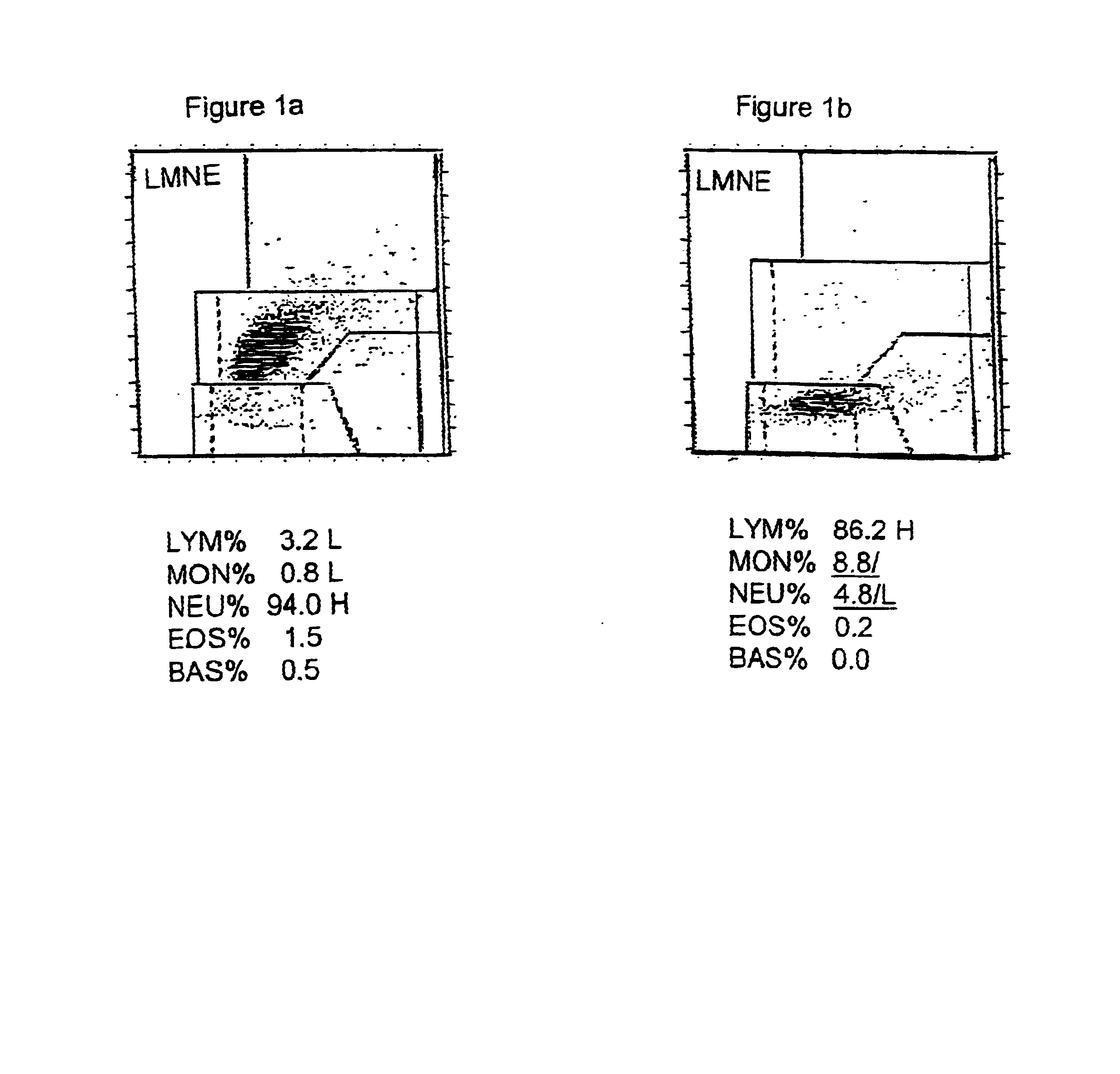
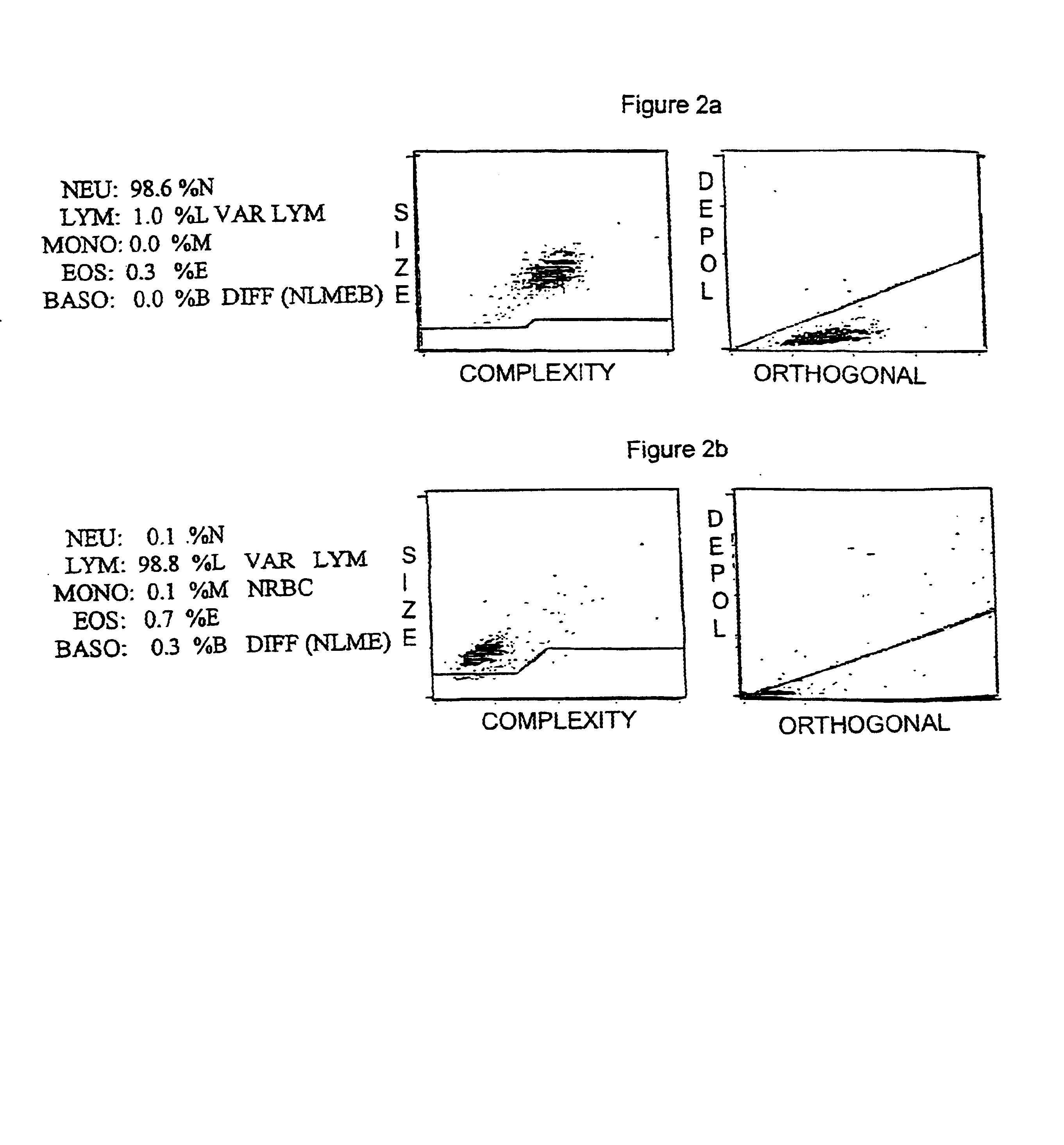
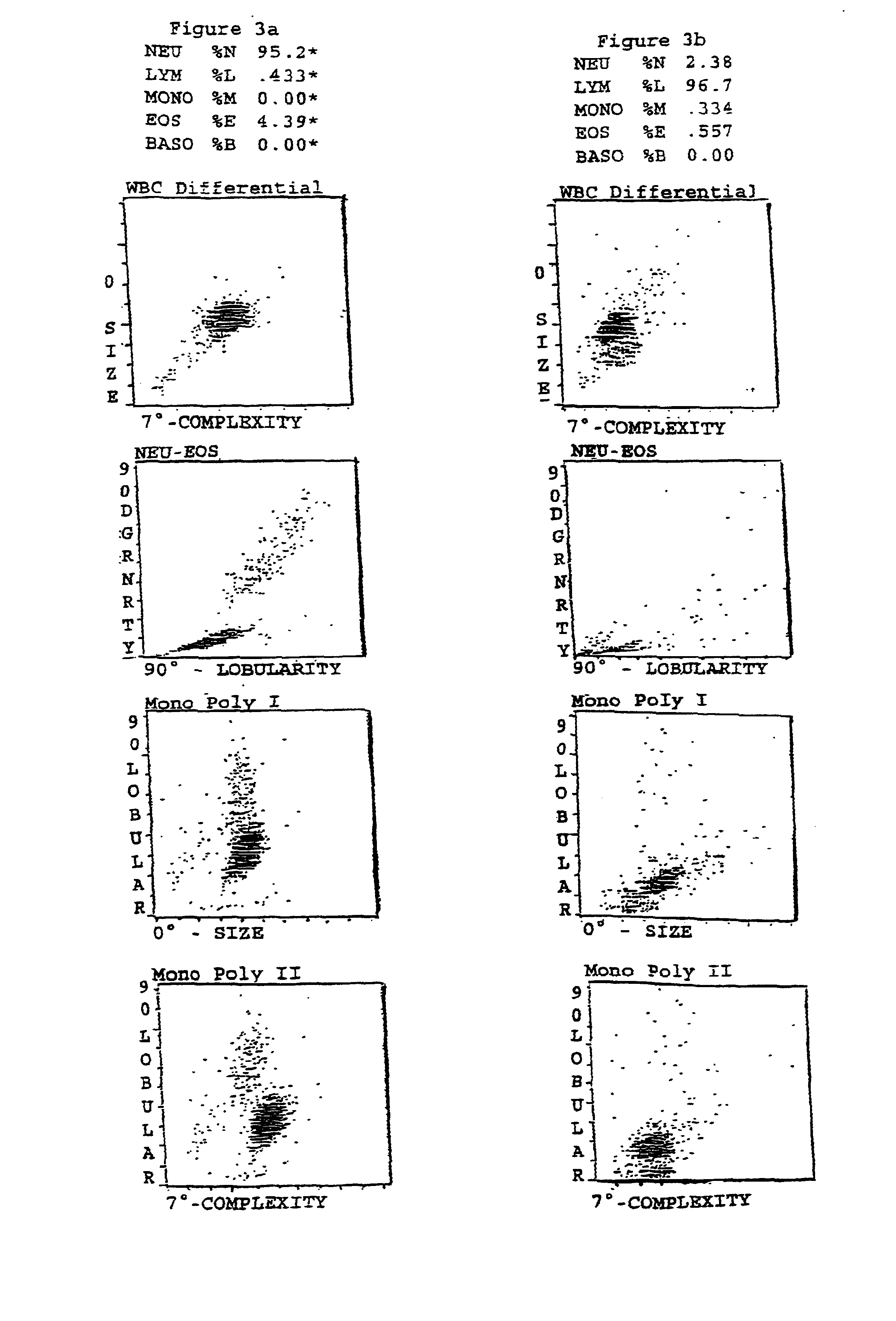
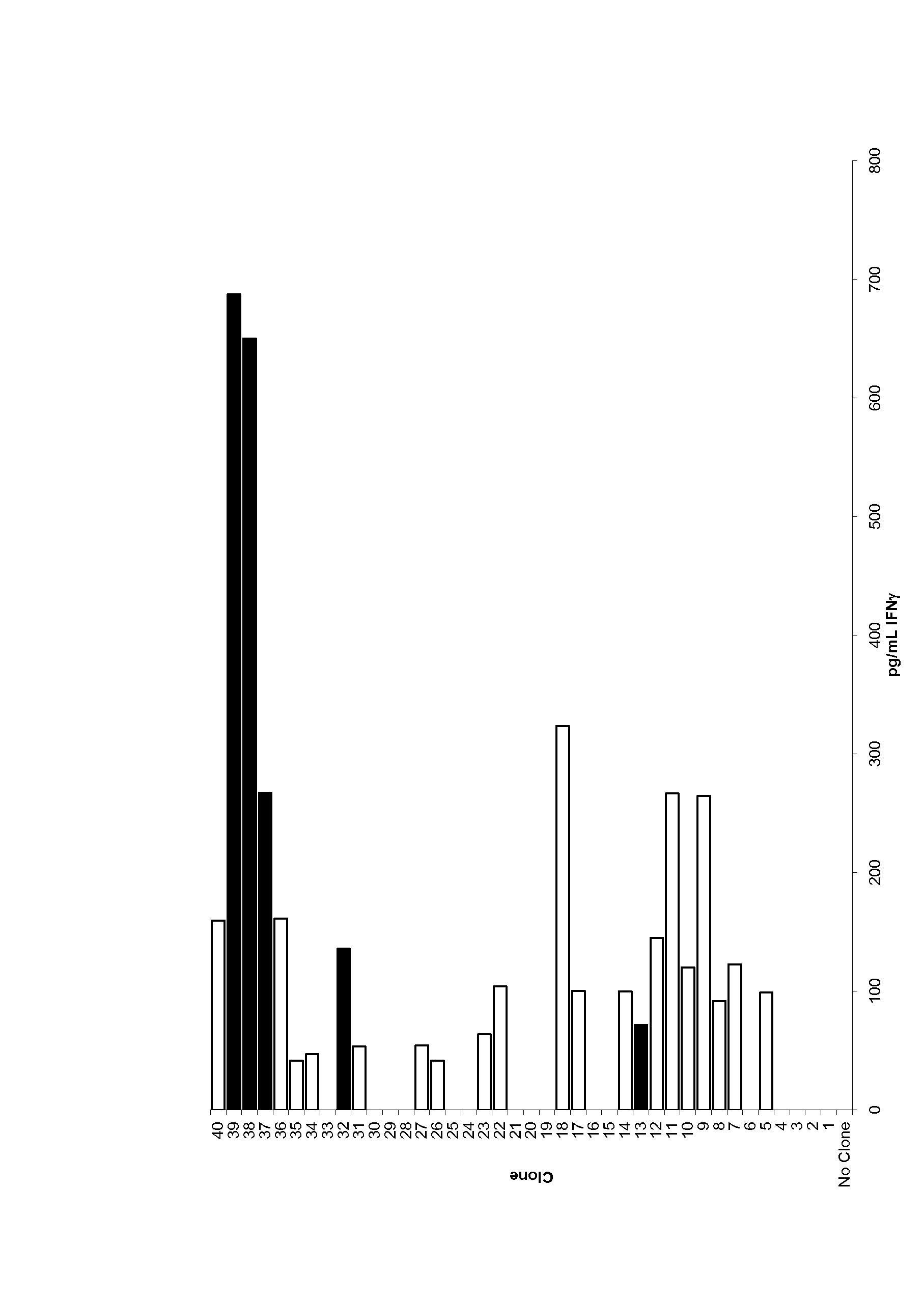
Patents
Literature
118 results about "Human lymphocyte" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:BIOGEN INC
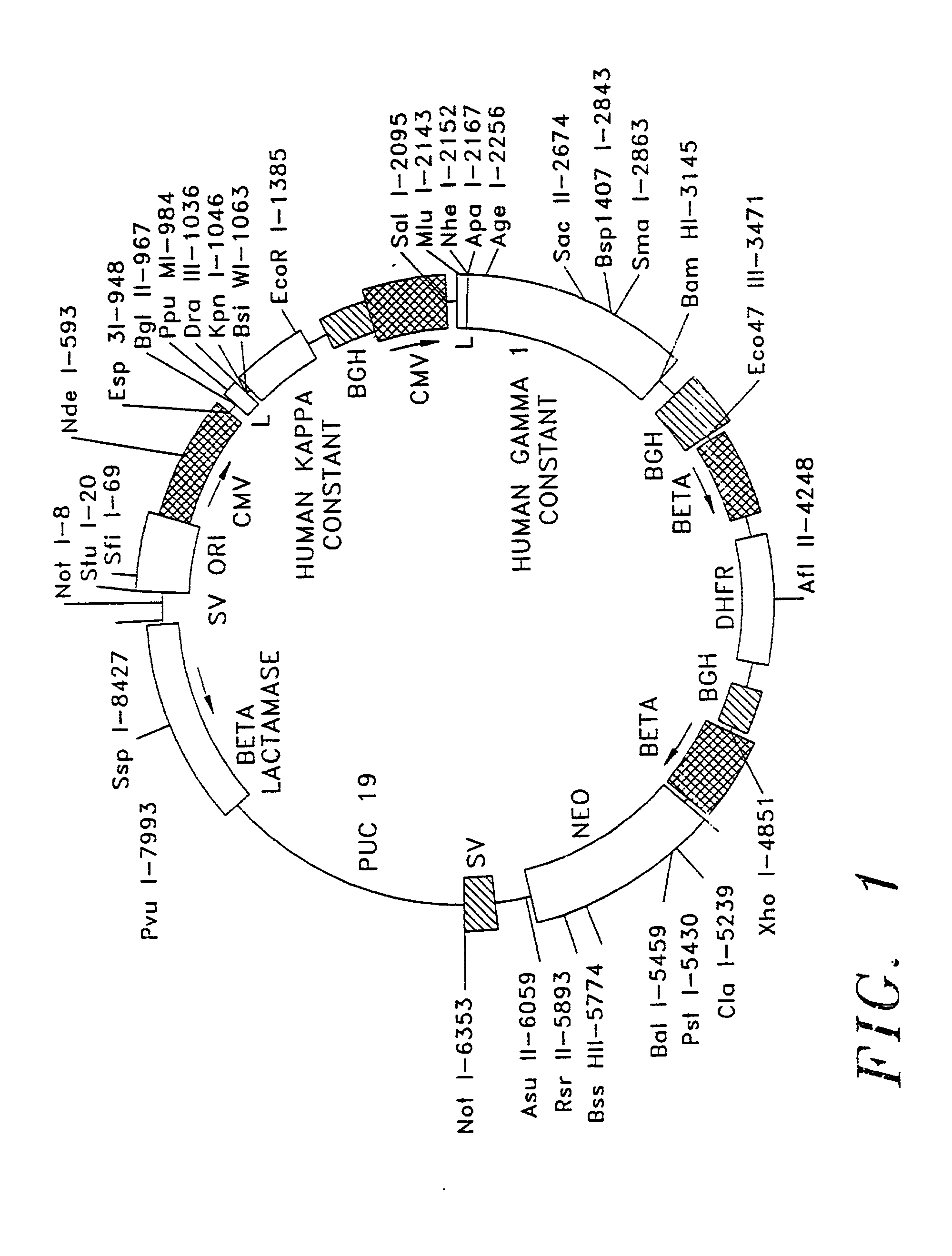
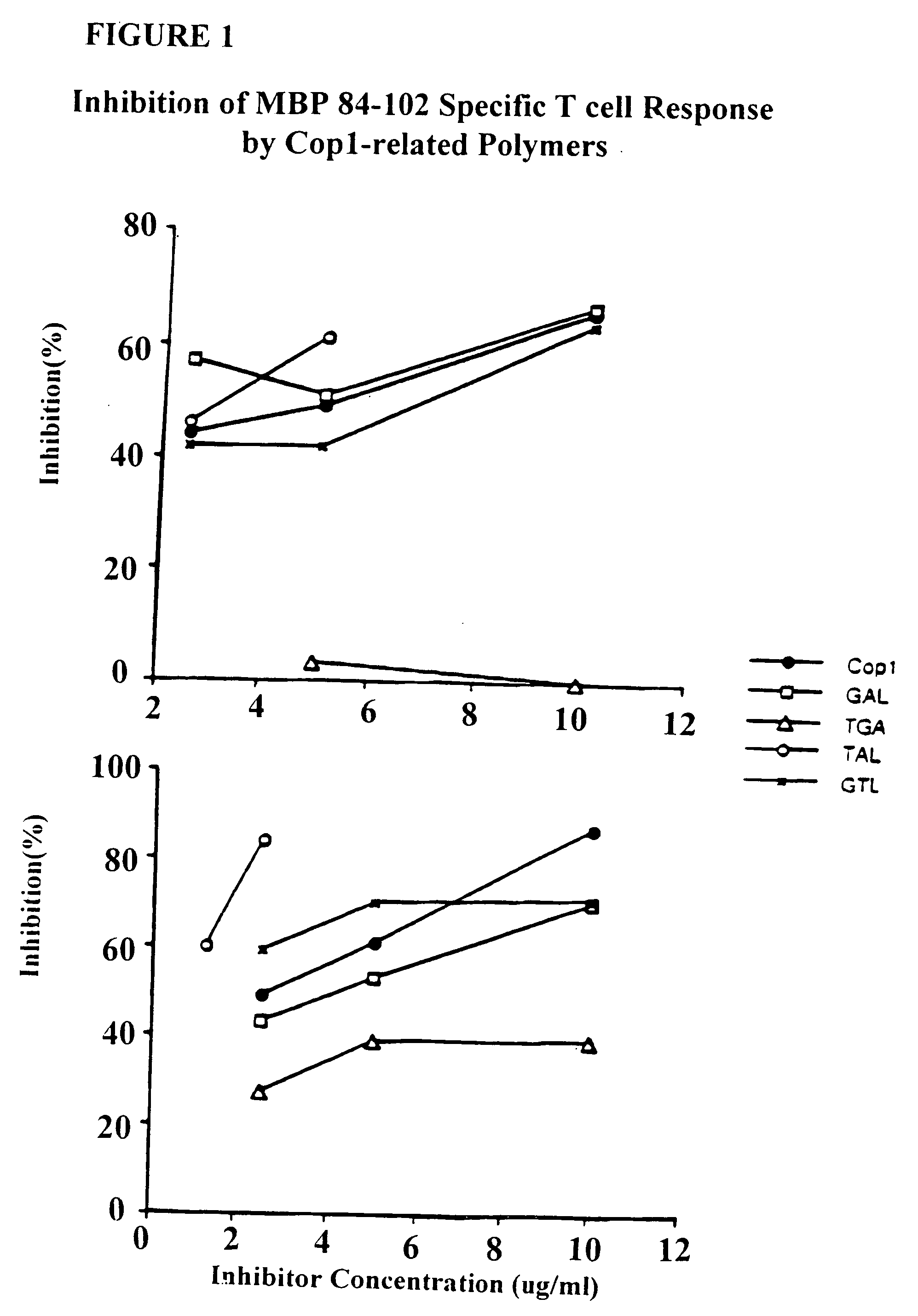
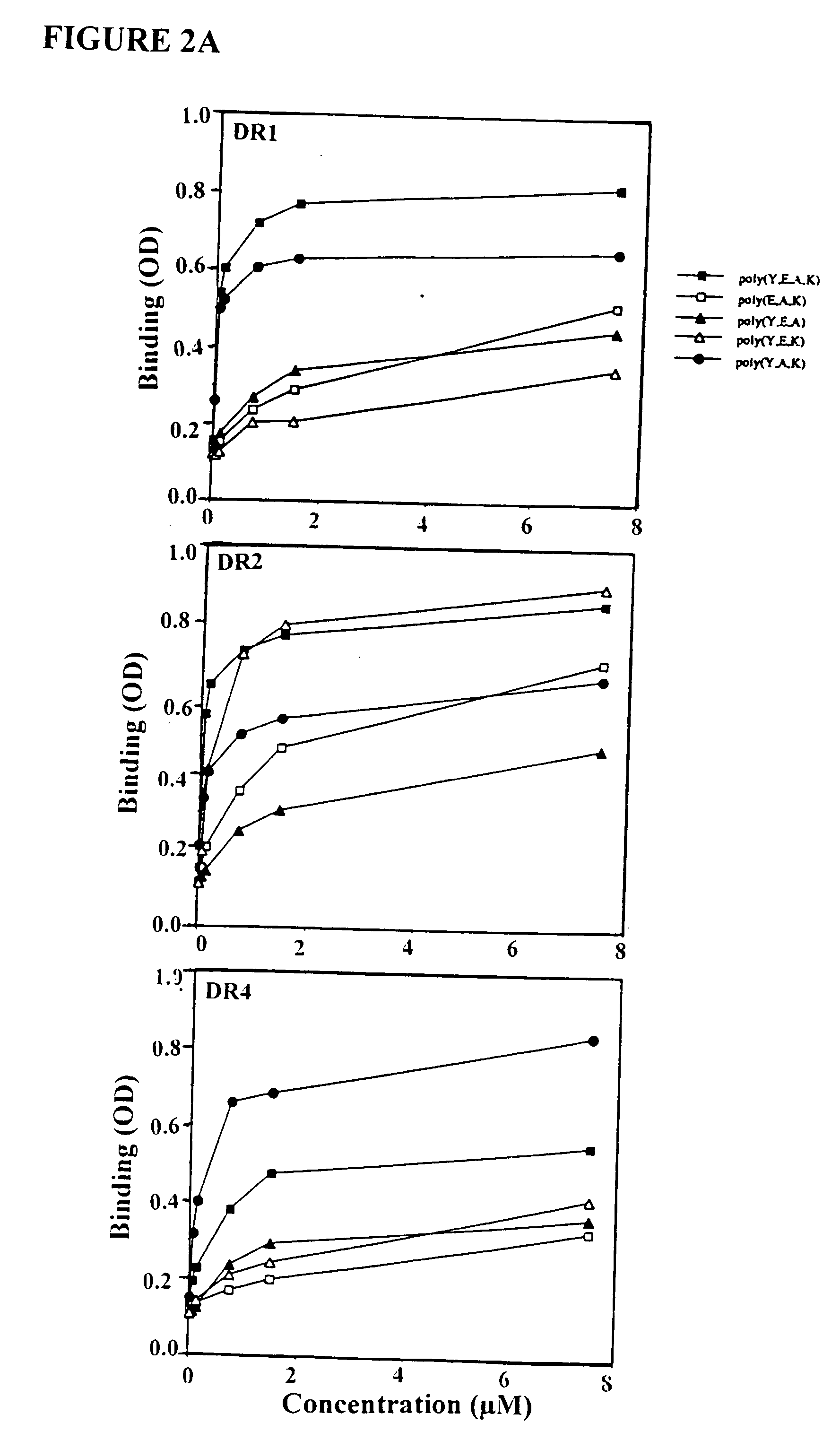
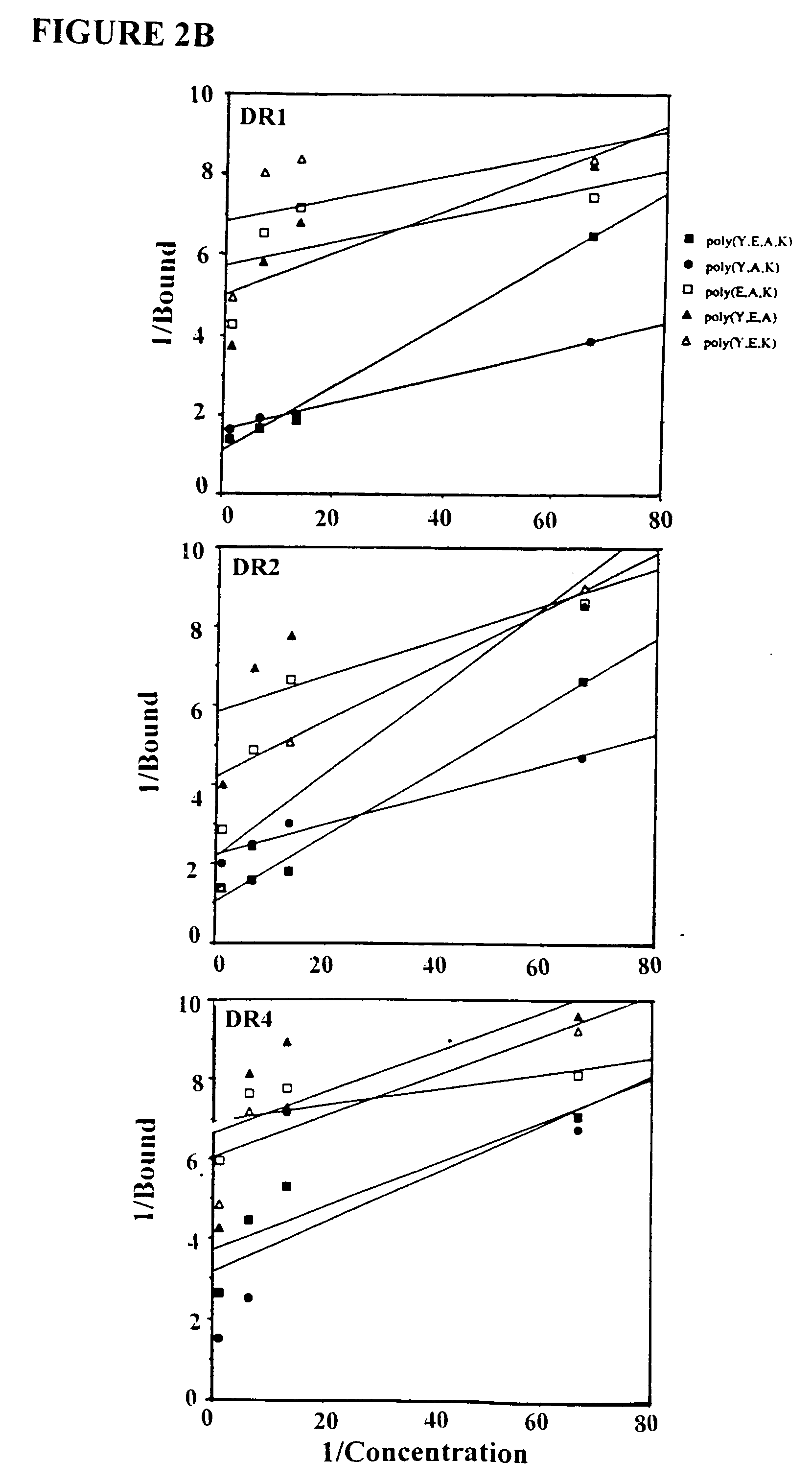
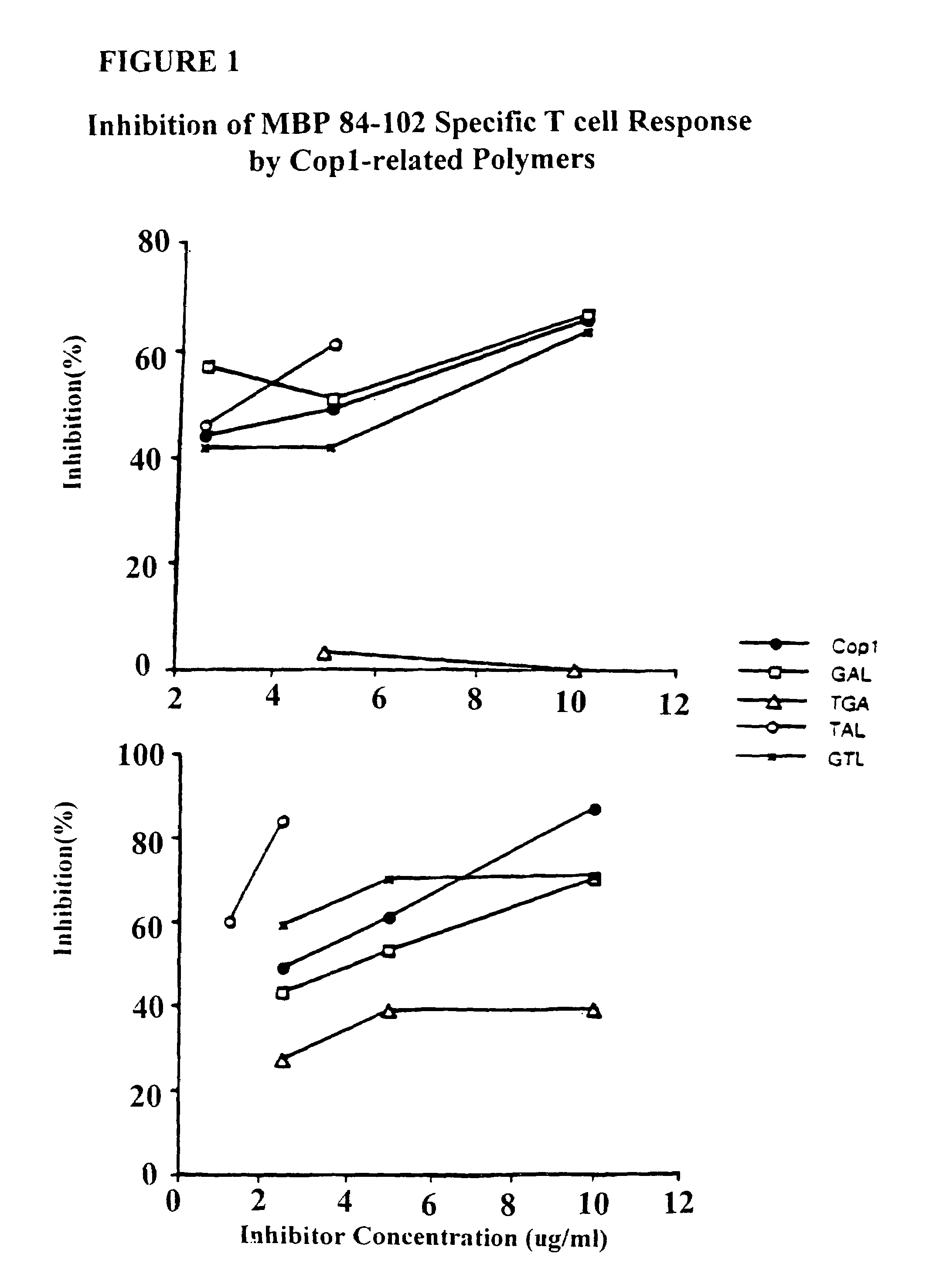
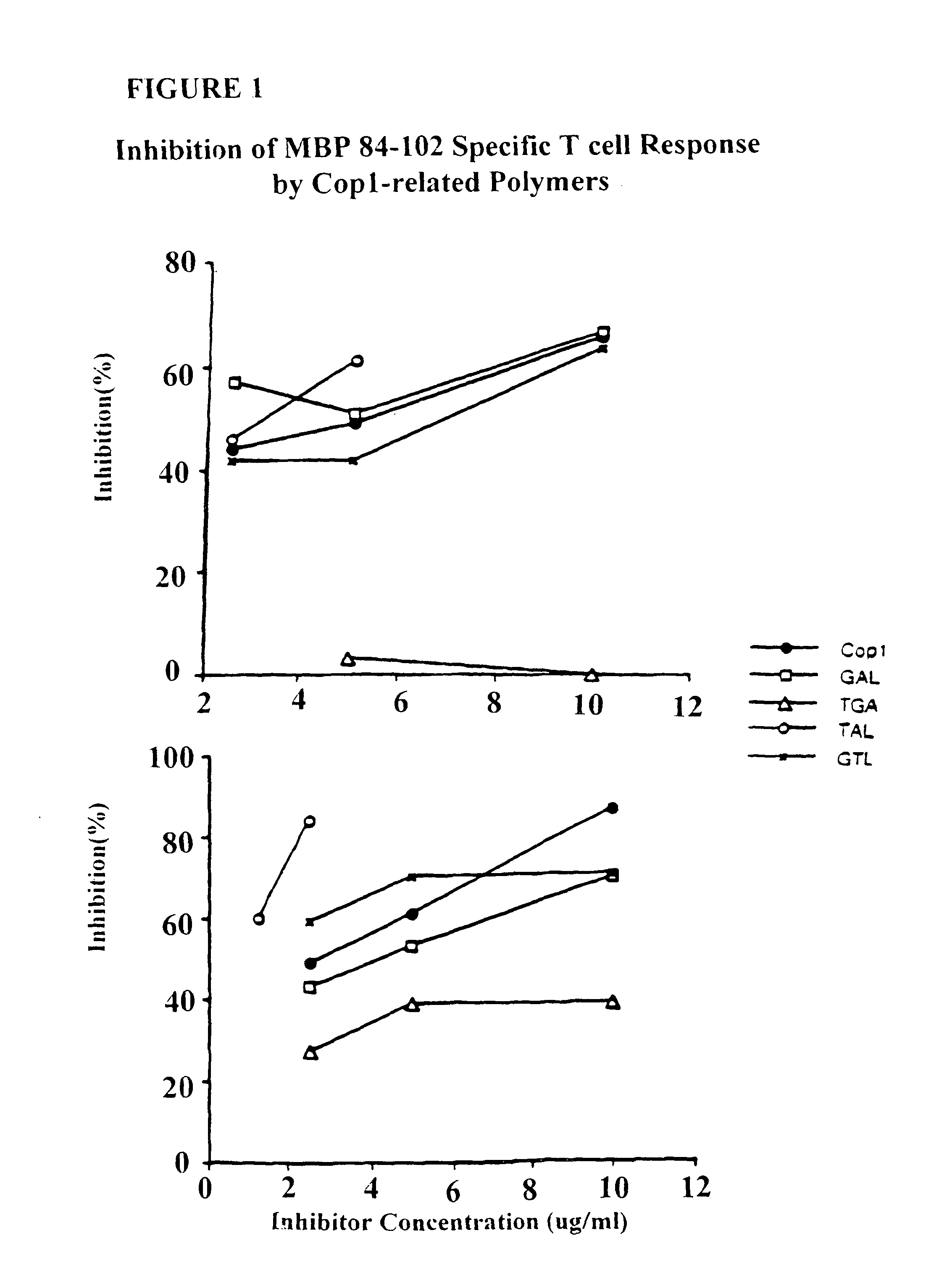
Treatment of autoimmune conditions with Copolymer 1 and related Copolymers
InactiveUS20070021341A1Stimulate growth and functioningAvoid attackOrganic active ingredientsSenses disorderAutoimmune conditionMammal
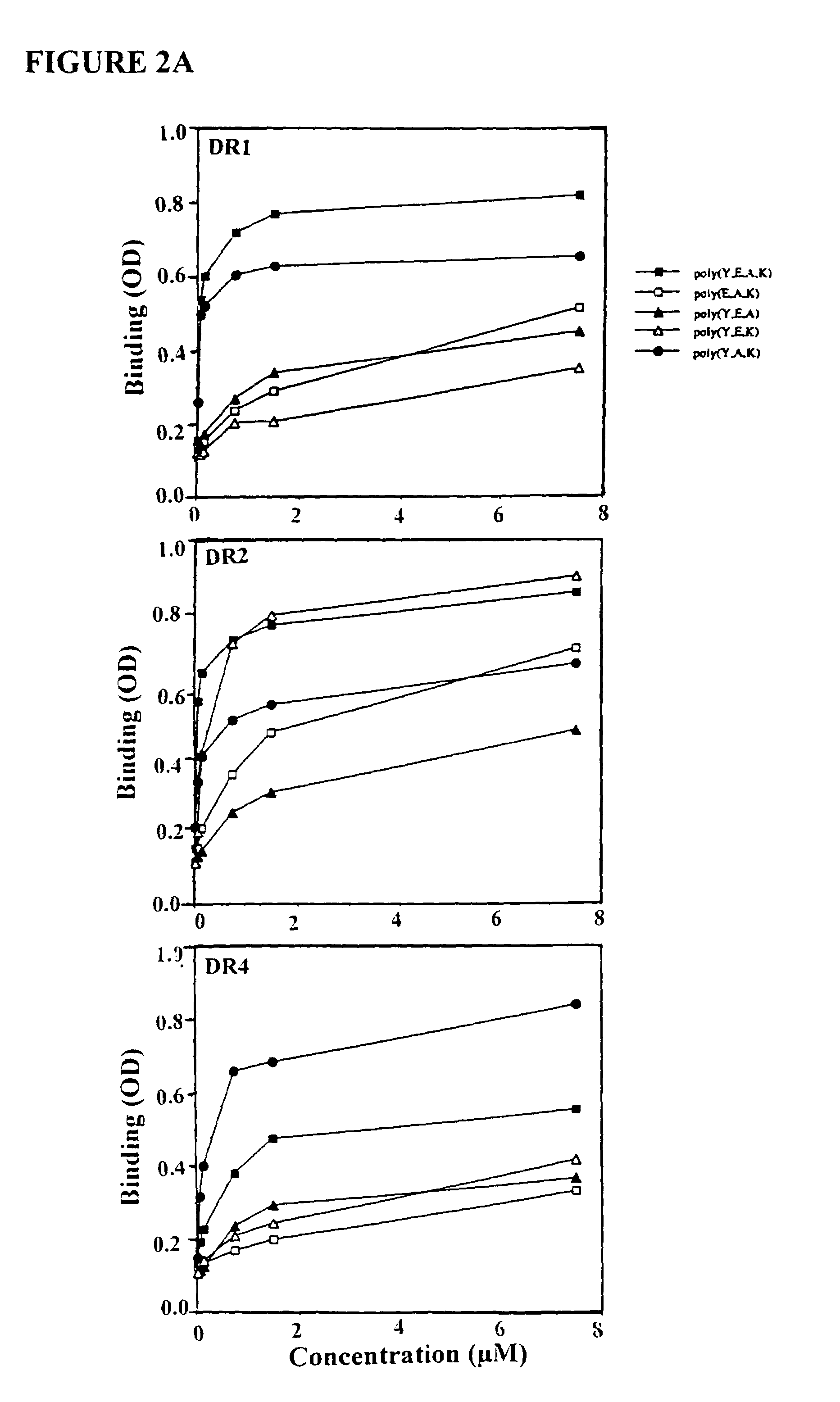
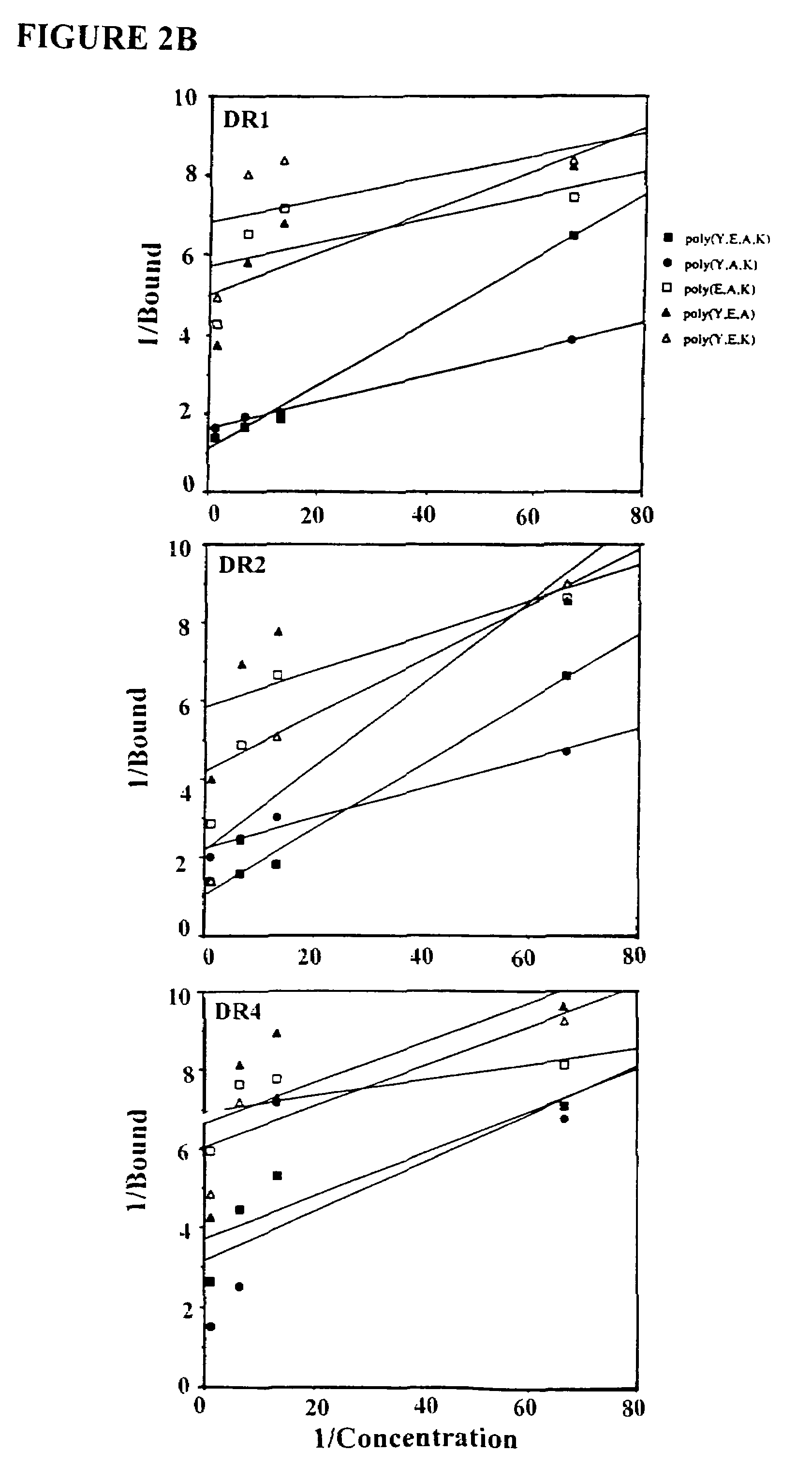
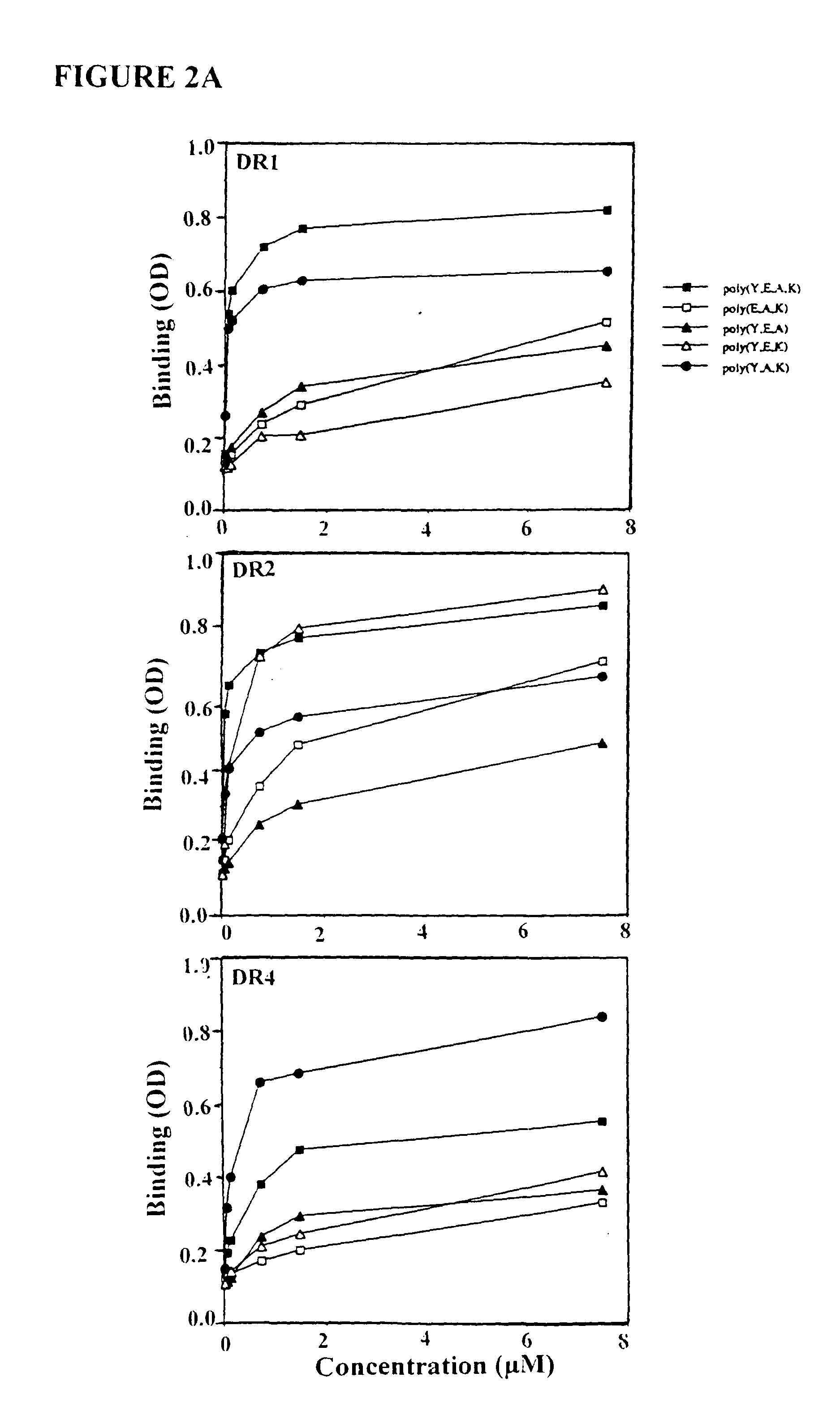
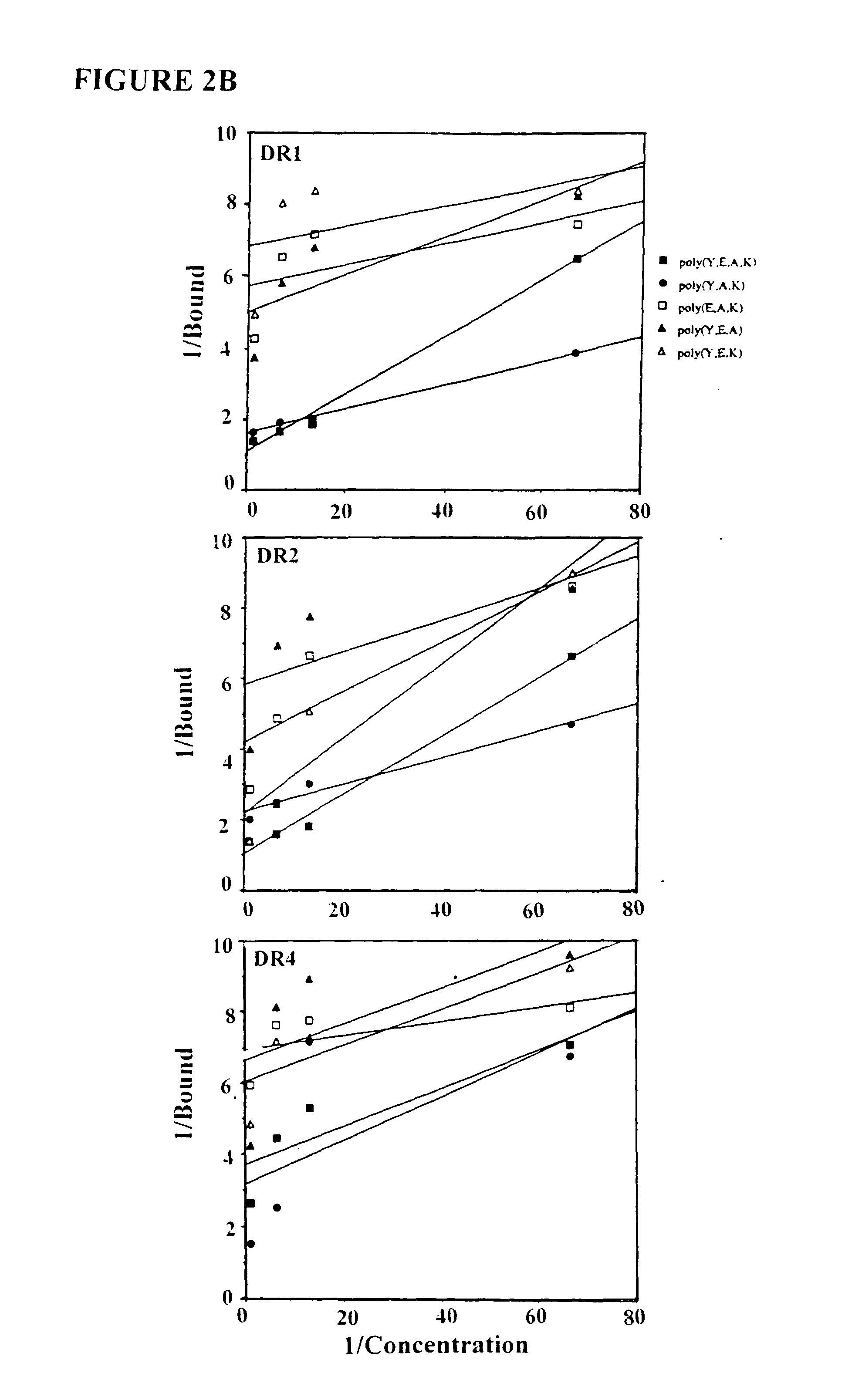
The present invention is directed to polypeptides containing at least three amino acids randomly joined in a linear array; wherein at least one of the three amino acids is an aromatic amino acid, at least one of the three amino acids is a charged amino acid and at least one amino acid is an aliphatic amino acid. In a preferred embodiment the polypeptide contains three or four of the following amino acids: tyrosine, alanine, glutamic acid or lysine. According to the present invention, the present polypeptides bind to antigen presenting cells, purified human lymphocyte antigens (HLA) and / or Copolymer 1-specific T cells. Moreover, according to the present invention, these polypeptides can be formulated into pharmaceutical compositions for treating autoimmune disease. The present invention further contemplates methods of treating an autoimmune disease in a mammal by administering a pharmaceutically effective amount of any one of the present polypeptides to the mammal.
