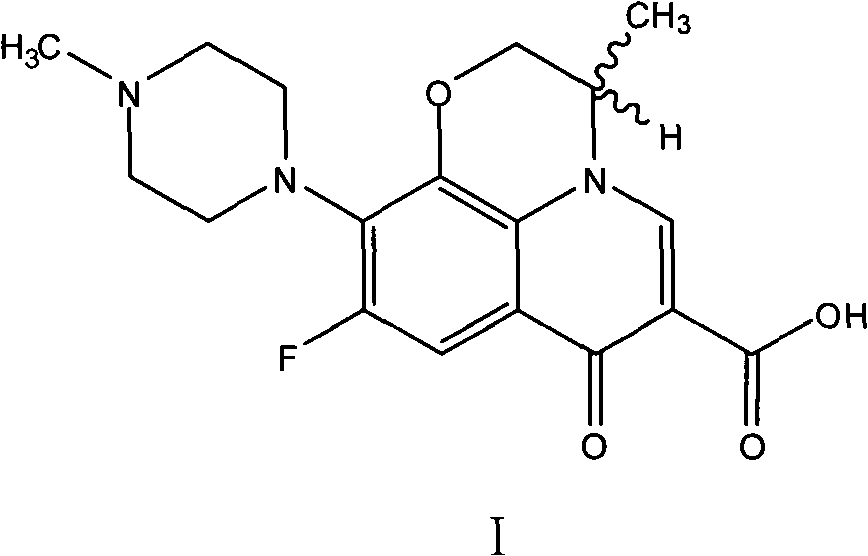
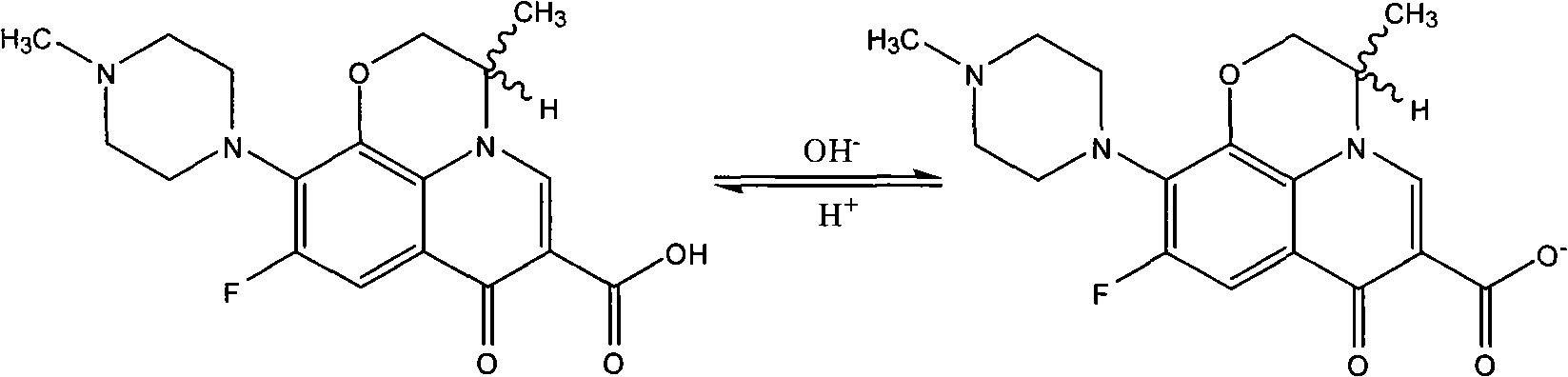
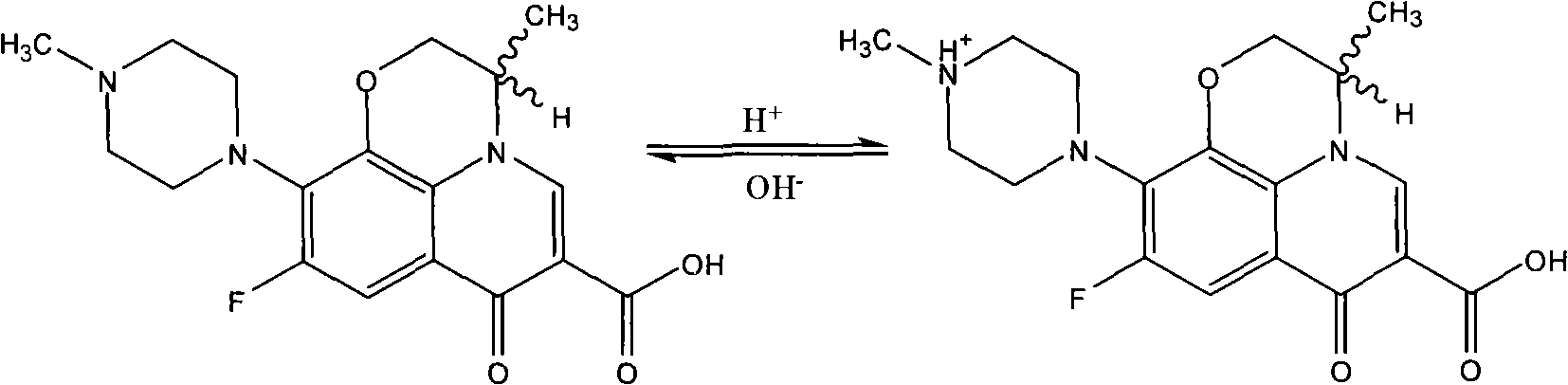
Patents
Literature
399 results about "Ofloxacin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat a variety of bacterial infections.
Ophthalmic, otic or nasal pharmaceutical composition and the use thereof
InactiveUS20100222308A1Effective treatmentPreventing increase of bacterial infection riskAntibacterial agentsBiocideInfective rhinitisNose
The invention provides an ophthalmic, otic or nasal pharmaceutical composition, comprising levofloxacin or the pharmaceutical acceptable salts thereof and loteprednol etabonate, wherein the weight ratio of loteprednol etabonate to levofloxacin is 1:0.2-5. The use of ophthalmic, otic or nasal pharmaceutical composition of the invention in preparation of the medication for treatment of conjunctivitis, keratitis, blepharitis, dacrycystitis, hordeolum, corneal ulcer and ocular infection accompanied with ophthalmitis and even inflammation of the surrounding tissues, to prevent increase of bacterial infection risks and the tissue inflammation of the infected area after the ophthalmic surgeries or ocular injuries, to treat or alleviate the bacterial infection in combination with the tissue inflammation of the infected area, or to treat tympanitis, otitis externa and infective rhinitis.
Owner:SHENZHEN REGOO LAB
Aerosolized fluoroquinolones and uses thereof
ActiveUS7838532B2Reduce riskHigh levelPowder deliveryHeavy metal active ingredientsAerosol drugsLevofloxacin
Owner:HORIZON ORPHAN LLC
Vanadate nanofiber photocatalyst and preparation method thereof
InactiveCN103272576AVisible light catalytic degradation ability is goodNarrow band gapWater/sewage treatment by irradiationCatalyst activation/preparationSolubilityBismuth vanadate
The invention relates to a vanadate nanofiber photocatalyst and a preparation method thereof. The nanofiber photocatalyst is pucherite, or silver vanadate, or a compound of pucherite and silver vanadate. Organic vanadic salt, organic bismuth salt and the like with good alcohol solubility are taken as precursor reactors, a spinning solution is prepared by a PVP (Polyvinyl Pyrrolidone) and alcohol system, electrospining is performed by an electrospining device, then high-temperature roasting is performed, and the vanadate nanofiber photocatalyst is obtained. A prepared vanadate nanofiber is in a monoclinic crystal phase, is 30-100nm in diameter, has a narrower band gap, shows good visible light catalytic degradation ability for ofloxacin, a contaminant in water, and can be separated from a solution quickly by precipitation.
Owner:QINGDAO AGRI UNIV
Pure levofloxacin hemihydrate and processes for preparation thereof
The invention relates to pure levofloxacin hemihydrate and a process for preparing pure levofloxacin hemihydrate. The invention also relates to pharmaceutical compositions that include the pure levofloxacin hemihydrate and use of said compositions for treating a patient in need of an antimicrobial therapy.
Owner:RANBAXY LAB LTD
Medicament composition for eyes or nose, and uses thereof
The present invention provides a combination of medicines for eyes or ears and nose, which comprises levofloxacin and loteprednol carbon ester; wherein, the weight ratio of the loteprednol carbon ester to the levofloxacin is between 1 to 0.2 and 1 to 5. The combination of medicine for eyes or ears and nose of the present invention is used for curing conjunctivitis, keratitis, blepharitis, dacryocystitis, hordeolum, corneal ulcer and eye infection with inflammation of eyes or even inflammation of tissue around eyes. The combination is also used for preventing bacterial infection risk after ophthalmology operation or eye injury and inflammation of the infected region, or the combination is used for curing or alleviating bacterial infection and tissue inflammation of the infected region after ophthalmology operation or eye injury, or cure tympanitis, otitis externa and infectious rhinitis.
Owner:SHENZHEN REGOO LAB
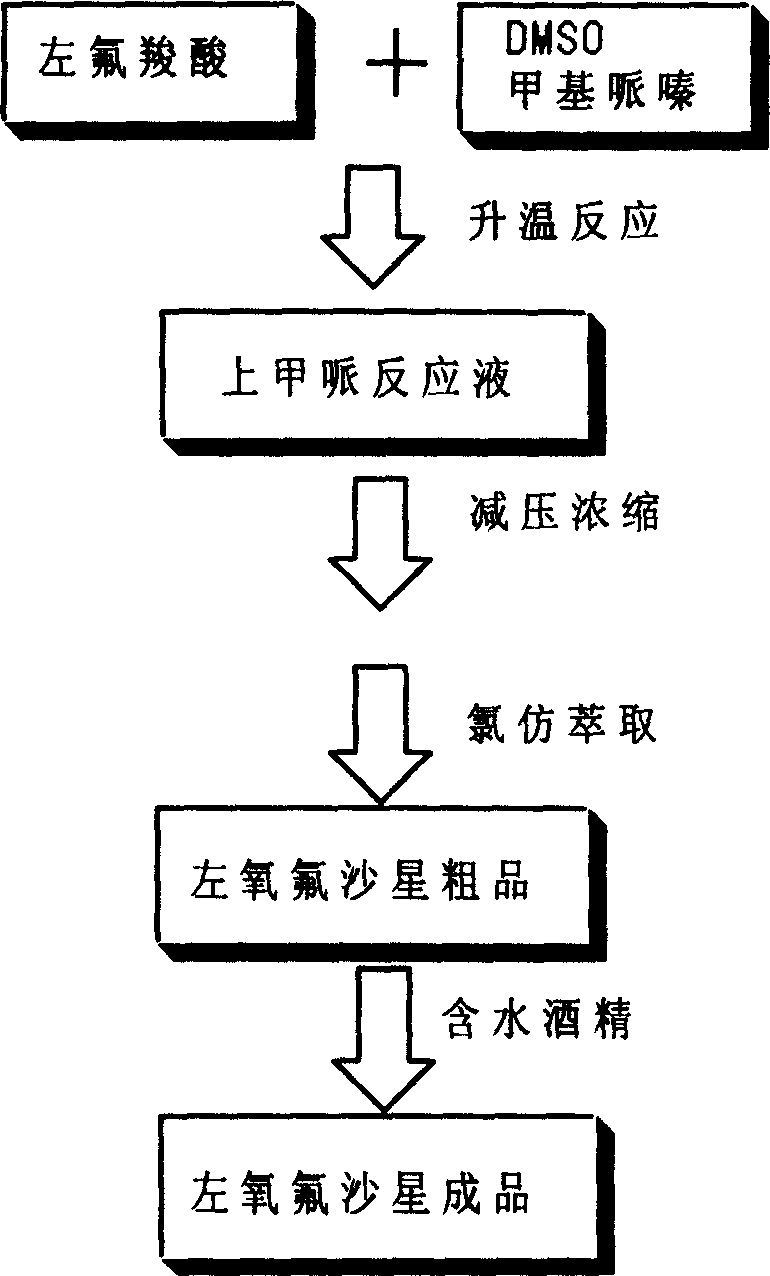
Left ofloxacin and Pidotimod compound preparation tech and its appts.
