CD20 chimeric antigen receptor T lymphocyte with apoptosis-inducing capability and carrying detecting tag and application of CD20 chimeric antigen receptor T lymphocyte
A technology of chimeric antigen receptors and lymphocytes, applied in the direction of receptors/cell surface antigens/cell surface determinants, genetically modified cells, cells modified by introducing foreign genetic materials, etc., can solve the problem of drug resistance in patients And other problems, to achieve the effect of increasing the safety switch sequence and strong killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
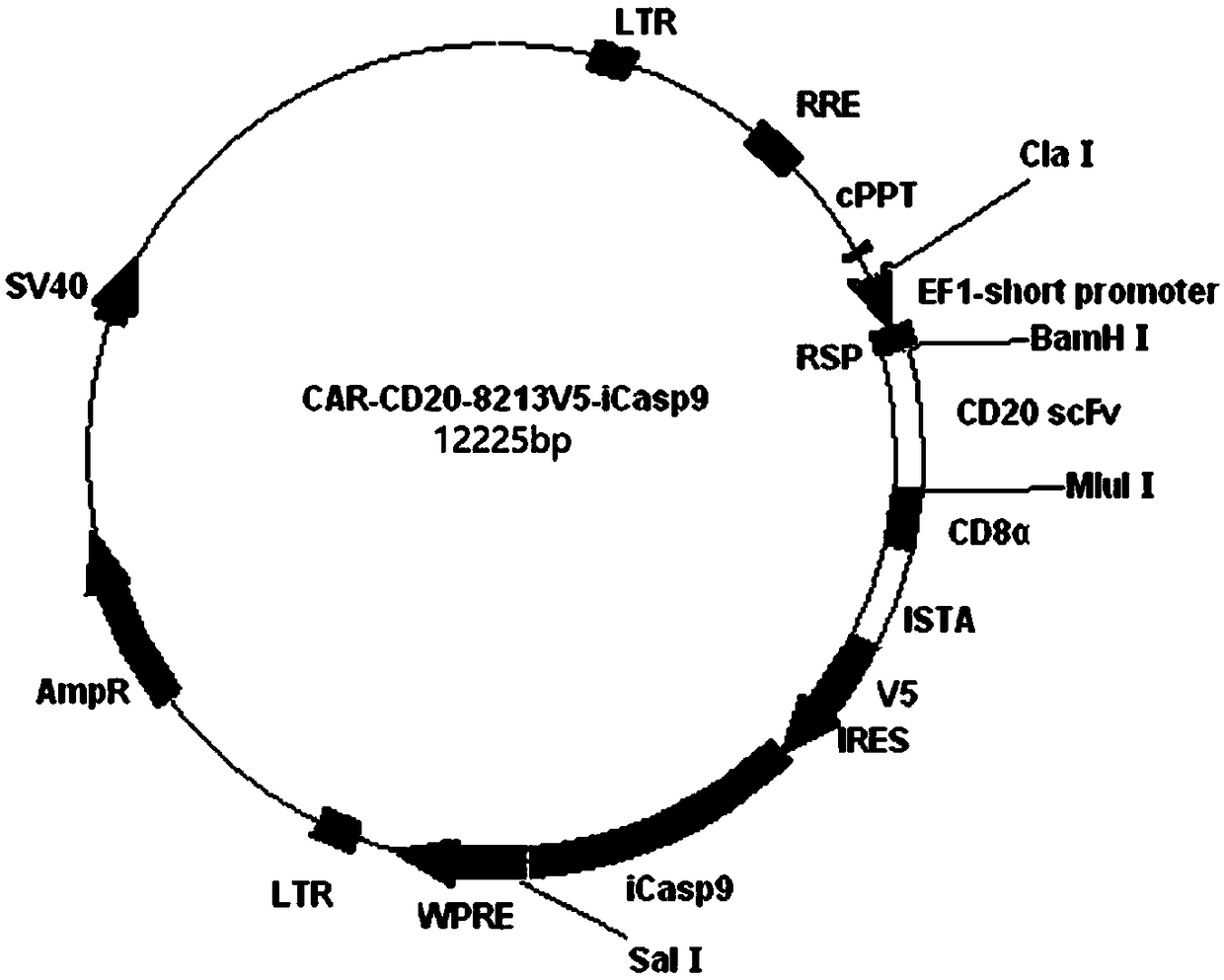
[0064] Example 1 Construction of lentiviral plasmid pWPTR-scFv(CD20)-CD8α-CD28-CD137-CD3ζ-V5-IRES-FKBP12(F36V)-Caspase9
[0065] The complete gene sequence scFv(CD20)-CD8α-CD28-CD137-CD3ζ-V5-IRES-FKBP12(F36V)-Caspase9 was synthesized by Wuhan Jinkairui Bioengineering Co., Ltd., cloned in the pUC57 plasmid, named pUC57-CAR20-V5- iCasp9 contains BamHI and SalI restriction sites at both ends. Which contains the sequence of the third generation CAR-CD20 fusion gene sequence (scFv (CD20) and intracellular signal transduction functional region sequence fusion gene sequence connected in sequence, scFv (CD20) contains the heavy chain variable region VH, Linker (Gly4er) 3 