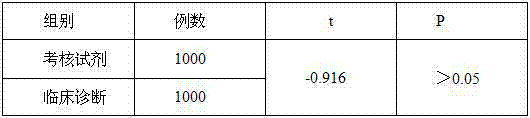
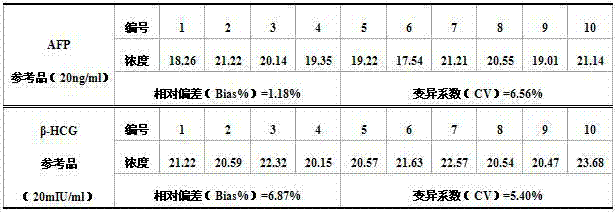
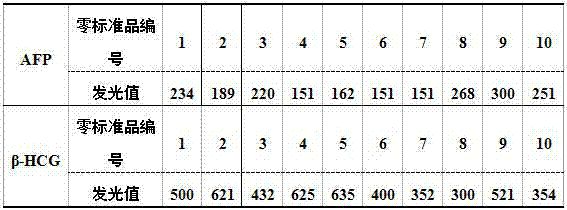
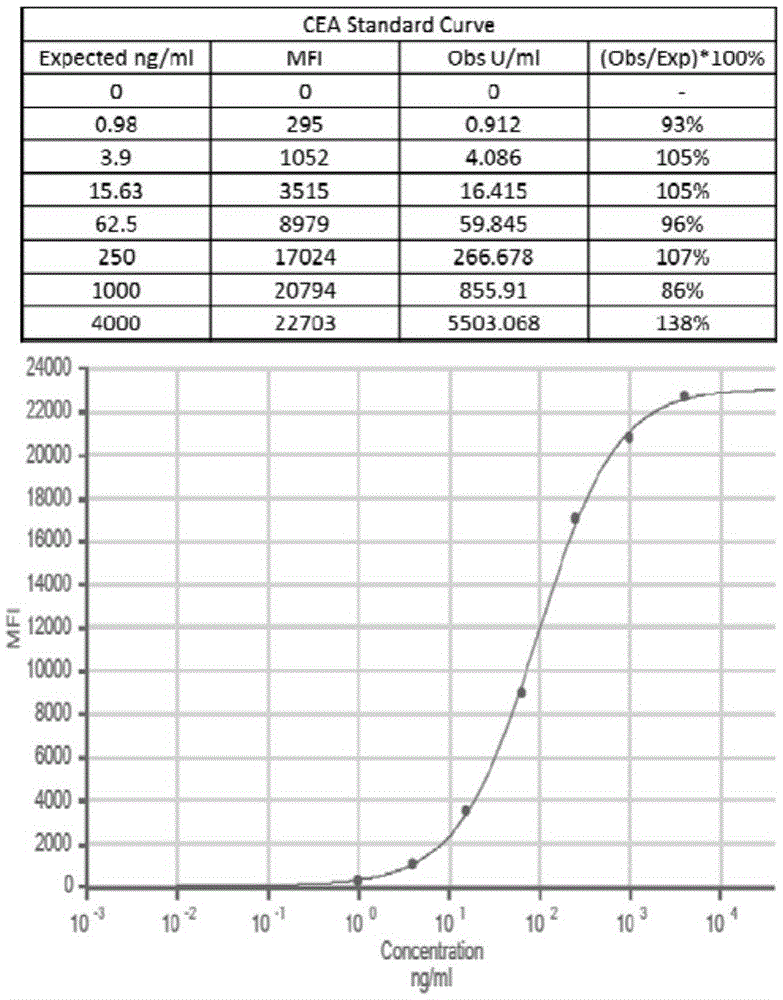
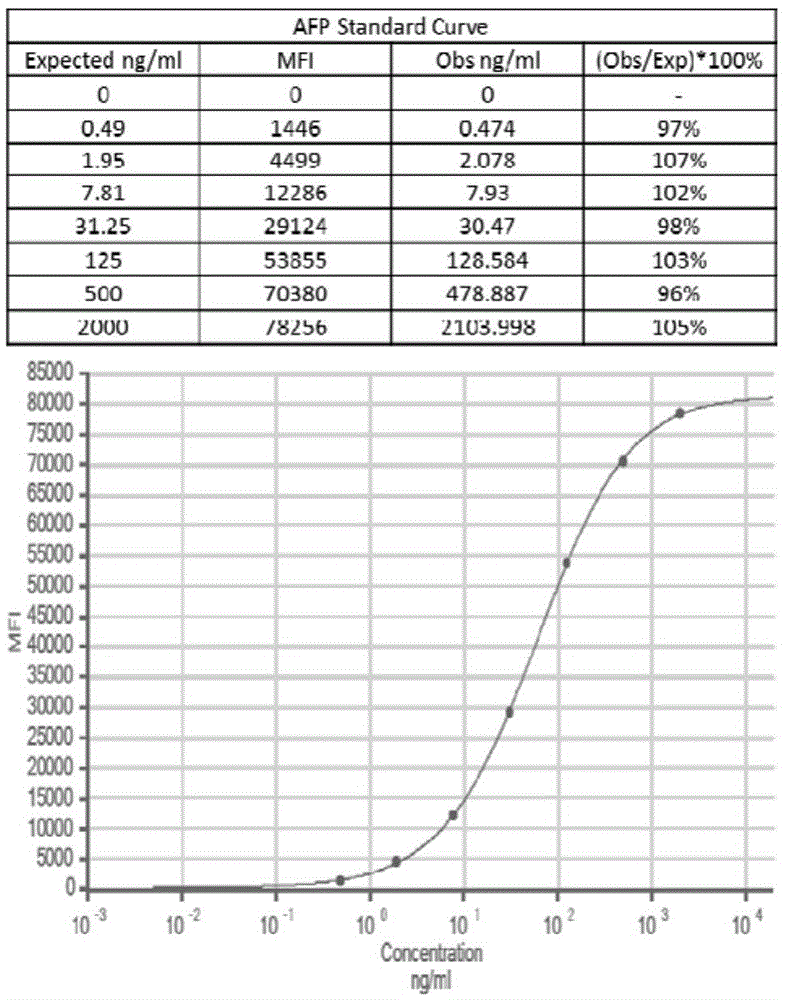
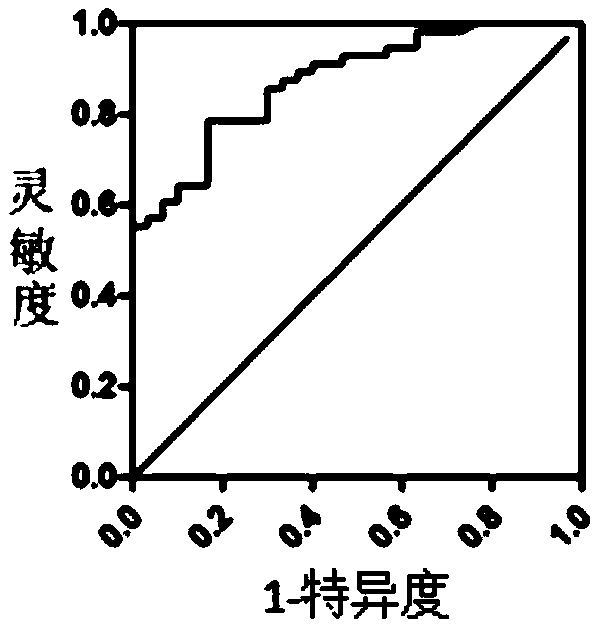
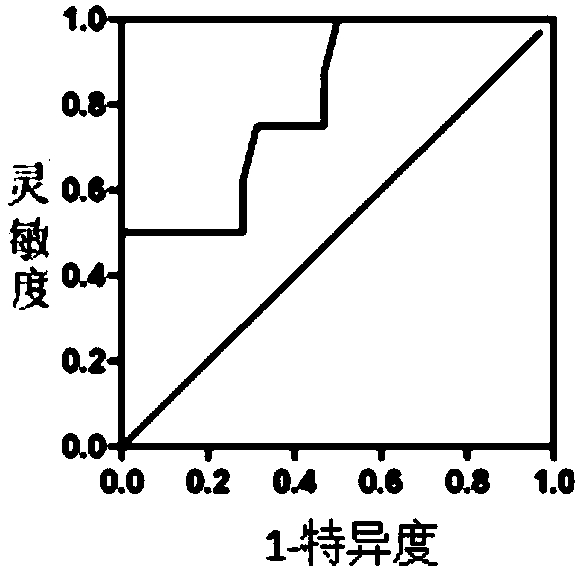
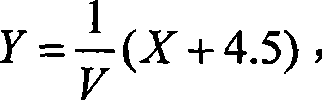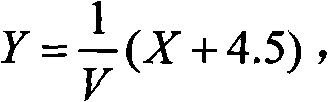Patents
Literature
45 results about "Fetal protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fetal proteins are high levels of proteins present during the fetal stage of development. Often related proteins assume similar roles after birth or in the embryo, in which case the fetal varieties are called fetal isoforms. Sometimes, the genes coding fetal isoforms occur adjacent to their adult homologues in the genome, and in those cases a locus control region often coordinates the transition from fetal to adult forms. In other cases fetal isoforms can be produced by alternate splicing using fetal exons to produce proteins that differ in only a portion of their amino acid sequence. In some situations the continuing expression of fetal forms can reveal the presence of a disease condition or serve as a treatment for diseases such as sickle cell anemia. Some well known examples include...
Preparation method of unlabelled photoelectrochemical alpha fetoprotein immunosensor
InactiveCN104569435AEasy to makeEasy to operateBiological testingMaterial electrochemical variablesChemical synthesisPhysical chemistry
The invention discloses a preparation method of an unlabelled photoelectrochemical alpha fetoprotein immunosensor, and belongs to the fields of novel nano functional materials and biosensors. On the basis of a titanium dioxide nano particle base, a trimetal alloy nano material in a dendrite nanorod form is prepared by a photoelectrochemical synthetic method, so that the prepared unlabelled photoelectrochemical alpha fetoprotein immunosensor for detecting alpha fetal protein is low in cost, high in sensitivity, good in specificity, fast in detection and simple to prepare.
Owner:UNIV OF JINAN
Alpha fetal protein (AFP) detection method based on fluorescent nano luminous and magnetic nano material
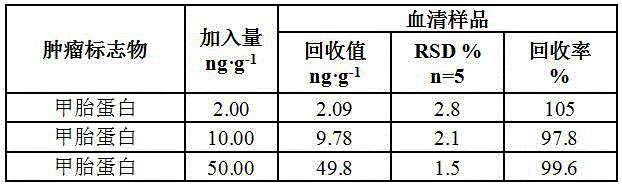
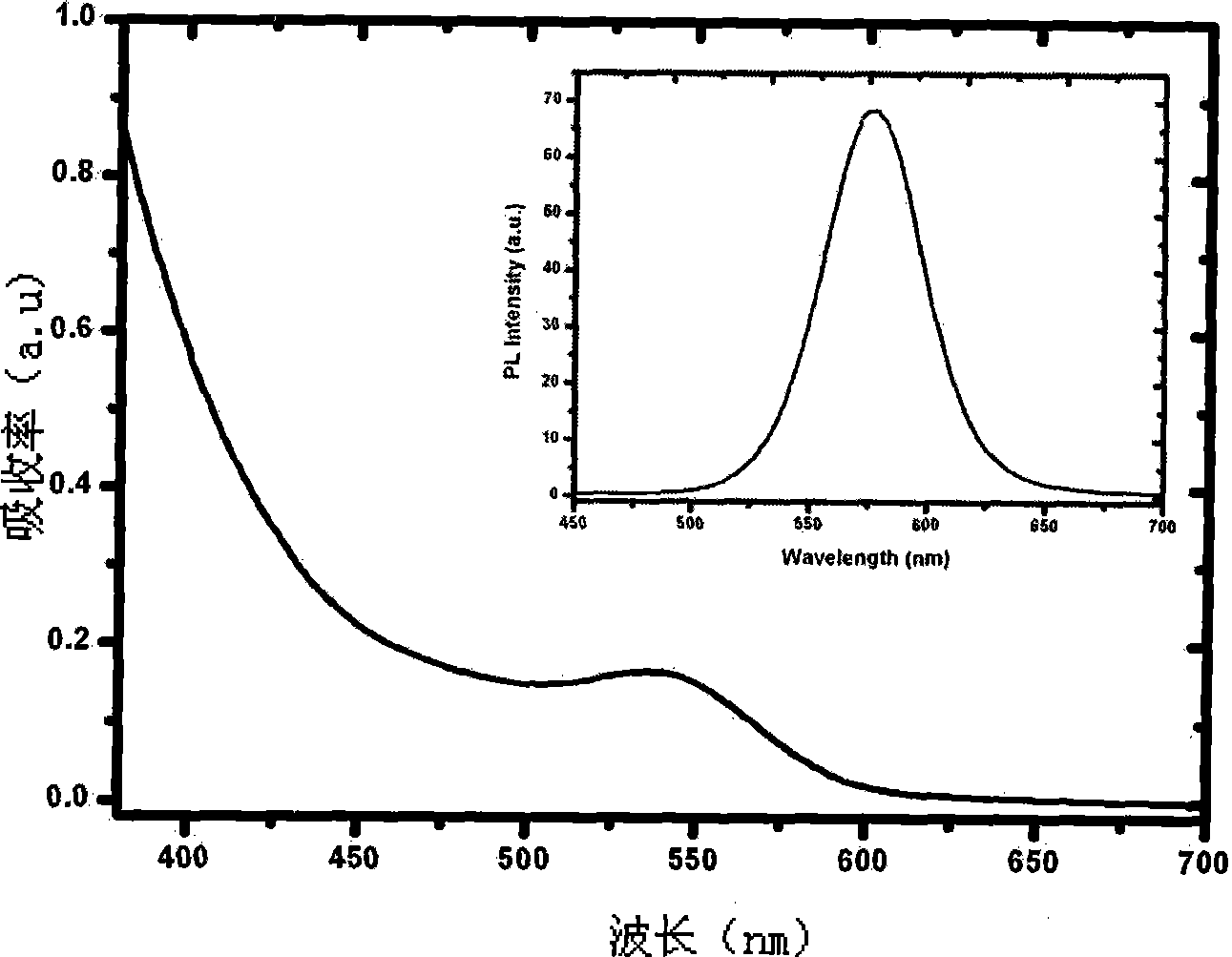
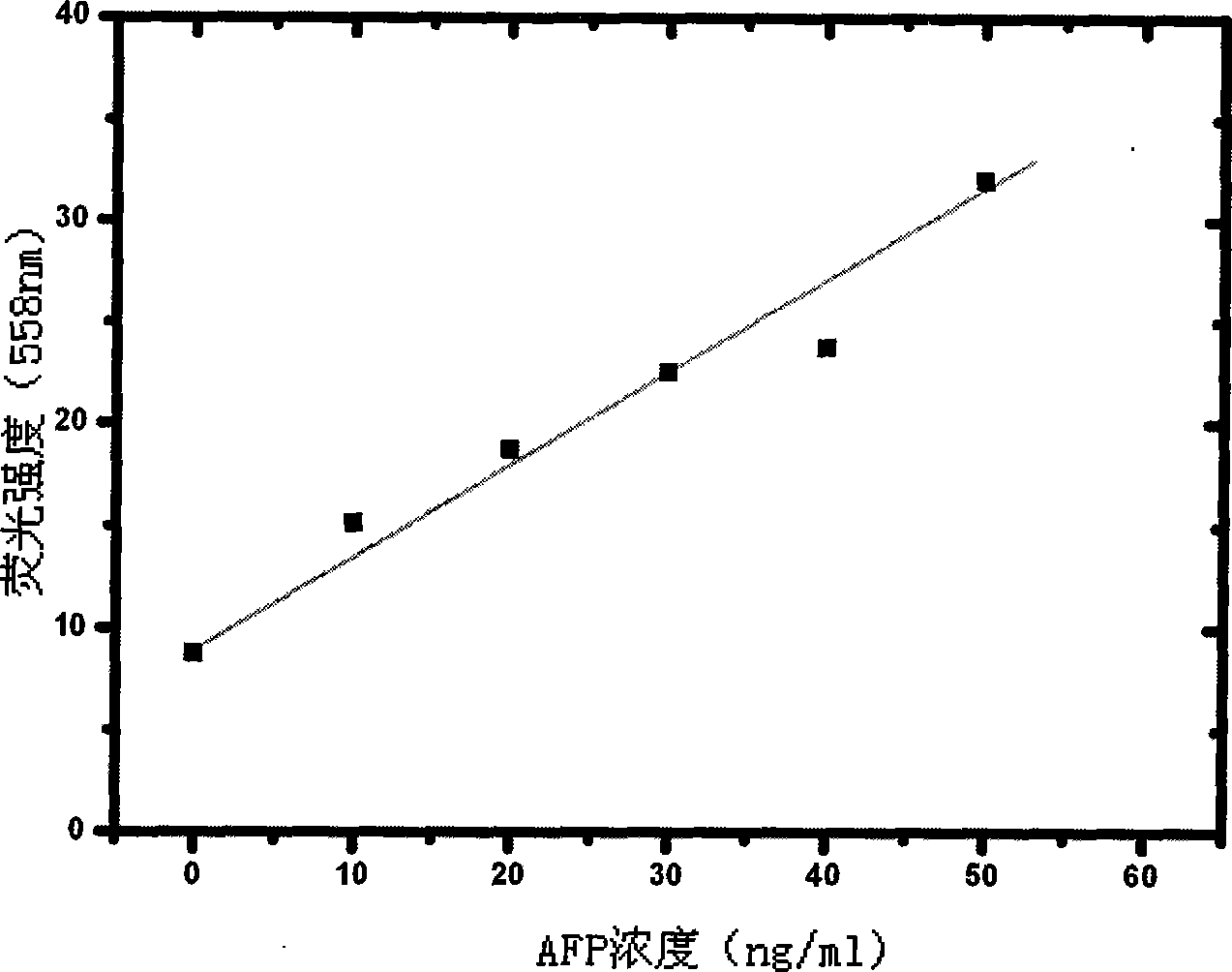
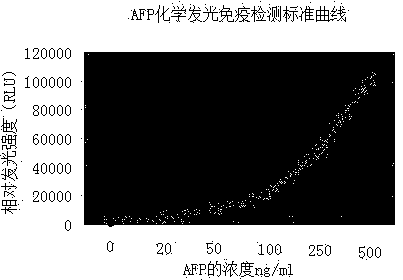
The invention relates to a method for determining the content of AFP in serum by using CdTe quantum dots and Fe3O4-Dextran nano particles, characterized by comprising the steps of (a) preparing Fe3O4-Dextran-first antibody complex; (b) preparing CdTe-second antibody complex; (c) drawing 'fluorescence intensity-AFP concentration' standard work curve, and calculating work equation; and (d) measuring the fluorescence intensity value for the work equation to determine the AFP content of the patents with liver cancer. The method is simple and has high sensitivity, can easily detect the disease for the early cancer patients so as to treat the patients in time, and furthermore, the method opens up a road for the clinic detection of liver cancer.
