Patents
Literature
92 results about "Medications therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
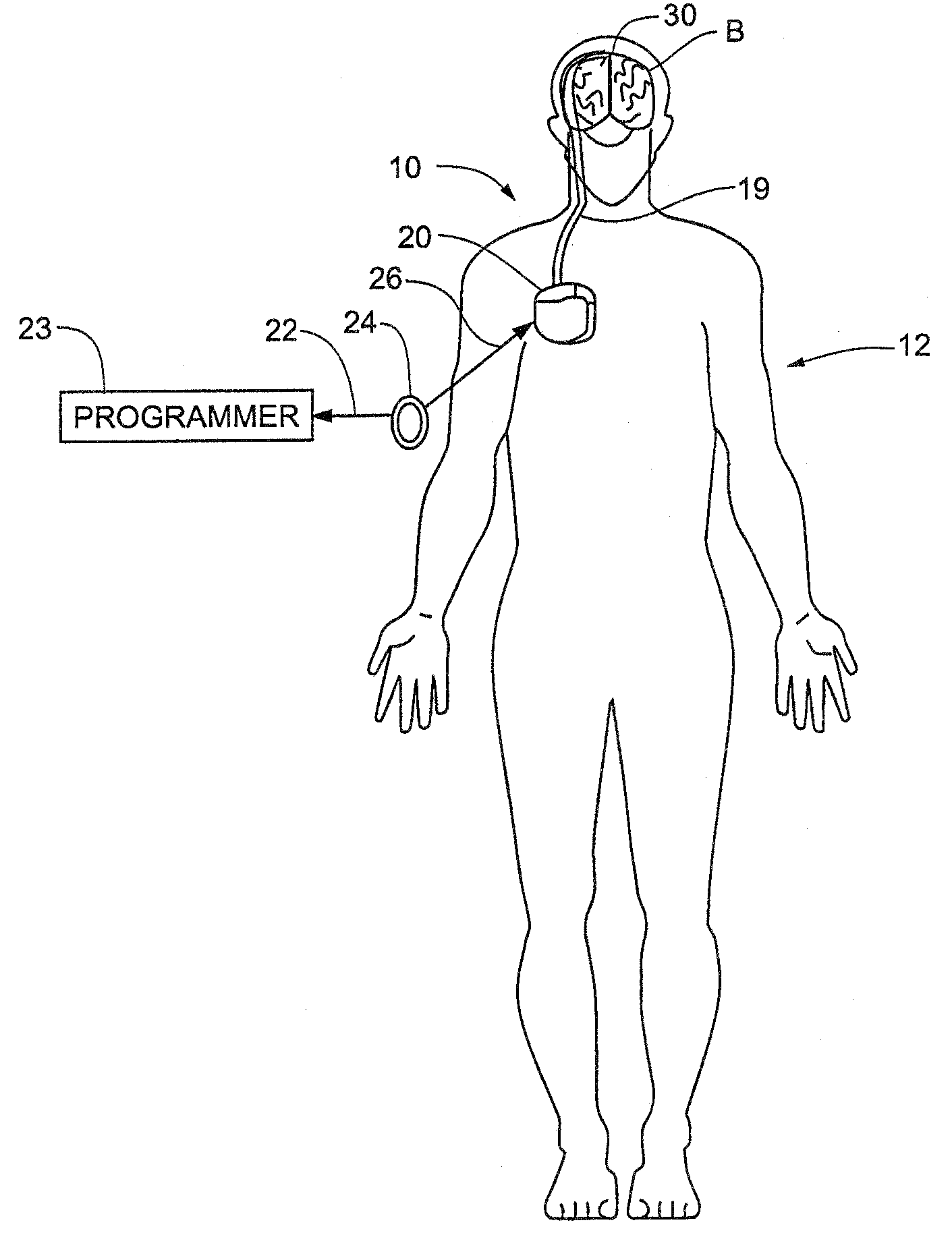
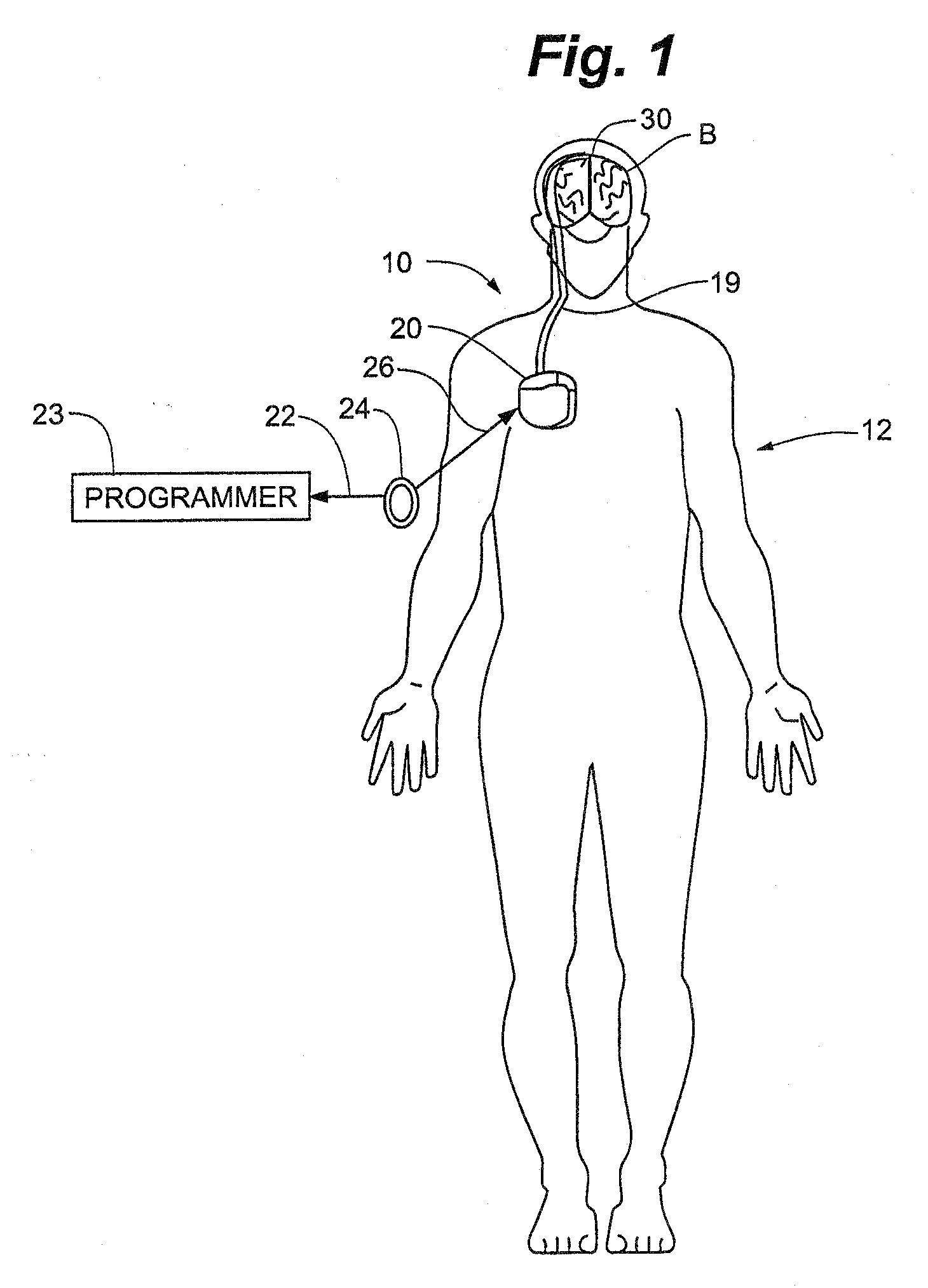
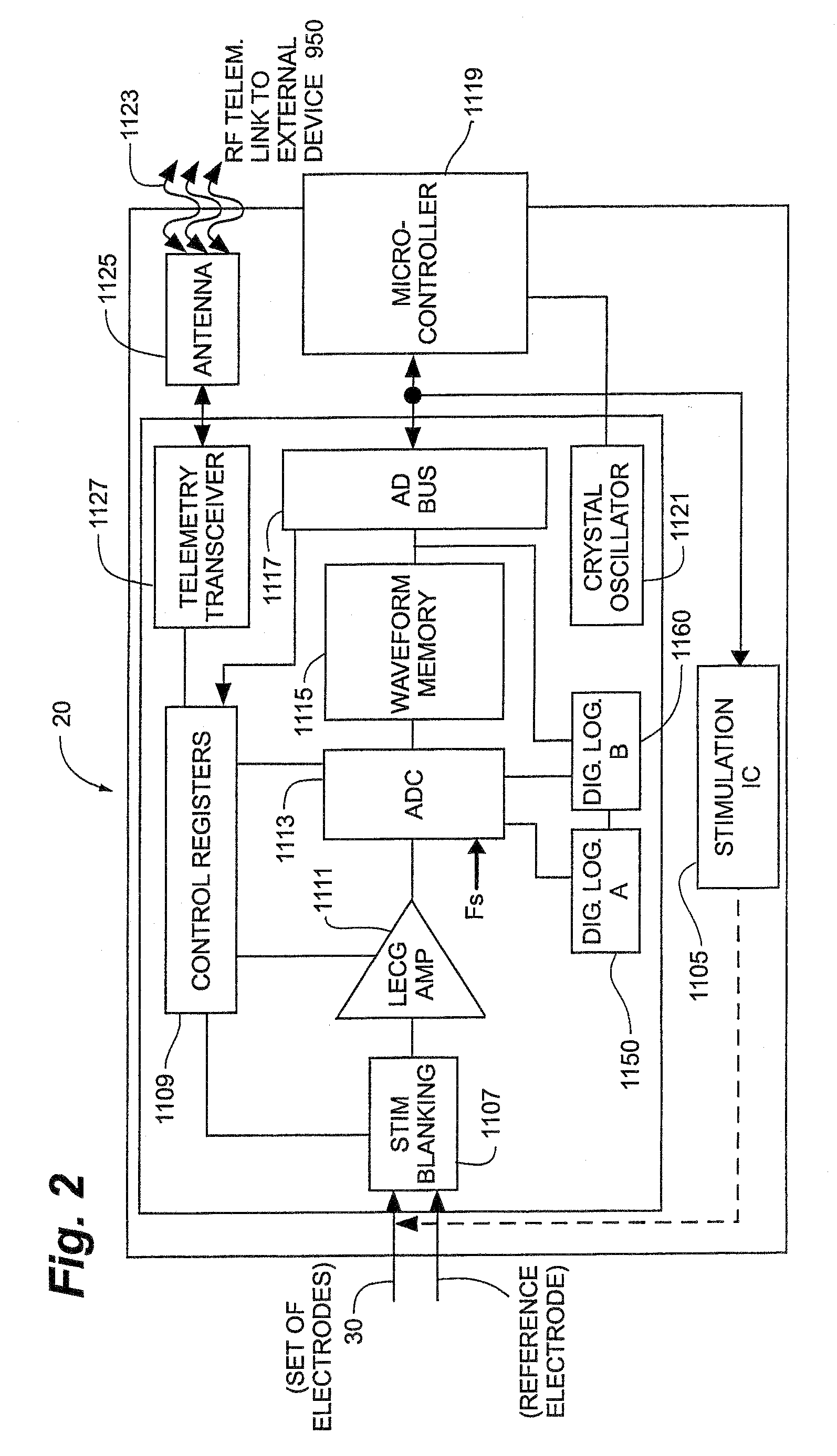
Method and Apparatus for the Treatment of Movement Disorders
A method, apparatus, and system for treating patients suffering from movement disorders having the ability to determine one or more biomarkers indicative of a disease state. In some embodiments, the biomarker may be used as a closed-loop feedback signal to control the delivery of therapy (such as electrical stimulation or drug therapy), and which may also be used as an indication of therapy effectiveness. One embodiment uses electrodes placed in the brain to measure EEG or local field potential (LFP) signals, from which the one or more biomarkers may be determined.
Owner:MEDTRONIC INC
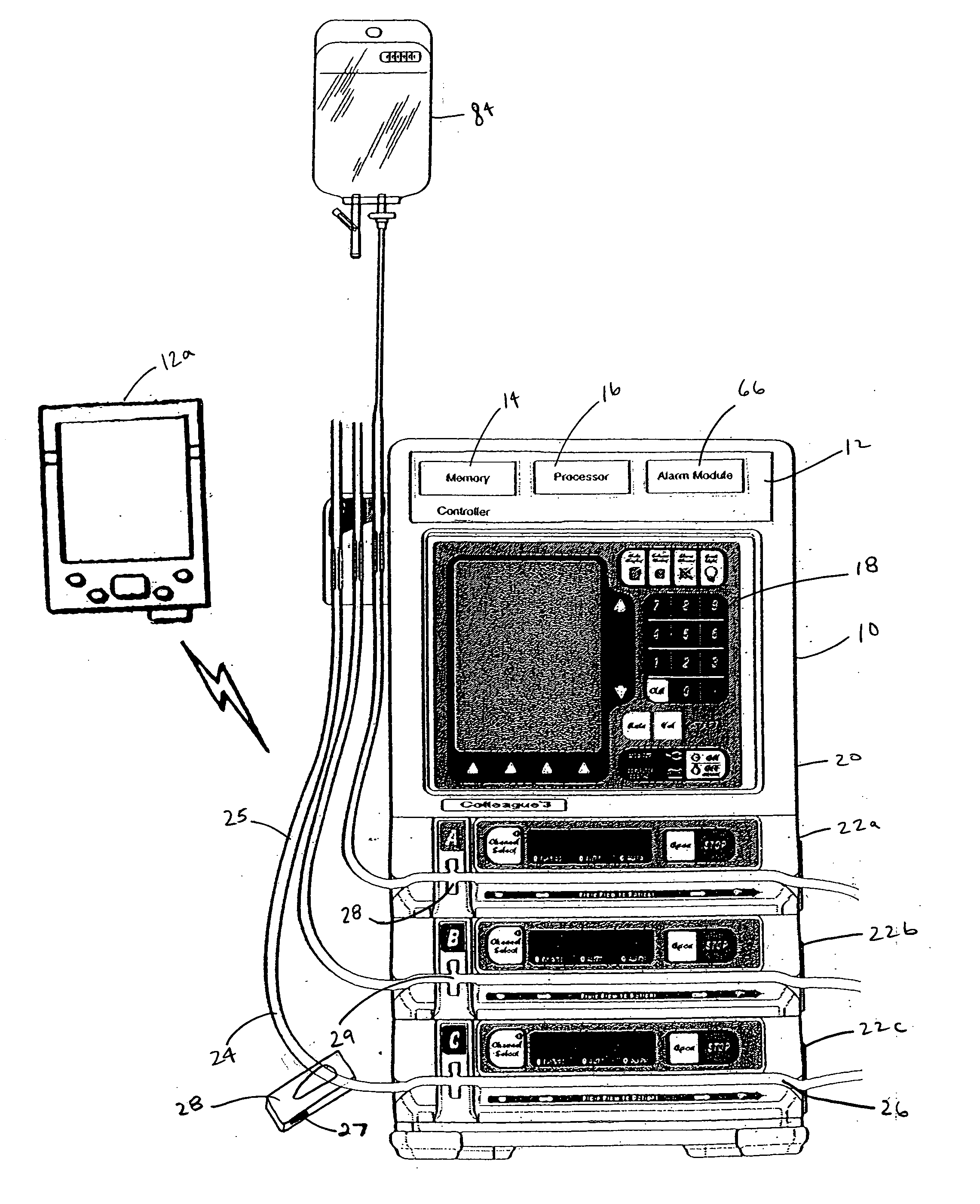
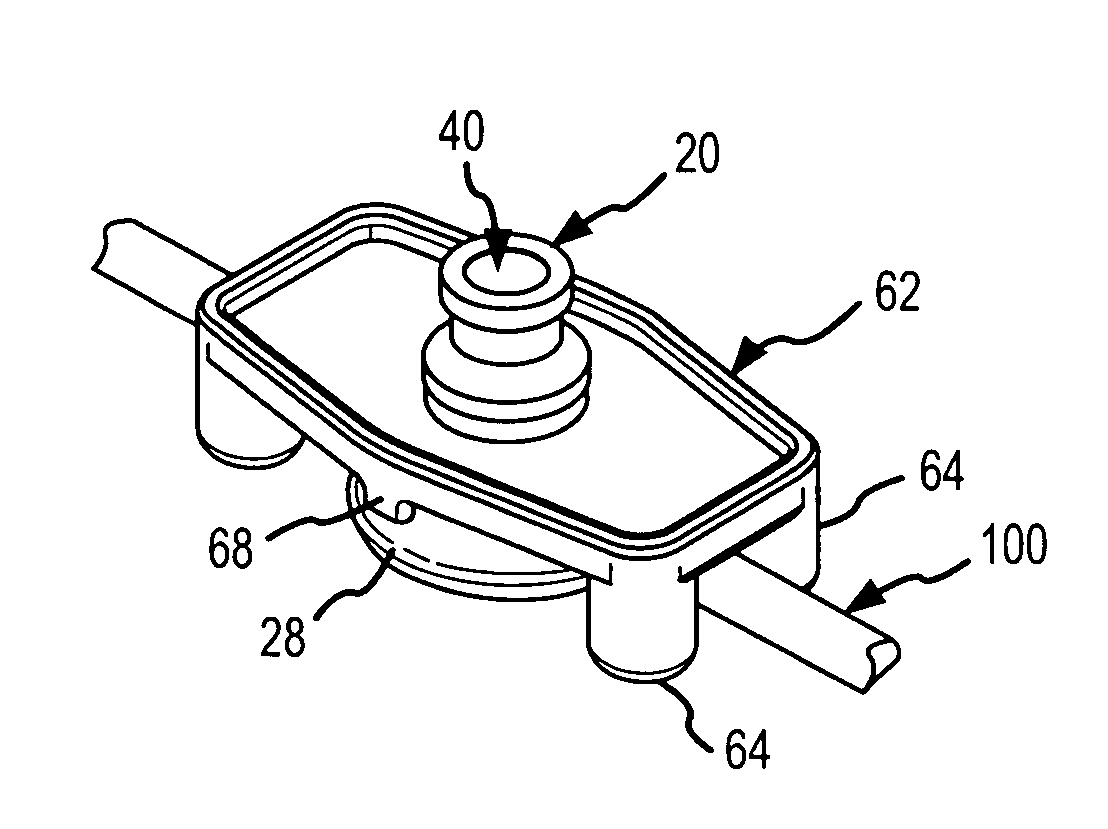
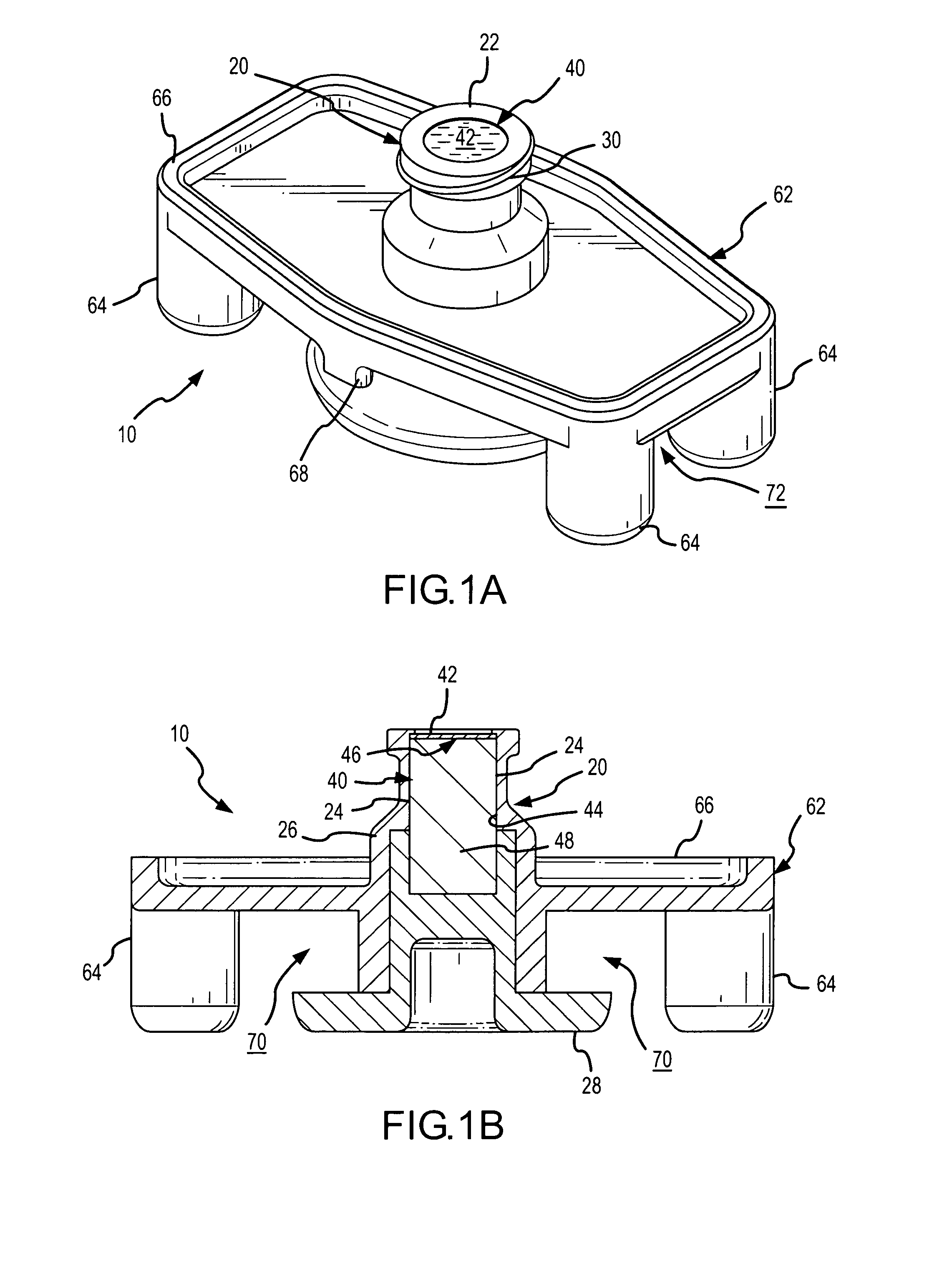
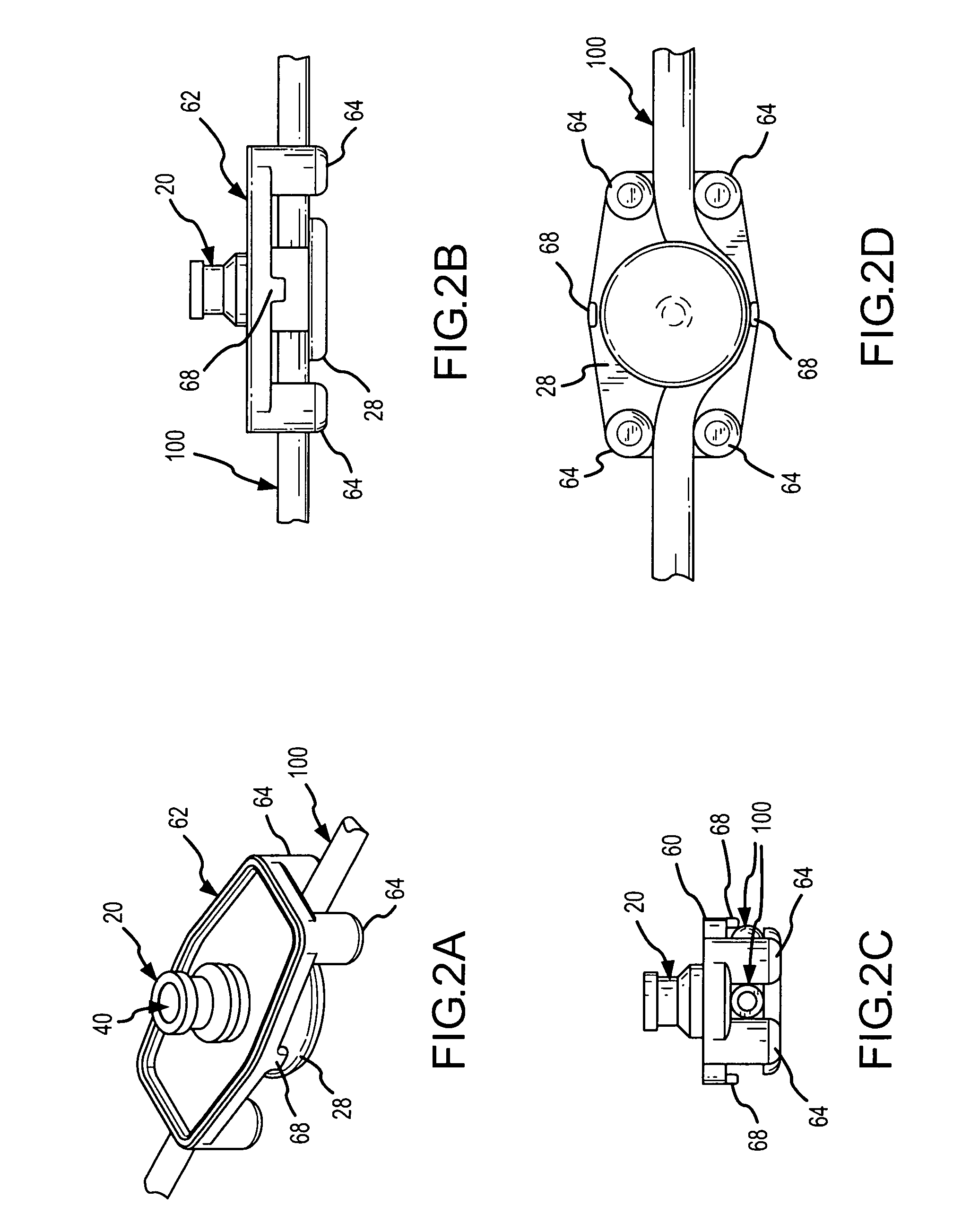
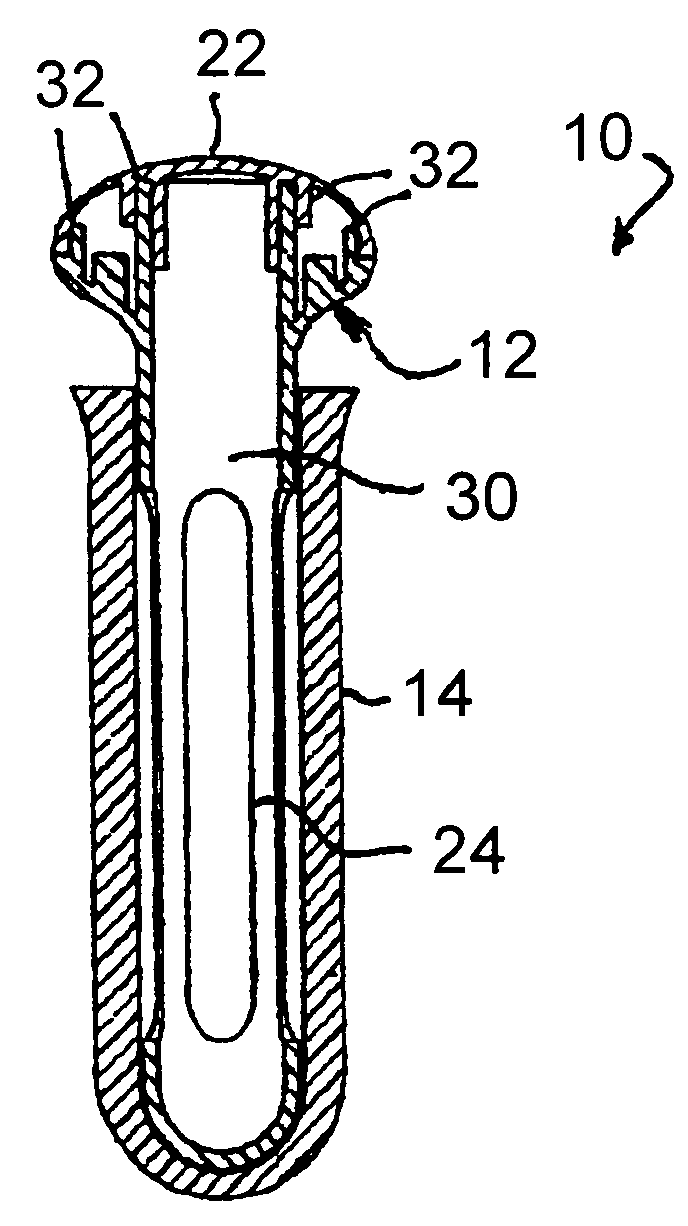
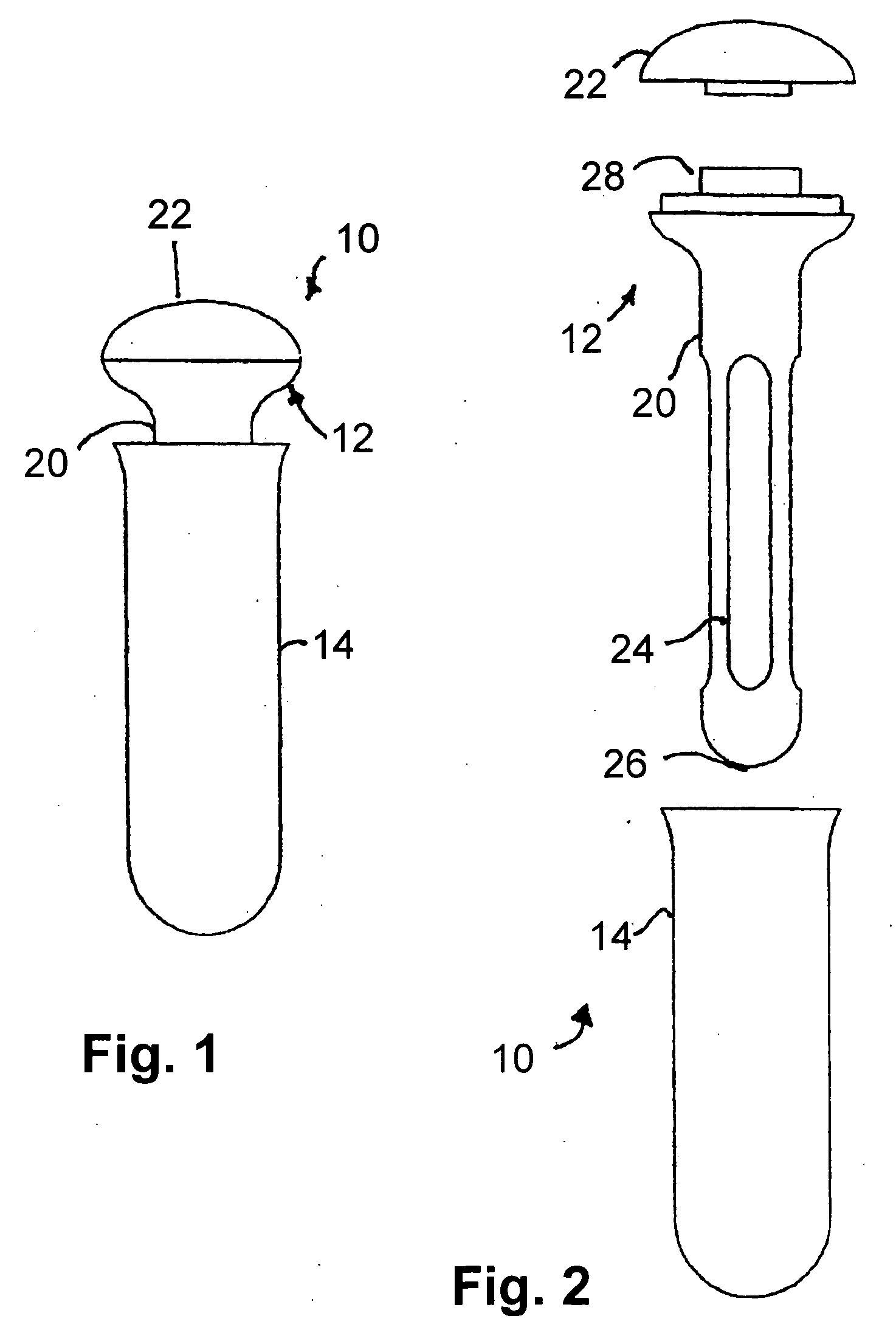
