Latanoprost eye drop
A technology for latanoprost and eye drops, which is applied in the directions of aerosol delivery, medical preparations of inactive ingredients, organic active ingredients, etc. Overcome the short residence time, improve water solubility, and inhibit the effect of decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
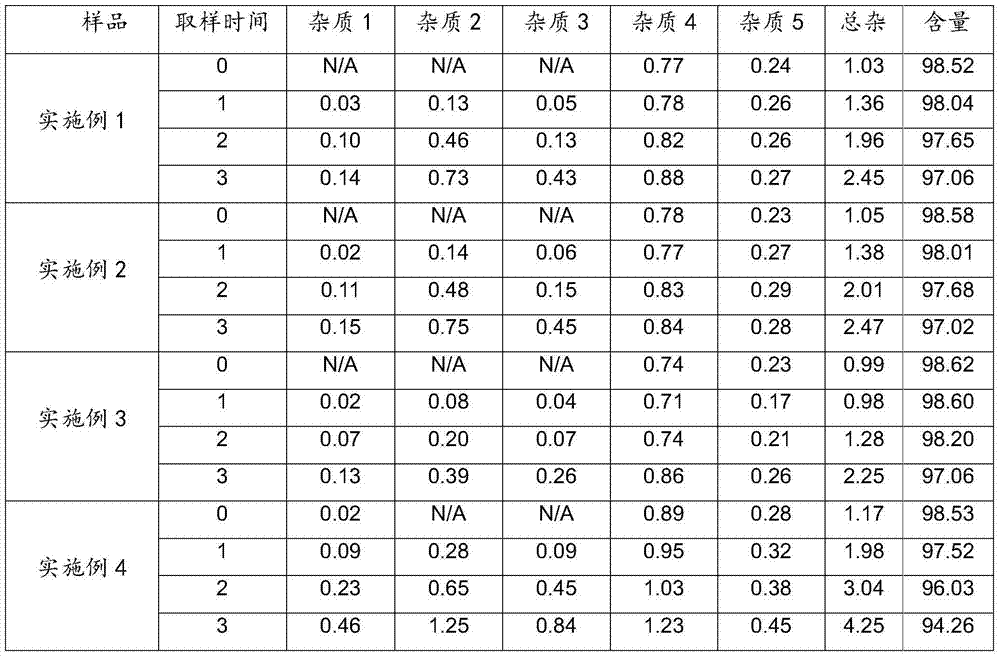
Embodiment 1
[0034] Latanoprost 0.005%, Tween-80 0.1%, Polycarbophil 0.9%, Disodium EDTA 0.1%, Sodium Dihydrogen Phosphate 0.5%, Disodium Hydrogen Phosphate 0.4%, Benzalkonium Chloride 0.02%, the balance is water;
[0035] Material name
Prescription ratio (%)
effect
Latanoprost
0.005
Main drug
Tween 80
0.1
Surfactant
Polycarbophil
0.9
thickener
0.1
0.5
Osmotic pressure and pH regulator
0.4
Osmotic pressure and pH regulator
0.002
preservatives
Water for Injection
add up to 100
[0036] The preparation method is as follows:
[0037] 1): Take out latanoprost with a capillary tube, accurately weigh latanoprost, benzalkonium chloride, and Tween-80 to the bottom of the beaker according to the prescription amount, then add a small amount of wat...
Embodiment 2
[0042] Latanoprost 0.005%, Tween-80 0.1%, Polycarbophil 0.1%, Poloxamer 0.2%, Disodium EDTA 0.1%, Sodium Dihydrogen Phosphate 0.5%, Disodium Hydrogen Phosphate 0.4%, the balance is water;
[0043] The preparation method is the same as in Example 1.
Embodiment 3
[0045] Latanoprost 0.005%, Tween-80 0.3%, Polycarbophil 0.05%, Poloxamer 0.2%, Disodium EDTA 0.15%, Sodium Dihydrogen Phosphate 0.4%, Disodium Hydrogen Phosphate 0.32%, benzalkonium chloride 0.02%, the balance is water;
[0046] Prepare with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Osmotic pressure | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com