Owner:YEDA RES & DEV CO LTD +1
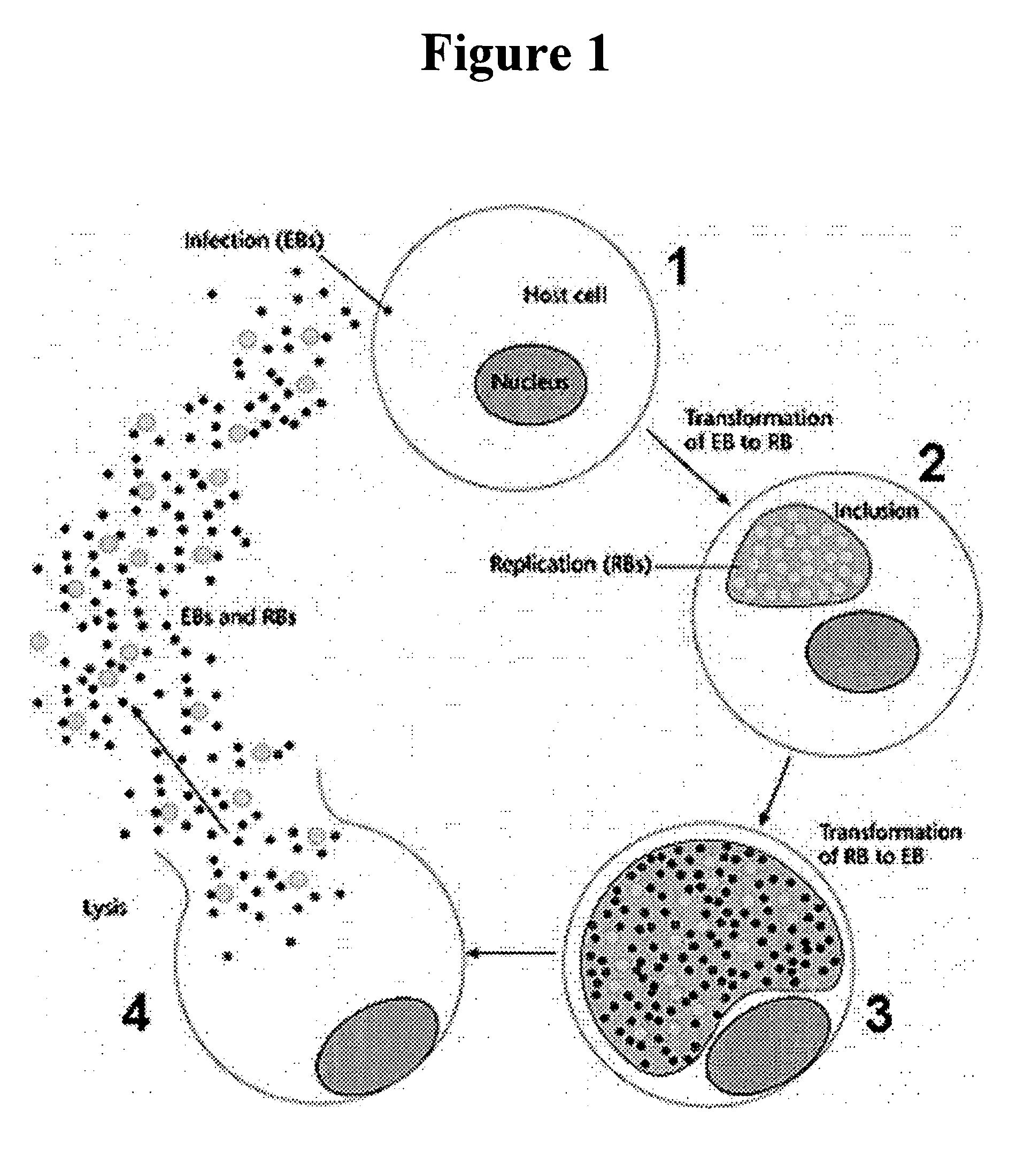
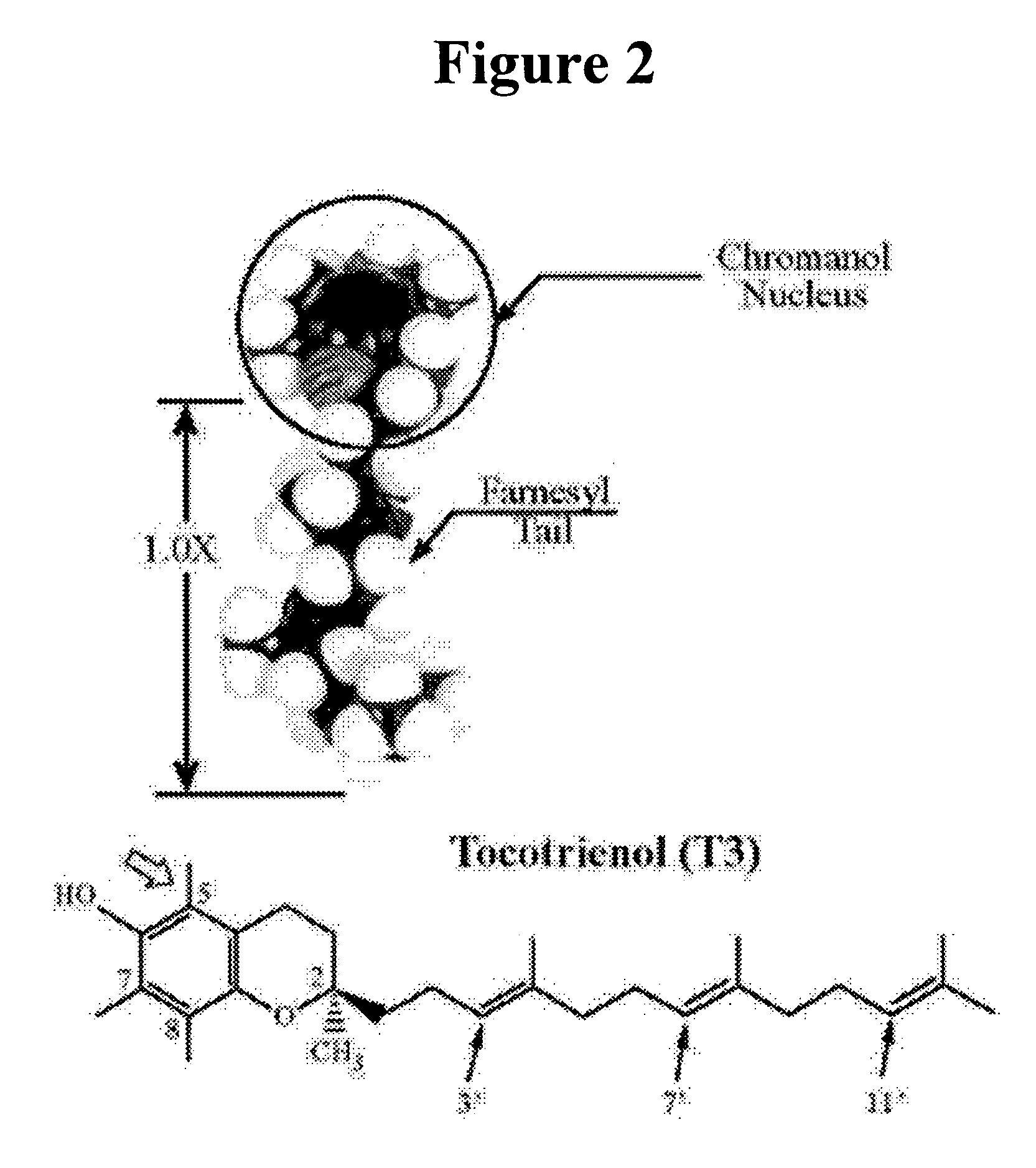
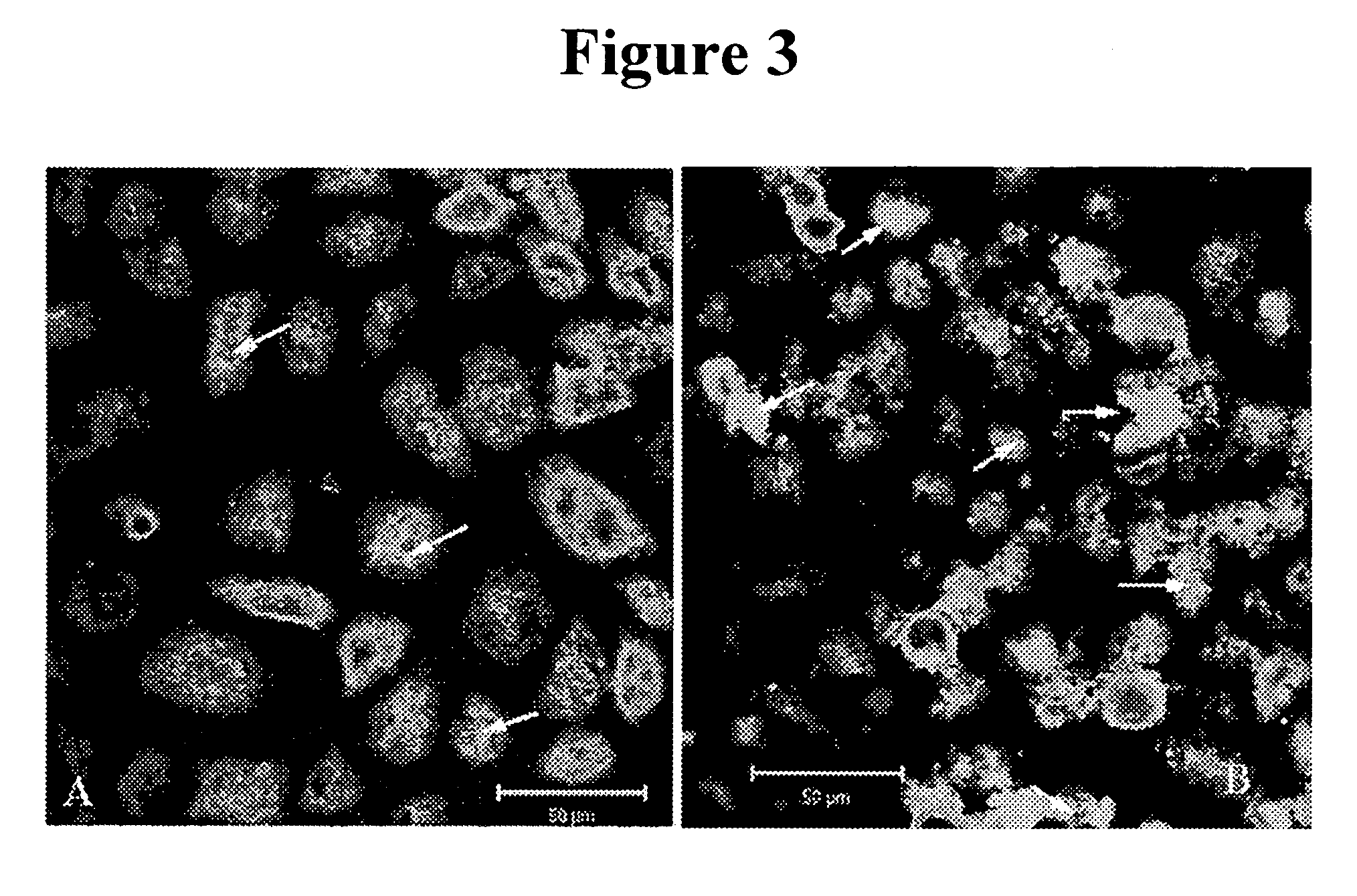
Vitamin E tocotrienols inhibition of intracellularly obligate pathogen Chlamydia and methods of use
InactiveUS20060241174A1Reducing Chlamydia-induced blindnessLower blood pressureAntibacterial agentsBiocideControl cellLymph
This invention reveals the beneficial use of vitamin E tocotrienols for inhibition of chlamydial infections. Chlamydial infection levels in mouse macrophages treated with tocotrienol were decreased >50%, with concomitant aberrant pathogen development. The number of large and small inclusions in tocotrienol-versus-control cells was decreased 3-fold and 2-fold, respectively. When treated with delta tocotrienol, Chlamydia in human lymphocytes was inhibited by at least 2.6-fold in 1.5 days. Dietary delta tocotrienol inhibited Chlamydia infection and persistence in hypercholesterolemic patients with a corresponding drop in LDL. These studies demonstrate that tocotrienol lowers cholesterol, thus preventing or diminishing the cholesterol hijacking by Chlamydia obligatory for its infectivity and replication. Therefore, hypolipidemic agents used to treat cardiovascular diseases, metabolic syndrome, and diabetes are used as monotherapies, or in combination with tocotrienol to treat Chlamydia.
Owner:AMERICAN RIVER NUTRITION
Treatment of autoimmune conditions with Copolymer 1 and related Copolymers
InactiveUS7425332B2Stimulate growth and functioningAvoid attackOrganic active ingredientsSenses disorderDiseaseHuman lymphocyte
Owner:YEDA RES & DEV CO LTD +1
Treatment of autoimmune conditions with Copolymer 1 and related copolymers
