Sustained release latanoprost implant
a technology of latanoprost and implant, which is applied in the field of biodegradable intraocular implants, can solve the problems of extremely difficult molecule incorporation into biodegradable implants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0110]
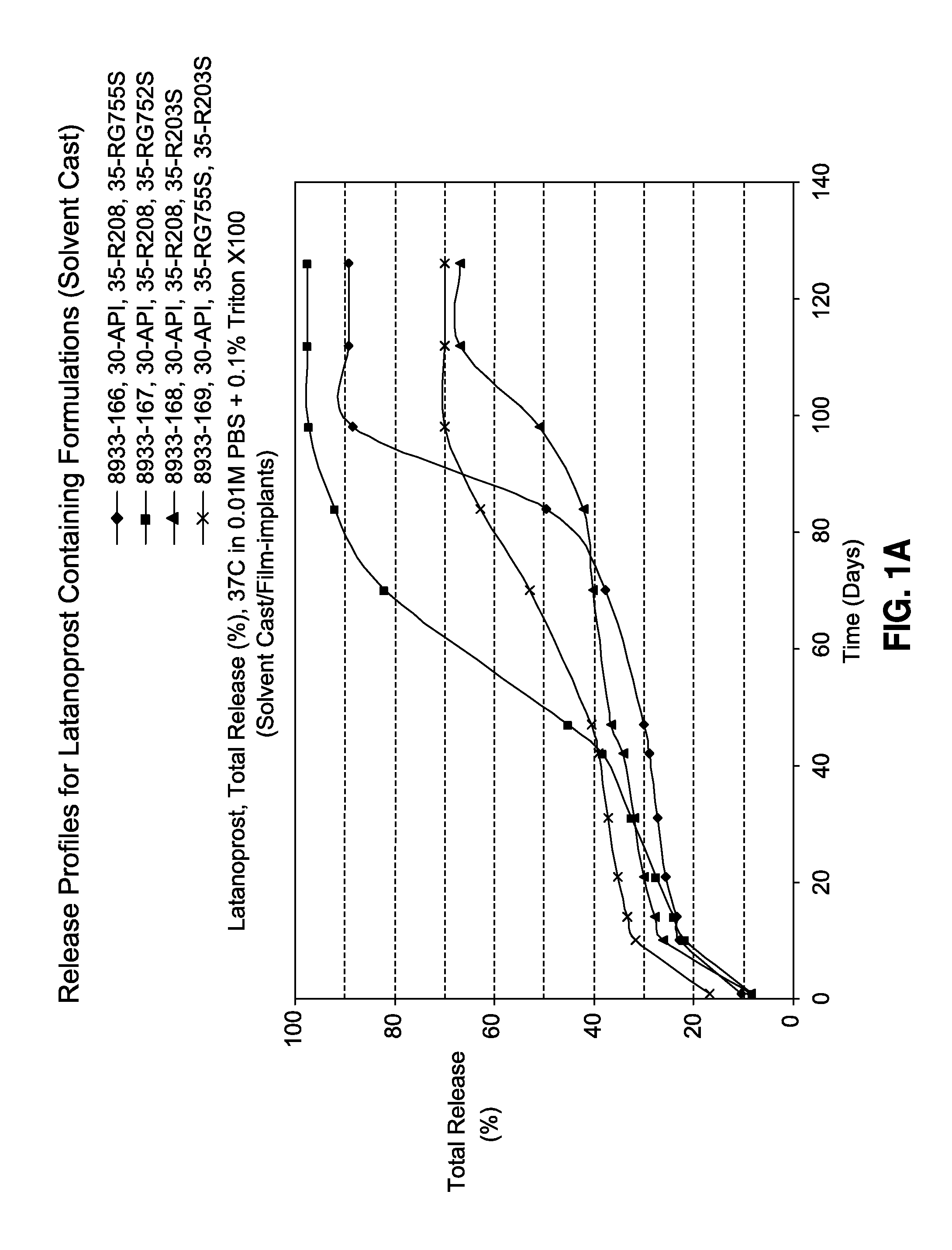
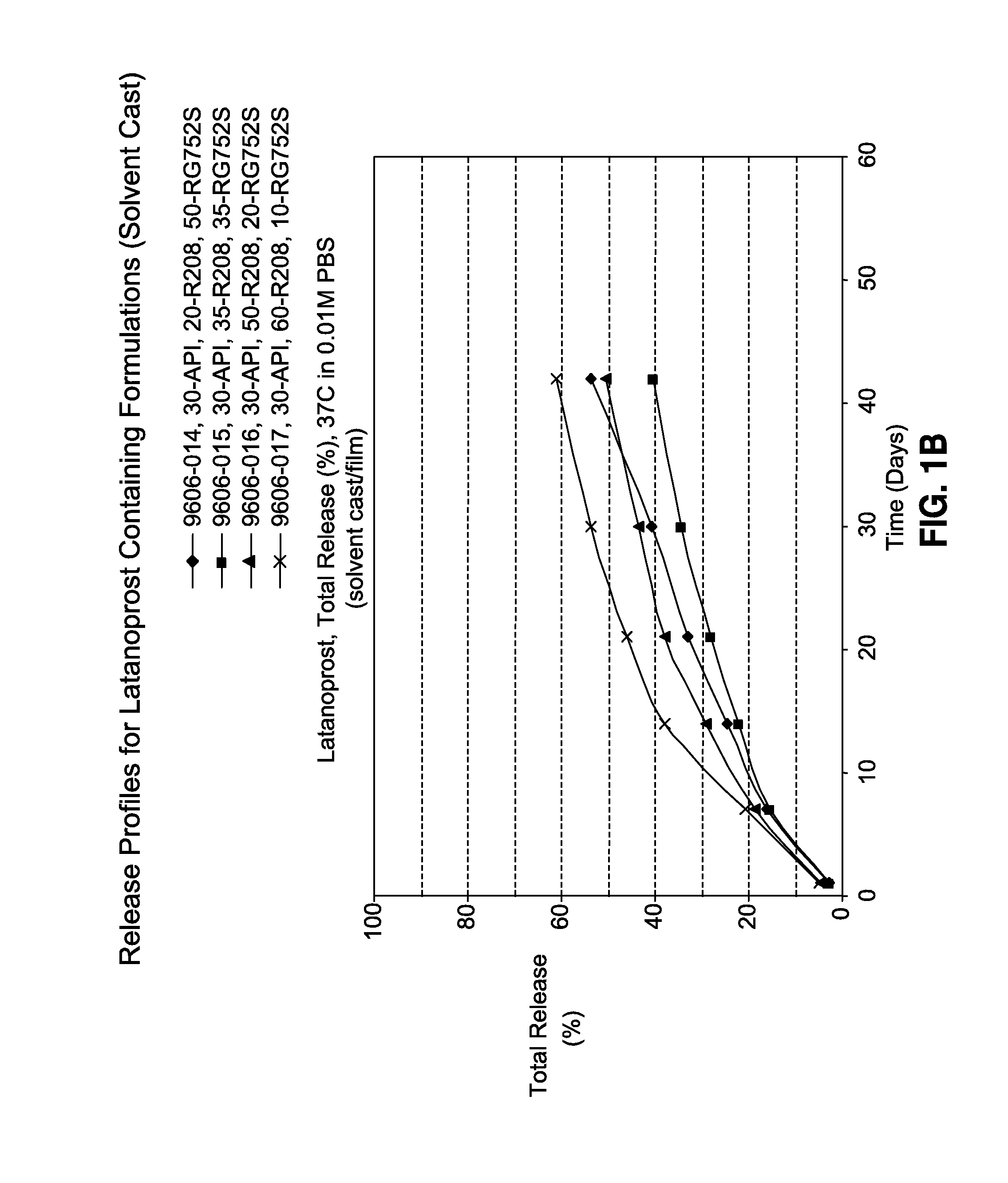
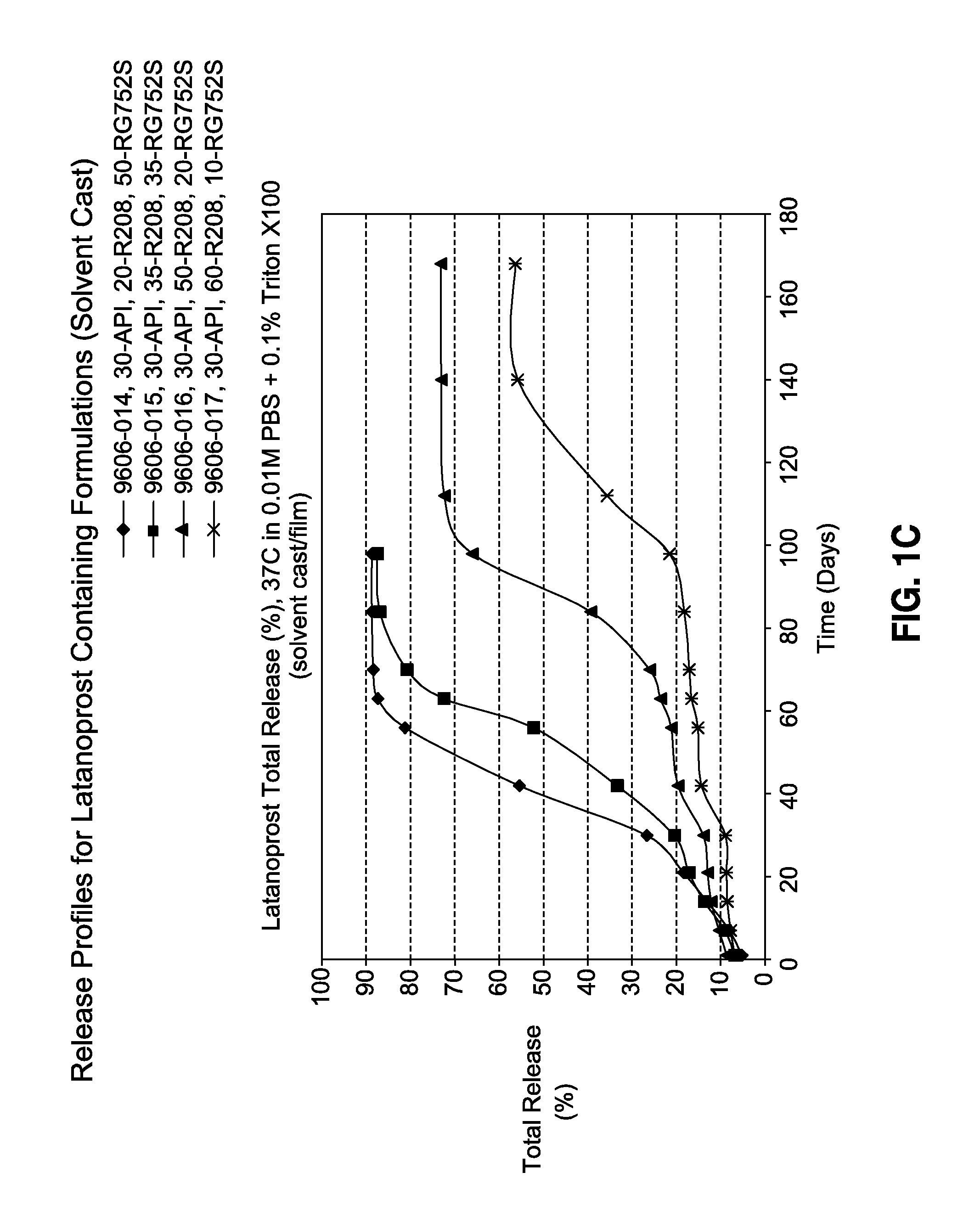
TABLE 1Latanoprost Containing Film Formulationsw / w, %ResomerResomerResomerResomerFormulation NoLatanoprostR208R203SRG752SRG755SR-2007-8933-16630350035R-2007-8933-16730350350R-2007-8933-16830353500R-2007-8933-16930035035R-2009-9606-01430200500R-2009-9606-01530350350R-2009-9606-01630500200R-2009-9606-01730600100
