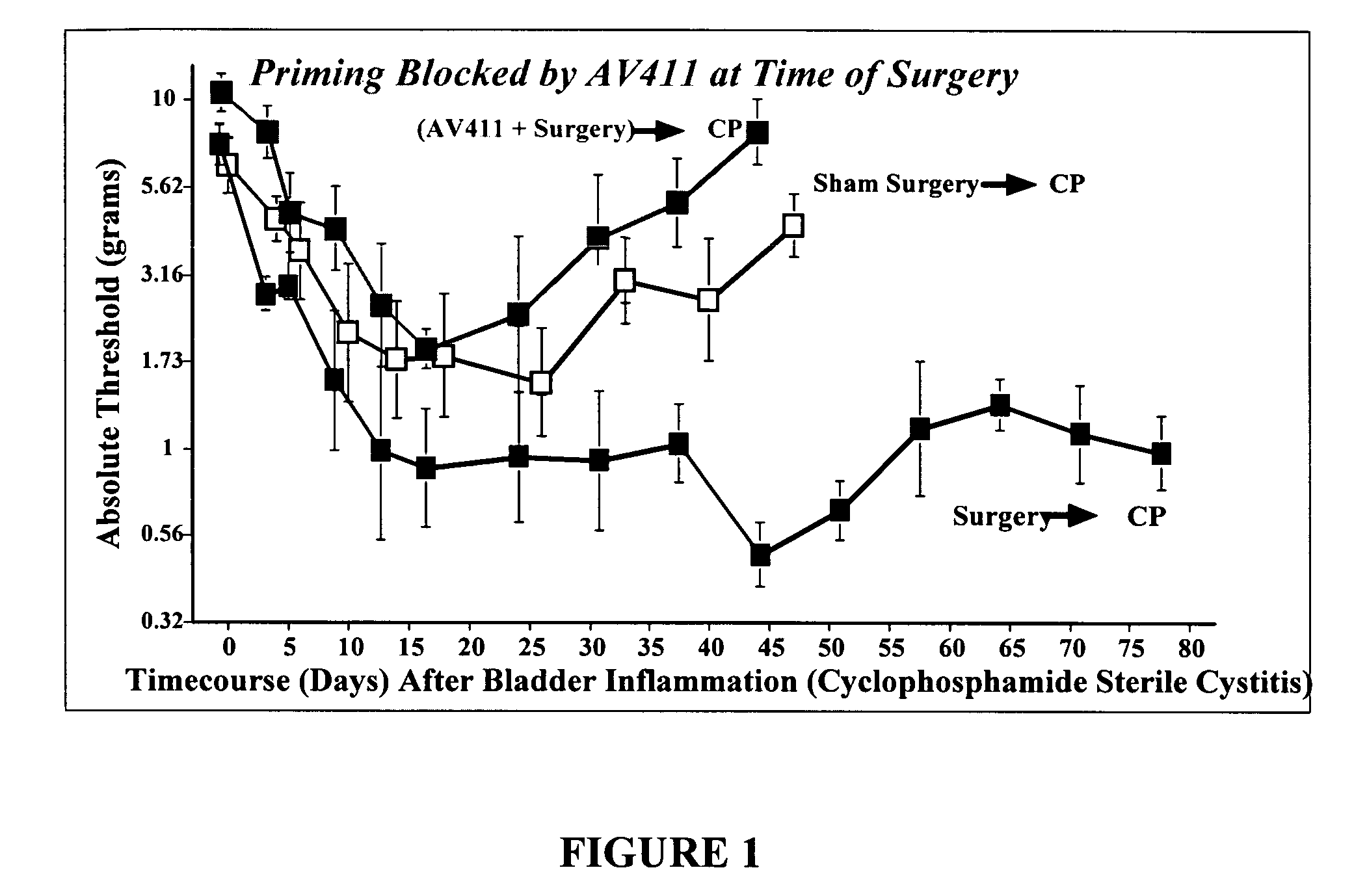
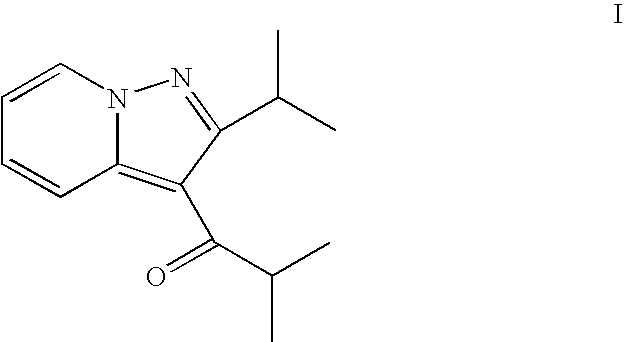
Use of a glial attenuator to prevent amplified pain responses caused by glial priming
InactiveUS20080287402A1Preventing and diminishing painBiocideNervous disorderInflammationPain responses
The use of a glial attenuator, such as ibudilast (3-isobutyryl-2-isopropylpyrazolo[1,5-a]pyridine), to prevent the negative consequences of glial priming is described. In particular, the present invention is directed to a method of treating a subject with ibudilast to prevent amplified pain responses to inflammation or injury as a result of glial priming following an initial glial activating event.
