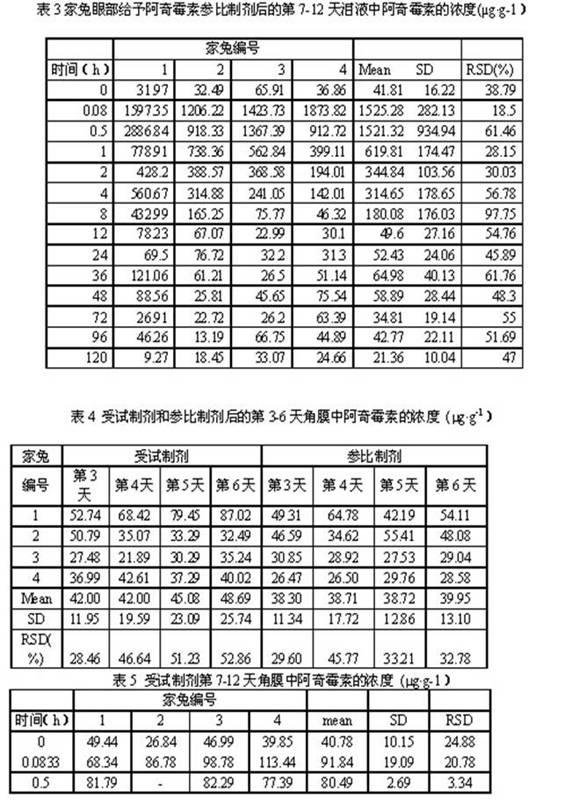
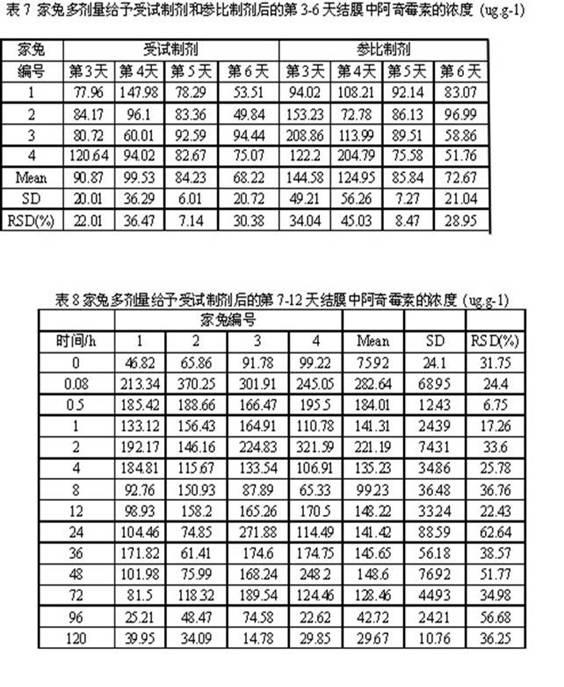
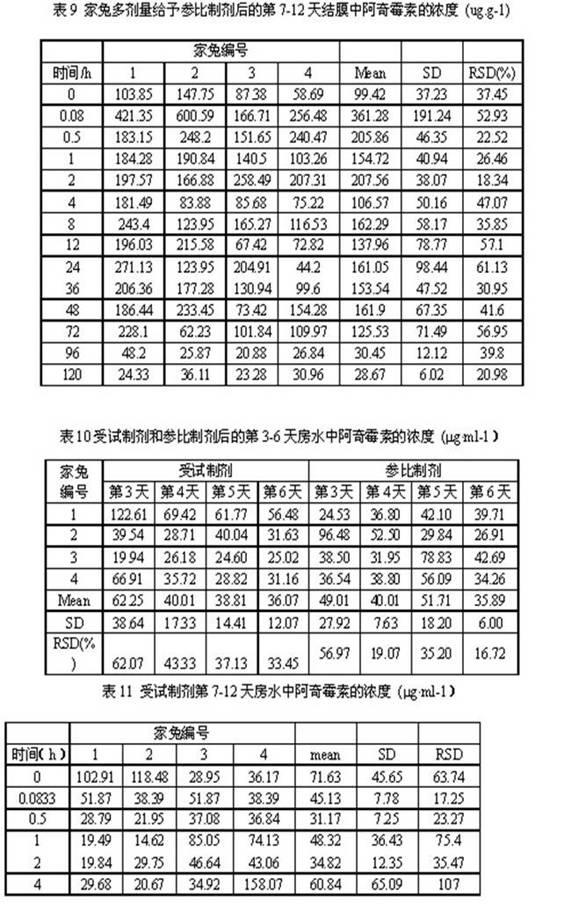
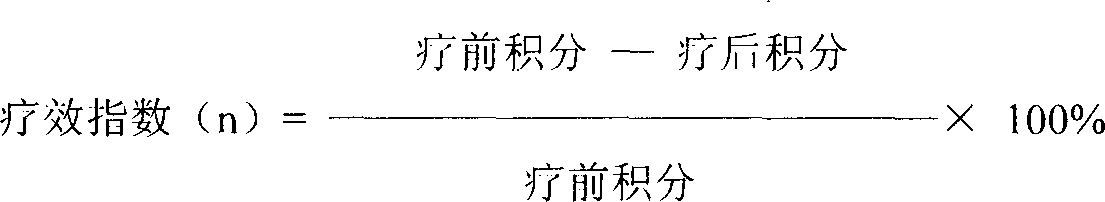
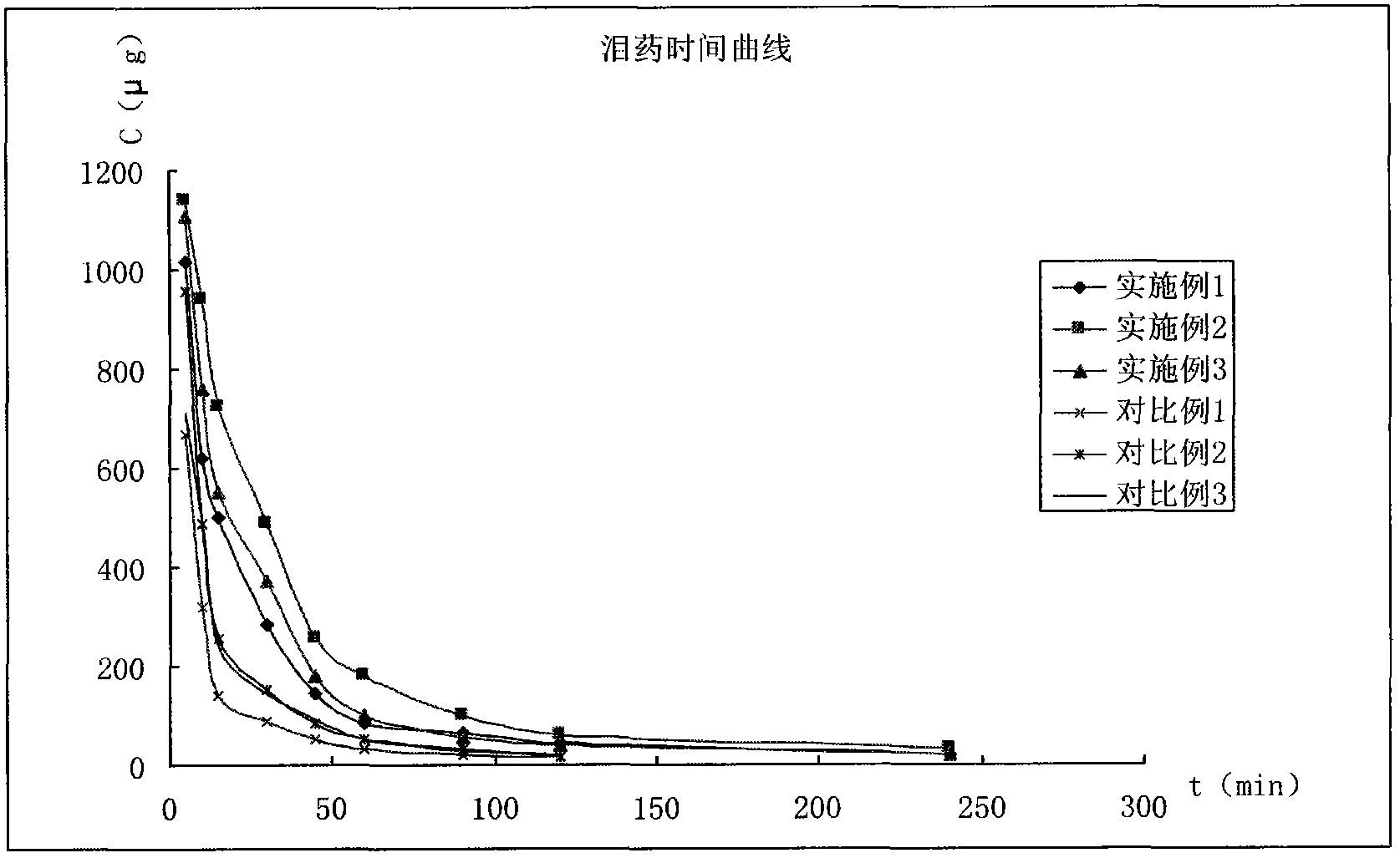
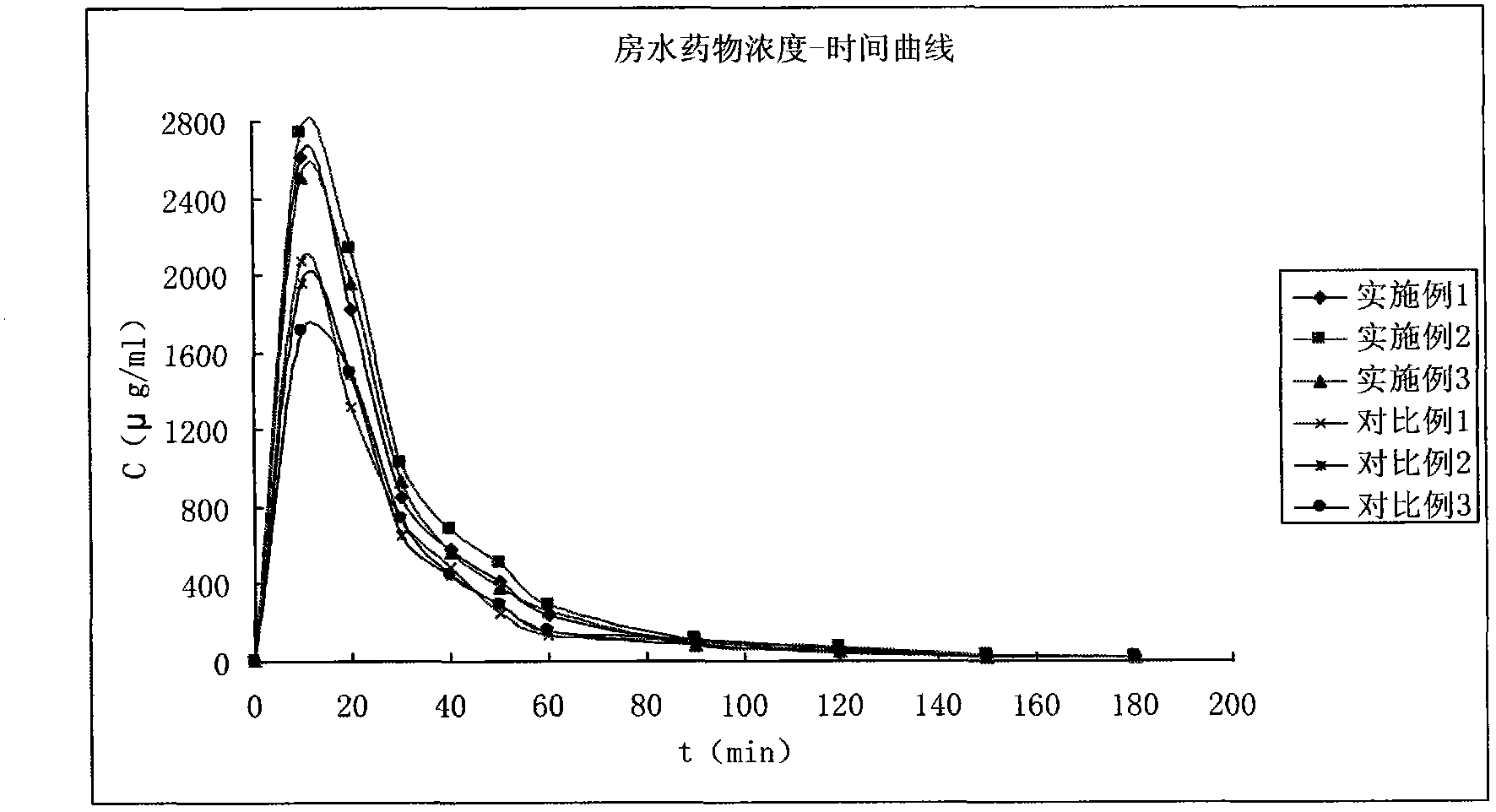
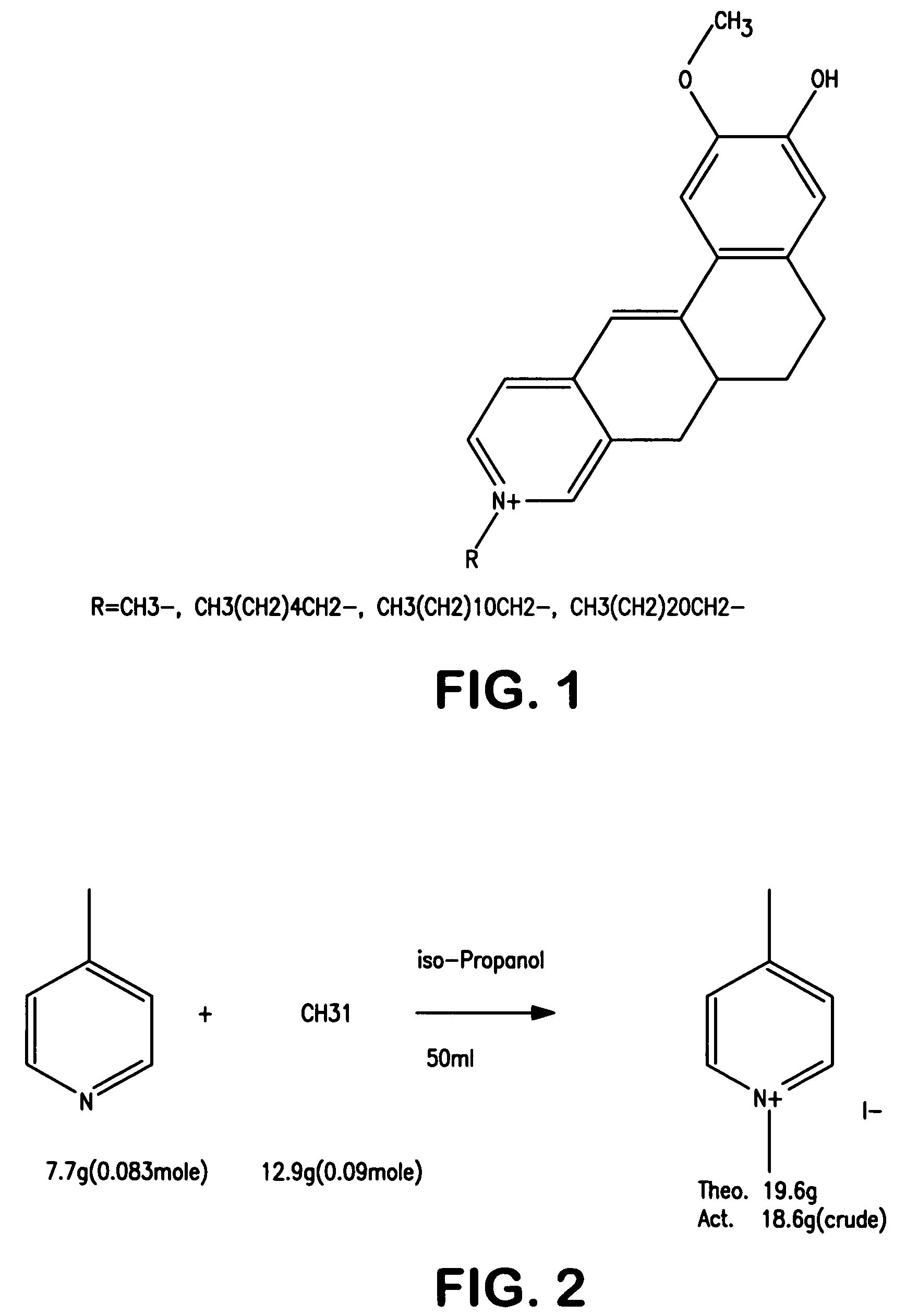
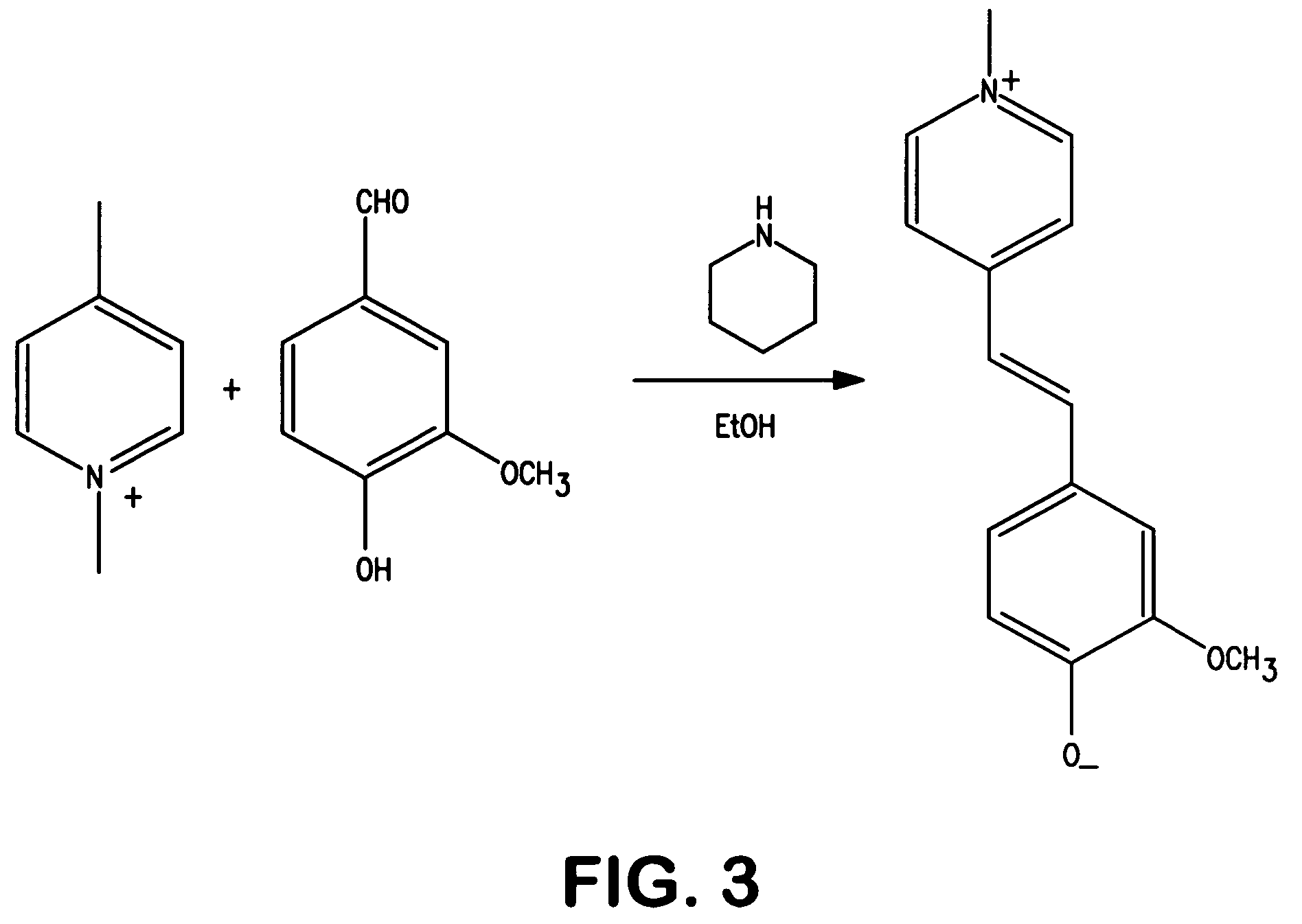
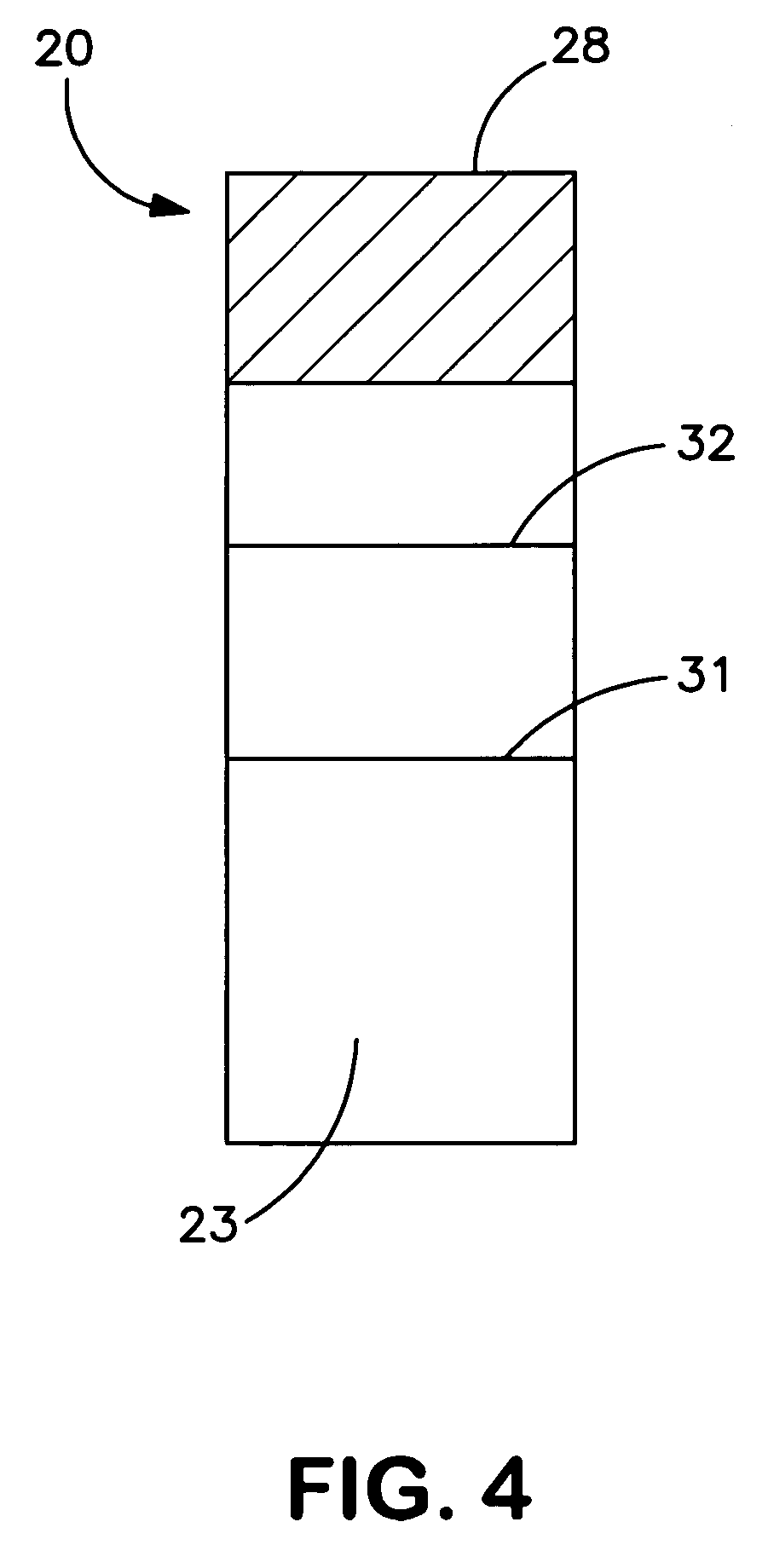
Patents
Literature
42 results about "Bacterial Conjunctivitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inflammation of the conjunctiva caused by a variety of bacterial agents.
A kind of azithromycin gel eye drop and its preparation process
ActiveCN102283799AStrong practical significanceStrong application valueAntibacterial agentsOrganic active ingredientsBacterial ConjunctivitisAntioxidant
The invention discloses azithromycin gel eye drops and a preparation process thereof. The eye drops are prepared from a main medicine, namely azithromycin and excipients such as an adhesive, a gel matrix, an isotonic regulator, a preservative, an antioxidant, a buffering agent and the like. The adhesive, namely polycarbophil in the eye drops can increase the biological adhesion of the eye drops, so that the detention time of medicines in eyes is further prolonged. The invention provides a practical, convenient and reliable ophthalmic preparation for treating bacterial conjunctivitis, and solves the problems that the detention time of medicines in an eye drop formulation in the eyes is short, the medicines are not easy to absorb, the bioavailability is low and the like.
Owner:北京乐维生物技术有限公司
In-vivo gel preparatino able to be dropped in eyes and its preparing process
InactiveCN1397272ALow toxicityLess irritatingSenses disorderPharmaceutical delivery mechanismTreatment effectIrritation
An eyedrops able to become gel after it is dropped in eye for treating bacterial conjuctivities, keratitis, keratohelcosis, dacryocystitis, etc is prepared from antibacterial infilammatino-relieving medicine, thickening agent, antiseptic, isotonic regulator, pH regulator and water. Its advantages are long stay time in eye, high curative effect, and low poison and irritation.
Owner:SHANGHAI XINGKANG PHARMA RES & DEV
Bear bile powder eye drop and its prepn
The eye drop is prepared with bear bile powder as medicine material and phosphate solution as buffering agent. It has the functions of bacteriostasis, resisting virus, strengthening eyesight, clearing away heat, etc. It is used in treating acute bacterial conjunctivitis, epidemic viral keratitis and has high curative effect and short treating period.
Owner:王春红
Gatifloxacin external and ophthalmic gel preparation
InactiveCN1448137ADegradableGood film formingOrganic active ingredientsSenses disorderOphthalmic Gel Dosage FormDisease
