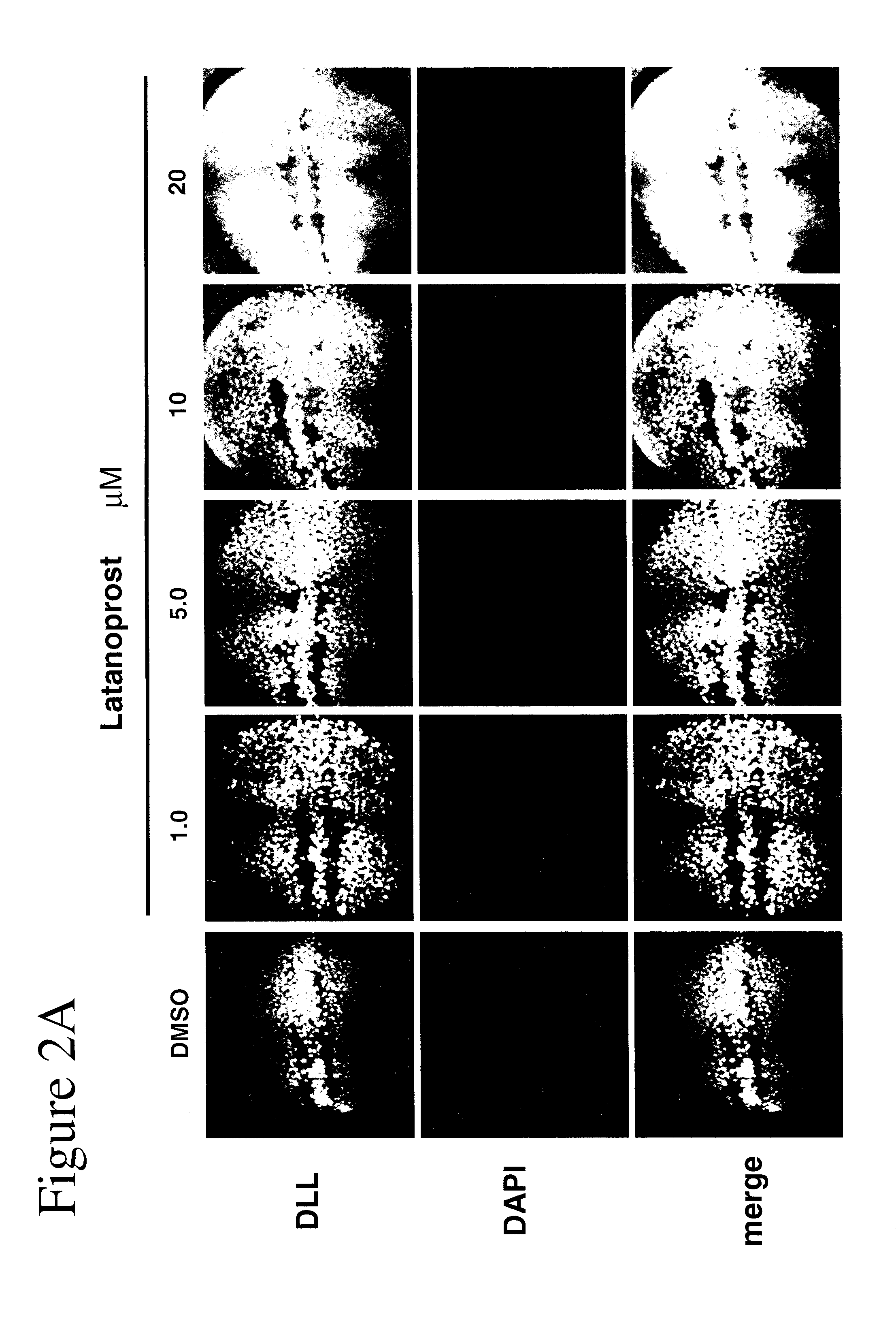
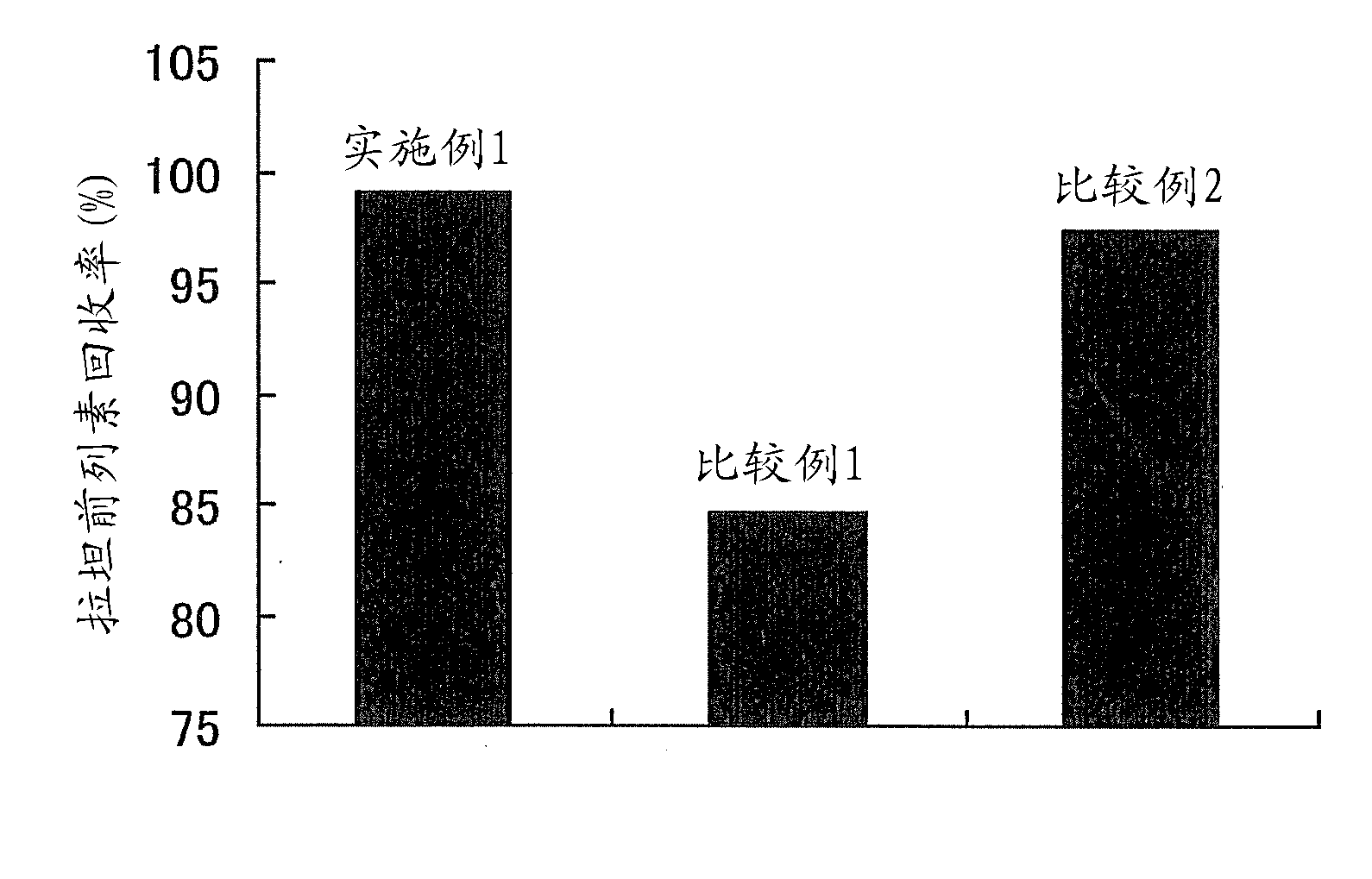
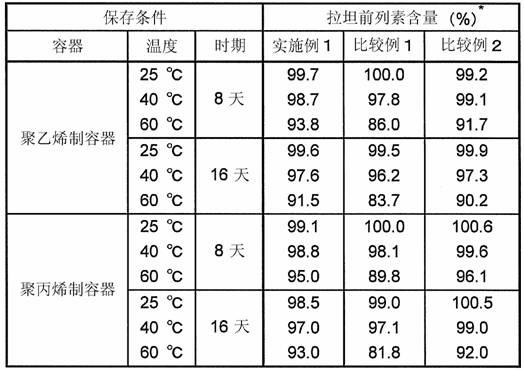
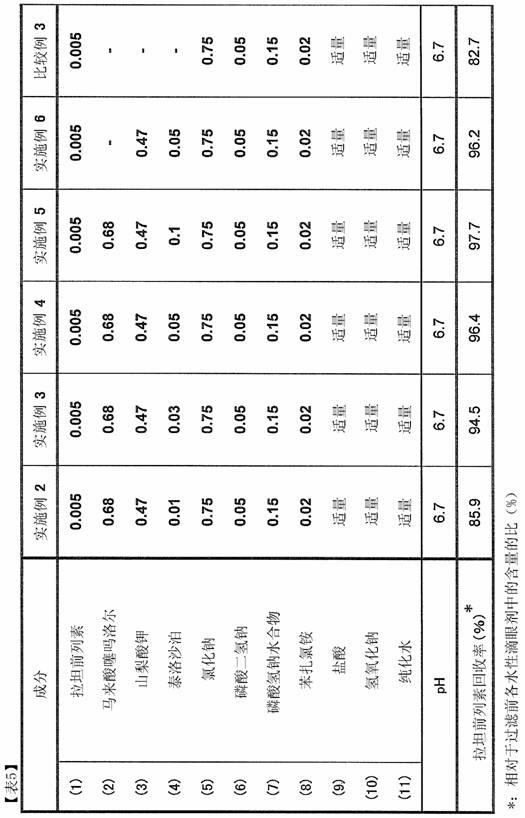
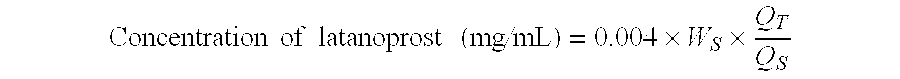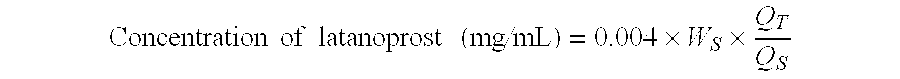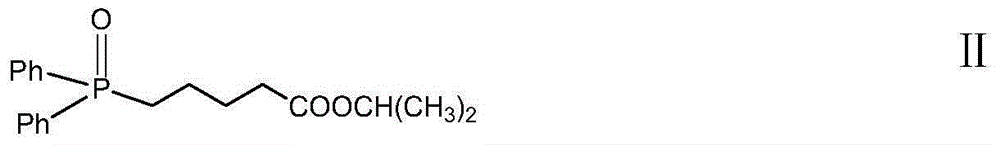Patents
Literature
70 results about "Latanoprost" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
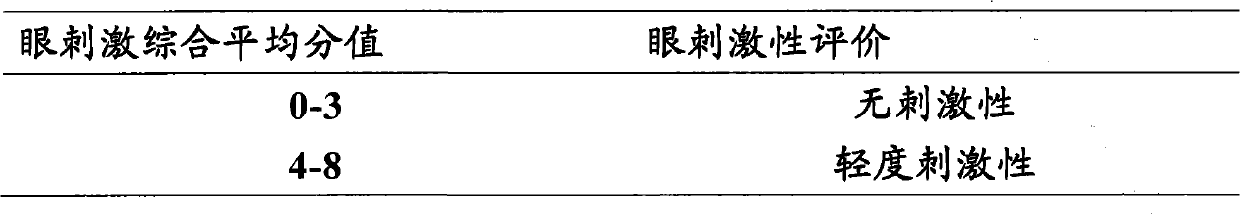
Latanoprost is used to treat high pressure inside the eye due to glaucoma (open angle type) or other eye diseases (e.g., ocular hypertension).
Method for treating ocular hypertension and glaucoma
Provided is a method for treating ocular hypertension and glaucoma with reduced side effects such as keratoconjunctive disorders and macular edema, which comprises administering an ophthalmic composition comprising latanoprost as an active ingredient thereof to a subject in need of said treatment, wherein the ophthalmic composition contains substantially no benzalkonium chloride.
Owner:SUCAMPO USA