[0111]Release studies were performed in triplicates as follows. The dried film was cut using 4-mm biopsy punch (approximately 2.0-mg), and was placed into a 10-mL vial containing 0.01M phosphate buffered saline (pH 7.4)+0.1% Triton X100. The samples were then transferred into a shaking water bath set at 37° C. and 50 rpm. At various time-points, the solution was removed and analyzed by HPLC for the amount of released latanoprost. The removed solution is replaced with fresh phosphate buffered saline solution. Drug release profiles are shown in FIGS. 1A-C.
[0112]The film-shaped implants made by solvent casting can be cut to any shape and dimension. One example is a disc shap...
example 2
[0113]This example prepares an extruded solid polymer implant containing latanoprost. While the above discussion relates mainly to the problems of incorporating latanoprost in a film oily drug substances (DS) are also very difficult to incorporate into hot-melt extruded implants because they exude the oily DS when heated. It is important to keep the extrusion temperature as low as possible to avoid loss and degradation of the DS. This can be overcome by using a select combination of polymers and a plasticizer like PEG that are compatible with the drug substance. The oily DS and PEG plasticize the polymers to a degree that allows the mixture to be extruded at a temperature where the DS is not degraded or lost. Suitable formulations are shown in Table 2.
[0114]The polymer implants were made by hot-melt extrusion using a mechanically driven ram microextruder, but they can also be made by direct compression or solvent casting. The implants are rod-shaped, but they can be made into any ge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com