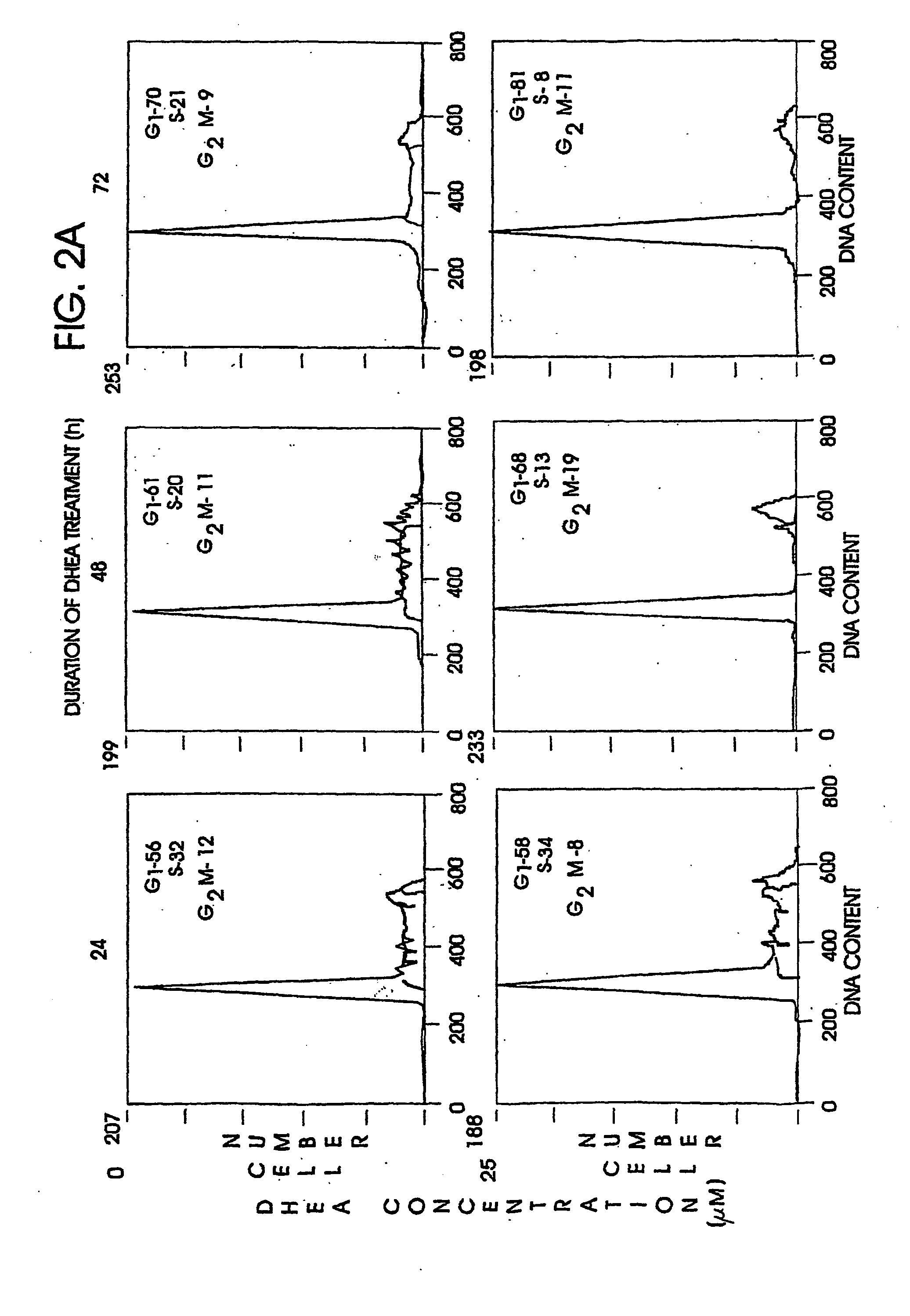
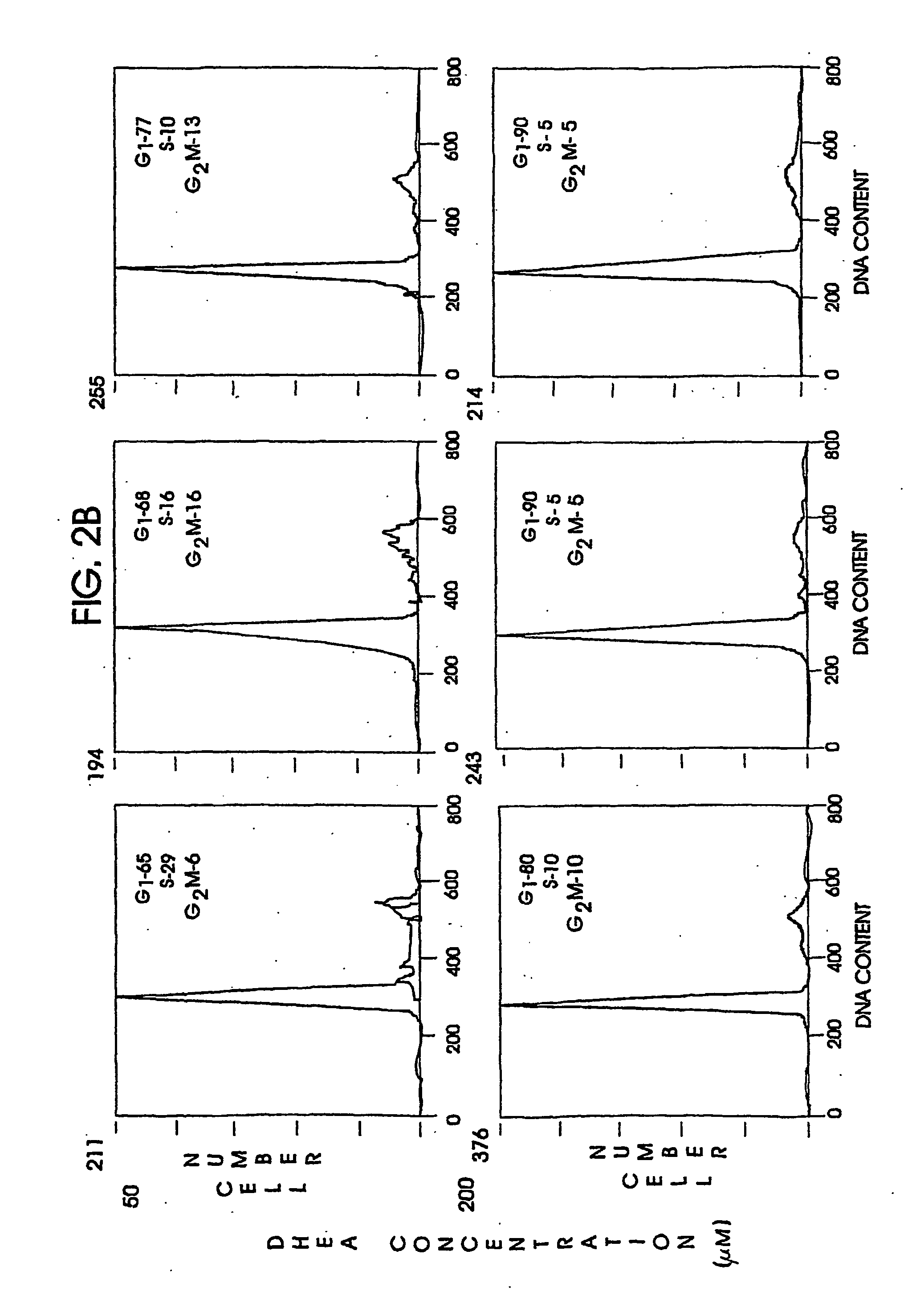
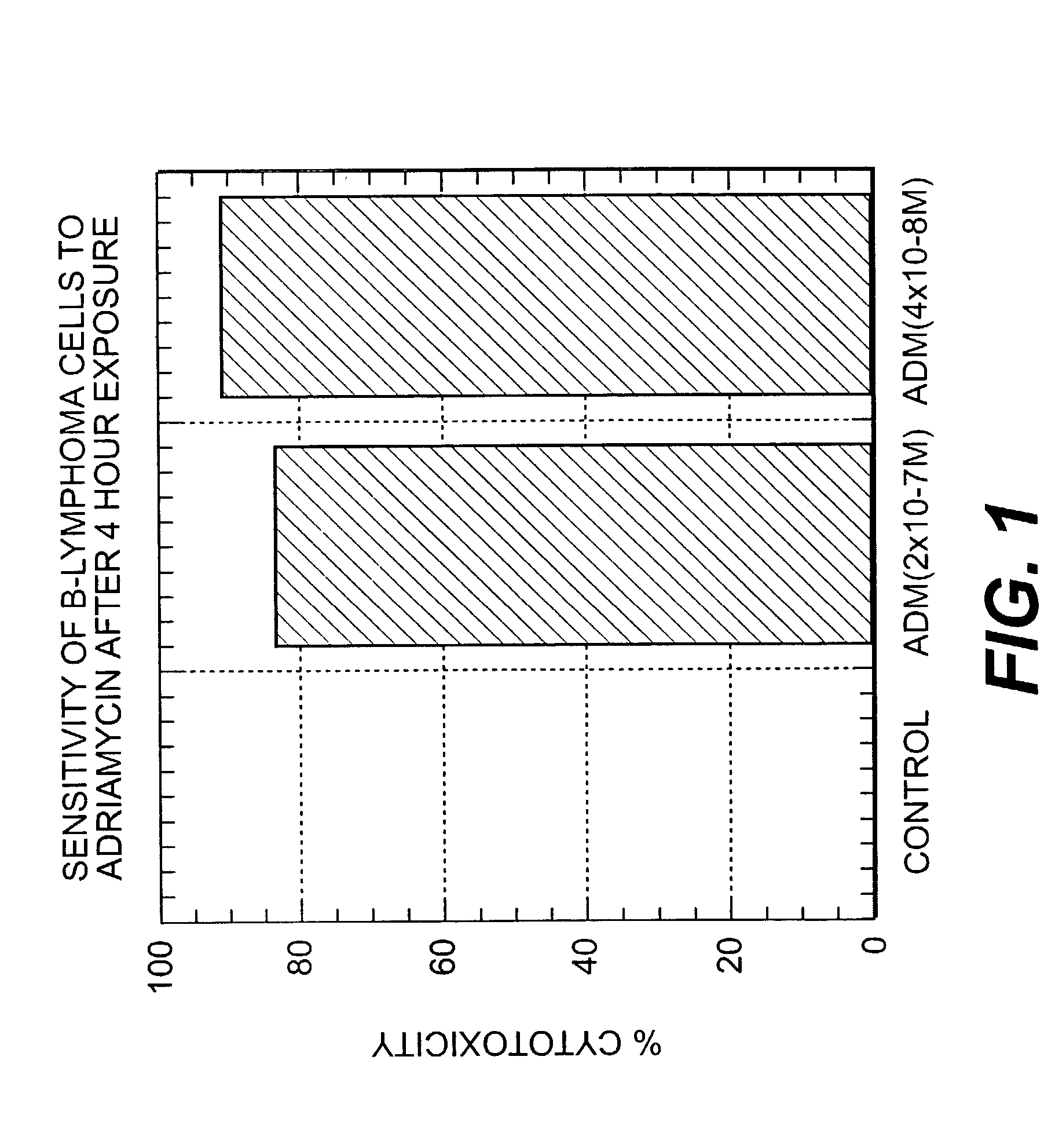
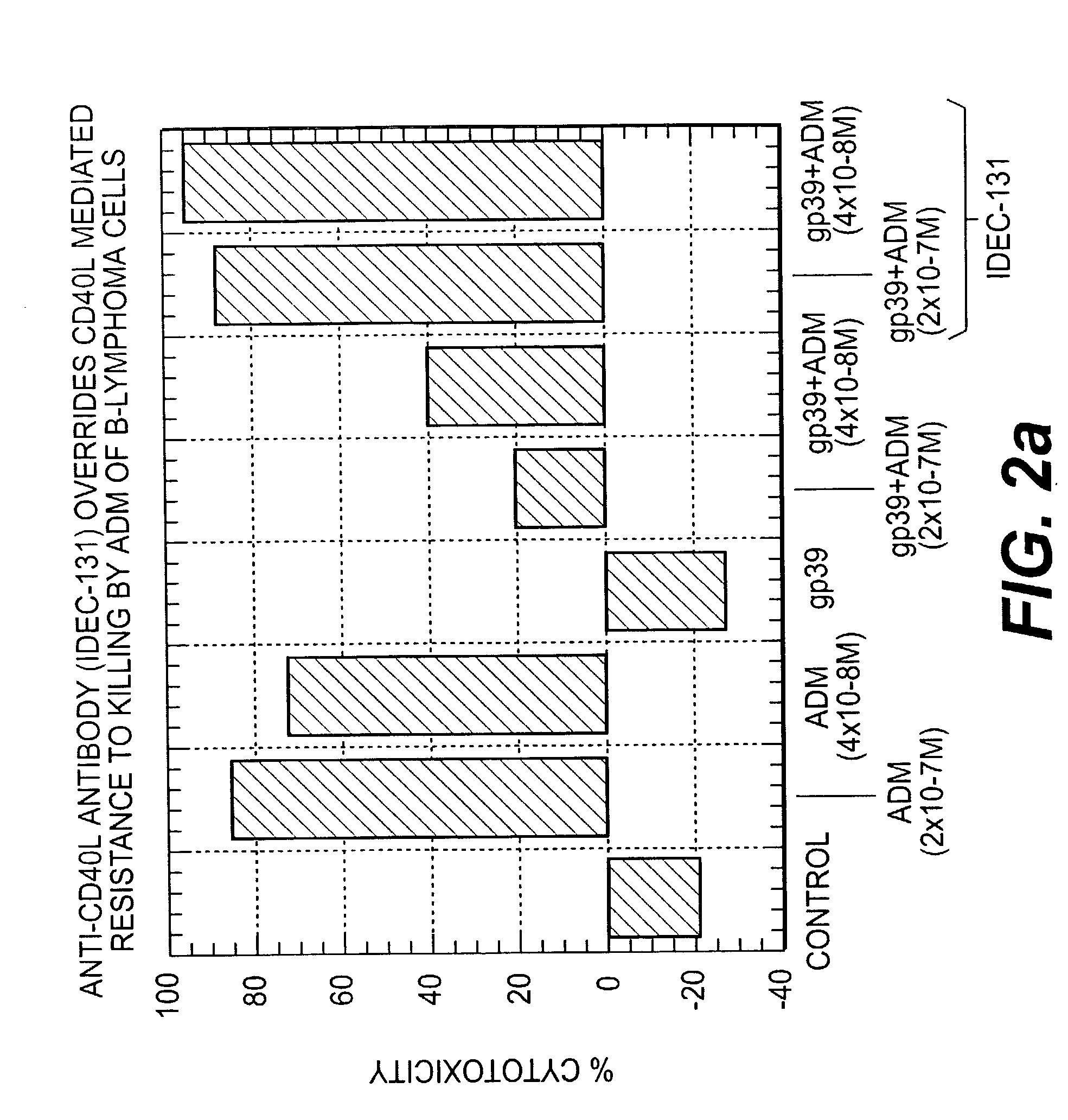
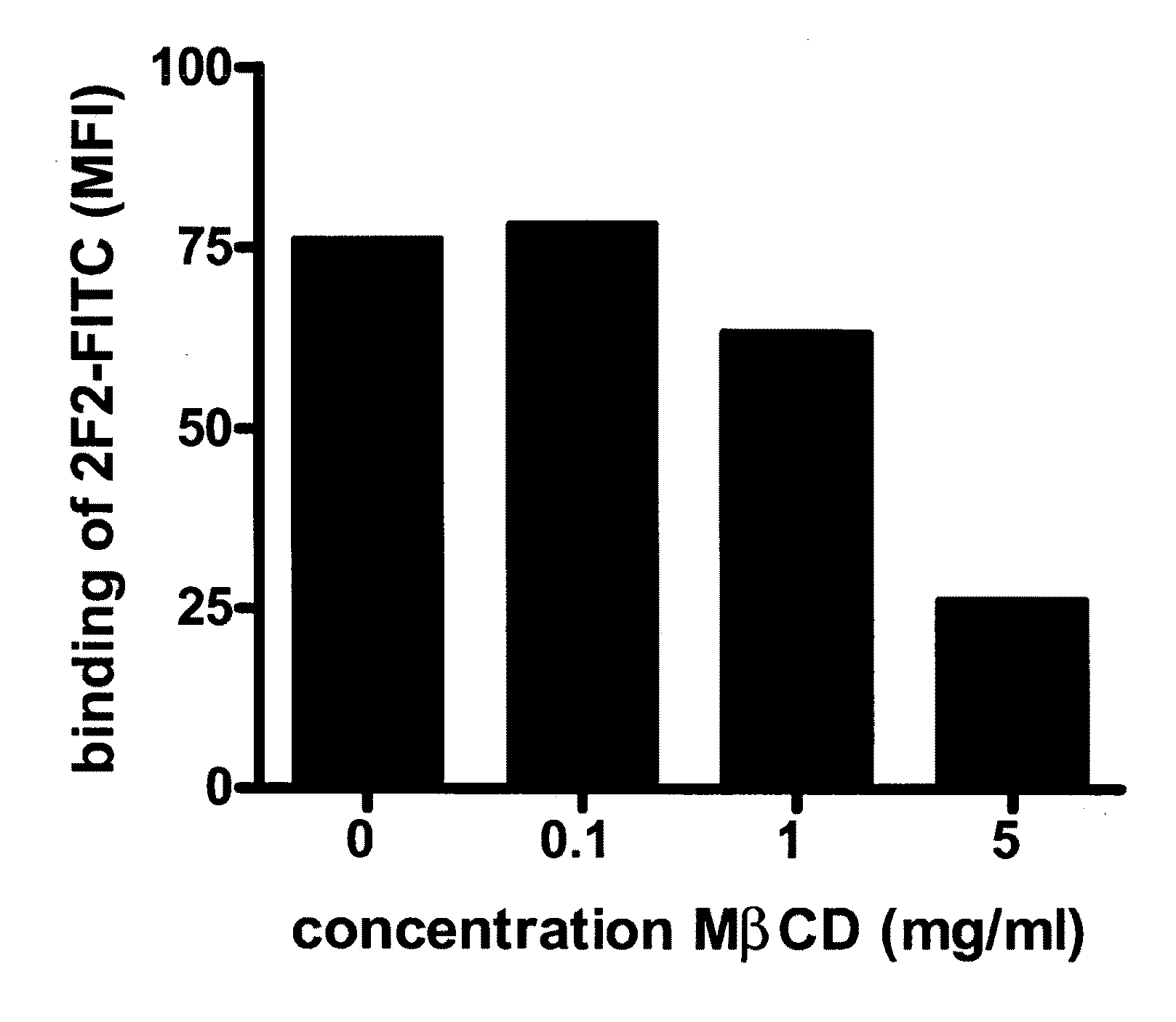
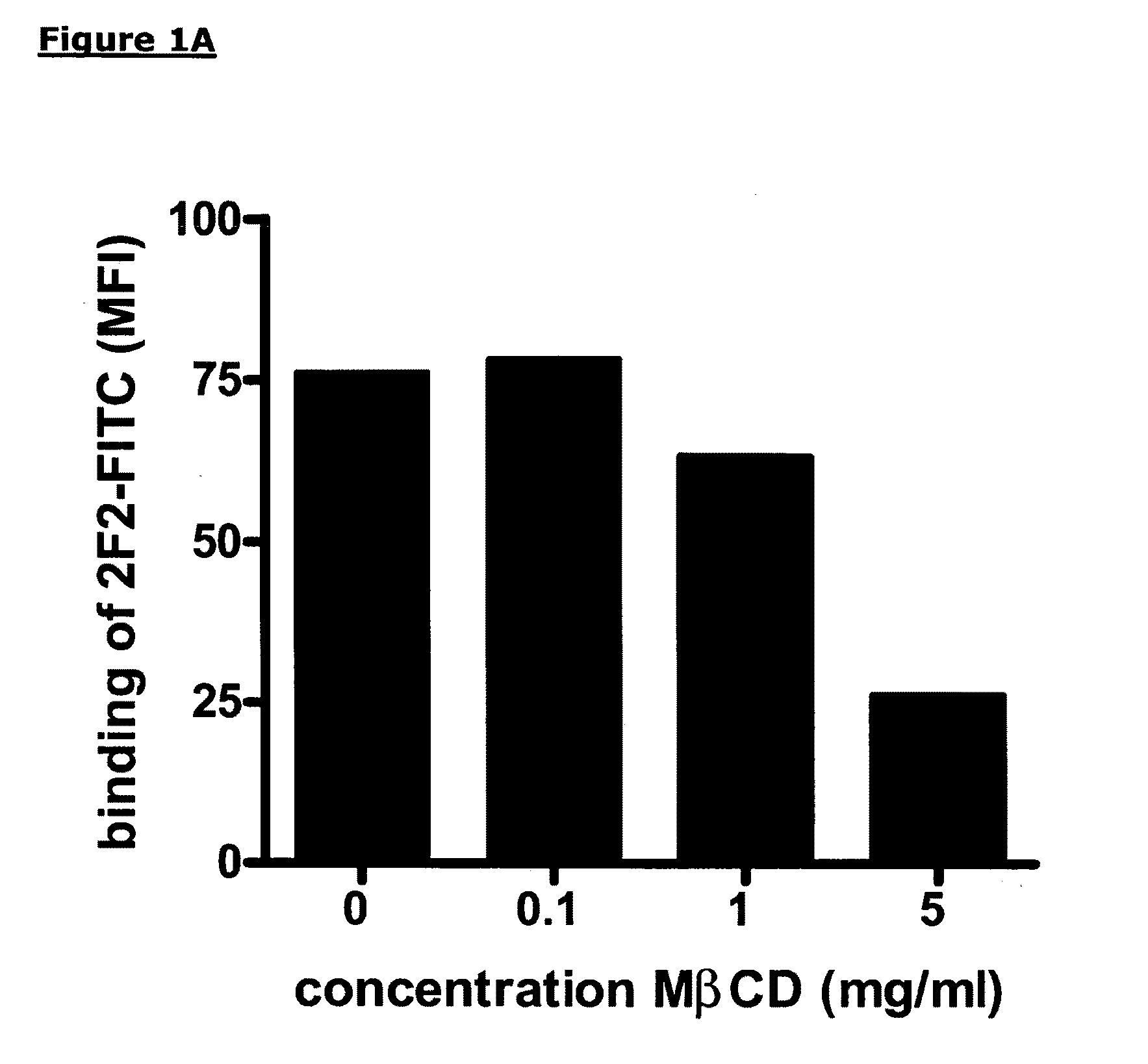
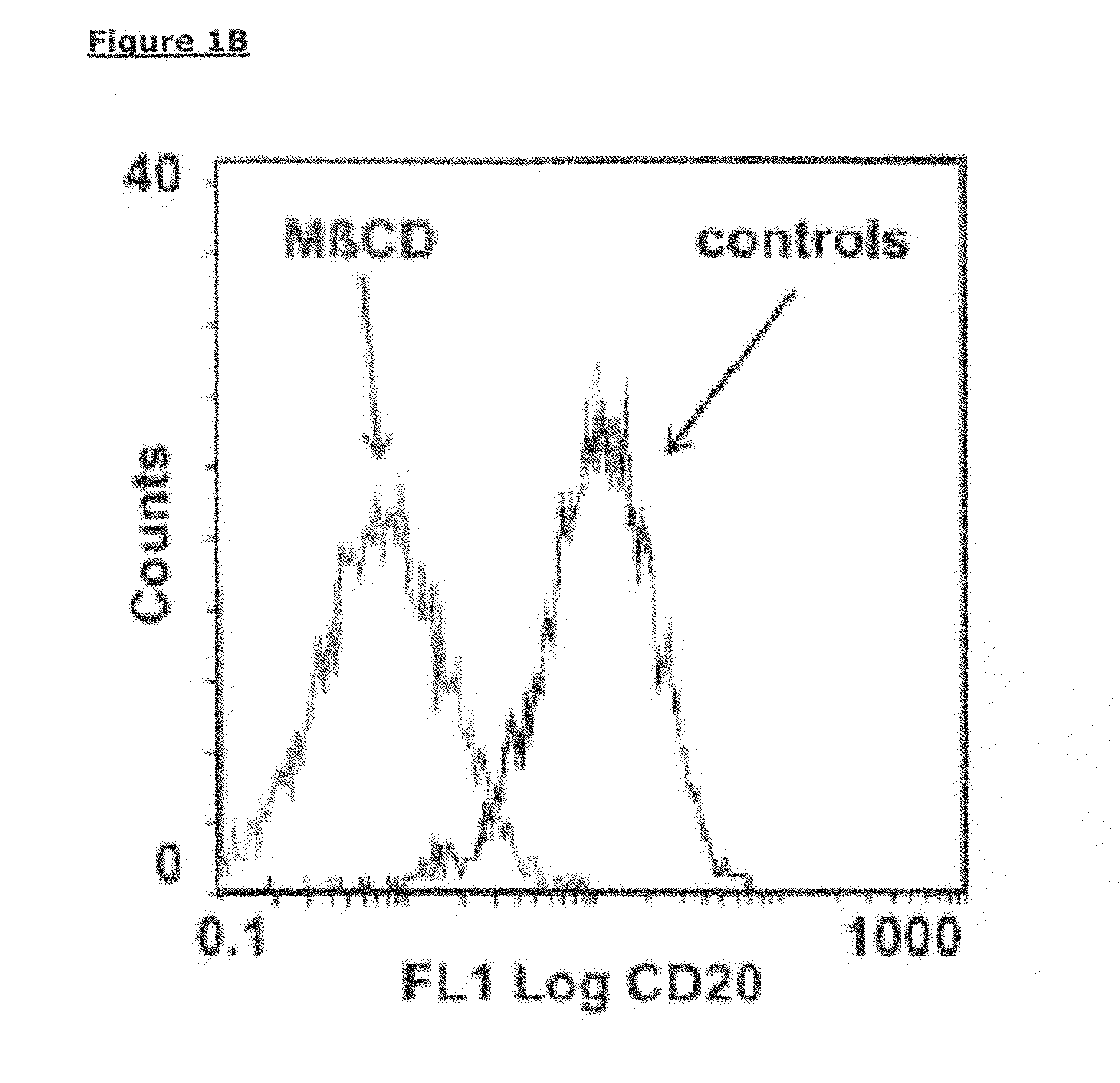
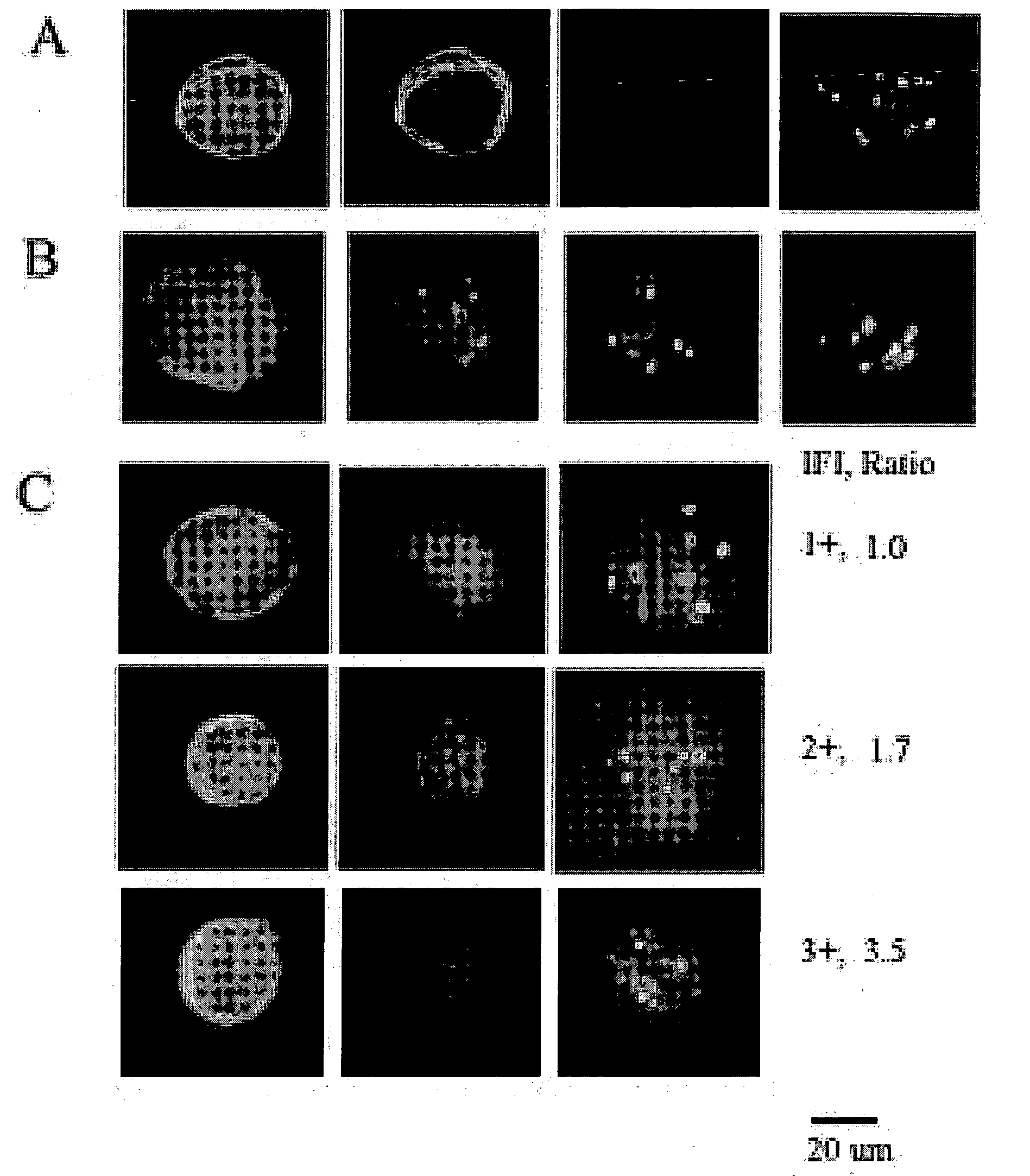
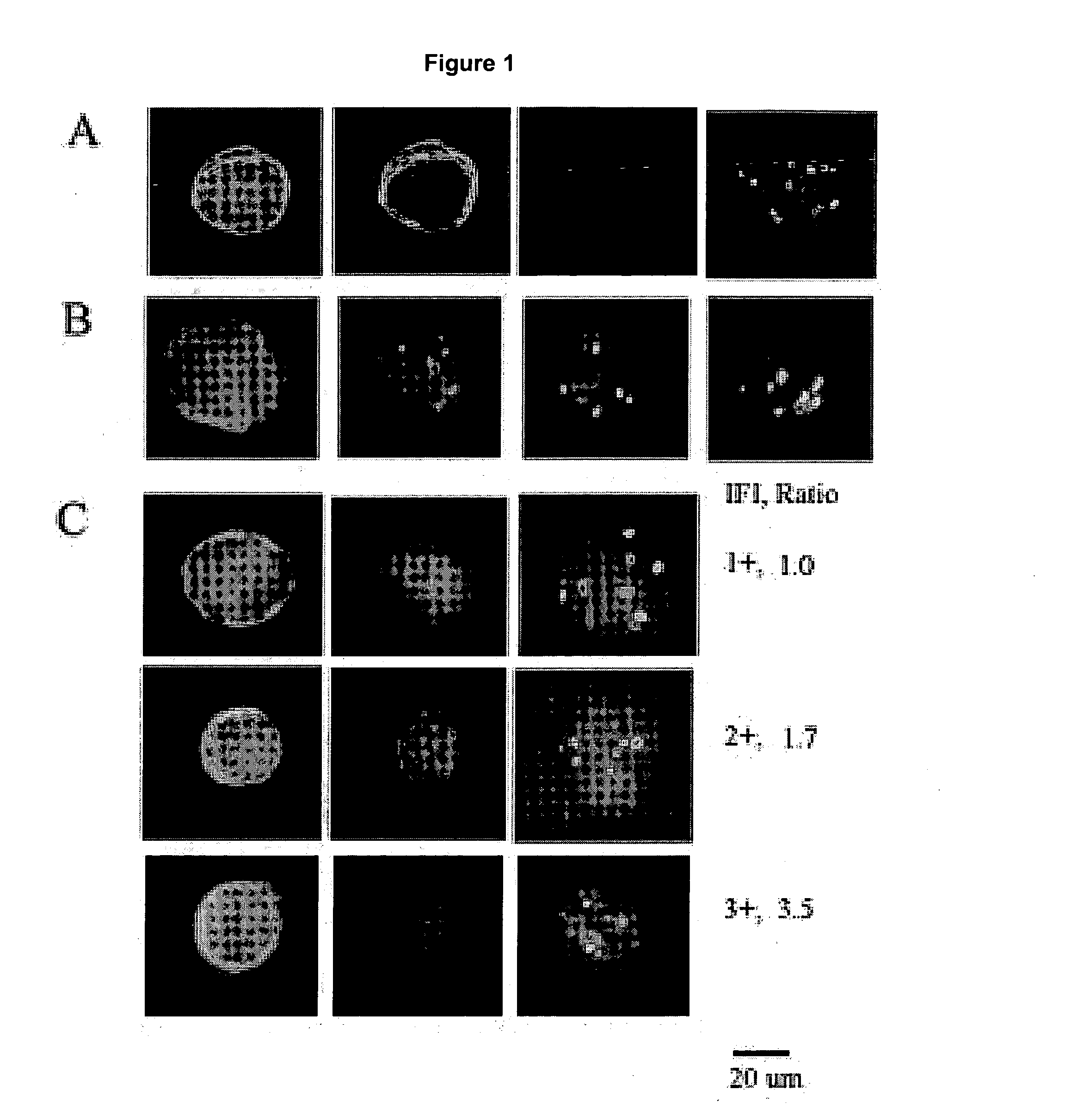
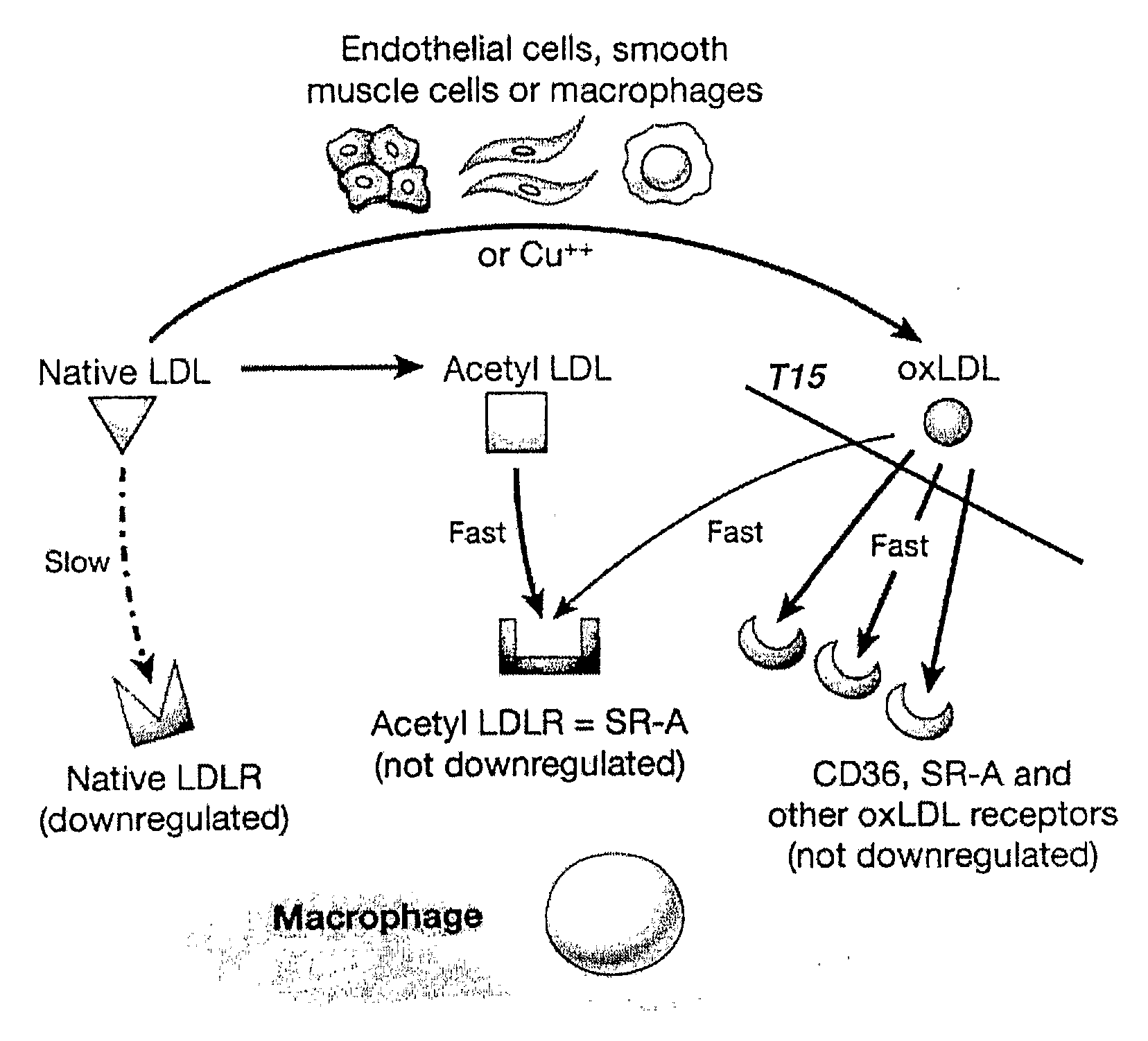
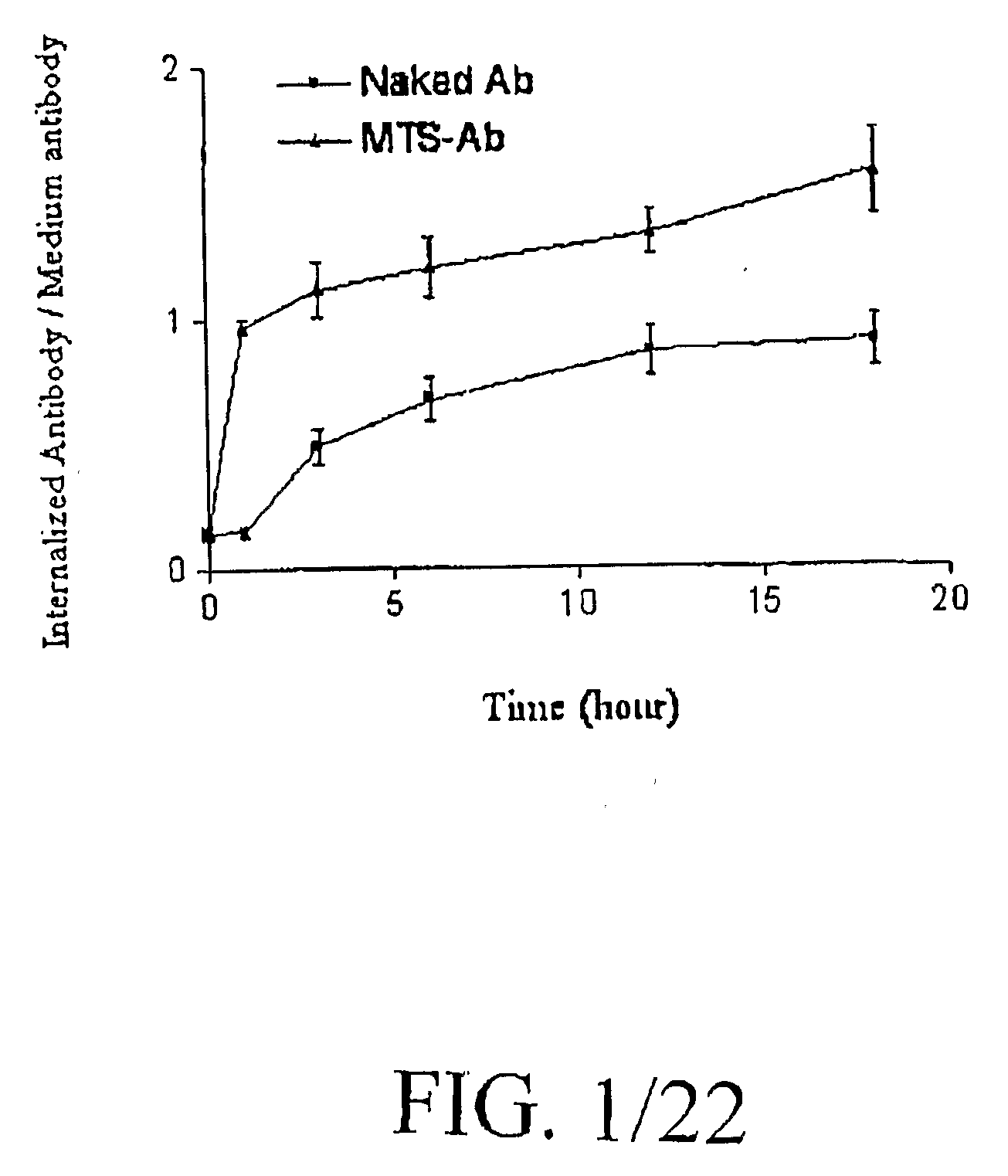
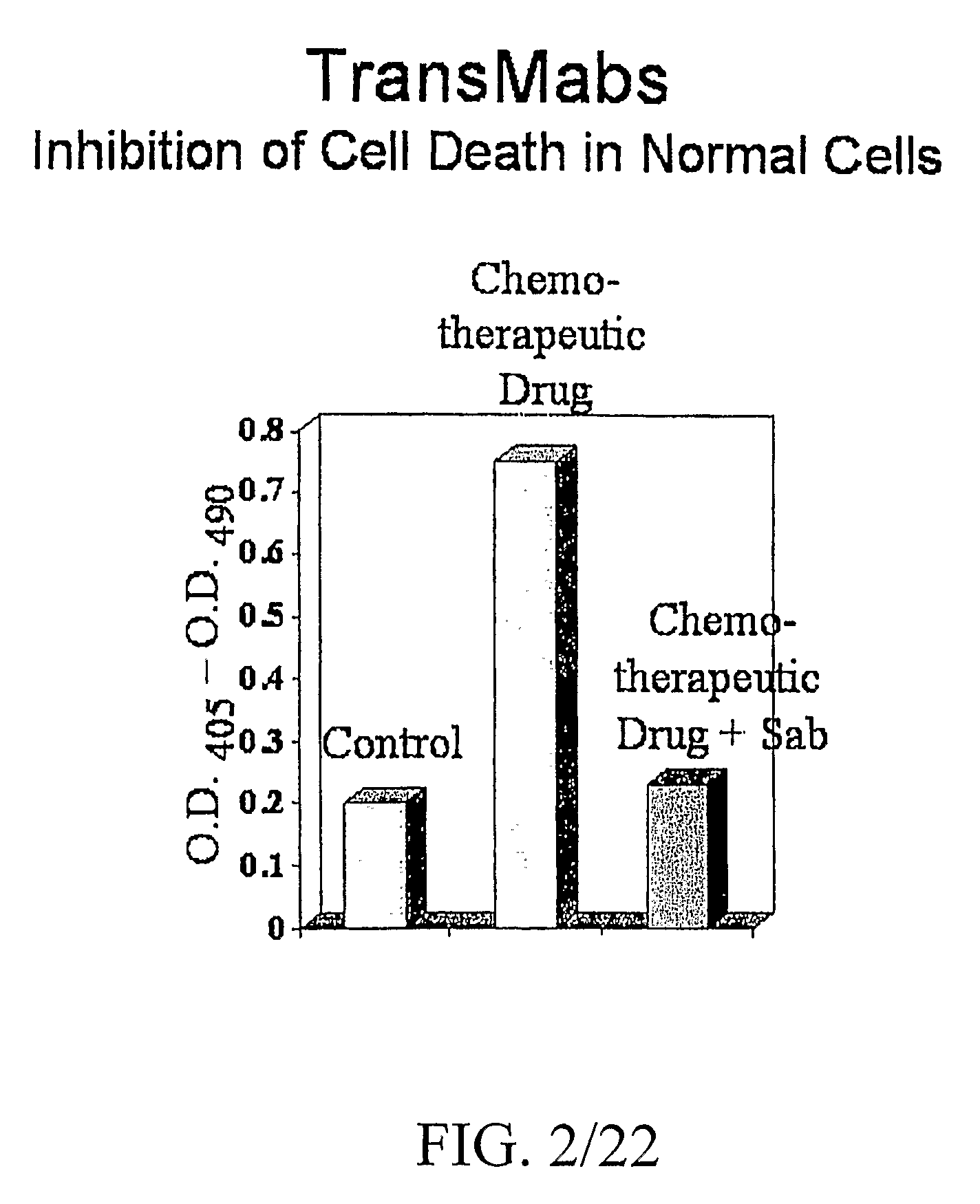
Patents
Literature
135 results about "Antibody therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAb) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, ...
Therapy via targeted delivery of nanoscale particles
InactiveUS20050090732A1Destroying inhibiting vascularityAntibacterial agentsNervous disorderDiseaseProstate cancer
Disclosed are compositions, systems and methods for treating a subject's body, body part, tissue, body fluid cells, pathogens, or other undesirable matter involving the administration of a targeted thermotherapy that comprises a bioprobe (energy susceptive materials that are attached to a target-specific ligand). Such targeted therapy methods can be combined with at least one other therapy technique. Other therapies include hyperthermia, direct antibody therapy, radiation, chemo- or pharmaceutical therapy, photodynamic therapy, surgical or interventional therapy, bone marrow or stem cell transplantation, and medical imaging, such as MRI, PET, SPECT, and bioimpedance. The disclosed therapies may be useful in the treatment of a variety of indications, including but not limited to, cancer of any type, such as bone marrow, lung, vascular, neuro, colon, ovarian, breast and prostate cancer, epitheleoid sarcomas, AIDS, adverse angiogenesis, restenosis, amyloidosis, tuberculosis, cardiovascular plaque, vascular plaque, obesity, malaria, and illnesses due to viruses, such as HIV.