Tea leaves is used as raw material, by using water or solvent, to extract tea-polyphenol, then settle, centrifugal separation, column absorbing, extracting by ethyl acetate, vacuum concentrating, drying to obtain crude tea-polyphenol. Said crude product is then dissolved in water, absorbed by resin column, washed-off by agent gradient adding-in, based-on standard flowing curve to obtain 7 monomer catechins with purity>99%. For this purpose, full automatic absorbing resin column is equiped with metering pump to adjust fed-in of water, solution and solvent, HPLC real-time monitoring and pick-correcting to washing-off liquid is achieved by proportional flow-splitter. Automatic back-wash and resin-exchanging are achieved by being equiped with over-flow hole, resin discharging hole and others.
Owner:胡绍海
Pithecellobium clypearia extracts and application of extract in preparation of medicines for treating methicillin-resistant staphylococcus aureus
ActiveCN103385912AHas a sensitizing effectRealize comprehensive utilizationAntibacterial agentsPlant ingredientsEthyl acetatePharmaceutical Substances
The invention discloses water, ethanol and ethanol aqueous extracts of traditional Chinese medicine pithecellobium clypearia and application of corresponding petroleum ether, ethyl acetate, normal butanol and a water extractants in preparation of medicines for treating methicillin-resistant staphylococcus aureus (MRSA) and antibiotics anti-MRSA sensitization medicines. Meanwhile, the invention further discloses a preparation method of the extracts or extractants. Experimental results show that water, 10% ethanol, 30% ethanol, 60% ethanol, 95% ethanol extracts of pithecellobium clypearia and corresponding ethyl acetate, normal butanol and water extractants have stronger anti-MRSA effect, wherein the activity of the ethyl acetate extracting part of the 60% ethanol extract of pithecellobium clypearia is the strongest, and the ethyl acetate extracting part of the 60% ethanol extract of pithecellobium clypearia has sensitization effect on erythrocin, ceftriaxone sodium, levofloxacin for treating MRSA.
Owner:HUACHENG PHARMA FACTORY GAUNGZHOU
Semiconductor photocatalyst and preparation method thereof
ActiveUS20200156053A1Catalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsHeterojunctionSolar light
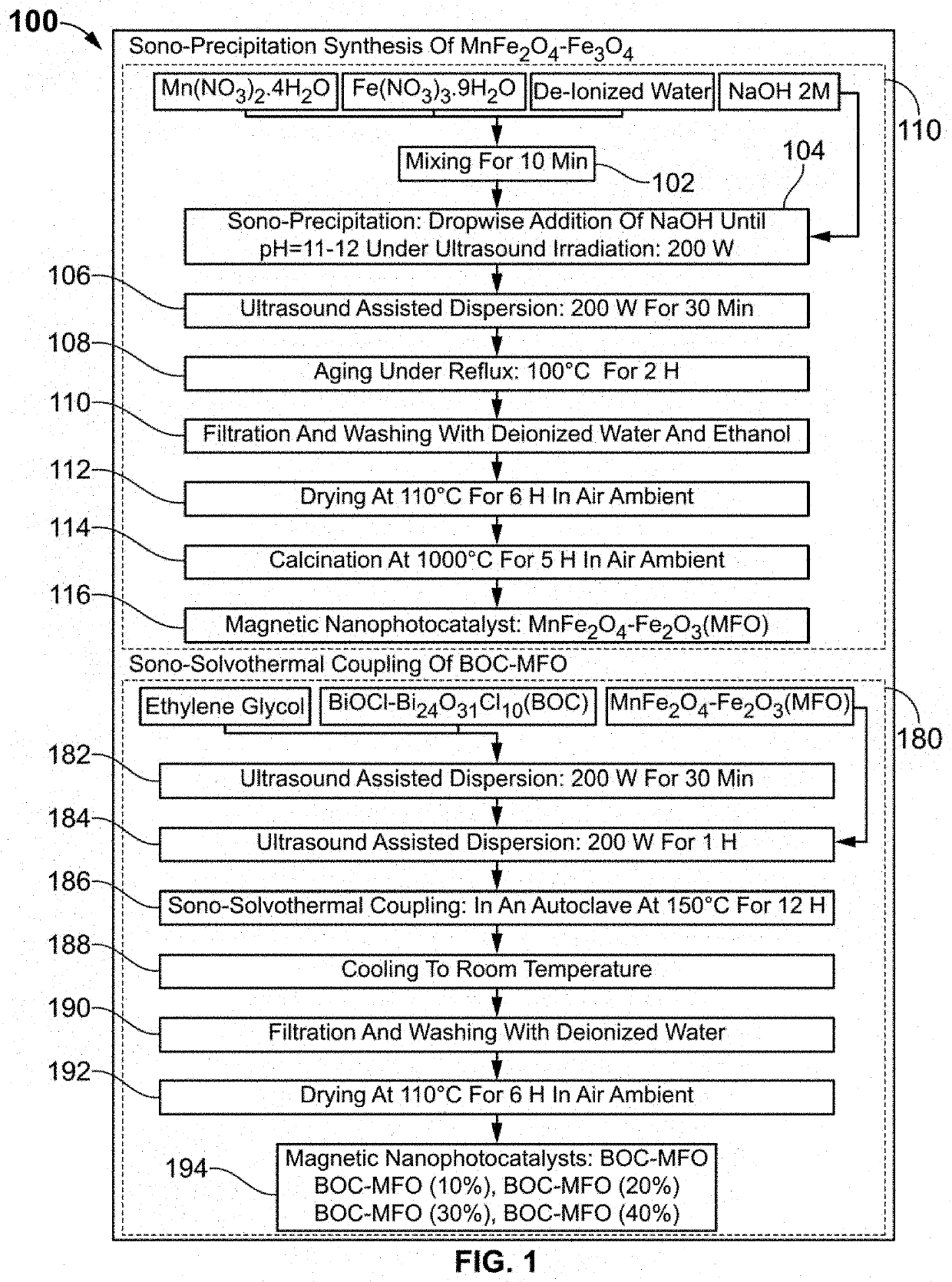
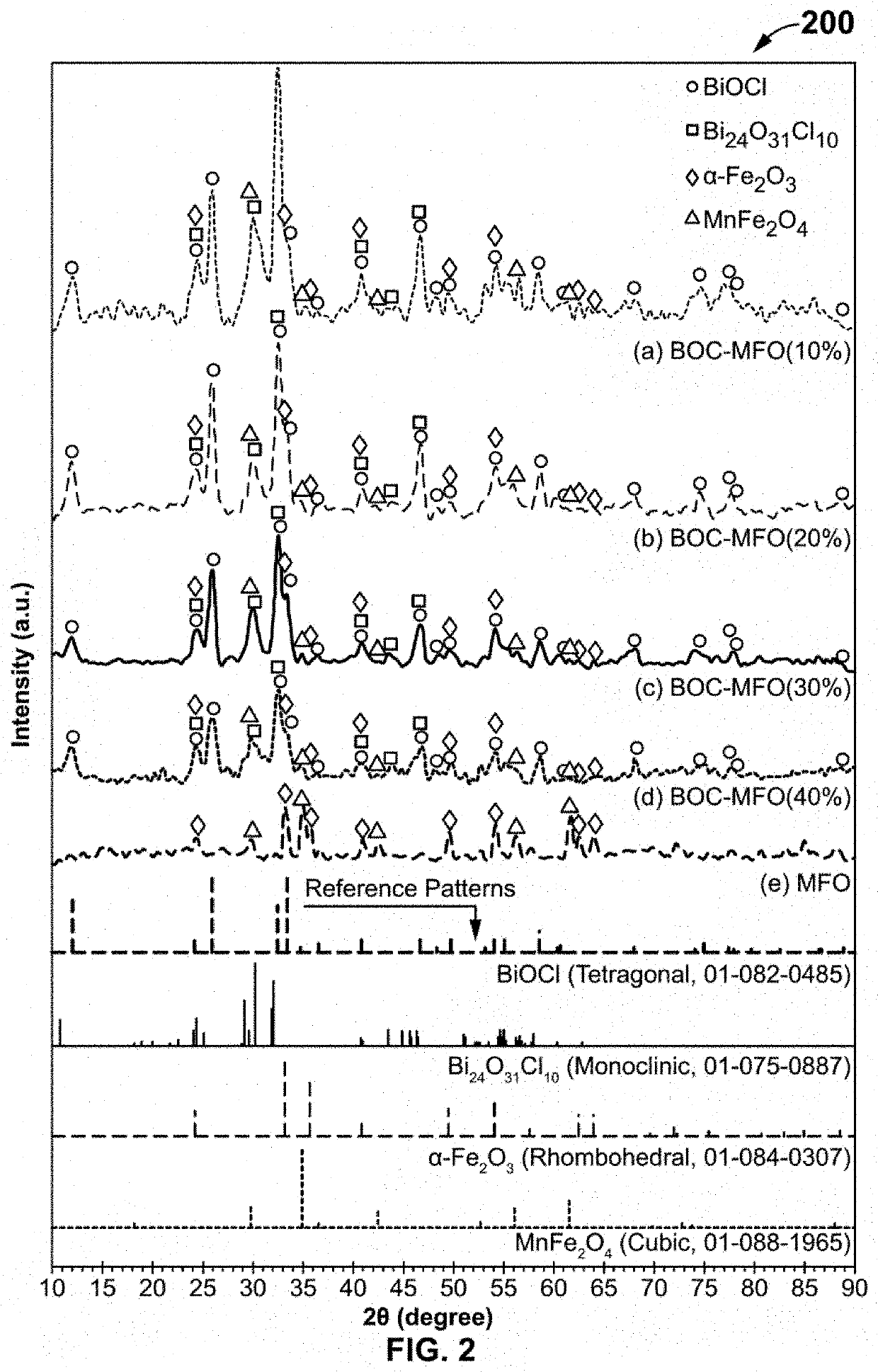
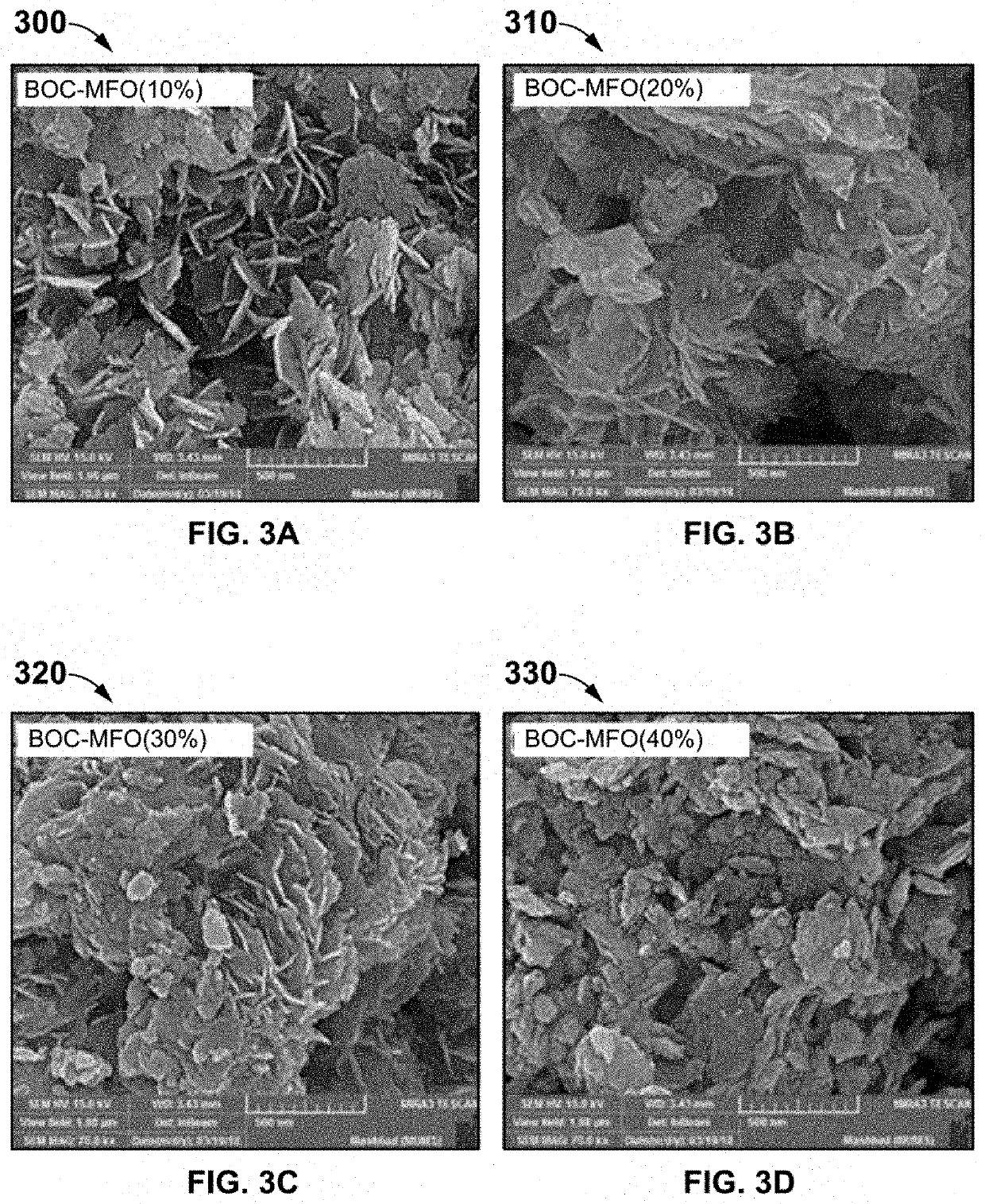
The present invention discloses a novel magnetic BiOCl—Bi24O31Cl10 / MnFe2O4—Fe2O3 semiconductor photocatalyst as a staggered multi-heterojunction nano-photocatalyst for pharmaceutical effluents remediation, and preparation method and use thereof. The semiconductor photocatalysts are at weighted ratios 9:1 4:1, 7:3 and 3:2 of BiOCl—Bi24O31Cl10 and MnFe2O4—Fe2O3 semiconductor. The BiOCl—Bi24O31Cl10 / MnFe2O4—Fe2O3 semiconductor photocatalyst with 10% MnFe2O4—Fe2O3 is a solar light activated photocatalyst for pharmaceutical effluents remediation. The pharmaceutical effluents include ofloxacin antibiotic. The mentioned semiconductor photocatalyst effectively removes the ofloxacin (OFL) antibiotic from polluted aqueous solution under simulated solar light, facilitates separation of photocatalyst from treated aqueous solution using magnetic property, enhances light absorption edge, improves intra-particle mass transfer, increases adsorption capacity and promotes efficient surface reactions, which includes: increasing the light absorption range, increasing quantum efficiency and reducing the recombination phenomenon.
