Patents
Literature
91 results about "Buffy coat" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
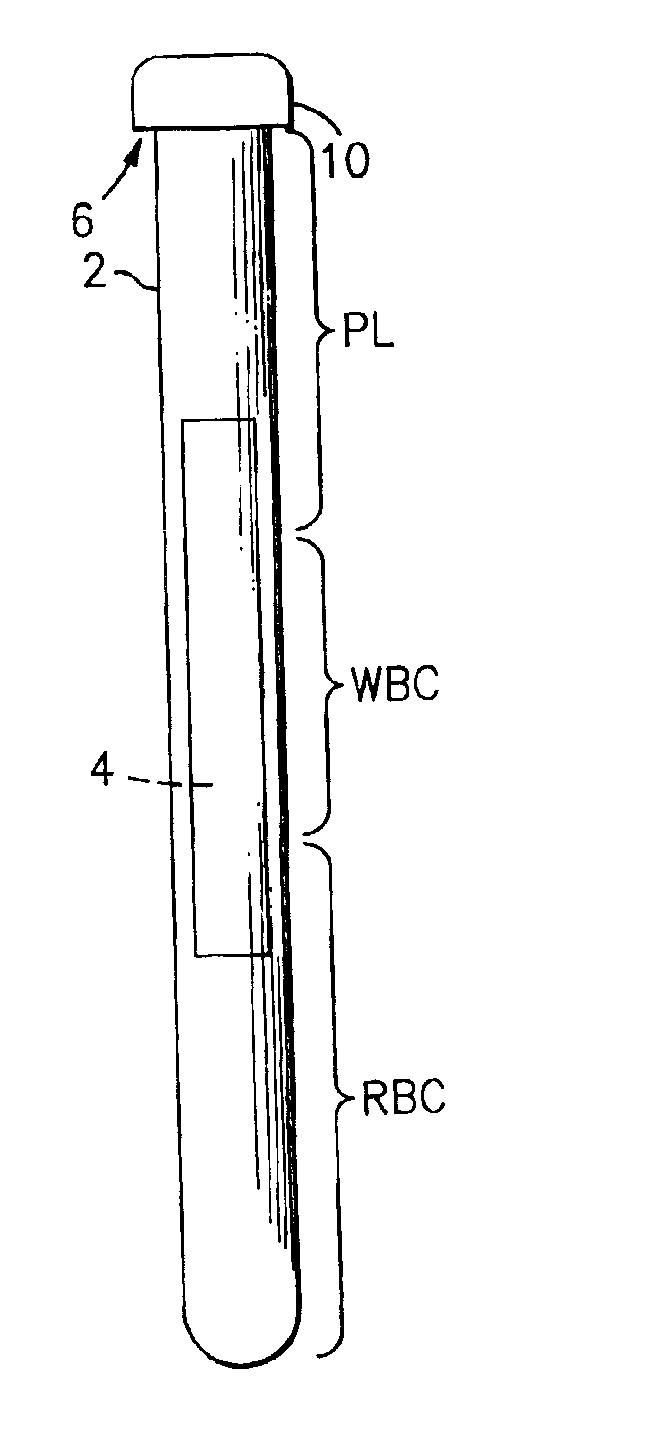
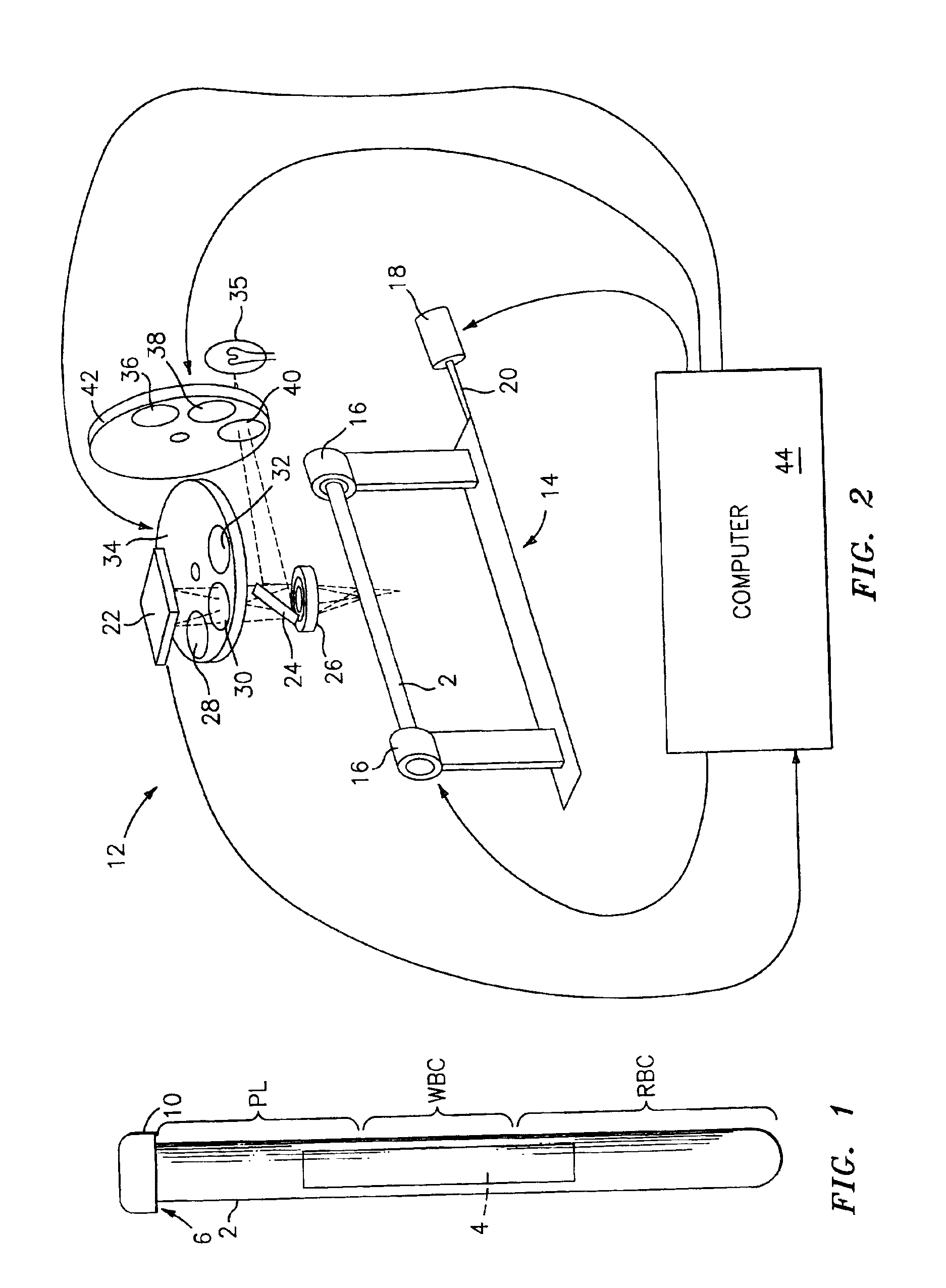
The buffy coat is the fraction of an anticoagulated blood sample that contains most of the white blood cells and platelets following density gradient centrifugation of the blood.
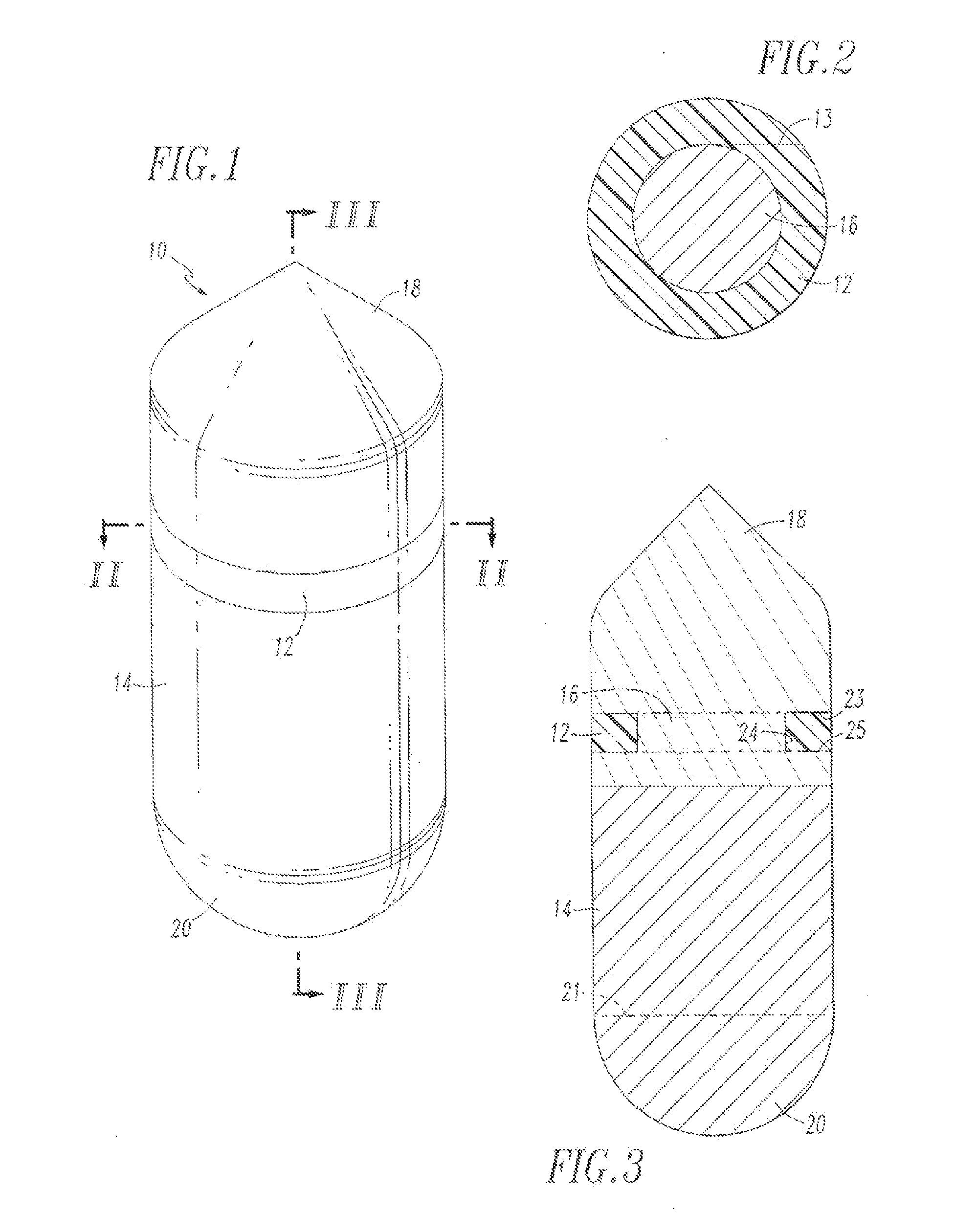
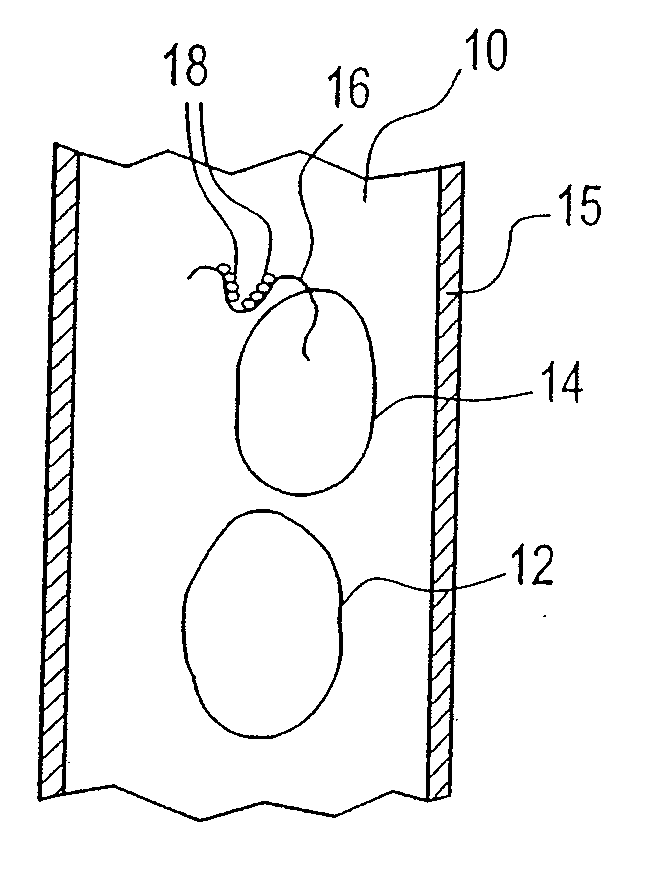
Methods and apparatus for isolating platelets from blood
InactiveUS20050186120A1Simple and fast preparationIncrease productionMedical devicesLaboratory glasswaresMedicineRed blood cell
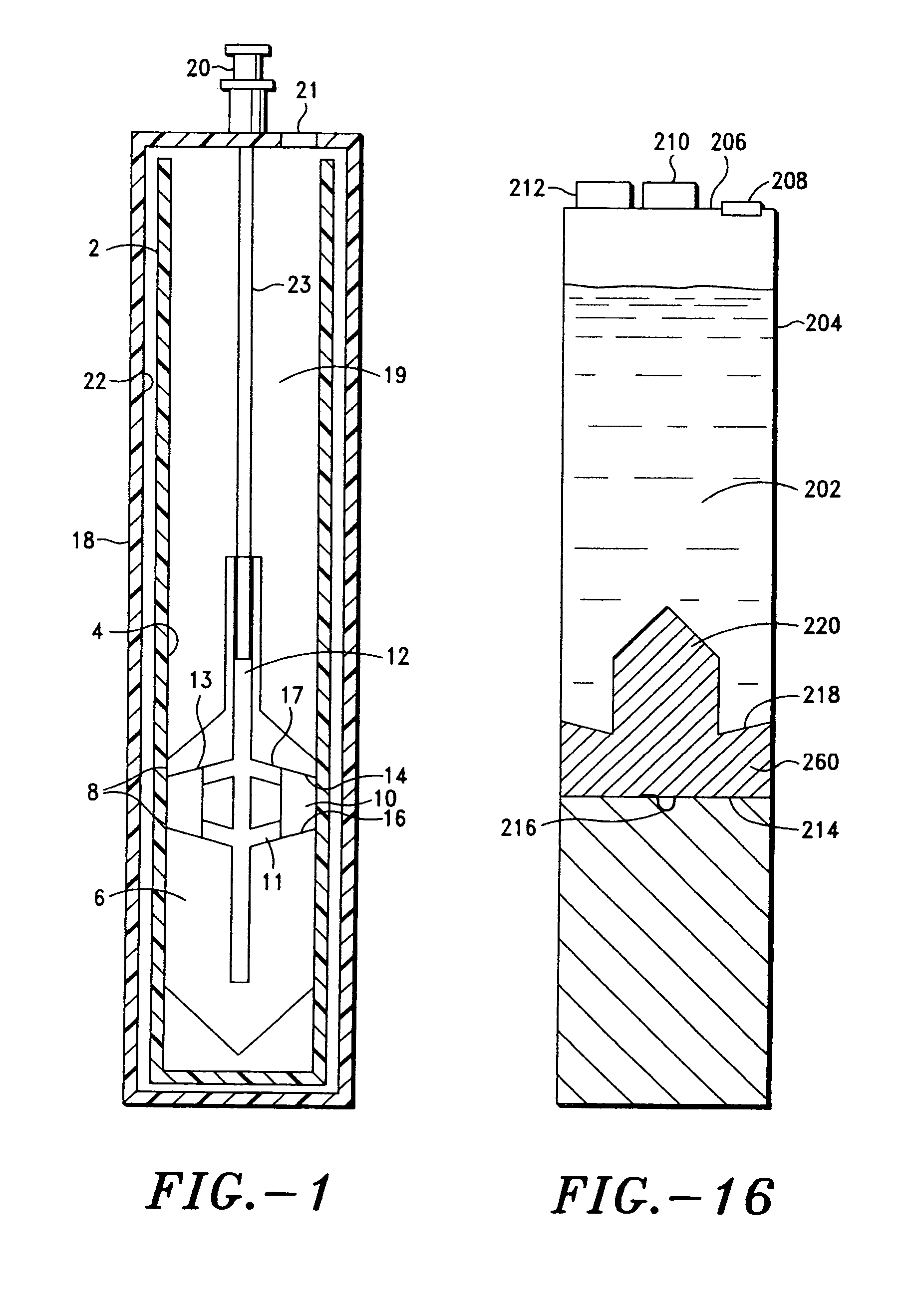
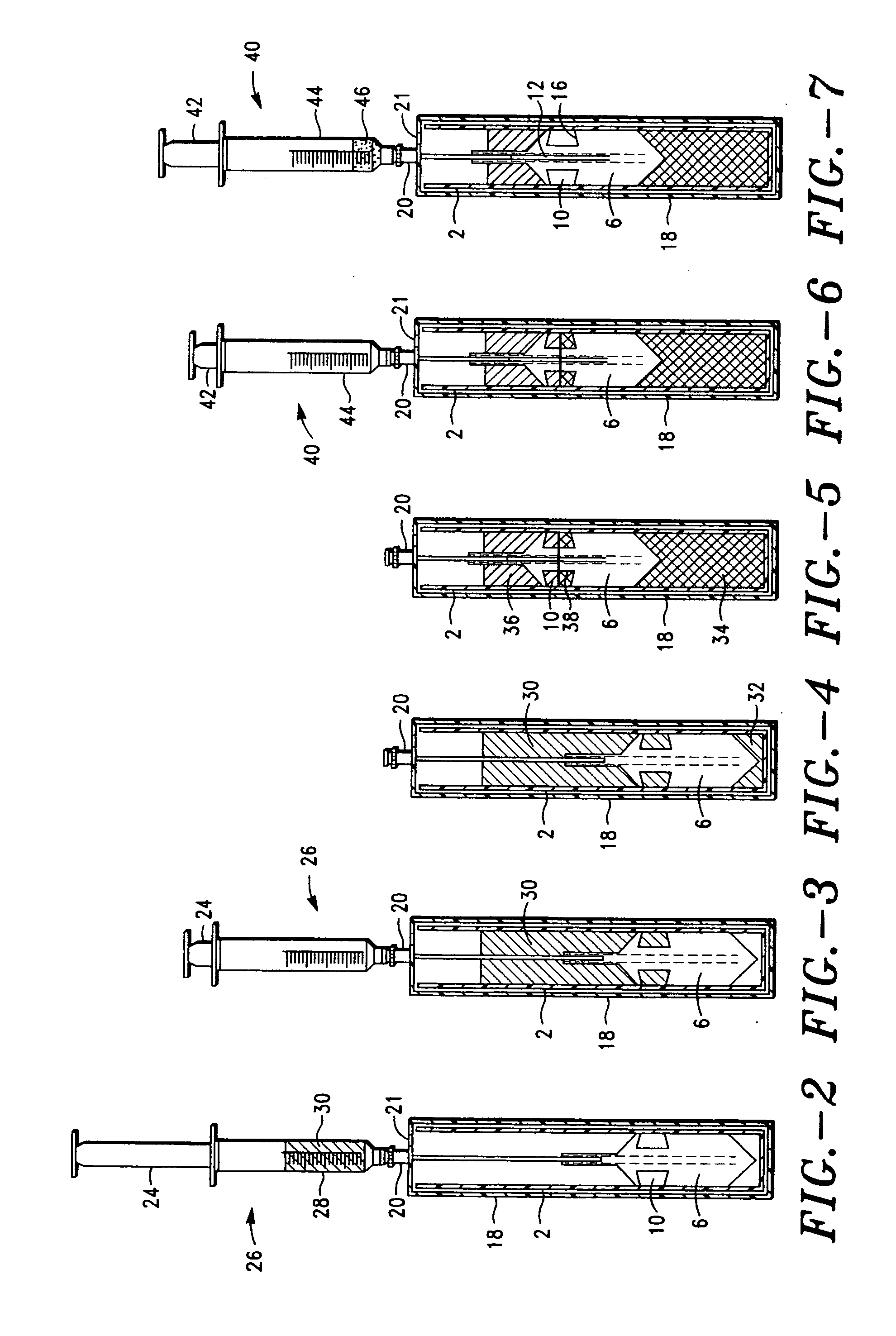
A platelet collection device comprising a centrifugal spin-separator container with a cavity having a longitudinal inner surface. A float in the cavity has a base, a platelet collection surface above the base, an outer surface. The float density is below the density of erythrocytes and above the density of plasma. The platelet collection surface has a position on the float which places it below the level of platelets when the float is suspended in separated blood. During centrifugation, a layer of platelets or buffy coat collects closely adjacent the platelet collection surface. Platelets are then removed from the platelet collection surface. Movement of a float having a density greater than whole blood through the sedimenting erythrocytes releases entrapped platelets, increasing the platelet yield.
Owner:DORIAN RANDEL +2
Methods and apparatus for isolating platelets from blood
InactiveUS20050196874A1Simple and fast preparationIncrease productionSurgeryVaccination/ovulation diagnosticsMedicineRed blood cell
A platelet collection device comprising a centrifugal spin-separator container with a cavity having a longitudinal inner surface. A float in the cavity has a base, a platelet collection surface above the base, an outer surface. The float density is below the density of erythrocytes and above the density of plasma. The platelet collection surface has a position on the float which places it below the level of platelets when the float is suspended in separated blood. During centrifugation, a layer of platelets or buffy coat collects closely adjacent the platelet collection surface. Platelets are then removed from the platelet collection surface. Movement of a float having a density greater than whole blood through the sedimenting erythrocytes releases entrapped platelets, increasing the platelet yield.