Owner:DONGHUA UNIV
AFP (alpha fetal protein) detection kit
ActiveCN104360085AQuick checkSimple and fast operationBiological testingTrue positive rateQuality control
The invention relates to the field of biological detection, and particularly relates to a detection kit for quantitatively detecting AFP (alpha fetal protein) and a preparation method and use of the detection kit. The invention provides a detection kit for quantitatively detecting AFP. The detection kit comprises a test paper card, wherein the test paper card comprises a bottom plate, and a sample pad, a gold-marking pad, a nitrocellulose membrane and an absorbent pad which are arranged on the surface of the bottom plate and are sequentially arranged from the loading end; the gold-marking pad comprises an AFP antibody; the nitrocellulose membrane is coated with a detection line and a quality control line; and the AFP antibody on the gold-marking pad is marked by virtue of fluorescent microspheres. The detection kit for quantitatively detecting the AFP, provided by the invention, is used for detecting the AFP through a fluorescent microsphere immune chromatography technology for the first time, has sensitivity and specificity, and has the advantages of being rapid and easy to operate, accurate in result, economical, applicable and the like.
Owner:ZYBIO INC
Magnetic particle chemiluminescence immune assay kit of tumor marker AFP (alpha fetal protein) and detection method thereof
InactiveCN104034892AEasy to separateAdequate and prompt responseChemiluminescene/bioluminescenceDisease diagnosisFluorescein isothiocyanateImmuno detection
The invention relates to a magnetic particle chemiluminescence immune assay kit of tumor marker AFP (alpha fetal protein) and a detection method of the magnetic particle chemiluminescence immune assay kit, which belongs to the technical field of the immune detection and analysis. An AFP monoclonal antibody marked by fluorescein isothiocyanate (FITC) and a monoclonal antibody marked by alkaline phosphatase (AP) are combined with an antigen to form a sandwiched immune compound of an FITC marked antibody-antigen-AP marked antibody, and the sandwiched immune compound is similar to a sandwich structure. Then the magnetic particles which are connected with the anti-FITC antibody are added, the specific binding of the anti-FITC antibody and the FITC enables antigen-antibody complex to be connected onto the magnetic particles, the magnetic particles are directly precipitated in an external magnetic field, and the compound formed in the immune reaction manner can be separated from other non-combined substances without centrifuging. According to the kit, the chemiluminescence is combined with the magnetic particles, a reaction system which is approximate to the homogenous phase is provided, and compared with the prior art, the kit has the advantages of high sensitivity, wide linear range, high speed and the like; moreover, the product cost is greatly reduced, and the application prospect on the aspects such as the clinical inspection is promising.
Owner:GUANGXI DOCTOR HAIYI INFORMATION TECH
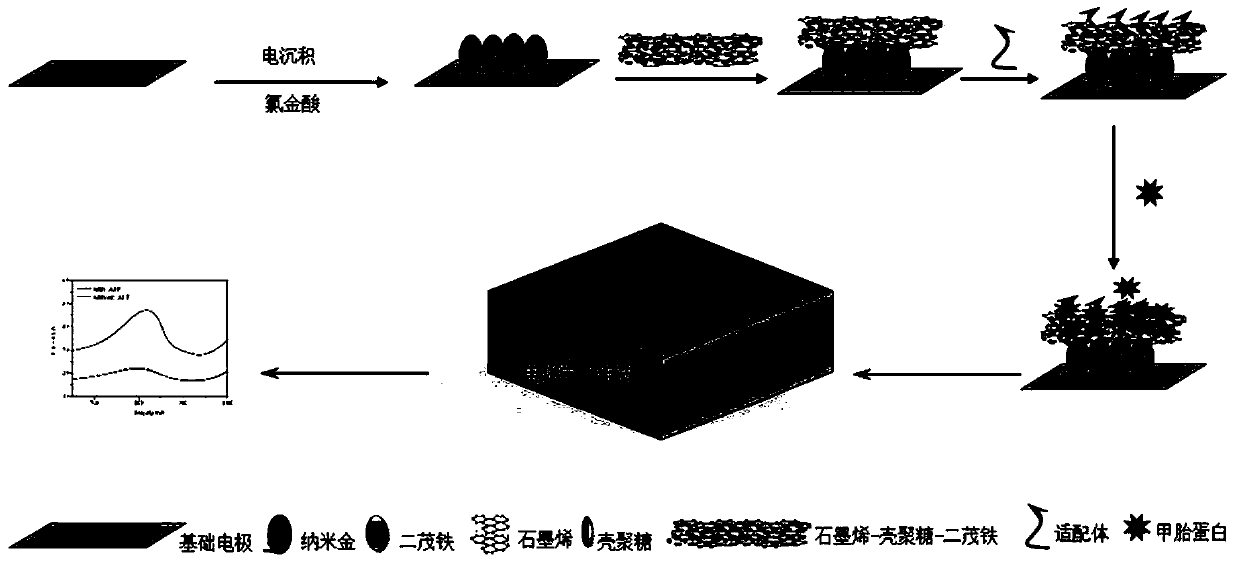
Method for detecting alpha-fetal protein on basis of RGO-CS-Fc/Au NPs nano composite material and aptamers
ActiveCN110146581AReduce distractionsHigh affinityMaterial electrochemical variablesScreen printed electrodeMaterials science