The Gatifloxacin gel preparation for external use and eye use has Gatifloxacin as main component and its supplementary material includes chitosan as gel substrate, iso-osmotic regulator, pH regulator, preservative, injection water, etc. The preparation has Gatifloxacin content of 0.1-3 wt% and chitosan content of 0.3-3 wt%. It has obvious anti-infection function and functions of speeding heal of wound, promoting epidermal growth, inhibiting formation of scar tissue, maintaining local medicine density for long term, etc. It is used in treating burns, scalds, skin infection, folliculitis, bacterial conjunctivitis, keratitis, etc.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Chinese medicinal composition for treating conjunctivitis and preparation method and application thereof
ActiveCN102836228ARemissionEasy to makeSenses disorderPlant ingredientsViral ConjunctivitisBacterial Conjunctivitis
The invention discloses a Chinese medicinal composition for treating conjunctivitis, belonging to the technical field of Chinese medicines. The Chinese medicinal composition is prepared from 15 parts of baikal skullcap root, 6 parts of honeysuckle flower, 10 parts of divaricate saposhnikovia root, 10 parts of virgate wormwood herb, 10 parts of szechuan lovage rhizome, 20 parts of dandelion, 20 parts of cicada shell, 10 parts of gentian, 5 parts of white paeony root, 5 parts of mint and 10 parts of liquoric root. The Chinese medicinal composition has good treatment effects on the aspect of treatment of inflammatory conjunctivitis, bacterial conjunctivitis, viral conjunctivitis and allergic conjunctivitis, and has a remarkable clinical popularization value.
Owner:NANTONG HONGCI PHARMA
Chinese-medicinal preparation and making method for treating acute corneitis and releasing visual fatigue
A Chinese medicine for treating acute conjunctivitis and keratitis and relaxing visual fatigue is prepared from bear gall and borneol. Its preparing process is also disclosed.
Owner:CHANGCHUN PUHUA PHARMA
Lomefloxacin hydrochloride eye drops and preparation method and application thereof
ActiveCN102670493AIncrease viscosityExtended stayAntibacterial agentsOrganic active ingredientsBacterial ConjunctivitisTherapeutic effect
The invention discloses lomefloxacin hydrochloride eye drops and a preparation method and application thereof. The lomefloxacin accounts for 0.3% of the active components. The preparation also comprises a tackifier, a buffer salt system, a wetting agent, an isoosmotic adjusting agent, a pH adjusting agent and a bacteriostatic agent, and is suitable for treating the external eye infections such asacute and chronic bacterial conjunctivitis, blepharitis, hordeolum, meibomiantis, dacryocystitis, keratitis, keratohelcosis and the like caused by sensitive