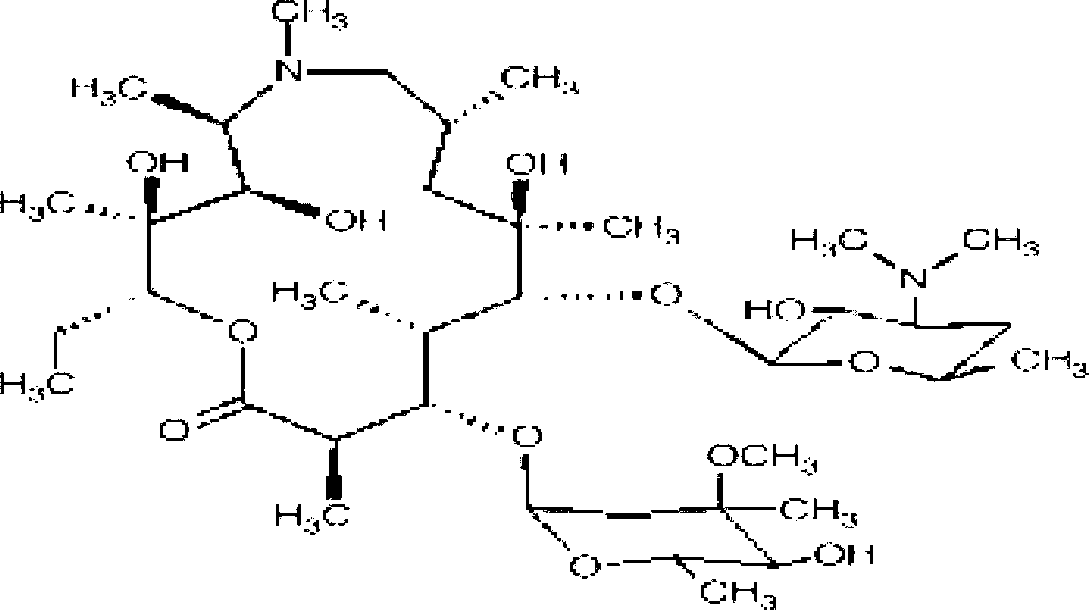
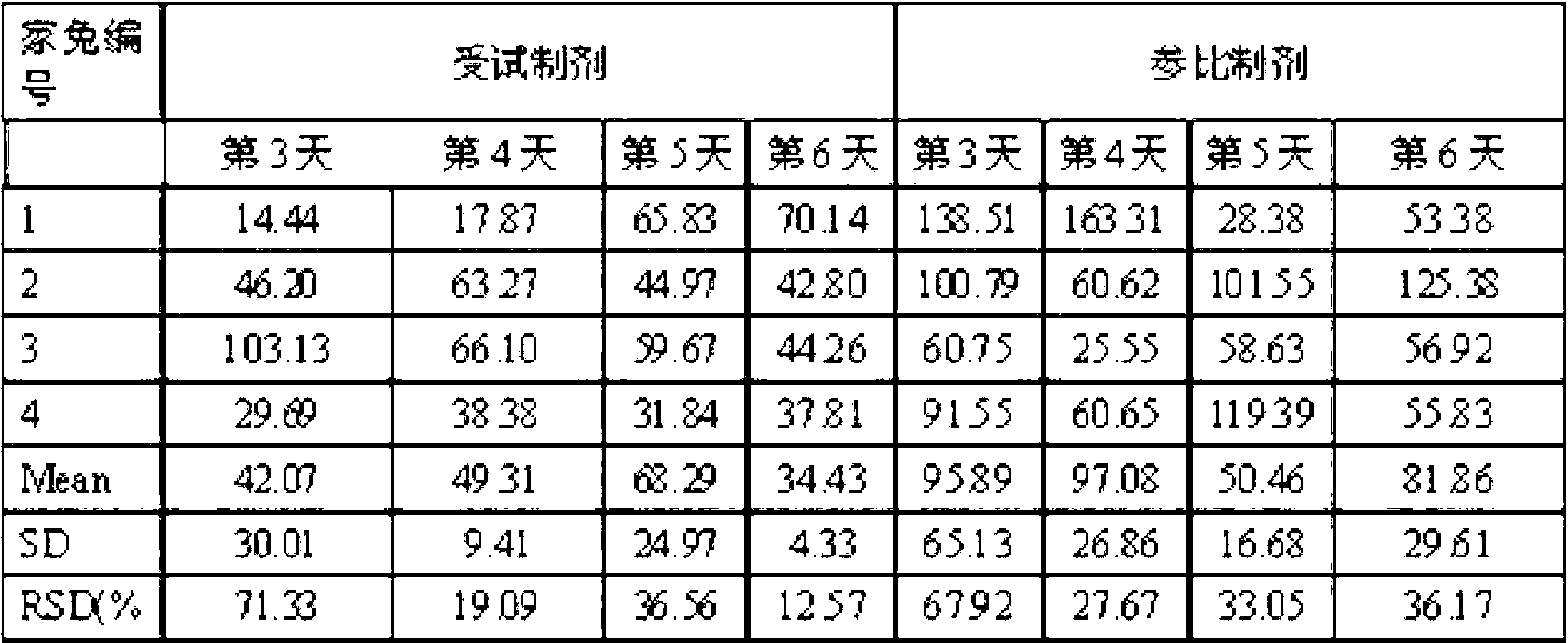
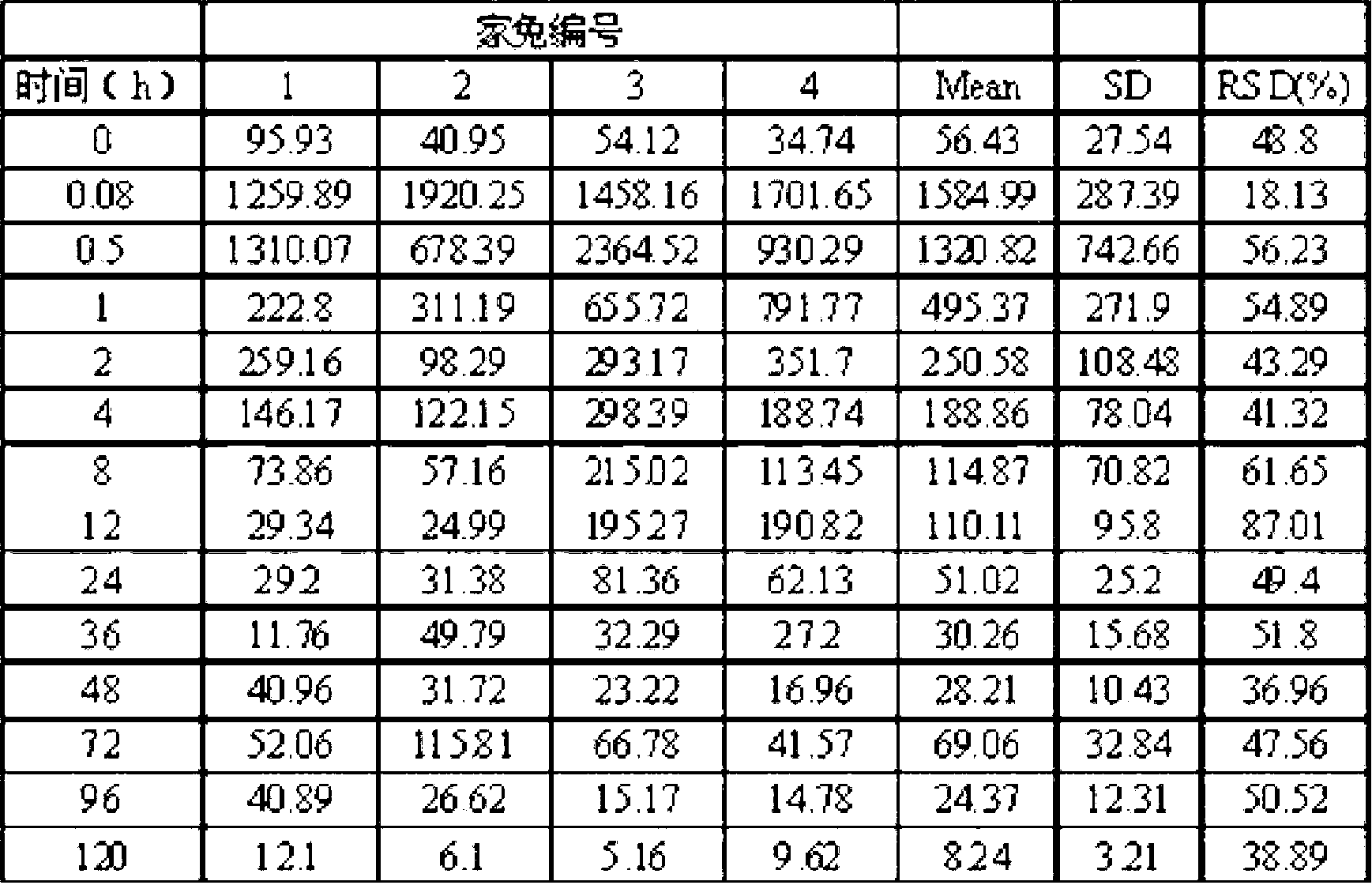
Azithromycin gel eye drops and preparation process thereof
A technology of azithromycin and eye drops, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problem of low bioavailability, poor drug absorption, and short retention time. problem, to achieve the effect of reducing drug loss, delaying drug release, and increasing residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Take by weighing 0.15g of azithromycin, stir and dissolve with appropriate amount of citric acid-sodium citrate buffer solution, then add 4.0g of sodium chloride, 0.1g of disodium edetate, and 0.05g of thimerosal solution into the above-mentioned drug solution, replenish Add an appropriate amount of water for injection and ultrasonically dissolve; add 0.01g polycarbophil, and shear with high-shear equipment at 10,000r / min for 1 minute to make polycarbophil fully swell and disperse; after polycarbophil is completely swelled