Patents
Literature
123 results about "Corneal epithelium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The corneal epithelium (epithelium corneæ anterior layer) is made up of epithelial tissue and covers the front of the cornea. It acts as a barrier to protect the cornea, resisting the free flow of fluids from the tears, and prevents bacteria from entering the epithelium and corneal stroma.
Defined serumfree medical solution for ophthalmology
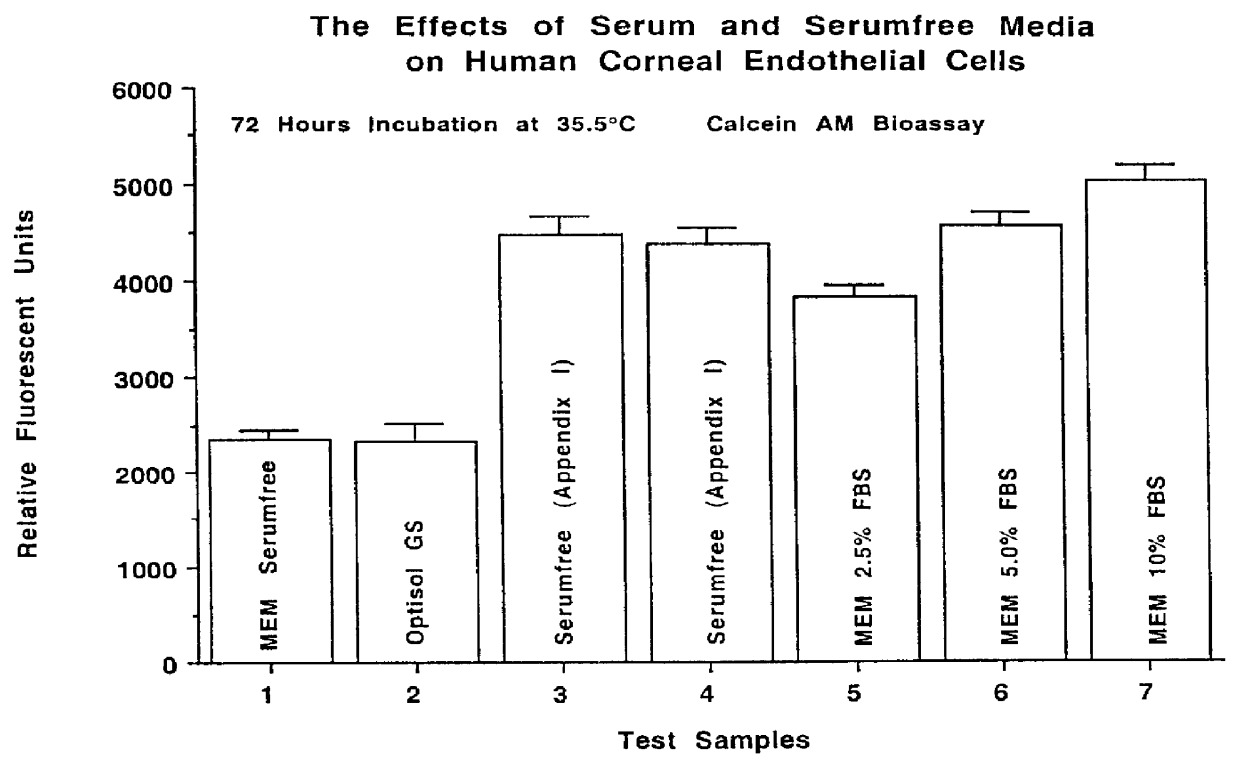
A defined serumfree medical solution for applications in Ophthalmology, that contains one or more cell nutrient supplements, and a growth factor(s) which maintains and enhances the preservation of eye tissues, including human corneal, retinal and corneal epithelial tissues at low to physiological temperatures (2 DEG C. to 38 DEG C.). This solution is composed of a defined aqueous nutrient and electrolyte solution, supplemented with a glycosaminoglycan(s), a deturgescent agent(s), an energy source(s), a buffer system(s), an antioxidant(s), membrane stabilizing agents, an antibiotic(s) and / or antimycotic agent(s), ATP or energy precursors, nutrient cell supplements, coenzymes and enzyme supplements, nucleotide precursors, hormonal supplements, non-essential amino acids, trace minerals, trace elements and a growth factor(s).
Owner:SKELNIK DEBRA L
Corneal onlays and methods of producing same
Corneal onlays and corneal onlay production methods are described. The present corneal onlays include a lens body. An example of a corneal onlay includes a lens body that includes a corneal epithelium-contactable anterior surface and a Bowman's membrane-contactable posterior surface. The lens body has an optical power from about −10 diopters to about +10 diopters, an optic zone diameter from about 5 mm to about 11 mm, a base curve from about 5 mm to about 12 mm, a center thickness from about 10 micrometers to about 300 micrometers, and an edge thickness from about 0 micrometers to about 120 micrometers. Methods include forming the present corneal onlays from polymeric materials.
Owner:FORSIGHT LABS
Biological Tissue Sheet, Method Of Forming The Same And Transplantation Method By Using The Sheet
InactiveUS20080039940A1Raise security concernsSimple structureSkin implantsEpidermal cells/skin cellsConjunctival EpitheliumCuticle
A biological tissue sheet which is expected as exerting a favorable therapeutic effect and a high safety in transplantation. The biological tissue sheet formed by (a) preparing in vivo-derived cells; (b) sowing the in vivo-derived cells on amniotic membrane; and (c) culturing and proliferating the in vivo-derived cells in the absence of any xenogeneic animal cells. As the cells of a biological origin, for example, cells originating in corneal epithelium, conjunctival epithelium, skin epidermis, hair follicle epithelium, oral mucosa, respiratory tract mucosa, or intestinal tract mucosa.
Owner:KOUJI HASHIMOTO +1
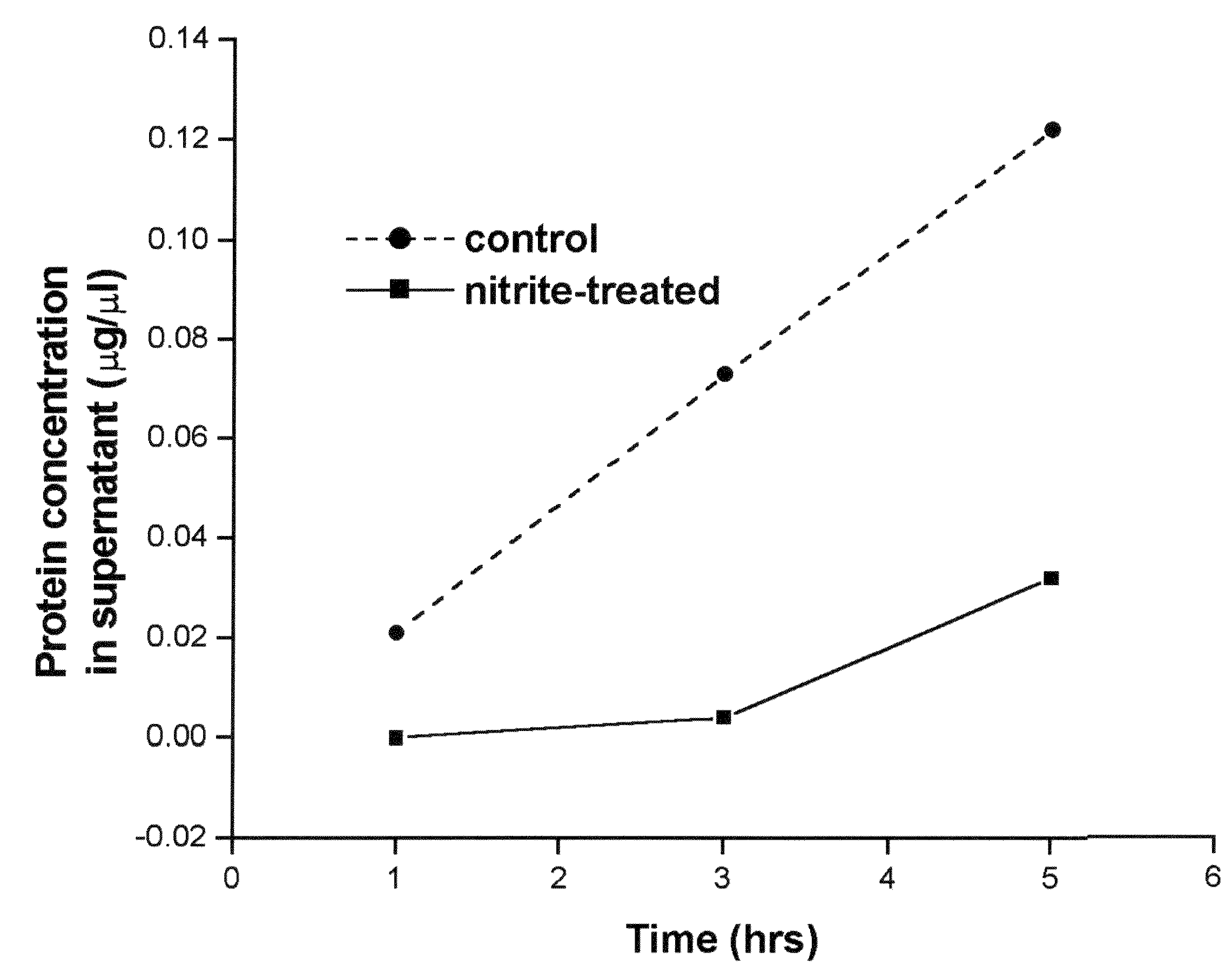
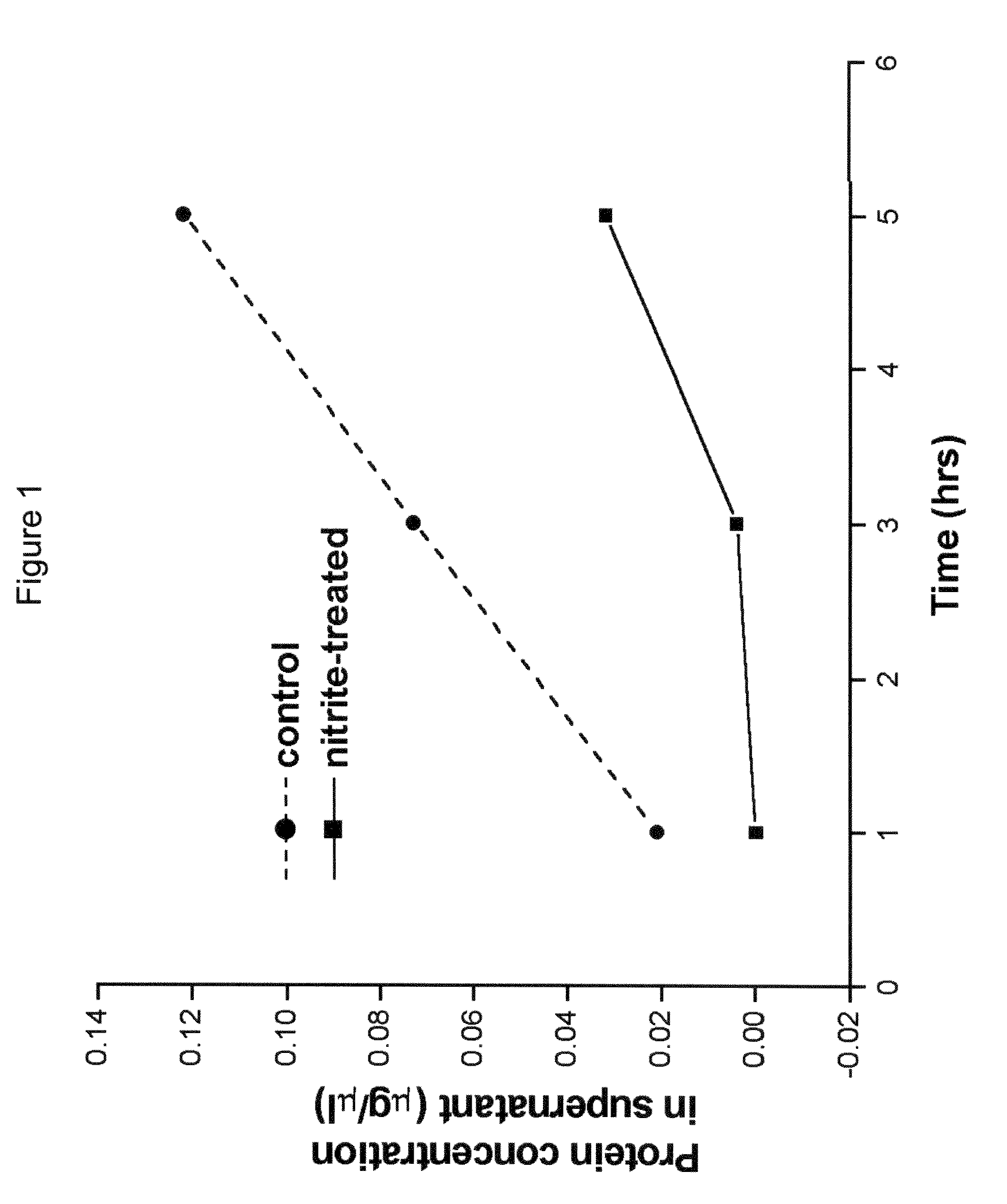
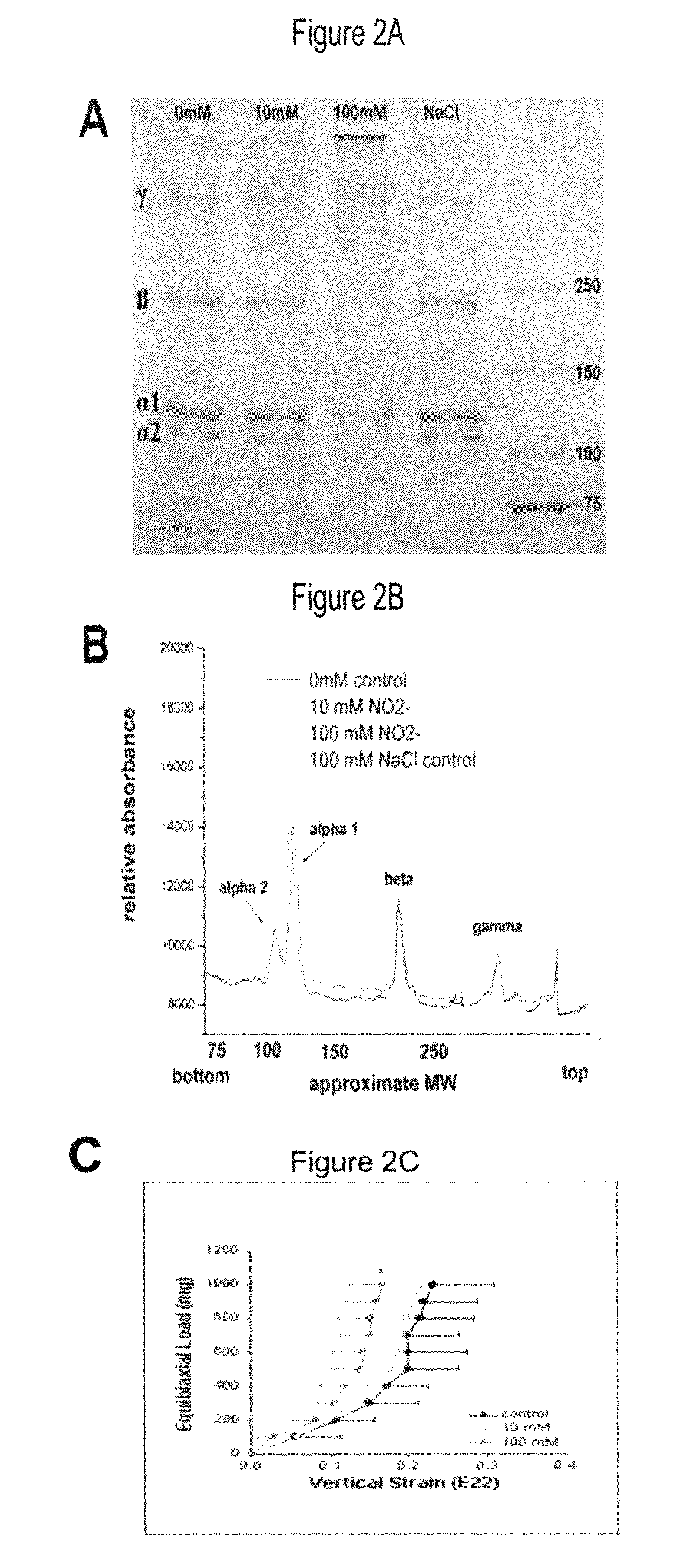
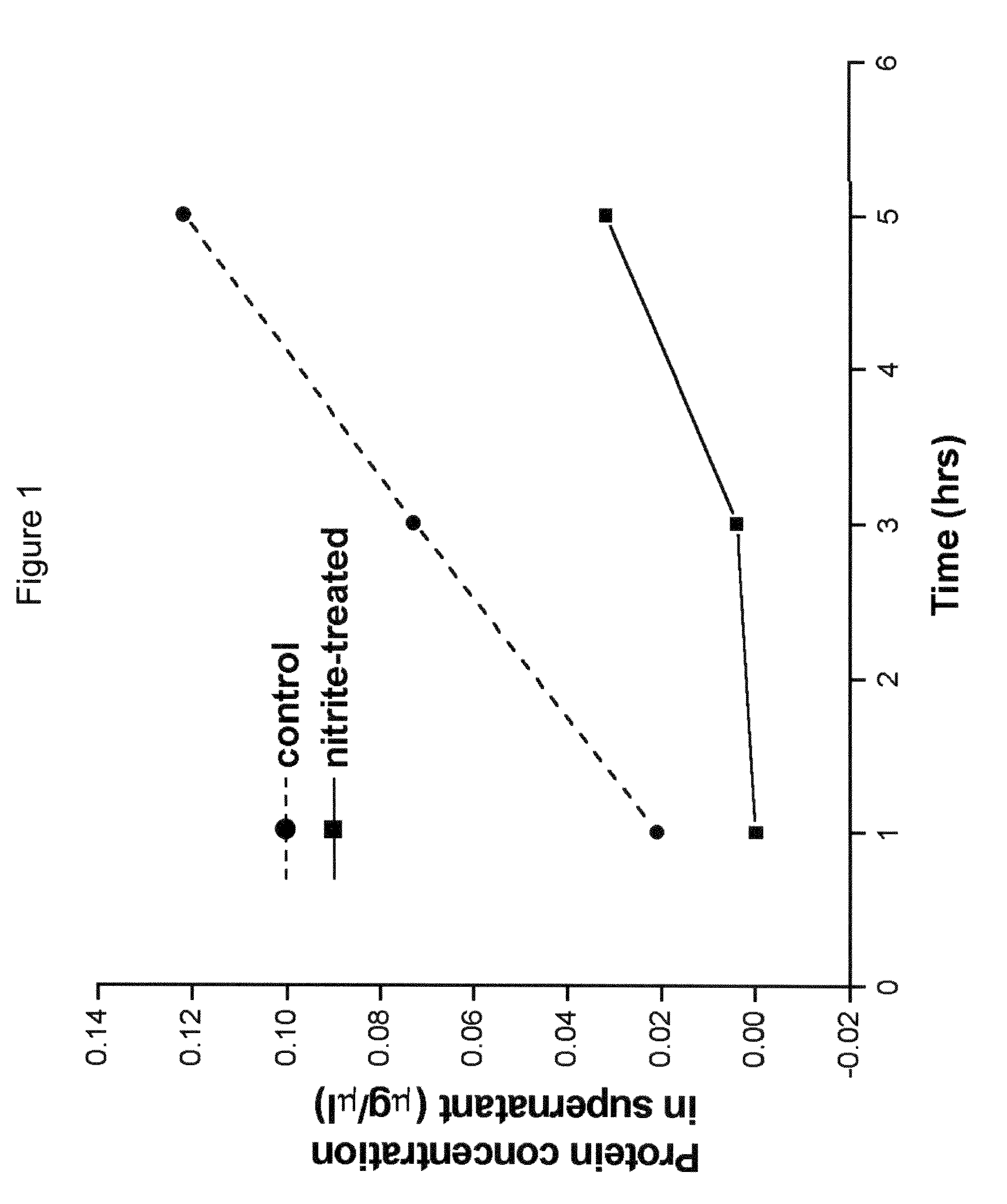
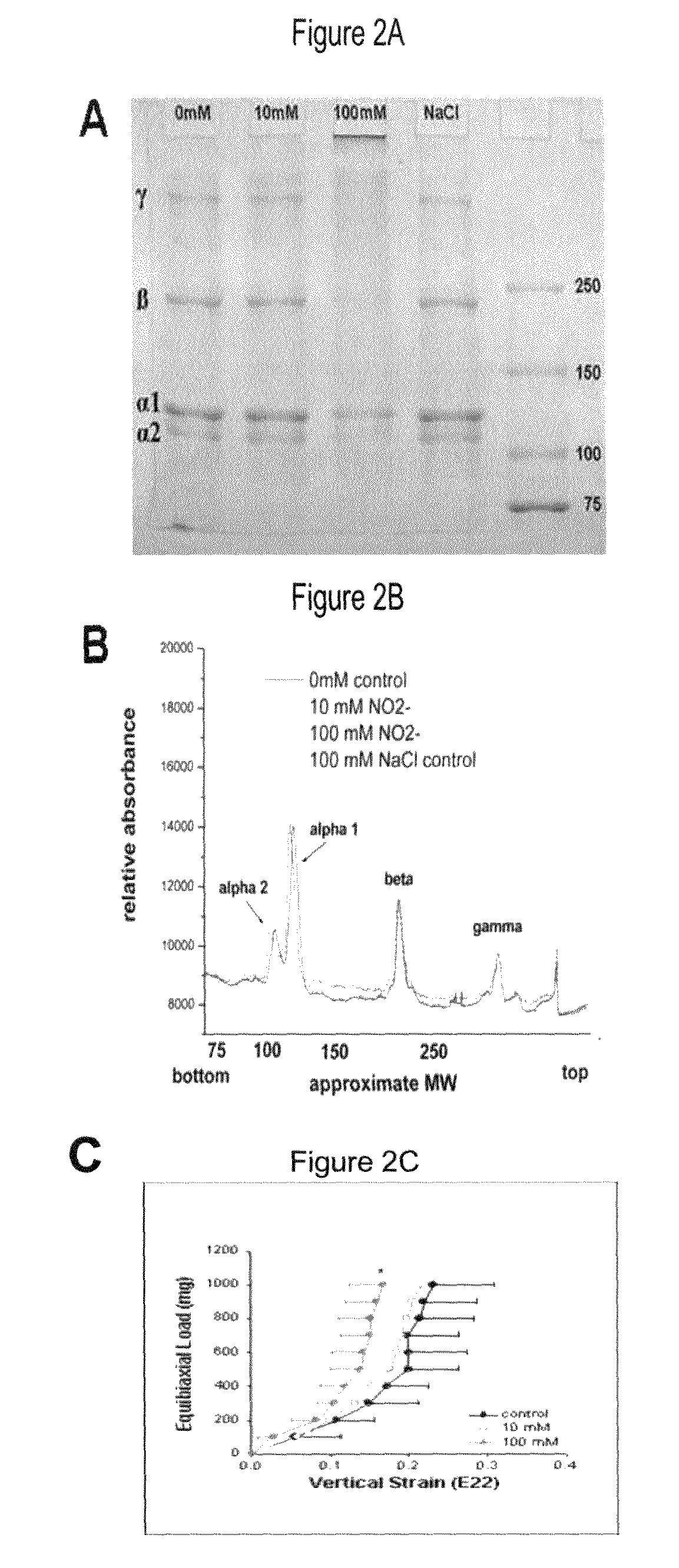
Method of stabilizing human eye tissue by reaction with nitrite and related agents such as nitro compounds