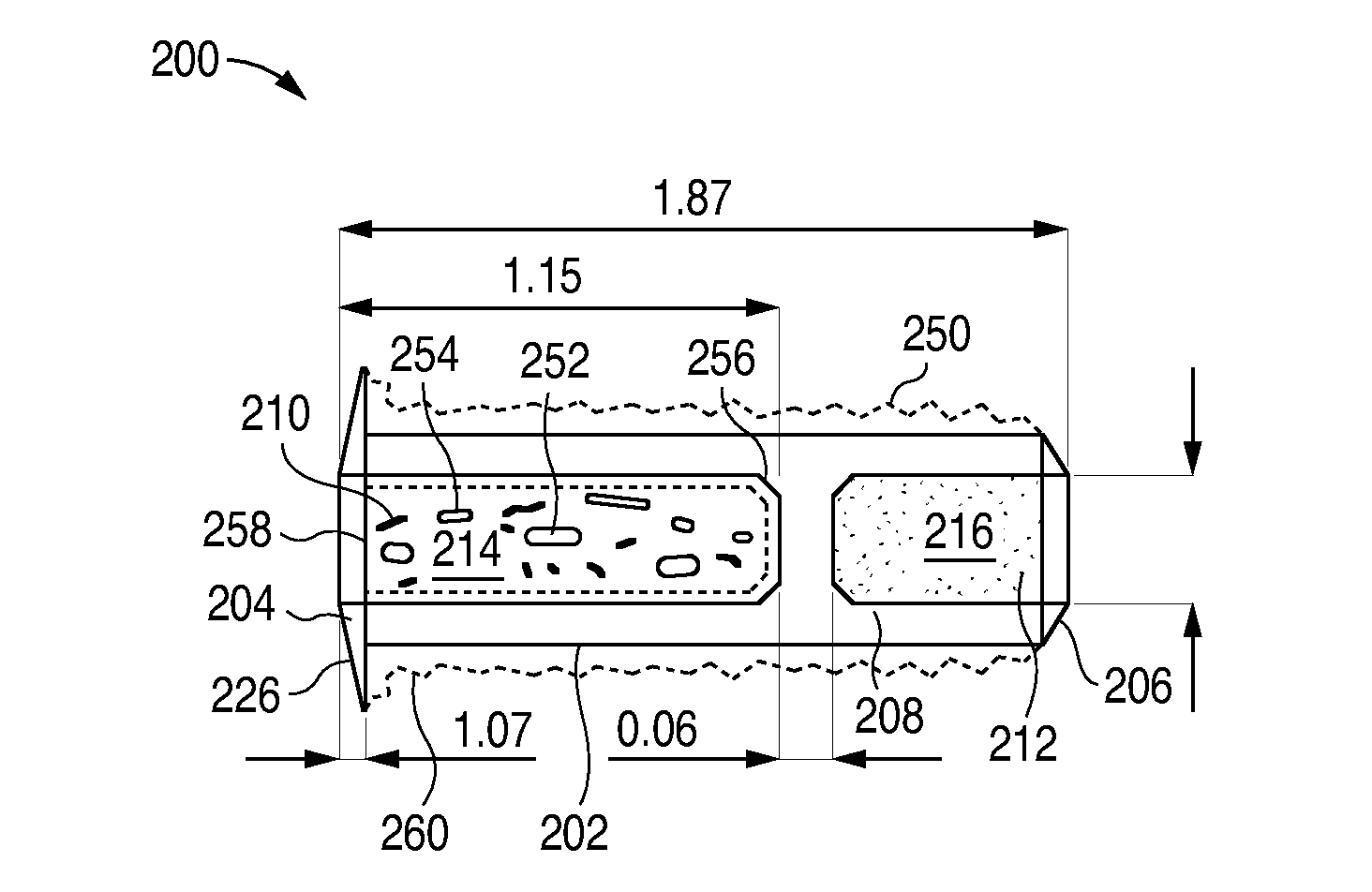
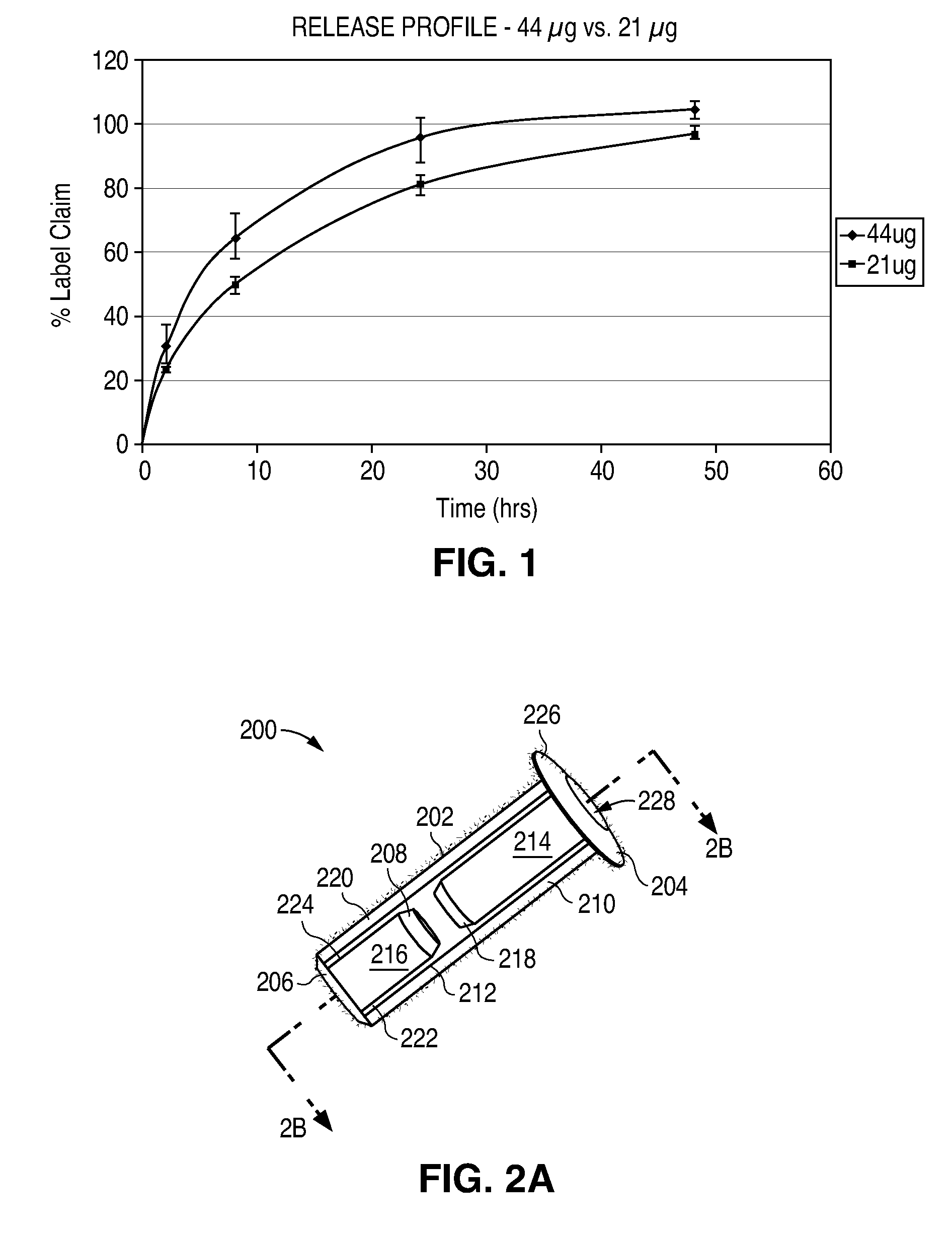
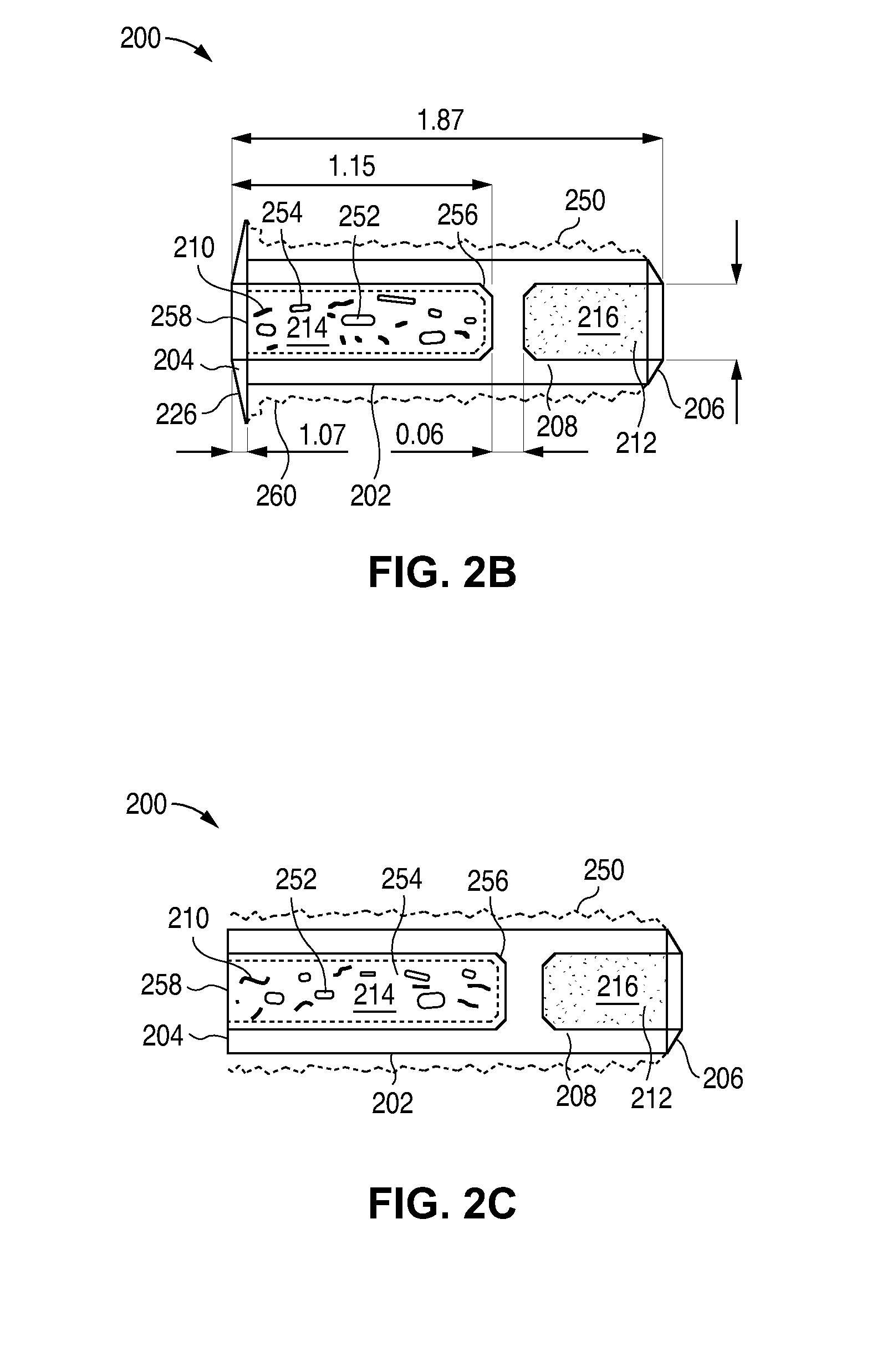
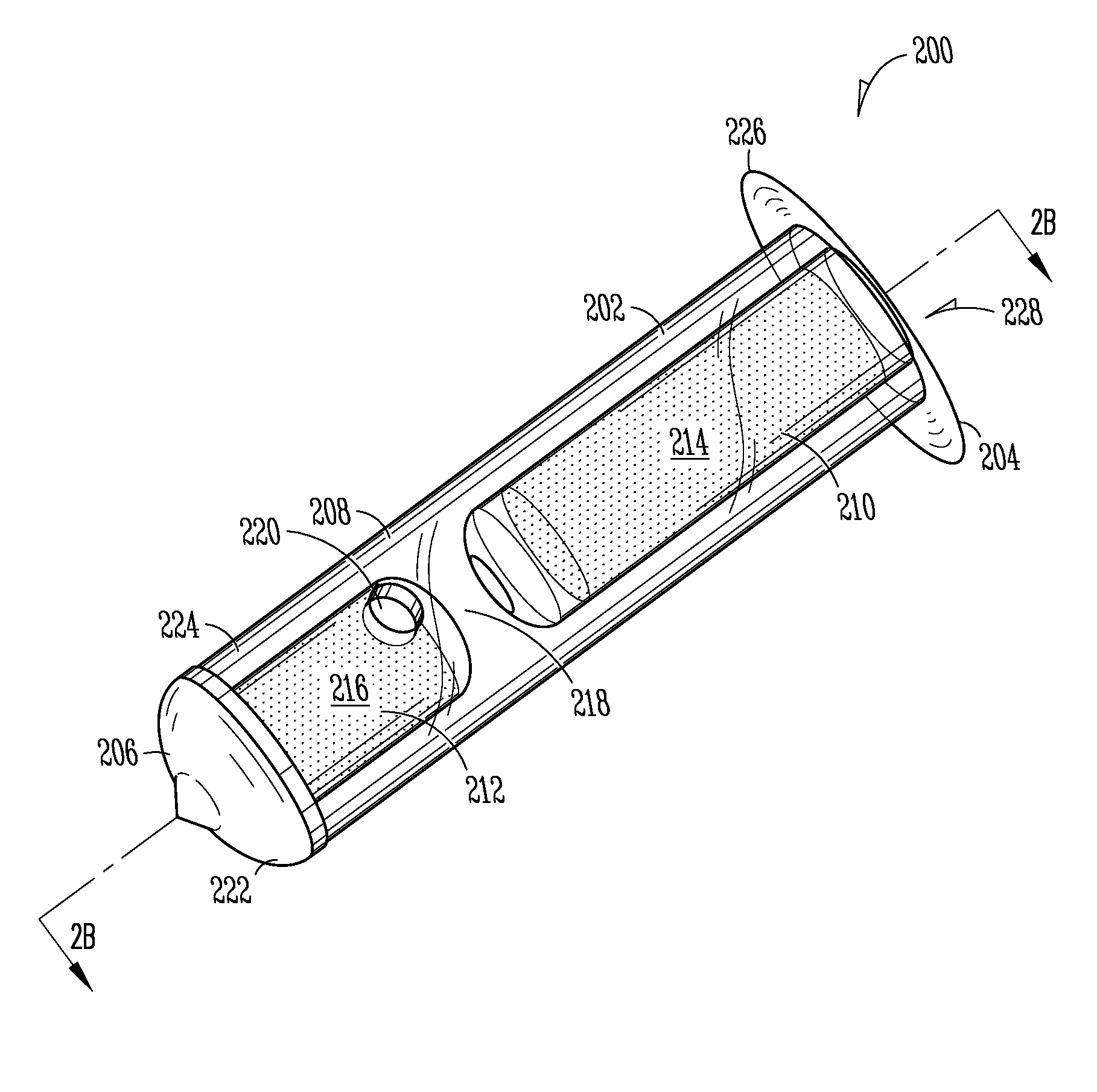
Sustained release delivery of active agents to treat glaucoma and ocular hypertension
ActiveUS20090280158A1Lower eye pressureReduction in patient noncomplianceBiocideSenses disorderLatanoprostActive agent
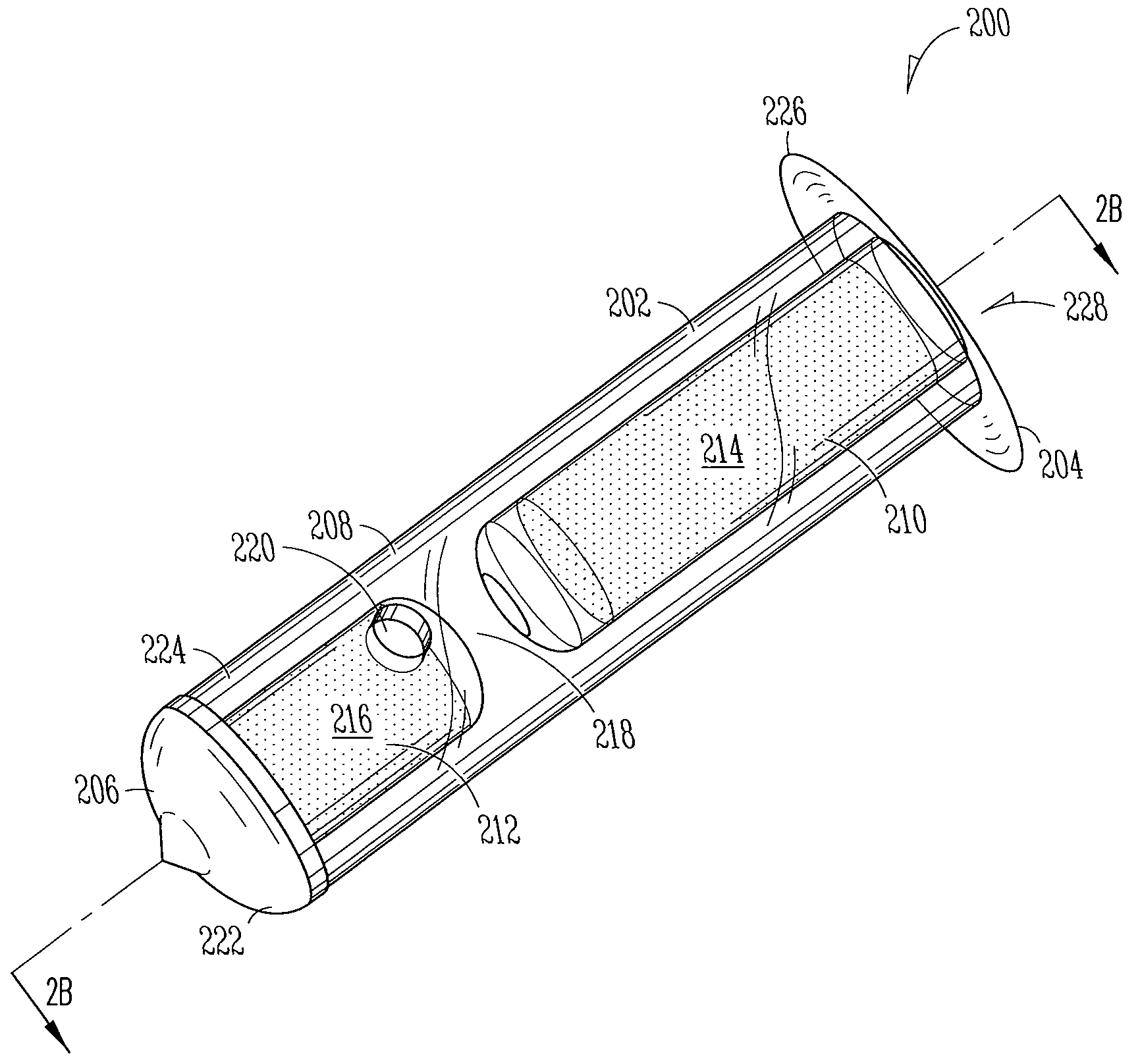
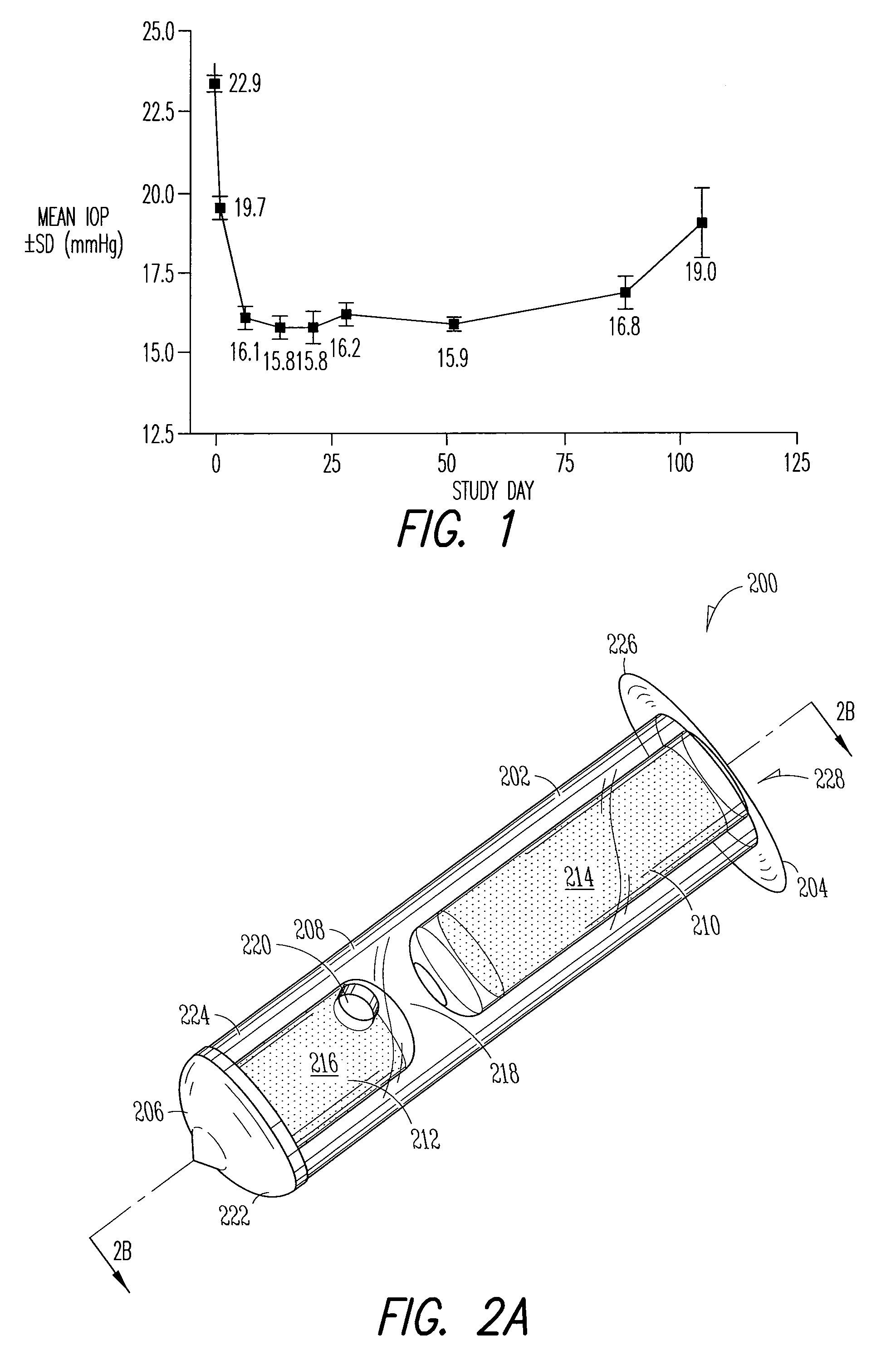
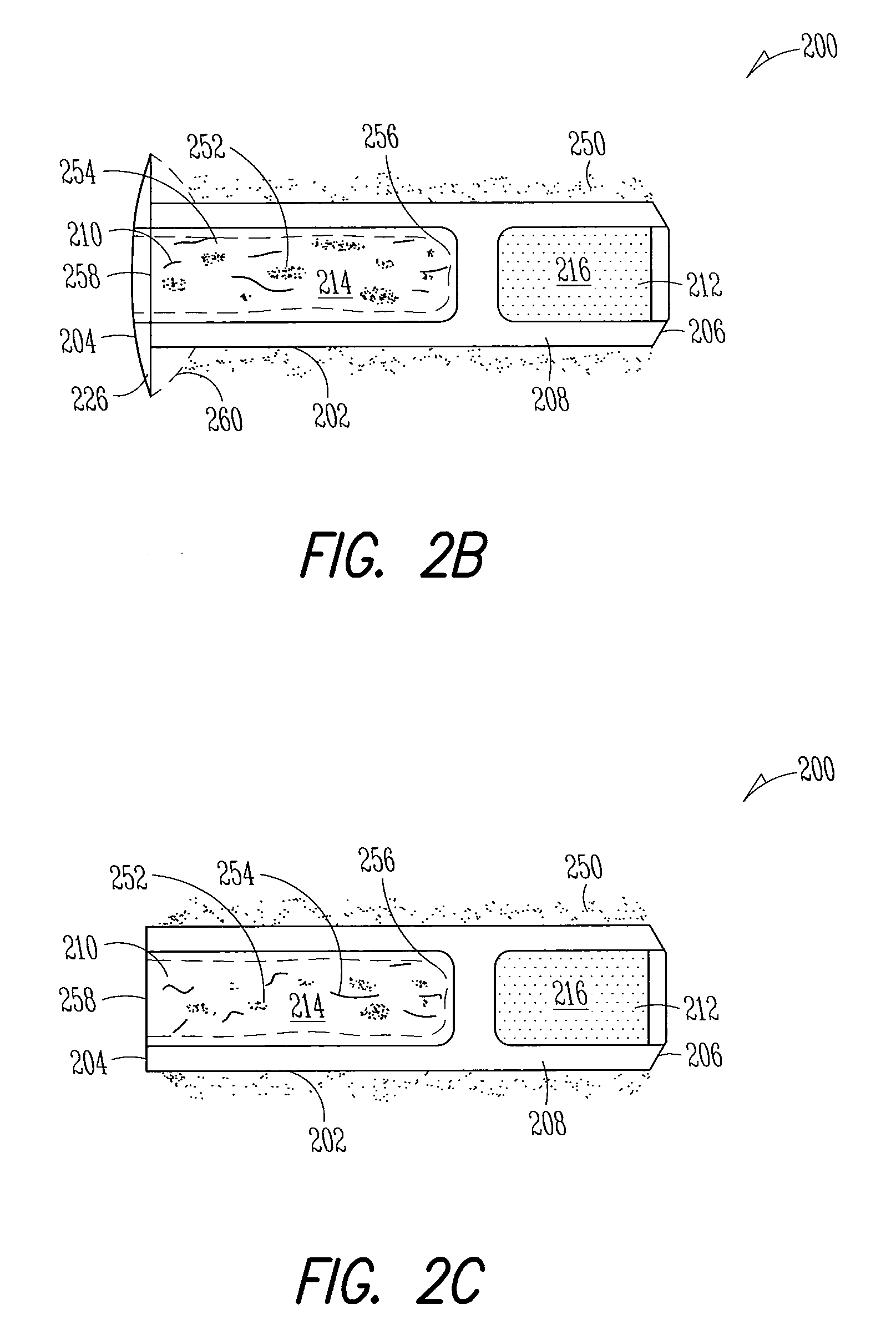
The methods described herein provide treatment of glaucoma, ocular hypertension, and elevated intraocular pressure with latanoprost or other therapeutic agent(s). Implant devices for insertion into a punctum of a patient provide sustained release of latanoprost or other therapeutic agent(s) that is maintained for 7, 14, 21, 30, 45, 60, or 90 days or more, thus avoiding patient noncompliance and reducing or lowering adverse events associated with eye drop administration of latanoprost or other therapeutic agent(s) and other therapeutic agent(s).
