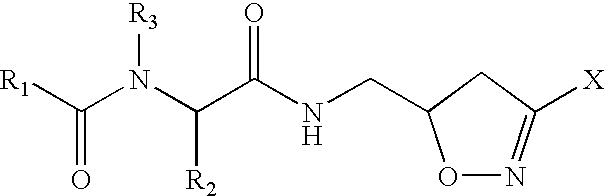
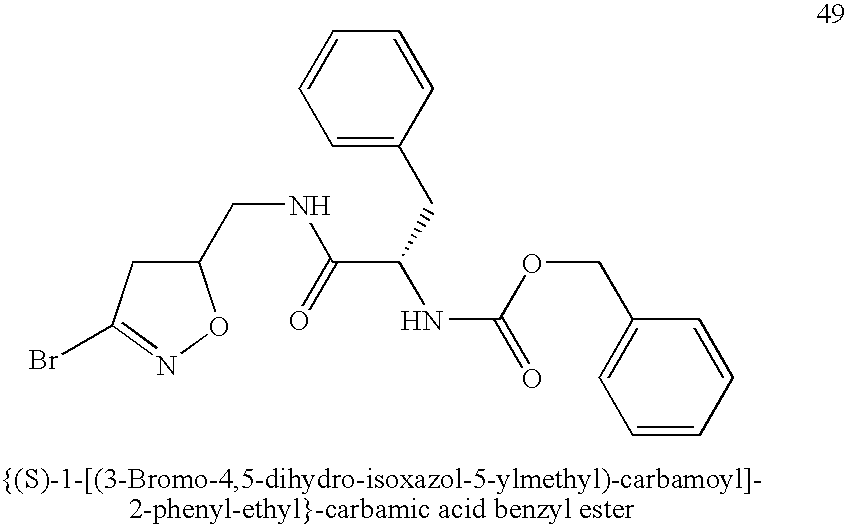
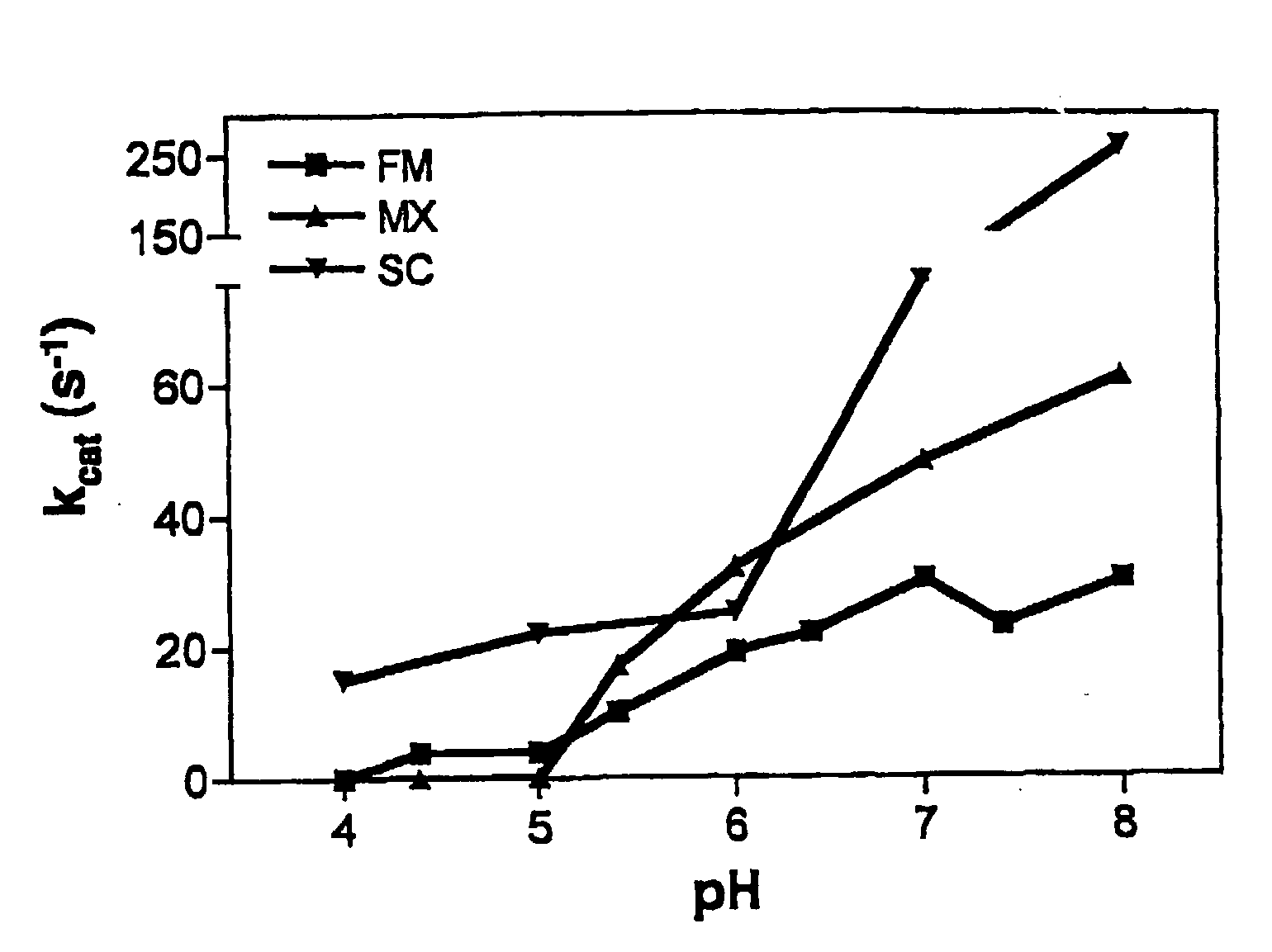
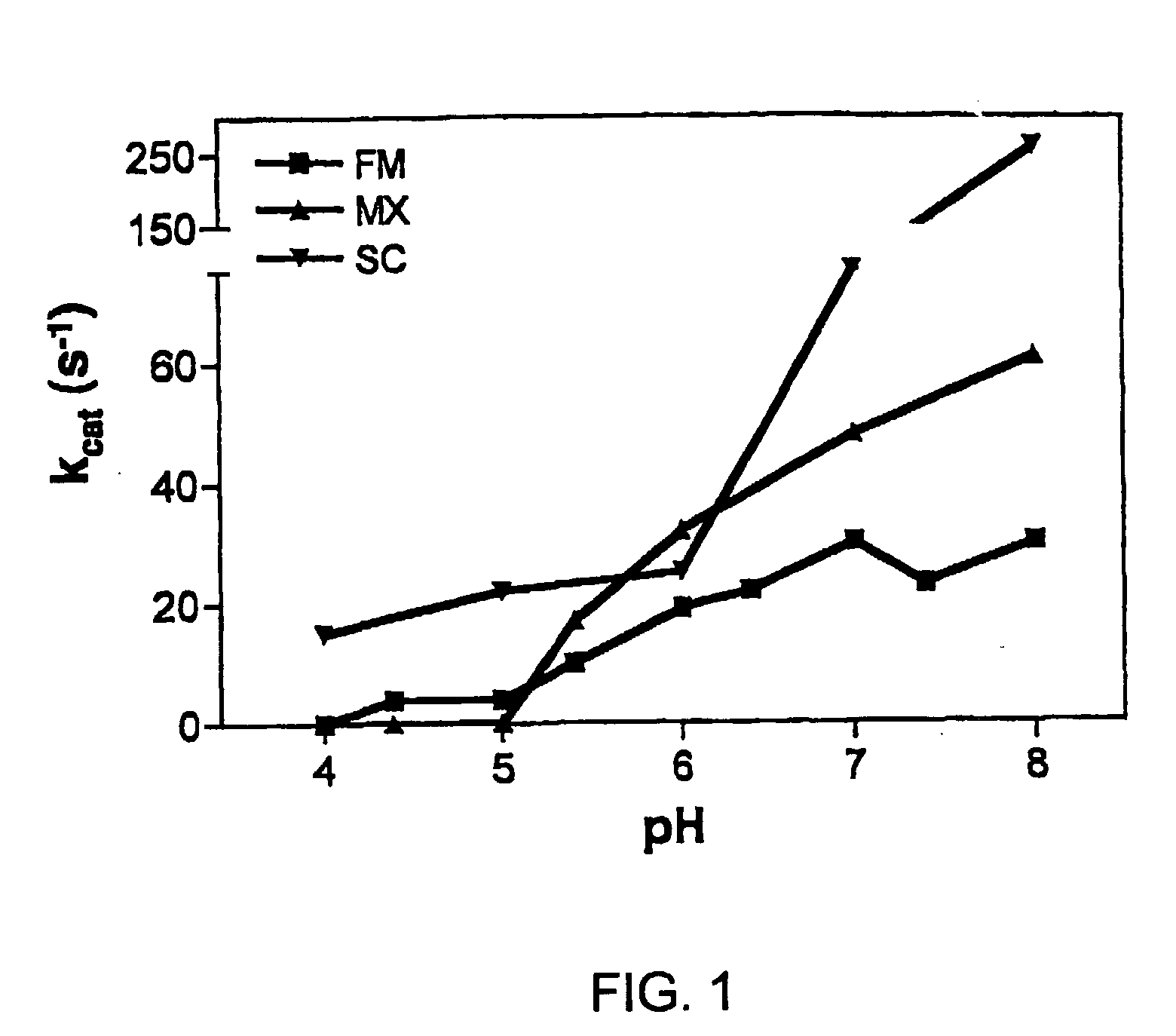
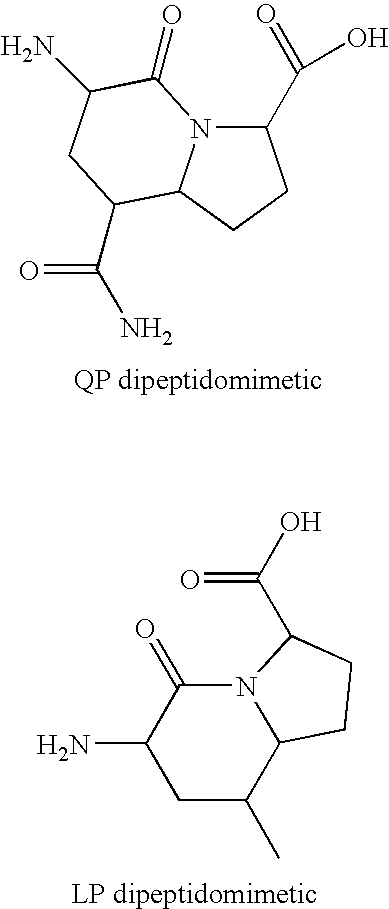
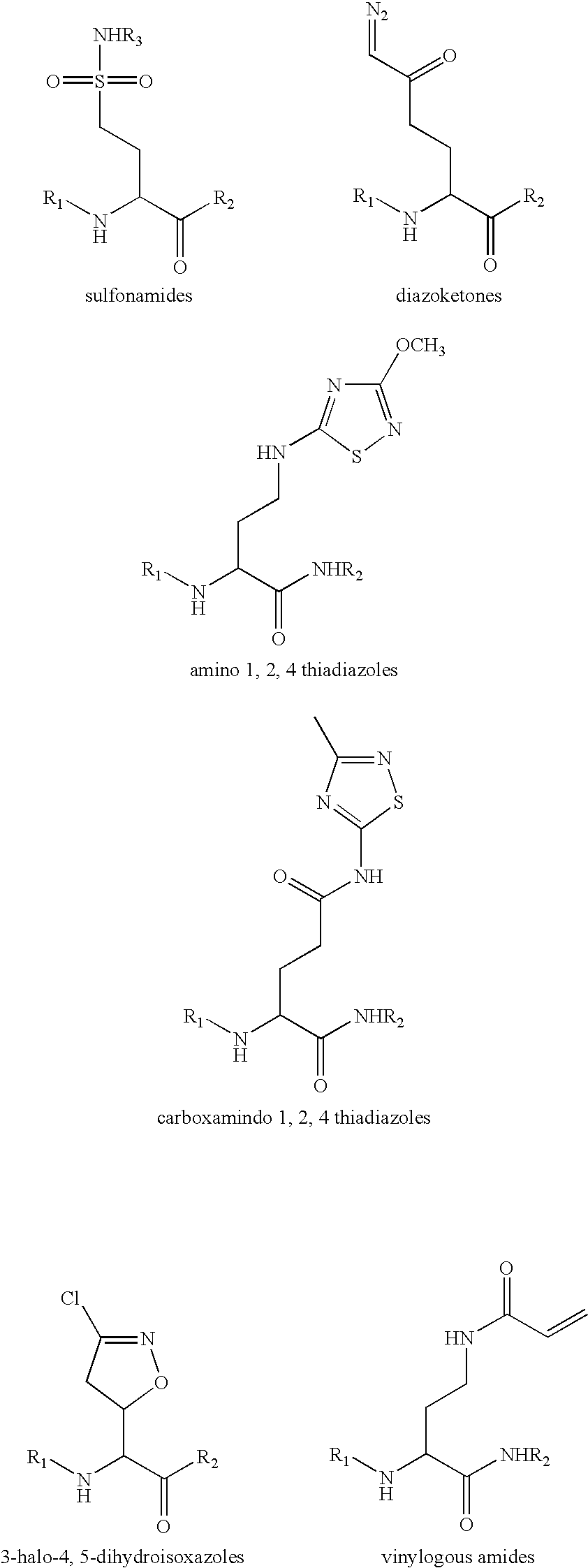
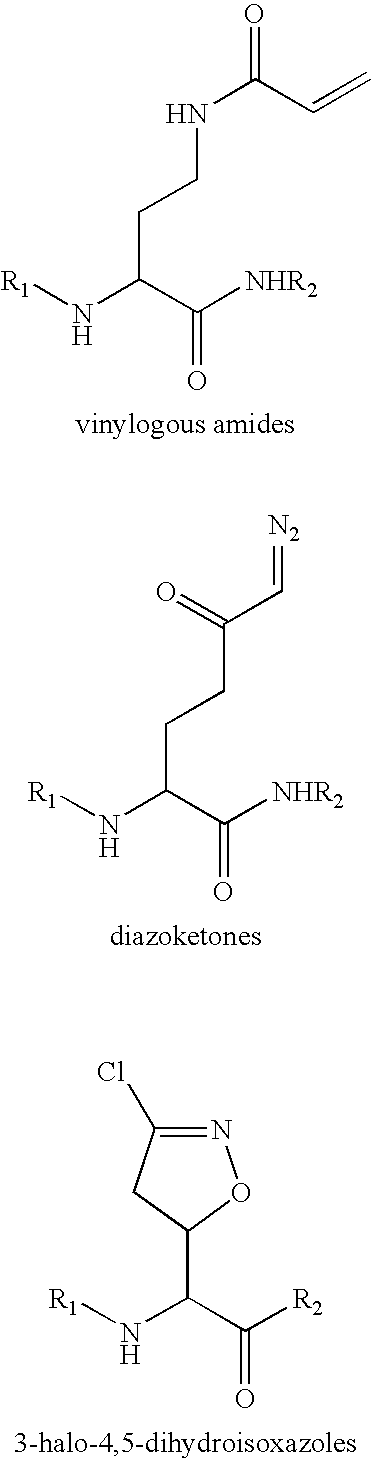
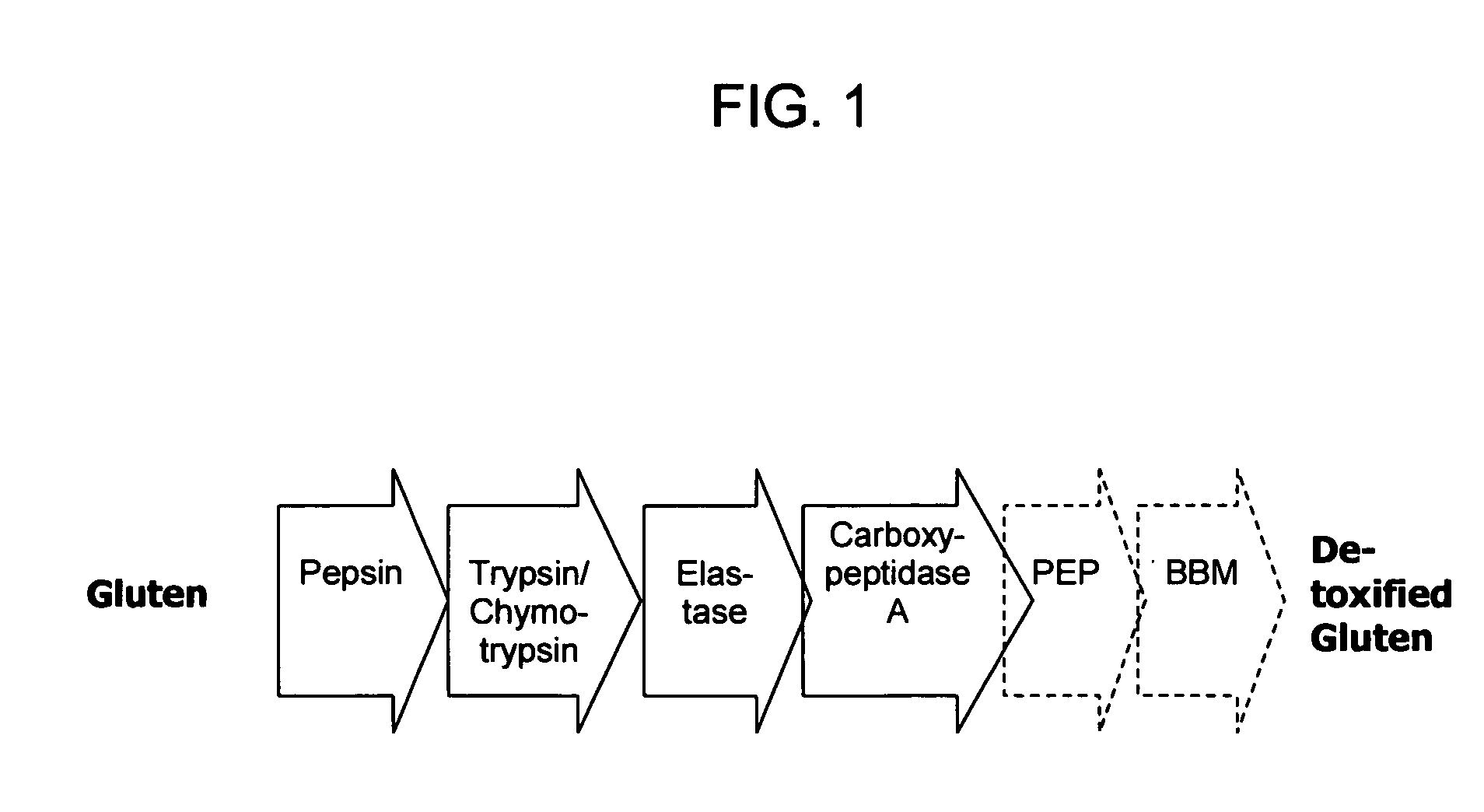
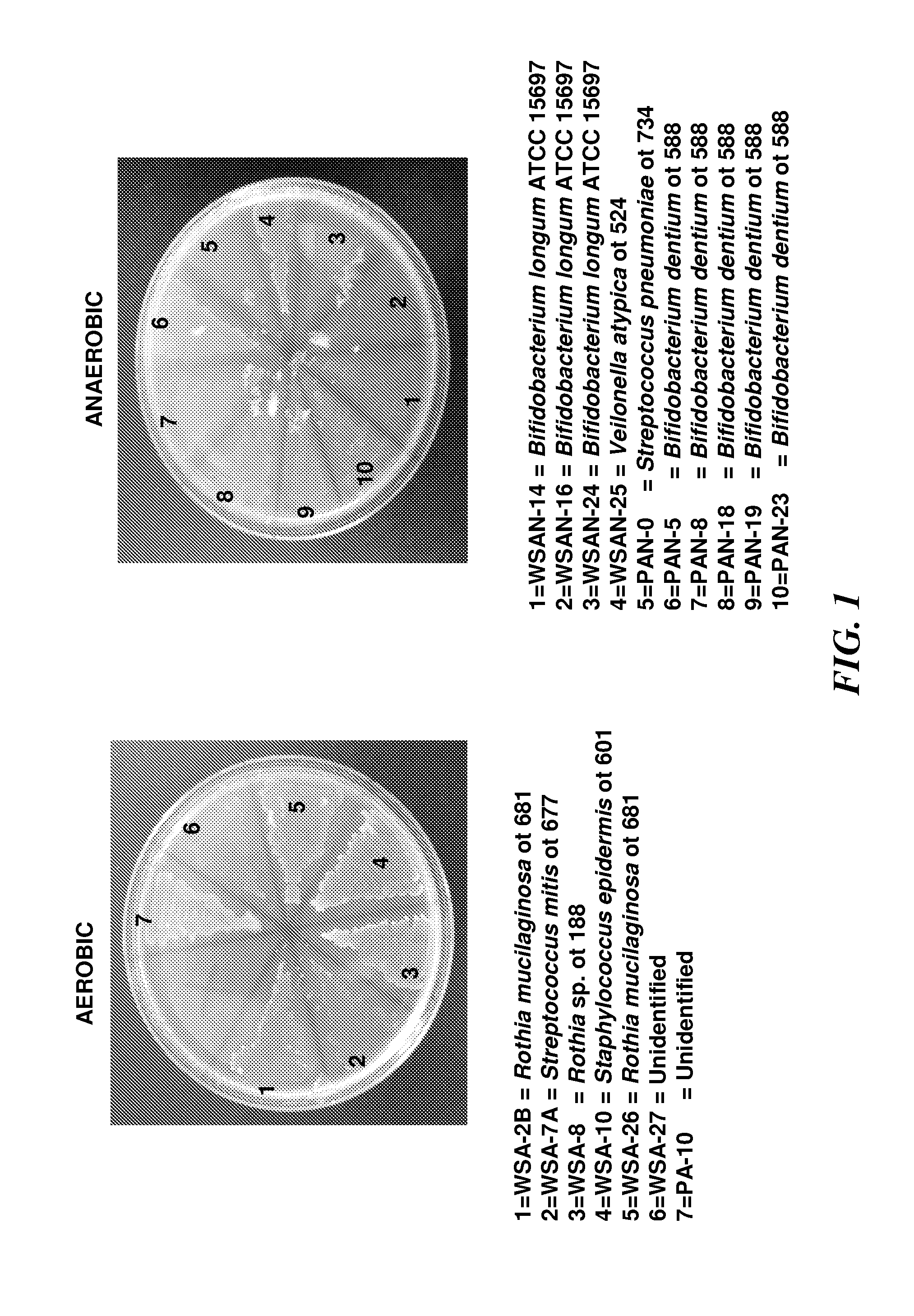
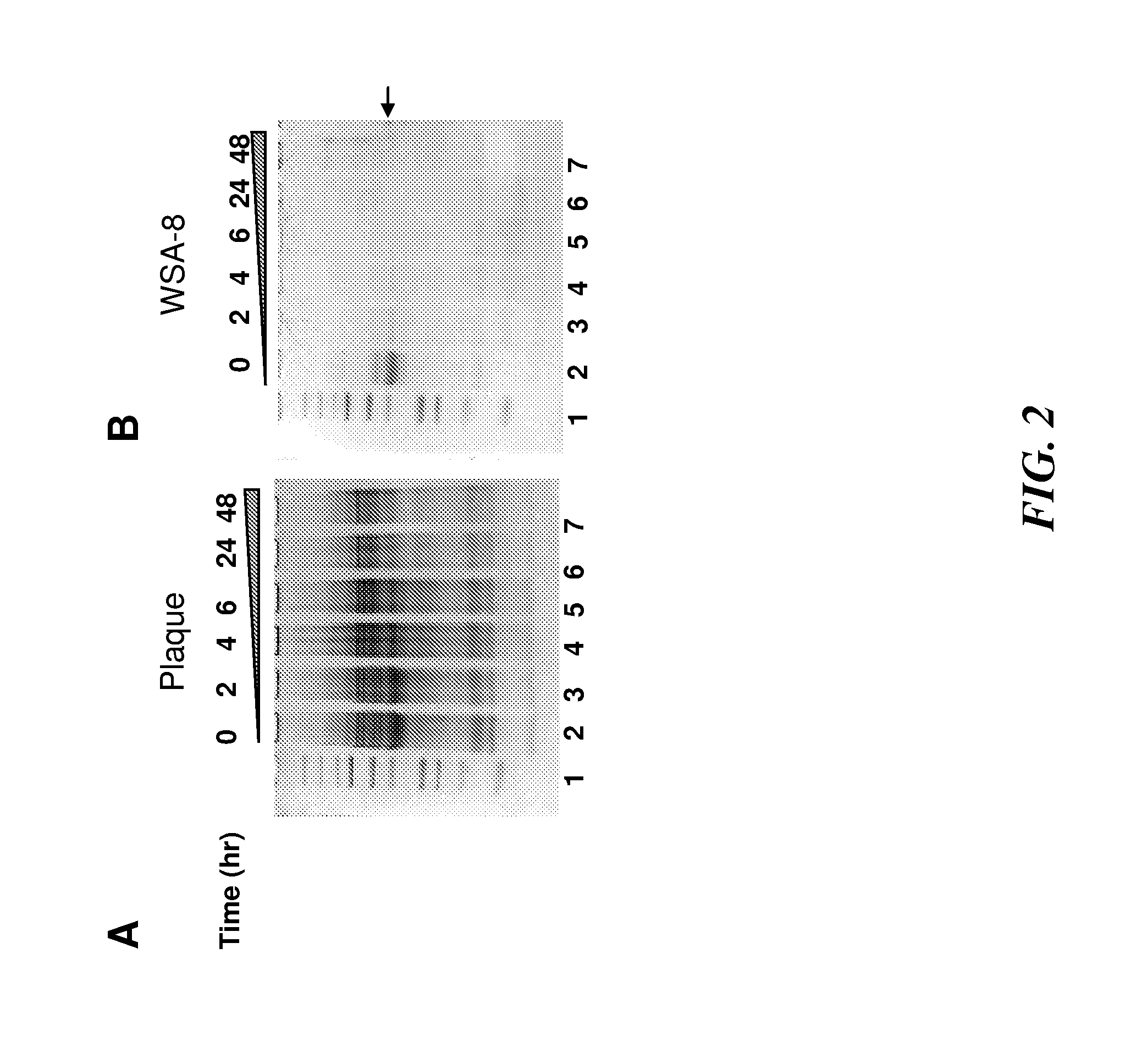
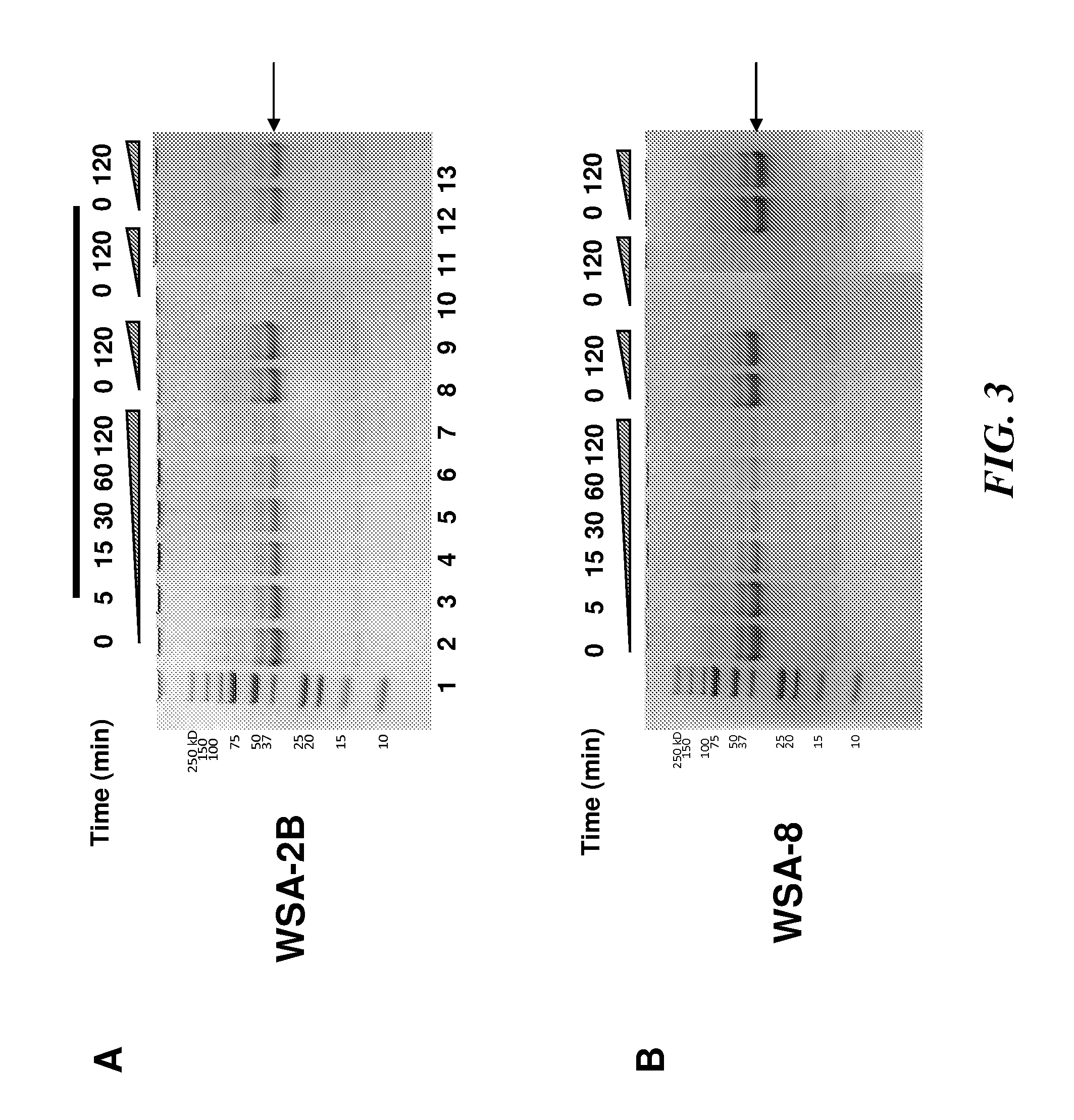
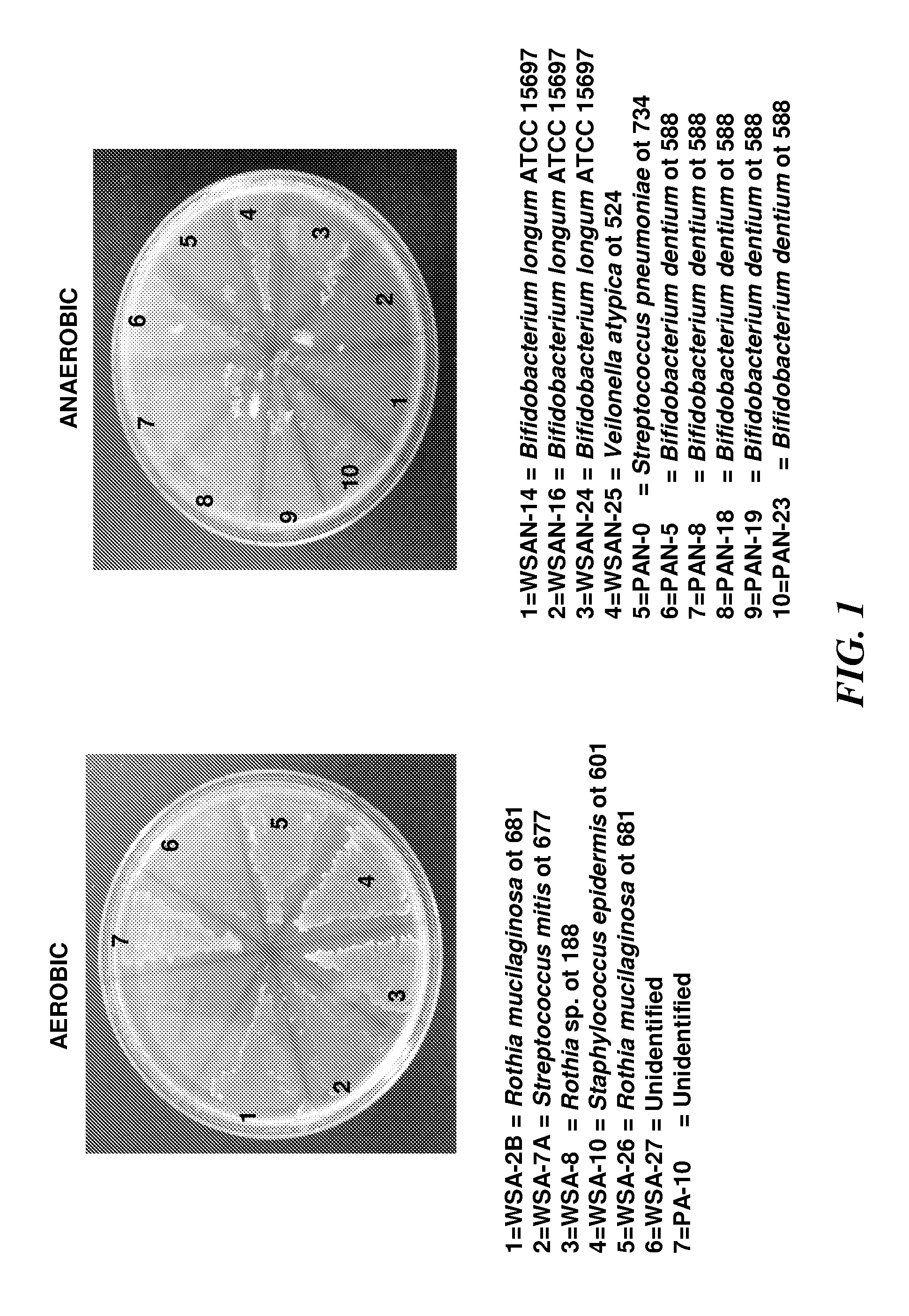
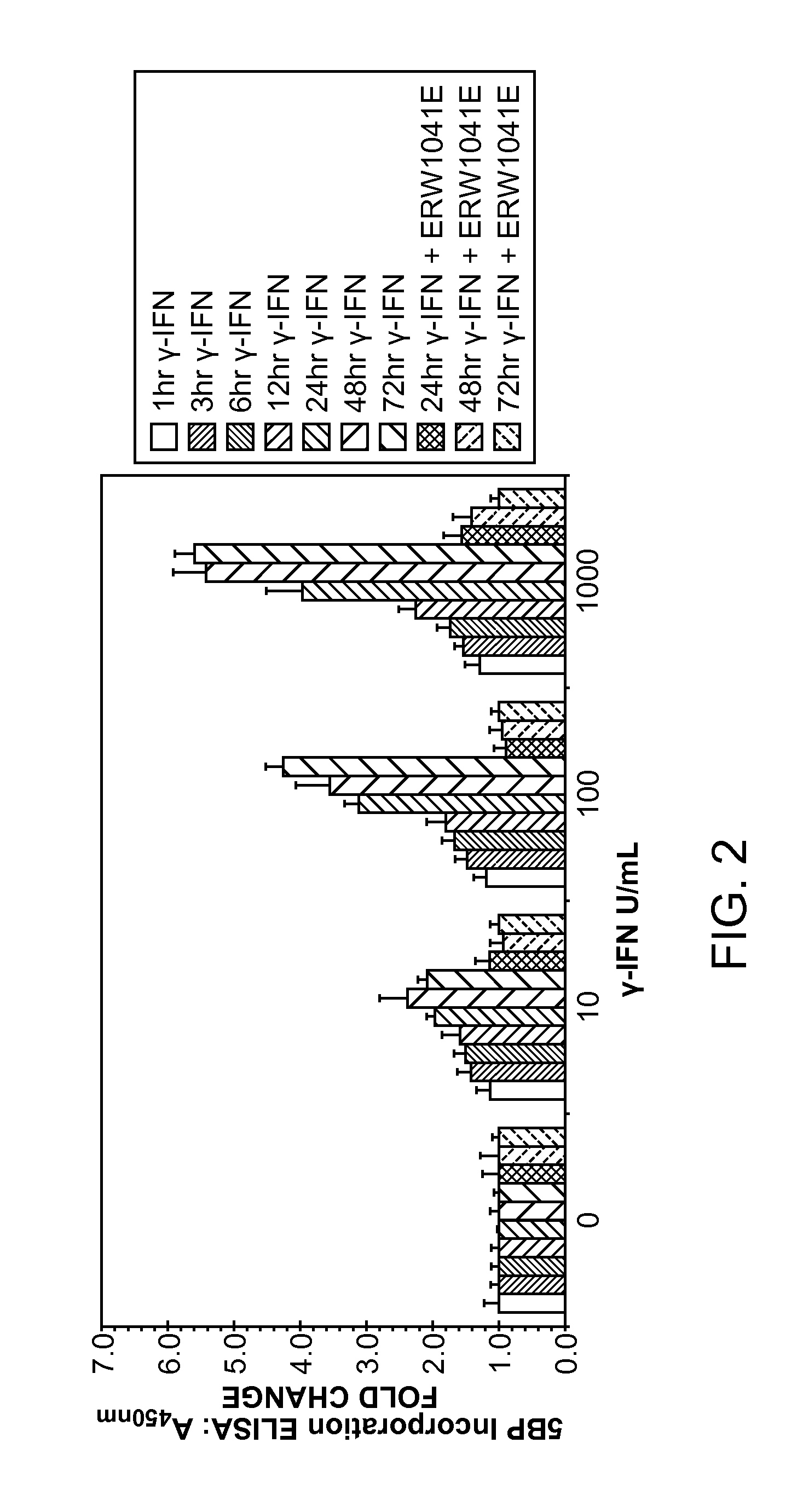
Patents
Literature
38 results about "Dermatitis herpetiformis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A chronic blistering skin disease.
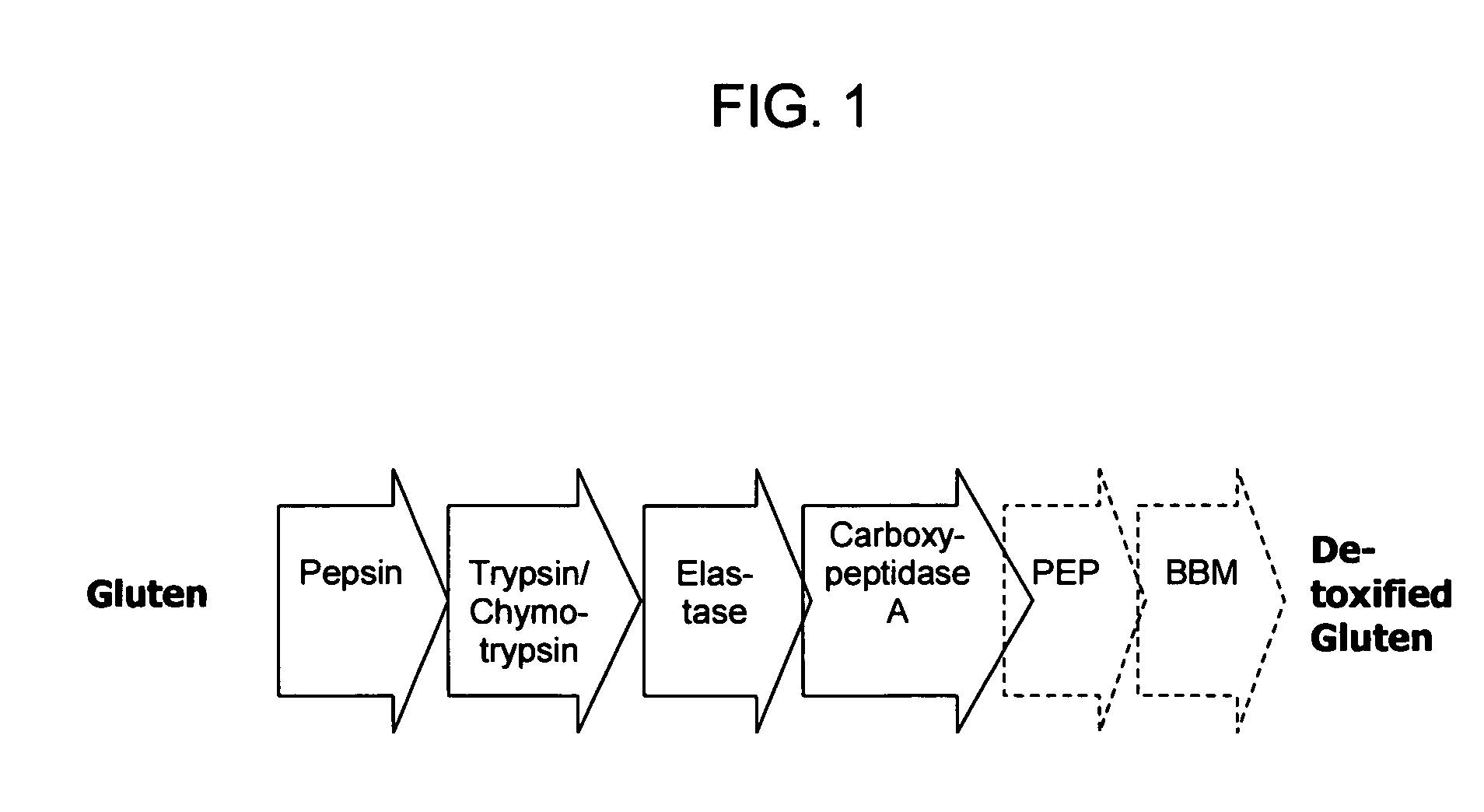
Enzyme treatment of foodstuffs for Celiac Sprue
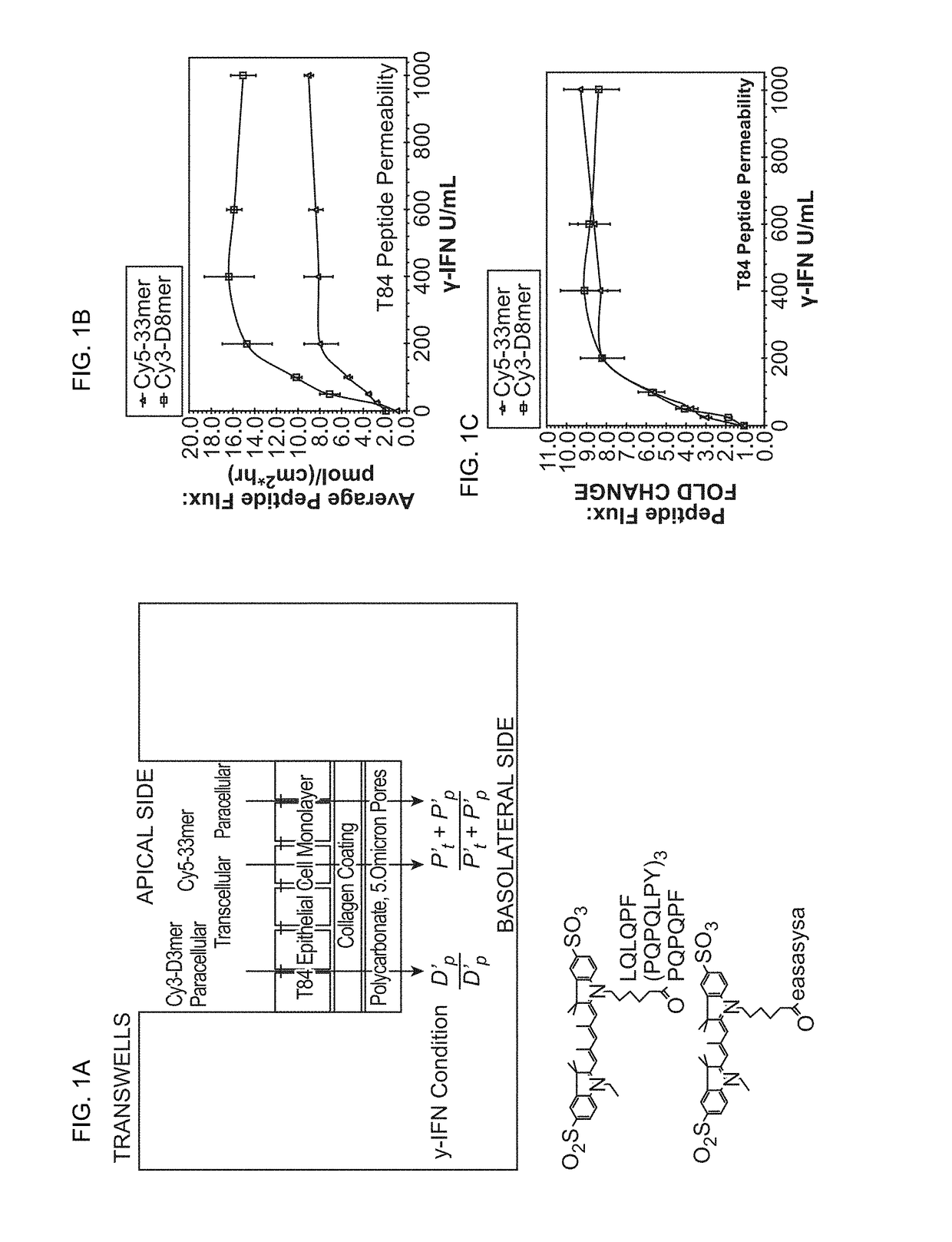
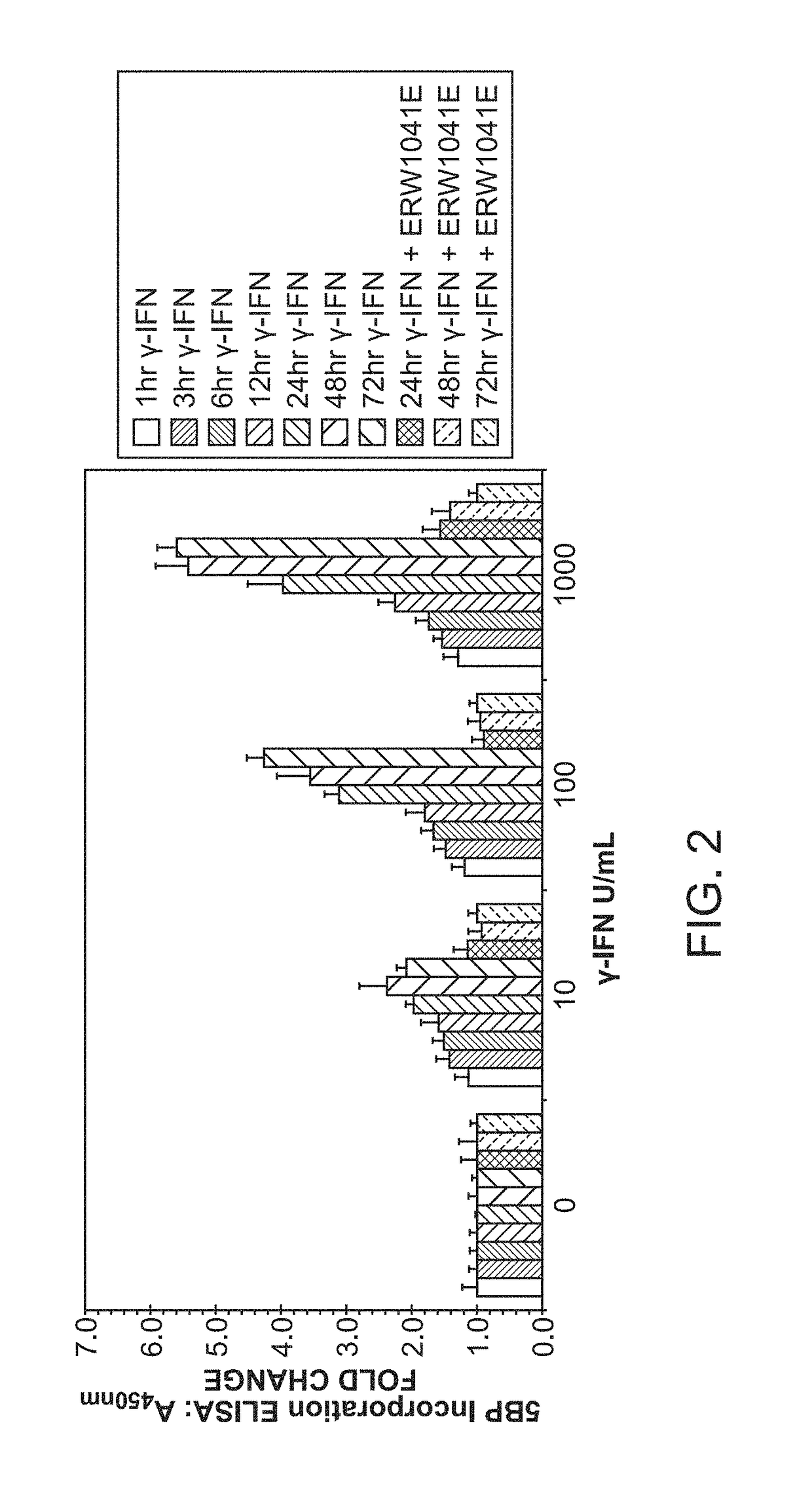
ActiveUS7303871B2Lower Level RequirementsAvoid toxicityPeptide/protein ingredientsHydrolasesDermatitis herpetiformisGluten
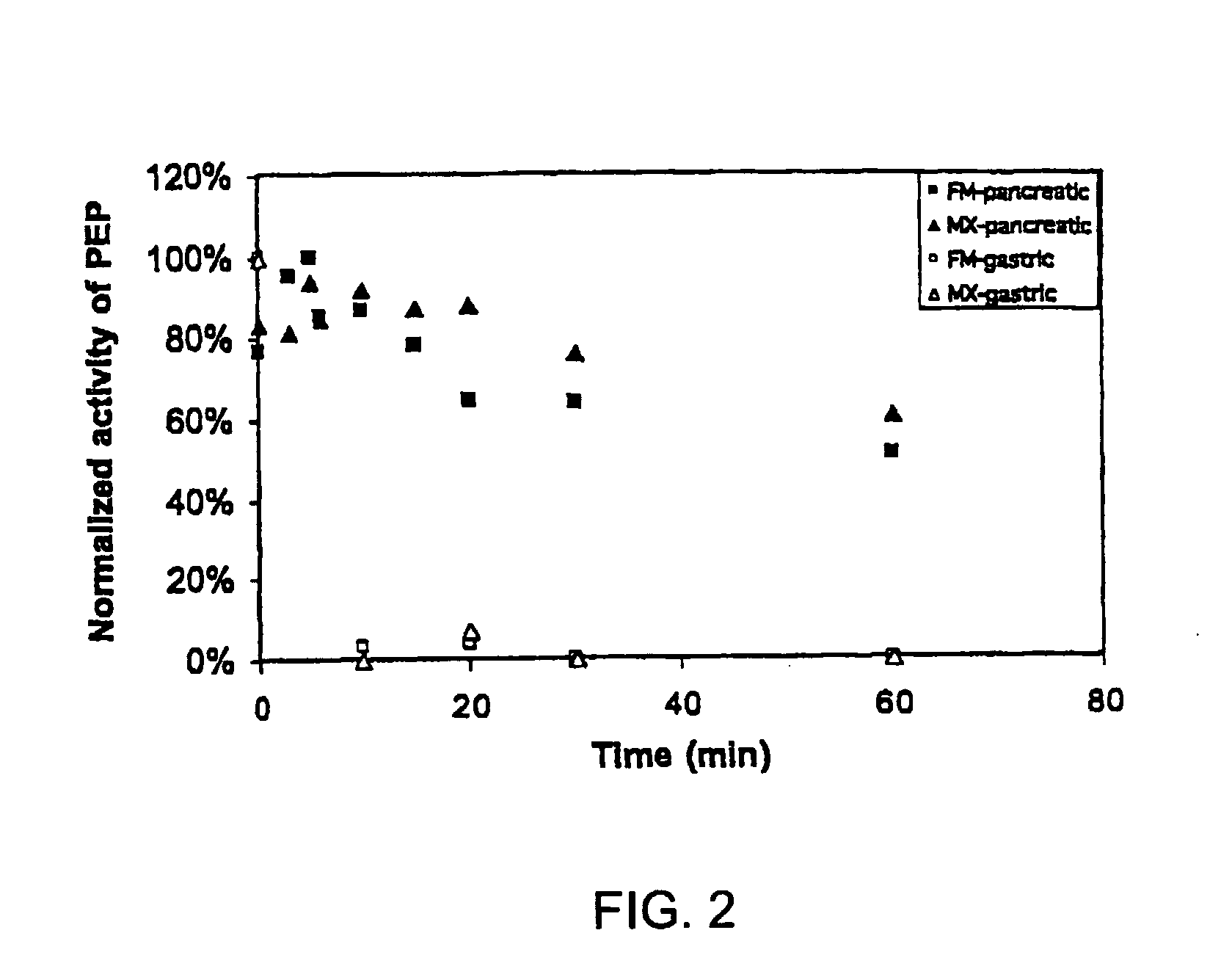