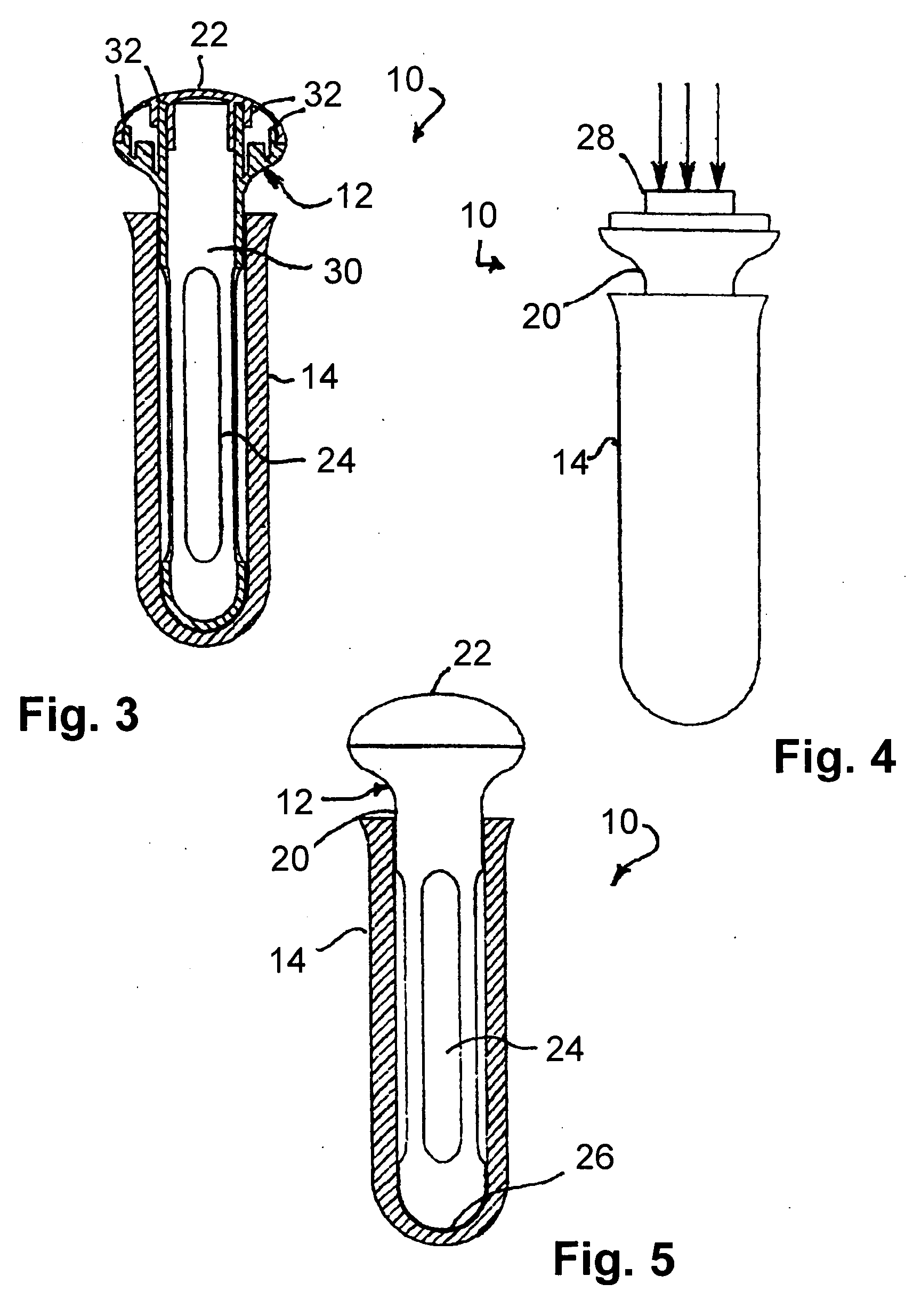
Apparatus and method for therapeutic delivery of medication
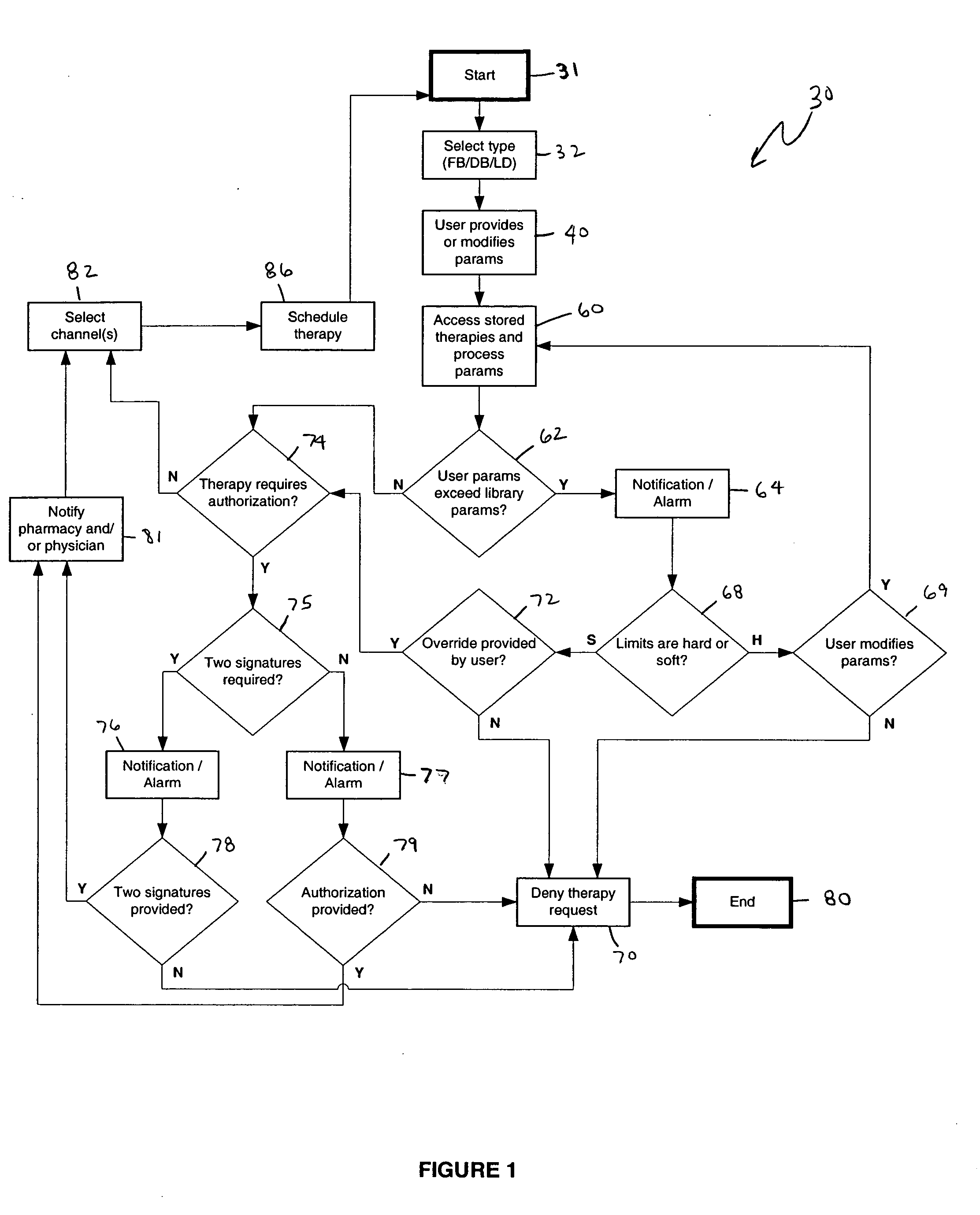
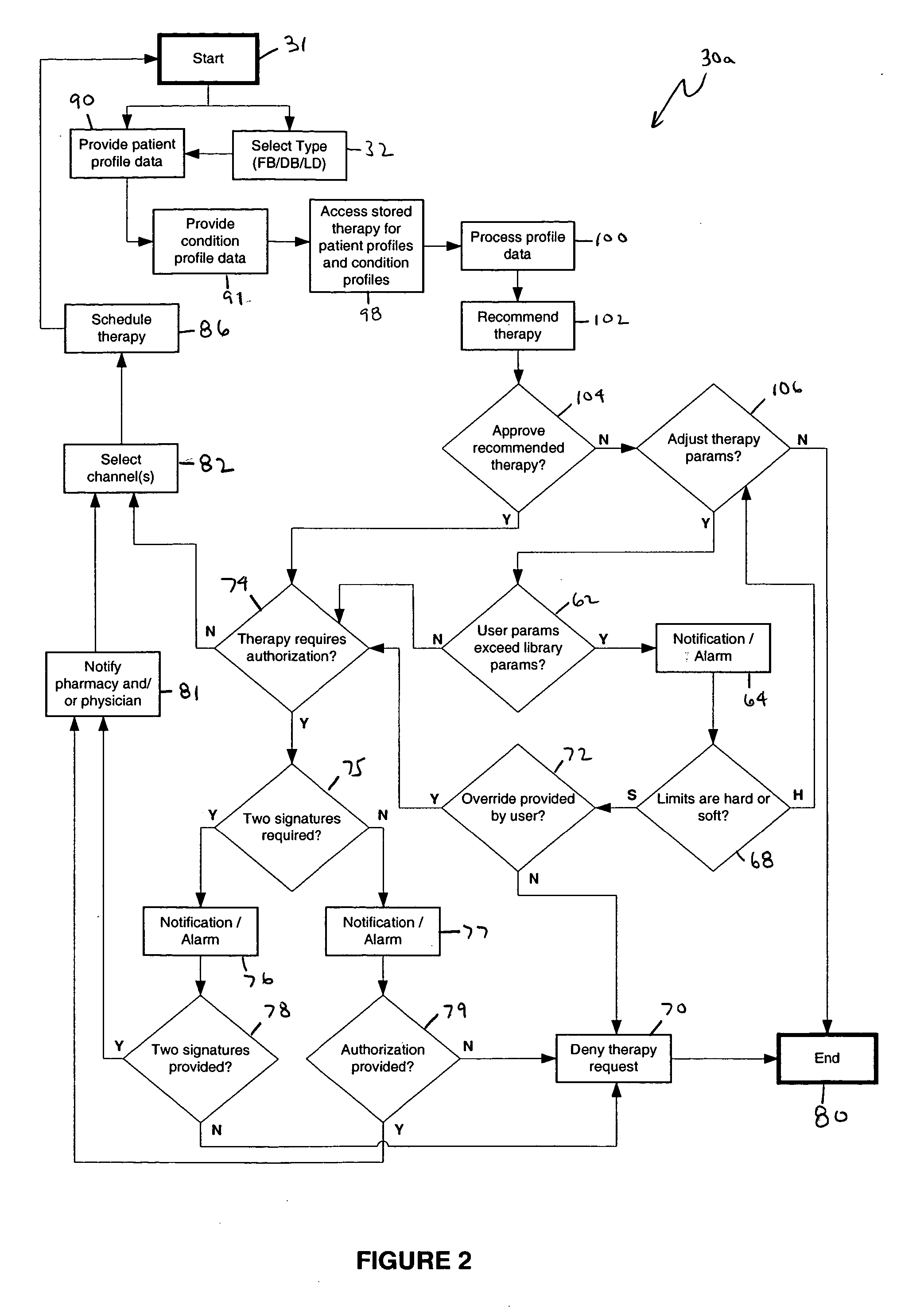
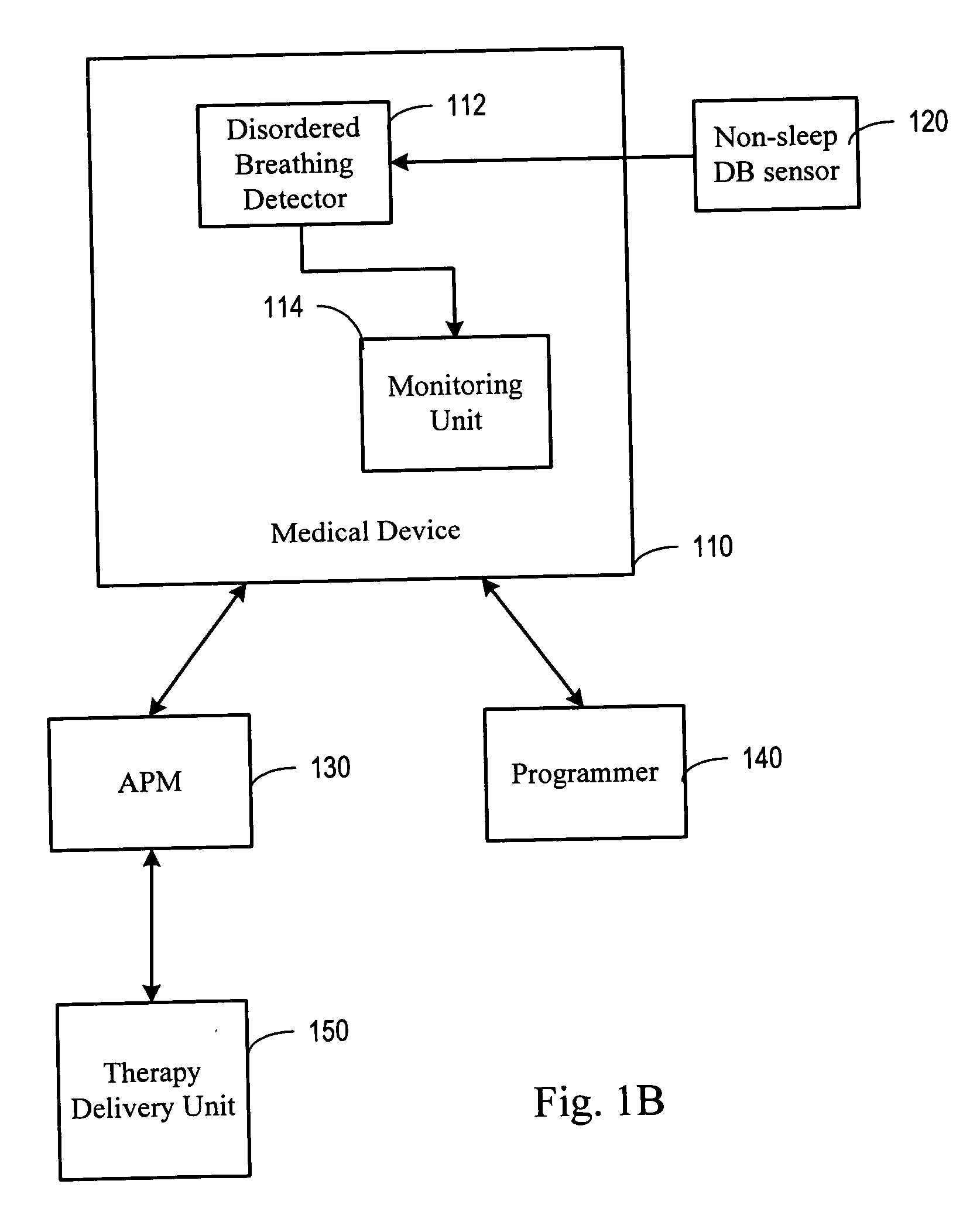
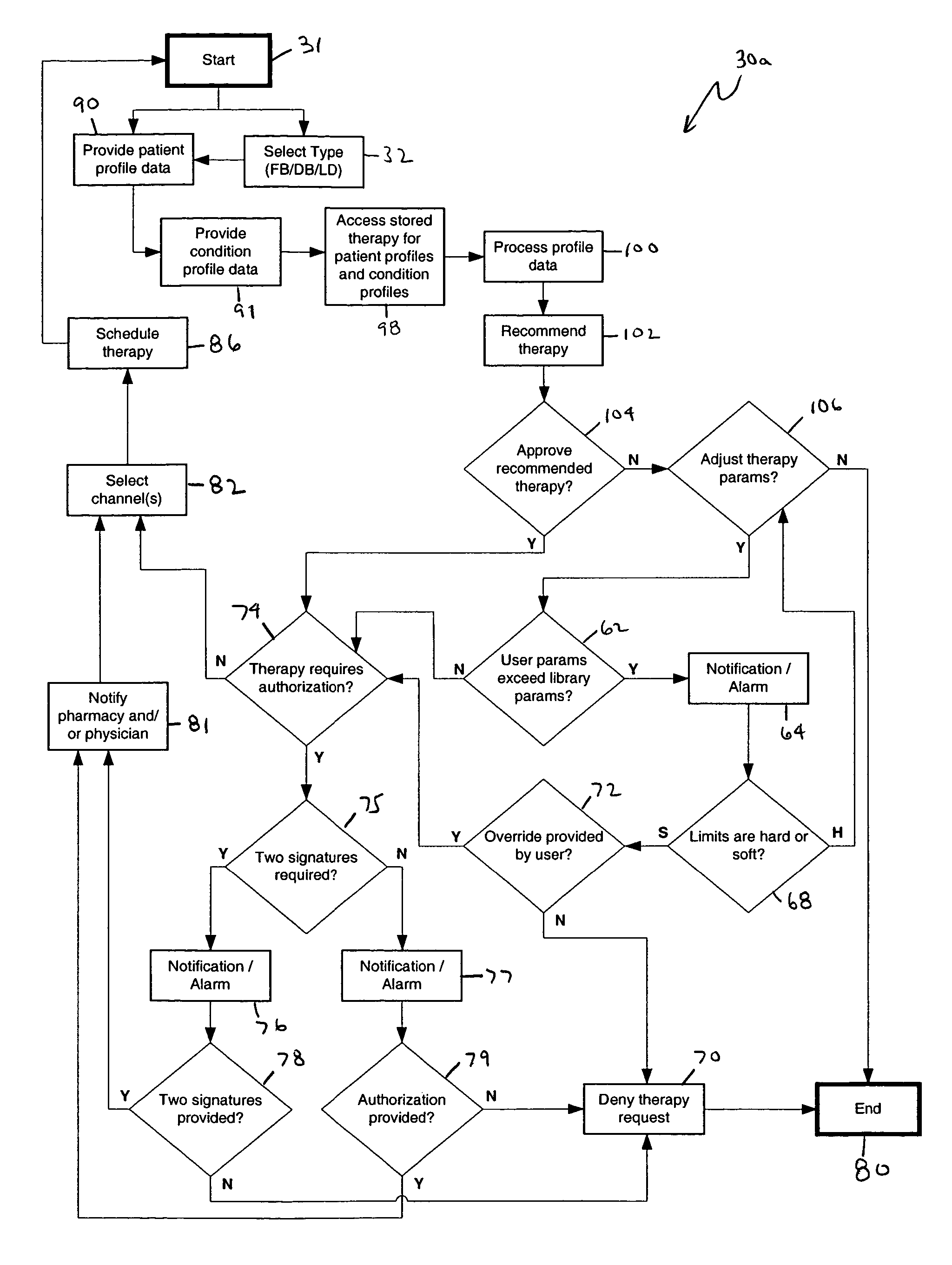
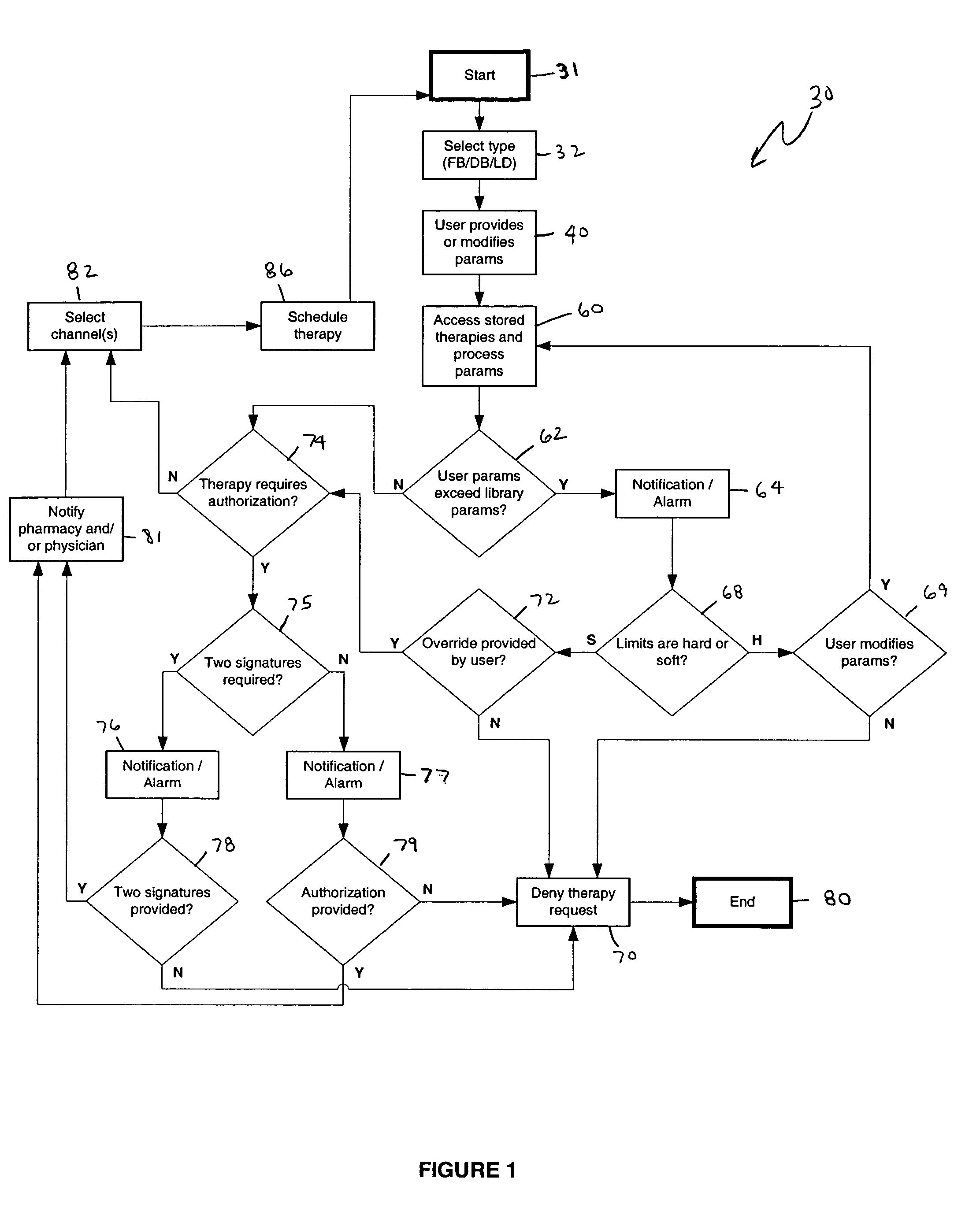
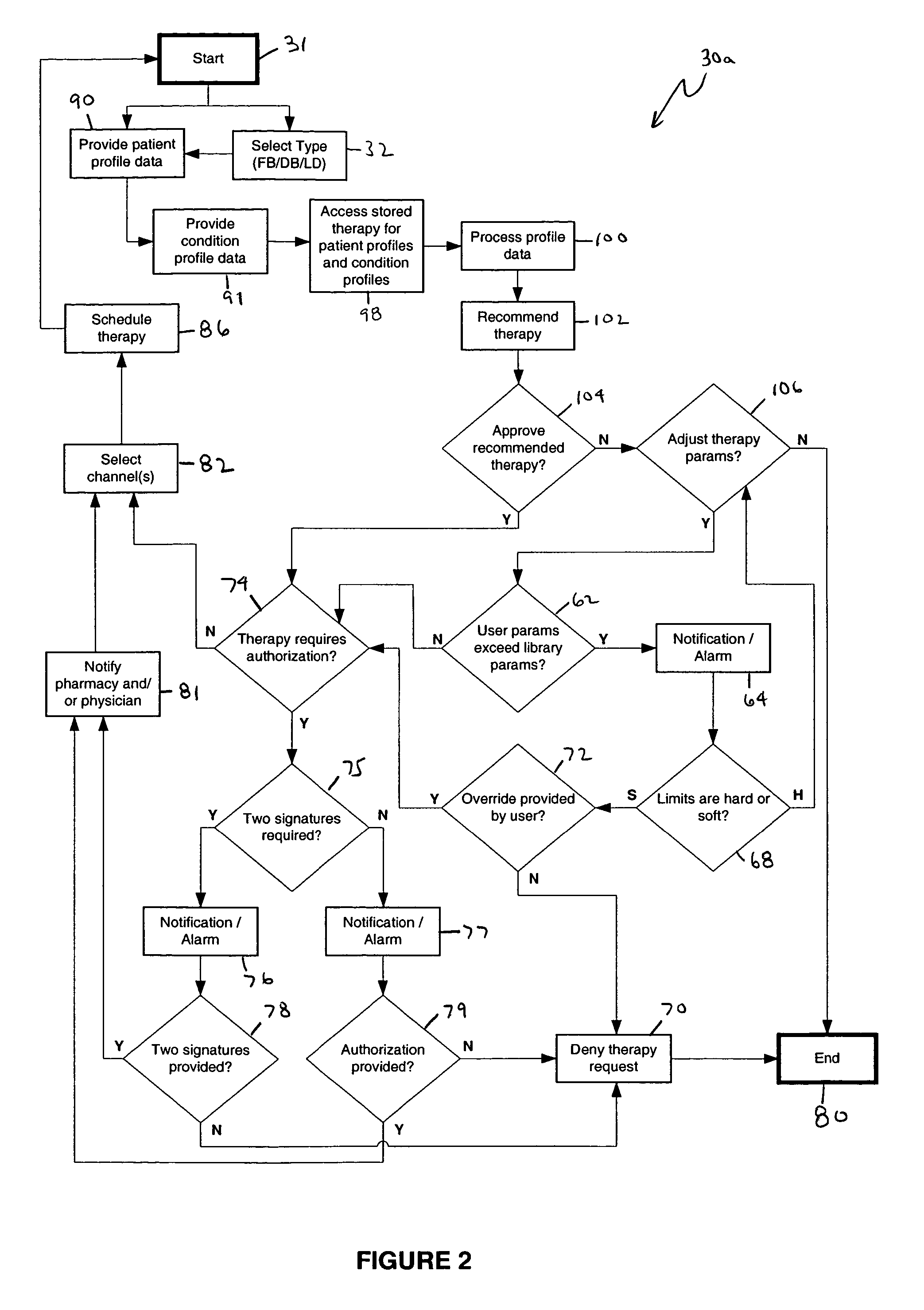
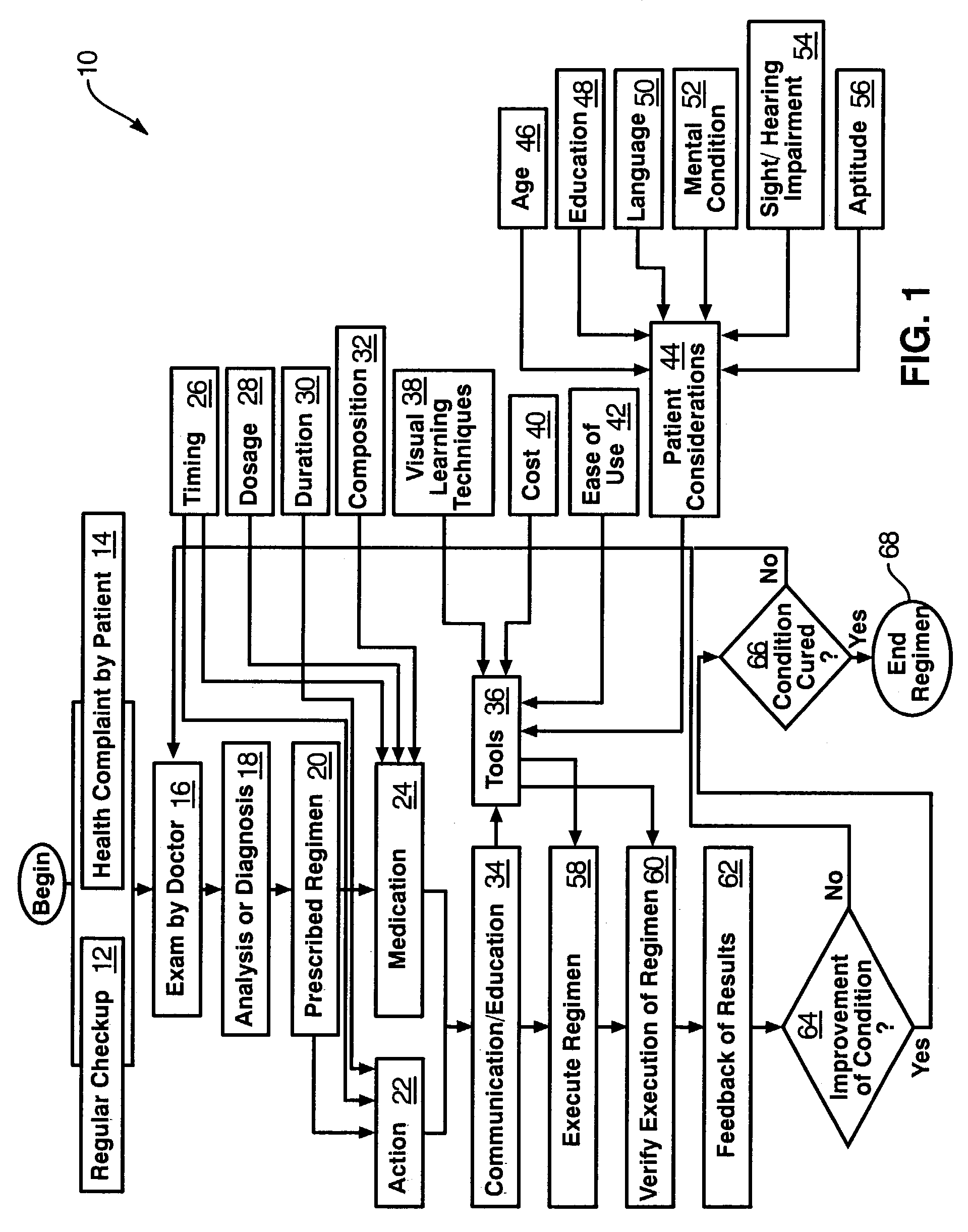
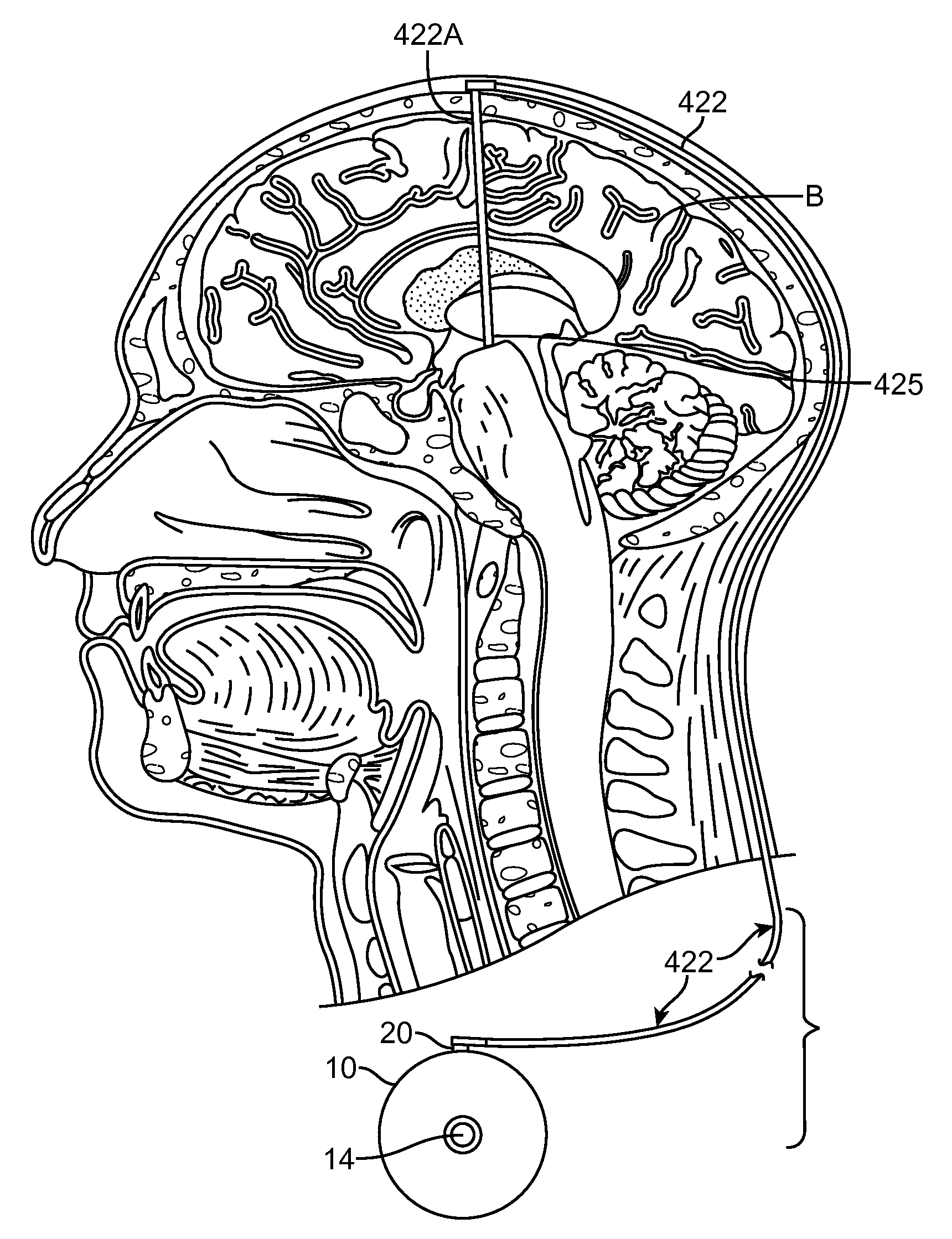
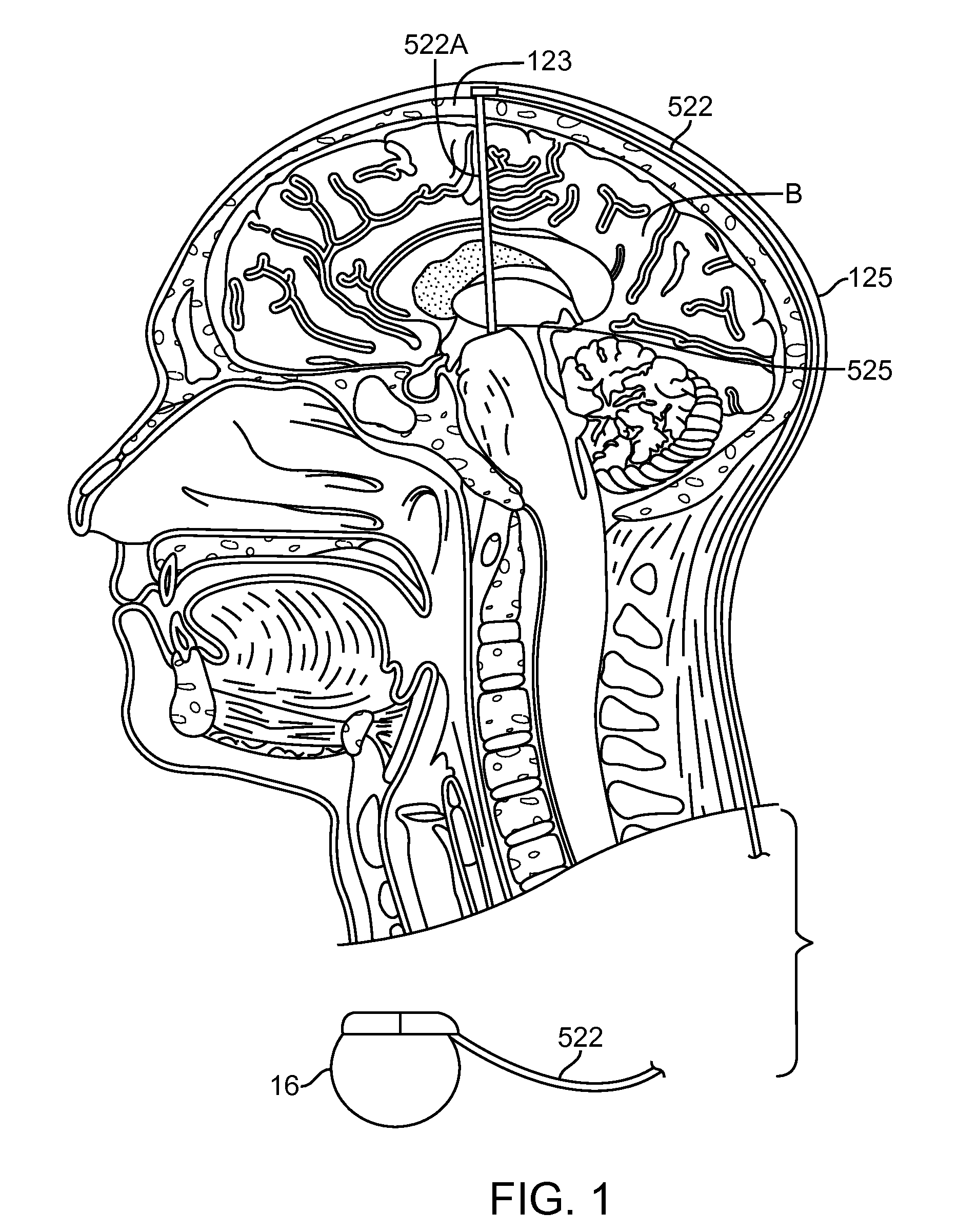
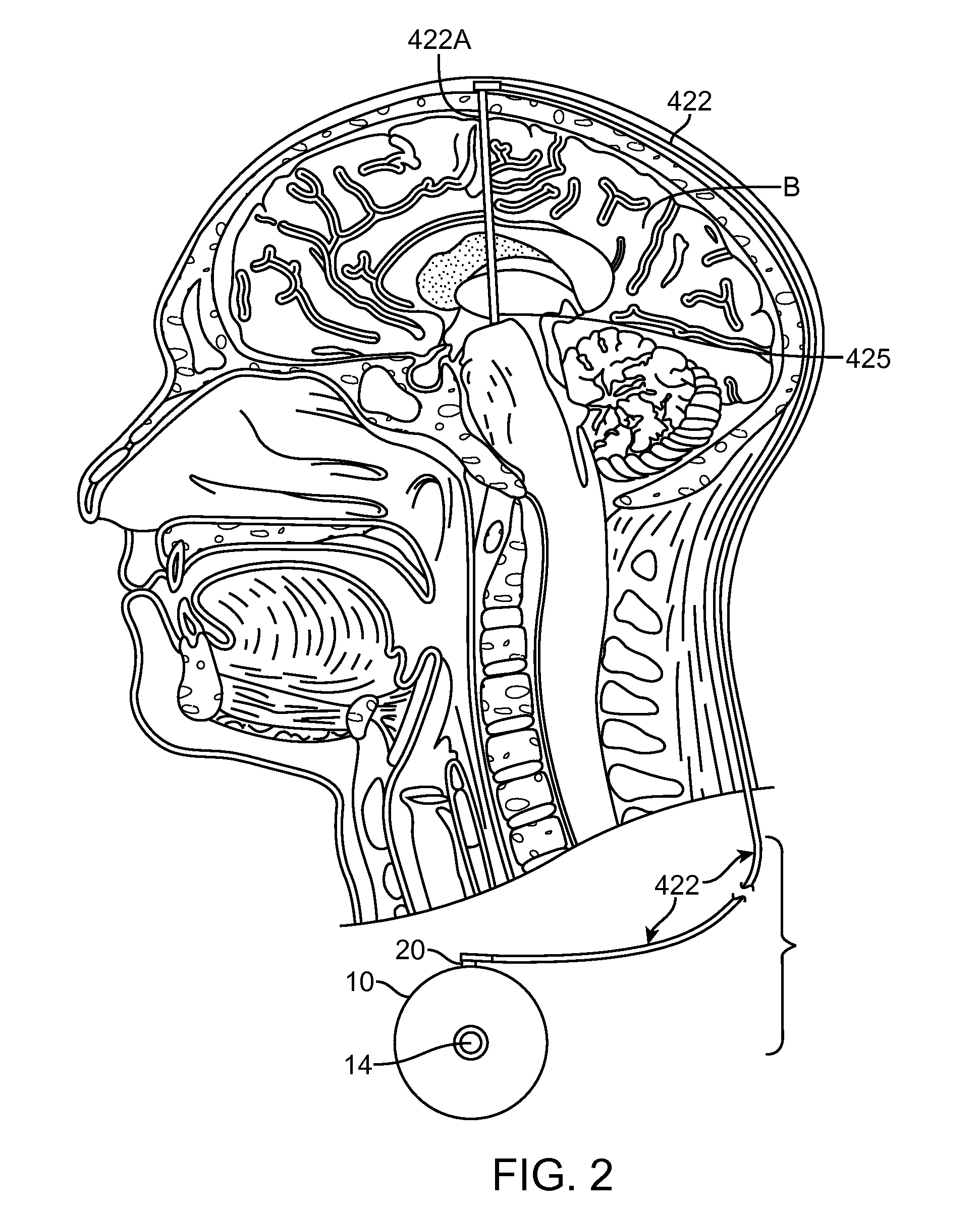
An apparatus, system and method of programming a medical therapy in a medical device are provided. Generally, the medical device 10 has a controller 12, a memory 14, a processor 16 and an input device 18. The memory 14 is preloaded with at least one of a plurality of patient profiles and condition profiles. The memory 14 is further preloaded with an associated medication therapy for a plurality of the profiles. The input device 18 receives profile data 90, 91 comprising at least one of a patient profile data and a condition profile data for a specific patient, and the processor 16 processes the received profile data 100 and provides as output one of the preloaded medication therapies based on the processed profile data 102.