Owner:NANOTX INC
Compositions, formulations and kit with anti-sense oligonucleotide and anti-inflammatory steroid and/or obiquinone for treatment of respiratory and lung disesase
InactiveUS20070021360A1Decreased airwayOrganic active ingredientsBiocideDiseaseAntiendomysial antibodies
A pharmaceutical composition and formulations comprise preventative, prophylactic or therapeutic amounts of an oligo(s) anti-sense to a specific gene(s) or its corresponding mRNA(s), and a glucocorticoid and / or non-glucocorticoid steroid or a ubiquinone or their salts. The agents, composition and formulations are used for treatment of ailments associated with impaired respiration, bronchoconstriction, lung allergy(ies) or inflammation, and abnormal levels of adenosine, adenosine receptors, sensitivity to adenosine, lung surfactant and ubiquinone, such as pulmonary fibrosis, vasoconstriction, inflammation, allergies, allergic rhinitis, asthma, impeded respiration, lung pain, cystic fibrosis, bronchoconstriction, COPD, RDS, ARDS, cancer, and others. The present treatment is effectively administered by itself for conditions without known therapies, as a substitute for therapies exhibiting undesirable side effects, or in combination with other treatments, e.g. before, during and after other respiratory system therapies, radiation, chemotherapy, antibody therapy and surgery, among others. Each of the agents of this invention may be administered directly into the respiratory system so that they gain direct access to the lungs, or by other effective routes of administration. A kit comprises a delivery device, the agents and instructions for its use.