Owner:HANUMAN
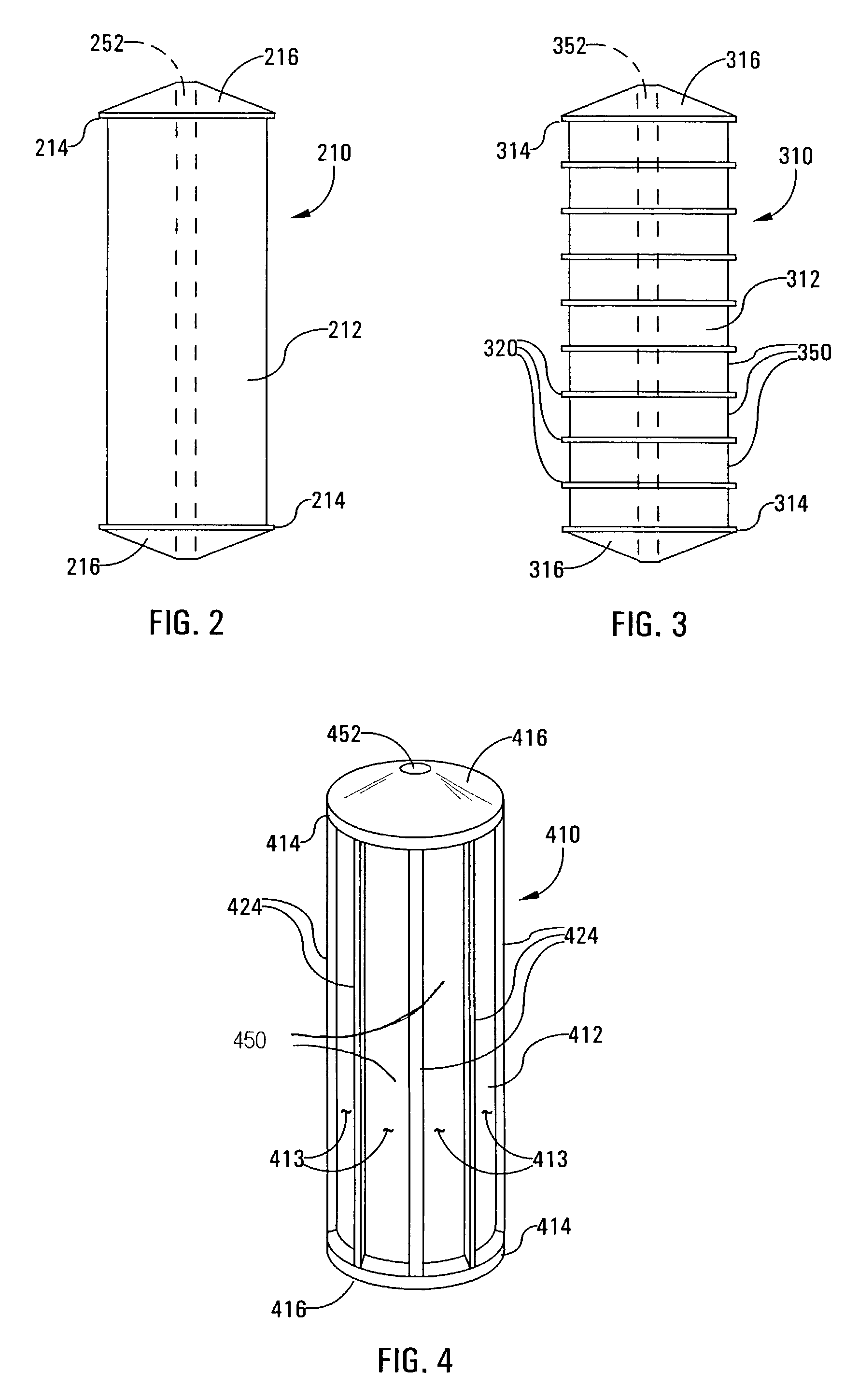
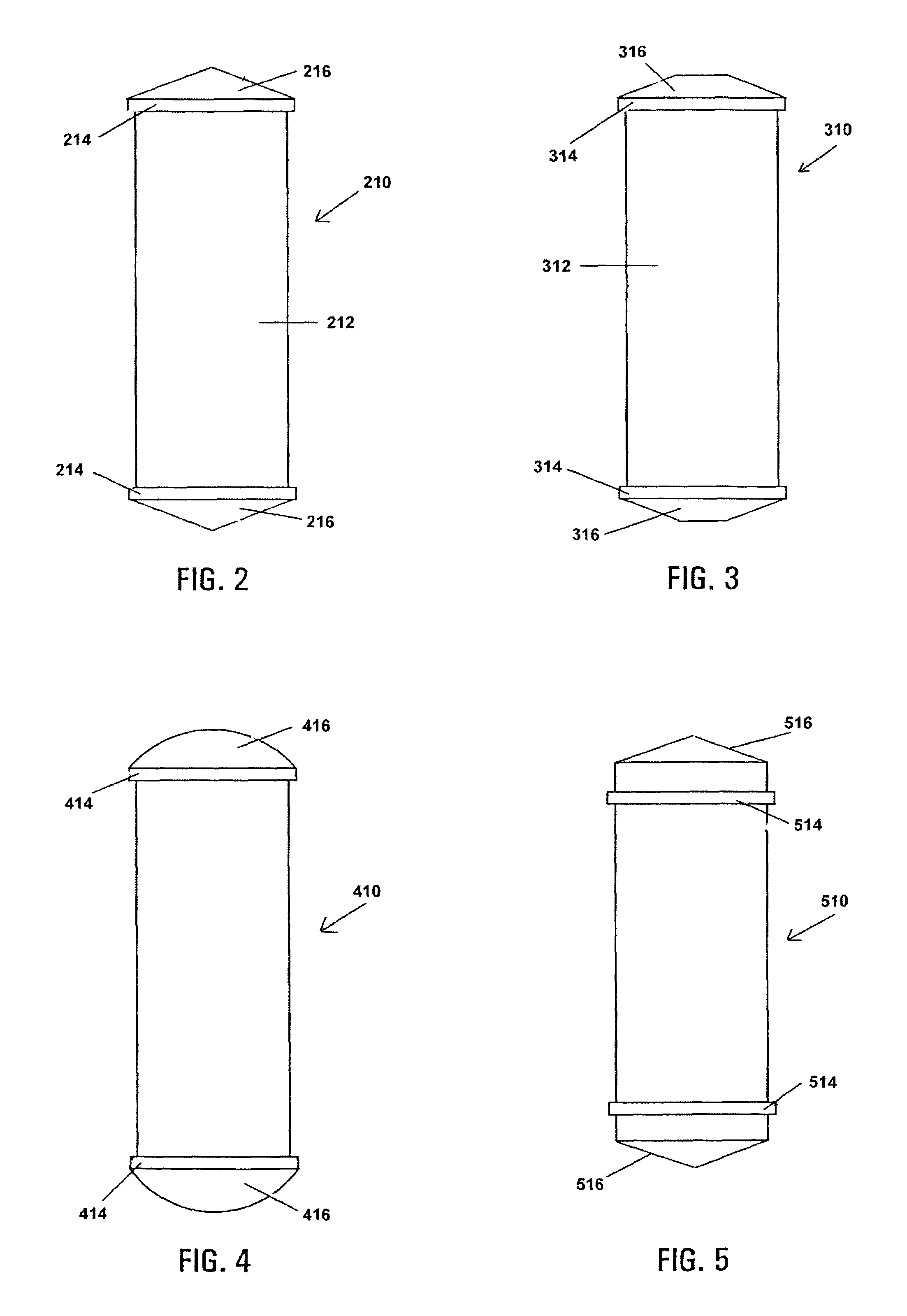
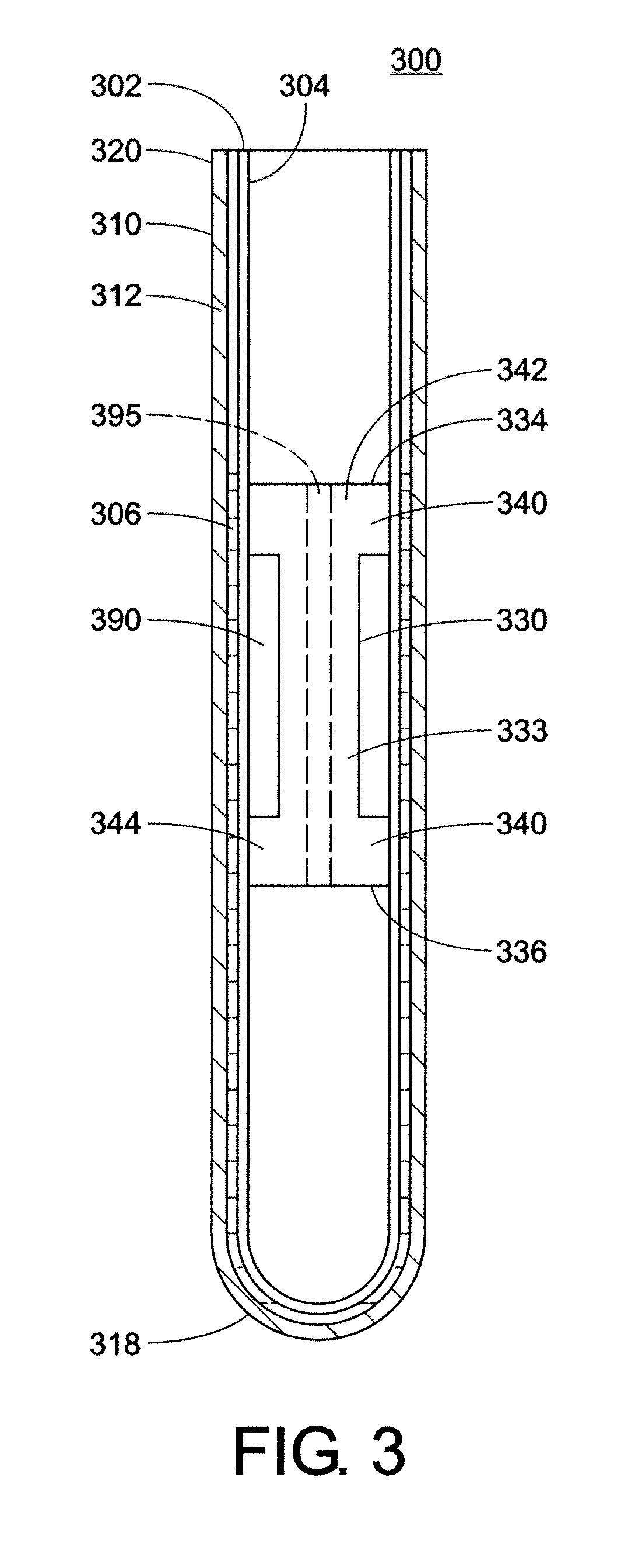
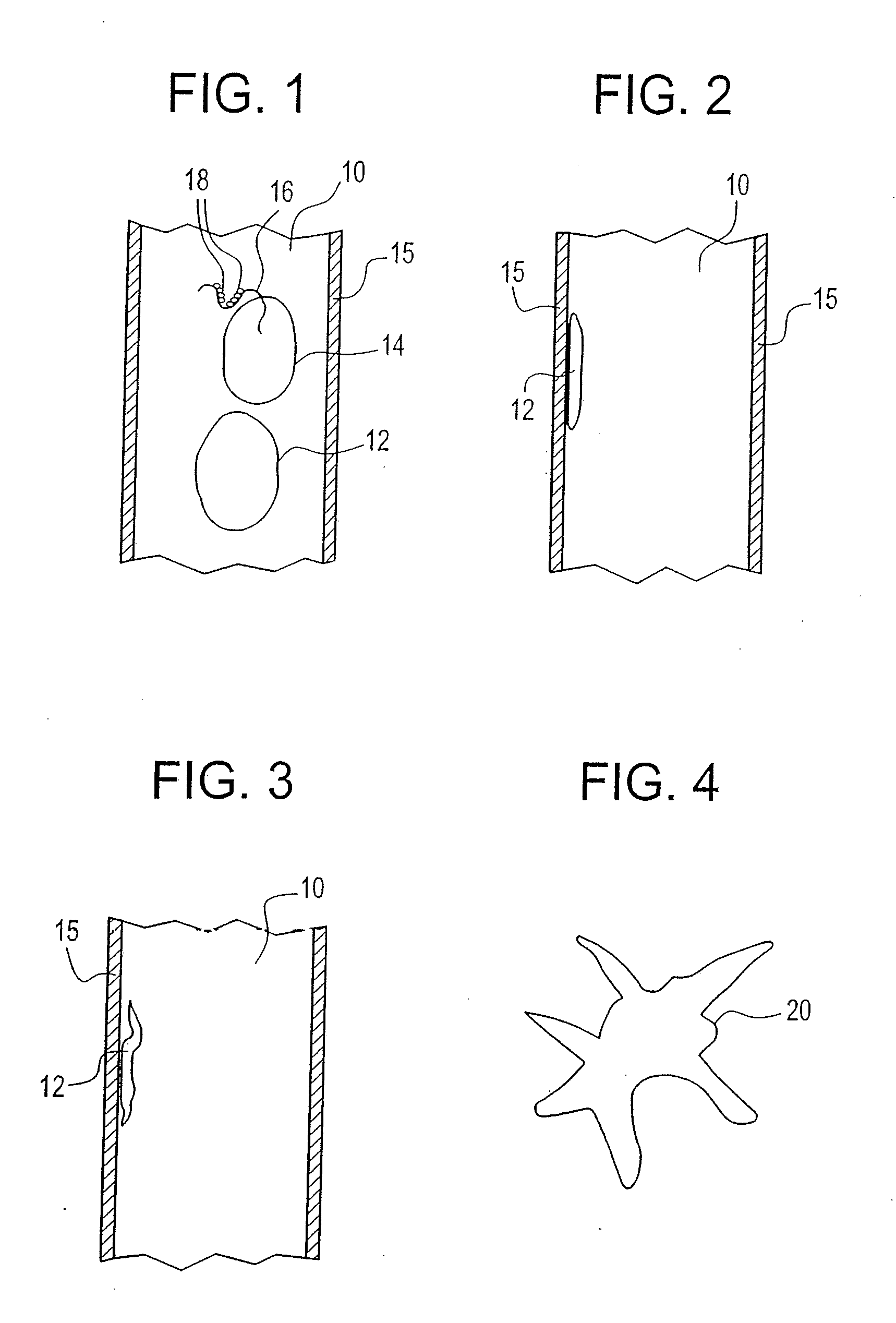
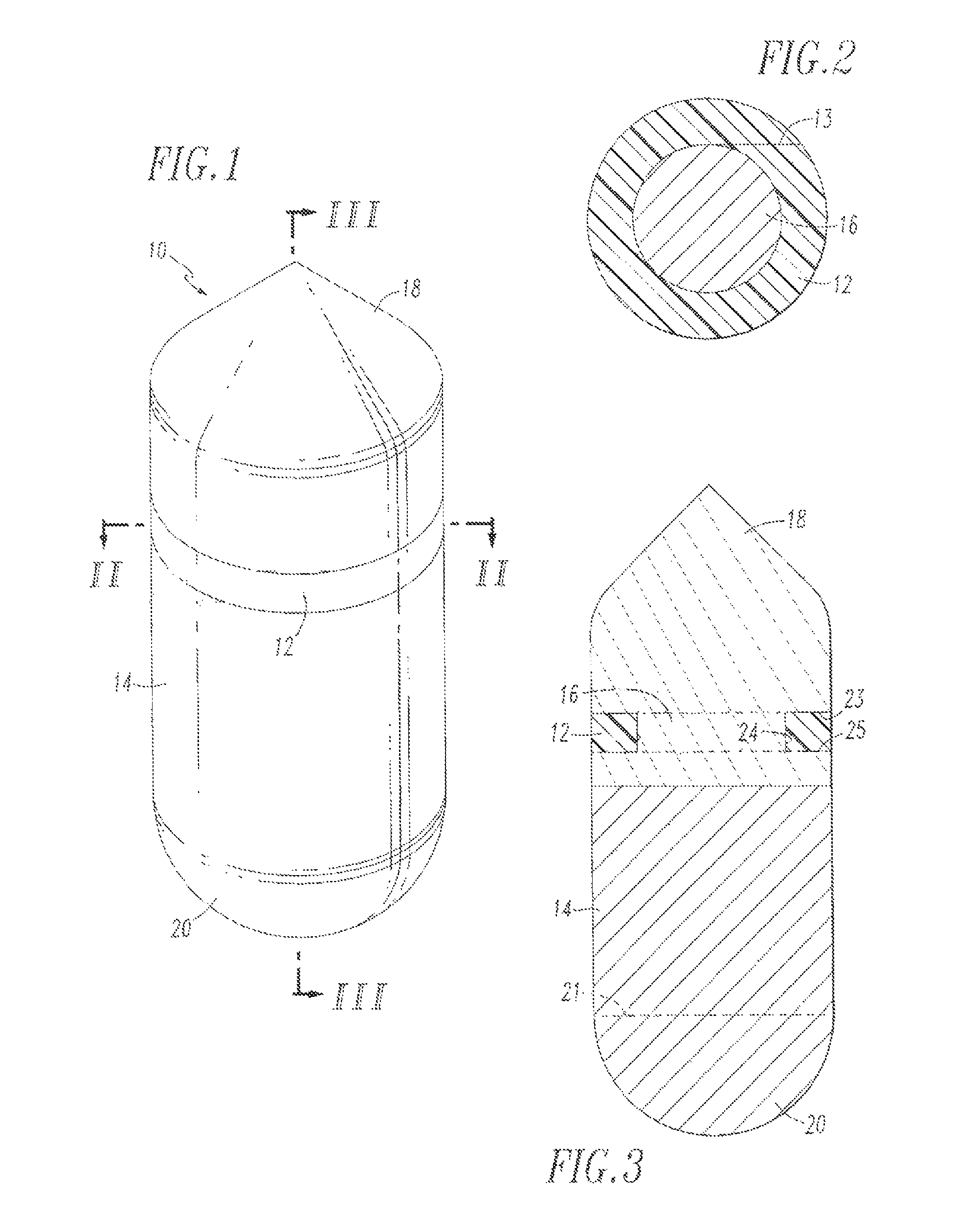
Buffy coat separator float system and method
InactiveUS7220593B2Enhance the imageWater/sewage treatment by centrifugal separationWithdrawing sample devicesCentrifugationDensity based
Owner:BATTELLE MEMORIAL INST
Buffy coat tube and float system and method
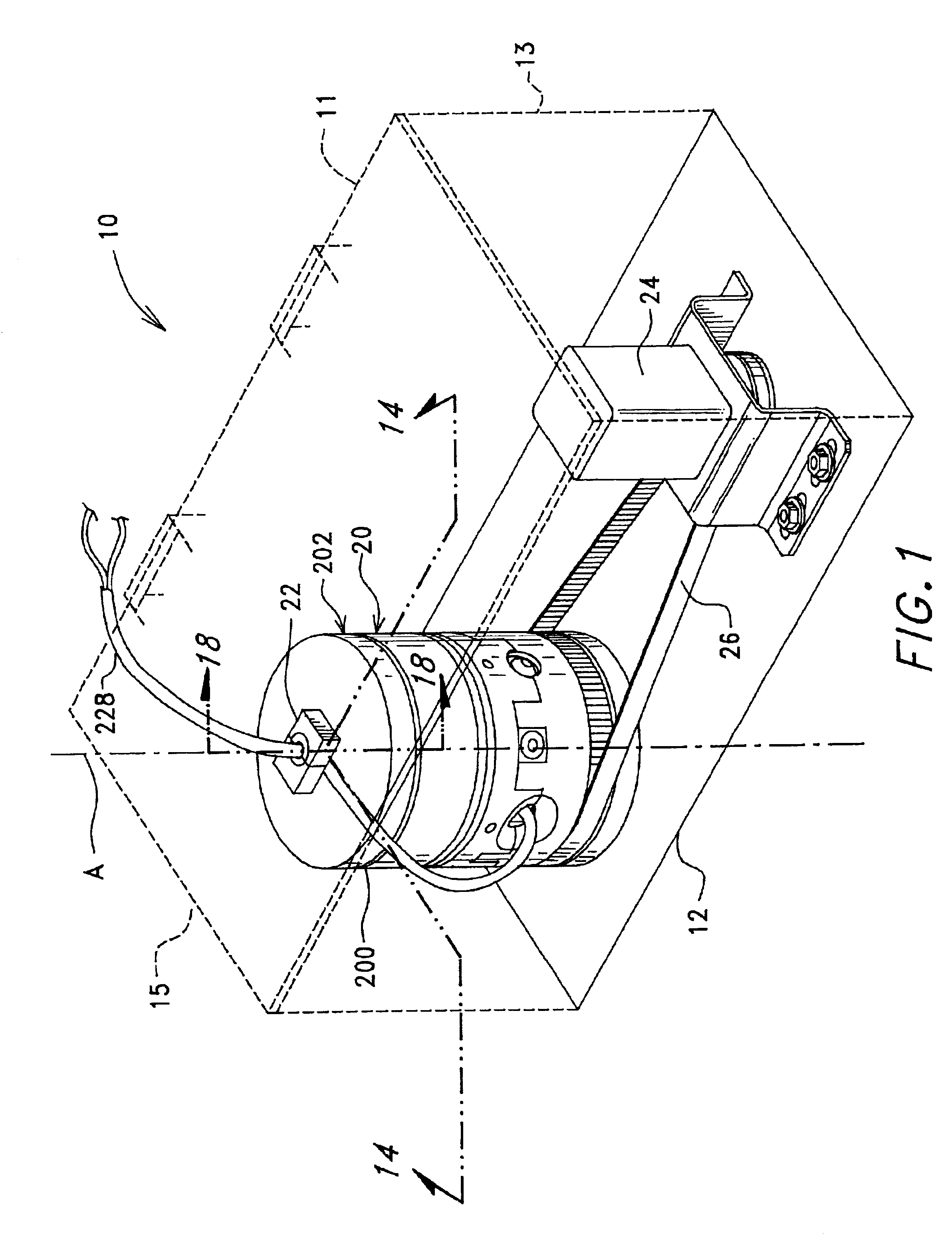
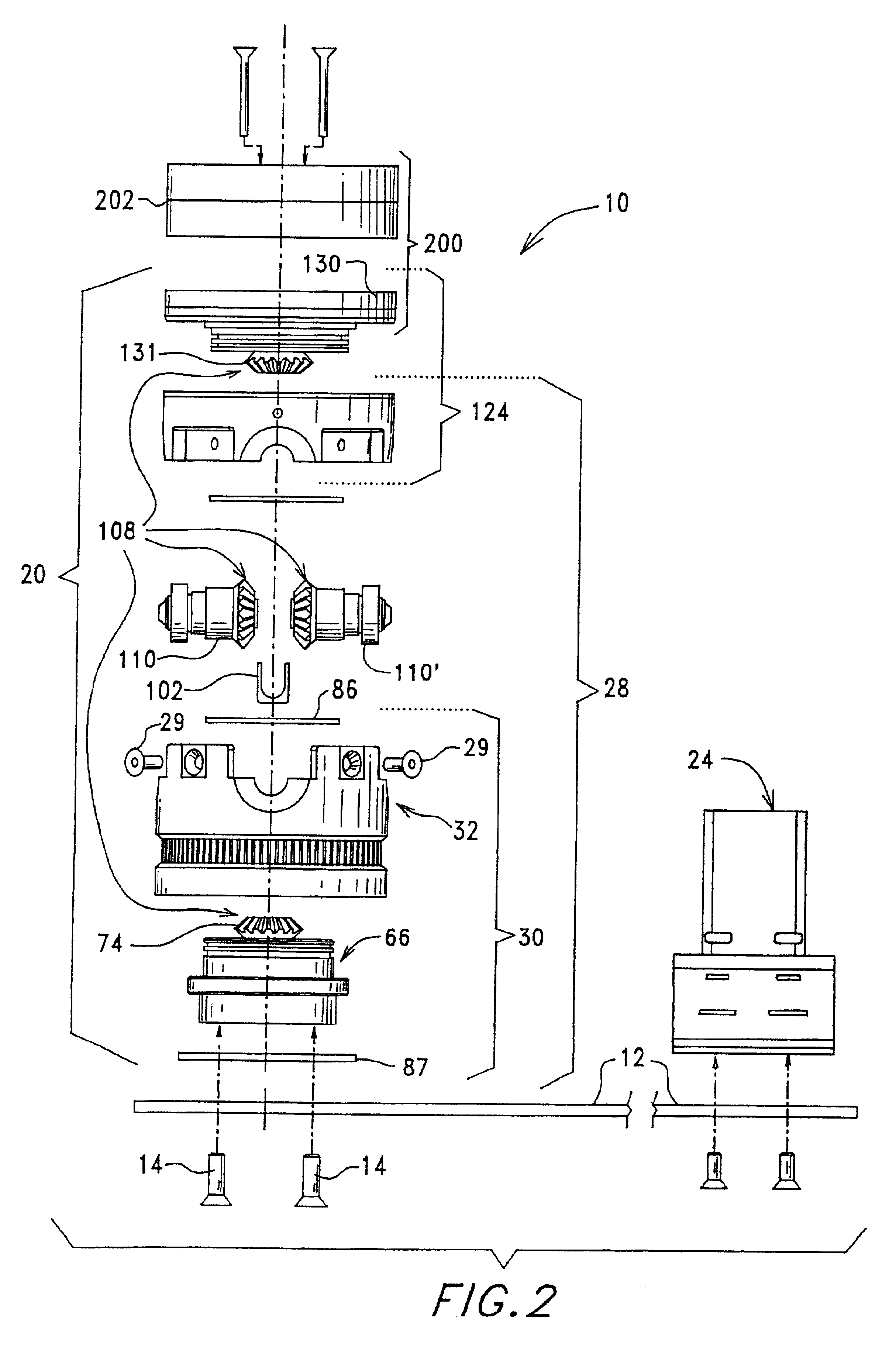
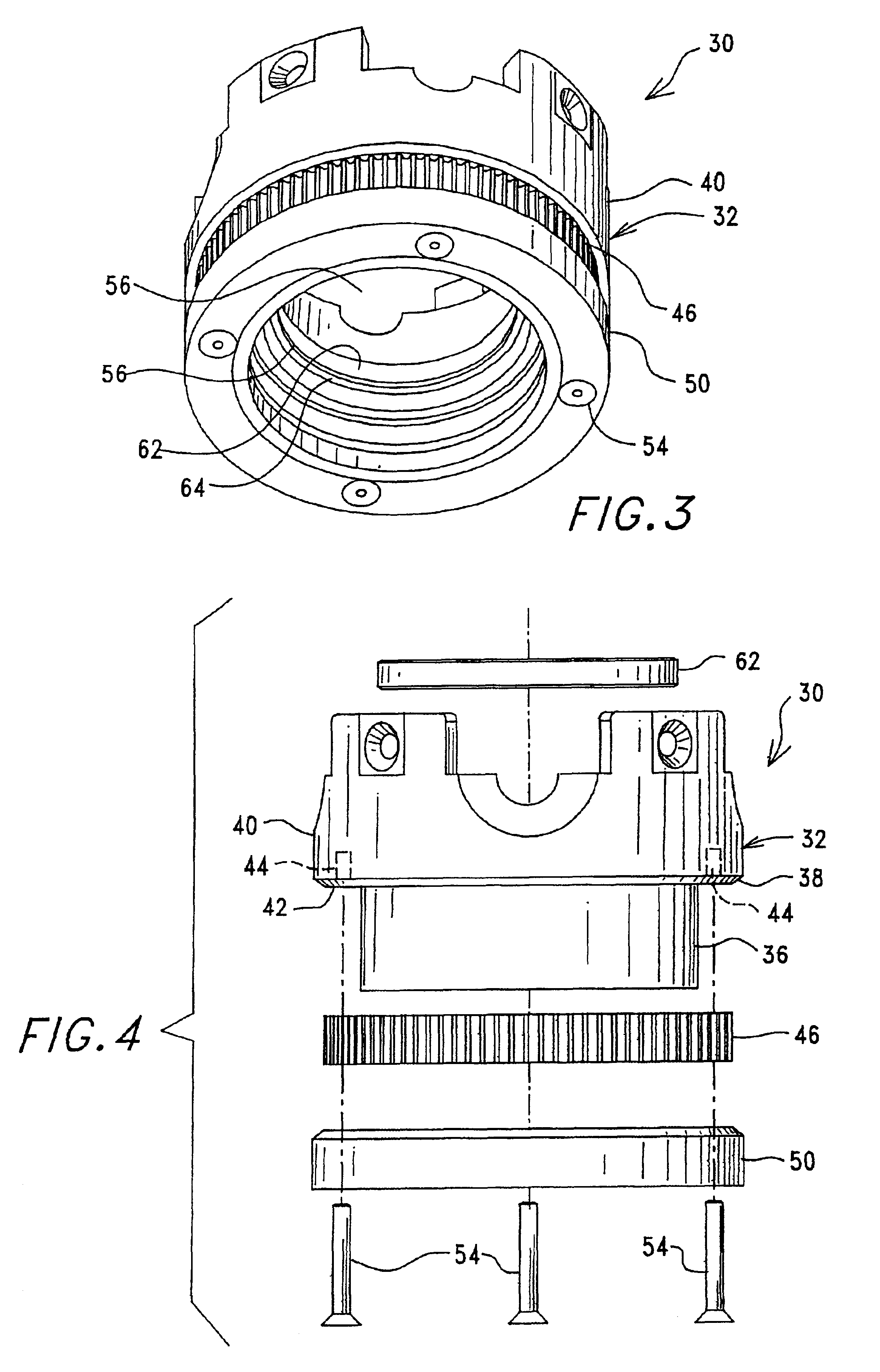
ActiveUS7074577B2Easy to separateReducing necessary cost of componentBioreactor/fermenter combinationsBiological substance pretreatmentsCentrifugationRed blood cell
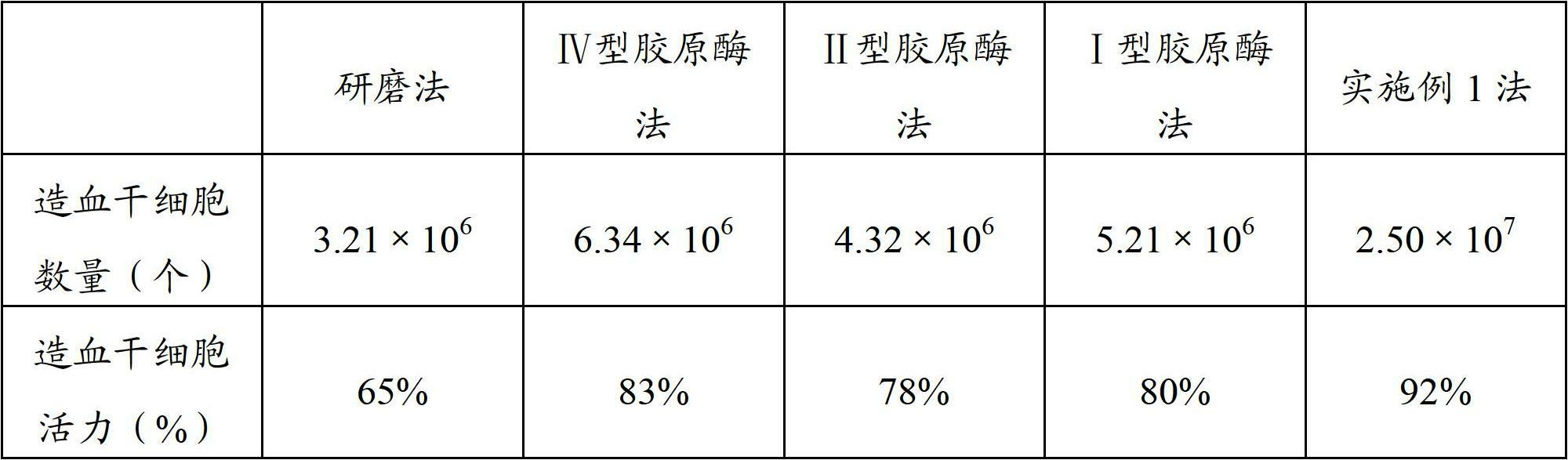
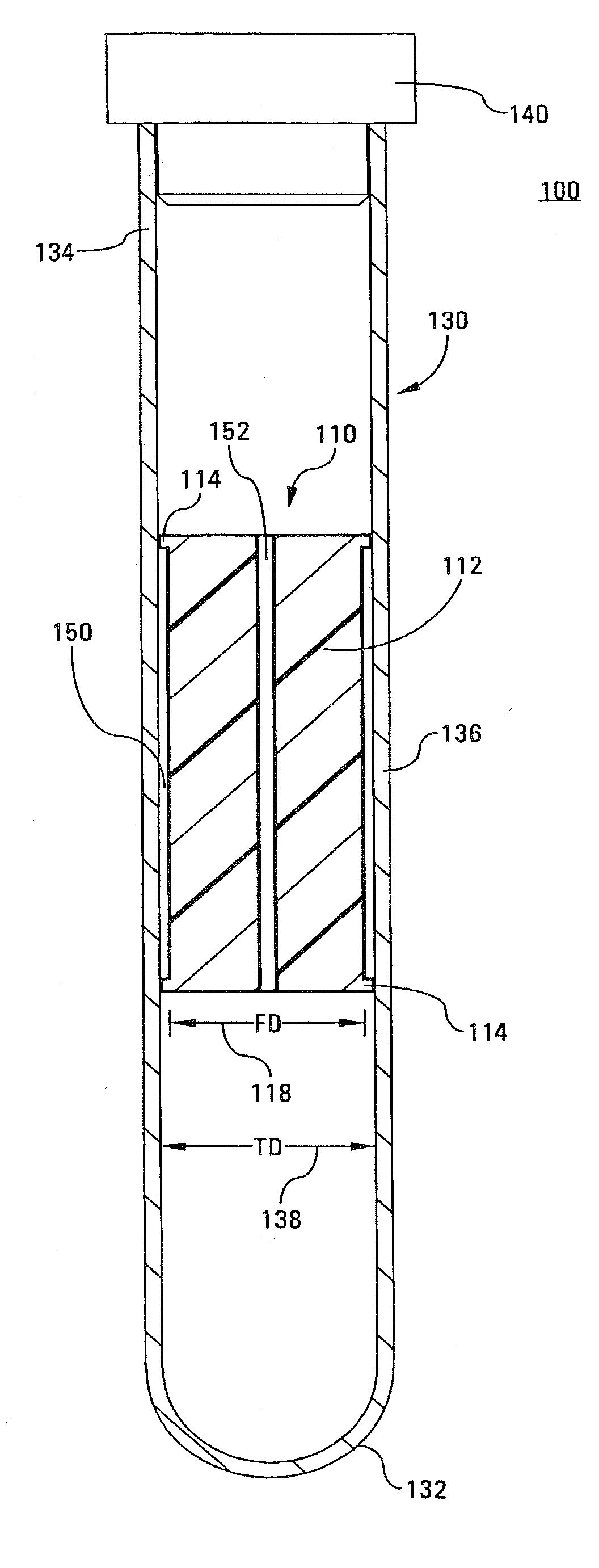
A tube and float system for use in separation and axial expansion of the buffy coat is provided. The system includes a transparent, or semi-transparent, flexible sample tube and a rigid separator float having a specific gravity intermediate that of red blood cells and plasma. The sample tube has an elongated sidewall having a first cross-sectional inner diameter. The float consists of a main body portion and one or more support members protruding from the main body portion to engage and support the sidewall of the sample tube. The main body portion and the support members of the float have a cross-sectional diameter less than that of the first cross-sectional inner diameter of the tube when the sample tube is expanded, such as by centrifugation. The main body portion of the float together with an axially aligned portion of the sidewall define an annular volume therebetween. The support members protruding from the main body portion of the float traverse said annular volume to produce one or more analysis areas. During centrifugation, the centrifugal force enlarges the diameter of the tube to permit density-based axial movement of the float in the tube. Thereafter, the centrifugal force is reduced to cause the tube sidewall to return to its first diameter, thereby capturing the float and trapping the buffy coat constituents in the analysis area. The buffy coat constituents can then be evaluated or measured.
