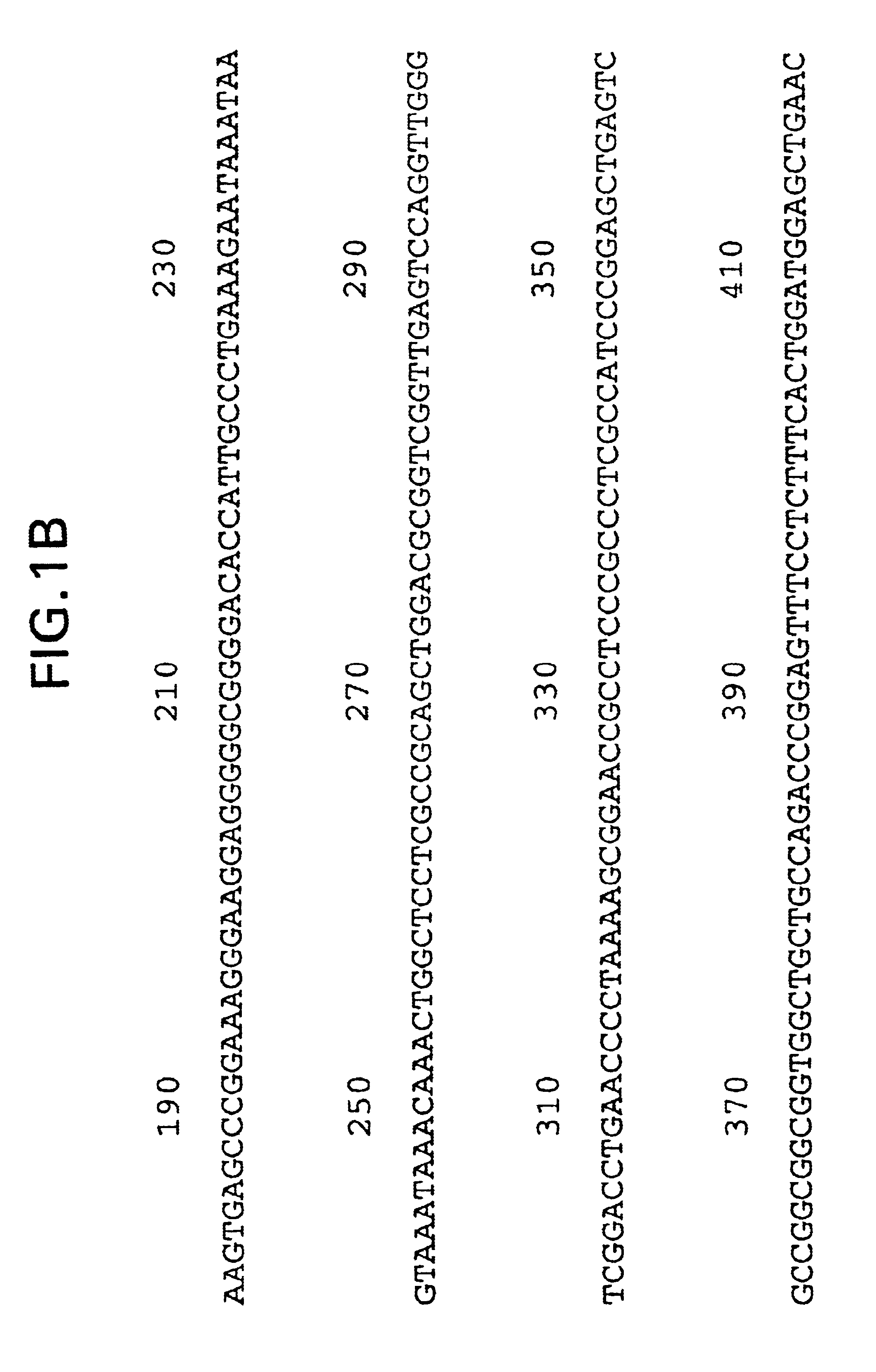
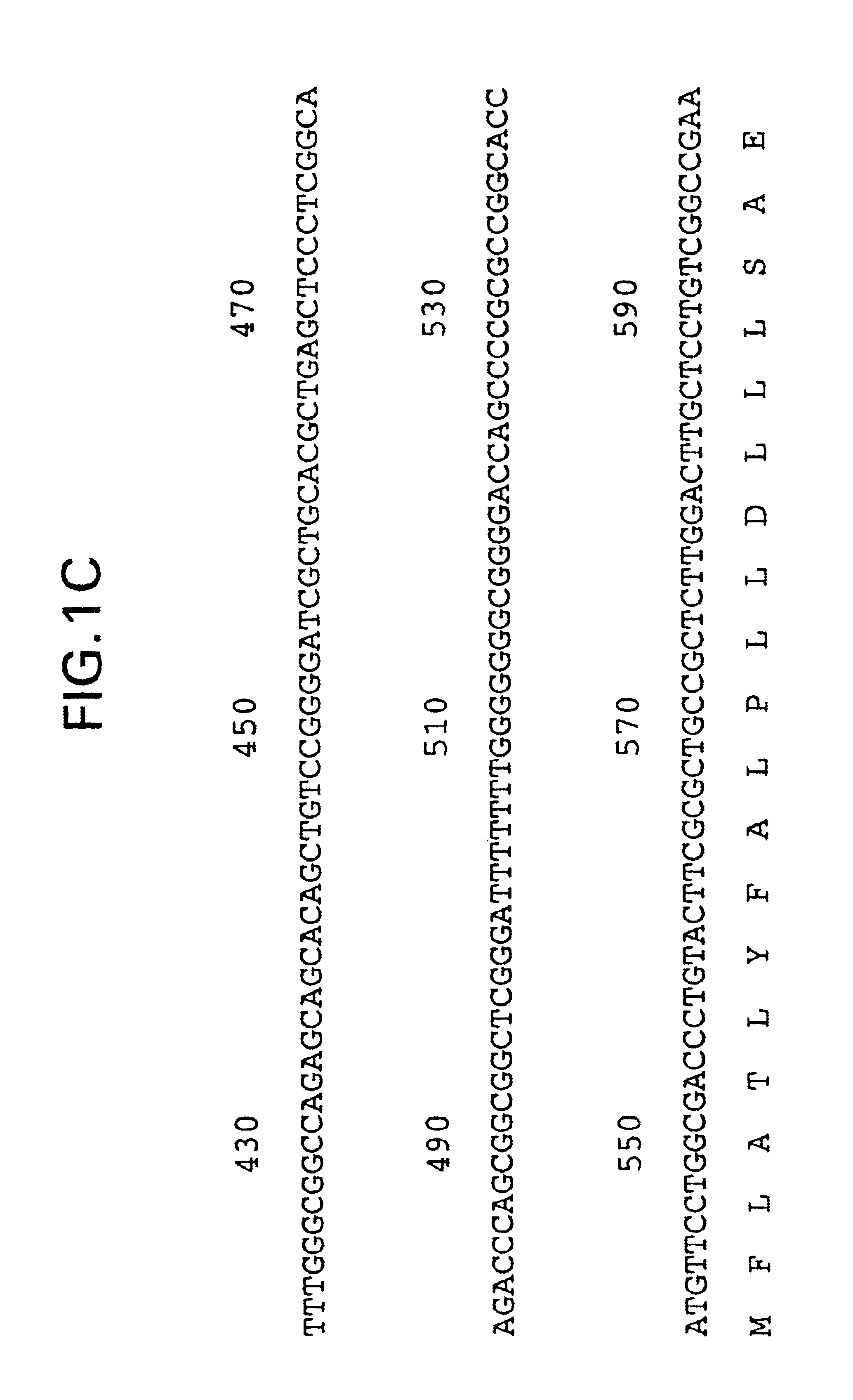
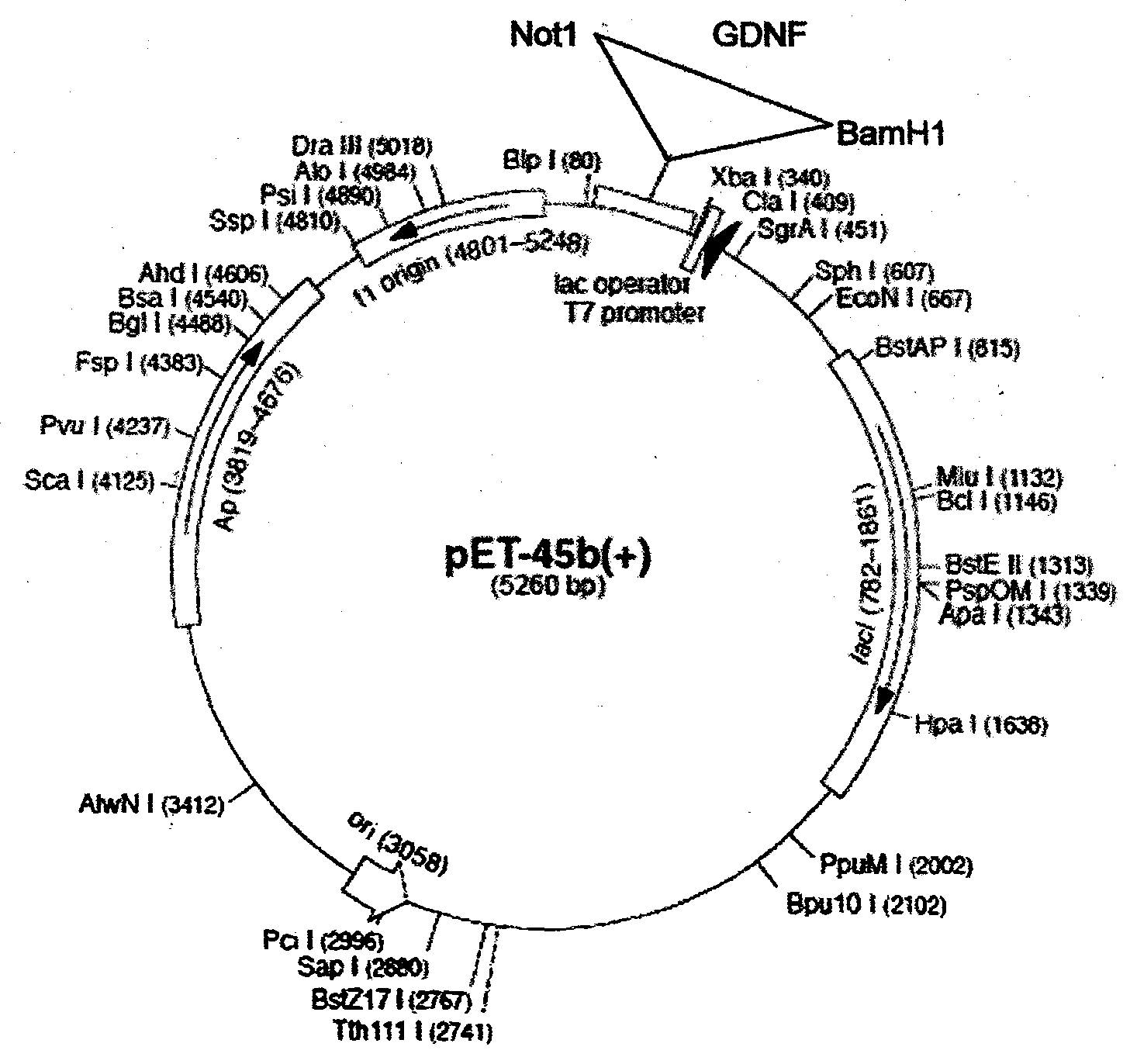
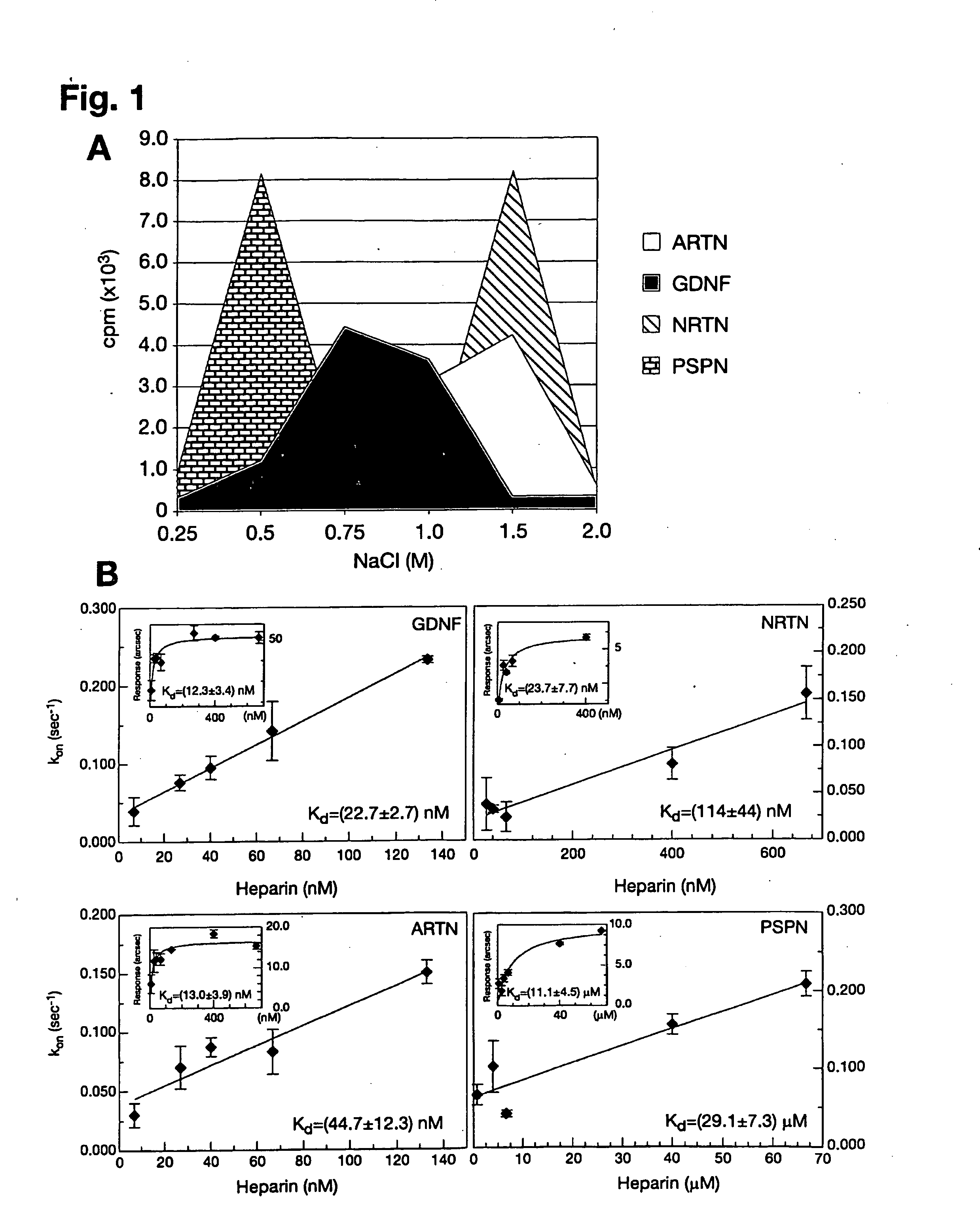
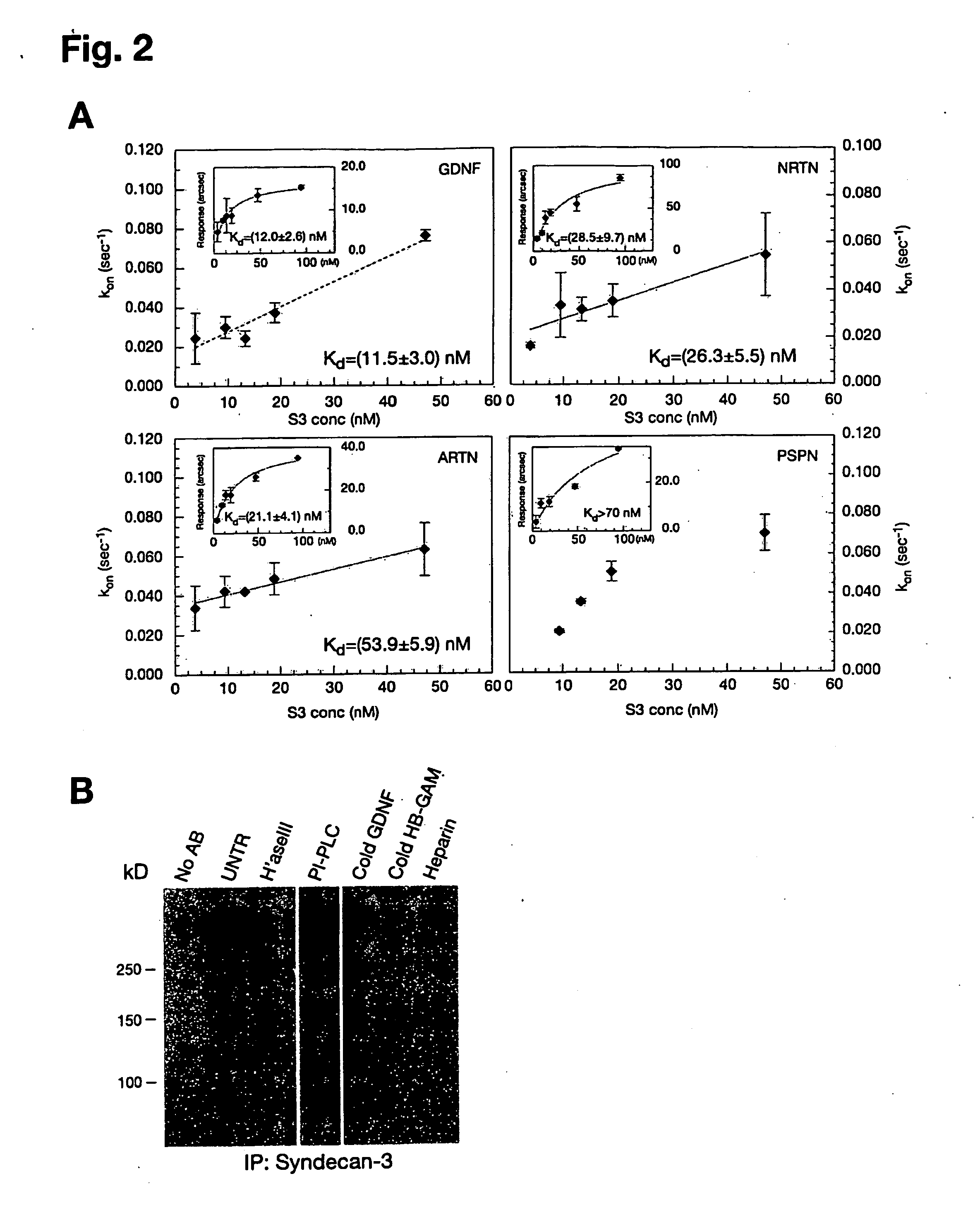
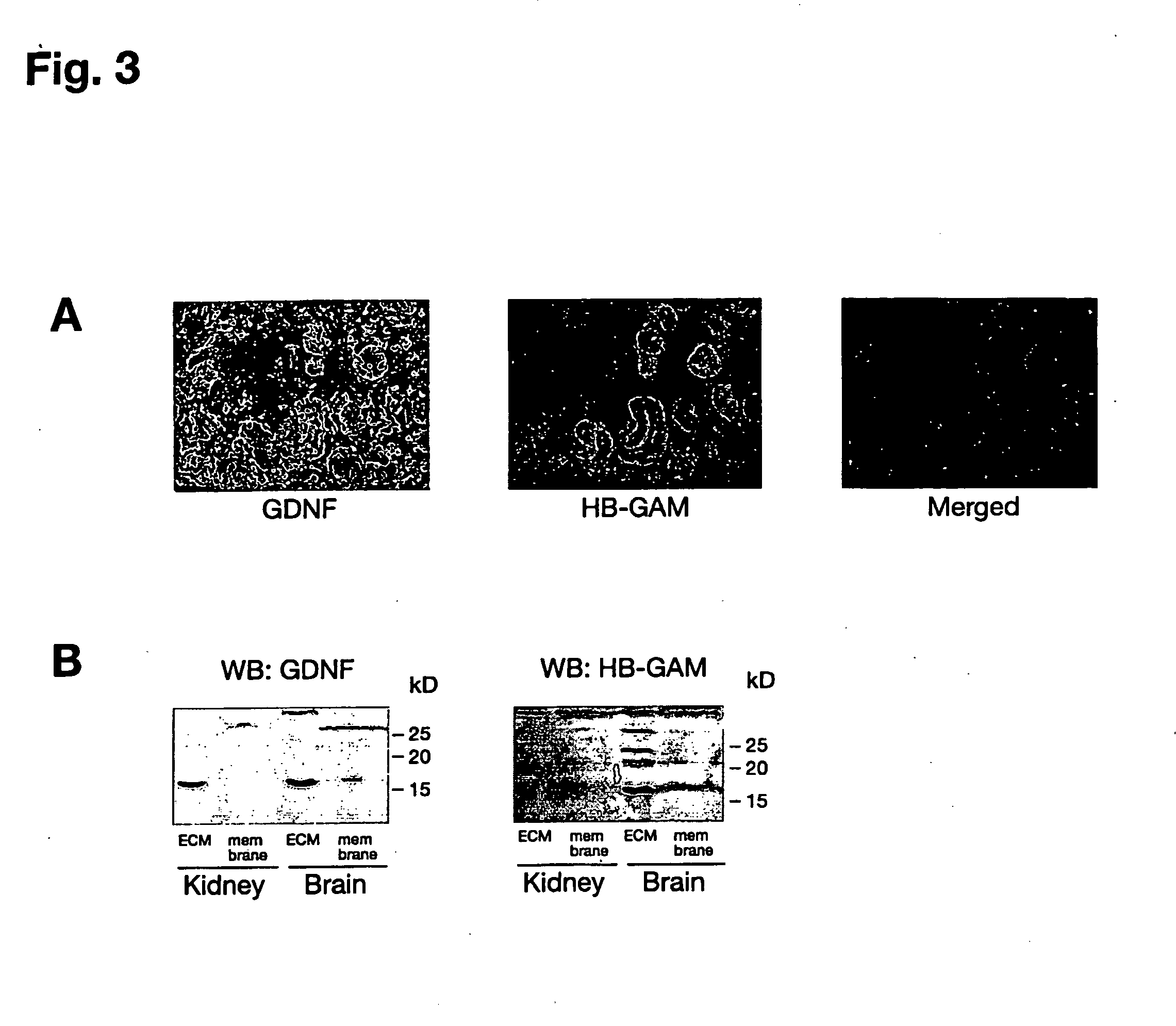
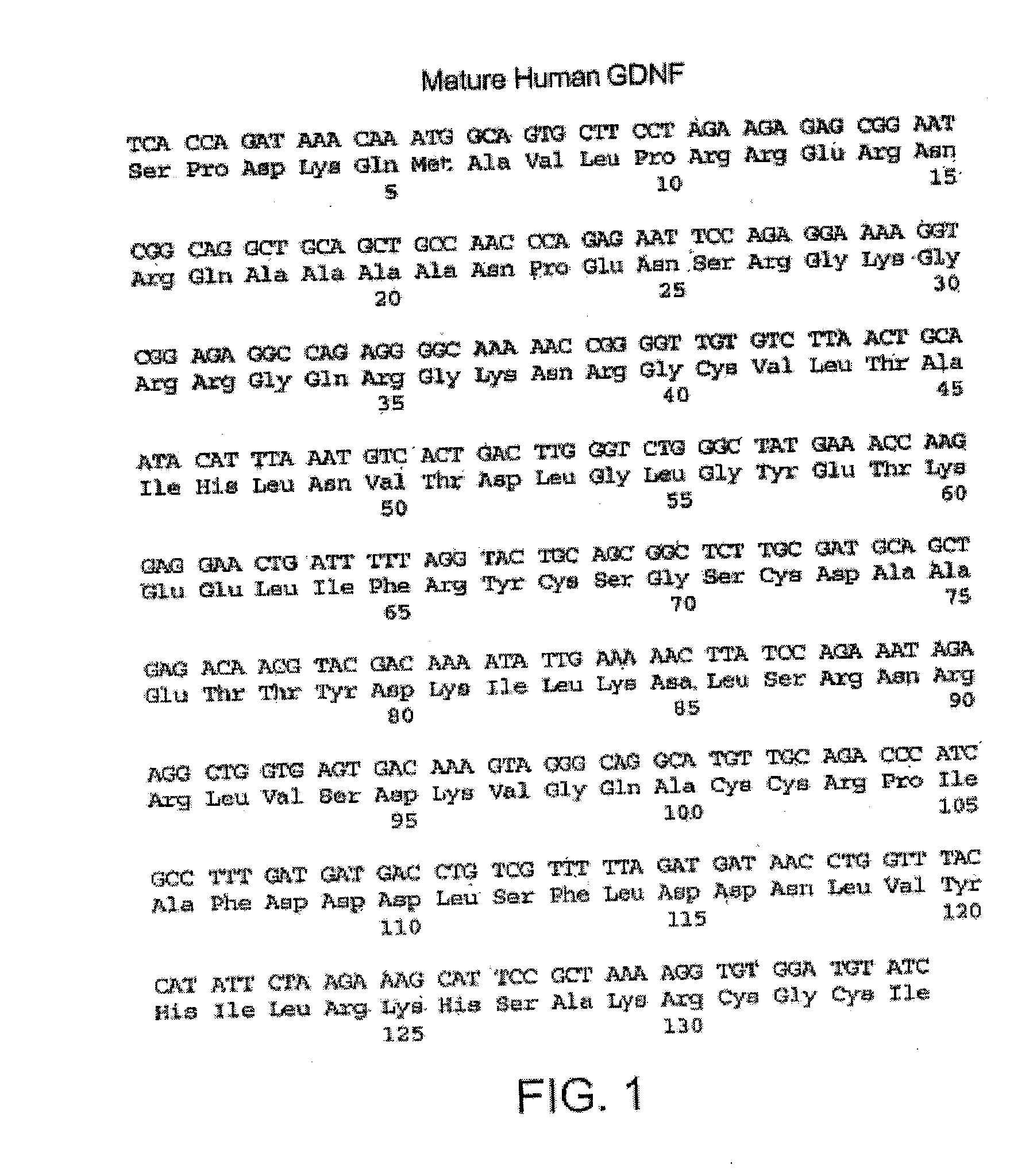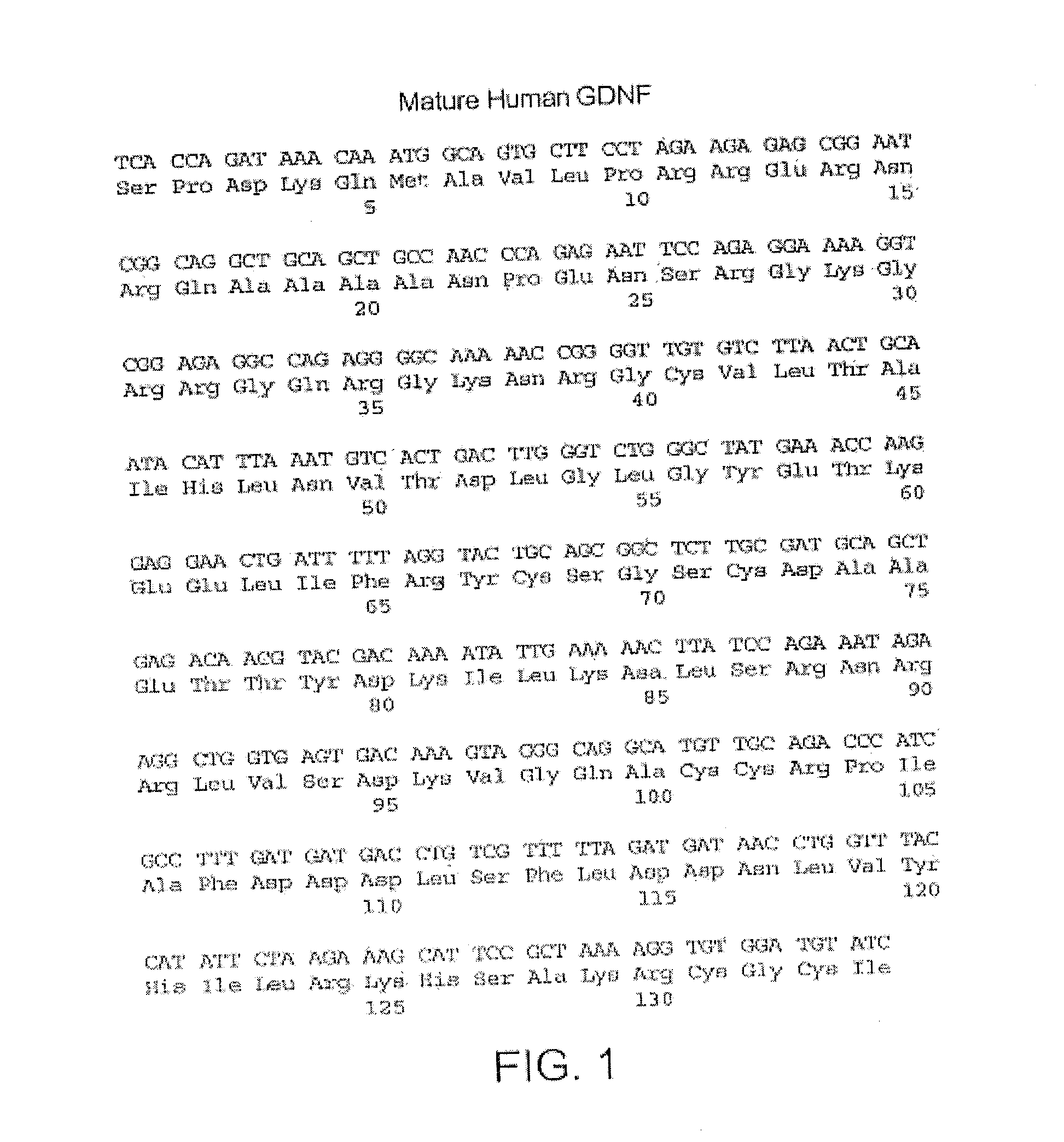Patents
Literature
45 results about "Glial cell line-derived neurotrophic factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
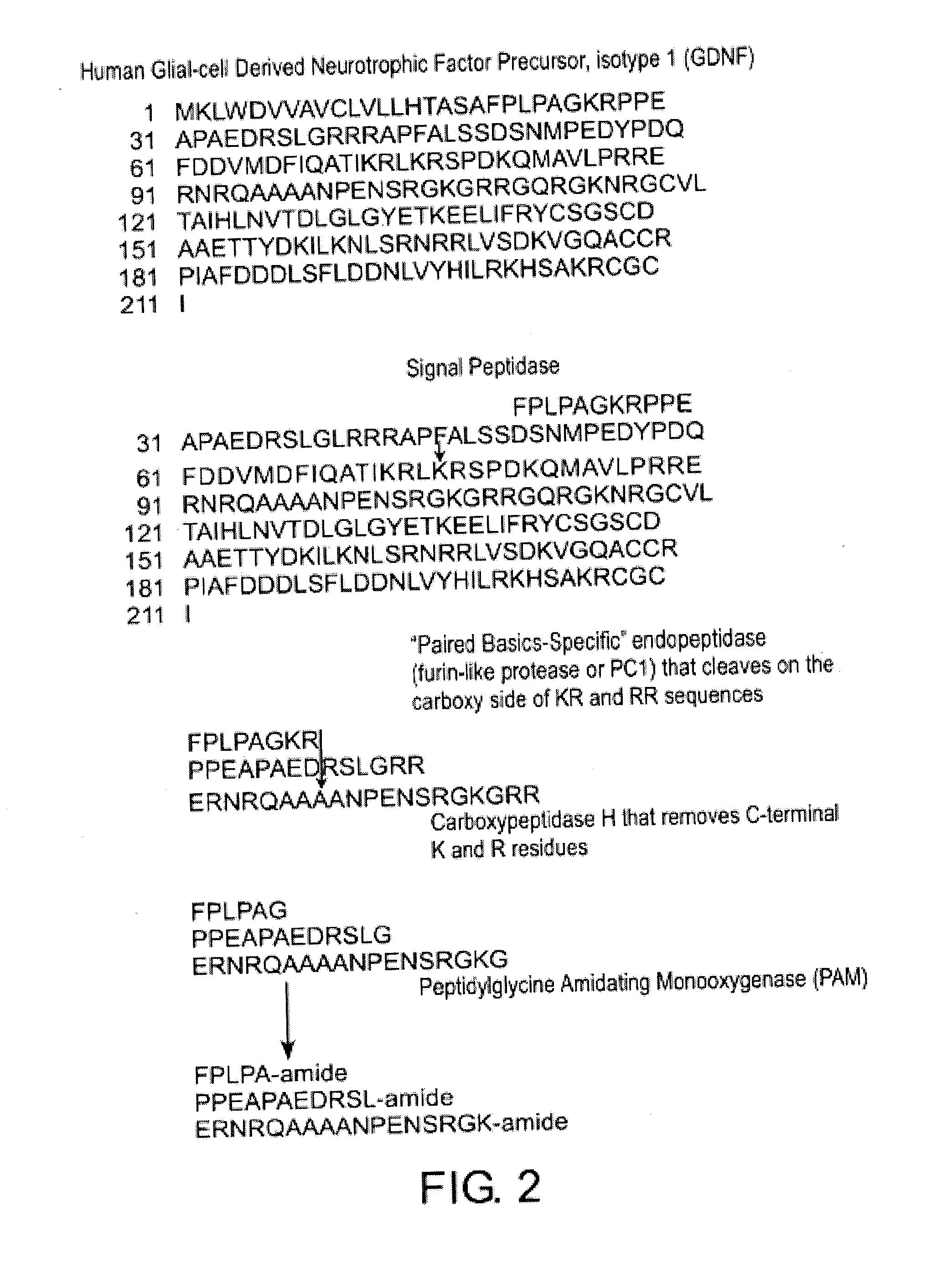
Glial cell-derived neurotrophic factor (GDNF) is a protein that, in humans, is encoded by the GDNF gene. GDNF is a small protein that potently promotes the survival of many types of neurons. It signals through GFRα receptors, particularly GFRα1.
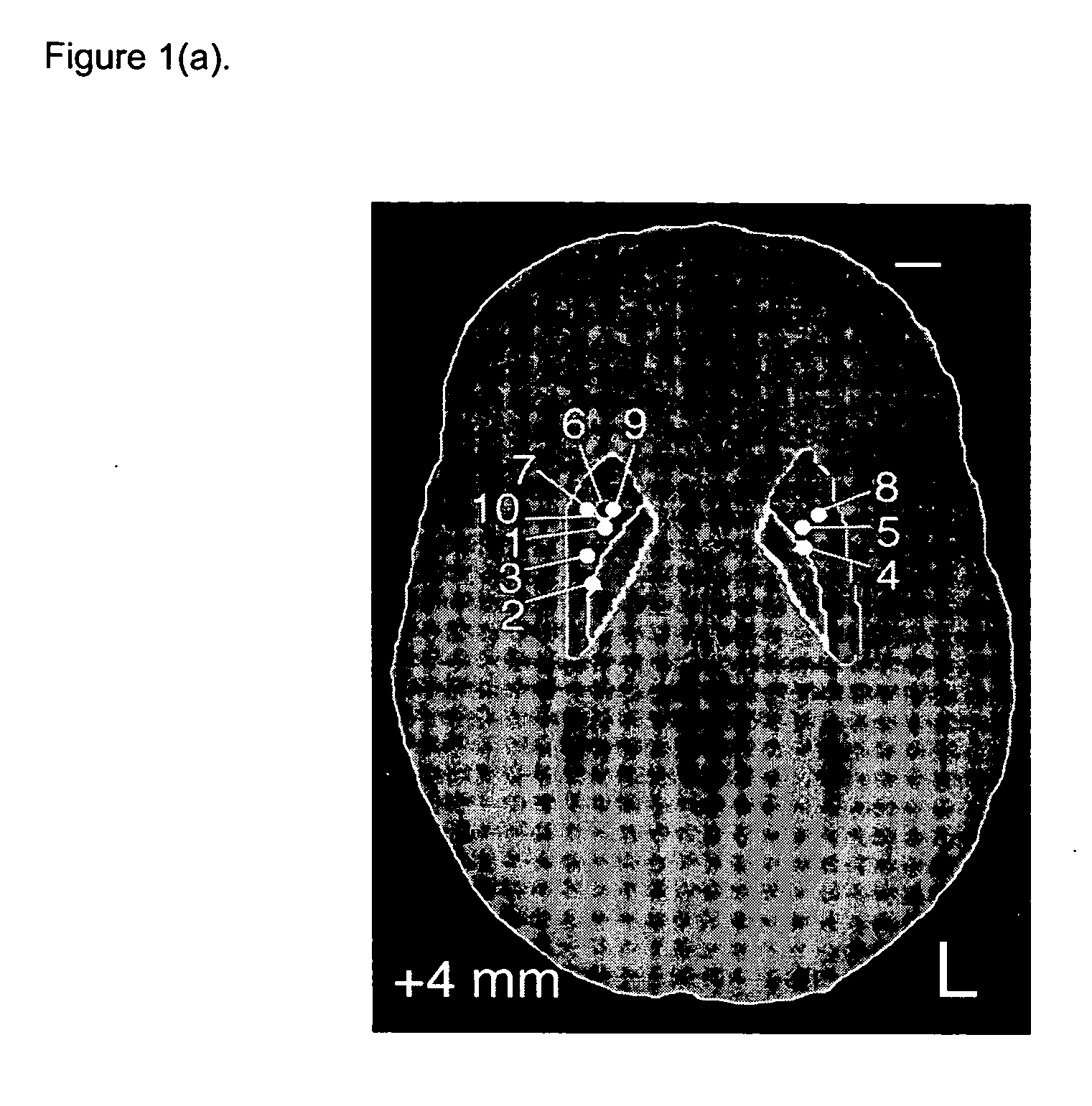
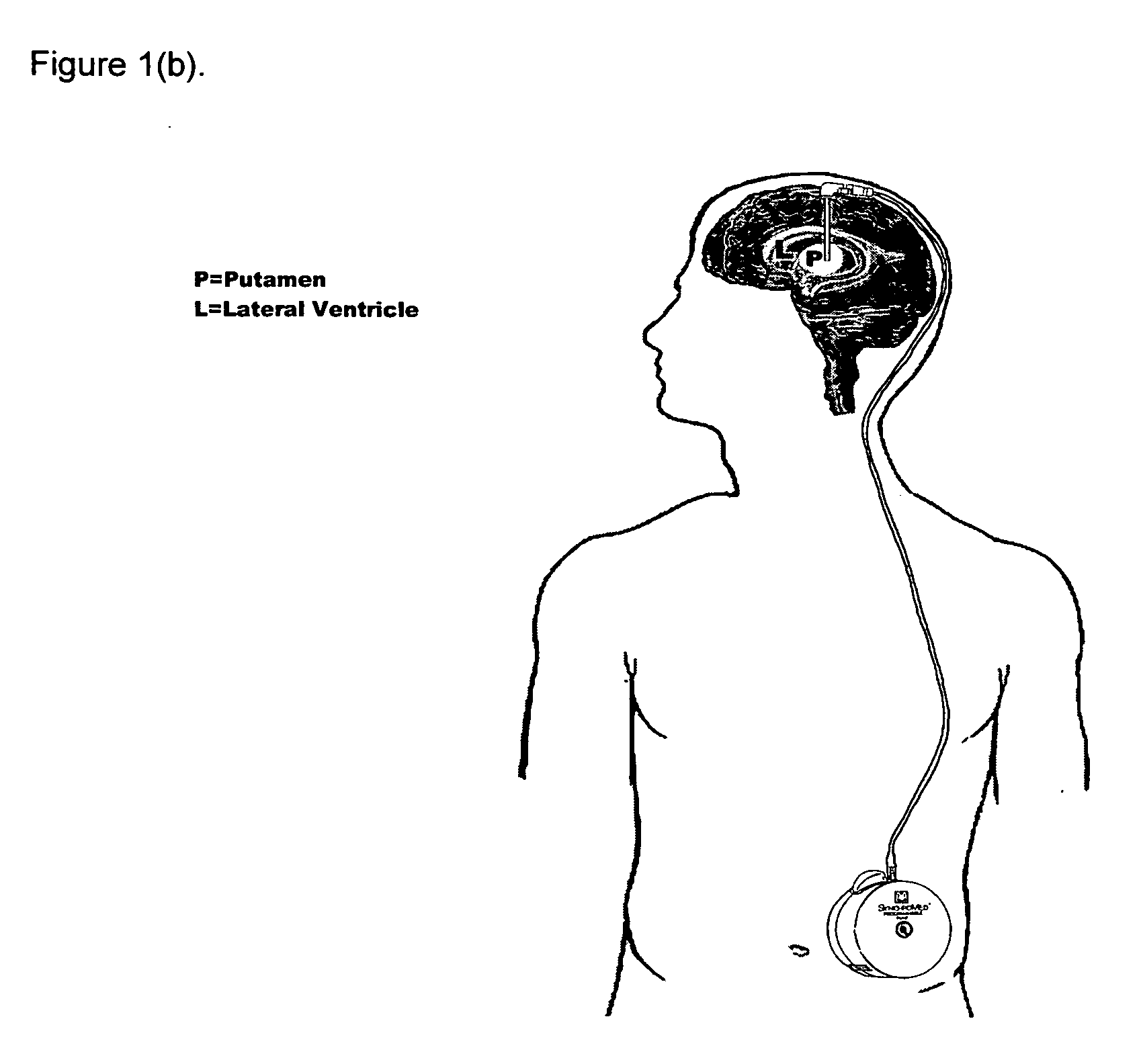
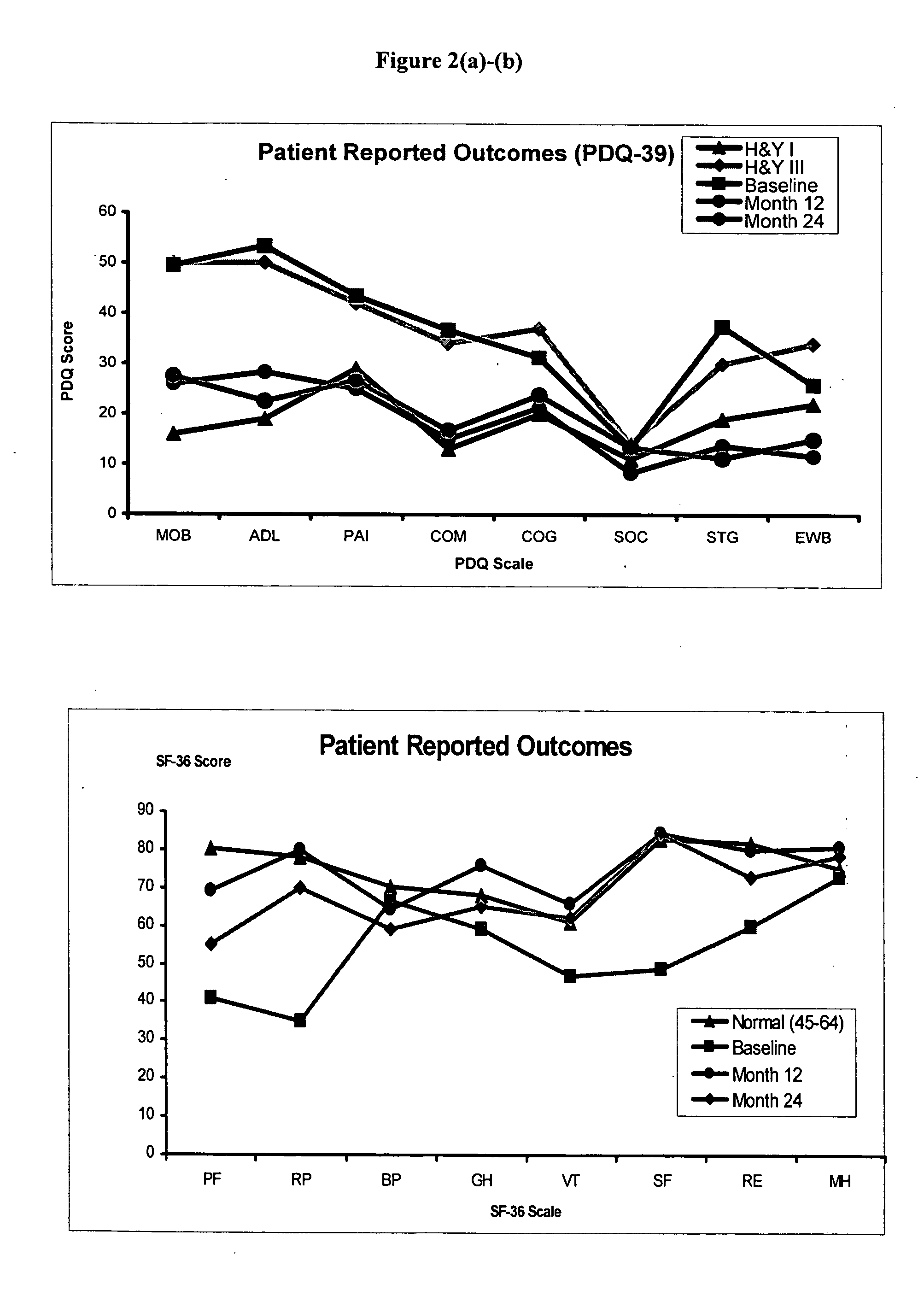
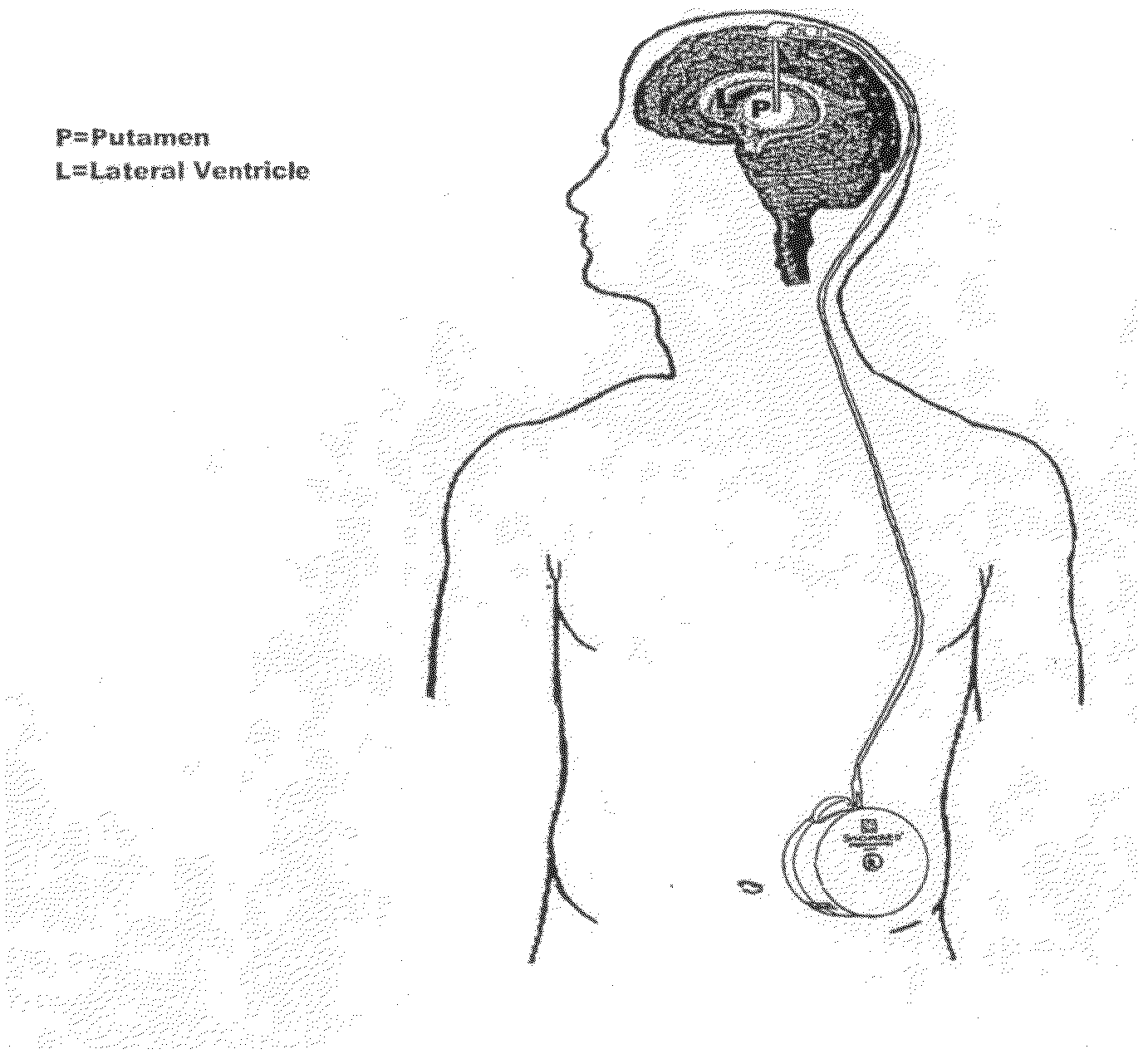
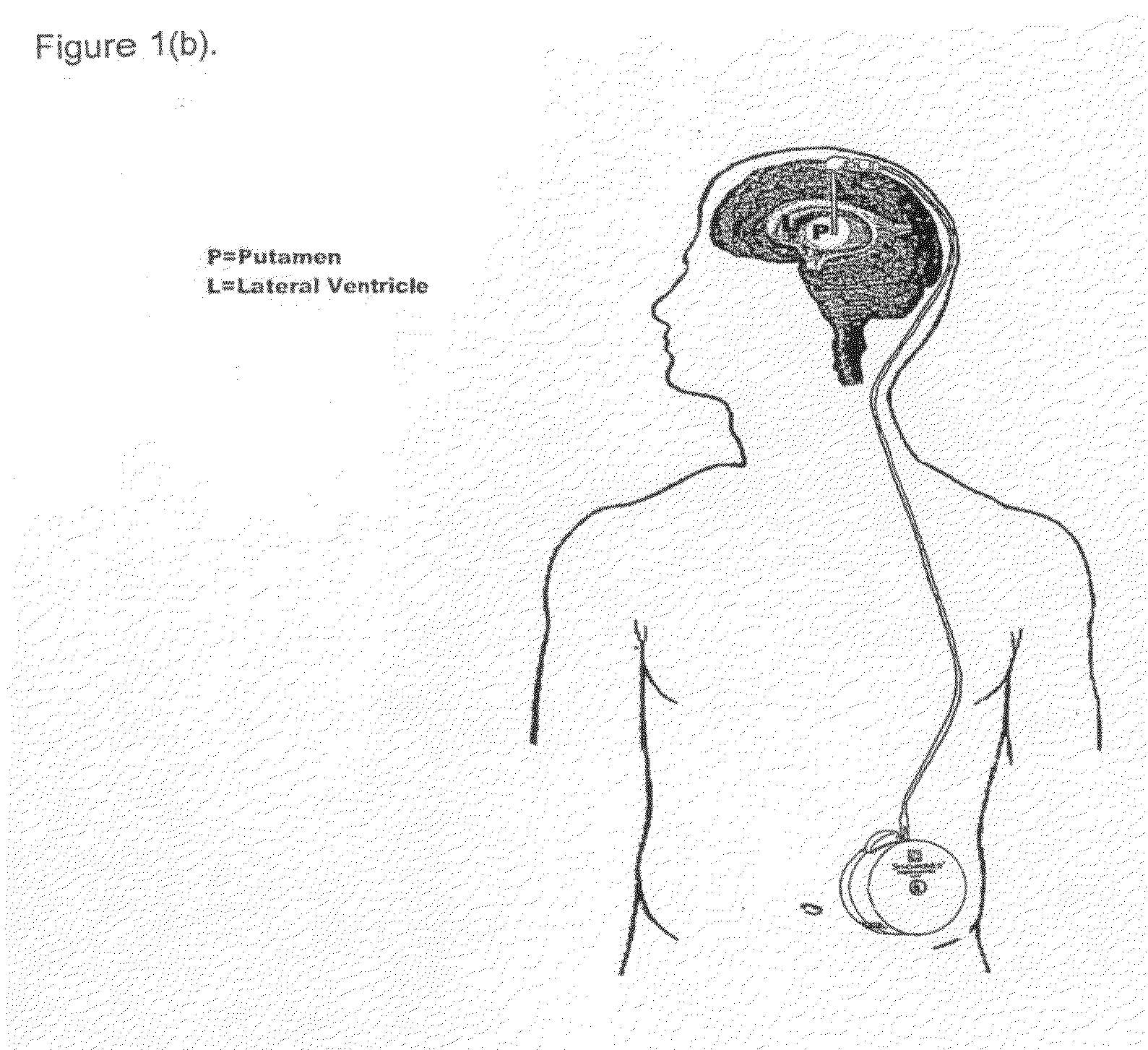
Method of treating Parkinson's disease in humans by convection-enhanced infusion of glial cell-line derived neurotrophic factor to the putamen
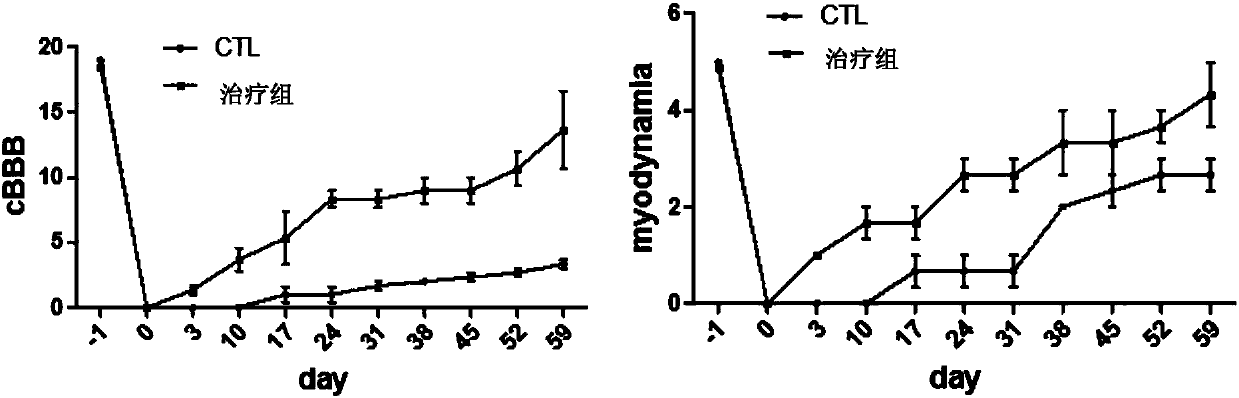
ActiveUS20050137134A1Impressive re-innervationDramatic effectNervous disorderPeptide/protein ingredientsGlial cell line-derived neurotrophic factorImplantable Pump
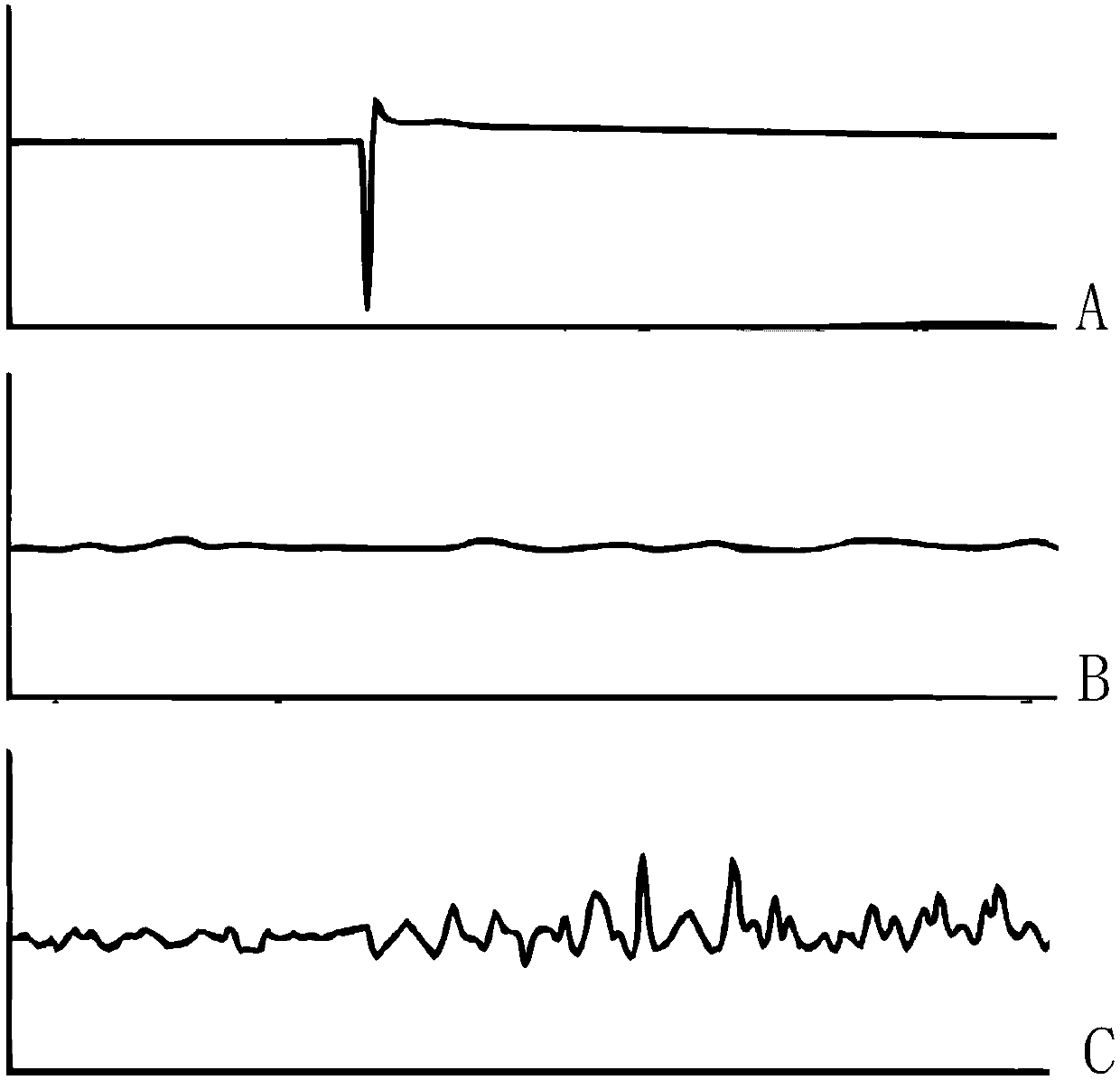
A method of treating Parkinson's disease in humans is disclosed, wherein glial cell-line derive neurotrophic factor (GDNF) is chronically administered directly to one or both putamen of a human in need of treatment thereof via convection-enhanced infusion using at least one implantable pump and at least one catheter. In one aspect of the present invention the GDNF is infused directly into one or both putamen through one or more indwelling intraparenchymal mutltiport brain catheters connected to one or moreimplantable pumps wherein the flow rate is pulsed.