Owner:MEDICINOVA INC
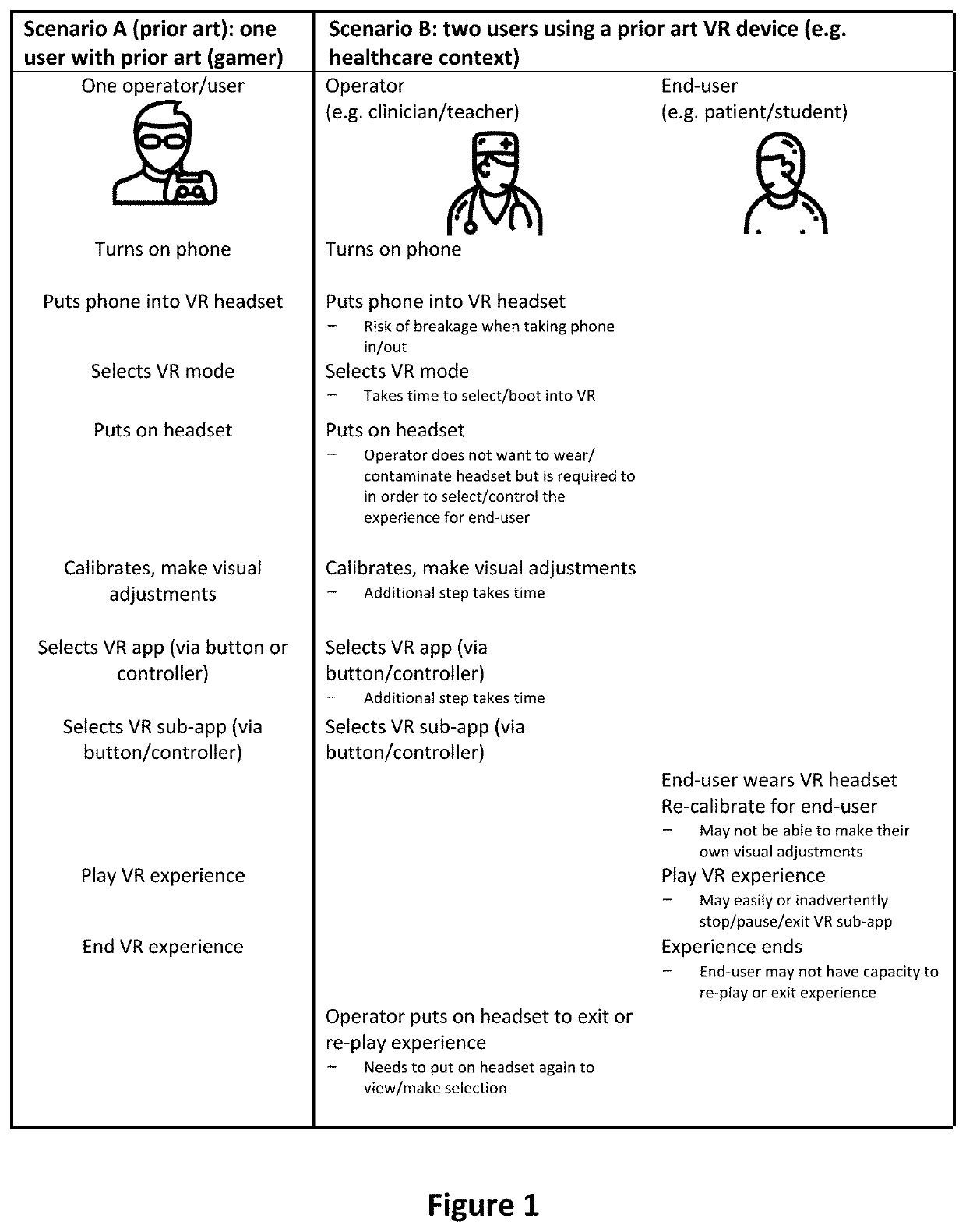
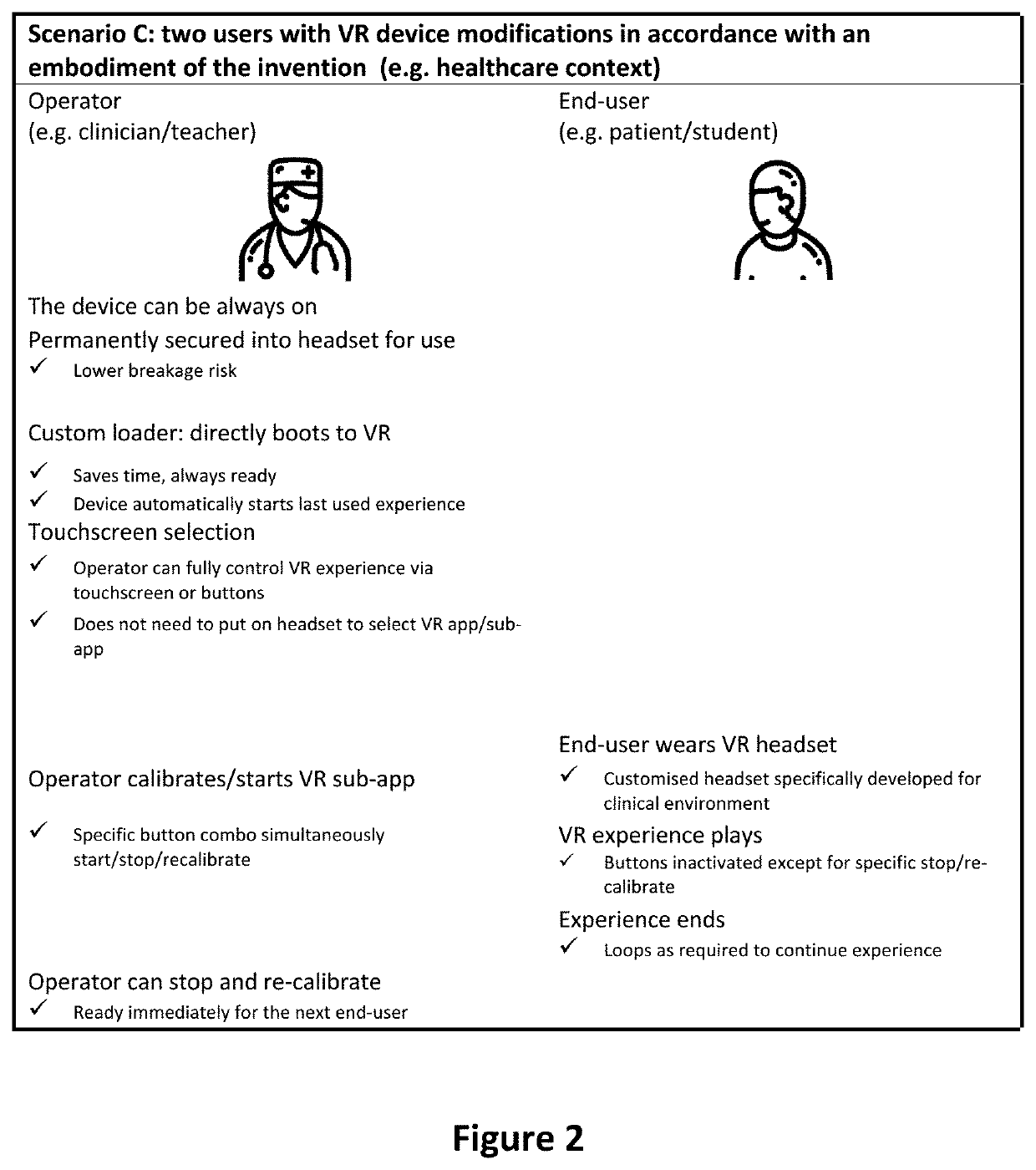
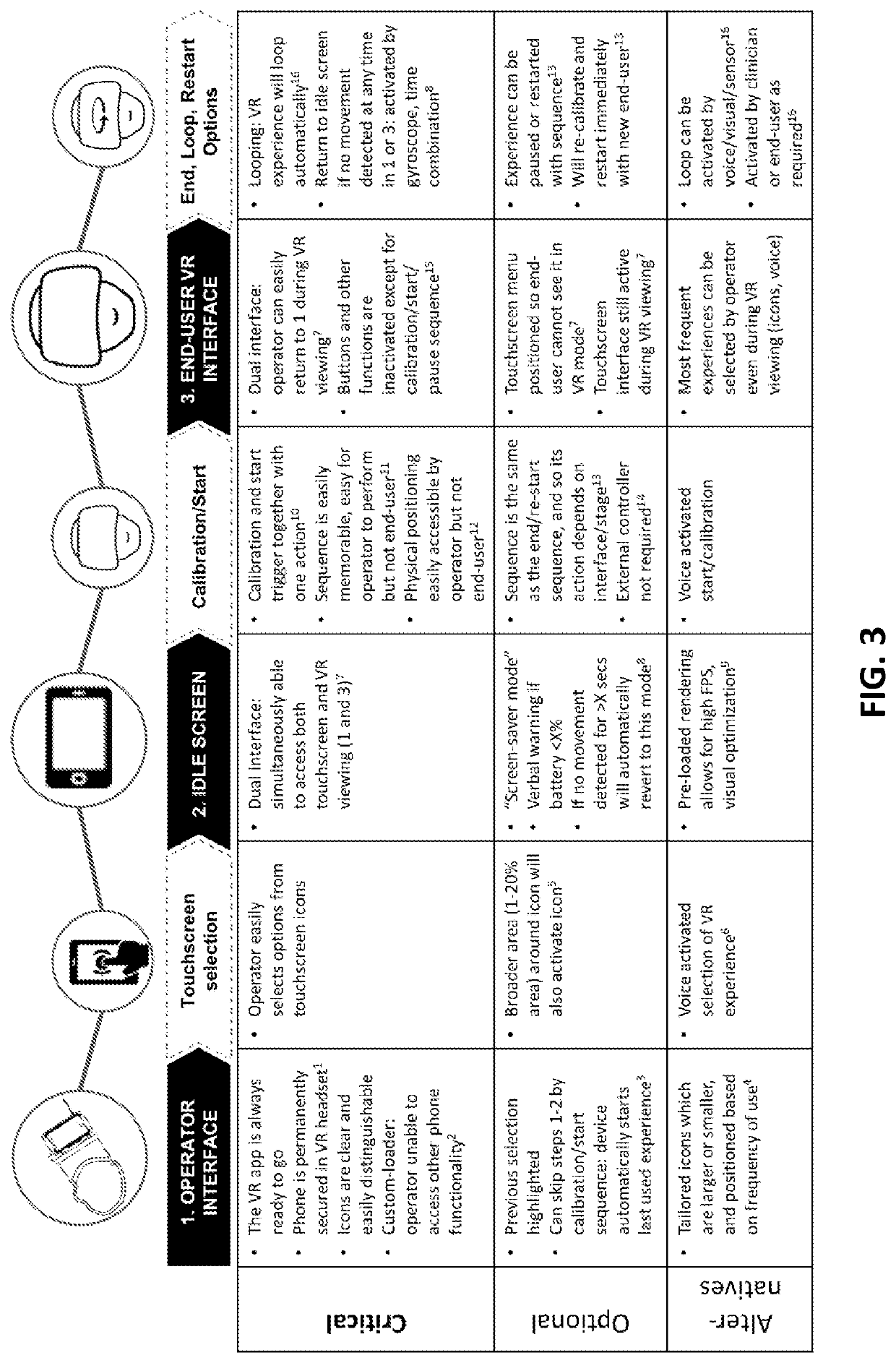
Virtual reality apparatus
PendingUS20220164025A1Input/output for user-computer interactionMedical devicesReoperative surgeryAnxiety
Provided is a virtual reality (VR) device, system and framework for generating VR continuum experience choreographed to a physical procedure incorporating at least one procedural action associated with a physical sensation and potentially inducing an anxiety or pain response. The VR continuum experience can modify perceptions of pain and anxiety associated with the procedure. The virtual reality device is configured to allow device control via a device user interface accessible to an operator other than the wearer (i.e. a medical practitioner), to allow the operator to control device calibration and virtual reality (VR) experience start while the apparatus is worn by the wearer, and to provide one or more VR experiences each associated with a physical procedure.