Owner:BAXTER INT INC +1
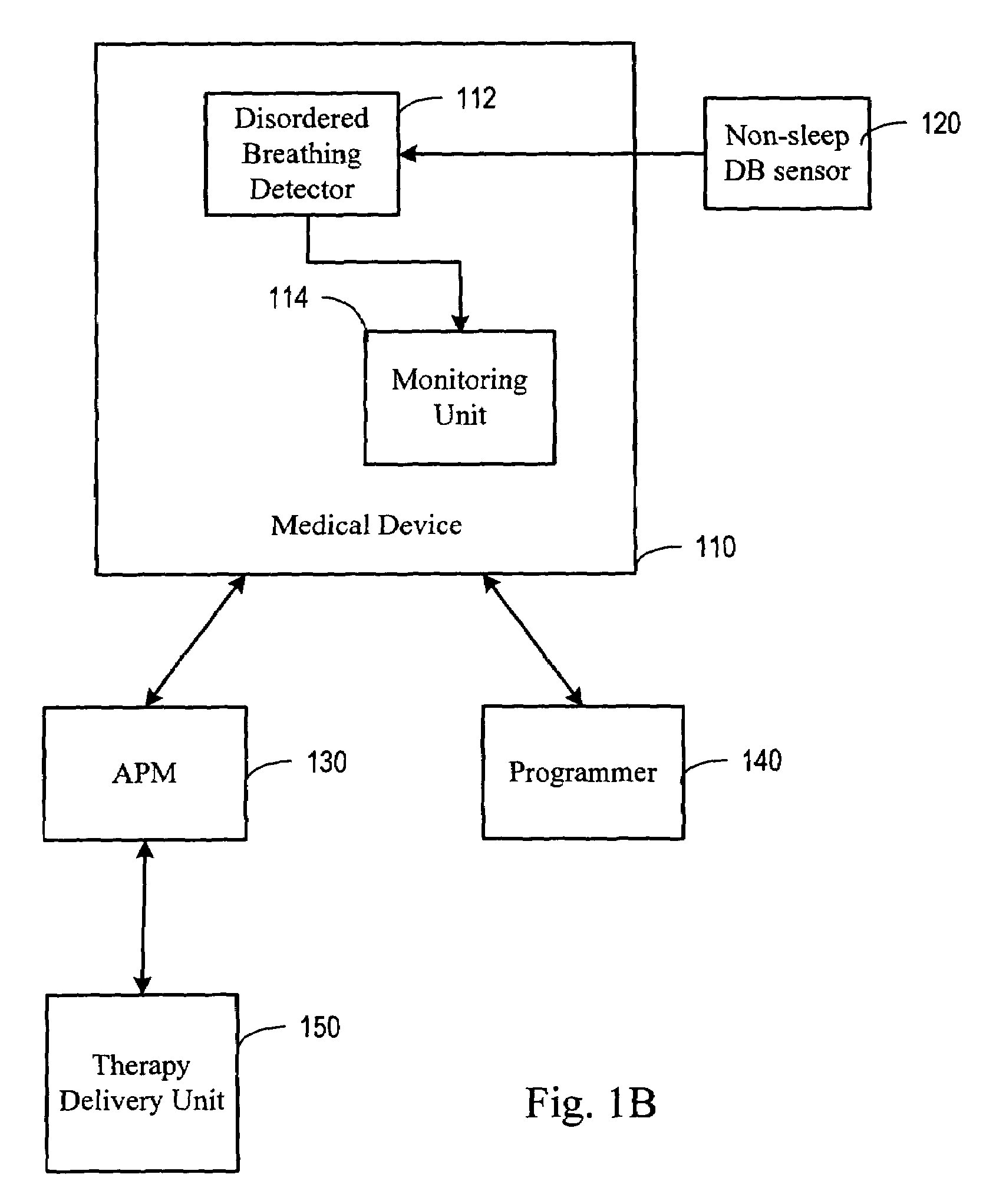
System and method for moderating a therapy delivered during sleep using physiologic data acquired during non-sleep
InactiveUS20050080461A1Inertial sensorsMedical devicesPositive airway pressureSleep disordered breathing
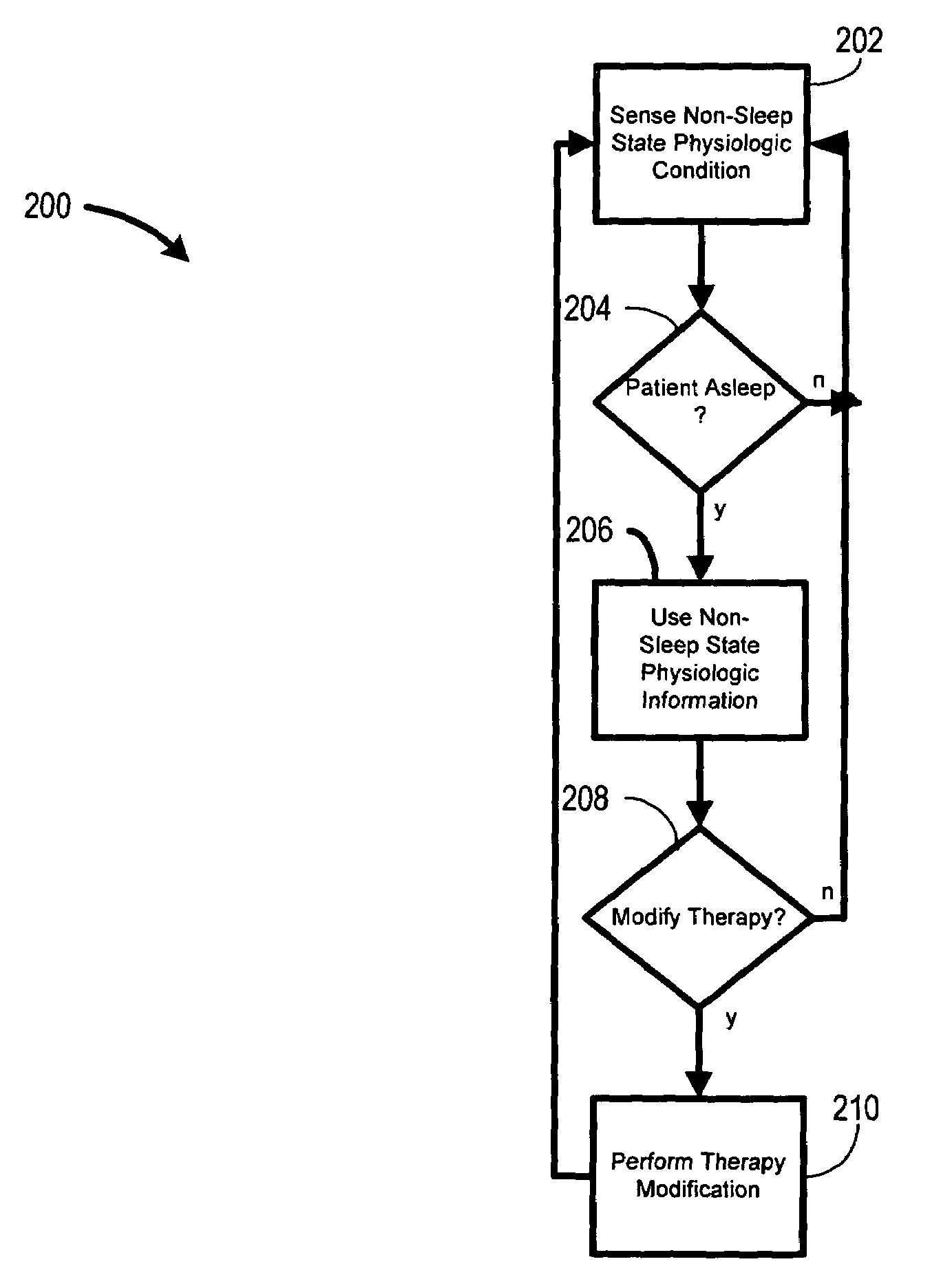
Systems and methods provide for gathering of patient related data during non-sleep periods and modulating a therapy delivered to the patient during sleep using the gathered data. Data associated with a patient is gathered while the patient is awake. A therapy delivered to the patient during patient sleep is adjusted using the acquired data. The therapy delivered to the patient may include one or more of a respiratory therapy, such as a positive airway pressure (xPAP) therapy, a sleep disordered breathing therapy, a cardiac rhythm management therapy, such as a cardiac overdrive pacing therapy, a medication therapy, or a drug delivery therapy. The therapy delivered to the patient may be optimized using the acquired data.