Owner:PARAPARI MOHAMMAD HAGHIGHI +2
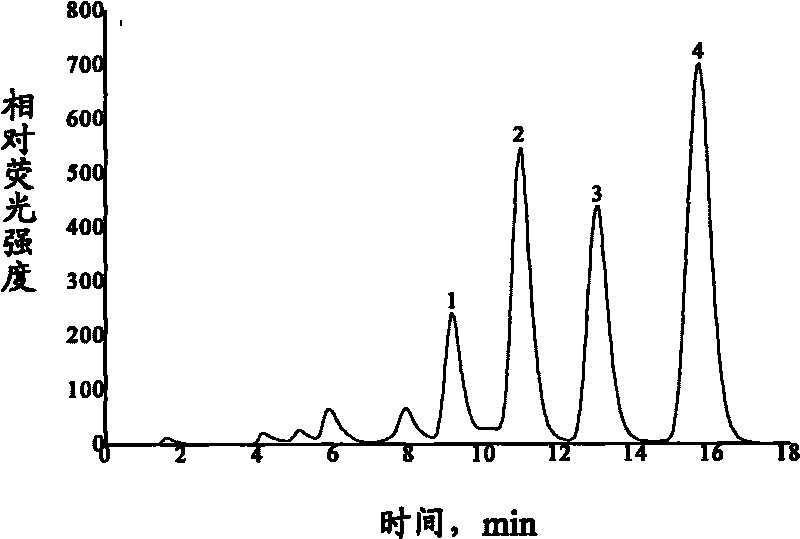
Solid phase extraction and HPLC-fluorescence detection method for fluoroquinolones antibiotics
InactiveCN101696964AMeet the testing requirementsReduce test costsIon-exchange process apparatusComponent separationAntibiotic YNorfloxacin
The invention discloses a solid phase extraction and HPLC-fluorescence detection method for fluoroquinolones antibiotics. The method comprises the following steps of passing a pretreated water sample through an activated solid phase extraction column; eluting the solid phase extraction column with a Na2EDTA aqueous solution; then vacuumizing; and finally, eluting the water sample for three times by a concentrated ammonia methanol solution with a concentrated ammonia volume percent concentration of 6 percent by 0.5-2mL each time. An obtained eluent is blow-dried to 0.1-0.4mL in air flow, water is added to reach a constant column of 1.0mL so as to obtain a test sample, and then HPLC-fluorescence detection is carried out. By adopting the solid phase extraction-HPLC-fluorescence detection technology and utilizing a conventional analytical instrument, the method can simultaneously measure the contents of four typical fluoroquinolones antibiotics of ofloxacin, norfloxacin, ciprofloxacin and enrofloxacin in the water sample. The method has simple operation and low cost and can be applied to the measurement of the ultratrace of four typical fluoroquinolones antibiotics in waste water and surface water.
Owner:ZHEJIANG UNIV
Preparation process of lavo-ofloxacin and ofloxacin
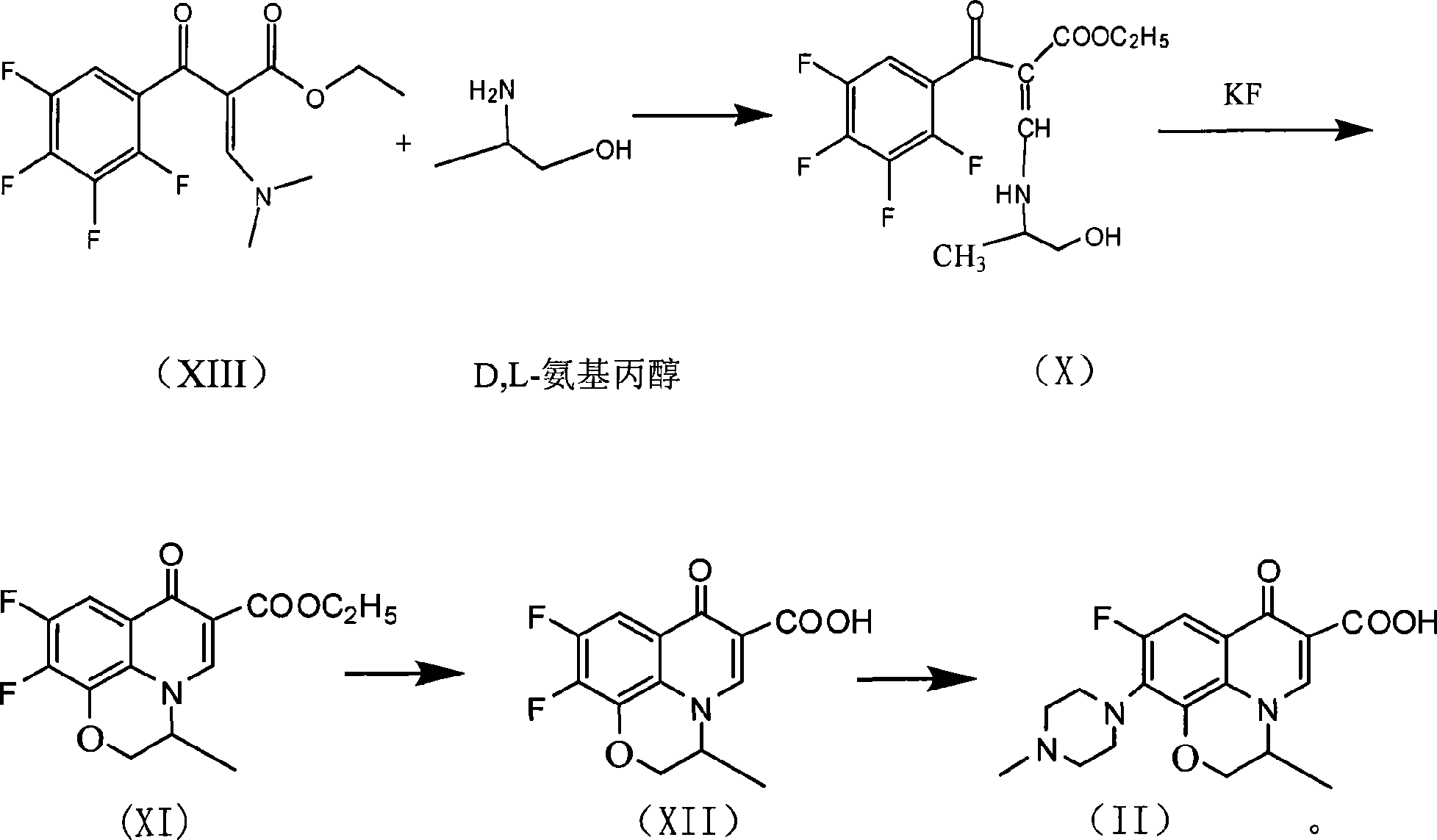
InactiveCN101519361AReduce generationIncrease production levelsOrganic compound preparationAntiinfectivesPotassium fluorideReaction temperature
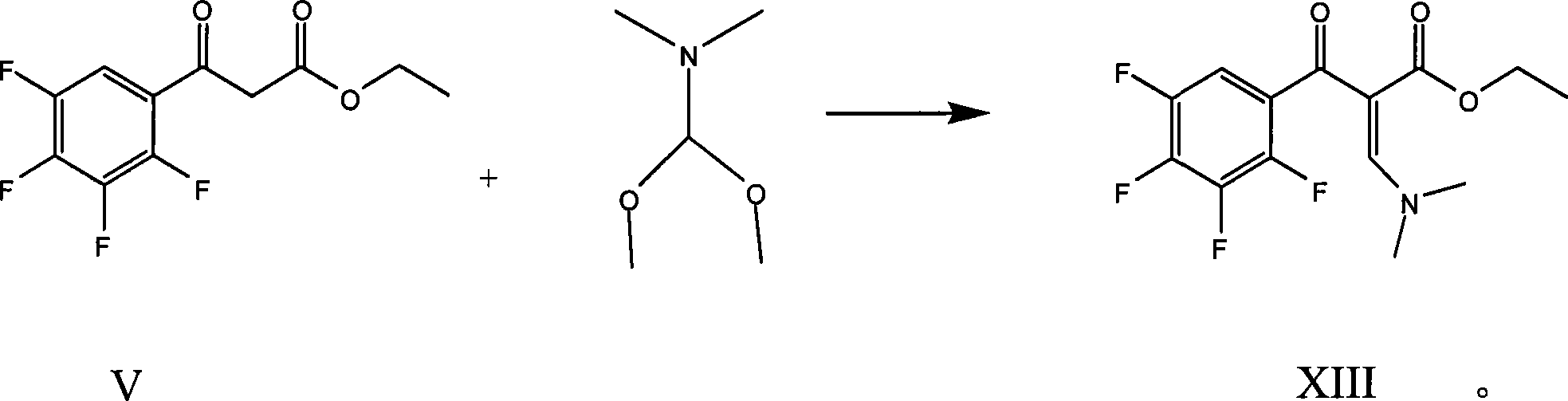
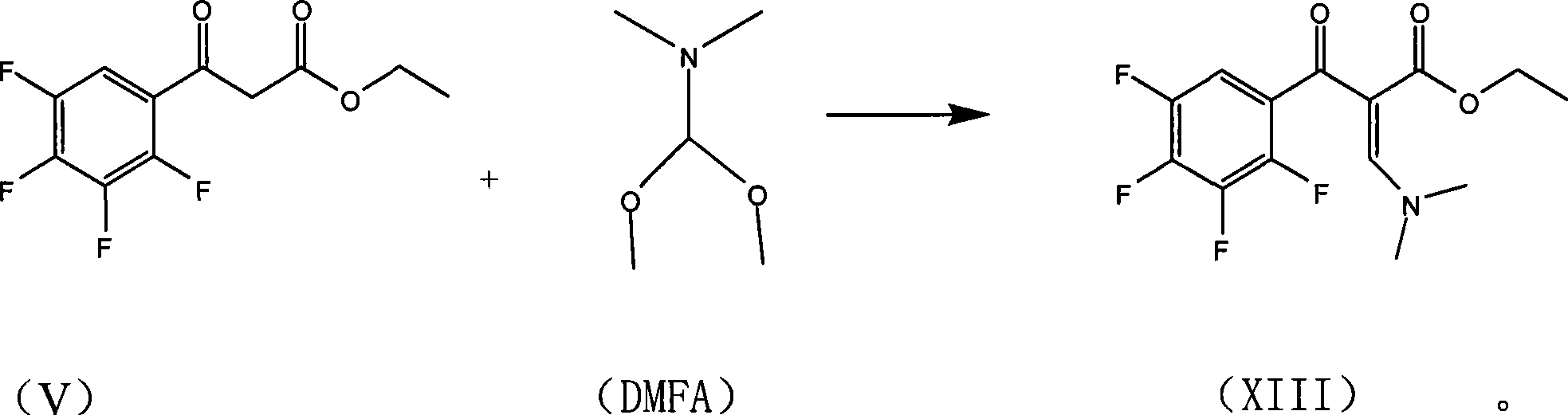
The invention relates to a preparation process of lavo-ofloxacin and ofloxacin which are anti-infectious medicaments, belonging to the synthetic process with tetrafluorobenzoic aid as raw material. The preparation method is characterized in that (2, 3, 4, 5-phenyl tetrafluoride formyl) ethyl acetate and DMFA react for 1.0-1.5h in toluene at 50-55 DEG C with the existence of acylating catalyst; the reaction product is washed by water, and an aqueous layer is separated; at 30-35 DEG C, L-amino propanol is dripped in an oil layer to carry out replacement reaction for 1.5-2.0h; toluene is decompressed, recovered and dried proper quantity of DMF is added to the oil layer for diluting; the diluted oil layer is dripped into back-flow DMF with the existence of anhydrous potassium fluoride to carry out back-flow reaction for 6h; DMF is recovered, water is added for centrifugation, acid is added to the obtained solid to be hydrolyzed to prepare lavo-perfluorocarboxylic acid, the lavo-perfluorocarboxylic acid reacts with N-methyl piperazine in DMSO at 90-110 DEG C by taking triethylamine as an acid-binding agent, and the lavo-ofloxacin is obtained after the fine purification of the product of reaction. The process improves the reaction condition of (2, 3, 4, 5-phenyl tetrafluoride formyl) ethyl acetate and DMFA, lowers the reaction temperature, shortens the reaction time and improves the reaction yield of lavo-fluoro ester serving as a reaction intermediate by 20 percent.