Owner:SMILEYSCOPE PTY LTD
Devices and methods for pain management
InactiveUS20080161356A1Efficient managementAdequate pain reliefBiocideNervous disorderWhole bodyPain management
The invention features devices and methods for the systemic delivery of fentanyl or a fentanyl congener (e.g., sufentanil) to treat pain. In the present invention, a drug formulation comprising fentanyl or a fentanyl congener is stored within a drug delivery device (e.g., contained in a reservoir or impregnated within a matrix within the controlled drug delivery device). The drug formulation comprises an amount of drug sufficient for treatment and is stable at body temperatures (i.e., no unacceptable degradation) for the entire pre-selected treatment period. The drug delivery devices store the drug formulation safely (e.g., without dose dumping), provide sufficient protection from bodily processes to prevent unacceptable degradation of the formulation, and release the drug formulation in a controlled fashion at a therapeutically effective rate to treat pain. In use, the drug delivery device is implanted in the subject's body at an implantation site, and the drug formulation is released from the drug delivery device to a delivery site. The delivery site may be the same as, near, or distant from the implantation site. Once released at the delivery site, the drug formulation enters the systemic circulation and is transported to the site of action in the body to modulate the pain response (e.g., the brain or other pain sensory location).
Owner:JOHNSON RANDOLPH MELLUS +1
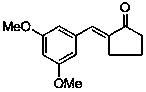
Application of (E)-2-(3,5-dimethoxybenzylidene)-cyclopentanone in preparing medicines used for treating pain diseases
ActiveCN103385866BObvious analgesic effectReduce pain responseOrganic active ingredientsNervous disorderThermal stimulationNeuralgia
The invention discloses an application of (E)-2-(3,5-dimethoxybenzylidene)-cyclopentanone in preparing medicines used for treating pain diseases. Related studies show that the compound has a significant abirritation, and can effectively reduce pain reactions caused by thermal stimulation and chemical stimulation. The compound can be used for preparing medicines used for relieving acute pains, headache, toothache, neuralgia, tumor-type pain, and pains caused by inflammatory diseases of muscles and bone joints, wherein the acute pains are caused by severe injury, burns, etc., and the pains caused by the inflammatory diseases of the muscles and the bone joints comprise rheumatic and rheumatoid arthritis pains, dysmenorrheal and other inflammatory pains, and neuropathic pains.
