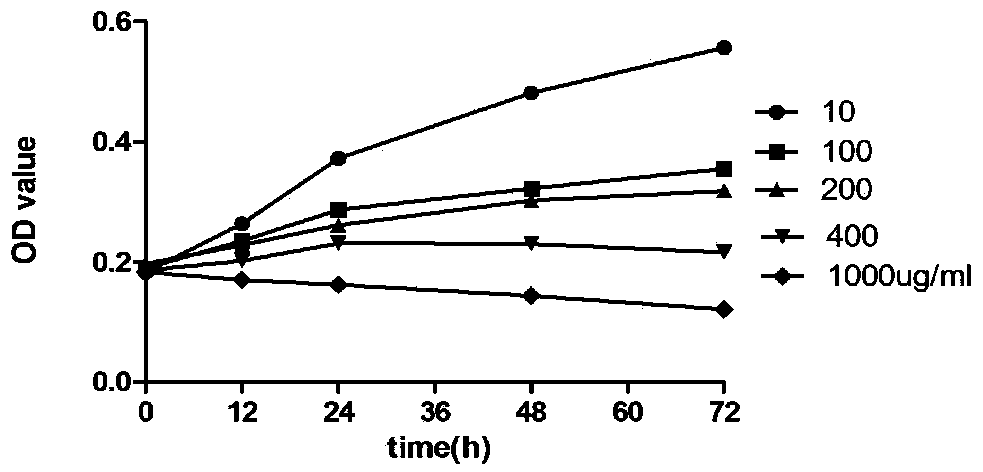
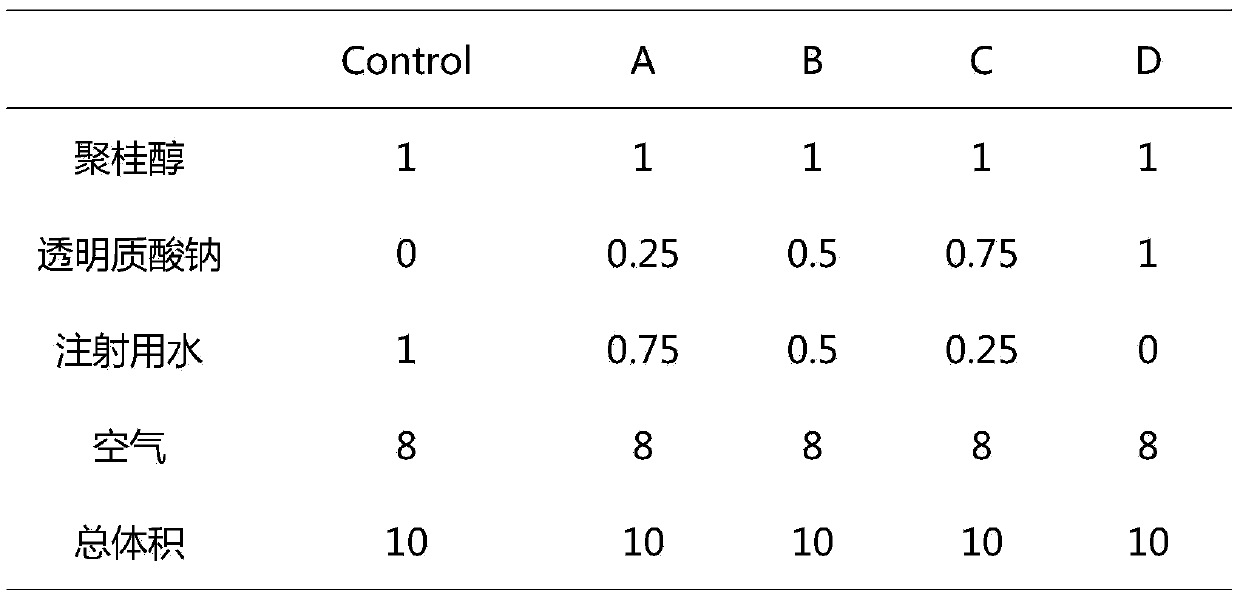
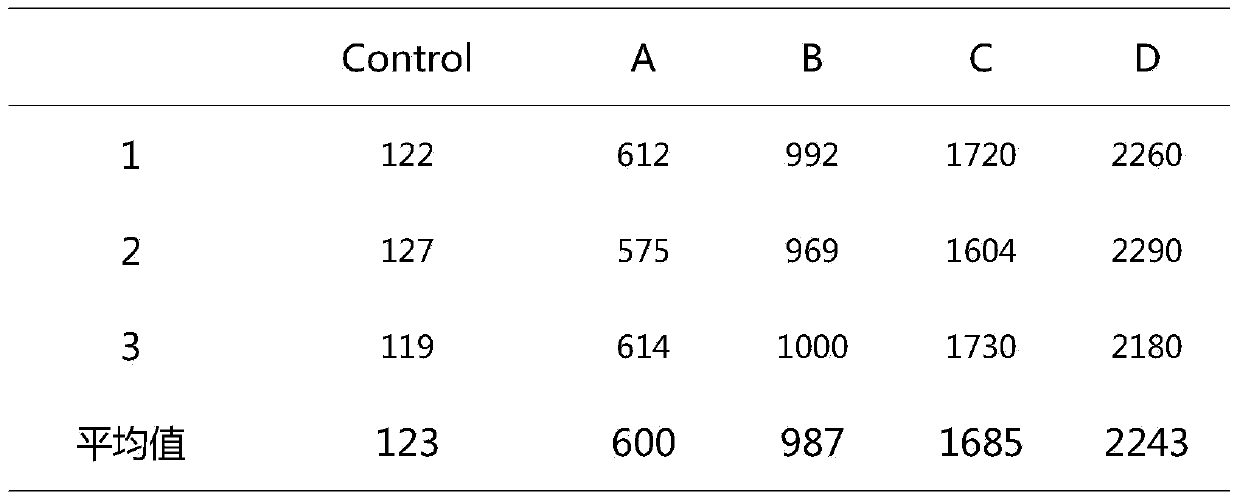
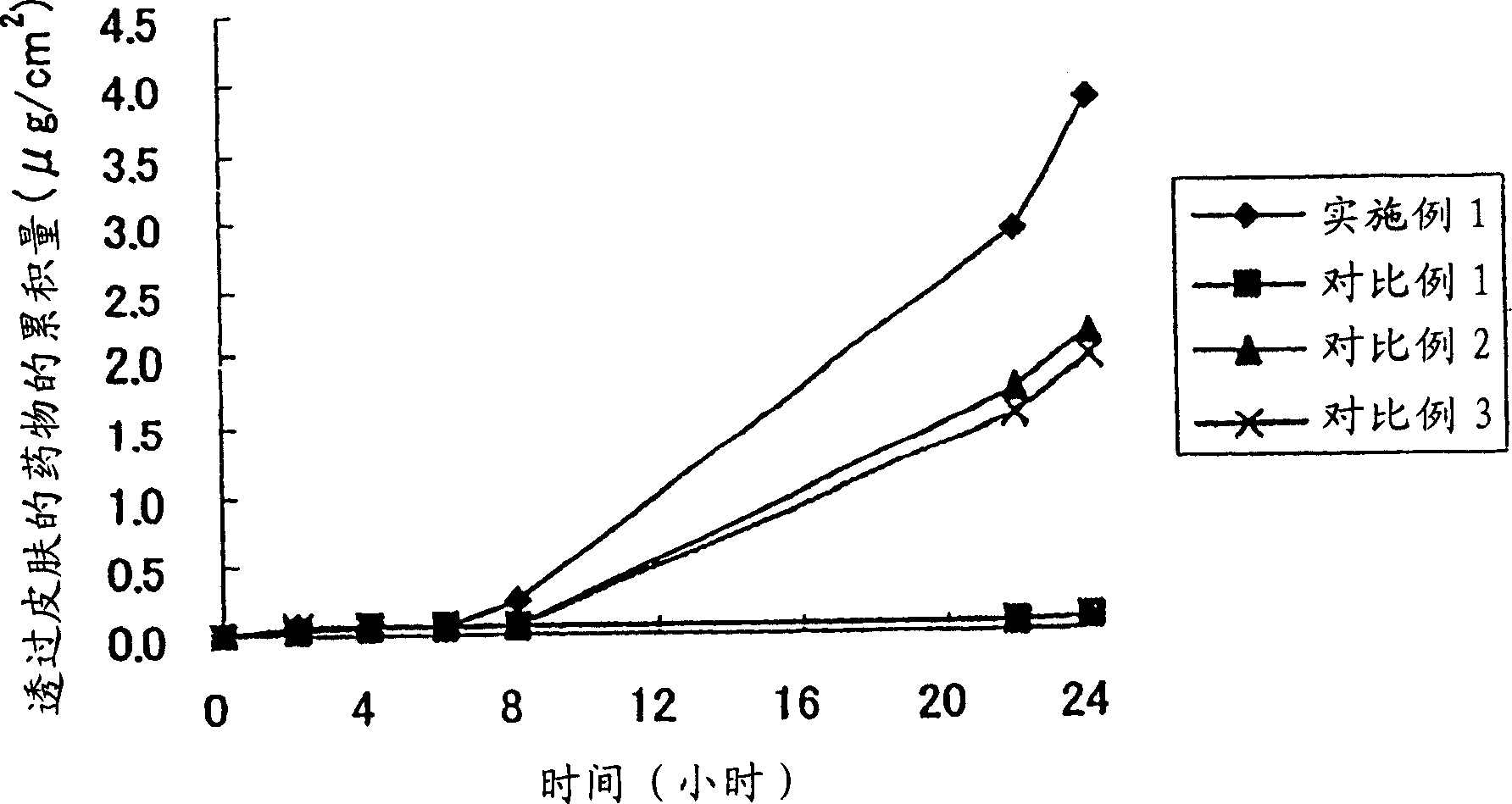
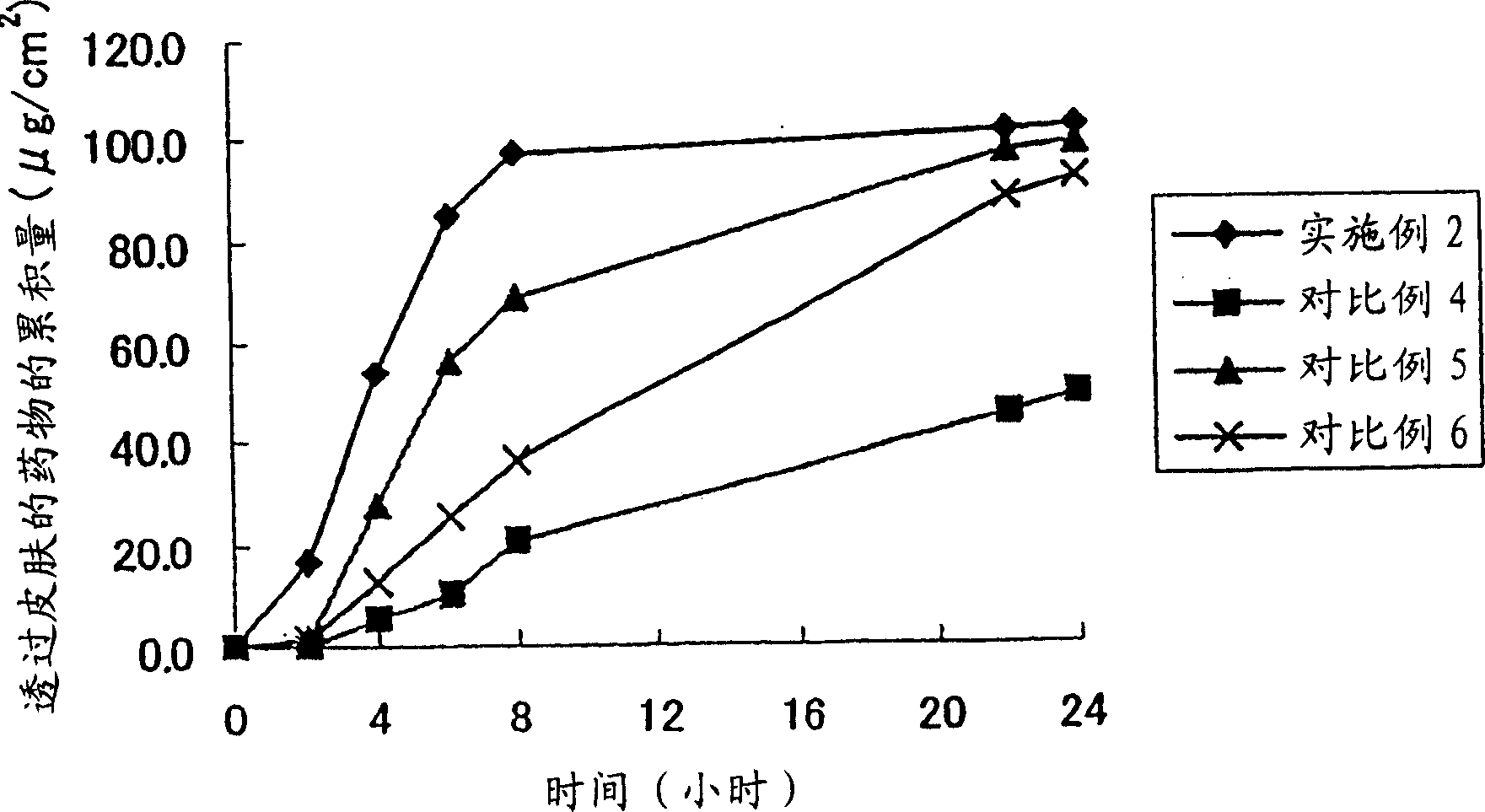
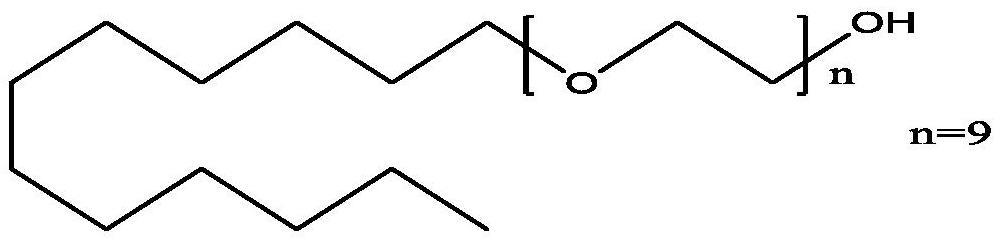
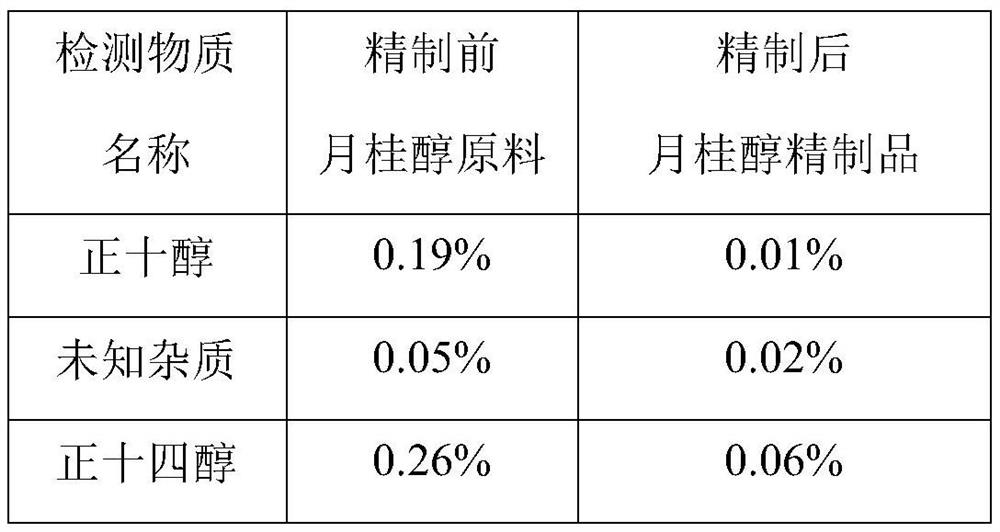
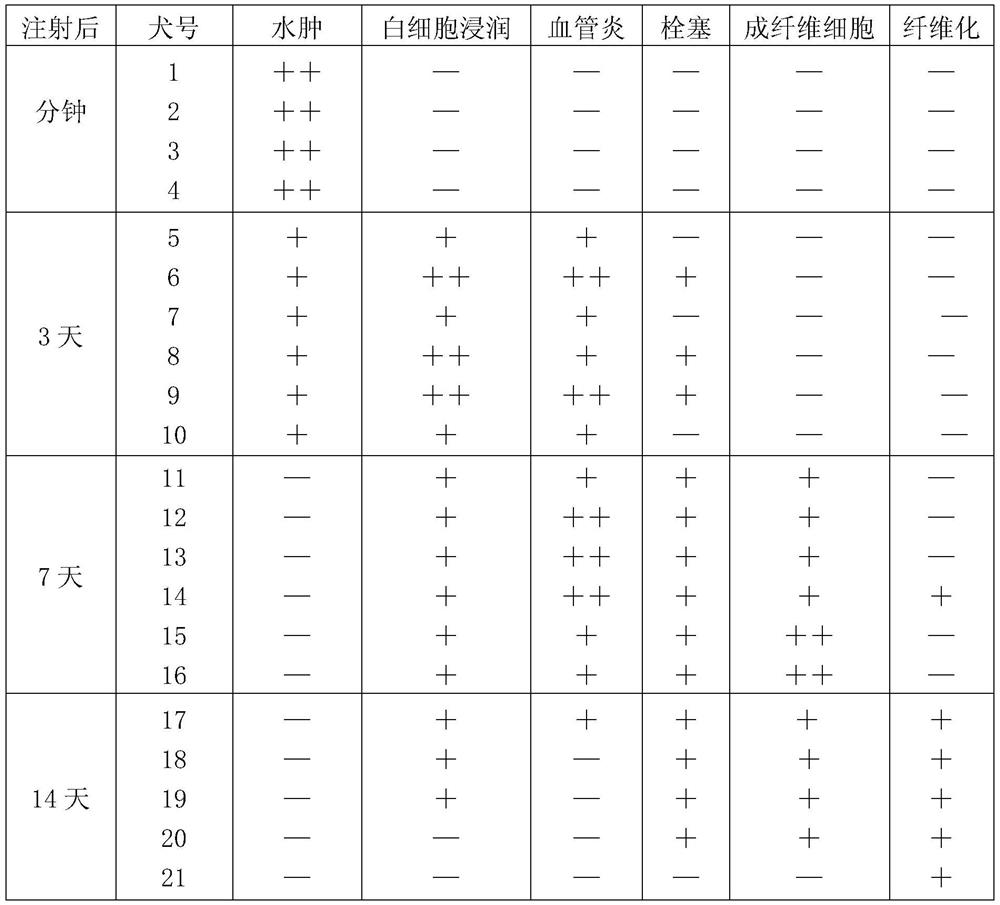