The invention relates to a method for detecting alpha-fetal protein on the basis of an RGO-CS-Fc / Au NPs nano composite material and aptamers. According to the method, the surface of a silk-screen printing electrode is modified with RGO-CS-Fc / Au NPs by means of an electro-deposition technology and an electrostatic adsorption effect; AFP aptamers are loaded on the surface of the RGO-CS-Fc / Au NPs bymeans of a nanotechnology and an intermolecular force, and the aptamers exist on the surface of the composite material in the form of single-chain structures due to the unstable spatial structures ofthe aptamers; after AFP is added into the surface of the electrode, the AFP can be specifically combined with the AFP aptamers, so that stable spatial structures can be generated, and therefore, the AFP and the aptamers can be orderly arranged on the surface of the electrode; a current value is detected through a DPV method, a relation curve of current and the concentration of the alpha-alpha protein is depicted; and therefore, the quantitative detection of the alpha-alpha protein is realized. The method has the advantages of simple operation, time-saving performance, low cost and low detection limit.
Owner:GUILIN UNIV OF ELECTRONIC TECH
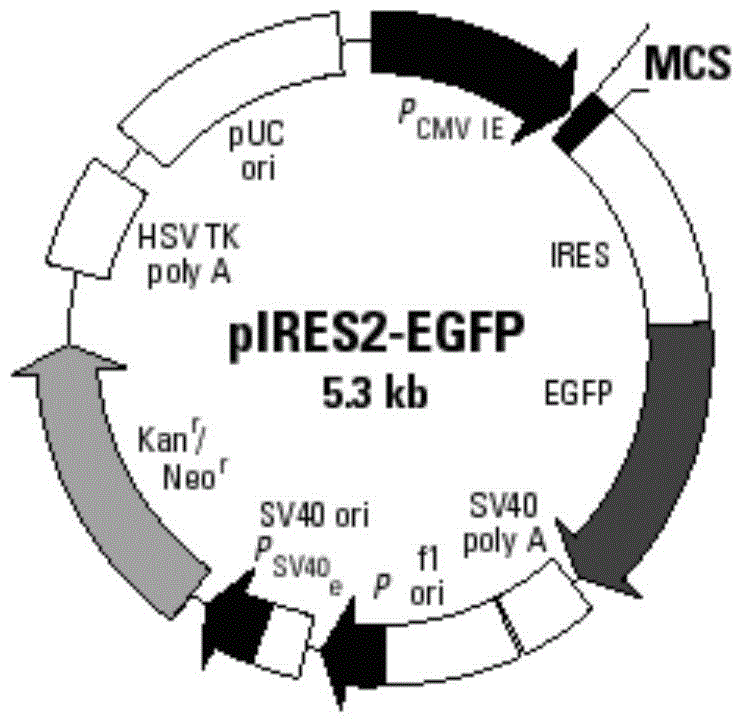
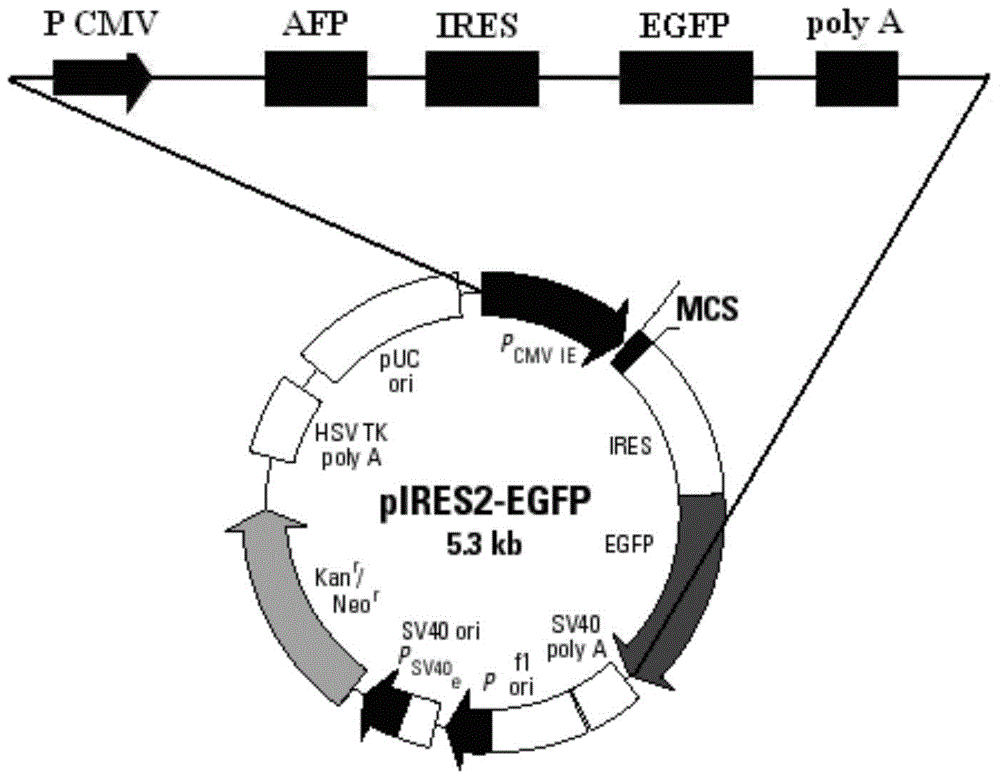
AFP and GM-CSF dual-gene co-expression recombinant vector as well as preparation method and application of recombinant vector
ActiveCN103555762AReduce the introductionGood immune regulationGenetic material ingredientsDigestive systemNucleotideGene coexpression
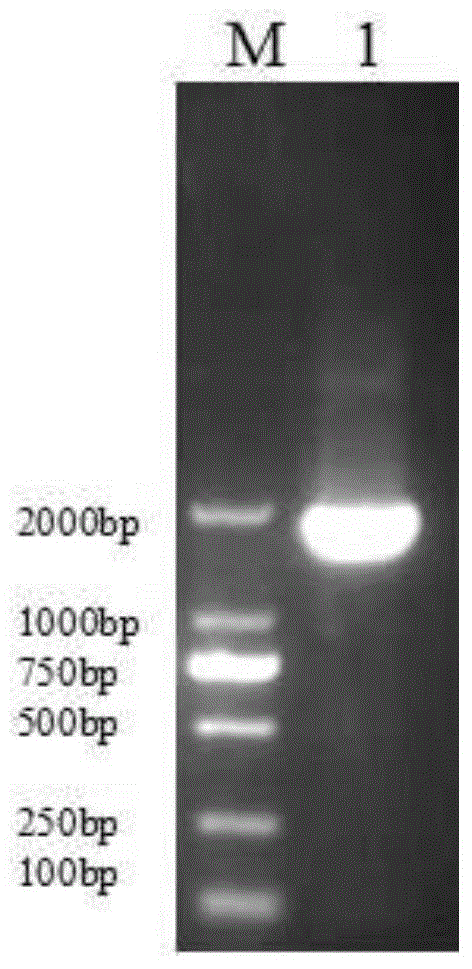
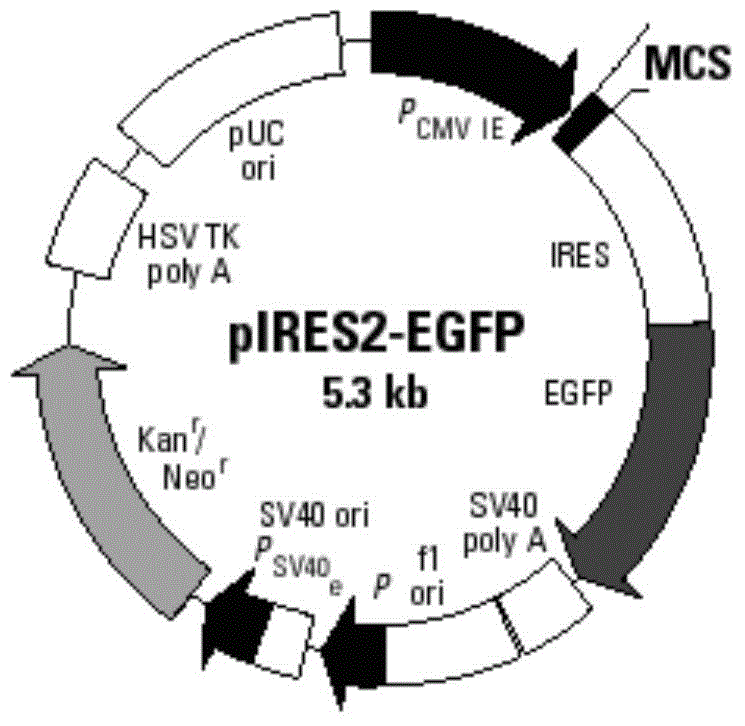
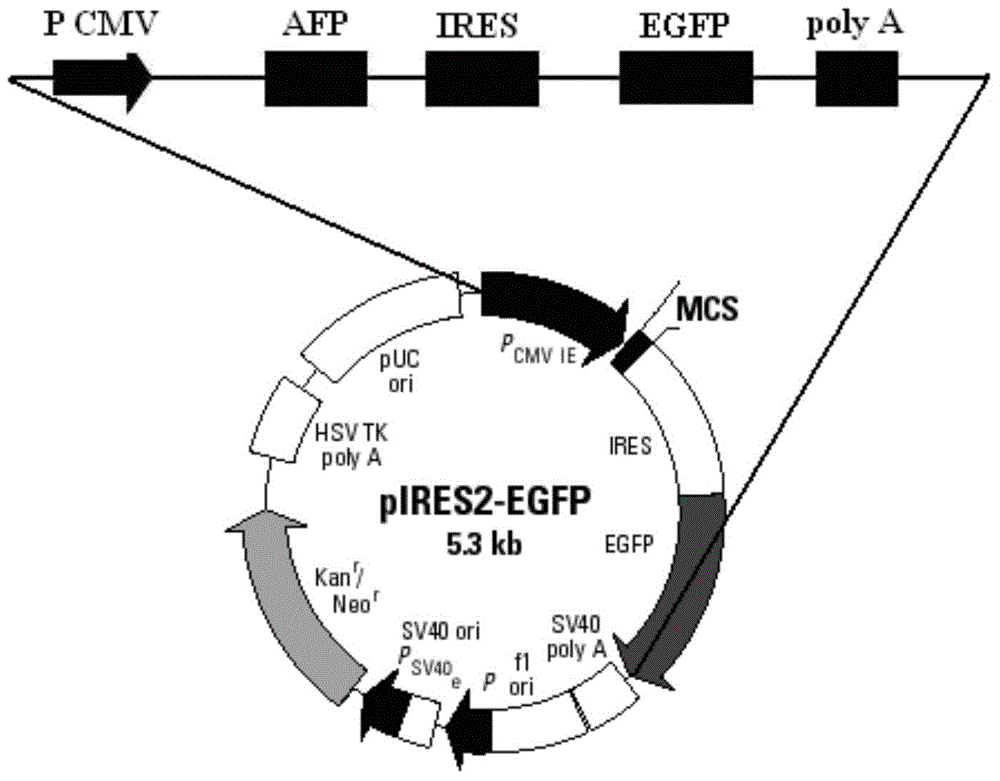
The invention discloses an AFP and GM-CSF dual-gene co-expression recombinant vector, which is sequentially linked with an AFP gene, an IRES sequence and a GM-CSF gene in a vector transcription direction, or is sequentially linked with a GM-CSF gene, an IRES sequence and an AFP gene in a vector transcription direction, wherein nucleotide sequence of the AFP gene is represented in SEQ ID NO: 1 in a sequence list, nucleotide sequence of the GM-CSF gene is represented in SEQ ID NO: 2 in the sequence list, and nucleotide sequence of the IRES sequence is represented in SEQ ID NO: 3 in the sequence list. The dual-gene co-expression recombinant vector, which links the AFP gene and the GM-CSF gene through the IRES sequence, can simultaneously express alpha fetal protein and a granulocyte-macrophage colony stimulating factor in a same vector; and the recombinant vector, in immunogene therapy of liver cancer, not only can develop an immune regulating function of a cell factor but also can generate a specific anti-tumor effect targeted for the liver cancer.
Owner:XINXIANG MEDICAL UNIV
High-sensitivity antenatal screening kit of Down's Syndrome in pregnant women at second trimester as well as preparation and detection methods of kit
ActiveCN102866254APrenatal screening is safe and non-invasiveHigh sensitivityMaterial analysisEnzyme bindingBiochemistry
The invention discloses a high-sensitivity antenatal screening kit of Down's Syndrome in pregnant women at second trimester as well as preparation and detection methods of the high-sensitivity antenatal screening kit. The kit comprises a kit body, AFP (Alpha Fetal Protein) and beta-HCG (Human Chorionic Gonadotropin) coated plates, AFP and beta-HCG standard products, AFP and beta-HCG quality control products, AFP and beta-HCG enzyme conjugates, sample diluent, light-emitting substrate liquids and concentrated scrubbing liquid. The preparation method comprises the following steps of: preparing