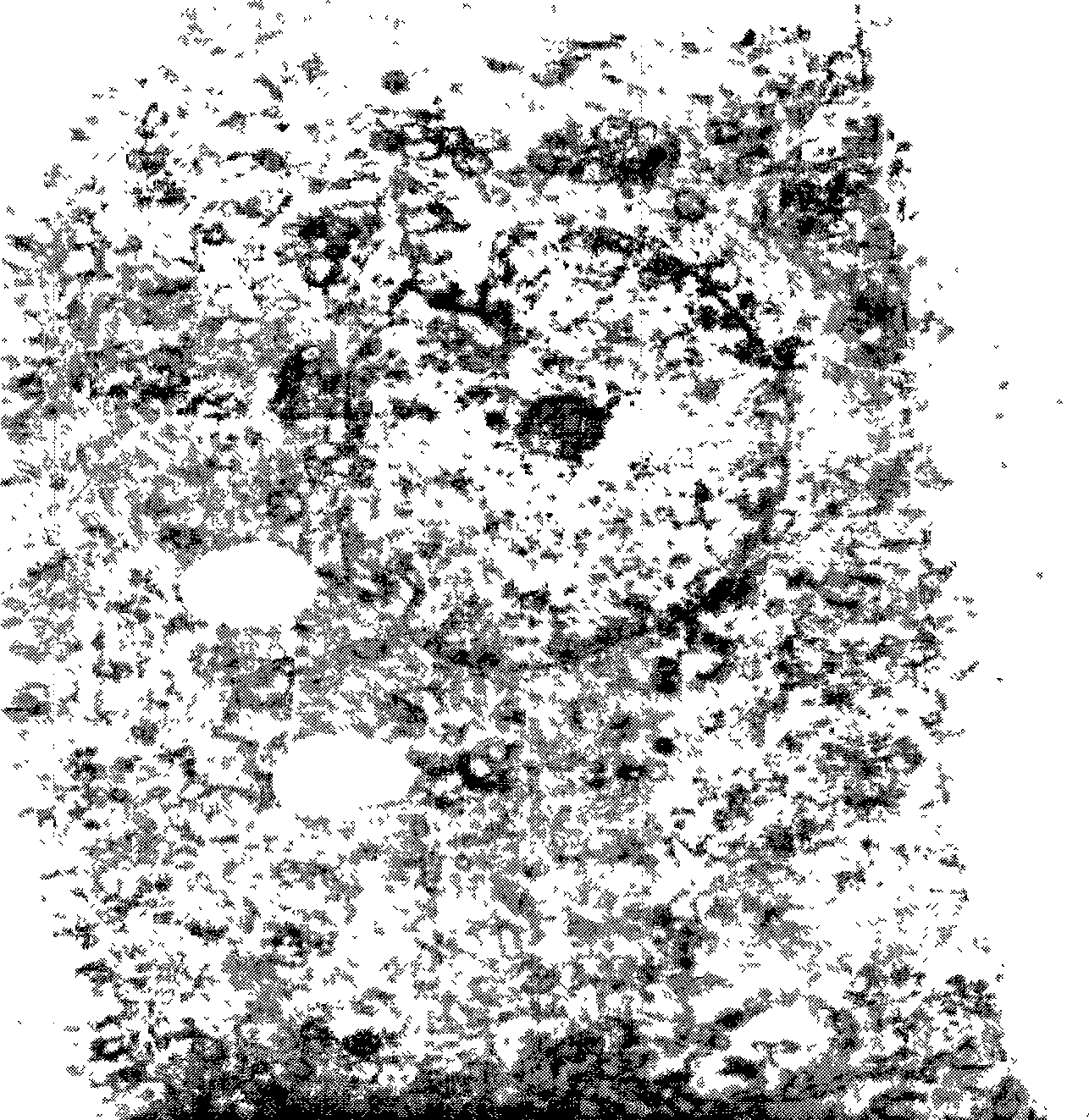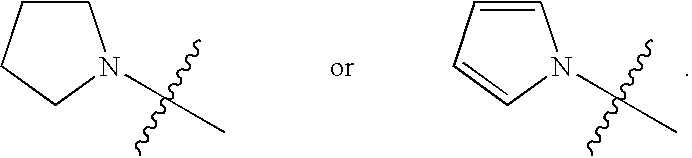Patents
Literature
93 results about "Corneal ulcer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
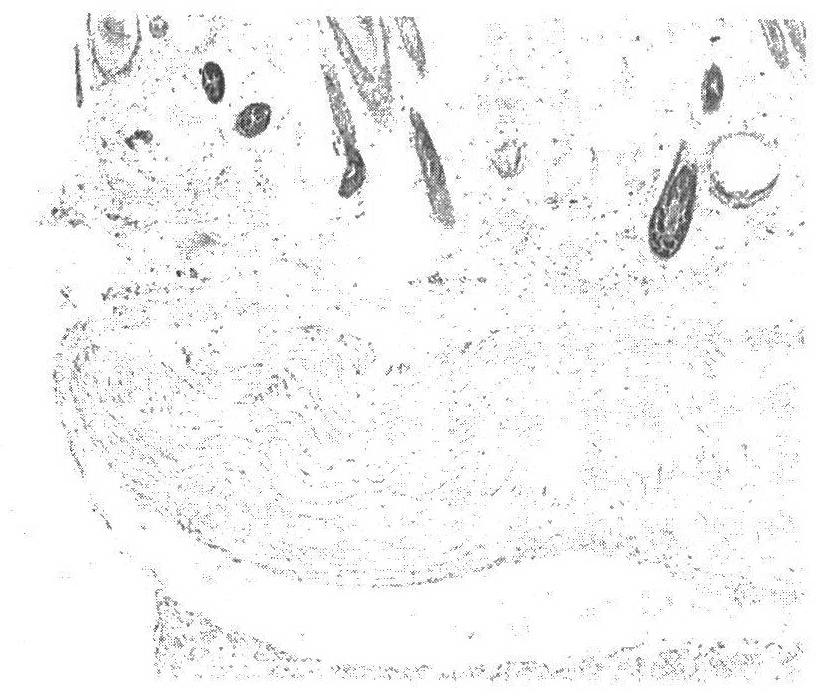
Corneal ulcer is an inflammatory or, more seriously, infective condition of the cornea involving disruption of its epithelial layer with involvement of the corneal stroma. It is a common condition in humans particularly in the tropics and the agrarian societies. In developing countries, children afflicted by Vitamin A deficiency are at high risk for corneal ulcer and may become blind in both eyes, which may persist lifelong. In ophthalmology, a corneal ulcer usually refers to having an infectious cause while the term corneal abrasion refers more to physical abrasions.
Therapeutic agent for keratoconjunctival disorder
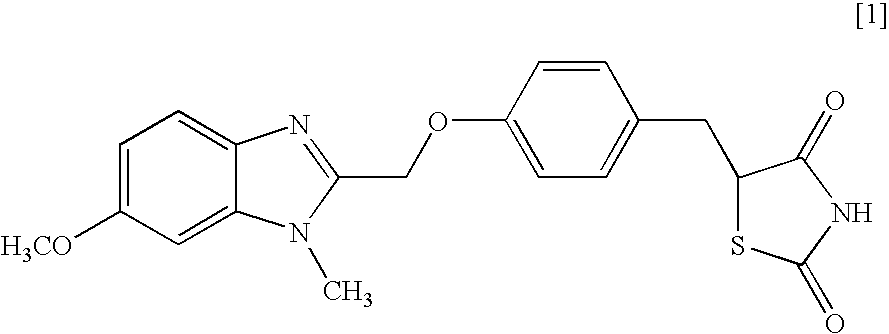
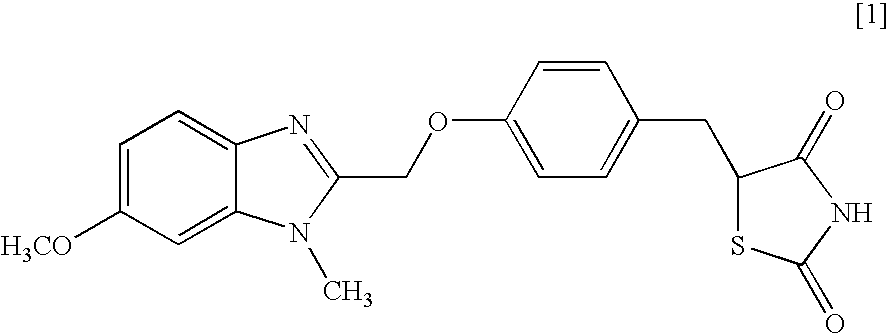
Object of the present invention is to search a novel pharmaceutical use of 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione being a condensed heterocyclic compound, or a salt thereof. 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione or a salt thereof can exert an excellent effect to promote healing in a dry eye model, and is useful as a therapeutic agent for keratoconjunctival disorders such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
Ophthalmic, otic or nasal pharmaceutical composition and the use thereof
InactiveUS20100222308A1Effective treatmentPreventing increase of bacterial infection riskAntibacterial agentsBiocideInfective rhinitisNose
The invention provides an ophthalmic, otic or nasal pharmaceutical composition, comprising levofloxacin or the pharmaceutical acceptable salts thereof and loteprednol etabonate, wherein the weight ratio of loteprednol etabonate to levofloxacin is 1:0.2-5. The use of ophthalmic, otic or nasal pharmaceutical composition of the invention in preparation of the medication for treatment of conjunctivitis, keratitis, blepharitis, dacrycystitis, hordeolum, corneal ulcer and ocular infection accompanied with ophthalmitis and even inflammation of the surrounding tissues, to prevent increase of bacterial infection risks and the tissue inflammation of the infected area after the ophthalmic surgeries or ocular injuries, to treat or alleviate the bacterial infection in combination with the tissue inflammation of the infected area, or to treat tympanitis, otitis externa and infective rhinitis.
Owner:SHENZHEN REGOO LAB
Method and application for preparing tissue engineering corneal carrier stent by utilizing fresh porcine cornea
ActiveCN104189957AReduce manufacturing costComplete structureProsthesisFreeze thawingBiocompatibility Testing
The invention discloses a method and application for preparing a tissue engineering corneal carrier stent by utilizing fresh porcine cornea. The method comprises the following steps: cutting a fresh porcine cornea for swelling, repeatedly freeze thawing and breaking cells, removing residual nucleic acid substances through DNA-RNA enzyme treatment, cross-linking, airing, and finally performing irradiation sterilization through ionization rays, thereby obtaining the product. The method is applied to carrier stents of tissue engineering corneal epithelium, stroma, endothelium, front board layer half cornea, rear board layer half cornea and full-layer board layer half cornea, so that the requirement on batch production of tissue engineering corneas is met. The carrier stent serves as a substitute of human corneal stroma to be used for clinical transplant and treatment of un-accumulated whole layer keratohelcosis. The method is applied to large-scale production and clinical popularization and application. The prepared carrier stent has the characteristics of high transparency, dense structure, high mechanical property, high biocompatibility with human corneal seed cells and the like. Therefore, the method is suitable for batch production, so that the requirements on lots of carrier stents for tissue engineering corneas are met.
Owner:青岛彩晖生物科技有限公司
Medicament composition for eyes or nose, and uses thereof
The present invention provides a combination of medicines for eyes or ears and nose, which comprises levofloxacin and loteprednol carbon ester; wherein, the weight ratio of the loteprednol carbon ester to the levofloxacin is between 1 to 0.2 and 1 to 5. The combination of medicine for eyes or ears and nose of the present invention is used for curing conjunctivitis, keratitis, blepharitis, dacryocystitis, hordeolum, corneal ulcer and eye infection with inflammation of eyes or even inflammation of tissue around eyes. The combination is also used for preventing bacterial infection risk after ophthalmology operation or eye injury and inflammation of the infected region, or the combination is used for curing or alleviating bacterial infection and tissue inflammation of the infected region after ophthalmology operation or eye injury, or cure tympanitis, otitis externa and infectious rhinitis.
