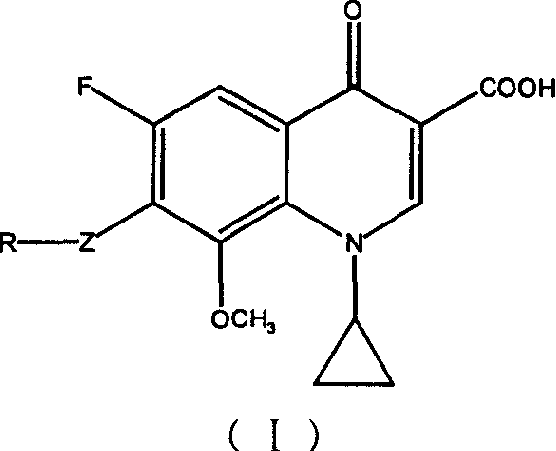
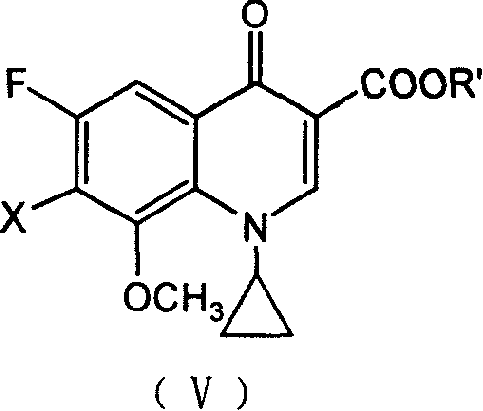
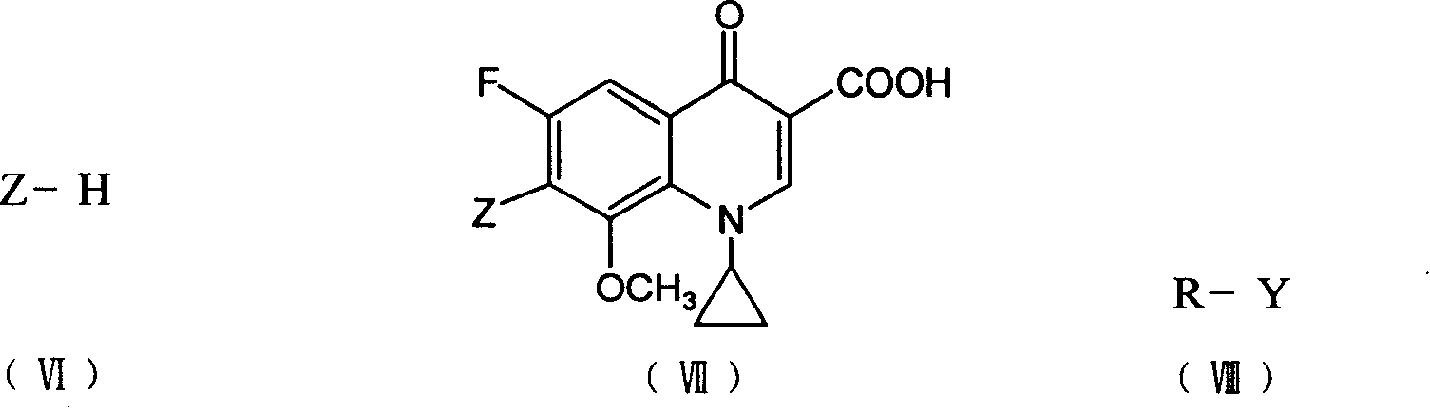
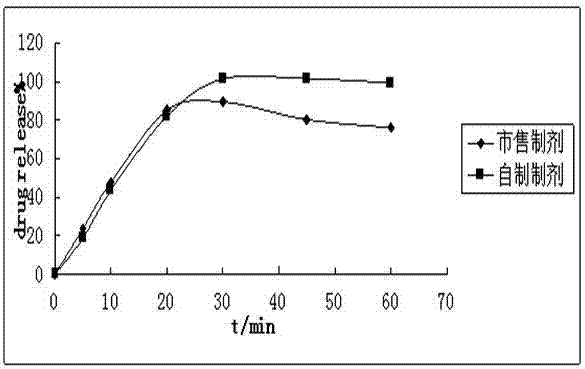
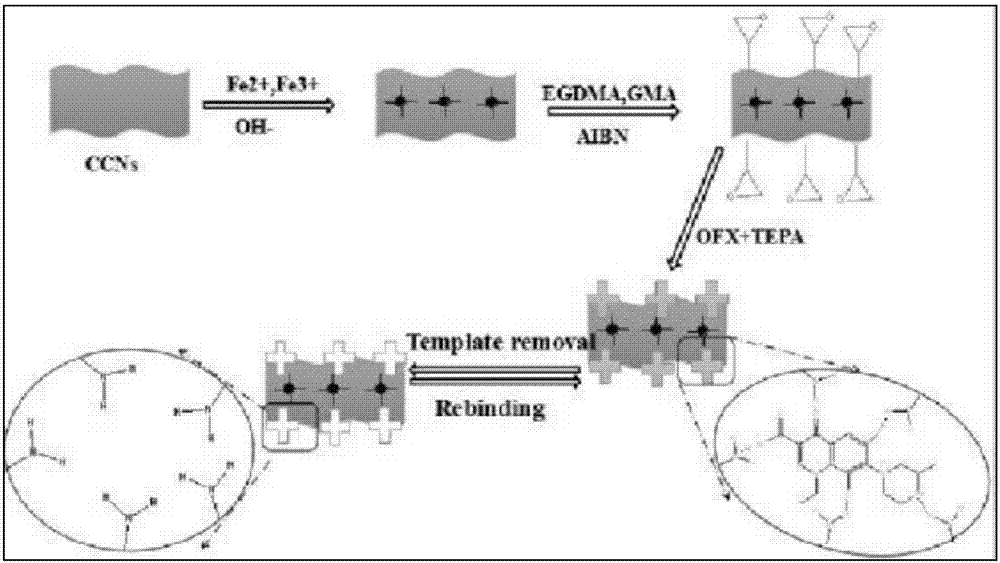
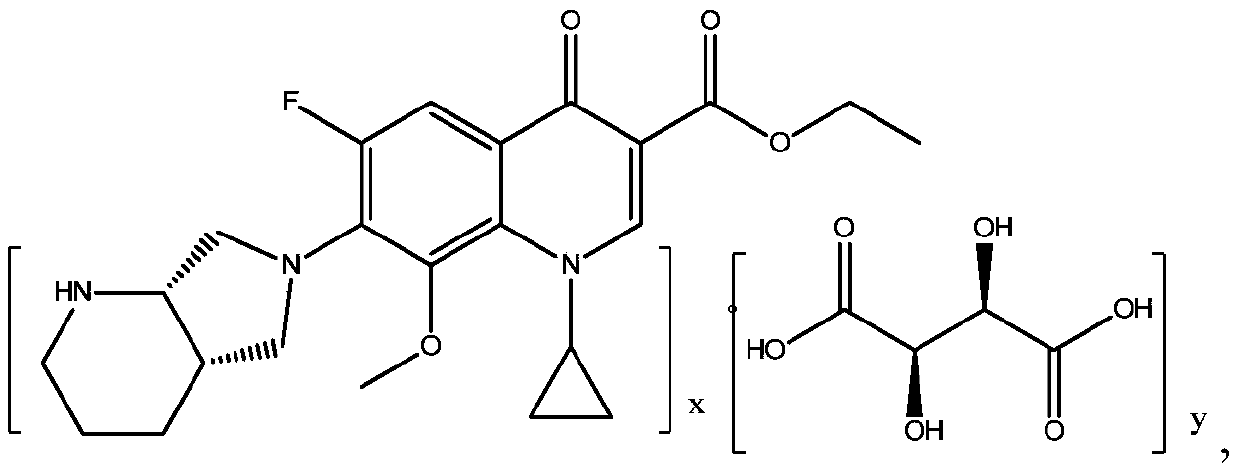
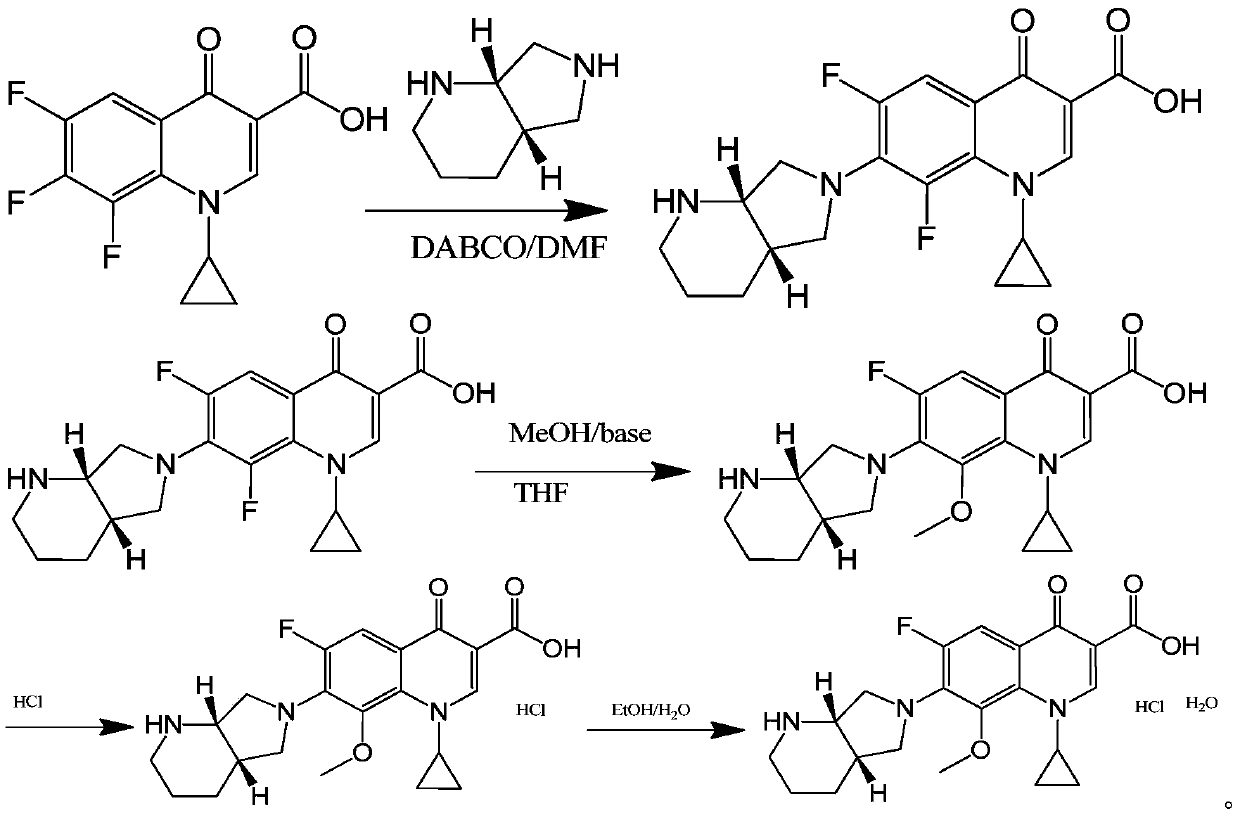
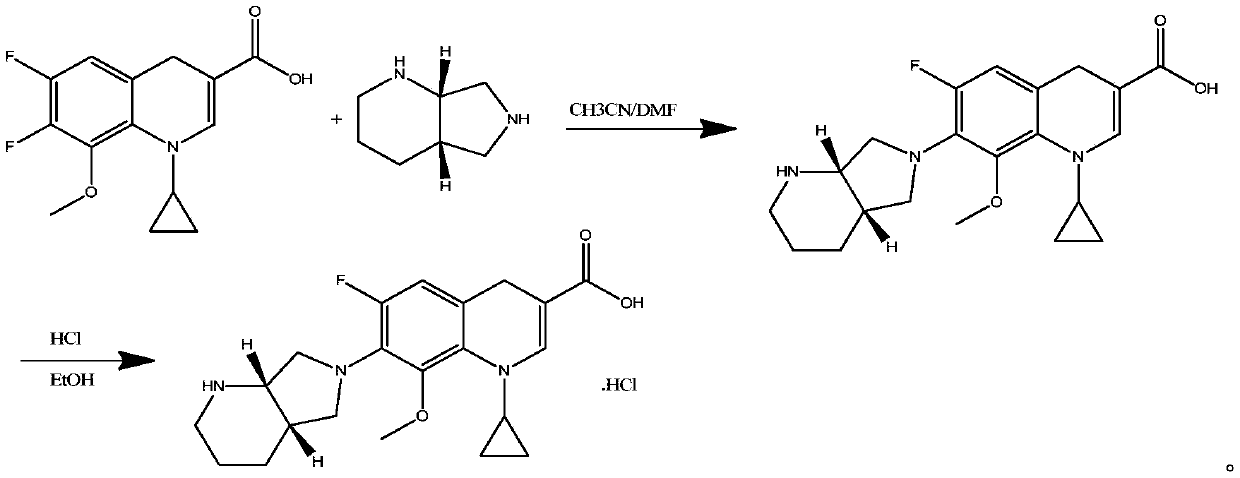
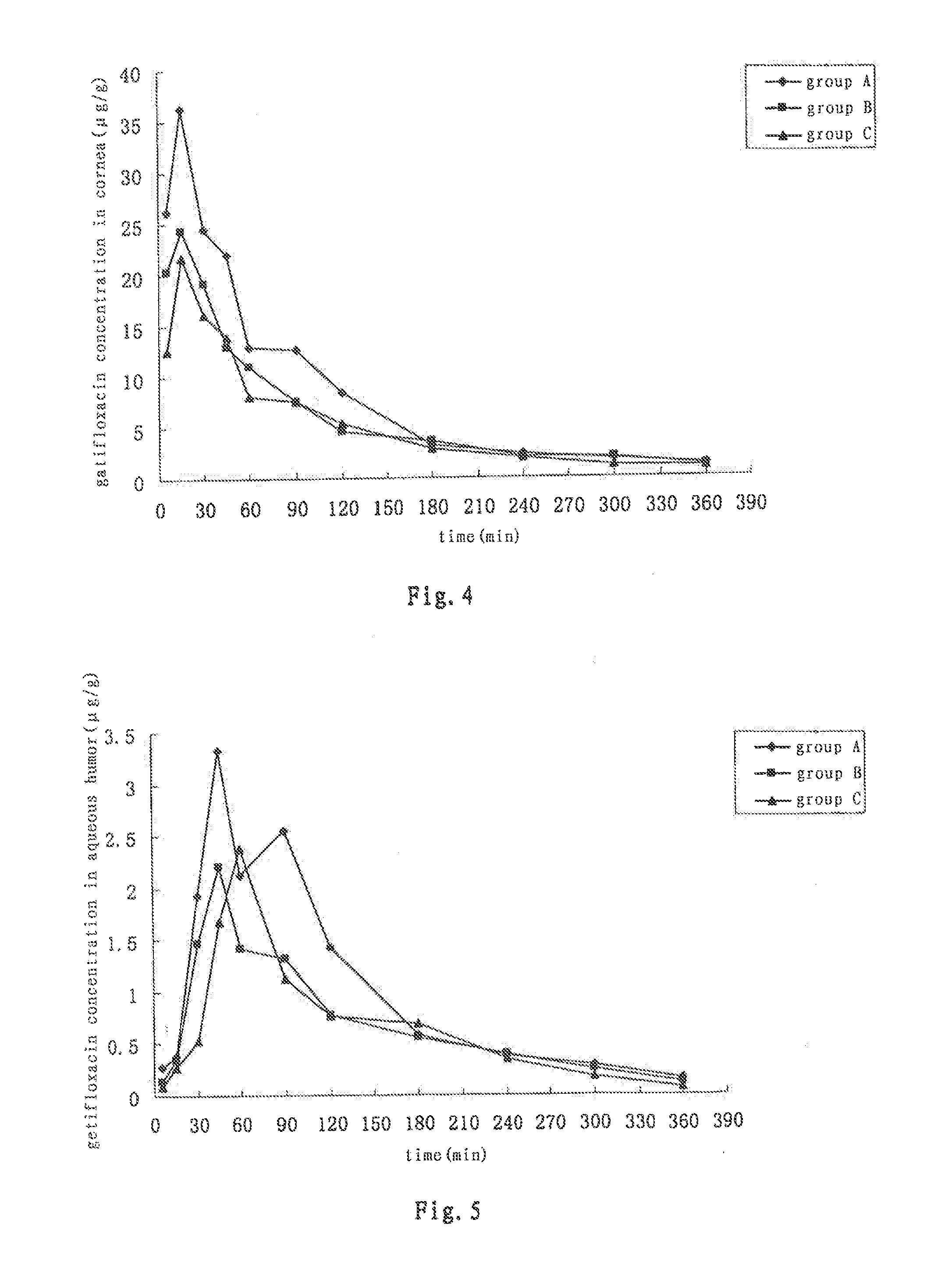
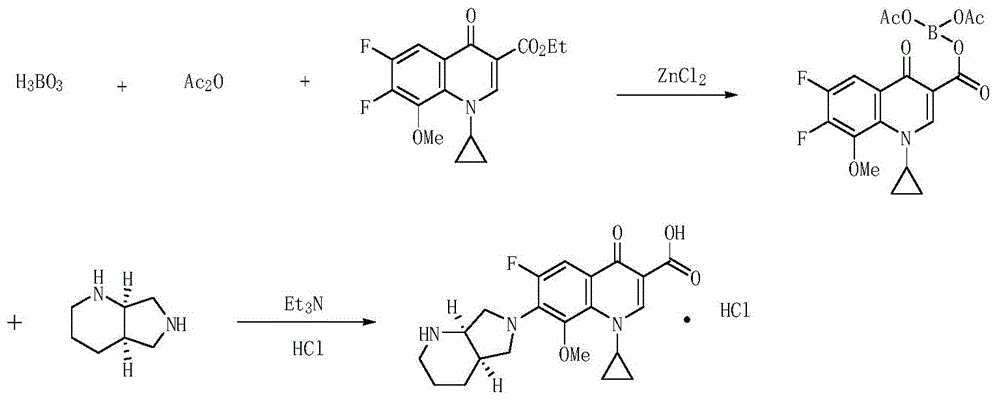
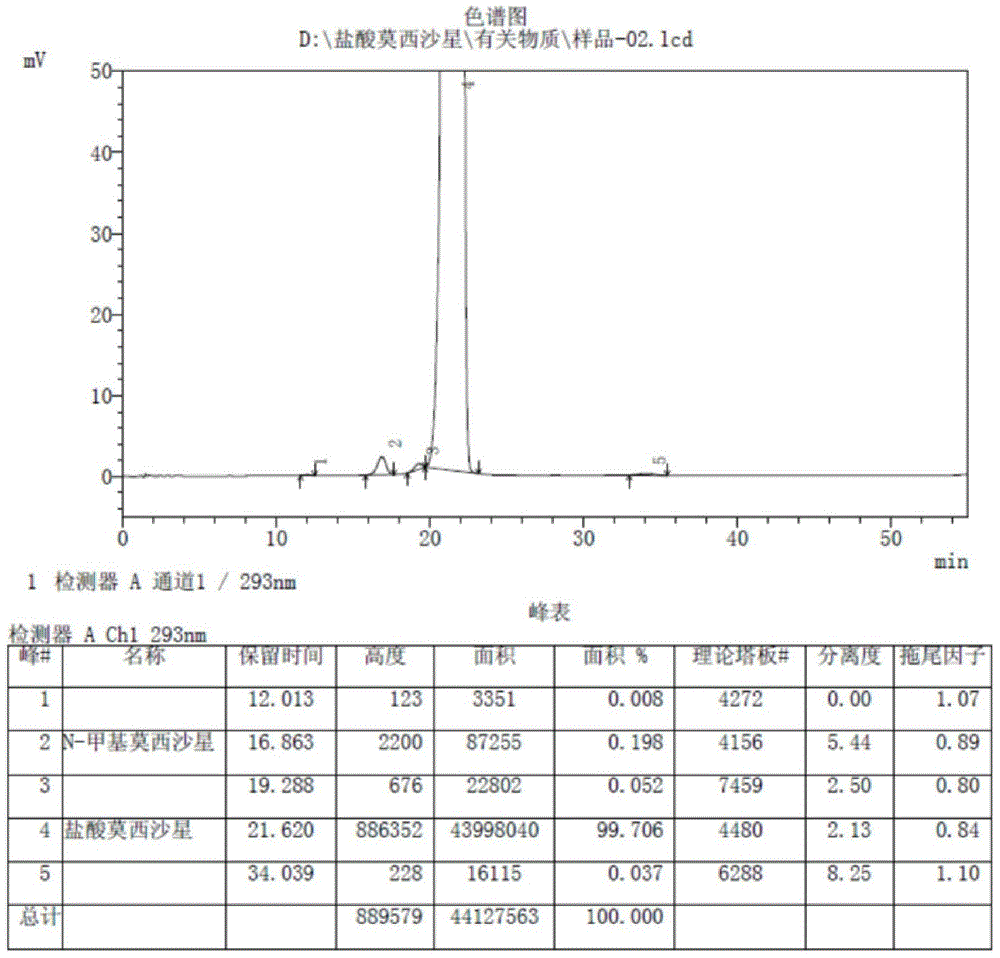
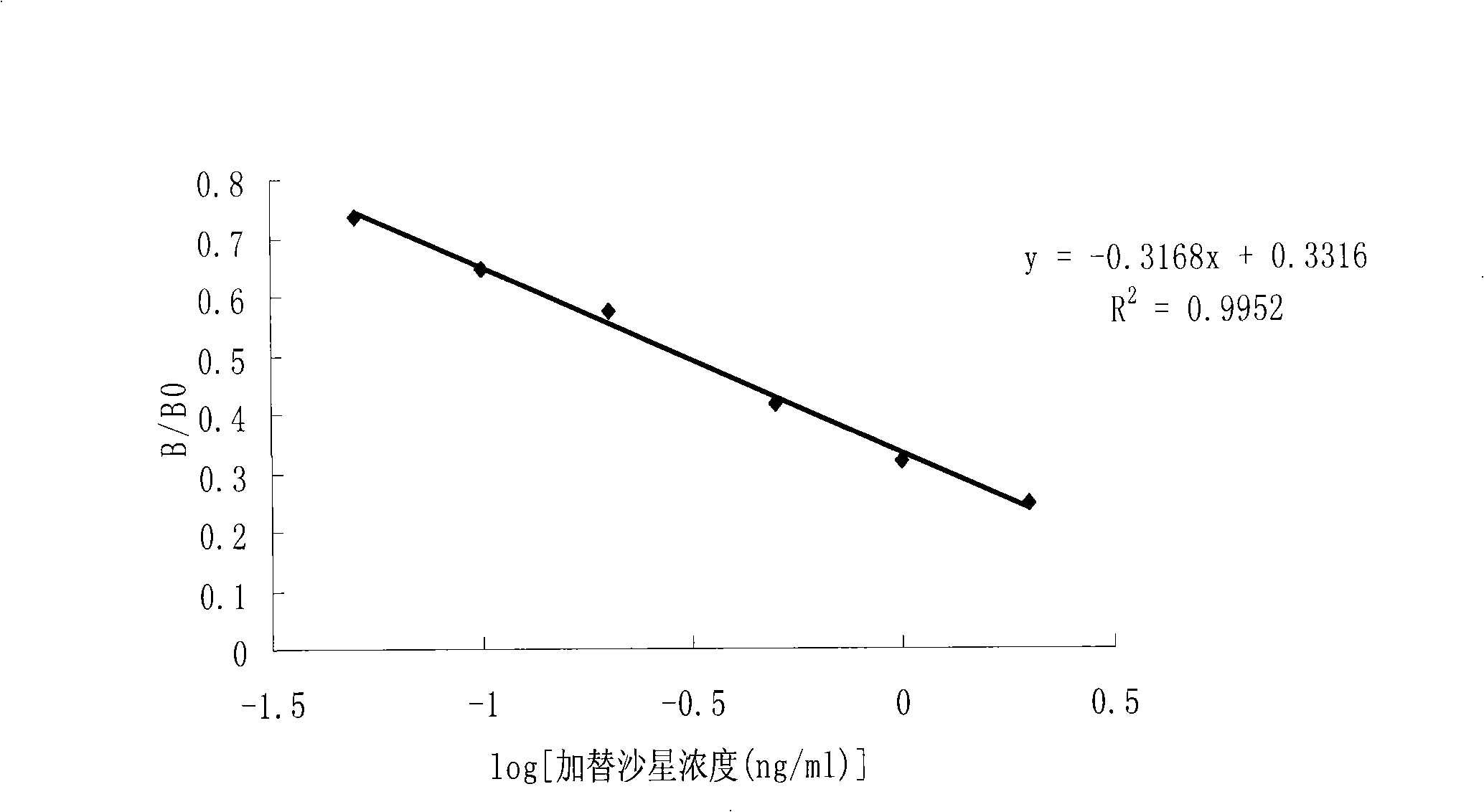Patents
Literature
115 results about "Gatifloxacin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is a quinolone antibiotic used for eye infections (such as conjunctivitis).
Ophthalmic composition containing gatifloxacin and lotepredenol etabonate and method of preparing the same
The invention discloses a turbid liquor eye composition with chlotiponuo and jiatishaxing and making method, which comprises the following parts: (a) 0. 2-2% chlotiponuo and salt with grain size below 50um, (b) 0. 2-1% jiatishaxing and salt, (c) 0. 5-2. 5% one or more non-ion polymer as suspension-assisting agent, (d) eye excipient agent. The making method comprises the following steps: grinding the chlotiponuo carbonate below 50um; sterilizing; adding jiatishaxing, non-ion polymer and other eye adjuvants to form an eye agent to treat and prevent opthalmitis and bacterial infection.
Owner:SHANDONG BAUSCH & LOMB FREDA PHARM CO LTD
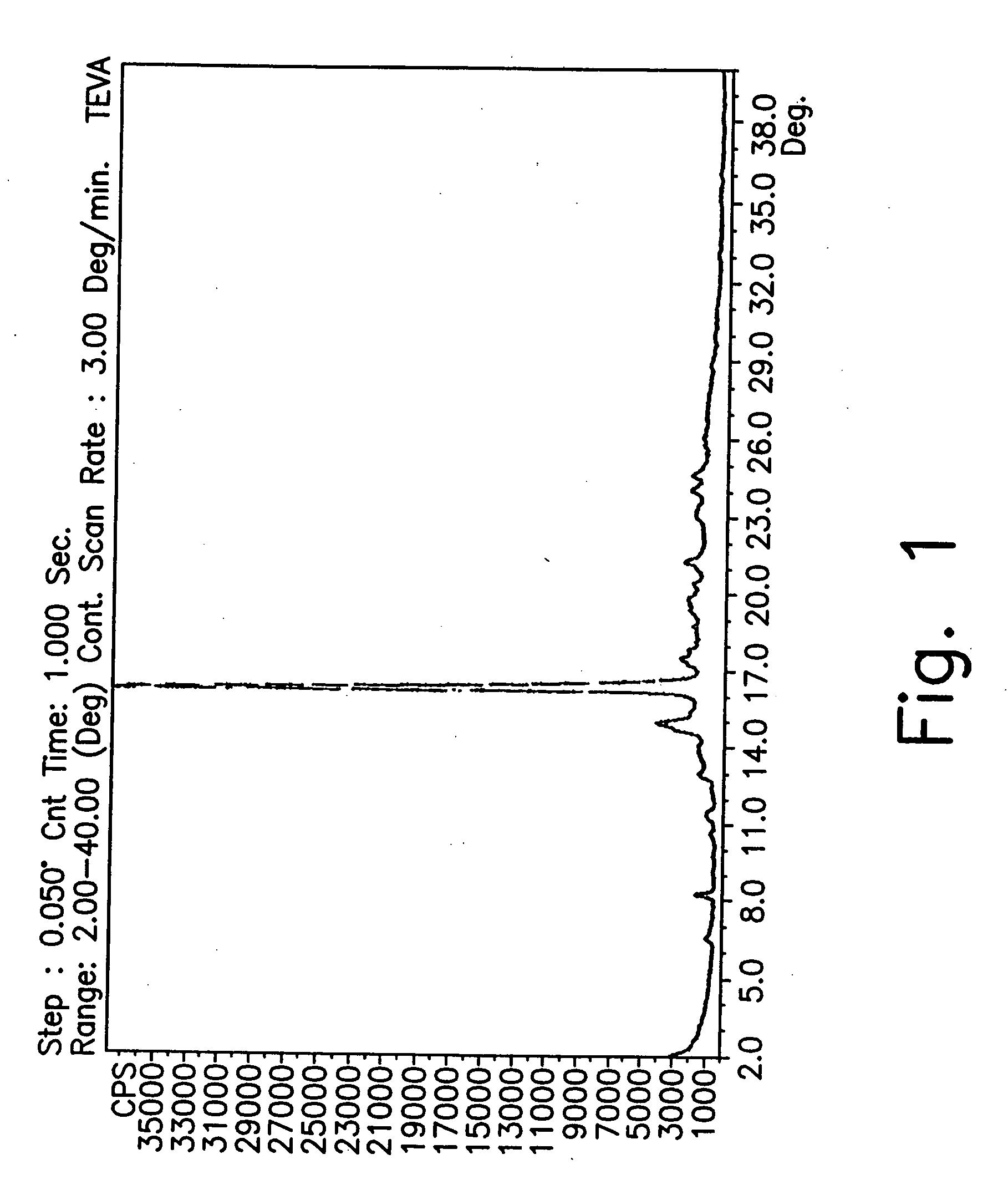
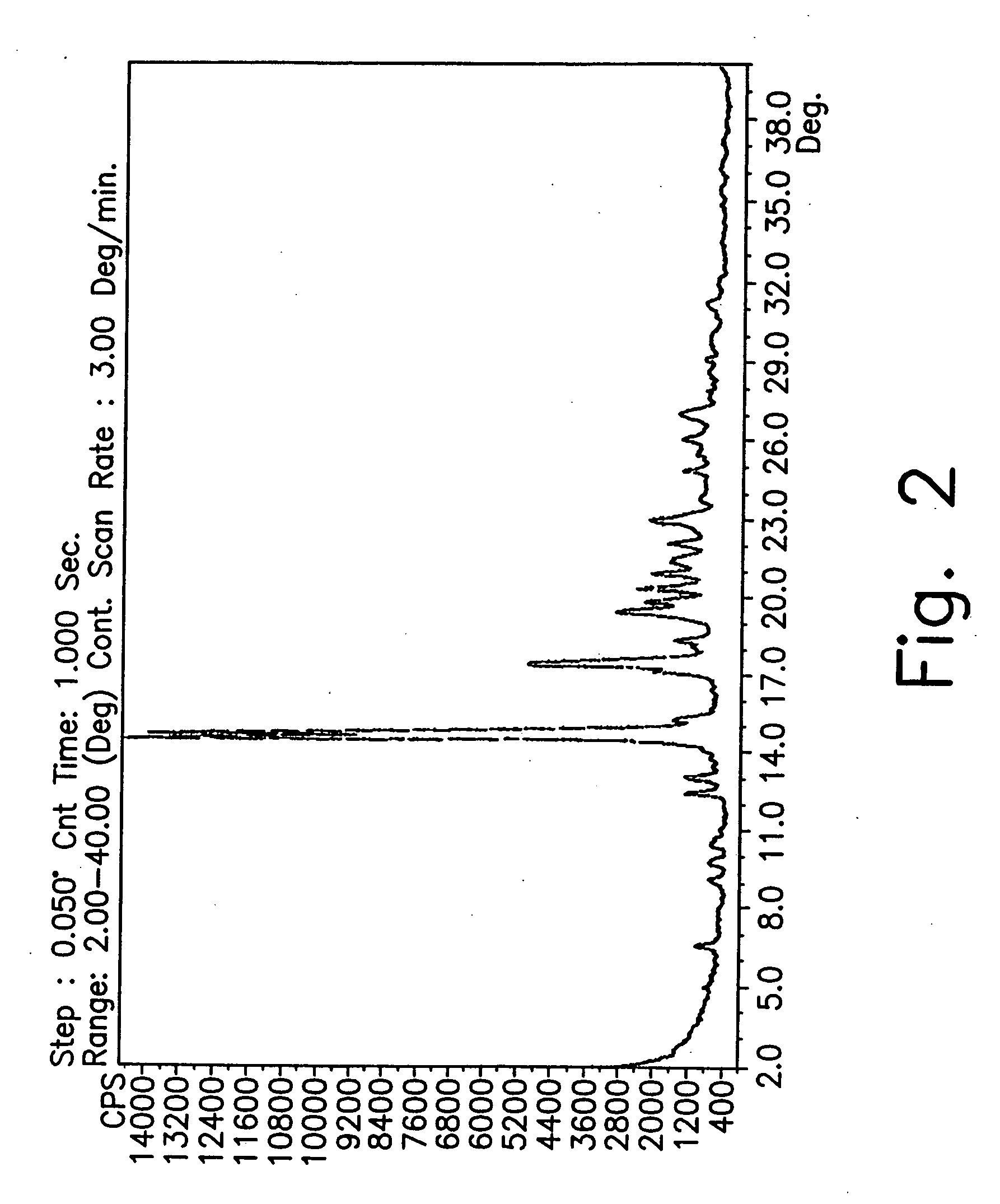
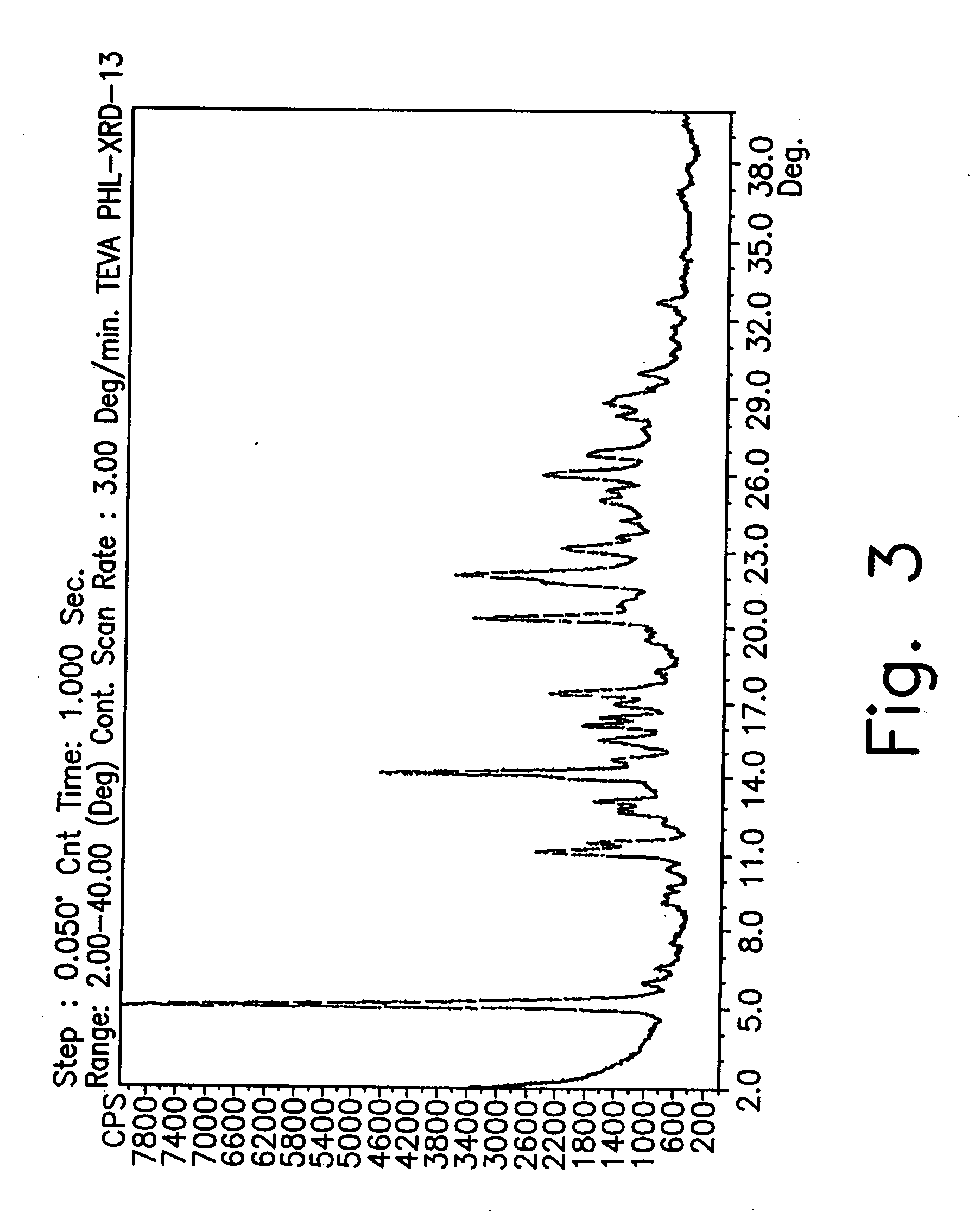
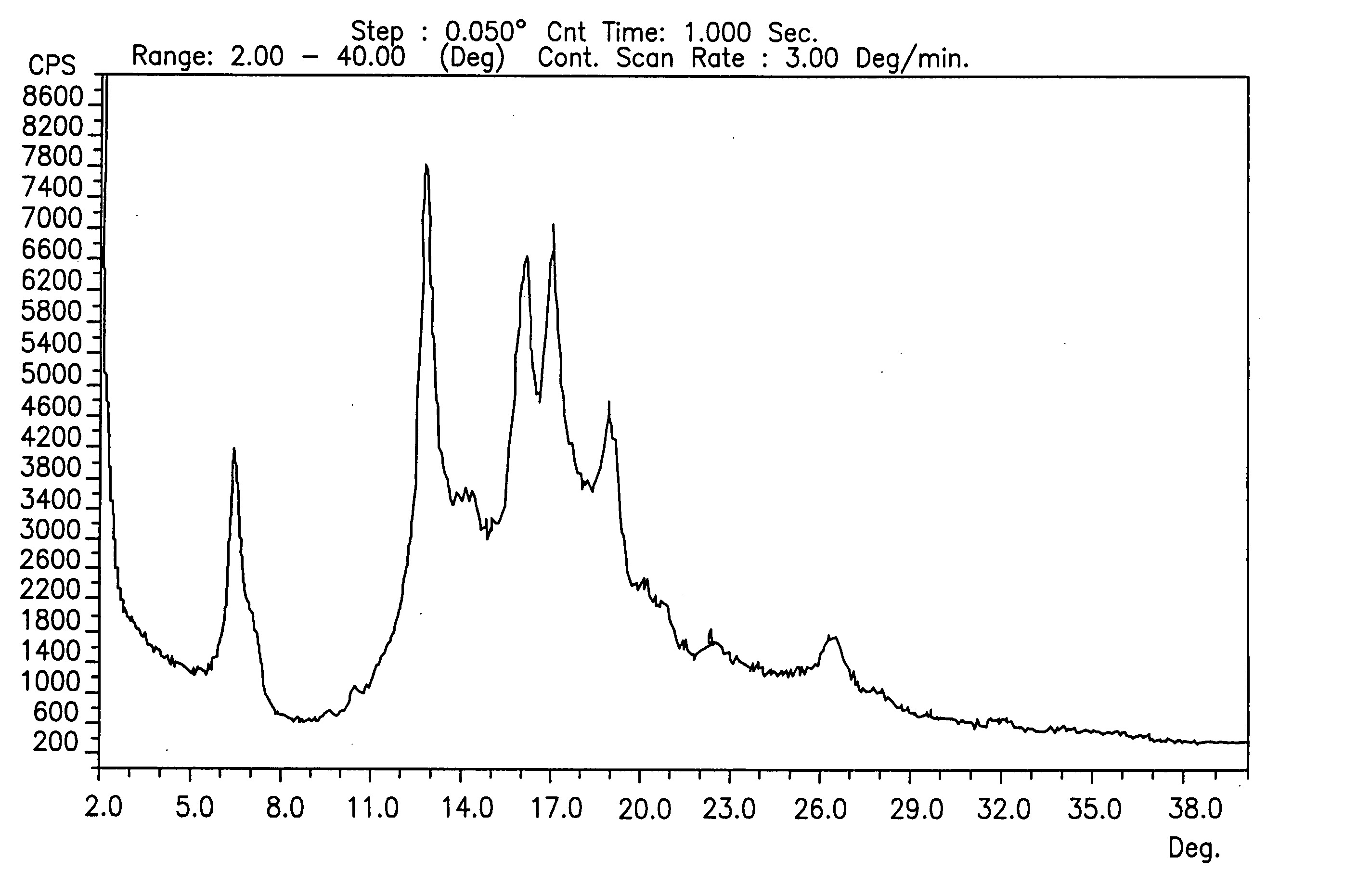