Owner:CARDIAC PACEMAKERS INC
Apparatus and method for therapeutic delivery of medication
Owner:BAXTER INT INC +1
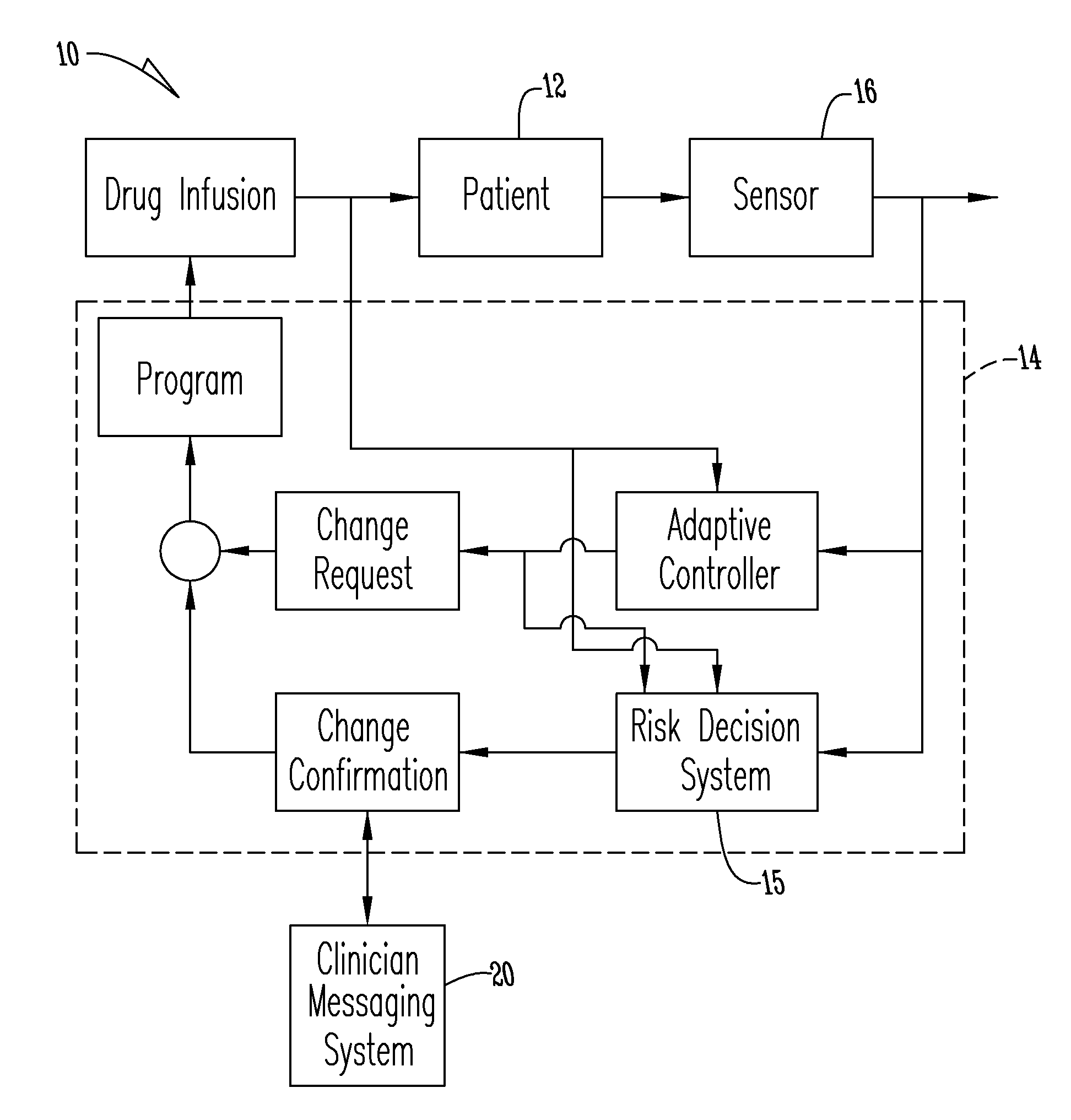
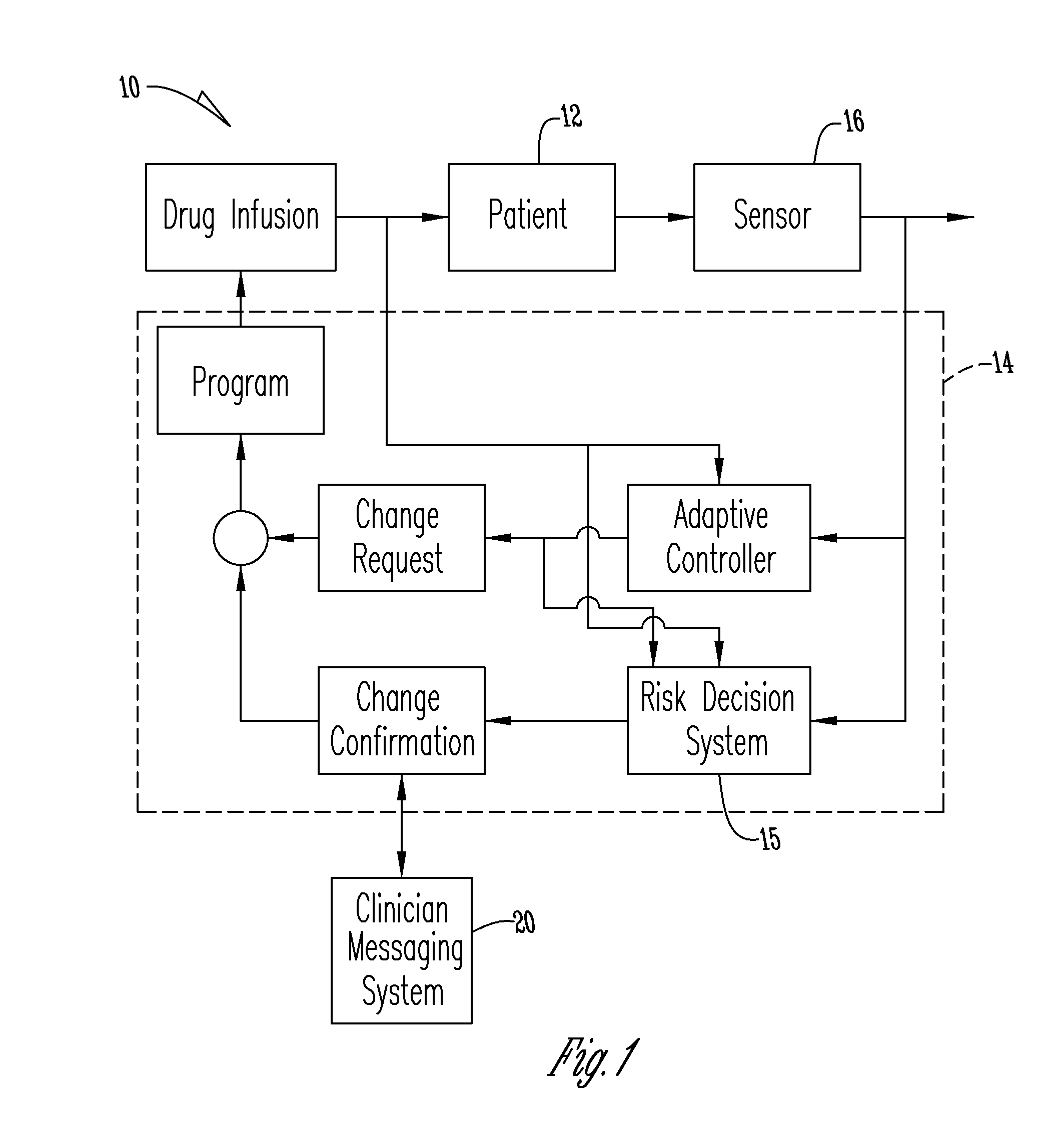
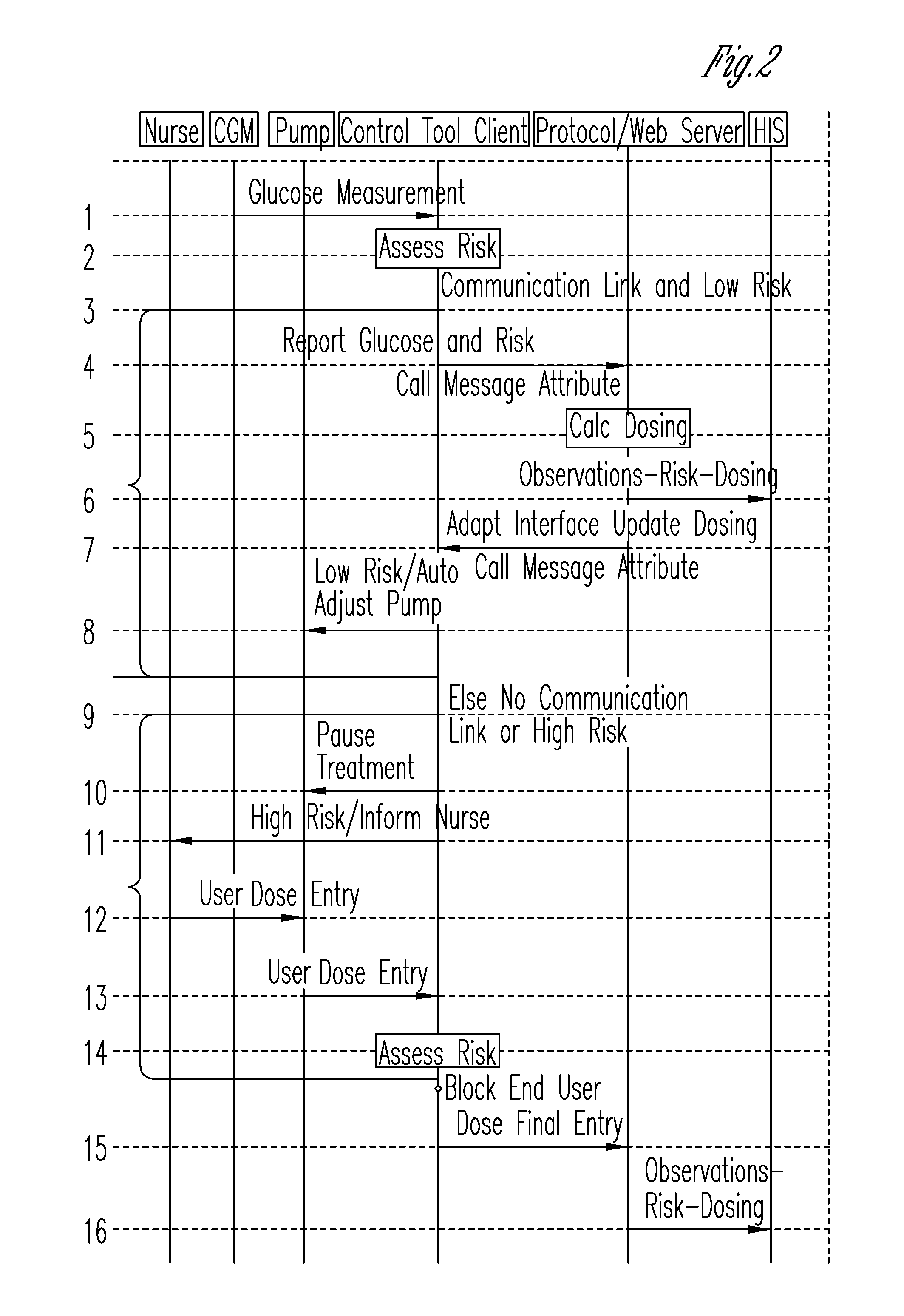
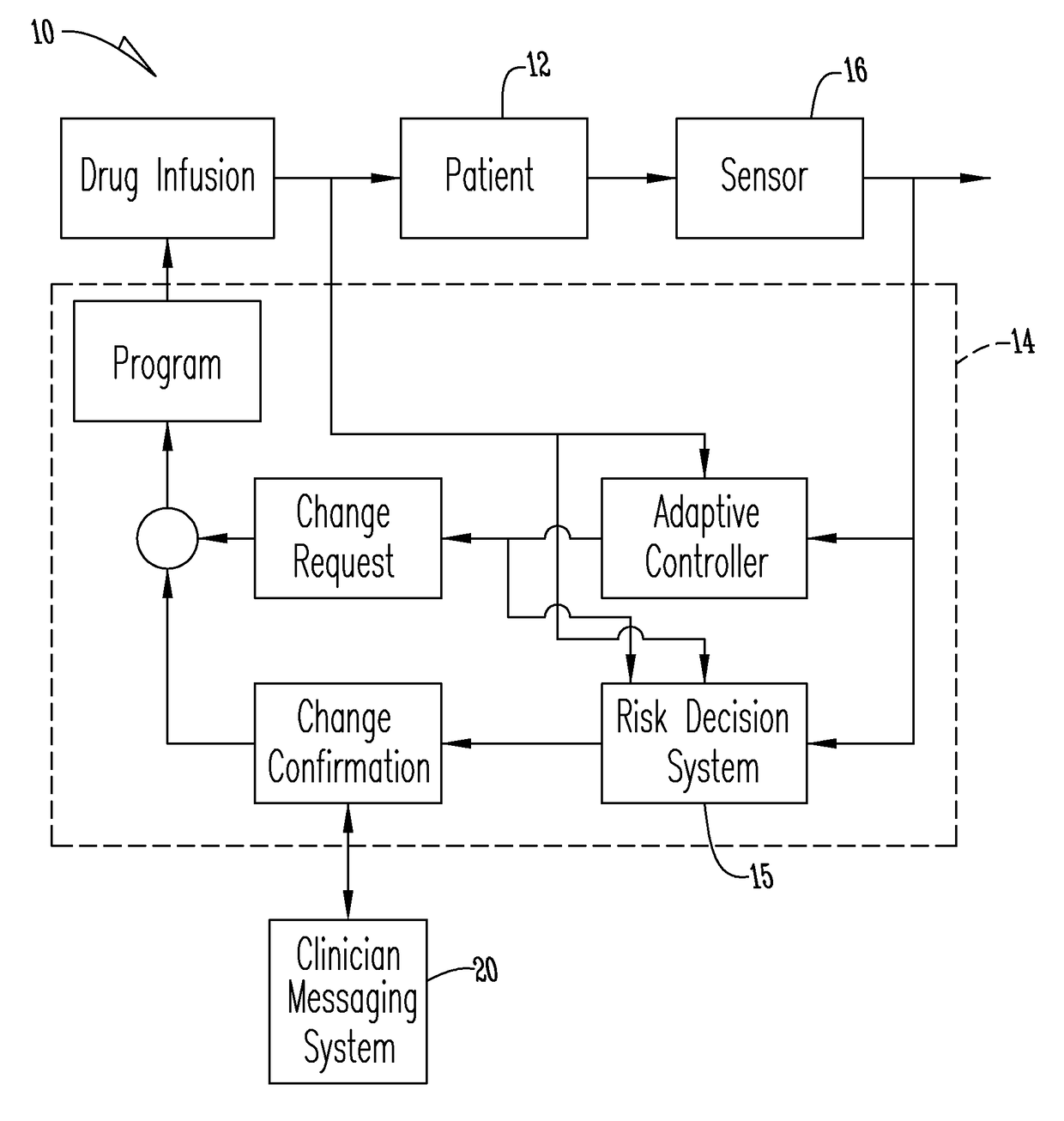
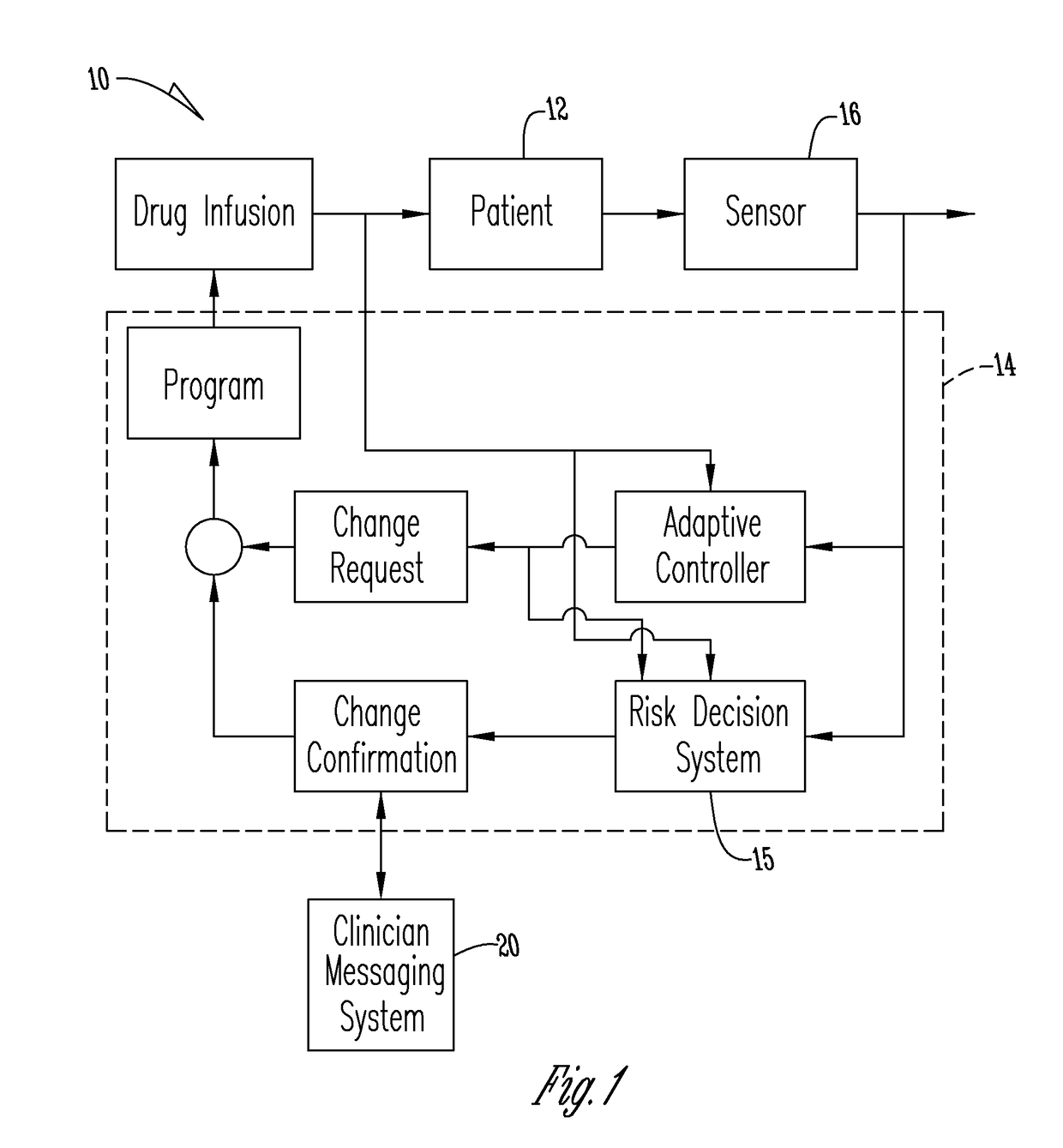
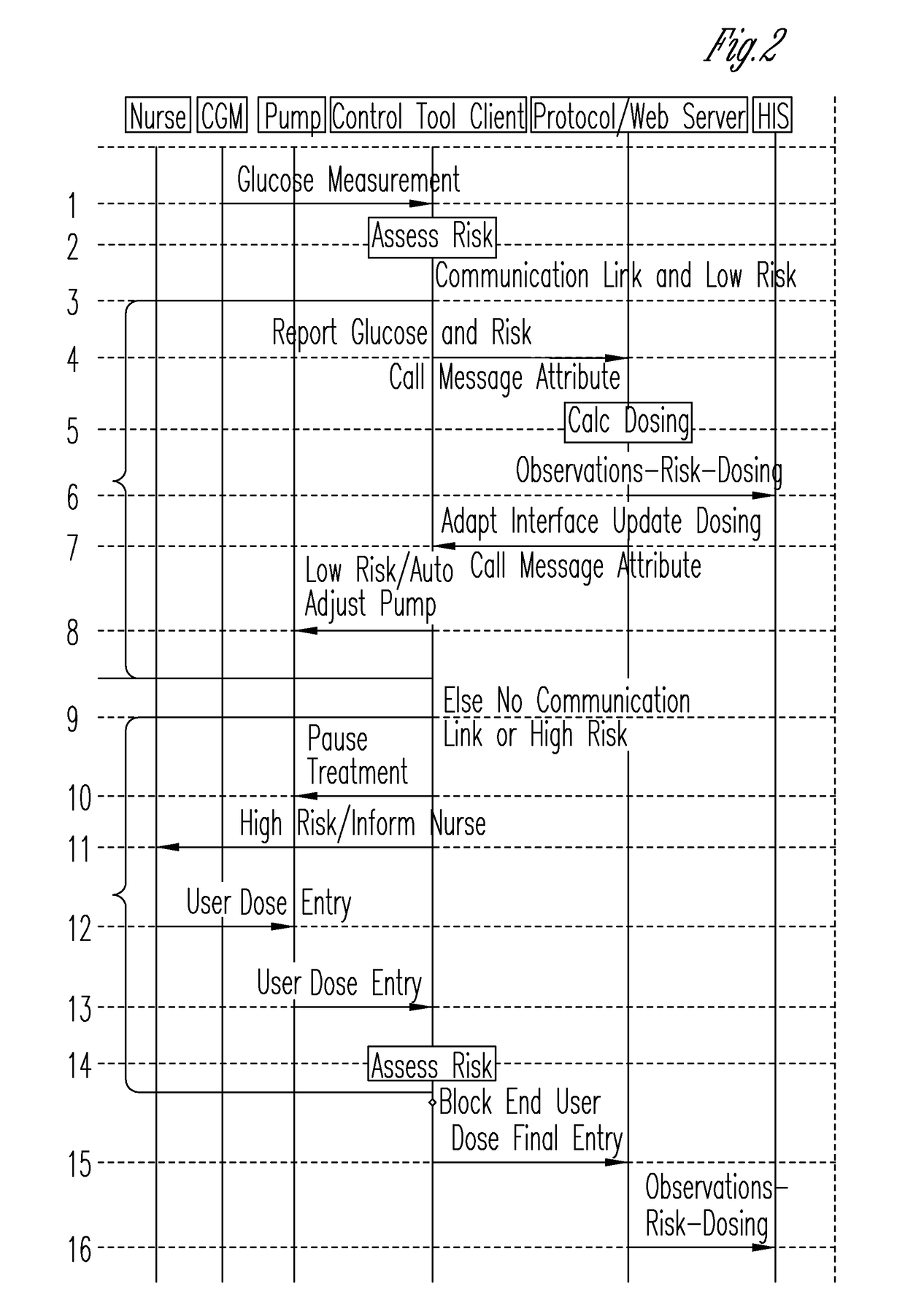
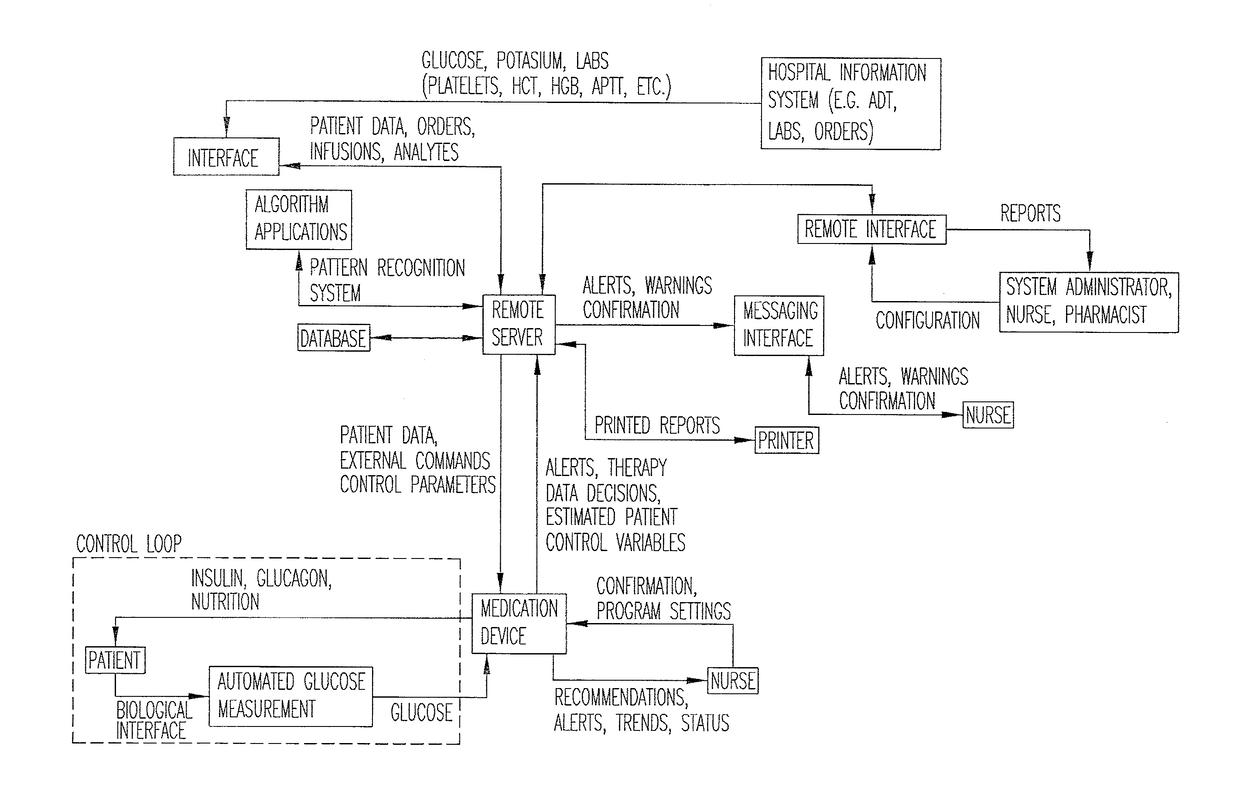
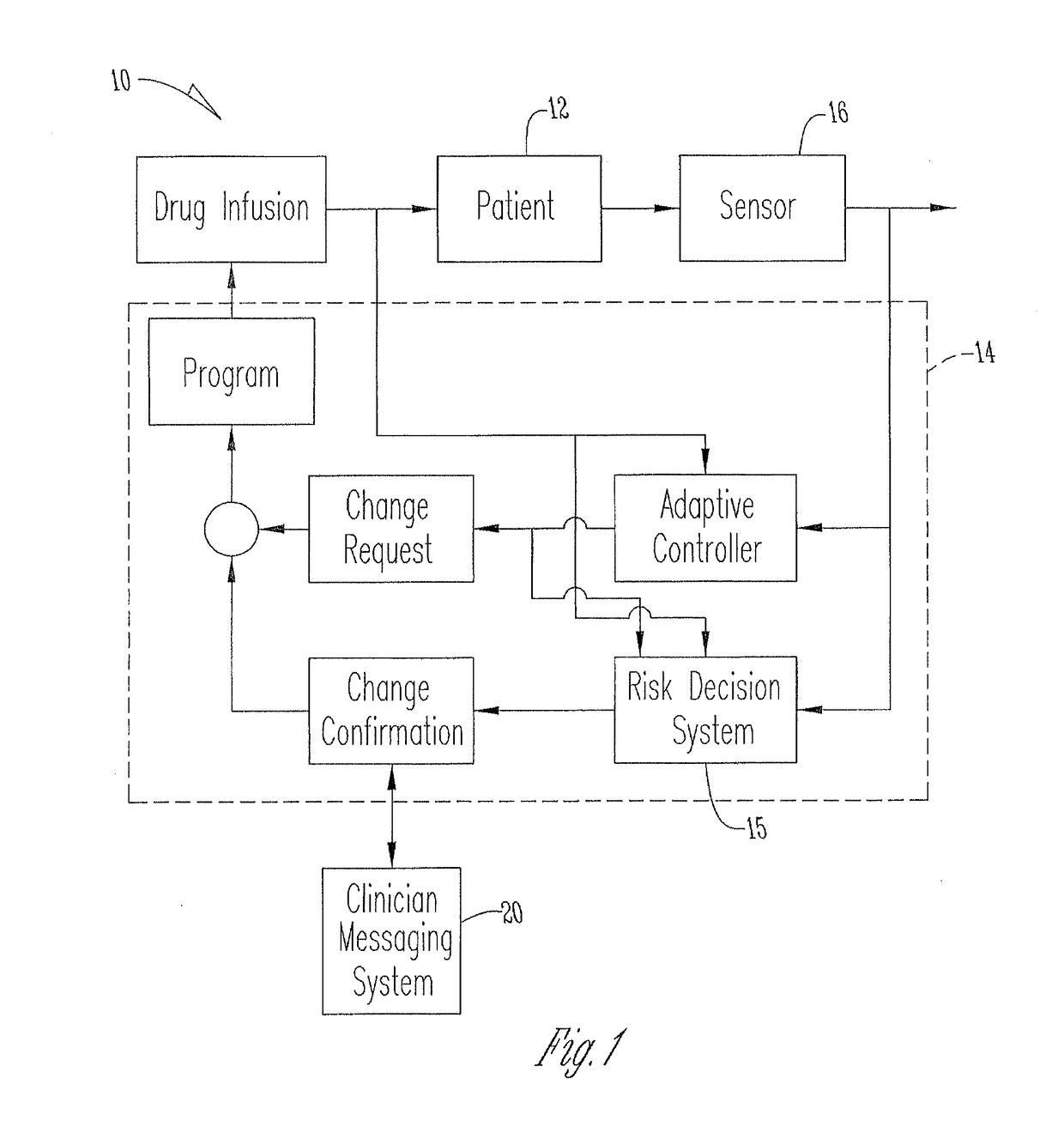
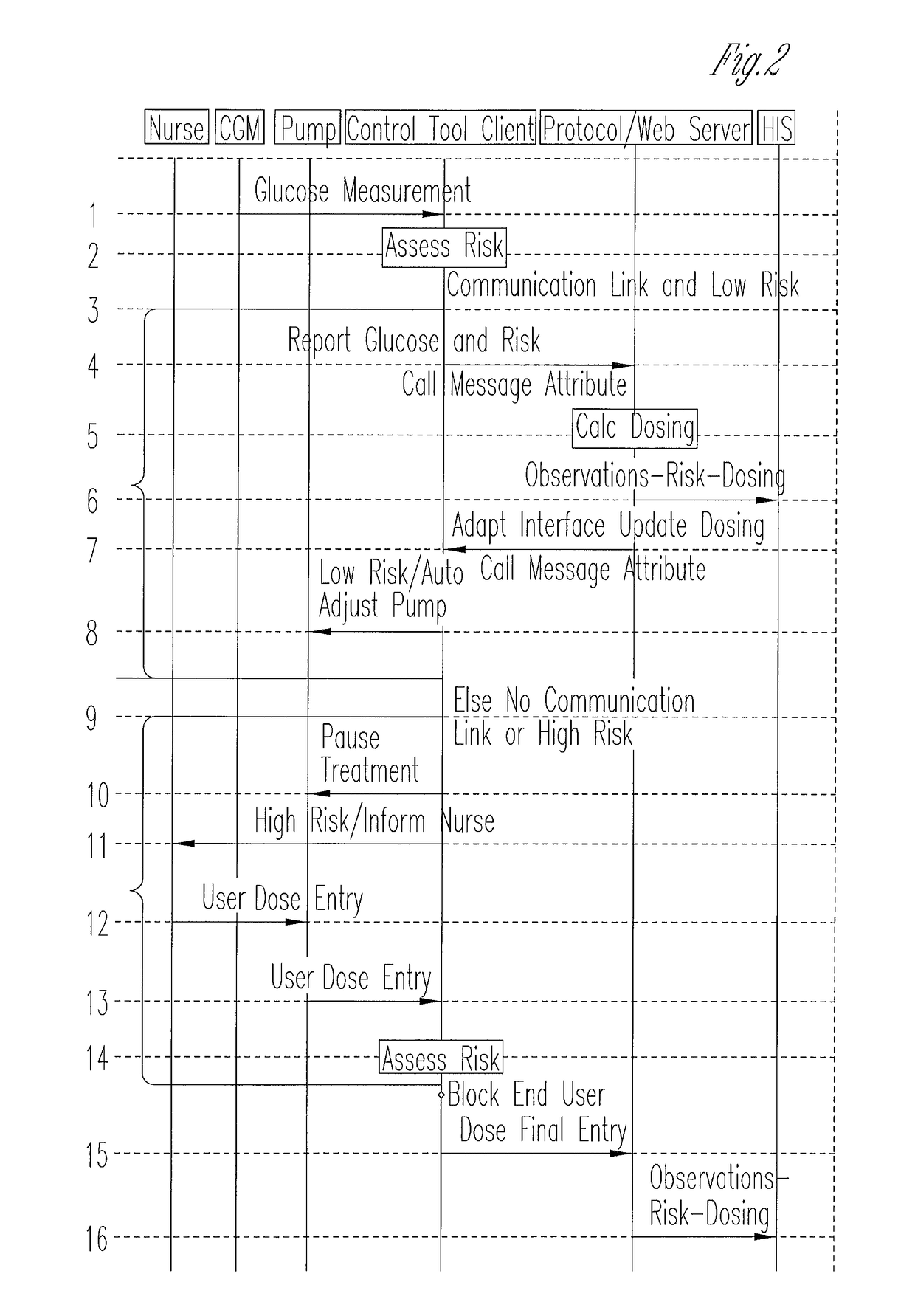
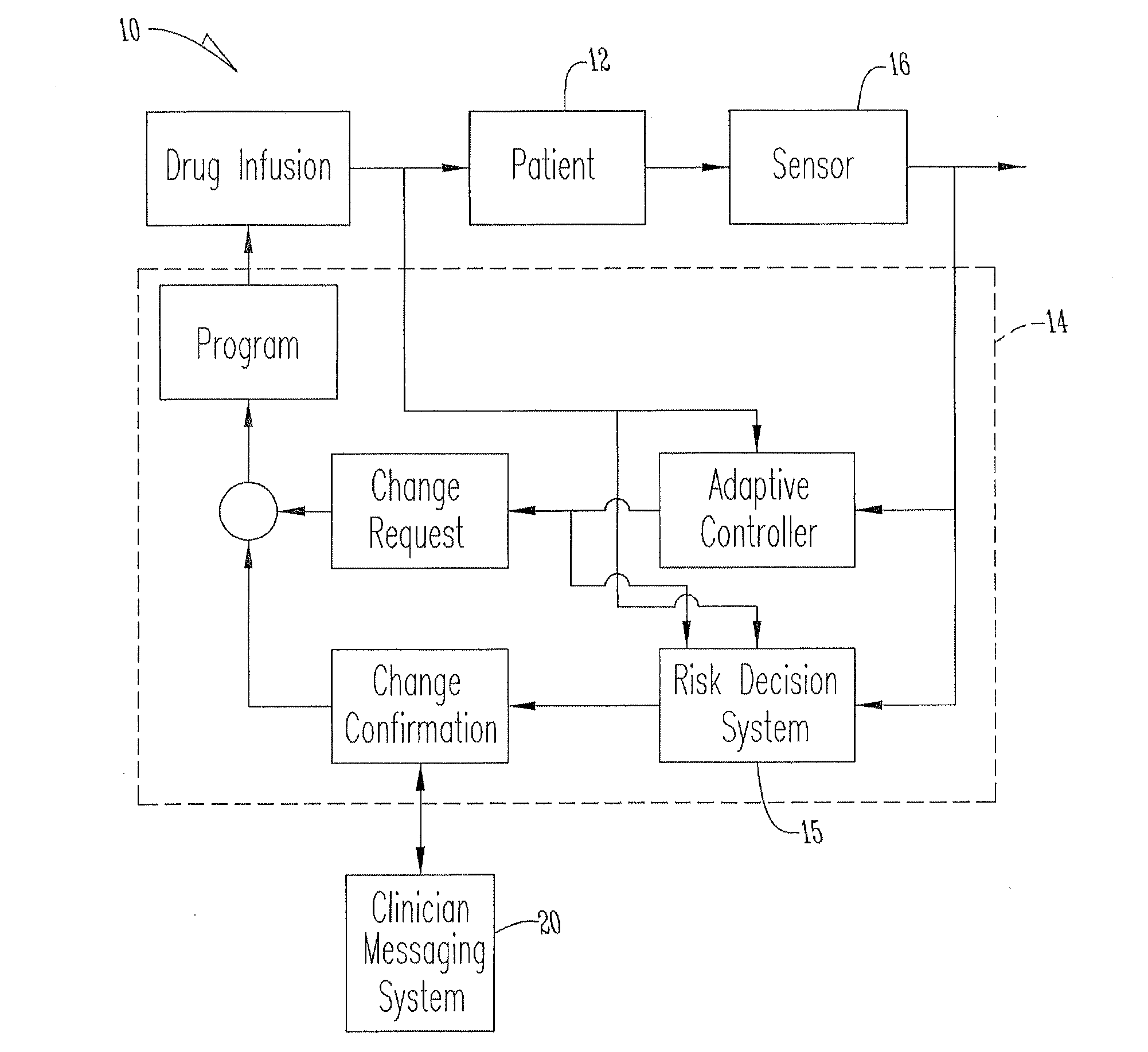
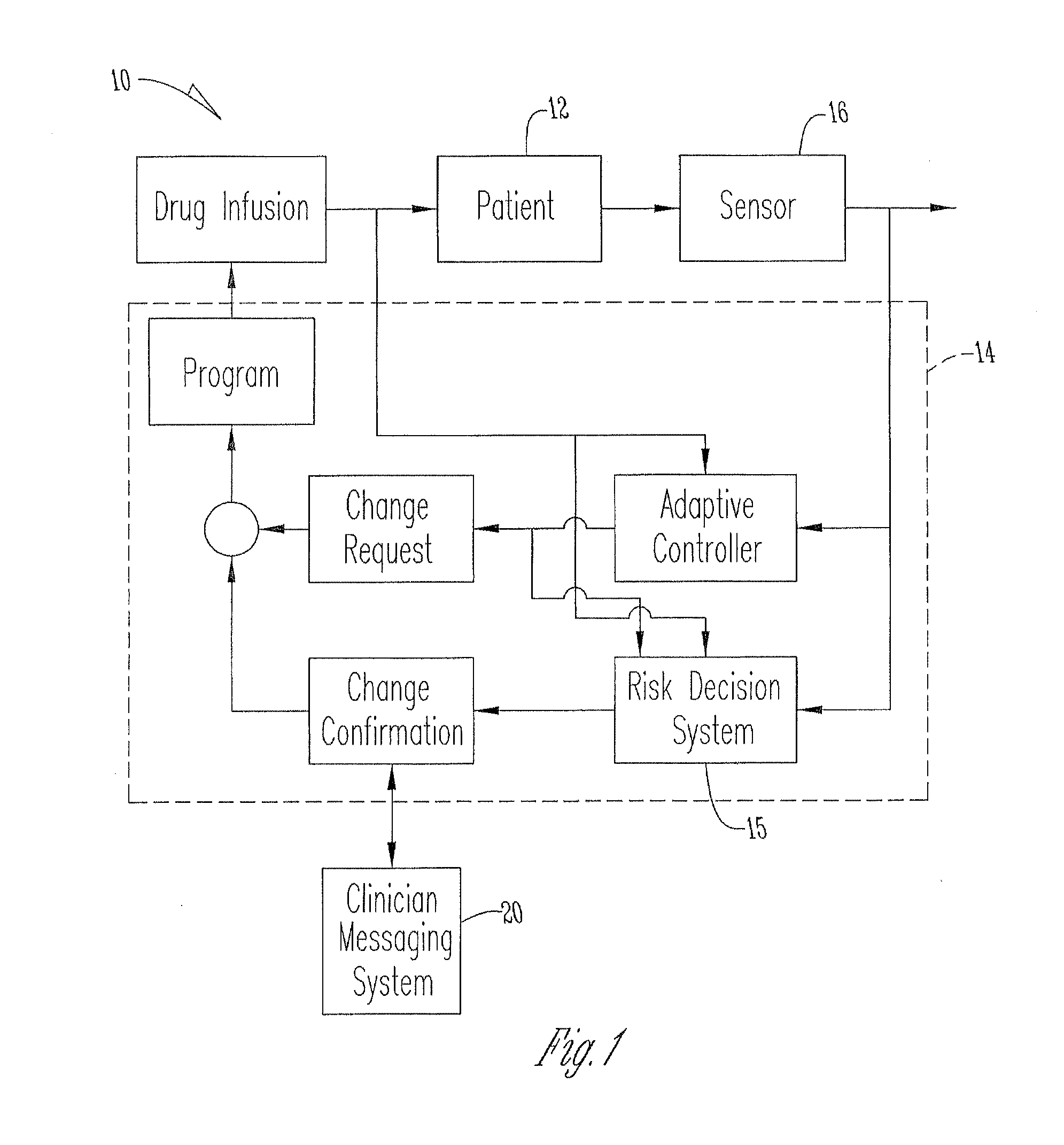
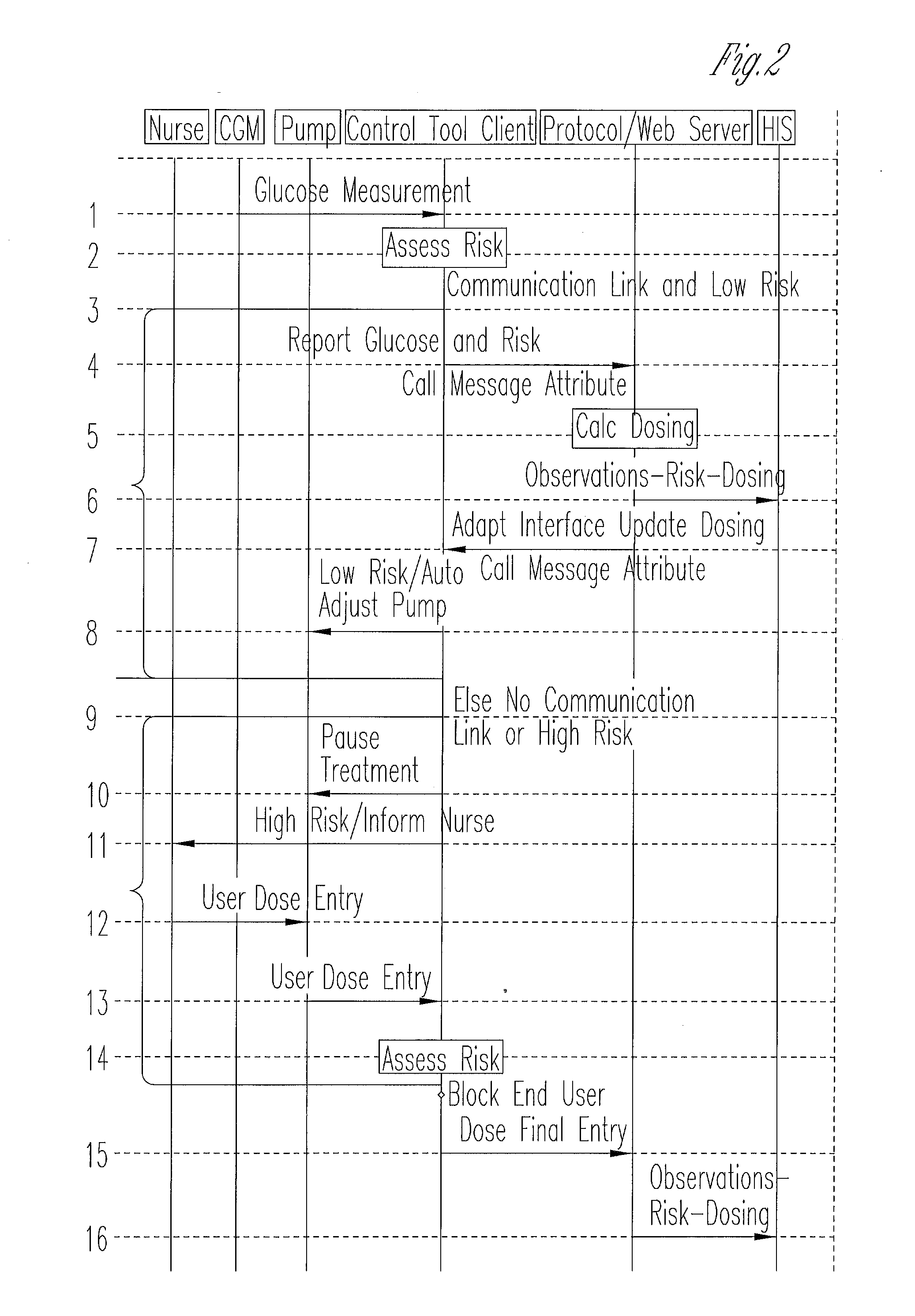
System for monitoring and delivering medication to a patient and method of using the same to minimize the risks associated with automated therapy
ActiveUS20130158504A1Potential riskRisk minimizationMedical simulationHealth-index calculationClosed loopMedical treatment
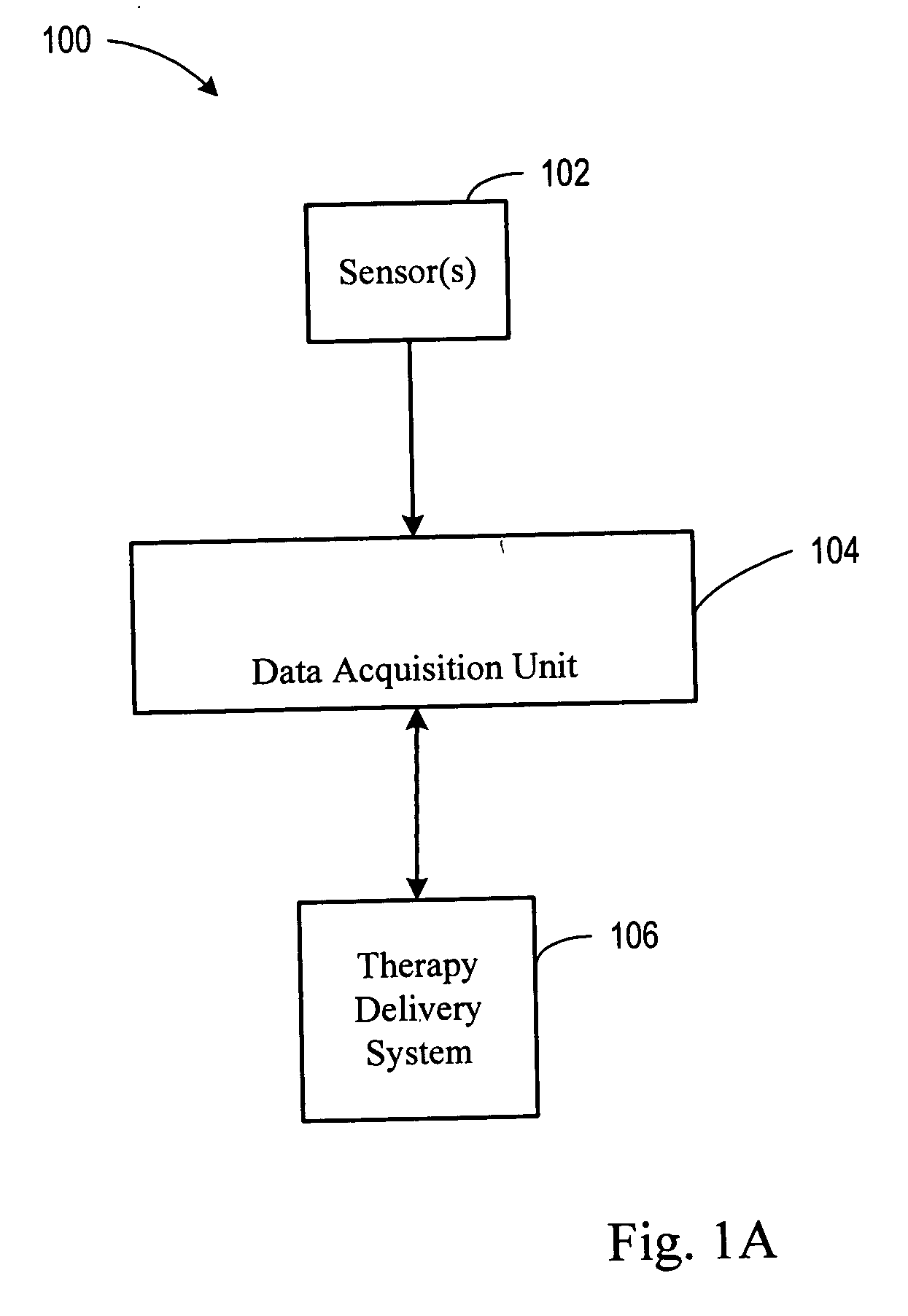
A system and method for monitoring and delivering medication to a patient includes a controller that has a control algorithm and a closed loop control that monitors the control algorithm. A sensor is in communication with the controller and monitors a medical condition. A rule base application in the controller receives data from the sensor and the closed loop control and compares the data to predetermined medical information to determine the risk of automation of therapy to the patient. The controller then provides a predetermined risk threshold where below the predetermined risk threshold automated closed loop medication therapy is provided. If the predetermined risk threshold is met or exceeded, automated therapy adjustments may not occur and user / clinician intervention is requested.
Owner:ICU MEDICAL INC
Items dispenser
ActiveUS20060259188A1Patient compliance is goodMinimizing direct oversightDrug and medicationsComputer-assisted medicine prescription/deliveryRegimenDrug regimen
An automated small items dispensing apparatus is disclosed. In a preferred embodiment, the invention includes a networked, programmable, automatic medication items dispensing apparatus for use by individual patients who are enrolled in a medication protocol or medication therapy regimen. The device is located separate from the patient's health care provider's facility, such as in the patient's home.
Owner:UNIVERSITY OF ROCHESTER