Owner:AMGEN INC +1
Polynucleotides encoding a neurotrophic factor receptor
The present invention relates to glial cell line-derived neurotrophic factor (GDNF), a potent neurotrophin that exhibits a broad spectrum of biological activities on a variety of cell types from both the central and peripheral nervous systems. The present invention involves the cloning and characterization of receptors for GDNF. Nucleic acid and amino acid sequences are described for GDNFR protein products. A hydrophobic domain with the features of a signal peptide is found at the amino terminus, while a second hydrophobic domain at the carboxy terminus is involved in the linkage of the receptor to the cell membrane. The lack of a transmembrane domain and cytoplasmic region indicates that GDNFR requires one or more accessory molecules in order to mediate transmembrane signaling. GDNFR mRNA is widely distributed in both nervous system and non-neural tissues, consistent with the similar distribution found for GDNF.
Owner:AMGEN INC
Glial cell line-Derived Neurotrophic Factor (GDNF) fusing penetrating peptides
The invention discloses an expression structure method for constructing Glial cell line-Derived Neurotrophic Factor (GDNF) fusing penetrating peptides, the purification of an expressed protein product and the significance thereof, which is mainly characterized in that (1) GDNF parent is a human source mature type; (2) the N-tail end of the GDNF is fused with PEP-1 penetrating peptides comprising the 21 amino acid; and the expression product of the constructed penetrating peptides GDNF fusing the gene expression plasmid has the membrane penetrating function and biological function of GDNF and can be clinically applied in the treatment of the related diseases.
Owner:广州市凯诺生物科技有限公司
Construction method of glial cell line-derived neurotrophic factor gene-modified embryo neural stem cell
InactiveCN103215225AViruses/bacteriophagesBiological testingGlial cell line-derived neurotrophic factorNeuronal degeneration
The invention relates to a biotechnology and especially relates to a construction method of a glial cell line-derived neurotrophic factor gene-modified embryo neural stem cell. The construction method comprises the following steps of 1, plasmid transformation, 2, plasmid digestion purification, 3, target gene and vector cloning, 4, recombinant plasmid packaging, 5, recombinant plasmid identification, 6, virus titer determination, 7, stem cell culture, 8, virus infection of the stem cells, 9, transgenic stem cell identification, 10, transgenic stem cell target-protein content determination, and 11, transgenic stem cell biological-activity detection. The glial cell line-derived neurotrophic factor gene-modified embryo neural stem cell has strong effects of promoting survival of dopaminergic neurons and retains characteristics of the original neural stem cell. The glial cell line-derived neurotrophic factor gene-modified embryo neural stem cell has good effects of treating motor neuron damage diseases and neuronal degeneration diseases.