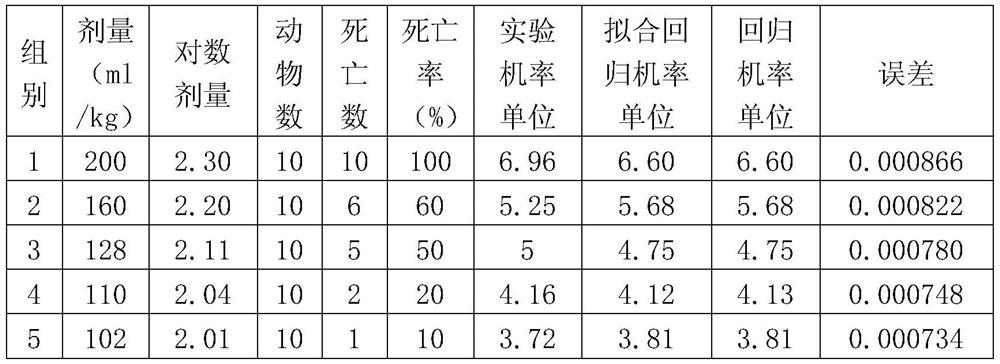
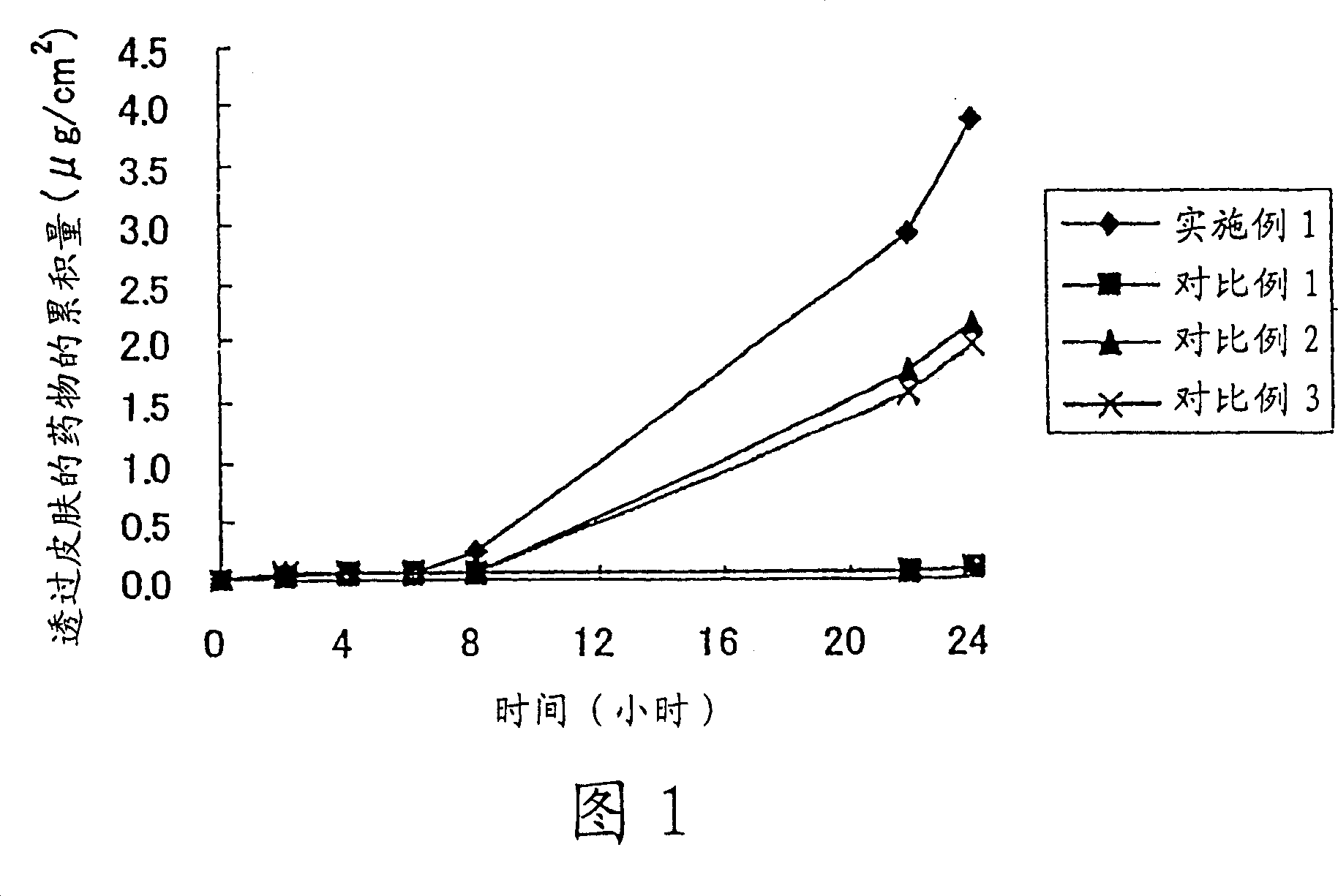
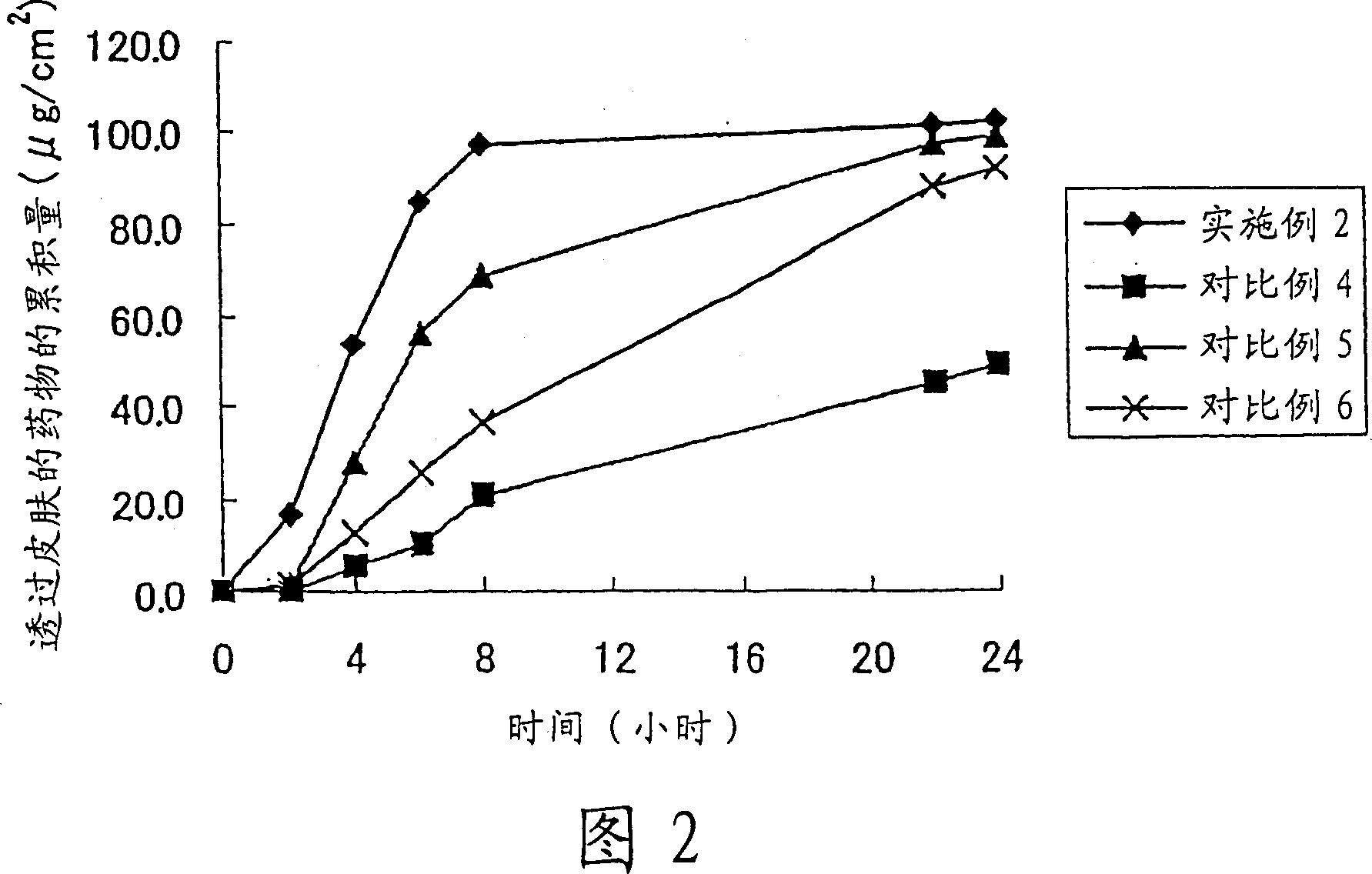
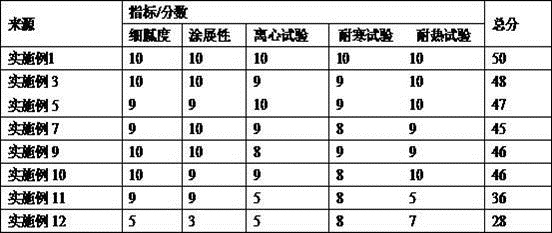
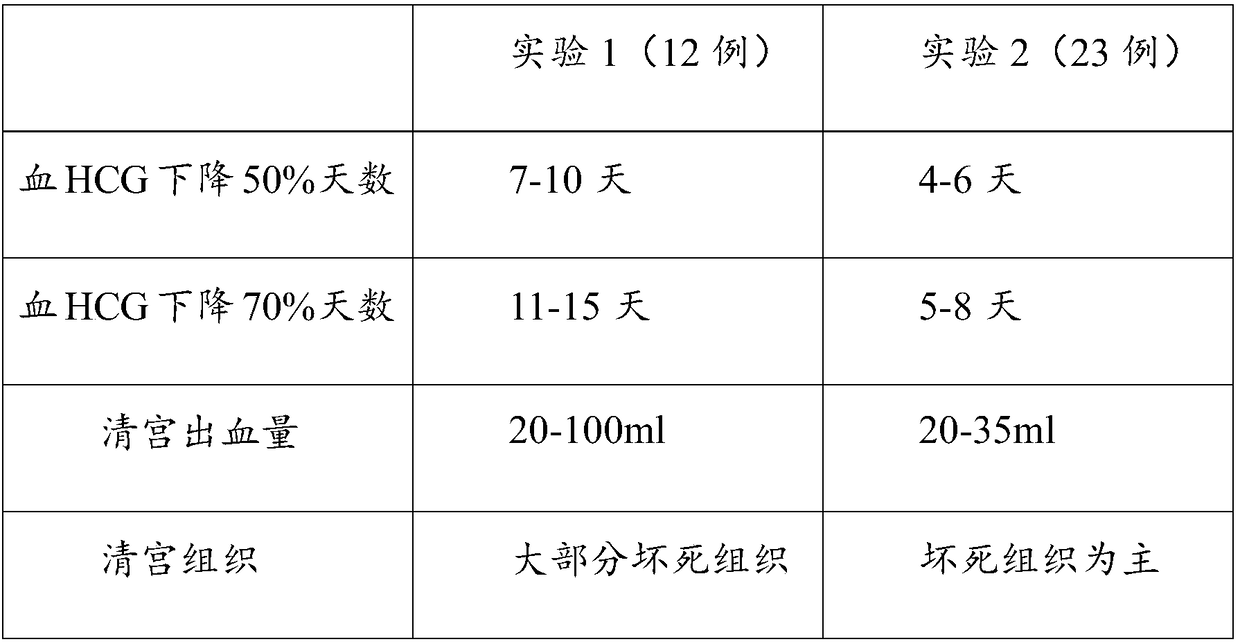
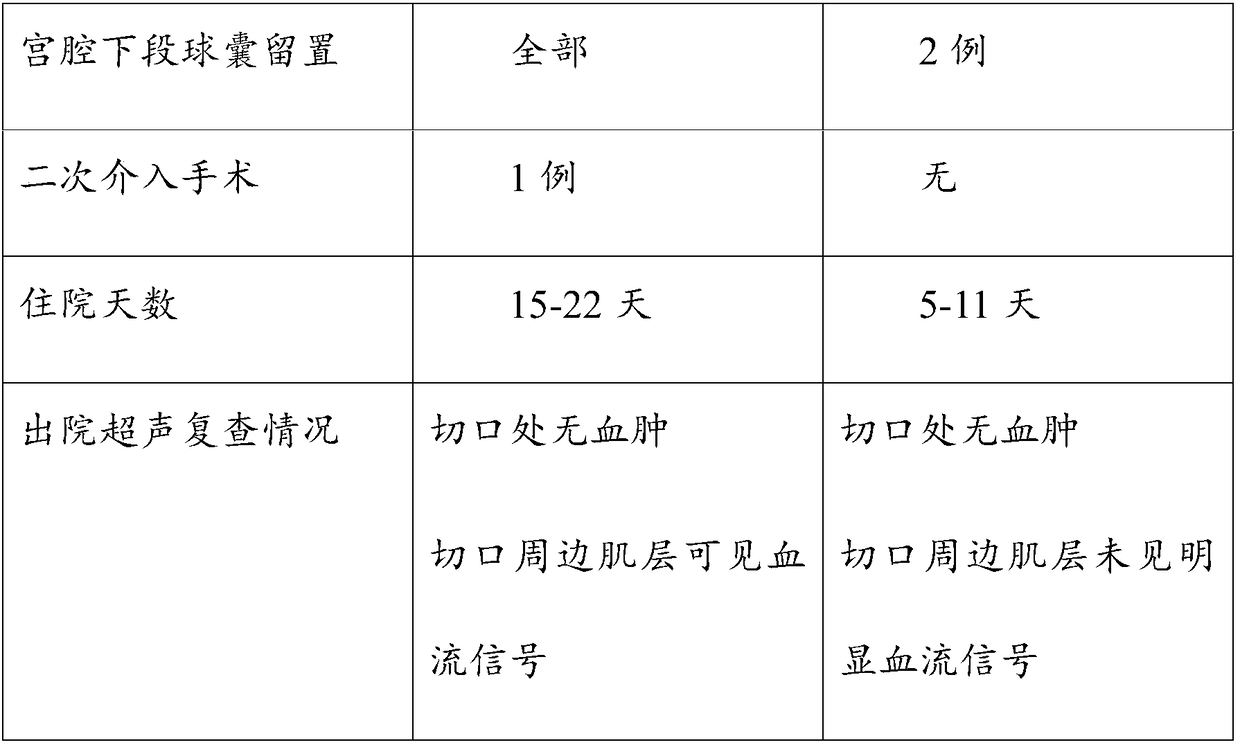
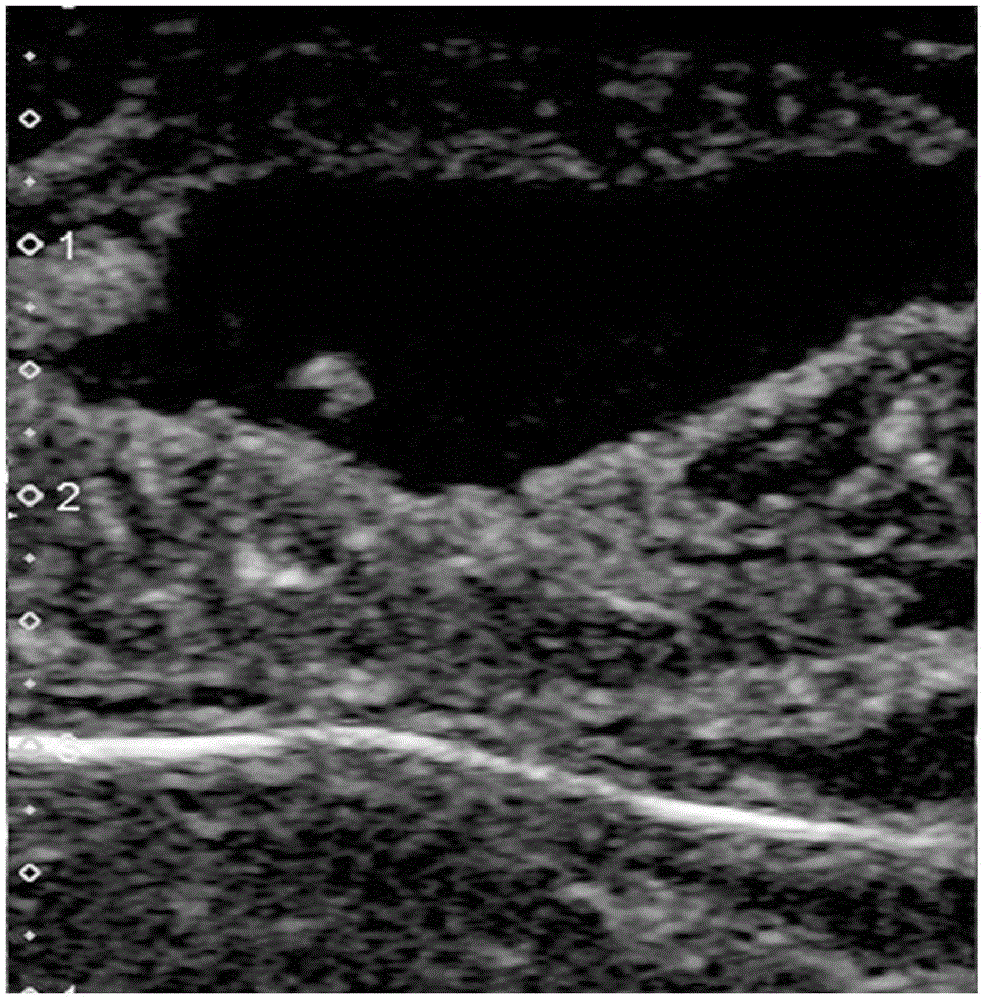
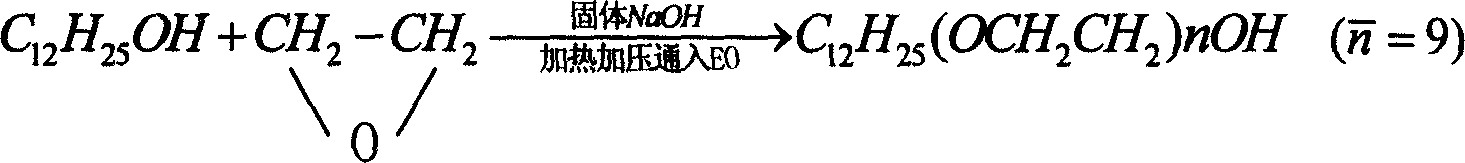Patents
Literature
42 results about "Lauromacrogol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
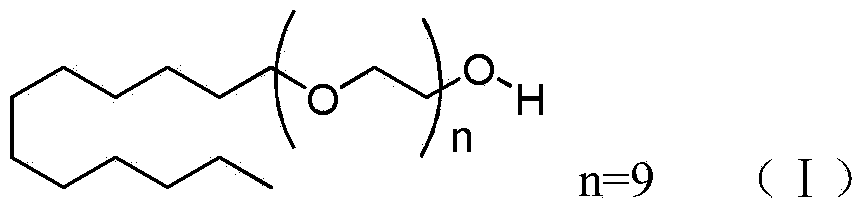
Lauromacrogol 400 is a medicine available in a number of countries worldwide. A list of US medications equivalent to Lauromacrogol 400 is available on the Drugs.com website.