InactiveUS20100298227A1Stimulate growth and functioningAvoid attackOrganic active ingredientsNervous disorderHuman lymphocyteAutoimmune condition
The present invention is directed to polypeptides containing at least three amino acids randomly joined in a linear array; wherein at least one of the three amino acids is an aromatic amino acid, at least one of the three amino acids is a charged amino acid and at least one amino acid is an aliphatic amino acid. In a preferred embodiment the polypeptide contains three or four of the following amino acids: tyrosine, alanine, glutamic acid or lysine. According to the present invention, the present polypeptides bind to antigen presenting cells, purified human lymphocyte antigens (HLA) and / or Copolymer 1-specific T cells. Moreover, according to the present invention, these polypeptides can be formulated into pharmaceutical compositions for treating autoimmune disease. The present invention further contemplates methods of treating an autoimmune disease in a mammal by administering a pharmaceutically effective amount of any one of the present polypeptides to the mammal.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE & YEDA RES
Rapid immunoselection cloning method
InactiveUS7119183B2Large amount of proteinImprove efficiencyPeptide librariesPeptide/protein ingredientsAntigenCDNA library
A simple and highly efficient method for cloning cDNAs including CD27 (SEQ ID NO:28) from mammalian expression libraries based on transient expression in mammalian host cells has been discovered. Novel expression vectors allowing highly efficient construction of mammalian cDNA libraries are disclosed. The cloning method of the invention which has been used to clone genes for cell surface antigens of human