System for monitoring and delivering medication to a patient and method of using the same to minimize the risks associated with automated therapy
ActiveUS10022498B2Potential riskRisk minimizationMedical simulationHealth-index calculationClosed loopCvd risk
A system and method for monitoring and delivering medication to a patient includes a controller that has a control algorithm and a closed loop control that monitors the control algorithm. A sensor is in communication with the controller and monitors a medical condition. A rule base application in the controller receives data from the sensor and the closed loop control and compares the data to predetermined medical information to determine the risk of automation of therapy to the patient. The controller then provides a predetermined risk threshold where below the predetermined risk threshold automated closed loop medication therapy is provided. If the predetermined risk threshold is met or exceeded, automated therapy adjustments may not occur and user / clinician intervention is requested.
Owner:ICU MEDICAL INC
Sterile docking apparatus and method
Owner:BAXTER ENGLEWOOD
System for monitoring and delivering medication to a patient and method of using the same to minimize the risks associated with automated therapy
ActiveUS9724470B2Potential riskRisk minimizationDrug and medicationsMedical devicesRemote systemClosed loop
A system and method for monitoring and delivering medication to a patient. The system includes a controller that has a control algorithm and a closed loop control that monitors the control algorithm. A sensor is in communication with the controller and monitors a medical condition. A rule based application in the controller receives data from the sensor and the closed loop control and compares the data to predetermined medical information to determine the risk of automation of therapy to the patient. A system monitor is also in communication with the controller to monitor system, remote system, and network activity and conditions. The controller then provides a predetermined risk threshold where below the predetermined risk threshold automated closed loop medication therapy is provided. If the predetermined risk threshold is met or exceeded, automated therapy adjustments may not occur and user / clinician intervention is requested.