Application of lauromacrogol combined hyaluronic acid in preparation of medicine for treating venous malformed foam sclerosis
InactiveCN103800278AExtended half-lifeSignificant clinical effectOrganic active ingredientsPharmaceutical delivery mechanismVeinCurative effect
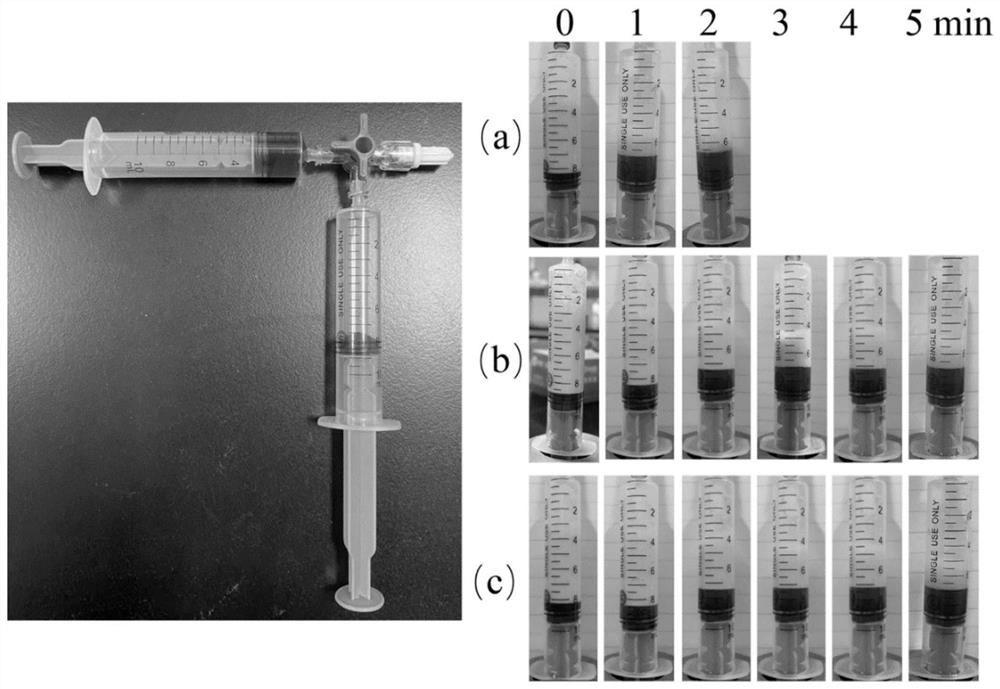
The invention discloses application of lauromacrogol combined hyaluronic acid in preparation of a medicine for treating venous malformed foam sclerosis. The lauromacrogol combined hyaluronic acid is prepared to form a foam hardening agent in particular application. The preparation method of lauromacrogol combined hyaluronic acid comprises the following steps: pumping lauromacrogol, hyaluronic acid and injection water by using an injector, and uniformly shaking and mixing; pumping sterile air by the other injector, wherein the two injectors are connected by a medical tee-joint valve; and feeding a plurality of times in a Tessari method, and mixing to form uniform and stable foam. According to the application, the foam hardening agent is prepared from the lauromacrogol combined hyaluronic acid and used for treating venous malformation, so that the half time of foam can be remarkably prolonged, and the curative effect can be improved. Currently, the method is successfully adopted in the stomatological department of Qilu Hospital of Shandong University, 20 cases with large-area venous malformation in maxillofacial region are treated, and the clinical effect is remarkable.
Owner:SHANDONG UNIV QILU HOSPITAL
Polycinnamic alcohol production formula and its preparation process
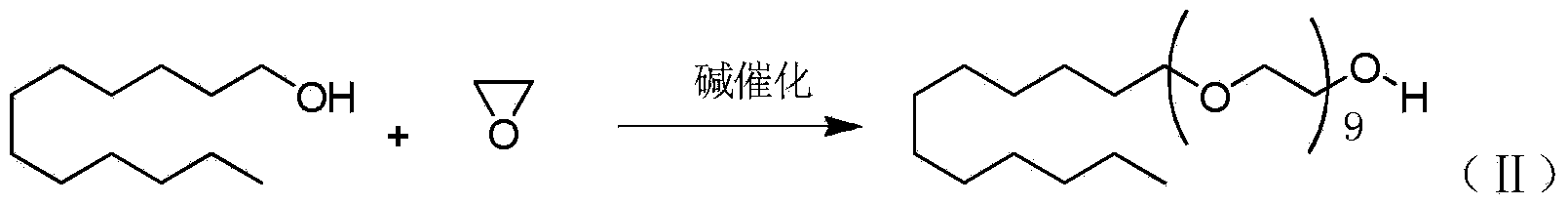
The present invention relates to chemical medicine preparation, and is the preparation process of lauromacrogol. The preparation process of the present invention adopts NaOH as catalyst to simplify operation and control of heavy metal content, HAC for neutralization in mild reaction condition to avoid oxidation of phosphoric acid at high temperature, and cloud point measuring method to control polymerization end point. Therefore, the synthesis process of the present invention is reasonable and can ensure stable quality of medicine material product.
Owner:SHAANXI TIANYU PHARMA
External gel for lauromacrogol and preparation method of external gel
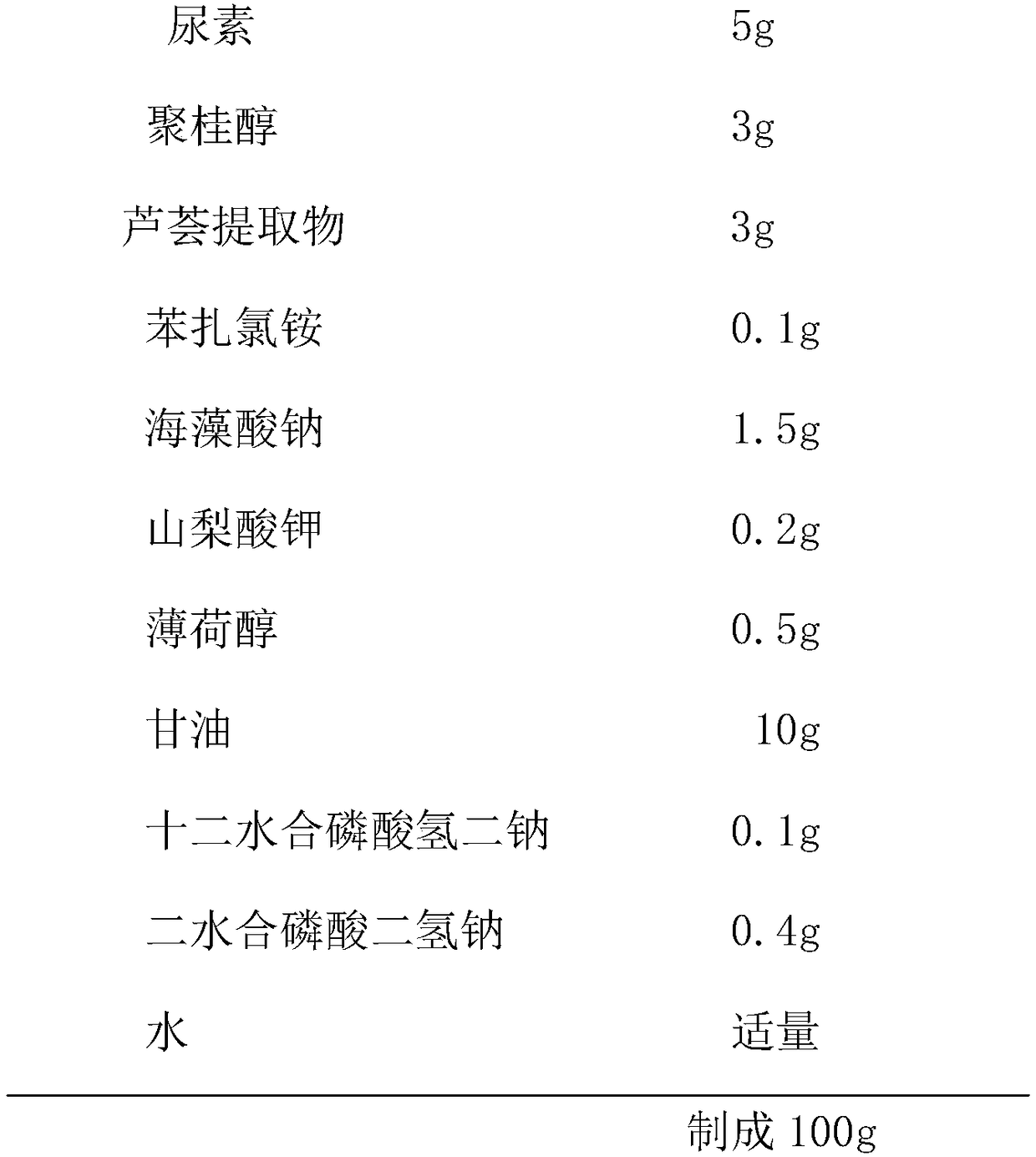
ActiveCN108853312AQuickly relieve itching and painWith convergenceAntipyreticAerosol deliveryInflammationDisodium hydrogen phosphate dodecahydrate
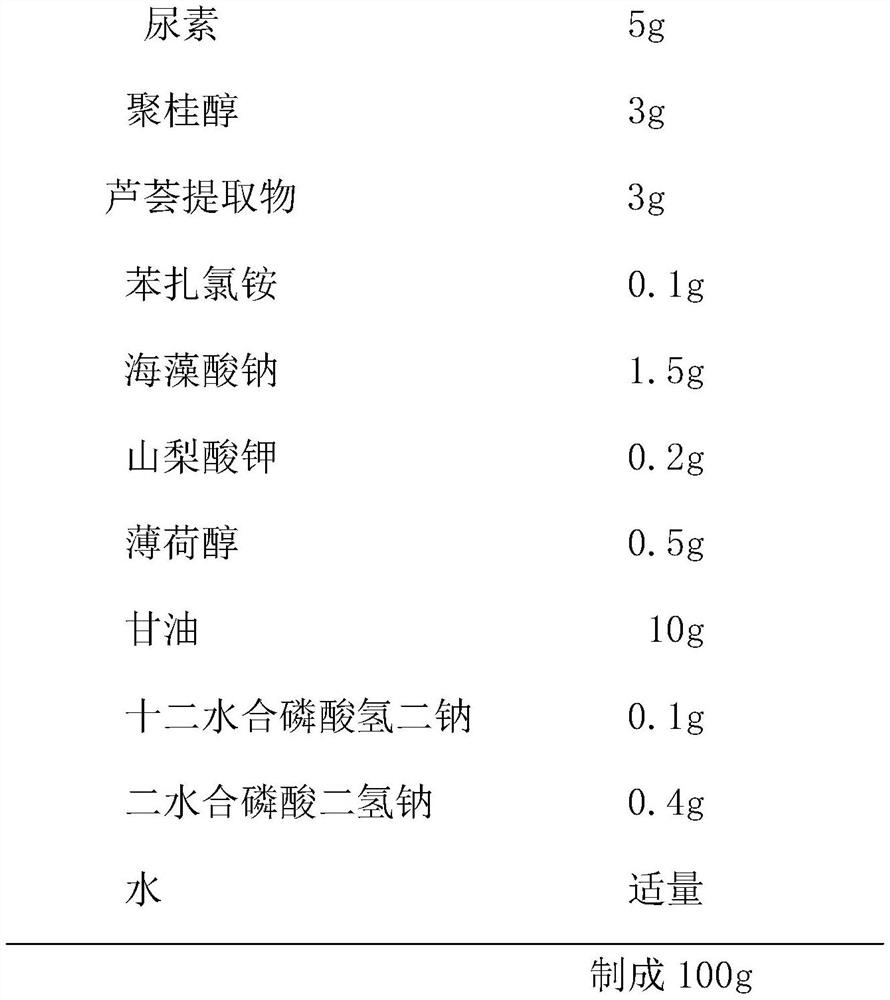
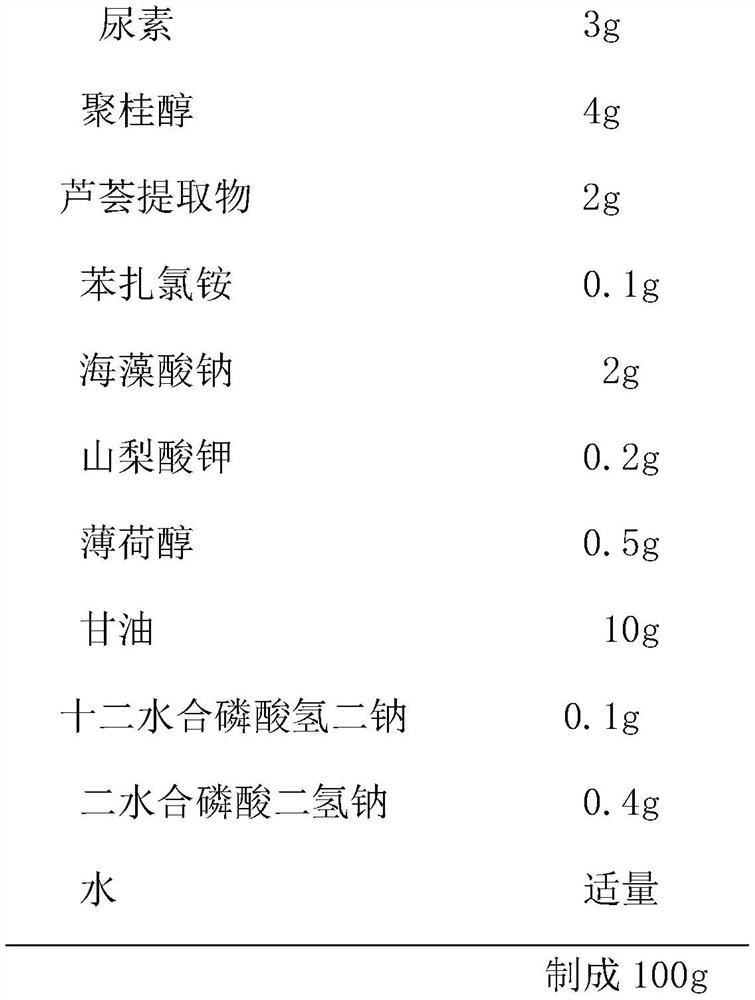
The invention relates to external gel for lauromacrogol and a preparation method of the external gel. The external gel is prepared from the components of urea, lauromacrogol, an aloe extract, benzalkonium chloride, sodium alginate, potassium sorbate, menthol, glycerinum, disodium hydrogen phosphate dodecahydrate, sodium hydrogen phosphate dihydrate and purified water. The external gel has the characteristics that lauromacrogol with the effects of quickly relieving itching and pain and diminishing inflammation and swelling is combined together with the aloe extract with the effects of refiningpores and repairing skin, the external gel adopts a gel film manner to treat patients suffering from burning of first degree and second degree, mosquito biting and bruise; and the external gel achieves the effects which are not reached by similar drugs for treating burning and mosquito biting at present, and no similar anti-inflammatory products or related inventions appear at present.
Owner:SHAANXI TIANYU PHARMA
Application of lauromacrogol injection as medicament for treating internal hemorrhoid
InactiveCN102198089AEliminate or reduce dilationEliminate or reduce congestionOrganic active ingredientsPharmaceutical delivery mechanismDiseaseFibrosis
The invention belongs to the field of the medical application of a lauromacrogol injection, and particularly relates to application of a lauromacrogol injection as a medicament for treating internal hemorrhoid. Internal hemorrhoid is one of hemorrhoids, which occurs above the pectinate line of the anal canal and is a commonly encountered disease having the highest incidence rate among the hemorrhoids; for majorities of people, internal hemorrhoid bleeding is a disease difficult to deal with; and when the internal hemorrhoid bleeds too much, secondary anemia can be caused. In the invention, the lauromacrogol injection is injected into the hemorrhoid, so that an aseptic inflammation is produced and is then gradually fiberized, thus realizing the treatment of the internal hemorrhoid. The lauromacrogol injection can wrap veins and arterioles in the hemorrhoid, so that a protective layer can be formed at the peripheries of the veins and arterioles, thereby preventing weak blood vessels from bleeding due to damage caused by defecation and other factors; fiber textures can occlude blood vessel cavities, eliminate or lessen the expansion and congestion of the veins and atrophy the hemorrhoid; because of the fiberization process, a relaxed mucous membrane can be fixed on the muscular wall thereunder again through the fiber textures, thereby eliminating the symptom.