Owner:孙勇
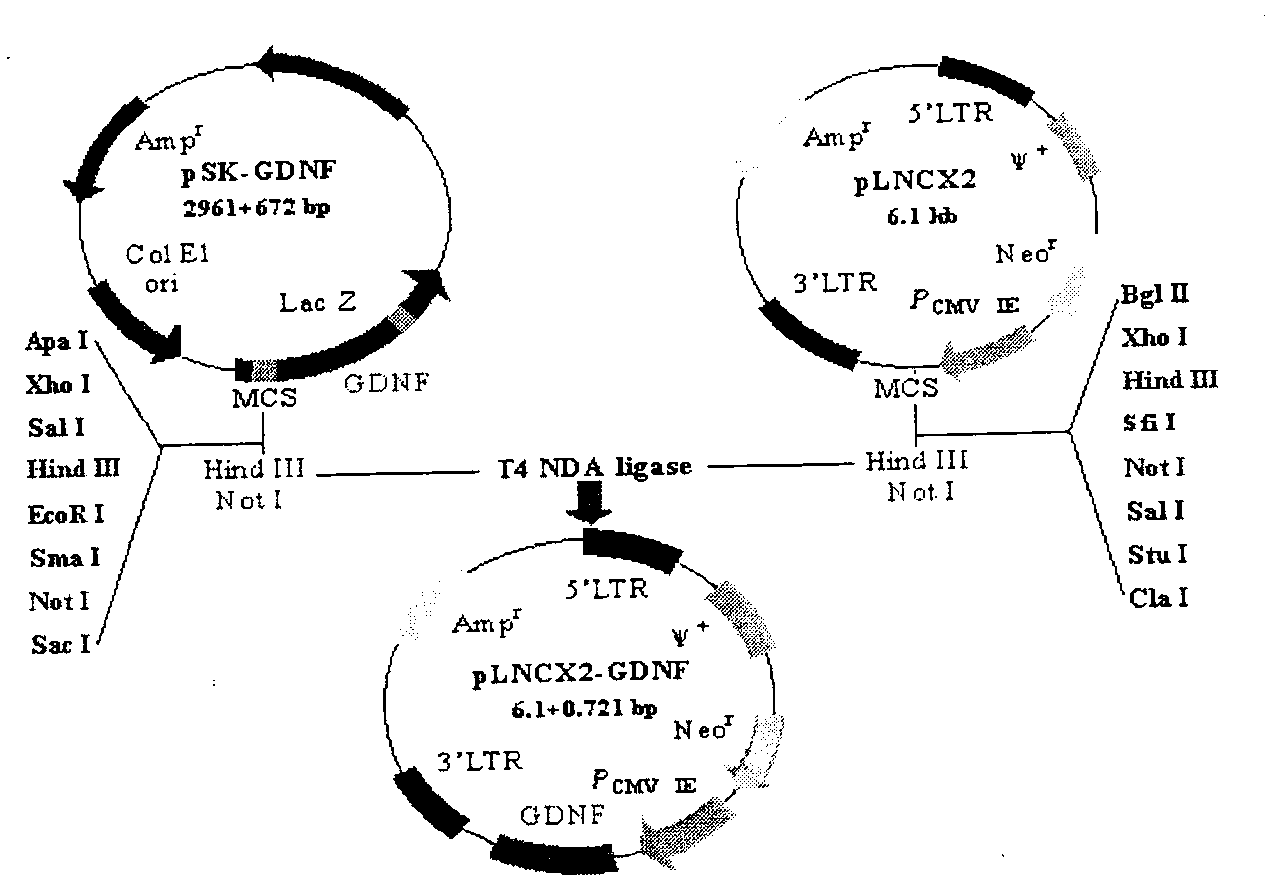
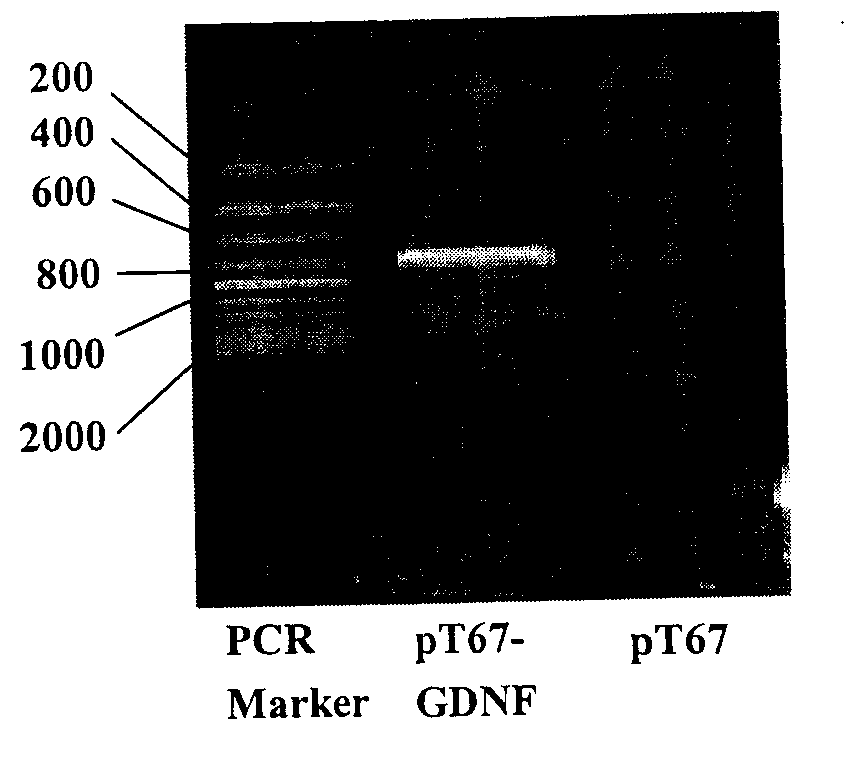
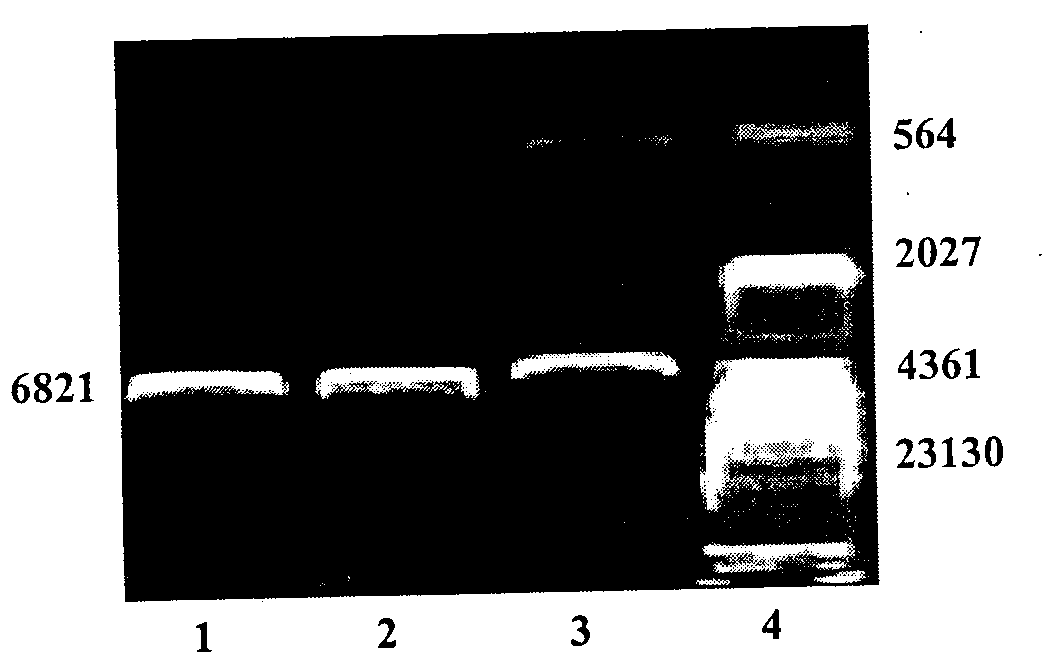
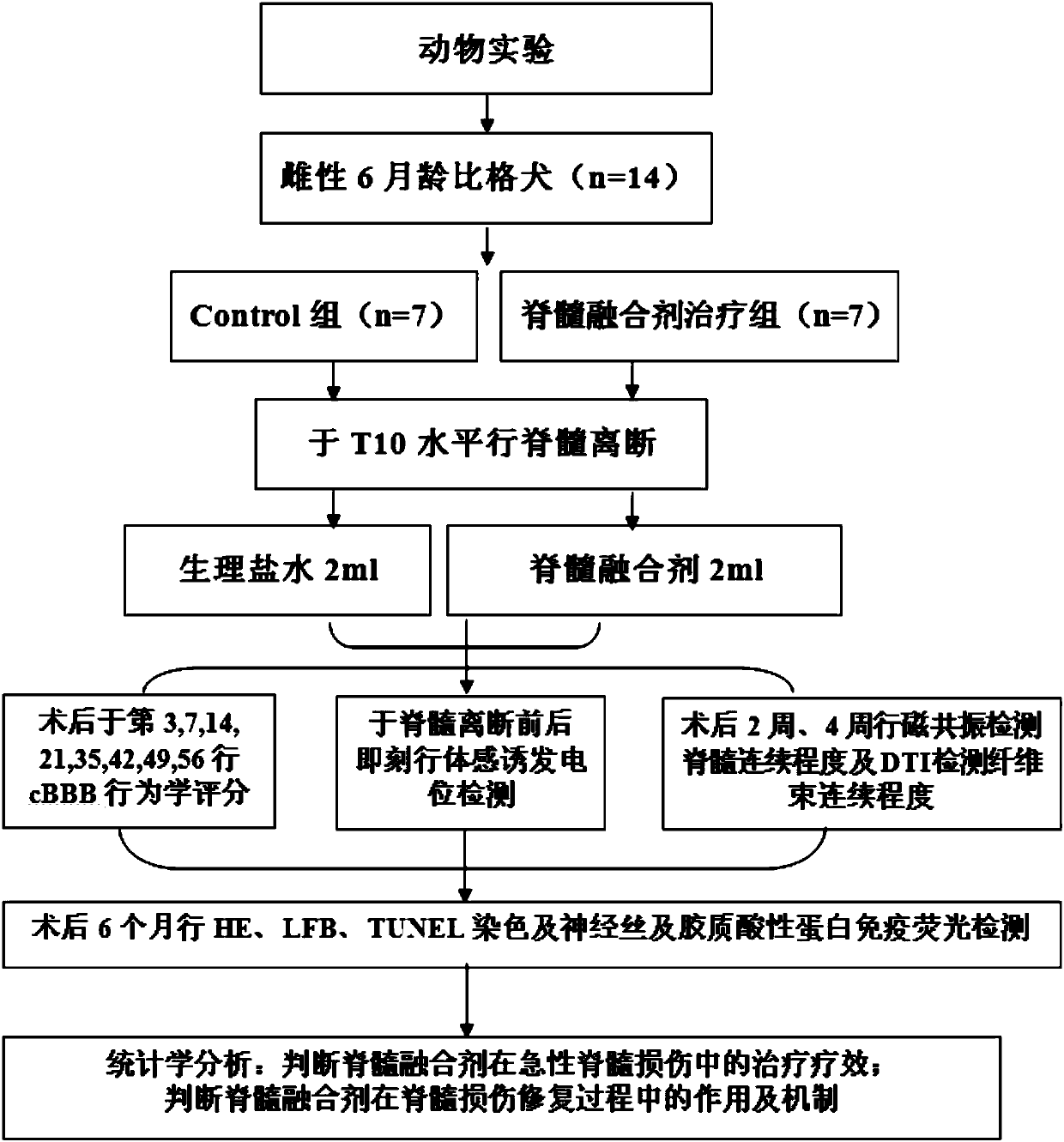
Spinal cord fusion agent, preparation method and use thereof
InactiveCN107596348AInhibit necrosisInhibit local inflammatioNervous disorderPeptide/protein ingredientsGrey matterGlial cell line-derived neurotrophic factor
The invention discloses a spinal cord fusion agent and a preparation method and use thereof. The spinal cord fusion agent disclosed by the invention is prepared from polyethylene glycol, bone mesenchymal stem cells, Schwann cells, glial cell lined derived neurotrophic factor (GDNF), salvianolic acid and nano graphene. Studies show that the use of the spinal cord fusion agent of the invention enables rapid reconnection of divided white matter fiber tracts and gray matter staple fibers to reconstitute spinal integrity and electrical conduction and is capable of recovering motion and sensory functions below the lesion level in a short period of time, thereby effectively reducing the incidence of paraplegia and complications thereof, and no relevant products exist at home and abroad. The spinal cord fusion agent disclosed by the invention can have a wide application prospect clinically.
Owner:HARBIN MEDICAL UNIVERSITY
Method for inducing neural differentiation
InactiveUS20050287665A1Safe and effective in changeFix bugsOrganic active ingredientsNervous disorderGlial cell line-derived neurotrophic factorBone Marrow Stem Cell
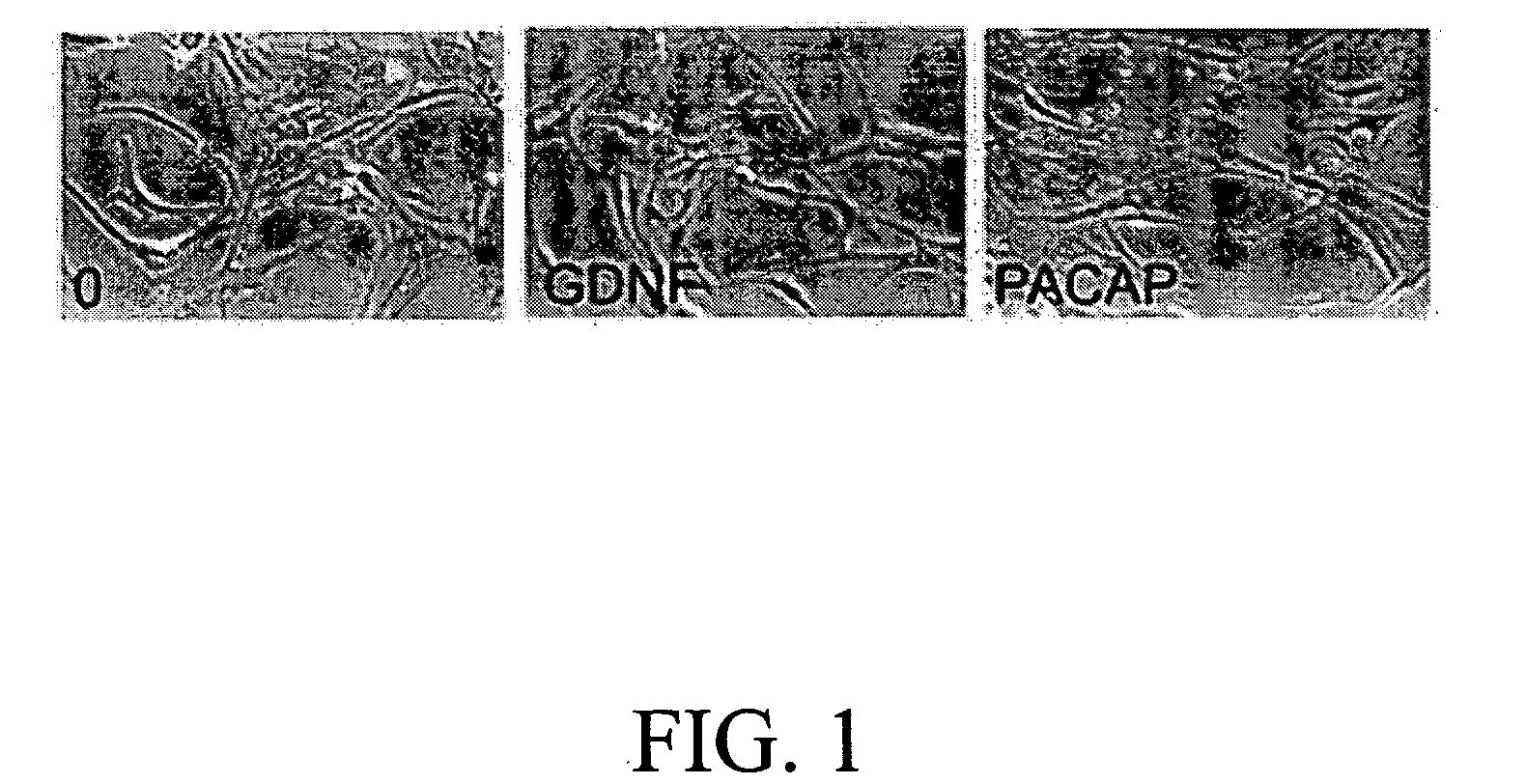
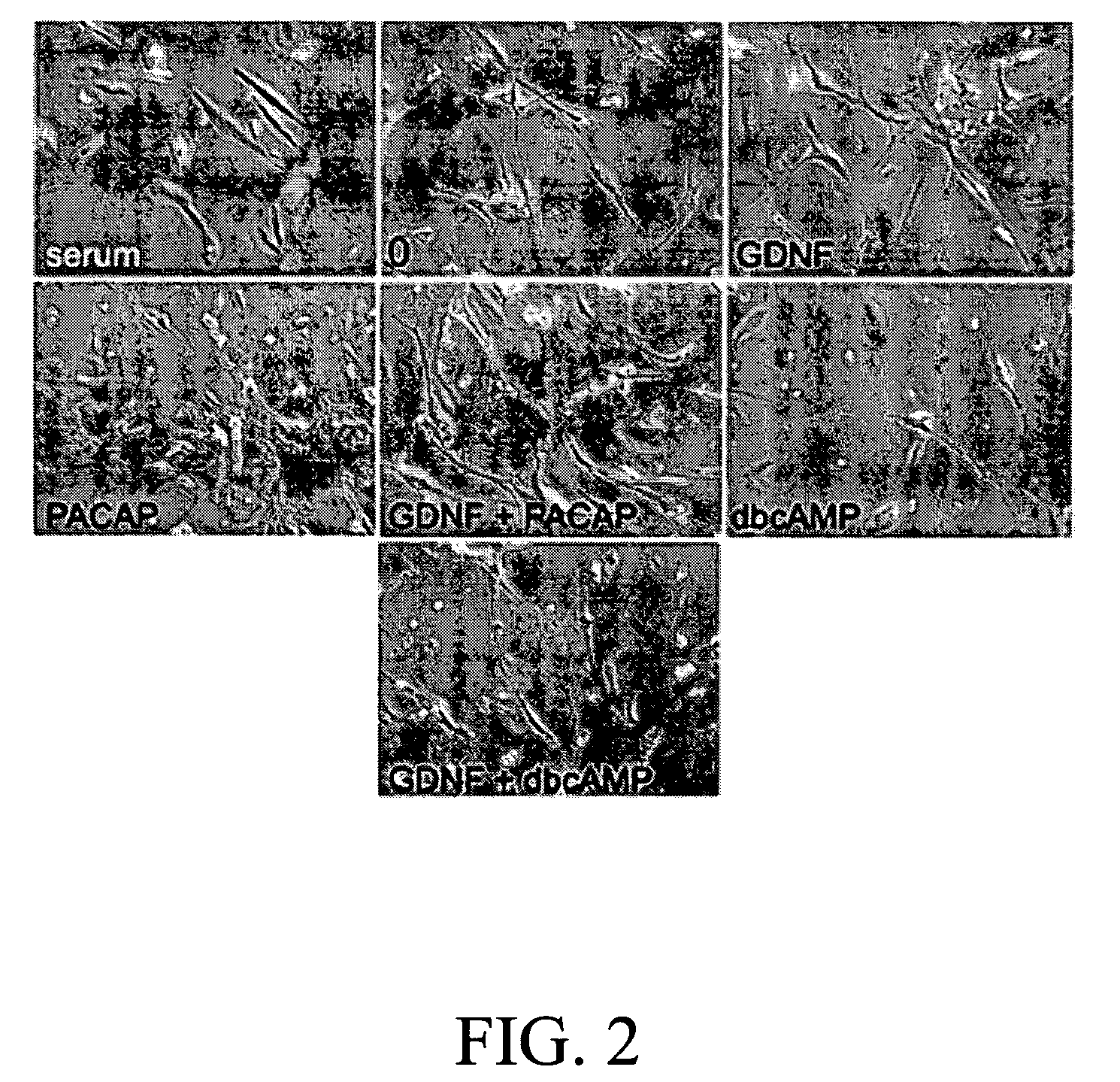
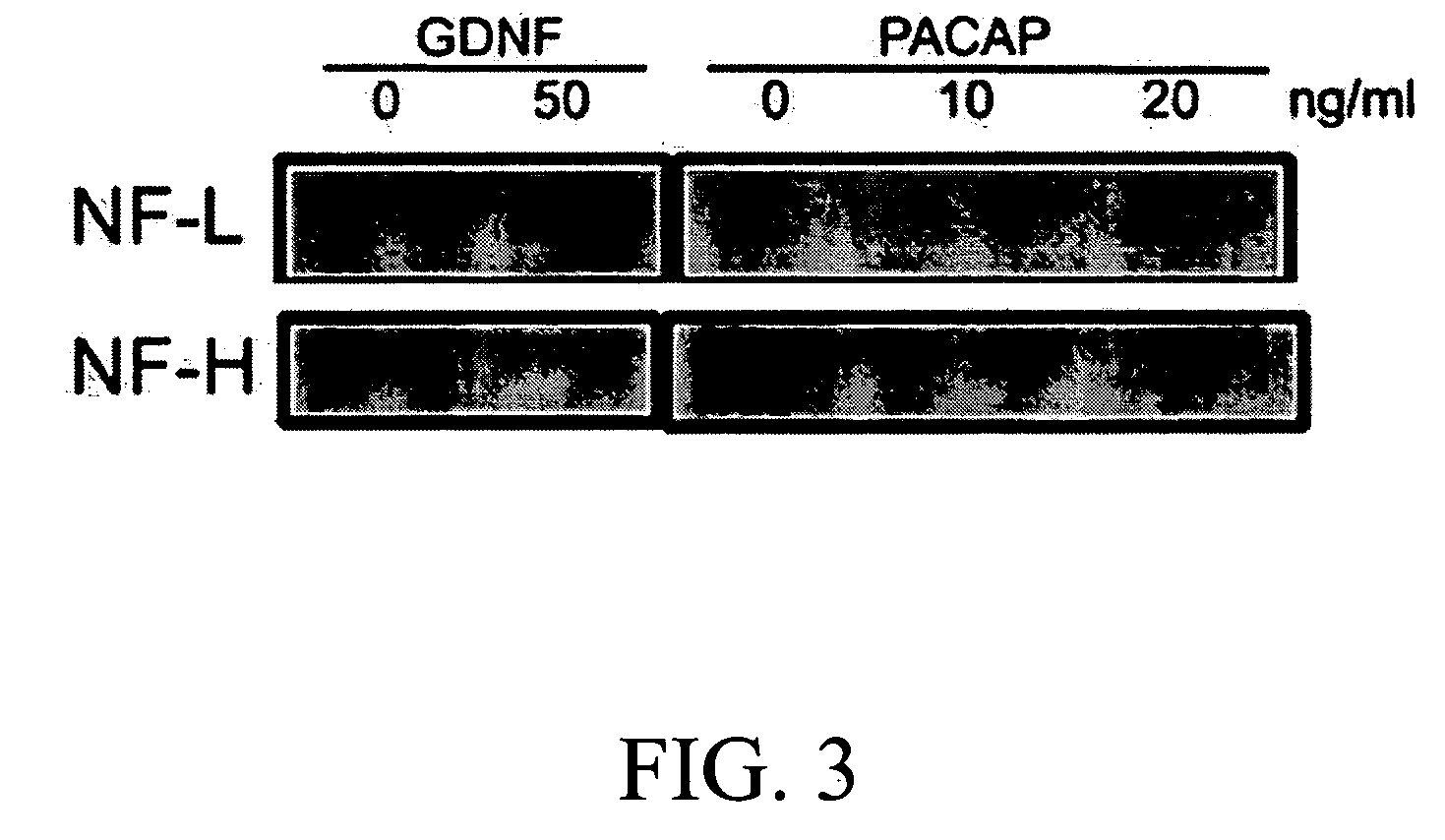
The present invention provides a method for inducing neural differentiation comprising treating a bone marrow stem cell with a neurotrophic factor and / or dibutyryl cAMP (dbcAMP), wherein the neurotrophic factor comprises glial cell line-derived neurotrophic factor (GDNF) or pituitary adenylate cyclase-activating polypeptide (PACAP).