Administering an effective dose of glutenase to a Celiac or dermatitis herpetiformis patient reduces levels of toxic gluten oligopeptides, thereby attenuating or eliminating the damaging effects of gluten.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Enzyme treatment of foodstuffs for Celiac Sprue
InactiveUS7320788B2Decrease in levelAvoid toxicityPeptide/protein ingredientsMetabolism disorderCoeliac sprueDermatitis herpetiformis
Administering an effective dose of glutenase to a Celiac or dermatitis herpetiformis patient reduces levels of toxic gluten oligopeptides, thereby attenuating or eliminating the damaging effects of gluten.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Enzyme treatment of foodstuffs for Celiac Sprue
InactiveUS20050249719A1Decrease in levelAvoid toxicityPeptide/protein ingredientsMetabolism disorderDermatitis herpetiformisGluten
Administering an effective dose of glutenase to a Celiac or dermatitis herpetiformis patient reduces levels of toxic gluten oligopeptides, thereby attenuating or eliminating the damaging effects of gluten.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Glutenase enzyme assays
InactiveUS7534426B2Decrease in levelAvoid toxicityBiocidePeptide/protein ingredientsDermatitis herpetiformisGluten
Administering an effective dose of glutenase to a Celiac or dermatitis herpetiformis patient reduces levels of toxic gluten oligopeptides, thereby attenuating or eliminating the damaging effects of gluten.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Therapeutic enzyme formulations and uses thereof in celiac sprue and/or dermatitis herpetoformis
InactiveUS7628985B2Decrease in levelAvoid toxicityOrganic active ingredientsPeptide/protein ingredientsDermatitis herpetiformisPharmaceutical formulation
Pharmaceutical formulations of glutenase enzymes are provided. The enzymes find particular use in the treatment of a Celiac or dermatitis herpetiformis patient.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Drug therapy for Celiac Sprue
Administering an effective dose of a tTGase inhibitor to a Celiac or dermatitis herpetiformis patient reduces the toxic effects of toxic gluten oligopeptides, thereby attenuating or eliminating the damaging effects of gluten.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Therapeutic Enzyme Formulations And Uses Thereof
