Complexes of prostaglandin derivatives and monosubstituted, charged beta-cyclodextrins
A technology of prostaglandins and derivatives, applied in the field of treatment of ocular hypertension and glaucoma, can solve problems such as itching, patient discomfort, burning, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Dissolution of Latanoprost
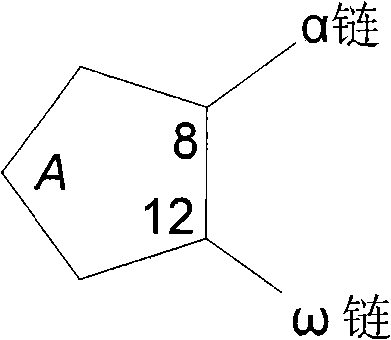
[0057] Libraries of unsubstituted and monosubstituted cyclodextrins were screened to identify those cyclodextrin derivatives with the highest ability to solubilize latanoprost. Complexation with cyclodextrin derivatives to latanoprost at a molar ratio of 5:1 in ultrapure water corresponds to a concentration of 115.58 mM and 23.12 mM for cyclodextrin derivatives and latanoprost, respectively. concentration. Because of the known sensitivity of latanoprost to light, high temperature, and oxidation, experiments were performed at room temperature in the dark and under nitrogen. The selectivity results are shown in Table 1. It has been found that with a single C in the glucose unit 6 Contains -NH in position 2 + -(CH 2 ) p -NH 3 + or -NH 2 + -(CH 2 ) p β-cyclodextrins with -OH (p is 2 to 6) type substituents lead to a significant increase in the water solubility of latanoprost, illustrative examples are mono-6-deoxy-6-diami...
Embodiment 2
[0061] Example 2: Latanoprost, bimatoprost and travoprost in 50 mM mono-6-deoxy-6-diaminopropyl-β-cyclodextrin, mono-6-deoxy-6-aminopropanol - Beta-cyclodextrin or solubility in water
[0062] Table 2: Dissolution of Prostaglandin Derivatives
[0063]
Embodiment 3
[0064] Example 3: Characterization of Complexation: Latanoprost-Cyclodextrin Complex
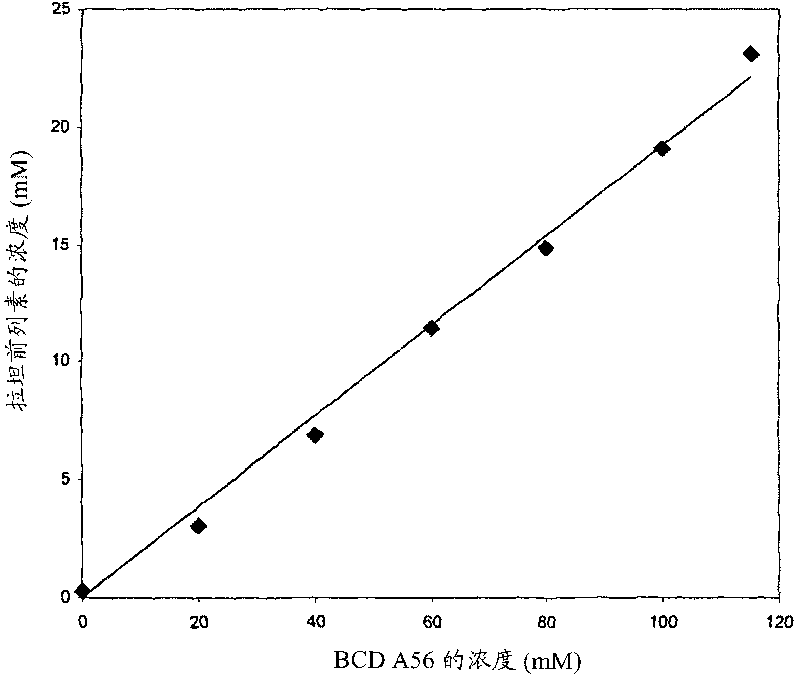
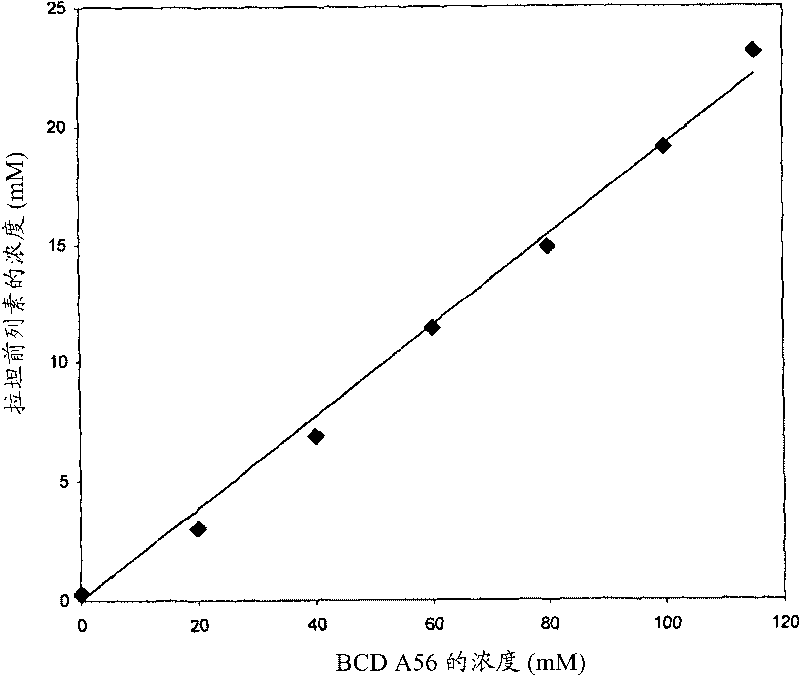
[0065] The minimum time required for the guest molecule, latanoprost, to reach maximum solubility in a solution containing 115 mM mono-6-deoxy-6-diaminopropyl-β-cyclodextrin was determined. After the addition of latanoprost, the resulting suspension was sonicated for 5 min and then stirred magnetically for 48 h at room temperature in the dark. Aliquots were removed at 0, 1, 3, 6, 12, 24 and 48 h. Each aliquot was filtered through a 0.45 μm PVDF (Millipore / Whatman) membrane, and the filtrate was diluted for quantitative analysis of latanoprost by HPLC. The results showed that the dissolution equilibrium was reached at about 24h. Subsequent experiments were performed after 24 h of equilibration.
[0066] Phase solubility diagrams were constructed to examine the increase in the solubility of latanoprost in the presence of increasing concentrations of mono-6-deoxy-6-diaminopropyl-β-cyclodextr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com