Sequence, light chain variable region VL, its nucleotide sequence is shown in Seq ID No. 1 in the sequence listing, and the amino acid sequence is shown in Seq ID No. 2 in the sequence listing. The intracellular signal transduction functional region sequence includes CD8α The hinge region and transmembrane region sequen...
Embodiment 2
[0068] Example 2 Packaging and concentration of chimeric antigen receptor lentivirus
[0069] The empty vector plasmid pWPT-GFP-V5 and the chimeric antigen receptor lentiviral expression plasmid CAR-CD20-8213V5-iCasp9 and the auxiliary plasmids psPAX2 and pMD2.G were extracted with a small plasmid extraction kit, and the plasmid concentration was determined by spectrophotometer. Inoculate 293T cells in a 10cm culture dish. When the cells are 60-80% full, use Lipofectamine with the expression plasmid and the two helper plasmids at a mass ratio of 4:2:1. TM 2000 Transfection Reagent co-transfects 293T packaging cells. Collect the virus supernatant in an EP tube at 48h and 72h after transfection, centrifuge at 2000g for 10min at 4°C, transfer the supernatant to a new EP tube, filter the virus supernatant with a 4.5μm filter; the filtered virus supernatant and 5× Mix PEG8000-NaCl in a 4:1 volume ratio, let stand at 4°C for 2h, then centrifuge at 10000g for 20min at 4°C, discard th...