Novel crystalline forms of gatifloxacin and processes for preparation
InactiveUS20060258677A1Organic active ingredientsOrganic chemistryMedicinal chemistryCrystallization
Provided are novel crystalline forms of gatifloxacin, some of which are DMSO solvates, and methods for making them.
Owner:TEVA PHARMA IND LTD
Novel crystalline forms of gatifloxacin
Provided are novel crystalline forms of gatifloxacin denominated forms A, B, C, D, E1, F, G, H, I, and J, and methods for their preparation. Also provided are methods for making known crystalline forms of gatifloxacin, in particular forms omega and T2RP.
Owner:NIDDAM HILDESHEIM VALERIE +3
Gatiflxacin eye gels based on HPMC medium and its preparing method
InactiveCN1562030AExtended stayNot easy to loseOrganic active ingredientsSenses disorderTreatment effectIrritation
An ocular Jiatishaxing gel for treating eyelid inflammation, stye, conjunctivitis, dacryocystitis, keratitis, corneal ulcer and trachoma is prepared from Jiatishaxing, HPMC as matrix, antiseptic, isotonic regulator, osmotic promoter, pH regulator and water through dissolving Jiatishaxing in water, adding others, stirring, regulating pH=5-9, filter and adding water.
Owner:SHENYANG PHARMA UNIVERSITY
Gatifloxacin external and ophthalmic gel preparation
InactiveCN1448137ADegradableGood film formingOrganic active ingredientsSenses disorderOphthalmic Gel Dosage FormDisease