lymphocytes, has general application in gene cloning. Cell surface antigens cloned according to the present invention have been purified, and the nucleotide and amino acid sequences determined. These antigens have diagnostic and therapeutic utility in immune-mediated infections in mammals, including humans.
Owner:THE GENERAL HOSPITAL CORP
Bispecific antibody capable of resisting B cell lymphoma and application thereof
InactiveCN102250245AHighlighting the role of anti-B-cell lymphomaFungiHybrid immunoglobulinsAntiendomysial antibodiesT lymphocyte
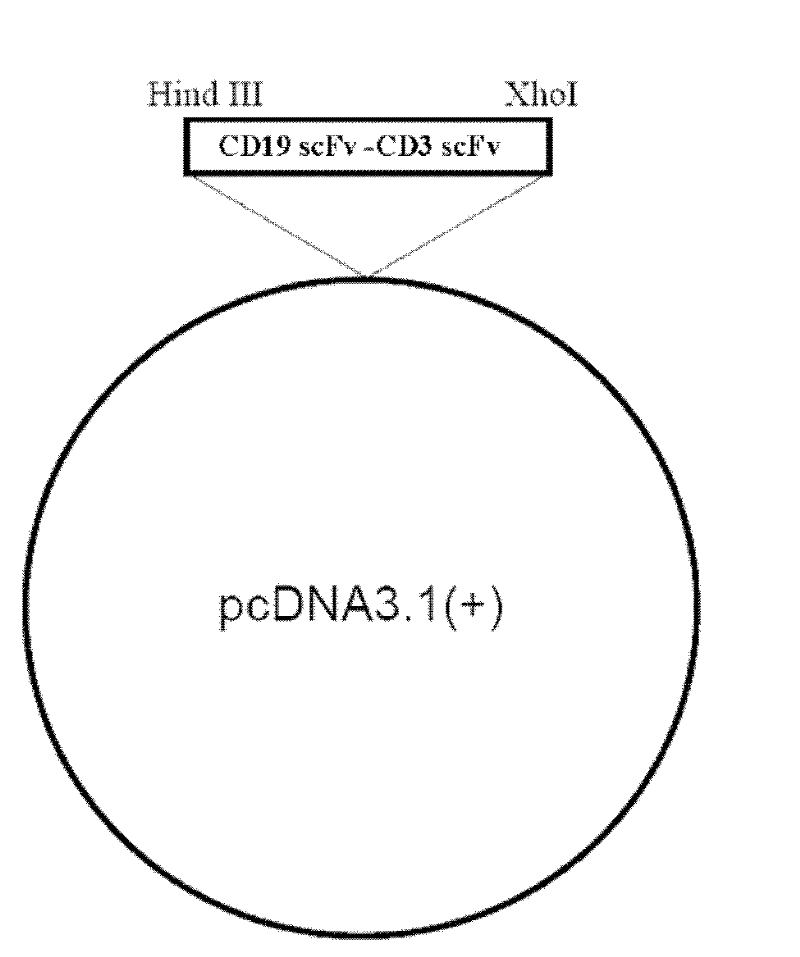
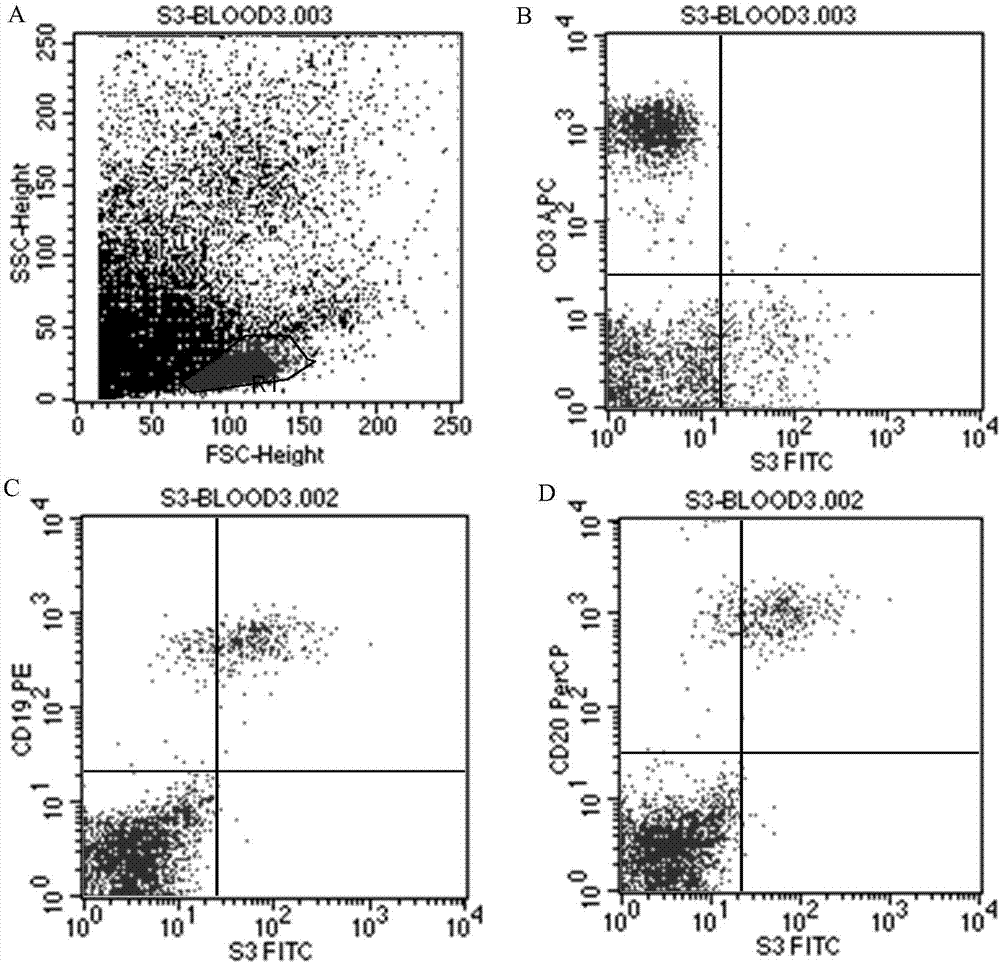
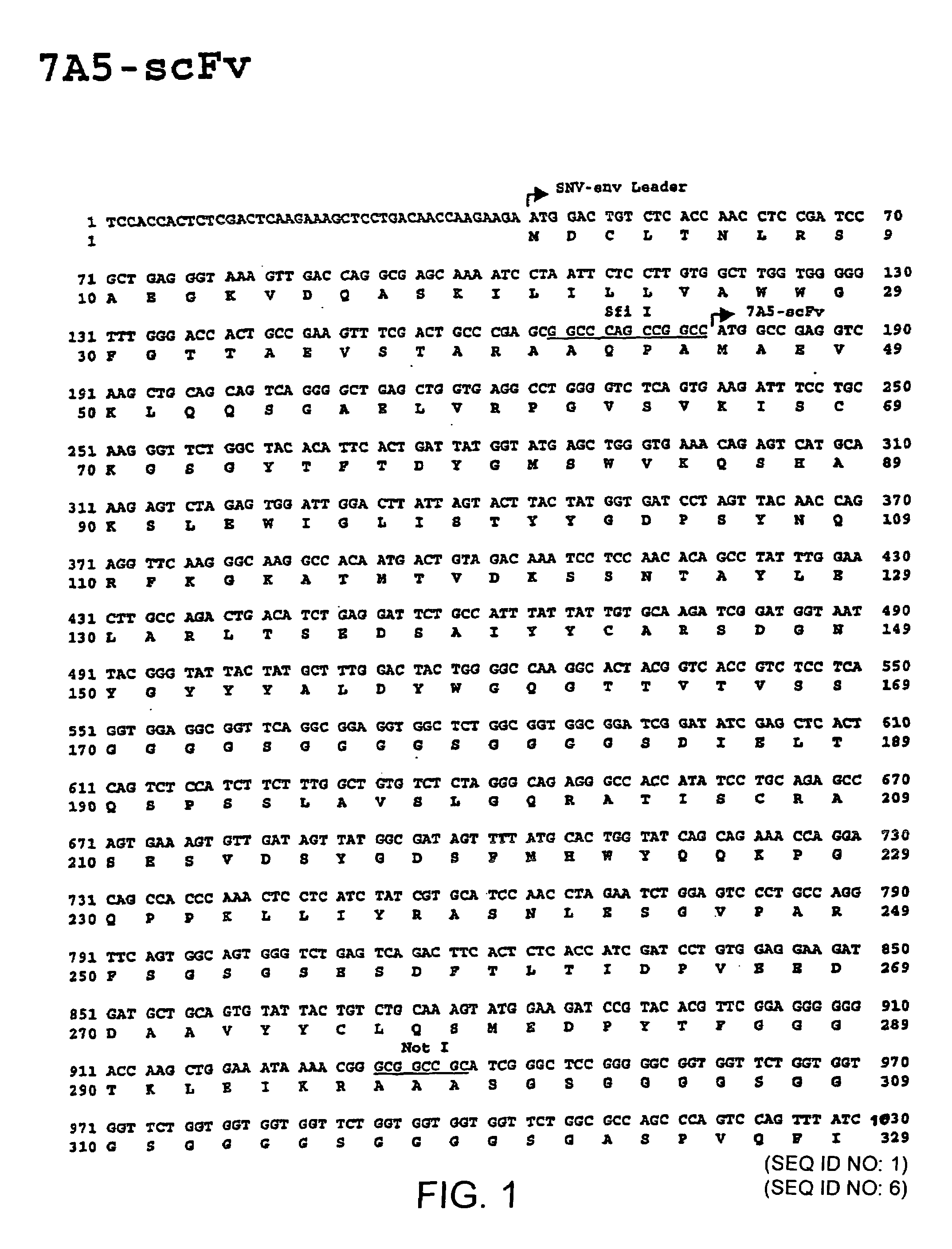
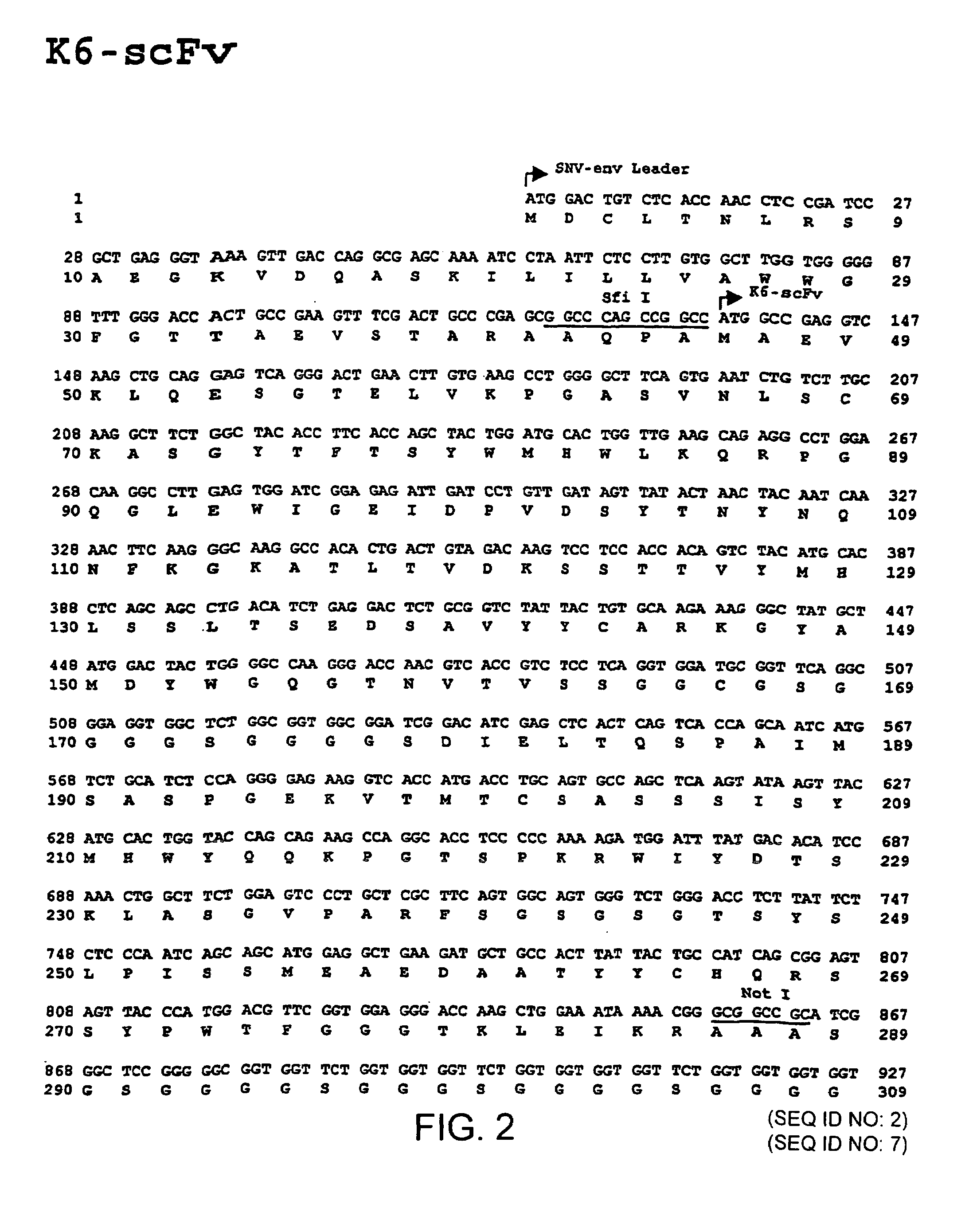
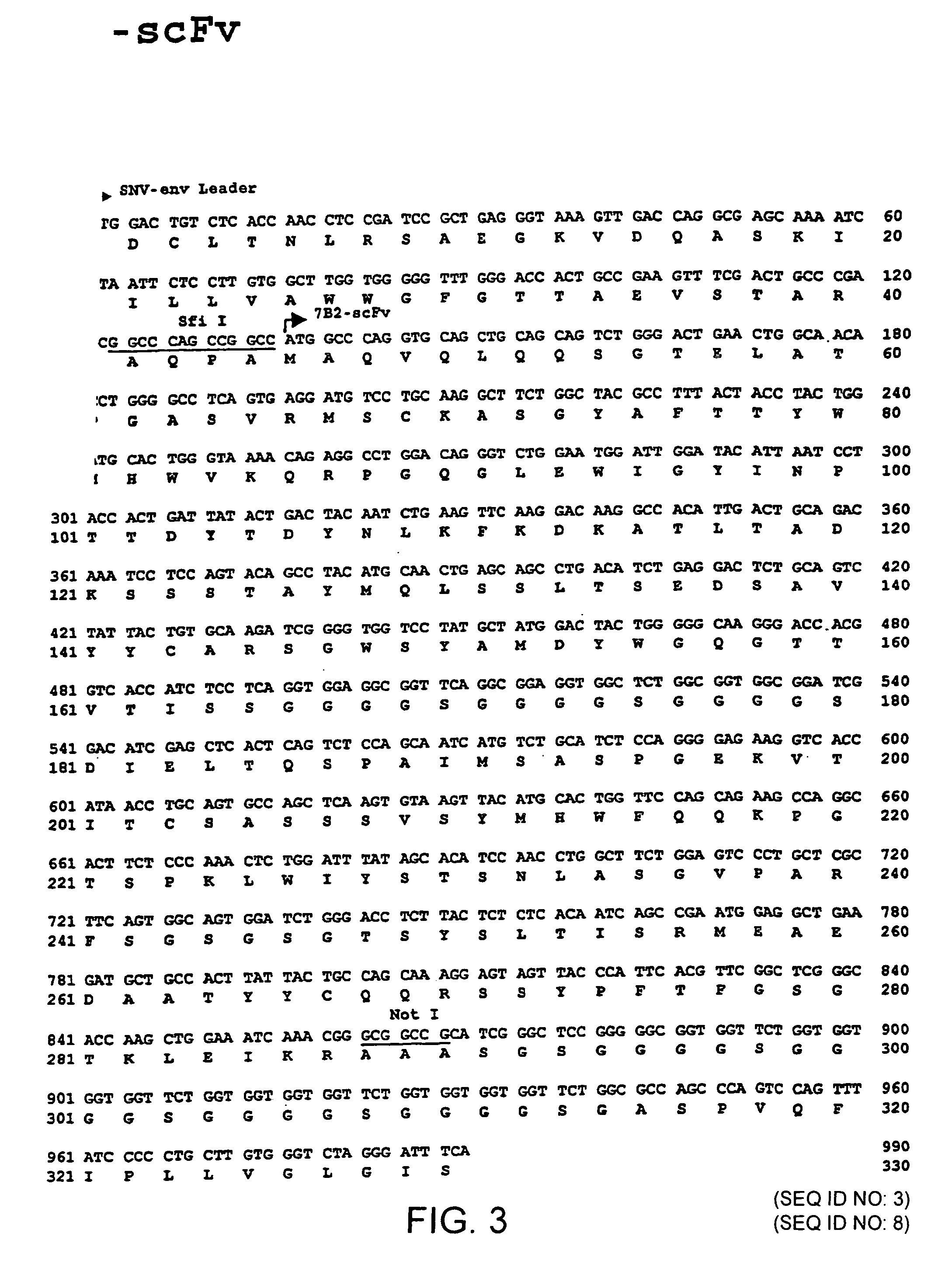
The invention relates to the technical fields of genetic engineering and protein engineering, in particular relates to DNA (deoxyribonucleic acid) for encoding recombinant fusion protein containing human CD19 antibody variable region and human CD3 antibody variable region fragments, fusion protein encoded by DNA, a production method of the fusion protein, pharmaceutical application of the fusion protein and a treatment method using the fusion protein. The invention provides bispecific antibody protein containing human CD19scFv and CD3scFv. The bispecific antibody protein can be combined with positive CD19 and CD3 positive cells, has good bioactivities in vivo and vitro, can activate human T lymphocyte, kill B lymphoma cells, and has good application prospects.
Owner:SICHUAN UNIV
Novel efficient method for separating human T-Lymphocytes by immunomagnetic beads
InactiveCN102719398AProlong survival timeEasy to operateBlood/immune system cellsHuman lymphocyteLymphocyte culture