Owner:SUZHOU UNIV
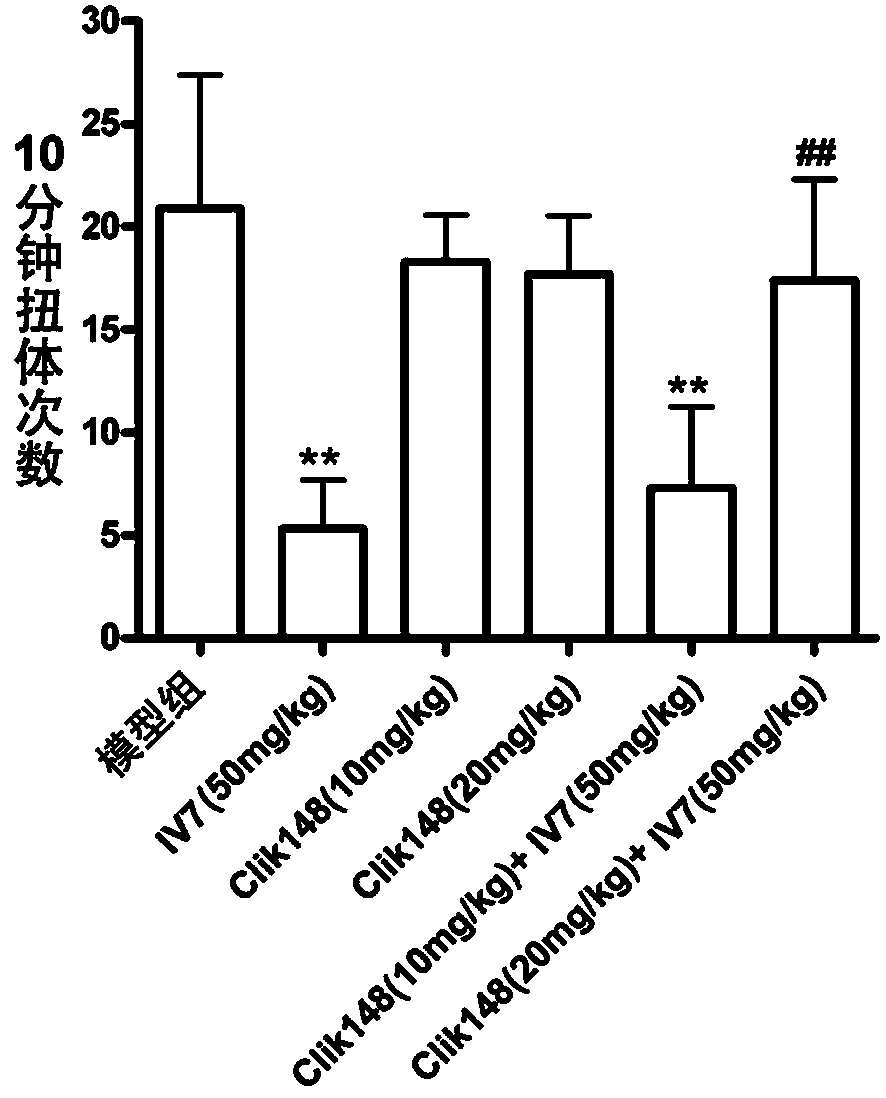
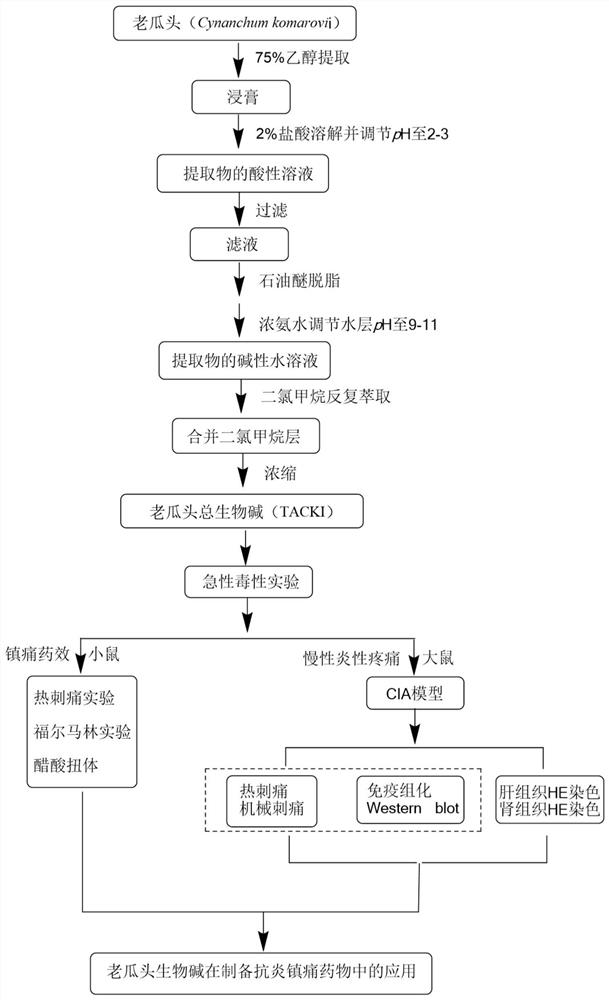
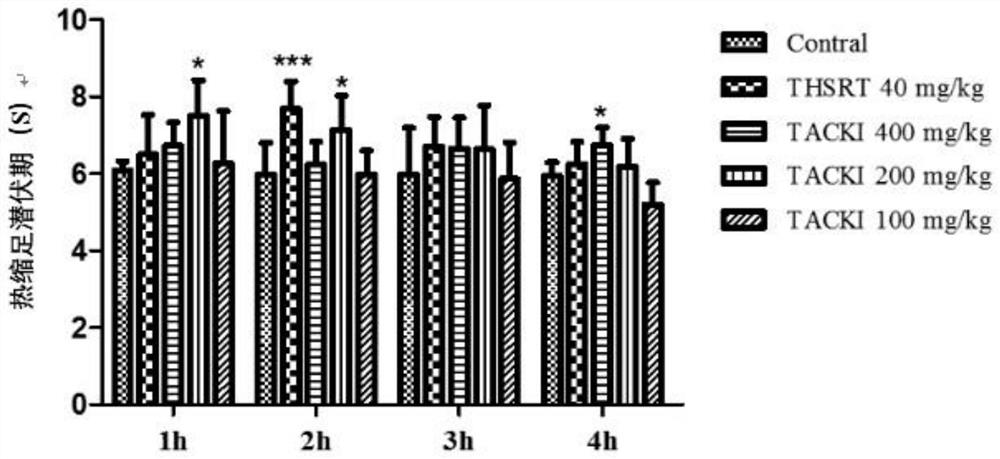
Application of total alkaloids of cynanchum komarovii in preparation of anti-inflammatory and analgesic drugs
PendingCN113476491AImprove pain responseInhibition releaseAntipyreticAnalgesicsInflammatory factorsAcetic acid
The invention discloses application of total alkaloids of cynanchum komarovii in preparation of anti-inflammatory and analgesic drugs. The research finds that the total alkaloids of cynanchum komarovii can prolong the thermal withdrawal latency period of a mouse in a thermal stabbing pain experiment, shorten the paw licking time of the mouse in a formalin pain experiment; and prolong the pain latency period of the mouse in an acetic acid writhing experiment, and reduce the writhing frequency, thereby effectively alleviating the pain response of the mouse. In addition, the total alkaloids of cynanchum komarovii can prolong the thermal withdrawal latency period and increase the mechanical withdrawal threshold of CIA rats, which indicates that the total alkaloids of cynanchum komarovii also have a mitigative effect on chronic inflammatory pain. Meanwhile, the total alkaloids of cynanchum komarovii can remarkably inhibit expression of inflammatory factors such as GFAP, NMDAR2B, TNF-a, IL-6 and IL-17, so that the total alkaloids of cynanchum komarovii can be used as a main component of anti-inflammatory and analgesic drugs, thus providing a new way for research and development of new drugs.
Owner:NINGXIA MEDICAL UNIV
Application of lauromacrogol injection in preparation of medicine for treating spontaneous pneumothorax
InactiveCN114288240ANo pain responseOrganic active ingredientsPharmaceutical delivery mechanismPleural cavityPneumothorax