Owner:SHAANXI TIANYU PHARMA
Transdermally absorbable donepezil-containing preparation
Disclosed is a transdermally absorbable donepezil-containing preparation which enables the administration of donepezil in a sustained manner for a long period and can achieve both the rapid increase in blood donepezil level and the sustained release of donepezil. The transdermally absorbable donepezil-containing preparation comprises an adhesive patch base material comprising a hydrophobic polymer and an absorption-enhancing agent and donepezil (an active ingredient) dissolved in the adhesive patch base material, wherein the absorption-enhancing agent comprises at least one component selected from lauryl alcohol, triethyl citrate, isopropyl myristate, cetyl lactate, oleyl alcohol, sorbitan monooleate, polyethylene glycol monostearate, lauromacrogol, N-methyl-2-pyrrolidone and triacetin.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Efficient painless drug for treating local tumor ablation
InactiveCN103191146AIncrease injection volumeImprove complianceHydroxy compound active ingredientsPharmaceutical delivery mechanismColor dopplerBlood flow
The invention discloses an efficient painless drug for treating local tumor ablation. The efficient painless drug is mixed solution of absolute ethyl alcohol and lauromacrogol, wherein the ratio of lauromacrogol is 0.1-99.9%, and the ratio of absolute ethyl alcohol is 99.9%-0.1% according to the volume fraction. The efficient painless drug for treating local tumor ablation, which is injected into local tumor under guide of an ultrasonic imaging technique, is the mixed solution of absolute ethyl alcohol and lauromacrogol according to the volume ratio of 19 / 1 to 99 / 1; and volume ratio of absolute ethyl alcohol to lauromacrogol preferably is 99 / 1 or 19 / 1; the efficient painless drug for treating local tumor ablation is applied to preparation of the drug for treating ablation by being injected to the tumor under guide of the ultrasonic imaging technique; the sufferer has no adverse reaction such as pain in an operation; and the postoperation color Doppler ultrasonic review finds out that the tumor echo is increased, blood flow signals are obviously reduced, and the indexes of a part of patients with rising alpha fetal protein are obviously reduced by review.
Owner:FUJIAN MEDICAL UNIV UNION HOSPITAL
Stable multilayer-film structural foaming agent and preparation method thereof
The invention particularly relates to a stable multilayer-film structural foaming agent and a preparation method of the stable multilayer-film structural foaming agent. The technical scheme is that the preparation method comprises steps as follows: getting 20 to 50wt% of main agent, 20 to 50wt% of assistant and 20 to 50wt% of foam stabilizer as the raw materials at 15 to 25 DEG C; then adding foam stabilizer of which the mass is 10 to 20 times that of the raw materials mentioned above; and agitating for 5 to 15 minutes, so as to obtain the stable multilayer-film structural foaming agent, wherein the main agent is one of sodium lauryl sulfate, sodium dodecyl benzene sulfonate, sodium dodecyl sulfate, alkylphenol ethoxylates and fatty alcohol-polyoxyethylene ether; the assistant is one of dodecanol, polyethylene glycol, setanol, octadecanol and lauromacrogol; and the foam stabilizer is one of sodium carboxymethylcellulose, gelatin, sodium carboxymethyl starch, arabia gum adhesive and hydroxyethyl cellulose. The preparation method has the characteristics of being simple, convenient to operate, and short in preparation time and the like; and the prepared stable multilayer-film structural foaming agent is relatively small in dimension, uniform in distribution, full in foaming and high in stability.
Owner:WUHAN UNIV OF SCI & TECH
Lauromacrogol refinement method
ActiveCN103922901ASuitable for large-scale industrial productionRaise quality standardsEther separation/purificationDistillationLauromacrogol
The invention relates to the field of pharmaceutical chemicals and in particular relates to a lauromacrogol refinement method. The method comprises the steps of performing reduced pressure distillation on a lauromacrogol crude product at certain temperature under certain pressure, filtering, adjusting the pH value of filter liquor, and performing reduced pressure distillation to obtain a high-purity lauromacrogol refinement product. The refinement method is simple and convenient to operate, mild in reaction condition and suitable for industrialized production and use.