pathogenic bacteria. The product is characterized in that: by adding the sodium hyaluronate with a tackifying effect and a moisturizing effect, the viscosity of the lomefloxacin hydrochloride eye drops is improved, the fluidics property thereof is changed, the residence time of the eye drops in the eyes is prolonged, the sufficient absorption of the medicine is ensured, the bioavailability is increased, the administration times are reduced, the compliance of a patient is improved, and an effective treatment effect is realized.
Owner:KANGYA OF NINGXIA PHARMA
Method for screening for bacterial conjunctivitis
InactiveUS20070140971A1Ultrasonic/sonic/infrasonic diagnosticsMicrobiological testing/measurementMicroorganismInfective conjunctivitis
A method for rapidly detecting infectious conjunctivitis in a host is provided. The method includes contacting an ocular test sample with a chromogen (e.g., Reichardt's dye) that exhibits a color change in the presence of a microbe. The present inventors have discovered that the extent of the color change may vary depending on whether the microbe is a bacteria or virus. Without intending to be limited by theory, the present inventors believe that the chromogen interacts with the peptidoglycan-based cell wall structure of bacteria to induce a color change that is even more apparent at infectious levels. It is believed that this interaction occurs to a much greater extent in bacteria than in viruses. Accordingly, although the chromogen may still undergo a color change in the presence of the viruses, it is typically to a much lesser extent. In this manner, the degree of color change of the chromogen may be used in the present invention as a mechanism for differentiating between viral and bacterial conjunctivitis.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Azithromycin-containing slow-released eye drops and preparation method thereof
InactiveCN103989660ASolve the problems of low water solubility and instabilityImprove stabilityAntibacterial agentsOrganic active ingredientsSolubilityEye/ear drops
The invention relates to slow-released eye drops with azithromycin as an effective ingredient and a preparation method thereof, and aims at solving the problems of poor water solubility of azithromycin and unstable physicochemical property of lipidosome. According to the preparation method, the emulsification evaporation-low-temperature curing method is used to prepare chitosan modified azithromycin solid lipid nanoparticles, so that the stability of lipid nanoparticles in storage is improved, slow release of azithromycin in eyes is also realized, and thus the medicine can fully act to realize the optimal treatment effect. The eye drops are mainly used for treating bacterial conjunctivitis.