Owner:ICU MEDICAL INC
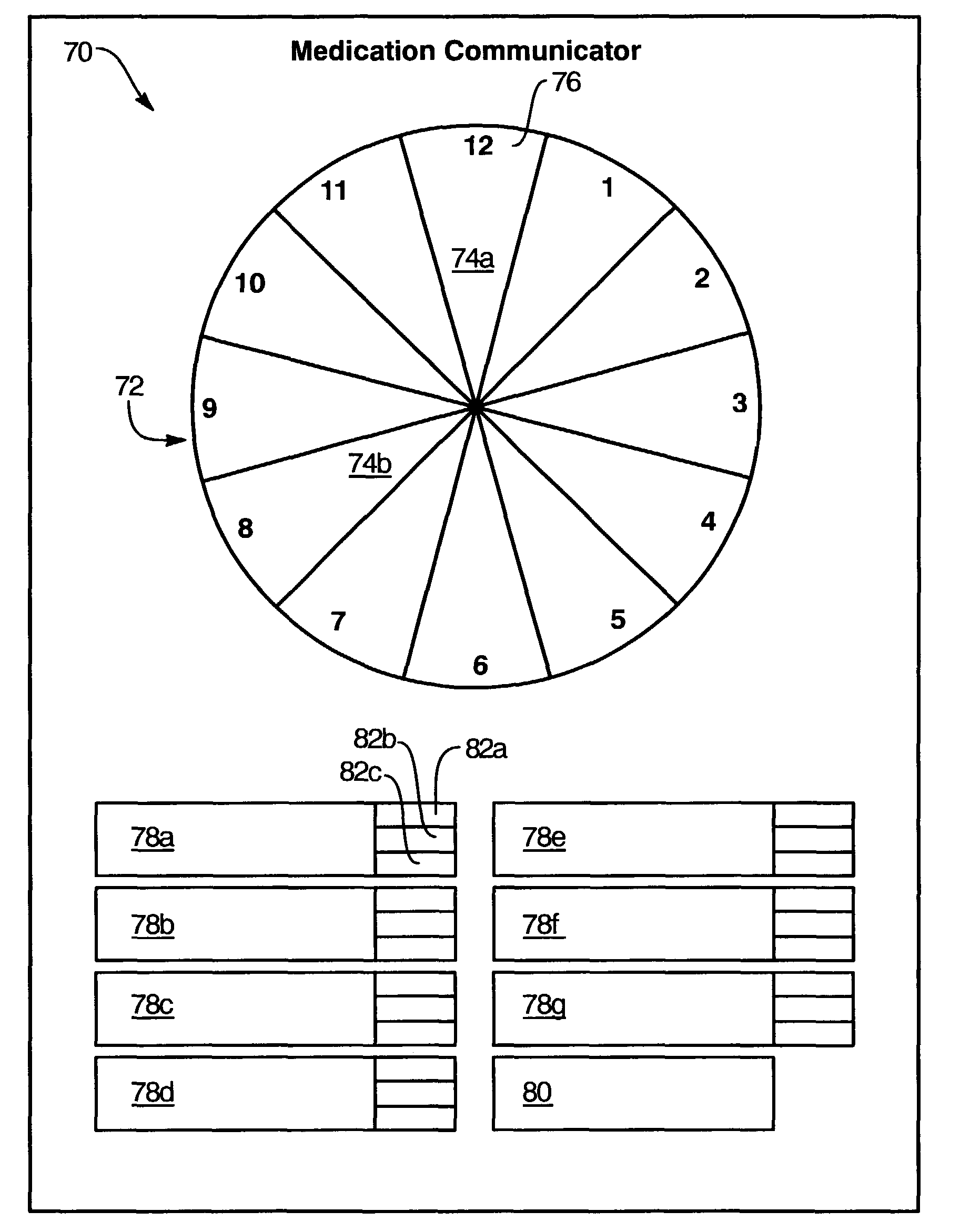
Medication regimen communicator apparatus and method
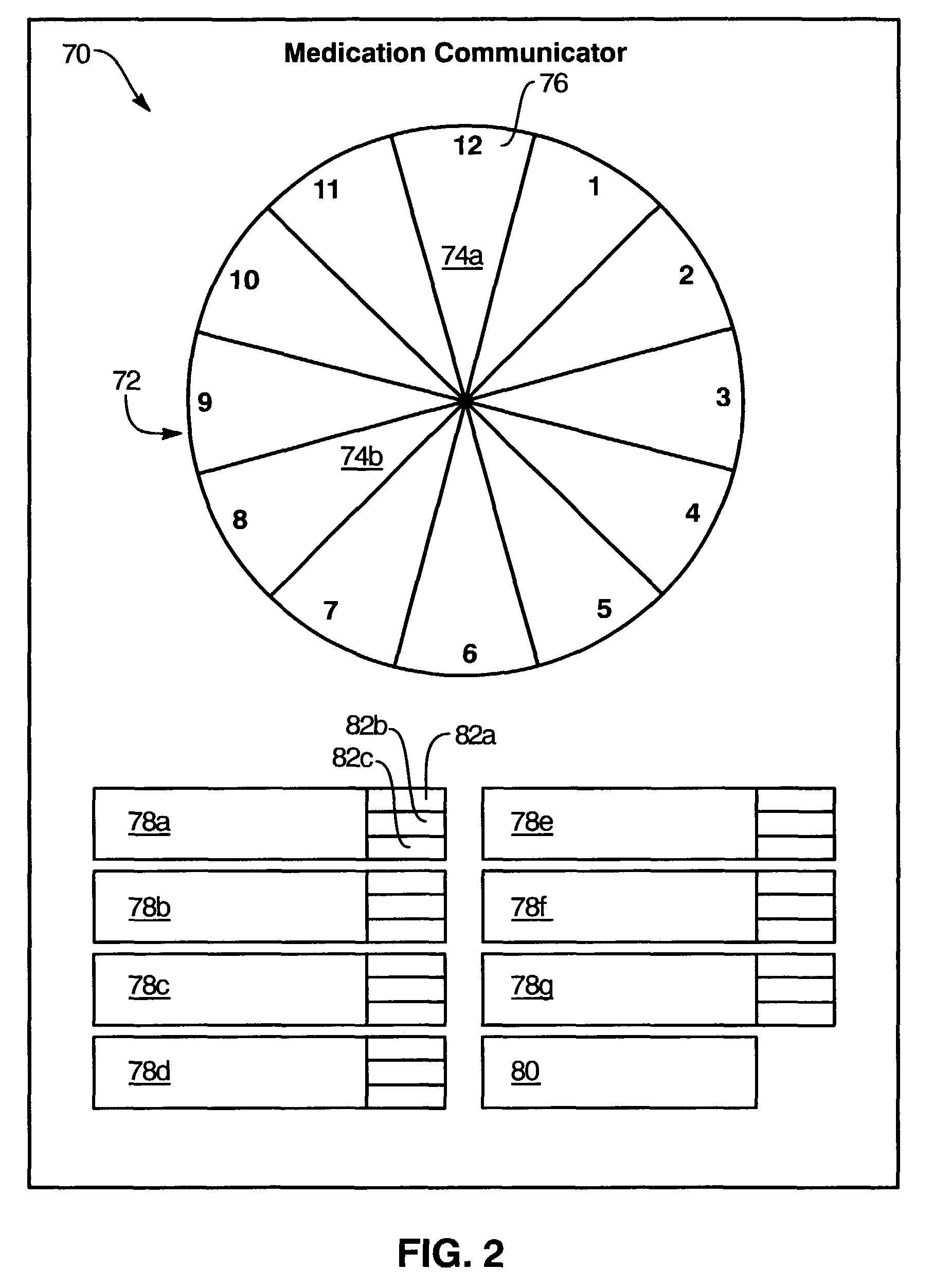
InactiveUS7773460B2Easy to understandImprove visualizationVisual indicationDrug and medicationsTeaching toolApplication time
A “medication communicator” chart is used as a teaching tool to educate patients and to schedule events corresponding to a prescribed medication regimen. The “medication communicator” chart includes a scheduling graph having the shape of a 12-hour or 24-hour analog clock. The scheduling graph is divided into regions corresponding to each hour of a day for scheduling event information. Fields, on the “medication communicator” chart, are receptive to labels communicating information corresponding to numerous medications in the medication regimen. Timing indicators may be applied to the regions of the scheduling graph to indicate consumption or application times of each of the medications.
Owner:HOLT LINDSAY
System and method for moderating a therapy delivered during sleep using physiologic data acquired during non-sleep
InactiveUS7757690B2Inertial sensorsMedical devicesPositive airway pressureSleep disordered breathing
Owner:CARDIAC PACEMAKERS INC
Drug therapy for Celiac Sprue
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Cognitive function within a human brain
ActiveUS8000795B2Improve cognitive functionHead electrodesWound drainsImplanted deviceCognitive diseases
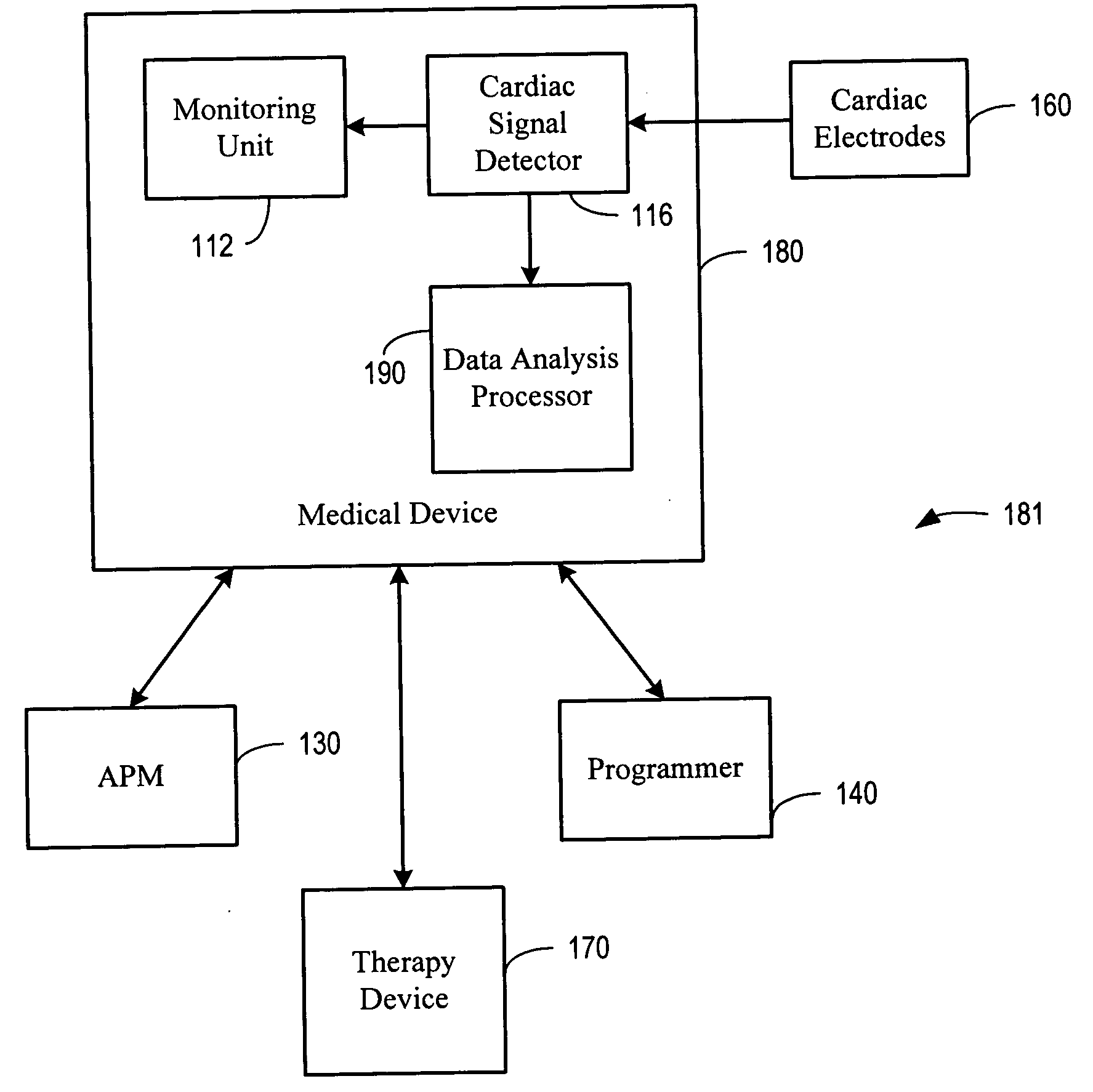
Methods and apparatus for improving cognitive function within a human. The invention utilizes an implanted device, such as an implantable signal generator or an implantable pump, to affect tissue elements within a Papez circuit of the human brain as well as tissue upstream or downstream from the Papez circuit. The implanted device delivers treatment therapy to thereby improve cognitive function by the human. A sensor may be used to detect various symptoms of the cognitive disorder. A microprocessor algorithm may then analyze the output from the sensor to regulate delivery of the stimulation and / or drug therapy.
Owner:FUNCTIONAL NEUROMODULATION
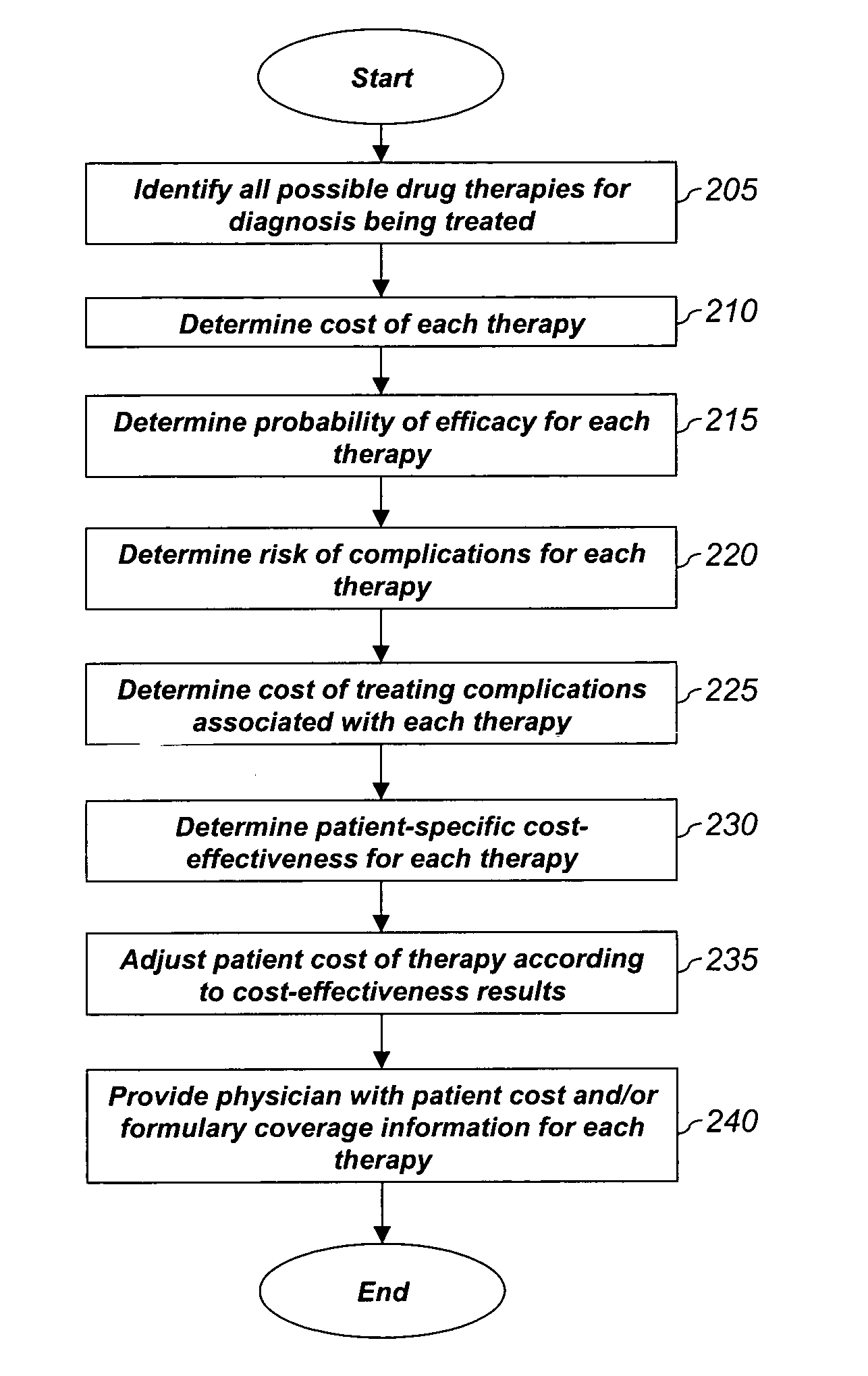
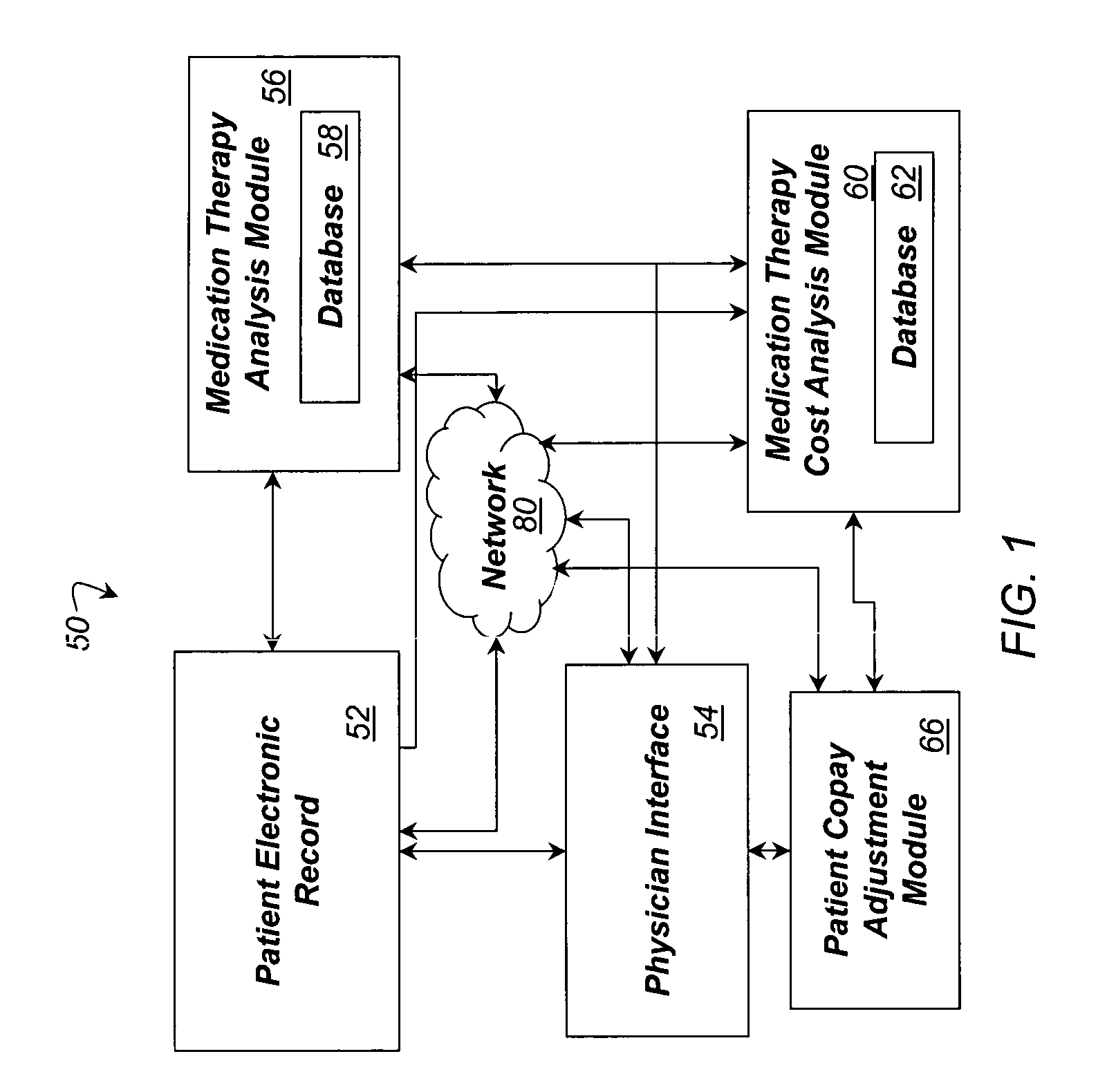
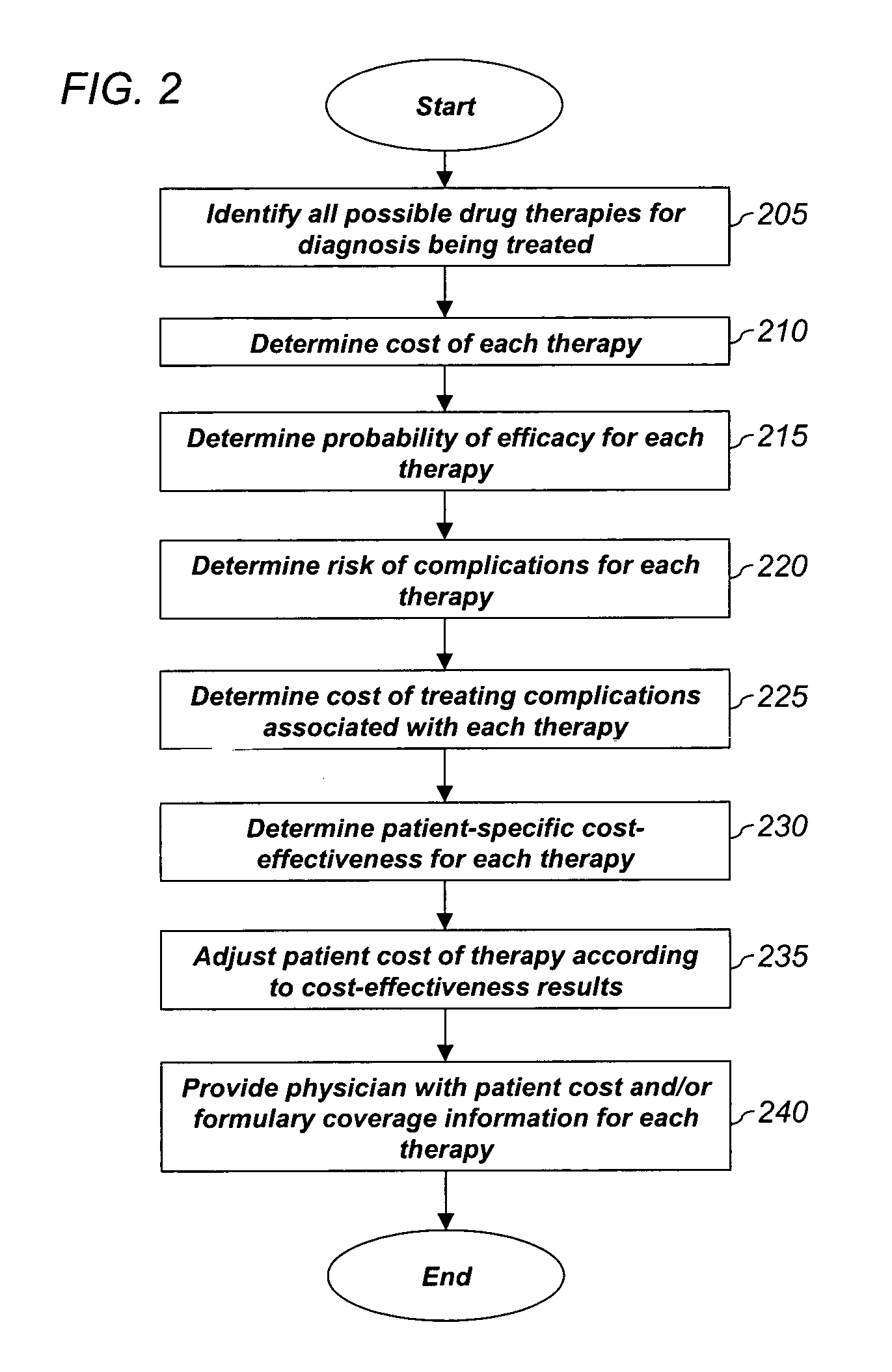
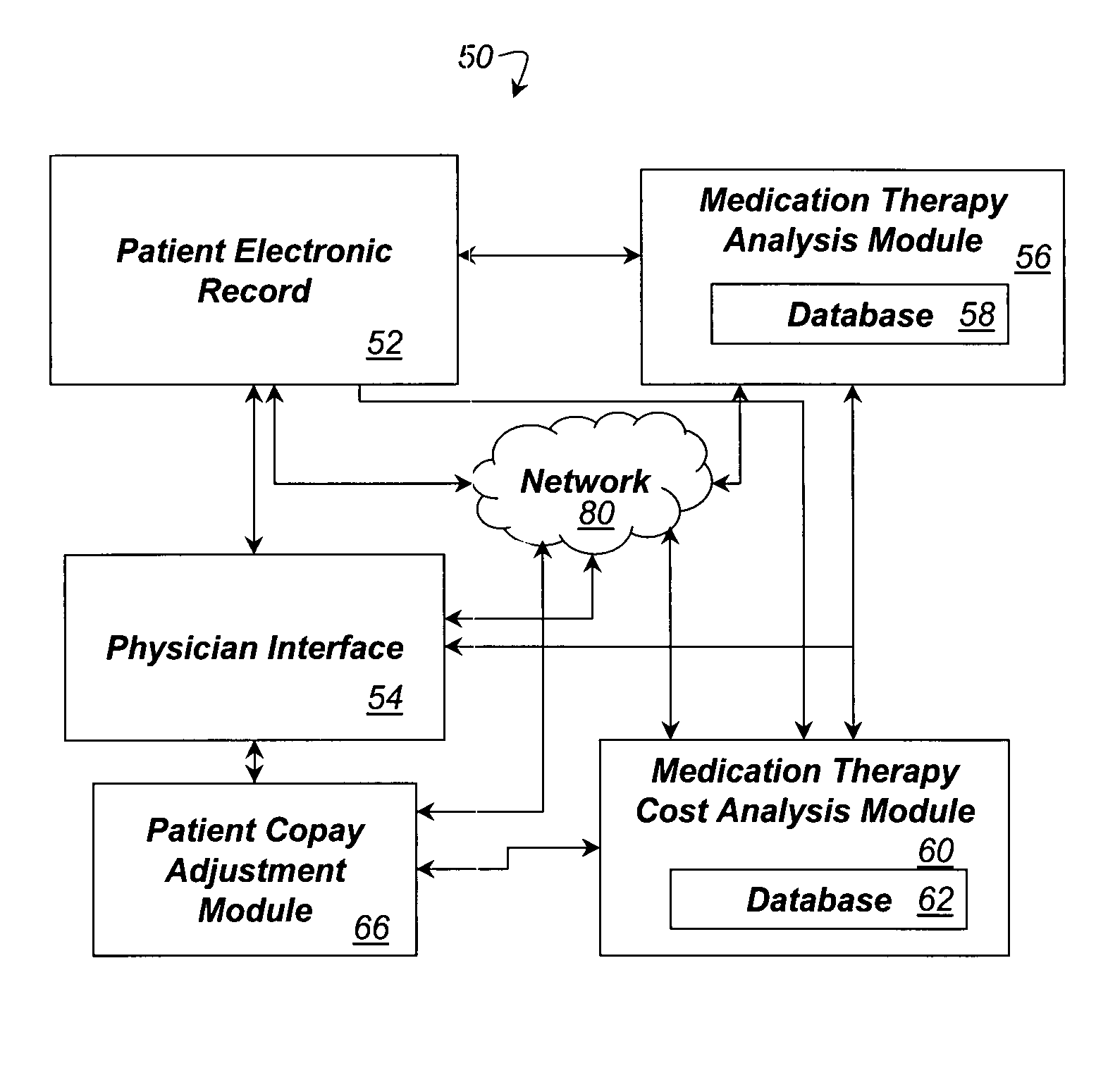
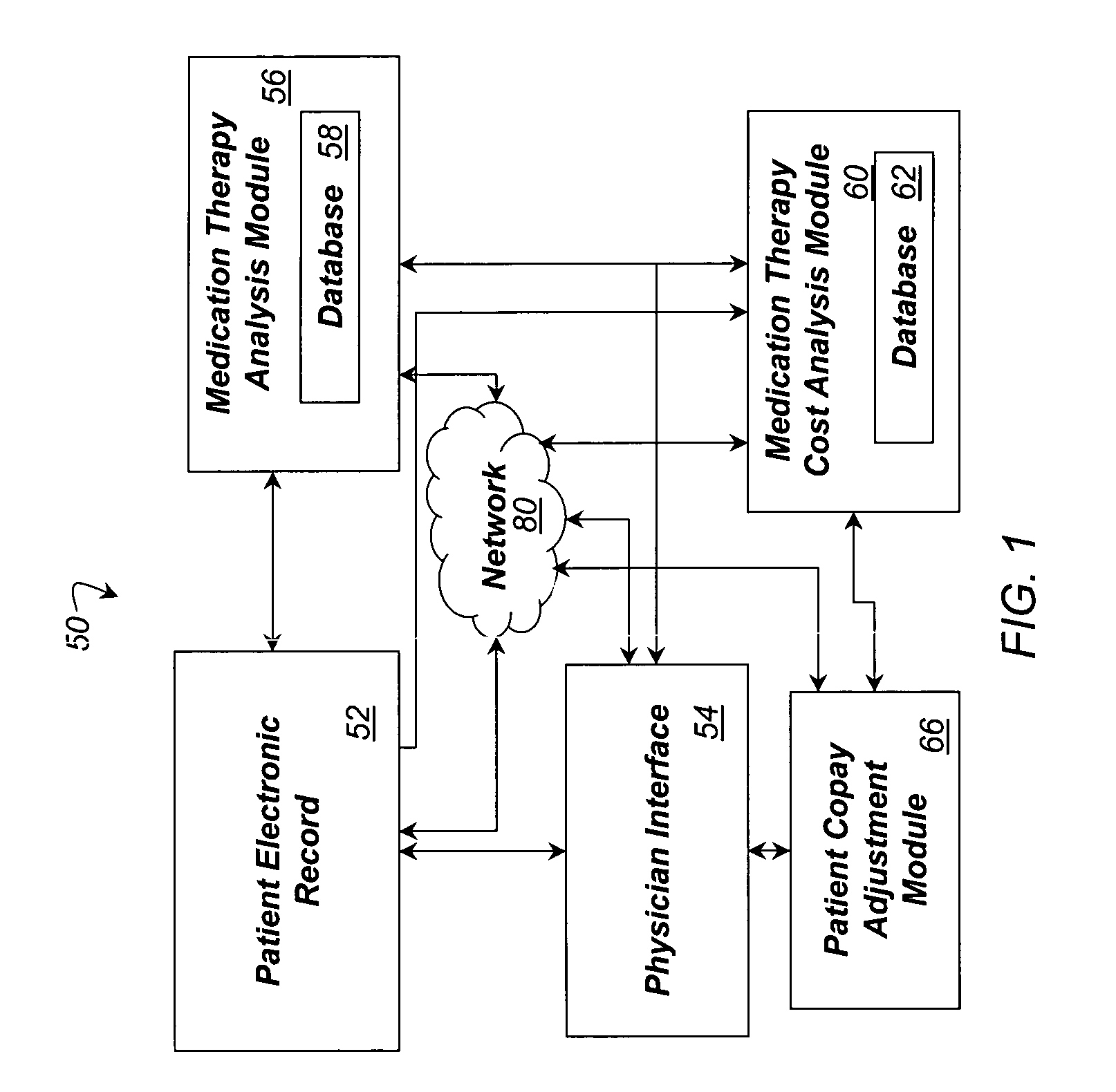
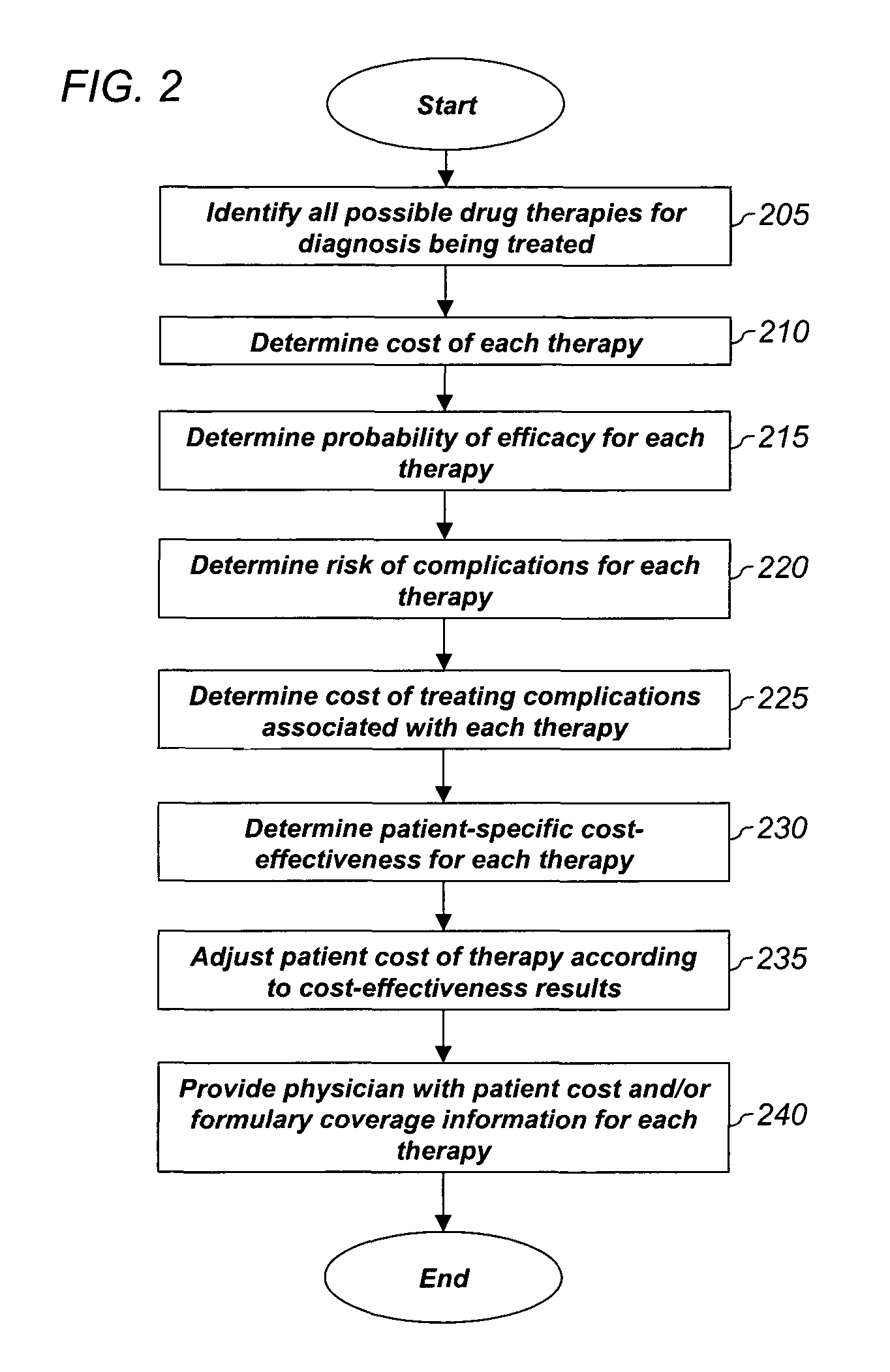
System and method for dynamic adjustment of copayment for medication therapy
System and method for the cost-effective use of medications, comprising dynamically adjusting the patient cost for a plurality of possible medication treatment therapies according to the cost-effectiveness of each possible medication therapy based on known patient attributes, and providing a physician with the dynamically determined patient cost of at least one of the possible medication treatment therapies.