Owner:EPIGENESIS PHARMA LLC
Immunoregulatory antibodies and uses thereof
InactiveUS20030103971A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD37CD23
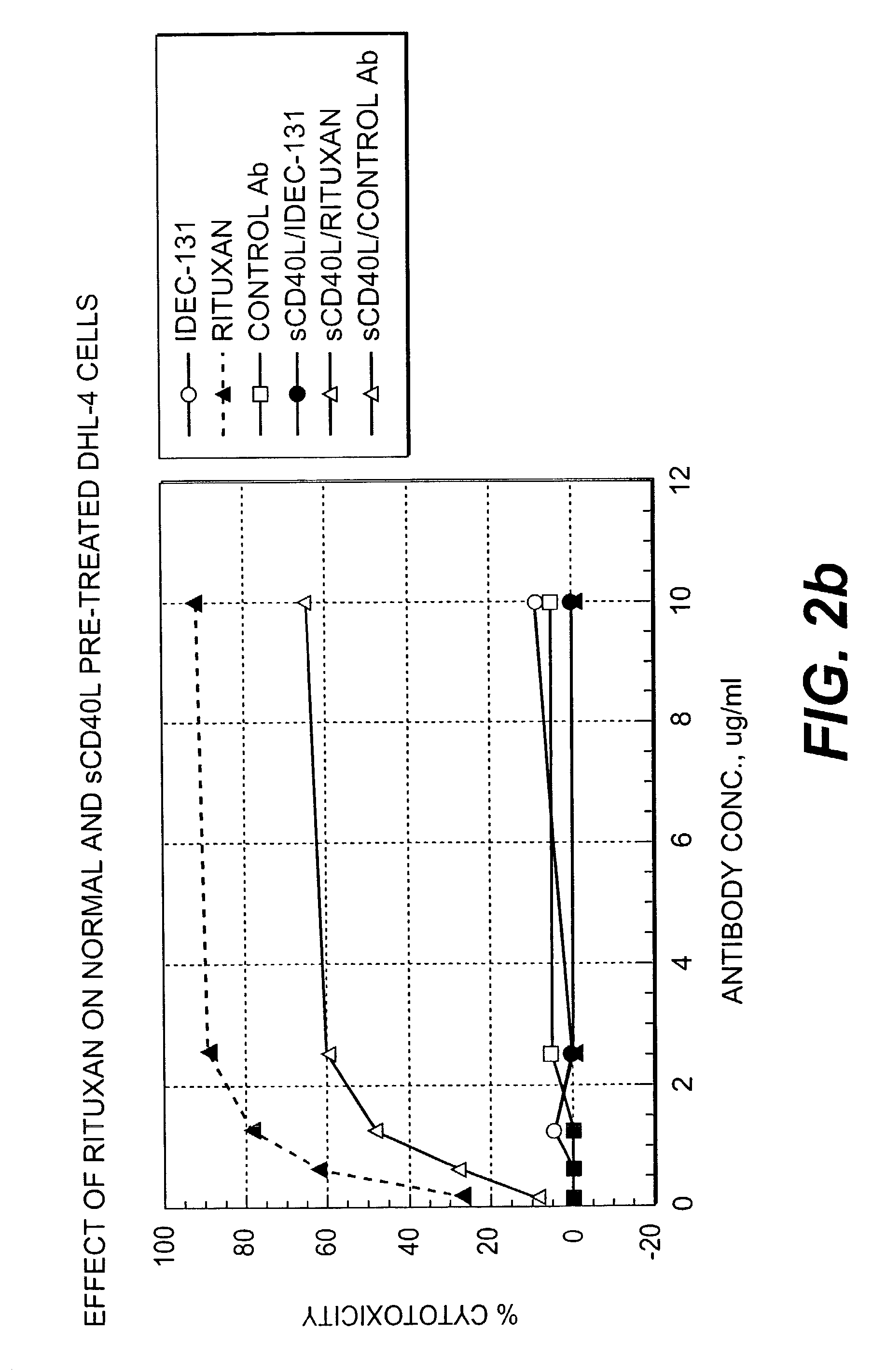
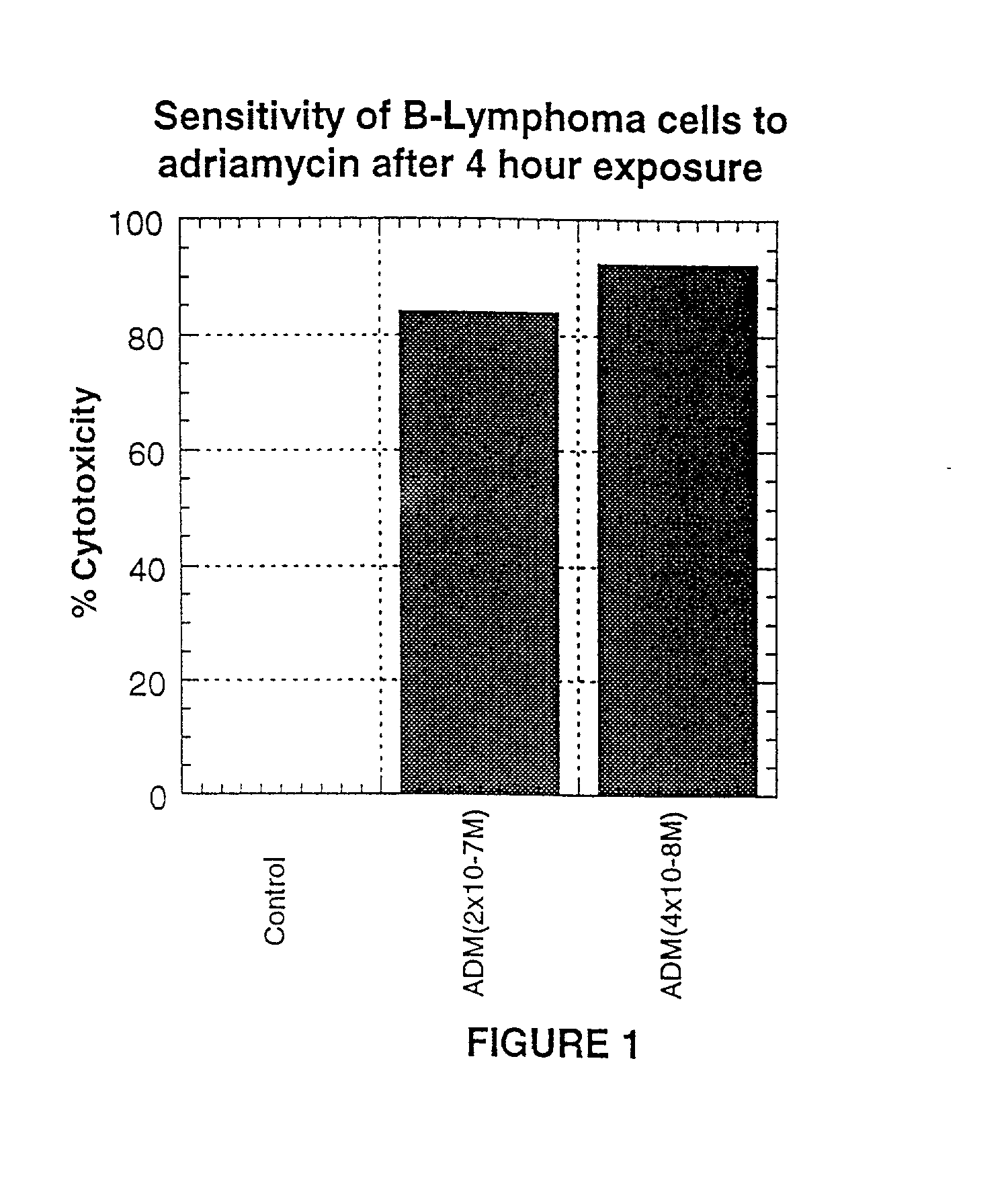
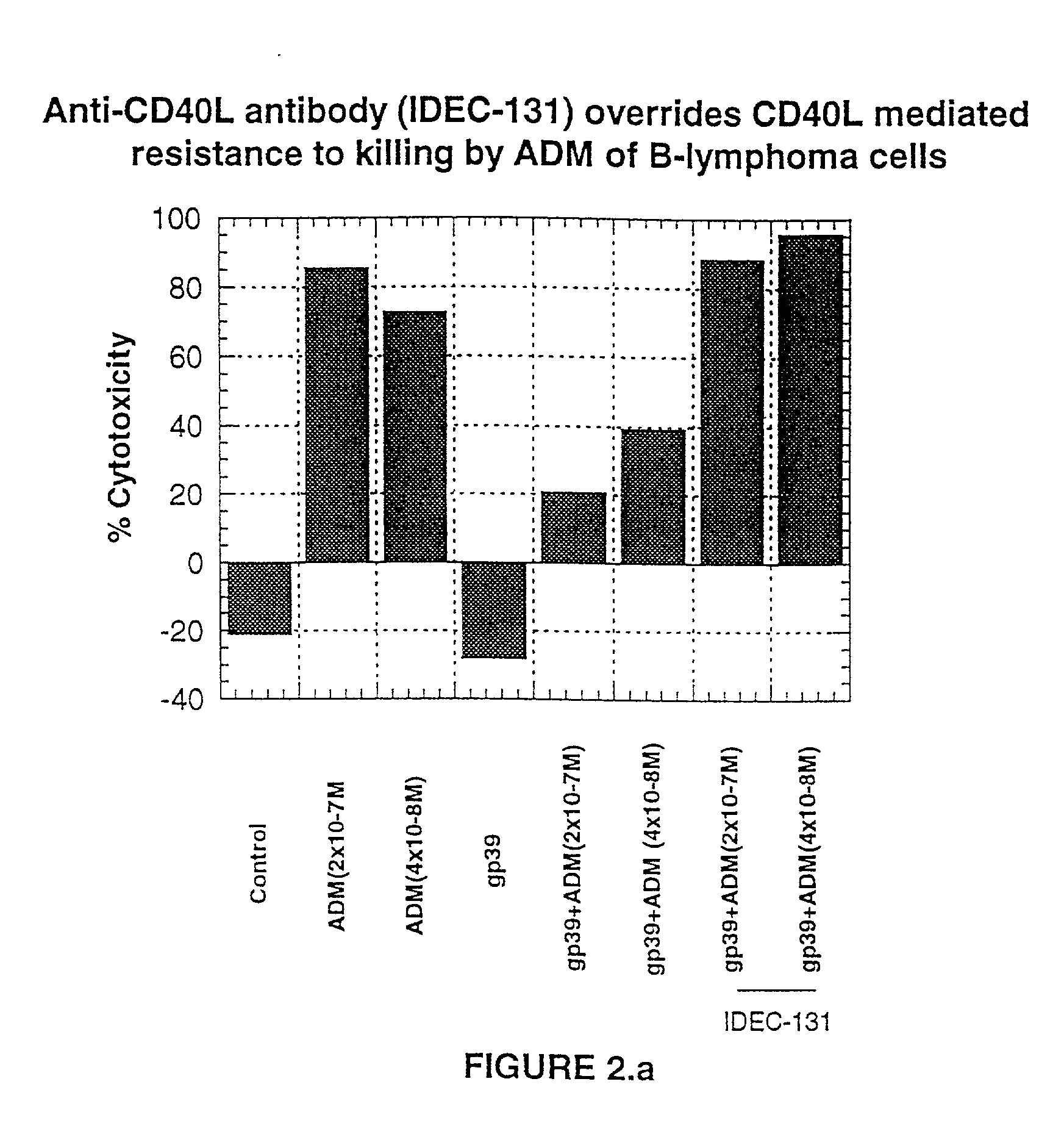
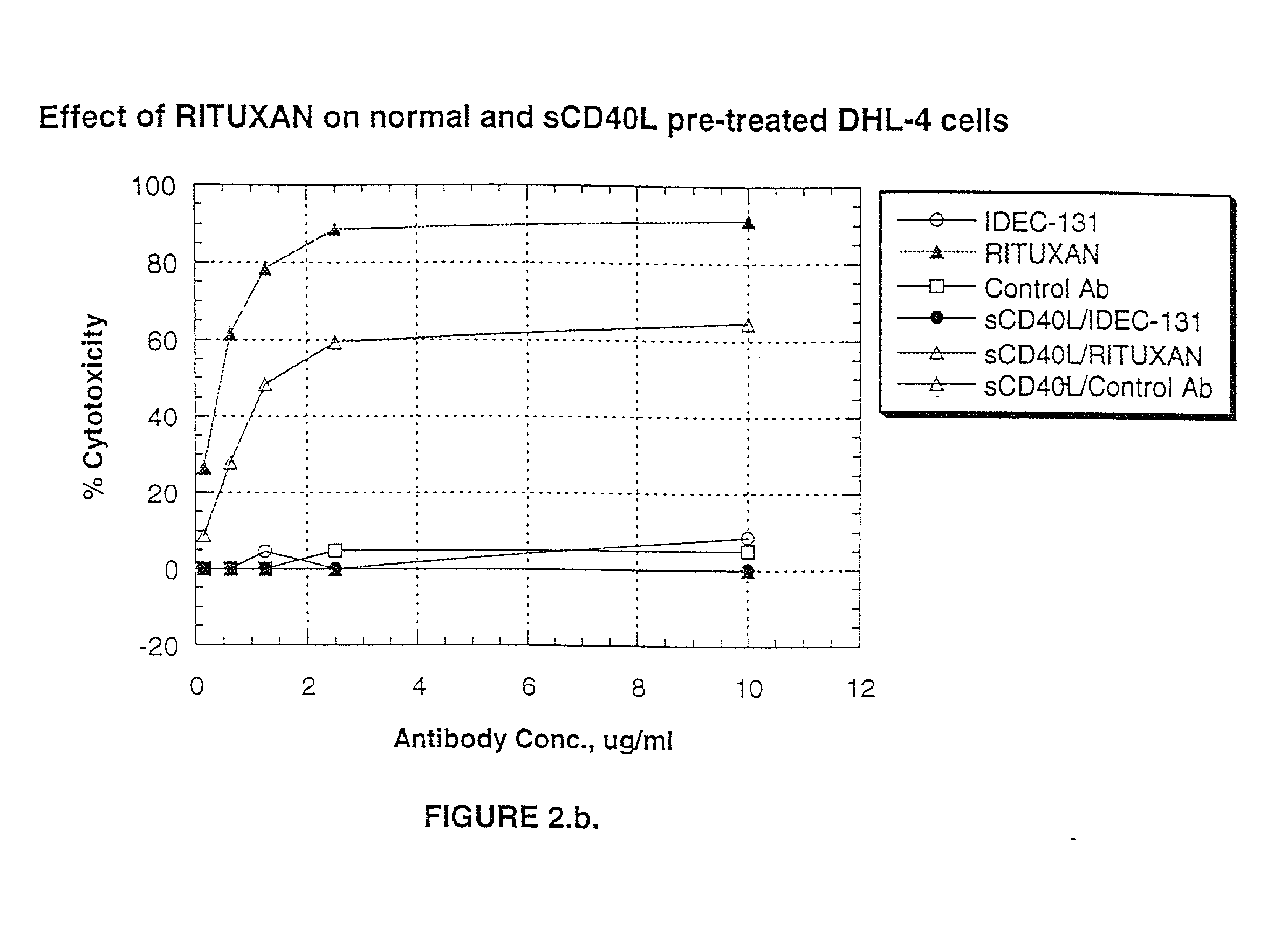
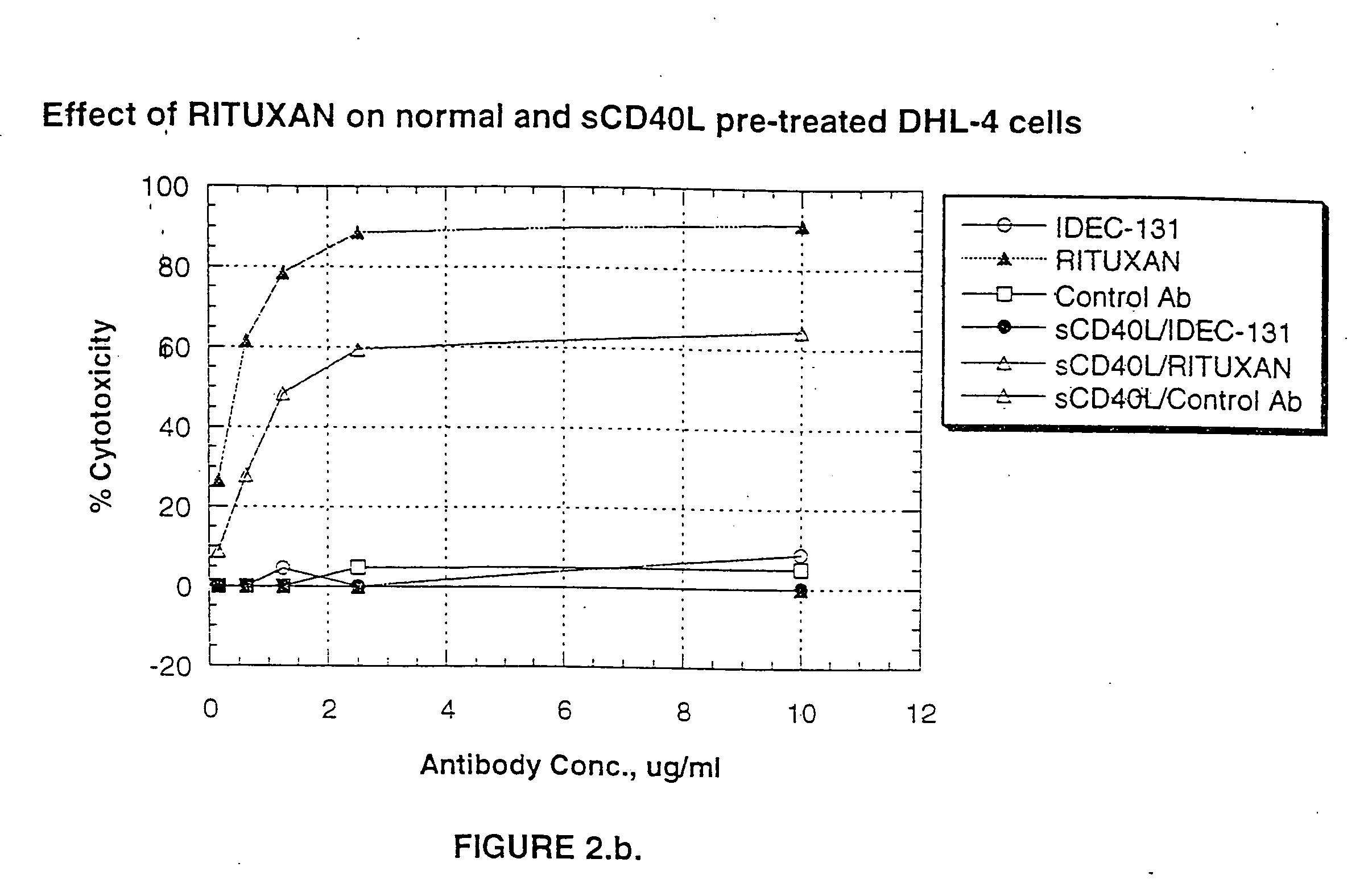
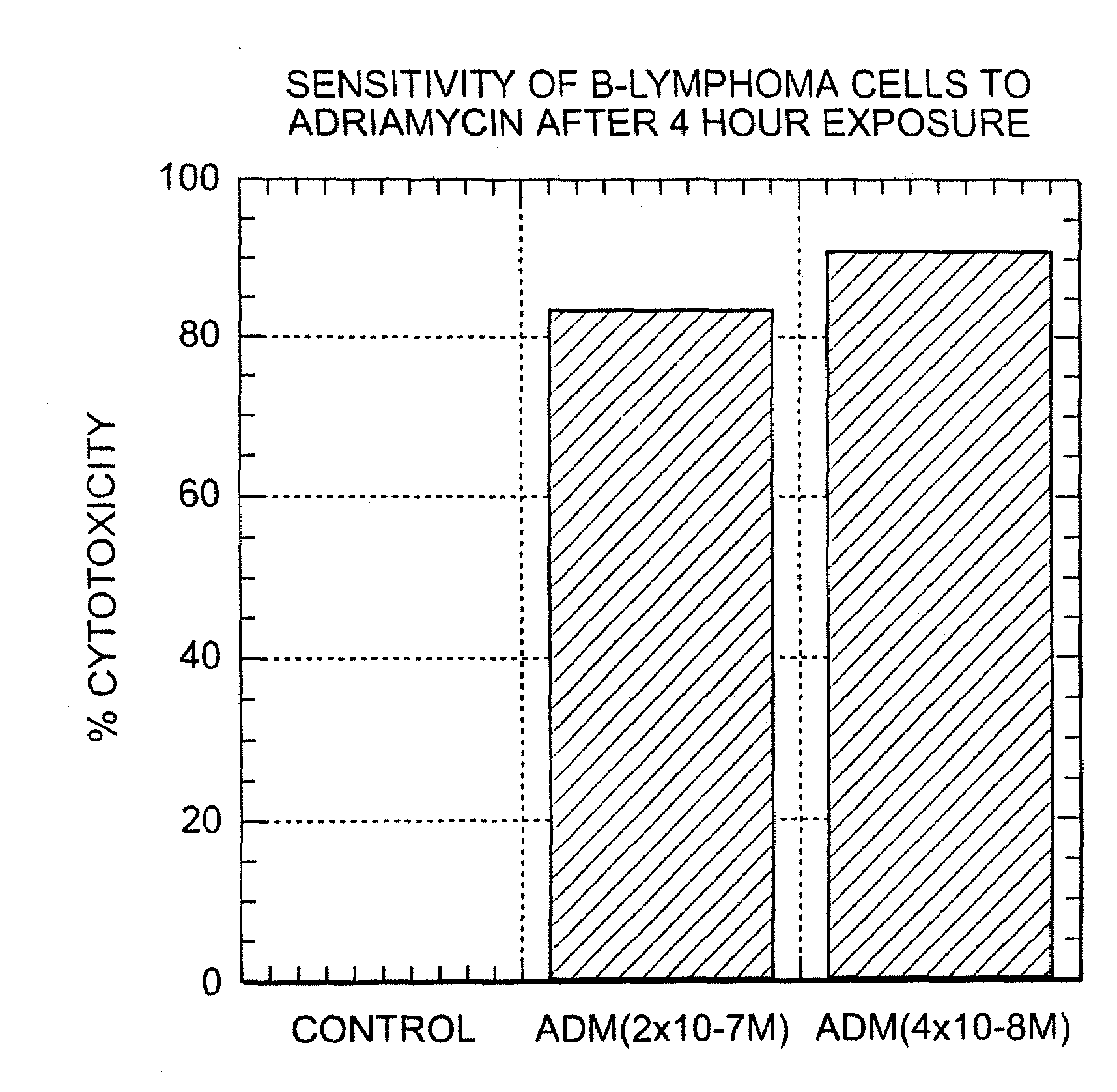
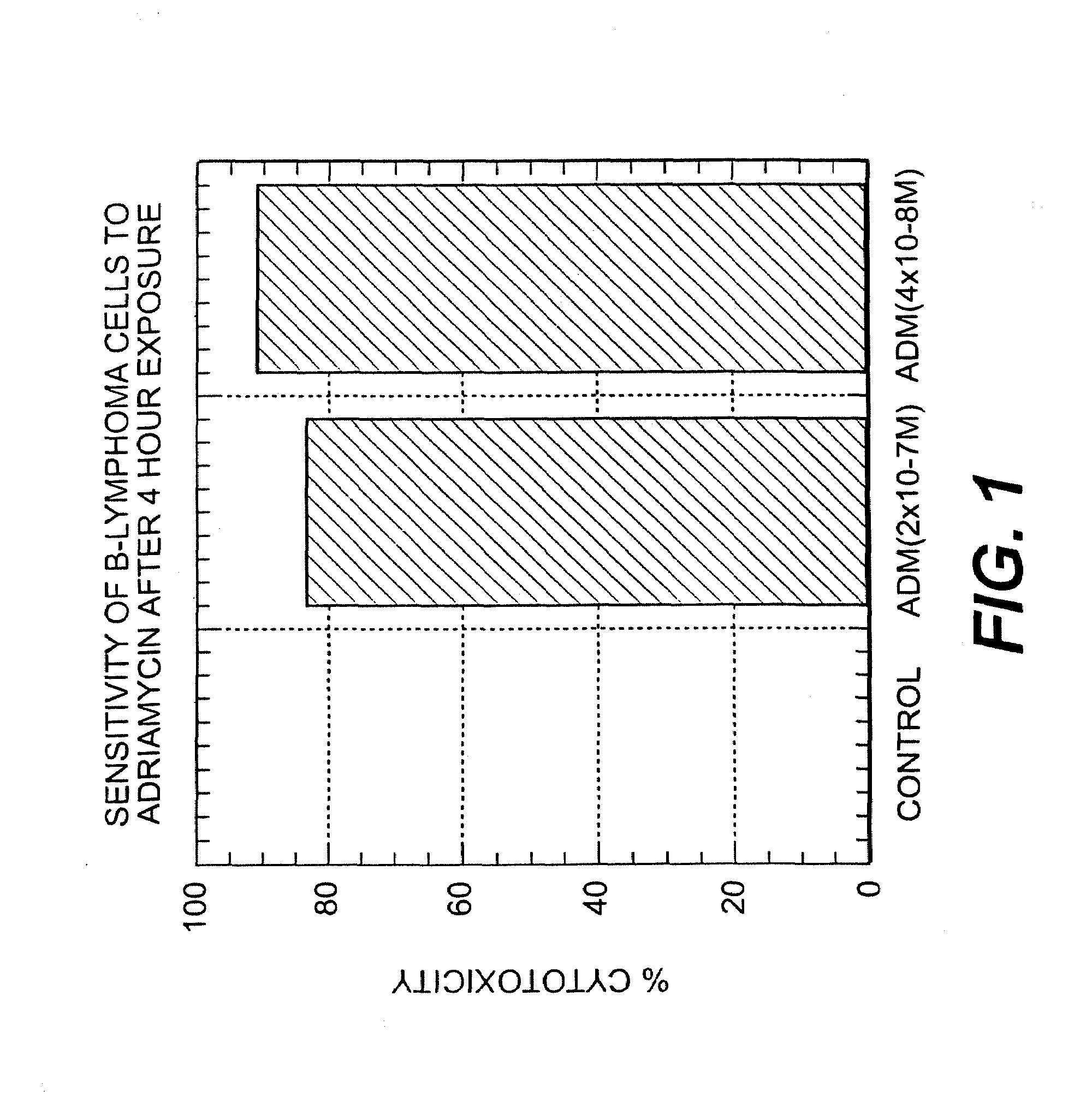
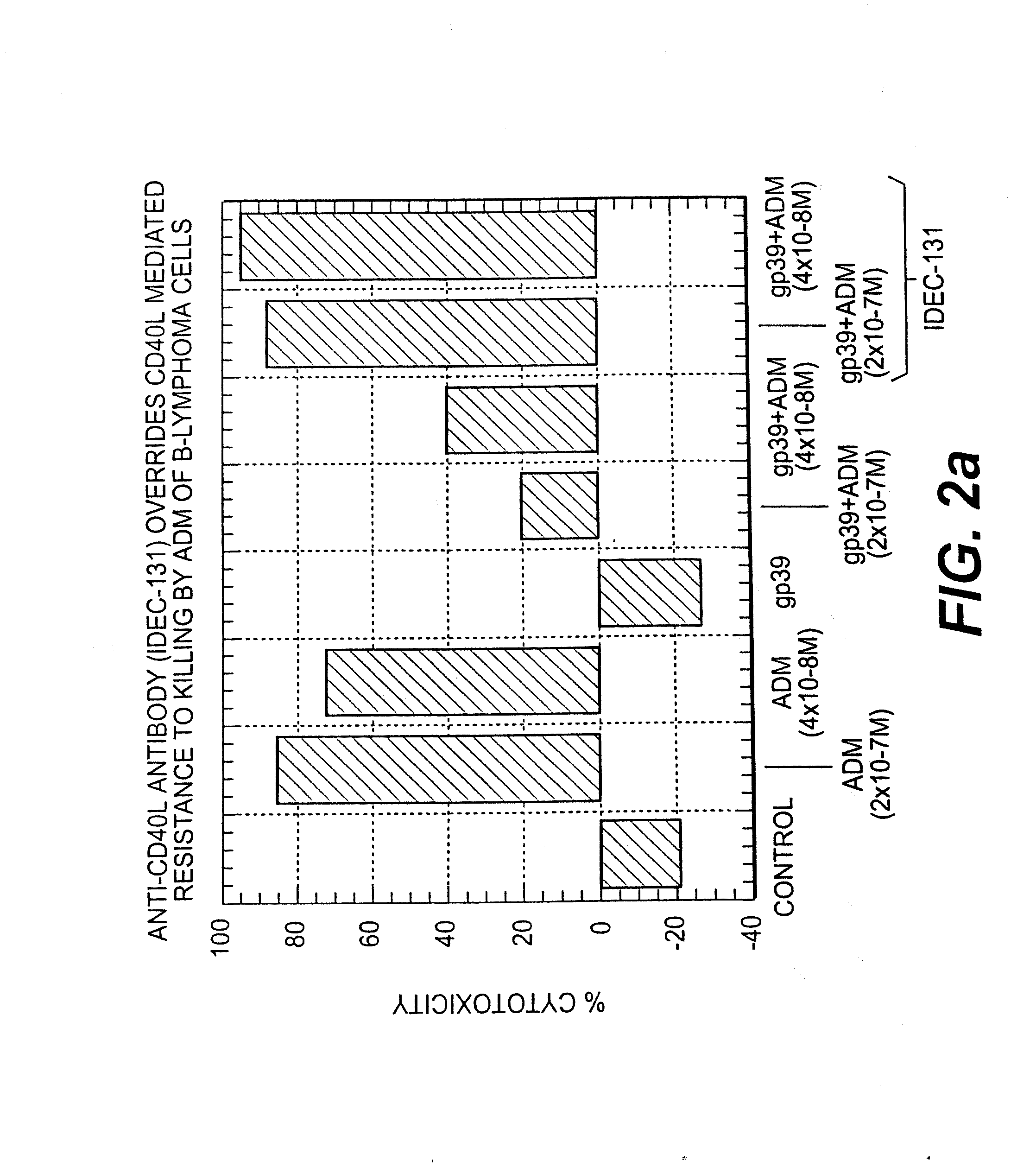
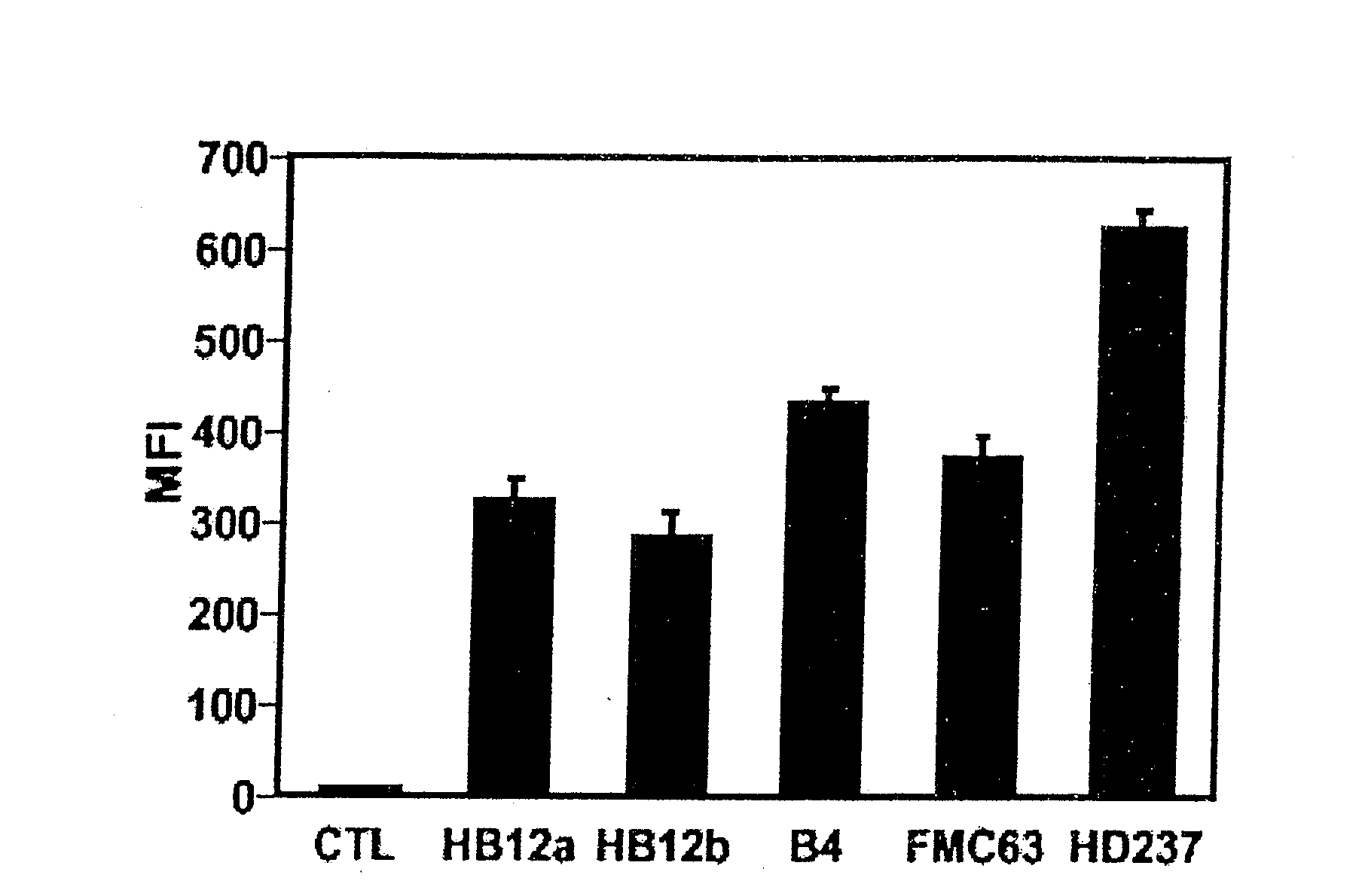
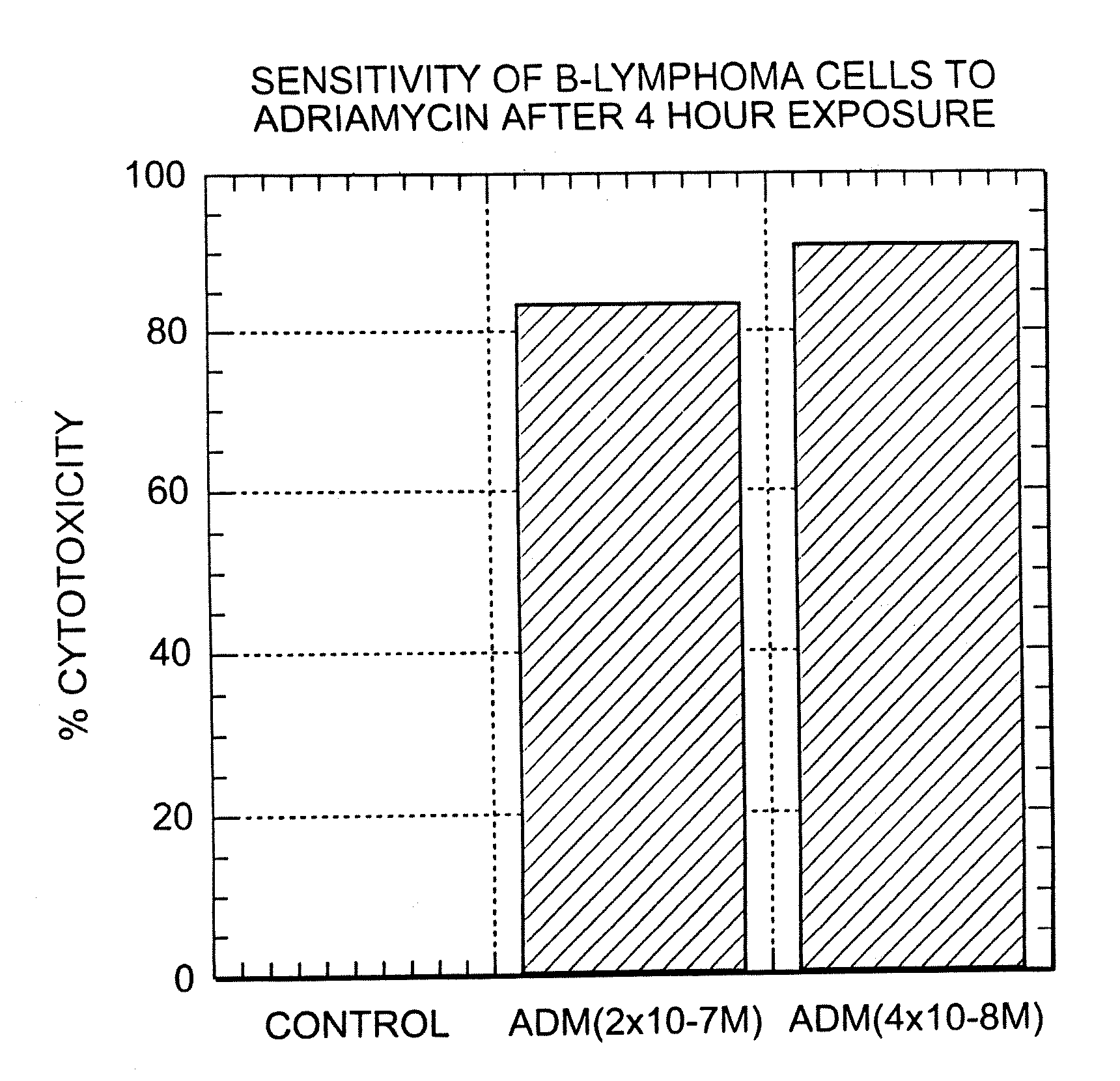
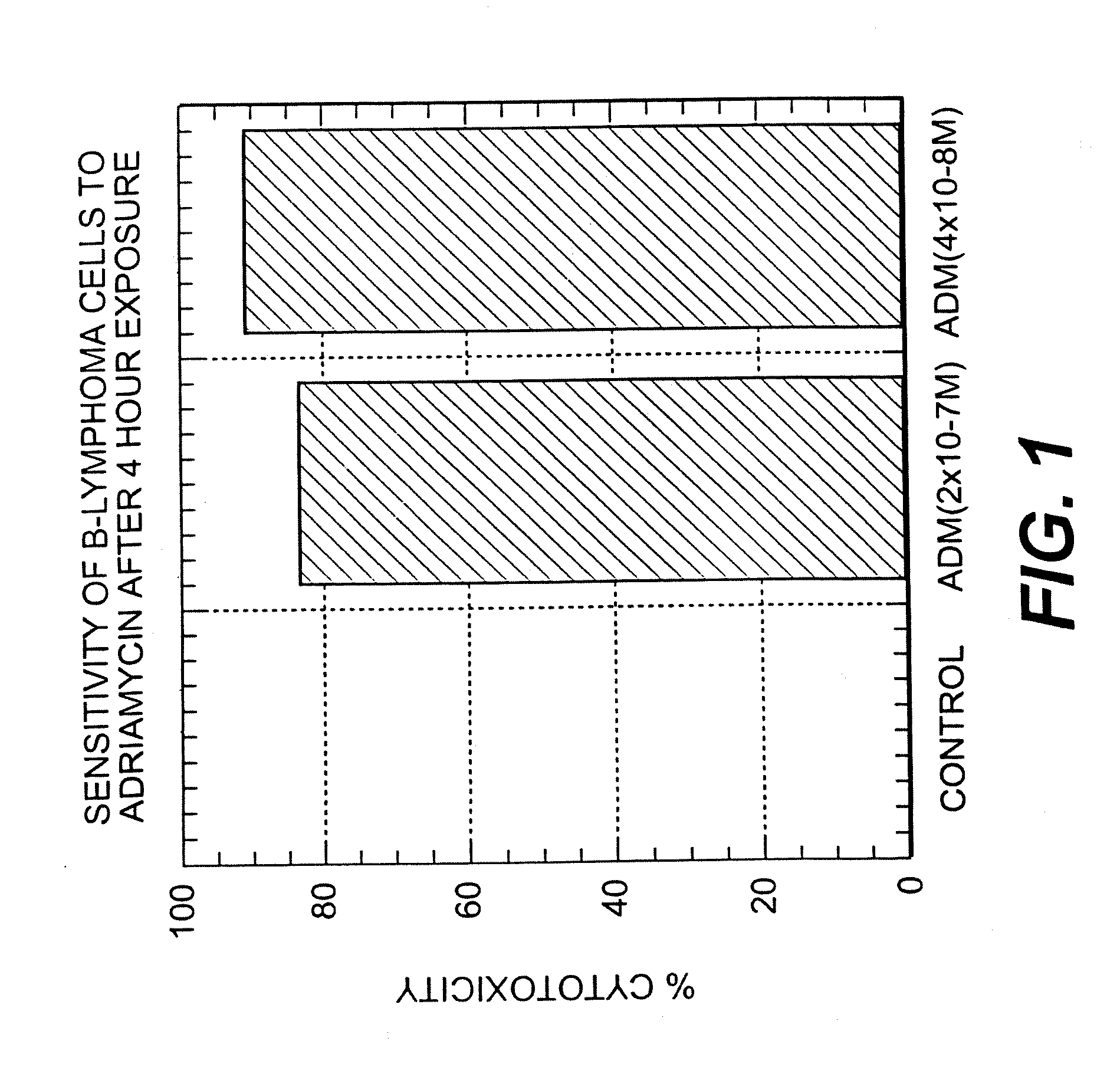
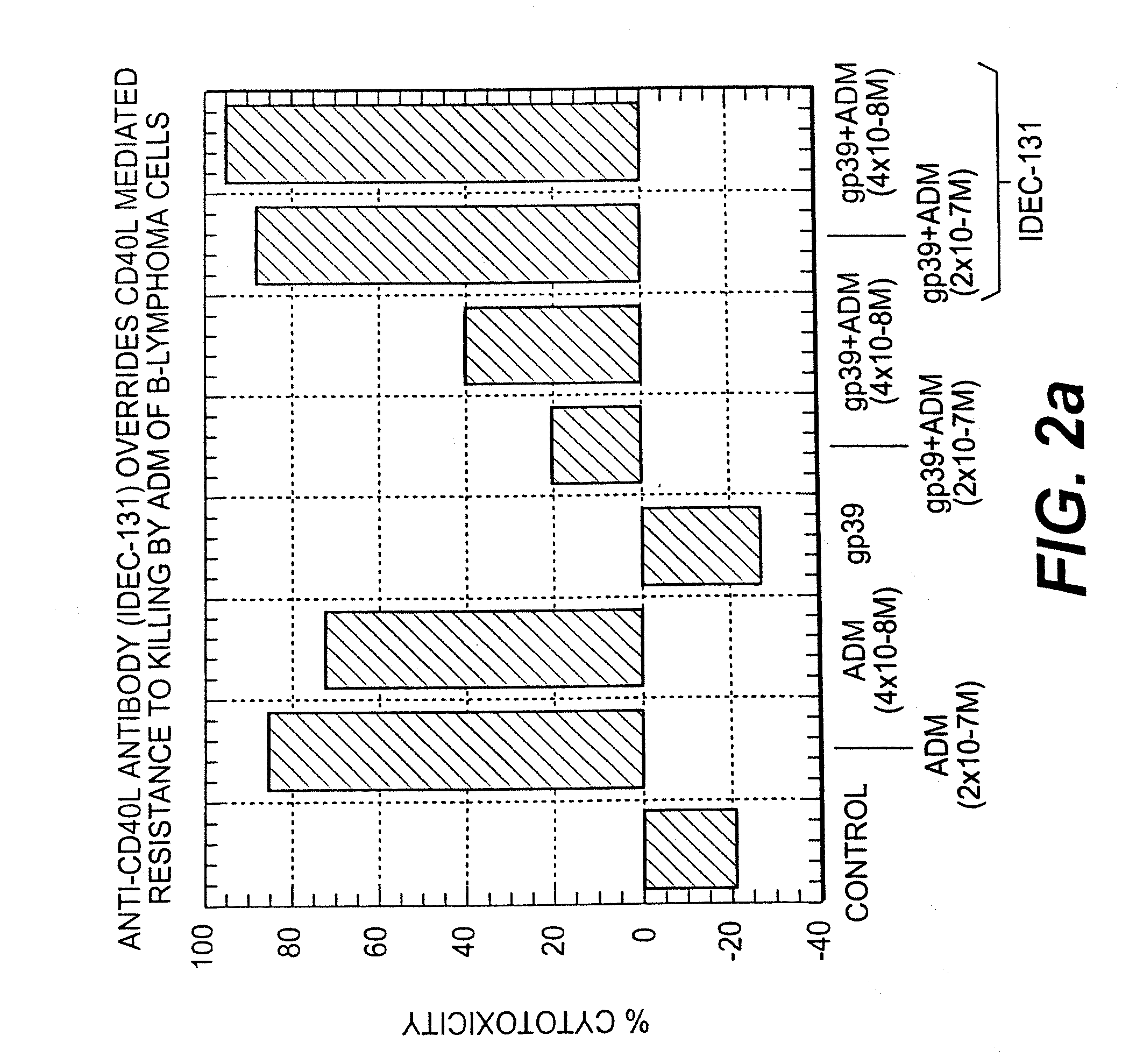
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Treatment of B cell malignancies using combination of B cell depleting antibody and immune modulating antibody related applications
InactiveUS20020028178A1Prevent and reduce proliferation of cellReduce and prevent proliferationRadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsCD20Antiendomysial antibodies