InactiveUS20080193436A1Decrease in levelAvoid toxicityOrganic active ingredientsPeptide/protein ingredientsDermatitis herpetiformisPharmaceutical formulation
Pharmaceutical formulations of glutenase enzymes are provided. The enzymes find particular use in the treatment of a Celiac or dermatitis herpetiformis patient.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Drug therapy for Celiac Sprue
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
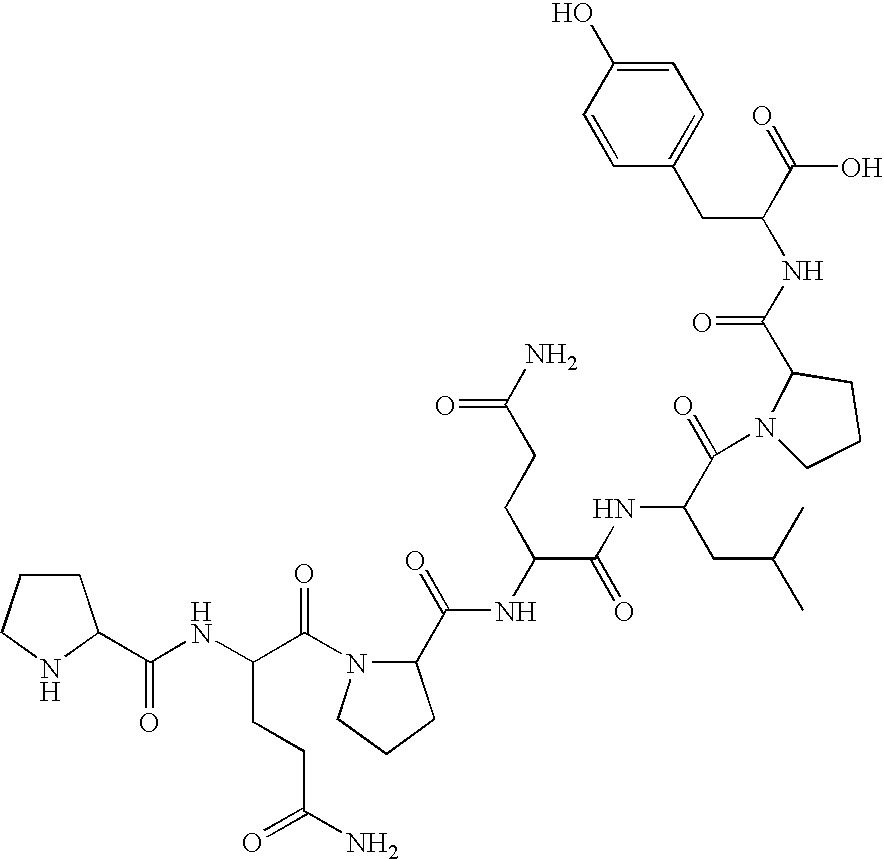
Prolyl endopeptidase mediated destruction of T cell epitopes in whole gluten
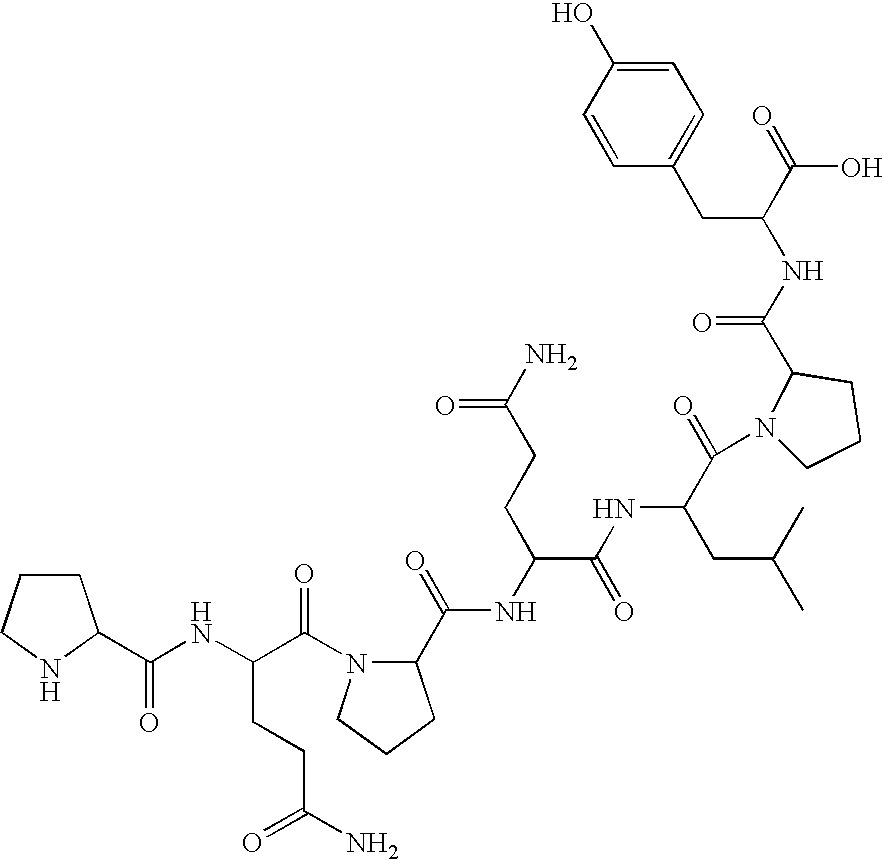
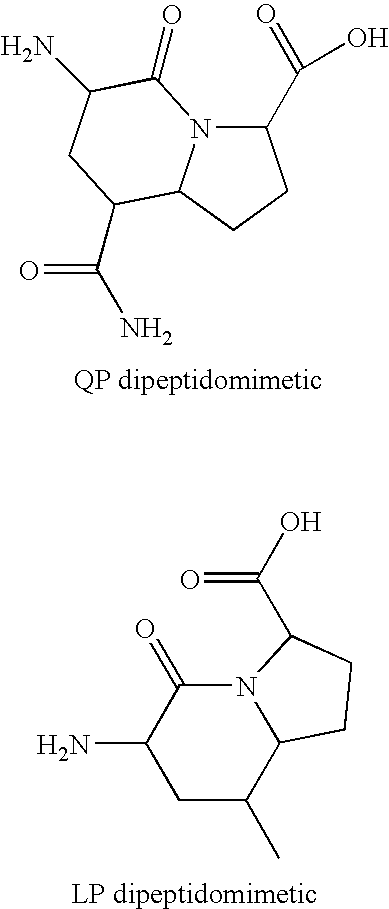
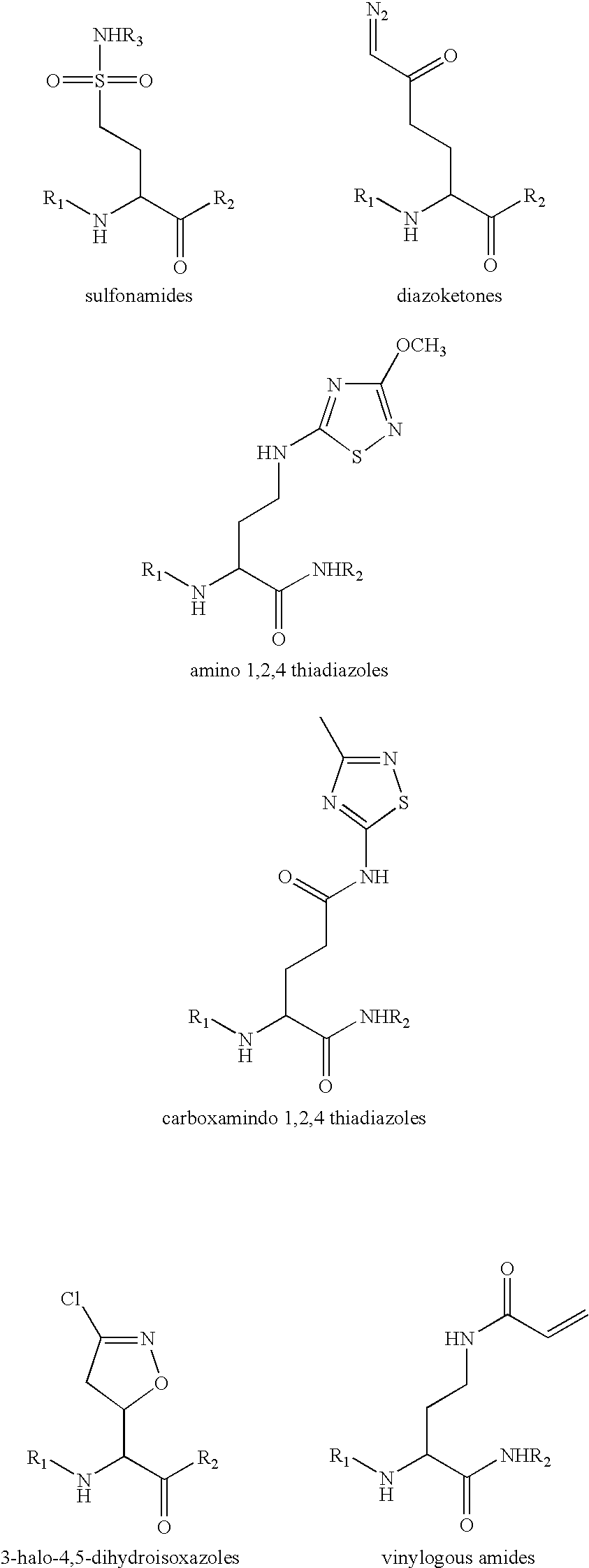
Celiac Sprue and / or dermatitis herpetiformis are treated by interfering with HLA binding of immunogenic gluten peptides. The antigenicity of gluten oligopeptides and the ill effects caused by an immune response thereto are decreased by administration of an HLA-binding peptide inhibitor. Such inhibitors are analogs of immunogenic gluten peptides and (i) retain the ability to bind tightly to HLA molecules; (ii) retain the proteolytic stability of these peptides; but (iii) are unable to activate disease-specific T cells.
