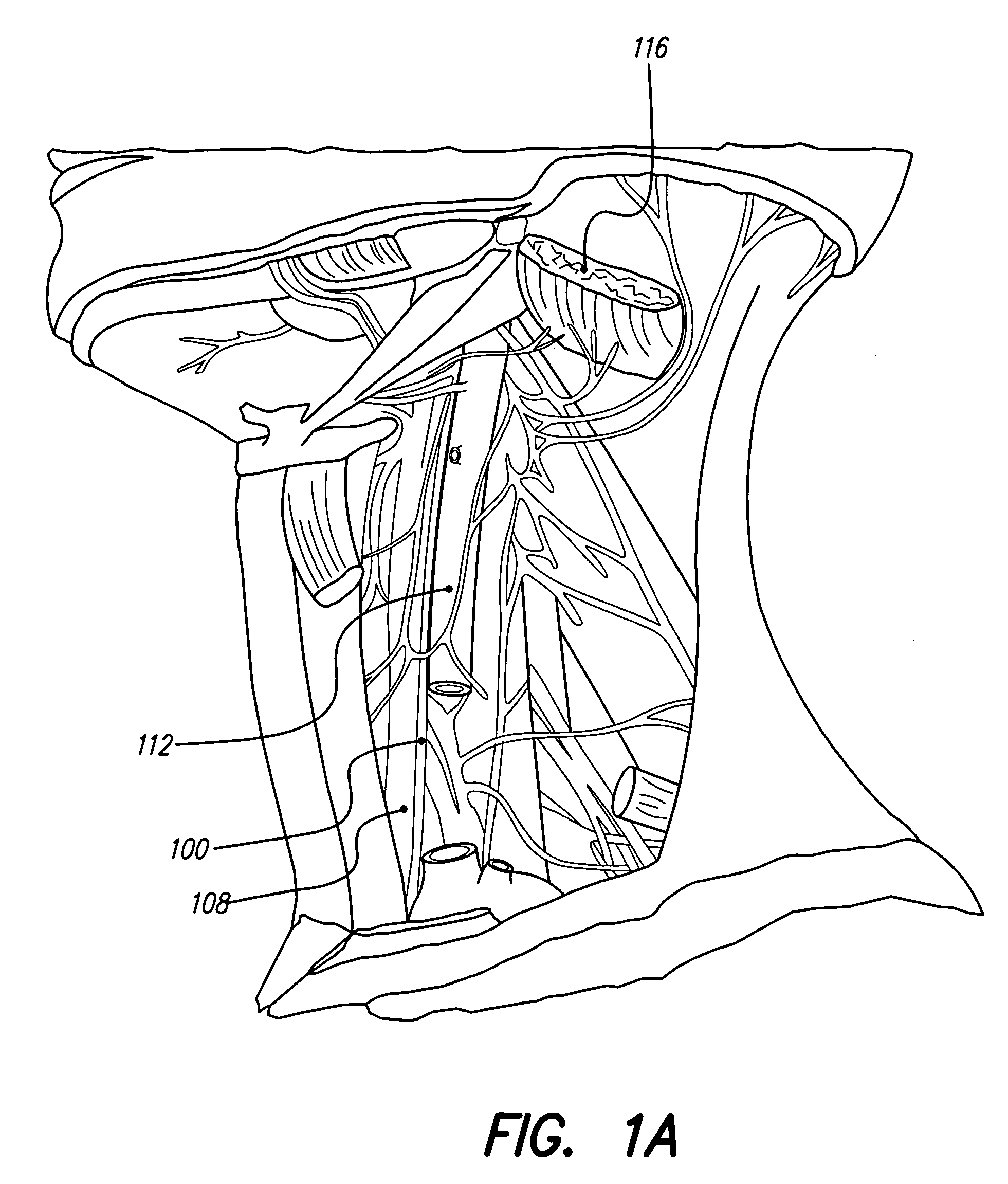
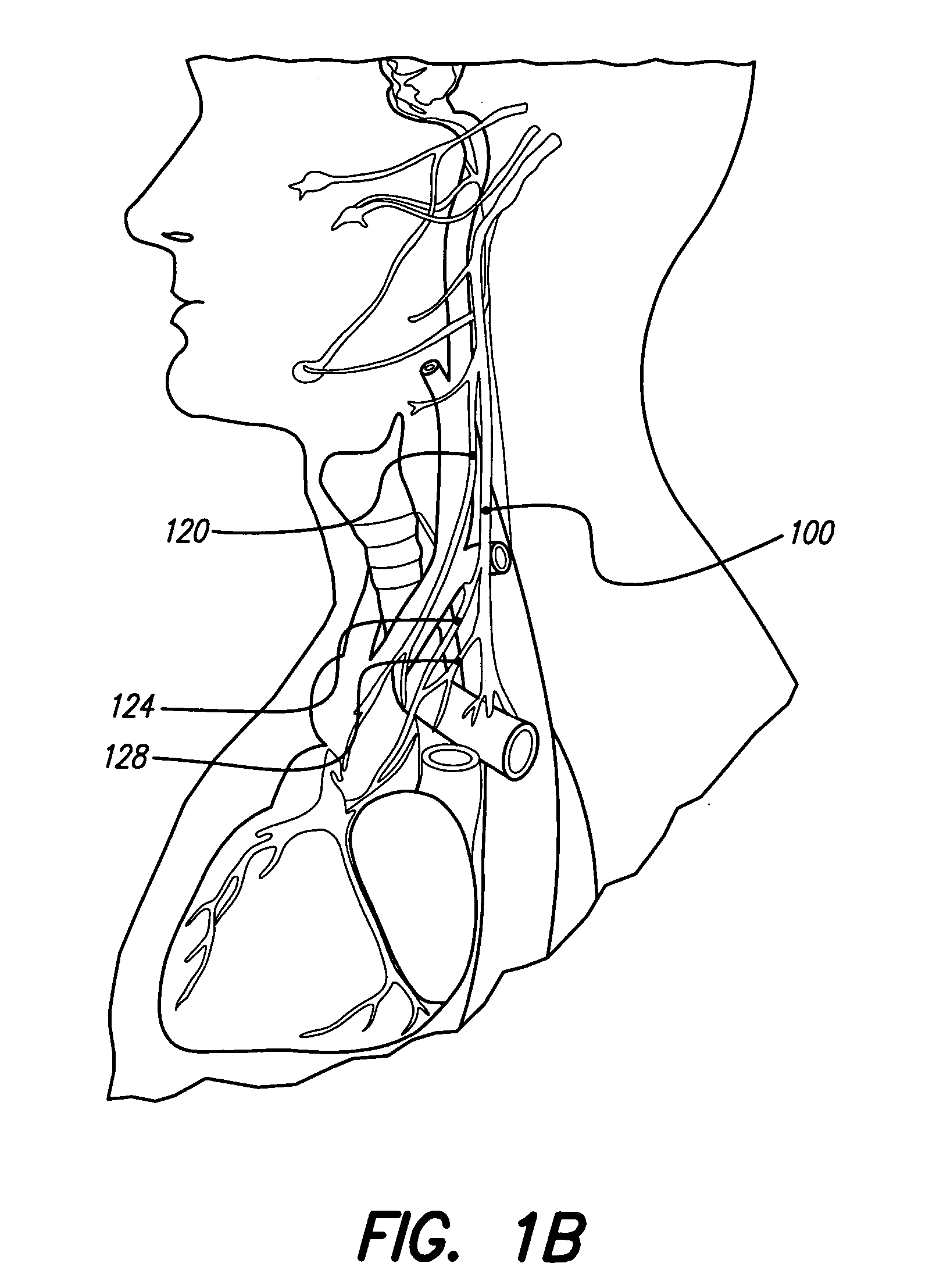
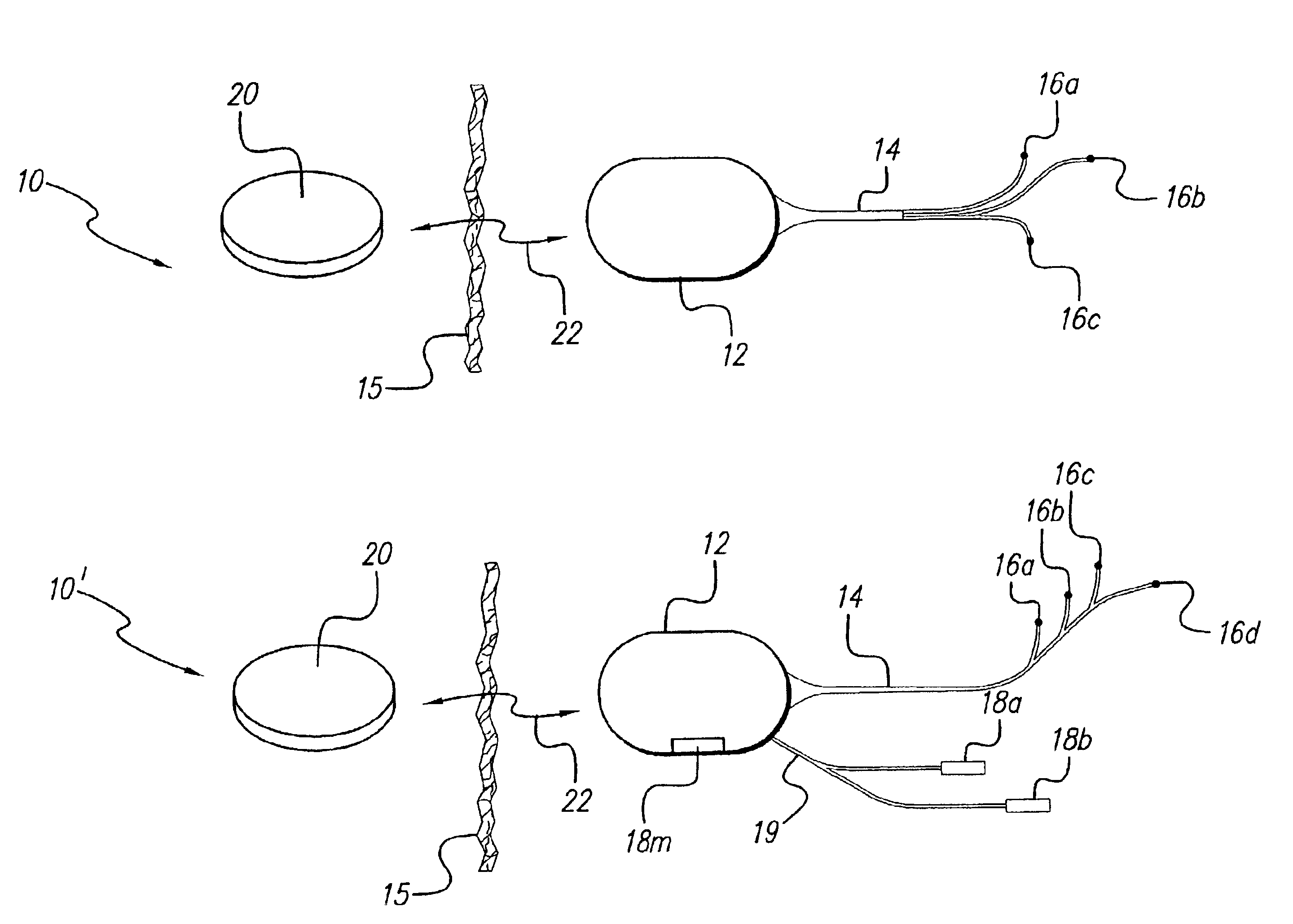
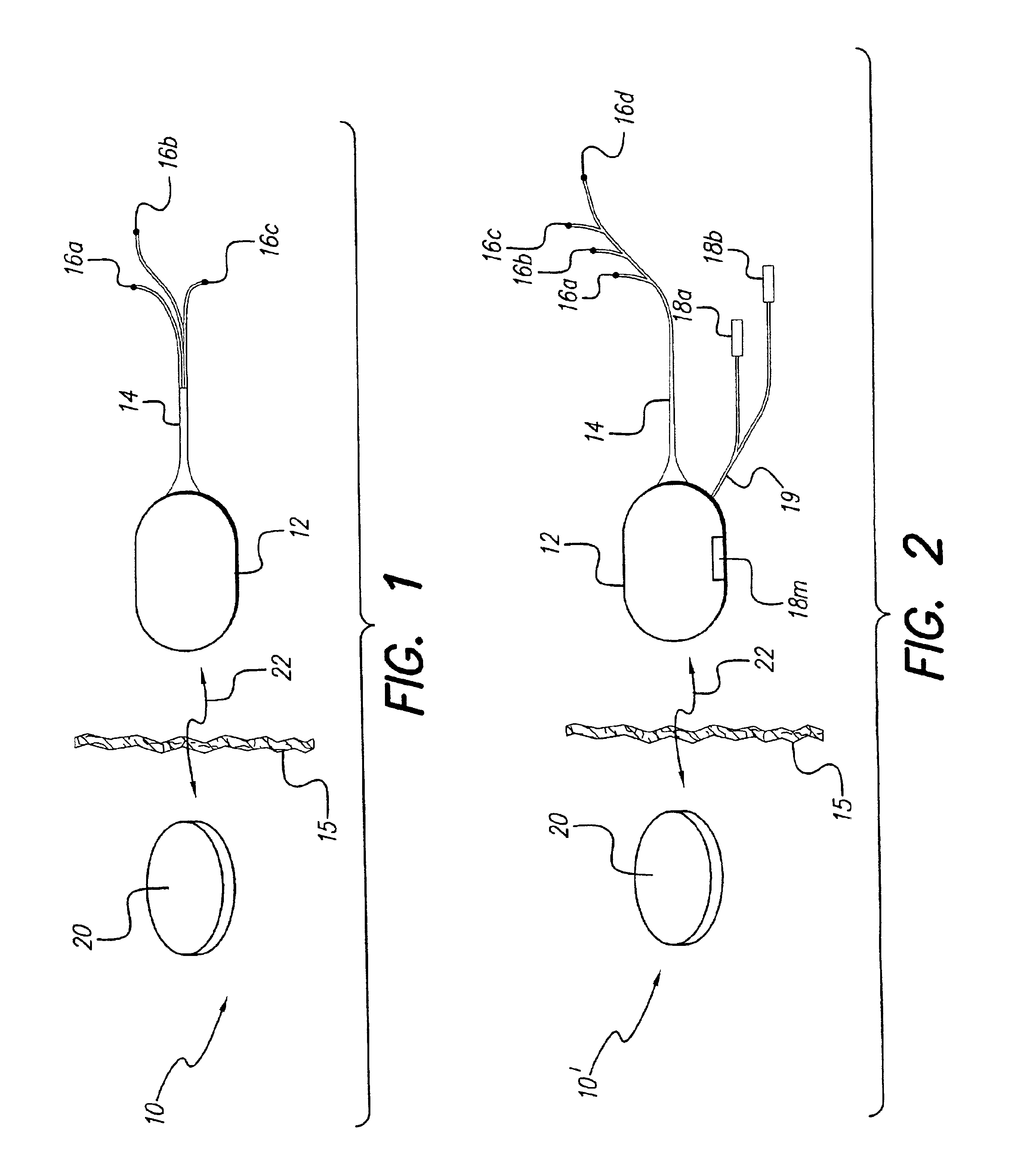
Patents
Literature
154 results about "Implantable Pump" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
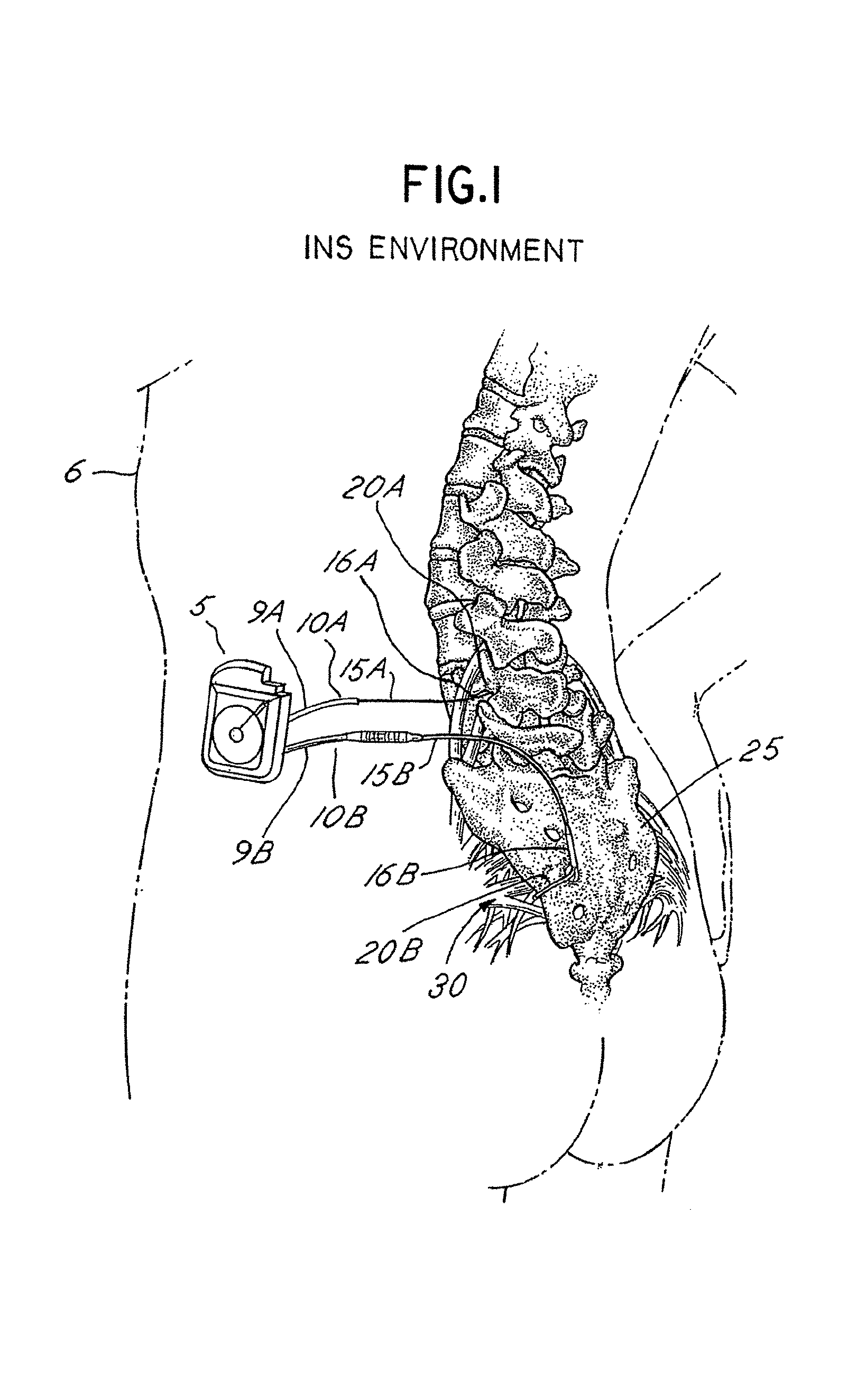
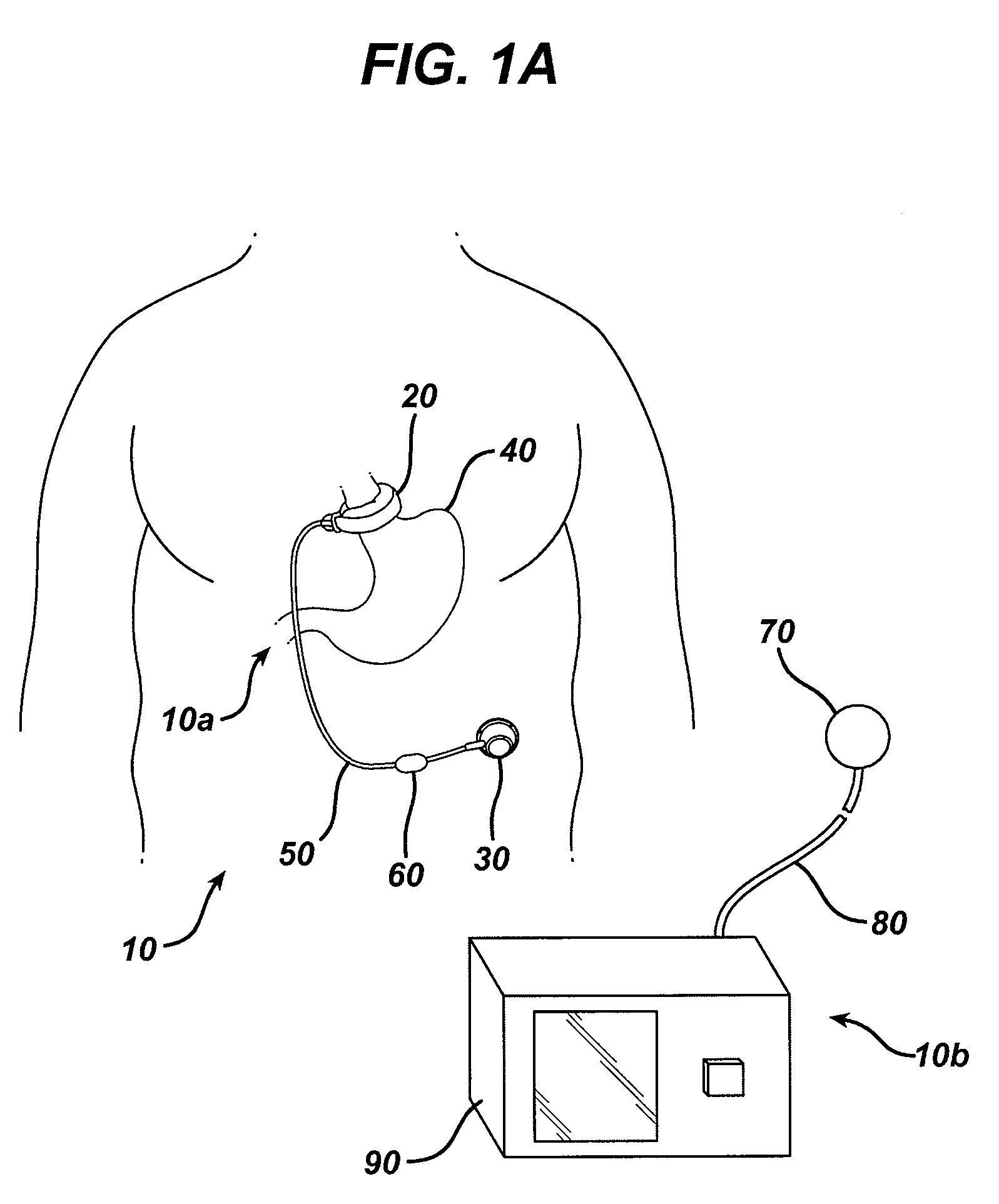
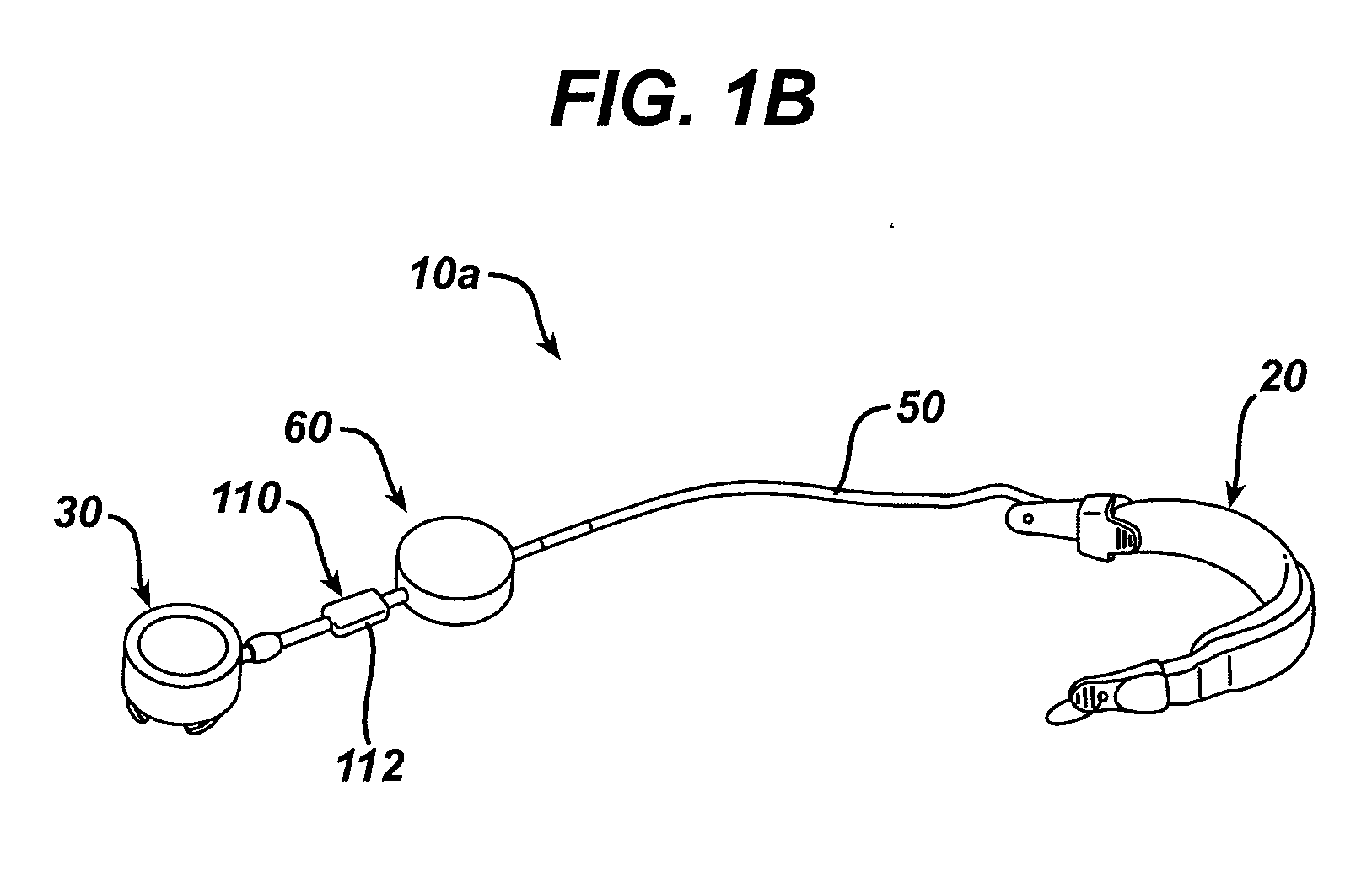
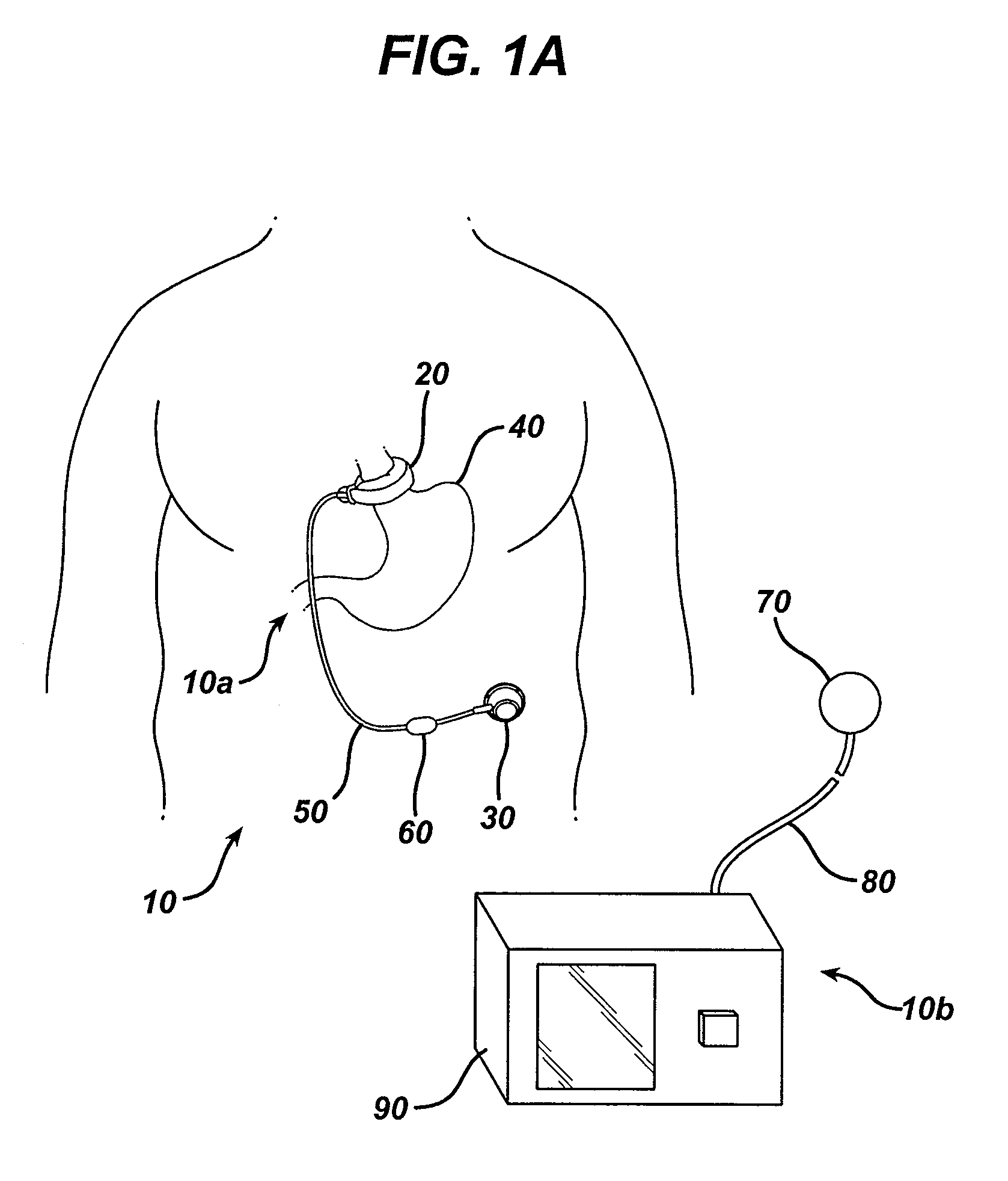
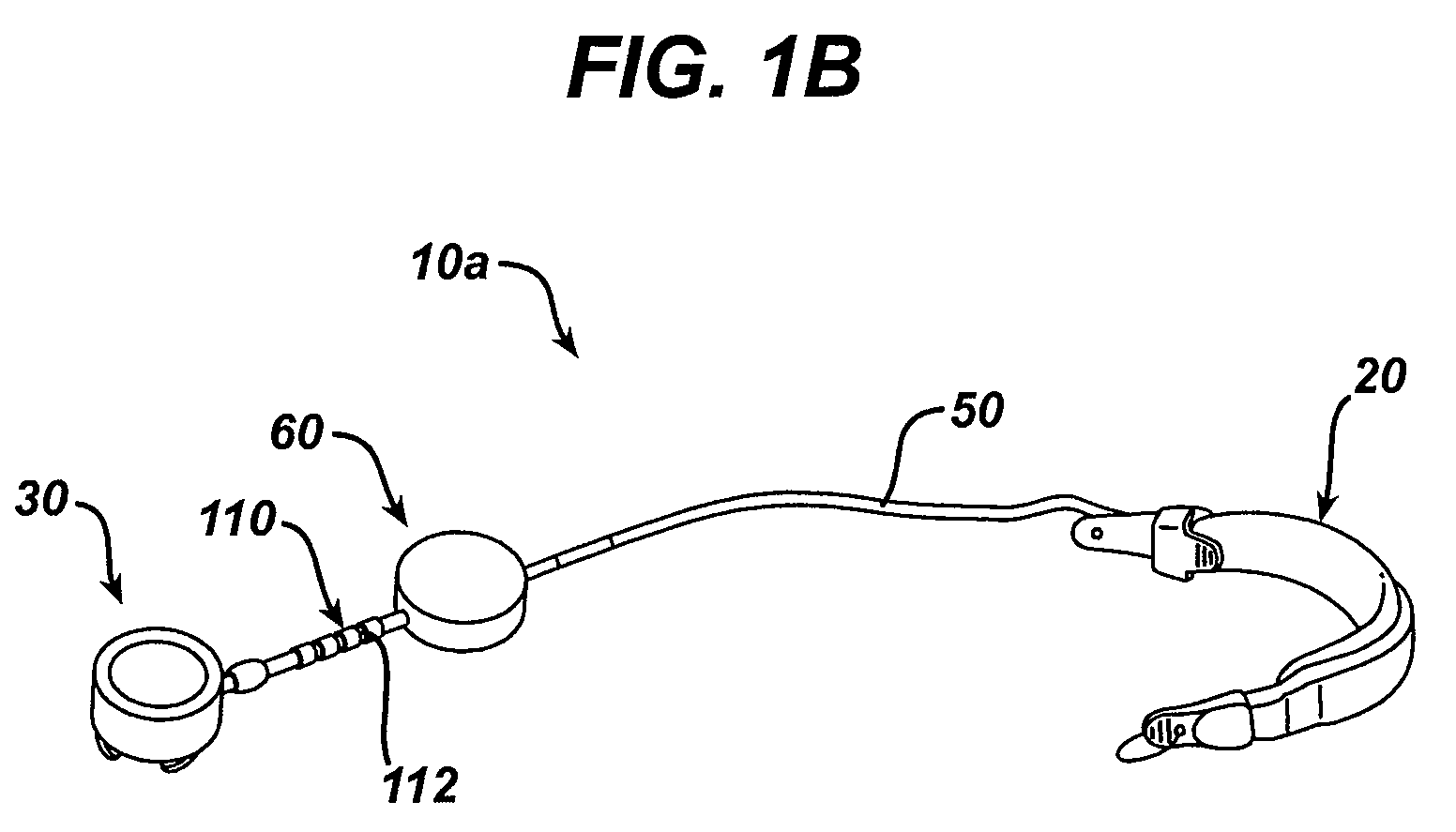
A small device installed under the skin to provide long-term controlled-rate delivery of drugs such as chemotherapeutic agents or analgesics. Delivery rate may be externally controlled or osmotically or peristaltically controlled with the aid of transcutaneous monitoring.