A method for stabilizing collagenous eye tissues by nitrite and nitroalcohol treatment. The topical stiffening agent contains sodium nitrite or a nitroalcohol in a buffered balanced salt solution and can be applied to the surface of the eye on a daily basis for a prolonged period. Application of the solution results in progressive stabilization of the corneal and scleral tissues through non-enzymatic cross-linking of collagen fibers. The compounds can penetrate into the corneal stroma without the need to remove the corneal epithelium. In addition, ultraviolet light is not needed to activate the cross-linking process. The resulting stabilization of corneal and scleral tissues can prevent future alterations in corneal curvature and has utility in diseases such as keratoconus, keratectasia, progressive myopia, and glaucoma.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Acellular cornea or acellular corneal stroma, preparation method and application thereof
InactiveCN101985051ARetain toughnessLow immunogenicityProsthesisFreeze thawingVaccine Immunogenicity
The invention discloses acellular cornea or acellular corneal stroma, a preparation method and application thereof. The method comprises the following steps of: (1) obtaining fresh animal full-thickness cornea or corneal stroma; (2) removing corneal epithelium, corneal endothelium and stroma cells, namely 1, soaking the full-thickness cornea or the corneal stroma in pure water at room temperature; 2, placing the soaked full-thickness cornea or corneal stroma into enzyme solution, digesting with oscillating, and washing with balanced salt solution with oscillating; and 3, repeating freeze-thaw processes of the full-thickness cornea or the corneal stroma for 4 to 8 times and washing with balanced salt solution with oscillating to obtain the acellular cornea or the acellular corneal stroma; (3) dehydrating; and (4) sterilizing and storing. In the method, the decellularization processing time of the cornea is short; the influence on the structure and the physiological property of the cornea is small; and the processed cornea has very low immunogenicity which is similar to the property of natural cornea. The acellular cornea or the acellular corneal stroma can be applied to artificial cornea construction of tissue engineering and also can serve as a medical material applied to corneal transplantation and refraction surgery.
Owner:JINAN UNIVERSITY
Hydrogel for intelligent desorption of cell sheet layer and application of hydrogel
ActiveCN102718928APromote rapid proliferationFast desorptionSurgeryProsthesisSolubilityFunctional monomer
Owner:TIANJIN CHANGHE BIOLOGICAL TECH
Method and application for preparing tissue engineering corneal carrier stent by utilizing fresh porcine cornea
ActiveCN104189957AReduce manufacturing costComplete structureProsthesisFreeze thawingBiocompatibility Testing
The invention discloses a method and application for preparing a tissue engineering corneal carrier stent by utilizing fresh porcine cornea. The method comprises the following steps: cutting a fresh porcine cornea for swelling, repeatedly freeze thawing and breaking cells, removing residual nucleic acid substances through DNA-RNA enzyme treatment, cross-linking, airing, and finally performing irradiation sterilization through ionization rays, thereby obtaining the product. The method is applied to carrier stents of tissue engineering corneal epithelium, stroma, endothelium, front board layer half cornea, rear board layer half cornea and full-layer board layer half cornea, so that the requirement on batch production of tissue engineering corneas is met. The carrier stent serves as a substitute of human corneal stroma to be used for clinical transplant and treatment of un-accumulated whole layer keratohelcosis. The method is applied to large-scale production and clinical popularization and application. The prepared carrier stent has the characteristics of high transparency, dense structure, high mechanical property, high biocompatibility with human corneal seed cells and the like. Therefore, the method is suitable for batch production, so that the requirements on lots of carrier stents for tissue engineering corneas are met.