Owner:UNIVERSITY OF OSLO +1
Drug therapy for celiac sprue
InactiveUS20060035838A1Salicyclic acid active ingredientsBiocideDermatitis herpetiformisPoisonous effects
A ministering an effective dose of a tTGase inhibitor to a Celiac or dermatitis herpetiformis patient reduces the toxic effects of toxic gluten oligopeptides, thereby attenuating or eliminating the damaging effects of gluten.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Prolyl endopeptidase mediated destruction of T cell epitopes in whole gluten
InactiveUS20060286601A1Peptide/protein ingredientsTransferasesDermatitis herpetiformisBinding peptide
Owner:UNIVERSITY OF OSLO +1
Enzyme treatment of foodstuffs for celiac sprue
ActiveUS8143210B2Decrease in levelAvoid toxicityIn-vivo radioactive preparationsPeptide/protein ingredientsDermatitis herpetiformisGluten
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Antibody therapy for treatment of diseases associated with gluten intolerance
InactiveUS20070184049A1Specific activityMetabolism disorderMilk immunoglobulinsGluten intoleranceDermatitis herpetiformis
The present invention includes a pharmaceutical compositions and methods for treating diseases associated with gluten intolerance in a patient, comprising: administering to the patient an effective amount of an antibody having specific activity against gluten or gluten-derived peptides. Such diseases include, for example, celiac disease and dermatitis herpetiformis.
Owner:CIRCLE 33 LLC
Rothia species glutamine endopeptidases and use thereof
InactiveUS20120230976A1Improve enzyme stabilityIncrease enzyme activityMilk preparationDough treatmentMedicineDermatitis herpetiformis
Disclosed are glutamine endopeptidase enzymes from Rothia sp. bacteria that are naturally associated with the oral cavity, formulations comprising the glutamine endopeptidase enzymes and the use thereof for the treatment, prevention of allergic reaction and diagnosis of gluten allergy related diseases such as Celiac Sprue, gluten allergy and / or dermatitis herpetiformis.
Owner:TRUSTEES OF BOSTON UNIV
Drug therapy for celiac sprue
InactiveUS20050256054A1Data processing applicationsPeptide/protein ingredientsDermatitis herpetiformisBinding peptide
Celiac Sprue and / or dermatitis herpetiformis arc treated by interfering with HLA binding of immunogenic gluten peptides. The antigenicity of gluten oligopeptides and the ill effects caused by an immune response thereto are decreased by administration of an HLA-binding peptide inhibitor. Such inhibitors are analogs of immunogenic gluten peptides and (i) retain the ability to bind tightly to HLA molecules; (ii) retain the protcolytic stability of these peptides; but (iii) are unable to activate disease-specific T cells.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Rothia species glutamine endopeptidases and use thereof
The invention relates to glutamine endopeptidase enzymes from Rothia spp. bacteria that are naturally associated with the oral cavity, formulations comprising the glutamine endopeptidase enzymes and the use thereof for the treatment, prevention of allergic reaction and diagnosis of gluten allergy related diseases such as Celiac Sprue, gluten allergy and / or dermatitis herpetiformis.
Owner:TRUSTEES OF BOSTON UNIV
Effect of prolyl endopeptidase on digestive-resistant gliadin peptides in vivo
InactiveUS20060002917A1Lower Level RequirementsAvoid toxicityPeptide/protein ingredientsMicrobiological testing/measurementDermatitis herpetiformisIn vivo
Administering an effective dose of glutenase to a Celiac or dermatitis herpetiformis patient reduces levels of toxic gluten oligopeptides, thereby attenuating or eliminating the damaging effects of gluten.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Enzyme treatment of foodstuffs for celiac sprue
InactiveUS20080145356A1Peptide/protein ingredientsImmunoglobulins against plantsMedicineDermatitis herpetiformis
Administering an effective dose of glutenase to a Celiac or dermatitis herpetiformis patient reduces levels of toxic gluten oligopeptides, thereby attenuating or eliminating the damaging effects of gluten.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
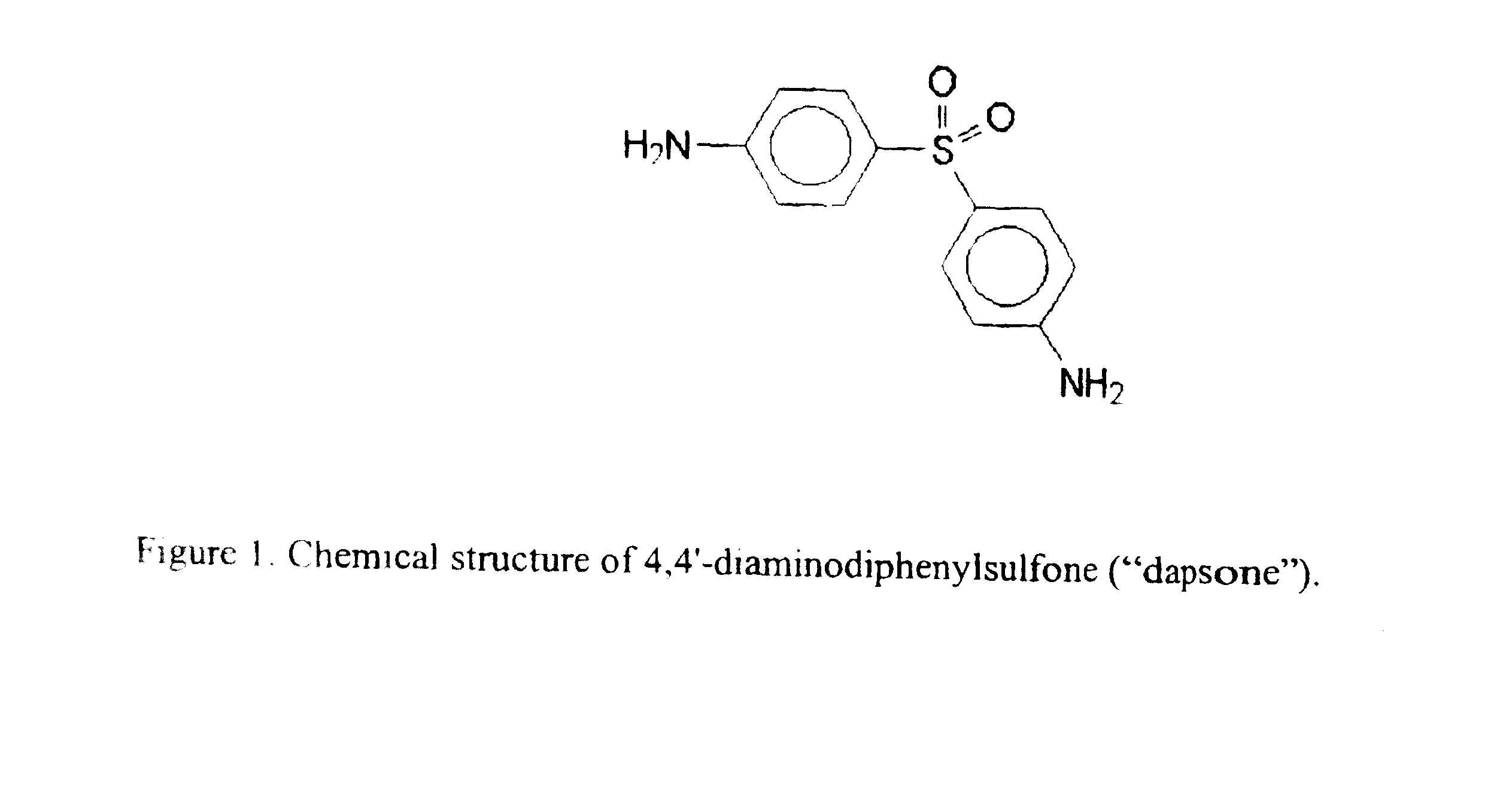
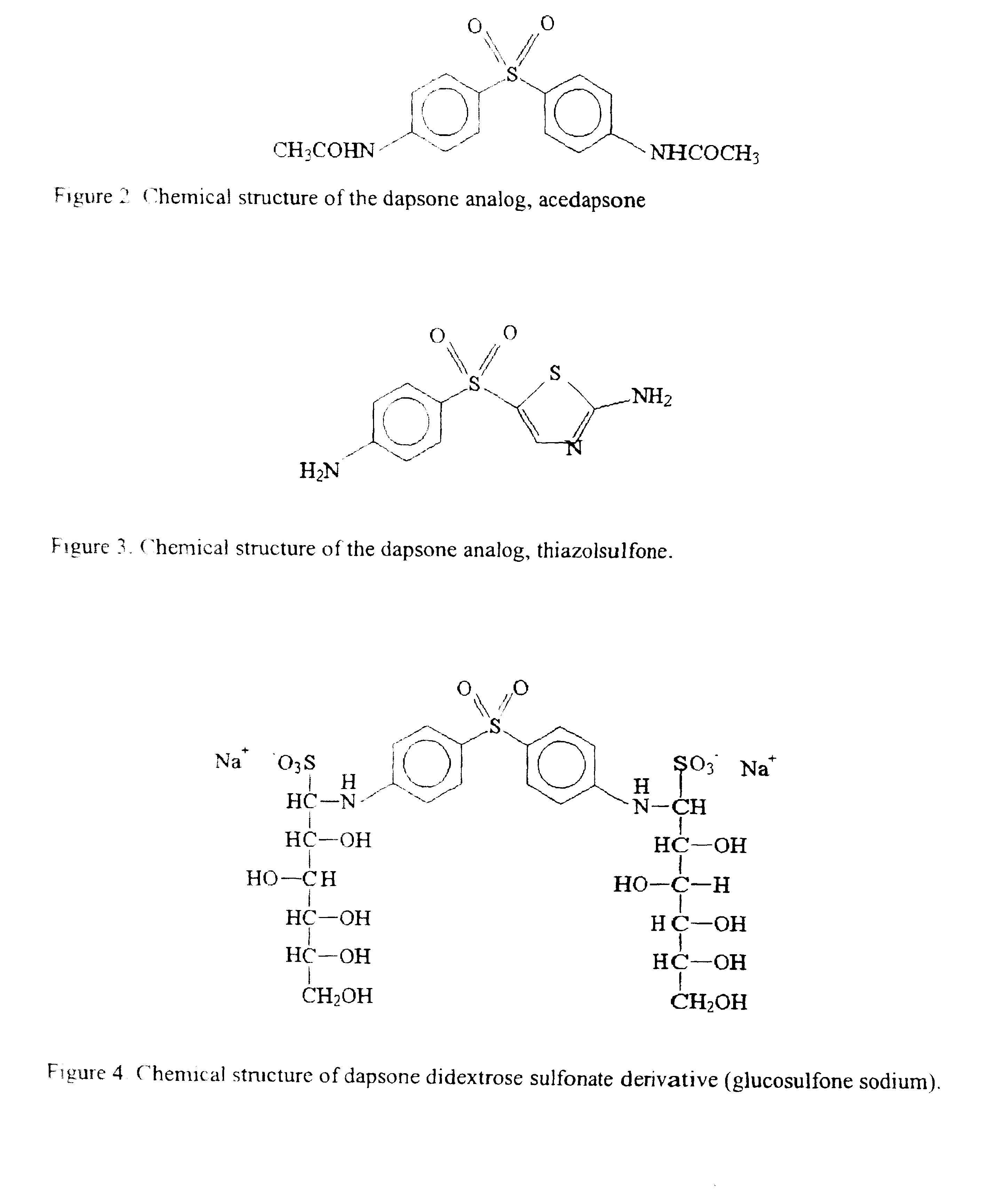
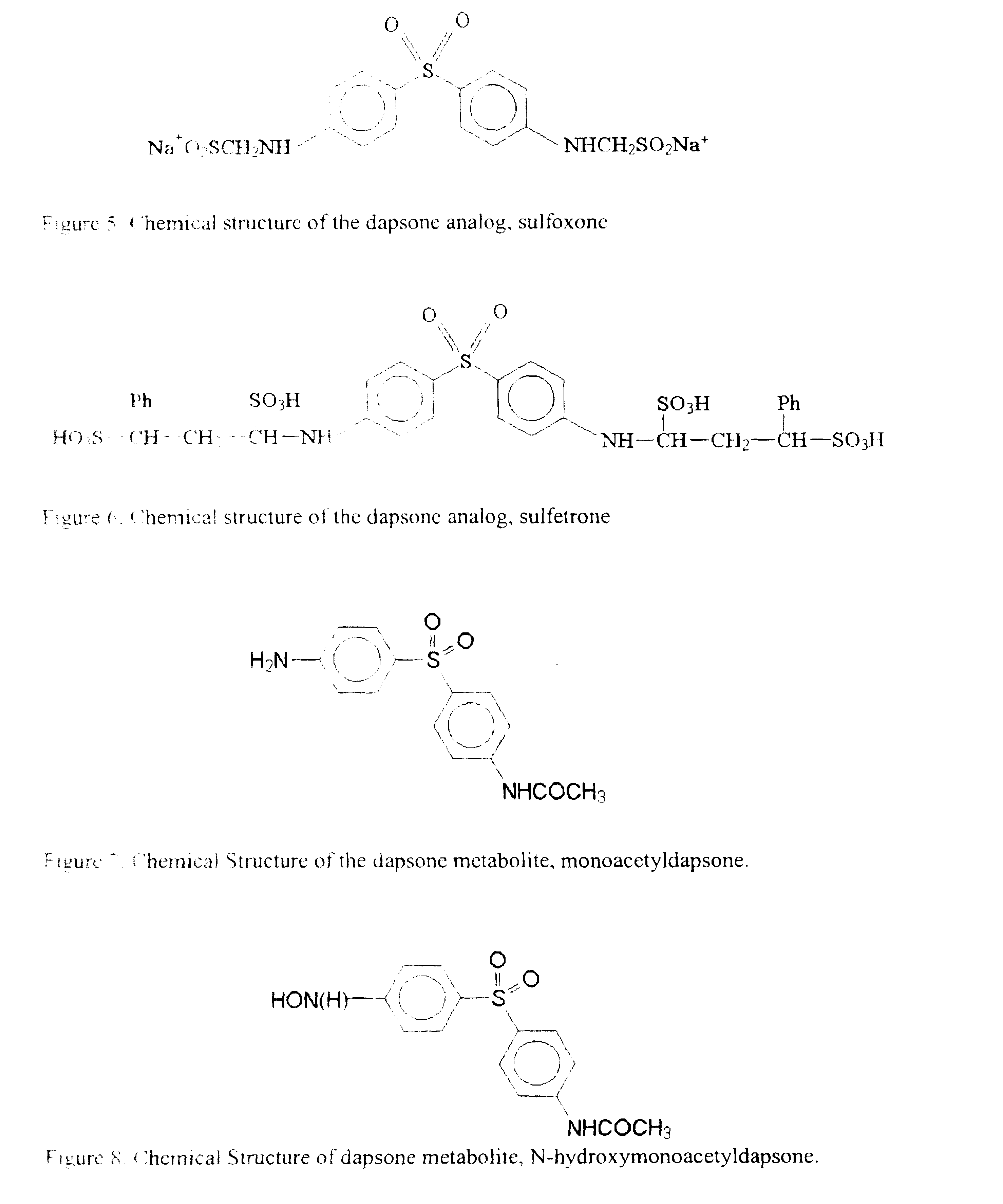
Galenical preparations of dapsone and related sulphones, and method of therapeutic and preventative treatment of disease
Dapsone and related sulfones are known to have therapeutic activity against leprosy, dermatitis herpetiformis, actinomycotic mycetoma, asthma, malaria, rheumatoid arthritis, Kaposiís sarcoma, pneumocystis carinií (pneumonia), subcorneal pustular dermatosis and cystic acne, in patients in need of such therapy. These sulfones are also known to have therapeutic activity against memory loss in patients in need of such therapy, including patients suffering from Alzheimer's disease and related neurodegenerative disorders. It has now been found that new, modified-release formulations of dapsone and related sulfones may also be used that decrease side effects and increase effectiveness of the drugs. New methods are disclosed utilizing certain formulations of dapsone and related sulfones that improve the therapeutic index of said drugs. Side effects of these drugs are known to those skilled in the art and include, but are not restricted to anorexia, psychosis, agranulocytosis, peripheral neuritis, hemolysis, methemoglobinemia, nausea, vomiting, headache, dizziness, tachycardia, nervousness, insomnia and skin disorders. Modified-release (as defined herein) formulations of dapsone have now been found to avoid some or all of these side effects, and to have more efficacy on potency.