Owner:WUHAN WUYAO SCI & TECH
Treatment of ocular inflammatory diseases using laquinimod
InactiveUS20130324574A1Improve oral bioavailabilityBiocideSenses disorderDiseaseViral Conjunctivitis
Disclosed is a method for treating an ocular inflammatory disease (OID), e.g., uveitis or conjunctivitis, comprising periodic administration of a therapeutically effective amount of laquinimod or a pharmaceutically acceptable salt thereof. Also provided is a pharmaceutical composition comprising laquinimod or a pharmaceutically acceptable salt thereof for use in treating a subject suffering from an OID, uveitis, bacterial conjunctivitis, viral conjunctivitis, an inflammation of the orbital tissue, the lacrimal apparatus, the eyelid, the cornea, the retina or the optic pathway. This application also provides a method for treating a subject suffering from an autoimmune disease-associated ocular inflammation comprising periodic ocular administration to the subject a therapeutically effective amount of laquinimod or a pharmaceutically acceptable salt, and an ocular pharmaceutical composition comprising laquinimod or a pharmaceutically acceptable salt thereof for use in treating an autoimmune disease-associated ocular inflammation.
Owner:TEVA PHARMA IND LTD
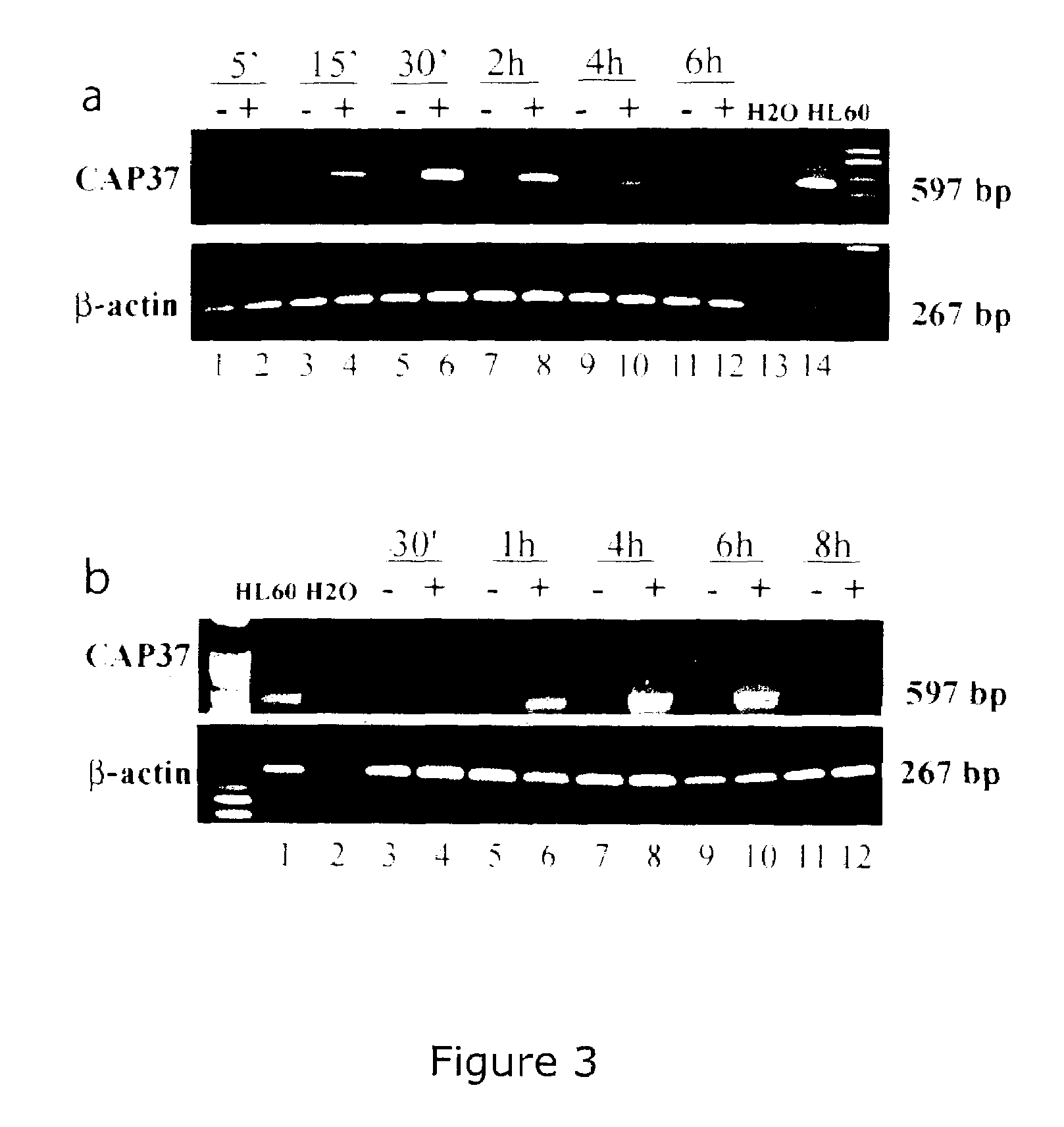
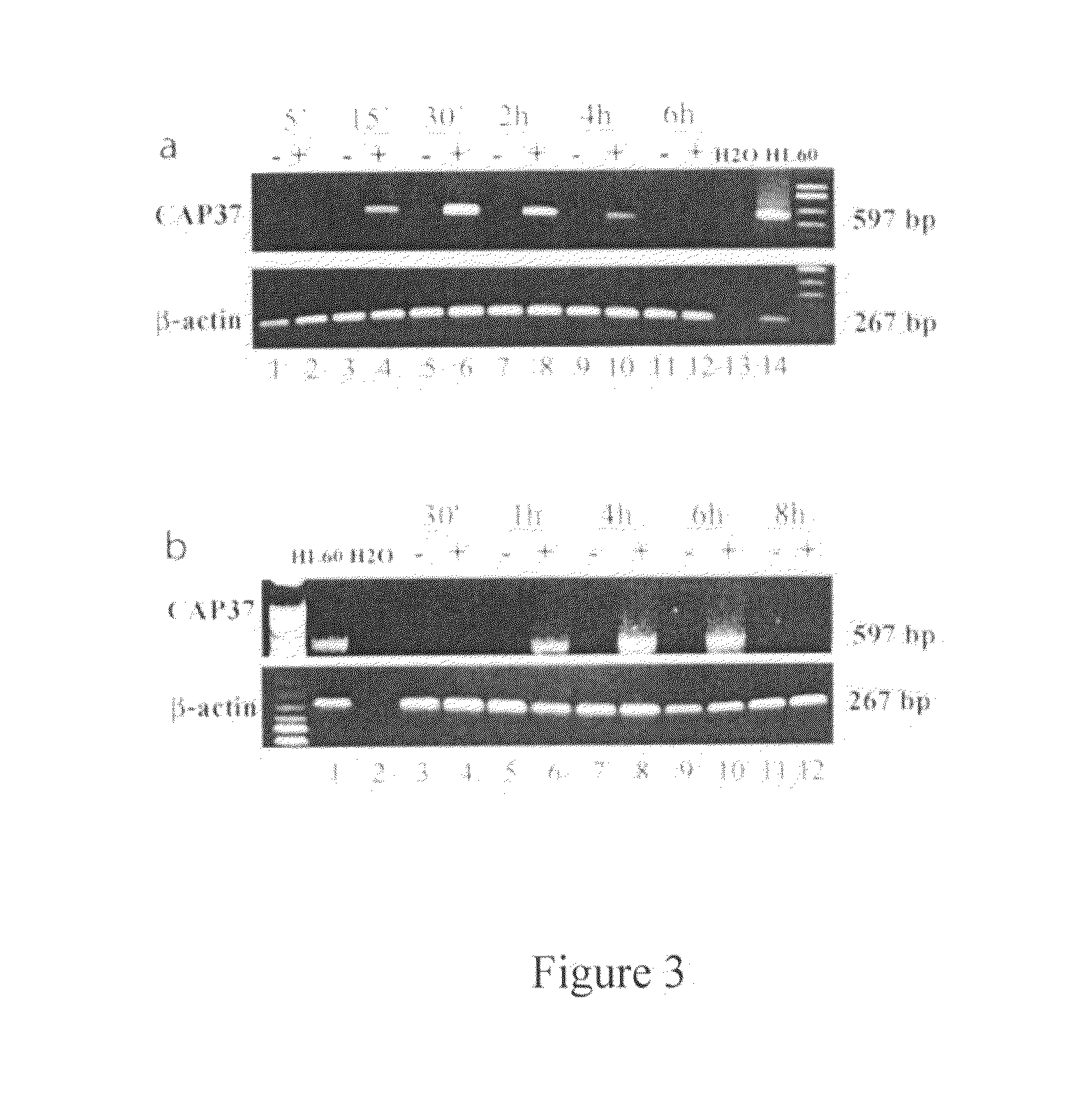
Treatment and inhibition of ocular infections and wounds by CAP37 and CAP37 peptides
ActiveUS7354900B2Promote healingAvoid infectionAntibacterial agentsBiocideBacterial ConjunctivitisMammal
A method for treating ocular conditions such as bacterial keratitis, bacterial conjunctivitis, corneal ulcers and wounds, endophthalmitis, and blebitis in mammals, by using a native, synthetic, or recombinant CAP37, or effective peptide portions thereof including CAP37 peptides 20-44, 23-42, 102-122, and 120-146 and monocysteine derivatives of peptides 20-44 and 23-42. The CAP37, peptides, and peptide derivatives can also be used to store, clean, sterilize, or coat contact lenses, and may be used in media for storing mammalian corneal transplants.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Treatment of ocular wounds and ulcers
InactiveUS20090233867A1Promote healingAvoid infectionAntibacterial agentsBiocideDiseaseBacterial Conjunctivitis
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Method for screening for bacterial conjunctivitis
InactiveUS7727513B2Ultrasonic/sonic/infrasonic diagnosticsMicrobiological testing/measurementBacteroidesMicroorganism
A method for rapidly detecting infectious conjunctivitis in a host is provided. The method includes contacting an ocular test sample with a chromogen (e.g., Reichardt's dye) that exhibits a color change in the presence of a microbe. The present inventors have discovered that the extent of the color change may vary depending on whether the microbe is a bacteria or virus. Without intending to be limited by theory, the present inventors believe that the chromogen interacts with the peptidoglycan-based cell wall structure of bacteria to induce a color change that is even more apparent at infectious levels. It is believed that this interaction occurs to a much greater extent in bacteria than in viruses. Accordingly, although the chromogen may still undergo a color change in the presence of the viruses, it is typically to a much lesser extent. In this manner, the degree of color change of the chromogen may be used in the present invention as a mechanism for differentiating between viral and bacterial conjunctivitis.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Azithromycin gel eye drops and preparation process thereof
ActiveCN102283799BStrong application valueAntibacterial agentsOrganic active ingredientsBacterial ConjunctivitisAdhesive
The invention discloses azithromycin gel eye drops and a preparation process thereof. The eye drops are prepared from a main medicine, namely azithromycin and excipients such as an adhesive, a gel matrix, an isotonic regulator, a preservative, an antioxidant, a buffering agent and the like. The adhesive, namely polycarbophil in the eye drops can increase the biological adhesion of the eye drops, so that the detention time of medicines in eyes is further prolonged. The invention provides a practical, convenient and reliable ophthalmic preparation for treating bacterial conjunctivitis, and solves the problems that the detention time of medicines in an eye drop formulation in the eyes is short, the medicines are not easy to absorb, the bioavailability is low and the like.