Owner:HENAN TOPFOND PHARMA
ssDNA aptamer for recognizing ofloxacin with specificity and application of ssDNA aptamer
ActiveCN104830866AHigh sensitivityEasy to manufactureBiological testingDNA/RNA fragmentationAptamerGraphene
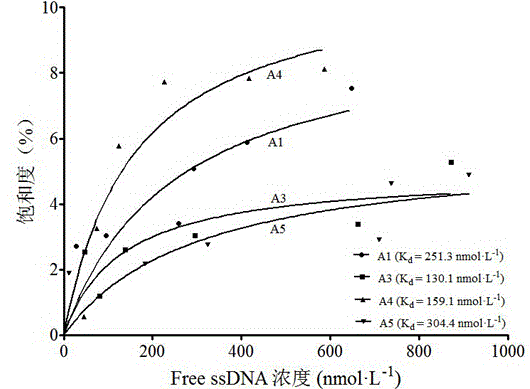
The invention discloses an ssDNA aptamer for recognizing ofloxacin with the specificity and application of the ssDNA aptamer and belongs to the field of biochemistry, molecular biology, analytical chemistry and combinatorial chemistry. According to the ssDNA aptamer and the application thereof disclosed by the invention, a method of screening an ofloxacin aptamer by utilizing a modified graphene oxide-SELEX technology (GO-SELEX) is provided to obtain four ssDNA aptamer sequences A1, A3, A4 and A5 with high affinity with the ofloxacin, so that a detection and recognition element which is good in stability, high in affinity and specificity, low in cost and easy for modification and marking is provided to detect residual ofloxacin.
Owner:JIANGNAN UNIV
Aerosolized fluoroquinolones and uses thereof
ActiveUS20100158957A1Reduce riskHigh levelAntibacterial agentsPowder deliveryAerosol drugsLevofloxacin
Disclosed herein are formulations of fluoroquinolones suitable for aerosolization and use of such formulations for aerosol administration of fluoroquinolone antimicrobials for the treatment of pulmonary bacterial infections. In particular, inhaled levofloxacin specifically formulated and delivered for bacterial infections of the lungs is described. Methods include inhalation protocols and manufacturing procedures for production and use of the compositions described.
Owner:HORIZON ORPHAN LLC
Composite collagen eye drops
ActiveCN1927392AKeep aliveMaintain adhesionOrganic active ingredientsSenses disorderTobramycinAntibiotic Y
Owner:GUANGZHOU TRAUER BIOTECH
Method for detecting antibiotic in environment sample
InactiveCN108318613AEfficient enrichmentRealize detectionComponent separationNorfloxacinSurface water
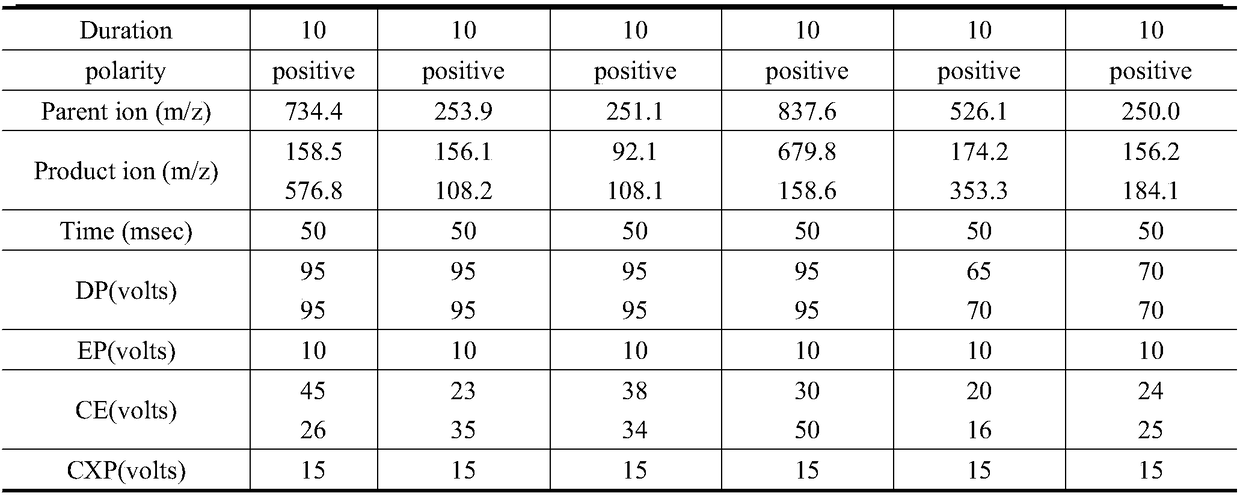
The invention relates to the technical field of environment detection and is suitable for measuring erythrocin, chloramphenicol, sulfamethoxazole, sulfadiazine, roxithromycin, cefotiam, pyridazol, norfloxacin, ofloxacin, tetracycline and doxycycline in sewage, surface water and bottom mud. After being sampled, a water sample and a mud sample are pretreated and detected through ultra-high performance liquid chromatography-triple quadrupole mass spectrometry, and qualitative diagnosis is conducted through the characteristic ion pair (m / z) of a target compound, a standard curve is drawn accordingto the response value and the corresponding concentration, and the external standard method is used in quantification, so that the contents of various antibiotics in the sample are obtained. The method is accurate, sensitive and simple, and is suitable for measuring the contents of 11 typical antibiotics in the sewage, the surface water and the mud at the same time.
Owner:四川国测检测技术有限公司
Method for preparing ofloxacin
ActiveCN101648960AFew reaction stepsShort reaction timeOrganic chemistryOrganic solventCarboxylic acid
The invention discloses a method for preparing ofloxacin. The invention aims to provide a method for preparing ofloxacin, which has short production period, less pollution, high raw material utilization rate, yield and purity, and simple and convenient operation. The method comprises the following steps: preparing 9,10-difluoro-2,3-dihydro-3-methyl-7-O-7H-pyridino[1,2,2-de]-[1,4]- benzoxazinyl-6-carboxylic acid by tetrafluorobenzoyl chloride as a raw material through a compounding method; and reacting with alkali in an organic solvent to obtain the ofloxacin. The invention has the advantages that the tetrafluorobenzoyl chloride reacts with 3-(2-R1-2-R2-4- methylimidazole alkyl) acrylic ester, the product is directly hydrolyzed during post processing and then is carried out the ring close;the reaction step is reduced, the reaction time is shortened, and the yield is as high as 85-90 percent. Organic or inorganic alkali is added in preparation, thereby reducing the feed content of methyl piperazine, reducing the reaction cost and having high yield.
Owner:CHENGDA PHARM CO LTD
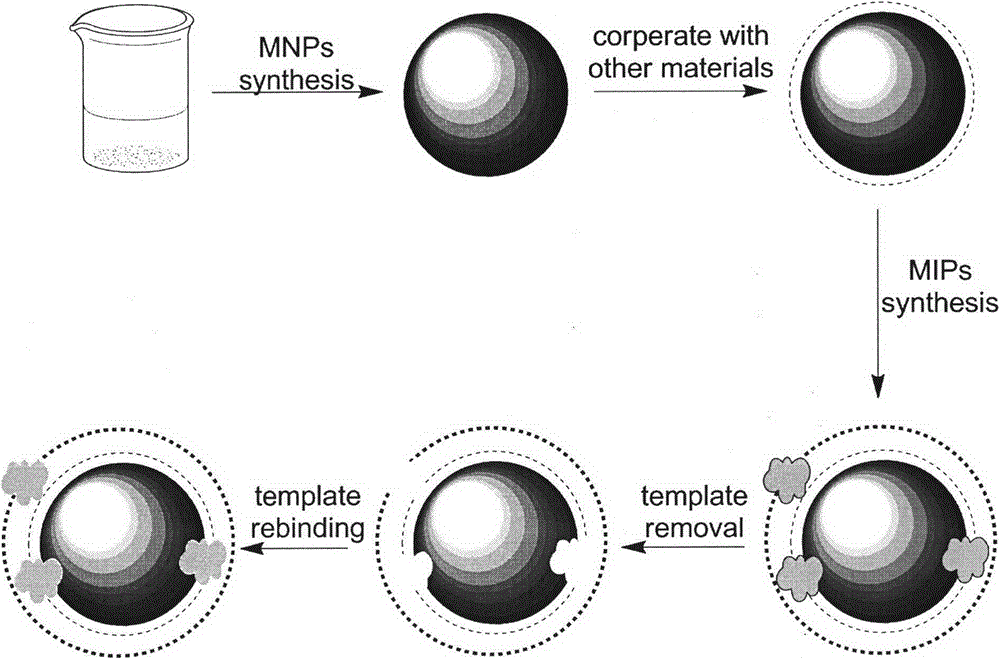
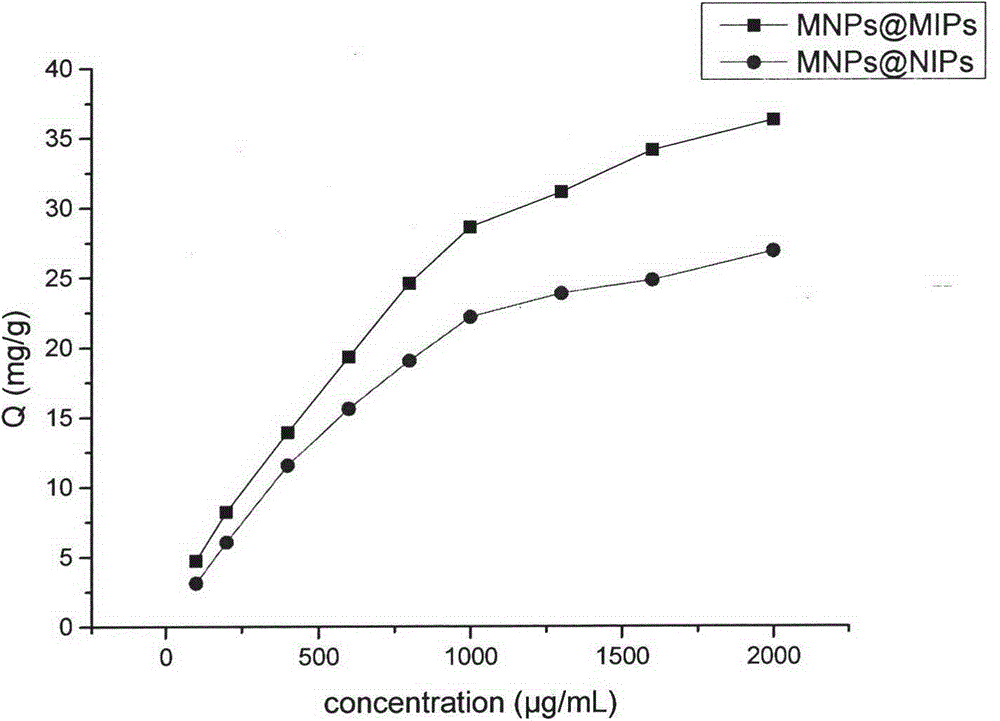
Preparation method of magnetic microspheres-based levofloxacin surface imprinted material
InactiveCN104788612AImprove adsorption capacityGood magnetic responseOther chemical processesAlkali metal oxides/hydroxidesSynthesis methodsMicrosphere
The invention relates to a preparation method of a magnetic microspheres-based levofloxacin surface imprinted material. The preparation process mainly comprises three steps: preparation of magnetic microspheres; coating of the magnetic microspheres with a molecularly imprinted material; and elution of template molecules. A synthetic method provided by the invention is simple. The prepared magnetic microspheres-based surface molecularly imprinted material has specific recognition function, high adsorption capacity and rapid adsorption ability of levofloxacin and has chiral resolution function of ofloxacin, has good magnetic response and high mechanical strength, can be used as an absorption filler or a coating material, also can be used in preparation of a molecular imprinting sensor and a chip, and is of great significance for researches on specific recognition, chiral resolution and highly sensitive detection of floxacin drugs.