Owner:青岛彩晖生物科技有限公司
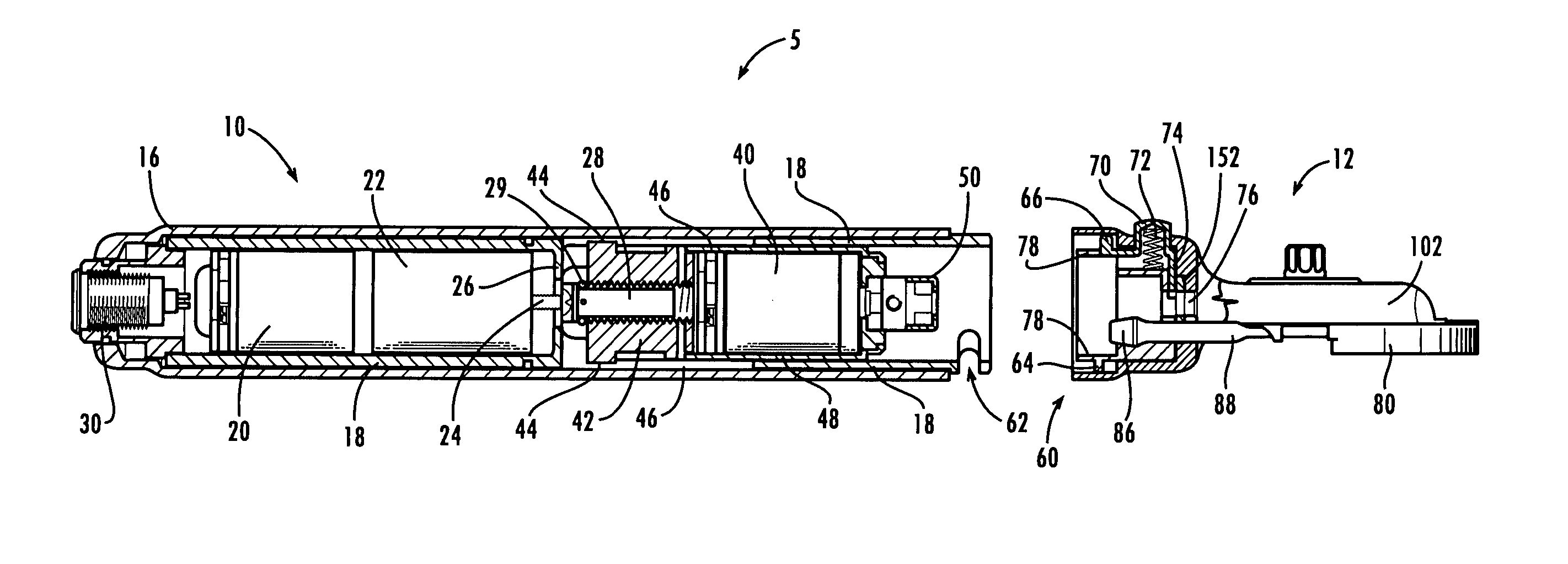
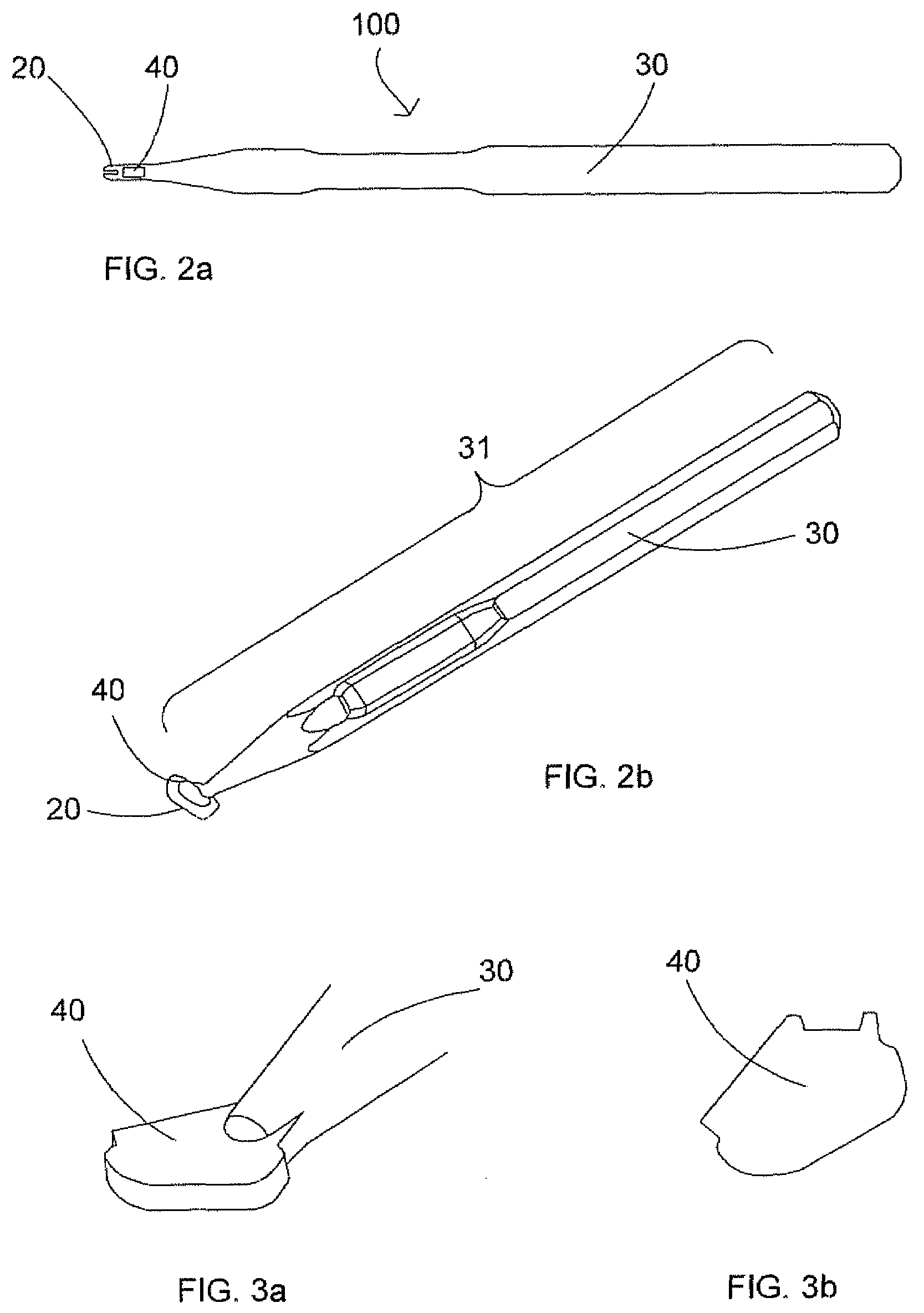
Device for separation of corneal epithelium
InactiveUS20050055041A1Simple and elegant in designSimple and elegant in and constructionEye surgeryEngineeringSurgery procedure
A drive tool for optical surgery includes a handpiece containing a traverse motor and an oscillating motor, wherein the oscillating motor is axially driven through the handpiece by the traverse motor. A head assembly including a suction ring is mounted to the handpiece, and a separator drive assembly is positioned therein for advancement imparted by the traverse motor and oscillation imparted by the oscillating motor.
Owner:SIGHTRATE
Preparation method and application thereof of acellular conjunctiva matrix
InactiveCN101590292AEasy to prepareGood biocompatibilityEye implantsDead animal preservationTreatment effectBiocompatibility Testing
The invention relates to a preparation method and application thereof of an acellular conjunctiva matrix used as a tissue engineering corneal scaffold material. The method comprises the following steps: removing cell components in a bulbar conjunctiva first; preparing the acellular conjunctiva matrix; and taking the acellular conjunctiva matrix as a tissue engineering corneal scaffold. A rabbit corneal epithelium or an endothelial cell can form a good cell single layer on the scaffold, can construct a tissue engineering corneal epithelium or endothelium, and can successfully transplant the tissue engineering corneal epithelium or endothelium onto rabbit animal model eyes. The degradation time of the built tissue engineering cornea is longer, rabbit eyes have no obvious immune reject reaction, and the therapeutic effects on the lack of corneal limbus stem cells or the decompensation of the corneal endothelial cell function are obvious. The method and the application thereof have the advantages of simple preparation method, good biocompatibility, low antigenicity, easy growth and proliferation of seed cells, slow degradation, extensive bulbar conjunctiva sources and wide application and development prospects; and the transparency can be maintained all the time in the training process and after transplantation.
Owner:SHANDONG EYE INST
Reconstruction method of tissue engineering human corneal epithelium
The invention relates to a reconstruction method of tissue engineering human corneal epithelium. The method comprises the following steps of: adopting a DMEM / F12 culture medium containing 20% calf serum to carry out the in vitro culture on human corneal epithelium cells to a logarithmic growth phase, and adopting trypsin and a trypsin-EDTA digestion method to obtain a digestive amniotic carrier bracket of which the epithelium is completely removed; and after the digestive amniotic carrier bracket of which the epithelium is removed is flatly laid in a plug-in Petri dish and is firmly and pasted in a drying way, inoculating the human corneal epithelium cells at the logarithmic growth phase suspended in the DMEM / F12 culture medium containing IV type collagen and 20% calf serum to the plug-inPetri dish flatly laid with the digestive amniotic carrier bracket of which the epithelium is removed, and carrying out the in vitro reconstruction on the tissue engineering human corneal epithelium by a gas-liquid interface culture method. The invention has scientific and reasonable process, the reconstructed tissue engineering human corneal epithelium can be used for mass production, a lot of demands of vast blind patients with corneal epithelium diseases for the tissue engineering human corneal epithelium in clinical corneal transplantation treatment can be met, and the costs of the in vitro reconstruction and clinical treatment of the tissue engineering human corneal epithelium are low.
Owner:OCEAN UNIV OF CHINA +1
Methods to increase permeability of corneal epithelium and destabilize stromal collagen fibril network
InactiveUS20110086802A1Reduce deliveryImprove stabilityBiocideSenses disorderNon invasiveCollagen fibril