Owner:北京乐维生物技术有限公司
Polyhexamethylene guanidine eye drops
InactiveCN104398534AInhibition of mitotic functionIncapacity to reproduceAntibacterial agentsOrganic active ingredientsBacterial ConjunctivitisMethyl cellulose
Polyhexamethylene guanidine eye drops relate to the technical field of eye drops, and comprise the following components by mass: 0.01%-0.1% of polyhexamethylene guanidine hydrochloride, 0.01% of a synergist, 0.1% of pharmaceutical grade hydroxy propyl methyl cellulose, 0.05% of PEG200 (polyethylene glycol), 0.05% of PEG6000, 0.05% of water soluble vitamin E, 0.01% of water-soluble mint, 0.1% of pure sodium chloride, and 99.53%-99.62% of pure water. Polyhexamethylene guanidine is used in the eye drops, and the polyhexamethylene guanidine eye drops are suitable for bacterial conjunctivitis, keratitis, keratohelcosis, dacryocystitis, postoperative infection and other external ocular infections, and is good in sterilization effect, mild in nature, and non-irritating.
Owner:卫国刚
Eye drops for treating conjunctivitis
InactiveCN103191215AGood treatment effectHas anti-inflammatory and eyesight-enhancing effectsSenses disorderPlant ingredientsEye/ear dropsBacterial Conjunctivitis
The invention relates to eye drops for treating conjunctivitis, which have the effect of treating the conjunctivitis by washing eyes to achieve anti-inflammatory treatment. The eye drops for treating the conjunctivitis are the externally applied eye drops which are prepared from the following traditional Chinese medicine raw materials: 13-17 parts of common jujube bark, 13-17 parts of mulberry bark, 8-12 parts of herb of ramose scouring rush, 8-12 parts of swordlike atractylodes rhizome, 10-14 parts of chrysanthemum and 8-12 parts of feather cockscomb seed. According to the invention, 319 patients (408 eyes) with the acute conjunctivitis in a group are observed after being subjected to conjunctival sac washing treatment with the eye drops disclosed by the invention so that the eye drops are proved to have significant curative effects against acute bacterial conjunctivitis.
Owner:高蓉
Eye drops with capillary artemisia, cape jasmine and baikal skullcap root, and its preparing method
InactiveCN1850191AHigh purityHigh claritySenses disorderPharmaceutical delivery mechanismDiseaseViral Conjunctivitis
The present invention relates to a kind of Yizhihuang eye drops and its preparation method. It can be used for curing the diseases of conjunctivitis, bacterial conjunctivitis, viral conjunctivitis, acute hemorrhagic conjunctivitis, neonatal bacterial conjunctivitis and chronic conjunctivitis and keratitis, etc. It is made up by using four Chinese medicinal materials of lonicera flower, scutellaria root, capillaries and gardenia fruit through a certain preparation process.
Owner:SINOPHARM A THINK PHARMA
Dendrobe eye drops and preparation method thereof
ActiveCN107308342ARaw materials are easy to getLow priceAntibacterial agentsSenses disorderDiseaseSide effect