Systems and Methods for Diabetes Management Using Consumer Electronic Devices
InactiveUS20080119705A1Facilitate communicationDrug and medicationsNutrition controlDiabetes managementContinuous glucose monitoring
The invention is embodied in a system for diabetes management including a medical device (MD) and a consumer electronic device (CED). The CED may be used to monitor and / or control the MD. In particular embodiments, the system may include a connector that plugs into the CED to allow communication between the MD and the CED. The medical device may be an external infusion device, implantable pump, glucose meter, glucose monitor, continuous glucose monitoring system, or the like. The CED may be any type of consumer electronic device including, but not limited to, cellular phones, personal digital assistants (PDAs), BlackBerry, Smartphones, pocketpc phones, mp3 players, radios, CD players, and the like.
Owner:MEDTRONIC MIMIMED INC
Treatment of movement disorders with drug therapy
ActiveUS7155279B2Increase excitementPrevent movementElectrotherapyDiagnosticsTherapeutic exerciseMedicine
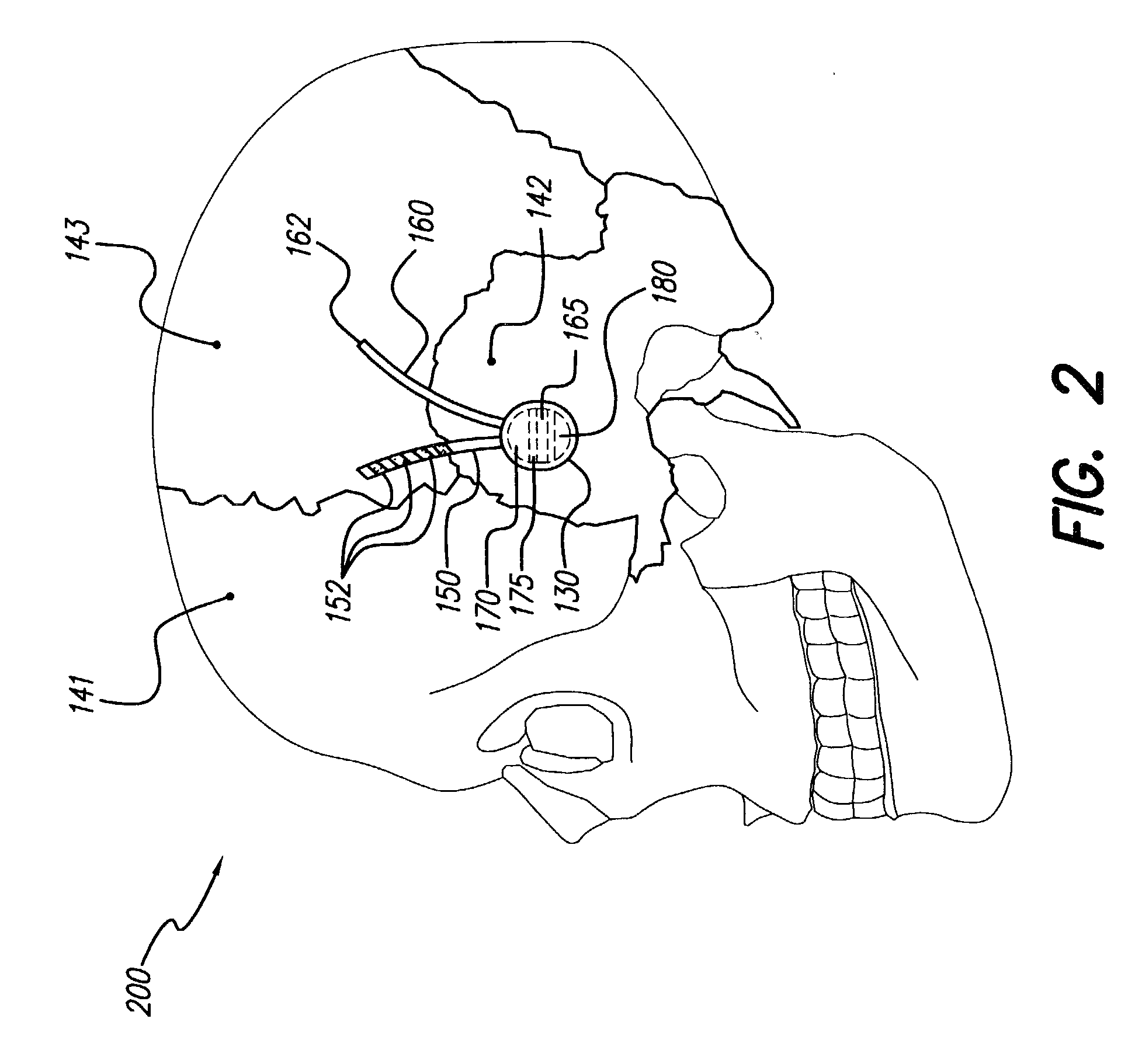
Introducing one or more stimulating drugs to the vagus nerve and / or one or more branches of the vagus nerve to treat movement disorders uses at least one implantable system control unit (SCU) with an implantable pump with at least one infusion outlet. Optional electrical stimulation may additionally be supplied by an implantable signal / pulse generator (IPG) with one or more electrodes. In certain embodiments, a single SCU provides one or more stimulating drugs and the optional electrical stimulation. In some embodiments, one or more sensed conditions are used to adjust stimulation parameters.
Owner:MEDTRONIC MIMIMED INC
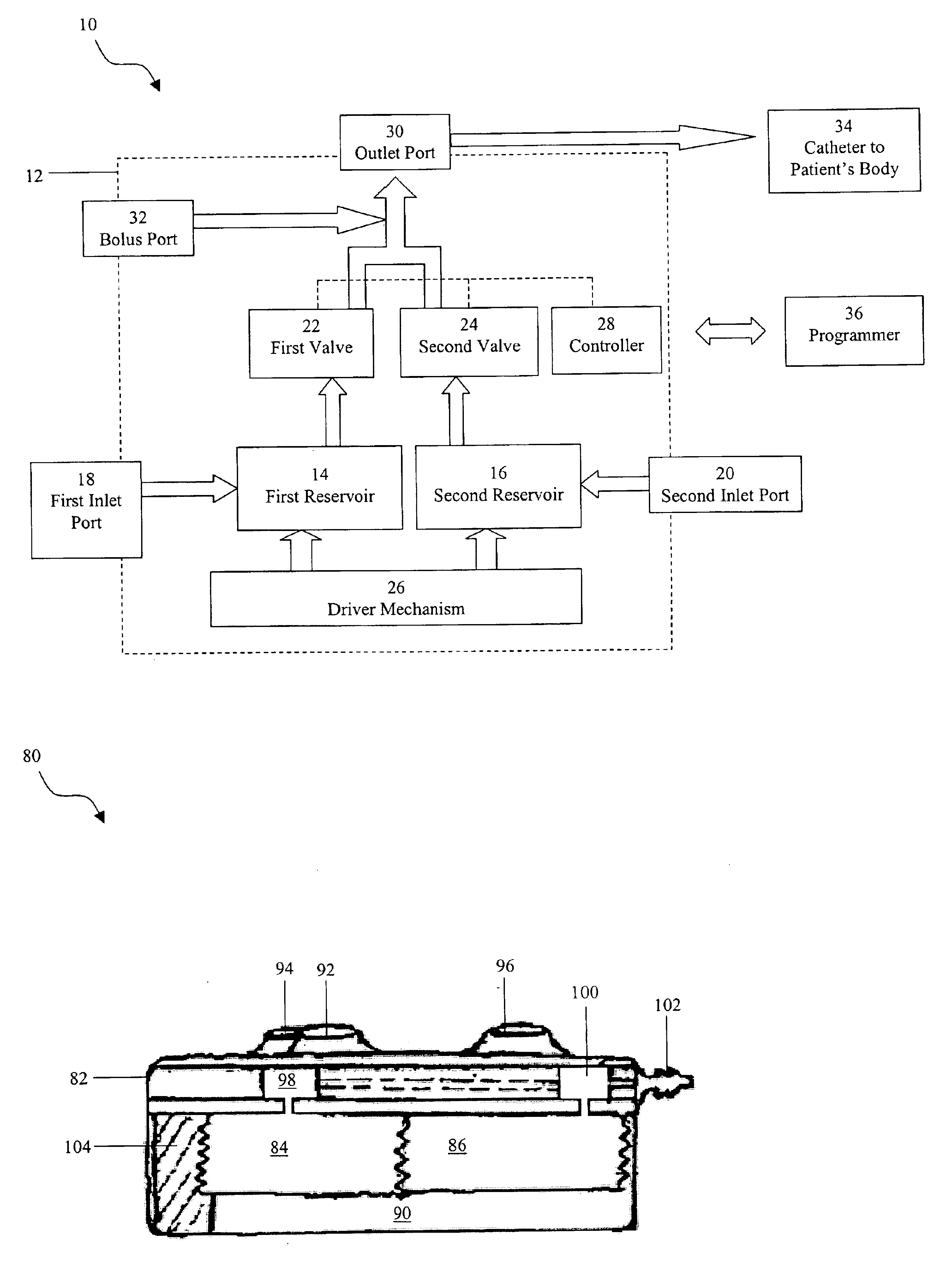
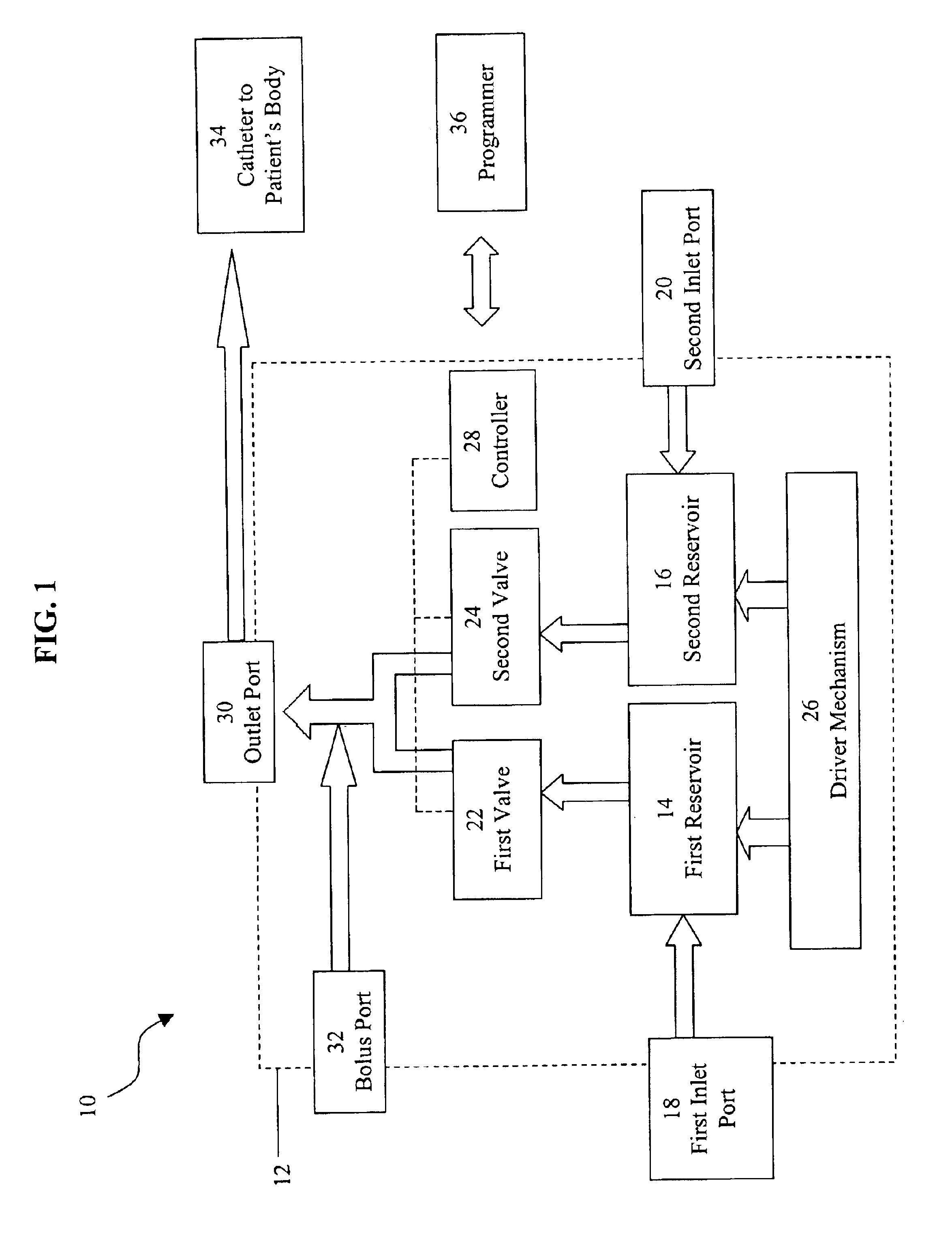
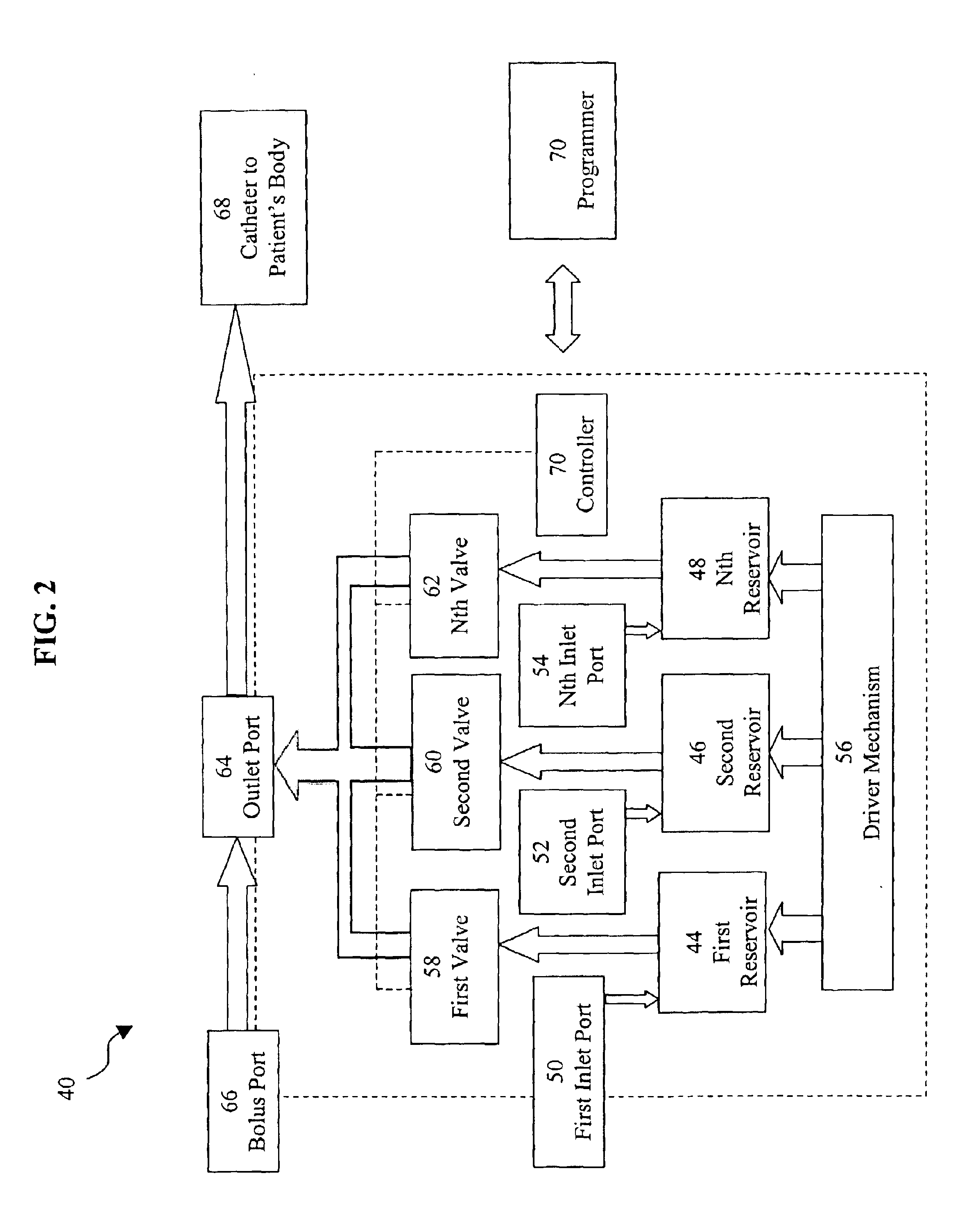
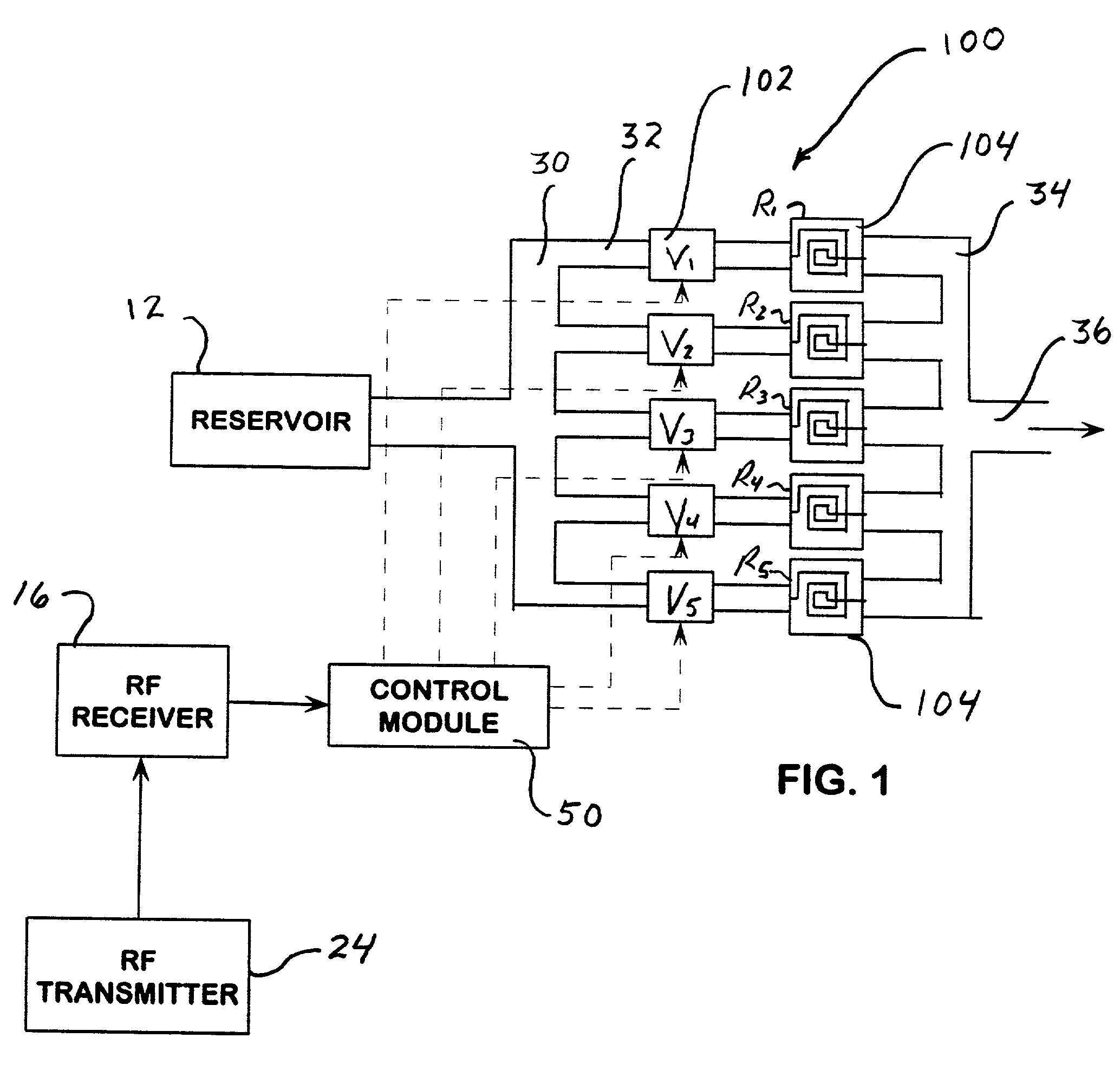
Programmable implantable pump with accessory reservoirs and multiple independent lumen catheter
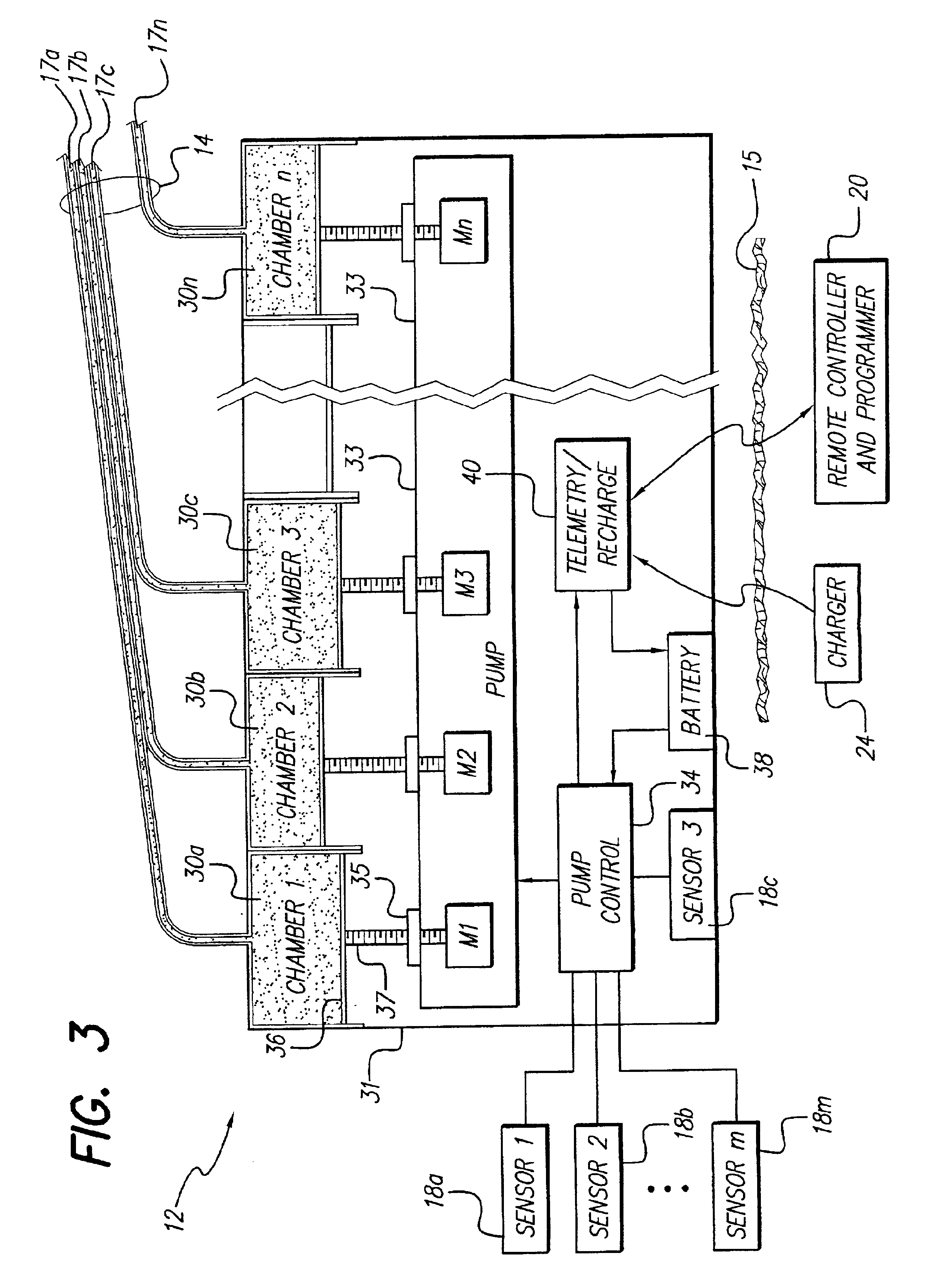
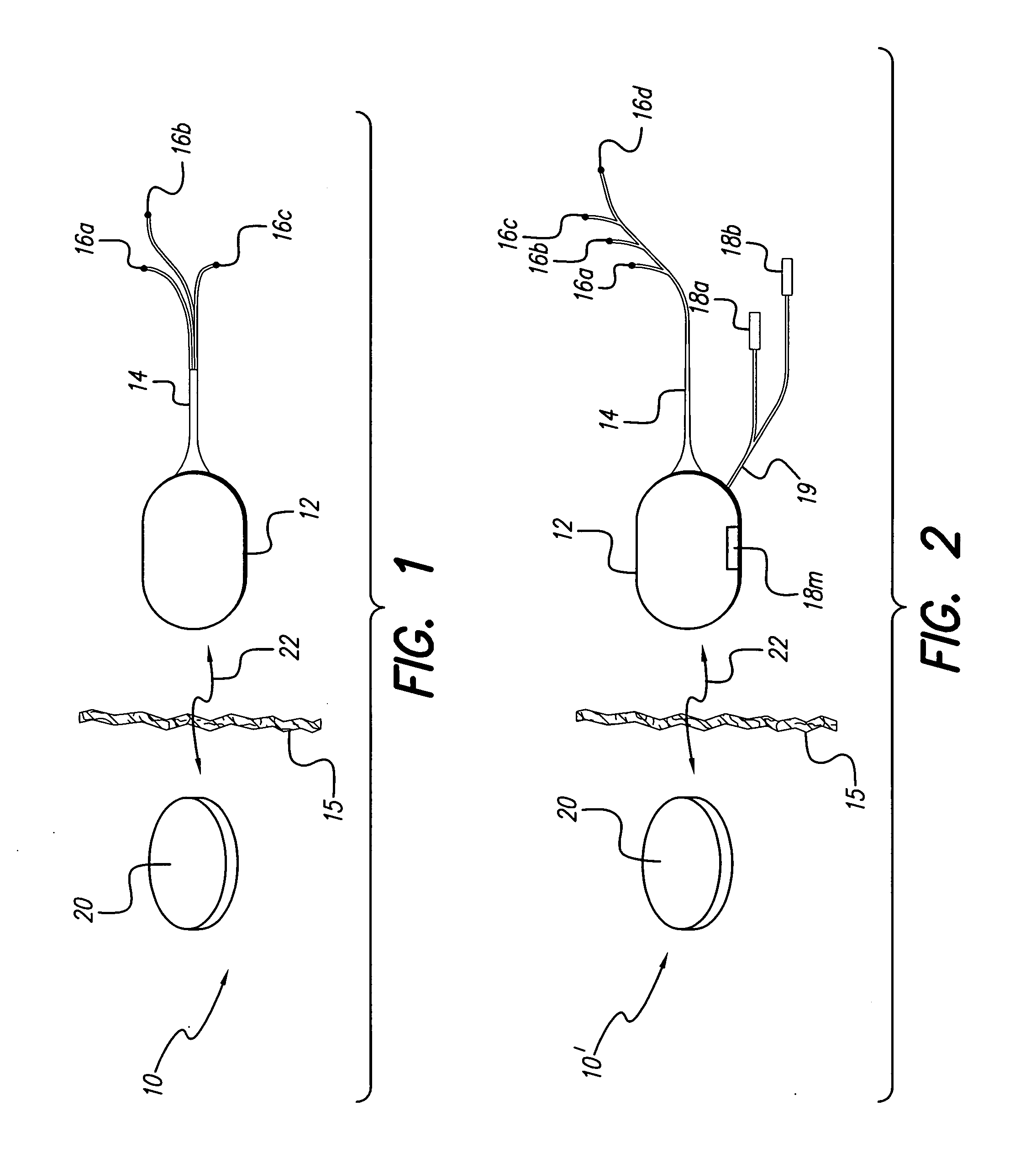
An implantable pump system includes: (1) an implantable pump having separate chambers or reservoirs, at least one of which is coupled to the pump so as to allow a programmable rate of delivery of the medication stored in the pump chamber or reservoir, the other chambers or reservoirs of which are at least capable of delivery of a bolus via a pressurized, and potentially independently programmable chamber or pumping mechanism; (2) a patient controller that enables the actuation of the pump so as to administer a bolus or programmed rate of the first, second, third, . . . or nth medication contained in the independent chambers or reservoirs coupled to the pump; and (3) a catheter having two or more reservoir-specific inlet ports directed into respective lumens of the catheter. In one embodiment, the distal tips of the respective lumens may be directed to different sites within the patient's body, thereby allowing site specific and independent delivery of the medications stored in the respective pump chambers or reservoirs to be administered to different body sites at independently controlled times and rates. In another embodiment, the distal tips of the respective lumens are directed, more or less, to the same body site or tissue region, thereby providing for the independent delivery of multiple medications to the same regions at independently controlled times and rates.