Owner:HENRICH CHENG
Chitosan artificial nerve graft containing neurotrophic factor and preparation method thereof
InactiveCN102343112AImprove adsorption capacityEasy to transportProsthesisGlial cell line-derived neurotrophic factorCross-link
The invention discloses a chitosan artificial nerve graft containing a neurotrophic factor and a preparation method thereof. The chitosan artificial nerve graft is prepared by processing chitosan into a nerve conduit with a porous structure and high tensile strength by virtue of weak acid dissolution, mold injection, freezing formation, neutralization and immobilization, cleaning, lyophilization and other processes under a condition of not adding any foaming agent or cross linking agent. The cell factor with a treatment effect in the nerve conduit is one or more of a basic fibroblast growth factor (bFGF), a brain derived neurotrophic factor (BDNF), a neurotrophic factor 3 (NT-3), a basic fibroblast cell growth factor (bFGF) and a glial-cell-line derived neurotrophic factor (GDNF) and the like. The product provided by the invention contains the neurotrophic factor, adopts degradable materials, and has excellent biocompatibility with a human body. The prepared product does not contain exogenous toxic or side-effect substances brought by a preparation process.
Owner:JIANGSU YITONG BIOTECHNOLOGY CO LTD
Method for separating human spermatogonial stem cells
InactiveCN102344909AHigh purityIncrease productionGerm cellsGlial cell line-derived neurotrophic factorSeminiferous tubule
The invention belongs to the field of biological engineering and provides a method for separating human spermatogonial stem cells. The method comprises the following steps of: mechanically separating a testicular tissue; processing the testicular tissue into a semi-liquid state; adding digestive enzyme solution I into the liquid testicular tissue in the semi-liquid state; digesting the testiculartissue into a single convoluted tubule; adding digestive enzyme solution II in the convoluted tubule; digesting the convoluted tubule into a single cell; separating reproductive cells from supportingcells in the single cell; separating G protein-coupled receptor 125 (GPR125) positive spermatogonial stem cells from glial cell line-derived neurotrophic factor (GDNF) family receptor alpha 1 (GFRA1)positive spermatogonial stem cells by adopting an immunomagnetic bead cell sorting method; and identifying the separated GPR125 positive spermatogonial stem cells and the GFRA1 positive spermatogonial stem cells by adopting an immune cell chemical method. By the method, a large number of the high-purity human spermatogonial stem cells are obtained, and sufficient cell sources are provided for theresearch of the human spermatogonial stem cells and the application of the human spermatogonial stem cells in regenerative medicine and reproductive medicine.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Recovery liquid and method for cryopreserved epidermal stem cells
PendingCN106520677APromote growthSuppression of aging gene expressionCulture processEpidermal cells/skin cellsRecovery methodGlial cell line-derived neurotrophic factor
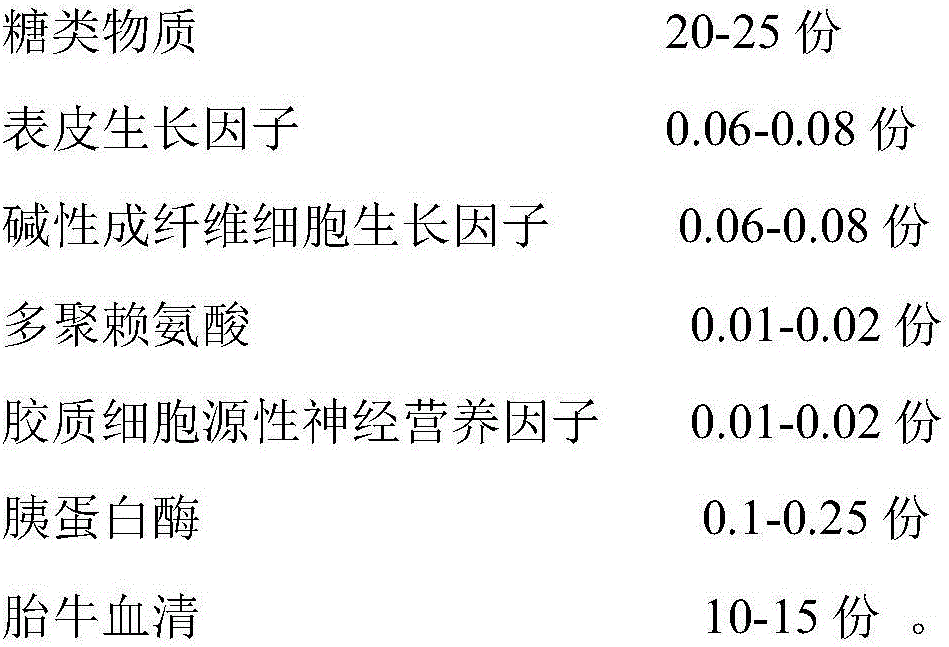
The invention discloses a recovery liquid and a recovery method for cryopreserved epidermal stem cells. According to the key point of the technical scheme, the recovery liquid is prepared from the following components in parts by weight: 20-25 parts of carbohydrates, 0.06-0.08 part of epidermal growth factors, 0.06-0.08 part of alkaline fibroblast growth factors, 0.01-0.02 part of polylysine, 0.01-0.02 part of glial cell line derived neurotrophic factors, 0.1-0.25 part of trypsin and 10-15 parts of fetal calf serum. According to the technical scheme, good recovery effect of the epidermal stem cells is achieved, and the growth environment of the epidermal stem cells is relatively proper.
Owner:浙江译美生物科技有限公司
Novel receptor for GDNF family ligands
InactiveUS20080057516A1Advantageous biodistributionReduce processing stepsBioreactor/fermenter combinationsCompound screeningDiseaseGlial cell line-derived neurotrophic factor
The present invention relates to an in vitro method for identifying a molecule, which interferes with the interaction between a glial cell-line derived neurotrophic factor family ligand (GFL) and a heparan sulfate proteoglycan (HSPG), by screening a library of molecules against a matrix anchored complex comprising at least one immobilized glial cell-line derived neurotrophic factor family ligand (GFL) and at least one heparan sulfate proteoglycan (HSPG), wherein the interfering molecule is isolated based on its capacity to replace a glial cell-line derived neurotrophic factor family ligand (GFL) in said anchored complex. The invention also relates to a complex for identifying such a molecule. The invention also relates to methods for preventing or delaying a neurodegenerative process as well as to method for prophylactic treatment or treatment of a disorder in the nervous system.
Owner:SAARMA MART +3
Nerve graft and nerve graft system using same
ActiveCN106822993AImprove match rateImplement category guidanceTissue regenerationProsthesisGlial cell line-derived neurotrophic factorDisease
The invention provides a nerve graft and a nerve graft system using the same, and relates to the technical field of medical engineering. The nerve graft provided by the invention comprises a sensory nerve bundle passage, a moving nerve bundle passage and a mixed nerve bundle passage; all nerve bundle passages are matched with the nerve bundles of the corresponding sections of normal nerve, and are respectively filled with NGF (nerve growth factor), GDNF (glial cell-line derived neurotrophic factor), and NGF and GDNF. The nerve graft provided by the invention is matched with the nerve section to be restored in appearance; the inside nerve bundle passage trend is also precisely matched with the nerve bundle to be restored. Secondly, different nerve nutrition factors are respectively loaded in the nerve graft; the promotion and the direction guide can be performed for the nerve bundles with different functions; the matching rate of the nerve graft and the receptor nerve can be improved; the classified guidance on different nerve bundles can be realized; the nerve restoration effect is improved. In addition, the invention also provides the nerve graft system using the nerve graft so as to treat complicated nerve defect diseases and patients.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +2
Method of Treating Parkinson's Disease in Humans by Convection-Enhanced Infusion of Glial Cell-Line Derived Neurotrophic Factor to the Putamen
ActiveUS20110245798A1Impressive re-innervationFunction increaseNervous disorderPeptide/protein ingredientsGlial cell line-derived neurotrophic factorMedicine
A method of treating Parkinson's disease in humans is disclosed, wherein glial cell-line derive neurotrophic factor (GDNF) is chronically administered directly to one or both putamen of a human in need of treatment thereof via convection-enhanced infusion using at least one implantable pump and at least one catheter. In one aspect of the present invention the GDNF is infused directly into one or both putamen through one or more indwelling intraparenchymal mutitiport brain catheters connected to one or moreimplantable pumps wherein the flow rate is pulsed.
Owner:AMGEN INC +1
Receptor for GDNF family ligands
InactiveUS8034572B2Advantageous biodistributionAvoid delayCompound screeningBioreactor/fermenter combinationsGlial cell line-derived neurotrophic factorNervous system
The present invention relates to an in vitro method for identifying a molecule, which interferes with the interaction between a glial cell-line derived neurotrophic factor family ligand (GFL) and a heparan sulfate proteoglycan (HSPG), by screening a library of molecules against a matrix anchored complex comprising at least one immobilized glial cell-line derived neurotrophic factor family ligand (GFL) and at least one heparan sulfate proteoglycan (HSPG), wherein the interfering molecule is isolated based on its capacity to replace a glial cell-line derived neurotrophic factor family ligand (GFL) in said anchored complex. The invention also relates to a complex for identifying such a molecule. The invention also relates to methods for preventing or delaying a neurodegenerative process as well as to method for prophylactic treatment or treatment of a disorder in the nervous system.
Owner:SAARMA MART +3
Gene capable of enhancing anti-inflammatory capability of human adipose-derived stem cells and application of gene
InactiveCN108441498AEasy treatmentOptimizing FibrosisNeuregulinsUrinary disorderGlial cell line-derived neurotrophic factorCell therapy
The invention discloses a gene capable of enhancing the anti-inflammatory capability of human adipose-derived stem cells (AMSCs) and application of the gene. A nucleotide sequence of the gene is shownas SEQ ID NO: 1. The invention further provides the application of the gene capable of enhancing the anti-inflammatory capability of the human adipose-derived stem cells to a preparation process of amedicine for treating kidney diseases. The gene is a glial cell line-derived neurotrophic factor GDNF. The GDNF modified AMSCs can be used for optimizing the effects of treating kidney fibrosis and delaying a renal failure progress of pure adipose-derived stem cells. According to the gene disclosed by the invention, related researches of the kidney diseases are carried out by applying the GDNF modified AMSCs for the first time, a stem cell therapy method is optimized and a novel thought of traditional cell therapy is explored.