The invention belongs to the technical field of medicine product processing, and in particular relates to dendrobe eye drops and a preparation method thereof. The dendrobe eye drops consist of the following components in percentage by mass: 40 to 65 percent of dendrobe, 10 to 23 percent of the root bark of the peony tree, 5 to 8 percent of rhizoma acori graminei, 2 to 10 percent of bighead atractylodes rhizome, 6 to 10 percent of fructus cnidii, 3 to 7 percent of biond magnolia flower, 2 to 5 percent of chitosan, 1 to 3 percent of vitamin B12 powder, 1 to 3 percent of vitamin B1 and 3 to 8 percent of normal saline. The dendrobe eye drops provided by the invention adopts the common traditional Chinese medicine components, is reasonable in compatibility and safe and reliable in medicine, has antibacterial and anti-inflammation effects, has remarkable treatment effect on eye diseases such as bacterial conjunctivitis and keratitis, and has the characteristics of exact curative effect, quick response, high curative ratio, no side effect and the like; and the preparation of the dendrobe eye drops is capable of storing effective components in the raw materials, reasonable in production process and suitable for industrialized production.
Owner:海南诗博丽生物科技有限公司 +1
Bear bile powder eye drop and its prepn
The eye drop is prepared with bear bile powder as medicine material and phosphate solution as buffering agent. It has the functions of bacteriostasis, resisting virus, strengthening eyesight, clearing away heat, etc. It is used in treating acute bacterial conjunctivitis, epidemic viral keratitis and has high curative effect and short treating period.
Owner:王春红
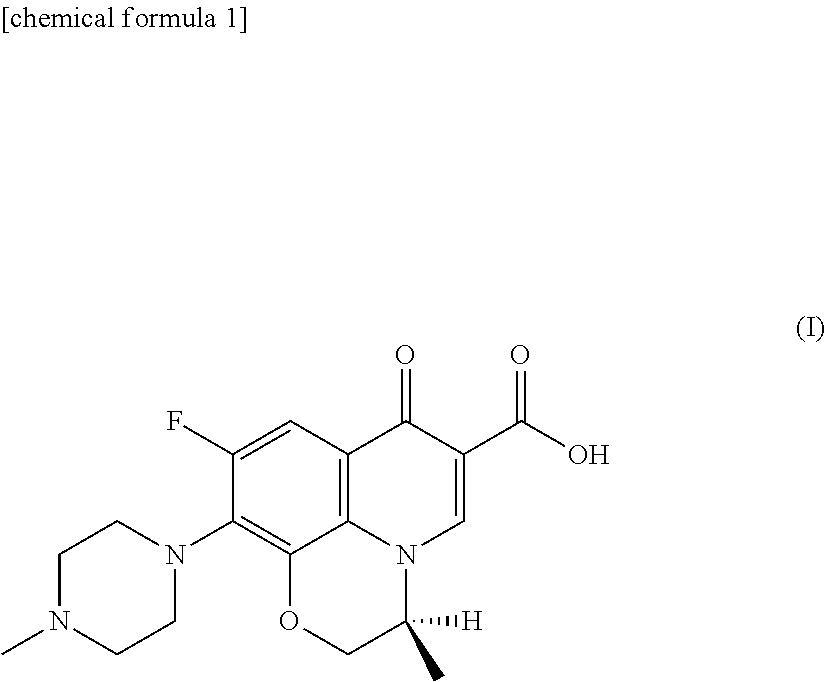
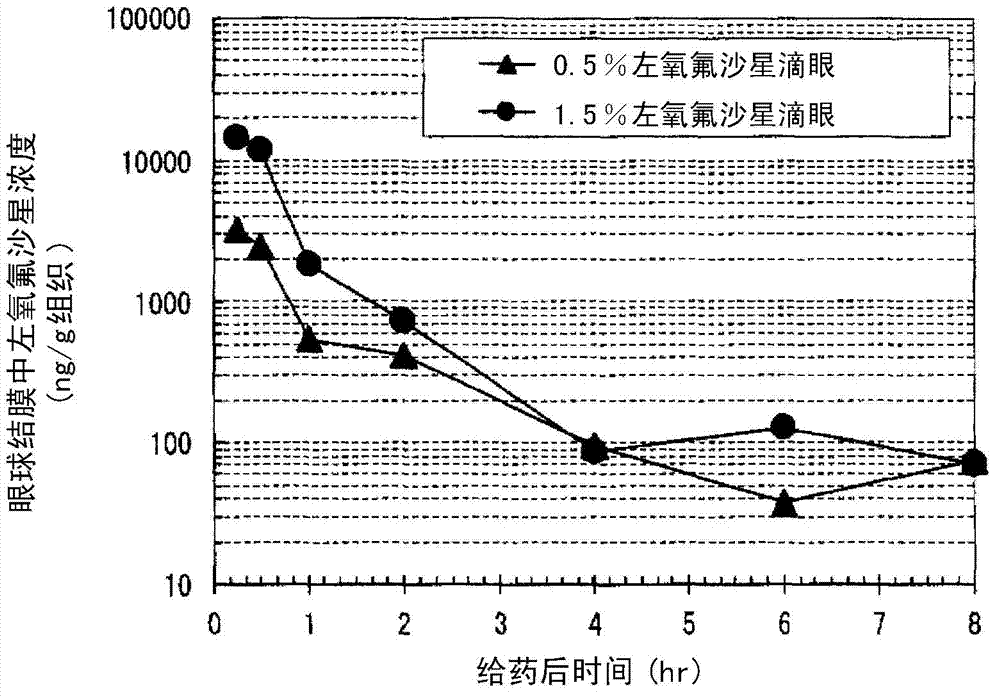
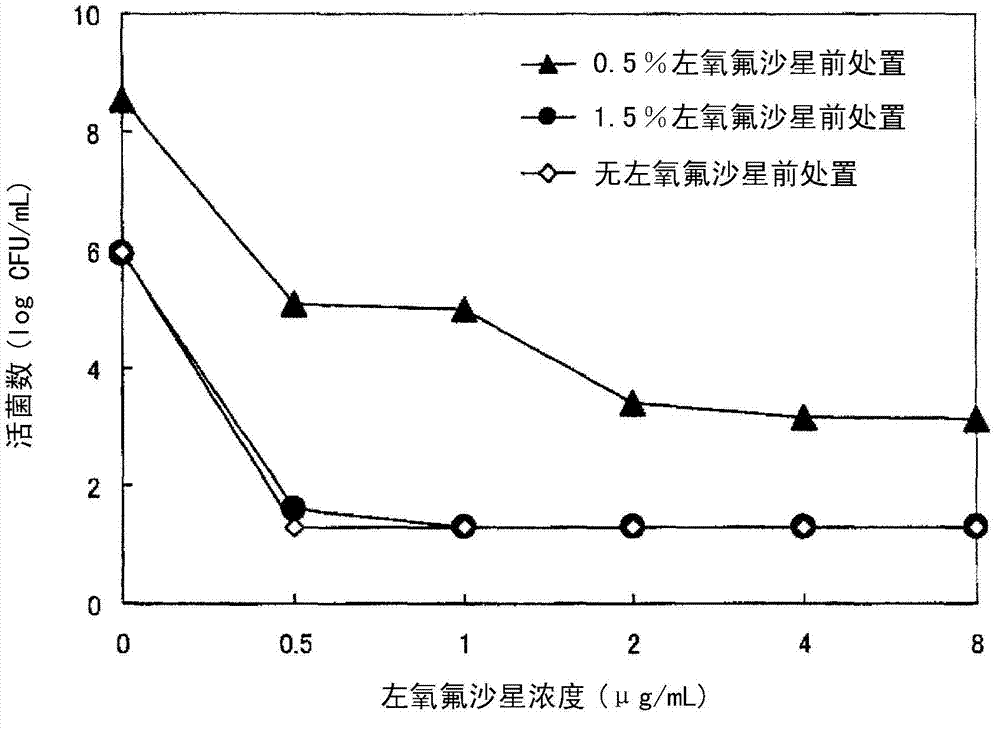
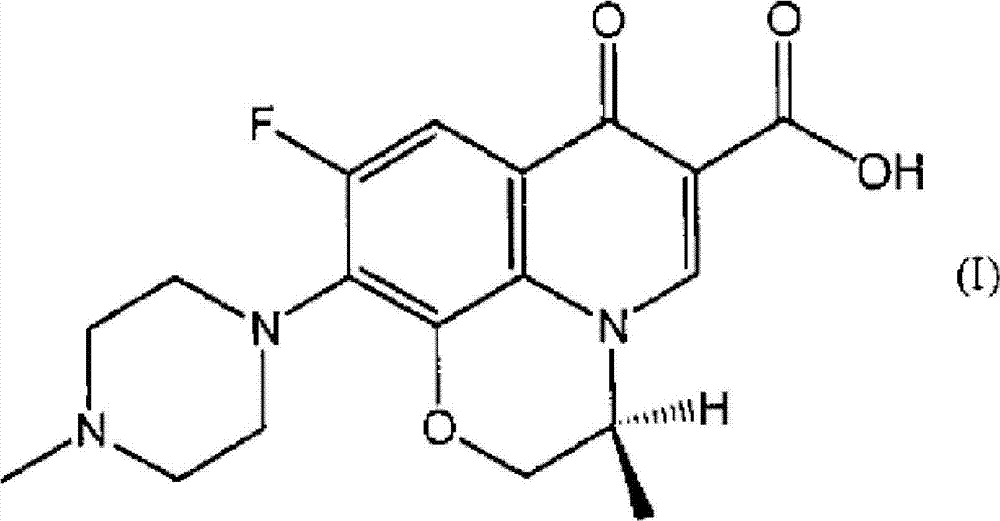
Ophthalmic solution for treating ocular infection comprising levofloxacin or salt thereof or solvate of the same, method for treating ocular infection, levofloxacin or salt thereof or solvate of the same, and use thereof
InactiveUS20120316158A1Reduce contact timeHigh frequencyAntibacterial agentsOrganic active ingredientsDosing regimenRegimen
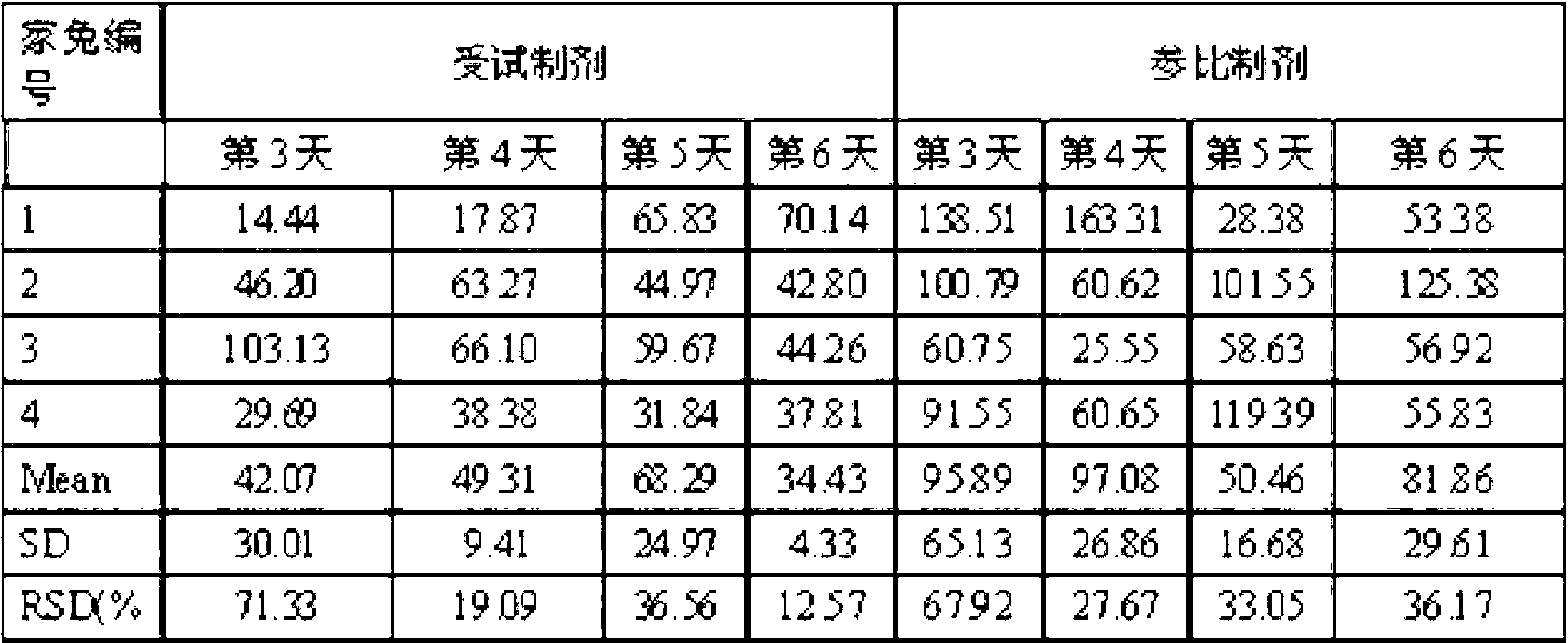
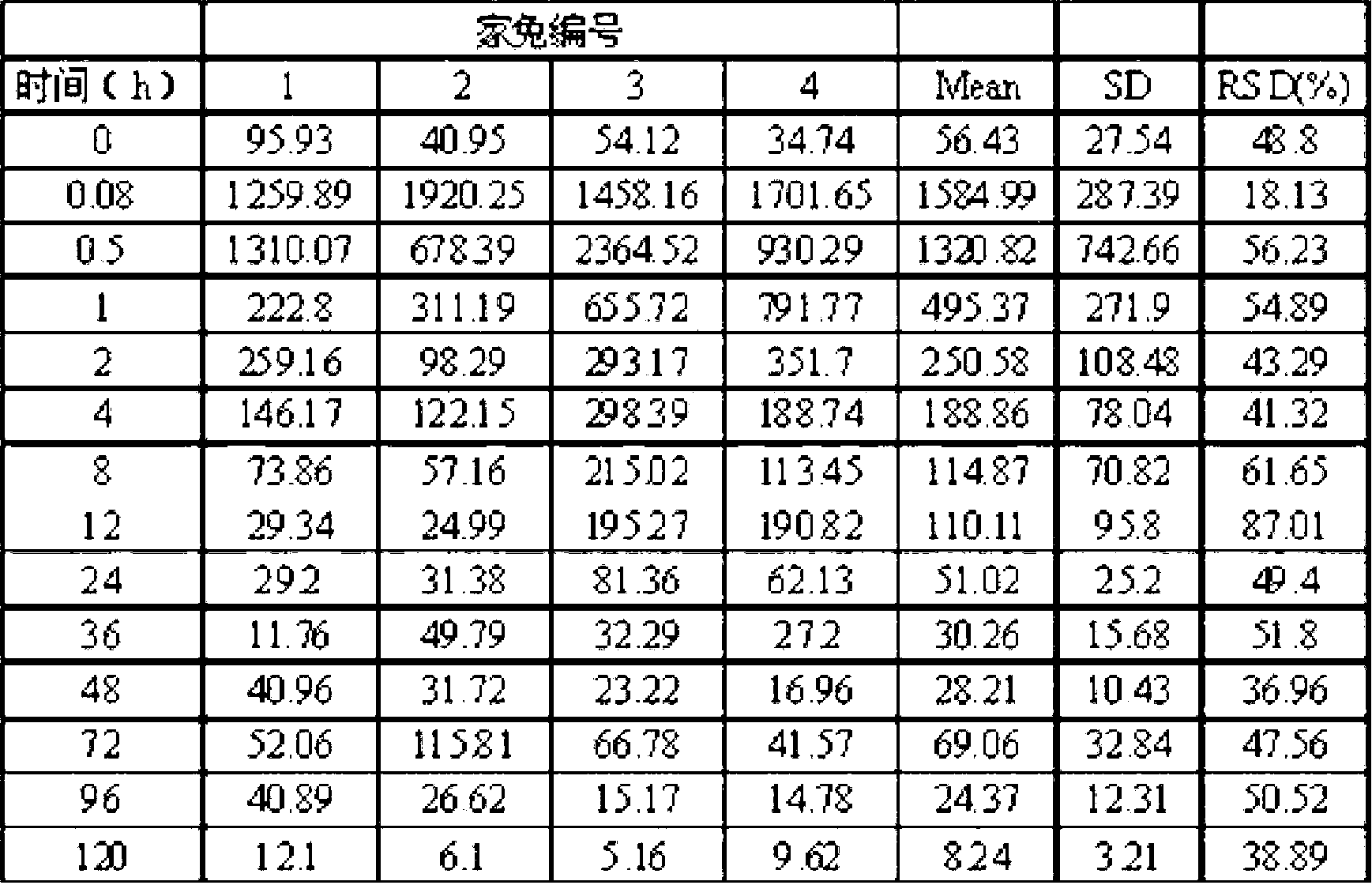
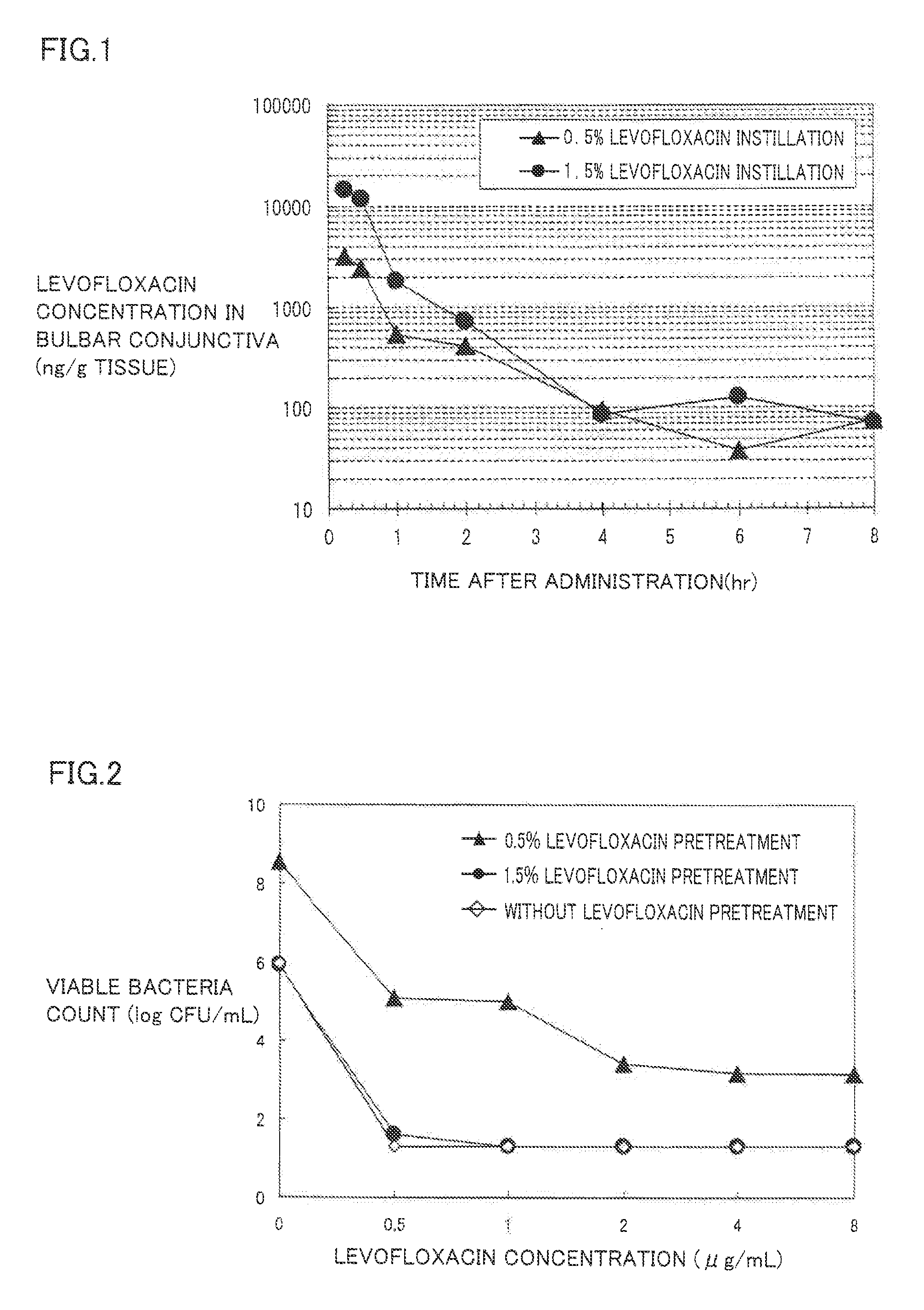
Instillation of a 1.5% (w / v) levofloxacin ophthalmic solution three times a day, which is the dosage or dose regimen of the present invention, has features to cure bacterial conjunctivitis in a shorter time than instillation of a 0.5% (w / v) ophthalmic solution three times a day, which is the conventional dosage or dose regimen, and not to increase the rate of occurrence of side effects. Curing the ocular infection in a short time leads to shortening of the duration of exposure of the ocular-infection-causing bacterium to levofloxacin. Therefore, the levofloxacin ophthalmic solution in the dosage or dose regimen of the present invention is eventually expected to suppress emergence of the resistant bacterium resulting from the long-term use of the levofloxacin ophthalmic solution in the conventional dosage or dose regimen. In addition, it is confirmed that the levofloxacin ophthalmic solution in the dosage or dose regimen of the present invention directly inhibits the ocular-infection-causing bacterium such as Staphylococcus aureus from becoming resistant to levofloxacin, which results from the short-term use of the levofloxacin ophthalmic solution in the conventional dosage or dose regimen.
Owner:SANTEN PHARMA CO LTD +1