Owner:BATTELLE MEMORIAL INST
Methods of isolating blood components using a microcentrifuge and uses thereof
InactiveUS6890728B2Small sizeQuantity maximizationOther blood circulation devicesGenetic material ingredientsCellular componentMuscle tissue
Methods and apparatuses for the separation a predetermined cellular component from a bodily fluid or tissue by centrifugation are provided. More specifically, this invention provides a method for the separation and isolation of one or more components, such as stem cells, buffy coat, bone marrow cells, erythrocytes, leukocytes, thrombocytes, serum, fluorescently labeled cells, and magnetically labeled cells, from whole blood, bone marrow, buffy coat, fat tissue, muscle tissue, and nerve tissue by centrifugation, wherein the components are isolated while the centrifuge is rotating.
Owner:ARTERIOCYTE MEDICAL SYST
Apparatus And Method For Separating And Concentrating Fluids Containing Multiple Components
An apparatus is disclosed that allows for separating and collecting a fraction of a sample. The apparatus, when used with a centrifuge, allows for the creation of at least three fractions in the apparatus. It also provides for a new method of extracting the buffy coat phase from a whole blood sample. A buoy system that may include a first buoy portion and a second buoy member operably interconnected may be used to form at least three fractions from a sample during a substantially single centrifugation process. Therefore, the separation of various fractions may be substantially quick and efficient.
Owner:BIOMET MFG CORP
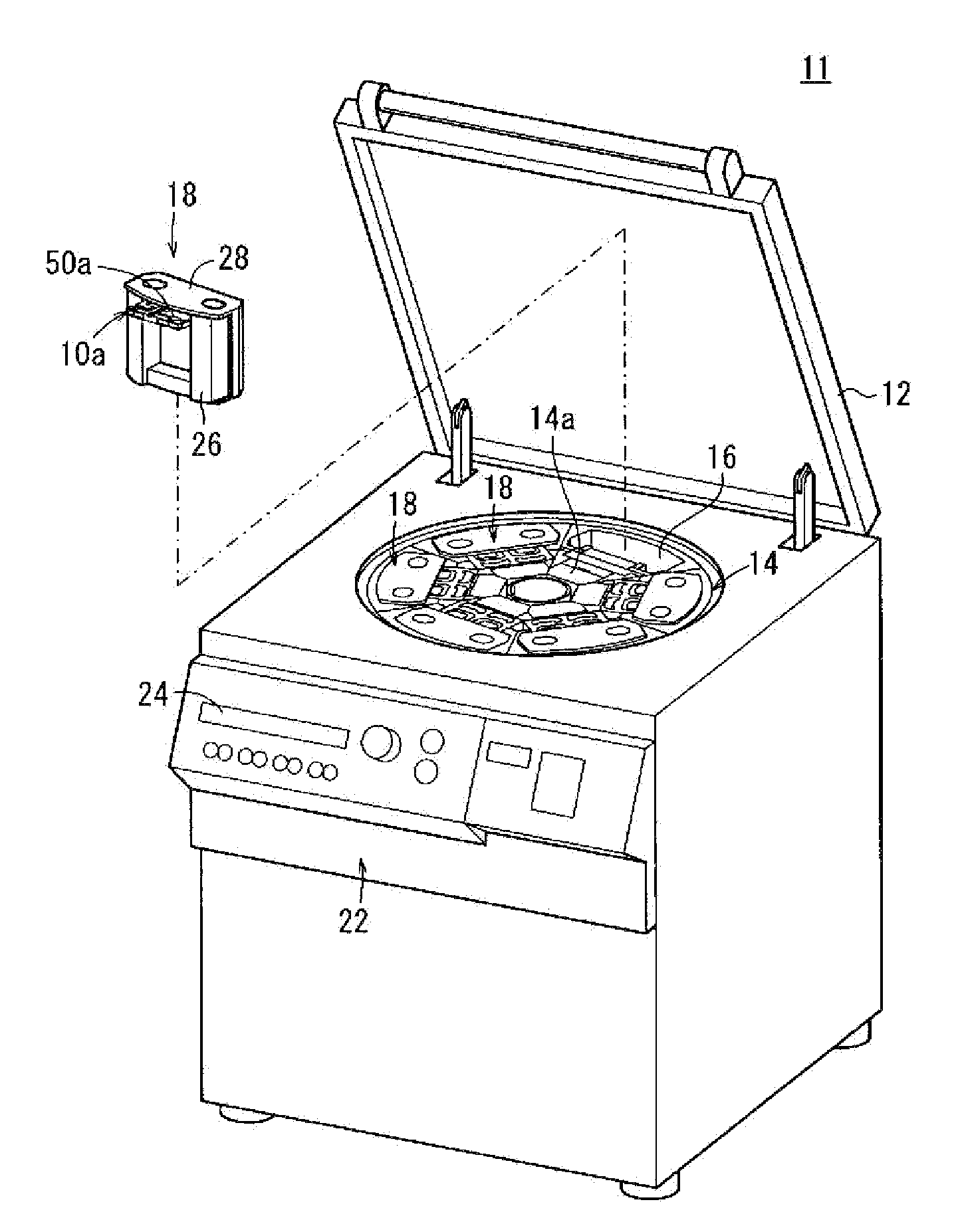
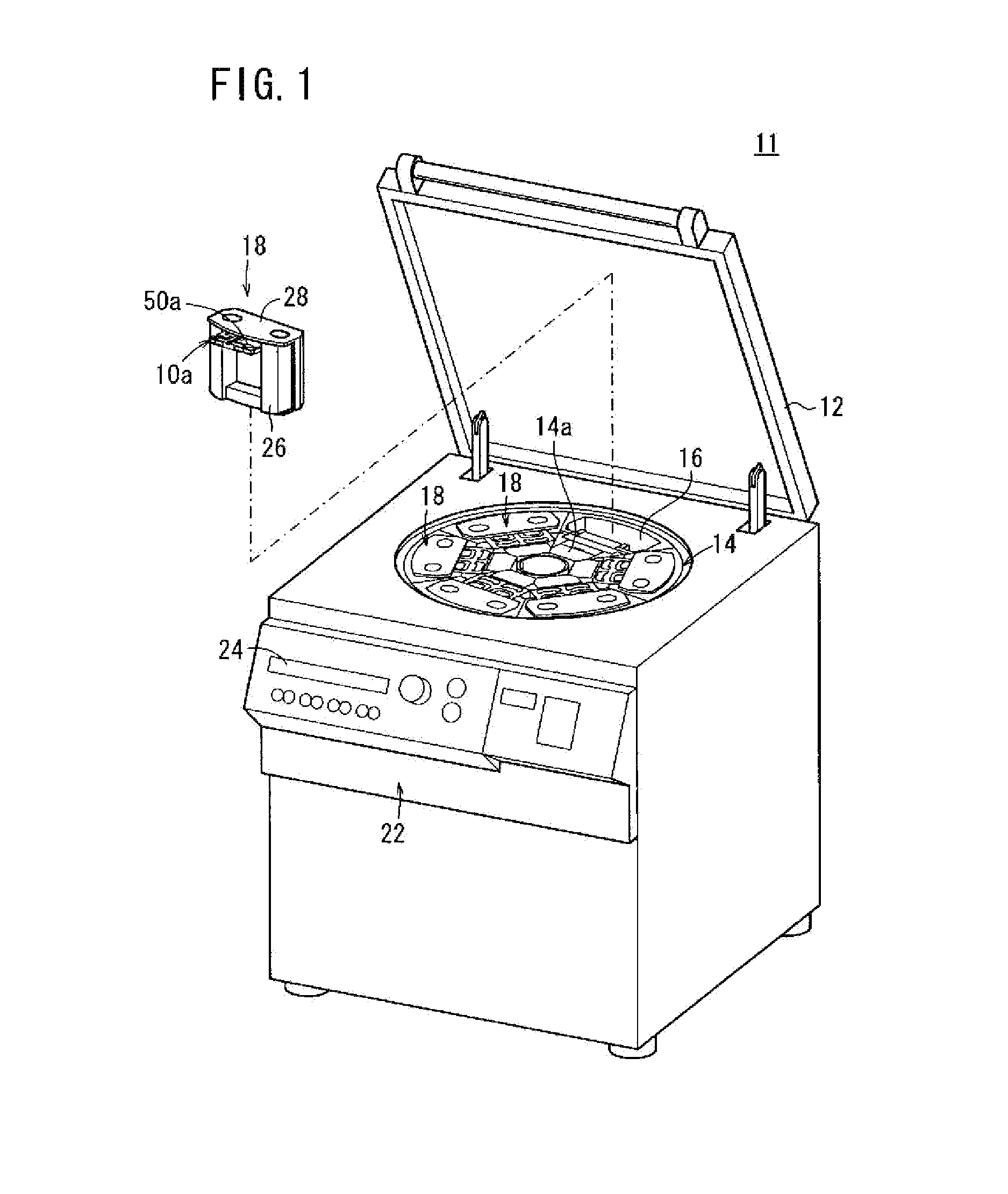
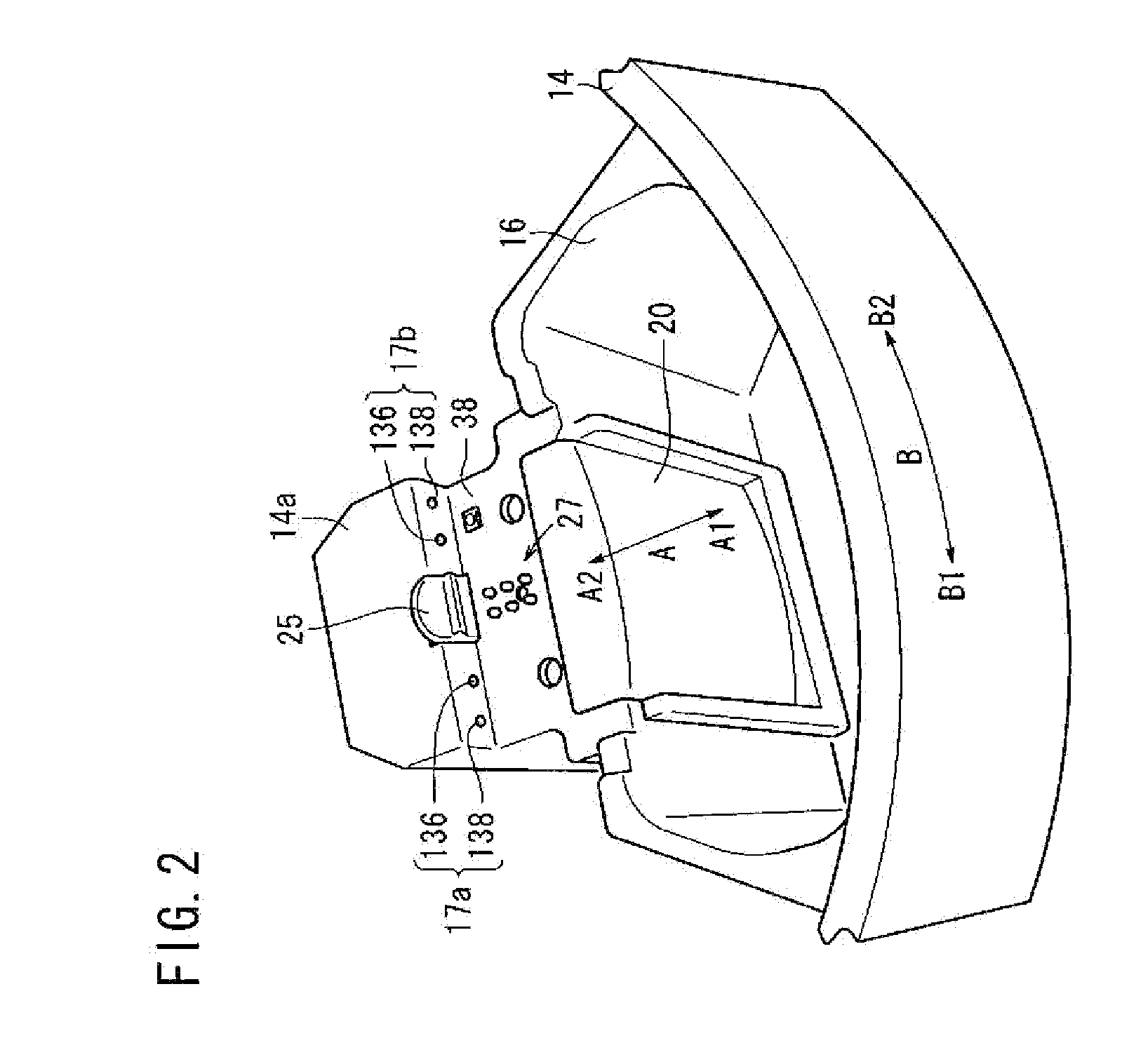
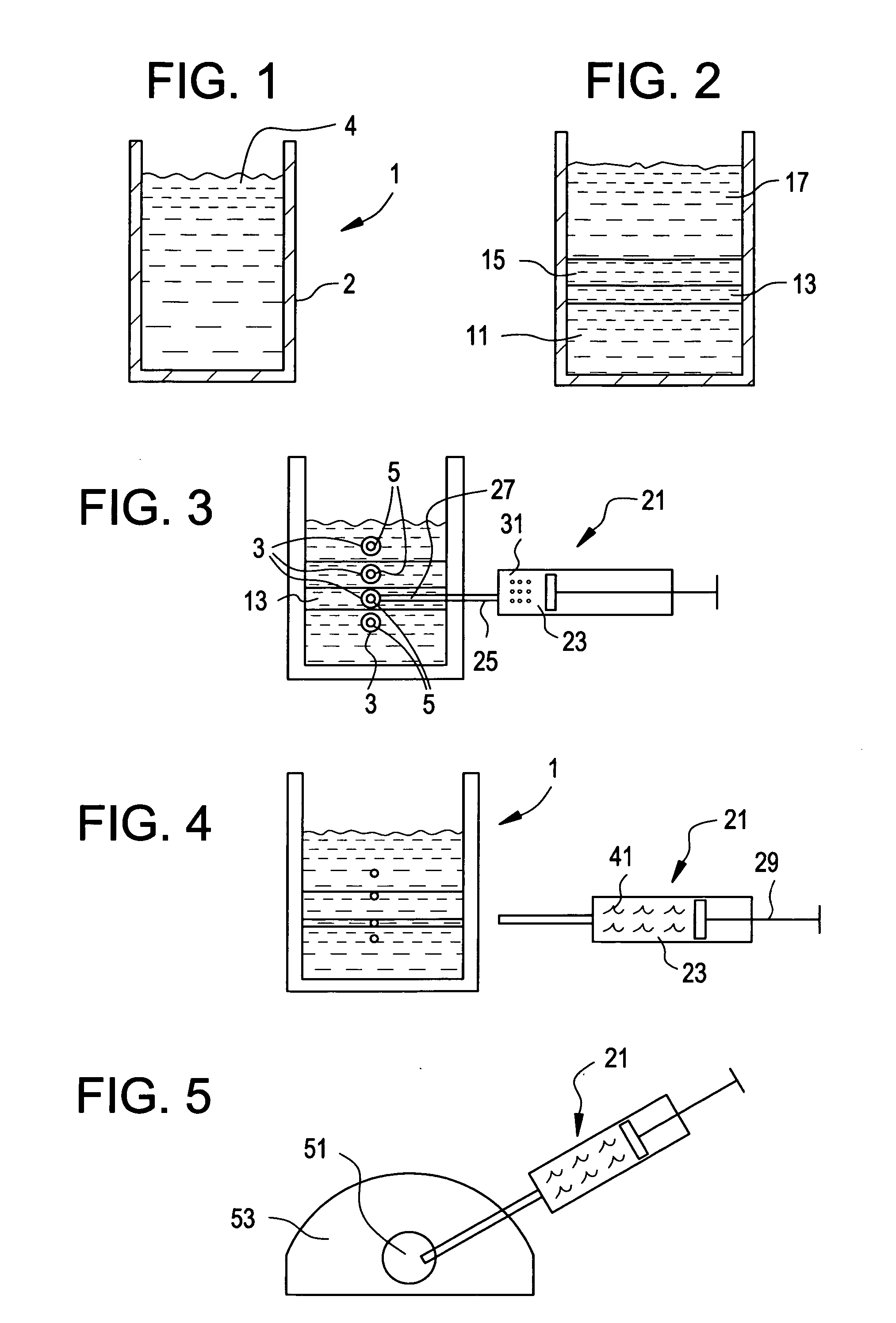
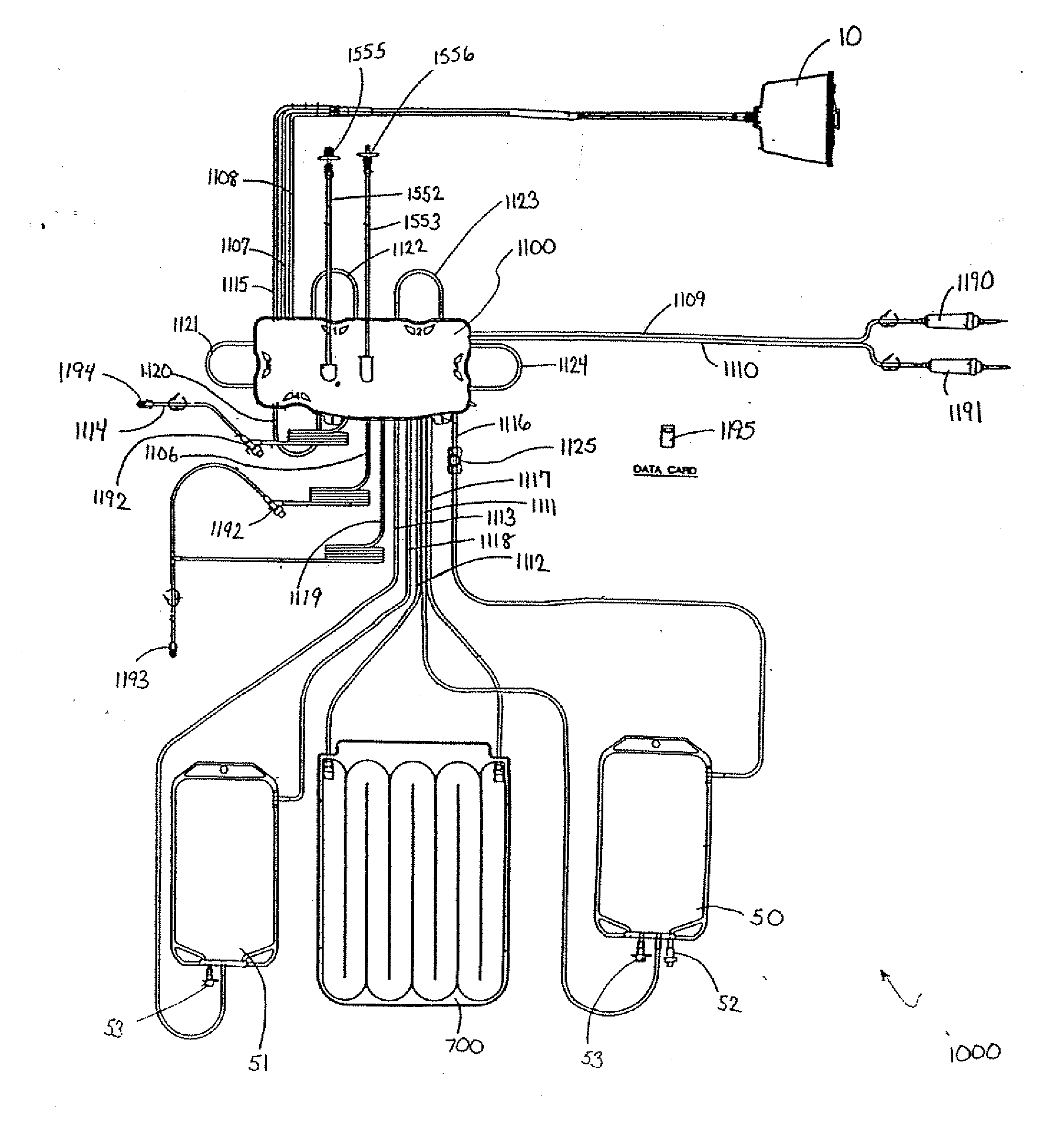
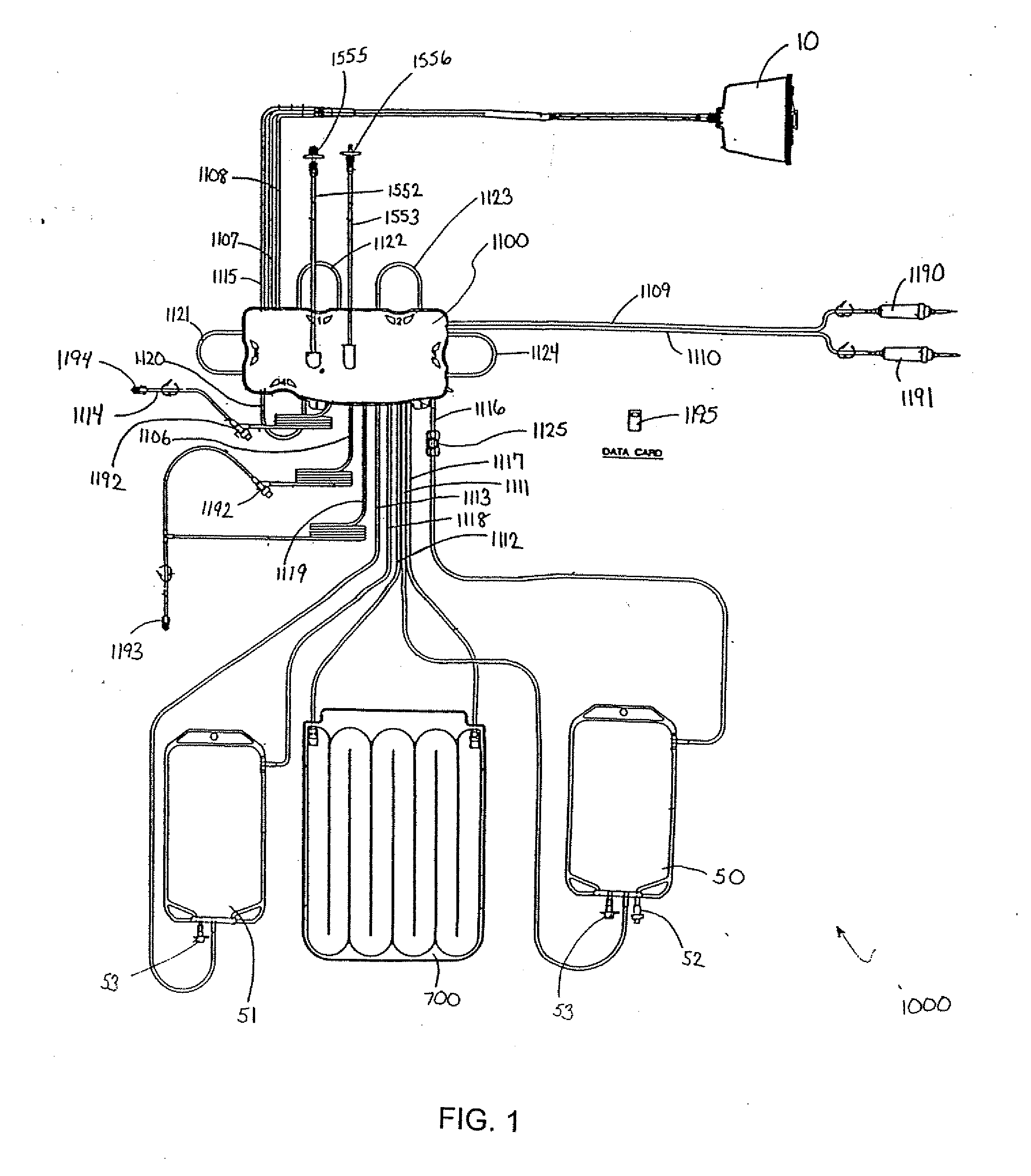
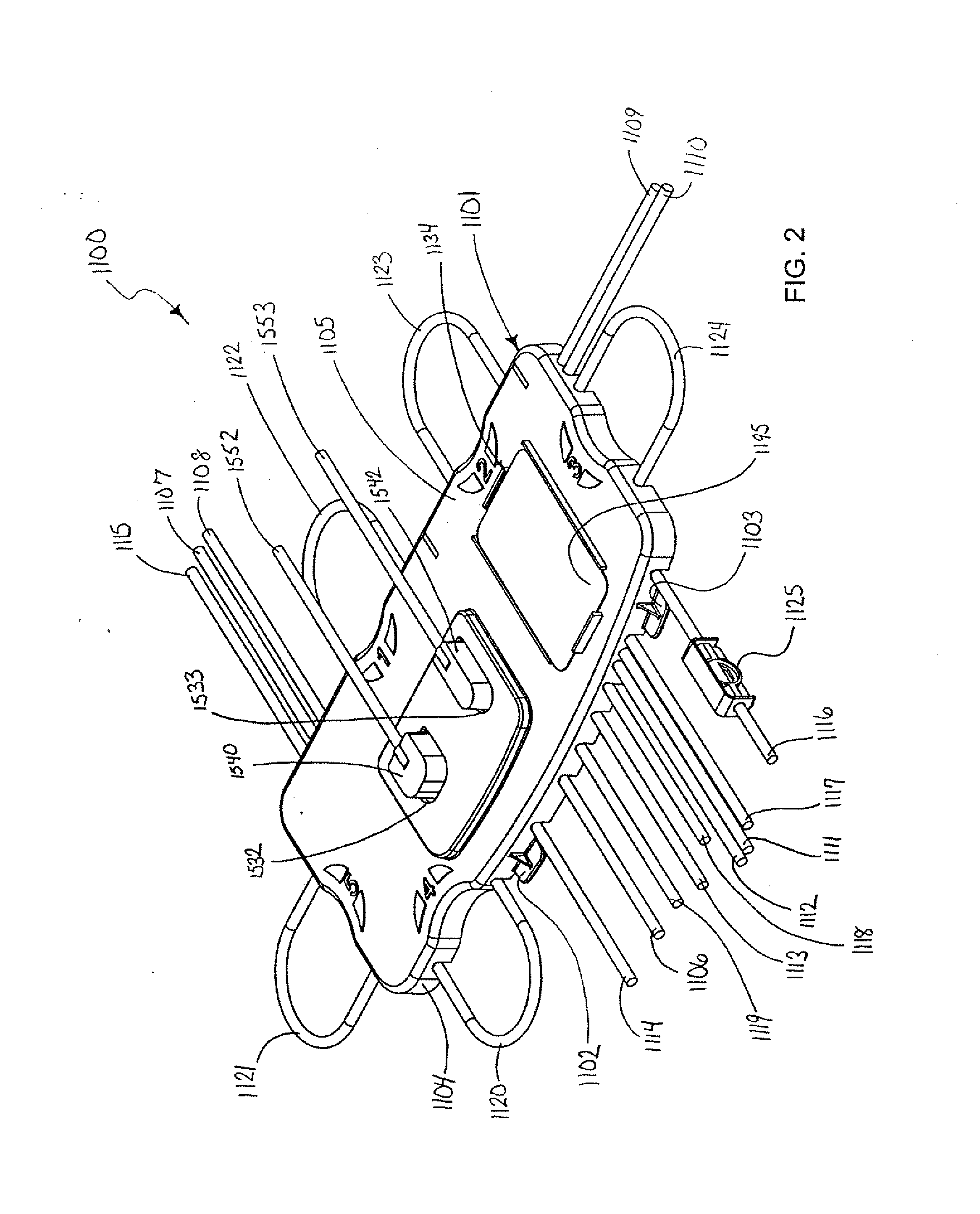
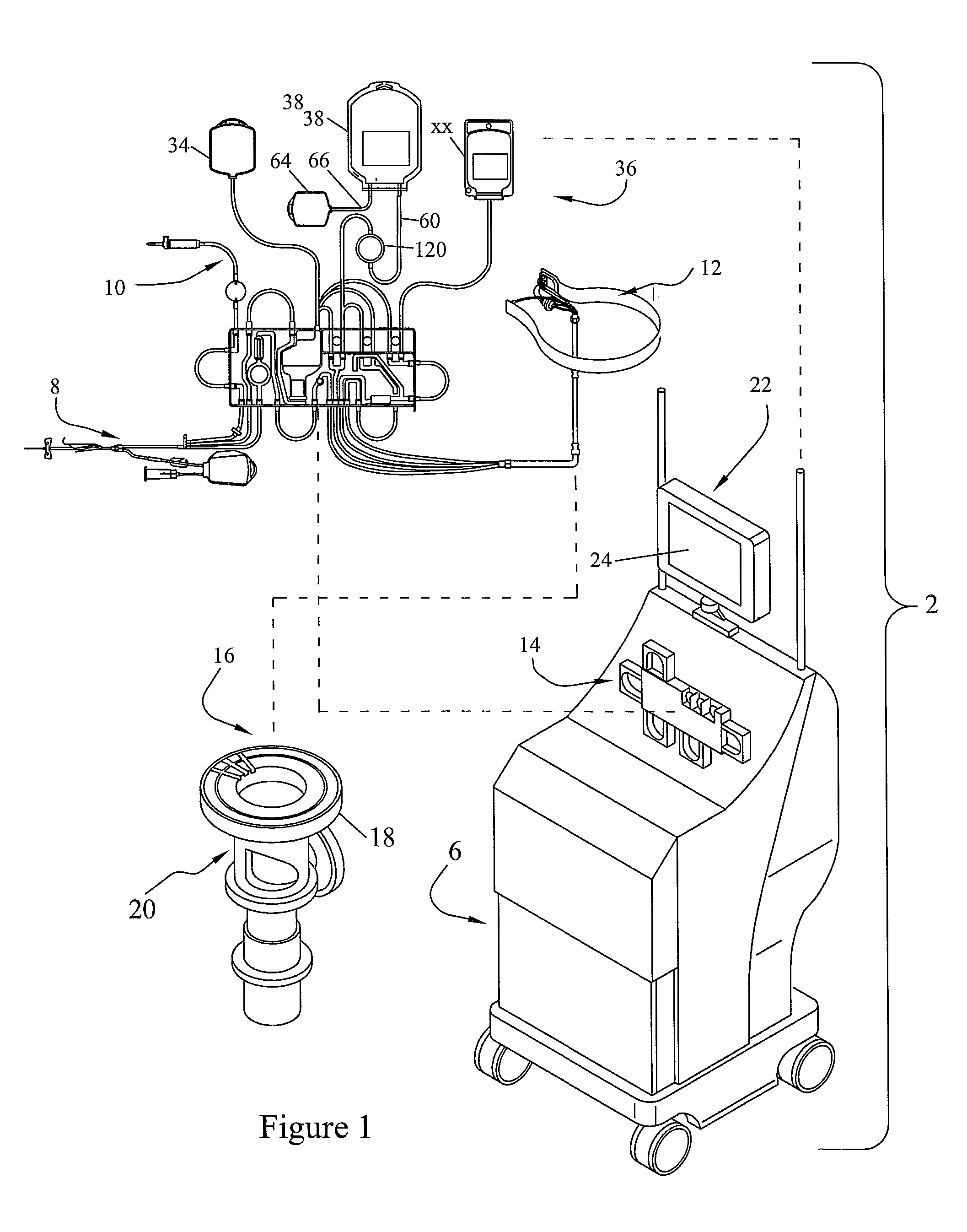
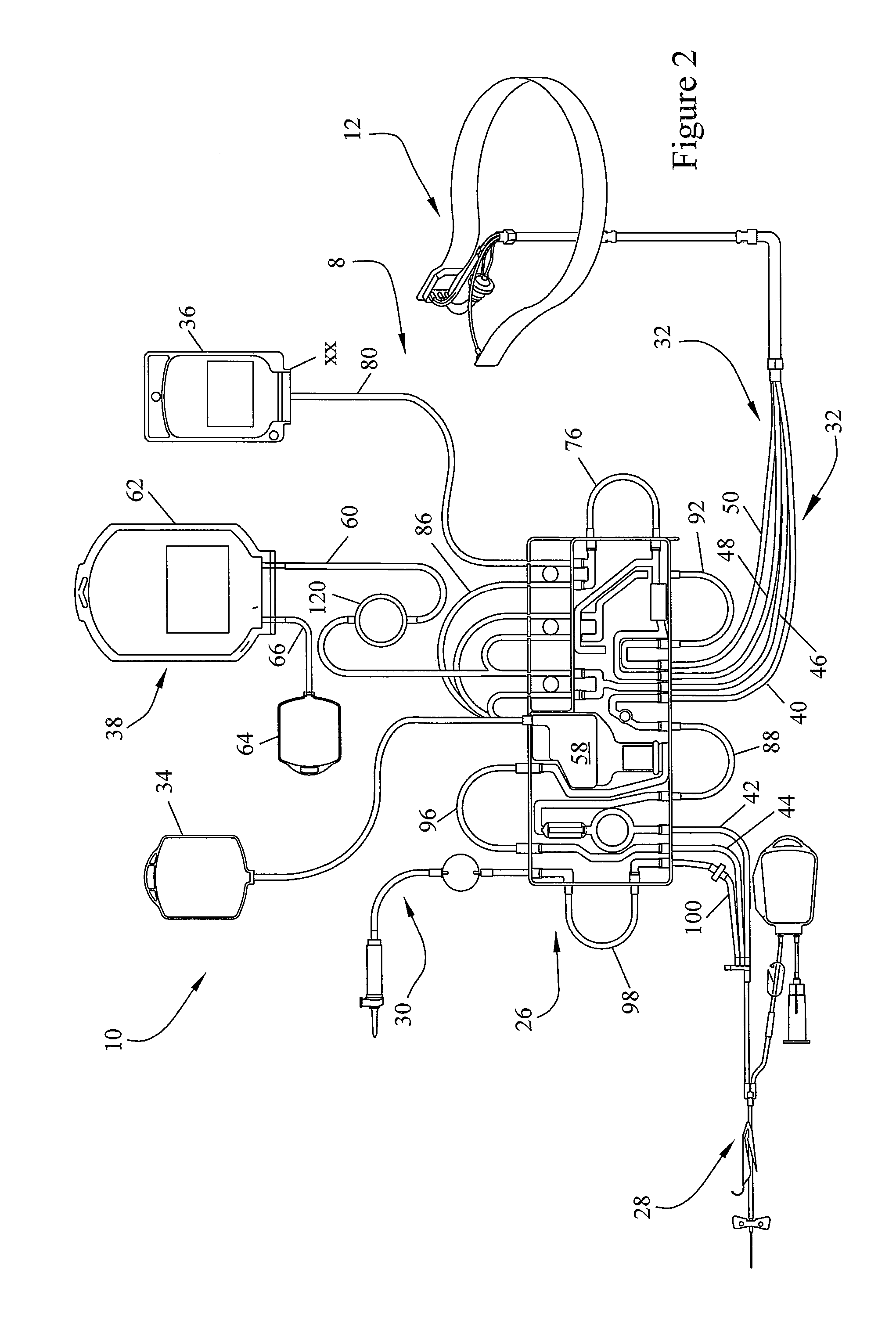
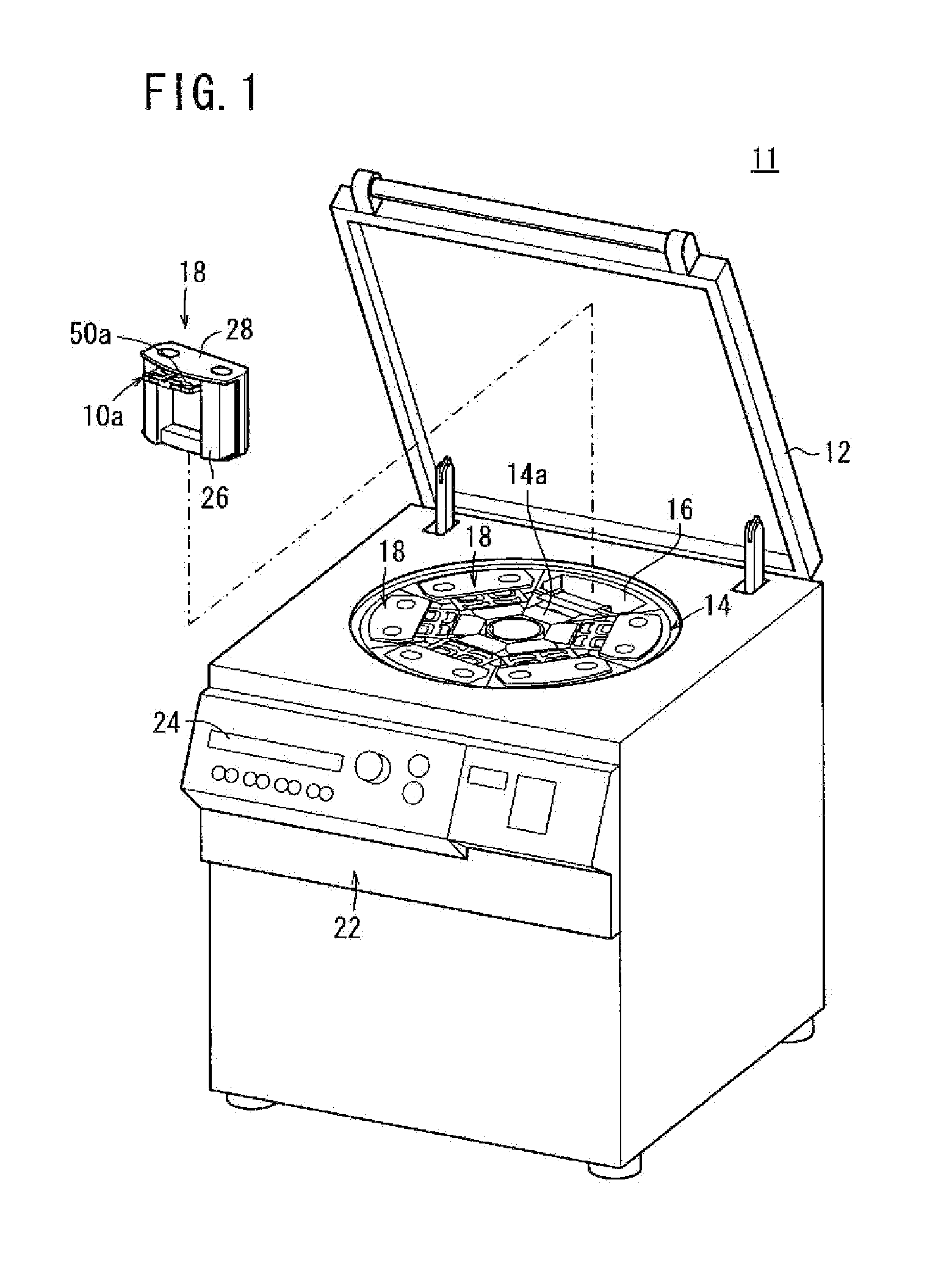
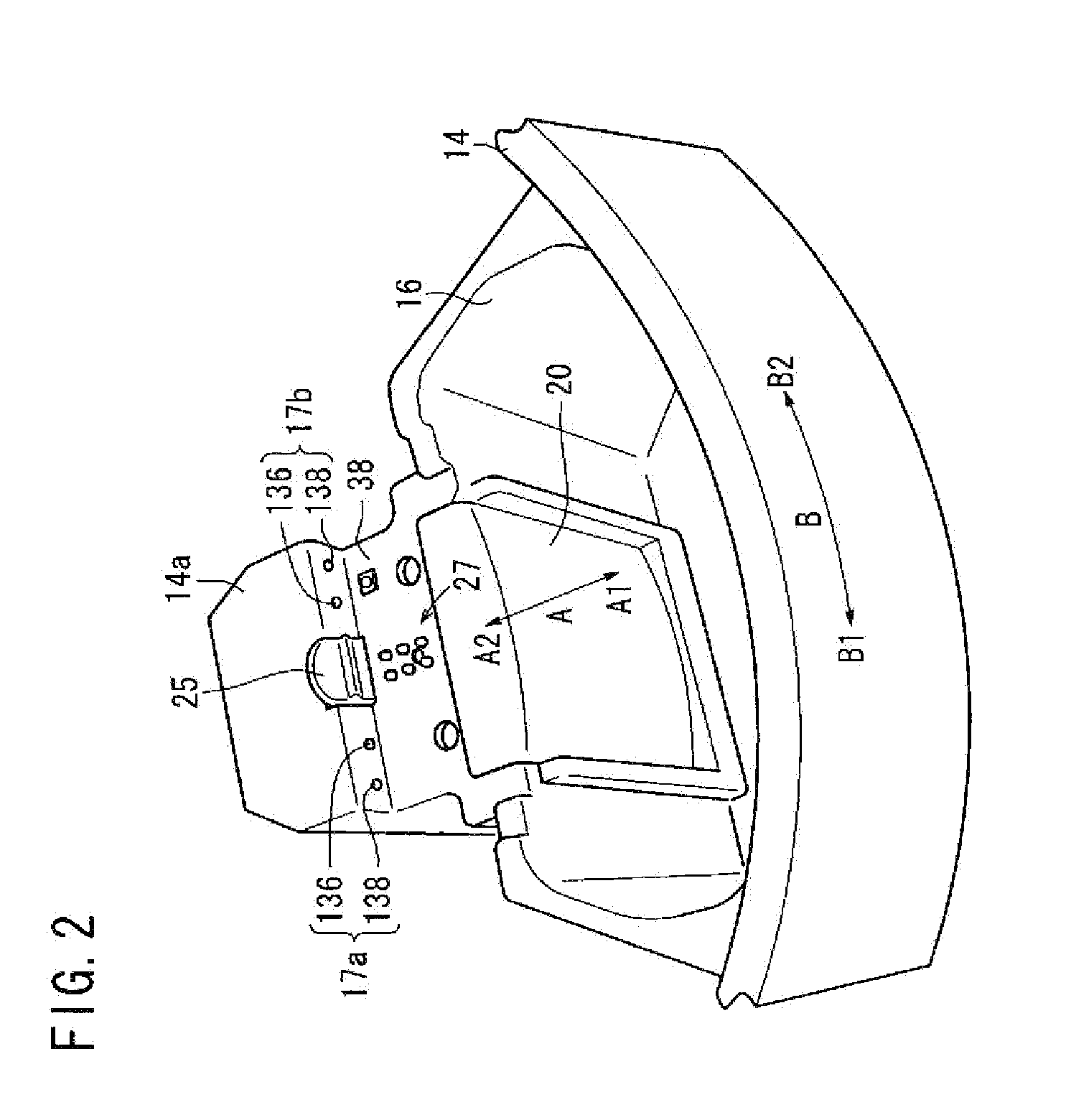
Centrifuge comprising a blood bag system with an upper and lower outlet
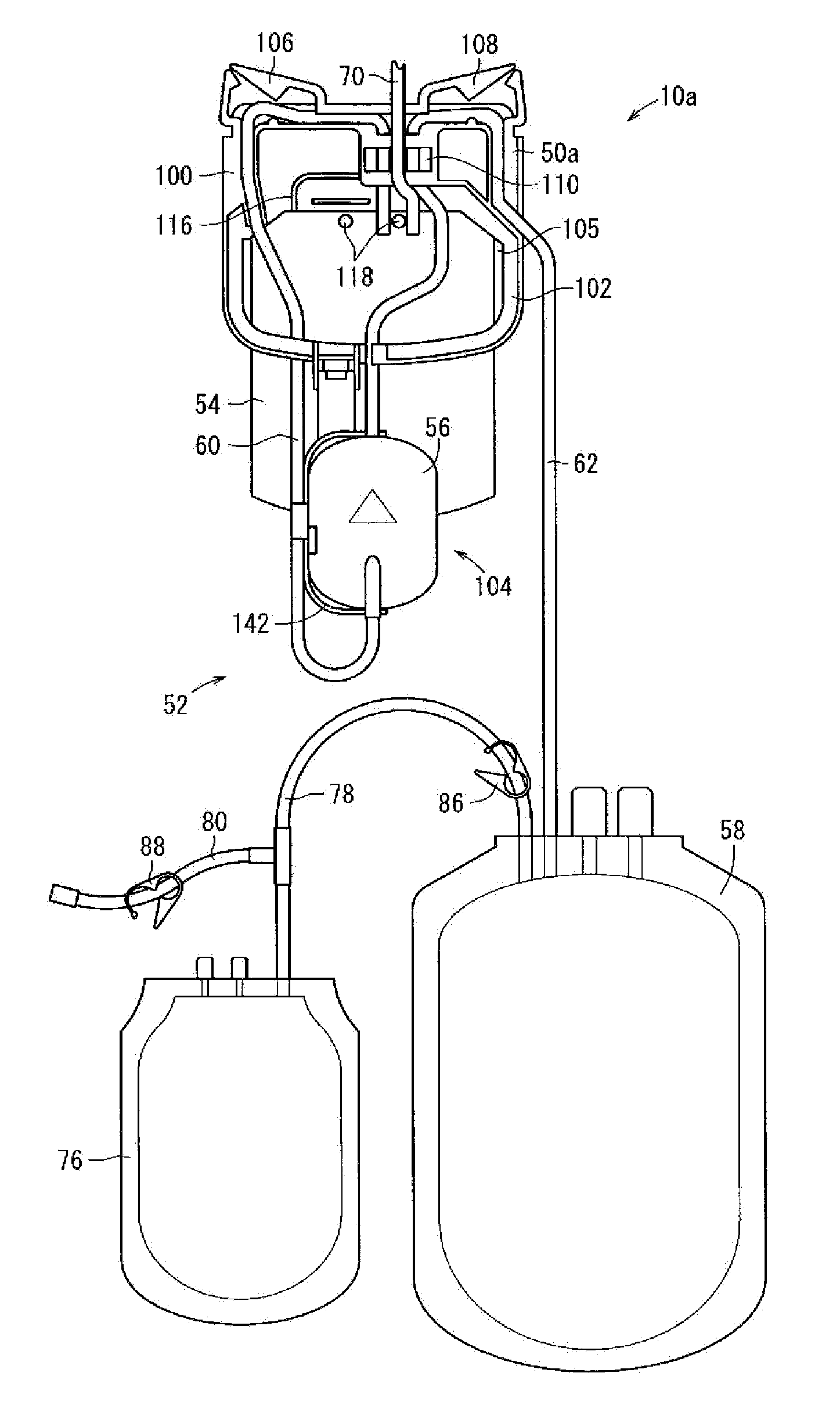