Owner:CHINA PHARM UNIV
Ophthalmic preparation of levofloxacin and prednisolone acetate and preparation method thereof
ActiveCN102085203ALess irritatingReduce secretionAntibacterial agentsOrganic active ingredientsOphthalmologyLevofloxacin
The invention relates to an ophthalmic preparation of levofloxacin and prednisolone acetate and a preparation method thereof. Particularly, the invention relates to an ophthalmic preparation containing levofloxacin and prednisolone acetate with an effective amount for treatment and / or prevention, a high polymer material, a surfactant, a complexing agent and water. The invention also relates to anophthalmic preparation containing the ophthalmic preparation provided by the invention and a medicinal excipient mixed with the ophthalmic preparation provided by the invention before application, and a preparation method of the ophthalmic preparation. The ophthalmic preparation provided by the invention not only has a favorable effect of treating eye diseases, but also has very low stimulation to the eyes.
Owner:SHENYANG XINGQI PHARM CO LTD
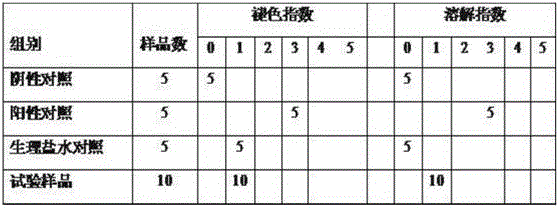
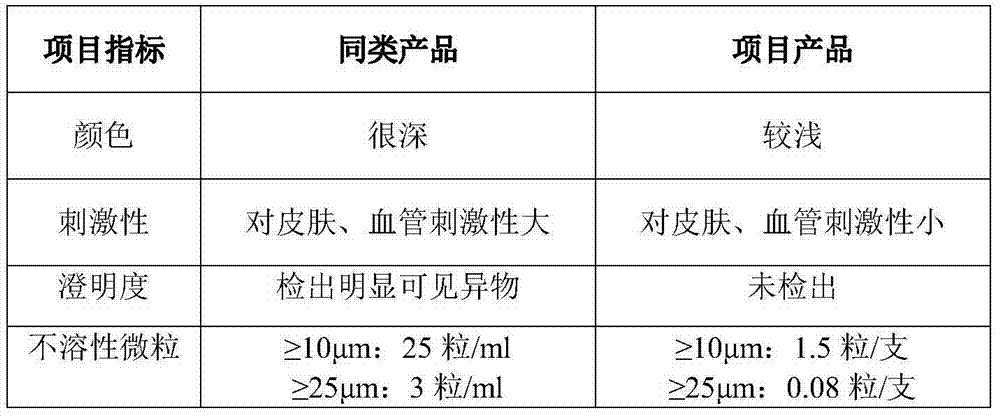
Ofloxacin injection and preparation process thereof
ActiveCN101693008AImprove solubilityPromote crystallizationAntibacterial agentsOrganic active ingredientsDrugs solutionAcetic acid
The invention discloses an ofloxacin injection which is prepared by ofloxacin, acetic acid, disodium tetracemate, propylene glycol and water for injection. The invention solves the problem that an ofloxacin injection hydro-acupuncture is easy to crystallize through adopting the acetic acid as cosolvent and improving the dissolvability of ofloxacin by adding the propylene glycol to regulate the polarity of solution. The injection provided by the invention has excellent quality stability, solves the problem of crystallization commonly existed in products of ofloxacin injection hydro-acupuncture, solves the weaknesses of instability and short retention period of injection, reduces the number of insoluble particles in medicine solution, and provides the effective guarantee for the safe use in clinics.
Owner:ANHUI FENGYUAN PHARM CO LTD
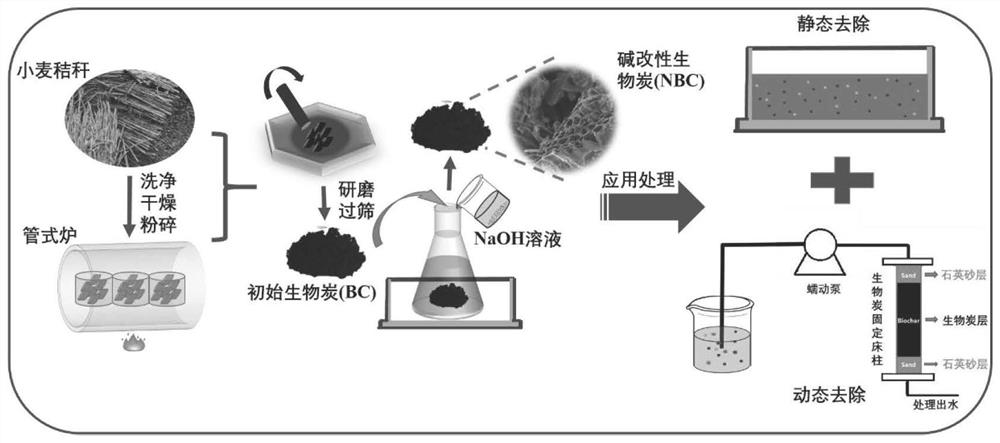
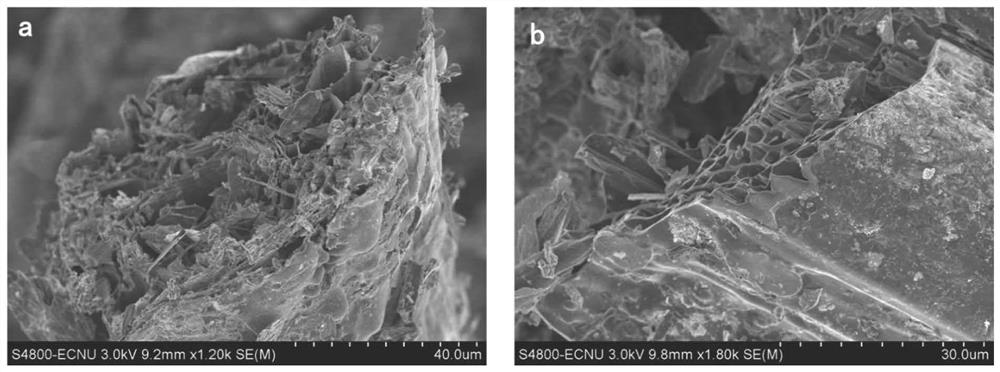
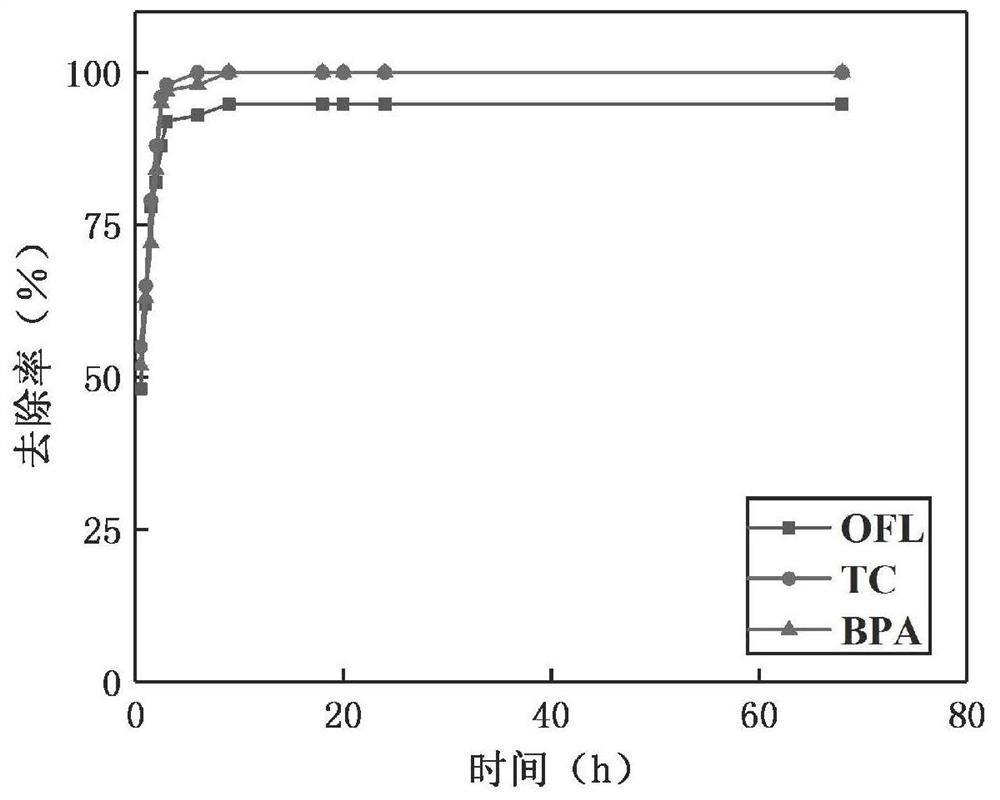
Preparation of alkali-modified biochar and application of alkali-modified biochar in removal of emerging pollutants in sewage
InactiveCN112934175AEfficient removalReduce secondary pollutionOther chemical processesBiofuelsFiltrationCarbonization
The invention discloses alkali modified biochar and a preparation method thereof, and a method for removing emerging pollutants in sewage by using the modified biochar. The method comprises the following steps: preparing initial biochar by using a pyrolysis carbonization method, dipping the initial biochar in an alkaline solution, oscillating and fully contacting, washing with ultrapure water until the pH value is neutral, and carrying out suction filtration and drying to prepare the alkali modified biochar. Compared with the initial biochar, the alkali modified biochar prepared by the invention has larger specific surface area and stronger hydrophobicity, and has higher adsorption capacity and better removal effect on emerging pollutants bisphenol A and antibiotics (tetracycline TC, ofloxacin OFL and the like). Meanwhile, pollutants adsorbed on the alkali modified biochar are not easy to desorb, so that secondary pollution is avoided. In conclusion, emerging pollutants in sewage can be effectively removed, meanwhile, secondary pollution of the alkali-modified biochar after adsorption is little, and the method has the advantages of being easy to operate, efficient, environmentally friendly, capable of saving cost and the like.