Owner:IMMUNE NETWORK
Method and means for detecting gluten-induced diseases
The invention is directed to a method of detecting gluten-induced diseases such as e.g. celiac disease and dermatitis herpetiformis. Said diseases are indicated by the presence of autoantibodies against tissue transglutaminase tTG in the blood The test is carried out on a whole blood sample using tTG liberated from the red blood cells of the blood sample as an autoantigen. The liberated autoantigens react with the autoantibodies in the sample and form antigen-antibody complexes, which are detected. The presence of such complexes indicates the disease. The invention is also directed to the use of the autoantigen in a test and to a test-kit.
Owner:MAKI MARKKU +1
Drug therapy for celiac sprue
Administering an effective dose of a tTGase inhibitor to a Celiac or dermatitis herpetiformis patient reduces the toxic effects of toxic gluten oligopeptides, thereby attenuating or eliminating the damaging effects of gluten.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Wheat Antigens and Peptides for Diagnosis of Wheat Induced Hypersensitivity
InactiveUS20140037661A1Improve permeabilityImprove inflammationPeptide/protein ingredientsAntiviralsAntigenWheat hypersensitivity
The present invention relates to the field of different wheat hypersensitivities, particularly with antigens and peptides for discrimination of different forms of these diseases. The invention relates to the identification of novel wheat allergens and the use thereof in therapy and diagnosis of celiac disease, dermatitis herpetiformis, and IgE-mediated allergy. Furthermore, the present invention provides the use of known peptides and proteins in therapy and diagnosis. The invention also relates to methods for diagnosis and treatment of celiac disease, dermatitis herpetiformis, and IgE-mediated allergy.
Owner:PHADIA AB
Diagnosis of gluten sensitive enteropathy and other autoimmunopathies
InactiveUS7781169B1Increased riskReduce the overall heightDisease diagnosisBiological testingInsulin dependentRheumatism
Method for diagnosis of autoimmune diseases of the GSE-type or associated with gluten sensitive enteropathy comprising taking a sample and testing the sample for antibodies against human tissue transglutaminase, tissue-specific transglutaminases, or other transglutaminases. It was found that autoimmune diseases other than celiac disease can be diagnosed and distinguished in this way, notably, dermatitis herpetiformis Duhring, Crohn's disease, Addison's disease, AI hemolytic anemia, AI thrombocytopenic purpura, AI thyroid diseases, atrophic gastritis—pernicious anemia, IgA nephropathy or IgA glomerulonephritis, myasthenia gravis, partial lipodystrophy, polymyositis, primary biliary cirrhosis, primary sclerosing cholangitis, recurrent pericarditis, relapsing polychondritis, rheumatoid arthritis, rheumatism, sarcoidosis, Sjögren's syndrome, SLE, splenic atrophy, type I (insulin-dependent) diabetes mellitus, diabetes mellitus of other types, ulcerative colitis, vasculitis (both systemic and cutaneous), vitiligo as well as autoimmune diseases associated with infertility, increased risk of abortion, or reduced fetal growth.
Owner:PAULSSON MATS +5
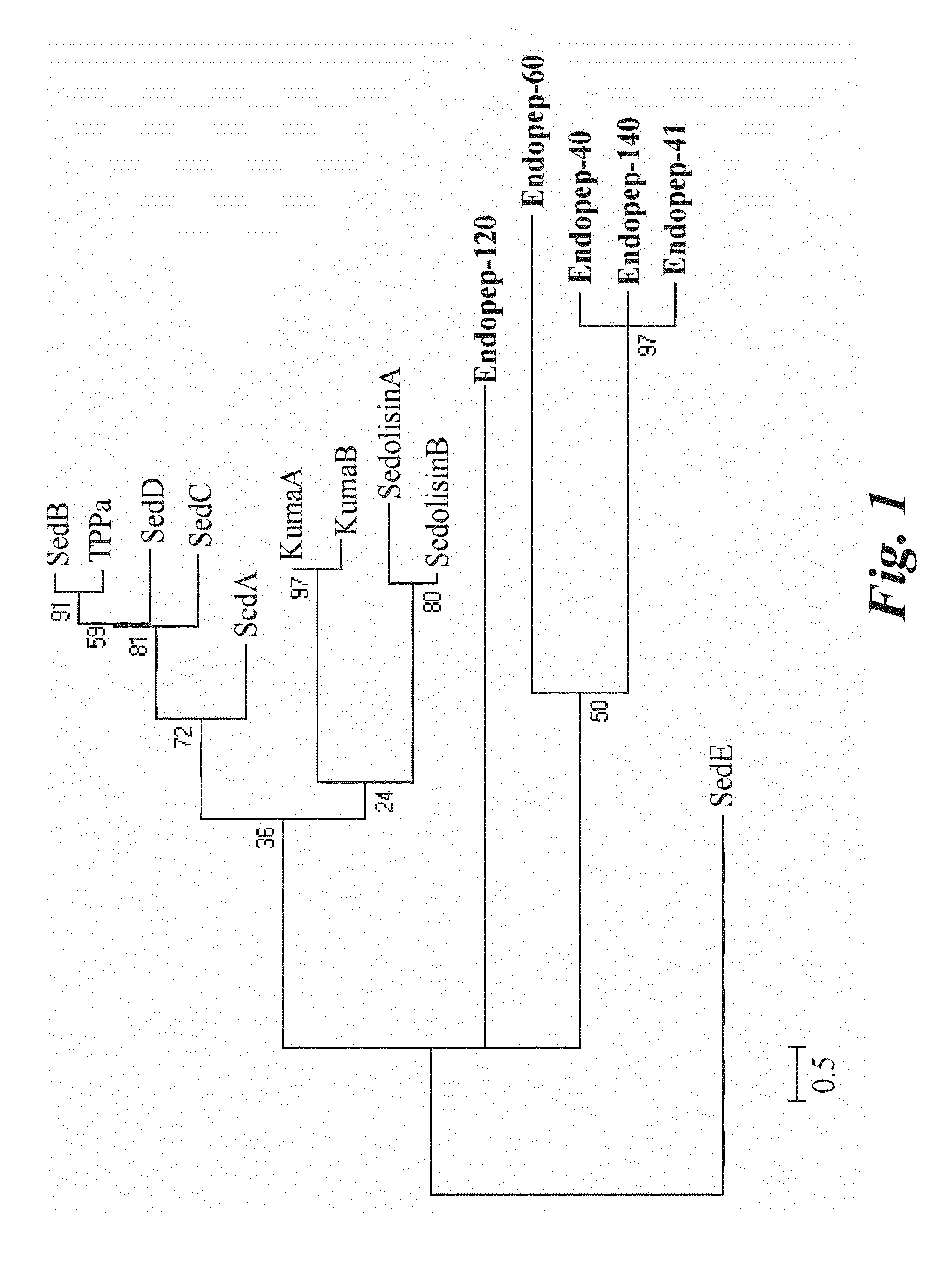
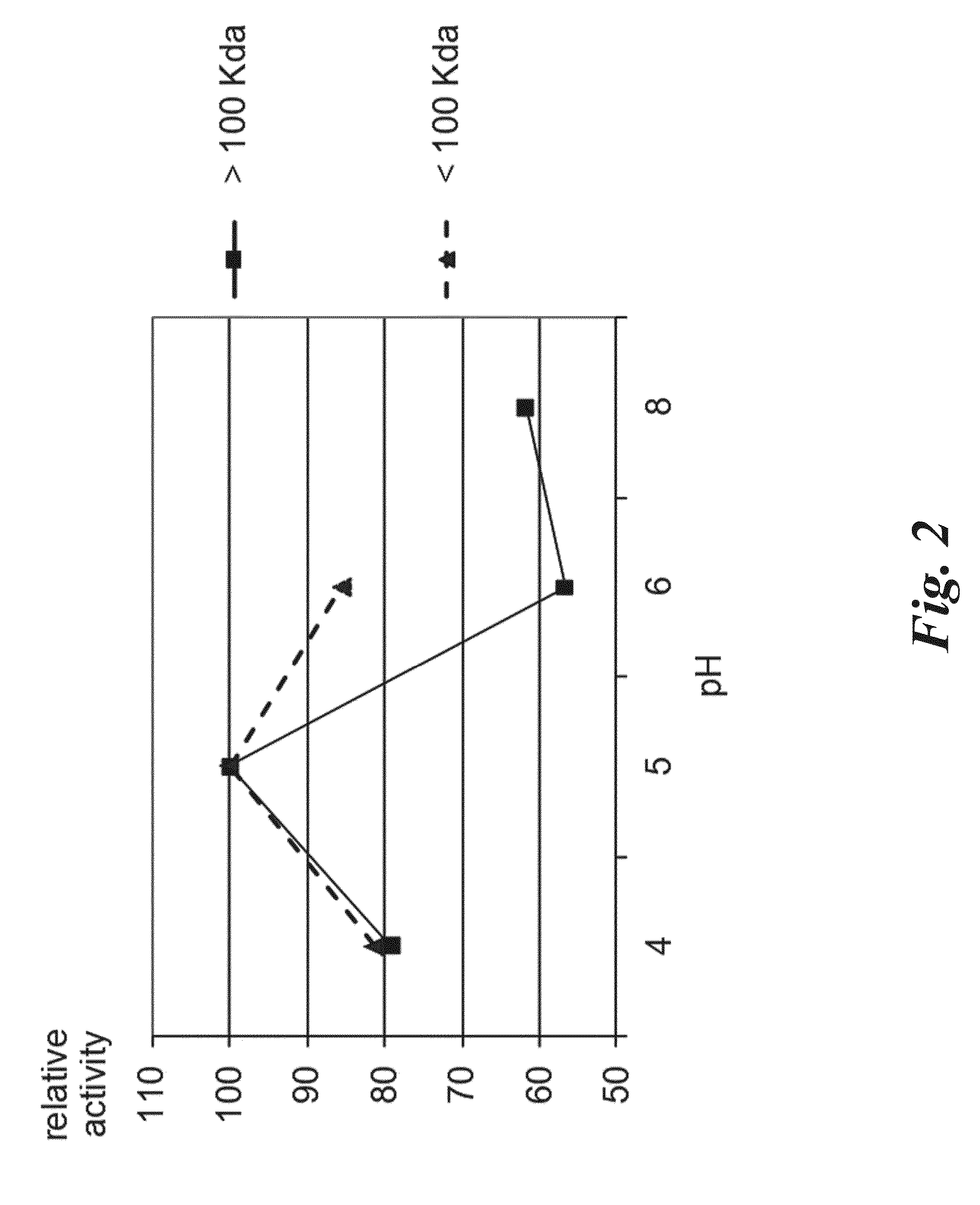
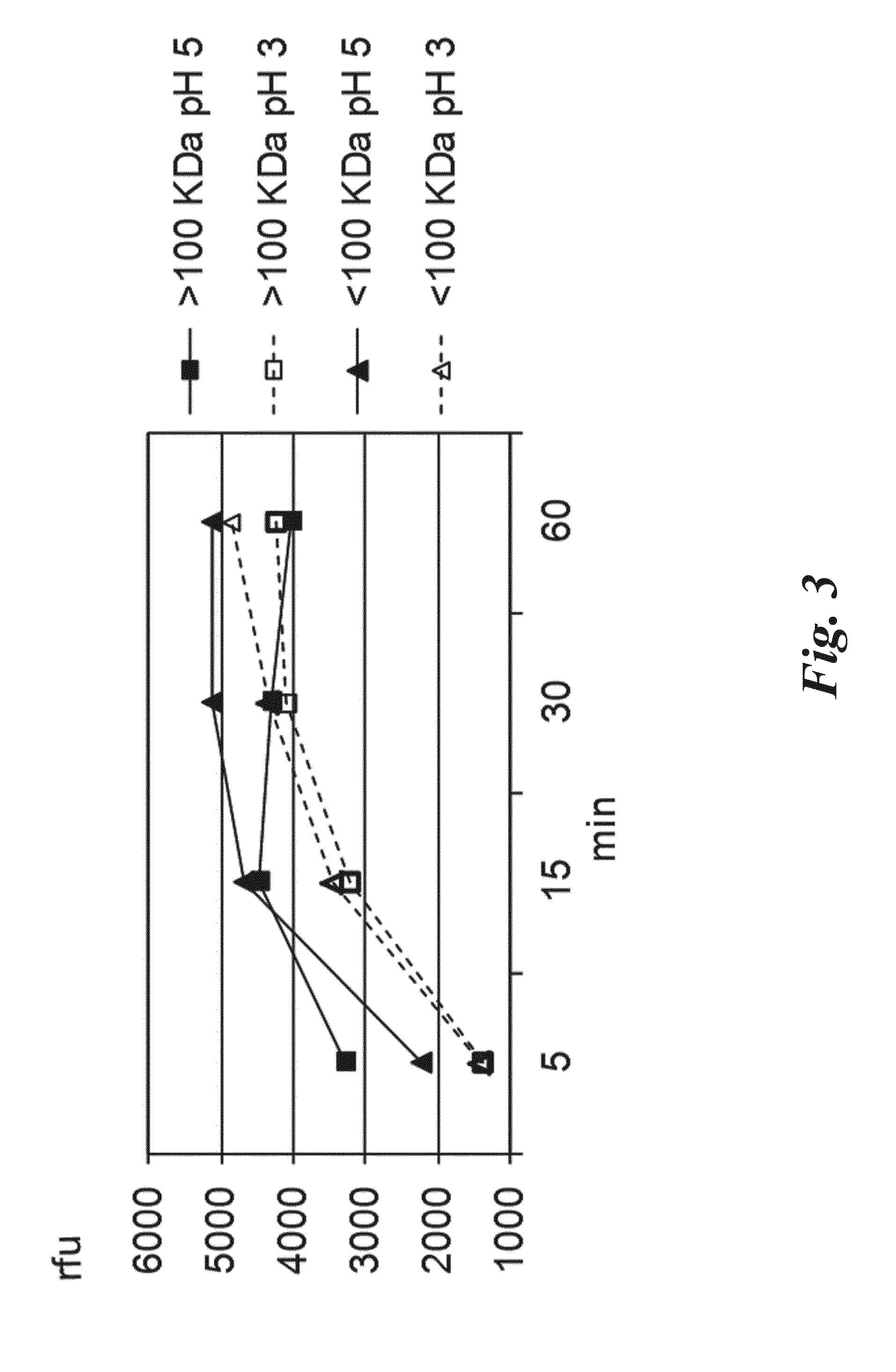
New Proteases Able to Hydrolyze Gluten Peptides and Proteins at Acidic PH, from the Actinomycete Actinoallomurus
InactiveUS20140356345A1Avoid symptomsEfficient productionMilk preparationSugar derivativesActinoallomurusPancrelipase
The invention relates to a new family of proteolytic enzymes having the ability to hydrolize at a p H between 3 and 8 gluten olygopeptides which are resistant to cleavage by gastric and pancreatic enzymes and whose presence in the intestinal lumen results in toxic effects. The enzymes have been identified as endopeptidases of the S8 / S53 family and are produced by an Actinoallomurus strain. The object of the invention includes also methods for producing enzymes composition comprising the endopeptidases by cultivation of native Actinoallomurus strains, mutants thereof, or recombinant host cells comprising nucleic acids codifying for the endopeptidases. Said nucleic acids constitute a further object of the invention. The enzyme compositions comprising at least one endopeptidase of the invention are useful for the treatment and / or prevention of celiac sprue, dermatitis herpetiformis and any other disorder associated with gluten intolerance as ingredients of pharmaceutical formulations or as additives of foods and drinks.