The invention relates to a centrifuge provided with a blood bag system having an upper and lower outlet for separation of blood constituents, containing a rotor which can be driven in a rotative manner about a hub, wherein at least one conventional whole blood bag equipped with an upper outlet and with a lower outlet can be positioned vertically in this blood bag, while the whole blood bag can be arranged on the rotor in such a way that one outlet of the whole blood bag is bent in a radial direction, and the opposite outlet of the whole blood bag is bent in the other radial direction, so that the entire whole blood bag and the outlets thereof adopt an approximately Z- or S-shaped form in its functional position. In this case it is preferred that the upper outlet of the whole blood bag lie radially toward the interior and the lower outlet of the whole blood bag lie radially toward the exterior of the whole blood bag. The whole blood bag is held in an insert, and the insert is in its turn held by an approximately sector-shaped cartridge, wherein a plurality of such cartridges is uniformly distributed by being arranged around the circumference of the rotor. One advantage of the centrifuge is that conventional blood bags can be used and recovery of individual blood constituents (plasma, erythrocytes, buffycoat) can be achieved with a significantly higher degree of efficiency and functionality, and with a high degree of purity.
Owner:ANDREAS HETTICH GMBH & CO KG
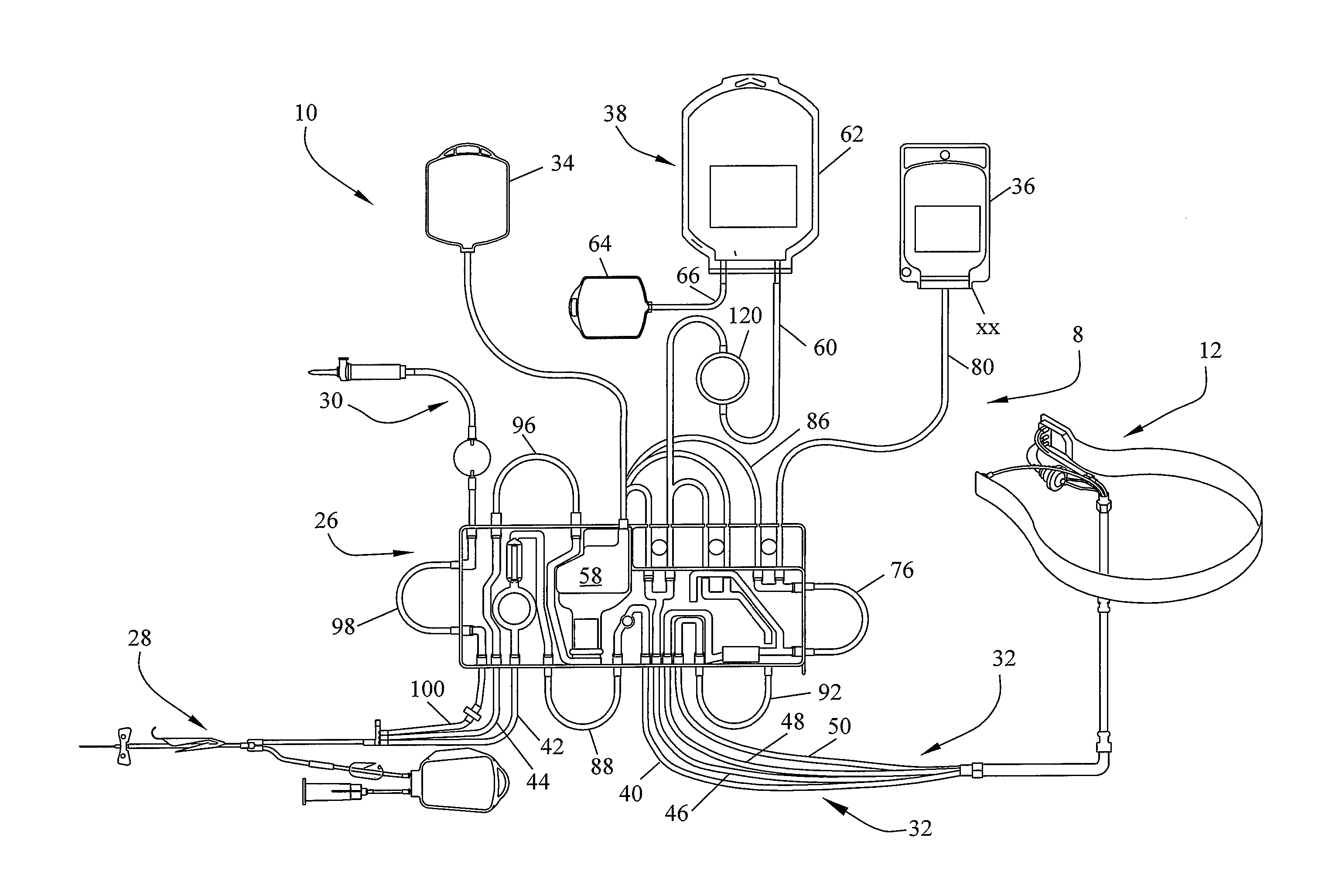
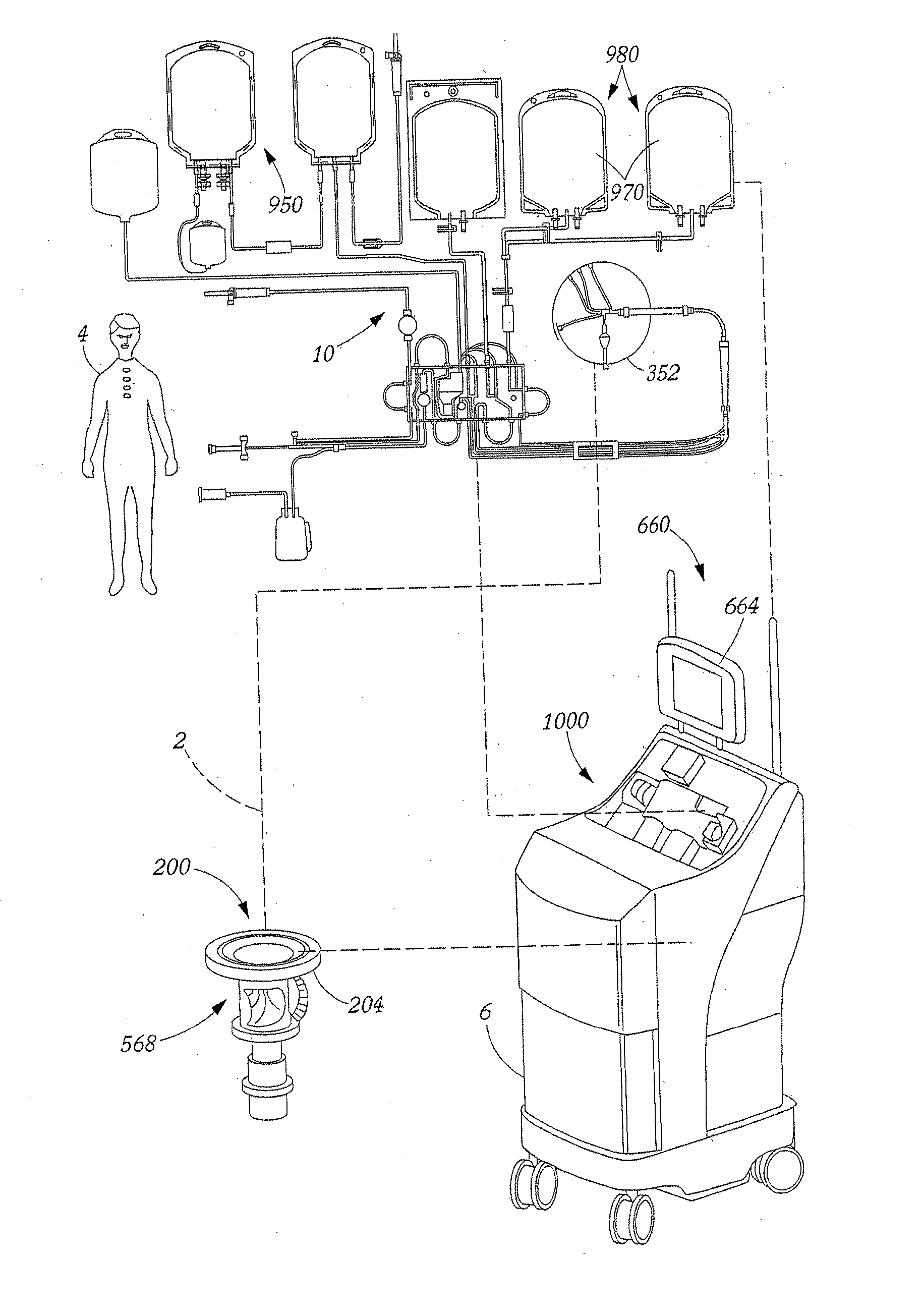
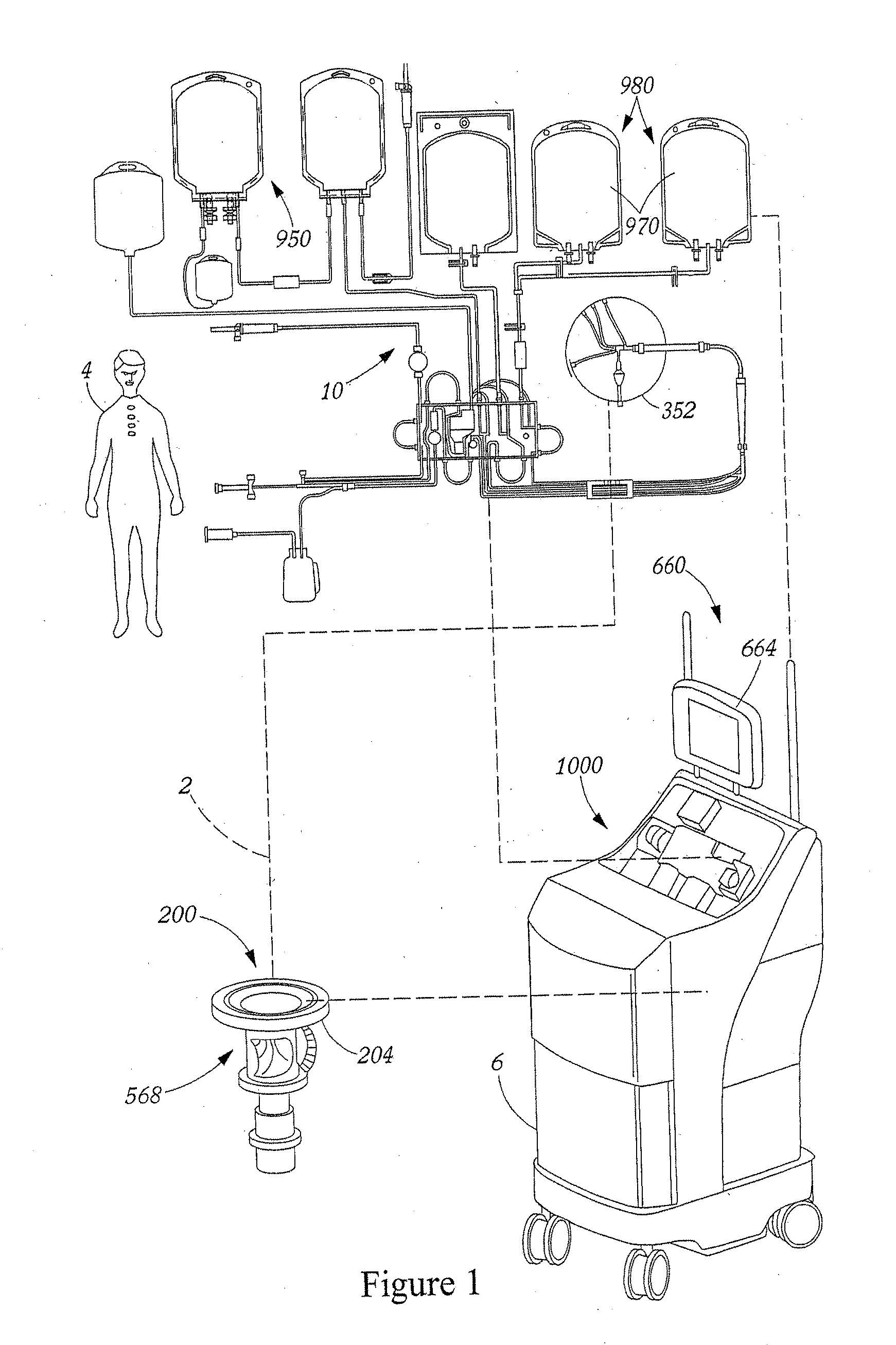
Method for collecting a desired blood component and performing a photopheresis treatment
ActiveUS7479123B2Improve efficiencyHigh yieldOther blood circulation devicesFlexible member pumpsDiseasePhotopheresis
An improved method for separating whole blood into components and collecting a desired blood component. The method allows a desired blood component to be subjected to centrifugal forces within a separator for prolonged periods of time, yielding a cleaner cut and higher yield of the desired blood component. Whole blood is drawn from a source and pumped into a separator, the undesired blood components are removed from the separator at rates so as to build up the desired blood component in the separator. The desired blood component is only removed after a predetermined amount of the desired blood component has built up in the separator. It is preferred that the desired blood component be buffy coat and that the method be used to perform photopheresis treatments. In another aspect, the invention is a method of performing a full photopheresis treatment to treat diseases in a reduced time, preferably less than about 70 minutes, and more preferably less than about 45 minutes.