Owner:NANJING CHIA TAI TIANQING PHARMA
Transdermal absorption preparation
InactiveCN1668334AImprove permeabilityGood touchAerosol deliveryDigestive systemSkin permeabilityLauromacrogol
A transdermal absorption promotion composition comprising the following components (a), (b), and (c) and a transdermal absorption preparation comprising the following components (a), (b), (c), and (d) are disclosed. (a) propylene glycol (b) a polyol fatty acid ester (c) lauromacrogol (d) a drug component The transdermal absorption promotion composition and transdermal absorption preparation not only exhibit an excellent transdermal absorption promotion effect, but also exhibit superior skin-permeability, even if a drug having a relatively high lipophilic property and poor transdermal absorbability is used, exhibit a favorable feeling of use, and are safe and stable.
Owner:HISAMITSU PHARM CO INC
Preparation technology of lauromacrogol
InactiveCN103570934AEasy to controlSimple processOrganic compound preparationHydroxy compound preparationActivated carbonAlcohol
The invention relates to a preparation technology of lauromacrogol. The preparation technology takes lauryl alcohol, potassium hydroxide, oxirane, absolute ethyl alcohol and activated carbon as raw materials. The preparation technology is simple; no special equipment is required; a solvent can be recycled, so that the industrial production cost is lowered; potassium hydroxide serves as a catalyst, so that operation is simplified; the content of heavy metal is controlled easily according to a medicinal quality standard requirement; lauromacrogol is high in yield.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Laurinol purification method and lauromacrogol preparation method
InactiveCN104649863AMeet raw material requirementsReduce self-aggregationOrganic active ingredientsOrganic compound preparationActivated carbonAlcohol
The invention relates to a preparation technology of lauromacrogol. The preparation technology takes lauryl alcohol, potassium hydroxide, oxirane, absolute ethyl alcohol and activated carbon as raw materials. The preparation technology is simple; no special equipment is required; a solvent can be recycled, so that the industrial production cost is lowered; potassium hydroxide serves as a catalyst, so that operation is simplified; the content of heavy metal is controlled easily according to a medicinal quality standard requirement; lauromacrogol is high in yield.
Owner:BEIJING WANCHENG WEIYE MEDICAL TECH
A method for purifying lauryl alcohol and a method for preparing lauryl alcohol
InactiveCN104649863BMeet raw material requirementsReduce self-aggregationOrganic active ingredientsOrganic compound preparationActivated carbonPurification methods
The invention relates to a preparation technology of lauromacrogol. The preparation technology takes lauryl alcohol, potassium hydroxide, oxirane, absolute ethyl alcohol and activated carbon as raw materials. The preparation technology is simple; no special equipment is required; a solvent can be recycled, so that the industrial production cost is lowered; potassium hydroxide serves as a catalyst, so that operation is simplified; the content of heavy metal is controlled easily according to a medicinal quality standard requirement; lauromacrogol is high in yield.
Owner:BEIJING WANCHENG WEIYE MEDICAL TECH
Application of lauromacrogol in preparing medicament for treating sublingual gland cyst
InactiveCN104257683ASave functionNo allergic reactionOrganic active ingredientsDigestive systemCystLauromacrogol
Owner:SHANDONG UNIV QILU HOSPITAL
Application of lauromacrogol injection as drug for curing cesarean scar pregnancy
InactiveCN105412009APromote formationReduce bleeding riskOrganic active ingredientsPharmaceutical delivery mechanismInjection siteFibrosis
The invention relates to the field of lauromacrogol injection drug application, and discloses application of lauromacrogol injection as a drug for curing cesarean scar pregnancy. The drug is applied according to the following steps: step (1), a focus scope, a muscular layer thickness and the blood supply situation of a focus location are determined by ultrasound contrast; step (2), the vagina is conventionally disinfected and a towel is spread at a lithotomy position of a patient; step (3), under ultrasonic guidance, the lauromacrogol injection is injected at a peripheral muscular layer of a gestational sac and around the gestational sac, until annular or flaky reinforcement of the gestational sac is seen under ultrasound and peripheral blood is sparse, wherein the specification of the lauromacrogol injection is that each 10ml of injection contains 100mg of lauromacrogol. According to the application of the lauromacrogol injection as the drug for curing cesarean scar pregnancy, the lauromacrogol injection is adhered to the inside of a vessel at the injection site, thus prompting a fibrosis strip to replace the pathological vessel, resulting in permanent occlusion of the vessel around the gestational sac, interdicting an open vessel at the pathological location, and greatly reducing bleeding risk in induced abortion operation.
Owner:张淑珍
Refining process of laurinol and process for preparing lauromacrogol by using refined product as raw material
ActiveCN113527060AReduce moistureEfficient removalEther/acetal active ingredientsEther preparation from oxiranesDrug utilisationLauromacrogol
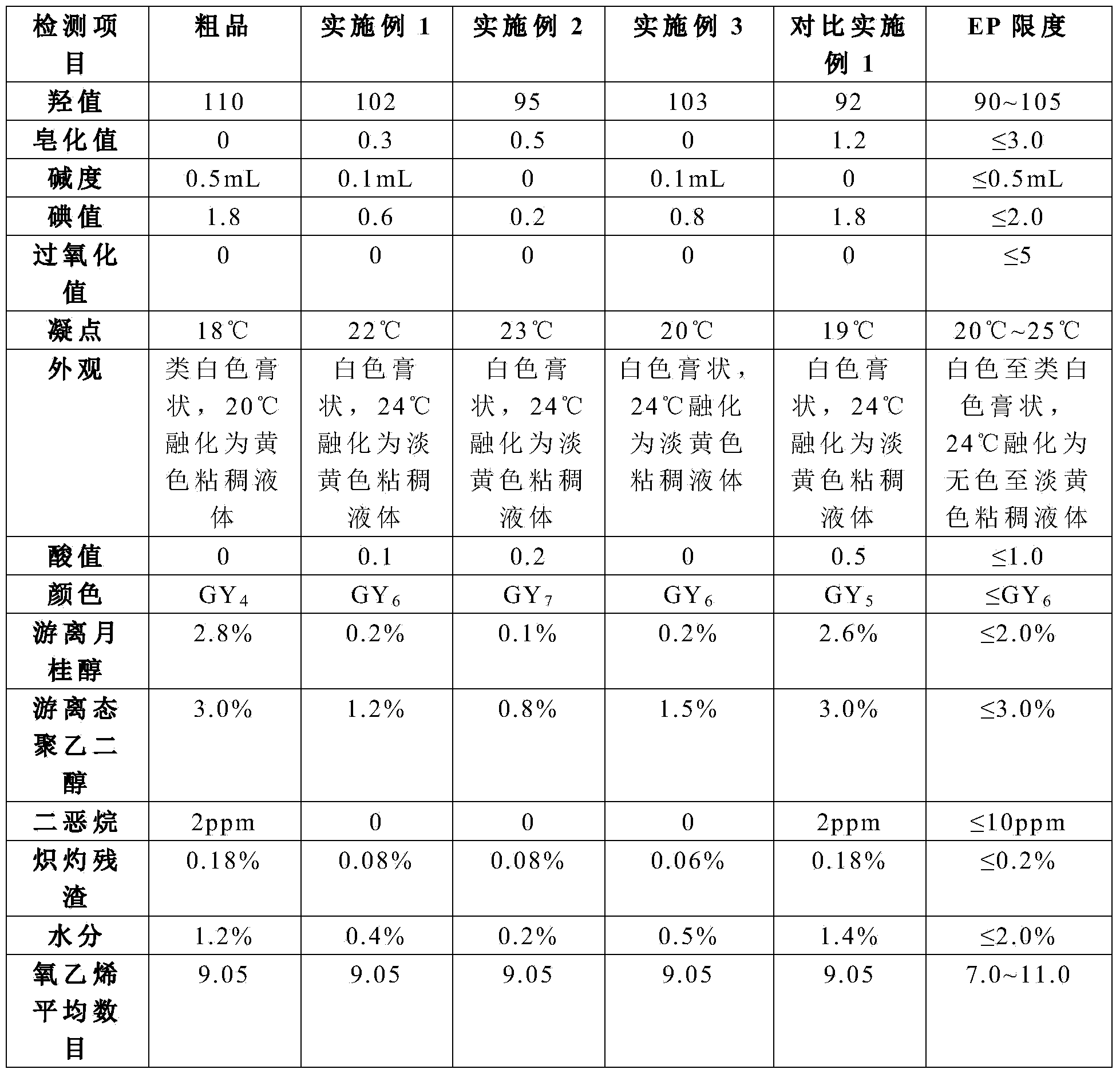
The invention relates to a refining process of laurinol and a process for preparing lauromacrogol by using a prepared laurinol refined product as a raw material. According to the preparation process of the lauromacrogol, impurities in the lauromacrogol are effectively reduced, a dehydration step in a lauromacrogol synthesis process is reduced, and energy consumption is reduced, so that the preparation process is suitable for industrial production; all indexes of the prepared lauromacrogol meet the requirements of EP and Chinese pharmacopoeia, the lauromacrogol has good oxidation resistance and high temperature resistance, can be stably stored at room temperature in the presence of air, and solves the problem that an existing lauromacrogol product needs to be stored at low temperature under the protection of inert gas, and the stability of the lauromacrogol is improved; and the lauromacrogol can be stored without special packaging, so that the production, storage and transportation costs are greatly reduced, effective support is provided for the production of the lauromacrogol preparation, the medication expenditure of patients is greatly reduced, and the lauromacrogol is beneficial to the majority of patients.
Owner:BEIJING ENCHENG KANGTAI BIOLOGICAL TECH
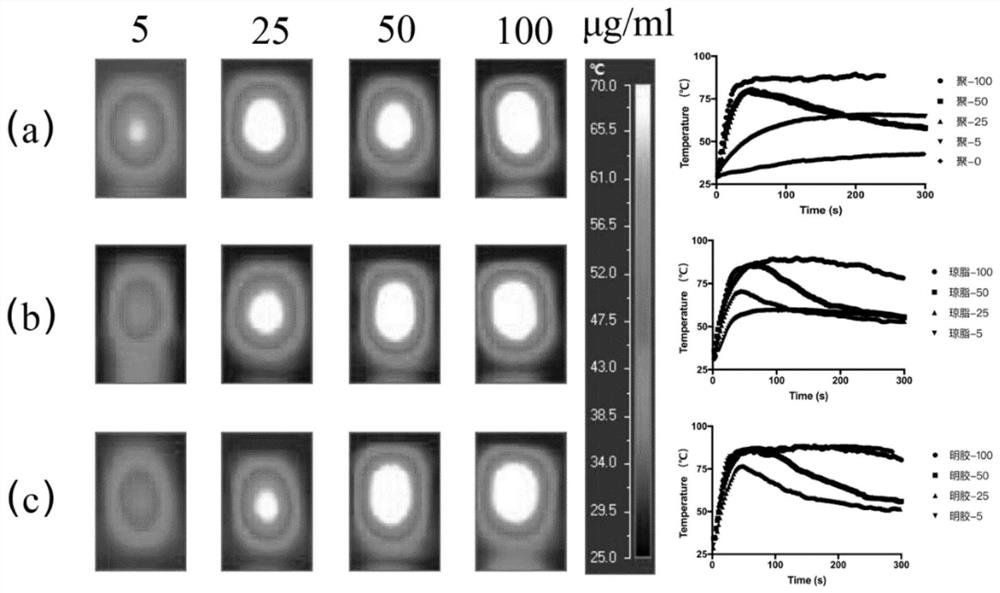
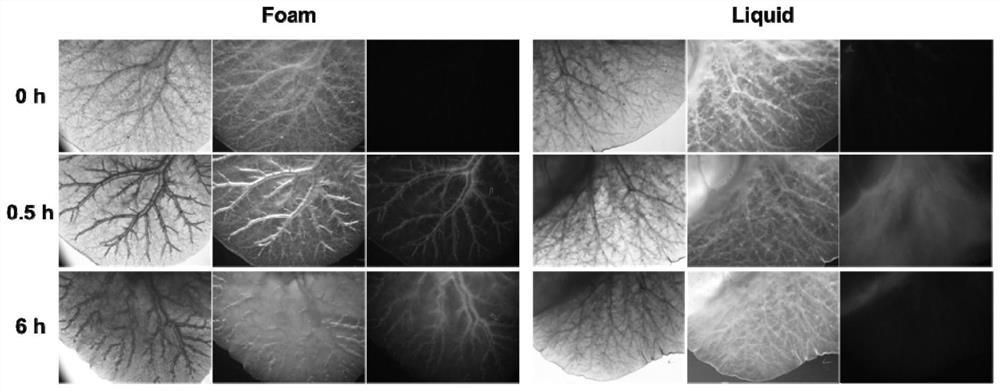
Modified lauromacrogol foam hardening agent as well as preparation method and application thereof
PendingCN113143955AImprove stabilityExtension of timeOrganic active ingredientsPhotodynamic therapyFormularyTherapeutic effect
The invention discloses a modified lauromacrogol foam hardening agent as well as a preparation method and application thereof. The preparation method comprises the following steps: firstly, mixing a lauromacrogol bulk drug with gelatin or agar powder with surface activity and foam stabilizing effect through an ultra-stable homogeneous formula technology to prepare a modified lauromacrogol mother solution, and obviously improving the foam stability and defoaming time of the foam hardening agent prepared from the modified lauromacrogol mother solution; subjecting the modified lauromacrogol mother liquor and various active ingredients including a preparation with a photosensitive (thermal) characteristic, a nuclear magnetic resonance imaging preparation, a chemotherapeutic drug, nuclide and the like to ultrasonic mixing to obtain a multifunctional improved lauromacrogol mixed preparation, and then preparing the multifunctional and visual lauromacrogol foam hardening agent under the action of a three-way valve device. The modified lauromacrogol foam hardener for varicose veins and tumors is locally administered in a minimally invasive mode, and then a good treatment effect on the varicose veins and the tumors is achieved in combination with a sclerotherapy and other therapies.