monoclonal antibody coated plates of AFP and beta-HCG; preparing the standard products and the quality control products of AFP and beta-HCG; and preparing the enzyme conjugates of AFP and beta-HCG; and preparing the sample diluent, a light-emitting substrate liquid A, a light-emitting substrate liquid B, the concentrated scrubbing solution and the like. The high-sensitivity antenatal screening kit of the Down's Syndrome in the pregnant women at the second trimester has the advantages that the purpose of the antenatal screening of the Down's Syndrome in the pregnant women at the second trimester is realized, and the accordance rate with the clinic diagnosis reaches more than 99.7%; and the kit is high in sensitivity, strong in the specificity and accurate and reliable in result.
Owner:山东康华生物医疗科技股份有限公司
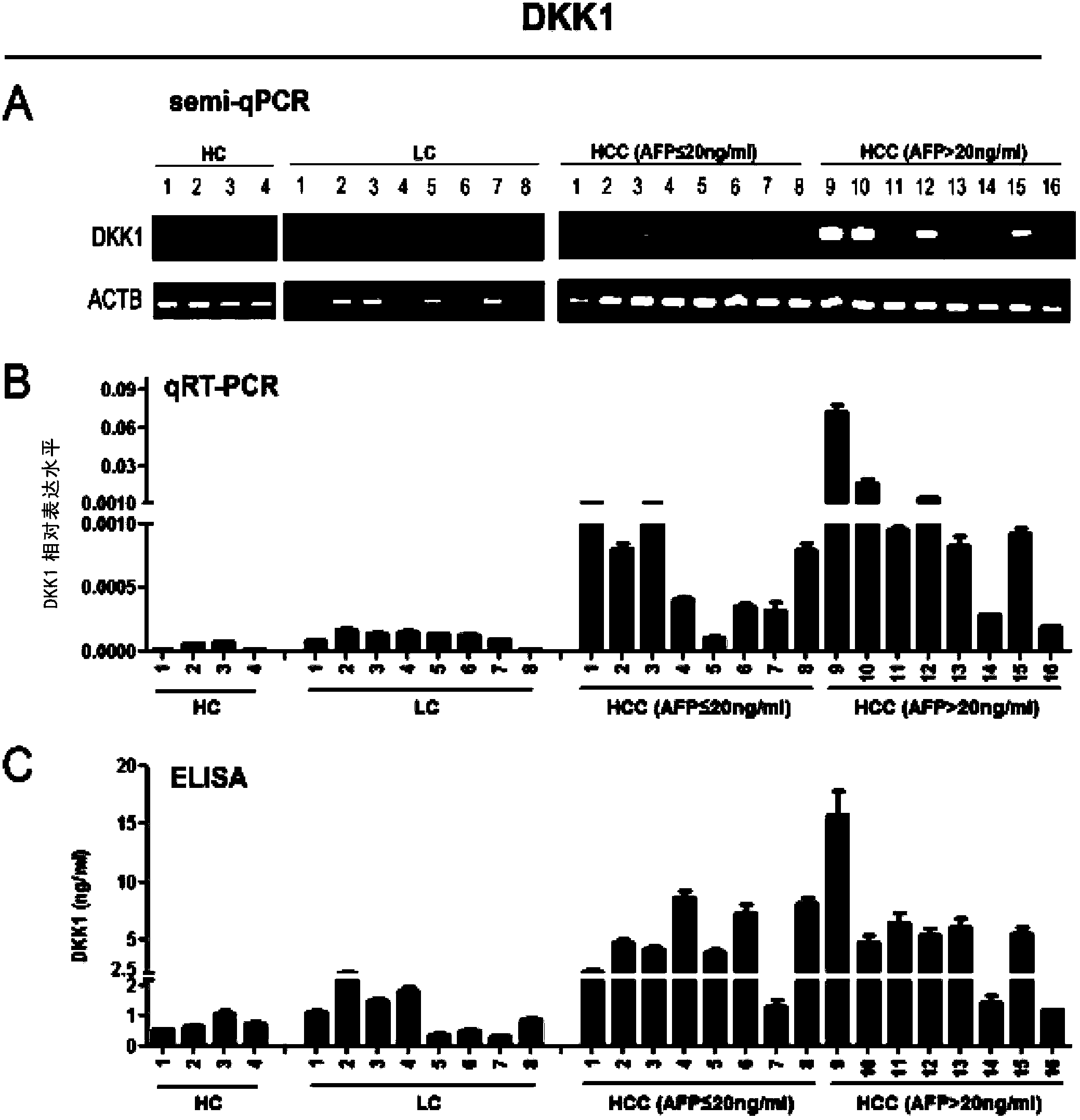
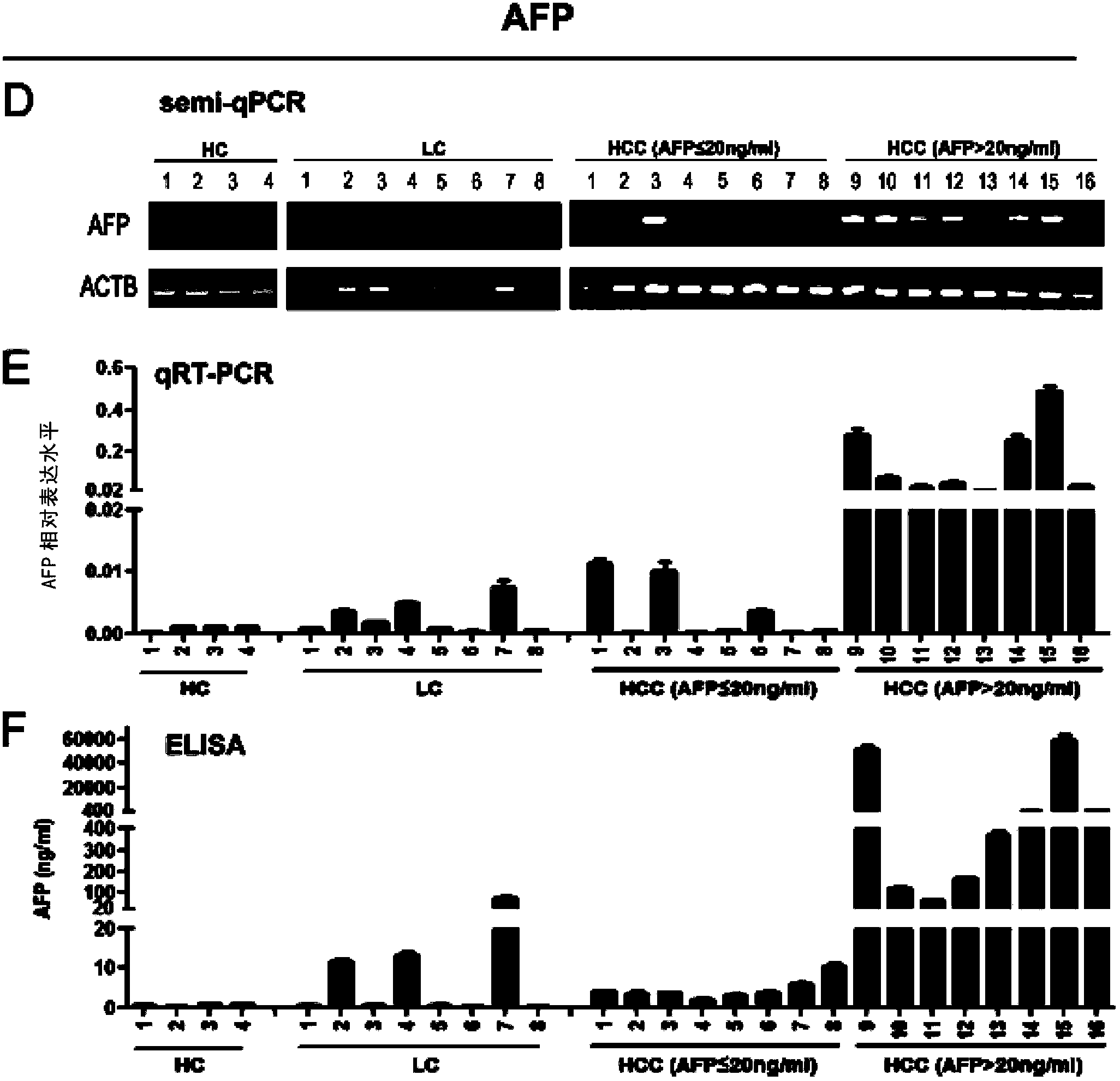
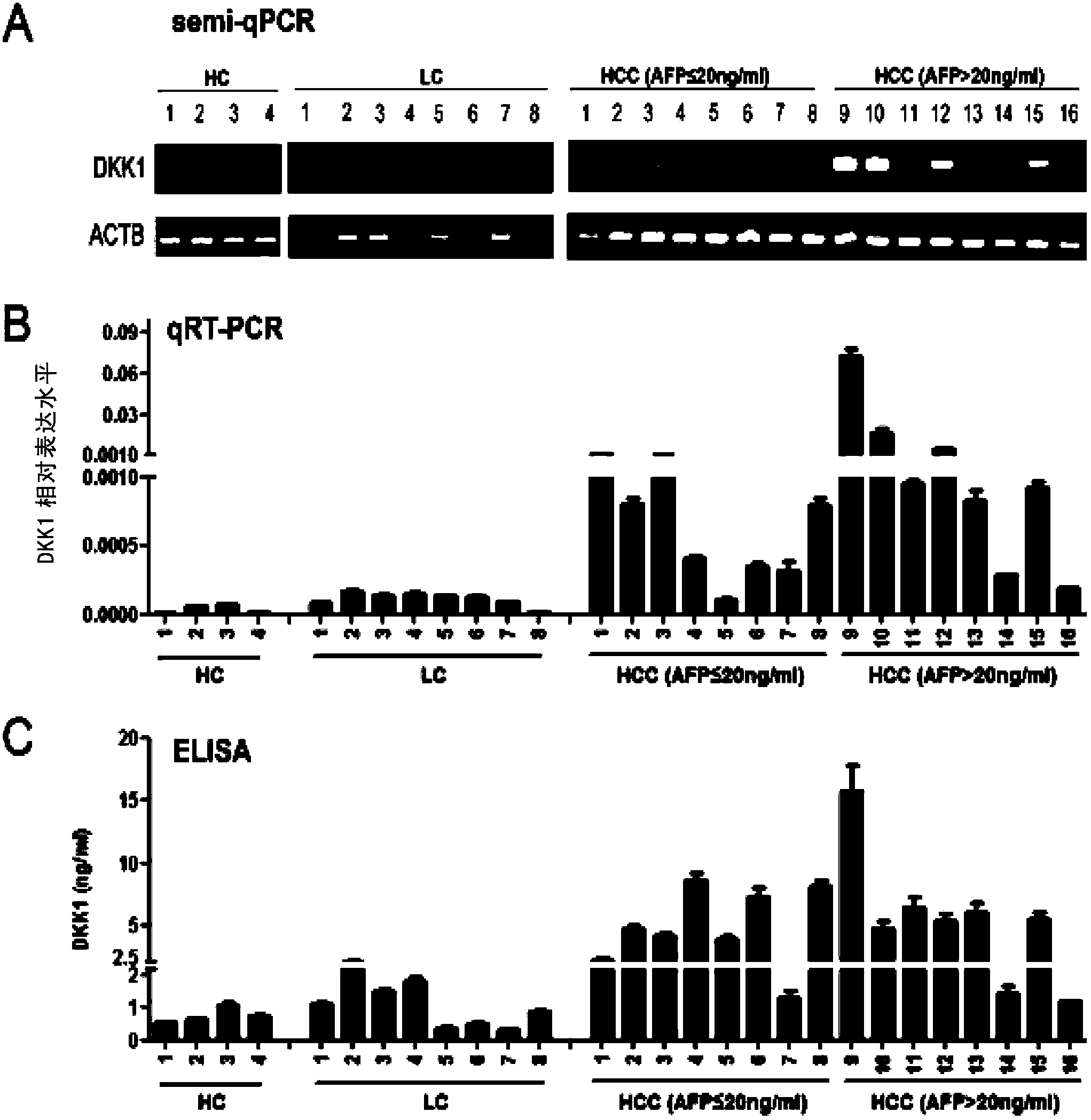
Application of DKK1 as diagnostic marker
InactiveCN103487580AMicrobiological testing/measurementBiological material analysisTrue positive rateDKK1
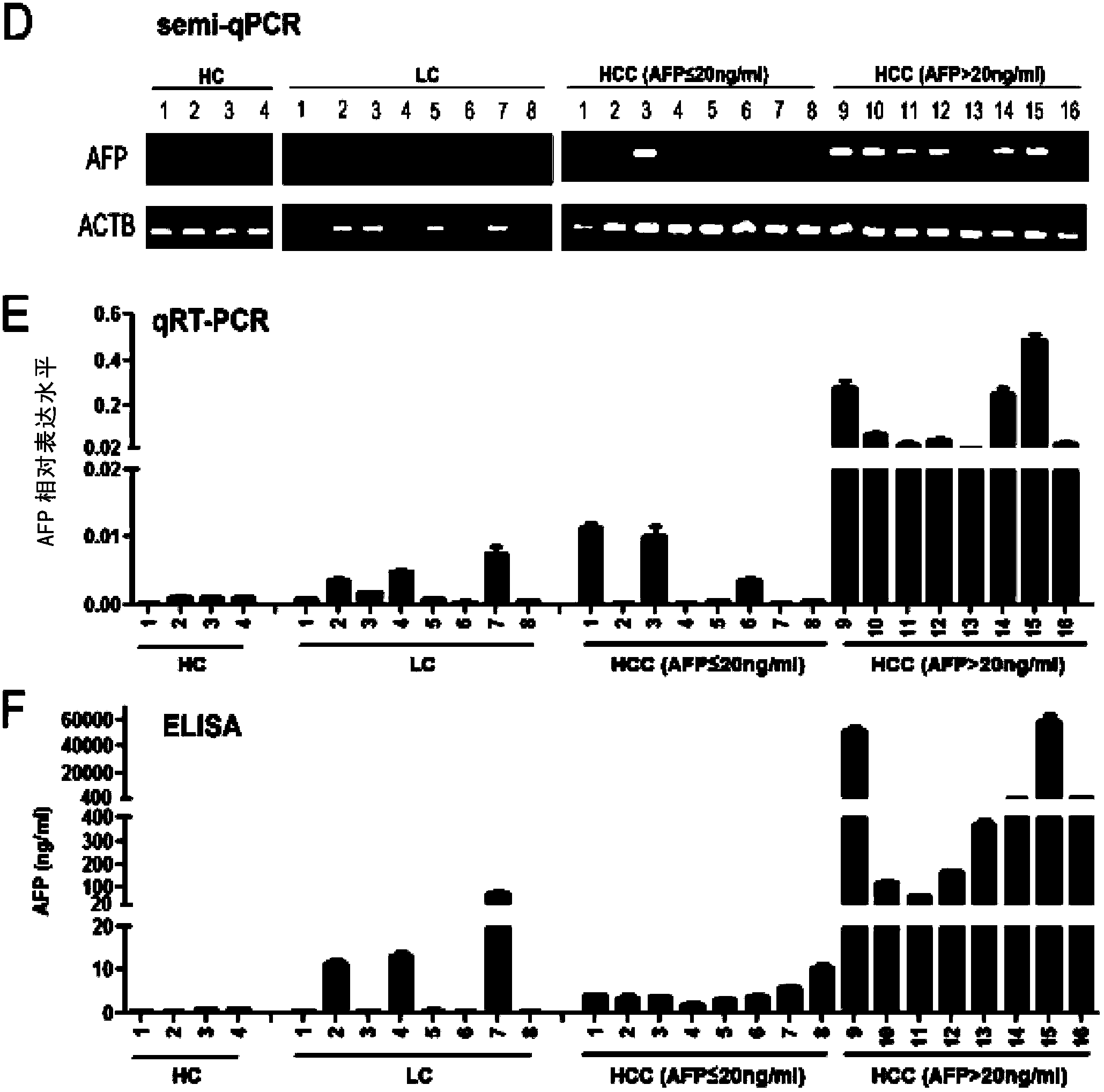
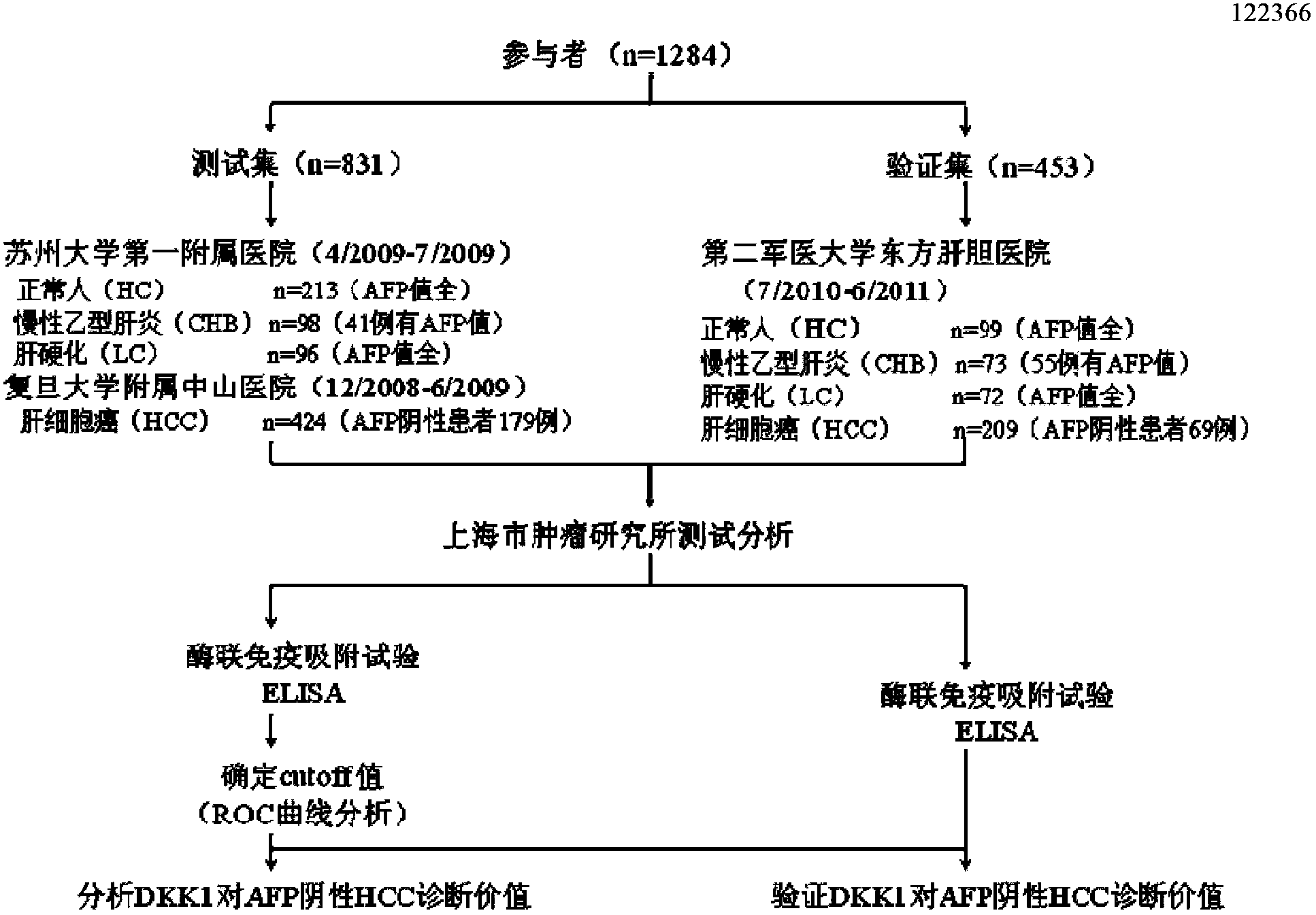
The invention discloses an application of DDK1 as a diagnostic marker. Through analysis of a large amount of hepatocellular carcinoma samples, the inventor analyzes the expressions of DKK1 and AFP, firstly discovers that serum DKK1 has a high sensitivity and specificity on diagnosis of hepatocellular carcinoma, especially diagnosis of alpha fetal protein negative hepatocellular carcinoma, identification of hepatocellular carcinoma and alpha fetal protein positive chronic liver disease, and / or diagnosis of hepatocellular carcinoma in the early stage or small hepatocellular carcinoma, and more especially the precision of clinical diagnosis can be largely increased by DKK1-AFP combined diagnosis.
Owner:SHANGHAI INST OF ONCOLOGY
Combined general check protein chip for early-stage cancers mainly comprising lung cancer
The invention discloses a combined general check protein chip for early-stage cancers mainly comprising lung cancer and belongs to the technical field of a protein chip. The combined general check protein chip for the early-stage cancers mainly comprising the lung cancer, provided by the invention, comprises a chip substrate and marker antibodies distributed on the chip substrate, wherein the chip substrate is an FAST protein chip substrate having a three-dimensional nitrocellulose basic material; one or more sample application module is arranged on the FAST protein chip substrate; the following marker antibodies are fixed on the sample application module: 14-3-3 theta, LAMR-1, TSGF (Tumor Specific Growth Factor), CEA (Cancer Embryo Antigen), SCC (Squamous Cell Carcinoma), CYFRA21-1 and positive control; the following marker antibodies are also fixed: AFP (Alpha Fetal Protein), CA242, MG-Ag, CA199, TPA (Tissue Polypeptide Antigen) and EA-IgA; and the following marker antibodies are further fixed: CA125, CA153, SF (Serum Ferritin) and PSA (Prostate Specific Antigen). According to the combined general check protein chip provided by the invention, the related indexes are highly integrated; the combined general check protein chip has the advantages of excellent stability, strong specificity, long retention period, high sensitivity, excellent repeatability, convenience in operation and low cost; the labor efficiency is increased; and a cancer patient is diagnosed and treated from the general check before clinical symptoms occur.