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN MA INC
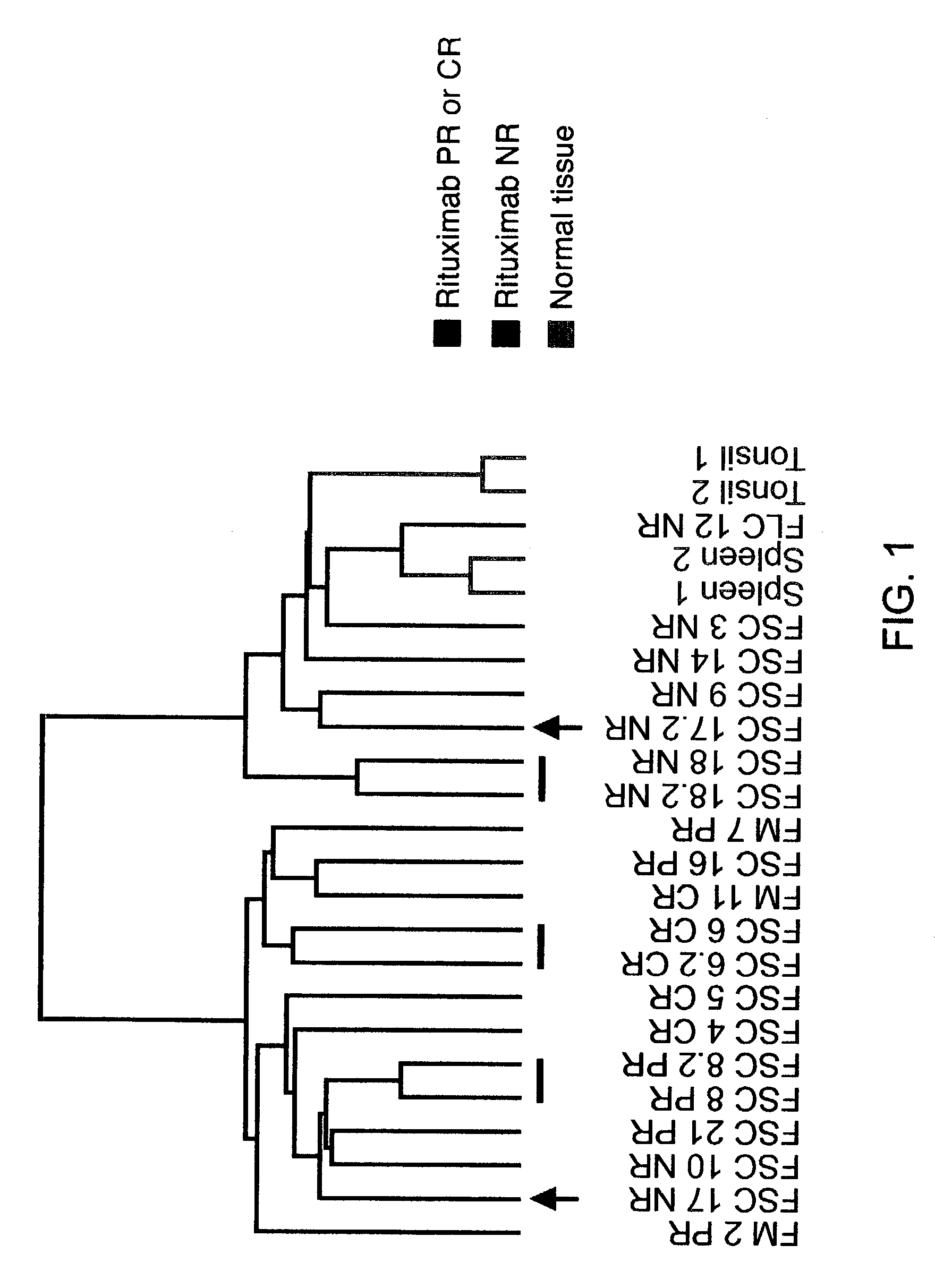
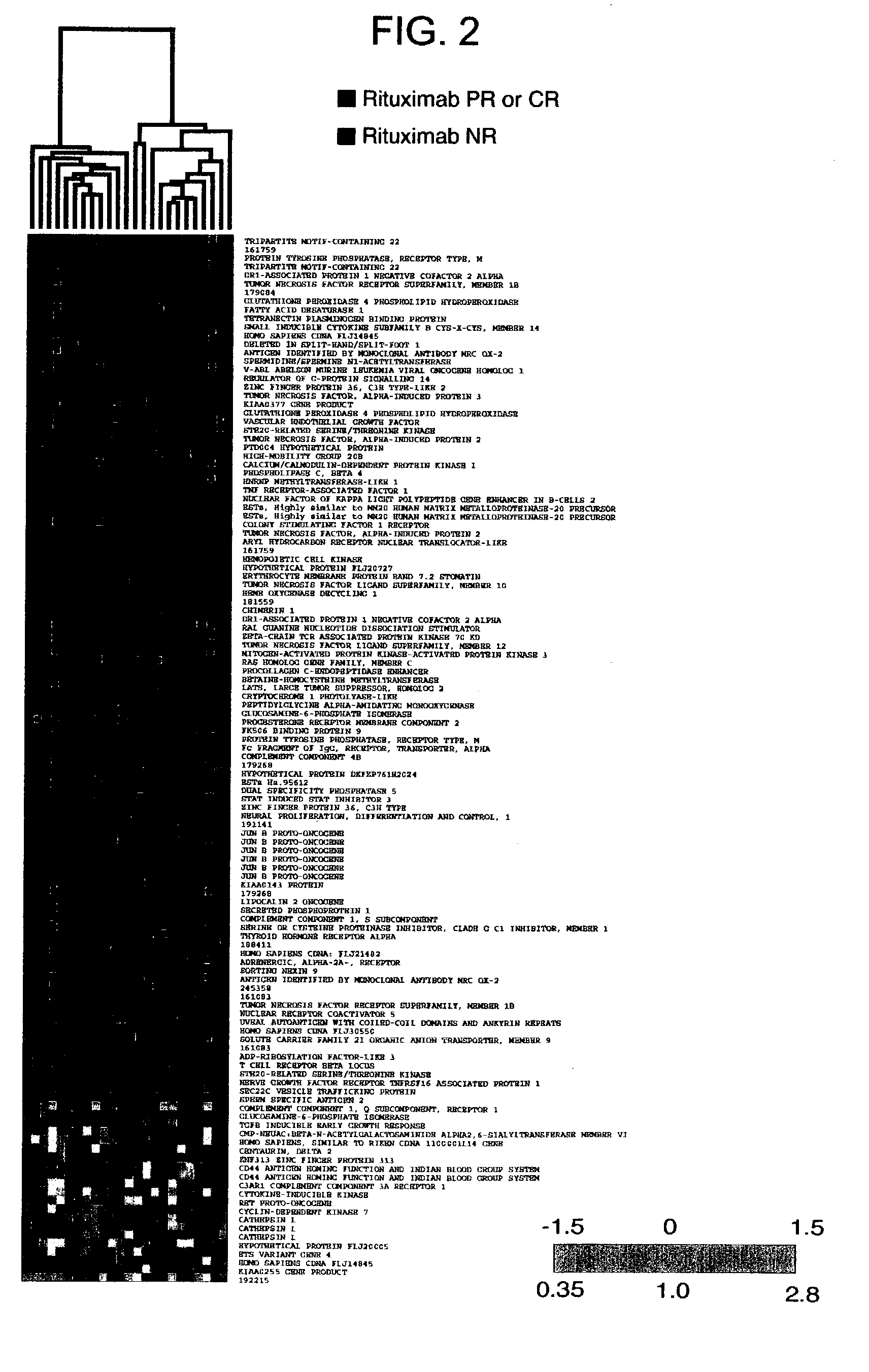
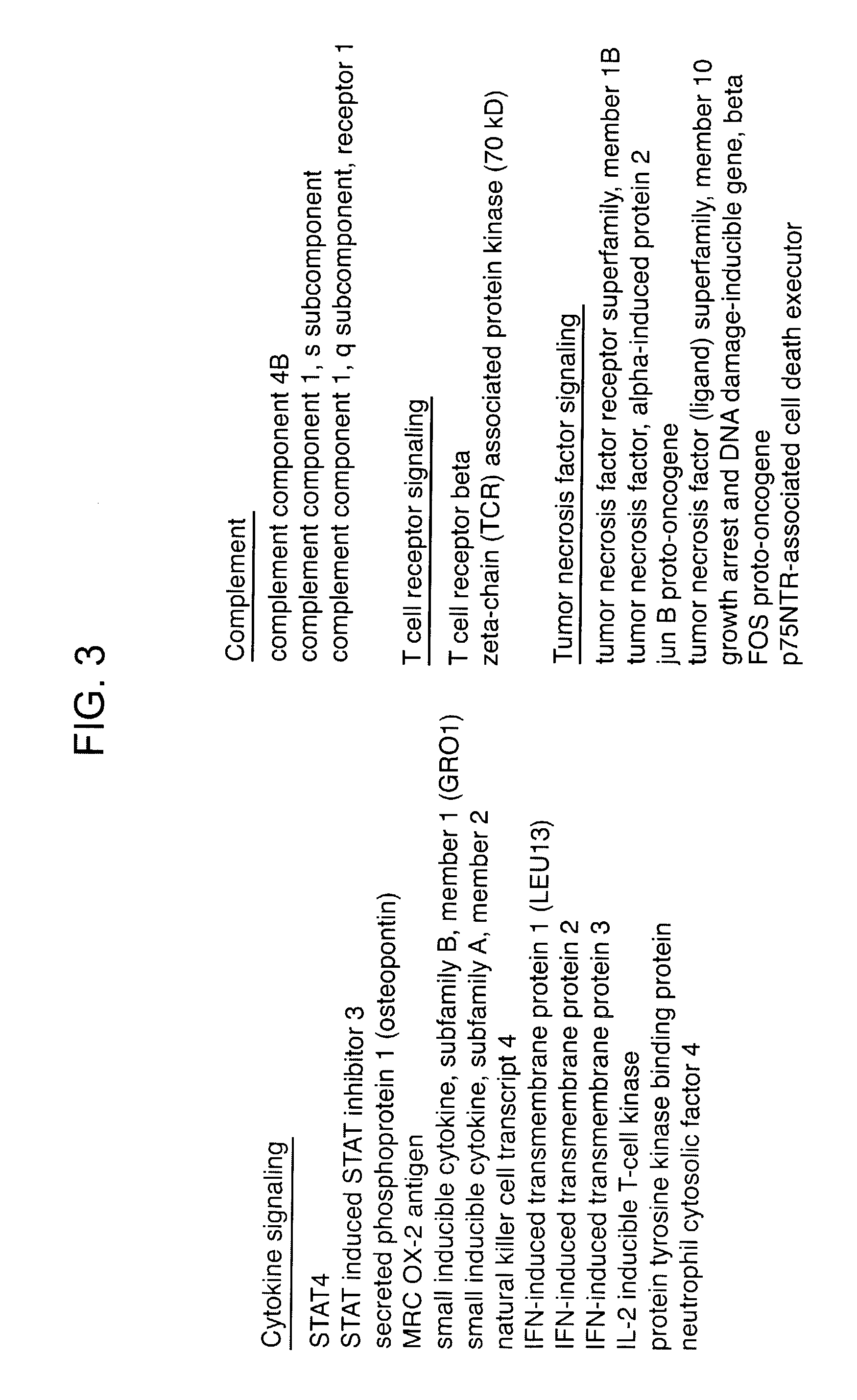
Methods and compositions for determining neoplastic disease responsiveness to antibody therapy
InactiveUS20030219818A1Microbiological testing/measurementDisease diagnosisFollicular lymphoma grade IIWilms' tumor
Methods are provided for determining whether a subject suffering from a neoplastic condition, e.g., non-Hodgkin's lymphoma (NHL), such as follicular lymphoma, is responsive to antineoplastic therapy, such as antibody therapy, e.g., Rituximab. In practicing the subject methods, an expression profile is obtained from the subject suffering from NHL and employed to determine whether the subject is responsive to antineoplastic therapy. In addition, reagents and kits thereof that find use in practicing the subject methods are provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