Owner:SHENZHEN REGOO LAB
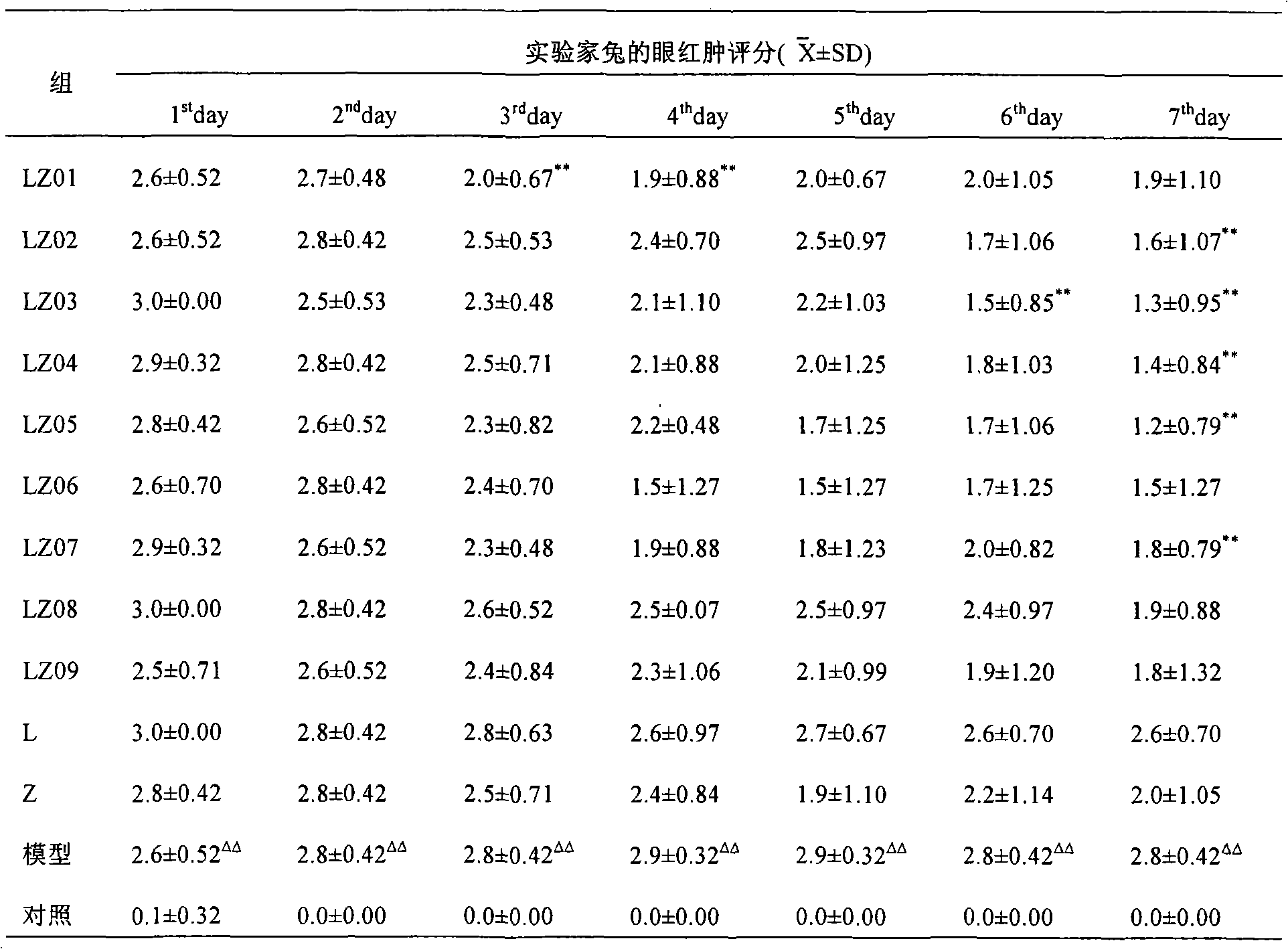
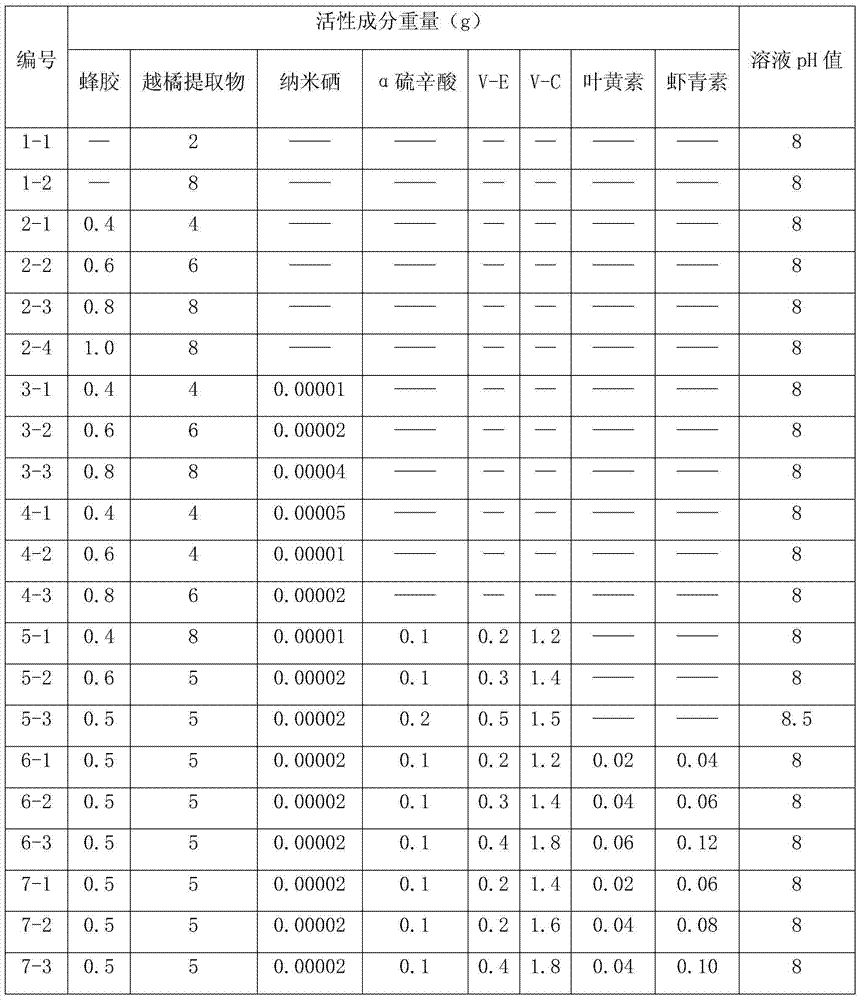
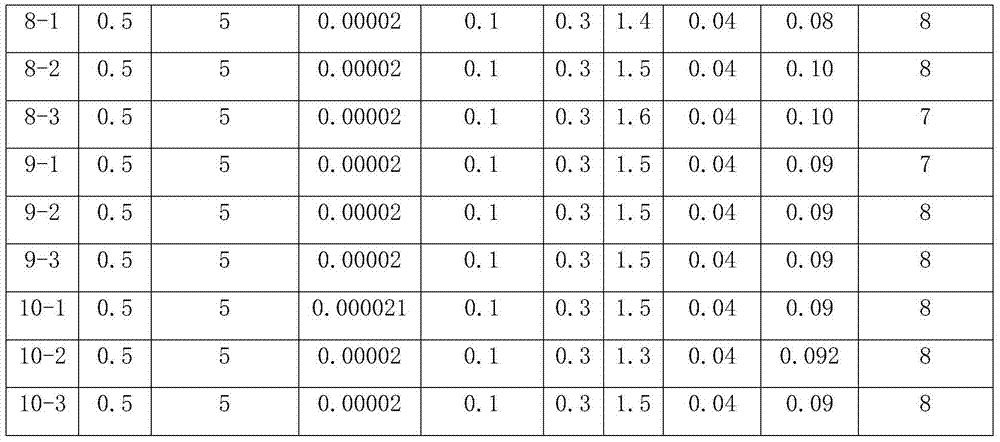
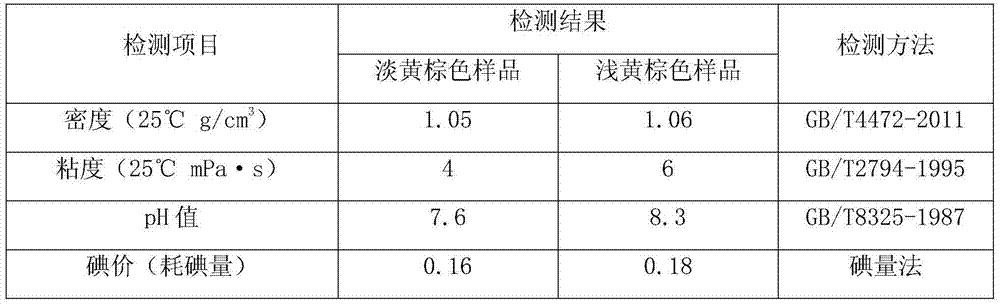
Cranberry extract eye ophthalmic preparation and preparation method and uses thereof
InactiveCN103860625AGood effectMeet the strong demand for eye protectionSenses disorderAnthropod material medical ingredientsLingonberry extractDisease
The invention relates to a cranberry extract eye ophthalmic preparation and a preparation method and uses thereof, and belongs to the technical field of eye ophthalmic preparations. The technical problem to be solved is to provide a cranberry extract eye ophthalmic preparation. The cranberry extract eye ophthalmic preparation comprises an active ingredient of cranberry extract. The cranberry extract eye ophthalmic preparation can effectively treat, prevent or alleviate cataract, glaucoma, vitreous opacity, macular degeneration, retinopathy, optic atrophy, keratitis, epiphora induced by wind, presbyopia, myopia, visual fatigue, blurred vision, conjunctival burn, conjunctivitis, corneal ulcer, palpebral ecgema, floaters or trachoma and other eye diseases. A novel preparation is provided for treating cataract, glaucoma, vitreous opacity, macular degeneration, retinopathy, optic atrophy, keratitis, epiphora induced by wind, presbyopia, myopia, visual fatigue, blurred vision, conjunctival burn, conjunctivitis, corneal ulcer, palpebral ecgema, floaters or trachoma and other eye diseases, and meets the strong demand of people for loving and protecting eyes.
Owner:CHENGDU JIANKABIN SCI & TECH
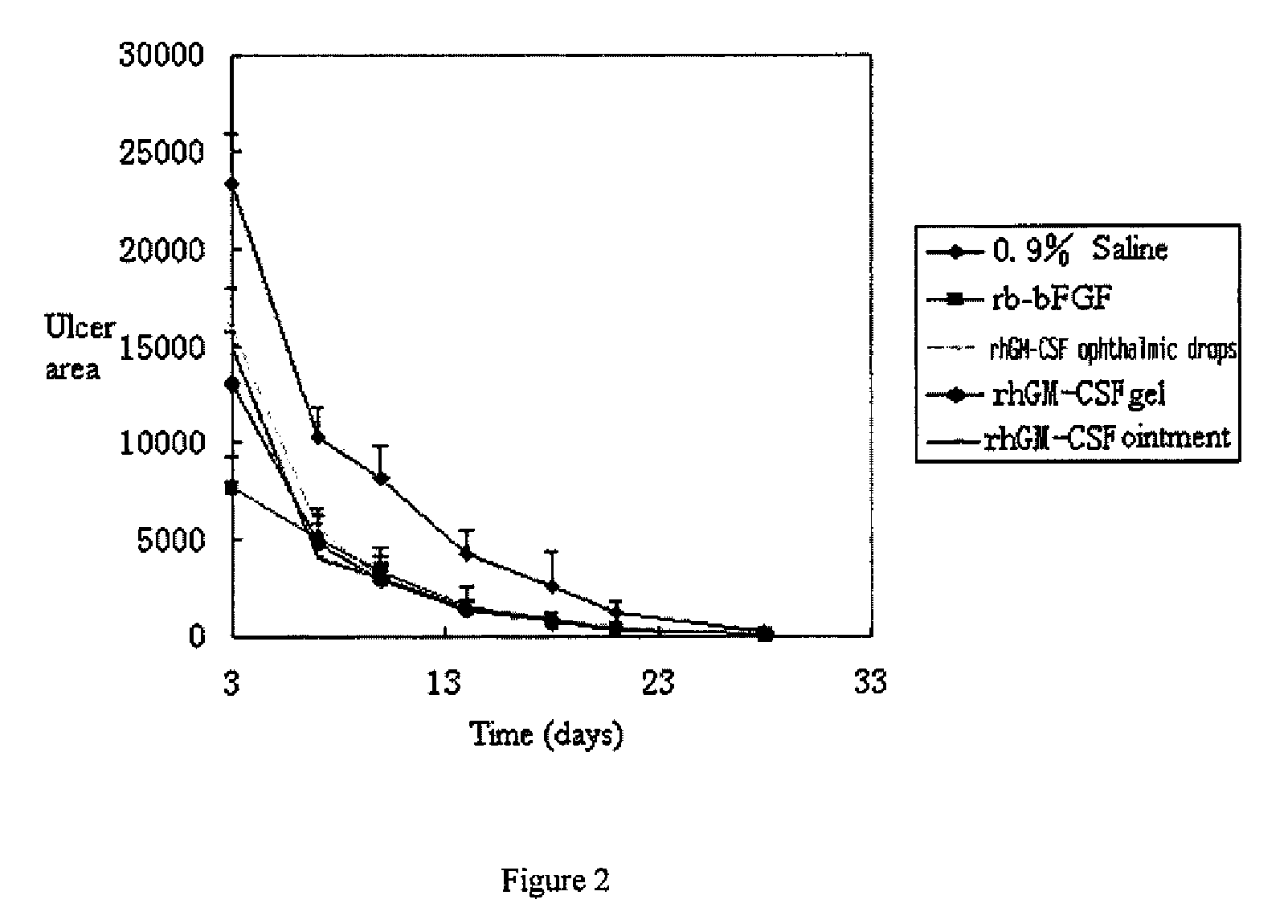
Ophthalmic hGM-CSF preparation
InactiveUS7858582B2Avoid excessive viscosityBenefit to drugSenses disorderPeptide/protein ingredientsGynecologyHuman growth hormone
Owner:CHANGCHUN GENESCIENCE PHARM CO LTD
Eye drops containing chitosan derivative
InactiveCN1403158AImprove bioavailabilityGood curative effectSenses disorderPharmaceutical non-active ingredientsClinical efficacyAdditive ingredient
The present invention relates to medicine technology, and is one kind of eye drops for treating conjunctivitis, keraritis, corneal ulcer, xerophthalma and other eye diseases. The eye drops contains water soluble chitosan derivative and medicinal active component, proper amount of pH controlling agent and osmotic pressure regulator. It has delayed releasing and long acting function and thus high biological medicine utilization and clinical curative effect.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Gatiflxacin eye gels based on HPMC medium and its preparing method
InactiveCN1562030AExtended stayNot easy to loseOrganic active ingredientsSenses disorderTreatment effectIrritation
An ocular Jiatishaxing gel for treating eyelid inflammation, stye, conjunctivitis, dacryocystitis, keratitis, corneal ulcer and trachoma is prepared from Jiatishaxing, HPMC as matrix, antiseptic, isotonic regulator, osmotic promoter, pH regulator and water through dissolving Jiatishaxing in water, adding others, stirring, regulating pH=5-9, filter and adding water.