Owner:马鞍山微因泰克生物科技有限公司
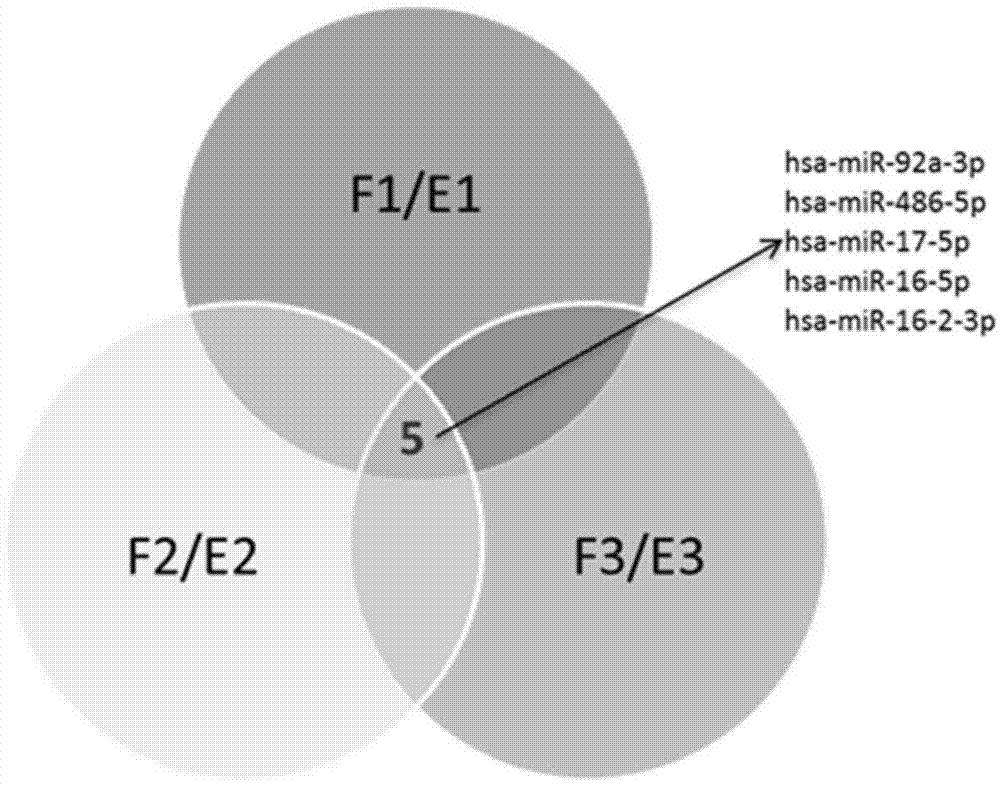
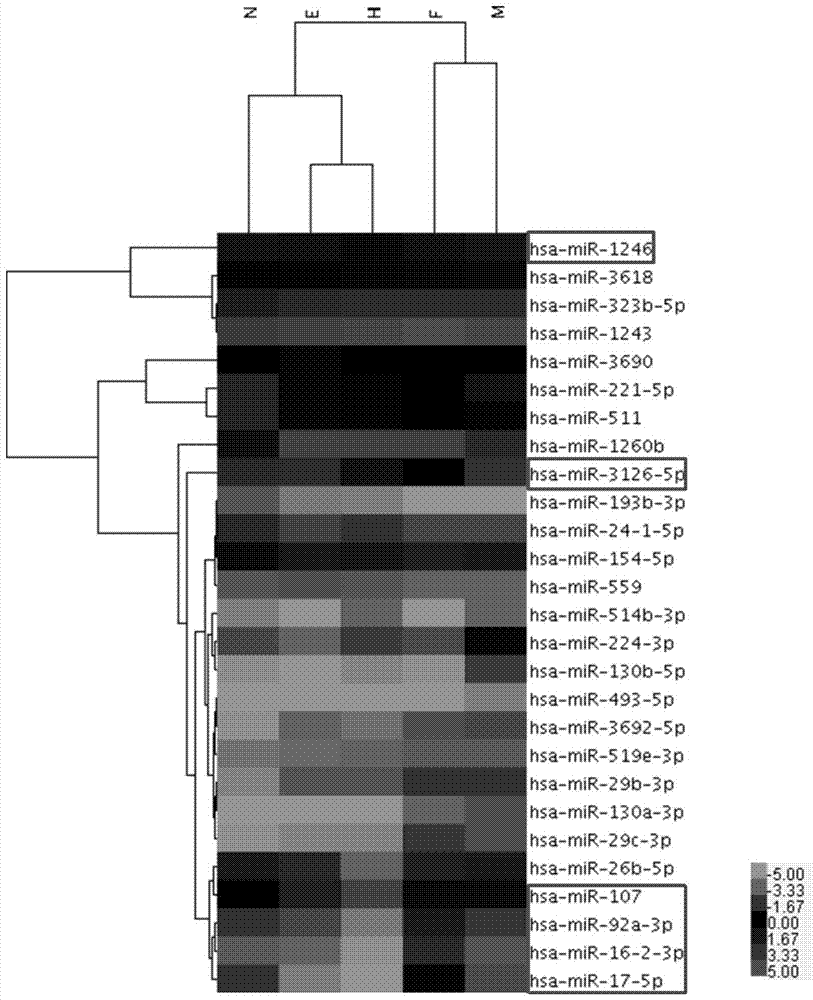
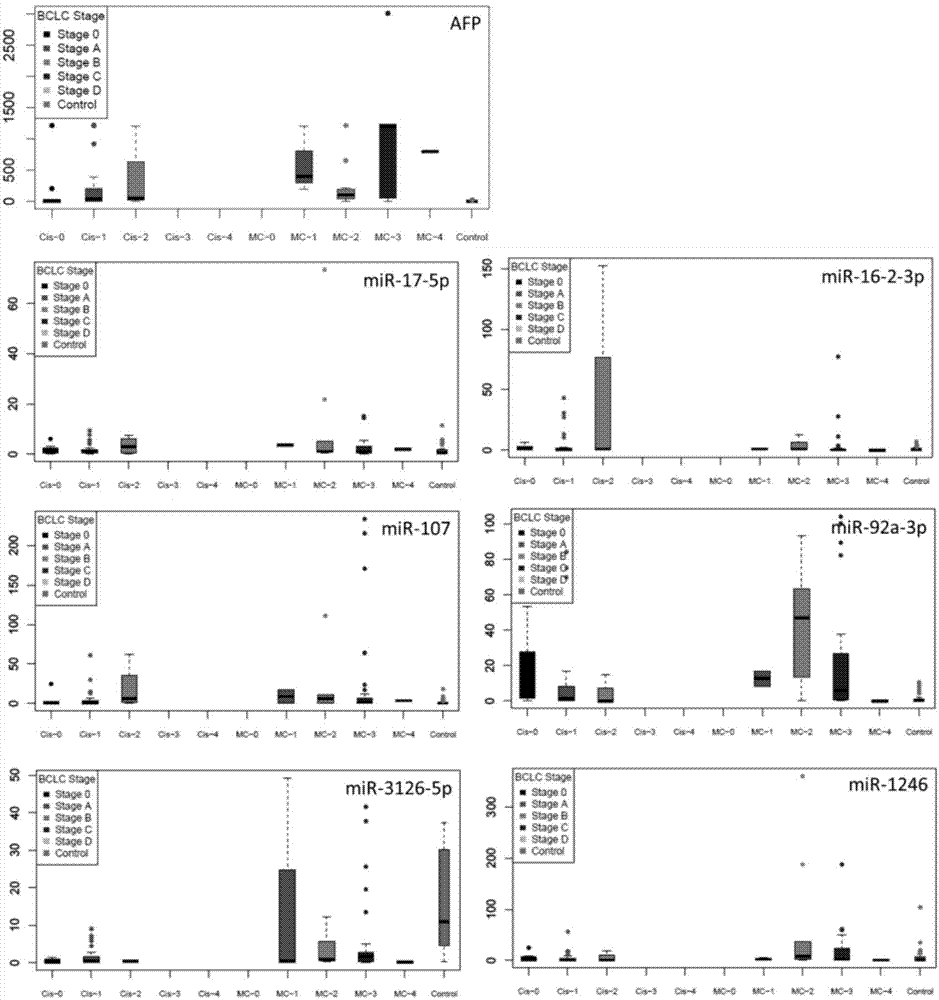
Application of serum microRNA as early diagnostic marker for hepatocellular carcinoma metastasis
The invention discloses an application of miR-107 combined with miR-1246 as an early diagnostic marker for hepatocellular carcinoma metastasis. Researches find that miR-107 combined with miR-1246 for diagnosis of early liver cancer metastasis is superior to alpha fetal protein (AFP), and has good sensitivity and specificity, and the miR-107 and miR-1246 combined with AFP diagnosis is more excellent. As to metastasis diagnosis of low alpha fetal protein secretion liver cancer (AFP is smaller than 200), the miR-1246 is superior to the APF, the miR-107 combined with miR-1246 diagnosis is much superior to the APF, and the miR-107 and miR-1246 combined with AFP are more excellent. On the basis, a primer, a kit and a detection method for detecting the miR-107 and the miR-1246 are developed, and the serum microRNA has a good development prospect and clinical application value. A sequence of the primer is shown in SEQ ID NO.3 and 4, and the kit is formed by a specific amplification primer and a required reagent for PCR amplification.
Owner:青岛瑞思德医学检验实验室有限公司
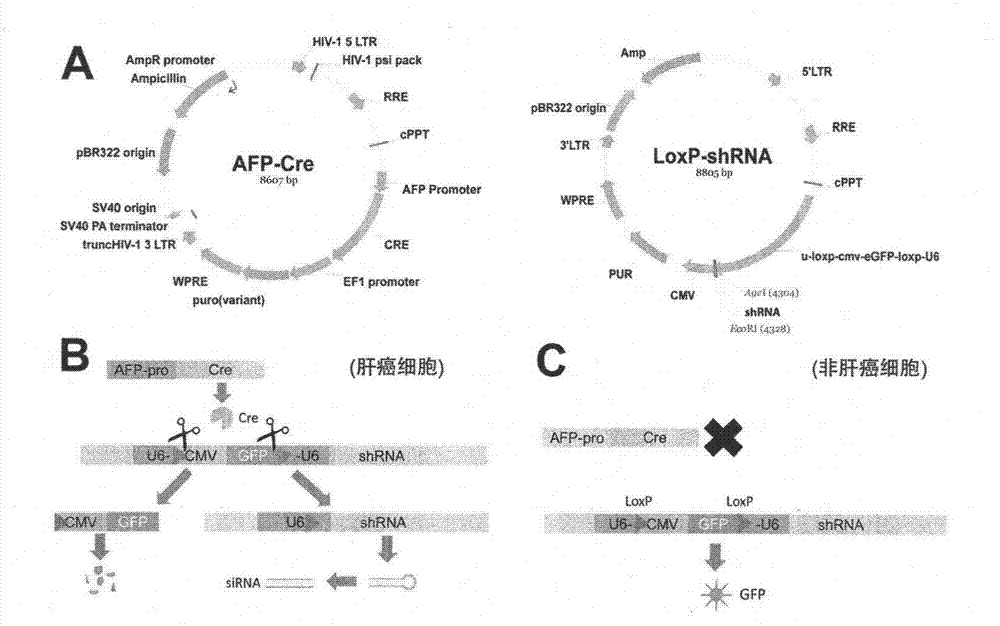
Liver cancer tissue specific RNA (Ribose Nucleic Acid) interference system, as well as construction method and application method thereof
InactiveCN103088064AExpand the scope of researchAvoid side effectsGenetic material ingredientsFermentationCre recombinaseBasic research
The invention provides a liver cancer tissue specific RNA (Ribose Nucleic Acid) interference system, as well as a construction method and an application method thereof. The liver cancer tissue specific RNA interference system comprises an AFP (Alpha Fetal Protein)-Cre vector and an LoxP-shRNA vector which are both constructed by lentiviral vectors, wherein the AFP-Cre vector contains an AFP promoter and a Cre recombinase gene placed at the downstream of the AFP promoter; the LoxP-shRNA vector contains a reconstructive H6 promoter and an shRNA sequence placed at the downstream of the U6 promoter; the reconstructive U6 promoter contained in the LoxP-shRNA vector is the U6 promoter in which a structure, capable of indicating whether the AFP-Cre / LoxP-shRNA system is in work, is inserted inside; and the structure, capable for indicating whether the AFP-Cre / LoxP-shRNA system is in work, is shown as LoxP-CMV-eGFP-LoxP. The RNA interference system, provided by the invention, has the characteristics of being high in liver cancer tissue specificity, long-lasting in effect, high in efficiency, excellent in indication performance, and simple and convenient in operation; and the liver cancer tissue specific RNA interference system enables the liver cancer RNA interferential targeted treatment of liver cancer, provides high convenience to relative research and analysis, and can be widely applied to the treatment of the liver cancer and the basic research.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Kit for detecting alpha fetal protein and preparation method of kit
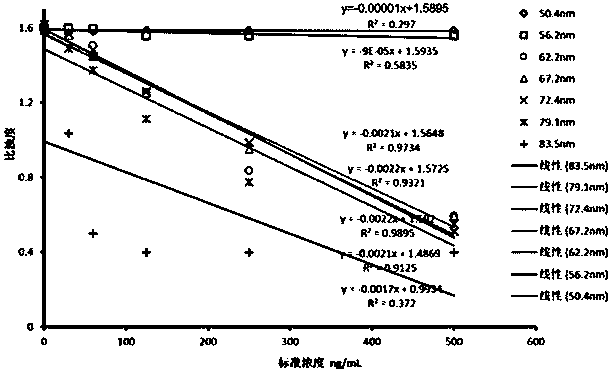
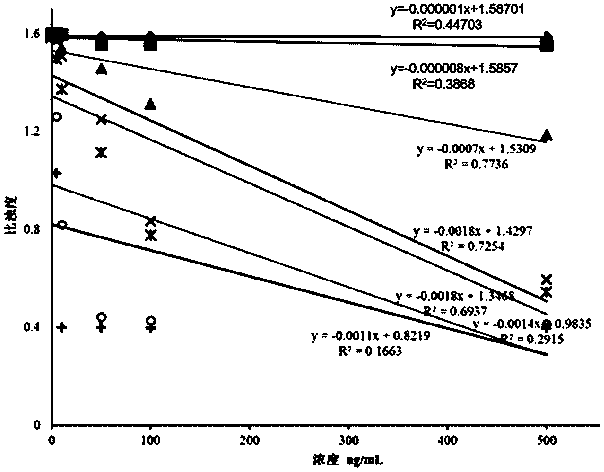
ActiveCN103954773AHigh sensitivityStrong specificityDisease diagnosisBiological testingAlpha-fetoproteinColloid
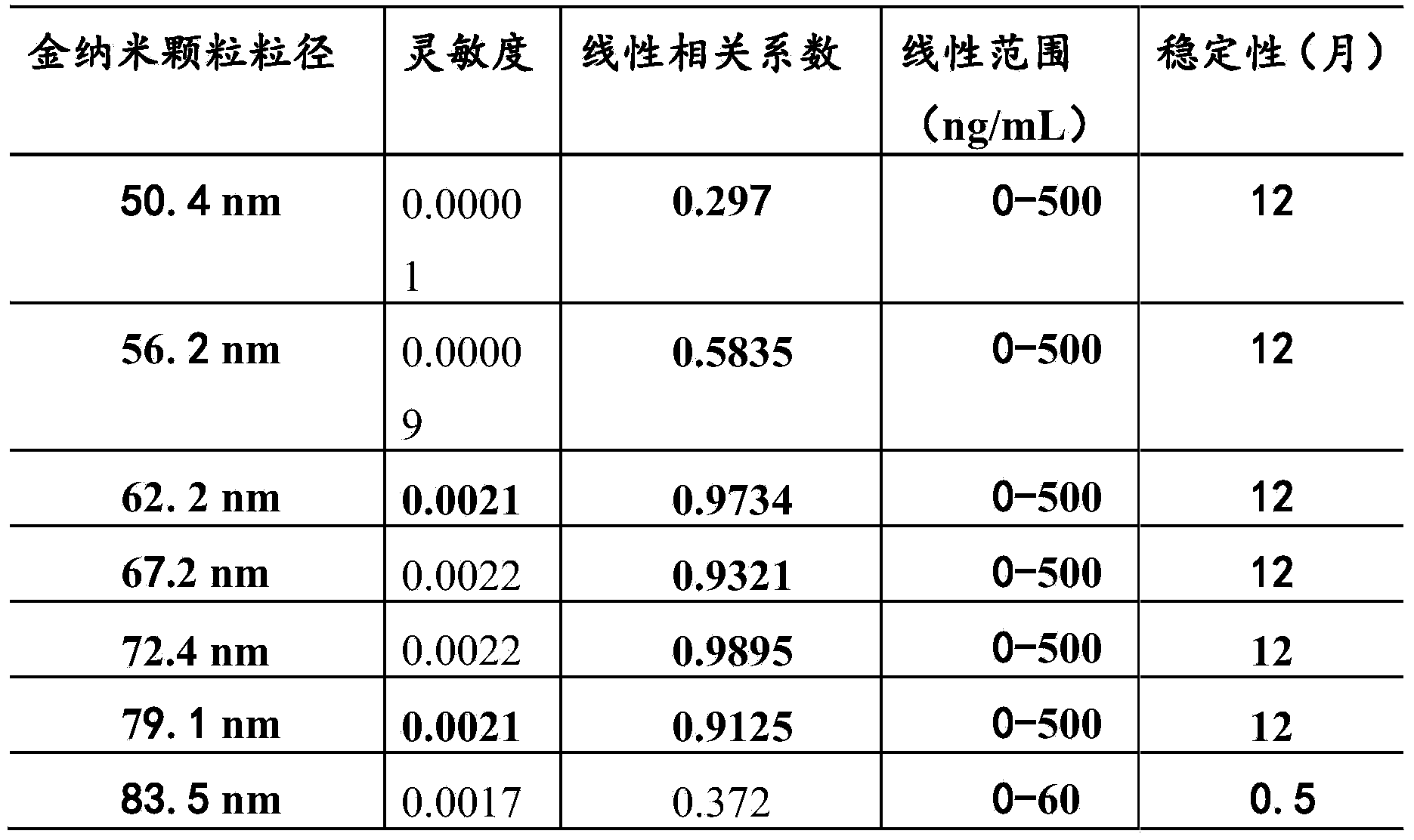
The invention provides a kit for detecting an alpha fetal protein. The kit is operated based on a colloidal gold immunoturbidimetry and comprises a reagent R2, wherein the Reagent R2 is a solution which contains gold nanoparticles marked with alpha fetal protein antibodies. The kit is characterized in that the particle diameters of the contains gold nanoparticles are 62.2-79.1 nomameters; the mass ratio of the contains gold nanoparticles to the alpha fetal protein antibodies is 50:(20-60). The invention also provides a preparation method of the kit. The kit provided by the invention has the characteristics of high sensitivity, high specificity, fastness in reaction and good stability, precipitations can not be generated after reaction, a biochemical analyzer is convenient to clean and the service life of the biochemical analyzer is prolonged.