The Gatifloxacin gel preparation for external use and eye use has Gatifloxacin as main component and its supplementary material includes chitosan as gel substrate, iso-osmotic regulator, pH regulator, preservative, injection water, etc. The preparation has Gatifloxacin content of 0.1-3 wt% and chitosan content of 0.3-3 wt%. It has obvious anti-infection function and functions of speeding heal of wound, promoting epidermal growth, inhibiting formation of scar tissue, maintaining local medicine density for long term, etc. It is used in treating burns, scalds, skin infection, folliculitis, bacterial conjunctivitis, keratitis, etc.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Water-soluble salt of aspartic acid carbostyril series antibacterial drugs and injection dosage forms thereof
The invention provides an aspartate quinolone antibiotics water-soluble salt and the injection formulation thereof, including sparfloxacin, gatifloxacin, rufloxacin, pefloxacin, tosufloxacin, moxifloxacin, and so on; the invention improves the water solubility of quinolone antibiotics and enhances the anti-bacterial effect of quinolone antibiotics. Compared with the oral liquid of quinolone drugs of the prior art, the invention has the advantages that: water solubility of the drug is good, as the drug enters the blood directly, the invention can not only achieve treatment function rapidly, but can also be absorbed by the human body fully, and the invention has significant effects in the two aspects of fast onset of action and low consumption. Compared with the injection of the quinolone drugs of the prior art, the invention has the advantages that: due to the existence of the L-aspartate, the antibacterial activity of quinolone drugs can be enhanced.