Owner:XIAMEN UNIV
Medicine for interventional treatment of uterine myoma
ActiveCN103768090AAvoid traumaPreserve uterine functionSynthetic polymeric active ingredientsAntineoplastic agentsBlood vesselPOLYOXYETHYLENE ETHER
The invention provides a medicine for interventional treatment of uterine myoma. The medicine belongs to a polyoxyethylene lauryl alcohol ether compound, called lauromacrogol for short; after lauromacrogol is injected under the envelope of an uterine myoma body, a layer of fibre textures is formed in the lower layer of the envelope; blood vessels are injured and permanently occluded by the fibre textures, so that the uterine myoma body is reduced and gradually disappears; uterine myoma is subjected to interventional treatment by utilizing the medicine, so that the functions of an uterus can be intactly retained; in addition, fertilization is not influenced; operative wound and a series of post-operative complications are avoided; the effect of the medicine disclosed by the invention is comparable with operation in the aspect of symptom improvement; by means of interventional treatment of uterine myoma by utilizing the medicine, the medicine disclosed by the invention is small in wound, rapid in recovery, free from being in hospital and easy for patients to accept; compared with the traditional operative treatment, interventional treatment of uterine myoma by utilizing the medicine is simple and convenient; furthermore, blood transfusion is generally unnecessary; the medicine disclosed by the invention has the advantages of being minimally invasive, repeatable, accurate for locating, high in curative effect, rapid in effect, few in complications and the like and brings a good development prospect for clinical treatment of uterine myoma.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
Litsea cubeba oil ointment with anti-inflammatory and antipruritic effects and preparation method of litsea cubeba oil ointment
InactiveCN112999264ASoft smellAntipruritic and anti-inflammatoryAerosol deliveryOintment deliveryLauromacrogolPharmaceutical medicine
The invention belongs to the field of traditional Chinese medicines, and particularly relates to a litsea cubeba oil ointment with anti-inflammatory and antipruritic effects and a preparation method of the litsea cubeba oil ointment. The litsea cubeba oil ointment is prepared from litsea cubeba oil, lauromacrogol and pharmaceutically acceptable additives, is derived from plant extracted essential oil, is soft in smell, has the effects of relieving itching and resisting inflammation, and is safe and convenient to use.
Owner:福建宸润生物科技有限公司
Application of hyaluronic acid combined with lauromacrogol in preparation of foam sclerosing medicine for treating venous malformation
InactiveCN103800278BExtended half-lifeSignificant clinical effectOrganic active ingredientsPharmaceutical delivery mechanismCurative effectLauromacrogol
The invention discloses application of lauromacrogol combined hyaluronic acid in preparation of a medicine for treating venous malformed foam sclerosis. The lauromacrogol combined hyaluronic acid is prepared to form a foam hardening agent in particular application. The preparation method of lauromacrogol combined hyaluronic acid comprises the following steps: pumping lauromacrogol, hyaluronic acid and injection water by using an injector, and uniformly shaking and mixing; pumping sterile air by the other injector, wherein the two injectors are connected by a medical tee-joint valve; and feeding a plurality of times in a Tessari method, and mixing to form uniform and stable foam. According to the application, the foam hardening agent is prepared from the lauromacrogol combined hyaluronic acid and used for treating venous malformation, so that the half time of foam can be remarkably prolonged, and the curative effect can be improved. Currently, the method is successfully adopted in the stomatological department of Qilu Hospital of Shandong University, 20 cases with large-area venous malformation in maxillofacial region are treated, and the clinical effect is remarkable.
Owner:SHANDONG UNIV QILU HOSPITAL
Mosquito-repelling and itching-relieving composition for children and preparation method of mosquito-repelling and itching-relieving composition
PendingCN112999120ASimple recipePleasant aromaCosmetic preparationsHydroxy compound active ingredientsBiotechnologyChemical synthesis
The invention belongs to the field of traditional Chinese medicine, and relates to a composition for repelling mosquitoes and relieving itching for children and a preparation method thereof.The composition is elaborately prepared from litsea cubeba oil and lauromacrogol, can overcome harm of chemically synthesized mosquito repellents to human beings and the environment, can be slowly released in the using process, has pleasant fragrance and can be used for repelling mosquitoes and relieving itching. And the mosquito-repellent and itching-relieving liquid soap is especially suitable for children to use.
Owner:福建宸润生物科技有限公司
Application of lauromacrogol injection in preparation of medicine for treating cardia laceration
InactiveCN114306375AContribute to thrombosisAchieve hemostatic effectPharmaceutical delivery mechanismPharmaceutical non-active ingredientsIntravascular Thrombus FormationSurgical treatment
The invention relates to an application of a lauromacrogol injection in preparation of a medicine for treating cardia laceration, solves the problems of large trauma and secondary bleeding in the prior art, and relates to an application of a lauromacrogol injection with low recurrence rate as a medicine for treating cardia laceration by replacing surgical treatment with medicine treatment. A formula of the lauromacrogol injection comprises 1g of lauromacrogol, 5ml of ethanol (96%) and the balance of water for injection, wherein the total volume is 100ml. According to the invention, surgical treatment is replaced by drug treatment, and after 1% lauromacrogol injection is locally injected to a patient, fibrosclerosis of local blood vessels can be changed, and thrombus formation in the blood vessels is facilitated, so that bleeding blood vessels are blocked to achieve a hemostatic effect. The cardia laceration treatment device is simple, convenient and effective in cardia laceration treatment, can be repeatedly operated, and can achieve an effective hemostatic effect.
Owner:SHAANXI TIANYU PHARMA
Application of lauromacrogol injection in preparation of medicine for treating bronchial cyst
InactiveCN114159388AAvoid high recurrence ratesOrganic active ingredientsPharmaceutical delivery mechanismSterile inflammationLauromacrogol
The invention relates to application of lauromacrogol injection in preparation of a medicine for treating bronchial cyst, and solves the problems that in the prior art, operative trauma is large, and part of special patients cannot tolerate operations and general anesthesia. According to the invention, the lauromacrogol injection with low recurrence rate is used as a medicament for treating bronchial cyst by replacing operative treatment with medicament treatment. A formula of the lauromacrogol injection comprises 1g of lauromacrogol, 5ml of ethanol and the balance of water for injection, wherein the total volume is 100ml. According to the invention, operation treatment is replaced by drug treatment, after the lauromacrogol injection is injected into the sac cavity of the bronchial cyst, sterile inflammation is generated, and the sac cavity is solidified, hardened, adhered, closed and gradually absorbed to disappear. According to the invention, patients with bronchial cyst do not suffer from pain during operation, can recover quickly and can be discharged from hospital for 3 days after operation, and the high recurrence rate of cyst caused by incomplete excision during operation is avoided.
Owner:SHAANXI TIANYU PHARMA
Transdermal absorption preparation
InactiveCN100341577CImprove permeabilityGood touchAerosol deliveryDigestive systemSkin permeabilityLauromacrogol
A transdermal absorption promotion composition comprising the following components (a), (b), and (c) and a transdermal absorption preparation comprising the following components (a), (b), (c), and (d) are disclosed. (a) propylene glycol (b) a polyol fatty acid ester (c) lauromacrogol (d) a drug component The transdermal absorption promotion composition and transdermal absorption preparation not only exhibit an excellent transdermal absorption promotion effect, but also exhibit superior skin-permeability, even if a drug having a relatively high lipophilic property and poor transdermal absorbability is used, exhibit a favorable feeling of use, and are safe and stable.
Owner:HISAMITSU PHARM CO INC
Polycinnamic alcohol facial cleanser suitable for skin care of psoriasis patients and preparation method thereof
InactiveCN112402294ASolving Daily Skin Care ConcernsHas a slight anesthetic effectCosmetic preparationsToilet preparationsPsoriasis patientGlycerol
The invention relates to a lauromacrogol facial cleanser suitable for skin care of psoriasis patients and a preparation method thereof. The facial cleanser comprises the following components in partsby weight: 0.1-1 part of lauromacrogol, 0.01-0.1 part of sodium hyaluronate and 5-10 parts of glycerol. The facial cleanser provided by the invention, as a nonionic surfactant, can take effects of emulsifying, dispersing, cleaning and wetting, and can solve the problem of daily skin care of the psoriasis patients, so that the facial cleanser can take effects of preserving moisture, removing dandruff and relieving itching; the lauromacrogol, which is especially contained in the formula, has a slight anesthetic effect; and the facial cleanser, as an external skin care preparation, not only can promote softening of skin cutin, but also can rapidly relieve itching, preserve moisture and repair skin.
Owner:陕西万友康年生物科技有限公司
Application of lauromacrogol injection in preparation of medicine for treating spontaneous pneumothorax
InactiveCN114288240ANo pain responseOrganic active ingredientsPharmaceutical delivery mechanismPleural cavityPneumothorax
The invention relates to application of lauromacrogol injection in preparation of a medicine for treating spontaneous pneumothorax, and solves the problem that special groups cannot tolerate operations and general anesthesia in the prior art. According to the invention, the lauromacrogol injection with low recurrence rate is used as the drug for treating spontaneous pneumothorax by replacing operative treatment with drug treatment. A formula of the lauromacrogol injection comprises 1g of lauromacrogol, 5ml of ethanol (96%) and the balance of water for injection, wherein the total volume is 100ml. According to the pleural cavity adhesive, operation treatment is replaced by medicine treatment, after the lauromacrogol injection is injected into the pleural cavity, the pleura is stimulated to generate aseptic inflammation, coagulation, sclerosis, adhesion and closure, the painful reaction of the pleural cavity is not caused, and the pleural cavity adhesive is an ideal pleural cavity adhesive.