Owner:BEIJING JIUJIAYI TECH
Use of serum markers containing alpha fetal protein (AFP), Golgi protein 73 (GP73) and carcinoembryonic antigen associated cell adhesion molecule 1 (CEACAM1) in diagnosis of liver diseases
The invention relates to use of serum markers containing alpha fetal protein (AFP), Golgi protein 73 (GP73) and carcinoembryonic antigen associated cell adhesion molecule 1 (CEACAM1) in diagnosis of liver diseases. Specifically, the invention provides use of reagents containing an associative part of at least one specific binding AFP, an associative part of at least one specific binding GP73 and an associative part of at least one specific binding CECAM1 protein in preparation of kits for diagnosis and / or prognosis of the liver diseases.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
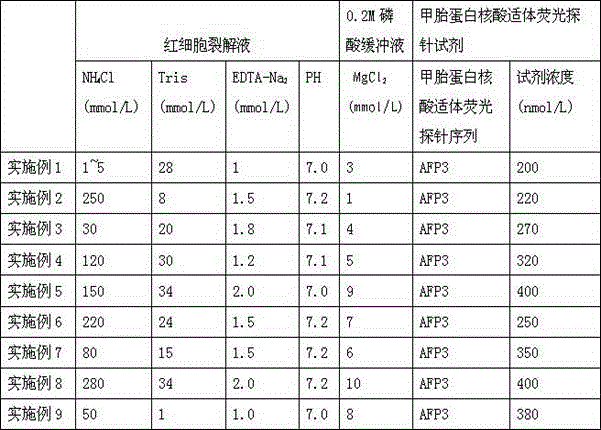
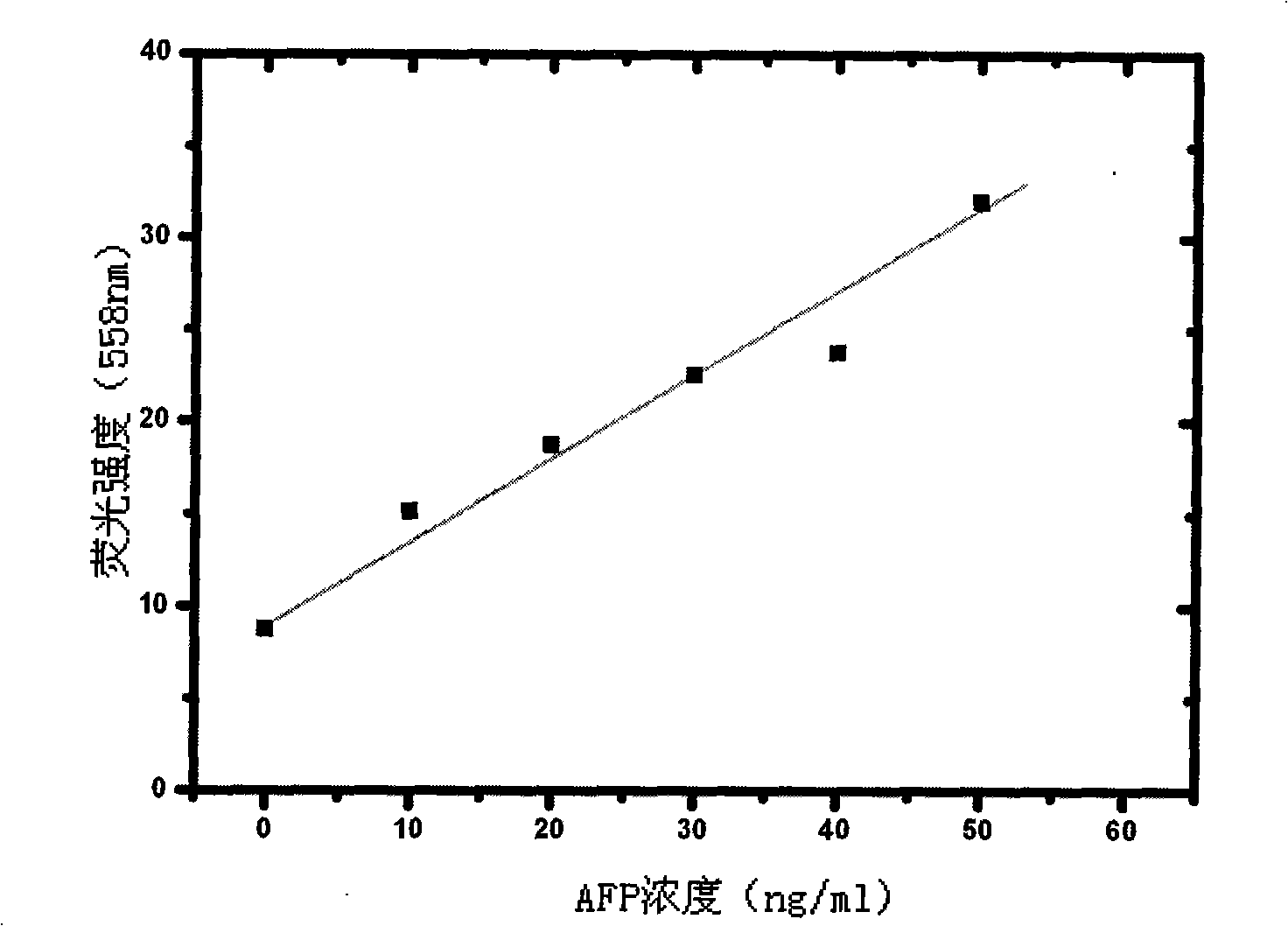
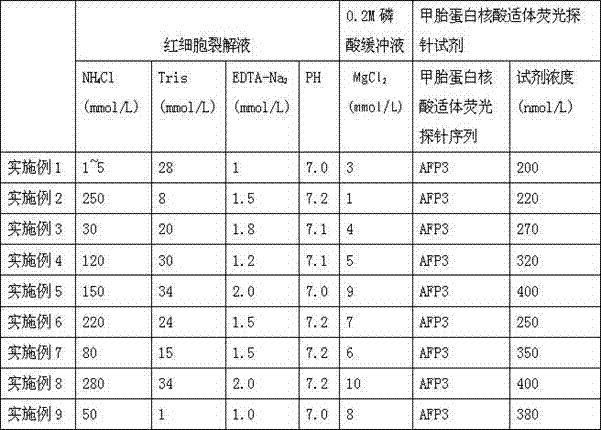
Alpha fetal protein kit based on aptamer fluorescent probe AFP3 and detection method thereof
InactiveCN105785034ANo workabilityNo separabilityBiological material analysisBiological testingRed blood cellBiology
The invention relates to an alpha fetal protein (AFP) kit based on an aptamer fluorescent probe, further relates to a method for measuring AFP concentration and reagent composition and components, and belongs to the technical field of medical detection and measurement. The kit mainly comprises the components of red blood cell lysis buffer, a phosphate buffer solution (PBS), an AFP standard and the AFP aptamer fluorescent probe. The AFP concentration is measured through blood sample splitting and mixed egg cultivation in conjunction with a fluorescent spectrophotometer. The alpha fetal protein kit has the advantages of simple sample treatment, easiness and convenience in operation, short detection time, high detection specificity, high flexibility, high detection result repeatability and the like.
Owner:GUANGZHOU HIGHER EDUCATION MEGA CENT HEALTH IND SCI & TECH PARK INVESTMENT MANAGEMENT
Artificial polypeptide capable of inducing bone mesenchymal stem cells to differentiate into hepatic cells and biological product of such artificial polypeptide
ActiveCN107056895AArtificial cell constructsDepsipeptidesAcidic Fibroblast Growth FactorBiologic Products
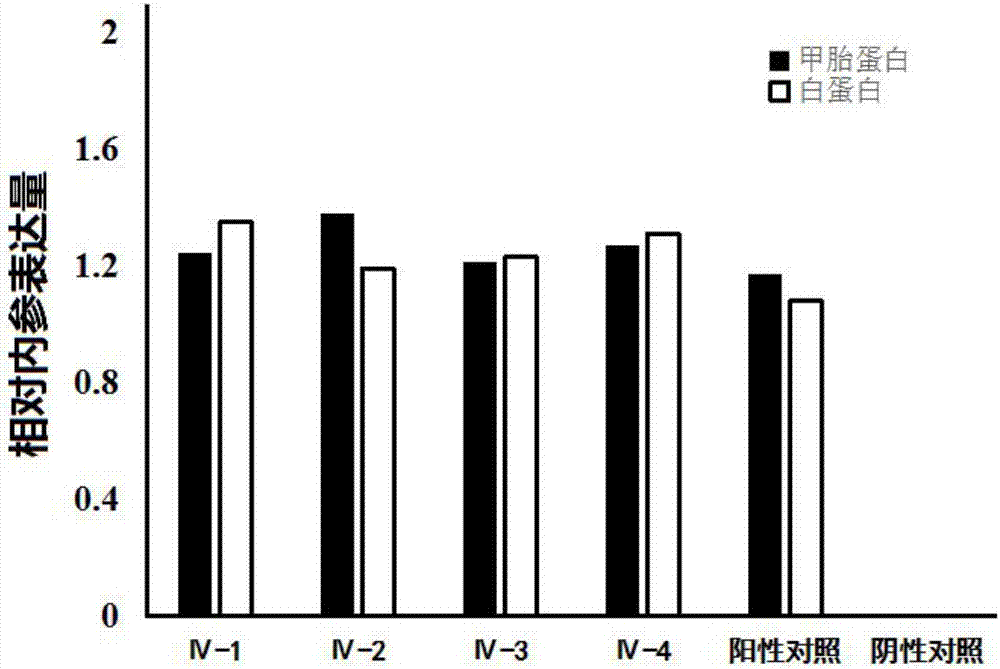
The invention discloses an artificial polypeptide capable of inducing bone mesenchymal stem cells to differentiate into hepatic cells and a biological product of such artificial polypeptide. The artificial polypeptide is a polypeptide containing an amino acid sequence shown in formula (IV): LGENQPDAK(Xa)PCFQEDPMA(Xb)GTDCTLMEIWN, (IV), wherein each of Xa and Xb is selected from M, Y, L, V, W or E. Professionals in the field know that a strong ability to induce the bone mesenchymal stem cells to differentiate into the hepatic cells is achieved in case of combination of acidic fibroblast growth factor, hepatocyte growth factor and oncostatin M, and after induced culture for 14 days, alpha fetal protein mRNA and albumin mRNA in the cells are in remarkably positive expression. It is proven that the artificial polypeptide with the same effect is capable of inducing the bone mesenchymal stem cells to differentiate into the hepatic cells, and accordingly the artificial polypeptide can be prepared into the biological product for inducing the bone mesenchymal stem cells to differentiate into the hepatic cells.
Owner:诺赛联合(北京)生物医学科技有限公司
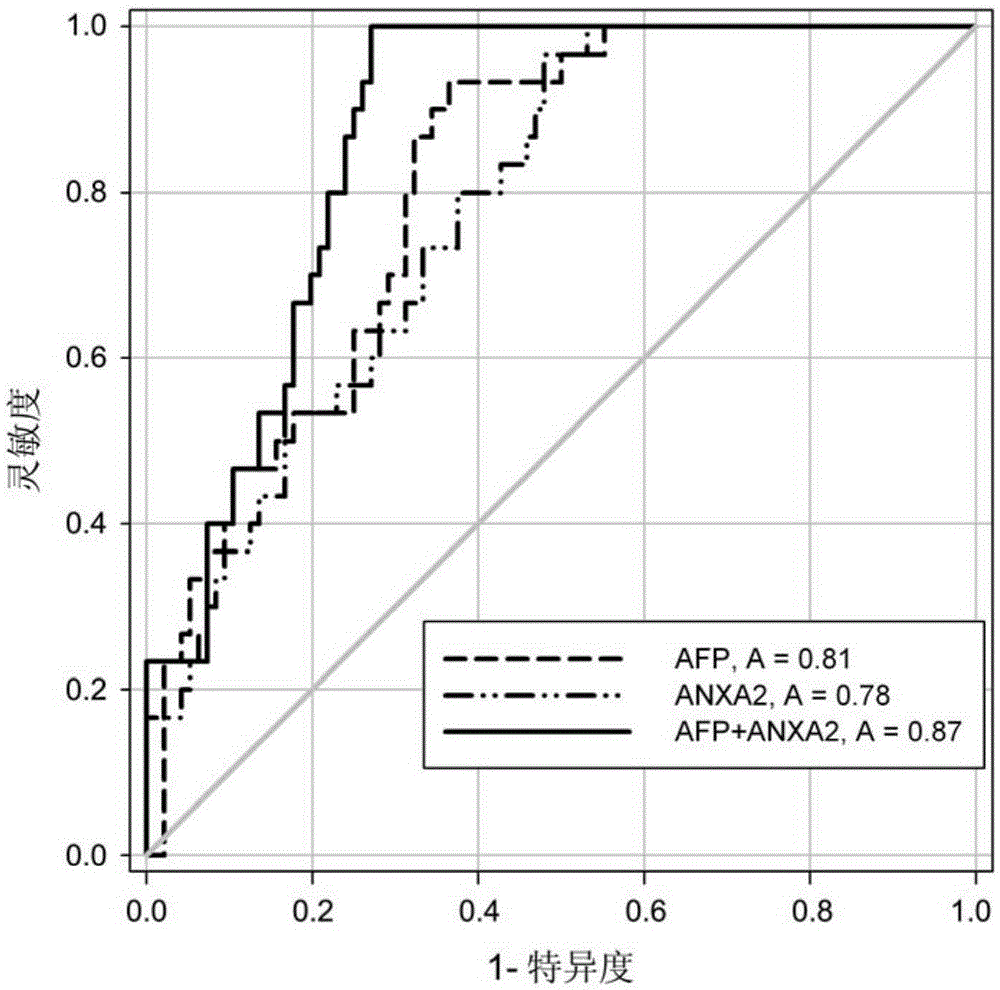
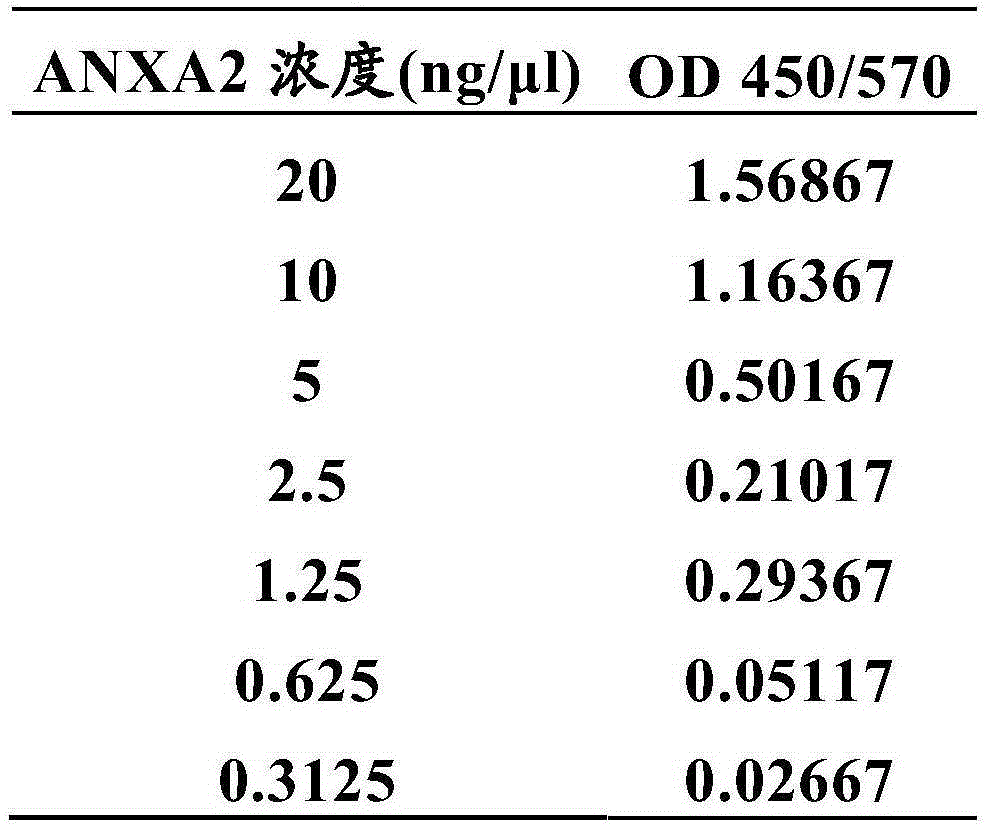
Blood serum detection method of annexin A2 as well as detection kit and application thereof
The invention relates to a blood serum detection method of annexin A2 as well as a detection kit and application thereof, and particularly relates to a method for detecting HCC (Hepatocellular Carcinoma) by independently using a new marker annexin A2 or detecting the HCC by the combination of the annexin A2 and AFP (Alpha Fetal Protein) as well as the detection kit and the application thereof.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI +1
AFP (Alpha Fetal Protein), GP (Golgi Protein)73 and PIVKA (Protein Induced By Vitamin K Absence)-II joint detection kit
ActiveCN104407155ASimple and fast operationThe result is accurateBiological testingFetal proteinGolgi protein
The invention relates to the field of biological detection, in particular to a detection kit for quantitatively detecting AFP (Alpha Fetal Protein), Golgi Protein-73GP73 and PIVKA (Protein Induced Vy Vitamin K Absence)-II or an antagonist-II as well as a preparation method and application of the detection kit. The detection kit comprises an AFP detection test paper card, a Golgi Protein-73GP73 detection test paper card and a PIVKA-II detection test paper card which are independent of one another, wherein each detection test paper card comprises a bottom plate, a sample pad, a gold-labeled pad, a nitrocellulose membrane and a water absorbing pad, wherein the sample pad, the gold-labeled pad, the nitrocellulose membrane and the water absorbing pad are positioned on the surface of the bottom plate and are arranged from the sample adding end in sequence. The kit provided by the invention has the benefits that the AFP, the GP73 and the PIVKA-II are firstly detected through a fluorescent microsphere immune chromatography, and both the sensitivity and the specificity are realized; the kit has the advantages of high quickness, simplicity and convenience in operation, accurate results, good economy and applicability and the like.