Owner:THE AFFILIATED HOSPITAL OF XUZHOU MEDICAL UNIV
Splice variants of gdnf and uses thereof
ActiveUS20100311653A1Organic active ingredientsNervous disorderBiological regulationGlial cell line-derived neurotrophic factor
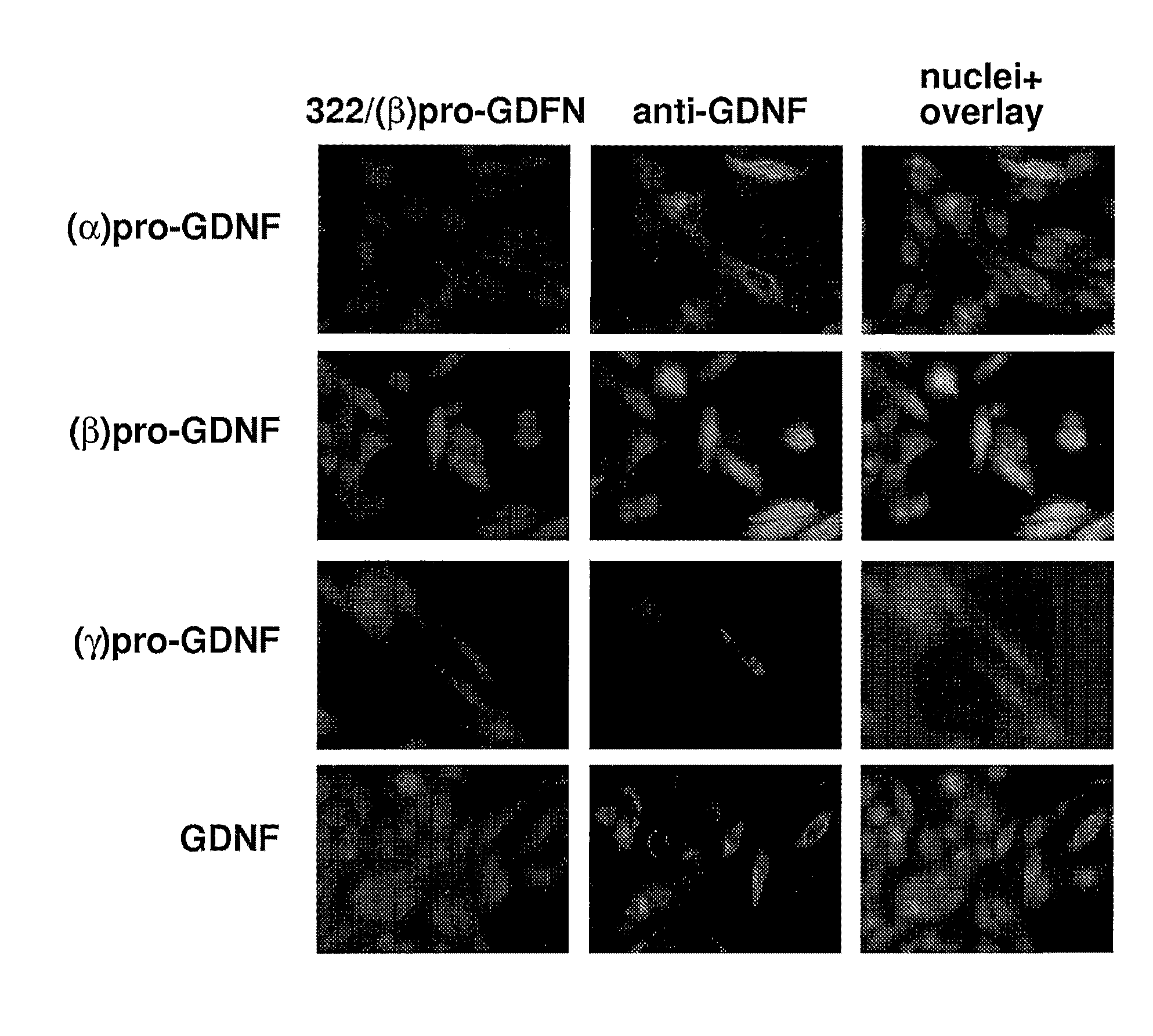
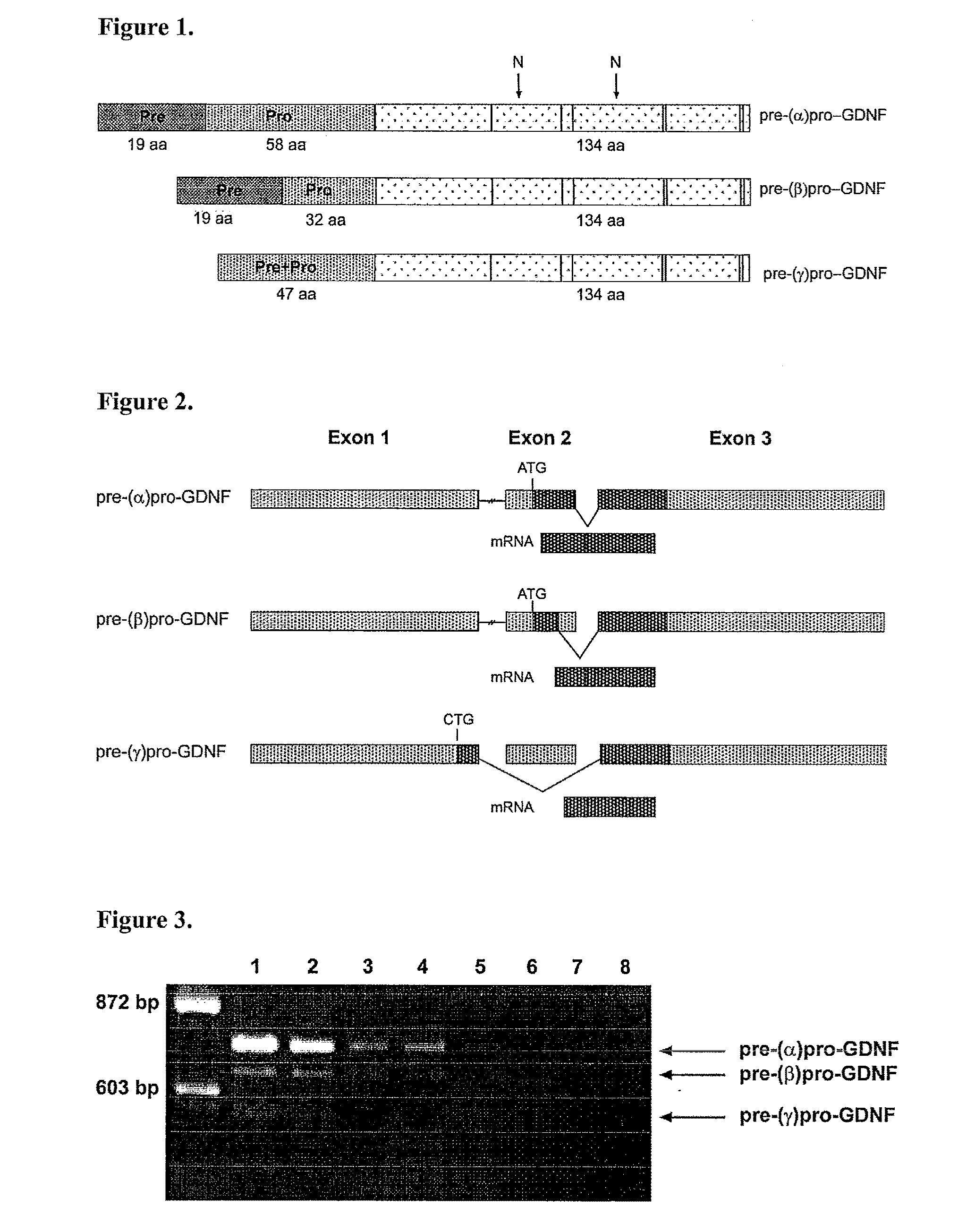
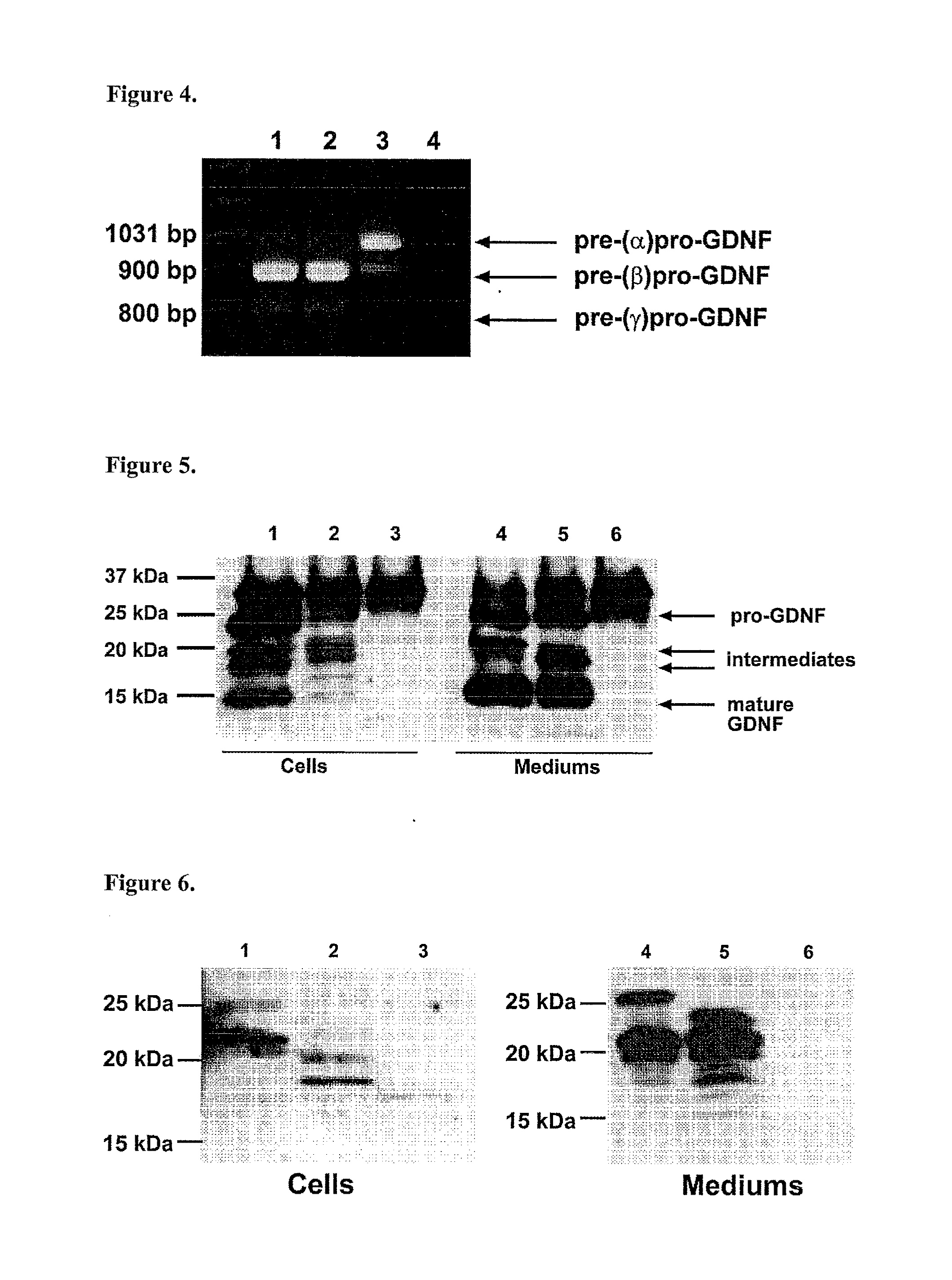
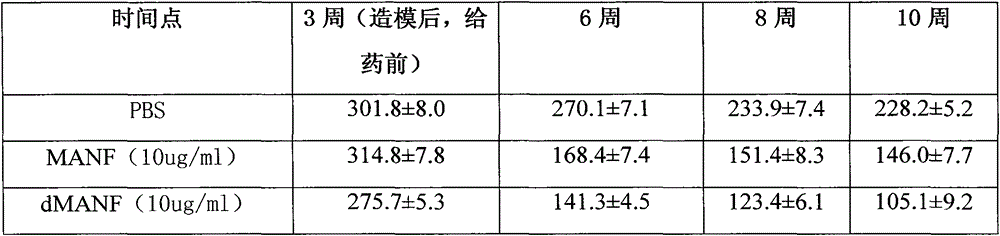
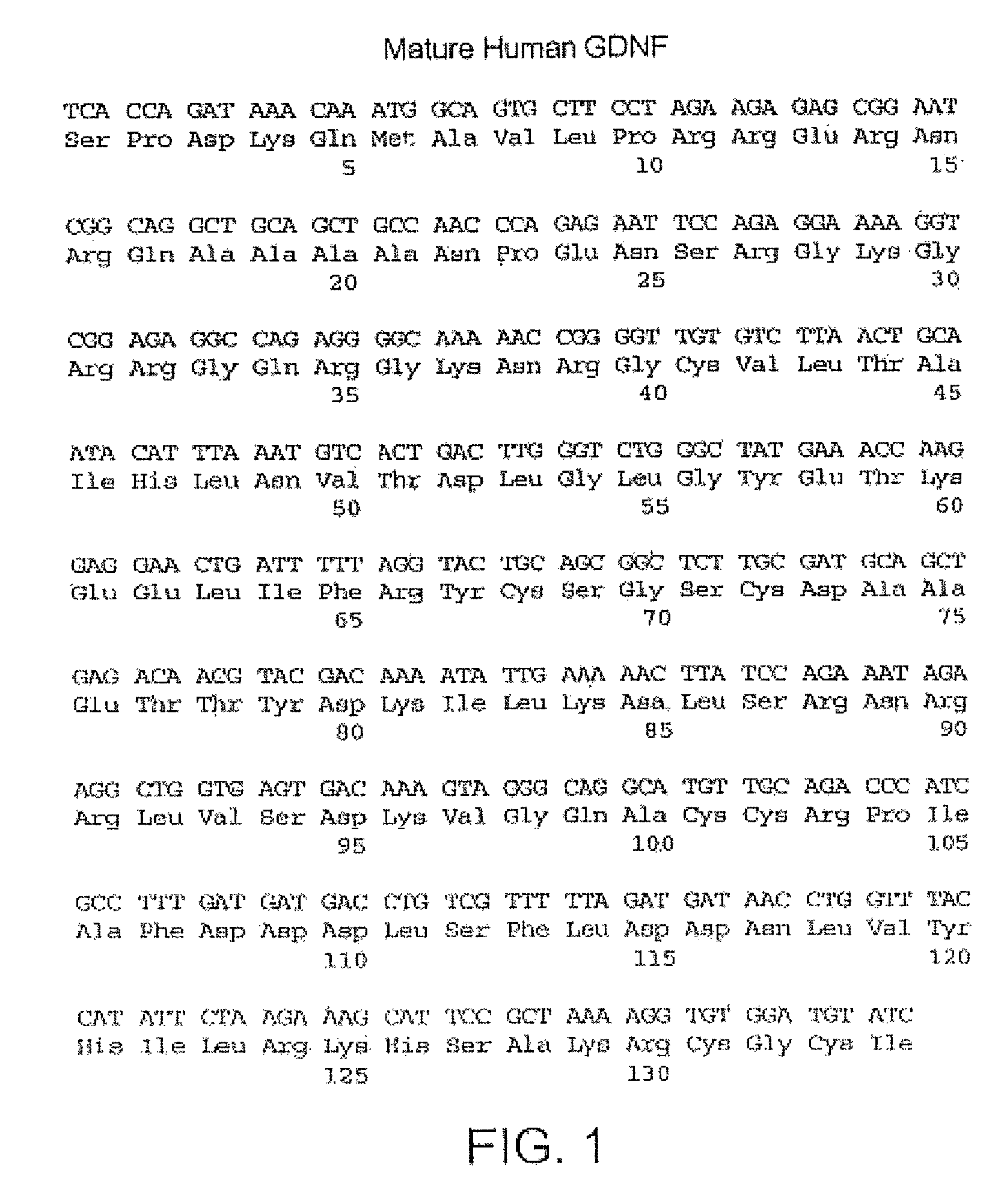
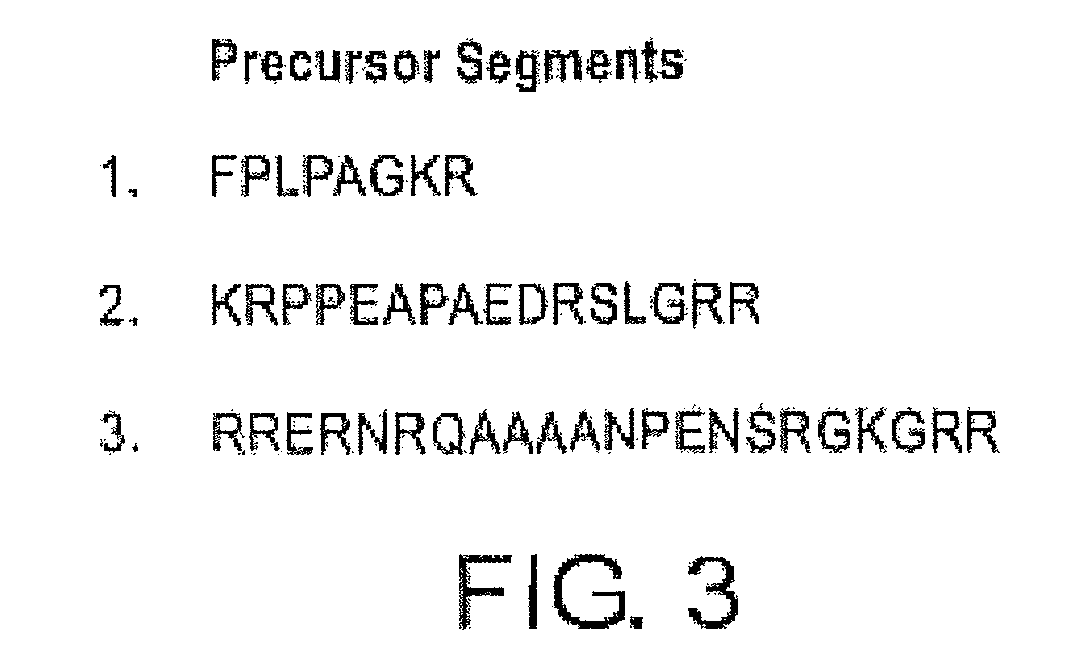
The present invention relates to Glial Cell Line-Derived Neurotrophic Factor (GDNF) protein and gene and is, in particular, directed to a novel splice variant of GDNF protein, which is encoded by a novel splice variant pre-(γ)pro-GDNF, and secreted under biological regulation.
Owner:NEVALAITA LIINA +1
Method for studying migration, proliferation and differentiation of brain endogenous neural stem cells
InactiveCN103940985ADisease diagnosisBiological testingGlial cell line-derived neurotrophic factorNeural cell
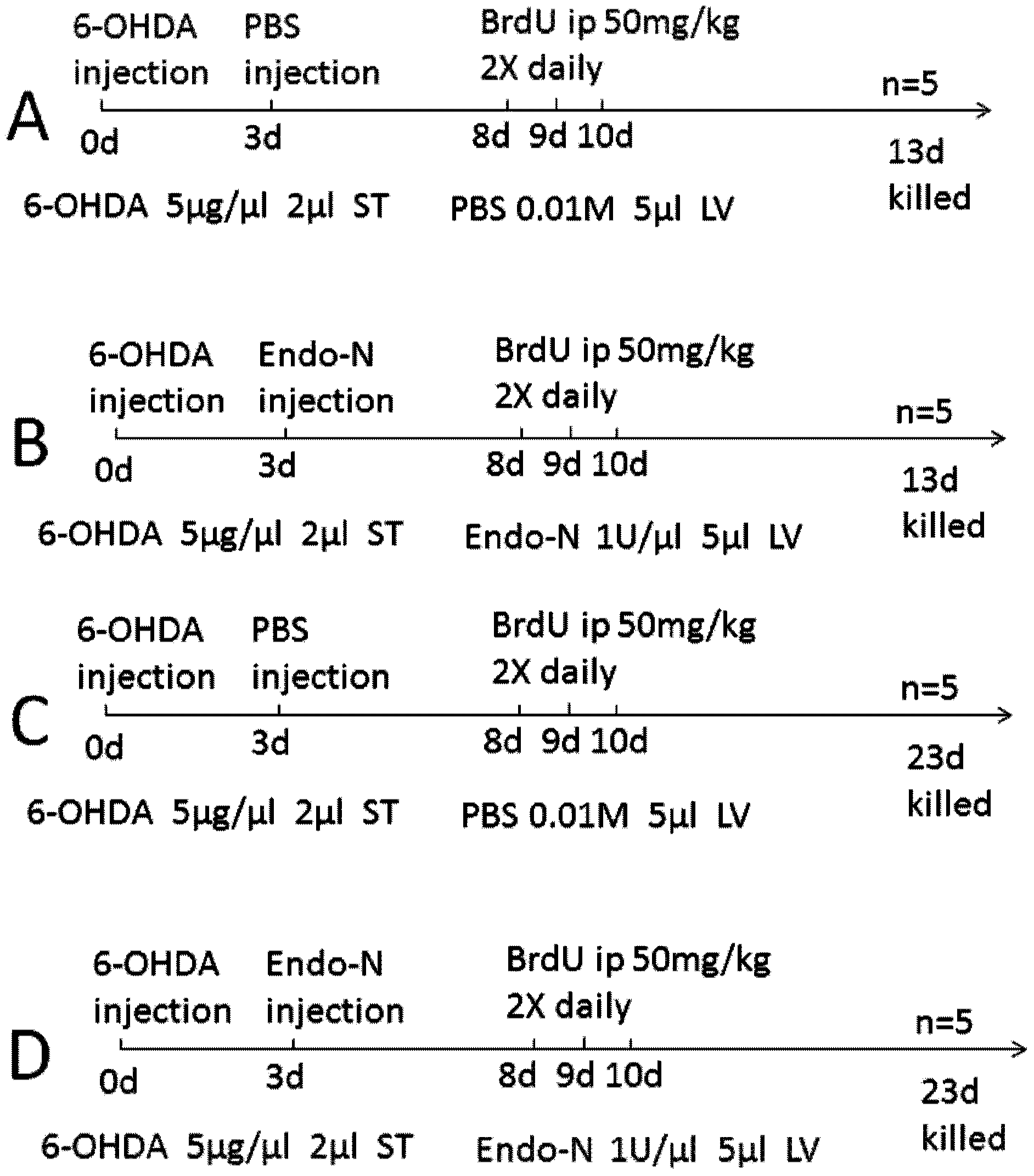
The invention relates to a method for studying migration, proliferation and differentiation of brain endogenous neural stem cells. The method comprises the following steps: (1) performing double-labeled immunofluorescent staining on an animal brain section by 5-bromndeoxyuridine and doublecortin, 5-bromndeoxyuridine and neuronal nuclei antigen as well as 5-bromndeoxyuridine and glial cell line-derived neurotrophic factor, and single-labeled immunofluorescent staining by sialyl neural cell adhesion molecules; and (2) carrying out statistic analysis on data and drawing by GraphPad Prism6.0 software, and performing one-way variance analysis on single-factor multi-group data of the 5-bromndeoxyuridine positive cells, wherein the animal brain section is subjected to the following treatment: stereotactically injecting a 6-hydroxydopamine solution into a corpus striatum of the right side of the brain, injecting a PSA (prostate-specific antigen) specificity lyase stock solution into the lateral ventricle, and then injecting a 5-bromndeoxyuridine solution in the abdominal cavity. An effective method for studying migration influence of the brain endogenous neural stem cells is provided.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Implantable Biocompatible Immunoisolatory Vehicle for Delivery of Gdnf
InactiveUS20080286250A1Reduce reduction reactionReduce riskBiocideSenses disorderGlial cell line-derived neurotrophic factorHuman cell
The present invention relates to devices comprising a composition of human cells secreting a therapeutically effective amount of GDNF (Glial cell-line-derived neurotrophic factor) encapsulated in a device comprising a core and a semipermeable membrane allowing for the diffusion of GDNF protein. The human cells are from one cell line.