Owner:SHENYANG PHARMA UNIVERSITY
In-vivo gel preparatino able to be dropped in eyes and its preparing process
InactiveCN1397272ALow toxicityLess irritatingSenses disorderPharmaceutical delivery mechanismTreatment effectIrritation
An eyedrops able to become gel after it is dropped in eye for treating bacterial conjuctivities, keratitis, keratohelcosis, dacryocystitis, etc is prepared from antibacterial infilammatino-relieving medicine, thickening agent, antiseptic, isotonic regulator, pH regulator and water. Its advantages are long stay time in eye, high curative effect, and low poison and irritation.
Owner:SHANGHAI XINGKANG PHARMA RES & DEV
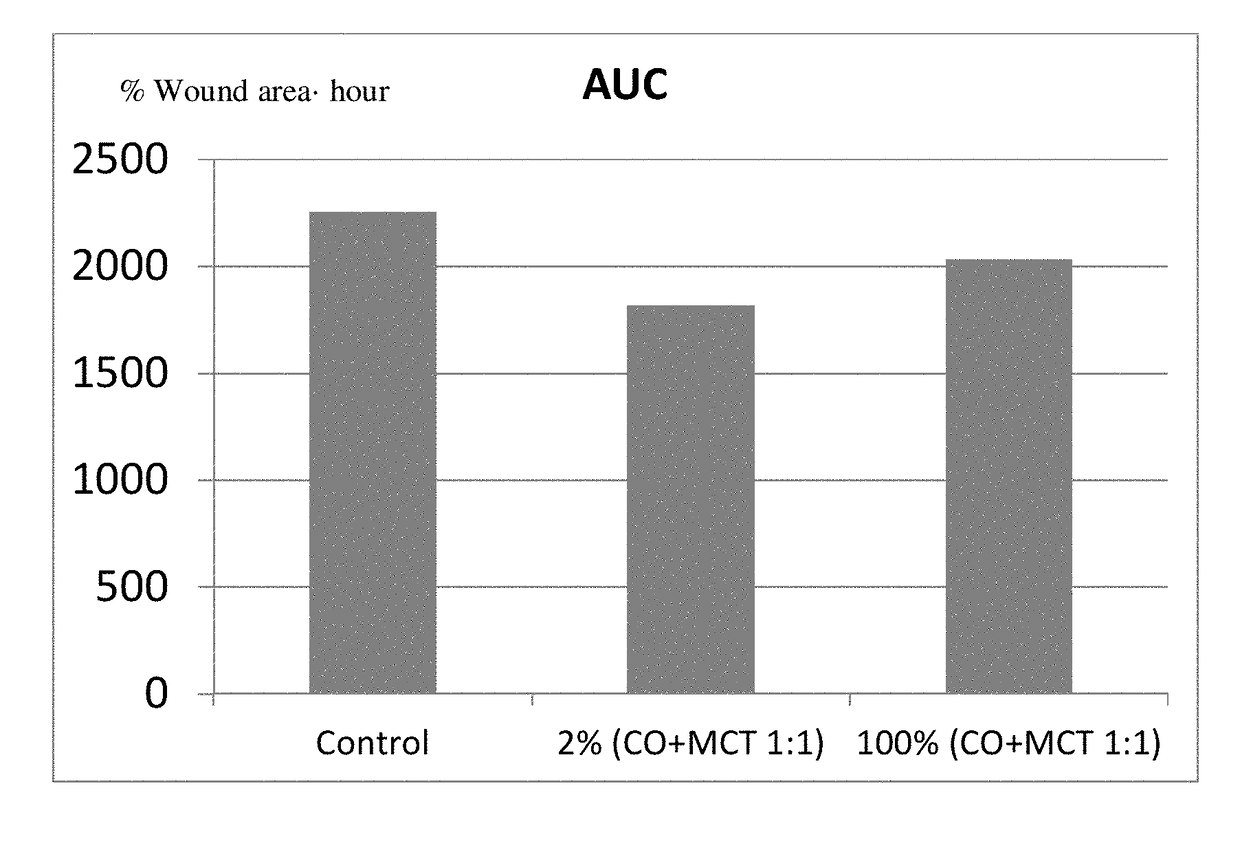
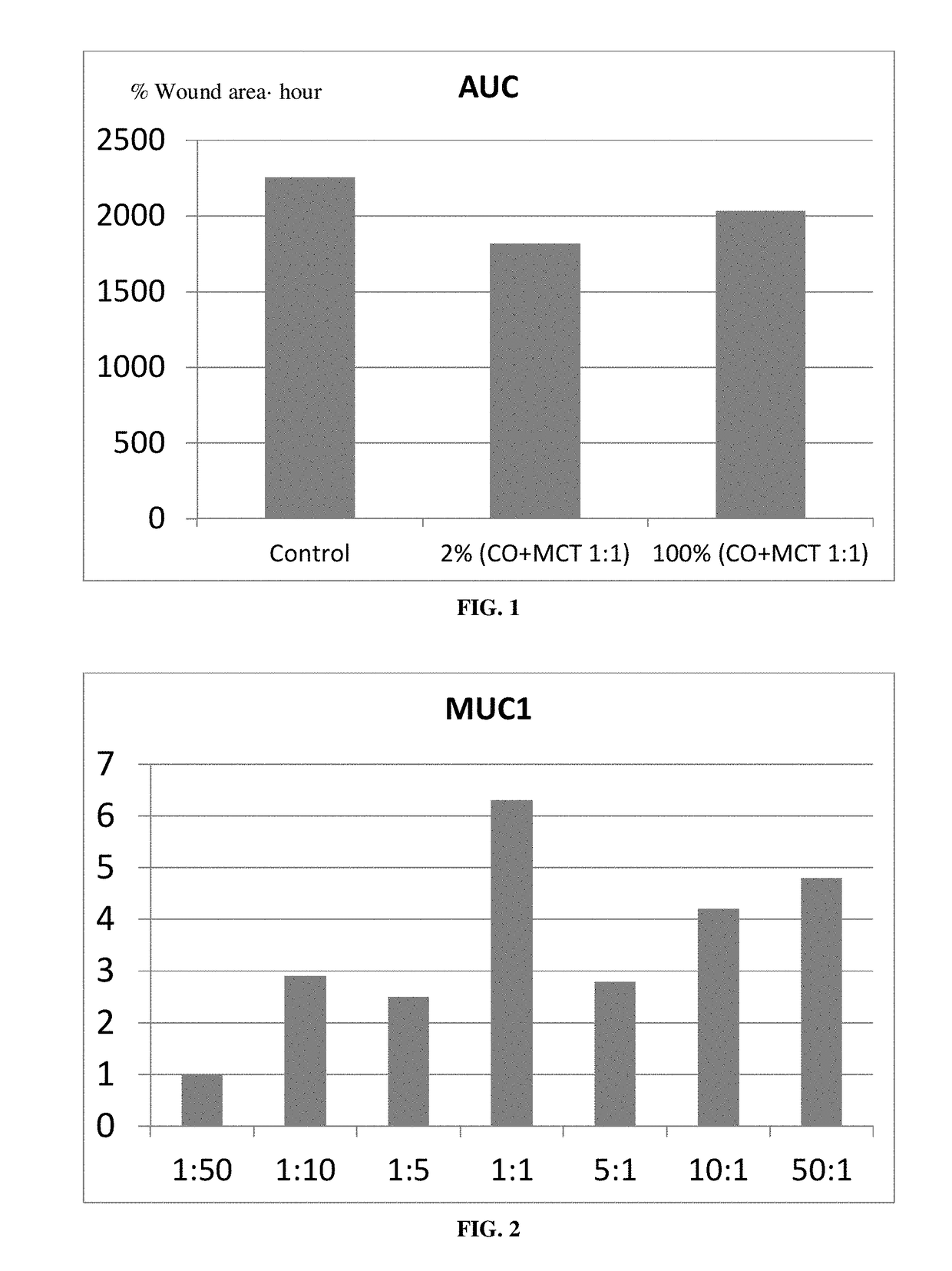
Ophthalmic compositions
ActiveUS20180008538A1High expressionSenses disorderAmide active ingredientsUveitisMedium-chain triglyceride
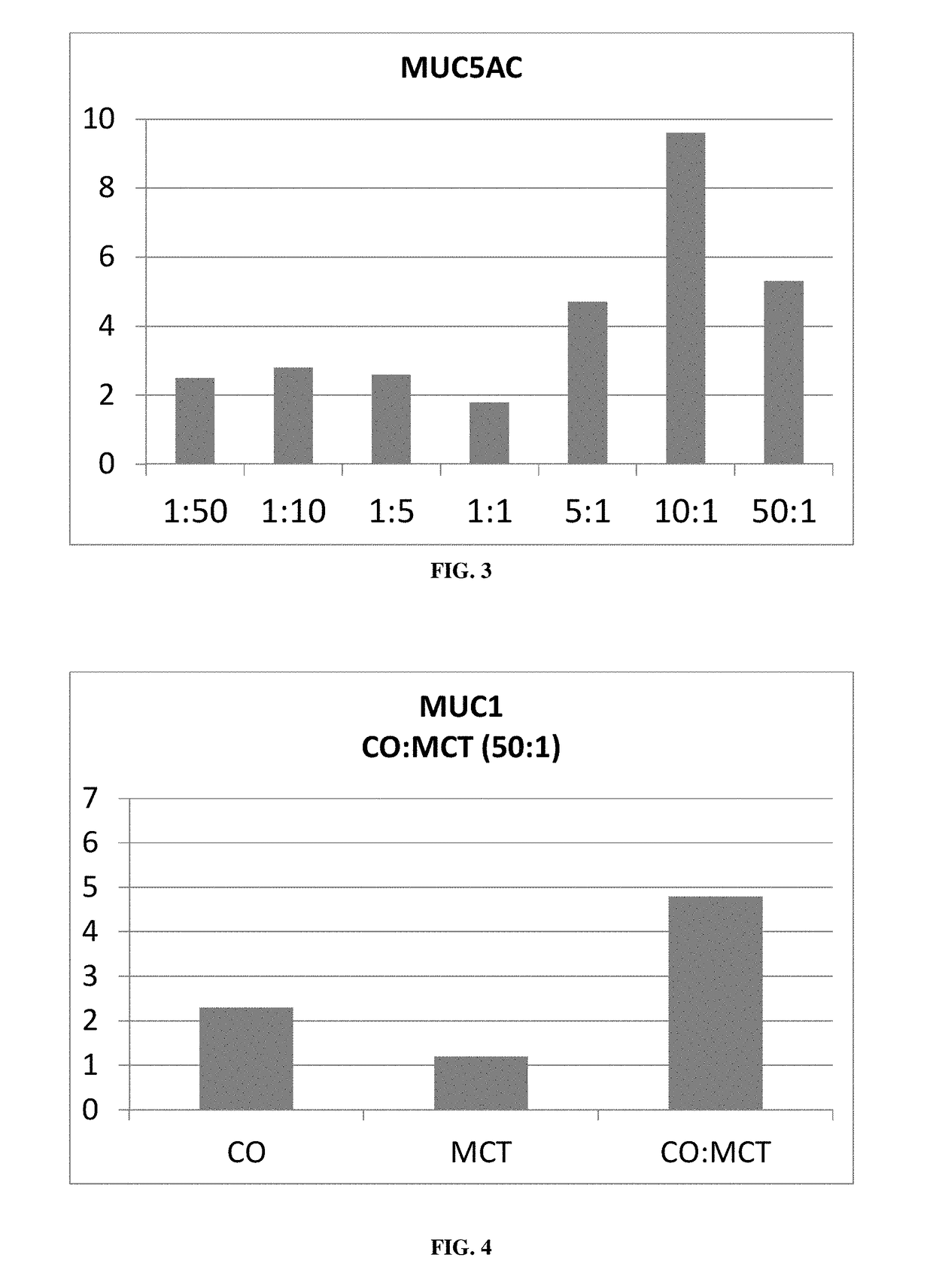
The present invention relates to a sterile ophthalmic composition comprising castor oil and a medium chain triglyceride, to its use in medicine, in particular for the treatment and / or prevention of an ocular disease selected from the group consisting of dry eye, conjunctivitis, dermatitis, blepharitis, entropion, floppy eyelid syndrome, thyroid ophthalmopathy, pterygium, conjunctivochalasis, epithelial damage induced by preservatives, epithelial or anterior chamber damage induced by ocular surgery, limbal cell deficiency, corneal ulcers induced by physical or chemical agents, keratitis, episcleritis and uveitis.