Methods of increasing the permeability of corneal epithelium to facilitate the diffusion of agents into the collagen fibrillar network of the stroma are provided. Used in combination, these methods open the epithelium to facilitate diffusion of stabilization molecules into the stroma and dissociate bridging molecules from stromal collagen fibers, thereby priming the collagen fibrillar network for restabilization by stabilization molecules. These methods can be used to increase the effectiveness and longevity of non-invasive corneal reshaping, such as orthokeratology, for correcting myopia, hyperopia and astigmatism.
Owner:EUCLID SYST CORP
Eye drops containing recombinant human keratinocyte growth factor-2 and application thereof in treating xeroma
InactiveCN101721358AImprove stabilityExtension of timeSenses disorderPeptide/protein ingredientsRecombinant Human Keratinocyte Growth FactorEye drop
The invention discloses eye drops with the function of relieving the symptom of xeroma, which are characterized by comprising a recombinant human keratinocyte growth factor-2, a protein protective agent, a thickening agent, an osmotic pressure regulator and a proper buffer system in a certain proportion; the invention also discloses a preparation method of the eye drops. Pharmacological experiments show that the eye drops can delay the rupture time of the lacrimal film, maintain the integrity of the ocular barrier, restore the ocular immunological network and relieve the symptom of the xeroma by restoring the corneal epithelium.
Owner:CHANGCHUN GROSTRE BIOLOGICAL TECH
Multipurpose Lens Care Solution with Benefits to Corneal Epithelial Barrier Function
A multipurpose lens care solution comprising 0.005 wt. % to 1 wt. % of an anionic biopolymer, and an antimicrobial agent selected from 0.5 ppm to 2 ppm of poly(hexamethylene biguanide), 0.5 ppm to 2 ppm polyquaternium-1, or 1 ppm to 4 ppm alexidine. The lens care solution exhibits a ZO-1 immunostaining of HCEpiC similar to phosphate buffered saline for after thirty minutes of contact time with the solution. The lens care solution will also have a transepithelial electrical resistance (TEER) of HCEpiC within a 25% difference or less than phosphate buffered saline in Ohm / cm2 after one hour of contact time with a 3:1 dilution (solution:DMEM), or the ECIS electrode arrays exhibit a 25% difference or less than phosphate buffered saline in Ohm after one hour of contact time with a 1:1 dilution (solution:DMEM).
Owner:BAUSCH & LOMB INC
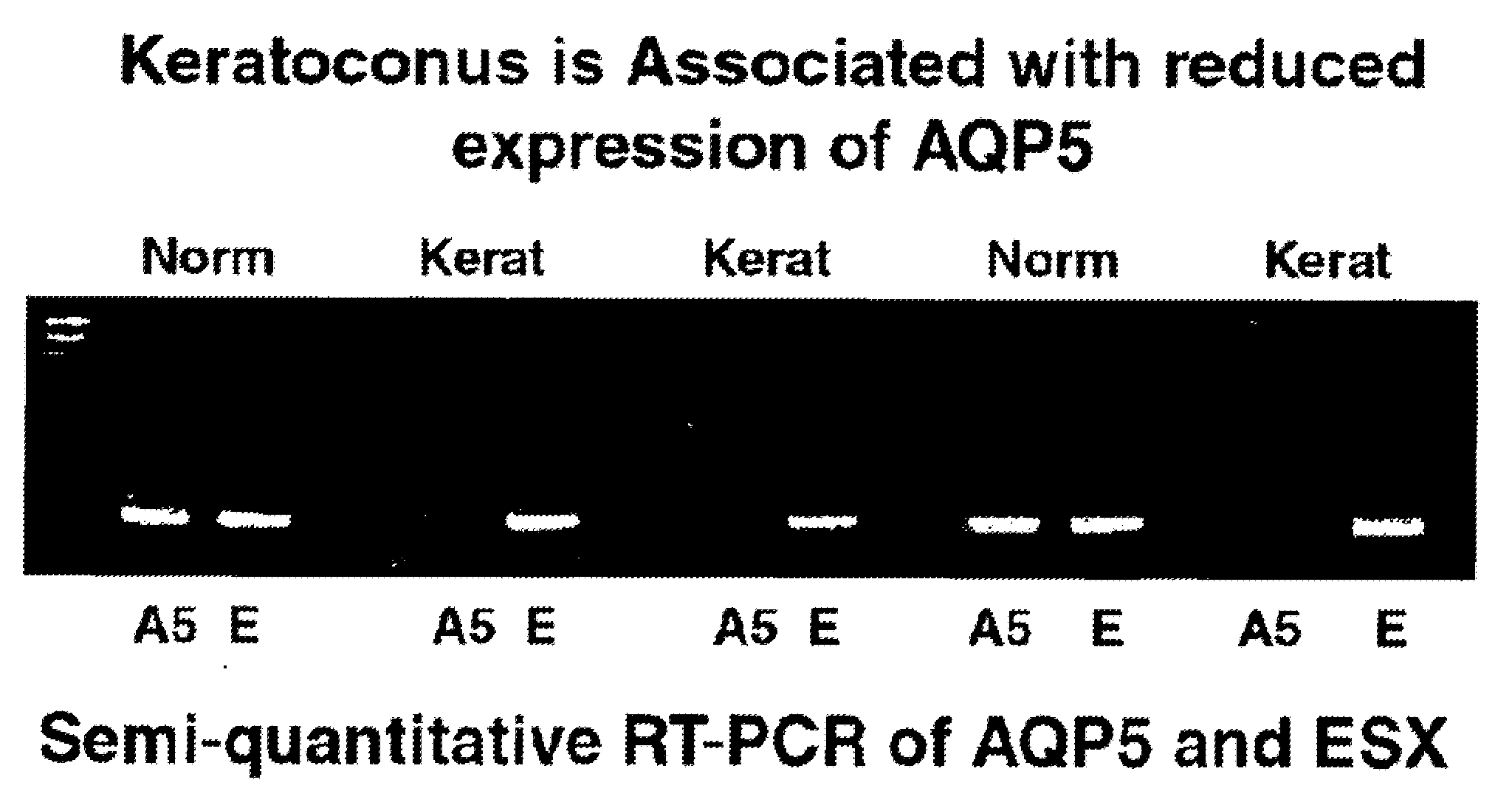
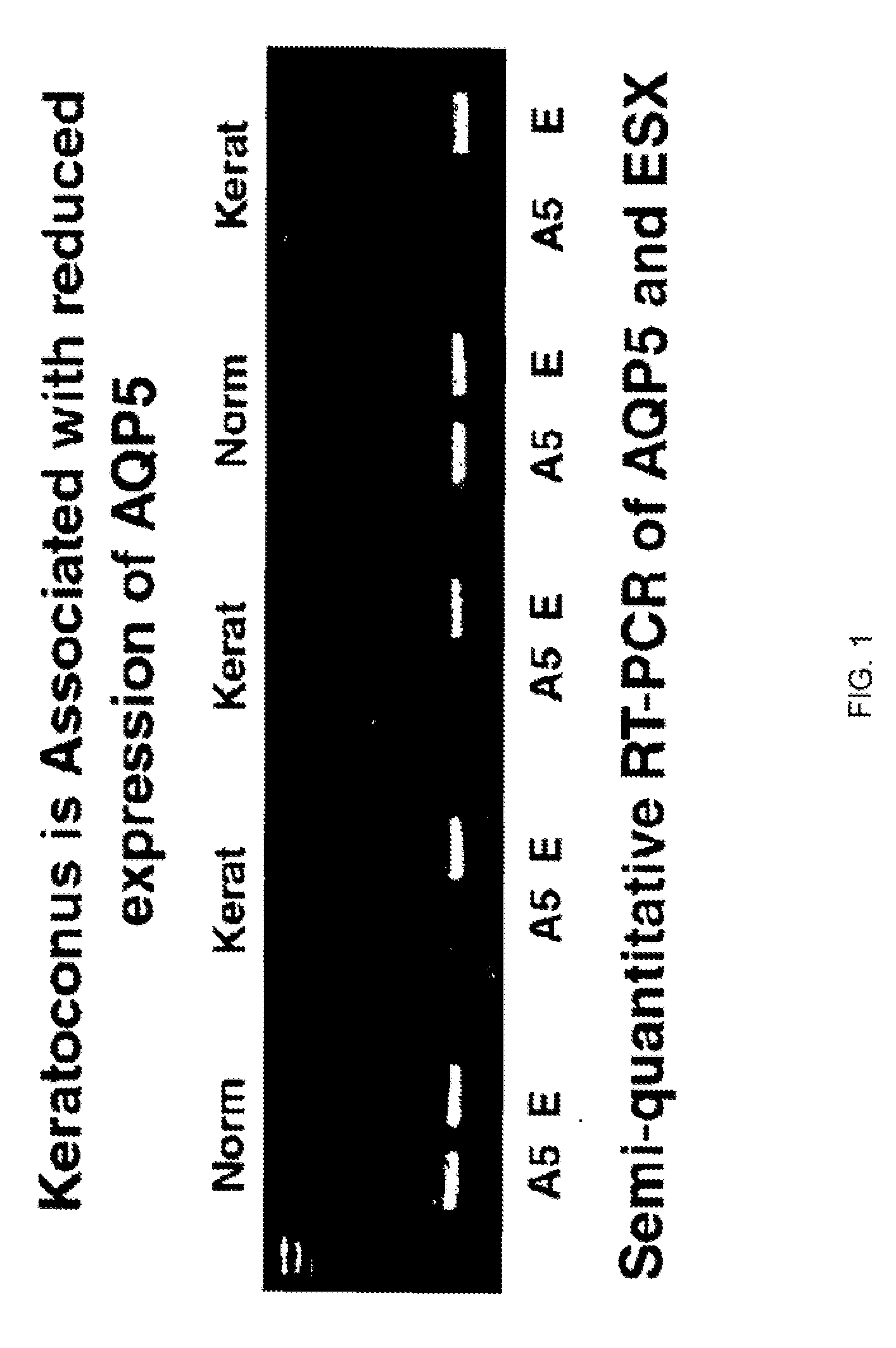
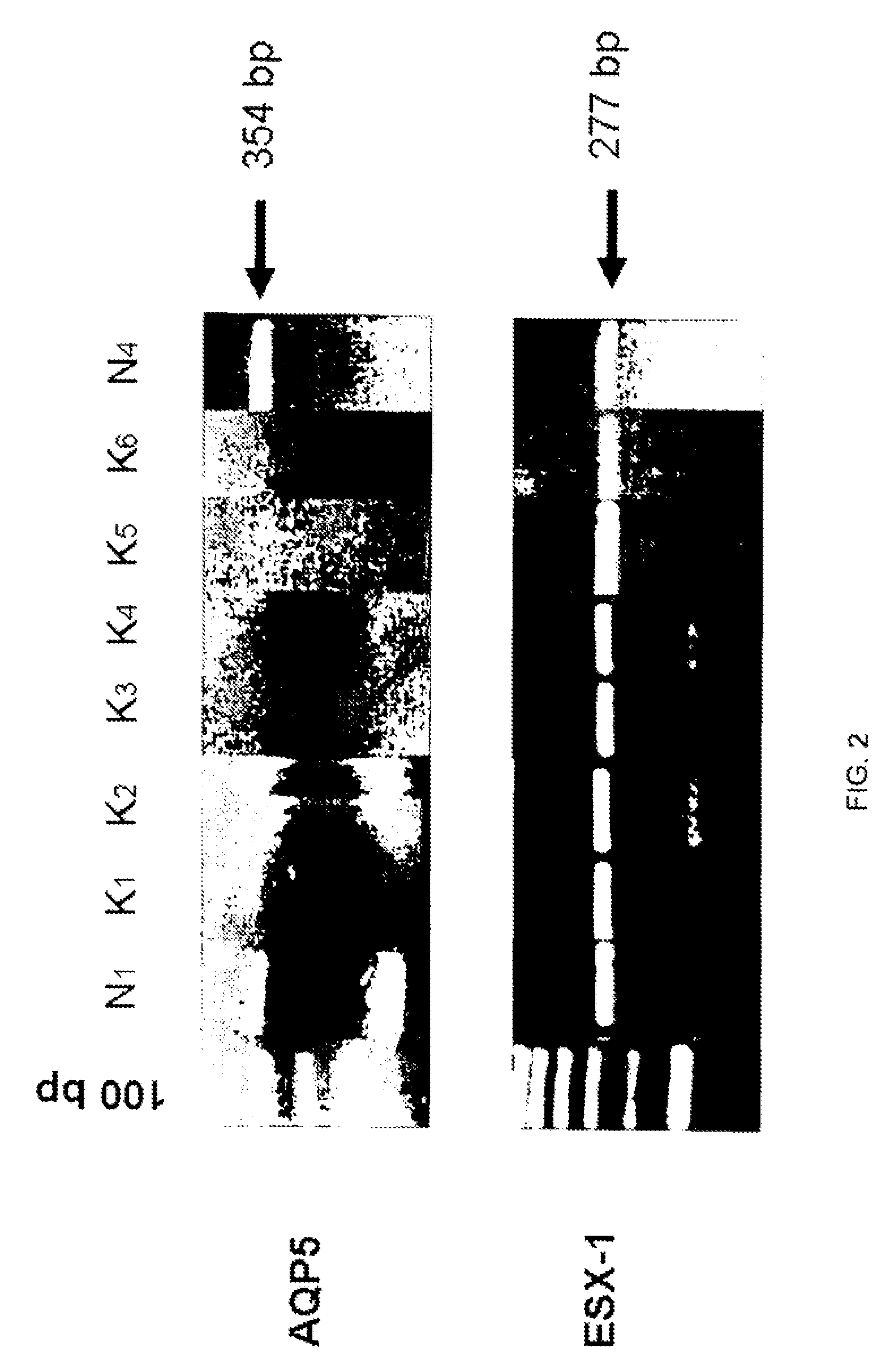
Compositions and methods for detecting keratoconus
InactiveUS20070248970A1Sugar derivativesMicrobiological testing/measurementConical corneaSub clinical
The invention provides a method and assay kits for detecting keratoconus, including sub clinical keratoconus, in a specimen. The method comprises assaying a specimen of corneal epithelium for the presence of an expression product of the AQP5 gene. The invention further provides a novel gene, KC6, that exhibits cornea-specific expression, as well as KC6-related molecules.
Owner:EYE BIRTH DEFECTS RES FOUND
Ophthalmic Composition Comprising Xanthan Gum and Glucose
ActiveUS20090269369A1Good treatment effectImprove usabilityBiocideOrganic active ingredientsD-GlucoseTherapeutic effect
The present invention provides an ophthalmic composition containing xanthan gum and glucose, which has a superior corneal epithelial disorder-treating effect.
Owner:SENJU PHARMA CO LTD
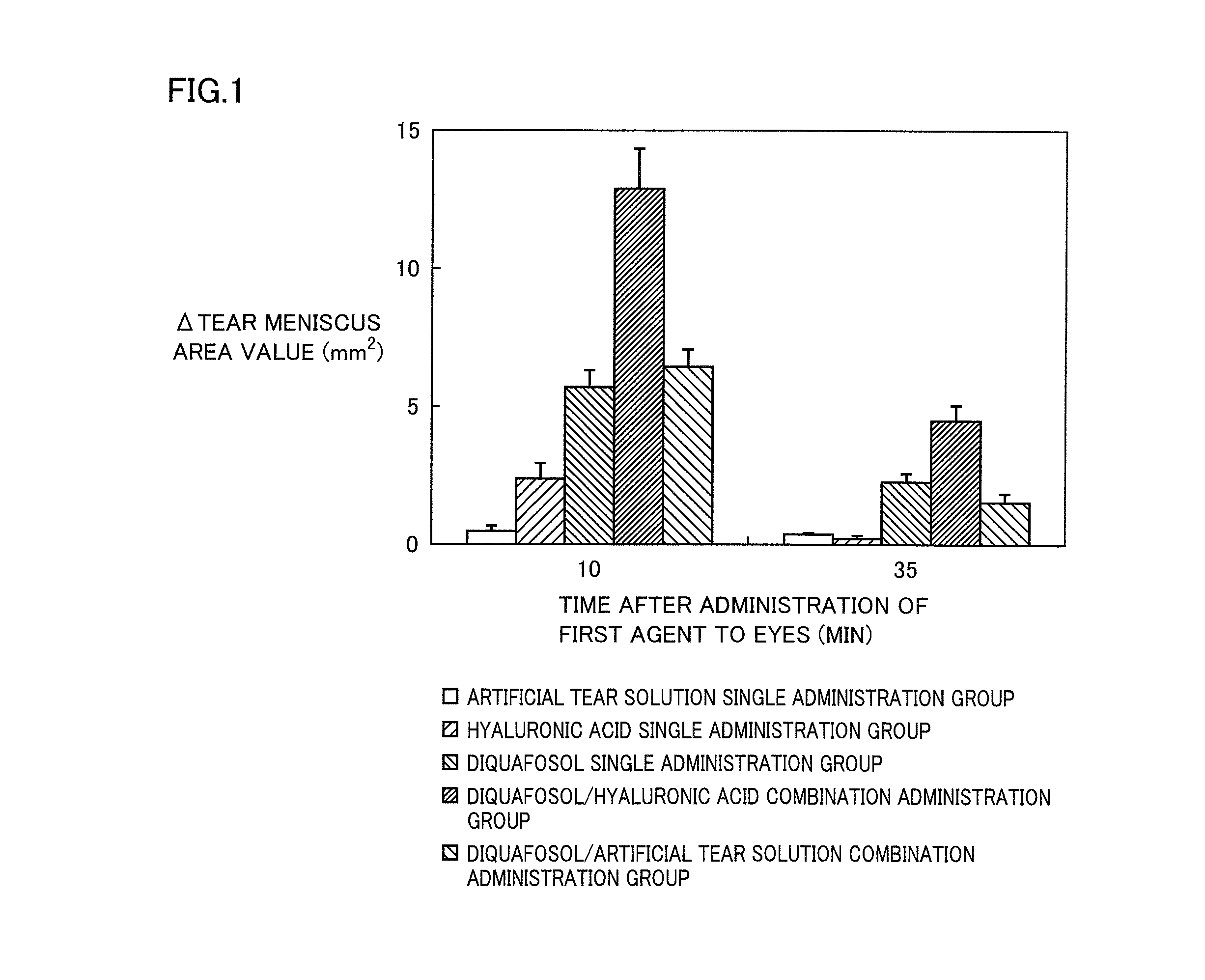
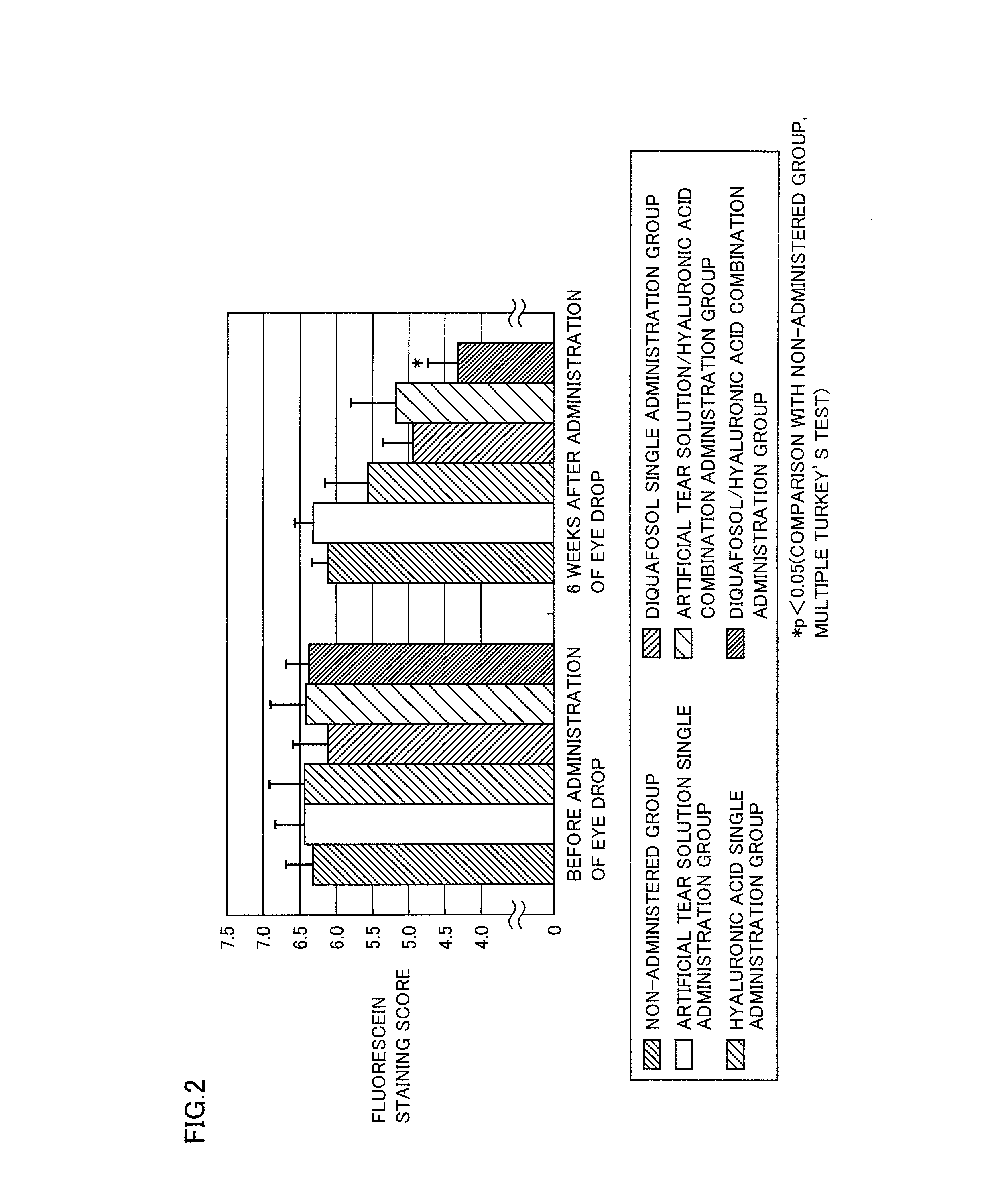
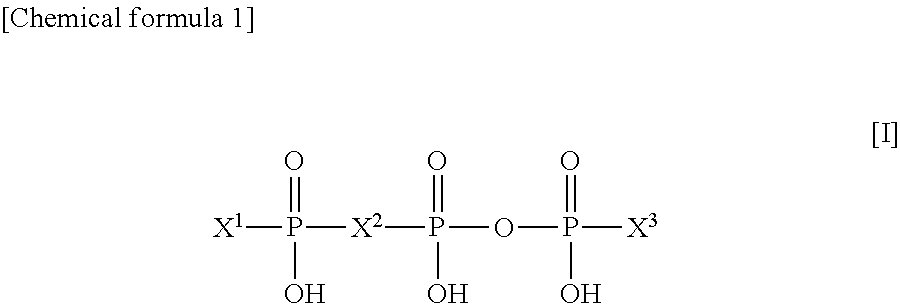
Agent for treatment of dry eye characterized by combining p2y2 receptor agonist and hyaluronic acid or salt thereof, method for treating dry eye, and use of the p2y2 receptor agonist and hyaluronic acid or salt thereof
InactiveUS20130172287A1Stimulating tear secretionImprove corneal epithelial disorderBiocideSenses disorderOphthalmic AgentsPharmaceutical drug
An agent for treatment of dry eye comprising a combination of a P2Y2 receptor agonist at a therapeutically effective concentration and hyaluronic acid or a salt thereof at a therapeutically effective concentration, which agent has a dosage form of an ophthalmic agent, can promote the secretion of tear remarkably and can improve corneal epithelial disorders remarkably, and is therefore expected to be a novel agent for treatment of dry eye.