Owner:SHENYANG WOSEN PHARMA INST
Chemical luminescence ELISA detection kit for gatifloxacin
InactiveCN101403752ASpecific detectionSpecific assessmentChemiluminescene/bioluminescencePhosphateElisa method
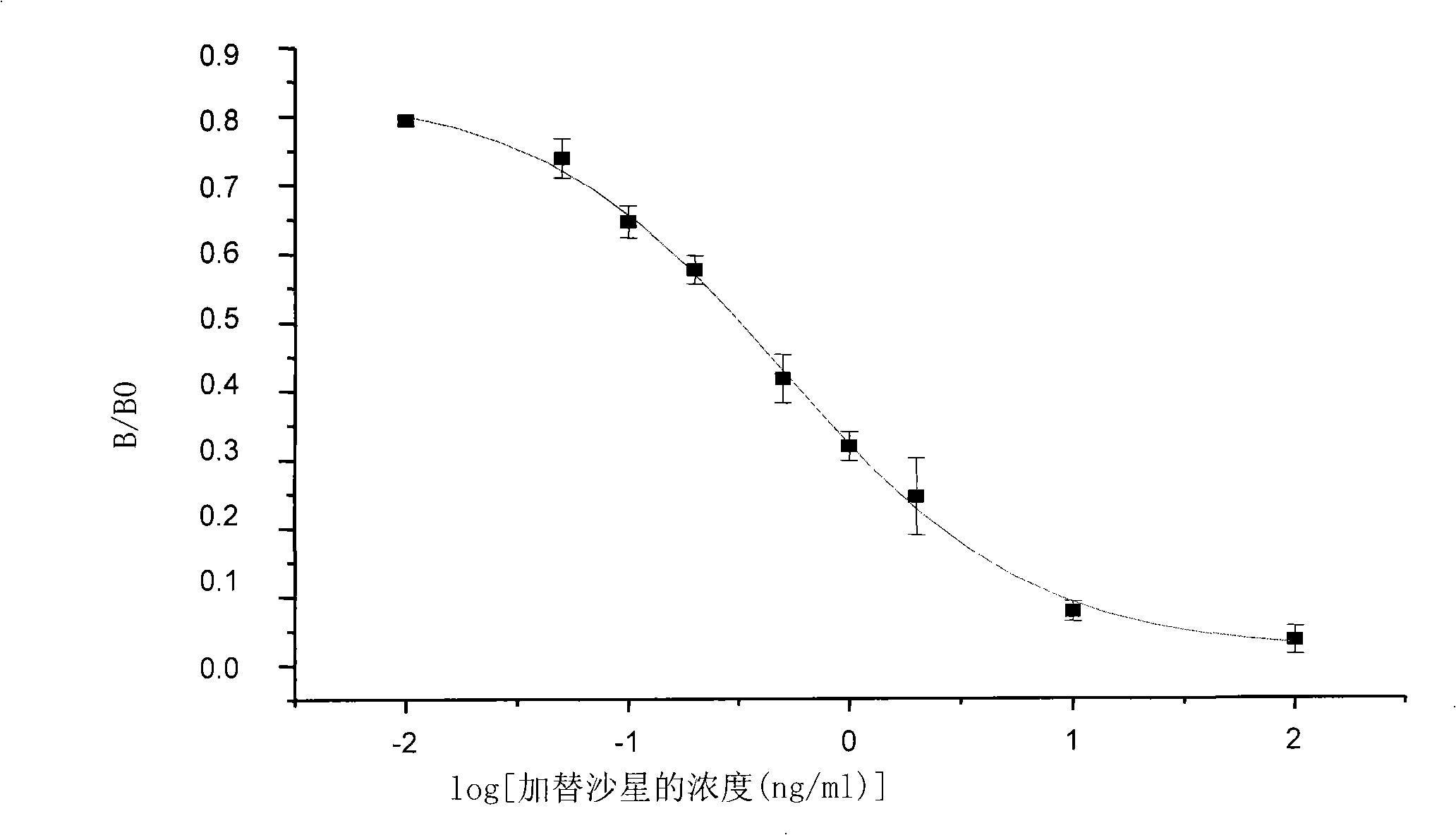
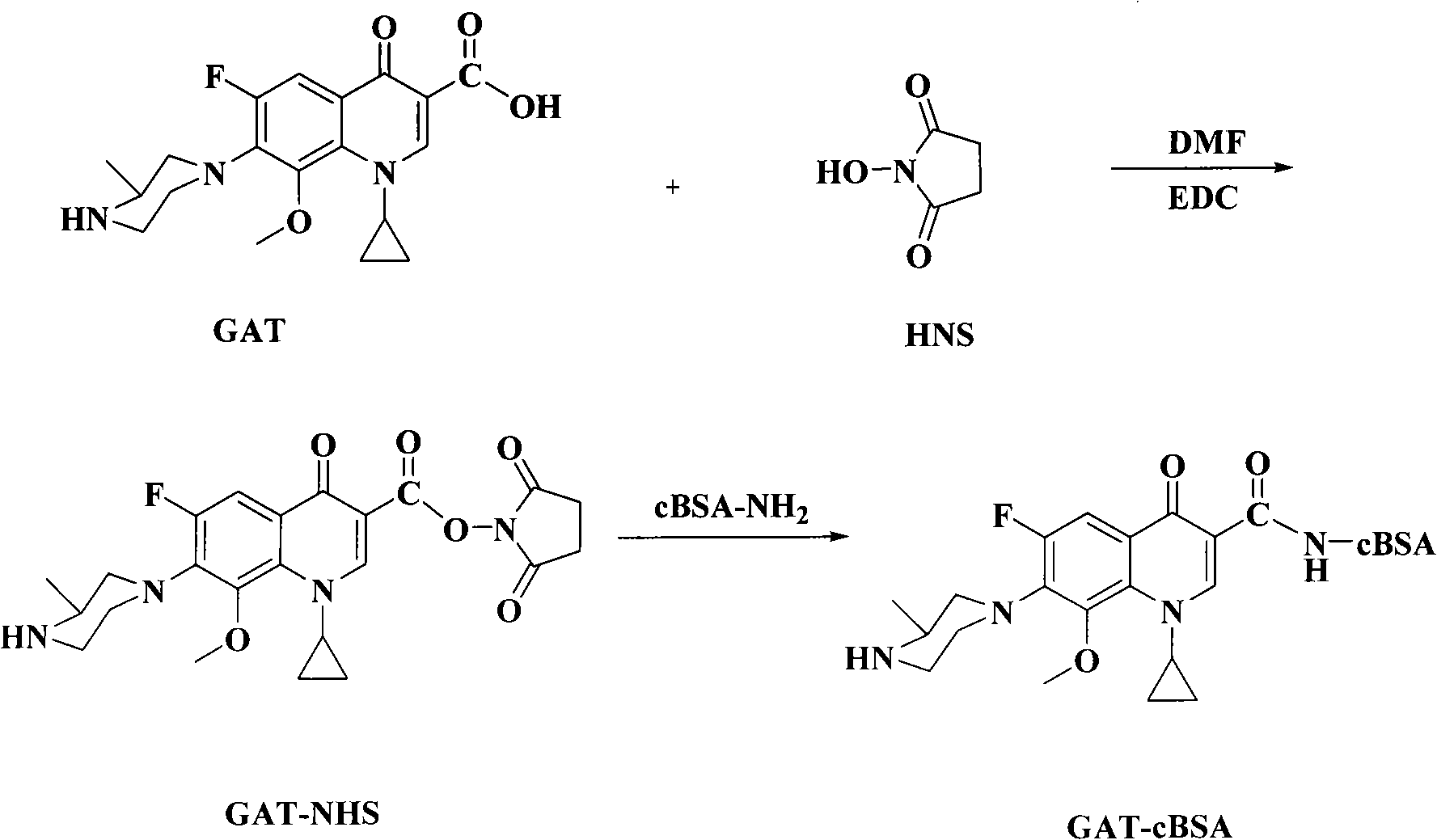
The invention discloses a chemical luminescence enzyme-linked immunoassay kit of gatifloxacin, comprising a kit body, an enzyme-marked plate and a reagent which are arranged in the kit body; wherein, all the holes of the enzyme-marked plate are coated with coating antigen; the coating antigen is prepared by the coupling of the gatifloxacin and ovalbumin; the reagent comprises a polyclonal antibody of the gatifloxacin, an enzyme-marked second antibody (namely, the antibody of horseradish peroxidase-marked goat anti-rabbit), a gatifloxacin series standard solution, a concentrated phosphate buffer, a concentrative cleaning solution and a luminescence solution. The kit has the advantages of high sensitiveness, good repeatability, simpleness, fastness and exactness; and compared with traditional colorimetric ELISA method, the sensitiveness can be improved by one magnitude and the method is expected to play an import role in the gatifloxacin residual detection of animal source foods (such as milk samples and animal tissue samples) and a urine sample.
Owner:SHANDONG UNIV
Ophthalmic Suspension for Ocular Use
InactiveUS20100063017A1More acceptabilityMore patient complianceAntibacterial agentsBiocidePrednisoloneGatifloxacin
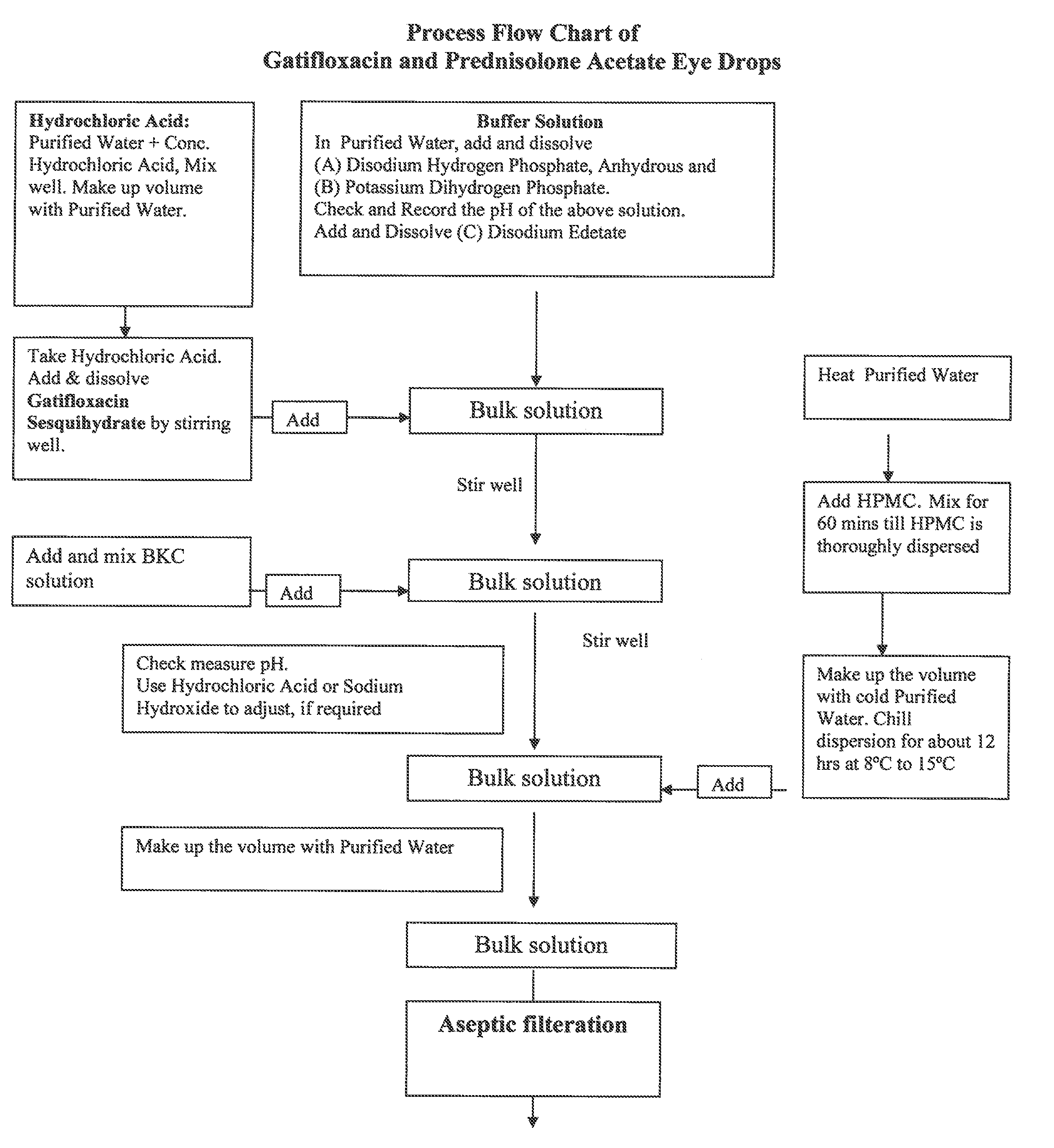
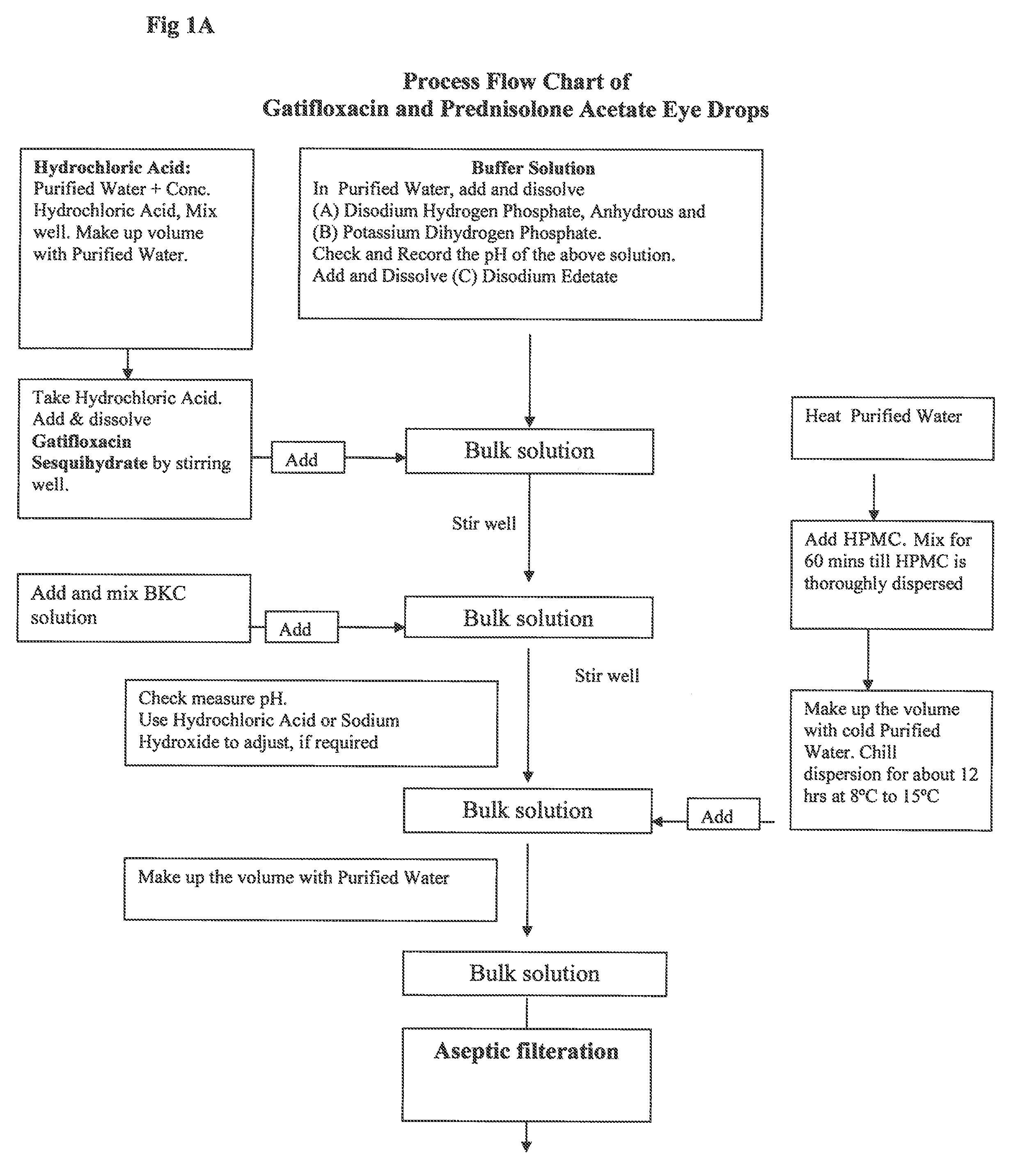
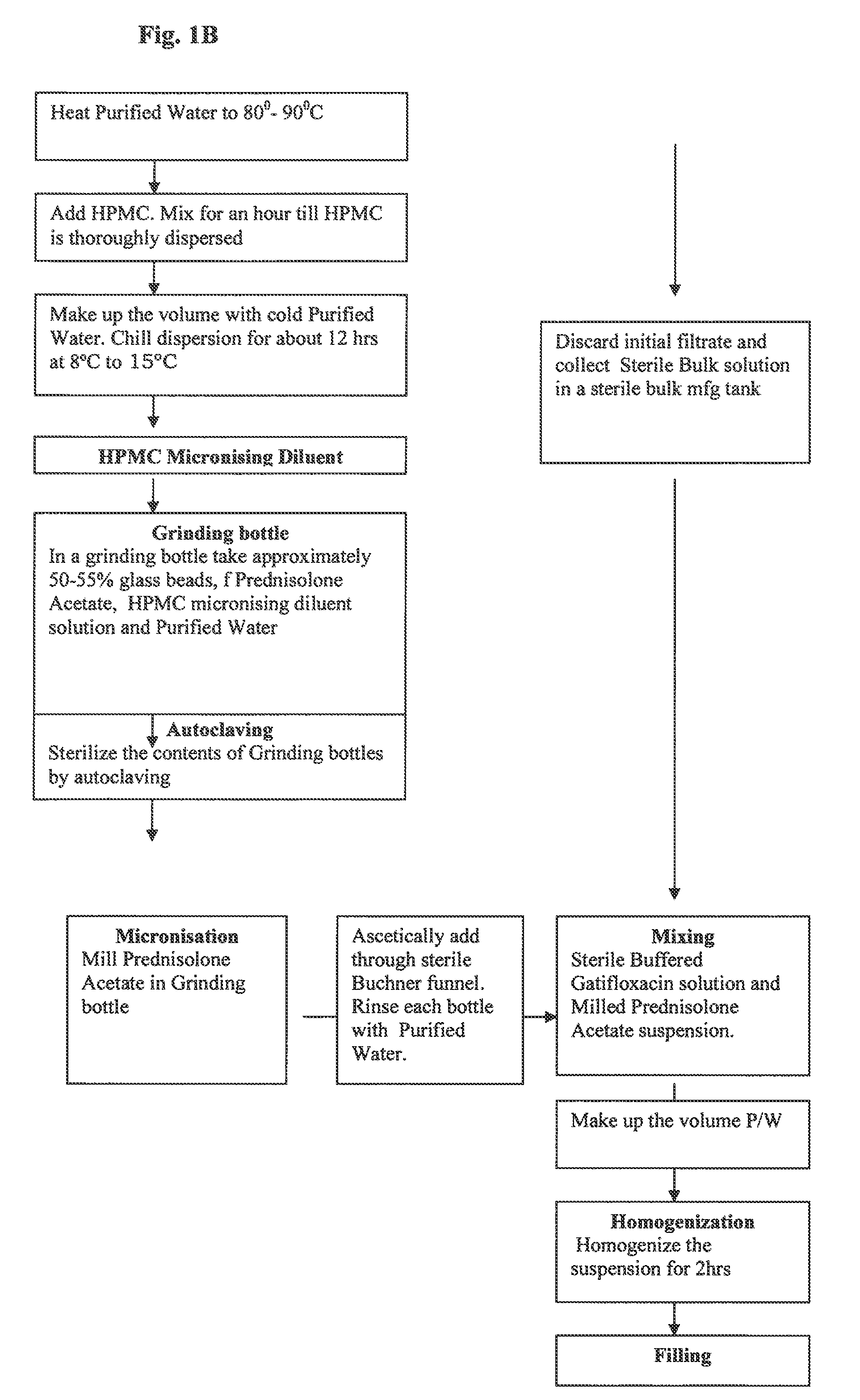
A gatifloxacin and prednisolone topical ophthalmic pharmaceutical compositions for prevention and treatment of ophthalmic bacterial infections and inflammatory conditions associated with pre-surgical and / or post surgical ocular surgeries.