Owner:MALLINCKRODT HOSPITAL PRODUCTS IP LTD
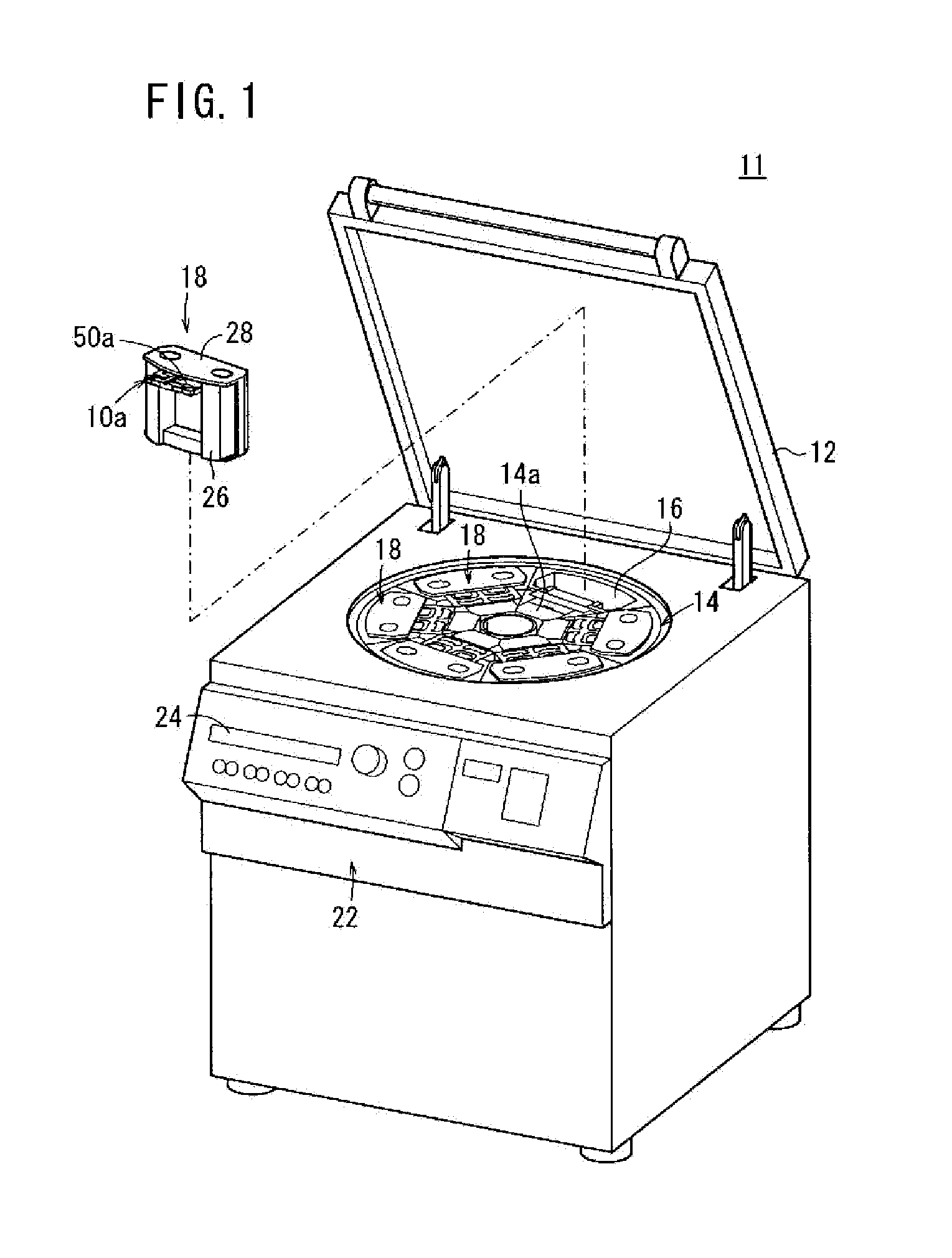
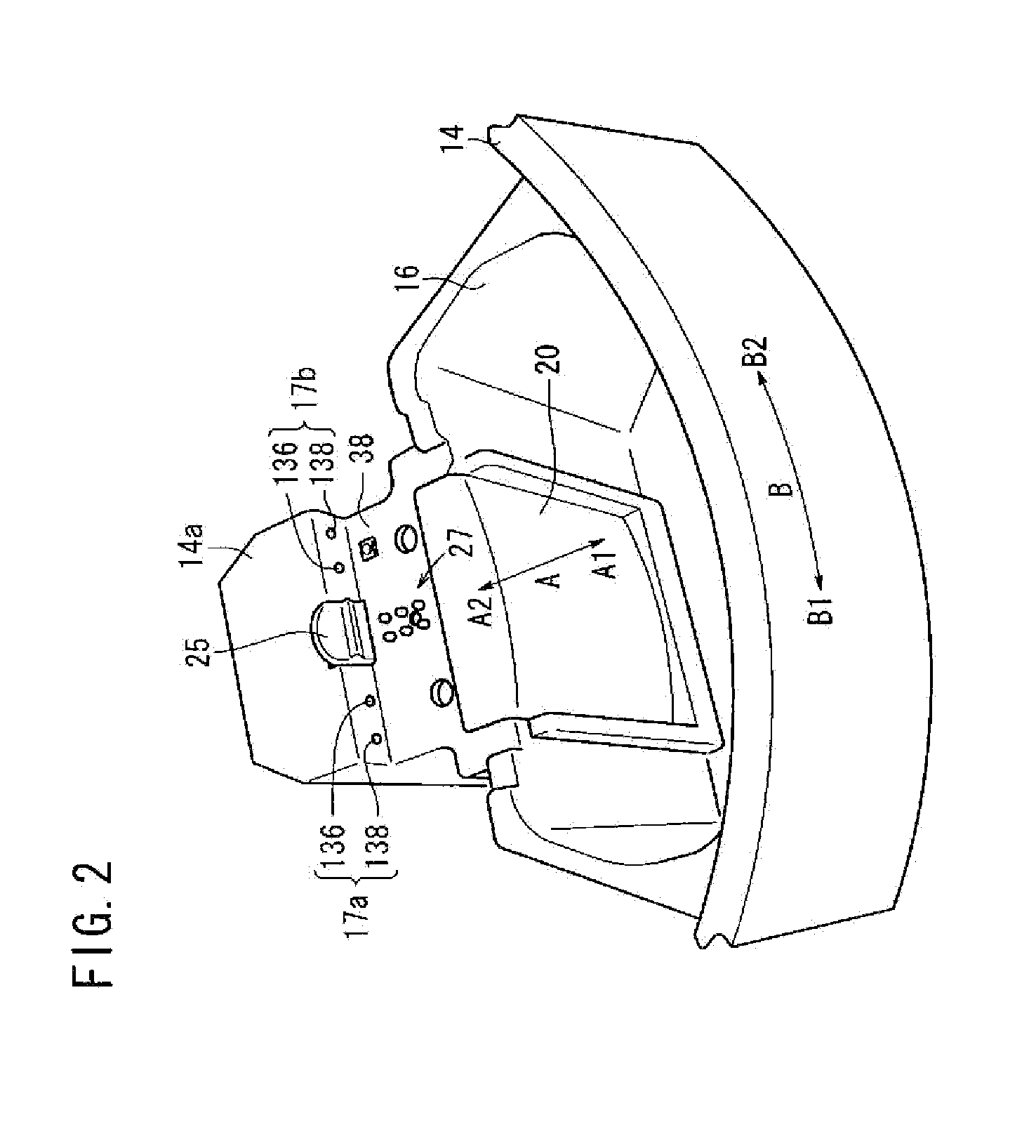
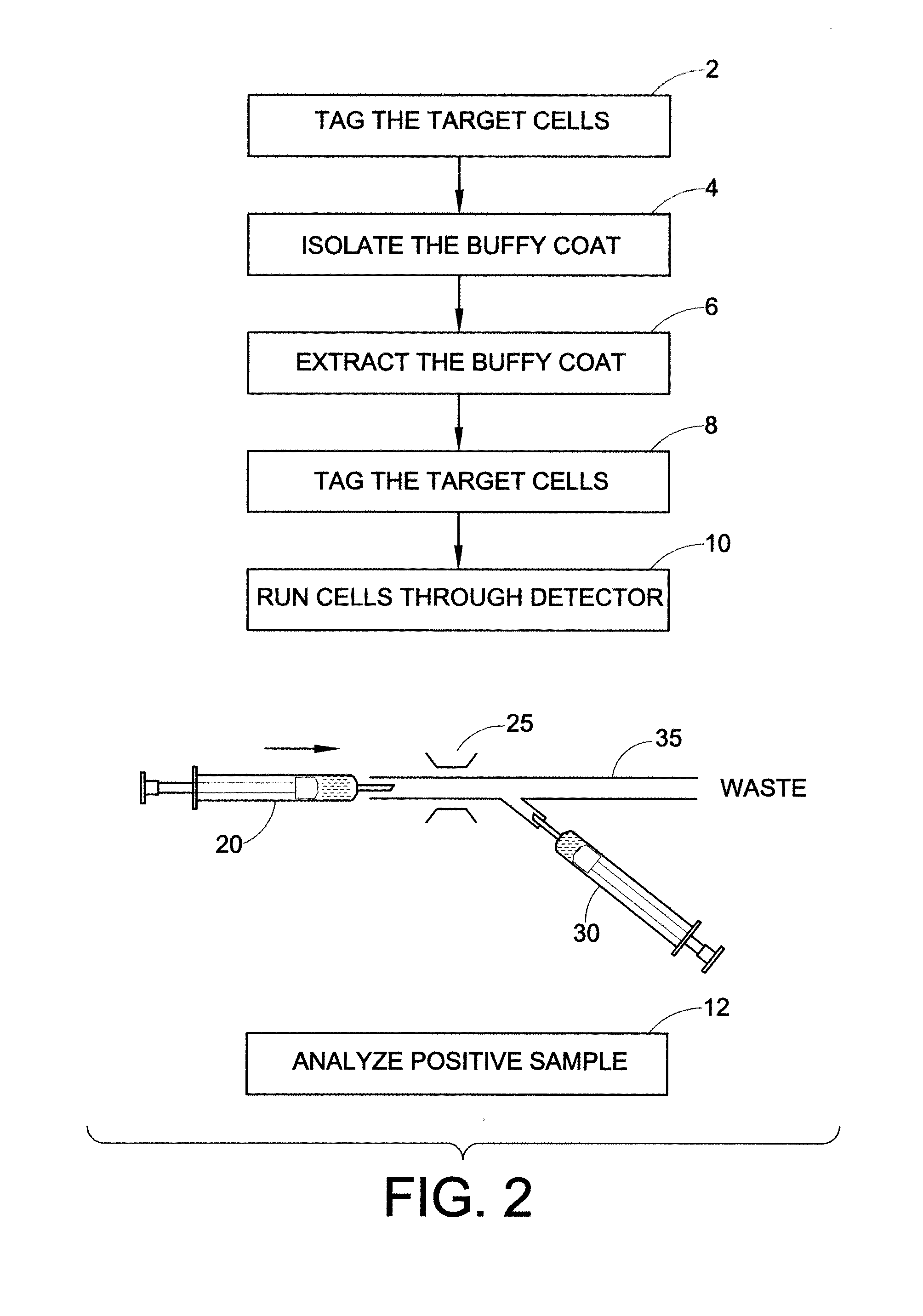
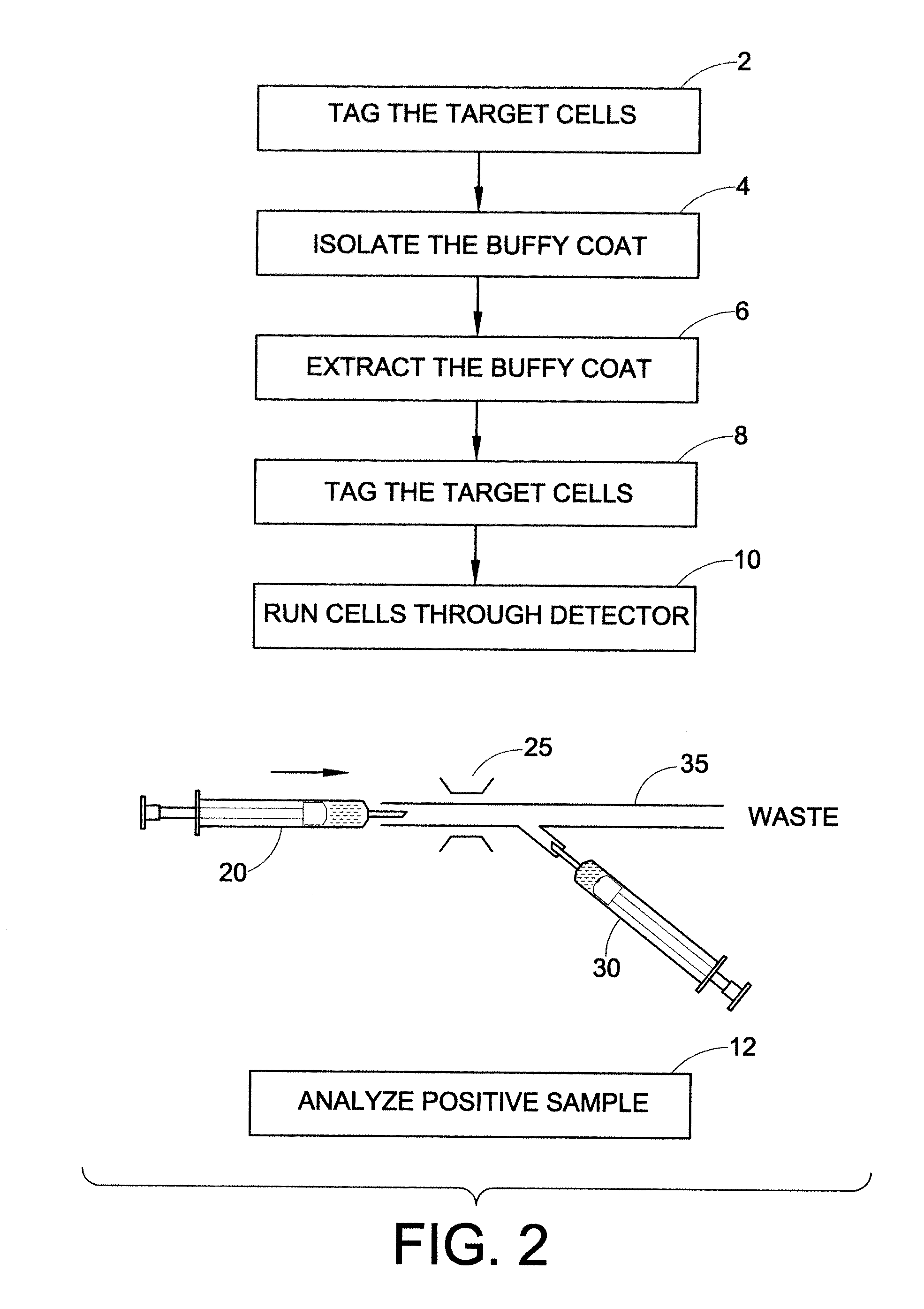
Method for the detection, identification, enumeration and confirmation of virally infected cells and other epitopically defined cells in whole blood
InactiveUS6911315B2Reduce the possibilityAvoid pollutionMicrobiological testing/measurementImmunoglobulins against virusesInfected cellFluorophore
A method for analyzing blood enables one to isolate, detect, enumerate and confirm under magnification the presence of target cells which have expressed surface epitopes that indicate intracellular infection by various viruses or other infectious agents, and also cells which have expressed surface epitopes that indicate the presence of non-infectious medical conditions. The analysis involves the examination of cells in the blood sample for the presence or absence of particular surface epitopes while the blood sample is disposed in a centrifuged blood sampling container. The epitopic analysis for the presence or absence of infected cells, or cells which indicate the presence of non-infectious medical conditions relies on the detection of known target expressed epitopes. The target epitopes on the target cell types are epitopes which are also known to be absent on normal circulating cells in the blood. Fluorophores or other labels with distinct wavelength emissions are coupled with specific binding agents such as lectins, antibodies, aptamers, or the like, which are directed against the target expressed epitopes. The epitopic analyses may be performed in or near the expanded buffy coat layer in the centrifuged blood sample. The epitopic analysis may be performed under magnification either visually and / or photometrically. The blood sampling container is sized to hold between about 1 and about 20 ml, preferably about 10 ml of blood, and contains an insert that occupies about 90-98% of the volume of the container bore in the area of the container where the target cells will, if present, be detected. The insert forces the target cells in question to reside in an annular space in the container which is adjacent to the circumference of the container bore. The entire analysis can be performed in a relatively short period of time which is typically a matter of minutes to single digit hours.
Owner:LEVINE ROBERT A +1
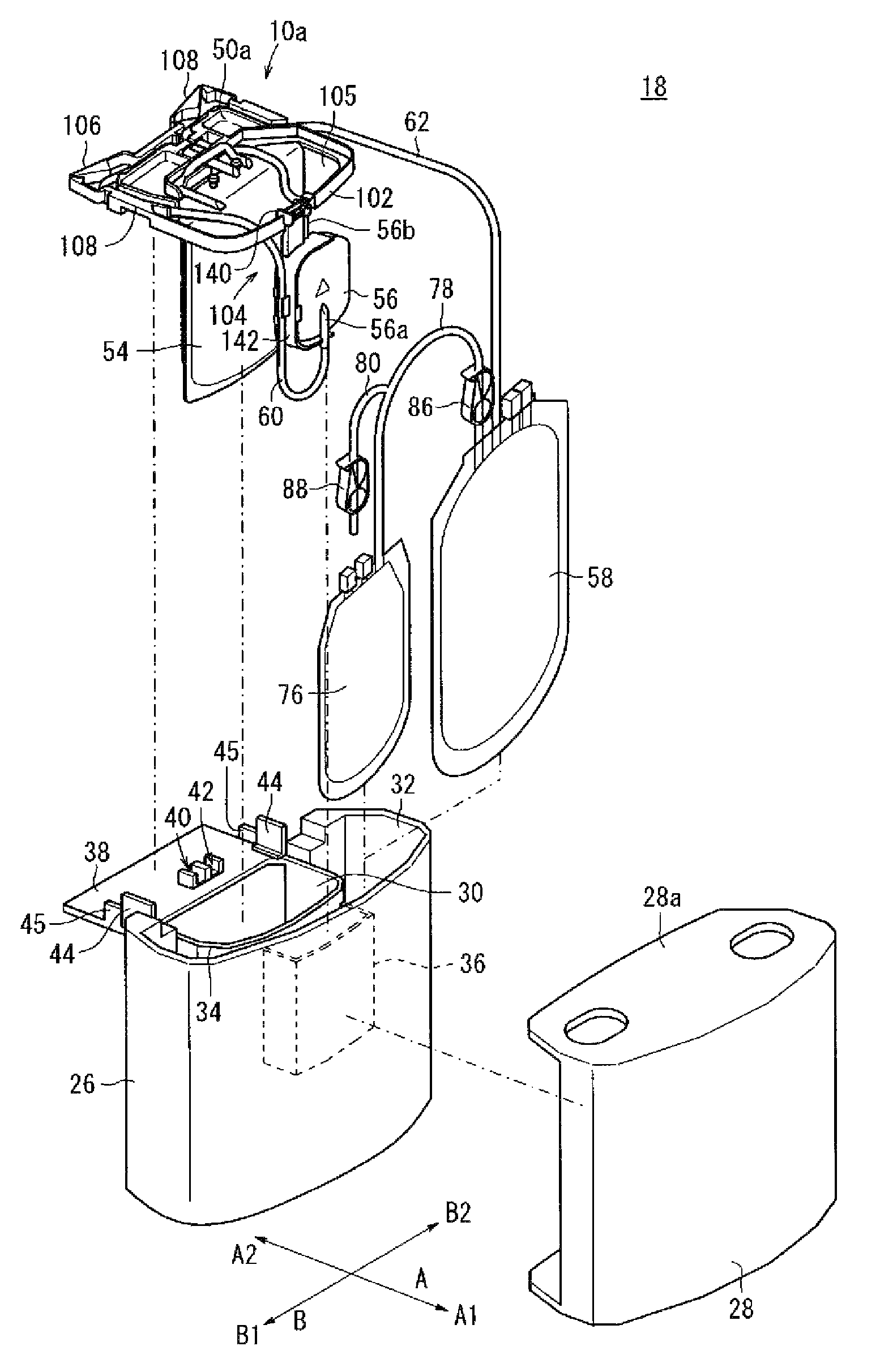
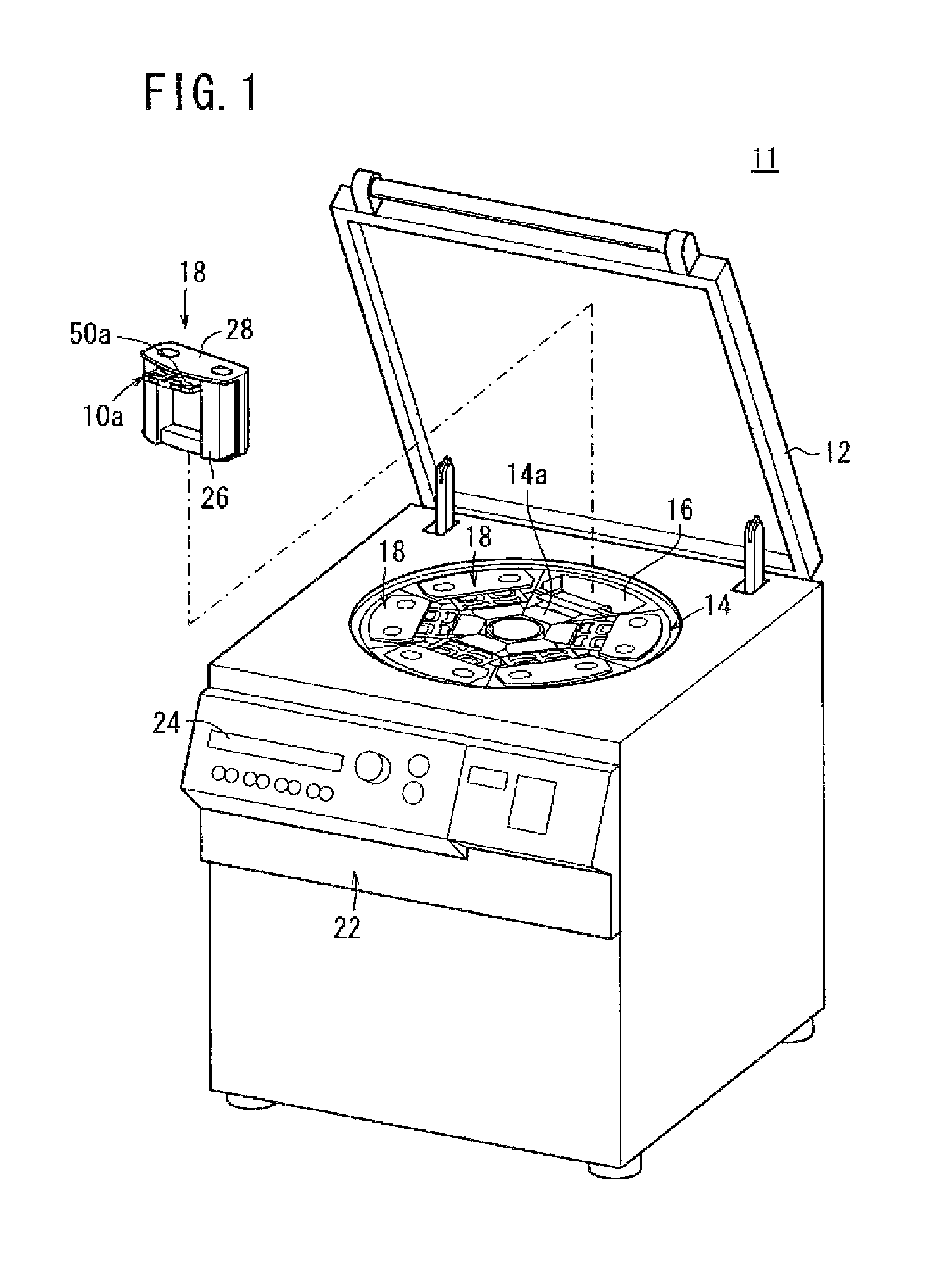
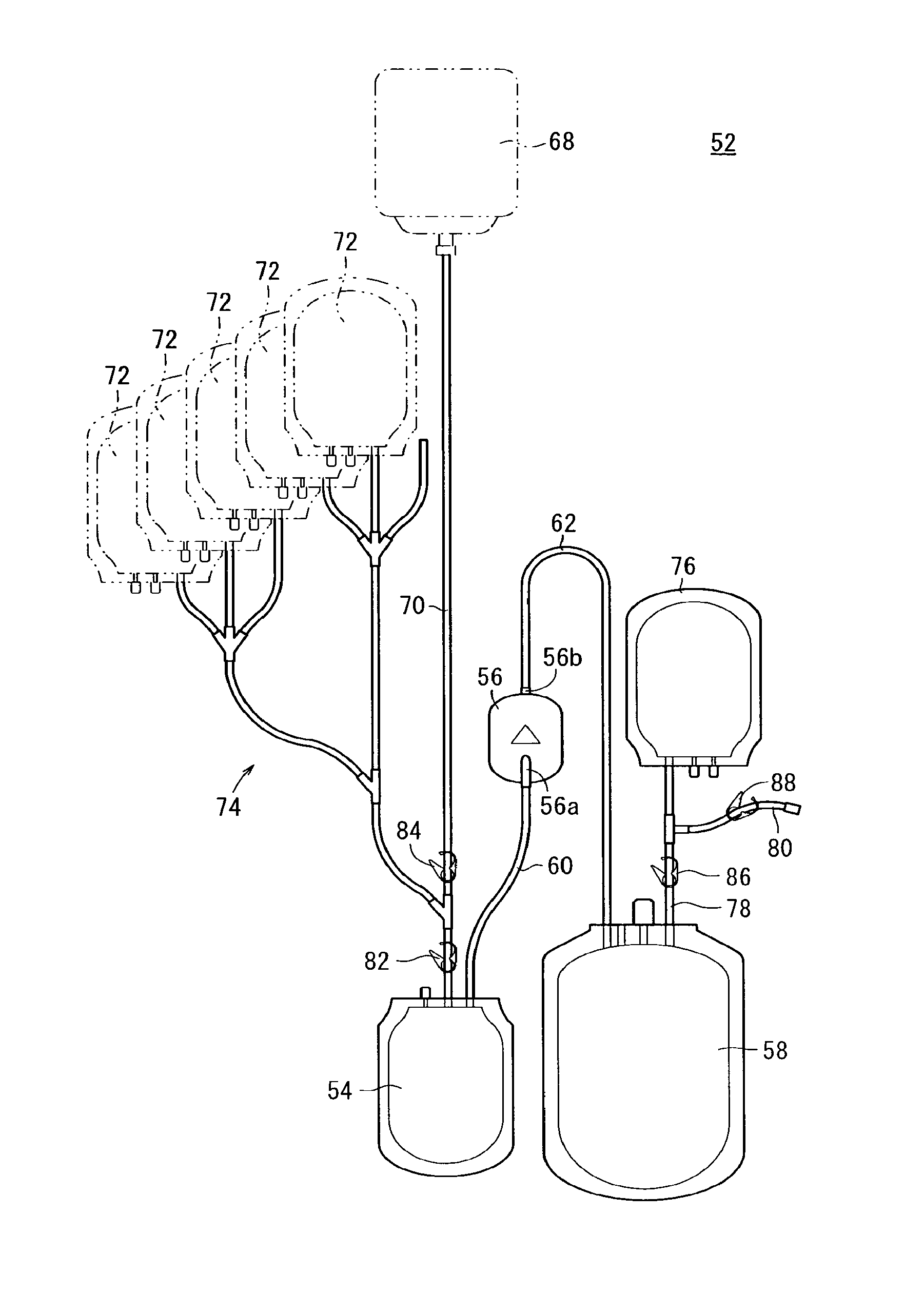
Blood bag system and cassette