Owner:MEDTRONIC MIMIMED INC
Stomach prosthesis
InactiveUS7037343B2Treating obesityFacilitate expedite mixing breaking downDiagnosticsSurgeryPylorusSmall intestine
An implantable stomach prosthesis is provided for surgically replacing or augmenting all or part of the antrum and / or pylorus of a stomach. The prosthesis controls the passage of food from the stomach to the small intestine. The prosthesis may be configured to churn ingested material and release it from the stomach through a prosthetic pyloric valve. At least one expandable member is arranged to be expanded to control the passage of food and / or to mimic the churning action of a patient's stomach. The prosthesis includes an outer support structure, a flexible inner member forming a conduit for the movement of material, and at least one expandable member located between the outer support structure and inner member. An implantable pump system is provided for inflating and deflating the expandable member(s).
Owner:PYTHON MEDICAL
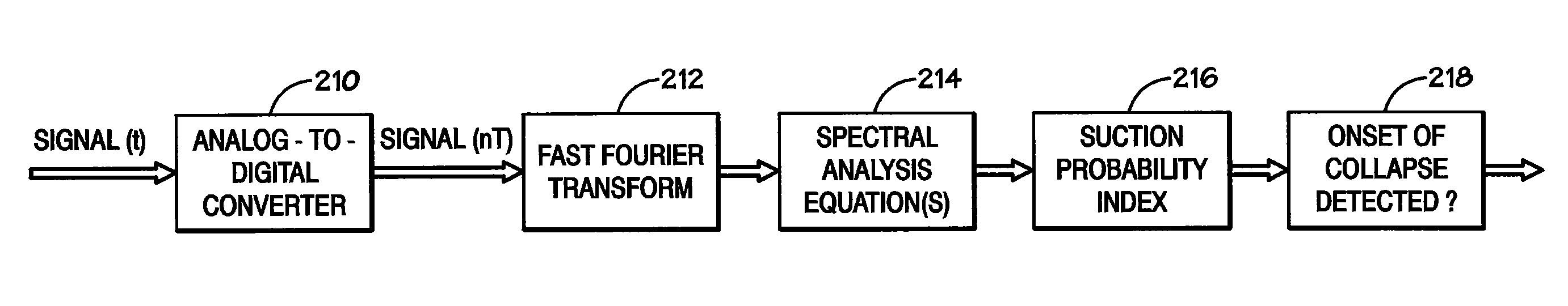
Method and system for detecting ventricular collapse
A pump system includes an implantable pump including a motor having a rotor and a stator. The stator includes a plurality of stator windings, and a motor controller is coupled to the motor to energize the windings so as cause the rotor to turn. A time-based system parameter of the pump is sampled and the system parameter is analyzed to calculate a suction probability index that provides an indication of the imminence of ventricle collapse.
Owner:RELIANTHEART
Troubleshooting accelerator system for implantable drug delivery pumps
InactiveUS6902544B2Efficient deliveryEfficiently containedPharmaceutical delivery mechanismMedical devicesControl valvesVALVE PORT
An implantable pump and methods for detecting leaks in an implantable pump are provided. In one embodiment, the implantable drug pump includes a housing having at least one inlet port and an outlet port formed therein. The outlet port is adapted to communicate with a catheter for delivering fluid to a patient's body, and the inlet port(s) are effective to deliver fluid into the housing. The housing further includes at least one reservoir disposed therein and effective to contain a fluid. In use, the pump preferably includes a fluid having one or more drugs disposed in at least one of the reservoirs, and a radiolucent fluid disposed in one of the reservoirs. A user programmable control mechanism is coupled to the drug pump and is effective to selectively control movement of the valves between the open and closed positions.
Owner:DEPUY SYNTHES PROD INC
Treatment of movement disorders by extra dural motor cortex stimulation
InactiveUS20060241717A1Increase excitementReduce excitementElectrotherapyArtificial respirationElectricityMedicine
System and methods for introducing one or more stimulating drugs and / or applying electrical stimulation to the cortex of the brain to treat movement disorders uses at least one implantable system control unit (SCU), specifically an implantable signal / pulse generator (IPG) or microstimulator with two or more electrodes in the case of electrical stimulation, and an implantable pump with one or more infusion outlets in the case of drug infusion. In certain embodiments, a single SCU provides both electrical stimulation and one or more stimulating drugs. In some embodiments, one or more sensed conditions are used to adjust stimulation parameters.
Owner:BOSTON SCI NEUROMODULATION CORP
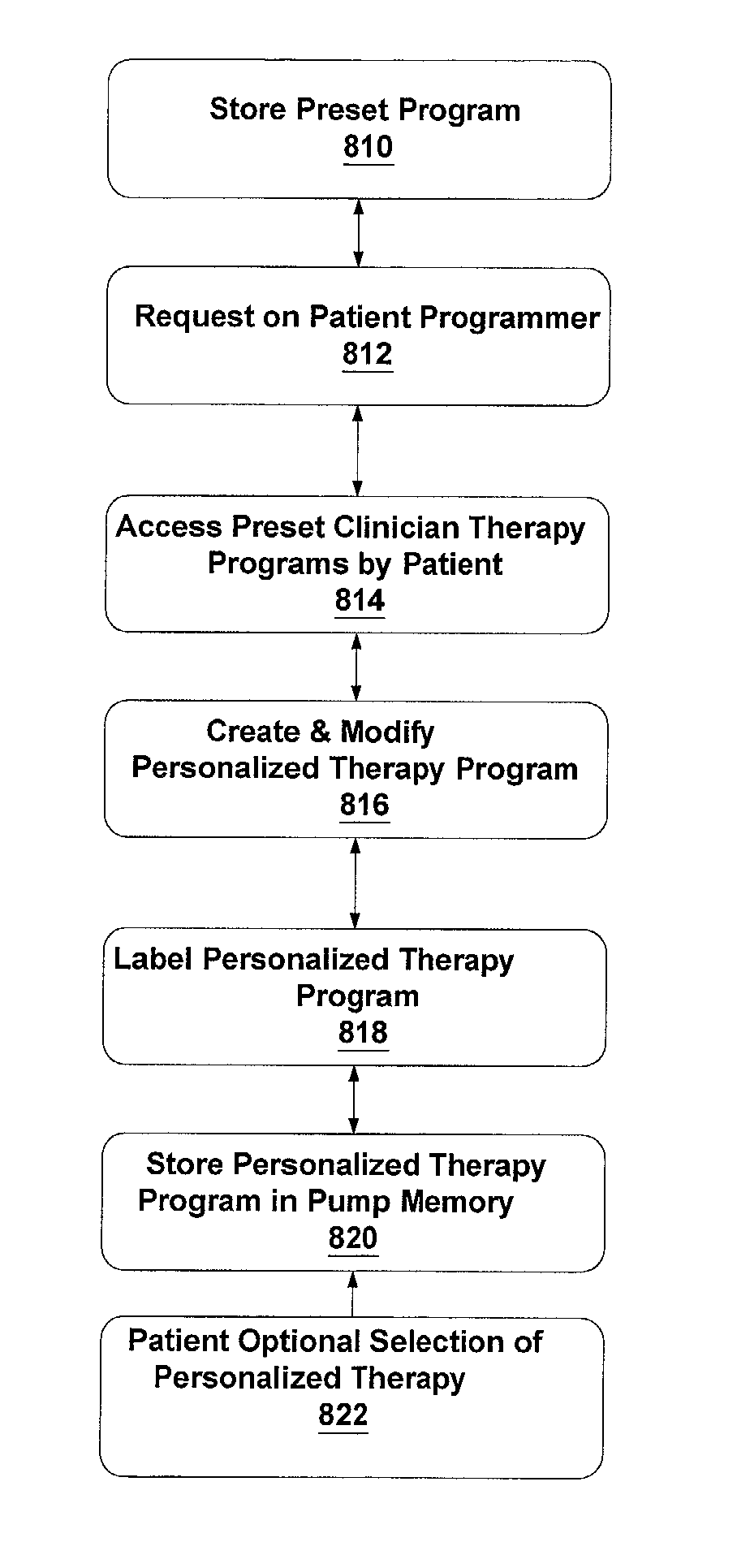
Patient directed therapy management
An method and system that allows a patient to access stored preset patient therapy programs, that are resident in a medical device such an implantable pump or a combination medical device having an implantable pump and a implantable neural stimulator, and to create personalized therapy programs or automatic timing therapy programs from preset therapy programs to accommodate the patient's particular activity. Alternatively, the patient can select and access stored preset patient therapy programs and combine at least two modified or unmodified preset therapy programs to create personalized therapy programs.
Owner:MEDTRONIC INC
Implantable sensors and implantable pumps and anti-scarring agents
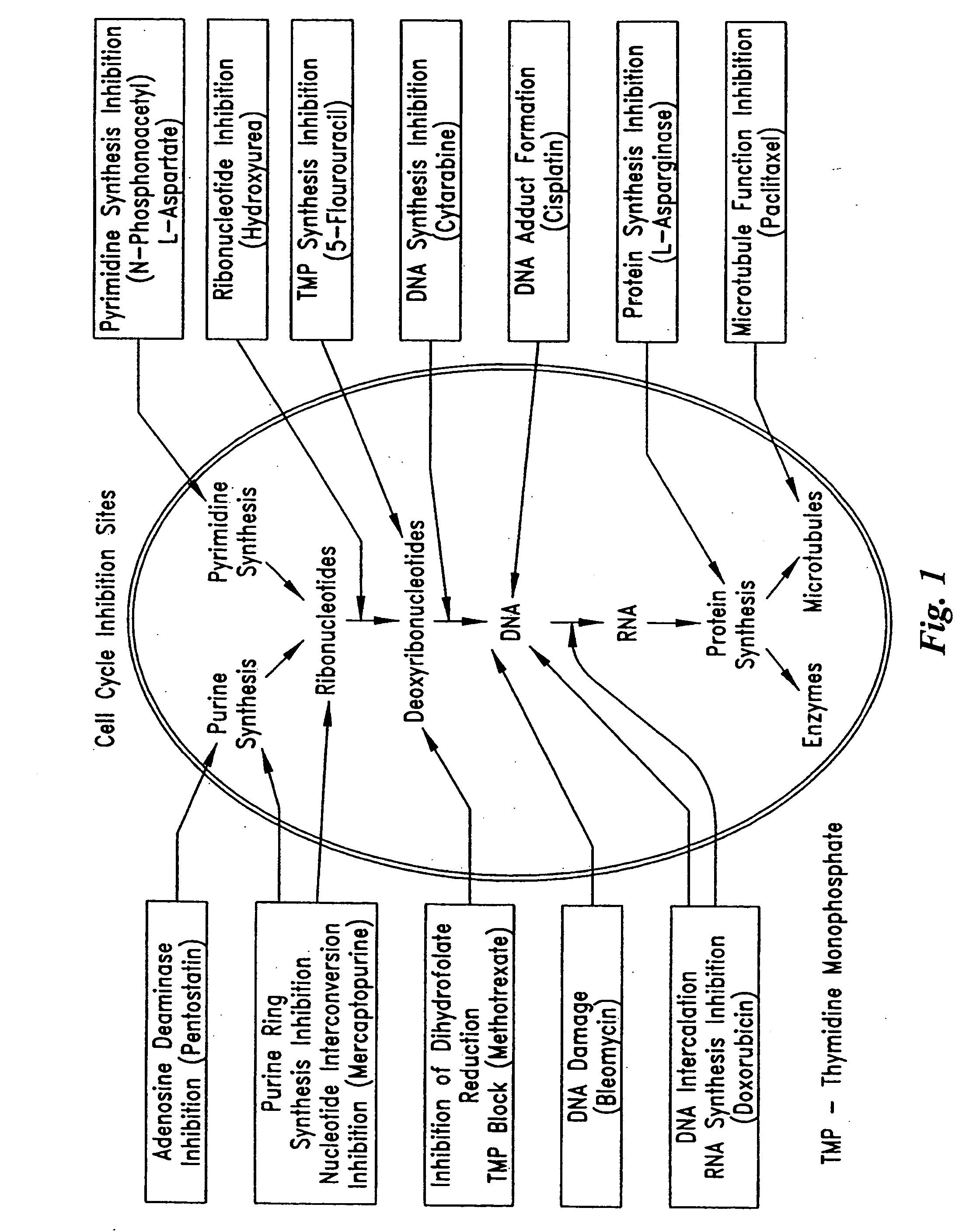
InactiveUS20050154374A1Function increaseImprove clinical outcomesPeptide/protein ingredientsAntipyreticMedicineCell Cycle Inhibition
Pumps and sensors for contact with tissue are used in combination with an anti-scarring agent (e.g., a cell cycle inhibitor) in order to inhibit scarring that may otherwise occur when the pumps and sensors are implanted within an animal.
Owner:ANGIOTECH INT AG (CH)
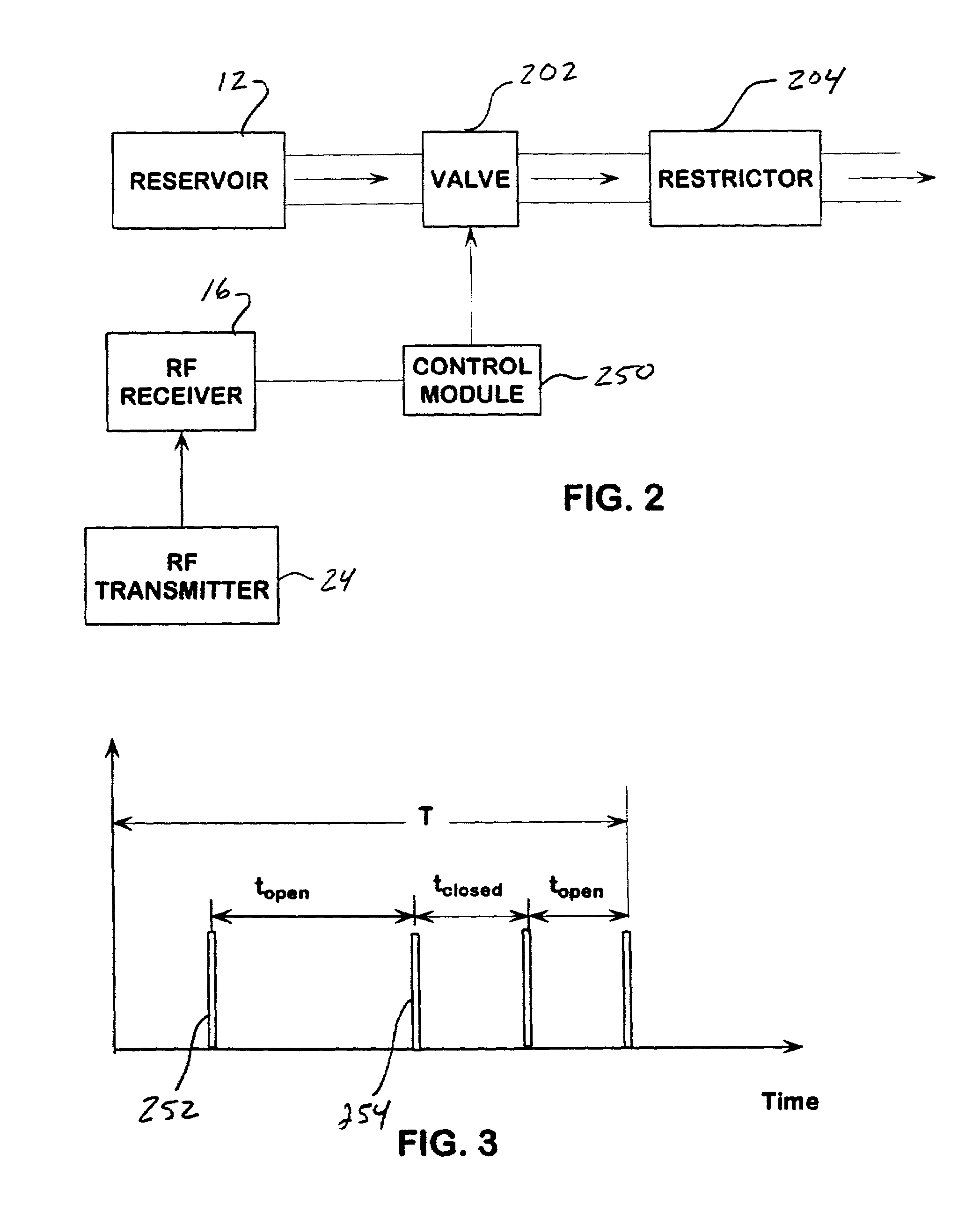
Passive flow control devices for implantable pumps
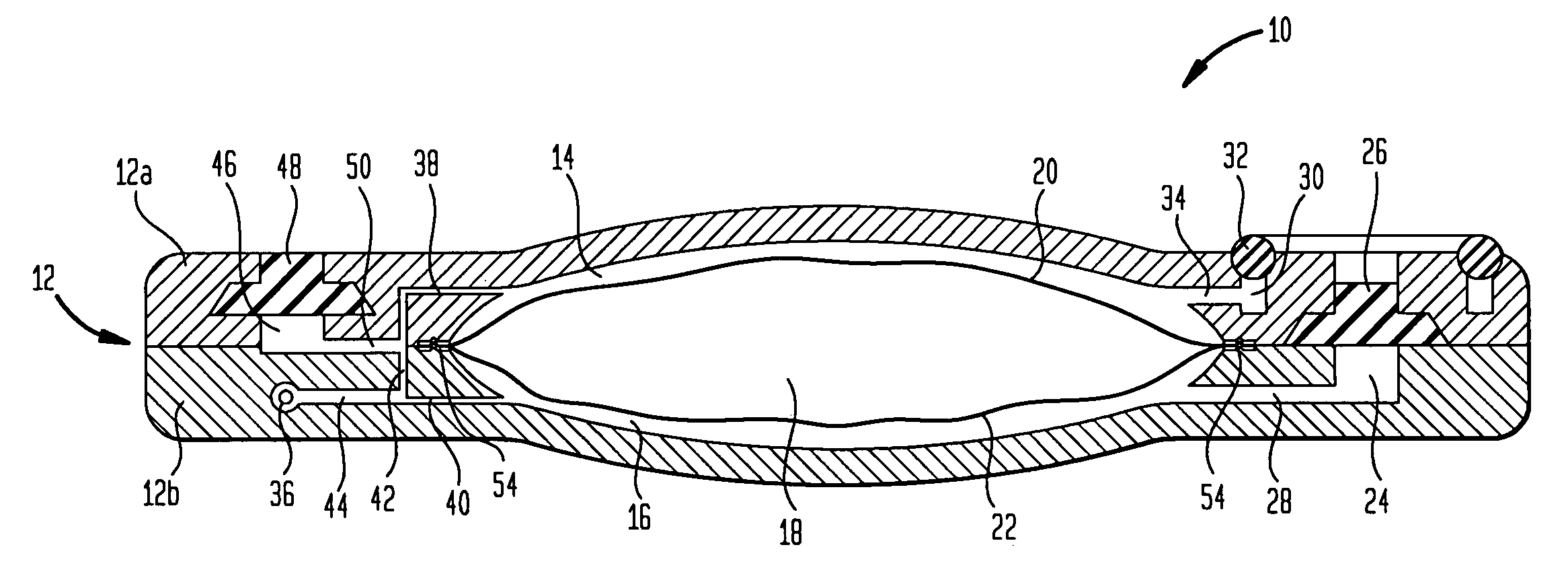
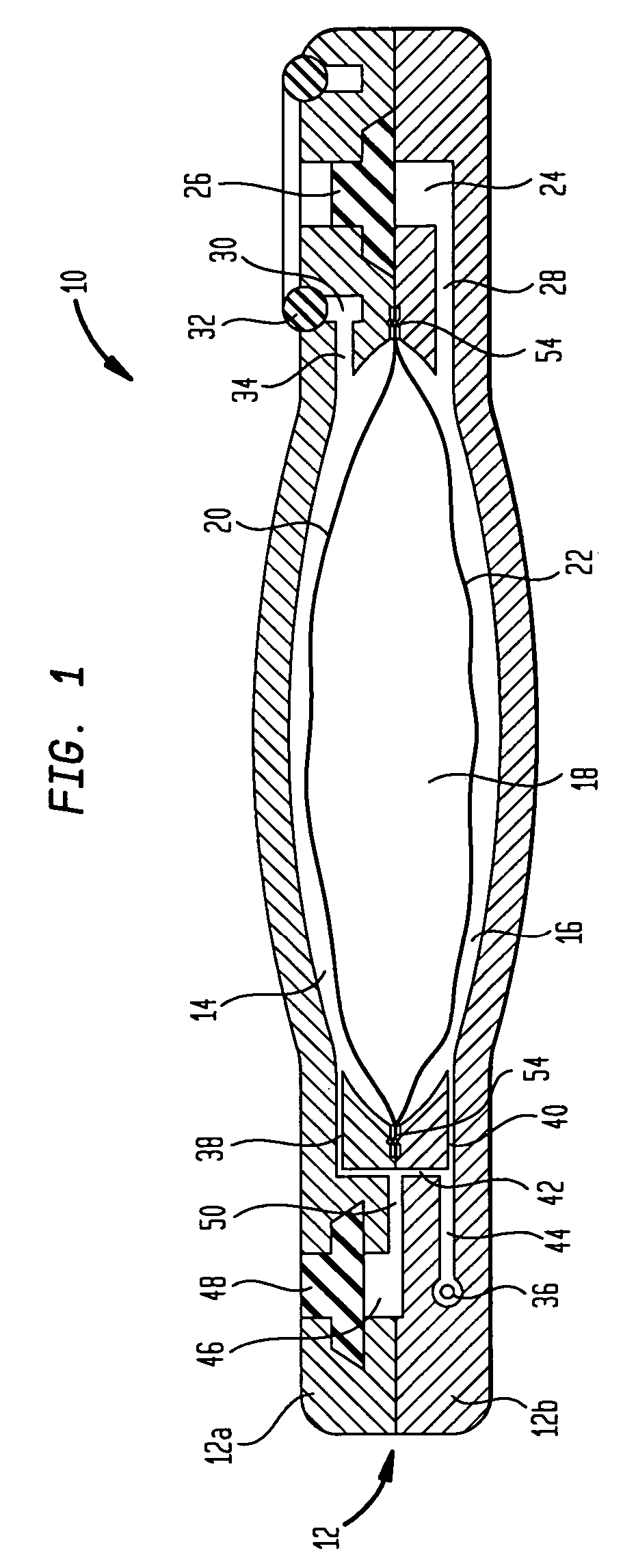
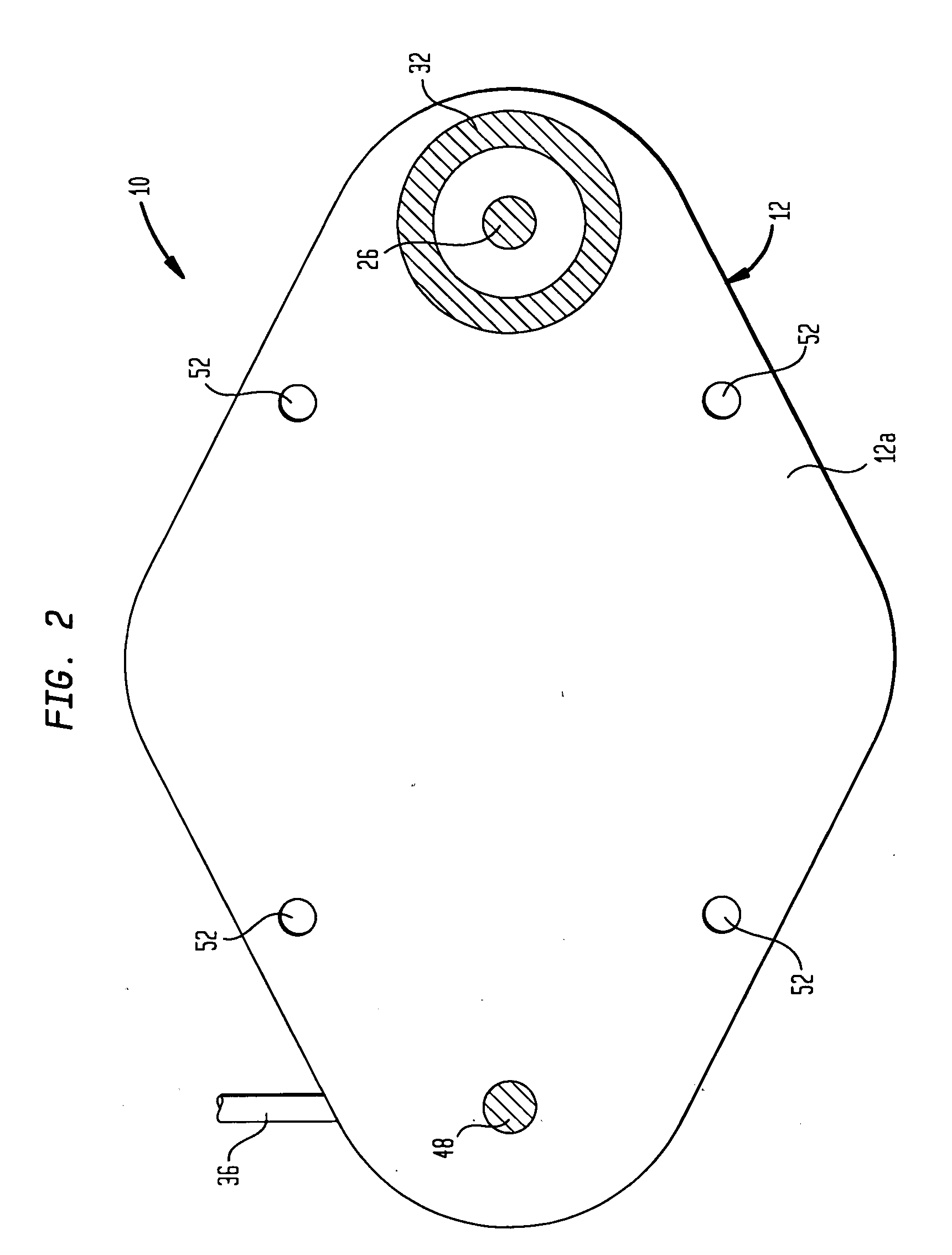
An implantable drug delivery device is provided with a passive flow control device is provided in the form of a valve which may assume two flow states. Flow control is achieved by duty cycling the valve using a control module which generates appropriate signals in response to an input telemetry signal corresponding to a desired flow rate. In another embodiment, a passively controlled bolus delivery device is provided to deliver a bolus of drug in addition to normal dosage.