Owner:NSGENE AS
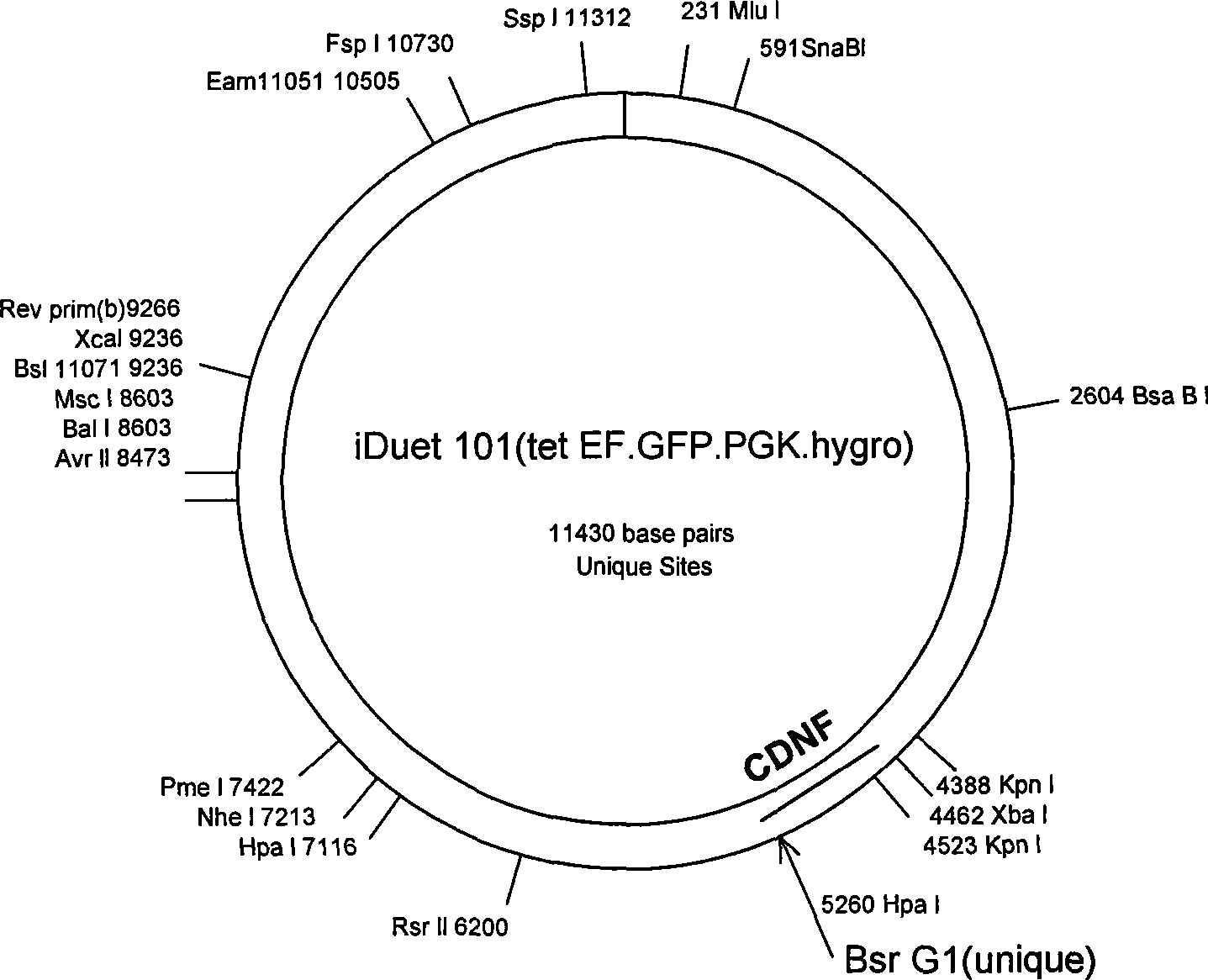
Deletion type recombinant human mesencephalic astrocyte-derived neurotrophic factor
InactiveCN105315359ANervous disorderPeptide/protein ingredientsGlial cell line-derived neurotrophic factorCiliary neurotrophic factor
The invention belongs to the field of biological medicines, particularly a deletion type recombinant human mesencephalic astrocyte-derived neurotrophic factor for treating Parkinson's disease. By carrying out partial delection mutation on an N terminal of the deletion type recombinant human mesencephalic astrocyte-derived neurotrophic factor, the obtained deletion type recombinant human mesencephalic astrocyte-derived neurotrophic factor has the characteristic of higher activity.
Owner:BEIJING NEWINER BIOTECH CO LTD
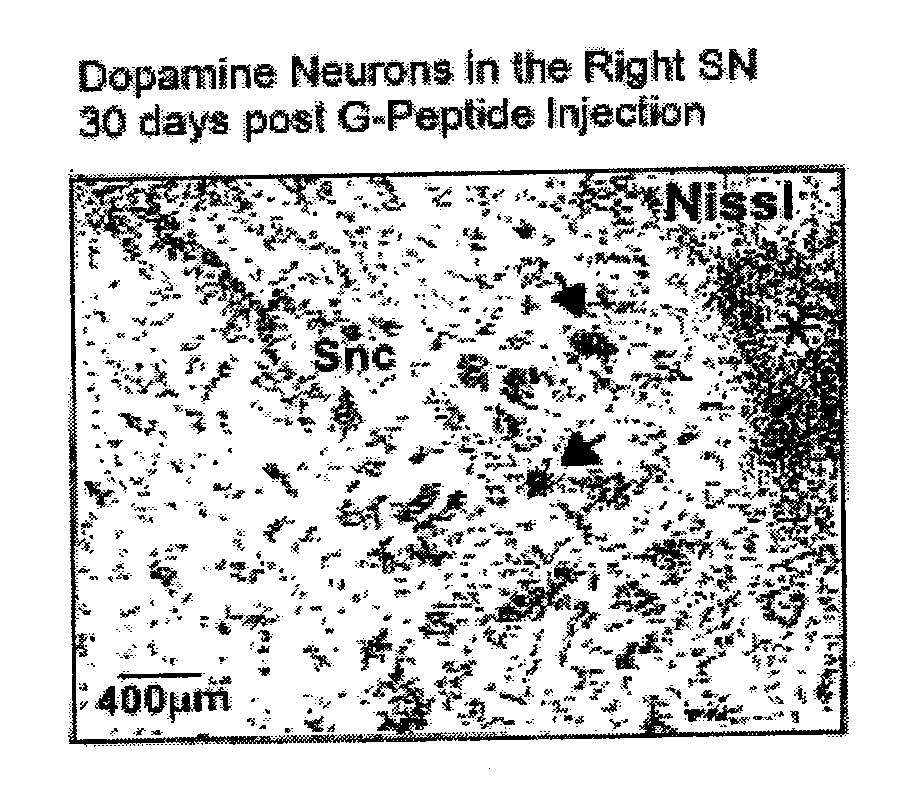
Amidated dopamine neuron stimulating peptide restoration of mitochondrial activity
ActiveUS9402875B2Relieve symptomsAmeliorate and inhibit effectNervous disorderPeptide/protein ingredientsDiseaseGlial cell line-derived neurotrophic factor
The present invention relates to the use of novel proteins, referred to herein as amidated glial cell line-derived neurotrophic factor (GDNF) peptides (or “Amidated Dopamine Neuron Stimulating peptides (ADNS peptides)”), for treating brain diseases and injuries that result in dopaminergic deficiencies and mitochodrial dysfunction, e.g., reduced complex I enzyme activity.
Owner:UNIV OF KENTUCKY RES FOUND
Method for separating human spermatogonial stem cells
InactiveCN102344909BHigh purityIncrease productionGerm cellsSeminiferous tubuleGlial cell line-derived neurotrophic factor
The invention belongs to the field of biological engineering and provides a method for separating human spermatogonial stem cells. The method comprises the following steps of: mechanically separating a testicular tissue; processing the testicular tissue into a semi-liquid state; adding digestive enzyme solution I into the liquid testicular tissue in the semi-liquid state; digesting the testicular tissue into a single convoluted tubule; adding digestive enzyme solution II in the convoluted tubule; digesting the convoluted tubule into a single cell; separating reproductive cells from supporting cells in the single cell; separating G protein-coupled receptor 125 (GPR125) positive spermatogonial stem cells from glial cell line-derived neurotrophic factor (GDNF) family receptor alpha 1 (GFRA1) positive spermatogonial stem cells by adopting an immunomagnetic bead cell sorting method; and identifying the separated GPR125 positive spermatogonial stem cells and the GFRA1 positive spermatogonial stem cells by adopting an immune cell chemical method. By the method, a large number of the high-purity human spermatogonial stem cells are obtained, and sufficient cell sources are provided for the research of the human spermatogonial stem cells and the application of the human spermatogonial stem cells in regenerative medicine and reproductive medicine.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
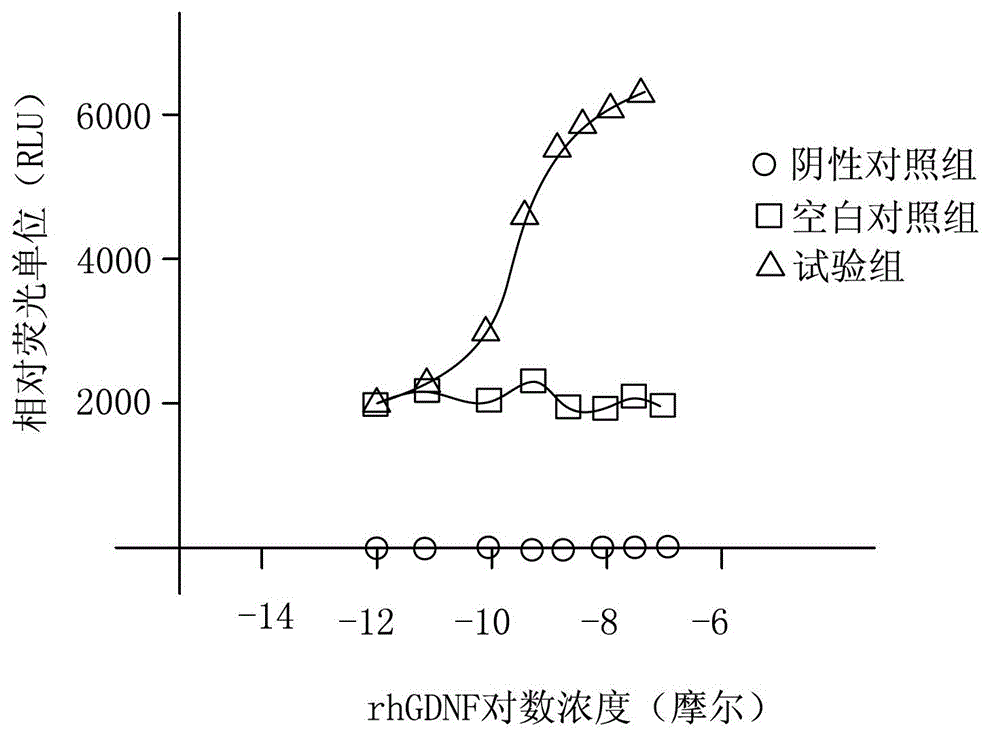
Cytological method for assaying activity of rhGDNF (recombinant human Glial cell line-Derived Neurotrophic Factor)
ActiveCN103146798AEffective assay activityAccurate and reliable measurement resultsMicrobiological testing/measurementGlial cell line-derived neurotrophic factorCytology
The invention relates to a cytological method for assaying the activity of an rhGDNF (recombinant human Glial cell line-Derived Neurotrophic Factor). The method comprises the following steps of: constructing expression plasmid pcDNA3.1-hGFR alpha 1, and then preparing an HEK293 cell which stably expresses hGFR alpha 1; constructing expression plasmid pcDNA3.1-hRET, and then preparing an HEK293 cell capable of simultaneously expressing hGFR alpha 1, hRET and a luciferase reporting system; adding the rhGDNF to be assayed into the HEK293 cell capable of simultaneously expressing hGFR alpha 1, hRET and the luciferase reporting system, and assaying fluorescence intensity F1 after culturing; meanwhile, arranging a blank control group in which the rhGDNF is not added, and assaying fluorescence intensity F2 after culturing; and comparing F1 with F2. The method has the advantages that the activity of the rhGDNF can be effectively assayed and the assayed result is accurate and reliable.
Owner:JIANGSU CELL TECH MEDICAL RES INST CO LTD
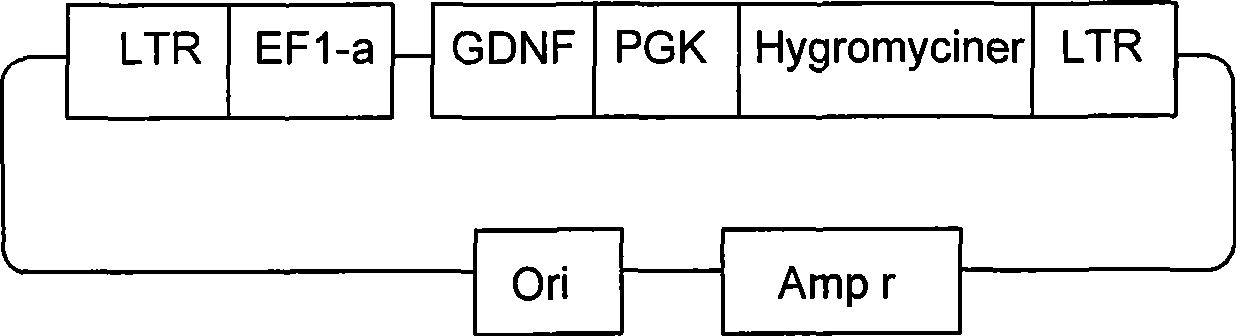
Retroviral vector for expression of glial cell line-derived neurotrophic factor and use thereof
InactiveCN101397573AMaintain biological propertiesNervous disorderGenetic material ingredientsGlial cell line-derived neurotrophic factorBiological property
The invention discloses a lentiviral vector for expressing the glial cell line-derived neurotrophic factor and applications thereof, which belong to the field of medical biotechnology. The preservation number of the lentiviral vector is CGMCC No.2086. The invention has the advantages: the lentiviral vector constructed by the invention has two promoter structures and can simultaneously express GDNF gene and hygromycin resistance gene, the lentiviral vector supernatant packaged by the transient transfeetion method can carry out high-efficiency transfection on the neural stem cells which divide relatively slowly, thus leading the GDNF gene to be integrated to the target cell genom and be stably expressed for a long time; the neural stem cells after transfection can remove the neural stem cells which are not successful for transfection by the screening of the hygromycin so as to obtain the uniform over-expression GDNF cell system; and the neural stem cells which carry out gene modification by the lentiviral vector can still keep the intrinsical biological characteristics.
Owner:XUANWU HOSPITAL OF CAPITAL UNIV OF MEDICAL SCI
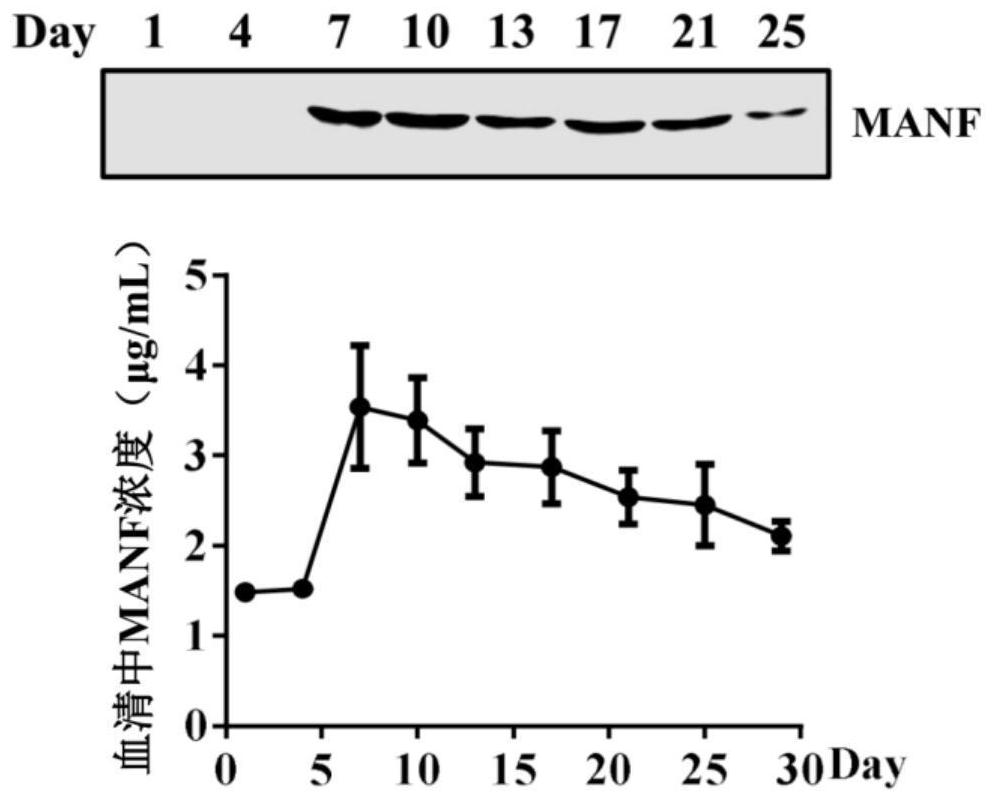
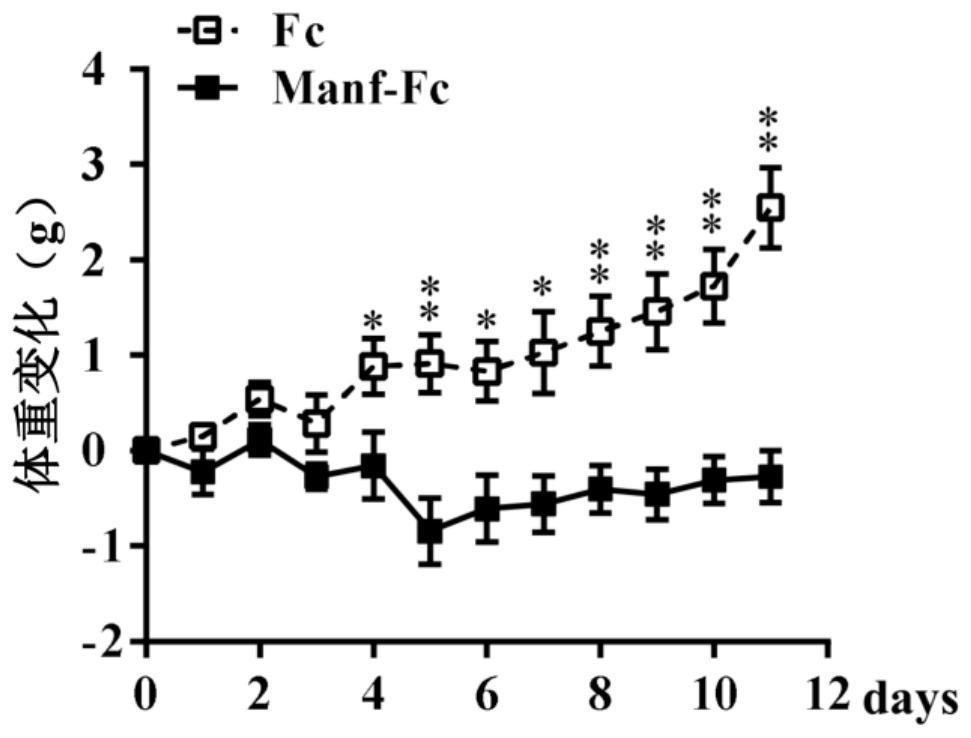
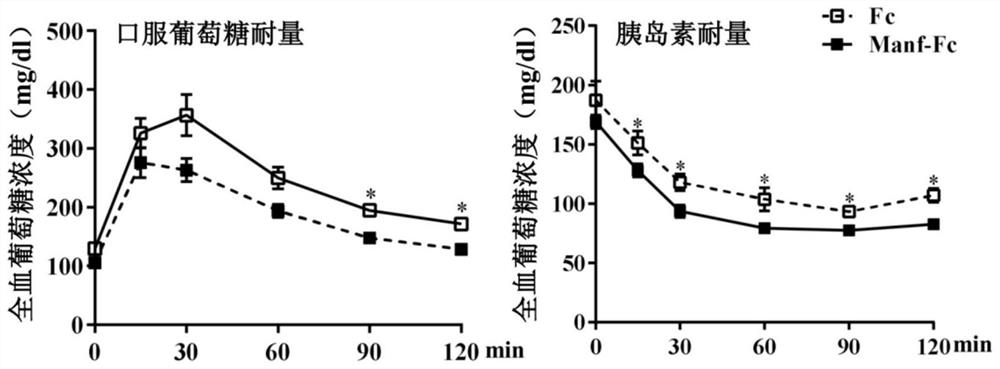
Fusion protein of midbrain astrocyte-derived neurotrophic factor for preventing and treating obesity
ActiveCN113292656AProlong half-life in vivoPeptide/protein ingredientsMetabolism disorderGlial cell line-derived neurotrophic factorDisease
The invention relates to a fusion protein of a midbrain astrocyte-derived neurotrophic factor for preventing and treating obesity, and belongs to the technical field of biological medicines. The invention provides a fusion protein of a midbrain astrocyte-derived neurotrophic factor. The fusion protein at least contains the following two fragments: an amino acid sequence of the midbrain astrocyte-derived neurotrophic factor and an amino acid sequence of an immune globulin Fc fragment. The fusion protein is good in stability and long in half-life period, can be used for treating obesity, diabetes mellitus and other metabolic diseases, and has a good application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Amidated Dopamine Neuron Stimulating Peptides for CNS Dopaminergic Upregulation
InactiveUS20110178025A1Hormone peptidesNervous disorderGlial cell line-derived neurotrophic factorDisease
The present invention relates to novel proteins, referred to herein as amidated glial cell line-derived neurotrophic factor (GDNF) peptides (or “Amidated Dopamine Neuron Stimulating peptides (ADNS peptides)”), that are useful for treating brain diseases and injuries that result in dopaminergic deficiencies.