Owner:ALLERGAN INC
Gatifloxacin molecularly imprinted polymer adsorbent and preparation process thereof
InactiveCN101829550AImprove enrichment effectGood choiceOther chemical processesPolymer scienceSorbent
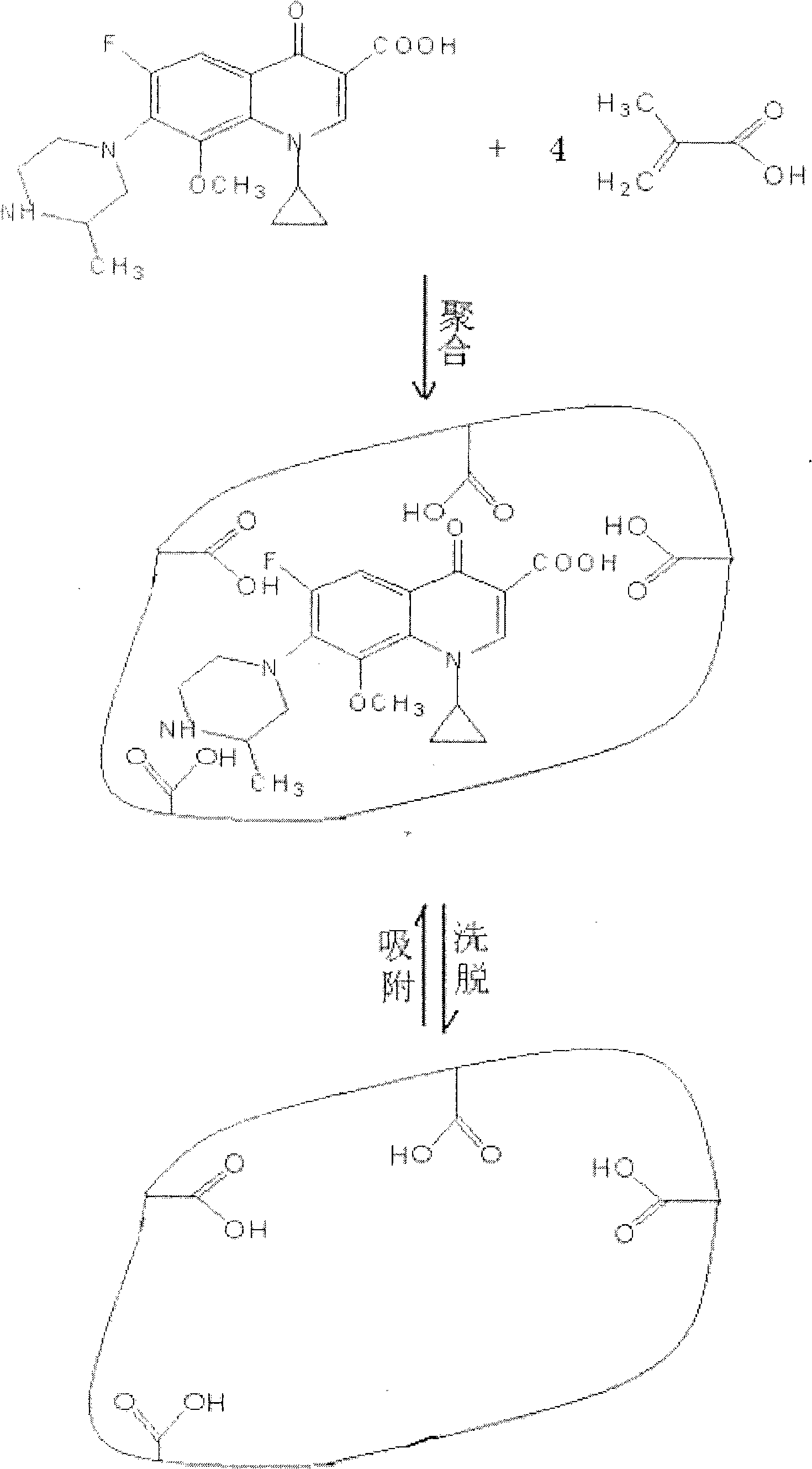
The invention discloses a gatifloxacin molecularly imprinted polymer adsorbent and preparation process thereof. The invention relates to an organic polymer compound, and aims to provide a gatifloxacin molecularly imprinted polymer adsorbent having good selectivity and adsorbability to the gatifloxacin, and preparation process thereof. The technical scheme of the invention is as follows: the gatifloxacin, methylacrylic acid, ethylene glycol dimethacrylate and azodiisobutyronitrile are subject to thermalpolymerization to generate porous spherical high polymer of which the specific surface area is not less than 90m<2> / g. The invention provides the process of the gatifloxacin molecularly imprinted polymer adsorbent described in preparation claim 1, the gatifloxacin, methylacrylic acid, ethylene glycol dimethacrylate and azodiisobutyronitrile are placed in the solvent of acetonitrile or trichloromethane at 50-70 DEG C to thermally initiate the gatifloxacin molecularly imprinted polymer adsorbent. The invention is used for detecting the content of the gatifloxacin.
Owner:HENAN NORMAL UNIV
7-substituted-8-methoxy fluoroquinolone carboxylic derivatives, preparing process, preparation and use thereof
InactiveCN1683339AHigh antibacterial activityAntibacterial agentsOrganic chemistryQuinoloneAcid derivative
The present invention relates to medicine chemistry and discloses 7-substituent-8-methoxy fluoro quinolone carboxylic derivative, i. e. 7-substituent-1-cyclopropyl-6-fluoro-1, 4-dihydro-8-methoxy-4-oxo-3-quinolone carboxylic acid and its salt; their preparation process and medicine composition for human and animal. The compounds of the present invention has antiseptic activity and safety higher than gatifloxacin and similar medicine.
Owner:南京澳新医药科技有限公司
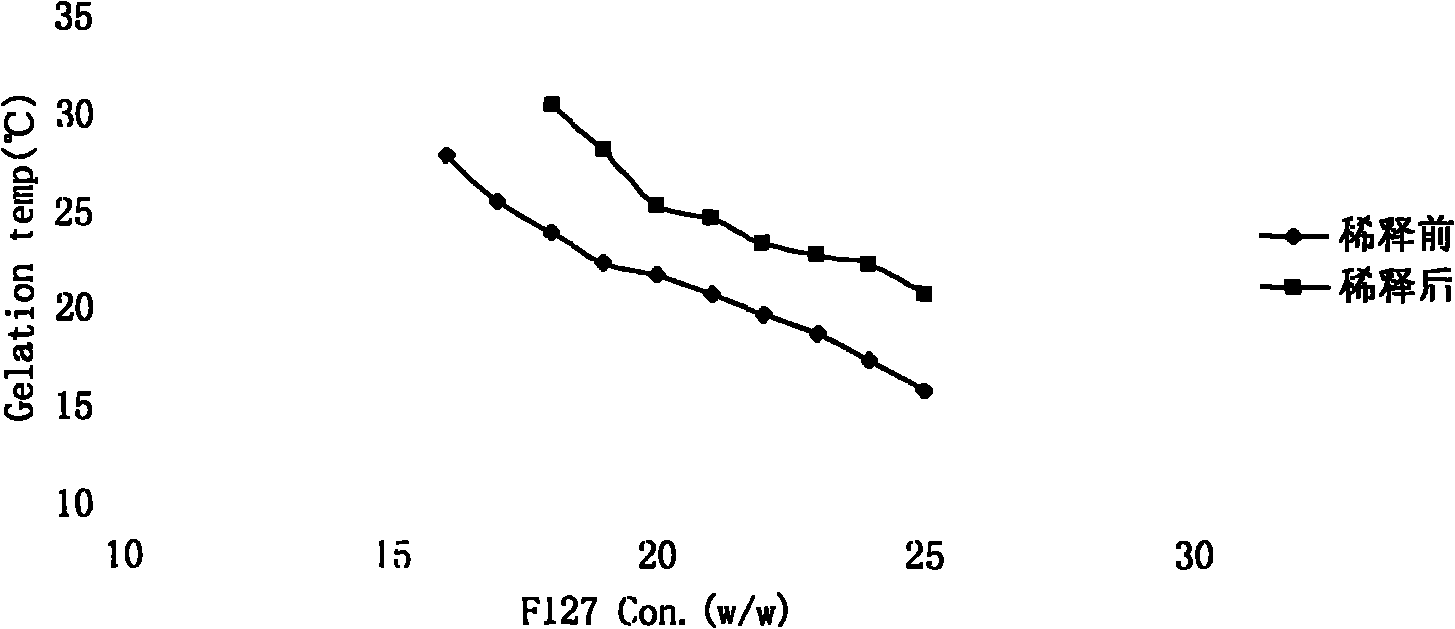
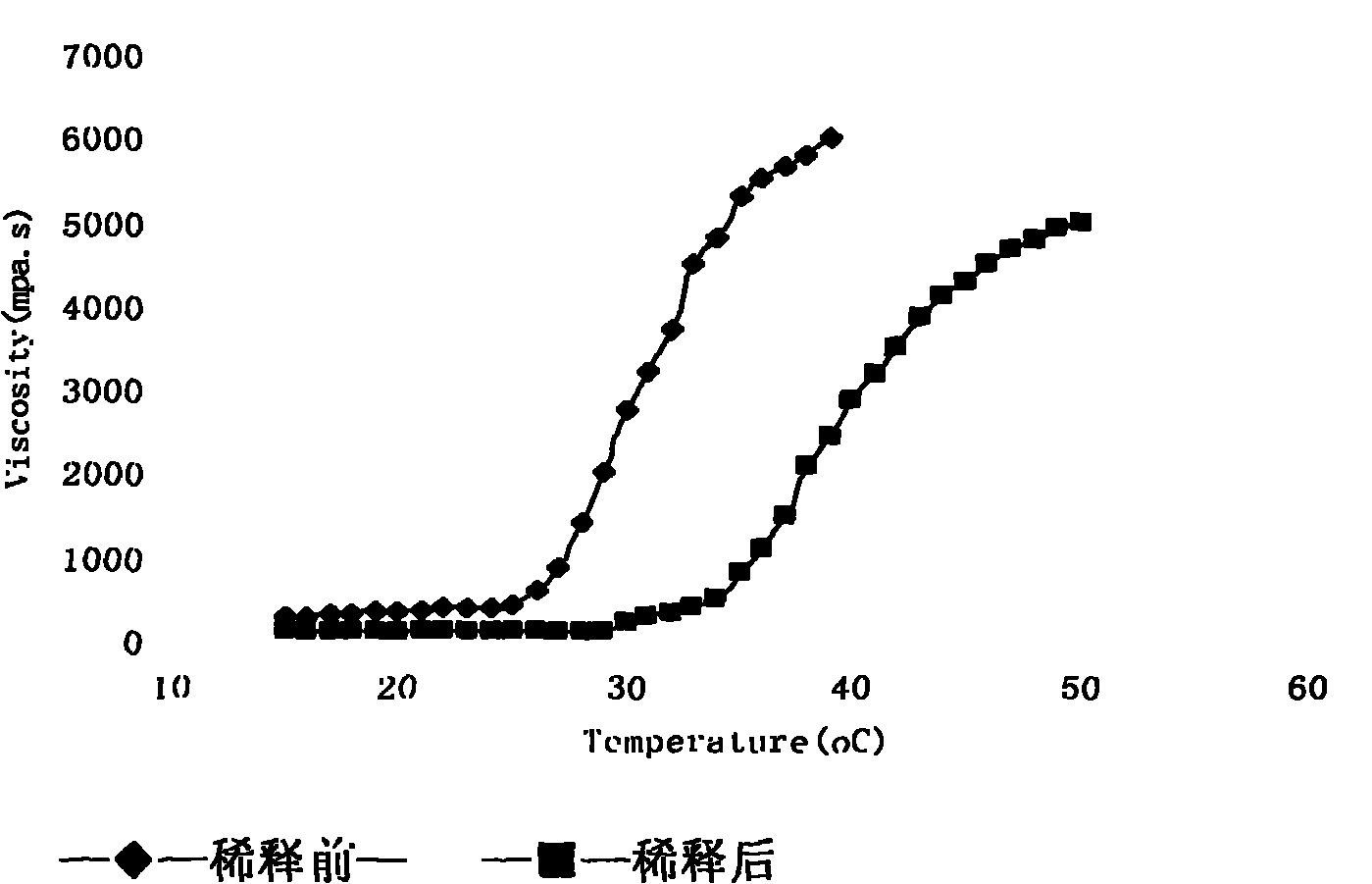
Preparation and application of gatifloxacin temperature and pH sensitive ophthalmic gel
InactiveCN101966143AExtended stayImprove bioavailabilityOrganic active ingredientsSenses disorderPhase variationCurative effect
The invention relates to the technical field of medicaments and a new gatifloxacin ophthalmic formulation, in particular to the preparation and application of gatifloxacin temperature and pH sensitive ophthalmic gel. The invention is characterized in that: the formulation is solution in a non-physiological state and is converted into gel in a physiological state through phase variation. The temperature sensitive ophthalmic gel is prepared by performing systematic observation on an auxiliary material and using poloxamer 407 and poloxamer 188 in a proper ratio. The pH sensitive ophthalmic gel is prepared by regulating the using amount of poloxamer 980 and hydroxypropyl methyl cellulose (HPMC). Due to the adoption of the formulation, the detention time of the medicaments on eyes is greatly prolonged, the bioavailability is enhanced and the curative effect is improved; and the formulation has ideal application prospects.