ActiveUS20150209495A1Readily and accuratelyEasy to carryOther blood circulation devicesPharmaceutical containersWhite blood cellCentrifugation
Owner:TERUMO KK
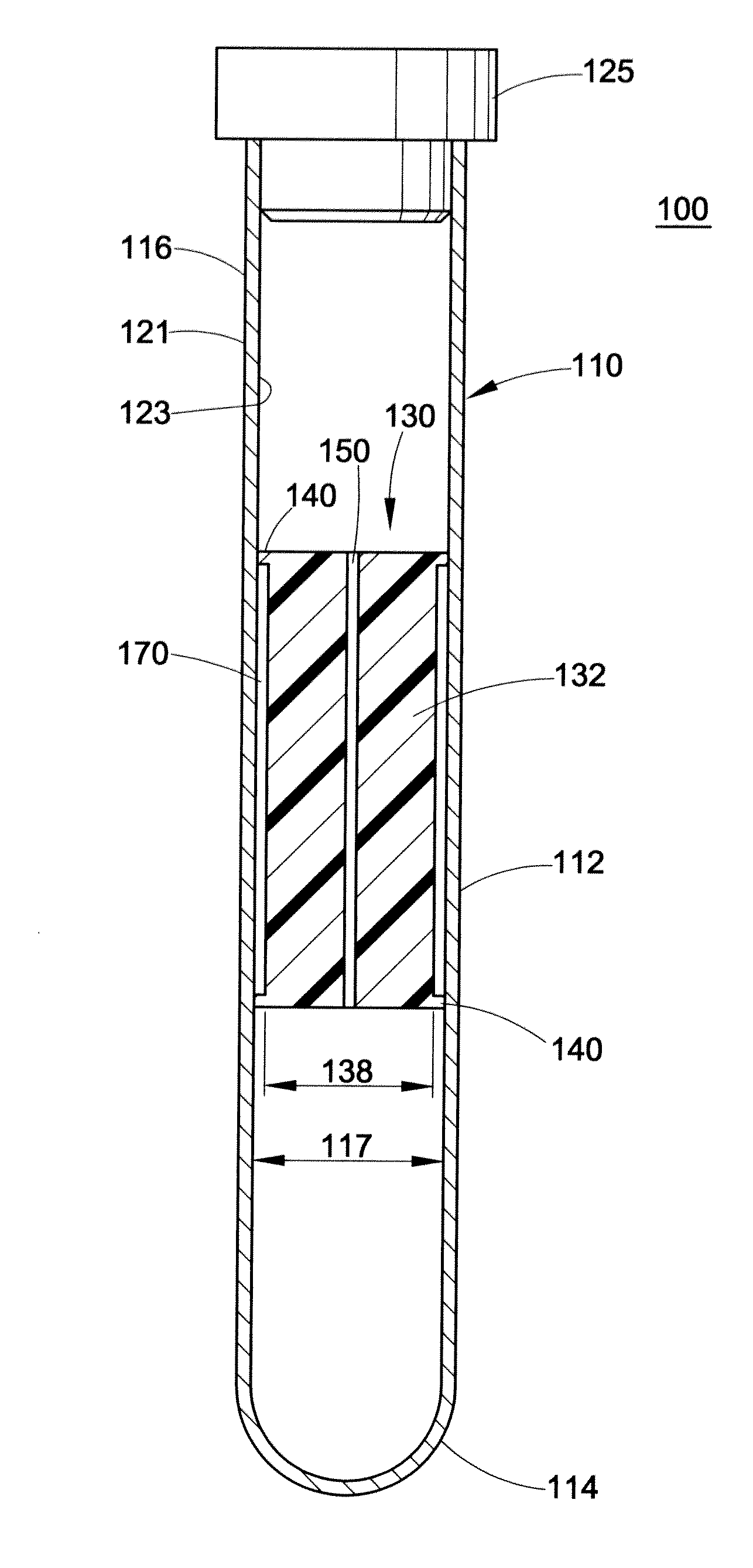
Buffy coat separator float systems and methods
InactiveUS20130017130A1Reduce speedReduce pressurePreparing sample for investigationDispersed particle separationCentrifugationBuffy coat
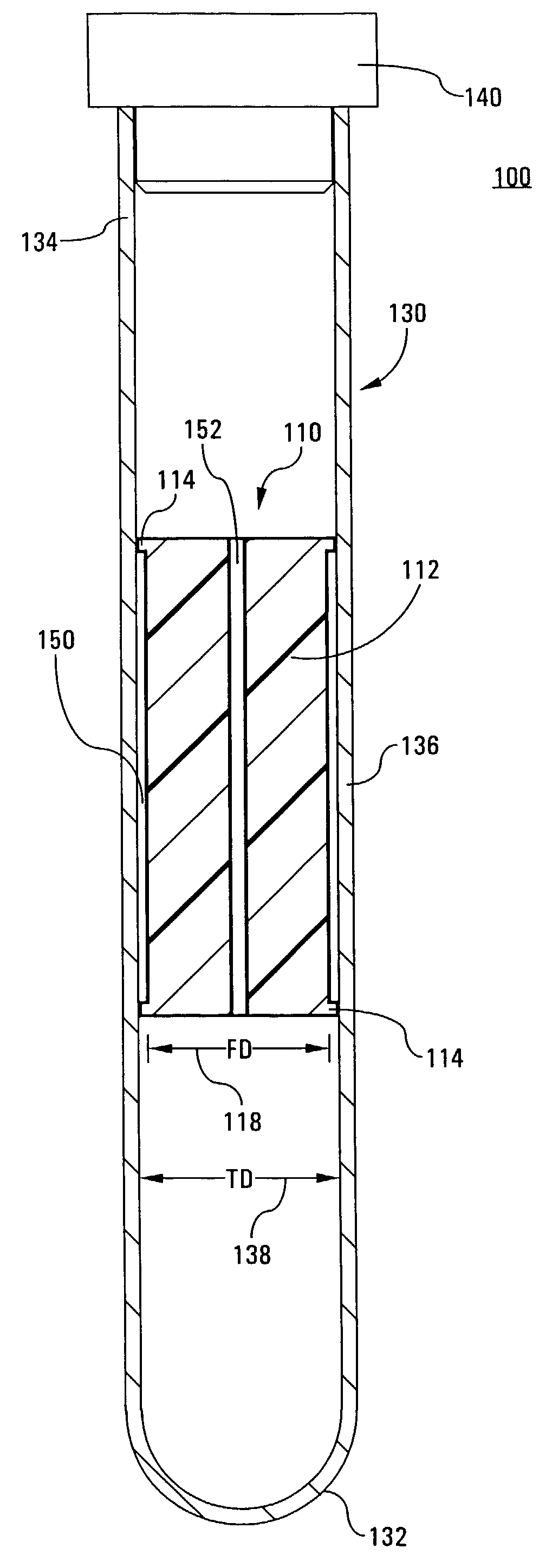
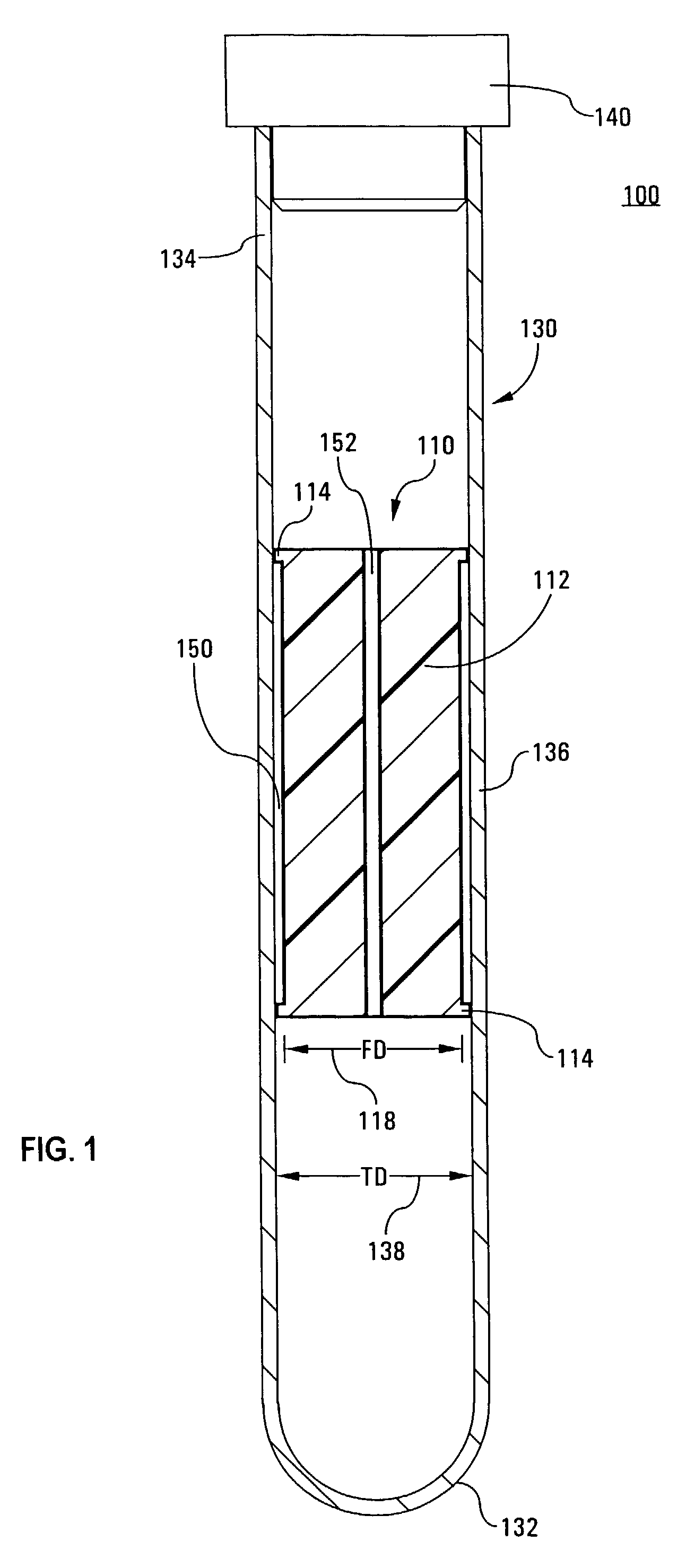
Tube and float systems for separation and axial expansion of the buffy coat are provided. Generally, the systems include a flexible sample tube and a rigid separator float having a specific gravity intermediate that of red blood cells and plasma. The sample tube has an elongated sidewall having a first cross-sectional inner diameter. The float has a main body portion and one or more support members protruding from the main body portion to engage and support the sidewall of the sample tube. During centrifugation, the centrifugal force enlarges the diameter of the tube to permit density-based axial movement of the float in the tube. After centrifugation is ended, the tube sidewall returns to its first diameter, thereby capturing the float and trapping the buffy coat constituents in an annular volume. Several different systems for capturing and retrieving the buffy coat constituents are described.
Owner:BATTELLE MEMORIAL INST
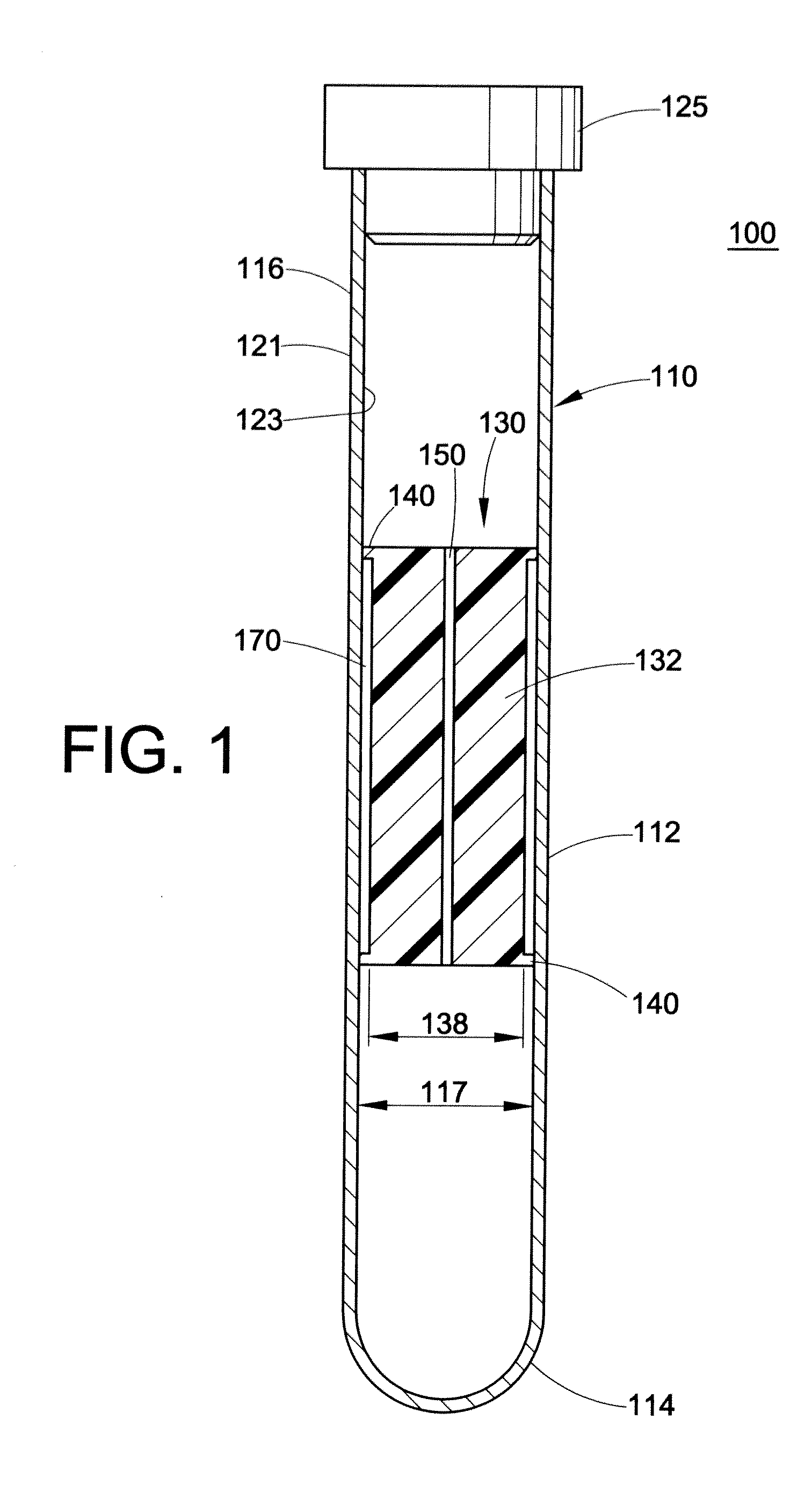
Buffy coat separator float systems and methods
InactiveUS20130095007A1Increase volumeReduce speedDispersed particle separationLaboratory glasswaresCentrifugationRed blood cell
Tube and float systems for separation and axial expansion of the buffy coat are provided. Generally, the systems include a flexible sample tube and a rigid separator float having a specific gravity intermediate that of red blood cells and plasma. The sample tube has an elongated sidewall having a first cross-sectional inner diameter. The float has a main body portion and one or more support members protruding from the main body portion to engage and support the sidewall of the sample tube. During centrifugation, the centrifugal force enlarges the diameter of the tube to permit density-based axial movement of the float in the tube. After centrifugation is ended, the tube sidewall returns to its first diameter, thereby capturing the float and trapping the buffy coat constituents in an annular volume. Several different systems for capturing and retrieving the buffy coat constituents are described.
Owner:BATTELLE MEMORIAL INST
Blood bag system and cassette
InactiveUS20110238029A1Readily and accuratelyEasy to carryDiagnosticsSurgeryCentrifugationWhite blood cell