Owner:UNIV OF KENTUCKY RES FOUND
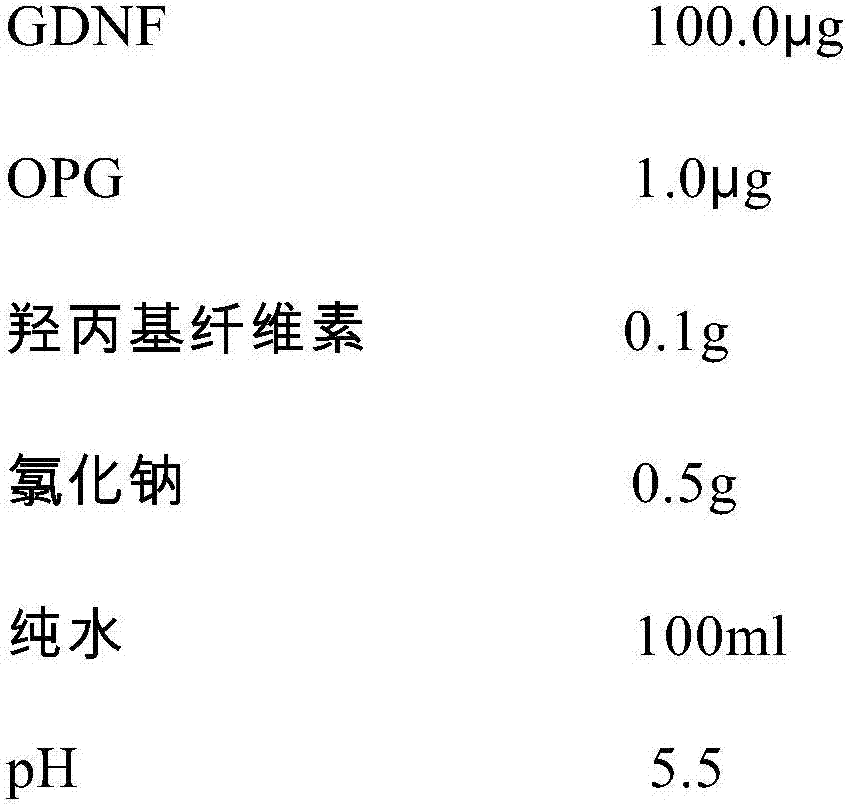
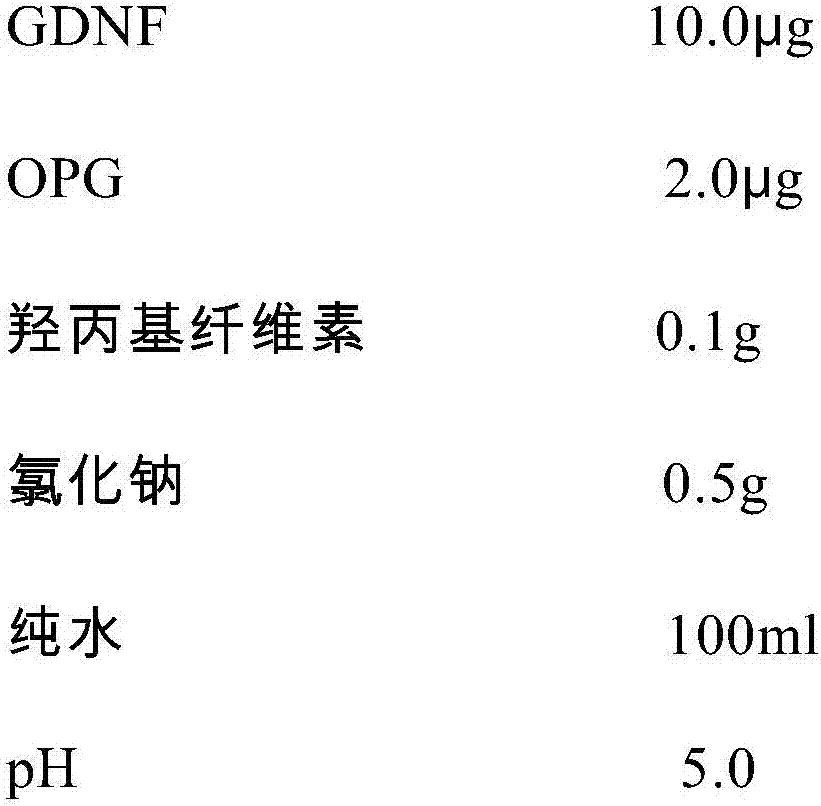
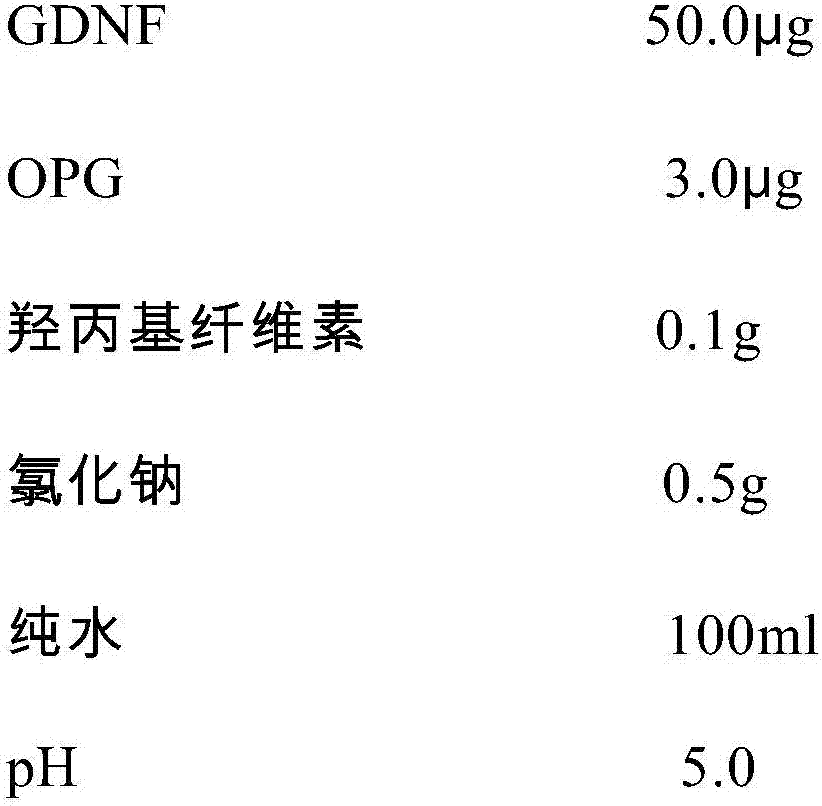
GDNF (glial cell line-derived neurotrophic factor)-containing medicinal composition for treating corneal epithelial damage
InactiveCN107137698APromote proliferationPromotes clonogenicitySenses disorderNervous disorderGlial cell line-derived neurotrophic factorDisease
The invention discloses a GDNF (glial cell line-derived neurotrophic factor)-containing medicinal composition for treating corneal epithelial damage. The medicinal composition contains GDNF (preferably human-sourced GDNF), and preferably further contains OPG (osteoprotegerin, preferably human-sourced OPG). The medicinal composition (preferably in a form of an eye drop) can not only promote the proliferation and the clonal formation ability of corneal limbal stem cells, but also can effectively promote damage repair on a corneal epithelium, including repair on sustained corneal epithelial damage, treatment to diabetic corneal lesions, treatment to neurogenic keratitis and treatment to corneal limbal stem cell deficiency.
Owner:PEKING UNIV THIRD HOSPITAL
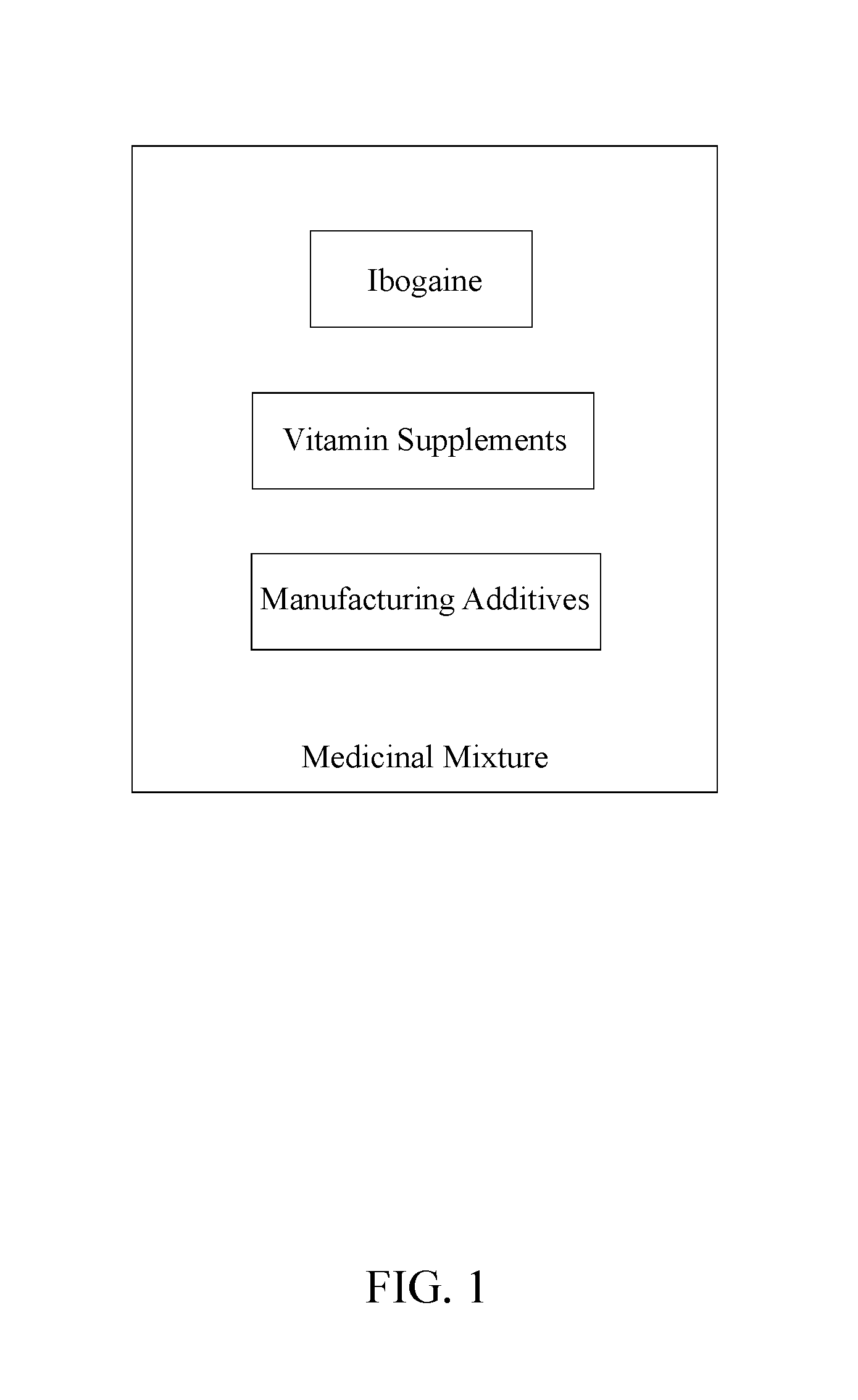
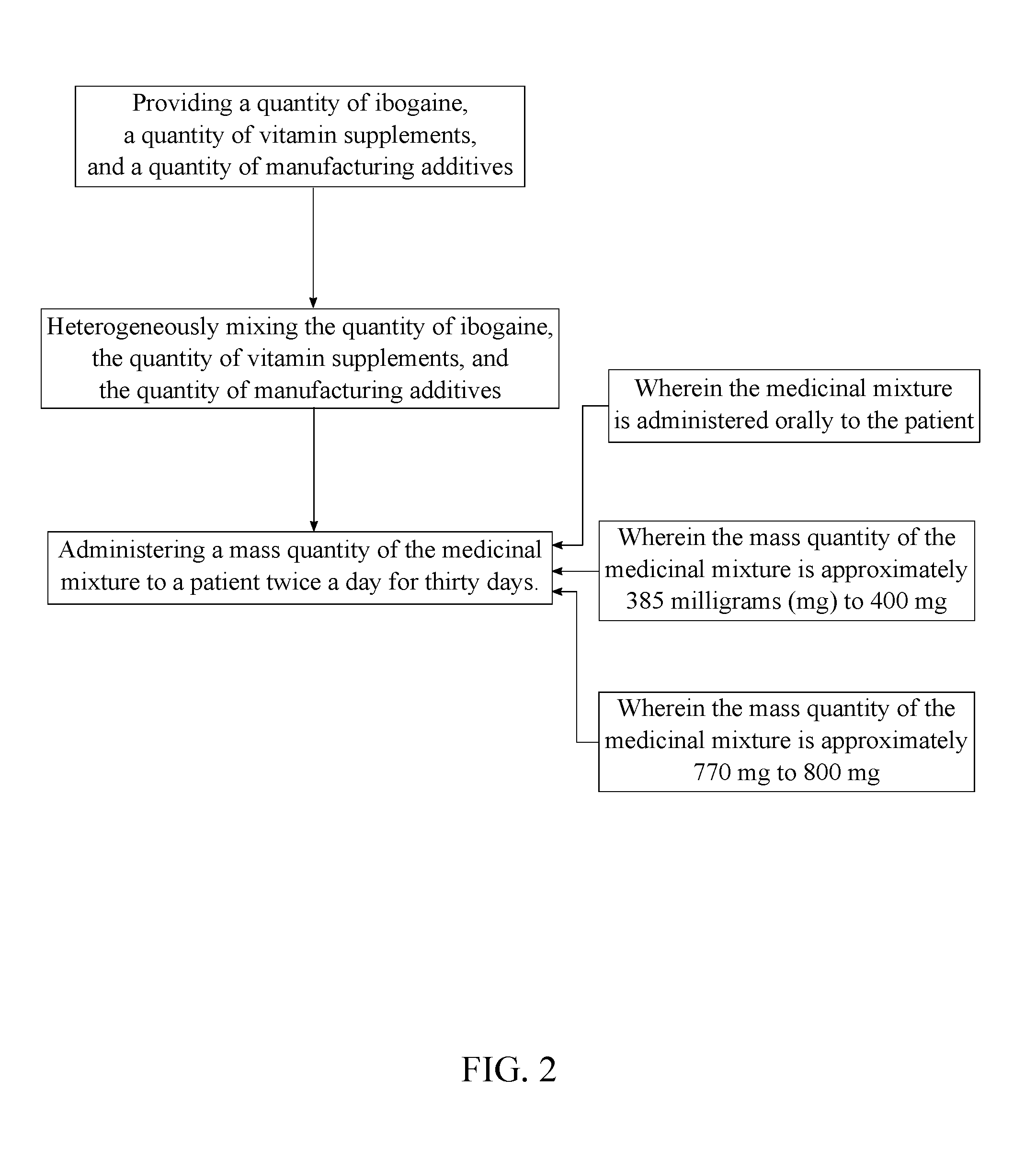
Medicinal Composition for Treating Symptoms Related to Parkinson's Disease
InactiveUS20160331759A1Pharmaceutical non-active ingredientsHeterocyclic compound active ingredientsGlial cell line-derived neurotrophic factorJuvenile parkinson's disease
A medicinal composition is administered orally twice a day to a patient to ameliorate the symptoms related to Parkinson's disease. The medicinal composition includes mixing a quantity of ibogaine, a quantity of vitamin supplements and a quantity of manufacturing additives into a heterogeneous medicinal mixture. The quantity of ibogaine, formulated as ibogaine HCl, other non-toxic salts of ibogaine (an alkaloid of the family apocynaceae), or noribogaine, its active metabolite, is an active ingredient to regulate endogenous glial cell line-derived neurotrophic factor (GDNF). The quantity of vitamin supplements promotes the general health and neurological functions. The quantity of manufacturing additives bonds the quantity of ibogaine and the quantity of vitamin supplements together, as well as, provides anti-adherent properties to prevent the medicinal mixture from adhering to manufacturing equipment.
Owner:PHYTOSTAN INT INC
Real-time fluorescence quantitative PCR (polymerase chain reaction) special primer and method for detecting GDNF (glial cell line-derived neurotrophic factor) splice variants 2,4
InactiveCN103614481AStrong specificityGood repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationGlial cell line-derived neurotrophic factorFluorescence
The invention relates to a real-time fluorescence quantitative PCR (polymerase chain reaction) special primer and method for detecting GDNF (glial cell line-derived neurotrophic factor) splice variants 2,4. The sequences of the primer are shown in SEQIDNO.1 and SEQIDNO.2. The fluorescence quantitative PCR method is implemented by taking a brain tissue cDNA (complementary deoxyribonucleic acid) sample as a template, setting a template-free control, and groping and optimizing condition parameters of a quantitative reaction system by using the primer, so that a reliable and sensitive quantitative detection method for splice variants is developed. According to the invention, the expression levels of a GDNF splice variant 2 (GenecodeNO.: NM_001190469.1) and a splice variant 4 (GenecodeNO.: NM_199231.2) can be detected; the primer and method disclosed by the invention have the advantages of strong specificity and high repeatability.
Owner:XUZHOU MEDICAL COLLEGE
Amidated Dopamine Neuron Stimulating Peptides for CNS Dopaminergic Upregulation
ActiveUS20140148393A1Hormone peptidesNervous disorderDiseaseGlial cell line-derived neurotrophic factor
The present invention relates to novel proteins, referred to herein as amidated glial cell line-derived neurotrophic factor (GDNF) peptides (or “Amidated Dopamine Neuron Stimulating peptides (ADNS peptides)”), that are useful for treating brain diseases and injuries that result in dopaminergic deficiencies.
Owner:UNIV OF KENTUCKY RES FOUND
Method for detecting GDNF (glial cell line-derived neurotrophic factor) gene promoter in glial cells
InactiveCN106048002AStable expressionGuaranteed survival rateMicrobiological testing/measurementGlial cell line-derived neurotrophic factorGAPDH Gene
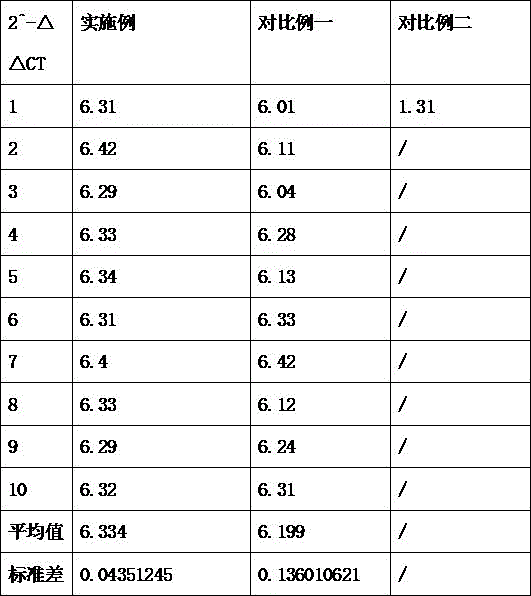
The invention discloses a method for detecting GDNF (glial cell line-derived neurotrophic factor) gene promoter in glial cells; comprising the steps: first, extracting glial cells; second, culturing the cells; third, treating with a drug; fourth, extracting mRNA; fifth, reacting: pre-denaturing at 95 DEG C for 30 s, denaturing at 95 DEG C for 20 S, annealing at 60 DEG C for 15 s, extending at 72 DEG C for 15 s, and amplifying by 40 loops; sixth, calculating: using mRNA expression of GAPDH gene as an internal reference for a sample, and calculating expression level of relative mRNA of a target gene in in various samples through a relative quantitation method (2- CT). Sequences of a primer include: GAPDH: F: 5' TCCCTCAAGATTGTCAGCAA3'; R: 5' AGATCCACAACGGATACATT3'; GDNF: F: 5' GACTTGGGTTTGGGCTACGA3'; R: 5' TGGTAAACCAGGCTGTCGTC3'. Through a treatment process of the invention, the expression level of the detected glial cell 2^- CT is higher; meanwhile, through variance comparison, the method of the invention enables more stable expression level of 2^- CT.
Owner:WENZHOU CENT HOSPITAL
Fusion proteins for delivery of gdnf and bdnf to the central nervous system
InactiveCN102459348APolypeptide with localisation/targeting motifNervous disorderGlial cell line-derived neurotrophic factorAmyotrophic lateral sclerosis
The present invention relates to a compound that includes a peptide vector, such as angiopep-2 which acts as a carrier across the blood-brain barrier, linked to glial-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), or a related molecule, such as an analog or a fragment thereof. The compounds of the invention may be used to treat any disease where increased neuronal survival or growth is desired, e.g., neurodegenerative diseases, such as Parkinson's disease or amyotrophic lateral sclerosis. Other diseases can be treated using the compounds include schizophrenia and depression.
Owner:ANGLACHEM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com