Owner:MEDTRONIC INC
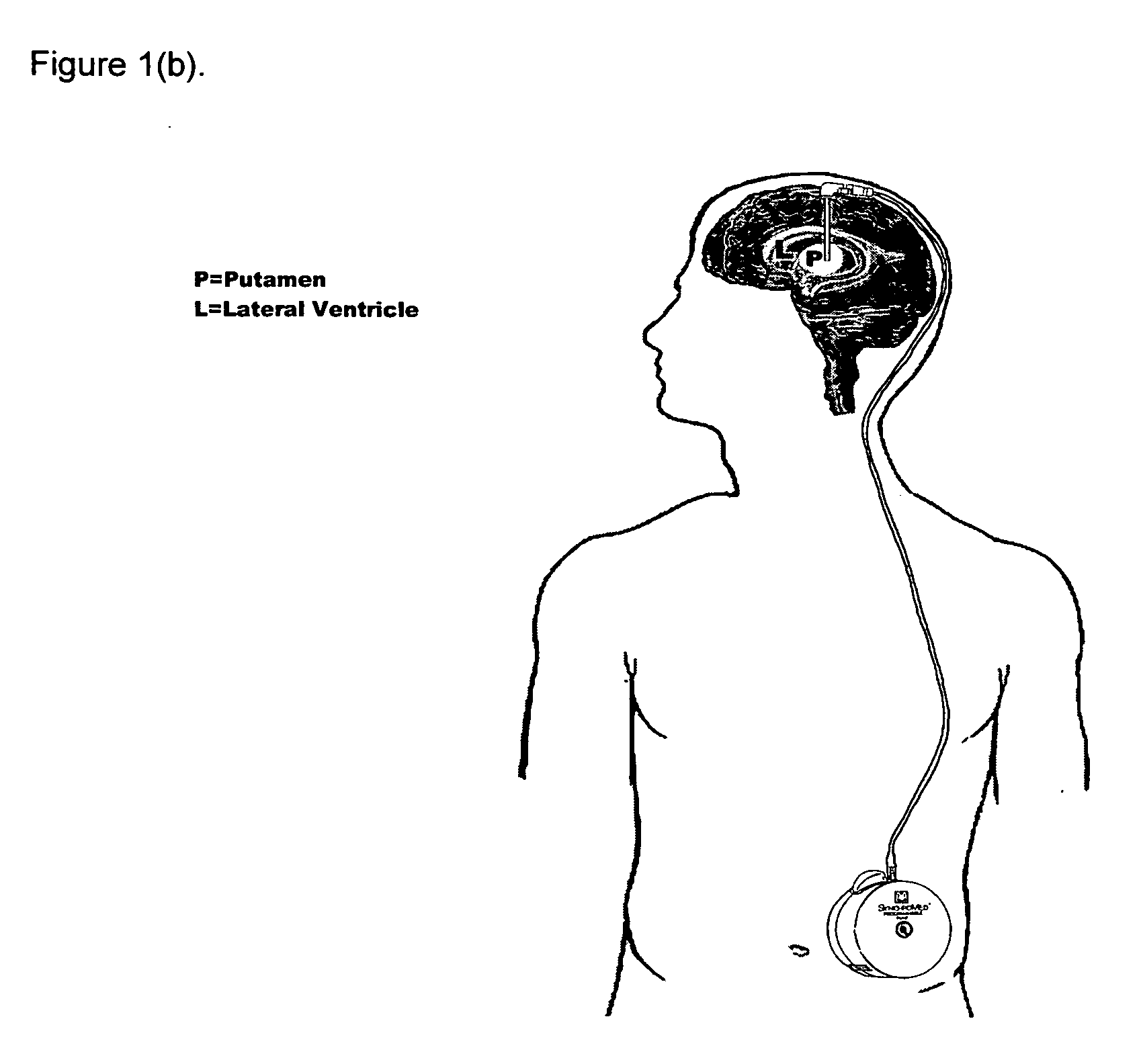
Method of treating Parkinson's disease in humans by convection-enhanced infusion of glial cell-line derived neurotrophic factor to the putamen
ActiveUS20050137134A1Impressive re-innervationDramatic effectNervous disorderPeptide/protein ingredientsGlial cell line-derived neurotrophic factorImplantable Pump
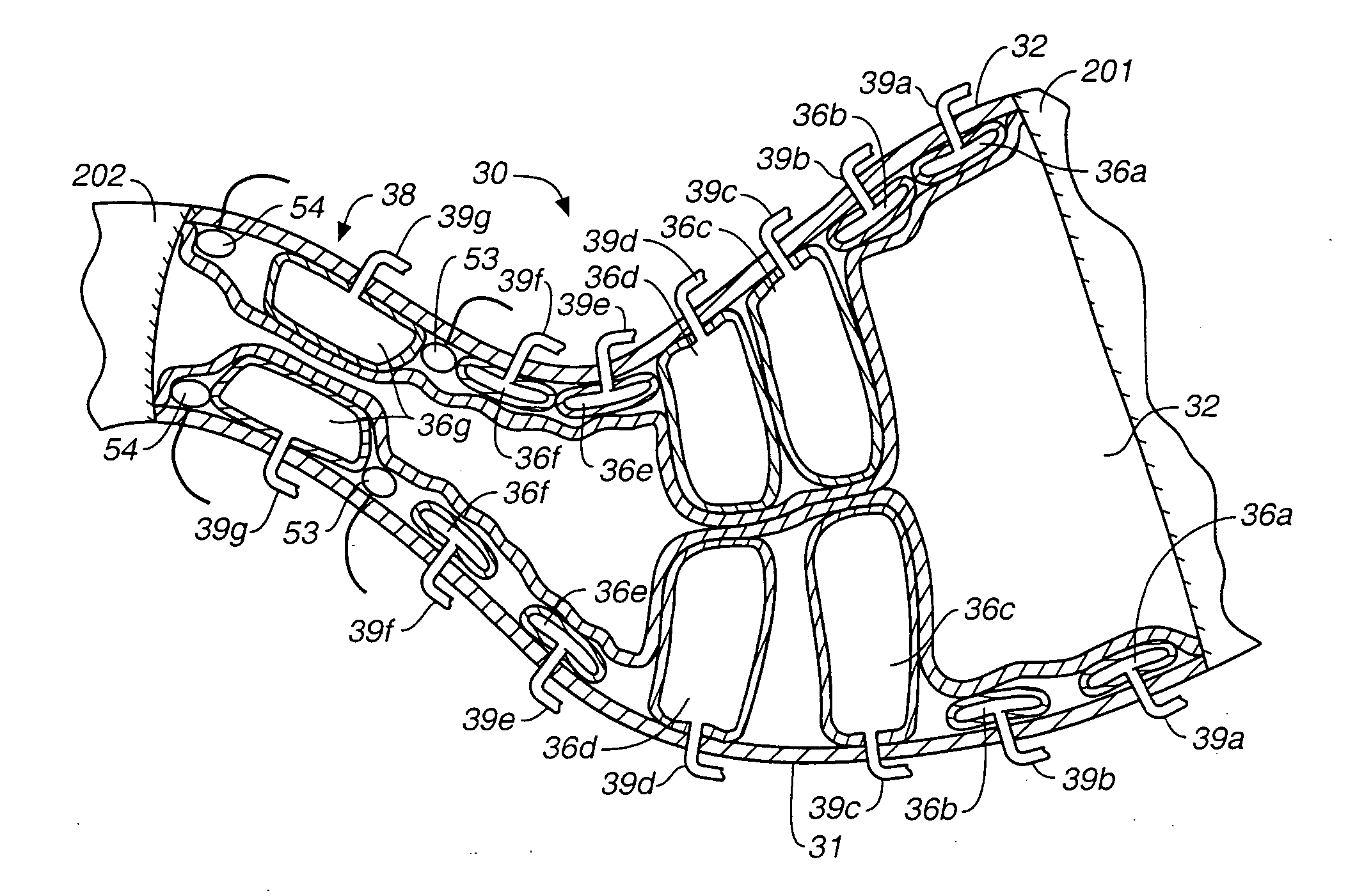
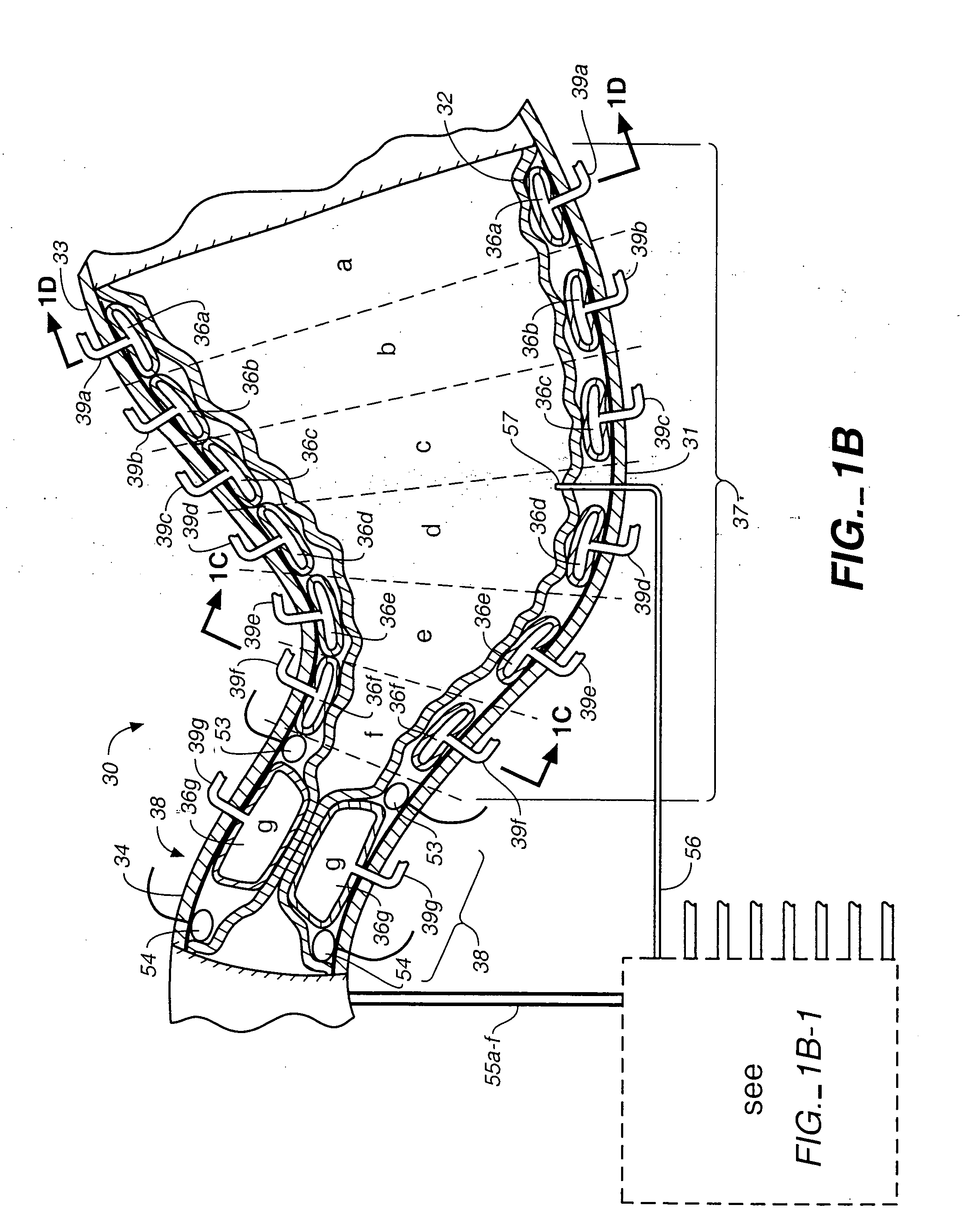
A method of treating Parkinson's disease in humans is disclosed, wherein glial cell-line derive neurotrophic factor (GDNF) is chronically administered directly to one or both putamen of a human in need of treatment thereof via convection-enhanced infusion using at least one implantable pump and at least one catheter. In one aspect of the present invention the GDNF is infused directly into one or both putamen through one or more indwelling intraparenchymal mutltiport brain catheters connected to one or moreimplantable pumps wherein the flow rate is pulsed.
Owner:AMGEN INC +1
Variable flow infusion pump system
InactiveUS20070112328A1Increase and decrease flow rateAlter shapeInfusion devicesPharmaceutical delivery mechanismEngineeringElectronics
Owner:AQULINX MEDICAL
Access port indicator for implantable medical device
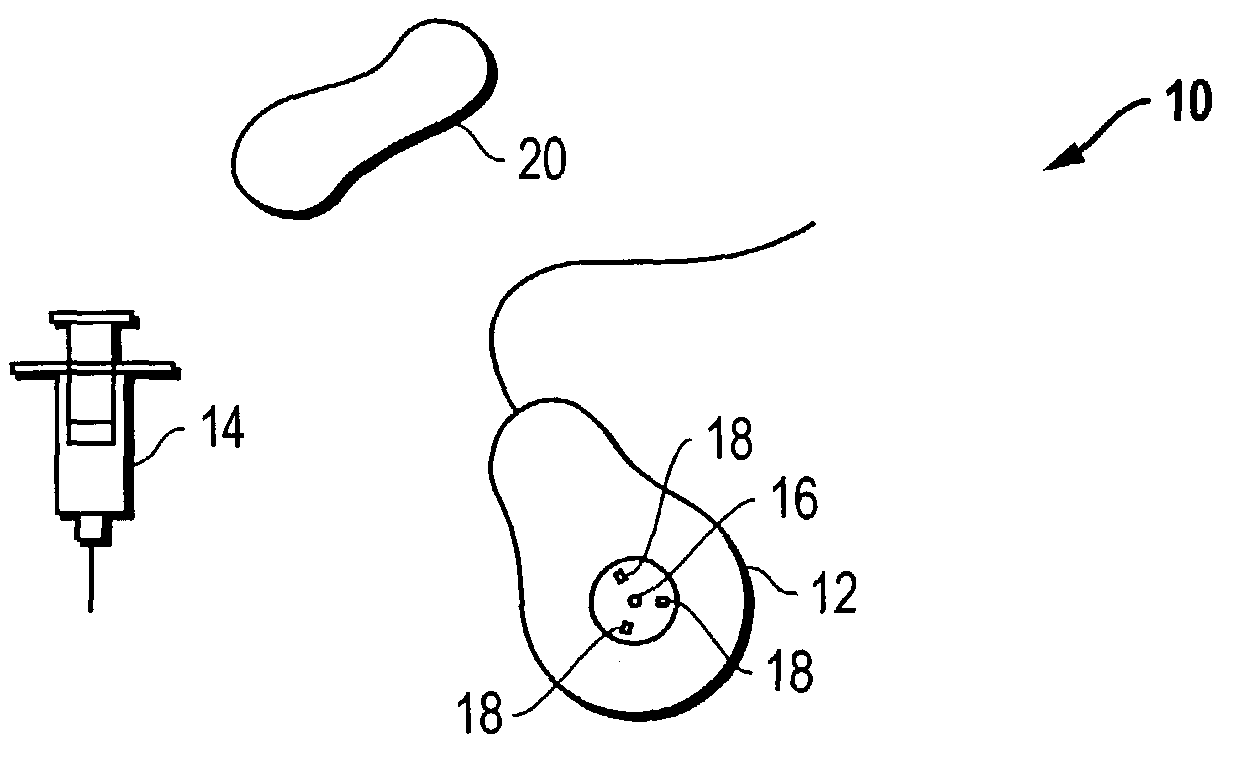
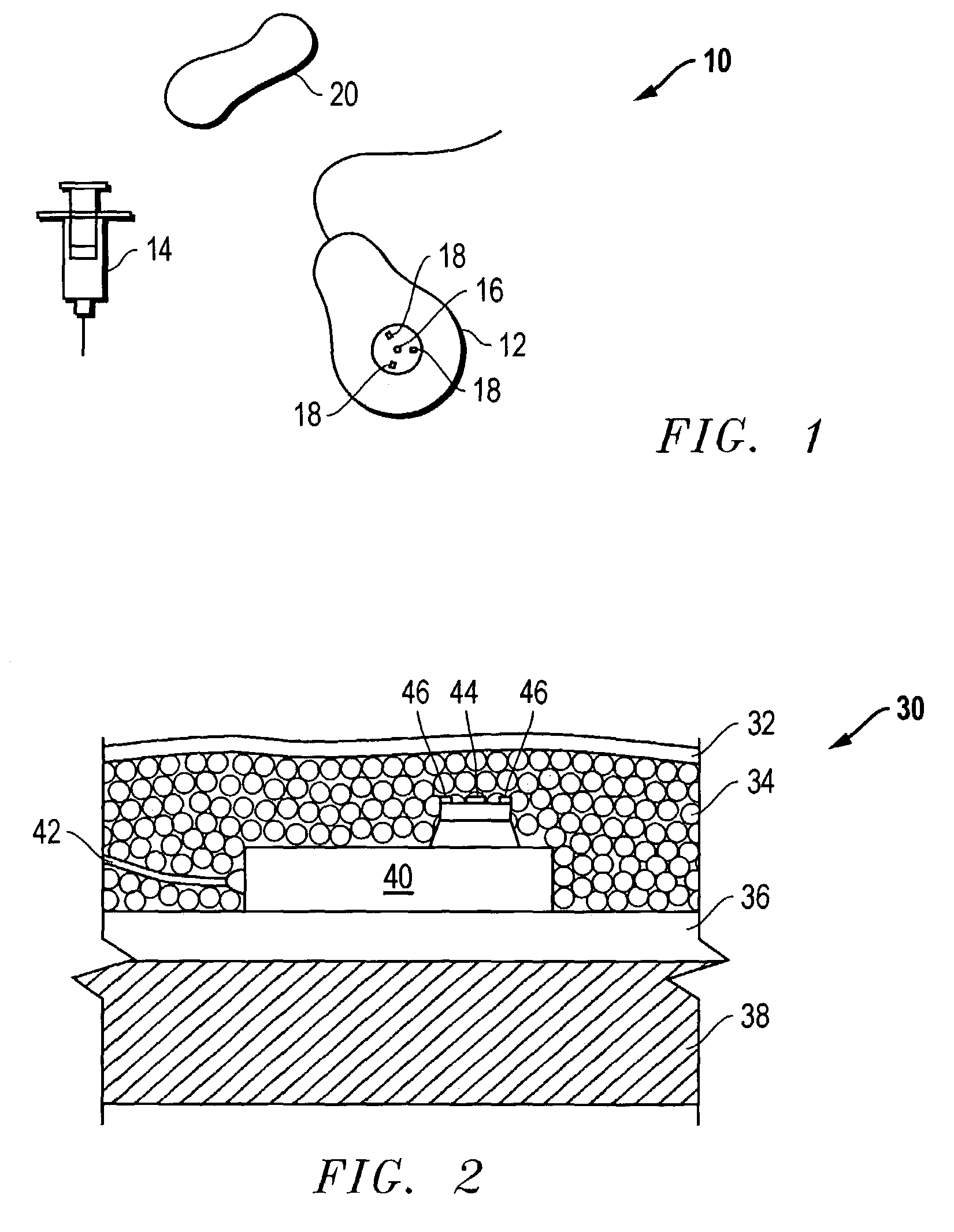
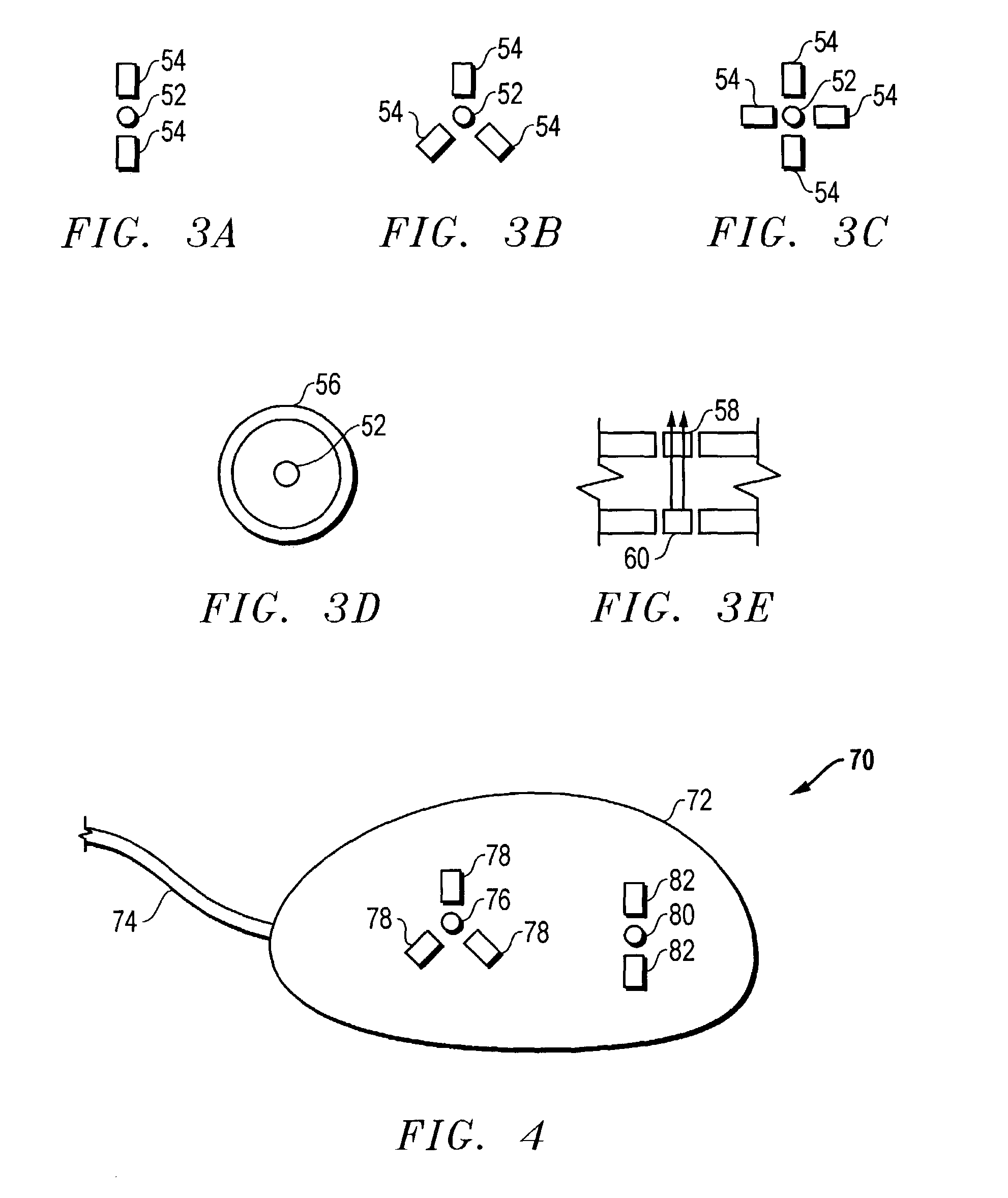
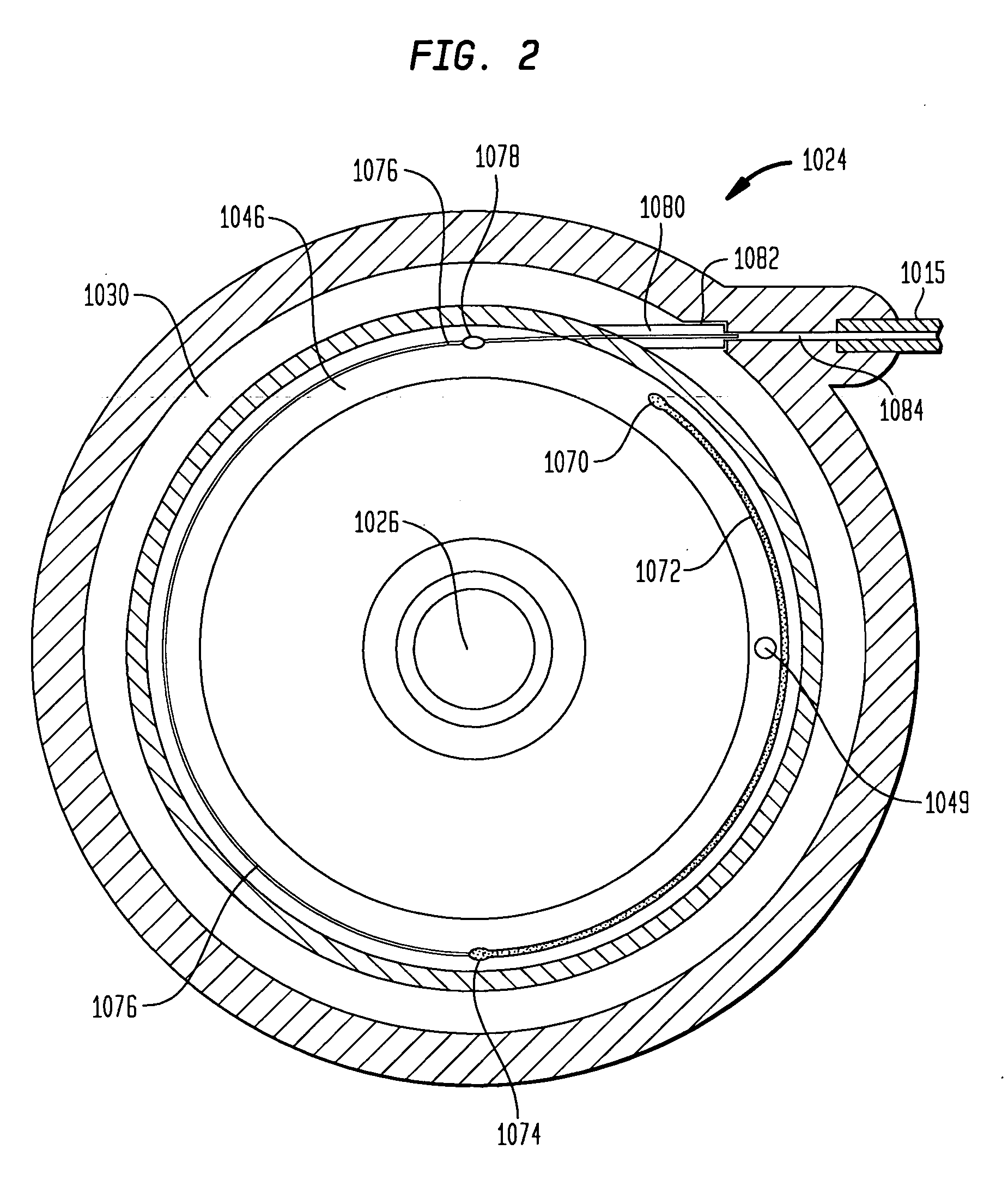
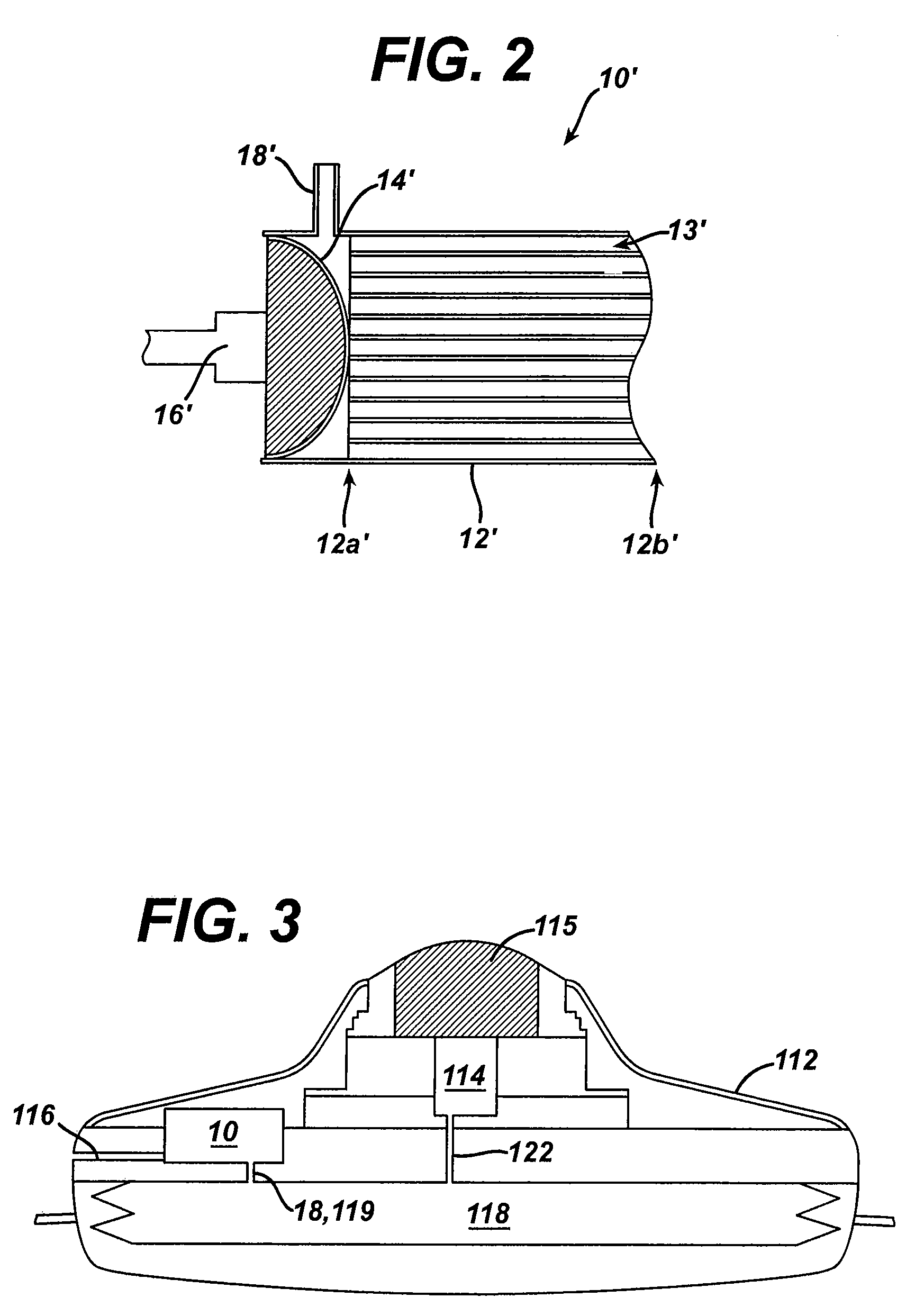
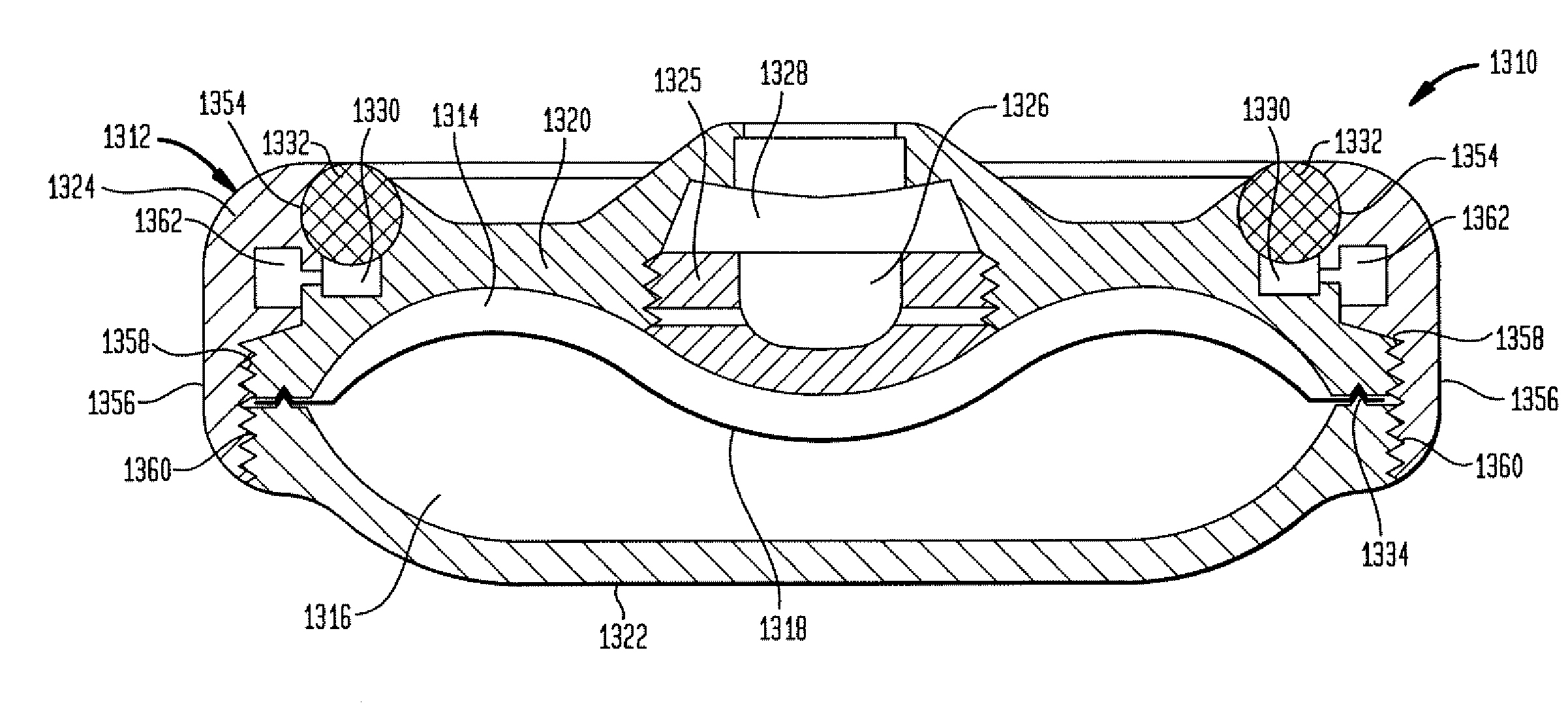
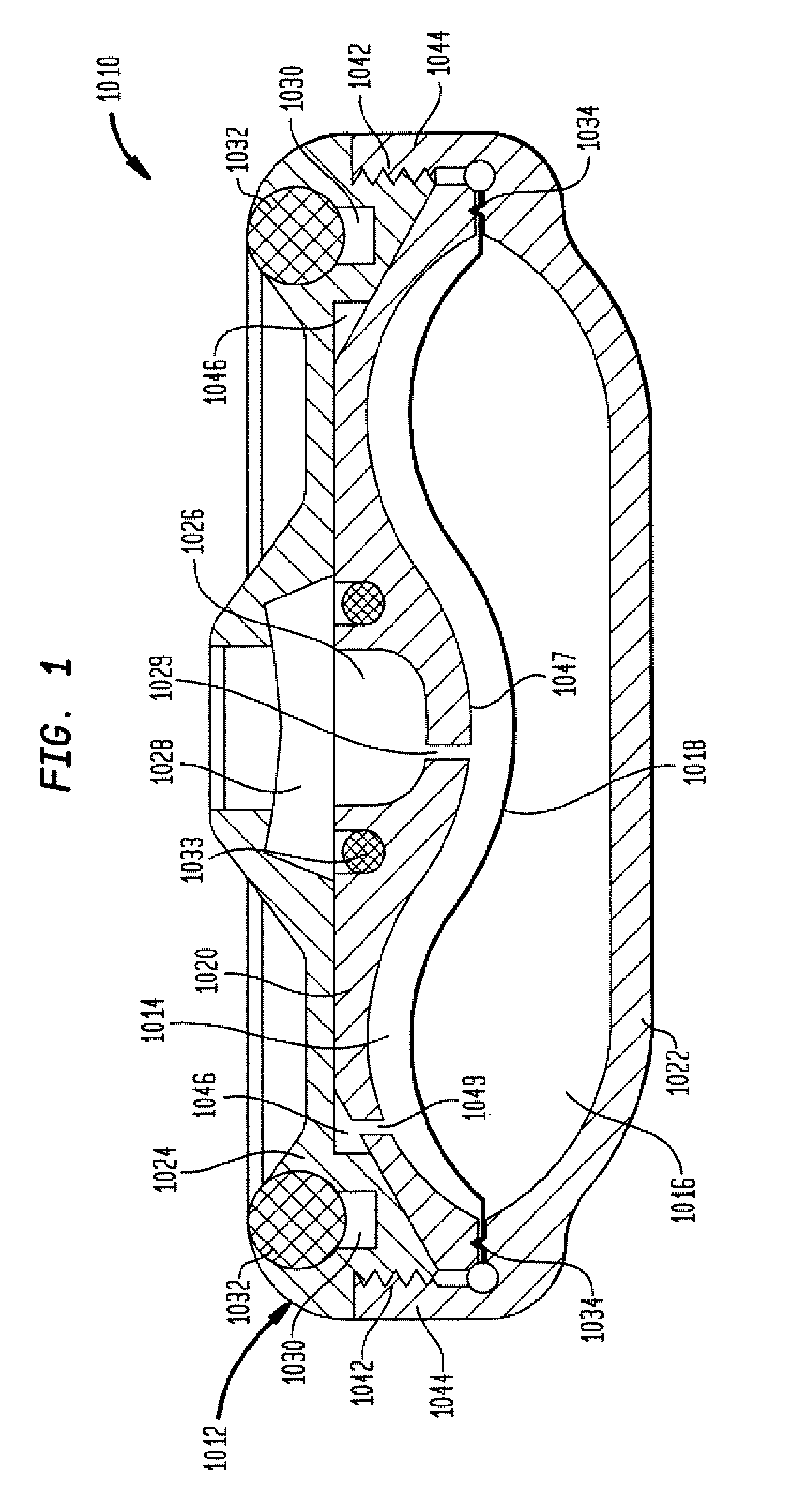
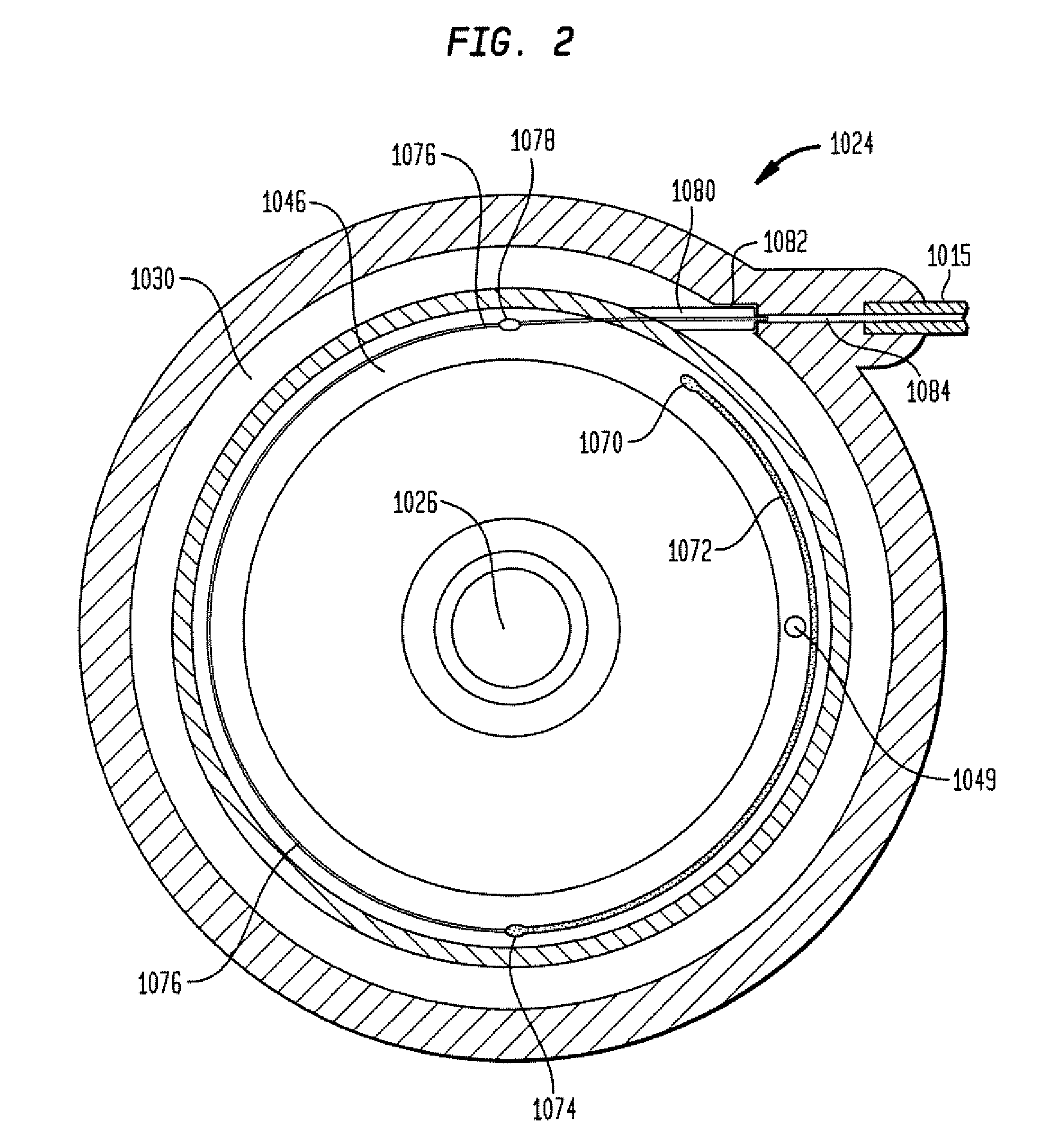
The invention is directed to an implantable pump. The implantable pump has a port with light emitters indicating the location of the port. The port can be useful in providing bolus injections or the refilling of reservoirs. The light emitters can be arranged in various forms about the port including linear, triangular, square, or circular, among others. If more than one port is located on a device, these ports can be differentiated by differing colors and arrangements of emitters. In addition, various circuitries can be used to activate the emitters. These circuitries can include a coil and capacitor arrangement that provide a separate power source from that of the pump.
Owner:ADVANCED NEUROMODULATION SYST INC
Programmable implantable pump with accessory reservoirs and multiple independent lumen catheter
An implantable pump system includes: an implantable pump having separate chambers or reservoirs; and a catheter having two or more reservoir-specific inlet ports directed into respective lumens of the catheter. In one embodiment, the distal tips of the respective lumens may be directed to different sites within the patient's body, thereby allowing site specific and independent delivery of the medications stored in the respective pump chambers or reservoirs to be administered to different body sites at independently controlled times and rates. In another embodiment, the distal tips of the respective lumens are directed, more or less, to the same body site or tissue region, thereby providing for the independent delivery of multiple medications to the same regions at independently controlled times and rates.