Owner:LAB SALVAT
Fresh amnion preservation fluid, fresh amnion preservation method and application thereof
InactiveCN101444202AExtended shelf lifeMorphologically normalDead animal preservationBatch processingPhosphate
The invention discloses a fresh amnion preservation fluid, a fresh amnion preservation method and application thereof. The fresh amnion preservation fluid is made by adding water for injection to 9.0-12.0g of DMEM culture medium, 10.0-25.0g of chondroitin sulfate, 0.5-1.0g of sodium hyaluronate, 4.0-5.0g of HEPES, 10.0-15.0g of dextran, 1.0-6.0ml of gentamycin, 24.0-30.0mg of dexamethasone, 10.0-15.0ml of non-essential amino acid, 0.50-0.95g of glutathione, 0.0-50.0ml of fetal calf serum to 1000ml. The fresh amnion preservation method comprises the following steps: separating the amnion by a blunt, rinsing the amnion clean, and then processing the amnion by an antibiotic; cutting and packaging the amnion into a container with the amnion preservation fluid; storing the amnion preservation fluid in a refrigerator with the temperature of 4 DEG C, and rinsing hands clean with aseptic phosphate buffer before use. The fresh amnion preservation fluid has the following advantages: 1. the amnion preservation fluid is only stored in the refrigerator with the temperature of 4 DEG C; 2. the shelf life is prolonged to at least 20-30 days; 3. the amnion preservation fluid can be in batch processing and centralized storage, and 4. the amnion preservation fluid can be applied to various amnion transplantation repairs, for example (1) ocular surface reconstruction as a conjunctival substitute, (2) amnion transplantation for treating symblepharon, and (3) treatment of corneal ulcer caused by various reasons and the like.
Owner:天津市医药科学研究所
8-Hydroxyquinoline compounds and methods thereof
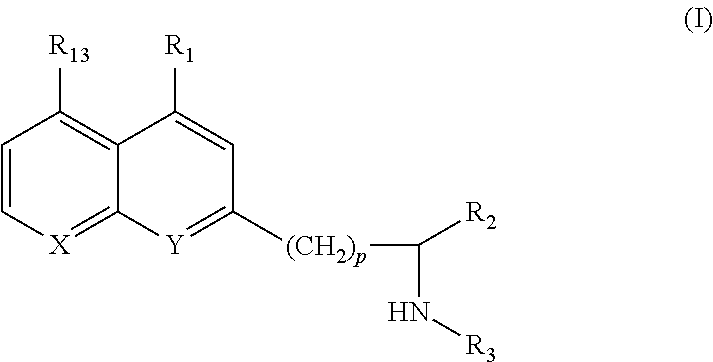
The present invention relates to 8-Hydroxyquinoline Compounds; compositions comprising an 8-Hydroxyquinoline Compound; and methods for treating or preventing a metalloproteinase-related disorder, such as, an arthritic disorder, osteoarthritis, malignant neoplasm, rheumatoid arthritis, asthma, chronic obstructive pulmonary disease, atherosclerosis, age-related macular degeneration, myocardial infarction, a corneal ulceration, an ocular surface disease, hepatitis, an aortic aneurysm, tendonitis, a central nervous system disorder, abnormal wound healing, angiogenesis, restenosis, cirrhosis, multiple sclerosis, glomerulonephritis, graft versus host disease, diabetes, an inflammatory bowel disease, shock, invertebral disc degeneration, stroke, osteopenia or a periodontal disease or comprising administering an effective dose of an 8-Hydroxyquinoline Compound to a mammal in need thereof.
Owner:WYETH LLC
Use of Urokinase Type Plasminogen Activator Inhibitors for the Treatment of Corneal Disorders
InactiveUS20110028397A1Effective treatmentImprove purification effectSenses disorderTripeptide ingredientsDiseaseCorneal ulcer
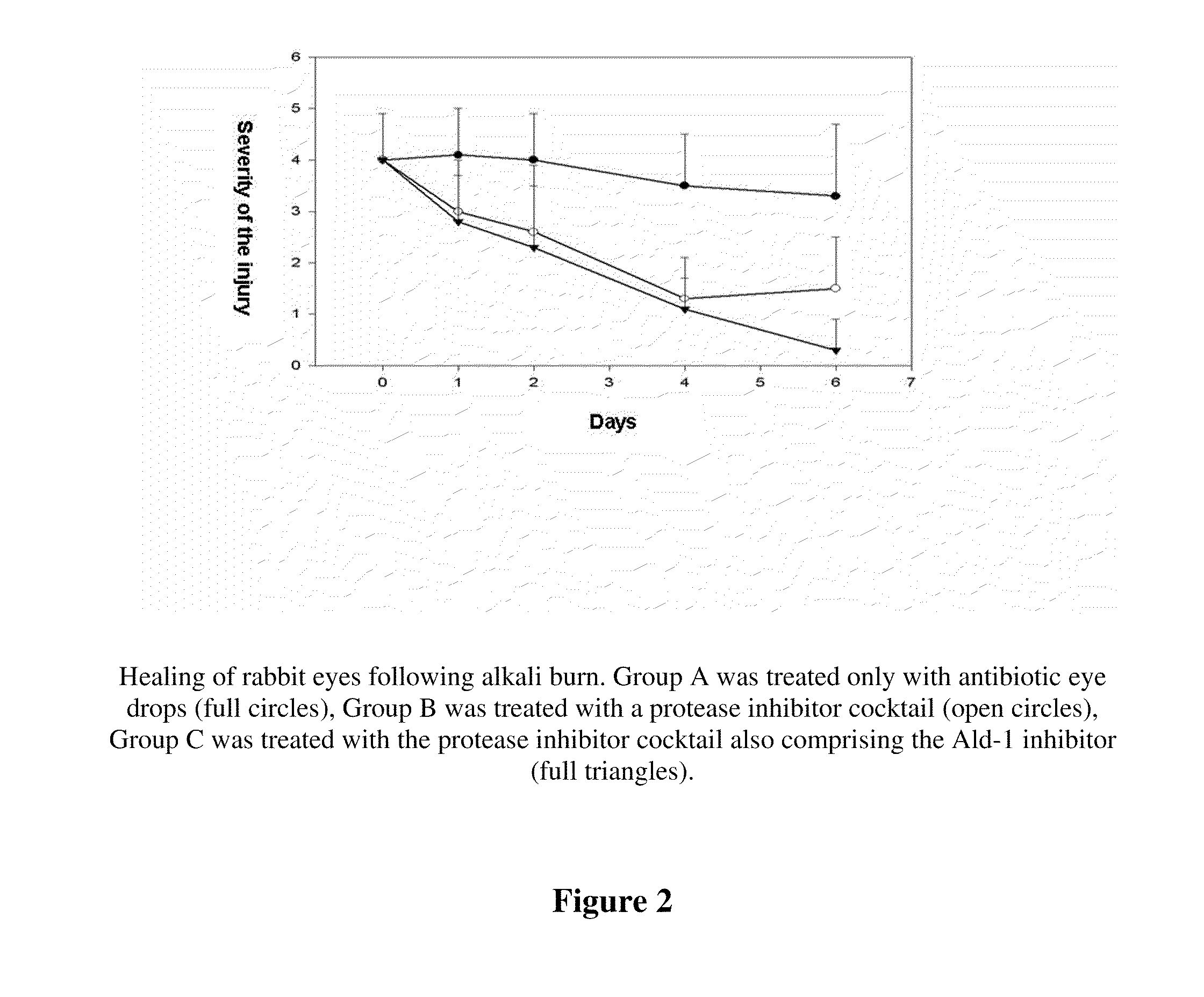
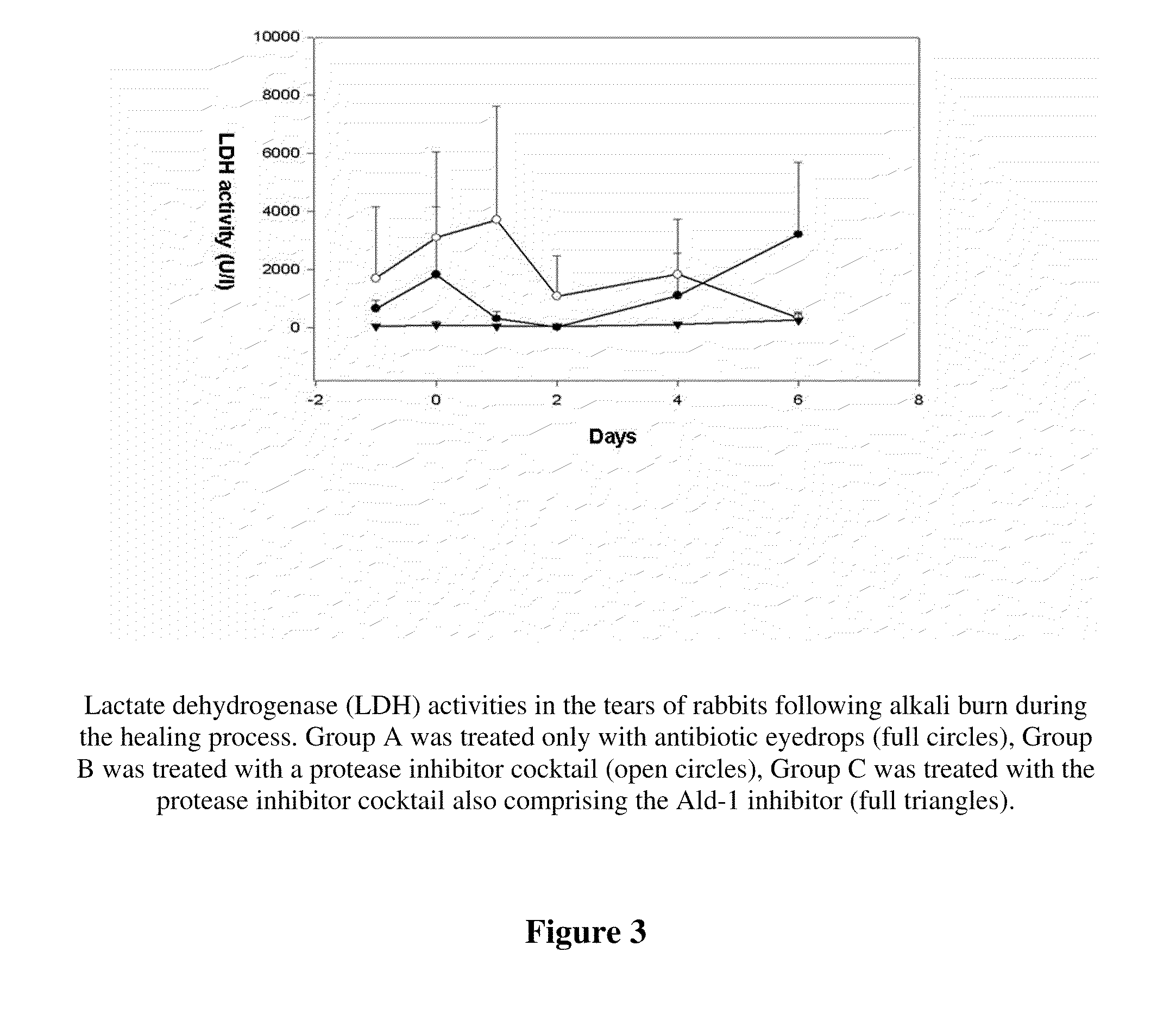
The invention concerns the use of inhibitors of the urokinase type of plasminogen activator (uPA) appearing in the anterior segment of the eye, for the treatment and prevention of corneal ulcers and other disorders. The invention further concerns pharmaceutical compositions, comprising inhibitors of uPA, preferably eye drops and eye ointments. The pharmaceutical compositions according to the invention preferably comprise PAI-2 protein or a derivative thereof retaining uPA-inhibiting capacity, or a tripeptide aldehyde inhibitor, preferably the D-Phe-Pro-Arg-aldehyde (Ald-1). The PAI-2 protein, used according to the invention, is preferably produced through bacterial expression, as a fusion protein.
Owner:UNIVERSITY OF DEBRECEN
Medical biological eye cold therapy dressing and preparation method thereof
ActiveCN104306430AKeep moistEnhance anti-inflammatorySenses disorderHydroxy compound active ingredientsPharmacologyDry eyes
The invention relates to the technical field of eye dressings and in particular relates to a medical biological eye cold therapy dressing and a preparation method thereof. The medical biological eye cold therapy functional dressing is prepared from the following components: sorbitol, hyaluronic acid, pearl powder, peppermint oil, borneol, a mother chrysanthemum extracting solution, cassia seed extractive and a balsam pear extracting solution. The medical biological eye cold therapy dressing has functions of diminishing inflammation and swelling, easing pain and keeping moist of eyes for a long time, is used for improving and auxiliary treating of symptoms of dry eyes, eyestrain, eye itching, eye swelling, black eyes, lacrimation, secretion increase, asthenopia, corneal ulcer and the like caused by reasons of myopia, cataract, presbyopia, glaucoma, excessive eye and the like and has function of improving amblyopia.