Owner:ZYBIO INC
Preparation method of a label-free photoelectrochemical alpha-fetoprotein immunosensor
InactiveCN104569435BEasy to makeEasy to operateBiological testingMaterial electrochemical variablesChemical synthesisFetuin b
The invention discloses a preparation method of an unlabelled photoelectrochemical alpha fetoprotein immunosensor, and belongs to the fields of novel nano functional materials and biosensors. On the basis of a titanium dioxide nano particle base, a trimetal alloy nano material in a dendrite nanorod form is prepared by a photoelectrochemical synthetic method, so that the prepared unlabelled photoelectrochemical alpha fetoprotein immunosensor for detecting alpha fetal protein is low in cost, high in sensitivity, good in specificity, fast in detection and simple to prepare.
Owner:UNIV OF JINAN
Hepatocellular carcinoma targeting gene expression element AP and applications thereof
InactiveCN101671670APrevent proliferationReplication blockGenetic material ingredientsDigestive systemFetal proteinGene expression
The invention provides a hepatocellular carcinoma targeting gene expression element AP and applications thereof, and the gene expression element AP has a nucleotide sequence indicated in SEQ ID No.1.After the expression element AP is guided into Alpha-fetoprotein positive tumor cells, the artificial microRNA aiming at DNA polymerase can be expressed in the cell and the expression of the DNA polymerase in the cell can be effectively inhibited, the copy of the DNA in the cell is further affected; the cell cycle of is blocked finally, the multiplication of the AFP positive hepatoma cell is specially inhibited and the expression element AP can be applied to the preparation of the targeting gene curing medicine of AFP (alpha fetal protein) positive liver cancer.
Owner:ZHEJIANG UNIV
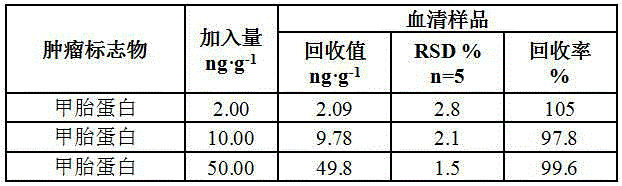
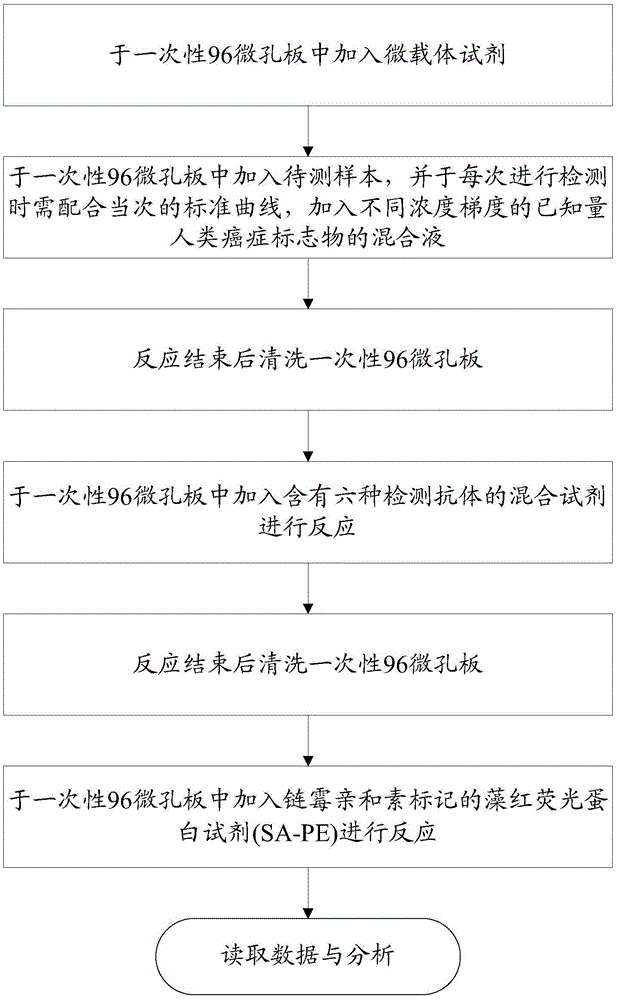
Human cancer maker detection kit and detection method
InactiveCN106885904AComprehensive test resultsEffective auxiliary clinical diagnosisBiological material analysisHuman cancerBinding site
The invention relates to a human cancer marker detection kit and a detection method. The detection kit includes microcarriers, coating antibodies and detection antibodies. There is at least one kind of microcarrier, and the microcarrier has coding information, and the coding information of microcarriers for different human cancer markers is different. Coating antibodies are used to coat microcarriers. Both the coating antibody and the detection antibody are selected from the antibodies of carcinoembryonic antigen, alpha-fetoprotein, prostate specific antigen, tumor antigen 19-9, tumor antigen 125 and tumor antigen 15-3 For at least one of the corresponding human cancer markers, the coating antibody and the detection antibody have different antigen binding sites. The detection kit can be used to detect up to six human cancer markers, thereby enriching the detection results and assisting clinical diagnosis more effectively.
Owner:江苏博铼生技医疗科技有限公司
Human serum AFP (Alpha Fetal Protein) negative liver cell cancer detection kit
ActiveCN108676880AAuxiliary diagnosis realizedImprove survival rateMicrobiological testing/measurementGAS5Hepatocellular carcinoma
The invention discloses a human serum AFP (Alpha Fetal Protein) negative liver cell cancer detection kit. A combined diagnosis kit is used for combined diagnosis on patients suffering from AFP negative hepatocellular carcinoma by detecting expression amounts of five RNAs (Ribonucleic Acids) of MiR-125b, MiR-205, SPRY4-IT1, GAS5-AS1 and circSMARCA5 in plasma by using a real-time quantitative PCR (Polymerase Chain Reaction) technique. The kit disclosed by the invention has the characteristics of high sensitivity, high specificity and high accuracy, patients suffering from AFP negative hepatocellular carcinoma can be effectively diagnosed, auxiliary diagnosis on early-stage hepatocellular carcinoma can be achieved, the survival rate of the patients can be increased, and the kit has remarkableclinical application values.
Owner:WUHAN UNIV
Recombinant plasmid and virus of tissue specificity and application thereof.
InactiveCN102533828AAvoid influenceGrowth inhibitionGenetic material ingredientsViruses/bacteriophagesNormal cellFetal protein
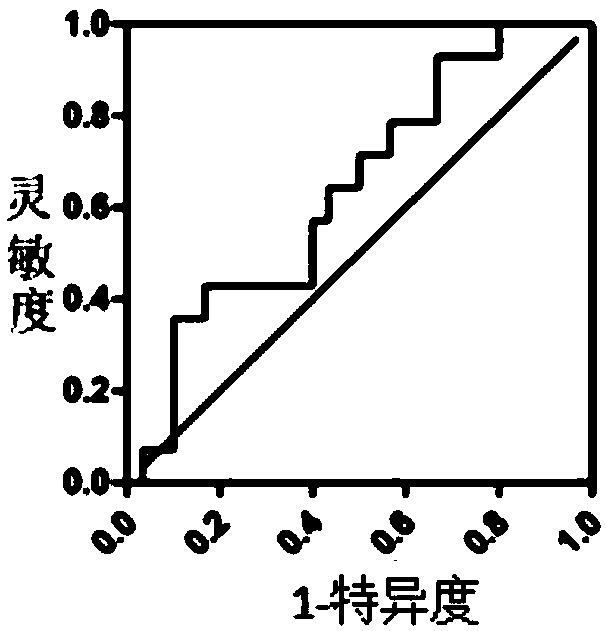
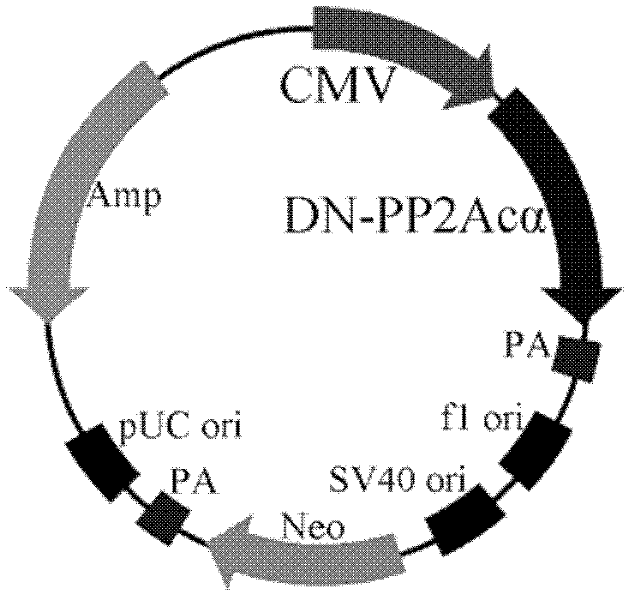
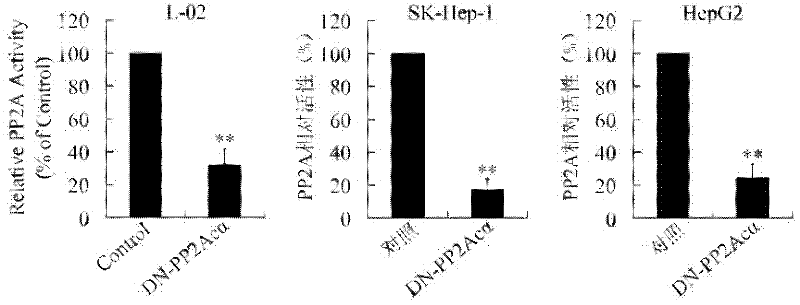
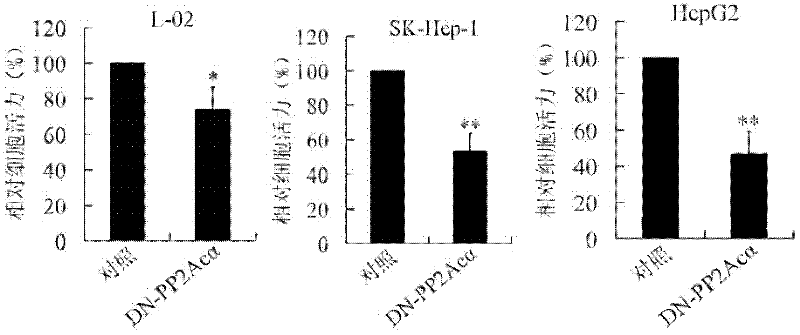
The invention relates to the biotechnology field, and discloses a recombinant plasmid, a recombinant virus plasmid, a recombinant virus of tissue specificity directing against hepatoma cells with positive AFP (Alpha Fetal Protein) and application in preparing drugs for treatment of hepatoma with positive AFP. In the invention, DN-PP2Ac expression is regulated by AFpg promoter of tissue specificity of hepatoma, so that DN-PP2Ac specificity is expressed in hepatoma cells with positive AFP, thereby preventing the effect on normal cells and having a good prospect of clinical application.