A blood bag system includes a BC pooling bag for centrifugation of a buffy coat, a filter for removing white blood cells from a supernatant liquid transferred from the BC pooling bag, a platelet preserving bag for reserving the supernatant liquid that has passed through the filter, a first tube connecting the BC pooling bag and an inlet of the filter, a second tube connecting the platelet preserving bag and an outlet of the filter, and a cassette to be fixed in the centrifugation and separation apparatus. The first tube and the second tube are disposed within the cassette. The cassette includes a first clamp section for closing and opening the first tube, and a second clamp section for closing and opening the second tube.
Owner:TERUMO KK
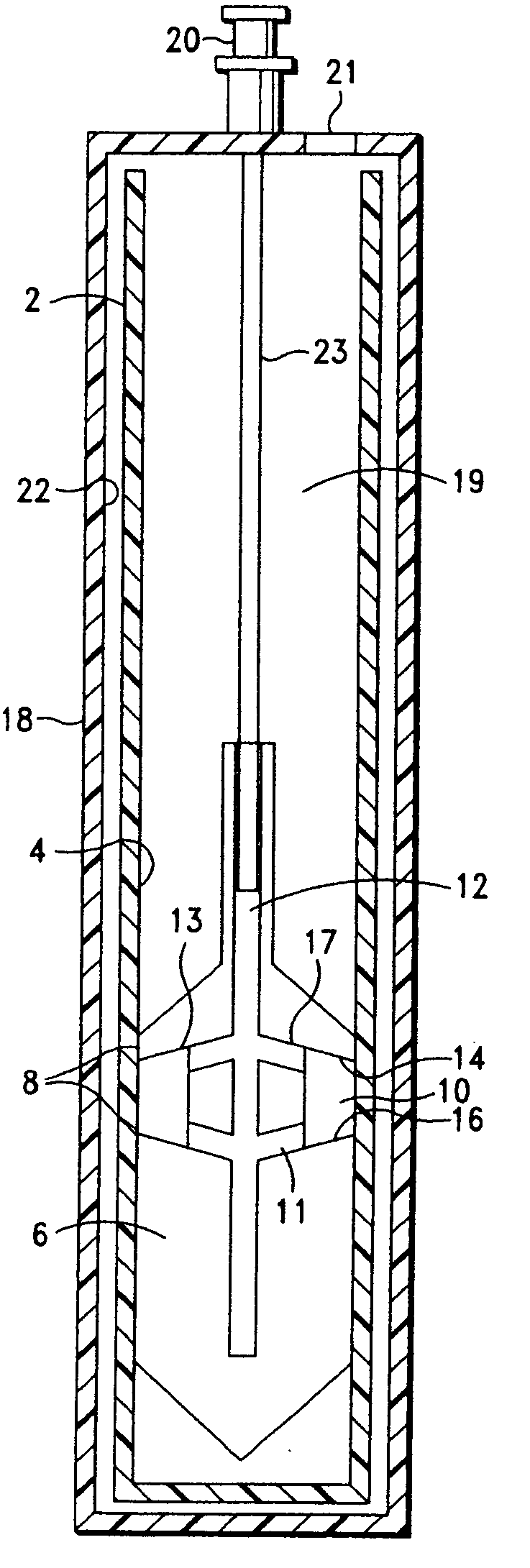
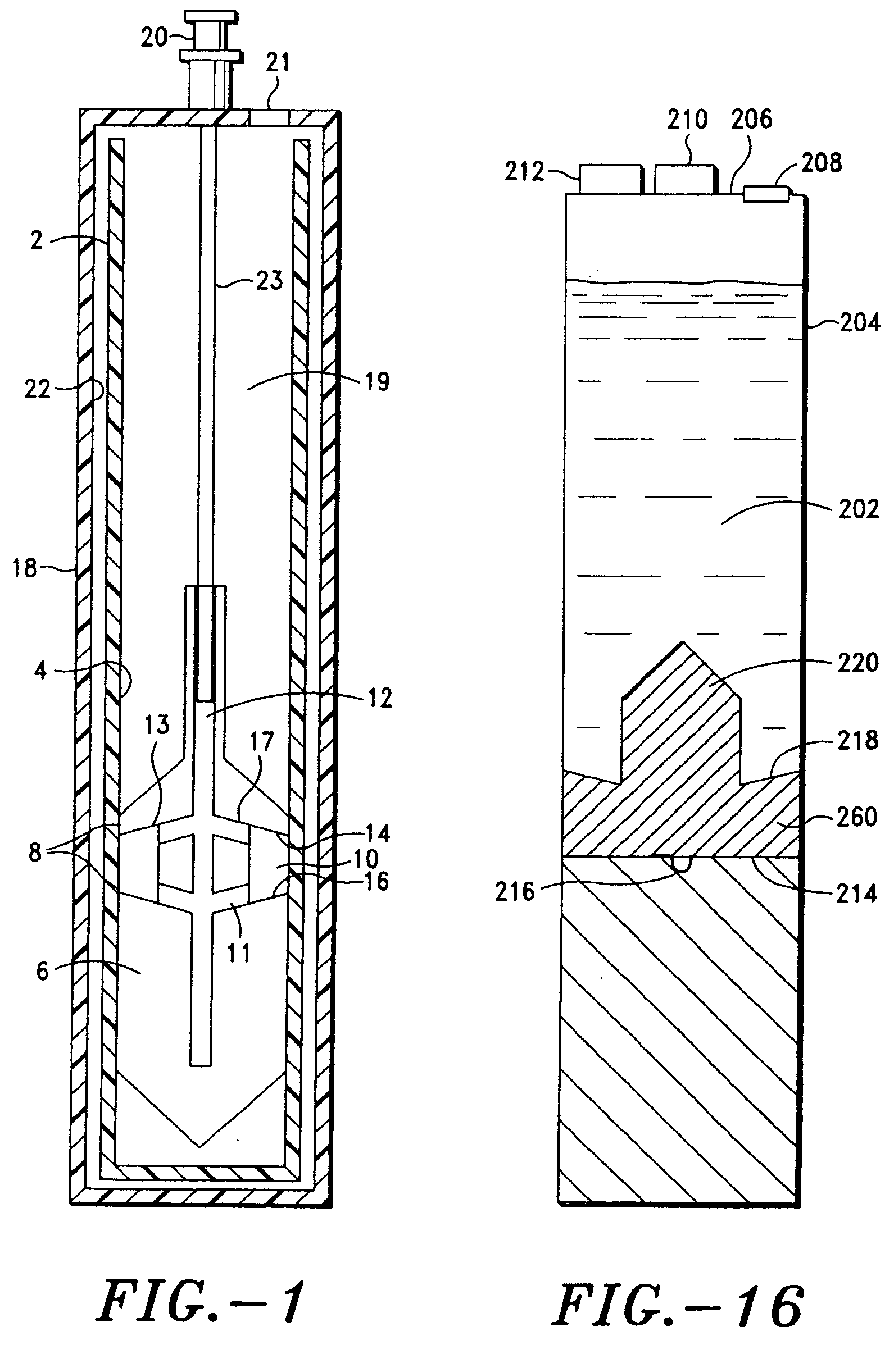
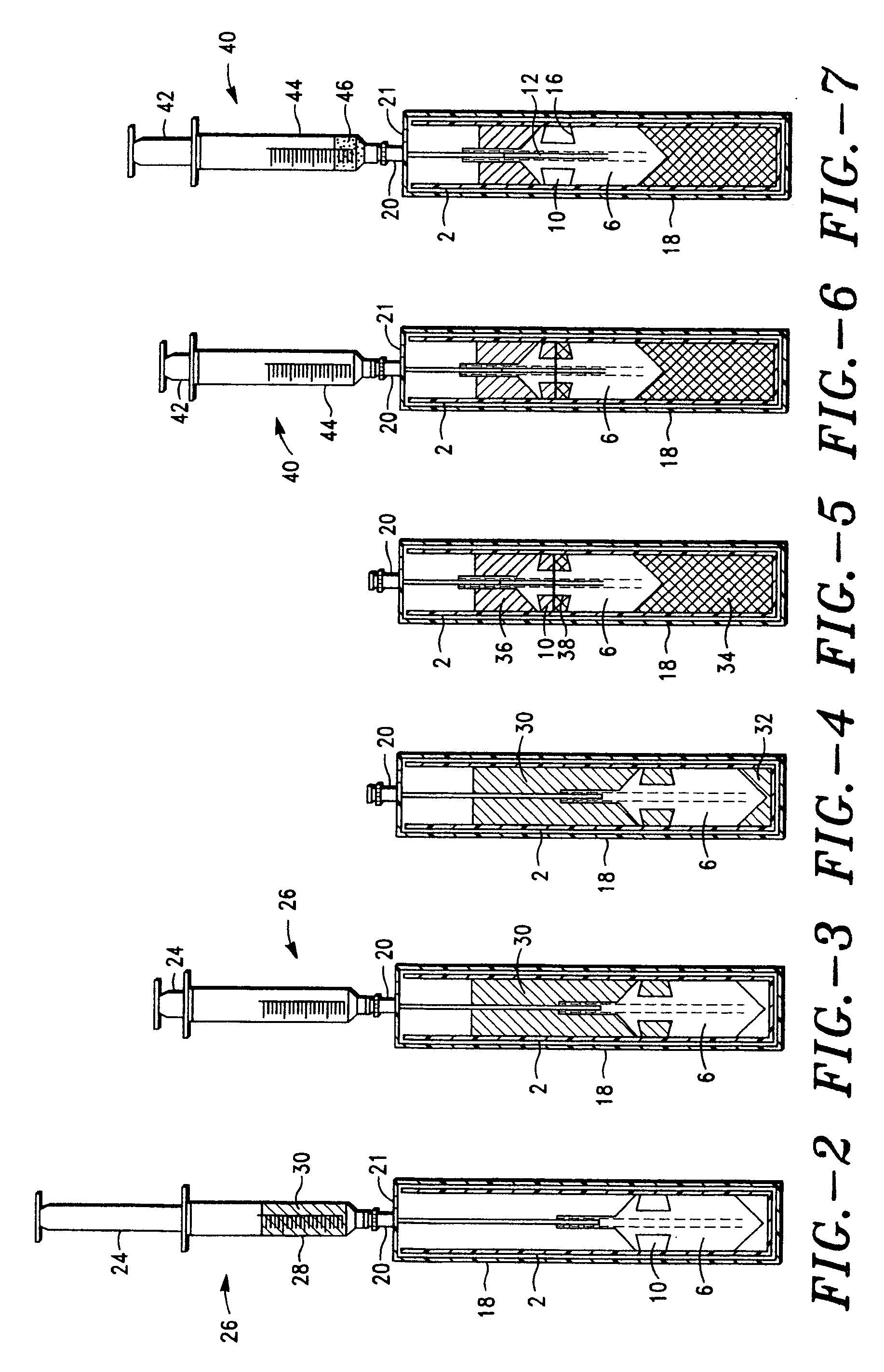
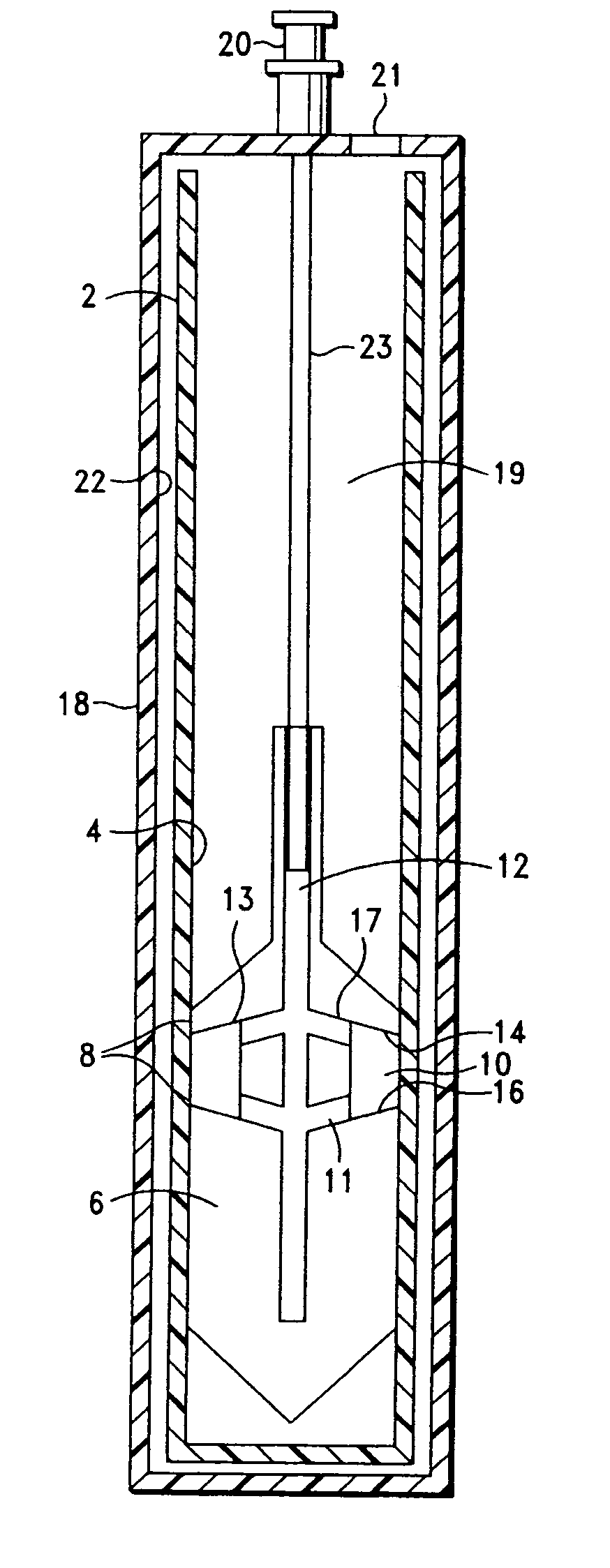
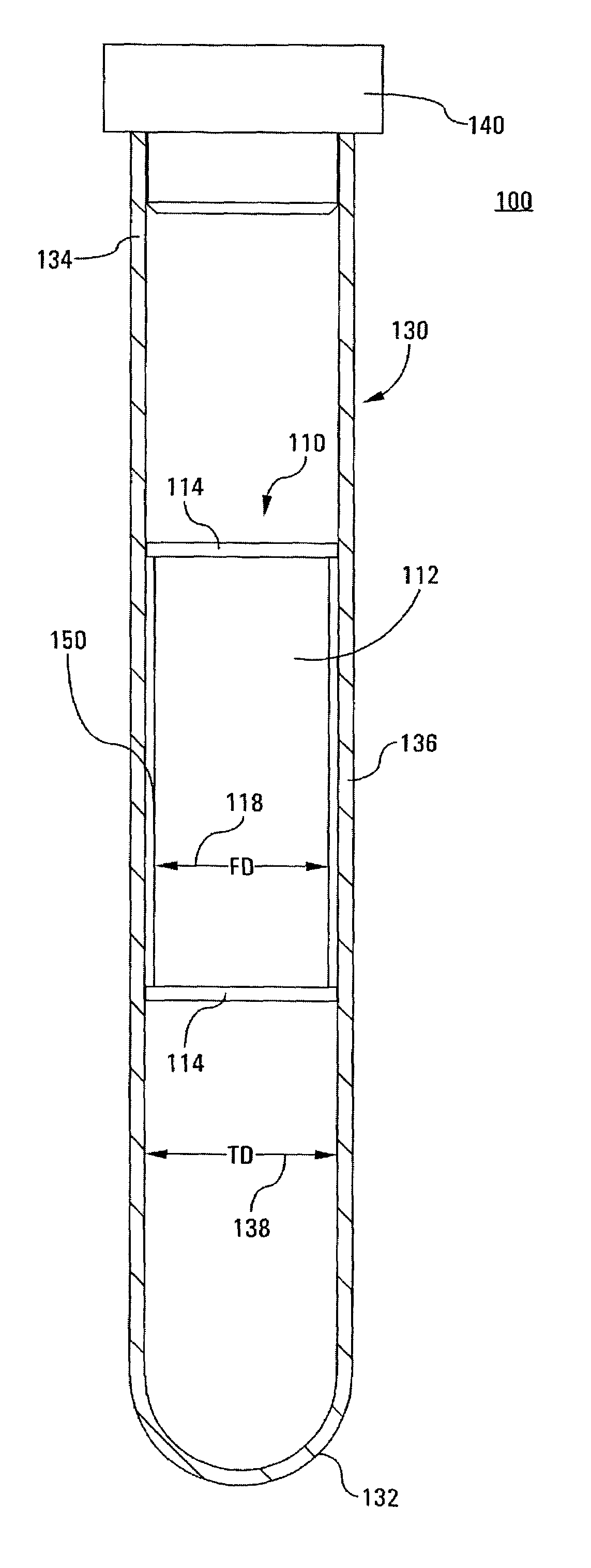
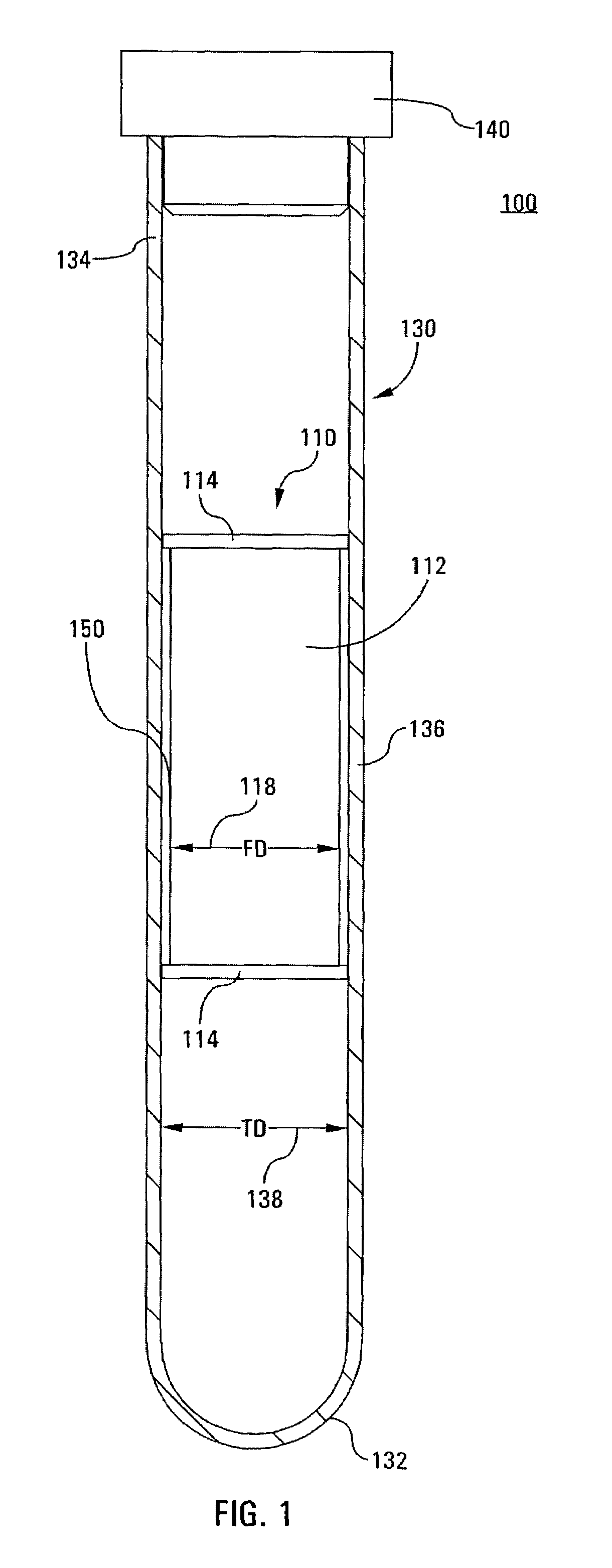
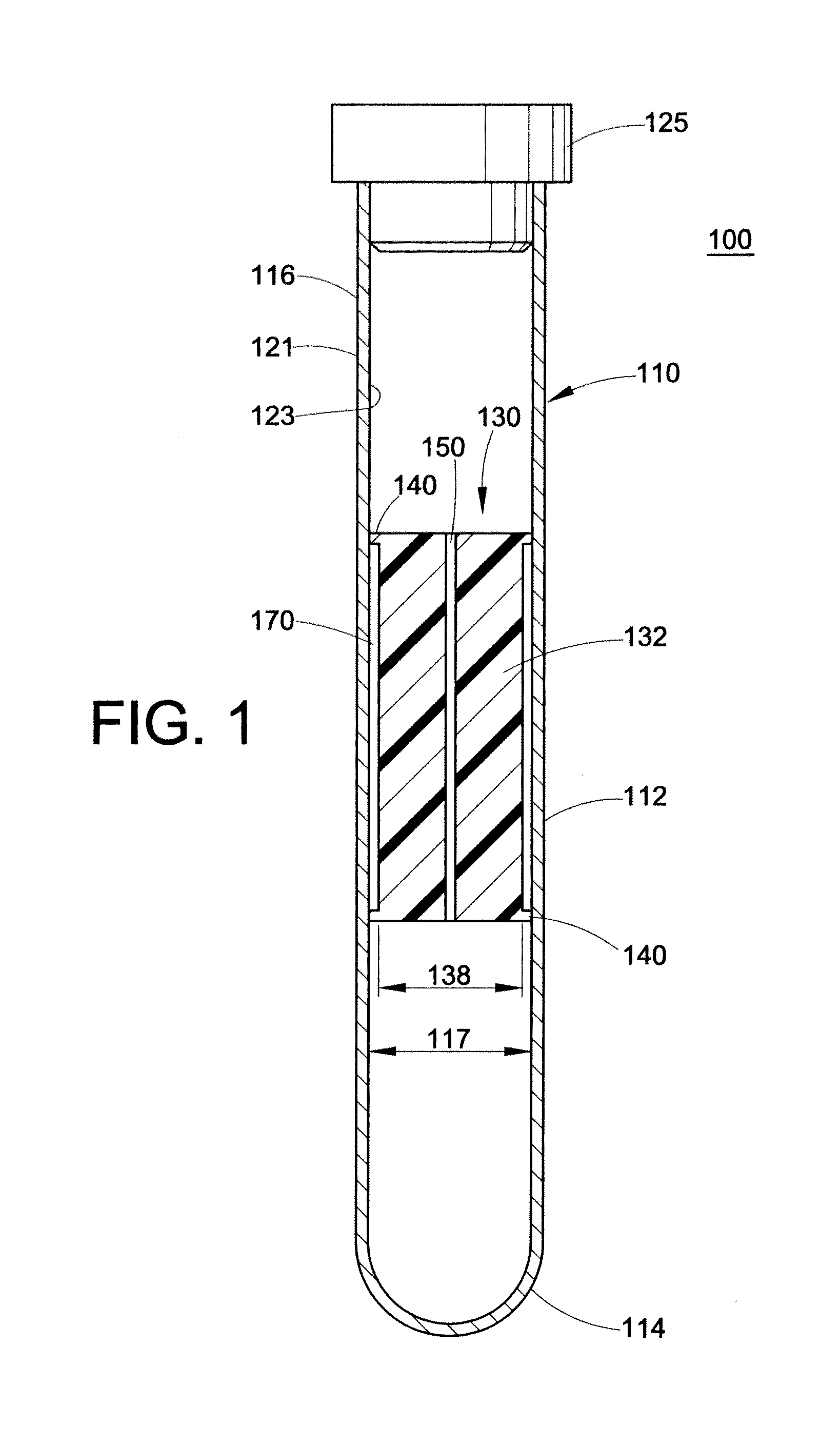
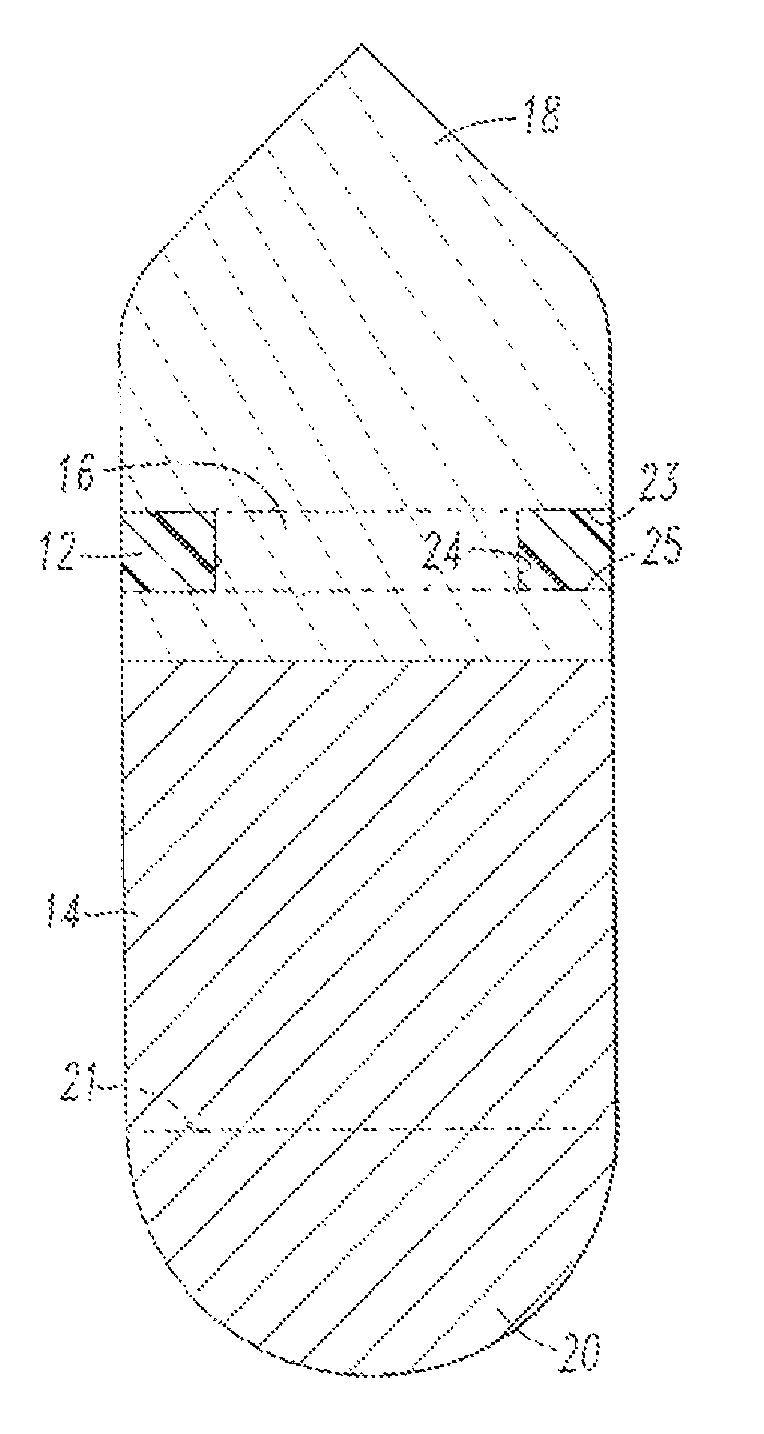
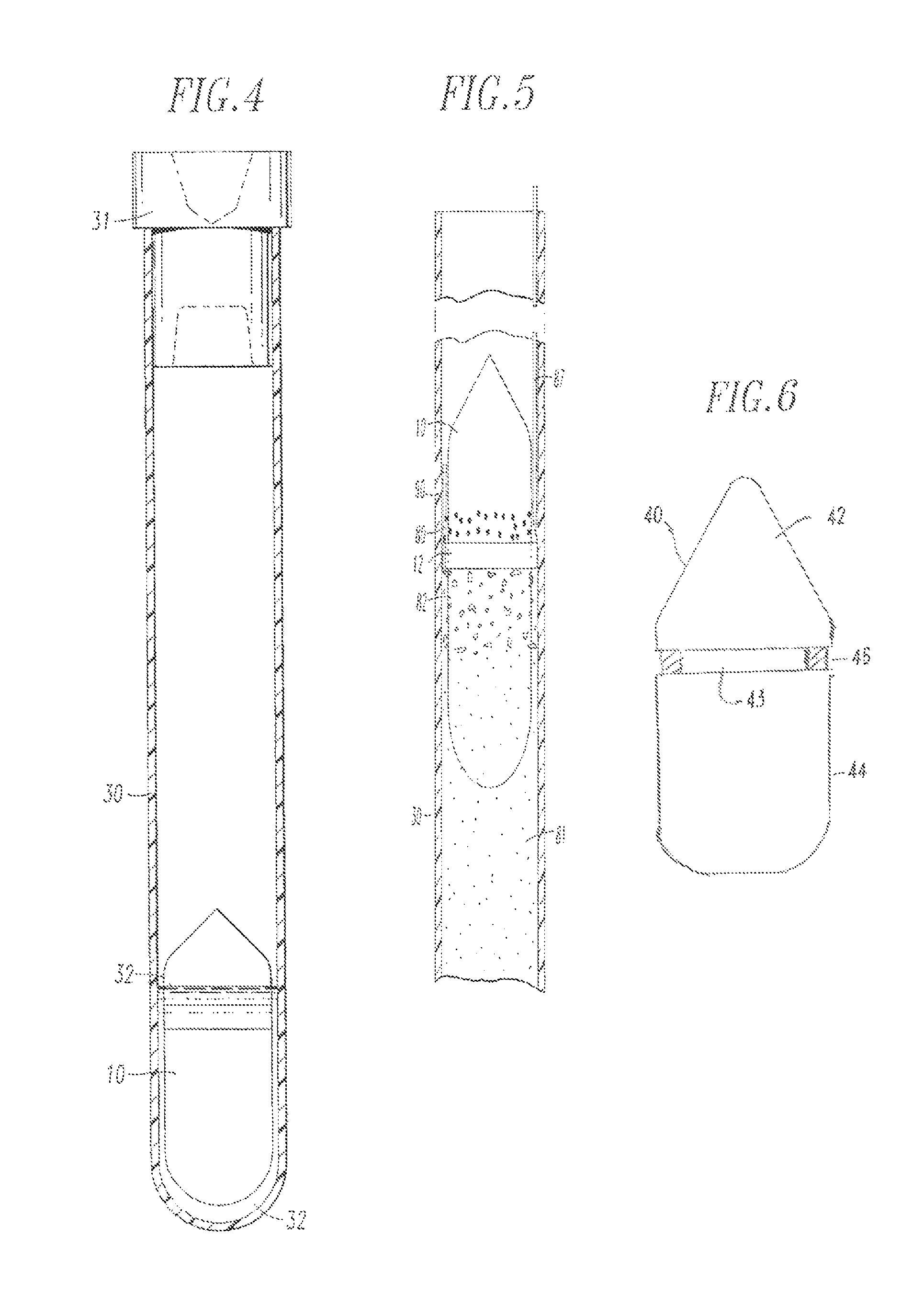
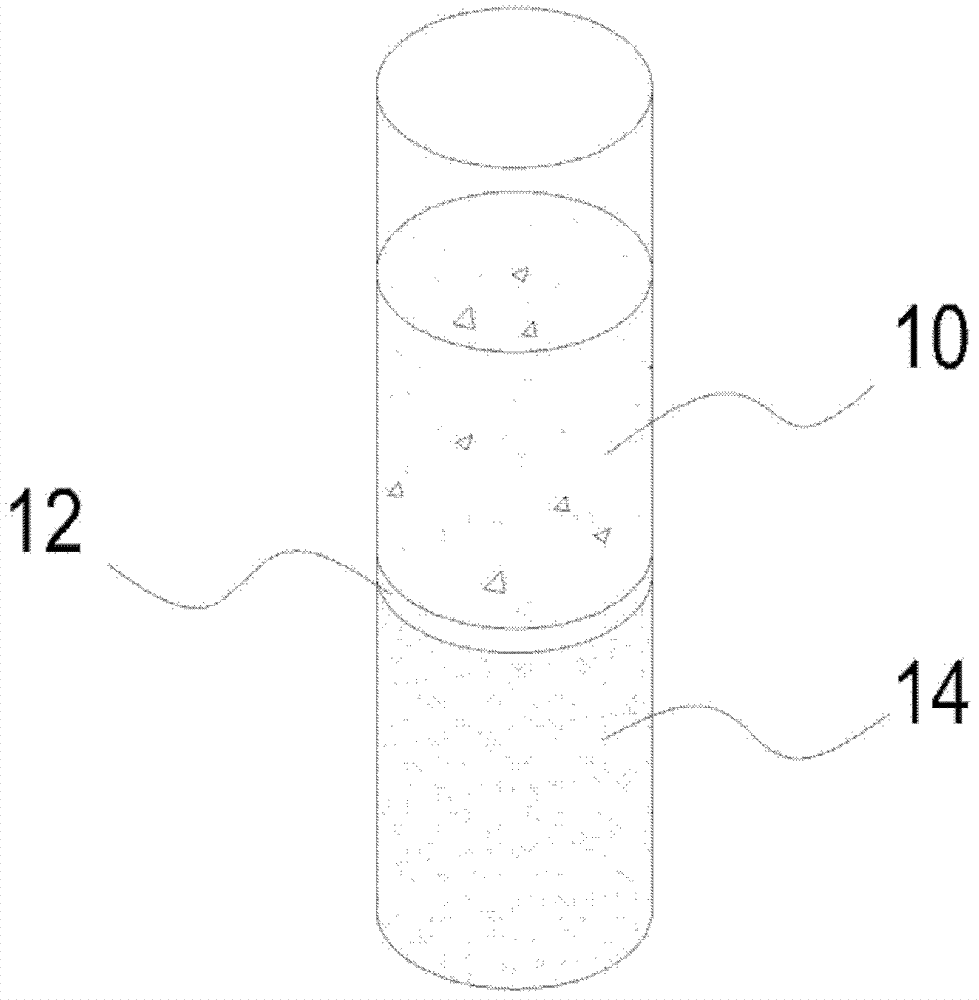
Integrated Blood Specimen Processor
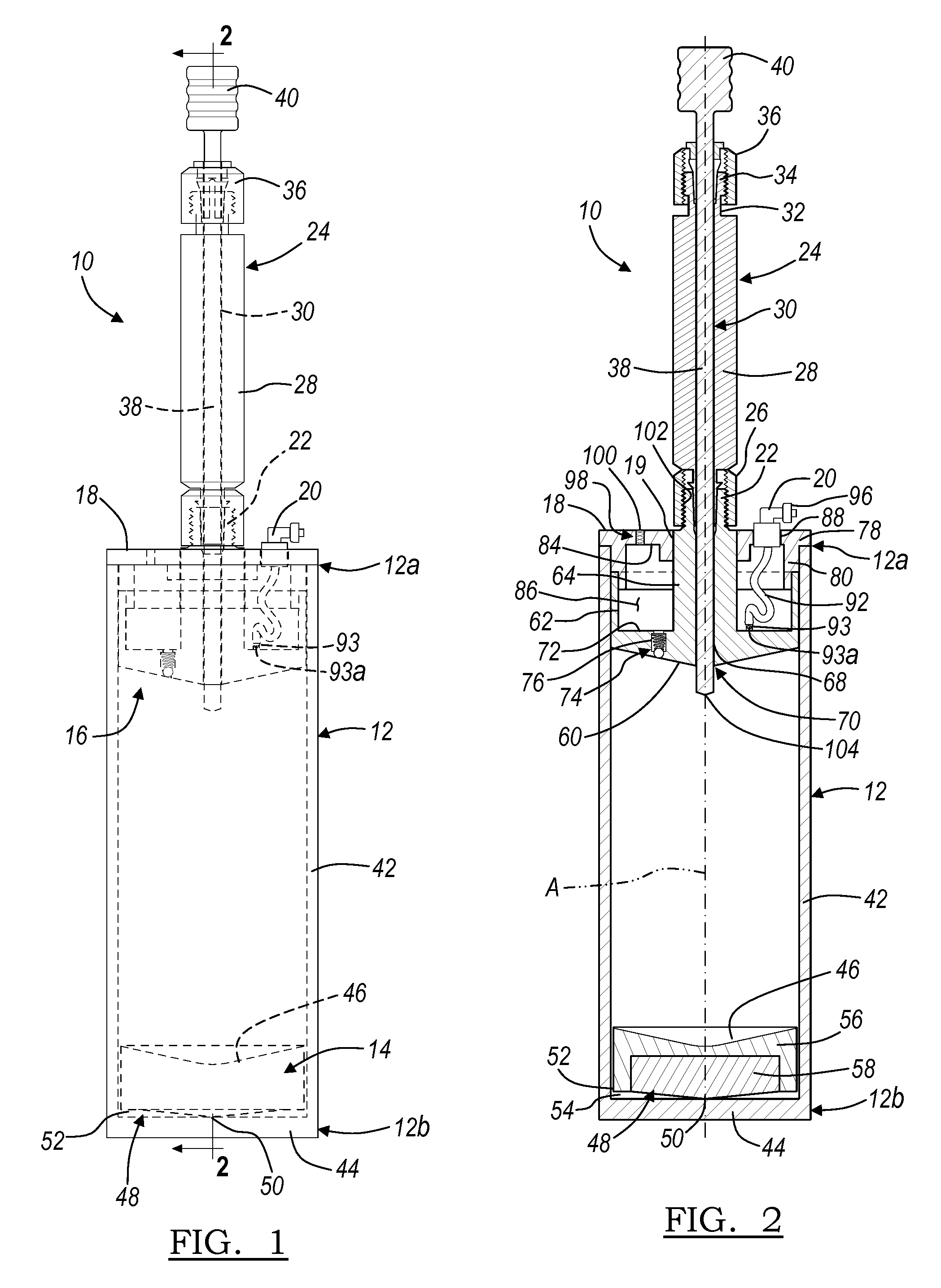
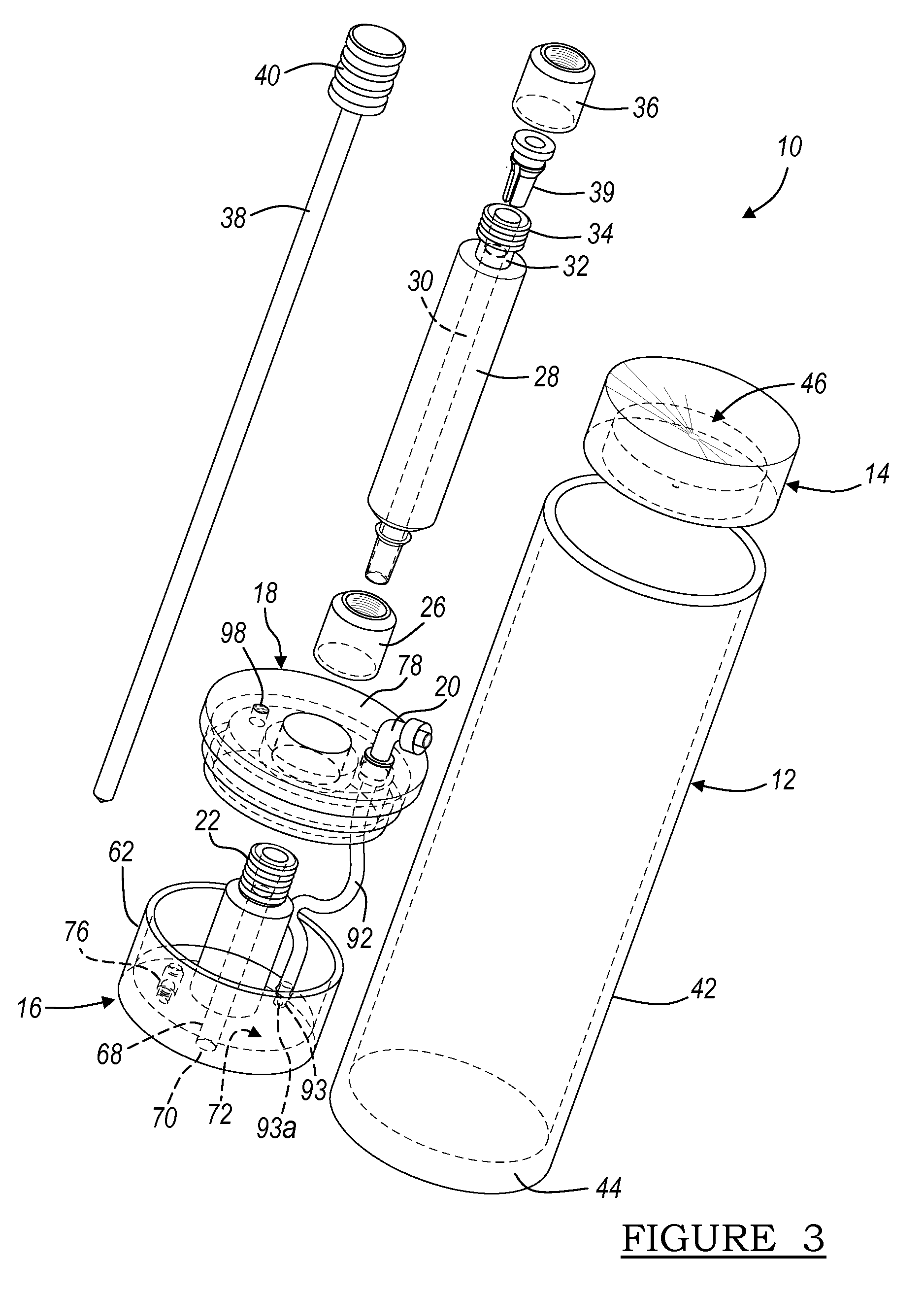
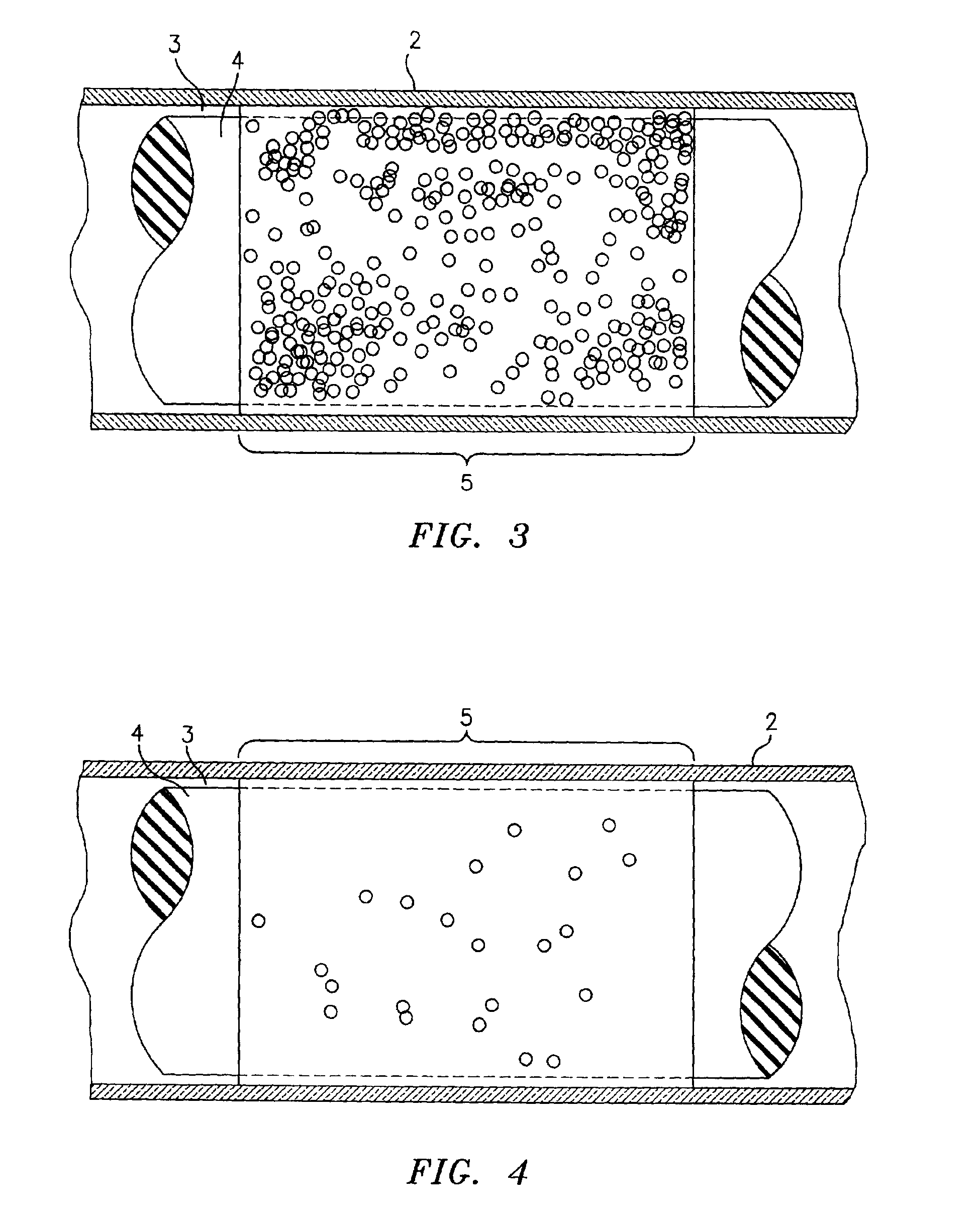
A blood specimen processor by means of which blood specimens are collected into a collection tube containing an anticoagulant and a molded buoy of predetermined density between 1.045 and 1.084 with an embedded water swellable o-ring that expands to form a robust seal in a leucocyte density gradient between the buoy's outer surface and tube's inner surface. The buoy is made of a first resin and a second resin, the first resin having a lower density than the second resin, the first resin and the second resin being present in such amounts and relative proportions so that the body has an overall density between 1.045 and 1.084 and preferably, a center of gravity below a geometric center of the body. When the blood specimen contained in the processor is centrifuged, the buoy is thrust through the separating blood and the developing interface of erythrocytes, leucocytes, and plasma. A reproducible gradient develops that builds a reproducible buffy coat ring around the upper trough at the junction of the edge of the separator buoy and the tube wall. The relative proportions of resin are controlled to create a separator buoy of a desired density so that after centrifugation the buffy coat or other cells of interest will be above the water swellable o-ring.