Owner:MATI THERAPEUTICS
Sustained release delivery of one or more agents
InactiveUS20100209477A1Improve permeabilityReduce the amount requiredBiocideOrganic active ingredientsCross-linkExcipient
The lacrimal implant delivery systems and methods described herein provide for controlled release of a therapeutic agent for the treatment of disease, including the treatment of glaucoma, ocular hypertension, or elevated intraocular pressure with latanoprost or other anti-glaucoma agents. Treatment of disease, including glaucoma, ocular hypertension, or elevated intraocular pressure with latanoprost or other anti-glaucoma agent in conjunction with penetration enhancer, such as benzalkonium chloride, and / or artificial tears is also provided. Also provided are implants containing a drug core emplacable in a punctum adjacent to an eye of a patient for controlled release of a therapeutic agent such as latanoprost for the treatment of glaucoma, the drug core containing a polymer such as cross-linked silicone, a therapeutic agent, and an excipient, wherein the excipient can increase the rate of release of the agent from the drug core, or can increase the drug loading in the core without loss of desirable homogeneity of the agent within the core, or can improve retention of the agent in the eye or in tear fluid, or can increase corneal penetration of the agent into the eye.
Owner:MATI THERAPEUTICS
Combination treatment of glaucoma
InactiveUS20090318549A1Lower eye pressureOrganic active ingredientsBiocideLatanoprostSustained Release Formulations
The methods described herein provide reduction of intraocular pressure by administering a sustained release formulation including latanoprost and a pharmaceutically acceptable vehicle and administering an eye drop adjunctive composition to the eye of a patient. The sustained release formulation can release latanoprost continuously for at least 90 days from a punctum plug delivery system. The eye drop adjunctive composition can also include latanoprost.
Owner:MATI THERAPEUTICS
Sustained release latanoprost implant
InactiveUS20120276186A1Preserve latanoprost activityOrganic active ingredientsBiocideLatanoprostProstate implant
The present invention provides a sustained release, biodegradable intraocular latanoprost implant for reducing elevated intraocular pressure in an individual in need thereof. The implant can be configured as a film (e.g., a rolled film) or extruded filament, either of which can be inserted into the eye of the individual to provide for extended release of latanoprost for several days. Upon insertion into the eye, a rolled film may unroll to provide a film having a high surface area to volume ratio for drug diffusion.
Owner:ALLERGAN INC
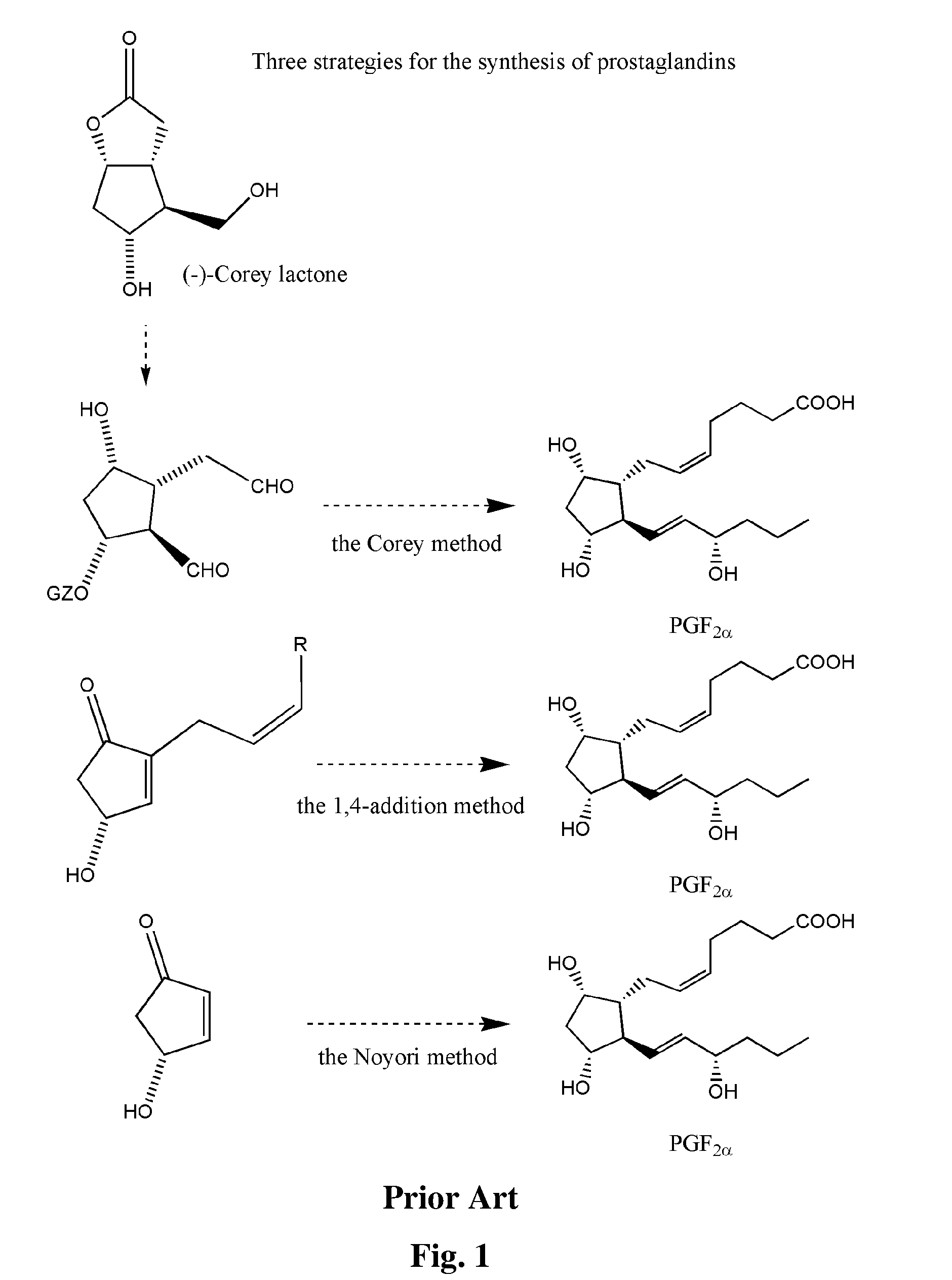
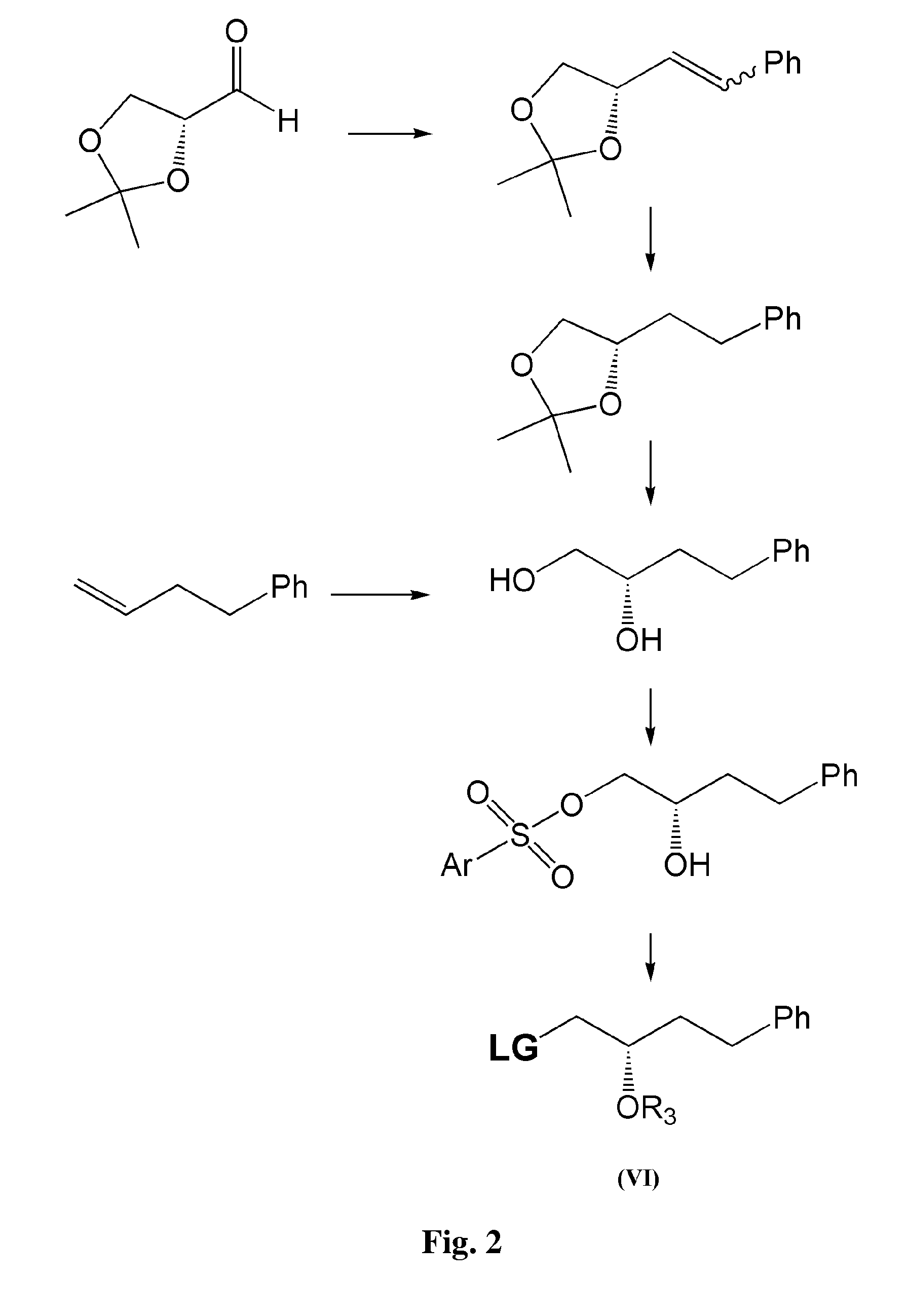
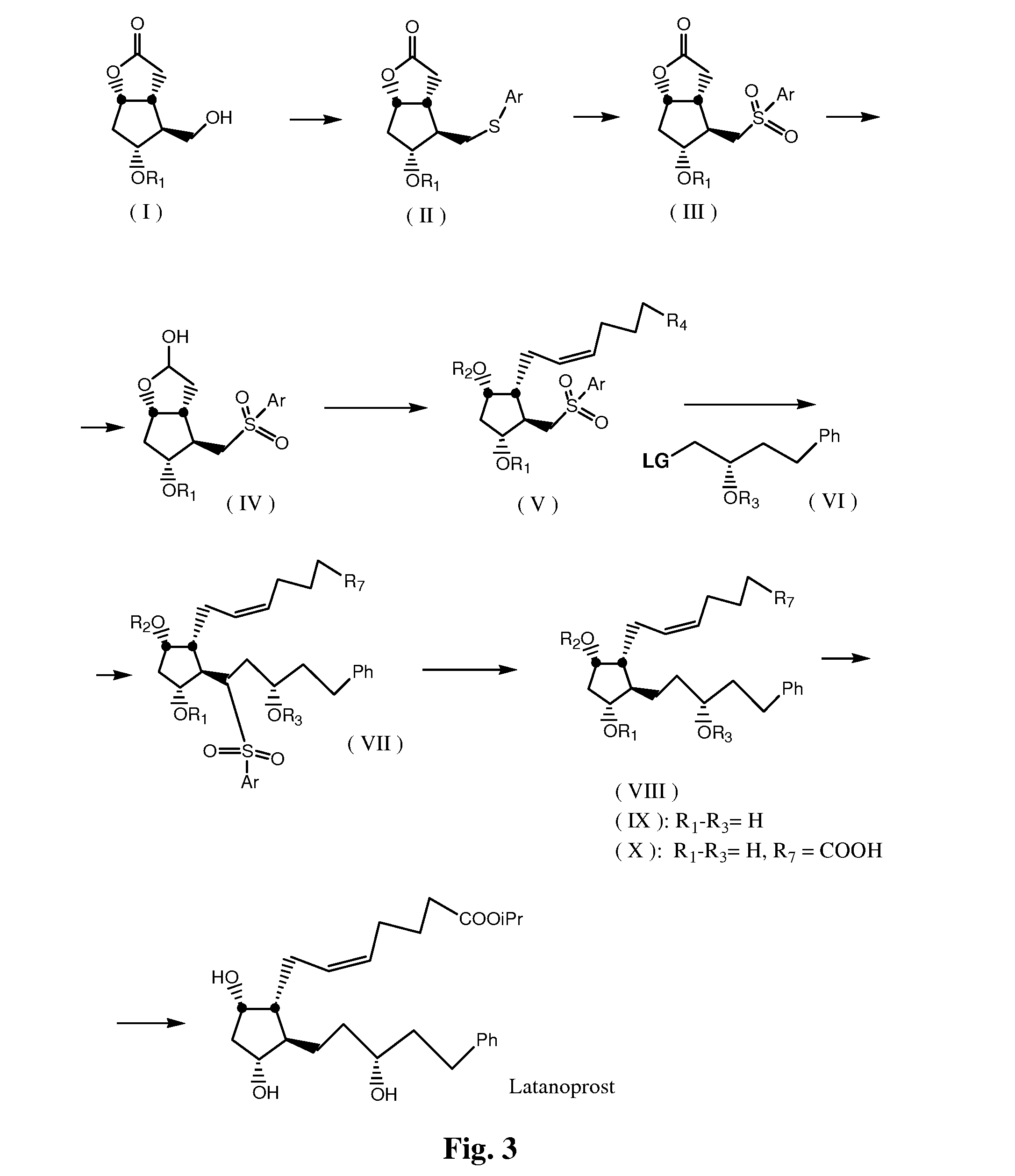
Process for the preparation of 17-phenyl-18,19,20-thinor-pgf 2a and its derivatives
InactiveUS7157590B2High yieldDesired purityOrganic compound preparationOrganic chemistry methodsLatanoprostOphthalmology
The present invention provides a new and effective process for the synthesis of 17-phenyl-18,19,20-trinor-PGF2α and its derivatives, including the anti-glaucoma drugs Bimatoprost and Latanoprost. The benefit of the present invention rises inter alia from the fact that a major intermediate involved in the synthesis of the above compounds may be isolated from a mixture containing also an undesired isomer, by crystallization. In addition, the undesired isomer may be oxidized to give the starting compound, which is then recycled.
Owner:FINETECH PHARMA
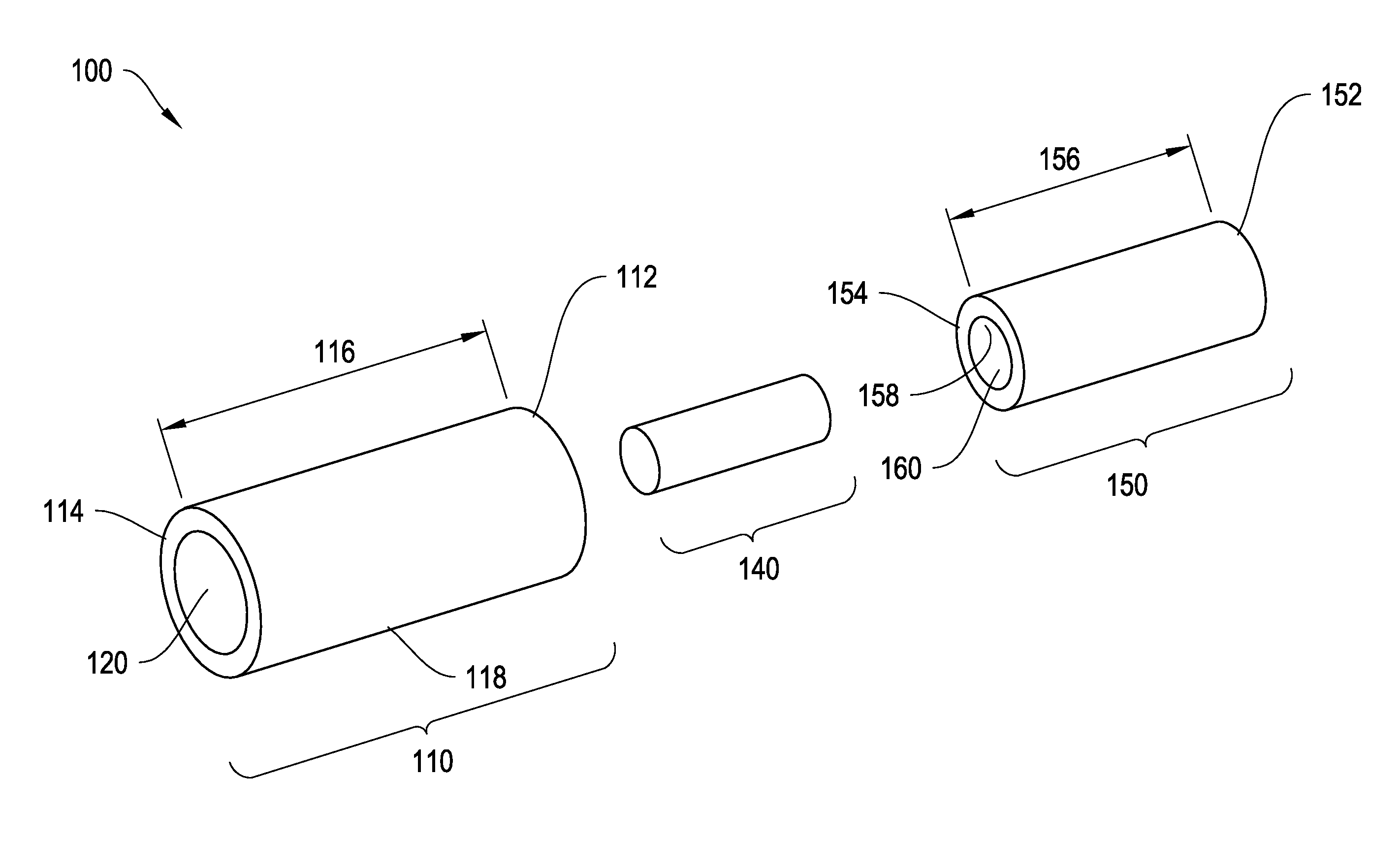
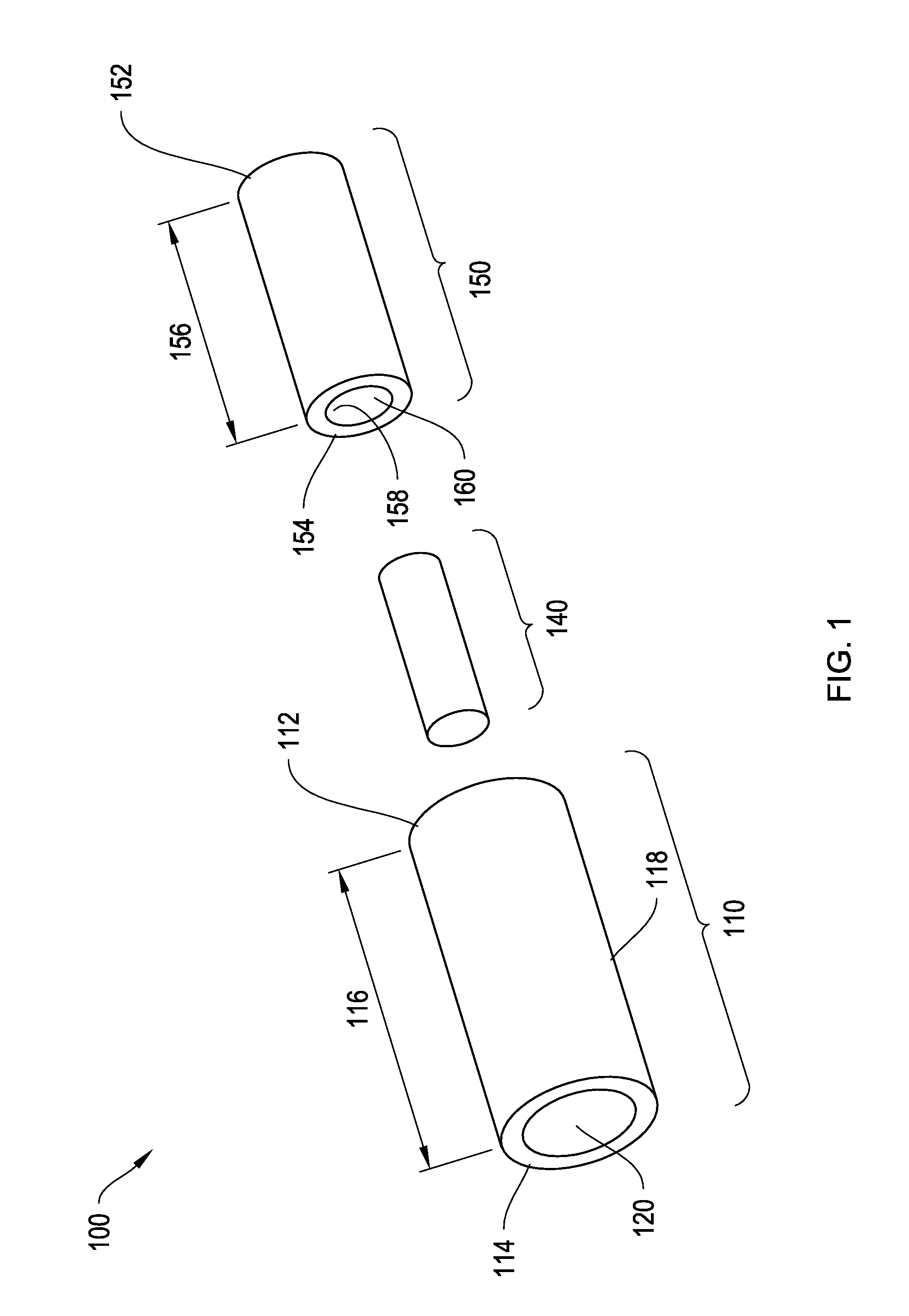
Two-piece injectable drug delivery device with heat-cured seal
The invention provides a drug delivery device for latanoprost or latanoprost acid. The device has a core of latanoprost or latanoprost acid which is surrounded by an internal and external sheath. The external sheath has a first cap that is permeable to latanoprost or latanoprost acid. The first cap may comprise polyvinyl alcohol (PVA), and the PVA may be heat cured. In certain aspects, there are one or more additional caps on the ends of the sheaths formed from one or more polymers. In certain aspects, one or more portions of the drug delivery device is substantially impermeable to latanoprost or latanoprost acid. In certain aspects, the latanoprost or latanoprost acid elutes through the first cap into a biological environment. The invention further provides methods for manufacturing the drug delivery device.