Owner:DONGGUAN DAQING MEDICAL DEVICES CO LTD +1
Composite collagen eye drops
ActiveCN1927392AKeep aliveMaintain adhesionOrganic active ingredientsSenses disorderTobramycinAntibiotic Y
Owner:GUANGZHOU TRAUER BIOTECH
8-hydroxyquinoline compounds and methods thereof
The present invention relates to 8-Hydroxyquinoline Compounds; compositions comprising an 8-Hydroxyquinoline Compound; and methods for treating or preventing a metalloproteinase-related disorder, such as, an arthritic disorder, osteoarthritis, malignant neoplasm, rheumatoid arthritis, asthma, chronic obstructive pulmonary disease, atherosclerosis, age-related macular degeneration, myocardial infarction, a corneal ulceration, an ocular surface disease, hepatitis, an aortic aneurysm, tendonitis, a central nervous system disorder, abnormal wound healing, angiogenesis, restenosis, cirrhosis, multiple sclerosis, glomerulonephritis, graft versus host disease, diabetes, an inflammatory bowel disease, shock, invertebral disc degeneration, stroke, osteopenia or a periodontal disease or comprising administering an effective dose of an 8-Hydroxyquinoline Compound to a mammal in need thereof.
Owner:WYETH LLC
Ophthalmic bacterial-infection resisting medicine for external use
ActiveCN101766628AGood intraocular penetrationInhibit or kill growthAntibacterial agentsSenses disorderDiseaseSide effect
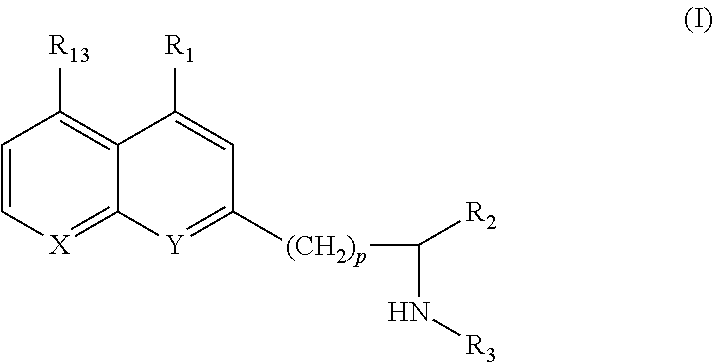
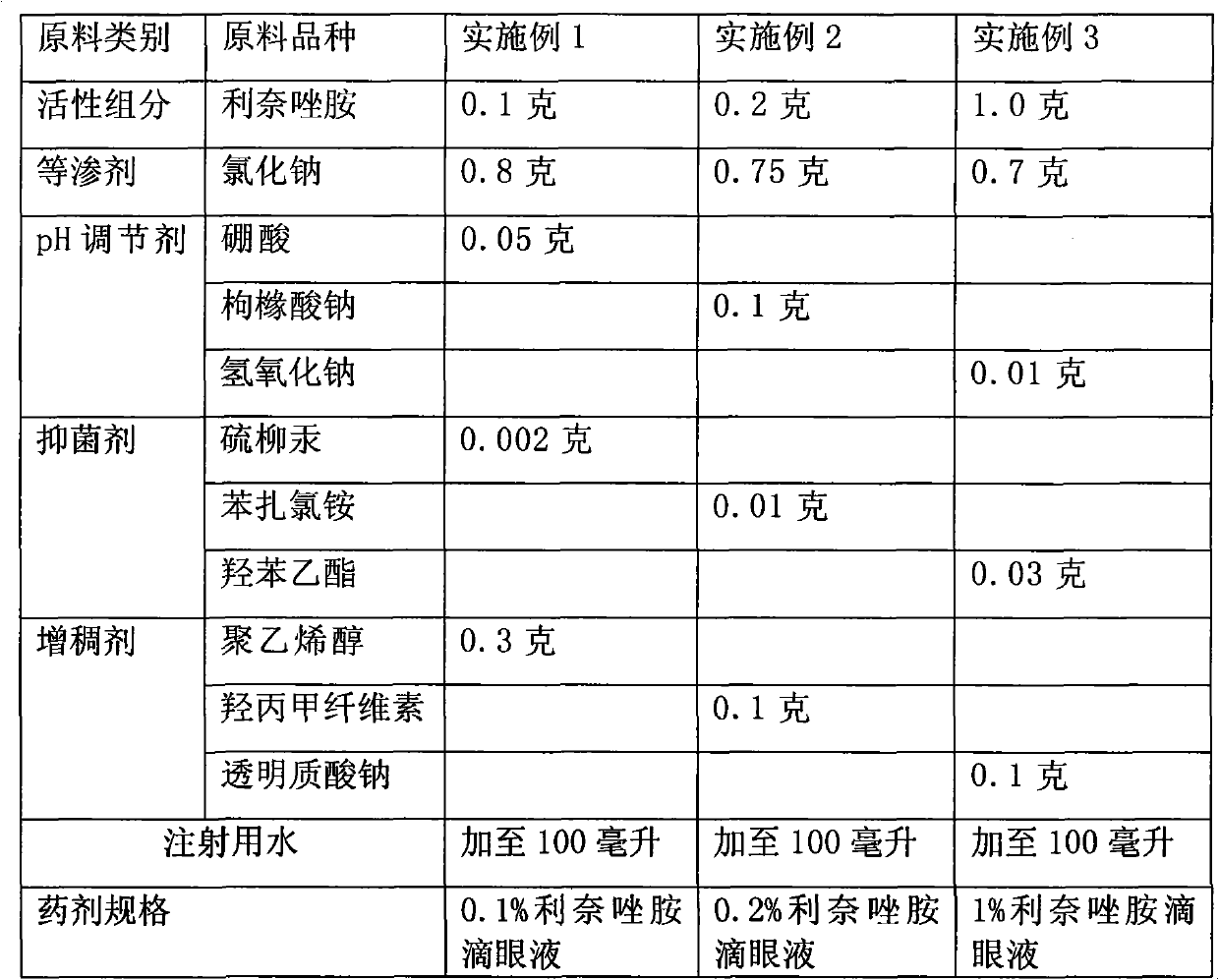
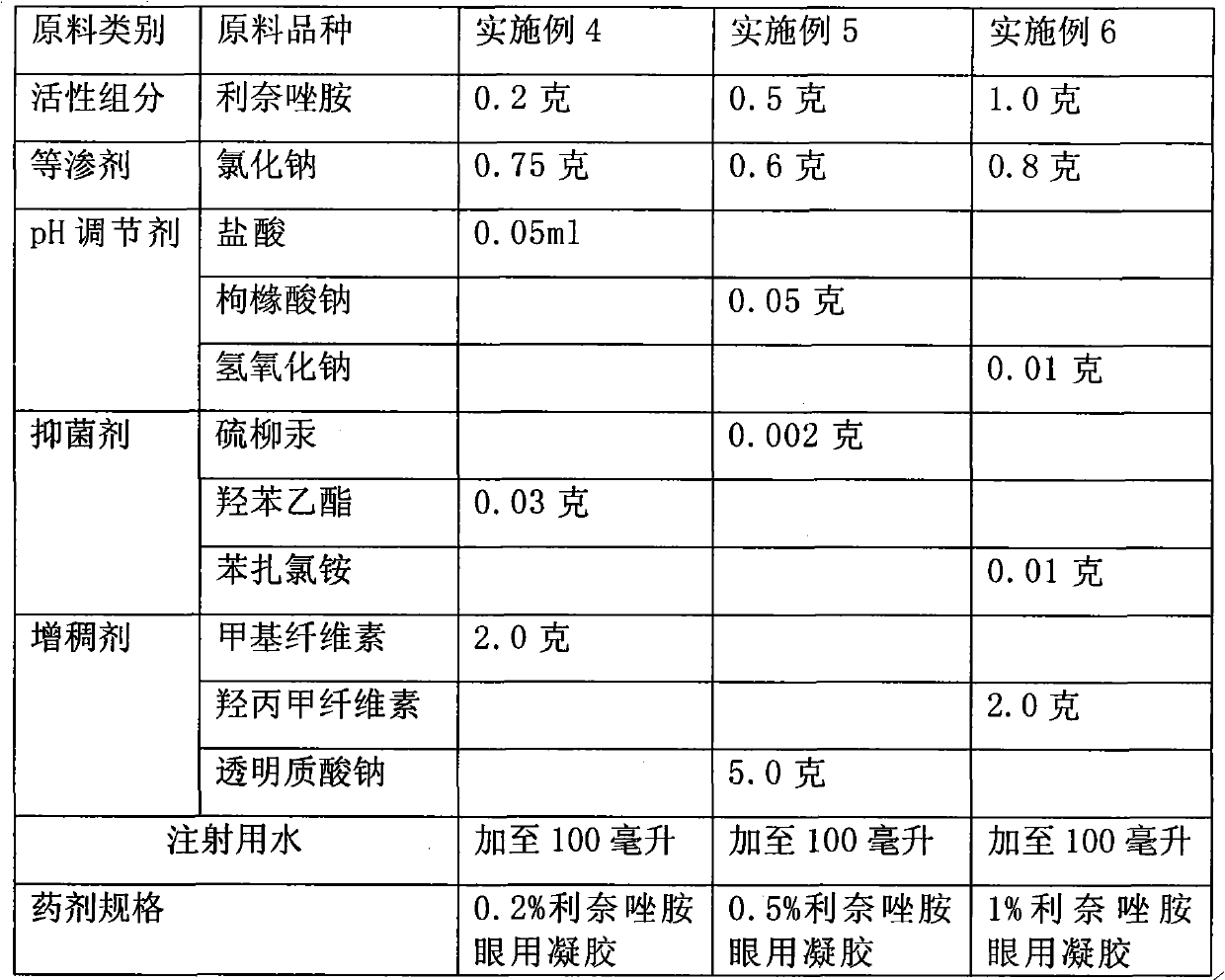
The invention provides ophthalmic bacterial-infection resisting medicine for external use. Linezolid is adopted as medicative raw materials to prepare eye drops, ophthalmic gel and eye ointments. The content of the linezolid in every 100 parts by weight of a medicament is within 0.1 to 1.0 parts by weight. The medicine is for external use at partial positions of eyes, and has good intraocular penetrability and mild toxic as well as side effects; moreover, the medicine is suitable for treating as well as preventing bacterial infection at partial positions of the eyes, including diseases of conjunctivitis, keratitis, keratohelcosis, iritis, ocular traumas, chemical injuries, ophthalmic postoperative infections and the like.
Owner:GUANGDONG WHOLEWIN TECH
Gatifloxacin external and ophthalmic gel preparation
InactiveCN1448137ADegradableGood film formingOrganic active ingredientsSenses disorderOphthalmic Gel Dosage FormDisease
The Gatifloxacin gel preparation for external use and eye use has Gatifloxacin as main component and its supplementary material includes chitosan as gel substrate, iso-osmotic regulator, pH regulator, preservative, injection water, etc. The preparation has Gatifloxacin content of 0.1-3 wt% and chitosan content of 0.3-3 wt%. It has obvious anti-infection function and functions of speeding heal of wound, promoting epidermal growth, inhibiting formation of scar tissue, maintaining local medicine density for long term, etc. It is used in treating burns, scalds, skin infection, folliculitis, bacterial conjunctivitis, keratitis, etc.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Therapeutic agent for keratoconjunctive disorders
ActiveUS20150290172A1Strongly suppressing keratoconjunctive collagen contractionOrganic active ingredientsSenses disorderSuperficial punctate keratopathyDisease
The present invention addresses the problem of providing a novel therapeutic agent for keratoconjunctive disorders. As a means for solving the problem, a therapeutic agent for keratoconjunctive disorders which contains a RARγ agonist as an active ingredient is provided. The therapeutic agent exhibits an excellent ameliorating effect in a keratoconjunctive disorder model, and is therefore useful as a therapeutic agent for keratoconjunctive disorders such as corneal ulcer, corneal epithelial abrasion, keratitis, dry eye, conjunctivitis, chronic superficial keratitis, corneal erosion, persistent corneal disorders, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, infectious keratitis, noninfectious keratitis, infectious conjunctivitis and noninfectious conjunctivitis. The therapeutic agent is also useful as a therapeutic agent for corneal scarring and conjunctival scarring both associated with keratoconjunctive disorders.