Owner:SHAANXI TIANYU PHARMA
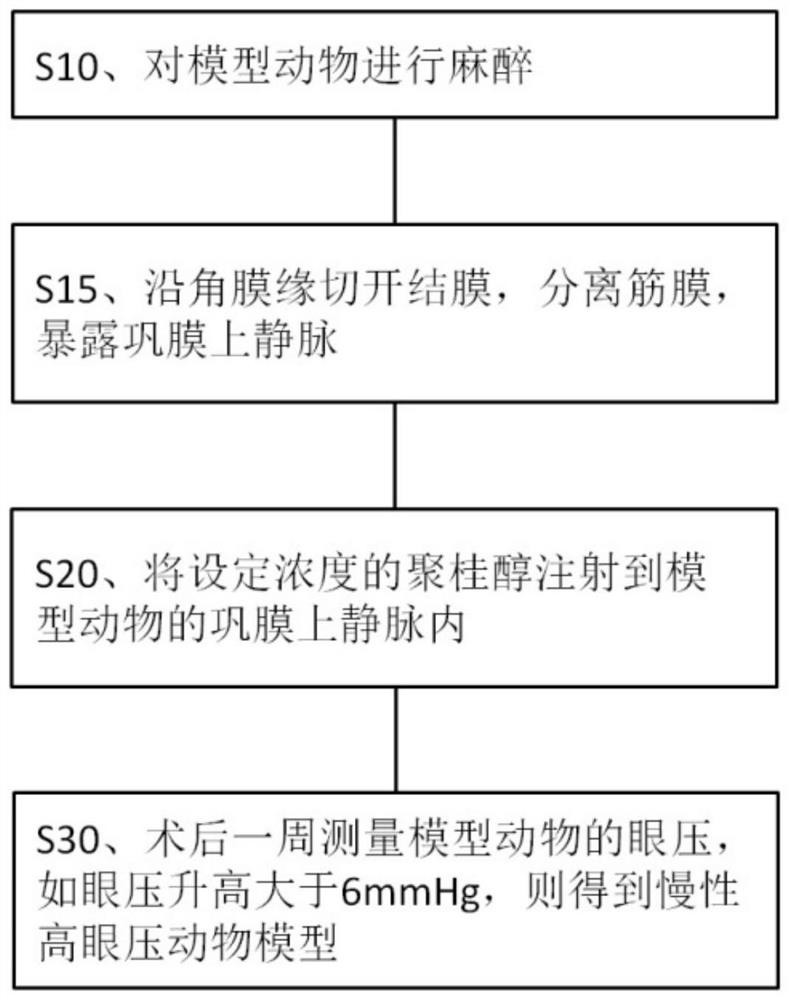
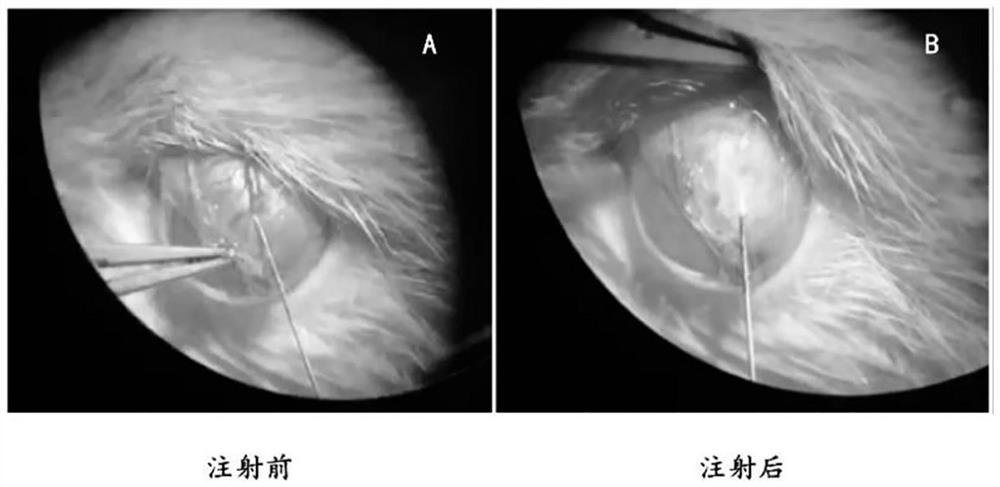
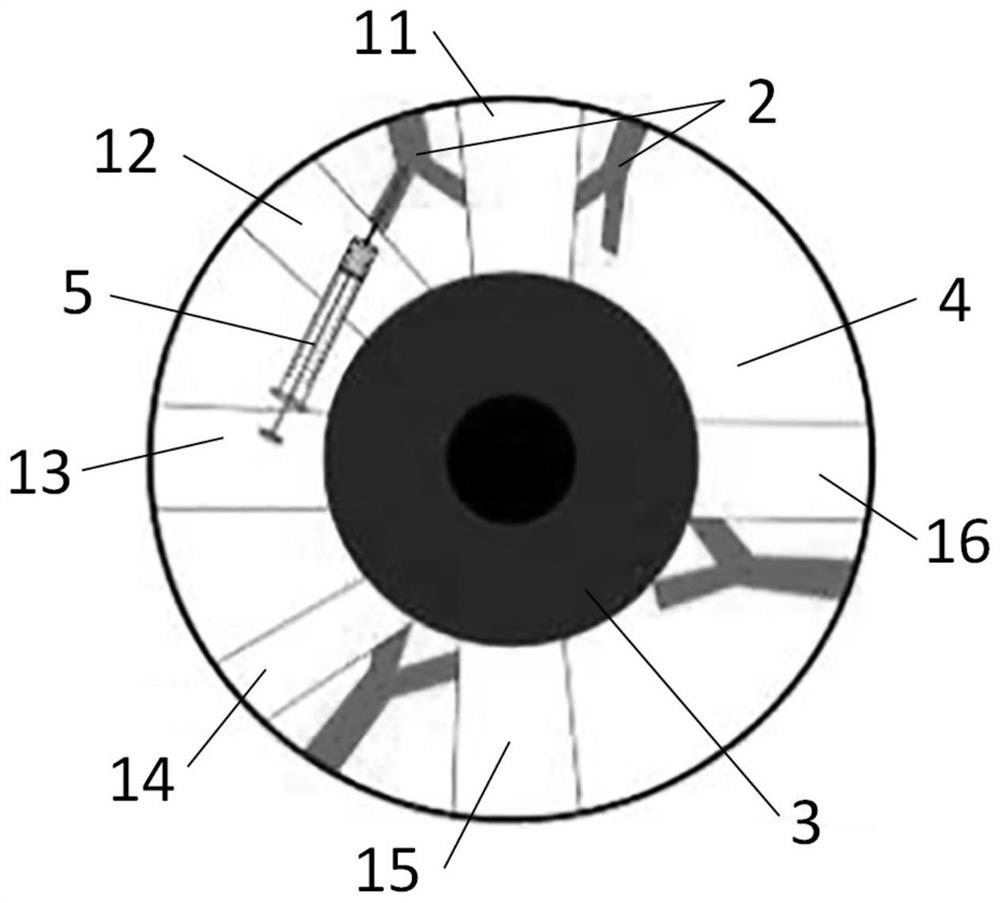
Application of lauromacrogol in preparation of chronic intraocular hypertension animal model and animal model
InactiveCN113273546APromote formationPlay a hemostatic effectAnimal husbandryIntraocular Pressure RiseIntra ocular pressure
The invention discloses application of lauromacrogol in preparation of a chronic intraocular hypertension animal model. The chronic intraocular hypertension animal model can be constructed by injecting lauromacrogol into suprascleral veins of a model animal, especially an animal model with chronic mild intraocular pressure rise. The model construction method is simple, the intraocular hypertension maintaining time is long, and complications do not exist. The chronic intraocular hypertension animal model can be used for simulating the chronic intraocular pressure rising process of open-angle glaucoma of people, is suitable for animal experiment research of screening glaucoma treatment drugs and glaucoma pathogenesis research, and has the application value of preclinical research.
Owner:CENT SOUTH UNIV
Anti-allergic, itching-relieving and anti-inflammatory pharmaceutical composition for skin and preparation method of anti-allergic, itching-relieving and anti-inflammatory pharmaceutical composition
InactiveCN112999263AFast absorptionGood curative effectAerosol deliveryInorganic non-active ingredientsUse medicationSide effect
The invention belongs to the field of traditional Chinese medicines, and relates to an anti-allergic, itching-relieving and anti-inflammatory pharmaceutical composition for skin and a preparation method thereof. The pharmaceutical composition comprises the following parts in percentage by weight: 1%-10% of litsea cubeba oil and 0.5%-5% of lauromacrogol, is derived from plants, is soft in smell and special in effect, has the characteristics of quicker absorption, good curative effect, convenience in use, no sticky feeling on skin during use, no irritation, safety, no toxic or side effect and the like, and increases the medication compliance of patients.
Owner:福建宸润生物科技有限公司
Application of lauromacrogol and methotrexate in preparation of medicine for treating cesarean scar pregnancy
InactiveCN108785324AAccelerated necrotic processFacilitate strippingSynthetic polymeric active ingredientsSexual disorderObstetricsEmbryo
The invention provides application of lauromacrogol and methotrexate in preparation of a medicine for treating cesarean scar pregnancy. The method comprises the following steps: inserting needle fromthe posterior fornix of uterus under ultrasonic guidance, enabling puncture needle to directly reach the gestational sac, extracting gestational sac fluid, and inactivating with lauromacrogol to causequick inactivation of the embryo; injecting methotrexate into the gestational sac and multiple surrounding points thereof. By virtue of drug combination, trophocyte necrocytosis can be effectively accelerated, trophoderm peel is facilitated, and HCG (Human Chorionic Gonadotropin) of blood is rapidly lowered. Additionally, multi-point injection is performed in a blood flow richness region of a peripheral muscle layer of the incision with the lauromacrogol, peripheral flow of the trophoderm is effectively blocked, the blood flow of the gestation sac and peripheral muscle layer is obviously reduced, the HCG of the blood is further lowered, and massive haemorrhage of uterine curettage, incision hematoma, perforation of uterus and other complications under ultrasonic guidance are obviously reduced.
Owner:盐城协和医院 +1
Efficient painless drug for treating local tumor ablation
InactiveCN103191146BIncrease injection volumeImprove complianceHydroxy compound active ingredientsPharmaceutical delivery mechanismColor dopplerBlood flow
The invention discloses an efficient painless drug for treating local tumor ablation. The efficient painless drug is mixed solution of absolute ethyl alcohol and lauromacrogol, wherein the ratio of lauromacrogol is 0.1-99.9%, and the ratio of absolute ethyl alcohol is 99.9%-0.1% according to the volume fraction. The efficient painless drug for treating local tumor ablation, which is injected into local tumor under guide of an ultrasonic imaging technique, is the mixed solution of absolute ethyl alcohol and lauromacrogol according to the volume ratio of 19 / 1 to 99 / 1; and volume ratio of absolute ethyl alcohol to lauromacrogol preferably is 99 / 1 or 19 / 1; the efficient painless drug for treating local tumor ablation is applied to preparation of the drug for treating ablation by being injected to the tumor under guide of the ultrasonic imaging technique; the sufferer has no adverse reaction such as pain in an operation; and the postoperation color Doppler ultrasonic review finds out that the tumor echo is increased, blood flow signals are obviously reduced, and the indexes of a part of patients with rising alpha fetal protein are obviously reduced by review.
Owner:FUJIAN MEDICAL UNIV UNION HOSPITAL
Lauromacrogol external gel and preparation method thereof
The invention relates to a lauromacrogol gel for external use and a preparation method thereof. The components include urea, lauromacrogol, aloe extract, benzalkonium chloride, sodium alginate, potassium sorbate, menthol, glycerin, dodecahydrate Disodium hydrogen phosphate, sodium dihydrogen phosphate dihydrate and purified water. The feature of the present invention is to combine lauromacrogol, which has rapid antipruritic and pain relief, anti-inflammatory and detumescent effects, and aloe vera extract, which has astringent and skin repairing effects, and is used in the form of a gel film to treat 1-2 degree burns and mosquitoes Bites and bruises patients; this is beyond the reach of similar drugs for the treatment of burns and mosquito bites, and there is no similar treatment of anti-inflammatory products or related inventions.
Owner:SHAANXI TIANYU PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com