Owner:SANTEN PHARMA CO LTD
Method of stabilizing human eye tissue by reaction with nitrite and related agents such as nitro compounds
InactiveUS8466203B2Impede structural integrityAvoid structureBiocideSenses disorderNitro compoundDisease
A method for stabilizing collagenous eye tissues by nitrite and nitroalcohol treatment. The topical stiffening agent contains sodium nitrite or a nitroalcohol in a buffered balanced salt solution and can be applied to the surface of the eye on a daily basis for a prolonged period. Application of the solution results in progressive stabilization of the corneal and scleral tissues through non-enzymatic cross-linking of collagen fibers. The compounds can penetrate into the corneal stroma without the need to remove the corneal epithelium. In addition, ultraviolet light is not needed to activate the cross-linking process. The resulting stabilization of corneal and scleral tissues can prevent future alterations in corneal curvature and has utility in diseases such as keratoconus, keratectasia, progressive myopia, and glaucoma.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
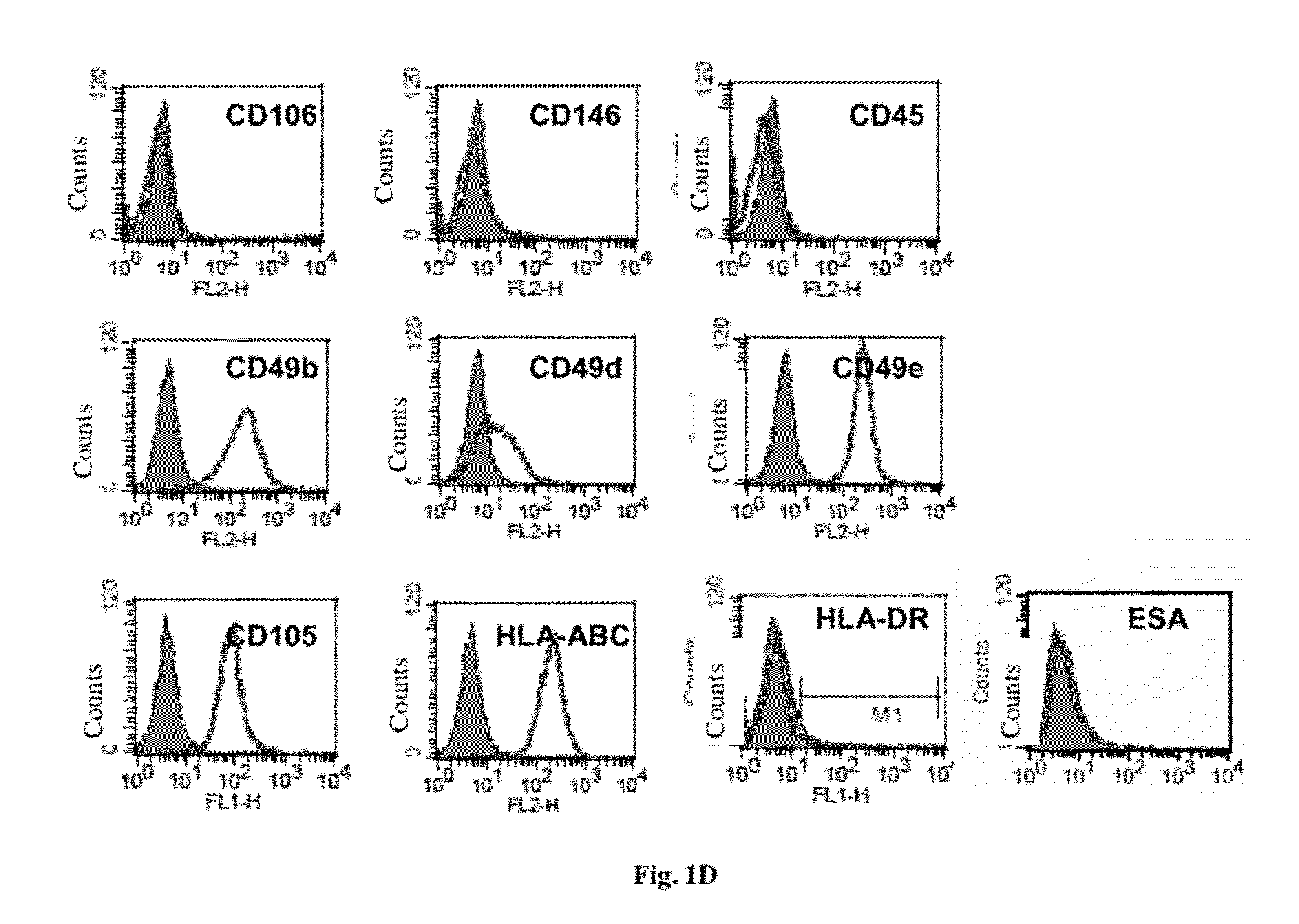
Cell population comprising orbital fat-derived stem cells (OFSCS) and their isolation and applications
The invention relates to a cell population comprising minimal volume of orbital fat-derived stem cells (OFSCs) and its isolation, purification, characterization and application. The OFSCs of the invention are capable of multilineage development and express at least CD90 and CD 105 but not hematopoietic and epithelial markers. The OFSCs have colony formation ability and multi-lineage differentiation ability. They possess at least osteogenic, chondrogenic and adipogenic differentiation capacity; besides mesodermal tri-linage differentiation, the OFSCs have corneal epithelial differentiation potential. Taking together, orbital fat tissues are a novel source for multi-potent stem cells which possess multiple therapeutic potential. Therefore, the OFSCs can be used in cell therapy and tissue engineering.
Owner:TAIPEI MEDICAL UNIV
Corneal epithelial sheet and process for producing the same
Owner:阿如布勒斯特有限公司 +1
Ophthalmic composition comprising xanthan gum and glucose
ActiveUS7875271B2Good treatment effectImprove usabilityBiocideSenses disorderD-GlucoseGlucose polymers
The present invention provides an ophthalmic composition containing xanthan gum and glucose, which has a superior corneal epithelial disorder-treating effect.
Owner:SENJU PHARMA CO LTD
Therapeutic agent for keratoconjunctive disorders
ActiveUS20150290172A1Strongly suppressing keratoconjunctive collagen contractionOrganic active ingredientsSenses disorderSuperficial punctate keratopathyDisease
The present invention addresses the problem of providing a novel therapeutic agent for keratoconjunctive disorders. As a means for solving the problem, a therapeutic agent for keratoconjunctive disorders which contains a RARγ agonist as an active ingredient is provided. The therapeutic agent exhibits an excellent ameliorating effect in a keratoconjunctive disorder model, and is therefore useful as a therapeutic agent for keratoconjunctive disorders such as corneal ulcer, corneal epithelial abrasion, keratitis, dry eye, conjunctivitis, chronic superficial keratitis, corneal erosion, persistent corneal disorders, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, infectious keratitis, noninfectious keratitis, infectious conjunctivitis and noninfectious conjunctivitis. The therapeutic agent is also useful as a therapeutic agent for corneal scarring and conjunctival scarring both associated with keratoconjunctive disorders.
Owner:YAMAGUCHI UNIV +1
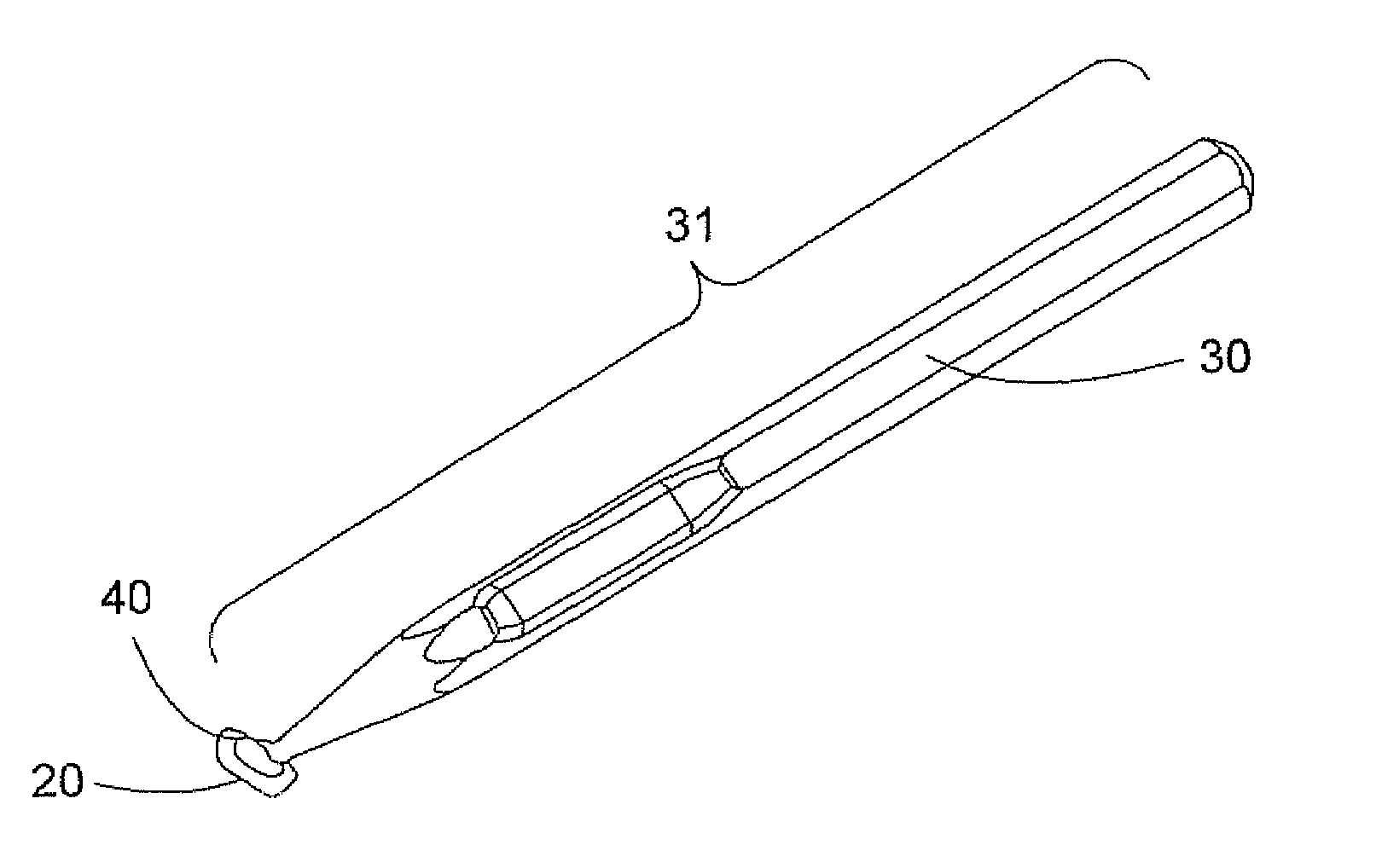
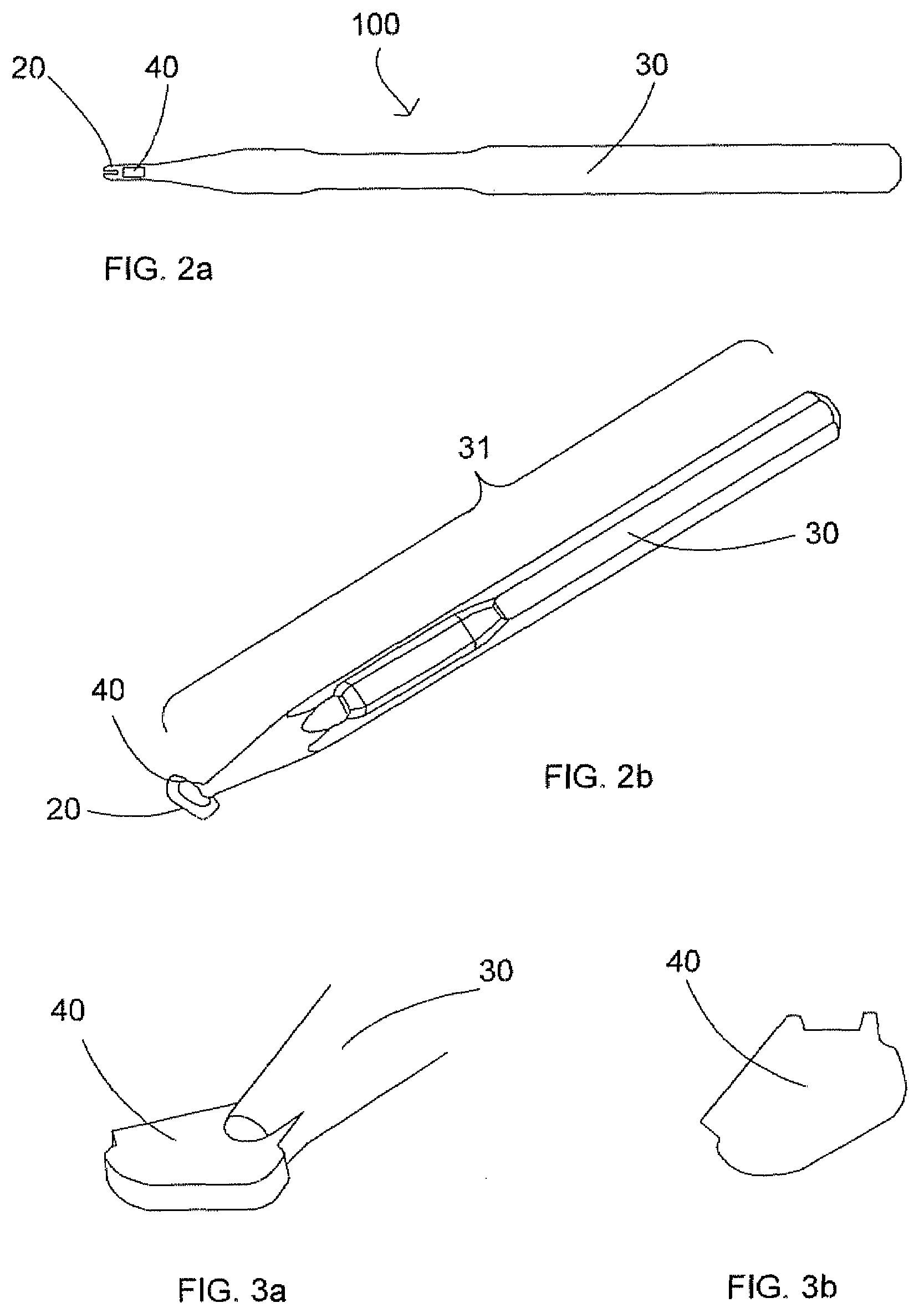
Instrument And Method For Scrubbing The Corneal Epithelium
A scrubbing instrument for scrubbing the human eye corneal epithelium, including a handle, having a grip portion, a scrubbing head having a curved main scrubbing surface, and a connection arrangement for enabling connection of the scrubbing head to the handle and disconnection of the scrubbing head from the handle, thus enabling the scrubbing head to serve as a sterile, disposable, single-use component.