Owner:MEDTRONIC MIMIMED INC
Implantable pump with infinitely variable resistor
InactiveUS20060259015A1Increase and decrease flow rateAlter shapeInfusion devicesPharmaceutical delivery mechanismMembrane configurationResistor
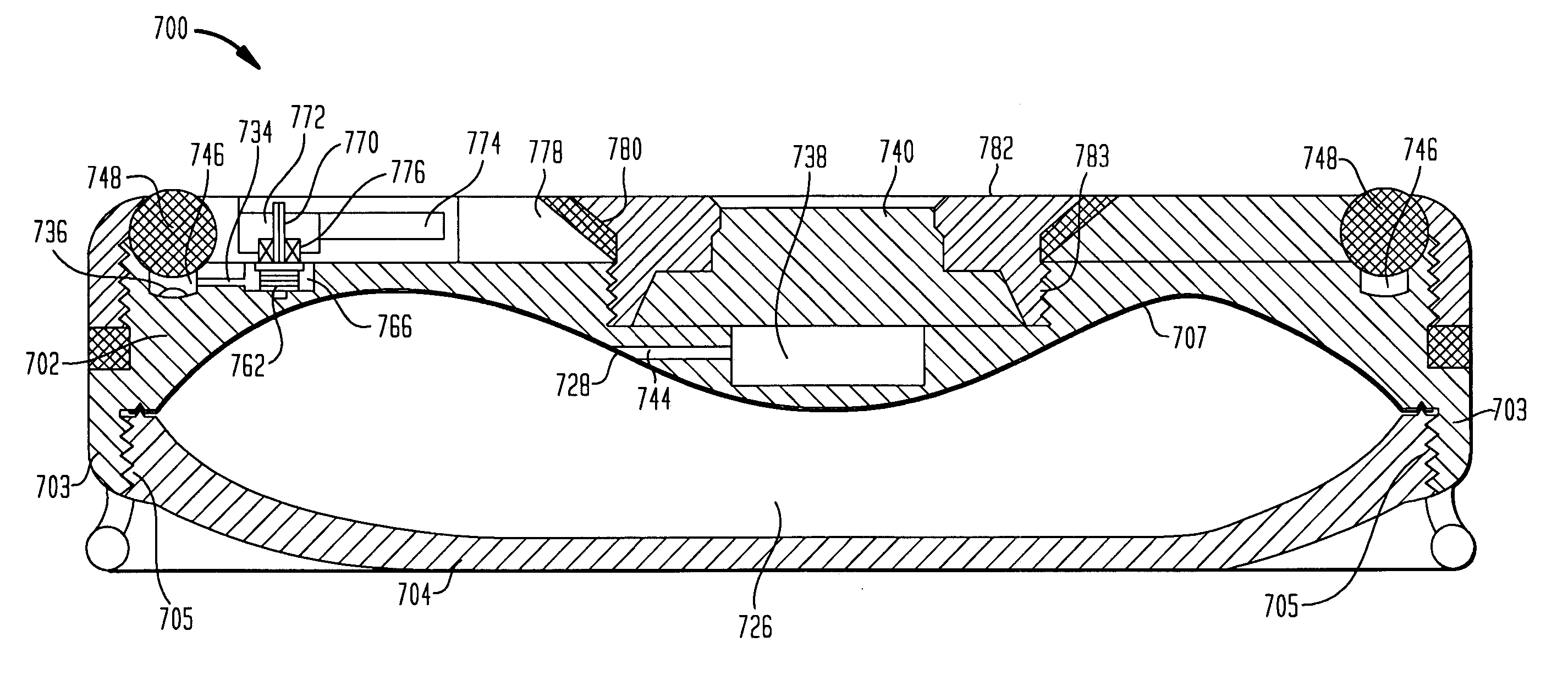
A variable hydraulic resistor for use with implantable pumps is disclosed. The variable hydraulic resistor according to the present invention is particularly useful in varying the flow rate of a medication fluid from an otherwise constant flow implantable pump. An implantable pump is also disclosed, which does not require a complicated clinching system or the like, and which may include an undulating membrane and chamber design to reduce the height of the pump.
Owner:AQULINX MEDICAL
Implantable pump apparatuses
InactiveUS20070106199A1Harvesting energyControl flowEye surgeryFlexible member pumpsDynamic motionEngineering
Owner:SOLTANPOUR DAVID P
Stomach peristalsis device and method
InactiveUS20060129237A1Facilitate expedite mixing breaking downPassage is slowedLigamentsMusclesPylorusPeristalsis
The invention relates to an implantable stomach prosthesis for surgically replacing or augmenting all or part of the antrum and / or pylorus of a stomach. The prosthesis controls the passage of food from the stomach to the small intestine. The prosthesis may be configured to chum ingested material and release it from the stomach through a prosthetic pyloric valve. At least one expandable member is arranged to be expanded to control the passage of food and / or to mimic the churning action of a patient's stomach. The prosthesis includes an outer support structure, a flexible inner member forming a conduit for the movement of material, and at least one expandable member located between the outer support structure and inner member. An implantable pump system is provided for inflating and deflating the expandable member(s).
Owner:PYTHON MEDICAL
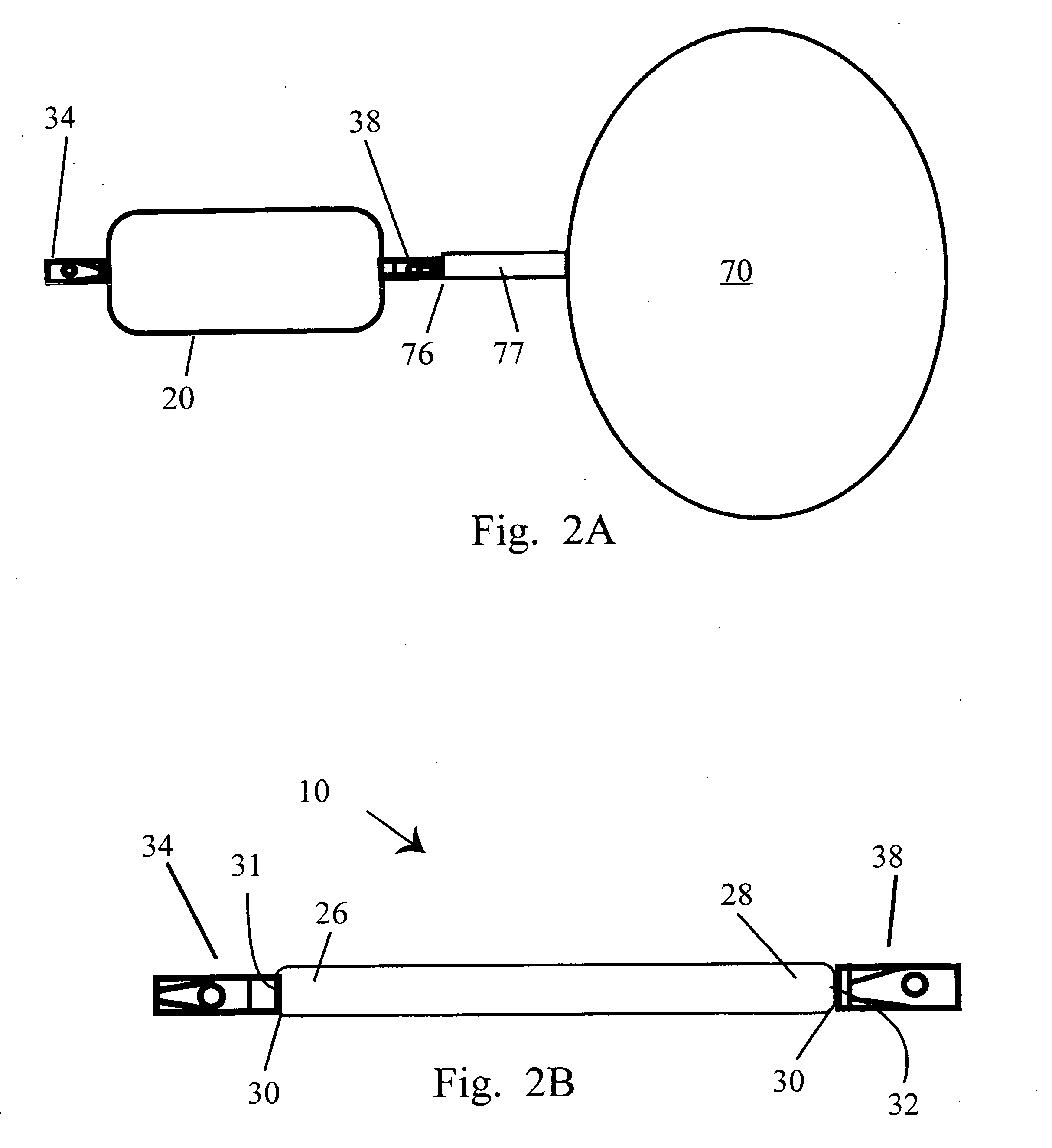
Automatically adjusting band system with MEMS pump
Devices and methods for forming a restriction in a patient are disclosed. In one exemplary embodiment, a restriction system is provided including an implantable restriction device, an implantable port in fluid communication with the implantable restriction device, and an implantable pump in fluid communication with the restriction device. In general, the implantable restriction device is adjustable and configured to form a restriction in a patient, and the implantable port is configured to receive fluid from a fluid source external to the patient. The implantable pump is a micro-electro-mechanical systems (MEMS) device effective to create pumping action to move fluid through the pump.
