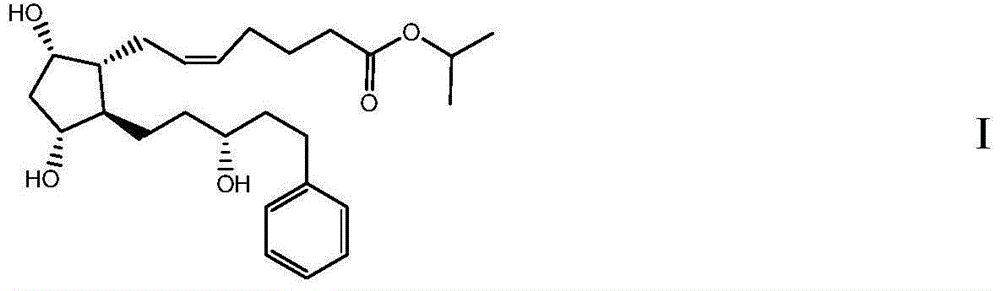
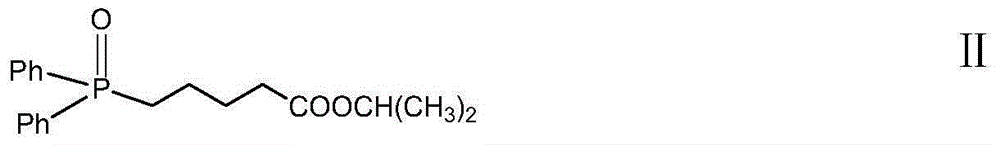High-purity Latanoprost, preparation method therefor and use of Latanoprost
A latanoprost and high-purity technology, which is applied in the field of high-purity latanoprost and its preparation, can solve the problems that the separation method is not efficient enough, perfect, complicated and difficult to achieve, and is beneficial to industrial production and preparation And the purification process is simple, the effect of removing impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Formula IV compound (intermediate 1, R 1 = H, R 2 =H) Preparation of a composition containing latanoprost
[0092]
[0093] In a four-necked bottle, N 2 For protection, put in 4-carboxybutyltriphenylphosphine bromide (247.6g) and THF (270ml), stir, cool to -5°C, add 1mol / L tetrahydrofuran solution (1039ml) of t-BuOK dropwise. The temperature is controlled at -5~0°C, after 1 hour of dripping, stir at this temperature for 40min after dripping, then raise the temperature to 10~15°C and stir for 60min, cool to -30~-20°C, add dropwise 1-1 (41.1g ) THF (410ml) solution, after 1.5h dropwise, maintain the temperature and stir overnight after dropwise. The next day, add saturated ammonium chloride solution (about 350g / L, 400ml) and ethyl acetate (1600ml), stir for 15min after the temperature rises to 10-20°C, separate the layers, and extract the aqueous layer with EA (400ml, 200ml). All organic layers were combined, washed successively with 700ml of saturated ammonium chl...
Embodiment 2
[0097] Formula IV compound (intermediate 1, R 1 = tert-butyldimethylsilyl, R 2 =H) Preparation of a composition containing latanoprost
[0098]
[0099] In a four-necked bottle, N 2 For protection, put in TPBA (247.6g) and THF (270ml), stir, cool to 0-5°C, add 1mol / L tetrahydrofuran solution (1040ml) of t-BuOK dropwise, and control the temperature at -5-0°C during the dropwise addition. After 1 hour of dropping, stir at this temperature for 10 minutes, then raise the temperature to 8-12°C and stir for 45 minutes, cool to -30--20°C, add intermediate 1-2 (47.0g) in THF (370ml) dropwise, After 1.5 hours of dripping, the temperature was maintained and stirred overnight. The next day, add saturated ammonium chloride solution (about 350g / L, 400ml) and EA (1600ml), rise to 10-20°C, stir for 15min, separate the layers, extract the aqueous layer with EA (400ml, 300ml), and combine all organic layer, washed successively with half-saturated ammonium chloride (about 180g / L), satura...
Embodiment 3
[0104] Formula IV compound (intermediate 1, R 1 = R 2 =tetrahydropyranyl) for the preparation of compositions containing latanoprost
[0105]
[0106] In a four-necked bottle, N 2 For protection, put in TPBA (247.6g) and THF (270ml), stir, cool to 0-5°C, add 1mol / L tetrahydrofuran solution (1040ml) of t-BuOK dropwise, and control the temperature at -5-0°C during the dropwise addition. After 1 hour of dropping, stir at this temperature for 10 minutes, then raise the temperature to 8-12°C and stir for 45 minutes, cool to -30--20°C, add intermediate 1-3 (51.0g) in THF (390ml) dropwise, After 1.5 hours of dripping, the temperature was maintained and stirred overnight. The next day, add half-saturated ammonium chloride solution (about 180g / L, 400ml) and EA (1600ml), rise to 10-20°C, stir for 15min, separate the layers, extract the aqueous layer with EA (400ml, 300ml), and combine all The organic layer was washed successively with half-saturated ammonium chloride (about 180g / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com