Owner:COLEMAN CHARLES M
Blood testing method
ActiveUS20050074893A1Accurate diagnosisWithdrawing sample devicesDead animal preservationConcentrations glucoseBlood plasma
A blood test method including (a) centrifuging a mammalian blood sample into a plasma and blood cells; (b) removing buffy coats from the blood cells to obtain red blood cells containing solutes confined therewithin; (c) washing the red blood cells with a buffered physiological saline solution and isolating the washed blood cells; (d) mixing the washed red blood cells with a buffered physiological saline solution to obtain a suspended liquid; (e) centrifuging the suspended liquid to remove a supernatant and to obtain a red blood layer; (f) mixing the red blood layer with a hypertonic solution and maintaining the resulting suspension at a temperature of 25 to 40° C. for a period of time sufficient for the solutes confined in the red blood cells to penetrate into the hypertonic solution; (g) centrifuging the suspension to obtain a supernatant containing the solutes; and (h) measuring the supernatant for at least one factor selected from a glucose concentration, a pyruvic acid concentration, a lactic acid concentration and an oxidation-reduction potential.
Owner:RELTEC MEDICAL DEVICES CORPORATION
Intradiscal production of autologous interleukin antagonist
InactiveUS20060057223A1Sufficient in vivo productionReduce inflammationMammal material medical ingredientsAntibody ingredientsBuffy coatIntervertebral disk
Administering a buffy coat and immunoglobulin into a disc to induce the production of interleukin receptor antagonist protein IRAP within the disc is disclosed.
Owner:DEPUY SYNTHES PROD INC +1
Method for collecting a desired blood component and performing a photopheresis treatment
ActiveUS20070100272A1BuildImprove efficiencyOther blood circulation devicesFlexible member pumpsPhotopheresisBlood component
An improved method for separating whole blood into components and collecting a desired blood component. The method allows a desired blood component to be subjected to centrifugal forces within a separator for prolonged periods of time, yielding a cleaner cut and higher yield of the desired blood component. Whole blood is drawn from a source and pumped into a separator, the undesired blood components are removed from the separator at rates so as to build up the desired blood component in the separator. The desired blood component is only removed after a predetermined amount of the desired blood component has built up in the separator. It is preferred that the desired blood component be buffy coat and that the method be used to perform photopheresis treatments. In another aspect, the invention is a method of performing a full photopheresis treatment to treat diseases in a reduced time, preferably less than about 70 minutes, and more preferably less than about 45 minutes.
Owner:MALLINCKRODT HOSPITAL PRODUCTS IP LTD
Methods and apparatus for collection of filtered blood components
InactiveUS20110152740A1Water/sewage treatment by centrifugal separationOther blood circulation devicesBlood collectionPeristaltic pump
A method and apparatus for red blood collection and filtration is provided wherein a red blood cell collection assembly provides for leukoreduction filtration concurrent with or soon after the red blood cell separation and collection procedure. After red blood cells have been collected, an unwanted blood component, e.g., buffy coat, is diverted into the red blood cell collect line. Operation of a peristaltic pump engaging pump loop allows insertion of a pre-determined quantity of buffy coat into the collect line. The buffy coat can therefore push red blood cells out of the filter and into a collect bag without diluting the collected red blood cells. Alternatively, gravitational force may be used to allow the unwanted blood component to displace red blood cells out of the filter.
Owner:TERUMO BCT
Blood bag system and cassette
In a blood bag system mounted in a centrifugation and separation apparatus, a filter is settable in different appropriate orientations at storage time and usage time, and first and second tubes and a filter are arranged respectively in appropriate orientations during use. The blood bag system includes a BC pooling bag for centrifugation of buffy coat, the filter for removing white blood cells from a supernatant liquid transferred from the BC pooling bag, a platelet preserving bag for reserving the supernatant liquid that has passed through the filter, the first tube connecting the BC pooling bag and an inlet of the filter, the second tube connecting the platelet preserving bag and an outlet of the filter, a cassette fixable in the centrifugation and separation apparatus, an attachment for holding the filter, and a hinge section tiltable relative to the cassette with reference to an axis in the circumferential direction.
Owner:TERUMO KK
Method and Apparatus for Leukoreduction of Red Blood Cells
InactiveUS20070118063A1Other blood circulation devicesMedical devicesBlood componentWhite blood cell
This invention relates to a method for the continuous separation of red blood cells from whole blood which includes the steps of removing the whole blood from a donor, continuously separating the whole blood into blood component layers within a separation vessel including at least a plasma layer, a buffy coat layer and a red blood cell layer, removing the plasma layer from the separation vessel, removing the buffy coat layer from the separation vessel, and removing red blood cells from the red blood cell layer which partially overlaps with the buffy coat layer to create a mononuclear cell (MNC)-reduced red blood cell layer in the separation vessel.
Owner:CARIDIANBCT
Methods for inducing the differentiation of blood monocytes into functional dendritic cells
ActiveUS20100267137A1Increase the number ofIncrease differentiationCulture processMedical devicesDendritic cellTransmittance
Methods are provided for treating blood monocytes to produce functional antigen presenting dendritic cells. An extracorporeal quantity of a subject's blood is treated to separate the blood into a plasma component containing proteins, a platelet component and a buffy coat component. A plastic treatment device is provided having plastic channels that allow transmittance of light to the interior of the plastic device and a light source that produces light of a wave length selected to activate the photoactivatable agent. The plasma component containing proteins is first pumped through the plastic treatment device, followed by the platelet component and finally the buffy coat component. The resulting treated cells may be incubated or reinfused directly to the subject.
Owner:YALE UNIV
Integrated blood specimen processor
InactiveUS8377395B2Bioreactor/fermenter combinationsBiochemistry cleaning apparatusWhite blood cellRed blood cell
A blood specimen processor by means of which blood specimens are collected into a collection tube containing an anticoagulant and a molded buoy of predetermined density between 1.045 and 1.084 with an embedded water swellable o-ring that expands to form a robust seal in a leucocyte density gradient between the buoy's outer surface and tube's inner surface. The buoy is made of a first resin and a second resin, the first resin having a lower density than the second resin, the first resin and the second resin being present in such amounts and relative proportions so that the body has an overall density between 1.045 and 1.084 and preferably, a center of gravity below a geometric center of the body. When the blood specimen contained in the processor is centrifuged, the buoy is thrust through the separating blood and the developing interface of erythrocytes, leucocytes, and plasma. A reproducible gradient develops that builds a reproducible buffy coat ring around the upper trough at the junction of the edge of the separator buoy and the tube wall. The relative proportions of resin are controlled to create a separator buoy of a desired density so that after centrifugation the buffy coat or other cells of interest will be above or below the water swellable o-ring.
Owner:COLEMAN CHARLES M
Blood bag system and cassette
ActiveUS20150209496A1Readily and accuratelyEasy to carryDiagnosticsSurgeryWhite blood cellCentrifugation
Owner:TERUMO KK
Non-animal source cell blood serum substitute and use thereof
ActiveCN101486996AReduce usageReduce riskArtificial cell constructsSkeletal/connective tissue cellsWhite blood cellAdditive ingredient
The invention provides a non-animal origin cell cultivating serum substitute (PLT) and applications thereof. The PLT is prepared by the following method: 3 to 5 volume units of O-typed buffy coats and 1 volume unit of AB-typed plasma are mixed; 300 to 400 grams of the mixture is centrifuged; a supernatant is extracted as 1 volume unit of platelet-rich plasma; the platelet-rich plasma is filtered so as to remove leucocyte, stored under cryopreservation at the temperature below minus 10 DEG C for standby, and unfrozen at the temperature of 30 to 37 DEG C before use, and 3000 to 5000 grams of the platelet-rich plasma is centrifuged so as to remove platelet membrane fragments, thus obtaining the non-animal origin cell cultivating serum substitute. The non-animal origin cell cultivating serum substitute has following essential advantages: the PLT can be used as fetal bovine serum substitute in cell cultivation, thus avoiding animal-origin ingredients in cell cultivation, quickening cell multiplication and consequently satisfying the requirements of clinical security and operation period shortening, and the non-animal origin cell cultivating serum substitute is applicable not only to mesenchymal stem cells, but also to the multiplication cultivation of other human origin cells.
Owner:ZHEJIANG UNIV
Methods and apparatus for collection of filtered blood components
A method and apparatus for red blood collection and filtration is provided wherein a red blood cell collection assembly provides for leukoreduction filtration concurrent with or soon after the red blood cell separation and collection procedure. After red blood cells have been collected, an unwanted blood component, e.g., buffy coat, is diverted into the red blood cell collect line. Operation of a peristaltic pump engaging pump loop allows insertion of a pre-determined quantity of buffy coat into the collect line. The buffy coat can therefore push red blood cells out of the filter and into a collect bag without diluting the collected red blood cells. Alternatively, gravitational force may be used to allow the unwanted blood component to displace red blood cells out of the filter.
Owner:TERUMO BCT
Placental hematopoietic stem cell and preparation method thereof and placental hematopoietic stem cell injection
ActiveCN102660499AIncrease the number ofHigh activityPharmaceutical delivery mechanismMammal material medical ingredientsHuman lymphocyteCentrifugation
The invention relates to the field of biology and discloses a placental hematopoietic stem cell and a preparation method thereof and a placental hematopoietic stem cell injection. The preparation method comprises the following steps of: after pretreating a placenta, digesting the treated placenta by using collagenase type IV digestive juice and collagenase type II digestive juice, then washing and filtering and respectively collecting first filtrate and first residue; digesting the first filter residue by using collagenase type I digestive juice and the collagenase type II digestive juice in the first step and then washing, filtering and collecting second filtrate; combining the filtrate from two times, centrifuging and discarding supernate, re-suspending and precipitating; adding a resuspension solution into a lymphocytes separation medium; and carrying out density gradient centrifugation, collecting a buffy coat cell solution on an intermediate layer and centrifuging and discarding supernate to obtain the placental hematopoietic stem cell. According to the placental hematopoietic stem cell and the preparation method thereof disclosed by the invention, collagenase type IV and collagenase type II are combined, collagenase type I and collagenase type II are combined and the hematopoietic stem cell is prepared by fully digesting the placenta according to the characteristics of different collagenases, so that the quantity and the vitality of the prepared hematopoietic stem cell are improved.
Owner:BOYALIFE
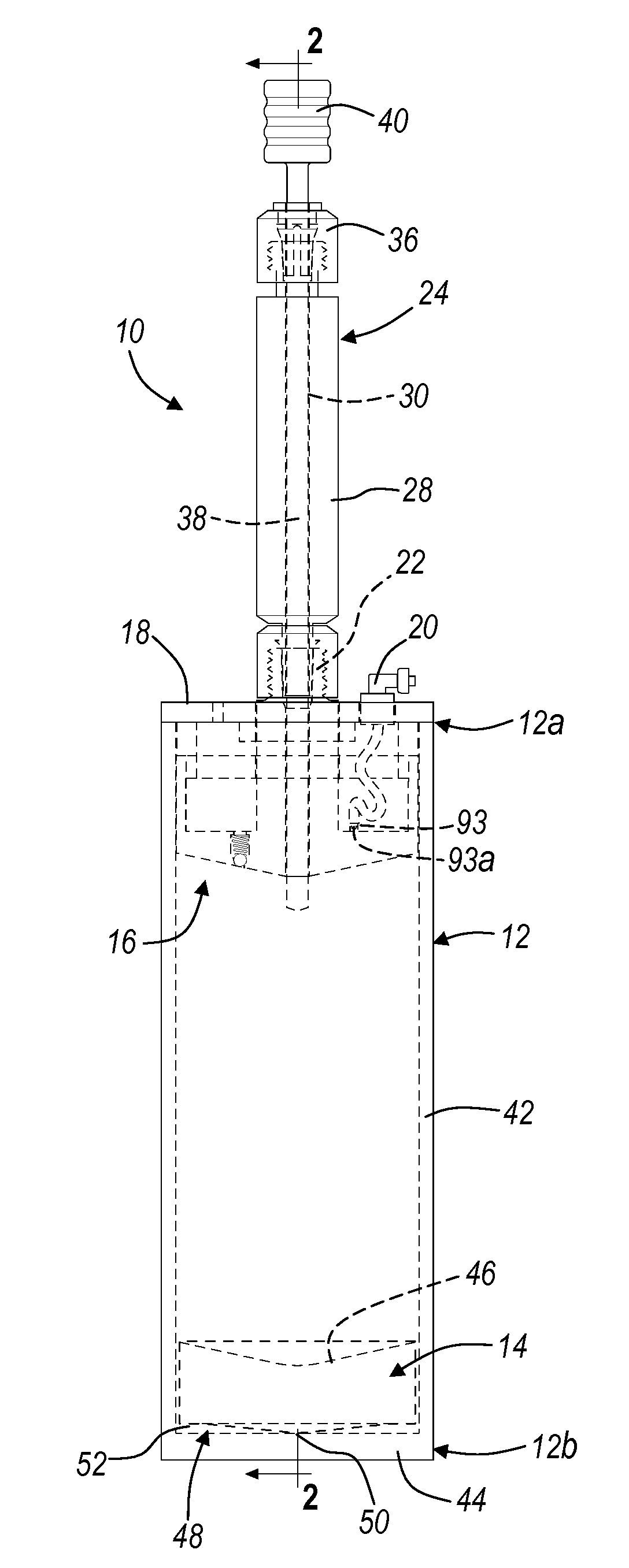
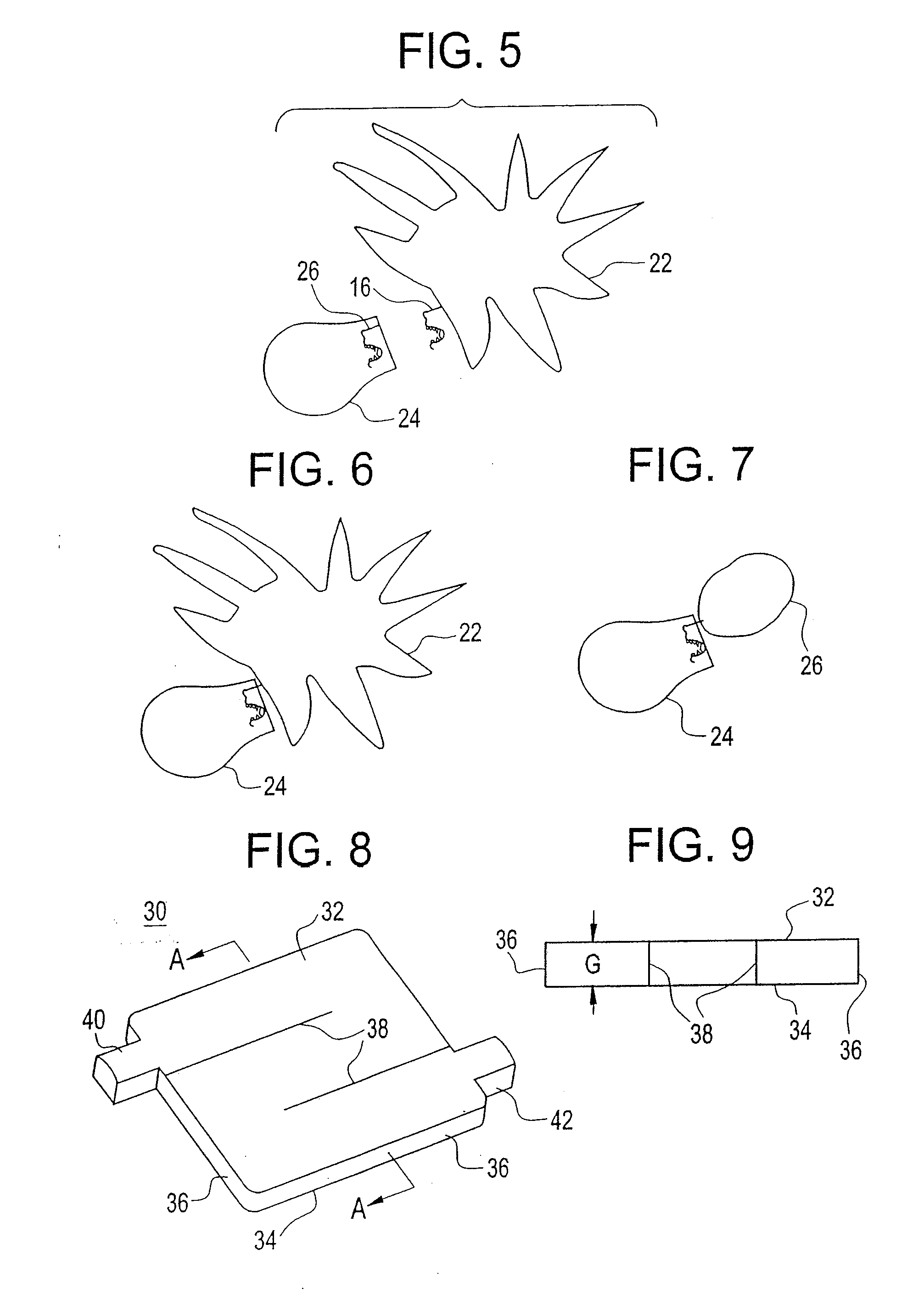
Buffy coat separator float system and method
InactiveUS20070092971A1Easy to separateReduced centrifugation speedWater/sewage treatment by centrifugal separationWithdrawing sample devicesCentrifugationDensity based
A tube and float system for use in separation and axial expansion of the buffy coat includes a transparent or semi-transparent, flexible sample tube and a rigid separator float having a specific gravity intermediate that of red blood cells and plasma. The float includes a main body portion of reduced diameter to provide a clearance gap between the inner wall of the sample tube and the float. One or more protrusions on the main body portion serve to support the flexible tube. During centrifugation, the centrifugal force causes the diameter of the flexible tube to expand and permit density-based axial movement of the float in the tube. The float further includes a pressure relief system to alleviate pressure build up in the trapped red blood cell blood fraction below the float, thereby preventing red blood cells from being forced into the annular gap containing the buffy coat layers.
Owner:BATTELLE MEMORIAL INST
Method of carrying out isolated culture on immune cells by virtue of peripheral blood
InactiveCN104480069AViability does not affectThe total amount is largeBlood/immune system cellsSerum free mediaFicoll
The invention discloses a method of carrying out isolated culture on immune cells by virtue of peripheral blood. The method comprises the following steps: centrifuging the peripheral blood for 10 min under a condition of 2000rpm and 4 DEG C, and collecting the upper-layer plasma to prepare a serum-free medium containing the plasma; adding Ficoll separation liquid and centrifuging; sucking a buffy coat, and washing to obtain PBMC; adding the serum-free medium in the PBMC, inoculating in the serum-free medium, and adding 20-300U / mL of IL-2 and 5-50ng / mL of IL-15; collecting the immune cells while culturing to the 13th to the 15th day. According to the method disclosed by the invention, the centrifuging time, the centrifuging rotational speed and the centrifuging temperature are adjusted, lots of the plasma can be obtained, and the stability of protein content in the plasma and no deficiencies of active factor ingredients can be ensured.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Blood bag system and cassette
ActiveUS20110230855A1Easy to carryAppropriate settingDiagnosticsSurgeryWhite blood cellCentrifugation
In a blood bag system mounted in a centrifugation and separation apparatus, a filter is settable in different appropriate orientations at storage time and usage time, and first and second tubes and a filter are arranged respectively in appropriate orientations during use. The blood bag system includes a BC pooling bag for centrifugation of buffy coat, the filter for removing white blood cells from a supernatant liquid transferred from the BC pooling bag, a platelet preserving bag for reserving the supernatant liquid that has passed through the filter, the first tube connecting the BC pooling bag and an inlet of the filter, the second tube connecting the platelet preserving bag and an outlet of the filter, a cassette fixable in the centrifugation and separation apparatus, an attachment for holding the filter, and a hinge section tiltable relative to the cassette with reference to an axis in the circumferential direction.
Owner:TERUMO KK
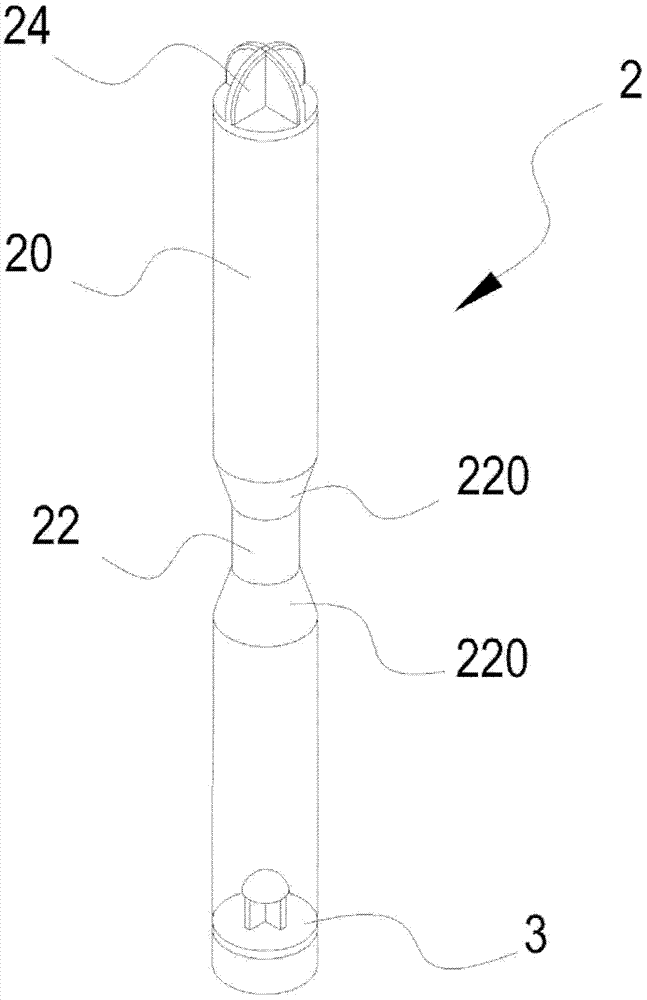
Platelet-rich blood plasma acquiring device
InactiveCN103111097AEasy accessObvious stratificationLiquid separationHigh concentrationWhite blood cell number
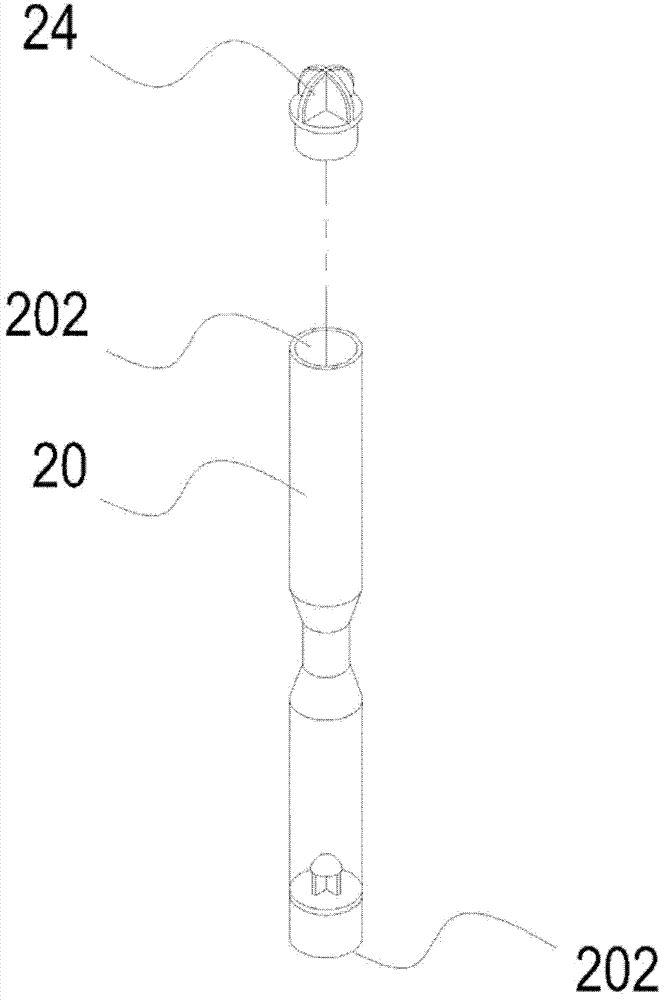
The invention relates to a platelet-rich blood plasma acquiring device. The device is composed of a tube with two ends respectively having an opening, a cover used for covering one opening, and a piston used for sealing the other opening. A narrow tube portion positioned in the middle section of the tube and having an internal diameter lower than the internal diameter of the tube uses the matching of the tube and a push bar to conveniently push the piston to move a buffy coat rich in platelets and leucocytes to the middle of the tube by the push bar after blood centrifuge. The narrow tube portion in the middle section of the tube makes the buffy coat more obvious, so a blood plasma having a high concentration of the platelets can be conveniently taken by a user. The cover positioned at one end of the tube makes the user easily open and operate.
Owner:MARIA VON MED BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com