Owner:ETHICON ENDO SURGERY INC
Automatically adjusting band system
Devices and methods for forming a restriction in a patient are disclosed. In one exemplary embodiment, a restriction system is provided including an implantable restriction device, an implantable port in fluid communication with the implantable restriction device, and an implantable pump in fluid communication with the restriction device. In general, the implantable restriction device is adjustable and configured to form a restriction in a patient, and the implantable port is configured to receive fluid from a fluid source external to the patient. The implantable pump has a plurality of actuators configured to change shape upon the application of energy thereto such that sequential activation of the plurality of actuators is effective to create pumping action to move fluid through the pump.
Owner:ETHICON ENDO SURGERY INC
Cognitive function within a human brain
ActiveUS20070060974A1Improve cognitive functionHead electrodesWound drainsHuman givensImplanted device
Methods and apparatus for improving cognitive function within a human. The invention utilizes an implanted device, such as an implantable signal generator or an implantable pump, to affect tissue elements within a Papez circuit of the human brain as well as tissue upstream or downstream from the Papez circuit. The implanted device delivers treatment therapy to thereby improve cognitive function by the human. A sensor may be used to detect various symptoms of the cognitive disorder. A microprocessor algorithm may then analyze the output from the sensor to regulate delivery of the stimulation and / or drug therapy.
Owner:FUNCTIONAL NEUROMODULATION
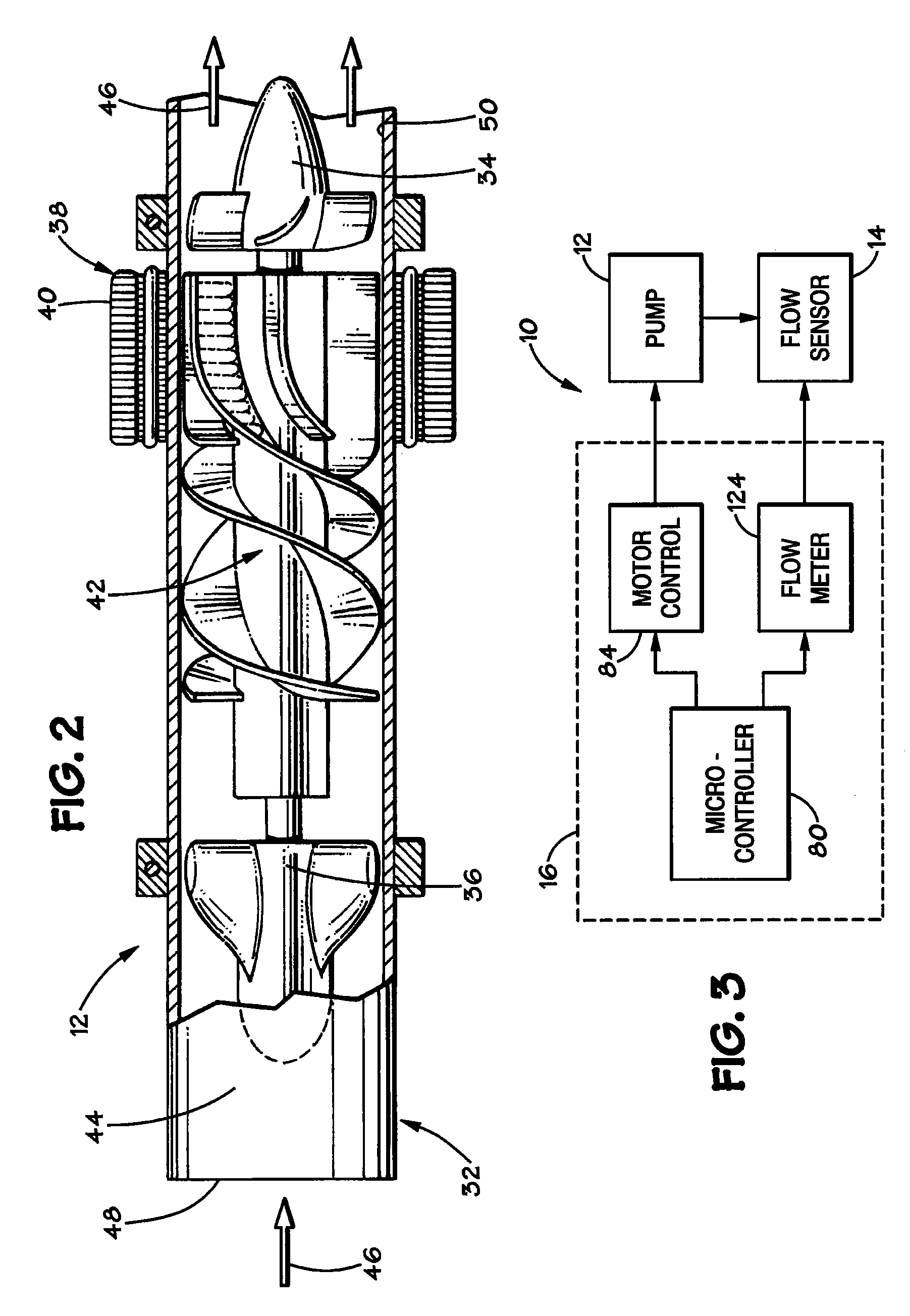
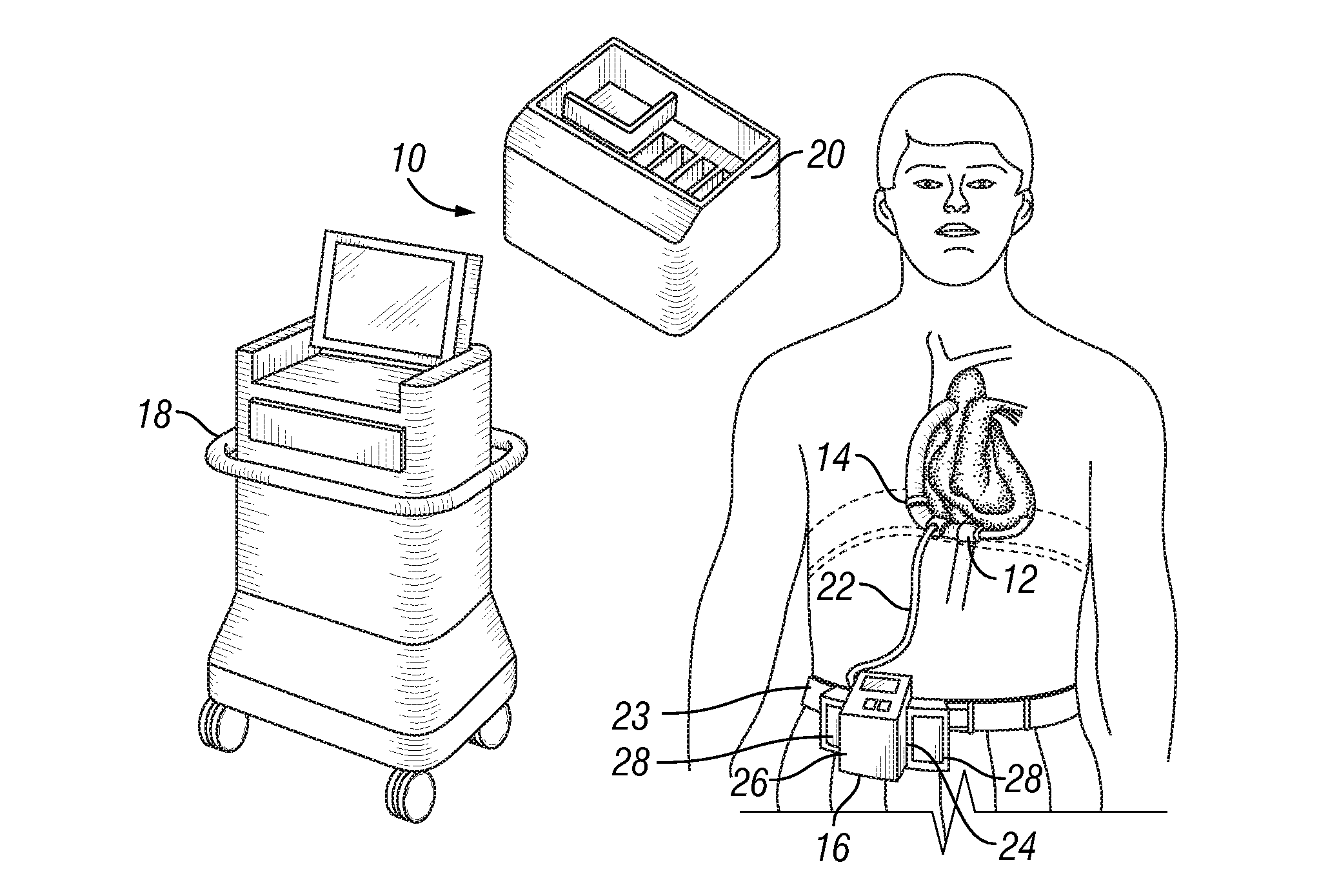
Blood pump system
A blood pump system and methods for the use and operation of such a blood system is described, wherein the blood pump system includes an implantable pump and an implantable flow measurement device. A processing device receives indications of a number of pump parameters such as pump voltage, pump current and pump speed. Flow rate is determined based on the pump parameters, and this determined flow rate is compared to the actual flow rate as measured by the flow measurement device. In certain embodiments, the flow measurement device may be periodically energized to make the comparison, then powered off to reduce power consumption. The time period in which the flow measurement device is powered off is based on the difference between the determined and the actual flow rates.
Owner:RELIANTHEART
Implantable pump with adjustable flow rate
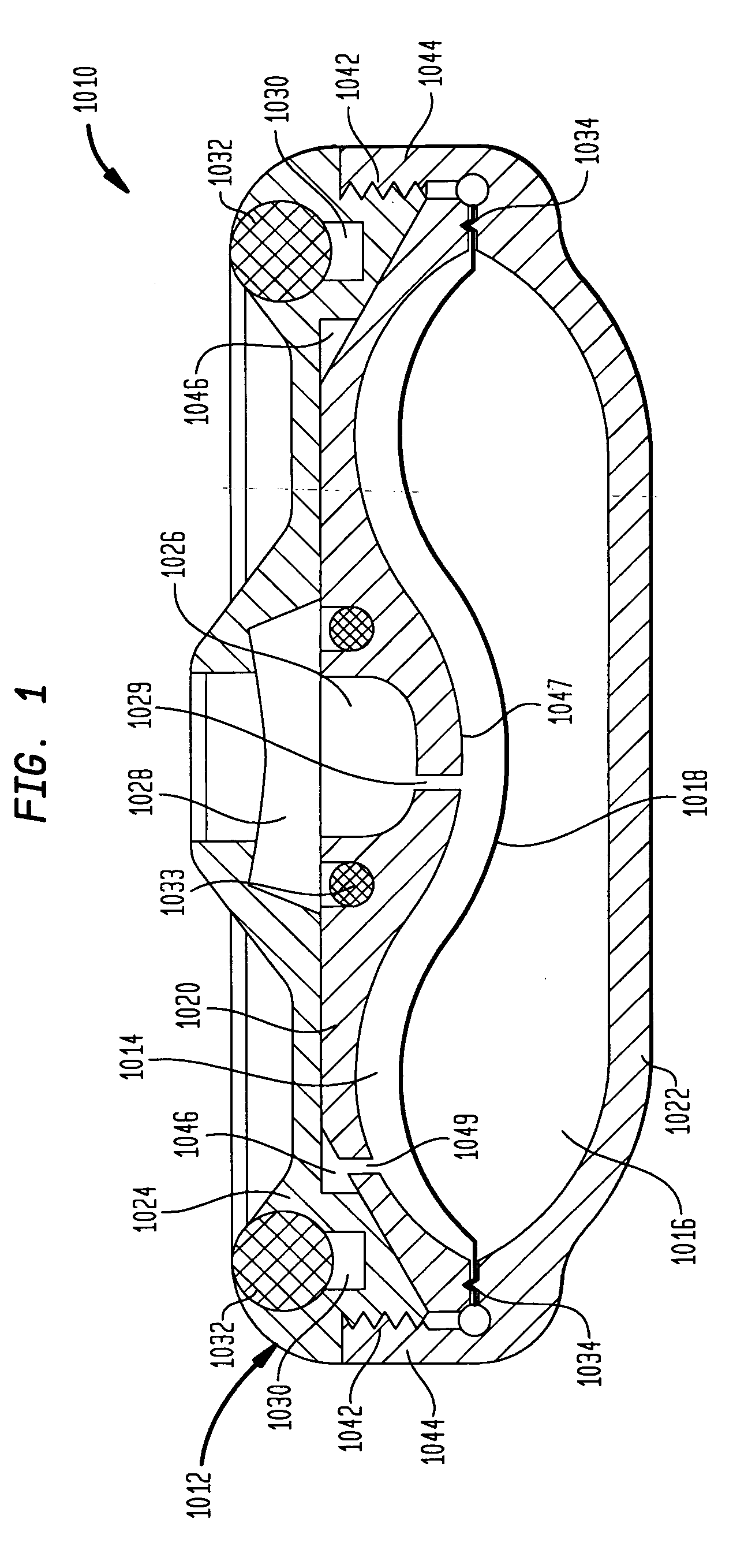
ActiveUS7367968B2Selection is limitedFlow adjustableDiaphragm valvesEngine diaphragmsStreamflowVALVE PORT
A valve that is adapted to control the flow rate of fluid flow from an implantable pump or other fluid delivery device is provided. In general, the valve includes a multi-lumen member that is adapted to receive fluid-flow therethrough, and a restrictor member that is coupled to the multi-lumen member such that the restrictor member is effective to selectively restrict at least a portion of one or more lumens in the multi-lumen member to thereby adjust the flow rate of fluid flowing through the multi-lumen member. The valve can be built into an implantable drug pump to control fluid flow exiting the pump, or alternatively the valve can disposed within a catheter or otherwise coupled to an outlet port in an implantable drug pump to control the flow rate of fluid exiting the drug pump.
Owner:CODMAN & SHURTLEFF INC
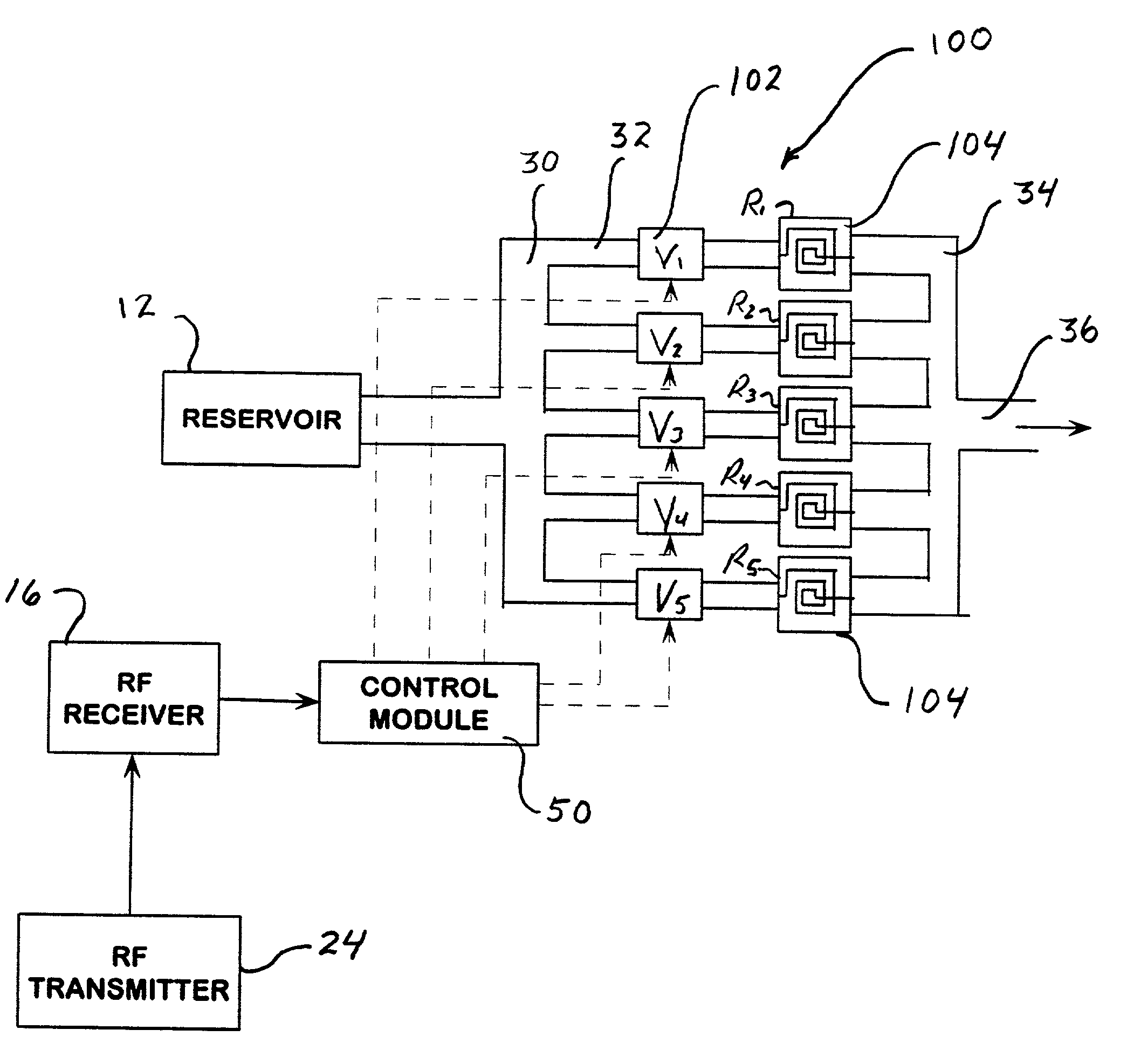
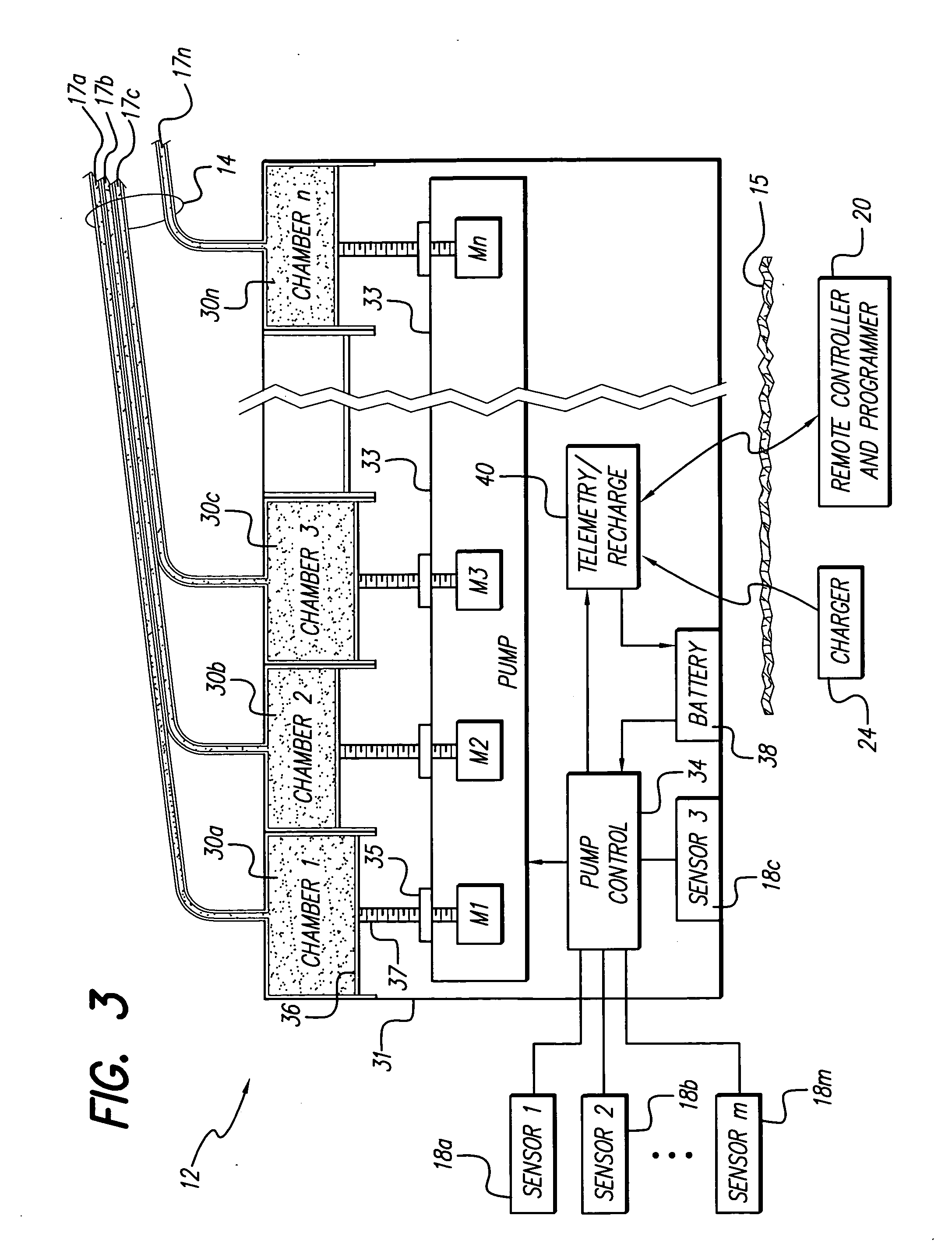
Multi-reservoir implantable pump with variable flow rate capabilities
InactiveUS20060271022A1Avoid medicationPharmaceutical delivery mechanismMedical devicesTraffic volumeImplantable Pump
A multiple reservoir or chambered implantable pumps is disclosed. The pump according to the present invention is particularly useful in allowing for multiple constant flow rates to be provided from an otherwise constant flow implantable pump. The pump is also useful in allowing for housing of multiple active substances. A multiple reservoir implantable pump is also disclosed, which has at least one chamber capable of providing a constant flow rate and at least one chamber capable of being utilized for patient controlled injections.
Owner:AQULINX MEDICAL
Variable flow infusion pump system
InactiveUS20100069892A1Increase and decrease flow rateFacilitate communicationInfusion devicesMedical devicesElectronicsElectronic equipment
An implantable infusion pump system is disclosed. The pump system preferably includes an implantable pump and a removable module. The module may provide for varying flow rates of fluid being dispensed from the pump or may provide for a constant flow rate of such fluid. In the case of varying flow rate capabilities, the module preferably includes one or more sensors to determine information relating to the flow rate, electronics for analyzing the flow rate information, and a mechanism for physically altering the flow rate. Methods of dispensing a medicament to a patient are also disclosed, as are variations of the pump system.
Owner:AQULINX MEDICAL
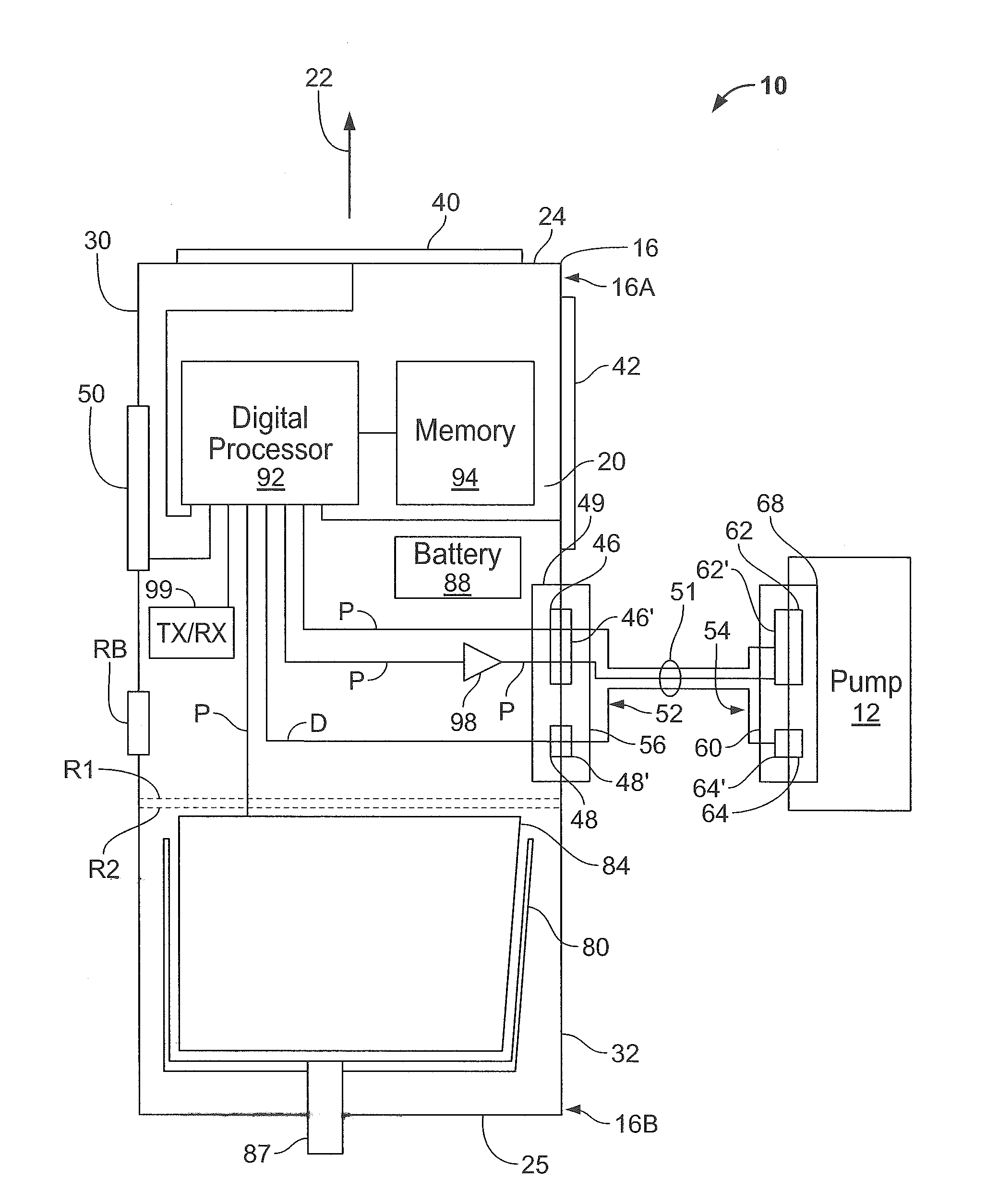
Controller and power source for implantable blood pump
ActiveUS20140194985A1Loss in efficiencyDigital data processing detailsControl devicesControl signalEmbedded system
Methods and apparatus for controlling the operation of, and providing power for and to, implantable ventricular assist devices which include a brushless DC motor-driven blood pump, are disclosed. In one embodiment, a control system for driving an implantable pump is provided. The digital processor is responsive to data associated with the operation of the pump received at the data transfer port, and from the program data stored in memory, (i) to determine therefrom, the identity of the pump, (ii) to determine therefrom, electrical characteristics and features of the identified pump, and (iii) to adaptively generate and apply to the data port, control signals for driving the identified pump. Latch mechanisms, an elongated flexible electrical cable with a strain relief segment, and a lower housing portion that is heavier than an upper housing portion, may also be provided with the control system.