Owner:NAZZARO MARTIN +2
Method Of Preparing A Latanoprost Opthalmic Solution And The Resulting Solution
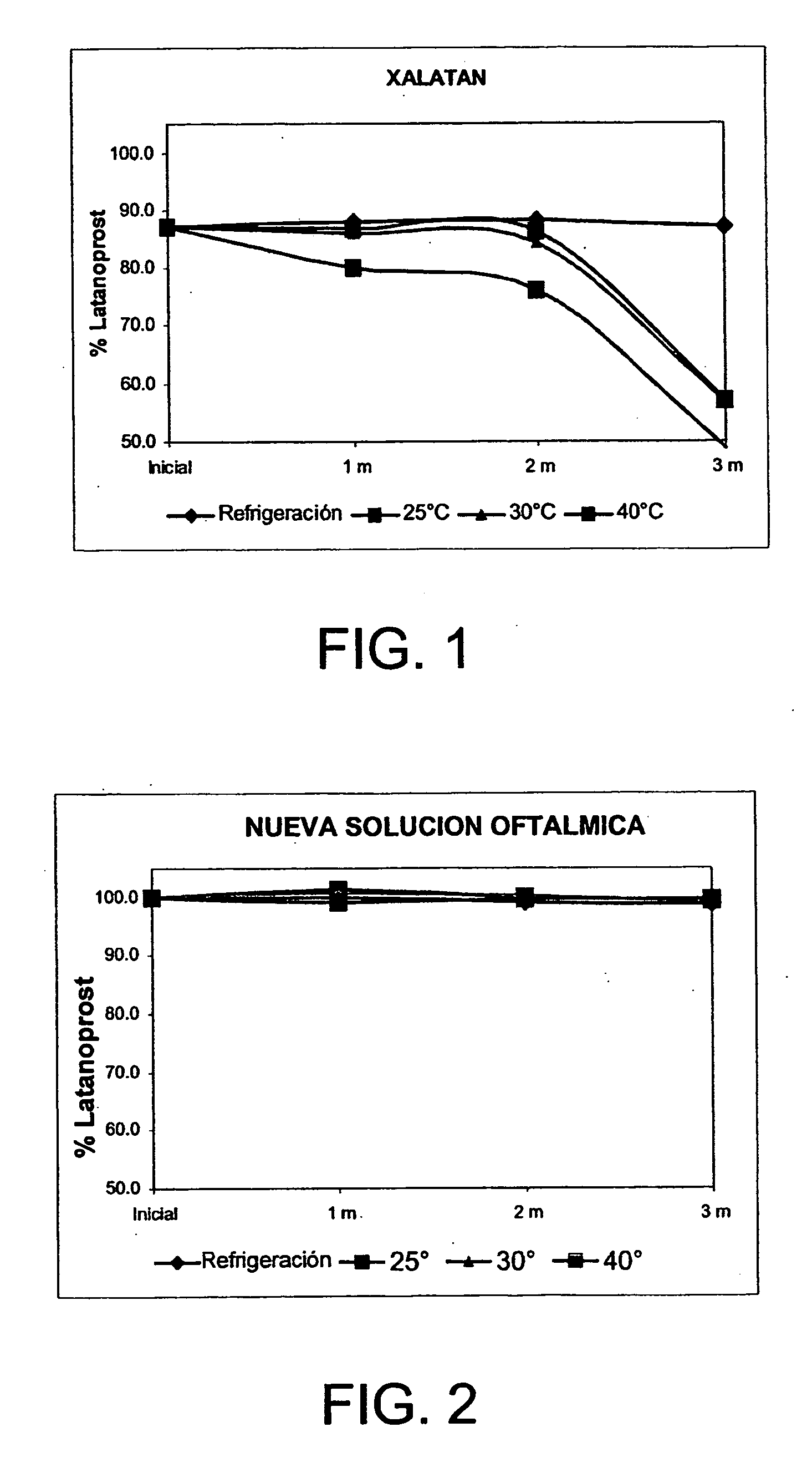
A method of solubilizing an analog active agent of the prostaglandin F2α, such as latanoprost, is described and a method of preparing an ophthalmic solution of the solubilized latanoprost for the treatment of distinct ocular ailments. This invention also refers to an ophthalmic aqueous solution resulting from the aforementioned method, which is characterized by its chemical stability at room temperature, its safety, and innocuousness and efficiency in the treatment of the patient. The new ophthalmic aqueous solution is distinguished because its pharmaceutical value is found in the handling of a vehicle of easy access that not only permits the solubility of latanoprost, but also promotes its chemical stability and a greater tolerance of the patient with its ophthalmic application for the treatment of the patient's ailment.
Owner:JIMENEZ BAYARDO ARTURO +3
Method for treating ocular hypertension and glaucoma
Provided is a method for treating ocular hypertension and glaucoma with reduced side effects such as keratoconjunctive disorders and macular edema, which comprises administering an ophthalmic composition comprising latanoprost as an active ingredient thereof to a subject in need of said treatment, wherein the ophthalmic composition contains substantially no benzalkonium chloride.