The invention establishes an operation method in T-Lymphocytes culture in vitro by immunomagnetic bead separation technique to maximize the separation of human T-Lymphocytes from the same volume of human peripheral blood to obtain a lot of purified T-Lymphocytes and cover the shortage that lymphocytes can be only primary cultured but not be subcultured to the greatest extent. The method is simple and practicable.
Owner:赵品楠
Hematology control composition including lymphocyte analogs and method for preparation and use
A control composition, and method of preparing a control composition, that includes stabilized, mammalian granulocytes having altered physical properties so that the granulocytes function as a human lymphocyte analogs when used on an automated blood cell analyzer.
Owner:BIO TECHNE
Antigen screening system
Owner:GENOCEA BIOSCI
Method for producing human antibodies with properties of agonist, antagonist, or inverse agonist
ActiveUS20050277173A1The process is simple and effectiveSimple methodAnimal cellsSugar derivativesSynthetic ImmunogensHuman lymphocyte
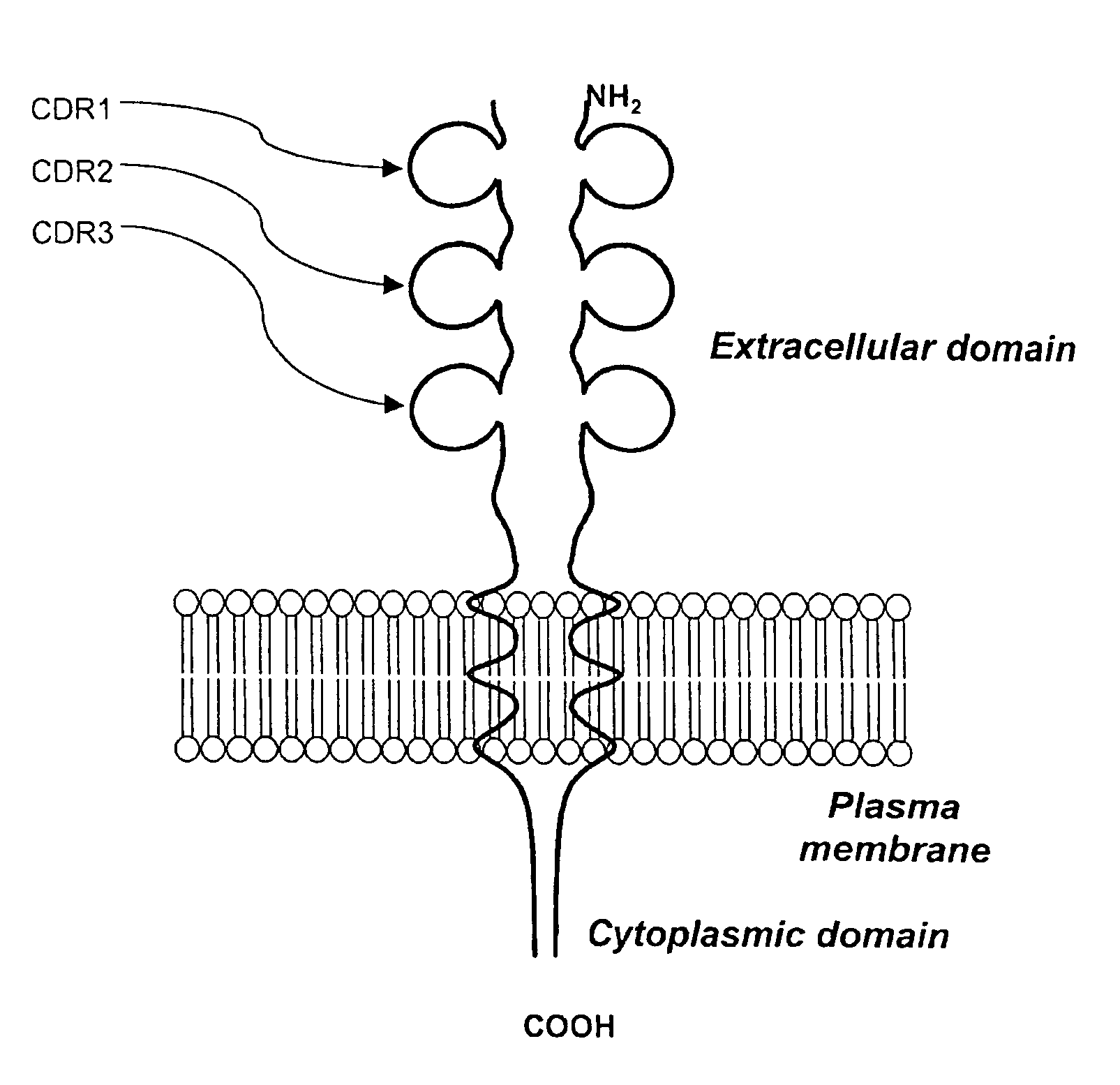
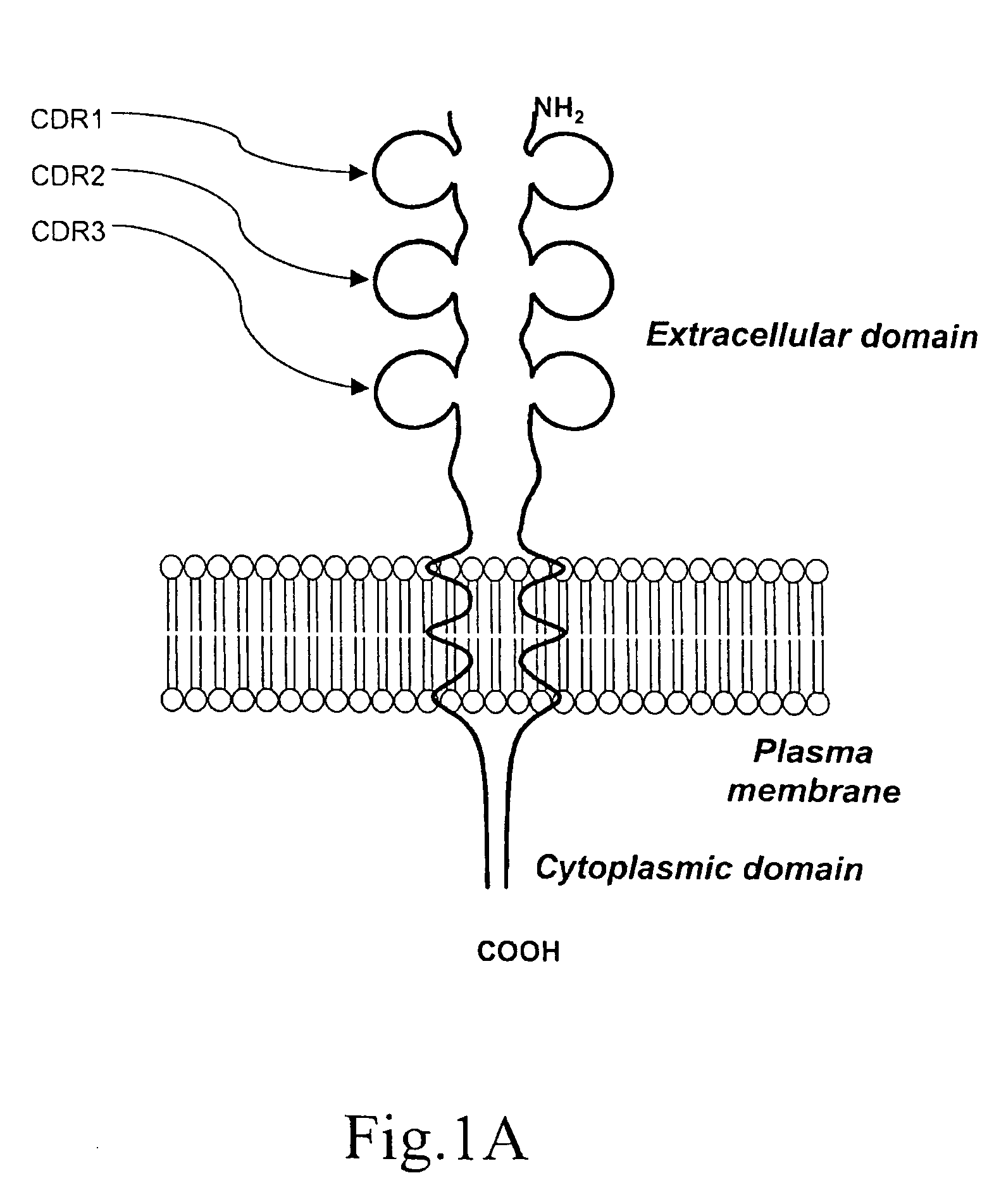
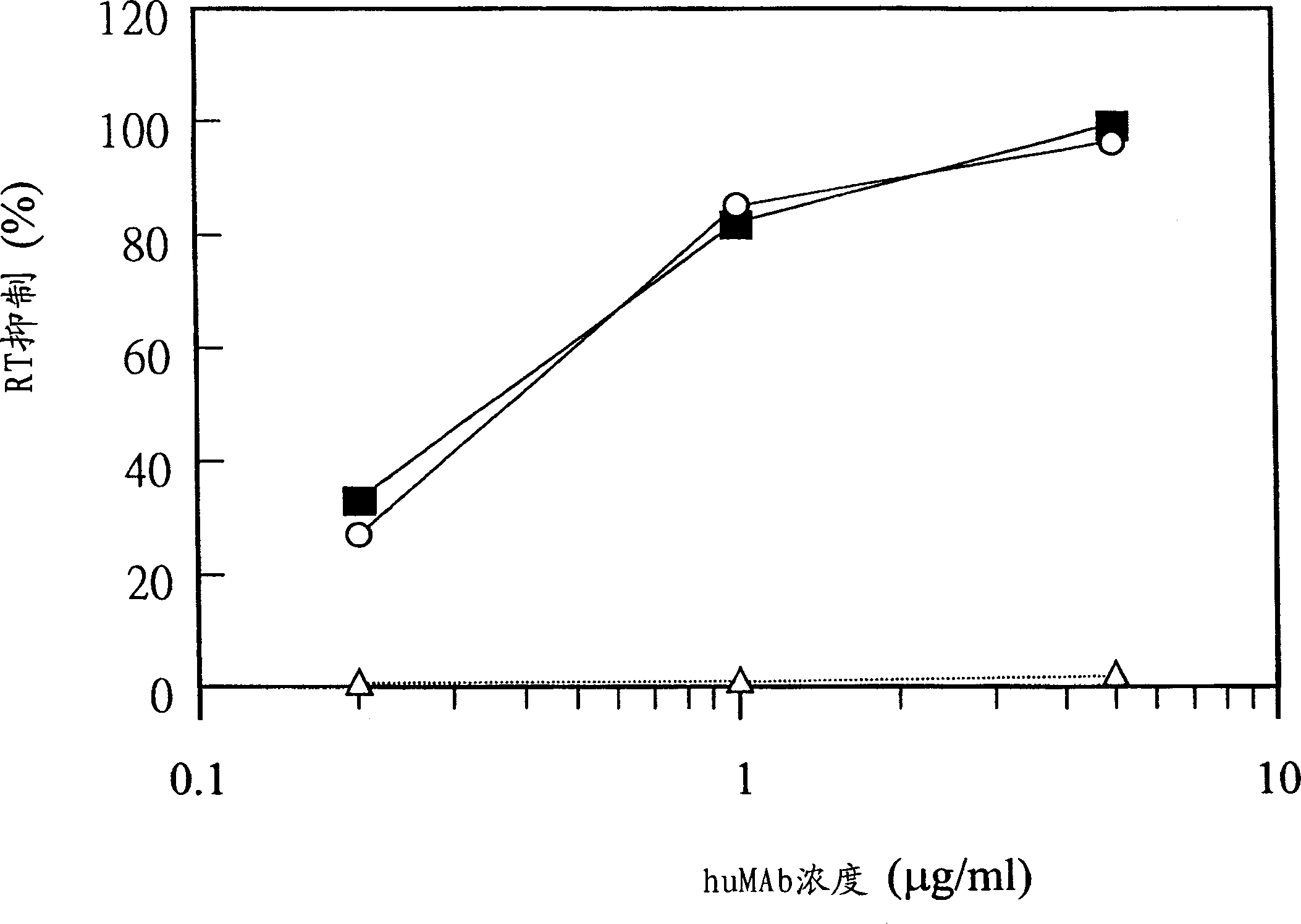
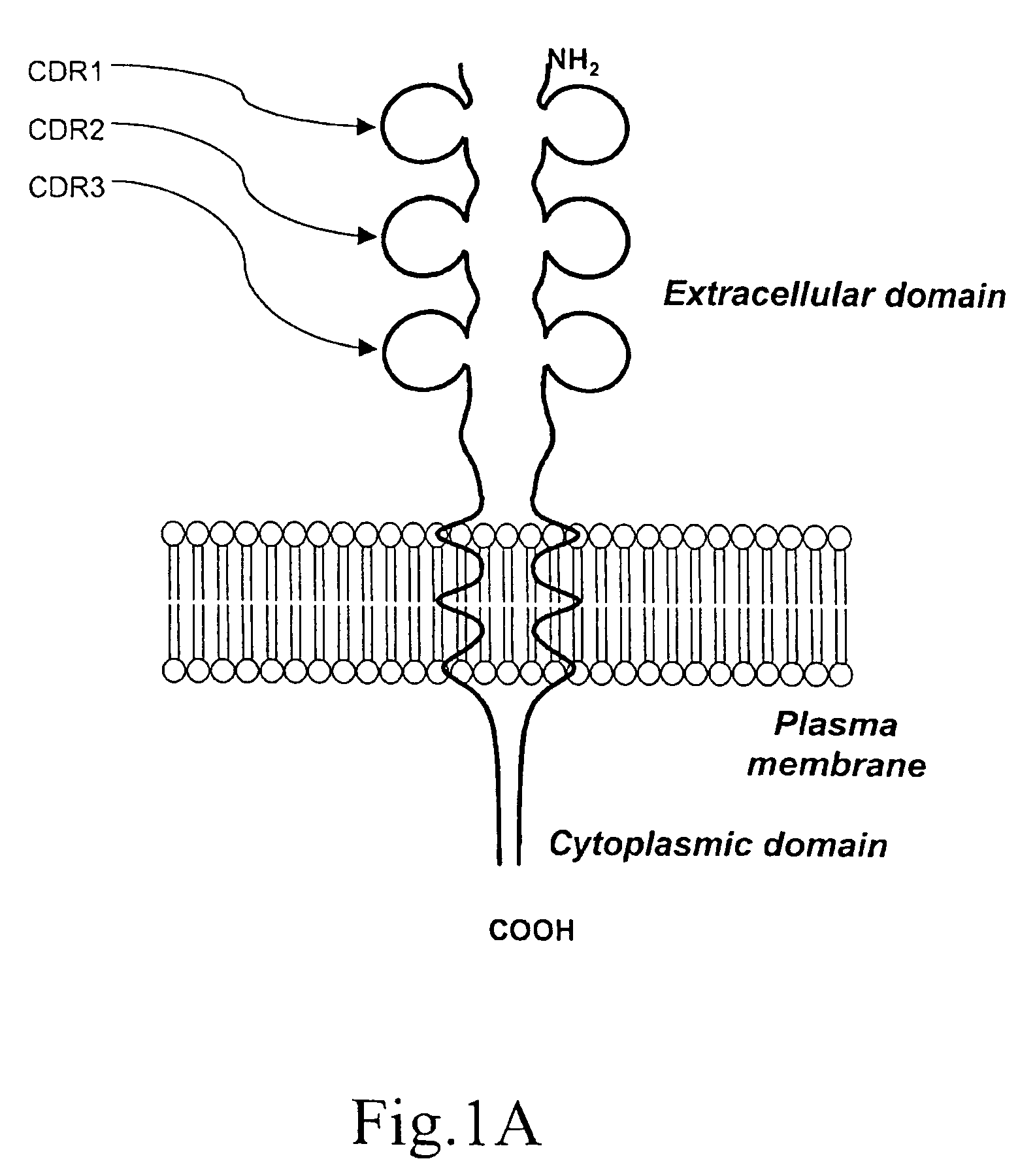
A method for obtaining agonist, antagonist and inverse agonist, to a given physiological receptor is disclosed. For the method, use is made of in silico design synthetic immunogens, which are caused to act in vitro on human lymphocyte-containing cell populations. A preferred receptor is human CD152, particularly the regions of CDRl, CDR2 and CDR3 that elicit antibodies serving as antagonist, inverse agonist and agonist, respectively. Also provided is a method in the treatment of human peripheral lymphocytes for use in the screening of CD152 ligands that yield pharmacological effects.