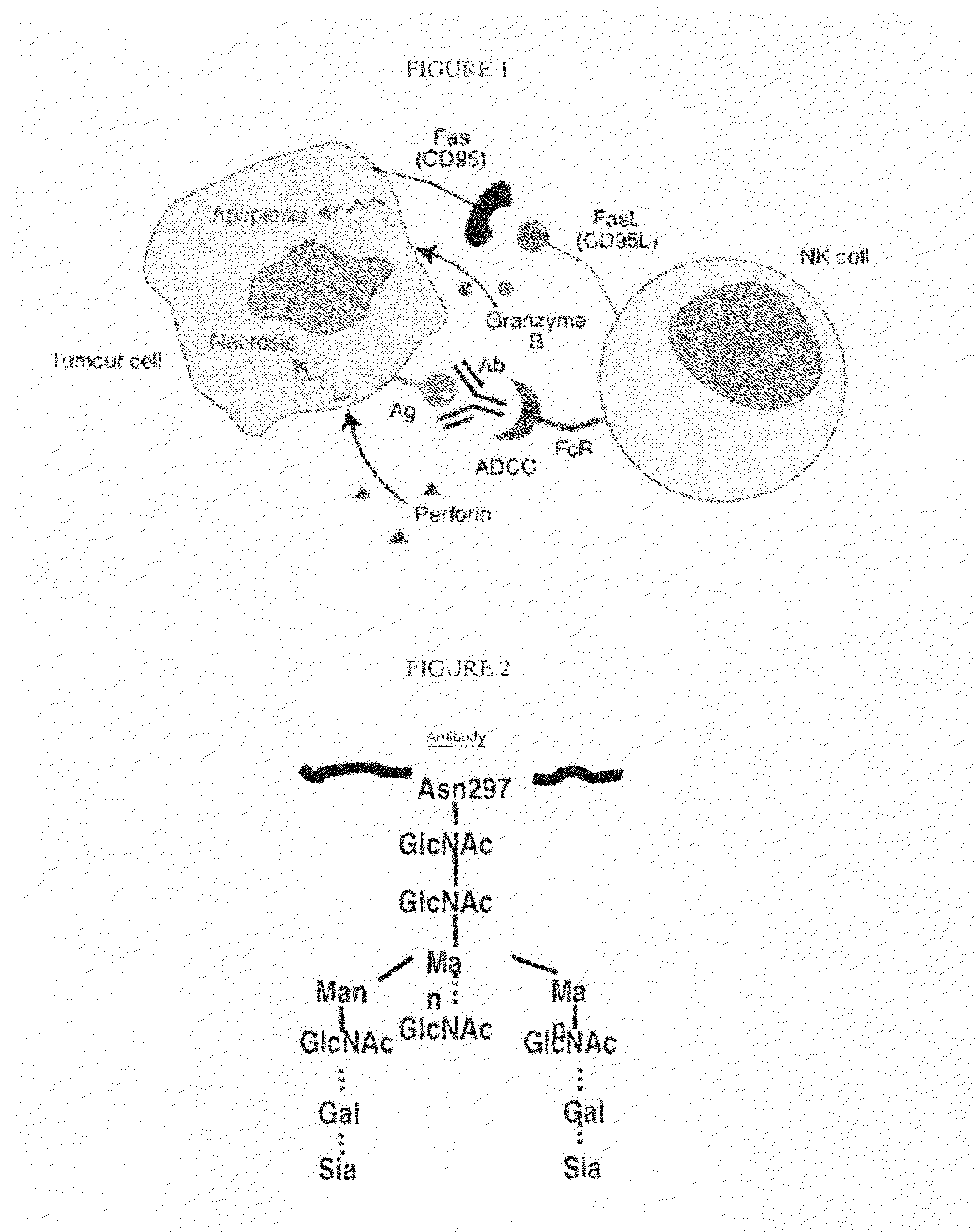
Glycosylation engineered antibody therapy
InactiveUS20100173323A1Good curative effectLow toxicityAntibody ingredientsImmunoglobulinsDiseaseDrug biological activity
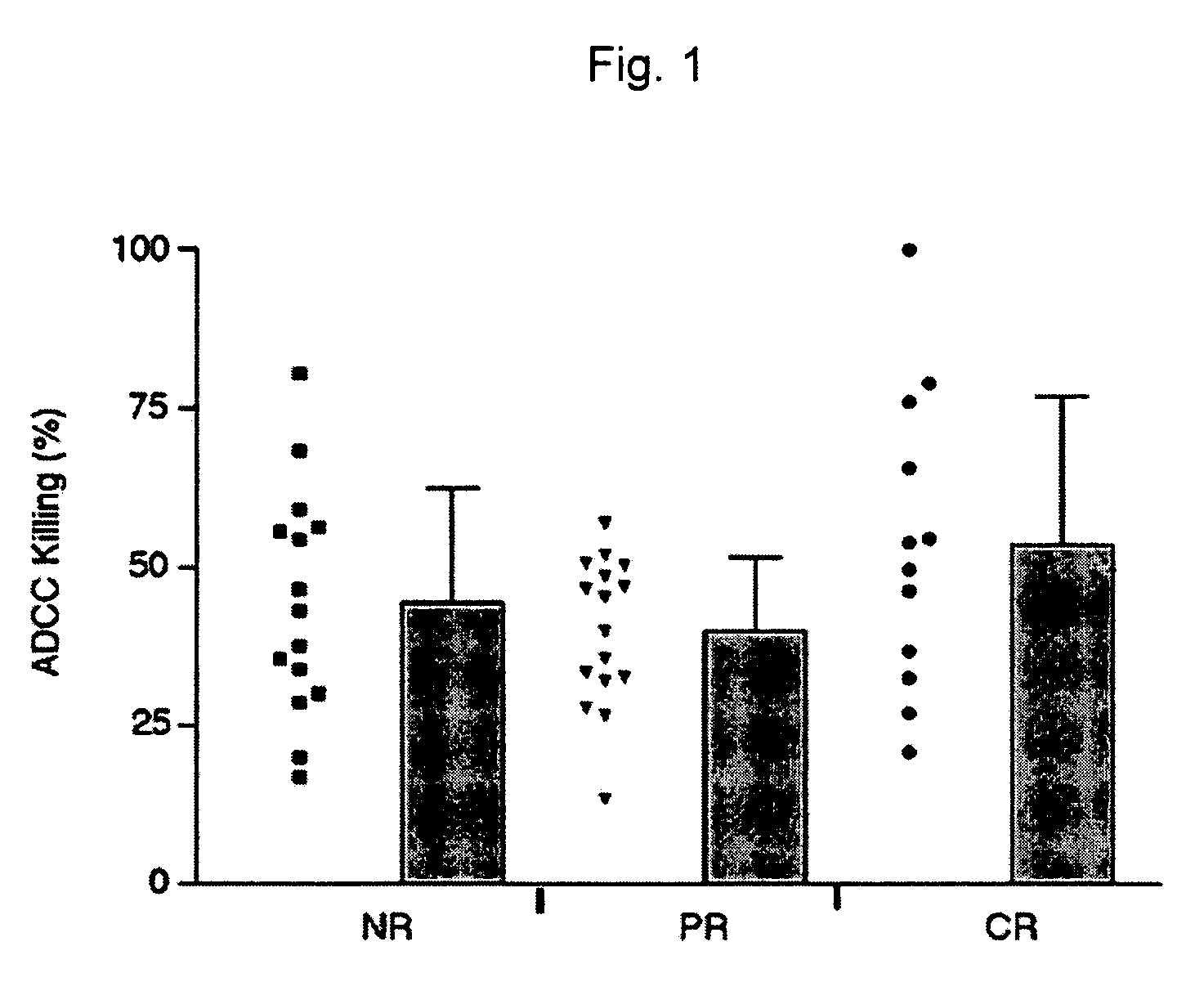
The instant invention is drawn to methods of generating a glycosylation-engineered antibody, and using the glycosylation-engineered antibody for treating a patient, particularly a cancer patient or a patient with an immune disease or disorder. The instant invention is also drawn to methods of generating a glycosylation-engineered antibody for use in the treatment of patients having a polymorphism that does not respond to conventional antibody therapy. The instant invention is also drawn to methods of improving the biological activity of an antibody by glycosylation engineering. The instant invention is also drawn to methods of modulating antibody-dependent cell-mediated cytoxicity (ADCC) using a glycosylation-engineered antibody.