Owner:SUCAMPO USA
Process for the preparation of Latanoprost
Disclosed is a process for the preparation of the anti-glaucoma drug Latanoprost, in good yield, in large amounts and with desired purity. Also disclosed are novel intermediates for the above process.
Owner:FINETECH PHARMA
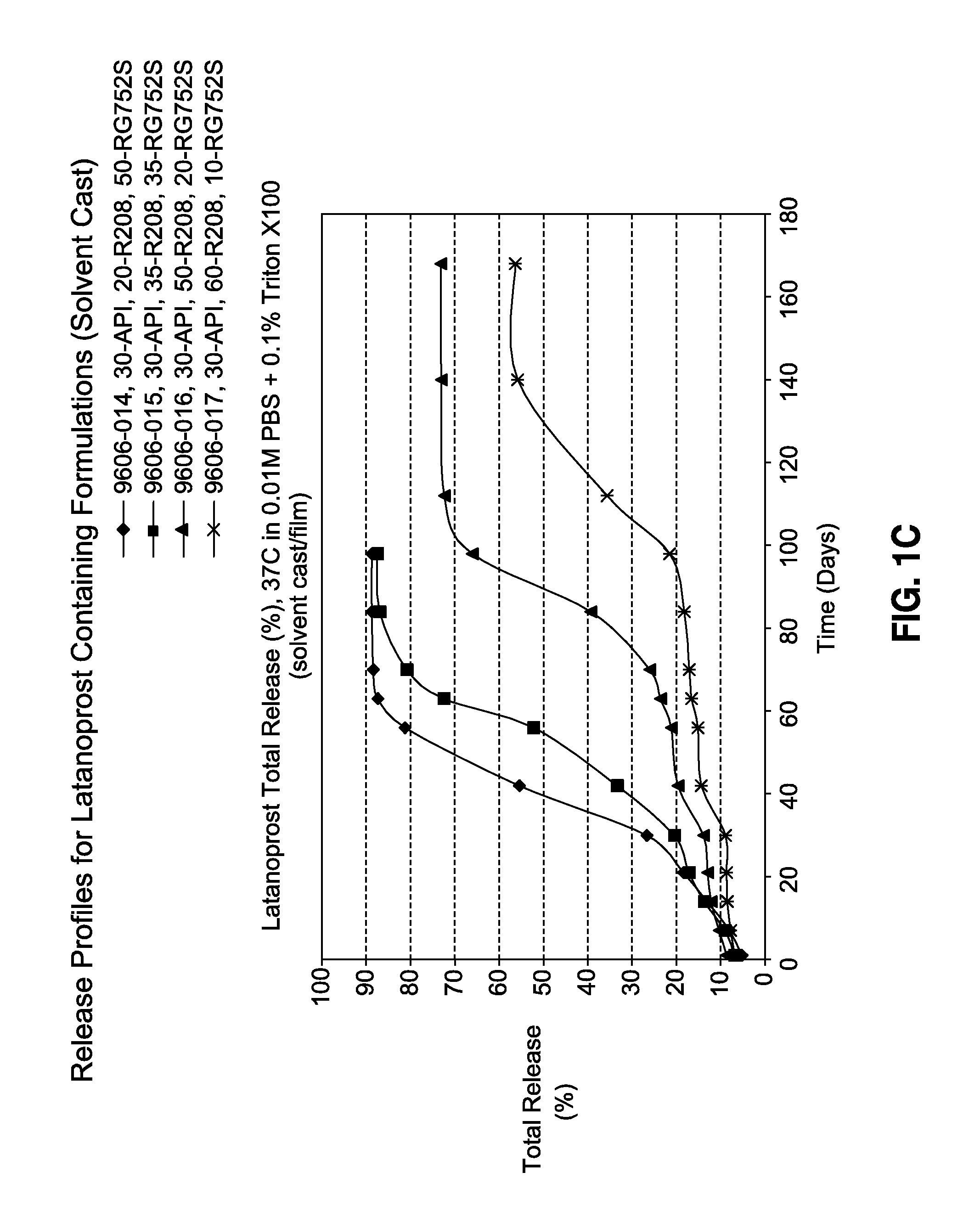
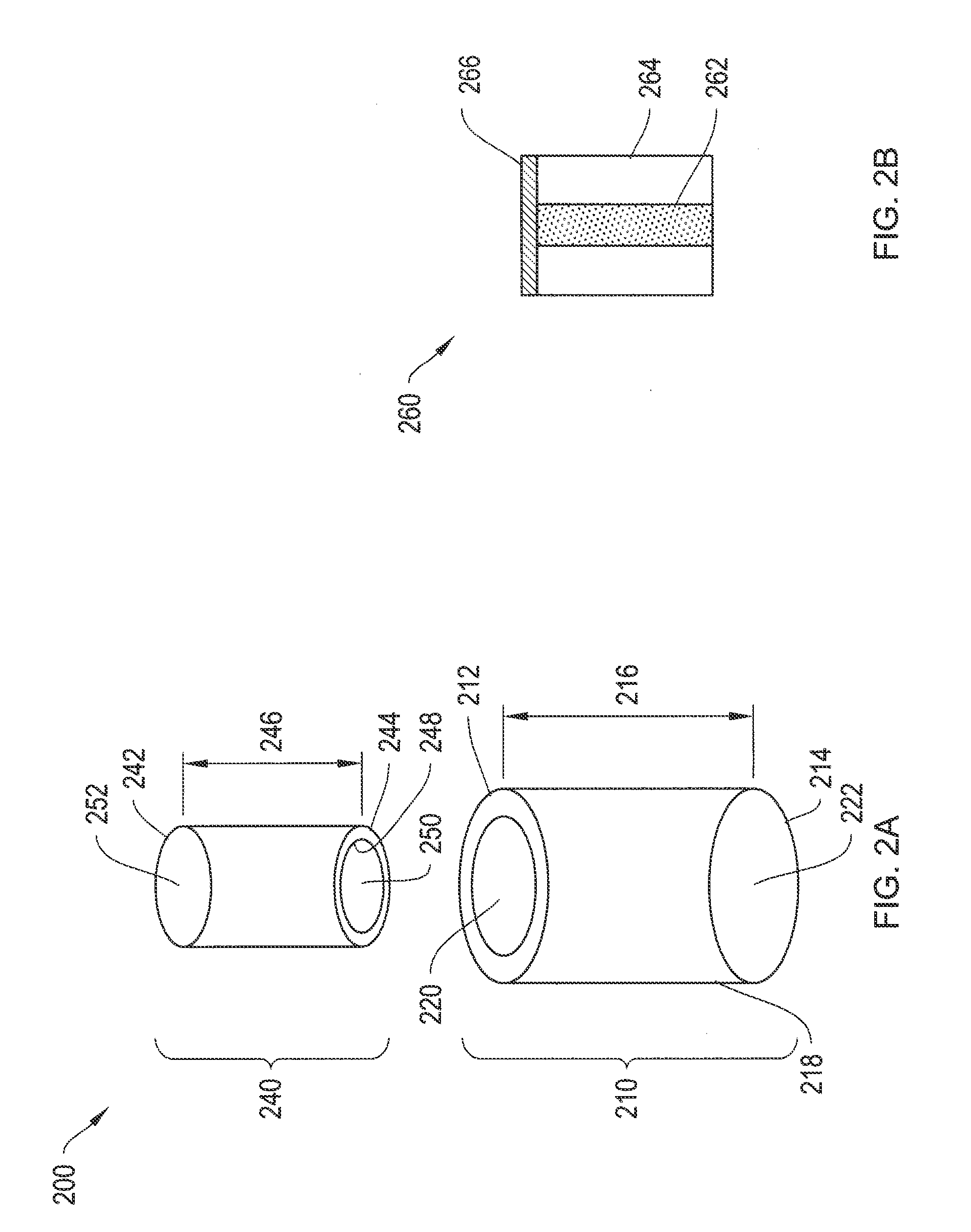
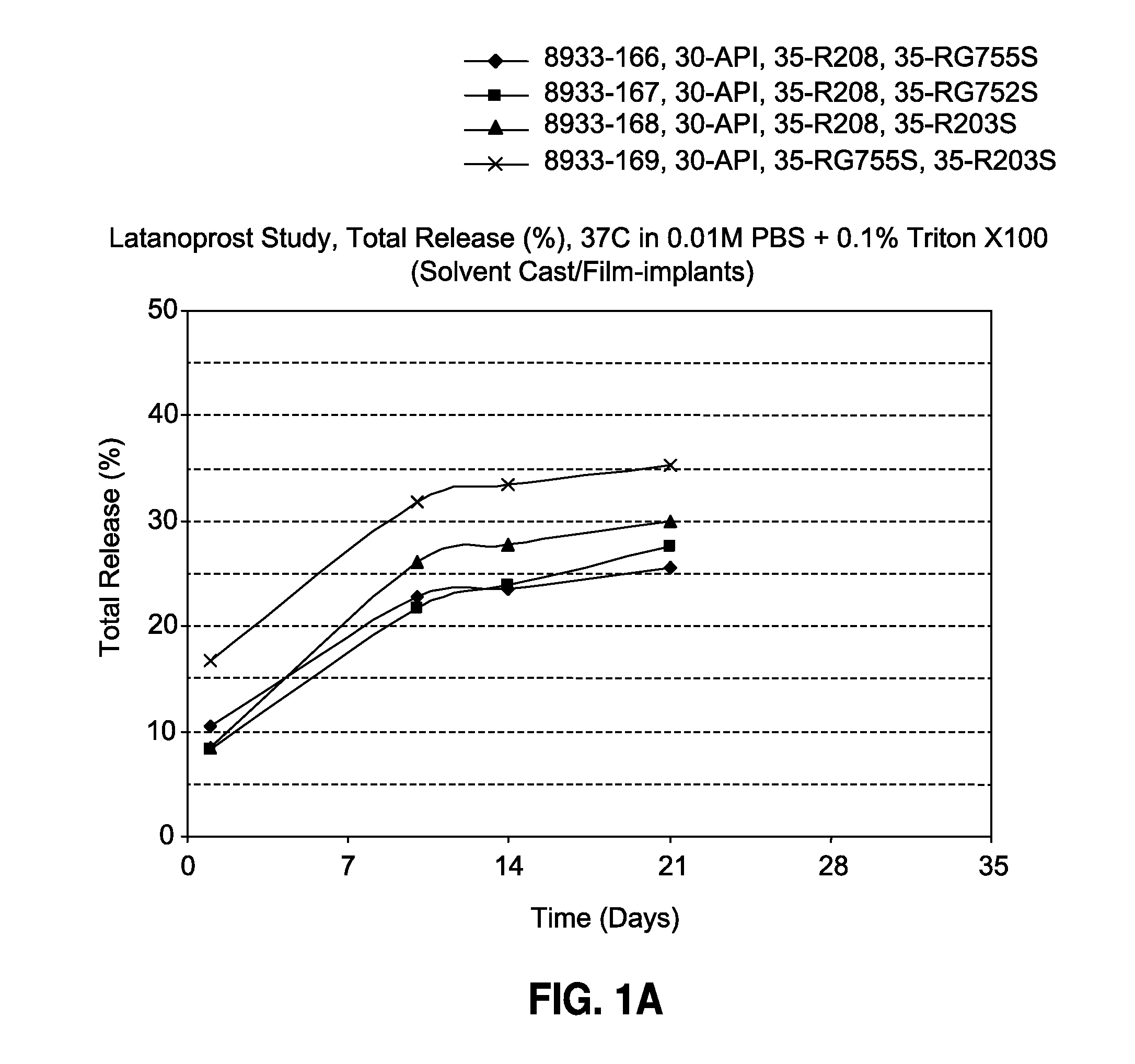
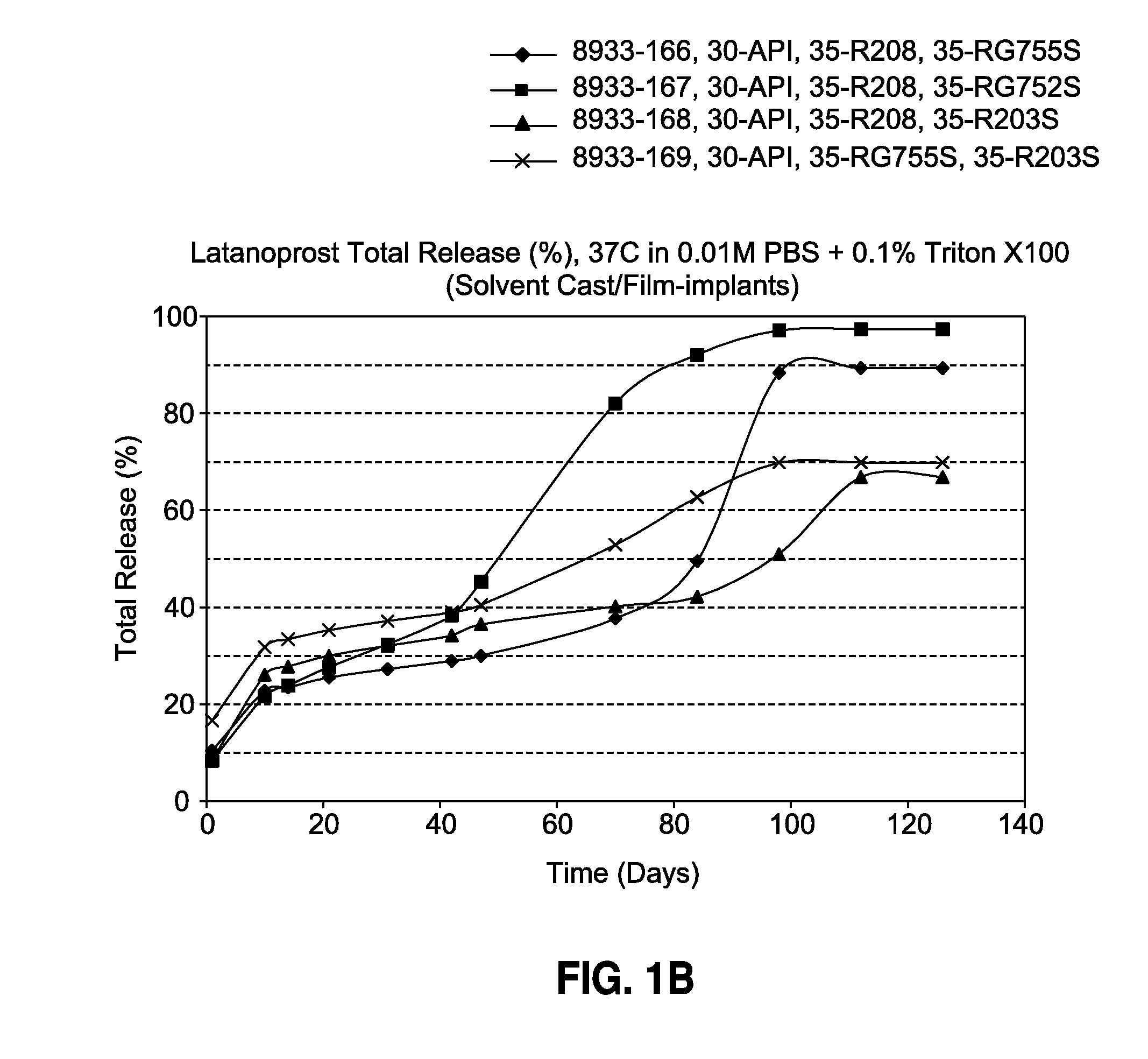
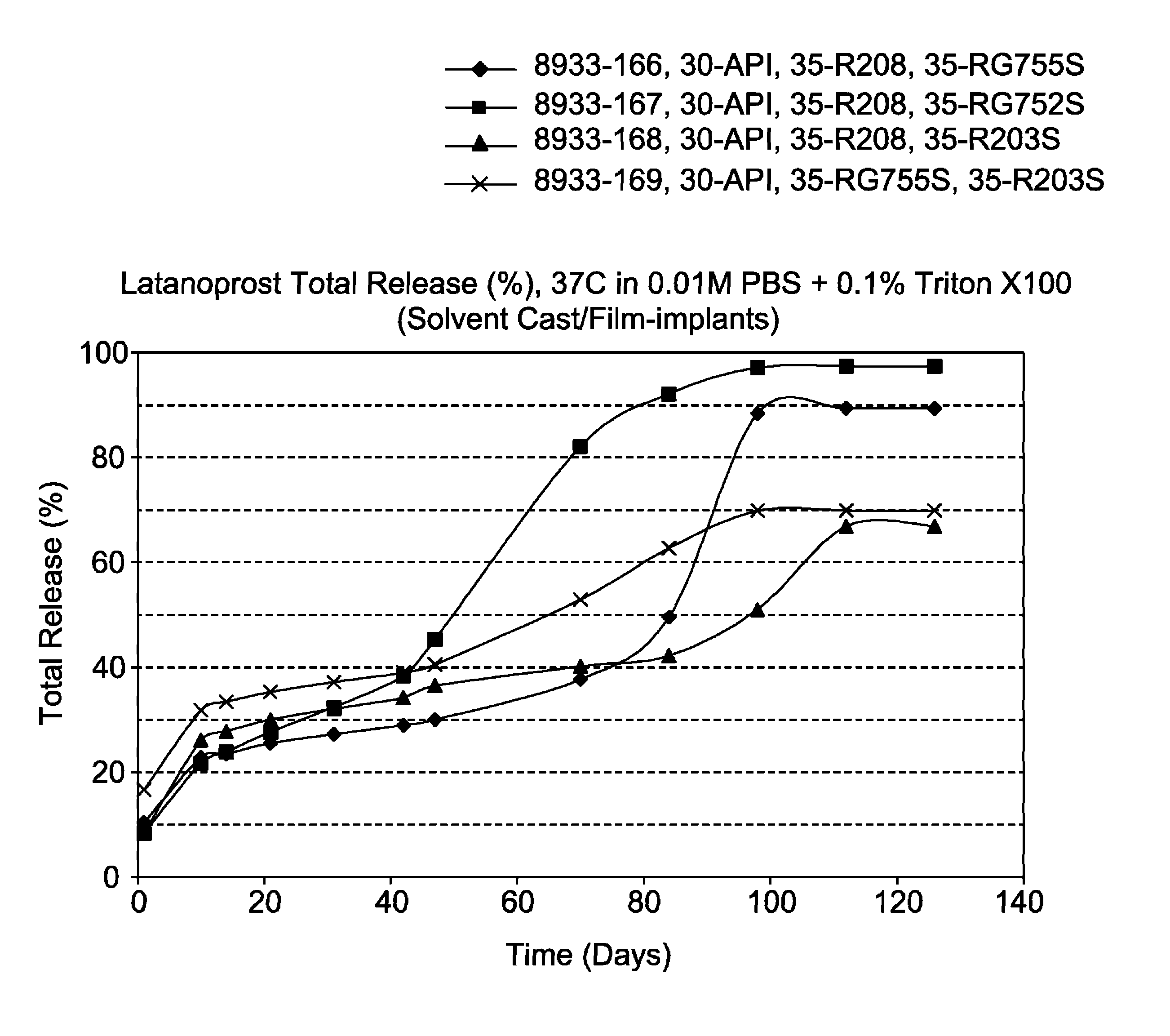
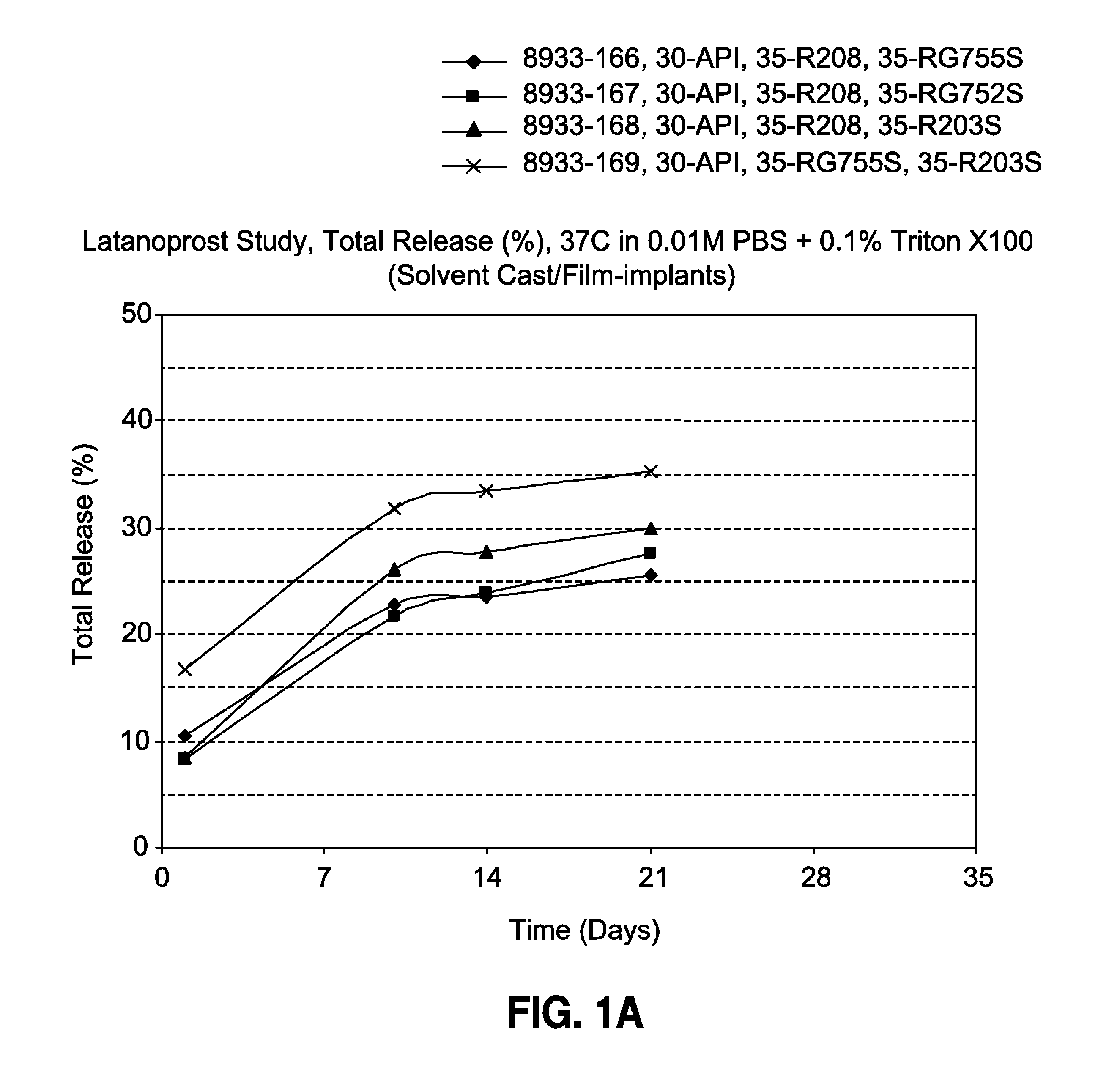
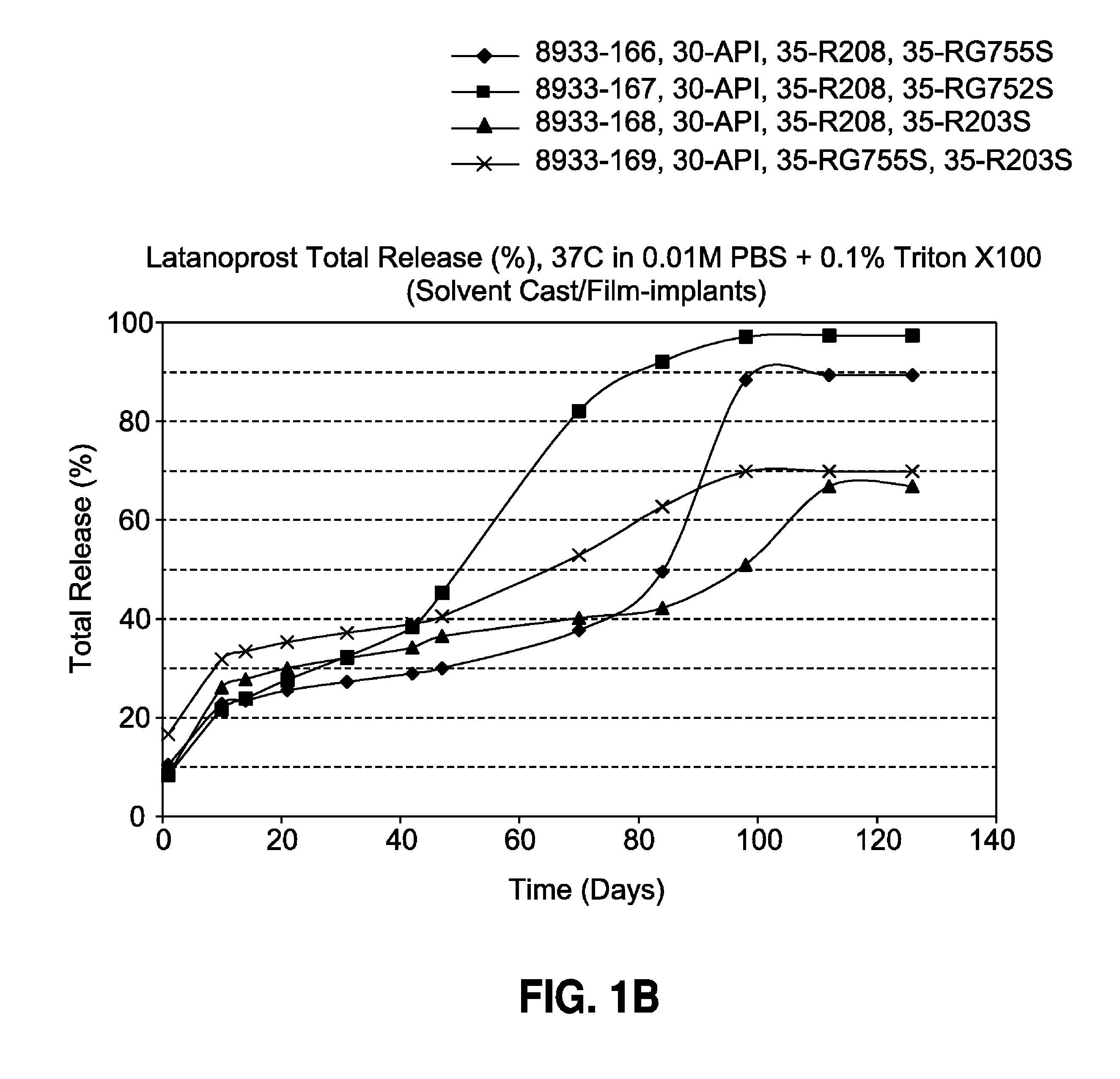
Solvent cast film sustained release latanoprost implant
The present invention provides a sustained release latanoprost implant in the form of a thin film comprising latanoprost incorporated in a biodegradable polymer matrix. Preferably, said implant is an intraocular implant comprising a thin film comprising latanoprost incorporated in a biodegradable polymer matrix wherein said implant is configured as a disc or a rolled film that can be inserted into the eye and unrolls to provide a film having a high surface area to volume ratio.