Owner:EAST CHINA NORMAL UNIV
Green synthesizing process for ofloxacin
The invention discloses a preparation method for ofloxacin. The prior method uses an organic solvent with higher boiling point and larger polarity, so unit consumption and energy consumption of raw materials are increased and heavy odor is caused; and the prior method adopts other alkaline matters except the reaction raw materials as acid-bonding agents, so solid waste is increased and salt and organic matters in discharged waste water are increased. The preparation method mixes difluorocarboxylic acid, N-methyl piperazine and a catalyst in a reaction solvent for piperazine condensation reaction, obtains a crude product of the ofloxacin after complete reaction and post treatment, and converts the crude product of the ofloxacin into the finished product of the ofloxacin through primary purification treatment; and the reaction solvent is water or a water solution of the N-methyl piperazine. The method takes the water as the reaction solvent and the N-methyl piperazine as the acid-bonding agent, and does not use any other organic solvent and acid-bonding agent to prepare the ofloxacin with good quality and high yield.
Owner:ZHEJIANG JINGXIN PHARMA +1
Aerosolized fluoroquinolones and uses thereof
ActiveUS20100040560A1Overcome resistancePrevent further resistanceAntibacterial agentsBiocideInhalationLevofloxacin
Disclosed herein are formulations of fluoroquinolones suitable for aerosolization and use of such formulations for aerosol administration of fluoroquinolone antimicrobials for the treatment of pulmonary bacterial infections. In particular, inhaled levofloxacin specifically formulated and delivered for bacterial infections of the lungs is described. Methods include inhalation protocols and manufacturing procedures for production and use of the compositions described.
Owner:HORIZON ORPHAN LLC
Medicine-carrying system of vitamin E-modified silicon-base hydrogel contact lens and preparation method of medicine-carrying system
ActiveCN107260655ALong release timeGood biocompatibilityOrganic active ingredientsSenses disorderMethacrylateBiocompatibility Testing
The invention discloses a medicine-carrying system of a vitamin E-modified silicon-base hydrogel contact lens as well as a preparation method and application of the medicine-carrying system. The preparation method comprises the following steps: (1) synthesizing an organosilicone prepolymer of a methacrylate terminal group through hydroxyl modification of the methacrylate terminal group, mixing reaction monomers, putting a mixture into a mold, and carrying out curing by virtue of a one-time mold pressing method, so as to obtain a silicon-base hydrogel contact lens; (2) soaking the prepared silicon-base hydrogel contact lens into a mixed solution of vitamin E and ethanol for modification, so as to obtain a vitamin E-modified silicon-base hydrogel contact lens; and (3) loading ofloxacin of different concentrations with the silicon-base hydrogel contact lens before being modified by vitamin E and the vitamin E-modified silicon-base hydrogel contact lens. The prepared medicine-carrying system of the vitamin E-modified silicon-base hydrogel contact lens is good in biocompatibility, high in light transmittance and relatively good in mechanical property and is capable of prolonging the drug release time.
Owner:南京美材科技有限公司
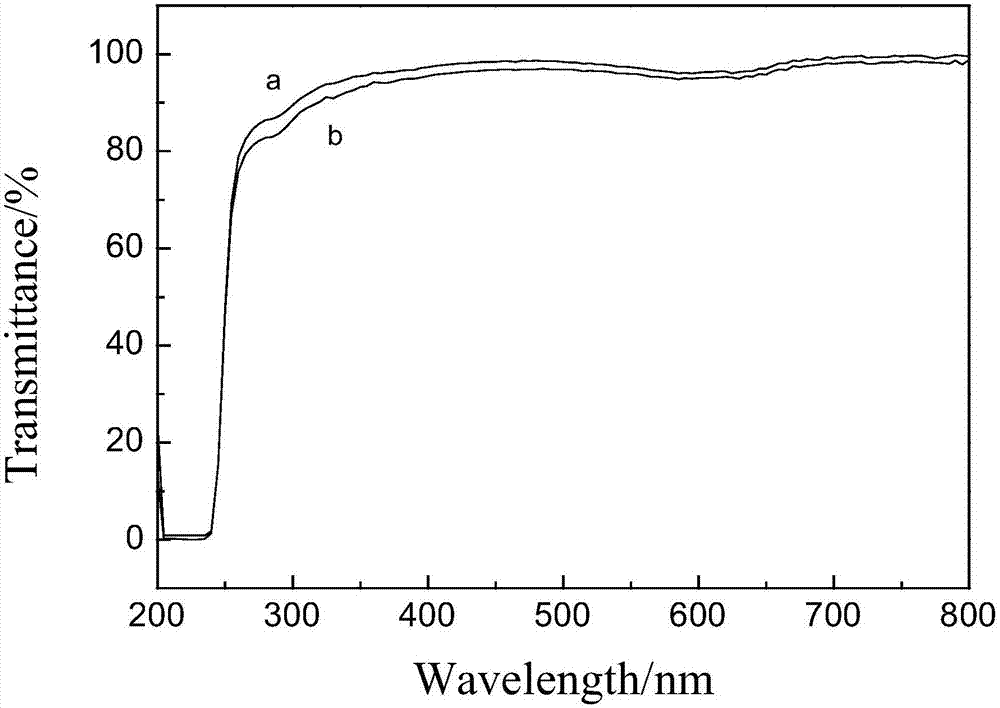
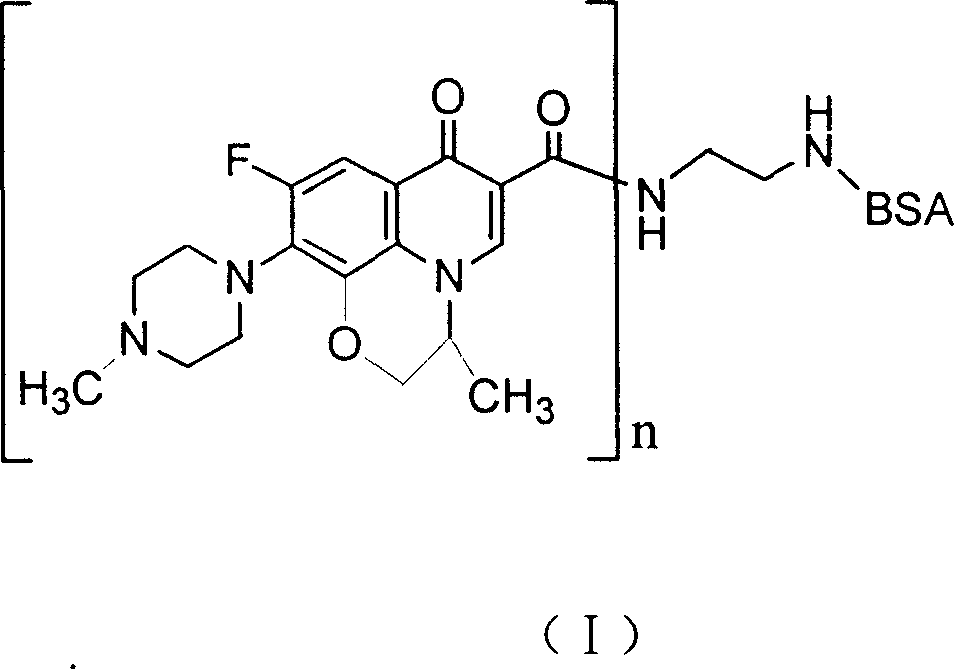
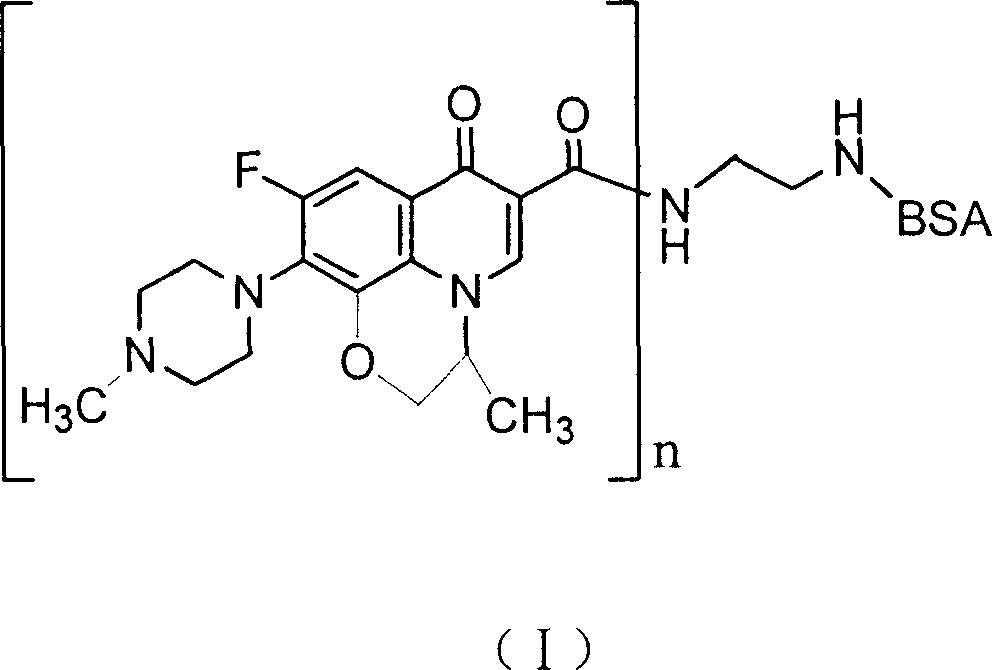
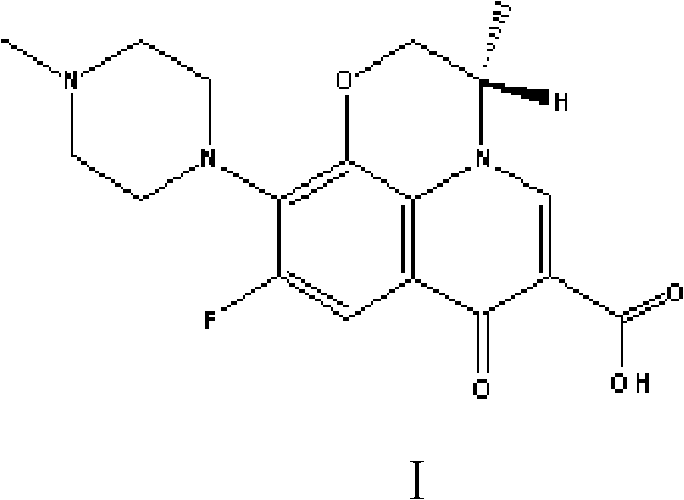
Ofloxacin couple and its preparing method and use
InactiveCN1827642ASimple methodShorten inspection timeImmunoglobulins against animals/humansPeptide preparation methodsBovine serum albuminEnzyme immunoassays
The invention discloses an ofloxacin coupling compound with general formula (I), which comprises coupling compounds of ofloxacin hapten and bovine serum albumin of carrier substance which can produce immunogen or egg albumin. Thereinto, n stands for molecular number of ofloxacin combined with a bovine serum albumin molecule, the said n is an integer between one and twenty, and BSA stands for bovine serum albumin with 6.6KDa-6.9KDa of molecular weight. The invention also discloses a process for preparing the said coupling compound, which consists of joining ofloxacin and carrier substance which can produce immunogen to obtain the said coupling compound which induces immune system of animals to produce antibody. By immuning white rabbits from New Zealand, the ofloxacin coupling compound of this invention has prepared antiserum with 1:512,000 of potency, of which the lowest check limit is 0.1ppb. The invention is characterized in that it is simple, rapid, specific, exact and so on, which provides a foundation for preparing enzyme immunoassay agent box of ofloxacin.