Owner:HUMORIGIN BIOTECH CORP
Shell-broken ganoderma spore powder and particle combination and preparation method thereof
InactiveCN107158043AKeep active ingredientsHigh content of active ingredientsPowder deliveryAntinoxious agentsRadiation hazardMaterials science
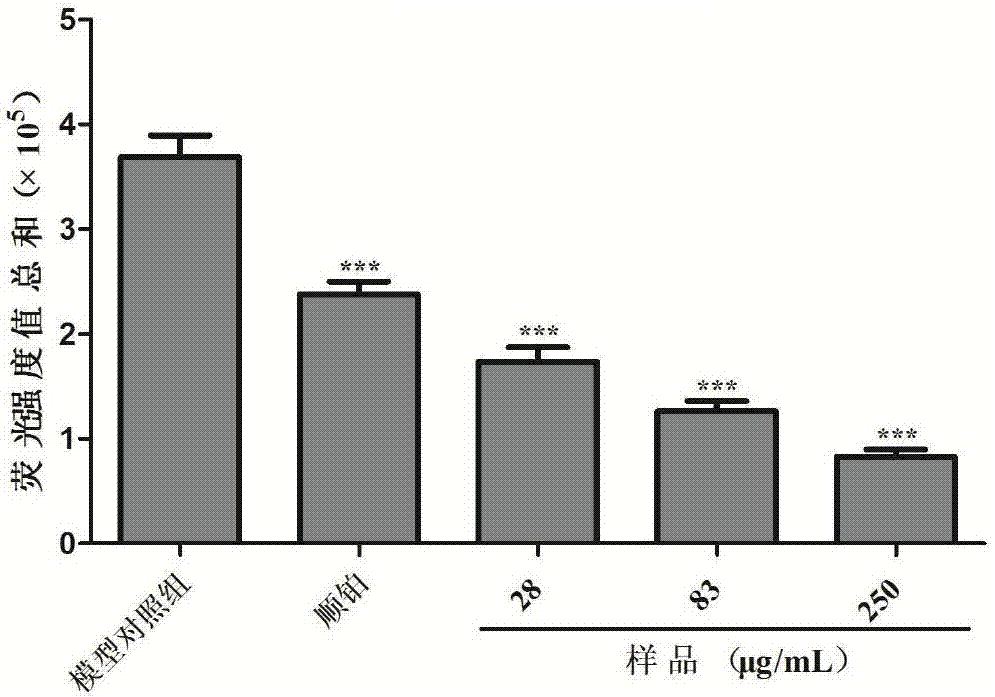
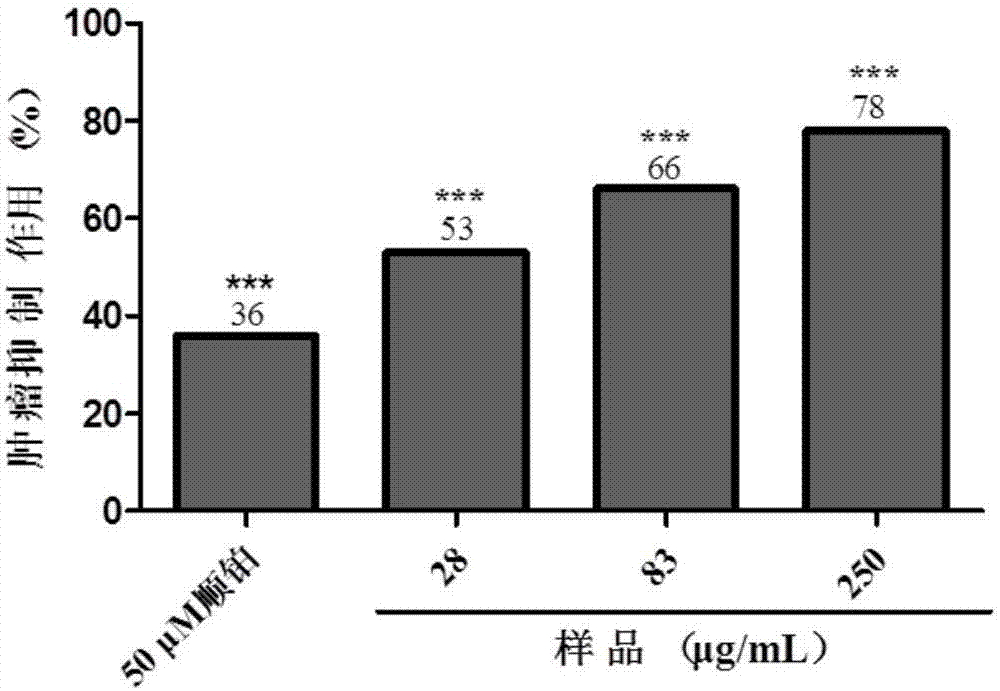
The invention discloses shell-broken ganoderma spore powder and particle combination and a preparation method thereof. Shell-broken ganoderma spore powder is extracted, centrifuged, concentrated, dried and crushed to obtain the shell-broken ganoderma spore powder and particle combination, the content of crude polysaccharide in the shell-broken ganoderma spore powder and particle combination is 10-20g / 100g, and the content of total triterpene in the shell-broken ganoderma spore powder and particle combination is 4-10g / 100g. The shell-broken ganoderma spore powder and particle combination has the advantages that immunities are enhanced, radiation hazards are reduced in an auxiliary manner, the shell-broken ganoderma spore powder is treated by the aid of a special process, the content of effective components are increased, nutrient components in the effective components can be effectively absorbed by human bodies, functions of the shell-broken ganoderma spore powder are effectively played, the shell-broken ganoderma spore powder is conveniently carried and taken, the shell-broken ganoderma spore powder and particle combination has an inhibiting effect on zebra fish human stomach cancer transplantation tumors, human lung cancer transplantation tumors and human lymphocyte cancer transplantation tumors, and the shell-broken ganoderma spore powder and particle combination has a strong inhibiting effect on the zebra fish human stomach cancer transplantation tumors and the human lymphocyte cancer transplantation tumors.
Owner:ZHEJIANG SHOUXIANGU PHARMA CO LTD +1
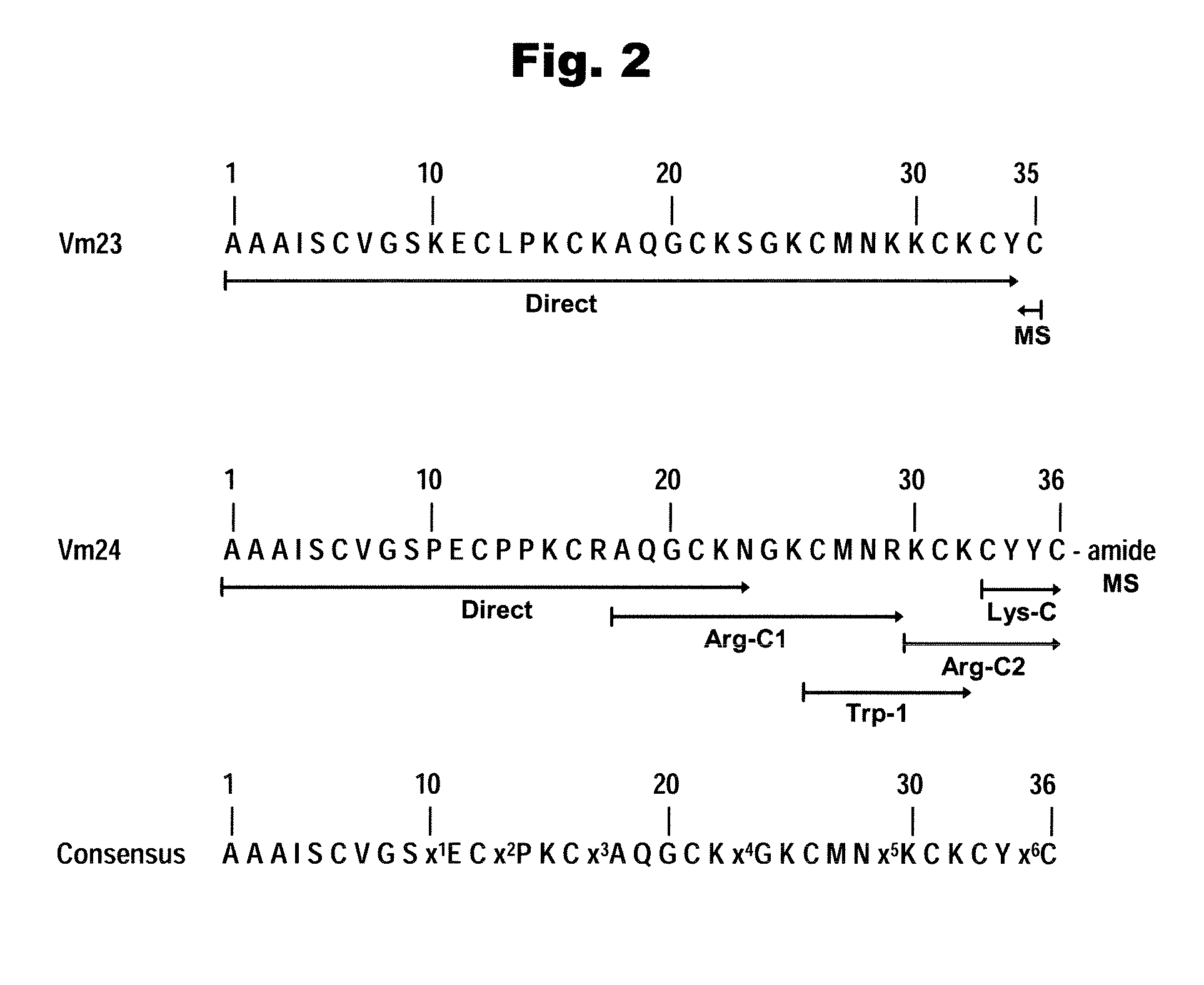
Vm23 and Vm24, two scorpion peptides that block human T-lymphocyte potassium channels (sub-type Kv1.3) w/High Selectivity and Decrease the in vivo DTH-responses in Rats
ActiveUS20110059064A1Considerable immunological responseConsiderable of immune responseSenses disorderNervous disorderChemical synthesisPotassium
Potassium channels Kv1.3 are known to be implicated in immunological diseases and graft rejections. Disclosed are peptides capable of blocking with high affinity and specificity potassium channels Kv1.3, their pharmaceutical compositions, and methods for their use to block Kv1.3 potassium channels, to treat various immunological conditions and to diagnostic applications. Methods for their chemical synthesis and correct folding are also disclosed. Exemplary peptides correspond to protein components (Vm23 and Vm24) isolated from the venom of the Mexican scorpion Vaejovis mexicanus smithi. Vm23 and Vm24 bind to hKv1.3 channels in an almost irreversible manner, showing a Kd value in the order of 3 picomolar range, when applied to human lymphocytes cultures in vitro. Vm24 was chemically synthesized and used in in vivo experiments to successfully treat sensitized rats (on the DTH-response). Neither Vm24 nor synthetic Vm24 is toxic to mice when injected at relatively high concentrations (assayed up to 10,000 micrograms per kilogram mouse body weight). These peptides (Vm24 and Vm23) and their functional equivalent analogs with at least 83% of sequence identity are lead compounds, candidates for the treatment of various immunological conditions and diagnostic applications.
Owner:UNIV NAT AUTONOMA DE MEXICO
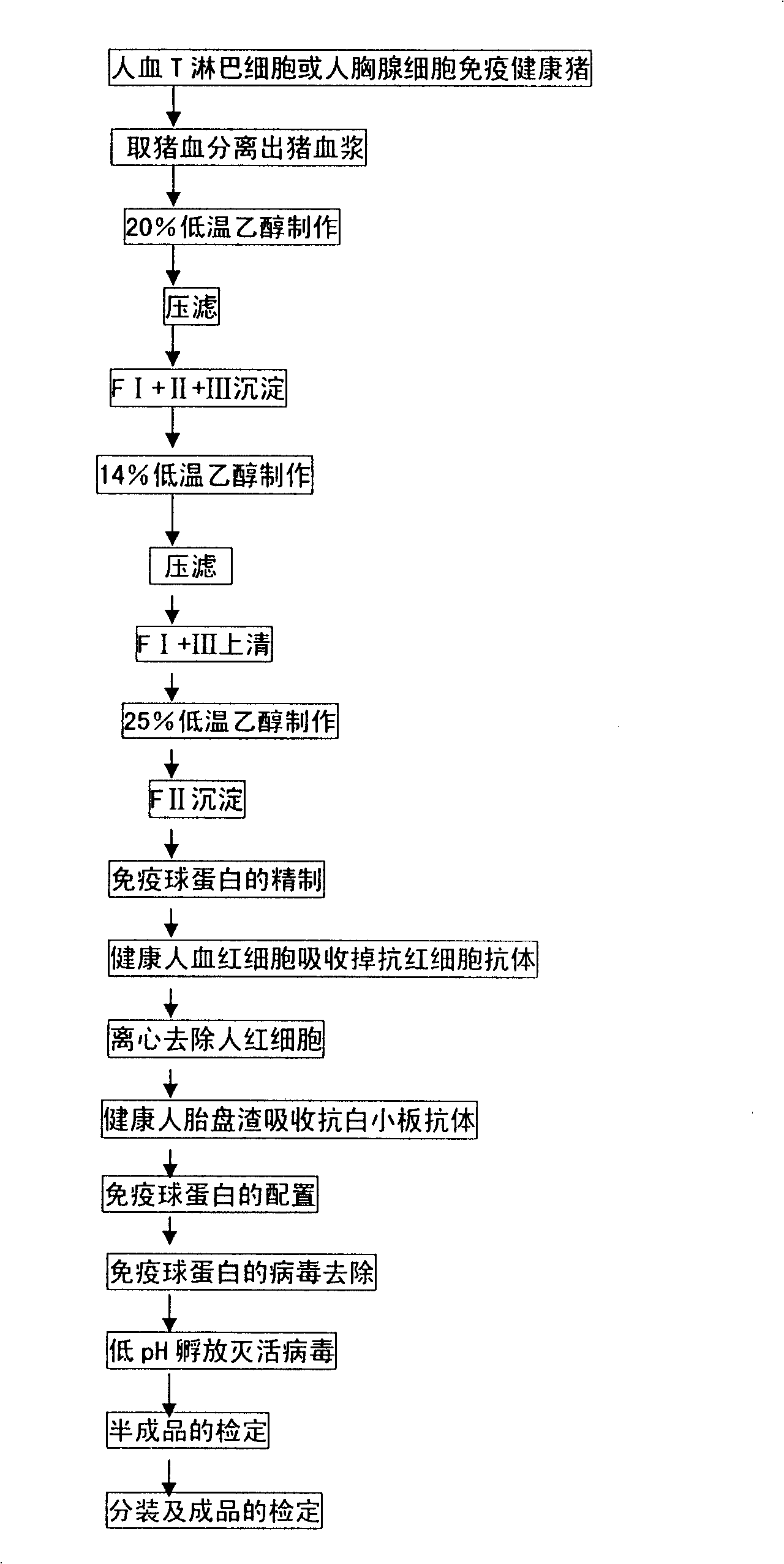
Method for preparing immune globulin against lymphocyte of human
ActiveCN101311191ALess side effectsEasy to collectAntibody ingredientsImmunoglobulinsVZV immune globulinLymphocyte immunoglobulin
The invention provides a preparation method for anti-lymphocyte immunoglobulin, which has the steps that human peripheral blood T lymphocytes or human thymocyte is used for immunizing healthy pigs for collecting and separating porcine plasma; after the manufacturing with 20 percent of ethanol, components I plus II plus III deposit is separated by a low-temperature high-speed centrifuge or a filter press; after the manufacturing with 14 percent of ethanol, supernatant fluid is separated and collected; after the manufacturing with 25 percent of ethanol and component II deposit is separated; subsequently, anti-T cell porcine immunoglobulin is obtained after the steps of refinement, configuration and virus inactivation of immunoglobulin, etc. Compared with the currently used ammonium sulfate precipitation method, the technology of the invention has the advantages of mass production, simple operation, easy command, short production period, little reagent usage, low cost, no pollution, and the like, which overcomes the disadvantages of the existing technology of hard realization of large-scale production, low yield, serious waste, environment pollution, high production cost, complex production process, various service chemical agents, long production period, high equipment occupation rate, etc.