Owner:UNIV OF MARYLAND BIOTECH INST +1
Treatment of B cell malignancies using combination of B cell depleting antibody and immune modulating antibody related applications
InactiveUS20050123540A1Radioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsCD37Combination therapy
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:IDEC PHARM CORP +1
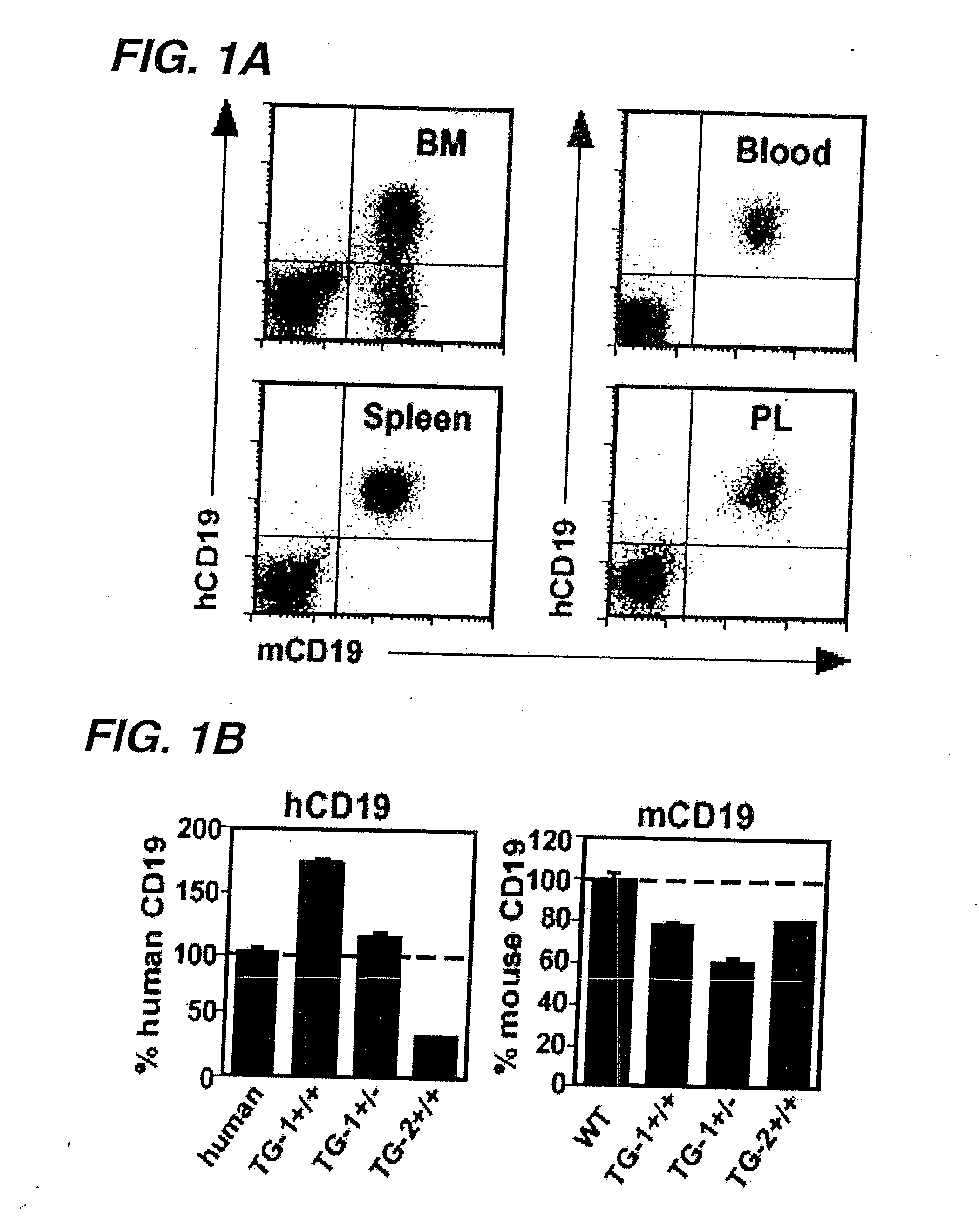
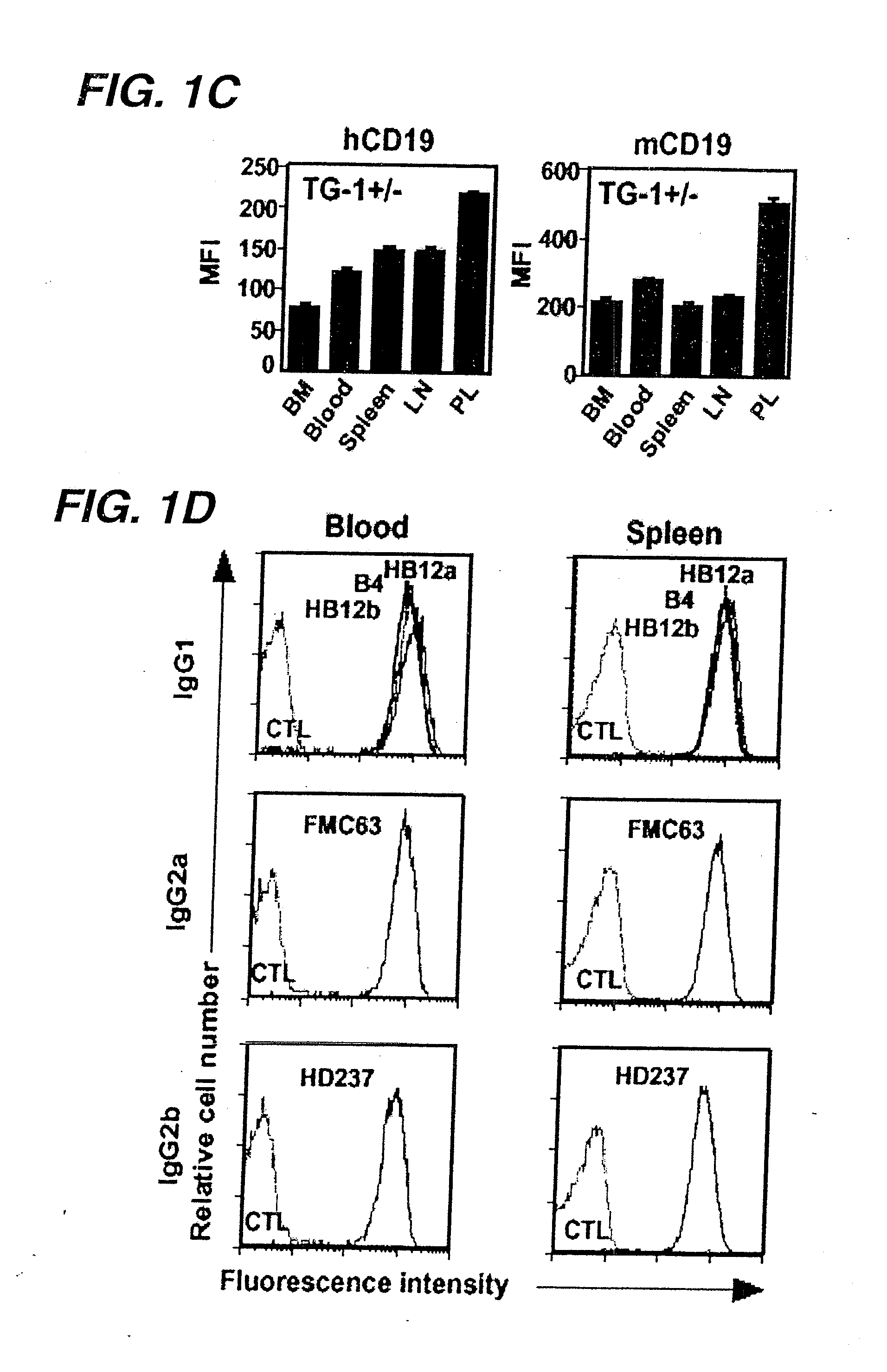
Anti-CD19 antibody therapy for transplantation
InactiveUS20060280738A1Efficient productionEfficiently depletedBiocidePhosphorous compound active ingredientsAntigenDisease
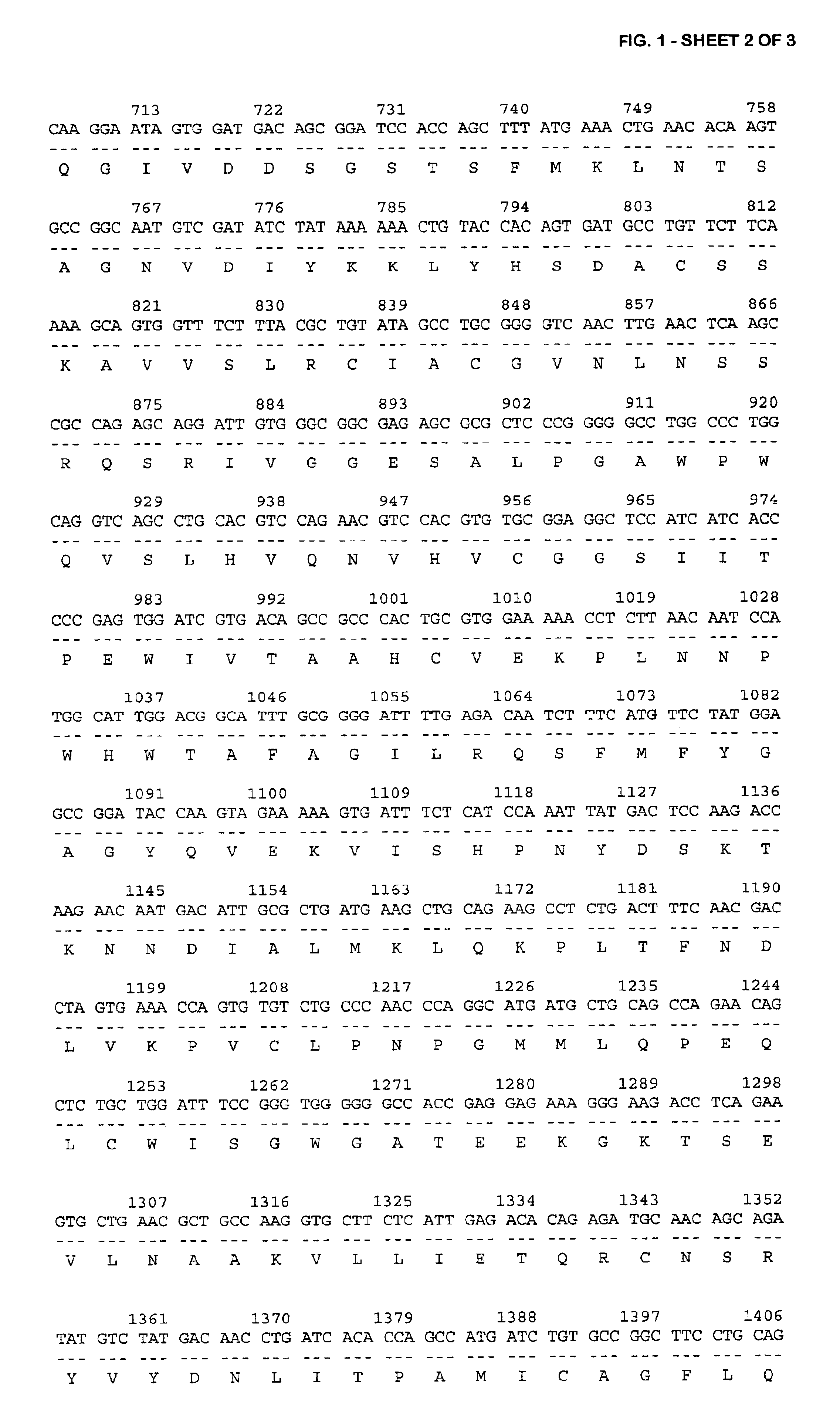
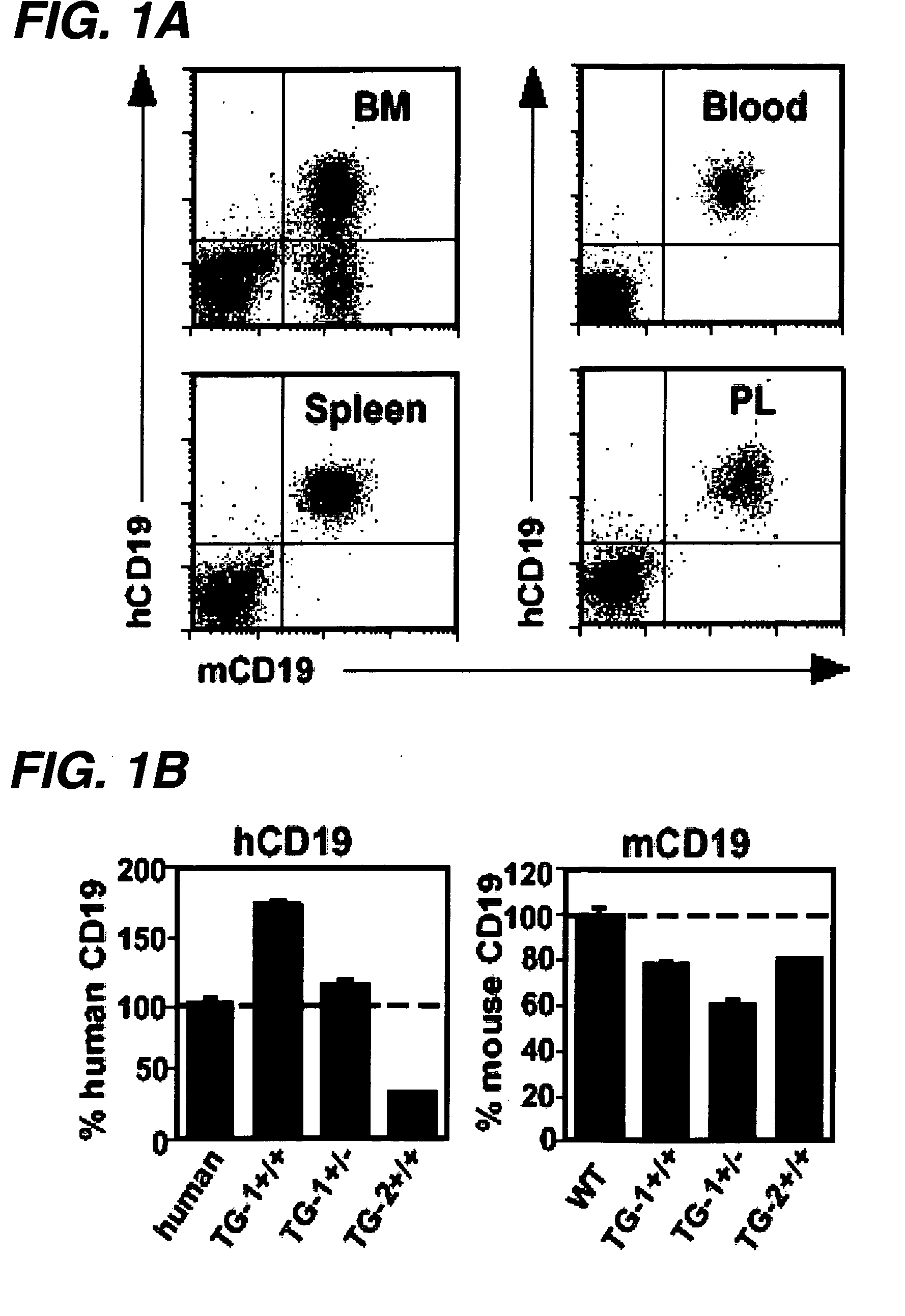
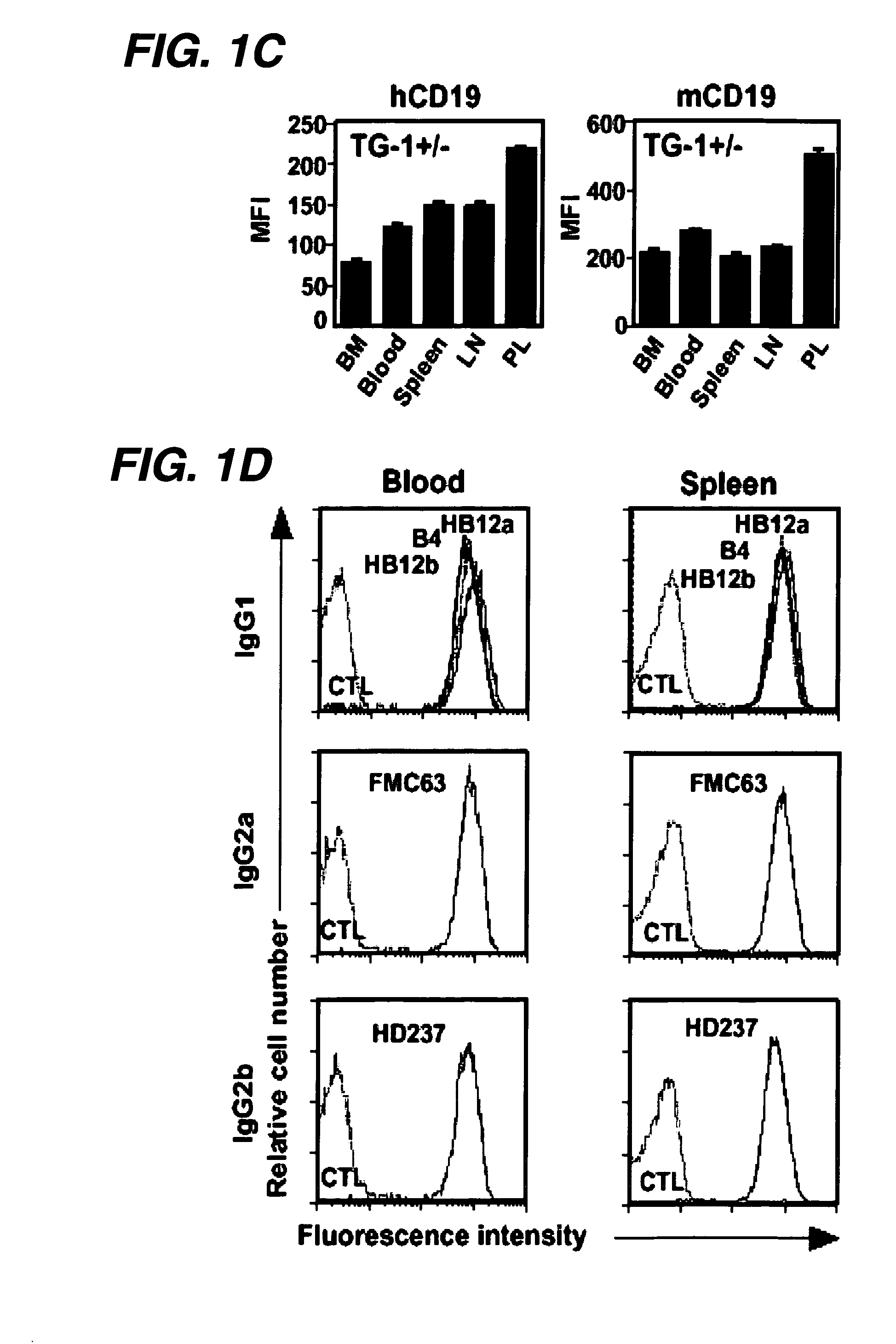
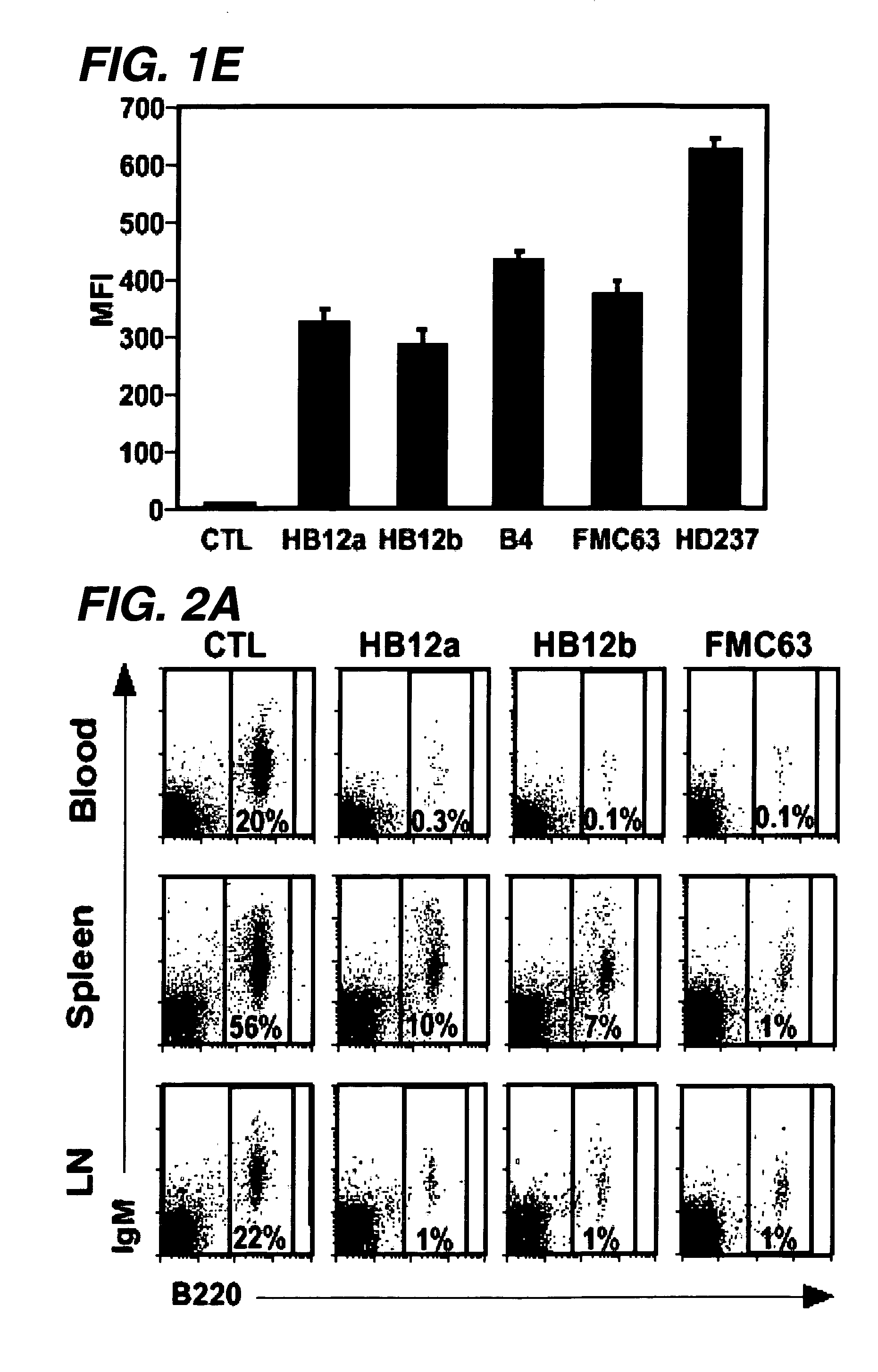
The invention relates to immunotherapeutic compositions and methods for the treatment and prevention of GVHD, humoral rejection, and post-transplantation lymphoproliferative disorder in human subjects using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD 19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
Novel antibody therapies
Antibody capable of mediating effector function which specifically binds to a multiple membrane spanning antigen or to an antigen which forms dimers or multimers (i) for use in combination with a cholesterol-increasing agent in the treatment of a disease or disorder associated with said antigen, wherein antibody-induced effector function has a beneficial effect on said disease or disorder or (ii) for use in the treatment of such disease or disorder, wherein the antibody is to be administered to a subject undergoing therapy with a cholesterol-lowering agent, such as a statin, and wherein the subject is withdrawn from treatment with the cholesterol-lowering agent prior to the administration of the antibody. Furthermore, a kit of parts comprising such antibody as well as a cholesterol-increasing agent.
Owner:GENMAB AS
Blood test to monitor the genetic changes of progressive cancer using immunomagnetic enrichment and fluorescence in situ hybridization (FISH)
InactiveUS20080113350A1Accurate measurementEasy accessMicrobiological testing/measurementLymphatic SpreadGenetic Change
Amplification and overexpression of theHER-2 oncogene in breast cancer is felt to be stable over the course of disease and concordant between the primary tumor and metastases. Therefore, patients with HER-2 negative primary tumors will rarely receive anti-HER-2 antibody therapy. A very sensitive blood test is used to capture circulating tumor cells (CTC's) and evaluate their HER-2 gene status by FISH evaluation. The HER-2 status of the primary tumor and corresponding CTC's is used to assess the ratio of CTC's as a reliable surrogate marker. HER-2 expression of 10 CTC's is sufficient to make a definitive diagnosis of the HER-2 gene status for the whole population of CTC's in patients with recurrent breast cancer.
Owner:JANSSEN DIAGNOSTICS LLC
Superantibody synthesis and use in detection, prevention and treatment of disease
InactiveUS20090208418A1Facilitate its translocationFunction increaseUltrasonic/sonic/infrasonic diagnosticsSurgeryPurineApoptosis
Superantibodies having enhanced autophilic, catalytic, and / or membrane-penetrating properties are prepared by affinity-based conjugation of a photoactivatable organic molecule to a target immunoglobulin. The photoactivatable organic molecule bears a chromophoric aromatic hydrocarbon moiety, which has affinity for the immunoglobulin. Upon photolysis, the organic molecule is covalently linked to the immunoglobulin. A preferred organic molecule is a peptide and a preferred aromatic hydrocarbon moiety is a tryptophan residue. The photoactivatable organic molecule need not bear a purine, pyrimidine or azido group to effect binding to the immunoglobulin and / or photoactivation. The superantibodies can enhance the potency and expand the targeting range of target antibodies. Autophilic superantibodies can promote apoptosis of target cells and / or enhance therapeutic efficacies in the treatment of patients with diseases or disorders responsive to antibody therapy. Exemplary of such diseases are atherosclerosis and cardiovascular disease. Membrane-penetrating superantibodies can prevent apoptosis by binding to intracellular anti-caspase signal proteins. Compositions containing the superantibodies, as well as methods of making and using them, are disclosed.
Owner:INNEXUS BIOTECHNOLOGY INT LTD
Immunoregulatory Antibodies and Uses Thereof
InactiveUS20070009519A1Convenient treatmentIn-vivo radioactive preparationsPharmaceutical containersCD37Combination therapy
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Tumor antigen useful in diagnosis and therapy of prostate and colon cancer
InactiveUS7037667B1Microbiological testing/measurementBiological material analysisCell membraneTumor antigen
Owner:AGENSYS
Anti-CD19 antibody therapy for autoimmune disease
InactiveUS20060263357A1Efficient productionEfficiently depletedAntipyreticMetabolism disorderAntigenImmunologic disorders
The invention relates to immunotherapeutic compositions and methods for the treatment of autoimmune diseases and disorders in human subjects using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
Prophylaxis and treatment of enterocolitis associated with Anti-ctla-4 antibody therapy
InactiveUS20070243184A1Reduce inflammationReduce morbidityOrganic active ingredientsGenetic material ingredientsEnterocolitisImmunotherapy
The present invention provides methods for reducing the incidence of adverse events related to immunotherapy. More specifically, the present invention provides methods for reducing the incidence of enterocolitis associated with anti-CTLA-4 antibody immunotherapy.
Owner:CEDARS SINAI MEDICAL CENT +2
Antibody therapy for use in the digestive tract
InactiveUS20130337018A1Milk immunoglobulinsPharmaceutical delivery mechanismAntibody therapyAntibody
In accordance with the invention, the development and use of antibodies within the digestive tract is provided. Antibodies are described that are used to treat disorders associated with altered permeability of the digestive tract. Antibodies are described with increased stability within the environment of the digestive tract. Antibodies are described with enhanced permeability to a compromised digestive tract.