Owner:HEARTWARE INC
Implantable pump with adjustable flow rate
ActiveUS20050054988A1Control flowSelection is limitedDiaphragm valvesEngine diaphragmsStreamflowVALVE PORT
A valve that is adapted to control the flow rate of fluid flow from an implantable pump or other fluid delivery device is provided. In general, the valve includes a multi-lumen member that is adapted to receive fluid-flow therethrough, and a restrictor member that is coupled to the multi-lumen member such that the restrictor member is effective to selectively restrict at least a portion of one or more lumens in the multi-lumen member to thereby adjust the flow rate of fluid flowing through the multi-lumen member. The valve can be built into an implantable drug pump to control fluid flow exiting the pump, or alternatively the valve can disposed within a catheter or otherwise coupled to an outlet port in an implantable drug pump to control the flow rate of fluid exiting the drug pump.
Owner:CODMAN & SHURTLEFF INC
Implantable pump
InactiveUS6991601B2Improved protrusion and groove systemFirmly graspTubular organ implantsNon-surgical orthopedic devicesImplantable Pump
Owner:BOSTON SCI SCIMED INC
Implantable digestive tract organ
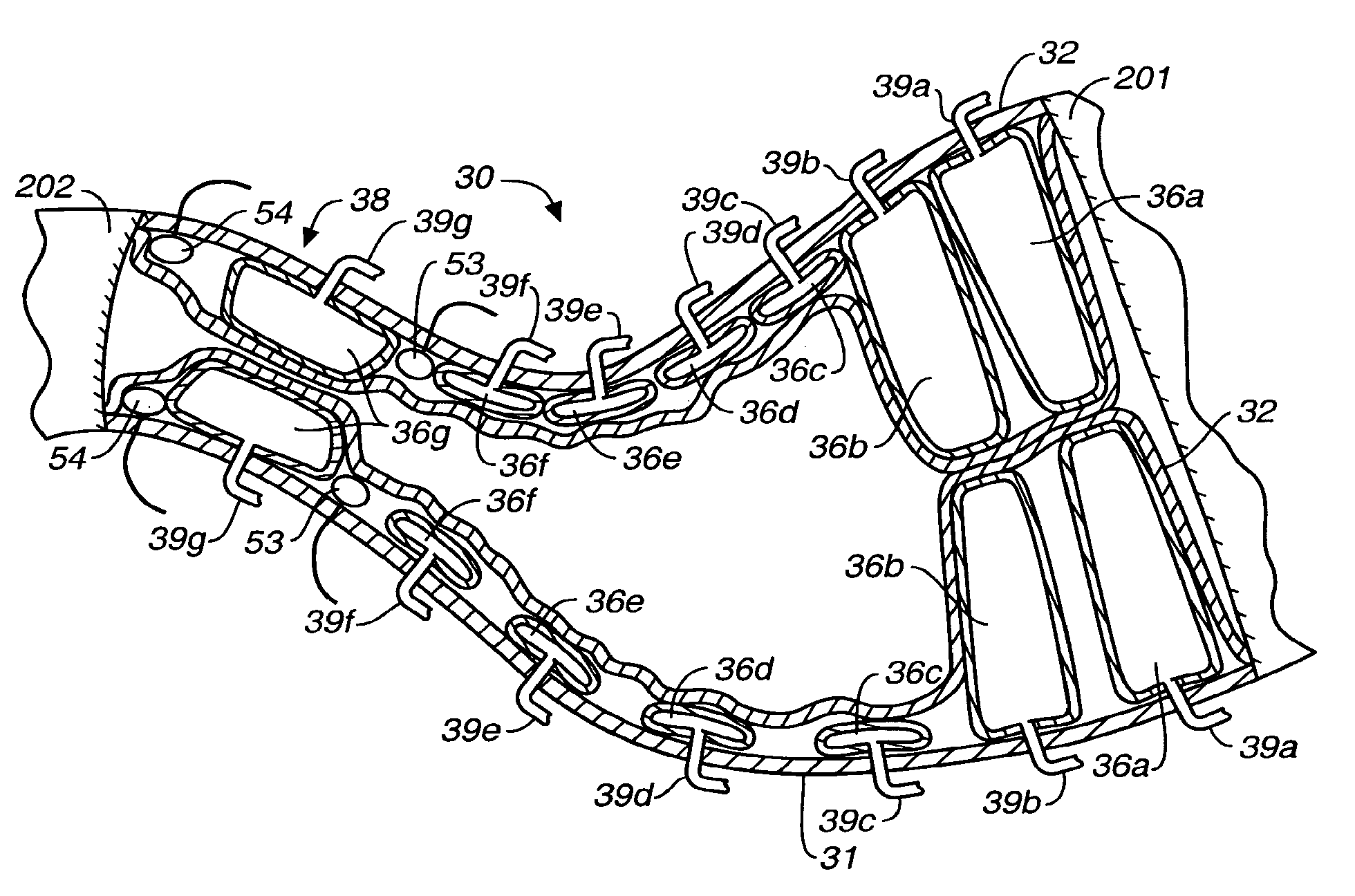
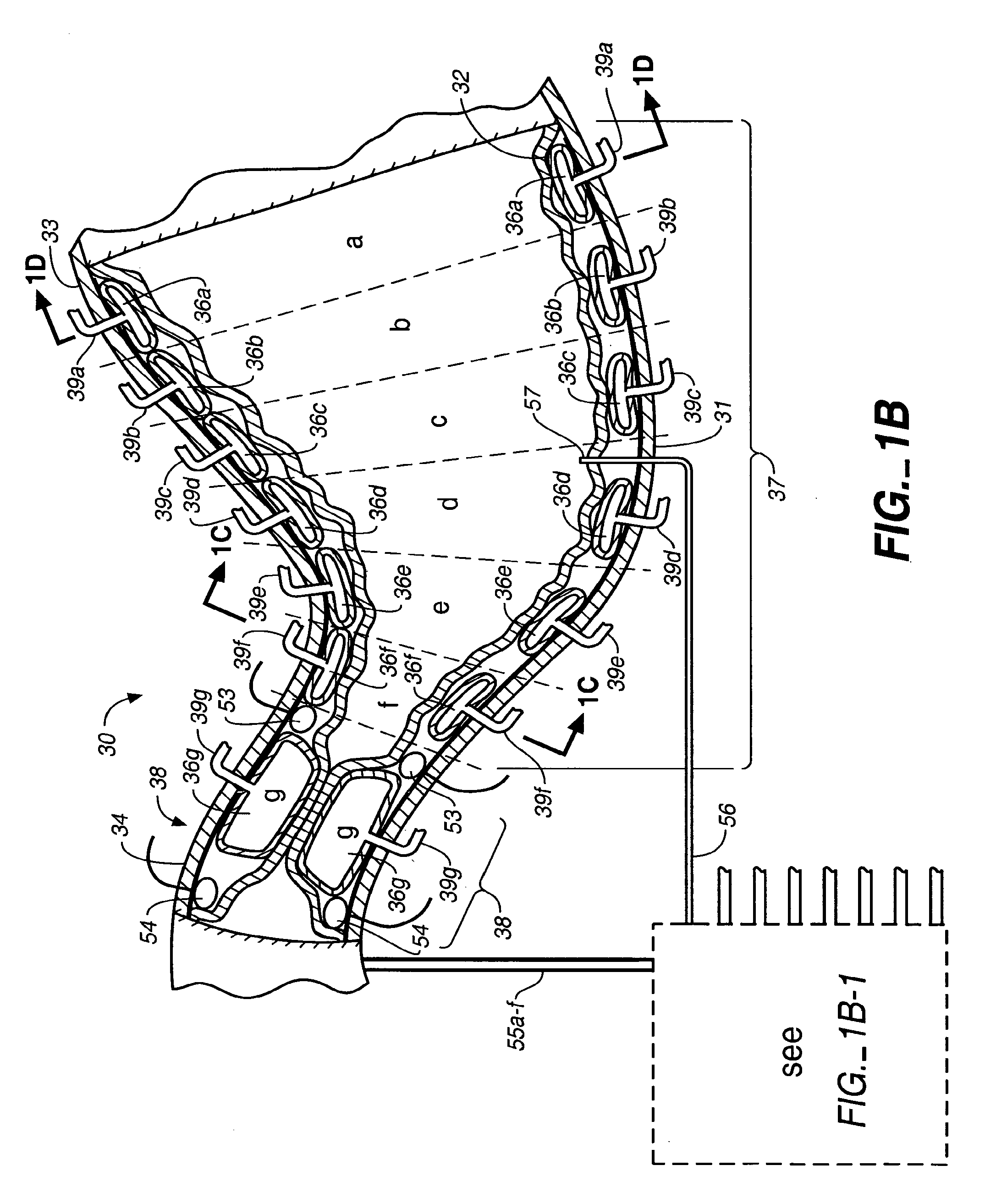
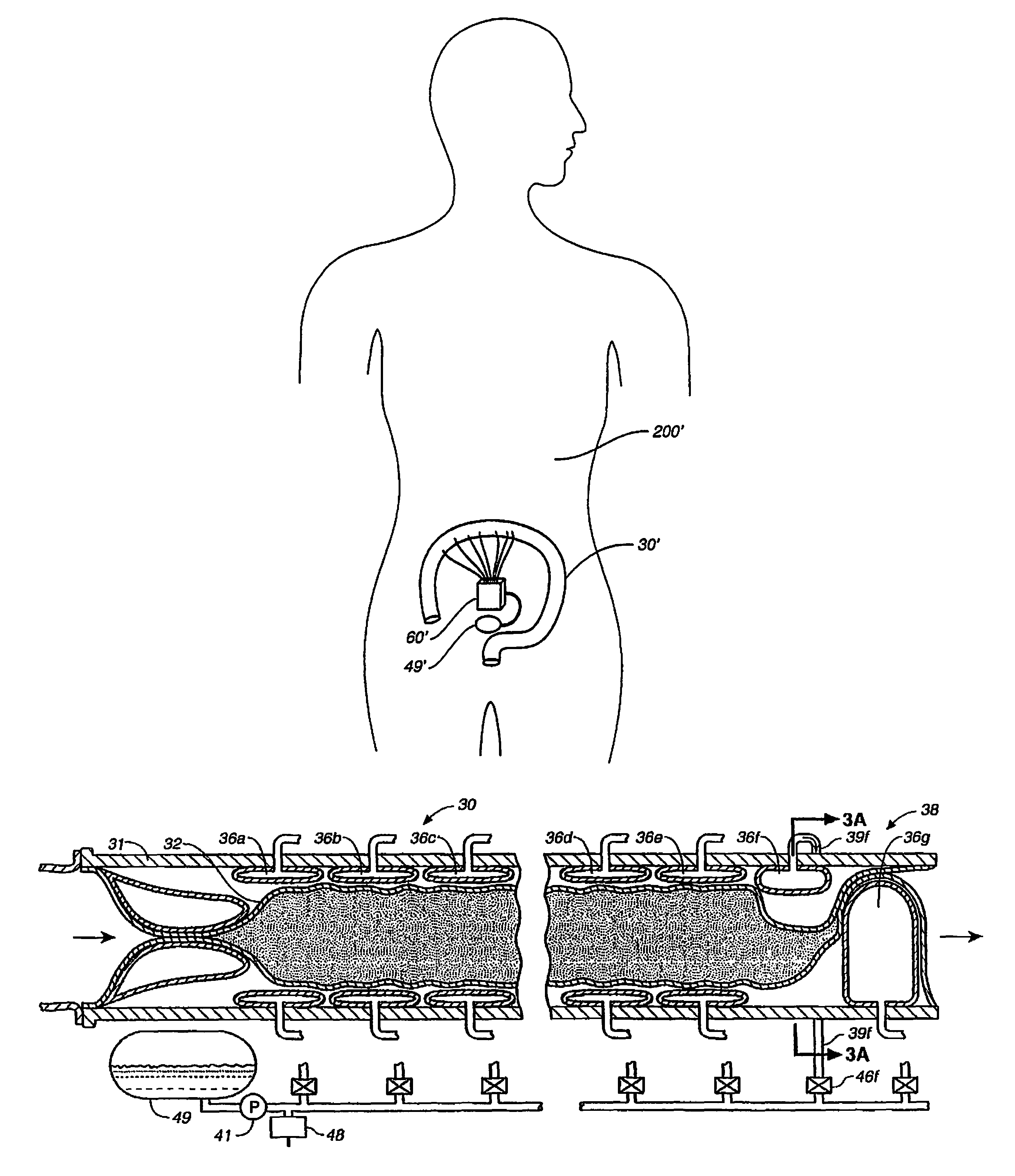
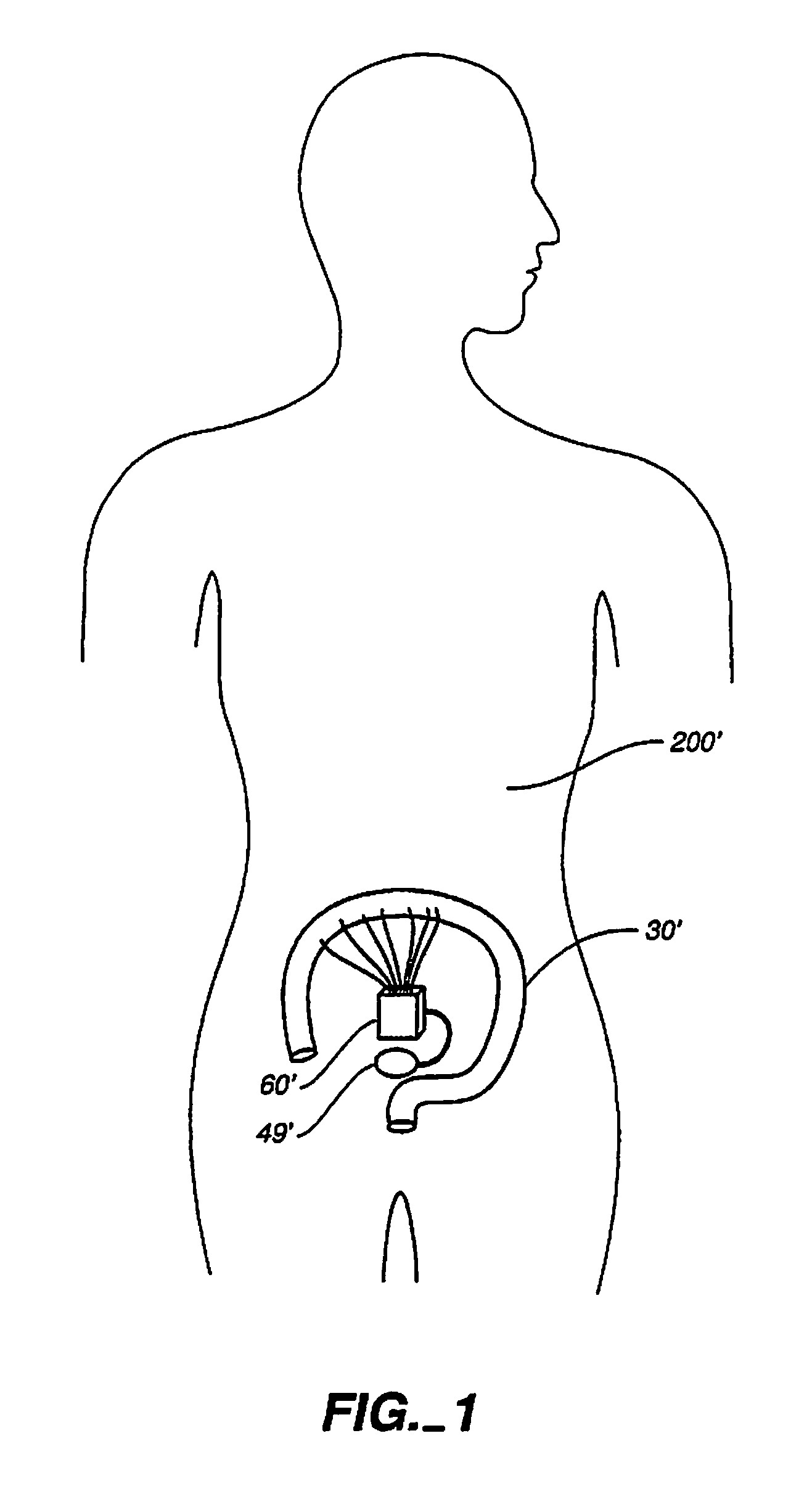
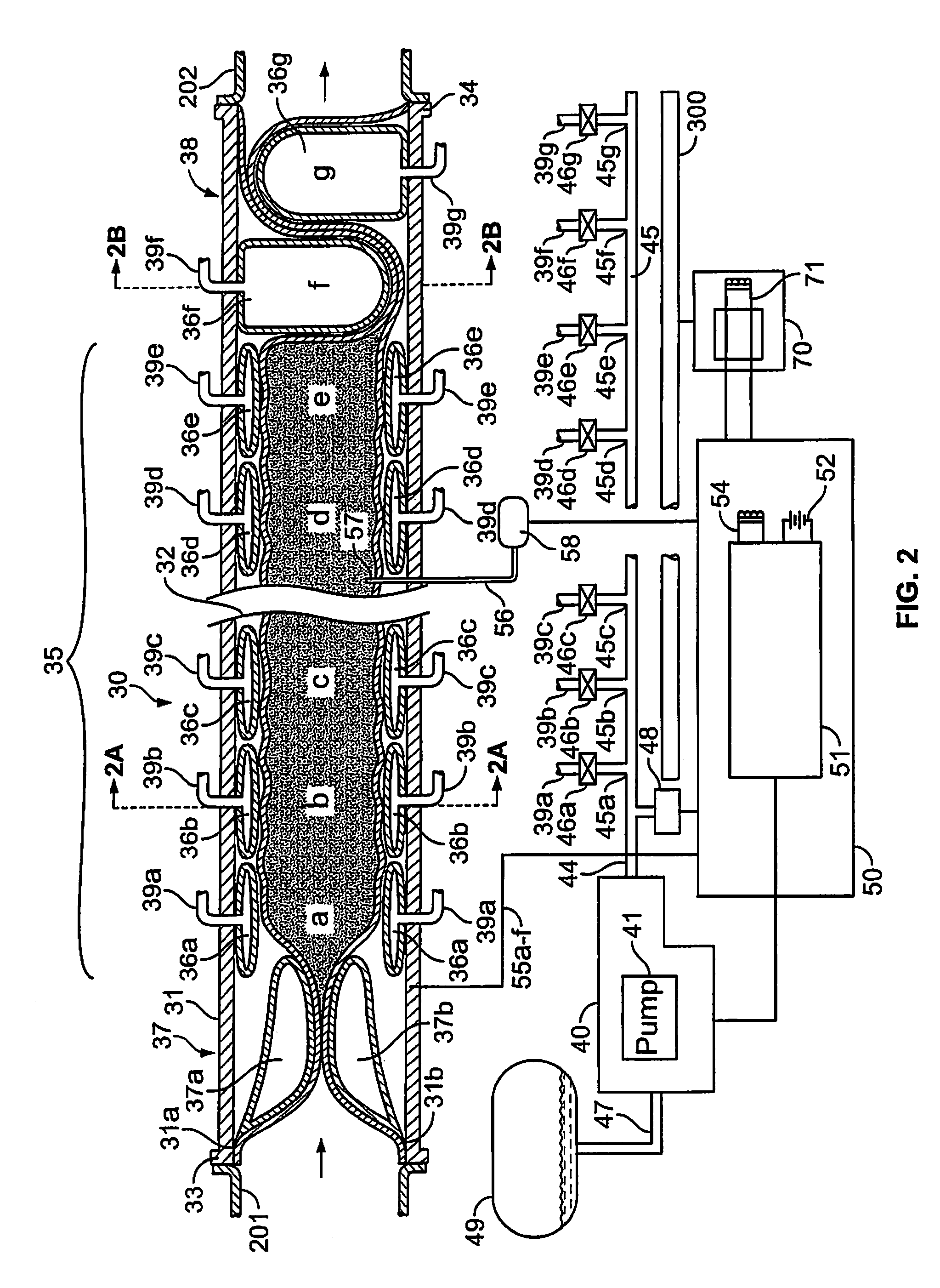
An implantable digestive organ is provided for the transport of materials through the digestive tract and in one particular application to an artificial large bowel for replacing all or part of a colon or large bowel. The prosthetic organ of one embodiment includes an outer support structure, an expandable member or members located within the outer support structure, and a flexible inner member forming a conduit for the passage of material. The flexible inner member is located within the outer member and the expandable member or members are located between the inner member and the outer support structure. The expandable members are expanded and contracted, or inflated and deflated to provide a pumping action that pumps the material through the organ. The prosthesis may also include valves or sphincters at the entrance and / or exit points of the organ where material moves into and out of the prosthesis. An implantable pump unit may be included for inflating and deflating the expandable members according to a desired sequence.
Owner:PYTHON MEDICAL
Connector for catheter attachment to an implantable pump
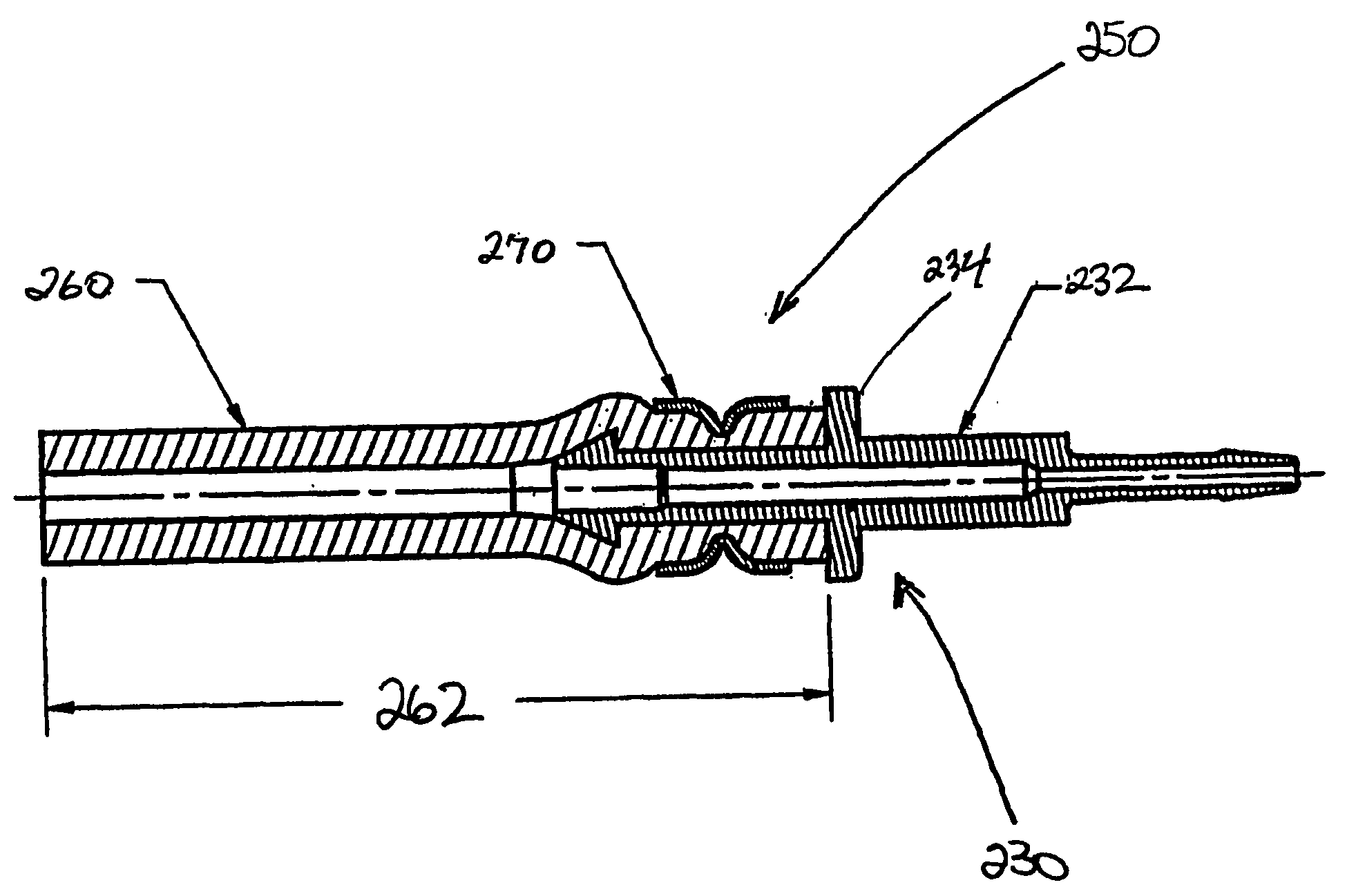
InactiveUS20050242579A1Effective flushingSecure and safe and effective mannerPressure infusionCatheterHydrophilic coatingCatheter device
A catheter system for locking a catheter to an implantable pump and for effectively flushing a catheter after implantation within a body. A locking component comprises an extension boot and catheter lock that together fluidly connect the catheter to the pump in a secure, safe and effective manner. A catheter component comprises a design having kink-resistant walls and a unique tip. A flushing component comprises a hub and stylet combination characterized by a hydrophilic coating on the stylet and a flush through hub to allow flushing of the stylet while inside the catheter.