Owner:FOND IST INSUBRICO DI RICERCA PER LA VITA
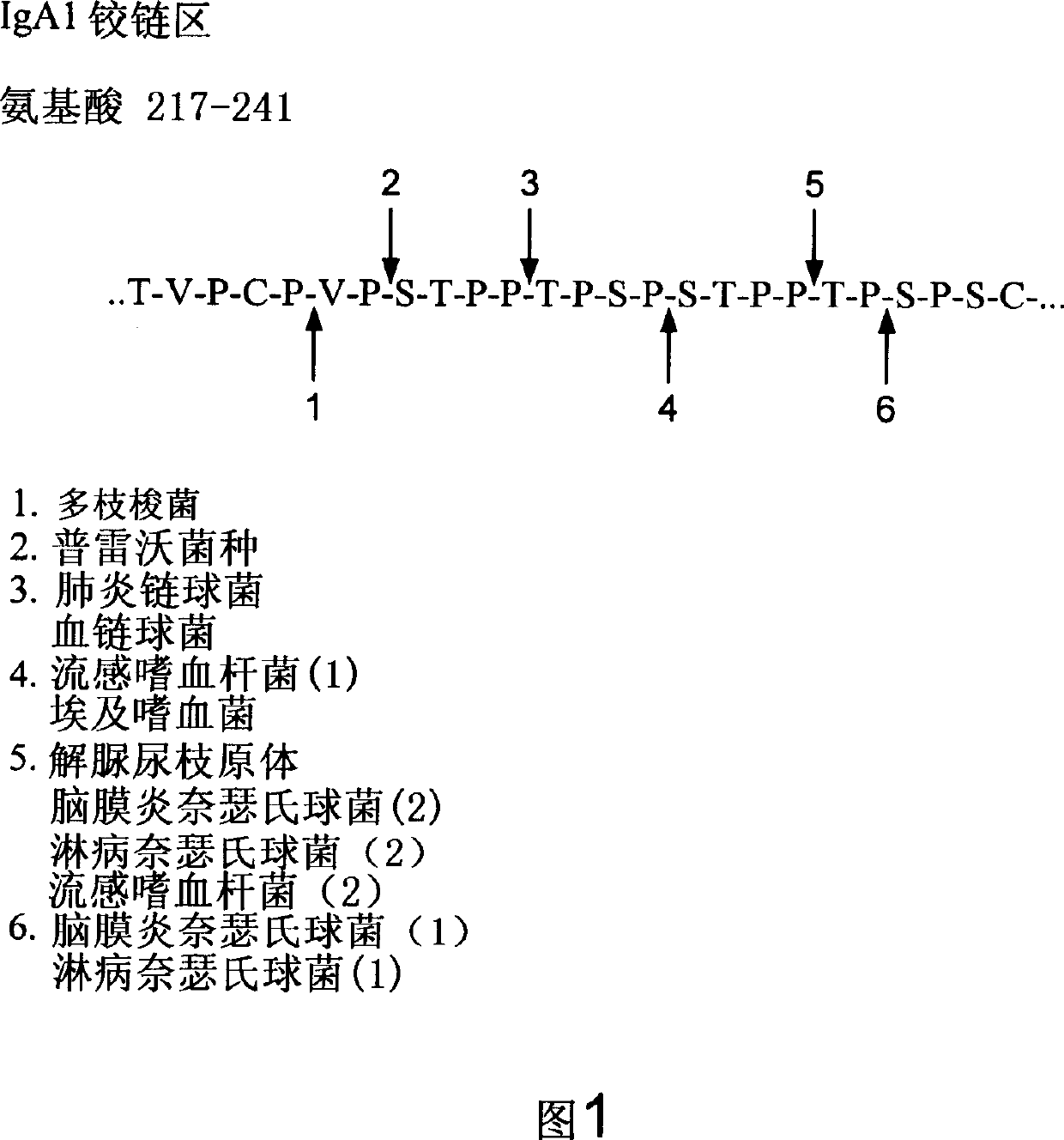
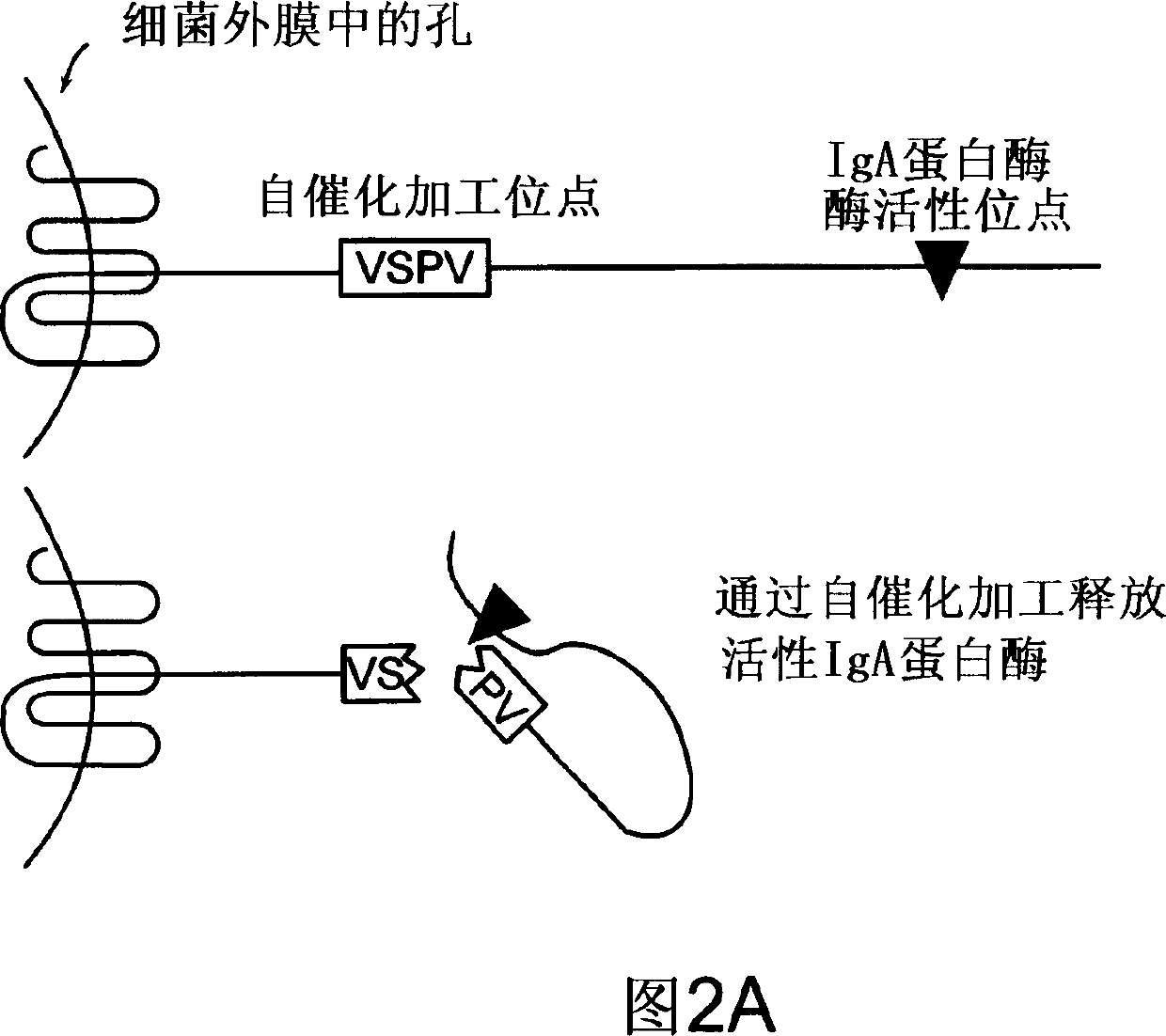
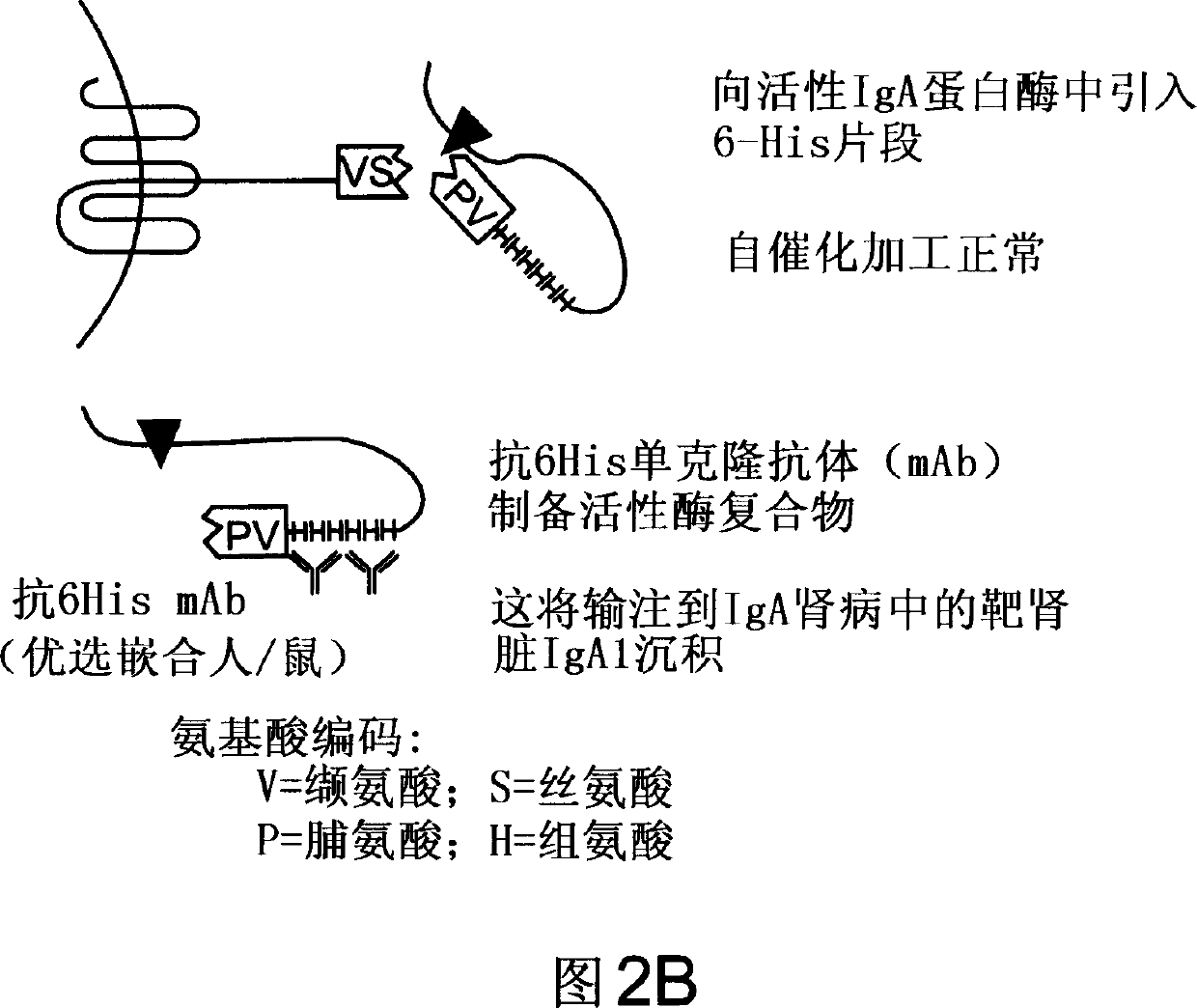
Treatment of IgA1 deposition diseases
The present invention discloses the use of bacterial IgA1 proteases to treat IgA1 deposition in tissue and organs. Bacterial IgA1 proteases specifically cleave IgA1 molecules and thus provide a means to specifically cleave and remove IgA1 depositions. Accordingly, therapeutic agents for the treatment of diseases characterized by IgA deposition are provided. In particular, therapeutic agents to treat IgA nephropathy, Dermatitis herpetiformis (DH), and Henoch-Schoenlein purpura (HS) are disclosed.
Owner:NEW ENGLAND MEDICAL CENT HOSPITALS
Herbal Composition and a Method of Making Thereof
InactiveUS20130266671A1Treatment safetyReduce usageBiocideCosmetic preparationsGranuloma annularePityriasis rosea
A topical composition is prepared using macerated leaves of St. Andrew's Cross (hypericum hypericoides). An aqueous extract of the leaves is mixed with ethanol to form a spray or to saturate a wet wipe and administer to a mammal skin for treatment a variety of skin conditions, including eczema, psoriasis, rosacea, cellulitis, contact dermatitis, seborrhoeic dermatitis, nummular dermatitis, dandruff, stasis dermatitis, perioral dermatitis, dermatitis herpetiformis, ecthyma, impetigo, shingles, urticaria, tinea pedis, tinea manuum, tinea cruris, sunburn, insect bites and stings, jellyfish stings, granuloma annulare, bullous pemphigoid, lichen planus, bed sore, inflammations caused by waterborne vectors, diaper rash, heat rash, pityriasis rosea, jungle rot, scleroderma, skin rashes and photo-aging.