Owner:ORCA SURGICAL
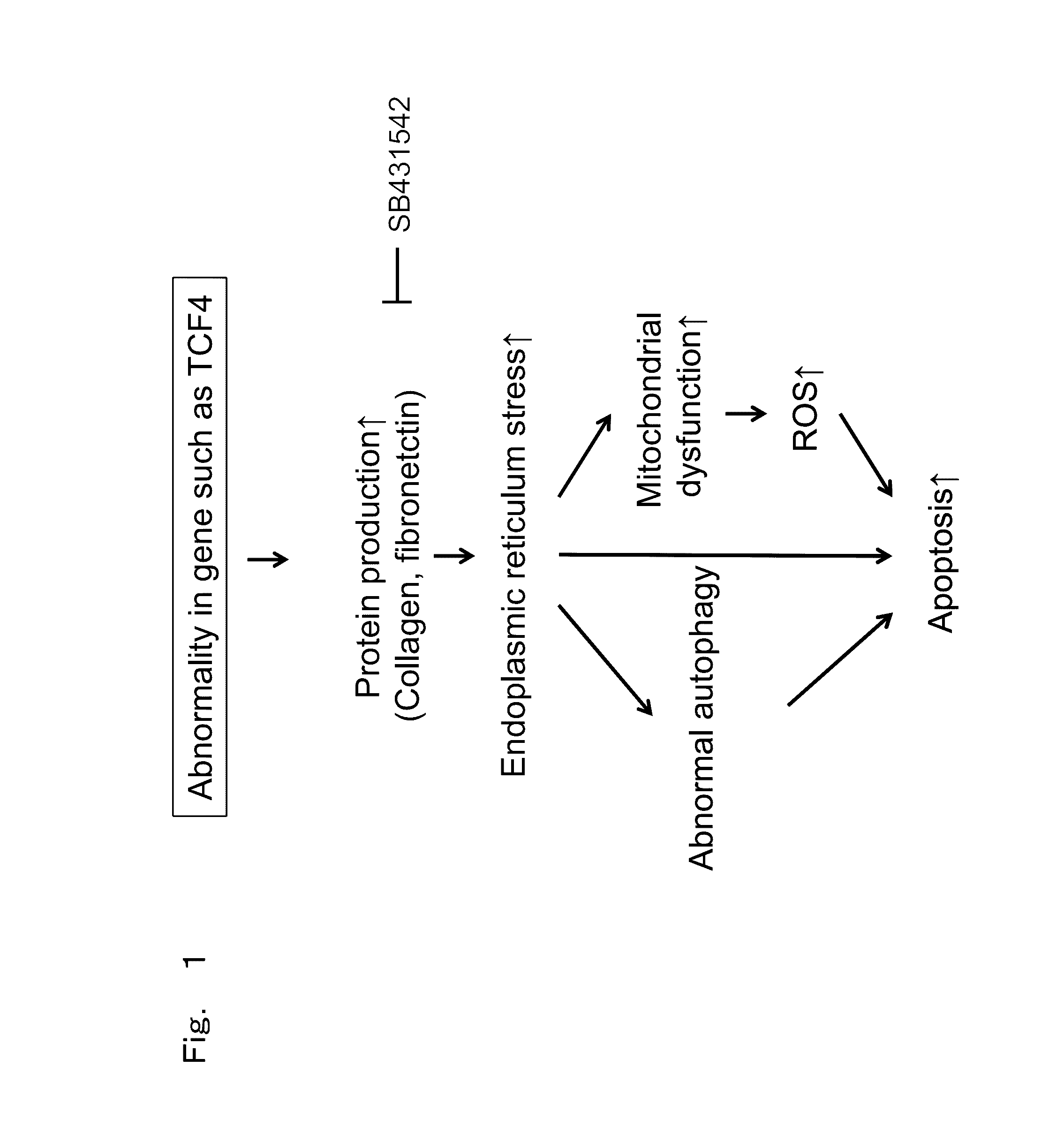
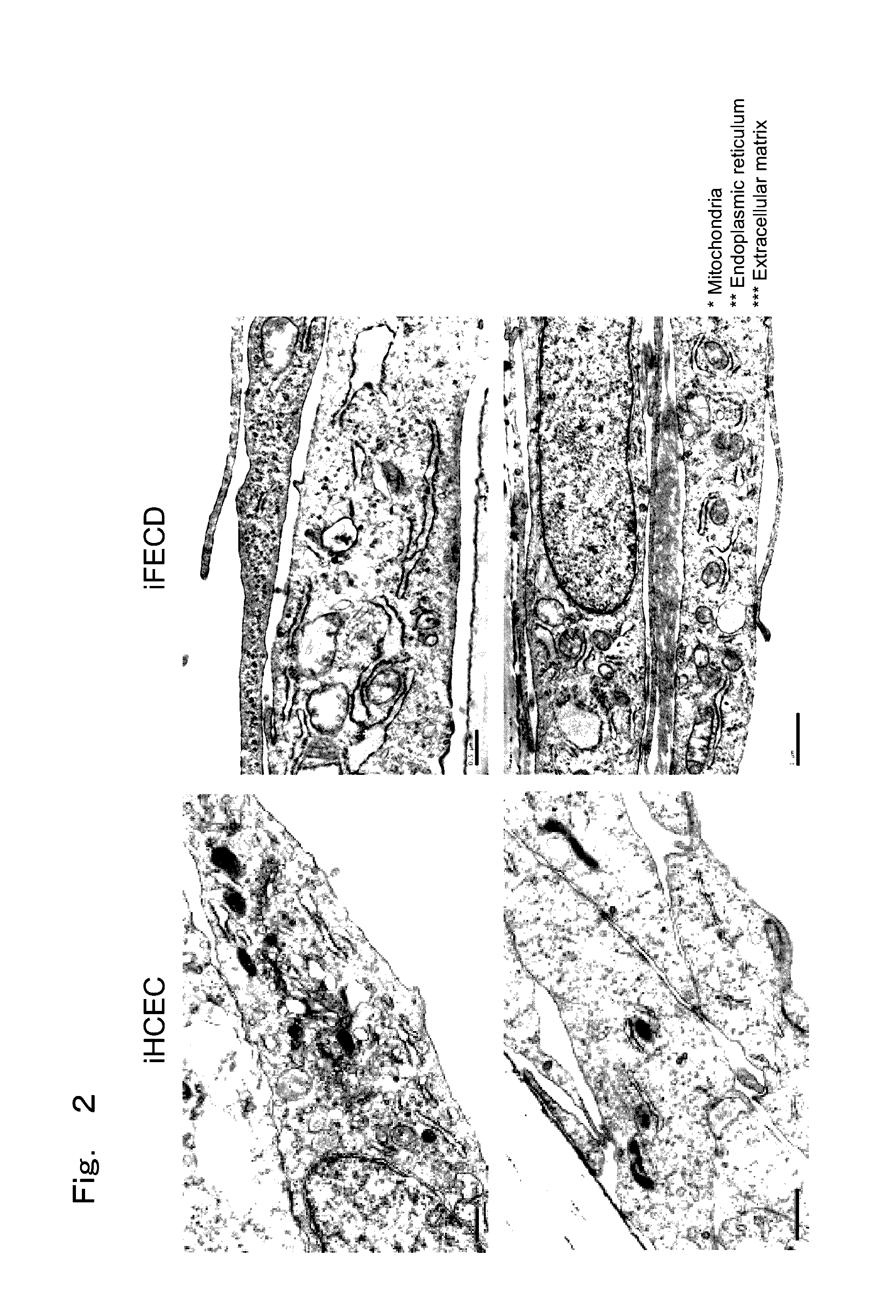
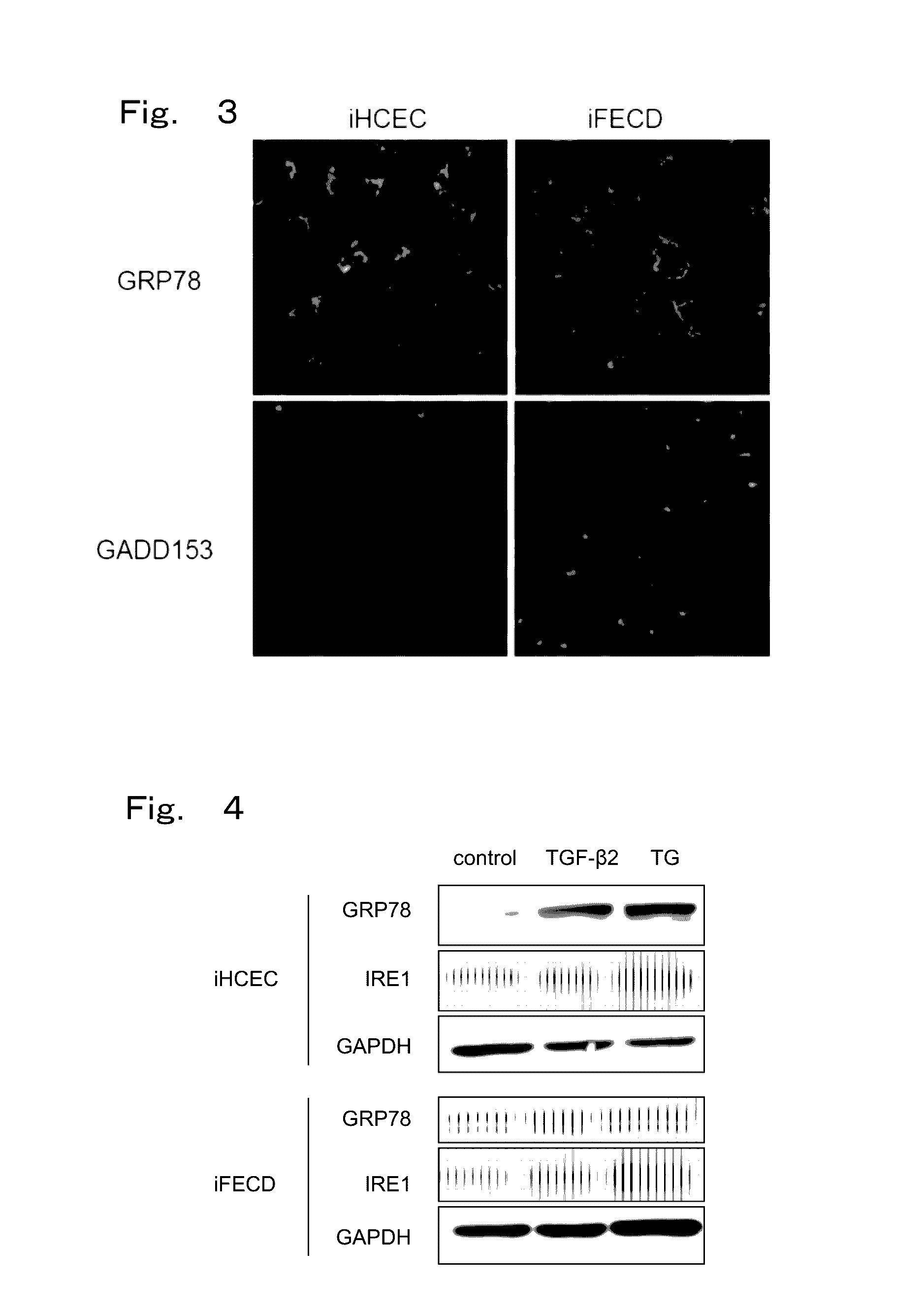
Therapeutic drug for diseases related to endoplasmic reticulum cell death in corneal endothelium
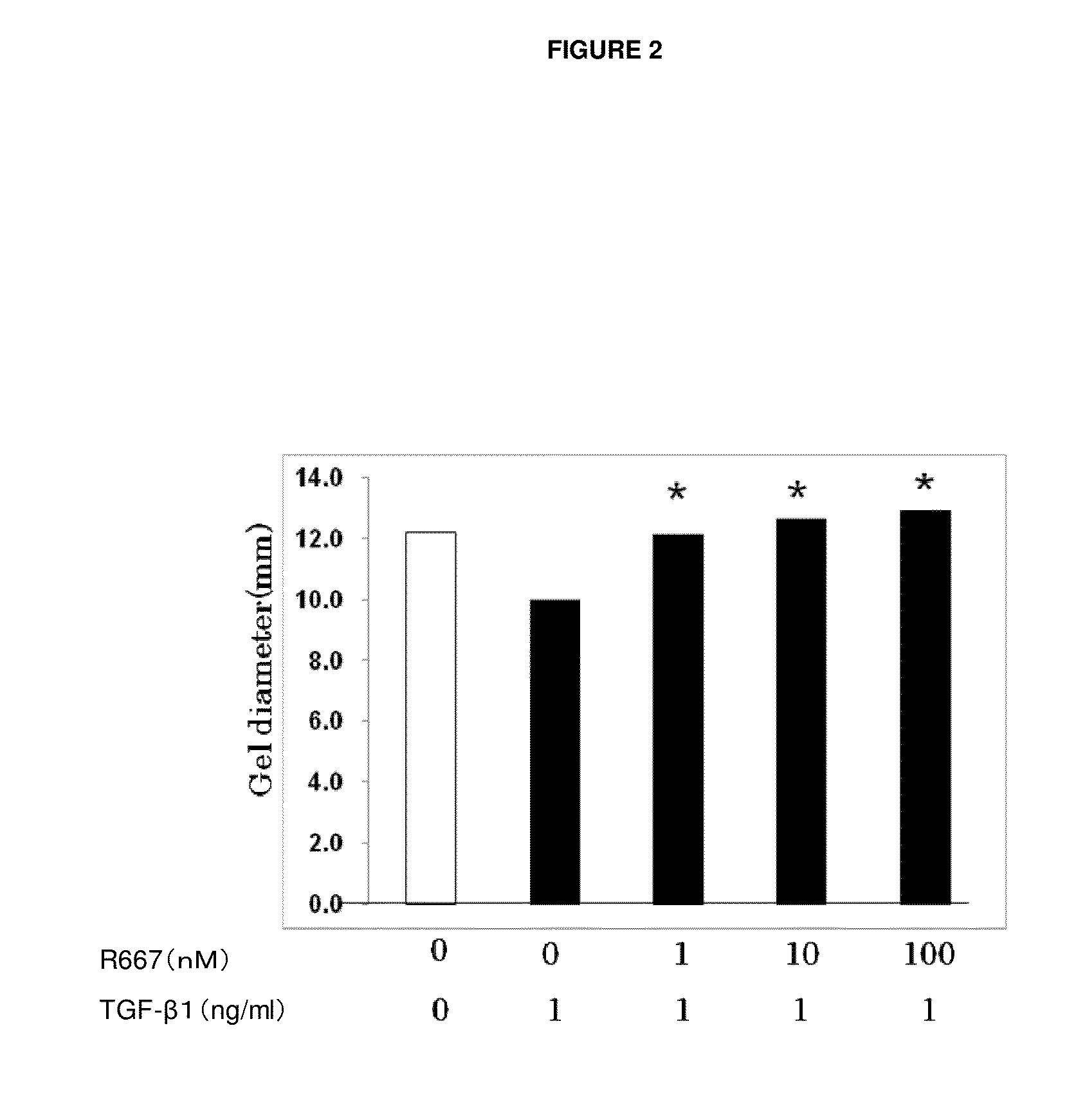
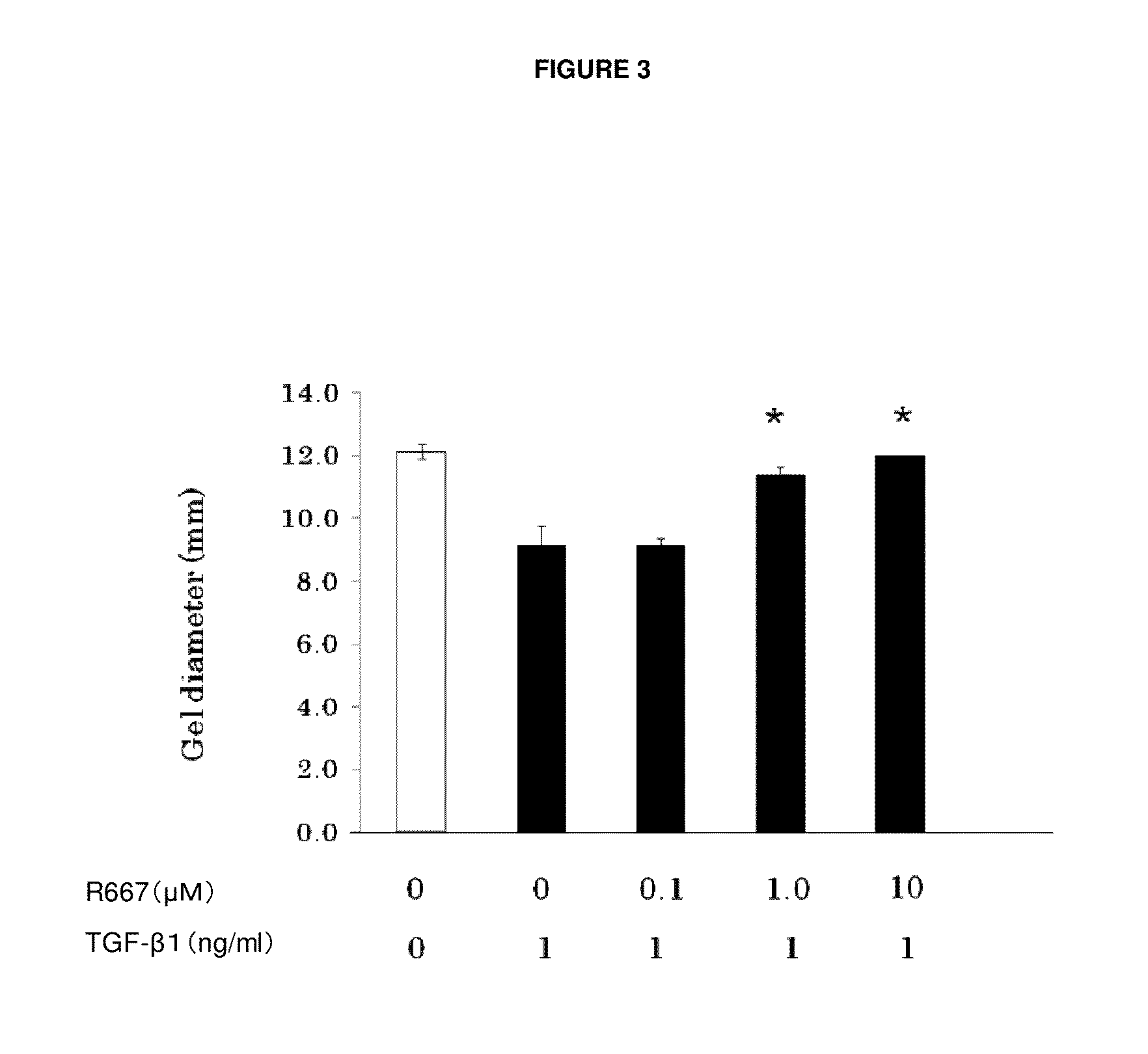
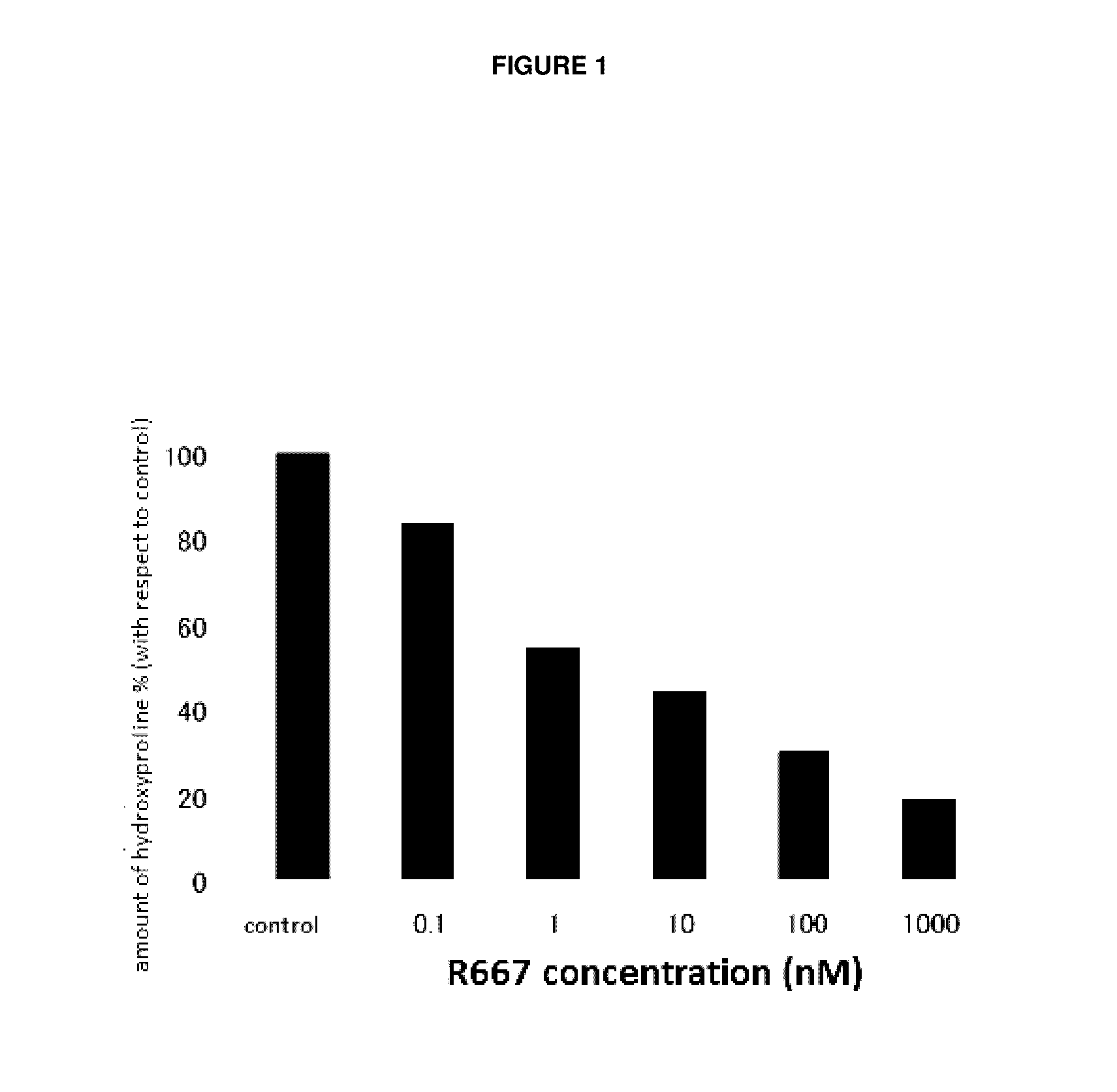
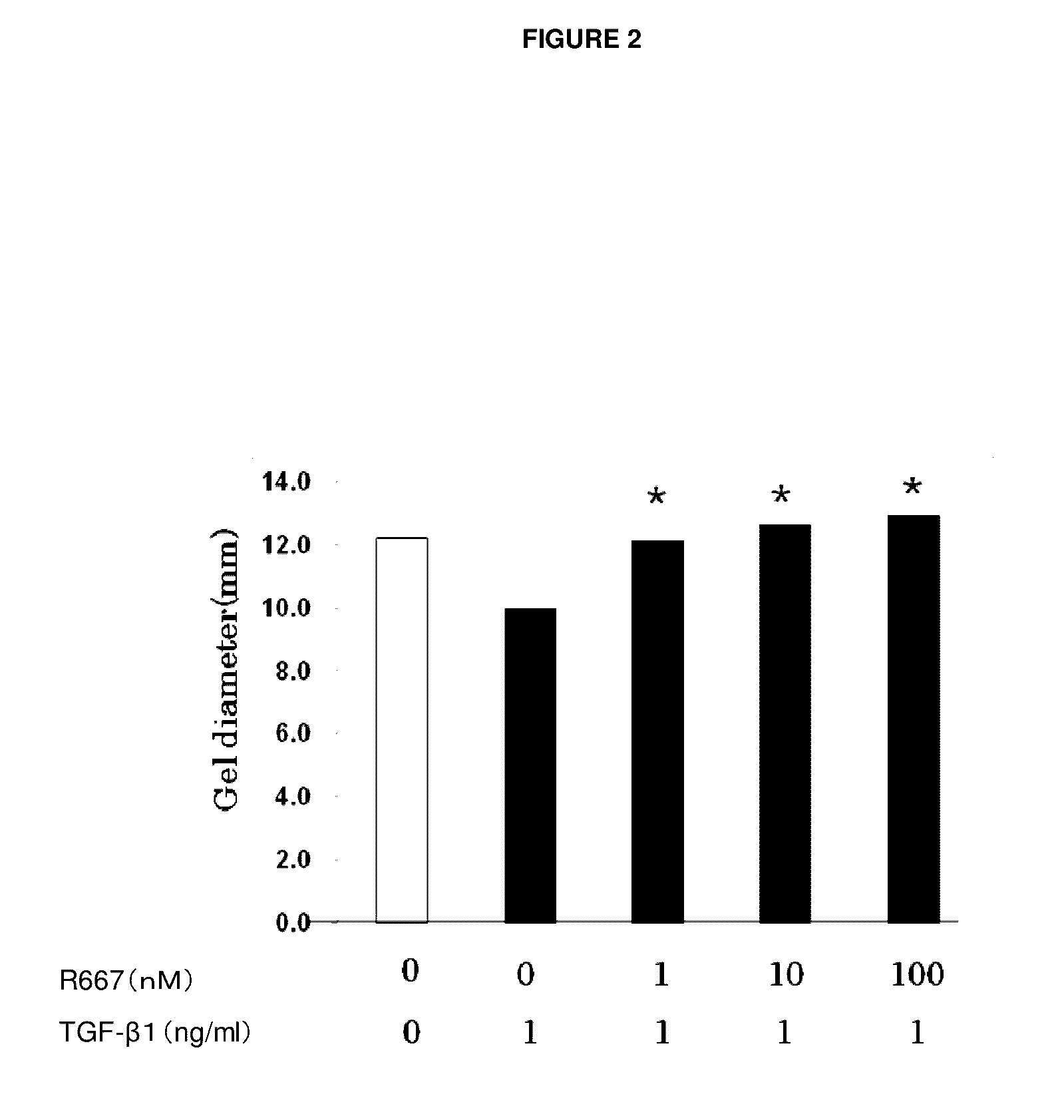
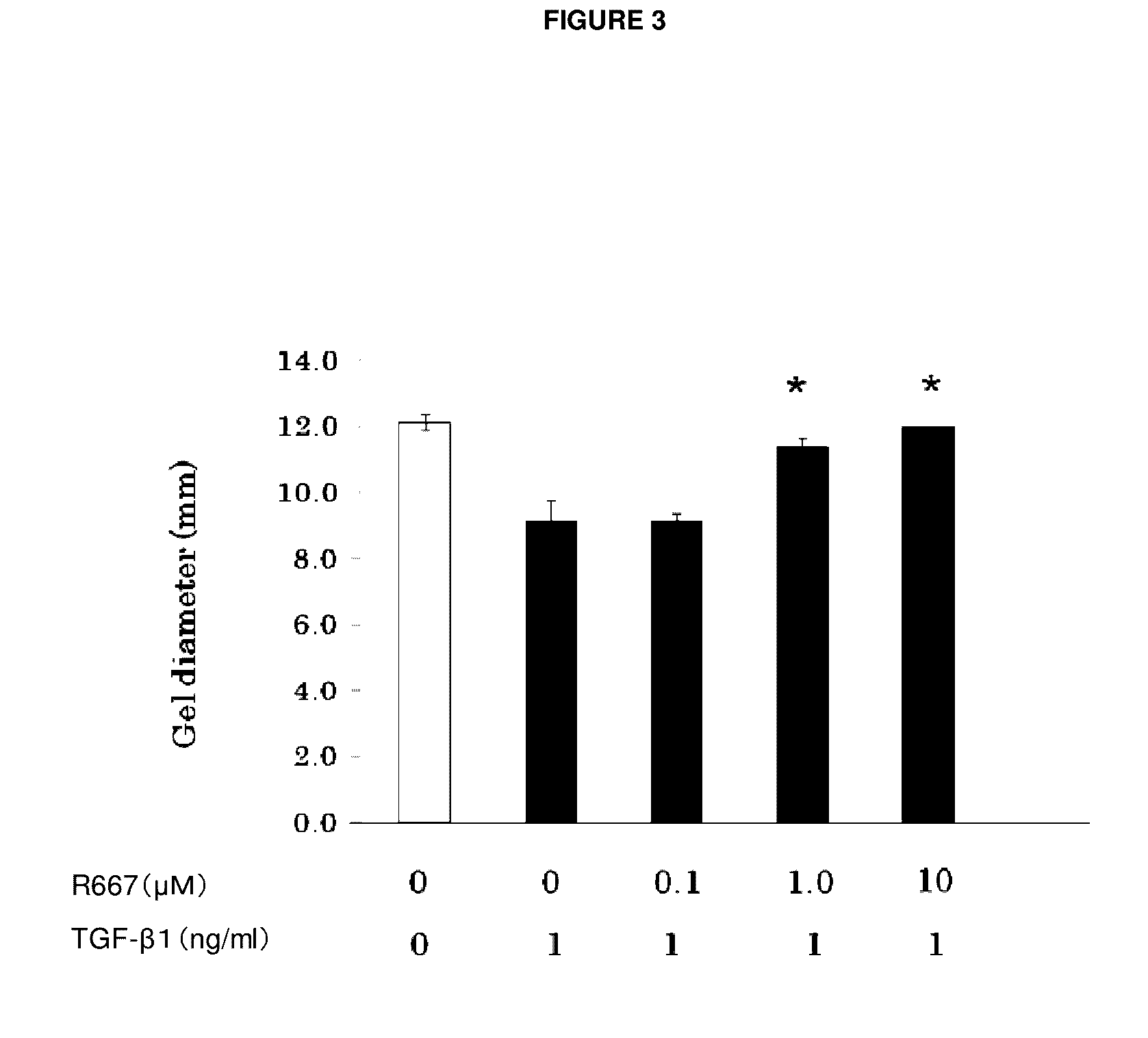
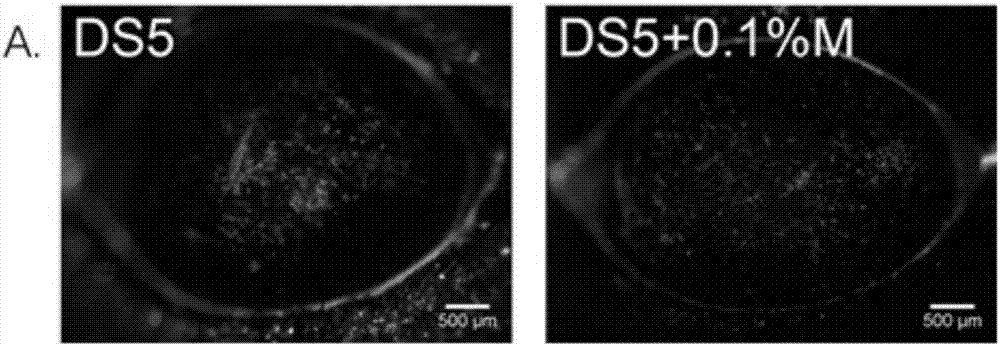
The present invention provides a treatment drug or prophylactic drug for diseases, disorders, or conditions related to endoplasmic reticulum (ER) stress. Specifically, the present invention provides a treatment drug or prophylactic drug for diseases, disorders, or conditions related to endoplasmic reticulum (ER) stress in the corneal epithelium, the drug containing a TGFβ-signal inhibitor. As a preferred TGFβ-signal inhibitor, the drug contains 4-[4-(1,3-benzodioxole-5-yl)-5-(2-pyridinyl)-1H-imidazole-2-yl]benzamide.