The invention relates to application of lauromacrogol injection in preparation of a medicine for treating spontaneous pneumothorax, and solves the problem that special groups cannot tolerate operations and general anesthesia in the prior art. According to the invention, the lauromacrogol injection with low recurrence rate is used as the drug for treating spontaneous pneumothorax by replacing operative treatment with drug treatment. A formula of the lauromacrogol injection comprises 1g of lauromacrogol, 5ml of ethanol (96%) and the balance of water for injection, wherein the total volume is 100ml. According to the pleural cavity adhesive, operation treatment is replaced by medicine treatment, after the lauromacrogol injection is injected into the pleural cavity, the pleura is stimulated to generate aseptic inflammation, coagulation, sclerosis, adhesion and closure, the painful reaction of the pleural cavity is not caused, and the pleural cavity adhesive is an ideal pleural cavity adhesive.
Owner:SHAANXI TIANYU PHARMA
Devices and methods for pain management
InactiveUS20080161357A1Efficient managementAdequate pain reliefBiocideNervous disorderWhole bodyPain management
The invention features devices and methods for the systemic delivery of fentanyl or a fentanyl congener (e.g., sufentanil) to treat pain. In the present invention, a drug formulation comprising fentanyl or a fentanyl congener is stored within a drug delivery device (e.g., contained in a reservoir or impregnated within a matrix within the controlled drug delivery device). The drug formulation comprises an amount of drug sufficient for treatment and is stable at body temperatures (i.e., no unacceptable degradation) for the entire pre-selected treatment period. The drug delivery devices store the drug formulation safely (e.g., without dose dumping), provide sufficient protection from bodily processes to prevent unacceptable degradation of the formulation, and release the drug formulation in a controlled fashion at a therapeutically effective rate to treat pain. In use, the drug delivery device is implanted in the subject's body at an implantation site, and the drug formulation is released from the drug delivery device to a delivery site. The delivery site may be the same as, near, or distant from the implantation site. Once released at the delivery site, the drug formulation enters the systemic circulation and is transported to the site of action in the body to modulate the pain response (e.g., the brain or other pain sensory location).
Owner:JOHNSON RANDOLPH MELLUS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com