Embodiment 3
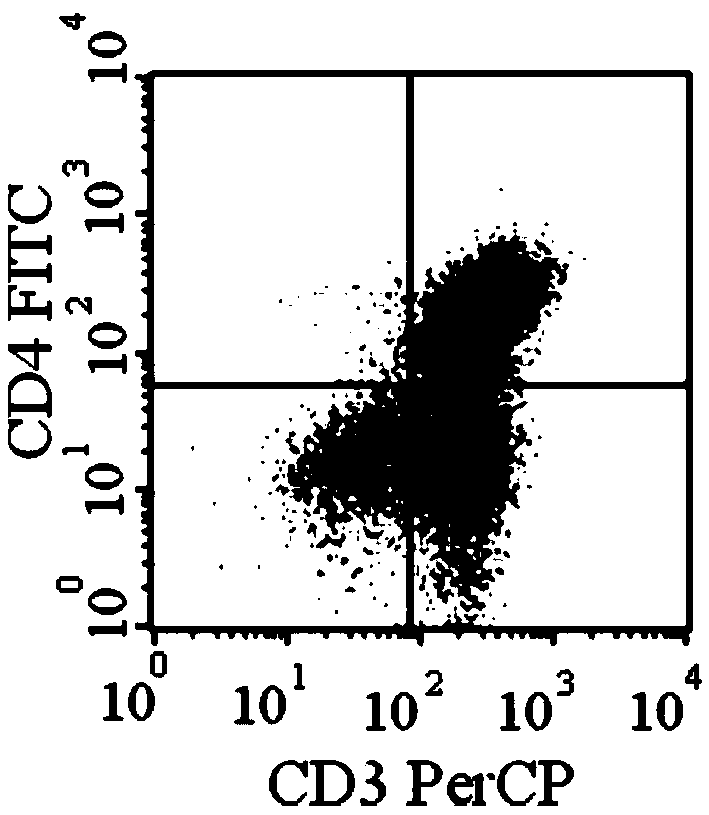
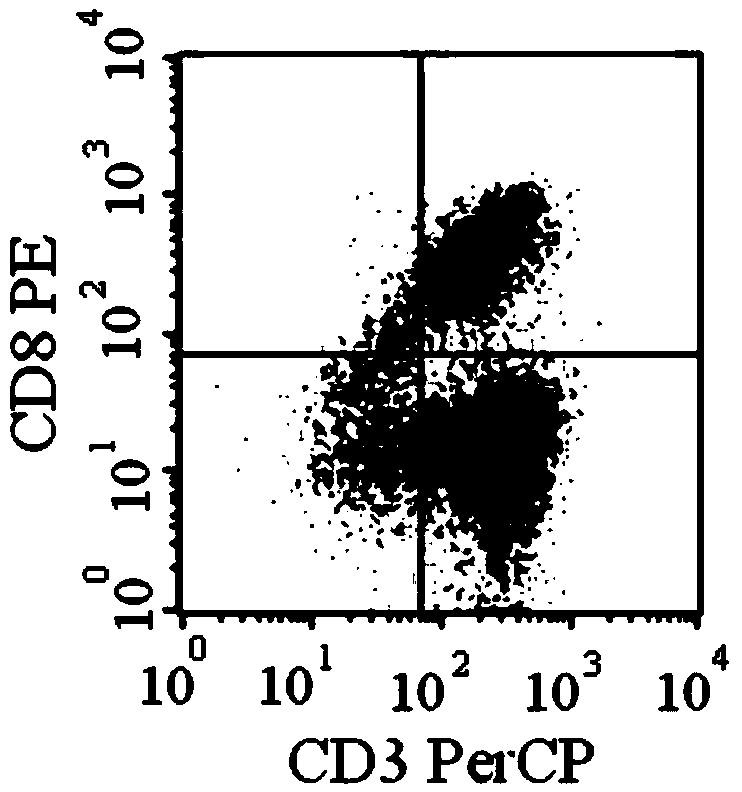
[0070] Example 3 Determination of chimeric antigen receptor lentiviral lentivirus titer by flow cytometry
[0071] On the first day, 1×10 6 / mL inoculate 293T cells in a 48-well culture plate, 200μL / well, 37℃, 5% CO 2 The culture is carried out in an incubator, and the culture medium contains DMEM with 10% fetal bovine serum. On the second day, 100 μL of culture supernatant was discarded from each well, and 100 μL of fresh culture medium was added, and the final concentration of polybrene was 8 μg / mL. Add 10μL / well of virus concentrate, 5-fold dilution, 4 gradients, two replicate wells, 37℃, 5% CO 2 Incubate in an incubator. After 48 hours of infection, trypsin digestion into a single cell suspension, fix it with 0.01% formaldehyde (prepared in PBS) for 10-15 min, ice bath with 0.1% saponin (saponin) for 15 min to permeate the membrane; reconstitute with an appropriate amount of buffer Hanging cells are 2×10 7 Pcs / ml, add 50μL of diluted primary antibody (mouse anti-V5 monoclonal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com