Owner:胡容峰
Gatifloxacin-containing aqueous liquid preparation
InactiveUS20090247543A1Improve intraocular penetrationImprove permeabilityAntibacterial agentsOrganic active ingredientsAdditive ingredientPhosphoric acid
There is provided an aqueous liquid preparation comprising Gatifloxacin or a pharmacologically acceptable salt thereof or a hydrate thereof, phosphoric acid or a salt thereof, and xanthan gum, wherein a pH thereof is 5.5 or more and less than 7.0. The aqueous liquid preparation has improved intraocular penetration of Gatifloxacin. Further, the formation of a precipitate during storage at a lower temperature and at the time of freezing and thawing of the aqueous liquid preparation is suppressed by incorporating at least one of the ingredient selected from the group consisting of nicotinamide, caffeine, methylglucamine, methyl parahydroxybenzoate and a salt thereof into the aqueous liquid preparation.
Owner:KYORIN PHARMA CO LTD
Ophthalmic medicine-carried amnion and preparation method thereof
InactiveCN102166376AThe content is easy to controlStable contentOrganic active ingredientsSenses disorderWound healingSide effect
The invention provides an ophthalmic medicine-carried amnion which comprises fibrin glue, fresh amnion or preserved amnion. The ophthalmic medicine-carried amnion is further characterized by comprising gatifloxacin-carried chitosan nanoparticles. A preparation method of the ophthalmic medicine-carried amnion comprises the steps of: preparing gatifloxacin-carried chitosan nanoparticles suspension liquid, preparing gatifloxacin-carried chitosan nanoparticles dry powder and preparing medicine-carried amnion. The gatifloxacin, the chitosan nanoparticles, the fibrin glue and the amnion can mutually play a role in synergetic beneficiation and multiple slow release, and can prolong the release of the contained medicine, so that the medicine is more durable in local effective concentration after the use of the amnion, and the medicine can not be frequently used; and the invention has the advantages of being low in allergic reaction rate, good in histocompatibility, free of side effects, capable of being absorbed gradually, healing the wound and filling in gaps, good for healing the wound, and capable of withdrawing a medicine slow release carrier without operation and the like.
Owner:CHONGQING MEDICAL UNIVERSITY
Gatifloxacin gels foreye and its preparing method
InactiveCN1562033AExtended stayImprove bioavailabilityOrganic active ingredientsSenses disorderMedicineGlycerol
Owner:深圳市华晖生物科技有限公司
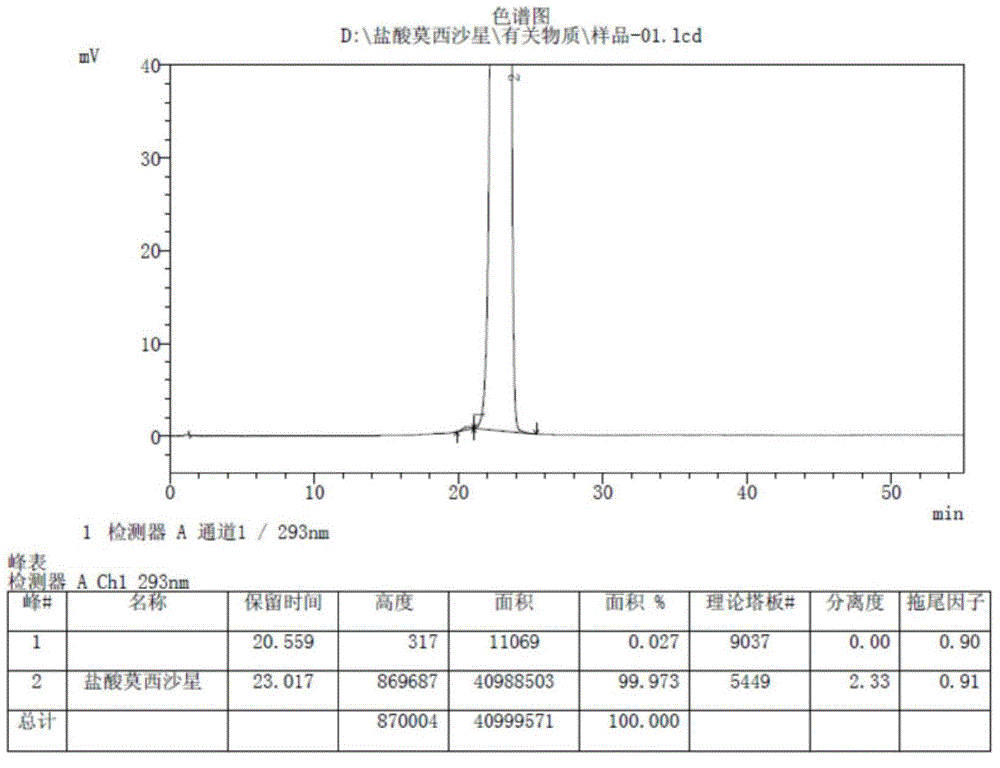
Method for preparing and purifying laevogyrate gatifloxacin
InactiveCN1660838ASimple and fast operationImprove product qualityOrganic chemistryPurification methodsBoric acid
A process for preparing and purifying levogatixacin includes such steps as reaction between cycloester quinolinecarboxylate and boric acid to generate a chelate, reacting on S-(+)-N-methylpiperazine in polar solvent, hydrolyzing and regulating pH value.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Process for preparing compound antituberculous preparation
InactiveCN102920707AReduce exposureImprove bioavailabilityAntibacterial agentsOrganic active ingredientsHydrazoneBULK ACTIVE INGREDIENT
The invention provides a process for preparing a compound antituberculous preparation. Active ingredients are rifampicin and isoniazide and one of mixtures of isoniazide + pyrazinamide and isoniazide + pyrazinamide + gatifloxacin. The process includes that the rifampicin and a part of pharmaceutic adjuvant are sieved and mixed to be subjected to dry granulating, or rifampicin and any other active ingredients except for isoniazide are mixed with corresponding amount of pharmaceutic adjuvant to be subjected to the dry granulating, then the isoniazide, residual active ingredients and corresponding quantity of pharmaceutic adjuvant are sieved and mixed to be subjected to dry granulating or wet granulating, and finally the residual pharmaceutic adjuvant and two types of granules are mixed for a secondary tabletting to obtain the preparation. The process for preparing the compound antituberculous preparation has the advantages that by means of granulation step by step, the contact between the rifampicin and isoniazide is reduced, the amount of impurity hydrazone generated through reaction between the isoniazide and the rifampicin can be effectively reduced, the bioavailability of the isoniazide and the rifampicin is greatly improved, and the medication effectiveness and safety for mass patients with tuberculosis are guaranteed.
Owner:SHENYANG PHARMA UNIVERSITY
Magnetic carboxylation nano-crystalline cellulose amino-functionalization surface molecularly imprinted polymer
ActiveCN107141406AHigh adsorptionIncrease surface areaOther chemical processesWater contaminantsCelluloseGlycidyl methacrylate
The invention belongs to the technical field of molecularly imprinted polymers, and particularly discloses a magnetic carboxylation nano-crystalline cellulose amino-functionalization surface molecularly imprinted polymer. Magnetic carboxylation nano-crystalline cellulose Fe3O4@CCNs serves as a carrier, ofloxacin OFX serves as template molecules, glycidyl methacrylate GMA serves as a functional monomer, and through a polymerization reaction, the magnetic carboxylation nano-crystalline cellulose amino-functionalization surface molecularly imprinted polymer Fe3O4@CCNs@MIPs is formed. Fluoroquinolone drugs in a water body can be selectively extracted, and include ofloxacin OFX, lomefloxacin LOM, gatifloxacin GAT, sarafloxacin SARA, marbofloxacin MARBO, orbifloxacin OBX, difloxacin DFX, ciprofloxacin CIP, enrofloxacin ENRO, sparfloxacin SPX, and moxifloxacin MFX, the recovery rate reaches 81.2%-93.7%, and the adsorption capacity reaches 33 mg / g after adsorption / elution are repeated seven times.
Owner:ZHEJIANG OCEAN UNIV
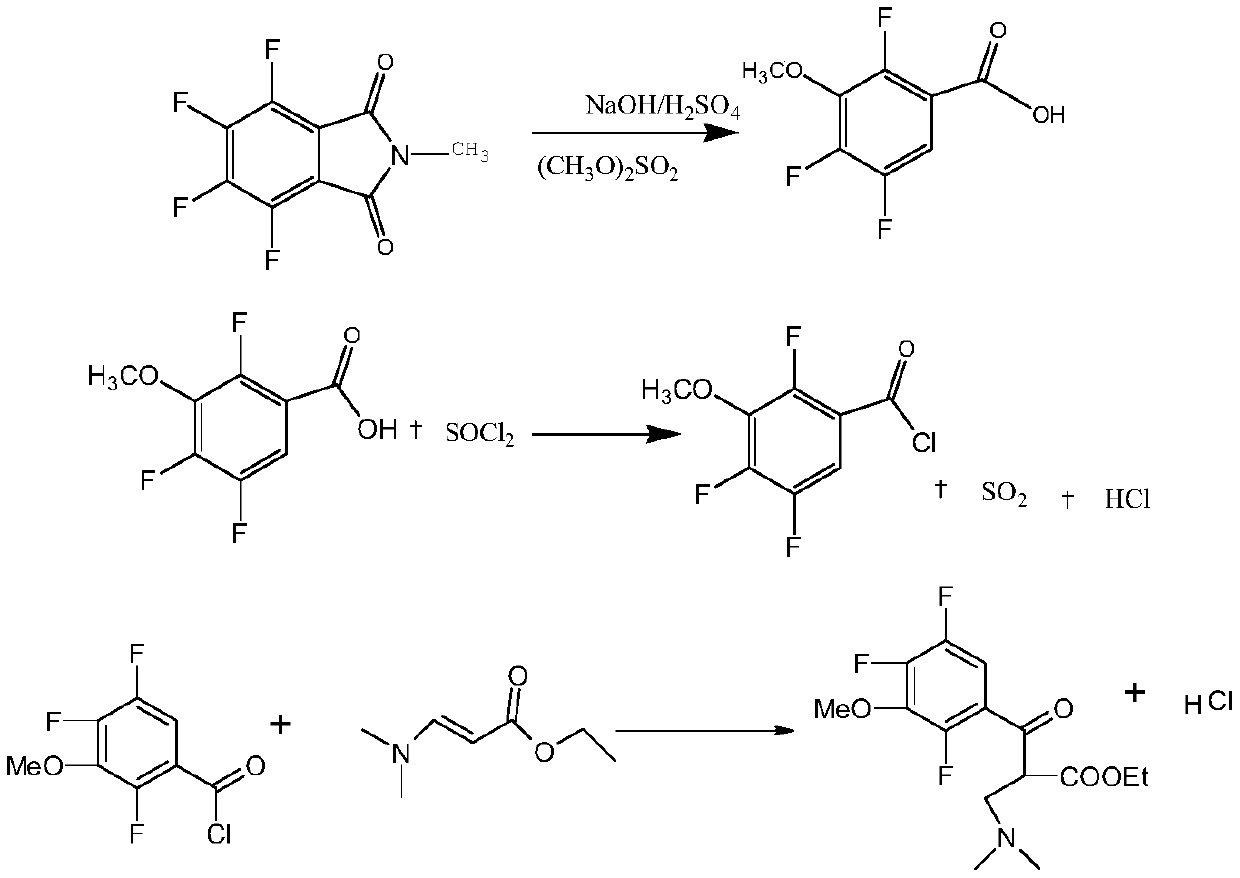
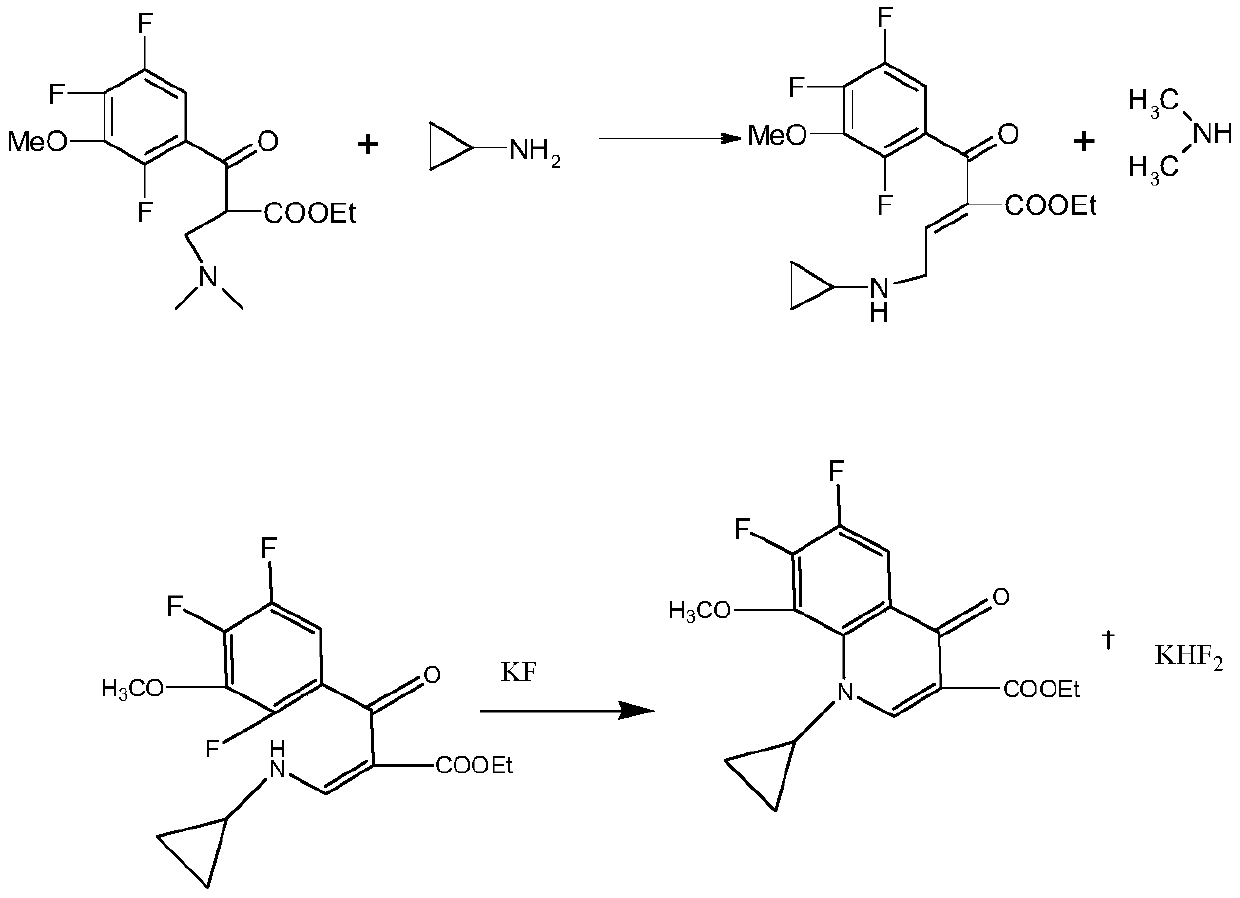
Synthesis method of gatifloxacin cyclic ester
The invention belongs to a production process of a pharmaceutical intermediate, and particularly discloses a synthesis method of gatifloxacin cyclic ester. The synthesis method comprises the followingsteps: performing hydrolysis, decarboxylation and methylation on 3, 4, 5, 6-tetrafluoro-N-methylphthalimide serving as a starting material to obtain 2,4,5-trifluoro-3-methoxybenzoyl chloride, coupling the 2,4,5-trifluoro-3-methoxybenzoyl chloride with N,N-ethyl dimethylaminoacrylate, then replacing with cyclopropylamine, and finally performing cyclization to produce the gatifloxacin cyclic esterunder the action of DMF and potassium fluoride. The reaction route is short, the raw materials are wide in source; furthermore, the reaction conditions are mild and easy to operate and control, whichreduces the consumption of the raw materials, facilitates the post-treatment and reduces the cost; potassium fluoride used instead of potassium carbonate in the reaction can not only reduce productionof exhaust gas, but also make the used potassium fluoride used continuously through adjustment by potassium hydroxide, and the cost is further reduced.
