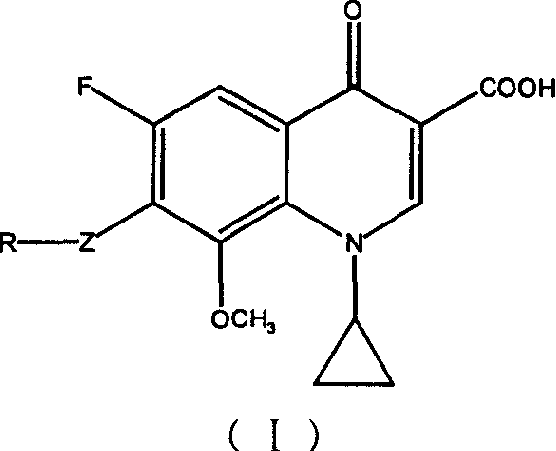
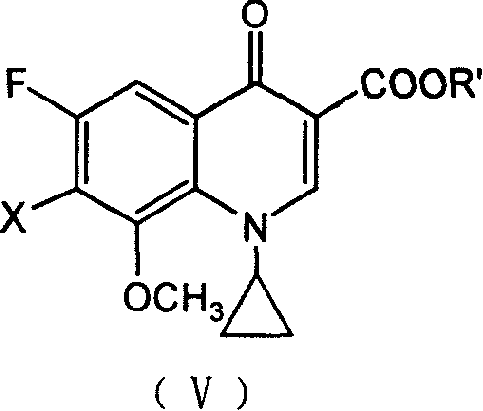
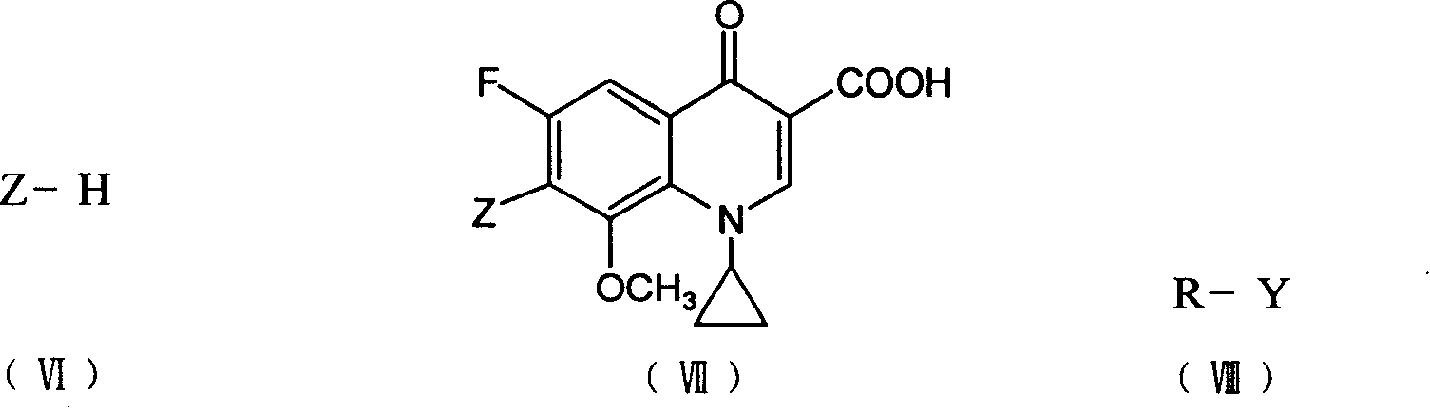
7-substituted-8-methoxy fluoroquinolone carboxylic derivatives, preparing process, preparation and use thereof
A methyl, representative technology, applied in the field of 7-substituted-8-methoxyfluoroquinolone carboxylic acid derivatives, can solve the problems of central nervous system toxicity and side effects, and achieve the effect of high antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] 1-cyclopropyl-6-fluoro-7-[(S)-3-methyl-4-methyl-1-piperazinyl]-1,4-dihydro-8-methoxy-4-oxo Dai-3-quinolinecarboxylic acid (No.1, supergafloxacin)
[0075] Mix 16.2g (22.3ml, 0.16mol) of triethylamine, 40.5ml of dimethylsulfoxide and 16.1g (0.16mol) of (S)-2-methylpiperazine, and add 33.92g (0.0802mol) of 1- Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate bisacetoxy borate (chelate), at about 50°C React for 5 hours, TLC detection, after the reaction is complete, distill under reduced pressure to dryness, add water and hydrolyze the residue, neutralize it with alkali, filter the precipitate, and recrystallize with ethanol to obtain light yellow crystals of levo-gatifloxacin hemihydrate 12.7g, yield 41.2%, mp189~191°C (dec).
[0076] Mix 18.5g (0.048mol) of levo-gatifloxacin hemihydrate and 108ml of 37% formaldehyde solution, heat slightly, add 180ml of formic acid, react at about 60°C for 6 hours, evaporate to dryness under reduced pressure, ...
Embodiment 2
[0084] 1-cyclopropyl-6-fluoro-7-[(S)-3-methyl-4-(5-methyl-2-oxo-1,3-dioxol-4-yl) Methyl-1-piperazinyl]-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid (No.2, saprofloxacin)
[0085] Levo-gatifloxacin hemihydrate is obtained in the same way as example 1, 15.4g (0.04mol) levo-gatifloxacin hemihydrate, 12g (0.08mol) 4-chloromethyl-5-methyl-1, 3-dioxol-2-ketone and 200ml N, N-dimethylformamide are mixed, add 4.8 grams of KHCO 3 , reacted at 0°C for 9 hours, evaporated to dryness under reduced pressure, washed the residue with water to obtain a yellow crude product, and obtained 14 g of light yellow powder by column chromatography, yield 71.8%, mp 165.2~166.4°C.
[0086] MS(EI, 70V, M / Z): 488[M+H] +
[0087] IR (KBr, cm -1 ): 3446, 2845, 1727, 1623, 1461, 1329, 1063
[0088] 1 H-NMR (dimethylsulfoxide-d 6 , δ, ppm): 1.13 (4H, m, C a,a′ -H), 1.35(3H, m, C 13 -CH 3 ), 8.72 (1H, S, C 2 -H), 7.80 (1H, d, C 5 -H), 3.74 (3H, S, OCH 3 ), 4.35 (1H, m, C b -H), 3.46-3.3...
Embodiment 3
[0093] 1-cyclopropyl-6-fluoro-7-[(R)-3-methyl-4-methyl-1-piperazinyl]-1,4-dihydro-8-methoxy-4-oxo Substituent-3-quinolinecarboxylic acid (No.3)
[0094] Mix 16.2g (22.3ml, 0.16mol) of triethylamine, 40.5ml of dimethylsulfoxide and 16.1g (0.16mol) of (R)-2-methylpiperazine, and add 33.92g (0.0802mol) of 1- Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinoline carboxylic acid diacetoxy borate (chelate) reacts at about 50°C After 5 hours, TLC detection, after the reaction was complete, distilled to dryness under reduced pressure, hydrolyzed the residue with water, neutralized to neutral with alkali, filtered the precipitate, and recrystallized with ethanol to obtain light yellow crystals of dextro-gatifloxacin hemihydrate 12.9g, yield 41.8%, mp187~189°C.
[0095] Dissolve 18.5g (0.048mol) of dextro-gatifloxacin hemihydrate in 200ml of acetonitrile, add 8.5ml of triethylamine, 6ml (0.096mol) of methyl iodide, react at room temperature for 10 hours, concentrate to dryn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com