Owner:INSET TECH
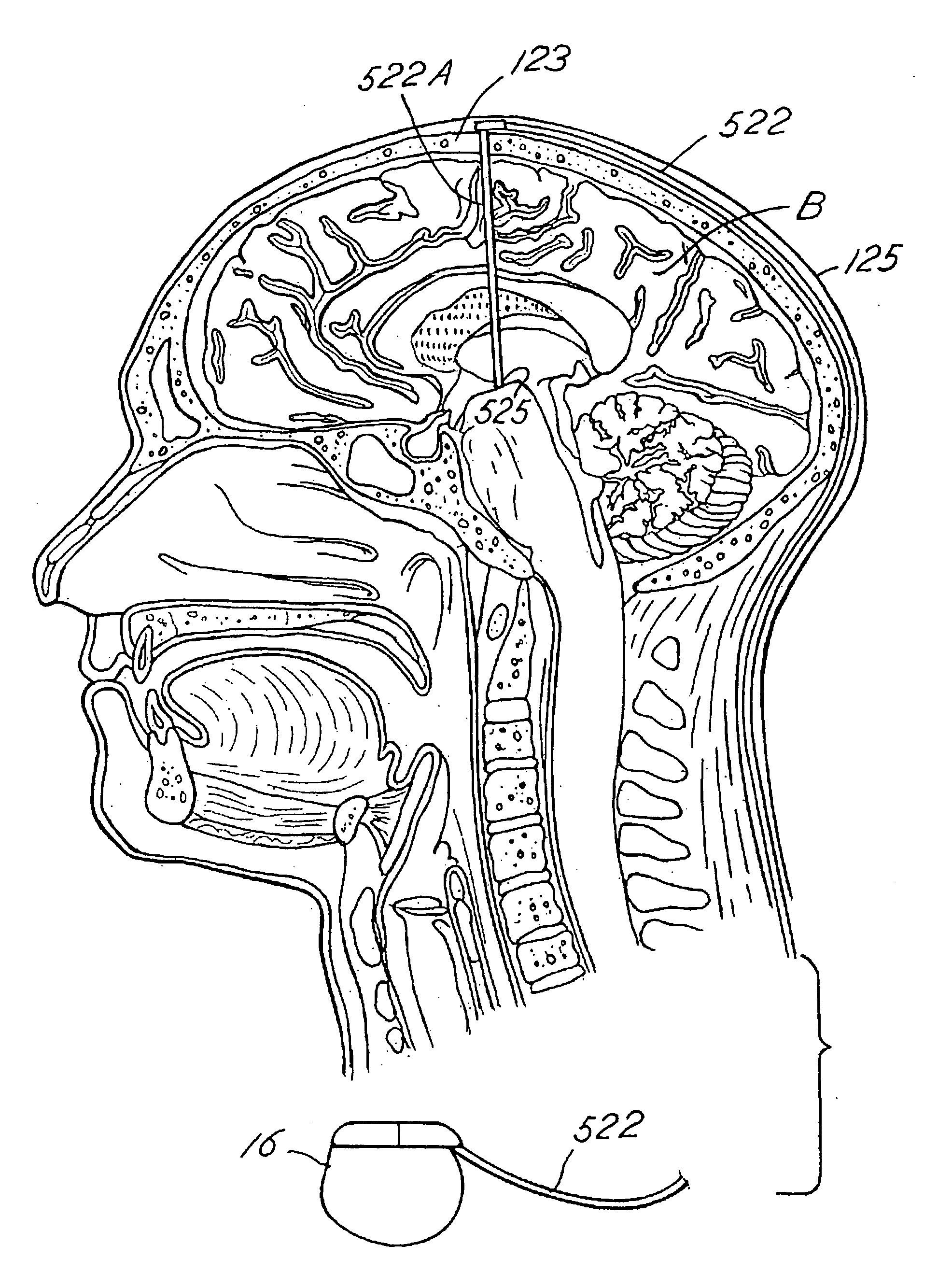
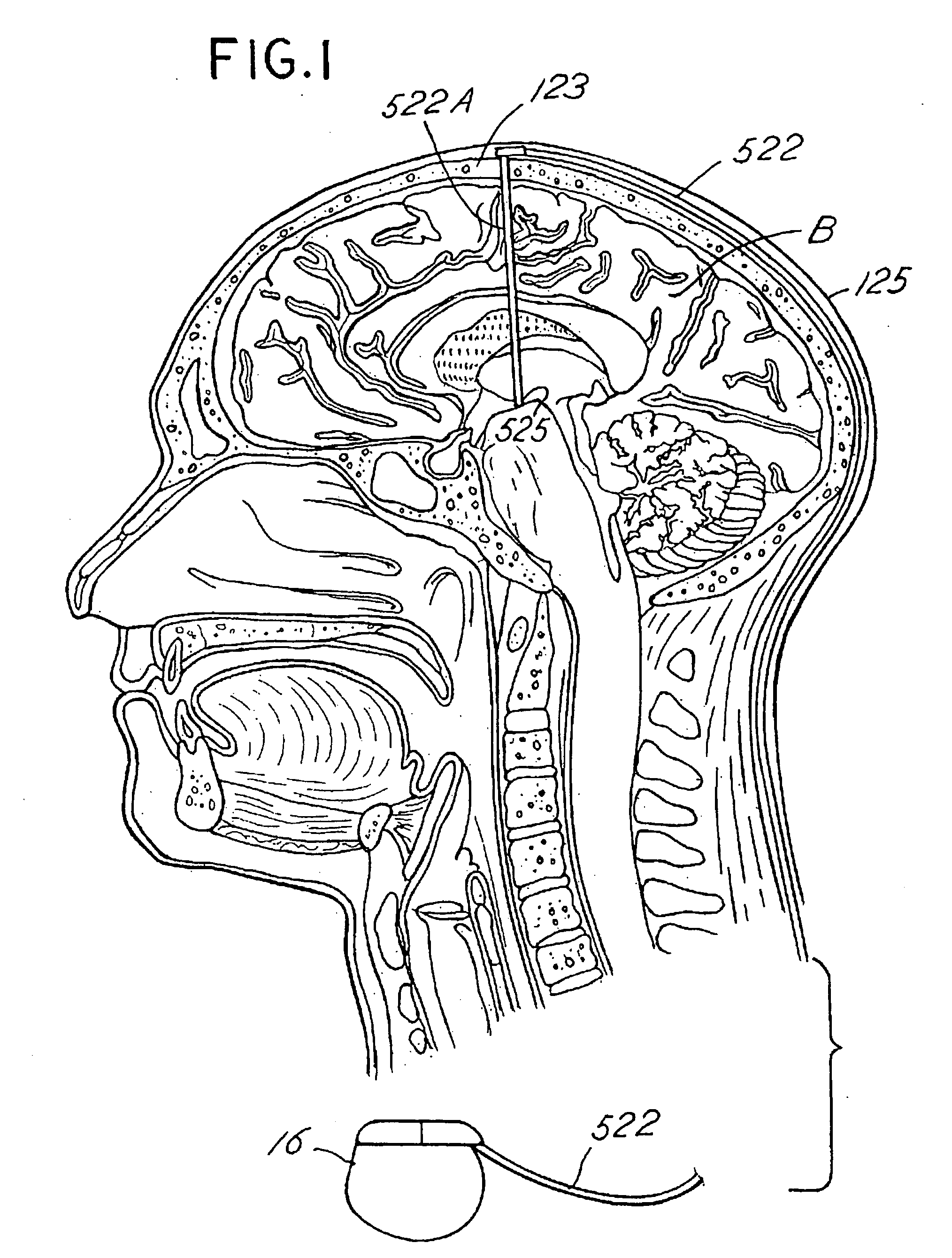
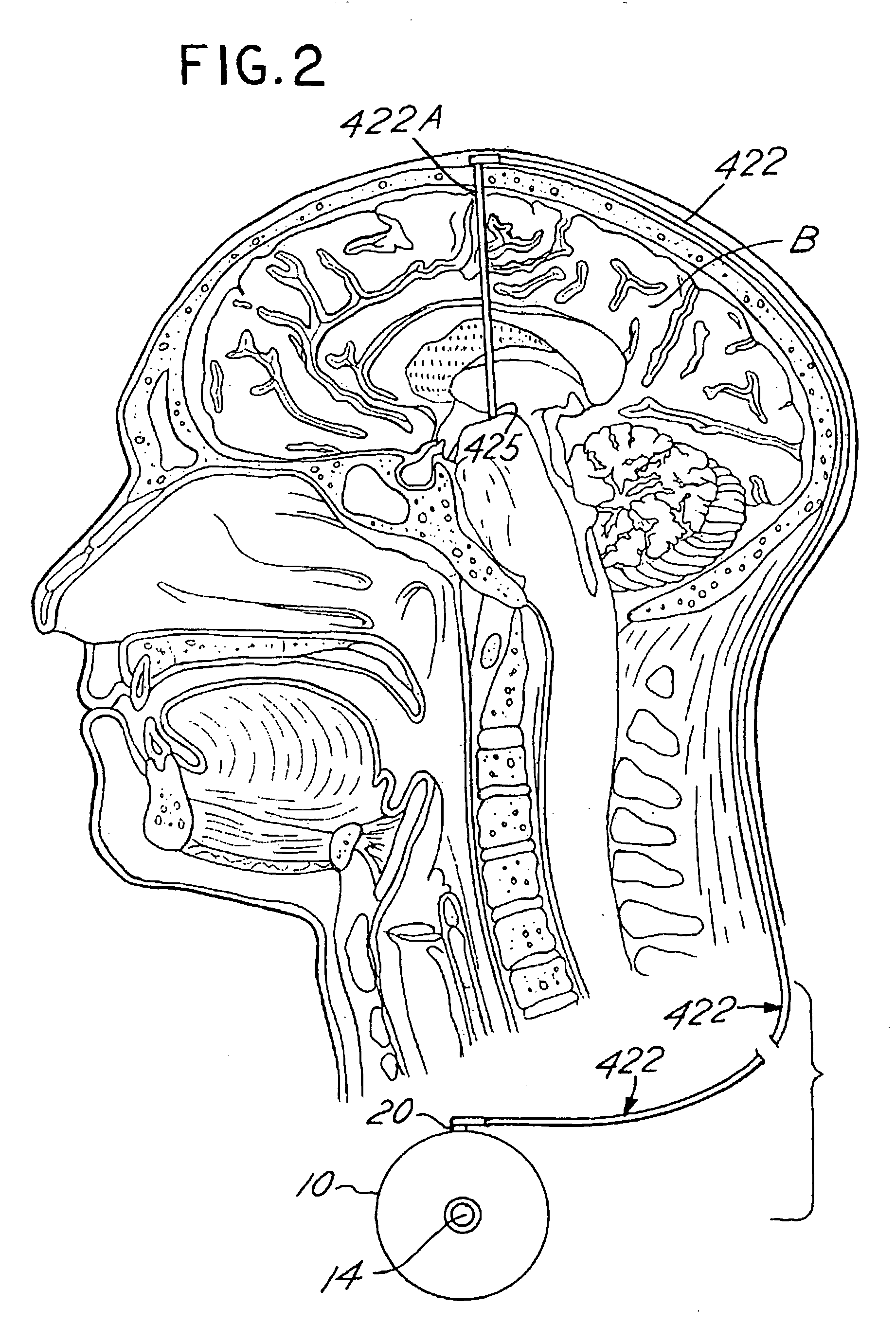
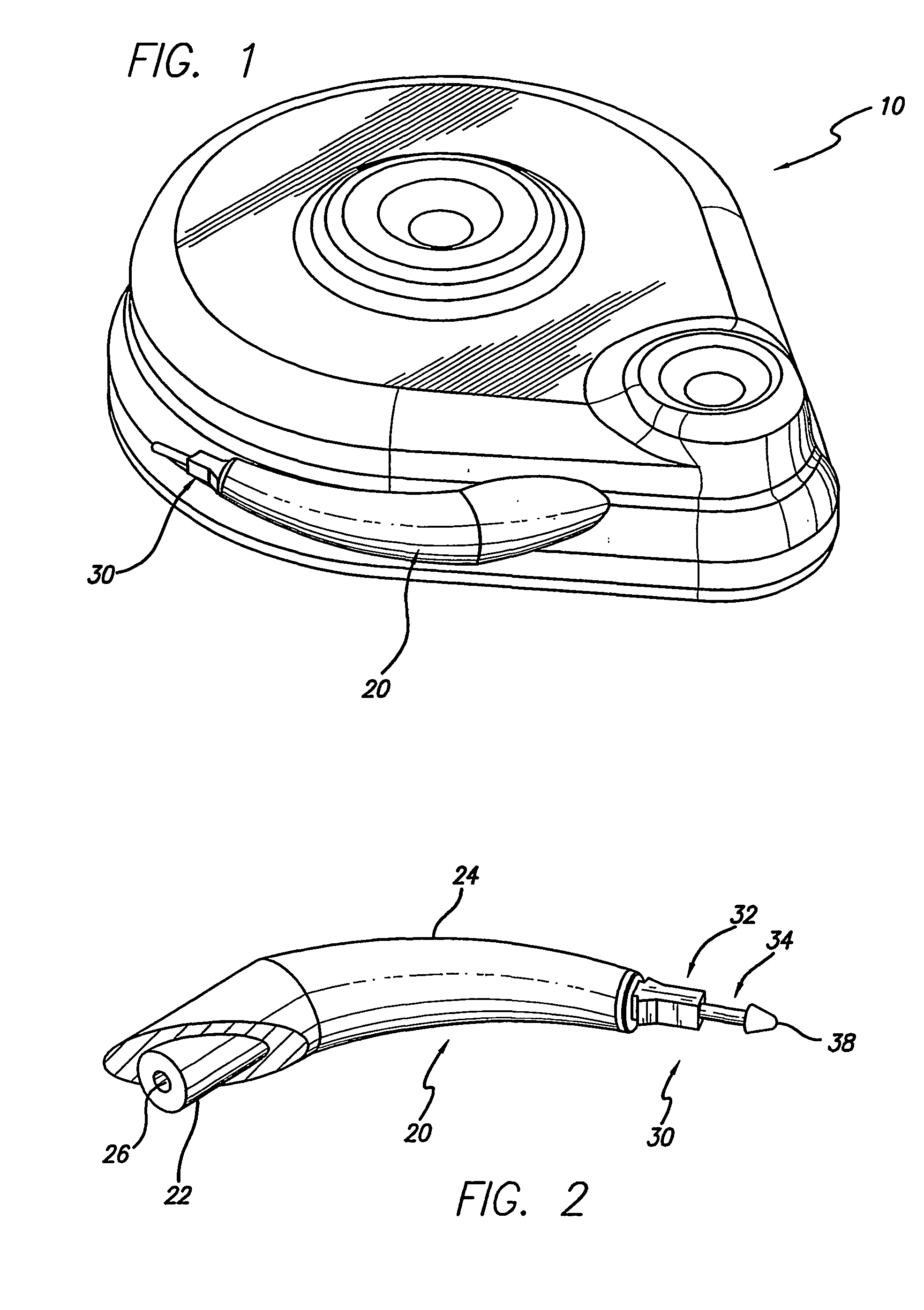
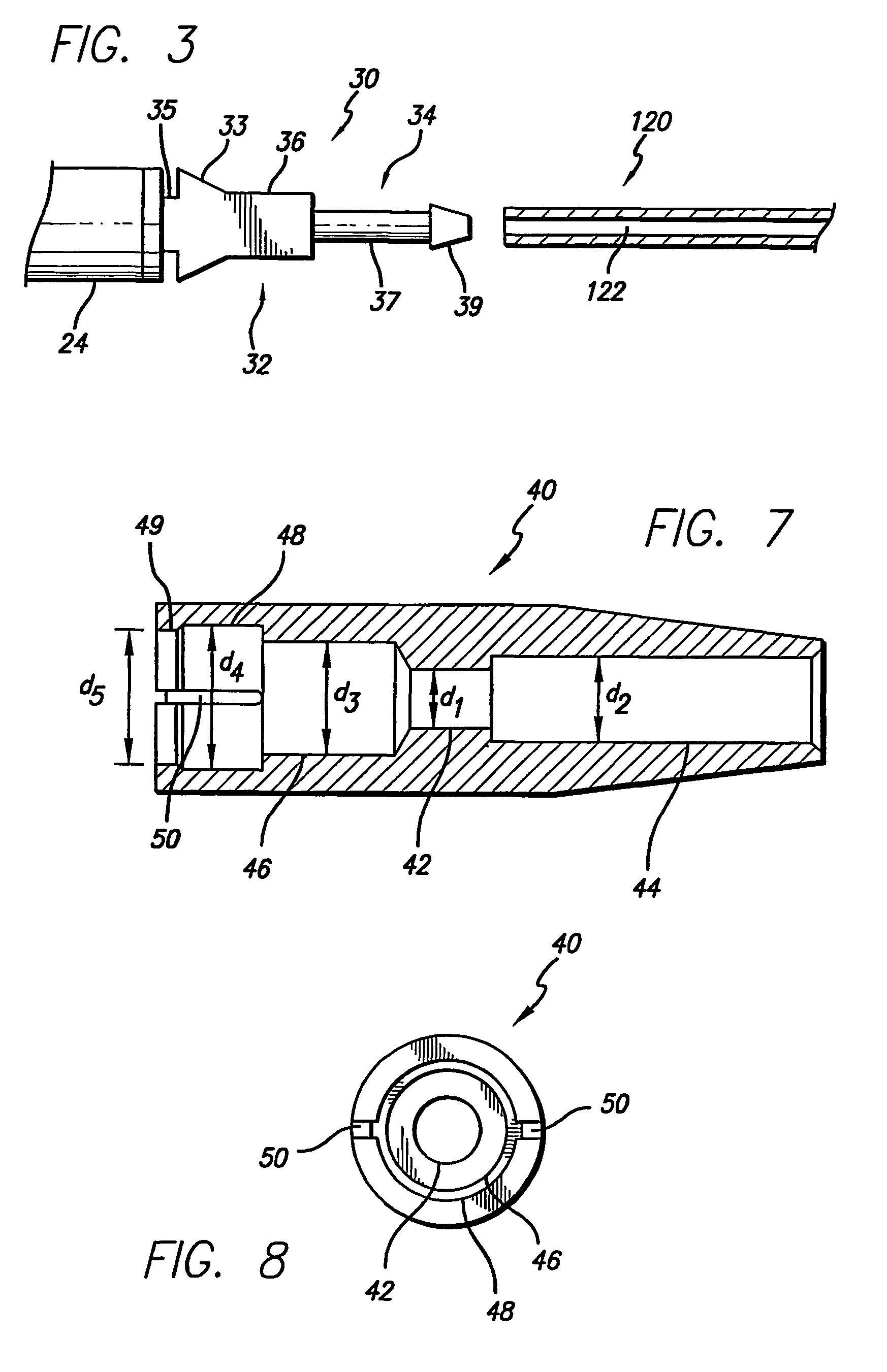
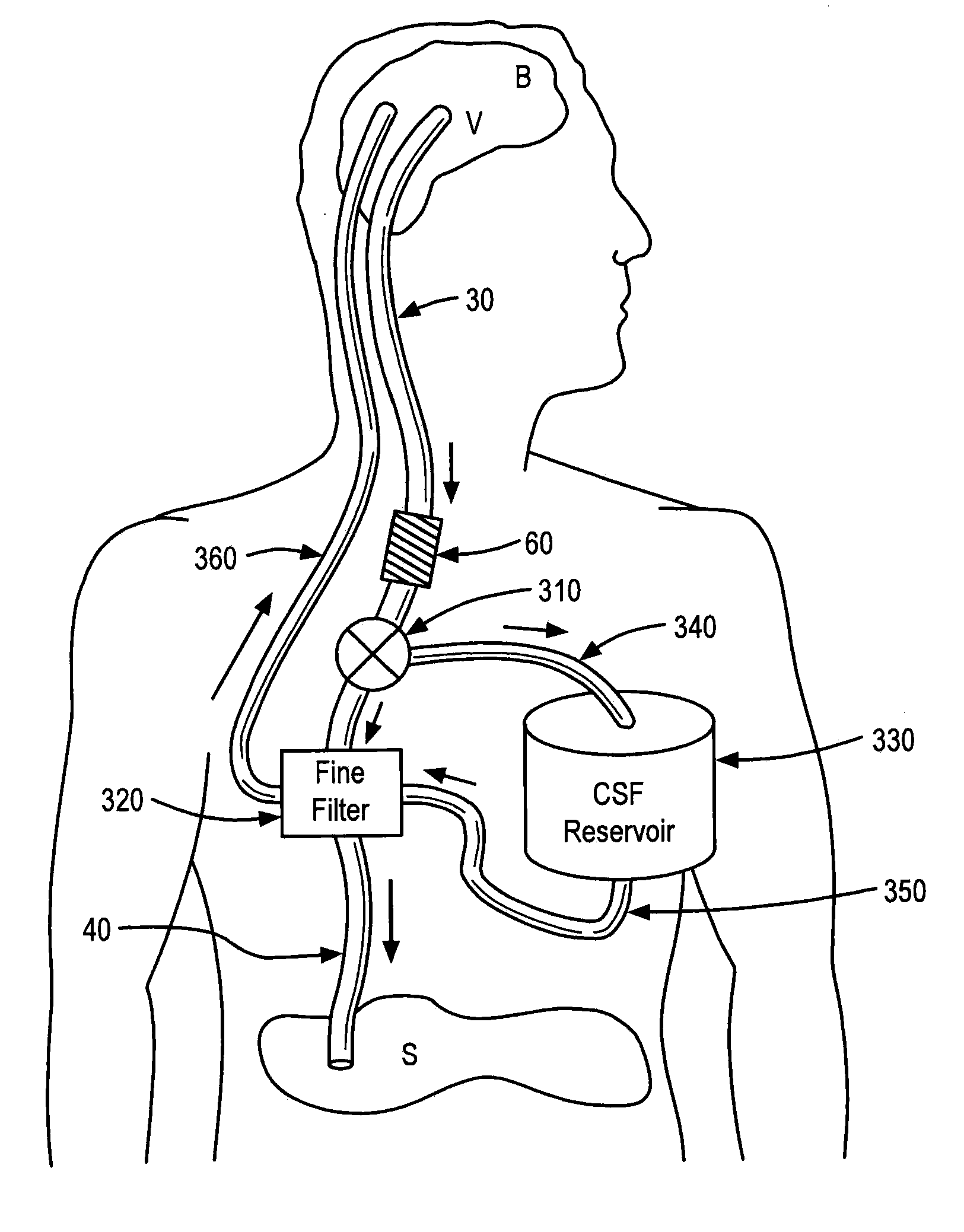
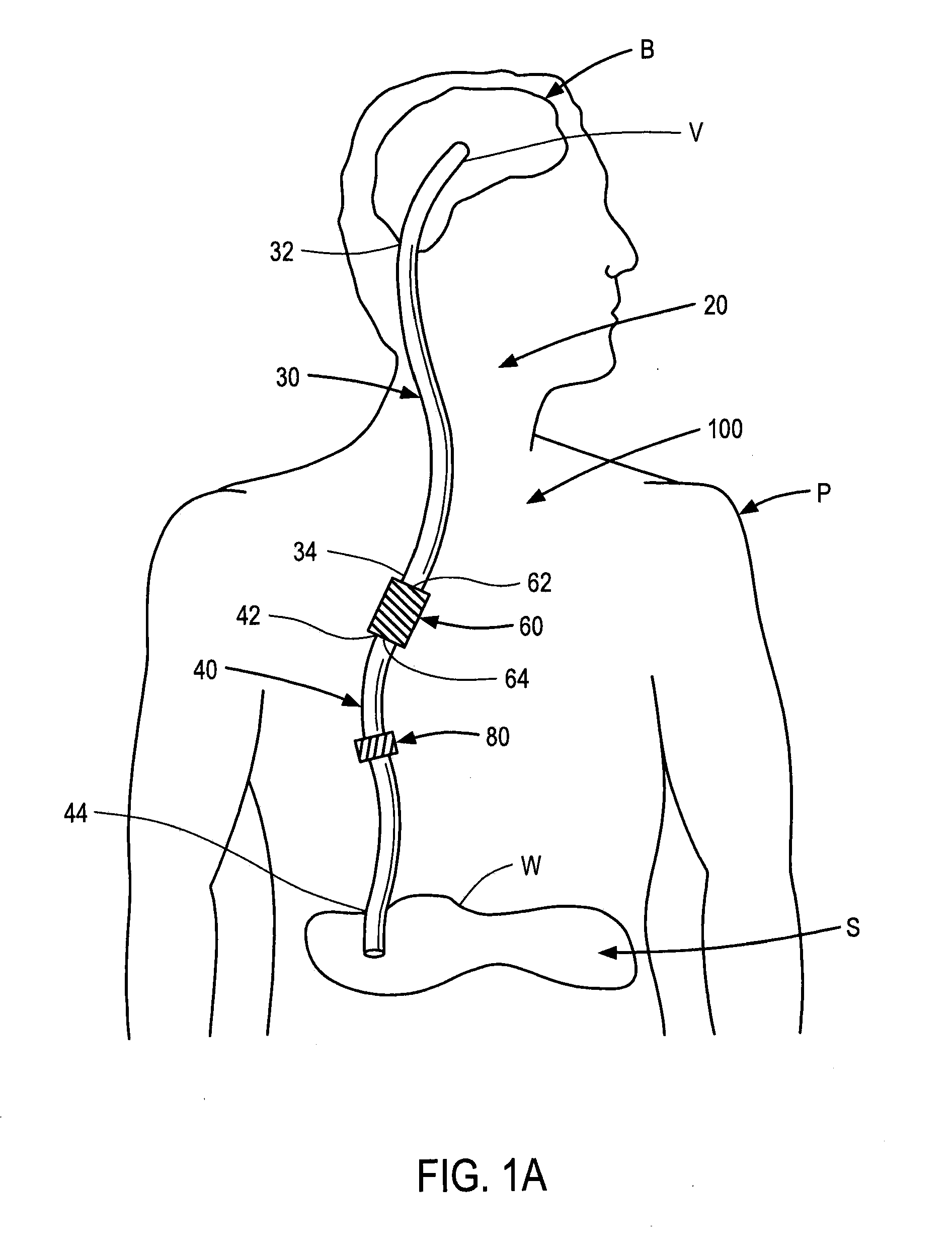
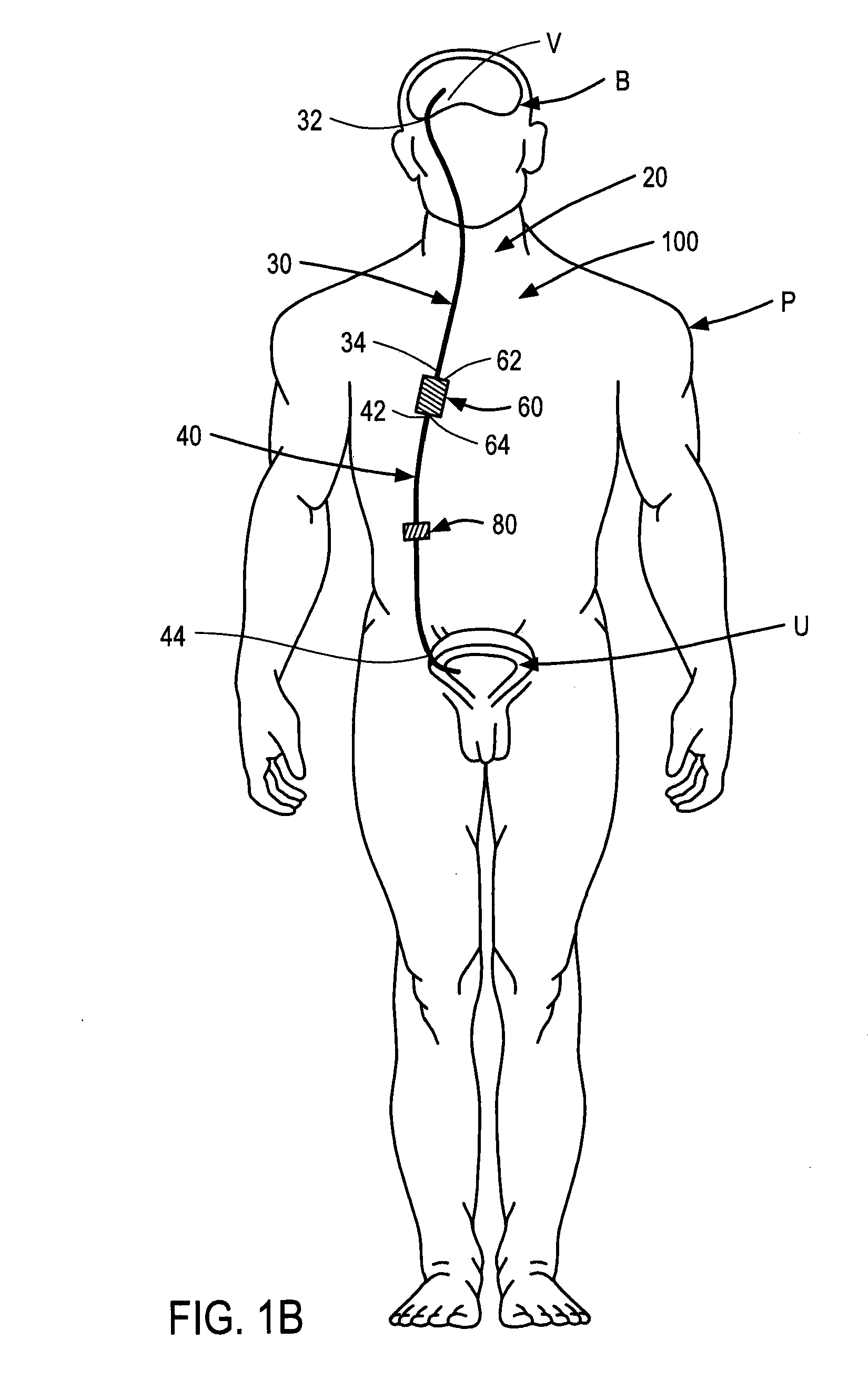
Systems and methods for moving and circulating fluid to treat alzheimer's disease
ActiveUS20150094644A1Reduce concentrationOvercomes drawbackWound drainsMedical devicesDiseaseCatheter device
A system for the treatment of Alzheimer's disease is provided by moving cerebrospinal fluid containing particles know to contribute to onset of Alzheimer's disease from a source of cerebrospinal fluid to the stomach or bladder, where the particles are safely digested by gastric acid or excreted, the system including an implantable pump, an inlet catheter, an outlet catheter, and a one-way valve. The system further includes at least one filter to filter harmful particles from the cerebrospinal fluid and return the filtered cerebrospinal fluid back to the source of cerebrospinal fluid, where the harmful particles blocked by the filter may be rinsed off the filter and transported to the stomach or bladder.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com