Owner:HUMANA INC
Drug therapy for celiac sprue
InactiveUS20060035838A1Salicyclic acid active ingredientsBiocideDermatitis herpetiformisPoisonous effects
A ministering an effective dose of a tTGase inhibitor to a Celiac or dermatitis herpetiformis patient reduces the toxic effects of toxic gluten oligopeptides, thereby attenuating or eliminating the damaging effects of gluten.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Drug therapy for celiac sprue
InactiveUS20050256054A1Data processing applicationsPeptide/protein ingredientsDermatitis herpetiformisBinding peptide
Celiac Sprue and / or dermatitis herpetiformis arc treated by interfering with HLA binding of immunogenic gluten peptides. The antigenicity of gluten oligopeptides and the ill effects caused by an immune response thereto are decreased by administration of an HLA-binding peptide inhibitor. Such inhibitors are analogs of immunogenic gluten peptides and (i) retain the ability to bind tightly to HLA molecules; (ii) retain the protcolytic stability of these peptides; but (iii) are unable to activate disease-specific T cells.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Method for determining drug dose for inhaled drug therapy
The invention is directed to a method for determining the total amount of liquid medicament containing an active drug substance to be aerosolized and inhaled by a patient in order to deliver a pharmaceutically effective amount (“PEA”) of said drug to the respiratory tract of said patient comprising: A) aerosolizing a measured amount of said medicament liquid containing a known amount of drug (“Aerosolized Dose”) using an aerosolization means connected to said patient via an inhalation tube and an exhalation tube; wherein said exhalation tube contains a filter means and wherein said Aerosolized Dose is less than said PEA of said drug; B) administering said aerosol to the respiratory tract of said patient via said inhalation tube; C) allowing the patient to exhale through said exhalation tube containing said filter wherein any exhaled drug is trapped on said filter; D) measuring the reflectance of the color on the filter media contained in said exhalation filter using a reflectance spectrophotometer to determine the amount of drug on said exhalation filter (“Exhaled Dose”); E) determining the actual dose delivered to said patient (“Delivered Dose”) as follows: Aerosolized Dose minus Trapped Dose minus Exhaled Dose=Delivered Dose, where the Trapped Dose is the amount of drug trapped in the inhalation tube; and F) calculating the total amount of liquid medicament to be aerosolized (“Total Aerosolized Dose”) by multiplying the PEA by the result of dividing the Delivered Dose by the Aerosolized Dose; wherein a solution or suspension of the drug is colored when viewed by the human eye or wherein the drug reacts with a reagent on the filter media of said filter means to produce a color.
Owner:ZIVENA
System for monitoring and delivering medication to a patient and method of using the same to minimize the risks associated with automated therapy
ActiveUS20150359966A1Potential riskRisk minimizationDrug and medicationsMedical devicesRemote systemClosed loop
A system and method for monitoring and delivering medication to a patient. The system includes a controller that has a control algorithm and a closed loop control that monitors the control algorithm. A sensor is in communication with the controller and monitors a medical condition. A rule based application in the controller receives data from the sensor and the closed loop control and compares the data to predetermined medical information to determine the risk of automation of therapy to the patient. A system monitor is also in communication with the controller to monitor system, remote system, and network activity and conditions. The controller then provides a predetermined risk threshold where below the predetermined risk threshold automated closed loop medication therapy is provided. If the predetermined risk threshold is met or exceeded, automated therapy adjustments may not occur and user / clinician intervention is requested.
Owner:ICU MEDICAL INC
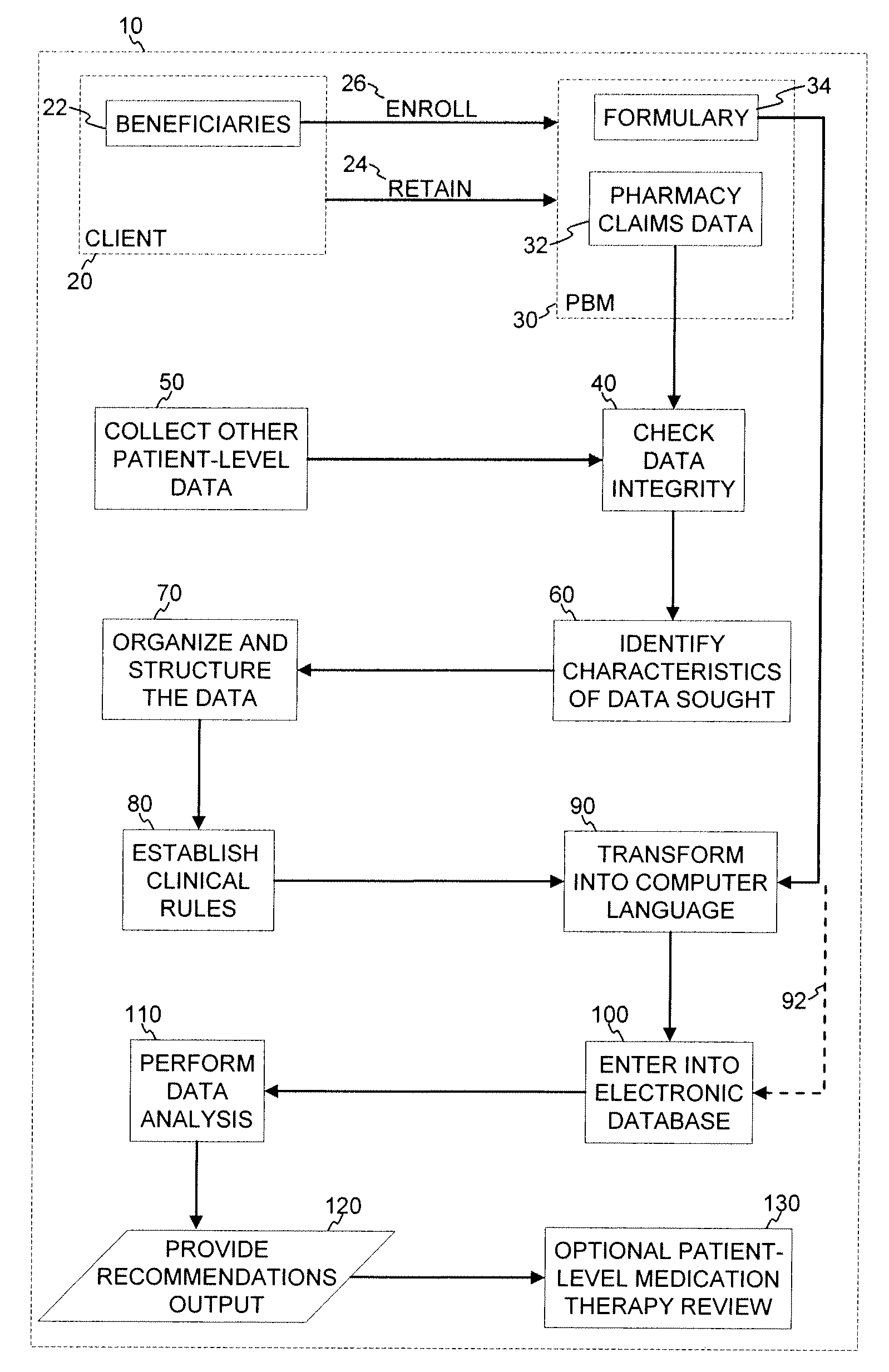
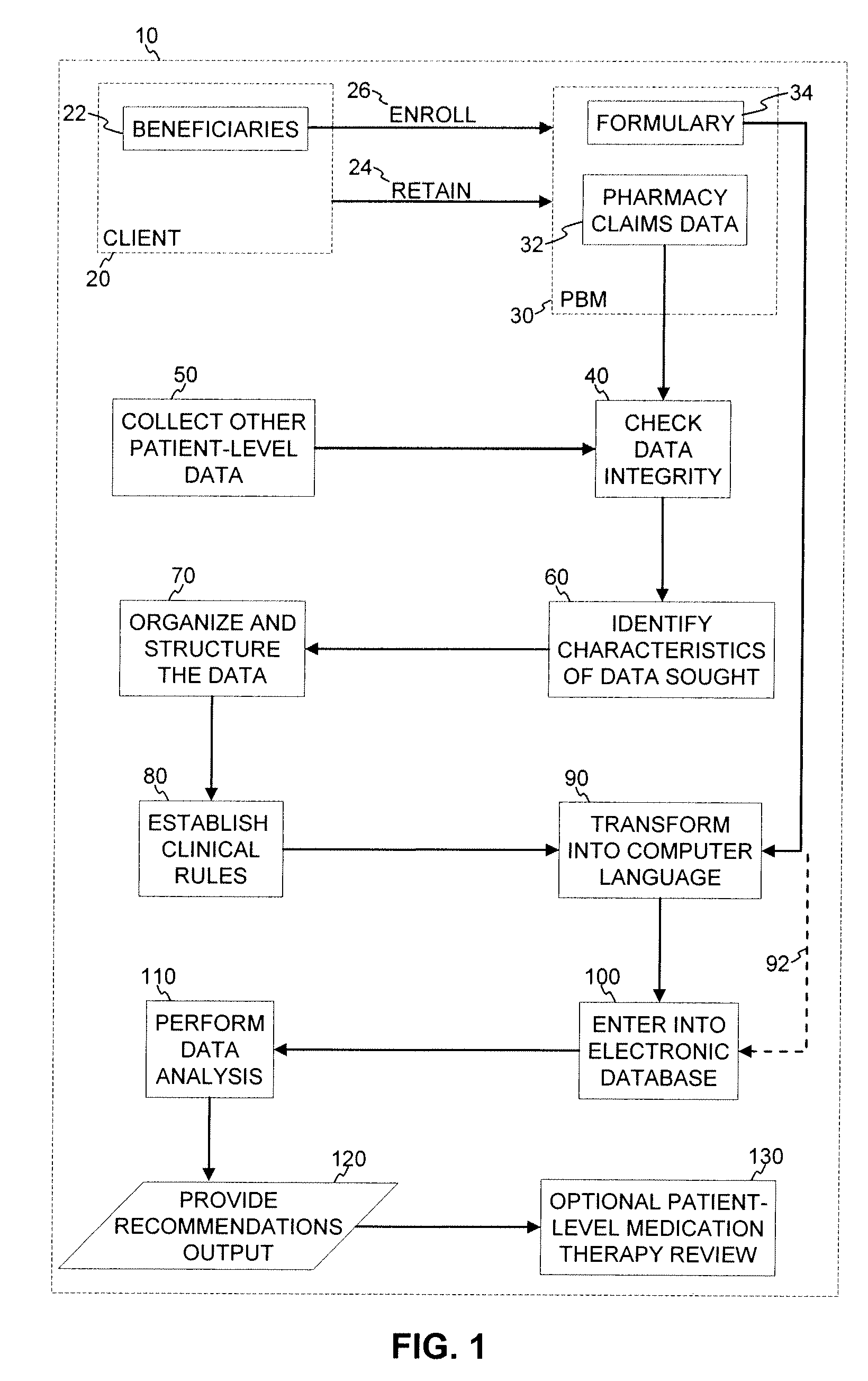
Medication Therapy Review Methods
InactiveUS20090265189A1Good for healthConvenient careMedical data miningDrug and medicationsData integrityCrowds
A method for population and patient-specific medication therapy review using a computer particularly adapted for a medication therapy management or health care delivery organization is provided, wherein the method includes at least the steps of: collecting patient claims data; reviewing the patient claims data for accuracy and data integrity; identifying particular characteristics sought within the patient claims data; organizing and structuring the patient claims data; establishing a set of clinical rules; transforming the set of clinical rules established into a computer language, and entering the transformed clinical rules into an electronic database; and analyzing the patient claims data by applying the clinical rules using a computer particularly adapted for a medication therapy management or health care delivery organization, thereby producing an analytical result. In further embodiments, the analytic results are used to evaluate the need for patient-level medication therapy review and, optionally, to actually conduct a patient-level medication therapy review based on the review of the analytic results.
Owner:PHARMMD SOLUTIONS
Genetic markers in the HLA-DQBI gene associated with an adverse hematological response to drugs
Genetic markers in the HLA-DQB1 gene associated with adverse hematological response to drug therapy are disclosed. Compositions and methods for detecting and using these HLA-DQB1 markers in a variety of clinical applications are disclosed. Such applications include methods for testing an individual for susceptibility for an adverse hematological response, methods of selecting the appropriate drug therapy for patients based on the presence or absence of a HLA-DQB1 marker, and products comprising a drug with hematological toxicity that are approved for treating patients lacking a genetic marker.
Owner:PGXHEALTH
Structure having nanoantenna and method for manufacturing same
The present invention relates to a structure having a nanoantenna, a method for manufacturing same, a drug delivery body having the same, a thermotherapy complex, a drug therapy device, and a thermotherapy device. The structure of the present invention has a nanoantenna pattern formed on the outer surface of a porous micro-container, thereby enabling wireless control from the outside, and when the structure is used as a drug delivery system and a thermotherapy complex, drug therapy and thermotherapy can be carried out at a desired application region inside a living body at a desired time. Also, the structure of the present invention enables transmission and reception of a wireless signal with an external controller through the nanoantenna, thereby enabling the detection of a signal inside the living body and the transmission of the signal to the external controller, and the discharge of a drug or nanowires according to a response signal transmitted from the external controller.
Owner:KOREA UNIV RES & BUSINESS FOUND
Genetic markers in the HLA-C gene associated with an adverse hematological response to drugs
Genetic markers in the HLA-C gene associated with adverse hematological response to drug therapy are disclosed. Compositions and methods for detecting and using these HLA-C markers in a variety of clinical applications are disclosed. Such applications include methods for testing an individual for susceptibility for an adverse hematological response, methods of selecting the appropriate drug therapy for patients based on the presence or absence of a HLA-C marker, and products comprising a drug with hematological toxicity that are approved for treating patients lacking a genetic marker.
Owner:PGXHEALTH
Enhancement of drug therapy by mirna
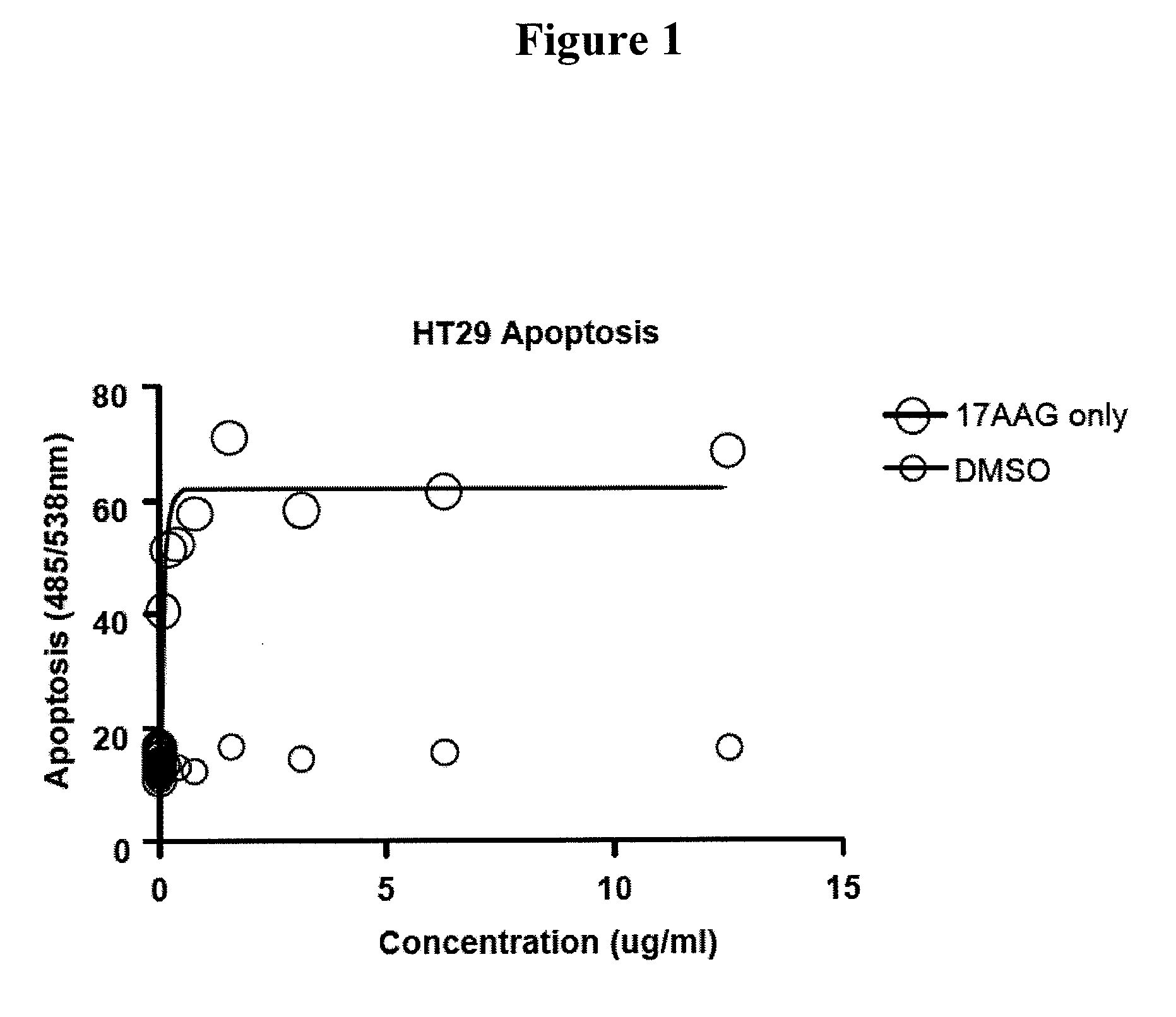
InactiveUS20090280167A1Strong therapeutic activityHigh activityOrganic active ingredientsNervous disorderPharmaceutical drugHsp Inhibitor
This invention provides methods and compositions for screening of microRNA capable of modulating gene expression in the apoptotic pathway in the presence of HSP90 inhibitor. The use of miRNA for enhancing the activity of therapeutic agents not limited to HSP90 inhibitor is also disclosed. The diagnostic use of miRNA for predicting response to therapy not limited to therapeutic agents is also disclosed. A method for the identification and therapeutic application of small molecules which are modulators of these nucleic acids are also included in this application
Owner:ABRAXIS BIOSCI LLC
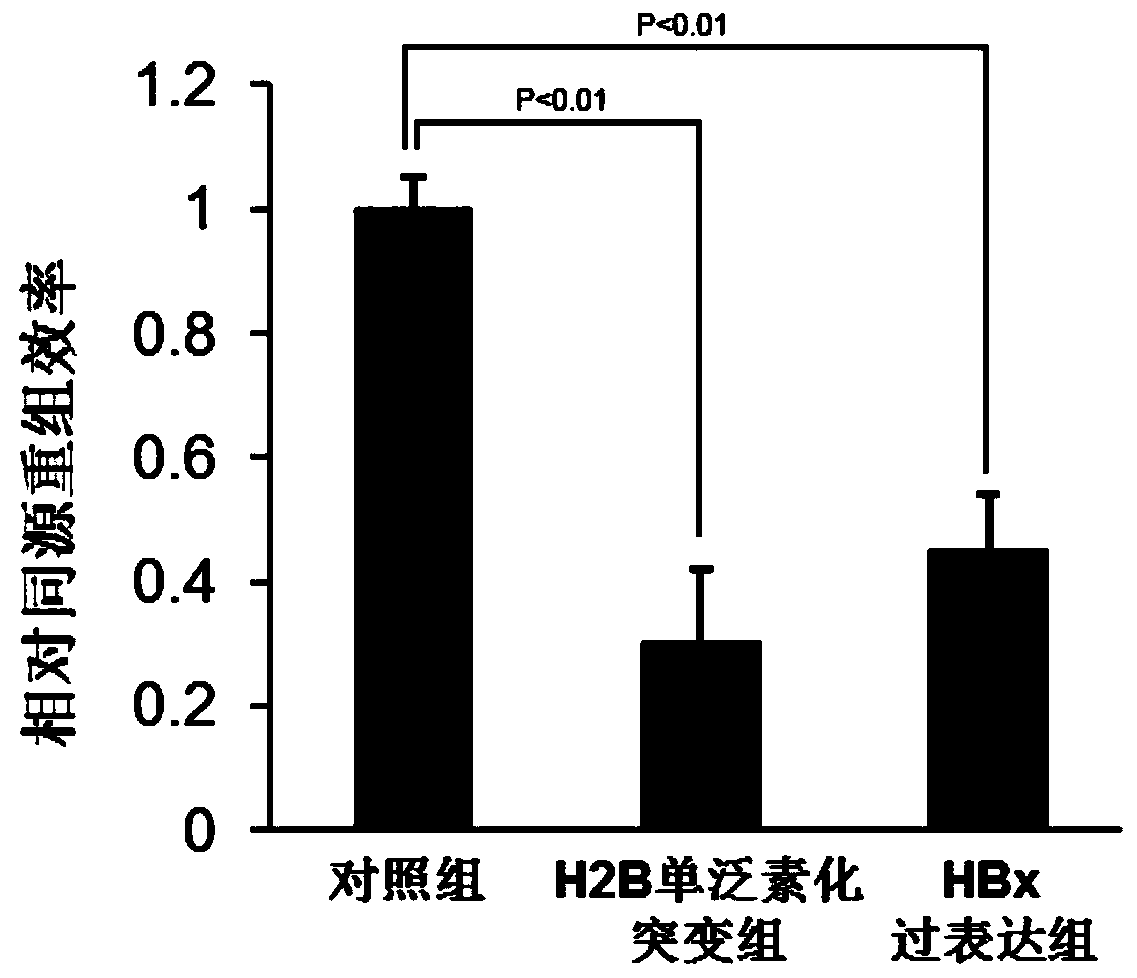
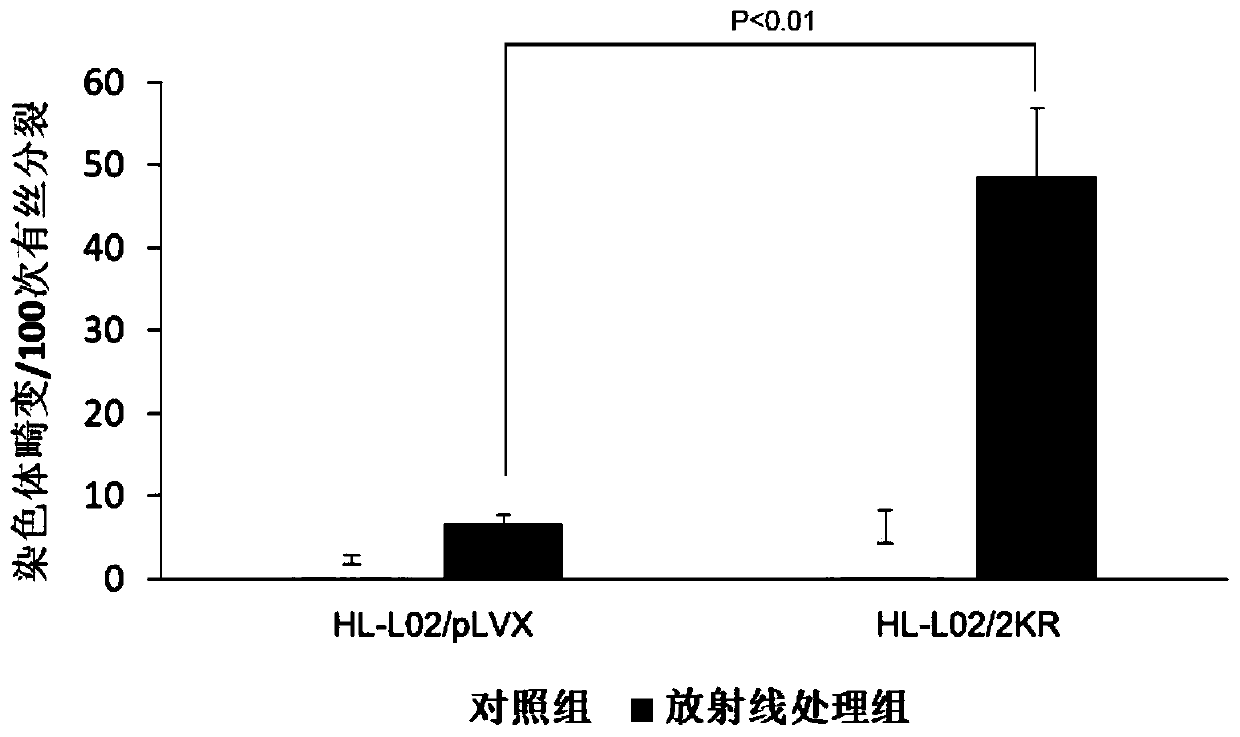
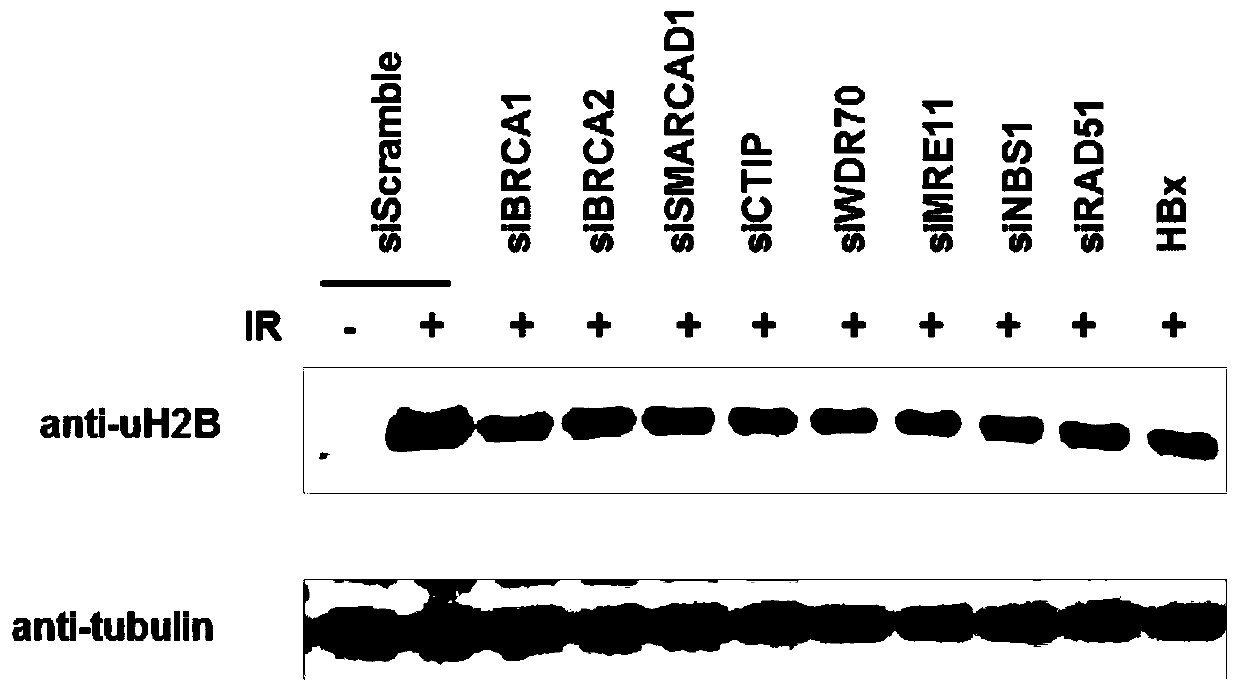
Application of histone H2B mono-ubiquitination for identifying homologous recombination repair defects
PendingCN110221070AConvenient for clinical operationGood clinical economicsMicrobiological testing/measurementMaterial analysisWilms' tumorLiver cancer
The invention relates to an application of histone H2B based mono-ubiquitination for detecting homologous recombination repair defects, in particular to a kit for detecting homologous recombination repair defects based on histone H2B mono-ubiquitination level and an application thereof. The application is to detect the defect of homologous recombination repair by detecting the histone H2B mono-ubiquitination level, and the result contributes to distinguishing the progress of related diseases and the responsiveness of homologous recombination targeted drug therapy such as PARP inhibitor and thelike, including but not limited to liver tissues or liver cancer with hepatitis B virus, and various tumors with homologous recombination gene mutation, expression and splicing body abnormality.
