Latanoprost-containing aqueous eye drops and method for inhibiting adsorption of latanoprost to resin
A technology for latanoprost and eye drops, which is applied in the field of inhibiting the adsorption of latanoprost to resin, can solve problems such as insufficient inhibition effect, and achieve the effects of improving thermal stability and inhibiting adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
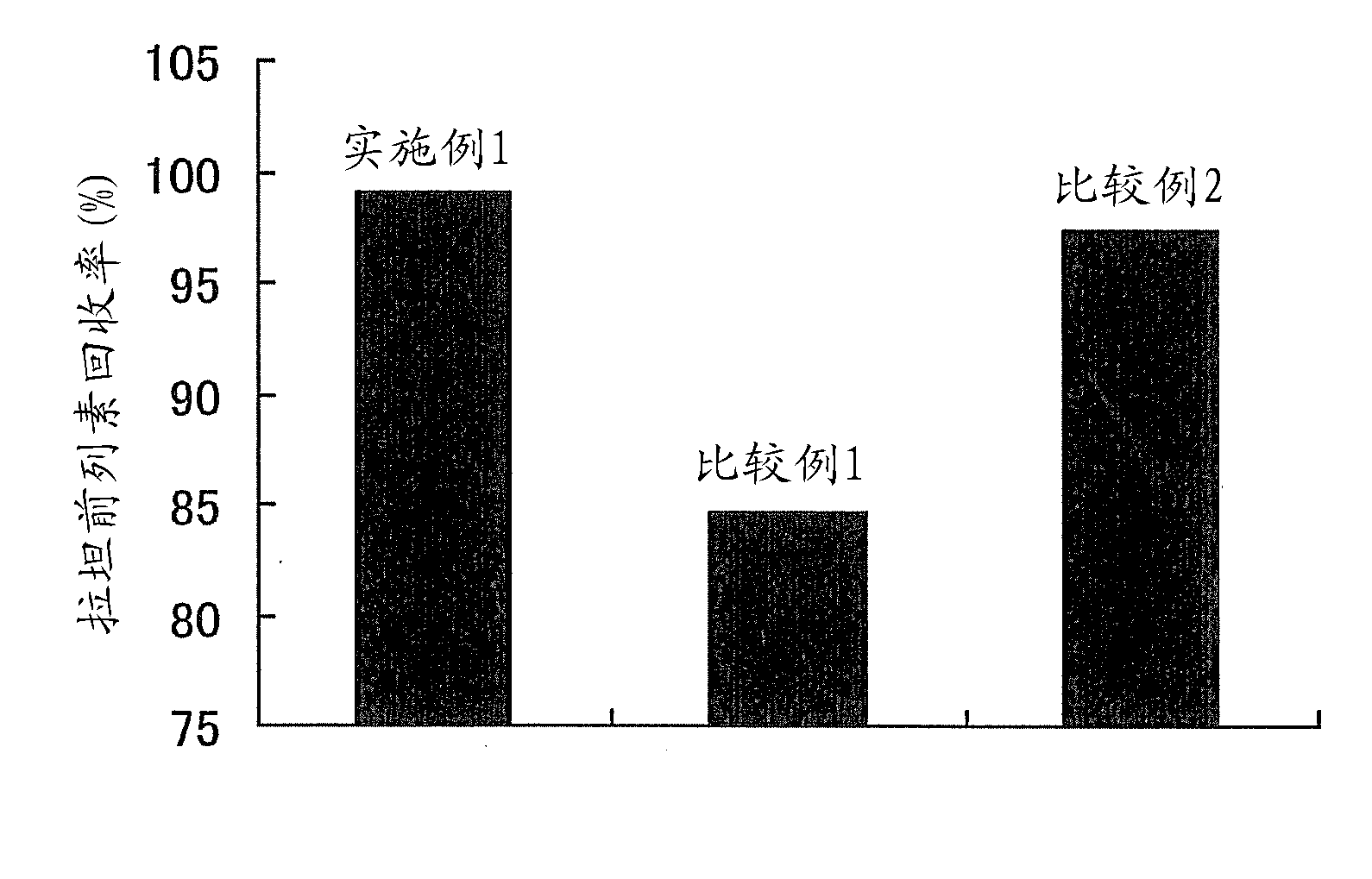
[0163] [Example 1] Aqueous eye drops
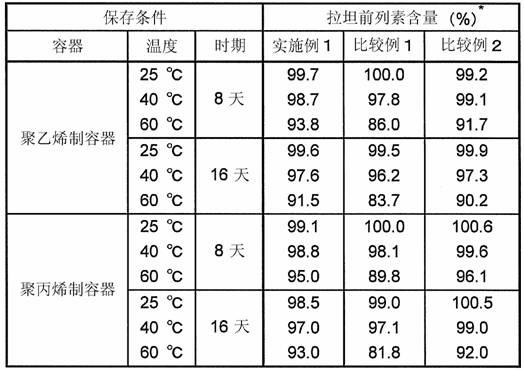
[0164] The formulation of the aqueous eye drops of Example 1 is shown in Table 1 together with the formulations of the aqueous eye drops of Comparative Examples 1 and 2. For their preparation, first, (1) in Table 1 was added to (8) (10% benzalkonium chloride solution), stirred and dissolved at about 50° C. to prepare a stock solution of latanoprost. Add (2)-(7) and an appropriate amount of (11) into another container, stir to dissolve, and prepare a base stock solution. Add all the basic stock solution to the latanoprost stock solution, stir well, add (9) and (10) to adjust the pH to 6.7, add (11) to make the total amount 1L.
[0165] 【Table 1】
[0166]
Embodiment 2~6
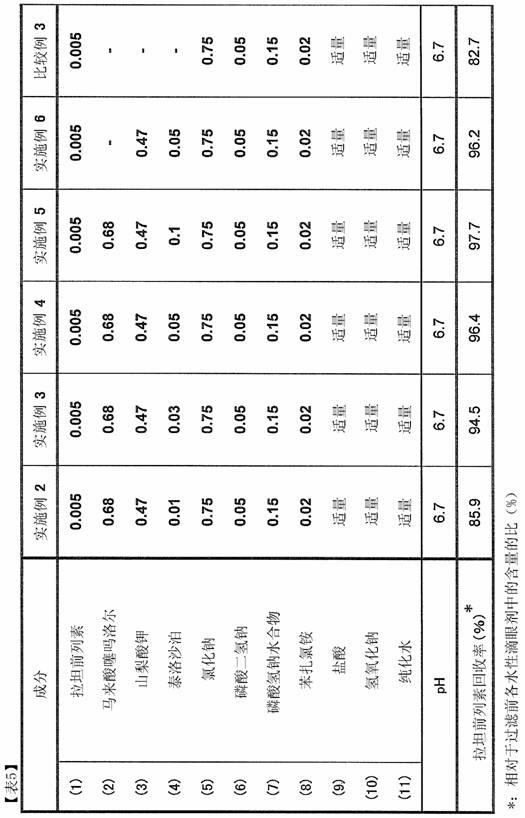
[0197] [Examples 2-6] Aqueous eye drops
[0198] The formulations of the aqueous eye drops of Examples 2 to 6 are shown in Table 5 together with the formulation of the aqueous eye drops of Comparative Example 3. In Table 5, the numerical values for each component are represented by content ((w / v)%). "Appropriate amount" means the amount required to make the pH of the aqueous eye drops 6.7 for hydrochloric acid and sodium hydroxide, and means the amount to make the total amount 100 (w / v)% for purified water. These aqueous eye drops were prepared in the same manner as in Example 1.
Embodiment 7~11
[0203] [Examples 7-11] Aqueous eye drops
[0204] In this example, in order to more accurately evaluate the effect of surfactants and sorbic acid on inhibiting the adsorption of latanoprost to the resin, a formulation without benzalkonium chloride interacting with sorbic acid was applied.
[0205] Table 6 shows the formulations of the aqueous eye drops of Examples 7-11 and Comparative Examples 10-13. In Table 7, the numerical values for each component are represented by content (w / v%).
[0206] The aqueous eye drops of Examples 7 to 11 and Comparative Examples 10 to 13 were prepared as follows: (1) to (10) in Table 6 were added to (14), stirred at about 80°C, and after dissolving, added to (12) and (13) Adjust the pH to 6.7, and use (14) to make the total amount 200 mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com