Owner:SHANDONG UNIV
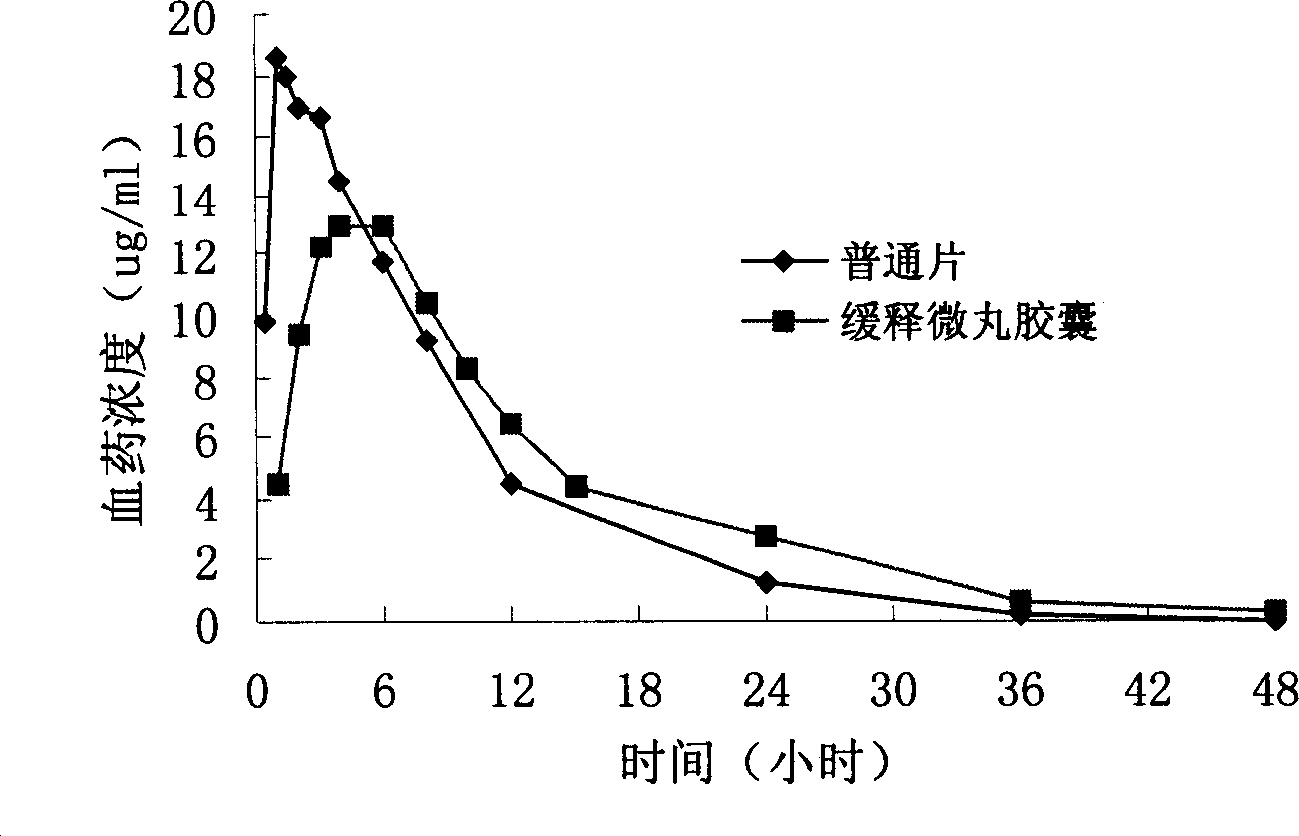
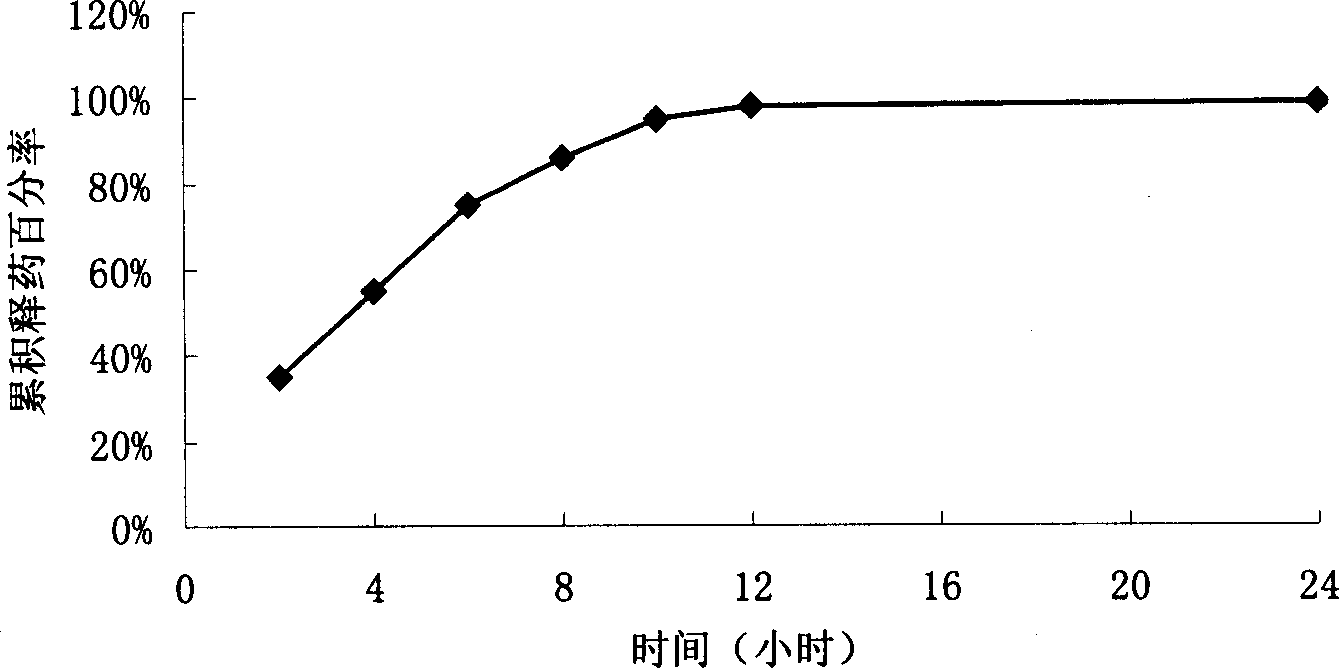
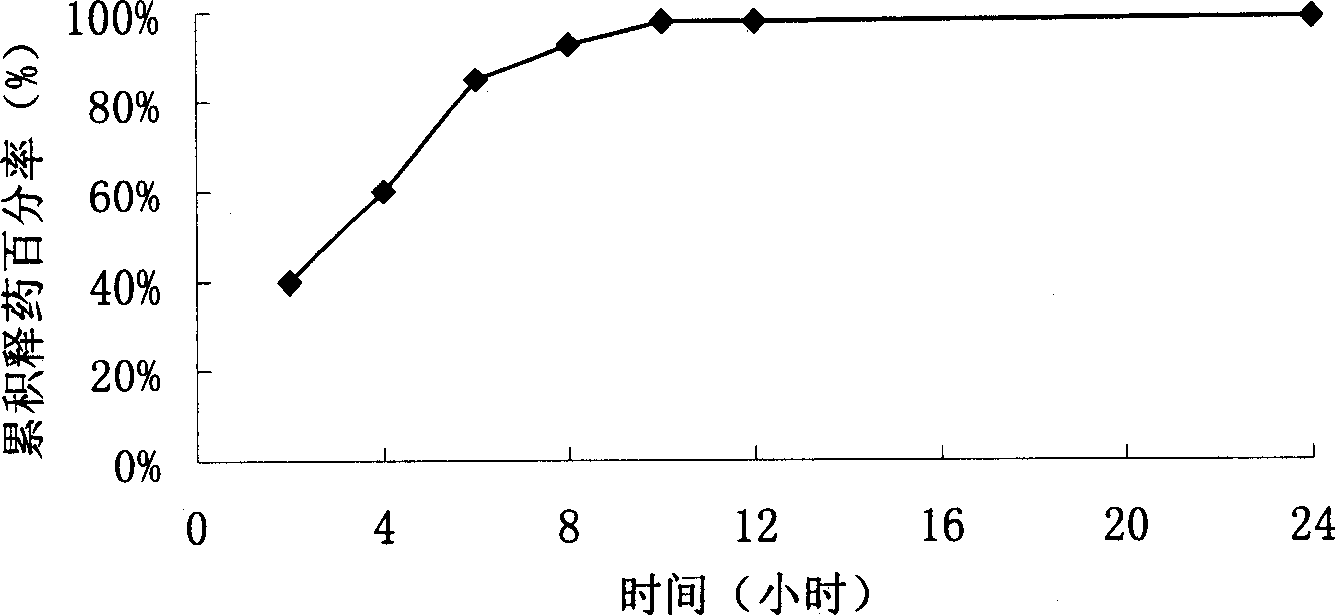
Levofloxacin slow release micropill, its preparation method and uses
InactiveCN1839846AFacilitated releaseReach plasma concentrationAntibacterial agentsOrganic active ingredientsLevofloxacinPharmaceutical formulation
The invention relates to a slow release micro-pellet preparation containing Levofloxacin, its preparation process and therapeutic use, wherein the micro-pellet preparation contains 0-40% of conventional medicinal particles and 60-100% of slow release medicinal micro-pellet, the micro-pellet contains medicinal core including Levofloxacin, and slow release coating layer whose content being 4-100% of the pellet core.