Owner:THE FIRST AFFILIATED HOSPITAL OF SOOCHOW UNIV
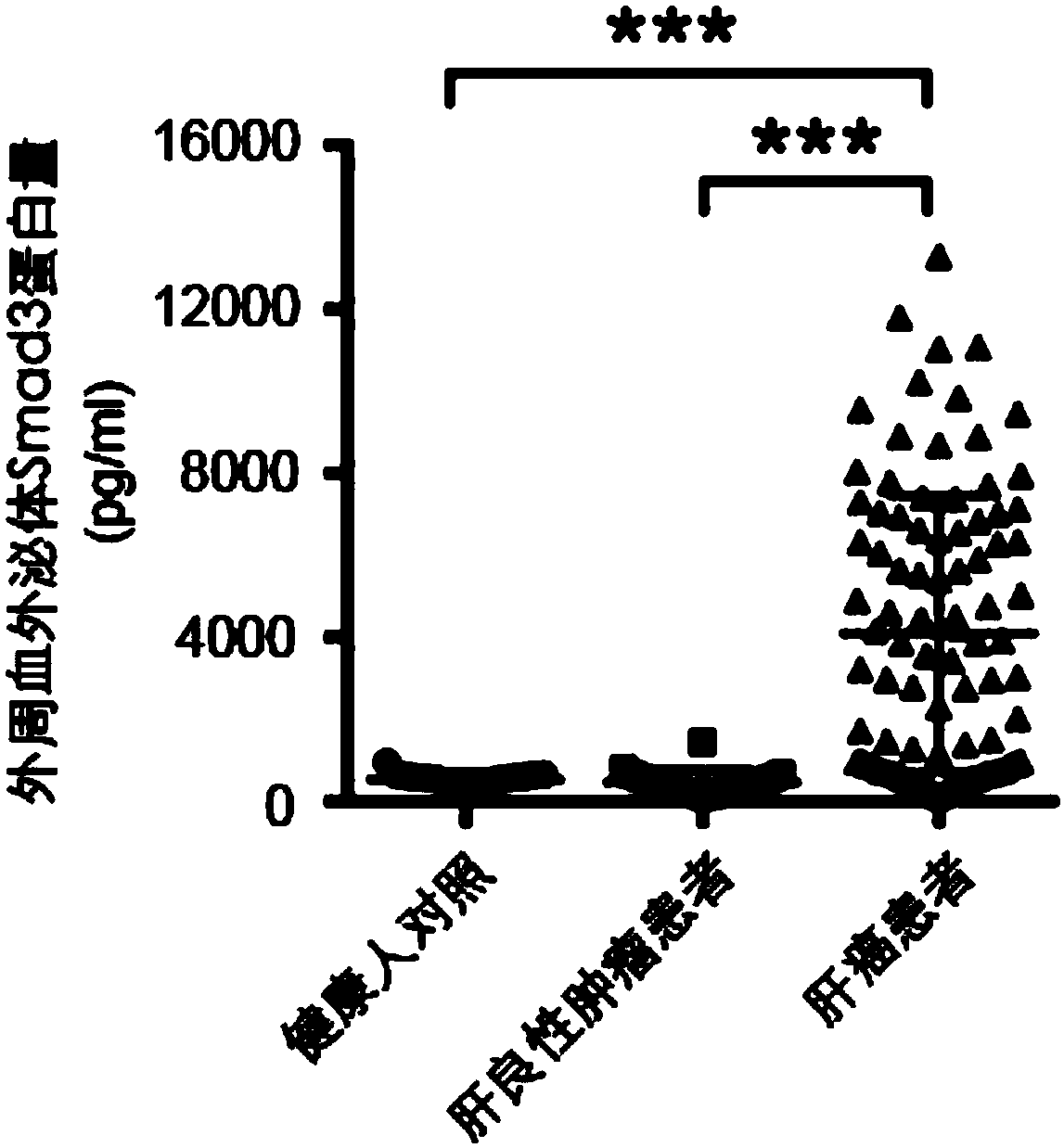
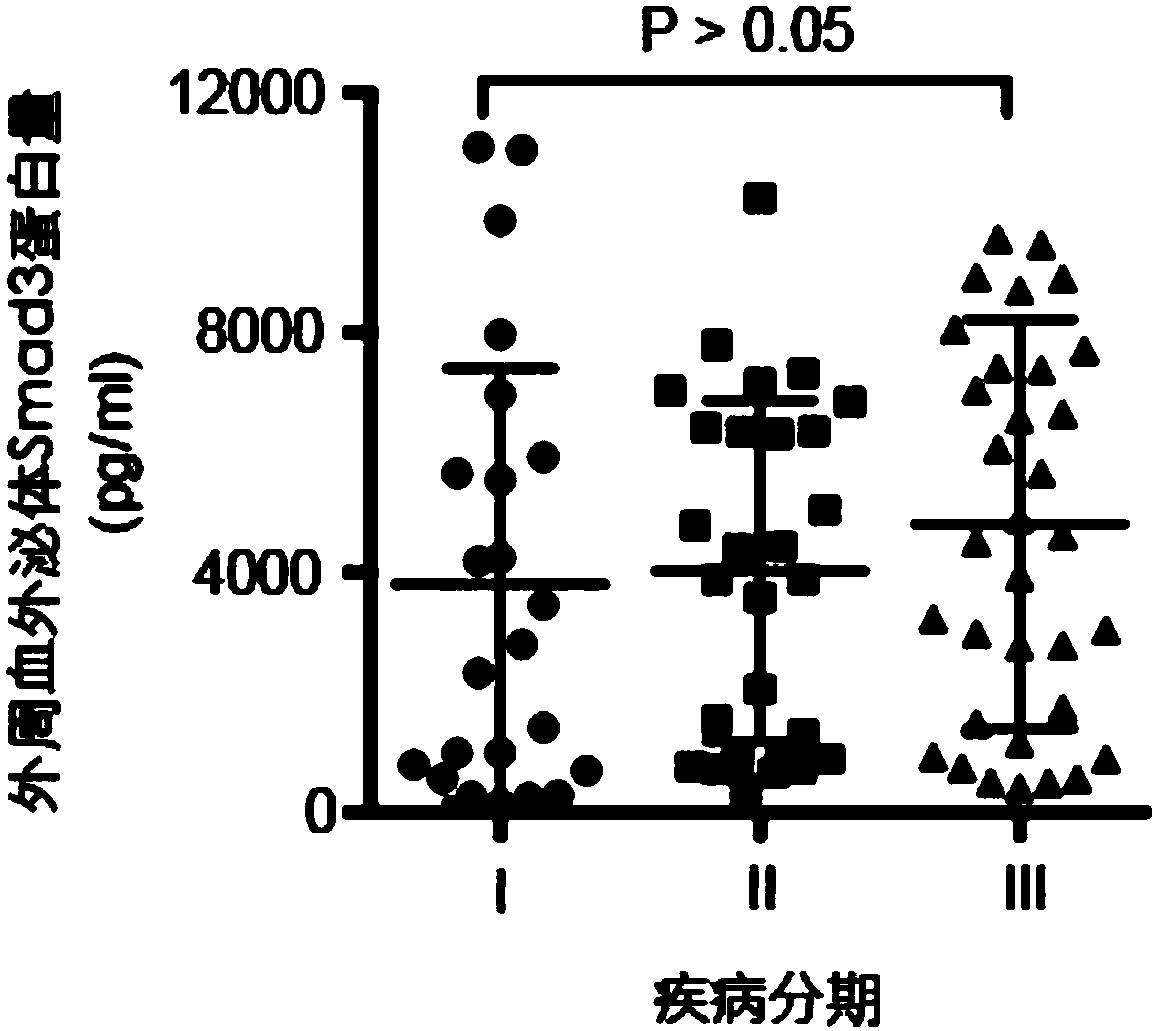
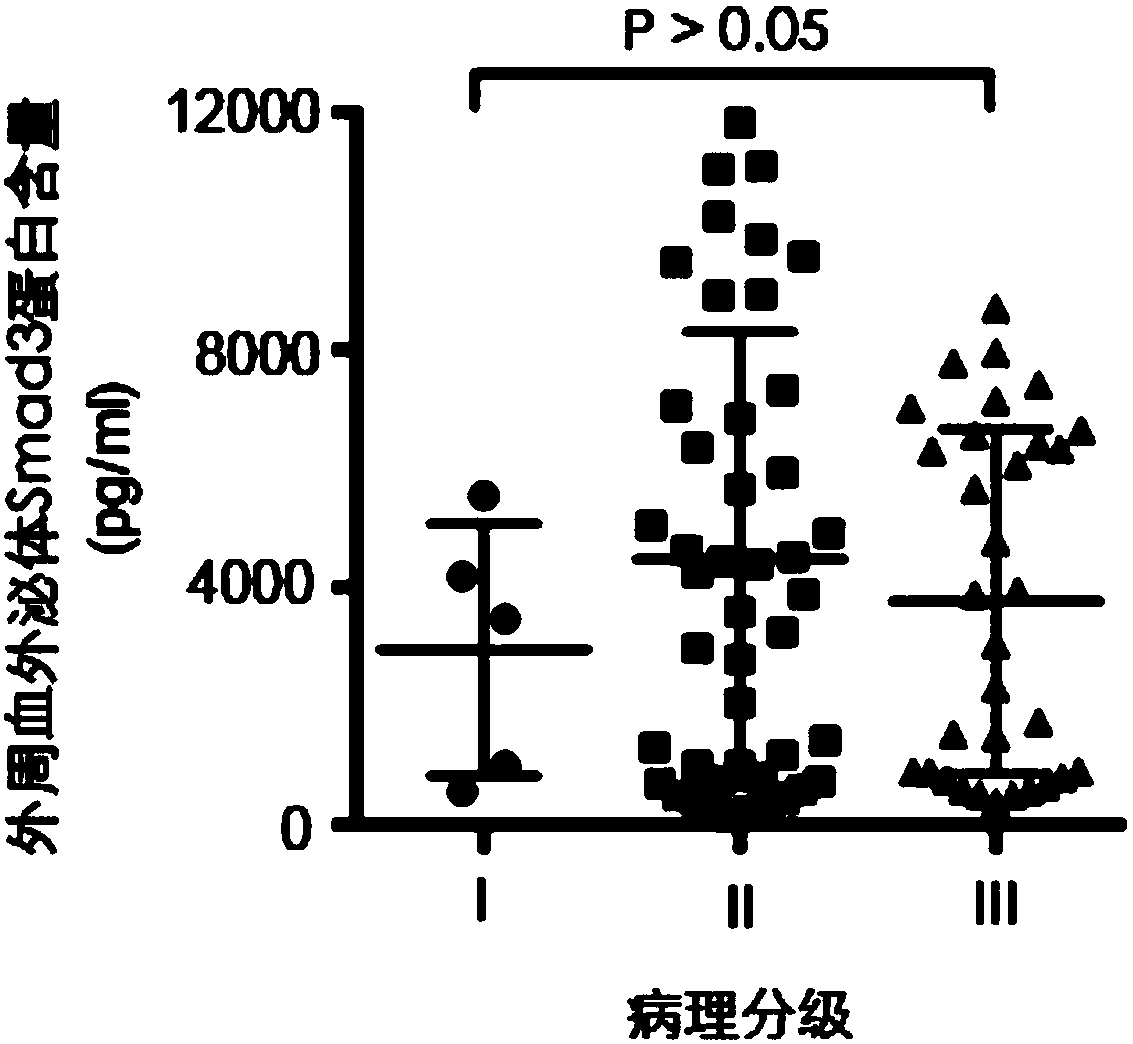
Use of Smad3 protein in peripheral blood exosome as molecular marker and liver cancer detection kit
The invention discloses use of a Smad3 protein in a peripheral blood exosome as a molecular marker for preparing a liver cancer detection kit and the liver cancer detection kit. A research of the invention shows that the Smad3 protein content of an exosome extracted from human peripheral blood is obviously increased in a liver cancer patient; the Smad3 protein used as a tumor marker for diagnosinga liver cancer obtains an area under the ROC curve of 0.888; in combination with an alpha fetal protein for diagnosing the liver cancer, the area under the ROC curve can be up to 0.975; therefore, the Smad3 in the peripheral blood exosome can be independently used as the tumor marker for diagnosing the liver cancer or can be jointed with the alpha fetal protein to be used as the tumor marker fordiagnosing the liver cancer, and also can be used for judging a postoperative recurrence risk of a liver cancer patient.
Owner:ZHEJIANG UNIV
Application of serum DDK1 in preparation of diagnosis reagent for alpha fetal protein negative hepatocellular carcinoma
The invention relates to an application of serum DDK1 in the preparation of a diagnosis reagent for alpha fetal protein negative hepatocellular carcinoma. Through analysis of a large amount of hepatocellular carcinoma samples, the inventor analyzes the expression situations of DKK1 and AFP from the samples, and firstly discovers that serum DKK1 has a high sensitivity and specificity on diagnosis of AFP negative hepatocellular carcinoma.
Owner:SHANGHAI INST OF ONCOLOGY
Hepatocellular carcinoma targeting gene expression element AP and applications thereof
InactiveCN101671670BPrevent proliferationReplication blockDNA/RNA fragmentationNucleotideWilms' tumor
The invention provides a hepatocellular carcinoma targeting gene expression element AP and applications thereof, and the gene expression element AP has a nucleotide sequence indicated in SEQ ID No.1. After the expression element AP is guided into Alpha-fetoprotein positive tumor cells, the artificial microRNA aiming at DNA polymerase can be expressed in the cell and the expression of the DNA polymerase in the cell can be effectively inhibited, the copy of the DNA in the cell is further affected; the cell cycle of is blocked finally, the multiplication of the AFP positive hepatoma cell is specially inhibited and the expression element AP can be applied to the preparation of the targeting gene curing medicine of AFP (alpha fetal protein) positive liver cancer.
Owner:ZHEJIANG UNIV
AFP and GM-CSF dual-gene co-expression recombinant vector as well as preparation method and application of recombinant vector
ActiveCN103555762BOvercoming reciprocal inhibitionGuarantee structureGenetic material ingredientsDigestive systemNucleotideAlpha-fetoprotein
The invention discloses an AFP and GM-CSF dual-gene co-expression recombinant vector, which is sequentially linked with an AFP gene, an IRES sequence and a GM-CSF gene in a vector transcription direction, or is sequentially linked with a GM-CSF gene, an IRES sequence and an AFP gene in a vector transcription direction, wherein nucleotide sequence of the AFP gene is represented in SEQ ID NO: 1 in a sequence list, nucleotide sequence of the GM-CSF gene is represented in SEQ ID NO: 2 in the sequence list, and nucleotide sequence of the IRES sequence is represented in SEQ ID NO: 3 in the sequence list. The dual-gene co-expression recombinant vector, which links the AFP gene and the GM-CSF gene through the IRES sequence, can simultaneously express alpha fetal protein and a granulocyte-macrophage colony stimulating factor in a same vector; and the recombinant vector, in immunogene therapy of liver cancer, not only can develop an immune regulating function of a cell factor but also can generate a specific anti-tumor effect targeted for the liver cancer.
Owner:XINXIANG MEDICAL UNIV
High-sensitivity antenatal screening kit of Down's Syndrome in pregnant women at second trimester as well as preparation and detection methods of kit
ActiveCN102866254BPrenatal screening is safe and non-invasiveHigh sensitivityMaterial analysisEnzyme bindingBiochemistry
The invention discloses a high-sensitivity antenatal screening kit of Down's Syndrome in pregnant women at second trimester as well as preparation and detection methods of the high-sensitivity antenatal screening kit. The kit comprises a kit body, AFP (Alpha Fetal Protein) and beta-HCG (Human Chorionic Gonadotropin) coated plates, AFP and beta-HCG standard products, AFP and beta-HCG quality control products, AFP and beta-HCG enzyme conjugates, sample diluent, light-emitting substrate liquids and concentrated scrubbing liquid. The preparation method comprises the following steps of: preparing monoclonal antibody coated plates of AFP and beta-HCG; preparing the standard products and the quality control products of AFP and beta-HCG; and preparing the enzyme conjugates of AFP and beta-HCG; and preparing the sample diluent, a light-emitting substrate liquid A, a light-emitting substrate liquid B, the concentrated scrubbing solution and the like. The high-sensitivity antenatal screening kit of the Down's Syndrome in the pregnant women at the second trimester has the advantages that the purpose of the antenatal screening of the Down's Syndrome in the pregnant women at the second trimester is realized, and the accordance rate with the clinic diagnosis reaches more than 99.7%; and the kit is high in sensitivity, strong in the specificity and accurate and reliable in result.
Owner:山东康华生物医疗科技股份有限公司
A colorimetric immunoassay for the detection of tumor markers for non-diagnostic purposes
Owner:上海塞力斯医学检验实验室有限公司
Alpha fetal protein (AFP) detection method based on fluorescent nano luminous and magnetic nano material
The invention relates to a method for determining the content of AFP in serum by using CdTe quantum dots and Fe3O4-Dextran nano particles, characterized by comprising the steps of (a) preparing Fe3O4-Dextran-first antibody complex; (b) preparing CdTe-second antibody complex; (c) drawing 'fluorescence intensity-AFP concentration' standard work curve, and calculating work equation; and (d) measuring the fluorescence intensity value for the work equation to determine the AFP content of the patents with liver cancer. The method is simple and has high sensitivity, can easily detect the disease for the early cancer patients so as to treat the patients in time, and furthermore, the method opens up a road for the clinic detection of liver cancer.
Owner:DONGHUA UNIV
Alpha-fetoprotein kit and detection method based on nucleic acid aptamer fluorescent probe afp3
InactiveCN105785034BSimple detection operationHigh sensitivityBiological material analysisBiological testingRed blood cellMedical testing
The invention relates to an alpha fetal protein (AFP) kit based on an aptamer fluorescent probe, further relates to a method for measuring AFP concentration and reagent composition and components, and belongs to the technical field of medical detection and measurement. The kit mainly comprises the components of red blood cell lysis buffer, a phosphate buffer solution (PBS), an AFP standard and the AFP aptamer fluorescent probe. The AFP concentration is measured through blood sample splitting and mixed egg cultivation in conjunction with a fluorescent spectrophotometer. The alpha fetal protein kit has the advantages of simple sample treatment, easiness and convenience in operation, short detection time, high detection specificity, high flexibility, high detection result repeatability and the like.
Owner:GUANGZHOU HIGHER EDUCATION MEGA CENT HEALTH IND SCI & TECH PARK INVESTMENT MANAGEMENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com