Owner:CIRCLE 33 LLC
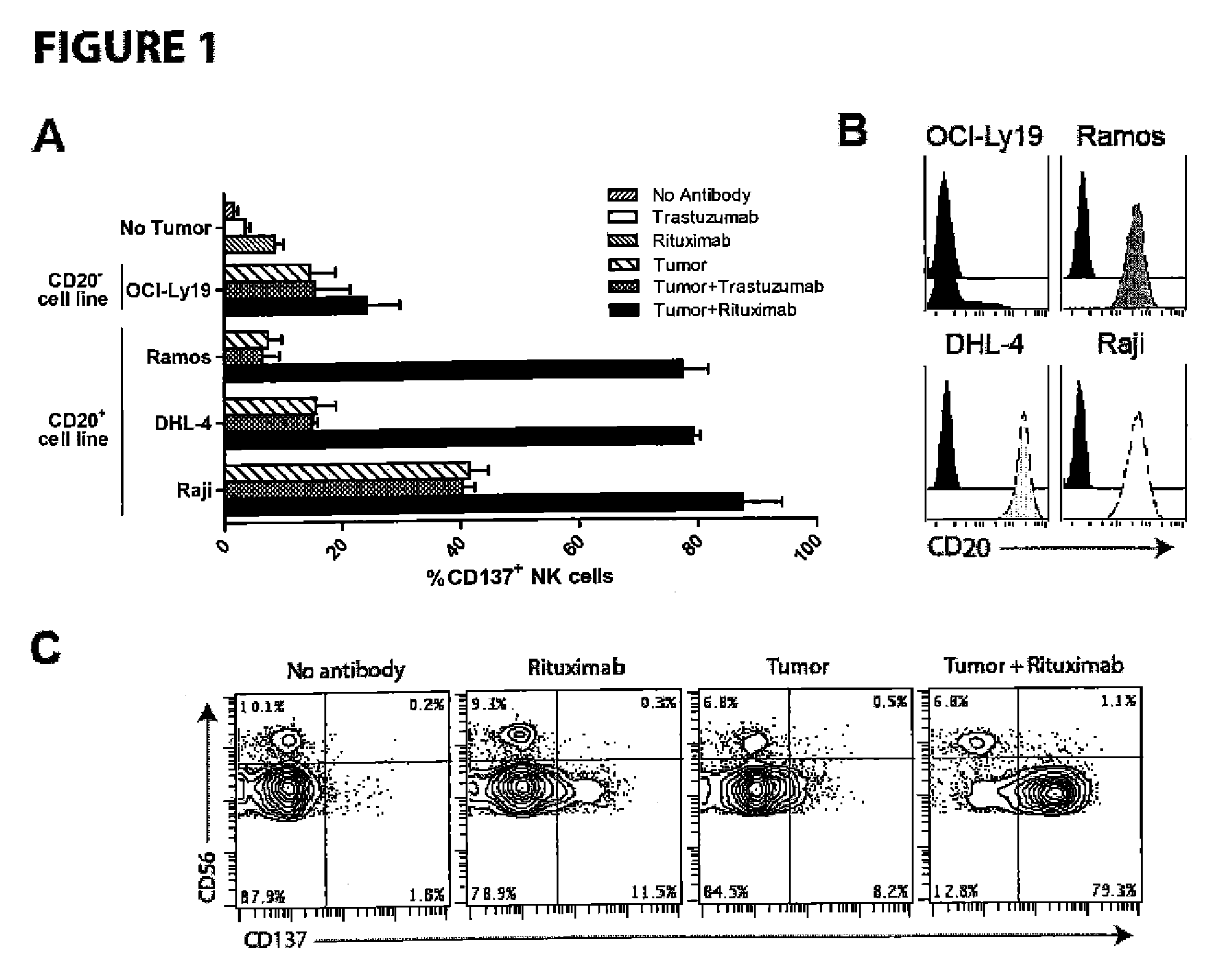
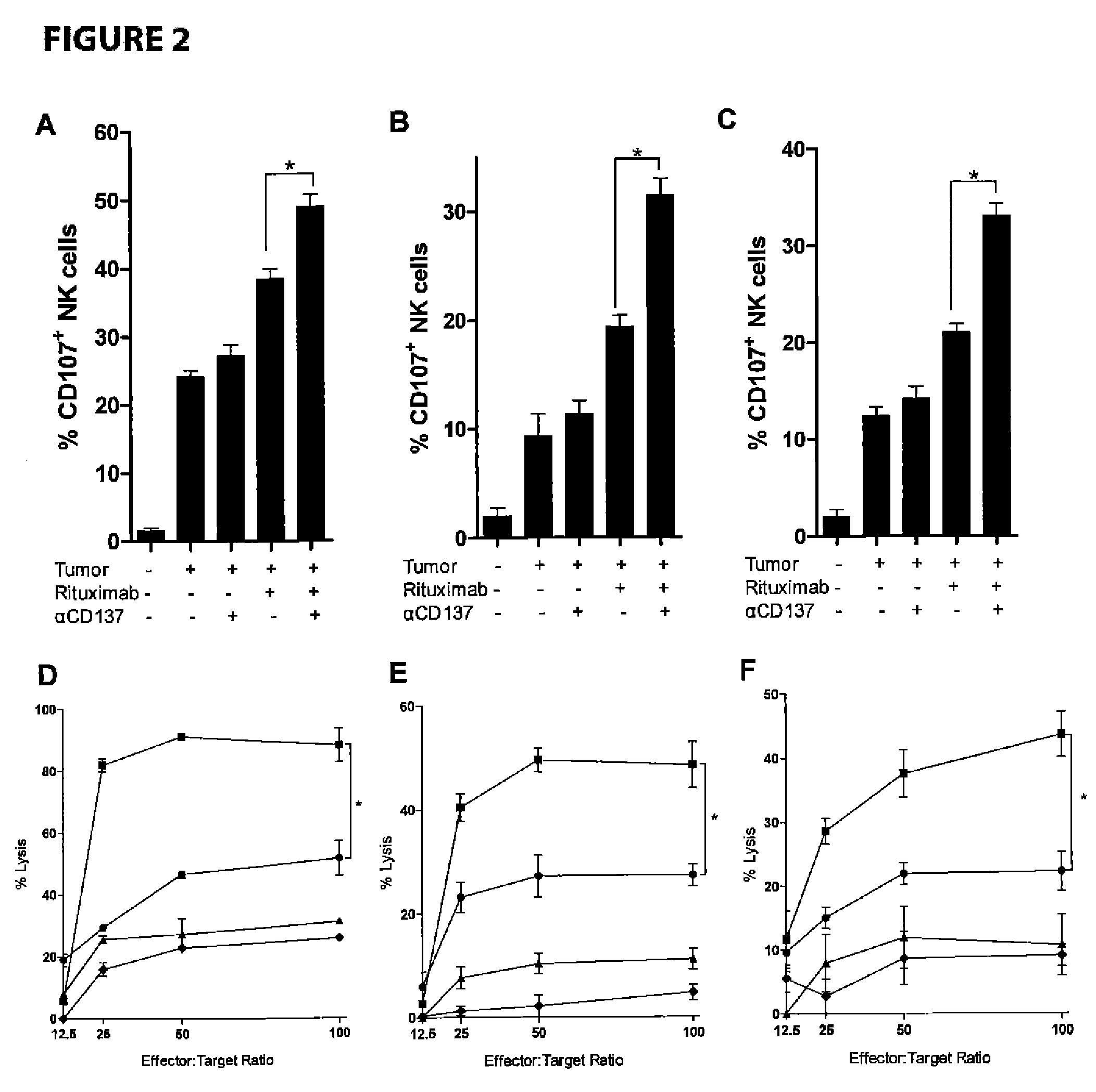
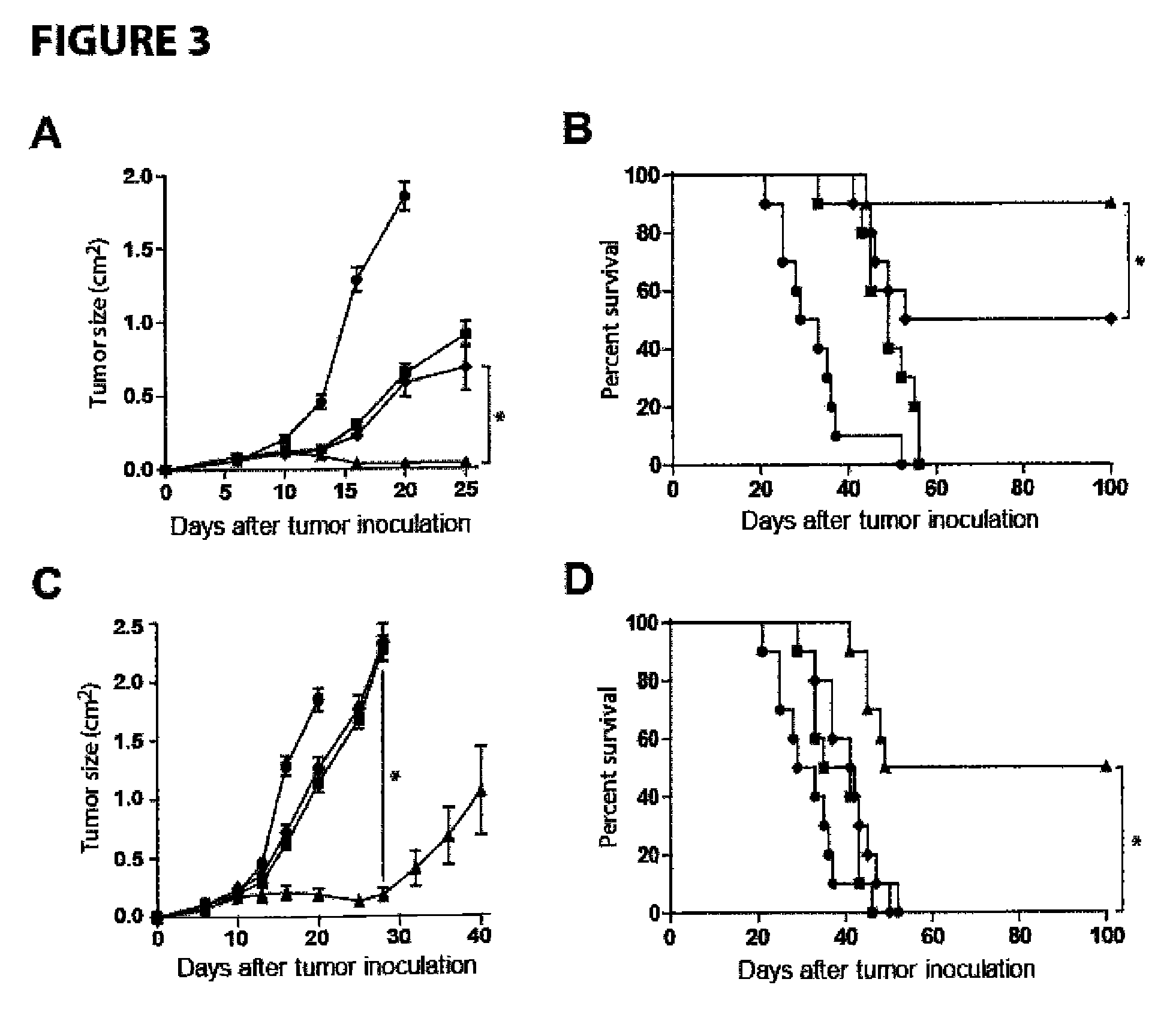
Methods for enhancing anti-tumor antibody therapy
InactiveUS9005619B2Improve anti-tumor effectEnhances target cell killingBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsCell Surface AntigensAgonist
Methods of enhancing the efficacy of antibody-directed cellular cytotoxicity (ADCC) for therapy directed to killing of tumor cells are disclosed. Cancer specific cell surface antigens are bound by monoclonal antibodies, thereby stimulating a cytotoxic T cell response characterized by an upregulation of cell surface expression of costimulatory molecules on the T cell. The ADCC response is augmented by the subsequent administration of a second antibody that is an agonist of the costimulatory molecule.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
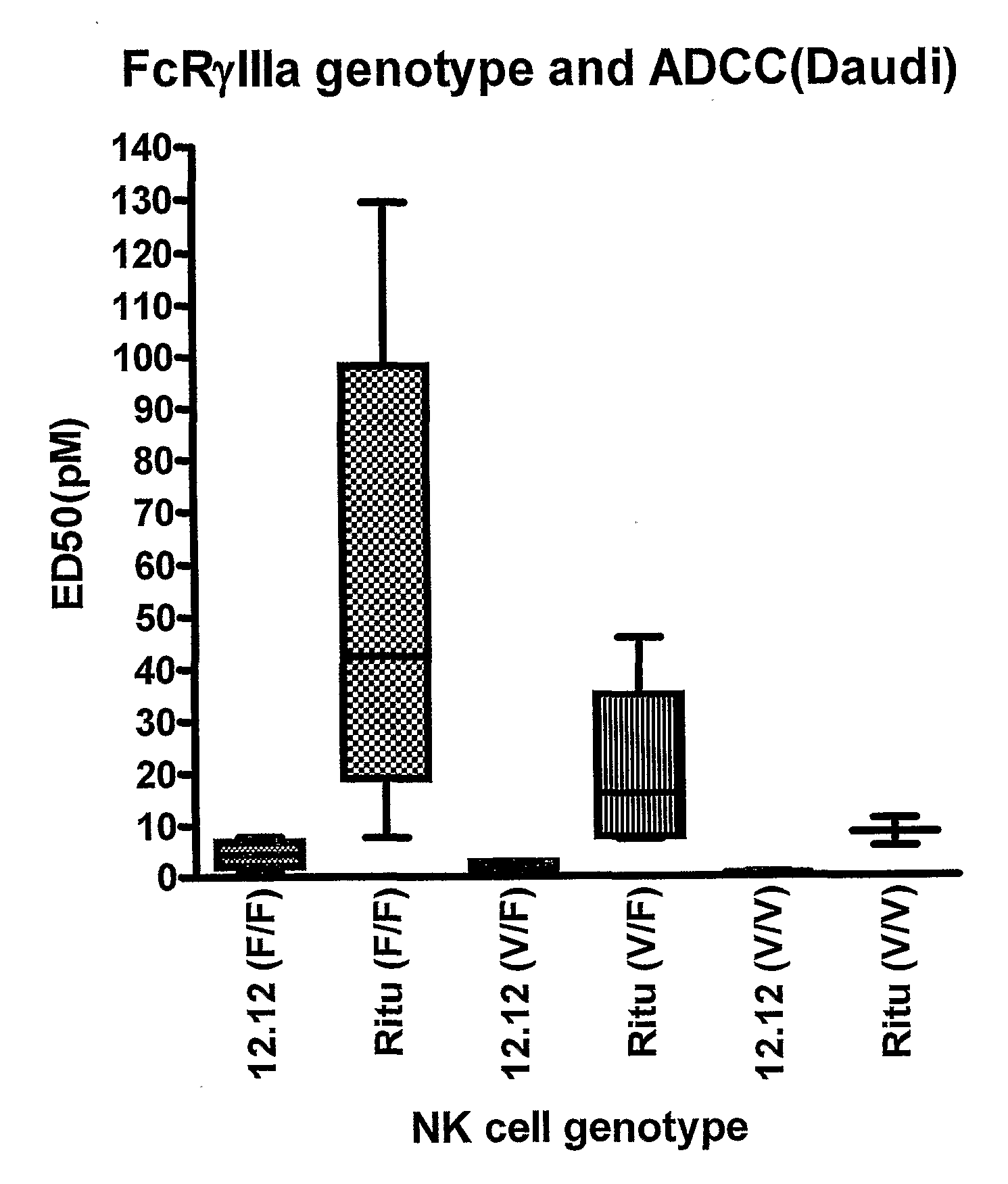
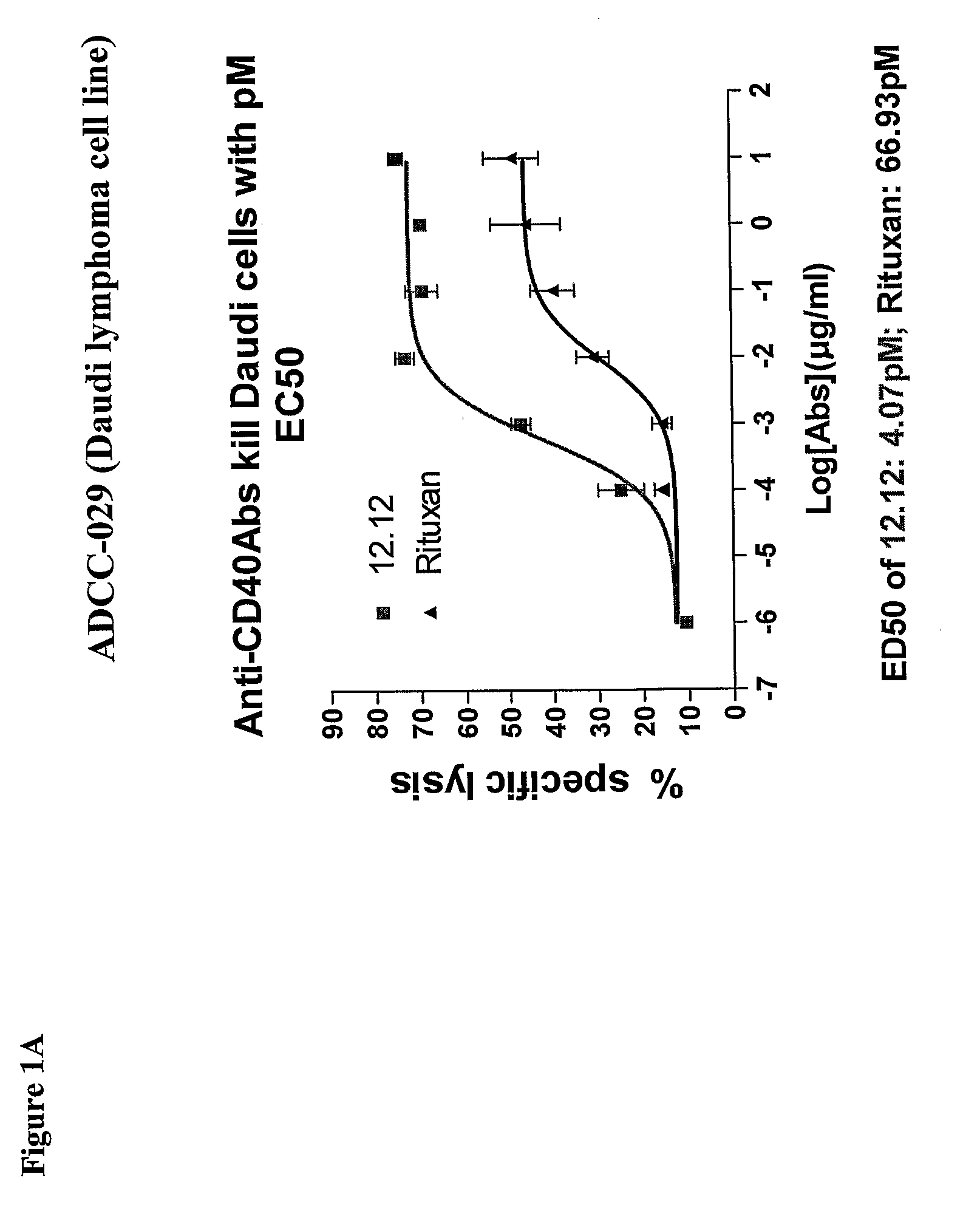
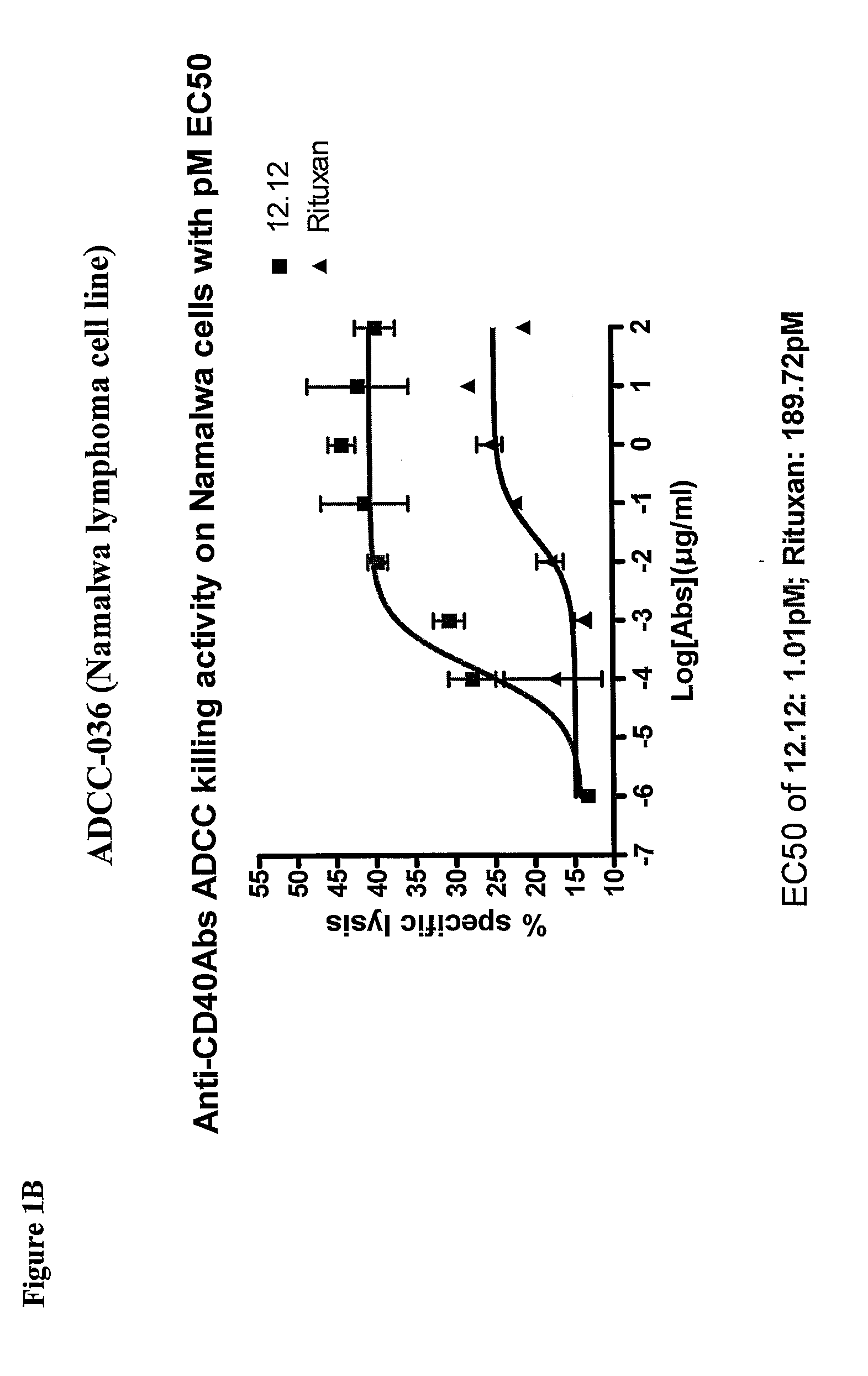
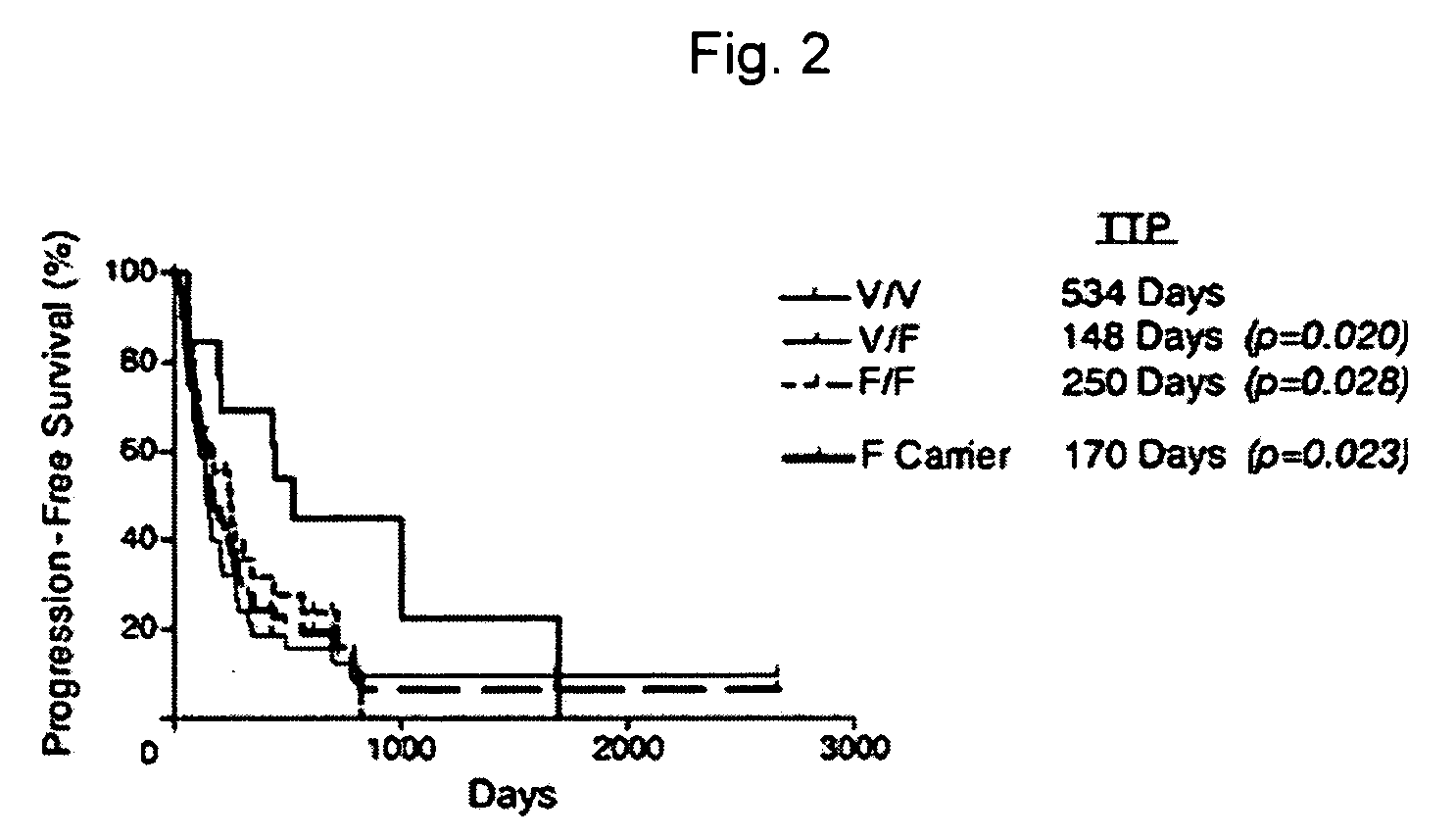
Uses of Anti-cd40 antibodies
Methods for treating a human patient for a cancer or pre-malignant condition that is associated with CD40-expressing cells are provided, where the human patient is heterozygous or homozygous for FcγRIIIa-158F (genotype V / F or F / F). Also provided are methods of inhibiting antibody production by B cells in a human patient who is heterozygous or homozygous for FcγRIIIa-158F (genotype V / F or F / F). The methods comprise administering to the human patient a therapeutically or prophylactically effective amount of an anti-CD40 antibody. Methods and kits for identifying a human patient with a cancer or pre-malignant condition that is treatable with an anti-CD40 antibody and which is refractory to treatment with rituximab (Rituxan®), as well as methods and kits for selecting an antibody therapy for treatment of a human patient having a cancer or pre-malignant condition that is refractory to treatment with rituximab (Rituxan®), are also provided. The methods of the present invention find use in treatment of cancers and pre-malignant conditions that are associated with CD40-expressing cells. These methods are particularly advantageous with respect to cancers and pre-malignant conditions that are associated with cells expressing both CD40 and CD20, as the methods enable the treatment of patients having a cancer or pre-malignant condition that is refractory to therapy with other oncotherapeutic agents such as anti-CD20 antibodies.