Owner:THE WEST CHINA SECOND UNIV HOSPITAL OF SICHUAN +1
Items dispenser
ActiveUS20090112361A1Patient compliance is goodMinimizing direct oversightDrug and medicationsComputer-assisted medicine prescription/deliveryRegimenDrug dispensing
Owner:UNIVERSITY OF ROCHESTER
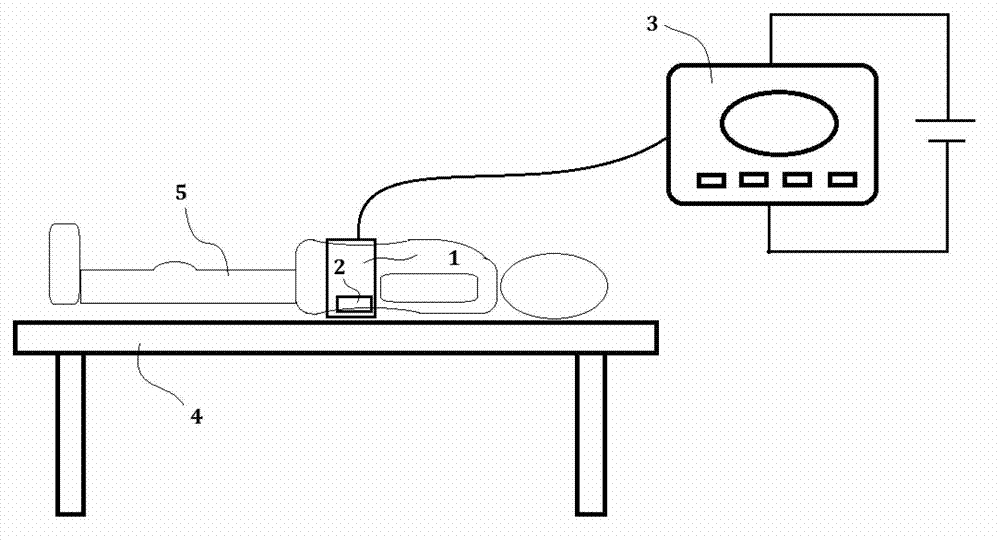
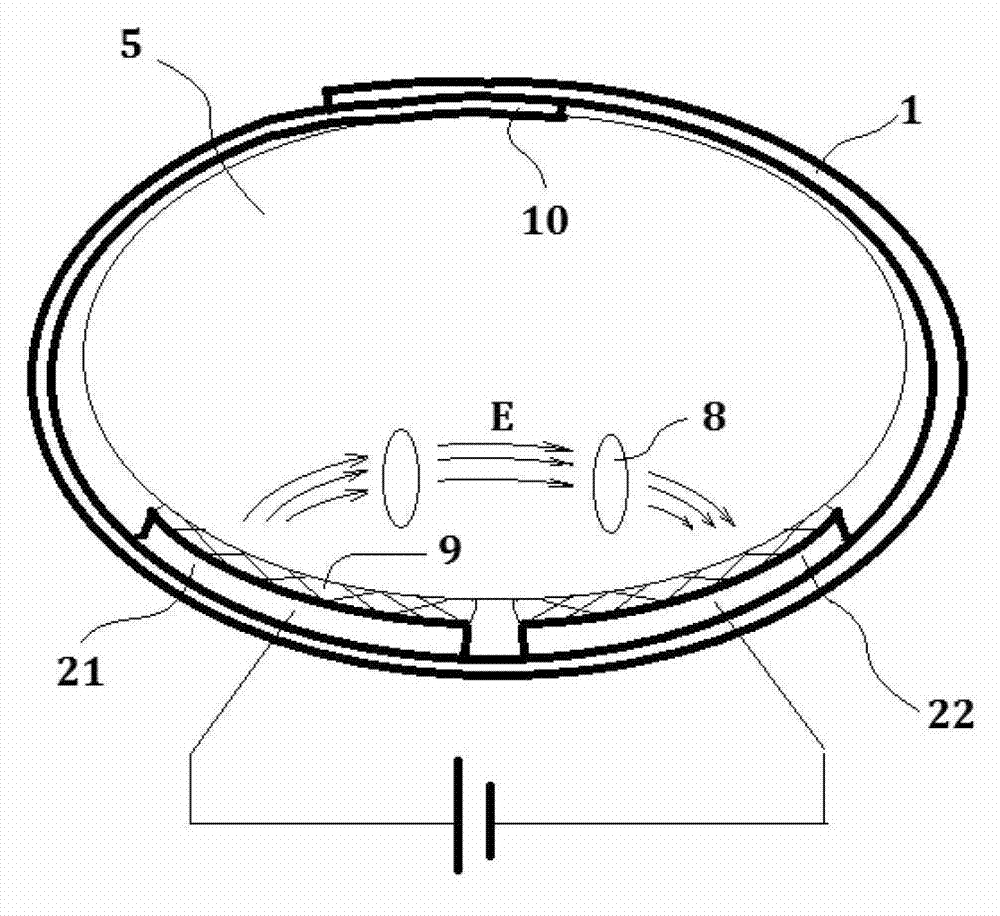
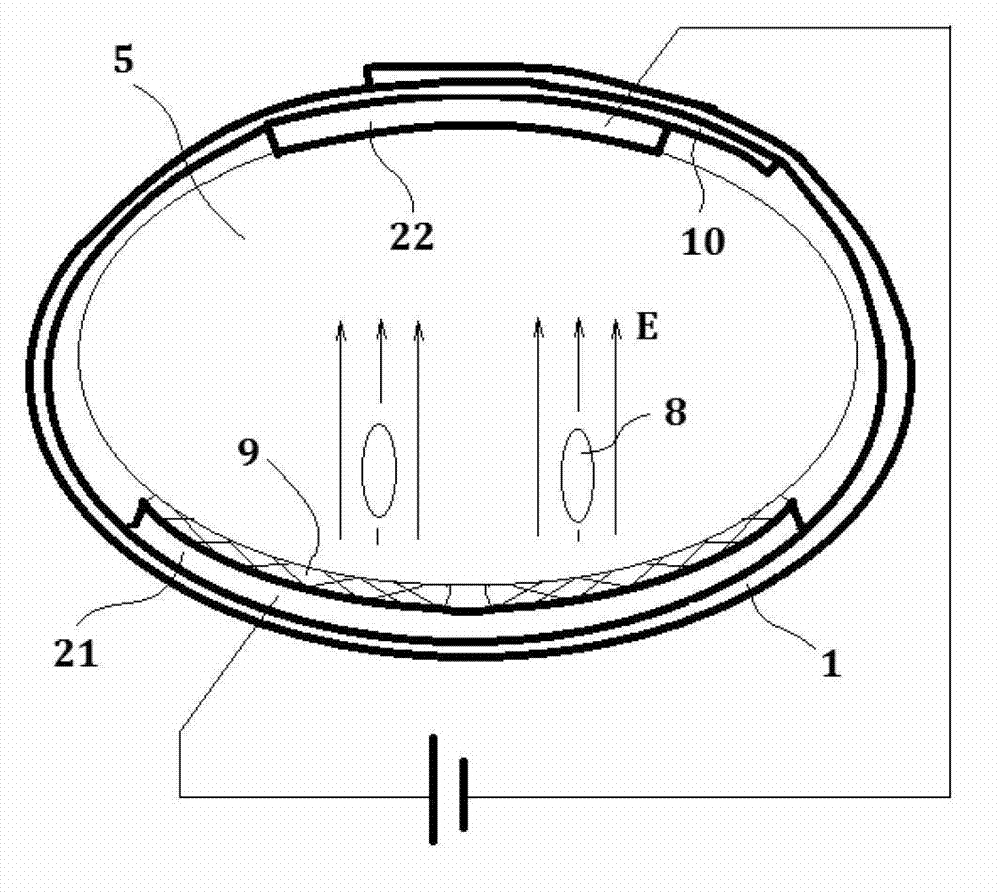
Medication therapy system for curing nephropathy in rehabilitation mode through targeted guiding of Chinese herbal medicinal ingredients into renal region
InactiveCN102861376AWith drug import functionActivate the immune systemElectrotherapyInanimate material medical ingredientsAdditive ingredientTherapeutic effect
Disclosed is a medication therapy system for curing nephropathy in a rehabilitation mode through targeted guiding of Chinese herbal medicinal ingredients into a renal region. The system comprises a renal region Chinese herbal medicinal ingredient leading-in instrument, a thermal therapy belt and a drug administration device, the renal region Chinese herbal medicinal ingredient leading-in instrument comprises a controller which can generate an electronic biological wave so as to comprehensively treat human body diseased tissue, the controller can reset output intensity and magnetic thermal therapy temperature to zero automatically and in advance when the renal region Chinese herbal medicinal ingredient leading-in instrument is powered on and control treatment time, treatment mode, output intensity, output frequency, magnetic thermal therapy starting and stopping and magnetic thermal therapy temperature adjustment, when the treatment timing is finished, the instrument is automatically stopped, the thermal therapy belt holds the drug administration device and encircles on the waist of a patient, a drug guiding electrode plate of the thermal therapy belt is communicated with the renal region Chinese herbal medicinal ingredient leading-in instrument through a guide line, and the drug administration device contains the Chinese herbal medicine. By means of the medication therapy system for curing the nephropathy in the rehabilitation mode through targeted guiding of Chinese herbal medicinal ingredients into the renal region, suffering of the patient caused by oral drugs is avoided, the therapeutic effect is good, the treatment cost is low, and a safe, simple, convenient, rapid and painless treatment method is achieved.
Owner:潍坊复能肾病研究院
Disposable cryotherapy device for the treatment of hemorrhoids with frozen healing media
The present invention provides a method and devices for the treatment of hemorrhoids. The method involves the combined delivery of cryotherapy and medication therapy. The devices described provide cryotherapy for immediate relief of pain, itching, reduction of swelling, reduction of existing bleeding, shrinkage of hemorrhoidal tissue, and promotes healing of affective tissues. Subsequent medication release from these devices provides sustained healing and protracted relief of symptoms. The devices may be disposable and may be used externally or internally, and may include an invasive or non-invasive applicator.
Owner:POHLER JERZY
System and method for dynamic adjustment of copayment for medication therapy
System and method for the cost-effective use of medications, comprising dynamically adjusting the patient cost for a plurality of possible medication treatment therapies according to the cost-effectiveness of each possible medication therapy based on known patient attributes, and providing a physician with the dynamically determined patient cost of at least one of the possible medication treatment therapies.
Owner:HUMANA INC
Systems for clinical trials
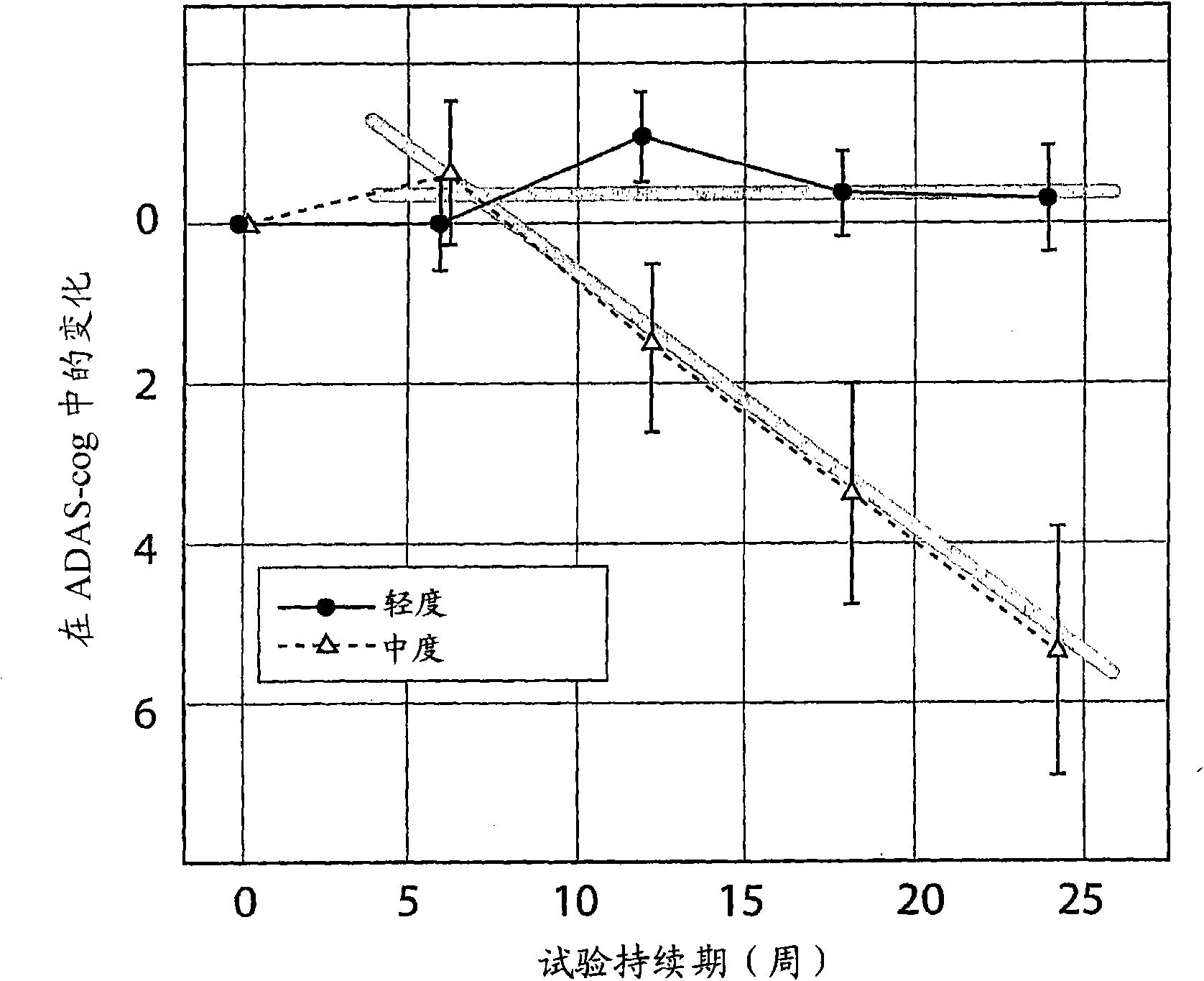
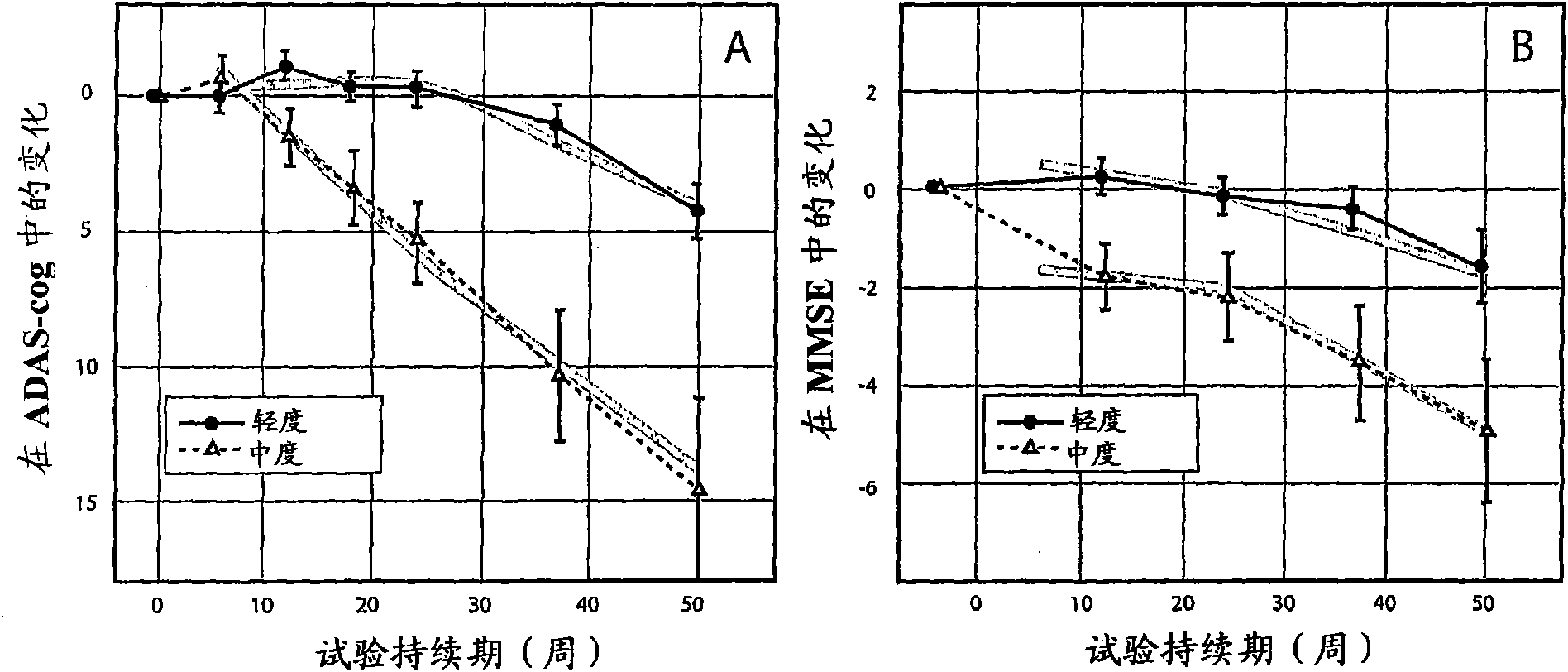
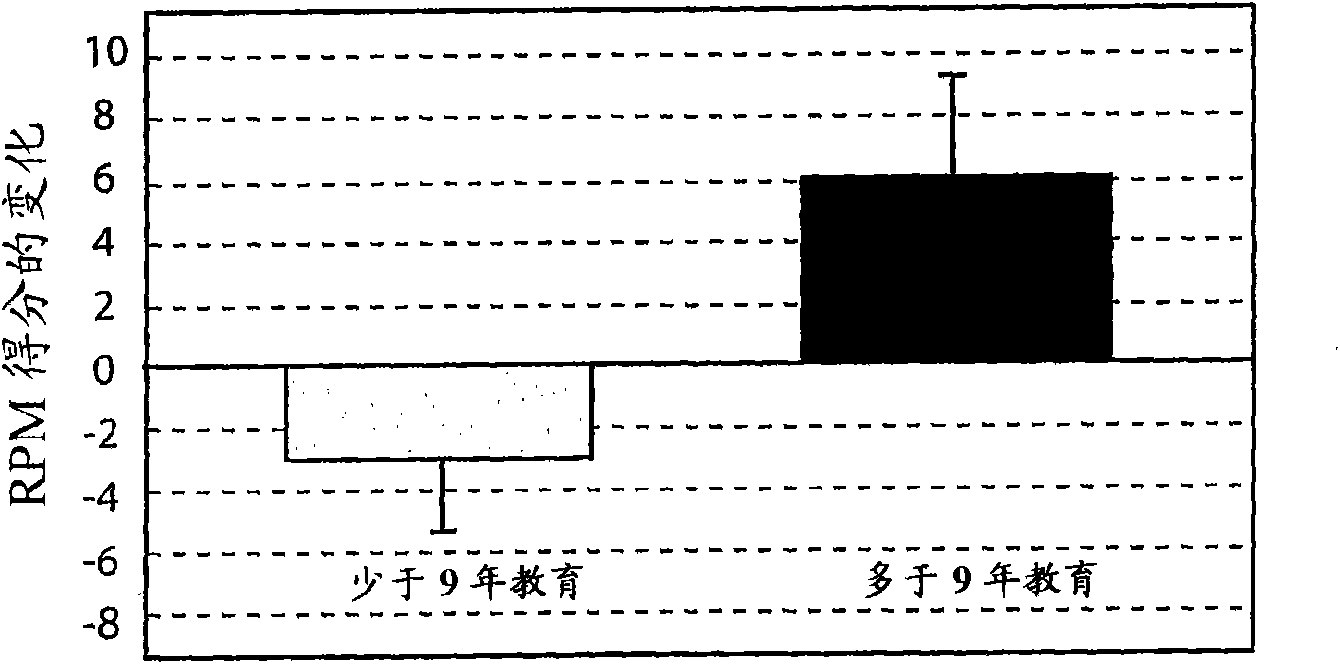
The invention provides methods and systems for assessing the efficacy of a pharmaceutical which is putatively disease modifying of a cognitive disorder, for use in the treatment or prophylaxis of that cognitive disorder, the method comprising the steps of: (1) stratifying a subject group into at least 2 sub-groups according to a baseline indicator of likely disease progression, (2) treating members of each subject group with the pharmaceutical for a treatment time frame, (3) deriving psychometric and optionally physiological outcome measures for each treated patient group, (4) comparing the outcomes at (3) with a comparator arm of said sub-groups which is optionally a placebo or minimal efficacy comparator arm, (5) using the comparison in (4) to derive an efficacy measure for the pharmaceutical. The methods and systems of the invention address problems such as low rate of decline over the treatment time-frame of patients who have mild-disease severity at baseline and biased withdrawal, particularly in the placebo / comparator treatment arm.
Owner:WISTA LAB LTD
Ultrasonic medication therapy comb
InactiveCN104174112ASimple structureExpand the scope of treatmentUltrasound therapyHair combsDiseaseTreatment effect
The invention provides an ultrasonic medication therapy comb to solve the problem that the existing medication comb has the function of medication only. The ultrasonic medication therapy comb comprises teeth, an inner shell, an outer shell, a control panel, an ultrasonic head and a medication hole. The teeth of metal are arranged at one end of the bottom of the outer shell; the teeth of plastic are arranged at the other end of the bottom of the outer shell. The ultrasonic head is disposed between the teeth of metal and the teeth of plastic. The control panel is arranged on the upper portion of the outer shell. The medication hole is arranged in the back of the outer shell. The inner shell is arranged within the outer shell. A drug passage is arranged in the inner shell; one end of the drug passage is connected with the medication hole; the other end of the drug passage is connected with the teeth of metal; the teeth of metal are hollow; a ball is disposed within each tooth of metal. The ultrasonic medication therapy comb is a novel medical, health-care, rehabilitation and physiotherapy instrument which is simple in structure and which integrates the traditional medicine and the modern high-tech; a novel therapy method is provided for the treatment of head skin diseases. The ultrasonic medication therapy comb has the advantages that the treatment range is widened, and treatment effect is improved.
Owner:LUOYANG ANPU BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com