Owner:KYOTO PREFECTURAL PUBLIC UNIV CORP +2
Therapeutic agent for keratoconjunctive disorders
ActiveUS9492431B2Strongly suppressing keratoconjunctive collagen contractionOrganic active ingredientsSenses disorderSuperficial punctate keratopathyInfectious Keratitis
The present invention addresses the problem of providing a novel therapeutic agent for keratoconjunctive disorders. As a means for solving the problem, a therapeutic agent for keratoconjunctive disorders which contains a RARγ agonist as an active ingredient is provided. The therapeutic agent exhibits an excellent ameliorating effect in a keratoconjunctive disorder model, and is therefore useful as a therapeutic agent for keratoconjunctive disorders such as corneal ulcer, corneal epithelial abrasion, keratitis, dry eye, conjunctivitis, chronic superficial keratitis, corneal erosion, persistent corneal disorders, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, infectious keratitis, noninfectious keratitis, infectious conjunctivitis and noninfectious conjunctivitis. The therapeutic agent is also useful as a therapeutic agent for corneal scarring and conjunctival scarring both associated with keratoconjunctive disorders.
Owner:YAMAGUCHI UNIV +1
Eye drop for treating eye dryness
ActiveCN107049938AProtect epithelial barrier functionRecovery quantityOrganic active ingredientsSenses disorderConjunctivaEye dryness
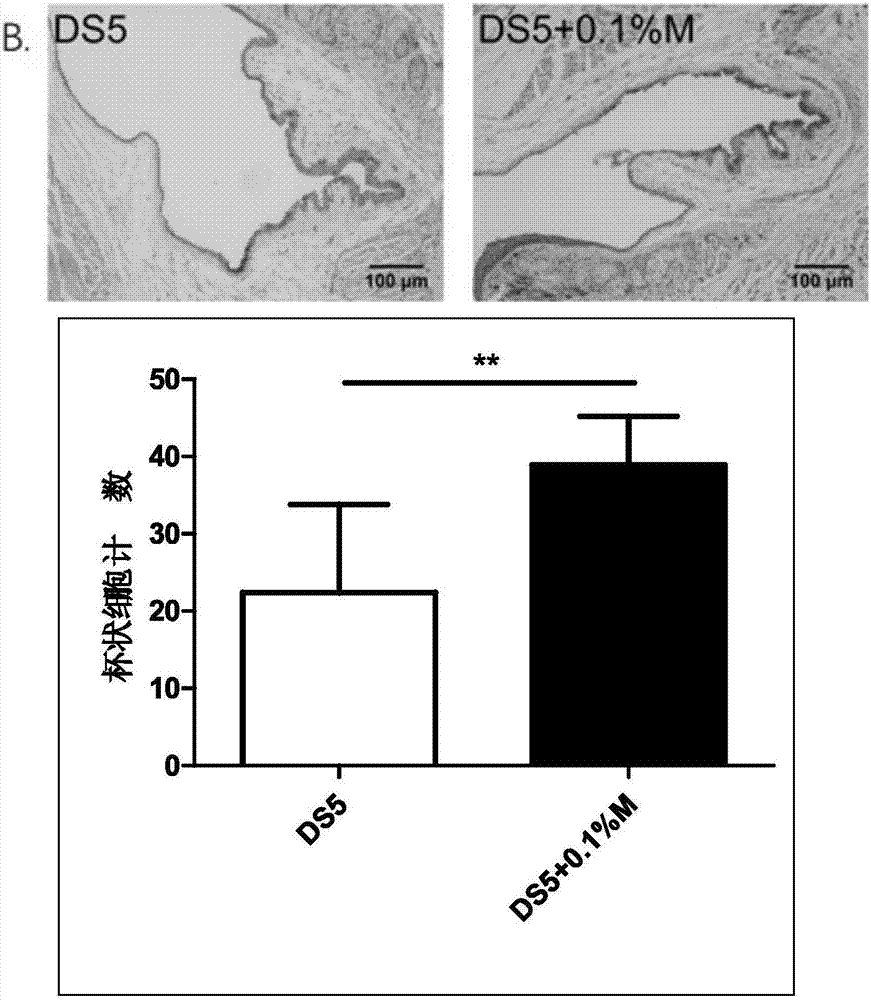
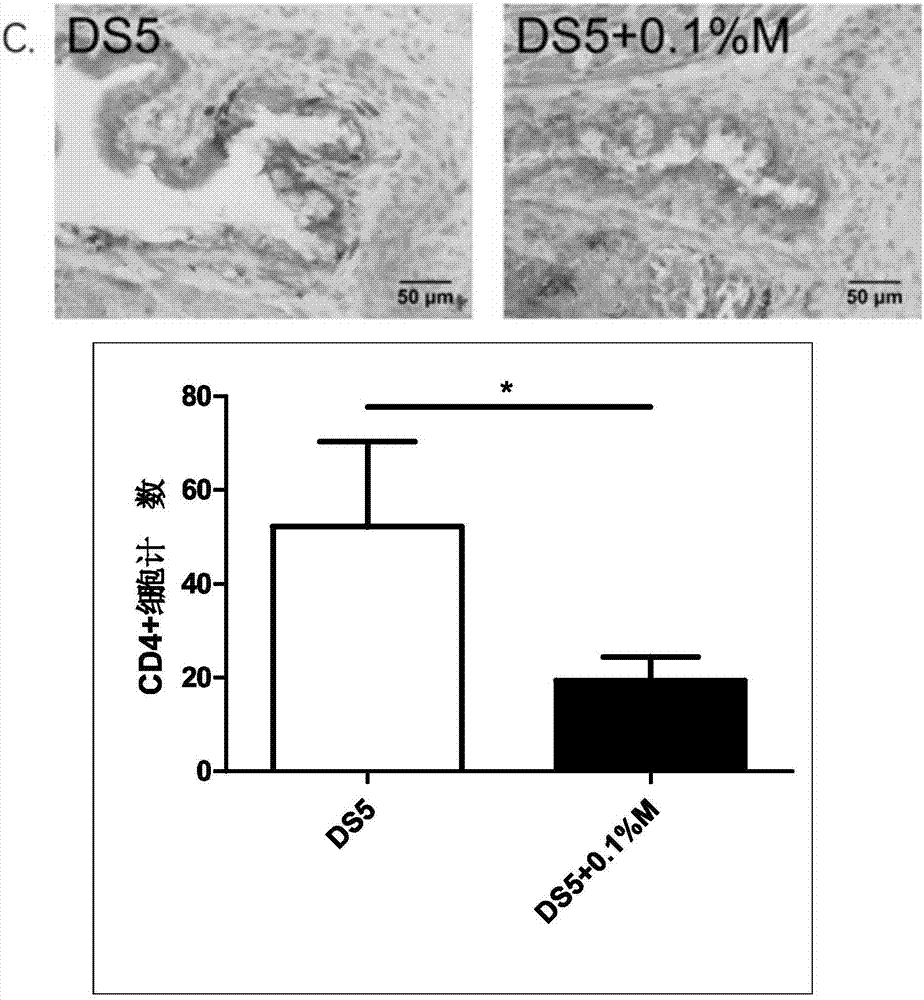
The invention discloses an eye drop for treating eye dryness. The pH value of the eye drop is 5.0-7.0, the eye drop comprises effective components of 5-hydroxy-1-beta-D-furanose-1H-imidazole-5-carboxamide, and specifically the eye drop consists of the following components in percentage by weight: 0.05-0.1% of 5-hydroxy-1-beta-D-furanose-1H-imidazole-5-carboxamide, a proper amount of a pH value adjusting agent, 0.01-3% of an iso-osmotic agent, 0.003-0.5% of a bacteriostatic agent, 0.001-0.5% of a stabilizer, 0.01-0.5% of a tackifier, 2-5% of a solubilizer and the balance of water. Through partial treatment of the eye drop, epithelial barrier functions of dry eye mouse cornea can be protected, the number of goblet cells of cornea can be recovered, and relatively good anti-inflammatory and immunity inhibition effects can be achieved.
Owner:EYE MEDICAL XIAMEN BIOTECHNOLOGY CO LTD
Artificial multiporous nanometer cornea made of carbon-polyvinyl alcohol hydrogel
InactiveCN1568908AOvercoming complicated proceduresOvercome precisionEye implantsFiberIntra ocular pressure
A porous nanometer carbon-polyvinyl alcohol hydrogel artificial cornea, which is used for patients of cornea diaphaneity descent and cornea blind caused by eye tissue diseases, consisting of an optical part and a supporting part, the optical part being made from transparent polyvinyl alcohol hydrogel, and the supporting part being made from black nanometer carbon materials. The optical part is provided with an upper and a lower surface, the supporting part enwraps the optical part, combines tightly with human cornea tissues, and is embedded in the optical part. The materials of the optical part protrude on the top and the bottom and overtops the supporting part at the joint of the two parts, and forms a trapezoid right-angle structure. The invention overcomes the shortcomings of existing techniques, and is capable of watertightness engomphosis with host cornea, preventing infection, degrowth of corneal epitheliums, antagonizing intra-ocular pressure, and preventing the forming of the back fibrous membrane of cornea. The artificial cornea has advantages of tenderness, good elasticity, certain tensile strength, convenience in surgical operation, and maximum decrease of complicating diseases.
Owner:深圳华明生物科技有限公司
Apparatus and Method for Removing Epithelium from the Cornea
ActiveUS20120087970A1Facilitating de-epithelializationDelaminationOrganic active ingredientsBiocideMedicineCornea surface
An apparatus and a method for removing epithelium from the cornea include a fluid agent for facilitating de-epithelialization of the cornea. A disc includes a biocompatible material operable for covering a predetermined zone of a cornea. The disc is hydrated by the fluid agent, wherein the hydrated disc is pliable for conforming to a surface of the cornea. An application of the hydrated disc to the cornea substantially constrains the fluid agent to the determined zone and softens a corneal epithelium enabling delamination of the epithelium from an underlying stroma.
Owner:NEWMAN LEONARD A
Instrument and method for scrubbing the corneal epithelium
Owner:ORCA SURGICAL
Sodium hyaluronate eye drops and a preparation method thereof
ActiveCN102697713AExtended burst timePromote healingSenses disorderHydroxy compound active ingredientsDisodium EdetateBoronic acid
The invention relates to the field of medicine and particularly relates to sodium hyaluronate eye drops and preparation method thereof. Every 100 ml of the sodium hyaluronate eye drops comprise the following components: 80 to 150 mg of sodium hyaluronate, 130 to 200 mg of boric acid, 10 to 20 mg of borax, 8 to 15 mg of edetate disodium, 80 to 160 mg of 6-amino caproic acid, 80 to 160 mg of mannitol, 600 to 800 mg of sodium chloride, 3 to 10 mg of benzalkonium chloride, and 8 to 15 mg of borneo camphor, wherein 100 ml of water for injection is added, and the pH value is regulated to 6.0 to 7.8. The eye drops provided by the invention can play the role of promoting healing of injuries of corneal epithelium, can prolong the break-up time remarkably and can better improve the symptoms of xerophthalmia. Meanwhile, the sodium hyaluronate eye drops have good stability at ambient temperature, and all figures are similar, which indicates that the preparation technology is steady and practicable; and if the eye drops are used within the available period, the indexes of HA-Na are all within the quality control range.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
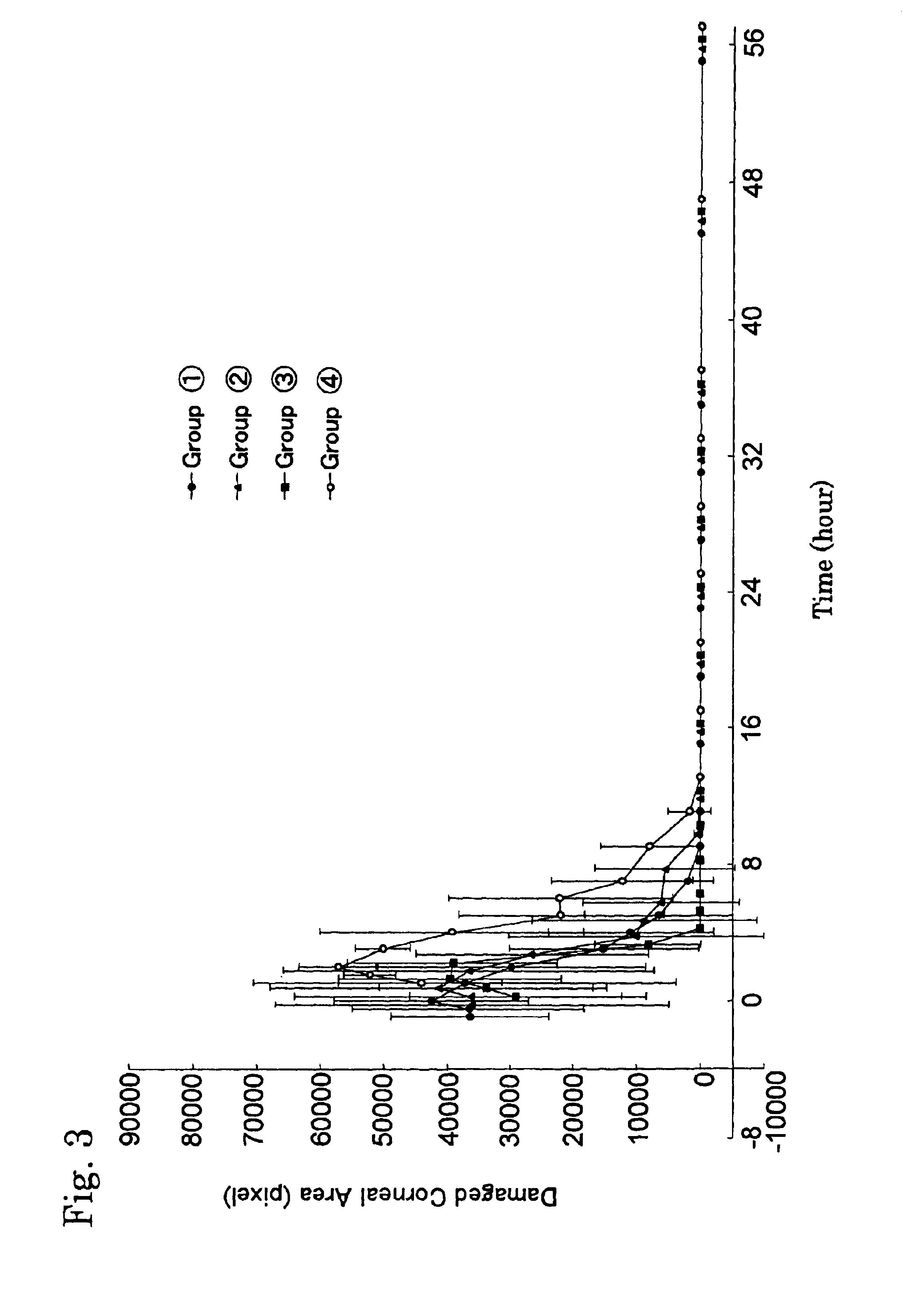
Experimental animals for evaluation of therapeutic effects on corneal epithelial damages
Owner:BIOCHEM & PHARMACOLOGICAL LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com