Owner:XOMA TECH LTD
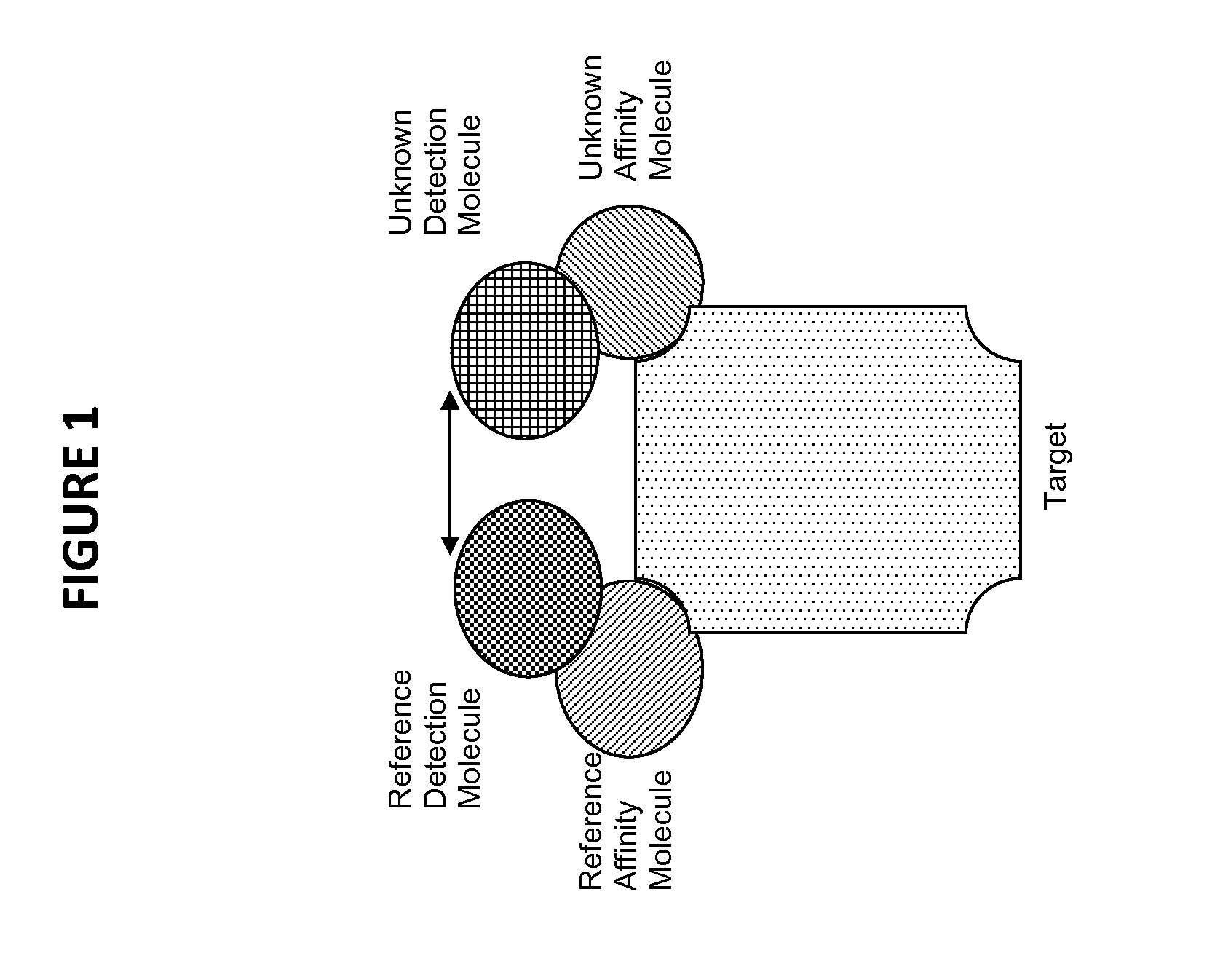
Novel method for the selection of specific affinity binders by homogeneous noncompetitive assay
The invention generally relates to the field of immunochemistry including antibody therapy, diagnostics, and basic research and specifically relates to the area of selecting affinity molecules such as natural antibodies, including artificial antibodies, antibody mimics, and aptamers. The invention relates particularly to a method of selecting affinity molecules using a homogeneous noncompetitive assay in a high throughput process.
Owner:DYNAMIC AFFINITY REAGENTS
Anti-light antibody therapy for inflammatory bowel disease
InactiveUS20130315913A1Increase currentCompounds screening/testingImmunoglobulins against cytokines/lymphokines/interferonsHerpes simplex virus DNAT lymphocyte
The present invention provides safe therapeutic doses of an antagonist of human LIGHT (lymphotoxin-like, exhibits inducible expression and competes with Herpes Virus Glycoprotein D for Herpes Virus Entry Mediator (HVEM), a receptor expressed by T lymphocytes), as well as methods of monitoring whether a therapeutic dose of an anti-LIGHT antagonist is safe.
Owner:SANOFI SA
Immunoregulatory antibodies and uses thereof
InactiveUS20080227198A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD37CD23
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Anti-cd19 antibody therapy for autoimmune disease
The invention relates to immunotherapeutic compositions and methods for the treatment of autoimmune diseases and disorders in human subjects using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
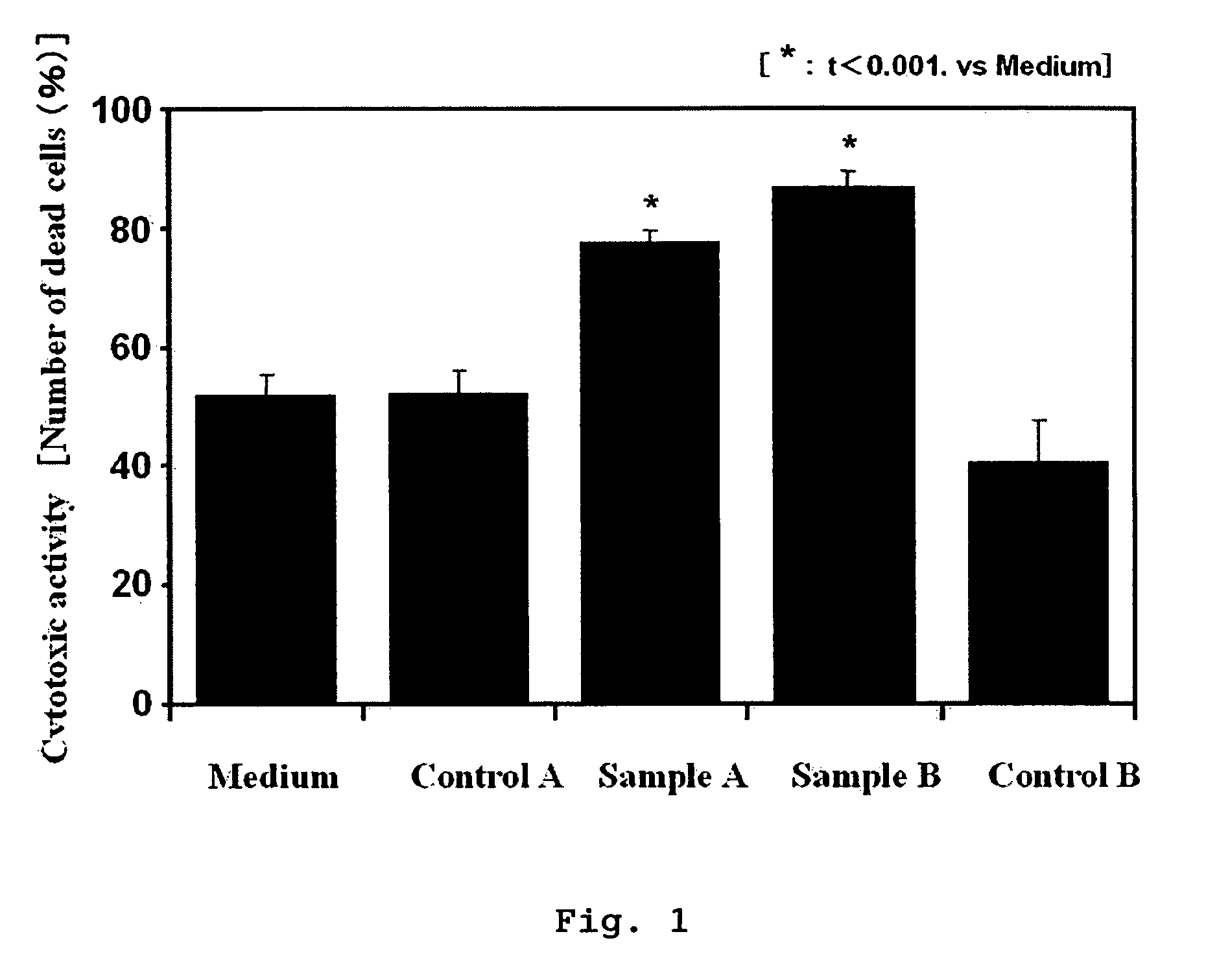
Drug for cancer therapy
InactiveUS7419667B2High cytotoxic activityHigh activityBiocidePeptide/protein ingredientsHydrolysateBULK ACTIVE INGREDIENT
A lactoferrin hydrolysate mixture or lactoferrin partial peptide that can be obtained by hydrolyzing lactoferrin with a hydrolytic enzyme and has an action of enhancing cytotoxic activity of an antibody drug in an antibody therapy of cancer is used as an active ingredient of a drug for enhancing cytotoxic activity of an antibody drug in an antibody therapy of cancer.
Owner:MORINAGA MILK IND CO LTD
Immunoregulatory antibodies and uses thereof
InactiveUS20080226626A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD37CD23
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Methods and systems for multi-antibody therapies
ActiveUS20100278830A1Simple processImprove effectivenessAntibacterial agentsAntimycoticsCancer cellToxin
The present invention relates to methods and systems for administering antibody therapeutic agents. The methods include administering one or more (e.g., two or three) binding agents, wherein each of the binding agents has a binding region that is specific to a portion of a disease agent and one or more copies of a tag. The binding agents can be specific to one or more portions of the same or different disease agents. The tag is the same for each of the binding agents. The methods include administering an anti-tag antibody, wherein the anti-tag antibody has an anti-tag region that is specific to the tag, and can have an immunoglobulin (e.g., IgA, IgD, IgE, IgG, and IgM.). Disease agents include bacterial proteins, viral proteins, cancer cells, and proteins or toxins produced therefrom. In particular, the present invention includes methods and systems for binding agents that are specific to neurotoxins that cause botulism.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
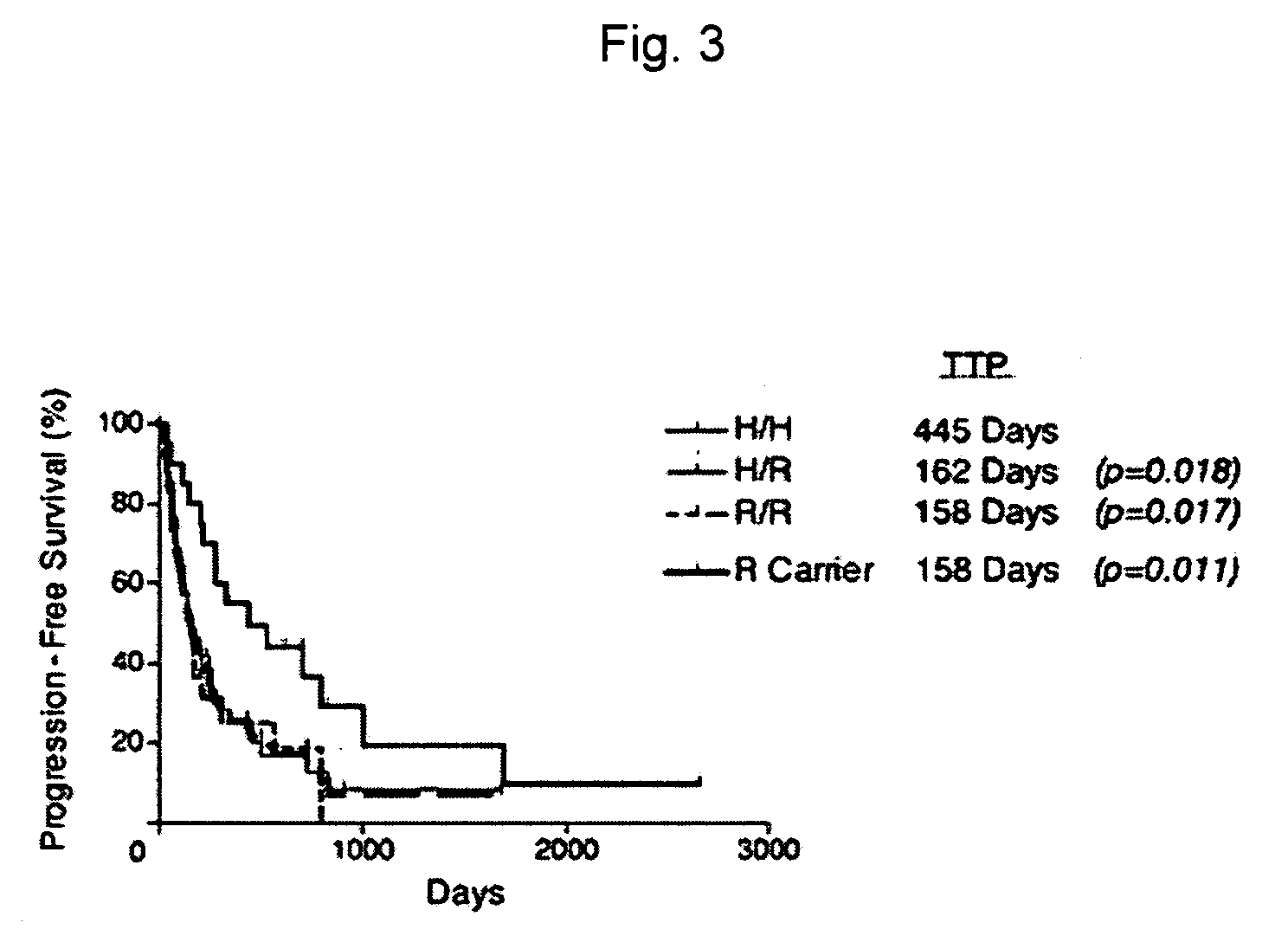
Methods and compositions for determining responsiveness to antibody therapy
ActiveUS20060008825A1Microbiological testing/measurementMedical automated diagnosisMedicineGenotyping
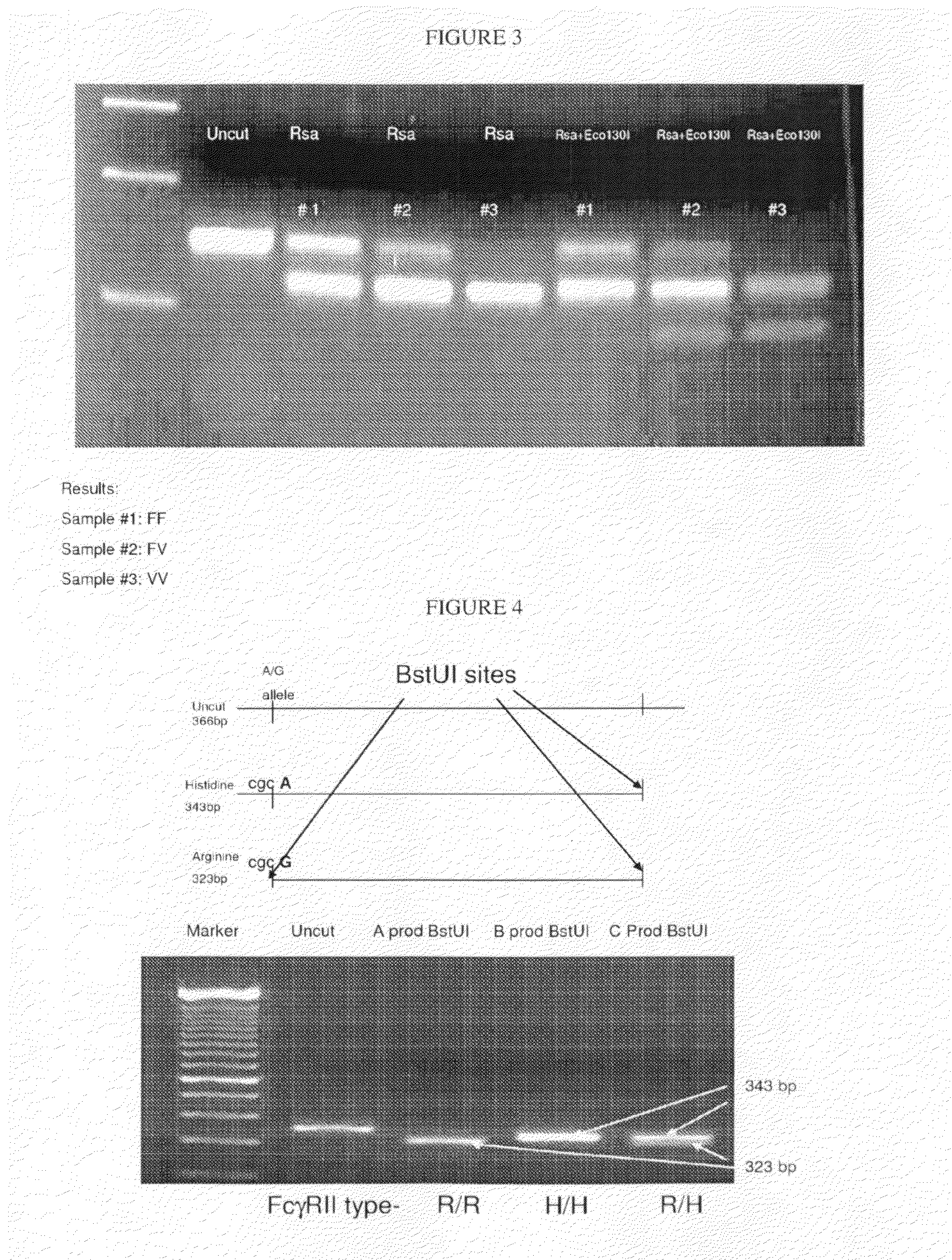
Methods and compositions are provided for determining whether a subject suffering from a neoplastic condition is responsive to an antineoplastic therapy, such as antibody therapy, e.g., Rituximab. In practicing the subject methods, the subject is genotyped to determine whether the subject has a least one favorable FcγR polymorphism, e.g., the 131 H / H genotype or the 158 V / V genotype. In addition, reagents, devices and kits thereof, that find use in practicing the subject methods are provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Novel methods and compositions to upregulate, redirect or limit immune responses to peptides, proteins and other bioactive compounds and vectors expressing the same
InactiveUS20050074449A1Deleterious immune responseAvoid immune responseBiocidePowder deliveryAntigenAutoimmune condition
Owner:NOVARTIS FARMA
Antibody therapy for treatment of diseases associated with gluten intolerance
InactiveUS20070184049A1Specific activityMetabolism disorderMilk immunoglobulinsGluten intoleranceDermatitis herpetiformis
The present invention includes a pharmaceutical compositions and methods for treating diseases associated with gluten intolerance in a patient, comprising: administering to the patient an effective amount of an antibody having specific activity against gluten or gluten-derived peptides. Such diseases include, for example, celiac disease and dermatitis herpetiformis.
Owner:CIRCLE 33 LLC
Antibody therapy for use in the digestive tract
In accordance with the invention, the development and use of antibodies within the digestive tract is provided. Antibodies are described that are used to treat disorders associated with altered permeability of the digestive tract. Antibodies are described with increased stability within the environment of the digestive tract. Antibodies are described with enhanced permeability to a compromised digestive tract.
Owner:CIRCLE 33 LLC
Anti-cd19 antibody therapy for transplantation
InactiveUS20090246195A1Reduce or deplete circulating B cellsReduce or deplete circulating immunoglobulin (Ig)Dead animal preservationAntibody ingredientsAntigenTherapeutic antibody
The invention relates to immunotherapeutic compositions and methods for the treatment and prevention of GVHD, humoral rejection, and post-transplantation lymphoproliferative disorder in human subjects using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com