Owner:内蒙古源宏精细化工有限公司
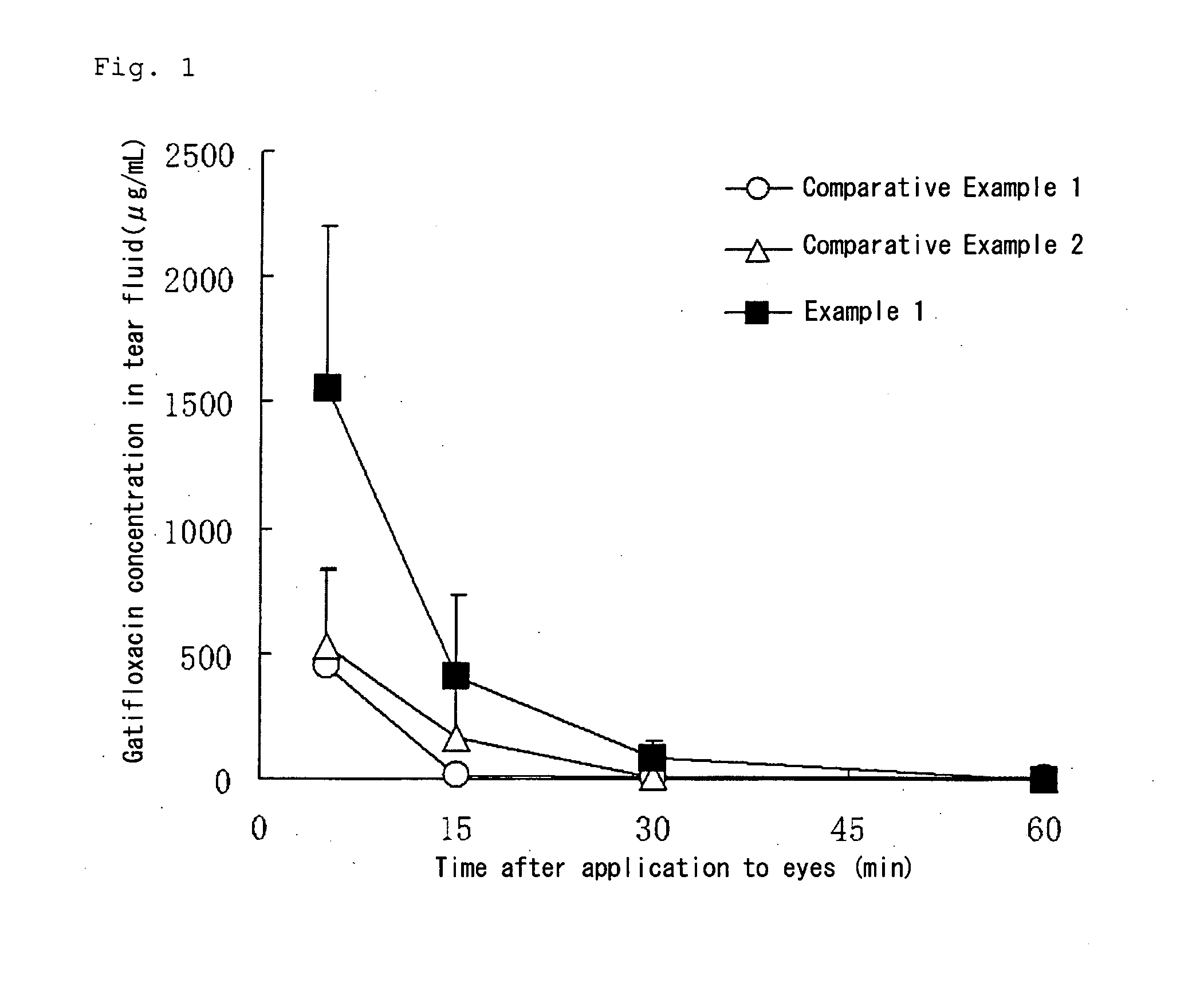
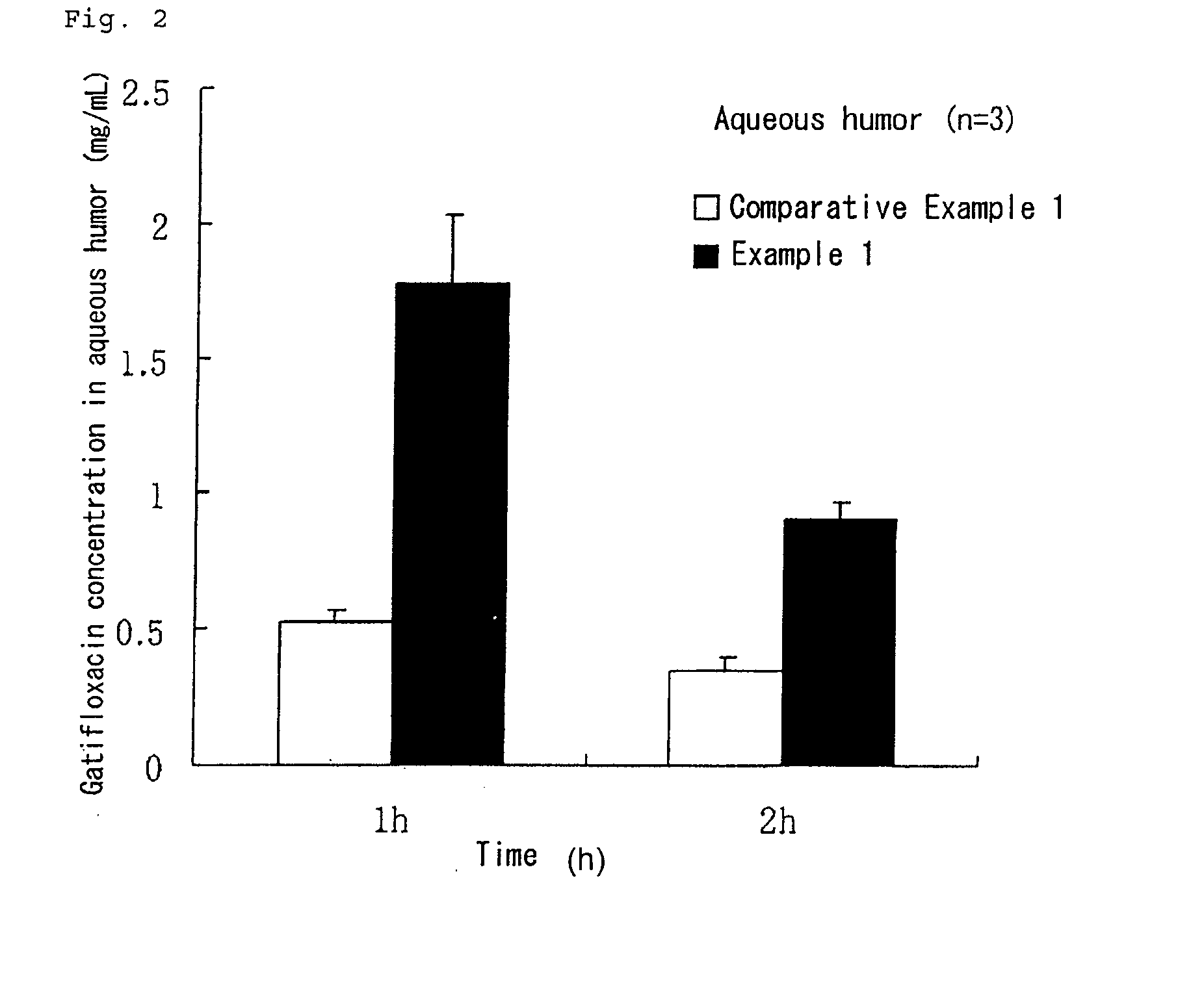
Aqueous liquid preparation having improved intraocular gatifloxacin penetration
InactiveUS20100035894A1Improve permeabilityLow viscosityAntibacterial agentsOrganic active ingredientsConjunctivaAqueous humor
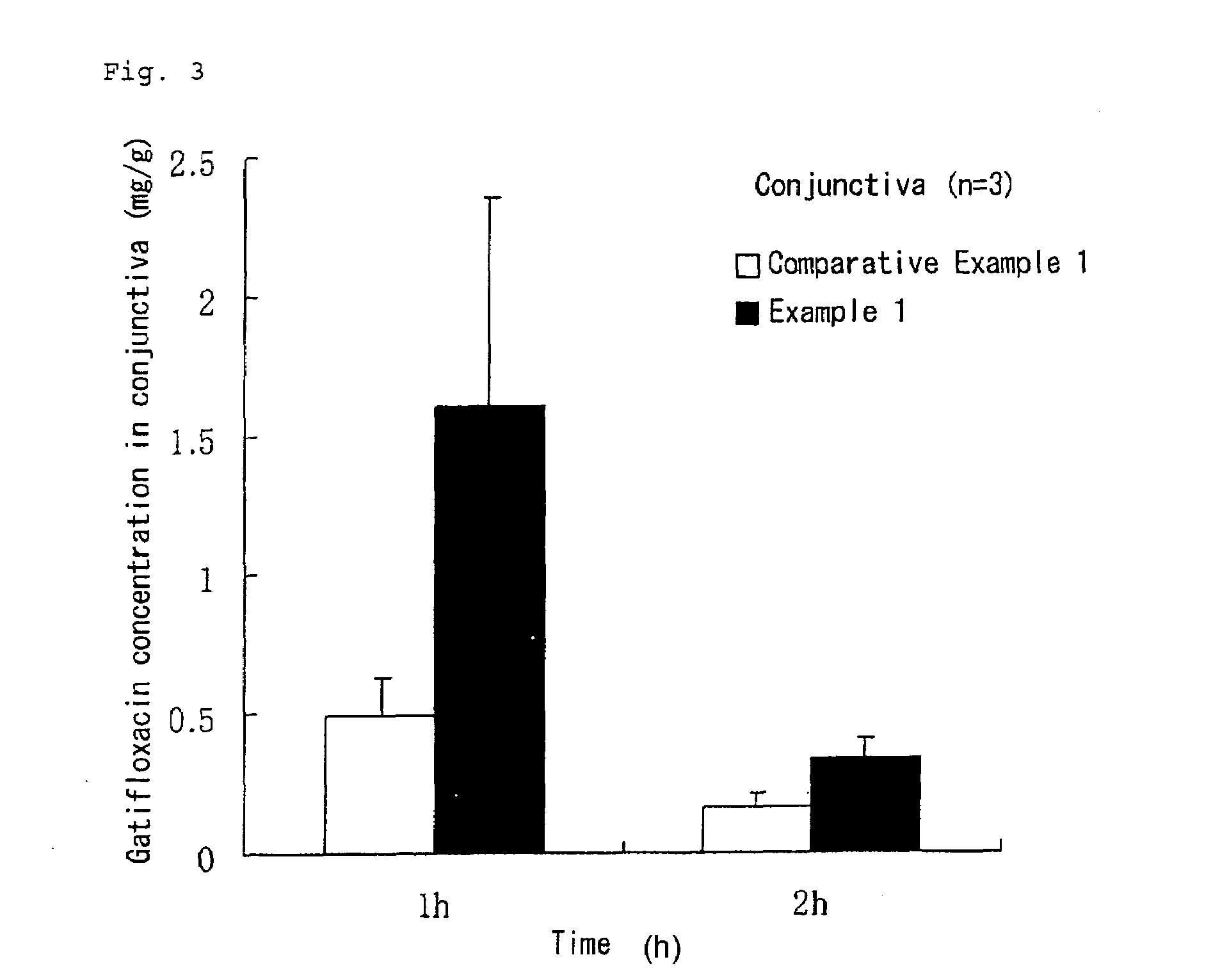
An object is to provide an ophthalmic aqueous preparation excellent in the retention of gatifloxacin in a tear fluid and the preparation of gatifloxacin into an aqueous humor and a conjunctiva. Another object is to prevent the formation of any precipitate and the reduction in viscosity in the aqueous liquid preparation. An aqueous liquid preparation comprising gatifloxacin, a pharmacologically acceptable salt thereof or a hydrate of gatifloxacin or the salt and at least 0.15 w / v % of xanthan gum enables to improve the retention and penetration of gatifloxacin. When at least 0.2 w / v % of sodium chloride is added to the aqueous liquid preparation, the formation of any precipitate and the reduction in viscosity in the aqueous liquid preparation can be prevented.
Owner:KYORIN PHARMA CO LTD +1
Compound antitubercular preparation containing gatifloxacin, and preparation method thereof
InactiveCN102198138APromote degradationReduced anti-TB efficacyAntibacterial agentsOrganic active ingredientsCurative effectTherapeutic effect
The invention relates to a new compound antitubercular preparation containing gatifloxacin, and a preparation method thereof. The compound antitubercular preparation comprises rifampicin, isoniazide, pyrazinamide and gatifloxacin. Each unit of preparation contains 100-150mg of rifampicin, 60-120mg of isoniazide, 200-400mg of pyrazinamide and 100-200mg of gatifloxacin. The addition of gatifloxacin in the preparation can shorten the course of treatment of tuberculosis and improve the treatment effect; and degradation of the rifampicin can be reduced through dry method granulating tabletting, thus improving the bioavailability of rifampicin and improving the curative effect of the medicament. The compound preparation can be prepared into common tablets, double-layer coated-core tablets or pellet-core tablets.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of moxifloxacin hydrochloride and intermediate of moxifloxacin hydrochloride
InactiveCN110194767AEfficient removalRemove basicOrganic compound preparationOrganic chemistry methodsNonaneFiltration
The invention provides a preparation method of moxifloxacin hydrochloride and an intermediate of moxifloxacin hydrochloride, and belongs to the technical field of heterocyclic compound. The preparation method comprises following steps: gatifloxacin intermediate, (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane, a reaction solvent, an organic base, and a Lewis acid are mixed, are reacted fully at a preset temperature, and are subjected to cooling filtering, an obtained filtrate is heated, L-(+)-tartaric acid is added, thermal insulation crystallization is carried out, cooling is carried out, centrifugation filtration, washing, and drying are carried out so as to obtain moxifloxacin ethyl ester tartrate; moxifloxacin ethyl ester tartrate is added into a hydrogen chloride containing solution, heatingis carried out, thermal insulation full reaction is carried out, crystallization is carried out, after cooling, centrifugation filtration, beating, and baking are carried out so as to obtain finishedproducts. According to the preparation method, one-pot reaction is realized, the selectivity and conversion rate are higher than those of disclosed methods, energy is saved, post-treatment is convenient, the preparation method is suitable for industrialized production, and HPLC>99.9%.