Owner:YAMAGUCHI UNIV +1
Compound capable of inhibiting zinc ion metalloproteinases
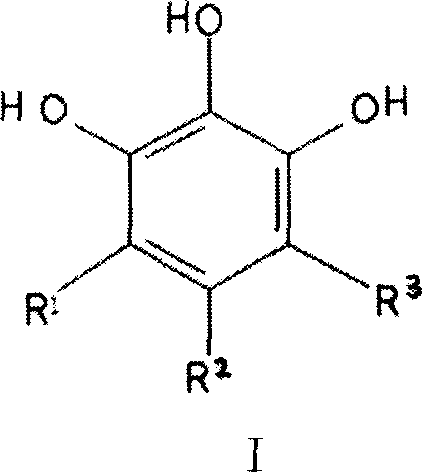
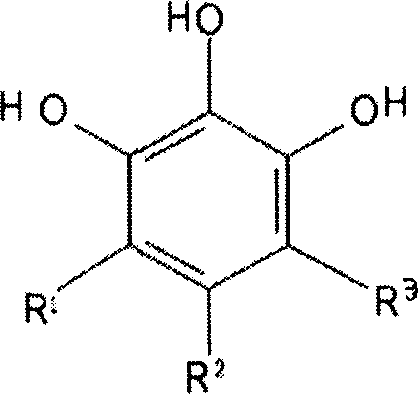
The invention discloses a 1,2,3-trihydroxy benzene and derivant or pharmacy acceptable salt for zinc ion metal-prolease inhibition, which is characterized by the following: theses compounds is used for selective depressant of zinc ion metal-prolease (such as MT1-MMP, gelatinase A, B and collagenase, matrilysins, metal-elastase and stromelysin-1); these depressants can adjust physiological and pathology course with MMPs, ADAMs, ADAM-TS such as vessel rebirth, wound healing, organ transplantation, fecundation course and reactivation capability, rebuilding bone and ache, which is used for treating cancer, cardiovascular disease, arthritis, periodontal disease, multiple sclerosis, inflammation, adenomyosis, cornea ulcer, bacterial meningitis, diabetic syndrome, kidney disease, nerve retrogression disease, AIDS, bleb, anaphylaxis, adenomyosis, osteoporosis, asthma and so on; theses blocking agents can be used for antisenescence, antibiosis, commercial manufacture addition agent of cell epimatrix, collagen product, cosmetics and make-up preparation, which can used for animals and other living bodies.
Owner:房学迅
Therapeutic agent for keratoconjunctive disorders
ActiveUS9492431B2Strongly suppressing keratoconjunctive collagen contractionOrganic active ingredientsSenses disorderSuperficial punctate keratopathyInfectious Keratitis
The present invention addresses the problem of providing a novel therapeutic agent for keratoconjunctive disorders. As a means for solving the problem, a therapeutic agent for keratoconjunctive disorders which contains a RARγ agonist as an active ingredient is provided. The therapeutic agent exhibits an excellent ameliorating effect in a keratoconjunctive disorder model, and is therefore useful as a therapeutic agent for keratoconjunctive disorders such as corneal ulcer, corneal epithelial abrasion, keratitis, dry eye, conjunctivitis, chronic superficial keratitis, corneal erosion, persistent corneal disorders, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, infectious keratitis, noninfectious keratitis, infectious conjunctivitis and noninfectious conjunctivitis. The therapeutic agent is also useful as a therapeutic agent for corneal scarring and conjunctival scarring both associated with keratoconjunctive disorders.
Owner:YAMAGUCHI UNIV +1
External preparation, method of producing the same and usage for the same
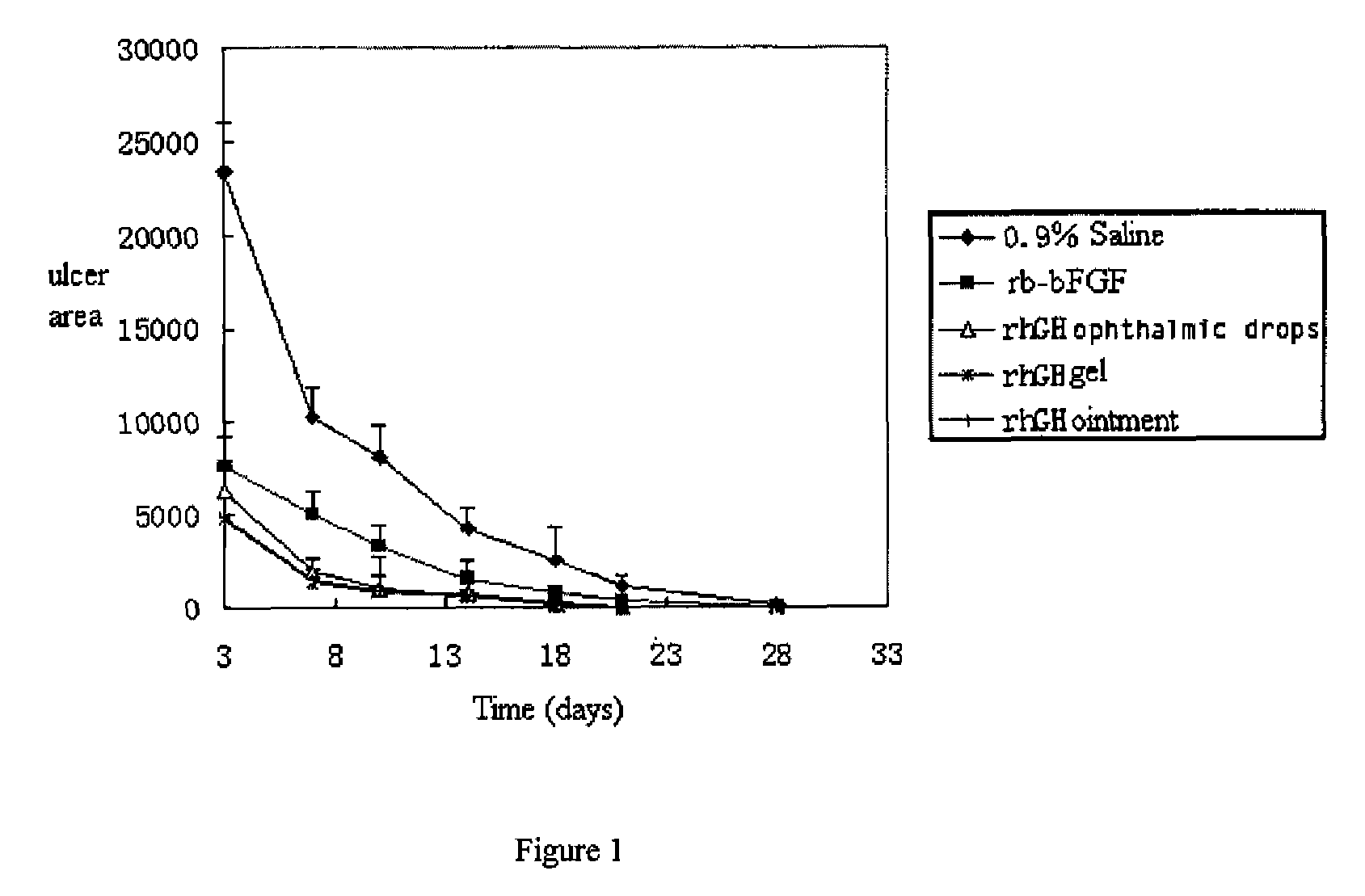
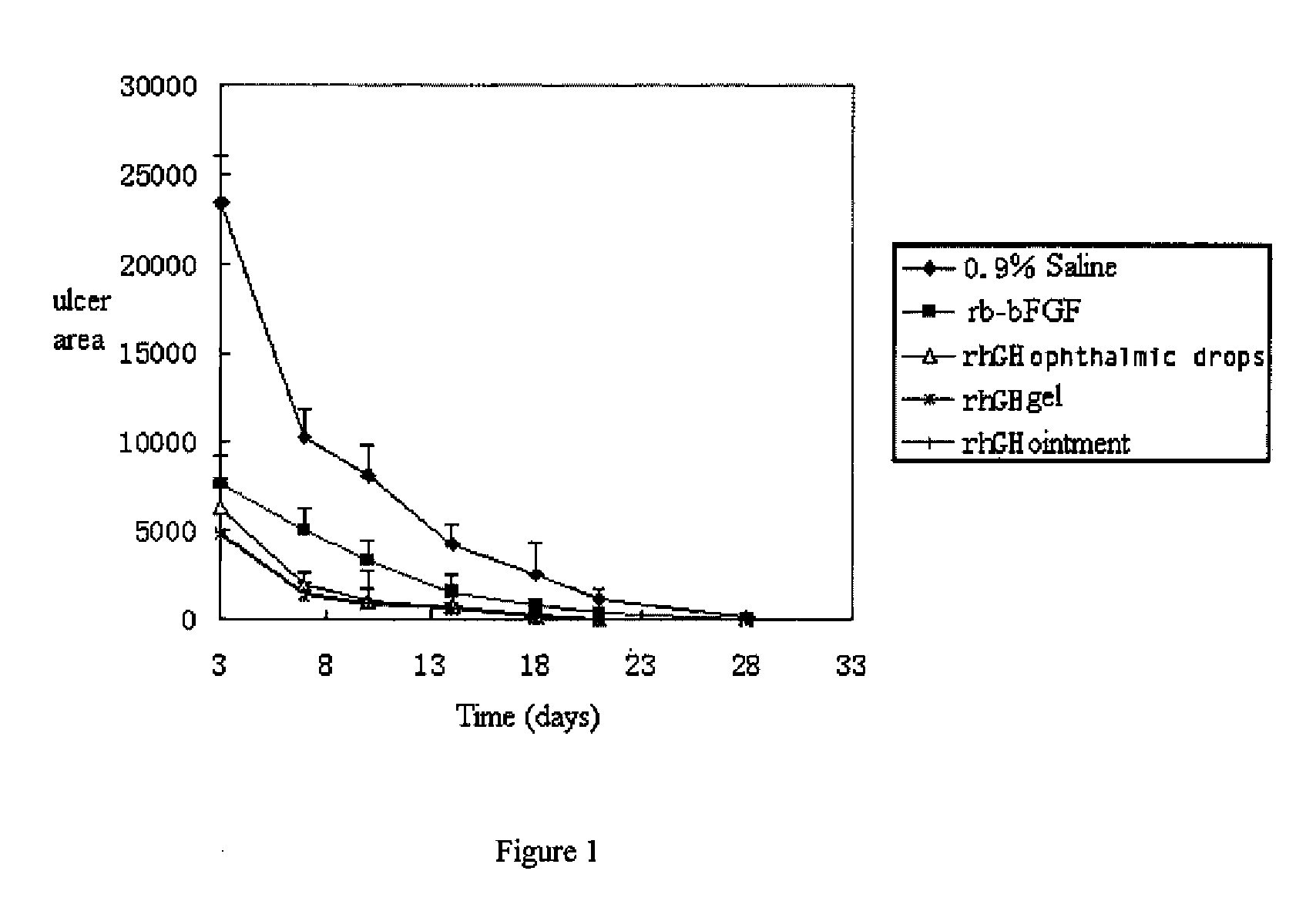
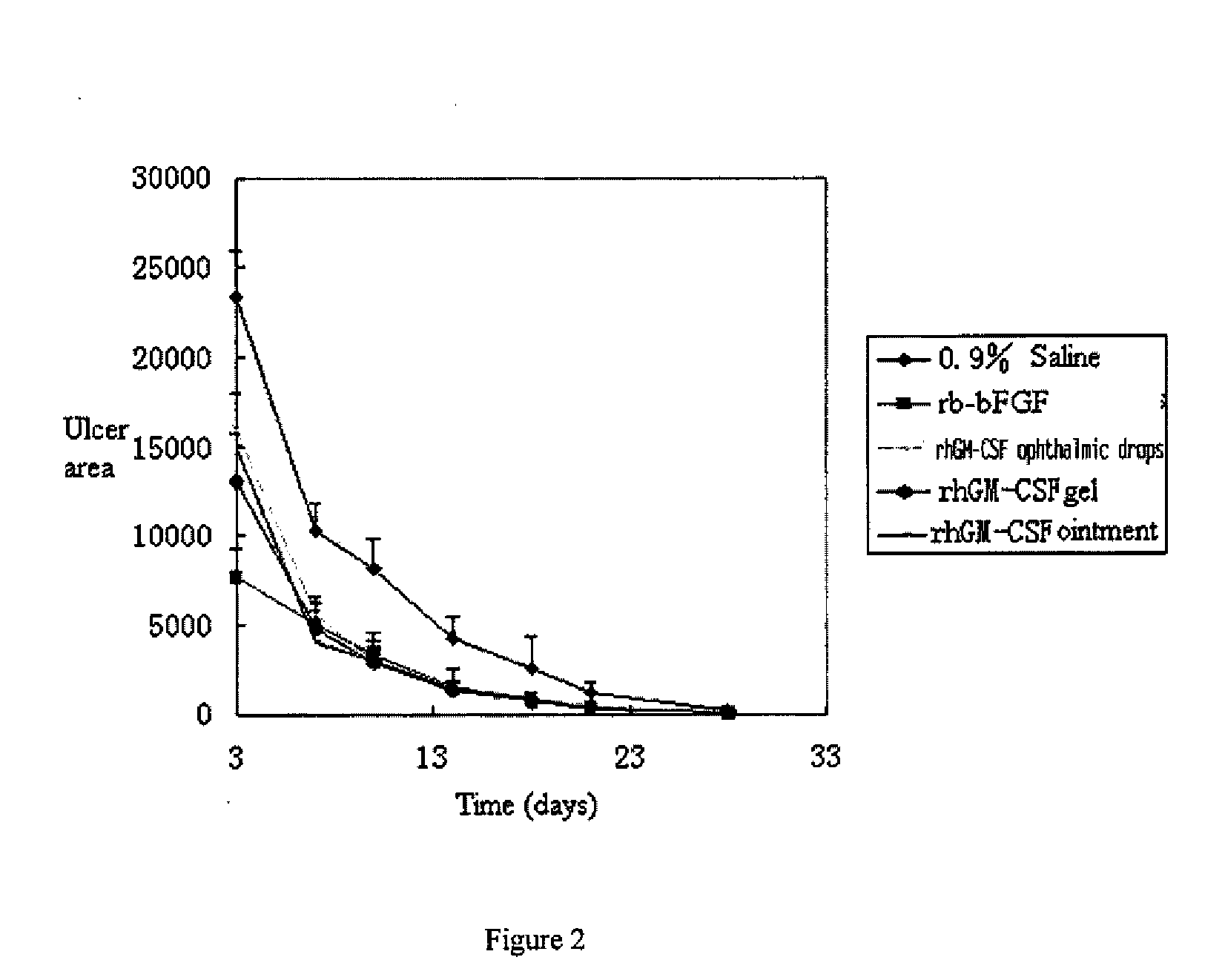
InactiveUS20090156482A1Facilitated DiffusionRegulate expressionOrganic active ingredientsSenses disorderHuman growth hormoneMedicine
The present invention provides an external preparation and the method for produce the same, in which said external preparation comprises recombinant human growth hormone or recombinant human granulocyte macrophage-stimulating factor and pharmaceutical acceptable carriers. The present invention also relates to application and usage method in preparing medicaments for treatment of various lesions and ulcer, especially corneal lesion and corneal ulcer.