and dispersed, cool to room temperature, adjust the pH to 6.0-6.5 with 1M sodium hydroxide solution; add 0.1 g of poloxamer 407 to the solution, and then transfer the solution to 4°C to completely dissolve poloxamer 407 and obtain clarification After the solution, add the full amount of water for injection, filter, subpackage; store at 2-8°C.
Embodiment 2
[0032]Example 2: Weigh 1.06g of azithromycin, 8.0g of mannitol, 0.1g of disodium edetate, and 0.05g of benzalkonium chloride solution, and dissolve them ultrasonically with an appropriate amount of citric acid-sodium citrate buffer and water for injection; add 0.05g polycarbophil, cut for 1 minute with high-shear equipment at 10000r / min to make polycarbophil fully swell and disperse; after polycarbophil is completely swollen and dispersed, cool to room temperature and oxidize with 1M Mix solution of sodium and potassium hydroxide (1:1 molar ratio) to adjust the pH to 6.0-6.5; add 15g of carbomer to the solution, and then transfer the solution to 4°C to completely dissolve the carbomer and obtain clarification After the solution, add water for injection to the full amount, filter, subpackage; store at 2-8°C.
Embodiment 3
[0033] Example 3: Weigh a mixture of 5.0 g of azithromycin, 2.4 g of glycerin, 6.0 g of disodium edetate, paraben A, B, C, and butyl ester (mass ratio is 70:10:10:10) 2.0 g, ultrasonically dissolve with an appropriate amount of citric acid-sodium citrate buffer and water for injection; add 2.5g of polycarbophil, and shear with high-shear equipment at 10000r / min for 2 minutes, so that the polycarbophil is fully swollen and dispersed; After the polycarbophil is completely swollen and dispersed, cool to room temperature, and adjust the pH to 6.0-6.5 with 1M sodium hydroxide solution; add 10.0 g of poloxamer 407 to the solution, and then transfer the solution to 4°C. Completely dissolve the poloxamer 407 to obtain a clear solution, add water for injection to the full amount, filter and sub-package; store at 2-8°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com