Owner:ZHEJIANG GUOBANG PHARMA
Preparation method for compound tilmicosin enteric-coated granules
ActiveCN104586875AIncrease payEffective infectionAntibacterial agentsOrganic active ingredientsOral glucoseDisease
The invention belongs to the technical field of veterinary medicines, and specifically relates to a preparation method for compound tilmicosin enteric-coated granules. The preparation method sequentially comprises the steps of: preparing materials, mixing the materials, granulating, preparing a coating solution, coating and the like, wherein the raw materials of the granules are tilmicosin, colistin, gatifloxacin, a sweetening agent, a flavouring agent, bromhexine hydrochloride, oral glucose, corn starch, saccharose powder, dextrin, talcum powder and starch slurry; the raw materials of the coating solution are HPMC, Tween-80, polyethylene glycol 6000, talcum powder and ethanol. The method disclosed by the invention enables the tilmicosin effective ingredient not to be broken in gastric acid, and to safely arrive at intestinal tracts to be absorbed; the obtained compound tilmicosin enteric-coated granules are capable of preventing and treating respiratory diseases such as mycoplasma diseases, pleuropneumonia and swine plague, capable of reducing the occurrences of bacterial diseases such as diarrhoea and salmonellosis, and capable of increasing the feed conversion.
Owner:ZHENGZHOU DOURIN VETERINARY TECH
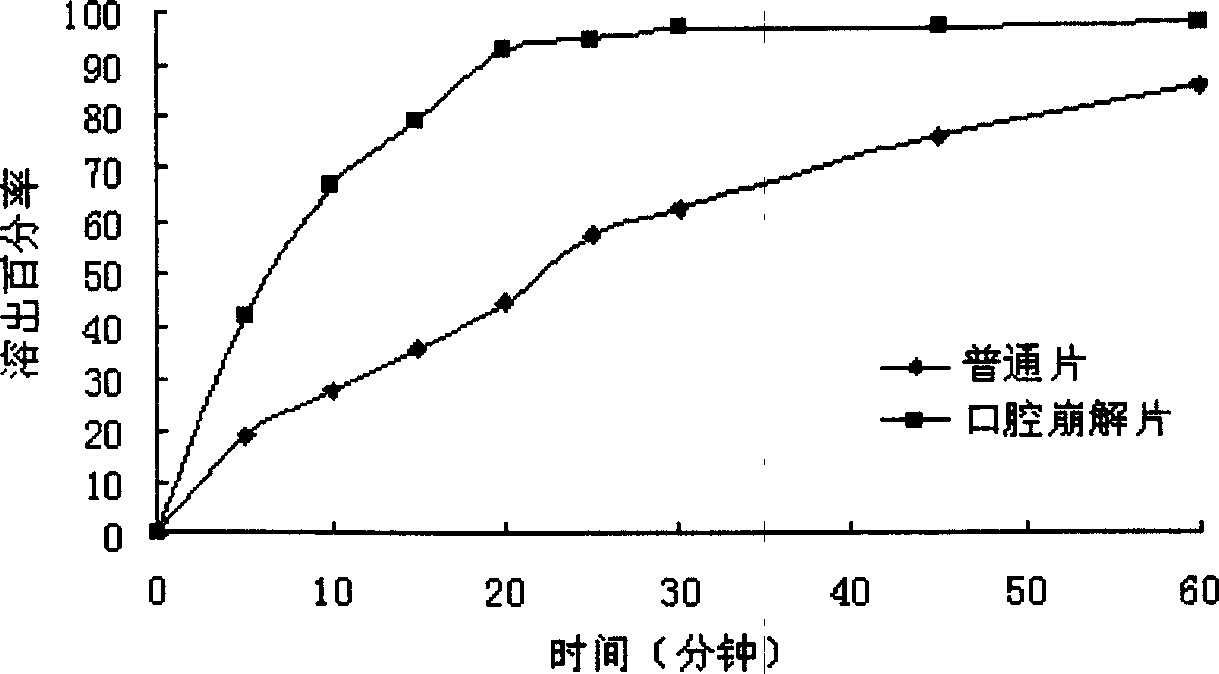
Oral gatifloxacin disintegrant and its preparing process
InactiveCN1857228APromote dissolutionQuickly exert the therapeutic effect of the whole bodyAntibacterial agentsOrganic active ingredientsAdhesiveDentistry
The present invention provides an oral gatifloxacin disintegrant and its preparation process. The oral gatifloxacin disintegrant contains gatifloxacin in 20-75 wt% and medicinal supplementary material in 25-80 wt%, and the medicinal supplementary material includes one or several of disintegrating agent, stuffing, wetting adhesive, lubricant and coating material. The preparation process of the oral gatifloxacin disintegrant includes coating medicine powder or medicine carrying micro pill and tabletting. The oral gatifloxacin disintegrant of the present invention has the advantages of fast medicine release, increased absorbing points and less local excitation on gastrointestinal tract and is suitable for taking without using water and by patient with dysphagia.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
Capsules of gatifloxacin, and technique of dry process
InactiveCN1872065AAvoid decompositionDisintegrates quicklyAntibacterial agentsOrganic active ingredientsCurative effectMagnesium stearate
A galtixacin capsule able to quickly take its high curative effect is prepared from galtixacin, filler, carboxyrnethyl starch sodium, magnesium stearate, etc through mixing galtixacin with filler and disintegrant, granulating, mixing with lubricant, filling the particles in capsules and aluminum-plastic packing.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
Ophthalmic gel of gatifloxacin and preparation method thereof
ActiveUS20130040960A1Reduced bioavailabilityPoor patient complianceAntibacterial agentsOrganic active ingredientsSodium hyaluronateGatifloxacin
An ophthalmic gel of gatifloxacin and the preparation method thereof are disclosed. The gel comprises gatifloxacin or its pharmaceutical salts, matrix and water. Said matrix is one or more selected from carbomer, mixture of carbomer and HPMC, and sodium hyaluronate.
Owner:SHENYANG XINGQI PHARM CO LTD
Production method of moxifloxacin hydrochloride
InactiveCN104860944ALow priceQuality improvementOrganic chemistryEthyl esterMoxifloxacin hydrochloride
The invention discloses a production method of moxifloxacin hydrochloride. The method comprises the following steps: with an intermediate 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid ethyl ester of gatifloxacin and (S,S)-2,8-diazacyclo bicycle[4,3,0] nonane as initial materials, chelating, condensing, and salifying to prepare the moxifloxacin hydrochloride. The production method has the beneficial effects that the production technology is relatively mild, easy to operate, free of a special requirement on equipment, relatively high in yield, and suitable for industrialized mass production.
Owner:INNER MONGOLIA NORTHEAST NO 6 PHARMA GRP CO LTD
Gatifloxacin ear drop and its prepn process
InactiveCN1931170AImprove Medication AdherenceAvoid drug resistanceAntibacterial agentsOrganic active ingredientsMedicineWhole body
The gatifloxacin ear drop is prepared with gatifloxacin or its salt as the main component and the supplementary materials including glycerin, ethanol and sterilized water for injection, pure water or distilled water. The present invention also discloses the preparation process of the gatifloxacin ear drop. As one fourth generation oxyquinolone type antibacterial medicine, the gatifloxacin ear drop has high bioavailability, reduced medicine taking times, less systemic untoward reaction, high patient's compliance and less drug resistance.
Owner:王廷春
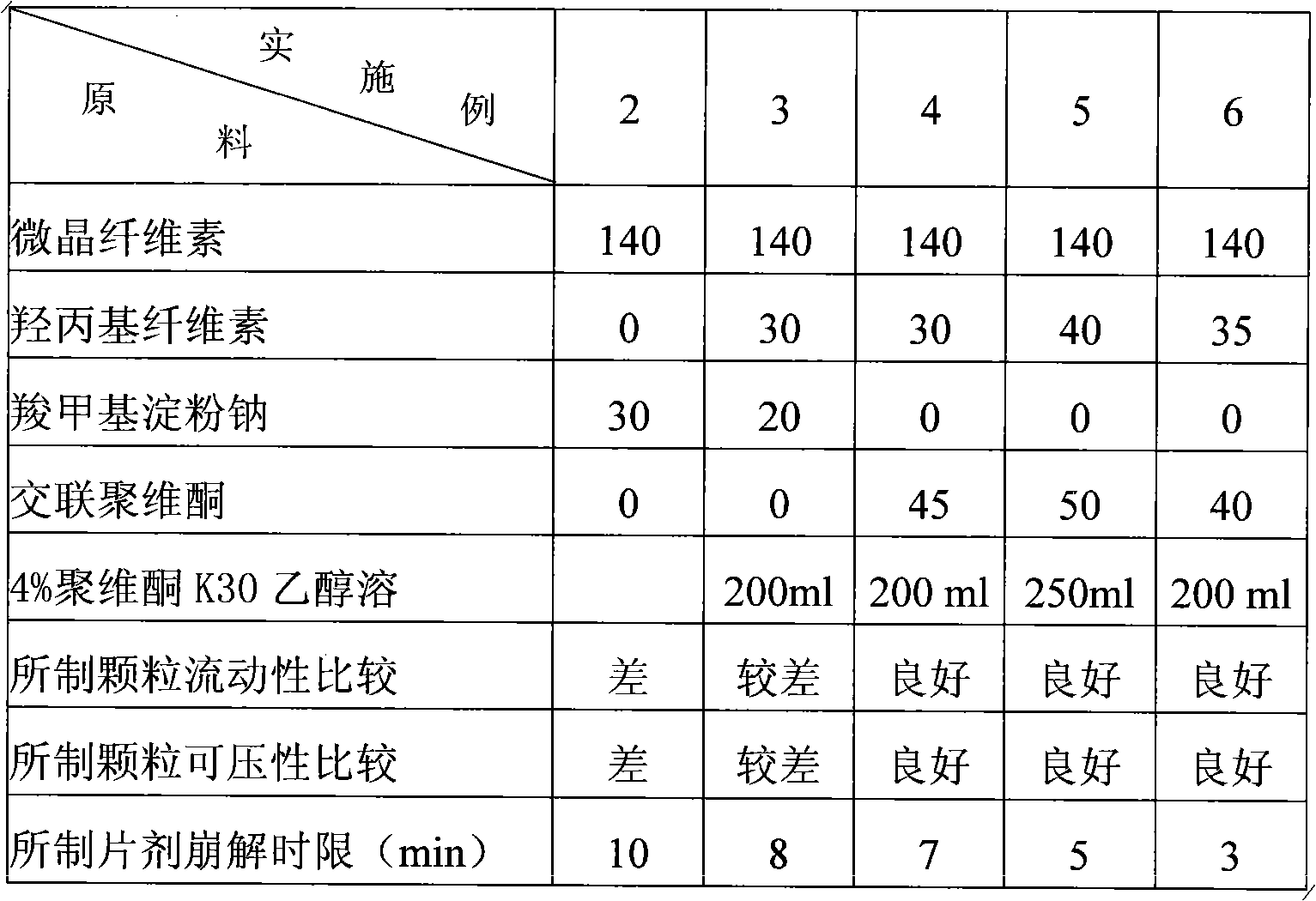
Gatifloxacin dispersible tablets and preparation method thereof
The invention discloses gatifloxacin dispersible tablets and a preparation method thereof. The dispersible tablets are prepared from the following raw materials and auxiliary materials in part by weight: 200 to 260 parts of gatifloxacin, 40 to 50 parts of crospovidone, 140 to 180 parts of microcrystalline cellulose, 30 to 40 parts of hydroxy propyl cellulose, 7 to 10 parts of stevia, 4.5 to 8 parts of magnesium stearate, and a proper amount of ethanol solution of povidoneK30; and the preparation method comprises the following steps of: taking the gatifloxacin, the microcrystalline cellulose, the hydroxy propyl cellulose, the stevia and the crospovidone according to the weight part ratio, respectively sieving by using a 80-mesh sieve, sieving by using a 60-mesh sieve, uniformly mixing, and preparing into a soft material by using the 4 percent ethanol solution of povidoneK30; sieving by using a 30-mesh sieve for granulation, performing forced air drying at the temperature of between 55 and 60 DEG C, and finishing the dry granules by using a 24-mesh sieve; and adding the crospovidone and the magnesium stearate according to the prescription, mixing, and tabletting. The tablets provided by the invention have good disintegration effect; and the granules prepared by the method have high fluidity and compressibility.
Owner:华北制药集团制剂有限公司
Compositions and Methods for Treating Eyes and Methods of Preparation
InactiveUS20180318319A1Patient compliance is goodMinimize the numberOrganic active ingredientsSenses disorderPhenylephrine hclBromfenac
Pharmaceutical compositions, methods for treating various issues of the eyes, and methods of preparing such compositions are described. These pharmaceutical compositions may be for treating glaucoma, in preparation of eye surgery, during eye surgery, various post-op care (e.g., after cataract surgery, laser eye surgery, and the like), for treating dry eyes, and / or for promoting eyelash growth. These pharmaceutical compositions may comprise such active ingredients (APIs) as: timolol, latanoprost, brimonidine tartrate, dorzolamide, moxifloxacin HCl, dexamethasone PO4, phenylephrine HCl, lidocaine HCl, ketorolac tromethamine, bromfenac, prednisolone PO4, gatifloxacin, amniotic cytokine extract (ACE), prostaglandin E2 (PGE2), and combinations thereof.
Owner:OCULAR SCI INC
Anti-quinolone antibiotic class specific monoclonal antibody hybridoma cell strain YH6 and application thereof
ActiveCN106520704AHigh sensitivityBiological material analysisMicroorganism based processesOrbifloxacinEnzyme linked immunoassay
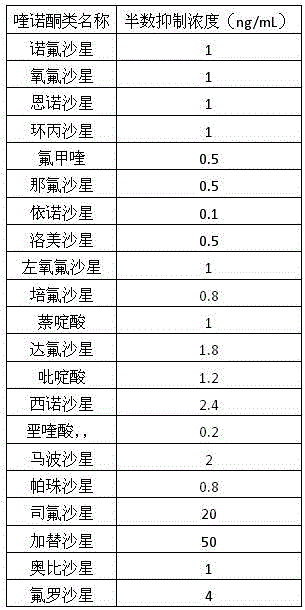
An anti-quinolone antibiotic class specific monoclonal antibody hybridoma cell strain YH6 and an application thereof belong to the technical field of immunochemistry. The monoclonal cell strain YH6 is preserved in China General Microbiological Culture Collection Center with the preservation number of CGMCC No.12024. A monoclonal antibody secreted by the YH6 is detected by indirect competitive enzyme-linked immunosorbent assay, and has cross reaction with the following 21 pyrethroids: norfloxacin, ofloxacin, enrofloxacin, ciprofloxacin, flumequine, nafloxacin, enoxacin, lomefloxacin, levofloxacin, pefloxacin, nalidixic acid, danofloxacin, pyridine acid, cinoxacin, oxolinic acid, marbofloxacin, pazufloxacin, sparfloxacin, gatifloxacin, orbifloxacin and fleroxacin, and the IC50 value of the monoclonal antibody is 0.1-50 ng / mL. The class specific monoclonal antibody can be used for developing colloidal gold immunochromatographic test strips and immunosensors, provides a raw material for the immunodetection of quinolone antibiotic residues in foods, and has practical application values.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com