Human serum AFP (Alpha Fetal Protein) negative liver cell cancer detection kit
A technology of GAS5-AS1 and SPRY4-IT1, applied in the biological field, can solve the problems of missed detection and lack of effective detection methods for AFP-negative HCC patients, and achieve the effect of improving the survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]Plasma was collected from HCC (HCC) patients and healthy controls, including 59 cases of HCC (age: 55±9; sex: 55 males, 4 females), whose serum AFP was lower than 20ng / ml, and 72 healthy controls (age: 52 ±10; sex: 65 males, 7 females). Extract total RNA from plasma. The RNA extraction kit (RP4002) of Beijing Biotech Biotechnology Co., Ltd. was used, and the operation was performed according to the kit instructions. The brief description is as follows: ①Place EDTA anticoagulated whole blood in a low-temperature centrifuge, centrifuge at 2000×g for 5 minutes at 4°C. Separate plasma and blood cells. ② Carefully draw the upper layer of plasma and transfer it to a 1.5ml RNase-free EP tube. Note that this step should avoid suctioning the middle buffy coat, which contains white blood cells and platelets, which will cause contamination of RNA from sources outside the plasma. ③ Put the plasma in the RNase-free centrifuge tube back into the cryogenic centrifuge, and centrifug...
Embodiment 2
[0025] [Example 2] reverse transcription
[0026] Take 1 μg of total RNA for reverse transcription, using Takara (Dalian Bao Biotechnology Co., Ltd.) PrimeScript TM The RTreagent Kit with gDNA Eraser kit was carried out according to the steps recommended by the reagent manufacturer, as follows: ① Prepare the reaction reagents in an ice box, and the reaction system is shown in Table 1:
[0027] Table 1 Reverse transcription first step system
[0028]
[0029]
[0030] After mixing, centrifuge the reaction fraction to the bottom of the tube. ②After incubating at 42°C for 2 minutes, put the EP tube on ice again, and add the following reaction components:
[0031] Table 2 Reverse transcription second step system
[0032]
[0033] After mixing, the reaction components were spotted to the bottom of the tube. ③Place the reaction solution in a PCR instrument, first react at 37°C for 15 minutes, then react at 85°C for 5s. The reverse transcription experiment is completed....
Embodiment 3
[0034] [Example 3] Real-time fluorescent quantitative PCR
[0035] 1. The cDNA samples obtained in Example 3 were diluted 10 times to configure real-time fluorescent quantitative PCR systems. The system configuration is shown in Table 3:
[0036] Table 3 Fluorescent quantitative PCR reaction system
[0037]
[0038] 2. The detection target non-coding RNA and the corresponding internal reference primer sequences in the above table are shown in Table 4:
[0039] Table 4 forward primer and reverse primer feature table
[0040]
[0041] 3. Add the configured qRT-PCR solution into a real-time fluorescent quantitative PCR instrument (Bio-Red) for reaction. The reaction conditions for detecting target RNA in the above table are shown in Table 5:
[0042] Table 5 Fluorescent quantitative PCR conditions
[0043]
[0044]
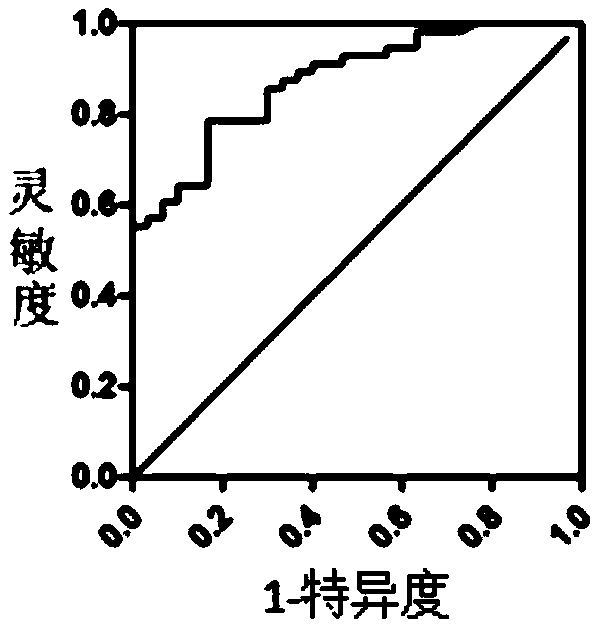
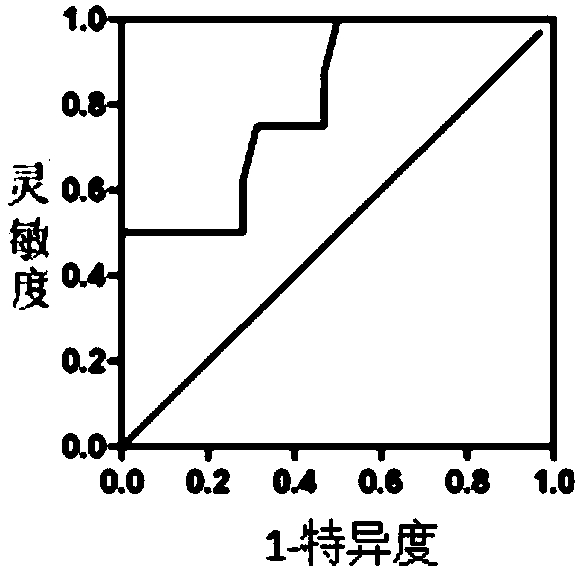
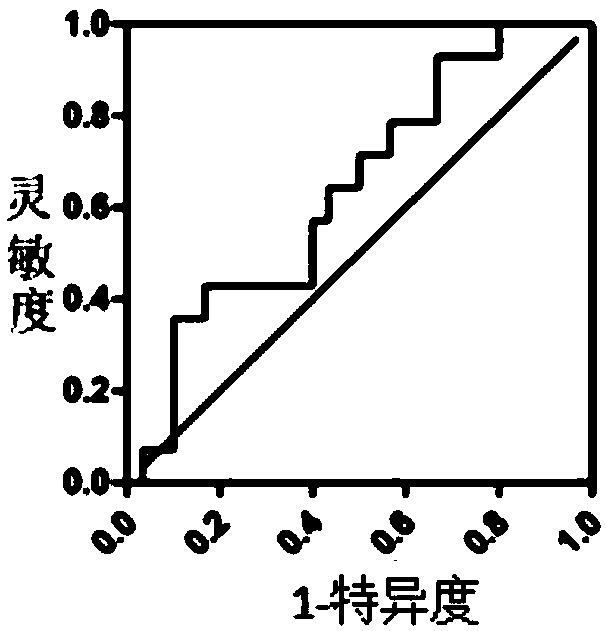
[0045] 4, data statistics: the present invention carries out statistical analysis of data by IBMSPSS Statistics 21, evaluates the diagnostic value of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com