Owner:武汉中生毓晋生物医药有限责任公司
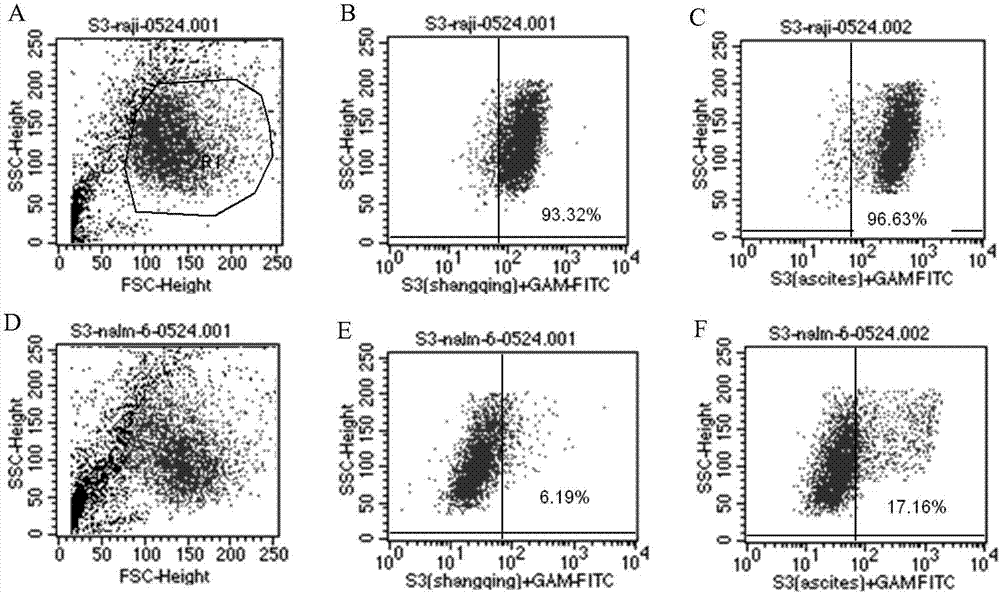
Antibody for resisting human CD79a extracellular terminal protein, coding gene and application
ActiveCN107488230AEfficient identificationHigh internalization rateOrganic active ingredientsTetrapeptide ingredientsDiseaseSequence analysis
The invention discloses an antibody for resisting human CD79a extracellular terminal protein, a coding gene and application. The antibody is characterized in that a human B cell lymphoma Raji cell line is used as an immunogen for immunizing mice; splenocyte is obtained and then is fused with a mouse myeloma cell to obtain a hybridoma cell; a monoclonal antibody for specifically aiming at the human CD79a extracellular terminal protein is obtained via screening; an amino acid sequence of the monoclonal antibody is obtained by sequence analysis; variable region sequence with a heavy chain and a light chain and CDR (Complementarity-Determining Region) sequences with light chains and heavy chains are obtained via analyzing; an chimeric antibody expressed via cloning can be used for effectively and specifically recognizing the human CD79a extracellular terminal protein; in addition, when the antibody acts on human B lymphocyte, the internalization rate is high; the antibody has a good prospect for preparing a targeted drug of targeted B lymphocyte and can be used for treating diseases associated with the B lymphocyte.
Owner:ZHEJIANG UNIV
Cell culture media containing N-acetyl-L-cysteine and uses thereof
InactiveUS6949382B2Stimulate lymphocyteKeeps antioxidant functionAnimal cellsMicrobiological testing/measurementHuman lymphocyteCysteine thiolate
The present invention provides cell culture media and methods useful for determining levels of intracellular function of glutathione or cysteine and for providing biochemical analysis of antioxidant function in human lymphocytes.
Owner:RES DEVMENT FOUND
Gene transfer in human lymphocytes using retroviral scFv cell targeting
Owner:BUNDESREPUBLIK DEUTSCHLAND
High efficiency fetal calf serum substitute
InactiveCN104830765AIncrease supplyAvoid the risk of spreading infectious diseasesBlood/immune system cellsCholesterolAllergy
The invention relates to a high efficiency fetal calf serum substitute. The high efficiency fetal calf serum substitute is composed of recombinant human albumin, recombinant human transferrin, galactose, lipid, a trace element compound and vitamins, a weight ratio of the recombinant human albumin to the recombinant human transferrin to the galactose is 2:50:(15-25), and the lipid is a cholesterol, linoleic acid and linolenic acid mixture. The fetal calf serum substitute provided by the invention avoids infectious disease propagation risk and foreign protein induced allergy, increases the in vitro culture survival rate and the increment multiple of human lymphocytes to 96% and 28 respectively, improves the supply of the lymphocytes, reduces the culture cost, and facilitates large scale use.
Owner:上海泰坦科技股份有限公司
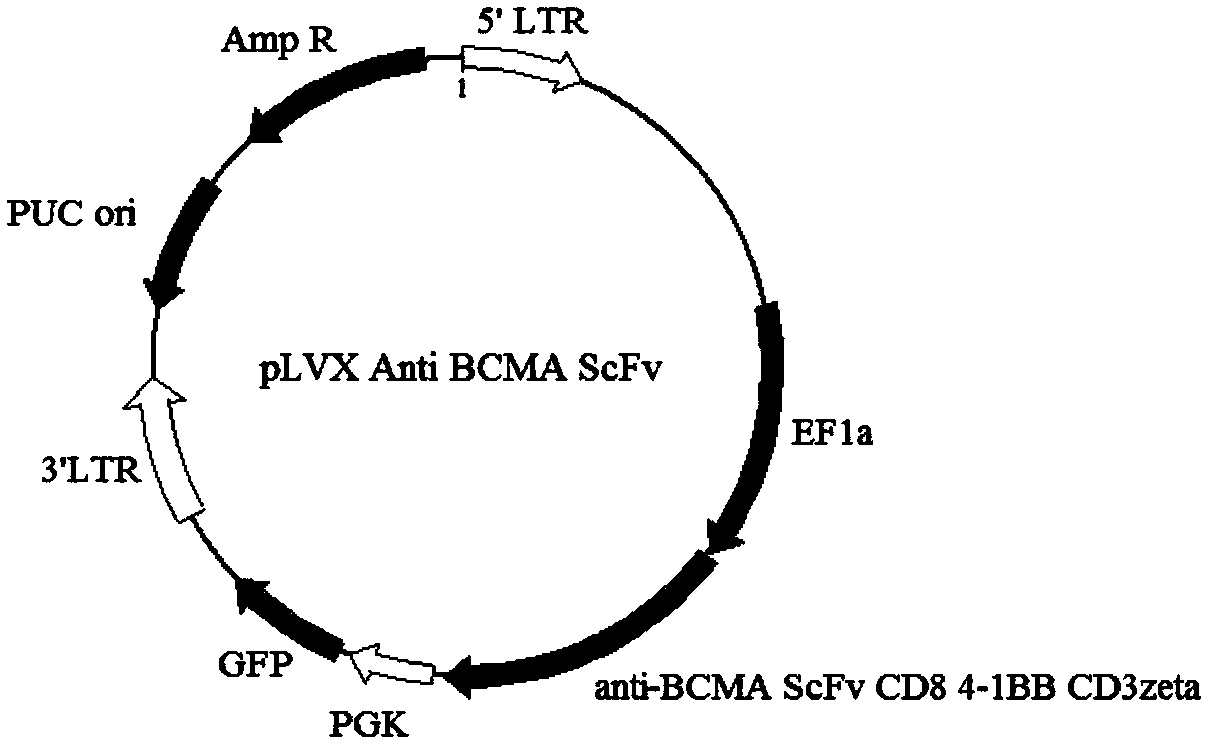
BCMA specific chimeric antigen receptor T cell and application thereof
ActiveCN109748968AGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSingle-Chain AntibodiesT cell
The invention discloses a specific chimeric antigen receptor (anti-BCMA scFv-CD8a-41BB-CD3 zeta) for human BCMA and application thereof. The chimeric antigen receptor is formed by a single-chain-antibody anti-BCMA scFv, a hinge region, a transmembrane region and an intracellular region in series. The chimeric antigen receptor is used for modifying human T lymphocyte, and the modified T lymphocyteis used for prevention and treatment of surface BCMA positive tumor and preparing of antitumor medicines.
Owner:广州因明生物医药科技股份有限公司
Quality-control product of drying human lymphocyte surface antigen and method for making same
InactiveCN101363846AImprove biological activityLong validity periodBiological testingHuman lymphocyteImmunofluorescence
The invention relates to a product for controlling the quality of lyophilized human lymphocyte surface antigens, which is a grey-white solid prepared by lyophilizing a cell suspension having a cell concentration of 400,000-600,000 / mL and containing lyophilized human lymphocytes immobilized by a neutral buffer formaldehyde solution and a protective agent. The mark values respectively are: CD<3> of 37.25-69.1%, CD<4> of 17.9-33.7%, CD<8> of 12.4-23.9%, and the ratio of CD<4> / CD<8> of 0.98-1.94. The invention also provides the preparation method of the quality control product. The product has the characteristics of good antigen storability and stable antigenicity, and can be used for the quality control reagent for detecting the lymphocyte surface mark antigenicity (CD series) by adopting the cell analyzer method, the immunofluorescence method, the immunoenzyme method, a SPA (Staphylococcus aureus A protein) rosettes method and the like.
Owner:甘肃省医学科学研究院
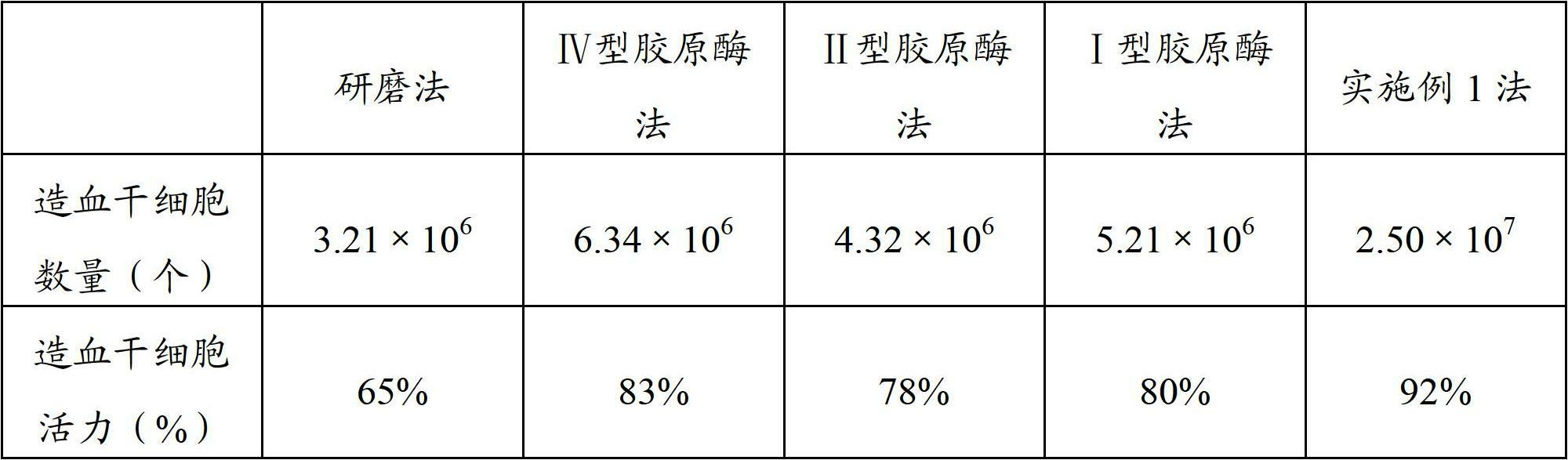
Placental hematopoietic stem cell and preparation method thereof and placental hematopoietic stem cell injection
ActiveCN102660499AIncrease the number ofHigh activityPharmaceutical delivery mechanismMammal material medical ingredientsHuman lymphocyteCentrifugation
The invention relates to the field of biology and discloses a placental hematopoietic stem cell and a preparation method thereof and a placental hematopoietic stem cell injection. The preparation method comprises the following steps of: after pretreating a placenta, digesting the treated placenta by using collagenase type IV digestive juice and collagenase type II digestive juice, then washing and filtering and respectively collecting first filtrate and first residue; digesting the first filter residue by using collagenase type I digestive juice and the collagenase type II digestive juice in the first step and then washing, filtering and collecting second filtrate; combining the filtrate from two times, centrifuging and discarding supernate, re-suspending and precipitating; adding a resuspension solution into a lymphocytes separation medium; and carrying out density gradient centrifugation, collecting a buffy coat cell solution on an intermediate layer and centrifuging and discarding supernate to obtain the placental hematopoietic stem cell. According to the placental hematopoietic stem cell and the preparation method thereof disclosed by the invention, collagenase type IV and collagenase type II are combined, collagenase type I and collagenase type II are combined and the hematopoietic stem cell is prepared by fully digesting the placenta according to the characteristics of different collagenases, so that the quantity and the vitality of the prepared hematopoietic stem cell are improved.
Owner:BOYALIFE
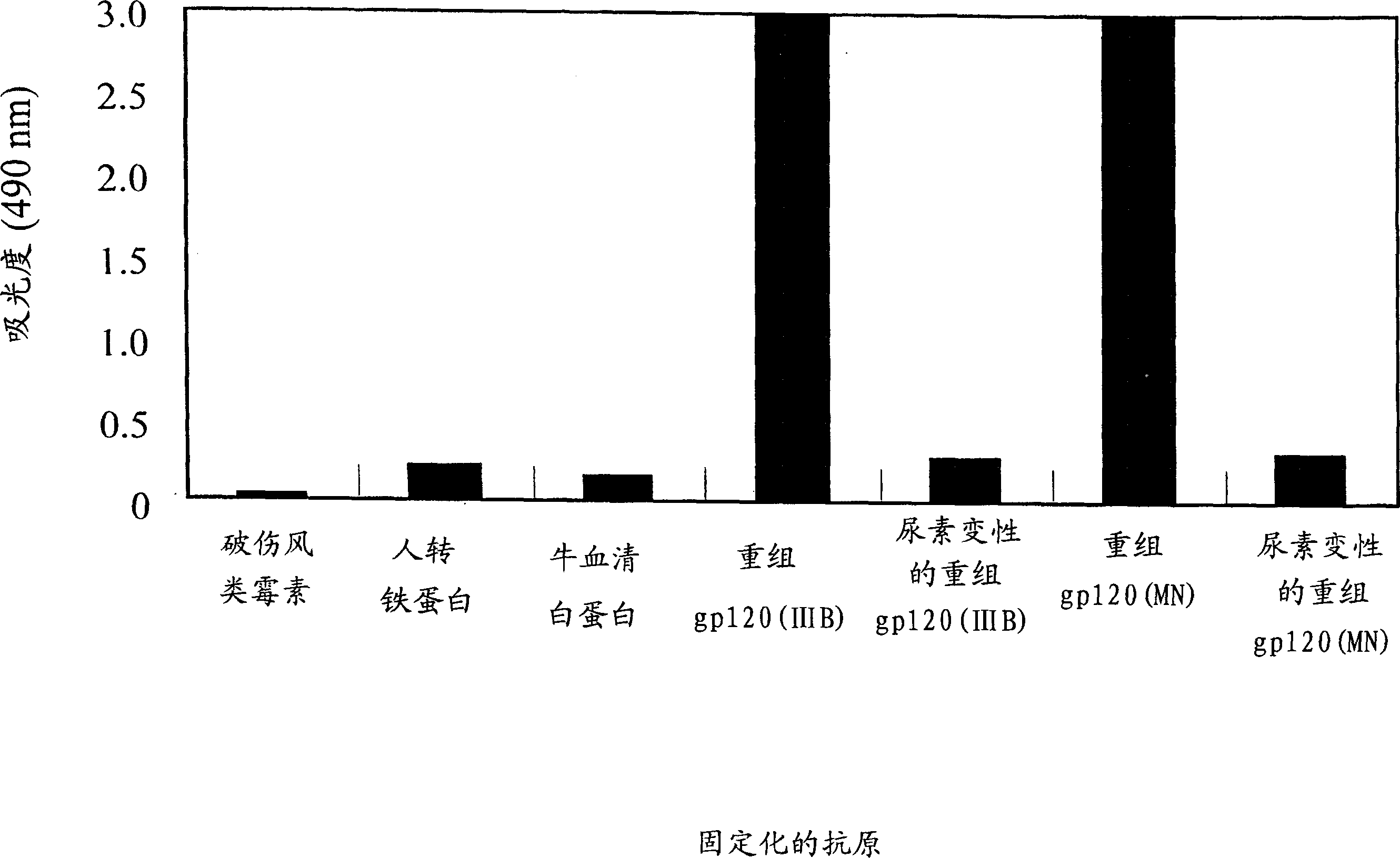
Preparation of full-length human antibody
InactiveCN1626669AImmunoglobulins against animals/humansImmunoglobulins against virusesAntigenHuman lymphocyte
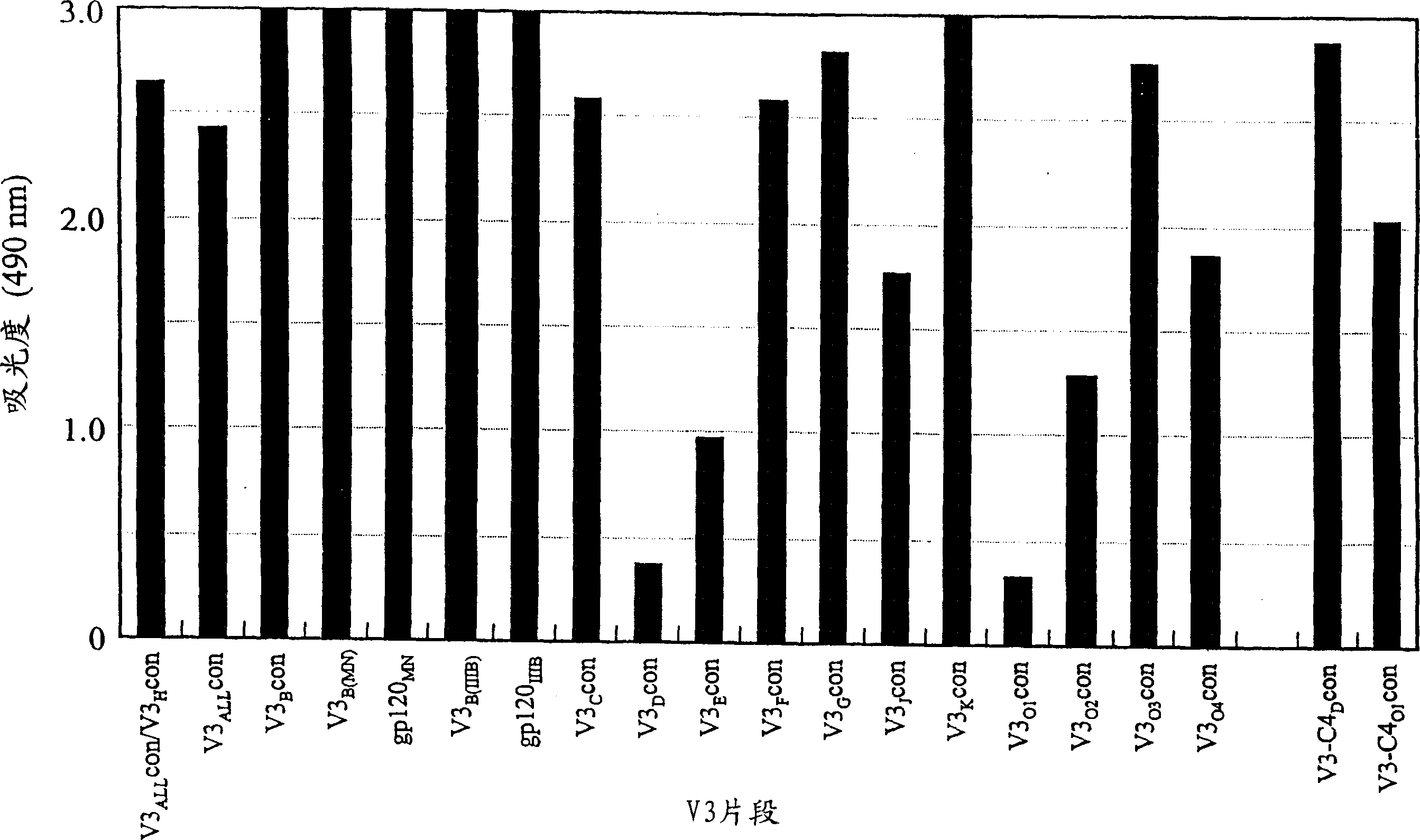
The present invention provides a method for producing a fully humanized antibody that recognizes a predetermined antigen and does not rely on a human donor who has been exposed to the antigen. To achieve this, lymphocytes from natural human donors are immunized in vitro with an antigen of interest, and antibody-producing cells directed against said antigen are then identified. Because lymphocytes immunize in vitro rather than in vivo, it is possible to modulate the antigen or antigen fragment recognized by the antibody. A preferred antigen is an HIV gp120 peptide, especially the co-receptor binding region of gp120.