Owner:CHINA PHARM UNIV +2
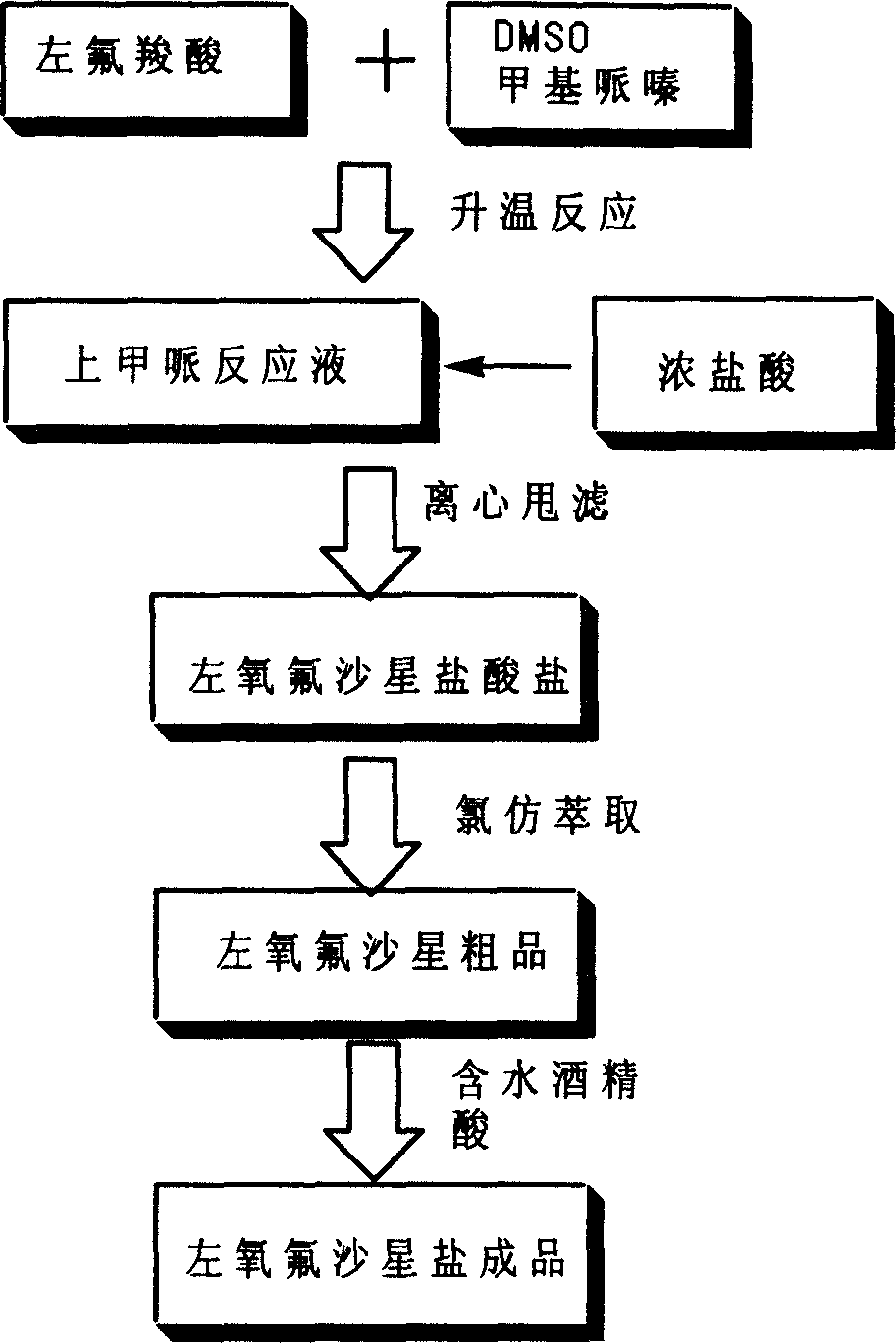
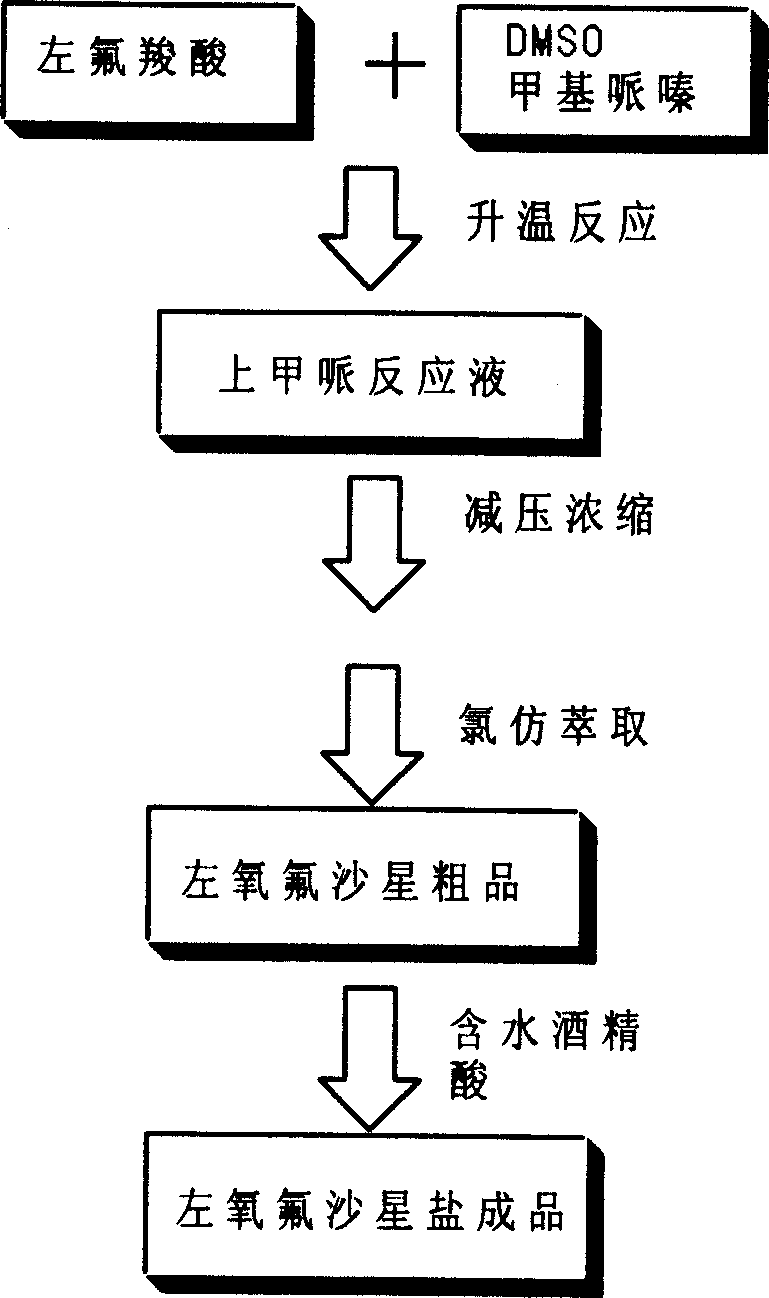
Post processing method for preparing levo-ofloxacin
The present invention provides an after-treatment method for preparation of levofloxacin. Said method is characterized by that after reaction with methylpipie it can directly recover solvent, and adopts the method of firstly salt-forming and then using alkali to make neutralization so as to effectively obtain levofloxacin with high purity.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Ophthalmic or otic and nasal composition containing difluprednate and lavo-ofloxacin and application thereof
InactiveCN101564395AAntibacterial agentsOrganic active ingredientsInfective rhinitisInfectious Rhinitis
The invention provides an ophthalmic or otic and nasal pharmaceutical composition, which comprises lavo-ofloxacin and salts thereof and difluprednate, wherein the weight ratio of the difluprednate to the lavo-ofloxacin is 1:1-1:10. The ophthalmic or otic and nasal medicament composition is applied to treating conjunctivitis, keratitis, eyelid inflammation, dacryocystitis, hordeolum, corneal ulcer and eye infections with inflammations of eyes or surrounding tissues, or preventing increased risk of bacterial infections and tissue inflammations of infected parts after ophthalmic surgeries or eye injuries, or treating or relieving the bacterial infections combined with tissue inflammations of the infected parts after the ophthalmic surgeries, or treating otitis media, otitis externa and infectious rhinitis.
Owner:SHANDONG INST OF PHARMA IND
Levofloxacin hydrochloride sustained-release eye drops
InactiveCN106236706AAvoid pollutionReduce risk of damageAntibacterial agentsOrganic active ingredientsSodium hyaluronateEye drop
The invention discloses levofloxacin hydrochloride sustained-release eye drops which are ophthalmic preparations prepared by using levofloxacin hydrochloride as a pharmacodynamic raw material, matching with sodium hyaluronate to play a role of a thickener, and then matching with a metal ion complexing agent pharmaceutically acceptable to local parts of eyes, an osmotic pressure regulator and a pH regulator, wherein per 100 parts by weight of a finished product preparation contains 0.30-1.00 part by weight of levofloxacin hydrochloride and 0.1-1.00 part by weight of sodium hyaluronate. The medicine liquid provided by the invention has high viscosity, prolongs the time of medicine staying in the eyes, improves the absorption of the medicine, and improves the bioavailability of the eye drops; no preservative is added to the eye drops provided by the invention, so as to improve the biological safety of the eye drops.
Owner:GUANGDONG WHOLEWIN TECH
Levofloxacin hydrochloride tablet and preparation method thereof
ActiveCN103520124AReduce alkalinityGuaranteed not to precipitateAntibacterial agentsOrganic active ingredientsAlkalinityAlcohol
The invention discloses a levofloxacin hydrochloride tablet and a preparation method of the levofloxacin hydrochloride tablet. The preparation is prepared by blending and tabletting medicine-containing particles, citric acidenteric particles and a lubricant, wherein the citric acidenteric particles is prepared by dissolving the citric acid and the polyvinyl acetatephthalic acid ester in ethyl alcohol. According to the invention, the citric acid is wrapped in an enteric material and is not dissolved in the stomach, the citric acid is dissolved and released in the intestines, so that the alkalinity of the intestinal juice is reduced, the levofloxacin hydrochloride is enabled not to be separated out, and therefore, the bioavailability of the medicine is improved.
Owner:NANJING REDWOOD FINE CHEM CO LTD
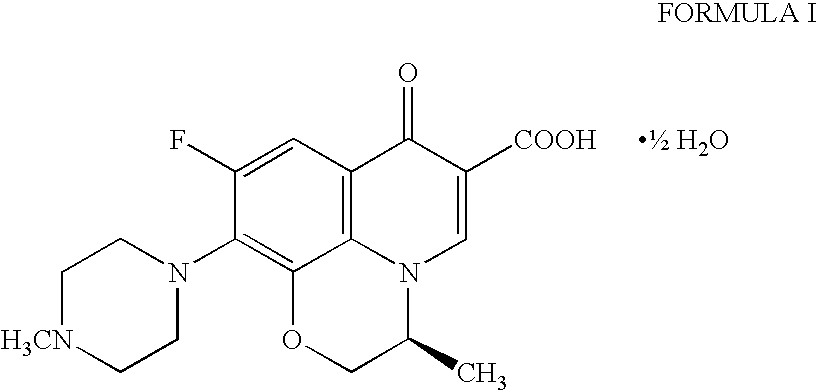
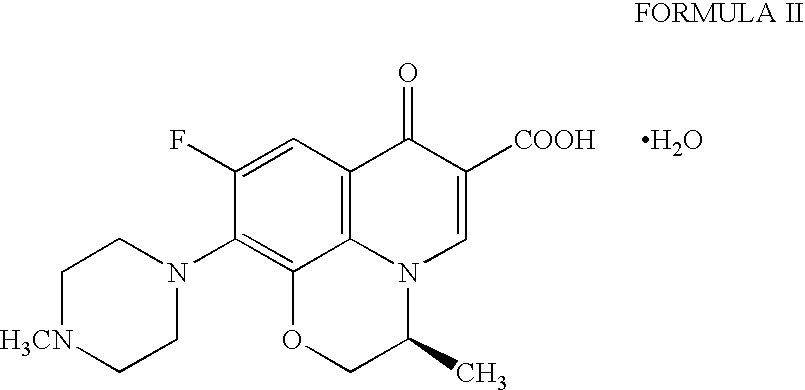
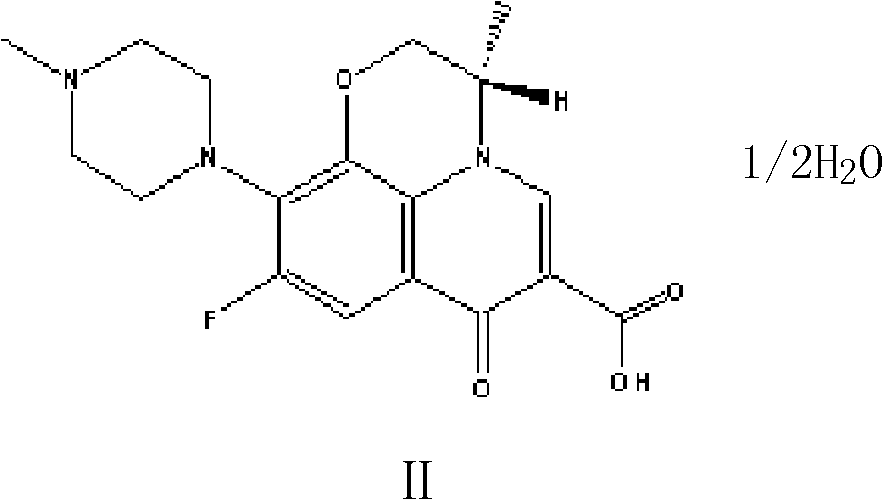
Preparation method of high-purity Levofloxacin semihydrate
The invention provides a preparation method of high-purity Levofloxacin semihydrate, which belongs to the technical field of the medicine synthesis, and solves the problems of the present preparation method that the prepared Levofloxacin semihydrate is difficult to recycle and has low purity and the used organic solvent is difficult to degrade. The preparation method comprises the following steps: a. preparing Levofloxacin salt; b. preparing Levofloxacin crude product II; c. preparing Levofloxacin semihydrate. The Levofloxacin semihydrate prepared by the preparation method has the advantages of high purity, good quality and appearance, high titration content, high recycling rate, low cost and good environmental-protection performance.
Owner:浙江东亚药业股份有限公司
Method for preparing levofloxacin hydrochloride sodium chloride injection
InactiveCN103479522ASubstance reductionHigh clarityAntibacterial agentsOrganic active ingredientsWater bathsSodium Chloride Injection
The invention provides a method for preparing levofloxacin hydrochloride sodium chloride injection. By researching a liquid medicine preparation method, medicinal charcoal pretreatment and a terminal sterilization process, product quality is ensured by the aid of technologies of a concentrated solution and diluted solution method, charcoal slurry preparation from medicinal charcoal, water bath sterilization and the like, production is facilitated, obtained finished products have higher stability, so that the storage life of the finished products can be prolonged, and clinical use effects are better.
Owner:HAINAN HOTMED TIANYA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com