Owner:ALLERGAN INC
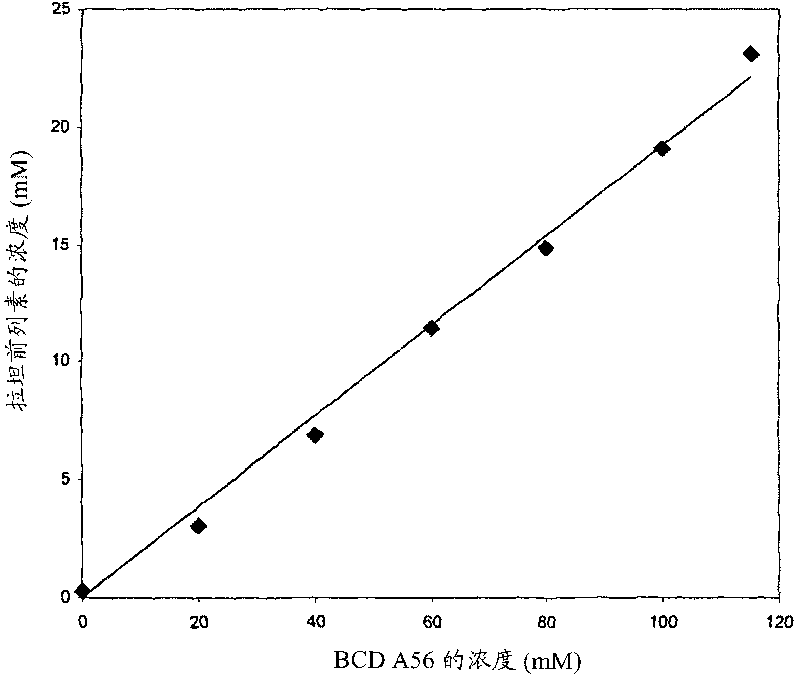
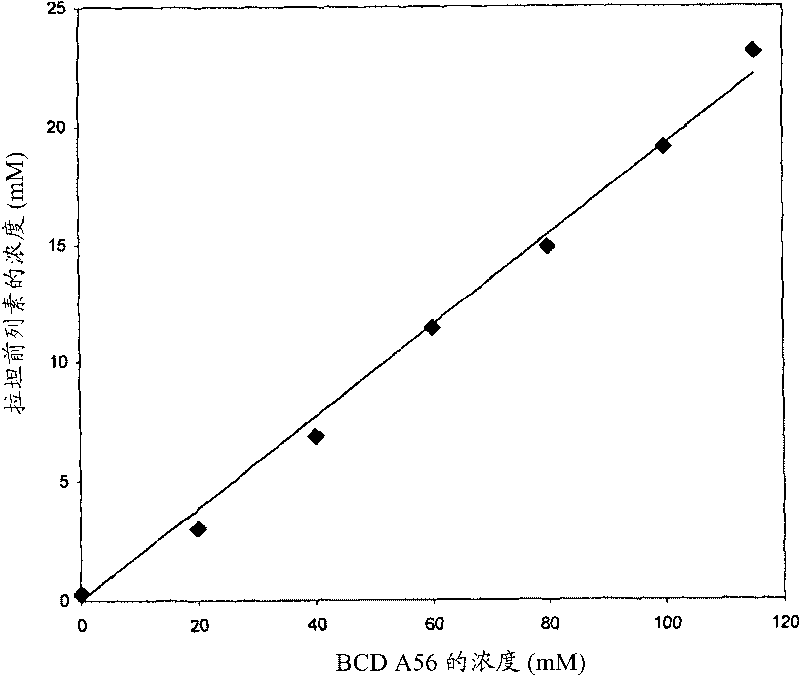
Composition Containing Sulfoalkyl Ether Cyclodextrin and Latanoprost
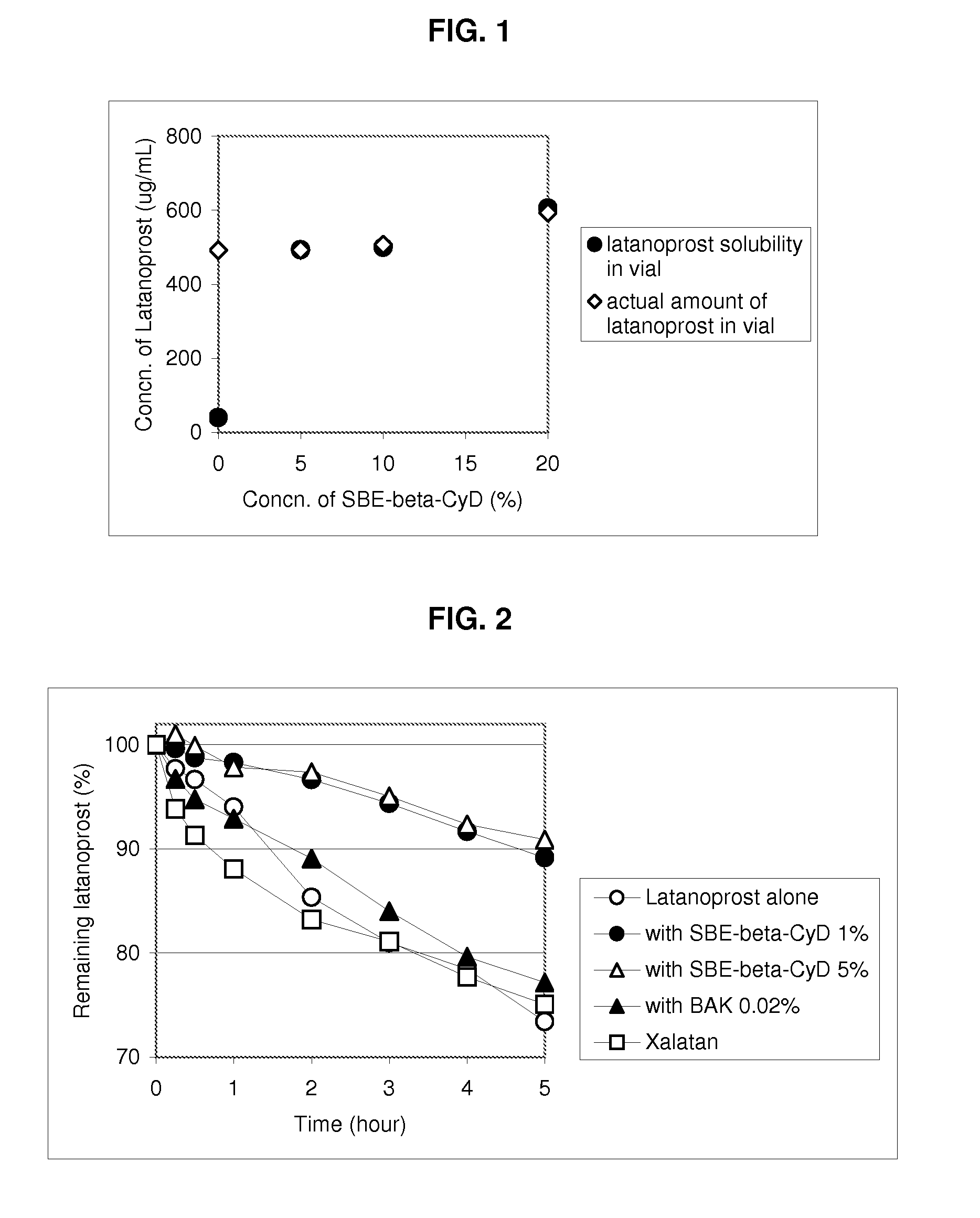
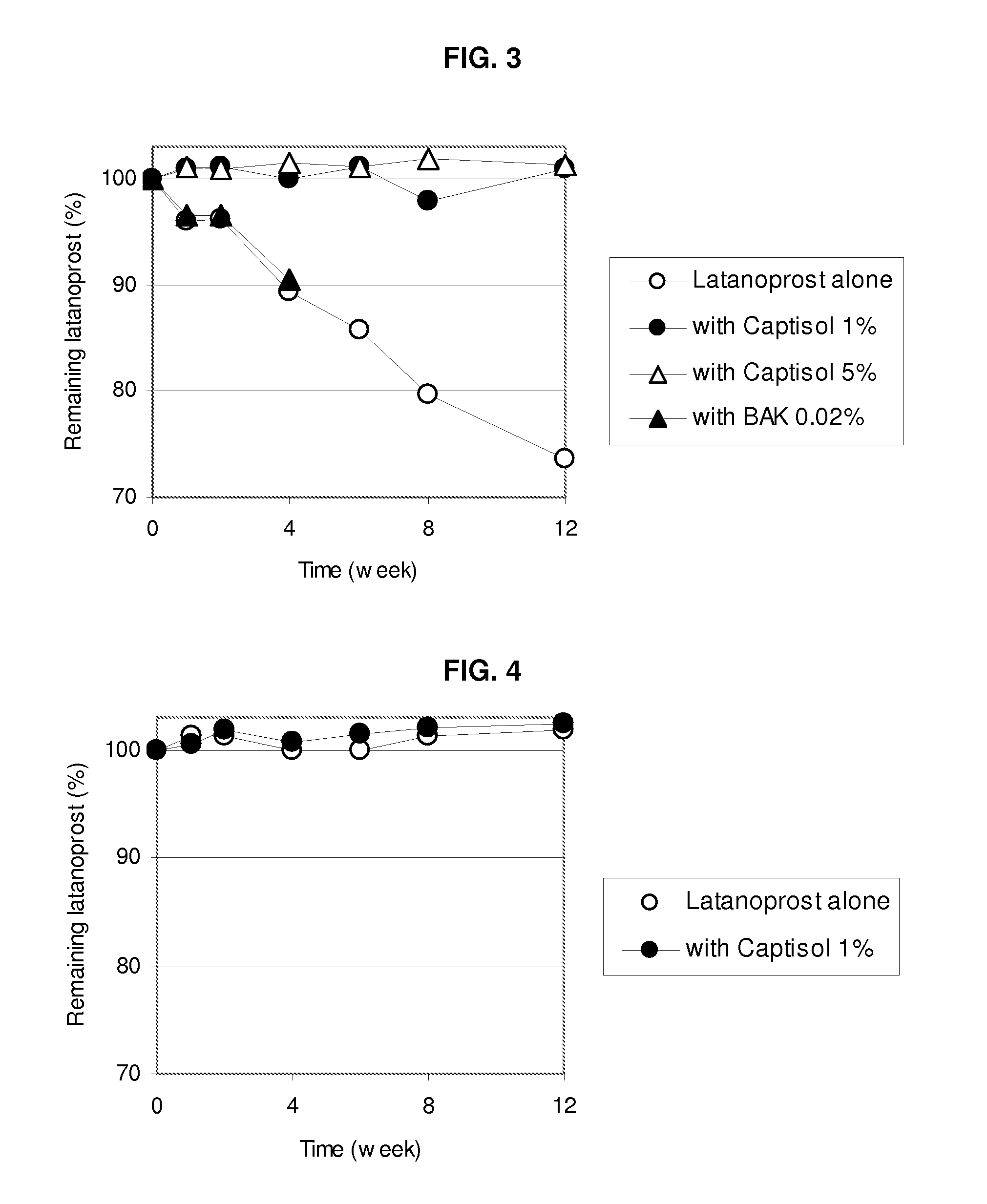
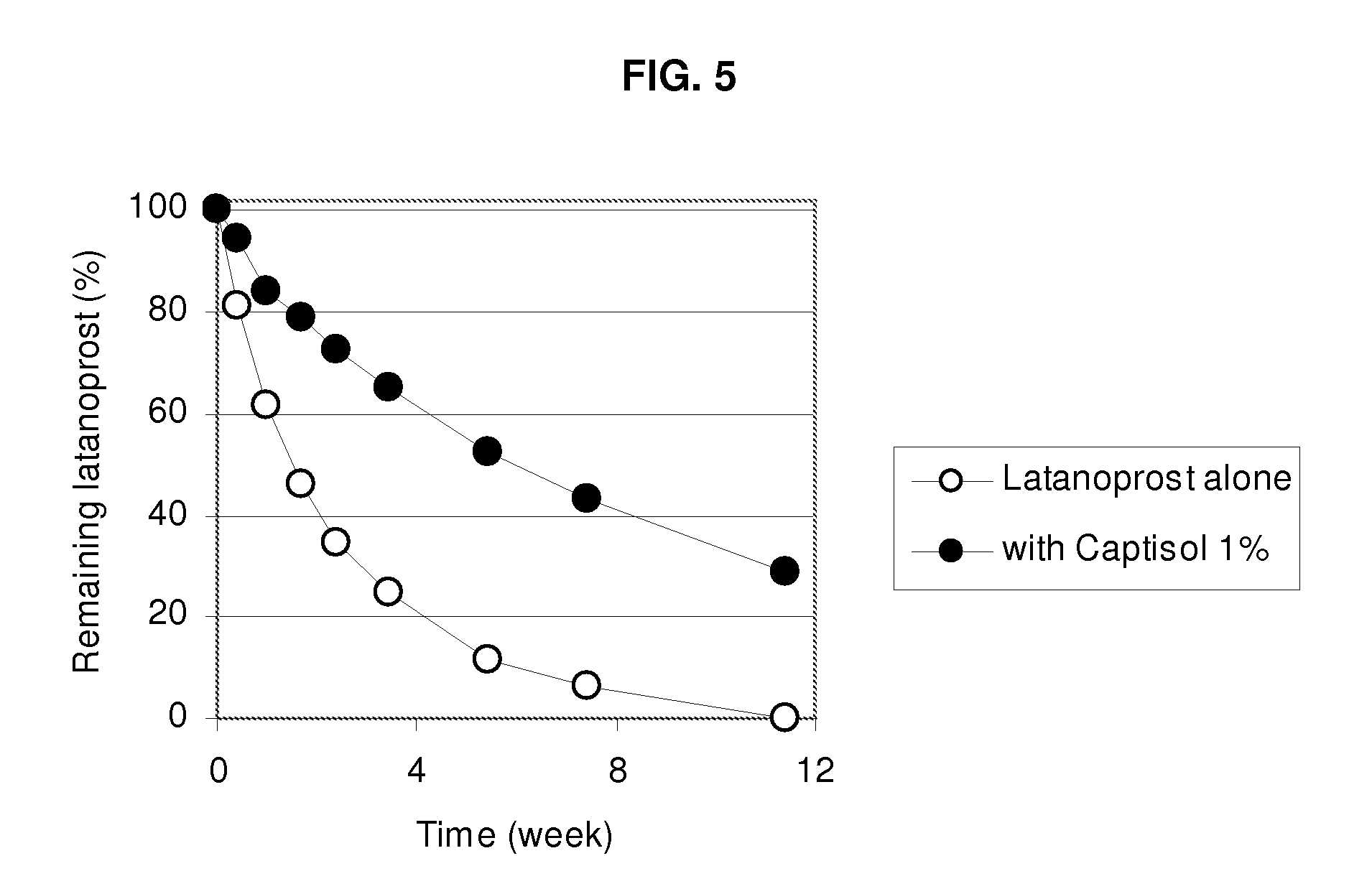
An aqueous composition of latanoprost and SAE-CD is provided. The composition possesses improved stability over otherwise similar compositions excluding SAE-CD. Methods of and systems for treating diseases, disorders, conditions or symptoms of the eye that are therapeutically responsive to latanoprost are also provided.
Owner:CYDEX PHARMACEUTICALS INC
Latanoprost-containing aqueous eye drops and method for inhibiting adsorption of latanoprost to resin
InactiveUS20120184552A1Inhibition of adsorptionImprove stabilityOrganic active ingredientsBiocideCarbon numberLatanoprost
The invention provides an aqueous eye drop containing latanoprost, a surfactant, and an aliphatic mono- or di-carboxylic acid having a carbon number of 3-10 or a salt thereof. In addition, the invention provides a method of suppressing adsorption of latanoprost to a resin in an aqueous solution, by adding a surfactant and an aliphatic mono- or di-carboxylic acid having a carbon number of 3-10 or a salt thereof.
Owner:SENJU PHARMA CO LTD
Eye drop preparation comprising latanoprost
InactiveUS20100120908A1Reduce contentLess difficulty in storingBiocideOrganic active ingredientsLatanoprostEye drop
Disclosed is an eye drop preparation comprising latanoprost, which is characterized in that the degradation of latanoprost in water can be prevented, the adsorption of latanoprost onto a plastic container can be prevented, and therefore the decrease in the latanoprost content can be prevented satisfactorily. The eye drop preparation comprises an eye drop composition comprising the following components (A)-(B) and packed in a plastic container: (A) latanoprost; and (B) a nonionic surfactant.
Owner:TEIKA PHARMA CO LTD
Process for preparation of 13,14-dihydro-pgf2 alpha derivatives
InactiveUS20080207926A1Group 4/14 element organic compoundsOrganic chemistry methodsBiologically active substancesSubstituent
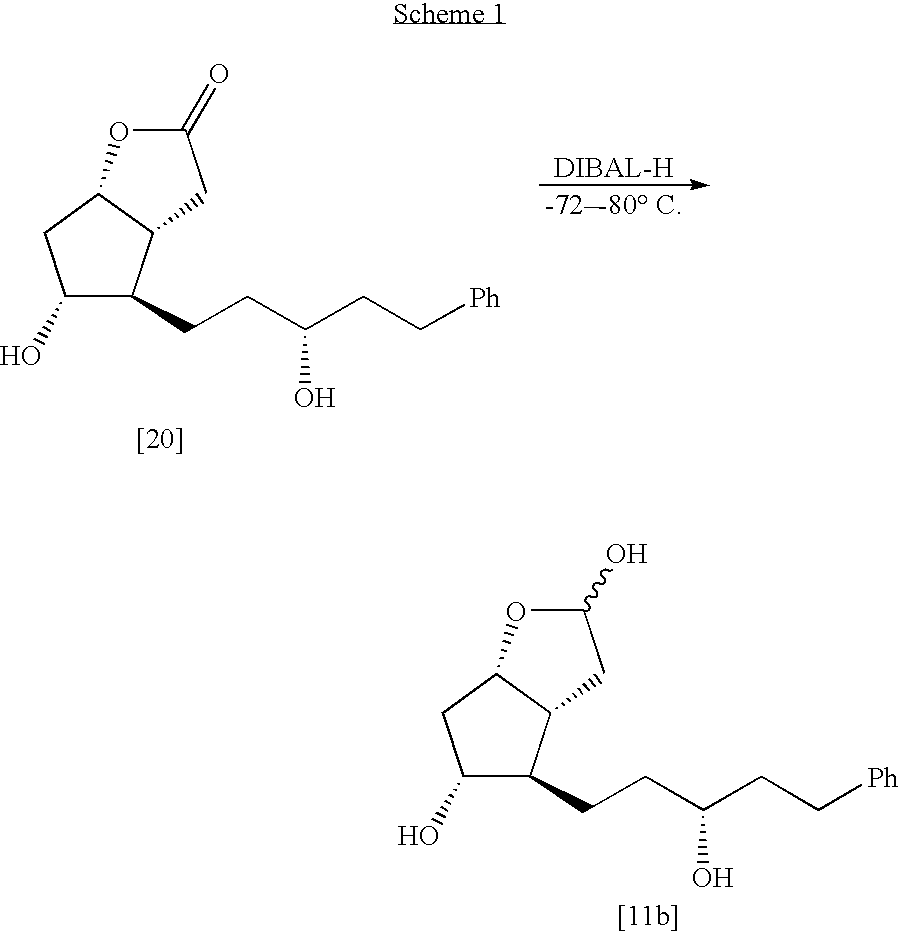
The invention relates to a process for the preparation of 13,14-dihydro-PGF2α derivatives of R or S configuration at carbon 15, represented by the general formula (I), wherein the identity of the substituents is defined in the description. Compounds of the formula (I) are valuable biologically-active substances or intermediates in the preparation thereof. The invention especially relates to the process for preparation of 13,14-dihydro-15(R)-17-substituted-18,19,20-trinor-PGF2α, i.e., latanoprost.
Owner:INSTITUT FARMACEUTYCZNY
Ophthalmic gel and preparation method thereof
ActiveCN102085175ALess irritatingReduce systemic adverse reactionsOrganic active ingredientsSenses disorderWhole bodyExcipient
The invention relates to an ophthalmic gel and a preparation method thereof, in particular to the ophthalmic gel. The ophthalmic gel comprises effective quantity of latanoprost and timolol or salts thereof for treatment and / or prevention, a gel matrix, a surfactant and water. The invention also comprises the ophthalmic gel, an ophthalmic preparation of a medicinal excipient mixed with the ophthalmic gel before being used, and the preparation method of the ophthalmic gel. The ophthalmic gel has the favorable effects of treating eye diseases, and has less adverse effects on a cardiovascular system and a respiratory system of the whole body.