Owner:CCL HLDG
Method for producing human antibodies to human CD152 with properties of agonist, antagonist, or inverse agonist
ActiveUS7494779B2Simple methodReduce allergic reactionsAnimal cellsSugar derivativesSynthetic ImmunogensHuman lymphocyte
A method for obtaining agonist, antagonist and inverse agonist, to a given physiological receptor is disclosed. For the method, use is made of in silico design synthetic immunogens, which are caused to act in vitro on human lymphocyte-containing cell populations. A preferred receptor is human CD152, particularly the regions of CDR1, CDR2 and CDR3 that elicit antibodies serving as antagonist, inverse agonist and agonist, respectively. Also provided is a method in the treatment of human peripheral lymphocytes for use in the screening of CD152 ligands that yield pharmacological effects.
Owner:HUMORIGIN BIOTECH CORP
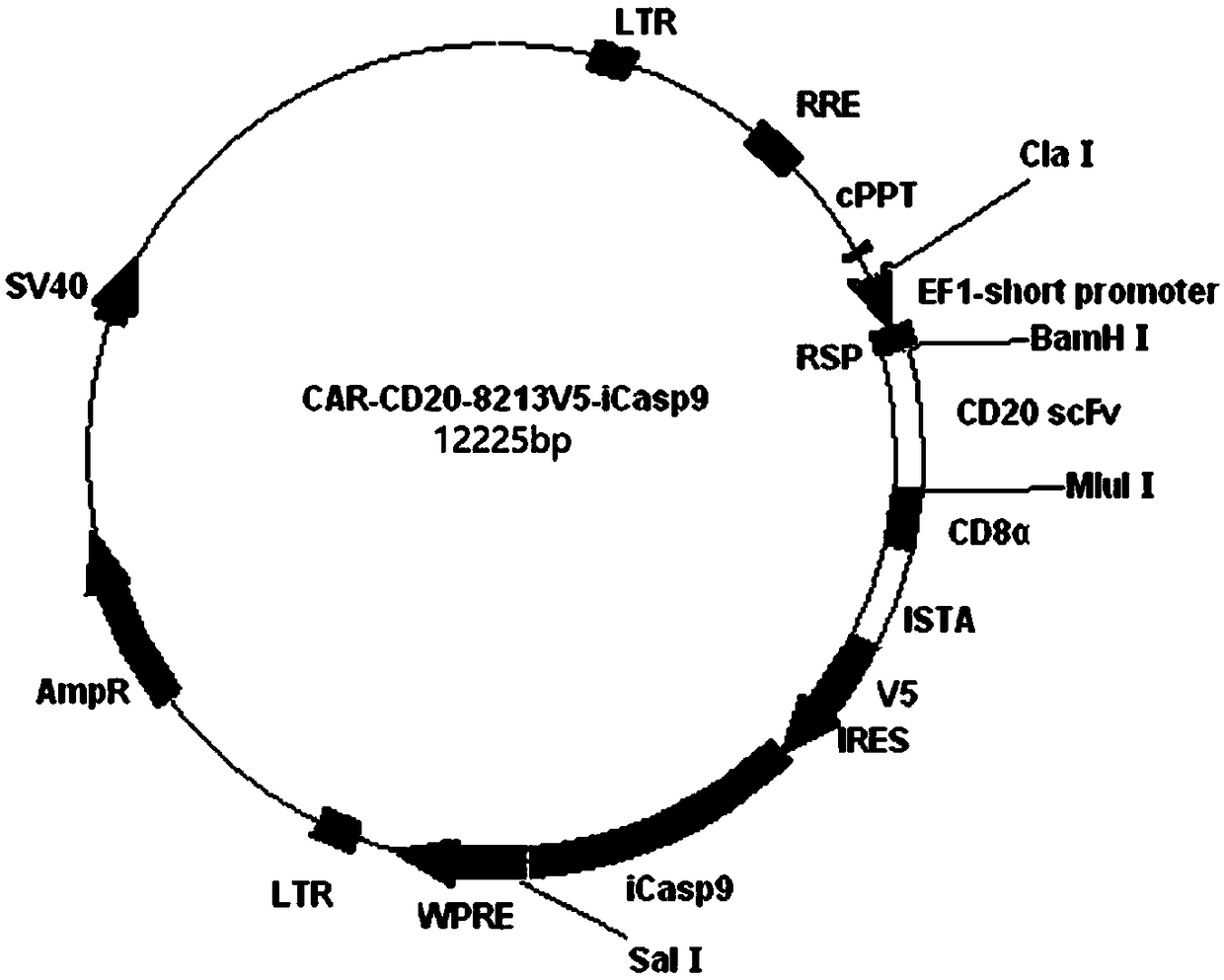
CD20 chimeric antigen receptor T lymphocyte with apoptosis-inducing capability and carrying detecting tag and application of CD20 chimeric antigen receptor T lymphocyte
InactiveCN109136244AHigh affinitySafe immunotherapyHydrolasesAntibody mimetics/scaffoldsCD20Single-Chain Antibodies
The invention provides a nucleic acid which can be expressed on a CD20 chimeric antigen receptor of the surface of a human lymphocyte and a tag fusion protein in a coded manner. The chimeric antigen receptor comprises an extracellular binding region, a transmembrane domain and an intracellular signal region which are connected sequentially and a tag protein which can be used for detecting CAR-T cells in a tracking manner, wherein the extracellular binding region comprises a single-chain antibody scFv(CD20) of the CD20 extracellular region specifically; and the nucleic acid also expresses a protein capable of inducing apoptosis in the human lymphocyte in a coding manner, the chimeric antigen receptor modified T lymphocyte apoptosis can be induced at any time, and side effects after immunotherapy are eliminated. The invention further provides T lymphocyte modified by protein expressed by the nucleic acid, the chimeric antigen receptor which can be directly detected is expressed on the surface, and the protein capable of inducing apoptosis is expressed intracellularly.
Owner:河北璋达生物科技有限公司
Method for preparing dendritic cell vaccine
InactiveCN102847145AIncrease multipleStrong specific lethalityBlood/immune system cellsAntibody medical ingredientsLiquid ChangeHuman lymphocyte
The invention relates to the technical field of biology, in particular to a method for preparing dendritic cell vaccine. The method is characterized by comprising the following preparation steps: (1) taking autologous blood of a patient, and separating red blood cells, mononuclear cells and plasma from separating medium of human lymphocyte; (2) adjusting concentration of the mononuclear cells; (3) adding the solution into a six-pore plate and performing adherence for 16 hours; (4) sucking and discharging non-adherent cells on the upper layer of each pore of the six-pore plate; (5) adding 3ml of dendritic cell (DC) culture medium, placing the solution in a carbon dioxide cultivating box with the concentration of carbon dioxide of 5% at the temperature of 37 DEG C to perform cultivation for 2-3 days; (6) performing half-quantity liquid changing; (7) enabling the concentration of the autologous tumor antigen in each pore of the six-pore plate to be 20 mu g / ml by loading the autologous tumor antigen in the sixth day; and (8) detecting the quantity and maturity of the matured DC cells after 24 hours. Compared with the prior art, the number of the matured DC cells at least reaches 1*107, and the maturity is larger than 85%.
Owner:FUDAN UNIV
Quality control quality of freeze-dried human lymphocyte surface antigen and method for preparing same
InactiveCN101358968AImprove biological activityLong validity periodBiological testingSurface markerHuman lymphocyte
The present invention relates to a freeze-dry quality control material of human lymphocyte surface antigens. The quality control material is cell suspension which consists of human lymphocytes solidified by a fixative and a protective agent and has a cell concentration of 4,000,000 to 6,000,000 per milliliter, and appears as off-white flocculent solid after being dried; and the marked values are 37.2 to 69.1 percent of CD3<+>, 17.9 to 33.7 percent of CD4<+>, 12.4 to 23.9 percent of CD8<+>, and 0.98 to 1.94 percent of CD4<+> / CD8<+>. Simultaneously, the invention also discloses a preparation method of the quality control material. The invention has the advantages of excellent antigen conservation and stable antigenicity, and can be used as the quality control agent which is used for detecting the antigenicity of cell surface markers (CD Series) in such method as the flow cytometry, the immunofluorescence, the mmunoenzyme, SPA (staphylococcal protein A) rosette and the like.
Owner:甘肃省医学科学研究院
Leukocyte extract and preparation method and application thereof
InactiveCN108567719AImprove securityHigh yieldCosmetic preparationsToilet preparationsPeripheral blood mononuclear cellWhite blood cell
The invention discloses a preparation method of leukocyte extract. Neonate cord blood is used directly as a raw material subjected to human lymphocyte separation density-gradient centrifugation to obtain PBMCs (peripheral blood mononuclear cells); amplification culture is performed via cord serum separated from the cord blood; centrifugal separating is performed to obtain supernate; leukocyte extract is acquired by ultra-filtration. The preparation method has no need for bovine serum, no heterogeneous animal proteins or stimulating factors are involved, and safety of application in human bodies is good. The leukocyte extract prepared via the method has rich natural human cellular factors, wherein bFGF (basic fibroblast growth factor) reaches 60 pg / ml and above, the yield is high, and the production cost is low. The invention also provides application of the leukocyte extract in the activation and restoration of cells. The leukocyte extract may be added to cosmetics to provide synergy with other components in cosmetic formulations; the leukocyte extract can cooperate to improve cell mitochondrial functionality, complete nutrients for cell metabolism are provided, and the cell activating function of the leukocyte extract is significantly improved.
Owner:上海蕙禾生物科技事务所
Acyl-hydrazone and oxadiazole compounds, pharmaceutical compositions containing the same and uses thereof
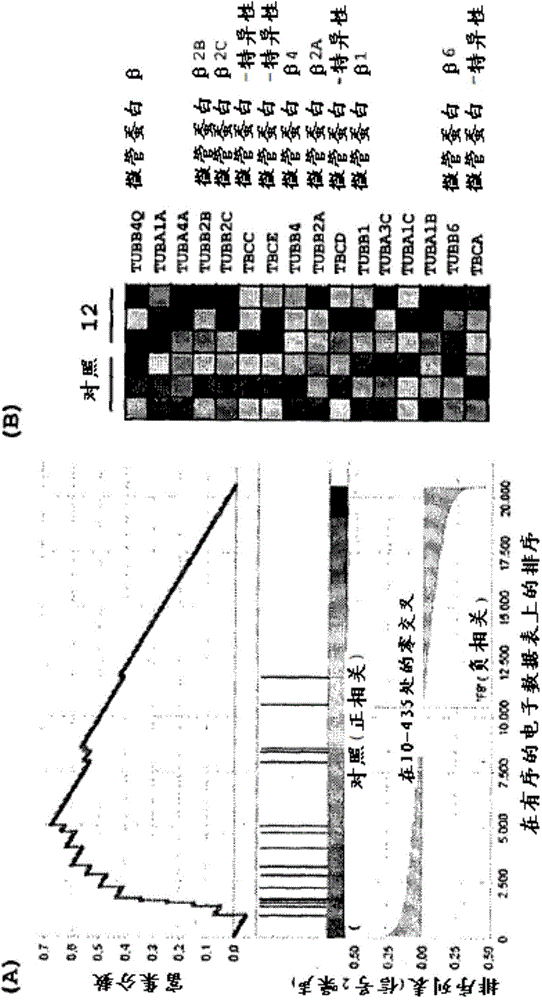
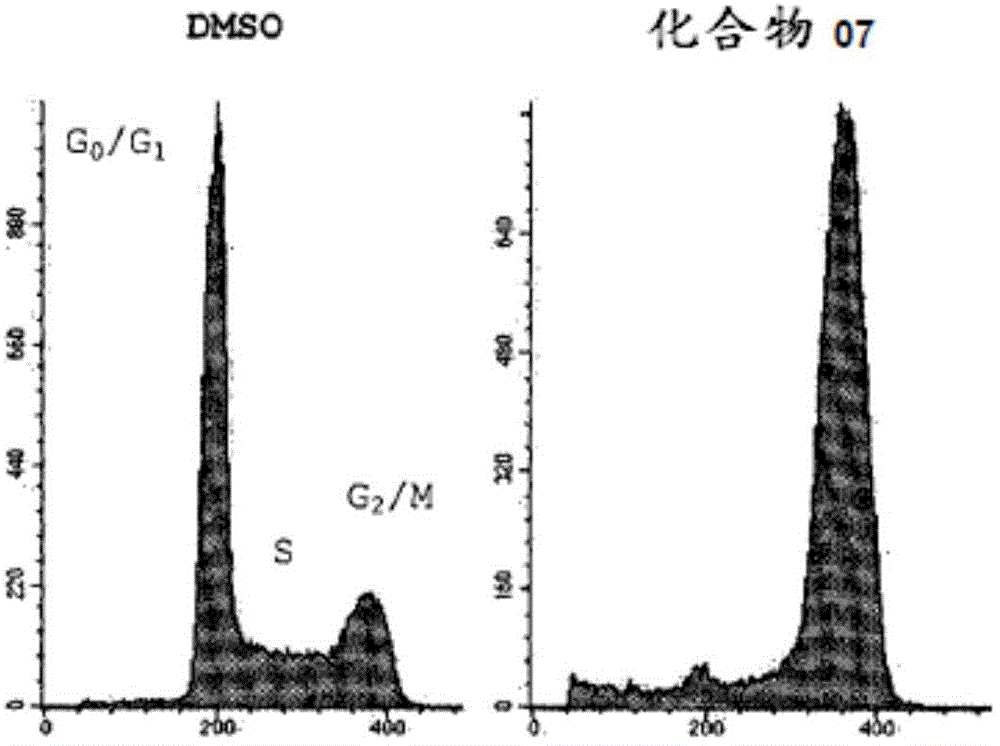
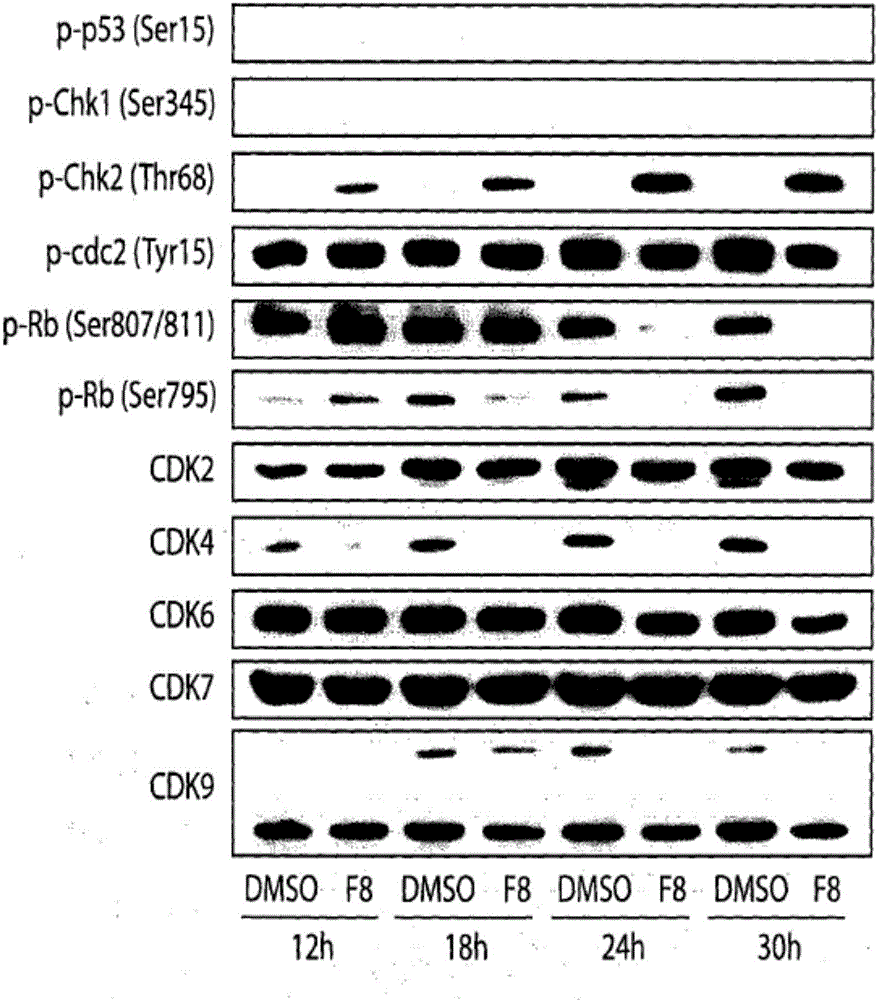
The present invention relates to acyl-hydrazone compounds, in particular 3,4,5-trimethoxyphenyl-hydrazide derivatives, as well as the oxadiazole analogs thereof and other similar compounds, and to the pharmaceutical use of the same for the treatment of various diseases associated with cell proliferation, such as leukemias, including acute lymphoblastic leukemia (ALL), tumours and inflammation. Acyl-hydrazones have been obtained having activity similar to that of the compound used as a standard in experiments (colchicine). The greater selectivity of the compounds according to the invention is an important feature, associated with fewer side effects than the pharmaceuticals used at present in clinical treatments. The synthetised acyl-hydrazones, more particularly the compounds 02 and 07, exhibited important anti-leukemic activity, which suggests 02 and 07 as candidates to pharmaceutical prototypes, or to pharmaceuticals for the treatment of leukemias, in particular acute lymphoblastic leukemia (ALL), tumours and other proliferative diseases, such as inflammation. The action mechanism of the most active compounds was determined by using DNA microarrays and subsequent tests indicated by the chip, besides selectivity studies in healthy human lymphocytes.
Owner:UNIVERSIDADE FEDERAL DE SANTA CATARINA
Animal origin-free culture medium capable of efficiently amplifying human lymphocytes
InactiveCN105602898AGood antitumor activityCulture processBlood/immune system cellsLipid formationPositive control
The invention discloses an animal origin-free culture medium capable of efficiently amplifying human lymphocytes. The animal origin-free culture medium comprises a base culture medium, a serum replacement and an amplification accelerant as well as lipids, trace element compounds and vitamins, wherein the serum replacement comprises recombinant human serum albumin, recombinant human transferrin, recombinant human insulin, lipids, trace element compounds and vitamins; the lipids include cholesterol, linoleic acid and linolenic acid; the trace element compounds include ferric nitrate, sodium selenite, copper sulfate and zinc sulfate; and the amplification accelerant is compound Ce phaloziellin N. The culture medium disclosed by the invention can remarkably increase the proliferation multiple of lymphocytes to promote the proliferation of lymphocytes, wherein the effect is basically consistent with that of a positive control group and better than that of a culture medium without amplification accelerant; and moreover, the culture medium can remarkably improve the tumor killing activity of lymphocytes, wherein the effect is better than that of the culture medium without amplification accelerant.
Owner:谷超
Freeze-dried human lymphocyte CD4 surface antigen quality control material and preparation method thereof
InactiveCN107576790AMeet needsSave on shipping costsDepsipeptidesBlood/immune system cellsHuman lymphocyteFreeze-drying
The invention aims to provide a freeze-dried human lymphocyte CD4 surface antigen quality control material and a preparation method thereof. The reference substance is subjected to surface or intracellular specific antibody labeling by peripheral lymphocytes and can be applied to direct detection of flow cytometry. Moreover, in the preparation process of the reference substance, the concentrationof specific cells is calibrated after positive cell counting of the specific antigen, so the reference substance can provide reference for relative counting detection of the flow cytometry and can also serve as a reference substance or a counting reference in absolute counting detection, particularly applied to reference and calibration of flow cytometry calculated by a volumetric method at different time, different equipment and different personnel.
Owner:巴德生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com