Owner:VAN ALLER ROBERT THOMAS +2
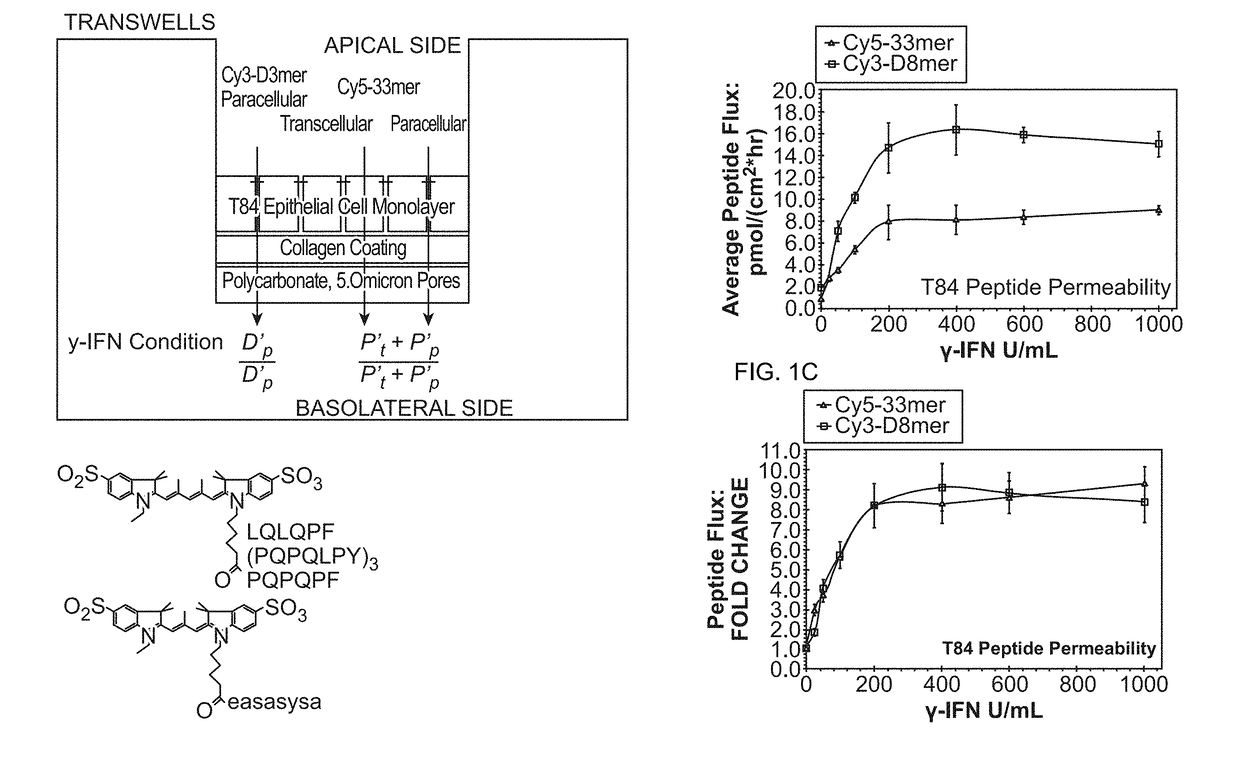
Modulation of Tissue Transglutaminase Activation in Disease
InactiveUS20140322278A1Improve throughputInhibition of activationBiocideOrganic chemistryGlutaminaseBiological activation
Compositions and methods are provided for modulating the physiological activation of tissue transglutaminase (TG2); which methods can include inhibiting the activation of TG2 associated with enteric inflammatory disorders, which disorders may include celiac disease, irritable bowel syndrome, Crohn's Disease, dermatitis herpetiformis, and the like. In other embodiments of the invention, methods are provided for reducing undesirable paracellular transport in enteric tissues, in particular the paracellular transport of molecules greater than about 500 mw, e.g. peptides, including without limitation immunogenic gluten peptides.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
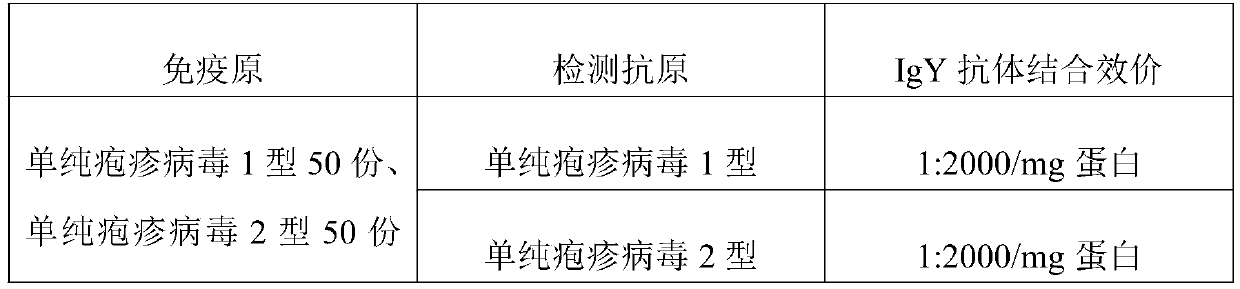
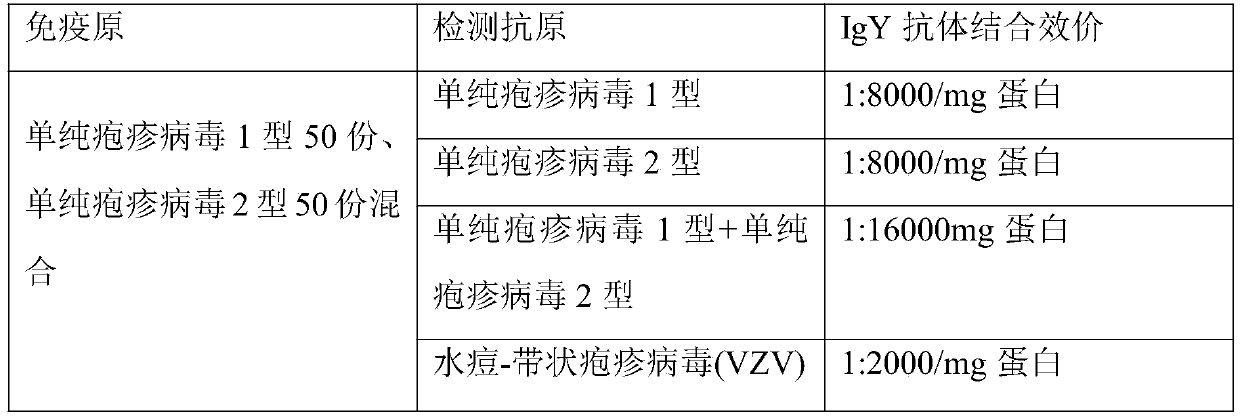
Preparation method and application of anti-herpes simplex virus composite IgY antibody
ActiveCN110669128ANo teratogenicNot mutagenicEgg immunoglobulinsImmunoglobulins against virusesDiseaseAntiendomysial antibodies
The invention discloses a preparation method and application of an anti-herpes simplex virus complex IgY antibody, and belongs to the technical field of herpes simplex virus resisting. The preparationmethod includes the following steps that step 1, an immunogen is prepared, specifically, a herpes simplex virus type 1 and a herpes simplex virus type 2 are taken and mixed and then the mixture is mixed with a Freund's complete adjuvant and a Freund's incomplete adjuvant separately and stirred and emulsified to prepare a water-in-oil Freund's complete adjuvant and Freund's incomplete adjuvant herpes simplex virus immunogen; and step 2, immunity is performed, specifically, the Freund's complete adjuvant immunogen is injected into laying hens. Application to ocular conjunctiva, a nasal cavity and an oral cavity is achieved, no limitations and side effects of systemic medication exist, the local application taking effect is quick, the dosage is low, the curative effect is good, the normal micro-ecological environment on the surface of the conjunctiva, the nasal cavity and the oral cavity is not absorbed and damaged, no toxic and side effects exist, and diseases of herpes labialis, dermatitis herpetiformis and herpes zoster caused by the herpes simplex virus type 1 and the herpes simplex virus type 2 and viruses with a common antigen with the herpes simplex virus type 1 and the herpessimplex virus type 2 are prevented and treated.
Owner:南京道大药业有限公司
Modulation of tissue transglutaminase activation in disease
Compositions and methods are provided for modulating the physiological activation of tissue transglutaminase (TG2); which methods can include inhibiting the activation of TG2 associated with enteric inflammatory disorders, which disorders may include celiac disease, irritable bowel syndrome, Crohn's Disease, dermatitis herpetiformis, and the like. In other embodiments of the invention, methods are provided for reducing undesirable paracellular transport in enteric tissues, in particular the paracellular transport of molecules greater than about 500 mw, e.g. peptides, including without limitation immunogenic gluten peptides.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
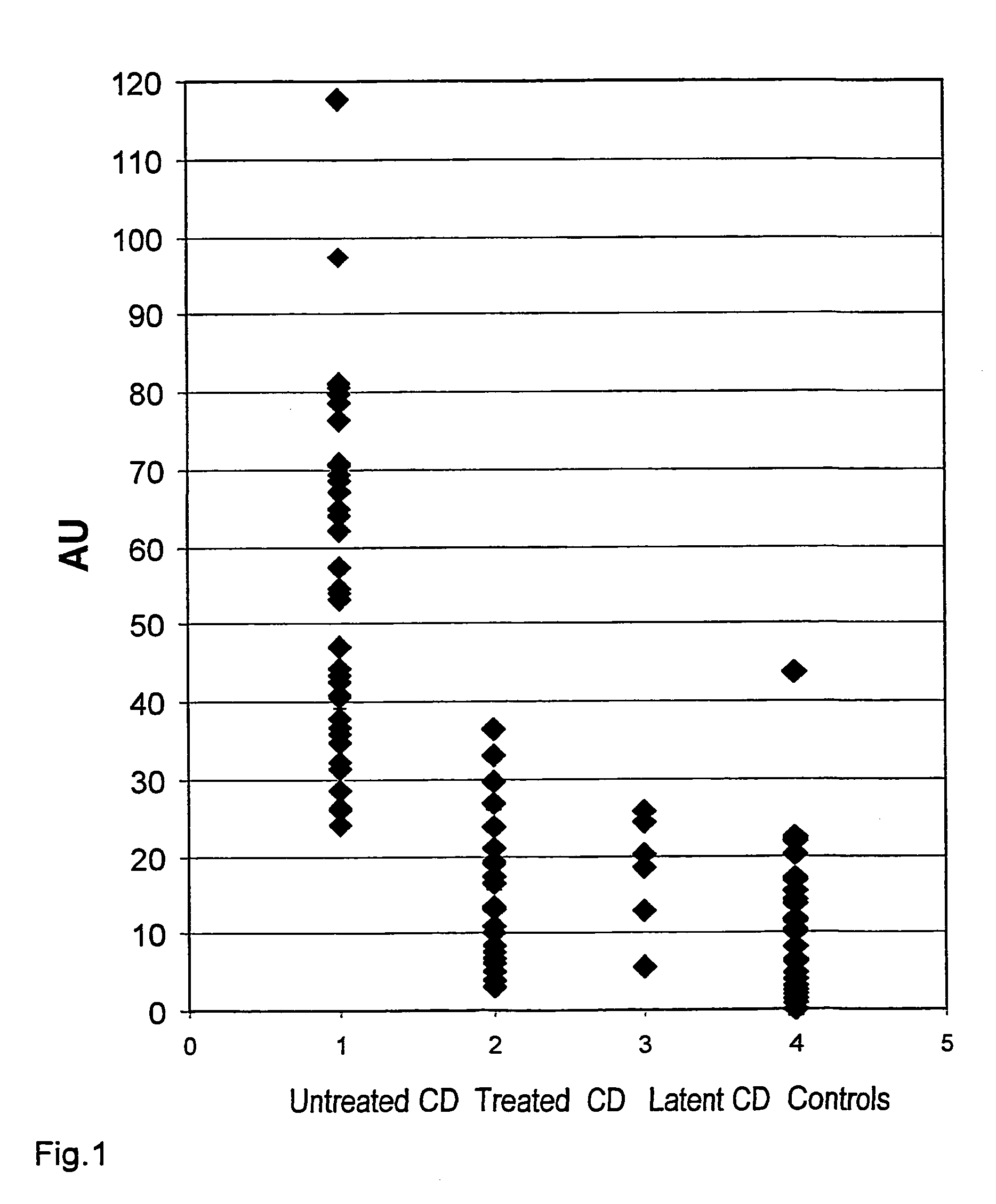
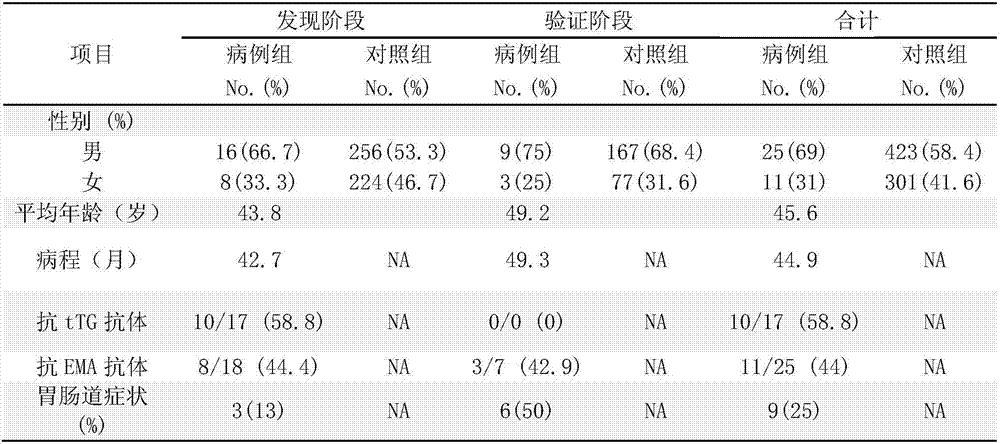
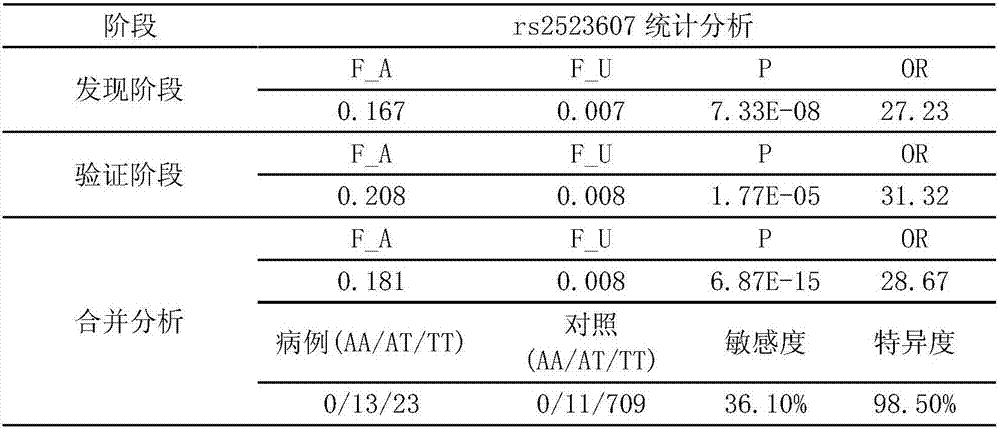
Application of single nucleotide polymorphism site rs2523607 to screening of patients suffering from dermatitis herpetiformis
The invention discloses application of single nucleotide polymorphism site rs2523607 to screening of patients suffering from dermatitis herpetiformis. A technical scheme protected by the invention is characterized in that application of a substance for detecting polymorphism or a gene type of the rs2523607 in a human genome to preparation of a product for screening the patients suffering from the dermatitis herpetiformis and application of the substance for detecting the polymorphism or the gene type of the rs2523607 in the human genome to preparation of a product for detecting single nucleotide polymorphism relevant to the dermatitis herpetiformis are provided. The substance for detecting the polymorphism or the gene type of the rs2523607 and other substances (for example, substances for detecting other single nucleotide polymorphism or gene types relevant to the dermatitis herpetiformis) are combined together to screen the patients suffering from dermatitis herpetiformis.
Owner:SHANDONG PROVINCIAL INST OF DERMATOLOGY & VENEREOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com