Owner:SHENYANG XINGQI PHARM CO LTD
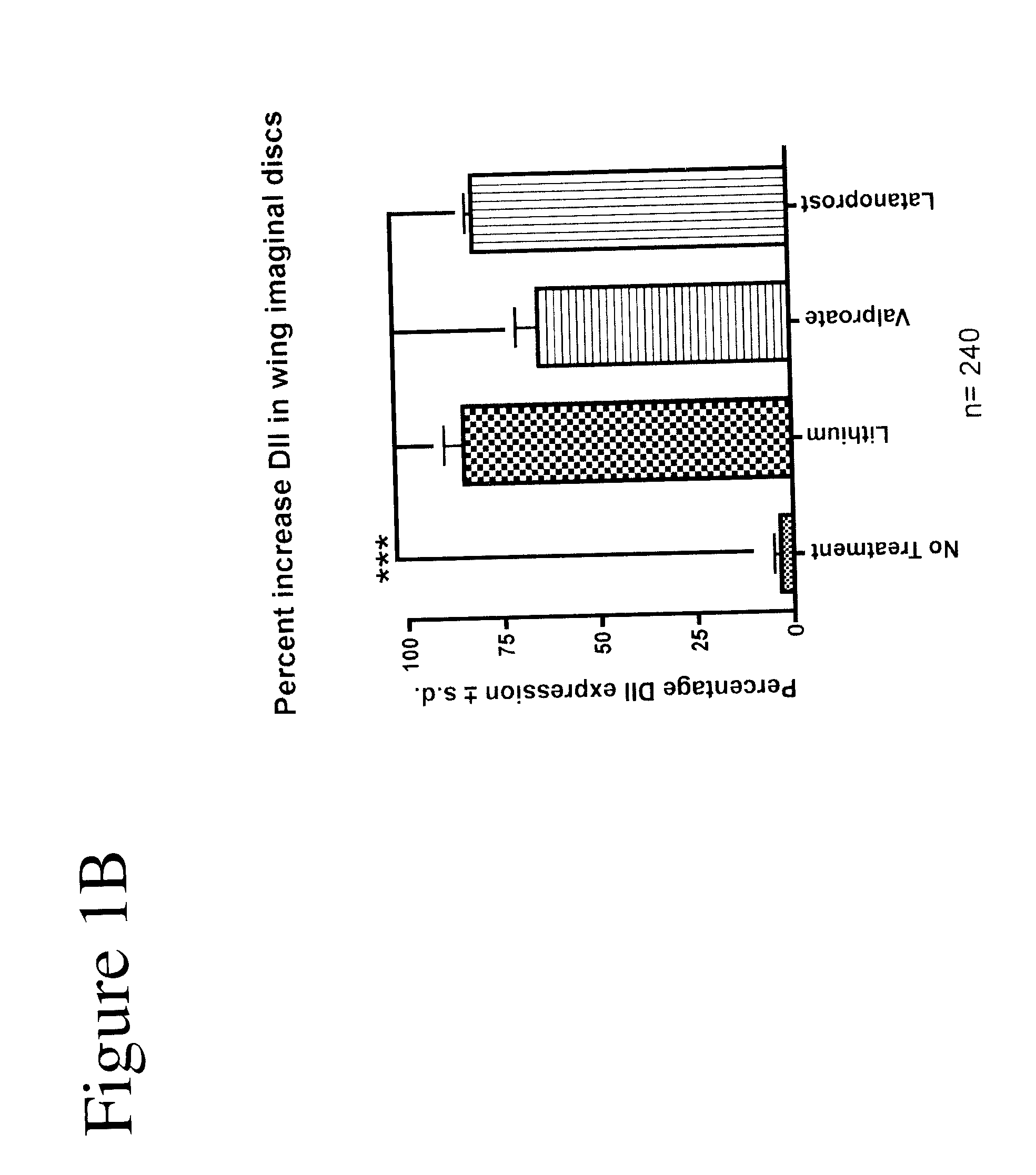
Compositions comprising a prostaglandin for treating neuropsychiatric conditions
ActiveUS20130309330A1Diminishment of extentAvoid spreadingBiocideNervous disorderLatanoprostBipolar mood disorder
The present invention relates to methods and compositions for the treatment of neuropsychiatric conditions (e.g., bipolar disorder) by administration of prostaglandin or prostaglandin derivatives (e.g., latanoprost) to a subject (e.g., a human).
Owner:NEUROTHERYX CANADA LTD
Latanoprost-containing aqueous eye drops and method for inhibiting adsorption of latanoprost to resin
InactiveCN102481301AImprove stabilityImprove thermal stabilityOrganic active ingredientsSenses disorderLatanoprostCarboxylic acid
Provided is a formulation of latanoprost-containing aqueous eye drops wherein decreases in effective latanoprost concentration due to adsorption to resin are inhibited and the stability of the latanoprost is improved. Also provided is a method for inhibiting adsorption of latanoprost to resin. The provided aqueous eye drops contain latanoprost, a surfactant, and a C3-10 aliphatic mono- or di-carboxylic acid or a salt thereof. The inclusion of a surfactant and either a C3-10 aliphatic mono- or di-carboxylic acid or a salt thereof allows the provision of a method for inhibiting the adsorption to resin of latanoprost in an aqueous solution.
Owner:SENJU PHARMA CO LTD
Method of treating blepharospasm with a prostaglandin derivative
InactiveUS20060089408A1Avoid treatmentEasy and convenience can be appliedBiocideElcosanoid active ingredientsLatanoprostChemical composition
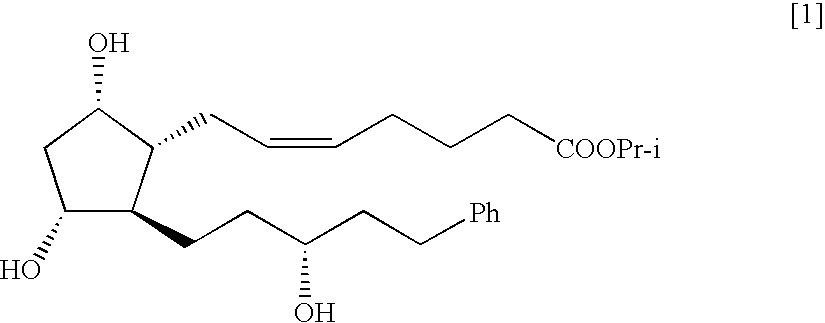
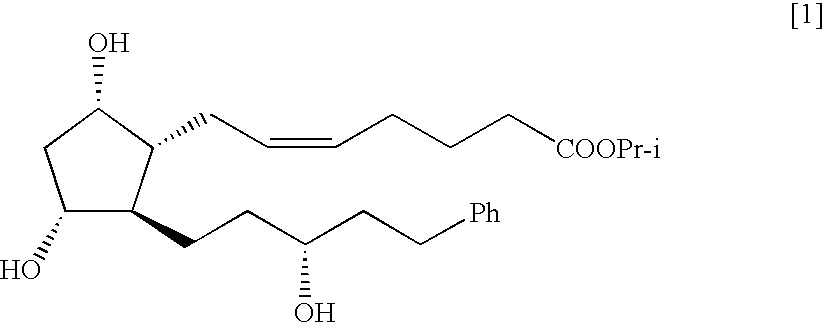
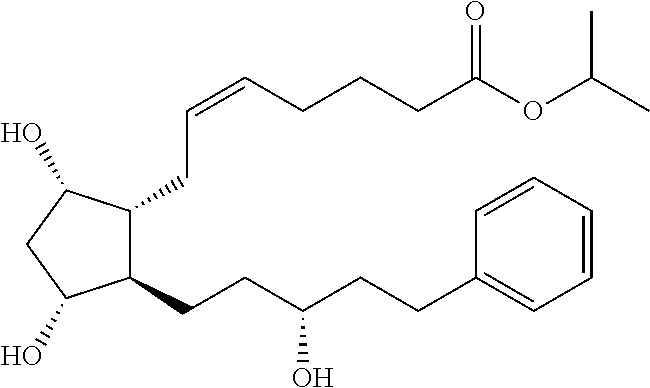
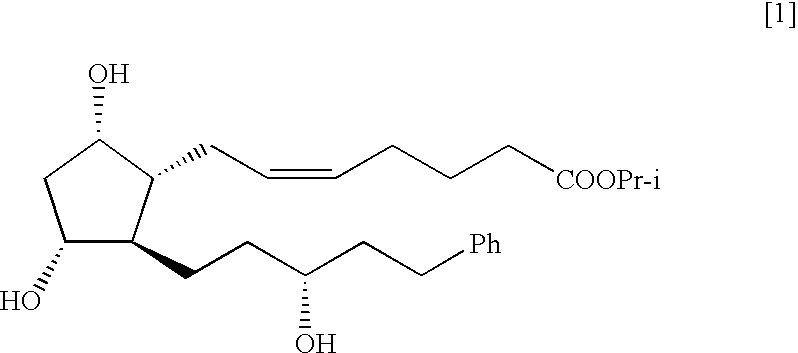
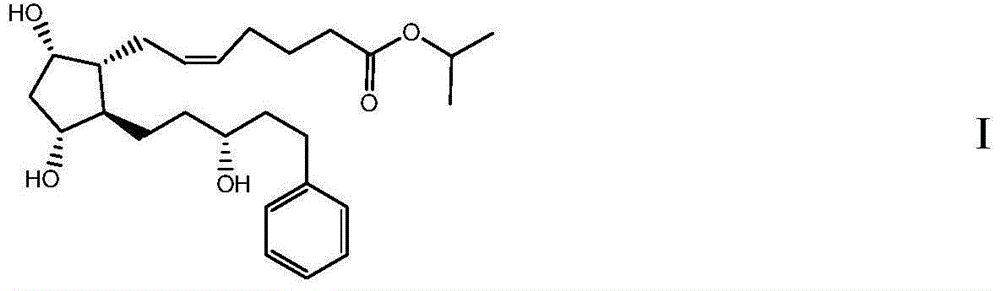
The present invention is a method of treating blepharospasm with a prostaglandin derivative, comprising the steps of (a) providing a predetermined amount of prostaglandin derivative; and (b) applying the prostaglandin derivative in a predetermined position. The prostaglandin derivative is preferably in the form of latanoprost wherein the chemical composition of latanoprost is a prostaglandin F.sub.2.alpha derivative which has the chemical name isopropyl-(Z)-7[(1R,2R,3R,5S)3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl I]cyclo-pentyl]-5-heptenoate. The dosage of the prostaglandin derivative is generally in the range of 1.5 ug to 4.5 ug which is equivalent to 1 to 3 drops aqueous solution for human eye
Owner:WEI TED C JR +1
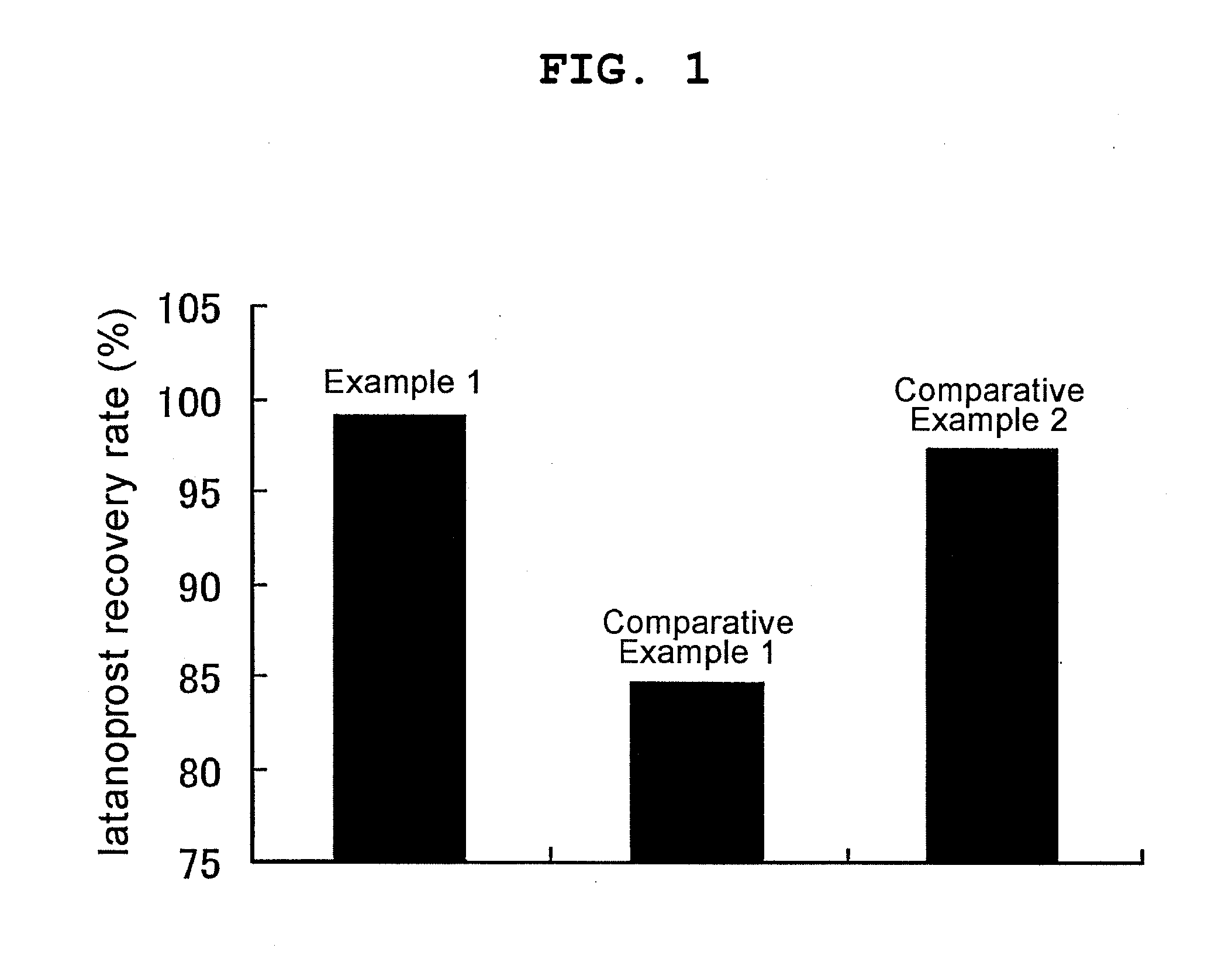
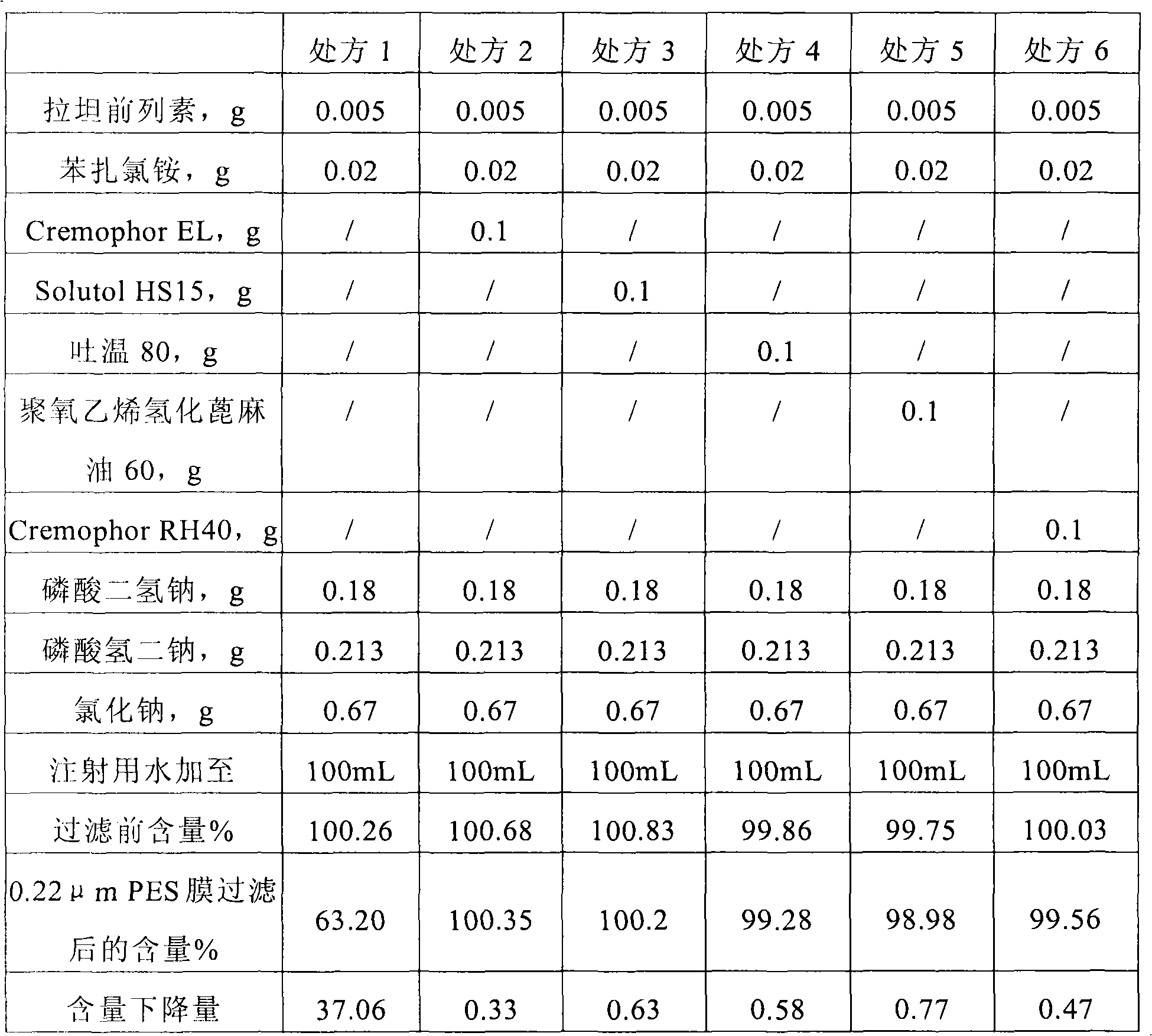
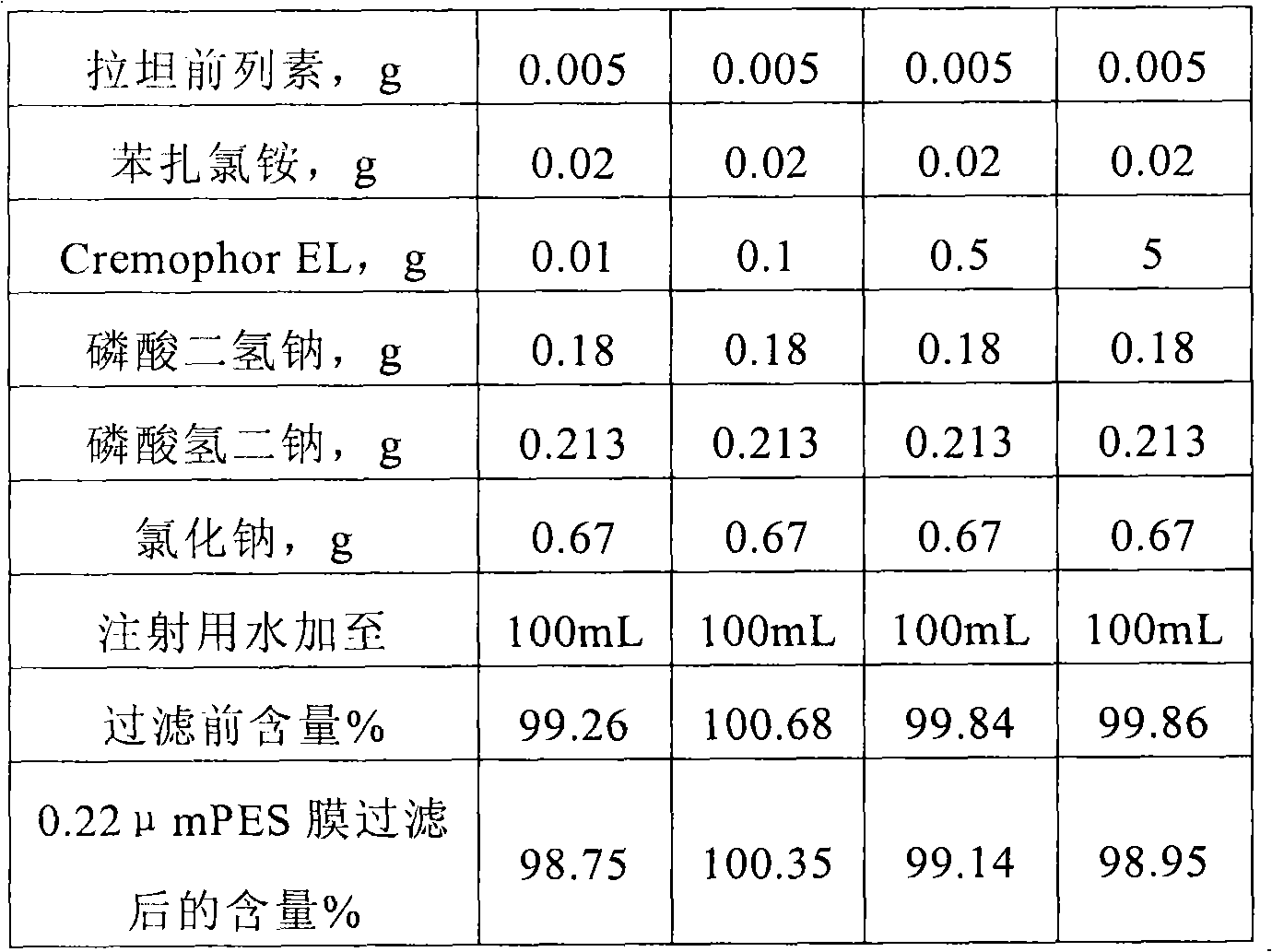
Non-ionic surface active agent-containing Latanoprost eye drop and preparation method thereof
ActiveCN102028697AImprove stabilitySolve the problem of being easily adsorbed by the filter membraneOrganic active ingredientsSenses disorderLatanoprostNon ionic
The invention provides a Latanoprost composition which comprises a therapeutically effective amount of Latanoprost, a non-ionic surface active agent and a pharmaceutically acceptable carrier. The invention also provides a method for preparing the Latanoprost composition. The Latanoprost composition can solve the problem that the Latanoprost eye drop is easily adsorbed by a filter membrane in the preparation process. Besides, the non-ionic surface active agent-containing Latanoprost eye drop and the preparation method thereof can improve the stability of the Latanoprost eye drop at high temperature, reduce the creation of related substances and lower the transport refrigeration requirements.