Eye drops for treating eye infection containing levofloxacin, salt thereof or solvate of same, method for treating eye infection, levofloxacin, salt thereof or solvate of same, and utilization thereof
InactiveCN102770141AImprove short-term cure rateInhibition of resistant bacteriaAntibacterial agentsOrganic active ingredientsSide effectBacterial Conjunctivitis
Disclosed are a method and dosage of eye drops containing 1.5% (w / v) of levofloxacin, characterized in that, when the eye drops are dropped into the eyes thrice a day, bacterial conjunctivitis can be healed within a short period of time, compared with the conventional case where 0.5% (w / v) eye drops are used thrice a day, without increasing the incidence of side effects. Healing of an eye infection within a short period of time contributes to shortening of the exposure time of a pathogenic bacterium causing the eye infection to levofloxacin. As a result, it is expected that, when used in the aforesaid method and dosage, the levofloxacin-containing eye drops inhibit the appearance of a resistant strain which is caused by the prolonged usage of levofloxacin-containing eye drops in the conventional method or dosage. Moreover, it is confirmed that, when used in the aforesaid method and dosage, the levofloxacin-containing eye drops directly inhibit the appearance of resistance, which is caused by the short-term usage of levofloxacin-containing eye drops in the conventional method or dosage, in pathogenic bacteria causative of eye infections such as Staphylococcus aureus.
Owner:SANTEN PHARMA CO LTD +1
Azithromycin eye drops
InactiveCN102579335AAvoid abuseImprove complianceOrganic active ingredientsSenses disorderBacterial ConjunctivitisStaphylococcus aureus
The invention discloses azithromycin eye drops. The azithromycin eye drops are a stable eye preparation which is prepared from azithromycin serving as a main active ingredient and proper auxiliary materials. The long-acting eye drops with mucosa adhesiveness prepared from the latest auxiliary materials at home and abroad have a medicine controlled-release system, the active ingredient stops in eyes for several hours to enhance the antibacterial activity of target tissues, so that the using frequency of the eye drops can be reduced, and the eye drops are convenient to use. The azithromycin eye drops are suitable for treating bacterial conjunctivitis caused by microbial sensitive strains such as G group corynebacterium, haemophilus influenzae, staphylococcus aureus, streptococcus mitis groups and streptococcus pneumoniae.
Owner:GUANGDONG WHOLEWIN TECH
Medicinal composition for treating bacterial conjunctivitis and keratitis and preparation and preparation method
InactiveCN1579402AEasy to useImprove inner qualityOrganic active ingredientsSenses disorderUse medicationMedicine
The invention provides a medicine compound and its manufacturing method, concretely, it is slow released eye preparation containing hydrochloric acid levo ofloxacin and its manufacturing method. The invention uses in situ gelling technology to produce the eye drip, the quality is stable, and it can maintain effect medicine density in a long time, upgrades the biological utilization rate, reduces the stimulation to eyes, reduces the medicine feeding frequency, the producing process is high controlled, the cost is low, it provides a new medicine for clinic.