Owner:CHANGCHUN GENESCIENCE PHARM CO LTD
Reagent kit for real time fluorescence quantitative PCR detection of bacillus pyocyaneus
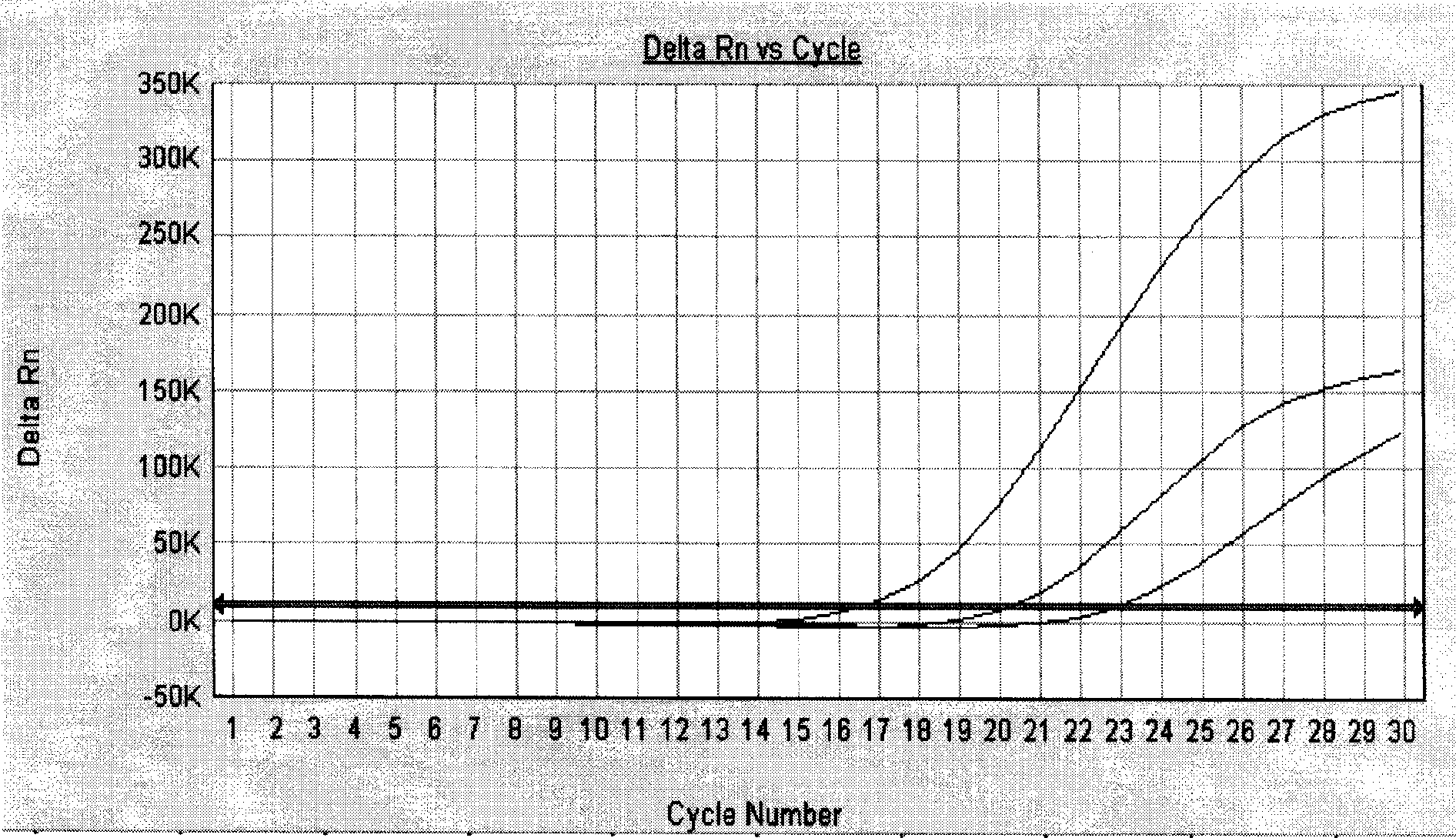
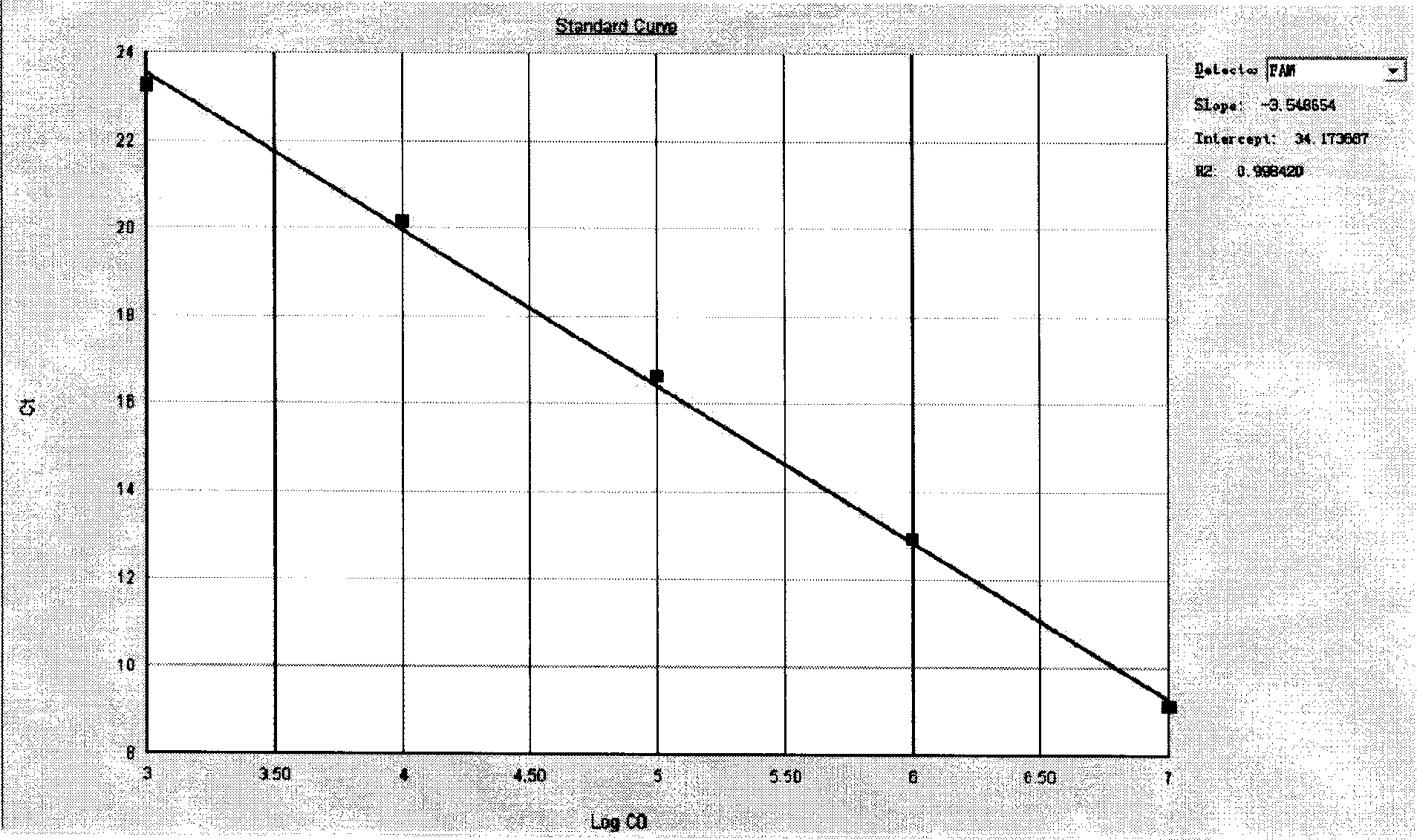
InactiveCN101429539AHigh sensitivityStrong specificityMicrobiological testing/measurementUpper urinary tract infectionFluorescence
The invention relates to a kit for detecting presence of pathogen pseudomonas aeruginosa in patient samples such as clinical bacterial pneumonia, corneal ulcer, urinary tract infection, septicemia and the like and samples such as cosmetics, environmental monitoring objects and the like, in particular to a kit for early and quickly diagnosing pseudomonas aeruginosa infection by a real-time fluorescence quantitative polymerase chain reaction technique.
Owner:DAAN GENE CO LTD
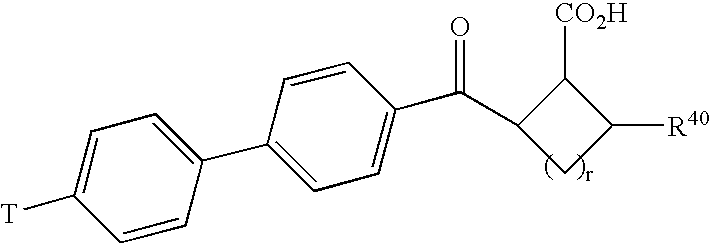
Inhibition of matrix metalloproteases by substituted biaryl oxobutyric acids
InactiveUS6911449B2Reduced activityObserved effectBiocideOrganic chemistryDiseaseAbnormal tissue growth
Owner:BAYER PHARMA CORP
Ophthalmic or otic and nasal composition containing difluprednate and lavo-ofloxacin and application thereof
InactiveCN101564395AAntibacterial agentsOrganic active ingredientsInfective rhinitisInfectious Rhinitis
The invention provides an ophthalmic or otic and nasal pharmaceutical composition, which comprises lavo-ofloxacin and salts thereof and difluprednate, wherein the weight ratio of the difluprednate to the lavo-ofloxacin is 1:1-1:10. The ophthalmic or otic and nasal medicament composition is applied to treating conjunctivitis, keratitis, eyelid inflammation, dacryocystitis, hordeolum, corneal ulcer and eye infections with inflammations of eyes or surrounding tissues, or preventing increased risk of bacterial infections and tissue inflammations of infected parts after ophthalmic surgeries or eye injuries, or treating or relieving the bacterial infections combined with tissue inflammations of the infected parts after the ophthalmic surgeries, or treating otitis media, otitis externa and infectious rhinitis.
Owner:SHANDONG INST OF PHARMA IND
Method for preparing cornea lamina material
ActiveCN102552979AStrong ability to pass onReduce the risk of contaminationProsthesisBiocompatibility TestingStem cell culture
The invention discloses a method for preparing a cornea lamina material. The method comprises the following steps of: carrying out accellular processing on a cornea-sclera complex of a mammal eyeball, culturing an amnion epithelial stem cell, inducing and secreting TSP (Thrombospondin)-1 and co-culturing accellular cornea stroma and the amnion epithelial stem cell, sizing, packaging and sterilizing, and the like. According to an obtained cornea lamina transplanting material, the promotion and the balance among the biocompatibility, the stability, the transparency, the mechanical strength and the bioactivity are realized; the integrity of cornea collagen molecules and a lamina structure can be kept to the largest extent, and compared with that prepared with the traditional method, the accellular cornea stroma prepared according to the method disclosed by the invention is larger in tensile strength and has the moisture content and the transparency close to those of normal cornea; and various cell growth factors and the TSP-1 are gathered, and the cornea lamina material has special effects of inflammatory resistance and vascularization resistance, has the capability of greatly enhancing the bioactivity of the cornea lamina material and can be widely used for repairing complicated ocular surface coloboma such as inflammation and corneal ulcer grown in a vascularization way.
Owner:SHAANXI RUISHENG BIOTECH
Preventive or therapeutic agent for keratoconjunctival disorder
InactiveUS20090105313A1Improve the improvement effectGood prevention effectBiocideSenses disorderConjunctivaDisease
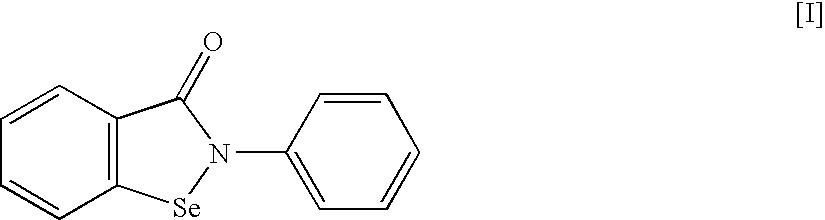
An object of the present invention is to provide a new medicinal use of 2-phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof. 2-Phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof exhibits an excellent prevention and improvement effect in corneal disorder models, and is therefore useful as a preventive or therapeutic agent for a keratoconjunctival disorder such as dry eye, superficial punctate keratopathy, corneal epithelial defects, corneal erosion, corneal ulcer, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, keratitis or conjunctivitis.
