Compositions and Methods for Treating Eyes and Methods of Preparation
a technology for eyes and compositions, applied in the field of compositions for treating eyes and methods for treating eyes, can solve problems such as increased patient cost and patient compliance, and achieve the effects of improving efficacy, minimal side effects, and increasing patient complian
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
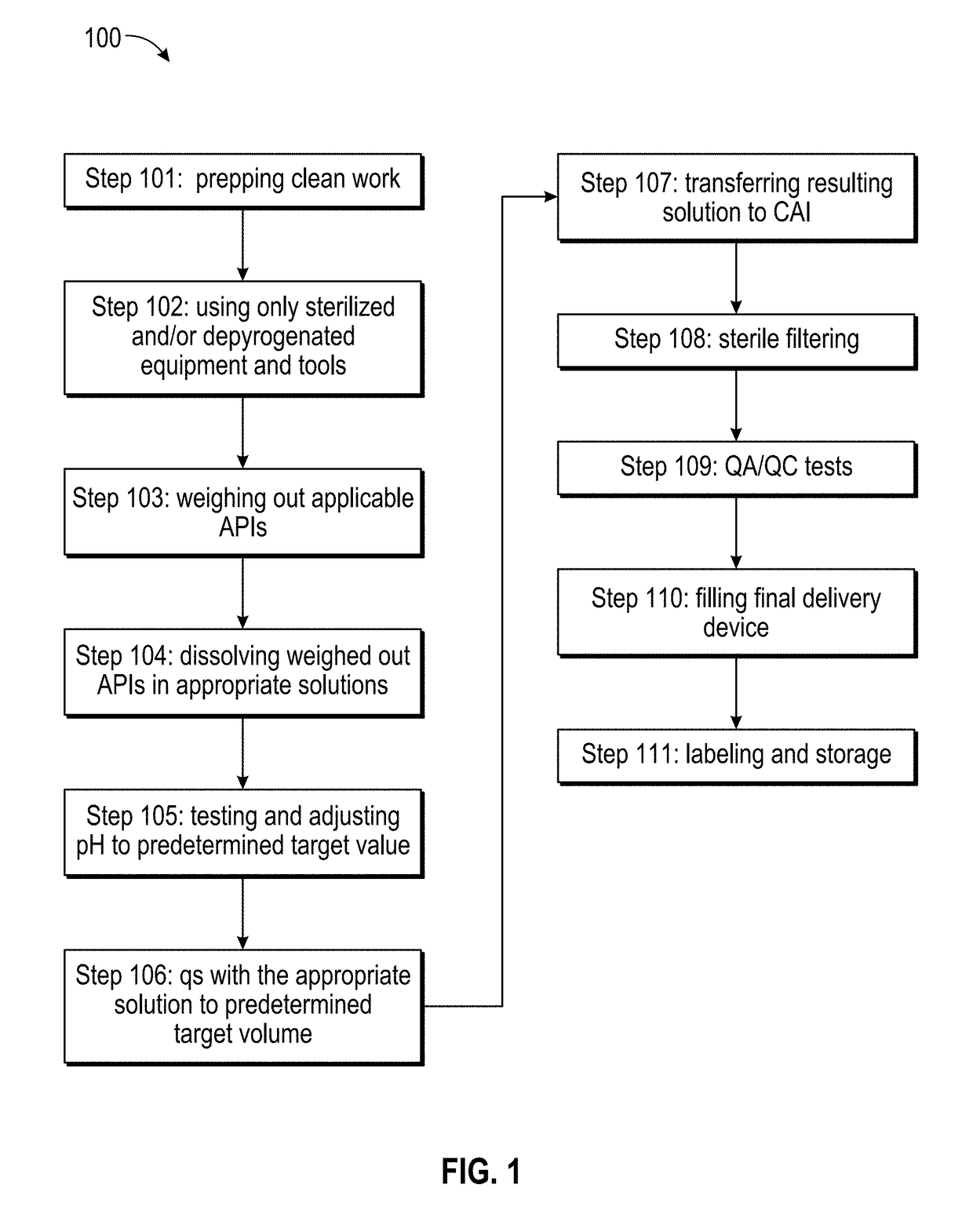
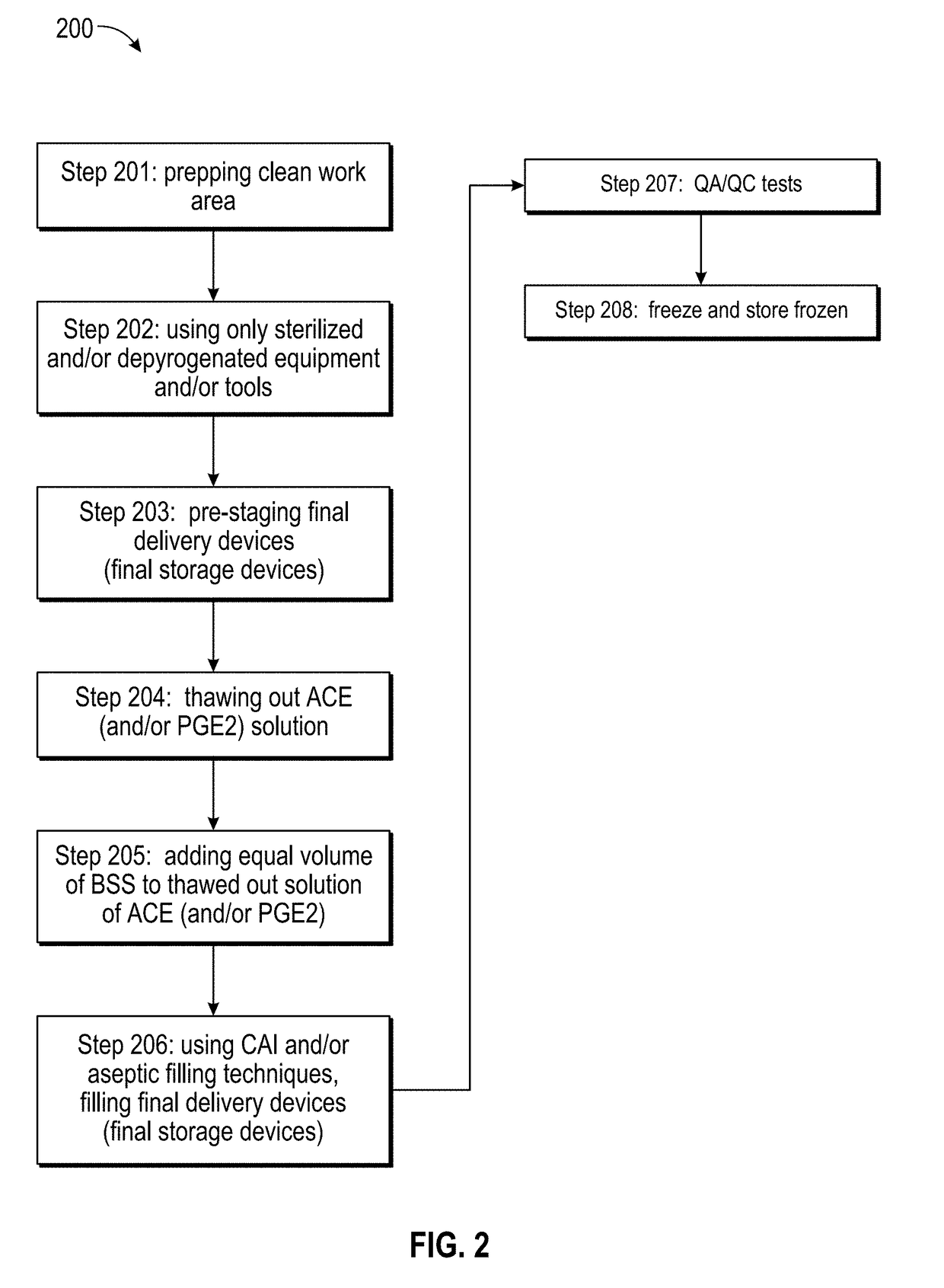
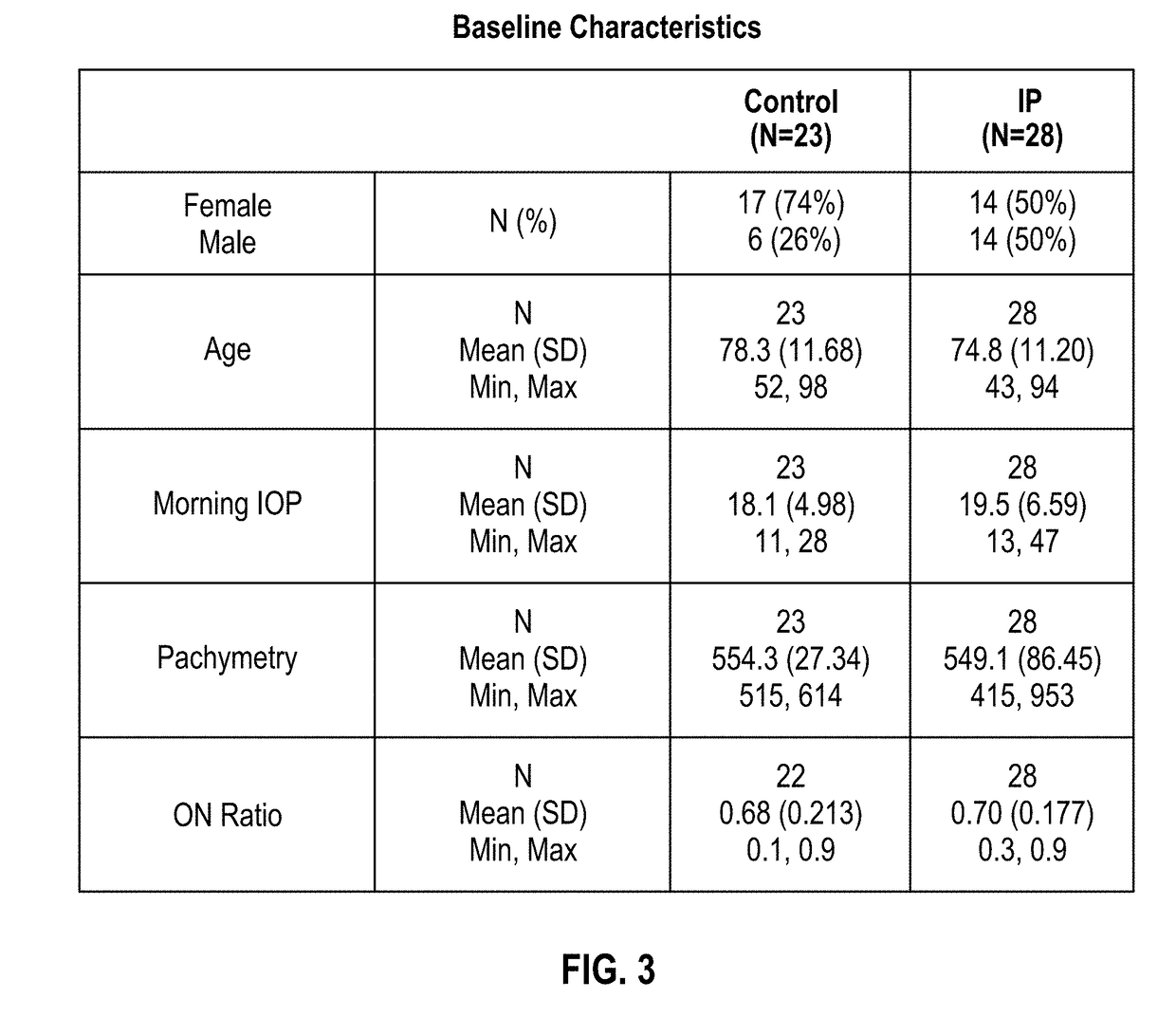
[0039]Below is a table listing at least fifteen separate and distinct pharmaceutical compositions by their respective APIs (active pharmaceutical ingredients), as well as listing at least one treatment purpose and at least one delivery device or method that may be contemplated embodiments of the present invention:
SampleSampleTreatmentDeliveryCompositions of APIsPurposeDevice / Method1Timolol 0.5%, Latanoprostfor treatingeye drops0.005%glaucoma2Timolol 0.5%, Brimonidinefor treatingeye dropsTartrate 0.2%, Dorzolamide 2%glaucoma3Timolol 0.5%, Brimonidinefor treatingeye dropsTartrate 0.2%, Dorzolamide 2%,glaucomaLatanoprost 0.005%4Moxifloxacin HCl 0.5%post-op care afterintra-cameralcataract surgeryinjection5Dexamethasone PO4 0.1%,post-op care afterintra-cameralMoxifloxacin HCl 0.5%cataract surgeryinjection6Dexamethasone PO4 0.1%,post-op care afterintra-cameralMoxifloxacin HCl 0.5%,cataract surgeryinjectionKetorolac Tromethamine 0.5%7Dexamethasone PO4 0.1%,post-op care afterintra-cameralMo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com