Owner:成都三明药物研究所
Ophthalmic composition as well as preparation method and application thereof
PendingCN114129590ASuppress generationImprove medication complianceAntibacterial agentsSenses disorderConjunctivaBacterial Conjunctivitis
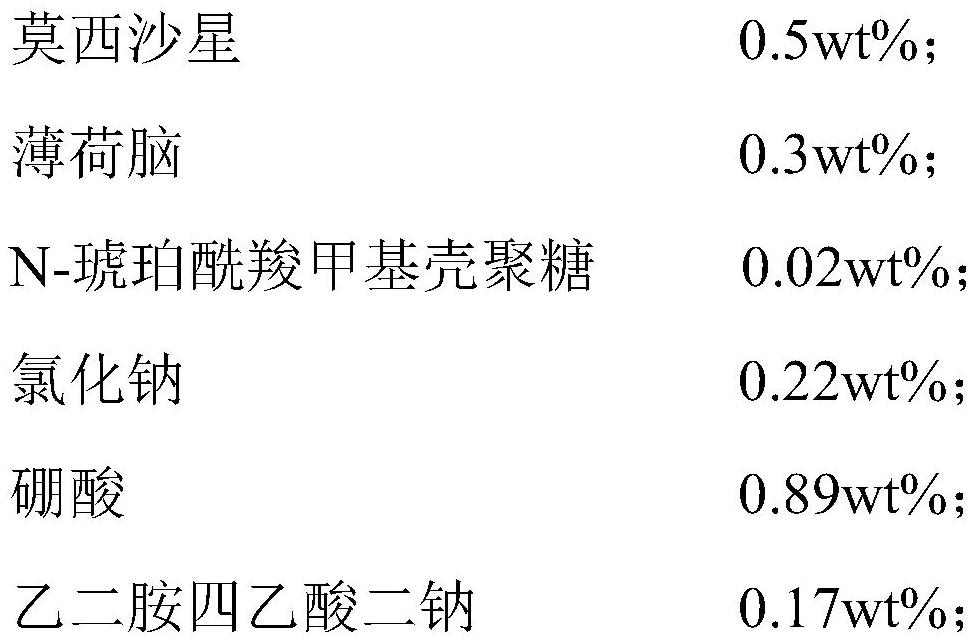
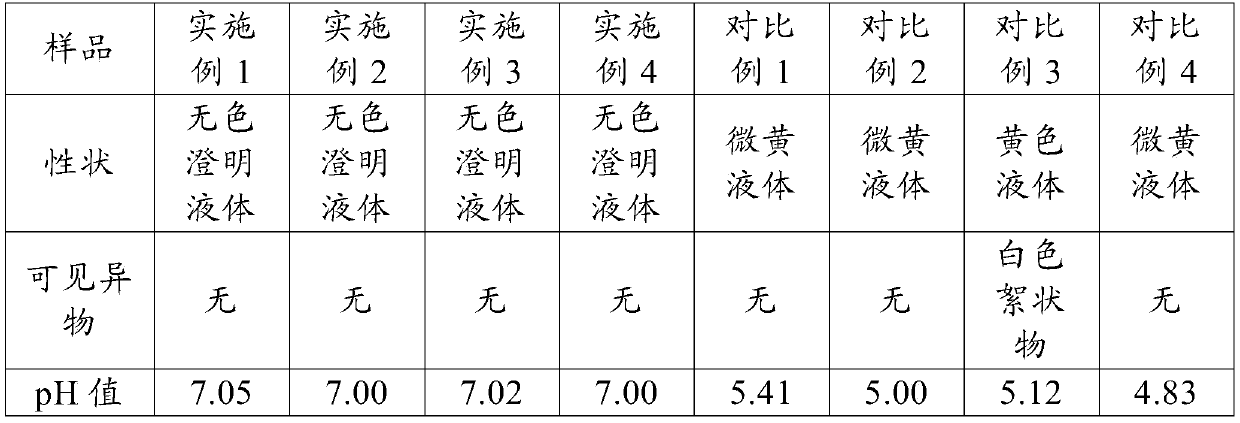
The invention relates to an ophthalmic composition. The ophthalmic composition comprises moxifloxacin and menthol and / or N-succinyl carboxymethyl chitosan. The invention also relates to a preparation method of the ophthalmic composition, which comprises the following steps: mixing the moxifloxacin, the menthol and / or the N-succinyl carboxymethyl chitosan, the osmotic pressure regulator, the pH regulator, the metal ion chelating agent, the surfactant and the water for injection to obtain the ophthalmic composition. The invention also relates to an application of the ophthalmic composition in preparation of a medicine for treating ocular inflammation, and the ocular inflammation comprises bacterial conjunctivitis caused by gram-positive bacterium and / or gram-negative bacterium infection. As eye drops, the ophthalmic composition provided by the invention not only can improve the treatment effect on bacterial conjunctivitis of a patient, but also can avoid adverse effects such as eye stimulation, conjunctival congestion and edema after administration, so that the administration compliance of the patient is improved, and the recovery time of the patient is shortened.
Owner:湖北远大天天明制药有限公司
Enoxacin ocular sustained release gelata and preparation thereof
InactiveCN1543952AExtended stayNot easy to loseAntibacterial agentsOrganic active ingredientsPreservativeFiltration
The invention relates to an eye use Enoxacin slow release gelling agent and its preparation method, which comprises the following constituents (by weight proportions), Enoxacin 0.1-1, and thickening agent 0.1-40, preservative agent 0.001-3, isotonic conditioning agent 0.1-10, pH=4.5-9 of pH modifier, and balancing water. The preparation process comprises dissolving the Enoxacin into water, adding thickening agent and stewing, charging preservative agent, isotonic conditioning agent, stirring to dissolve, regulating pH=4.5-9 with pH modifier, subjecting the solution to filtration by microporous filtering film, watering to total amount from the filter.
Owner:SHENYANG PHARMA UNIVERSITY
Dendrobium eye drops for treating conjunctivitis and keratitis caused by bacterial infection
ActiveCN107308342BRaw materials are easy to getLow priceAntibacterial agentsSenses disorderBacterial ConjunctivitisVitamin B12
Owner:海南诗博丽生物科技有限公司 +1
Traditional Chinese medicine eye drops
InactiveCN112402369AAntibacterial and anti-inflammatoryGood treatment effectAntibacterial agentsSenses disorderMedicinal herbsBacterial Conjunctivitis
The invention discloses traditional Chinese medicine eye drops, and relates to the technical field of eye drops. The traditional Chinese medicine eye drops consist of the following raw materials: 15-25 parts of ofloxacin and 10-20 parts of borneol flake-like crisp crystals as main components, and 3-6 parts of mannitol, 5-8 parts of disodium edetate and 2-5 parts of benzalkonium bromide as auxiliary materials, and the raw materials and the auxiliary materials are mixed and processed to form the traditional Chinese medicine eye drops. By selecting natural medicines and aiming at etiology and pathogenesis of bacterial infection such as bacterial conjunctivitis, bacterial keratitis, pinkeye and bacterial endophthalmitis caused by sensitive bacteria, all the medicinal materials have respectiveeffects and are mutually coordinated, common traditional Chinese medicine components are adopted, the raw materials are easy to obtain and low in price, the medicines are safe and reliable, and the effects of resisting bacteria, diminishing inflammation and the like are achieved. The traditional Chinese medicine eye drops have a remarkable treatment effect on bacterial infection eye diseases suchas bacterial conjunctivitis, bacterial keratitis, pinkeye and bacterial endophthalmitis, and have the characteristics of being exact in curative effect, quickly taking effect, and being high in cure rate, no side effect and the like.
Owner:李强德
Eye drops with capillary artemisia, cape jasmine and baikal skullcap root, and its preparing method
InactiveCN100417399CHigh purityHigh claritySenses disorderPharmaceutical delivery mechanismDiseaseViral Conjunctivitis
The present invention relates to a kind of Yizhihuang eye drops and its preparation method. It can be used for curing the diseases of conjunctivitis, bacterial conjunctivitis, viral conjunctivitis, acute hemorrhagic conjunctivitis, neonatal bacterial conjunctivitis and chronic conjunctivitis and keratitis, etc. It is made up by using four Chinese medicinal materials of lonicera flower, scutellaria root, capillaries and gardenia fruit through a certain preparation process.
Owner:SINOPHARM A THINK PHARMA
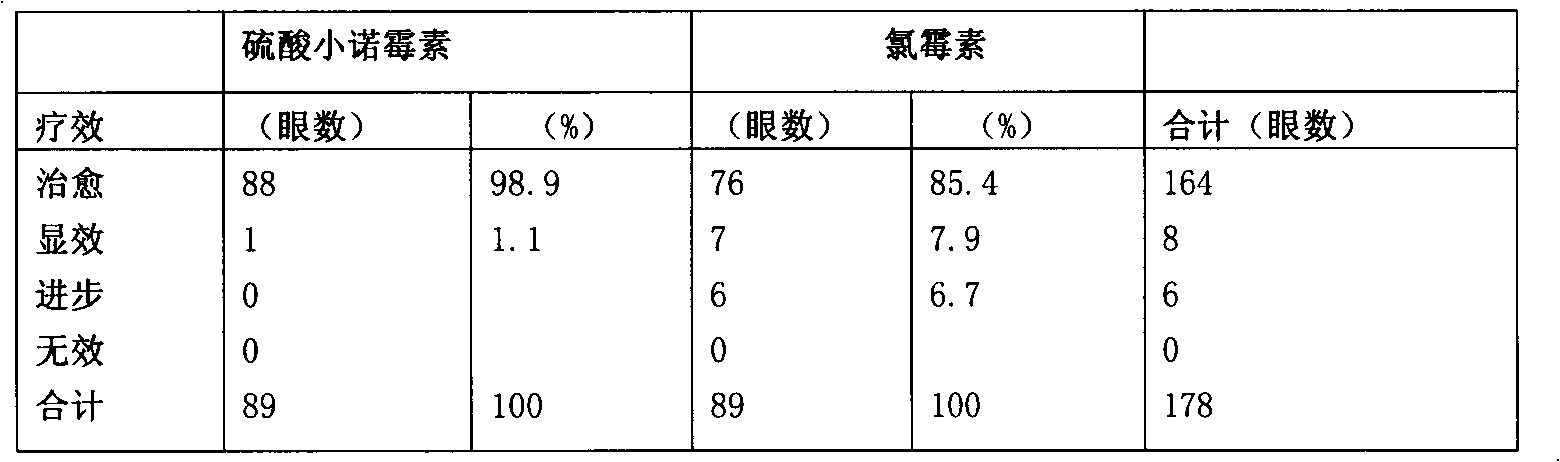
Micronomicin sulfate eye drop and preparation method thereof
ActiveCN101647775BHigh cure rateSmall toxicityOrganic active ingredientsSenses disorderDiseaseSide effect
The invention relates to a micronomicin sulfate eye drop with micronomicin sulfate as a main component. The micronomicin sulfate eye drop can be used for curing eye diseases including bacterial conjunctivitis and the like, and contains micronomicin sulfate, sodium chloride, sodium metabisulfite and water for injection. The micronomicin sulfate eye drop also contains hydroxy propyl cellulose for achieving better curative effect. A preparation method comprises the following steps: adding the components into the water for injection, fully stirring and dissolving, adjusting a pH value to 6.8-7.2 through 0.3-0.5mol of sodium hydroxide aqueous solution, then adding a needed amount of water for injection, and filtering and sterilizing through a 0.22-micron microporous filtering film. The eye drop has a high curative ratio for the eye diseases, small side effects, simple process and equipment of the preparation method and little investment.
Owner:LIAONING QIANLIMING PHARMA GROUP
Enoxacin ocular sustained release gelata and preparation thereof
InactiveCN100563657CExtended stayNot easy to loseAntibacterial agentsOrganic active ingredientsGel preparationPreservative
The invention relates to an enoxacin ophthalmic sustained-release gel and a preparation method thereof, which has the characteristics of maintaining effective drug concentration for a long time and good compatibility. It is composed of the following components: component content (weight%) enoxacin 0.1-1, thickener 0.1-40, preservative 0.001-3, isotonic regulator 0.1-10, pH regulator to adjust the pH value The amount is 4.5-9, the balance of water. It is to dissolve enoxacin in water, add a thickener and let it stand overnight, then add preservatives and isotonic regulators, stir to dissolve, adjust the pH to 4.5-9 with a pH regulator, and pass the solution through a microporous membrane It is prepared by filtering and adding water from the filter to the total amount. This ophthalmic preparation is suitable for treating bacterial conjunctivitis, keratitis and other eye infections.
Owner:SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com