Owner:SHANGHAI XINYI JINZHU PHARMA
Process for the preparation of latanoprost
InactiveUS20030149294A1Organic active ingredientsOrganic compound preparationLatanoprostPhotochemistry
Disclosed is a novel process for the preparation of the anti-glaucoma drug Latanoprost, in good yield, in large amounts and with desired purity. Also disclosed are novel intermediates for the above process.
Owner:FINETECH PHARMA
Eye-drop preparation and use thereof
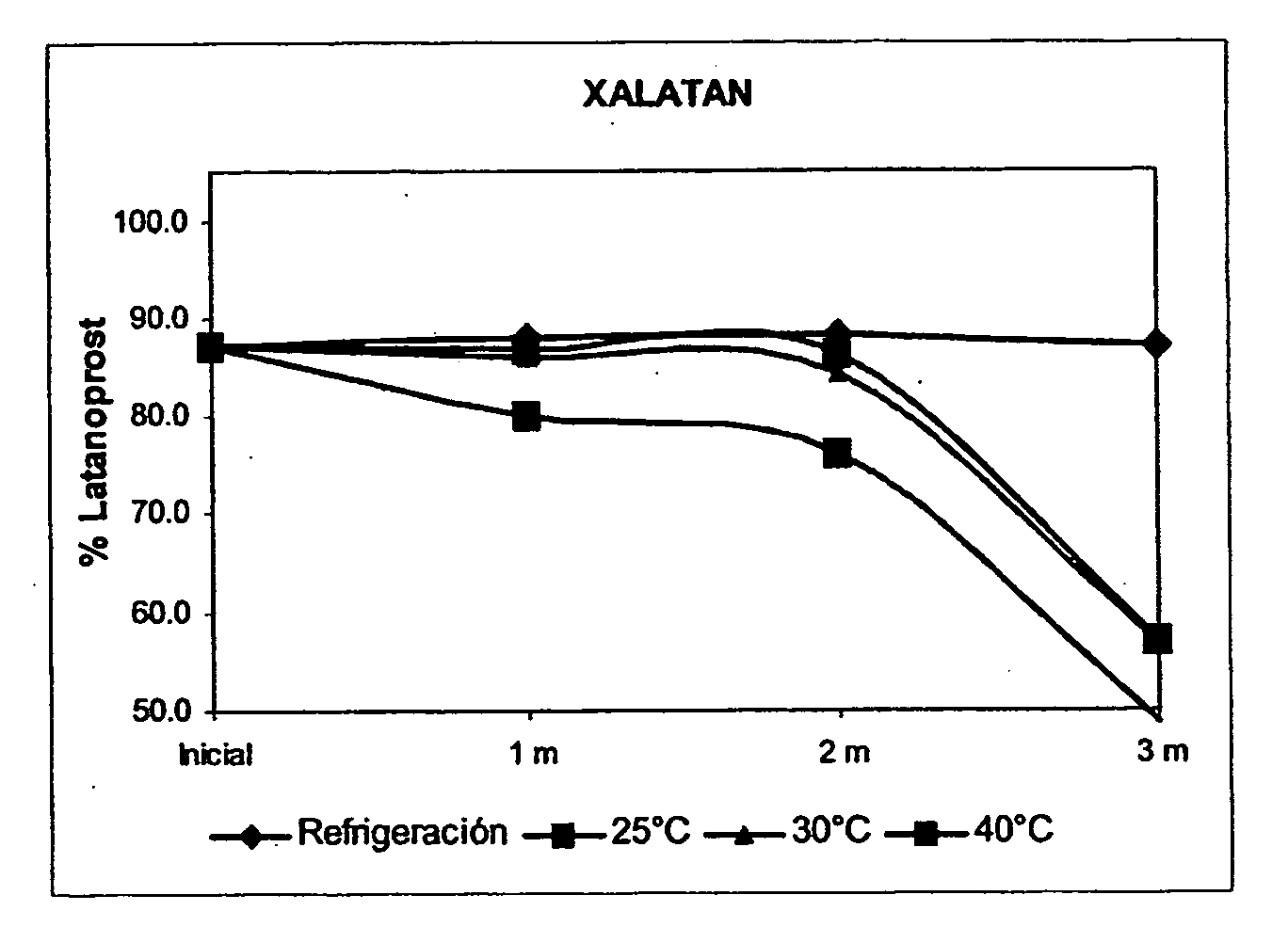
InactiveUS20110105558A1Effective controlImprove stabilityBiocideSenses disorderLatanoprostDecomposition
Disclosed is a technique for preventing the decomposition of latanoprost (a thermally unstable substance) contained in an eye-drop solution to stabilize the eye-drop solution. Specifically disclosed is an eye-drop preparation comprising latanoprost and carteolol hydrochloride. By adding carteolol hydrochloride to an eye-drop solution containing latanoprost which is a thermally unstable substance and is likely to be adhered on the surface of a container, it becomes possible to prevent the decomposition of latanoprost in the eye-drop solution and also prevent the loss of latanoprost caused by the adsorption of latanoprost on the surface of a container.
Owner:OTSUKA PHARM CO LTD
Composition for ocular topical administration treatment ocular hypertension and glaucoma
InactiveUS20120232139A1Reduce the amount requiredLower eye pressureOrganic active ingredientsBiocideSide effectBenzalkonium chloride
The present invention provides a composition for ocular topical administration for treating ocular hypertension and glaucoma, comprising latanoprost as an active ingredient, and (a) a polyol and / or sugar alcohol, (b) a nonionic surface active agent, and (c) an edetic acid compound. The composition of the present invention comprises lower amount of preservatives such as benzalkonium chloride comparative to the conventional product and therefore, can reduce incidence of the adverse side effects caused by the preservatives. In addition, the composition of the present invention can be stored stably at room temperatures for a long term.
Owner:SUCAMPO
Prostaglandin and prostamide drug delivery systems and intraocular therapeutic uses thereof
Biocompatible, bioerodible implants and microspheres include latanoprost and a biodegradable polymer effective, when placed intraocular (such as into the subtenon space) to treat glaucoma.
Owner:ALLERGAN INC
Latanoprost eye drop
InactiveCN104288092AGood water solubilityAvoid decompositionOrganic active ingredientsSenses disorderLatanoprostAntioxidant
The invention discloses a latanoprost-containing in situ gel eye drop. The eye drop contains an active component latanoprost, an antioxidant, a surfactant, a gel matrix, an adhesive, a pH adjusting agent and an optional antiseptic. The eye drop has good stability, can avoid the drug concentration reduction due to the adsorption of a container, and solves the defects of short retention time of drugs in eyes and low bioavailability of ordinary eye drops.
Owner:SUNSHINE LAKE PHARM CO LTD
Solvent cast film sustained release latanoprost implant
The present invention provides a sustained release latanoprost implant in the form of a thin film comprising latanoprost incorporated in a biodegradable polymer matrix. Preferably, said implant is an intraocular implant comprising a thin film comprising latanoprost incorporated in a biodegradable polymer matrix wherein said implant is configured as a disc or a rolled film that can be inserted into the eye and unrolls to provide a film having a high surface area to volume ratio.
Owner:ALLERGAN INC
Complexes of prostaglandin derivatives and monosubstituted, charged beta-cyclodextrins
The present invention relates to water-soluble, non-covalent complexes of a group of prostaglandin derivatives including latanoprost and monosubstituted, charged ss- cyclodextrins, as well as uses of these complexes in therapeutic compositions that are administered topically for treating intraocular hypertension and glaucoma.
Owner:眼科药物股份有限公司 +1
Ophthalmic compositions comprising latanoprost for use in the treatment of ocular diseases
PendingUS20200268648A1Reduced systemic exposureReduce adverse effectsOrganic active ingredientsSenses disorderIntra ocular pressureAlkane
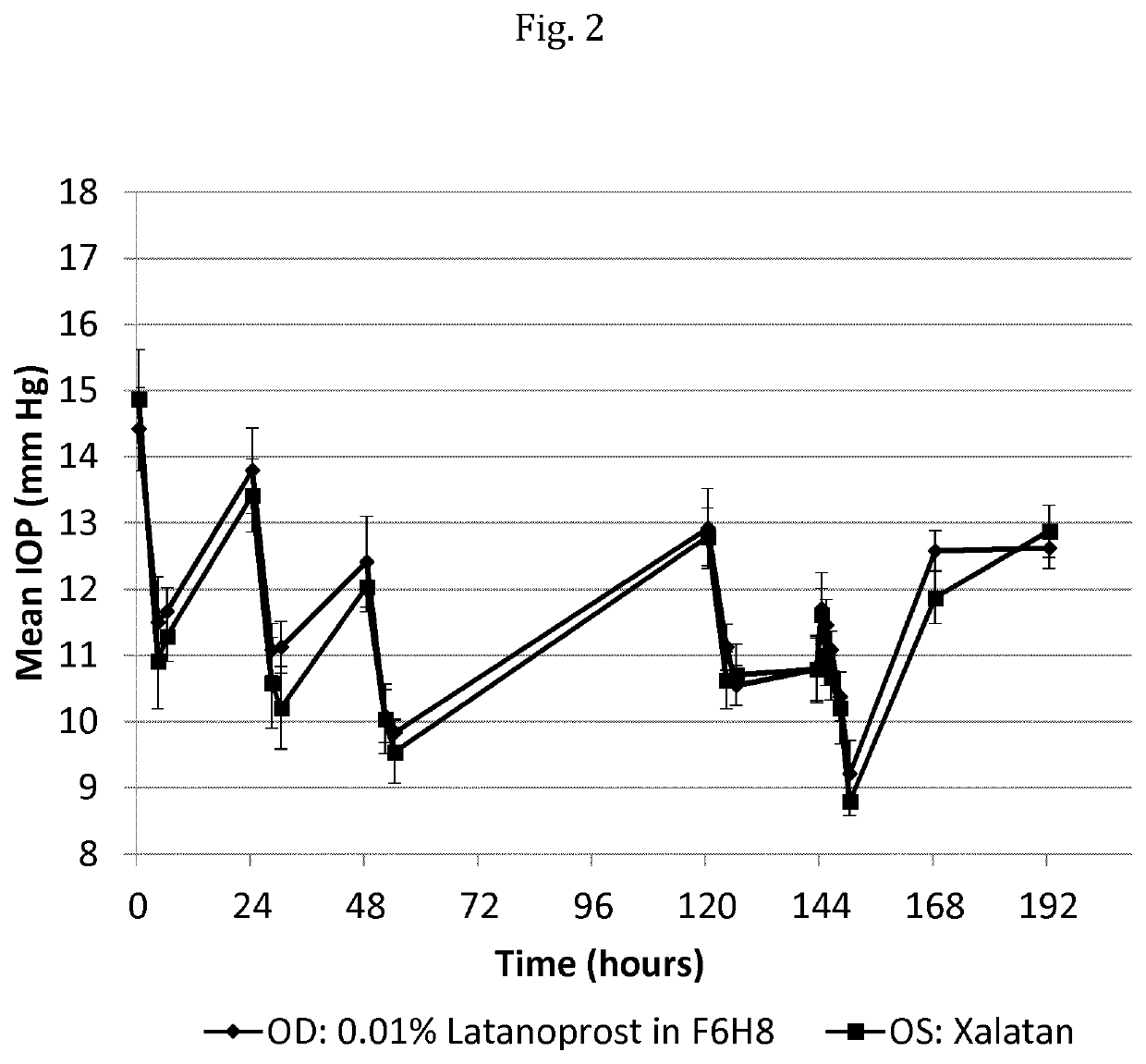
The invention provides a pharmaceutical composition for use in the prevention or therapy of glaucoma, increased intraocular pressure, ocular hypertension and / or a symptom associated therewith, wherein—the composition comprises latanoprost and a liquid vehicle comprising a semifluorinated alkane; and—the composition is administered to the eye of a subject; and—the amount of latanoprost administered in a single dose per eye is in the range of from about 0.5 to 1.4 μg.
Owner:NOVALIQ GMBH
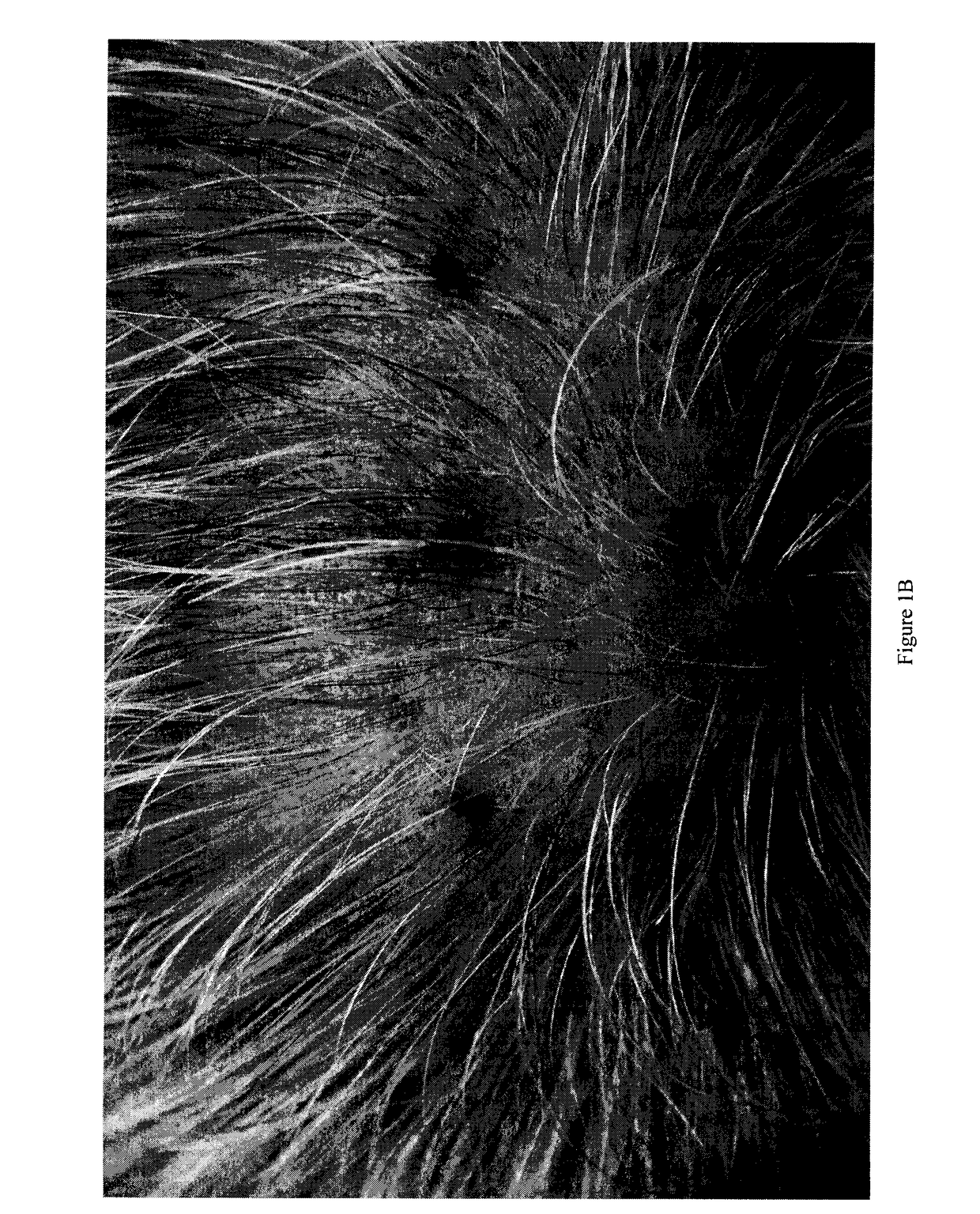
Compositions for Reducing Hair Loss and/or Increasing Hair Regrowth
InactiveUS20170100319A1Reduces hair lossIncreases hair regrowthOrganic active ingredientsCosmetic preparationsLatanoprostMedicine
The invention relates to a composition comprising 2% to 5% minoxidil, 0.01% to 15% finasteride and 0.01% to 15% of a prostaglandin analogue. In one embodiment, the prostaglandin analogue is latanoprost. In a preferred embodiment, the composition comprises 5% minoxidil, 0.1% finasteride and 0.03% latanoprost. The invention also relates to the use of the said composition to reduce hair loss and / or increase regrowth of hair in a human subject.
Owner:TRIPLE HAIR
High-purity Latanoprost, preparation method therefor and use of Latanoprost
InactiveCN106467465ALittle side effectsHigh yieldOrganic active ingredientsGroup 4/14 element organic compoundsLatanoprostWittig reaction
The invention discloses high-purity Latanoprost, a preparation method therefor and use of the Latanoprost. According to the high-purity Latanoprost disclosed by the invention, the content of an impurity with a structure represented by a formula II shown in the description in the high-purity Latanoprost is not higher than 0.1%. The preparation method comprises the steps: (1) subjecting a compound represented by a formula IV shown in the description (an intermediate 1 of the Latanoprost) to a wittig reaction, so as to obtain a compound represented by a formula V shown in the description (an intermediate 2 of the Latanoprost); (2) subjecting the compound V to washing and purifying by a ammonium chloride solution, then, carrying out dehydroxylation protection or not, and carrying out a reaction with iodo-isopropane, so as to obtain a crude raw pharmaceutical material of the Latanoprost; and (3) loading a sample of the raw pharmaceutical material of the Latanoprost to a silica-gel column, carrying out eluting with an eluate, and carrying out chromatographic purification, thereby obtaining the high-purity Latanoprost.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com