Owner:SANTEN PHARMA CO LTD
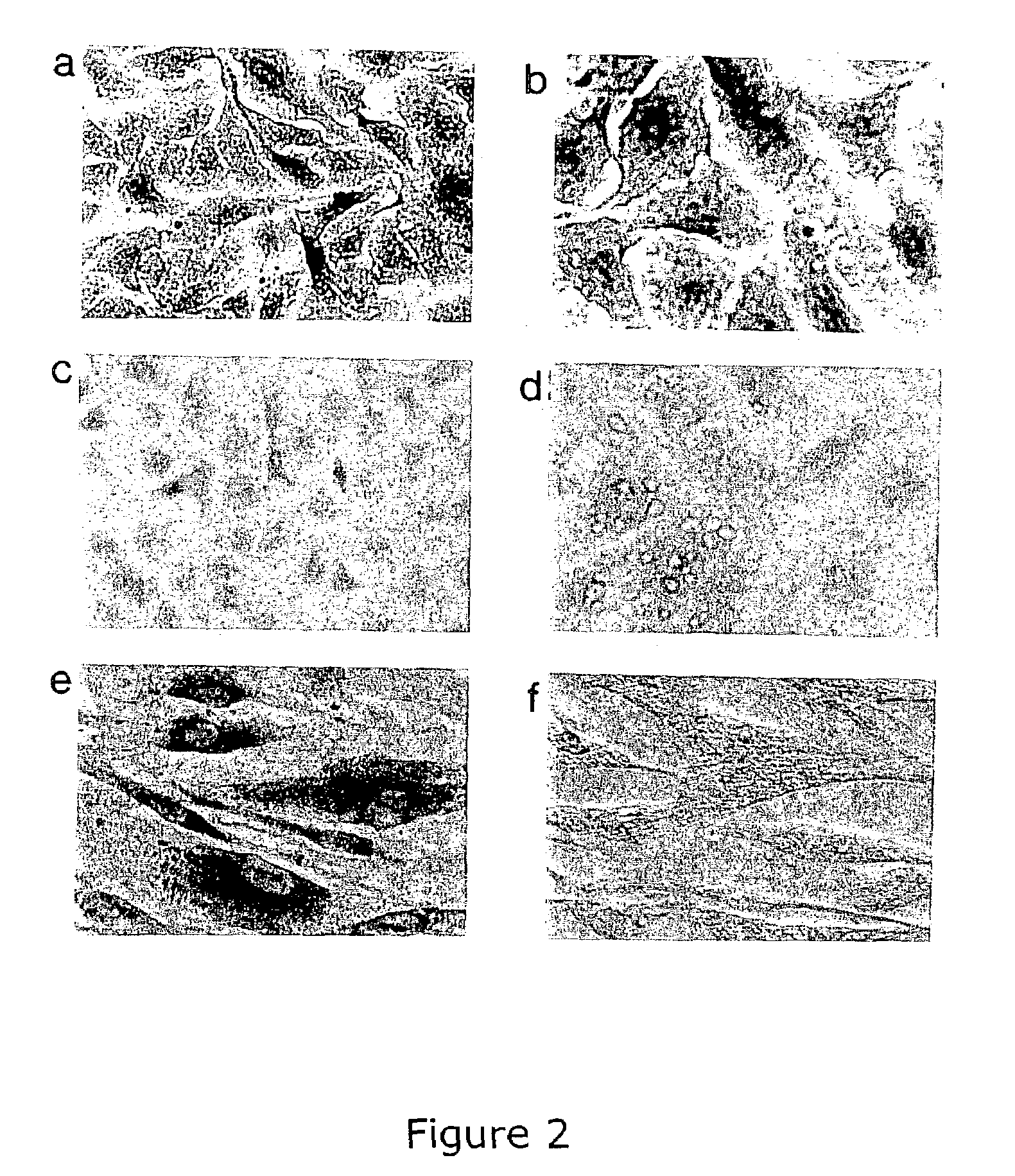
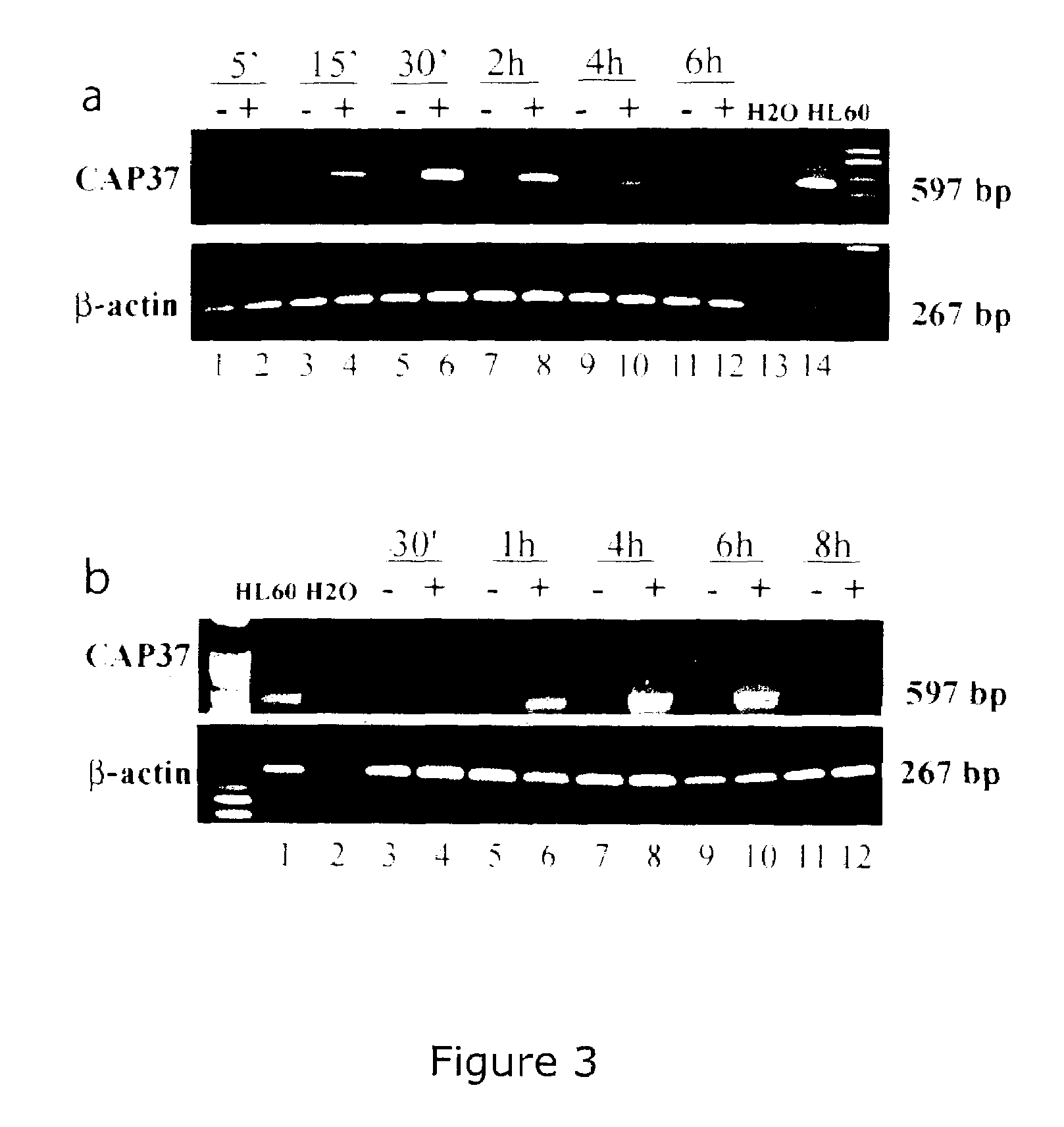
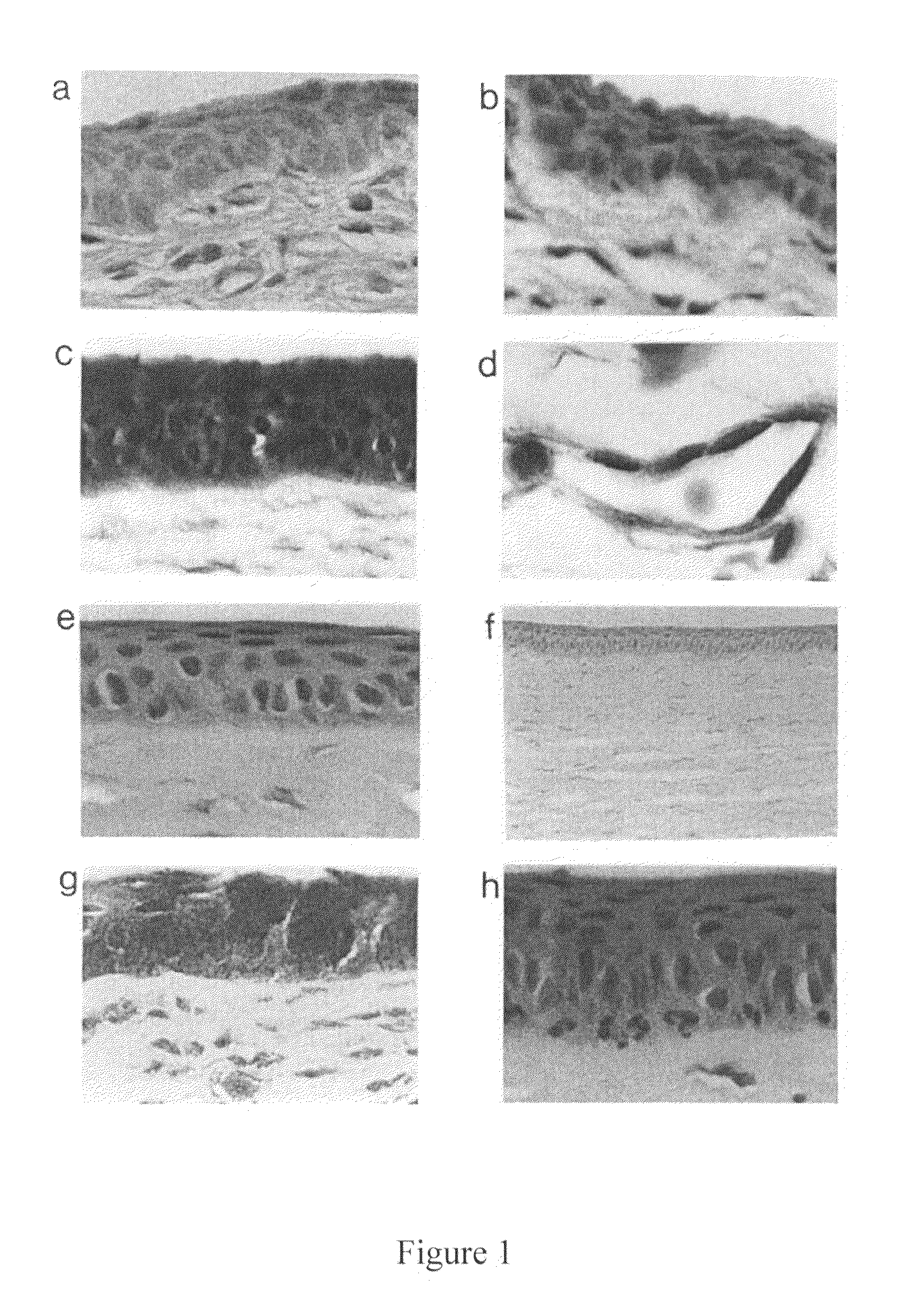
Treatment and inhibition of ocular infections and wounds by CAP37 and CAP37 peptides
ActiveUS7354900B2Promote healingAvoid infectionAntibacterial agentsBiocideBacterial ConjunctivitisMammal
A method for treating ocular conditions such as bacterial keratitis, bacterial conjunctivitis, corneal ulcers and wounds, endophthalmitis, and blebitis in mammals, by using a native, synthetic, or recombinant CAP37, or effective peptide portions thereof including CAP37 peptides 20-44, 23-42, 102-122, and 120-146 and monocysteine derivatives of peptides 20-44 and 23-42. The CAP37, peptides, and peptide derivatives can also be used to store, clean, sterilize, or coat contact lenses, and may be used in media for storing mammalian corneal transplants.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Therapeutic agent for keratoconjunctival disorder
Object of the present invention is to search a novel pharmaceutical use of 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy) benzyl] thiazolidine-2, 4-dione being a condensed heterocyclic compound, or a salt thereof. 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy) benzyl] thiazolidine-2, 4-dione or a salt thereof can exert an excellent effect to promote healing in a dry eye model, and is useful as a therapeutic agent for keratoconjunctival disorders such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
Treatment of ocular wounds and ulcers
InactiveUS20090233867A1Promote healingAvoid infectionAntibacterial agentsBiocideDiseaseBacterial Conjunctivitis
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com